-
工业废水中有毒重金属镉对水体的污染是一个世界性的环境问题。快速的工业化严重地促进了有毒重金属镉向河流的释放。与有机污染物不同,重金属镉不可生物降解,且可以持续地积聚在生物体内,对人体及其他物种造成严重的伤害,如众所周知的痛痛病。因此,必须在工业废水排出之前将水体中的镉移除[1-3]。我国钢铁工业水污染物排放标准(GB 13456-2012)规定,镉离子排放前浓度须低于0.1 mg·L−1[4]。截至目前,有多种物理化学技术被用来去除水中重金属离子,包括化学沉淀、离子交换、化学氧化/还原、反渗透、膜技术和吸附等。在这些技术中,吸附技术由于具有快速、高效、易于操作、成本低、吸附剂种类多和适用性强等特点,得到了广泛的应用[5-7]。其中农业和工业固体废物[8-11]、蒙脱土和高岭土[12]、壳聚糖[13]、高分子材料[3, 14]等吸附材料都显示了较好的从废水中吸附汞、铅、铜等重金属离子的能力,但对镉的去除往往表现不佳。如何更高效地去除重金属镉,开发新型的镉吸附剂,还需要深入的研究。
随着20世纪末纳米技术的发展,基于纳米材料吸附重金属离子的新技术被广泛应用[15-16]。纳米吸附剂具有比表面积大、特异性强、反应活性高、操作简单、无内扩散阻力等优点,是一种去除水溶液中重金属离子的高效吸附剂[17-18]。近年来,含硫化合物已被广泛用于去除重金属[19-20],因其原料来源广泛,廉价易得,且易于合成,在厌氧环境中处理效率高等优点在治理地下水和土壤重金属污染问题上引起广泛关注[21]。其中已有研究报道硫化亚铁可有效去除重金属[22],但它具有热力学稳定性差,容易遭受腐蚀并且对镉的吸附量较低等缺点[23]。而硫化锰(MnS)作为重要的金属硫化物,具有较强的还原能力,且光催化量子效率相比其他材料显著增大,是极具潜力的半导体材料[24-25]。然而,到目前为止,尽管对于纳米MnS的制备及其性质已有许多报道,但尝试使用MnS去除Cd2+的研究还鲜有报道。而且有研究显示Mn2+的存在会抑制水稻根部对于Cd2+的吸收[26]。因此,研究MnS对Cd2+的去除潜力是十分必要的。
本研究首先采用共沉淀法厌氧合成了MnS纳米颗粒,并对其进行表征,探讨了MnS纳米粒子对于水中重金属Cd的吸附性能,包括MnS对于水中重金属Cd的去除效率、吸附动力学和吸附等温线,最后探讨了MnS的除镉机制,为MnS在镉污染环境修复中的应用提供参考。
-
本研究中用到的四水合硝酸镉、一水合硫酸锰、九水合硫化钠、氢氧化钠(98%)、浓硝酸(68%)等试剂来自于国药集团化学试剂有限公司,以上试剂均为分析纯。
-
参照共沉淀法[17],在厌氧条件下制备硫化锰纳米材料。在52.5 mL去离子水中持续曝纯度为99%的氮气30 min,去除水中的溶解氧。随后用曝过氮气的去离子水制备MnSO4和Na2S溶液。在氮气全程曝气的状态下,取10 mL化学计量浓度为0.043 mol·L−1的MnSO4溶液于三口烧瓶中,使用分液漏斗滴加5 mL化学计量浓度为0.085 mol·L−1的Na2S溶液,并通过磁力搅拌器混合溶液。合成后,使用去离子水洗涤3次,并用高速离心机分离固体和液体,在60 ℃条件下真空干燥24 h后,研磨得到MnS纳米颗粒。
-
通过批量吸附实验分别研究了吸附动力学、吸附等温线和混合阳离子对吸附效果的影响。(25±1) ℃下,MnS纳米颗粒投加量为0.1 g·L−1。吸附动力学实验中镉初始浓度为60 mg·L−1,吸附等温线实验中镉初始浓度为10~600 mg·L−1,在探究混合阳离子对吸附的影响实验中,使用了初始浓度为1 mg·L−1且包含了Cu2+、Pb2+、Cd2+、Ca2+和Mg2+的混合阳离子溶液。用0.1 mol·L−1 HNO3和NaOH调节溶液pH为6.0±0.1。将悬浊液置于超声波水浴振荡器(80 kHz)中5 min,随后在恒温振荡箱(120 r·min−1)中磁力搅拌。每隔一定时间,吸取5 mL溶液,待反应结束后,离心吸取5 mL上清液,用0.22 µm的微孔滤膜过滤,测定各离子浓度。所有吸附实验均重复3次。
-
X射线衍射(XRD)用于分析样品的晶相,采用X射线衍射仪(X'Pert Powder PANalytical,荷兰),Cu Kα射线(λ=0.154 nm,40 kV,40 mA),扫描角度为5°~90°,扫描速度为0.13(°)·s−1。将硫化锰纳米颗粒填充到样品槽中,并用压制法将表面压平,用盖玻片除去多余的粉末后进行测定。
激光粒度分析仪(Mastersizer 2000)通过对颗粒群的衍射,反映出各颗粒级的分布丰度,测定主要粒度大小。
使用ASAP 2020装置(Micrometrics,USA),在−196 ℃下,测量氮气(N2)吸附解吸等温曲线来确定硫化物纳米颗粒的孔径、孔体积和表面积。通过分析N2等温线的数据并且基于Brunauer-Emmett-Teller(BET)方法计算其比表面积。孔体积由相对压力为0.95时吸附的氮量确定。平均孔宽度由Stoeckli和Ballerini提出的方程[27]计算。
使用带有EDS元素分析功能的高分辨透射电子显微镜(JEM-2100,日本)观察硫化锰纳米颗粒的吸附前后的晶体尺寸形貌和元素组成。透射电镜样品制备如下:称取约1 mg样品于20 mL乙醇中,在80 kHz下超声30 min后,取一滴上清液置于铜网的碳膜上,常温下在空气中干燥。
-
1)扫描电镜和能谱分析。MnS纳米颗粒的扫描电镜如图1所示。可以看出,MnS纳米颗粒呈球状,尺寸较小,大小不一,平均粒径为100 nm。由粒度分析得出,MnS纳米颗粒的主要粒度分布在169.8 nm,这与扫描电镜呈现的结果一致。从MnS纳米颗粒的能谱分析可以得出,除合成过程中无法完全避免的氧原子外无其他杂质。元素O、S、Mn的原子百分比分别为30.78%、26.27%、42.95%。
2) BET比表面积和孔径大小分析。MnS纳米颗粒具有比较大的BET比表面积(30.56 m2·g−1),平均孔体积(0.14 cm3·g−1)以及平均孔径(37.39 nm)。纳米颗粒的BET比表面积越大,表示活性位点越多,其吸附能力就越强。同时,孔径越大,水蒸气分子扩散阻力就越小,同时颗粒的大孔径也有利于吸附过程中放出的热量扩散到环境中,从而有利于吸附过程的进行[28]。
-
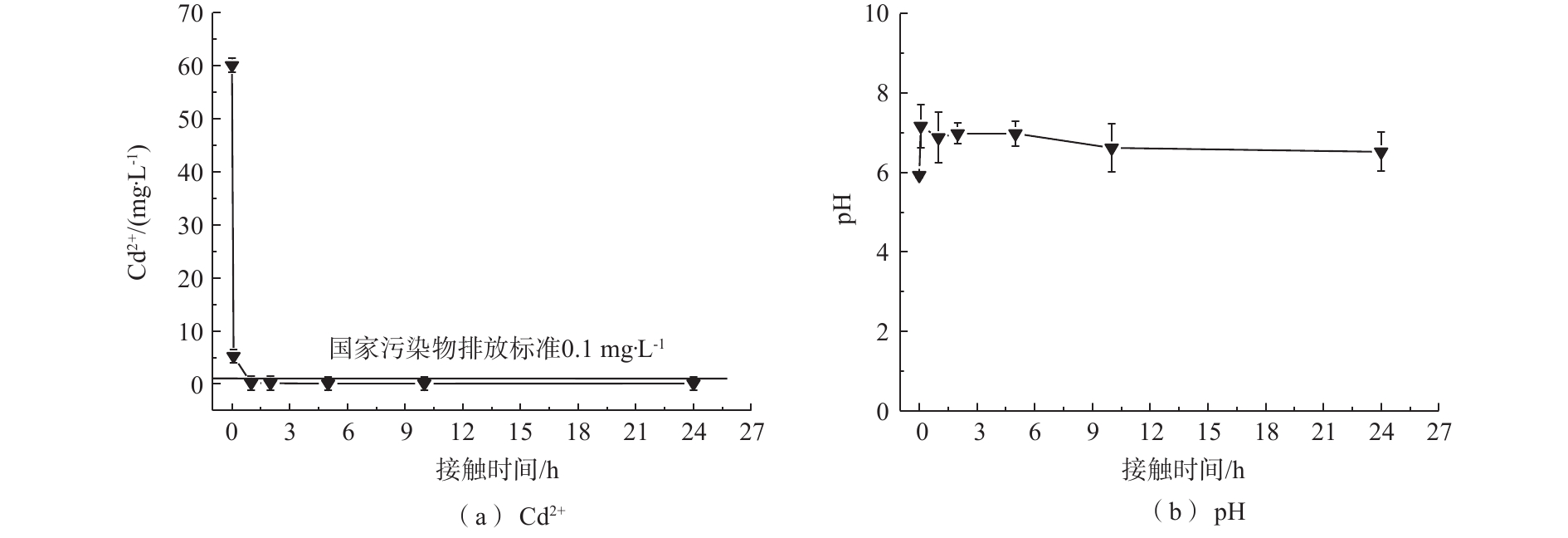
1)吸附时间对镉去除率的影响。为模拟工厂废水浓度[29],在Cd2+初始浓度为60 mg·L−1、吸附剂投加量为1 g·L−1的条件下,MnS纳米颗粒对于Cd2+的去除率如图2(a)所示。可以看出,MnS纳米粒子对于Cd2+的去除率均随着时间的增加而增加,Cd2+在溶液中的浓度快速下降并于5 h内达到我国钢铁工业水污染物排放标准(<0.1 mg·L−1),后期趋于缓慢直至平衡。MnS在初始浓度为60 mg·L−1的条件下的平衡时间为10 h。在MnS处理下的Cd2+浓度达到平衡状态时为0.08 mg·L−1。从图2(b)可以看出,MnS在反应过程中pH稳定,对水体环境扰动较少。
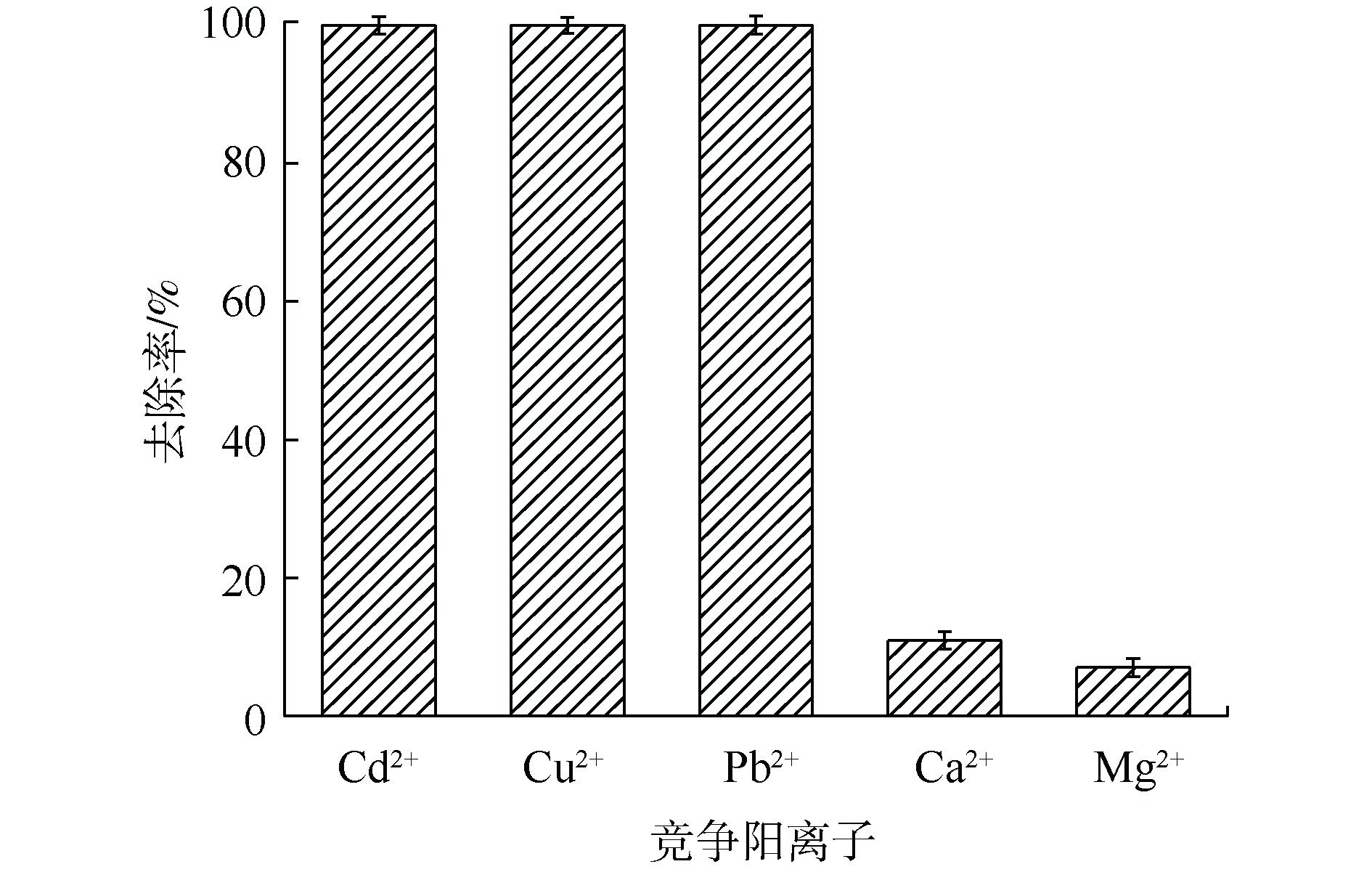
2)混合金属阳离子对镉吸附效率的影响。在实际应用中,废水中会含有多种不同竞争阳离子(如Cu2+、Pb2+、Ca2+和Mg2+等),因此,探究竞争离子对于吸附效率的影响尤为重要。如图3所示,在多种竞争阳离子共存条件下,MnS纳米颗粒对Cd2+在反应后依然可以取得接近100%的去除率,并且可以同时去除多种重金属离子。而在自然界中,大量存在的Ca2+和Mg2+对MnS纳米颗粒去除水溶液中重金属离子的影响较小,这有利于MnS纳米颗粒在实际环境中的应用。
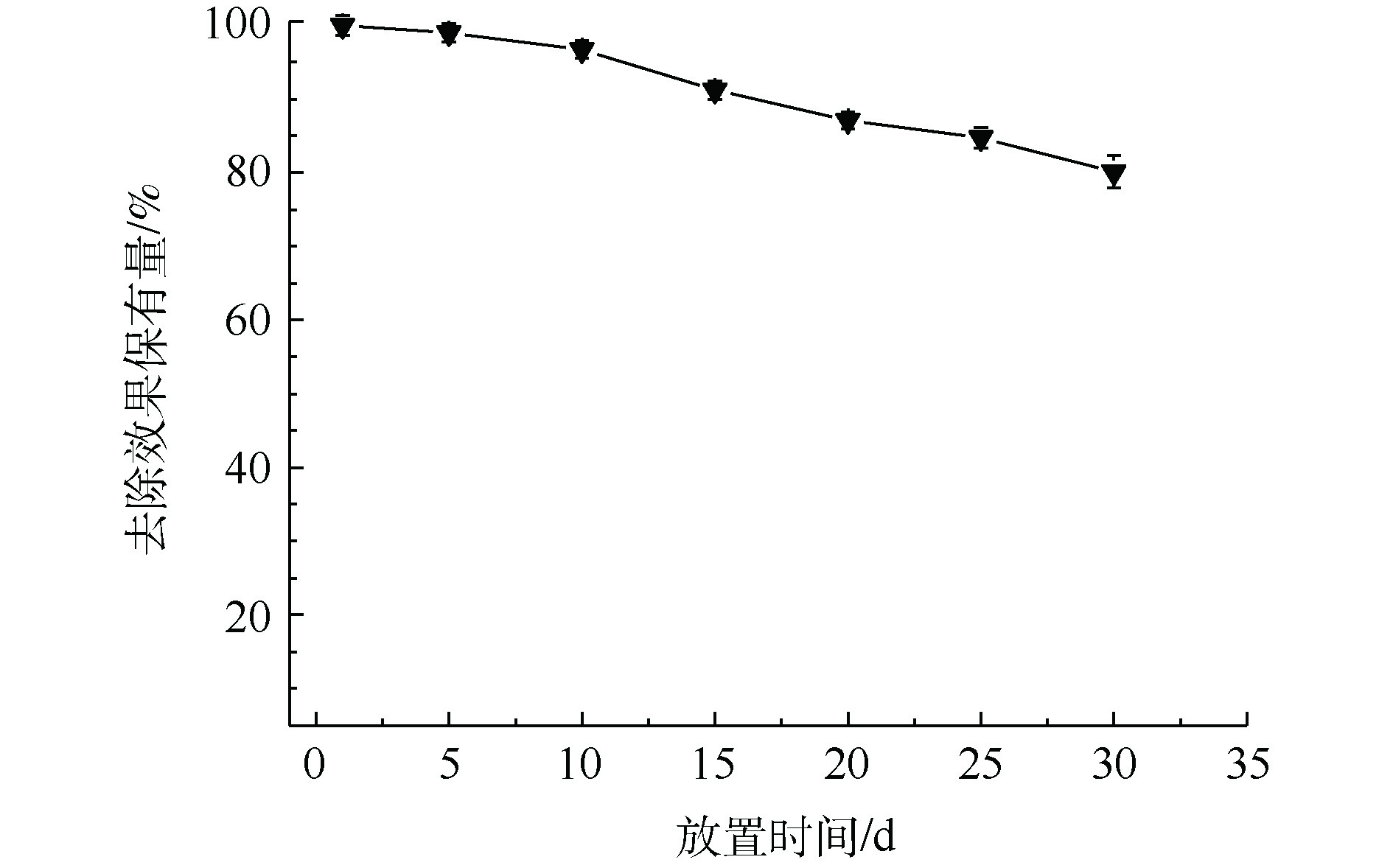
3) MnS纳米颗粒稳定性分析。Mn为过渡金属,MnS在空气中容易被氧化,从而影响去除效果。如图4所示,将MnS纳米颗粒在空气中放置30 d后,其对于Cd2+的去除率缓慢下降。与新鲜合成的MnS纳米颗粒相比,在空气中放置了30 d后的MnS纳米颗粒依然保有80%左右的去除能力。
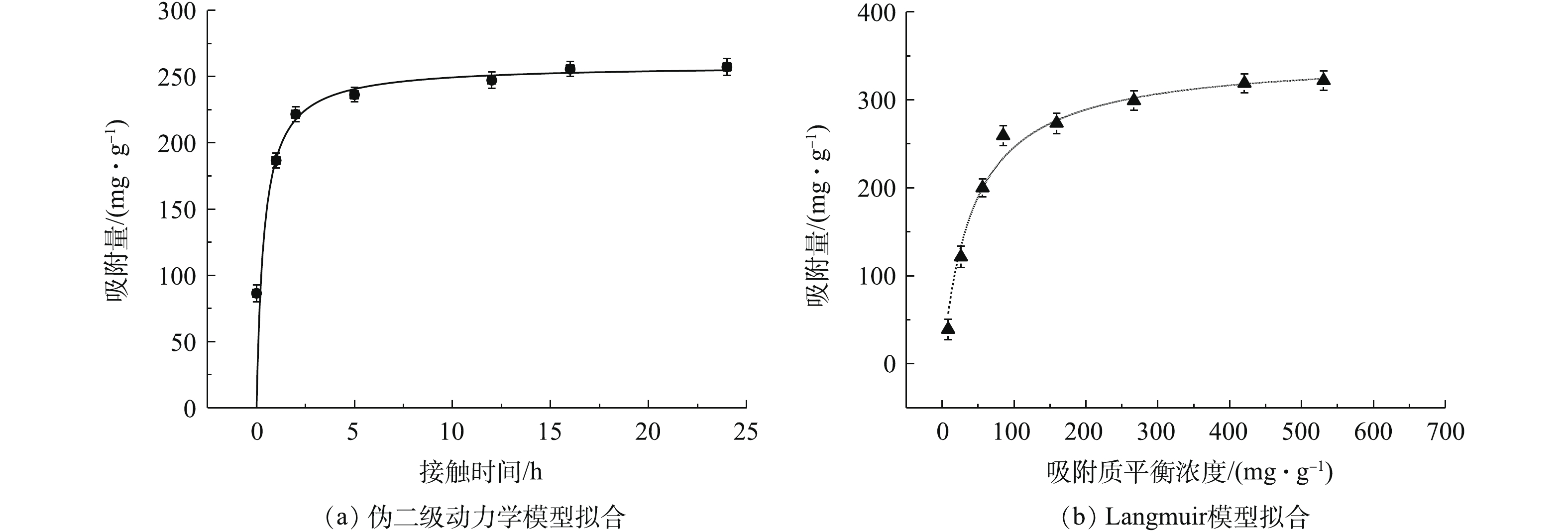
4)吸附动力学和等温线分析。实验结果采用伪一级和二级动力学模型进行拟合,方程见式(1)和式(2)。
式中:k1、k2为伪一级、伪二级吸附速率常数,g·(mg·h)−1;qt、qe为t时刻、吸附反应平衡时反应材料对金属离子的吸附量,mg·g−1;t为反应时间,h。将反应时间为24 h的MnS纳米颗粒去除Cd2+的实验结果与2种动力学模型拟合,并计算出各个吸附动力学模型的参数,结果见表1。
由图5和表1可知,MnS纳米粒子吸附剂与伪二级动力学的线性拟合度较好,对镉的吸附动力学行为较符合伪二级吸附速率模型,表明化学吸附可能是MnS纳米颗粒吸附镉的速率控制步骤[30]。
如表1所示,MnS纳米颗粒的吸附分别采用Langmuir和Freundlich模型进行拟合。Langmuir模型用于描述吸附过程是吸附剂均质表面的单层吸附,且吸附质分子之间没有相互作用;Freundlich模型是一个经验方程,一般用于描述异质表面的多层化学吸附[25]。
Langmuir吸附等温线模型见式(3)。
式中:qe、qmax为吸附量和最大吸附量,mg·g−1;KL为Langmuir吸附常数,L·mg−1;Ce为吸附质平衡浓度,mg·L−1。
Freundlich吸附等温线模型见式(4)。
式中:qe为平衡吸附量,mg·g−1;Ce为吸附质平衡浓度,mg·L−1;KF为与吸附有关的常数;n为常数。
吸附等温线拟合相关参数如表1所示,MnS的Langmuir等温线模型的拟合结果优于Freundlich等温线模型(R2>0.98)。MnS纳米粒子吸附剂对于Cd2+的吸附过程与Langmuir等温线模型有更好的相关性,更符合Langmuir等温线模型描述的吸附过程。因此,MnS对于Cd2+的吸附过程发生在单分子层,吸附类型主要以单分子层吸附为主。从Langmuir模型中拟合出MnS的最大吸附量为349.6 mg·g−1,高于大多数已报道的吸附剂对于镉的吸附量(表2)。说明MnS是一种具有高吸附量的Cd2+吸附材料,具有很大的应用潜力。
-
1) X射线衍射图谱。图6给出了硫化锰纳米颗粒的XRD谱图,由图6中MnS吸附前的XRD图谱可知,硫化锰纳米颗粒被成功合成。MnS纳米颗粒XRD图谱中含有位于25.78°、27.69°、29.33°、38.19°和50.00°的主要衍射峰,与MnS的XRD标准谱JCPDS#01-089-4089相对应。但由于Mn为过渡金属,反应生成过程中不可避免地产生了常见的杂峰(如Mn2O3和Mn3O4[41])。吸附镉后,XRD图谱上可以明显地检测到CdS峰的生成,这表明MnS纳米粒子与水溶液中的Cd2+反应生成CdS沉淀,从而去除多余的镉。由于锰氧化物对于水中重金属也有吸附作用[42],因此,合成过程中生成的部分锰氧化物也有可能参加了镉的吸附反应,对取得的高吸附量做出了一定贡献。
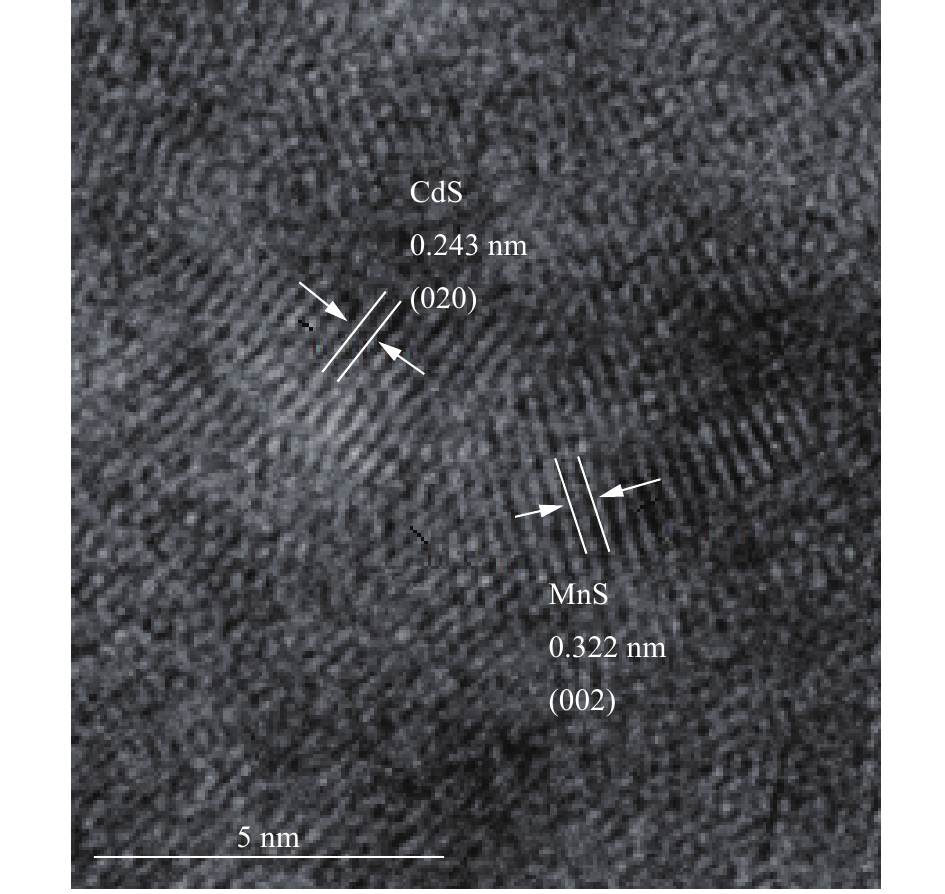
2)高分辨透射电镜(HR-TEM)图像分析。通过高分辨透射电镜图对硫化锰纳米粒子与水溶液中的镉反应前后的形貌进行分析,图7清晰地显示了MnS纳米吸附剂的晶格条纹,说明制备的吸附剂结晶度较高。在MnS与Cd反应后的HR-TEM图像上,可以观察到与MnS晶体(002)和CdS晶体(020)平面分别对应的间距为0.322 nm和0.243 nm的晶格条纹,这与XRD结果相对应。
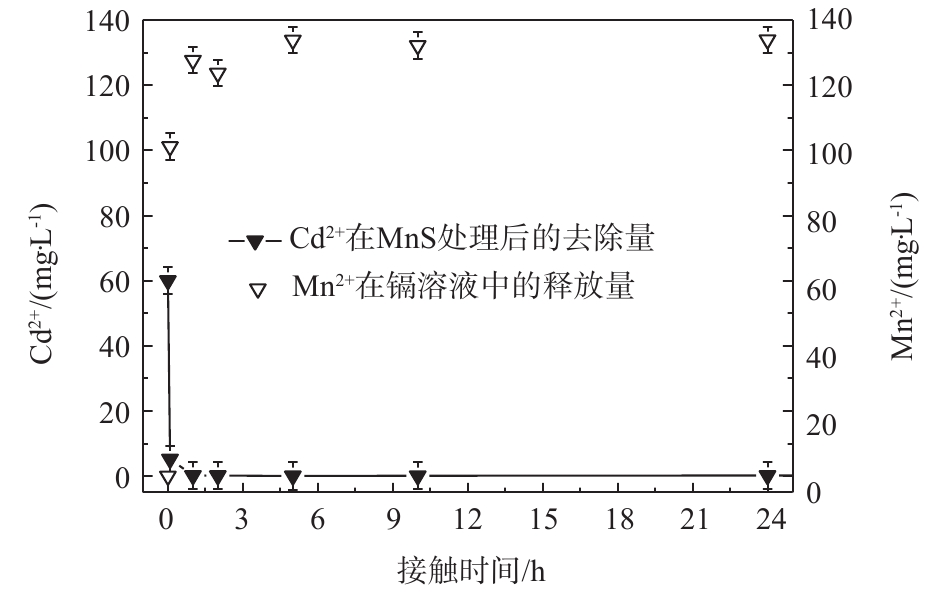
3)锰离子释放量。MnS随着时间变化所释放的金属阳离子释放量展现出与溶液中镉离子去除量相同的趋势。如图8所示,MnS在Cd2+初始浓度为60 mg·L−1的条件下释放出大量Mn2+,到达平衡后,Cd2+的去除量和Mn2+释放量分别为133 mg·L−1和60 mg·L−1。通过比较硫化锰纳米吸附剂对于Cd2+的去除和其自身阳离子释放量可以得出,MnS释放出较多的Mn2+,从而取得较大的去除率。
通过实验数据分析,硫化锰纳米粒子与硫化亚铁纳米颗粒相比有着较大的镉离子吸附量,其原因可能是MnS纳米颗粒与FeS纳米颗粒相比(比表面积为7.64 m2·g−1[43])具有较大的比表面积,其颗粒尺寸也较小。此外,考虑到金属硫化物的KSP序列MnS>FeS>NiS>CoS>ZnS>CdS>PbS>CuS>Ag2S[44],MnS纳米颗粒在水中比较容易溶解;MnS在反应过程中Mn2+释放量较大,其离子交换能力和水中有效硫释放能力较强,同时锰氧化物也有着吸附重金属离子的能力,这也是MnS纳米颗粒的镉去除能力较可观的原因。图8中数据分别除以各自的分子质量后得出Mn2+释放量和Cd2+吸附量的摩尔比为4.56∶1,Mn2+释放量远大于Cd2+吸附量。纯锰氧化物对于镉的平均吸附量为131 mg·g−1[45],EDS半定量分析MnS含量为61%,计算得出锰氧化物对于Cd的去除贡献率为15%。因此,MnS纳米颗粒高效除镉的原因是化学吸附形成CdS沉淀为主,锰氧化物吸附作用为辅。
-
1)本研究成功合成了硫化锰纳米颗粒。电镜结果显示MnS纳米颗粒呈球状,尺寸较小,平均粒径为100 nm。MnS纳米颗粒的BET比表面积为30.56 m2·g−1,孔径为37.38 nm左右。
2) MnS纳米颗粒对Cd2+的吸附动力学数据均较好地符合伪二级动力学模型;对Cd2+的吸附过程较好地符合Langmuir吸附等温模型,是一个以单分子层吸附为主的化学吸附过程。
3) MnS纳米颗粒展现了优越的吸附能力,在模拟工厂废水Cd2+初始浓度为60 mg·L−1的条件下,可以在5 h达到<0.1 mg·L−1的国家规定排放标准。吸附等温线结果表明,使用Langmuir拟合的MnS最大饱和吸附量为349.6 mg·g−1。MnS在反应过程中pH稳定,对水体环境扰动较少。在多种重金属离子共存的情况下,仍可以达到接近100%的Cd2+去除率,且在空气中放置30 d仍保有80%的去除效果,说明MnS是一种具有高吸附量的Cd2+吸附材料,具有非常大的应用潜力。
4)MnS纳米颗粒高效除镉主要是由于化学吸附形成CdS沉淀导致的。
High-efficiency removal of heavy metal cadmium by manganese sulfide nanoparticles
- Received Date: 15/03/2019
- Available Online: 01/01/2020
-
Key words:
- adsorbent /
- cadmium /
- heavy metal /
- metal sulfide /
- water treatment
Abstract: The problem of cadmium pollution in China is becoming more and more serious. The development of high-efficiency cadmium adsorbent is an important technology to solve the problem of environmental cadmium pollution. Manganese sulfide nanoparticles were synthesized by coprecipitation, the adsorption behavior of manganese sulfide nanoparticles on cadmium was studied. The morphology, chemical composition and removal mechanism of cadmium were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), high resolution transmission electron microscopy (HR-TEM), the surface area (BET) and other methods. The results show that MnS nanoparticles were spherical, with an average particle size of 100 nm and a specific surface area of 30.56 m2·g−1. The adsorption kinetics data of Cd2+ by MnS nanoparticles accorded well with the pseudo-second-order kinetics model; the adsorption data of Cd2+ by MnS nanoparticles was in good agreement with the Langmuir adsorption isotherm model, indicating that the above adsorption was a mainly monolayer chemical adsorption. The maximum cadmium adsorption capacity of MnS determined by Langmuir model was 349.6 mg·g−1, which was at the forefront among many cadmium adsorption materials. For the treatment of simulated wastewater, MnS nanoparticles could reduce the concentration of cadmium from 60 mg·L−1 to below the national discharge line (<0.1 mg·L−1) within 5 h, and the water pH during the adsorption process remained stable, and slight interference to water bodies occurred. In the solution of coexistence of various heavy metal ions, the Cd2+ removal rate still reached nearly 100%. MnS was a relatively stable absorbent with 80% the removal efficiency left after 30 days exposing in the air. The main reason for high-efficiency removal of cadmium was identified as CdS precipitate formation through the high ion exchange capacity.







 DownLoad:
DownLoad:
