-
近年来,随着经济建设的高速发展,城镇人口急剧增加,污水排放量和污染负荷不断增大,从而导致污水处理厂出水排放的受纳水体水质不断恶化[1-3]。2015年4月,国务院印发的《水污染行动防治计划》中明确要求,敏感区域城镇污水处理设施应全面达到一级A排放标准[4]。因此,提标改造已成为污水处理厂满足愈发严格的出水排放标准的必然选择之一[5]。然而,在实际污水处理厂提标改造过程中,由于对运行参数变化导致的运行效率改变机制认识不清,盲目选择微生物种群结构作为响应指标,导致在提标改造关键参数及工艺的选择上也存在一定的盲目性[6-7]。因此,明确运行参数变化对运行效率产生影响的根本原因,对目前污水处理厂提标改造具有重要的理论意义。
从污染物降解途径来看,限速酶是物质转化最根本的原因之一。如在氮素转化过程中,氨单加氧酶(AMO)和羟胺氧化还原酶(HAO)是硝化反应的限速酶[8],硝酸盐原酶(NR)和亚硝酸盐还原酶(NIR)是反硝化反应的限速酶[9-11]。一直以来,关于生物脱氮过程中关键酶的研究主要集中在酶的纯化和反应机理上[12-14],近年来,对于酶活性在污水处理过程中的作用才逐步展开。LI等[15]初步分析了与TN去除相关的关键酶种类;CALDERON等[16]阐述了酶活性水平与运行参数变化之间的关系;PAN等[17]探讨了污水处理系统脱氮过程中NR和NIR的特性。然而,这些研究主要集中在实验室小试规模。事实上,实际污水处理厂运行过程比实验室小试装置更加复杂。因此,有必要对实际污水处理厂关键酶活性与污染物去除率之间的关系进行深入研究。
氧化沟是城市污水处理的3大典型工艺之一[18],在中国,从20世纪80年代以来,氧化沟工艺一直被广泛采用[19]。本研究以Orbal氧化沟为研究对象,分析2种运行模式下活性污泥中微生物种群结构、功能微生物含量、关键酶活性及污染物去除效率,并对其相互关系进行了探讨,目的是揭示影响实际污水处理厂污染物去除率的根本原因,以期为实际污水处理厂提标改造提供参考。
-
PCR产物回收纯化试剂盒、实时荧光定量PCR反应试剂盒;磷酸钾(K3PO4)、硫酸铵((NH4)2SO4)、细胞色素C(C42H52FeN8O6S2)、醋酸钠(CH3COONa)、羟胺(NH2OH)、甲基紫(C24H28N3)、硝酸钠(NaNO3)、双对氯苯基三氯乙烷((ClC6H4)2CH(CCl3))均为分析纯。
核酸自动提取仪(Tanbead,北京九宇金泰生物技术有限公司);聚丙烯酰胺凝胶电泳仪(Bio-Rad,伯乐生命医学产品(上海)有限公司);凝胶成像系统(Bio-Rad,伯乐生命医学产品(上海)有限公司);测序仪(ABI 3730XL,爱普拜斯应用生物系统贸易(上海)有限公司);实时荧光定量PCR仪(SteponePlus,爱普拜斯应用生物系统贸易(上海)有限公司);冷冻离心机(Biofuge Stratos,赛默飞世尔科技(中国)有限公司);溶氧仪(CellOx325,德国WTW中国技术服务中心);pH计(SenTix 41-3,德国WTW中国技术服务中心);温度计(WTW-Multi 340i,德国WTW中国技术服务中心);紫外可见分光光度计(UV-1700,岛津企业管理(中国)有限公司)。
-
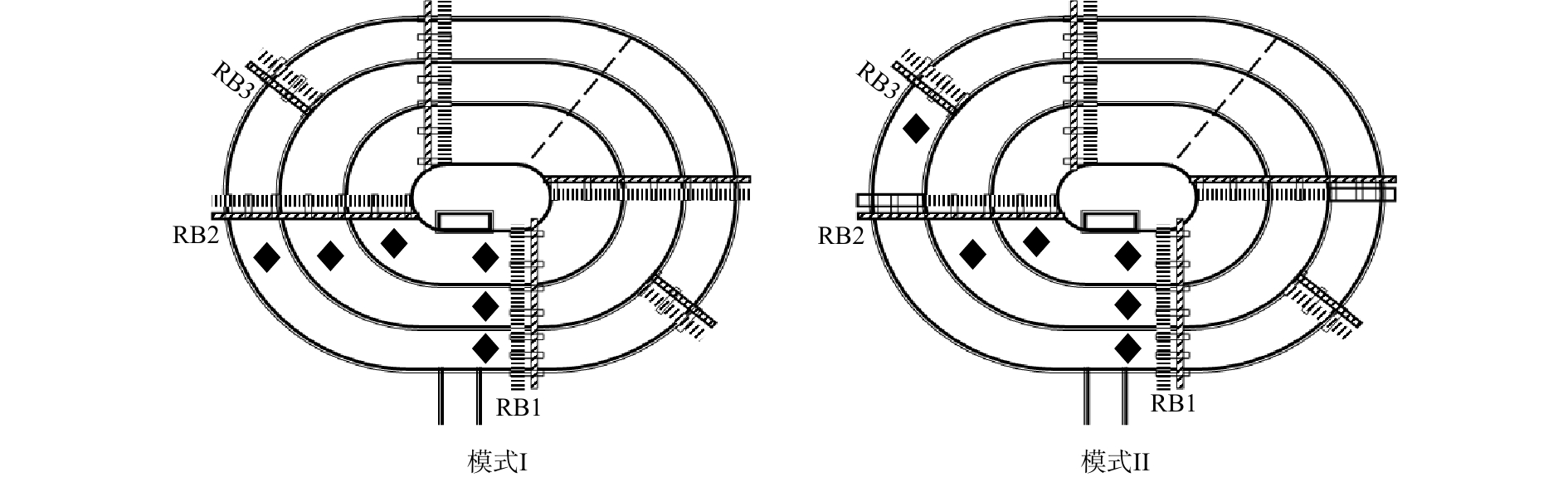
本实验在河南省某市的一个实际污水处理厂进行,该污水厂主体采用Orbal氧化沟工艺,污水处理量为4×104 m3·d−1,水力停留时间为10 h,污泥龄为12 d。实验分别在2种模式下进行,每种模式的运行周期为1年,进水水质如表1所示。2种运行模式的主要区别在于沟道内转刷开启数量不同,模式I的外、中、内沟道转刷开启数量分别为6、4、4个;模式Ⅱ的沟道转刷开启数量分别为4、4、4个。2种模式下的污泥浓度、污泥负荷、COD负荷及
NH+4 -N负荷均相近,模式I的污泥浓度、污泥负荷、COD负荷及NH+4 -N负荷分别为3 015 mg·L−1、0.13 kg·(kg·d)−1、0.35 kg·(m3·d)−1和3.80×10−2 kg·(m3·d)−1;模式Ⅱ的污泥浓度、污泥负荷、COD负荷及NH+4 -N负荷分别为2 965 mg·L−1、0.13 kg·(kg·d)−1、0.34 kg·(m3·d)−1和3.80×10−2 kg·(m3·d)−1。每周监测不同模式下进出水水质及沟道内溶解氧变化,测试位置如图1所示(包括转刷后1 m和下一个转刷前1 m)。同时,在每年6月和12月,分别采集沟道内活性污泥样品,用于微生物种群、功能微生物含量及关键酶活性分析。 -
分别采用PCR-DGGE技术、实时荧光定量PCR技术定性、定量分析不同运行模式下活性污泥微生物种群结构及功能微生物含量[20-23];采用分光光度法测定不同运行模式下关键酶活性,一个单位的酶活性(U)定义为:1 g活性污泥中,1 h转化1 mg催化底物所需酶的量[24-26];采用文献中的方法[27]测定不同运行模式下污水厂的进出水水质[27]。
-
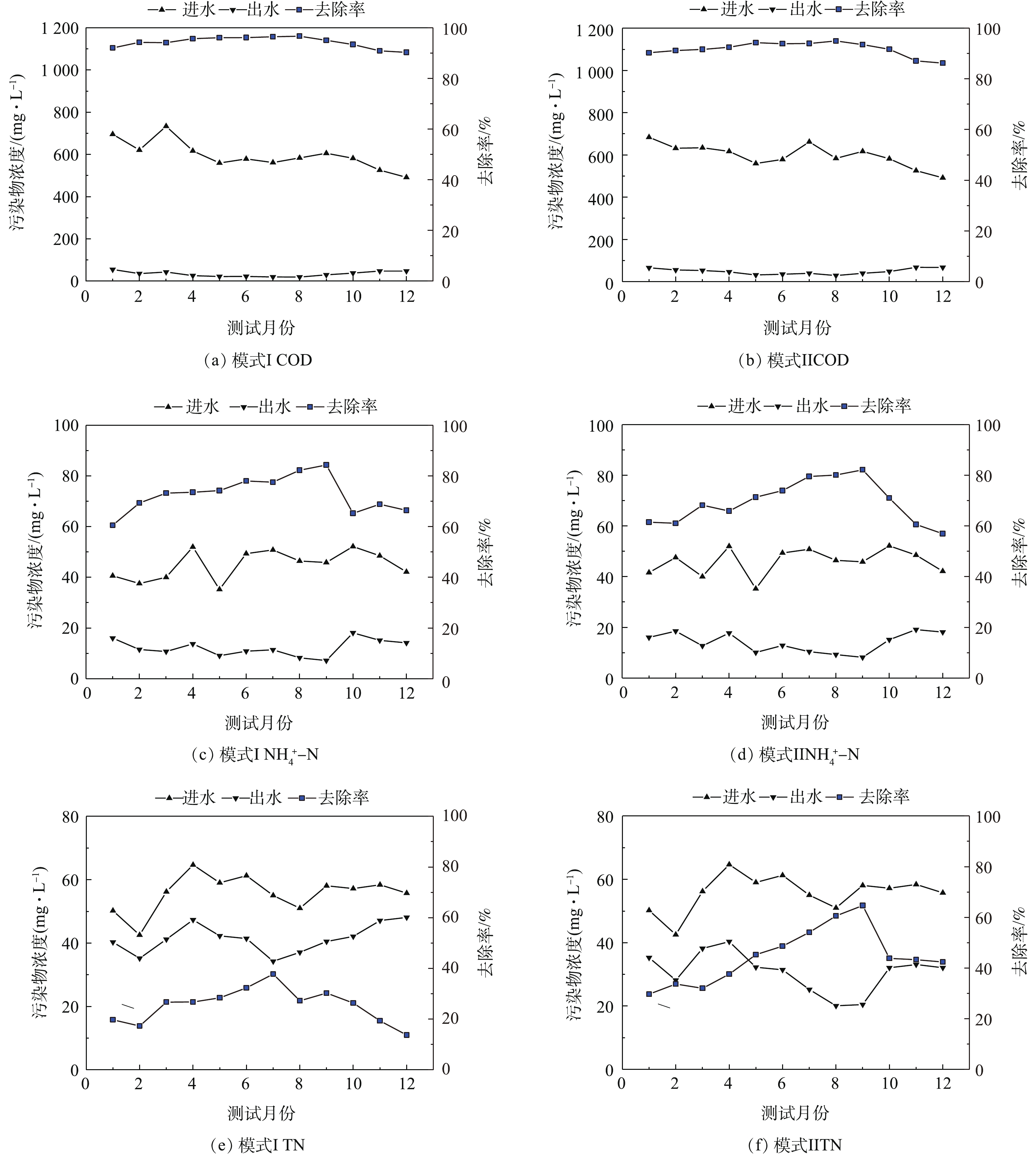
在2种运行模式下,该厂进出水中COD、
NH+4 -N和TN的监测结果见图2。从图2可以看出,在模式I和模式Ⅱ下,COD的平均去除率分别为(94.28±2.19)%和(91.79±2.77)%;NH+4 -N的平均去除率分别为(72.80±7.07)%和(69.36±8.45)%;TN的平均去除率分别为(25.50±6.83)%和(44.67±10.96)%。同时,图2中结果表明,除冬季外,其余季节在模式Ⅱ运行条件下,COD、NH+4 -N和TN的去除率均明显高于模式I。在4—10月,模式I和模式Ⅱ的COD的平均去除率分别为(96.08±0.87)%和(94.17±0.73)%;NH+4 -N的平均去除率分别为(81.38±3.47)%和(80.59±1.39)%,TN的平均去除率分别为(31.77±5.41)%和(59.81±5.33)%。 -
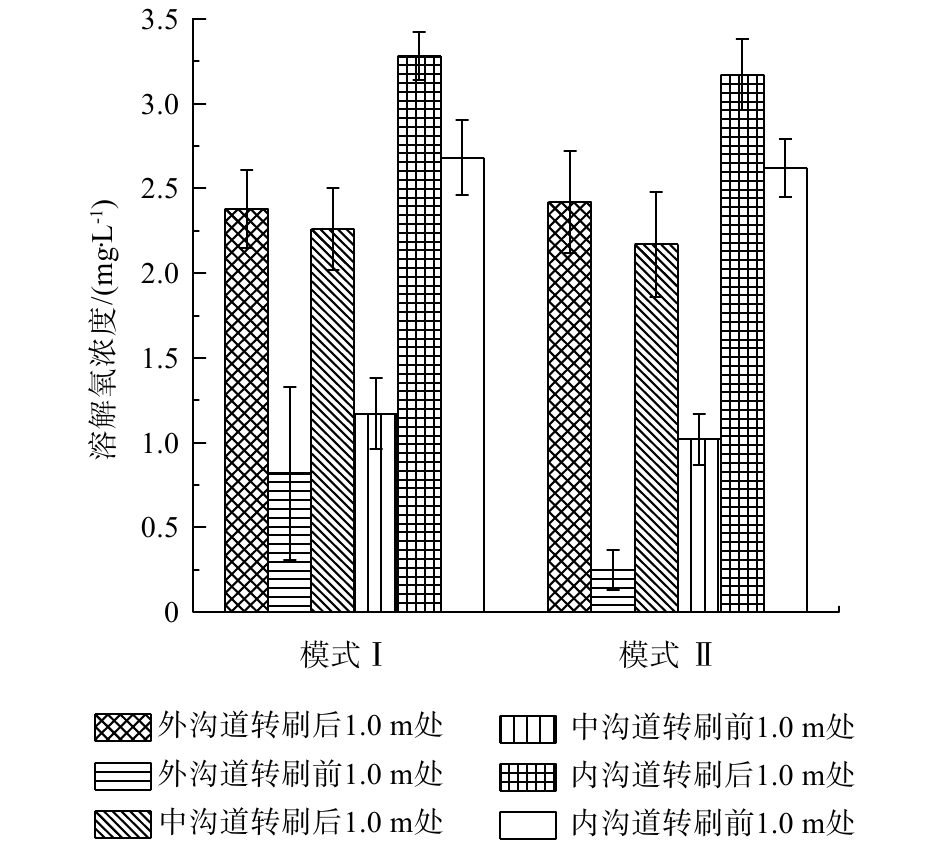
在2种运行模式下,分别对Orbal氧化沟3个沟道不同位置处DO浓度进行测定,结果见图3。可以看出,DO浓度在转刷前和转刷后有明显不同,特别是在外侧沟道。模式I条件下,转刷后1 m处,外渠道的DO浓度为(2.28±0.3) mg·L−1,在下一个转刷前1 m处,外渠道的DO浓度为(0.80±0.1) mg·L−1。在模式Ⅱ条件下,转刷后1 m处外渠道的DO浓度为(2.03±0.4) mg·L−1,在下一个转刷前1 m处,外渠道的DO浓度为(0.16±0.1) mg·L−1。
-
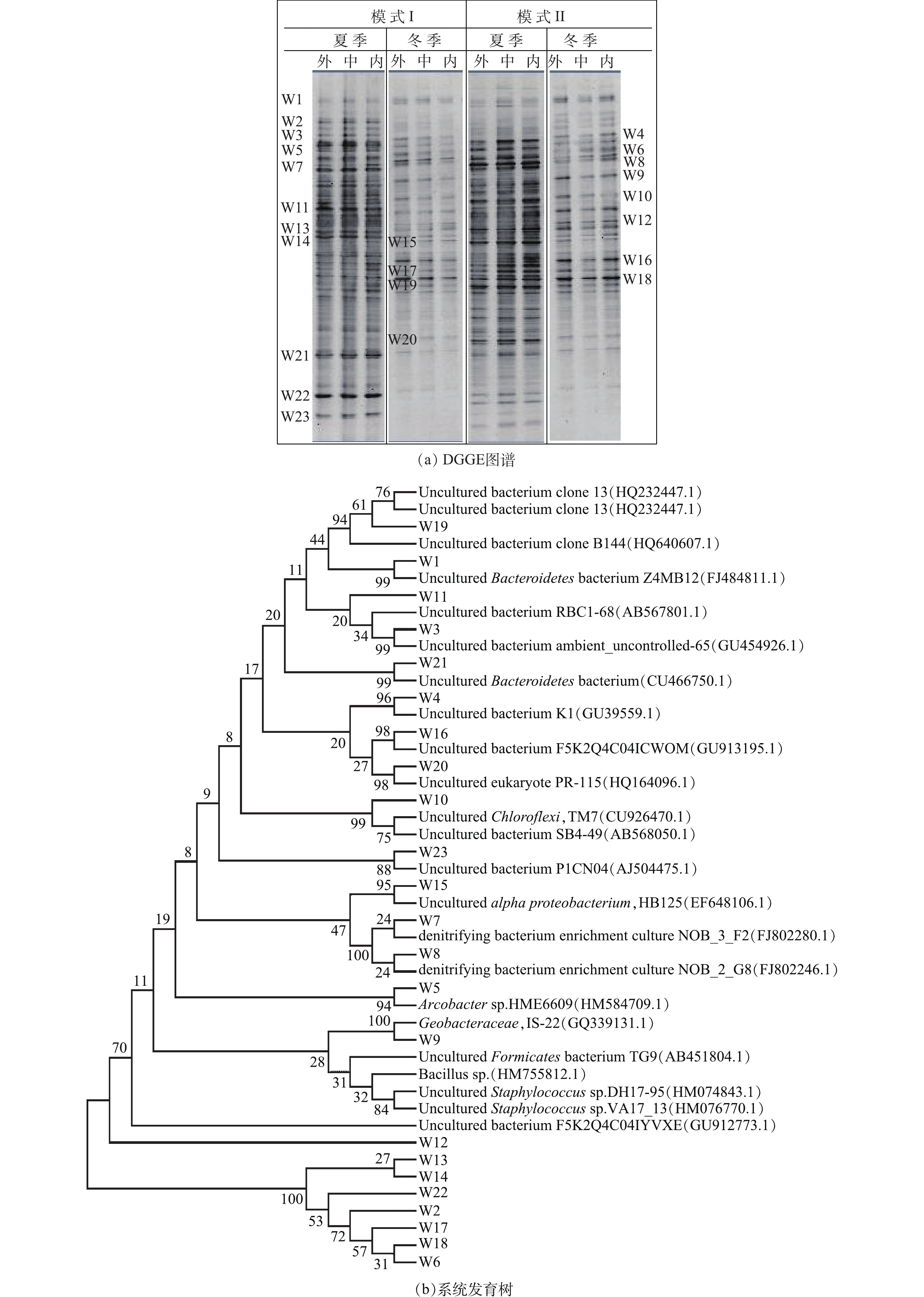
在2种运行模式下,DGGE图谱见图4。可以看出,各沟道内的微生物种群结构基本类似(图4(a))。夏季时,模式I外、中、内沟道香农指数分别为3.76、3.79和3.83,模式Ⅱ外、中、内沟道香农指数分别为3.81、3.97和3.97。冬季时,模式I外、中、内沟道香农指数分别为3.01、3.11和3.15,模式Ⅱ外、中、内沟道香农指数分别为3.05、3.02和3.11。并且,在2种模式下各个沟道中均有主条带W4~W19存在。比对结果显示,所有测得序列97%~100%程度上均与先前确定的16S rRNA基因序列具有同源性,分别隶属于拟杆菌门、变形杆菌门、绿弯菌门和厚壁菌门[28-30](图4(b))。
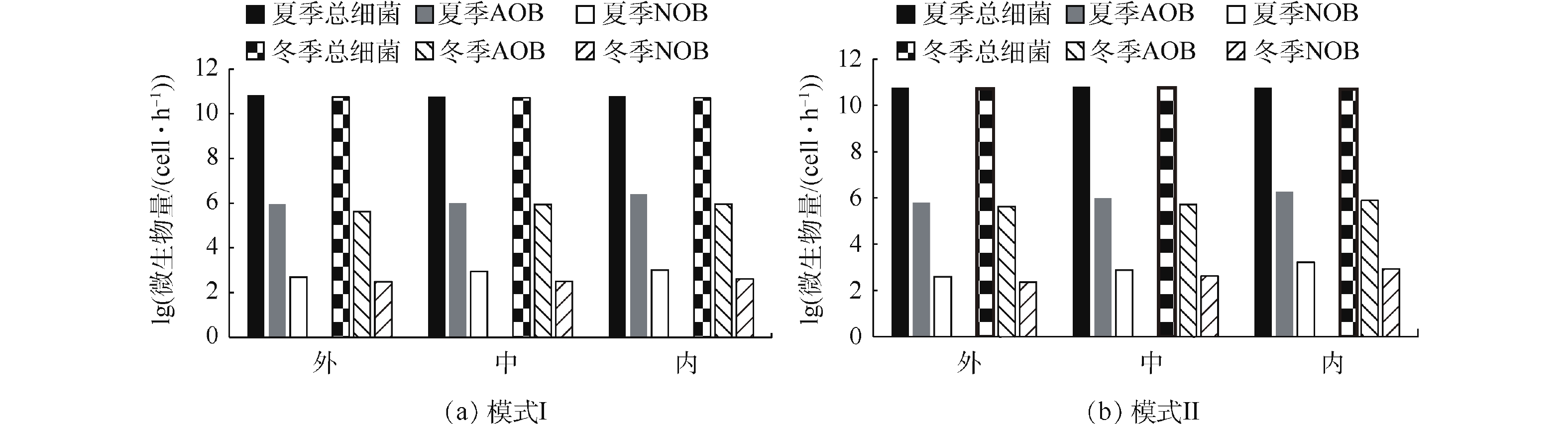
功能微生物氨氧化菌AOB和硝化细菌NOB定量检测结果见图5。可以看出,无论夏季还是冬季,总细菌、AOB和NOB的含量在模式I和模式Ⅱ下均呈现相似趋势。夏季时,在模式I下,Orbal氧化沟外、中、内沟道中总细菌含量分别为6.70×1010、5.80×1010、5.96×1010 cells·g−1(以干污泥含量计),AOB含量分别为8.98×105、1.02×106、2.52×106 cells·g−1(以干污泥含量计),NOB含量分别为4.89×102、8.88×102、1.02×103 cells·g−1(以干污泥含量计);而在模式Ⅱ下,外、中、内沟道中总细菌含量分别为5.84×1010、6.19×1010、5.88×1010 cells·g−1(以干污泥含量计),AOB含量分别为6.25×105、9.88×105、1.80×106 cells·g−1(以干污泥含量计),NOB含量分别为3.96×102、7.69×102、1.66×103 cells·g−1(以干污泥含量计)。冬季时,Orbal氧化沟3个沟道内总细菌、AOB、NOB含量均略低于夏季。在模式I下,外、中、内沟道中总细菌含量分别为5.58×1010、5.21×1010、5.07×1010 cells·g−1(以干污泥含量计),AOB含量分别为4.25×105、8.85×105、9.26×105 cells·g−1(以干污泥含量计),NOB含量分别为3.10×102、3.23×102、4.15×102 cells·g−1(以干污泥含量计);而在模式Ⅱ下,外、中、内沟道中总细菌含量分别为5.26×1010、5.61×1010、5.12×1010 cells·g−1(以干污泥含量计),AOB含量分别为4.23×105、5.26×105、7.68×105 cells·g−1(以干污泥含量计),NOB含量分别为2.26×102、4.21×102、8.52×102 cells·g−1(以干污泥含量计)。从AOB和NOB在总细菌中所占的相对比例来看,模式I和模式Ⅱ条件下也呈现相似结果。在模式I下,AOB和NOB的比例分别是7.62×10−6~4.23×10−5和8.19×10−9~1.71×10−8;在模式Ⅱ下,AOB和NOB的比例分别是9.38×10−6~3.06×10−5和7.50×10−9~2.82×10−8。
理论上,活性污泥中的微生物种群会随着污水处理运行参数的变化而发生变化。因此,微生物种群结构变化常用来解释运行参数调节后污水处理效果发生变化这一现象[31]。本实验是在一个实际污水处理厂展开,水质监测结果发现,当外沟道转刷开启数量减少后,污水处理厂TN去除效率明显提升。然而,2种运行模式下微生物种群结构和功能微生物含量却呈现高度相似现象。这与HASHIMOTO等[32]提出的活性污泥中细菌群落结构在实际污水处理系统中是相对稳定的这一结论是相符的。当然,本实验在同一污水处理厂展开,进水水质的稳定也是2种运行模式下细菌种群结构未发生明显改变的重要原因之一,而这一结论也与ZHOU等[33]在实际污水处理厂的研究结果相符。因此,在实际污水处理厂中,仅选取微生物种群来解释运行参数变化引起运行效率提升的原因是远远不够的。
-
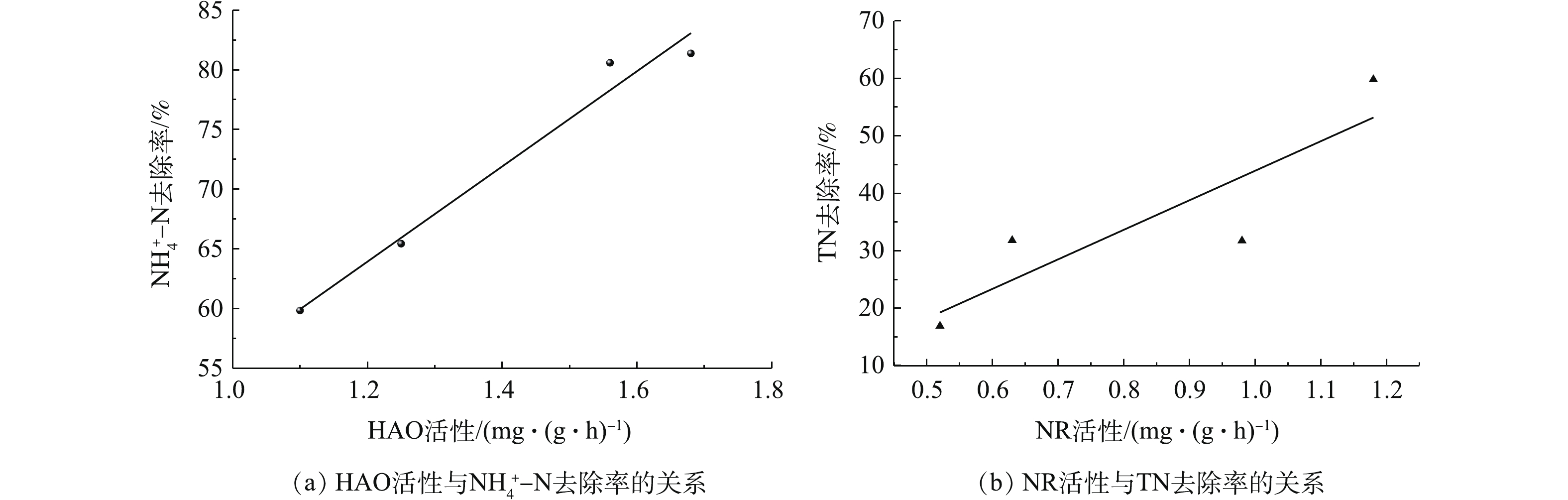
在夏季和冬季,分别采集2种运行模式下3个沟道内活性污泥样品,进行关键酶HAO和NR的活性分析。结果显示,在同一运行模式下,与中、内沟道相比,HAO活性在外沟道中最低。相反,NR活性在外沟道中最高。并且,HAO和NR的酶活性在夏季都高于冬季。外沟道中,在模式Ⅱ条件下NR活性明显高于模式Ⅰ。在模式Ⅰ下,夏季和冬季的NR活性(以羟胺计)分别为1.58 mg·(g·h)−1和0.80 mg·(g·h)−1;而模式Ⅱ下,夏季和冬季的NR活性分别为2.27 mg·(g·h)−1和1.07 mg·(g·h)−1。内沟道中,模式I和模式Ⅱ条件下的HAO活性并无明显区别。在模式I下,夏季和冬季的HAO活性(以羟胺计)分别为2.17 mg·(g·h)−1和1.56 mg·(g·h)−1;而在模式Ⅱ下,夏季和冬季的HAO活性分别为2.05 mg·(g·h)−1和1.42 mg·(g·h)−1。分析结果表明,外侧沟道转刷开启数量的减少,直接对其中关键酶NR的活性产生了影响。在模式Ⅱ下,冬季和夏季外侧沟道内NR活性分别比模式I下提高了25%和30%。与此同时,该水厂出水中TN的去除率也由模式I的(25.50±6.83)%提高到了模式Ⅱ的(44.67±10.96)%。综合分析关键限速酶HAO、NR与TN、
NH+4 -N去除的关系,结果表明,HAO和NR活性与NH+4 -N和TN的去除均呈正相关关系,斯皮尔曼相关系数r分别为0.99(P=0.01)和0.88(P=0.12)(图6)。也就是说,改变污水厂运行参数,生物处理单位中关键酶活性随之发生变化,进而改变污染物的去除率。进一步深入分析发现,减少Orbal氧化沟外侧沟道转刷开启数量,其沟道中缺氧或厌氧区段明显延长。供氧量的减少直接改变了外侧沟道局部的微环境条件。而这种微环境条件的改变,在不影响其微生物种群结构的前提下,直接提升了沟道内关键酶活性,进而提升了污水出水水质。这与赵群英等[34]关于DO含量变化对污水出水水质具有明显影响的研究结论是一致的。也就是说,在实际污水处理厂中,改变运行参数后,相对于微生物种群结构和功能微生物含量而言,关键酶活性的响应更为快速灵敏。然而,本研究对关键酶活性的分析仅仅是酶粗提取物的分析,并且仅在一家污水处理厂进行。如要将该研究结果用于解析实际污水处理厂运行参数变化对处理效率影响的机制时,需要进行更为精准且全面的研究。例如,结合更多实际污水处理厂的研究,综合分析多种运行参数变化后其关键酶的响应过程;同时,设计小型批量研究实验,对提取的关键酶进行纯化,进而分析不同运行参数条件下关键酶的响应关系。 -
1)减少Orbal氧化沟外侧沟道转刷开启数量,可有效地提高实际污水处理厂TN的去除率。
2)转刷开启数量减少后,Orbal氧化沟外侧沟道内溶解氧含量降低,缺氧或厌氧区明显延长,局部微环境发生改变。
3)在此过程中,微生物种群及功能微生物含量保持稳定,未发生明显变化。关键酶NR活性随转刷开启数量的减少而升高。并且关键酶NR活性与TN去除效率呈正相关关系。本研究为实际污水处理厂提标改造参数及工艺选择提供了参考。
| 模式 | COD/(mg·L−1) |  | TN/(mg·L−1) | TP/(mg·L−1) | SS/(mg·L−1) | pH |
| I | 492~734 | 35.25~48.52 | 42.56~61.25 | 2.25~4.15 | 100~325 | 6.80~7.20 |
| II | 490~684 | 36.75~47.56 | 45.75~60.25 | 2.65~4.75 | 120~280 | 6.70~7.20 |







 DownLoad:
DownLoad: