-
焦化废水产生于高温焦炭生产、气体净化和副产品回收过程,其化学成分复杂,含有大量的酚类物质、氰化物、硫化物、杂环化合物和多环芳烃等[1]. 因此,为了控制和减少其对环境的不利影响,通常采用物化预处理、生物处理、深度处理和回用处理相结合的组合工艺处理焦化废水[2]. 在处理过程中会产生大量污泥,而废水中的一部分难降解有机物会被污泥吸附[3]. 因此,焦化废水处理过程中产生的污泥如果不经妥善处置,会对环境造成危害. 污泥通过高温热解转化为高附加值的污泥炭是解决污泥处理处置问题的一个有发展前景的途径. 通过热解不仅能去除污泥中的有害污染物,杀灭污泥中的病原体,还能回收资源和能源. 污泥经过高温作用不仅形成了导电石墨碳,还形成了具有氧化还原活性的含氧官能团以及过渡金属及其氧化物等[4]. 污泥炭能够作为电子供体、受体和媒介,充当电子转移介质进一步提高催化性能,催化活化过硫酸盐、过氧化氢(H2O2)和臭氧等氧化剂生成硫酸根自由基(SO4-·)、羟基自由基(·OH)、超氧自由基(·O2-)和单线态氧非自由基(1O2)等[5 − 6].
在以过氧化氢(H2O2)为氧化剂的高级氧化体系中,研究者发现污泥炭的金属、杂原子掺杂的碳结构以及官能团可以作为电子转移的介质,使H2O2分解产生·OH,降解水体中的有机污染物[7]. 催化湿式过氧化物氧化 (CWPO) 工艺可以在温和的反应条件下高效降解废水中的难降解污染物[8]. 近年来,关于市政污泥热解炭化制备催化剂并用于CWPO体系中降解有机污染物的研究逐渐增多[6, 9]. 然而,工业废水处理过程中产生的污泥制备生物炭催化剂的研究相对较少. 焦化废水中含有的多种成分使焦化污泥中的元素种类更丰富,可能有利于其制备的污泥炭性质的改善[3]. 氯离子是高盐工业废水中的主要阴离子,能影响CWPO反应中生成的自由基种类,降低催化剂的活性,从而影响有机物的降解. 在制革和染料制造废水中,经常可以检测到高浓度的NaCl(>50 g·L−1),但是其对有机物降解过程的影响还未有很好的解释[10]. 因此,研究Cl-对有机物降解的影响对处理实际废水具有重要意义.
本研究以焦化废水处理厂再生水处理段排放的污泥为原料制备生物炭催化剂,采用硝酸低温改性方法,提高其催化性能,以间甲酚为模型污染物,研究在催化湿式过氧化氢反应体系中污泥炭催化剂对氯介质下间甲酚降解的催化活性和稳定性,并分析间甲酚的降解过程,考察氯离子对间甲酚降解的影响.
-
间甲酚标准品(中国药品生物制品检定所),甲醇为色谱纯(国药集团化学试剂有限公司),过氧化氢、硝酸、氯化钠、亚硫酸钠等药品为分析纯(天津科密欧化学试剂有限公司),实验用水为超纯水.
-
焦化污泥取自山西省某工业园区的焦化废水处理厂中的再生水处理环节. 依次用超纯水和乙醇冲洗焦化污泥,再自然晾干,之后置于烘箱中105 ℃处理24 h,待冷却后,得到干燥后的焦化污泥,记为CSS. 随后,干燥的焦化污泥CSS在炭化转炉中进行炭化处理,反应条件为:300 mL·min−1的N2气氛下,5 ℃·min−1速率升温至800 ℃,焙烧2 h,炭化转炉的转速为5 r·min−1. 待冷却后,记为未改性焦化污泥炭CSSC-0. 未改性焦化污泥炭用6 mol·L−1的HNO3在0 ℃改性24 h,之后用超纯水冲洗至冲洗液为中性,得到改性焦化污泥炭,记为CSSC-N.
-
配置浓度为100 mg·L−1的间甲酚废水溶液100 mL,加入250 mL锥形瓶中,分别再加入不同污泥催化剂,置于水浴振荡器上. 以120 r·min−1的速度振荡混合,进行吸附实验. 待吸附平衡后,加入过氧化氢,进行催化降解反应,每隔一段时间进行取样并立即加入Na2SO3抑制反应进行. 用0.45 μm滤膜过滤后分析间甲酚和TOC的浓度. 为保证实验数据准确性,实验做3个平行样,实验结果为3个平行样的平均值.
催化降解反应条件为:温度为30 ℃、pH为3、过氧化氢投加量为1.85 g·L−1、催化剂投加量为0.75 g·L−1. 吸附实验中,分别投加3种催化剂:CSS、CSSC-0、CSSC-N. 降解实验中,除上述3种催化剂外,再加入一组无催化剂的实验作为对照.
-
样品中的金属元素含量用PerkinElmer 7300DV电感耦合等离子体发射光谱(ICE-OES)测定. 样品的比表面积及孔隙结构用麦克ASAP2460测定. 用日本理学Rigaku Ultima Ⅳ型X射线衍射仪(XRD)分析晶型结构,用PerkinElmer Spectrum Two红外光谱仪(FT-IR)分析催化剂表面官能团. 用Thermo ESCALAB 250XI型X射线光电子能谱(XPS)分析样品中元素价态和结合形态,用ZEISS Gemini 300型扫描电镜(SEM)结合X射线能谱(EDS)和元素面分布技术(EDS-mapping)观察样品表面形貌和元素组成. 间甲酚检测采用液相色谱法(Aglient HP1100G1312A). 色谱柱:Bioband GP120-C18(250 mm× 4.6 mm,5 μm),流动相为80%的甲醇和20%的超纯水,流速为1.0 mL·min−1,进样量20 μL. 用ShimadzuRF-6000荧光分光光度计(3D-EEMs)分析间甲酚的降解过程. 用Bruker Solarix 15T 傅立叶变换离子回旋共振质谱(FT-ICR-MS),分析间甲酚的降解中间产物.
-
由表1可见,焦化污泥CSS含碳量为13.27%,铁含量高达28.32%,这与焦化废水混凝处理阶段加入含铁混凝剂有关. CSS经过炭化后转化为CSSC-0,金属元素含量升高,碳含量增加. CSSC-0经过硝酸改性后转化为CSSC-N,Ca含量大幅度降低,这与SEM--EDS mapping结果一致,说明酸溶解了氧化钙、碳酸钙等含钙物质. 此外,由于硝酸的氧化作用,CSSC-N中的氧含量增加. 改性后的焦化污泥炭中的铁含量稍有降低,但仍然在较高的水平(30.14%),保证了催化剂的活性组分含量.
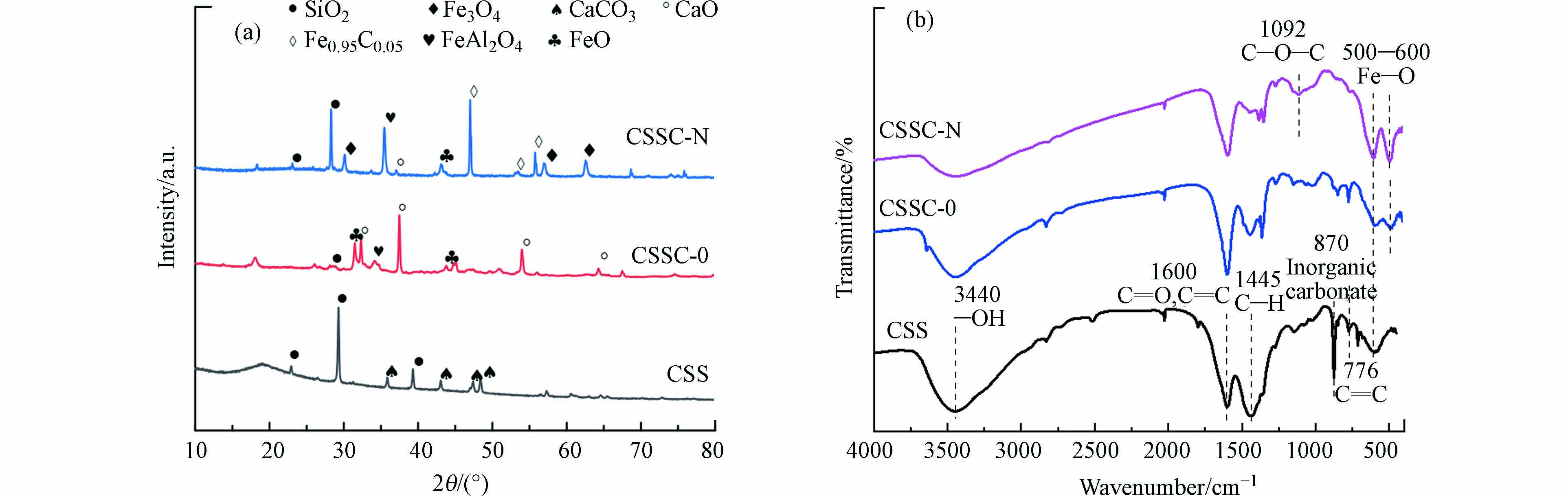
对焦化污泥和污泥炭样品进行XRD分析. 如图1(a)所示,焦化污泥CSS中含CaCO3和SiO2. 经过炭化后,焦化污泥中的CaCO3转化为CaO[11],且焦化污泥炭中出现FeO和FeAl2O4物相. 经过硝酸低温改性后的焦化污泥炭CSSC-N的XRD谱图中出现Fe3O4和Fe0.95C0.005的特征峰,这可能是因为在硝酸溶液中,污泥炭中的物质发生反应,生成了新的物相. 改性后的污泥炭中铁物相种类更丰富,有利于CWPO反应中的Fe(Ⅱ)和Fe(Ⅲ)的循环,铁铝尖晶石相FeAl2O4已被证实具有催化活性,对酸性环境具有一定耐受性[12 − 13]. 因此,铁物相的增加有利于催化H2O2分解产生·OH,促进间甲酚的降解. 图1(b)红外谱图显示,位于3440、1445、1092、870、776 cm−1处的峰分别属于—OH、C—H、C—O—C、无机碳酸盐、C═C的振动[3, 14 − 16]. 而位于500—600 cm-1范围的特征峰表明Fe—O官能团的存在[15, 17]. 焦化污泥通过炭化和改性后,C—H、C═C键的振动减弱,无机碳酸盐消失,Fe—O特征峰增强. 这说明C被氧化,与O结合形态增多,含铁官能团增多,与XRD和后文中的SEM EDS-mapping结果一致.
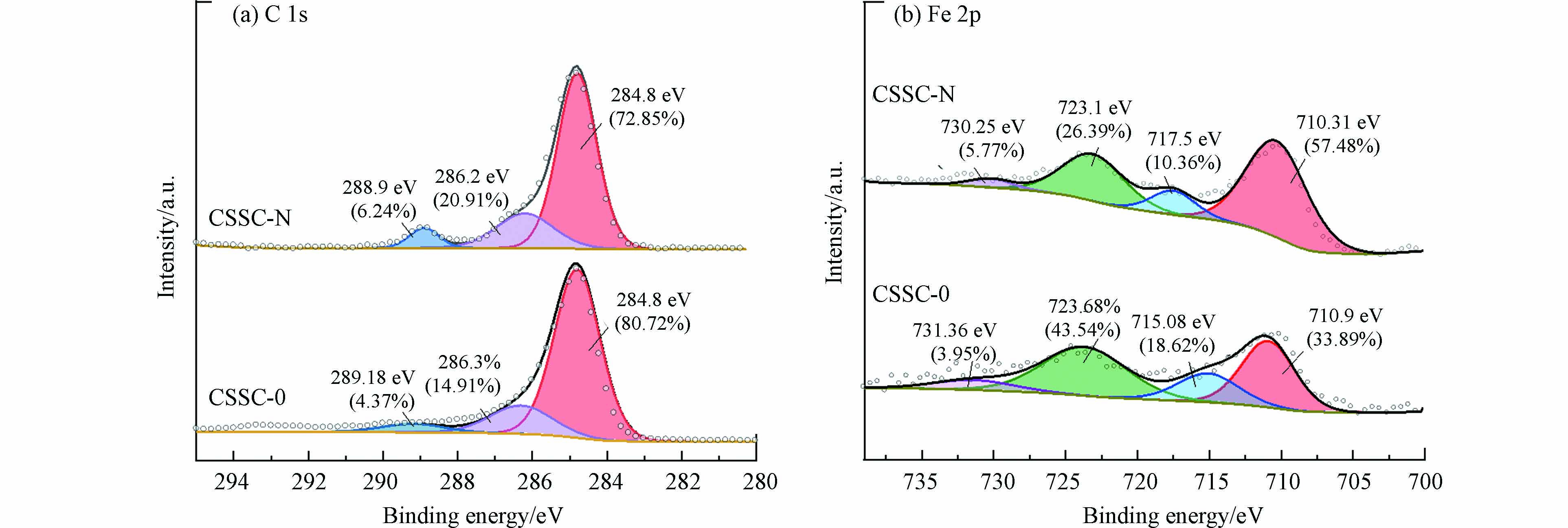
为研究焦化污泥炭表面特性和官能团分布,用XPS分析其C1s和Fe2p. 如图2(a)所示,将C1s分为3种类型的峰,分别为C—C(石墨态碳,284.8 eV),C—O(酚羟基及醚类碳,286.2—286.3 eV)和碳酸盐(或碳酸氢盐,288.9—289.18 eV)[18 − 19]. 通过硝酸低温改性后的焦化污泥炭中,石墨态碳被氧化,其含量减少,而与氧结合的碳含量增多. 污泥炭上的含氧官能团能充当电子介质将电子转移至H2O2发生反应,从而促进·OH的生成,达到降解有机污染物的目的[20]. 如图2(b)所示,Fe2p可分为4种峰:FeO(710.31—710.9 eV),α-Fe2O3(715.08—717.5 eV),γ-Fe2O3 (723.1—723.68 eV)和Fe3O4 (730.25 —731.36 eV)[21 − 23]. 经过改性后,FeO在焦化污泥炭表面上的含量增加. Fe2O3的含量减少,可能是由于原本包裹在Fe(Ⅱ)上的Fe2O3或其他氧化物被酸溶解,暴露在催化剂表面的Fe(Ⅱ)成为催化剂活性位点,使焦化污泥炭的催化性能得到提高.
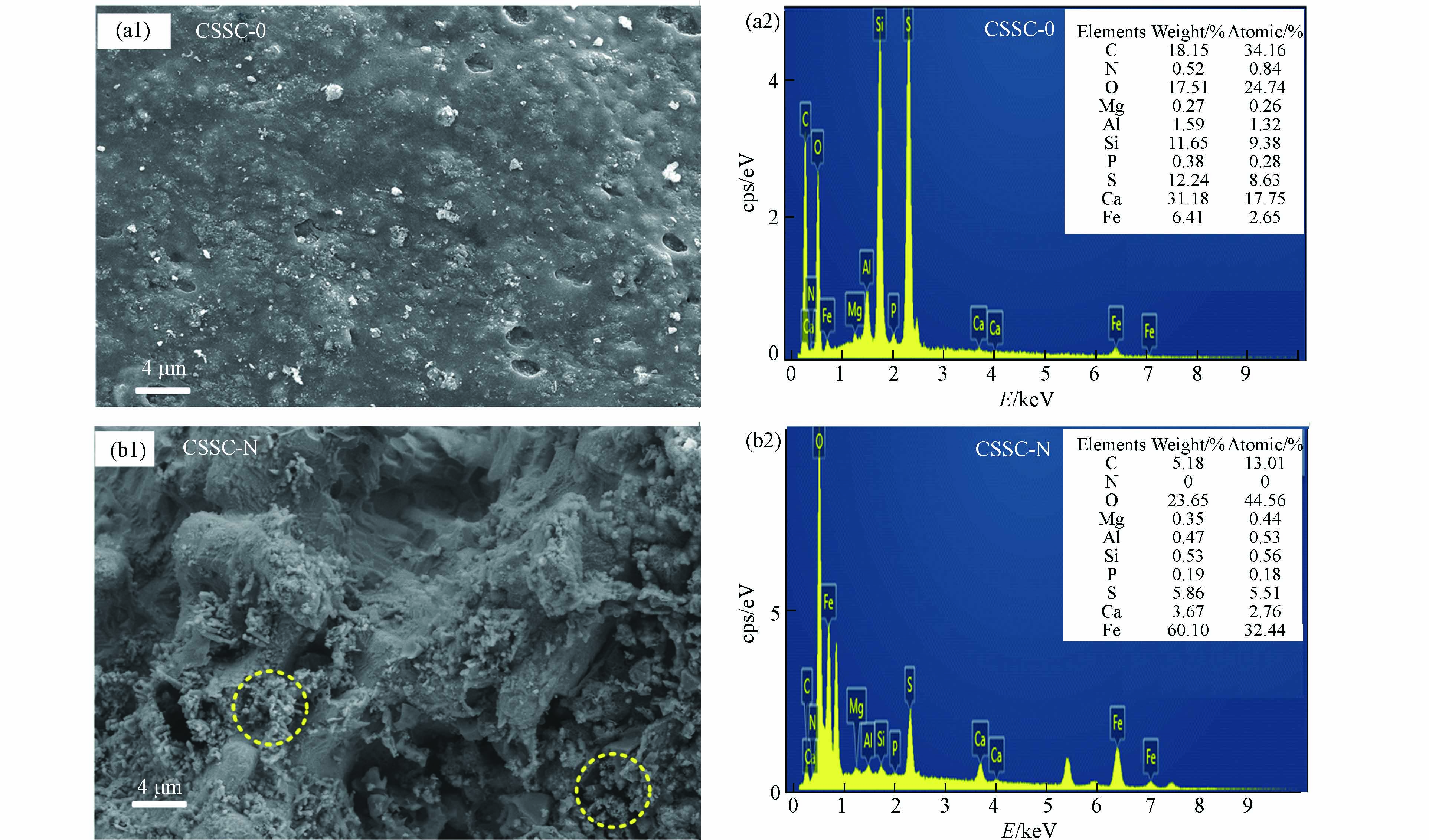
由图3中的SEM图可观察催化剂的表观形貌特征,未改性的焦化污泥炭CSSC-0中的表面形态较光滑(图3(a1)),而改性后的焦化污泥炭CSSC-N表面粗糙多孔(图3(b1)),且具有铁氧化化物形态结构,这与前面XRD、元素分析等表征结果一致. 由EDS-mapping分析催化剂表面元素组成(图3(a2)和(b2)),通过改性后的焦化污泥炭表面元素组成发生了明显变化,表面铁含量由6.51%增加至60.10%. 说明通过改性后,表面含碳、钙等元素的物质被硝酸淋溶,而铁化物转移到催化剂表面. 结合表1中焦化污泥和污泥炭中铁的总含量结果,说明CSSC-N中的铁主要分布在催化剂表面,而CSSC-0中的铁主要分布在催化剂内部. 除铁含量外,铁在催化剂结构上的分布对催化剂的活性具有重要的影响[24]. 表面铁含量越多,活性位点越多. 越易催化过氧化氢产生·OH,从而氧化降解有机污染物[25].
-
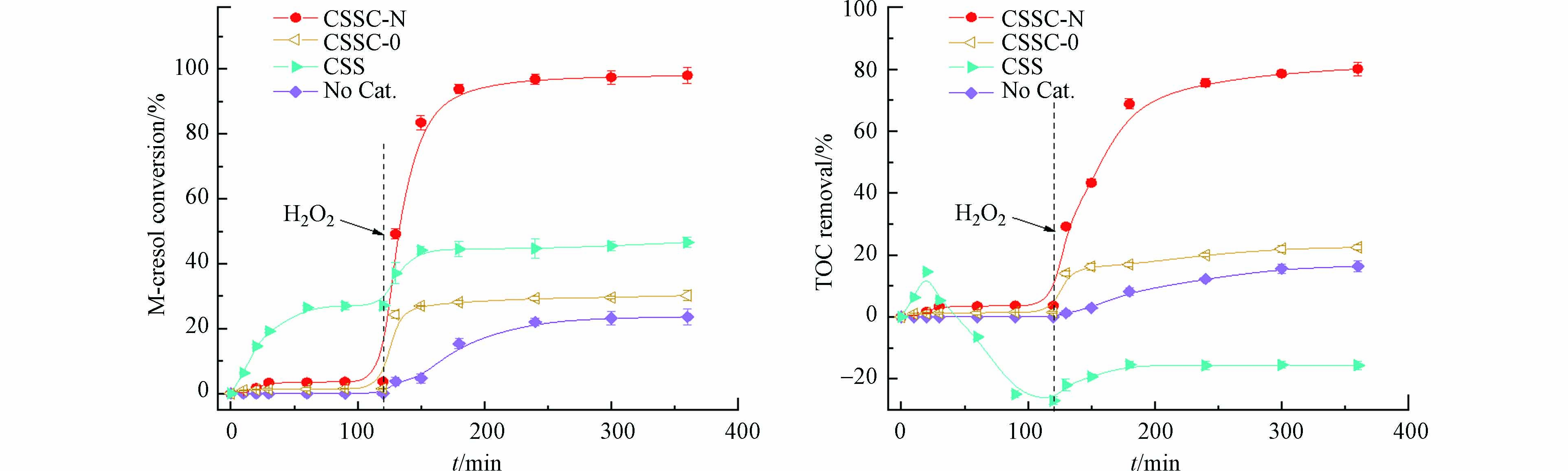
在催化降解反应前,在反应体系中不加H2O2,进行吸附反应. 由图4可知,前120 min,焦化污泥炭CSSC-0、CSSC-N对间甲酚的吸附去除率很小,去除率低于5%,因此吸附作用可以忽略. 而焦化污泥CSS对间甲酚的吸附作用较强,去除率达27.1%,这是因为CSS具有较高的比表面积(表1)和较丰富的官能团—OH(图1(b))等. 通过改性后的焦化污泥炭的比表面积显著减少,且官能团—OH减少,吸附作用很小,主要起催化作用[9, 26]. 如图4所示,在CSS吸附初期,TOC浓度由于间甲酚的快速吸附去除而降低,但随着CSS中的有机物成分逐渐溶解到液相中,TOC浓度增加,TOC去除率变为负值.
待吸附稳定后,在反应体系中加入H2O2,进行间甲酚降解反应. 在没加催化剂的体系中,降解反应发生较慢,最终间甲酚和TOC的去除率分别为23.6%和16.4%. 而加入CSS作为催化剂的体系中,间甲酚总去除率(吸附和降解)为46.6%,主要为吸附作用. 加入H2O2后,体系中的TOC去除率稍有增加,但由于CSS中有机物的大量溶出,TOC去除率仍为负值. 因此,污泥直接作为吸附剂或催化剂使用时,易将本身含有的有机物转移到液相中造成水体污染[27 − 28]. 未改性焦化污泥炭CSSC-0的催化降解效率较低,对间甲酚和TOC的去除率分别为30.2%和22.5%,远低于改性焦化污泥炭CSSC-N(98.1%和80.1%). 改性后的焦化污泥炭物相、官能团和表观形貌都发生了显著变化,有利于催化活性的提高.
-
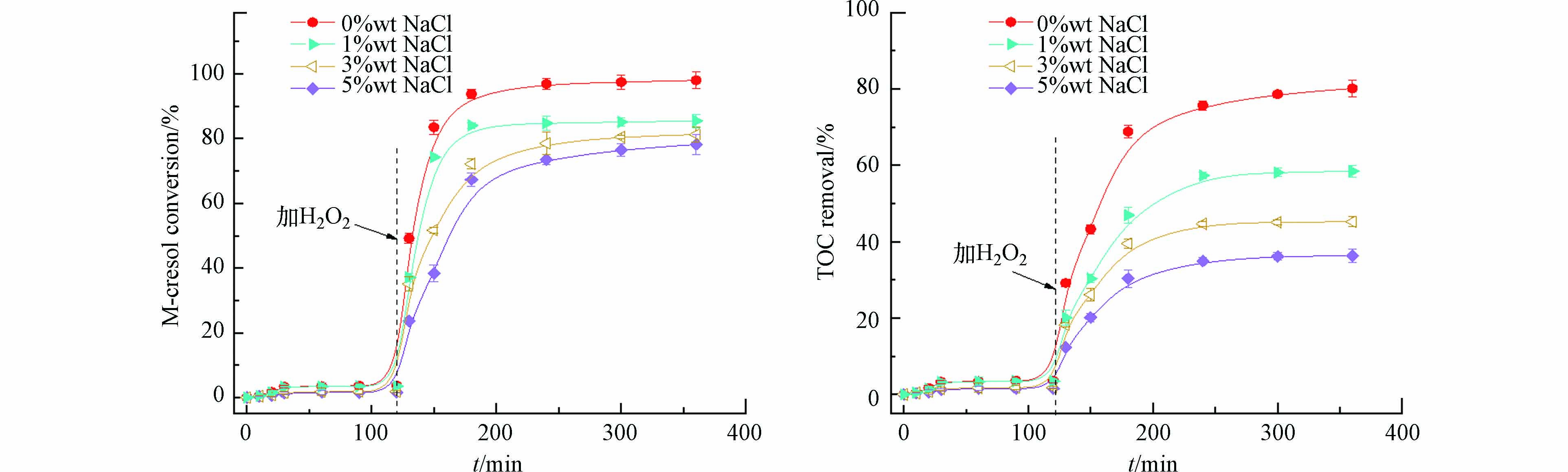
使用H2O2作为氧化剂的高级氧化体系中,处理含氯有机废水时,Cl-的亲电子能力非常强,能与·OH发生反应,影响有机物降解过程[29]. 以CSSC-N为催化剂进行CWPO反应,如图5所示,随着NaCl浓度升高,间甲酚的去除率逐渐降低. 在5%wt NaCl介质下,间甲酚的去除率仍然高达78.2%. 如加入NaCl对于TOC去除率影响更显著,NaCl浓度越高,TOC去除率越低. 当NaCl浓度为5%wt时,TOC的去除率仅为36.3%. 说明加入NaCl后,影响了焦化污泥炭CSSC-N对间甲酚的降解过程.
-
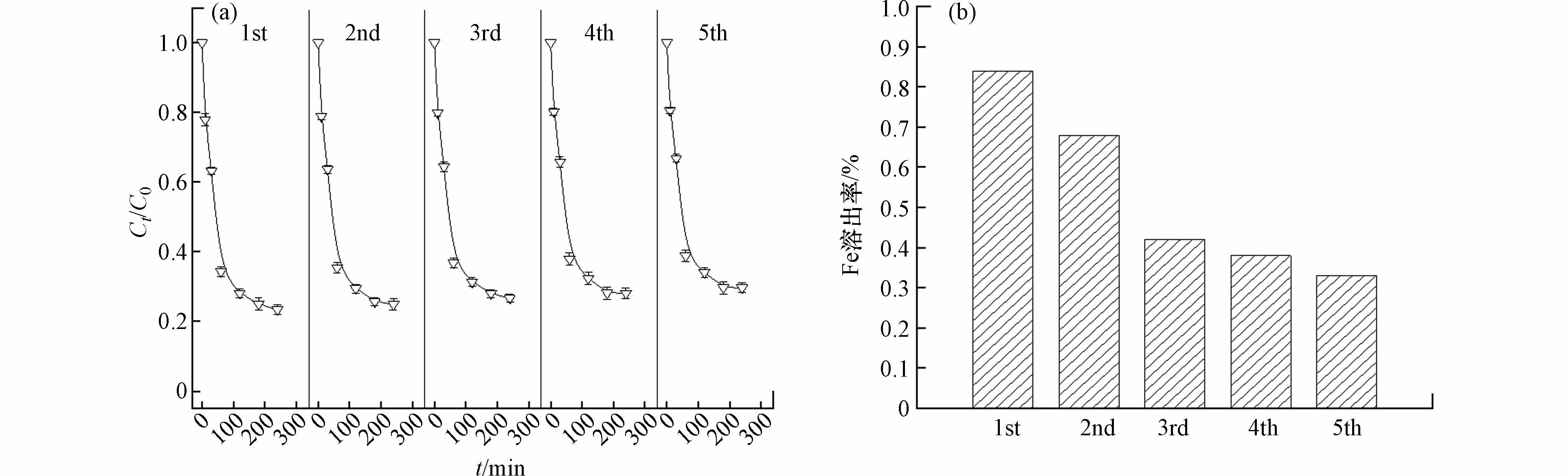
在5%wt NaCl介质下,对焦化污泥炭CSSC-N的催化性能进行稳定性考察,进行了5次重复利用实验,催化效率和铁溶出率的结果如图6所示. 在稳定性考察实验中,间甲酚的降解率均高于70%,且催化活性组分铁的溶出率均低于0.84%. 与其他载铁催化剂的铁溶出率(>5%)相比[5,26],CSSC-N催化剂在催化反应中的铁溶出率较低. 通过氯介质下的催化活性与稳定性考察实验,证明CSSC-N催化剂具有一定的抗氯性能,不仅催化活性较高,且具有较强的重复利用性.
-
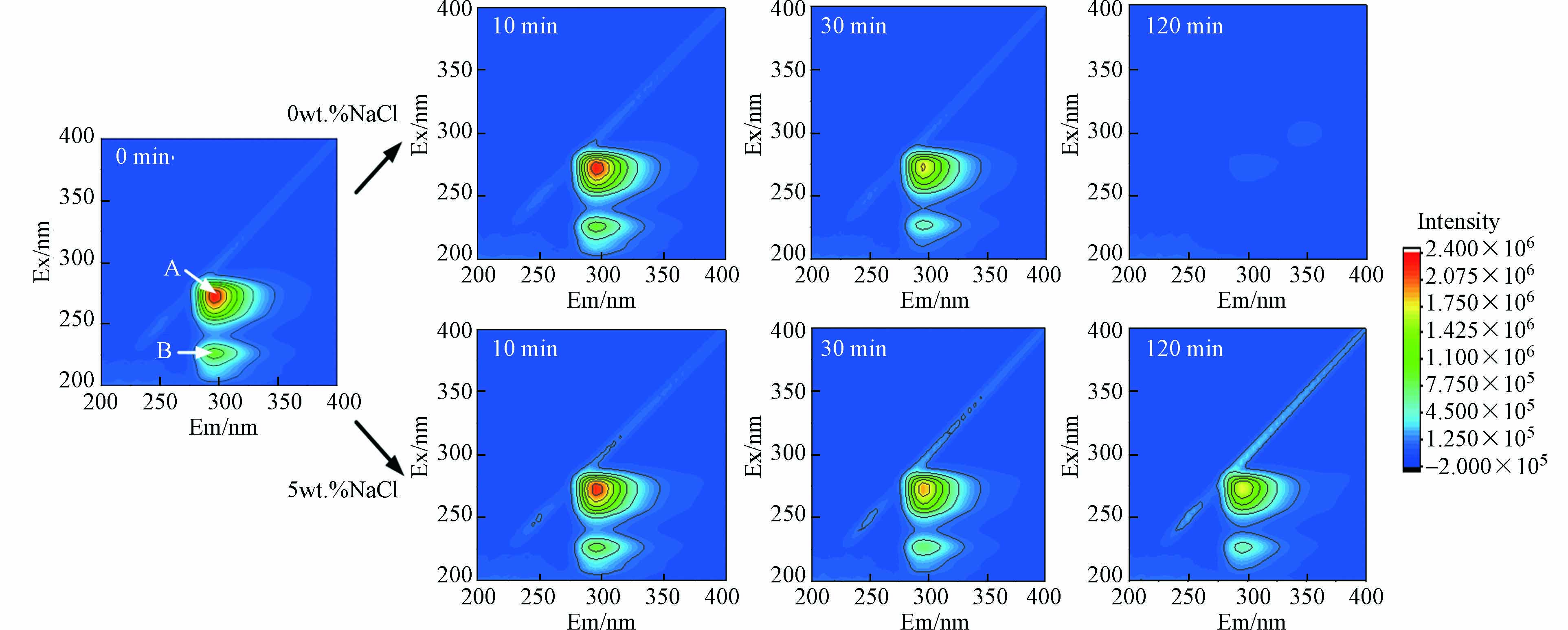
用三维荧光光谱分析CWPO体系中间甲酚的降解过程. 如图7所示,反应未开始时,间甲酚的荧光区域集中在两个主要的峰A(Ex/Em= 273/296 nm)和B(Ex/Em=225/296 nm)[30]. 在不加NaCl体系中,随着反应进行,峰A和B强度逐渐减弱,表明间甲酚的特征结构被破坏,发生降解. 催化降解反应120 min时,间甲酚几乎完全降解,与图5结果一致. 在加入5%wt NaCl反应体系中,峰A和B强度也逐渐降低,但120 min时仍较明显. 然而,图5中催化反应120 min时(总反应时间为240 min),间甲酚的去除率高达73.5%. 这可能是因为在氯介质下生成了氯代副产物(表2),虽然间甲酚去除率较高,但氯代副产物在三维荧光中仍然具有特征峰A和B,且TOC去除率较低(图5).
用FT-ICR-MS检测分析氯介质下间甲酚降解的中间产物,数据处理由软件DataAnalysis 4.2(Bruker, Daltonics GmbH, Bremen, Germany)完成. 检测到的中间产物共15种(P1—P15),如表2所示. 间甲酚甲基的邻位和对位被·OH攻击,导致苯环羟基化,生成二羟基甲苯(P3),随后转化为醌类物质(P4),P4进一步氧化为P5,随后发生开环反应生成链状化合物(P6—P9),有机链被进一步氧化,生成易降解的小分子酮和羧酸(P10—P15). 然而,氯介质下产生的P1(C7H5ClO3)和P2(C7H4Cl2O3)为氯代副产物,属于难降解有机物,导致TOC的降解率降低,影响了间甲酚的降解过程.
-
(1)以焦化污泥制备生物炭,采用低温硝酸改性提高其催化性能. 焦化污泥(CSS)比表面积大,表面官能团丰富,吸附效果较好. 而通过炭化和改性后,比表面积和官能团数量显著降低,焦化污泥炭CSSC-0和CSSC-N的吸附作用很弱.
(2)改性焦化污泥炭CSSC-N对间甲酚和TOC的去除率明显高于未改性焦化污泥炭CSSC-0和焦化污泥CSS. 催化剂CSSC-N表面含有丰富的铁元素,且具有多种铁物相,有利于CWPO反应中铁的循环和催化剂活性位点的提供.
(3)Cl-对CSSC-N催化降解间甲酚具有抑制作用,随着Cl-浓度的升高,间甲酚和TOC的去除率均有所降低,而TOC去除率降低更为显著. CSSC-N具有一定的抗氯性能,催化活性和稳定性较好.
(4)通过三维荧光光谱和FT-ICR MS分析间甲酚的降解过程和中间产物,表明Cl-的存在影响了间甲酚的降解过程,产生了更难降解的氯代副产物.
焦化污泥基生物炭在氯介质下催化降解间甲酚的性能
Catalytic degradation of m-cresol by coking sludge based biochar in chloride medium
-
摘要: 以焦化污泥制备生物炭,并在硝酸溶液中低温条件下进行改性,研究其在氯介质下对间甲酚降解的效率与机理. 焦化污泥(CSS)具有较大的比表面积和丰富的官能团,其吸附作用较强,对间甲酚的吸附去除率为27.1%. 而通过炭化和改性的焦化污泥炭的比表面积显著降低,官能团数量减少,吸附作用极弱. 在催化降解过程中,改性焦化污泥炭(CSSC-N)对间甲酚和总有机碳(TOC)的去除率分别为98.1%和80.1%,远高于未改性的焦化污泥炭(CSSC-0),主要归因于CSSC-N中具有多种含铁物质,有利于铁循环,且表面铁含量高达60.10%,能提供丰富的催化活性组分. Cl-对间甲酚和TOC的去除率有抑制作用,随着Cl-浓度的升高,去除率降低. 当废水中的NaCl为5%wt时,在催化重复实验中间甲酚的去除率均高于70%. CSSC-N具有一定的抗氯性能,在氯介质下具有较高的催化活性和稳定性. 三维荧光光谱分析和中间产物鉴定结果表明Cl-的存在影响了间甲酚的降解过程,生成了难降解的氯代副产物.
-
关键词:
- 催化湿式过氧化氢氧化 /
- 焦化污泥 /
- 生物炭 /
- 间甲酚 /
- 氯介质.
Abstract: A biochar was prepared from coking sludge and modified with nitric acid at low temperature. Afterwards, the degradation efficiency and mechanism of m-cresol in chloride medium was studied. Coking sludge (CSS) had a large specific surface area and was rich in functional groups, so its adsorption effect was strong. The adsorption removal rate of m-cresol with CSS was 27.1%. However, the adsorption effect of the coking sludge carbon through carbonization and modification was very weak, because the specific surface area and the number of functional groups were significantly reduced. In the catalytic degradation process, the removal rates of m-cresol and Total organic carbon (TOC) with the modified coking sludge carbon (CSSC-N) were 98.1% and 80.1%, respectively, which were much higher than those with the unmodified coking sludge carbon (CSSC-0). That was mainly because there were a variety of iron-containing substances in CSSC-N which was conducive to iron circulation, and the surface iron content was as high as 60.10%, providing catalytic active components. The removal rate of m-cresol and TOC was inhibited by Cl-, which was deceased with an increase of Cl- concentration. When the concentration of NaCl in wastewater was 5% wt, the removal rates of m-cresol during the catalytic repeat experiment were all above 70%. Therefore, CSSC-N with the chorine-resistant property had a high catalytic activity and stability in chlorine medium. Based on the results of three-dimensional fluorescence spectrometry analysis and intermediate products identification, the presence of Cl- affected the degradation process of m-cresol and chlorine-containing intermediates were produced.-
Key words:
- catalytic wet peroxide oxidation /
- coking sludge /
- biochar /
- m-cresol /
- chloride medium.
-
焦化废水产生于高温焦炭生产、气体净化和副产品回收过程,其化学成分复杂,含有大量的酚类物质、氰化物、硫化物、杂环化合物和多环芳烃等[1]. 因此,为了控制和减少其对环境的不利影响,通常采用物化预处理、生物处理、深度处理和回用处理相结合的组合工艺处理焦化废水[2]. 在处理过程中会产生大量污泥,而废水中的一部分难降解有机物会被污泥吸附[3]. 因此,焦化废水处理过程中产生的污泥如果不经妥善处置,会对环境造成危害. 污泥通过高温热解转化为高附加值的污泥炭是解决污泥处理处置问题的一个有发展前景的途径. 通过热解不仅能去除污泥中的有害污染物,杀灭污泥中的病原体,还能回收资源和能源. 污泥经过高温作用不仅形成了导电石墨碳,还形成了具有氧化还原活性的含氧官能团以及过渡金属及其氧化物等[4]. 污泥炭能够作为电子供体、受体和媒介,充当电子转移介质进一步提高催化性能,催化活化过硫酸盐、过氧化氢(H2O2)和臭氧等氧化剂生成硫酸根自由基(SO4-·)、羟基自由基(·OH)、超氧自由基(·O2-)和单线态氧非自由基(1O2)等[5 − 6].
在以过氧化氢(H2O2)为氧化剂的高级氧化体系中,研究者发现污泥炭的金属、杂原子掺杂的碳结构以及官能团可以作为电子转移的介质,使H2O2分解产生·OH,降解水体中的有机污染物[7]. 催化湿式过氧化物氧化 (CWPO) 工艺可以在温和的反应条件下高效降解废水中的难降解污染物[8]. 近年来,关于市政污泥热解炭化制备催化剂并用于CWPO体系中降解有机污染物的研究逐渐增多[6, 9]. 然而,工业废水处理过程中产生的污泥制备生物炭催化剂的研究相对较少. 焦化废水中含有的多种成分使焦化污泥中的元素种类更丰富,可能有利于其制备的污泥炭性质的改善[3]. 氯离子是高盐工业废水中的主要阴离子,能影响CWPO反应中生成的自由基种类,降低催化剂的活性,从而影响有机物的降解. 在制革和染料制造废水中,经常可以检测到高浓度的NaCl(>50 g·L−1),但是其对有机物降解过程的影响还未有很好的解释[10]. 因此,研究Cl-对有机物降解的影响对处理实际废水具有重要意义.
本研究以焦化废水处理厂再生水处理段排放的污泥为原料制备生物炭催化剂,采用硝酸低温改性方法,提高其催化性能,以间甲酚为模型污染物,研究在催化湿式过氧化氢反应体系中污泥炭催化剂对氯介质下间甲酚降解的催化活性和稳定性,并分析间甲酚的降解过程,考察氯离子对间甲酚降解的影响.
1. 材料与方法(Materials and methods)
1.1 试剂
间甲酚标准品(中国药品生物制品检定所),甲醇为色谱纯(国药集团化学试剂有限公司),过氧化氢、硝酸、氯化钠、亚硫酸钠等药品为分析纯(天津科密欧化学试剂有限公司),实验用水为超纯水.
1.2 焦化污泥炭的制备
焦化污泥取自山西省某工业园区的焦化废水处理厂中的再生水处理环节. 依次用超纯水和乙醇冲洗焦化污泥,再自然晾干,之后置于烘箱中105 ℃处理24 h,待冷却后,得到干燥后的焦化污泥,记为CSS. 随后,干燥的焦化污泥CSS在炭化转炉中进行炭化处理,反应条件为:300 mL·min−1的N2气氛下,5 ℃·min−1速率升温至800 ℃,焙烧2 h,炭化转炉的转速为5 r·min−1. 待冷却后,记为未改性焦化污泥炭CSSC-0. 未改性焦化污泥炭用6 mol·L−1的HNO3在0 ℃改性24 h,之后用超纯水冲洗至冲洗液为中性,得到改性焦化污泥炭,记为CSSC-N.
1.3 实验方法
配置浓度为100 mg·L−1的间甲酚废水溶液100 mL,加入250 mL锥形瓶中,分别再加入不同污泥催化剂,置于水浴振荡器上. 以120 r·min−1的速度振荡混合,进行吸附实验. 待吸附平衡后,加入过氧化氢,进行催化降解反应,每隔一段时间进行取样并立即加入Na2SO3抑制反应进行. 用0.45 μm滤膜过滤后分析间甲酚和TOC的浓度. 为保证实验数据准确性,实验做3个平行样,实验结果为3个平行样的平均值.
催化降解反应条件为:温度为30 ℃、pH为3、过氧化氢投加量为1.85 g·L−1、催化剂投加量为0.75 g·L−1. 吸附实验中,分别投加3种催化剂:CSS、CSSC-0、CSSC-N. 降解实验中,除上述3种催化剂外,再加入一组无催化剂的实验作为对照.
1.4 检测方法
样品中的金属元素含量用PerkinElmer 7300DV电感耦合等离子体发射光谱(ICE-OES)测定. 样品的比表面积及孔隙结构用麦克ASAP2460测定. 用日本理学Rigaku Ultima Ⅳ型X射线衍射仪(XRD)分析晶型结构,用PerkinElmer Spectrum Two红外光谱仪(FT-IR)分析催化剂表面官能团. 用Thermo ESCALAB 250XI型X射线光电子能谱(XPS)分析样品中元素价态和结合形态,用ZEISS Gemini 300型扫描电镜(SEM)结合X射线能谱(EDS)和元素面分布技术(EDS-mapping)观察样品表面形貌和元素组成. 间甲酚检测采用液相色谱法(Aglient HP1100G1312A). 色谱柱:Bioband GP120-C18(250 mm× 4.6 mm,5 μm),流动相为80%的甲醇和20%的超纯水,流速为1.0 mL·min−1,进样量20 μL. 用ShimadzuRF-6000荧光分光光度计(3D-EEMs)分析间甲酚的降解过程. 用Bruker Solarix 15T 傅立叶变换离子回旋共振质谱(FT-ICR-MS),分析间甲酚的降解中间产物.
2. 结果与讨论 (Results and discussion)
2.1 焦化污泥和污泥炭的基本性质
由表1可见,焦化污泥CSS含碳量为13.27%,铁含量高达28.32%,这与焦化废水混凝处理阶段加入含铁混凝剂有关. CSS经过炭化后转化为CSSC-0,金属元素含量升高,碳含量增加. CSSC-0经过硝酸改性后转化为CSSC-N,Ca含量大幅度降低,这与SEM--EDS mapping结果一致,说明酸溶解了氧化钙、碳酸钙等含钙物质. 此外,由于硝酸的氧化作用,CSSC-N中的氧含量增加. 改性后的焦化污泥炭中的铁含量稍有降低,但仍然在较高的水平(30.14%),保证了催化剂的活性组分含量.
表 1 焦化污泥炭样品的基本理化性质Table 1. Physicochemical properties of the coking sludge carbon samples样品 Samples C/% wt H/%wt O/%wt N/%wt S/%wt Fe/%wt Al/%wt Ca/%wt SBET/(m2·g−1) Vp/(cm3·g−1) CSS 13.27 2.54 19.21 1.72 6.57 28.32 0.91 12.16 86.024 0.154 CSSC-0 21.43 1.27 16.71 1.02 0.61 32.96 1.47 13.25 0.0807 0.00293 CSSC-N 17.69 0.65 26.57 0.98 4.82 30.14 0.33 2.36 9.665 0.0228 对焦化污泥和污泥炭样品进行XRD分析. 如图1(a)所示,焦化污泥CSS中含CaCO3和SiO2. 经过炭化后,焦化污泥中的CaCO3转化为CaO[11],且焦化污泥炭中出现FeO和FeAl2O4物相. 经过硝酸低温改性后的焦化污泥炭CSSC-N的XRD谱图中出现Fe3O4和Fe0.95C0.005的特征峰,这可能是因为在硝酸溶液中,污泥炭中的物质发生反应,生成了新的物相. 改性后的污泥炭中铁物相种类更丰富,有利于CWPO反应中的Fe(Ⅱ)和Fe(Ⅲ)的循环,铁铝尖晶石相FeAl2O4已被证实具有催化活性,对酸性环境具有一定耐受性[12 − 13]. 因此,铁物相的增加有利于催化H2O2分解产生·OH,促进间甲酚的降解. 图1(b)红外谱图显示,位于3440、1445、1092、870、776 cm−1处的峰分别属于—OH、C—H、C—O—C、无机碳酸盐、C═C的振动[3, 14 − 16]. 而位于500—600 cm-1范围的特征峰表明Fe—O官能团的存在[15, 17]. 焦化污泥通过炭化和改性后,C—H、C═C键的振动减弱,无机碳酸盐消失,Fe—O特征峰增强. 这说明C被氧化,与O结合形态增多,含铁官能团增多,与XRD和后文中的SEM EDS-mapping结果一致.
为研究焦化污泥炭表面特性和官能团分布,用XPS分析其C1s和Fe2p. 如图2(a)所示,将C1s分为3种类型的峰,分别为C—C(石墨态碳,284.8 eV),C—O(酚羟基及醚类碳,286.2—286.3 eV)和碳酸盐(或碳酸氢盐,288.9—289.18 eV)[18 − 19]. 通过硝酸低温改性后的焦化污泥炭中,石墨态碳被氧化,其含量减少,而与氧结合的碳含量增多. 污泥炭上的含氧官能团能充当电子介质将电子转移至H2O2发生反应,从而促进·OH的生成,达到降解有机污染物的目的[20]. 如图2(b)所示,Fe2p可分为4种峰:FeO(710.31—710.9 eV),α-Fe2O3(715.08—717.5 eV),γ-Fe2O3 (723.1—723.68 eV)和Fe3O4 (730.25 —731.36 eV)[21 − 23]. 经过改性后,FeO在焦化污泥炭表面上的含量增加. Fe2O3的含量减少,可能是由于原本包裹在Fe(Ⅱ)上的Fe2O3或其他氧化物被酸溶解,暴露在催化剂表面的Fe(Ⅱ)成为催化剂活性位点,使焦化污泥炭的催化性能得到提高.
由图3中的SEM图可观察催化剂的表观形貌特征,未改性的焦化污泥炭CSSC-0中的表面形态较光滑(图3(a1)),而改性后的焦化污泥炭CSSC-N表面粗糙多孔(图3(b1)),且具有铁氧化化物形态结构,这与前面XRD、元素分析等表征结果一致. 由EDS-mapping分析催化剂表面元素组成(图3(a2)和(b2)),通过改性后的焦化污泥炭表面元素组成发生了明显变化,表面铁含量由6.51%增加至60.10%. 说明通过改性后,表面含碳、钙等元素的物质被硝酸淋溶,而铁化物转移到催化剂表面. 结合表1中焦化污泥和污泥炭中铁的总含量结果,说明CSSC-N中的铁主要分布在催化剂表面,而CSSC-0中的铁主要分布在催化剂内部. 除铁含量外,铁在催化剂结构上的分布对催化剂的活性具有重要的影响[24]. 表面铁含量越多,活性位点越多. 越易催化过氧化氢产生·OH,从而氧化降解有机污染物[25].
2.2 焦化污泥炭的催化性能研究
在催化降解反应前,在反应体系中不加H2O2,进行吸附反应. 由图4可知,前120 min,焦化污泥炭CSSC-0、CSSC-N对间甲酚的吸附去除率很小,去除率低于5%,因此吸附作用可以忽略. 而焦化污泥CSS对间甲酚的吸附作用较强,去除率达27.1%,这是因为CSS具有较高的比表面积(表1)和较丰富的官能团—OH(图1(b))等. 通过改性后的焦化污泥炭的比表面积显著减少,且官能团—OH减少,吸附作用很小,主要起催化作用[9, 26]. 如图4所示,在CSS吸附初期,TOC浓度由于间甲酚的快速吸附去除而降低,但随着CSS中的有机物成分逐渐溶解到液相中,TOC浓度增加,TOC去除率变为负值.
待吸附稳定后,在反应体系中加入H2O2,进行间甲酚降解反应. 在没加催化剂的体系中,降解反应发生较慢,最终间甲酚和TOC的去除率分别为23.6%和16.4%. 而加入CSS作为催化剂的体系中,间甲酚总去除率(吸附和降解)为46.6%,主要为吸附作用. 加入H2O2后,体系中的TOC去除率稍有增加,但由于CSS中有机物的大量溶出,TOC去除率仍为负值. 因此,污泥直接作为吸附剂或催化剂使用时,易将本身含有的有机物转移到液相中造成水体污染[27 − 28]. 未改性焦化污泥炭CSSC-0的催化降解效率较低,对间甲酚和TOC的去除率分别为30.2%和22.5%,远低于改性焦化污泥炭CSSC-N(98.1%和80.1%). 改性后的焦化污泥炭物相、官能团和表观形貌都发生了显著变化,有利于催化活性的提高.
2.3 焦化污泥炭在氯介质下的催化性能
使用H2O2作为氧化剂的高级氧化体系中,处理含氯有机废水时,Cl-的亲电子能力非常强,能与·OH发生反应,影响有机物降解过程[29]. 以CSSC-N为催化剂进行CWPO反应,如图5所示,随着NaCl浓度升高,间甲酚的去除率逐渐降低. 在5%wt NaCl介质下,间甲酚的去除率仍然高达78.2%. 如加入NaCl对于TOC去除率影响更显著,NaCl浓度越高,TOC去除率越低. 当NaCl浓度为5%wt时,TOC的去除率仅为36.3%. 说明加入NaCl后,影响了焦化污泥炭CSSC-N对间甲酚的降解过程.
2.4 催化稳定性考察
在5%wt NaCl介质下,对焦化污泥炭CSSC-N的催化性能进行稳定性考察,进行了5次重复利用实验,催化效率和铁溶出率的结果如图6所示. 在稳定性考察实验中,间甲酚的降解率均高于70%,且催化活性组分铁的溶出率均低于0.84%. 与其他载铁催化剂的铁溶出率(>5%)相比[5,26],CSSC-N催化剂在催化反应中的铁溶出率较低. 通过氯介质下的催化活性与稳定性考察实验,证明CSSC-N催化剂具有一定的抗氯性能,不仅催化活性较高,且具有较强的重复利用性.
2.5 间甲酚的催化降解过程
用三维荧光光谱分析CWPO体系中间甲酚的降解过程. 如图7所示,反应未开始时,间甲酚的荧光区域集中在两个主要的峰A(Ex/Em= 273/296 nm)和B(Ex/Em=225/296 nm)[30]. 在不加NaCl体系中,随着反应进行,峰A和B强度逐渐减弱,表明间甲酚的特征结构被破坏,发生降解. 催化降解反应120 min时,间甲酚几乎完全降解,与图5结果一致. 在加入5%wt NaCl反应体系中,峰A和B强度也逐渐降低,但120 min时仍较明显. 然而,图5中催化反应120 min时(总反应时间为240 min),间甲酚的去除率高达73.5%. 这可能是因为在氯介质下生成了氯代副产物(表2),虽然间甲酚去除率较高,但氯代副产物在三维荧光中仍然具有特征峰A和B,且TOC去除率较低(图5).
表 2 氯介质下间甲酚催化降解中间产物Table 2. Intermediates generated during the catalytic degradation of m-cresol in chlorine medium产物Product 分子式Molecular formula 分子结构Molecular structure 检测值Measurement value (m/z) 理论值Theoretical value (m/z) 离子类型Ionic types P1 C7H5ClO3 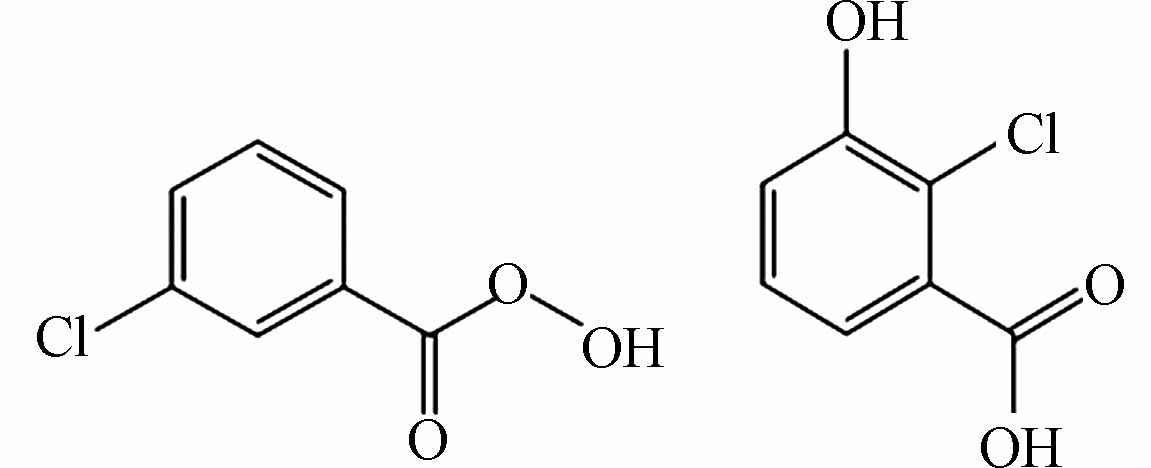
170.9853 170.9584 [M-H]- P2 C7H4Cl2O3 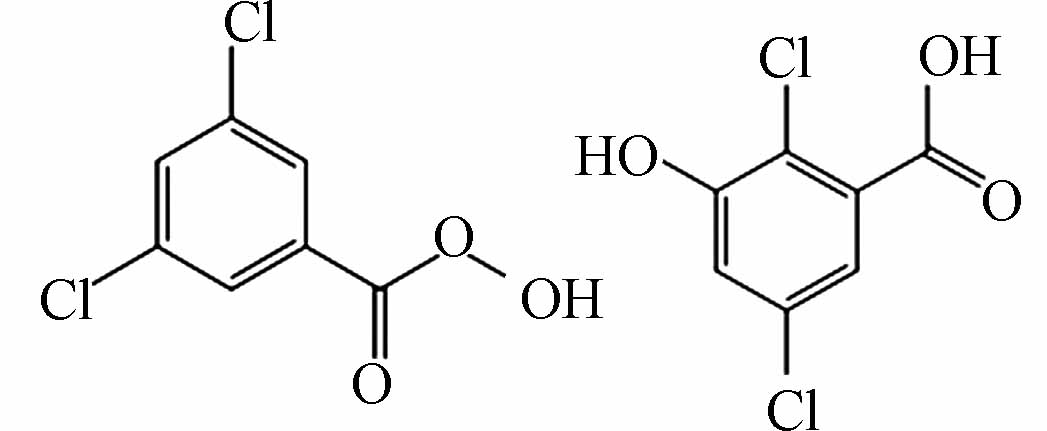
205.9463 205.9465 [M-H]- P3 C7H8O2 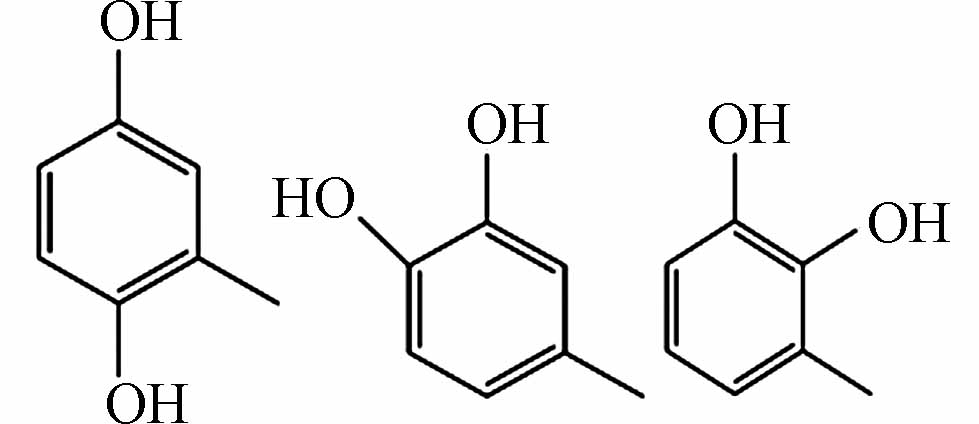
183.06616 183.06628 [M+CH3COOH-H]- P4 C7H6O2 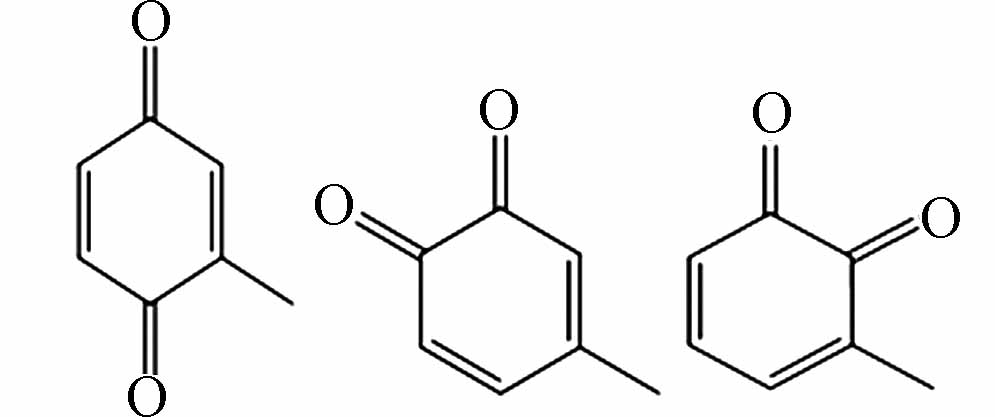
243.06613 243.06628 [2M-H]- P5 C7H10O2 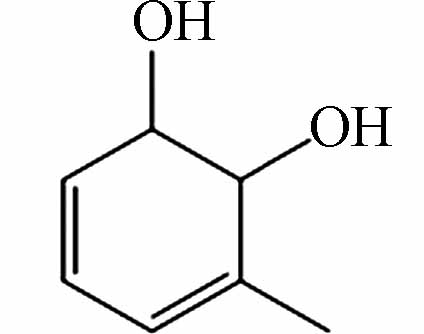
185.08181 185.08193 [M+CH3COOH-H]- P6 C7H12O2 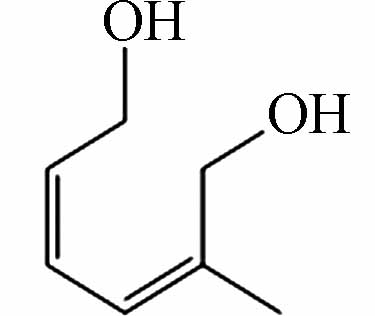
255.15986 255.16018 [2M-H]- P7 C6H10O 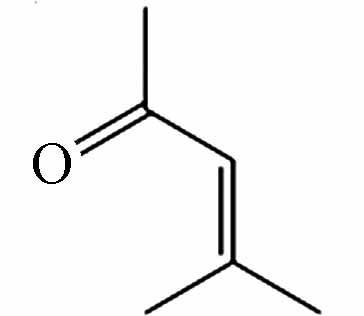
157.08685 157.08701 [M+CH3COOH-H]- P8 C6H12O 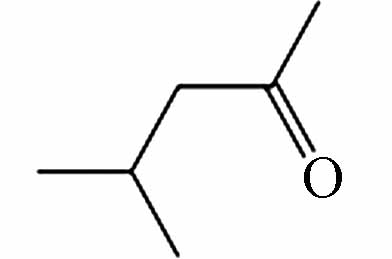
199.17017 199.17036 [2M-H]- P9 C5H10O 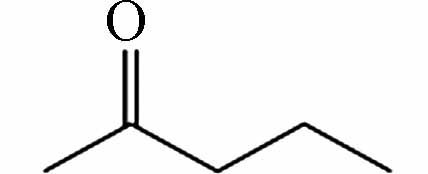
171.13892 171.13904 [2M-H]- P10 C5H6O3 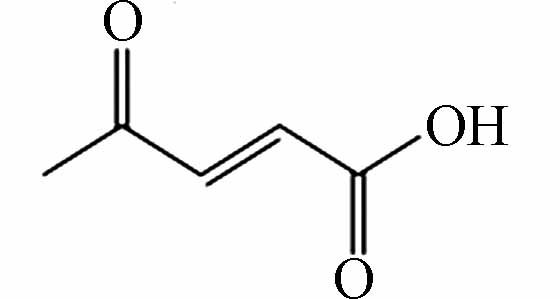
227.05587 227.05610 [2M-H]- P11 C5H8O3 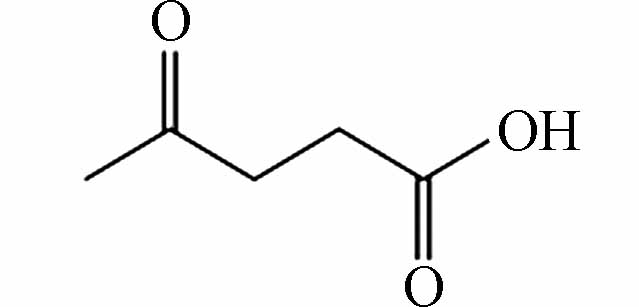
231.08721 231.08740 [2M-H]- P12 C4H8O 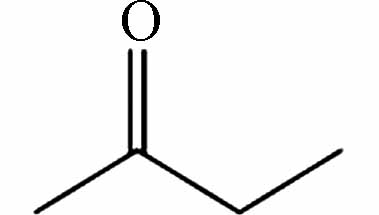
71.05016 71.05023 [M-H]- P13 C3H4O3 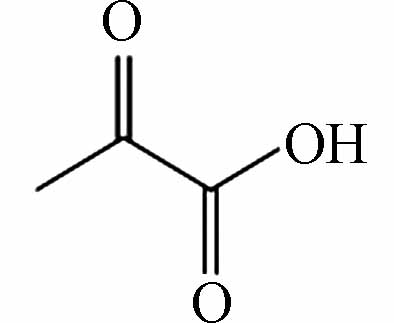
87.00875 87.00876 [M-H]- P14 C2H4O3 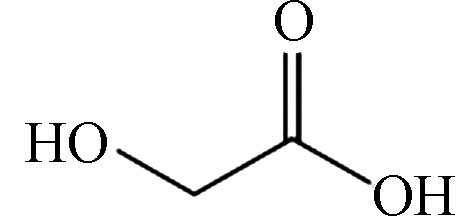
121.01457 121.01424 [M+CH3COOH-H]- P15 CH2O2 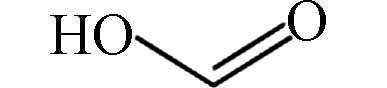
91.00382 91.00368 [M+CH3COOH-H]- 用FT-ICR-MS检测分析氯介质下间甲酚降解的中间产物,数据处理由软件DataAnalysis 4.2(Bruker, Daltonics GmbH, Bremen, Germany)完成. 检测到的中间产物共15种(P1—P15),如表2所示. 间甲酚甲基的邻位和对位被·OH攻击,导致苯环羟基化,生成二羟基甲苯(P3),随后转化为醌类物质(P4),P4进一步氧化为P5,随后发生开环反应生成链状化合物(P6—P9),有机链被进一步氧化,生成易降解的小分子酮和羧酸(P10—P15). 然而,氯介质下产生的P1(C7H5ClO3)和P2(C7H4Cl2O3)为氯代副产物,属于难降解有机物,导致TOC的降解率降低,影响了间甲酚的降解过程.
3. 结论(Conclusion)
(1)以焦化污泥制备生物炭,采用低温硝酸改性提高其催化性能. 焦化污泥(CSS)比表面积大,表面官能团丰富,吸附效果较好. 而通过炭化和改性后,比表面积和官能团数量显著降低,焦化污泥炭CSSC-0和CSSC-N的吸附作用很弱.
(2)改性焦化污泥炭CSSC-N对间甲酚和TOC的去除率明显高于未改性焦化污泥炭CSSC-0和焦化污泥CSS. 催化剂CSSC-N表面含有丰富的铁元素,且具有多种铁物相,有利于CWPO反应中铁的循环和催化剂活性位点的提供.
(3)Cl-对CSSC-N催化降解间甲酚具有抑制作用,随着Cl-浓度的升高,间甲酚和TOC的去除率均有所降低,而TOC去除率降低更为显著. CSSC-N具有一定的抗氯性能,催化活性和稳定性较好.
(4)通过三维荧光光谱和FT-ICR MS分析间甲酚的降解过程和中间产物,表明Cl-的存在影响了间甲酚的降解过程,产生了更难降解的氯代副产物.
-
表 1 焦化污泥炭样品的基本理化性质
Table 1. Physicochemical properties of the coking sludge carbon samples
样品 Samples C/% wt H/%wt O/%wt N/%wt S/%wt Fe/%wt Al/%wt Ca/%wt SBET/(m2·g−1) Vp/(cm3·g−1) CSS 13.27 2.54 19.21 1.72 6.57 28.32 0.91 12.16 86.024 0.154 CSSC-0 21.43 1.27 16.71 1.02 0.61 32.96 1.47 13.25 0.0807 0.00293 CSSC-N 17.69 0.65 26.57 0.98 4.82 30.14 0.33 2.36 9.665 0.0228 表 2 氯介质下间甲酚催化降解中间产物
Table 2. Intermediates generated during the catalytic degradation of m-cresol in chlorine medium
产物Product 分子式Molecular formula 分子结构Molecular structure 检测值Measurement value (m/z) 理论值Theoretical value (m/z) 离子类型Ionic types P1 C7H5ClO3 170.9853 170.9584 [M-H]- P2 C7H4Cl2O3 205.9463 205.9465 [M-H]- P3 C7H8O2 183.06616 183.06628 [M+CH3COOH-H]- P4 C7H6O2 243.06613 243.06628 [2M-H]- P5 C7H10O2 185.08181 185.08193 [M+CH3COOH-H]- P6 C7H12O2 255.15986 255.16018 [2M-H]- P7 C6H10O 157.08685 157.08701 [M+CH3COOH-H]- P8 C6H12O 199.17017 199.17036 [2M-H]- P9 C5H10O 171.13892 171.13904 [2M-H]- P10 C5H6O3 227.05587 227.05610 [2M-H]- P11 C5H8O3 231.08721 231.08740 [2M-H]- P12 C4H8O 71.05016 71.05023 [M-H]- P13 C3H4O3 87.00875 87.00876 [M-H]- P14 C2H4O3 121.01457 121.01424 [M+CH3COOH-H]- P15 CH2O2 91.00382 91.00368 [M+CH3COOH-H]- -
[1] ZHANG W H, WEI C H, CHAI X S, et al. The behaviors and fate of polycyclic aromatic hydrocarbons (PAHs) in a coking wastewater treatment plant[J]. Chemosphere, 2012, 88(2): 174-182. doi: 10.1016/j.chemosphere.2012.02.076 [2] FEI Y H, ZHAO D, LIU Y, et al. Feasibility of sewage sludge derived hydrochars for agricultural application: Nutrients (N, P, K) and potentially toxic elements (Zn, Cu, Pb, Ni, Cd)[J]. Chemosphere, 2019, 236: 124841. doi: 10.1016/j.chemosphere.2019.124841 [3] ZHANG F Z, WU K Y, ZHOU H T, et al. Ozonation of aqueous phenol catalyzed by biochar produced from sludge obtained in the treatment of coking wastewater[J]. Journal of Environmental Management, 2018, 224: 376-386. doi: 10.1016/j.jenvman.2018.07.038 [4] 赵迎新, 麻泽浩, 杨知凡, 等. 污泥生物炭催化高级氧化过程进展[J]. 化工进展, 2021, 40(7): 3984-3994. doi: 10.16085/j.issn.1000-6613.2020-1658 ZHAO Y X, MA Z H, YANG Z F, et al. Progress of advanced oxidation process catalyzed by sludge biochar[J]. Chemical Industry and Engineering Progress, 2021, 40(7): 3984-3994 (in Chinese). doi: 10.16085/j.issn.1000-6613.2020-1658
[5] TU Y T, XIONG Y, TIAN S H, et al. Catalytic wet air oxidation of 2-chlorophenol over sewage sludge-derived carbon-based catalysts[J]. Journal of Hazardous Materials, 2014, 276: 88-96. doi: 10.1016/j.jhazmat.2014.05.024 [6] YU Y, HUANG F, HE Y D, et al. Heterogeneous Fenton-like degradation of ofloxacin over sludge derived carbon as catalysts: Mechanism and performance[J]. Science of the Total Environment, 2019, 654: 942-947. doi: 10.1016/j.scitotenv.2018.11.156 [7] GU L, ZHU N W, ZHOU P. Preparation of sludge derived magnetic porous carbon and their application in Fenton-like degradation of 1-diazo-2-naphthol-4-sulfonic acid[J]. Bioresource Technology, 2012, 118: 638-642. doi: 10.1016/j.biortech.2012.05.102 [8] CHEN Y D, HO S H, WANG D W, et al. Lead removal by a magnetic biochar derived from persulfate-ZVI treated sludge together with one-pot pyrolysis[J]. Bioresource Technology, 2018, 247: 463-470. doi: 10.1016/j.biortech.2017.09.125 [9] YU L, LIU Y K, WEI H Z. A review: Preparation of sludge derived carbons and their performance in wastewater treatment[J]. Desalination and Water Treatment, 2020, 201: 169-182. doi: 10.5004/dwt.2020.26146 [10] FAN S S, WANG Y, WANG Z, et al. Removal of methylene blue from aqueous solution by sewage sludge-derived biochar: Adsorption kinetics, equilibrium, thermodynamics and mechanism[J]. Journal of Environmental Chemical Engineering, 2017, 5(1): 601-611. doi: 10.1016/j.jece.2016.12.019 [11] YU Y, WEI H Z, YU L, et al. Catalytic wet air oxidation of m-cresol over a surface-modified sewage sludge-derived carbonaceous catalyst[J]. Catalysis Science & Technology, 2016, 6(4): 1085-1093. [12] TAO S Y, LIANG S, CHEN Y, et al. Enhanced sludge dewaterability with sludge-derived biochar activating hydrogen peroxide: Synergism of Fe and Al elements in biochar[J]. Water Research, 2020, 182: 115927. doi: 10.1016/j.watres.2020.115927 [13] GAN Q, HOU H J, LIANG S, et al. Sludge-derived biochar with multivalent iron as an efficient Fenton catalyst for degradation of 4-Chlorophenol[J]. Science of the Total Environment, 2020, 725: 138299. doi: 10.1016/j.scitotenv.2020.138299 [14] YANG Y Q, CUI M H, REN Y G, et al. Towards understanding the mechanism of heavy metals immobilization in biochar derived from Co-pyrolysis of sawdust and sewage sludge[J]. Bulletin of Environmental Contamination and Toxicology, 2020, 104(4): 489-496. doi: 10.1007/s00128-020-02801-4 [15] DAI H W, XU S Y, CHEN J X, et al. Oxalate enhanced degradation of Orange II in heterogeneous UV-Fenton system catalyzed by Fe3O4@γ-Fe2O3 composite[J]. Chemosphere, 2018, 199: 147-153. doi: 10.1016/j.chemosphere.2018.02.016 [16] TIAN S Q, LIU Y L, JIA L R, et al. Insight into the oxidation of phenolic pollutants by enhanced permanganate with biochar: The role of high-valent manganese intermediate species[J]. Journal of Hazardous Materials, 2022, 430: 128460. doi: 10.1016/j.jhazmat.2022.128460 [17] WANG S Z, WANG J L. Kinetics of PMS activation by graphene oxide and biochar[J]. Chemosphere, 2020, 239: 124812. doi: 10.1016/j.chemosphere.2019.124812 [18] ZHOU J H, SUI Z J, ZHU J, et al. Characterization of surface oxygen complexes on carbon nanofibers by TPD, XPS and FT-IR[J]. Carbon, 2007, 45(4): 785-796. doi: 10.1016/j.carbon.2006.11.019 [19] CHEN X N, WANG X H, FANG D. A review on C1s XPS-spectra for some kinds of carbon materials[J]. Fullerenes, Nanotubes and Carbon Nanostructures, 2020, 28(12): 1048-1058. doi: 10.1080/1536383X.2020.1794851 [20] PI Z J, LI X M, WANG D B, et al. Persulfate activation by oxidation biochar supported magnetite particles for tetracycline removal: Performance and degradation pathway[J]. Journal of Cleaner Production, 2019, 235: 1103-1115. doi: 10.1016/j.jclepro.2019.07.037 [21] 余丽, 刘允康, 卫皇曌, 等. 酸改性颗粒污泥炭催化降解左氧氟沙星机制[J]. 中国环境科学, 2021, 41(10): 4695-4702. doi: 10.19674/j.cnki.issn1000-6923.2021.0354 YU L, LIU Y K, WEI H Z, et al. Mechanism of the catalytic degradation of levofloxacin by acid-modified granular sludge carbons[J]. China Environmental Science, 2021, 41(10): 4695-4702 (in Chinese). doi: 10.19674/j.cnki.issn1000-6923.2021.0354
[22] NIE X, LI G Y, LI S S, et al. Highly efficient adsorption and catalytic degradation of ciprofloxacin by a novel heterogeneous Fenton catalyst of hexapod-like pyrite nanosheets mineral clusters[J]. Applied Catalysis B: Environmental, 2022, 300: 120734. doi: 10.1016/j.apcatb.2021.120734 [23] SUN C, ZHOU R, E J, et al. Ascorbic acid-coated Fe3O4 nanoparticles as a novel heterogeneous catalyst of persulfate for improving the degradation of 2, 4-dichlorophenol[J]. RSC Advances, 2016, 6(13): 10633-10640. doi: 10.1039/C5RA22491H [24] YU L, WANG L, LIU Y K, et al. Pyrolyzed carbon derived from red soil as an efficient catalyst for cephalexin removal[J]. Chemosphere, 2021, 277: 130339. doi: 10.1016/j.chemosphere.2021.130339 [25] YAO C X, JIN C Y, WANG S Z, et al. Analysis of the degradation of m-cresol with Fe/AC in catalytic wet peroxide oxidation enhanced by swirl flow[J]. Chemosphere, 2022, 298: 134356. doi: 10.1016/j.chemosphere.2022.134356 [26] WEN H F, GU L, YU H X, et al. Radical assisted iron impregnation on preparing sewage sludge derived Fe/carbon as highly stable catalyst for heterogeneous Fenton reaction[J]. Chemical Engineering Journal, 2018, 352: 837-846. doi: 10.1016/j.cej.2018.07.106 [27] DEVI P, SAROHA A K. Utilization of sludge based adsorbents for the removal of various pollutants: A review[J]. Science of the Total Environment, 2017, 578: 16-33. doi: 10.1016/j.scitotenv.2016.10.220 [28] SMITH K M, FOWLER G D, PULLKET S, et al. Sewage sludge-based adsorbents: A review of their production, properties and use in water treatment applications[J]. Water Research, 2009, 43(10): 2569-2594. doi: 10.1016/j.watres.2009.02.038 [29] 刘宇程, 杨冰, 李沁蔓, 等. Cl-和pH对高级氧化工艺去除含盐废水中有机物的影响及机理[J]. 环境工程学报, 2021, 15(5): 1487-1499. LIU Y C, YANG B, LI Q M, et al. Effects and mechanism of Cl- and pH on organic matter removal in salt-containing wastewater treatment by advanced oxidation processes[J]. Chinese Journal of Environmental Engineering, 2021, 15(5): 1487-1499 (in Chinese).
[30] 刘屹. NH2OH/Fe2+/PMS体系对间甲酚的降解效能及机理研究[D]. 太原: 太原理工大学, 2019. LIU Y. Research on the degradation efficiency and mechanism of M-cresol by NH2OH/Fe2+/PMS system[D]. Taiyuan: Taiyuan University of Technology, 2019 (in Chinese).
-






 DownLoad:
DownLoad: