-
印染废水是工业废水中占比较大并且难以处理的废水之一. 大量印染废水的排放会造成水体、空气和土壤的污染,对生态环境的和谐共生造成严重的威胁,因此对有机染料废水的处理问题亟待解决[1]. 有机染料废水的常用处理方法有生物法[2]、物理法[3 − 8]、化学法[9 − 13],其中化学法中光催化氧化法应用研究最为广泛[14 − 17]. 光催化氧化法是利用紫外光或可见光照射半导体材料,使其与水体在接触的界面上产生的大量的空穴-电子,从而参与到氧化还原反应,在反应过程中产生的羟基自由基(·OH)和超氧自由基(·O2−)等能够破坏染料分子结构,进一步降解成无害小分子水、二氧化碳等[18 − 22]. 随着光催化基础研究的不断深入,推动光催化剂的研发与应用,其性能的优劣直接影响到光的利用率,因而其制备、改性提高催化剂的性能成为该领域的一个研究热点[23 − 26].
在常见的半导体催化剂中(如TiO2、CdS、WO3、Fe2O3、g-C3N4等),TiO2价格低廉、无毒,环境友好,化学稳定性好和耐光腐蚀性较强,因此在光催化领域内研究最多,是最具潜力的半导体光催化材料之一. 然而,TiO2禁带宽度大,仅能吸收约4%的太阳光,限制其在各个领域的应用. 并且TiO2激发产生的光生电子和空穴极易复合,使得该体系的量子效率不高[27 − 30]. 为提如高其量子效率,可以采用不同的策略如:离子掺杂、半导体复合等[31 − 32],并将它们组合在一起形成更多光吸收体系,以提高光催化效率. 光催化降解有机物废水是一种高效节能的催化氧化技术,对环境保护发挥重要作用. Zeng等[33]报道一种新型掺氟SnO2微球催化剂,在光照条件下高效去除废水Cr(VI)和甲基橙. Yu等[34]采用水热法制备出具有特殊层状形貌的SiO2/BiOCl材料,在降解苯酚、双酚A和罗丹明B等有机污染物方面表现出优异的光催化活性和稳定性. 此外TiO2表面的活性物质能够吸附有机染料并利用在水体中产生的强氧化基团羟基自由基等将有机物氧化为CO2、H2O及无机小分子等无害物质[35 − 39]. Bamwenda等[40]采用不同的方法分别制备了Pt/TiO2和Au/TiO2,探究其在乙醇溶液中的制氢效果. Zhang等[41]通过Pt、Au、Pd负载TiO2,研究其对酸性绿B的降解效果. Castro[42]认为,由于TiO2需要在近紫外区域受到激发,从而实现电子-空穴的分离,因此,在可见光的激发下染料会向TiO2的导电带中注入电子,从而实现催化氧化与光敏化的双重效应. Zangeneh等[43]在TiO2中加入窄带隙的CdS,发现TiO2的光催化吸收光谱红移,催化活性提高,分析发现当可见光的能量足够使CdS的价带电子向导带跃迁时,光生空穴将停滞在其价带上,由此延长电子与空穴的复合时间,提高了光生载流子的分离效率. Guo等[44]通过紫外-可见光谱表征,证实了C-N共掺杂的TiO2在紫外光下具有良好的光催化性能. Wu等[45]通过实验也发现,TiO2共掺C-N后其光催化活性有较大的提升. Wu等[46]研究发现,TiO2掺杂F与N后降解效果优于单掺N和F的TiO2. Wang等[47]通过溶胶-凝胶法制备了N-F掺杂TiO2,发现该催化剂降解苯酚的效果较好,可能是由于N与F的协同作用.
近年来,二硫化钼(MoS2)作为2D层状材料在过渡金属硫族化合物中的应用也取得了重大进展. MoS2的光吸收波长范围宽,可弥补其它半导体材料对可见光吸收低的缺点. 纳米MoS2具有更大的比表面积,可以暴露更多的催化活性位点. 在MoS2单层结构内,其类型类似于石墨烯结构,上层和下层由S组成,中层由Mo构成,两个S之间由共价键连接,每个Mo周围连接有6个S原子,因而一个S周围就有3个Mo原子,即为S-Mo-S三个原子层堆积而成. MoS2原子层间是较弱的范德华力,因此在反应中更容易剥离形成层状的二维结构,单层与单层之间的间距大致为0.65 nm[48]. Faglia等[49]通过磁控溅射方法在剥离得到的二维MoS2层片上,成功地沉积出一维垂直排列的ZnO纳米棒,并评价了其光致发光(PL)性能. Hsu等[50]采用热蒸镀法制备了MoS2/ZnO异质结,并对其光电导和光响应性能进行了分析,揭示了MoS2/ZnO异质结在光电子器件中的应用前景. Krishnan等[51]采用二步水热还原方法制备出对亚甲基蓝(MB)具有较好的光催化降解能力的MoS2/ZnO纳米复合物.
TiO2的导带电势高于MoS2,当电子被激发时MoS2迅速接收来自TiO2导带上的光生电子,从而促进TiO2中光生电子-空穴对的分离,提高其分离效率. 因而构建二氧化钛/二硫化钼异质结在光催化反应中具有一定发展前景. 例如,Zhou等[52]通过构建三维分层的异质结结构,当负载一定量的二硫化钼时,催化性能最佳;Zheng等[53]研究表明,在TiO2纳米管上复合MoS2所形成的异质结结有较好的光敏性、更好的光催化性能,成功提升了光催化活性和光电流响应. 此外,构建异质结是一种半导体复合改性的常见方法,由于不同半导体之间的能带结构不同,光生载流子的跃迁和运动发生变化从而促进电子空穴的分离,改善光催化剂的催化活性. Chen等[54]通过构建ZnTiO3/Zn2Ti3O8/ZnO三元Z型异质结光催化剂改善电子空穴的分离和迁移,使其在降解有机污染物和还原重金属Cr(Ⅵ)离子方面表现出优异的性能. 并研究了非贵金属(Cu NPs)沉积ZnTiO3/Zn2Ti3O8/ZnO,能进一步提高其在重金属Cr(Ⅵ)还原、染料降解和催化产氢的效能[55].
因此,MoS2复合TiO2,将可能减小带隙、增强TiO2对可见光的吸收能力和拓宽光响应范围,形成异质结降低光生载流子复合率,改善TiO2的催化性能. 本文通过沉淀胶溶法和超声辅助法制备出具有良好光催化性能的MoS2/F-TiO2异质结,并研究其吸附-光催化性能,为处理污水中的有机物提供一种策略.
-
所有化学品均为分析纯. 硫酸钛(Ti(SO4)2国药集团化学试剂有限公司),氢氟酸(HF成都市科龙化工试剂厂),氨水(NH3·H2O重庆万盛川东化工有限公司),二硫化钼(MoS2 山东西亚化学工业有限公司),无水乙醇(CH3CH2OH重庆川东化工有限公司),罗丹明B(RhB山东西亚化学工业有限公司),试剂溶液均为超纯水配置.
-
称取4.8 g的硫酸钛于烧杯中,加入100 mL去离子水搅拌至完全溶解,在持续搅拌状态下滴加一定量的氢氟酸(F:Ti=20:1),继续搅拌10 min后,缓慢滴加氨水调节溶液pH=8—9,并继续搅拌30 min后,用去离子水离心洗涤数次,在60 ℃下干燥,将干燥后的样品研磨得到固体粉末. 在马弗炉中煅烧,程序为:升温速率2 ℃·min−1,550 ℃保温2 h,随炉冷却至室温得到F-TiO2,以上方法不加氢氟酸得到纯TiO2.
-
称取数份适量的”1.1”所制备的样品放入烧杯中,加入无水乙醇35 mL磁力搅拌30 min,再加入不同比例二硫化钼粉体,将烧杯放入超声清洗仪超声60 min,使MoS2与F-TiO2结合. 放入60 ℃烘箱中干燥得到不同MoS2质量分数的MoS2/F-TiO2复合材料. MoS2在MoS2/F-TiO2复合材料中质量分数(%)分别为0.5%、1.0%和2.0%,分别标记为0.5%MoS2/F-TiO2、1.0%MoS2/F-TiO2和2.0%MoS2/F-TiO2.
-
称取数份0.02 g光催化剂于石英管中,分别量取25 mL 10 mg·L−1的罗丹明B溶液在石英管中,将其一起放入光催化反应仪器中. 先在黑暗条件下处理30 min,然后混匀溶液用离心机(4000 r·min−1)离心5 min,用紫外可见分光光度计测量其吸光度,纯罗丹明B溶液的吸光度为初始吸光度(A0). 然后开灯调节光催化反应仪的功率使金卤灯的功率达到300 W,光降解反应中每隔一定时间取样离心并测其吸光度,该时间点溶液吸光度记为(At),利用公式(1)计算罗丹明B的降解率,以此反应催化剂光催化性能.
-
通过扫描电子显微镜测试样品的表观形貌(SEM,Regulus 8100,日本日立公司);采用X射线衍射仪(XRD,DX-2700,丹东浩元仪器有限公司)研究样品的晶相组成(Cu Kα,步进角度0.05°,工作电压/电流为40 kV/40 mA);使用Brunauer-Emmett-Teller比表面分析仪(BET,ASAP2460,Micromeritics)分析粉末样品比表面积和孔结构;通过紫外可见分光光度计(UV-Vis-Abs,UV2700,日本岛津公司)分析固体样品的光吸收特性并计算禁带宽度;采用X射线光电子能谱(XPS,Escalab 250 Xi,美国赛默飞世尔科技公司)表征样品的元素组成和和化合价态;通过傅里叶变换红外光谱(FTIR,Thermo Thermo Nicolet iS5,美国赛默飞世尔科技公司)分析样品中官能团结构.
-
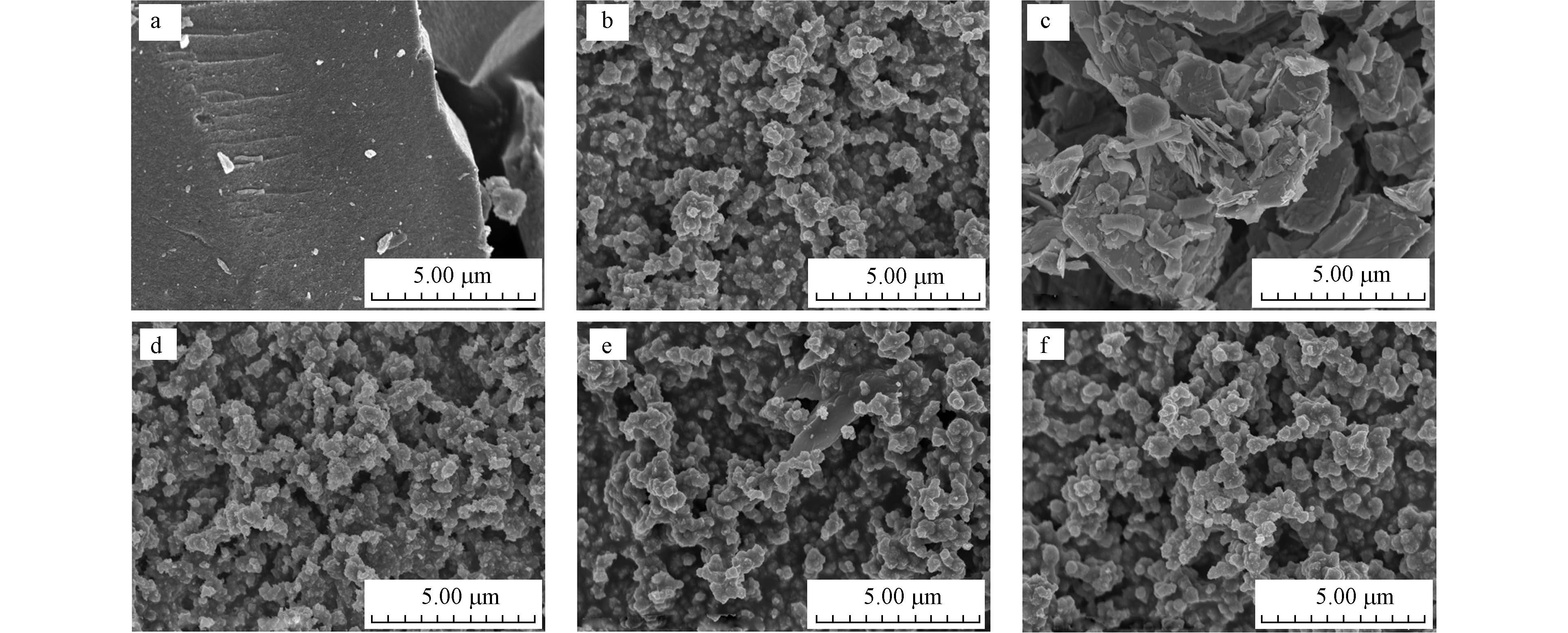
图1为纯TiO2、F-TiO2、MoS2和不同质量比例MoS2/F-TiO2的扫描电子显微镜图片(SEM). 如图1a、b,纯TiO2为表面较为光滑的块状结构,F-TiO2形貌不规则,由大小较为均一的颗粒聚集而且表面粗糙,使其具有更大比表面积,提供更多的催化反应活性位点. 这可能是由于锐钛矿的高能(001)晶面对F−的选择性吸附可使TiO2生长成尺寸较小的材料. 从图1c可知,MoS2为片层状的集聚体,片与片之间堆积非常紧密,片层的表面较为光滑平整. 图1d、e、f分别为0.5%、1.0%、2.0%的二硫化钼负载氟化二氧化钛的MoS2/F-TiO2样品形貌. 由图可知,随着MoS2负载量增加,MoS2对F-TiO2形貌结构影响明显: 0.5%MoS2/F-TiO2的颗粒堆积现象明显,颗粒团聚使尺寸增大;当随着MoS2含量增多有利于减弱颗粒团聚现象,这使MoS2/F-TiO2比表面积增大,暴露更多的反应活性位点促进光催化反应. 因为MoS2加入降低F-TiO2的表面能,有利于颗粒物的分散.
-
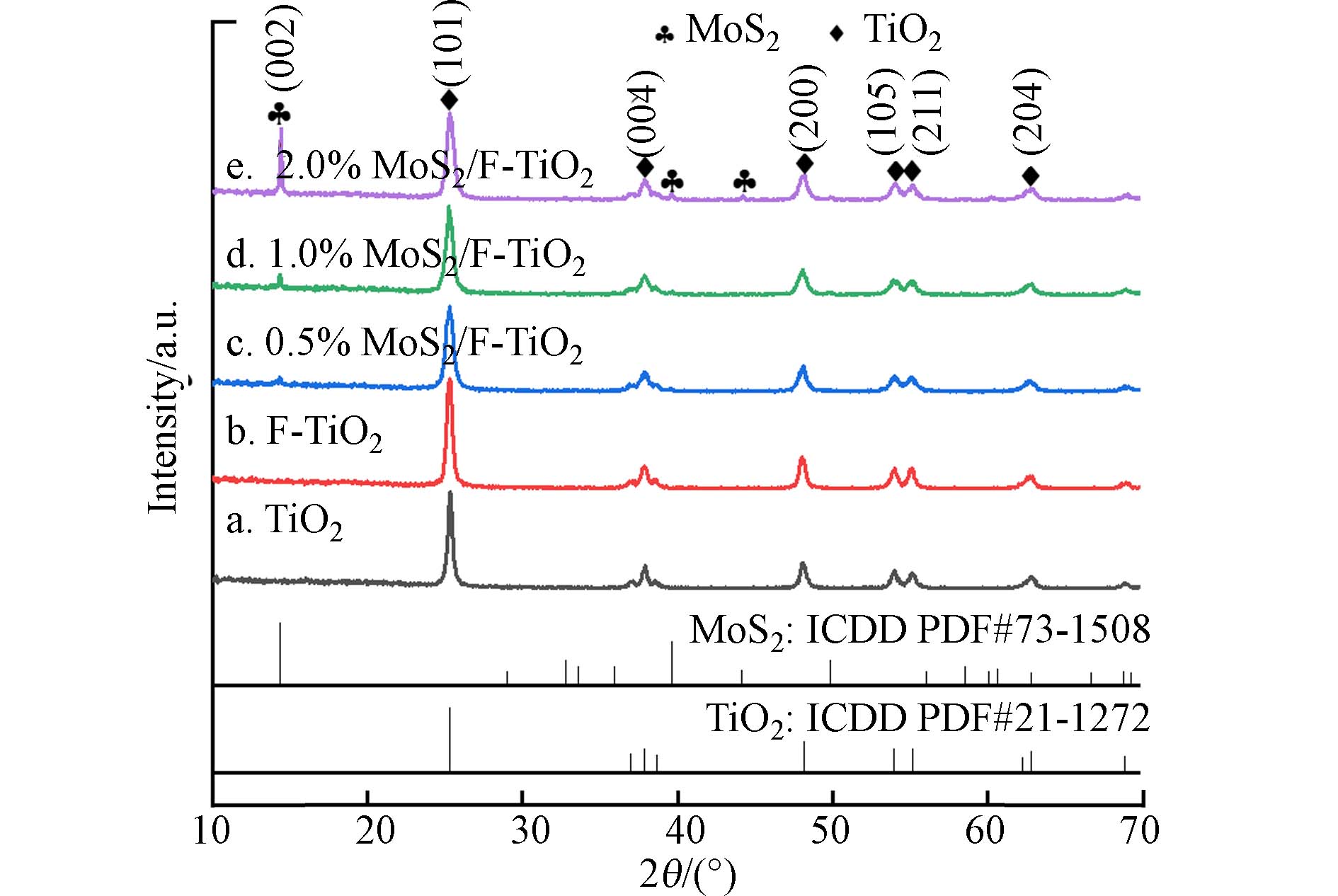
图2为TiO2和不同质量比MoS2/F-TiO2样品的XRD图,通过与锐钛矿TiO2(PDF#21-1272)和MoS2(PDF#73-1508)标准卡片对比分析Ti-O化合物和Mo-S化合物来确定样品的物相成分. 由图2可知,在衍射角2θ在25.3°、37.8°、48°、53.9°、55°和62.7°附近观察到较为明显的锐钛矿相TiO2衍射峰,分别对应的晶面为(101)、(004)、(200)、(105)、(211)和(204);在2θ为14.1°附近观察到MoS2特征衍射峰对应的晶面为(002),表明MoS2成功负载于F-TiO2. 由图2可知,样品中以锐钛矿相的二氧化钛为主,且结晶程度较好. 随着MoS2含量的增加,锐钛矿TiO2的XRD的谱线没有明显改变,但出现MoS2的特征衍射峰强度相应增强. 在图2中a、b、c、d曲线上并未检查到氟化合物的衍射峰,说明氟离子掺入并未影响锐钛矿相的形成. 由XRD图和Scherrer公式(2)可计算晶粒尺寸,结果如表1所示. 改性后的F-TiO2晶粒尺寸均大于纯TiO2的晶粒尺寸, 1.0%MoS2/F-TiO2样品晶粒度最大. 其中F-TiO2晶粒尺寸大于纯TiO2,说明氟的修饰可以促进TiO2晶粒的生长并提高结晶度. 此外,氟化改性的TiO2晶格产生缺陷,F−进入TiO2晶格内部,因此F-TiO2化学性质更为活泼,结晶度提高,晶粒尺寸更大 (D为晶粒平均厚度,K为Scherrer常数,λ为X射线波长,β为半峰高,θ为布拉格角)。
-
图3A为不同MoS2/F-TiO2样品的紫外可见吸收光谱(UV-Vis-Abs). 由图3可知,改性TiO2后在紫外光区和可见光区有明显区别. 首先,纯TiO2吸收光谱的阈值波长(λg)为427.88 nm,F-TiO2吸收光谱的阈值波长为390.83 nm,并且0.5%、1.0%和2.0%MoS2/F-TiO2的吸收光谱的阈值波长分别为392.69 nm、394.54 nm和398.61 nm. 其次,F-TiO2、MoS2/F-TiO2与纯TiO2相比,紫外吸收边带均发生蓝移;与纯TiO2吸收光谱的阈值波长相比,F-TiO2蓝移约37.05 nm,表明氟的加入会增加光响应的阈值,从而改善光催化剂对光的吸收能力,增加其光催化活性.
氟掺杂使二氧化钛吸收带边蓝移原因可能是:F−的加入改变了TiO2的能带结构,使得TiO2的带隙能有一定的降低,氟化后的二氧化钛在可见光范围内吸收性增加,是由于F−进入二氧化钛晶格内部替代O2−,所产生的电价使得Ti从Ti4+变成Ti3+,生成的Ti3+降低了禁带宽度[56]. 在光的照射下,一定时间内能激发产生较多的光生电子-空穴,有利于光催化活性的提高. MoS2对F-TiO2的改性使之紫外吸收边带产生红移. 可以解释为:二硫化钼与氟化二氧化钛形成异质结后,带有能量的光照使得MoS2上电子发生跃迁,二硫化钼的导带低于二氧化钛,由此光生电子能更多的转移到而二氧化钛的导带上,光生空穴留在MoS2的价带上,进而高效的降低电子-空穴的复合率. 窄带隙的MoS2与F-TiO2复合后,禁带宽度减小,带隙结构发生一定的变化,以上两点限制了MoS2/F-TiO2对可见光的响应范围[57]. MoS2/F-TiO2相较于F-TiO2产生红移,F-TiO2相较于纯TiO2产生蓝移,说明制备的改性二氧化钛材料同时具有氟和二硫化钼性质,因此MoS2/F-TiO2在390—450 nm范围对光的吸收较明显.
图3B为F-TiO2和不同MoS2/F-TiO2的(αhv)1/2和光电子能量关系图,通过研究发现纯TiO2带隙能量为2.73 eV,F-TiO2带隙能量为3.06 eV,0.5%、1.0%和2.0%MoS2/F-TiO2带隙能分别为3.04 eV、3.01 eV和2.96 eV. 由图3分析可知,随着MoS2含量的增加,催化剂的阈值波长(λg)增大,光电子能量减小,即带隙能减小,这与前文分析一致.
-
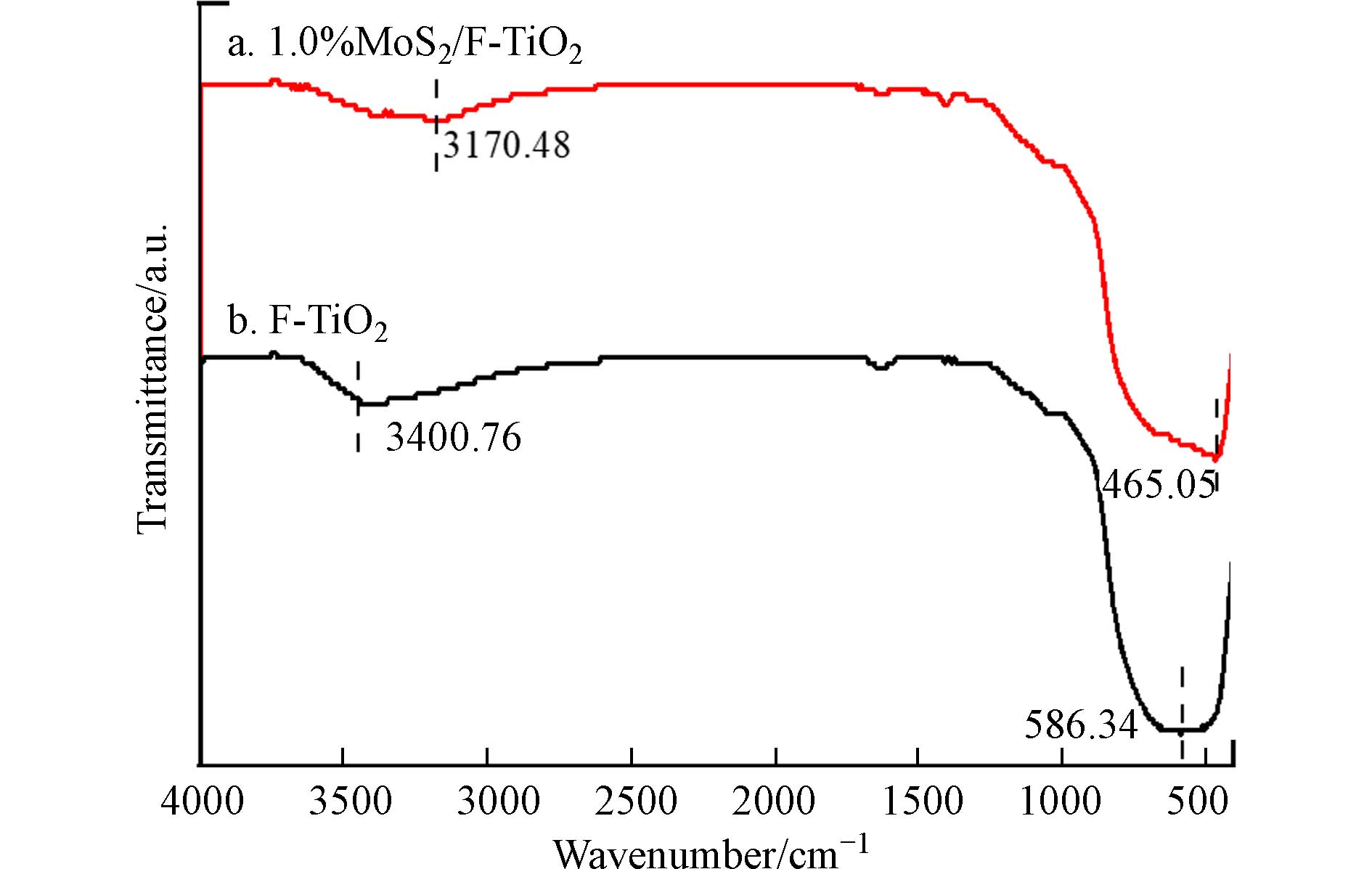
对F-TiO2和1.0%MoS2/F-TiO2样品进行傅里叶变换红外光谱分析(FTIR),结果如图4所示. 二硫化钼的特征峰位置主要在800—400 cm−1范围内,a曲线465.05 cm−1处的特征峰可能是由于Mo—S键振动引起的,表明二硫化钼成功负载在F-TiO2上. b曲线586.34 cm−1处出现的峰可归属于F—Ti键的伸缩振动,为说明F−在二氧化钛表面可能是以化学吸附状态存在,也可能是F−进入晶格取代O的位置存在[58]. 在3600—3400 cm−1处左右的宽吸收带,是TiO2表面羟基基团或吸附水分子中O—H键的伸缩振动引起的,而1635 cm−1处的吸收峰为水分子的弯曲振动导致. 总之,两曲线吸收峰相似,而F-TiO2的吸收强度更高,这可能是MoS2负载在F-TiO2表面抑制了羟基自由基的产生. MoS2与TiO2的吸收峰也在700—500 cm−1处左右,二者的特征吸收峰叠加,从而使得1.0%MoS2/F-TiO2在500 cm−1左右处的吸收强度增大.
-
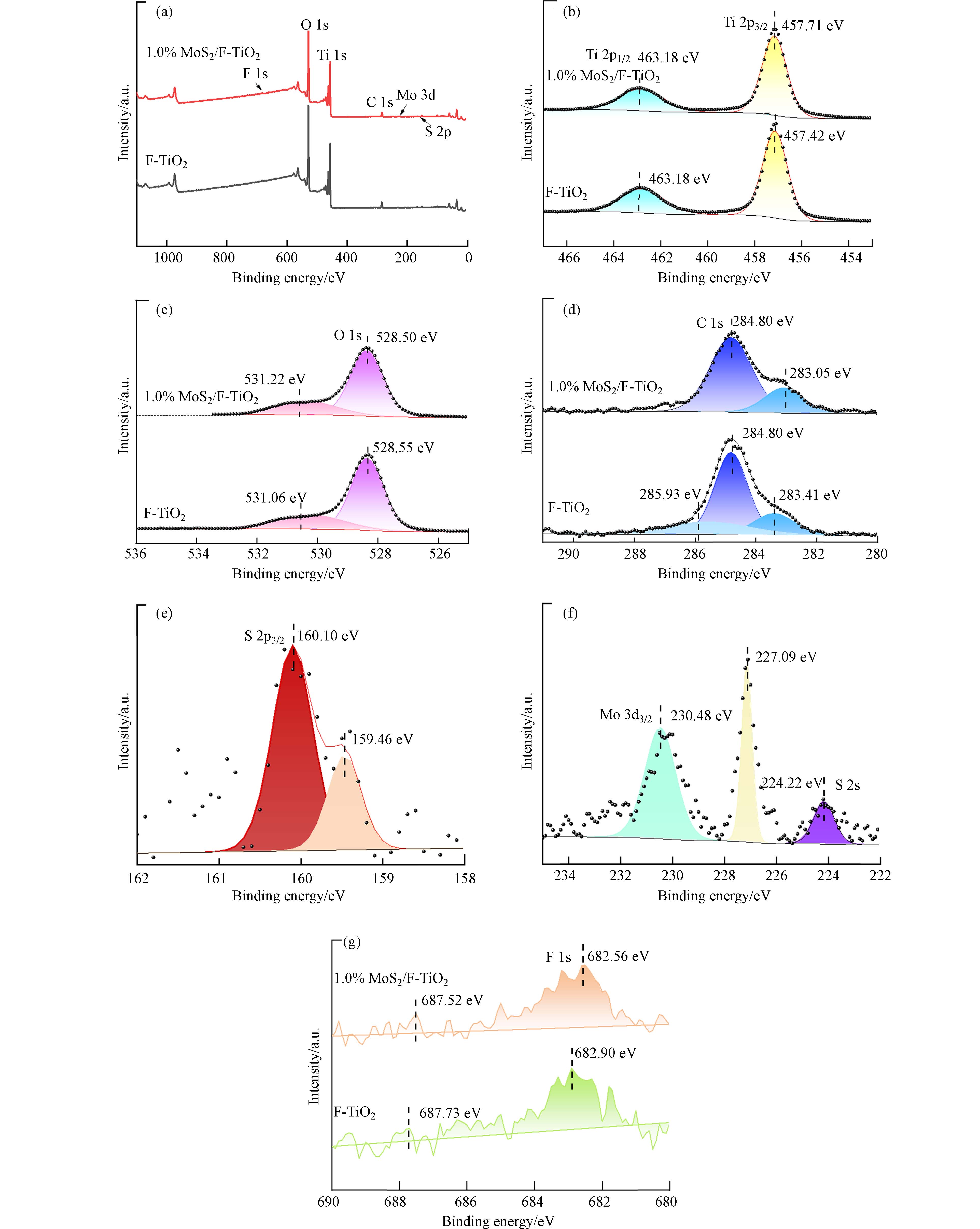
通过X射线光电子能谱(XPS)研究1.0% MoS2/F-TiO2、F-TiO2表面元素组成及价态,结果见图5. 图5a为样品元素全谱,在全谱图中F-TiO2和1.0% MoS2/F-TiO2中均含有C和F元素,C元素是样品测试过程中引入的,F元素的峰在F-TiO2和1.0% MoS2/F-TiO2全谱图中均不明显. 此外,在MoS2/F-TiO2的XPS全谱可见其中含有一定量的S和Mo.
图5b为Ti元素XPS分谱图,两个样品均出现了两个特征峰,在自旋轨道Ti 2p1/2处峰值相同均为463.18 eV,在Ti元素的自旋轨道为Ti 2p3/2处的结合能分别为457.71 eV和 457.42 eV,在457 eV左右处的峰归属于Ti3+而463 eV左右处的峰归属于Ti4+[59]. 并且样品的Ti元素的结合能大于标准Ti的结合能,说明Ti主要以Ti4+形式存在[60]. 与F-TiO2相比,1.0% MoS2/F-TiO2在自旋轨道为Ti 2p3/2处的结合能增加了0.29 eV,说明MoS2的修饰对Ti4+周围化学键环境有较大影响. 氟元素对二氧化钛的结合能本身就有较大的影响,这是由于吸附在TiO2表面的F−有较高的电负性,能强有力的吸引Ti原子周围的电子,F−在进入TiO2晶格中会取代O的位置,形成Ti—F键,由此使得Ti原子的电子云密度降低,故Ti 2p3/2处结合能增加.
图5c为O元素XPS分谱,MoS2/F-TiO2与F-TiO2相比,在O 1s轨道的结合能减小了0.05 eV,在O 2s轨道的结合能增加了0.16 eV. 可能是因为MoS2与F-TiO2复合后,导致O原子周围的电子云密度增加,从而使O 1s轨道处Ti原子的结合能减小. 通过拟合,531.22 eV处的峰可归属为羟基氧[31],MoS2/F-TiO2的峰强度要比F-TiO2的峰强度高,其原因可能是MoS2与F-TiO2复合后有利于其表面羟基氧的产生,这可能是MoS2对其改性,一部分进入晶格内部,一部分对F-TiO2表面进行修饰,导致表面对羟基吸附性增强,延长空穴寿命产生较多的具有高催化活性的·OH. 图5d为测试时加入污染碳源的分谱,通过284.8 eV处C峰位校正各谱线. 由图5g可知,MoS2/F-TiO2和F-TiO2均出现F元素的两个特征峰,与F-TiO2相比,MoS2/F-TiO2两个特征峰结合能都有一定程度的降低,原因是片状MoS2复合F-TiO2,其异质结结构导致其结合能降低[61]. 由图5e,图5f可知Mo 3d3/2、Mo 3d5/2的结合能分别是230.48 eV、227.09 eV,图中位于224.22 eV和160.10 eV特征峰分别对应S 2p3/2和S 2s. 根据报道,纯MoS2中Mo 3d5/2、S 2p1/2和S 2p3/2的结合能分别位于232.5 eV、229.eV和163.3 eV. 而与纯MoS2相比,MoS2/F-TiO2异质结中O 3d5/2、S 2P1/2和S 2P3/2的结合能都向更低结合能方向移动,峰移动可归因于MoS2与F-TiO2异质结导致结合能降低,也表明MoS2和F-TiO2是以化学键结合的方式复合而成.
-
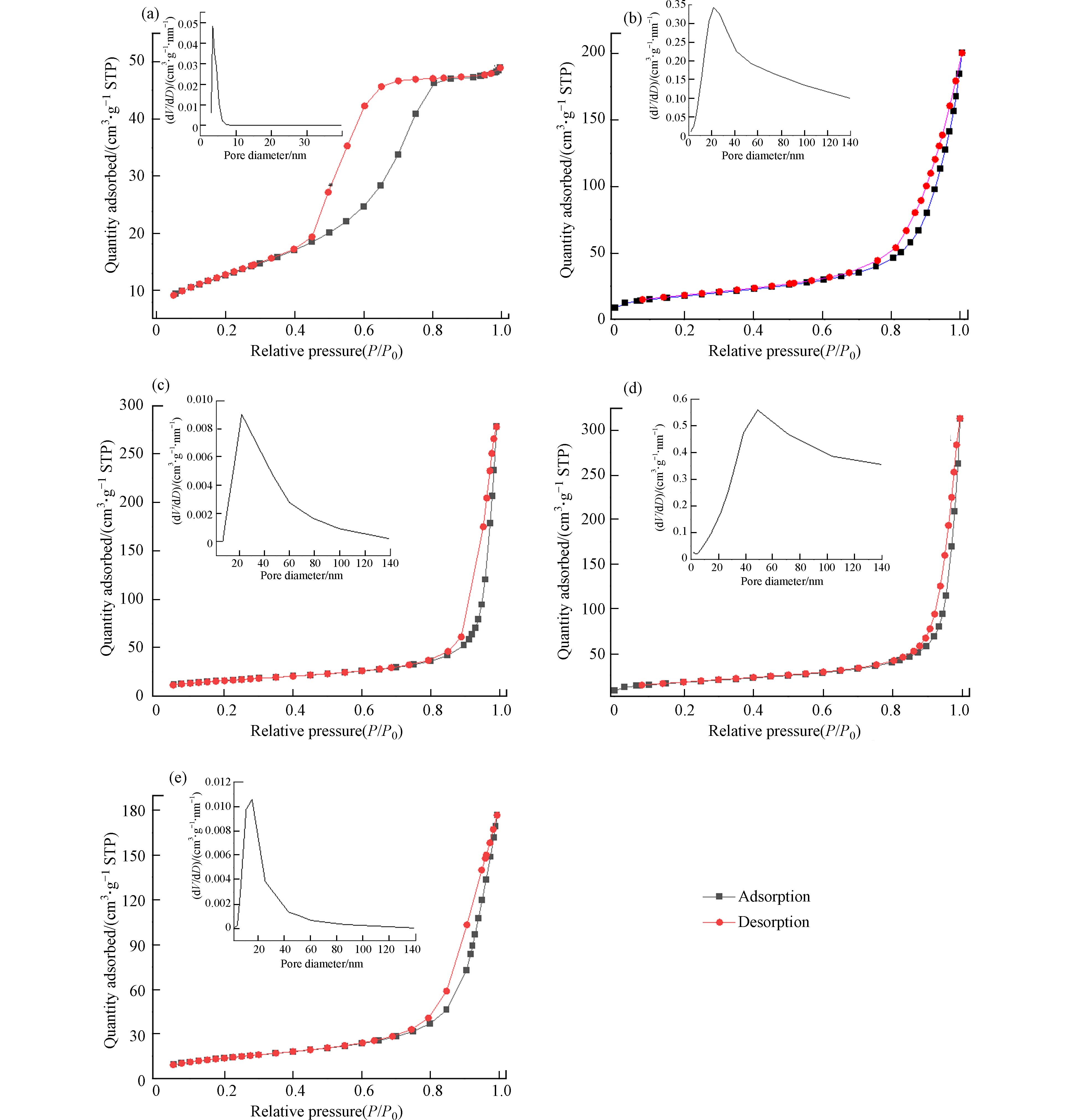
图6为TiO2,F-TiO2和不同MoS2/F-TiO2样品的N2吸附-脱附等温曲线图及孔径分布图. 图6a为纯TiO2的N2吸附-脱附等温曲线均属于第Ⅳ型的H2型,表面TiO2的孔径是相对较宽的墨水瓶型孔材料. 图6b—6e可知样品的N2吸附-脱附等温曲线均属于第Ⅲ型的H3型,且都存在滞后环(F-TiO2: P/P0=0.50; MoS2/F-TiO2: P/P0=0.80),说明两者材料均为介孔材料,结构不规则可能存在裂缝或狭缝. 此外,根据曲线类型(Ⅲ)可知材料存在大孔结构,没有可识别的单分子层形成,并且材料与染料(Rh B)的相互作用力弱,因此在饱和压力点(P/P0=1)的吸附量是有限的. 这证实在光催化降解过程中1%MoS2/F-TiO2样品暗处理出现的吸附现象. 由图6a子图的孔径分布可知,TiO2的孔径很小约为5 nm ,图6bF-TiO2孔径分布均匀,其孔径主要分布在10—40 nm范围内,并且约21.04 nm的介孔为主,因为F−能降低TiO2的表面能,使其从块状形貌变成颗粒状具有更好的分散性,造成F-TiO2比表面积更大. 图6c中MoS2/F-TiO2的孔径分布图显示孔径范围较宽,但以介孔49.82 nm为主并且大孔(>50 nm)数量增多. 从图6c—6e的孔径分布图可知,随着MoS2含量的增加MoS2/F-TiO2的孔径先增大后减小,表明只有适量MoS2负载F-TiO2才能增大比表面积.
表2中1%MoS2/F-TiO2和F-TiO2的比表面积相近,表明少量MoS2负载F-TiO2时对比表面积影响较小,随着MoS2含量增多比表面积逐渐减小;1%MoS2/F-TiO2的孔径和孔容积大于纯F-TiO2,说明MoS2会促进F-TiO2介孔长大使大孔数量增多,但是过量的MoS2比表面积、孔容和孔径减小. 综上所述,MoS2/F-TiO2和F-TiO2相对于纯TiO2具有大量的孔结构,使材料在光催化反应过程中产生更多的反应活性位点,有利提高光催化性能.
-
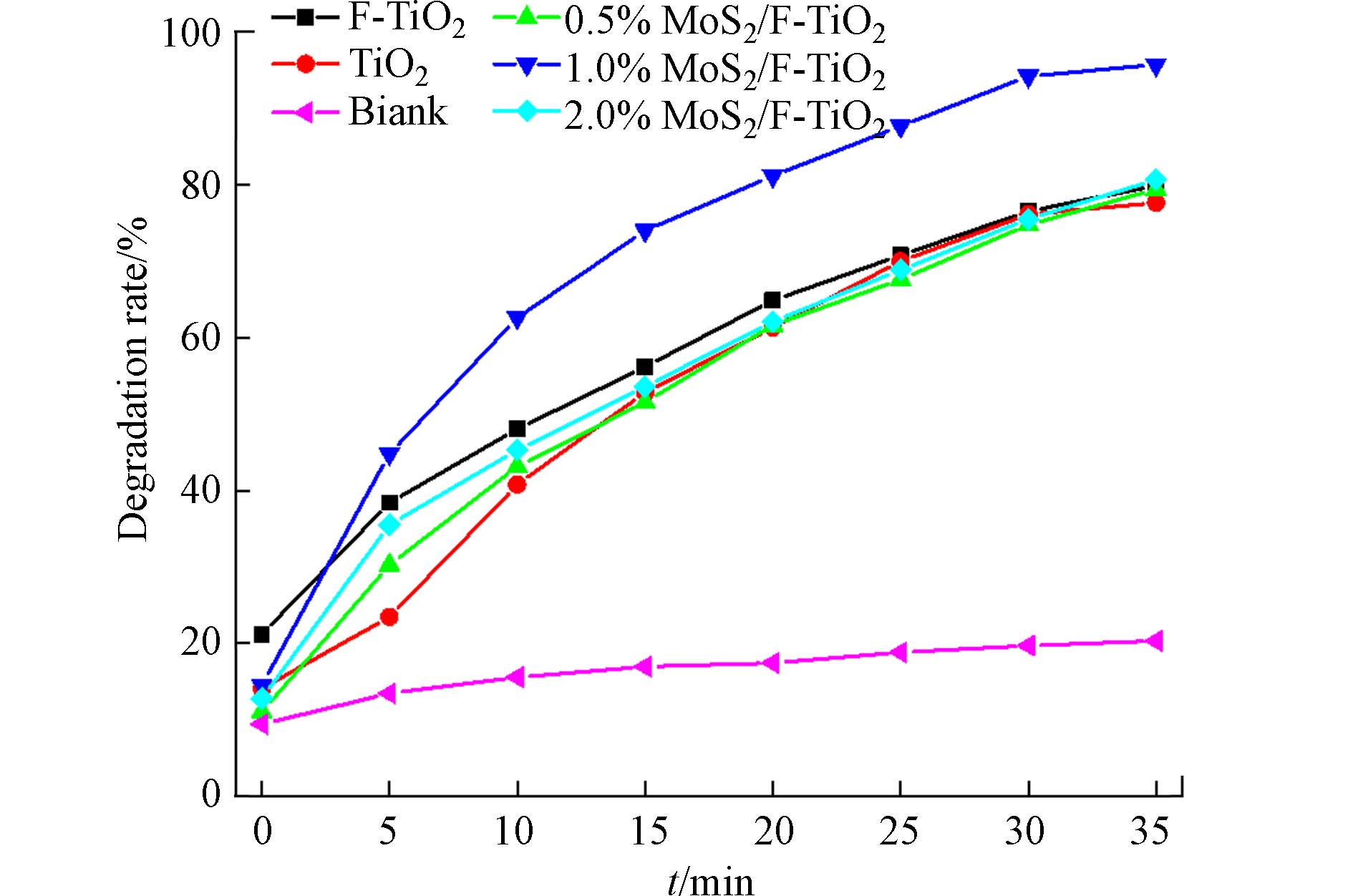
图7为不同比例MoS2/F-TiO2光催化剂和空白对照组,在相同光照条件下对罗丹明B(Rh B)的降解率随时间的变化曲线图. 由图7可知,不同光催化剂经过35 min后光催化后对Rh B的降解率顺序为:1%>2%>F-TiO2>0.5%>TiO2>空白. 在金卤灯照射条件下,Rh B(空白对照组)随着时间的变化,自身有一定的降解效果但不明显. 在30 min暗反应后,F-TiO2对罗丹明B的脱色效果最好,其原因可能是氟化后的TiO2颗粒变小并且存在孔结构,对罗丹明B有较好的吸附效果,吸附率达到20%左右. 光催化剂对罗丹明B的降解主要发生在样品表面,暗反应时吸附在表面的染料优先被降解,多孔结构增加样品的比表面积,二者有效提高了光催化剂和染料的接触,显著增加了光催化剂的光催化效果,提高光催化性能. 经过35 min后光催化反应,1%MoS2/F-TiO2降解率最好达到95.73%,其后依次为2%MoS2/F-TiO2(80.68%)、F-TiO2(79.92%)、0.5%MoS2/F-TiO2(79.42%)、TiO2(77.67%). 1.0%MoS2/F-TiO2对该染料的降解率最高,这表明使用适量MoS2与F-TiO2进行复合改性,可有效提高对罗丹明B的光催化降解效率.
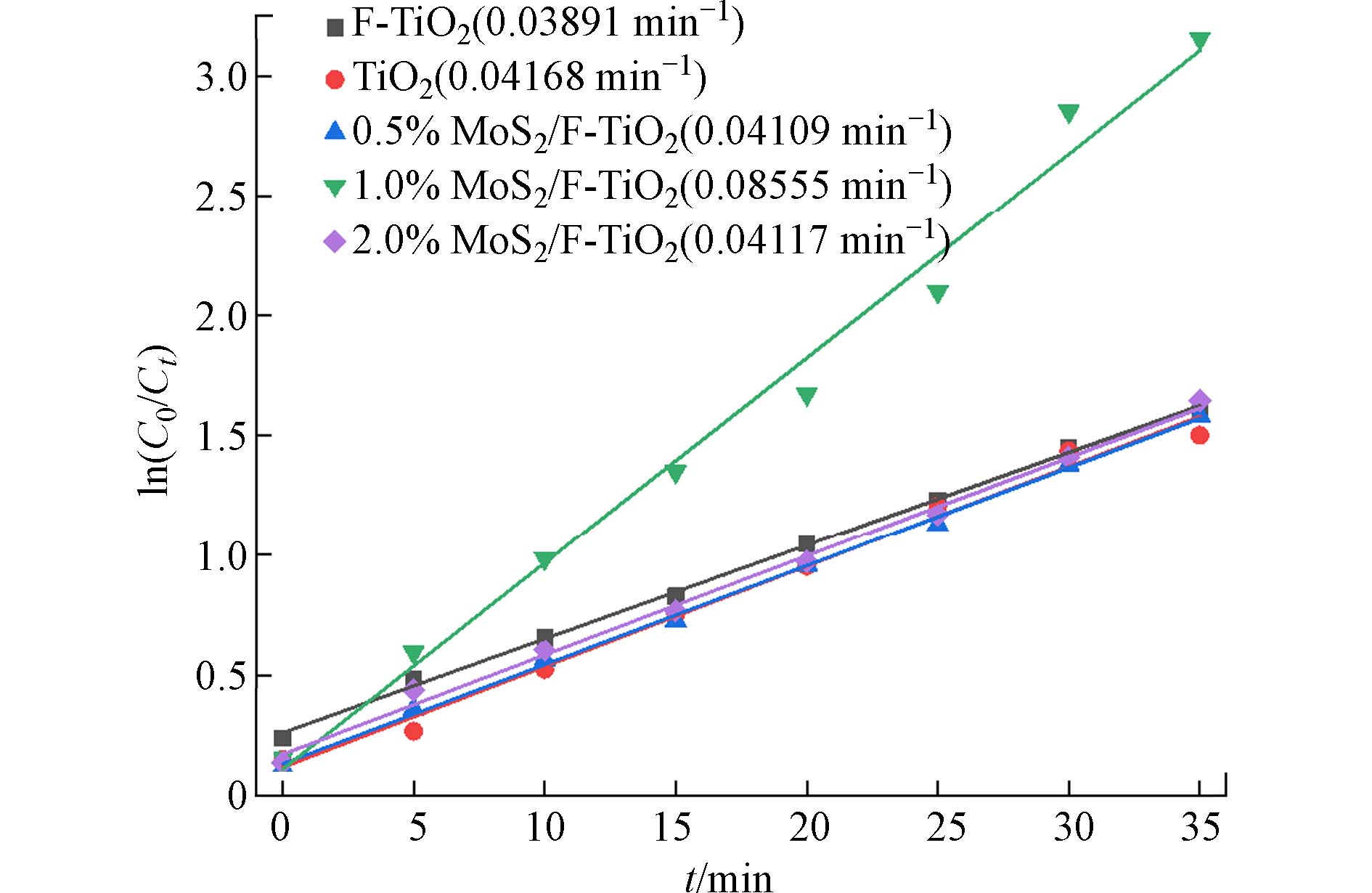
在研究中,通过构建MoS2/F-TiO2异质结材料,较为明显的提高对罗丹明B的降解能力,表明MoS2对F-TiO2的改性,一定程度上提高了TiO2、F-TiO2的光催化能力. 其原因有以下几点:一是氟对二氧化钛改性后使其对染料具有一定的吸附能力,而光催化降解反应发生在材料表面,因而光催化剂在光照作用下直接在其表面与吸附的染料进行反应,缩短了反应接触时间,加快了降解效率. 二是F-TiO2颗粒尺寸较小,比表面积大,光催化反应时与罗丹明B溶液的接触面积大,光催化活性高. 三是层片状的MoS2与颗粒状的F-TiO2形成异质结,该异质结吸收光谱的阈值波长(λg)发生红移,紫外光区和可见光区的吸收强度增大,有效的增强了光催化活性,提高了光催化降解效率. 研究一级反应动力学,如图8所示,用ln(C0/Ct)绘制光催化降解动力学曲线(C0:Rh B溶液初始浓度,Ct:降解过程中在t时Rh B溶液的浓度),结果表明ln(C0/Ct)—t有较好的线性关系,根据公式ln(C0/Ct)=Kt计算反应速率常数,其中最大与最小反应速率常数K分别为0.08555 min−1(1.0%MoS2/F-TiO2),0.03896 min−1(F-TiO2). 上述结果表明,F-TiO2和1.0%MoS2/F-TiO2对罗丹明B的光催化降解遵循Langmuir-Hinshelwood一级动力学模型,改性后MoS2/F-TiO2光催化活性得到提高.
-
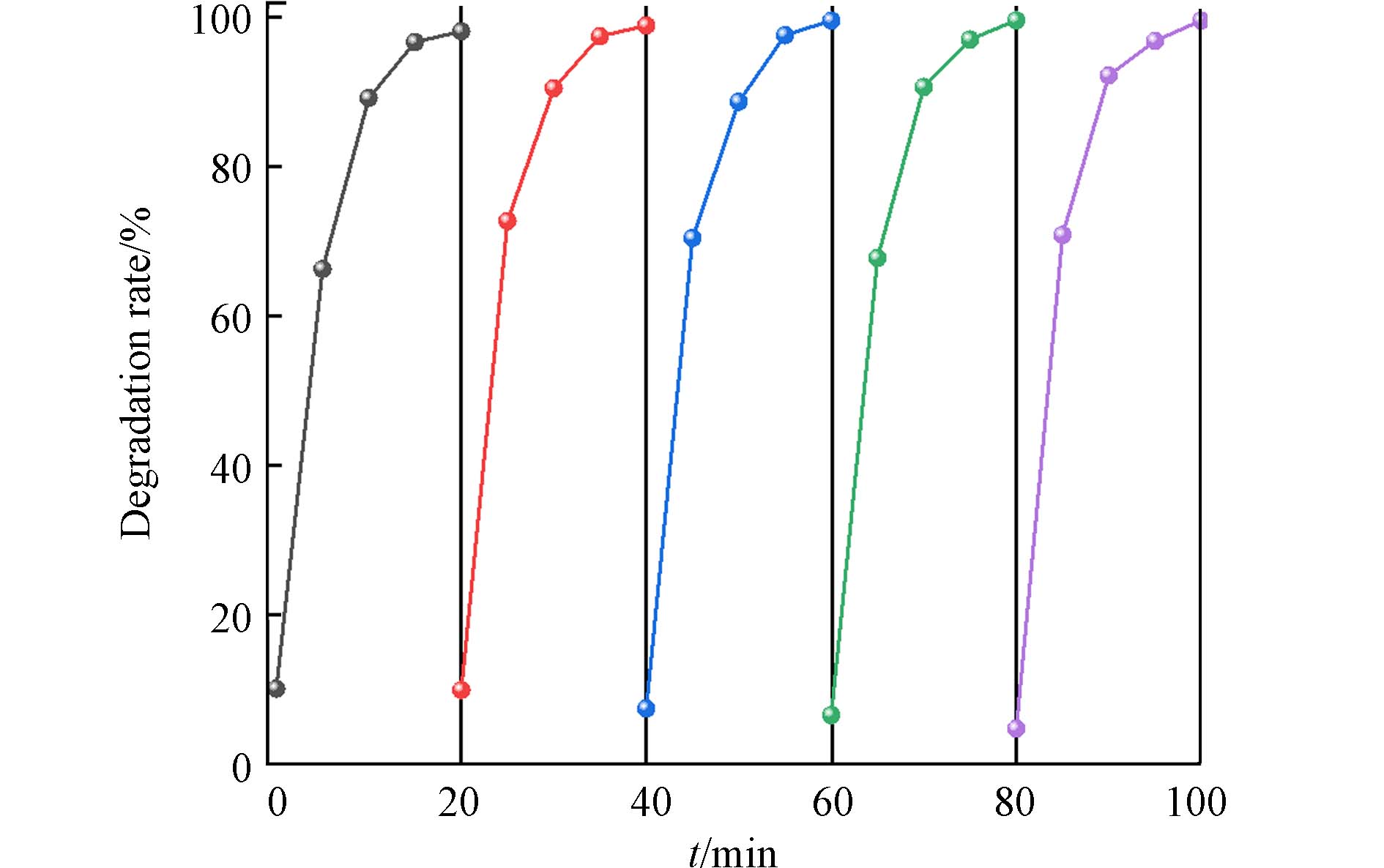
在实际应用中,光催化剂的稳定性是评价材料的光催化性能的一个重要指标. 为了检验自制的光催化剂的重复使用性能,在光催化实验后回收1.0%MoS2/F-TiO2催化剂,再次利用回收的光催化剂在金卤灯下对罗丹明B进行降解. 如图9所示,在相同时间下,罗丹明B首次被1.0%MoS2/F-TiO2降解的降解率为98.95%,连续对罗丹明B降解,第2—5次的降解率分别为98.16%、 99.61%、99.61% 99.62%. 在进行暗处理时,首次吸附可达到9.95%,第5次后为4.79%,吸附性能出现降低,可能是吸附在F-TiO2上的罗丹明B染料在光催化降解时未降解完全,占据了部分氟化二氧化钛吸附活性位点,但光催化降解效果仍然较好,由此可知,1.0%MoS2/F-TiO2光催化剂具有良好的循环使用性能.
-
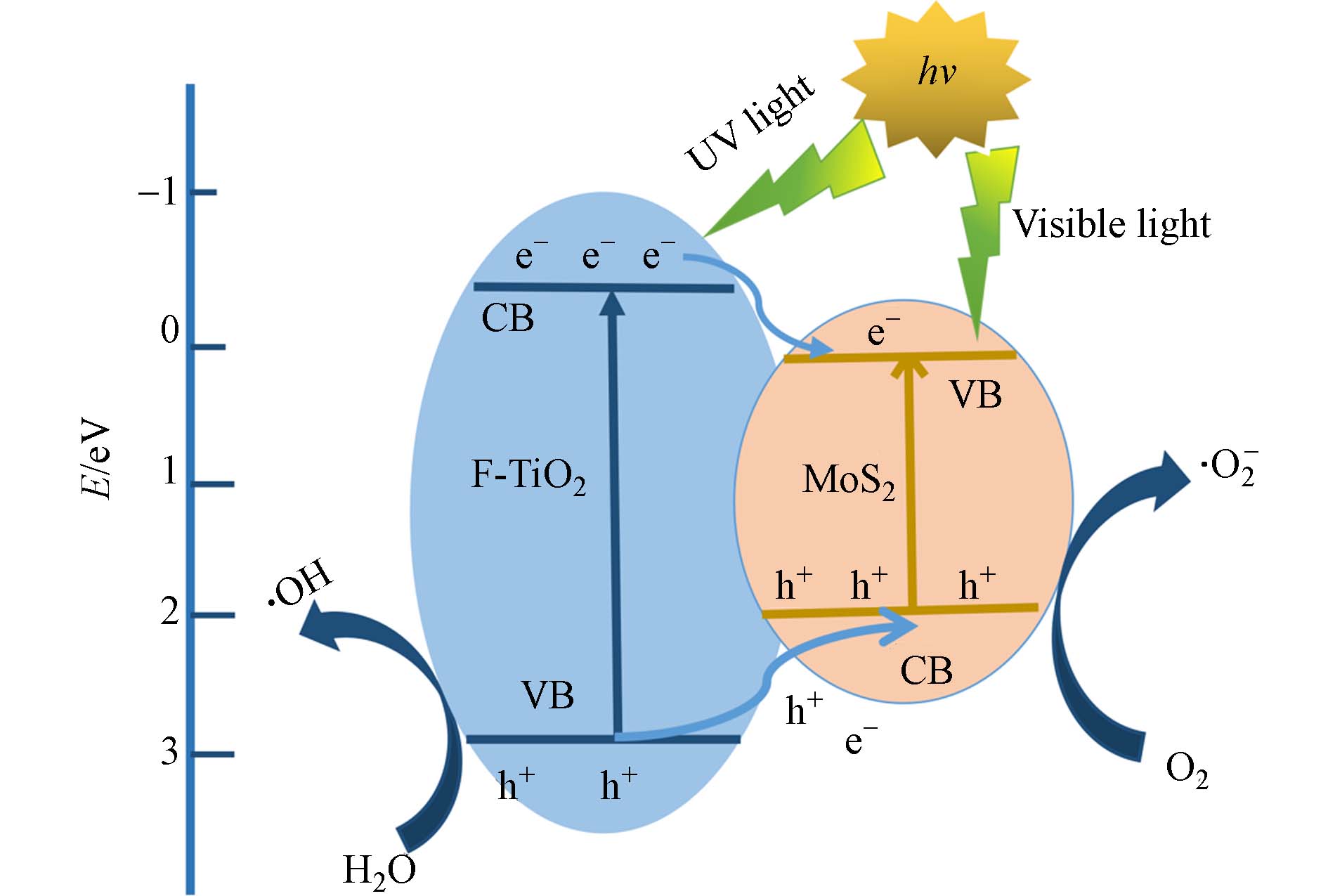
MoS2/F-TiO2对罗丹明B的降解如图10所示. TiO2为n型半导体,MoS2为p型半导体,MoS2的Eg为1.2 —1.9 eV,TiO2的Eg为2.73 eV,两者构成p-n型异质结. 异质结光催化剂与有机染料反应时,会破坏有机染料的官能团结构,将其分解成无害化的CO2和H2O等,因此MoS2/F-TiO2异质结是一种显著提高催化剂光催化性的科学策略. 由图10可知,二硫化钼导带(CB)比二氧化钛低但价带(VB)比二氧化钛高,当催化剂吸收大于Eg的能量后,价带上的电子产生跃迁至导带,同时在价带上留下相等数量的空穴. 此外MoS2价带上的电子被激发,跃迁到TiO2价带上,由于二氧化钛具有更低的导带电势,MoS2导带上跃迁电子更易迁移到二氧化钛导带,在价带上留下等量空穴,从而使电子与空穴分离. 整个光催化反应过程产生的光生电子会在MoS2和TiO2上转移,延长光生空穴滞留的时间,降低空穴复合的效率,有效提高催化剂光催化性能. 产生的光生电子和空穴在电场的作用或扩散作用下分别迁移到催化剂的表面,形成大量具有还原能力的电子和具有氧化能力的空穴累积与吸附在半导体表面的染料分子发生氧化还原反应. 光生电子具有强还原性,很容易被表面吸附的分子捕获,可将H+还原成H2也可以与O2相互作用将其还原为超氧自由基(·O2),超氧自由基(·O2)可进一步与H+产生羟基自由基(·OH ). 同时空穴具有较强的氧化性,可将OH−、H2O反应氧化成羟基自由基(·OH ). 两者将染料分子最终分解为CO2、H2O和其他无机物,但羟基自由基(·OH )在降解过程中发挥主要作用,发生的主要反应如下所示.
-
为了提高TiO2光催化活性,通过沉淀胶溶法制备了氟改性的多孔颗粒状TiO2 (F-TiO2),再通过超声将MoS2与F-TiO2复合构建了吸附-光催化一体的MoS2/F-TiO2异质结,MoS2/F-TiO2异质结的构建能有效抑制光生载流子复合,提高光生电子和空穴分离率,进而提高光催化性能. 经过35 min,1.0%MoS2/F-TiO2对罗丹明B的降解率高达95.73%,显著高于纯TiO2和F-TiO2. 此外,MoS2/F-TiO2复合材料具有良好的循环稳定性. 构建异质结结不仅可以增加材料对光的吸收范围,优化材料光催化性能,也为半导体的改性提升光催化效果,为处理污水中的有机物提供一种参考.
MoS2/F-TiO2异质结的制备及光降解罗丹明B性能研究
Study on preparation of MoS2/F-TiO2 heterojunction and its photodegradation performance on rhodamine B
-
摘要: 构建异质结型光催化剂是提高半导体光催化性能的有效方法之一,同时也是光催化处理有机废水的重要技术策略. 采用沉淀胶溶法制备出F-TiO2,并与 MoS2复合构建兼具吸附-光催化性能的MoS2/F-TiO2异质结. 采用扫描电子显微镜(SEM)、比表面积分析仪(BET)、X射线衍射仪(XRD)、傅里叶变换红外光谱仪(FTIR)、紫可见吸收光谱(UV-Vis-Abs)、X射线光电子能谱仪(XPS)等技术分别对MoS2/F-TiO2复合材料进行表征,研究了MoS2/F-TiO2的光催化性能. 结果表明,1.0%MoS2/F-TiO2在降解有机污染物罗丹明B(RhB) 溶液过程中表现出优异的光催化活性和稳定性. MoS2和F-TiO2形成异质结催化剂有效抑制光生载流子复合,提高光生电子和空穴分离率和寿命,从而产生更多羟基自由基和超氧自由基以提高光催化效率;并且1.0%MoS2/F-TiO2异质结具有较大的比表面积,可增加催化反应的活性位点和有机物在材料表面的吸附,使其35 min后降解率高达95.73%,显著高于纯TiO2和F-TiO2. 此外,MoS2/F-TiO2复合材料具有良好的循环稳定性,经过5次重复降解测试,其对RhB的降解率仍保持在90%以上.Abstract: Constructing heterojunction photocatalysts is one of the effective methods to improve the photocatalytic performance of semiconductors, and is also an important strategy for photocatalytic treatment of organic wastewater. F-TiO2 was prepared by the precipitation-peptization method, followed by coupling with MoS2 to construct a MoS2/F-TiO2 heterojunction with the integrated adsorption-photocatalysis performance. Scanning electron microscope (SEM), specific surface area analyzer (BET), X-ray diffractometer (XRD), fourier transform infrared spectrometer (FTIR), ultraviolet visible absorption spectrum (UV-Vis-Abs) and X-ray photoelectron spectroscopy (XPS) were used to characterize the obtained composites, and the photocatalytic activity of MoS2/F-TiO2 heterojunction was investigated. The results show that 1.0% MoS2/F-TiO2 exhibit excellent photocatalytic activity and stability in the degradation of organic pollutant Rhodamine B (RhB) solution. The heterojunction catalyst formed by MoS2 and F-TiO2 effectively inhibits the recombination of photo generated charge carriers, improves the separation rate and lifetime of photo generated electrons and holes, and generates more hydroxyl and superoxide radicals to improve photocatalytic efficiency. 1.0% MoS2/F-TiO2 heterojunction has a large specific surface area, which can increase the active sites of catalytic reactions and the adsorption of organic compounds on the material surface, resulting in a degradation rate of 95.73% after 35 min, significantly higher than pure TiO2 and F-TiO2. In addition, MoS2/F-TiO2 composites have a good cyclic stability, and the degradation rate of RhB still remains above 90% after 5 repeated degradation tests.
-
Key words:
- F-TiO2 /
- MoS2 /
- heterojunction /
- organic dye /
- adsorption-photocatalysis.
-
表 1 TiO2, F-TiO2和不同MoS2/F-TiO2的晶粒度
Table 1. The grain size of TiO2, F-TiO2 and different MoS2/F-TiO2
试样Samples 晶粒尺寸/nmCrystallite size TiO2 7.06 F-TiO2 13.24 0.5%MoS2/F-TiO2 13.40 1.0%MoS2/F-TiO2 16.49 2.0%MoS2/F-TiO2 14.34 表 2 TiO2,F-TiO2和MoS2/F-TiO2的的BET数据
Table 2. BET data of TiO2,F-TiO2 and MoS2/F-TiO2.
试样Samples 比表面积/ (m2·g−1)Surface area 孔容/ (cm3·g−1)Pore volume 孔径/ nmPore diameter TiO2 46.244 0.075 4.282 F-TiO2 62.545 0.310 18.453 0.5%MoS2/F-TiO2 57.536 0.430 28.062 1%MoS2/F-TiO2 61.636 0.485 32.473 2%MoS2/F-TiO2 50.912 0.273 18.496 -
[1] KATSOYIANNIS I A, LAMMEL G, SAMARA C, et al. Innovative aspects of environmental chemistry and technology regarding air, water, and soil pollution[J]. Environmental Science and Pollution Research, 2021, 28(42): 58958-58968. doi: 10.1007/s11356-021-15370-8 [2] GAO L, GU J D. A new unified conceptual framework involving maintenance energy, metabolism and toxicity for research on degradation of organic pollutants[J]. International Biodeterioration & Biodegradation, 2021, 162: 105253. [3] NIE H Q, YANG X H, YANG S L, et al. The enhanced catalytic decomposition behaviors of RDX by using porous activated carbon loaded with nanosized metal oxides[J]. Journal of Thermal Analysis and Calorimetry, 2023, 148(10): 4255-4266. doi: 10.1007/s10973-023-11987-8 [4] YU H X, DING D, ZHAO S L, et al. Co/N co-doped porous carbon as a catalyst for the degradation of RhB by efficient activation of peroxymonosulfate[J]. Environmental Science and Pollution Research, 2023, 30(4): 10969-10981. [5] WANG P T, ZHANG C, WU L L, et al. MoF(ZB)/Potassium citrate-derived porous carbon composite and its Electrochemical Properties[J]. Russian Journal of Electrochemistry, 2023, 59(4): 299-312. doi: 10.1134/S1023193523040134 [6] WANG Y, LIANG X, WU X L, et al. Preparation of N-doped porous carbon matrix in a solid-liquid coexisted NaCl template and its applications in Li-S batteries[J]. Ionics, 2023, 29(1): 183-191. doi: 10.1007/s11581-022-04801-2 [7] MAHMOODI N M, SALEHI R, ARAMI M. Binary system dye removal from colored textile wastewater using activated carbon: Kinetic and isotherm studies[J]. Desalination, 2011, 272(1-3): 187-195. doi: 10.1016/j.desal.2011.01.023 [8] DJILANI C, ZAGHDOUDI R, DJAZI F, et al. Adsorption of dyes on activated carbon prepared from apricot stones and commercial activated carbon[J]. Journal of the Taiwan Institute of Chemical Engineers, 2015, 53: 112-121. doi: 10.1016/j.jtice.2015.02.025 [9] WANG Y N, ZHANG C, ZENG Y Q, et al. Ag and MoFs-derived hollow Co3O4 decorated in the 3D g-C3N4 for creating dual transferring channels of electrons and holes to boost CO2 photoreduction performance[J]. Journal of Colloid and Interface Science, 2022, 609: 901-909. doi: 10.1016/j.jcis.2021.11.153 [10] OH W C, AREEROB Y. Photocatalytic CO2 reduction with new band gap energy evaluation from spectroscopic relationship of graphene-Mg2CuSnCoO6 composite bridged with organics[J]. Physica E:Low-dimensional Systems and Nanostructures, 2021, 134: 114864. doi: 10.1016/j.physe.2021.114864 [11] HUANG Y L, BAI J, ZHOU G T, et al. Improved intrinsic emission efficiency and photocatalysis of Nb2WO8 by Li+-doping[J]. Journal of Alloys and Compounds, 2021, 881: 160679. doi: 10.1016/j.jallcom.2021.160679 [12] ZHANG G X, ZHANG H M, WANG R F, et al. Preparation of Ga2O3/ZnO/WO3 double s-scheme heterojunction composite nanofibers by electrospinning method for enhancing photocatalytic activity[J]. Journal of Materials Science:Materials in Electronics, 2021, 32: 7307-7318. doi: 10.1007/s10854-021-05441-4 [13] LIU Y, LIN Y T, LIU Z Y, et al. Self-generating CoFe2O4 as electric channel in z-scheme CoO(111)/CoFe2O4/Fe2O3 photocatalyst for synchronous photocatalytic degradation and hydrogen production[J]. Materials Science in Semiconductor Processing, 2022, 140: 106382. doi: 10.1016/j.mssp.2021.106382 [14] CHEN P, LI M L, PENG Q M, et al. Direct evidence for the electron-hole pair mechanism by studying the organic magneto-electroluminescence based on charge-transfer states[J]. Organic Electronics, 2012, 13(10): 1774-1778. doi: 10.1016/j.orgel.2012.05.019 [15] TORUBAEV Y V, SKABITSKY I V, ROZHKOV A V, et al. Highly polar stacking interactions wrap inorganics in organics: lone-pair-π-hole interactions between the PdO4 core and electron-deficient arenes[J]. Inorganic Chemistry Frontiers, 2021, 8(23): 4965-4975. doi: 10.1039/D1QI01067K [16] YAO S S, LI Y Y, XUE L H, et al. Photoluminescent properties of the monoclinic Ba2MgSi2O7: Eu2+ phosphor prepared by the combustion-assisted synthesis method[J]. Physica Status Solid(a), 2010, 207(9): 2164-2169. doi: 10.1002/pssa.200925524 [17] EL-NEWEHY M H, KIM H Y, KHATTAB T A, et al. Development of highly photoluminescent electrospun nanofibers for dual-mode secure authentication[J]. Ceramics International, 2022, 48(3): 3495-3503. doi: 10.1016/j.ceramint.2021.10.128 [18] HU X Y, ZHANG Q, NAN H S, et al. Heterojunction Cu2O/RGO/BiVO4 ternary nanocomposites with enhanced photocatalytic activities towards degradation of rhodamine B and tetracycline hydrochloride[J]. New Journal of Chemistry, 2019, 43(46): 18240-18250. doi: 10.1039/C9NJ04351A [19] 吕鲲, 张庆竹. 纳米二氧化钛光催化技术与大气污染治理[J]. 中国环境科学, 2018, 38(3): 852-861. doi: 10.3969/j.issn.1000-6923.2018.03.007 LV K, ZHANG Q Z. Nano-TiO2 photocatalytic technology and atmospheric pollution control[J]. China Environmental Science, 2018, 38(3): 852-861 (in Chinese). doi: 10.3969/j.issn.1000-6923.2018.03.007
[20] KAMPOURI S, STYLIANOU K C. Dual-functional photocatalysis for simultaneous hydrogen production and oxidation of organic substances[J]. ACS Catalysis, 2019, 9(5): 4247-4270. doi: 10.1021/acscatal.9b00332 [21] HUMAYUN M, ULLAH H, USMAN M, et al. Perovskite-type lanthanum ferrite based photocatalysts: preparation, properties, and applications[J]. Journal of Energy Chemistry, 2022, 66: 314-338. doi: 10.1016/j.jechem.2021.08.023 [22] ARIFIN K, YUNUS R M, MINGGU L J, et al. Improvement of TiO2 nanotubes for photoelectrochemical water splitting: review[J]. International Journal of Hydrogen Energy, 2021, 46(7): 4998-5024. doi: 10.1016/j.ijhydene.2020.11.063 [23] YAN J W, SHI L. The preparation of 0D/2D P‐doped MnxCd1‐ xS/Ni2P photocatalyst and its photocatalytic activity of pure water splitting for H2[J]. International journal of energy research, 2022, 46(6): 8218-8228. doi: 10.1002/er.7724 [24] ZHAO X P, LI J C, LIU J C, et al. Recent progress of preparation of branched poly(lactic acid) and its application in the modification of polylactic acid materials[J]. International journal of biological macroMolecules, 2021, 193(Pt A): 874-892. [25] ZHANG L, HUANG K L, CHEN H, et al. Preparation and modification of Y2Cu2O5/Y2O3 photocatalysts for H2 evolution under simulated sunlight irradiation[J]. Advanced Materials Research, 2011, 239(242): 3001-3004. [26] WU H, WANG P, WANG Z, et al. Preparation of EDTA modified cotton fiber iron complex and catalytic properties for aqueous Cr(VI) reduction and dye degradation[J]. Water Practice and Technology, 2021, 16(3): 1000-1011. doi: 10.2166/wpt.2021.047 [27] ROSHINI R A, KANNAN E S. Pinched hysteresis as a regeneration marker for the semiconductor photocatalyst[J]. Materials Chemistry and Physics, 2022, 275: 125247. doi: 10.1016/j.matchemphys.2021.125247 [28] KAUSHIK B, RANA P, RAWAT D, et al. Magnetically separable type-II semiconductor based ZnO/MoO3 photocatalyst: a proficient system for heteroarenes arylation and rhodamine B degradation under visible light[J]. New Journal of Chemistry, 2022, 46(18): 8478-8488. doi: 10.1039/D2NJ00906D [29] ARENAS L T, VILLIS P C M, ARGUELLO J, et al. Niobium oxide dispersed on a carbon-ceramic matrix, SiO2/C/Nb2O5, used as an electrochemical ascorbic acid sensor[J]. Talanta, 2010, 83(1): 241-248. doi: 10.1016/j.talanta.2010.09.014 [30] LIN Y, PAN D M, LUO H. Hollow direct Z-Scheme CdS/BiVO4 composite with boosted photocatalytic performance for RhB degradation and hydrogen production[J]. Materials Science in Semiconductor Processing, 2021, 121: 105453. doi: 10.1016/j.mssp.2020.105453 [31] REN Y W, XING S, WANG J, et al. Weak-light-driven Ag-TiO2 photocatalyst and bactericide prepared by coprecipitation with effective Ag doping and deposition[J]. Optical Materials, 2022, 124: 111993. doi: 10.1016/j.optmat.2022.111993 [32] DONG L, WANG X F, WANG P, et al. A one-step solvothermal synthesis of the topological insulator Bi2Te3 nanorod-modified TiO2 photocatalyst for enhanced H2-evolution activity[J]. Journal of Materials Chemistry C, 2022, 10(16): 6402-6410. doi: 10.1039/D2TC00594H [33] ZENG D B, YU C L, FAN Q Z, et al. Theoretical and experimental research of novel fluorine doped hierarchical Sn3O4 microspheres with excellent photocatalytic performance for removal of Cr(VI) and organic pollutants[J]. Chemical Engineering Journal, 2020, 391: 123607. doi: 10.1016/j.cej.2019.123607 [34] YU C L, HE H B, LIU X Q, et al. Novel SiO2 nanoparticle-decorated BiOCl nanosheets exhibiting high photocatalytic performances for the removal of organic pollutants[J]. Chinese Journal of Catalysis, 2019, 40(8): 1212-1221. doi: 10.1016/S1872-2067(19)63359-0 [35] YANG J F, SHI C Y, DONG Y H, et al. Efficient hydrogen generation of vector Z-scheme CaTiO3/Cu/TiO2 photocatalyst assisted by cocatalyst Cu nanoparticles[J]. Journal of Colloid and Interface Science, 2022, 605: 373-384. doi: 10.1016/j.jcis.2021.07.106 [36] DU Y C, LIU M C, GUO L J. Numerical investigation on the optical properties of TiO2 photocatalyst suspension by light scattering model of particulate aggregates[J]. Journal of Photonics for Energy, 2021, 11(1): 016501. [37] MISHRA S, SUNDARAM B, MUTHUKUMAR S. Advanced oxidation process for leachate treatment: A critical review[M]. Advanced Oxidation Processes for Wastewater Treatment. Boca Raton: CRC Press, 2022: 169-182. [38] Di PAOLA A, BELLARDITA M, PALMISANO L. Brookite, the least known TiO2 photocatalyst[J]. Catalysts, 2013, 3(1): 36-73. doi: 10.3390/catal3010036 [39] ZHANG J W, FU D F, XU Y D, et al. Optimization of parameters on photocatalytic degradation of chloramphenicol using TiO2 as photocatalyst by response surface methodology[J]. Journal of Environmental Sciences, 2010, 22(8): 1281-1289. doi: 10.1016/S1001-0742(09)60251-5 [40] BAMWENDA G R, TSUBOTA S, NAKAMURA T, et al. Photoassisted hydrogen production from a water-ethanol solution: a comparison of activities of Au TiO2 and Pt TiO2[J]. Journal of Photochemistry and Photobiology A:Chemistry, 1995, 89(2): 177-189. doi: 10.1016/1010-6030(95)04039-I [41] ZHANG Y, CRITTENDEN J C, HAND D W, et al. Fixed-bed photocatalysts for solar decontamination of water[J]. Environmental Science & Technology, 1994, 28(3): 435-442. [42] CASTRO C A, CENTENO A, GIRALDO S A. Iron promotion of the TiO2 photosensitization process towards the photocatalytic oxidation of azo dyes under solar-simulated light irradiation[J]. Materials Chemistry and Physics, 2011, 129(3): 1176-1183. doi: 10.1016/j.matchemphys.2011.05.082 [43] ZANGENEH H, ZINATIZADEH A A, ZINADINI S, et al. A novel L-Histidine (C, N) codoped-TiO2-CdS nanocomposite for efficient visible photo-degradation of recalcitrant compounds from wastewater[J]. Journal of Hazardous Materials, 2019, 369: 384-397. doi: 10.1016/j.jhazmat.2019.02.049 [44] GUO Y, GUO T, CHEN J H, et al. Synthesis of C-N-S co-doped TiO2 mischcrystal with an isobandgap characteristic and its photocatalytic activity under visible light[J]. Catalysis Science & Technology, 2018, 8(16): 4108-4121. [45] WU Y C, JU L S. Annealing-free synthesis of CN co-doped TiO2 hierarchical spheres by using amine agents via microwave-assisted solvothermal method and their photocatalytic activities[J]. Journal of Alloys and Compounds, 2014, 604: 164-170. doi: 10.1016/j.jallcom.2014.03.023 [46] WU Y M, XING M Y, TIAN B Z, et al. Preparation of nitrogen and fluorine co-doped mesoporous TiO2 microsphere and photodegradation of acid orange 7 under visible light[J]. Chemical Engineering Journal, 2010, 162(2): 710-717. doi: 10.1016/j.cej.2010.06.030 [47] WANG X B, QIN Y L, ZHU L H, et al. Nitrogen-doped reduced graphene oxide as a bifunctional material for removing bisphenols: synergistic effect between adsorption and catalysis[J]. Environmental Science & Technology, 2015, 49(11): 6855-6864. [48] RYU G H, LEE J, KIM N Y, et al. Line-defect mediated formation of hole and Mo clusters in monolayer molybdenum disulfide[J]. 2D Materials, 2016, 3(1): 014002. doi: 10.1088/2053-1583/3/1/014002 [49] FAGLIA G, FERRONI M, Le DANG T T L, et al. Vertically coupling ZnO nanorods onto MoS2 flakes for optical gas sensing[J]. Chemosensors, 2020, 8(1): 19. doi: 10.3390/chemosensors8010019 [50] HSU H P, LIN D Y, LU G T, et al. Optical and electrical transport properties of ZnO/MoS2 heterojunction p-n structure[J]. Materials Chemistry and Physics, 2018, 220: 433-440. doi: 10.1016/j.matchemphys.2018.09.002 [51] KRISHNAN U, KAUR M, KAUR G, et al. MoS2/ZnO nanocomposites for efficient photocatalytic degradation of industrial pollutants[J]. Materials Research Bulletin, 2019, 111: 212-221. doi: 10.1016/j.materresbull.2018.11.029 [52] ZHOU W J, YIN Z Y, DU Y P, et al. Synthesis of few-layer MoS2, nanosheet-coated TiO2, nanobelt heterostructures for enhanced photocatalytic activities[J]. Small, 2013, 9(1): 140-147. doi: 10.1002/smll.201201161 [53] ZHENG L X, HAN S C, LIU H, et al. Hierarchical MoS2 nanosheet@ TiO2 nanotube array composites with enhanced photocatalytic and photocurrent performances[J]. Small, 2016, 12(11): 1527-1536. doi: 10.1002/smll.201503441 [54] CHEN F Y, YU C L, WEI L F, et al. Fabrication and characterization of ZnTiO3/Zn2Ti3O8/ZnO ternary photocatalyst for synergetic removal of aqueous organic pollutants and Cr(Ⅵ) ions[J]. Science of The Total Environment, 2020, 706: 136026. doi: 10.1016/j.scitotenv.2019.136026 [55] CHEN F Y, LIU X Q, ZHOU W Q, et al. The distinct role of non-noble metal Cu NPs deposition in boosting the overall photocatalytic performance over a ternary Zn-based photocatalyst system[J]. Journal of Alloys and Compounds, 2021, 875: 160068. doi: 10.1016/j.jallcom.2021.160068 [56] ZHAO B, WANG X, ZHANG Y Z, et al. Synergism of oxygen vacancies, Ti3+ and N dopants on the visible-light photocatalytic activity of N-doped TiO2[J]. Journal of Photochemistry and Photobiology A:Chemistry, 2019, 382: 111928. doi: 10.1016/j.jphotochem.2019.111928 [57] CASTAÑEDA C, SANTOS D, HERNÁNDEZ J S, et al. Efficient NiO/F–TiO2 nanocomposites for 4-chlorophenol photodegradation[J]. Chemosphere, 2023, 315: 137606. doi: 10.1016/j.chemosphere.2022.137606 [58] ZHANG L Y, YANG J J, ZHOU B Q, et al. Fluorinated and bayberry tannin modified titanium dioxide and its photocatalytic performance[J]. Russian Journal of Physical Chemistry A, 2022, 96(14): 3264-3273. doi: 10.1134/S0036024423020309 [59] GOSTEAU J, ARRAS R, CHEN P, et al. Spin-orbit effects in ferroelectric PbTiO3 under tensile strain[J]. Physical Review B, 2021, 103(2): 024416. doi: 10.1103/PhysRevB.103.024416 [60] GUO S D. Importance of spin-orbit coupling in power factor calculations for half-Heusler ANiB (A= Ti, Hf, Sc, Y; BSn, Sb, Bi)[J]. Journal of Alloys and Compounds, 2016, 663: 128-133. doi: 10.1016/j.jallcom.2015.12.139 [61] MIAO F J, SUN B, TAO B R, et al. MoS2/Ag/TiO2 for photoanode of dye sensitized solar cells[J]. Journal of Materials Research and Technology, 2022, 20: 781-790. doi: 10.1016/j.jmrt.2022.07.134 -




 下载:
下载: