-
随着人类社会的发展和工业化进程的加快,环境污染问题已经严重影响到人类正常的生活和生产. 解决环境污染问题主要在于如何高效地处理污染物从而达到环境修复的目的. 吸附法作为一种操作简单、经济成本低且高效迅速的技术,是去除环境污染物的重要方法之一. 应用吸附法的关键主要在于对吸附剂的选择. 活性炭[1 − 2]、沸石[3 − 5]、生物炭[6 − 7]、氮化碳材料[8 − 9]、金属有机框架材料[10-11]、石墨烯基材料(graphene-based materials) [12 − 13]、层状双金属氢氧化物(layered double hydroxide,LDHs) [14 − 15]以及有机多孔材料[16 − 17]等均是应用于环境污染物去除的吸附剂.
共价有机框架(covalent organic frameworks,COFs)材料作为一种新型有机多孔材料,具有如密度低、良好的热稳定性和化学稳定性、大比表面和丰富的组成单元等众多优良特性,使得其自Yaghi等[18]报道后就引起了科研工作者的广泛关注. 经过众多科研工作者十几年来的努力,COF材料的构筑单体、拓扑结构、合成方法、功能应用等方面得到了瞩目的发展. 在这其中,COF通过引入带电基团可以为后续修饰带来更多的可操作性,因此离子型共价有机框架(ionic covalent organic frameworks,iCOFs)材料进入科研工作者的视野. iCOF除了具有COF材料的优良特性外,关键在于其含有数量众多的离子基团,能与客体分子的特定结构产生较强静电作用. 另外,通过改变离子基团的类型也可对其表面积、孔隙孔径以及性质进行调控,从而进一步扩充其应用领域.
自2015年Peng等[19]首次报道了具有电负性的磺酸基iCOF以来,iCOF材料已被广泛应用于分离[20 − 21]、质子传导[22 − 23]、催化[24 − 25]、生物医学[26 − 27]等诸多领域. 本文从iCOF的结构、合成方法以及在环境修复中的应用等角度综述了近年来离子型共价有机框架材料的研究进展,并对其目前存在的挑战和应用前景进行展望.
-
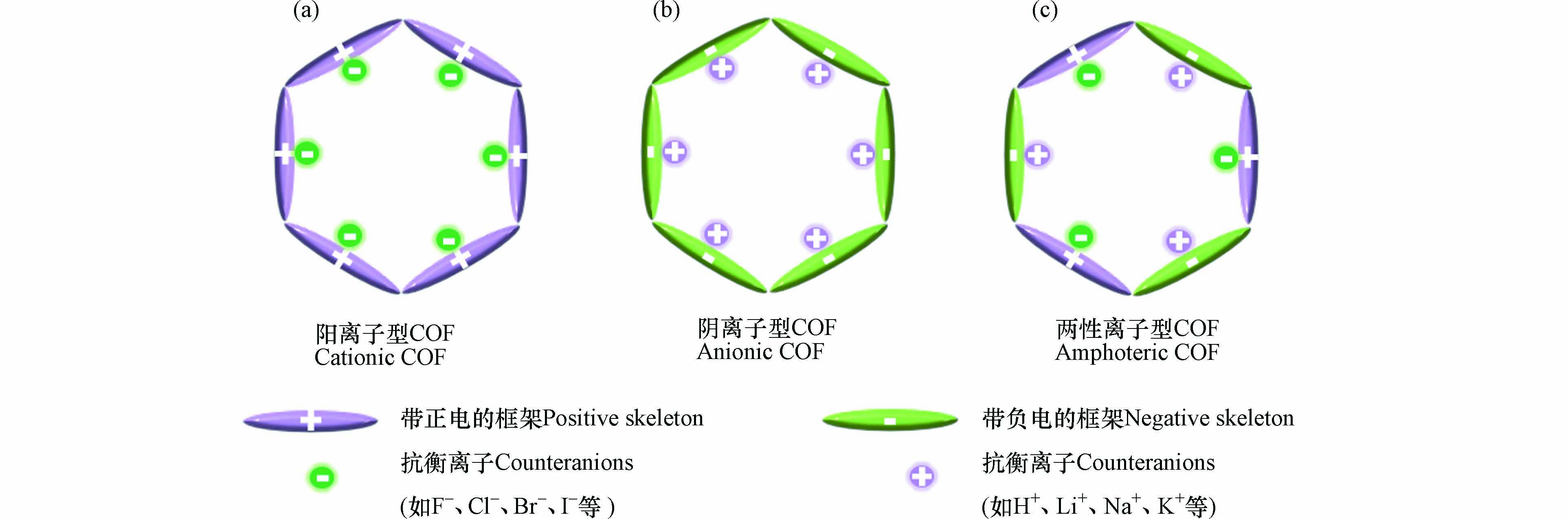
iCOF是晶体多孔结构与电离特性结合的产物,对其结构的探究能更好的解释其性能特征,这对功能性材料的设计也具有重要意义. 根据iCOF框架的离子性质,一般将iCOF分为带正电的阳离子型COF和带负电的阴离子型COF. 阳离子型COF具有的正电荷框架通常是通过引入的杂环化合物或脂肪族化合物中的氮原子来实现. 为了保持电中性,同时需要引入如F−、Cl−、Br−、I−等负离子组成的抗衡离子(如图1a所示). 此外,根据正电荷框架中离子部分所处的位置的不同,阳离子COF又可以分为三类(离子部分位于主链上、侧链上或者节点处). 相反,阴离子型COF由负电荷框架以及如H+、Li+、Na+、K+等抗衡阳离子构成(如图1b所示). 此外,2021年Fu等[28]合成出一种框架内部同时具有阴离子和阳离子的COF. 这种两性离子型COF的框架内分布有大量的正负电荷(如图1c所示),特殊的拓扑结构使其具有良好的结晶度和较高的比表面积,为COF的性能改进和机理探索等多个层面带来了深远的影响.
-
构建iCOF通常有两种方法:直接合成法和合成后修饰法. 直接合成法顾名思义就是指直接使用离子单体合成. 预先选择离子单体作为核心结构单元,将其与中性连接剂进行聚合反应,两者通过共价键连接成预设的拓扑结构,从而得到最终产物. 直接合成法的优势在于能够预设离子位置以及控制最终产物的电荷密度. 此外,直接合成法所得的iCOF结构孔道较为完整,且产率比较高. 但由于离子单体之间固有的静电排斥作用限制,用于直接合成法的离子单体种类较少.
不能通过直接合成法合成的iCOF可以用合成后修饰法(post-synthesis modification,PSM)来合成.通常有两种合成思路,除了直接将带电的离子基团引入中性框架外,另一种方法是将中性活性基团引入框架后再将其转化为离子基团,后者最大的优势在于赋予COF新功能特性的同时不改变共价有机框架材料的基本框架结构,但也存在中性活性基团转化率较低,导致最终产物电荷密度较低的缺点.
-
常用的直接合成法主要包括热溶剂合成法、机械化学合成法以及微波合成法. 热溶剂合成法作为最主要的合成方法,已经广泛用于多种iCOF的合成. 在密闭且高温的条件下,将离子单体和中性连接剂放入容器内,经过3 d或者更长的反应时间,从而得到最终产物. 在合成过程中,密封容器内的压力、温度、反应时间、溶剂的比例、催化剂用量等都会对最终产物的结晶度有影响. 因此,想要通过热溶剂合成法来合成iCOF需要大量的前期工作来寻找最佳的反应条件和适宜的溶剂. 此外,由于iCOF对合成条件异常敏感,通过热溶剂合成法合成出的不同批次的iCOF之间也存在差异.
机械化学合成法作为一种简单、环保、经济的方法,具体操作是将离子单体和中性连接剂放在研钵内,不加溶剂或者加入少量溶剂,在室温下通过不断研磨从而得到最终产物. 2016年,Peng等[29]将1,3,5-三甲酰基间苯三酚(1,3,5-triacylphloroglucinol,Tp)分别与两种不同的磺酸基单体在均三甲苯/二氧六烷/乙酸混合溶剂下混合研磨,合成出两种iCOF材料(NUS-9和NUS-10). 通过此法合成出的iCOF材料孔隙度和结晶度一般较低.
微波合成法是指通过微波辐射来实现对反应体系温度的均匀升高,从而提高能量传导效率,最终使反应速率明显提高的一种方法. 利用微波合成法制备的iCOF材料往往具有较高的纯度、更窄的粒径分布和更为均匀的形态等优异特性. 2009年,Campbell等[30]首次采用微波合成法制备出两种iCOF(COF-5和COF-102). 与传统的热溶剂合成法的产物相比,COF-5和COF-102这两种iCOF材料表现出更高的表面积. 除此之外,微波合成法还具有节省能源的优点,能极大地缩短反应时间.
-
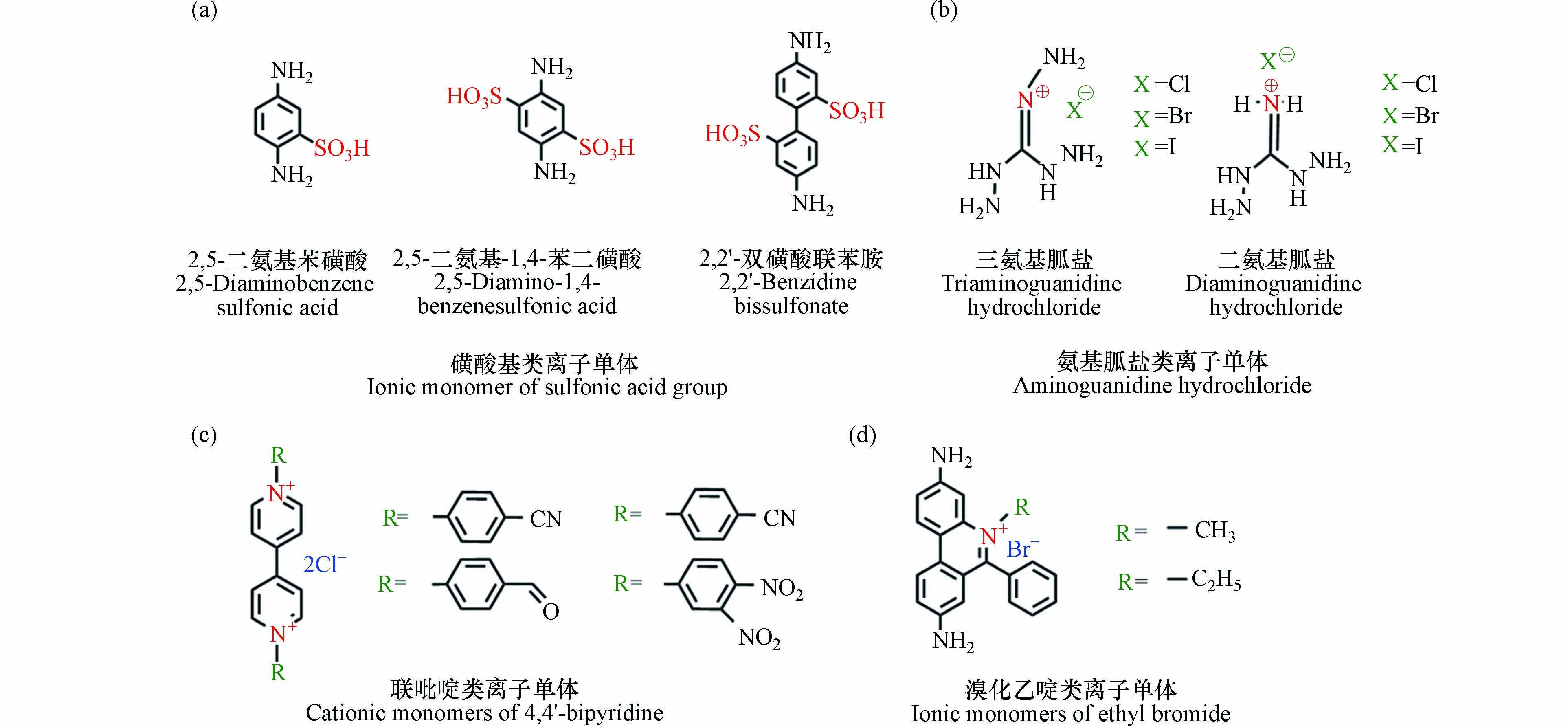
直接合成法中常用的离子单体如图2所示,包括磺酸基类离子单体(图2a),氨基胍盐酸盐类离子单体(图2b),联吡啶类离子单体(图2c)以及溴化乙啶类离子单体(图2d).
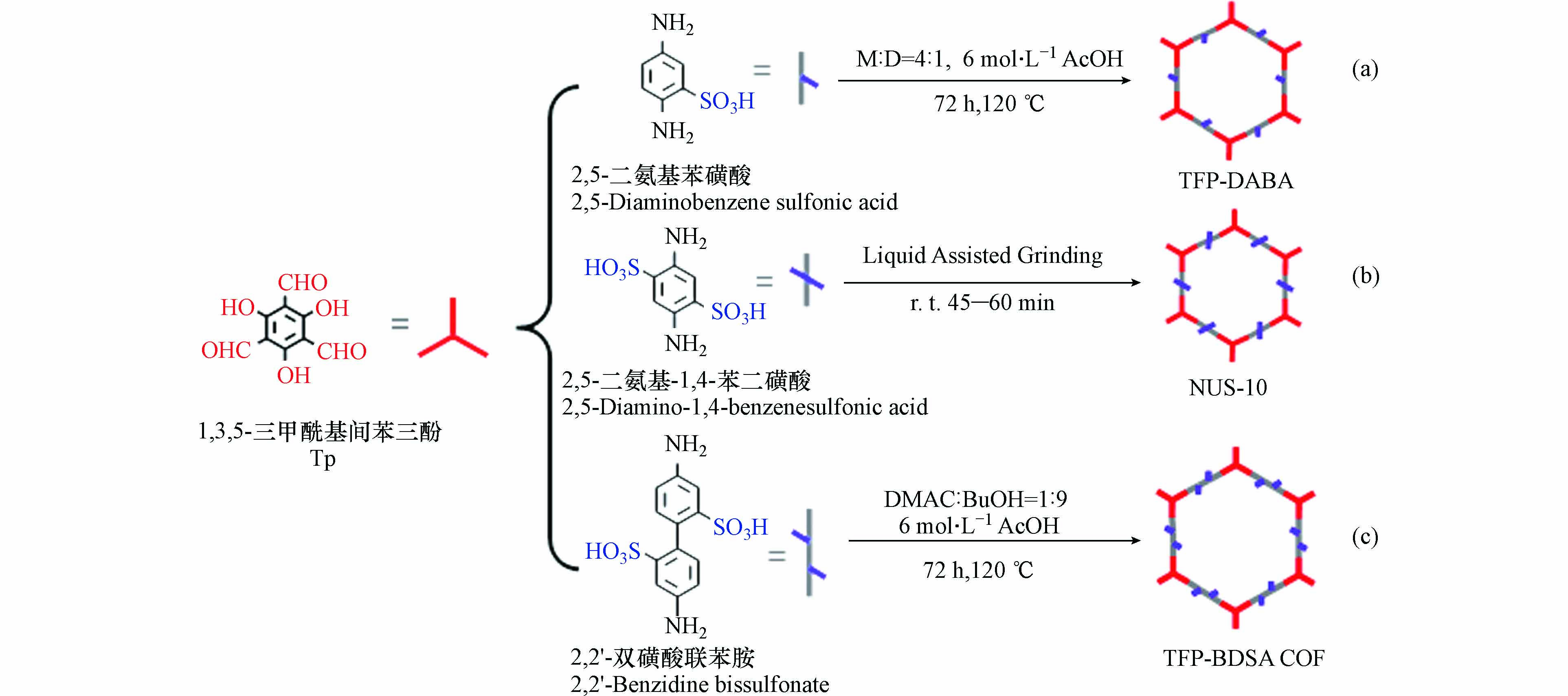
2015年Peng等[19]以2,5-二氨基苯磺酸为离子单体,Tp为中性连接剂,在120 ℃条件下通过热溶剂合成法得到了一种含有磺酸基的离子型COF(TFP-DABA),如图3(a)所示. 这种具有电负性的磺酸基COF成为近年来研究的热点. 2016年,Chandra等[31]在无水条件下证实TFP-DABA具有超高的质子导电性,为质子的高效传递提供了可能. 同年,Peng等[29]以2,5-二氨基-1,4-苯二磺酸为单体,得到另一种含有磺酸基的离子型COF(NUS-10),如图3(b)所示. 与TFP-DABA的合成单体相比,NUS-10的合成单体拥有两个磺酸基,因此NUS-10在导电性方面也更胜一筹. Chen等[32]将TFP-DABA结构中磺酸基所含的H+置换为Na+,得到了阴离子COF膜材料(TpPa-SO3Na). 这种膜材料在吸附有机染料方面展现出极强的性能,进一步扩展了磺酸基COF的应用范围. Yan等[33]以2,2'-双磺酸联苯胺为单体,制备出另一种磺酸基离子型COF(TFP-BDSA COF),如图3(c)所示. 由于2,2'-双磺酸联苯胺含有两个苯环结构,与TFP-DABA和 NUS-10相比,其孔径进一步扩大,可达2.3 nm. 大孔径使其对多种阳离子染料展现出优异的选择性吸附和分离功能.
氨基胍盐类离子单体主要包括二氨基胍盐和三氨基胍盐(图2b所示),通常与含有醛基的连接剂用于合成iCOF. Mitra等[34]用三氨基胍盐(TGx)和Tp通过热溶剂合成法合成出一种具有自剥离性质的iCOF(TpTGx, x=Cl-、Br-、I-),该材料能与带负电荷的细菌膜磷脂双分子层产生静电相互作用从而破坏细胞结构,因此对细菌具有良好的抑制效果. 2019年,Da等[35]用TGCl和2,5-二羟基对苯二甲酸(Dha)合成出的DhaTGCl具有高稳定性、有序的疏水孔通道和密度极高的阳离子位点等独特优势,能有效去除放射性离子.
杂环和脂肪族化合物中的氮原子稳定性好且易于合成,是将阳离子引入COF框架的良好候选物质. 作为常见的富氮杂环化合物,含有4,4'-联吡啶结构的离子单体(图2c所示)在iCOF合成中得到了十分广泛的应用. Hao等[36]以1,1’-双(4-甲酰基苯基)-4,4’-二氯化联吡啶作为核心框架,制备出PS-COF-1具有大量的联吡啶结构,对99TcO4−展现出优异的吸附性能. 探究发现,吸附驱动力主要来源于联吡啶基团与99TcO4−的静电吸引力. Yu等[20]报道了一种二维阳离子型COF材料(PC-COF),含有丰富的联吡啶基团,因此能够在极低浓度条件下从水中吸收甲基橙、酸性绿25等有机染料并使其浓度降低至10−8 M以下. Buyukcakir等[37]制备出离子型共价三嗪骨架(cCTF)具有联吡啶结构,独特的产物选择性能快速地将CO2转化为对应的环状碳酸盐,为解决CO2造成的温室效应提供了可能.
溴化乙啶(ethidium bromide,EB)具有易于调节的溴离子结构(图2d所示),常直接被用于合成iCOF. 2016年,Ma等首次[38]用EB和Tp得到一种离子型COF材料(EB-COF). EB-COF结构中的Br-可通过离子交换作用置换为F-、Cl-和I-等,通过控制抗衡离子来控制孔道孔径,该材料能用于新型释放载体的制备. 2021年,Deng等[39]将EB-COF制备成2D纳米片材料,通过机械分层加载技术能将其用于二氯喹啉酸(QNC)的释放,有望成为一种新型高效的农药制剂. 除了能用于制备2D材料外,EB也可用于3D阴离子COF的合成. Li等[40]将EB和一种四面体中性连接剂制备出一种具有高结晶度和孔隙度的正电荷三维COF材料,该材料对核废料和有机染料具有极强的吸附能力.
-
随着对iCOF的深入研究,科研工作者发现直接合成法并不能合成所有的目标产物. 某些活性基团由于自身与COF的整体框架不相匹配或反应条件较为苛刻,不能通过简单的“自下而上”直接合成法引入框架中. 因此需要通过合成后修饰法(post-synthesis modification,PSM)来合成. PSM是指通过化学转化或修饰COF框架内预先建立的官能团,不改变其基本拓扑结构却能赋予COF新特性的一种方法.
常用的PSM合成思路是直接在中性COF材料中引入带电基团. Hu等[41]通过离子交换先后将Mn2+和2,2-联吡啶基团直接引入到一种COF材料内(DhaTab),分别设计合成出两类iCOF([Mn(bpy)2]-DhaTab、[SO3Mn]-DhaTab). Mu等[42]在中性COF中引入甜菜碱基团从而制备出一种特殊的两性离子COF([BE]X%-TDCOFs),这种材料可精确控制CO2的还原,具有较高的产率和选择性. 除引入离子基团外,具有多功能性和高活性的离子液体(ionic liquids,ILs)也常被选择引入至中性COF内. Dong等[43]通过将离子液体引入COF通道壁上从而构建出一种具有催化活性的iCOF([Et4NBr]50%-Py-COF). 该材料对CO2具有极高的吸附量,是将CO2转化为甲酰胺的催化剂. 此外,Li等[44]在前人合成磺酸基离子型COF的基础上将其与离子液体浸渍,通过结合蚕丝纳米纤维(silk nanofibrils,SNFs)制备出一种COF/SNF复合膜材料,该复合膜具有极高的电导率.
除了直接引入带电基团外,将COF本身的活性基团活化成离子基团也是常用的思路. 活性基团的转化率一般较低,因此合成出的iCOF电荷密度通常较低. 联吡啶结构具有易电离的特性,Aiyappa[45]将中性COF浸泡在甲醇醋酸钴溶液中,搅拌后使联吡啶活化,从而生成了一种iCOF(Co-TpBpy). 该材料具有极强的催化性,经多次循环使用后仍旧具有极高活性. Mi等将含有联吡啶结构的中性COF与1,2-二溴乙烷进行季胺化反应,将联吡啶结构转化为更稳定的顺式构型,此举增加了该COF的稳定性. 随后用连二亚硫酸钠(Na2S2O4)进一步还原该产物,生成的iCOF(Py-BPy+·COF)可用于医学中的光声成像和光热治疗.
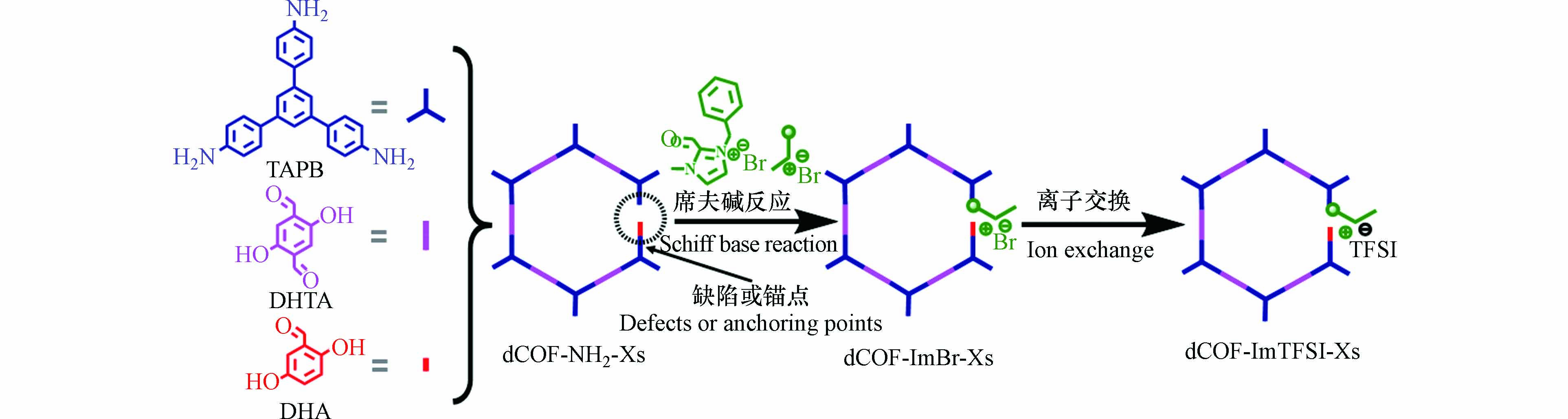
除了上述的两种合成后修饰思路外,预先设计出具有缺陷的COF,随后引入带电的活性基团填补缺陷,这也是一种PSM思路. 这种方法最初是在MOF合成中实现的,预设缺陷的MOF材料在引入新的活性基团后获得了许多新的功能. 基于此,Li等[46]以1,3,5-三(4-氨基苯基)苯(TAPB)为连接剂,2,5-二羟基对苯二甲醛(DHTA)和2,5-二羟基苯甲醛(DHA)为单体,通过DHA缺失的一个醛基预留出缺陷,将一种含咪唑基的离子引入得到dCOF-ImTFSI-Xs,如图4所示. 该材料不仅具有完整的孔通道,能为离子传输提供途径,而且还拥有咪唑基等阳离子和TFSI−等阴离子能进行更好的离子传导,在303 K-423 K内均可作为一种良好电解质.
-
有机污染物如甲基橙、刚果红等有机染料以及抗生素和有机氟化物等,放射性核素如235,238U(Ⅵ)、232Th(Ⅳ)以及99Tc(Ⅶ)等,重金属如Cr(Ⅵ)、Pb(Ⅱ)和Hg(Ⅱ)等这些有毒有害物质均易通过食物链在生物体内积聚,从而对人体健康构成威胁. iCOF作为吸附剂去除环境污染物的相关进展及其相互作用机理总结于表1中.
-
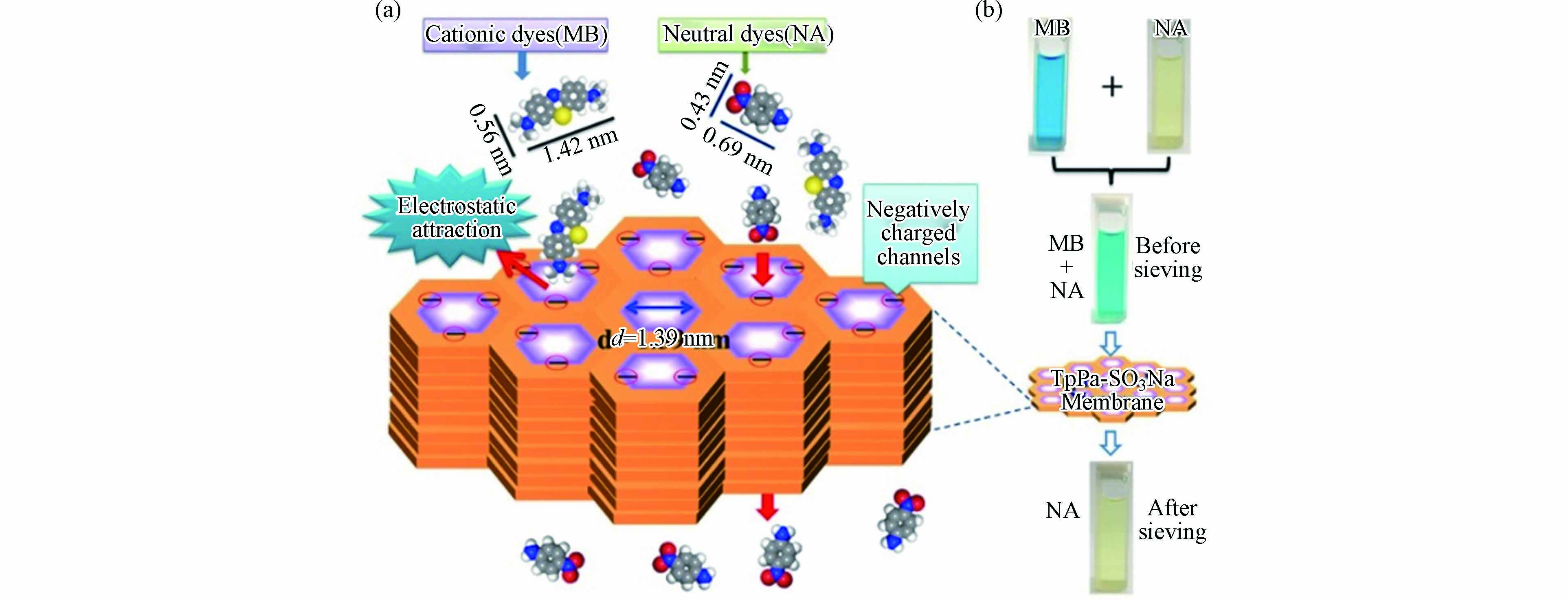
水、土壤、湖泊中的有机染料在含量较低的水平下也能对人体健康构成巨大威胁. 有机颜料在水中通常以离子形式存在,因此可以通过静电吸引将其吸附,从而达到去除的目的. Chen等[32]用制备出具有磺酸基团的二维共价有机框架膜材料(TpPa-SO3Na)用来吸附MB、碱性橙2等有机染料. 阳离子有机污染物通过其微孔通道时,除了会受到与负电荷通道产生的静电作用力外,在进入微孔通道前还会受到刚性孔径大小的影响(图5a所示). 这两种因素使得该阴离子共价有机框架膜对阳离子有机染料具有较好的分离特性. 将亚甲基蓝(MB)和对硝基苯胺(NA)混合后通过该膜材料,选择性吸附效果如图5b所示,正电的MB基本完全被吸附,而中性的NA仍存在溶液中. 除MB外,该膜材料对溴化乙啶(EB)等阳离子有机染料也具有高拦截率,同时能保持良好的溶剂渗透性. Dang等[47]通过在COF中引入咪唑基来增强与有机染料的静电吸引,从而提高吸附量. 该材料对阴离子及中性染料表现出极低的吸附量,而对MB、碱性橙2等阳离子染料吸附量分别达到2865.3 mg·g−1和597.9 mg·g−1. 实验结果证明其具有的电荷选择性是选择性吸附的关键.
Dey等[48]合成出4种具有不同孔径大小的阳离子型COF膜材料(Tp-Tta、Tp-Ttba、Tp-Bpy、Tp-Azo). 这4种材料均具有类似半透膜的性质,对水等溶剂表现出优异的渗透性,对刚果红(CR)、亮蓝G(BB)等阴离子有机染料产生静电吸引将其拦截,从而达到去除分离的目的. Yu等[20]以1,1’-双(4-甲酰苯基)-4,4’-二氯化联吡啶(BFBP2+•2Cl−)为单体制备出一种二维阳离子型COF(PC-COF),其在处理有机污染物的过程中表现出了优良的特性. 由于富含大量联吡啶结构,PC-COF能与有机染料产生强烈的静电相互作用. 在低浓度条件下依旧能够从水中吸收如甲基橙(MO)、酸性绿25(AG-25)、直接红棕M(DFBM)、靛蓝胭脂红(IC)等多种阴离子有机染料,并使其浓度降低至10−8 mol·L−1. 对其吸附机理的探讨表明吸附驱动力主要来源于COF材料中阳离子联吡啶基团与有机染料阴离子的静电吸引力. 此外,所含的“硬碱”氯离子与有机染料之间的离子交换也增强了吸附作用.
除有机染料外,水体环境中还存在着如抗生素、有机氟化物、双酚A等其他有机污染物. 这些物质对人体具有很高的毒性,很容易通过食物链在生物体内积累,从而对人体造成伤害. 氟喹诺酮类药物(fluoroq-uinolones,FQs)在人体内代谢不完全导致在水环境中能检测出其残余物. Jiang等[49]合成出一种离子型COF(Tp-MTABs)将其用于吸附FQs,30 s内就能达到吸附平衡. 实验证明在多种竞争离子和高盐度天然海水的复杂环境中,Tp-MTABs对FQs仍具有较高的选择性. Tp-MTABs能进行自剥离从而形成二维离子共价有机纳米片材料,促使其暴露更多的离子结合位点,因此能增强与目标离子间的静电相互作用和π-π相互作用,达到更好的吸附效果. Li等[50]使用Fe3O4@TpBD去除双酚A(BPA),在5 min内就能达到吸附平衡,吸附量可达161 mg·g−1. 实验探究发现,BPA与Fe3O4@TpBD的苯环结构之间存在π-π相互作用和氢键作用,是导致BPA吸附的主要原因. 类似机理如Liu等[51]用醛基和肼基对COF进行功能化后应用于废水中BPA的去除,去除率达到97%. Da等[52]通过使用不同的中性连接剂来提高层之间的π-π堆积效率,减少层之间强烈的电荷排斥,从而实现提高材料结晶度的目的. 根据此策略合成出的阳离子型COF(TFPT-TGCl-iCOF)对农药中间体2,4-二氯苯酚的最高吸附量可达893 mg·g−1. 除了静电相互作用、π-π堆积和氢键作用外,疏水相互作用也能影响吸附过程. Wang等[53]通过亚胺缩合反应制备的COF1具有疏水性,对同样具有疏水性的六氟环氧丙烷二聚酸(GenX)和六氟环氧丙烷三聚酸(HFPO-TA)具有特异的选择吸附. 在疏水作用和静电吸引的双重作用下,对GenX和HFPO-TA吸附量分别为684 mg·g−1和1214 mg·g−1,表现出优异的吸附能力.
总的来说,iCOF吸附有机污染物的能力不仅与材料的结构性质和孔径通道大小有关,也和污染物分子的结构和大小有关. 通过密度泛函计算可以有效探究有机污染物与iCOF之间的相互作用机制,两者之间的作用主要以静电相互作用、π-π相互作用和氢键作用为主. 但如何尽可能地提高这些相互作用力从而扩展iCOF在处理有机污染物的应用仍旧是一个挑战.
-
核工业的快速发展伴随着大量核废料的产生. 核废料中常见的放射性核素主要包括235,238U(Ⅵ)、232Th(Ⅳ)以及99Tc(Ⅶ)等. 放射性核素流动性高,易溶于水,可以对人体的中枢神经和内分泌系统造成伤害,大剂量或长时间接触会对人体造成明显伤害,增加患癌风险. 从废水中高效捕获和去除放射性核素是解决核废料造成的环境问题的主要途径之一. 近年来,众多iCOF广泛地应用于放射性核素.
根据硬软酸碱(HSAB)理论,含氧基团对放射性核素的亲和力一般较好,可以用于放射性核素的去除. Xiong[54]将一种离子型COF材料([NH4]+[COF-SO3]−)用于吸附铀酰,该材料的合成步骤如图6所示. 将2,5-二氨基苯磺酸和Tp合成的COF-SO3H经氨水氨化后,磺酸基(−SO3H)失去H成为−SO3−基团,−SO3−基团能与U(Ⅵ)、Th(Ⅳ)等放射性离子产生配位作用,与此同时NH4+可以与放射性离子进行离子交换. 在两种作用下,该材料对铀酰的吸附量最高可达851 mg·g−1. 其同时对232Th(Ⅳ)也具有极强的吸附效果. [[NH4]+[COF-SO3]−]具有选择性高、pH适用范围广、循环性能好等优点,有望成为处理核废料中放射性核素的高效吸附材料. 此外,Li等[55]合成出一种含有羧基(−COOH)的离子型COF(JUC-505-COOH),羧基与废水中的铀离子能产生较强的配位键,在较短的时间内就能吸附大部分铀离子. 由于该材料在低pH条件下表现的极强耐酸性,其在实际铀污染修复方面展现出巨大潜力.
除含氧基团外,具有含氮基团的COF也能对放射性核素产生极强的吸附效果. 吡啶结构作为一种常用的富氮配体,结构中的N原子能与99Tc(Ⅶ)形成稳定的配位键. 因此含有吡啶结构的COF可用于吸附高锝酸根离子. 99Tc(Ⅶ)主要以高锝酸根(99TcO4−)的形态存在于核废料中. Hao等[36]合成出具有丰富联吡啶基团结构的PS-COF-1,对99TcO4−的吸附驱动力主要来源于联吡啶基团与99TcO4−的静电吸引力. 此外联吡啶中的“硬碱”氯离子与99TcO4−的离子交换也起到重要的作用. He等[56]以1,1’-双(4-氨基苯基)-4,4’-二氯化联吡啶(Viologen-NH2)作为核心框架,制备出的SCU-COF-1与PS-COF-1的结构相比,吡啶结构的数量相似,但增加了大量的亚氨基(—NH—)和羟基(—OH). 这两类官能团促使其吸附能力得到了提升. 由于99TcO4−具有极高的辐射性,因此在实验中通常用电荷密度相同、阴离子交换性能相似且不具备放射性的铼酸根(ReO4−)替代. Li等[57]报道的另一种富含吡啶结构的阳离子COF(SCU-CPN-1)对ReO4−具有极快的吸附速率,在30s内就能去除溶液中超过99%的ReO4−. 与之对比的两种商用交换树脂(A532E、A530E)在5 min内分别只能去除32%和38%的ReO4−,且至少需要120 min才能达到吸附平衡.
含氮基团中除吡啶外,咪唑基团与99TcO4−之间的结合能也很高,咪唑基团也是该类型COF常选择的基团之一. 通过引入咪唑基团增强材料中的正电荷密度,增大材料与目标离子之间的静电作用力,相应地能使吸附量提高. 考虑到电荷排斥和空间位阻的影响,Li等[58]将吸附能力最强的1,3,5-三(咪唑基)苯与空间位阻较小的1,3,5-三(溴甲基)苯制备得到SCU-CPN-2. 该材料同时具有极高的正电荷密度和极强的抗辐射性能,对ReO4−的最高吸附量可达1467 mg·g−1. Jiao[59]用三(4-咪唑基苯基)胺合成出QUST-iPOP-1,该材料在利用咪唑基团拉高电荷密度的同时,还具有高孔隙率和宽孔径分布的特点,对水中ReO4−表现了极高的吸附性能.
使用iCOF作为吸附剂来处理放射性核素的污染已经得到广泛研究. 科研人员往往通过iCOF的官能团来实现其目的. —COOH、—OH、—CHO等含氧官能团对放射性核素具有很好的亲和力,但由于其选择性不如含氮官能团,因此实际效果并不理想. 而吡啶和联吡啶、酰胺肟基团等含氮官能团对放射性核素具有很好的选择性,因此常用于萃取U(Ⅵ)、Tc(Ⅶ)在内的多种核素. 除此之外,核废水通常具有高酸性或高碱性、强辐射性等,因此在极性条件下COF的稳定性以及可重复利用性也需考虑.
-
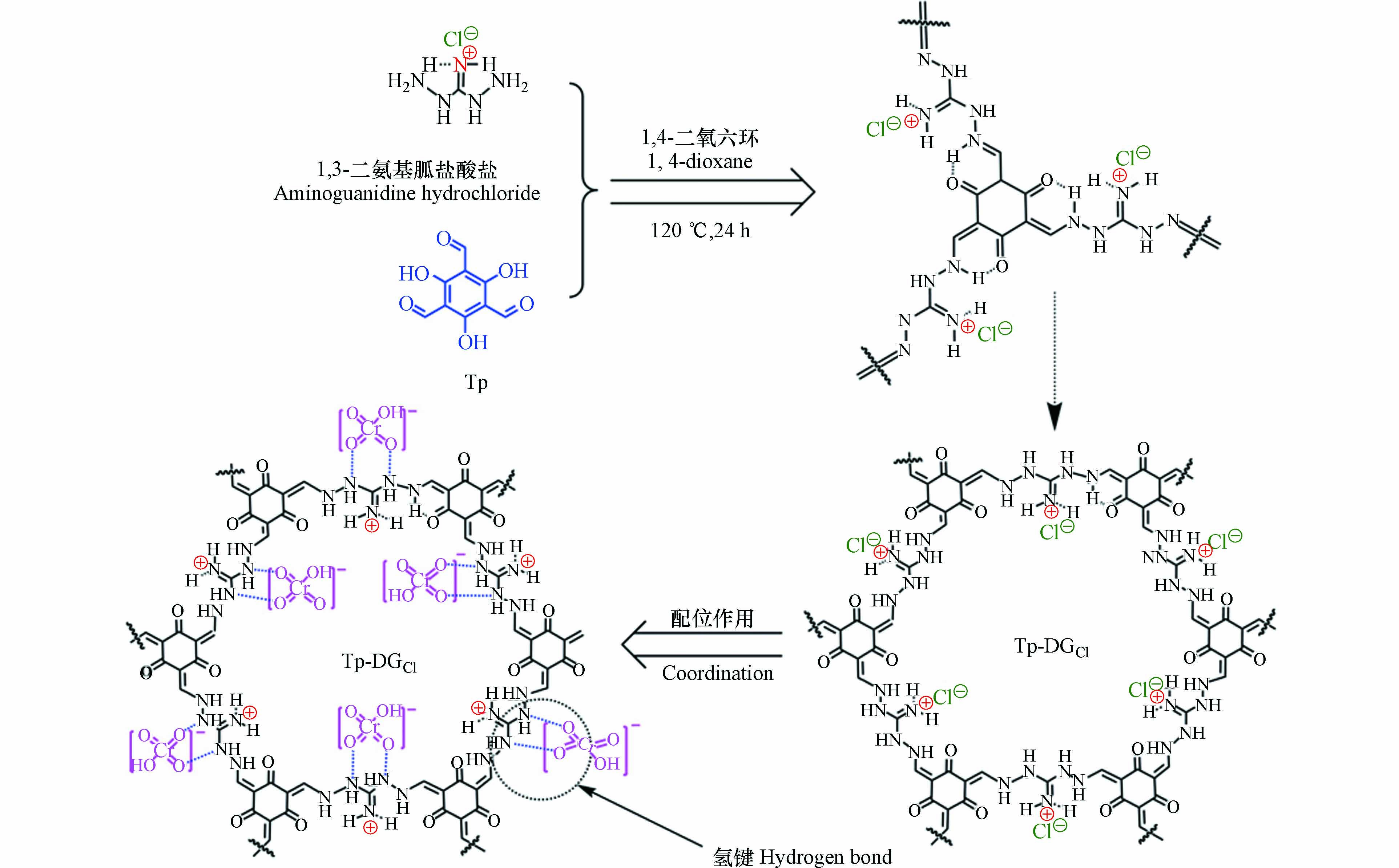
工业发展会导致大量重金属离子排入水体. 这些重金属离子会通过食物链转移到动植物体内. 重金属离子不仅影响动植物的生长发育,而且还会给人类健康带来一系列负面影响. Cr(Ⅵ)对人的呼吸道和皮肤均有刺激,对人体具有致癌和致突变的作用. 氨基胍盐类离子单体中存在的胍基与Cr(Ⅵ)之间会产生静电吸引,而结构中存在的羟基、氨基等能与Cr(Ⅵ)产生氢键,这两种因素使得含氨基胍盐的COF对Cr(Ⅵ)有极强的吸附能力,因此氨基胍盐常用于制备吸附Cr(Ⅵ)的iCOF材料. Li等[60]以1,3-二氨基胍盐酸盐为单体合成出一种具有二氨基胍基团的iCOF材料(BT-DGCl),将该材料用于吸附Cr2O72−. 对Cr2O72−的理论吸附量为BT-DGCl中总氯化物含量的一半. 因为在与Cr2O72−交换的过程中,每2个Cl−与1个Cr2O72−进行交换. 在多种离子共存的条件下该材料依具有良好的选择性和快速吸附动力学,几分钟内能使Cr2O72−的浓度降低多个数量级. Zhuang等[61]以1,3-二氨基胍盐酸盐为单体合成出含有β-酮烯胺键的iCOF(Tp-DGCl). 通过提高材料中二氨基胍的密度,使其对目标离子具有更高的负载能力和亲和力. Tp-DGCl对Cr(Ⅵ)的最大吸附量可达336.04 mg·g−1. 如图7所示,通过Cl−与Cr(Ⅵ)的离子交换作用和氢键相互作用来实现对Cr(Ⅵ)的快速高效吸附. 5 min内该材料对Cr2O72−的吸附就能到达最大吸附容量的一半,这与结构中含有较多的胍基有关. 另外,结构中的β-酮烯胺键结构极大地增大材料的表面积,增强了对目标离子的吸附.
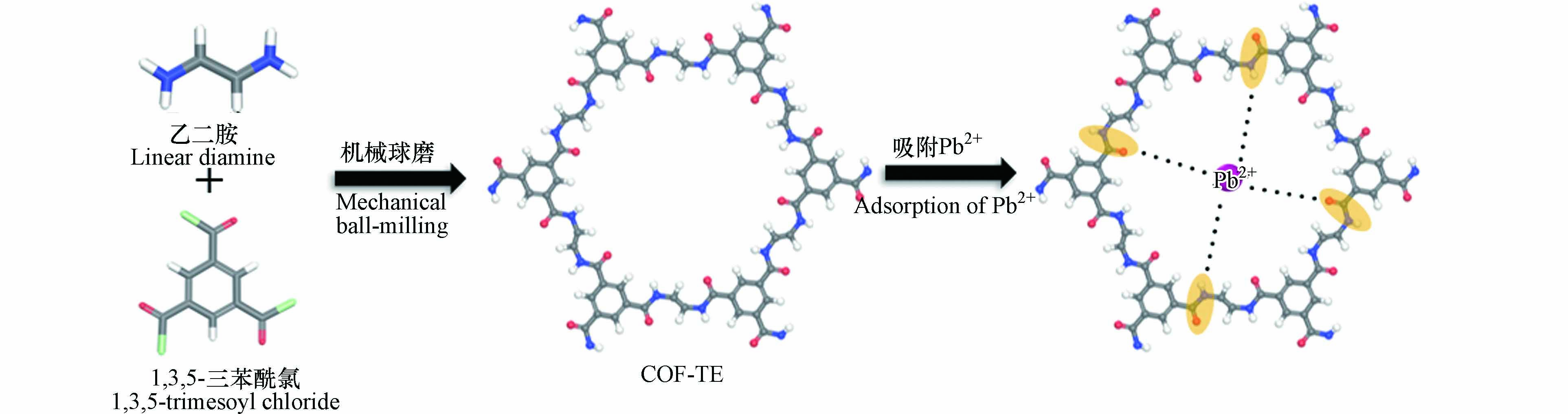
Pb(Ⅱ)会导致食欲不振、恶心、呕吐、贫血等症状,能对人体的神经、消化、心血管和内分泌等造成严重的危害. 基于分子结构设计策略原则,Li等[62]通过酰氯和氨基的聚合反应制备了两种酰胺基COF(COF-TP和COF-TE). COF-TP和COF-TE分别由对苯二胺、乙二胺与1,3,5-三甲苯甲酰氯合成. 如图8所示,COF-TE中的酰胺基能与Pb(Ⅱ)产生配位作用,表现出良好的吸附性. 在较高pH的条件下,COF-TE表现出比COF-TP更好的吸附效果. 这是由于COF-TE不但具有较多的负电荷,也存在更多的以酰胺基为代表的吸附位点,对Pb(Ⅱ)具有更高的吸附能力. Gupta[63]设计合成出一种框架内含有二甲基胺盐(DMA+)的iCOF-1,实验表明iCOF-1对DMA+和Pb2+的相互作用能分别为−24.5 kJ·mol−1 和−39.2 kJ·mol−1,说明iCOF-1能与Pb2+产生更强的吸附作用. iCOF-1通过离子交换过程将DMA+置换为Pb2+,从而达到去除分离的目的.
汞(Hg)对人体的呼吸系统、皮肤、血液以及眼睛都具有毒性,能对人体造成精神障碍、震颤以及肾脏损害等影响. Li等[64]将由草酰二肼(ODH)和Tp制备出的一种烷基胺类iCOF用于去除水中的Hg(Ⅱ). 由于具有不可逆的烯醇-酮互变结构,且分子内N—H键与O═C键之间存在氢键,因此其结晶度和化学稳定性都得到增强. 同时,该材料存在大量周期排列的含氮基团和含氧基团,有利于增强与Hg(Ⅱ)之间的亲和性及协同作用. 实验表明该材料对Hg(Ⅱ)的吸附量最高可达1692 mg·g−1. 此研究为构建用于环境修复的烷基iCOF提供了可能.
以上结果表明,含N、O、S的官能团与重金属离子之间存在较强的作用力. 在适宜的条件下,这些官能团对重金属离子具有极强的吸附能力,但对目标金属离子的选择性普遍较差. 考虑到构成iCOF的有机配体可以提供特殊的活性位点,进而与目标离子之间形成稳定的作用. 因此,从iCOF的合成入手可以有效解决这一问题:通过选择特殊的有机配体从而实现对目标金属离子的选择性吸附. 除了从合成入手外,通过引入特殊官能团或与其他纳米材料结合,同样也能提高iCOF的选择吸附能力. 因此,构建具有特殊官能团的iCOF材料有利于吸附性能的提高.
-
iCOF作为一种新兴材料,自2015年出现至今发展迅速. 由于其具有的电离特性以及晶体多孔材料的性质,因此具有巨大的应用前景. 本文综述了iCOF的结构和合成方法,以及其在环境修复领域展现出的独特优势和极高的应用价值,并对其相互机理进行了探究. iCOF对不同的环境污染物具有较高的吸附能力. 与污染物之间的静电吸引是其用于环境修复的重要机理. 在修复过程中,iCOF内部的活性离子基团与污染物基团进行置换或代替,使污染物滞留在iCOF框架内从而达到吸附分离的目的. 除静电吸引外,用特殊官能团进行表面修饰或引入特殊活性位点能有效提高对目标污染物分子的选择性去除. 近年来iCOF被广泛应用于污染物的去除.
iCOF在环境修复领域展现出极高应用价值的同时,还有许多亟需解决的问题. (1)有机单体价格昂贵导致iCOF的合成成本很高且合成条件苛刻导致其不能大规模生产. 随着技术的发展,iCOF以较低成本、大规模地合成出来是毋庸置疑的. (2)iCOF合成之路仍需探索. 科研人员仍需筛选合适的合成单体和连接剂,丰富其拓扑结构以及所含官能团的种类. (3)如何对整体框架和离子基团的位置进行设计,有效地增强其在复杂环境中的稳定性和重复利用性以及对目标污染物的高效选择性仍是未来的主要挑战. (4)带电基团的引入导致iCOF发生静电排斥和堵塞孔道,因此其在结晶度和孔隙率方面的表现不理想. 如能将机器学习算法和人工智能应用于iCOF的合成,将有利于开发出高结晶度、高孔隙度以及缺陷少的iCOF. (5)将iCOF应用于环境修复无疑会将其释放到环境中. 因此还应考虑iCOF在自然水系统中的毒性,特别是其在食物链中的富集和最终对人体的毒性. 随着技术的快速发展,在不久的将来,iCOF在环境修复中一定具有巨大的应用潜力.
离子型共价有机框架材料的合成及其在环境修复应用的研究进展
Progress in the synthesis of ionic covalent organic frameworks and their application in environmental remediation
-
摘要: 离子型共价有机框架(ionic covalent organic frameworks,iCOFs)材料是框架内或孔道中带有电荷的共价有机框架(COF)材料. 除了具有低密度、易于调节的孔道结构、比表面积大、优异的热稳定性和化学稳定性等传统优点外,其内部的离子基团作为作用位点能与电性相反的基团结合,从而在质子传导、催化、生物医学等领域展现出巨大的应用价值. 尤其在环境修复领域,iCOF已被广泛应用于去除有机污染物、放射性核素和重金属等污染物. 本文从iCOF的结构与合成方法出发,综述了其对环境污染物的选择性吸附,并对相互作用机理进行探究,最后对研究前景进行展望.Abstract: Ionic covalent organic frameworks (iCOFs) are a kind of covalent organic framework containing ionic groups in the structural framework or microporous channels. In addition to the advantages of low density, tunable pore structure, large specific surface area, excellent thermal and chemical stability, etc., iCOFs can be combined with opposite charged groups, so as to exhibit great applications in proton conduction, catalysis, biomedicine and other fields. Especially in the field of environmental remediation, iCOF have been widely used in the removal of organic contaminants, radionuclides and heavy metal ions. In this review, the structure, the synthesis methods and the application of iCOF in environmental pollutants have been discussed and especially, the underlying removal mechanism has been highlighted. Finally, the opportunities and challenges of iCOFs in environmental remediation were analyzed in detail to understand their future developing prospects.
-
Key words:
- ionic covalent organic framework /
- adsorption /
- organic pollutants /
- radionuclide /
- heavy metal ions.
-
长江上游地区是我国重要的经济发展带,位于长江上游的中大型城市多以丘陵地貌为主,具有地势落差大、雨污收集速度快、地下水渗入率高等雨污排放特征[1-3]。城市污水处理厂是雨污治理的重要基础设施。一方面,污水处理厂减少了有机污染物、氮磷等污染物的排放,在水环境污染物控制中发挥重要作用[4-6]。城市污水处理厂累计削减的有机污染物 (以COD计) 占全国减排总量的70%以上,且占比逐年增加[7];另一方面,城市污水处理厂需消耗大量电能。污水处理厂的用电量约占全社会用电量的2.6‰[8],且占比逐年上升。此外,城市污水处理厂在深度脱氮除磷中,需使用大量的有机碳源和混凝剂[9-11]。
随着城市污水水量增加和污染物种类增多,污水处理厂面临严峻挑战。为保证出水水质达标,污水处理厂会考虑延长处理工艺单元链和增加了药剂的投加量,致使其运行成本增加[12-13]。本研究拟基于长江上游西南丘陵城市污水处理厂的运行优化需求,以泸州作为长江上游西南丘陵城市的代表,分析2020—2022年泸州中心区城市污水处理厂的电力、混凝剂和碳源使用量和使用效率,并与同期全国城市污水处理厂运行情况比较,以期掌握西南典型丘陵城市污水处理厂的用电量和药剂使用量特征,为污水处理厂运行优化提供参考。
1. 污水处理厂数据来源与运行特征
1.1 数据来源
本研究中泸州中心区5座城市污水处理厂的运行用电量、药剂投加量和进水与出水水质由泸州市住房和城乡建设局、泸州市兴泸水务 (集团) 股份有限公司提供,全国污水处理厂的运行数据来自住建部全国城镇污水处理管理信息系统和《中国城镇污水处理与再生利用发展报告 (1978-2020) 》。污水处理厂的用电量和药剂投加量为月累积用量;进水和出水水质指标为月均数据;统计期 (2020年1月—2022年4月) 内中位值用于定量讨论水质特征,5th~95th概率分布 (将数据按大小顺序排列的5%和95%分位值) 和箱式图用于表示水质数据分布特征。
1.2 泸州市城区污水处理厂基本情况
泸州市位于长江上游丘陵地区,其城市污水和初期雨水经由排水管网进入污水处理厂处理后再排入河流中。该市中心区共有5座城市污水处理厂,依次为纳溪污水处理厂、鸭儿凼污水处理厂、二道溪污水处理厂、城东污水处理厂和城南污水处理厂。5座污水处理厂的设计污水处理量、主体处理工艺和主要耗能单元如表1所示。
表 1 泸州中心区5座城市污水处理厂概况Table 1. Overview of the five urban wastewater treatment plants in the central area of Luzhou污水处理厂 设计污水处理量/(104 m3·d−1) 主体处理工艺和主要能耗单元 纳溪污水处理厂 2.75 曝气沉砂池-A2/O改良工艺-紫外消毒渠 鸭儿凼污水处理厂 8.0 曝气沉砂池-MBR-紫外消毒渠 二道溪污水处理厂 10.0 曝气沉砂池-A2/O-反硝化滤池-紫外消毒渠 城东污水处理厂 5.0 曝气沉砂池-A2/O改良工艺-D型滤池-紫外消毒渠 城南污水处理厂 5.0 曝气沉砂池-A2/O改良工艺-D型滤池-紫外消毒渠 注:A2/O改良工艺即在厌氧池前端增加了预反硝化单元。 1.3 污水处理厂处理水量与进出水水质
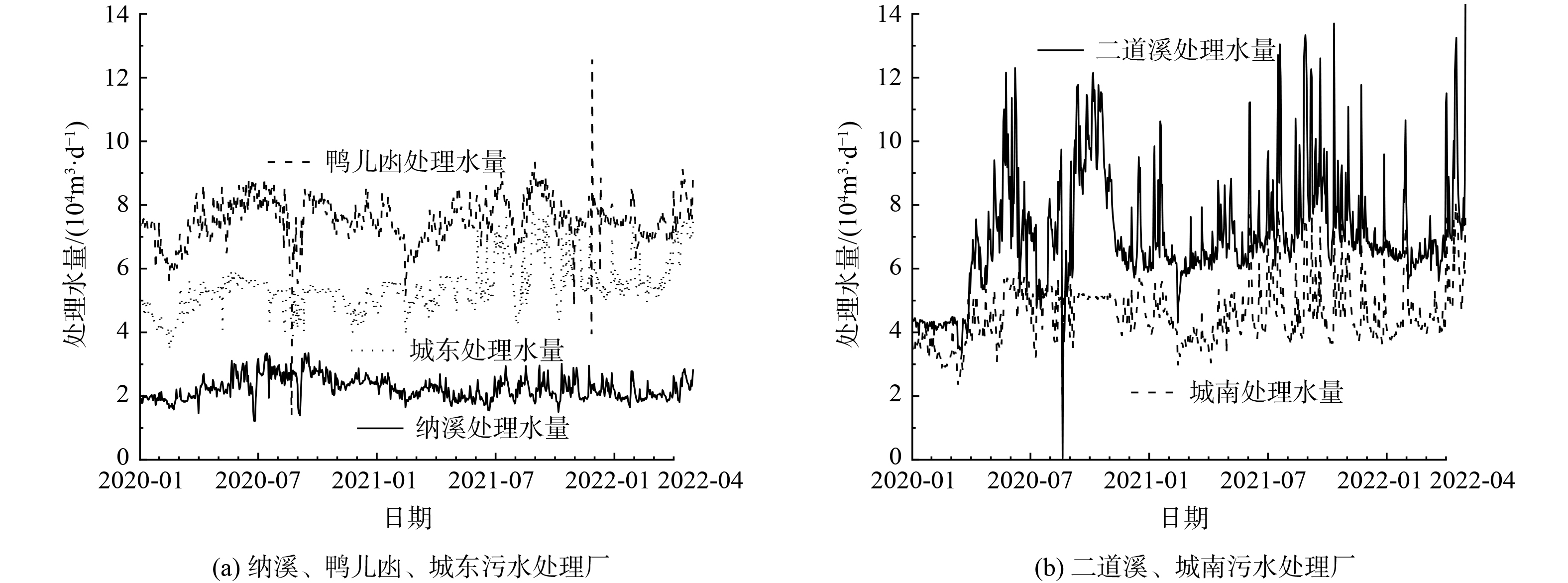
污水处理厂的处理水量和运行负荷如图1所示。一方面,污水处理厂水量突然升高情况较多,与该地区的降雨频繁密切关联;另一方面,水量随季节性波动明显,二道溪、城东和城南污水处理厂在7—9月份的水量较大[14]。从2021年开始,城南污水处理厂和城东污水处理厂处理水量长期大于设计处理量,主体工艺处于高负荷运行状态。此外,表2展示了5座污水处理厂进出水水质,出水符合《城镇污水处理厂污染物排放标准》 (GB 18918-2002) 一级A排放标准,但距离地表Ⅲ类水相关指标限值尚有一定差距。
表 2 泸州中心区城市污水处理厂进水和出水水质特征 (2020年1月—2022年4月)Table 2. Quality characteristics of inlet and outlet water of urban wastewater treatment plants in the central area of Luzhou (2020-01—2022-04)污水 处理厂 BOD5/(mg·L−1) TN/(mg·L−1) [NH3-N]/(mg·L−1) TP/(mg·L−1) 进水 出水 进水 出水 进水 出水 进水 出水 纳溪污水处理厂 198.47±60.79 12.03±3.87 27.80±6.67 9.57±1.10 18.70±5.72 0.61±0.45 2.79±0.72 0.21±0.06 鸭儿凼污水处理厂 197.01±55.66 11.77±1.79 29.85±5.18 7.83±1.54 23.21±2.95 0.51±0.32 3.40±1.01 0.24±0.06 二道溪污水处理厂 231.52±55.01 11.26±2.61 29.85±5.18 7.83±1.54 28.39±9.40 0.04±0.39 3.54±0.85 0.25±0.09 城东污水处理厂 182.33±78.28 19.86±3.55 33.18±8.68 10.20±1.21 25.69±6.69 0.04±0.28 3.28±1.08 0.31±0.03 城南污水处理厂 216.65±65.50 11.97±2.99 28.97±7.55 9.25±1.49 20.73±6.04 0.03±0.03 2.50±0.77 0.24±0.07 2. 结果与讨论
2.1 污水处理厂运行用电量与用电效率
1) 运行用电量。用电量是污水处理厂的主要运行成本之一,亟需识别西南典型丘陵城市污水处理厂的用电特征和用电效率[15-16]。总用电量 (Q,104 kW·h) 、单位处理水量的用电量 (QV,kW·h·m−3) 和单位污染物去除量的用电量 (QCOD和QNH3-N,分别指去除污水中单位质量有机污染物 (以COD计) 或NH3-N的用电量,kW·h·kg−1) ,常用于表示用电特征和用电效率,计算方法如式 (1)~(3) 。
Qv(kW⋅h⋅m−3)=总用电量(kW⋅h)处理水量(m3) (1) Qc(kW⋅h⋅kg−3)=总用电量(kW⋅h)COD削减量(kg) (2) QNH3−N(kW⋅h⋅kg−3)=总用电量(kW⋅h)NH3−N削减量(kg) (3) 泸州中心区5座城市污水处理厂的总用电量Q为20.0~110.9 104 kW·h (5th~95th) ,如图2 (a) 所示。鸭儿凼污水处理厂的Q值最大 (中位值105.75 104 kW·h) ,其次为二道溪污水处理厂 (中位值 85.10 104 kW·h) 。城东和城南污水处理厂的处理水量和主体处理工艺相同,但城南污水处理厂Q的中位值为51.01 104 kW·h ,高于城东污水处理厂 (中位值47.61 104 kW·h) 。在污水处理厂的实际运行当中,曝气是主要的耗能工艺。此外,5座城市污水处理厂均在三级处理流程中设置了紫外消毒渠处理单元,这也会增加污水处理厂的总用电量。伴随着污水处理工艺链的延长,电耗的总量和来源也逐渐增加。
泸州中心区5座城市污水处理厂的QV为0.25~0.52 kW·h·m−3 (5th~95th) ,如图2(b)所示。鸭儿凼的QV值最高 (中位值0.46 kW·h·m−3) ,与其膜生物反应器 (membrane bioreactor,MBR) 处理工艺能耗较高有关[17]。城东污水处理厂的QV值最低 (中位值0.28 kW·h·m−3) ,且显著低于城南污水处理厂 (中位值 0.36 kW·h·m−3) 。城东污水处理厂与城南污水处理厂的处理水量相近、工艺相同,QV差异表明进水水质、运营操作等因素会导致用电效率的差异。
泸州中心区城市污水处理厂的QCOD和QNH3-N分别如图2(c)和图2(d)所示。QCOD为1.12~3.55 kW·h·kg−1 (5th~95th) 。其中,鸭儿凼污水处理厂的QCOD最高 (中位值2.58 kW·h·kg−1) ,城东污水处理厂QCOD稍高于城南污水处理厂且分布范围较大 (中位值1.94 kW·h·kg−1) 。QNH3-N为9.64~30.6 kW·h·kg−1 (5th~95th) 。鸭儿凼污水处理厂的QNH3-N最大 (中位值20.57 kW·h·kg−1) ,而城东污水处理厂的QNH3-N最小 (中位值11.23 kW·h·kg−1) ,且显著低于城南污水处理厂 (中位值 17.39 kW·h·kg−1)。
2) 运行用电效率。分析2020—2021年全国5 799座城镇污水处理厂处理用电量 (图3(a)) 。QV为0.16~1.20 kW·h·m−3 (5th~95th) ,中位值和平均值分别为0.36和0.47 kW·h·m−3。80%的污水处理厂的QV低于0.60 kW·h·m−3。此外,泸州中心区5座城市污水处理厂的QV均优于全国平均值,但差于前28%的污水处理厂。其中,鸭儿凼、二道溪和城南污水处理厂的QV差于国内50%以上的污水处理厂。由此说明,泸州中心城区城市污水处理厂的用电效率整体较高,但仍可通过处理工艺优化和升级进一步提高用电效率[18-19]。
泸州是典型的西南丘陵城市。进一步比较其与西南地区和其他地区的用电效率,结果如图3(b)所示。华北地区的QV值最高 (中位值 0.61 kW·h·m−3) ,西南地区、西北地区和华东地区的QV值为中等 (中位值 0.36~0.51 kW·h·m−3) 。东北地区、华中地区和华南地区的QV值最低 (中位值 0.27~0.36 kW·h·m−3) 。与各地区典型运行效率相比,泸州市中心城区5座污水处理厂的QV优于华北地区、西北地区和西南地区的QV。各地区Qv的存在较显著的差异,与污水进水水质、处理工艺和气候紧密关联[20-21]。
为增加运行用电效率,污水处理厂从厂区设计方面应该结合所处的地形特点,在处理单元高程布置中采用合理的布局,以减少提升泵的使用。此外,鼓风机和搅拌器是主要耗能设备,从选购的角度来说,应考虑实际处理水量和水质,选择型号适宜的设备;从布置的角度来说,精准曝气以增加溶解氧的传质速率可提升运行用电效率[22]。
2.2 污水处理厂除磷混凝剂用量与使用效率
1) 除磷混凝剂用量。泸州市中心城区污水处理厂投加的混凝药剂为聚合硫酸铁 (polyferric sulfate,PFS) 。2020—2022年5座城市污水处理厂的单位处理水量的PFS投加量 (PFSV,mg·L−1) 和单位总磷 (TP) 去除量的PFS投加量 (PFSTP,mg·mg−1) 如图4所示。鸭儿凼和二道溪污水处理厂的PFSV较高,为48.42~53.57 mg·L−1;城东和城南污水处理厂的PFSV较低,为25.11~26.98 mg·L−1。
 图 4 泸州中心区城市污水处理厂单位处理水量的PFS投加量 (PFSV) 和单位总磷去除量的PFS投加量 (PFSTP) 时间变化和统计分布 (2020年1月—2022年4月)Figure 4. Temporal variation and statistical distribution of PFS dosage per unit treated water volume (PFSV) and PFS dosage per unit total phosphorus removal (PFSTP) of urban wastewater treatment plants in the central area of Luzhou (2020-01—2022-04)
图 4 泸州中心区城市污水处理厂单位处理水量的PFS投加量 (PFSV) 和单位总磷去除量的PFS投加量 (PFSTP) 时间变化和统计分布 (2020年1月—2022年4月)Figure 4. Temporal variation and statistical distribution of PFS dosage per unit treated water volume (PFSV) and PFS dosage per unit total phosphorus removal (PFSTP) of urban wastewater treatment plants in the central area of Luzhou (2020-01—2022-04)PFSTP与进水和出水水质密切相关。泸州中心城区污水处理厂出水TP均满足一级A排放要求。进一步分析发现,泸州中心城区5座污水处理厂的PFSTP为2.67~30.41 mg·mg−1 (5th~95th) 。城东污水处理厂PFS投加质量分数较低,但同时PFSTP仅为9.41 mg·mg−1;鸭儿凼不仅PFS投加质量分数较高,同时PFSTP达到19.16 mg·mg−1。这与鸭儿凼污水处理厂二级出水的磷形态 (偏磷酸盐或溶解态为主) 等有关,需进一步开展水质分析来提高除磷工艺优化[23-24]。值得注意的是,城东污水处理厂和城南污水处理厂的工艺和设计规模相同,但城南污水处理厂的PFSTP显著较高,为13.34 mg·mg−1。这可能是由于工艺操作和水中磷形态不同。如PFS对偏磷酸盐的去除效果较差,对磷形态分析和操作条件优化将有助于提高除磷效率和混凝剂投加效率[25]。
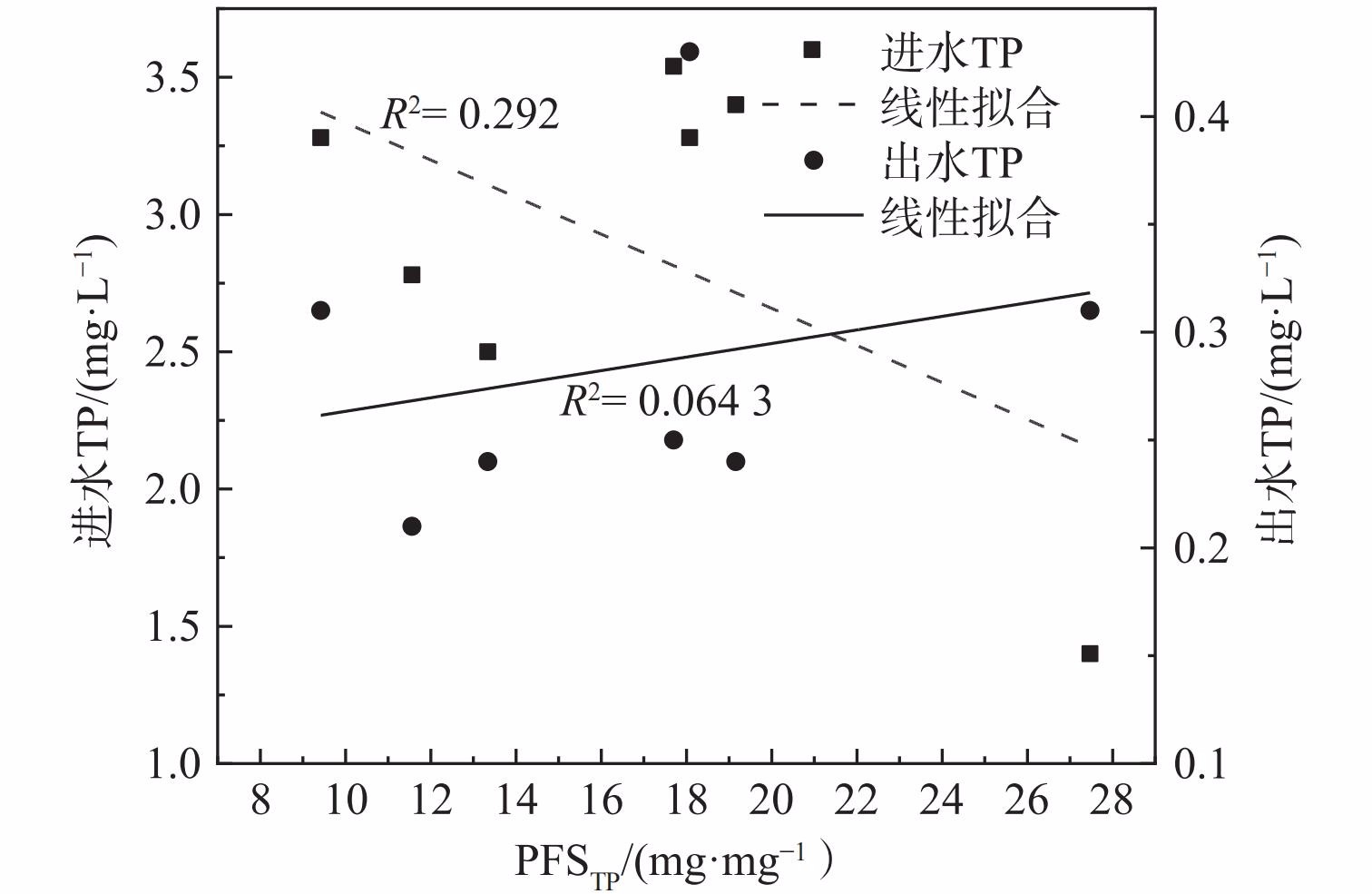
2) 除磷混凝剂使用效率。比较泸州市中心城区5座污水处理厂与国内其他污水处理厂的混凝剂用量,结果如表3所示。北京市吴家村污水处理厂和上海市城投污水处理厂投加的混凝剂为PFS,但其PFSTP是泸州市中心城区污水处理厂的1.36和2.06倍;昆明市第三污水处理厂投加的混凝剂为聚合氯化铝 (polyaluminium chloride,PAC) ,单位TP去除量的PAC投加量 (PACTP) 为11.1 mg·mg−1。混凝剂使用效率比较结果表明,泸州市中心城区的PFS使用效率较高;由于PFS与PAC的性质差异,混凝剂的处理效率和使用效率存在差异。后续可基于混凝剂的单位除磷效率,进行混凝剂种类、操作条件和投加量等工艺条件优化[26]。污水处理厂的PFSTP与进出水TP关系如表3和图5所示,拟合结果表明其线性相关性较差。这说明污水处理厂的在三级处理化学除磷过程中,混凝剂使用效率PFSTP与进出水TP线性不相关[27]。
表 3 典型城市污水厂处理厂混凝剂药耗水平Table 3. Coagulant consumption level in a typical urban wastewater treatment plants2.3 污水处理厂运行碳源投加量
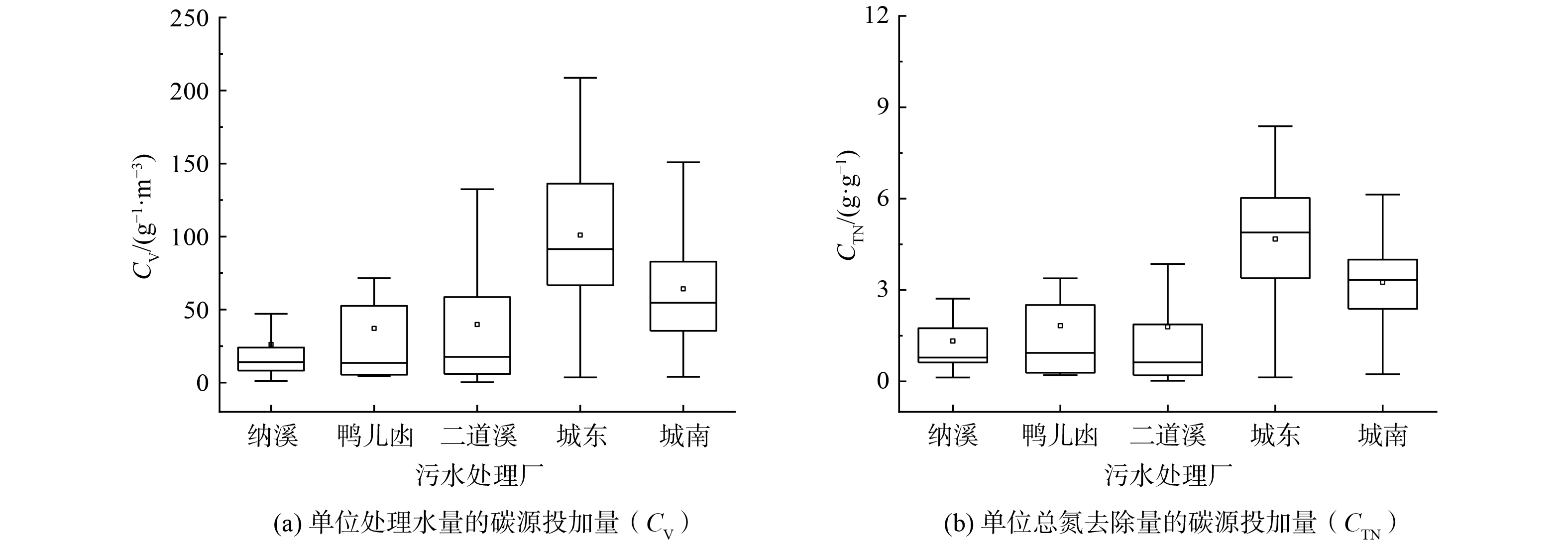
1) 碳源投加量。为实现高效除氮,污水处理厂通常需要补充外部碳源[30-31]。如图6 (a) 所示,泸州市中心区5座城市污水处理厂单位处理水量的碳源投加量 (CV,折合COD计) 为1.45~161.38 g·m−3 (5th~95th) 。污水处理厂之间CV差别较大,其中纳溪污水处理厂投加的碳源较少 (中位值14.04 g·m−3) ,城东污水处理厂投加的碳源较多 (中位值 96.43 g·m−3) 。污水处理厂的CV差异与进水碳源、形态、污水处理工艺等因素相关。在泸州市的中心区污水处理厂中,单位总氮 (TN) 去除量对应碳源投加量 (CTN,以COD计) 为0.13~6.55 g·g−1 (5th~95th) (图6 (b) )。污水处理厂间的CTN差异规律与CV相似。城东污水处理厂的CTN较高 (中位值为4.88 g·g−1) ,二道溪污水处理厂较低 (中位值为0.60 g·g−1) 。
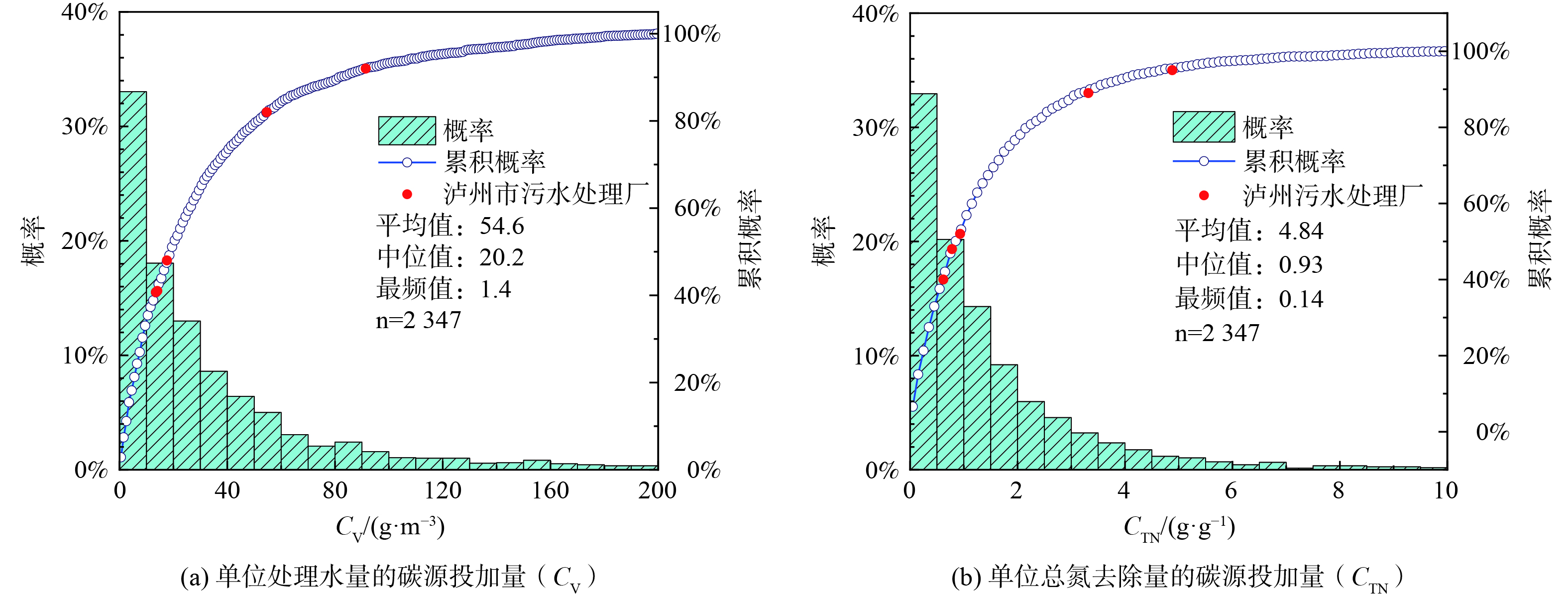
2) 碳源使用效率。2020年全国2 347座城镇污水处理厂的CV和CTN如图7所示,CV为1.4~154.2 g·m−3 (5th~95th) ,中位值为20.2 g·m−3 (图7(a)) ; CTN为0.04~4.67 g·g−1 (5th~95th) ,中间值为4.84 g·g−1 (图7(b)) 。泸州中心区的5座污水处理厂CV和CTN均差于国内50%以上的污水处理厂,这说明泸州市这5座污水处理厂的碳源使用效率仍有较大提升空间。
为探究碳源使用效率CTN与进出水TN的相关性,对CTN与进出水TN进行线性拟合,结果如图8所示,这说明污水处理厂在外加碳源增强反硝化脱氮过程中,碳源使用效率CTN与进出水TN线性不相关。为提升碳源的使用效率或减少碳源的使用量,可考虑从运行优化、工艺升级与协同两个方面入手。以往污水处理厂投加外加碳源是凭借经验控制投加量,这会导致在维持出水TN达标的情况下,部分碳源可能浪费且增加水体BOD5。而精准加碳可提升碳源的使用效率,可通过对硝酸盐和亚硝酸盐质量浓度的在线监测和反馈调节来实现精准加碳。此外,工艺升级与协同可减少碳源的使用量,采用短程硝化工艺使得氮元素以亚硝酸根的形式存在,进而减少还原过程中的碳源投加量。此外,反硝化滤池工艺的增加和协同不仅可减少碳源在A2/O工艺的投加量,还可维持出水TN稳定性。
3. 结论
1) 西南丘陵城市泸州市中心区城市污水处理厂的电力、混凝剂和碳源的用量具有显著季节特征,夏季降雨较多时的用电量和药剂用量较高。2) 在用电量方面,泸州市中心城区污水处理厂的QV为0.25~0.52 kW·h·m−3,用电效率较好,位于全国28%~66%。其中,鸭儿凼污水处理厂的QV较高 (中位值 0.46 kW·h·m−3) ,与其膜生物处理工艺有关。城东和城南污水处理厂的设计处理水量和工艺相同,但QV和QNH3~N差异较大,主要原因为进水中污染物的种类和[NH3-N]差异较大。因此,污水处理厂用电量、用电效率与水量相关,也与进水出水水质、操作条件等相关。3) 在药剂投加量方面,泸州市中心城区污水处理厂以PFS为除磷混凝剂,PFSTP效率较高,为2.67~30.41 mg·mg−1;泸州市中心区城市污水处理厂的碳源使用率较低,位于全国44%~95%,需通过对进水中硝酸盐与亚硝酸盐质量浓度进行监测、升级脱氮工艺和外加碳源精准投加等方式来提升碳源使用效率。
-
表 1 iCOF去除环境污染物及其影响吸附因素
Table 1. Removal of environmental pollutants by iCOF and its influence on adsorption factors
iCOF 材料iCOF 污染物Pollutants 相互作用机理Interaction mechanism 参考文献Reference TpPa-SO3Na MB,EB,MO 静电相互作用和COF材料的孔径大小 [32] ImI@TpBd-(SO3)2 MB、碱性橙2 静电相互作用 [47] Tp-Bpy RhB,CR,BB 与含氮活性位点之间的静电相互作用 [48] PC-COF MO, DFBM, AG-25, IC 与结构中联吡啶基团之间的静电相互作用以及阴离子交换选择性 [20] Tp-MTABs FQs 与含氮基团之间的静电相互作用和π-π 相互作用 [49] Fe3O4 @TpBD BPA π-π 相互作用和氢键 [50] TFPT-TGCl-iCOF 2,4-dichlorophenol 与含氮基团之间的配位相互作用 [52] COF1 GenX, HFPO-TA 静电相互作用和疏水作用 [53] [[NH4]+[COF-SO3]−] U(Ⅵ)、Th(Ⅳ) 与-SO3H基团之间的配位相互作用以及 阴离子交换选择性 [54] JUC-505-COOH U(Ⅵ) 与-COOH基团之间的配位相互作用 [55] PS-COF-1 Tc(Ⅶ) 与联吡啶基团之间的配位相互作用以及阳离子交换选择性 [56] PS-COF-1 Tc(Ⅶ) 与联吡啶基团之间的配位相互作用以及氢键 [36] SCU-CPN-1 Re(Ⅶ) 与联吡啶基团之间的配位相互作用以及氢键 [57] SCU-CPN-2 Tc(Ⅶ) 与联吡啶基团之间的配位相互作用以及氢键 [58] QUST-iPOP-1 Tc(Ⅶ) 与COF框架之间的静电相互作用 [59] BT-DGCl Cr(Ⅵ) 与COF框架之间的静电相互作用和阴离子交换选择性 [60] Tp-DGCl Cr(Ⅵ) 与COF框架之间的静电相互作用和氢键 [61] COF-TP、COF-TE Pb(Ⅱ) 与-NHR 基团之间的配位相互作用和静电相互作用 [62] iCOF-1 Pb(Ⅱ) 与COF框架之间的静电相互作用和阳离子交换选择性 [63] TpODH Hg(Ⅱ) 与-NH 基团和-CO基团之间的配位相互作用以及氢键 [64] -
[1] ONDON B S, SUN B, YAN Z Y, et al. Microwave preparation of modified activated carbons for phenol adsorption in aqueous solution[J]. Advanced Materials Research, 2013, 726/727/728/729/730/731: 1883-1889. [2] HAO W M, BJÖRKMAN E, LILLIESTRÅLE M, et al. Activated carbons prepared from hydrothermally carbonized waste biomass used as adsorbents for CO2[J]. Applied Energy, 2013, 112: 526-532. doi: 10.1016/j.apenergy.2013.02.028 [3] JIANG N, SHANG R, HEIJMAN S G J, et al. High-silica zeolites for adsorption of organic micro-pollutants in water treatment: A review[J]. Water Research, 2018, 144: 145-161. doi: 10.1016/j.watres.2018.07.017 [4] ALVER E, METIN A Ü. Anionic dye removal from aqueous solutions using modified zeolite: Adsorption kinetics and isotherm studies[J]. Chemical Engineering Journal, 2012, 200/201/202: 59-67. [5] YOUSEF R I, EL-ESWED B, AL-MUHTASEB A H. Adsorption characteristics of natural zeolites as solid adsorbents for phenol removal from aqueous solutions: Kinetics, mechanism, and thermodynamics studies[J]. Chemical Engineering Journal, 2011, 171(3): 1143-1149. doi: 10.1016/j.cej.2011.05.012 [6] PAN X Q, GU Z P, CHEN W M, et al. Preparation of biochar and biochar composites and their application in a Fenton-like process for wastewater decontamination: A review[J]. Science of the Total Environment, 2021, 754: 142104. doi: 10.1016/j.scitotenv.2020.142104 [7] DING Z H, HU X, WAN Y S, et al. Removal of lead, copper, cadmium, zinc, and nickel from aqueous solutions by alkali-modified biochar: Batch and column tests[J]. Journal of Industrial and Engineering Chemistry, 2016, 33: 239-245. doi: 10.1016/j.jiec.2015.10.007 [8] LI Q, ZHANG N, YANG Y, et al. High efficiency photocatalysis for pollutant degradation with MoS2/C3N4 heterostructures[J]. Langmuir: the ACS Journal of Surfaces and Colloids, 2014, 30(29): 8965-8972. doi: 10.1021/la502033t [9] WANG W, FANG J J, SHAO S F, et al. Compact and uniform TiO2@g-C3N4 core-shell quantum heterojunction for photocatalytic degradation of tetracycline antibiotics[J]. Applied Catalysis B: Environmental, 2017, 217: 57-64. doi: 10.1016/j.apcatb.2017.05.037 [10] KIM S, LEE J, SON Y, et al. Study of the dye adsorption kinetics ofMetal–organic frameworks in aqueous media[J]. Bulletin of the Korean Chemical Society, 2020, 41(8): 843-850. doi: 10.1002/bkcs.12076 [11] SINGH A, SINGH A K, LIU J Q, et al. Syntheses, design strategies, and photocatalytic charge dynamics of metal–organic frameworks (MOFs): A catalyzed photo-degradation approach towards organic dyes[J]. Catalysis Science & Technology, 2021, 11(12): 3946-3989. [12] SINGH P, SHANDILYA P, RAIZADA P, et al. Review on various strategies for enhancing photocatalytic activity of graphene based nanocomposites for water purification[J]. Arabian Journal of Chemistry, 2020, 13(1): 3498-3520. doi: 10.1016/j.arabjc.2018.12.001 [13] LAI K C, LEE L Y, HIEW B Y Z, et al. Environmental application of three-dimensional graphene materials as adsorbents for dyes and heavy metals: Review on ice-templating method and adsorption mechanisms[J]. Journal of Environmental Sciences, 2019, 79: 174-199. doi: 10.1016/j.jes.2018.11.023 [14] ZUBAIR M, IHSANULLAH I, ABDUL AZIZ H, et al. Sustainable wastewater treatment by biochar/layered double hydroxide composites: Progress, challenges, and outlook[J]. Bioresource Technology, 2021, 319: 124128. doi: 10.1016/j.biortech.2020.124128 [15] KANG D J, YU X L, TONG S R, et al. Performance and mechanism of Mg/Fe layered double hydroxides for fluoride and arsenate removal from aqueous solution[J]. Chemical Engineering Journal, 2013, 228: 731-740. doi: 10.1016/j.cej.2013.05.041 [16] ZHU L L, SHEN D K, LUO K H. A critical review on VOCs adsorption by different porous materials: Species, mechanisms and modification methods[J]. Journal of Hazardous Materials, 2020, 389: 122102. doi: 10.1016/j.jhazmat.2020.122102 [17] HUANG N, ZHAI L P, XU H, et al. Stable covalent organic frameworks for exceptional mercury removal from aqueous solutions[J]. Journal of the American Chemical Society, 2017, 139(6): 2428-2434. doi: 10.1021/jacs.6b12328 [18] CÔTÉ A P, BENIN A I, OCKWIG N W, et al. Porous, crystalline, covalent organic frameworks[J]. Science, 2005, 310(5751): 1166-1170. doi: 10.1126/science.1120411 [19] PENG Y W, HU Z G, GAO Y J, et al. Synthesis of a sulfonated two-dimensional covalent organic framework as an efficient solid acid catalyst for biobased chemical conversion[J]. ChemSusChem, 2015, 8(19): 3208-3212. doi: 10.1002/cssc.201500755 [20] YU S B, LYU H, TIAN J, et al. A polycationic covalent organic framework: A robust adsorbent for anionic dye pollutants[J]. Polymer Chemistry, 2016, 7(20): 3392-3397. doi: 10.1039/C6PY00281A [21] SKORJANC T, SHETTY D, GÁNDARA F, et al. Remarkably efficient removal of toxic bromate from drinking water with a porphyrin-viologen covalent organic framework[J]. Chemical Science, 2019, 11(3): 845-850. [22] ASHRAF S, ZUO Y M, LI S, et al. Crystalline anionic germanate covalent organic framework for high CO2 selectivity and fast Li ion conduction[J]. Chemistry (Weinheim an Der Bergstrasse, Germany), 2019, 25(59): 13479-13483. [23] LI Z, LIU Z W, MU Z J, et al. Cationic covalent organic framework based all-solid-state electrolytes[J]. Materials Chemistry Frontiers, 2020, 4(4): 1164-1173. doi: 10.1039/C9QM00781D [24] XIAO G J, LI W Q, CHEN T, et al. Application of electron-rich covalent organic frameworks COF-JLU25 for photocatalytic aerobic oxidative hydroxylation of arylboronic acids to phenols[J]. European Journal of Organic Chemistry, 2021, 2021(29): 3986-3991. doi: 10.1002/ejoc.202100173 [25] CHEN Y X, ZHANG M, ZHANG S Z, et al. Copper-decorated covalent organic framework as a heterogeneous photocatalyst for phosphorylation of terminal alkynes[J]. Green Chemistry, 2022, 24(10): 4071-4081. doi: 10.1039/D2GC00754A [26] GUAN Q, ZHOU L L, LI W Y, et al. Covalent organic frameworks (COFs) for cancer therapeutics[J]. Chemistry (Weinheim an Der Bergstrasse, Germany), 2020, 26(25): 5583-5591. [27] MI Z, YANG P, WANG R, et al. Stable radical cation-containing covalent organic frameworks exhibiting remarkable structure-enhanced photothermal conversion[J]. Journal of the American Chemical Society, 2019, 141(36): 14433-14442. doi: 10.1021/jacs.9b07695 [28] FU Y, WU Y, CHEN S H, et al. Zwitterionic covalent organic frameworks: Attractive porous host for gas separation and anhydrous proton conduction[J]. ACS Nano, 2021, 15(12): 19743-19755. doi: 10.1021/acsnano.1c07178 [29] PENG Y W, XU G D, HU Z G, et al. Mechanoassisted synthesis of sulfonated covalent organic frameworks with high intrinsic proton conductivity[J]. ACS Applied Materials & Interfaces, 2016, 8(28): 18505-18512. [30] CAMPBELL N L, CLOWES R, RITCHIE L K, et al. Rapid microwave synthesis and purification of porous covalent organic frameworks[J]. Chemistry of Materials, 2009, 21(2): 204-206. doi: 10.1021/cm802981m [31] CHANDRA S, KUNDU T, DEY K, et al. Interplaying intrinsic and extrinsic proton conductivities in covalent organic frameworks[J]. Chemistry of Materials, 2016, 28(5): 1489-1494. doi: 10.1021/acs.chemmater.5b04947 [32] CHEN T F, LI B, HUANG W B, et al. Highly crystalline ionic covalent organic framework membrane for nanofiltration and charge-controlled organic pollutants removal[J]. Separation and Purification Technology, 2021, 256: 117787. doi: 10.1016/j.seppur.2020.117787 [33] YAN Y H, WU S M, IAN Y L, et al. Sulfonic Acid-functionalized Spherical Covalent Organic Framework with Ultrahigh Capacity for the Removal of Cationic Dyes[J]. Chemical Journal of Chinese Universities-Chinese 2021, 42: 956-964. [34] MITRA S, KANDAMBETH S, BISWAL B P, et al. Self-exfoliated guanidinium-based ionic covalent organic nanosheets (iCONs)[J]. Journal of the American Chemical Society, 2016, 138(8): 2823-2828. doi: 10.1021/jacs.5b13533 [35] DA H J, YANG C X, YAN X P. Cationic covalent organic nanosheets for rapid and selective capture of perrhenate: An analogue of radioactive pertechnetate from aqueous solution[J]. Environmental Science & Technology, 2019, 53(9): 5212-5220. [36] HAO M J, CHEN Z S, YANG H, et al. Pyridinium salt-based covalent organic framework with well-defined nanochannels for efficient and selective capture of aqueous 99TcO4−[J]. Science Bulletin, 2022, 67(9): 924-932. doi: 10.1016/j.scib.2022.02.012 [37] BUYUKCAKIR O, JE S H, TALAPANENI S N, et al. Charged covalent triazine frameworks for CO2 capture and conversion[J]. ACS Applied Materials & Interfaces, 2017, 9(8): 7209-7216. [38] MA H P, LIU B L, LI B, et al. Cationic covalent organic frameworks: A simple platform of anionic exchange for porosity tuning and proton conduction[J]. Journal of the American Chemical Society, 2016, 138(18): 5897-5903. doi: 10.1021/jacs.5b13490 [39] DENG X L, ZHAO P Y, ZHOU X M, et al. Excellent sustained-release efficacy of herbicide quinclorac with cationic covalent organic frameworks[J]. Chemical Engineering Journal, 2021, 405: 126979. doi: 10.1016/j.cej.2020.126979 [40] LI Z L, LI H, GUAN X Y, et al. Three-dimensional ionic covalent organic frameworks for rapid, reversible, and selective ion exchange[J]. Journal of the American Chemical Society, 2017, 139(49): 17771-17774. doi: 10.1021/jacs.7b11283 [41] HU H, YAN Q Q, WANG M, et al. Ionic covalent organic frameworks for highly effective catalysis[J]. Chinese Journal of Catalysis, 2018, 39(9): 1437-1444. doi: 10.1016/S1872-2067(18)63065-7 [42] MU Z J, DING X S, CHEN Z Y, et al. Zwitterionic covalent organic frameworks as catalysts for hierarchical reduction of CO2 with amine and hydrosilane[J]. ACS Applied Materials & Interfaces, 2018, 10(48): 41350-41358. [43] DONG B, WANG L Y, ZHAO S, et al. Immobilization of ionic liquids to covalent organic frameworks for catalyzing the formylation of amines with CO2 and phenylsilane[J]. Chemical Communications (Cambridge, England), 2016, 52(44): 7082-7085. doi: 10.1039/C6CC03058K [44] LI P, CHEN J, TANG S K. Ionic liquid-impregnated covalent organic framework/silk nanofibril composite membrane for efficient proton conduction[J]. Chemical Engineering Journal, 2021, 415: 129021. doi: 10.1016/j.cej.2021.129021 [45] AIYAPPA H B, THOTE J, SHINDE D B, et al. Cobalt-modified covalent organic framework as a robust water oxidation electrocatalyst[J]. Chemistry of Materials, 2016, 28(12): 4375-4379. doi: 10.1021/acs.chemmater.6b01370 [46] LI Z, LIU Z W, LI Z Y, et al. Defective 2D covalent organic frameworks for postfunctionalization[J]. Advanced Functional Materials, 2020, 30(10): 1909267. doi: 10.1002/adfm.201909267 [47] DANG M, DENG Q L, TIAN Y Y, et al. Synthesis of anionic ionic liquids@TpBd-(SO3)2 for the selective adsorption of cationic dyes with superior capacity[J]. RSC Advances, 2020, 10(9): 5443-5453. doi: 10.1039/C9RA10035K [48] DEY K, PAL M, ROUT K C, et al. Selective molecular separation by interfacially crystallized covalent organic framework thin films[J]. Journal of the American Chemical Society, 2017, 139(37): 13083-13091. doi: 10.1021/jacs.7b06640 [49] JIANG W, CUI W R, LIANG R P, et al. Zwitterionic surface charge regulation in ionic covalent organic nanosheets: Synergistic adsorption of fluoroquinolone antibiotics[J]. Chemical Engineering Journal, 2021, 417: 128034. doi: 10.1016/j.cej.2020.128034 [50] LI Y, YANG C X, YAN X P. Controllable preparation of core-shell magnetic covalent-organic framework nanospheres for efficient adsorption and removal of bisphenols in aqueous solution[J]. Chemical Communications (Cambridge, England), 2017, 53(16): 2511-2514. doi: 10.1039/C6CC10188G [51] LIU Z S, WANG H W, OU J J, et al. Construction of hierarchically porous monoliths from covalent organic frameworks (COFs) and their application for bisphenol A removal[J]. Journal of Hazardous Materials, 2018, 355: 145-153. doi: 10.1016/j.jhazmat.2018.05.022 [52] DA H J, YANG C X, QIAN H L, et al. A knot-linker planarity control strategy for constructing highly crystalline cationic covalent organic frameworks: Decoding the effect of crystallinity on adsorption performance[J]. Journal of Materials Chemistry A, 2020, 8(25): 12657-12664. doi: 10.1039/D0TA01037E [53] WANG W, ZHOU Z M, SHAO H P, et al. Cationic covalent organic framework for efficient removal of PFOA substitutes from aqueous solution[J]. Chemical Engineering Journal, 2021, 412: 127509. doi: 10.1016/j.cej.2020.127509 [54] XIONG X H, YU Z W, GONG L L, et al. Ammoniating covalent organic framework (COF) for high-performance and selective extraction of toxic and radioactive uranium ions[J]. Advanced Science (Weinheim, Baden-Wurttemberg, Germany), 2019, 6(16): 1900547. [55] LI Z Y, ZHU R M, ZHANG P L, et al. Functionalized polyarylether-based COFs for rapid and selective extraction of uranium from aqueous solution[J]. Chemical Engineering Journal, 2022, 434: 134623. doi: 10.1016/j.cej.2022.134623 [56] HE L W, LIU S T, CHEN L, et al. Mechanism unravelling for ultrafast and selective 99TcO4 - uptake by a radiation-resistant cationic covalent organic framework: A combined radiological experiment and molecular dynamics simulation study[J]. Chemical Science, 2019, 10(15): 4293-4305. doi: 10.1039/C9SC00172G [57] LI J, DAI X, ZHU L, et al. 99TcO4− remediation by a cationic polymeric network[J]. Nature Communications, 2018, 9(1): 1-11. doi: 10.1038/s41467-017-02088-w [58] LI J, CHEN L, SHEN N N, et al. Rational design of a cationic polymer network towards record high uptake of 99TcO4− in nuclear waste[J]. Science China Chemistry, 2021, 64(7): 1251-1260. doi: 10.1007/s11426-020-9962-9 [59] JIAO S S, DENG L M, ZHANG X H, et al. Evaluation of an ionic porous organic polymer for water remediation[J]. ACS Applied Materials & Interfaces, 2021, 13(33): 39404-39413. [60] LI P, DAMRON J T, BRYANTSEV V S, et al. Guanidinium-based ionic covalent-organic nanosheets for sequestration of Cr(Ⅵ) and As(V) oxoanions in water[J]. ACS Applied Nano Materials, 2021, 4(12): 13319-13328. doi: 10.1021/acsanm.1c02845 [61] ZHUANG X Q, HAO J, ZHENG X S, et al. High-performance adsorption of chromate by hydrazone-linked guanidinium-based ionic covalent organic frameworks: Selective ion exchange[J]. Separation and Purification Technology, 2021, 274: 118993. doi: 10.1016/j.seppur.2021.118993 [62] LI G L, YE J R, FANG Q L, et al. Amide-based covalent organic frameworks materials for efficient and recyclable removal of heavy metal lead (II)[J]. Chemical Engineering Journal, 2019, 370: 822-830. doi: 10.1016/j.cej.2019.03.260 [63] GUPTA K M, ZHANG K, JIANG J W. Efficient removal of Pb2+ from aqueous solution by an ionic covalent–organic framework: Molecular simulation study[J]. Industrial & Engineering Chemistry Research, 2018, 57(18): 6477-6482. [64] LI Y, WANG C, MA S J, et al. Fabrication of hydrazone-linked covalent organic frameworks using alkyl amine as building block for high adsorption capacity of metal ions[J]. ACS Applied Materials & Interfaces, 2019, 11(12): 11706-11714. 期刊类型引用(15)
1. 尹旭冉,姜维,柴敏,刘宇. 固定化蟹壳生物炭制备及吸附Pb~(2+)的研究. 工业水处理. 2024(01): 103-111 .  百度学术
百度学术
2. 刘晨阳,单悦,李久义,魏婷,张忠国. 海藻酸钠基吸附材料制备及其脱氮除磷研究进展. 现代化工. 2024(02): 25-31+36 .  百度学术
百度学术
3. 柳富杰,何倩,苏龙,蒋才云. 海藻酸钠表面功能化磁性生物炭对亚甲基蓝的吸附特性. 无机盐工业. 2024(02): 65-73 .  百度学术
百度学术
4. 王其鹏,张达娟,黄莺,王泽斌,张树林,张志华. 固定化小球藻去除氮磷的研究. 水处理技术. 2023(02): 62-65 .  百度学术
百度学术
5. 王继豪,冯秀娟,黄哂莅,邹彦鋆. 固定化硫酸盐还原菌颗粒去除含Cr~(6+)废水的性能研究. 应用化工. 2022(03): 696-700+705 .  百度学术
百度学术
6. 赵希强,张健,孙爽,王文龙,毛岩鹏,孙静,刘景龙,宋占龙. 生物质炭改性微球去除化工废水中无机磷的性能研究. 化工学报. 2022(05): 2158-2173 .  百度学术
百度学术
7. 刘金金,马兴冠. 固定化小球藻处理条件对低碳氮比生活污水处理的影响. 绿色科技. 2022(12): 53-56 .  百度学术
百度学术
8. 冯锦坤,邵维龙,孙玉玲,牟晋华,吕冬伟. 固定化小球藻对鸡场污水处理效果的研究. 黑龙江畜牧兽医. 2022(16): 44-48+54 .  百度学术
百度学术
9. 肖映瑱,徐小琳,汤伟华,侯勇博,郭德亮. 脱色菌藻聚生体系代谢组及固定化分析. 石河子大学学报(自然科学版). 2022(05): 555-563 .  百度学术
百度学术
10. 芦尚德,刘婧婧,冯一平,赵鹏,徐仰仓. 固定化小球藻产氧及光合速率的研究. 生物技术通报. 2021(03): 92-98 .  百度学术
百度学术
11. 刘金金. 微藻的固定化和分离技术及其在污水处理中的应用. 辽宁化工. 2021(06): 836-839 .  百度学术
百度学术
12. 陈艺香. 小球藻吸附制革废水中铬生物材料研究. 皮革制作与环保科技. 2020(03): 86-89 .  百度学术
百度学术
13. 韩嘉碧,吴慧芳,庄子孟,陆俊鑫,高瑞. 固定化微生物技术用于废水处理的研究进展. 江西化工. 2020(04): 50-51 .  百度学术
百度学术
14. 王雁秋,彭万里,梁如冰. 固定化Pseudomonas citronellae SJTE-3菌剂对水中炔雌醇的去除作用. 农业环境科学学报. 2020(08): 1803-1810 .  百度学术
百度学术
15. 吴义诚,欧阳云祥,许荣焕,苏晓东,代智能,傅海燕. 纳米Al_2O_3介导的固定化小球藻燃料电池产电性能分析. 厦门理工学院学报. 2020(05): 16-20 .  百度学术
百度学术
其他类型引用(12)
-






 DownLoad:
DownLoad: