-
城镇化的快速发展加快了工业发展的同时,也带来城市环境问题,如土壤中重金属含量较高[1]. 在哥本哈根的城市土壤研究中发现,与农业土壤相比,城市土壤的Cd、Cu、Pb浓度升高了5—27倍[2];对中国香港的城市公园土壤的大规模调查显示,城市土壤中Cu和Pb的平均浓度(分别为24.8 mg·kg−1和90 mg·kg−1)至少比农村土壤高出2倍和10倍[3-4];对浙江绍兴的城乡梯度蔬菜生产基地土壤质量研究中发现,土壤中的Cu和Pb均有向市区积累的趋势,市区土壤的Cu和Pb浓度约为农村土壤的2倍[5]. 此外,城镇化的快速发展也增加了城市废弃物的产生,如污水厂的污泥、城市园林废弃物、城郊农作物秸秆、生活垃圾等. 目前这些城市废弃物常用的处理方法主要是填埋和焚烧[6]:本课题组之前的研究发现生活垃圾制备成生物炭后,降低了城市土壤的重金属有效态含量,有望用来降低城市土壤重金属的环境风险[7]. 生物炭作为一种稳定的碳储存方式,在土壤、水体和污染修复等方面得到了广泛的关注[8-9]. 生物炭是由生物质在低氧或缺氧的环境下热解产生的,具有较大的比表面积、较高的阳离子交换容量以及丰富的官能团[10]. 而生物炭源溶解性有机质(dissolved organic matter,DOM)是一类组成复杂、物化性质活跃的聚合体,可能含有低分子量物质,如游离氨基酸、糖等;同时含有各种类型的大分子组分,如酶、氨基糖、多酚、腐殖酸和其他混合物,并且具有一定的亲水性和芳香性[11-12],是具有高活性官能团的有机化合物的非均相混合物[13-14],可以直接参与重金属的络合,这与DOM自身的高芳香族化合物和丰富的羟基、苯酚等有机官能团有关[15].
DOM的组成和结构是影响DOM与重金属络合的主要因素,如DOM分子大小对金属的结合性能影响不同,沉积物DOM中的高分子量(>1 kDa)的类腐殖酸表现出比低分子量(<1 kDa)更高的金属络合能力(条件稳定常数5.39>4.87)[16];而雨水径流DOM中分子量小的组分(<1 kDa)与金属离子的络合作用则较强[17]. 此外,DOM的疏水性和亲水性,也可能会影响DOM与重金属的结合能力. 分馏对于表征DOM的疏水性和亲水性至关重要. 分馏有多种方法,其中树脂法较常见. 树脂是一种中性吸附剂,能够有效吸附疏水化合物从而实现对DOM的分馏[18-19]. 具有反相保留和离子交换吸附能力的固相萃取柱,因含有聚合物而对疏水性和部分透明性化合物均有较好的吸附作用,能够吸附树脂无法吸附的弱酸性和弱碱性化合物,具有更高的分馏效应,目前已成功用于含油废水DOM的分离[20],然而,固相萃取柱应用于生物炭源DOM的分馏尚鲜见报道. 因此本研究拟对生活垃圾生物炭DOM进行固相萃取分馏成4个组分,探究不同萃取组分对重金属Cu和Pb的络合作用.
三维荧光光谱联合平行因子分析(three-dimensional excitation emission matrix with parallel factor analysis,3DEEM-PARAFAC)和同步荧光光谱(synchronous fluorescence,SF)是阐明金属离子与荧光团相互作用机制的高效、快速且灵敏的方法[21],此外,二维相关光谱(two-dimensional correlation spectroscopy,2D-COS)分析进一步提供了分子结构随金属添加的变化信息和顺序,因此,利用这些光谱技术进行多光谱研究,可以更深入细致地研究DOM中不同分馏组分与重金属的结合机理.
本研究的主要目的是:(1)使用具有反相保留和离子交换吸附能力的固相萃取柱,对生活垃圾生物炭DOM进行分馏,并利用三维荧光光谱对馏分进行荧光表征;(2)利用三维荧光光谱和同步荧光光谱,借助荧光淬灭实验研究DOM的4个组分与Cu和Pb的结合特性;(3)结合2D-COS分析和多参数模拟,研究疏水中性组分和Cu的络合能力、顺序和参数.
-
生物炭的制备:将厨余米饭、菜叶、骨头和果皮以4:4:1:1的比例混合均匀后烘干,升温速率为10 ℃·min−1,终温为800 ℃制备成生活垃圾生物炭. 生物炭的基本性质:DOC浓度为2320 mg·kg−1,pH值为10.6,水溶性盐分为6.15 mS·cm−1,Cu和Pb浓度分别为33.9 mg·kg−1和6.96 mg·kg−1.
两种固相萃取柱Waters Oasis MAX(mixed anion exchange reversed-phase adsorbent,混合型阴离子交换反相吸附剂,200 mg)和Waters Oasis MCX(mixed cation exchange reversed-phase adsorbent,混合型阳离子交换反相吸附剂,200 mg)购自美国Waters公司. 乙腈(LC-MS级)购自美国Fisher Scientific 公司. 盐酸、甲酸、氢氧化铵、氢氧化钠和盐酸等分析级试剂购自国药集团.
-
将生物炭研磨后过100目筛网,按照1:10的固液比加入超纯水,以250 r·min−1在25 ℃振荡24 h,在3000 r·min−1离心20 min,上清液过0.45 μm玻璃纤维滤膜,得到的滤液即为DOM.
本研究中,使用两种固相萃取柱Waters Oasis MCX和Waters Oasis MAX,将DOM分馏成4个组分:疏水酸性组分(hydrophobic-acidic component,HOA)、疏水中性组分(hydrophobic-neutral component,HON)、疏水碱性组分(hydrophobic-base component,HOB)和亲水性组分(hydrophilic substance,HIS). MAX柱含有带季胺基团的聚二乙烯基苯-N-乙烯基吡咯烷酮,对酸性化合物具有较高的选择性和灵敏度,是一种在pH (0—14)范围内稳定的混合型强阴离子交换、水可浸润性吸附剂;MCX柱含有磺化的聚二乙烯基苯-N-乙烯基吡咯烷酮,对碱性化合物具有较高的选择性和灵敏度.
4个组分的分馏步骤[20]如下:(1)用10 mL乙腈和10 mL超纯水先后冲洗活化MAX和MCX柱;(2)向10 mL DOM溶液中加入适量的1 mol·L−1盐酸,酸化至pH = 2;(3)用真空泵将酸化的DOM溶液过MAX柱,接着用10 mL 2%甲酸洗涤MAX柱,在室温下将MAX柱氮吹至干,然后用10 mL乙腈洗脱MAX柱,萃取物为HON-1组分;(4)用10 mL含5%氢氧化铵的乙腈进一步洗脱MAX柱,洗脱液为HOA组分;(5)取第(3)步的萃余液,用1 mol·L−1氢氧化钠调pH至12;(6)用真空泵将碱化的DOM溶液过MCX柱,洗脱液为HIS;(7)用10 mL的5%氢氧化铵洗涤MCX柱,在室温下用氮气吹干MCX柱,接着用10 mL乙腈洗脱,萃取物为HON-2组分;(8)用10 mL含2%甲酸的乙腈进一步洗脱MCX柱,萃取液为HOB;(9)最后,将HON-1和HON-2合并为HON,(10)将4个组分在50 ℃下氮吹干燥后用超纯水定容至10 mL.
-
实验1:将定容后的4个组分,稀释至10 mg·L−1(以C计,避免内滤效应);接着分别取10 mL加入离心管中,分别滴加Cu和Pb溶液,使离心管中Cu和Pb浓度在100 μmol·L−1;将离心管在恒温振荡器(25 ℃)中以250 r·min−1转速避光振荡24 h,反应平衡后进行同步荧光光谱和3D-EEM光谱测定.
实验2:根据实验1的结果,选择淬灭效果最显著的HON组分,和Cu继续进行荧光猝灭实验. 向HON组分溶液(C: 10 mg·L−1)中,滴加Cu母液,使Cu的浓度分别为0、20、50、100、150、200 μmol·L−1,后续步骤同实验1.
-
溶解性有机碳(dissolved organic carbon,DOC)浓度用总有机碳分析仪(TOC V-CPH,Elementar)测定. 同步荧光光谱由荧光分光光度计(Lumina,Thermo)测定,激发波长200—450 nm,发射波长和激发波长差值固定为60 nm,扫描速度300 nm·min−1. EEM光谱使用荧光分光光度计(F-4600,日立)测定:激发波长范围220—450 nm,间隔5 nm;发射波长范围为260—600 nm,间隔1 nm;狭缝宽度均为5 nm,扫描速度12000 nm·min−1.
-
EEM数据利用Matlab 2016b中的Dreem工具箱进行拉曼散射和瑞丽散射的修正. 采用荧光区域积分法对光谱进行定量分析[22-23],将光谱划分为5个区域:区域 Ⅰ 类酪氨酸(Ex/Em = 220—250/260—330 nm)、区域 Ⅱ 类色氨酸(Ex/Em = 220—250/330—380 nm)、区域 Ⅲ 类富里酸物质(Ex/Em = 220—250/>380 nm)、区域 Ⅳ 微生物代谢产物(Ex/Em = 250—280/<380 nm)和区域 Ⅴ 类腐殖酸物质(Ex/Em = >280/>380 nm). 利用Matlab 2016b计算每个荧光区域的积分体积
Fi,n ,代表各区域的有机物质的相对含量;各区域的积分体积占总积分体积的比例为Pi,n . 采用Excel 2017和Origin 2021分析处理数据,采用Matlab 2016b绘制三维荧光图. -
利用Ryan和Weber提出的络合模型对猝灭过程进行拟合[24]. 该模型是基于1:1静态猝灭模型,假设引起荧光猝灭反应的结合点位与重金属离子是按照1:1的形式结合成有机金属复合物,如式(1)所示:
其中,
F0 和F分别代表初始的荧光峰强度和不同重金属浓度Cs 下的荧光峰强度,Fend 为络合能力达到饱和时的荧光强度,CL 为DOM荧光组分中有效配位体的浓度(mol·L−1),即最大结合容量,K是条件稳定常数. 为避免常规的3参数非线性拟合会忽略CL 值[25],将将公式(1)转化为双参数方程,转化后的方程见式(2):式中,
|Fend−F0| 和α为拟合参数,运用Origin 2021软件对式(1)和(2)进行非线性拟合. 此外,猝灭荧光占初始荧光的比例f按式(3)计算: -
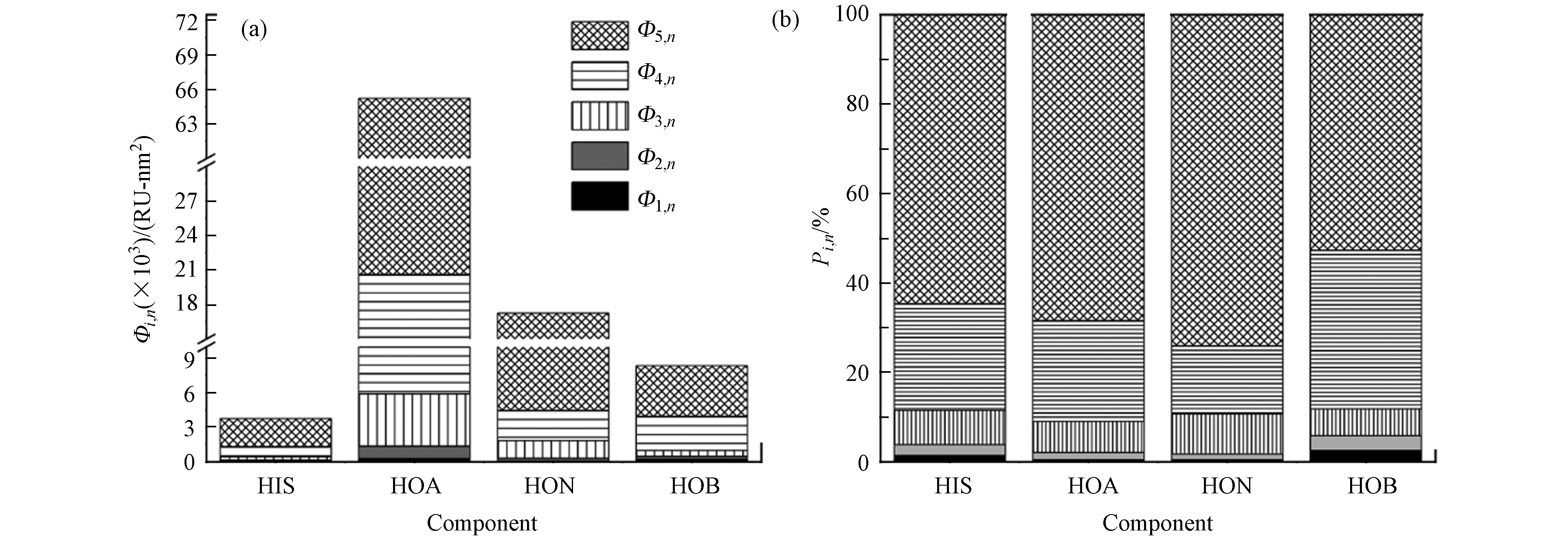
DOC的浓度代表了DOM中可溶性有机碳的含量. 4个组分(HOA、HON、HOB和HIS)在50 ℃下氮吹干燥后用超纯水定容至10 mL后,溶液中DOC的浓度分别为8.44、96.39、17.46、27.45 mg·L−1,分馏前DOC浓度为232.31 mg·L−1,回收率约64.46%. 因此,城市生活垃圾生物炭中DOM由81.67%(以DOC值计算)的疏水组分和18.33%的亲水组分组成;其中疏水组分中,HOA、HON和HOB分别占5.64%、64.37%和11.66%;这表明DOM中疏水中性物质占一半以上. Fang等[20]用同样的方法对炼油厂废水的DOM进行分馏,HOA、HON和HOB分别为22.0%、36.7%和13.9%,疏水组分占72.6%,与本文的研究结果基本一致. 而Li等[26]利用XAD-8树脂对炼油厂废水DOM进行分馏得到同样4个组分,其中疏水性组分占比43%(HOA、HON和HOB分别占34%、3%和6%). XAD-8树脂是一种中性吸附剂,能够有效吸附疏水化合物;而本实验使用的两种固相萃取柱含有聚二乙烯基苯-N-乙烯基吡咯烷酮聚合物,对疏水性和部分透明性化合物均有吸附作用,MCX柱中的磺酸基和MAX柱中的季胺基能够吸附XAD-8树脂无法吸附的弱酸性和弱碱性化合物;因此,与XAD-8分馏工艺相比,采用两种固相萃取柱对DOM的萃取效率更高.
-
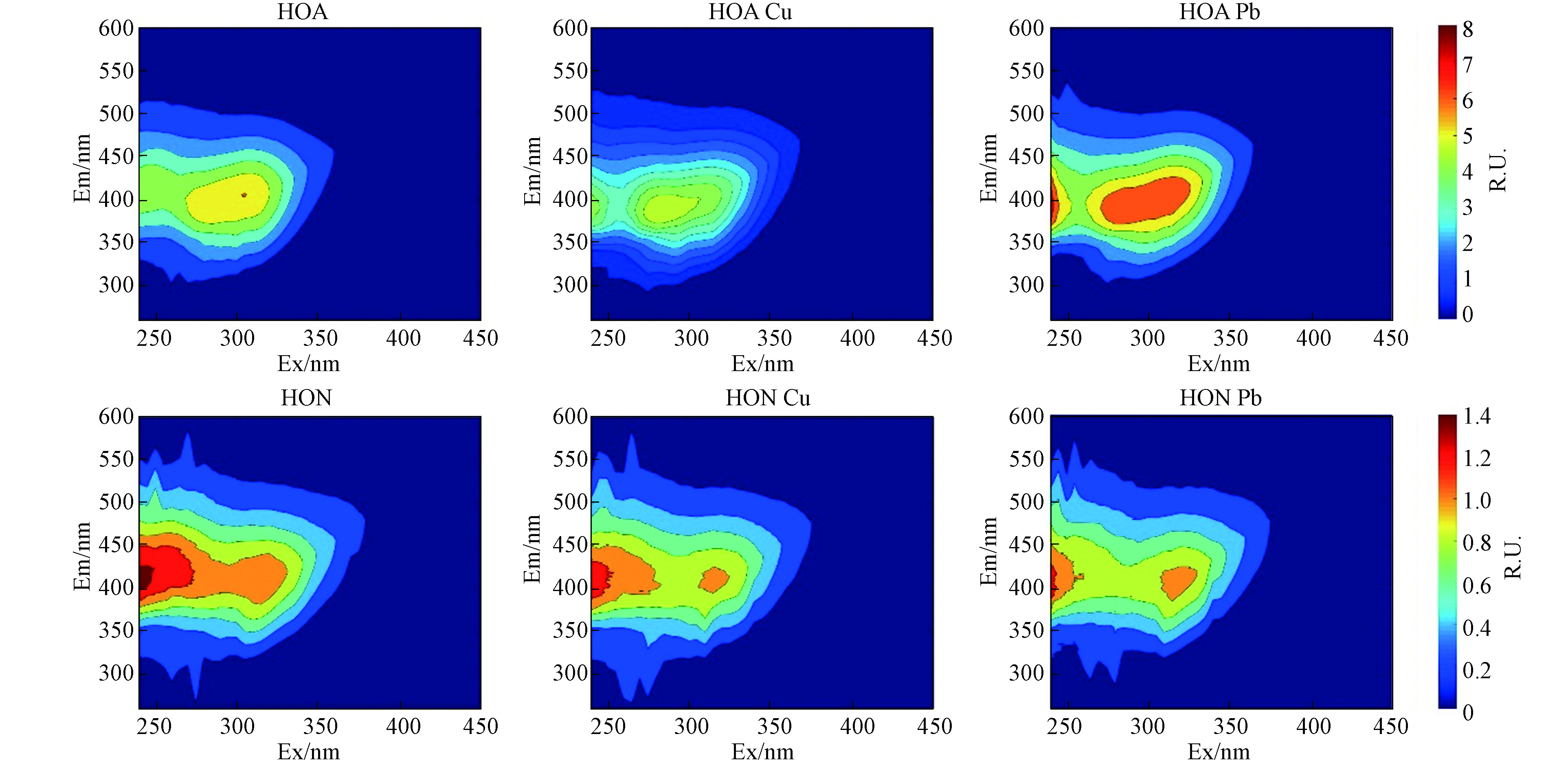
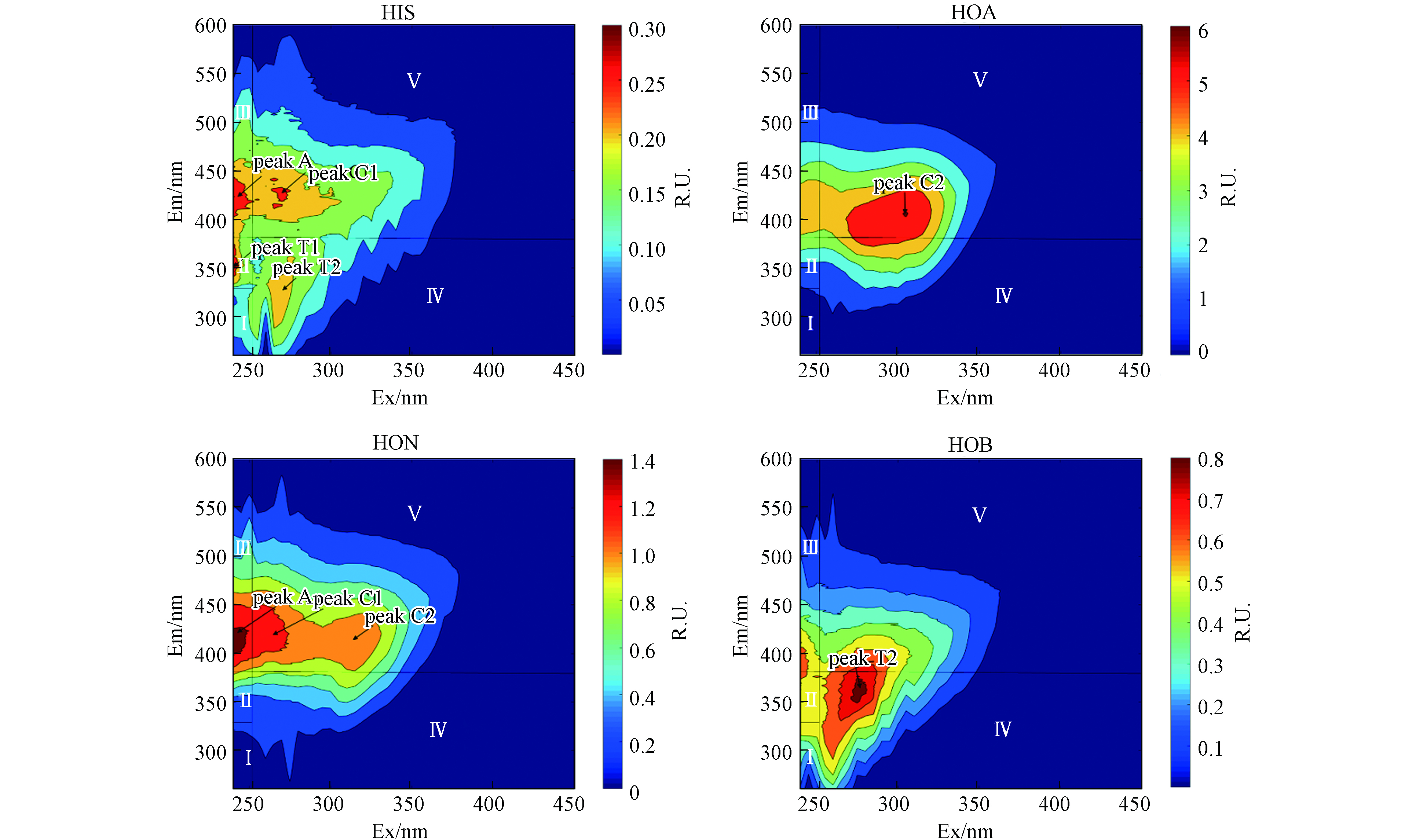
荧光EEM数据广泛用于类蛋白质、类富里酸和腐殖酸物质的测定并评估其对环境的影响[27]. 为了避免内滤效应,将HON、HOB和HIS 按DOC稀释到10 mg·L−1;HOA不稀释,DOC浓度为8.44 mg·L−1;用荧光分光光度计测定EEM光谱. HIS组分的EEM图像中主要观察到4个峰,分布在区域Ⅱ的T1(Ex/Em=240/354 nm)为类色氨酸物质,区域Ⅲ的A(Ex/Em=240/425 nm)为类富里酸物质,区域Ⅳ的T2(Ex/Em=270/341 nm)为微生物代谢产物和区域Ⅴ的C1(Ex/Em=270/425 nm)为类腐殖酸物质. HOA组分中值观察到C2(Ex/Em=305/405 nm),为类腐殖酸物质;HON组分观察到3个峰,A(Ex/Em=240/406 nm)为类富里酸物质,C1(Ex/Em=275/422 nm)和peak C2(Ex/Em=315/412 nm)为类腐殖酸物质;HOB组分只在Ⅳ内观察到T2(Ex/Em=275/358 nm),为微生物代谢产物(图1).
分别对4个组分(HIS、HOA、HON、HOB)进行三维荧光区域积分,每个组分的分区图见图1,丰度分布图见图2a. 为便于比较,4个组分的含碳量均为10 mg·L−1 C. HIS、HOA、HON、HOB 的总积分体积分别为3743、65209、17240、8352 RU-nm2;由此可见4个组分的总荧光强度由高到低依次为:HOA>HON>HOB>HIS(图2a). 4个组分中,Ⅰ、Ⅱ、Ⅲ、Ⅳ和Ⅴ 分别占比0.42%—2.53%、1.34%—3.31%、5.98%—8.94%、15.27%—35.54%、52.65%—74.03%(图2). 由此可见,4个组分中,区域Ⅴ类腐殖酸物质占比最大,其次是区域Ⅳ微生物代谢产物,再其次为区域Ⅲ类富里酸物质,而类芳香蛋白物质(Ⅰ和Ⅱ区)占比最低.
-
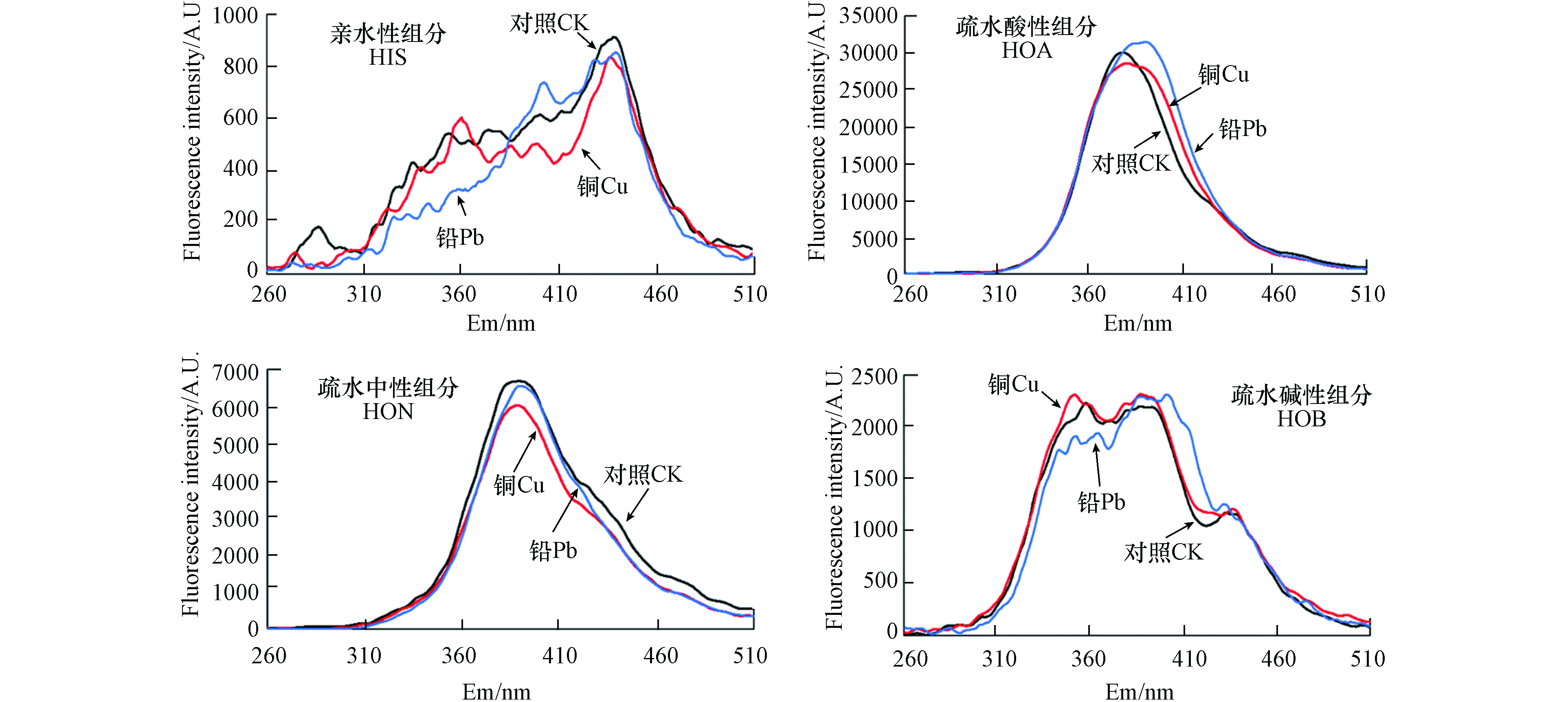
同步荧光光谱能够表明淬灭剂对DOM分子构象的影响,提供荧光基团微环境的相关信息[28]. 加入100 μmol·L−1 Cu后,HON组分在波长260—510 nm范围内,荧光均有不同程度的猝灭;在389 nm处,荧光强度降低幅度最大,为9.82%. 而加入100 μmol·L−1 Pb后,发射波长260—510 nm范围内,荧光亦有不同程度的猝灭;在389 nm处,荧光强度降低幅度最大(1.96%)(图3). 由此可见,Cu对HON组分的络合作用强于Pb,而这与Cu的半径和顺磁性有关[29],金属离子对相同有机配体的特殊亲和力可能是由于电子壳在不同金属离子中的分布不同以及接受电子对的能力不同而产生的[30]. 前人研究表明,同步荧光光谱按照发射波长分为3个区域,分别为类蛋白质物质区域(250—300 nm)、类富里酸物质区域(300—380 nm)和类腐殖酸物质区域(380—600 nm)[31-32];加入Cu和Pb后,HON组分在389 nm处荧光强度降低幅度最大,这说明Cu和Pb更易与HON组分中的类腐殖酸物质络合.
HOA组分主峰在发射波长Em=378 nm处荧光值最大,加入100 μmol·L−1 Cu后,荧光强度降低4.98%;而加入Cu后荧光强度主峰发生红移,至发射波长Em=380 nm处荧光值最大,荧光强度降低4.56%(图3). 而加入100 μmol·L−1 Pb后,在Em=378 nm处荧光强度增加4.80%;主峰红移至发射波长Em=390 nm处,荧光强度增加12.92%;这可能是因为Pb自身存在荧光贡献. HIS与HOB组分中同步荧光光谱相对紊乱,Cu和Pb荧光猝灭规律难以阐述(图3).
由表1和图4可知,在HON组分中,Cu对A、C1、C2 的3个峰的荧光猝灭率分别为9.82%、14.48%和18.02%;Pb对A、C1、C2 的3个峰的荧光猝灭率分别为6.79%、19.27%和6.94%(图4);因此,Pb对HON组分的猝灭趋势与Cu一致,除C1外,猝灭程度略低于Cu.
加入Cu和Pb后,HON组分中的 C1峰的Ex值从275 nm移到295 nm,发生了红移,这可能是因为HON组分中可络合的芳香环增加[33]. HOA组分中Cu对C2的猝灭率为17.55%(图4),荧光猝灭率略低于HON组分;Pb对C2略有增强,从6.04 R.U.增加到6.97 R.U.
-
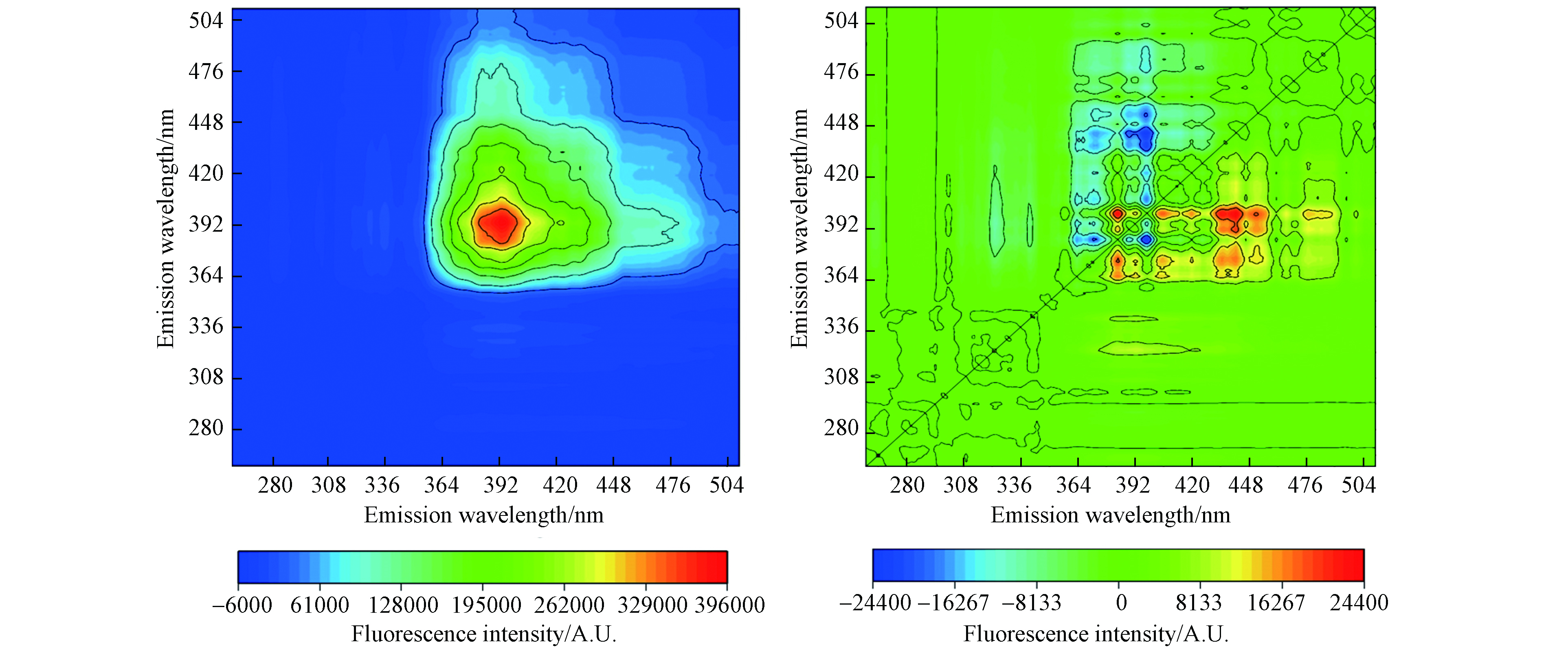
对同步荧光图谱进行2D-COS分析以揭示HON的荧光组分与 Cu 的络合顺序(图5). 沿着同步图的对角线仅在发射波长397 nm处观察到一个正自相关峰,说明该峰相对应的类腐殖酸物质荧光变化强度最为显著,对角线下方397/(323—340)nm处的正交叉峰表明类富里酸物质和类腐殖酸物质的光谱变化方向一致,随Cu浓度升高而降低(图5a)[34].
在异步图(图5b)的对角线下方观察到2个正交叉峰(397/323—340 nm和405—450/383 nm)和1个负交叉峰(397/383 nm). 根据Noda规则[35],如果同步图中λ1/λ2处的交叉峰为正,异步图中也为正,表明λ1处的强度变化优于λ2处发生;若异步图中为负,则表明λ2处的强度变化优于λ1处发生. 基于Noda规则,对2D-COS图的分析可知Cu结合顺序为:405—450 nm > 383 nm > 397 nm > 323—340 nm. 总体而言,类腐殖酸物质在Cu与HON组分的络合反应中占主导地位,这是因为类腐殖酸物质具有较高的芳香性,从而比类富里酸物质表现出更高的金属亲和力[36-37].
-
3个峰(A、C1和C2)的荧光猝灭程度和Cu的剂量-效应关系,用式(2)的双参数方程进行拟合(图6). HON组分中3个峰的荧光强度为:A> C2> C1; 3个峰(A、C1和C2)的荧光强度都随着Cu浓度的增加而显著降低,猝灭效果明显. 条件稳定常数(lgK)用于表征DOM对重金属络合的稳定性.
使用式(2)拟合出HON中3个峰与Cu的lgK(表2),3个荧光峰的拟合度较高(R2>0.95, P<0.001). lgK值大小顺序为C2> A > C1,说明C2与Cu的络合作用最稳定,这可能归因于C2所含的芳香羟基以及酚羟基可以与Cu相互作用形成稳定的环状结构[38]. C2的
f 值大于A和C1,这说明C2处的类腐殖酸物质具有更高的Cu结合能力[39]. 类腐殖酸物质的荧光峰可以被Cu猝灭有不少报道,Cu和沉积物DOM中类腐殖质络合的lgK值为4.87—5.39[16];Cu与污水污泥DOM 中类腐殖酸物质lgK值在3.82—6.86之间[40],与我们的研究结果一致. -
采用两种固相萃取柱进行萃取分馏和荧光猝灭滴定研究了城市垃圾生物炭衍生的DOM中不同组分与Cu的络合作用. 利用三维荧光光谱和同步荧光光谱结合二维相关分析,探究了DOM的4个组分与重金属的络合能力和顺序,主要结论如下:
(1)固相萃取实验把DOM分馏后得到4个组分:HOA、HON、HOB和HIS,根据DOC分析DOM主要由疏水性物质构成(81.67%),其中疏水中性物质占主要部分(64.37%).
(2)在4组分的三维荧光光谱中主要发现5个荧光峰:A(Ex/Em=240/425 nm,为类富里酸物质)、C1和C2(Ex/Em=270/425 nm和Ex/Em=315/412 nm,为类腐殖酸物质)、T1(Ex/Em=240/354 nm,为类色氨酸物质)和T2(Ex/Em=275/358 nm,为微生物代谢产物). 在HON组分中明显观察到3个荧光峰A、C1和C2.
(3)加入100 μmol·L−1 Cu后,在Em=260—510 nm范围内,HON组分的荧光均有不同程度的猝灭;在389 nm处,荧光强度降低幅度最大,达到9.82%;而加入100 μmol·L−1 Pb后,在Em=389 nm处,荧光强度降低幅度虽然最大,但仅为1.96%;这说明HON组分更易与Cu络合.
(4)2D-COS分析表明,Cu对HON组分的络合顺序为:405—450 nm > 383 nm > 397 nm > 323—340 nm,因此与类富里酸物质相比,Cu优先与类腐殖酸物质络合.
(5)类富里酸物质(A)和类腐殖酸物质(C2)的lgK分别为3.97和4.10,这说明类腐殖酸物质具有更高的Cu结合能力.
生活垃圾生物炭源溶解性有机质分馏和络合重金属的作用
Fractionation of dissolved organic matter from domestic waste biochar and its complexation with heavy metals
-
摘要: 为揭示生活垃圾衍生的溶解性有机质的成分及其与重金属的相互作用机制,利用固相萃取与分馏技术,将生活垃圾衍生的溶解性有机质进行分馏,并采用荧光猝灭滴定与光谱分析探究各馏分与Cu的络合作用. 结果表明,分馏共得到4种组分,分别为疏水酸性组分、疏水中性组分、疏水碱性组分和亲水性组分,按C含量分别占5.64%、64.37%和11.66%和18.33%. 在这4个组分的三维荧光光谱中观察到5个荧光峰:A(Ex/Em = 240/425 nm,类富里酸物质)、C1和C2(Ex/Em = 270/425 nm和Ex/Em = 315/412 nm,类腐殖酸物质)、T1(Ex/Em = 240/354 nm,类色氨酸物质)和T2(Ex/Em = 275/358 nm,微生物代谢产物);其中在疏水中性组分中观察到3个荧光峰A、C1和C2. Cu对疏水中性组分的络合顺序为:405—450 nm >383 nm >397 nm >323—340 nm;Ryan-Weber模型拟合表明络合稳定常数(lgK):C2(4.10)>A(3.97)>C1(3.91);这表明与Cu络合时类腐殖酸物质优先于类富里酸物质. 总体来说,生活垃圾衍生的溶解性有机质的主要成分为疏水中性组分,与Cu络合可以改变疏水中性组分的分子微环境,从而改变Cu的形态和归趋,影响其生物有效性和生态毒性.Abstract: In order to reveal the composition and interaction mechanism with heavy metals, the dissolved organic matter derived from domestic waste biochar was processed by solid-phase extraction and fractionation techniques, and the complexation of each fraction with Cu was explored by fluorescence quenching titration and spectral analyses. The results showed that four components were obtained by fractionation, and they were hydrophobic- acidic component, hydrophobic-neutral component, hydrophobic-base component, and hydrophilic substance, accounting for 5.64%, 64.37%, 11.66% and 18.33% respectively according to the content of C. Five fluorescence peaks were observed in the three-dimensional fluorescence spectra of these four components: A (Ex/Em = 240 /425 nm, fulvic acid like substance), C1 and C2 (Ex/Em = 270/425 nm and Ex/Em = 315 /412 nm, humic acid like substance), T1 (Ex/Em = 240/354 nm, tryptophan like substance) and T2 (Ex/Em = 275/358 nm, microbial metabolite). In addition, three fluorescence peaks (A, C1 and C2) were observed in the hydrophobic-neutral component. The complexation order of Cu with hydrophobic-neutral components was 405—450 nm > 383 nm > 397 nm > 323—340 nm, and the Ryan Weber model fitting showed that the complexation stability constant (lgK) was C2 (4.10) > A (3.97) > C1 (3.91). Furthermore, it shows that humic-acid like substances are superior to fulvic-acid like ones when being complexed with Cu. In general, the main components of dissolved organic matter derived from domestic waste are hydrophobic-neutral components and its complexation with Cu can change the molecular microenvironment of the hydrophobic-neutral component, and mediate the morphology and fate of Cu in term of bioavailability and ecotoxicity.
-
表 1 100 μmol·L−1 Cu和Pb的添加后HOA和HON组分的荧光峰特征
Table 1. Fluorescent peak characteristics of HOA and HON with the addition of Cu and Pb of 100 μmol·L−1
组分Component A峰 Peak A C1峰 Peak C1 C2峰 Peak C2 (Ex/Em)/nm 荧光强度/R.U.Fluorescence intensity (Ex/Em)/nm 荧光强度/R.U.Fluorescence intensity (Ex/Em)/nm 荧光强度/R.U.Fluorescence intensity HOA — — — — 305/405 6.040 HOA+Cu 240/392 4.500 260/389 3.355 280/383 4.980 HOA+Pb 240/394 7.796 260/396 4.922 305/402 6.970 HON 240/414 1.517 275/422 1.126 315/412 1.182 HON+Cu 240/405 1.368 295/408 0.963 315/401 0.969 HON+Pb 240/415 1.414 255/413 1.024 320/408 1.100 表 2 HON组分中荧光峰强度与Cu络合作用的拟合参数
Table 2. Fitting parameters for fluorescence peak intensity and Cu complexation in the HON fraction
峰 Peak Fend-F0/(R.U.) F0/(R.U.) f Fend/(R.U.) R2a lgK CL (×10−4)/(mol·L−1) R2b P c A 0.83 1.50 55.55 0.67 0.96 3.97 1.70 0.97 <0.001 C1 0.56 1.06 52.73 0.50 0.98 3.91 2.74 0.98 <0.001 C2 1.07 1.20 89.05 0.13 0.96 4.10 6.59 0.93 <0.001 注:a式(2)拟合的回归系数;b式(1)拟合的回归系数;c式(1)和式(2)的显著性检验值,由于均为P <0.001,故未分开. Notes: a regression coefficient fitted by equation (2); b regression coefficient fitted by equation (1); c significance test values for equation (1) and equation (2), which are not separated due to both two values with P < 0.001. -
[1] ZHOU N, CHEN H G, XI J T, et al. Biochars with excellent Pb(II) adsorption property produced from fresh and dehydrated banana peels via hydrothermal carbonization [J]. Bioresource Technology, 2017, 232: 204-210. doi: 10.1016/j.biortech.2017.01.074 [2] LI L J, HOLM P E, MARCUSSEN H, et al. Release of cadmium, copper and lead from urban soils of Copenhagen [J]. Environmental Pollution, 2014, 187: 90-97. doi: 10.1016/j.envpol.2013.12.016 [3] WONG C S C, LI X D, THORNTON I. Urban environmental geochemistry of trace metals [J]. Environmental Pollution, 2006, 142(1): 1-16. doi: 10.1016/j.envpol.2005.09.004 [4] LI X D, POON C S, LIU P S. Heavy metal contamination of urban soils and street dusts in HongKong [J]. Applied Geochemistry, 2001, 16(11/12): 1361-1368. [5] ZHANG M K, WANG M Q, LIU X M, et al. Characterization of soil quality under vegetable production along an urban-rural gradient [J]. Pedosphere, 2003, 13(2): 173-180. [6] 于洋, 崔胜辉, 林剑艺, 等. 城市废弃物处理温室气体排放研究: 以厦门市为例 [J]. 环境科学, 2012, 33(9): 3288-3294. YU Y, CUI S H, LIN J Y, et al. Study on greenhouse gas emissions from urban waste disposal system: A case study in Xiamen [J]. Environmental Science, 2012, 33(9): 3288-3294(in Chinese).
[7] 吴文雨, 唐剑锋, 郑思俊, 等. 城市源生物炭对城市土壤溶解性有机质及重金属有效态的影响[J]. 应用技术学报(已接收). WU W Y, TANG J F, ZHENG S J, et al. The effect of urban biochar on urban soil DOM characteristics and heavy metal availability [J]. Journal of Technology(Received) (in Chinese).
[8] AHMAD M, RAJAPAKSHA A U, LIM J E, et al. Biochar as a sorbent for contaminant management in soil and water: A review [J]. Chemosphere, 2014, 99: 19-33. doi: 10.1016/j.chemosphere.2013.10.071 [9] ZHOU X, CHEN Z H, LI Z R, et al. Impacts of aeration and biochar addition on extracellular polymeric substances and microbial communities in constructed wetlands for low C/N wastewater treatment: Implications for clogging [J]. Chemical Engineering Journal, 2020, 396: 125349. doi: 10.1016/j.cej.2020.125349 [10] JIA L X, WU W Z, ZHANG J, et al. Insight into heavy metals (Cr and Pb) complexation by dissolved organic matters from biochar: Impact of zero-valent iron [J]. Science of the Total Environment, 2021, 793: 148469. doi: 10.1016/j.scitotenv.2021.148469 [11] SUN J L, DROSOS M, MAZZEI P, et al. The molecular properties of biochar carbon released in dilute acidic solution and its effects on maize seed germination [J]. Science of the Total Environment, 2017, 576: 858-867. doi: 10.1016/j.scitotenv.2016.10.095 [12] DONG X L, MA L Q, GRESS J, et al. Enhanced Cr(Ⅵ) reduction and As(Ⅲ) oxidation in ice phase: Important role of dissolved organic matter from biochar [J]. Journal of Hazardous Materials, 2014, 267: 62-70. doi: 10.1016/j.jhazmat.2013.12.027 [13] LI M, ZHANG A F, WU H M, et al. Predicting potential release of dissolved organic matter from biochars derived from agricultural residues using fluorescence and ultraviolet absorbance [J]. Journal of Hazardous Materials, 2017, 334: 86-92. doi: 10.1016/j.jhazmat.2017.03.064 [14] LI G, KHAN S, IBRAHIM M, et al. Biochars induced modification of dissolved organic matter (DOM) in soil and its impact on mobility and bioaccumulation of arsenic and cadmium [J]. Journal of Hazardous Materials, 2018, 348: 100-108. doi: 10.1016/j.jhazmat.2018.01.031 [15] SOJA G, WIMMER B, ROSNER F, et al. Compost and biochar interactions with copper immobilisation in copper-enriched vineyard soils [J]. Applied Geochemistry, 2018, 88: 40-48. doi: 10.1016/j.apgeochem.2017.06.004 [16] XU H C, ZOU L, GUAN D X, et al. Molecular weight-dependent spectral and metal binding properties of sediment dissolved organic matter from different origins [J]. Science of the Total Environment, 2019, 665: 828-835. doi: 10.1016/j.scitotenv.2019.02.186 [17] 于振亚, 杜晓丽, 高参, 等. 道路雨水径流溶解性有机物与重金属结合作用分析 [J]. 环境科学学报, 2018, 38(8): 3004-3011. doi: 10.13671/j.hjkxxb.2018.0225 YU Z Y, DU X L, GAO C, et al. Complexation between heavy metals and dissolved organic matters in road stormwater runoffs [J]. Acta Scientiae Circumstantiae, 2018, 38(8): 3004-3011(in Chinese). doi: 10.13671/j.hjkxxb.2018.0225
[18] WASKA H, KOSCHINSKY A, RUIZ CHANCHO M J, et al. Investigating the potential of solid-phase extraction and Fourier-transform ion cyclotron resonance mass spectrometry (FT-ICR-MS) for the isolation and identification of dissolved metal-organic complexes from natural waters [J]. Marine Chemistry, 2015, 173: 78-92. doi: 10.1016/j.marchem.2014.10.001 [19] LEENHEER J A. Comprehensive approach to preparative isolation and fractionation of dissolved organic carbon from natural waters and wastewaters [J]. Environmental Science & Technology, 1981, 15(5): 578-587. [20] FANG Z, HE C, LI Y Y, et al. Fractionation and characterization of dissolved organic matter (DOM) in refinery wastewater by revised phase retention and ion-exchange adsorption solid phase extraction followed by ESI FT-ICR MS [J]. Talanta, 2017, 162: 466-473. doi: 10.1016/j.talanta.2016.10.064 [21] AFTAB B, SHIN H S, HUR J. Exploring the fate and oxidation behaviors of different organic constituents in landfill leachate upon Fenton oxidation processes using EEM-PARAFAC and 2D-COS-FTIR [J]. Journal of Hazardous Materials, 2018, 354: 33-41. doi: 10.1016/j.jhazmat.2018.04.059 [22] ZHOU J, WANG J J, BAUDON A, et al. Improved fluorescence excitation-emission matrix regional integration to quantify spectra for fluorescent dissolved organic matter [J]. Journal of Environmental Quality, 2013, 42(3): 925-930. doi: 10.2134/jeq2012.0460 [23] CHEN W, WESTERHOFF P, LEENHEER J A, et al. Fluorescence excitation-emission matrix regional integration to quantify spectra for dissolved organic matter [J]. Environmental Science & Technology, 2003, 37(24): 5701-5710. [24] RYAN D K, WEBER J H. Fluorescence quenching titration for determination of complexing capacities and stability constants of fulvic acid [J]. Analytical Chemistry, 1982, 54(6): 986-990. doi: 10.1021/ac00243a033 [25] LUSTER J, LLOYD T, SPOSITO G, et al. Multi-wavelength molecular fluorescence spectrometry for quantitative characterization of copper(Ⅱ) and aluminum(Ⅲ) complexation by dissolved organic matter [J]. Environmental Science & Technology, 1996, 30(5): 1565-1574. [26] LI Y Y, XU C M, CHUNG K H, et al. Molecular characterization of dissolved organic matter and its subfractions in refinery process water by Fourier transform ion cyclotron resonance mass spectrometry [J]. Energy & Fuels, 2015, 29(5): 2923-2930. [27] 曹昌丽, 梁梦琦, 何桂英, 等. 城镇化河流溶解性有机质的荧光特性与水质相关性: 以宁波市北仑区芦江为例 [J]. 环境科学, 2018, 39(4): 1560-1567. CAO C L, LIANG M Q, HE G Y, et al. Fluorescent dissolved organic matter and its correlation with water quality in a urban river: A case study of the Lujiang river in Beilun, Ningbo [J]. Environmental Science, 2018, 39(4): 1560-1567(in Chinese).
[28] BI H N, TANG L, GAO X, et al. Spectroscopic analysis on the binding interaction between tetracycline hydrochloride and bovine proteins β-casein, α-lactalbumin [J]. Journal of Luminescence, 2016, 178: 72-83. doi: 10.1016/j.jlumin.2016.05.048 [29] LI W W, ZHANG F F, YE Q, et al. Composition and copper binding properties of aquatic fulvic acids in eutrophic Taihu Lake, China [J]. Chemosphere, 2017, 172: 496-504. doi: 10.1016/j.chemosphere.2017.01.008 [30] IRVING H, WILLIAMS R J P. Order of stability of metal complexes [J]. Nature, 1948, 162(4123): 746-747. doi: 10.1038/162746a0 [31] HUR J, KIM G. Comparison of the heterogeneity within bulk sediment humic substances from a stream and reservoir via selected operational descriptors [J]. Chemosphere, 2009, 75(4): 483-490. doi: 10.1016/j.chemosphere.2008.12.056 [32] NGUYEN H V M, HUR J, SHIN H S. Changes in spectroscopic and molecular weight characteristics of dissolved organic matter in a river during a storm event [J]. Water, Air, & Soil Pollution, 2010, 212(1/2/3/4): 395-406. [33] HE X S, XI B D, WEI Z M, et al. Spectroscopic characterization of water extractable organic matter during composting of municipal solid waste [J]. Chemosphere, 2011, 82(4): 541-548. doi: 10.1016/j.chemosphere.2010.10.057 [34] HUR J, LEE B M. Characterization of binding site heterogeneity for copper within dissolved organic matter fractions using two-dimensional correlation fluorescence spectroscopy [J]. Chemosphere, 2011, 83(11): 1603-1611. doi: 10.1016/j.chemosphere.2011.01.004 [35] NODA I, OZAKI Y. Two-dimensional correlation spectroscopy: applications in vibrational and optical spectroscopy[M]. Chichester, UK: John Wiley & Sons, Ltd, 2004. [36] CHEN W, HABIBUL N, LIU X Y, et al. FTIR and synchronous fluorescence heterospectral two-dimensional correlation analyses on the binding characteristics of copper onto dissolved organic matter [J]. Environmental Science & Technology, 2015, 49(4): 2052-2058. [37] BAKEN S, DEGRYSE F, VERHEYEN L, et al. Metal complexation properties of freshwater dissolved organic matter are explained by its aromaticity and by anthropogenic ligands [J]. Environmental Science & Technology, 2011, 45(7): 2584-2590. [38] YUAN D H, GUO X J, WEN L, et al. Detection of Copper (II) and Cadmium (II) binding to dissolved organic matter from macrophyte decomposition by fluorescence excitation-emission matrix spectra combined with parallel factor analysis [J]. Environmental Pollution, 2015, 204: 152-160. doi: 10.1016/j.envpol.2015.04.030 [39] CHEN B F, ZHAO M, LIU C, et al. Comparison of copper binding properties of DOM derived from fresh and pyrolyzed biomaterials: Insights from multi-spectroscopic investigation [J]. Science of the Total Environment, 2020, 721: 137827. doi: 10.1016/j.scitotenv.2020.137827 [40] XING J, XU G R, LI G B. Analysis of the complexation behaviors of Cu(II) with DOM from sludge-based biochars and agricultural soil: Effect of pyrolysis temperature [J]. Chemosphere, 2020, 250: 126184. doi: 10.1016/j.chemosphere.2020.126184 -






 下载:
下载: