-
我国是纺织品生产和出口大国,根据世界贸易组织的统计数据,我国在世界纺织品出口中所占份额在2019年升至39.2%。每生产1 kg的纺织产品大约需要100—200 L的高质量水,因而会产生大量印染废水[1]。印染废水通常具有高色度(50—2500 度)、高化学需氧量(COD)(150—30000 mg·L−1)、较高总溶解固体(TDS)(1500—12000 mg·L−1)和硫酸盐(500—1000 mg·L−1)以及可生化性较低的水质特征[2—3]。染色过程中添加的硫酸钠等染色辅助药剂,导致印染废水中无机离子较高。此外,在染色工艺过程使用的染料中主要发色基团为偶氮基(—N=N—)、羰基(—C=O)、硝基(—N=O)、醌基、以及诸如胺基、羧基、磺酸基、羟基等助色基团,染料中有 60%—70% 为难降解的偶氮染料,所有染料中有15%—20%会被排放到环境中进而造成严重环境危害[4—5]。根据《纺织染整工业废水治理工程技术规范(HJ 471—2020)》,印染废水处理工艺普遍为混凝-沉淀/气浮-水解酸化-好氧-深度处理或间接排放,常规处理后的深度处理工艺或回用处理工艺一般为混凝沉淀法、化学氧化法、膜分离法、曝气生物滤池法、生物活性炭法、过滤法、吸附法等工艺中的一种或几种工艺组合。化学氧化法对难降解残余有机物有较好的去除效果,但无法去除无机离子,而膜处理工艺因其优异的有机、无机污染物去除效果及易于规范化操作管理,在实际工程中得到了推广应用[6—10]。膜污染是膜工艺正常运行的重要控制要素,人们对膜污染特征与污染机制做了众多研究[3, 11—12],反渗透RO膜污染一般分为颗粒和胶体污染、无机和结垢污染、有机污染和生物污染四大类[13],具体的膜污染特征与处理水质密切相关。
目前人们对实际染整废水深度处理过程中,有机组分和无机组分特征及其变化规律的研究不足,尤其对溶解性有机物(DOM)组分的变化特征及其来源研究较少,对染整废水深度处理过程中水质特征与RO膜污染的内在联系还缺乏统一认识。因此本研究针对采用典型常规处理工艺(即混凝—沉淀—好氧)+深度处理工艺的浙江某染整废水处理厂,重点考察该厂超滤(UF)+反渗透(RO)深度处理工艺过程中,各单元出水的无机离子组成与DOM组分的变化特征,并进行了DOM的来源解析,研究结果可为深入了解实际染整废水深度处理过程中的水质变化特征,以及下一步探讨实际染整废水处理过程中的水质特征与膜污染机制内在联系提供科学参考依据。
-
浙江某印染厂染整废水采用混凝沉淀+SBR工艺为主要生化处理工艺单元,生化出水进入超滤(UF)+反渗透(RO)工艺进行深度处理。其中UF单元采用PVDF中空纤维膜(艾格清TM(XGREEN),江苏),孔径0.03 μm,操作压力0.2 MPa;RO单元采用陶氏公司的BW30FR-400/34 膜组件,操作压力1.0—1.2 MPa。深度处理工艺处理水量为5000 t·d−1,回用率约为50%。
本研究于2021年1月—4月间对该处理工艺的生化出水、RO进水(即UF出水)、RO出水、RO浓水和RO清洗水(在线清水清洗工艺出水)5个取样点进行水样采集,分别标记为S1、S2、S3、S4、S5。各取样点共采集样品20次,用于溶解性有机物的三维荧光光谱平行因子分析,同时取其中4个批次样品用于考察常规污染物、阴离子、阳离子、溶解性有机物等水质特征。所采集样品4 ℃运输及保存.
-
水样pH和电导率(EC)采用便携式水质分析仪(WTW, Welheim,Germany)测定,浊度采用哈希2100AN 型台式浊度仪测定。氨氮(
NH+4−N )、亚硝氮(NO−2−N )、硝氮(NO−3−N )、磷酸盐(PO3−4−P )、TP、溶解性总固体(TDS)、色度等指标依据《水和废水监测分析方法》进行测定[14];总氮(TN)与总有机碳(total organic carbon,TOC)由 TOC-VCPH 分析仪 (Shimadzu, Japan) 测定;COD通过哈希预制管消解及DR2800分光光度计 (HACH, USA) 测定。采用Lowry法测定蛋白质(protein)[15],改进Lowry法测定腐殖酸(humic acid, HA)[16],Dubois法测定多糖(polysaccharide)[17]。通过阴离子色谱(Ion Chromatography,USA)对
SO2−4 、Cl− 进行测定,并采用双指标滴定法测定HCO−3 和CO2−3 [14] ;通过电感耦合等离子体发射光谱仪(Inductively Coupled Plasma Optical Emission Spectrometer,ICP-OES,USA)检测水样中的Na、K、Ca、Mg、Al、Fe、Si、Mn。 -
UV254 由紫外-可见分光光度计测定(Spectrum Lab 752sp,Lengquang Tech,China);COD通过哈希预制管消解及DR2800分光光度计 (HACH,USA) 测定。以UV254与TOC的比值作为SUVA,代表水样芳香度大小[18]。采用三维荧光光谱仪(3D-EEMs,HITACHI, Japan)对水样中溶解性有机物DOM进行分析。用HITACHI-F4700荧光光谱仪连续扫描,测量激发波长(200—400 nm)和发射波长(220—550 nm)的荧光强度,生成三维激发发射荧光矩阵。激发和发射狭缝宽度设置为5 nm。
-
采用Origin2018软件进行作图。通过SPSS 20.0软件进行Pearson相关分析。采用如下指标考察RO膜单元的处理效果:RO浓缩倍数= RO浓水/RO进水;RO截留量= RO进水-RO出水。在Matlab2020中使用dreem6.0及N-Way工具箱对各取样点的水样进行EEMs分析。样品减去水空白的散射校正荧光得到实际荧光强度。将所有EEM归一化为拉曼单位(R.U.)[19],减少微弱荧光峰被掩盖的几率[20]。将Ex<225 nm的荧光值剪切掉,以最小化光谱噪声对PARAFAC建模产生的残留荧光的干扰[21]。通过二分法中组分的相似程度和最优平方和误差来判断最佳组分数。组分的确定使用在线Openfluor数据库进行比对。荧光参数FI(荧光指数)、HIX(腐殖化指数)、BIX(自生源指数)、Fn355(代表类腐殖物质相对浓度水平)、Fn280(代表类蛋白物质相对浓度水平)、r(A/C)(代表腐殖质组分的腐殖化程度)和r(T/C)(代表类蛋白组分与类腐殖质组分的比值)等指数的计算公式如下:
式中,A为紫外光区类腐殖物质荧光峰;C为可见光区类腐殖物质荧光峰;T为类蛋白质物质荧光峰[22]。
-
染整废水生化出水pH为7.32±0.35,由于RO膜的脱盐作用,RO出水pH降为6.91±0.48(表1)。进出水电导率(EC)的变化是反映RO膜截留性能的重要指标,可以作为考察印染废水是否可回用的一个参考指标[23]。RO进水EC在5810—7540 μs·cm−1之间,TDS在3635—5280 mg·L−1之间,表明RO进水可能因染整生产工艺的调整影响,含盐量不稳定;RO出水EC稳定在289—391 μs·cm−1之间,TDS在107.5—297.5 mg·L−1之间,展现出良好稳定的脱盐效果。RO对色度也有良好的去除效果,平均去除率分别为(88.9%±0.6%)。生化出水正磷和总磷仅为(0.09±0.01) mg·L−1和(0.19±0.08) mg·L−1,且在RO出水则均被未检出。生化出水TN、
NH+4−N 、NO−3−N 和NO−2−N 分别为(13.07±5.44) mg·L−1、(0.92±0.76) mg·L−1、(5.27±2.29) mg·L−1、(0.15±0.09) mg·L−1,超滤单元对氮的去除效果不明显,而RO单元去除率达到(84.2%—90.3%),RO出水分别为(1.59±0.46) mg·L−1、(0.16±0.10) mg·L−1、(0.71±0.17) mg·L−1、(0.01±0.01) mg·L−1。所有水样的TN均大于NH+4−N 、NO−3−N 和NO−2−N 之和,表明染整废水深度处理过程中持续性存在有机氮,推测主要是印染废水中常使用的偶氮染料及其代谢产物残留。偶氮染料含有一个或多个—N=N—基团的多环芳香族化合物,在污水处理过程中厌氧、兼氧、好氧条件下,由多种酶的作用先降解为芳香胺再被分解为CO2和H2O[24-26]。偶氮染料为难生物降解类有机物[27],因此,偶氮染料及其代谢产物可残留在RO出水中,成为有机氮的组成部分。染整废水深度处理过程中生化出水COD、TOC、腐殖酸、蛋白质、多糖分别为(94±4) mg·L−1、(11.95±5.55) mg·L−1、(49.13±6.01) mg·L−1、(27.26±11.64) mg·L−1、(14.85±4.71) mg·L−1(图1),超滤单元对上述有机物去除率为12.0%—61.5%,而RO单元的去除效果更显著,为83.8%—100%。RO出水可以满足《纺织染整工业水污染物排放标准》(GB4287-2012)和《城市污水再生利用工业用水水质标准》(GB/T19923-2005)的要求。
通常认为DOM的SUVA越大芳香度越高,SUVA>4 L·mg−1·m−1 为高芳香度、高分子量和高疏水性的DOM,SUVA<2 L·mg−1·m−1 为低芳香度、低分子量和亲水性的DOM[18]。染整废水生化出水、RO进水、浓水、清洗水的DOM均为高芳香度(SUVA>4 L·mg−1·m−1)。染整废水在深度处理过程中,SUVA呈持续下降趋势,RO出水SUVA仅为0.92 L·mg−1·m−1,表明RO处理单元对染整废水中高芳香度、高分子量、疏水性DOM有良好的去除效果。
在深度处理过程中,RO浓水对蛋白质、多糖、腐殖质、有机氮的浓缩倍数分别为3.32、1.98、1.62、1.45倍;RO清洗水中蛋白质、多糖、腐殖质、有机氮分别为RO截留量的2.44、1.98、1.80、1.18倍,这一结果表明,相比而言RO单元对蛋白质、多糖的富集和清洗效果优于对腐殖质、有机氮的富集和清洗效果。
-
印染工艺中常需添加NaCl、Na2SO4等药剂辅助染色过程,因而印染废水中常含有大量的无机离子[27],而离子组成直接影响到RO膜污染特征,因此本研究考察了染整废水深度处理过程主要阴、阳离子的分布特征,如表2所示。
染整废水中主要阳离子为Na,其次为Ca,再次是K;阴离子均以
Cl− 、SO2−4 和HCO−3 为主,这一水质特征与江苏某印染废水处理厂RO处理单元的水质特征相似[8]。RO膜对主要阴离子Cl− 、SO2−4 和HCO−3 的去除率分别为95.3%、98.6%和91.8%;浓缩倍数在1.37—1.55之间。所有水样均未检出Mn离子(未在表2中显示)。在所有检测出的阳离子中Mg的含量最低,RO进水仅为(0.80±1.05) mg·L−1。RO进水中Ca、Si、Fe分别为(52.17±1.79) mg·L−1、(12.00±1.33) mg·L−1、(2.45±0.55) mg·L−1,这些离子有促使RO膜表面形成钙垢、硅垢和铁垢的可能性[12, 28]。RO单元对Fe、Si和Al的去除率相对较低,分别为55.4%、79.8%和83.6%,对K、Ca、Na、Mg的去除率则达到96.3%—100%。已有研究也发现Fe[29]、Si[30]的RO去除效果低于其他离子,但均未给出合理解释,这一现象还需要做进一步研究分析。RO浓水对Mg的浓缩倍数最高,为6.92,对其他阳离子的浓缩倍数均在1.71—2.09。RO清洗水中Fe和Al的浓度为RO截留量的0.51倍和1.06倍,而Ca和Si分别为膜截留量的1.27倍和1.66倍。这一结果表明,易于造成RO膜表面结垢中的阳离子中,Fe和Al相比Ca和Si更难被清洗下来。已有研究发现Fe比其他元素更易沉积在RO膜上[12],而本研究结果表明Fe比其他元素更难被洗脱,因此下一步应重点关注染整废水中的Fe在RO膜污染过程中的行为特征。
已有研究发现,RO膜表面结垢物中,CaSO4结垢、CaCO3结垢、Si-Ca协同作用、Ca-腐殖酸协同作用等为常见的污染物形成机制,清洗水中有协同作用的各种离子之间存在显著相关关系[31],但本研究RO清洗水中各离子相关分析发现,所有阴离子均和阳离子之间不存在显著相关关系,各阳离子与腐殖酸、蛋白质、多糖组分也未发现显著相关关系,这一方面说明实际染整废水RO工艺中RO膜表面的离子结垢较为复杂,另一方面也与RO清洗水样品为实际处理工艺的在线清水清洗而非药剂清洗水样品,未能达到膜清洗最佳效果有关。此外,RO膜结垢与离子组成、离子强度、温度和膜表面性能(如粗糙度、表面电荷等)等多种因素有关,在后续研究中仍需做进一步研究。
-
DOM是引起RO膜污染的重要原因之一。本研究采用三维荧光光谱通过平行因子分析对染整废水深度处理工艺各水样做进一步解析,得到4个荧光峰(图2,表3):A峰(Ex/Em: 235/335,235/340,235/345),色氨酸类芳香蛋白荧光峰[32];B峰(Ex/Em: 225/285),酪氨酸类芳香蛋白荧光峰[33]; C峰(Ex/Em: 255/455),富里酸类含芳香环基团荧光峰[20];D峰 (Ex/Em: 275/320,280/320),溶解性微生物产物(SMP)[34]。本研究未发现腐殖酸类物质荧光峰,仅发现富里酸类物质荧光峰,已有研究表明印染废水中的荧光物质中,腐殖质类物质主要以富里酸为主[35]。占比较大的富里酸类C峰,主要由染整废水处理过程中微生物新陈代谢产物的荧光物质产生[36]。
生化出水荧光物质的荧光峰强度由大到小顺序为:色氨酸类>富里酸类>SMP>酪氨酸类(表3),根据各峰的荧光强度做相对百分比分析,四类荧光物质占比分别为33.3%、28.0%、22.7%和16.0%。薛菲菲等[37]研究也发现,印染废水二级出水的荧光物质主要为色氨酸类、富里酸类和溶解性微生物产物,而酪氨酸类和腐殖酸类物质只占总有机物的20%—25%。在检测出的荧光峰中,A峰色氨酸和B峰酪氨酸均可在城市污水中被检出[38],表明这些荧光物质可能部分来源于生活污水中常见有机物的代谢产物。其中的Ex/Em 230/340 nm和275/320 nm附近的荧光峰也可在印染废水中检出,这部分荧光物质可能来源于未降解的分散染料以及阳离子染料未降解完全产生的芳香族化合物[39—40]。有研究认为,Ex/Em 230/338 nm和275/320 nm两个荧光峰与印染工艺中广泛使用的染料分散剂MF有关[41]。MF的主要荧光结构是萘,主要成分是磺化萘甲醛缩合物(SNFC),SNFC 具有高度水溶性,在生物处理过程中难以消除[42],因此在染整废水的RO出水中仍可检出。
经过UF单元后,色氨酸类、酪氨酸类和SMP荧光峰强度均显著下降,UF出水中上述3种DOM分别占39.0%、30.7%和30.3%,而富里酸类荧光峰则未被检出,表明超滤单元对四类DOM尤其对富里酸类物质有一定的去除效果。已有研究对火电厂废水UF-RO工艺处理过程中的DOM三维荧光光谱分析结果发现,RO出水除色氨酸和酪氨酸类荧光峰外,还检出SMP类和腐殖酸类荧光峰[43],但本研究RO出水仅检出色氨酸类(Ex/Em=235/335 nm)和酪氨酸类(Ex/Em=225/285 nm)蛋白荧光峰,分别为53.3%和46.7%,这或与本研究中RO进水的有机物组分和浓度比火电厂废水更高有关。RO出水中的Ex/Em=235/335 nm荧光峰与前文所述的染料相关荧光峰接近,推测RO出水中仍为染料相关荧光峰,这也与RO出水中检测出有机氮(染料及其代谢物相关)的结论所对应。染整废水深度处理过程中SMP类荧光物质全部被RO膜截留,在RO浓水中检出。RO浓水中色氨酸类、酪氨酸类和SMP分别占比40.4%、28.9%和30.7%,荧光强度为RO进水的2.58—2.84倍。RO清洗水中,荧光物质仍以色氨酸类、酪氨酸类、SMP为主,未测出富里酸类物质荧光峰。
对DOM组分各荧光峰值与蛋白质、多糖、腐殖质、TOC、COD和有机氮(即TN与无机氮之差)做Peason相关分析,结果表明色氨酸类荧光峰与蛋白质显著相关(P<0.05),SMP荧光峰与蛋白质和有机氮显著相关(P<0.05),其他荧光峰与上述有机物则无显著相关关系。这表明染整废水UF-RO深度处理过程中,溶解性有机物里的蛋白质类有机物与荧光组分密切相关,而多糖类、腐殖质类有机物与荧光类DOM的关系相对较弱;荧光性溶解性微生物产物不但与蛋白质类有机物有关,还可能受到有机氮(即染料类物质的代谢产物)的影响。
-
将不同取样点样品的各荧光峰做相关性分析,可以探讨水样中荧光类物质的同源性或同结构相似性[35, 44]。染整废水深度处理过程中,A峰与B峰(P<0.01)和D峰(P<0.05)荧光强度显著相关,表明水中残留的色氨酸类、酪氨酸类、溶解性微生物产物可能来源相似或相似结构;C峰与其它峰均无显著相关,说明本染整废水厂染整废水中的腐殖质类物质与其他DOM组分来源不同且结构差异较大。
进一步通过FI(荧光指数)、BIX(自生源指数)和HIX(腐殖化指数)等荧光参数对DOM的来源进行解析。荧光指数FI<1.4表示DOM中的腐殖质主要来源于植物、土壤有机质的分解与浸出的陆源为主,并具有较高的芳香度,FI>1.9表示腐殖质主要由水体中的微生物和藻类活动引起的内生源为主[45];当自生源指数BIX>1时,为新近生物或细菌引起的自生源为主[46];腐殖化指数HIX常用来表征DOM的腐殖化程度,HIX>4时腐殖化程度较高,HIX的值越大则DOM的腐殖化程度越高,DOM越稳定[47]。此外,Fn280代表类蛋白物质相对浓度水平,Fn355代表类腐殖物质相对浓度水平,Fn280和Fn355两指标分别用来表征自生源和陆源对水体DOM 组分的贡献[48];r(A/C)为紫外光区类腐殖物质荧光峰与可见光区类腐殖物质的荧光峰之比,可用来衡量 DOM 中腐殖质组分的腐殖化程度[49],r(A/C)的值越高,水中DOM 中稳定腐殖组分比重越低。r(T/C)值是类蛋白荧光与类腐殖质荧光的比值,用来评价水质受人类活动污染的程度,r(T/C)>2.0时表示人为引入的污染影响较大[50] 。
由图3(a)可知,所有水样BIX>1,其中RO出水BIX最低,仅RO出水FI<1.9,其他水样FI>1.9,表明水样中荧光物质以微生物活动引起的自生源类及新释放到DOM中的有机物为主,相比而言,RO出水中除了自生源类DOM之外,陆源输入的DOM也占据较大比例。所有水样HIX<4.0,生化出水的HIX最大(1.75),其他水样则在0.27—0.29之间[图3(b)],表明所有水样的腐殖化程度偏低,腐殖质主要为微生物或水生细菌来源[47],水样中生化出水的腐殖化程度最高,结构相对最稳定难以降解。所有取样点的水样Fn280均显著高于Fn355[图3(c)],表明水样中类蛋白质组分高于类腐殖质组分,呈现出较强的自生源特征[44]。需要注意的是,由于偶氮染料的代谢产物和溶解性微生物产物中含有丰富的蛋白质类荧光团,尤其是芳香胺的代谢产物与蛋白质类荧光峰相似[25],因此本研究染整废水DOM中BIX指数高、Fn280/Fn355高的成因也可能与水中染料及其代谢产物的残留有关。水样r(A/C)值的排序为生化出水>RO进水>RO清洗水>RO浓水>RO出水[图3(d)],表明UF和RO单元处理后,水中稳定的腐殖质组分越来越多。所有水样r(T/C)>4.0,RO出水r(T/C)最低,RO浓水的r(T/C)最高,表明RO浓水中类蛋白组分占比显著高于RO出水中的类蛋白组分占比。
各水样对比而言,染整废水生化出水类腐殖质组分占比最低,但腐殖化程度最高,类蛋白组分占比最高;RO进水和清洗水的荧光组分类型较为接近,二者与生化出水相比,类腐殖质组分比重降低,腐殖化程度降低,类蛋白组分占比增加;RO浓水与RO进水/RO清洗水相比较而言,类腐殖质组分占比略低,腐殖化程度相似,类蛋白组分占比略高;相比而言,RO出水类蛋白组分占比最低,类腐殖质组分占比也最低,但腐殖化程度与RO进水、清洗水、浓水相似。除RO出水外其他水样均以内源输入为主,说明RO出水中的类腐殖质既有生化系统中微生物活动产生的自生源输入,也有生产用水(自然水体来源)中原有携带的植物、土壤有机质的分解产物与浸出物的陆源输入,而且陆源输入的这部分荧光物质,腐殖化程度相对较低,难以被RO工艺截留。
-
(1)染整废水深度处理工艺过程中,UF-RO深度处理单元对常规污染指标去除效果显著,但RO出水中仍存在有机氮。UF单元对COD、TOC、腐殖酸、多糖和蛋白质的去除率为12.0%—61.5%,RO单元的去除率为83.8%—100%。所有水样的腐殖化程度偏低,腐殖质主要为微生物或水生细菌来源,经过UF和RO单元处理后,稳定的腐殖质组分占比增加。RO单元对染整废水中高芳香度、高分子量、疏水性DOM有显著去除效果。
(2)染整废水中主要阳离子为Na、Ca和K,阴离子以
Cl− 、SO2−4 和HCO−3 为主;RO单元对Fe、Si和Al的去除率为55.4%—83.6%,对K、Ca、Na、Mg的去除率达到96.3%—100%;RO浓水中Mg的浓缩倍数最高;RO膜上Fe和Al相比Ca和Si更难被洗脱。(3)染整废水生化出水荧光物质的荧光峰强度顺序为:色氨酸类>富里酸类>溶解性微生物产物>酪氨酸类。UF单元可显著截留富里酸类DOM,RO出水则仅存在色氨酸类 (Ex/Em=235/335 nm)和酪氨酸类(Ex/Em=225/285 nm)蛋白荧光峰,其中Ex/Em=235/335 nm荧光峰为染料相关的荧光峰。
(4)除RO出水外其他水样均以内源输入为主,RO出水中的类腐殖质既有生化系统中微生物活动产生的自生源输入,也有自然水体中原有携带的植物、土壤有机质的分解产物与浸出物的陆源输入,其中陆源输入的DOM腐殖化程度相对较低,难以被RO工艺截留。
某实际染整废水深度处理过程中无机组分与溶解性有机物的变化
Variation of inorganic components and dissolved organic matter during advanced treatment of a full-scale dyeing wastewater treatment plant
-
摘要: 近年来超滤(UF)-反渗透(RO)组合工艺逐渐在印染废水深度处理中得到推广应用,但目前对实际染整废水深度处理过程中无机与有机组分变化特征的研究仍有不足。研究考察了某实际染整废水的UF-RO深度处理工艺过程中无机离子、溶解性有机物(DOM)组分特征与来源解析。结果表明,实际染整废水深度处理工艺过程中,UF-RO工艺对常规污染物去除显著,但RO出水中仍存在有机氮;该工艺可显著去除染整废水中高芳香度、高分子量、疏水性的DOM,而陆源输入、腐殖化程度相对较低的DOM可透过RO膜。染整废水中主要阳离子为Na、Ca和K,阴离子为
Cl− 、SO2−4 和HCO−3 ,RO单元对Fe、Si和Al的去除率为55.4%—83.6%,对K、Ca、Na、Mg的去除率达到96.3%—100%;RO膜对Mg的浓缩倍数最高,RO膜上 Fe和Al相比Ca和Si更难被洗脱。UF单元可显著截留富里酸类DOM,RO出水则仅存在色氨酸类(Ex/Em=235/335 nm)和酪氨酸类(Ex/Em=225/285 nm)荧光峰,其中Ex/Em=235/335 nm峰为染料相关的荧光峰。除RO出水外,其它水样DOM均以内源输入为主,RO出水则兼具自生源输入和陆源输入特征。这些研究结果可以为人们进一步了解实际染整废水深度处理过程中水质变化特征及RO膜性能提供参考价值。Abstract: The ultrafiltration (UF)-reverse osmosis (RO) process has gradually been applied in the advanced treatments of dyeing wastewater in recent years. However, there is still a gap in the characteristics of the inorganic and organic components of dyeing wastewater in the full-scale advanced treatment process. Therefore, the composition and occurrence of the inorganic ions and dissolved organic matter (DOM), and the source of DOM during a full-scale UF-RO advanced treatment process of dyeing wastewater were investigated in this study. Results show that the UF-RO process could significantly remove the conventional contaminants, while still with organic nitrogen in the RO permeate. The DOM with higher aromaticity, molecular weight and hydrophobic characters were significantly rejected during the advanced treatment, whereas the terrestrial input, lower humification DOM were permeable in the RO system. The dominant anions were Na, Ca and K, withCl− ,SO2−4 andHCO−3 as the main cations in the dyeing wastewater. The removal rates of Fe, Si and Al by the RO unit were 55.4%—83.6%, and 96.3%—100% for that of K, Ca, Na, Mg, respectively. The concentration factor of Mg was the highest for the RO system. Fe and Al were harder to be cleaned from the fouling RO membrane surface than Ca and Si. The fulvic acid-like DOM could be rejected perfectly by the UF unit, and only tryptophan protein-like (Ex/Em=235/335 nm) and tyrosine protein-like (Ex/Em=225/285 nm) DOM were detected in the RO product water. Moreover, DOM with fluorescence peak at Ex/Em=235/335 nm in the RO product water were related to dyestuffs. Endogenous derived DOM were dominant in the water samples other than RO product water, while both endogenous derived and terrestrial derived DOM were mainly in the RO product water. This research can provide a valuable reference for further understanding of the dyeing wastewater characteristics during the full-scale advanced treatment and the performance of the RO system. -
我国铅锌工业总规模大,常年位于世界第一,仅2018年,精Zn产量达5.68×106 t,约占全球总量的40%,我国Zn冶炼主流工艺为“焙烧—浸出—电积”,其生产过程中产生大量含Zn、Cd和As等的冶炼废渣,统计表明,平均生产1 t Zn,产生0.96 t废渣[1],废渣历史积存量和年新增量大,难以得到有效的消纳利用,通常采用无害化填埋、堆置储存等方式进行处置。在长期的堆置过程中,受风蚀、淋溶和浸蚀等作用影响,废渣中的重金属释放,对周边人群健康和土壤、地下水等生态环境造成严重威胁[2]。
稳定化是废渣常见的无害化处理方式,通过加入稳定剂降低重金属的迁移性,而稳定剂的选择是关键。目前,常用稳定剂一般包括有机、无机和生物质型3种[3]。其中,无机型药剂因对重金属稳定效果好而广被应用[4-6],其对重金属主要是通过化学键合、物理包容、吸附或形成惰性沉淀物等作用进行稳定[7-8]。目前,研究应用多以含硫、磷、铁、钙、镁等药剂为主[7-11],但多集中在Zn、Pb等个别污染指标,而针对废渣中As、Cd等其他多污染物共存的系统化研究还较为欠缺,特别是个别稳定剂对废渣中As反而存在活化作用则较少关注,受介质类型、污染程度、稳定剂种类、投加量、配伍等因素的影响,不同药剂实际稳定化效果还须进行综合比对和考证。
本研究以湖南某大型冶炼企业渣场堆存的铅锌冶炼废渣为研究对象,采用Na2S·9H2O、(NH4)2HPO4、Na3PO4·12H2O、CaO、MgO为稳定化药剂,并进行了药剂配伍研究,以水浸提法[12]模拟废渣堆存过程的浸蚀淋溶影响,考察了不同药剂对Zn、Cd、Cu、As的综合稳定化效果及其对环境的影响,以期为国内铅锌冶炼废渣的无害化处置提供参考。
1. 材料与方法
1.1 供试材料
本研究所用工业废渣取自湖南某大型冶炼企业典型渣堆场。将堆场上性状明显类似的废渣进行现场机械开挖和预混,运至具防渗结构的预存场进行自然风干、人工除杂、混匀、磨碎后,过4 mm筛,取筛下物于小型卧式搅拌机中再次混匀后,用于稳定化实验。
废渣污染特性如表1所示,与《污水综合排放标准》(GB 8978-1996) [13]1级最高允许排放浓度相比,废渣H2O浸出Zn、Cd和Cu超标,分别超标6.60、10.10、1.76倍,废渣pH为2.40,呈强酸性。
表 1 供试废渣污染特性Table 1. Pollution characteristics of waste slag供试废渣 总量/(mg·kg−1) H2O浸出/(mg·L−1) GB 8978-1996 I级/(mg·L−1) Zn 4 631 38 5.0 Cd 2 188 1.11 0.1 Cu 1 572 5.51 2.0 As 1 442 0.25 0.5 Pd 4 752 0.02 1.0 Cr 106 0.05 1.5 稳定化药剂包括Na2S·9H2O、(NH4)2HPO4、Na3PO4·12H2O、CaO、MgO,均为分析纯。
1.2 实验方法与分析方法
称量1 000.00 g(干质量)废渣置于敞口玻璃容器中,将Na2S·9H2O、(NH4)2HPO4和Na3PO4·12H2O 分别配成200 mL的水剂,按废渣Zn、Cd、Cu、As等的理论水浸出量计算药剂添加量,药剂与元素浸出摩尔比设计为2∶1、4∶1、8∶1、16∶1,对应药剂投加质量分数如表2所示,边加药剂边充分混匀搅拌,搅拌时间20 min,CaO和MgO按粉剂添加,根据前期Zn、Cd稳定化实验结果,3种药剂复配配方按质量比Na2S·9H2O∶(NH4)2HPO4∶Na3PO4·12H2O=2∶1∶3进行药剂配伍,设置空白对照(CK),每处理设3次重复,控制水∶渣(质量比)=1:4左右,室温养护7 d后,进行废渣pH、毒性浸出测试。
表 2 稳定化实验设计Table 2. Design of heavy metals stabilization experiment% 实验处理 Na2S·9H2O (NH4)2HPO4 Na3PO4·12H2O CaO MgO 1 0.30 — — — — 2 0.60 — — — — 3 1.21 — — — — 4 2.42 — — — — 5 — 0.16 — — — 6 — 0.32 — — — 7 — 0.64 — — — 8 — 1.28 — — — 9 — — 0.46 — — 10 — — 0.92 — — 11 — — 1.84 — — 12 — — 3.68 — — 13 — — — 0.40 — 14 — — — 0.60 — 15 — — — 1.00 — 16 — — — — 0.40 17 — — — — 0.60 18 — — — — 1.00 19 0.60 — — 0.40 — 20 1.21 — — 0.40 — 21 2.42 — — 0.40 — 22 — 0.32 — 0.40 — 23 — 0.64 — 0.40 — 24 — 1.28 — 0.40 — 25 0.40 0.20 0.60 — — 26 0.80 0.40 1.20 — — 27 1.60 0.80 2.40 — — 空白对照 — — — — — 注:“—”表示未添加,配伍药剂添加时先加CaO。 使用酸度计(pHs-3C型,上海仪电科学仪器股份有限公司)测定供试废渣的pH,测定方法采用《固体废物 腐蚀性测定 玻璃电极法》(GB/T 15555.12-1995)[14];重金属总量测试的前处理采用石墨炉三酸(体积比为HNO3∶HF∶HClO4=3∶2∶2)消解法;毒性浸出采用水浸法[12];消解液和水浸出液中重金属含量采用电感耦合等离子体发射光谱仪(ICP-MS 7500,美国Agilent公司)测定。
修复效果评估根据式(1)~式(3)进行计算。
η(M)=(C0−Ct)/C0×100% (1) η(Zn,Cd)=(η(Zn)+η(Cd))/2 (2) η=(η(Zn)+η(Cd)+η(Cu)+η(As))/4 (3) 式中:η(M)为M元素的稳定率;C0、Ct分别为废渣在稳定化前和稳定化后的元素水浸出浓度,mg·L−1;η(Zn, Cd)为Zn和Cd这2种重金属的稳定率均值;η为Zn、Cd、Cu、As 4种元素的综合稳定率均值。
2. 结果与讨论
2.1 单一稳定剂
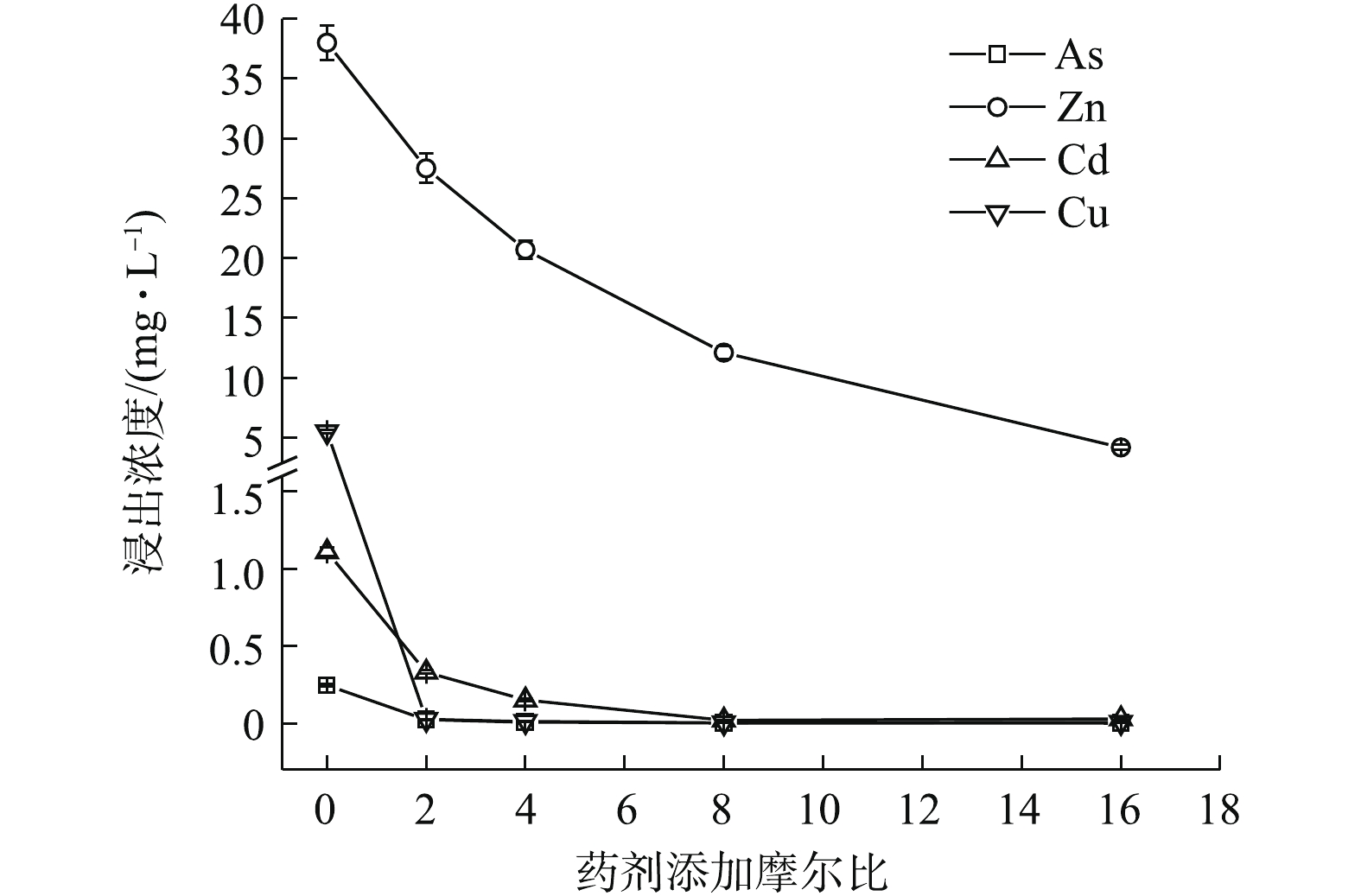
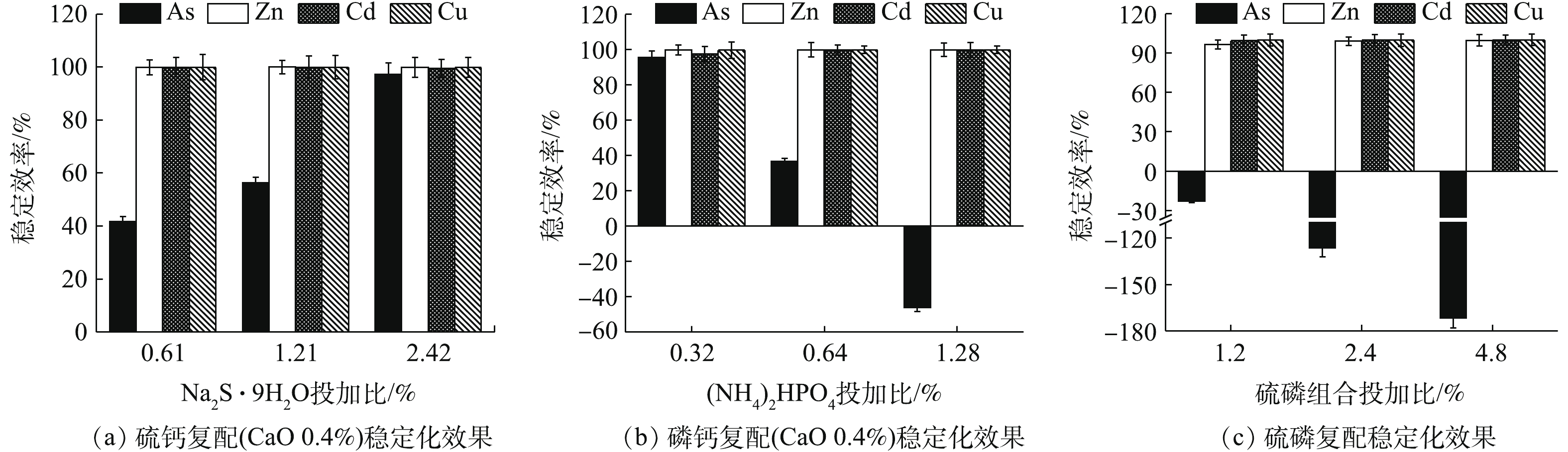
Na2S·9H2O、(NH4)2HPO4、Na3PO4·12H2O均能明显降低4种金属元素的水浸出浓度(图1~图3)。如图1所示,随Na2S·9H2O投加量的增加,各金属元素浸出均呈降低的趋势,Zn、Cd和Cu的稳定率分别为27.63%~88.97%、70.08%~98.05%、99.52%~99.85%。其中,药剂与4种金属元素浸出总量的摩尔比为8 (投加1.21%)时,Cd的浸出浓度由CK的1.11 mg·L−1降至0.02 mg·L−1,达到GB 8978-1996中Cd (0.1 mg·L−1)限标;摩尔比16(投加2.42%)时,Zn浸出才达标,由CK的38 mg·L−1降至4.19 mg·L−1,此时η(Zn)为88.97%、η(Cd)为97.35%,η(Zn, Cd)为93.16%,Na2S·9H2O对3种重金属稳定效果突出,总体稳定效应大小依次为Cu>Cd>Zn,这与其提供的S2-与Zn2+、Cd2+和Cu2+形成金属硫化物沉淀有关[15]。这种难溶物溶解度很低,形成沉淀的先后顺序一般为CuS>CdS>ZnS[16],随投加量的增加,其水解过程中产生的OH−也有利于重金属的稳定。此外,各处理使As浸出降低了90.24%~99.11%,说明其与As形成的惰性沉淀物在应对水浸滤风险能力方面也很显著,但在稳定化过程中有少许H2S逸出。
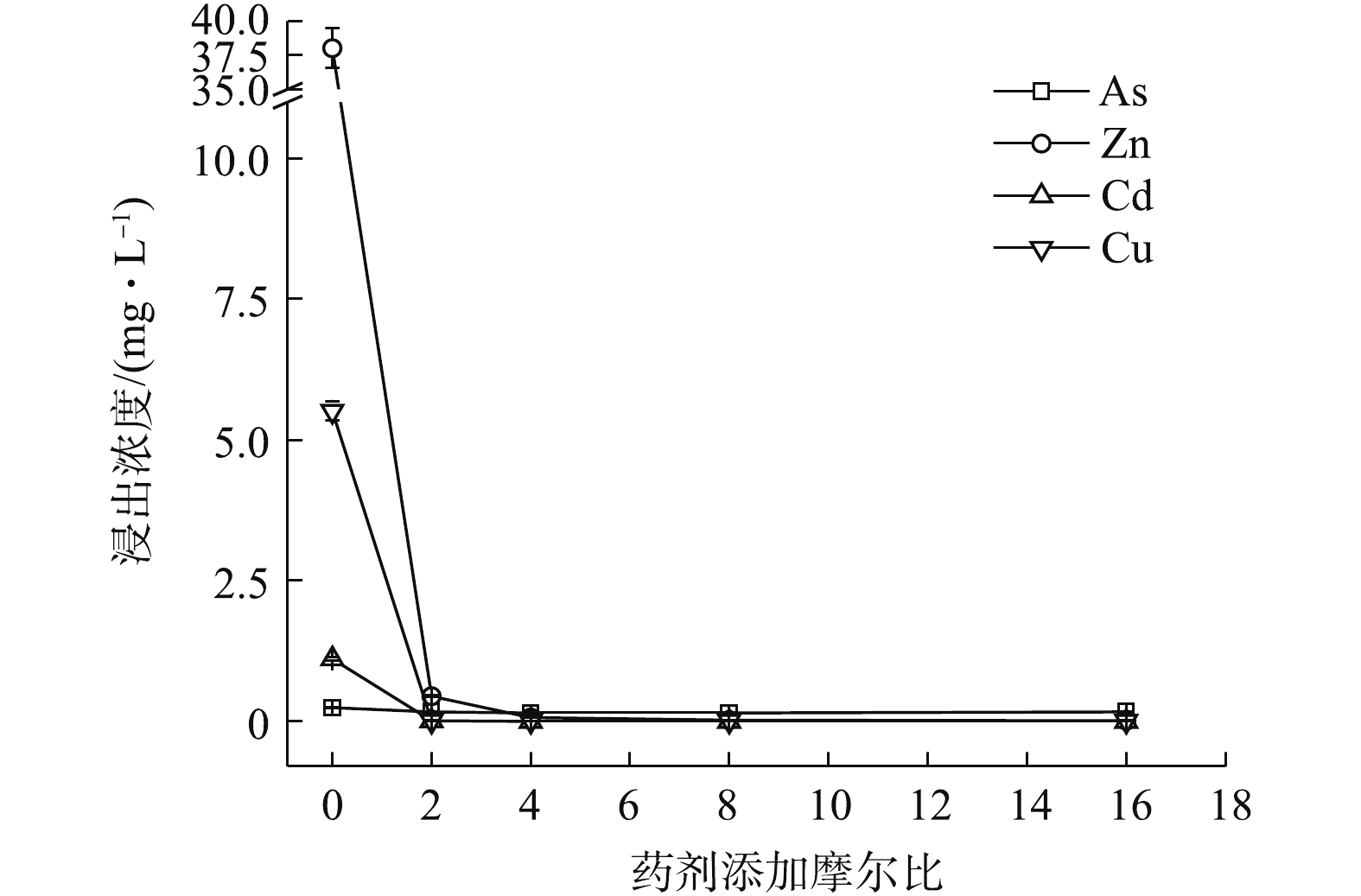
(NH4)2HPO4可明显降低重金属的水浸出毒性。如图2所示,随(NH4)2HPO4投加量的增加,Zn、Cd和Cu浸出浓度均呈不断降低的趋势,稳定率分别为59.52%~99.46%、57.97~99.23%、98.85~99.93%,而As浸出则呈现先降低后回升的趋势,药剂摩尔比≥8后,As浸出浓度开始升高并被活化,药剂摩尔比升高至16时,As浸出浓度被活化1.74倍,浓度为0.68 mg·L−1,超出0.5 mg·L−1限标的36%,药剂摩尔比为4时,As浸出降至最低0.016 mg·L−1,η(As)高达93.52%,但此时Zn和Cd浸出分别降至8.47 mg·L−1和0.23 mg·L−1,并未达标。直到药剂摩尔比为8(投加量为0.64%)时,才使以上4种金属元素均达标,Zn和Cd分别降至0.224 mg·L−1和0.01 mg·L−1,η(Zn)和η(Cd)分别为99.41%和99.08%,η(Zn, Cd)为99.25%,4种元素综合稳定率η达到最高值,为87.42%。因此,0.64%的(NH4)2HPO4投加量综合稳定效果最好,但须控制投加剂量,以免高量添加对As过度活化。
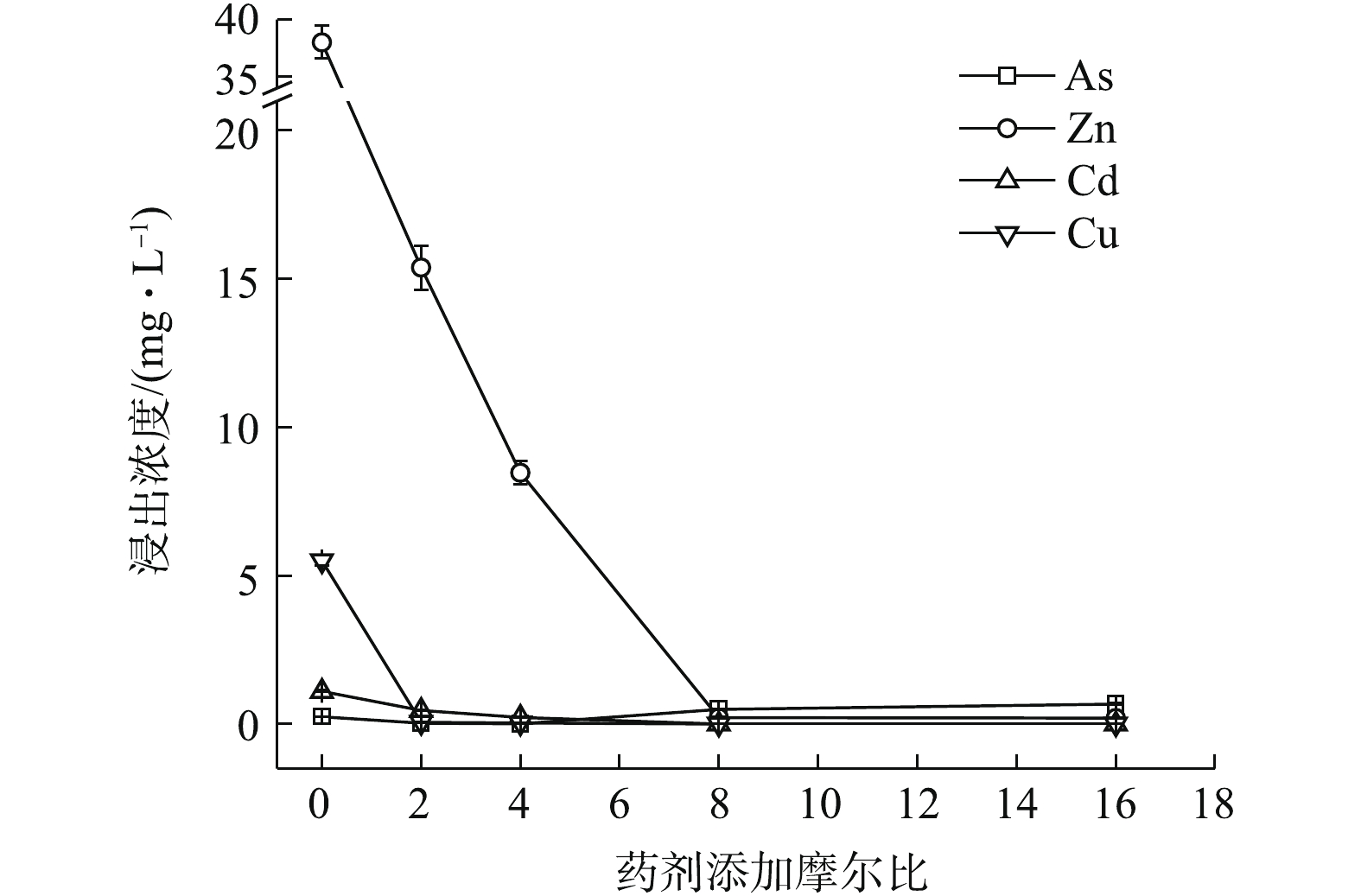
如图3所示,Na3PO4·12H2O稳定化效果突出,最低药剂摩尔比为2 (投加量为0.46%)时,可使4种元素均能达标,Zn、Cd、Cu和As浸出分别降至0.446、0.014、0.004、0.169 mg·L−1,稳定率分别为98.83%、98.70%、99.93%、31.58%,4元素综合稳定率η可达82.26%。其中,η(Zn)和η(Cd)分别为98.83%和98.70%,η(Zn, Cd)为98.76%,稳定率均高于同摩尔比条件下的(NH4)2HPO4(η(Zn) 59.52%,η(Cd) 57.97%,η(Zn, Cd) 58.94%)和Na2S·9H2O(η(Zn)27.63%,η(Cd) 70.08%,η(Zn, Cd) 48.85%),在Na3PO4·12H2O的各处理组中,η(As)均可稳定在34.92%左右。磷酸盐对Cd、Cu等的固定主要是通过与金属发生表面络合吸附和共沉淀等作用所致[8],对Zn主要以诱导或直接吸附[8]以及少量溶解性无定形沉淀反应[17]为主,并可促进Zn的可氧化态向残渣态转变[18]。而P与As化学性质相似,一般认为,
PO3−4 和HPO2−4 会与AsO3−4 形成竞争吸附,使As的流动性增强,但本研究采用单一Na3PO4·12H2O处理强酸性废渣,并未对As产生明显的活化作用,其具体原因尚须进一步的深入研究。由图1~图3可知,对Zn的稳定化效果顺序依次为Na3PO4·12H2O>(NH4)2HPO4>Na2S·9H2O,对Cd和Cu的稳定化效果依次为Na3PO4·12H2O>Na2S·9H2O>(NH4)2HPO4,对As的稳定化效果依次为Na2S·9H2O>(0.16~0.32)% (NH4)2HPO4>Na3PO4·12H2O。其中,(NH4)2HPO4易对As产生活化作用,从药剂投加量角度来看,使4种金属元素同时达标的综合稳定效应依次为0.46% Na3PO4·12H2O(摩尔比为2,η=82.26%)>0.64% (NH4)2HPO4(摩尔比为8,η=87.42%)>2.42% Na2S·9H2O (摩尔比为16,η=96.36%),因此,Na3PO4·12H2O对Zn和Cd稳定效果均最为突出,对As活化效果不明显,且低剂量添加不易导致溶解P过量,因此,在3种药剂中,Na3PO4·12H2O最适合单独处理强酸性废渣。
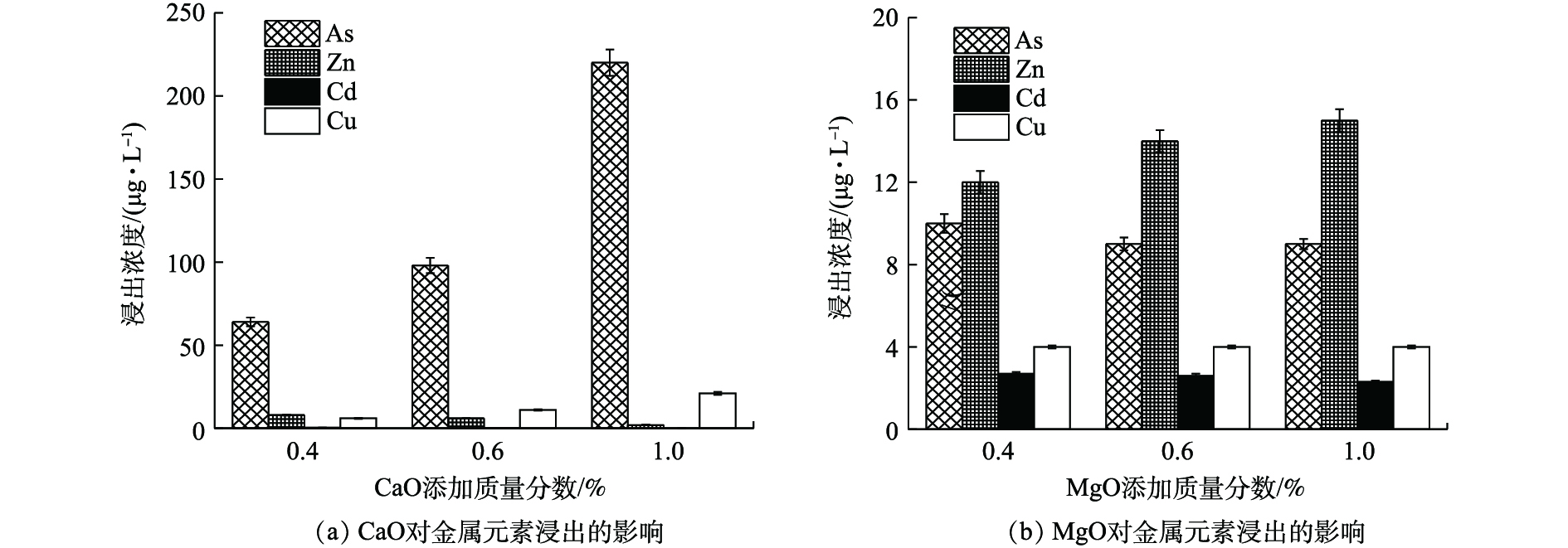
如图4(a)所示,(0.4~1)% CaO处理后,废渣中Zn、Cd、Cu、As的浸出浓度分别降至<10、<0.50、<25、<220 μg·L−1,远低于GB 8978-1996最高限值。其中,Zn、Cd、Cu稳定率均高于96%,这与CaO提高废渣pH、重金属氢氧化物沉淀增多、黏土物质等对重金属吸附性增强[19]、提供的钙离子与金属离子发生同晶替代[20]等作用有关。η(As)则为10.93%~74.09%,低量CaO更有利于As的稳定,CaO对As的稳定作用主要与强氧化性和适当pH条件下易形成CaHAsO4和Ca3(AsO4)2沉淀[21]有关。但本研究表明,当CaO≤1%,随CaO量的增加,As浸出浓度逐渐升高,这可能由于随着OH−浓度增高,负电荷对As的竞争吸附作用逐渐加强,最终导致As的迁移性有所增强[22]。
如图4(b)所示,MgO使废渣中Zn、Cd、Cu、As的浸出浓度分别降至≤15、<3、<5、≤10 μg·L−1,η均高于98%,其中η(As)均高于95%,明显优于CaO,这可能与MgO比表面积大,表面具有镁氧基(Mg―O)活泼反应基团,对重金属的吸附、沉淀等作用更强有关[23]。
由图4可知,CaO、MgO均可作为高效稳定剂,Ca2+、Mg2+虽均会与砷酸根离子形成复杂的络合沉淀物,但CaO的添加会使As浸出反升,4种元素综合稳定效应为0.4% MgO(η=98.90%)>0.4% CaO(η=93.48%)。
2.2 无机-无机组合处理
硫钙、磷钙和硫磷组合处理结果如图5所示。各类组合对废渣中的Zn、Cd和Cu的稳定化效果均较为明显,稳定率均高于95%,硫化物的添加有助于As的稳定,磷酸盐过量则对As的稳定化效果产生一定的拮抗作用。由图5(a)可知,0.4% CaO与3种不同投加比的Na2S·9H2O组合处理后,4种元素浸出浓度均达标,Zn、Cd和Cu的稳定率均高于99%,Zn浸出浓度均低于0.07 mg·L−1,Cd和Cu浸出浓度均低于0.01 mg·L−1,随Na2S·9H2O投加量的增加,η(As)明显增强。与单一Na2S·9H2O相比,各处理的硫钙组合均提高了Zn和Cd的稳定效果,其中,η(Zn)从45.53%~88.97%升至99.80%以上,说明CaO的加入有助于增强阳离子金属的稳定效果,协同增效作用明显,但同时一定程度上降低了As的稳定效果,η(As)从96.36%~99.11%降至41.70%~97.17%。在Na2S·9H2O组合比为0.61%和1.21%时,As的稳定化效果反而不如2种药剂单独使用时的效果,不仅未达到协同稳定As的效应,反而弱化了As的稳定效果,这与王浩等[3]的研究结果类似。但CaO与最高投加比(2.42%)Na2S·9H2O组合时,稳定As的能力依次为Na2S·9H2O>硫钙组合>CaO,组合处理对4种元素的综合稳定率η高达99.08%,这也说明硫钙组合稳定As的效果优于CaO,究其原因主要为2个方面:一方面,低量CaO利于降低As的浸出;另一方面,存在碱性条件使As流动性增强的风险[24-25],而硫钙组合对As的稳定化水平则随Na2S·9H2O投加量的增加而增强,在控制低剂量CaO防止因过量而活化As的同时,可使As2S3等更稳定的沉淀作用逐渐占优,As的稳定性增强。综上所述,硫钙组合有利于增强单一Na2S·9H2O对Zn的稳定效果,高剂量时对As的稳定效果优于单一CaO,且对4种元素的综合稳定效果均强于单一处理时的效果,组合处理中先加CaO也大大减少了H2S的逸出,有助于环境友好性,因此,与CaO和Na2S·9H2O单一处理相比,硫钙组合更占优势。
由图5(b)可知,低剂量的0.4% CaO+0.32% (NH4)2HPO4使4种金属元素的稳定率均高于95%,η高达98.13%,优于单一CaO(η 93.48%)和单一(NH4)2HPO4(η 87.42%),与单一(NH4)2HPO4相比,组合处理进一步降低了Zn和As的浸出浓度,但随着组合处理中(NH4)2HPO4投加量的增加,η(As)明显降低,甚至为负值,0.4% CaO+1.28% (NH4)2HPO4处理组的η(As)降为−46.15%。磷钙组合主要弱化了单一(NH4)2HPO4对As的活化作用,CaO与低量(NH4)2HPO4组合更有助于4种金属元素的综合稳定。
考虑到Na2S·9H2O和磷酸盐分别对As和Zn稳定能力强的特点,硫磷复配后的稳定化效果如图5(c)所示。在投加1.2%~4.8%复配剂后,Zn、Cd和Cu的稳定率均高于96%,但As均被活化,η(As)为−171.66%~−23.08%,其中,控制药剂投加量为1.2%,4种金属元素均达标,η仅为68.07%,2.4%和4.8%处理后As浸出超标。硫磷组合剂对As活化性较强,须控制投加量≤1.2%。
3. 结论
1)单一硫化物和磷酸盐对重金属的稳定化结果表明,3种药剂中Na3PO4·12H2O对重金属的稳定效果最好,Na2S·9H2O对As的稳定效果最好,(NH4)2HPO4易对As产生活化作用,从药剂投加量角度考虑,与GB 8978-1996最高允许排放浓度相比,达标稳定4种金属元素综合稳定化效应次序依次为0.46% Na3PO4·12H2O(η 82.26%)>0.64% (NH4)2HPO4(η 87.42%)>2.42% Na2S·9H2O(η 96.36%),Na3PO4·12H2O最优。
2)单一钙基、镁基和无机配伍对重金属的稳定化结果表明,单一MgO(投加比0.4%时,η(Zn) 99.97%,η(Cd) 99.76%)或CaO处理即可使之达标,硫钙组合提高了单一Na2S·9H2O对Zn、Cd的稳定化水平,η(Zn)从45.53%~88.97%升至99.80%以上,0.4% CaO与2.42% Na2S·9H2O的组合对As稳定效果优于单一CaO,对4种金属元素η高达99.08%,优于单一处理;磷钙和磷硫配伍中磷酸盐过量易活化As。各处理满足4种金属元素均达标的综合稳定效应大小顺序依次为0.4% MgO>0.4% CaO>(0.4% CaO+0.61% Na2S·9H2O)>(0.4% CaO+0.32% (NH4)2HPO4)>1.2% (Na2S·9H2O∶(NH4)2HPO4∶Na3PO4·12H2O=2∶1∶3)。
3) MgO、Na3PO4·12H2O、硫钙组合为优选稳定剂,CaO过量和组合剂中磷酸盐过量均不利于As的同时稳定。
-
表 1 常规无机水质指标变化特征
Table 1. Regular characteristics of dyeing wastewater samples
采样点Sampling spots pH 色度Colourity 浊度/NTUTurbidity 电导率/(μs·cm−1) Conductivity TDS/(mg·L−1) TP/(mg·L−1) PO3−4-P NH+4-N NO−2-N NO−3-N TN/(mg·L−1) S1 7.32±0.35 150±38 1.78±1.63 6563±926 4177±877 0.19±0.08 0.09±0.01 0.92±0.76 0.15±0.09 5.27±2.29 13.07±5.44 S2 6.95±0.28 45±10 0.54±0.16 6698±904 4564±1078 0.02±0.01 0.01±0.00 1.60±2.01 0.13±0.01 4.49±1.34 12.62±1.78 S3 6.91±0.48 5±0 0.12±0.01 350±68 193±84 0 0 0.16±0.10 0.01 0.71±0.17 1.59±0.46 S4 7.48±0.14 55±6 0.57±0.50 11340±2855 8343±3134 0.17±0.01 0.05±0.03 3.10±4.13 0.18±0.02 6.70±1.50 19.28±4.45 S5 7.52±0.33 78±22 0.77±0.44 8573±883 5312±1092 0.05±0.01 0.02±0.003 2.35±2.99 0.13±0.05 5.23±1.27 14.44±2.86 注:所有数据为4次采样的均值±标准差. Note: All data are calculated from results of four sampling events (mean ± standard deviation). 表 2 主要阴阳离子组成
Table 2. Anions and cations composition in dyeing wastewater samples
采样点 Na/(mg·L−1) Al/(mg·L−1) Ca/(mg·L−1) K/(mg·L−1) Fe/(mg·L−1) Mg/(mg·L−1) Si/(mg·L−1) Cl−/(mg·L−1) SO42-/(mg·L−1) HCO3−/(mg·L−1) S1 1264.43±182.47 4.41±7.07 38.86±7.86 24.81±0.24 2.62±2.35 0.72±1.09 13.01±1.83 827.69±508.72 788.09±473.08 516.59±225.20 S2 1259.94±191.28 13.62±11.10 52.17±1.79 24.92±0.77 2.45±0.55 0.80±1.05 12.00±1.33 980.28±381.53 849.49±334.98 381.25±196.88 S3 46.36±3.51 2.23±2.50 0.30±0.40 49.88±17.44 1.09±1.23 0.00±0.00 2.43±1.67 45.77±29.09 12.24±8.32 31.24±7.30 S4 2255.83±615.78 23.69±7.41 100.16±33.36 0.75±0.41 5.12±3.56 5.51±4.15 20.53±4.09 1612.68±852.62 1441.73±750.81 619.53±199.94 S5 1639.95±154.72 5.83±2.34 65.62±7.14 33.99±6.14 1.44±1.44 2.09±1.79 15.85±0.86 1348.12±192.41 1319.25±90.82 520.41±206.15 注:所有数据为4次采样的均值±标准差. Note: All data are calculated from results of four sampling events (mean ± standard deviation). 表 3 各水样荧光峰位置与荧光峰强度
Table 3. The locations and intensities of fluorescence peaks of dyeing wastewater samples
采样点Sampling spots A峰(色氨酸类)Peak A (Tryptophan-like) B峰(酪氨酸类)Peak A (Tyrosine -like) C峰(富里酸类)Peak A (Fulvic-like) D峰(SMP类)Peak A (SMP -like) Ex/Em 荧光峰强度Fluorescence intensity Ex/Em 荧光峰强度Fluorescence intensity Ex/Em 荧光峰强度Fluorescence intensity Ex/Em 荧光峰强度Fluorescence intensity S1 235/340 1243 225/285 598 255/455 1047 280/320 850 S2 235/345 690 225/285 544 — — 275/320 536 S3 235/335 263 225/285 230 — — — — S4 235/340 1960 225/285 1402 — — 280/320 1487 S5 235/345 809 225/285 661 — — 275/320 548 -
[1] YIN H, GUO H F, QIU P W, et al. Case analysis on textile wastewater subjected to combined physicochemical–biological treatment and ozonation [J]. Desalination and Water Treatment, 2017, 66: 140-148. doi: 10.5004/dwt.2017.1619 [2] YASEEN D A, SCHOLZ M. Textile dye wastewater characteristics and constituents of synthetic effluents: A critical review [J]. International Journal of Environmental Science and Technology, 2019, 16(2): 1193-1226. doi: 10.1007/s13762-018-2130-z [3] TAN Y J, SUN L J, LI B T, et al. Fouling characteristics and fouling control of reverse osmosis membranes for desalination of dyeing wastewater with high chemical oxygen demand [J]. Desalination, 2017, 419: 1-7. doi: 10.1016/j.desal.2017.04.029 [4] HOLKAR C R, JADHAV A J, PINJARI D V, et al. A critical review on textile wastewater treatments: Possible approaches [J]. Journal of Environmental Management, 2016, 182: 351-366. [5] DONKADOKULA N Y, KOLA A K, NAZ I, et al. A review on advanced physico-chemical and biological textile dye wastewater treatment techniques [J]. Reviews in Environmental Science and Bio/Technology, 2020, 19(3): 543-560. doi: 10.1007/s11157-020-09543-z [6] AL-KDASI A, IDRIS A, SAED K, et al. Treatment of textile wastewater by advanced oxidation processes- A review [J]. Global Nest Journal, 2004, 6(1): 222-230. [7] GUNAWAN F M, MANGINDAAN D, KHOIRUDDIN K, et al. Nanofiltration membrane cross-linked by m-phenylenediamine for dye removal from textile wastewater [J]. Polymers for Advanced Technologies, 2019, 30(2): 360-367. doi: 10.1002/pat.4473 [8] 朱利杰, 范云双, 谢康, 等. 印染废水RO浓水水质分析 [J]. 中国环境科学, 2019, 39(11): 4646-4652. doi: 10.3969/j.issn.1000-6923.2019.11.020 ZHU L J, FAN Y S, XIE K, et al. Analysis of reverse osmosis concentrates from printing and dyeing wastewater treatment [J]. China Environmental Science, 2019, 39(11): 4646-4652(in Chinese). doi: 10.3969/j.issn.1000-6923.2019.11.020
[9] ZHENG L, WANG X J, WANG X Z. Reuse of reverse osmosis concentrate in textile and dyeing industry by combined process of persulfate oxidation and lime-soda softening [J]. Journal of Cleaner Production, 2015, 108: 525-533. doi: 10.1016/j.jclepro.2015.09.027 [10] 朱薛妍, 郑银萍, 俞三传, 等. 浸没式纳滤印染废水深度处理研究 [J]. 水处理技术, 2013, 39(4): 93-96. doi: 10.3969/j.issn.1000-3770.2013.04.023 ZHU X Y, ZHENG Y P, YU S C, et al. Treatment of dyeing and printing wastewater through submerged nanofiltration [J]. Technology of Water Treatment, 2013, 39(4): 93-96(in Chinese). doi: 10.3969/j.issn.1000-3770.2013.04.023
[11] 谭玉珺, 张泽田, 吴乾元, 等. 印染废水反渗透脱盐系统运行性能及膜污堵特性 [J]. 环境科学, 2018, 39(5): 2249-2255. doi: 10.13227/j.hjkx.201707020 TAN Y J, ZHANG Z T, WU Q Y, et al. Operating characteristics and fouling characteristics of a RO membrane system for desalination of dyeing wastewater [J]. Environmental Science, 2018, 39(5): 2249-2255(in Chinese). doi: 10.13227/j.hjkx.201707020
[12] JIANG S X, LI Y N, LADEWIG B P. A review of reverse osmosis membrane fouling and control strategies [J]. Science of the Total Environment, 2017, 595: 567-583. doi: 10.1016/j.scitotenv.2017.03.235 [13] LIN W C, ZHANG Y T, LI D Y, et al. Roles and performance enhancement of feed spacer in spiral wound membrane modules for water treatment: A 20-year review on research evolvement [J]. Water Research, 2021, 198: 117146. doi: 10.1016/j.watres.2021.117146 [14] 国家环境保护总局. 水和废水监测分析方法(第四版)[M]. 北京: 中国环境科学出版社, 2002. State Environmental Protection Administration. Water and Wastewater Monitoring and Analysis Methods-4th Edition [M]. Beijing: China Environment Science Press, 2002(in Chinese).
[15] LOWRY O, ROSEBROUGH N, FARR A L, et al. Protein measurement with the folin phenol reagent [J]. Journal of Biological Chemistry, 1951, 193(1): 265-275. doi: 10.1016/S0021-9258(19)52451-6 [16] VAKONDIOS N, KOUKOURAKI E E, DIAMADOPOULOS E. Effluent organic matter (EfOM) characterization by simultaneous measurement of proteins and humic matter [J]. Water Research, 2014, 63: 62-70. doi: 10.1016/j.watres.2014.06.011 [17] DUBOIS M, GILLES K A, HAMILTON J K, et al. Colorimetric method for determination of sugars and related substances [J]. Analytical Chemistry, 1956, 28(3): 350-356. doi: 10.1021/ac60111a017 [18] ATES N, KITIS M, YETIS U. Formation of chlorination by-products in waters with low SUVA—correlations with SUVA and differential UV spectroscopy [J]. Water Research, 2007, 41(18): 4139-4148. doi: 10.1016/j.watres.2007.05.042 [19] LAWAETZ A J, STEDMON C A. Fluorescence intensity calibration using the Raman scatter peak of water [J]. Applied Spectroscopy, 2009, 63(8): 936-940. doi: 10.1366/000370209788964548 [20] 陈诗雨, 李燕, 李爱民. 溶解性有机物研究中三维荧光光谱分析的应用 [J]. 环境科学与技术, 2015, 38(5): 64-68,73. CHEN S Y, LI Y, LI A M. Application of three-dimensional fluorescence spectroscopy in the study of dissolved organic matter [J]. Environmental Science & Technology, 2015, 38(5): 64-68,73(in Chinese).
[21] MENDOZA W G, WEISS E L, SCHIEBER B, et al. Controls on the distribution of fluorescent dissolved organic matter during an under-ice algal bloom in the western Arctic Ocean [J]. Global Biogeochemical Cycles, 2017, 31(7): 1118-1140. doi: 10.1002/2016GB005569 [22] COBLE P G. Marine optical biogeochemistry: The chemistry of ocean color [J]. Chemical Reviews, 2007, 107(2): 402-418. doi: 10.1021/cr050350+ [23] 章君. 膜技术在印染废水深度处理中的工程应用及效益分析[D]. 杭州: 浙江工商大学, 2015. ZHANG J. The engineering application with membrane technology in advanced treatment of printing and dyeing wastewater & its benefit analysis[D]. Hangzhou: Zhejiang Gongshang University, 2015(in Chinese).
[24] PANDEY A, SINGH P, IYENGAR L. Bacterial decolorization and degradation of azo dyes [J]. International Biodeterioration & Biodegradation, 2007, 59(2): 73-84. [25] SARATALE R G, SARATALE G D, CHANG J S, et al. Bacterial decolorization and degradation of azo dyes: A review [J]. Journal of the Taiwan Institute of Chemical Engineers, 2011, 42(1): 138-157. doi: 10.1016/j.jtice.2010.06.006 [26] 张庆华, 陈国涛, 冯琳琳, 等. 混合菌群对偶氮染料的脱色降解研究进展 [J]. 应用与环境生物学报, 2020, 26(2): 469-478. doi: 10.19675/j.cnki.1006-687x.2019.06009 ZHANG Q H, CHEN G T, FENG L L, et al. Research progress on microbial decolorization and degradation of azo dyes [J]. Chinese Journal of Applied and Environmental Biology, 2020, 26(2): 469-478(in Chinese). doi: 10.19675/j.cnki.1006-687x.2019.06009
[27] CHEN S Q, LI M, MA X Y, et al. Influence of inorganic ions on degradation capability of Fe-based metallic glass towards dyeing wastewater remediation [J]. Chemosphere, 2021, 264: 128392. doi: 10.1016/j.chemosphere.2020.128392 [28] PANDEY S R, JEGATHEESAN V, BASKARAN K, et al. Fouling in reverse osmosis (RO) membrane in water recovery from secondary effluent: A review [J]. Reviews in Environmental Science and Bio/Technology, 2012, 11(2): 125-145. doi: 10.1007/s11157-012-9272-0 [29] OZBEY-UNAL B, OMWENE P I, YAGCIOGLU M, et al. Treatment of organized industrial zone wastewater by microfiltration/reverse osmosis membrane process for water recovery: From lab to pilot scale [J]. Journal of Water Process Engineering, 2020, 38: 101646. doi: 10.1016/j.jwpe.2020.101646 [30] RAHARDIANTO A, GAO J B, GABELICH C J, et al. High recovery membrane desalting of low-salinity brackish water: Integration of accelerated precipitation softening with membrane RO [J]. Journal of Membrane Science, 2007, 289(1/2): 123-137. [31] LI Y F, LI M C, XIAO K, et al. Reverse osmosis membrane autopsy in coal chemical wastewater treatment: Evidences of spatially heterogeneous fouling and organic-inorganic synergistic effect [J]. Journal of Cleaner Production, 2020, 246: 118964. doi: 10.1016/j.jclepro.2019.118964 [32] OSBURN C L, OVIEDO-VARGAS D, BARNETT E, et al. Regional groundwater and storms are hydrologic controls on the quality and export of dissolved organic matter in two tropical rainforest streams, Costa rica [J]. Journal of Geophysical Research:Biogeosciences, 2018, 123(3): 850-866. doi: 10.1002/2017JG003960 [33] CHEN M L, JUNG J, LEE Y K, et al. Surface accumulation of low molecular weight dissolved organic matter in surface waters and horizontal off-shelf spreading of nutrients and humic-like fluorescence in the Chukchi Sea of the Arctic Ocean [J]. Science of the Total Environment, 2018, 639: 624-632. doi: 10.1016/j.scitotenv.2018.05.205 [34] WÜNSCH U J, MURPHY K R, STEDMON C A. The one-sample PARAFAC approach reveals molecular size distributions of fluorescent components in dissolved organic matter [J]. Environmental Science & Technology, 2017, 51(20): 11900-11908. [35] 蔡华玲, 宁寻安, 陈晓晖, 等. 印染外排废水中溶解性有机质的荧光特性 [J]. 环境化学, 2021, 40(5): 1592-1601. doi: 10.7524/j.issn.0254-6108.2020010402 CAI H L, NING X N, CHEN X H, et al. Fluorescence characteristics of dissolved organic matter in textile-dyeing effluents [J]. Environmental Chemistry, 2021, 40(5): 1592-1601(in Chinese). doi: 10.7524/j.issn.0254-6108.2020010402
[36] CHEN W, WESTERHOFF P, LEENHEER J A, et al. Fluorescence excitation-emission matrix regional integration to quantify spectra for dissolved organic matter [J]. Environmental Science & Technology, 2003, 37(24): 5701-5710. [37] 薛菲菲. 珠江三角洲典型印染废水处理设施出水中残余有机物及其生物毒性研究[D]. 广州: 广东工业大学, 2019 XUE F F. Residual micro organic pollutants and their biotoxicity of the effluent from the textile wastewater treatment plants at Pearl River Delta[D]. Guangzhou: Guangdong University of Technology. 2019(in Chinese).
[38] 郑璐, 许光明, 陈俊, 等. 污水厂深度处理过程中有机物三维荧光光谱的平行因子分析研究 [J]. 环境科学与管理, 2015, 40(10): 89-91,96. doi: 10.3969/j.issn.1673-1212.2015.10.021 ZHENG L, XU G M, CHEN J, et al. Organic matter removal based on 3D fluorescence spectroscopy and parafac analysis during wastewater advanced treatment process [J]. Environmental Science and Management, 2015, 40(10): 89-91,96(in Chinese). doi: 10.3969/j.issn.1673-1212.2015.10.021
[39] 王士峰. 印染废水三维荧光特征的研究[D]. 绵阳: 西南科技大学, 2015. WANG S F. Study on the three-dimensional fluorescence characteristics of the printing and dyeing wastewater[D]. Mianyang: Southwest University of Science and Technology, 2015(in Chinese).
[40] 黄振荣, 程澄, 汤久凯, 等. 印染废水处理厂排水中有机物的荧光方法表征 [J]. 光谱学与光谱分析, 2017, 37(10): 3118-3121. HUANG Z R, CHENG C, TANG J K, et al. Characterization of organic matters in the effluent of dyeing and printing wastewater treatment plants with fluorescence method [J]. Spectroscopy and Spectral Analysis, 2017, 37(10): 3118-3121(in Chinese).
[41] CHENG C, WU J, YOU L D, et al. Novel insights into variation of dissolved organic matter during textile wastewater treatment by fluorescence excitation emission matrix [J]. Chemical Engineering Journal, 2018, 335: 13-21. doi: 10.1016/j.cej.2017.10.059 [42] ALTENBACH B, GIGER W. Determination of benzene- and naphthalenesulfonates in wastewater by solid-phase extraction with graphitized carbon black and ion-pair liquid chromatography with UV detection [J]. Analytical Chemistry, 1995, 67(14): 2325-2333. doi: 10.1021/ac00110a002 [43] ZHENG L B, YU D W, WANG G, et al. Characteristics and formation mechanism of membrane fouling in a full-scale RO wastewater reclamation process: Membrane autopsy and fouling characterization [J]. Journal of Membrane Science, 2018, 563: 843-856. doi: 10.1016/j.memsci.2018.06.043 [44] 孙伟, 胡泓, 赵茜, 等. 达里诺尔湖水体DOM荧光特征及其来源解析 [J]. 环境科学研究, 2020, 33(9): 2084-2093. doi: 10.13198/j.issn.1001-6929.2020.03.26 SUN W, HU H, ZHAO Q, et al. Fluorescence characteristics and source analysis of dissolved organic matter in Dali-nor Lake [J]. Research of Environmental Sciences, 2020, 33(9): 2084-2093(in Chinese). doi: 10.13198/j.issn.1001-6929.2020.03.26
[45] MCKNIGHT D M, BOYER E W, WESTERHOFF P K, et al. Spectrofluorometric characterization of dissolved organic matter for indication of precursor organic material and aromaticity [J]. Limnology and Oceanography, 2001, 46(1): 38-48. doi: 10.4319/lo.2001.46.1.0038 [46] HUGUET A, BALMANN H R D, PARLANTI E. Fluorescence spectroscopy applied to the optimisation of a desalting step by electrodialysis for the characterisation of marine organic matter [J]. Journal of Membrane Science, 2009, 326(1): 186-196. doi: 10.1016/j.memsci.2008.09.051 [47] HUGUET A, VACHER L, RELEXANS S, et al. Properties of fluorescent dissolved organic matter in the Gironde Estuary [J]. Organic Geochemistry, 2009, 40(6): 706-719. doi: 10.1016/j.orggeochem.2009.03.002 [48] ZHANG Y L, LIU M L, QIN B Q, et al. Photochemical degradation of chromophoric-dissolved organic matter exposed to simulated UV-B and natural solar radiation [J]. Hydrobiologia, 2009, 627(1): 159-168. doi: 10.1007/s10750-009-9722-z [49] CORY R M, MILLER M P, MCKNIGHT D M, et al. Effect of instrument-specific response on the analysis of fulvic acid fluorescence spectra [J]. Limnology and Oceanography:Methods, 2010, 8(2): 67-78. doi: 10.4319/lom.2010.8.67 [50] JIANG T, SKYLLBERG U, BJÖRN E, et al. Characteristics of dissolved organic matter (DOM) and relationship with dissolved mercury in Xiaoqing River-Laizhou Bay Estuary, Bohai Sea, China [J]. Environmental Pollution, 2017, 223: 19-30. doi: 10.1016/j.envpol.2016.12.006 -












 DownLoad:
DownLoad: