-
海洋生物污损是指海洋微生物、海洋植物和海洋动物大量附着生长在船舶、人工岛等海洋人工设施上,并对人类的经济活动造成危害的现象[1]。为防止或减少船舶生物污损,一般对船舶采取防污损处理技术,即在船体接触海水的表面涂上含防污杀生剂的涂料[2-3]。这些防污杀生剂会在海水中缓慢而均匀释放出来,从而抑制海洋污损生物的附着和生长[4]。
有机锡化合物是20世纪世界范围内使用最多的防污杀生剂。然而,这类化合物在水环境中持久性很强并对多种海洋非靶标生物具有严重毒性影响,甚至会威胁人类的健康[5-7]。因此,国际海事组织通过了《国际控制船舶有害防污底系统公约》,从而禁止了有机锡的使用[6]。公约已于2008年9月17日正式生效,成为强制性标准[8]。尽管有机锡已经被禁止了十多年,但由于其在全球禁令之前被广泛用于船舶,海洋中大量累积该类物质,并能在海洋中持久存在。此外,一些老旧船舶仍有可能将该类物质释放到海洋环境中。因此,有机锡化合物对水体的污染以及对水生生态环境的危害仍然是全球关注的问题[6].
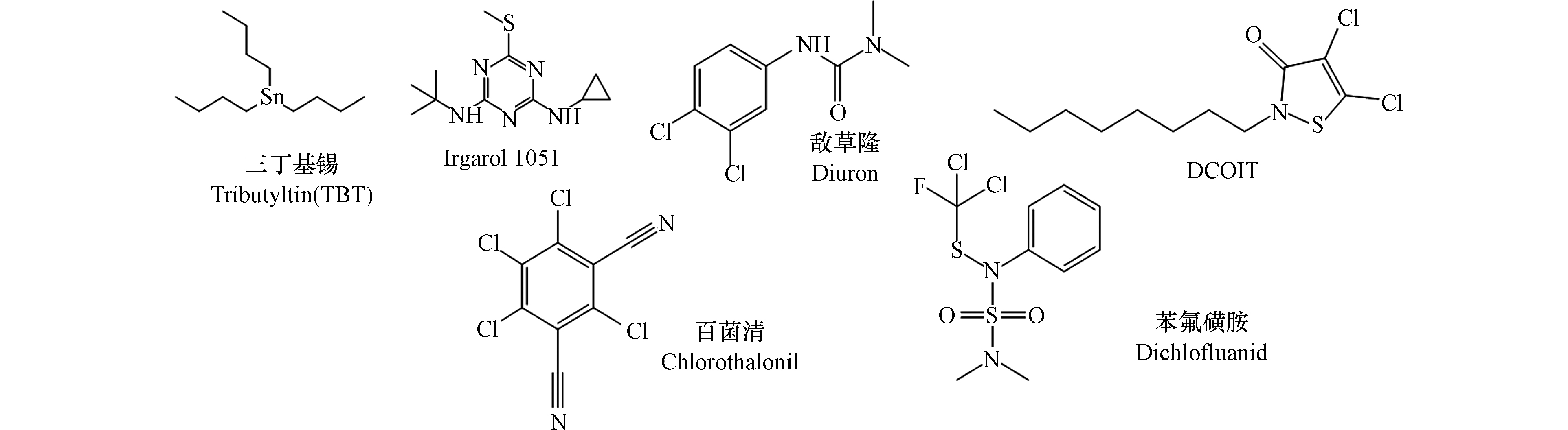
随着有机锡的禁用,新型的船舶防污杀生剂如Irgarol 1051、敌草隆(diuron)、DCOIT、百菌清(chlorothalonil)和苯氟磺氨(dichlofluanid)等[7, 9-13] 在全球范围内大量使用。而新型的船舶生物杀伤剂对非靶标水生生物的毒性问题仍然存在,已有研究证明Irgarol 1051和敌草隆即使在ng·L−1浓度水平上亦会对海洋生物造成危害[14-15],这给海洋环境带来了新的威胁。因此,美国和欧盟已禁止将Irgarol 1051和敌草隆用于船舶防污涂料。
近年来随着航海事业的迅速发展,船舶防污杀生剂被广泛使用已导致在世界范围内的许多港口和码头的水、沉积物和生物体中都检出了较高的浓度。然而,水环境中微量船舶防污杀生剂浓度就会在许多非靶标生物中引发毒性影响[1, 16],并对当地的水生生态环境产生潜在的危害。基于此,本文全面综述了三丁基锡、Irgarol 1051、敌草隆、DCOIT、百菌清和苯氟磺胺等6种典型船舶防污杀生剂的基本性质、对非靶标水生生物的毒性效应、以及在全球水环境中的分布特征。
-
有机锡防污剂主要包括二丁基锡(DBT)、三丁基锡(TBT)和三苯基锡(TPT)等[17]。其中,TBT是应用最为广泛的船舶防污杀生剂,其使用可以追溯到上世纪60年代,至上世纪90年代后期,TBT的全球年产量高达4000吨[1]。海水中TBT降解的半衰期从6 d到4个月不等,而沉积物中的半衰期为1—4年甚至可以超过20年[18]。由于其疏水性强、半衰期长,易在沉积物中富集,会对海洋环境造成长期的潜在危害。在环境介质当中,三丁基锡可以通过脱去与锡原子相连的有机基团而变成毒性相对小的新有机锡化合物甚至无机锡化合物。降解过程通常是非生物降解和生物降解的综合作用的结果。
-
Irgarol 1051 (2-(Tert-butylamino)-4-cyclopropylamino)-6-(methylthio)-1,3,5- triazine) 是一种微溶、中等亲脂性的三嗪类除草剂,是最常被用于控制船体上的污垢的化合物之一[19]。Irgarol 1051 主要通过抑制光合生物体的光系统II(PSII)中的电子传递,从而防止船体表面水生植物生长[1]。Irgarol 1051主要溶解在海水中,沉积物中的含量较低。根据多介质逸度模型计算结果表明,约95%的Irgarol 1051存在于海水中,只有4.4%进入到沉积物中[19]。Irgarol 1051在海水中的半衰期约为100—350 d,沉积物约为100—200 d,在海水中主要进行生化和光化学降解,其降解产物主要包括M1 (2-methylthio-4-tert-butylamino-6-amino-s-triazine)、M2 (3-[4-tertbutylamino-6-methylthiol-s-triazin-2-ylamino]-propionaldehyde)和M3 (N'-di-tert-butyl-6-methylthiol-s-triazine-2,4- diamine)。
-
敌草隆(1-(3,4-Dichlorophenyl)-3,3-dimethylurea1)是一种广泛用作除草剂的苯基脲,自上世纪80年代以来一直用于船舶防污杀生剂。与Irgarol 1051相比,敌草隆除了会抑制PSII中的电子传递外,还会降低浮游和附生微藻的叶绿素a的水平[1]。敌草隆在海水中具有良好持久性和稳定性,其半衰期为30—365 d,而在沉积物中的持久性则相对较低约为14 d[20]。与Irgarol 1051相似,敌草隆主要存在于水体中,不易在沉积物中富集[21]。
-
DCOIT (4,5-Dichloro-2-n-octyl-3-(2H)-isothiazolin-3-one)是一种异噻唑啉酮,是一种广泛用作防污漆中的广谱增效生物杀伤剂[6]。因其在海洋环境中可快速降解,DCOIT在欧盟被批准作为新型生物杀灭产品,并在商业上得到推广。然而,近期研究证明DCOIT在全球不同地区水域环境下的降解速度并不相同,其海水半衰期在 1—13 d之间[6, 22],具体取决于环境条件,例如阳光、溶解氧或温度。DCOIT倾向于与沉积物强烈结合,海洋沉积物可以作为DCOIT的储存库[22]。DCOIT在天然海水中经历快速降解并与沉积物强烈结合,从而降低其生物利用度,从而降低其生物积累潜力[19]。
-
百菌清(2,4,5,6-Tetrachloroisophthalonitrile)是一种异黄酮类有机氯,在防污涂料中主要用作杀菌剂,涂料和粘结剂的防护剂[23]。在全球有机锡禁令后,百菌清在防污涂料中的使用量大大增加,其在水生系统中的降解途径为还原脱氯、氧化脱氯和水解[24]。百菌清对水体颗粒物和沉积物的亲和性较高,易在水体有机相中富集。微生物活动是海洋环境中百菌清降解的主要过程,其半衰期为1.8—8 d,在水中的持久性较低。
-
苯氟磺胺(n′-Dimethyl-n-phenylsulphamide)是一种杀真菌剂,主要用于防污涂料中的增强杀菌剂,是英国卫生和安全署批准的一种具有高水解降解化合物。苯氟磺胺半衰期较短,在海水中约为3—18 h,在水中高度不稳定会发生水解并迅速转化为DMSA[25]。在沉积物中半衰期小于10 h[26],因此不易沉积物中积累。
船舶防污杀生剂的理化性质如表1所示,6种典型的船舶防污杀生剂的结构图如图1所示。
-
TBT是美国环境保护署(US EPA)认定的金属有机内分泌干扰物,也是人为排放到水生环境中毒性最强的化学物质之一。研究发现TBT在极低的浓度时,对藻类、无脊椎动物、甲壳类动物和鱼类等水生生物都具有较高的毒性[5]。TBT可以防止藻类、异养微生物的复合混合物的光合作用和繁殖,对藻类和周丛植物的最大无影响浓度值(NOEC)分别为33 μg·L−1和8 μg·L−1[20]。TBT对长牡蛎和紫贻贝也有很高的毒性,在1 μg·L−1的剂量下暴露4—5 d,其幼虫的生长就会受到破坏[1]。TBT对许多甲壳类动物毒性也很大,例如拉帕耳虾、日本虎虾、锯齿白虾和大型水蚤等[27]。此外,有研究表明幼鱼对TBT非常敏感,通常在低浓度下ng·L−1就表现出严重毒性效应[28]。TBT可在一些水生生物体内积累,并造成腹足类物种的性逆转[29],对鲸类动物具有免疫抑制作用[30]、鱼类具有致肥效应[31]、双壳类贝壳具有致畸作用[32]。当人体皮肤接触TBT、或食用被TBT污染的鱼类,亦会对人类健康产生危害。人体暴露于TBT可能会产生细胞免疫、神经毒性、诱变性和致癌性,导致生殖和免疫系统疾病[17, 33-34]。
-
Irgarol 1051通过抑制光合作用和损害叶绿体内的电子传递而发挥其防污作用[35]。Irgarol 1051对藻类、无脊椎动物、甲壳类和鱼类都具有毒性影响。在对多种海藻的试验中,Irgarol 1051表现出非常显著的毒性,其中锯齿状岩藻对Irgarol 1051最不敏感,细毛壳藻最为敏感[36-37]。研究还调查了Irgarol 1051对幼虾和成虾的致死和亚致死效应,并在暴露后48 h观察到死亡现象。草虾幼体在3000 μg·L−1浓度组暴露96 h后死亡率为87%;成虾在24 h后死亡率为73%,且在72 h后全部死亡[38]。此外,Irgarol 1051对其他甲壳类动物也具有毒性效应[38]。Irgarol 1051对鱼类毒性效应相对较小,其中 杂色鲤鱼、白纹伊蚊、黑斑鱼、大腹鱼的致死浓度均大于1500 μg·L−1[29, 33]。
-
与Irgarol 1051相似,敌草隆对藻类、无脊椎动物、甲壳类动物和鱼类的都具有一定的毒性影响。在4000 μg·L−1浓度组暴露96 h后,棘突线虫的存活率降低50%[39]。绿藻的96 h的EC50值范围为2.4—37 μg·L−1。此外,敌草隆对薄毛藻、月桂藻、小球藻等海藻亦具有较强的毒性[36-37]。敌草隆对敌草隆对鱼类的肝脏、肾脏和性腺也有一定的影响。与绿藻相比,鱼类暴露在较高浓度的敌草隆时其毒性阈值相对较低。总体而言,敌草隆对水生生物毒性的敏感性为藻类>无脊椎动物>甲壳类动物>鱼类。人体接触敌草隆后,会刺激皮肤和眼睛,并产生致癌作用。此外,敌草隆会导致肝脏和脾脏的异常,导致血液中高铁血红蛋白的形成,进而影响体内激素的运输和释放[40]。
-
DCOIT的毒性在不同营养级的物种中均有详细的记录,包括细菌、浮游植物、浮游动物和鱼类等[22]。研究发现DCOIT对多种水生生物具有急性毒性,但是对其进行的大量毒理学试验中没有观察到慢性毒性作用[41]。DCOIT对海胆胚胎的最低观察效应浓度LOEC值为10−8 μg·L−1,NOEC值为10−9 μg·L−1。海洋青鳉鱼慢性暴露28 d后,其LOEC值为0.76 μg·L−1,并具有内分泌干扰作用[22]。一种海洋动物鹦鹉螺在添加DCOIT的沉积物中暴露14 d后,其LC50值为110 ng·g−1干重[42]。DCOIT易通过细胞膜和细胞壁扩散,导致细胞氧化应激和细胞内Ca2+水平升高,并使细胞坏死。此外,DCOIT可以破坏细胞骨架成分的组装和组织,从而改变吞噬细胞的细胞形态,极大地阻碍了吞噬活性[22]。
-
百菌清被报道对甲壳动物、无脊椎动物和鱼类等几种海洋物种具有高度有效性。有研究表明,百菌清对褐背飞虱、肠毒虱和贻贝等3种海洋无脊椎动物早期发育阶段存在较强的毒性作用,会导致胚胎毒性、幼虫抑制和死亡[43]。许多甲壳动物物种亦被研究发现对百菌清非常敏感,如细尾对虾、双口对虾、杜比亚西足亚目和大型水蚤等。此外,对于草虾的3个生活阶段(胚胎、幼虫、成虫)的研究表明,草虾胚胎对百菌清的敏感性最低,成虫次之,而幼虫的敏感性最高[44]。鱼类对百菌清也表现出很高的敏感性,暴露96 h后的LC50可达8.2—76 μg·L−1[45-46]。此外,百菌清还具有遗传毒性,影响雄性蜇鱼的生育能力[47]。
-
苯氟磺胺对水体无脊椎动物具有毒性,但其毒性作用相较于其他5种船舶防污杀生剂则相对较小[48]。暴露于苯氟磺胺后,石斑鱼胚胎发育的EC10和EC50值分别为277 μg·L−1和627 μg·L−1,幼鱼生长的EC10值为206 μg·L−1;蓝鳍金线虫胚胎发育的EC10值为49 μg·L−1,EC50值为74 μg·L−1[43]。然而,因为苯氟磺胺在水中可迅速发生水解,它的毒性可能是由其降解产物引起的[49]。
结合有关船舶防污杀生剂对非靶标水生生物毒性的现有数据来看,TBT是一种毒性较强的内分泌干扰化合物,并且会影响所有营养级别的生物体,Irgarol 1051、敌草隆、百菌清和DCOIT是对生产者最具毒性的杀生剂,而苯氟磺胺对海洋非靶标生物毒性相对较低。其对海洋非靶标生物的敏感性可归纳为TBT> Irgarol 1051>DCOIT>敌草隆>百菌清>苯氟磺胺。船舶防污杀生剂对各种非靶标海洋生物的具体毒性数据见表2。现有的大多数研究数据是指基于致死终点和生产者生长抑制的短期接触,缺乏长期接触的慢性毒性数据,而且全球范围内鲜有关于船舶防污杀生剂沉积物毒性数据,以及多种海洋防污杀伤剂的联合毒性的研究。
-
由于船舶防污杀生剂的广泛使用,在船舶航行、停泊和清洁期间,其表面的船舶防污杀生剂与水体都有直接接触,这些化合物会直接渗出到水环境中。近年来船舶防污杀生剂已在全球沿海地区的水体、沉积物和生物体中被广泛检测到。
-
国际海事组织于2008年通过了《控制船舶有害防污底系统国际公约》禁止了TBT的使用,因此在许多海洋区域水体的TBT浓度呈下降的趋势,但其造成的污染及危害仍不可忽视。近15年有关水体中TBT的报道主要集中在亚洲和欧洲沿海港口,如亚洲韩国[11, 64]、印度[65]、沙特[66]以及中国[67]等沿海。全球水体中TBT检测到的最高浓度是在亚洲沙特沿海地区,达到了1900 ng·L−1。我国水体中TBT污染也比较严重,其中在中国三峡库区最高浓度为222 ng·L−1。欧洲的南亚得里亚海[68- 69]、波兰格丁尼亚港[8]、西班牙北海岸以及希腊海岸[70]均被广泛检测到,浓度范围为0.3—380 ng·L−1。在南美洲的巴西沿海[71]、非洲的开普敦港口[72]、尼日利亚拉各斯港[8]都检测到了TBT。在全球范围内,亚洲沿海水域是TBT污染最为严重的区域。
在目前使用的新型船舶防污杀生剂中,Irgarol 1051是全世界水体环境中检出频率最高的,在全球各大洲均有被检出。在北美洲的美国佛罗里达州[73]、加利福尼亚游艇码头[74]、夏威夷瓦胡岛[75]、以及加拿大沿海[76]都检测到Irgarol 1051,浓度范围为1—254 ng·L−1。在南美洲的巴西沿海[10, 77-78]、巴拿马沿海[79]以及桑托斯港[80]都有检出,浓度范围在1.3—4800 ng·L−1,污染程度高于北美地区。欧洲地区,Irgarol 1051在意大利那不勒斯海湾的浓度范围为0.8—134.5 ng·L−1[13],在丹麦码头的浓度为1.78 ng·L−1[81],在法国布列塔尼的浓度为14 ng·L−1[82],瑞典沿海水域的浓度为0.2—8.0 ng·L−1[83]。在欧洲沿海进行了较多的检测,检测的浓度偏低,污染程度相比与南美洲和北美洲较轻,这主要与欧洲制定的相关控制措施有关。在亚洲地区,Irgarol 1051在伊朗波斯湾布什尔浓度范围为1.0—63.4 ng·L−1[84],在韩国沿海最高浓度为318.5 ng·L−1[64],在日本沿海不同的水域的检出浓度存在较大的空间差异,其中日本神户港的浓度达到了1850 ng·L−1,日本内海的浓度仅为2 ng·L−1 [85]。在中国水域鲜有针对Irgarol 1051污染现状的研究,仅2005年在中国香港沿海水域有报道,浓度范围为100—1600 ng·L−1,显示了极高的污染水平[86]。在非洲地区,坦桑尼亚桑给巴尔岛Irgarol 1051的浓度范围为1.35—15.44 ng·L−1[80]。在大洋洲地区,澳大利亚沿海水域Irgarol 1051的浓度范围为5—6 ng·L−1,污染水平相比较与其他几个大洲较低[87]。
敌草隆是另一种在全球水体中检出率较高的船舶防污杀生剂。在北美洲的加利福尼亚游艇码头浓度范围为2—68 ng·L−1[74]。在南美洲地区,巴西圣马科斯湾的最高浓度为22 ng·L−1[10],巴拿马沿海的浓度范围为2.7—70 ng·L−1[79]。在欧洲地区,意大利[88]、阿尔巴尼亚[88]、西班牙[89]等国家的沿海地区均有检测到敌草隆。在大洋洲澳大利亚河口中的浓度范围为1—96.7 ng·L−1[87]。在亚洲地区,敌草隆的污染较为严重,韩国沿海的最高浓度为1360 ng·L−1[90],日本港口的最高浓度为2120 ng·L−1。此外在马来西亚和伊朗布什尔也有敌草隆的检出,浓度范围分别为1—285 ng·L−1[21]和4.8—29 ng·L−1[84]。当前,中国则缺乏对敌草隆的关注,仅对长江三角洲水体有报道,浓度范围为1.7—107 ng·L−1[91]。
DCOIT、百菌清、苯氟磺胺也是应用广泛的船舶防污杀生剂。DCOIT在中国[91]、韩国[11, 90]、日本[92]、西班牙[93]、德国[70]、英国[94]、和丹麦[95]等国家的海水中均有不同程度的检出。其中,在西班牙加泰罗尼亚码头的浓度高达 3700 ng·L−1;在丹麦沿海的最高浓度283 ng·L−1;在中国长江三角洲的浓度范围为13.7—226 ng·L−1,污染程度高于其他国家。百菌清和苯氟磺胺仅在卢森堡[96]、韩国[64]、泰国[97]、加拿大[98]、希腊[70]和日本[92]的沿海水域有报道。其中,在日本沿海的污染最为严重,百菌清和苯氟磺胺的最高值分别达到了1380 ng·L−1和760 ng·L−1。
现阶段的新型船舶防污杀生剂的浓度数据缺乏变化趋势的研究,多为散点式,缺乏系统性。总体而言,码头、造船厂和船舶航线所在区域是船舶防污杀生剂污染较为关注的区域,并检测到了较高浓度的污染物,而其他水域的研究则想对较少。基于空间分布情况,有关水环境中船舶防污杀生剂的报道多集中于亚洲和欧洲地区,美洲地区次之,而非洲和大洋洲报道相对较少。其中,亚洲地区的韩国和日本因具有较发达的造船业,因此该地区水域船舶防污杀生剂的污染也最为严重;南美洲地区的污染也较为严重,可能跟缺乏针这些物种管理控制措施有关;欧洲、北美洲因有严格的环境法规,因此污染情况则相对较轻。基于时间分布情况,近15年来水体中船舶防污杀生剂污染程度呈现出下降的趋势。水体中船舶防污杀生剂的浓度没有显著的季节变化趋势,但与船运旺季和航行船只数量有关。此外,水体中船舶防污杀生剂污染还受水动力学的影响,在水交换速率低、稀释能力有限的地方污染浓度较高。近海水体中船舶防污杀生剂的分布特征见表3。
-
沉积物作为水环境的一个重要组成部分,对有机污染物在水环境中的迁移、转化、归趋和生态效应等环境行为起着重要作用。进入水环境中的船舶防污杀生剂会吸附在水体的颗粒物上后通过沉降作用进入到沉积物中。由于不同船舶防污杀生剂的有机碳吸附系数(Koc)不同,其在沉积物中的富集能力亦有不同。
有机锡化合物的疏水性较高,因此TBT易富集在沉积物中,在全球许多沿海区域沉积物中都有一定的积累。南美洲地区的巴拿马[9]、委内瑞拉[122]、秘鲁[123]、智利[124]和巴西[71]沿海,这些地区的TBT的污染较为严重,一些研究报告称TBT在当地部分地区仍在被使用[71]。在亚洲的中国[103, 125-126]、韩国[11, 127]、印度[65]和日本[91]沿海地区被广泛检出,最高浓度为2304 ng·g−1在韩国造船厂沉积物中检测到。在TBT禁止使用后,亚洲沿海沉积物中的TBT呈现下降的趋势,例如在沙特沿海沉积物中TBT污染状况下降了48倍[66]。在中国,王晓萌等[128]评估了2012—2013年我国近岸12个疏浚物倾倒区的沉积物中有机锡污染情况,浓度范围在4.83—1334 ng·g−1干重。与我国其他海域相比,疏浚物倾倒区的沉积物中TBT污染程度较为严重,部分倾倒区的TBT污染呈现显著的历史性输入特征。在欧洲的德国[69]、法国[129]、葡萄牙[130]、德国[69]等国家的沿海沉积物中均有检出,浓度范围为8.3—4738 ng·g−1干重,污染程度比亚洲地区高。在非洲地区,南非开普敦港口沉积物中的浓度范围为10—829 ng·g−1干重[72],尼日利亚格拉斯港雨季的浓度范围为0.01—7.56 ng·g−1干重,旱季为ND.—2.98 ng·g−1干重[8]。
lrgarol 1051 在水体中主要以自由溶解态的形式存在,不易吸附到水体颗粒相和沉降物中。然而,lrgarol 1051在全球水体沉积物中也有一定程度检出,如美国佛罗里达州0.3—8.9 ng·g−1干重[74],巴西桑托斯港1.0—89.7 ng·g−1干重[10]、巴拿马沿海0.08—2.8 ng·g−1干重[79]、丹麦码头20 ng·g−1干重[81]、韩国沿海0.02—230 ng·g−1干重[127]、印度尼西亚沿海61—76 ng·g−1干重[131]。
敌草隆在全球各大洲沉积物中均有检出。其中,美国和澳大利亚的污染程度相对较轻,浓度范围分别为<0.3—4.2 ng·g−1干重[74]和0.3—11.0 ng·g−1干重[132];南美洲和亚洲的污染程度较为严重,在阿根廷巴伊亚布兰卡河口浓度高达7800 ng·g−1干重[33],而日本沿海的平均浓度达到了897 ng·g−1干重[113]。在欧洲地区,敌草隆在意大利南亚得里亚海[68]地区的浓度为1.9—583 ng·g−1干重。
沉积物中DCOIT、百菌清、苯氟磺胺研究相对较少,这主要是由于这3种化合物的半衰期较短,在水环境中容易被降解。韩国[127]、印度尼西亚[131]、日本[92, 116]、丹麦[95]、美国[74]、巴拿马[9]和巴西[133]等国家的沿海沉积物中均发现了DCOIT,最高浓度为281 ng·g−1 干重。百菌清在韩国(1.2—1065 ng·g−1干重)和巴西(<0.1—9.2 ng·g−1干重)的港口沉积物中有报道[127]。在印度尼西亚沿海(<0.4—80 ng·g−1干重)[131]、巴西桑托斯港(< 2.1—16 ng·g−1干重)[133]和丹麦码头((1.98 ± 1.77) ng·g−1干重)[81]的沉积物中发现了苯氟磺胺的存在。
尽管水体中TBT的浓度呈现逐年下降的趋势,并低于新型船舶防污杀生剂的浓度,但其在沉积物中浓度仍显著高于新型船舶防污杀生剂。这主要是由于TBT在沉积物中降解缓慢,并具有较低水溶性和较高的沉积物/水分配系数,水体中的TBT也倾向于吸附在沉积物中。沉积物中有机物含量会影响其对船舶防污杀生剂的吸附能力,从而影响船舶防污杀生剂的分配[134]。然而,港口的疏浚工作会扰动富集在沉积物中的污染物质,使其重新进入水体中,造成二次污染。近海沉积物船舶防污杀生剂分布特征见表4。
-
TBT可通过生物富集作用,在水生生物体中进行积累,其对软体动物和鱼类的生物富集因子(BCF)高达7000[145],对生物体产生了不利影响。目前已经有大量文献报道了TBT在水生生物体内的分布情况,在多种类型的水生生物体中均有检出,如海草(46 ng·g−1,按Sn算)[9]、腹足类动物(190 ng·g−1,按Sn算)[124]、鱼类(53—330 ng·g−1,按Sn算)[71, 105]。阿拉伯湾捕获的8种重要经济鱼类中均有检测到TBT,表明这种化合物能在鱼体组织中的进行累积,并对人群造成潜在的危害[66]。处于食物链高营养级的哺乳动物和鸟类也受到有机锡污染的威胁,在海洋哺乳动物和海鸟的组织、器官屮均有检出,其中,韩国水域中小须鲸和海豚体内三丁基锡浓度分别为15.7—297 ng·g−1和59—412 ng·g−1[30]。
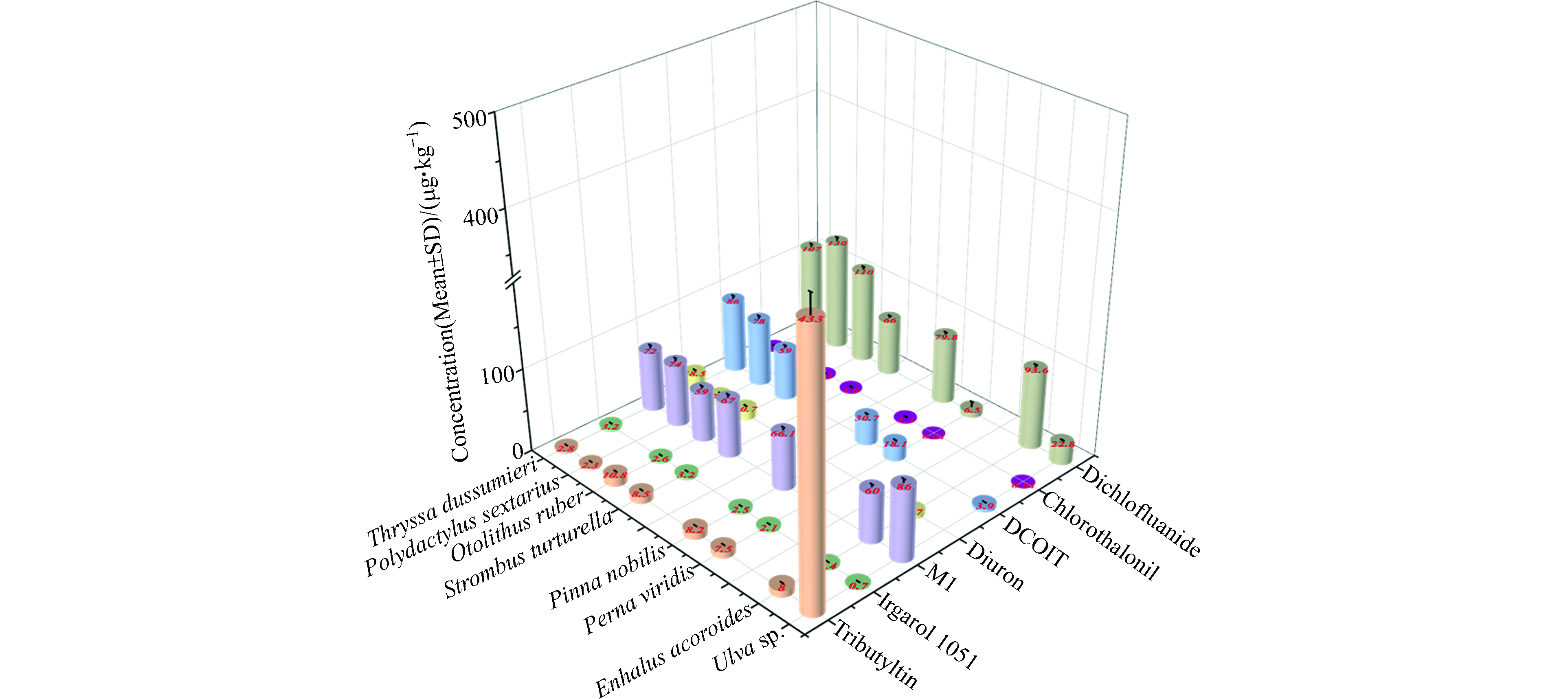
相比较与TBT,其余5种新型船舶防污杀生剂在水生生物体中的报道相对较少,缺乏完整食物链的研究。仅对2020年在马来西亚部分海洋生物进行了系统研究,研究物种包括石莼(Ulva sp.)和海菖蒲(Enhalus acoroides)等水生植物,翡翠贻贝(Perna viridis)和大蛤蜊海丝(Pinna nobilis)等贝类,以及大菱鲆(Strombus turturella)、红牙䱛(Otolithus ruber)、六指马鲅(Polydactylus sextarius)和杜氏棱鳀(Thryssa dussumieri)等鱼类[145]。其中,Irgarol 1051和百菌清在8种物种中的浓度分布较为均一,浓度范围分别为0.7—3.2 g·kg−1和1.2—1.6 g·kg−1;而Irgarol 1051的代谢产物(2-methylthio-4-tert-butylamino-6-amino-s-triazine,M1)浓度是其母体化合物的10—100倍,这主要是由于M1较强的生物富集能力和较长的环境半衰期,易在水生生物体中积累[73, 145]。敌草隆在海菖蒲的浓度仅为4.7 g·kg−1,其在鱼体中的浓度相对较高,达到了7.7—18.5 g·kg−1。DCOIT和苯氟磺胺在水生生物体中呈现出一定的生物放大特征,即在低营养级的水生植物中污染程度相对较低,在高营养级的贝类和鱼类中具有相对较高的浓度。
TBT是在生物体中检测到的最高的船舶防污杀生剂,其次是敌草隆和Irgarol 1051。化合物的正辛醇-水分配系数(KOW)与其生物体的富集浓度之间并无显著的相关性。与生物体一样,水体和沉积物中检测到的最高浓度的船舶防污杀生剂也是TBT、Irgarol 1051和敌草隆,表明影响生物体中的船舶防污杀生剂浓度的主要是其在水环境中的污染程度。船舶防污杀生剂不进会对水生生物造成影响,并会饮食摄入进而对人体健康产生危害。海洋生物体中船舶防污杀生剂的分布特征见图2。
-
船舶防污杀生剂在保护船舶、人工岛等海洋人工设施上免受生物污损的同时,会对非靶标水生生物产生严重的毒性作用。其中,TBT会影响所有营养级的水生生物。近15年,全球水环境中广泛检测出了船舶防污杀生剂,其污染程度整体呈逐年下降的趋势,但在港口、造船厂、渔港和码头等船舶活动频繁地区污染情况仍较为严重。进入到水环境中的船舶防污杀生剂会对水体生态环境造成了长期的影响。
近年来,我国水运发展迅速,发达的修造船业务和繁忙水上运输活动造成船舶防污杀生剂的释放量也随之增加。然而,我国对这些污染物的研究起步较晚,但已有研究表明有机锡化合物已经对我国海洋环境产生严重的影响,而新型的船舶防污杀生剂在中国水环境中尚未开展系统的研究,其生态风险亦未知。因此对我国沿海和内河水环境中船舶防污杀生剂的环境分布情况调查、环境行为的研究、以及对水生生物的毒性效应和环境风险的评价是非常重要和必要的。
因此在今后的研究中,建议把重点放在:(1)分布特征研究。当前环境分布研究是断点式的缺乏周期性和系统性。因此需要开发不同环境介质中快速船舶防污杀生剂分析方法;周期性的对重点水域进行全面的船舶防污杀生剂污染程度调查,并分析其时空变化趋势和主要的污染来源。(2)环境行为研究。排放到水体中的船舶防污杀生剂并不是稳定不变的,在物理、化学以及生物作用下会进行一系列的迁移转化过程。目前国内外针对这类污染物在水体环境中迁移转化过程尚未开展系统的研究。(3)毒理学研究。船舶防污杀生剂及其降解产物对多种水生生物的毒性效应仍然是未知的,并且排放到水体中的船舶防污杀生剂并不是单一的,往往是多种化合物的联合使用,也可能不同的船只在同一地点排放出不同的多种船舶防污杀生剂,这就需要探究这些污染物对水生生物的联合毒性作用。(4)生态风险评价研究。当前国内外针对这类污染物的生态风险评价研究还在起步阶段缺乏系统性,建议结合环境化学、环境毒理学、生态风险评估模型等学科的理论与方法,更加深入的研究不同水域环境中船舶防污杀生剂生态风险水平。(5)保护措施研究。研究已表明船舶防污杀生剂对海洋环境产生了威胁因此应加强对船舶防污杀生剂污染源的管理,制定合理保护措施消减其进入环境的总量;研究和发展船舶防污杀生剂以及其降解产物的处理技术;同时研究对生态环境危害小的新型绿色船舶防污涂料替代品。
船舶防污杀生剂的水生生物毒性和近海海域分布特征研究进展
Occurrence and aquatic organism toxicity of antifouling biocides in coastal environment
-
摘要: 近年来,有关船舶防污杀生剂在水环境中的分布及生态影响已经引起了人们的广泛关注。本文对2005—2020年间全球水环境中6种典型船舶防污杀生剂(三丁基锡、Irgarol 1051、DCOIT、敌草隆、百菌清、苯氟磺胺)研究工作进行了调研和梳理,总结了典型船舶防污杀生剂的基本性质、非靶标水生生物的毒性效应、以及在水体环境中的分布特征。毒性作用结果显示,三丁基锡(TBT)是一种毒性很强的内分泌干扰化合物,会影响所有营养级的水生生物,并可能通过饮食摄入受污染的海产品进而对人体健康产生影响。Irgarol 1051、敌草隆、百菌清和DCOIT仅对生产者具有较强的毒性,而苯氟磺胺对水生生物的毒性则相对较小。对水生生物毒性的敏感性可归纳为TBT > Irgarol 1051 > DCOIT > 敌草隆 > 百菌清 > 苯氟磺胺。水环境中主要的船舶防污杀生剂为TBT、Irgarol 1051和敌草隆。亚洲和南美洲沿海地区的污染最为严重,并主要集中在港口、造船厂、渔港和码头等船舶活动频繁地区。尽管TBT已经禁止使用了10多年,但在韩国造船厂附近的沉积物中浓度高达2304 ng·g−1。在水环境中和选定的海洋生物中检测到较高的船舶防污杀生剂证明了这些化合物在航运系统的广泛应用,并对海洋生态系统造成威胁。因此,未来的工作中需要对船舶防污杀生剂进行持续的调查并结合各地区水生生物毒性特性,进行系统的生态风险评价,为制定切实可行的海洋生态环境保护措施提供科学依据。Abstract: In recent years, the distribution and ecological effects of marine antifouling biocides in the aquatic environment have attracted a lot of attention. In this paper, we investigated and reviewed six typical marine antifouling biocides in the global water environment during 2005—2020, and summarized the basic properties, toxic effects on non-target aquatic organisms, and distribution characteristics of typical marine antifouling biocides in the water environment. The toxicological effects showed that tributyltin (TBT) is a highly toxic endocrine-disrupting compound that affects aquatic organisms at all trophic levels and may affect human health through dietary intake of contaminated seafood. Irgarol 1051, diuron, chlorothalonil and DCOIT are only highly toxic to producers, while dichlofluanid is relatively less toxic to aquatic organisms. The sensitivity of toxicity to aquatic organisms can be summarized as TBT > Irgarol 1051 > DCOIT > diuron > chlorothalonil > dichlofluanid. The major marine antifouling biocides in the aquatic environment are TBT, Irgarol 1051 and diuron. The pollutions in Asia and South America are most serious, especilly in the ports, shipyards, and docks with frequently ship activities. Although TBT has been banned for more than 10 years, the level in the shipyards sediment were as high as 2304 ng·g−1 in Korea. The detection of high levels of ship antifouling biocides in the aquatic environment and in selected marine organisms attests to the widespread use of these compounds in shipping systems and the threat they pose to marine ecosystems. Therefore, future work is needed to conduct a systematic ecological risk evaluation by continuously investigating ship antifouling biocides and combining them with the toxicity characteristics of aquatic organisms in each region, so as to provide a scientific basis for developing practical marine ecological protection measures.
-
Key words:
- antifouling biocides /
- toxic effect /
- environmental distribution
-
海洋生物污损是指海洋微生物、海洋植物和海洋动物大量附着生长在船舶、人工岛等海洋人工设施上,并对人类的经济活动造成危害的现象[1]。为防止或减少船舶生物污损,一般对船舶采取防污损处理技术,即在船体接触海水的表面涂上含防污杀生剂的涂料[2-3]。这些防污杀生剂会在海水中缓慢而均匀释放出来,从而抑制海洋污损生物的附着和生长[4]。
有机锡化合物是20世纪世界范围内使用最多的防污杀生剂。然而,这类化合物在水环境中持久性很强并对多种海洋非靶标生物具有严重毒性影响,甚至会威胁人类的健康[5-7]。因此,国际海事组织通过了《国际控制船舶有害防污底系统公约》,从而禁止了有机锡的使用[6]。公约已于2008年9月17日正式生效,成为强制性标准[8]。尽管有机锡已经被禁止了十多年,但由于其在全球禁令之前被广泛用于船舶,海洋中大量累积该类物质,并能在海洋中持久存在。此外,一些老旧船舶仍有可能将该类物质释放到海洋环境中。因此,有机锡化合物对水体的污染以及对水生生态环境的危害仍然是全球关注的问题[6].
随着有机锡的禁用,新型的船舶防污杀生剂如Irgarol 1051、敌草隆(diuron)、DCOIT、百菌清(chlorothalonil)和苯氟磺氨(dichlofluanid)等[7, 9-13] 在全球范围内大量使用。而新型的船舶生物杀伤剂对非靶标水生生物的毒性问题仍然存在,已有研究证明Irgarol 1051和敌草隆即使在ng·L−1浓度水平上亦会对海洋生物造成危害[14-15],这给海洋环境带来了新的威胁。因此,美国和欧盟已禁止将Irgarol 1051和敌草隆用于船舶防污涂料。
近年来随着航海事业的迅速发展,船舶防污杀生剂被广泛使用已导致在世界范围内的许多港口和码头的水、沉积物和生物体中都检出了较高的浓度。然而,水环境中微量船舶防污杀生剂浓度就会在许多非靶标生物中引发毒性影响[1, 16],并对当地的水生生态环境产生潜在的危害。基于此,本文全面综述了三丁基锡、Irgarol 1051、敌草隆、DCOIT、百菌清和苯氟磺胺等6种典型船舶防污杀生剂的基本性质、对非靶标水生生物的毒性效应、以及在全球水环境中的分布特征。
1. 船舶防污杀生剂的基本性质(Basic properties of marine antifouling biocides)
1.1 三丁基锡
有机锡防污剂主要包括二丁基锡(DBT)、三丁基锡(TBT)和三苯基锡(TPT)等[17]。其中,TBT是应用最为广泛的船舶防污杀生剂,其使用可以追溯到上世纪60年代,至上世纪90年代后期,TBT的全球年产量高达4000吨[1]。海水中TBT降解的半衰期从6 d到4个月不等,而沉积物中的半衰期为1—4年甚至可以超过20年[18]。由于其疏水性强、半衰期长,易在沉积物中富集,会对海洋环境造成长期的潜在危害。在环境介质当中,三丁基锡可以通过脱去与锡原子相连的有机基团而变成毒性相对小的新有机锡化合物甚至无机锡化合物。降解过程通常是非生物降解和生物降解的综合作用的结果。
1.2 Irgarol 1051
Irgarol 1051 (2-(Tert-butylamino)-4-cyclopropylamino)-6-(methylthio)-1,3,5- triazine) 是一种微溶、中等亲脂性的三嗪类除草剂,是最常被用于控制船体上的污垢的化合物之一[19]。Irgarol 1051 主要通过抑制光合生物体的光系统II(PSII)中的电子传递,从而防止船体表面水生植物生长[1]。Irgarol 1051主要溶解在海水中,沉积物中的含量较低。根据多介质逸度模型计算结果表明,约95%的Irgarol 1051存在于海水中,只有4.4%进入到沉积物中[19]。Irgarol 1051在海水中的半衰期约为100—350 d,沉积物约为100—200 d,在海水中主要进行生化和光化学降解,其降解产物主要包括M1 (2-methylthio-4-tert-butylamino-6-amino-s-triazine)、M2 (3-[4-tertbutylamino-6-methylthiol-s-triazin-2-ylamino]-propionaldehyde)和M3 (N'-di-tert-butyl-6-methylthiol-s-triazine-2,4- diamine)。
1.3 敌草隆
敌草隆(1-(3,4-Dichlorophenyl)-3,3-dimethylurea1)是一种广泛用作除草剂的苯基脲,自上世纪80年代以来一直用于船舶防污杀生剂。与Irgarol 1051相比,敌草隆除了会抑制PSII中的电子传递外,还会降低浮游和附生微藻的叶绿素a的水平[1]。敌草隆在海水中具有良好持久性和稳定性,其半衰期为30—365 d,而在沉积物中的持久性则相对较低约为14 d[20]。与Irgarol 1051相似,敌草隆主要存在于水体中,不易在沉积物中富集[21]。
1.4 DCOIT
DCOIT (4,5-Dichloro-2-n-octyl-3-(2H)-isothiazolin-3-one)是一种异噻唑啉酮,是一种广泛用作防污漆中的广谱增效生物杀伤剂[6]。因其在海洋环境中可快速降解,DCOIT在欧盟被批准作为新型生物杀灭产品,并在商业上得到推广。然而,近期研究证明DCOIT在全球不同地区水域环境下的降解速度并不相同,其海水半衰期在 1—13 d之间[6, 22],具体取决于环境条件,例如阳光、溶解氧或温度。DCOIT倾向于与沉积物强烈结合,海洋沉积物可以作为DCOIT的储存库[22]。DCOIT在天然海水中经历快速降解并与沉积物强烈结合,从而降低其生物利用度,从而降低其生物积累潜力[19]。
1.5 百菌清
百菌清(2,4,5,6-Tetrachloroisophthalonitrile)是一种异黄酮类有机氯,在防污涂料中主要用作杀菌剂,涂料和粘结剂的防护剂[23]。在全球有机锡禁令后,百菌清在防污涂料中的使用量大大增加,其在水生系统中的降解途径为还原脱氯、氧化脱氯和水解[24]。百菌清对水体颗粒物和沉积物的亲和性较高,易在水体有机相中富集。微生物活动是海洋环境中百菌清降解的主要过程,其半衰期为1.8—8 d,在水中的持久性较低。
1.6 苯氟磺胺
苯氟磺胺(n′-Dimethyl-n-phenylsulphamide)是一种杀真菌剂,主要用于防污涂料中的增强杀菌剂,是英国卫生和安全署批准的一种具有高水解降解化合物。苯氟磺胺半衰期较短,在海水中约为3—18 h,在水中高度不稳定会发生水解并迅速转化为DMSA[25]。在沉积物中半衰期小于10 h[26],因此不易沉积物中积累。
船舶防污杀生剂的理化性质如表1所示,6种典型的船舶防污杀生剂的结构图如图1所示。
表 1 船舶防污杀生剂的理化性质Table 1. Physicochemical properties of marine antifouling biocides船舶防污杀生剂Antifouling biocides CAS 分子量Relative molecular mass 分子式Molecular formula lg Kow 海水半衰期/d Half-lives in water 沉积物半衰期/d Half-Lives in sediment 溶解度/(mg·L−1)Solubility in water 毒性作用方式Toxicity mode of action 三丁基锡 1461-22-9 290.05 C12H27ClSn 4.1 6—120 440—1500 5—50 内分泌干扰效应 Irgarol 1051 28159-98-0 253.37 C11H19N5S 2.38—4.1 100—350 100—200 6.0—7.0 抑制PSⅡ电子传递 敌草隆 330-54-1 233.09 C9H10Cl2N2O 2.82 30—365 14 35—42 抑制PSⅡ电子传递 百菌清 1897-45-6 265.91 C8Cl4N2 2.64—4.4 1.8—8 <1—8 0.6—0.9 抑制线粒体电子转运 DCOIT 64359-81-5 282.23 C11H17Cl2NOS 2.85 1—13 <1 14 抑制电子传递 苯氟磺胺 1085-98-9 333.23 C9H11Cl2FN2O2S2 2.8—3.7 0.12—0.75 7 0.006—1.3 抑制线粒体电子转运 2. 船舶防污杀生剂的水生生物毒性(Aquatic toxicity of antifouling biocides)
2.1 三丁基锡的毒性
TBT是美国环境保护署(US EPA)认定的金属有机内分泌干扰物,也是人为排放到水生环境中毒性最强的化学物质之一。研究发现TBT在极低的浓度时,对藻类、无脊椎动物、甲壳类动物和鱼类等水生生物都具有较高的毒性[5]。TBT可以防止藻类、异养微生物的复合混合物的光合作用和繁殖,对藻类和周丛植物的最大无影响浓度值(NOEC)分别为33 μg·L−1和8 μg·L−1[20]。TBT对长牡蛎和紫贻贝也有很高的毒性,在1 μg·L−1的剂量下暴露4—5 d,其幼虫的生长就会受到破坏[1]。TBT对许多甲壳类动物毒性也很大,例如拉帕耳虾、日本虎虾、锯齿白虾和大型水蚤等[27]。此外,有研究表明幼鱼对TBT非常敏感,通常在低浓度下ng·L−1就表现出严重毒性效应[28]。TBT可在一些水生生物体内积累,并造成腹足类物种的性逆转[29],对鲸类动物具有免疫抑制作用[30]、鱼类具有致肥效应[31]、双壳类贝壳具有致畸作用[32]。当人体皮肤接触TBT、或食用被TBT污染的鱼类,亦会对人类健康产生危害。人体暴露于TBT可能会产生细胞免疫、神经毒性、诱变性和致癌性,导致生殖和免疫系统疾病[17, 33-34]。
2.2 Irgarol 1051的毒性
Irgarol 1051通过抑制光合作用和损害叶绿体内的电子传递而发挥其防污作用[35]。Irgarol 1051对藻类、无脊椎动物、甲壳类和鱼类都具有毒性影响。在对多种海藻的试验中,Irgarol 1051表现出非常显著的毒性,其中锯齿状岩藻对Irgarol 1051最不敏感,细毛壳藻最为敏感[36-37]。研究还调查了Irgarol 1051对幼虾和成虾的致死和亚致死效应,并在暴露后48 h观察到死亡现象。草虾幼体在3000 μg·L−1浓度组暴露96 h后死亡率为87%;成虾在24 h后死亡率为73%,且在72 h后全部死亡[38]。此外,Irgarol 1051对其他甲壳类动物也具有毒性效应[38]。Irgarol 1051对鱼类毒性效应相对较小,其中 杂色鲤鱼、白纹伊蚊、黑斑鱼、大腹鱼的致死浓度均大于1500 μg·L−1[29, 33]。
2.3 敌草隆的毒性
与Irgarol 1051相似,敌草隆对藻类、无脊椎动物、甲壳类动物和鱼类的都具有一定的毒性影响。在4000 μg·L−1浓度组暴露96 h后,棘突线虫的存活率降低50%[39]。绿藻的96 h的EC50值范围为2.4—37 μg·L−1。此外,敌草隆对薄毛藻、月桂藻、小球藻等海藻亦具有较强的毒性[36-37]。敌草隆对敌草隆对鱼类的肝脏、肾脏和性腺也有一定的影响。与绿藻相比,鱼类暴露在较高浓度的敌草隆时其毒性阈值相对较低。总体而言,敌草隆对水生生物毒性的敏感性为藻类>无脊椎动物>甲壳类动物>鱼类。人体接触敌草隆后,会刺激皮肤和眼睛,并产生致癌作用。此外,敌草隆会导致肝脏和脾脏的异常,导致血液中高铁血红蛋白的形成,进而影响体内激素的运输和释放[40]。
2.4 DCOIT的毒性
DCOIT的毒性在不同营养级的物种中均有详细的记录,包括细菌、浮游植物、浮游动物和鱼类等[22]。研究发现DCOIT对多种水生生物具有急性毒性,但是对其进行的大量毒理学试验中没有观察到慢性毒性作用[41]。DCOIT对海胆胚胎的最低观察效应浓度LOEC值为10−8 μg·L−1,NOEC值为10−9 μg·L−1。海洋青鳉鱼慢性暴露28 d后,其LOEC值为0.76 μg·L−1,并具有内分泌干扰作用[22]。一种海洋动物鹦鹉螺在添加DCOIT的沉积物中暴露14 d后,其LC50值为110 ng·g−1干重[42]。DCOIT易通过细胞膜和细胞壁扩散,导致细胞氧化应激和细胞内Ca2+水平升高,并使细胞坏死。此外,DCOIT可以破坏细胞骨架成分的组装和组织,从而改变吞噬细胞的细胞形态,极大地阻碍了吞噬活性[22]。
2.5 百菌清的毒性
百菌清被报道对甲壳动物、无脊椎动物和鱼类等几种海洋物种具有高度有效性。有研究表明,百菌清对褐背飞虱、肠毒虱和贻贝等3种海洋无脊椎动物早期发育阶段存在较强的毒性作用,会导致胚胎毒性、幼虫抑制和死亡[43]。许多甲壳动物物种亦被研究发现对百菌清非常敏感,如细尾对虾、双口对虾、杜比亚西足亚目和大型水蚤等。此外,对于草虾的3个生活阶段(胚胎、幼虫、成虫)的研究表明,草虾胚胎对百菌清的敏感性最低,成虫次之,而幼虫的敏感性最高[44]。鱼类对百菌清也表现出很高的敏感性,暴露96 h后的LC50可达8.2—76 μg·L−1[45-46]。此外,百菌清还具有遗传毒性,影响雄性蜇鱼的生育能力[47]。
2.6 苯氟磺胺的毒性
苯氟磺胺对水体无脊椎动物具有毒性,但其毒性作用相较于其他5种船舶防污杀生剂则相对较小[48]。暴露于苯氟磺胺后,石斑鱼胚胎发育的EC10和EC50值分别为277 μg·L−1和627 μg·L−1,幼鱼生长的EC10值为206 μg·L−1;蓝鳍金线虫胚胎发育的EC10值为49 μg·L−1,EC50值为74 μg·L−1[43]。然而,因为苯氟磺胺在水中可迅速发生水解,它的毒性可能是由其降解产物引起的[49]。
结合有关船舶防污杀生剂对非靶标水生生物毒性的现有数据来看,TBT是一种毒性较强的内分泌干扰化合物,并且会影响所有营养级别的生物体,Irgarol 1051、敌草隆、百菌清和DCOIT是对生产者最具毒性的杀生剂,而苯氟磺胺对海洋非靶标生物毒性相对较低。其对海洋非靶标生物的敏感性可归纳为TBT> Irgarol 1051>DCOIT>敌草隆>百菌清>苯氟磺胺。船舶防污杀生剂对各种非靶标海洋生物的具体毒性数据见表2。现有的大多数研究数据是指基于致死终点和生产者生长抑制的短期接触,缺乏长期接触的慢性毒性数据,而且全球范围内鲜有关于船舶防污杀生剂沉积物毒性数据,以及多种海洋防污杀伤剂的联合毒性的研究。
表 2 船舶防污杀生剂对非靶标海洋生物的毒性Table 2. Toxicity of marine antifouling biocides to non-target marine organisms船舶防污杀生剂Antifouling biocides 生物Organism 物种Species 毒性终点Endpoint 数值/(mg·L−1)Value 参考文献Reference 三丁基锡 藻类 班氏金针菜 48 h EC50 0.00016 [20] 藻类 卵囊藻 72 h EC50 0.003 [50] 无脊椎动物 普通海胆 48 h EC50 0.000309 [5] 无脊椎动物 玻璃海鞘 48 h EC50 0.0071 [5] 甲壳类动物 凶猛片钩虾 96 h LC50 0.0094 [51] 甲壳类动物 猛水蚤 96 h LC50 0.018 [51] 甲壳类动物 长臂虾 24 h LC50 0.0223 [43] 甲壳类动物 阿玛猛水蚤 96 h LC50 0.013 [43] 甲壳类动物 宽水蚤 72 h LC50 0.0006 [5] 甲壳类动物 溞 48 h EC50 0.00001 [52] 鱼类 青鳉 96 h LC50 0.025 [51] 鱼类 罗非鱼 96 h LC50 0.0038 [28] 鱼类 金头鲷 24 h LC50 0.0283 [28] Irgarol 1051 藻类 栅藻 48 h EC50 0.00113 [1] 藻类 角毛藻 72 h EC50 0.0011 [50] 无脊椎动物 普通海胆 48 h EC50 4.021 [43] 无脊椎动物 贻贝 48 h EC50 1.54 [41] 无脊椎动物 中间球海胆 48 h EC50 1.05 [38] 无脊椎动物 黄海胆 48 h EC50 0.4125 [48] 甲壳类动物 凶猛片钩虾 96 h LC50 1 [48] 甲壳类动物 草虾 96 h LC50 1.52 [38] 鱼类 虹鳟 96 h LC50 3.22 [53] 鱼类 大海鲢 96 h LC50 0.79 [53] 鱼类 美洲原银汉鱼 96 h LC50 3.5 [1] 鱼类 蓝鳃太阳鱼 96 h LC50 1.58 [1] 鱼类 斑马鱼 96 h LC50 2.6 [1] 鱼类 栅藻 96 h LC50 4 [1] 敌草隆 藻类 赫氏圆石藻 72 h EC50 0.002 [51] 藻类 等鞭金藻 96 h EC50 0.00373 [51] 藻类 骨条藻 96 h EC51 0.0103 [51] 藻类 多毛藻 96 h EC52 0.00042 [54] 藻类 杜氏藻 24 h EC50 0.035 [54] 刺胞类动物 海月水母 10 d LC50 0.00478 [15] 甲壳类动物 剑水蚤 96 h LC50 11 [15] 甲壳类动物 锯齿长臂虾 24 h LC50 3.04 [15] 甲壳类动物 卤虫 24 h LC50 10.3 [55] 甲壳类动物 卤虫 48 h LC50 6.14 [56] 甲壳类动物 卤虫 72 h LC50 2.76 [56] 甲壳类动物 汤氏纺锤水蚤 48 h LC50 1.08 [56] 甲壳类动物 糠虾期 96 h LC50 0.589 [1] 甲壳类动物 小甲壳动物 96 h LC50 7.06 [57] 棘皮动物 普通海胆 48 h EC50 2.39 [57] 棘皮动物 喇叭海胆 24 h EC50 3.33 [57] 鱼类 青鳉鱼 96 h LC50 7.8 [57] 鱼类 大海鲢 96 h LC50 0.89 [57] DCOIT 藻类 小球藻 24 h EC50 0.089 [58] 藻类 赫氏艾密里藻 72 h EC50 0.0004 [43] 藻类 三角褐指藻 72 h EC50 0.04 [43] 藻类 球等鞭金藻 72 h EC50 0.032 [59] 双壳类 鸟蛤 96 h LC50 0.325 [59] 甲壳类动物 红虾 96 h LC50 0.016 EPA 甲壳类动物 汤氏纺锤水蚤 72 h EC50 0.038 EPA 甲壳类动物 卤虫 48 h LC50 0.318 EPA 甲壳类动物 糠虾 96 h LC50 0.005 EPA 甲壳类动物 沙蟹 96 h LC50 1.31 [57] 甲壳类动物 剑水蚤 24 h EC50 0.03 [57] 甲壳类动物 长臂虾 96 h LC50 1.31 [57] 甲壳类动物 剑水蚤 24 h LC50 0.077 [57] 甲壳类动物 拟糠虾 96 h LC50 0.008 [41] 甲壳类动物 日本对虾 96 h LC50 0.013 [41] 棘皮动物 普通海胆 48 h LC50 0.025 [41] 棘皮动物 球海胆幼虫 48 h EC50 0.012 [41] 棘皮动物 黄海胆幼虫 53 h EC50 0.001 [60] 鱼类 红鳍东方鲀 96 h LC50 0.006 [60] 鱼类 杂色鱂 96 h LC50 0.023 [60] 鱼类 真鲷 96 h LC50 0.005 [60] 鱼类 底鳉 96 h LC50 0.005 [60] 百菌清 藻类 多毛藻 96 h IC50 0.064 [1] 环节动物 华美盘管虫 48 h LC50 0.012 [1] 甲壳类动物 指虾蛄 48 h LC50 0.56 [1] 甲壳类动物 指虾蛄 96 h LC50 0.14 [51] 甲壳类动物 指虾蛄 48 h EC50 0.17 [51] 甲壳类动物 剑水蚤 24 h LC50 0.098 [51] 甲壳类动物 剑水蚤 96 h LC50 0.091 [51] 甲壳类动物 凶猛片钩虾 96 h LC50 0.067 [51] 甲壳类动物 卤虫 48 h LC50 2.68 [51] 甲壳类动物 草虾 96 h LC50 0.153 [61] 甲壳类动物 草虾 48 h LC50 0.204 [62] 甲壳类动物 桃红对虾 96 h LC50 0.162 [14] 甲壳类动物 剑水蚤 24 h EC50 0.016 [62] 甲壳类动物 日本囊对虾 96 h LC50 0.29 [62] 鱼类 青鳉 96 h LC50 0.11 [55] 鱼类 杂色鱂 96 h LC50 0.032 [57] 鱼类 三刺鱼 96 h LC50 0.027 [57] 鱼类 黄尾平口石首鱼 48 h LC50 0.032 [57] 鱼类 底鳉 96 h LC50 0.06 [57] 苯氟磺胺 藻类 三角褐指藻 72 h EC50 0.193 [57] 藻类 糖海带 3 h NOEC 0.00001 [63] 甲壳类动物 汤氏纺锤水蚤 48 h LC10 0.017 [63] 甲壳类动物 卤虫 48 h LC50 154 [63] 棘皮动物 普通海胆 48 h EC50 0.282 EPA 棘皮动物 黄海胆 53 h EC50 0.177 [14] 鱼类 舌齿鲈 96 h EC50 0.015 [48] 鱼类 黄尾平口石首鱼 24 h LC50 0.032 [48] 3. 全球水环境中船舶防污杀生剂的分布特征(Distribution characteristics of antifouling biocides in the global water environment)
由于船舶防污杀生剂的广泛使用,在船舶航行、停泊和清洁期间,其表面的船舶防污杀生剂与水体都有直接接触,这些化合物会直接渗出到水环境中。近年来船舶防污杀生剂已在全球沿海地区的水体、沉积物和生物体中被广泛检测到。
3.1 船舶防污杀生剂在水体中的分布特征
国际海事组织于2008年通过了《控制船舶有害防污底系统国际公约》禁止了TBT的使用,因此在许多海洋区域水体的TBT浓度呈下降的趋势,但其造成的污染及危害仍不可忽视。近15年有关水体中TBT的报道主要集中在亚洲和欧洲沿海港口,如亚洲韩国[11, 64]、印度[65]、沙特[66]以及中国[67]等沿海。全球水体中TBT检测到的最高浓度是在亚洲沙特沿海地区,达到了1900 ng·L−1。我国水体中TBT污染也比较严重,其中在中国三峡库区最高浓度为222 ng·L−1。欧洲的南亚得里亚海[68- 69]、波兰格丁尼亚港[8]、西班牙北海岸以及希腊海岸[70]均被广泛检测到,浓度范围为0.3—380 ng·L−1。在南美洲的巴西沿海[71]、非洲的开普敦港口[72]、尼日利亚拉各斯港[8]都检测到了TBT。在全球范围内,亚洲沿海水域是TBT污染最为严重的区域。
在目前使用的新型船舶防污杀生剂中,Irgarol 1051是全世界水体环境中检出频率最高的,在全球各大洲均有被检出。在北美洲的美国佛罗里达州[73]、加利福尼亚游艇码头[74]、夏威夷瓦胡岛[75]、以及加拿大沿海[76]都检测到Irgarol 1051,浓度范围为1—254 ng·L−1。在南美洲的巴西沿海[10, 77-78]、巴拿马沿海[79]以及桑托斯港[80]都有检出,浓度范围在1.3—4800 ng·L−1,污染程度高于北美地区。欧洲地区,Irgarol 1051在意大利那不勒斯海湾的浓度范围为0.8—134.5 ng·L−1[13],在丹麦码头的浓度为1.78 ng·L−1[81],在法国布列塔尼的浓度为14 ng·L−1[82],瑞典沿海水域的浓度为0.2—8.0 ng·L−1[83]。在欧洲沿海进行了较多的检测,检测的浓度偏低,污染程度相比与南美洲和北美洲较轻,这主要与欧洲制定的相关控制措施有关。在亚洲地区,Irgarol 1051在伊朗波斯湾布什尔浓度范围为1.0—63.4 ng·L−1[84],在韩国沿海最高浓度为318.5 ng·L−1[64],在日本沿海不同的水域的检出浓度存在较大的空间差异,其中日本神户港的浓度达到了1850 ng·L−1,日本内海的浓度仅为2 ng·L−1 [85]。在中国水域鲜有针对Irgarol 1051污染现状的研究,仅2005年在中国香港沿海水域有报道,浓度范围为100—1600 ng·L−1,显示了极高的污染水平[86]。在非洲地区,坦桑尼亚桑给巴尔岛Irgarol 1051的浓度范围为1.35—15.44 ng·L−1[80]。在大洋洲地区,澳大利亚沿海水域Irgarol 1051的浓度范围为5—6 ng·L−1,污染水平相比较与其他几个大洲较低[87]。
敌草隆是另一种在全球水体中检出率较高的船舶防污杀生剂。在北美洲的加利福尼亚游艇码头浓度范围为2—68 ng·L−1[74]。在南美洲地区,巴西圣马科斯湾的最高浓度为22 ng·L−1[10],巴拿马沿海的浓度范围为2.7—70 ng·L−1[79]。在欧洲地区,意大利[88]、阿尔巴尼亚[88]、西班牙[89]等国家的沿海地区均有检测到敌草隆。在大洋洲澳大利亚河口中的浓度范围为1—96.7 ng·L−1[87]。在亚洲地区,敌草隆的污染较为严重,韩国沿海的最高浓度为1360 ng·L−1[90],日本港口的最高浓度为2120 ng·L−1。此外在马来西亚和伊朗布什尔也有敌草隆的检出,浓度范围分别为1—285 ng·L−1[21]和4.8—29 ng·L−1[84]。当前,中国则缺乏对敌草隆的关注,仅对长江三角洲水体有报道,浓度范围为1.7—107 ng·L−1[91]。
DCOIT、百菌清、苯氟磺胺也是应用广泛的船舶防污杀生剂。DCOIT在中国[91]、韩国[11, 90]、日本[92]、西班牙[93]、德国[70]、英国[94]、和丹麦[95]等国家的海水中均有不同程度的检出。其中,在西班牙加泰罗尼亚码头的浓度高达 3700 ng·L−1;在丹麦沿海的最高浓度283 ng·L−1;在中国长江三角洲的浓度范围为13.7—226 ng·L−1,污染程度高于其他国家。百菌清和苯氟磺胺仅在卢森堡[96]、韩国[64]、泰国[97]、加拿大[98]、希腊[70]和日本[92]的沿海水域有报道。其中,在日本沿海的污染最为严重,百菌清和苯氟磺胺的最高值分别达到了1380 ng·L−1和760 ng·L−1。
现阶段的新型船舶防污杀生剂的浓度数据缺乏变化趋势的研究,多为散点式,缺乏系统性。总体而言,码头、造船厂和船舶航线所在区域是船舶防污杀生剂污染较为关注的区域,并检测到了较高浓度的污染物,而其他水域的研究则想对较少。基于空间分布情况,有关水环境中船舶防污杀生剂的报道多集中于亚洲和欧洲地区,美洲地区次之,而非洲和大洋洲报道相对较少。其中,亚洲地区的韩国和日本因具有较发达的造船业,因此该地区水域船舶防污杀生剂的污染也最为严重;南美洲地区的污染也较为严重,可能跟缺乏针这些物种管理控制措施有关;欧洲、北美洲因有严格的环境法规,因此污染情况则相对较轻。基于时间分布情况,近15年来水体中船舶防污杀生剂污染程度呈现出下降的趋势。水体中船舶防污杀生剂的浓度没有显著的季节变化趋势,但与船运旺季和航行船只数量有关。此外,水体中船舶防污杀生剂污染还受水动力学的影响,在水交换速率低、稀释能力有限的地方污染浓度较高。近海水体中船舶防污杀生剂的分布特征见表3。
表 3 近海水体中船舶防污杀生剂的分布特征Table 3. Distribution characteristics of antifouling biocides in offshore waters船舶防污杀生剂Antifouling biocides 采样点Sampling point 采样时间Sampling time 浓度范围(中值)/ (ng·L−1)Concentration range (median) 参考文献Reference 三丁基锡 西班牙北海 2006 0.3—17.0 (4.6) [5] 印度果阿海 2006 0.5—126.2 (19) [99] 中国台湾高雄港 2006 90—480 (285) [67] 印度东海 2007 4—55 (14) [65] 日本沿海 2007 11.0—74.0 (15.5) [5] 威尼斯泻湖 2007 27.4—65.8 (46.6) [100] 希腊沿海 2007 ND.—191.9 (20.3) [70] 印度西海 2008 0.1—103 (16.2) [65] 中国厦门沿海 2008 5.28—384 (16) [101] 格丁尼亚港口 2009 12.9—110.2 (60.7) [102] 巴西南部沿海 2009—2010 ND.—175 (76) [71] 巴西东南部沿海 2009—2010 ND.—175 (54) [71] 韩国西部沿海 2010 ND.—63.4 (7.02) [64] 韩国东部沿海 2010 ND.—18.1 (3.69) [64] 韩国南部沿海 2010 ND.—51.2 (2.16) [64] 沙特沿海 2010 140—1900 [66] 中国台湾沿海 2011 16.8—273.3(52.7) [103] 意大利南亚得里亚海 2012 12—110 (50) [68] 阿尔巴尼亚沿海 2012 5—44 (24) [68] 波兰格丁尼亚港 2012 10.32—191.7 (25.9) [104] 南非开普敦港 2013 0.1—111.3 (9.7) [72] 中国台湾高雄港德国和波罗的海中部沿海 20142015 50.7—93.5 (11.6)2.5—380(95.5) [105][69] 韩国蔚山沿海 2014—2015 < 1—51.8 (13.3) [71] 韩国釜山沿海 2014—2015 < 1—69.9 (22) [71] 韩国光阳市沿海 2014—2015 3.25—24.6 (8.35) [71] 尼日利亚拉各斯港雨季 2016—2018 ND.—52.8 (7.8) [8] 尼日利亚拉各斯港旱季 2016—2018 ND.—6 (0.3) [8] 中国三峡 2018 21.0—222.1 (67) [106] 斯里兰卡沿海 2020 21.8—310.4 (166.1) [107] Irgarol 1051 中国香港沿海 2005 110—1620 (640) [86] 佛罗里达州 迈阿密码头 2008 20—66 (35) [73] 佛罗里达州 基拉戈港 2008 7—102 (35) [73] 美国加利福尼亚海域 2008 2—254 (67) [74] 澳大利亚沿海 2008 ND.—6 (5) [74] 西班牙大加那利亚海港 2008—2009 2.4—146.5 (51.6) [108] 巴西南部沿海 2009 <1.3—21 (4) [78] 韩国渔港 2009—2010 0.9—14.1 (4.0) [109] 意大利那不勒斯海湾 2010 0.8—134.5 (27.2) [13] 意大利拉斯佩齐亚湾 2010 <0.2—9.7 (4.1) [13] 韩国(马山、行岩、高贤)海湾 2010 ND.—11.5 (4.6) [90] 韩国西海 2010 ND.—63.37 (11.82) [64] 韩国东海 2010 ND.—51.15 (1.07) [64] 韩国南海 2010 ND.—18.06 (8.29) [64] 美国夏威夷瓦胡岛海域 2011 <17—283 (58.6) [75] 意大利南亚得里亚海 2012 0.6—16.1 (9.2) [68] 阿尔巴尼亚南亚得里亚海 2012 <0.2—9.3 (3.3) [68] 法国布列塔尼 2012 0—14 (6) [82] 马来西亚沿海 2012 ND.—2021 (471) [110] 日本库尔港 2012 ND.—740 (59) [85] 日本广岛港 2012 ND.—1050 (27) [85] 日本尾道港 2012 ND.—140 (22) [85] 日本神户港 2012 ND.—1850 (36) [85] 法国阿卡雄湾 2013 2.5—22 (12) [111] 波斯湾布什尔港口 2013 <1.0—63 (13.1) [84] 意大利阿普利亚海域 2014 0.5—18.1 (5.8) [88] 阿尔巴尼亚海域 2014 0.5—1.2 (0.8) [88] 巴西伊塔基港 2014 10—4800 (370) [77] 加拿大 五大湖流域 2016 ND.—3 (1) [76] 坦桑尼亚桑给巴尔岛海域 2016 1.35—15.44 (4.11) [80] 巴拿马沿海 2016 <0.3—5 (2.6) [79] 韩国光阳、釜山和蔚山海湾 2016 < 0.12—2.05 (0.15) [11] 日本濑户内海 2016 ND.—9 (3) [112] 韩国沿海 2016 ND.—318.5 (13.6) [23] 瑞典沿海水域 2017 <0.2—8.0 (0.25) [83] 巴西圣马科斯湾 2018 ND.—89.5 (15.6) [10] 丹麦码头 2019 ND.—1.78 (0.66) [81] 敌草隆 加利福尼亚海域 2008 <2—68 (35) [74] 西班牙大加那利岛港口 2008—2009 2.4—203.7 (74.4) [108] 巴西里约热内卢格兰德岛沿海 2009 <1.3—6 [10] 韩国海湾、渔港和港口 2009—2010 13—1360 (200) [90] 意大利那不勒斯湾 2010 <1.0—34.8 (22.2) [13] 意大利拉斯佩齐亚湾 2010 <1.0—28.2 (11.7) [13] 巴西伊塔基港 2011 <6—7800 [77] 比利时北海 2011 ND.—263 (108) [113] 布列塔尼维莱因湾 2012 ND.—88 (35) [82] 阿尔巴尼亚南亚得里亚海 2012 1.9—93.9 (33.3) [68] 意大利南亚得里亚海 2012 12.4—583.5 (68.9) [68] 日本库尔港 2012 ND.—1780 (59) [85] 日本广岛港 2012 ND.—1700 (23) [85] 日本尾道港 2012 ND.—2120 (61) [85] 日本神户港 2012 ND.—2030 (32) [85] 马来西亚半岛沿海 2012 1—285 (43) [21] 西班牙东北部沿海 2012 2.4—818 (99.7) [89] 法国阿卡雄湾 2013 5—40 (20) [111] 波斯湾布什尔港口 2013 ND.—29.1 (13.6) [84] 意大利阿普利亚沿海 2014 ND.—160.0 (15.8) [88] 阿尔巴尼亚沿海 2014 ND.—15.0 (7.4) [88] 澳大利亚布里斯班入海口 2015 1.0—56.8 (28.9) [87] 澳大利亚亚拉入海口 2015 1—38.8 (19.9) [87] 澳大利亚悉尼入海口 2015 15.1—96.7 (55.9) [87] 中国长三角洲 2016 1.7—107.2 (14.8) [91] 巴拿马沿海 2016 <2.7—70 (35.6) [79] 巴西圣马科斯湾 2016 <1.4—22 (7.5) [10] 韩国蔚山沿海 2016 2.41—90.4 (24.1) [11] 韩国釜山沿海 2016 < 0.31—41.9 (16.3) [11] 韩国光阳市沿海 2016 11.9—96.2 (29.4) [11] 日本西部濑户内海 2016 ND.—535 (307) [112] 韩国沿海 2016 ND.—55.2 (20) [23] 西班牙巴斯克沿海 2017 4—81 (14) [114] DCOIT 英国南安普顿 2000 <1 [94] 德国沿海 2002 ND.—49 [70] 日本大阪港 2002—2003 <0.3—4 [115] 丹麦沿海 2004 <5—283 [95] 日本迈祖鲁湾 2007 <0.3 [116] 韩国沿海 2009—2010 ND.—6 [90] 日本广岛湾 2010 0.1—53 (3.2) [117] 日本沿海 2010 1—3700 [117] 韩国蔚山沿海 2016 < 0.16 [11] 韩国釜山沿海 2016 < 0.16 [11] 韩国光阳市沿海 2016 <0.16—2.44 (0.17) [11] 韩国沿海 2016 ND.—144.5 (14.3) [90] 中国长江三角洲 2016 13.73—226.23 (66.05) [91] 百菌清 希腊沿海 2002 ND.—63 (30) [70] 加拿大沿海 2007 ND.—80 (40) [98] 日本沿海 2008 ND.—1.1 (0.5) [118] 卢森堡 2010 ND.—25 (11) [96] 韩国西部沿海 2010 (29.78) [64] 韩国东部沿海 2010 (8.49) [64] 韩国南部沿海 2010 (29.77) [64] 日本沿海 2010 ND.—1380 (68) [92] 韩国沿海 2013 ND.—318.5 (13.6) [23] 泰国北部沿海 2014 20—410 (105) [97] 苯氟磺胺 英国南海 2006 <1 [119] 南加州圣地亚哥 2006 <10 [120] 美国维尔京群岛 2006 <1 [121] 澳大利亚珀斯沿海 2008 <100 [13] 意大利沿海 2010 <1.0 [13] 卢森堡 2010 ND.—45 (7) [96] 韩国西部沿海 2010 (21.77) [64] 韩国东部沿海 2010 (8.5) [64] 韩国南部沿海 2010 (78) [64] 日本沿海 2010 1—760 (110) [13] 西班牙沿海 2013 <30 [13] 韩国沿海 2013 ND.—318.5 (13.6) [23] 澳大利亚东海岸 2015 <0.1 [87] 丹麦码头 2019 ND.—19.78 [81] ND.,未检出. ND., not detected. 3.2 船舶防污杀生剂在沉积物中的分布特征
沉积物作为水环境的一个重要组成部分,对有机污染物在水环境中的迁移、转化、归趋和生态效应等环境行为起着重要作用。进入水环境中的船舶防污杀生剂会吸附在水体的颗粒物上后通过沉降作用进入到沉积物中。由于不同船舶防污杀生剂的有机碳吸附系数(Koc)不同,其在沉积物中的富集能力亦有不同。
有机锡化合物的疏水性较高,因此TBT易富集在沉积物中,在全球许多沿海区域沉积物中都有一定的积累。南美洲地区的巴拿马[9]、委内瑞拉[122]、秘鲁[123]、智利[124]和巴西[71]沿海,这些地区的TBT的污染较为严重,一些研究报告称TBT在当地部分地区仍在被使用[71]。在亚洲的中国[103, 125-126]、韩国[11, 127]、印度[65]和日本[91]沿海地区被广泛检出,最高浓度为2304 ng·g−1在韩国造船厂沉积物中检测到。在TBT禁止使用后,亚洲沿海沉积物中的TBT呈现下降的趋势,例如在沙特沿海沉积物中TBT污染状况下降了48倍[66]。在中国,王晓萌等[128]评估了2012—2013年我国近岸12个疏浚物倾倒区的沉积物中有机锡污染情况,浓度范围在4.83—1334 ng·g−1干重。与我国其他海域相比,疏浚物倾倒区的沉积物中TBT污染程度较为严重,部分倾倒区的TBT污染呈现显著的历史性输入特征。在欧洲的德国[69]、法国[129]、葡萄牙[130]、德国[69]等国家的沿海沉积物中均有检出,浓度范围为8.3—4738 ng·g−1干重,污染程度比亚洲地区高。在非洲地区,南非开普敦港口沉积物中的浓度范围为10—829 ng·g−1干重[72],尼日利亚格拉斯港雨季的浓度范围为0.01—7.56 ng·g−1干重,旱季为ND.—2.98 ng·g−1干重[8]。
lrgarol 1051 在水体中主要以自由溶解态的形式存在,不易吸附到水体颗粒相和沉降物中。然而,lrgarol 1051在全球水体沉积物中也有一定程度检出,如美国佛罗里达州0.3—8.9 ng·g−1干重[74],巴西桑托斯港1.0—89.7 ng·g−1干重[10]、巴拿马沿海0.08—2.8 ng·g−1干重[79]、丹麦码头20 ng·g−1干重[81]、韩国沿海0.02—230 ng·g−1干重[127]、印度尼西亚沿海61—76 ng·g−1干重[131]。
敌草隆在全球各大洲沉积物中均有检出。其中,美国和澳大利亚的污染程度相对较轻,浓度范围分别为<0.3—4.2 ng·g−1干重[74]和0.3—11.0 ng·g−1干重[132];南美洲和亚洲的污染程度较为严重,在阿根廷巴伊亚布兰卡河口浓度高达7800 ng·g−1干重[33],而日本沿海的平均浓度达到了897 ng·g−1干重[113]。在欧洲地区,敌草隆在意大利南亚得里亚海[68]地区的浓度为1.9—583 ng·g−1干重。
沉积物中DCOIT、百菌清、苯氟磺胺研究相对较少,这主要是由于这3种化合物的半衰期较短,在水环境中容易被降解。韩国[127]、印度尼西亚[131]、日本[92, 116]、丹麦[95]、美国[74]、巴拿马[9]和巴西[133]等国家的沿海沉积物中均发现了DCOIT,最高浓度为281 ng·g−1 干重。百菌清在韩国(1.2—1065 ng·g−1干重)和巴西(<0.1—9.2 ng·g−1干重)的港口沉积物中有报道[127]。在印度尼西亚沿海(<0.4—80 ng·g−1干重)[131]、巴西桑托斯港(< 2.1—16 ng·g−1干重)[133]和丹麦码头((1.98 ± 1.77) ng·g−1干重)[81]的沉积物中发现了苯氟磺胺的存在。
尽管水体中TBT的浓度呈现逐年下降的趋势,并低于新型船舶防污杀生剂的浓度,但其在沉积物中浓度仍显著高于新型船舶防污杀生剂。这主要是由于TBT在沉积物中降解缓慢,并具有较低水溶性和较高的沉积物/水分配系数,水体中的TBT也倾向于吸附在沉积物中。沉积物中有机物含量会影响其对船舶防污杀生剂的吸附能力,从而影响船舶防污杀生剂的分配[134]。然而,港口的疏浚工作会扰动富集在沉积物中的污染物质,使其重新进入水体中,造成二次污染。近海沉积物船舶防污杀生剂分布特征见表4。
表 4 近海沉积物船舶防污杀生剂分布特征Table 4. Distribution characteristics of antifouling biocides for ships in offshore sediments船舶防污杀生剂Antifouling biocides 采样点Sampling point 采样时间Sampling time 浓度范围(中值)/(ng·g−1)Concentration range (median) 参考文献Reference 三丁基锡 葡萄牙 2005 0.2—72 (41) [130] 印度东海岸 2007 2.7—351.0 (65.8) [65] 印度西海岸 2008 11.0—943.4 (131.6) [65] 中国台湾高雄港 2009 1.4—76.8 (30.7) [125] 巴西东南部 2009—2010 ND.—279 (33) [71] 巴西东北部 2011 8.2—929.7 (150.8) [135] 中国台湾沿海 2011 9—1866.6 (23.6) [103] 撒丁岛 2011—2012 204.3 [136] 突尼斯沿海 2011—2012 16.7 [136] 葡萄牙沿海 2011—2012 7.1 [136] 秘鲁沿海 2012 143.2—469.0 (195.2) [123] 波兰格丁尼亚港 2012 134—6741 (4400) [104] 法国卡马格港 2012 13.7—1947.1 (120) [129] 印度尼西亚沿海 2012 160—3502 [131] 中国三峡水库 2012—2013 3.3—17.0 (9.2) [137] 中国近岸倾倒区 2012—2013 4.83—1334 [128] 南非开普敦港口 2013 10—829 (27) [72] 阿根廷巴伊亚布兰卡 2013 7.68—100.8 (57.1) [138] 中国南杭州湾 2013 ND.—42.2 (22.4) [126] 韩国蔚山沿海 2014—2015 < 0.1—34.5 (2.73) [127] 韩国釜山沿海 2014—2015 < 0.1—56.9 (6.54) [127] 韩国光阳市沿海 2014—2015 < 0.1—36.7 (3.41) [127] 韩国造船厂 2014—2015 < 0.1—2304 (172) [11] 委内瑞拉加勒比海 2015 156.3—1417.8 [122] 德国沿海 2015 1.97—8.3 (2.5) [69] 泼罗的海中部 2015 2.41—380 (95.5) [69] 中国台湾高雄港航道 2015 3.3—307.1 (81.4) [105] 中国长江口 2015—2016 ND.—28.8 [139] 智利沿海 2016 3.3—334.6 (4.38) [124] 尼日利亚拉各斯港雨季 2016—2018 0.01—7.56 (6.24) [8] 尼日利亚拉各斯港旱季 2016—2018 ND.—2.98 (2.230) [8] 智利北海 2017 247.9—1651.7 (531.8) [124] 波罗的海南部沿海 2018 10.1—5325.7 (334.6) [140] 南非沿海 2018 932.4 [141] 桑托斯港 2020 1.4—1886.8 (383.9) [134] 斯里兰卡沿海 2020 15.6—111.1 (63.3) [107] Irgarol 1051 美国加利福尼亚 2008 <0.3—8.9 (1.4) [74] 印度尼西亚 2012 61—76 (63) [131] 伊朗沿海 2013 ND.—35.4 (6.8) [84] 巴西帕托斯 2014 ND.—17.8 [142] 韩国沿海 2015 ND.—230 (39) [127] 巴西圣马科斯湾 2016 ND.—9.2 (1.2) [10] 韩国蔚山沿海 2016 < 0.02—1.61 (0.14) [11] 韩国釜山沿海 2016 < 0.02—5.04 (0.59) [11] 韩国光阳市沿海 2016 < 0.02—7.79 (0.68) [11] 巴拿马沿海 2016 ND.—2.8 [79] 巴拿马沿海地区 2018 0.08—2.8 (0.35) [9] 巴西东北部 2019 1.0—89.7 (12) [143] 丹麦码头 2019 20.13 ± 9.11 [81] 南美洲桑托斯港 2020 < 0.5 [134] 敌草隆 加利福尼亚游艇码头 2008 <0.3—4.2 (1.0) [74] 澳大利亚昆士兰州北部 2008 0.3—11.0 (7.8) [132] 韩国沿海 2009—2010 2.3—62.3 (31) [109] 印度尼西亚 2012 410—740 (574) [131] 阿根廷巴伊亚布兰韩国沿海 20132015 78006.9—46 (39) [33][127] 巴西圣马科斯湾 2016 ND.—15.0 (5.4) [10] 韩国蔚山湾 2016 0.42—9.05 (4.01) [127] 韩国釜山湾 2016 < 0.06—60.7 (12.1) [127] 韩国光阳市沿海 2016 < 0.06—140 (17.8) [127] 日本濑户内海 2016 897 ± 215 (1280) [112] 巴拿马运河 2018 14.1 ± 1.3 [9] 巴西东北部沿海 2019 <5.0—55.2 (10.2) [143] 南美洲桑托斯港 2020 < 0.5—9.9 (2.3) [134] DCOIT 丹麦沿海 2004 <20 [95] 越南沿海 2006 0.09—2.4 (1.3) [22] 日本大田湾 2007 <0.04—150 (1.8) [92] 日本迈祖鲁湾 2007 <0.04—7.2 [117] 印度尼西亚 2012 53—110 (79) [131] 美国沿海 2013 <0.3—4.2 [74] 韩国沿海 2015 ND.—281 (61) [127] 韩国蔚山沿海 2016 < 0.03—2.17 (0.65) [11] 韩国釜山沿海 2016 < 0.03—110 (11.1) [11] 韩国光阳市 2016 < 0.03—117 (9.48) [11] 巴西西南沿海码头 2017 0.13 ± 0.03 [144] 巴拿马沿海 2018 <0.38—81.6 (5.7) [9] 南美洲桑托斯港 2020 < 0.2—74.6 (8.5) [134] 百菌清 韩国沿海 2015 1.2—1065 (22) [127] 南美洲桑托斯港 2020 < 0.1—9.2 (1.4) [134] 苯氟磺胺 印度尼西亚 2012 <0.4—80 (45) [131] 丹麦码头 2019 1.98 ± 1.77 [81] 南美洲桑托斯港 2020 < 2.1—16.0 (0.7) [134] ND.,未检出. ND., not detected. 3.3 船舶防污杀生剂在水生生物体中的分布特征
TBT可通过生物富集作用,在水生生物体中进行积累,其对软体动物和鱼类的生物富集因子(BCF)高达7000[145],对生物体产生了不利影响。目前已经有大量文献报道了TBT在水生生物体内的分布情况,在多种类型的水生生物体中均有检出,如海草(46 ng·g−1,按Sn算)[9]、腹足类动物(190 ng·g−1,按Sn算)[124]、鱼类(53—330 ng·g−1,按Sn算)[71, 105]。阿拉伯湾捕获的8种重要经济鱼类中均有检测到TBT,表明这种化合物能在鱼体组织中的进行累积,并对人群造成潜在的危害[66]。处于食物链高营养级的哺乳动物和鸟类也受到有机锡污染的威胁,在海洋哺乳动物和海鸟的组织、器官屮均有检出,其中,韩国水域中小须鲸和海豚体内三丁基锡浓度分别为15.7—297 ng·g−1和59—412 ng·g−1[30]。
相比较与TBT,其余5种新型船舶防污杀生剂在水生生物体中的报道相对较少,缺乏完整食物链的研究。仅对2020年在马来西亚部分海洋生物进行了系统研究,研究物种包括石莼(Ulva sp.)和海菖蒲(Enhalus acoroides)等水生植物,翡翠贻贝(Perna viridis)和大蛤蜊海丝(Pinna nobilis)等贝类,以及大菱鲆(Strombus turturella)、红牙䱛(Otolithus ruber)、六指马鲅(Polydactylus sextarius)和杜氏棱鳀(Thryssa dussumieri)等鱼类[145]。其中,Irgarol 1051和百菌清在8种物种中的浓度分布较为均一,浓度范围分别为0.7—3.2 g·kg−1和1.2—1.6 g·kg−1;而Irgarol 1051的代谢产物(2-methylthio-4-tert-butylamino-6-amino-s-triazine,M1)浓度是其母体化合物的10—100倍,这主要是由于M1较强的生物富集能力和较长的环境半衰期,易在水生生物体中积累[73, 145]。敌草隆在海菖蒲的浓度仅为4.7 g·kg−1,其在鱼体中的浓度相对较高,达到了7.7—18.5 g·kg−1。DCOIT和苯氟磺胺在水生生物体中呈现出一定的生物放大特征,即在低营养级的水生植物中污染程度相对较低,在高营养级的贝类和鱼类中具有相对较高的浓度。
TBT是在生物体中检测到的最高的船舶防污杀生剂,其次是敌草隆和Irgarol 1051。化合物的正辛醇-水分配系数(KOW)与其生物体的富集浓度之间并无显著的相关性。与生物体一样,水体和沉积物中检测到的最高浓度的船舶防污杀生剂也是TBT、Irgarol 1051和敌草隆,表明影响生物体中的船舶防污杀生剂浓度的主要是其在水环境中的污染程度。船舶防污杀生剂不进会对水生生物造成影响,并会饮食摄入进而对人体健康产生危害。海洋生物体中船舶防污杀生剂的分布特征见图2。
4. 结论与展望(Conclusion and prospect)
船舶防污杀生剂在保护船舶、人工岛等海洋人工设施上免受生物污损的同时,会对非靶标水生生物产生严重的毒性作用。其中,TBT会影响所有营养级的水生生物。近15年,全球水环境中广泛检测出了船舶防污杀生剂,其污染程度整体呈逐年下降的趋势,但在港口、造船厂、渔港和码头等船舶活动频繁地区污染情况仍较为严重。进入到水环境中的船舶防污杀生剂会对水体生态环境造成了长期的影响。
近年来,我国水运发展迅速,发达的修造船业务和繁忙水上运输活动造成船舶防污杀生剂的释放量也随之增加。然而,我国对这些污染物的研究起步较晚,但已有研究表明有机锡化合物已经对我国海洋环境产生严重的影响,而新型的船舶防污杀生剂在中国水环境中尚未开展系统的研究,其生态风险亦未知。因此对我国沿海和内河水环境中船舶防污杀生剂的环境分布情况调查、环境行为的研究、以及对水生生物的毒性效应和环境风险的评价是非常重要和必要的。
因此在今后的研究中,建议把重点放在:(1)分布特征研究。当前环境分布研究是断点式的缺乏周期性和系统性。因此需要开发不同环境介质中快速船舶防污杀生剂分析方法;周期性的对重点水域进行全面的船舶防污杀生剂污染程度调查,并分析其时空变化趋势和主要的污染来源。(2)环境行为研究。排放到水体中的船舶防污杀生剂并不是稳定不变的,在物理、化学以及生物作用下会进行一系列的迁移转化过程。目前国内外针对这类污染物在水体环境中迁移转化过程尚未开展系统的研究。(3)毒理学研究。船舶防污杀生剂及其降解产物对多种水生生物的毒性效应仍然是未知的,并且排放到水体中的船舶防污杀生剂并不是单一的,往往是多种化合物的联合使用,也可能不同的船只在同一地点排放出不同的多种船舶防污杀生剂,这就需要探究这些污染物对水生生物的联合毒性作用。(4)生态风险评价研究。当前国内外针对这类污染物的生态风险评价研究还在起步阶段缺乏系统性,建议结合环境化学、环境毒理学、生态风险评估模型等学科的理论与方法,更加深入的研究不同水域环境中船舶防污杀生剂生态风险水平。(5)保护措施研究。研究已表明船舶防污杀生剂对海洋环境产生了威胁因此应加强对船舶防污杀生剂污染源的管理,制定合理保护措施消减其进入环境的总量;研究和发展船舶防污杀生剂以及其降解产物的处理技术;同时研究对生态环境危害小的新型绿色船舶防污涂料替代品。
-
表 1 船舶防污杀生剂的理化性质
Table 1. Physicochemical properties of marine antifouling biocides
船舶防污杀生剂Antifouling biocides CAS 分子量Relative molecular mass 分子式Molecular formula lg Kow 海水半衰期/d Half-lives in water 沉积物半衰期/d Half-Lives in sediment 溶解度/(mg·L−1)Solubility in water 毒性作用方式Toxicity mode of action 三丁基锡 1461-22-9 290.05 C12H27ClSn 4.1 6—120 440—1500 5—50 内分泌干扰效应 Irgarol 1051 28159-98-0 253.37 C11H19N5S 2.38—4.1 100—350 100—200 6.0—7.0 抑制PSⅡ电子传递 敌草隆 330-54-1 233.09 C9H10Cl2N2O 2.82 30—365 14 35—42 抑制PSⅡ电子传递 百菌清 1897-45-6 265.91 C8Cl4N2 2.64—4.4 1.8—8 <1—8 0.6—0.9 抑制线粒体电子转运 DCOIT 64359-81-5 282.23 C11H17Cl2NOS 2.85 1—13 <1 14 抑制电子传递 苯氟磺胺 1085-98-9 333.23 C9H11Cl2FN2O2S2 2.8—3.7 0.12—0.75 7 0.006—1.3 抑制线粒体电子转运 表 2 船舶防污杀生剂对非靶标海洋生物的毒性
Table 2. Toxicity of marine antifouling biocides to non-target marine organisms
船舶防污杀生剂Antifouling biocides 生物Organism 物种Species 毒性终点Endpoint 数值/(mg·L−1)Value 参考文献Reference 三丁基锡 藻类 班氏金针菜 48 h EC50 0.00016 [20] 藻类 卵囊藻 72 h EC50 0.003 [50] 无脊椎动物 普通海胆 48 h EC50 0.000309 [5] 无脊椎动物 玻璃海鞘 48 h EC50 0.0071 [5] 甲壳类动物 凶猛片钩虾 96 h LC50 0.0094 [51] 甲壳类动物 猛水蚤 96 h LC50 0.018 [51] 甲壳类动物 长臂虾 24 h LC50 0.0223 [43] 甲壳类动物 阿玛猛水蚤 96 h LC50 0.013 [43] 甲壳类动物 宽水蚤 72 h LC50 0.0006 [5] 甲壳类动物 溞 48 h EC50 0.00001 [52] 鱼类 青鳉 96 h LC50 0.025 [51] 鱼类 罗非鱼 96 h LC50 0.0038 [28] 鱼类 金头鲷 24 h LC50 0.0283 [28] Irgarol 1051 藻类 栅藻 48 h EC50 0.00113 [1] 藻类 角毛藻 72 h EC50 0.0011 [50] 无脊椎动物 普通海胆 48 h EC50 4.021 [43] 无脊椎动物 贻贝 48 h EC50 1.54 [41] 无脊椎动物 中间球海胆 48 h EC50 1.05 [38] 无脊椎动物 黄海胆 48 h EC50 0.4125 [48] 甲壳类动物 凶猛片钩虾 96 h LC50 1 [48] 甲壳类动物 草虾 96 h LC50 1.52 [38] 鱼类 虹鳟 96 h LC50 3.22 [53] 鱼类 大海鲢 96 h LC50 0.79 [53] 鱼类 美洲原银汉鱼 96 h LC50 3.5 [1] 鱼类 蓝鳃太阳鱼 96 h LC50 1.58 [1] 鱼类 斑马鱼 96 h LC50 2.6 [1] 鱼类 栅藻 96 h LC50 4 [1] 敌草隆 藻类 赫氏圆石藻 72 h EC50 0.002 [51] 藻类 等鞭金藻 96 h EC50 0.00373 [51] 藻类 骨条藻 96 h EC51 0.0103 [51] 藻类 多毛藻 96 h EC52 0.00042 [54] 藻类 杜氏藻 24 h EC50 0.035 [54] 刺胞类动物 海月水母 10 d LC50 0.00478 [15] 甲壳类动物 剑水蚤 96 h LC50 11 [15] 甲壳类动物 锯齿长臂虾 24 h LC50 3.04 [15] 甲壳类动物 卤虫 24 h LC50 10.3 [55] 甲壳类动物 卤虫 48 h LC50 6.14 [56] 甲壳类动物 卤虫 72 h LC50 2.76 [56] 甲壳类动物 汤氏纺锤水蚤 48 h LC50 1.08 [56] 甲壳类动物 糠虾期 96 h LC50 0.589 [1] 甲壳类动物 小甲壳动物 96 h LC50 7.06 [57] 棘皮动物 普通海胆 48 h EC50 2.39 [57] 棘皮动物 喇叭海胆 24 h EC50 3.33 [57] 鱼类 青鳉鱼 96 h LC50 7.8 [57] 鱼类 大海鲢 96 h LC50 0.89 [57] DCOIT 藻类 小球藻 24 h EC50 0.089 [58] 藻类 赫氏艾密里藻 72 h EC50 0.0004 [43] 藻类 三角褐指藻 72 h EC50 0.04 [43] 藻类 球等鞭金藻 72 h EC50 0.032 [59] 双壳类 鸟蛤 96 h LC50 0.325 [59] 甲壳类动物 红虾 96 h LC50 0.016 EPA 甲壳类动物 汤氏纺锤水蚤 72 h EC50 0.038 EPA 甲壳类动物 卤虫 48 h LC50 0.318 EPA 甲壳类动物 糠虾 96 h LC50 0.005 EPA 甲壳类动物 沙蟹 96 h LC50 1.31 [57] 甲壳类动物 剑水蚤 24 h EC50 0.03 [57] 甲壳类动物 长臂虾 96 h LC50 1.31 [57] 甲壳类动物 剑水蚤 24 h LC50 0.077 [57] 甲壳类动物 拟糠虾 96 h LC50 0.008 [41] 甲壳类动物 日本对虾 96 h LC50 0.013 [41] 棘皮动物 普通海胆 48 h LC50 0.025 [41] 棘皮动物 球海胆幼虫 48 h EC50 0.012 [41] 棘皮动物 黄海胆幼虫 53 h EC50 0.001 [60] 鱼类 红鳍东方鲀 96 h LC50 0.006 [60] 鱼类 杂色鱂 96 h LC50 0.023 [60] 鱼类 真鲷 96 h LC50 0.005 [60] 鱼类 底鳉 96 h LC50 0.005 [60] 百菌清 藻类 多毛藻 96 h IC50 0.064 [1] 环节动物 华美盘管虫 48 h LC50 0.012 [1] 甲壳类动物 指虾蛄 48 h LC50 0.56 [1] 甲壳类动物 指虾蛄 96 h LC50 0.14 [51] 甲壳类动物 指虾蛄 48 h EC50 0.17 [51] 甲壳类动物 剑水蚤 24 h LC50 0.098 [51] 甲壳类动物 剑水蚤 96 h LC50 0.091 [51] 甲壳类动物 凶猛片钩虾 96 h LC50 0.067 [51] 甲壳类动物 卤虫 48 h LC50 2.68 [51] 甲壳类动物 草虾 96 h LC50 0.153 [61] 甲壳类动物 草虾 48 h LC50 0.204 [62] 甲壳类动物 桃红对虾 96 h LC50 0.162 [14] 甲壳类动物 剑水蚤 24 h EC50 0.016 [62] 甲壳类动物 日本囊对虾 96 h LC50 0.29 [62] 鱼类 青鳉 96 h LC50 0.11 [55] 鱼类 杂色鱂 96 h LC50 0.032 [57] 鱼类 三刺鱼 96 h LC50 0.027 [57] 鱼类 黄尾平口石首鱼 48 h LC50 0.032 [57] 鱼类 底鳉 96 h LC50 0.06 [57] 苯氟磺胺 藻类 三角褐指藻 72 h EC50 0.193 [57] 藻类 糖海带 3 h NOEC 0.00001 [63] 甲壳类动物 汤氏纺锤水蚤 48 h LC10 0.017 [63] 甲壳类动物 卤虫 48 h LC50 154 [63] 棘皮动物 普通海胆 48 h EC50 0.282 EPA 棘皮动物 黄海胆 53 h EC50 0.177 [14] 鱼类 舌齿鲈 96 h EC50 0.015 [48] 鱼类 黄尾平口石首鱼 24 h LC50 0.032 [48] 表 3 近海水体中船舶防污杀生剂的分布特征
Table 3. Distribution characteristics of antifouling biocides in offshore waters
船舶防污杀生剂Antifouling biocides 采样点Sampling point 采样时间Sampling time 浓度范围(中值)/ (ng·L−1)Concentration range (median) 参考文献Reference 三丁基锡 西班牙北海 2006 0.3—17.0 (4.6) [5] 印度果阿海 2006 0.5—126.2 (19) [99] 中国台湾高雄港 2006 90—480 (285) [67] 印度东海 2007 4—55 (14) [65] 日本沿海 2007 11.0—74.0 (15.5) [5] 威尼斯泻湖 2007 27.4—65.8 (46.6) [100] 希腊沿海 2007 ND.—191.9 (20.3) [70] 印度西海 2008 0.1—103 (16.2) [65] 中国厦门沿海 2008 5.28—384 (16) [101] 格丁尼亚港口 2009 12.9—110.2 (60.7) [102] 巴西南部沿海 2009—2010 ND.—175 (76) [71] 巴西东南部沿海 2009—2010 ND.—175 (54) [71] 韩国西部沿海 2010 ND.—63.4 (7.02) [64] 韩国东部沿海 2010 ND.—18.1 (3.69) [64] 韩国南部沿海 2010 ND.—51.2 (2.16) [64] 沙特沿海 2010 140—1900 [66] 中国台湾沿海 2011 16.8—273.3(52.7) [103] 意大利南亚得里亚海 2012 12—110 (50) [68] 阿尔巴尼亚沿海 2012 5—44 (24) [68] 波兰格丁尼亚港 2012 10.32—191.7 (25.9) [104] 南非开普敦港 2013 0.1—111.3 (9.7) [72] 中国台湾高雄港德国和波罗的海中部沿海 20142015 50.7—93.5 (11.6)2.5—380(95.5) [105][69] 韩国蔚山沿海 2014—2015 < 1—51.8 (13.3) [71] 韩国釜山沿海 2014—2015 < 1—69.9 (22) [71] 韩国光阳市沿海 2014—2015 3.25—24.6 (8.35) [71] 尼日利亚拉各斯港雨季 2016—2018 ND.—52.8 (7.8) [8] 尼日利亚拉各斯港旱季 2016—2018 ND.—6 (0.3) [8] 中国三峡 2018 21.0—222.1 (67) [106] 斯里兰卡沿海 2020 21.8—310.4 (166.1) [107] Irgarol 1051 中国香港沿海 2005 110—1620 (640) [86] 佛罗里达州 迈阿密码头 2008 20—66 (35) [73] 佛罗里达州 基拉戈港 2008 7—102 (35) [73] 美国加利福尼亚海域 2008 2—254 (67) [74] 澳大利亚沿海 2008 ND.—6 (5) [74] 西班牙大加那利亚海港 2008—2009 2.4—146.5 (51.6) [108] 巴西南部沿海 2009 <1.3—21 (4) [78] 韩国渔港 2009—2010 0.9—14.1 (4.0) [109] 意大利那不勒斯海湾 2010 0.8—134.5 (27.2) [13] 意大利拉斯佩齐亚湾 2010 <0.2—9.7 (4.1) [13] 韩国(马山、行岩、高贤)海湾 2010 ND.—11.5 (4.6) [90] 韩国西海 2010 ND.—63.37 (11.82) [64] 韩国东海 2010 ND.—51.15 (1.07) [64] 韩国南海 2010 ND.—18.06 (8.29) [64] 美国夏威夷瓦胡岛海域 2011 <17—283 (58.6) [75] 意大利南亚得里亚海 2012 0.6—16.1 (9.2) [68] 阿尔巴尼亚南亚得里亚海 2012 <0.2—9.3 (3.3) [68] 法国布列塔尼 2012 0—14 (6) [82] 马来西亚沿海 2012 ND.—2021 (471) [110] 日本库尔港 2012 ND.—740 (59) [85] 日本广岛港 2012 ND.—1050 (27) [85] 日本尾道港 2012 ND.—140 (22) [85] 日本神户港 2012 ND.—1850 (36) [85] 法国阿卡雄湾 2013 2.5—22 (12) [111] 波斯湾布什尔港口 2013 <1.0—63 (13.1) [84] 意大利阿普利亚海域 2014 0.5—18.1 (5.8) [88] 阿尔巴尼亚海域 2014 0.5—1.2 (0.8) [88] 巴西伊塔基港 2014 10—4800 (370) [77] 加拿大 五大湖流域 2016 ND.—3 (1) [76] 坦桑尼亚桑给巴尔岛海域 2016 1.35—15.44 (4.11) [80] 巴拿马沿海 2016 <0.3—5 (2.6) [79] 韩国光阳、釜山和蔚山海湾 2016 < 0.12—2.05 (0.15) [11] 日本濑户内海 2016 ND.—9 (3) [112] 韩国沿海 2016 ND.—318.5 (13.6) [23] 瑞典沿海水域 2017 <0.2—8.0 (0.25) [83] 巴西圣马科斯湾 2018 ND.—89.5 (15.6) [10] 丹麦码头 2019 ND.—1.78 (0.66) [81] 敌草隆 加利福尼亚海域 2008 <2—68 (35) [74] 西班牙大加那利岛港口 2008—2009 2.4—203.7 (74.4) [108] 巴西里约热内卢格兰德岛沿海 2009 <1.3—6 [10] 韩国海湾、渔港和港口 2009—2010 13—1360 (200) [90] 意大利那不勒斯湾 2010 <1.0—34.8 (22.2) [13] 意大利拉斯佩齐亚湾 2010 <1.0—28.2 (11.7) [13] 巴西伊塔基港 2011 <6—7800 [77] 比利时北海 2011 ND.—263 (108) [113] 布列塔尼维莱因湾 2012 ND.—88 (35) [82] 阿尔巴尼亚南亚得里亚海 2012 1.9—93.9 (33.3) [68] 意大利南亚得里亚海 2012 12.4—583.5 (68.9) [68] 日本库尔港 2012 ND.—1780 (59) [85] 日本广岛港 2012 ND.—1700 (23) [85] 日本尾道港 2012 ND.—2120 (61) [85] 日本神户港 2012 ND.—2030 (32) [85] 马来西亚半岛沿海 2012 1—285 (43) [21] 西班牙东北部沿海 2012 2.4—818 (99.7) [89] 法国阿卡雄湾 2013 5—40 (20) [111] 波斯湾布什尔港口 2013 ND.—29.1 (13.6) [84] 意大利阿普利亚沿海 2014 ND.—160.0 (15.8) [88] 阿尔巴尼亚沿海 2014 ND.—15.0 (7.4) [88] 澳大利亚布里斯班入海口 2015 1.0—56.8 (28.9) [87] 澳大利亚亚拉入海口 2015 1—38.8 (19.9) [87] 澳大利亚悉尼入海口 2015 15.1—96.7 (55.9) [87] 中国长三角洲 2016 1.7—107.2 (14.8) [91] 巴拿马沿海 2016 <2.7—70 (35.6) [79] 巴西圣马科斯湾 2016 <1.4—22 (7.5) [10] 韩国蔚山沿海 2016 2.41—90.4 (24.1) [11] 韩国釜山沿海 2016 < 0.31—41.9 (16.3) [11] 韩国光阳市沿海 2016 11.9—96.2 (29.4) [11] 日本西部濑户内海 2016 ND.—535 (307) [112] 韩国沿海 2016 ND.—55.2 (20) [23] 西班牙巴斯克沿海 2017 4—81 (14) [114] DCOIT 英国南安普顿 2000 <1 [94] 德国沿海 2002 ND.—49 [70] 日本大阪港 2002—2003 <0.3—4 [115] 丹麦沿海 2004 <5—283 [95] 日本迈祖鲁湾 2007 <0.3 [116] 韩国沿海 2009—2010 ND.—6 [90] 日本广岛湾 2010 0.1—53 (3.2) [117] 日本沿海 2010 1—3700 [117] 韩国蔚山沿海 2016 < 0.16 [11] 韩国釜山沿海 2016 < 0.16 [11] 韩国光阳市沿海 2016 <0.16—2.44 (0.17) [11] 韩国沿海 2016 ND.—144.5 (14.3) [90] 中国长江三角洲 2016 13.73—226.23 (66.05) [91] 百菌清 希腊沿海 2002 ND.—63 (30) [70] 加拿大沿海 2007 ND.—80 (40) [98] 日本沿海 2008 ND.—1.1 (0.5) [118] 卢森堡 2010 ND.—25 (11) [96] 韩国西部沿海 2010 (29.78) [64] 韩国东部沿海 2010 (8.49) [64] 韩国南部沿海 2010 (29.77) [64] 日本沿海 2010 ND.—1380 (68) [92] 韩国沿海 2013 ND.—318.5 (13.6) [23] 泰国北部沿海 2014 20—410 (105) [97] 苯氟磺胺 英国南海 2006 <1 [119] 南加州圣地亚哥 2006 <10 [120] 美国维尔京群岛 2006 <1 [121] 澳大利亚珀斯沿海 2008 <100 [13] 意大利沿海 2010 <1.0 [13] 卢森堡 2010 ND.—45 (7) [96] 韩国西部沿海 2010 (21.77) [64] 韩国东部沿海 2010 (8.5) [64] 韩国南部沿海 2010 (78) [64] 日本沿海 2010 1—760 (110) [13] 西班牙沿海 2013 <30 [13] 韩国沿海 2013 ND.—318.5 (13.6) [23] 澳大利亚东海岸 2015 <0.1 [87] 丹麦码头 2019 ND.—19.78 [81] ND.,未检出. ND., not detected. 表 4 近海沉积物船舶防污杀生剂分布特征
Table 4. Distribution characteristics of antifouling biocides for ships in offshore sediments
船舶防污杀生剂Antifouling biocides 采样点Sampling point 采样时间Sampling time 浓度范围(中值)/(ng·g−1)Concentration range (median) 参考文献Reference 三丁基锡 葡萄牙 2005 0.2—72 (41) [130] 印度东海岸 2007 2.7—351.0 (65.8) [65] 印度西海岸 2008 11.0—943.4 (131.6) [65] 中国台湾高雄港 2009 1.4—76.8 (30.7) [125] 巴西东南部 2009—2010 ND.—279 (33) [71] 巴西东北部 2011 8.2—929.7 (150.8) [135] 中国台湾沿海 2011 9—1866.6 (23.6) [103] 撒丁岛 2011—2012 204.3 [136] 突尼斯沿海 2011—2012 16.7 [136] 葡萄牙沿海 2011—2012 7.1 [136] 秘鲁沿海 2012 143.2—469.0 (195.2) [123] 波兰格丁尼亚港 2012 134—6741 (4400) [104] 法国卡马格港 2012 13.7—1947.1 (120) [129] 印度尼西亚沿海 2012 160—3502 [131] 中国三峡水库 2012—2013 3.3—17.0 (9.2) [137] 中国近岸倾倒区 2012—2013 4.83—1334 [128] 南非开普敦港口 2013 10—829 (27) [72] 阿根廷巴伊亚布兰卡 2013 7.68—100.8 (57.1) [138] 中国南杭州湾 2013 ND.—42.2 (22.4) [126] 韩国蔚山沿海 2014—2015 < 0.1—34.5 (2.73) [127] 韩国釜山沿海 2014—2015 < 0.1—56.9 (6.54) [127] 韩国光阳市沿海 2014—2015 < 0.1—36.7 (3.41) [127] 韩国造船厂 2014—2015 < 0.1—2304 (172) [11] 委内瑞拉加勒比海 2015 156.3—1417.8 [122] 德国沿海 2015 1.97—8.3 (2.5) [69] 泼罗的海中部 2015 2.41—380 (95.5) [69] 中国台湾高雄港航道 2015 3.3—307.1 (81.4) [105] 中国长江口 2015—2016 ND.—28.8 [139] 智利沿海 2016 3.3—334.6 (4.38) [124] 尼日利亚拉各斯港雨季 2016—2018 0.01—7.56 (6.24) [8] 尼日利亚拉各斯港旱季 2016—2018 ND.—2.98 (2.230) [8] 智利北海 2017 247.9—1651.7 (531.8) [124] 波罗的海南部沿海 2018 10.1—5325.7 (334.6) [140] 南非沿海 2018 932.4 [141] 桑托斯港 2020 1.4—1886.8 (383.9) [134] 斯里兰卡沿海 2020 15.6—111.1 (63.3) [107] Irgarol 1051 美国加利福尼亚 2008 <0.3—8.9 (1.4) [74] 印度尼西亚 2012 61—76 (63) [131] 伊朗沿海 2013 ND.—35.4 (6.8) [84] 巴西帕托斯 2014 ND.—17.8 [142] 韩国沿海 2015 ND.—230 (39) [127] 巴西圣马科斯湾 2016 ND.—9.2 (1.2) [10] 韩国蔚山沿海 2016 < 0.02—1.61 (0.14) [11] 韩国釜山沿海 2016 < 0.02—5.04 (0.59) [11] 韩国光阳市沿海 2016 < 0.02—7.79 (0.68) [11] 巴拿马沿海 2016 ND.—2.8 [79] 巴拿马沿海地区 2018 0.08—2.8 (0.35) [9] 巴西东北部 2019 1.0—89.7 (12) [143] 丹麦码头 2019 20.13 ± 9.11 [81] 南美洲桑托斯港 2020 < 0.5 [134] 敌草隆 加利福尼亚游艇码头 2008 <0.3—4.2 (1.0) [74] 澳大利亚昆士兰州北部 2008 0.3—11.0 (7.8) [132] 韩国沿海 2009—2010 2.3—62.3 (31) [109] 印度尼西亚 2012 410—740 (574) [131] 阿根廷巴伊亚布兰韩国沿海 20132015 78006.9—46 (39) [33][127] 巴西圣马科斯湾 2016 ND.—15.0 (5.4) [10] 韩国蔚山湾 2016 0.42—9.05 (4.01) [127] 韩国釜山湾 2016 < 0.06—60.7 (12.1) [127] 韩国光阳市沿海 2016 < 0.06—140 (17.8) [127] 日本濑户内海 2016 897 ± 215 (1280) [112] 巴拿马运河 2018 14.1 ± 1.3 [9] 巴西东北部沿海 2019 <5.0—55.2 (10.2) [143] 南美洲桑托斯港 2020 < 0.5—9.9 (2.3) [134] DCOIT 丹麦沿海 2004 <20 [95] 越南沿海 2006 0.09—2.4 (1.3) [22] 日本大田湾 2007 <0.04—150 (1.8) [92] 日本迈祖鲁湾 2007 <0.04—7.2 [117] 印度尼西亚 2012 53—110 (79) [131] 美国沿海 2013 <0.3—4.2 [74] 韩国沿海 2015 ND.—281 (61) [127] 韩国蔚山沿海 2016 < 0.03—2.17 (0.65) [11] 韩国釜山沿海 2016 < 0.03—110 (11.1) [11] 韩国光阳市 2016 < 0.03—117 (9.48) [11] 巴西西南沿海码头 2017 0.13 ± 0.03 [144] 巴拿马沿海 2018 <0.38—81.6 (5.7) [9] 南美洲桑托斯港 2020 < 0.2—74.6 (8.5) [134] 百菌清 韩国沿海 2015 1.2—1065 (22) [127] 南美洲桑托斯港 2020 < 0.1—9.2 (1.4) [134] 苯氟磺胺 印度尼西亚 2012 <0.4—80 (45) [131] 丹麦码头 2019 1.98 ± 1.77 [81] 南美洲桑托斯港 2020 < 2.1—16.0 (0.7) [134] ND.,未检出. ND., not detected. -
[1] AMARA I, MILED W, SLAMA R B, et al. Antifouling processes and toxicity effects of antifouling paints on marine environment. A review [J]. Environmental Toxicology and Pharmacology, 2018, 57: 115-130. doi: 10.1016/j.etap.2017.12.001 [2] ABBOTT A, ABEL P D, ARNOLD D W, et al. Cost-benefit analysis of the use of TBT: The case for a treatment approach [J]. Science of the Total Environment, 2000, 258(1/2): 5-19. [3] XIE Q Y, PAN J S, MA C F, et al. Dynamic surface antifouling: Mechanism and systems [J]. Soft Matter, 2019, 15(6): 1087-1107. doi: 10.1039/C8SM01853G [4] TORNERO V, HANKE G. Chemical contaminants entering the marine environment from sea-based sources: A review with a focus on European seas [J]. Marine Pollution Bulletin, 2016, 112(1/2): 17-38. [5] ANTIZAR-LADISLAO B. Environmental levels, toxicity and human exposure to tributyltin (TBT)-contaminated marine environment. A review [J]. Environment International, 2008, 34(2): 292-308. doi: 10.1016/j.envint.2007.09.005 [6] DAFFORN K A, LEWIS J A, JOHNSTON E L. Antifouling strategies: History and regulation, ecological impacts and mitigation [J]. Marine Pollution Bulletin, 2011, 62(3): 453-465. doi: 10.1016/j.marpolbul.2011.01.012 [7] CASTRO Í B, MACHADO F B, de SOUSA G T, et al. How protected are marine protected areas: A case study of tributyltin in Latin America [J]. Journal of Environmental Management, 2021, 278: 111543. doi: 10.1016/j.jenvman.2020.111543 [8] BASHEERU K A, OKORO H K, ADEKOLA F A, et al. Speciation and quantification of organotin compounds in Lagos harbour, Nigeria [J]. International Journal of Environmental Analytical Chemistry, 2020: 1-20. [9] BATISTA-ANDRADE J A, CALDAS S S, BATISTA R M, et al. From TBT to booster biocides: Levels and impacts of antifouling along coastal areas of Panama [J]. Environmental Pollution, 2018, 234: 243-252. doi: 10.1016/j.envpol.2017.11.063 [10] VIANA J L M, DINIZ M D S, SANTOS S R V D, et al. Antifouling biocides as a continuous threat to the aquatic environment: Sources, temporal trends and ecological risk assessment in an impacted region of Brazil [J]. Science of the Total Environment, 2020, 730: 139026. doi: 10.1016/j.scitotenv.2020.139026 [11] LAM N H, JEONG H H, KANG S D, et al. Organotins and new antifouling biocides in water and sediments from three Korean Special Management Sea Areas following ten years of tributyltin regulation: Contamination profiles and risk assessment [J]. Marine Pollution Bulletin, 2017, 121(1/2): 302-312. [12] LEE S, LEE Y W. Determination of the concentrations of alternative antifouling agents on the Korean coast [J]. Marine Pollution Bulletin, 2016, 113(1/2): 253-257. [13] ANSANELLI G, MANZO S, PARRELLA L, et al. Antifouling biocides (Irgarol, Diuron and dichlofluanid) along the Italian Tyrrhenian coast: Temporal, seasonal and spatial threats [J]. Regional Studies in Marine Science, 2017, 16: 254-266. doi: 10.1016/j.rsma.2017.09.011 [14] JUNG S M, BAE J S, KANG S G, et al. Acute toxicity of organic antifouling biocides to phytoplankton Nitzschia pungens and zooplankton Artemia larvae [J]. Marine Pollution Bulletin, 2017, 124(2): 811-818. doi: 10.1016/j.marpolbul.2016.11.047 [15] DUPRAZ V, STACHOWSKI-HABERKORN S, MÉNARD D, et al. Combined effects of antifouling biocides on the growth of three marine microalgal species [J]. Chemosphere, 2018, 209: 801-814. doi: 10.1016/j.chemosphere.2018.06.139 [16] MARTINS S E, FILLMANN G, LILLICRAP A, et al. Review: ecotoxicity of organic and organo-metallic antifouling co-biocides and implications for environmental hazard and risk assessments in aquatic ecosystems [J]. Biofouling, 2018, 34(1): 34-52. doi: 10.1080/08927014.2017.1404036 [17] GAO J M, FU P T, CHEN X L, et al. Fate simulation and risk assessment of TBT and TPhT considering water level fluctuations in the TGR before and after AFS Convention implementation in China [J]. Environmental Sciences Europe, 2020, 32(2): 23-36. [18] HASSAN A T, QURBAN M, MANIKANDAN K, et al. Assessment of the organotin pollution in the coastal sediments of the Western Arabian Gulf, Saudi Arabia [J]. Marine Pollution Bulletin, 2019, 139: 174-180. doi: 10.1016/j.marpolbul.2018.12.041 [19] CASTRO Í B, WESTPHAL E, FILLMANN G. Third generation antifouling paints: New biocides in the aquatic environment [J]. Química Nova, 2010, 34(6): 1021-1031. [20] ARRHENIUS A, BACKHAUS T, GRÖNVALL F, et al. Effects of three antifouling agents on algal communities and algal reproduction: Mixture toxicity studies with TBT, Irgarol, and Sea-Nine [J]. Archives of Environmental Contamination and Toxicology, 2006, 50(3): 335-345. doi: 10.1007/s00244-005-1057-9 [21] ALI H R, ARIFIN M M, SHEIKH M A, et al. Contamination of diuron in coastal waters around Malaysian Peninsular [J]. Marine Pollution Bulletin, 2014, 85(1): 287-291. doi: 10.1016/j.marpolbul.2014.05.049 [22] CHEN L G, LAM J C W. SeaNine 211 as antifouling biocide: A coastal pollutant of emerging concern [J]. Journal of Environmental Sciences, 2017, 61: 68-79. doi: 10.1016/j.jes.2017.03.040 [23] LEE S, LEE D, LEE Y W. Determination of five alternative antifouling agents found along the Korean coasts [J]. Water Environment Research, 2017, 89(7): 622-628. doi: 10.2175/106143017X14902968254511 [24] CIMA F, BRAGADIN M, BALLARIN L. Toxic effects of new antifouling compounds on tunicate haemocytes: I. Sea-Nine 211™ and chlorothalonil [J]. Aquatic Toxicology, 2008, 86(2): 299-312. doi: 10.1016/j.aquatox.2007.11.010 [25] van WEZEL A P, van VLAARDINGEN P. Environmental risk limits for antifouling substances [J]. Aquatic Toxicology, 2004, 66(4): 427-444. doi: 10.1016/j.aquatox.2003.11.003 [26] THOMAS K V, MCHUGH M, HILTON M, et al. Increased persistence of antifouling paint biocides when associated with paint particles [J]. Environmental Pollution, 2003, 123(1): 153-161. doi: 10.1016/S0269-7491(02)00343-3 [27] KARLSSON J, BREITHOLTZ M, EKLUND B. A practical ranking system to compare toxicity of anti-fouling paints [J]. Marine Pollution Bulletin, 2006, 52(12): 1661-1667. doi: 10.1016/j.marpolbul.2006.06.007 [28] DIMITRIOU P, CASTRITSI-CATHARIOS J, MILIOU H. Acute toxicity effects of tributyltin chloride and triphenyltin chloride on gilthead seabream, Sparus aurata L., embryos [J]. Ecotoxicology and Environmental Safety, 2003, 54(1): 30-35. doi: 10.1016/S0147-6513(02)00008-8 [29] de CASTRO Í B, de MEIRELLES C A O, MATTHEWS-CASCON H, et al. Imposex in endemic volutid from northeast Brazil (Mollusca: Gastropoda) [J]. Brazilian Archives of Biology and Technology, 2008, 51(5): 1065-1069. doi: 10.1590/S1516-89132008000500024 [30] CHOI M, MOON H B, AN Y R, et al. Accumulation of butyltin compounds in cetaceans from Korean coastal waters [J]. Marine Pollution Bulletin, 2011, 62(5): 1120-1123. doi: 10.1016/j.marpolbul.2011.03.013 [31] MEADOR J P, SOMMERS F C, COOPER K A, et al. Tributyltin and the obesogen metabolic syndrome in a salmonid [J]. Environmental Research, 2011, 111(1): 50-56. doi: 10.1016/j.envres.2010.11.012 [32] PARMENTIER K F V, VERHAEGEN Y, de WITTE B P, et al. Tributyltin: A bottom–up regulator of the Crangon crangon population? [J]. Frontiers in Marine Science, 2019, 6: 633. doi: 10.3389/fmars.2019.00633 [33] QUINTAS P Y, ARIAS A H, OLIVA A L, et al. Organotin compounds in Brachidontes rodriguezii mussels from the bahía Blanca Estuary, Argentina [J]. Ecotoxicology and Environmental Safety, 2017, 145: 518-527. doi: 10.1016/j.ecoenv.2017.07.052 [34] OLUSHOLA SUNDAY A, ABDULLAHI ALAFARA B, GODWIN OLADELE O. Toxicity and speciation analysis of organotin compounds [J]. Chemical Speciation & Bioavailability, 2012, 24(4): 216-226. [35] ZHANG A Q, ZHOU G J, LAM M H W, et al. Toxicities of Irgarol 1051 derivatives, M2 and M3, to two marine diatom species [J]. Ecotoxicology and Environmental Safety, 2019, 182: 109455. doi: 10.1016/j.ecoenv.2019.109455 [36] HALL L W Jr, ANDERSON R D, KILLEN W D, et al. The relationship of Irgarol and its major metabolite to resident phytoplankton communities in a Maryland Marina, river and reference area [J]. Marine Pollution Bulletin, 2009, 58(6): 803-811. doi: 10.1016/j.marpolbul.2009.02.002 [37] HALL L W Jr, KILLEN W D, ANDERSON R D, et al. Ecological risk of Irgarol 1051 and its major metabolite in coastal California marinas and reference areas [J]. Marine Pollution Bulletin, 2009, 58(5): 702-710. doi: 10.1016/j.marpolbul.2008.12.019 [38] KEY P B, CHUNG K W, HOGUET J, et al. Effects of the anti-fouling herbicide Irgarol 1051 on two life stages of the grass shrimp, Palaemonetes pugio [J]. Journal of Environmental Science and Health, Part B, 2008, 43(1): 50-55. doi: 10.1080/03601230701734865 [39] DELORENZO M E, FULTON M H. Comparative risk assessment of permethrin, chlorothalonil, and diuron to coastal aquatic species [J]. Marine Pollution Bulletin, 2012, 64(7): 1291-1299. doi: 10.1016/j.marpolbul.2012.05.011 [40] TEKIN Z, ÖZTÜRK ER E, GÜNKARA Ö T, et al. A novel determination method for diuron in seaweed samples: Combination of quadruple isotope dilution strategy with liquid chromatography - quadrupole time of flight - tandem mass spectrometry for superior accuracy and precision [J]. Journal of Chromatography A, 2020, 1611: 460612. doi: 10.1016/j.chroma.2019.460612 [41] WANG H, LI Y, HUANG H H, et al. Toxicity evaluation of single and mixed antifouling biocides using the Strongylocentrotus intermedius sea urchin embryo test [J]. Environmental Toxicology and Chemistry, 2011, 30(3): 692-703. doi: 10.1002/etc.440 [42] CHEN L G, ZHANG W P, YE R, et al. Chronic exposure of marine medaka (Oryzias melastigma) to 4, 5-dichloro-2-n-octyl-4-isothiazolin-3-one (DCOIT) reveals its mechanism of action in endocrine disruption via the hypothalamus-pituitary-gonadal-liver (HPGL) axis [J]. Environmental Science & Technology, 2016, 50(8): 4492-4501. [43] BELLAS J. Comparative toxicity of alternative antifouling biocides on embryos and larvae of marine invertebrates [J]. Science of the Total Environment, 2006, 367(2/3): 573-585. [44] KEY P B, MEYER S L, CHUNG K W. Lethal and sub-lethal effects of the fungicide chlorothalonil on three life stages of the grass shrimp, Palaemonetes pugio [J]. Journal of Environmental Science and Health, Part B, 2003, 38(5): 539-549. doi: 10.1081/PFC-120023512 [45] GALLO A, TOSTI E. Reprotoxicity of the antifoulant chlorothalonil in ascidians: An ecological risk assessment [J]. PLoS One, 2015, 10(4): e0123074. doi: 10.1371/journal.pone.0123074 [46] van SCOY A R, TJEERDEMA R S. Environmental fate and toxicology of chlorothalonil [J]. Reviews of Environmental Contamination and Toxicology, 2014, 232: 89-105. [47] LOPES F C, VARELA A S Jr, CORCINI C D, et al. Impacts of the biocide chlorothalonil on biomarkers of oxidative stress, genotoxicity, and sperm quality in guppy Poecilia vivipara [J]. Ecotoxicology and Environmental Safety, 2020, 188: 109847. doi: 10.1016/j.ecoenv.2019.109847 [48] XU X, WANG X, LI Y, et al. Acute toxicity and synergism of binary mixtures of antifouling biocides with heavy metals to embryos of sea urchin Glyptocidaris crenularis [J]. Human & Experimental Toxicology, 2011, 30(8): 1009-1021. [49] HAMWIJK C, SCHOUTEN A, FOEKEMA E M, et al. Monitoring of the booster biocide dichlofluanid in water and marine sediment of Greek marinas [J]. Chemosphere, 2005, 60(9): 1316-1324. doi: 10.1016/j.chemosphere.2005.01.072 [50] FERNANDEZ-ALBA A R, PIEDRA L, MEZCUA M, et al. Toxicity of single and mixed contaminants in seawater measured with acute toxicity bioassays [J]. TheScientificWorldJOURNAL, 2002, 2: 686157. [51] BAO V W W, LEUNG K M Y, QIU J W, et al. Acute toxicities of five commonly used antifouling booster biocides to selected subtropical and cosmopolitan marine species [J]. Marine Pollution Bulletin, 2011, 62(5): 1147-1151. doi: 10.1016/j.marpolbul.2011.02.041 [52] HERNANDO M D, EJERHOON M, FERNÁNDEZ-ALBA A R, et al. Combined toxicity effects of MTBE and pesticides measured with Vibrio fischeri and Daphnia magna bioassays [J]. Water Research, 2003, 37(17): 4091-4098. doi: 10.1016/S0043-1354(03)00348-8 [53] KEY P B, HOGUET J, CHUNG K W, et al. Lethal and sublethal effects of simvastatin, irgarol, and PBDE-47 on the estuarine fish, Fundulus heteroclitus [J]. Journal of Environmental Science and Health, Part B, 2009, 44(4): 379-382. doi: 10.1080/03601230902801083 [54] BELLAS J, BEIRAS R, MARIÑO-BALSA J C, et al. Toxicity of organic compounds to marine invertebrate embryos and larvae: A comparison between the sea urchin embryogenesis bioassay and alternative test species [J]. Ecotoxicology (London, England), 2005, 14(3): 337-353. doi: 10.1007/s10646-004-6370-y [55] KOUTSAFTIS A, AOYAMA I. Toxicity of four antifouling biocides and their mixtures on the brine shrimp Artemia salina [J]. Science of the Total Environment, 2007, 387(1/2/3): 166-174. [56] AVELELAS F, MARTINS R, OLIVEIRA T, et al. Efficacy and ecotoxicity of novel anti-fouling nanomaterials in target and non-target marine species [J]. Marine Biotechnology (New York, N. Y. ), 2017, 19(2): 164-174. doi: 10.1007/s10126-017-9740-1 [57] de CAMPOS B G, FIGUEIREDO J, PERINA F, et al. Occurrence, effects and environmental risk of antifouling biocides (EU PT21): Are marine ecosystems threatened? [J]. Critical Reviews in Environmental Science and Technology, 2021: 1-32. [58] ARRHENIUS Å, BACKHAUS T, HILVARSSON A, et al. A novel bioassay for evaluating the efficacy of biocides to inhibit settling and early establishment of marine biofilms [J]. Marine Pollution Bulletin, 2014, 87(1/2): 292-299. [59] BELLAS J. Toxicity of the booster biocide Sea-Nine to the early developmental stages of the sea urchin Paracentrotus lividus [J]. Aquatic Toxicology, 2007, 83(1): 52-61. doi: 10.1016/j.aquatox.2007.03.011 [60] FIGUEIREDO J, OLIVEIRA T, FERREIRA V, et al. Toxicity of innovative anti-fouling nano-based solutions to marine species [J]. Environmental Science:Nano, 2019, 6(5): 1418-1429. doi: 10.1039/C9EN00011A [61] BELLAS J. Prediction and assessment of mixture toxicity of compounds in antifouling paints using the sea-urchin embryo-larval bioassay [J]. Aquatic Toxicology, 2008, 88(4): 308-315. doi: 10.1016/j.aquatox.2008.05.011 [62] DELORENZO M E, SERRANO L. Individual and mixture toxicity of three pesticides;atrazine, chlorpyrifos, and chlorothalonil to the marine phytoplankton species Dunaliella tertiolecta [J]. Journal of Environmental Science and Health. Part. B, Pesticides, Food Contaminants, and Agricultural Wastes, 2003, 38(5): 529-538. [63] CARTEAU D, VALLÉE-RÉHEL K, LINOSSIER I, et al. Development of environmentally friendly antifouling paints using biodegradable polymer and lower toxic substances [J]. Progress in Organic Coatings, 2014, 77(2): 485-493. doi: 10.1016/j.porgcoat.2013.11.012 [64] LEE S, CHUNG J, WON H, et al. Analysis of antifouling agents after regulation of tributyltin compounds in Korea [J]. Journal of Hazardous Materials, 2011, 185(2/3): 1318-1325. [65] GARG A, MEENA R M, JADHAV S, et al. Distribution of butyltins in the waters and sediments along the coast of India [J]. Marine Pollution Bulletin, 2011, 62(2): 423-431. doi: 10.1016/j.marpolbul.2010.12.003 [66] AL-SHATRI M A, NUHU A A, BASHEER C, et al. Assessment of tributyltin and triphenyltin compounds and their main degradation products in Saudi coastal waters [J]. Arabian Journal for Science and Engineering, 2015, 40(10): 2959-2967. doi: 10.1007/s13369-015-1673-2 [67] MENG P J, LIN J D, LIU L L. Aquatic organotin pollution in Taiwan [J]. Journal of Environmental Management, 2009, 90: S8-S15. doi: 10.1016/j.jenvman.2008.06.008 [68] MANZO S, ANSANELLI G, PARRELLA L, et al. First evaluation of the threat posed by antifouling biocides in the Southern Adriatic Sea [J]. Environmental Science. Processes & Impacts, 2014, 16(8): 1981-1993. [69] ABRAHAM M, WESTPHAL L, HAND I, et al. TBT and its metabolites in sediments: Survey at a German coastal site and the central Baltic Sea [J]. Marine Pollution Bulletin, 2017, 121(1/2): 404-410. [70] SAKKAS V A, KONSTANTINOU I K, LAMBROPOULOU D A, et al. Survey for the occurrence of antifouling paint booster biocides in the aquatic environment of Greece [J]. Environmental Science and Pollution Research International, 2002, 9(5): 327-332. doi: 10.1007/BF02987576 [71] dos SANTOS D M, TURRA A, de MARCHI M R R, et al. Distribution of butyltin compounds in Brazil's southern and southeastern estuarine ecosystems: Assessment of spatial scale and compartments [J]. Environmental Science and Pollution Research International, 2016, 23(16): 16152-16163. doi: 10.1007/s11356-016-6720-3 [72] OKORO H K, FATOKI O S, ADEKOLA F A, et al. Spatio-temporal variation of organotin compounds in seawater and sediments from Cape Town harbour, South Africa using gas chromatography with flame photometric detector (GC-FPD) [J]. Arabian Journal of Chemistry, 2016, 9(1): 95-104. doi: 10.1016/j.arabjc.2013.05.014 [73] FERNANDEZ M V, GARDINALI P R. Risk assessment of triazine herbicides in surface waters and bioaccumulation of Irgarol and M1 by submerged aquatic vegetation in Southeast Florida [J]. Science of the Total Environment, 2016, 541: 1556-1571. doi: 10.1016/j.scitotenv.2015.09.035 [74] SAPOZHNIKOVA Y, WIRTH E, SCHIFF K, et al. Antifouling biocides in water and sediments from California marinas [J]. Marine Pollution Bulletin, 2013, 69(1/2): 189-194. [75] KNUTSON S, DOWNS C A, RICHMOND R H. Concentrations of Irgarol in selected marinas of Oahu, Hawaii and effects on settlement of coral larval [J]. Ecotoxicology (London, England), 2012, 21(1): 1-8. doi: 10.1007/s10646-011-0752-8 [76] METCALFE C D, HELM P, PATERSON G, et al. Pesticides related to land use in watersheds of the Great Lakes Basin [J]. Science of the Total Environment, 2019, 648: 681-692. doi: 10.1016/j.scitotenv.2018.08.169 [77] DINIZ L G R, JESUS M S, DOMINGUEZ L A E, et al. First appraisal of water contamination by antifouling booster biocide of 3rdGeneration at itaqui harbor (são luiz - maranhão - Brazil) [J]. Journal of the Brazilian Chemical Society, 2014, 25(2): 380-388. [78] DOMINGUEZ L A E, CALDAS S S, PRIMEL E G, et al. The influence of salinity and matrix effect in the determination of antifouling biocides in estuarine waters of Patos lagoon (southern Brazil) [J]. Journal of the Brazilian Chemical Society, 2014, 25(7): 1302-1310. [79] BATISTA-ANDRADE J A, CALDAS S S, de OLIVEIRA ARIAS J L, et al. Antifouling booster biocides in coastal waters of Panama: First appraisal in one of the busiest shipping zones [J]. Marine Pollution Bulletin, 2016, 112(1/2): 415-419. [80] SHEIKH M A, JUMA F S, STAEHR P, et al. Occurrence and distribution of antifouling biocide Irgarol-1051 in coral reef ecosystems, Zanzibar [J]. Marine Pollution Bulletin, 2016, 109(1): 586-590. doi: 10.1016/j.marpolbul.2016.05.035 [81] KONING J T, BOLLMANN U E, BESTER K. The occurrence of modern organic antifouling biocides in Danish marinas [J]. Marine Pollution Bulletin, 2020, 158: 111402. doi: 10.1016/j.marpolbul.2020.111402 [82] CAQUET T, ROUCAUTE M, MAZZELLA N, et al. Risk assessment of herbicides and booster biocides along estuarine continuums in the Bay of Vilaine area (Brittany, France) [J]. Environmental Science and Pollution Research International, 2013, 20(2): 651-666. doi: 10.1007/s11356-012-1171-y [83] GUSTAVSSON B M, MAGNÉR J, CARNEY ALMROTH B, et al. Chemical monitoring of Swedish coastal waters indicates common exceedances of environmental thresholds, both for individual substances as well as their mixtures [J]. Marine Pollution Bulletin, 2017, 122(1/2): 409-419. [84] SALEH A, MOLAEI S, SHEIJOONI FUMANI N, et al. Antifouling paint booster biocides (Irgarol 1051 and diuron) in marinas and ports of Bushehr, Persian Gulf [J]. Marine Pollution Bulletin, 2016, 105(1): 367-372. doi: 10.1016/j.marpolbul.2016.02.037 [85] BALAKRISHNAN S, TAKEDA K, SAKUGAWA H. Occurrence of diuron and irgarol in seawater, sediments and planktons of seto inland sea, Japan [J]. Geochemical Journal, 2012, 46(3): 169-177. doi: 10.2343/geochemj.1.0163 [86] LAM K H, CAI Z W, WAI H Y, et al. Identification of a new Irgarol-1051 related s-triazine species in coastal waters [J]. Environmental Pollution, 2005, 136(2): 221-230. doi: 10.1016/j.envpol.2005.01.014 [87] ANSANELLI G, PARRELLA L, di LANDA G, et al. Risk assessment of selected priority pollutants coming from boating activities [J]. Environmental Monitoring and Assessment, 2016, 188(7): 435. doi: 10.1007/s10661-016-5419-8 [88] KÖCK-SCHULMEYER M, GINEBREDA A, GONZÁLEZ S, et al. Analysis of the occurrence and risk assessment of polar pesticides in the Llobregat River Basin (NE Spain) [J]. Chemosphere, 2012, 86(1): 8-16. doi: 10.1016/j.chemosphere.2011.08.034 [89] ANIM A K, THOMPSON K, DUODU G O, et al. Pharmaceuticals, personal care products, food additive and pesticides in surface waters from three Australian east coast estuaries (Sydney, Yarra and Brisbane) [J]. Marine Pollution Bulletin, 2020, 153: 111014. doi: 10.1016/j.marpolbul.2020.111014 [90] PENG Y, FANG W D, KRAUSS M, et al. Screening hundreds of emerging organic pollutants (EOPs) in surface water from the Yangtze River Delta (YRD): Occurrence, distribution, ecological risk [J]. Environmental Pollution, 2018, 241: 484-493. doi: 10.1016/j.envpol.2018.05.061 [91] KIM N S, SHIM W J, YIM U H, et al. Assessment of TBT and organic booster biocide contamination in seawater from coastal areas of South Korea [J]. Marine Pollution Bulletin, 2014, 78(1/2): 201-208. [92] HARINO H, YAMAMOTO Y, EGUCHI S, et al. Concentrations of antifouling biocides in sediment and mussel samples collected from Otsuchi Bay, Japan [J]. Archives of Environmental Contamination and Toxicology, 2007, 52(2): 179-188. doi: 10.1007/s00244-006-0087-2 [93] DOLORES HERNANDO M, PIEDRA L, BELMONTE Á, et al. Determination of traces of five antifouling agents in water by gas chromatography with positive/negative chemical ionisation and tandem mass spectrometric detection [J]. Journal of Chromatography A, 2001, 938(1/2): 103-111. [94] THOMAS K V, BROOKS S. The environmental fate and effects of antifouling paint biocides [J]. Biofouling, 2010, 26(1): 73-88. doi: 10.1080/08927010903216564 [95] STEEN R J C A, ARIESE F, van HATTUM B, et al. Monitoring and evaluation of the environmental dissipation of the marine antifoulant 4, 5-dichloro-2-n-octyl-4-isothiazolin-3-one (DCOIT) in a Danish Harbor [J]. Chemosphere, 2004, 57(6): 513-521. doi: 10.1016/j.chemosphere.2004.06.043 [96] MEYER B, PAILLER J Y, GUIGNARD C, et al. Concentrations of dissolved herbicides and pharmaceuticals in a small river in Luxembourg [J]. Environmental Monitoring and Assessment, 2011, 180(1/2/3/4): 127-146. [97] SANGCHAN W, BANNWARTH M, INGWERSEN J, et al. Monitoring and risk assessment of pesticides in a tropical river of an agricultural watershed in northern Thailand [J]. Environmental Monitoring and Assessment, 2014, 186(2): 1083-1099. doi: 10.1007/s10661-013-3440-8 [98] XING Z S, CHOW L, COOK A, et al. Pesticide application and detection in variable agricultural intensity watersheds and their river systems in the maritime region of Canada [J]. Archives of Environmental Contamination and Toxicology, 2012, 63(4): 471-483. doi: 10.1007/s00244-012-9789-9 [99] GARG A, MEENA R M, BHOSLE N B. Distribution of butyltins in waters and sediments of the Mandovi and Zuari estuaries, west coast of India [J]. Environmental Monitoring and Assessment, 2010, 165(1/2/3/4): 643-651. [100] BERTO D, GIANI M, BOSCOLO R, et al. Organotins (TBT and DBT) in water, sediments, and gastropods of the southern Venice lagoon (Italy) [J]. Marine Pollution Bulletin, 2007, 55(10/11/12): 425-435. [101] WANG X H, HONG H S, ZHAO D M, et al. Environmental behavior of organotin compounds in the coastal environment of Xiamen, China[J]. Marine Pollution Bulletin, 2008, 57(6/7/8/9/10/11/12): 419-424. [102] RADKE B, WASIK A, JEWELL L L, et al. The speciation of organotin compounds in sediment and water samples from the port of Gdynia [J]. Soil and Sediment Contamination:an International Journal, 2013, 22(6): 614-630. doi: 10.1080/15320383.2013.756448 [103] LIU L L, WANG J T, CHUNG K N, et al. Distribution and accumulation of organotin species in seawater, sediments and organisms collected from a Taiwan mariculture area[J]. Marine Pollution Bulletin, 2011, 63(5/6/7/8/9/10/11/12): 535-540. [104] RADKE B, WASIK A, JEWELL L L, et al. Seasonal changes in organotin compounds in water and sediment samples from the semi-closed Port of Gdynia [J]. Science of the Total Environment, 2012, 441: 57-66. doi: 10.1016/j.scitotenv.2012.09.006 [105] SHUE M F, CHEN T C, BELLOTINDOS L M, et al. Tributyltin distribution and producing androgenic activity in water, sediment, and fish muscle [J]. Journal of Environmental Science and Health. Part. B, Pesticides, Food Contaminants, and Agricultural Wastes, 2014, 49(6): 432-438. [106] GAO J M, CHEN X L, REN C R, et al. Organotins in the aquatic media of secondary anabranches in the Three Gorges Reservoir Region, China [J]. Chemosphere, 2019, 217: 232-242. doi: 10.1016/j.chemosphere.2018.10.204 [107] BANDARA K R V, CHINTHAKA S D M, YASAWARDENE S G, et al. Modified, optimized method of determination of Tributyltin (TBT) contamination in coastal water, sediment and biota in Sri Lanka [J]. Marine Pollution Bulletin, 2021, 166: 112202. doi: 10.1016/j.marpolbul.2021.112202 [108] SÁNCHEZ-RODRÍGUEZ Á, SOSA-FERRERA Z, SANTANA-DEL PINO Á, et al. Probabilistic risk assessment of common booster biocides in surface waters of the harbours of Gran Canaria (Spain) [J]. Marine Pollution Bulletin, 2011, 62(5): 985-991. doi: 10.1016/j.marpolbul.2011.02.038 [109] KIM N S, HONG S H, AN J G, et al. Distribution of butyltins and alternative antifouling biocides in sediments from shipping and shipbuilding areas in South Korea [J]. Marine Pollution Bulletin, 2015, 95(1): 484-490. doi: 10.1016/j.marpolbul.2015.03.010 [110] ALI H R, ARIFIN M M, SHEIKH M A, et al. Occurrence and distribution of antifouling biocide Irgarol-1051 in coastal waters of Peninsular Malaysia [J]. Marine Pollution Bulletin, 2013, 70(1/2): 253-257. [111] MAI H, MORIN B, PARDON P, et al. Environmental concentrations of irgarol, diuron and S-metolachlor induce deleterious effects on gametes and embryos of the Pacific oyster, Crassostrea gigas [J]. Marine Environmental Research, 2013, 89: 1-8. doi: 10.1016/j.marenvres.2013.04.003 [112] KAONGA C C, TAKEDA K, SAKUGAWA H. Concentration and degradation of alternative biocides and an insecticide in surface waters and their major sinks in a semi-enclosed sea, Japan [J]. Chemosphere, 2016, 145: 256-264. doi: 10.1016/j.chemosphere.2015.11.100 [113] WILLE K, CLAESSENS M, RAPPÉ K, et al. Rapid quantification of pharmaceuticals and pesticides in passive samplers using ultra high performance liquid chromatography coupled to high resolution mass spectrometry [J]. Journal of Chromatography A, 2011, 1218(51): 9162-9173. doi: 10.1016/j.chroma.2011.10.039 [114] MIJANGOS L, ZIARRUSTA H, ROS O, et al. Occurrence of emerging pollutants in estuaries of the Basque Country: Analysis of sources and distribution, and assessment of the environmental risk [J]. Water Research, 2018, 147: 152-163. doi: 10.1016/j.watres.2018.09.033 [115] KONSTANTINOU I K, ALBANIS T A. Worldwide occurrence and effects of antifouling paint booster biocides in the aquatic environment: A review [J]. Environment International, 2004, 30(2): 235-248. doi: 10.1016/S0160-4120(03)00176-4 [116] EGUCHI S, HARINO H, YAMAMOTO Y. Assessment of antifouling biocides contaminations in maizuru bay, Japan [J]. Archives of Environmental Contamination and Toxicology, 2010, 58(3): 684-693. doi: 10.1007/s00244-009-9394-8 [117] MOCHIDA K, HANO T, ONDUKA T, et al. Spatial analysis of 4, 5-dichloro-2-n-octyl-4-isothiazolin-3-one (Sea-Nine 211) concentrations and probabilistic risk to marine organisms in Hiroshima Bay, Japan [J]. Environmental Pollution, 2015, 204: 233-240. doi: 10.1016/j.envpol.2015.05.012 [118] YAMAMOTO A, MIYAMOTO I, KITAGAWA M, et al. Analysis of chlorothalonil by liquid chromatography/mass spectrometry using negative-ion atmospheric pressure photoionization [J]. Analytical Sciences, 2009, 25(5): 693-697. doi: 10.2116/analsci.25.693 [119] CRESSWELL T, RICHARDS J P, GLEGG G A, et al. The impact of legislation on the usage and environmental concentrations of Irgarol 1051 in UK coastal waters [J]. Marine Pollution Bulletin, 2006, 52(10): 1169-1175. doi: 10.1016/j.marpolbul.2006.01.014 [120] SAPOZHNIKOVA Y, WIRTH E, SCHIFF K, et al. Antifouling pesticides in the coastal waters of Southern California [J]. Marine Pollution Bulletin, 2007, 54(12): 1972-1978. doi: 10.1016/j.marpolbul.2007.09.026 [121] CARBERY K, OWEN R, FRICKERS T, et al. Contamination of Caribbean coastal waters by the antifouling herbicide Irgarol 1051 [J]. Marine Pollution Bulletin, 2006, 52(6): 635-644. doi: 10.1016/j.marpolbul.2005.10.013 [122] PAZ-VILLARRAGA C A, CASTRO Í B, MILOSLAVICH P, et al. Venezuelan Caribbean Sea under the threat of TBT [J]. Chemosphere, 2015, 119: 704-710. doi: 10.1016/j.chemosphere.2014.07.068 [123] CASTRO Í B, IANNACONE J, SANTOS S, et al. TBT is still a matter of concern in Peru [J]. Chemosphere, 2018, 205: 253-259. doi: 10.1016/j.chemosphere.2018.04.097 [124] BATISTA R M, CASTRO I B, FILLMANN G. Imposex and butyltin contamination still evident in Chile after TBT global ban [J]. Science of the Total Environment, 2016, 566/567: 446-453. doi: 10.1016/j.scitotenv.2016.05.039 [125] DONG C D, CHEN C F, CHEN C W. Composition and source of butyltins in sediments of Kaohsiung Harbor, Taiwan [J]. Estuarine, Coastal and Shelf Science, 2015, 156: 134-143. doi: 10.1016/j.ecss.2014.08.002 [126] CHEN C Z, CHEN L, LI F P, et al. Urgent caution to trace organometal pollution: Occurrence, distribution and sources of methyltins, butyltins and phenyltins in sediments from South Hangzhou Bay, China [J]. Environmental Pollution, 2019, 246: 571-577. doi: 10.1016/j.envpol.2018.12.037 [127] LEE M R N, KIM U J, LEE I S, et al. Assessment of organotin and tin-free antifouling paints contamination in the Korean coastal area [J]. Marine Pollution Bulletin, 2015, 99(1/2): 157-165. [128] WANG X M, KONG L N, CHENG J Y, et al. Distribution of butyltins at dredged material dumping sites around the coast of China and the potential ecological risk [J]. Marine Pollution Bulletin, 2019, 138: 491-500. doi: 10.1016/j.marpolbul.2018.11.043 [129] BRIANT N, BANCON-MONTIGNY C, ELBAZ-POULICHET F, et al. Trace elements in the sediments of a large Mediterranean Marina (Port Camargue, France): Levels and contamination history [J]. Marine Pollution Bulletin, 2013, 73(1): 78-85. doi: 10.1016/j.marpolbul.2013.05.038 [130] SOUSA A C A, OLIVEIRA I B, LARANJEIRO F, et al. Organotin levels in Nazaré canyon (west Iberian Margin, NE Atlantic) and adjacent coastal area [J]. Marine Pollution Bulletin, 2012, 64(2): 422-426. doi: 10.1016/j.marpolbul.2011.11.013 [131] MAGNUSSON M, HEIMANN K, RIDD M, et al. Pesticide contamination and phytotoxicity of sediment interstitial water to tropical benthic microalgae [J]. Water Research, 2013, 47(14): 5211-5221. doi: 10.1016/j.watres.2013.06.003 [132] HARINO H, ARIFIN Z, RUMENGAN I F M, et al. Distribution of antifouling biocides and perfluoroalkyl compounds in sediments from selected locations in Indonesian coastal waters [J]. Archives of Environmental Contamination and Toxicology, 2012, 63(1): 13-21. doi: 10.1007/s00244-011-9747-y [133] SOROLDONI S, VIEIRA da SILVA S, CASTRO Í B, et al. Antifouling paint particles cause toxicity to benthic organisms: Effects on two species with different feeding modes [J]. Chemosphere, 2020, 238: 124610. doi: 10.1016/j.chemosphere.2019.124610 [134] ABREU F E L, LIMA da SILVA J N, CASTRO Í B, et al. Are antifouling residues a matter of concern in the largest South American Port? [J]. Journal of Hazardous Materials, 2020, 398: 122937. doi: 10.1016/j.jhazmat.2020.122937 [135] MACIEL D C, CASTRO Í B, de SOUZA J R B, et al. Assessment of organotins and imposex in two estuaries of the northeastern Brazilian coast [J]. Marine Pollution Bulletin, 2018, 126: 473-478. doi: 10.1016/j.marpolbul.2017.11.061 [136] ANASTASIOU T I, CHATZINIKOLAOU E, MANDALAKIS M, et al. Imposex and organotin compounds in ports of the Mediterranean and the Atlantic: Is the story over? [J]. Science of the Total Environment, 2016, 569/570: 1315-1329. doi: 10.1016/j.scitotenv.2016.06.209 [137] GAO J M, ZHANG K, CHEN Y P, et al. Occurrence of organotin compounds in river sediments under the dynamic water level conditions in the Three Gorges Reservoir Area, China [J]. Environmental Science and Pollution Research International, 2015, 22(11): 8375-8385. doi: 10.1007/s11356-014-3986-1 [138] QUINTAS P Y, OLIVA A L, ARIAS A, et al. Seasonal changes in organotin compounds in sediments from the Bahía Blanca Estuary [J]. Environmental Earth Sciences, 2016, 75(8): 1-13. [139] CHEN C Z, CHEN L, XUE R, et al. Correction to: Spatiotemporal variation and source apportionment of organotin compounds in sediments in the Yangtze Estuary [J]. Environmental Sciences Europe, 2019, 31: 43. doi: 10.1186/s12302-019-0224-y [140] FILIPKOWSKA A, KOWALEWSKA G. Butyltins in sediments from the Southern Baltic coastal zone: Is it still a matter of concern, 10 years after implementation of the total ban? [J]. Marine Pollution Bulletin, 2019, 146: 343-348. doi: 10.1016/j.marpolbul.2019.06.050 [141] van GESSELLEN N, BOUWMAN H, AVERBUJ A. Imposex assessment and tributyltin levels in sediments along the Atlantic coast of South Africa [J]. Marine Environmental Research, 2018, 142: 32-39. doi: 10.1016/j.marenvres.2018.09.016 [142] SOROLDONI S, CASTRO Í B, ABREU F, et al. Antifouling paint particles: Sources, occurrence, composition and dynamics [J]. Water Research, 2018, 137: 47-56. doi: 10.1016/j.watres.2018.02.064 [143] VIANA J L M, dos SANTOS S R V, dos SANTOS FRANCO T C R, et al. Occurrence and partitioning of antifouling booster biocides in sediments and porewaters from Brazilian northeast [J]. Environmental Pollution, 2019, 255: 112988. doi: 10.1016/j.envpol.2019.112988 [144] SOROLDONI S, ABREU F, CASTRO Í B, et al. Are antifouling paint particles a continuous source of toxic chemicals to the marine environment? [J]. Journal of Hazardous Materials, 2017, 330: 76-82. doi: 10.1016/j.jhazmat.2017.02.001 [145] MUKHTAR A, ZULKIFLI S Z, MOHAMAT-YUSUFF F, et al. Distribution of biocides in selected marine organisms from South of Johor, Malaysia [J]. Regional Studies in Marine Science, 2020, 38: 101384. doi: 10.1016/j.rsma.2020.101384 -




 DownLoad:
DownLoad: