-
进入环境中的重金属具有高毒性、持久性、不可生物降解性和生物累积性等的特点,不仅会威胁生态系统的安全,而且还可以通过食物链危及人群的身体健康[1-4]。底泥是重金属的重要载体。随着全球城镇化和工业化进程的加快,大量的重金属排入湖泊、水库和河流等地表水体,最终汇集到底泥中,造成水体底泥的重金属污染[1-4]。当外界环境条件合适时,底泥中累积的重金属会释放出来进入上覆水体中,成为水体重金属污染的内源,对水体造成二次污染[3-4]。目前,水体底泥的重金属污染已成为世界范围内的一个重要环境问题[5-6]。因此,控制水体污染底泥中重金属的释放刻不容缓。
水体底泥重金属释放控制技术可分为两大类:异位和原位控制技术[5-7]。原位覆盖/改良技术,即将修复材料覆盖到底泥-水界面上方或直接添加进底泥中,被认为是一种极具应用前景的水体底泥重金属释放控制技术[5-7]。该技术具有运行简单、修复成本低、修复速度快和环境友好等的优点[5-7]。选择合适的修复材料,对于利用原位覆盖/改良技术控制底泥中重金属释放而言是非常重要的。目前,许多重金属污染底泥的修复材料已引起了人们的关注,包括磷灰石[8]、生物炭[9]、碳纳米管[10]、零价铁[10]、沸石[11]和铁氧化物[12]等。
铜(Cu)和铅(Pb)是两种底泥中广泛存在的重金属。沸石是一种含水的碱或碱土金属铝硅酸盐矿物,不仅来源广泛、价格低廉,而且对水中Cu2+和Pb2+等重金属阳离子的吸附性能较好[13-14]。Xiong等研究发现,2 cm厚天然沸石覆盖层对底泥中Pb释放的抑制率可达85.7%[11]。方解石是一种分布很广的碳酸钙矿物。研究发现,方解石对水中Cu2+和Pb2+等重金属阳离子的去除性能较好[15-17]。另外,最近的研究发现,采用铁盐对方解石进行改性所获得的铁改性方解石(铁方解石)是一种非常有应用前景的用于控制水体底泥磷释放的活性覆盖材料[18]。考虑到铁氧化物对水中Cu2+和Pb2+等重金属阳离子具有较好的吸附能力[19-20],因此天然沸石和铁方解石的联合覆盖预计也可用于水体底泥中Cu和Pb等重金属释放的控制。但是,目前对天然沸石和铁方解石联合覆盖控制水体底泥Cu和Pb释放效果及机制的了解尚不十分清楚。
为此,本研究分析了天然沸石和铁方解石对水中Cu2+和Pb2+的吸附特性,考察了天然沸石和铁方解石联合覆盖对上覆水中Cu和Pb浓度动态变化的影响,辨析了联合覆盖对上覆水-底泥垂向剖面上有效态Cu和Pb分布特征的影响,观察了联合覆盖对底泥-水界面Cu和Pb扩散通量的影响,分析了被联合覆盖层所吸附Cu和Pb的赋存形态,探讨了天然沸石和铁方解石联合覆盖对底泥中Cu和Pb释放的控制机制,以期为联合利用天然沸石和铁方解石作为覆盖材料控制水体底泥中重金属释放提供科技支撑。
-
底泥取自上海市浦东新区上海海洋大学校园景观水体,自然风干并过100 目筛。氯化铁、硝酸铅、硝酸铜、硝酸、乙酸、盐酸羟胺、过氧化氢、乙酸、乙酸铵、氢氧化钠和盐酸等化学药剂均购自国药集团化学试剂有限公司,均为分析纯。天然沸石(NZ)来自浙江省缙云县某矿山。方解石(CA)来自浙江省长兴县某矿山。Chelex DGT(薄膜扩散梯度)装置由南京智感环境科技有限公司提供。实验所用的水为去离子水。分别采用Cu(NO3)2·3H2O和Pb(NO3)2配制硝酸铜和硝酸铅的储备液,再通过稀释的方式获得硝酸铜和硝酸铅的使用液。使用液中铜和铅的浓度均以元素铜和铅计。
-
铁方解石(FeCA)的制备步骤为:称取20 g 过200 目筛的方解石粉末放入到500 mL 锥形瓶中,然后再向该锥形瓶中加入5 g FeCl3·6H2O,随后再向该锥形瓶中加入100 mL 去离子水并摇匀;接着,将该锥形瓶放置到磁力搅拌器上,再用1 mol·L−1氢氧化钠溶液以稳定的速度滴入到锥形瓶中,将悬浮液的pH值调节至10,然后继续搅拌反应1 h;随后采用离心分离的方式获得固体材料,再采用去离子水清洗材料5遍;再将所获得的固体材料放入到105℃的烘箱中进行烘干,烘干后将材料研磨收集即得到FeCA,并用自封袋密封备用。
-
称取2 kg的风干底泥置于容器中;分别称取4.53 g Cu(NO3)2·3H2O(等同于向底泥中添加600 mg·kg−1的Cu)和3.20 g Pb(NO3)2(等同于向底泥中添加1000 mg·kg−1的Pb)置于同一个烧杯中,再加入500 mL去离子水,配成含硝酸铜和硝酸铅的混合溶液;然后将该混合溶液倒入到含有风干底泥的容器中并加以搅拌,接着分2次加入 500 mL去离子水;再手动搅拌40 min,然后静置过夜,制备得到重金属污染底泥。
-
通过批量吸附实验研究溶液初始浓度对NZ和FeCA去除水中Cu2+的影响。实验步骤为:将25 mg NZ或FeCA加入到50 mL玻璃瓶中,再加入25 mL硝酸铜溶液,溶液的初始pH值设置为6,再将锥形瓶放置于水浴振荡器中恒温振荡24 h,温度设为25 ℃,转数为250 r·min−1;反应结束后离心分离获得上清液,再用原子火焰法测定上清液中铜的浓度。对于NZ吸附铜离子实验,初始浓度分别为5、10、15、20、25、30 mg·L−1。对于FeCA吸附水中铜离子实验,初始浓度分别为5、10、15、20、25、30、35、40、50 mg·L−1。
通过批量吸附实验考察吸附剂投加量对NZ吸附水中Cu2+的影响。实验步骤为:分别称取质量为15、20、25、30、40、50、60 mg的NZ置于一系列的100 mL锥形瓶中,再加入pH值为6、浓度为10 mg·L−1的硝酸铜溶液50 mL,再置于水浴振荡器中振荡反应24 h;反应时间结束后,通过离心分离的方式获取上清液;然后采用原子火焰法测定上清液中残留的铜浓度。对于吸附剂投加量对FeCA去除水中铜离子的影响实验,吸附剂质量分别为15、20、25、30、40、50、60 mg,初始浓度为20 mg·L−1,实验步骤同上。
-
通过批量吸附实验考察初始浓度对NZ和FeCA吸附水中Pb2+的影响。对于NZ的吸附实验,溶液体积为25 mL,吸附剂投加量为25 mg,溶液pH值为6,初始Pb浓度分别为75、100、125、150、200 mg·L−1,反应时间为24 h。对于FeCA的吸附实验,溶液体积为100 mL,吸附剂投加量为10 mg,溶液初始pH值为6,初始Pb浓度分别为10、20、40、60、80、100、150、200 mg·L−1,反应时间为24 h。
通过批量吸附实验考察吸附剂投加量对NZ和FeCA吸附水中Pb2+的影响。对于NZ的吸附实验,溶液体积为50 mL,初始Pb浓度为10 mg·L−1,初始pH值为6,吸附剂投加量分别为15、20、25、30、40、50、60 mg,反应时间为24 h。对于FeCA的吸附实验,溶液体积为50 mL,初始Pb浓度为20 mg·L−1,初始pH值为6,吸附剂投加量分别为15、20、25、30、40、50、60 mg,反应时间为24 h。
NZ和FeCA对水中Cu(II)和Pb(II)的单位吸附量(Qe,mg·g−1)和去除率(RE,%)分别采用公式(1)和(2)进行计算。
式中,C0和Ce分别代表初始时刻和平衡时刻水中Cu(Ⅱ)或Pb(Ⅱ)的浓度(mg·L−1);V代表Cu(Ⅱ)或Pb(Ⅱ)溶液的体积(L);M代表NZ或FeCA的投加量(g)。
-
称取634 g 湿的重金属污染底泥置于1.3 L玻璃瓶中,并对玻璃瓶中底泥进行以下处理:① 底泥不进行任何处理(对照组);② 将20 g NZ覆盖于底泥-水界面上方,并铺设均匀(沸石覆盖组);③ 将20 g FeCA均匀铺设到底泥-水界面上方(铁方解石覆盖组);④ 先将20 g NZ覆盖到底泥-水界面上方,再将20 g FeCA均匀铺设到沸石覆盖层上方(联合覆盖组)。配制含10 mmol·L−1 NaCl、1 mmol·L−1 NaHCO3和1 mmol·L−1 CaCl2的溶液,并将其pH值调节至7.5,然后采用亚硫酸钠氧化法去除溶液中的溶解氧(DO),使 DO浓度低于 0.5 mg·L−1。将配制好的溶液加入到玻璃瓶中,直至上覆水位到达玻璃瓶的瓶口,然后塞上橡胶塞,并用白凡士林涂抹封口,营造底泥处于缺氧状态的环境。每间隔一段时间采用便携式溶氧仪测定上覆水中DO 浓度,并采用原子吸收法测定上覆水中Cu和Pb的浓度。培养339 d之后,插入Chelex DGT测定上覆水和底泥中DGT有效态Cu和Pb的浓度。
DGT膜中积累的Cu或Pb的质量(m,mg)根据公式(3)进行计算[21]。
式中,Cl为HNO3提取液中Cu或Pb的浓度(mg·L−1);Vgel是DGT凝胶的体积(L);Vacid为HNO3提取液的体积(L);fe为Cu或Pb的提取率。
DGT有效态Cu或Pb浓度(CDGT,mg·L−1)根据公式(4)进行计算[21]。
式中,∆g为扩散层和滤膜的厚度(cm);Dd为Cu或Pb的扩散系数(cm2·s−1);A为DGT膜暴露面积(cm2);T为DGT放置时间(d)。
底泥-水界面Cu或Pb的表面扩散速率[J,µg·(m2·d)−1]采用以下公式进行计算[22]:
式中,Dw为Cu或Pb在水中的扩散系数(cm2·s−1);Ds为Cu或Pb在底泥中的扩散系数(cm2·s−1);
(∂CDGT∂xw)(x=0) 和(∂CDGT∂xs)(x=0) 分别为Cu或Pb在水相和底泥相中的浓度梯度[µg·(L·mm)−1]。Ds可根据以下公式进行计算[22]:底泥培养实验结束之后,收集覆盖材料,并采用改进的BCR连续提取法[4]对覆盖材料中Cu和Pb的赋存形态进行分析。该方法将重金属的形态定义为可交换态(F1)、可还原态(F2)、可氧化态(F3)和残渣态(F4)4种形态。取0.5 g覆盖材料置于50 mL离心管中,然后分别采用不同的提取剂对覆盖材料中各形态Cu和Pb进行分级提取,再采用原子吸收法测定提取液中各形态Cu和Pb的含量。另外,本研究也采用改进的BCR连续提取法[4]对底泥中Cu和Pb的赋存形态进行分析。
-
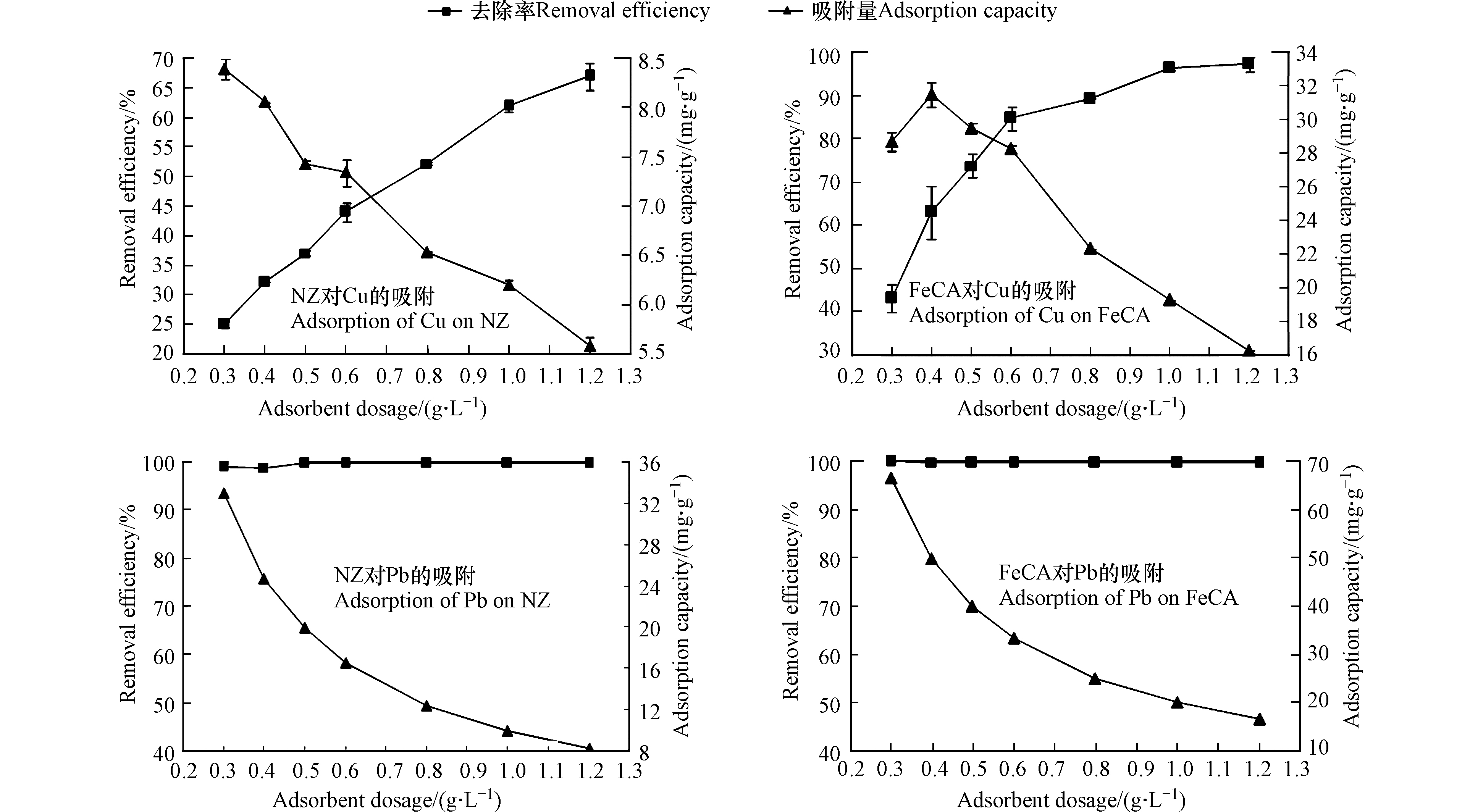
图1是吸附剂投加量对NZ和FeCA去除水中Cu(Ⅱ)和Pb(Ⅱ)的影响。从图1可见,吸附剂投加量由0.3 g·L−1逐渐增加到1.2 g·L−1时,NZ对水中Cu(Ⅱ)的去除率由25.2%增加到67.0%,而NZ对水中Cu(Ⅱ)的单位吸附量则由8.39 mg·g−1逐渐下降到5.58 mg·g−1。当吸附剂投加量由0.3 g·L−1逐渐增加到1.2 g·L−1时,FeCA对水中Cu(Ⅱ)的去除率逐渐从43.0%增加到97.2%,这与吸附剂投加量影响NZ对水中Cu(Ⅱ)去除率的规律类似。与NZ略微不同的是,随着吸附剂投加量由0.3 g·L−1逐渐增加到1.2 g·L−1时,FeCA对水中Cu(Ⅱ)吸附量先略微增加(当吸附剂投加量从0.3 g·L−1增加到0.4 g·L−1时,吸附量从28.7 mg·g−1增加到31.5 mg·g−1)而后持续下降(当吸附剂投加量从0.4 g·L−1增加到1.2 g·L−1时,吸附量从31.5 mg·g−1下降到16.2 mg·g−1)。NZ和FeCA对水中Cu(Ⅱ)去除率随着吸附剂投加量增加而增加的原因主要是:吸附剂投加量越大,提供的吸附剂活性点位越多,更有利于水中的Cu2+被吸附剂所吸附去除[23]。NZ和FeCA对水中Cu(Ⅱ)单位吸附量随着吸附剂投加量增加而降低的原因主要是:吸附剂投加量的增加导致了吸附剂表面上活性位点的聚集以及吸附质扩散路径长度的增加[23]。从图1中还可见,当吸附剂投加量由0.3 g·L−1增加到1.2 g·L−1时,NZ和FeCA对水中Pb(Ⅱ)的去除率基本保持不变,分别位于98.6%—99.7%和99.7%—99.9%。此外,当吸附剂投加量由0.3 g·L−1增加到1.2 g·L−1时,NZ和FeCA对水中Pb(Ⅱ)的单位吸附量则会逐渐下降,分别由33.0 mg·g−1下降到8.30 mg·g−1和由66.6 mg·g−1下降到16.6 mg·g−1。由以上分析可见,NZ和FeCA对水中Cu(Ⅱ)和Pb(Ⅱ)均具有较强的吸附去除能力。
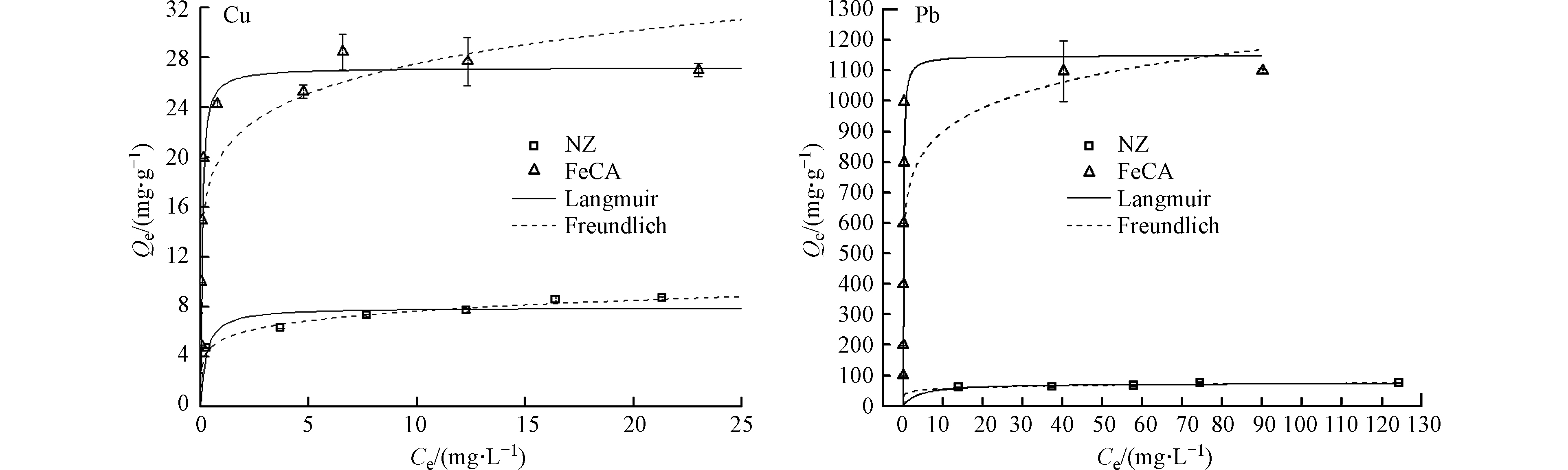
图2为NZ和FeCA对水中Cu(Ⅱ)和Pb(Ⅱ)的吸附等温线。由图2可见,NZ和FeCA对水中Cu(Ⅱ)的单位吸附量随着吸附平衡浓度的增加而增加,直至达到吸附饱和或者接近饱和。随着水中Pb(Ⅱ)平衡浓度的逐渐增加,NZ和FeCA对Pb(Ⅱ)的单位吸附量逐渐增加直至达到吸附饱和或者接近饱和。进一步采用Langmuir [式(7)][24]和Freundlich [式(8)][24]等温吸附模型对图2中的实验数据进行拟合分析,所获得模型参数值列于表1。根据模型参数值计算得到的Langmuir和Freundlich等温线也列于图2。
式中,Qe代表吸附剂对水中吸附质的吸附量(mg·g−1);Ce代表水中残留的吸附质浓度;QMax代表吸附剂对水中吸附质的最大单分子层吸附量(mg·g−1);KL代表Langmuir常数(L·mg−1);KF和1/n均代表Freundlich常数。
从表1中可见,与Langmuir模型相比,NZ对水中Cu(Ⅱ)和Pb(Ⅱ)的等温吸附行为更适合采用Freundlich模型加以描述。与之相反的是,FeCA对水中Cu(Ⅱ)和Pb(Ⅱ)的等温吸附行为则更适合采用Langmuir模型加以描述,说明FeCA对水中Cu(Ⅱ)和Pb(Ⅱ)的吸附属于单分子层吸附。根据Langmuir等温吸附模型,NZ对水中Cu(Ⅱ)和Pb(Ⅱ)的最大单分子层吸附量分别为7.98 mg·g−1和75.4 mg·g−1,接近根据实验确定的最大吸附量(Cu:8.69 mg·g−1;Pb:75.7 mg·g−1)。FeCA对水中Cu(Ⅱ)和Pb(Ⅱ)的最大单分子层吸附量分别为27.2 mg·g−1和1150 mg·g−1,接近根据实验确定的最大吸附量(Cu:28.4 mg·g−1;Pb:1099 mg·g−1)。这说明,采用Langmuir等温吸附模型预测NZ和FeCA对水中Pb和Cu的最大吸附量是可靠的。很显然,FeCA对水中Cu(Ⅱ)和Pb(Ⅱ)的最大单分子层吸附量高于NZ。这进一步说明了FeCA对水中Cu(Ⅱ)和Pb(Ⅱ)的吸附能力强于NZ。
表2为国内外先前文献报道的沸石、碳酸盐基材料和铁氧化物对水中Cu(Ⅱ)和Pb(Ⅱ)的最大单位吸附量。从表2中可见,本研究所用的天然沸石对水中Cu(Ⅱ)的最大吸附量(7.98 mg·g−1)小于先前文献报道的天然沸石(26.24 mg·g−1),也小于先前文献报道的人工合成沸石(57.803—202.76 mg·g−1)。另外,本研究所用的天然沸石对水中Pb(Ⅱ)的最大吸附量(75.4 mg·g−1)介于先前文献报道的天然沸石的最大吸附量(14.2—89.096 mg·g−1)之间,但小于先前文献报道的人工合成沸石最大吸附量(109.890—441.336 mg·g−1)。虽然天然沸石对水中Cu(Ⅱ)和Pb(Ⅱ)的最大吸附量小于人工合成沸石,但是前者的成本通常是小于后者的。因此,从性价比的角度看,应用天然沸石作为覆盖材料控制底泥中Cu和Pb的释放预计是更有希望的。虽然铁方解石对水中Cu(Ⅱ)的最大吸附量小于先前文献报道的碳酸盐基材料(大理石),也小于人工合成的沸石,但是铁方解石对水中Pb(Ⅱ)的最大吸附量明显大于先前文献报道的天然沸石、人工合成沸石和碳酸盐基材料(碳酸盐基尾矿渣和球霰石型碳酸钙)。所以,从吸附容量的角度看,利用铁方解石作为覆盖材料控制底泥中Pb的释放是很有希望的。
先前研究[23,32-33]已对沸石吸附水中Cu2+和Pb2+等重金属阳离子的机制开展了研究,结果发现,阳离子交换和静电吸引是沸石吸附水中Cu2+和Pb2+等重金属阳离子的重要作用机制。因此,笔者推测,本研究所用NZ吸附水中Cu2+和Pb2+的主要机制涉及阳离子交换和静电吸引。Tang等研究发现,方解石溶解和含铜沉淀物形成是方解石去除水中Cu2+的重要机制[34]。Song等研究发现,方解石去除水中Cu2+的机制主要为表面吸附和化学沉淀作用[35]。内层配合物形成是铁氧化物吸附水中Cu2+和Pb2+的重要机制[36]。所以,笔者推测,铁方解石去除水中Cu2+和Pb2+的主要机制涉及表面吸附和化学沉淀。
-
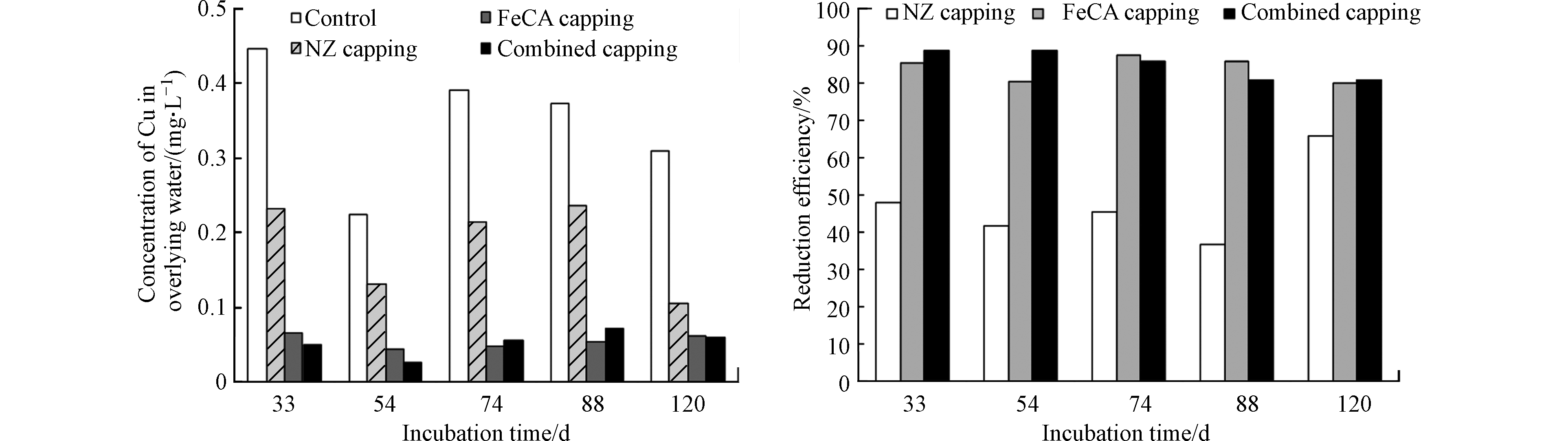
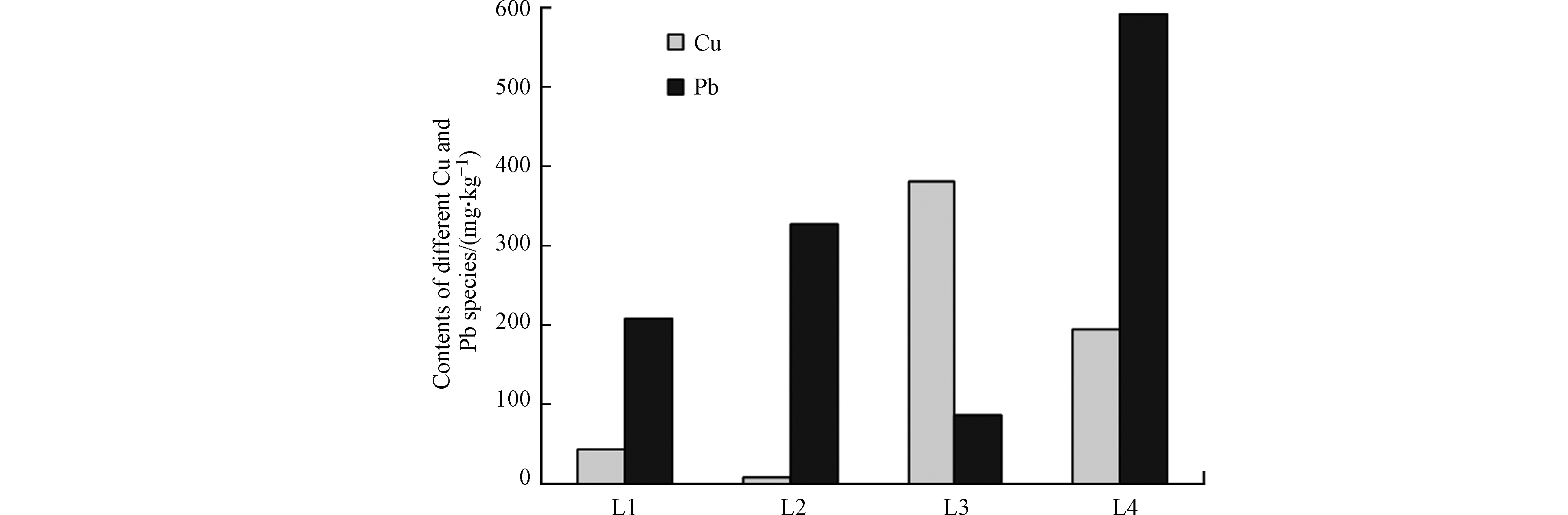
本研究所用底泥中可交换态、可还原态、可氧化态和残渣态Cu的含量分别为43.5、10.2、381 mg·kg−1和196 mg·kg−1,可交换态、可还原态、可氧化态和残渣态Pb的含量分别为209、326、86.9、592 mg·kg−1。在采用BCR法提取的4种形态重金属中,可交换态重金属被认为是最容易释放和最具生物有效性的[4]。可还原态重金属主要是指与铁锰氧化物/氢氧化物所结合的重金属,在缺氧条件下容易被释放出来[4]。本研究所用底泥中F1+F2态Cu和Pb含量分别为53.7 mg·kg−1和535 mg·kg−1。这说明本研究所用底泥中Cu和Pb存在释放的风险。底泥培养期间对照组、NZ覆盖组、FeCA覆盖组和联合覆盖组上覆水中DO浓度的最大值分别为0.65、0.17、0.79、0.25 mg·L−1。这说明,底泥培养期间各反应器中上覆水均处于缺氧状态。图3为对照组、NZ覆盖组、FeCA覆盖组和联合覆盖组上覆水中Cu浓度随培养时间变化而变化的规律以及NZ覆盖、FeCA覆盖和联合覆盖对上覆水中Cu的去除率。从中可见,对照组上覆水中Cu浓度位于0.223—0.447 mg·L−1。这说明缺氧条件下底泥中Cu会释放出来进入上覆水中。NZ覆盖组上覆水中Cu浓度为0.105—0.236 mg·L−1,低于对照组。计算得到的NZ覆盖对上覆水中Cu的削减率为37%—66%。这表明,天然沸石覆盖可以有效降低底泥中Cu向上覆水体中的释放。通常,底泥中物质向上覆水体释放的过程包括两个基本过程:(1)底泥中物质先释放进入间隙水中;(2)间隙水中溶解态物质通过分子扩散机制穿过底泥-水界面向上覆水迁移[37-38]。天然沸石对水中Cu2+具有较好的吸附能力(图1和图2)。底泥间隙水中Cu2+穿越底泥-水界面时可被天然沸石覆盖层所吸附,进而导致底泥-水界面Cu扩散通量的下降,从而导致上覆水中Cu浓度的下降。从图3中还可见,FeCA覆盖组上覆水中Cu的浓度为0.044—0.066 mg·L−1,低于对照组。计算得到的FeCA覆盖对上覆水中Cu的削减率为80%—86%。这说明,铁方解石覆盖可有效降低底泥中Cu向上覆水体中的释放风险。铁方解石对水中Cu具有较好的吸附能力(图1和图2)。铁方解石覆盖层可通过吸附作用拦截底泥间隙水中Cu向上覆水体的扩散,从而降低上覆水中Cu的浓度。铁方解石覆盖对上覆水中Cu的削减率高于天然沸石。这说明铁方解石覆盖控制底泥中Cu释放的效果优于天然沸石。联合覆盖组上覆水中Cu的浓度明显低于对照组,天然沸石和铁方解石联合覆盖对上覆水中Cu的削减率为81%—89%。由此可见,天然沸石和铁方解石联合覆盖同样可以有效控制底泥中Cu向上覆水体中的释放。这主要归功于联合覆盖层中天然沸石和铁方解石对底泥中Cu释放的拦截作用。另外,NZ/FeCA联合覆盖对底泥中Cu向上覆水体中释放的效率要高于单独的NZ覆盖。
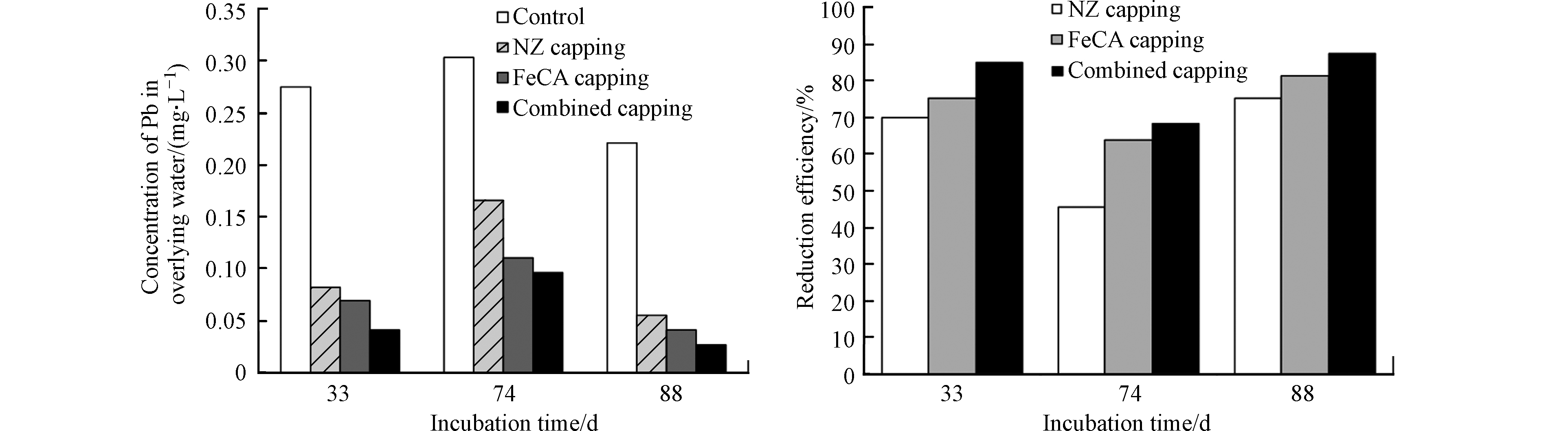
图4为各实验组上覆水中Pb浓度随培养时间变化而变化的规律以及NZ覆盖、FeCA覆盖和联合覆盖对上覆水中Pb的去除率。
从图4中还可见,对照组上覆水中Pb的浓度为0.220—0.303 mg·L−1,说明底泥中Pb可被释放出来进入上覆水中。与对照组相比,NZ覆盖组和FeCA覆盖组上覆水中Pb的浓度更低。NZ覆盖和FeCA覆盖对上覆水中Pb的削减率分别为45.5%—75.0%和63.6%—81.3%。显然,前者对上覆水中Pb的削减率低于后者。这说明NZ覆盖和FeCA覆盖均可有效控制底泥中Pb向上覆水体中的释放,且后者控制底泥中Pb向上覆水体中释放的效果优于前者。NZ对水中Pb2+具有一定的吸附能力,而FeCA对水中Pb2+的吸附能力强(图1和2)。NZ覆盖和FeCA覆盖之所以能有效控制底泥中Pb释放的主要原因之一是:NZ或FeCA覆盖层会吸附底泥间隙水中溶解态Pb,从而阻止了底泥间隙水中溶解态Pb向上覆水体中的扩散。从图4还可见,NZ和FeCA联合覆盖层同样可以有效地阻止底泥中Pb向上覆水体中的释放,导致上覆水中Pb的浓度明显低于对照组。NZ/FeCA联合覆盖层对上覆水中Pb2+的削减率为68.2%—87.5%。这主要归功于联合覆盖层中NZ和FeCA对底泥中Pb释放的拦截作用。另外,NZ/FeCA联合覆盖控制底泥中Pb向上覆水体中释放的效率要高于单独的NZ覆盖。
-
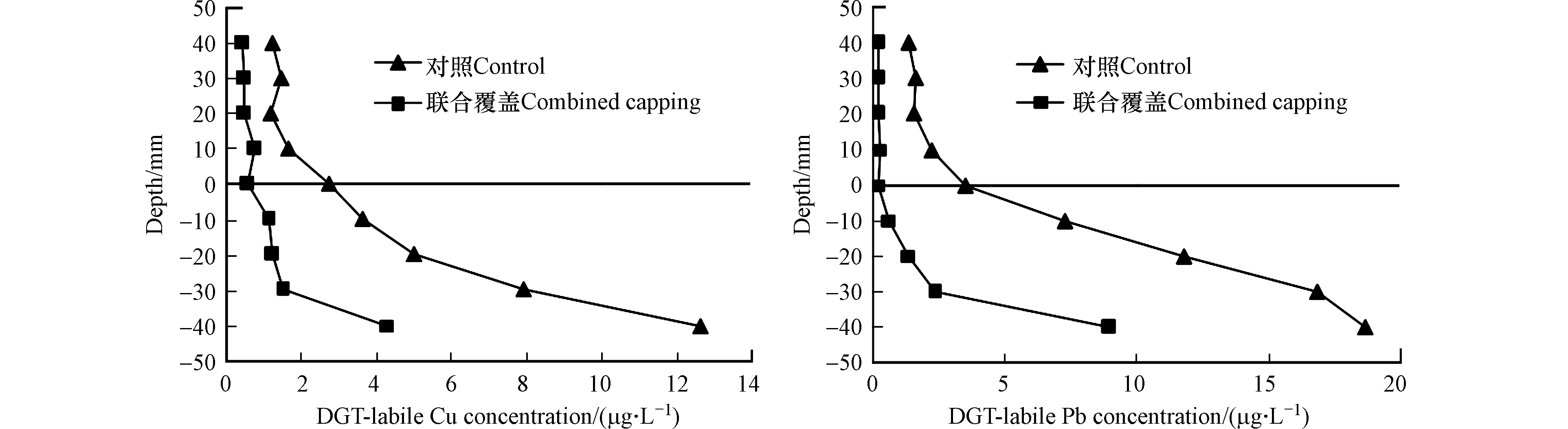
图5是对照组和联合覆盖组上覆水-底泥垂向剖面上DGT有效态Cu和Pb的分布特征。从中可见,对照组中底泥-水界面附近DGT有效态Cu和Pb浓度呈现显著的梯度变化。当深度从20 mm下降到0 mm时(正值表示底泥-水界面上方),上覆水中DGT有效态Cu和Pb浓度分别从1.20 µg·L−1增加到2.72 µg·L−1和从1.55 µg·L−1增加到3.48 µg·L−1。当深度从0 mm下降到-40 mm(负号表示底泥-水界面下方)时,底泥中DGT有效态Cu和Pb浓度分别由2.72 µg·L−1急剧增加到12.7 µg·L−1和由3.48 µg·L−1急剧增加到18.6 µg·L−1。这证实了本研究所用底泥中Cu和Pb会逐渐从下层底泥向上层底泥迁移,而后穿越底泥-水界面再向上覆水体中释放。对于联合覆盖组而言,不同深度处上覆水中DGT有效态Cu和Pb的浓度水平差异很小,但是不同深度处底泥中DGT有效态Cu和Pb的浓度仍存在的差异。当深度从−30 mm下降到−40 mm时,底泥中DGT有效态Cu浓度会由1.49 µg·L−1增加到4.31 µg·L−1。当深度从0 mm下降到−40 mm时,底泥中DGT有效态Pb浓度会从0.219 µg·L−1增加到8.95 µg·L−1。这说明,NZ和FeCA联合覆盖作用下底泥中Cu和Pb仍存在向间隙水和上覆水中释放的风险。
从图5中还可以看出,联合覆盖组上覆水中DGT有效态Cu和Pb浓度明显低于对照组。计算确定的联合覆盖对上覆水中DGT有效态Cu和Pb的削减率分别为54.3%—78.5%和84.1%—93.7%。这说明NZ和FeCA联合覆盖可有效降低底泥中Cu和Pb向上覆水体中释放的风险。
与对照组相比,联合覆盖组底泥中DGT有效态Cu和Pb浓度明显低。产生这种现象的原因推测是:利用DGT对底泥中有效态金属的测量结果不仅体现了间隙水中溶解态金属的浓度,而且体现了底泥中弱吸附态金属向液相的补给能力[39];在联合覆盖作用下,NZ和FeCA会吸附间隙水中溶解态Cu和Pb,可导致间隙水中溶解态Cu和Pb浓度的降低;当间隙水中溶解态Cu和Pb浓度下降后,底泥表面弱吸附态Cu和Pb会释放出来,以弥补间隙水中损失的溶解性Cu和Pb;NZ和FeCA对间隙水中Cu和Pb的吸附速率预计会大于底泥向间隙水的补充速率,从而导致联合覆盖作用下底泥中DGT有效态Cu和Pb浓度明显低于无覆盖条件下的浓度。计算确定的联合覆盖对底泥中有效态Cu和Pb的削减率分别为69.3%—81.2%和51.9%—91.7%。这说明,NZ和FeCA联合覆盖可以有效降低底泥中Cu和Pb向间隙水中释放的风险。
间隙水和上覆水之间溶解态物质浓度差大小是影响间隙水中溶解性物质向上覆水中扩散的重要因素。联合覆盖作用下底泥中DGT有效态Cu和Pb浓度的下降,导致了底泥中Cu和Pb向间隙水中释放风险的降低,进而可降低底泥间隙水中Cu和Pb的浓度,从而可降低间隙水和上覆水之间Cu和Pb的浓度差,最终导致了联合覆盖组上覆水中Cu和Pb浓度明显低于对照组。由此可见,NZ和FeCA联合覆盖层对底泥中DGT有效态Cu和Pb的削减作用,对于其控制底泥中Cu和Pb向上覆水体中释放而言是至关重要的。
根据公式(5)和(6),利用图5中的实验数据,计算得到对照组底泥-水界面Cu和Pb的扩散速率分别为11.3 µg·(m2·d)−1和35.9 µg·(m2·d)−1,而联合覆盖组底泥-水界面Cu和Pb的扩散速率分别为1.17 µg·(m2·d)−1和2.92 µg·(m2·d)−1。这说明,无覆盖条件下底泥中Cu和Pb可通过分子扩散机制穿越底泥-水界面进入上覆水中,并且联合覆盖条件下底泥中的Cu和Pb也可通过分子扩散机制穿越底泥-水界面进入上覆水中,分子扩散是无覆盖和联合覆盖作用下底泥中Cu和Pb向上覆水体中释放的重要机制。进一步计算确定,天然沸石和铁方解石联合覆盖对底泥-水界面Cu和Pb扩散速率的削减率分别为89.7%和91.9%。这说明,天然沸石和铁方解石的联合覆盖可有效降低底泥-水界面Cu和Pb的扩散速率。因此,天然沸石和铁方解石的联合覆盖对底泥-水界面Cu和Pb扩散速率的削减,是其控制底泥中Cu和Pb释放进入上覆水体中的重要作用机制。
-
分析覆盖层中重金属的形态分布特征,进而评估被覆盖层所吸附重金属的稳定性,对于利用活性覆盖技术控制底泥中重金属释放是很重要的。这是因为,如果被覆盖层所吸附的重金属会重新释放的话,那么活性覆盖技术控制底泥中重金属释放的长效性将很难得到保证。因此,分析NZ和FeCA联合覆盖层中Cu和Pb的形态分布特征,进而评估被该联合覆盖层所吸附Cu和Pb的稳定性,是非常必要的。从图6可见,联合覆盖层中各形态Cu含量从大到小依次为:可氧化态Cu(381 mg·kg−1)>残渣态Cu(196 mg·kg−1)>可交换态Cu(43.5 mg·kg−1)>可还原态Cu(10.2 mg·kg−1)。进一步计算确定,联合覆盖层中可氧化态、残渣态、可交换态和可还原态Cu占总可提取态Cu的比例分别为60.4%、31.1%、6.89%和1.62%。从图6中还可以看出,联合覆盖层中各形态Pb含量从大到小依次为残渣态Pb(592 mg·kg−1)>可还原态Pb(326 mg·kg−1)>可交换态Pb(209 mg·kg−1)>可氧化态Pb(86.9 mg·kg−1)。进一步计算确定,联合覆盖层中残渣态、可还原态、可交换态和可氧化态Pb占总可提取Pb的比例分别为48.8%、26.9%、17.2%和7.16%。培养实验结束之后,联合覆盖层中含一定数量的Cu和Pb,这证实了NZ和FeCA对底泥间隙水中溶解性Cu和Pb的吸附作用是联合覆盖层控制底泥中Cu和Pb释放的重要机制。
固体材料中重金属的生物可利用性和迁移能力与其赋存形态是密切相关的[40-42]。F1态金属对外界环境变化非常敏感,在偏酸性和中性条件下通常很容易释放出来[40-42]。F2态金属被认为在水体氧化还原电位降低或缺氧条件下较容易被释放出来[40-42]。F3态金属稳定性较高[42]。在强氧化条件下,F3态金属存在重新释放的可能性[42-43]。F4态金属通常情况下很难被释放出来[42]。联合覆盖层中F1和F2态Cu之和占总可提取态Cu的比例仅为8.51%。这说明被NZ和FeCA所吸附的Cu大部分(91.49%)以较为稳定或非常稳定的形态存在,重新释放的可能性很低。联合覆盖层中F1和F2态Pb之和占总可提取态Pb的比例为44.1%,而F3和F4态Pb之和占总可提取态Pb的比例为55.9%。由此可见,被NZ和FeCA联合覆盖层所吸附的Pb中超过半数以较为稳定或非常稳定的形态存在,被NZ和FeCA联合覆盖层所吸附的Pb总体上释放风险较低。需要指出的,F1+F2态Pb占总可提取态Pb的比例仍达到了44.1%。这说明,NZ和FeCA联合覆盖层吸附底泥中Pb饱和后,对其进行回收处理仍然是很有必要的。
-
(1)与Langmuir模型相比,NZ对水中Cu(Ⅱ)和Pb(Ⅱ)的等温吸附数据更适合采用Freundlich模型加以描述;与Freundlich模型相比,Langmuir模型更适合用于描述FeCA对水中Cu(Ⅱ)和Pb(Ⅱ)的等温吸附数据;根据Langmuir模型预测得到的NZ和FeCA对水中Cu(Ⅱ)的最大单分子层吸附量分别为7.98 mg·g−1和27.2 mg·g−1,NZ和FeCA对水中Pb(Ⅱ)的最大分子层吸附量分别为75.4 mg·g−1和1150 mg·g−1。
(2)NZ单独覆盖、FeCA单独覆盖和NZ/FeCA联合覆盖均可以有效地降低底泥中Cu和Pb向上覆水体中释放的风险,并且NZ和FeCA的联合覆盖控制底泥中Cu和Pb向上覆水体中释放的效率要高于NZ的单独覆盖。
(3)NZ和FeCA联合覆盖不仅可有效地降低上覆水-底泥垂向剖面上DGT有效态Cu和Pb的浓度,而且可降低底泥-水界面Cu和Pb的扩散通量。
(4)被NZ和FeCA联合覆盖层所吸附的Cu的存在形式为可氧化态(60.4%)>残渣态(31.1%)>可交换态(6.89%)>可还原态(1.62%),而Pb的存在形式则为残渣态(48.8%)>可还原态(26.9%)>可交换态(17.2%)>可氧化态(7.16%)。
(5)NZ和FeCA联合覆盖对底泥中DGT有效态Cu和Pb以及底泥-水界面Cu和Pb扩散通量的削减,是其控制底泥中Cu和Pb向上覆水体中释放的关键。
天然沸石和铁改性方解石联合覆盖对底泥铜和铅释放的控制效果及机制
Efficiency and mechanism of combined capping system using natural zeolite and iron-modified calcite for controlling copper and lead release from sediment
-
摘要: 本研究考察了天然沸石和铁改性方解石联合覆盖对水体底泥中铜(Cu)和铅(Pb)释放的控制效果,并探讨了相关的控制作用机制。结果发现,天然沸石(NZ)和铁改性方解石(FeCA)对水中Cu2+和Pb2+具有较好的吸附能力;与Langmuir模型相比,NZ对水中Cu2+和Pb2+的等温吸附行为更适合采用Freundlich模型加以描述;与Freundlich模型相比,Langmuir模型更适合用于描述FeCA对水中Cu2+和Pb2+的等温吸附行为;根据Langmuir模型计算确定的NZ和FeCA对水中Cu2+的最大单分子层吸附量分别为7.98 mg·g−1和27.2 mg·g−1,而NZ和FeCA对水中Pb2+的最大单分子层吸附量分别为75.4 mg·g−1和1150 mg·g−1。显然,FeCA对水中Cu2+和Pb2+的吸附能力优于NZ。NZ单独覆盖、FeCA单独覆盖以及NZ/FeCA联合覆盖均可以显著地降低底泥中Cu和Pb向上覆水体中释放的风险,且NZ和FeCA的联合覆盖控制底泥中Cu和Pb向上覆水体中释放的效率高于NZ的单独覆盖。NZ和FeCA联合覆盖不仅可以有效地降低上覆水-底泥垂向剖面上DGT(薄膜梯度扩散技术)有效态Cu和Pb的浓度,而且可以降低底泥-水界面Cu和Pb扩散通量。被NZ和FeCA联合覆盖层所吸附的Cu主要以可氧化态和残渣态这两种形态存在(占总量的91.5%),而Pb的存在形式则为残渣态(48.8%)>可还原态(26.9%)>可交换态(17.2%)>可氧化态(7.16%)。NZ和FeCA联合覆盖对底泥中DGT有效态Cu和Pb的削减,对于其控制底泥中Cu和Pb向上覆水体中释放是至关重要的。Abstract: This study investigated the efficiency and mechanism of a combined active capping system using natural zeolite (NZ) and iron-modified calcite (FeCA) for the interception of copper (Cu) and lead (Pb) released from sediment. The results revealed that NZ and FeCA exhibited good adsorption performance towards Cu(Ⅱ) and Pb(Ⅱ) in water. The adsorption isotherm data of Cu(Ⅱ) and Pb(Ⅱ) on NZ complied better with the Freundlich model than the Langmuir model, while those of Cu(Ⅱ) and Pb(Ⅱ) on FeCA was well consistent with the Langmuir model. Based on the Langmuir isotherm model, the predicted maximum Cu(Ⅱ) adsorption capacities for NZ and FeCA were 7.98 mg·g−1 and 27.2 mg·g−1, respectively, and the predicted maximum Pb(Ⅱ) adsorption capacities for NZ and FeCA were 75.4 mg·g−1 and 1150 mg·g−1, respectively. It is clear that the Cu(Ⅱ) or Pb(II) adsorption ability for FeCA was higher than that for NZ. Single capping with NZ or FeCA and the combination capping could all greatly reduce the risk of Cu and Pb release from sediment into the overlying water. The controlling efficiency of Cu and Pb by the NZ/FeCA combined capping was higher than that by the single NZ capping. Combined capping not only resulted in the reduction of DGT (diffusive gradient in thin-films technique)-labile Cu and Pb concentrations in the profile of sediment and overlying water, but also led to the decrease of the diffusion flux of Cu and Pb through the interface between sediment and overlying water. Oxidable and residual Cu (91.5% of total Cu) are two major forms of adsorbed Cu inthe NZ/FeCA combined capping layer. The percentages of different Pb species in the total Pb adsorbed by the NZ/FeCA combined capping layer decreased in the order of residual Pb (48.8%) > reducible Pb (26.9%)> exchangeable Pb (17.2%) >oxidable Pb (7.16%). Our results highlighted the reduction of DGT-labile Cu and Pb concentrations in sediment by the combined capping using NZ and FeCA is key to the control of Cu and Pb liberation from the sediment into the overlying water by the NZ/FeCA combined capping.
-
Key words:
- sediment /
- natural zeolite /
- iron-modified calcite /
- combined capping /
- lead /
- copper
-
近年来,我国自然水体中砷和磷复合污染案例屡见不鲜。砷(As)及其化合物主要通过人类工农业生产及地球化学循环而进入地表及地下水中。长期饮用高砷水会对人类的生理健康造成严重的危害,故地表水砷污染导致的饮用水健康风险问题受到人们的广泛关注[1-2]。因此,世界卫生组织(WHO)、美国环境保护署(EPA)等众多组织将饮用水中的砷含量限定为10 μg·L−1以下[3-5]。此外,水体富营养化也对我国各类水体(主要是湖泊、水库及河流城市河段)造成了严重威胁,而磷是引起水体富营养化的主要因子之一[6-8]。有研究表明,环境中磷和砷存在着竞争关系,砷容易置换出磷,从而被细胞吸收导致中毒[9-10]。因此,从环境治理、资源回收等角度考虑,有必要对污染水体中的砷和磷进行控制。
在现有砷、磷去除技术中,吸附法作为较成熟的除砷及除磷方法之一,具有处理效果好、经济安全、操作简单等特点,在小城镇及农村等分散供水地区水处理技术中具有明显优势[11-12]。近年来,新型吸附剂的开发成为国内外学者的研究重点。其中,介孔材料具有均一的孔径、较大的比表面积、稳定的骨架结构和易于修饰等优点,在环境治理领域已得到广泛的运用[13-15]。目前,已有研究报道吸附法单独去除水中的磷或砷时均有良好的效果[16-17],但是,对于砷、磷共存情况下对吸附剂性能的研究还较少。因此,本研究通过镧金属掺杂对介孔材料进行改性,合成了La-MCM-41吸附剂,考察了其同步去除砷、磷复合污染的性能,以期为同步去除环境水体中砷和磷提供参考。
1. 材料与方法
1.1 实验原料
十六烷基三甲基溴化铵(C19H42BrN, CTAB)、正硅酸乙酯(C8H20O4Si, TEOS)、硝酸镧(La(NO3)3·xH2O)、磷酸二氢钾(KH2PO4)、钼酸铵((NH4)6Mo7O24)、L-抗坏血酸(C6H8O6)、酒石酸氧锑钾(C4H4KO7Sb·0.5H2O)、乙二胺四乙酸二钠(C10H14N2O8Na2)、甲酸(CH2O2)、硫脲(CH4N2S)、氢氧化钠(NaOH)均为分析纯。
1.2 MCM-41的制备
本研究模板剂采用十六烷基三甲基溴化铵(CTAB),硅源采用正硅酸乙酯(TEOS),并按照TEOS∶CTAB∶NaOH∶H2O = 1∶0.1∶0.24∶100的摩尔配比进行实验。具体步骤:将NaOH溶于去离子水中,加入相应量的CTAB,36 ℃下磁力搅拌至溶液澄清,剧烈搅拌下逐滴加入TEOS;调节溶液pH = 9~11,混合体系剧烈搅拌2 h,将所得乳白色凝胶转入聚四氟乙烯晶化反应釜中,120 ℃下晶化24 h;取出冷却至室温,过滤、洗涤、干燥,得MCM-41原粉;将其置于马弗炉中,升温至550 ℃,焙烧6 h,制得MCM-41介孔材料。
1.3 La-MCM-41的制备
本研究模板剂采用十六烷基三甲基溴化铵(CTAB),硅源采用正硅酸乙酯(TEOS),硝酸镧为镧源,以La/Si = 0.03的量掺杂金属离子。具体步骤同MCM-41的制备。
1.4 样品表征
采用X射线衍射(德国布鲁克公司D8ADVANCE型,Cu靶,扫描角度为1°~10°)对介孔材料晶体结构进行测定;采用比表面积与孔隙度吸附仪测定介孔材料改性前后比表面积、孔容及孔径分布(美国麦克公司ASAP2460型);采用扫描电子显微镜观察介孔材料改性前后微观形貌(FEI公司NANO SEM430型)。
1.5 单独吸附体系
分别配制As(V)、
PO3−4 -P(以下简称P)浓度均为20 mg·L−1的溶液,取50 mL As(V)或P溶液倒入具塞锥形瓶中,吸附剂投加量为0.01 g,调节溶液pH为5.0。将各锥形瓶置于恒温振荡箱中,调节温度为15、25、35、45 ℃,在转速为150 r·min−1条件下,振荡24 h,反应过程中保持pH稳定。在反应结束后,取水样,过0.45 μm滤膜,采用氢化物发生-原子荧光分光光度法测定As(V)浓度,钼酸铵-抗坏血酸比色法测定P浓度1.6 同步吸附体系
配制As(V)、P浓度均为20 mg·L−1的混合溶液[18-19],取50 mL混合溶液倒入具塞锥形瓶中,吸附剂投加量为0.01 g,调节溶液pH为5.0。将锥形瓶置于恒温振荡箱中,调节温度为15、25、35、45 ℃,在转速为150 r·min−1条件下,振荡24 h,反应过程中保持pH稳定。反应结束后,取水样,过0.45 μm滤膜,采用日本岛津公司ICPS-7510 PLUS电感耦合等离子体原子发射光谱仪分别测定As(V)和P的浓度。
2. 结果与讨论
2.1 结构表征
2.1.1 X射线衍射
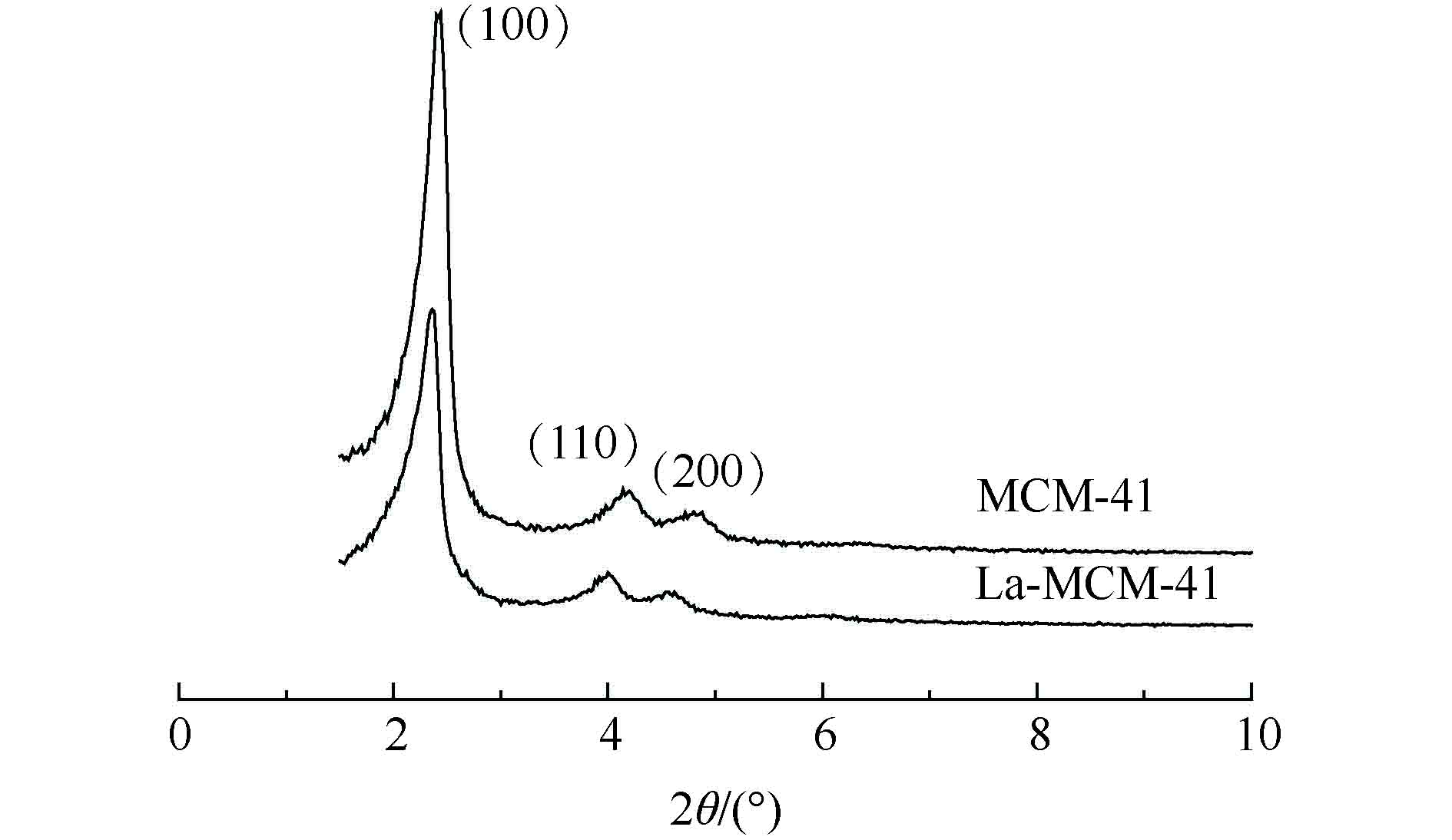
图1是MCM-41及La-MCM-41的XRD衍射图。由图1可见,2组材料在小角范围内都有3个明显的衍射峰。其中,在2θ=2.2°附近有1个较强的衍射峰,对应于介孔分子筛(100)晶面;在2θ=4.0°、4.3°附近出现2个强度相对较弱的衍射峰,分别对应于介孔分子筛(110)、(200)晶面。这与已有报道[20-21]中关于介孔材料的结构特征峰位置的结果一致,说明本研究制备的MCM-41和La-MCM-41都具有长程有序的六方相介孔结构特征,掺杂镧之后的介孔材料和MCM-41具有相似的结构特性。经La改性后的MCM-41的特征峰强度有明显的减弱,而且相应衍射峰2θ角向低角度偏移,这表明,骨架中镧的引入会改变分子筛的表面结构,从而降低了材料的有序性。
2.1.2 比表面积及孔结构分析
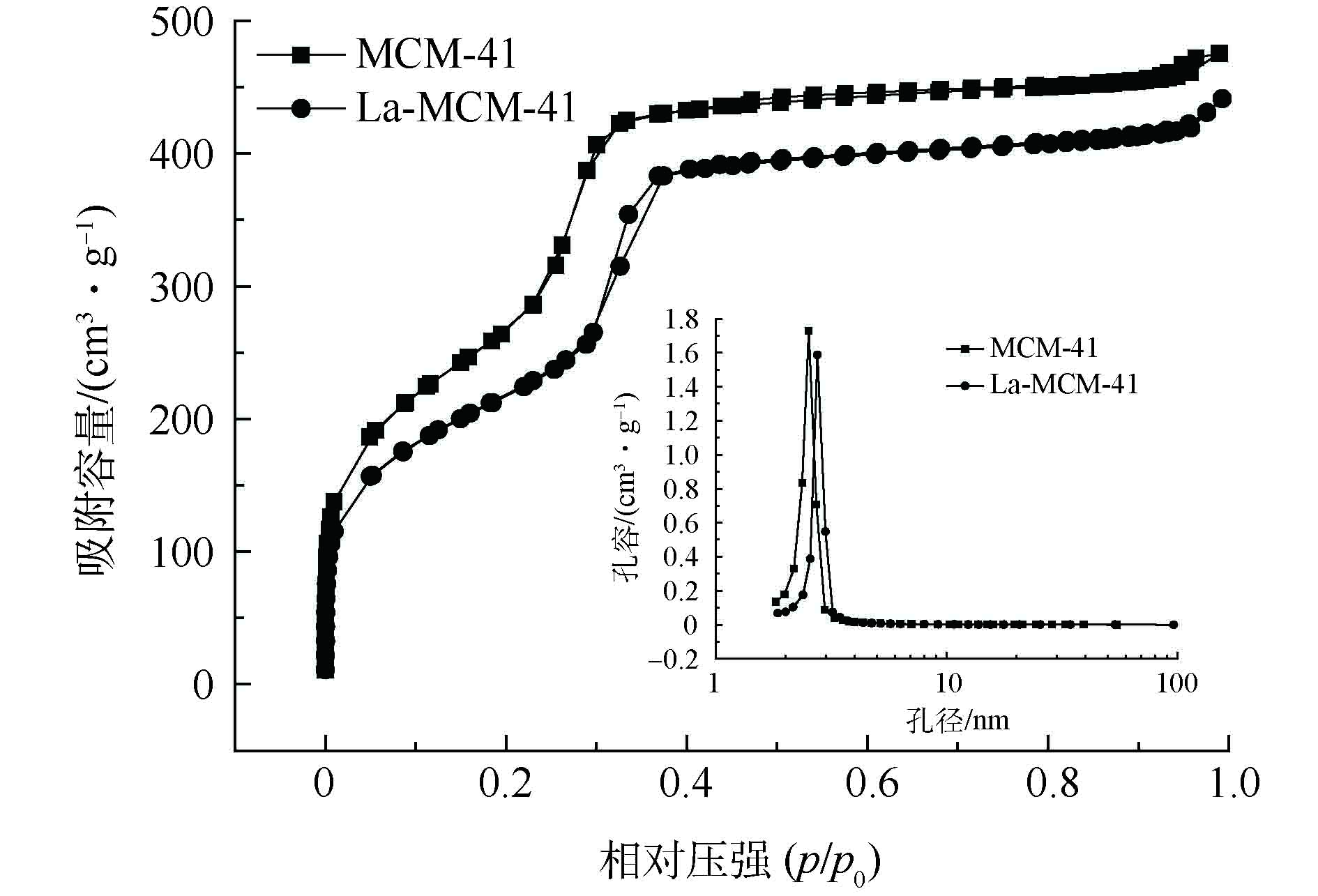
通过N2-吸脱附测试,可以表征多孔材料孔道结构等信息。MCM-41和La-MCM-41的N2吸脱附曲线和孔径分布如图2所示。2种材料的N2吸脱附等温线均为Ⅳ型[22],属于典型的介孔材料吸附曲线,镧掺杂后也没有明显改变介孔材料的结构特征,但La-MCM-41的比表面积和孔容积都有所减小,而孔径有所增加。其中比表面积从1 193.68 m2·g−1减小到812.27 m2·g−1,孔容从0.71 cm3·g−1减小到0.64 cm3·g−1,孔径从2.38 nm增加到3.18 nm,这是因为镧掺杂后占据了部分孔道,影响了原有孔道结构[23]。
2.1.3 扫描电镜
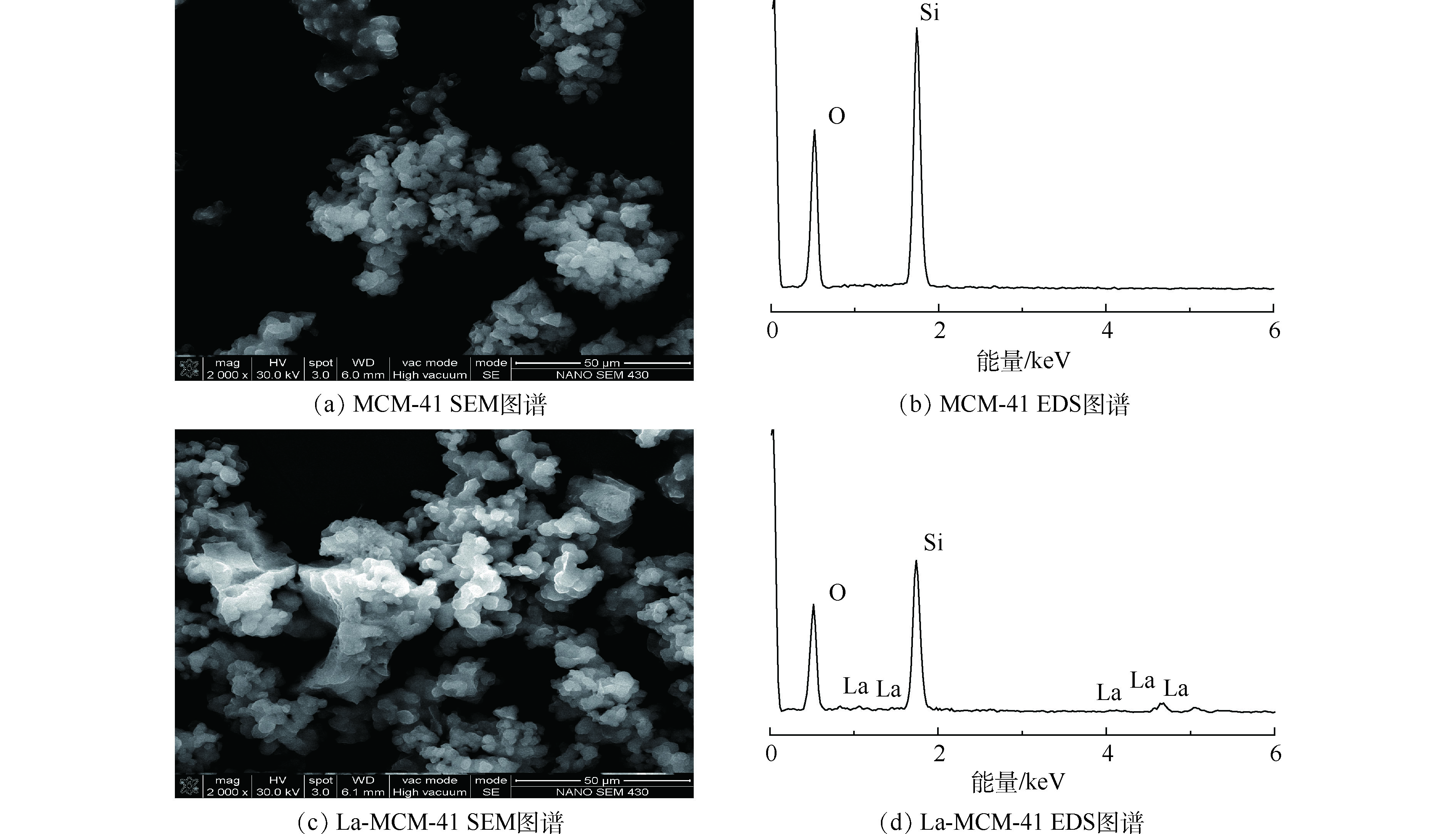
图3为MCM-41和La-MCM-41的扫描电镜(SEM)和X射线能谱分析图(EDS)。从图3中可以看出,MCM-41和La-MCM-41的颗粒外形均呈现颗粒状,改性并未改变介孔材料的形貌特征。对比二者发现,MCM-41介孔吸附材料颗粒粒径较小,团聚后呈现一些不规则的形状结构。La-MCM-41介孔分子筛外形轮廓粗糙,并出现了团聚后的大颗粒。改性前后EDS图也呈现出差别,改性后的吸附剂硅含量减少,同时出现镧元素,说明镧成功取代硅,掺杂到介孔材料中。
2.2 吸附剂的筛选
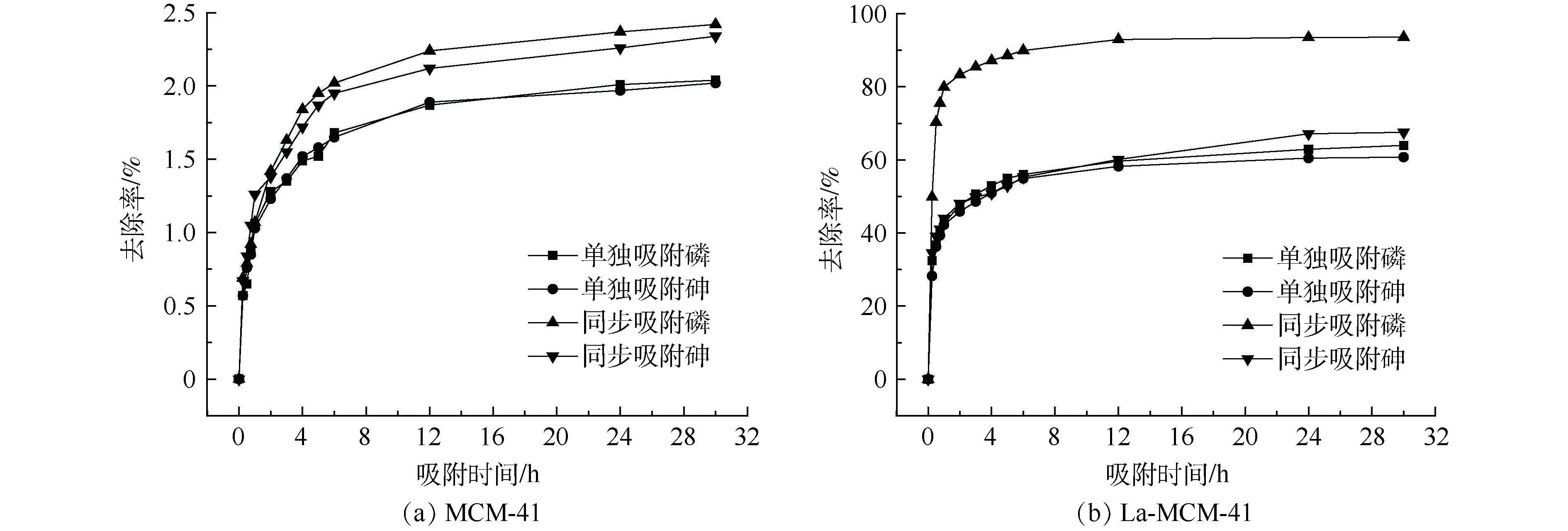
本研究考察了吸附剂改性前后对砷、磷吸附效果的影响,结果如图4所示。未经过改性的介孔吸附剂MCM-41对溶液中的砷、磷几乎没有吸附作用,单一吸附和同步吸附体系中砷、磷的去除率均低于2.5%。这主要是因为,未经过改性的MCM-41本身几乎不含活性位点;而经过改性的La-MCM-41吸附剂对砷、磷具有良好的吸附效果,吸附去除率大大提高。由此可见,介孔吸附材料对砷、磷吸附效果的提升是由于La元素的加入,La的加入使得吸附剂上活性吸附位点增加,故对砷、磷的去除率也提高。且与其他常用吸附剂相比(见表1),本研究的La-MCM-41对砷、磷的吸附效果更突出[24-28],表明La-MCM-41是一种能够同步去除砷、磷的良好吸附剂。
表 1 不同吸附剂去除As(V)和P性能的比较Table 1. Comparison of As(V) and P adsorption by different adsorbents2.3 吸附动力学研究
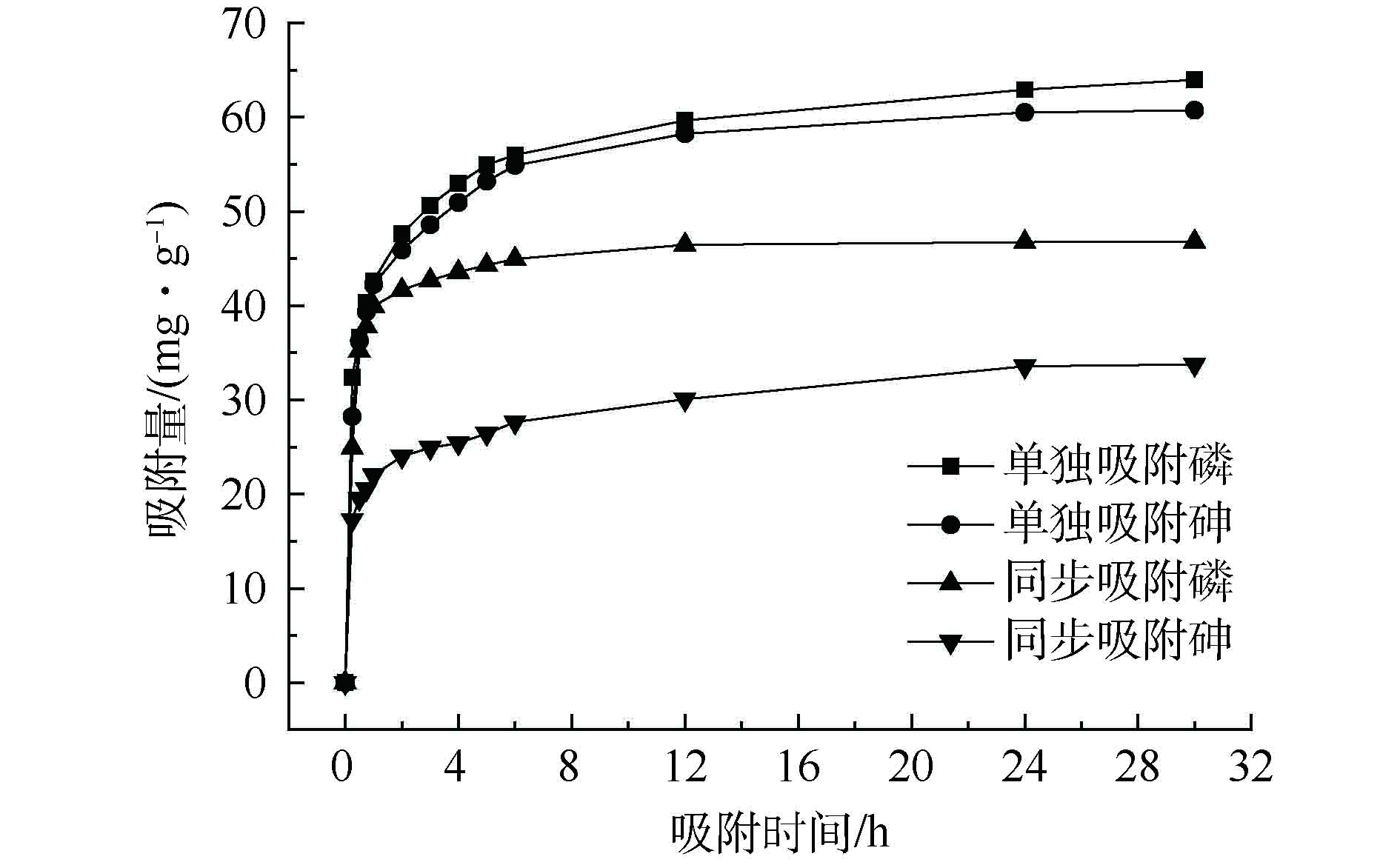
图5是在25 ℃条件下La-MCM-41对目标污染物的动力学吸附结果。La-MCM-41对目标污染物的吸附过程可分为2个阶段:快速反应阶段,由于溶液与吸附剂表面的浓度梯度较大,吸附剂对目标污染物的吸附量快速上升;慢速反应阶段,由于吸附剂表面位点被大量占据,目标污染物吸附量升高速度缓慢,到24 h基本达到平衡,随着平衡随着吸附时间的增加,吸附剂上的吸附点位被不断占用,吸附速率减慢从而达到平衡。从图5中还可发现,单一吸附体系中的吸附量均要高于同步吸附体系,其中,P的吸附量高于As(V)。
采用准一级和准二级动力学吸附模型对实验数据进行了拟合[29]。准一级和准二级动力学模型的数学方程如式(1)和式(2)所示。
ln(Qe−Qt)=lnQe−k1t(1) tQt=1k2Qe2+tQe(2) 式中:k1为准一级动力学速率常数,min−1;k2为准二级动力学速率常数,g·(mg·min)−1;t为反应时间,min;Qe为反应平衡时吸附剂对吸附质的吸附量,mg·g−1;Qt为反应时间t时吸附剂对吸附质的吸附量,mg·g−1。
结合表2及图5可看出,准一级动力学方程R2 > 0.9,准二级动力学方程R2 > 0.99,准二级动力学方程的拟合性更好,并且准二级动力学方程模拟得到的平衡吸附量更加接近于实际测定值,综合来看,La-MCM-41对As(V)、P的吸附更加符合准二级动力学模型。
表 2 La-MCM-41同步去除As(V)和P的动力学参数Table 2. Kinetic models parameters for simultaneous removal of As(V) and P by La-MCM-41温度/℃ 吸附类型 准一级动力学 准二级动力学 Qe/(mg·g−1) k1 R2 Qe/(mg·g−1) k2 R2 15 同步吸附P 10.38 0.160 3 0.965 46.08 0.094 0.997 15 同步吸附As 18.87 0.175 4 0.962 34.36 0.018 0.997 25 同步吸附P 12.08 0.252 8 0.903 47.17 0.118 0.999 25 同步吸附As 17.73 0.177 7 0.945 34.48 0.028 0.997 35 同步吸附P 13.57 0.363 3 0.992 48.31 0.165 0.999 35 同步吸附As 16.07 0.179 7 0.949 34.72 0.041 0.998 45 同步吸附P 14.11 0.695 2 0.991 50 0.222 0.999 45 同步吸附As 13.77 0.180 4 0.926 34.72 0.054 0.998 采用阿累尼乌斯方程计算反应活化能[30],阿累尼乌斯方程如式(3)所示。
K=Ae−EaRT (3) 两边取对数改写为式(4)。
lnK=−EaRT+lnA (4) 式中:K为温度为T时的反应速度常数,min−1;Ea为反应活化能,kJ·mol−1;T为热力学温度,K;R为摩尔气体常数,8.314 J·(mol·K)−1;A为指前因子。
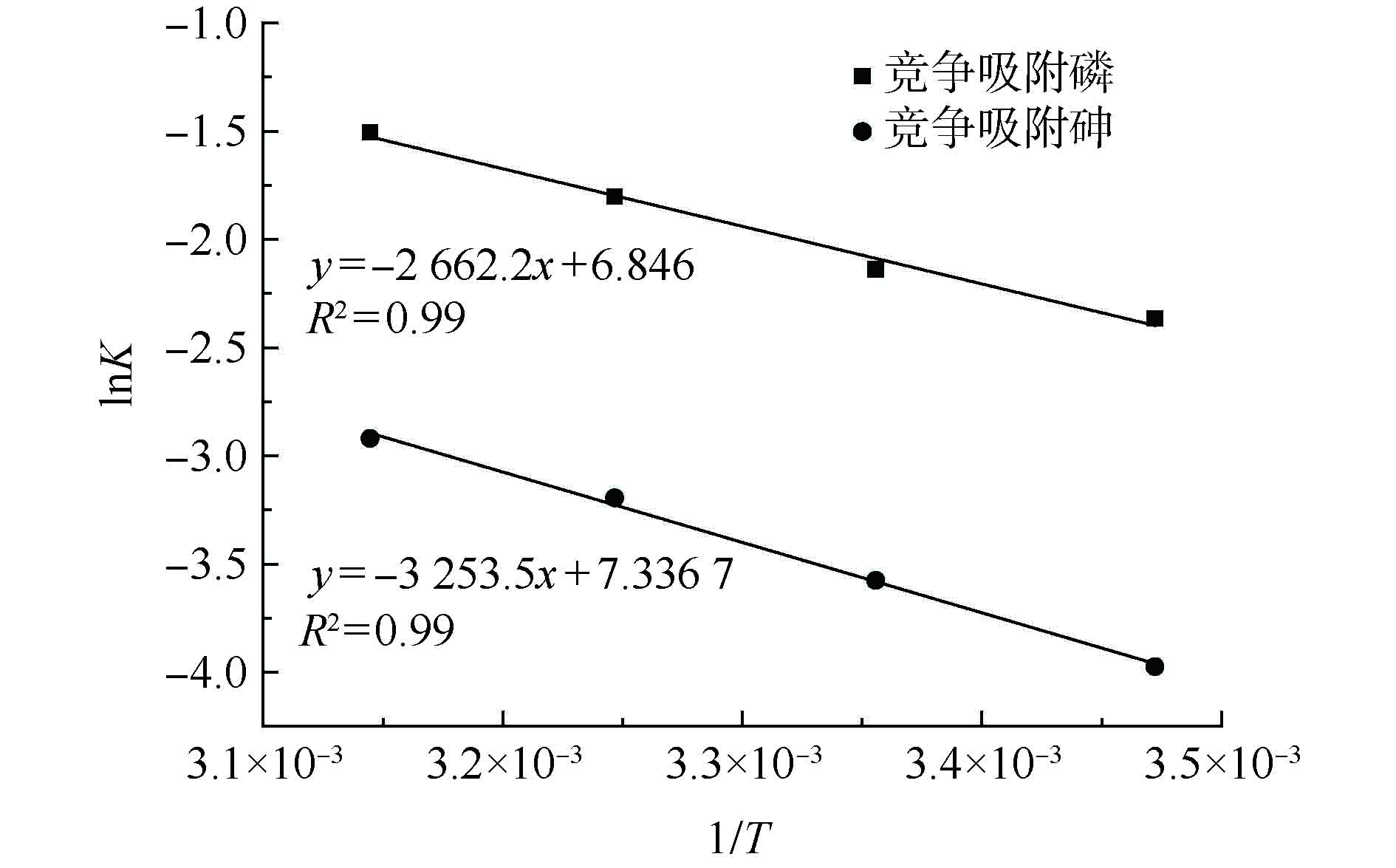
图6是同步吸附条件下La-MCM-41对P、As(V)吸附的阿累尼乌斯方程的拟合情况。根据图6中拟合曲线的直线方程,得出活化能Ea,La-MCM-41对砷和磷的同步吸附活化能分别为27.05 kJ·mol−1和22.68 kJ·mol−1。对比二者可发现,在同步吸附条件下,La-MCM-41对As(V)的吸附活化能稍高于对P的吸附活化能。这说明La-MCM-41对As(V)的吸附需要更多的能量来越过能垒,而对P的吸附相对更容易发生。因此,在同步吸附条件下,磷更容易被吸附在La-MCM-41的位点上,在同步吸附体系中占据优势地位。
2.4 La-MCM-41 对 As(V)、P 的吸附等温线
图7考察了25 ℃下La-MCM-41分别对不同初始浓度的As(V)、P的吸附效果。由图7可知,La-MCM-41对As(V)、P的等温吸附线呈现类似的变化过程,吸附量随着初始浓度的增加而增加,当初始浓度增大到一定程度时,吸附曲线增长速率减缓;达到平衡时,同步吸附时P的最大吸附容量可达到160.07 mg·g−1,As(V)的最大吸附容量为126.75 mg·g−1。两者吸附量的差异可能是因为砷酸根离子的分子大小比磷酸根离子大,砷酸根比磷酸根离子的直径大,因此,单位表面积的砷酸根离子的吸附量会低于磷酸根离子。上述结果与茹春云[30]的报道结果相似。
分别采用Langmuir方程和Freundlich方程[31-32]对吸附等温线进行拟合,拟合所得参数如表3所示。
表 3 La-MCM-41吸附As(V)和P的等温吸附模型参数Table 3. Isotherm parameters for As(V) and P adsorption onto La-MCM-41吸附类型 Langmuir Freundlich Qm/(mg·g−1) KL/(L·mg−1) R2 n KF/(mg·g−1) R2 单独吸附P 192.3 0.09 0.99 2.39 26.66 0.989 单独吸附As 163.93 0.14 0.982 2.73 24.75 0.988 同步吸附P 188.68 0.075 0.983 2.39 24.61 0.993 同步吸附As 133.33 0.075 0.979 2.38 17.3 0.987 Ceqe=Ceqm+1qmKL(5) lnqe=lnKF+1nlnCe(6) 式中:Ce为平衡时溶液浓度,mg·L−1;qe为平衡吸附量,mg·g−1;qm为吸附剂最大吸附量,mg·g−1;KL为Langmuir平衡常数,L·mg−1;KF为Freundlich吸附常数;1/n为浓度常数,其值越接近1,说明吸附等温线的线性程度越高,1/n值小于1,说明吸附过程属于优惠吸附。
La-MCM-41对As(V)、P的等温吸附数据拟合结果均能较好地符合Langmuir方程和Freundlich方程,除单独吸附P,吸附对Freundlich方程的拟合性更好,故推测La-MCM-41对As(V)和P的吸附介于单分子层和多分子层吸附。同时根据公式(7)可知,RL值均介于0与1之间,说明此吸附过程为有利吸附,且RL值随着浓度的增加而减小,更加说明提高砷和磷的浓度有利于吸附的进行。
RL=11+KLC0 (7) 式中:C0为起始砷(磷)浓度,mg·L−1;KL为Langmuir平衡常数,L·mg−1。
从表3可以看出,单一吸附体系和同步吸附体系下P的最大吸附量和KF值均大于As(V)的最大吸附量和KF值,说明La-MCM-41对P的吸附能力优于对As(V)的吸附能力。
3. 结论
1)采用水热合成法成功制备了吸附容量大,选择性较好的除砷、磷吸附剂La-MCM-41,吸附剂具有稳定的二维六方介孔结构,比表面积为812.27 m2·g−1、孔容为0.64 cm3·g−1、平均孔径为3.18 nm。
2)La-MCM-41对砷、磷的吸附能够在12 h内达到平衡。通过动力学研究分析可发现,在同步吸附体系中,La-MCM-41对2种污染物质的吸附能力具有一定的差距,其对磷的吸附能力比砷强,磷在同步吸附中占据优势,该过程符合准二级动力学模型。
3)采用Langmuir和Frreundlich方程对等温吸附数据进行拟合分析发现,La-MCM-41对磷、砷的吸附过程介于单分子层与多分子层之间,且在2种体系中n值均大于1,说明La-MCM-41对磷、砷的吸附均为优惠型吸附。相比单一吸附体系,同步吸附体系中La-MCM-41对2种离子的饱和吸附量均有不同程度的降低,其中As(V)吸附量下降较多。
-
图 3 各实验组上覆水中Cu浓度随培养时间变化而变化的规律以及NZ覆盖、FeCA覆盖和联合覆盖对上覆水中Cu的去除率
Figure 3. Change in Cu concentration of overlying water in control, natural zeolite (NZ) capping, iron-modified calcite (FeCA) capping, and NZ/FeCA combined capping columns and the reduction efficiencies of overlying water Cu by the NZ capping, FeCA capping and NZ/FeCA combined capping
图 4 各实验组上覆水中Pb浓度随培养时间变化而变化的规律以及NZ覆盖、FeCA覆盖和联合覆盖对上覆水中Pb的去除率
Figure 4. Change in Pb concentration of overlying water in control, natural zeolite (NZ) capping, iron-modified calcite (FeCA) capping, and NZ/FeCA combined capping columns and the reduction efficiencies of overlying water Pb by the NZ capping, FeCA capping and NZ/FeCA combined capping
表 1 天然沸石和铁方解石吸附水中Cu(Ⅱ)和Pb(Ⅱ)的等温线模型参数拟合结果
Table 1. Fitting results of isotherm models for Cu(Ⅱ) and Pb(Ⅱ) uptake by natural zeolite and iron-modified calcite
等温吸附模型Isotherm model 参数Parameter 天然沸石Natural zeolite 铁方解石Iron-modified calcite Cu(Ⅱ) Pb(Ⅱ) Cu(Ⅱ) Pb(Ⅱ) Langmuir QMAX/(mg·g−1) 7.98 75.4 27.2 1150 KL/(L·mg−1) 4.29 0.252 17.4 9.29 R2 0.685 0.515 0.983 0.722 Freundlich KF 5.40 43.8 20.3 685 1/n 0.153 0.114 0.132 0.119 R2 0.968 0.741 0.770 0.454 表 2 国内外沸石、碳酸盐基材料和铁氧化物对水中Cu(Ⅱ)和Pb(Ⅱ)的最大吸附量
Table 2. The maximum Cu(Ⅱ) and Pb(Ⅱ) uptake capacities for zeolite, carbonate-based material and iron oxide reported in previous literatures
序号Number 吸附剂名称Adsorbent name Cu(Ⅱ)最大吸附量/(mg·g−1)Copper maximum adsorption capacity Pb(Ⅱ)最大吸附量/(mg·g−1)Lead maximum adsorption capacity 确定方法Determination method 参考文献Reference 1 天然沸石 — 14.2 Langmuir模型 [13] 2 天然沸石 26.24(0.410 mmol·g−1) 89.096(0.430 mmol·g−1) Langmuir-Freundlich模型 [14] 3 FAU型沸石 57.803 109.890 Langmuir模型 [25] 4 NaA型沸石 202.76 — Langmuir模型 [26] 5 NaY型沸石 — 431.60 Langmuir模型 [27] 6 钙霞石型沸石 133.18 (2.081 mmol·g−1) 441.336 (2.130 mmol·g−1) Langmuir模型 [28] 7 碳酸盐基尾矿渣 — 832 实验 [29] 8 大理石 222.84 — Langmuir模型 [30] 9 球霰石型碳酸钙 — 499.86 实验 [31] 10 NZ 7.98 75.4 Langmuir模型 本研究 11 FeCA 27.2 1150 Langmuir模型 本研究 -
[1] 张志, 张润宇, 王立英, 等. 淡水沉积物中重金属生物有效性的研究进展 [J]. 地球与环境, 2020, 48(3): 385-394. ZHANG Z, ZHANG R Y, WANG L Y, et al. Research advance in bioavailability of heavy metals in freshwater sediment [J]. Earth and Environment, 2020, 48(3): 385-394(in Chinese).
[2] 杨颖, 孙文, 刘吉宝, 等. 北运河流域沙河水库沉积物重金属分布及生态风险评估 [J]. 环境科学学报, 2021, 41(1): 217-227. YANG Y, SUN W, LIU J B, et al. Distribution and ecological risk assessment of heavy metals in sediments of Shahe Reservoir in Northern Canal Basin [J]. Acta Scientiae Circumstantiae, 2021, 41(1): 217-227(in Chinese).
[3] 柳肖竹, 刘群群, 王文静, 等. 水力扰动对河口沉积物中重金属再释放的影响 [J]. 生态与农村环境学报, 2020, 36(11): 1460-1467. LIU X Z, LIU Q Q, WANG W J, et al. Effect of hydraulic disturbance on Re-release of heavy metals in estuarine sediments [J]. Journal of Ecology and Rural Environment, 2020, 36(11): 1460-1467(in Chinese).
[4] YUAN H Z, YIN H B, YANG Z, et al. Diffusion kinetic process of heavy metals in lacustrine sediment assessed under different redox conditions by DGT and DIFS model [J]. Science of the Total Environment, 2020, 741: 140418. doi: 10.1016/j.scitotenv.2020.140418 [5] PENG W H, LI X M, XIAO S T, et al. Review of remediation technologies for sediments contaminated by heavy metals [J]. Journal of Soils and Sediments, 2018, 18(4): 1701-1719. doi: 10.1007/s11368-018-1921-7 [6] ZANG F, WANG S L, NAN Z R, et al. Immobilization of Cu, Zn, Cd and Pb in mine drainage stream sediment using Chinese loess [J]. Chemosphere, 2017, 181: 83-91. doi: 10.1016/j.chemosphere.2017.04.070 [7] WANG M M, REN L S, WANG D Y, et al. Assessing the capacity of biochar to stabilize copper and lead in contaminated sediments using chemical and extraction methods [J]. Journal of Environmental Sciences, 2019, 79: 91-99. doi: 10.1016/j.jes.2018.11.007 [8] DENG R, HUANG D L, XUE W J, et al. Eco-friendly remediation for lead-contaminated riverine sediment by sodium lignin sulfonate stabilized nano-chlorapatite [J]. Chemical Engineering Journal, 2020, 397: 125396. doi: 10.1016/j.cej.2020.125396 [9] YANG Y Y, YE S J, ZHANG C, et al. Application of biochar for the remediation of polluted sediments [J]. Journal of Hazardous Materials, 2021, 404: 124052. doi: 10.1016/j.jhazmat.2020.124052 [10] CAI C Y, ZHAO M H, YU Z, et al. Utilization of nanomaterials for in situ remediation of heavy metal(loid) contaminated sediments: A review [J]. Science of the Total Environment, 2019, 662: 205-217. doi: 10.1016/j.scitotenv.2019.01.180 [11] XIONG C H, WANG D Y, TAM N F, et al. Enhancement of active thin-layer capping with natural zeolite to simultaneously inhibit nutrient and heavy metal release from sediments [J]. Ecological Engineering, 2018, 119: 64-72. doi: 10.1016/j.ecoleng.2018.05.008 [12] LI X C, YANG Z Z, ZHANG C, et al. Effects of different crystalline iron oxides on immobilization and bioavailability of Cd in contaminated sediment [J]. Chemical Engineering Journal, 2019, 373: 307-317. doi: 10.1016/j.cej.2019.05.015 [13] RAKHYM A B, SEILKHANOVA G A, KURMANBAYEVA T S. Adsorption of lead (II) ions from water solutions with natural zeolite and chamotte clay [J]. Materials Today:Proceedings, 2020, 31: 482-485. doi: 10.1016/j.matpr.2020.05.672 [14] PERIĆ J, TRGO M, VUKOJEVIĆ MEDVIDOVIĆ N. Removal of zinc, copper and lead by natural zeolite-a comparison of adsorption isotherms [J]. Water Research, 2004, 38(7): 1893-1899. doi: 10.1016/j.watres.2003.12.035 [15] WANG J M, ZHAO J, QIN X Z, et al. Theoretical study of adsorption mechanism of heavy metals As and Pb on the calcite (104) surface [J]. Materials Today Communications, 2021, 26: 101742. doi: 10.1016/j.mtcomm.2020.101742 [16] WEN T, ZHAO Y L, ZHANG T T, et al. Effect of anions species on copper removal from wastewater by using mechanically activated calcium carbonate [J]. Chemosphere, 2019, 230: 127-135. doi: 10.1016/j.chemosphere.2019.04.213 [17] ROUFF A A, REEDER R J, FISHER N S. Electrolyte and pH effects on Pb(Ⅱ)-calcite sorption processes: The role of the PbCO03(aq) complex [J]. Journal of Colloid and Interface Science, 2005, 286(1): 61-67. doi: 10.1016/j.jcis.2005.01.053[18] BAI X Y, LIN J W, ZHANG Z B, et al. Interception of sedimentary phosphorus release by iron-modified calcite capping [J]. Journal of Soils and Sediments, 2021, 21(1): 641-657. doi: 10.1007/s11368-020-02754-5 [19] FIELDH R, WHITAKERA H, HENSONJ A, et al. Sorption of copper and phosphate to diverse biogenic iron (oxyhydr)oxide deposits [J]. Science of the Total Environment, 2019, 697: 134111. doi: 10.1016/j.scitotenv.2019.134111 [20] LIU J, ZHU R L, MA L Y, et al. Adsorption of phosphate and cadmium on iron (oxyhydr)oxides: A comparative study on ferrihydrite, goethite, and hematite [J]. Geoderma, 2021, 383: 114799. doi: 10.1016/j.geoderma.2020.114799 [21] GAO L, LI R, LIANG Z B, et al. Mobilization mechanisms and toxicity risk of sediment trace metals (Cu, Zn, Ni, and Pb) based on diffusive gradients in thin films: A case study in the Xizhi River basin, South China [J]. Journal of Hazardous Materials, 2021, 410: 124590. doi: 10.1016/j.jhazmat.2020.124590 [22] SUNH R, GAOB, GAOL, et al. Using diffusive gradients in thin films (DGT) and DGT-induced fluxes in sediments model to assess the dynamic release of copper in sediment cores from the Three Gorges Reservoir, China [J]. Science of the Total Environment, 2019, 672: 192-200. doi: 10.1016/j.scitotenv.2019.03.400 [23] CHEN Y, ARMUTLULU A, SUN W L, et al. Ultrafast removal of Cu(II) by a novel hierarchically structured faujasite-type zeolite fabricated from lithium silica fume [J]. Science of the Total Environment, 2020, 714: 136724. doi: 10.1016/j.scitotenv.2020.136724 [24] TRAN H N, YOU S J, HOSSEINI-BANDEGHARAEI A, et al. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review [J]. Water Research, 2017, 120: 88-116. doi: 10.1016/j.watres.2017.04.014 [25] JOSEPH I V, TOSHEVA L, DOYLE A M. Simultaneous removal of Cd(Ⅱ), Co(Ⅱ), Cu(Ⅱ), Pb(Ⅱ), and Zn(Ⅱ) ions from aqueous solutions via adsorption on FAU-type zeolites prepared from coal fly ash [J]. Journal of Environmental Chemical Engineering, 2020, 8(4): 103895. doi: 10.1016/j.jece.2020.103895 [26] DJAMEL N, SAMIRA A. Mechanism of Cu2+ ions uptake process by synthetic NaA zeolite from aqueous solution: Characterization, Kinetic, intra-crystalline diffusion and thermodynamic studies [J]. Journal of Molecular Liquids, 2021, 323: 114642. doi: 10.1016/j.molliq.2020.114642 [27] BU N J, LIU X M, SONG S L, et al. Synthesis of NaY zeolite from coal gangue and its characterization for lead removal from aqueous solution [J]. Advanced Powder Technology, 2020, 31(7): 2699-2710. doi: 10.1016/j.apt.2020.04.035 [28] QIU W, ZHENG Y. Removal of lead, copper, nickel, cobalt, and zinc from water by a cancrinite-type zeolite synthesized from fly ash [J]. Chemical Engineering Journal, 2009, 145(3): 483-488. doi: 10.1016/j.cej.2008.05.001 [29] XIONG B W, ZHANG T T, ZHAO Y L, et al. Utilization of carbonate-based tailings to remove Pb(Ⅱ) from wastewater through mechanical activation [J]. Science of the Total Environment, 2020, 698: 134270. doi: 10.1016/j.scitotenv.2019.134270 [30] GUIMARÃES T, PAQUINI L D, LYRIO FERRAZ B R, et al. Efficient removal of Cu(Ⅱ) and Cr(Ⅲ) contaminants from aqueous solutions using marble waste powder [J]. Journal of Environmental Chemical Engineering, 2020, 8(4): 103972. doi: 10.1016/j.jece.2020.103972 [31] LIN P Y, WU H M, HSIEH S L, et al. Preparation of vaterite calcium carbonate granules from discarded oyster shells as an adsorbent for heavy metal ions removal [J]. Chemosphere, 2020, 254: 126903. doi: 10.1016/j.chemosphere.2020.126903 [32] LI J C, LI M, Song Q, et al. Efficient recovery of Cu(Ⅱ) by LTA-zeolites with hierarchical pores and their resource utilization in electrochemical denitrification: Environmentally friendly design and reutilization of waste in water [J]. Journal of Hazardous Materials, 2020, 394: 122554. doi: 10.1016/j.jhazmat.2020.122554 [33] HONG M, YU L Y, WANG Y D, et al. Heavy metal adsorption with zeolites: The role of hierarchical pore architecture [J]. Chemical Engineering Journal, 2019, 359: 363-372. doi: 10.1016/j.cej.2018.11.087 [34] TANG H M, XIAN H Y, HE H P, et al. Kinetics and mechanisms of the interaction between the calcite (10.4) surface and Cu2+-bearing solutions [J]. Science of the Total Environment, 2019, 668: 602-616. doi: 10.1016/j.scitotenv.2019.02.232 [35] SONG X W, CAO Y W, BU X Z, et al. Porous vaterite and cubic calcite aggregated calcium carbonate obtained from steamed ammonia liquid waste for Cu2+ heavy metal ions removal by adsorption process [J]. Applied Surface Science, 2021, 536: 147958. doi: 10.1016/j.apsusc.2020.147958 [36] SHI M Q, MIN X B, KE Y, et al. Recent progress in understanding the mechanism of heavy metals retention by iron (oxyhydr)oxides [J]. Science of the Total Environment, 2021, 752: 141930. doi: 10.1016/j.scitotenv.2020.141930 [37] 雷沛, 张洪, 王超, 等. 沉积物-水界面污染物迁移扩散的研究进展 [J]. 湖泊科学, 2018, 30(6): 1489-1508. doi: 10.18307/2018.0602 LEI P, ZHANG H, WANG C, et al. Migration and diffusion for pollutants across the sediment-water interface in lakes: A review [J]. Journal of Lake Sciences, 2018, 30(6): 1489-1508(in Chinese). doi: 10.18307/2018.0602
[38] LIN J W, HE S Q, ZHANG H H, et al. Effect of zirconium-modified zeolite addition on phosphorus mobilization in sediments [J]. Science of the Total Environment, 2019, 646: 144-157. doi: 10.1016/j.scitotenv.2018.07.281 [39] 房煦, 罗军, 高悦, 等. 梯度扩散薄膜技术(DGT)的理论及其在环境中的应用Ⅱ: 土壤与沉积物原位高分辨分析中的方法与应用 [J]. 农业环境科学学报, 2017, 36(9): 1693-1702. doi: 10.11654/jaes.2017-0454 FANG X, LUO J, GAO Y, et al. Theory and application of diffusive gradients in thin-films in the environment: High-resolution analysis and its applications in soils and sediments [J]. Journal of Agro-Environment Science, 2017, 36(9): 1693-1702(in Chinese). doi: 10.11654/jaes.2017-0454
[40] ZHANG T, LI L J, XU F, et al. Assessing the remobilization and fraction of cadmium and lead in sediment of the Jialing River by sequential extraction and diffusive gradients in films (DGT) technique [J]. Chemosphere, 2020, 257: 127181. doi: 10.1016/j.chemosphere.2020.127181 [41] 江涛, 林伟稳, 曹英杰, 等. 梅江流域清凉山水库沉积物重金属污染、生态风险评价及来源解析 [J]. 环境科学, 2020, 41(12): 5410-5418. JIANG T, LIN W W, CAO Y J, et al. Pollution and ecological risk assessment and source apportionment of heavy metals in sediments of Qingliangshan reservoir in the Meijiang basin [J]. Environmental Science, 2020, 41(12): 5410-5418(in Chinese).
[42] 姜时欣, 翟付杰, 张超, 等. 伊通河(城区段)沉积物重金属形态分布特征及风险评价 [J]. 环境科学, 2020, 41(6): 2653-2663. JIANG S X, ZHAI F J, ZHANG C, et al. Speciation distribution and risk assessment of heavy metals in sediments from the Yitong river city area [J]. Environmental Science, 2020, 41(6): 2653-2663(in Chinese).
[43] 徐晨, 王沛芳, 陈娟, 等. 望虞河西岸河网重金属污染特征及生态风险评价 [J]. 环境科学, 2019, 40(11): 4914-4923. XU C, WANG P F, CHEN J, et al. Contaminant characteristics and ecological risk assessments of heavy metals from river networks in the western area of the Wangyu river [J]. Environmental Science, 2019, 40(11): 4914-4923(in Chinese).
-






 下载:
下载: