-
蓝藻水华在世界各地许多湖库水体频繁发生,其代谢产生的微囊藻毒素(microcystins,MCs)引发的水源地和饮用水安全问题长期以来受到各地政府和学者的持续关注,至今已报道检测出百余种MCs的异构体。其中,Microcystin-LR是一类具强致癌效应的肝毒素,天然水体中Microcystin-LR大部分以细胞结合态、少部分以溶解态存在,一般条件下极难降解,可通过水生生物富集和食物链进入人体,对健康造成严重威胁。目前,在世界卫生组织(WHO)和我国《生活饮用水卫生标准》(GB 5749-2012)中,Microcystin-LR是唯一被明确管制的MCs指标,其最高浓度限值均为1 μg·L−1,因此,对水中Microcystin-LR的去除或无害化降解成为研究热点。已报道水中Microcystin-LR的去除方法包括物理吸附法[1-2]、化学氧化法[3-4]、微生物降解法等[5-6]。物理吸附(如活性炭)对于低浓度污染去除效率高,但无法从根本上去除Microcystin-LR,并存在二次污染的潜在风险;化学氧化法(如臭氧、高锰酸钾等)反应速度快,但矿化率低,反应过程可能产生具诱发癌变的基因毒性中间副产物;微生物降解法温和安全,但反应时间长、稳定可控性差。近十多年来对于水中难降解微污染有机物的研究,光催化技术因其具有降解高效彻底、环境友好等优点在水处理领域发展迅速[7],包括用于降解水中Microcystin-LR的研究也逐渐深入。
自1972年起,FUJISHIMA [8]发现,在TiO2电极上将水光解产生氢气的现象后,TiO2日益成为最具发展前景的半导体光催化剂,引发了广泛研究兴趣,并在环境污染物降解、清洁能源制取等多领域取得良好的研究进展[7,9]。但由于TiO2光谱响应范围窄(仅对紫外光响应)、光生电子-空穴对(e−-h+)易复合等缺点的制约,无法满足其在环保领域提升高效节能处理技术的发展需求。为促进TiO2光催化响应活性和效率,学者们对TiO2开展大量相关的掺杂元素(氮、硫等非金属,铜、铋等金属)改性与负载复合材料(硅藻土、活性炭)等研究,结果表明可不同程度提高可见光降解处理效率[10]。特别是氮元素掺杂对提高TiO2的可见光响应活性已从理论到对多种有机污染物降解实验效果被反复验证有效[11]。氧化石墨烯(GO)作为一种单层二维碳纳米材料,是碳原子以sp2杂化轨道组成的六角型平面结构,具有比表面积大、吸附性能好、表面富含多类含氧官能团,电荷迁移率高等特性,在光催化领域将其作为负载材料引入成为近年来关注热点。已证实GO负载不仅增强了材料的分散性、减缓了团聚现象,还能促进价带电子激发跃迁至导带,提高光生载流子的迁移速率,有效降低e−-h+的复合率,使光催化活性得到提高[12-13]。
目前关于GO负载改性TiO2的降解研究主要集中在染料废水的处理,在净水领域对微污染有机物的处理研究还较少见。因此,本研究基于氮掺杂改性TiO2(N-TiO2)在可见光下降解Microcystin-LR的前期研究基础[14],通过GO负载N-TiO2一步水热法合成N-TiO2/rGO复合材料,探究了二者协同效应进一步提高可见光催化Microcystin-LR的降解减毒和反应机理,深化改性TiO2的可见光催化方法理论,以期为蓝藻水华发生期水源地与水厂原水绿色高效去除Microcystin-LR污染提供参考。
-
钛酸丁酯(分析纯,阿拉丁),氧化石墨烯(GO≥96.6%,图灵进化科技),无水乙醇、浓盐酸、尿素(分析纯,西陇化工股份有限公司),Microcystin-LR标样(Microcystin-LR>95%,中国科学院水生生物研究所),三氟乙酸、对苯二甲酸、乙二胺四乙酸二钠(分析纯,国药集团化学试剂有限公司),甲醇(色谱纯,国药集团化学试剂有限公司)。
场发射扫描电子显微镜(Regulus 8100,HITACHI),氮气吸附-脱附仪(BELSORP-Mini Ⅱ,MicrotracBEL),X射线粉末衍射仪(D8 Advance,Bruker),显微共聚焦拉曼光谱仪(DXR2xi,Thermo Scientific),傅里叶变换红外光谱仪(Nicolet iS10,Thermo Scientific),紫外-可见漫反射光谱仪(U-4100,HITACHI),高效液相色谱仪(Agilent 1260,Agilent),总有机碳分析仪(TOC-VCPH,Shimadzu)。
-
取适量GO置于无水乙醇中,超声15 min使其分散均匀;将10 mL钛酸丁酯和50 mL无水乙醇缓慢滴入其中,搅拌混合均匀,再滴入适量尿素溶液,搅拌30 min后,滴加浓盐酸调节pH至2;再将10 mL H2O缓慢滴入,持续搅拌1 h。将混合液转移至100 mL水热反应釜中,置于电热鼓风干燥箱180℃反应12 h,冷却至室温,并用去离子水、无水乙醇洗涤,80 ℃干燥,研磨备用。另外,实验中用于对比的纯TiO2(不添加GO及尿素)和N-TiO2(不添加GO)的制备条件及流程与上述一致。
-
采用扫描电子显微镜(SEM)观察材料微观形貌、颗粒结构与尺寸,扫描电压为5.0 kV,样品的真空度小于2.7×10−5 Pa;采用氮气吸附-脱附仪(BET)分析材料比表面积、孔容和孔径等信息;采用X射线粉末衍射仪(XRD)分析材料晶型结构,使用Cu-Kα射线(λ=0.154 06 nm),扫描范围为5°~80°;显微共聚焦拉曼光谱(Raman)用于研究材料的分子振动和分子结构;傅里叶变换红外光谱(FT-IR)用于分析材料的化学键和官能团结构,设置波长为400~4 000 cm−1,分辨率4 cm−1,扫描32次;紫外-可见漫反射光谱仪(UV-vis DRS)用于测试材料在紫外-可见光谱频区的吸收性能曲线,扫描波长范围200~800 nm,以BaSO4作参比。
-
1)不同材料光催化降解Microcystin-LR实验。为比较不同材料对Microcystin-LR降解效果的影响,实验设置TiO2、6%N-TiO2(N-TiO2)和6%N-TiO2/5%GO(N-TiO2/rGO)可见光下催化降解Microcystin-LR,其中光催化材料投加量为1 g·L−1,Microcystin-LR溶液初始浓度为1 mg·L−1。暗反应30 min达到吸附-脱附平衡后在氙灯可见光(300 W,420 nm<λ<800 nm)下反应90 min,期间每隔15 min取样,3 000 r·min−1离心5 min,上清液过0.22 μm滤膜后用高效液相色谱测定。
检测条件:色谱柱 SB-C18 ( 5 μm,4.6 mm×150 mm,Agilent 1260);流动相为 V (体积分数为0.1%三氟乙酸溶液):V (甲醇) = 30:70;柱温40℃;流速0.8 mL·min−1;检测波长239 nm;进样量10 μL。
2)自由基捕获实验。通过自由基捕获实验探究Microcystin-LR光催化降解过程中起主要作用的活性物种,分析降解机理。羟基自由基(·OH)清除剂为1 mmol·L−1的对苯二甲酸(PTA),光生空穴(h+)清除剂为1 mmol·L−1的乙二胺四乙酸二钠(EDTA-2Na)。通过比较含有清除剂和不含清除剂的三组实验中Microcystin-LR去除效果的差异来判断两种主要活性物种发挥的作用,以自由基的贡献率(式(1))来表示。
式中:ƞ1为不含清除剂的Microcystin-LR降解率,%;ƞ2为含有清除剂(PTA或EDTA-2Na )的Microcystin-LR降解率,%;ƞ为贡献率,%。
3)降解动力学分析。采用L-H动力学模型(式(2))拟合Microcystin-LR的可见光催化降解过程,探究不同材料对反应速率的影响。
式中:C0为Microcystin-LR溶液的初始质量浓度,mg·L−1;C为Microcystin-LR溶液的实时质量浓度,mg·L−1;k为降解速率常数,min−1;t为光催化反应时间,min。
4)降解矿化率分析。通过测定反应过程中总有机碳(TOC)的浓度变化,探究Microcystin-LR在光降解过程中的深度氧化矿化水平,以矿化率θ (式(3))来表示其矿化程度。
式中:θ为矿化率,%;C0为Microcystin-LR溶液的初始TOC含量,mg·L−1;C为Microcystin-LR溶液的实时TOC含量,mg·L−1。
-
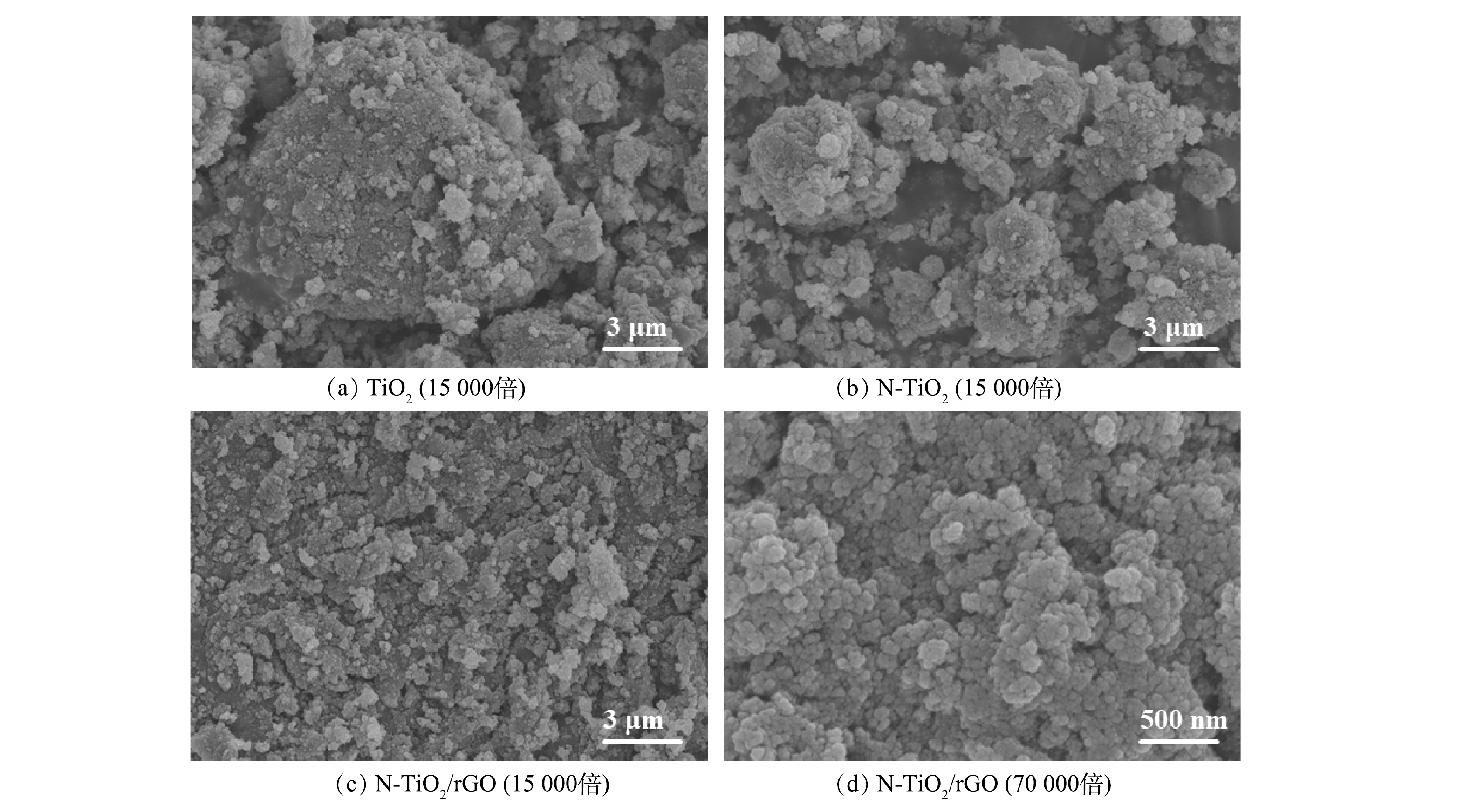
1) SEM分析。图1是不同放大倍数下TiO2、N-TiO2和N-TiO2/rGO的扫描电镜图。由图1(a)可见,纯TiO2形状不规则、颗粒粒径较大,大小不均的TiO2颗粒附着多呈块状且相互粘结集聚,团聚现象明显; N掺杂后的N-TiO2颗粒粒径减小,颗粒大小差异也显著缩小,材料整体分散性提高(图1(b))。GO载体表面原有的含氧官能团提供活性位点利于N-TiO2粒子与其键合,促进N-TiO2粒子的分散与生成。因此,负载后的N-TiO2/rGO(图1(c)~(d))颗粒更加均匀细小,N-TiO2粒子紧密附着在GO片层表面,分散较均匀且轮廓分明,证明N-TiO2粒子成功负载。相比于TiO2和 N-TiO2,N-TiO2/rGO颗粒之间分布较疏松均匀,几乎没有出现块状或团聚态颗粒。一方面,这是由于N的掺杂会部分替换晶格氧,消耗TiO2晶体生长时所需的能量,从而使颗粒粒径减小;另一方面,GO二维六角型平面结构良好的导电性促使颗粒间的静电作用力减弱[15],其平面晶格含有大量褶皱也同时为TiO2提供丰富附着点位[16],可有效克服晶体团聚,进而增强分散性、增大比表面积,易于有机污染物在N-TiO2/rGO复合材料上充分吸附,通过促进光生电子和空穴的产生与转移,强化其在纳米复合材料上的光催化降解反应进程和效率[17]。
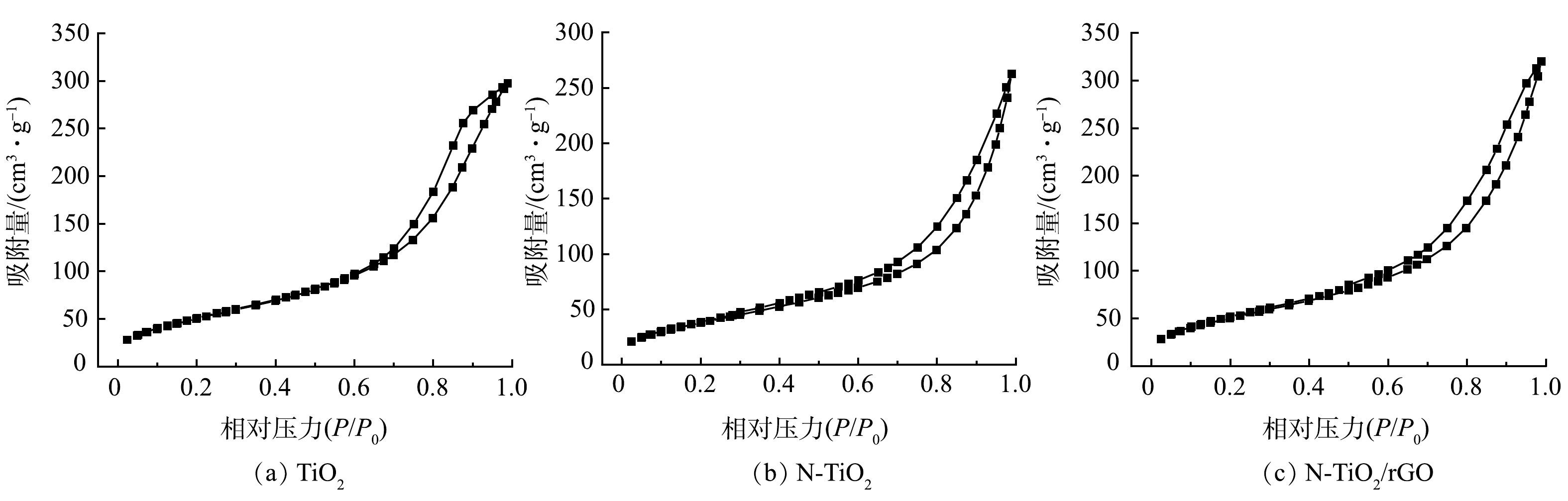
2) BET分析。采用氮气吸附-脱附法测试光催化材料的孔径结构变化,结果如图2所示。TiO2、N-TiO2和N-TiO2/rGO 3种光催化材料的比表面积、孔容及孔径见表1。分析图2,TiO2、N-TiO2和N-TiO2/rGO的吸附等温线均属于Ⅳ型等温线,为中孔材料,有滞后环伴随出现。等温线在末端出现吸附回滞环,说明在孔中会发生毛细凝聚现象,毛细凝聚会使其吸附中止,阻止更深入层次的吸附。图2(a)中TiO2在等温线末端的回滞环凸起更加明显,这也是其吸附性能较差从而导致光催化活性低的原因之一[18]。
相比GO、TiO2和N-TiO2,N-TiO2/rGO的比表面积、孔容和孔径均有所增大(表1)。GO负载后,N-TiO2/rGO的比表面积从101.055 m2·g−1提升至265.124 m2·g−1,增加了1.6倍。这表明N和GO的加入减小了TiO2纳米微粒的团聚程度,这也是N-TiO2/rGO具有更大比表面积的原因,进一步验证了前述SEM的表征结果。此外,N-TiO2/rGO相对于纯TiO2,孔容由0.173 cm3·g−1增至0.561 cm3·g−1,孔径由3.939 nm增至7.822 nm,这可能是N-TiO2与GO水热过程脱除部分氧官能团促进了孔隙的生成。N-TiO2/rGO更加丰富的介孔结构,能增加吸附容量并提供更多反应活性位点,更大的比表面可有效提高反应体系分子的传递能力和载流子的迁移率[19],从而提高光催化反应速率。
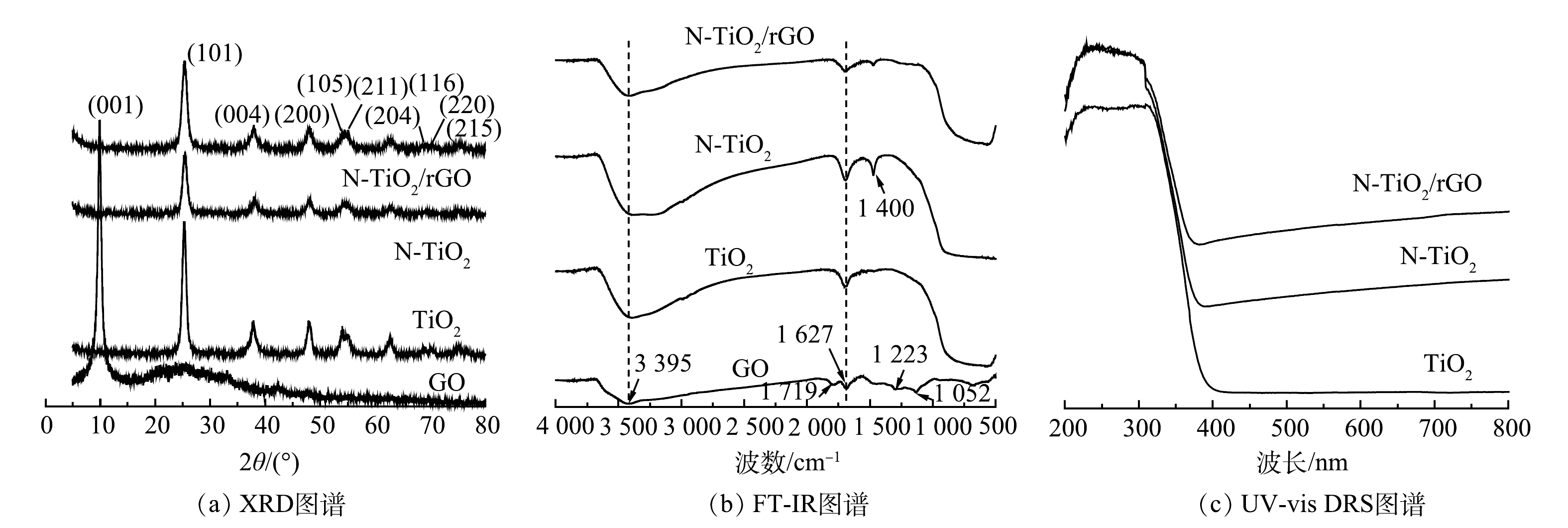
3) XRD、FT-IR、 UV-vis DRS光谱分析。水热合成纯TiO2、N-TiO2和N-TiO2/rGO样品的XRD、FT-IR、UV-vis DRS分析结果如图3。由图3(a)对比锐钛矿型标准卡片(JCPDS No.21-1272)可知,三者在25.39°处均出现主衍射峰,对应(101)晶面,同时在37.98°(004)、48.09°(200)、54.42°(105)、55.05°(211)、63.01°(204)、69.22°(116)、69.85°(220)、75.32°(215)出现一系列特征衍射峰,未发现TiO2其他相的衍射峰,表明3种催化剂均为锐钛矿型[20],N掺杂与GO负载对TiO2晶型结构没有明显影响。GO负载TiO2后,其在9.97°的特征衍射峰(001)消失,表明此时GO已经被还原,大部分含氧官能团消失。这进一步证实GO的负载没有破坏TiO2 的原物相结构,并且未出现新的衍射峰,其原因可能是GO在水热处理过程中材料组成遭到破坏,被还原成rGO,而其在24.5°处的(002)特征衍射峰又被TiO2锐钛矿相在25.39°处(101)特征衍射峰所掩盖[21]。图中均未出现明显N的特征峰,推测是由于材料中N组分的含量较少,且N原子的离子半径与O原子的较为接近,可能以置换的方式与TiO2晶体相结合,故未被检测出来。
图3(b)显示了GO、TiO2、N-TiO2和N-TiO2/rGO纳米复合物的FT-IR光谱。4种催化剂在1 627 cm−1和3 395 cm−1处均出现较明显的特征峰,归属于吸附在TiO2表面水分子的-OH弯曲和伸缩振动峰,同时1 627 cm−1处特征峰也是GO的sp2结构的C=C骨架伸缩振动峰。从GO图谱还可以看到,1 719 cm−1处出现清晰的C=O伸缩振动峰,来自于GO片层边缘羧基和羰基;1 052 cm−1和1 223 cm−1处的特征峰分别对应烷氧基和环氧基的C—O伸缩振动[11],表明GO含有大量含氧官能团。TiO2、N-TiO2和N-TiO2/rGO在400~900 cm−1处出现TiO2的Ti—O—Ti伸缩振动[22],说明TiO2自身没有发生明显变化;对比发现掺杂N后在1 400 cm−1处出现新的特征峰,可能是—NH2基团吸收峰,结合XRD分析,推测N原子进入TiO2晶格内取代部分O,在400~900 cm−1处形成Ti—O—N结构,可增强对可见光的吸收阈值。与GO相比,N-TiO2/GO在1 719 cm−1处的C=O伸缩振动、1 052 cm−1处烷氧基及1 223 cm−1处环氧基的C—O伸缩振动峰几乎都消失,说明GO在水热过程中发生了一定还原,大量含氧官能团被移除;同时,表面残余的部分含氧官能团有助于TiO2纳米颗粒与GO片层的结合,形成Ti—O—C键[23],与Ti—O—Ti同时存在。以上都表明,GO与N-TiO2已成功形成复合材料。
从TiO2、N-TiO2和N-TiO2/rGO的紫外-可见漫反射的吸收光谱(图3(c))中可明显看出,TiO2仅在紫外光区有明显吸收,在可见光区域几乎没有响应,而N-TiO2和N-TiO2/rGO的光吸收阈值显著增大,吸收边带发生明显红移。结合XRD和FT-IR的表征结果分析其原因:一方面,N原子进入晶体内部形成Ti—O—N结构,有利于产生氧空位,缩窄带隙,从而可减小电子发生跃迁时所需能量;另一方面,负载的GO片层可与TiO2结合形成Ti—O—C键[24-25],可加快光生电子的转移。通过UV vis-DRS一级积分曲线分别得到TiO2、N-TiO2和N-TiO2/rGO材料光吸收阈值λ为385、400和418 nm,根据Eg(eV)=1 240/λ计算得到相应的带隙值分别为3.22、3.10和2.97 eV。N-TiO2/rGO复合材料带隙变窄归因于N掺杂和GO的引入,使得TiO2的可见光响应范围拓宽。
4) Raman分析。为比较TiO2及其改性后复合材料的分子振动,研究碳原子在GO表面的无序排列和缺陷,对TiO2、N-TiO2和GO、N-TiO2/rGO材料进行了拉曼光谱表征分析(图4)。在143(Eg)、397(B1g)、517(A1g/B1g)和638(Eg) cm−1处出现的4个典型峰对应于锐钛矿相TiO2的特征峰[24],表明三者存在锐钛矿相,N掺杂未使TiO2的特征峰发生偏移,印证了XRD的表征结果(图3(a))。GO和N-TiO2/rGO在1 350 cm−1和1 580 cm−1处显示出GO的特征D峰和G峰,分别与sp3和sp2杂化碳原子的Raman振动峰相一致[26],这表明N-TiO2与GO已很好地结合。D峰表示GO边缘缺陷引起的无定型结构,G峰对应GO结构的有序状态,两峰的比值ID/IG反映结构的缺陷程度[27]。计算得出,GO和N-TiO2/rGO的ID/IG分别为0.98和1.01,与GO相比,N-TiO2/rGO的ID/IG更高。这表明GO与TiO2复合引入了sp3缺陷,GO的sp3杂化碳原子增加,推测这是GO与TiO2界面间的强相互作用(如Ti-O-C键)所致,即GO水热反应后被还原,大部分含氧官能团被移除,碳原子的化学状态发生了显著变化[24,26,28]。
-
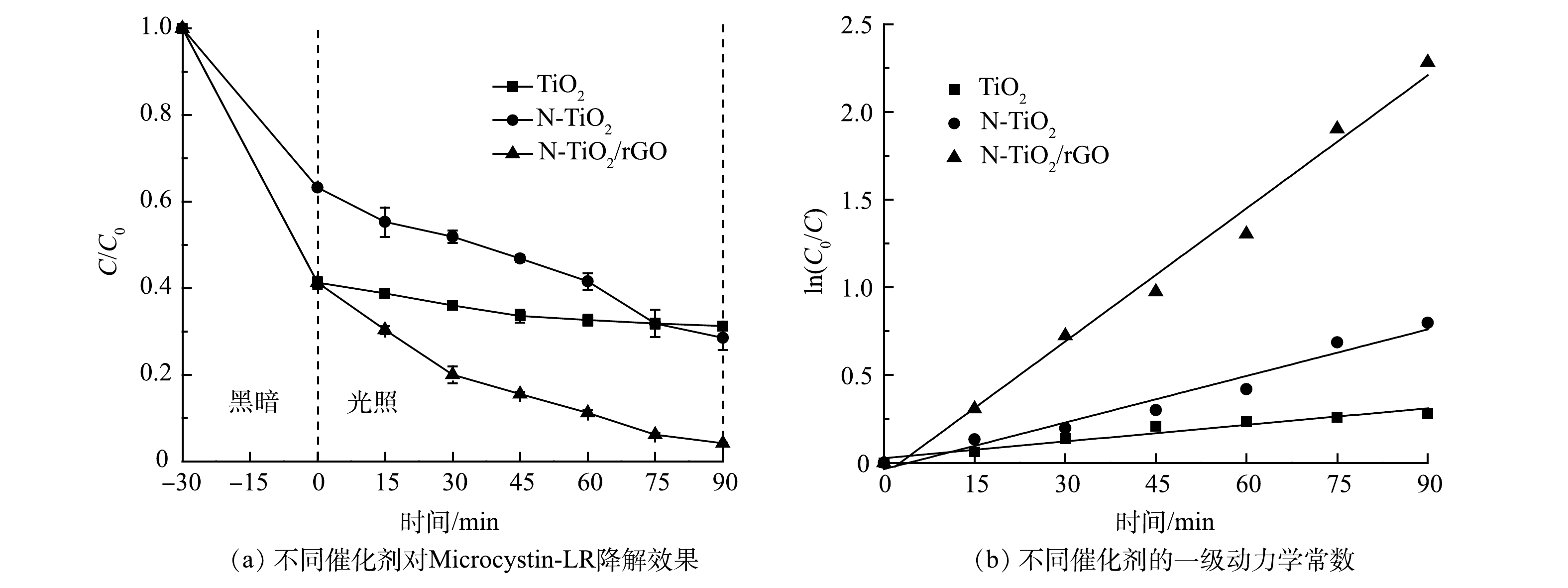
1)不同材料可见光催化降解Microcystin-LR及其动力学分析。由图5(a)可见,纯TiO2对Microcystin-LR光反应90 min后,去除率仅有24.2%;与纯TiO2相比,N-TiO2和N-TiO2/rGO对Microcystin-LR的降解效果均有显著提升,降解率分别达到54.9%和89.8%。根据UV-Vis DRS表征结果,N-TiO2/rGO的禁带宽度最小,光子利用率高,同时由于GO的负载,生成的光生电子-空穴对可以更快传递到光催化材料表面,与Microcystin-LR溶液反应生成·OH和O2−(式4),随后·OH与h+将Microcystin-LR氧化,生成中间产物或最终产物(式5)[29]。结合BET分析结果可知,N-TiO2/rGO的比表面积为265.1 m2·g−1,远大于N-TiO2(200.1 m2·g−1)和TiO2(101.0 m2·g−1),而较大的比表面积能够将Microcystin-LR有效吸附在材料表面,促进界面反应的进行。因界面反应过程是光催化反应过程的速率控制步骤,故N-TiO2/rGO对Microcystin-LR有较为优异的降解效果。
图5(b)的拟合结果说明,Microcystin-LR光降解反应过程的动力学拟合曲线符合准一级动力学方程。采用L-H模型拟合动力学方程,结果见表2。可见,TiO2光降解Microcystin-LR反应速率常数k仅为0.003 2 min−1,N-TiO2和N-TiO2/rGO分别提高1.78倍和6.91倍,达到0.008 9 min−1和0.025 3 min−1。结合BET分析,N-TiO2/rGO拥有更大的比表面积,能够提供更多的活性吸附位点;同时,反应体系中·OH和·O2−等具有强氧化性的活性物质浓度增大,使界面反应的效率得到提高,从而促进光催化效率。
2) Microcystin-LR降解矿化率分析。由图6可见,当N-TiO2/rGO投加量为1 g·L−1、Microcystin-LR初始质量浓度为1 mg·L−1时,光催化90 min后Microcystin-LR的降解率为89.8%,矿化率为81.8%。这表明,在光催化降解过程中,N-TiO2/rGO能够将大部分Microcystin-LR分子矿化为CO2、H2O以及少量的小分子有机化合物。
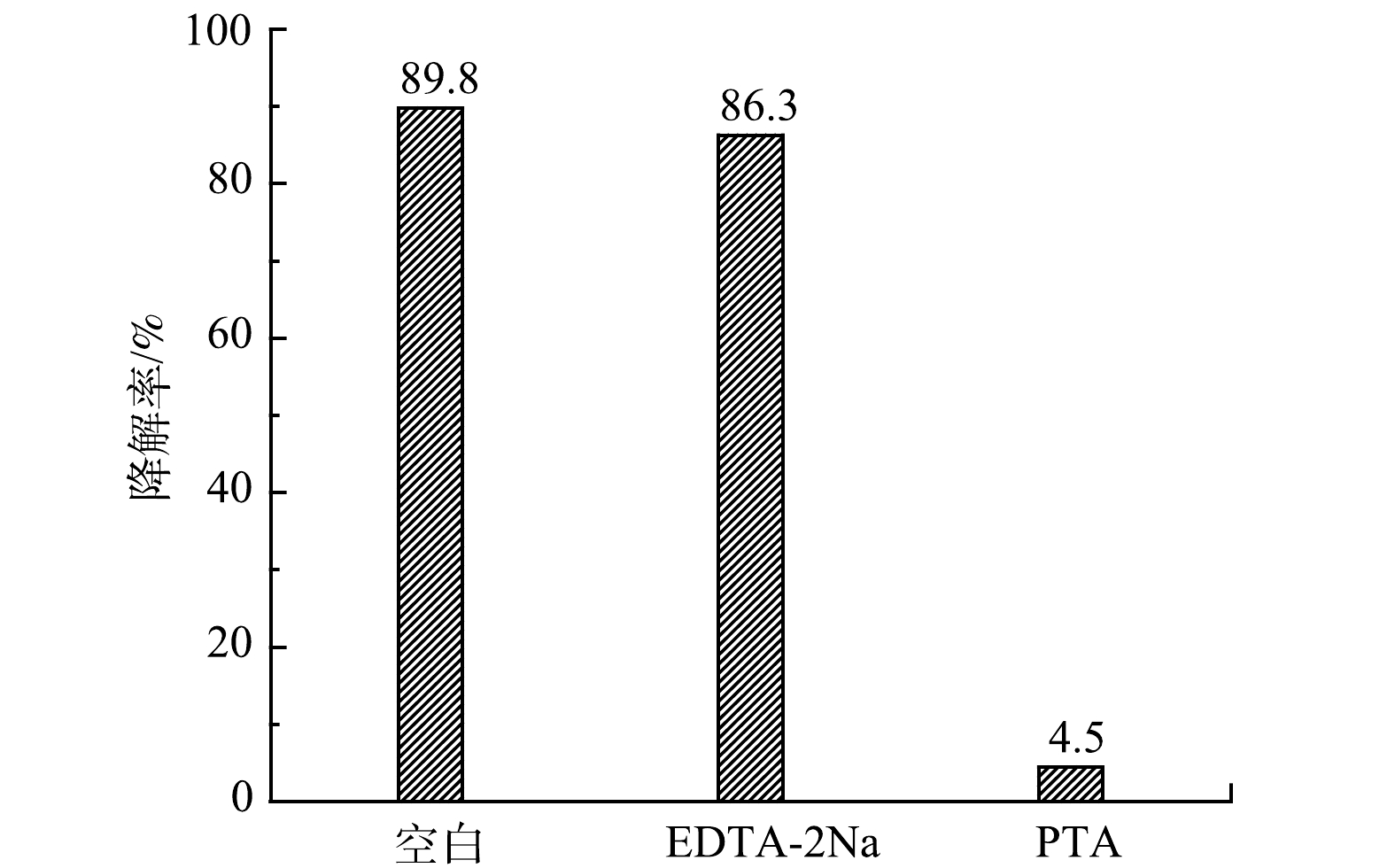
3) N-TiO2/rGO光催化降解Microcystin-LR的机理分析。N-TiO2/rGO复合光催化材料价带上的e−被激发跃迁到导带生成光生电子,同时在价带生成对应的光生空穴(h+),通过与水中O2氧化反应生成超氧自由基(·O2−),并进一步反应生成强氧化性的羟基自由基(·OH)。因此,光催化过程对有机物起主要作用的活性物种为羟基自由基(·OH)、空穴(h+)和超氧自由基(·O2−)等。为探究N-TiO2/rGO对Microcystin-LR光催化降解过程各类活性物种的贡献程度,采用竞争动力学实验,以对苯二甲酸(PTA)和乙二胺四乙酸二钠(EDTA-2Na)分别为·OH、h+的清除剂[30],比较含有与不含清除剂反应体系中Microcystin-LR的降解效果以及·OH/h+与Microcystin-LR竞争反应的影响程度。PTA是·OH的高效清除剂,反应体系中加入过量PTA,将优先捕获体系中生成的·OH并形成几乎没有氧化能力的二羟基-PTA,阻断·OH对Microcystin-LR的氧化降解;反应体系中过量EDTA-2Na快速将 h+还原,抑制h+氧化Microcystin-LR的反应,由此可间接获得相应活性氧物种在反应体系中的贡献。如图7所示,PTA的加入使得Microcystin-LR的降解率由89.8%锐减至4.5%;而 EDTA-2Na对光降解的抑制效果不明显,反应中加入捕获h+的EDTA-2Na后,Microcystin-LR的降解率仍达86.3%。由此,根据清除剂加入前后Microcystin-LR的光降解率差异推算·OH和h+对Microcystin-LR可见光催化降解的贡献率分别为95.0%和3.9%。此外,反应中间过程产生的超氧自由基(·O2−)等因存在时间短,氧化性较·OH和h+明显弱,贡献率仅约1%。因此,在TiO2/rGO复合光催化Microcystin-LR过程中,·OH起了主导性的贡献作用。许文泽等[31]在采用二氧化钛光催化氧化阿散酸中也证实,·OH是发挥主要氧化作用的物种。
有研究报道[32],光激发诱导产生的·OH通过攻击Microcystin-LR分子Adda支链上共轭双键及肽环结构等降解复杂的大分子,并进一步发生裂环分解成多个小分子物质,最后矿化为H2O和CO2。其中Adda支链共轭双键、苯环和甲氧基优先被·OH攻击,C4-C5和C6-C7共轭双键断裂氧化分别生成醛和酮;C9位甲氧基被氧化成甲酸,在酸性环境中可以水解,将Adda侧链从肽链中分离出来[33-35],因此,通过·OH的氧化分解可实现Microcystin-LR的降解减毒。
-
1)采用水热法一步还原制备N-TiO2/rGO光催化复合材料,最优制备条件为:水热反应温度180℃、掺N量6%、GO负载量5%。相较TiO2、N-TiO2,复合材料N-TiO2/rGO颗粒粒径小,分散性、层次性、多孔性优,晶型仍以锐钛矿相为主,光生载流子分离效率高,可见光催化活性好。
2)投加1 g·L−1的N-TiO2/rGO复合光催化剂,可见光照射90 min后,对初始质量浓度为1 mg·L−1的Microcystin-LR降解率达 89.8%,反应符合准一级动力学方程,降解速率常数为0.025 3 min−1。
3) ·OH 是Microcystin-LR光催化降解的主要活性物种,其对降解的贡献率达95.0%,而h+的贡献率仅为3.9%;·OH通过对Microcystin-LR分子Adda侧链共轭双键、苯环和甲氧基的攻击,实现降解和减毒。
N-TiO2/rGO纳米材料可见光下催化降解微囊藻毒素-LR的效果
Visible-light photodegradation of Microcystin-LR by N-TiO2/rGO nanomaterials
-
摘要: 以尿素、钛酸丁酯、氧化石墨烯为原料,采用水热法制备了N-TiO2/rGO复合纳米光催化剂,通过SEM、BET、XRD、Raman、FT-IR、UV-vis DRS等分析方法对催化剂进行了表征和分析,研究了该复合材料在可见光下对水中Microcystin-LR的降解动力学及相关反应机理。结果表明,相较未负载的N-TiO2,N-TiO2/rGO对可见光的响应范围显著拓宽,表面颗粒分布均匀,比表面积增大,其多层立体孔隙结构能有效克服团聚现象,光生载流子分离效率提升。在水热温度为180℃、掺N比为6%(质量百分比)及GO负载量为5%(质量百分比)的制备条件下,可获得光催化活性最优的N-TiO2/rGO复合纳米材料;投加1 g·L−1该材料,在可见光下对水中初始质量浓度为 1 mg·L−1 Microcystin-LR催化反应90 min后,降解率可达89.8%,反应速率常数为0.025 3 min−1。自由基捕获实验结果表明,·OH对光催化反应降解Microcystin-LR中起主导作用,贡献率为95.0 %,而空穴(h+)贡献率仅占3.9 %。Abstract: In this study, urea (nitrogen source), butyl titanate (precursor) and GO (carrier) were taken as raw materials, the novel photocatalysts of N-TiO2/rGO were synthesized by hydrothermal method for efficient degradation of Microcystin-LR (MC-LR). Then N-TiO2/rGO was characterized by SEM, BET, XRD, Raman, FT-IR and UV-vis DRS. Furthermore, the mechanism and reaction kinetics of MC-LR degradation in water by N-TiO2/rGO under visible light were studied. The results showed that, compared to unloaded N-TiO2, the compound photocatalyst of N-TiO2/rGO widened the visible-light response range, showed more evenly distribution of surface particles, and increased the specific surface area. In addition, the agglomeration behaviour of N-TiO2/rGO decreased obviously due to the multilayer three-dimensional pores structure, and the photocarries separation efficiency greatly improved. The optimum preparation conditions of N-TiO2/rGO occurred at hydrothermal temperature of 180℃, 6 wt% N-doped and 5 wt% GO-loaded, which exhibited the best visible light photocatalytic activity. The degradation efficiency of MC-LR (1 mg·L−1 of initial concentration) in water achieved 89.8% after 90 minutes photocatalysis at N-TiO2/rGO dosage of 1 g·L−1, and the reaction rate constant was 0.025 3 min−1. Free radical trapping experiments demonstrated that ·OH radical played the dominant role on the photocatalytic degradation of MC-LR, it contribution accounted for 95.0%, while the contribution of hole (H+) only accounted for 3.9%.
-
Key words:
- Microcystin-LR /
- N-doped /
- TiO2 /
- GO /
- visible light photocatalysis
-
蓝藻水华在世界各地许多湖库水体频繁发生,其代谢产生的微囊藻毒素(microcystins,MCs)引发的水源地和饮用水安全问题长期以来受到各地政府和学者的持续关注,至今已报道检测出百余种MCs的异构体。其中,Microcystin-LR是一类具强致癌效应的肝毒素,天然水体中Microcystin-LR大部分以细胞结合态、少部分以溶解态存在,一般条件下极难降解,可通过水生生物富集和食物链进入人体,对健康造成严重威胁。目前,在世界卫生组织(WHO)和我国《生活饮用水卫生标准》(GB 5749-2012)中,Microcystin-LR是唯一被明确管制的MCs指标,其最高浓度限值均为1 μg·L−1,因此,对水中Microcystin-LR的去除或无害化降解成为研究热点。已报道水中Microcystin-LR的去除方法包括物理吸附法[1-2]、化学氧化法[3-4]、微生物降解法等[5-6]。物理吸附(如活性炭)对于低浓度污染去除效率高,但无法从根本上去除Microcystin-LR,并存在二次污染的潜在风险;化学氧化法(如臭氧、高锰酸钾等)反应速度快,但矿化率低,反应过程可能产生具诱发癌变的基因毒性中间副产物;微生物降解法温和安全,但反应时间长、稳定可控性差。近十多年来对于水中难降解微污染有机物的研究,光催化技术因其具有降解高效彻底、环境友好等优点在水处理领域发展迅速[7],包括用于降解水中Microcystin-LR的研究也逐渐深入。
自1972年起,FUJISHIMA [8]发现,在TiO2电极上将水光解产生氢气的现象后,TiO2日益成为最具发展前景的半导体光催化剂,引发了广泛研究兴趣,并在环境污染物降解、清洁能源制取等多领域取得良好的研究进展[7,9]。但由于TiO2光谱响应范围窄(仅对紫外光响应)、光生电子-空穴对(e−-h+)易复合等缺点的制约,无法满足其在环保领域提升高效节能处理技术的发展需求。为促进TiO2光催化响应活性和效率,学者们对TiO2开展大量相关的掺杂元素(氮、硫等非金属,铜、铋等金属)改性与负载复合材料(硅藻土、活性炭)等研究,结果表明可不同程度提高可见光降解处理效率[10]。特别是氮元素掺杂对提高TiO2的可见光响应活性已从理论到对多种有机污染物降解实验效果被反复验证有效[11]。氧化石墨烯(GO)作为一种单层二维碳纳米材料,是碳原子以sp2杂化轨道组成的六角型平面结构,具有比表面积大、吸附性能好、表面富含多类含氧官能团,电荷迁移率高等特性,在光催化领域将其作为负载材料引入成为近年来关注热点。已证实GO负载不仅增强了材料的分散性、减缓了团聚现象,还能促进价带电子激发跃迁至导带,提高光生载流子的迁移速率,有效降低e−-h+的复合率,使光催化活性得到提高[12-13]。
目前关于GO负载改性TiO2的降解研究主要集中在染料废水的处理,在净水领域对微污染有机物的处理研究还较少见。因此,本研究基于氮掺杂改性TiO2(N-TiO2)在可见光下降解Microcystin-LR的前期研究基础[14],通过GO负载N-TiO2一步水热法合成N-TiO2/rGO复合材料,探究了二者协同效应进一步提高可见光催化Microcystin-LR的降解减毒和反应机理,深化改性TiO2的可见光催化方法理论,以期为蓝藻水华发生期水源地与水厂原水绿色高效去除Microcystin-LR污染提供参考。
1. 材料与方法
1.1 试剂与仪器
钛酸丁酯(分析纯,阿拉丁),氧化石墨烯(GO≥96.6%,图灵进化科技),无水乙醇、浓盐酸、尿素(分析纯,西陇化工股份有限公司),Microcystin-LR标样(Microcystin-LR>95%,中国科学院水生生物研究所),三氟乙酸、对苯二甲酸、乙二胺四乙酸二钠(分析纯,国药集团化学试剂有限公司),甲醇(色谱纯,国药集团化学试剂有限公司)。
场发射扫描电子显微镜(Regulus 8100,HITACHI),氮气吸附-脱附仪(BELSORP-Mini Ⅱ,MicrotracBEL),X射线粉末衍射仪(D8 Advance,Bruker),显微共聚焦拉曼光谱仪(DXR2xi,Thermo Scientific),傅里叶变换红外光谱仪(Nicolet iS10,Thermo Scientific),紫外-可见漫反射光谱仪(U-4100,HITACHI),高效液相色谱仪(Agilent 1260,Agilent),总有机碳分析仪(TOC-VCPH,Shimadzu)。
1.2 N-TiO2/rGO复合材料制备
取适量GO置于无水乙醇中,超声15 min使其分散均匀;将10 mL钛酸丁酯和50 mL无水乙醇缓慢滴入其中,搅拌混合均匀,再滴入适量尿素溶液,搅拌30 min后,滴加浓盐酸调节pH至2;再将10 mL H2O缓慢滴入,持续搅拌1 h。将混合液转移至100 mL水热反应釜中,置于电热鼓风干燥箱180℃反应12 h,冷却至室温,并用去离子水、无水乙醇洗涤,80 ℃干燥,研磨备用。另外,实验中用于对比的纯TiO2(不添加GO及尿素)和N-TiO2(不添加GO)的制备条件及流程与上述一致。
1.3 表征方法
采用扫描电子显微镜(SEM)观察材料微观形貌、颗粒结构与尺寸,扫描电压为5.0 kV,样品的真空度小于2.7×10−5 Pa;采用氮气吸附-脱附仪(BET)分析材料比表面积、孔容和孔径等信息;采用X射线粉末衍射仪(XRD)分析材料晶型结构,使用Cu-Kα射线(λ=0.154 06 nm),扫描范围为5°~80°;显微共聚焦拉曼光谱(Raman)用于研究材料的分子振动和分子结构;傅里叶变换红外光谱(FT-IR)用于分析材料的化学键和官能团结构,设置波长为400~4 000 cm−1,分辨率4 cm−1,扫描32次;紫外-可见漫反射光谱仪(UV-vis DRS)用于测试材料在紫外-可见光谱频区的吸收性能曲线,扫描波长范围200~800 nm,以BaSO4作参比。
1.4 光催化降解实验
1)不同材料光催化降解Microcystin-LR实验。为比较不同材料对Microcystin-LR降解效果的影响,实验设置TiO2、6%N-TiO2(N-TiO2)和6%N-TiO2/5%GO(N-TiO2/rGO)可见光下催化降解Microcystin-LR,其中光催化材料投加量为1 g·L−1,Microcystin-LR溶液初始浓度为1 mg·L−1。暗反应30 min达到吸附-脱附平衡后在氙灯可见光(300 W,420 nm<λ<800 nm)下反应90 min,期间每隔15 min取样,3 000 r·min−1离心5 min,上清液过0.22 μm滤膜后用高效液相色谱测定。
检测条件:色谱柱 SB-C18 ( 5 μm,4.6 mm×150 mm,Agilent 1260);流动相为 V (体积分数为0.1%三氟乙酸溶液):V (甲醇) = 30:70;柱温40℃;流速0.8 mL·min−1;检测波长239 nm;进样量10 μL。
2)自由基捕获实验。通过自由基捕获实验探究Microcystin-LR光催化降解过程中起主要作用的活性物种,分析降解机理。羟基自由基(·OH)清除剂为1 mmol·L−1的对苯二甲酸(PTA),光生空穴(h+)清除剂为1 mmol·L−1的乙二胺四乙酸二钠(EDTA-2Na)。通过比较含有清除剂和不含清除剂的三组实验中Microcystin-LR去除效果的差异来判断两种主要活性物种发挥的作用,以自由基的贡献率(式(1))来表示。
η=(η1−η2)/η1×100% (1) 式中:ƞ1为不含清除剂的Microcystin-LR降解率,%;ƞ2为含有清除剂(PTA或EDTA-2Na )的Microcystin-LR降解率,%;ƞ为贡献率,%。
3)降解动力学分析。采用L-H动力学模型(式(2))拟合Microcystin-LR的可见光催化降解过程,探究不同材料对反应速率的影响。
lnC=−kt+lnC0 (2) 式中:C0为Microcystin-LR溶液的初始质量浓度,mg·L−1;C为Microcystin-LR溶液的实时质量浓度,mg·L−1;k为降解速率常数,min−1;t为光催化反应时间,min。
4)降解矿化率分析。通过测定反应过程中总有机碳(TOC)的浓度变化,探究Microcystin-LR在光降解过程中的深度氧化矿化水平,以矿化率θ (式(3))来表示其矿化程度。
θ=(C0−C)/C0×100% (3) 式中:θ为矿化率,%;C0为Microcystin-LR溶液的初始TOC含量,mg·L−1;C为Microcystin-LR溶液的实时TOC含量,mg·L−1。
2. 结果与讨论
2.1 催化材料表征分析
1) SEM分析。图1是不同放大倍数下TiO2、N-TiO2和N-TiO2/rGO的扫描电镜图。由图1(a)可见,纯TiO2形状不规则、颗粒粒径较大,大小不均的TiO2颗粒附着多呈块状且相互粘结集聚,团聚现象明显; N掺杂后的N-TiO2颗粒粒径减小,颗粒大小差异也显著缩小,材料整体分散性提高(图1(b))。GO载体表面原有的含氧官能团提供活性位点利于N-TiO2粒子与其键合,促进N-TiO2粒子的分散与生成。因此,负载后的N-TiO2/rGO(图1(c)~(d))颗粒更加均匀细小,N-TiO2粒子紧密附着在GO片层表面,分散较均匀且轮廓分明,证明N-TiO2粒子成功负载。相比于TiO2和 N-TiO2,N-TiO2/rGO颗粒之间分布较疏松均匀,几乎没有出现块状或团聚态颗粒。一方面,这是由于N的掺杂会部分替换晶格氧,消耗TiO2晶体生长时所需的能量,从而使颗粒粒径减小;另一方面,GO二维六角型平面结构良好的导电性促使颗粒间的静电作用力减弱[15],其平面晶格含有大量褶皱也同时为TiO2提供丰富附着点位[16],可有效克服晶体团聚,进而增强分散性、增大比表面积,易于有机污染物在N-TiO2/rGO复合材料上充分吸附,通过促进光生电子和空穴的产生与转移,强化其在纳米复合材料上的光催化降解反应进程和效率[17]。
2) BET分析。采用氮气吸附-脱附法测试光催化材料的孔径结构变化,结果如图2所示。TiO2、N-TiO2和N-TiO2/rGO 3种光催化材料的比表面积、孔容及孔径见表1。分析图2,TiO2、N-TiO2和N-TiO2/rGO的吸附等温线均属于Ⅳ型等温线,为中孔材料,有滞后环伴随出现。等温线在末端出现吸附回滞环,说明在孔中会发生毛细凝聚现象,毛细凝聚会使其吸附中止,阻止更深入层次的吸附。图2(a)中TiO2在等温线末端的回滞环凸起更加明显,这也是其吸附性能较差从而导致光催化活性低的原因之一[18]。
表 1 GO、TiO2、N-TiO2和N-TiO2/rGO的比表面积、孔容和孔径Table 1. Specific surface area, pore volume and pore size of GO, TiO2, N-TiO2 and N-TiO2/rGO样品 比表面积/(m2·g−1) 孔容/(cm3·g−1) 孔径/nm GO 6.125 0.019 12.421 TiO2 101.055 0.173 3.939 N-TiO2 200.127 0.427 6.874 N-TiO2/rGO 265.124 0.561 7.822 相比GO、TiO2和N-TiO2,N-TiO2/rGO的比表面积、孔容和孔径均有所增大(表1)。GO负载后,N-TiO2/rGO的比表面积从101.055 m2·g−1提升至265.124 m2·g−1,增加了1.6倍。这表明N和GO的加入减小了TiO2纳米微粒的团聚程度,这也是N-TiO2/rGO具有更大比表面积的原因,进一步验证了前述SEM的表征结果。此外,N-TiO2/rGO相对于纯TiO2,孔容由0.173 cm3·g−1增至0.561 cm3·g−1,孔径由3.939 nm增至7.822 nm,这可能是N-TiO2与GO水热过程脱除部分氧官能团促进了孔隙的生成。N-TiO2/rGO更加丰富的介孔结构,能增加吸附容量并提供更多反应活性位点,更大的比表面可有效提高反应体系分子的传递能力和载流子的迁移率[19],从而提高光催化反应速率。
3) XRD、FT-IR、 UV-vis DRS光谱分析。水热合成纯TiO2、N-TiO2和N-TiO2/rGO样品的XRD、FT-IR、UV-vis DRS分析结果如图3。由图3(a)对比锐钛矿型标准卡片(JCPDS No.21-1272)可知,三者在25.39°处均出现主衍射峰,对应(101)晶面,同时在37.98°(004)、48.09°(200)、54.42°(105)、55.05°(211)、63.01°(204)、69.22°(116)、69.85°(220)、75.32°(215)出现一系列特征衍射峰,未发现TiO2其他相的衍射峰,表明3种催化剂均为锐钛矿型[20],N掺杂与GO负载对TiO2晶型结构没有明显影响。GO负载TiO2后,其在9.97°的特征衍射峰(001)消失,表明此时GO已经被还原,大部分含氧官能团消失。这进一步证实GO的负载没有破坏TiO2 的原物相结构,并且未出现新的衍射峰,其原因可能是GO在水热处理过程中材料组成遭到破坏,被还原成rGO,而其在24.5°处的(002)特征衍射峰又被TiO2锐钛矿相在25.39°处(101)特征衍射峰所掩盖[21]。图中均未出现明显N的特征峰,推测是由于材料中N组分的含量较少,且N原子的离子半径与O原子的较为接近,可能以置换的方式与TiO2晶体相结合,故未被检测出来。
图3(b)显示了GO、TiO2、N-TiO2和N-TiO2/rGO纳米复合物的FT-IR光谱。4种催化剂在1 627 cm−1和3 395 cm−1处均出现较明显的特征峰,归属于吸附在TiO2表面水分子的-OH弯曲和伸缩振动峰,同时1 627 cm−1处特征峰也是GO的sp2结构的C=C骨架伸缩振动峰。从GO图谱还可以看到,1 719 cm−1处出现清晰的C=O伸缩振动峰,来自于GO片层边缘羧基和羰基;1 052 cm−1和1 223 cm−1处的特征峰分别对应烷氧基和环氧基的C—O伸缩振动[11],表明GO含有大量含氧官能团。TiO2、N-TiO2和N-TiO2/rGO在400~900 cm−1处出现TiO2的Ti—O—Ti伸缩振动[22],说明TiO2自身没有发生明显变化;对比发现掺杂N后在1 400 cm−1处出现新的特征峰,可能是—NH2基团吸收峰,结合XRD分析,推测N原子进入TiO2晶格内取代部分O,在400~900 cm−1处形成Ti—O—N结构,可增强对可见光的吸收阈值。与GO相比,N-TiO2/GO在1 719 cm−1处的C=O伸缩振动、1 052 cm−1处烷氧基及1 223 cm−1处环氧基的C—O伸缩振动峰几乎都消失,说明GO在水热过程中发生了一定还原,大量含氧官能团被移除;同时,表面残余的部分含氧官能团有助于TiO2纳米颗粒与GO片层的结合,形成Ti—O—C键[23],与Ti—O—Ti同时存在。以上都表明,GO与N-TiO2已成功形成复合材料。
从TiO2、N-TiO2和N-TiO2/rGO的紫外-可见漫反射的吸收光谱(图3(c))中可明显看出,TiO2仅在紫外光区有明显吸收,在可见光区域几乎没有响应,而N-TiO2和N-TiO2/rGO的光吸收阈值显著增大,吸收边带发生明显红移。结合XRD和FT-IR的表征结果分析其原因:一方面,N原子进入晶体内部形成Ti—O—N结构,有利于产生氧空位,缩窄带隙,从而可减小电子发生跃迁时所需能量;另一方面,负载的GO片层可与TiO2结合形成Ti—O—C键[24-25],可加快光生电子的转移。通过UV vis-DRS一级积分曲线分别得到TiO2、N-TiO2和N-TiO2/rGO材料光吸收阈值λ为385、400和418 nm,根据Eg(eV)=1 240/λ计算得到相应的带隙值分别为3.22、3.10和2.97 eV。N-TiO2/rGO复合材料带隙变窄归因于N掺杂和GO的引入,使得TiO2的可见光响应范围拓宽。
4) Raman分析。为比较TiO2及其改性后复合材料的分子振动,研究碳原子在GO表面的无序排列和缺陷,对TiO2、N-TiO2和GO、N-TiO2/rGO材料进行了拉曼光谱表征分析(图4)。在143(Eg)、397(B1g)、517(A1g/B1g)和638(Eg) cm−1处出现的4个典型峰对应于锐钛矿相TiO2的特征峰[24],表明三者存在锐钛矿相,N掺杂未使TiO2的特征峰发生偏移,印证了XRD的表征结果(图3(a))。GO和N-TiO2/rGO在1 350 cm−1和1 580 cm−1处显示出GO的特征D峰和G峰,分别与sp3和sp2杂化碳原子的Raman振动峰相一致[26],这表明N-TiO2与GO已很好地结合。D峰表示GO边缘缺陷引起的无定型结构,G峰对应GO结构的有序状态,两峰的比值ID/IG反映结构的缺陷程度[27]。计算得出,GO和N-TiO2/rGO的ID/IG分别为0.98和1.01,与GO相比,N-TiO2/rGO的ID/IG更高。这表明GO与TiO2复合引入了sp3缺陷,GO的sp3杂化碳原子增加,推测这是GO与TiO2界面间的强相互作用(如Ti-O-C键)所致,即GO水热反应后被还原,大部分含氧官能团被移除,碳原子的化学状态发生了显著变化[24,26,28]。
2.2 N-TiO2/rGO催化降解Microcystin-LR的性能与机理
1)不同材料可见光催化降解Microcystin-LR及其动力学分析。由图5(a)可见,纯TiO2对Microcystin-LR光反应90 min后,去除率仅有24.2%;与纯TiO2相比,N-TiO2和N-TiO2/rGO对Microcystin-LR的降解效果均有显著提升,降解率分别达到54.9%和89.8%。根据UV-Vis DRS表征结果,N-TiO2/rGO的禁带宽度最小,光子利用率高,同时由于GO的负载,生成的光生电子-空穴对可以更快传递到光催化材料表面,与Microcystin-LR溶液反应生成·OH和O2−(式4),随后·OH与h+将Microcystin-LR氧化,生成中间产物或最终产物(式5)[29]。结合BET分析结果可知,N-TiO2/rGO的比表面积为265.1 m2·g−1,远大于N-TiO2(200.1 m2·g−1)和TiO2(101.0 m2·g−1),而较大的比表面积能够将Microcystin-LR有效吸附在材料表面,促进界面反应的进行。因界面反应过程是光催化反应过程的速率控制步骤,故N-TiO2/rGO对Microcystin-LR有较为优异的降解效果。
h++OH−→⋅OH,e−+O2→⋅O−2+H+→H2O2+e−→⋅OH (4) ⋅OH+MC−LR→MC−LR′+H2Oh++MC−LR→MC−LR′→中间产物/最终降解产物 (5) 图5(b)的拟合结果说明,Microcystin-LR光降解反应过程的动力学拟合曲线符合准一级动力学方程。采用L-H模型拟合动力学方程,结果见表2。可见,TiO2光降解Microcystin-LR反应速率常数k仅为0.003 2 min−1,N-TiO2和N-TiO2/rGO分别提高1.78倍和6.91倍,达到0.008 9 min−1和0.025 3 min−1。结合BET分析,N-TiO2/rGO拥有更大的比表面积,能够提供更多的活性吸附位点;同时,反应体系中·OH和·O2−等具有强氧化性的活性物质浓度增大,使界面反应的效率得到提高,从而促进光催化效率。
表 2 不同材料可见光催化降解Microcystin-LR的动力学拟合方程Table 2. Kinetic fitting equations of visible-light photocatalytic degradation of Microcystin-LR by different materials材料 准一级动力学方程 R2 k/min-1 TiO2 ln(C0/C)=0.003 2x+0.026 6 0.926 4 0.003 2 N-TiO2/rGO ln(C0/C)=0.008 9x−0.036 1 0.957 9 0.008 9 N-TiO2/rGO ln(C0/C)=0.025 3x−0.067 0 0.986 1 0.025 3 2) Microcystin-LR降解矿化率分析。由图6可见,当N-TiO2/rGO投加量为1 g·L−1、Microcystin-LR初始质量浓度为1 mg·L−1时,光催化90 min后Microcystin-LR的降解率为89.8%,矿化率为81.8%。这表明,在光催化降解过程中,N-TiO2/rGO能够将大部分Microcystin-LR分子矿化为CO2、H2O以及少量的小分子有机化合物。
3) N-TiO2/rGO光催化降解Microcystin-LR的机理分析。N-TiO2/rGO复合光催化材料价带上的e−被激发跃迁到导带生成光生电子,同时在价带生成对应的光生空穴(h+),通过与水中O2氧化反应生成超氧自由基(·O2−),并进一步反应生成强氧化性的羟基自由基(·OH)。因此,光催化过程对有机物起主要作用的活性物种为羟基自由基(·OH)、空穴(h+)和超氧自由基(·O2−)等。为探究N-TiO2/rGO对Microcystin-LR光催化降解过程各类活性物种的贡献程度,采用竞争动力学实验,以对苯二甲酸(PTA)和乙二胺四乙酸二钠(EDTA-2Na)分别为·OH、h+的清除剂[30],比较含有与不含清除剂反应体系中Microcystin-LR的降解效果以及·OH/h+与Microcystin-LR竞争反应的影响程度。PTA是·OH的高效清除剂,反应体系中加入过量PTA,将优先捕获体系中生成的·OH并形成几乎没有氧化能力的二羟基-PTA,阻断·OH对Microcystin-LR的氧化降解;反应体系中过量EDTA-2Na快速将 h+还原,抑制h+氧化Microcystin-LR的反应,由此可间接获得相应活性氧物种在反应体系中的贡献。如图7所示,PTA的加入使得Microcystin-LR的降解率由89.8%锐减至4.5%;而 EDTA-2Na对光降解的抑制效果不明显,反应中加入捕获h+的EDTA-2Na后,Microcystin-LR的降解率仍达86.3%。由此,根据清除剂加入前后Microcystin-LR的光降解率差异推算·OH和h+对Microcystin-LR可见光催化降解的贡献率分别为95.0%和3.9%。此外,反应中间过程产生的超氧自由基(·O2−)等因存在时间短,氧化性较·OH和h+明显弱,贡献率仅约1%。因此,在TiO2/rGO复合光催化Microcystin-LR过程中,·OH起了主导性的贡献作用。许文泽等[31]在采用二氧化钛光催化氧化阿散酸中也证实,·OH是发挥主要氧化作用的物种。
有研究报道[32],光激发诱导产生的·OH通过攻击Microcystin-LR分子Adda支链上共轭双键及肽环结构等降解复杂的大分子,并进一步发生裂环分解成多个小分子物质,最后矿化为H2O和CO2。其中Adda支链共轭双键、苯环和甲氧基优先被·OH攻击,C4-C5和C6-C7共轭双键断裂氧化分别生成醛和酮;C9位甲氧基被氧化成甲酸,在酸性环境中可以水解,将Adda侧链从肽链中分离出来[33-35],因此,通过·OH的氧化分解可实现Microcystin-LR的降解减毒。
3. 结论
1)采用水热法一步还原制备N-TiO2/rGO光催化复合材料,最优制备条件为:水热反应温度180℃、掺N量6%、GO负载量5%。相较TiO2、N-TiO2,复合材料N-TiO2/rGO颗粒粒径小,分散性、层次性、多孔性优,晶型仍以锐钛矿相为主,光生载流子分离效率高,可见光催化活性好。
2)投加1 g·L−1的N-TiO2/rGO复合光催化剂,可见光照射90 min后,对初始质量浓度为1 mg·L−1的Microcystin-LR降解率达 89.8%,反应符合准一级动力学方程,降解速率常数为0.025 3 min−1。
3) ·OH 是Microcystin-LR光催化降解的主要活性物种,其对降解的贡献率达95.0%,而h+的贡献率仅为3.9%;·OH通过对Microcystin-LR分子Adda侧链共轭双键、苯环和甲氧基的攻击,实现降解和减毒。
-
表 1 GO、TiO2、N-TiO2和N-TiO2/rGO的比表面积、孔容和孔径
Table 1. Specific surface area, pore volume and pore size of GO, TiO2, N-TiO2 and N-TiO2/rGO
样品 比表面积/(m2·g−1) 孔容/(cm3·g−1) 孔径/nm GO 6.125 0.019 12.421 TiO2 101.055 0.173 3.939 N-TiO2 200.127 0.427 6.874 N-TiO2/rGO 265.124 0.561 7.822 表 2 不同材料可见光催化降解Microcystin-LR的动力学拟合方程
Table 2. Kinetic fitting equations of visible-light photocatalytic degradation of Microcystin-LR by different materials
材料 准一级动力学方程 R2 k/min-1 TiO2 ln(C0/C)=0.003 2x+0.026 6 0.926 4 0.003 2 N-TiO2/rGO ln(C0/C)=0.008 9x−0.036 1 0.957 9 0.008 9 N-TiO2/rGO ln(C0/C)=0.025 3x−0.067 0 0.986 1 0.025 3 -
[1] JEONG B, OH M S, PARK H M, et al. Elimination of microcystin-LR and residual Mn species using permanganate and powdered activated carbon: Oxidation products and pathways[J]. Water Research, 2017, 114: 189-199. doi: 10.1016/j.watres.2017.02.043 [2] LIU Y L, WALKER H W, LENHART J J. Adsorption of microcystin-LR onto kaolinite, illite and montmorillonite[J]. Chemosphere, 2019, 220: 696-705. doi: 10.1016/j.chemosphere.2018.12.137 [3] KIM M S, LEE C, et al. Ozonation of microcystins: Kinetics and toxicity decrease[J]. Environmental Science & Technology, 2019, 53: 6427-6435. [4] CHANG J, CHEN Z, WANG Z, et al. Ozonation degradation of microcystin-LR in aqueous solution: Intermediates, byproducts and pathways[J]. Water Research, 2014, 63: 52-61. doi: 10.1016/j.watres.2014.06.007 [5] LI J M, LI R H, LI J. Current research scenario for microcystins biodegradation-A review on fundamental knowledge, application prospects and challenges[J]. Science of the Total Environment, 2017, 595: 615-632. doi: 10.1016/j.scitotenv.2017.03.285 [6] KUMAR P, HEGDE K, BRAR S K, et al. Biodegradation of microcystin-LR using acclimatized bacteria isolated from different units of the drinking water treatment plant[J]. Environmental Pollution, 2018, 242: 407-416. doi: 10.1016/j.envpol.2018.07.008 [7] CHEN D J, CHENG Y L, ZHOU N, et al. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review[J]. Journal of Cleaner Production, 2020, 268: 121725. doi: 10.1016/j.jclepro.2020.121725 [8] FUJISHIMA A, HONDA K. Photolysis-decomposition of water at the surface of an irradiated semiconductor[J]. Nature, 1972, 238(5385): 37-38. [9] WANG Y Y, CHEN Y X, BARAKAT T, et al. Recent advances in non-metal doped titania for solar-driven photocatalytic/photoelectrochemical water-splitting[J]. Journal of Energy Chemistry, 2022, 66: 529-559. doi: 10.1016/j.jechem.2021.08.038 [10] DONG H R, ZENG G M, TANG L, et al. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures[J]. Water Research, 2015, 79: 128-146. doi: 10.1016/j.watres.2015.04.038 [11] SHEYDAEI M, SHIADEH H R K, AYOUBI-FEIZ B, et al. Preparation of nano N-TiO2/graphene oxide/titan grid sheets for visible light assisted photocatalytic ozonation of cefixime[J]. Chemical Engineering Journal, 2018, 353: 138-146. doi: 10.1016/j.cej.2018.07.089 [12] LIN L, WANG H Y, XU P. Immobilized TiO2-reduced graphene oxide nanocomposites on optical fibers as high performance photocatalysts for degradation of pharmaceuticals[J]. Chemical Engineering Journal, 2017, 310(2): 389-398. [13] YAN Z D, YANG X N, LYNCH L, et al. Comparative evaluation of the mechanisms of toxicity of graphene oxide and graphene oxide quantum dots to blue-green algae microcystis aeruginosa in the aquatic environment[J]. Journal of Hazardous Materials, 2021, 27: 127898. [14] 陈俊伟, 李丽丽, 王菲凤, 等. N-TiO2/硅藻土负载型纳米材料可见光催化降解水中Microcystin-LR[J]. 福建师范大学学报(自然科学版), 2019, 35(6): 51-59. [15] PRASAD C, LIU Q Q, TANG H, et al. An overview of graphene oxide supported semiconductors based photocatalysts: Properties, synthesis and photocatalytic applications[J]. Journal of Molecular Liquids, 2020, 297: 111826. doi: 10.1016/j.molliq.2019.111826 [16] 任建, 李光照, 韩锐, 等. 溶胶-凝胶法原位制备还原氧化石墨烯/二氧化钛复合材料及光催化性能[J]. 功能材料, 2019, 50(7): 7185-7190. [17] SUN X Q, JI S D, WANG M Q, et al. Fabrication of porous TiO2-RGO hybrid aerogel for high-efficiency, visible-light photodegradation of dyes[J]. Journal of Alloys and Compounds, 2020, 819: 153033. doi: 10.1016/j.jallcom.2019.153033 [18] LUI G, LIAO J, DUAN A, et al. Graphene-wrapped hierarchical TiO2 nanoflower composites with enhanced photocatalytic performance[J]. Journal of Materials Chemistry A, 2013, 1(39): 12255-12262. doi: 10.1039/c3ta12329d [19] ZHOU Y J, Li J Z, LIU C Y, et al. Construction of 3D porous g-C3N4/AgBr/rGO composite for excellent visible light photocatalytic activity[J]. Applied Surface Science, 2018, 458: 586-596. doi: 10.1016/j.apsusc.2018.07.121 [20] WANG P, ZHAN S, XIA Y, et al. The fundamental role and mechanism of reduced graphene oxide in rGO/Pt-TiO2 nanocomposite for high-performance photocatalytic water splitting[J]. Applied Catalysis B:Environmental, 2017, 207: 335-346. doi: 10.1016/j.apcatb.2017.02.031 [21] 陈越, 何大伟, 王永生, 等. 水热法制备二氧化钛纳米管-石墨烯复合光催化剂及其光催化性能[J]. 发光学报, 2019, 40(2): 177-182. [22] ZHANG L, ZHANG Q H, XIE H Y, et al. Electrospun titania nanofibers segregated by graphene oxide for improved visible light photocatalysis[J]. Applied Catalysis B:Environmental, 2017, 201: 470-478. doi: 10.1016/j.apcatb.2016.08.056 [23] CALZA P, HADJICOSTAS C, SAKKAS V A, et al. Photocatalytic transformation of the antipsychotic drug risperidone in aqueous media on reduced graphene oxide-TiO2 composites[J]. Applied Catalysis B:Environmental, 2016, 183: 96-106. doi: 10.1016/j.apcatb.2015.10.010 [24] ZHOU X, ZHOU S Q, MA F Z, et al. Synergistic effects and kinetics of rGO-modified TiO2 nanocomposite on adsorption and photocatalytic degradation of humic acid[J]. Journal of Environmental Management, 2019, 235: 293-302. [25] 张宏忠, 秦小青, 王明花. 石墨烯/TiO2复合物的制备及其光催化性能[J]. 环境工程学报, 2016, 10(1): 169-174. doi: 10.12030/j.cjee.20160127 [26] WANG P, WANG J, WANG X F, et al. One-step synthesis of easy-recycling TiO2-rGO nanocomposite photocatalysts with enhanced photocatalytic activity[J]. Applied Catalysis B: Environmental, 2013, 132–133: 452-459. [27] LIU S, LIU C, WANG W, et al. Unique photocatalytic oxidation reactivity and selectivity of TiO2-graphene nanocomposites[J]. Nanoscale, 2012, 4(10): 3193-3200. doi: 10.1039/c2nr30427a [28] SUBODH, CHAUDHARY K, PRAKASH K, et al. TiO2 nanoparticles immobilized organo-reduced graphene oxide hybrid nanoreactor for catalytic applications[J]. Applied Surface Science, 2020, 509: 144902. doi: 10.1016/j.apsusc.2019.144902 [29] MOHSIN N, MOKREMA M, JIHO K, et al. Photodegradation of Microcystin-LR using graphene-TiO2/sodium alginate aerogels[J]. Carbohydrate Polymers, 2018, 199: 109-118. doi: 10.1016/j.carbpol.2018.07.007 [30] XU S B, JIANG L, YANG H G, et al. Structure and photocatalytic activity of polythiophene/TiO2 composite particles prepared by photoinduced polymerization[J]. Chinese Journal of Catalysis, 2011, 32(3): 536-545. [31] 许文泽, 杨春风, 李静, 等. 二氧化钛光催化氧化阿散酸[J]. 环境科学, 2016, 37(1): 193-197. [32] HE X H, WANG A Z, WU P, et al. Photocatalytic degradation of microcystin-LR by modified TiO2 photocatalysis: A review[J]. Science of the Total Environment, 2020, 743: 140694. doi: 10.1016/j.scitotenv.2020.140694 [33] 杨静, 陈登霞, 邓安平, 等. 掺氮二氧化钛可见光照射降解微囊藻毒素-LR[J]. 中国科学:化学, 2010, 40(11): 1688-1696. [34] HU X, HU X, TANG C, et al. Mechanisms underlying degradation pathways of microcystin-LR with doped TiO2 photocatalysis[J]. Chemical Engineering Journal, 2017, 330: 355-371. doi: 10.1016/j.cej.2017.07.161 [35] FOTIOU T, TRIANTIS T M, KALOUDIS T, et al. Photocatalytic degradation of Microcystin-LR and off-odor compounds in water under UV-A and solar light with a nanostructured photocatalyst based on reduced graphene oxide-TiO2 composite. Identification of intermediate products[J]. Industrial Engineering Chemistry Research, 2013, 52(39): 13991-14000. doi: 10.1021/ie400382r -




 下载:
下载: