-
我国市政污水处理厂的日处理规模已超2×108 m3,市政污水的能耗高达2.5 GW(按每m3污水处理能耗0.3 kWh计算)[1]。污水中蕴含的能量中,约50%以化学能(有机物COD)的形式转入剩余污泥,因此,回收利用污泥COD的能量对降低市政污水处理行业的能耗和碳排放至关重要[2-3]。厌氧消化(anaerobic digestion, AD)是实现污泥减量化、稳定化和能量回收的主流技术之一。目前,我国已建成并运行的市政污泥厌氧消化工程处理规模超9 000 t∙d−1(按80%含水率的脱水污泥计),但仅占我国需处理市政污泥总量的7%左右,与英国(75%)、美国(60%)、欧盟国家(30%~50%)的差距明显。这表明,污泥厌氧消化亟需高效的过程强化(process intensification, PI)技术以提高甲烷产率[4]。
电产甲烷是一种基于微生物电解池(microbial electrolysis cell, MEC)促进有机物降解和产甲烷的PI技术[5]。在较低的外加电压条件下,阳极电活性微生物(electroactive bacteria, EAB)氧化分解有机物产生电子、质子和二氧化碳(CO2),同时,阴极微生物可通过直接或间接电子传递的途径。MEC-AD可加速污泥有机物水解、显著提高甲烷产率,并实现沼气生物品位升级,从而与传统AD工艺相比有较大的技术优势[6-8]。在MEC-AD中,阳极EAB与阴极产甲烷古菌通常以氢气为电子载体或依靠直接胞外电子传递机制进行种间电子传递[9]。此外,EAB与产甲烷古菌亦可利用具有导电性或氧化还原活性的碳材料,以提高产甲烷过程的种间电子传递效率[10-12]。这些非溶解性的粒状材料易与微生物形成活性颗粒,能进行厘米级别的远距离电子传递,远超依靠扩散机制的可溶性电子载体/穿梭体(例如甲酸、核黄素、绿脓素等,电子传递距离1~100 μm)[13-16]。
有研究表明,基于生物质中低温(300~500 ℃)热解过程形成的生物炭可通过富含醌基的表面官能团进行电子交换或内部类石墨结构的导电碳层进行直接电子传递[17-18]。YIN等[19]发现,通过在MEC-AD体系中投加适量的污泥基生物炭,污泥挥发性固体(VS)去除率提高了17.9%,甲烷产量提高了24.7%。然而,在更接近实际应用的连续式MEC-AD体系中,投加污泥炭是否具有长期促进效果,以及如何影响体系内微生物群落结构和产甲烷代谢途径,仍有待进一步探索。
基于上述原因,本研究拟构建并在连续进料运行模式下运行污泥MEC-AD系统,以污泥热解制备的生物炭为碳材料,研究投加污泥炭对污泥MEC-AD产甲烷的改善情况。在确定了最佳外加电压之后,考察污泥炭在不同有机负荷条件下对甲烷产率和体系稳定性的提高程度。最后,利用宏基因组手段分析体系内微生物种群结构与特定代谢功能,以揭示污泥炭提高MEC-AD甲烷产率的微生物学机制,为污泥炭强化MEC-AD技术应用于污泥厌氧消化处理工艺提供参考。
-
MEC-AD装置进料底物为城镇污水处理厂剩余污泥,取自上海市闵行区水质净化厂二沉池。污泥取回实验室后经1 mm格筛过滤、高速离心(4 000 r∙min−1, 2 min)处理后置于4 ℃保存备用。MEC-AD接种污泥取自实验室长期运行的污泥高温厌氧消化罐。剩余污泥与接种污泥的主要理化性质见表1。
污泥炭由上述剩余污泥热解制备而成。污泥于105 ℃烘干48 h,球磨至平均粒径50 μm,然后将污泥颗粒干粉置于管式炉中,在无氧条件下热解1.5 h,热解温度为500 ℃,热解期间始终保持氮气循环,以保证无氧环境。待冷却至室温后,污泥炭在去离子水中浸泡72 h,离心过滤后去除上清液以去除有毒有害物质,待105 ℃烘干24 h后置于干燥皿内备用。污泥炭的主要理化性质为,pH7.61、BET比表面积41.8 m2∙g−1、总灰分质量分数66.5%。灰分的主要元素质量分数如表2所示,H/C摩尔比与O/C摩尔比分别为0.07与0.11,电子供给能力(EDC)与电子接收能力(EAC)分别达到0.116与0.754 meq∙g−1。
-
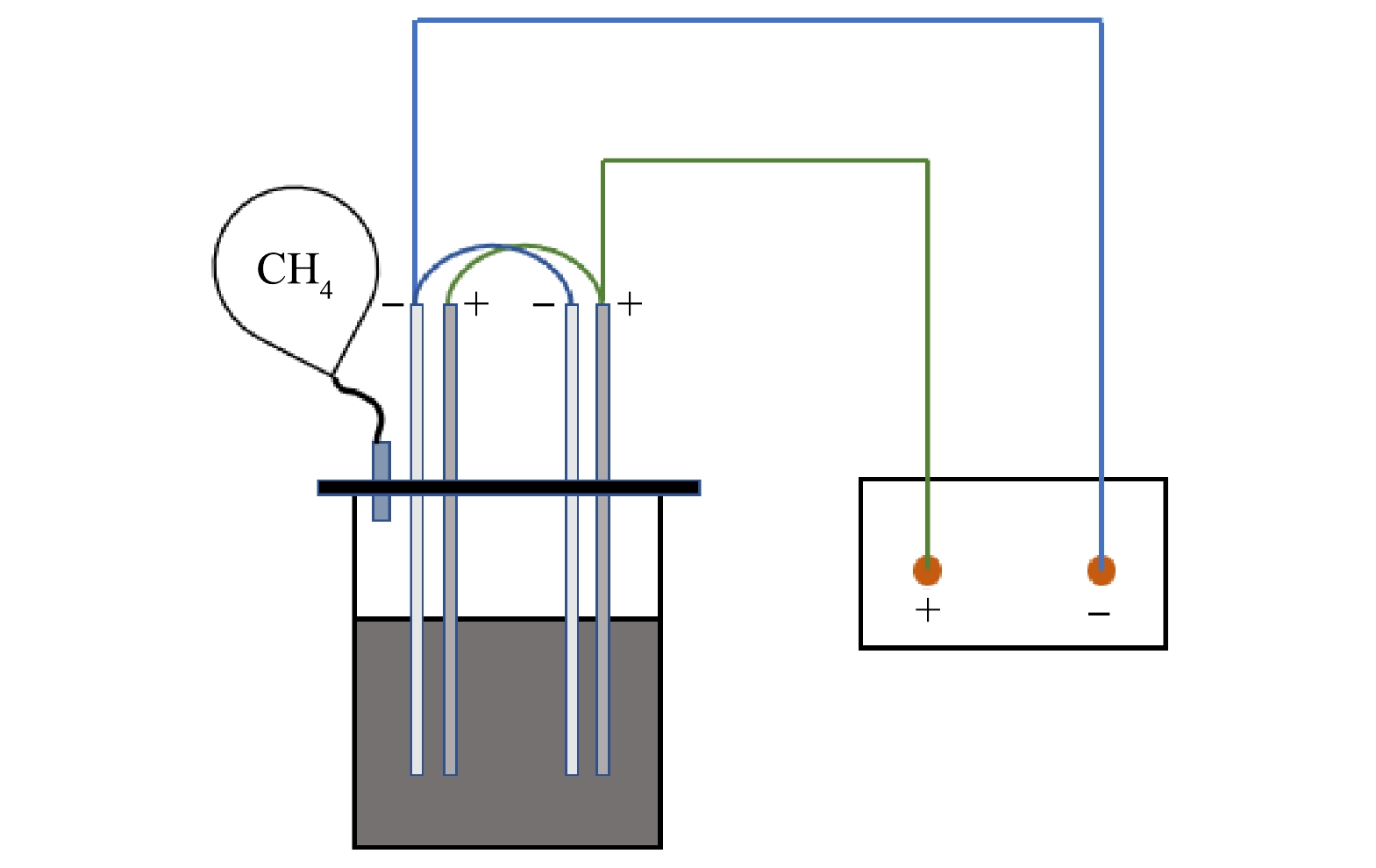
MEC-AD反应器为单室结构(图1),工作体积为1.0 L,装置采用一对碳毡双电极作为阳极和阴极,电极之间使用铜线连接,电极尺寸为12 cm× 8 cm,电极板间距为1.5 cm,供电采用直流稳流稳压电源(远方WY3101,杭州)。反应器使用循环水域控温,采用磁力转子搅拌以获得良好的传质与传热。反应器顶部设有1个气体出口和2个液体进出料口。
-
MEC-AD反应器采用连续式运行模式,每24 h进行进出料1次,实验期间温度和搅拌速率分别为(55 ± 1)℃和100 r∙min−1。反应器启动阶段,接种污泥与底物污泥按总固体含量(TS)1∶4的比例加入反应器,初始TS为2.0%。在第一阶段实验中,设有3组反应器,水力停留时间(HRT)为10 d,有机负荷率(OLR)为2.5 g∙(L∙d)−1,污泥炭的初始投加量为18 g∙L−1(剂量0.9 g∙g−1)。至沼气产率趋于稳定后,污泥炭每24 h随进料补充,投加量与初始剂量保持一致。实验组MEBC0.5和MEBC1.5的外加电压分别为0.5和1.5 V,另设一组无外加电压的对照组BC。在第2阶段实验中,设有2组反应器,分别是未加污泥炭的对照组ME和投加污泥炭的实验组MEBC,它们的外加电压采用第一阶段实验中的最佳电压。第2阶段实验条件设置如表3所示。运行周期Ⅰ~Ⅲ的HRT分别为10、5和2.5 d,进料为剩余污泥。运行周期Ⅳ的HRT恢复至5 d;为考察污泥炭对MEC-AD体系稳定性的影响,进料为剩余污泥+甘油混合底物(1∶1基于COD)。
-
总固体含量(TS)、挥发性固体含量(VS)和化学需氧量(总COD、SCOD)参照《城镇污水处理厂污泥泥质 GB 24188-2009》[21]和《水质化学需氧量测定 HJ 828-2017》[22]。
挥发性脂肪酸使用气相色谱测定(GC-2010,岛津,日本)。1 mL沼液经0.45 μm膜过滤后与100 μL的3%磷酸溶液混合,确保pH<4.0。测试条件为:色谱柱DB-FFAP,FID火焰离子化检测器,载气N2流速为30 mL∙min−1,进样口与检测器温度分别为200与250 ℃。
沼气体积采用排水法测定。保持水溶液pH不超过4.3以防止CO2吸收,沼气体积数据经标准化(273 K,1.01×105 Pa)后记录。
沼气成分(CH4、CO2、H2)采用气相色谱法(GC-7890,安捷伦,美国)测定。测试条件为:色谱柱G3591,TCD热导检测器柱箱温度80 ℃,载气He流速为40 mL∙min−1,进样口与检测器温度分别为200与250 ℃。
微生物多样性和宏基因组学分析。待第二阶段实验周期Ⅲ完成后(70 d),从阴极生物膜上取1 mL污泥样品进行DNA抽提。对DNA剪切后筛选长度为300 bp的片段进行建库。利用PCR扩增进行文库模板的富集,通过NaOH碱变性产生单链 DNA片段。随后进入Illumina Hiseq测序,对测序产生的短读序列(reads)进行质量剪切后优化序列进行拼装,筛选> 300 bp的重叠群(contig)作为最后的拼装结果[20],并将核酸长度>100 bp的基因翻译为氨基酸序列。最后,参考NCBI_NR数据库和KEGG数据库对比该非冗余基因集序列,分别进行分类和功能注释[23]。
-
外加电压对MEC-AD反应器运行效果的影响如图2所示。在反应器运行初期,3组反应器中的SCOD累积浓度均在2 d达到峰值(3 469 mg∙L−1, BC; 3 858 mg∙L−1, MEBC0.5; 4 066 mg∙L−1, MEBC1.5),之后随着产酸产甲烷过程的进行在10 d后趋于稳定,平均浓度由低到高分别为MEBC1.5 < MEBC0.5 < BC。这表明高电压可在促进污泥水解的同时提高可溶性有机物的降解。MEC-AD阳极反应产生的微量氧气(4H2O → 8H++8e−+2CO2)有利于兼养细菌的增殖从而加速有机物的降解转化[24]。此外,阴极富集的嗜氢产甲烷古菌消耗有机物水解酸化过程中产生的分子氢(H2),也可促进VFAs的乙酸化过程。MEBC0.5与MEBC1.5的电流密度分别为(1.16±0.29)与(3.83±0.99) A∙m−2。MEBC1.5的消化液pH值略高于MEBC0.5和BC,其原因可能是,高电压促进了电产甲烷的质子消耗(CO2+8H++8e− → CH4+H2O)。反应器运行期间,MEBC0.5与MEBC1.5的氢分压显著高于对照组BC,这表明MEC阴极反应的电子被用于还原质子生成H2,但MEBC实验组的高氢分压并未导致酸累积或降低甲烷产率。在反应器稳定运行时期(31~40 d),甲烷产率由高到底分别为MEBC1.5 > MEBC0.5 > BC。BC、MEBC0.5与MEBC1.5的沼气甲烷体积分数分别达到65.6%、72.3%与73.9%,外加电压0.5 V和1.5 V将沼气甲烷体积分数提高了10.2% (p<0.05)和12.6% (p<0.01)。这表明MEC生物阴极微生物群可通过氢还原CO2进行沼气原位品位升级,提高甲烷产量[25]。综上所述,第一阶段的实验结果表明,外加电压1.5 V的污泥炭耦合MEC-AD运行效果最佳。
-
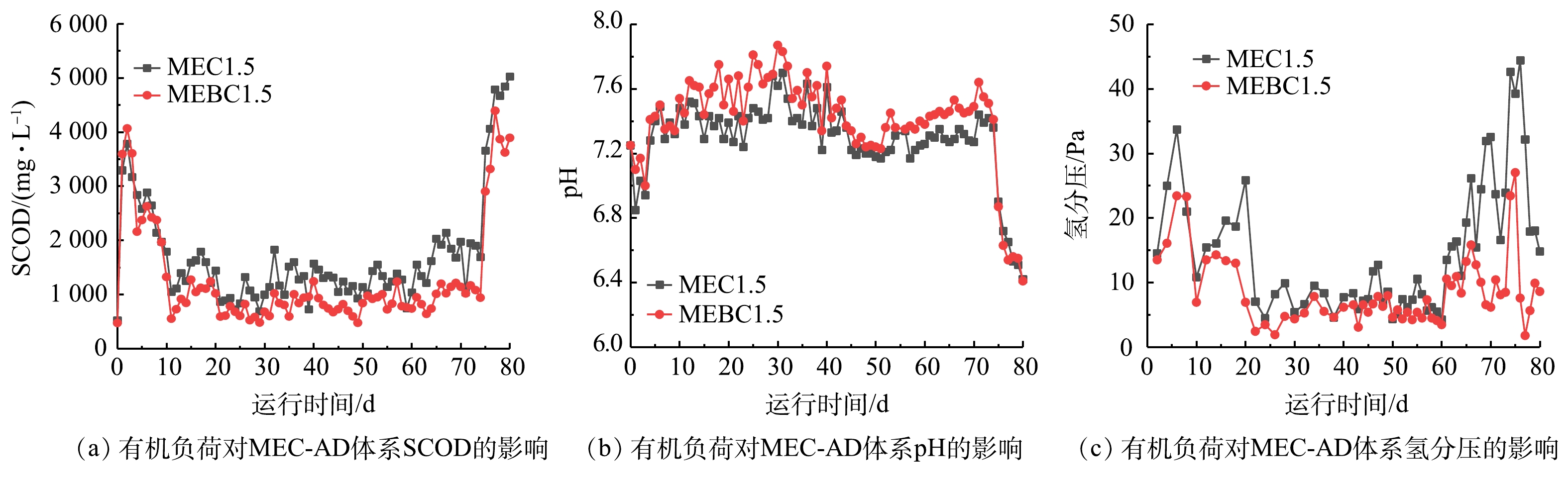
第2阶段实验考察了外加电压保持1.5 V时,不同有机负荷率条件下污泥炭对MEC-AD运行效果的影响,结果如图3所示。随着HRT的缩短以及OLR的增加,体系甲烷产率呈现先升高后降低的趋势。当HRT从10 d缩短至5 d,ME1.5与MEBC1.5的甲烷产率和相应的沼气甲烷体积分数分别有显著提升,这表明投加污泥炭可同时提高MEC-AD体系的甲烷产量并实现原位沼气品位提升。HRT进一步缩短至2.5 d后,ME1.5与MEBC1.5的甲烷产率与周期Ⅱ相比,分别下降了16.7%与22.3%。同时,相应的沼气甲烷体积分数也降低至73.1%±3.7%与76.5%±4.3%。VFAs质量浓度是反映AD系统稳定度的重要参数,ME1.5与MEBC1.5的总VFAs质量浓度在周期Ⅲ期间上升至(352±107)与(170±46) mg∙L−1,其主要组分为正丁酸、丙酸与乙酸。HO等[26]指出,污泥高温AD在HRT由3 d缩短至2 d出现的酸累积和甲烷产量下降主要由产甲烷古菌的洗脱所导致。在本研究中,虽然在HRT=2.5 d时观察到了酸累积现象,但其程度还未引起系统崩溃。由此可见,MEC-AD的碳毡电极表面滞留微生物的能力可有效缓解在短HRT高负荷运行中产甲烷古菌流失的问题[27]。MEC-AD体系内部的电流密度随着OLR的增加而升高,且在整个污泥消化实验周期,MEBC1.5的电流密度均显著高于ME1.5。电流密度的升高可能与多个因素相关,包括阳极氧化反应的有机物降解、EAB菌群的产电活性以及阴极电产甲烷[28]。因此,投加污泥炭可能促进以上的单个或多个代谢过程。此外,在周期Ⅰ~Ⅲ中,污泥炭并未明显改善沼气甲烷纯度(图3(a)),但显著提高了周期Ⅰ与周期Ⅱ的甲烷产率。由于有机物在水解、产酸、产甲烷阶段均可能释放CO2,这表明污泥炭可能同步强化了有机物厌氧降解和乙酸裂解产甲烷过程(CH3COOH → CH4+CO2),而并非选择性强化氢还原CO2产甲烷(CO2+H2 → CH4+H2O)。当HRT缩短至2.5 d时,ME1.5与MEBC1.5两组的甲烷产率差异不显著。这可能与产甲烷古菌的大量流失有关,尤其是乙酸营养型产甲烷古菌(例如甲烷八叠球菌属Methanosarcina)。
SCOD反映了污泥颗粒性大分子有机物的水解发酵与产甲烷的平衡情况(图4(a))。在反应器启动10 d后,SCOD的释放与消耗保持平衡并趋于稳定。在周期Ⅰ~III的稳定期,MEBC1.5的SCOD始终低于ME1.5。这表明污泥炭可促进SCOD的转化利用,亦与甲烷产率数据的总体趋势保持一致。MEC-AD系统消化液的pH基本保持在弱碱性范围(7.4~7.8,图4(b)),由于剩余污泥具有较高的碱度和较好的缓冲性能,周期Ⅲ的少量酸累积并未引起显著的pH下降。ME1.5和MEBC1.5的稳定期氢分压分别为(9.0±3.9) Pa与(4.9±1.8) Pa、(6.8±1.8) Pa与(4.9±1.1) Pa以及(25.3±6.9) Pa与(10.9±3.9) Pa(图4(c)),在高OLR运行的II~III周期,MEBC1.5的平均氢分压比ME1.5分别降低了26.0% (p<0.01)与56.9% (p<0.001)。这表明投加污泥炭可显著促进污泥MEC-AD的氢营养型产甲烷过程,从而强化产氢产乙酸菌-嗜氢产甲烷古菌的互营共生代谢机制[19]。
周期Ⅳ为污泥/甘油共消化的实验周期。相较于污泥消化的周期Ⅱ,虽然ME1.5与MEBC1.5的甲烷产率分别有小幅提高,但沼气甲烷体积分数却有所降低。这主要是因为:甘油无需经过溶胞-水解等限速步骤即可进入发酵产酸阶段,期间产生大量CO2,但伴随而来的酸累积效应可能导致体系不稳定甚至崩溃。基于ME1.5和MEBC1.5的SCOD、VFAs、pH等多个参数的变化情况来看,甘油的快速代谢转化已引起了体系不稳定,而投加污泥炭可显著缓解酸累积问题从而维持MEC-AD的运行效果。由厌氧发酵细菌代谢转化甘油而快速累积的VFAs为互养酸氧化细菌-嗜氢产甲烷古菌等功能菌群提供了丰度的代谢底物,此时MEBC1.5的甲烷产率显著高于ME1.5(图3),从两实验组在VFA浓度与氢分压的显著差异来看(图4),污泥炭的引入提高了VFAs的转化利用率,并大幅降低了体系氢分压,从而提高了甲烷产量。
-
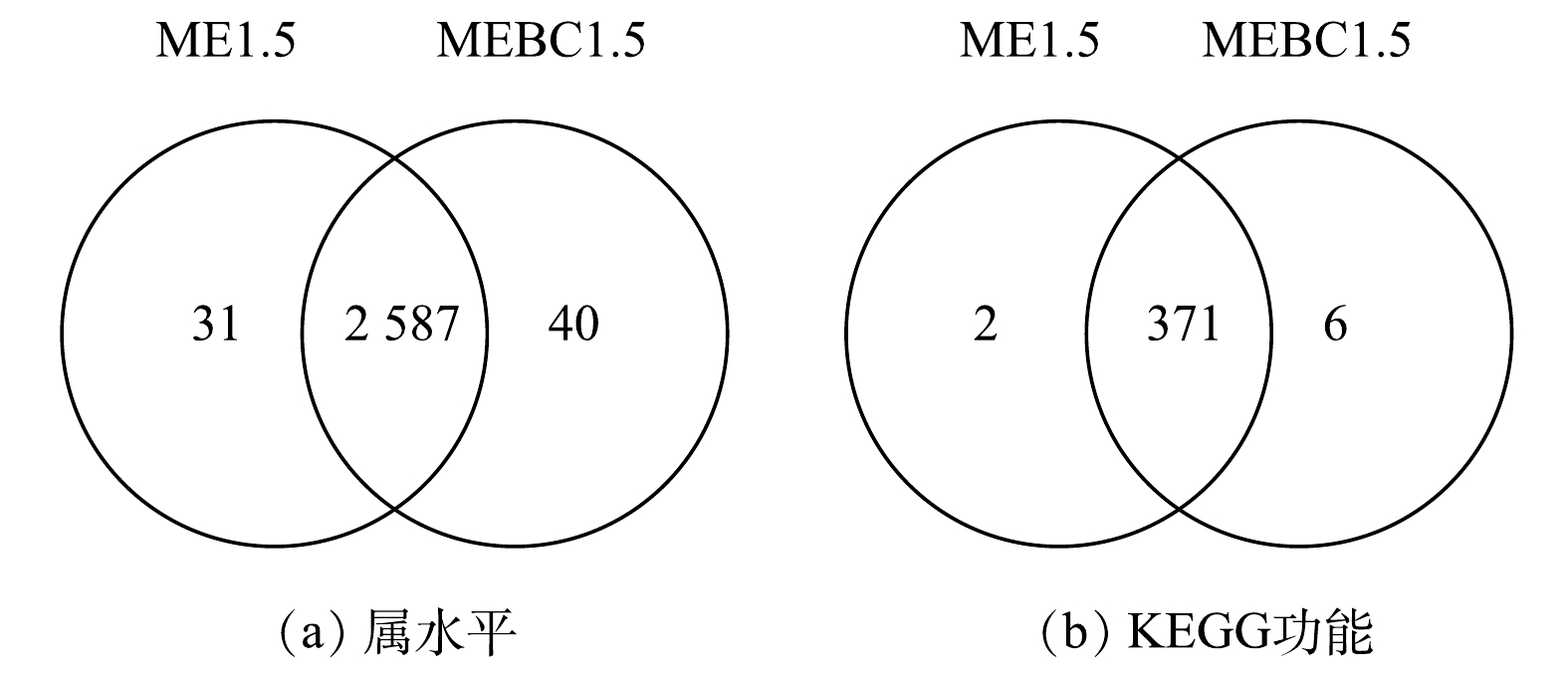
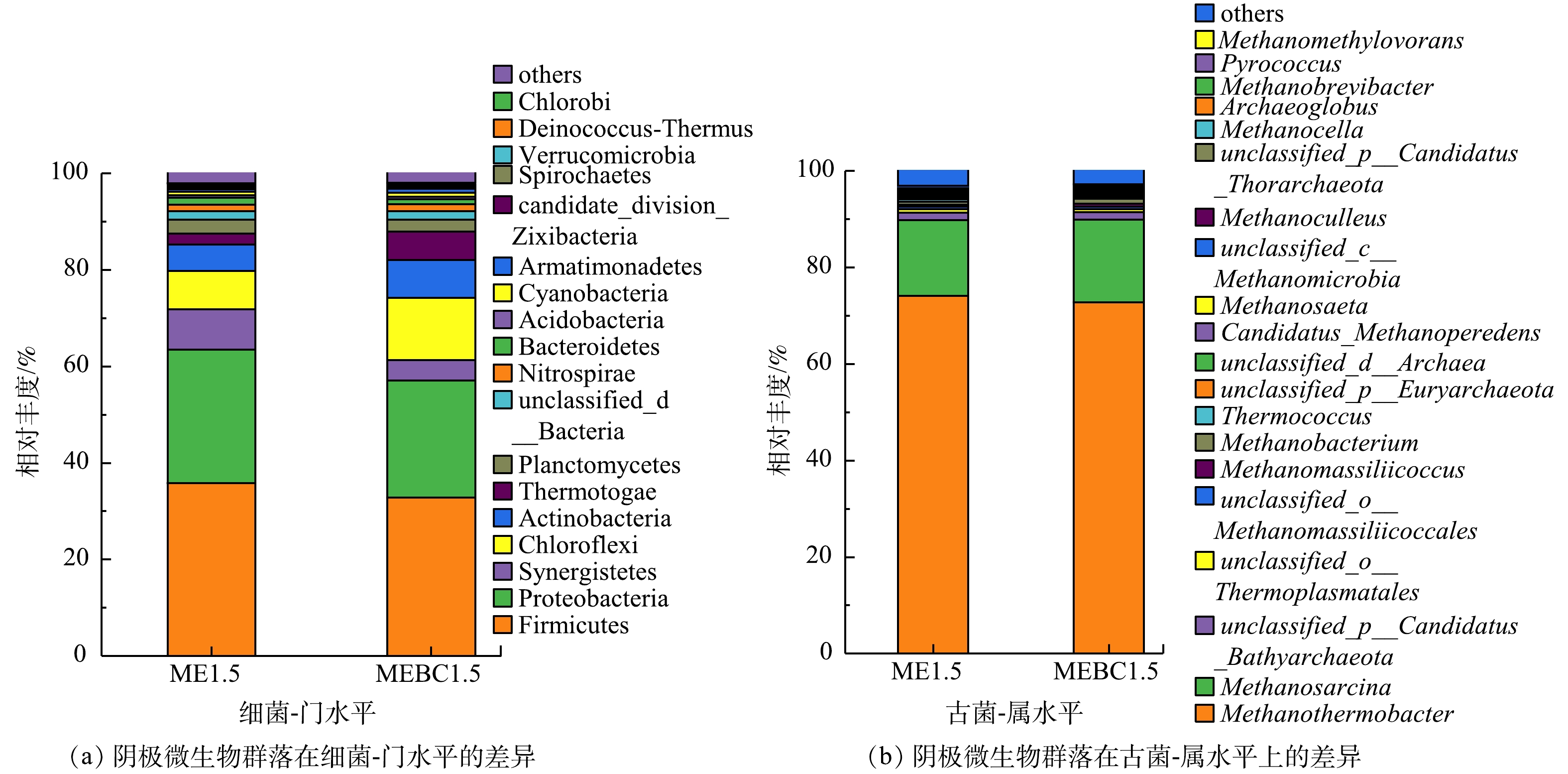
生物阴极微生物在基因属水平和KEGG功能注释水平上的Venn图显示,ME1.5与MEBC1.5阴极微生物在基因水平上和KEGG功能注释水平上分别共有2 587个属和371个代谢通路,共有率分别为97.3%和97.8%。这表明污泥炭未显著改变MEC-AD阴极微生物群落的组成和代谢功能(图5)。ME1.5与MEBC1.5生物阴极细菌组成如图6所示,优势门包括Firmicutes、Proteobacteria、Chloroflexi、Synergistetes、Actinobacteria和Thermotogae。在生物阴极细菌属水平上,投加污泥炭使得优势菌属Coprothermobacter、Fervidobacterium、Bellilinea的相对丰度分别显著增加34.1%、186.6%、130.5%,然而Anaerobaculum的丰度则下降了186.6%。Coprothermobacter是有机质高温AD系统富集程度较高的菌属,主要参与蛋白质组分的发酵和产乙酸代谢过程[29],而Bellilinear则是隶属于Chloroflexi门的丙酸互营降解产甲烷菌属[30]。甲烷热杆菌Methanothermobacter和甲烷八叠球菌Methanosarcina是生物阴极的优势古菌属。甲烷热杆菌为嗜氢产甲烷菌,极易成为生物阴极的优势产甲烷古菌以利用阴极产生的H2还原CO2生成甲烷[6]。此外,投加污泥炭提高了代谢底物最为广泛的甲烷八叠球菌的相对丰度,这可能有利于乙酸裂解途径。
-
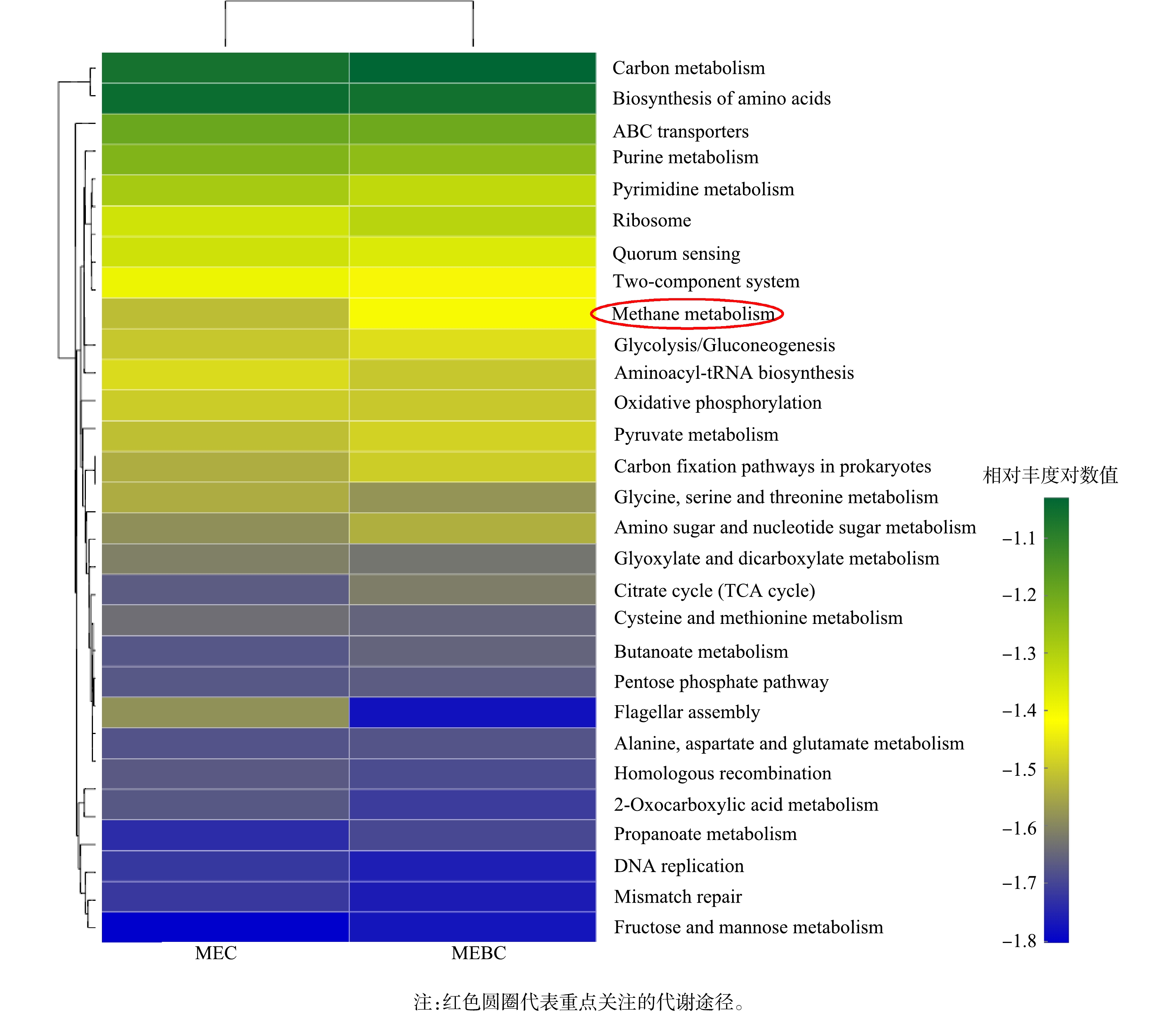
在两组生物阴极微生物KEGG代谢通路一级分类中,编码“Carbon Metabolism”功能的基因丰度最高,分别为67.2%(ME1.5)和68.3%(MEBC1.5),其次为“Genetic Information Processing”、“Environmental Information Processing”和“Cellular Processes”。 “Carbon Metabolism”类别主要包括“Carbohydrate metabolism”、“Amino acid metabolism”、“Energy metabolism”和“Nucleotide metabolism”,在ME1.5与MEBC1.5中分别为14.6%、10.3%、7.2%、6.4%与15.3%、10.1%、8.3%、5.9%。在本研究中,消化对象为污泥,其主要成分为蛋白质和多糖,因此,其碳水化合物代谢和氨基酸代谢功能基因所占比例较大。在KEGG代谢通路三级分类中,基因丰度差异如图7所示。生物阴极主要参与Carbon metabolism、Biosynthesis of amino acids、Purine metabolism、Pyrimidine metabolism、Methane metabolism、ABC transporters等过程。相较于对照组ME1.5,投加污泥炭促使MEC-AD阴极微生物实现以上代谢过程的相关基因丰度变化的幅度为+3.1%、−0.5%、+8.0%、+6.8%、+43.1%、−4.5%。以上结果表明,投加污泥炭对于生物阴极产甲烷代谢过程的影响最大(+43.1%)。
在产甲烷过程中,MEC-AD生物阴极微生物表达的酶包括EC 6.2.1.1(乙酸-辅酶A连接酶)、EC 2.8.4.1(辅酶B-磺乙基巯基转移酶)、EC 1.2.99.5(甲酰甲烷呋喃脱氢酶)、EC 2.1.1.86(四氢甲烷蝶呤 S-甲基转移酶)、EC 1.12.98.1(辅酶 F420)、EC 1.8.98.1(甲基吩嗪:CoB-CoM异二硫化物还原酶)。投加污泥炭对MEC-AD产甲烷过程关键酶的相关基因丰度影响如表4所示。ME1.5生物阴极的产甲烷过程主要通过H2还原CO2代谢途径(hydrogenotrophic methanogenesis, KEGG module M00567),投加污泥炭同时强化了H2还原CO2途径和乙酸裂解途径的产甲烷过程(acetoclastic methanogenesis, KEGG module M00357)。这与前文2.3节所述的可兼顾氢营养型和乙酸营养型的Methanosarcina菌属在MEBC1.5中的富集结果一致。污泥炭的投加可促进生物阳极的有机物氧化降解过程,从而提高MEC-AD体系内部电流密度,并通过富集嗜氢产甲烷菌强化了生物阴极电产甲烷过程。鉴于污泥炭本身的弱导电性,它们可能主要通过表面具有氧化还原能力的官能团反复供给、接受电子的机制来强化间接电产甲烷[4,31]。
-
1)在污泥炭耦合MEC-AD污泥产甲烷体系中,相较于无外加电压和低电压 (0.5 V),高电压 (1.5 V)可促进污泥SCOD释放与降解速率,提高甲烷产率。
2)在不同有机负荷运行条件下,在HRT为10 d与5 d的运行周期,投加污泥炭的实验组MEBC1.5的污泥厌氧消化甲烷产率均显著高于对照组ME1.5,但当HRT缩短至2.5 d,MEBC1.5与ME1.5的甲烷产率并无显著差异;在污泥-甘油共消化体系中,通过强化产氢产乙酸菌-嗜氢产甲烷古菌的互营共生代谢,污泥炭可有效缓解酸累积抑制,从而可提高甲烷产率。
3)宏基因组分析结果表明,投加污泥炭提高了优势菌属Coprothermobacter、Fervidobacterium、Bellilinea以及代谢底物广泛的优势产甲烷古菌Methnosarcina的相对丰度;在产甲烷代谢通路方面,投加污泥炭使得表达乙酸裂解途径和H2还原CO2途径的关键酶的相关基因丰度获得明显增加。
污泥炭强化微生物电解池提高污泥厌氧消化甲烷产率与系统稳定性
Sludge-derived biochar enhanced microbial electrolysis cell for improving biomethane productivity and system stability in anaerobic digestion of waste activated sludge
-
摘要: 投加污泥炭可有效改善利用微生物电解池厌氧消化(MEC-AD)甲烷产率偏低的问题,但其对MEC-AD体系的长期影响仍未知。研究了不同运行条件(外加电压、有机负荷率OLR)下,污泥炭对MEC-AD在连续进料运行模式下产甲烷效能的提高。首先,基于甲烷产率、电流密度等指标,确定最佳外加电压为1.5 V;然后,在相同外加电压(1.5 V)、不同OLR的条件下,比较了对照组(ME1.5)和投加污泥炭的实验组(MEBC1.5)的产甲烷和系统稳定性,OLR通过水力停留时间(HRT)控制。结果表明,当HRT从10 d逐步缩短至2.5 d,ME1.5与MEBC1.5的甲烷产率呈现先上升后下降的变化趋势,且MEBC1.5的甲烷产率始终高于ME1.5。这表明污泥炭在不同OLR运行条件下均可改善甲烷产率。微生物群落多样性分析结果表明,对比ME1.5与MEBC1.5,投加污泥炭使得生物阴极优势菌属Coprothermobacter、Fervidobacterium、Bellilinea、Methanosarcina的相对丰度分别增加了34.1%、186.6%、130.5%、9.5%。KEGG通路分析结果表明,MEBC1.5中乙酸裂解途径和H2还原CO2途径产甲烷代谢过程相关基因的丰度均有所提高。本研究结果可为污泥炭强化市政污泥MEC-AD产甲烷效能和相关的微生物机制提供参考。Abstract: Adding sludge-derived biochar has been demonstrated to improve the methanogenic performance of microbial electrolysis cell for anaerobic digestion (MEC-AD) of waste activated sludge (WAS) via short-term batch experiments. However, its long-term effect is still unclear. This paper investigated how sludge-derived biochar improved the performance of MEC-AD under continuous operation for producing methane from WAS with different operating conditions. First, optimal external voltage was determined to be 1.5 V based on the methane production rates and current densities. Next, to investigate the effect of biochar on methanogenic performance of MEC-AD, the system performance was compared between ME1.5 (control) and MEBC1.5 (with biochar addition) in terms of methane productivity and process stability. The reactors were operated under different organic loading rates (OLRs), which were maintained by adjusting the hydraulic retention time (HRT). With the HRT gradually decreased from 10 d to 2.5 d, the methane production rates in ME1.5 and MEBC1.5 showed a trend of first increasing and then decreasing, and the methane production rates in MEBC1.5 was always higher than ME1.5. This indicated that biochar could enhanced the methanogenic performance under high-rate operation. The relative abundances of dominant genera on biocathode, Coprothermobacter, Fervidobacterium, Bellilinea, and Methanosarcina were increased by 34.1%, 186.6%, 130.5% and 9.5%, respectively. The KEGG pathway analysis showed that the abundances of genes associated with both acetoclastic and hydrogenotrophic methanogenesis pathways both increased. This study demonstrated the stimulatory effect of sludge-derived biochar in MEC-AD for biomethane production from WAS and also elucidated the related microbial metabolism.
-
表 1 剩余污泥和接种污泥的主要理化性质
Table 1. Main characteristics of the waste activated sludge and inoculum
污泥种类 pH TS/(g∙L−1) VS/(g∙L−1) COD/(mg∙L−1) SCOD/(mg∙L−1) 剩余污泥 6.97 ± 0.03 30.2 ± 0.5 23.3 ± 0.3 29 800 ± 600 350 ± 60 接种污泥 7.61 ± 0.13 40.3 ± 0.7 33.2 ± 0.4 35 800 ± 2410 2 540 ± 210 表 2 污泥基生物炭灰分的主要元素质量分数
Table 2. Contents of main elements in sludge-derived biochar ash
% S Si Fe Al K Na Ca Mg Zn Ni Co 0.28 16.42 8.63 2.41 2.33 4.75 1.35 0.11 0.19 0.01 0.01 表 3 第二阶段各实验周期的运行参数
Table 3. Operation conditions for phase 2 experiment
运行周期 实验时间 进料底物 HRT/d OLR/(g∙(L∙d)−1) I 第1~40天 剩余污泥 10.0 2.5 II 第41~60天 剩余污泥 5.0 5.0 III 第61~70天 剩余污泥 2.5 10.0 IV 第71~80天 剩余污泥+甘油 5.0 8.0 表 4 ME1.5与MEBC1.5生物阴极微生物产甲烷过程关键酶的丰度差异
Table 4. Difference in abundance of key enzymes involving methanogenesis between ME1.5 and MEBC1.5
酶编号 酶名称 ME1.5 MEBC1.5 1.12.98.1 coenzyme F420 hydrogenase 3812 11526 1.12.98.2 5,10-methenyltetrahydromethanopterin hydrogenase 22 76 1.2.99.5 formylmethanofuran dehydrogenase 15952 17506 1.5.98.1 methylenetetrahydromethanopterin dehydrogenase 614 2068 1.5.98.2 5,10-methylenetetrahydromethanopterin reductase 678 2084 1.8.98.1 CoB-CoM heterodisulfide reductase 2850 8990 2.1.1.86 tetrahydromethanopterin S-methyltransferase 2846 11724 2.1.1.246 [methyl-Co(III) methanol-specific corrinoid protein]:coenzyme M methyltransferase 184 774 2.1.1.247 [methyl-Co(III) methylamine-specific corrinoid protein]:coenzyme M methyltransferase 54 262 2.3.1.169 acetyl-CoA decarbonylase/synthase, complex subunit beta 686 474 2.3.1.101 formylmethanofuran-tetrahydromethanopterin N-formyltransferase 4394 4274 2.3.1.8 phosphate acetyltransferase 302 444 2.7.2.1 acetate kinase 5452 2758 2.8.4.1 coenzyme-B sulfoethylthiotransferase 3766 13934 3.5.4.27 methenyltetrahydromethanopterin cyclohydrolase 812 2244 6.2.1.1 acetate-CoA ligase 2702 9338 -
[1] QU J, WANG H, WANG K, et al. Municipal wastewater treatment in China: Development history and future perspectives[J]. Frontiers of Environmental Science & Engineering, 2019, 13(6): 88. [2] MCCARTY P L, BAE J, KIM J. Domestic wastewater treatment as a net energy producer: Can this be achieved?[J]. Environmental Science & Technology, 2011, 45(17): 7100-7106. [3] ALLOUL A, GANIGUE R, SPILLER M, et al. Capture-ferment-upgrade: A three-step approach for the valorization of sewage organics as commodities[J]. Environmental Science & Technology, 2018, 52(12): 6729-6742. [4] 于亚梅, 沈雁文, 朱南文, 等. 生物炭和石墨的电化学性质对剩余污泥厌氧消化产甲烷的影响[J]. 环境工程学报, 2020, 14(3): 807-820. doi: 10.12030/j.cjee.201908046 [5] CHENG S, CALL D F, LOGAN B E, et al. Direct biological conversion of electrical current into methane by electromethanogenesis[J]. Environmental Science & Technology, 2009, 43: 3953-3958. [6] LIU W, CAI, GUO Z, WANG L, et al. Microbial electrolysis contribution to anaerobic digestion of waste activated sludge, leading to accelerated methane production[J]. Renewable Energy, 2016, 91: 334-339. doi: 10.1016/j.renene.2016.01.082 [7] ZHAO Z, ZHANG Y, QUAN X, et al. Evaluation on direct interspecies electron transfer in anaerobic sludge digestion of microbial electrolysis cell[J]. Bioresource Technology, 2016, 200: 235-244. doi: 10.1016/j.biortech.2015.10.021 [8] WANG X T, ZHAO L, CHEN C, et al. Microbial electrolysis cells (MEC) accelerated methane production from the enhanced hydrolysis and acidogenesis of raw waste activated sludge[J]. Chemical Engineering Journal, 2021, 413: 127472. doi: 10.1016/j.cej.2020.127472 [9] FU Q, KURAMOCHI Y, FUKUSHIMA N, et al. Bioelectrochemical analyses of the development of a thermophilic biocathode catalyzing electromethanogenesis[J]. Environmental Science & Technology, 2015, 49(2): 1225-1232. [10] REN G, CHEN P, YU J, et al. Recyclable magnetite-enhanced electromethanogenesis for biomethane production from wastewater[J]. Water Research, 2019, 166: 115095. doi: 10.1016/j.watres.2019.115095 [11] BAEK G, KIM J, KIM J, et al. Individual and combined effects of magnetite addition and external voltage application on anaerobic digestion of dairy wastewater[J]. Bioresource Technology, 2020, 297: 122443. doi: 10.1016/j.biortech.2019.122443 [12] QIN X, LU X, CAI T, et al. Magnetite-enhanced bioelectrochemical stimulation for biodegradation and biomethane production of waste activated sludge[J]. Science of the Total Environment, 2021, 789: 147859. doi: 10.1016/j.scitotenv.2021.147859 [13] STORCK T, VIRDIS B, BATSTONE D J. Modelling extracellular limitations for mediated versus direct interspecies electron transfer[J]. Multidisciplinary Journal of Microbial Ecology, 2016, 10(3): 621-31. [14] GLASSER N R, SAUNDERS S H, NEWMAN D K, et al. The colorful world of extracellular electron shuttles[J]. Annual Review of Microbiology, 2017, 71: 731-751. doi: 10.1146/annurev-micro-090816-093913 [15] BAI Y, MELLAGE A, CIRPKA O A, et al. AQDS and redox-active NOM enables microbial Fe(III)-mineral reduction at cm-scales[J]. Environmental Science & Technology, 2020, 54(7): 4131-4139. [16] LOVLEY D, MALVANKAR N S, VARGAS M, et al. Tunable metallic-like conductivity in microbial nanowire networks[J]. Nature Nanotechnology, 2011, 6(9): 573-579. [17] KAPPLER A, WUESTNER M L, RUECKER A, et al. Biochar as an electron shuttle between bacteria and Fe(III) minerals[J]. Environmental Science & Technology Letters, 2014, 1(8): 339-344. [18] SUN T, LEVIN B D, GUZMAN J J, et al. Rapid electron transfer by the carbon matrix in natural pyrogenic carbon[J]. Nature Communications, 2017, 8: 14873. doi: 10.1038/ncomms14873 [19] YIN C, SHEN Y, YUAN R, et al. Sludge-based biochar-assisted thermophilic anaerobic digestion of waste-activated sludge in microbial electrolysis cell for methane production[J]. Bioresource Technology, 2019, 284: 315-324. doi: 10.1016/j.biortech.2019.03.146 [20] LAM, LUO D H, RUIBANG, et al. MEGAHIT: An ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph[J]. Bioinformatics, 2015, 31(10): 1674-1676. [21] 中华人民共和国国家质量监督检验检疫总局, 中国国家标准化管理委员会. 城镇污水处理厂污泥泥质: GB 24188-2009[S]. 北京: 中国环境科学出版社, 2007. [22] 中华人民共和国环境保护部. 水质化学需氧量重铬酸盐法: HJ 828-2017[S]. 北京: 中国环境科学出版社, 2017. [23] JUHL J L, PHILIPPE J, MICHAEL K, et al. NOG: Automated construction and annotation of orthologous groups of genes[J]. Nucleic Acids Research, 2008, 36: 250-254. [24] NGUYEN D, WU Z, SHRESTHA S, et al. Intermittent micro-aeration: New strategy to control volatile fatty acid accumulation in high organic loading anaerobic digestion[J]. Water Research, 2019, 166: 115080. doi: 10.1016/j.watres.2019.115080 [25] CAI W, LIU W, YANG C, et al. Biocathodic methanogenic community in an integrated anaerobic digestion and microbial electrolysis system for enhancement of methane production from waste sludge[J]. ACS Sustainable Chemistry & Engineering, 2016, 4(9): 4913-4921. [26] HO D P, JENSEN P D, BATSTONE D J, et al. Methanosarcinaceae and acetate-oxidizing pathways dominate in high-rate thermophilic anaerobic digestion of waste-activated sludge[J]. Applied and Environmental Microbiology, 2013, 79(20): 6491-6500. doi: 10.1128/AEM.01730-13 [27] DE VRIEZE J, DEVOOGHT A, WALRAEDT D, et al. Enrichment of methanosaetaceae on carbon felt and biochar during anaerobic digestion of a potassium-rich molasses stream[J]. Applied Microbiology and Biotechnology, 2016, 100(11): 5177-5187. doi: 10.1007/s00253-016-7503-y [28] LOGAN B E, ROSSI R, RAGAB A, et al. Electroactive microorganisms in bioelectrochemical systems[J]. Nature Reviews Microbiology, 2019, 17(5): 307-319. doi: 10.1038/s41579-019-0173-x [29] KUNATH B J, DELOGU F, NAAS A E, et al. From proteins to polysaccharides: Lifestyle and genetic evolution of coprothermobacter proteolyticus[J]. Multidisciplinary Journal of Microbial Ecology, 2019, 13(3): 603-617. [30] YAMADA T, IMACHI H, OHASHI A, et al. Bellilinea caldifistulae gen. nov., sp. nov. and Longilinea arvoryzae gen. nov., sp. nov., strictly anaerobic, filamentous bacteria of the phylum Chloroflexi isolated from methanogenic propionate-degrading consortia[J]. International Journal of Systematic and Evolutionary Microbiology, 2007, 57(10): 2299-2306. doi: 10.1099/ijs.0.65098-0 [31] LÜ C, SHEN Y, LI C, et al. Redox-active biochar and conductive graphite stimulate methanogenic metabolism in anaerobic digestion of waste activated sludge: Beyond the direct interspecies electron transfer[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(23): 12626-12636. -




 下载:
下载: