-
全球能源危机和环境污染的加剧使得储能技术的发展受到了相当大的关注[1]。锂离子电池(lithium-ion battery, LIB)作为具潜力和竞争力的储能技术之一,以其能量密度高、自放电率低、无记忆效应、应用温度范围广和环境友好等诸多优点[2],被广泛应用于通信(3C便携电子设备[3]、5G基站[4])、交通(动力/混动车[5])、军事(无人机[6])、医疗(植入器官[7])和大型储能领域(清洁能源电网系统[8])等。随着锂离子电池应用范围扩大,社会供求量逐年急速增长[9]。2019年,锂离子电池的全球出货量约达163 GW·h[10],随着锂离子电池在各领域应用规模的进一步扩大,市场需求将持续上升,预计2025年全球产量将超过439 GW·h[11]。同时,据锂离子电池的寿命(动力车5~8 a,3C设备2~3 a)预测,至2020年我国将产生约5.0×108 kg的废旧锂离子电池。由于先进能源材料的开发和储能硬件的更新换代,而产生的大量废旧电池又将带来新的问题和挑战。
资源性与危险性并存是锂离子电池的典型特征。尽管锂离子电池不含Hg、Cd、Pb等毒害性较大的重金属元素,然而也并非绝对的无污染,破损电池中电解液会在空气中蒸发形成含水分的HF气体。不像铅酸电池已经形成完善的回收模式,事实上,95%的废锂离子电池由于回收难度和成本问题没有得到妥善处置,若这些大量废旧电池不被合理处置,会严重浪费宝贵资源,也会对人群健康、环境带来重大风险[12-13]。此外,锂离子电池的初次生产[14-16]和原材料的加工过程[17]对环境亦有破坏性影响,如锂元素的开采需要大量矿砂、矿藏或含矿物卤水。相比于初次生产,开采1 t原料需要开采近250 t矿砂,而再生产仅需回收约28 t废旧电池[14-16, 18]。废锂离子电池不仅含有锂元素,还含有大量价值材料,尤其是正极部分被认为是锂电体系中最具经济价值的部分[19-20]。正极材料主要是一元或多元含锂的活性物质[21],所含的Li、Co、Ni和Mn等价值金属元素远高于一般矿砂[22]。而作为集流体的铜箔、铝箔也极具回收价值。因此,锂离子电池的绿色资源化回收与高效再利用对环境保护、人类健康、经济和资源保护都有重要意义。
近年来,研究人员在开发高性能锂离子电池电极材料方面作出了巨大努力。然而,目前商用锂离子电池以及下一代可充电电池的可持续性问题却并没有得到高度的重视,而且尚缺乏正极材料绿色高效资源化回收再利用技术的系统总结。ZHANG等[23-24]总结了锂电池的主流回收工艺,即湿法冶金工艺,介绍了工艺方法、生命周期评价及金属分离层次等方面的内容。HUANG等[25]对该工艺后续的电池再制造进行了归纳。KIM等[26]对韩国废电池的处理及其材料的流动进行了分析。BOXALL等[27]对澳大利亚在锂电回收方面的创新潜力进行了评价。ZENG等[28]对废旧锂电池回收利用的现状和问题进行了简单概括,但尚未形成完善的体系并缺少对资源化回收过程中能耗、环境影响的评估。目前,对于正极材料资源化回收再利用的综述,仍局限于对于传统主流方法的总结,而忽略了对先进技术的探索与引进。传统主流工艺尽管较为成熟,但其繁琐低效、耗能耗材及不可避免的二次污染问题,是推进高效回收工作的瓶颈,也是制约废旧锂离子电池回收产业化发展的重要因素。
本文从实验室基础研究到工业应用的视角,梳理了废旧锂离子电池的正极材料资源化回收再利用的研究现状及问题,着重分析了以微波热处理为核心的先进便捷技术在回收过程中的应用及优势,通过能耗、效率、经济性、功能性和环境影响等诸多方面比较,总结了微波热处理技术在优化杂质降解、强化碳热还原、提升浸出效率、再生材料过程中的显著优势和发展潜力,旨在为锂离子电池正极材料回收处理行业的未来发展以及3R[21]和4H[11]绿色高效的能源材料回收体系的构建提供参考。
全文HTML
-
近年来,微波热技术因其先进便捷的特点在快速加热领域广泛应用。除了食品[29]、医疗[30]、农林业[31]、生物质[32-33]等行业,还被广泛应用于电子废物处理领域[34]。微波加热的众多特点使其在废旧锂离子电池高效资源化回收过程中具备较好的应用前景。
微波与材料之间的交互作用是微波热处理的一大特点[35-37]。微波电磁场对极性分子的作用使微波能在处理过程中被介电材料吸收。如图1(a),交变磁场使杂乱的极性分子趋于定向排列,导致其频繁地进行平移和转向的分子运动,引起的分子间摩擦损失会将电磁能量转换为热量;同时,交互过程中还伴随着弱分子间键断裂和新键生成的热效应[32],这是微波加热的机理(见图1(b))。不同于传统模式,微波加热可以辐射并穿透材料,整体上产生相对均匀的温度分布。而传统加热模式(见图1(c))则是从表面传热到内部,即所谓表面传热。这种方式下,材料表面的温度比起内部会高很多。因此,微波热处理被认为是传统电加热处理的一种更为先进的高效节能替代技术[35]。
-
材料的介电性能是影响微波与材料间交互作用及加热效果的重要因素。一般由复合介电常数
ε 来表示(见式(1))。式中:
ε 为复合介电常数,F·m−1;ε′ 和ε′′ 分别代表其实部和虚部,实部ε′ 相当于通常的介电常数,决定了材料的储电能力,而虚部ε′′ 亦称为损耗因数,反映了微波能到热能的转换效率;μ 为材料的复合磁导率,H·m−1,同样由实部μ′ 和虚部μ′′ 构成(见式(2));两者的损耗正切即tanδ 和tanδm (见式(3))是材料电磁能量损耗的指标。因此,具有适当
ε′ 值和较高ε′′ 值(即高tanδ 值)的材料被认为是良好的微波受体。通常,回收获得的正极材料含有碳质、含Li的金属氧化物或盐、附着的有机质及载体铝箔,他们均是影响微波加热/热解的重要物质。目前文献中提及的锂电相关材料在2.45 GHz微波场下的吸波性能归纳在表1和表2中。其中,碳质是理想的微波吸收剂,能够有效耦合微波作用[38]。而金属氧化物或盐和附着有机物是对微波的吸收相对较弱,铝箔则在一定程度上反射微波。碳材料(如木碳、碳黑和活性碳)的介电损耗角正切范围为0.1~0.8,比常见“微波良好受体”蒸馏水(约为0.1)的吸波能力更好。微波加热与碳材料组合已在一些材料的热处理中得到应用[46-49]。一般而言,这些材料吸波能力相对较差,不是被微波穿透,就是介电性能不理想,故需要通过与具有高吸波性的材料(碳材料[48-49]或金属氧化物[38, 47])掺杂,以便实现更高温度的广泛热解。相比于金属氧化物,由于碳材料的成本低且易获得,通常被用来作为吸波介质[46]。而正极材料自身既具备金属氧化物,又含有碳质,因此,在回收过程中应用微波加热/热解处理是非常有效的[38]。
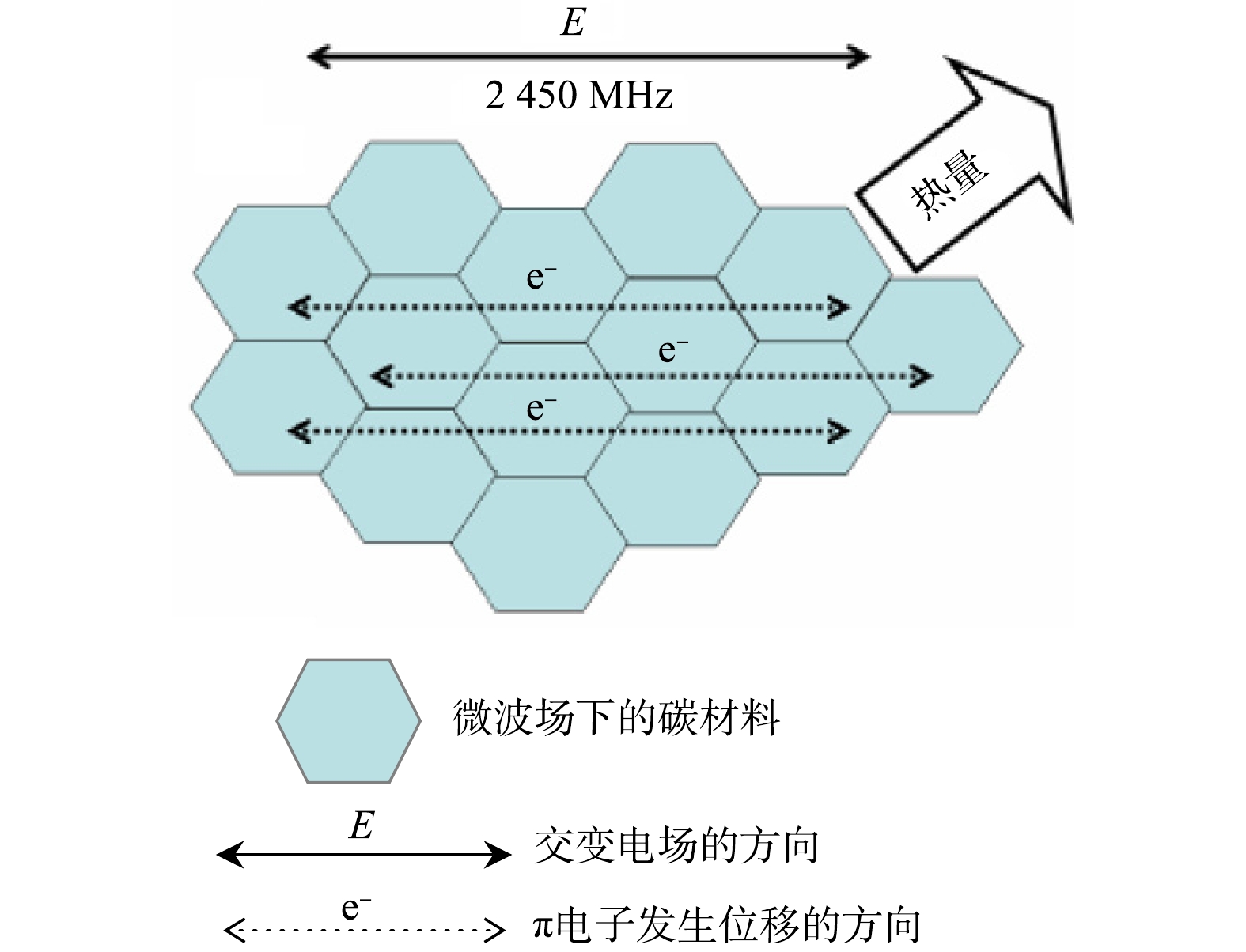
机理研究表明,微波与碳质的交互作用既可通过偶极再取向和离子传导机制[50]发生,也可通过麦克斯韦-瓦格纳极化和传导组合机制[46]发生。后者情况下,碳质相内会出现随微波场流动的电流,即碳材料中的π电子(可自由地在有限区域内移动的带电粒子)从其平衡位置发生位移,导致电介质极化[51]。MENENDEZ等[46]提出微波加热下碳材料相内电子位移机理(见图2),并证明在碳材料的微波加热过程中还可能会发生额外的电离现象[46, 51]。在某些情况下,一些π电子的动能增加可使相邻π电子从碳材料上“跳”出来,造成周围气氛的电离[46]。这种现象在宏观水平被认为是火花或电弧,在微观水平则被认为是热点或等离子体。热点或等离子体现象可能会在掺杂了碳的微波加热过程中产生额外热效果。热点效应既带来了加速局部升温的优点,又存在加热不均而产生局部烧结现象的风险,为此,一些学者对微波处理碳及碳升温进行了数值模拟[42]。
-
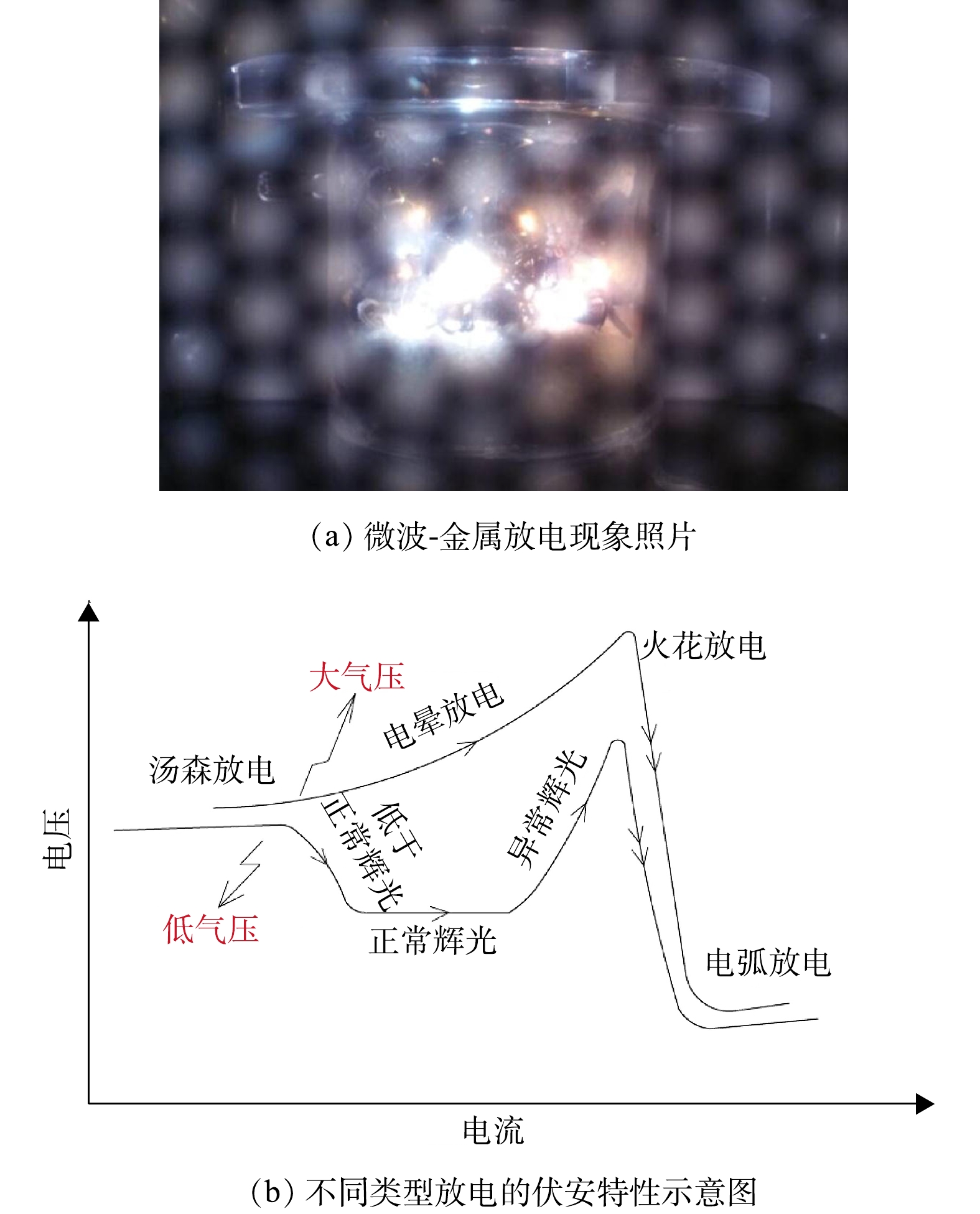
微波和金属的交互作用又是另一种有意义的现象。一般情况下,作为导体的金属会产生电磁屏蔽现象[52],故被金属包裹的材料因接受不到电磁波而不能被加热,或是加热效率大大降低。但由于正极材料的活性物质包覆在铝箔外表面上,因此,这种现象并不明显[53]。事实上,金属在交变电磁场的作用下还会产生涡流,从而产生大量热量。但当金属被破碎或表面被粗糙处理而产生尖锐边缘、尖端或微观不规则结构后,再通过微波辐射会发生独特的放电现象[52](见图3),产生高温热点(最高可达3 000 ℃[54])。同时,伴随高密度等离子体的释放[52],其中包含的大量高能电子和活性基团会通过互相碰撞产生活性自由基,而这些高能粒子会影响化学反应过程和产物组分。
值得注意的是,当光滑金属在屏蔽微波而产生的局部电磁场时,电荷在其表面重新排布而产生电势差。当电势差足够大时,可能会击穿空气产生电弧;若此时金属与微波腔内壁链接,则产生电弧可能会将其损坏,甚至损坏微波炉中的磁控管等其他电子零部件。因此,研究时应将金属置于透波绝缘容器中。
总的来说,微波热处理以其自身独特的加热方式及与各材料间的耦合交互效应,在代替传统热处理回收电池技术中具有无限潜力,可应用于电池回收预处理阶段热解黏结剂以脱箔解离,亦可用于金属分离回收阶段加强碳热还原反应,以及材料的再生以及新材料的制备过程。相比于传统热处理法回收锂离子电池[55],微波热处理过程工艺简单、能源利用率高、产生废物少、占地面积小、处理成本低、时间短。此外,微波技术环境友好,可使用清洁和可再生电力,不产生“三废”,基本可实现废物的近零排放及废旧锂电池的绿色能源化和资源化回收利用,故具有很好的发展前景。
1.1. 微波与碳质的交互作用
1.2. 微波与金属的交互作用
-
为实现正极材料的初步分离和金属箔粗选,需进行回收预处理[56-57]。在工业预处理中,通常会对完全放电的废电池进行批量机械破碎,并利用诸如粒径、密度、磁性和疏水性等特性进行物理分离,初步获得粗细不同的组分。其中,粗组分为聚合物塑料、金属外壳和箔,通过磁选工艺可去除金属外壳,通过密度分离工艺可分离塑料和金属箔;细组份主要是“黑色活性物质”——正极涂层的活性物质和碳质,可通过泡沫浮选利用其疏水性将碳质从亲水金属氧化物中分离。但由于物理分离的机械分选法[58]无法消除正极材料“黑色活性物质”中的聚合含氟黏性剂(PVDF、PTFE),故无法完全分离这些组分。此类黏性剂用于将正极活性物质、导电剂和有机溶剂等混合黏性在集流体铝箔上,使它们之间具有较强黏性。然而,在预处理时,这些组分难以分离且降解时具有毒气污染。目前,虽然制造商们已意识到此问题,生产中正将正极黏性剂由含氟黏性剂替代为黏性剂(如水溶性羧甲基纤维素CMC和丁苯橡胶SBR),研究中也转向水基、纤维素基、木质素基正极黏性剂的研究[21]。而预处理中黏性剂的去除仍是回收正极材料的纯化和完全分离价值组分的关键和首要难点。
-
为了在预处理中消除聚合黏性剂,释放活性物质和碳,完全分离出价值组分,通常采用溶剂溶解法[59-60]、NaOH溶解法[61]、超声辅助湿法[58]和热处理法[62-63]等方法进一步脱箔解离(见表3)。其中,溶剂溶解法[59-60]基于黏性剂的极性,采用同样具有极性的有机溶剂(如NMP和DMF)进行溶解实现分离;NaOH溶解法[61]则是利用铝箔的碱溶性,将不溶于碱液的正极活性物质与之分离,但该法导致铝回收困难,碱废水排放污染,弊端突出。超声处理[58]可结合上述溶剂,或使用中性洗涤剂或清水等减小污染源,通过外加能量减小两者之间的黏结力,诱导更快分层,但效率提升不理想。由于破碎后的颗粒尺寸和分离程度对后续回收再生有重要影响。上述湿法预处理得到的粗细组分易产生混凝,在后续处理中增加了活性物质流失可能性。此外,还有共性的废液污染问题,故在绿色回收体系中应尽避免大规模使用酸碱试剂。
ZHANG等[64]对预处理方法的影响进行了研究,发现相比于湿法,热法预处理可利用不同组分的热解机理特性,有效分步降解黏性剂或碳质等,以分离出所需的活性物质,实现一步脱箔分离,操作简单,有利于后续回收再生处理。相比于湿法的废液后处理,热处理产生的废气更容易加以控制,甚至可在无氧条件下被冷凝回收,且处理成本更低。有研究发现,粘合剂PVDF的热解温度大约为350 ℃,而碳质则约为600 ℃[56]。为了研究热法预处理实现活性物质脱箔解离效果的热解温度影响,SUN等[63]进行了多组LCO正极材料真空热解实验,当温度达到600 ℃后,正极活性物质易脱落,该温度点的真空脱箔解离效果最佳。然而,应注意到当温度继续升高至约700 ℃时,铝箔开始脆化、熔化。这时,高温会导致金属碎裂,黑色活性物质被铝污染。因此,若能在预处理中应用并优化热解法热处理,在提高脱箔解离效果的同时,控制能耗和过程污染,则该方法可为材料的回收提供新的思路。
-
微波热解作为传统热法处理的绿色替代方法,在脱箔解离过程中可发挥独特的作用。首先,微波热处理可通过其本身促使材料“由内而外”和整体加热的独特优势,直接在正极材料内部将微波能转化为热能,使被加热的材料本身成为发热源,则不会造成大部分材料的破坏,从而减少资源浪费。同时,该过程为非接触加热,无需热传导,从而避免了高能耗的问题。DIAZ等[53]对废三元正极材料进行了微波降解的机理研究,发现微波辅助热解增强了降解过程的动力学。实验中,微波热解提供的加热速率约为每秒3.5 ℃,比传统的热解速度快约20倍。同时,微波处理时无需引入其他物质和热量即可实现快速加热,可大大减少废弃物的重量,并可避免引入其他能源带来的环境污染。
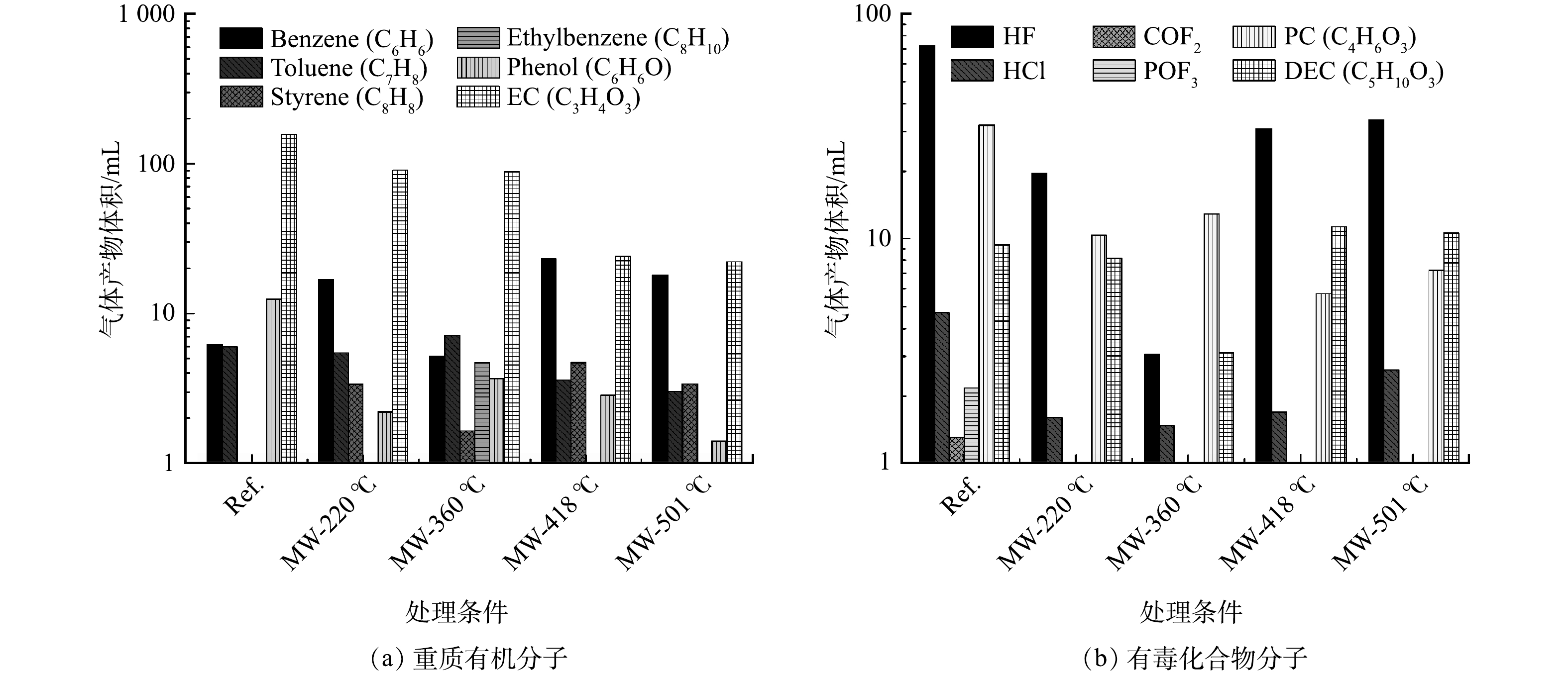
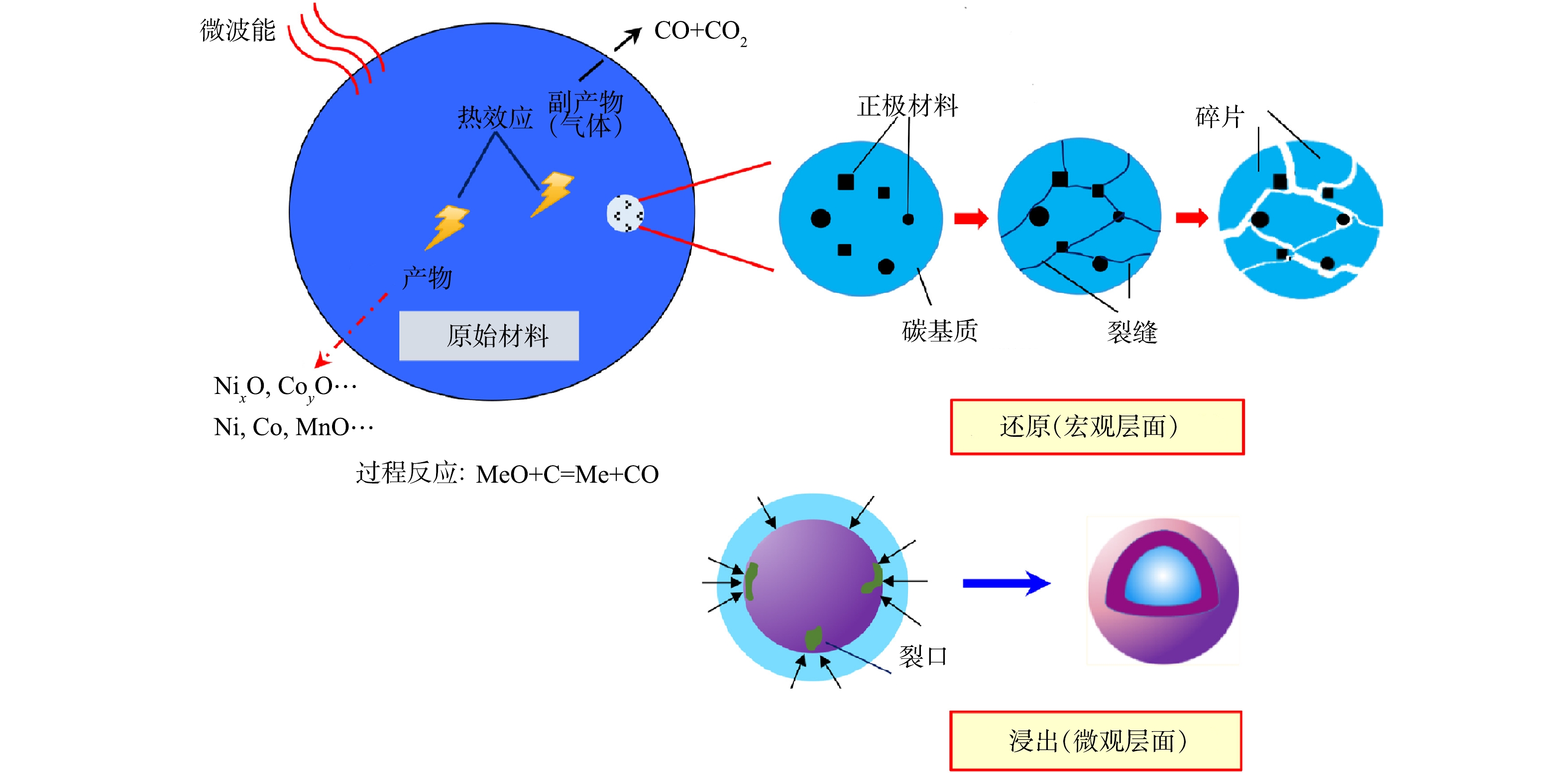
更重要的是,微波通过与不同介电特性的物质进行交互作用产生的独特效果,可使正极材料中的不同物质(如电解质、聚合物黏结剂、碳质、金属箔以及过渡金属氧化活性物质)有选择性地被加热,从而达到特定的加热目的。与传统热解相比,微波热解可产生特殊降解反应,有效控制降解过程污染物的生成。在微波下黏性剂、电解液中的长链分子将被断裂成短分子,从而可减少重分子和有毒有害大分子气体的产量(见图4)。对于含有Ni和\或Al的正极材料(如镍钴铝Li(NixCoyAlz)O(NCA)、镍钴锰Li(NixCoyMnz)O(NMC)),可将其作为催化剂在微波下催化这种额外的降解反应,使传统处理生成的有害电解质和有机气体能进一步在充满自由基的蒸汽气氛下重整为CO和H2。此外,汪永威等[65]的微波热处理废旧锂电池专利里也提到,在微波系统中更容易实现气体产物的冷凝回收。由于微波系统结构简单,可外接尾部处理装置,当热解温度达到有机溶剂和黏性剂发生分解反应的温度时,可直接将产物中凝固点不同的小分子化合物在不同温度下进行冷却分离。
微波加热的即时性和瞬控性可瞬间达到热解过程开停和升温的目的,过程操作便捷、易于控制,特别适合锂离子电池正极材料回收热处理工艺中的规范化、自动化应用。殷衡[66]将微波技术应用于先进等离子体激发器上,通过高功率的电流激发空气产生微波等离子体,等离子体所携带的大量能量被正极材料吸收而激发形成急剧膨胀的新等离子体,从而产生冲击波,该冲击波作用于黏性剂,使得正极材料从铝箔表面被剔除,直接实现了对正极活性材料及铝箔的分别回收。微波等离子体所携带的大量活性粒子(如离子、电子、激发态的原子及自由基等)可分解材料中的有机黏性剂,并将碳质导电剂氧化为二氧化碳气体,从而可一步实现对正极活性材料的提纯。
2.1. 黏结剂的降解工艺
2.2. 微波辅助热解黏性剂降解处理
-
正极材料的资源化回收包括活性物质的直接回收和金属或其氧化物的分离回收。对于生产不合格而非使用后废弃的电池,正极活性物质结构和性质没有改变,可脱箔解离后直接回收再加以修复[67]。而对于有使用损耗的正极材料,通常通过分离、提纯金属进行冶金处置。工业和实验室的冶金工艺有热(火)法、湿法、生物法这3类,其对比见表4。由于热法冶金具有能耗高、金属回收率低、废气烟尘污染严重等问题,而湿法冶金具有回收周期过长、试剂消耗量大、废水污染等问题。目前,工业上通常采用湿法-热法联合冶金工艺,将常用作预处理的热法冶金与传统的湿法冶金相配合,在处理现有问题上是行之有效的[68]。作为一种新型的热解手段,微波技术辅助热法或湿法冶金技术在节能省时、高效可控方面优于传统工艺。其特殊的热作用机制在冶金应用中具有很大的发展潜力,特别是对于热法冶金,采用微波处理技术加以辅助优化,可加强热效应和碳热还原机制,有效调控金属分离过程,从而为正极材料金属分离与资源化回收的高效规模化应用带来可能。
-
传统热法冶金技术尽管存在环境污染、高能耗成本以及金属回收率和种类有限等不足,但这是提取高价值过渡金属(如Co和Ni)的常用工艺。通常,传统热法是在高温下将正极活性物质还原为Li、Co、Mn、Ni等过渡金属单质或其简单氧化物的合金形式。但由于热传递经历了从表面到内部的过程,还原反应过程首先发生在活性物质的固相表面,还原产物随之包覆其表面从而阻碍反应进一步发生,导致反应时间过长,金属还原不充分,不利于后续的浸出过程。然而,微波技术的加热特点却可以有效解决该问题,并且在加热过程中,由于其容积加热和选择性加热的特性,还原反应速度更快,明显降低能耗和污染[85-86]。更重要的是,微波加热可以有效改善材料不同部分温度不均衡加热的问题,保证了还原反应的充分性。
利用微波热处理技术可有效强化碳热还原过程,从而提升还原金属的效果。目前,微波强化碳热还原对金属分离效果的提升已经在从红土镍矿中提取金属Ni和Co的相关研究中得到证实[87-91]。通常,碳材料(如石墨、活性碳、焦碳等)是非常理想的活性微波吸收材料[92]。碳材料在微波场中会产生较好的热效应,催化碳热还原反应,为冶金过程提供所需热量,且碳质材料又恰好就存在于电极材料中,耦合微波辐射作用可以迅速提高反应温度,实现便捷有效的金属分离。热力学研究表明,正极材料粉末可以在碳质存在的情况下被碳热还原为金属单质或简单金属氧化物的形式[93]。
在微波辅助碳热还原的研究中,PINDAR等[93]认为,使用负极回收的石墨恰好可以作为还原剂,配合微波强化碳热还原,可实现无额外添加的有效循环冶金回收过程。该研究者的实验最终以金属单质或其氧化物的形式实现了69%钴、20%锰、43%镍、82%锂的整体回收。这些产物可作为电极再生材料的潜在前体。相关研究结果也证明了基于微波碳热还原的工艺在回收产品质量和成本方面是成功的。值得注意的是,在与商业活性碳对比中,从负极中回收的原生石墨显示出了对正极活性物质具有更好的还原性,从而证明直接回收利用原生石墨,配合微波辅助碳热还原正极材料,促进金属的整体回收是高效可行的。
为了探索和对比传统和微波辅助热法在正极材料碳热还原过程中的效果,PINDAR和SUNIL等[93-97]对不同正极材料(纯粉末、混合粉末)在2种加热模式下进行了多组热法还原实验,并通过Box-Behnken、Jandler等数值模型计算了获得最佳回收效果的处理条件,见表5。经对比发现,2种加热模式下Li的回收率相当,且微波处理明显降低了过程中的质量损失。微波条件可明显提升Ni的还原效果,但对于Co和Mn并没有明显效果,这是总产率略逊于传统方法的原因。但采用微波时只需较少的还原剂量,成本、时间和能耗被大幅降低。总体来说,微波辅助碳热还原利大于弊。此外,通过优化石墨含量、加热时长、磁化强度,微波辅助还可达到更好的效果。
此外,作为正极材料活性物质的金属氧化物本身也可作为良好的微波吸收剂,能有效吸收微波能,提高材料加热速率,提升热-湿法回收金属的效益。针对微波促进碳热还原反应以提升回收效益的情况,ZHAO等[38]在微波强化还原过程中,研究了正极粉体在25~900 ℃下的微波吸波性能以及微波加热对回收过程的影响,发现正极材料在上述温度范围内具有良好的微波吸收性能。尤其是与碳混合时,材料的吸波性能得到明显改善。随着还原反应的进行,材料的介电性能从600 ℃开始迅速增加,证明微波加热可以促进碳热还原反应,从而促进有价金属及其氧化物的有效回收,为微波还原焙烧分离有价金属的基础研究提供了参考。
-
目前,大多数冶金回收工艺属于湿法回收的范畴,以此法分离回收得到的足够纯度价值金属不仅可以用于重新合成锂离子电池正极材料,还可用于一系列其他应用,如合成CoFe2O4或MnCo2O4[98-100]。湿法冶金处理常见的浸出剂有硫酸[75, 101-102]、盐酸[61, 74-76]或硝酸[75, 103]等无机酸。JOULIE等[75]研究了正极材料在硫酸、盐酸和硝酸溶液中的浸出特性,发现盐酸的浸出效果最好,Li、Ni、Co和Al等金属几乎可以完全浸出。但浸出过程中清洁性硫酸体系优于盐酸体系[75-76],盐酸浸出虽然节省成本,但处理过程中产生的氯气会造成不必要的大气污染和刺激性危害[76]。为降低无机强酸浸可能造成的水污染,研究人员认为可使用柠檬酸、抗坏血酸等有机酸作为它们的绿色替代[77-80]。LI等[79]测试了2类酸的浸出过程:无机酸(H2SO4和HCl)和有机酸(柠檬酸),证实了柠檬酸中钴和锂的浸出效果比2种无机酸更好,回收了超过96%的Co和近100%的Li。尽管如此,有机酸高昂的成本限制了其进一步的工业应用。在力求污染最小化的探索中,研究人员发现基于湿法的机械化学处理[104-105]通过将正极材料在密封球磨系统中与添加剂(如氯化合物或络合剂)进行密封共研磨,产生水溶性钴盐,然后通过纯水浸工艺很容易分离回收得到目标金属。这种工艺不使用腐蚀性酸或强氧化剂,绿色环保,在工业发展上前景可观。
正极材料在湿法冶金中的研究最早集中在一元材料LCO的浸出和再生上[106]。微波处理对湿法冶金的强化在“宏观层面”的主要体现是通过微波独特的加热方式,由内而外强化分子间运动,从而促进正极材料在溶剂中溶解度的增强。PATIL等[107]研究了利用微波辅助溶解法快速从正极材料LCO中回收价值金属Li和Co。在传统湿法工艺中,使用酸剂需要在80 ℃下处理至少6 h,可最多提取94%Li和91%Co,但在微波辅助湿法溶解过程中,仅在25 min内就溶解了87%Li和85%Co,这证明了微波照射25 min可得到与常规方法处理300 min相同的溶解度,回收效率得到了有效提升。
目前,湿法冶金的焦点已转至多种过渡金属的多元材料(如LNMO、NMC)上[108-109]。然而,这些多元正极材料中价态较高的过渡金属Ni、Co、Mn与O之间的化学键Me—O很强,不利于浸出分离[110-111]。因此,有必要将这些金属进行从高到低的价态还原,以提高其在水溶液中的溶解度。在酸浸体系的湿法冶金过程中,常添加H2O2[14, 101, 112]、NaHSO3[113]或葡萄糖等还原剂,以提高高价态金属的浸出率。此外,也可通过热处理对材料进行预先还原,将热法预处理还原与湿法冶金联合起来,这在处理高价态金属难以浸出分离的问题上被证明是有效和可持续的[24, 114]。同时,现有研究已有利用微波热处理技术提高预先还原效果的成果案例[115]。
微波处理对湿法冶金预处理的强化在“微尺度层面”的体现,主要涉及到碳热还原过程中正极材料的晶体结构、粒子分解和孔隙分布的变化。如图5所示,在微波加热下,材料晶体的晶格会从有序状态变为无序状态,同时,粒子的晶格之间会形成裂纹。这是由于在组分复杂的正极材料不同部位的微波热效应具有差异,从而引起了粒子晶格之间的热应力。相同地,还原产物的表面上也会出现裂痕,促使粒子间大量孔隙的形成,从而增加正极材料的整体孔隙率,有利于提高正极材料与湿法试剂之间的固液接触面积。FU等[115]将经过微波碳热还原处理的与未经还原处理的正极材料酸浸效果进行了比较,发现在微波还原处理下,Li、Ni、Co和Mn的回收效率分别可提升约10%、20%、20%和35%。由于微波辐射下产生热效应降低了粒子间团聚程度,改善电极晶体结构的无序性,提高了还原浸出反应活性,从而证实了微波辅助可有效强化金属从正极材料的浸出效果。
因此,大部分研究证实微波辅助技术对于湿法的金属浸出与分离有明显的强化效果:宏观层面上,微波从材料内部增强分子热运动,提升溶解度和反应速率;微尺度层面上,微波降低了材料粒子团聚程度,形成晶格间裂纹,促使还原浸出过程更加充分。
-
针对应用微波辅助技术实现湿法冶金过程中的工艺简化、控制成本能耗、提升效率及收益的目标,PATIL等[107]就传统和微波辅助工艺中产生的能耗和成本进行了对比分析(见表6)。假设传统热法-湿法过程中所需加热功率为300~500 W[116],若采用微波辅助溶解,则所用功率仅为180 W。传统热法-湿法在大约360 min内从正极材料中溶解大于85%的Li和Co,而微波辅助湿法在大约25 min内溶解几乎相同量的Li和Co。因此,传统热-湿法工艺的总成本约为14.675美元,微波辅助工艺仅需约1.4265美元,微波辅助加热-湿法冶金比传统工艺更为经济。
除锂离子电池外,碱性废电池的资源化高效回收[117]中也出现了几乎零浪费的微波辅助湿法冶金案例。与传统湿法工艺相比,微波辅助湿法大大减少了反应时间,保持了较高的金属浸出率,提高了湿法回收过程的经济性。
-
利用细菌等微生物回收价值金属的生物浸出技术被认为是一种最有望取代传统湿法冶金技术,亦可用于锂离子电池回收过程中的金属回收。目前,该法已成功应用于矿业[118-119],一定程度上可将其作为湿法冶金和焦化冶金工艺的补充[120-121],尤其对于Co和Ni很难分离,需要添加额外的溶剂萃取步骤时。生物法利用微生物选择性地从正极消化金属氧化物[81],并减少这些氧化物产生金属纳米颗粒[122-123]。目前,主要培育的微生物有嗜酸氧化亚铁硫杆菌[81-83]、氧化硫硫杆菌[82]等。
除了生物质在湿法浸出方面催化作用,生物质热解与正极材料分解两者耦合反应可促进金属的进一步分离。研究人员发现,微波热解生物质的应用在锂离子电池正极材料回收领域具有潜力。相比于传统加热热解,微波热解生物质制得的生物炭质往往具有更大的比表面积和更多孔隙[124],更适合作为回收热分解过程中碳热反应的还原剂。但是,在加热过程中对于微波和正极材料的兼容性、生物质热解与正极材料分解以及两者耦合反应的研究尚不多见。
生物质和微波的协同作用具有不确定性。生物质的种类、密度、湿度、温度都会对其吸波特性产生很大影响[125]。通常来说,在500 ℃以下,大多数生物质表现出较差的吸波特性[126],但其热解后生成的生物炭质却可理想地吸收微波能。另外,前面的研究表明,正极材料在较低的温度下有着较为出色的吸波特性,尤其是在2 450 MHz的微波功率下[38],如果混合起来进行微波处理,可补偿生物质较差吸波特性的缺陷。因此,在微波热解生物质回收正极材料的应用中,需要控制生物质的量以达到最佳的回收效果。
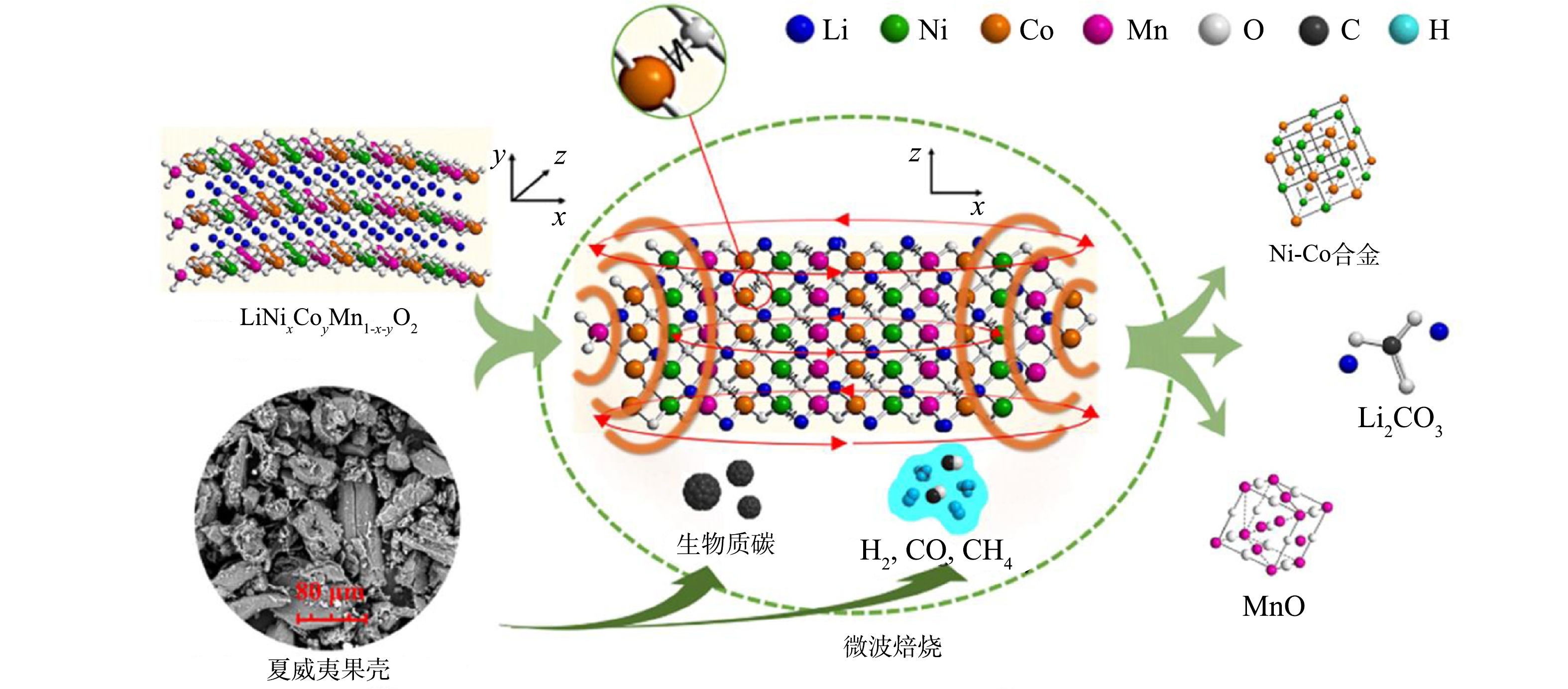
ZHAO等[84]借助微波加热和生物质热解两者的优势,利用当地生产的夏威夷果壳作为有效生物质,在混合粉末生物质量占24%的最优情况下,成功将常规热法下1 000 ℃才分解的三元正极材料Li(Ni1/3Co1/3Mn1/3)O2热解温度降低到300 ℃,实现了在较低温度下自发开始的生物质-正极材料偶合反应(见图6),有效提升了回收效率。值得注意的是,由于炭质和焦炭的复杂组成以及辐射-热转换机制的不同,微波加热下的“热点效应”造成的不均匀加热现象在一定程度上会导致材料的损耗。
3.1. 微波加热对正极材料的碳热还原
3.2. 微波强化湿法回收正极材料中的金属
3.3. 微波辅助热法-湿法工艺的经济性
3.4. 微波辅助生物法回收金属
-
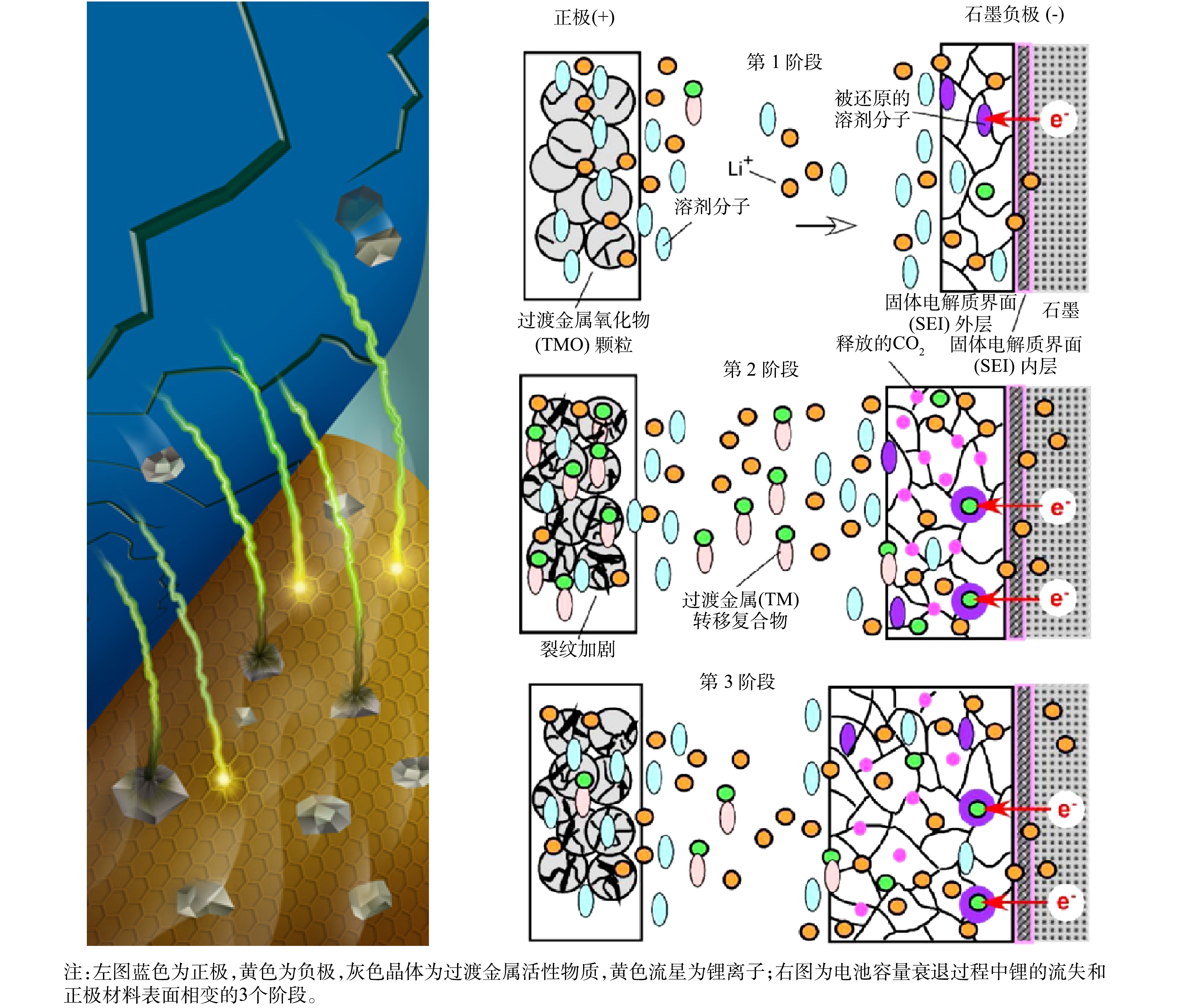
正确认识正极材料衰减的原因,对正极材料的资源化再生和循环利用工艺的探索和优化具有指导意义。锂离子电池老化报废的原因主要是电池容量的衰减,即氧化作用导致的电池内阻的增加[127-130]。而正极材料与电池容量衰减之间具有着密切的关系(见图7)。具体针对正极材料的衰减而言,主要原因有2点:1)锂的流失,即正极材料中的锂随着电池的循环无法完全回到正极,导致电池容量降低;2)活性物质的损失,如正极材料表面相变,即与电解液接触的材料表面更易发生相变,导致结构变化引起的较低的锂离子传导率,极化增大,容量衰减[131]。
-
在锂离子电池的正极材料回收过程中,热法冶金需要极高的处理温度和复杂的提纯过程。湿法冶金也需要腐蚀性酸液将金属元素溶出后逐步沉淀。这2种方法不仅耗能大,步骤复杂,而且会造成环境污染。更重要的是,两种工艺都会在一定程度上破坏正极材料的颗粒结构,从而浪费了材料中大量的有价值物质,除非通过控制并优化条件才能再合成出性能理想的正极材料。
直接再生法摒弃了热法和湿法冶金中破坏颗粒的不足,直接通过预处理过程将正极材料分离出来,然后对衰减后的正极材料进行补锂处理。经处理再生后的正极材料具有高容量,高倍率性能和稳定的循环性能,使其可直接被用于新生电池。有研究发现,对废正极三元材料进行直接煅烧球墨补锂处理,是使其恢复良好性能和层状结构的有效途径[132]。但张维民等[133]在一次煅烧球墨过程中发现,Li并没有进入材料的晶格中去,即不是有效重生过程,当材料接触电解液之后,锂盐必然会大部分溶解于电解液中,不利于电池容量的恢复。为了解决此问题,该团队公开了一种固相煅烧-球墨-煅烧的方法再生正极材料。经过二次煅烧,Li可以进入到材料晶格中去,可大量减少其溶解于电解液的量,恢复三元材料该有的晶格,使其容量有一个较大幅度的提升,电化学性能从而得到极大改善。
二次煅烧过程的反应机理:由于正极材料中存在与Li+直径相近的Ni2+,因而易占据Li+的3a位置而发生阳离子混排现象,阳离子混排数量直接影响到材料的电化学性能[134]。Ni2+在Li层不仅降低了放电比容量,而且会阻碍Li+扩散,这种无序状态会使电化学性能变差。同时,电池使用过程可能会造成正极材料层状结构的塌陷。由于Ni2+占据了Li+的位置,导致Li+无法进入,正极材料逐渐向前驱体转变。经过一次煅烧除去杂质后,作为前驱体与锂盐均匀混合球磨,再次煅烧,使Li+再次进入到三元材料的晶格中去,减少了阳离子混排数量,重塑了材料层状结构,恢复了其电化学性能。
虽然直接再生法流程简单、方便快捷、成本最小化,但目前工艺单一,无法完全保证再生材料的循环性能,且对再生材料形貌和电化学性能影响因素的研究及机理的探析尚不足,因此,目前难以实现工业化规模化的应用。目前,广泛采取间接再生法对回收正极进行补锂优化再生(见表7)。间接再生法包括水热法[135-136]、固相法[56, 137-138]、共沉淀法[108-109, 139]、溶胶凝胶法[140-141]、微波辅助法[142]等。
固相烧结法[56, 138]工艺简单,若预处理过程获得的粉末混合不均匀会再生出结构缺陷的LiCoO2晶体,导致材料性能不佳。因为活性物质中未完全去除的黏性剂在热解时产生的HF易与原粉末反应生成HCoO2,并进一步歧化反应再生杂质Co3O4,导致正极材料容量和循环性能并不理想。
水热法以其无额外杂质引入等诸多优点,在再生、活化衰减的正极活性物质方面得以推广应用,但是,实际生产条件必须是高压。SHI等[135]认为,采用共晶熔融盐作为锂源可以有效降低再生过程的压力条件,并首次实现在常压条件下低温共晶熔融盐法再生正极材料,成功将衰减的三元层状材料表面因相变产生的岩盐相(rock salt)转变回层状(layered)结构,同时复原了锂含量、晶体结构、材料表面结构及其电化学性能。该方法也可被用于再生如LMO、LFP等正极材料,以及钠离子基正极材料。该方法在锂电池回收领域具有良好应用潜力,也为能源材料再生苛刻条件的解决提供独特思路。
沉淀法和溶胶-凝胶法是较常用三元正极材料(如NCA、NMC)再生制备方法。多元材料含有多种性质相似、难以分离的过渡金属,可将冶金回收混合金属溶液直接再生循环制备,避免了复杂的深度分离。再生产物的电化学性能很大程度上受这些金属离子的分布均匀性影响。如果直接采用固相法烧结处理,很难实现这些离子理想混合和均匀分布,难以合成性能和稳定性良好的正极材料;不同于固相法,沉淀法能将金属离子均匀分散在溶液中,并通过沉淀达到原子尺度的液态均匀混合。此外,溶胶-凝胶法也可实现回收到金属离子的均匀混合,从而达到循环利用和再生正极活性物质的目的[141]。
-
无论是锂离子正极材料的初次制备还是修复再生,都是一个繁琐的过程,通常需要几天到一周的时间。而使用微波方法可同时加速几种电极材料的合成,合成时间甚至减少至30 min[142]。此外,制备材料的过程不仅可以单独使用微波处理[143-144],关于微波辅助溶胶-凝胶法[145]、微波辅助水热/溶剂热法[146]的报道也有很多。与常规的材料再生处理方法相比,微波处理有许多优势,包括但不局限于加热速度快、省时节能、能将能量直接耦合到材料中等优点。作为一种新兴的高效能源手段,微波可辅助正极材料进行优化再生,甚至可以利用微波辅助法将间接回收的金属氧化物合成新纳米正极材料,以提高结晶性和控制更佳的晶型结构。因此,有效微波技术不仅是正极材料再生过程中的一种高效低成本的方法,而且还为合成传统方法无法获得的新结构材料提供了可能性。
虽然使用微波辅助进行正极材料再生的研究尚不足,但微波辅助进行正极材料的制备研究已较成熟,可为微波辅助材料再生提供参考。表8是微波制备正极材料归纳的对照表,其中列出了制备不同正极材料使用的不同微波方法、处理过程及结果参数。
基于微波加热辅助固相烧结工艺,赵新兵等[157]在废正极材料中添加铁源和磷源,以及可选择性地添加新的LFP原料,再加入碳源高能球磨,经干燥后得到回收废料预烧料,将回收废料预烧料在保护性气氛下利用微波烧结或固相烧结处理得到LFP。该制备过程环保、无污染,简单易行,产品利用率高,工序简单,可做到生产过程零排放。相对于单纯的固相法耗能耗时、颗粒不均匀、稳定性不佳等缺陷,通过微波处理可有效改良失效活性物质的复原再生效果。
LI等[158]认为,在微波处理过程中活性碳催化的还原气氛是材料再生效果提升的关键。活性碳作为微波吸收体,不仅可以聚集微波能产生局部热点,加快升温速率,产生碳热还原效应,还能在升温时氧化形成还原气氛。有研究结果显示,在这种气氛中再生LFP正极活性物质,有利于防止Fe2+氧化,合成出单相纯净的LFP。同时,相比于固相高温合成材料,通过应用微波技术可大大缩短合成时间。当处理时间为14 min时,使用充电率0.25 C进行充放电循环测试,放电比容量可达96 mA·h·g−1,整体性能毫不逊于同等方法下使用固相高温再生的正极材料。综上所述,微波技术的有效应用在简化工艺和工业化方面具有较好前景。
合理有效地应用微波技术使合成传统方法无法获得的新结构材料成为可能。近年来,微波技术在循环合成新的正极替代材料方面有了新突破。如被认为是未来正极新材料的磷酸钴锂(LCP),其操作电压比当前采用的LFP要高,其能量密度也较高,可达到800 W·h·kg−1[159-161]。然而,这种颇具前景的正极材料产量很少,属于能源密集型产品,其生产效能并不高,且使用传统合成方法需要900 ℃以上极高温度条件[162]。此外,在高温条件下,合成出材料的结晶尺寸也各有不同,材料晶体的结构与形貌很难控制。
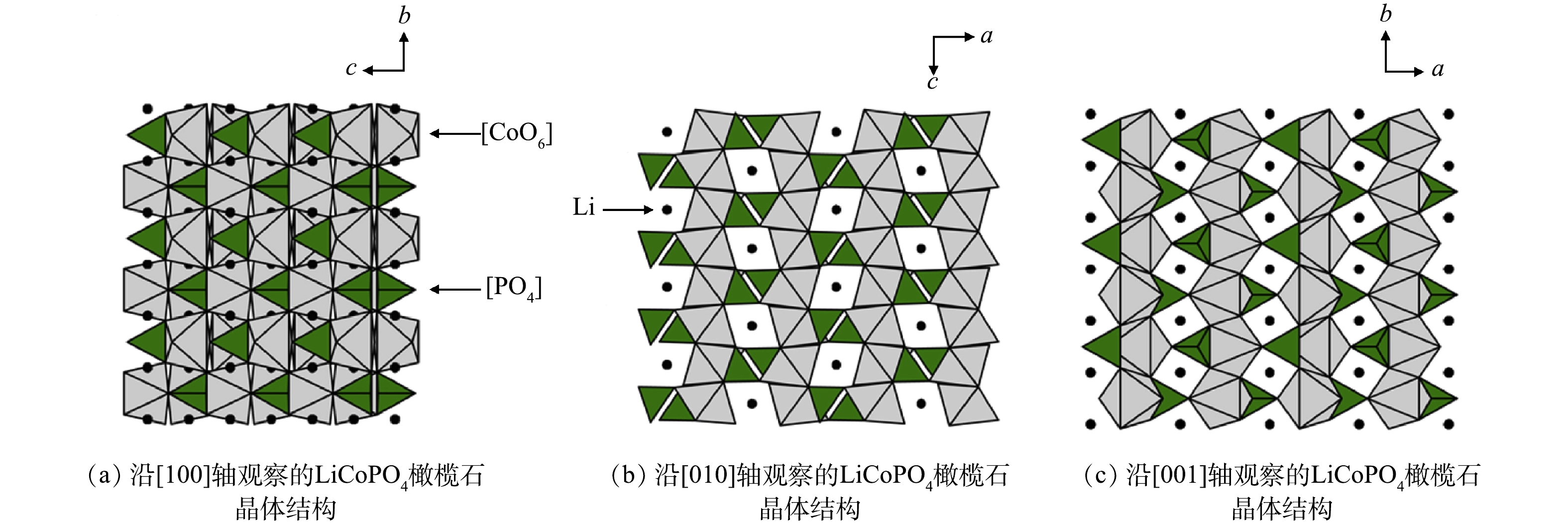
针对以上问题,LUDWIG等[162-163]采用一步微波辅助溶剂热法,在中等温度(250 ℃即可触发结晶反应)下耗费30 min成功生产出高纯度LCP。采用体积比为1∶1的水/醇混合溶剂,借助微波独特的加热方式可控制材料晶体的粒径和形貌,以优化材料的电化学性能。微波辐射下合成的LiCoPO4橄榄石晶体结构如图8所示。锂离子扩散性能增强的各向异性晶体取向具有较高的初始放电容量和重量能密度(在0.1 C和677 W·h·kg−1下获得的LCP最高可达141 mA·h·g−1),具有良好的倍率性能,循环寿命为20次。
4.1. 微波对正极材料直接再生与间接再生的影响
4.2. 微波技术在正极材料再生过程中的应用
-
1)相比于传统工艺,在预处理热解过程中,微波辅助热解诱导高能位点,通过靶向换能机制大幅提高降解速度,可最大限度地缩短处理时间、降低能耗;通过选择性热解,可有效减少重分子、有毒分子气体等危险产物的生成;通过有效耦合利用氧化性物质,在等离子体产生自由基的催化作用下,能够轻易分解有机黏结剂,还能在一定程度上活化正极材料。
2)在金属分离和资源化回收过程中,正极粉末金属氧化物和负极回收的石墨能作为良好的微波吸收剂,有效吸收微波能,促进热法冶金过程中的碳热还原反应,高效实现金属单质或其简单化合物的整体回收;微波辅助技术可大幅度简化湿法冶金步骤,降低对还原剂的需求,提高粉末溶解度,在有效节约成本的同时快速回收金属,从而提高经济效益。
3)在材料再生和循环利用过程中,微波辅助再生技术可通过辅助高温烧结提高材料的结晶性,修复循环衰减后的成分缺陷和结构缺陷,利用独特的加热特点调控设计性能更优化的新晶体形态,实现电池正极材料活性组分的资源化回收利用。
-
1)微波加热可即时控制功率升降的速率,但却难以实时准确地测量和控制微波反应过程中正极材料内部不同位置的温度,因而难以控制实时加热速率。这是因为:外置的红外温度测量设备只能读取加热系统的表面温度,且光纤探头的测温上限通常低于微波升温的温度范围[164]。当加热速率失控时,会对微波对材料的处理过程以及产物质量产生较大的影响。
2)微波加热的独特方式在带来效益的同时,也会一定程度上引起一些负面影响。例如,在较高微波场强度下或者当材料过度暴露于微波场时,由于正极材料的复杂物质组成及不同组分的辐射-热转换机制不同,容易产生局部“热点效应”造成不均匀加热现象[29],尤其是金属铝箔较容易会受到微波自热作用,在短时内产生局部放电和高温的物理现象,从而导致其熔化、破碎以至黑色活性物质被金属铝污染[38]。
因此,需要合理优化微波处理过程中温度监测和时间控制,以避免材料的损耗和对产物的非预期影响。基于上述1)、2)两点,通过定制微波回转反应器或微波流化床对正极材料进行搅动,并耦合配备更精准、快速的温度-功率联控程序,可有效解决物料测温、加热不均匀和热失控问题。此外,采用连续可调的新型微波源,如固态微波源系统,可实现对微波源输出功率和处理时间的精细控制。
3)如果材料中的吸波物质残留在腔内,会分散和消耗微波能并在腔内形成高温热点,这会很快损坏反应器和腔体,因此,每次进行处理过后需要进行清理腔体。此外,为了高温保护,始终需要在持续工作的微波源周围提供水循环。因此,使用微波前后应做好实验准备,注意操作规范,以避免不必要的损失和伤害。
-
针对微波辅助正极材料回收工艺在实验室和工程应用中出现的缺陷和问题,一些研究者通过数值模拟对微波热处理的热效应和腔内温度分布进行了仿真模拟、预测推断和验证研究,以更深层次地认知微波对整个加热体系的影响因素,从而进一步改进与优化工艺运行条件。
通常情况下,加热材料的复介电常数会随温度、时间、空间发生变化,且反应过程伴随着热量的转移,体系温度难以恒定。因此,微波过程数值模拟比一般过程更为复杂。CHATTERJEE等[165]综合考虑了转动、自然对流、微波功率等因素对微波加热旋转圆柱体温度分布的影响,证明了在保证微波源均匀的情况下,旋转的物体的加热温度是均匀的。DATTA等[166]通过数值分析发现,自然对流对微波加热效果有一定影响,并会对加热材料产生明显的温度差异。ROUSSY等[167]研究了微波流化反应器中气固混合态材料的温度分布。BASAK等[168]研究了微波对二维圆柱形“固-液”相变过程温度场的影响,发现在固体表面热非均匀性及热点更为明显。CHA-UM等[169]分析了对不同介电常数液体进行微波加热时的温度分布,该研究对微波辅助水热/溶剂热正极材料再生制备有一定指导意义。此外,CHA-UM等[170]还以水和介孔材料为对象,研究了体积、介电性质、空间位置对能量损耗及升温的影响。
值得一提的是,当有些材料处于某种状态时,对微波功率较为敏感,导致功率的微小变化都会引起材料温度发生较大改变,这被称为“热失控现象”。大量研究表明[171-172],热失控对于微波技术的应用是不利的,会严重烧毁材料,甚至损坏处理容器。许多研究者[173-176]也从数值计算入手,讨论了微波加热的热失控现象,并提出了热失控的有效解决措施。其中,KRIEGSMANN等[173]提出,使用“先大后小”的变功率加热方式可以有效避免热失控。微波热处理过程的数值模拟对于认识微波的特殊热效应,控制内部热点、避免热失控,解决工程实践问题,以及确保技术的有效性和安全性等具有重要意义和参考价值。
5.1. 微波辅助正极材料回收的优势
5.2. 微波辅助正极材料回收的局限性
5.3. 微波处理工艺的数值模拟与工况优化
-
微波热处理技术以其选择性、整体性、即时性、瞬控性等特点,在优化杂质降解反应、增强降解过程动力学、强化碳热还原过程、提升浸出效果、高效再生利用等诸多方面体现优势,在回收体系各阶段呈现正面效益,被认为是资源化处理废锂电正极材料有前途的应用之一。尽管目前微波技术在锂电材料回收再生领域实现工业化规模化应用仍有一段距离,但微波技术已经在实验室基础研究中展现出突出的优势。若将微波技术与直接再生法结合,充分发挥两者的优势,将在很大程度上简化回收过程,加快整个锂电绿色回收处理行业的规模化进程,从而对锂电正极材料回收体系的未来发展提供有益参考,对经济、资源和环境保护也具有重要意义。





 DownLoad:
DownLoad: