-
四环素类抗生素是由人工合成或者在放线菌繁殖过程中产生的代谢产物中被提取的一种能够抑制或者杀灭微生物的化合物,其中盐酸四环素(tetracycline hydrochloride, TCH)是一种典型的四环素类抗生素,被广泛应用于制药和畜牧养殖等行业[1-4]。由于TCH不能被动物完全代谢,未代谢的TCH可通过粪便和尿液排泄到水环境中,对土壤和水体会造成一定程度的污染。当人类饮用含有一定浓度TCH的水时,会出现胃肠道刺激、呕吐、腹泻和肾功能衰竭等症状[5-6]。因此,有效去除土壤和水体中的TCH是一个亟待解决的问题。
目前报道的对废水中抗生素类污染物的处理方法包括生物法、光催化法、吸附法和膜分离法等[7-8]。其中,光催化技术因其成本低、环境友好、降解效率高和反应稳定性好等优点受到广泛关注[9]。光催化原理为:当半导体材料被能量大于或等于其禁带宽度的光子激发时,在其价带和导带的位置分别形成具有较强氧化性的光生空穴和较强还原性的光生电子,其又可与水、OH−、氧气分子等反应生成羟基自由基(∙OH)、超氧自由基(
⋅O−2 )等活性含氧物质。在光生电子、光生空穴或活性含氧物质的作用下,难以降解的大分子有机污染物会被降解成低毒或无毒的小分子化合物,甚至直接降解成水和二氧化碳等无机物[10]。TiO2具有催化活性高、价格便宜、稳定性好、无毒及无二次污染等优点,被广泛应用于光催化领域[11-12]。近年来,研究者制备了多种不同形貌的纳米TiO2材料。其中,三维分层结构在增加比表面积和提高光收集能力方面具有优异性能,已受到广泛关注[13]。海胆状TiO2纳米结构作为一种典型的三维分层结构光催化剂,已经被应用于光催化降解染料和酚类有机化合物等。FAN等[14]通过钛酸四丁酯的水解作用制备了具有高光催化活性的海胆状TiO2纳米结构,在紫外光照射下对RhB进行降解取得了显著的效果。YU等[15]通过草酸钛钾的水解作用制备了海胆状TiO2纳米结构,对甲基橙和苯酚的降解效果明显优于商用P25。GUO等[16]研制了碳量子点均匀修饰的海胆状TiO2微球,其可以高效降解苯酚和亚甲基蓝。然而,在TiO2纳米材料中光生电子-空穴对的复合率较高,制约了其广泛的实际应用,如何进一步提高其性能成了一大难题[17]。已有研究[18-19]表明,TiO2与其他半导体(如ZnO、Cu2O、CeO2等)耦合构建异质结构是抑制光生电子-空穴对快速复合的有效方法。苏海英等[20]以TiCl3为TiO2钛源,二聚氰胺为g-C3N4前驱体,制备了g-C3N4/TiO2复合材料,其对布洛芬具有显著的降解效果。YU等[19]制备的空心TiO2@g-C3N4/Co3O4复合材料对四环素和甲基橙具有较好的降解效果,最终在60 min内降解了91.6%的四环素和97.8%的甲基橙。除此之外,吸附与光催化的协同作用能够更好地去除土壤和水体中的有机污染物[21-23]。因此,构筑具有良好吸附能力的异质结构是进一步提高TiO2纳米材料光催化性能的有效方法。基于此,我们制备了一种用于高效去除废水中TCH的海胆状TiO2/ZnO异质结复合材料,通过SEM、TEM、XRD、XPS及气体吸脱附等方式对复合材料进行了表征分析,并探索了ZnO的负载量对光催化性能的影响;随后检测了TCH降解过程中的中间降解产物变化,并基于自由基淬灭实验推测了TCH降解过程中可能的反应机制。
全文HTML
-
所用材料及试剂:二甘醇,草酸钛钾,尿素,六水合硝酸锌,六亚甲基四胺,TCH,对苯醌(BQ),异丙醇(IPA),乙二胺四乙酸二钠(EDTA-2Na) (AR,阿拉丁试剂);去离子水(实验室自制)。所用仪器:聚四氟乙烯反应釜(南京瑞尼克);X射线衍射仪(XRD,X'Pert PROMPD,Panalytical B. V.);X射线光电子能谱仪(XPS,美国Thermo公司,ESCALAB250Xi);扫描电子显微镜(SEM,德国卡尔蔡司,Gemini500);透射电子显微镜(TEM, 日本JEOL公司,JEM-2100F);气体吸附仪(美国康塔,Autosorb-IQ3);紫外可见分光光度计(上海光谱仪器,SP-1920UV);300 W氙灯光源(北京中教金源,CEL-S350);总有机碳/总氮分析仪(德国耶拿,multi N/C 3100);液相色谱-飞行时间质谱仪(美国waters,ACQUITY UPLC Premler XE)。
-
在100 mL烧杯中加入20 mL去离子水、60 mL二甘醇、0.8 g草酸钛钾和2.4 g尿素,并磁力搅拌均匀,然后将上述混合溶液转移到聚四氟乙烯反应釜中以180 ℃水热反应12 h,所得产物经离心、去离子水和无水乙醇依次洗涤、60 ℃干燥过夜,再置于马弗炉中,以5 ℃·min−1升温至500 ℃并保温煅烧1 h,最后获得海胆状TiO2微球。
-
在100 mL烧杯中加入一定质量的六水合硝酸锌、20 mL 0.05 mol·L−1六亚甲基四胺的水溶液和60 mL去离子水搅拌均匀,再加入0.2 g已经制备好的海胆状TiO2微球,继续搅拌均匀,然后转移到聚四氟乙烯反应釜中100 ℃反应3 h,所得产物经离心、去离子水和无水乙醇依次洗涤、60 ℃干燥过夜,最后获得TiO2/ZnO异质结复合材料(TZ)。通过设置硝酸锌的质量为30、60、90和120 mg,制备出一系列不同ZnO含量的TiO2/ZnO复合物,分别记为TZ30、TZ60、TZ90和TZ120。
-
将10 mg的TiO2微球或TiO2/ZnO复合材料投入20 mL质量浓度为100 mg·L−1的TCH水溶液中(催化剂用量为0.5 g·L−1)。在黑暗条件下,磁力搅拌1 h实现光催化剂对TCH的吸附。吸附剂的吸附量根据式(1)进行计算。
式中:q为吸附剂的吸附量(质量分数),mg·g−1;m为吸附剂的投放质量,g;V为TCH溶液的体积,L;C0为TCH的初始质量浓度,mg·L−1;C1为反应一段时间后溶液中TCH的质量浓度,mg·L−1。
达到吸附-解吸平衡之后,用装有中心波长为365 nm紫外带通滤光片的氙灯作为紫外光光源照射4 h,每隔0.5 h取出0.3 mL样品,使用装有0.22 μm滤膜的注射器过滤掉光催化剂,然后利用紫外可见分光光度计在357 nm波长下测量TCH溶液的吸光度,依此计算其质量浓度。TCH的去除率根据式(2)进行计算。最后,通过准一级反应动力学方程(式(3))对TCH的光降解过程进行研究。
式中:R为去除率;C0为TCH的初始质量浓度,mg·L−1;C2为光照一段时间后溶液中TCH的质量浓度,mg·L−1。
式中:k为反应速率常数,min−1;t为光照时间,min。
1.1. 实验材料与测试仪器
1.2. 海胆状TiO2微球的制备
1.3. TiO2/ZnO异质结复合材料的制备
1.4. 光催化实验
-
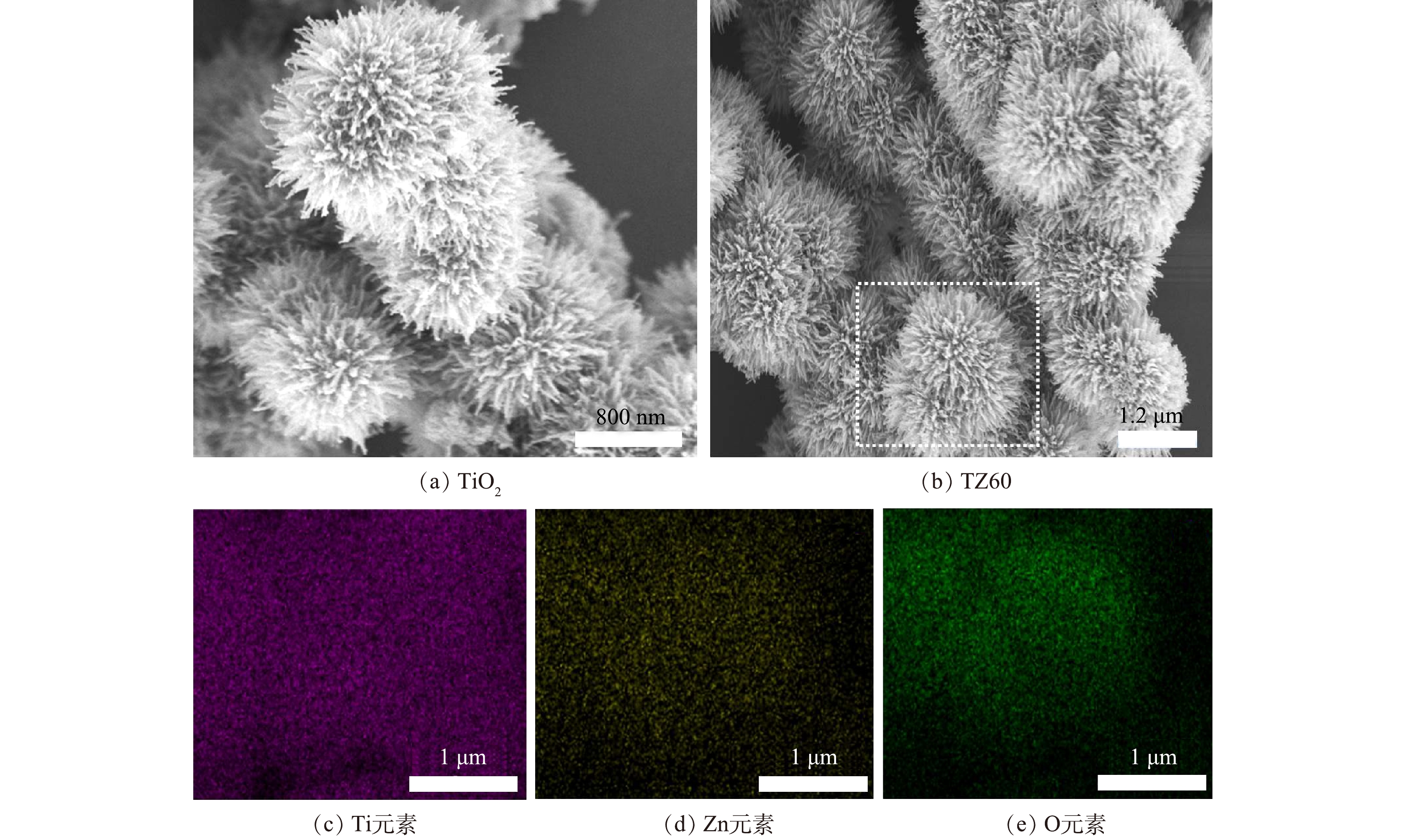
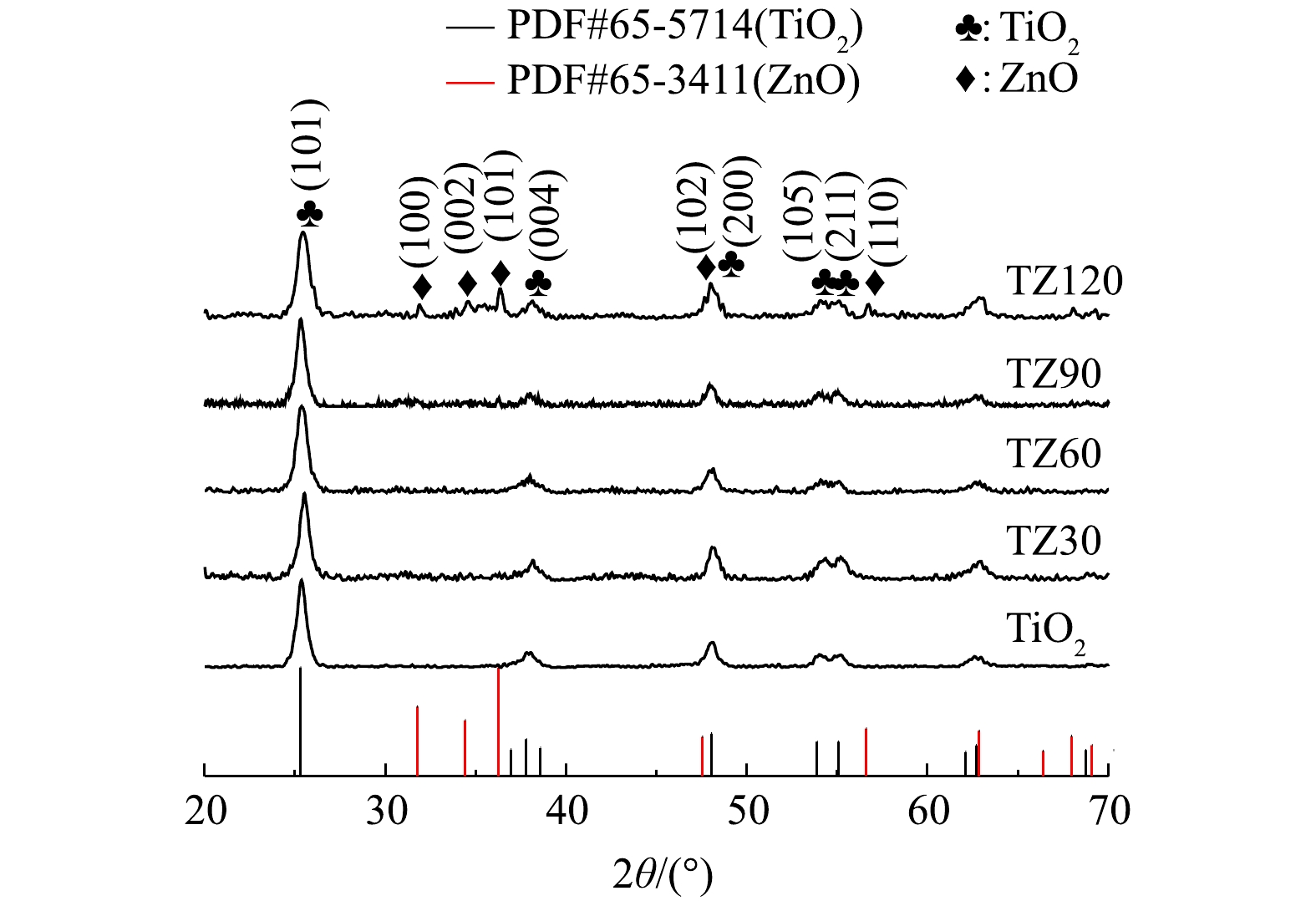
如图1所示,在TiO2微球和不同TiO2/ZnO复合材料的谱图中均检测到良好的晶面:25.3°(101)、37.8°(004)、48.0°(200)、54.0°(105)、55.1°(211);其与锐钛矿TiO2的标准卡片上的衍射特征峰位置一致,说明所有制备的材料中TiO2均为良好的锐钛矿晶相。然而,在复合材料TZ30、TZ60和TZ90中没有检测到ZnO的衍射特征峰,只在ZnO含量最高的TZ120里检测到了较弱的纤锌矿相ZnO的特征峰,位于31.9°、34.7°、36.2°、47.5°和56.6°,分别对应晶面(100)、(002)、(101)、(102)和(110)。这是因为ZnO的含量较低,故导致XRD仪器不容易检测到ZnO的特征峰。
-
因为ZnO的含量较低,故在XRD谱图中不容易判断ZnO的存在,因此,进一步通过XPS测试来进行元素及元素化合态的表征,结果如图2所示。由图2(a)可见,复合材料TZ60的XPS图中出现了C1s、Ti2p、O1s、Zn2p的特征峰,说明TZ60中含有Ti、Zn、O 3种元素,C元素的存在是由于XPS测试系统中会带来C元素的污染。图2(b)~(d)分别为Zn2p、Ti2p、O1s的高分辨XPS图像。由图2(b)可以看出,与纯ZnO材料相比,TZ60中Zn2p的2个特征峰均向右偏移了0.3 eV,分别位于1 021.88 eV和1 045.18 eV处,其对应氧化态Zn2+的2p3/2与2p1/2态。这说明所制备的复合材料中TiO2和ZnO形成了界面效应[24]。由图2(c)可以看出,与纯TiO2相比,TZ60中Ti2p的2个峰均向右偏移了0.3 eV,分别位于458.48 eV和464.18 eV,其对应氧化态Ti4+的2p3/2与2p1/2态。由图2(d)可见,O1s的特征峰由材料的晶格氧和表面羟基中的氧引起,但晶格氧起主导作用[25]。与纯TiO2相比,TZ60的峰(529.7 eV)向右偏移了0.4 eV。综合上述结果可判断,TiO2/ZnO异质结复合材料被成功制备。
-
如图3(a)所示,所制备的TiO2纳米结构的外部分布着许多纳米带毛刺,整体呈类海胆的球状,直径为1.7 μm左右。由图3(b)可以看出,所制备的复合材料保留了原TiO2的海胆状形貌,但可能由于ZnO的粒径较小,故无法明显观察到ZnO纳米颗粒,为此对虚线所围区域进行了EDS能谱和元素分布测试来判断Zn元素的存在及分布情况。由图3(c)~(e)可以看出,TZ60中含有Ti、Zn、O元素,这与XPS的结果一致,再一次验证了TiO2/ZnO复合材料的成功制备。
-
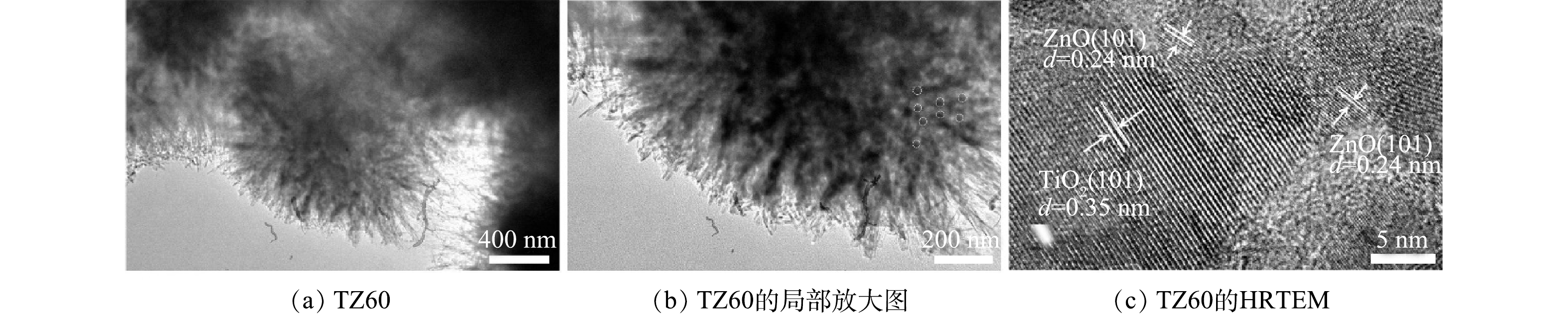
由图4(a)和图4(b)可以看出,TZ60为海胆状微球,与SEM表征结果吻合;微球的内部由小纳米颗粒聚集而成,外部由纳米带搭接而成。由图4(c)可以看出,存在2种不同的晶格条纹,条纹间距分别为0.35 nm和0.24 nm,分别对应于锐钛矿相TiO2的(101)晶面和纤锌矿ZnO的(101)晶面。综合上述SEM和TEM表征结果,可以判断ZnO纳米颗粒紧密地分布在海胆状TiO2微球的表面,说明TiO2/ZnO复合微球被成功制备。另外,因ZnO的导带和价带电位均比TiO2的导带和价带电位更负[25],TiO2与ZnO接触后形成II型异质结,因此,所制备的复合微球可以有效抑制光生电子-空穴对的复合并延长其寿命。
-
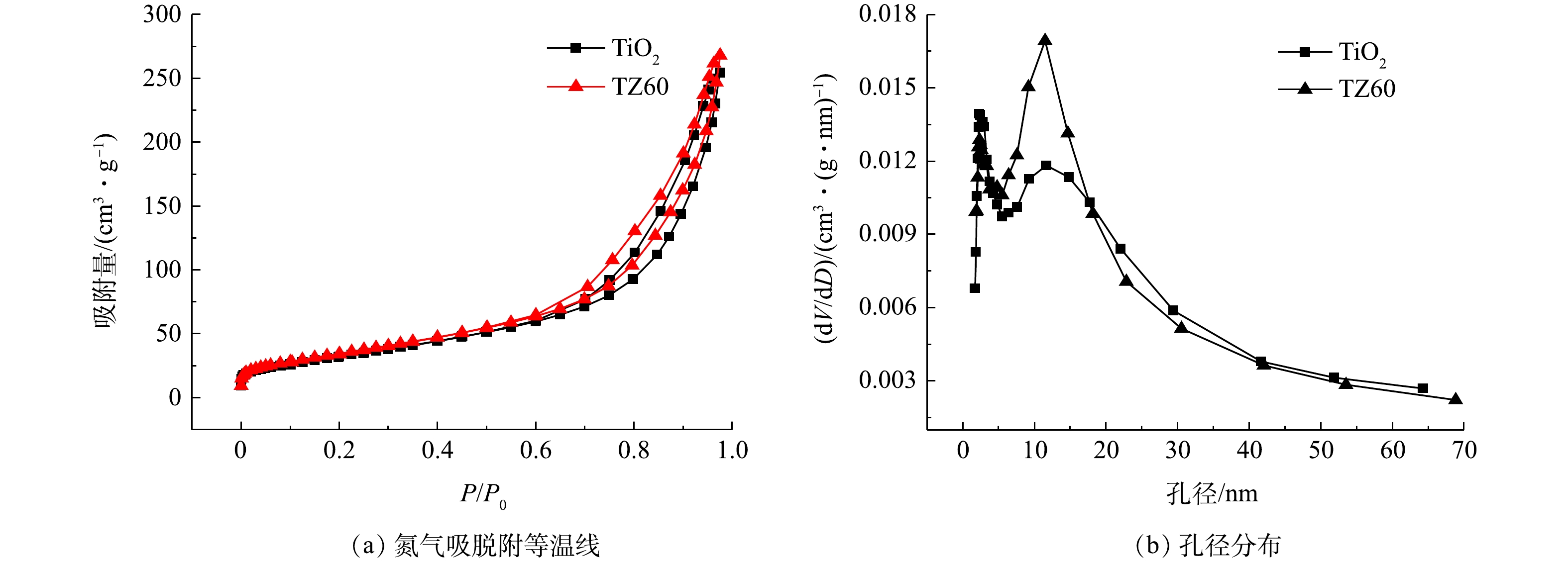
如图5(a)所示,进一步测试了所制备的TiO2和TZ60复合微球的氮气吸脱附等温线,可以发现其均是典型的IV型等温线,并在相对压力为0.5处出现了明显的毛细管凝聚现象。由于毛细管凝聚作用,吸脱附等温线不重合,呈现出H3型回滞环[26]。这与SEM图像展示的海胆状微球外部的纳米带之间形成的狭缝孔相一致。通过计算得到,TiO2和TZ60微球的BET比表面积分别为123 m2·g−1和131 m2·g−1,孔体积分别为0.40 cm3·g−1和0.42 cm3·g−1(表1)。图5(b)为TiO2和TZ60微球的BJH模型孔径分布,可以看出他们的孔径分布大致相同,均具有较宽的范围,并在3 nm和11 nm左右出现峰值,这归因于微球的内部是聚集的小纳米颗粒,外部是交织的纳米带。较大的比表面积可为光催化反应提供更多的活性位点,介孔分布能够保证有机污染物分子向催化剂的内部扩散,使得所有活性位点均能得到有效利用,从而提升光催化性能[13]。
-
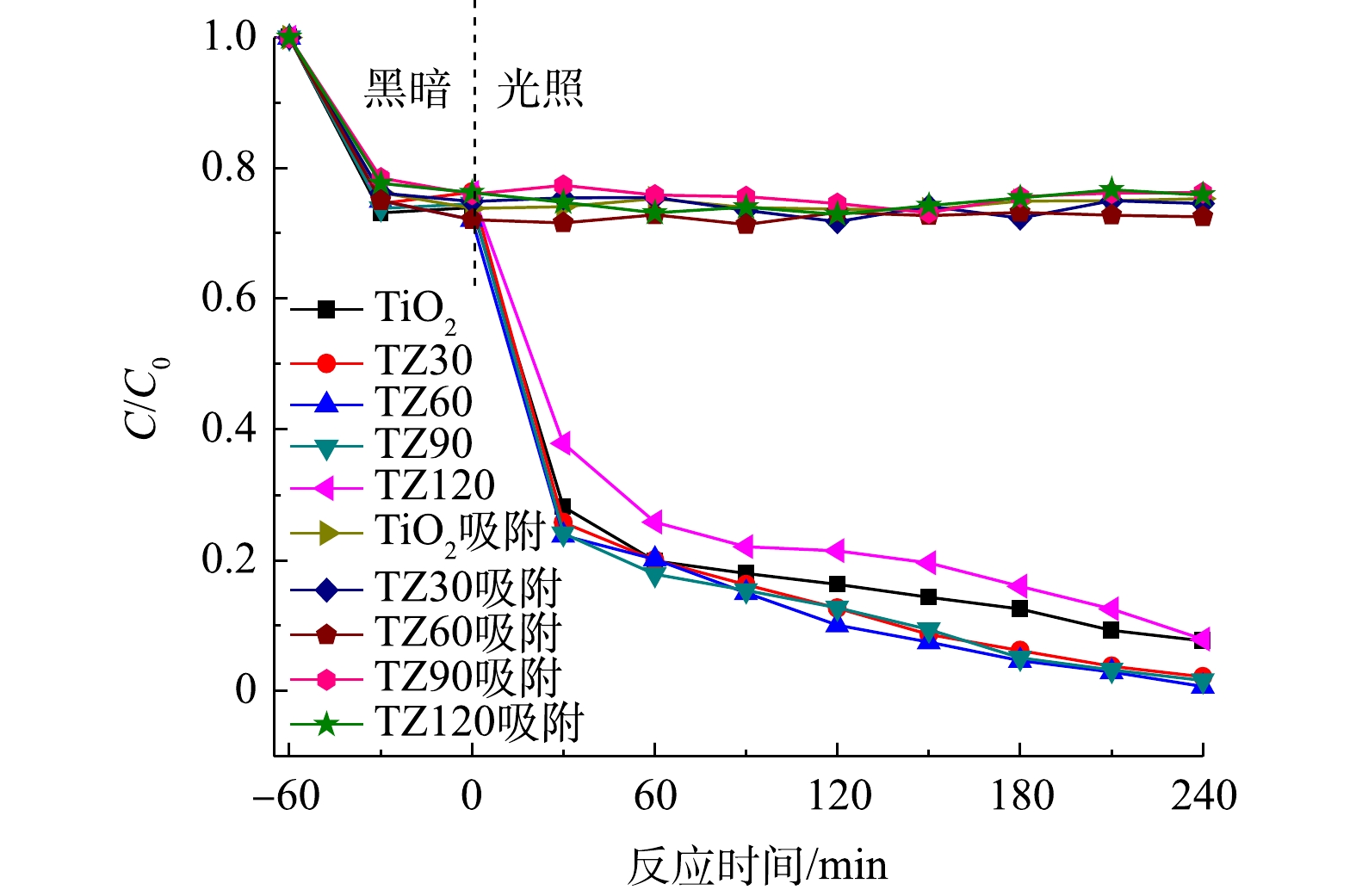
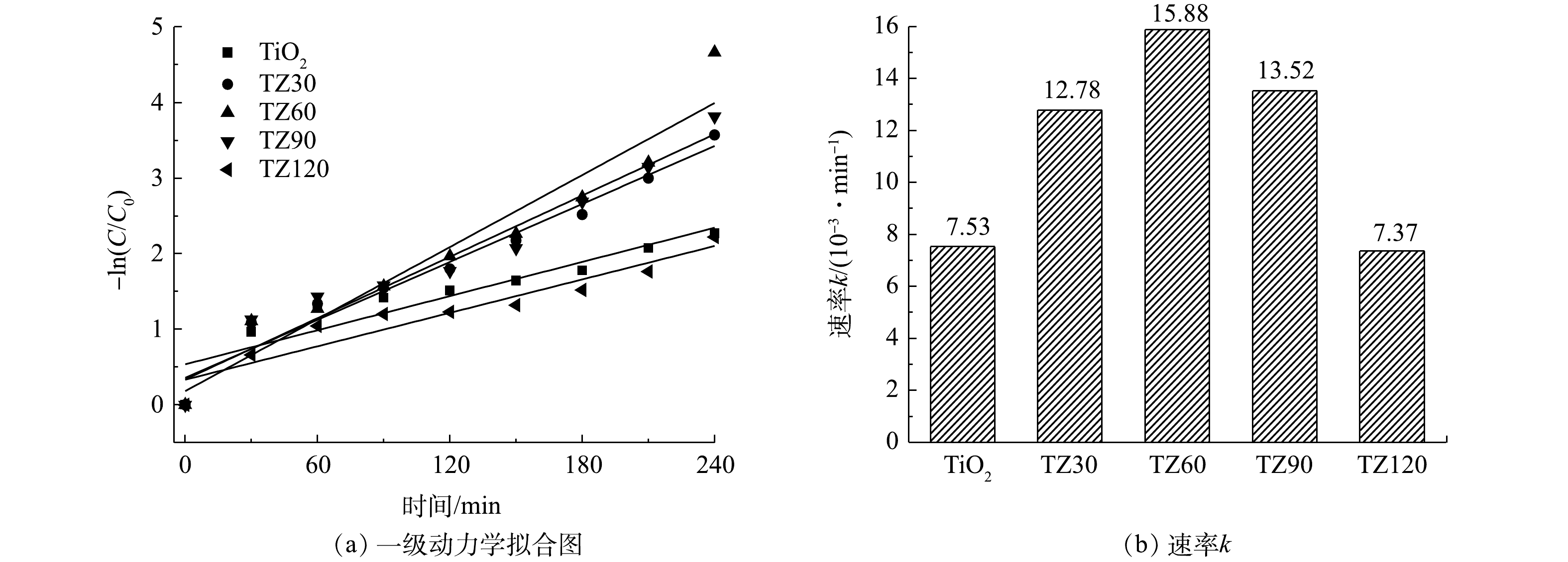
由图6可以看出,所有材料对TCH均具有良好的吸附能力,并且相差不大。根据式(1)计算可知,平均吸附量为51 mg·g−1,这归因于材料的较大比表面积。但各材料对TCH的光降解能力有一定差异,其中TZ60具有最强的光催化降解能力。
在光照之后,TCH的去除率通过式(2)进行计算。在光照4 h之后,所有的材料对TCH均具有良好的光降解效果。TiO2微球对TCH的去除率约为92%;随着ZnO纳米颗粒与TiO2微球的复合,ZnO与TiO2之间形成异质结加快光生电子-空穴对的分离,从而进一步提高对TCH的去除率,其中TZ60的降解效果最佳,TCH去除率高达99.3%;随着ZnO含量的进一步增加,TCH去除率反而出现下降的趋势,这可能是由于复合过多的ZnO纳米颗粒会减少材料表面降解TCH的活性位点[27]。
采用准一级反应动力学方程(式(3))对TCH的光降解过程进行了模拟,拟合曲线如图7所示。可以看出,TZ60的反应速率常数最优,约为0.015 88 min−1。上述结果表明,TZ60复合微球具有最佳的光催化降解性能,ZnO的负载量最适宜,ZnO与TiO2之间形成的异质结能够有效加快光生电子-空穴对的分离,抑制光生电子-空穴对的复合,进而协同提升降解效果。
-
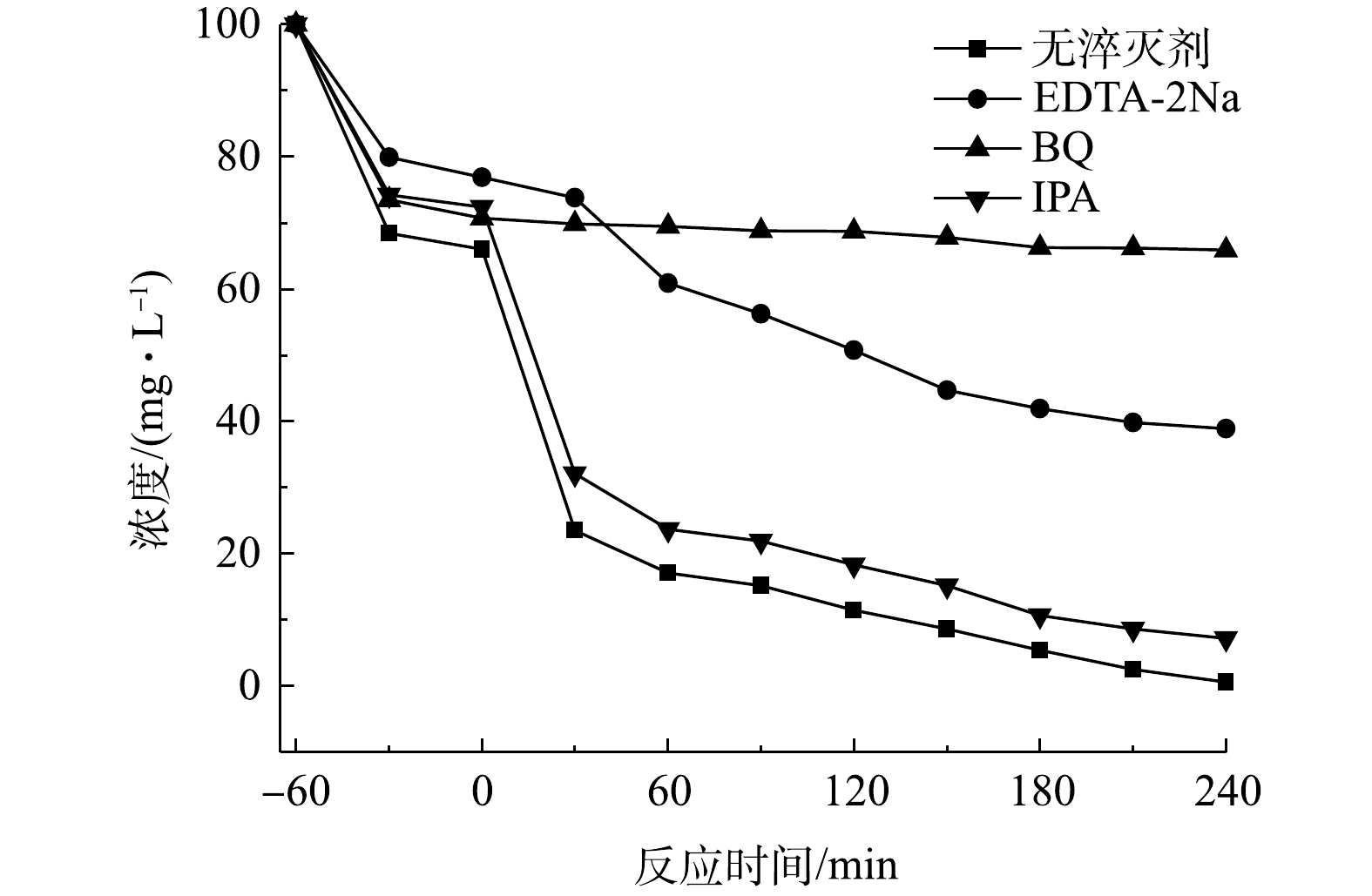
以上结果说明,所制备的TiO2/ZnO复合材料具有优异的光催化降解性能。为了进一步研究其光催化机理,通过自由基淬灭实验来揭示TiO2/ZnO复合材料在紫外光照下可能的降解机理。如图8所示,当加入空穴(h+)的淬灭剂EDTA-2Na和超氧自由基(
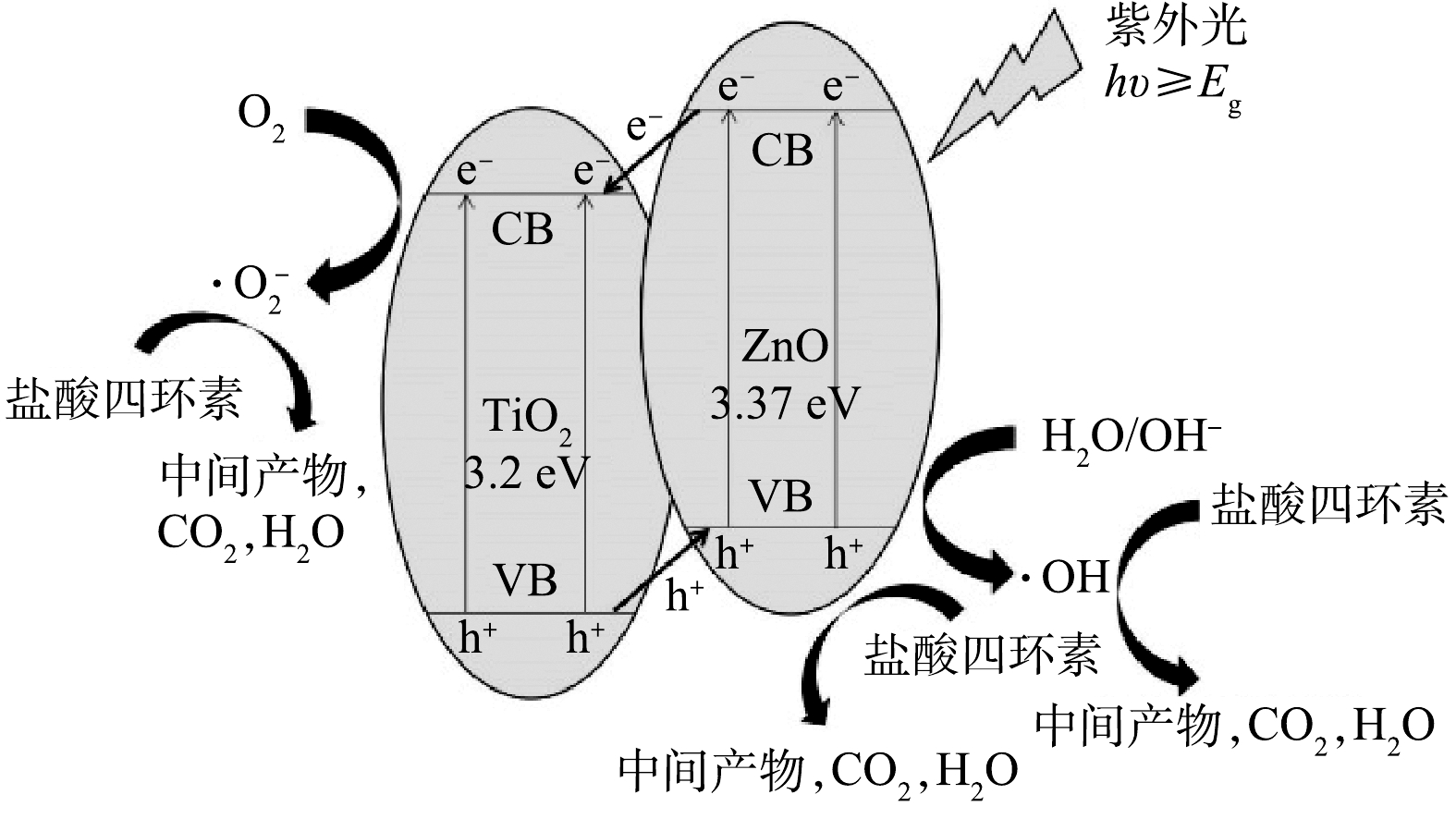
⋅O−2 )的淬灭剂BQ时,对TCH的光降解效果均有明显的降低,而加入羟基自由基(∙OH)的淬灭剂IPA时,光降解效果仅有轻微降低。上述结果说明,在反应体系中h+和⋅O−2 是主要的反应活性物质,∙OH也起到了重要的作用。图9为TiO2/ZnO复合材料光催化降解TCH的机理图。由于ZnO的导带和价带电位比TiO2的导带和价带电位更低,因此,TiO2与ZnO接触后形成II型异质结。在紫外光照下,ZnO和TiO2均会产生光生电子-空穴对。由于异质结的作用,ZnO导带上的电子(e−)很容易转移到TiO2导带上,TiO2价带上的h+很容易转移到ZnO价带上,这样就实现了光生电子-空穴对的快速分离,从而有效抑制光生电子-空穴对的复合[28]。TiO2导带上的e−可以与其表面吸附的氧分子反应,形成
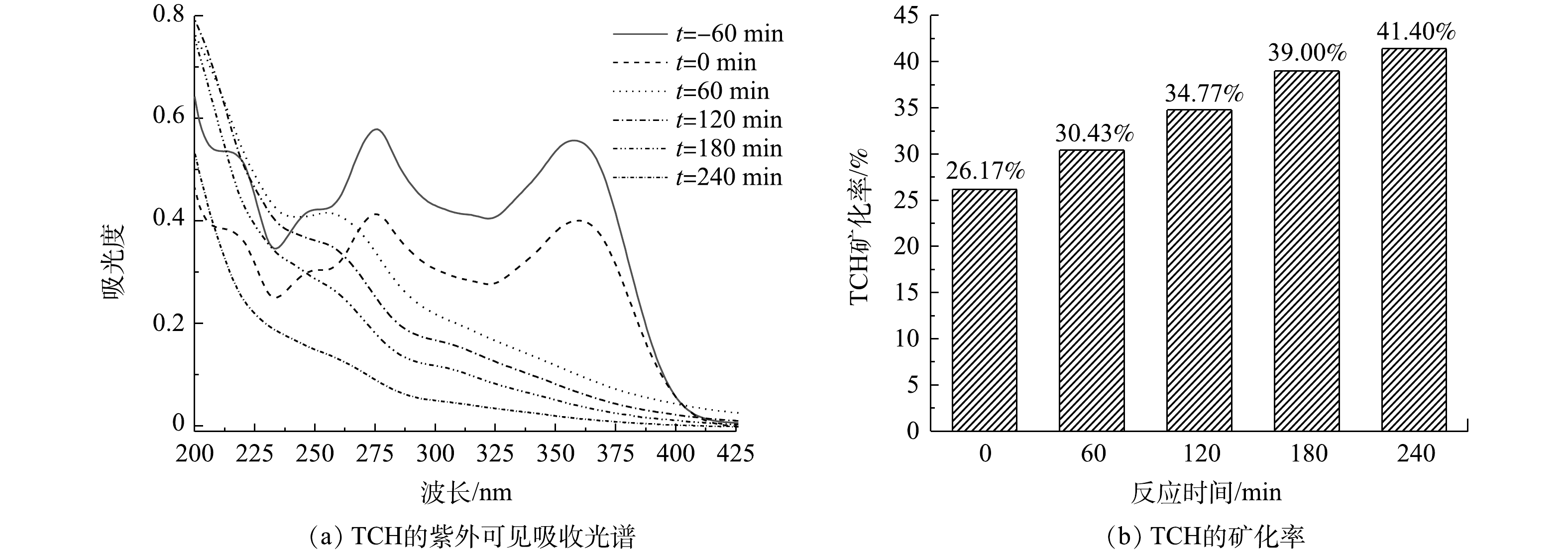
⋅O−2 ,ZnO价带上的h+能够与有机物、水、OH−等反应,形成∙OH。光生电子-空穴对的快速分离产生更多的h+和e−,进一步促进⋅O−2 和∙OH的形成,从而改善催化剂的光催化性能。图10(a)为光照不同时间后,TCH溶液的紫外可见吸收光谱。可以看出,随光照时间的增加,TCH的特征吸收峰(357 nm)的强度逐渐降低,位于275 nm和220 nm处的吸收峰消失,并在260 nm附近出现了新的吸收峰,表明TCH得到有效降解,并且同时生成了新物质。另外,测量了TCH溶液的总有机碳(TOC)的含量,并计算了TCH的矿化率,结果如图10(b)所示。可以看出,随着光照时间的增长,TCH的矿化率逐渐增加。光照4 h后,TCH的矿化率为41.4%,结合图6所示的光降解效果,表明仅有41.4%的TCH被矿化为CO2、H2O等无机物,剩余部分均被降解为小分子有机物。
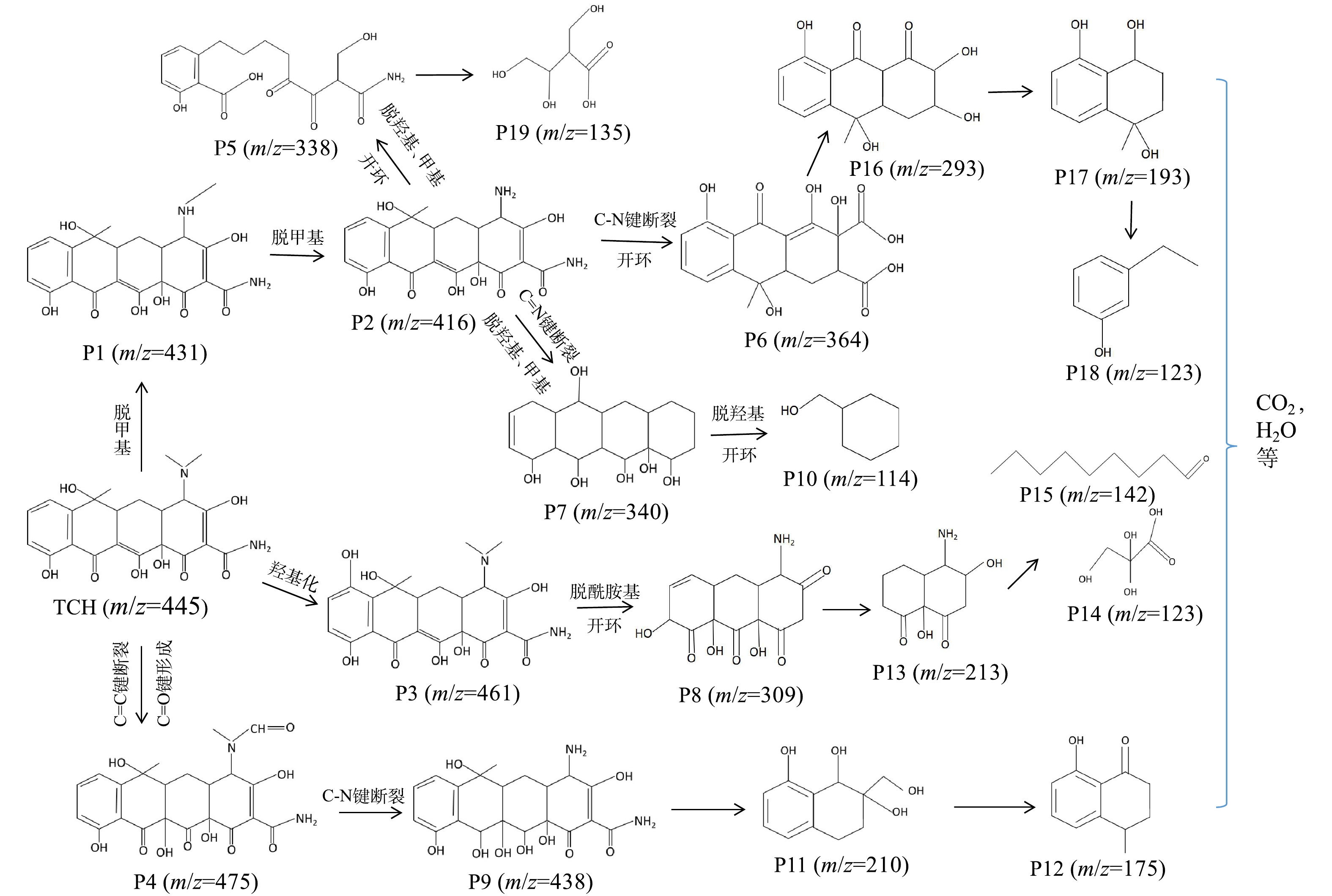
为了研究TCH的降解途径,利用液相色谱-飞行时间质谱仪检测了不同时间光照后TCH溶液中中间降解产物的质荷比。分别检测到质荷比(m/z)为475、461、438、431、416、364、340、338、309、293、210、193、175、142、135、114等一系列中间降解产物,依此可以推断TCH的可能降解途径。如图11所示,TCH的反应路径有3条:脱甲基生成中间产物P1(m/z=431)和P2(m/z=416),羟基化生成中间产物P3(m/z=461),碳-碳双键断裂和碳-氧双键形成,生成中间产物P4(m/z=475);产物P2会进一步发生脱甲基、脱羟基、开环、碳-氮键断裂等过程形成产物P5(m/z=338)、P6(m/z=364)、P7(m/z=340);产物P3会发生脱酰胺基、开环等过程形成产物P8(m/z=309)。产物P4的碳-氮键断裂形成P9(m/z=438)。最后,上述中间产物会被进一步氧化成小分子有机物、CO2和H2O等。
2.1. XRD表征
2.2. XPS表征
2.3. SEM表征
2.4. TEM表征
2.5. 氮气吸脱附实验
2.6. TCH吸附及光降解效果
2.7. 光催化机理
-
1)制备的海胆状TiO2微球和TiO2/ZnO复合微球具有较大的比表面积和适宜的介孔分布,对TCH均具有良好的吸附能力。
2) 60 mg六水合硝酸锌与0.2 g TiO2微球混合制备的复合微球(TZ60)具有最佳的光催化降解性能,对TCH的去除率最高,且其光降解过程的反应速率常数最大。
3)在催化剂TZ60用量为0.5 g·L−1的条件下,经过4 h紫外光照射后,对初始质量浓度为100 mg·L−1的TCH的去除率和矿化率分别为99.3%和41.4%;反应中,TCH被降解为小分子有机物或矿化为CO2、H2O等无机物,其中h+和
⋅O−2 是主要活性物质。








 下载:
下载: