-
含镍废渣和废水的排放会污染地下水。受镍污染的地下水作为饮用水源时会危害人体健康[1],主要是对皮肤、神经和心脑血管系统等造成损害[2],1990年国际癌症研究所确定Ni(Ⅱ)及其化合物对人体具有致癌性[3]。因此,我国对水中Ni(Ⅱ)的标准也越来越严格,《地下水质量标准》(GB/T 14848-2017)中规定,I类水中Ni(Ⅱ)含量不能超过2 μg·L−1[4]。有研究者发现,Ni(Ⅱ)在长江水体中暴露的质量浓度均值为168.14 μg·L−1,其中采样点最高检出质量浓度为480 μg·L−1,在海河和珠江流域平均暴露质量浓度也超标,分别为11.11 μg·L−1和15.87 μg·L−1[5]。目前,去除水中Ni(Ⅱ)的方法包括化学沉淀法、电解法、离子交换法、膜分离法和吸附法等[6-10]。其中,吸附法操作简单、成本低且不易造成二次污染,是目前去除水中Ni(Ⅱ)的主要方法之一。
目前,用于去除水中Ni(Ⅱ)的吸附剂主要有活性炭[11]、多壁碳纳米管[12]、石墨烯[13]和沸石[14]等。其中,多壁碳纳米管(MWCNTs)是一种表面分布均匀,具有独特中空多层结构和高化学稳定性的新型碳纳米材料,被广泛应用于水中重金属的吸附研究[15]。但MWCNTs作为纳米材料在溶液中易团聚[16],从而导致其对水中Ni(Ⅱ)的吸附效能有限。因此,为了提高MWCNTs在水中的分散性和吸附效果,有必要对MWCNTs进行改性。有研究者发现,改性MWCNTs的最常用方法为表面氧化法[17]。LI等[12]用H2O2、HNO3和KMnO4氧化处理后的MWCNTs去吸附去除水中Cd2+,结果表明,氧化处理后MWCNTs的孔容和比表面增加,吸附能力增强,且经KMnO4氧化后的MWCNTs对水中Cd2+的吸附量最大,为11.0 mg·g−1。FARGHALI等[17]用H2O2和HNO3混合物制备氧化MWCNT,对Ni(Ⅱ)的去除率可达83%。这主要是由于H2O2和HNO3氧化作用可以破坏MWCNT的内管空间,甚至部分打开尖端并将MWCNT分解成较小的碎片,改善了MWCNT的分散性,并且氧化处理后MWCNT表面上的含氧官能团(例如—COOH,—OH和—C=O)的数量大大增加,可以提供更多化学吸附位点。不难看出,氧化改性MWCNTs主要是通过常用的氧化剂,使原本疏水的MWCNTs表面产生大量的亲水性含氧官能基团(羧基、羟基和酯类)[18]。亲水性含氧官能基团一方面能在溶液中发生解离从而降低MWCNTs表面的Zeta电位值,进而增强其对带正电金属离子(Cu2+、Cd2+、Zn2+和Ni2+等)的静电吸引力[19];另一方面可降低MWCNTs颗粒之间的范德华力,从而提高MWCNTs在水中的分散性[20]。同时,为了进一步提高氧化MWCNTs在水中的分散性,有研究人员采用超声的方法,主要通过在溶液中生成的微小气泡瞬间爆破,产生大的能量冲击波使通过范德华力粘结在一起的MWCNTs团族分离[21]。
鉴于上述原因,本研究旨在通过超声氧化的方法研制出一种水中Ni(Ⅱ)的高效吸附剂——超声氧化多壁碳纳米管(MWCNTs-Mn),该方法一方面可提高其在水中的分散性,另一方面可增强其对水中Ni(Ⅱ)的吸附效能。首先,通过单因素变量法优化MWCNTs-Mn的制备条件;然后采用动力学和等温吸附考察了MWCNTs-Mn对水中Ni(Ⅱ)的吸附效能;最后基于表面物化特性表征探究了MWCNTs-Mn对水中Ni(Ⅱ)的吸附机理,以期为水中Ni(Ⅱ)的有效去除方法提供参考。
全文HTML
-
本研究选用的MWCNTs购自深圳市纳米港有限公司,管径为50 nm,使用前需要在60 ℃干燥箱中干燥24 h待用。本研究所用化学试剂有高锰酸钾、硝酸、过氧化氢、六水合硫酸镍、柠檬酸铵、丁二酮肟、EDTA、氨水、碘和碘化钾,均购自北京蓝弋化工产品有限责任公司。
-
本研究分别采用H2O2、HNO3和KMnO4对MWCNTs进行氧化改性。首先,将1.5 g的MWCNTs加入到250 mL一定浓度的氧化剂中,将混合液封口后,在恒温水浴超声仪中超声分散,设置水浴温度为15~35 ℃、超声时间为1.5~2.5 h,然后将混合液抽滤,得到固相的超声氧化MWCNTs,用去离子水洗净后置于60 ℃恒温干燥箱中干燥24 h,固体样品研磨待用。
为了进一步提高超声氧化MWCNTs对水中Ni(Ⅱ)的吸附量,本研究优化了其制备条件,主要包括氧化剂种类(H2O2、HNO3和KMnO4)、氧化剂投加量(2.10、1.43、0.75 g)、超声氧化时间(0.5、1.5、2.5 h)和超声氧化温度(15、25、35 ℃)。
-
本研究中动力学和等温吸附实验均参照HOU等[22]研究中使用的方法。其中,动力学吸附实验中Ni(Ⅱ)的初始质量浓度为150 mg·L−1,等温吸附实验中Ni(Ⅱ)的初始质量浓度依次为1、5、10、20、50、100和150 mg·L−1,吸附温度为25 ℃。本研究水中Ni(Ⅱ)的检测方法采用丁二酮肟分光光度法(GB 11910-1989)。每次实验都设置平行样,且实验误差控制在1%~3%。
-
本研究分别采用Autosorb-iQ仪器(美国Quanta-chrome公司)、场发射扫描电子显微镜(SEM)、vario MACRO cube元素分析仪和傅里叶变换红外光谱仪(Nicolet IS10美国尼高力公司)对超声氧化前后的MWCNTs进行表观形貌分析、BET比表面积和孔容孔径测定、元素分析以及表面含氧官能团分析。
1.1. 材料与试剂
1.2. 超声氧化MWCNTs的制备与优化
1.3. Ni(Ⅱ)的吸附
1.4. MWCNTs的表征
-
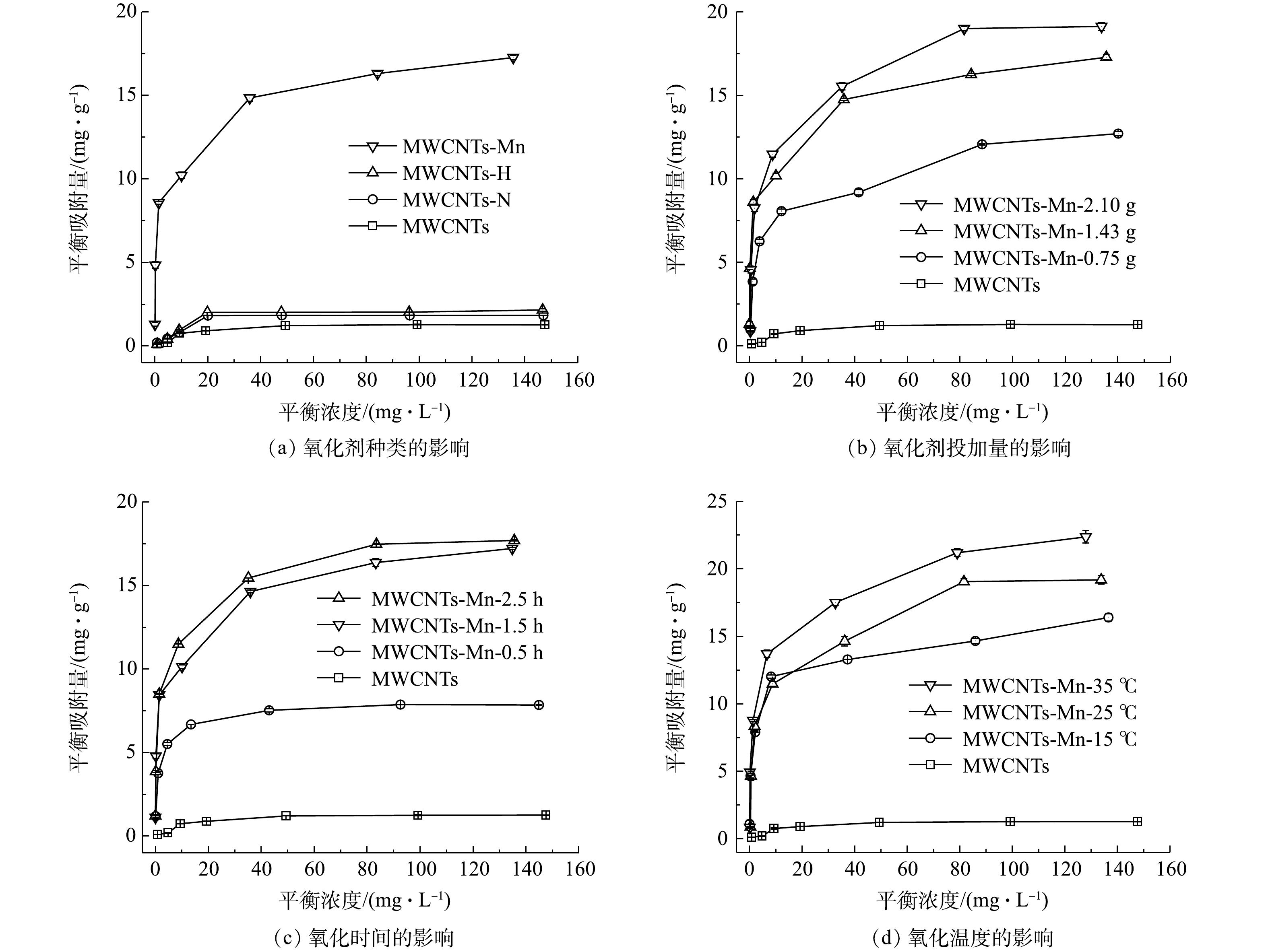
分别考察了不同氧化剂种类、氧化剂投加量、超声氧化时间和超声氧化温度对超声氧化MWCNTs吸附水中Ni(Ⅱ)的效能影响。由图1(a)可知,当氧化剂投加量为1.43 g、超声氧化时间为1.5 h和超声氧化温度为25 ℃时,在3种氧化剂中,经KMnO4氧化后的MWCNTs-Mn对Ni(Ⅱ)的吸附量最大(17.27 mg·g−1),是H2O2氧化后MWCNTs-H的8.15倍,是HNO3氧化后MWCNTs-N的9.39倍,是MWCNTs的13.59倍。这可能是由于:一方面,与H2O2和HNO3相比,经KMnO4氧化后MWCNTs表面的含氧官能基团含量更多,可提高其对Ni(Ⅱ)的吸附强度;另一方面,KMnO4氧化过程中产生了少量的MnO2,而MnO2本身就具有较大的比表面积和良好的吸附性能,可进一步增强MWCNTs-Mn对Ni(Ⅱ)的吸附作用。这与LI等[12]的研究结果是一致的,即当采用H2O2、HNO3和KMnO4分别氧化处理MWCNTs时,经KMnO4氧化后的MWCNTs对Cd2+的吸附量最大,为11 mg·g−1,而Ni2+与Cd2+具有相似的吸附特性。
由图1(b)可知,当氧化剂为KMnO4、超声氧化时间为1.5 h和超声氧化温度为25 ℃时,在氧化剂投加量从0 g增大到2.10 g的过程中,MWCNTs-Mn对Ni(Ⅱ)的吸附量逐渐增加;且当氧化剂投加量为2.10 g 时,MWCNTs-Mn对Ni(Ⅱ)的吸附量达到最大值(19.14 mg·g−1),明显高于氧化剂投加量为1.43 g和0.75 g时的吸附量(17.30 mg·g−1和12.71 mg·g−1)。这说明,当KMnO4的投加量增加时,MWCNTs的氧化程度提高,使其表面产生的含氧官能基团含量增加,从而可增加其对水中Ni(Ⅱ)的吸附量。
由图1(c)可知,当氧化剂为KMnO4、氧化剂投加量为2.10 g和超声氧化温度为25 ℃时,在超声氧化时间为2.5 h时,MWCNTs-Mn对Ni(Ⅱ)的吸附量最大(17.71 mg·g−1),当超声氧化时间分别减少到1.5 h和0.5 h时,MWCNTs-Mn对Ni(Ⅱ)的吸附量逐渐减小,分别为17.23和7.85 mg·g−1。以上结果说明,MWCNTs-Mn对Ni(Ⅱ)的吸附量随着氧化时间的增加而增加。这与叶智新等[23]的研究结果是一致的,即在使用酰胺化/氧化碳纳米管去除三价砷时,当氧化时间从2 h 增加到4 h时,吸附量从4 mg·g−1提高到了13 mg·g−1。
由图1(d)可以看出,当氧化剂为KMnO4、氧化剂投加量为2.10 g和超声氧化时间为2.5 h时,在超声氧化温度由 15 ℃升高到 35 ℃的过程中,MWCNTs-Mn对Ni(Ⅱ)的吸附量逐渐增加,且吸附速率逐渐升高,说明MWCNTs-Mn吸附去除水中Ni(Ⅱ)的反应属于吸热反应。当超声氧化温度为 35 ℃时,MWCNTs-Mn对Ni(Ⅱ)的吸附量达到最大,为22.37 mg·g−1。这主要是由于温度升高可以提高分子的活化能,分子扩散速率升高,从而促进反应进行。
综合以上结果可知,MWCNTs-Mn的最佳制备条件为:氧化剂KMnO4投加量为2.1 g,氧化时间为2.5 h,氧化温度为35 ℃,此时,MWCNTs-Mn对Ni(Ⅱ)的吸附量最大为22.37 mg·g−1(C0=150 mg·L−1),比氧化前提高了16.61倍。对比4种不同的制备条件可以看出,氧化剂种类对氧化效果影响最大,其次是氧化时间。这主要是由于MWCNTs-Mn的氧化程度越高,其表面的亲水性官能基团越多,分散性能越好,对水中Ni(Ⅱ)的吸附效果越好[20];此外,超声也可提高MWCNTs-Mn在水中的分散性,有利于MWCNTs-Mn对水中Ni(Ⅱ)的吸附。
-
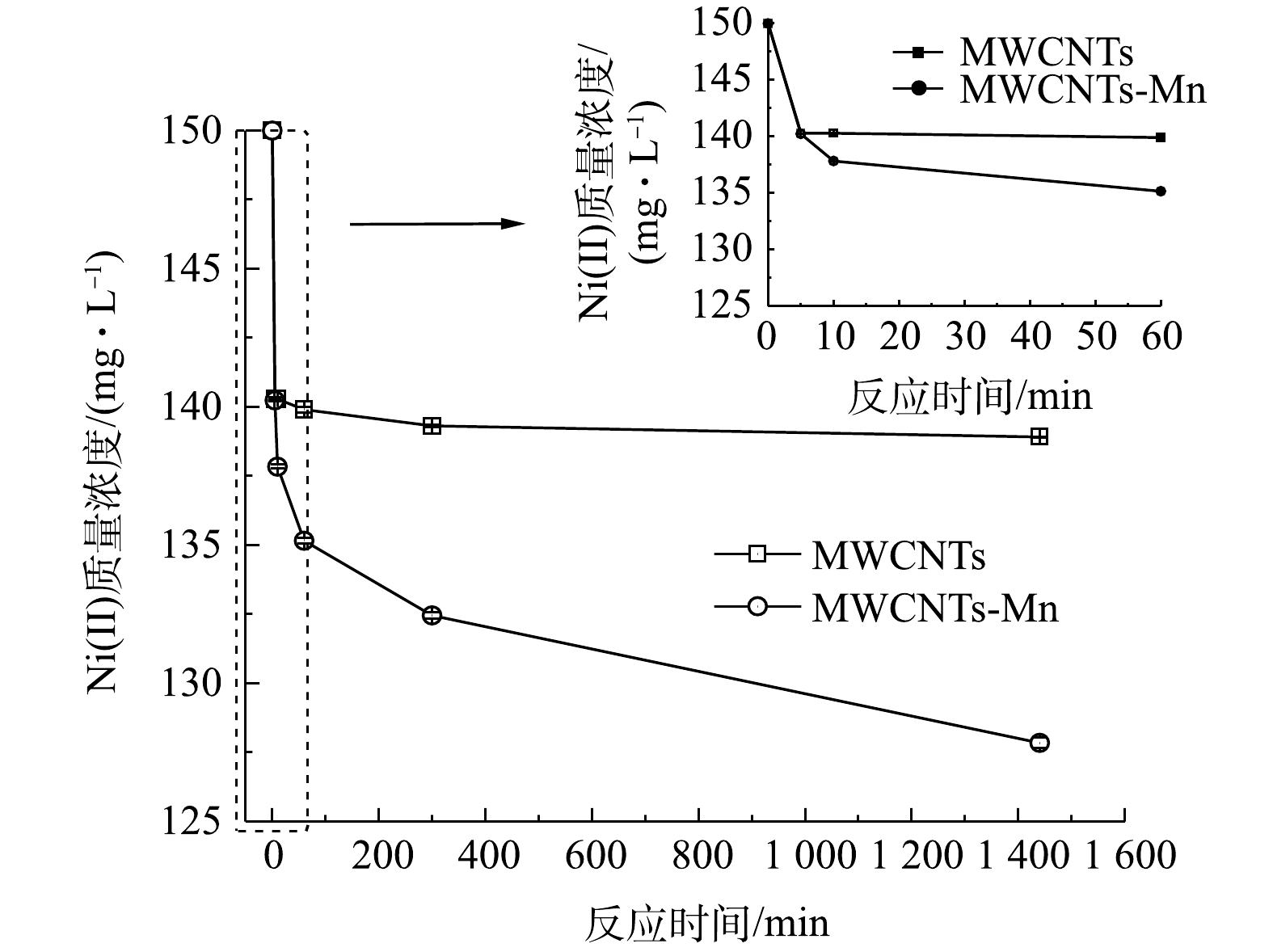
1)动力学吸附效能。为了进一步考察MWCNTs-Mn在超声氧化前后对水中Ni(Ⅱ)的吸附效能,本研究分析了MWCNTs-Mn对水中Ni(Ⅱ)的动力学吸附变化规律,如图2所示。由图2可以看出,当水中Ni(Ⅱ)的初始质量浓度为150 mg·L−1时,MWCNTs-Mn对水中Ni(Ⅱ)的吸附速率均高于MWCNTs;且随着吸附时间的增加,MWCNTs-Mn在超声氧化前后对水中Ni(Ⅱ)的吸附速率均逐渐降低。尤其需要关注的是,当吸附时间小于等于5 min时,超声氧化前后的MWCNTs对Ni(Ⅱ)的吸附速率最快;而当吸附时间大于5 min时,超声氧化前后的MWCNTs对Ni(Ⅱ)的吸附速率逐渐减慢。同时,MWCNTs-Mn对Ni(Ⅱ)的去除率明显提高,吸附平衡时去除率从超声氧化前的7.39%提高到14.77%。
为了进一步研究MWCNTs超声氧化前后的动力学吸附过程,通过准一阶动力学和准二阶动力学方程进行拟合[24],拟合结果如表1所示。由表1可以看出,准二阶动力学吸附模型可以更好地描述MWCNTs-Mn对Ni(Ⅱ)的吸附过程(R2=0.997 1),说明 MWCNTs-Mn对水中的Ni(Ⅱ)主要以化学吸附为主。此外,超声氧化后的MWCNTs-Mn对Ni(Ⅱ)的吸附速率常数k2(0.533 9)比超声氧化前(0.046 7)大幅提高,说明MWCNTs-Mn对Ni(Ⅱ)的吸附速率明显增加。这可能是由于化学吸附过程会涉及离子交换过程,或者MWCNTs-Mn上的含氧官能基团与Ni(Ⅱ)形成了化学键,这均有利于MWCNTs-Mn对水中Ni(Ⅱ)的吸附[25]。
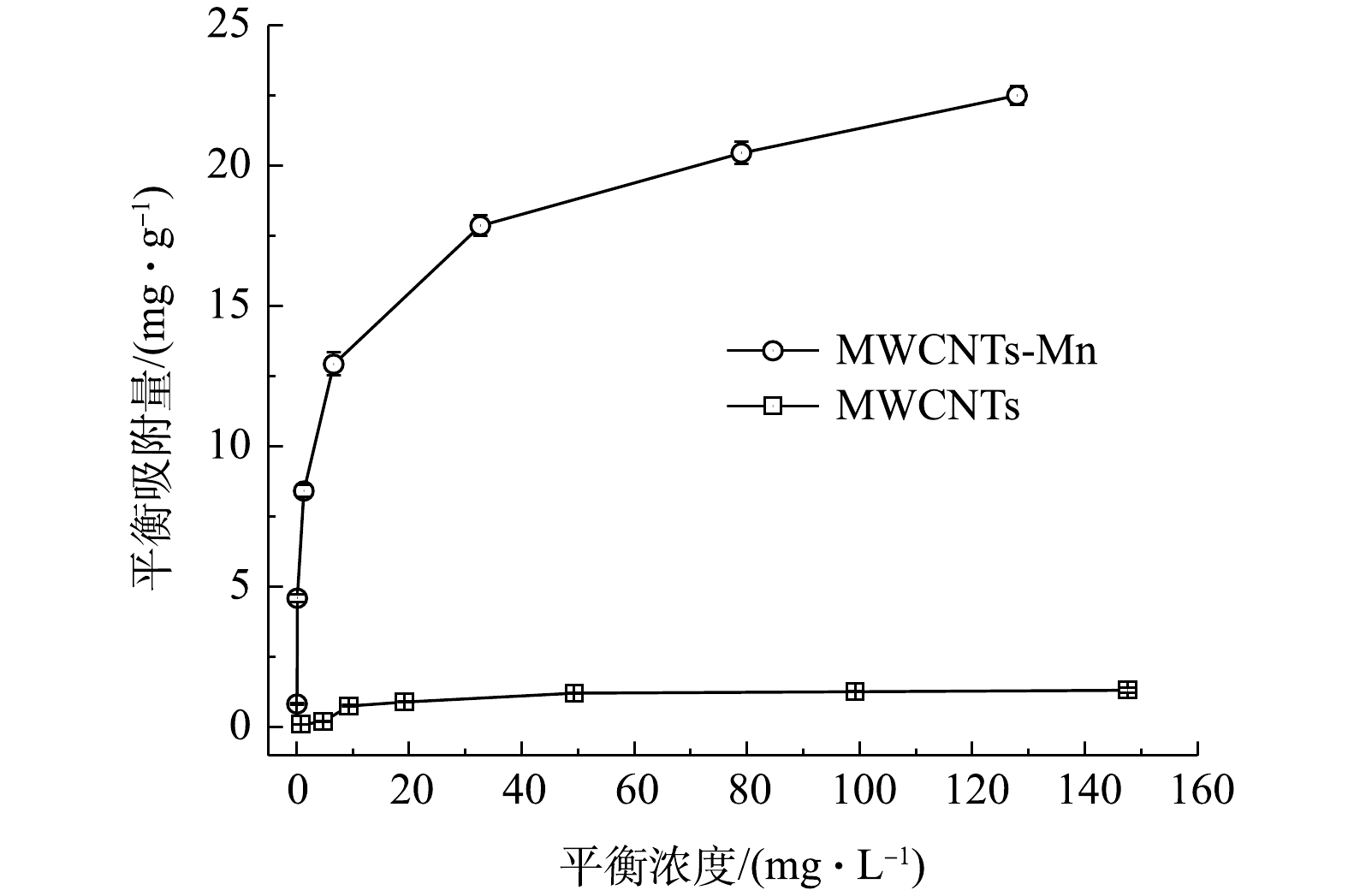
2)等温吸附效能。图3为超声氧化前后的MWCNTs对水中Ni(Ⅱ)的等温吸附曲线。由图3可以看出,MWCNTs-Mn对Ni(Ⅱ)的吸附量随着Ni(Ⅱ)的平衡浓度的增加而增加。当水中Ni(Ⅱ)平衡浓度低于15 mg·L−1时,吸附量呈直线上升趋势,吸附速率较快;当平衡浓度高于15 mg·L−1时,吸附量随平衡浓度的增加而增加缓慢;当吸附达到平衡时,MWCNTs-Mn的吸附量(22.37 mg·g−1)明显高于MWCNTs的吸附量(1.32 mg·g−1)。
为考察超声氧化前后MWCNTs吸附水中Ni(Ⅱ)的热力学机理,本研究分别采用Langmuir和Freundlich等温吸附模型对等温吸附过程进行拟合[24],结果见表2。由表2可以看出,Langmuir等温吸附模型能较好地描述MWCNTs-Mn对水中Ni(Ⅱ)的吸附过程(R2为0.996 2),说明MWCNTs-Mn表面均一,各处吸附性能相同,对Ni(Ⅱ)的吸附属于单分子层吸附。同时,拟合参数n>1,说明MWCNTs-Mn对水中Ni(Ⅱ)的吸附属于易吸附过程[26]。此外,Langmuir模型也可以较好地描述MWCNTs对Ni(Ⅱ)的吸附过程(R2为0.982 3),说明其对Ni(Ⅱ)的吸附也属于单分子层吸附。
以上结果表明,超声氧化MWCNTs-Mn能有效提高其对水中Ni(Ⅱ)的吸附效能,其最大吸附量可达到22.37 mg·g−1(表2),比超声氧化前增加了21.10 mg·g−1,亦高于其他研究中改性MWCNTs对水中Ni(Ⅱ)的吸附量[27-29]。CHEN等[27]制备的MWCNT/Fe2O3复合材料对水中Ni(Ⅱ)的吸附量为9.18 mg·g−1;AHMADI等[28]使用H2O2氧化改性MWCNT,其对水中Ni(Ⅱ)的吸附量为14.20 mg·g−1;SHIN等[29]制备的氮掺杂磁性MWCNT对水中Ni(Ⅱ)的吸附量为8.06 mg·g−1。
-
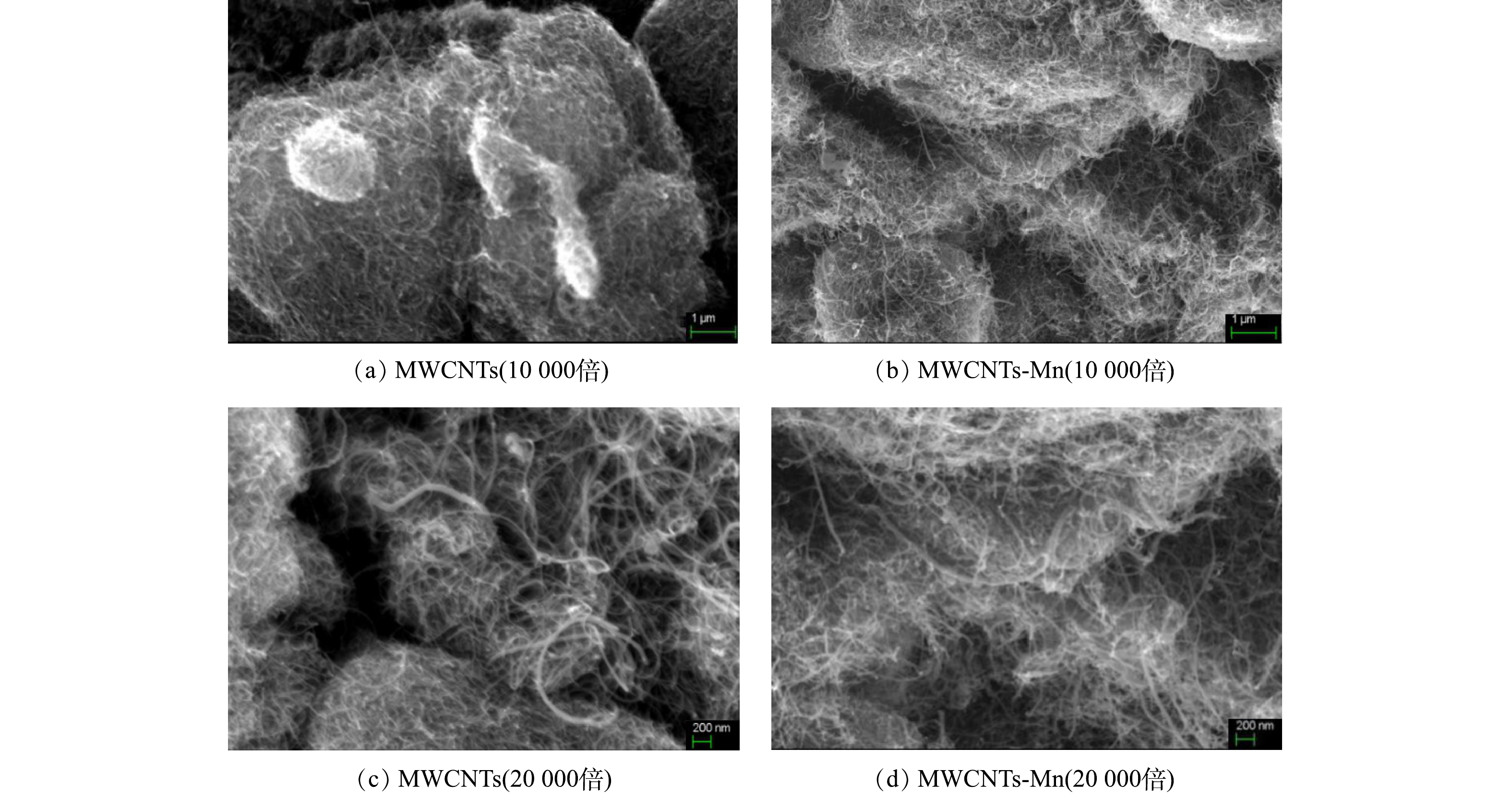
1) SEM分析。为了进一步研究MWCNTs在超声氧化前后的表面形态分布情况,本研究对比分析了超声氧化前后的MWCNTs的扫描电镜图像,如图4所示。如图4可知,超声氧化处理后的MWCNTs-Mn表面较为分散,呈层状分布,每层的厚度在0.5~1 μm(图4(b),图4 (d));而超声氧化前的MWCNTs则相互聚集且缠绕在一起,呈团状分布,团体的直径为4~6 μm,明显大于超声氧化后。这一结果说明超声氧化增强了MWCNTs-Mn的分散性,这可能是由于超声产生的微小气泡瞬间爆破,该能量冲击波对周围的碳纳米管簇起作用,从而使得通过范德华力粘结在一起的团族分散[30-31]。
2) BET比表面积及孔容孔径分布。表3为超声氧化前后MWCNTs的比表面积及孔容孔径分布情况。由表3可以看出,MWCNTs-Mn的总孔容量比超声氧化前减少了1.969 cm3·g−1,其中中孔孔容减少最多,为1.965 cm3·g−1。这可能是由于含氧官能基团占据了空位,或者氧化过程中生成的MnO2负载到MWCNTs表面,从而造成其部分孔道在反应过程中被堵塞。这与DAIFULLAH等[32]和LI等[12]研究结果是一致的。DAIFULLAH等[32]发现,经过KMnO4改性后的活性炭会有一些MnO2附着在其表面。LI等[12]使用H2O2、HNO3和KMnO4氧化处理的碳纳米管(CNT)去吸附去除水中Cd2+,对Cd2+的吸附量分别为2.6、5.1和11.0 mg·g−1,其中KMnO4氧化的CNT的吸附容量突然增加,这可能是由于负载在CNT上的残留MnO2颗粒的吸附作用所致,由此可以推测,本实验中MWCNT-Mn表面有MnO2沉积。
3)元素分析。表4为超声氧化前后MWCNTs的元素分析情况。由表4可以看出,在超声氧化后,MWCNTs-Mn表面的O含量明显增加,由超声氧化前的1.40 %增加到14.08 %,这说明超声氧化后MWCNTs-Mn表面的含氧官能基团增加,这与图5的结果一致。
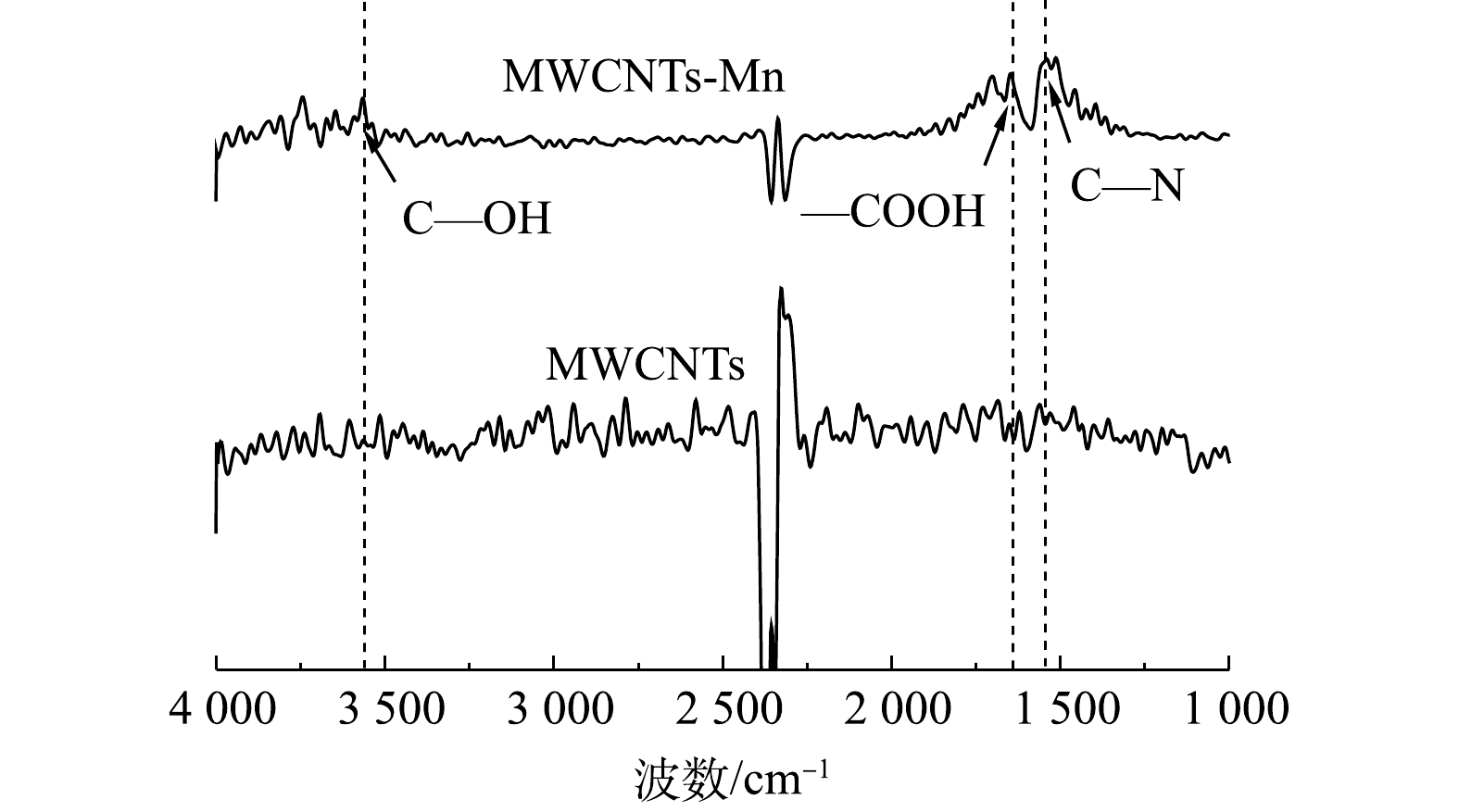
4)傅里叶变换红外光谱分析。图5为超声氧化前后MWCNTs的红外吸收光谱图。其中,波数3 200~3 600 cm−1为C—OH(羟基)的伸缩振动锋,波数1 650~1 750 cm−1为—COOH(羧基)的伸缩振动锋,波数1 690~1 590 cm−1为C—N(氰基)的伸缩振动锋。由图5可以看出,经过超声氧化处理,MWCNTs-Mn表面引入了大量的亲水性官能基团(羟基、羧基)。这一方面可提高MWCNTs-Mn在水中的分散性;另一方面,可提高MWCNTs-Mn对水中Ni(Ⅱ)的吸附效能。这与CHEN等[33]的研究结果一致,即通过X 射线吸收光谱证实了Cu2+和Pb2+ 与MWCNTs表面的羧基或者羟基能够形成内层配合物,且MWCNTs对金属离子的吸附能力随其表面含氧官能团(羧基、羟基和内酯基等)数量的增加而增强。
综上所述,通过对MWCNTs-Mn的扫描电镜分析、比表面积、孔容孔径分布、元素分析和红外光谱分析发现,MWCNTs-Mn的总孔容量减少了72.64%,O元素含量提高了12.68%,说明亲水性官能基团填充在MWCNTs-Mn表面孔隙内。亲水性含氧官能基团一方面能在溶液中发生解离从而降低MWCNTs表面的Zeta电位值,进而增强其对Ni(Ⅱ)的静电吸引力;另一方面可降低MWCNTs颗粒之间的范德华力,从而提高MWCNTs在水中的分散性[19]。同时,本研究采用超声的方法,通过在溶液中生成的微小气泡瞬间爆破,产生大的能量冲击波使通过范德华力粘结在一起的MWCNTs团族分离[20],从而可提高MWCNTs-Mn在水中的分散性,这些均有利于提高MWCNTs-Mn对水中Ni(Ⅱ)的吸附能力。
2.1. 超声氧化MWCNTs的制备条件优化
2.2. MWCNTs-Mn对Ni(Ⅱ)的吸附效能
2.3. 超声氧化MWCNTs对Ni(Ⅱ)的吸附机理
-
1)本实验获得了MWCNTs- Mn的最佳制备条件,即KMnO4为氧化剂、氧化剂投加量为2.1 g、超声氧化时间为2.5 h、超声氧化温度为35 ℃;此时MWCNTs-Mn对水中Ni(Ⅱ)的吸附量最大,为 22.37 mg·g−1,比超声氧化前提高了16.61倍,且优于其他常见的吸附剂。
2) MWCNTs-Mn对水中Ni(Ⅱ)的吸附过程符合准二阶动力学模型(R2=0.997 1)和Langmuir模型(R2=0.996 2),说明超声氧化后MWCNTs-Mn表面均一,对Ni(Ⅱ)的吸附属于单分子层吸附和化学吸附。
3)超声氧化后MWCNTs-Mn的总孔容量降低了72.64 %,O元素含量提高了12.68 %,亲水性含氧官能基团(羟基和羧基)含量增加,推测可能是这些含氧官能团和超声的综合作用提高了MWCNTs-Mn对Ni(Ⅱ)的吸附效能。





 下载:
下载: