-
传统的非均相芬顿催化剂材料多以铜基或铁基掺杂其他金属元素合成,因而存在工艺复杂、合成成本高、失活催化剂作为固体废弃物难以处理等问题。然而,自然界中的天然矿物本身具有低成本易处理的优点,故本研究以天然矿石作为异相芬顿催化剂来解决芬顿工艺现阶段存在的问题。天然矿物主要形成于火山喷发和地震等地壳运动,已被证明具有优异的催化性能[1-3],并在生产应用过程中扮演重要作用。例如,黄铜矿和赤铁矿已被证明在催化降解有机污染物和染料分子中有较高的活性[4-7];天然磁黄铁矿活化过氧硫酸盐可用于高效降解二丁基二硫代磷酸铵[8];天然磁性闪锌矿和黑钨矿曾作为可见光光催化剂在有机物降解和病原微生物灭活方面展现出优异的效果[9-10]。因此,开发和研究天然矿石作为异相芬顿催化剂活化过氧化氢降解废水中的有机污染物具有重要的应用价值。
本研究选取中国河南省矿区的一种天然辉铜矿,利用特定的物理化学手段对天然矿石进行了改良优化,从而实现天然矿石内部活性物质的有效暴露,进而获得更高效的催化效果[11],故以此物质作为一种非均相芬顿催化剂使用。鉴于含苯酚及其衍生物废水较强的水生态毒性,我国已将其列为重点解决的有害废水之一[12-14],因此,以苯酚为目标污染物来评价天然矿石催化剂活性具有重要意义。相比于传统经过复杂工序合成的非均相芬顿催化剂[15],利用物理改性的天然矿石具有经济高效、处理工艺简单、产量丰富和易再生等优势,为非均相芬顿催化氧化的拓展应用开辟了新思路。
全文HTML
-
苯酚(C6H5OH)、甲醇(CH3OH)、氯化钠(NaCl)购于阿拉丁、无水硫酸钠(Na2SO4)购于阿拉丁、硝酸钠(NaNO3)购于阿拉丁、无水乙醇(C2H5OH),以上为分析纯试剂,硫酸(H2SO4)、硝酸(HNO3)、30%过氧化氢(H2O2),DMPO(5,5-dimethyl-1-pyrroline N-oxide,>97%)。
研究所用矿石取自中国河南省某矿区。首先将矿石简单物理破碎,再经行星式球磨仪在400 r·min−1下球磨得到矿石粉末。取0.5 g矿石粉末均匀分散于10 mL现配制的王水中,室温下以450 r min−1搅拌反应12 h。所得反应液经100倍稀释后用0.45 μm滤膜过滤去除杂质最后利用电感耦合等离子体发射光谱仪(ICP-OES,Agilent Technologies 5110VDV,USA)检测溶液中金属元素的含量。经计算,实验所用矿石中铁、铜和钼的元素含量分别为2.66%、1.44%和0.04%。
-
在200 mL的烧杯中,改性后的矿石粉末被均匀分散于100 mL质量浓度为100 mg·L−1的模拟苯酚废水中,然后加入过氧化氢以开始反应。在预设的时间间隔内利用注射器取1.5mL反应液并用0.45 μm滤膜过滤,最后加入1 mL甲醇以淬灭自由基,终止反应进行。以上实验未经特殊说明,实验条件均保持不变且重复3次。
-
反应后溶液中苯酚浓度和总有机碳浓度分别采用高效液相色谱(Waters,Alliance e2695,USA)和总有机碳分析仪(Shimadzu,TOC-L,Japan)测定,色谱柱为C18柱,色谱柱温度为25 ℃,流动相为水∶甲醇(40%∶60%),流速为1 mL·min−1,检测波长为270 nm[16]。
利用高分辨蔡司场发射扫描电子显微镜(GEMINISEM 500)和X射线能谱分析(EDS)观察矿石形貌进行表征分析,采用max-2500 X射线衍射仪(XRD)通过Cu-Kα辐射,在40 kV和40 mA的操作条件下对矿石表面的晶形结构进行分析。采用赛默氏公司Al-Kα源的250XI能谱仪进行X射线光电子能谱(XPS)分析不同球磨时间和反应前后矿石表面元素变化。
以5,5-二甲基-1-吡咯啉-N-氧化物(DMPO)作为羟基自由基的捕获剂,并利用X波段电子自旋共振(ESR)谱仪(JEOL, JES FA-200)进行自由基检测。
1.1. 实验试剂和材料
1.2. 降解实验
1.3. 仪器分析及方法
-
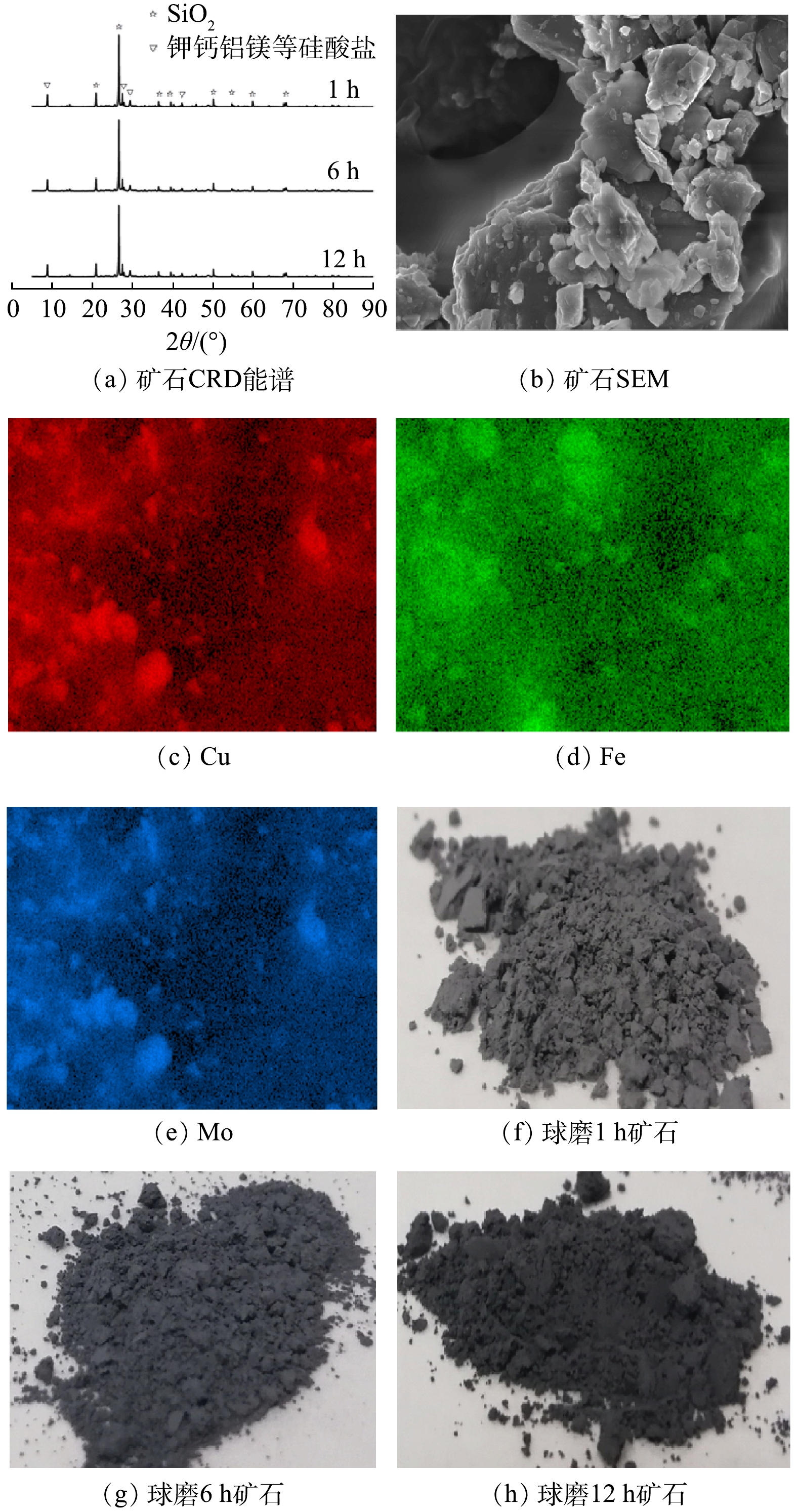
图1(a)为天然矿石在不同球磨时间下的XRD谱图,对比标准卡片(JCPDS 99-0088)可知,(100)、(011)、(110)、(102)、(111)、(200)、(201)、(112)、(122)、(121)和(031)主要为二氧化硅衍射峰,其中(100)和(011)晶面的衍射峰最强。此外,图1(a)中还有一些其他金属硅酸盐的晶面峰。通过比较不同球磨时间样品XRD谱图可知,球磨时间对矿石表面物相没有显著影响。这是由于天然矿石中Fe和Cu等金属元素含量较少,其化合物的特征衍射峰易被SiO2掩盖导致。图1(b)为天然矿石表面微观形貌图,可以看出,天然矿石呈现不规则形状,表面光滑,分布较为分散;由EDS光谱元素映射结果可知,催化剂表面含有较为明显的Fe、Mo和Cu元素映射,证明矿石表面有铁、铜和钼金属元素存在。综上所述,催化剂中主要成分为二氧化硅和少量的金属元素,因此,在其作为异相芬顿催化剂失活后方便处理,不会带来二次污染。
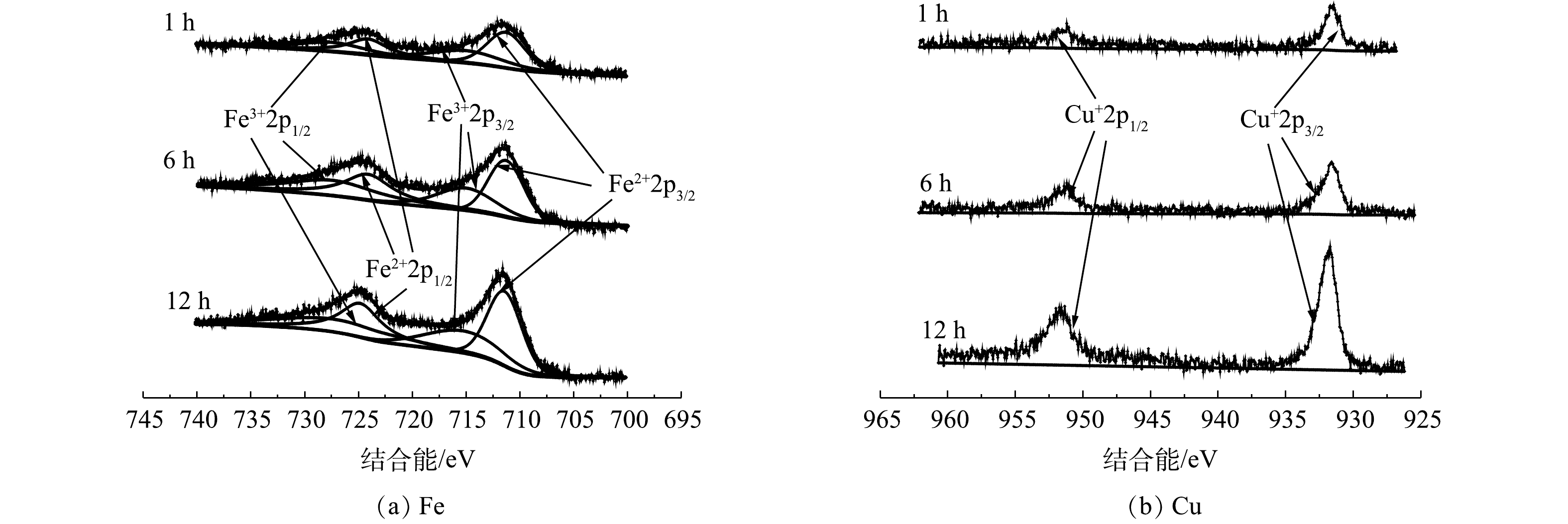
为了进一步研究矿石表面的化学组成,对催化剂材料做了XPS光谱表征分析。根据以往文献报道结果[17-18],在Fe2p轨道的XPS能谱中,715.0 eV和727.4 eV的峰对应于Fe(Ⅲ)的Fe2p1/2和Fe2p3/2轨道,911.3 eV和924.1 eV的峰分别归属于Fe(Ⅱ)的2p1/2和2p3/2轨道。在Cu2p轨道的XPS能谱中,932.0 eV和952.0 eV可以与Cu(Ⅰ)的2p1/2轨道的结合能相匹配[19]。如图2所示,随着球磨时间的增加,Fe2p和Cu2p峰值也随之增加,这表明球磨处理有利于提高矿石催化剂表面Fe和Cu元素的暴露量,进而增加了催化剂的活性位点。
-
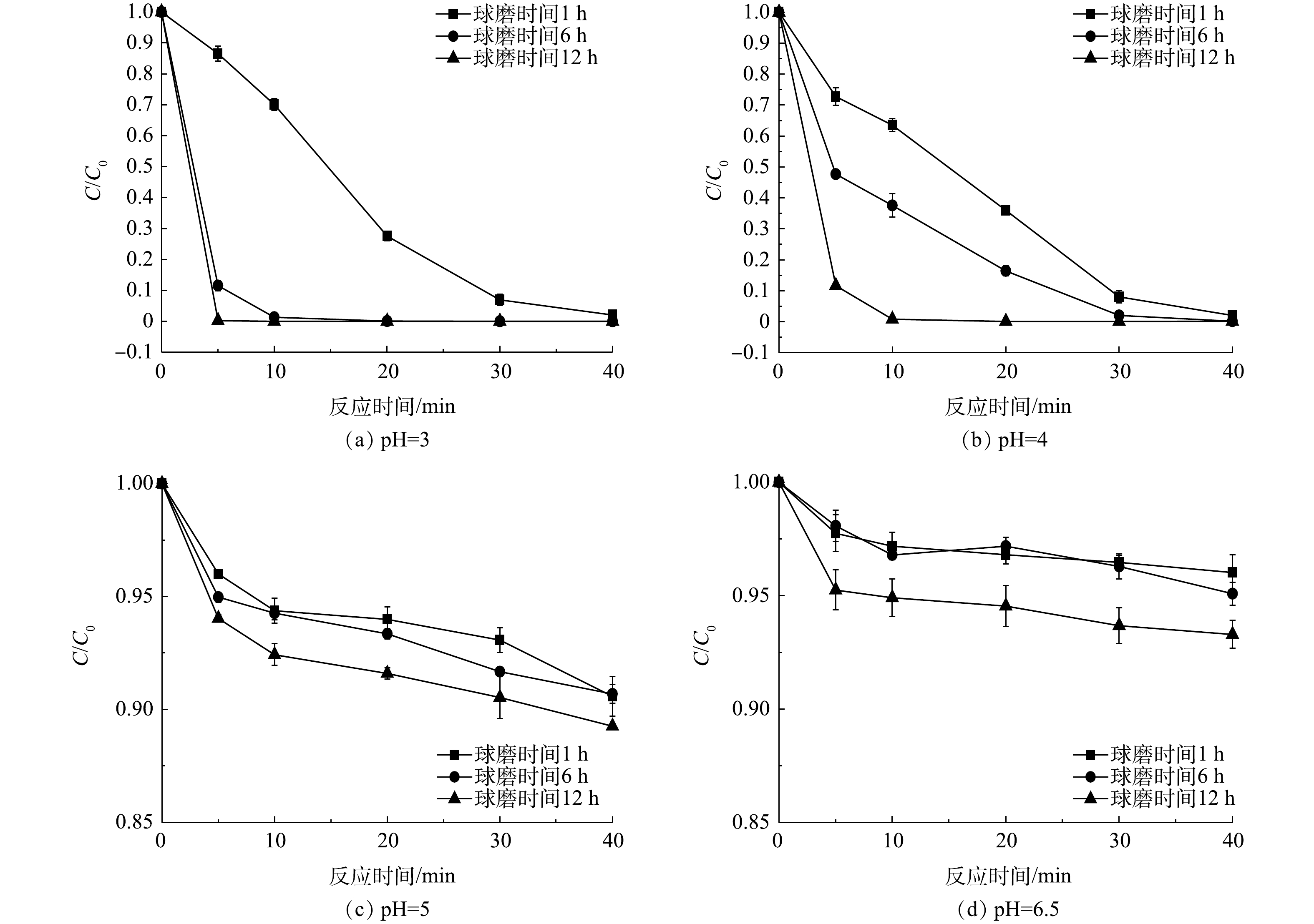
1)催化剂的加工条件对降解效果的影响。如图1(f)~图1(h)所示,在表征和矿石的粉碎过程中发现,随着球磨时间的延长,矿石粉末的颜色由灰色逐渐加深至黑色,并伴随着Cu(Ⅰ)含量的提高。为了进一步探究球磨时间对矿石催化剂催化性能的影响,利用不同球磨时间下的矿石催化剂进行了苯酚的降解实验。如图3(a)所示,球磨1、6和12 h的矿石催化剂对苯酚均有显著的降解效果,可分别在40、10和5 min内去除99%以上的苯酚。此外,不同球磨时间的矿石催化降解速度有明显差异,苯酚的降解速度随着球磨时间的延长而逐渐加快。
2)反应条件对降解效果的影响。为探究溶液pH对矿石催化降解苯酚效果的影响,分别在pH为3、4、5和6.5的条件下进行了苯酚降解实验。如图3所示,在pH为3和4下苯酚降解速度较快,经过40 min反应,溶液中苯酚的剩余量不足1%;当pH为5和6.5时,降解速度缓慢,说明在此条件下矿石的催化活性降低,不利于·OH的生成[20]。
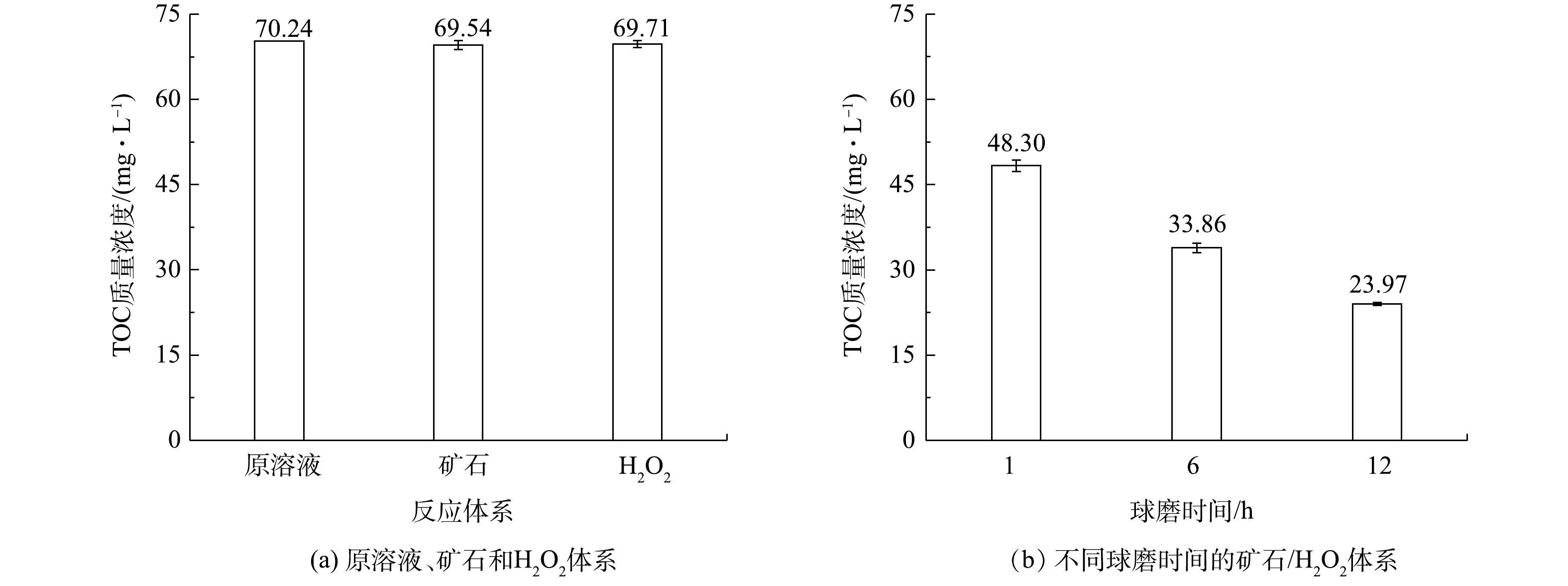
为了进一步探究天然矿石催化剂对有机污染物的矿化情况,检测了降解后苯酚废水中的TOC含量。如图4所示,反应40 min后,当反应体系中仅有矿石催化剂或过氧化氢时,溶液中的TOC含量没有明显下降,表明单独的矿石催化剂或过氧化氢对苯酚几乎没有矿化。然而,当催化剂和过氧化氢同时存在时,同苯酚降解实验的结果一致,球磨12 h的催化剂降解效果最好,TOC的剩余质量浓度为23.97 mg·L−1,矿化率可达66%。
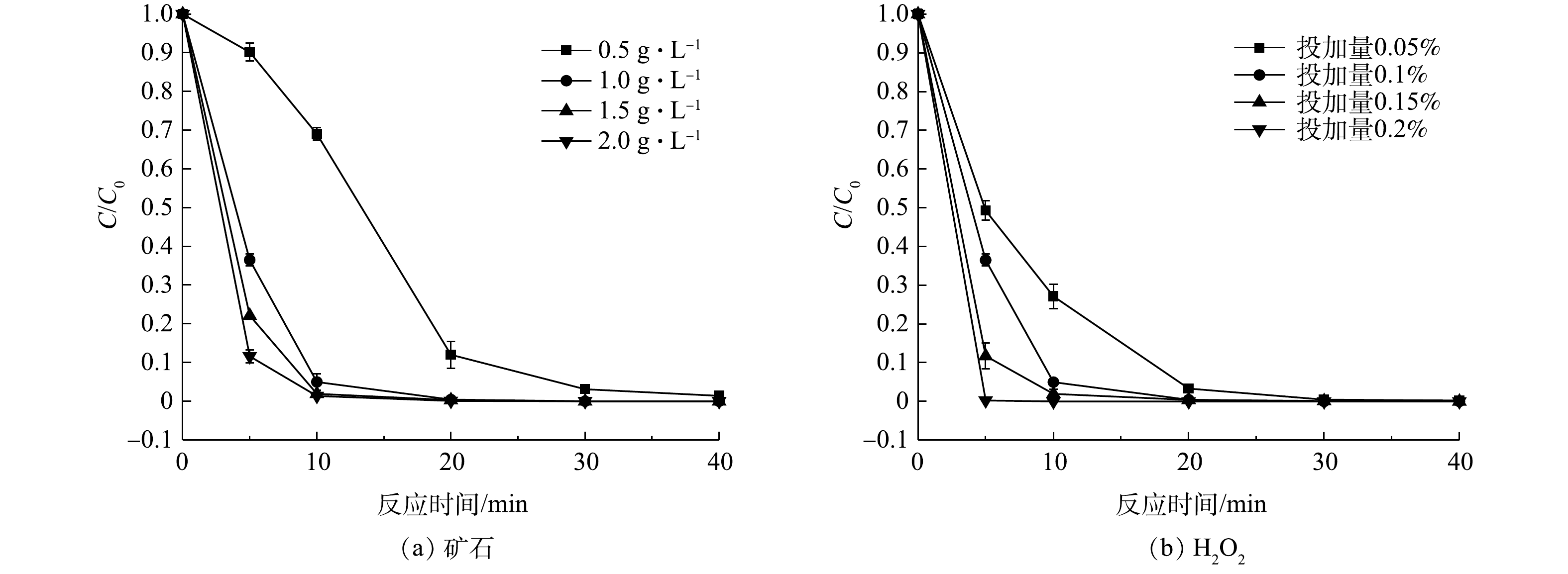
3)过氧化氢及催化剂投加量对催化降解效果的影响。催化剂浓度的增加可以为过氧化氢的活化提供更多的活性位点[20],从而可显著促进活性自由基的产生,而活性自由基是降解有机污染物的关键,故优化催化剂和过氧化氢的投加量有利于节省废水处理成本。因此,研究矿石催化剂的投加量和过氧化氢初始浓度对苯酚降解的影响具有重要意义。如图5(a)所示,当催化剂用量由0.5 g·L−1增加到1.0 g·L−1时,苯酚去除率到达95%所需的时间由40 min缩短到10 min;继续提高用量由1.5 g·L−1和2.0 g·L−1时,苯酚的降解速度没有明显地提升,这是由于受到了溶液中过氧化氢浓度影响,抑制了·OH的生成速度。
图5(b)反映了在过氧化氢在0.05%~0.2%时,过氧化氢投加量对苯酚去除率的影响。与催化剂加入量的影响趋势相似,增加过氧化氢浓度可显著提高苯酚去除率。当过氧化氢投加量为0.05%时,30 min苯酚的去除达到99%以上;当过氧化氢投加量到0.1%、0.15%、0.2%时,对苯酚的去除率分别为99%(20 min)、97%(10 min)、99%(5 min)。根据以上结果,可确定1.0 mg·L−1铜钼矿和0.1%过氧化氢投加量为最优条件,该实验条件既能提高经济效益又能保证较高的降解效果。
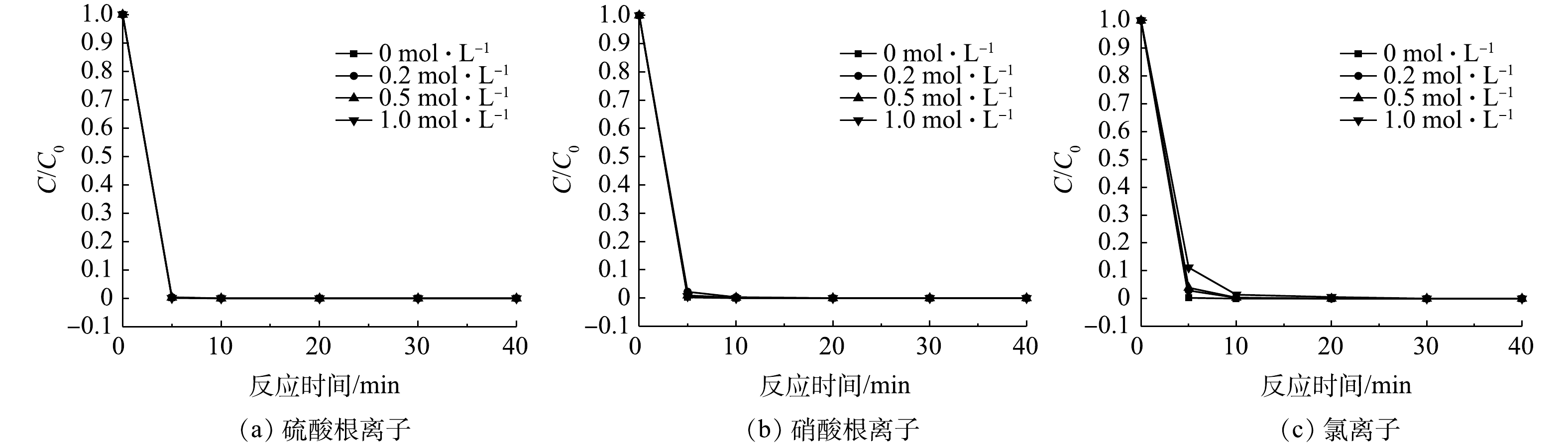
4)对实际高盐模拟废水的处理效果。在实际工业废水处理中,高盐废水的处理是芬顿工艺的一大难题。针对这一问题,探究了常规阴离子对催化剂催化能力的影响。图6为在pH为3时,不同浓度的氯离子、硫酸根离子和硝酸根离子对苯酚去除率影响。虽然硫酸根离子和硝酸根离子投加量不断增加,但苯酚的降解速度没有发生明显变化,这表明硫酸根离子和硝酸根离子不会影响矿石催化剂的催化效果。此外,如图6(c)所示,随着氯离子浓度的提高,苯酚降解速度逐渐减缓。这是由于氯离子在溶液中会同有机物分子竞争·OH,对·OH有一定的淬灭作用[21]。但经过10 min的反应,苯酚的去除率依然可以达到99%以上。综合以上结果可以判断,此矿石催化剂可用于处理高盐有机废水。
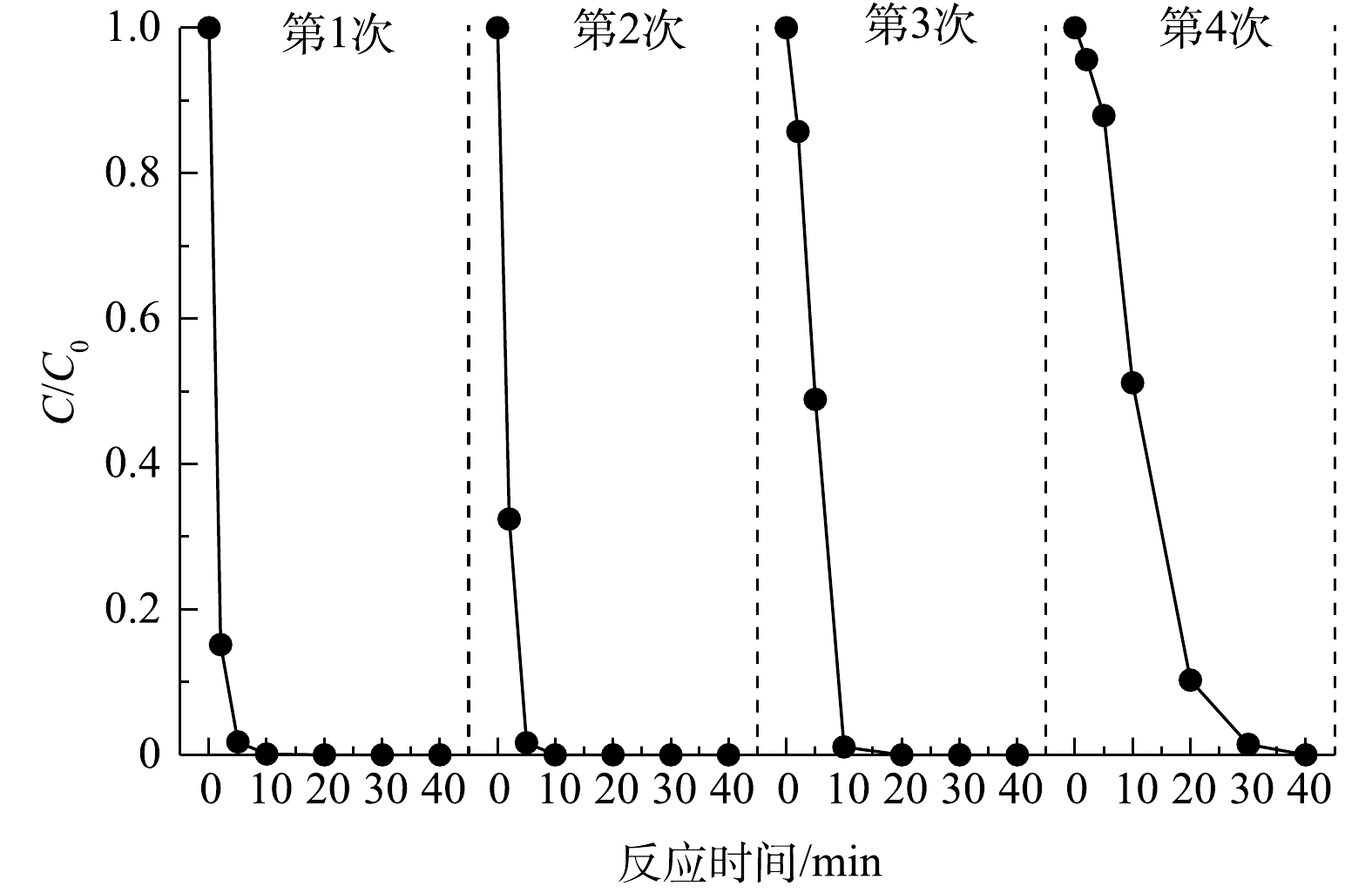
5)反应稳定性分析。为了考察天然矿石催化效果的稳定性,在相同条件下进行了4次重复使用回收实验。每次反应40 min后,通过离心分离的方式回收使用过的矿石催化剂,并用去离子水反复清洗3次以备下次使用。如图7所示,重复使用4次后,反应40 min后苯酚的去除率仍能达到99%,说明矿石催化剂具有良好的稳定性。但随着使用次数的进一步增加,苯酚去除率逐渐降低。其原因主要有2点:首先,每次使用时,催化剂活性位点上的Cu(Ⅰ)和Fe(Ⅱ)在催化过氧化氢的过程中被氧化成Cu(Ⅱ)和Fe(Ⅲ);其次,在清洗过程中,催化剂表面活性物质流失和表面结构变化也会影响对苯酚的催化降解效果[22]。
-
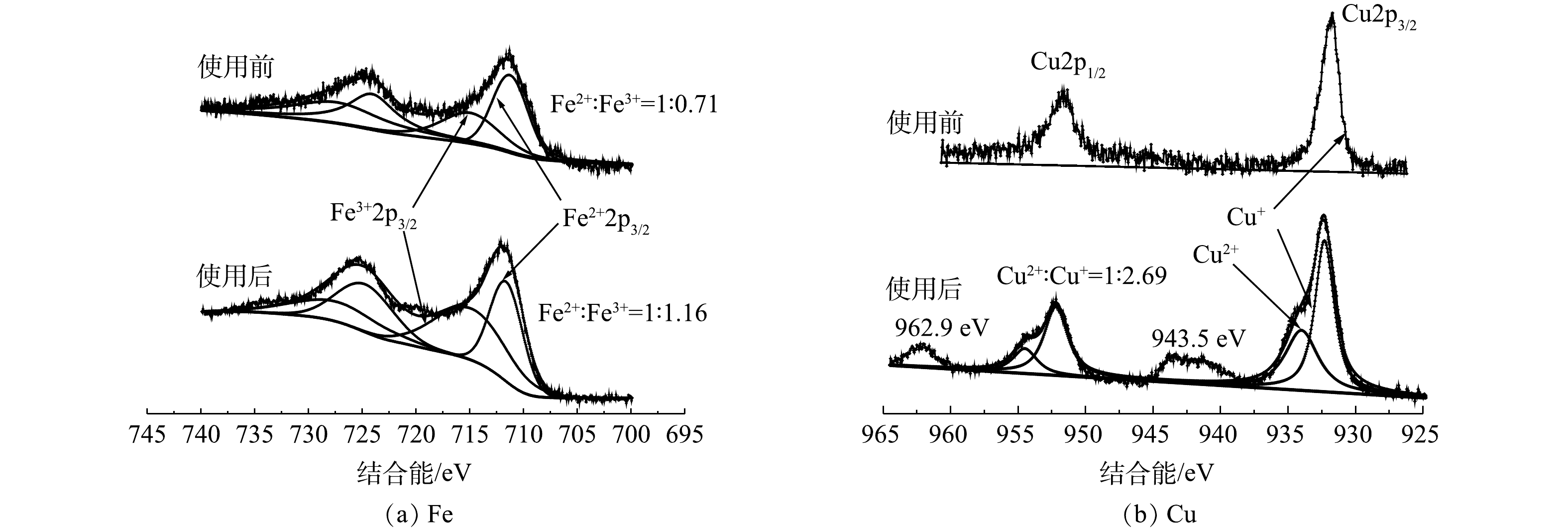
为了探究此天然矿石作为非均相芬顿催化剂催化过氧化氢降解有机污染物的机理,对不同球磨时间和使用前后的矿石进行了XPS光谱分析对比。由图8可知,通过对比使用前后的Fe2p轨道XPS能谱,可发现Fe(Ⅱ)和Fe(Ⅲ)的比例从1∶0.71下降到1∶1.16。这表明在发生催化反应时,Fe(Ⅱ)失去电子被氧化为Fe(Ⅲ),导致样品中Fe(Ⅲ)含量增高,证明Fe(Ⅱ)是天然矿石中发挥催化效果的活性成分之一。此外,通过对比使用前后矿石Cu2p轨道 XPS能谱,可发现使用后的矿石XPS能谱中出现了933.7 eV和954.9 eV峰,说明催化过程中存在Cu(Ⅱ)。XPS能谱在943.5 eV和962.9 eV处的强卫星峰也表明,催化剂表面生成了Cu(Ⅱ)[23]。因此,Cu(Ⅰ)也是催化剂中的催化活性物质之一。这与以往文献中报道的人工合成铜基和铁基催化剂活化过氧化氢有优异催化效果的结论一致[24-25],Cu(Ⅰ)和Fe(Ⅱ)在活化过氧化氢的过程中失去电子被氧化为Cu(Ⅱ)和Fe(Ⅲ)。因此,本实验结果可证明Cu(Ⅰ)和Fe(Ⅱ)是天然矿石上发挥催化效果的活性物质。
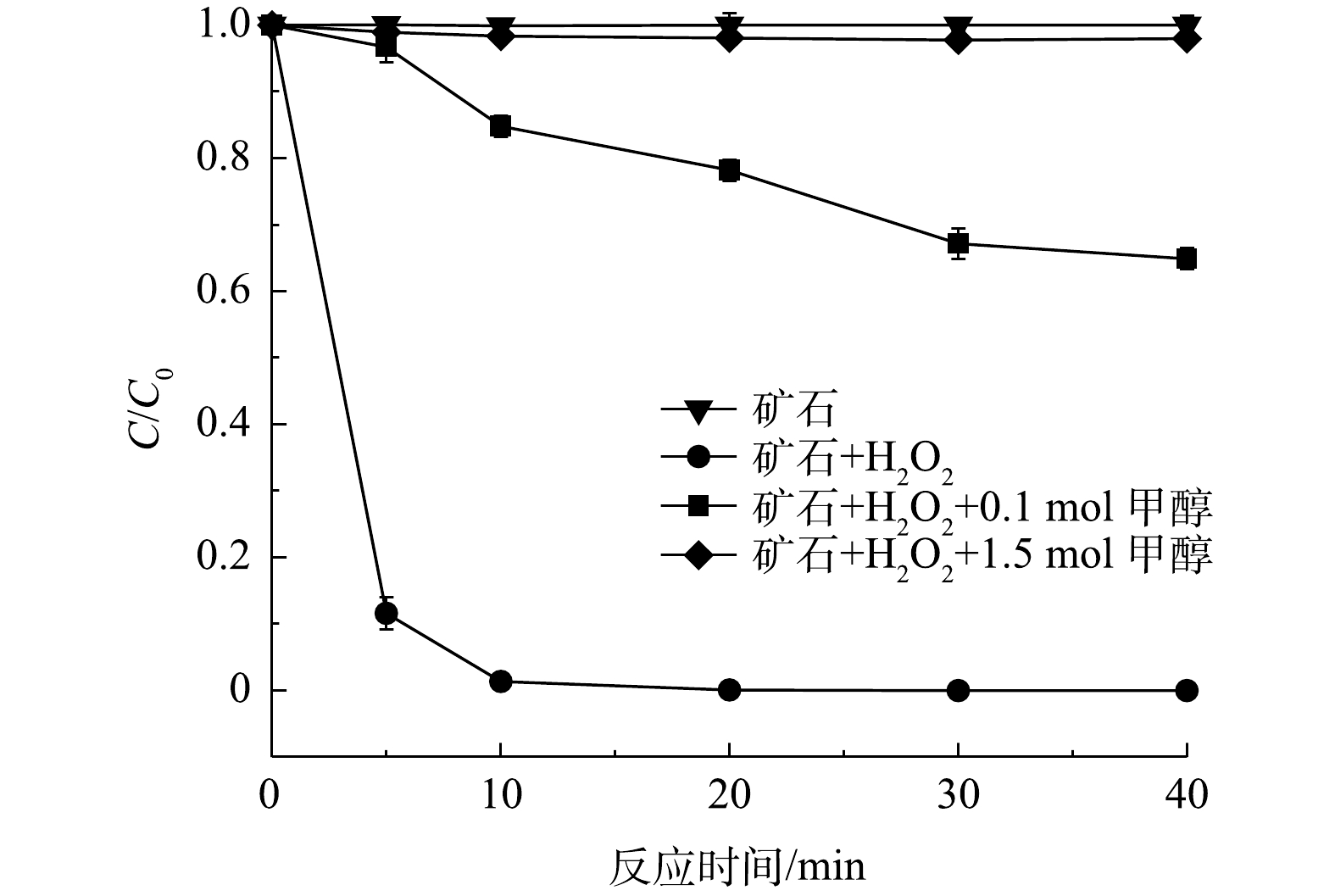
在100 mL体系中分别加入0.1 mol和1.5 mol甲醇。如图9所示,甲醇能有效抑制苯酚的降解,甲醇加量越大,抑制效果越明显。这是由于甲醇的竞争作用,大量活性自由基氧化降解甲醇,阻隔了活性自由基与苯酚的接触和氧化。上述结果也证明矿石催化剂活化过氧化氢过程中有大量活性自由基生成。
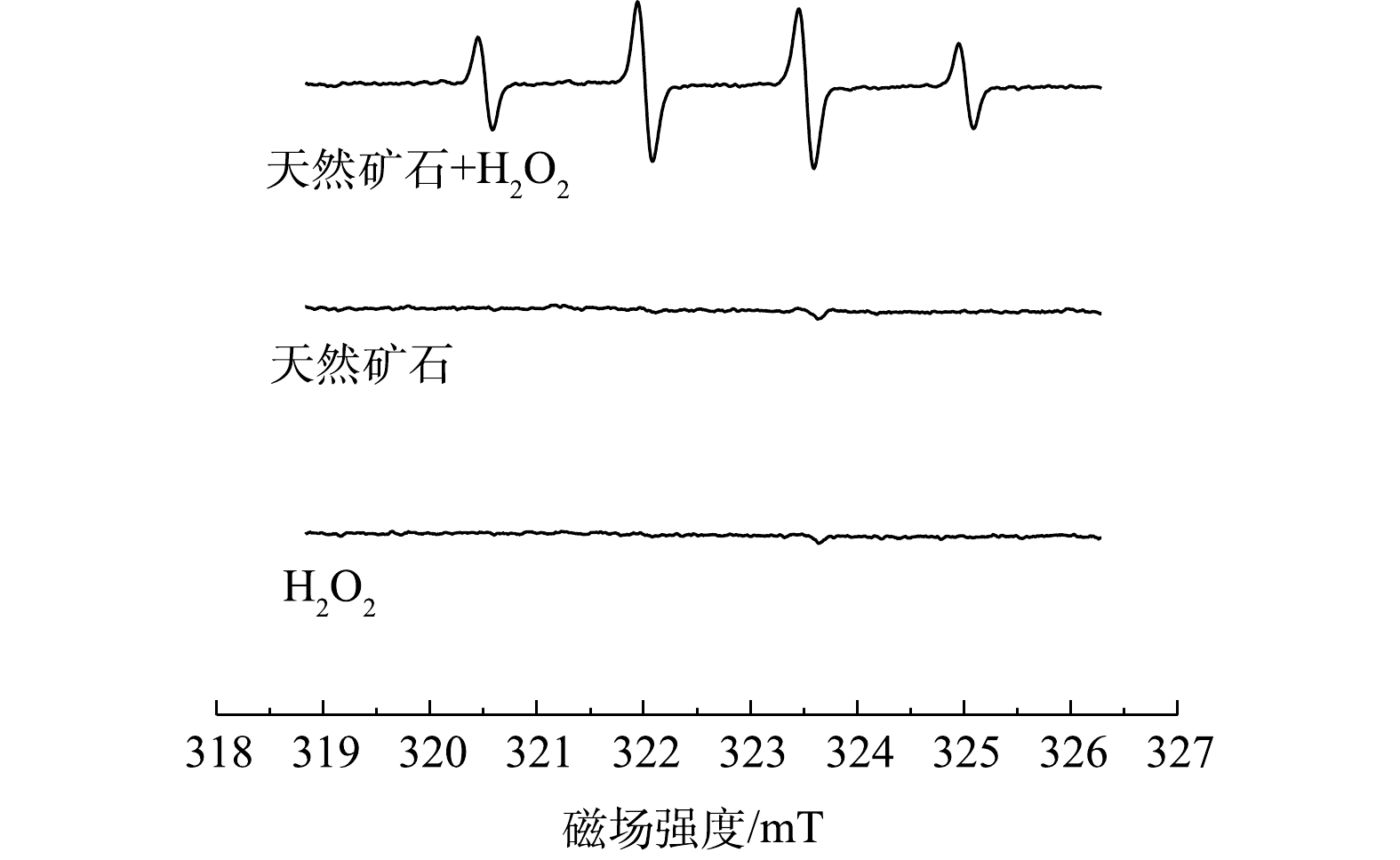
为了进一步鉴定降解过程中生成的自由基,以DMPO为捕获剂,通过电子自旋共振能谱(ESR)对活性种进行了鉴定。图10为单独催化剂吸附、单独过氧化氢氧化和在两者协同氧化条件下的ESR谱图。当溶液中只有矿石催化剂或过氧化氢时,仅有非常微弱的·OH信号峰出现。这是由于矿石中活性位点与水和溶解氧反应会产生少量的过氧化氢,过氧化氢分解产生微量·OH所导致的[26]。当催化剂与过氧化氢共同存在时,溶液中出现强度比为1∶2∶2∶1的信号峰,这与·OH的信号峰一致[27-28]。以上结果表明,矿石催化剂与过氧化氢体系中可产生大量的·OH,证明反应中生成的活性物质为·OH。
2.1. 催化材料表征分析
2.2. 催化降解效果分析
2.3. 机理分析
-
1)消解实验、SEM-EDS和XPS光谱均能证明天然矿石中有低价态的Cu、Fe等金属元素,球磨时间越长,矿石表面铜元素和铁元素含量越高,表明经过物理化学改性后天然矿石可作为高效的非均相芬顿催化剂使用;并且矿石中主要物质为SiO2,失活后方便处理,不会带来二次污染。
2)天然矿石作为异相芬顿催化剂活化过氧化氢降解苯酚废水的效果显著。高盐条件对其降解效果的影响较弱,可实际应用于高盐有机废水的处理。此外,经过优化,确定过氧化氢和矿石催化剂的投加量分别为1 g·L−1和0.1%,该配比可大幅降低实际应用的成本。
3)反应后矿石表面有Cu(Ⅱ)出现,Fe(Ⅱ)和Fe(Ⅲ)的比例从1∶0.71下降到1∶1.16,证明Cu(Ⅰ)和Fe(Ⅱ)是天然矿石中发挥催化效果的活性物质。加入甲醇会抑制苯酚的降解,说明·OH是苯酚降解过程中的主要活性自由基,ESR结果进一步证明了反应中·OH的存在。



 下载:
下载: