-
四环素(TC)是一种典型的抗生素,被广泛应用于人体治疗、畜牧业和水产养殖[1]。由于不能被人类和动物完全降解,摄入的TC只能通过粪便和尿液进入环境,经常在地表水、地下水,甚至饮用水中被检测到[2]。持续存在的TC会对生态环境和人体健康造成极大的危害。因此,去除水中的四环素显得尤为重要。不同于高级氧化法、生物处理法、膜过滤法和吸附法[3-6],光催化降解法以其高效、低能耗、绿色环保等优点,在污水处理中表现出良好的应用前景[7]。
金属有机骨架(MOFs)在光催化领域展现出的优异性能而备受关注[8-10]。MIL-88A(Fe)因其很强的可见光响应且安全无毒,被应用于光催化废水处理中[11]。但是,MIL-88A(Fe)由于有机配体的存在导致光催化剂光生电子-空穴对快速复合[12],且其较低的比表面积影响了其对污染物的吸附效果[13],从而限制了其光催化活性。已有研究利用氧化石墨烯(GO)促进MOFs的光生电荷的转移和传输,改善了材料的光催化性能,并且增强MOFs的稳定性[14]。但GO表面官能团有限[15],不能为MOFs晶体提供充足的生长位点,无法提供更多的吸附活性位点,这些都将阻碍其对水中污染物的高效吸附。离子液体(ILs)是一种具有良好稳定性、电导性、安全性的绿色溶剂[16],可以提升复合材料的分散性、导电性、吸附性能[17-19]等。此外,可以通过调节阴离子和阳离子的组成或在常规ILs的基础上,引入特定的基团(氨基)来获得功能化的离子液体以提供丰富的官能团,例如被用于酸性气体吸收的含胺基离子液体[TETAH]+[Ac]−[20]。据调研,目前尚未见[TETAH]+[Ac]−辅助生长IL/GO/MOFs用于光催化降解水中四环素的报道。
在本研究中,采用[TETAH]+[Ac]−辅助生长手段制备了IL/GO/88A,详细考察了其表面形态、晶体结构以及电子和光学性能,充分评估了IL/GO/88A对TC的光催化降解的性能;同时,提出了吸附-光催化协同机理以解释TC可能的降解路径,以期为新型MOFs光催化复合材料的开发及其在水污染控制领域的应用提供参考。
全文HTML
-
三亚乙基四胺(TETA, C6H18N4)、冰乙酸(HAc, CH3COOH)、六水合三氯化铁(FeCl3·6H2O)、反丁烯二酸(C4H4O4)、四环素(C22H24N2O8)、异丙醇((CH3)2CHOH)、对苯醌(C6H4O2)、乙醚(C4H10O)、乙醇(C2H5OH)、甲醇(CH3OH)、氢氧化钠(NaOH)、盐酸(HCl)均为分析级纯,氧化石墨烯(GO)为实验室自制,实验用水为去离子水。
-
用恒压漏斗将0.2 mol·L−1 TETA溶液添加到等摩尔浓度的HAc溶液中,并在<15 ℃条件下搅拌6 h,所得溶液用乙醚萃取,在80 ℃下真空浓缩,直至达到无水状态,得到离子液体[TETAH]+[Ac]−。
将MOFs前驱体1.5 mmol FeCl3·6H2O和1.5 mmol C4H4O4,投加到7.5 mL乙醇水溶液(V乙醇∶V水 = 1∶2)中,直到固体完全溶解。然后,将混合物转移到25 mL聚四氟乙烯内衬,密封在不锈钢高压釜中,并在65 ℃下加热12 h。待自然冷却后,分别用去离子水和无水乙醇洗涤纯化24 h。最后,将样品过滤并在50 ℃真空干燥12 h,收集橘红色粉末MIL-88A(Fe)[21]。
将5.9 mg GO、1.5 mmol [TETAH]+[Ac]−和相同摩尔质量的MOFs前驱体投加到乙醇水溶液,重复上述MIL-88A(Fe)的制备步骤,得到棕色粉末IL/GO/88A。作为对照,制备未添加ILs的复合材料,标记为GO/88A。
-
本研究中,光催化剂表征所采用的仪器包括X射线衍射仪(Bruker D8 Advance,德国布鲁克公司)、傅里叶变换红外光谱(NICOLET 5700,美国尼高力仪器公司)、扫描电子显微镜(Merlin,德国卡尔·蔡司公司)、全自动比表面及孔隙度分析仪(ASAP 2460,美国麦克公司)、紫外可见分光光度计(UV-3600,日本岛津公司)、电化学工作站(CHI 760D,上海辰华仪器有限公司)。
-
实验装置以300 W Xe灯(λ > 420 nm)为实验光源,250 mL夹套烧杯为反应器。实验过程中,开启循环冷却水使装置温度稳定在(25 ± 2) ℃,将催化剂投加到装有200 mL TC溶液的反应器中,用0.1 mol·L−1氢氧化钠和盐酸溶液调节反应溶液的pH,在黑暗条件下通过磁力搅拌60 min使催化剂达到吸附-解吸平衡,然后开启Xe灯照射悬浮液180 min,每个照射间隔采样2.0 mL并通过0.22 μm过滤器,使用紫外可见分光光度计(U-2910,日本日立公司)分析滤液中TC浓度,检测波长为357 nm。TC的去除率公式见式(1)。
式中:η为TC去除率;C0为TC的初始浓度,mg·L−1,Ct为t时刻对应的TC浓度, mg·L−1。
1.1. 实验原料
1.2. 催化剂的制备
1.3. 材料的表征仪器
1.4. TC的降解实验
-
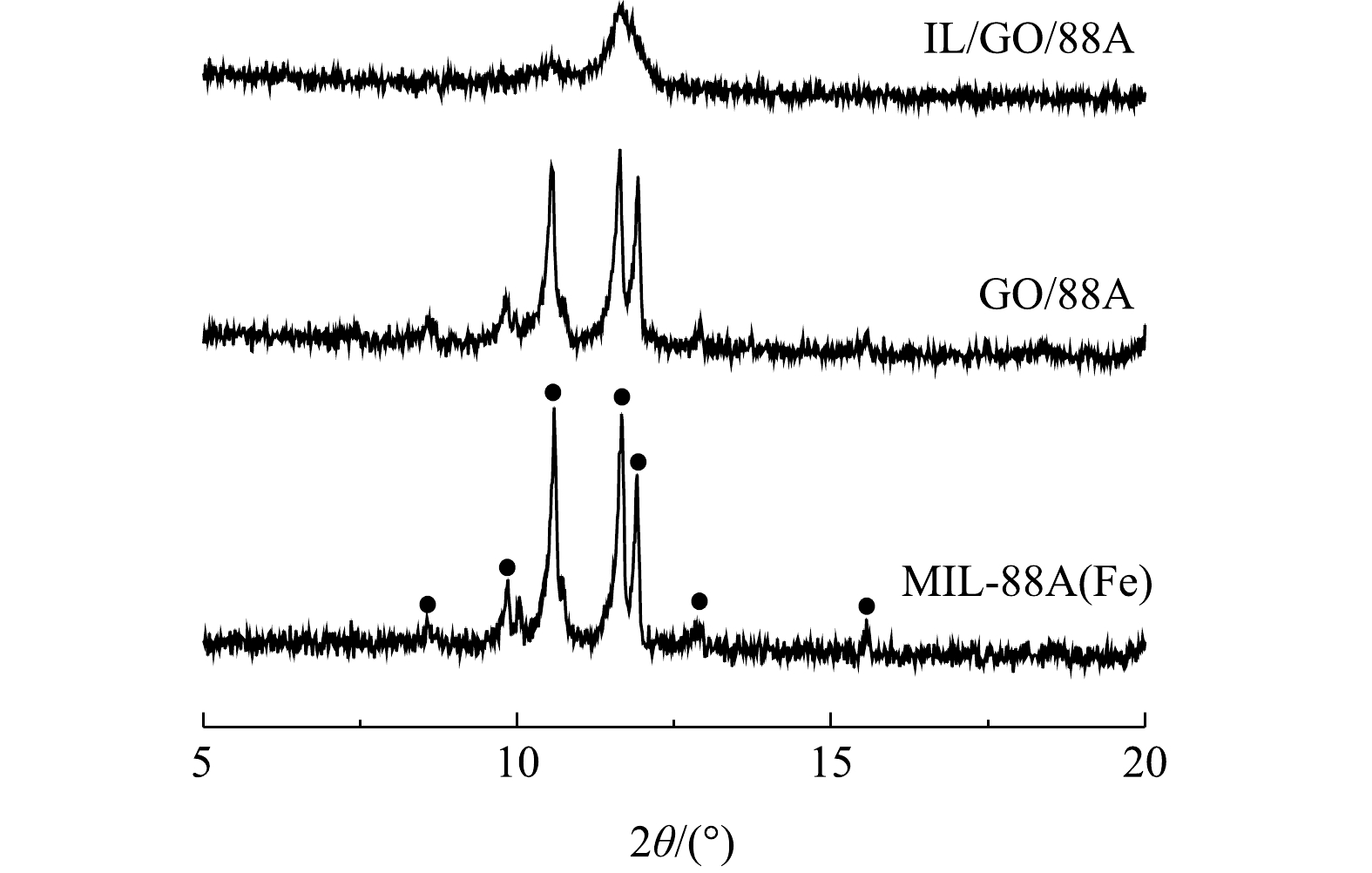
1) XRD分析。图1为MIL-88A(Fe)、GO/88A和IL/GO/88A的XRD图谱。由图1可以看出,MIL-88A(Fe)在2θ 为8.6°、9.9°、10.5°、11.7°、11.9°、12.8°、15.6°处具有强衍射峰,与先前的报道结果相符[22],表明MIL-88A(Fe)合成成功。GO/88A表现出与MIL-88A(Fe)相似的特征峰,但峰强略有减弱,这说明GO的引入阻止了MIL-88A(Fe)的适当结晶,从而导致MOFs结构的轻微变形。在IL/GO/88A的衍射图中,其峰强显著弱于MIL-88A(Fe)和GO/88A,这可能是由于MIL-88A(Fe)和GO被ILs包裹而被屏蔽。
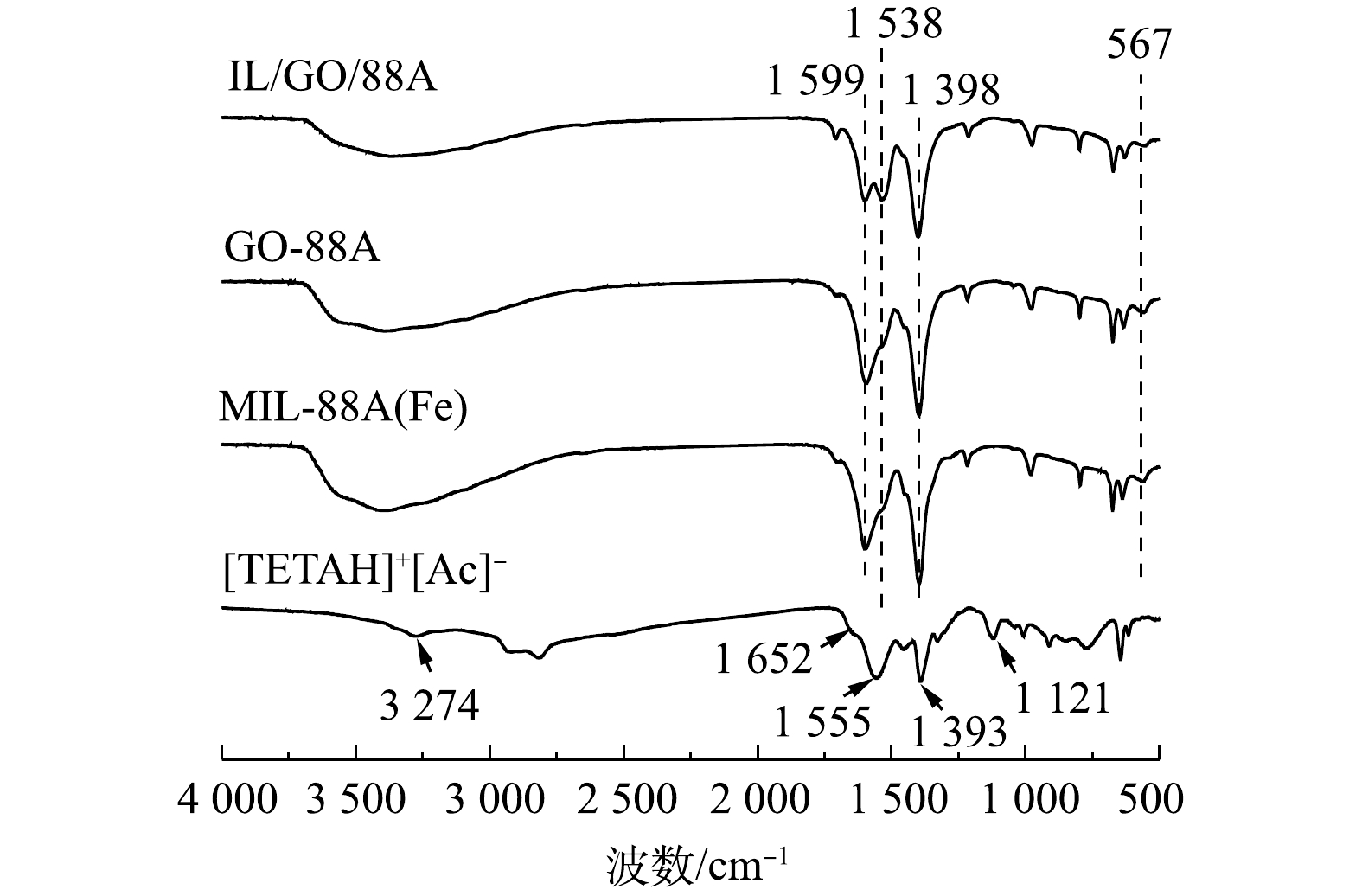
2) FTIR分析。[TETAH]+[Ac]−、MIL-88A(Fe)、GO/88A和IL/GO/88A的红外光谱如图2所示。由图2可见,在[TETAH]+[Ac]−中,3 274 cm−1附近较宽的吸收峰是—NH2的伸缩振动峰,1 555 cm−1是N—H的弯曲振动,1 121 cm−1为C—N的拉伸振动峰,1 652 cm−1和1 393 cm−1分别对应—COO的不对称伸缩振动和对称伸缩振动峰[23]。对于MIL-88A(Fe),1 599 cm−1和1 398 cm−1处的2个较强的峰分别来自羧基的不对称和对称振动。此外,在567 cm−1处的峰可归因于金属有机骨架的Fe—O振动,在3 000~3 500 cm−1处的较高波数处有1个较大的凸起,这来自样品对水的吸附[24]。GO/88A表现出与母体MIL-88A(Fe)相似的光谱,而IL/GO/88A在1 538 cm−1处新增了N—H的峰,表明ILs已成功引入复合材料中,并且不影响MIL-88A(Fe)单元的构成。
3) SEM分析。图3展示了MIL-88A(Fe)、GO/88A、IL/GO/88A的微观形貌。图3(a)为MIL-88A(Fe)晶体,其呈现出表面略微粗糙的棒状结构,长度为400 nm左右。在GO/88A中(图3(b)),可看到MIL-88A(Fe)单元紧密地覆盖在GO的片层上,并且两者被完整地保留。在添加ILs后,图3(c)中IL/GO/88A的晶型发生变化,尺寸减小,表面粗糙,这可归因于高度分散的ILs使MOFs快速成核,并包裹在MOFs和GO的表面。
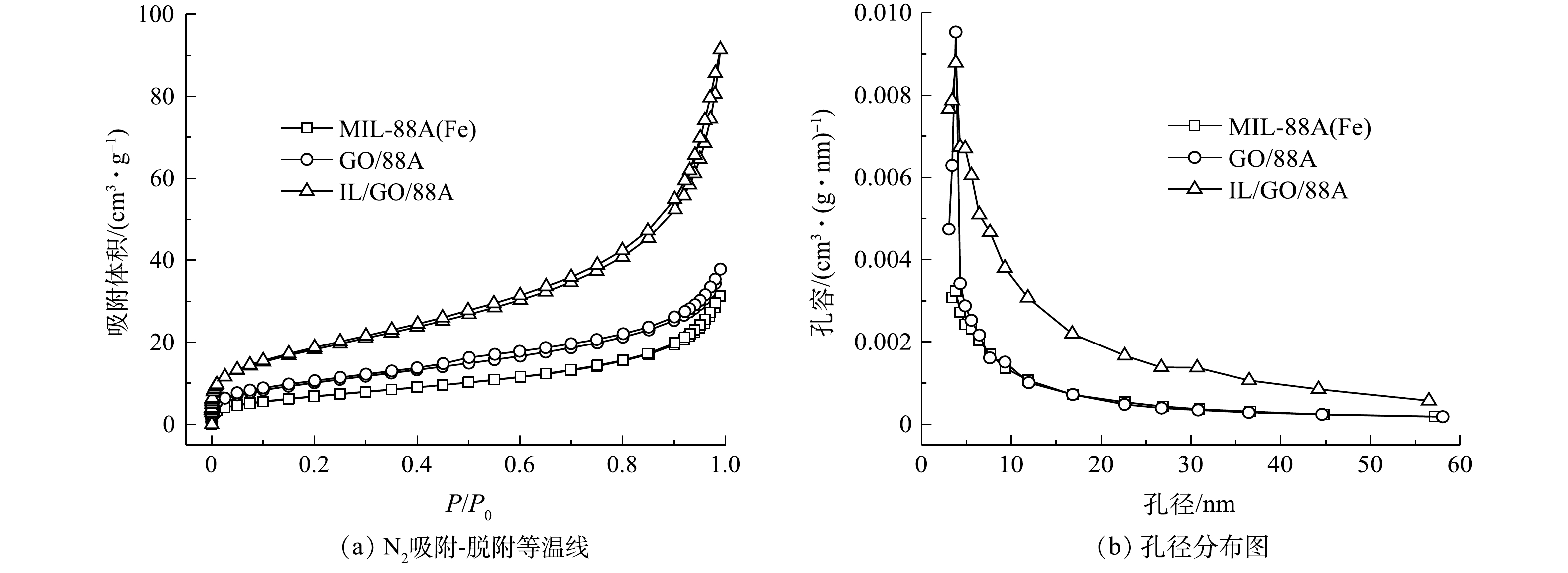
4) N2吸附-脱附分析。图4是MIL-88A(Fe)、GO/88A、IL/GO/88A的N2吸附-脱附等温线和孔径分布图。由图4(a)可以看出,3种材料的N2吸附-脱附等温线均为典型的Ⅳ型等温线,且具有明显的回滞环,表明存在介孔[25]。这说明ILs和GO的引入不会改变材料的吸附特征。经计算得出,IL/GO/88A的比表面积为66.22 m2·g−1,高于MIL-88A(Fe)的25.17 m2·g−1和GO/88A的36.98 m2·g−1,这可归因于IL/GO/88A的尺寸减小和表面粗糙。由相应的孔径分布结果(图4(b))可知,MIL-88A(Fe)、GO/88A、IL/GO/88A的平均孔径分别为3.827、3.826、3.812 nm。通常,较高的比表面积将暴露更多的活性位点,介孔结构可以促进反应物和活性物质的运输,从而促进材料的催化反应。这说明IL/GO/88A具有更加优异的光催化性能。
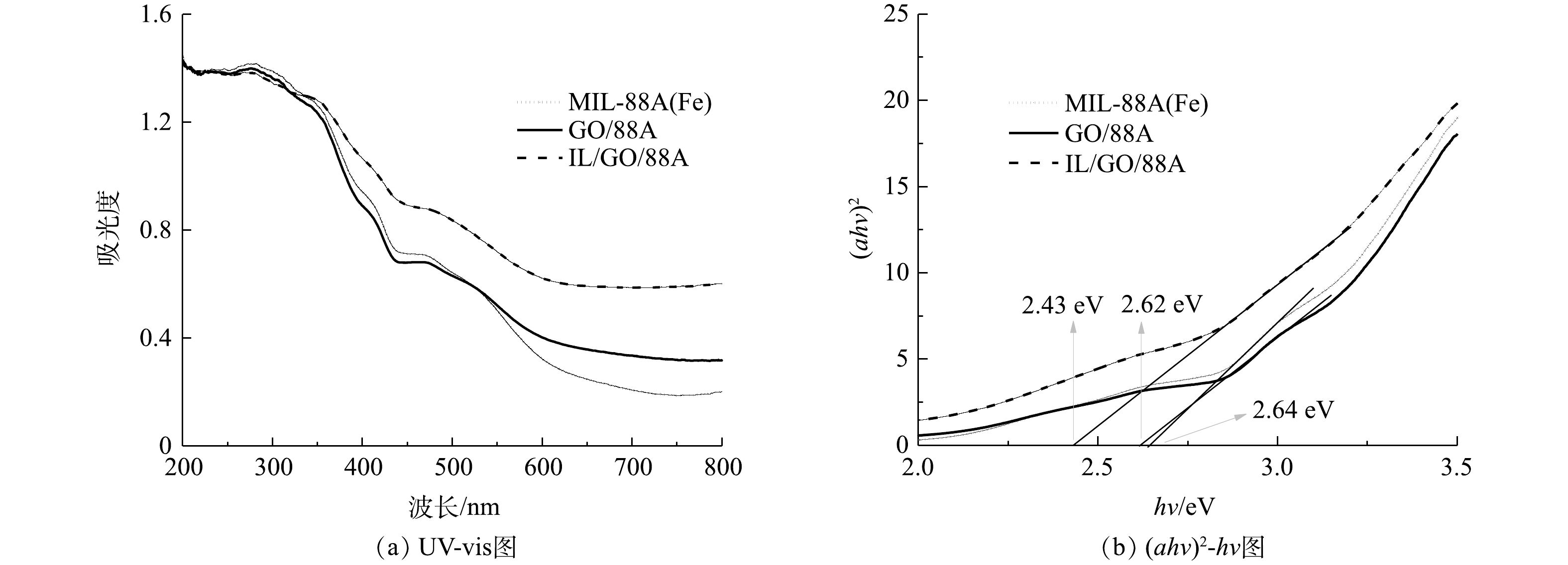
5) UV-vis分析。MIL-88A(Fe)、GO/88A、IL/GO/88A的光响应能力如图5(a)所示。MIL-88A(Fe)在200~600 nm处显示出显著的光吸收。经GO结合,GO/88A在600~800 nm处光吸收能力有所改善。当材料中引入ILs后,IL/GO/88A在可见光区的光吸收强度明显增强,且观察到其吸收带边发生了红移。根据禁带公式,可以计算出光催化剂的带隙能(图5(b))[26],其中MIL-88A(Fe)、GO/88A、IL/GO/88A的带隙能分别为2.64、2.62和2.43 eV。以上结果证明,通过引入ILs能够有效降低光催化材料的禁带宽度,拓宽光吸收范围,从而提高其对可见光的利用效率,这可进一步提升催化剂的光催化活性。
6) EIS分析。为了进一步证实上述结论,对MIL-88A(Fe)、GO/88A、IL/GO/88A进行了电化学阻抗测量,以评估光催化剂光生电子和空穴的分离和转移效率。奈奎斯特阻抗图的半圆直径与电解质溶液和工作电极界面之间的电荷转移电阻一致,较小的半径对应较低的电阻[27]。在图6中,与MIL-88A(Fe)和GO/88A相比,IL/GO/88A表现出最小的阻抗电弧半径,这表明具有导电性的ILs提高了光生电子-空穴对的分离效率。因此,IL/GO/88A独特的运输性质将通过阻碍电荷重组来改善其光催化性能。
-
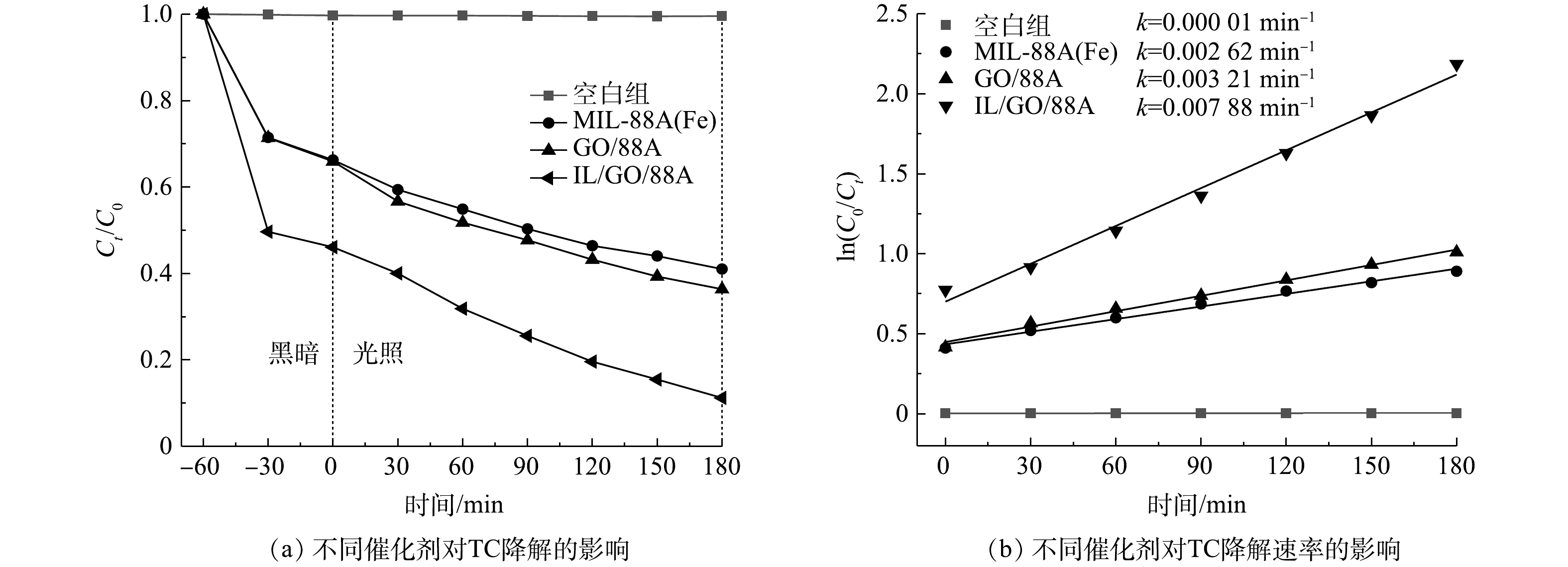
1)催化剂的活性。进行了光催化剂对TC(20 mg·L−1 TC,溶液pH为5.7,催化剂投加量为0.30 g·L−1)的光降解实验。由图7(a)中空白组可见,在没有催化剂的条件下,TC几乎不降解。在MIL-88A(Fe)体系中TC的光催化效率可达59.0%。对于GO/88A,其对TC的降解率为63.6%,这说明少量GO的修饰,可以改善MIL-88A(Fe)的光催化活性。相比之下,IL/GO/88A表现出更高的吸附性能和降解效率,在光照180 min后TC的去除率达88.8%。这是由于IL/GO/88A更大的比表面积能够吸附更多的TC分子,增强的光响应能力能够促进光催化活性,从而使其提高对TC的去除率。由ln(C0/Ct)与辐照时间之间的线性关系可以推断,TC的光降解动力学拟合曲线遵循准一级动力学模型[28]。如图7(b)所示,IL/GO/88A体系对TC的光催化降解速率最大(k=0.007 88 min−1),分别为MIL-88A(Fe)(k=0.002 62 min−1)和GO/88A(k=0.003 21 min−1)的3.01倍和2.45倍,这再次证明ILs、GO和MIL-88A(Fe)的复合作用显著优化了材料的光催化性能。
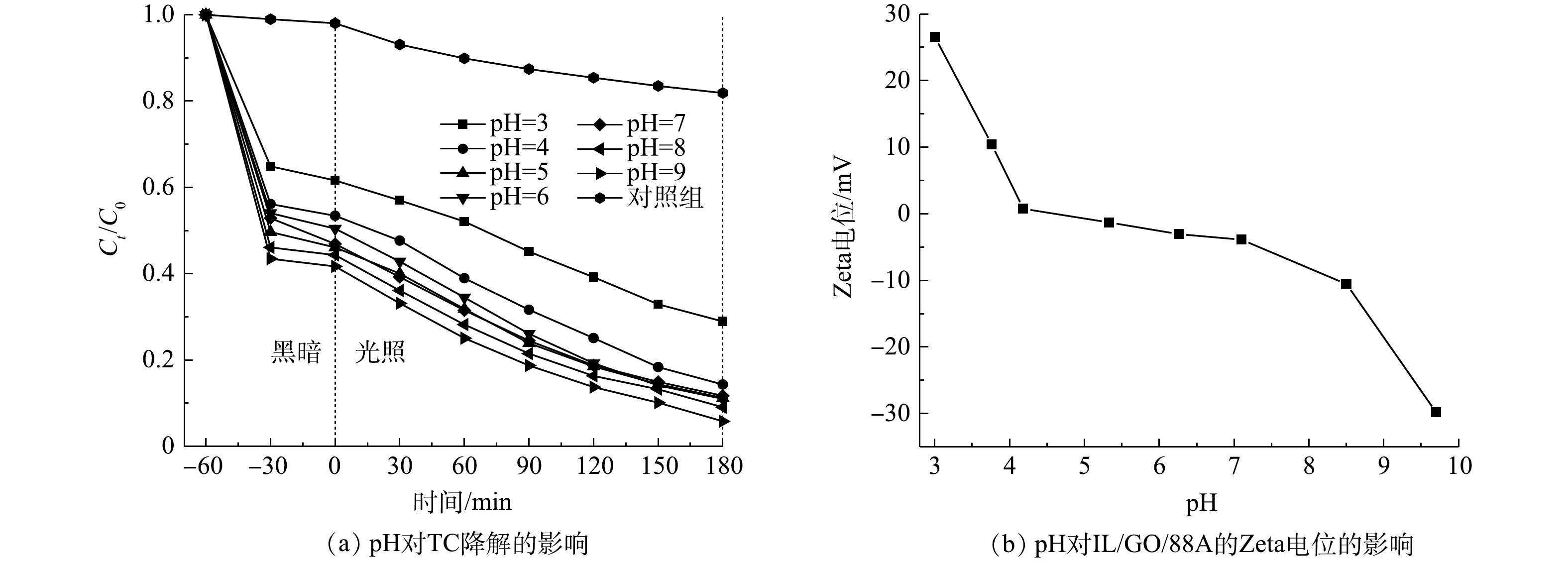
2) pH的影响。在不同的pH环境下,IL/GO/88A对TC的光催化降解如图8(a)所示(TC浓度为20 mg·L−1,催化剂投加量为0.30 g·L−1)。可以看出,在pH为3.0时,TC的降解率仅有71.1%;随着pH从4.0增加到9.0,TC的降解率也由85.7%逐渐增加到94.2%。图8(b)展示了IL/GO/88A的Zeta电位与pH的关系。在pH为3.0 时,IL/GO/88A表面带正电荷,会和同样带正电的TC产生静电排斥,因此,IL/GO/88A难以捕获TC从而阻碍了光催化进程。当pH为4.0~7.0时,IL/GO/88A接近电中性,此时其与TC之间不存在静电作用。而TC在碱性条件下同样可被高效去除,这是由于TC的水解作用和光解作用(图8(a)中的对照组,pH为9.0,不投加催化剂)所致。综上所述,pH会引起光催化剂和目标污染物之间作用力,从而影响其吸附能力,进而影响光催化剂的活性。由于TC在水溶液中的pH稳定在5.7±0.1,且IL/GO/88A在该pH条件下可以保持对TC较高的去除率,因此,后续实验不再调整溶液的pH。
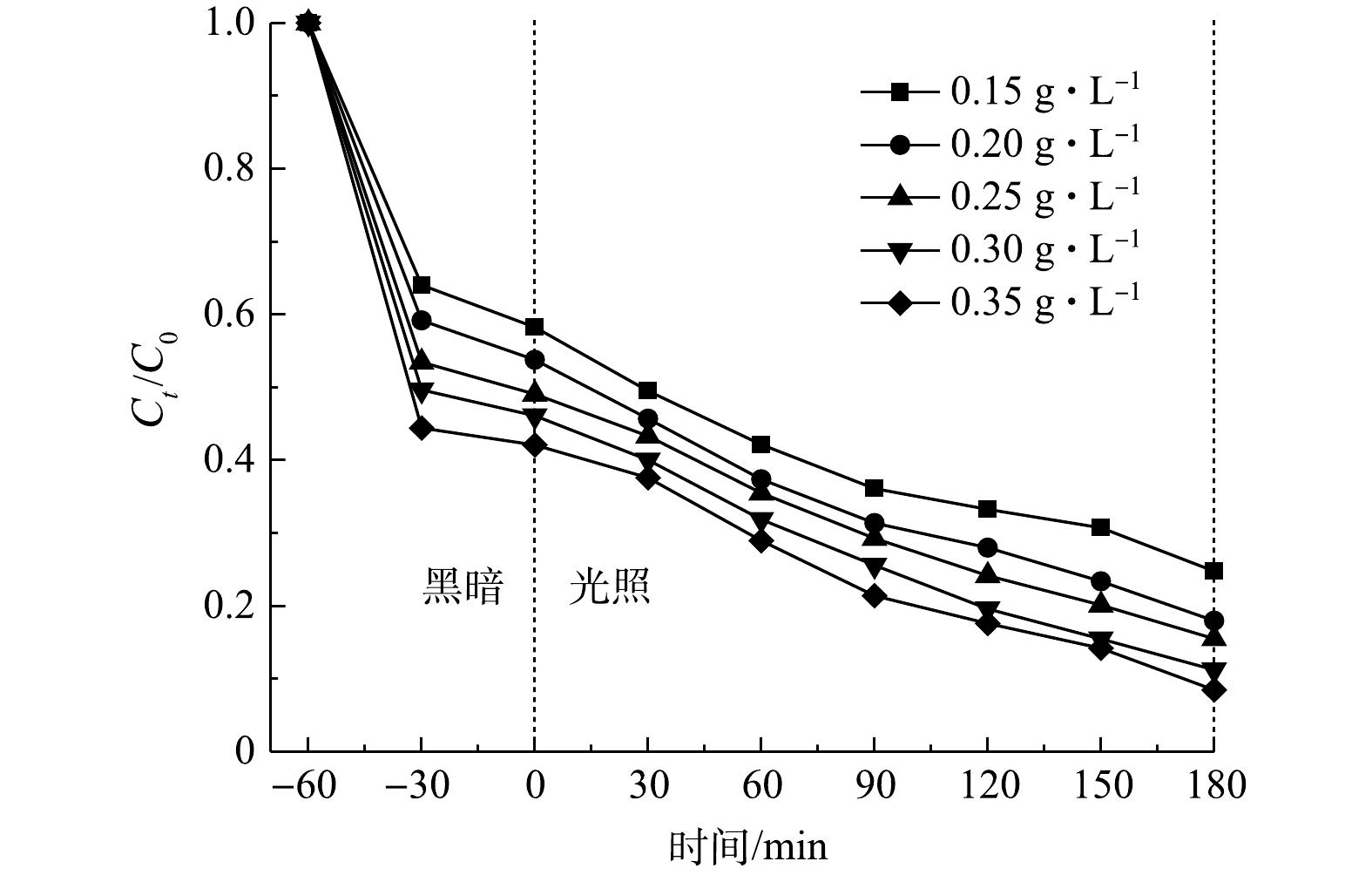
3)催化剂投加量的影响。考察了投加量为0.10~0.35 g·L−1的复合光催化剂对TC降解的影响(TC浓度为20 mg·L−1,溶液pH为5.7),结果如图9所示。随着催化剂投加量的增加,TC的去除效率也逐渐提高。这是由于催化剂增加了与TC的接触面积,提供了更多吸附和催化降解TC的活性位点。此外,当催化剂的投加量从0.30 g·L−1增加到0.35 g·L−1时,TC的去除率由88.8%提升到91.5%,其增幅较小。这可能是高浓度的催化剂一方面可导致对光的屏蔽效应,从而阻碍光催化[29],另一方面可造成催化剂的团聚,无法充分发挥其吸附作用[30]。考虑到去除效率和经济性,在后续实验中选择0.30 g·L−1的催化剂投加量进行TC的降解。
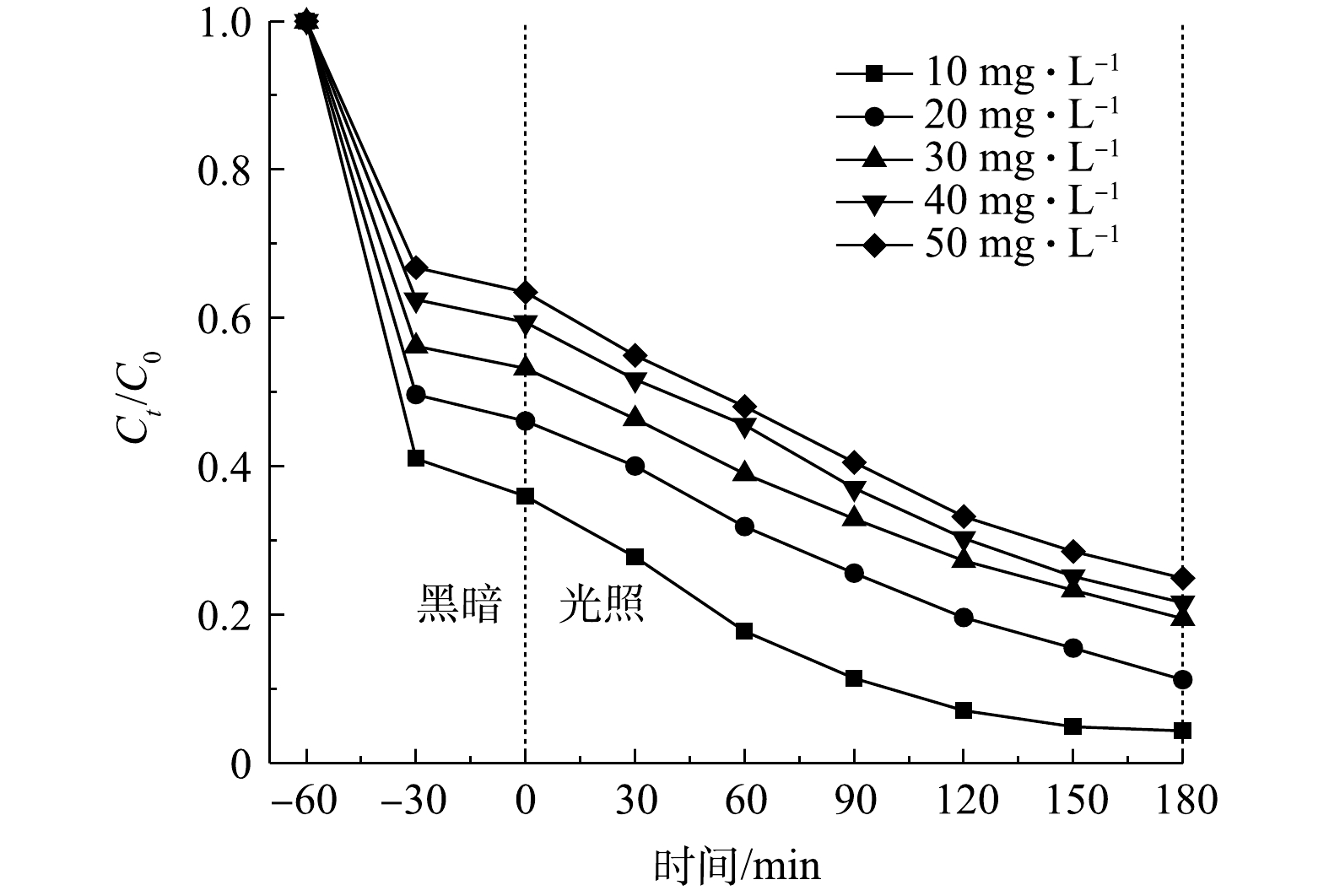
4) TC初始浓度的影响。分别考察TC初始浓度在10~50 mg·L−1内对光催化降解的影响(pH为5.7,催化剂投加量为0.30 g·L−1),结果如图10所示。显然,TC浓度在吸附阶段起着重要的作用,当TC的初始浓度从10 mg·L−1提高到50 mg·L−1时,IL/GO/88A在黑暗反应阶段的吸附率由64.0%降低到36.5%。在光照180 min后,50 mg·L−1 TC的光催化降解率仅有75.1%,10 mg·L−1 TC的光催化降解率最高达95.7%。这是由于催化剂上的吸附位点有限,随着TC浓度的升高,吸附位点被吸附在催化剂表面的TC分子所占据[31]。此外,在光催化过程中,TC降解生成了其他中间体,从而与TC产生了竞争关系[32]。鉴于四环素在实际废水中的浓度非常低,因此,本研究中所使用的光催化剂在低浓度TC下进行光催化实验具有现实意义。
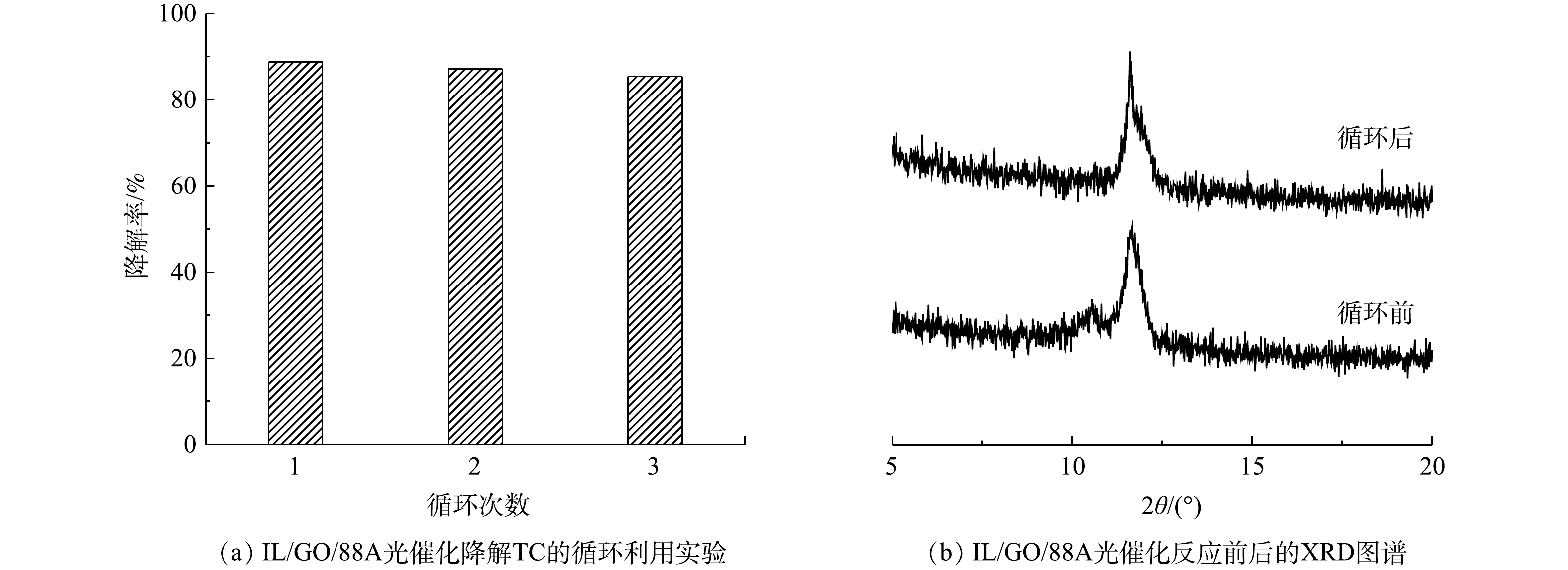
5)循环利用和稳定性分析。在实际工程应用中,材料的稳定性和回收价值是评价其性能的重要指标。针对IL/GO/88A,考察了其在光催化降解水中TC的重复利用性能(TC浓度为20 mg·L−1,pH为5.7,催化剂投加量为0.30 g·L−1)。每次反应结束后通过过滤回收样品,并用水和乙醇洗涤3次,然后在50 ℃下真空干燥6 h。如图11(a)所示,可以看出,IL/GO/88A对TC的去除率略有下降,但仍保持在86%左右,表明材料的光催化活性没有发生明显的变化,具有良好的循环利用性能。此外,通过对比反应前后IL/GO/88A的XRD图谱(图11(b)),在2θ为10.5°附近的峰强减弱,这可能是由于材料经过多次反应和洗涤引起的。但主要特征峰基本一致,这说明复合材料具有较好的稳定性。
-
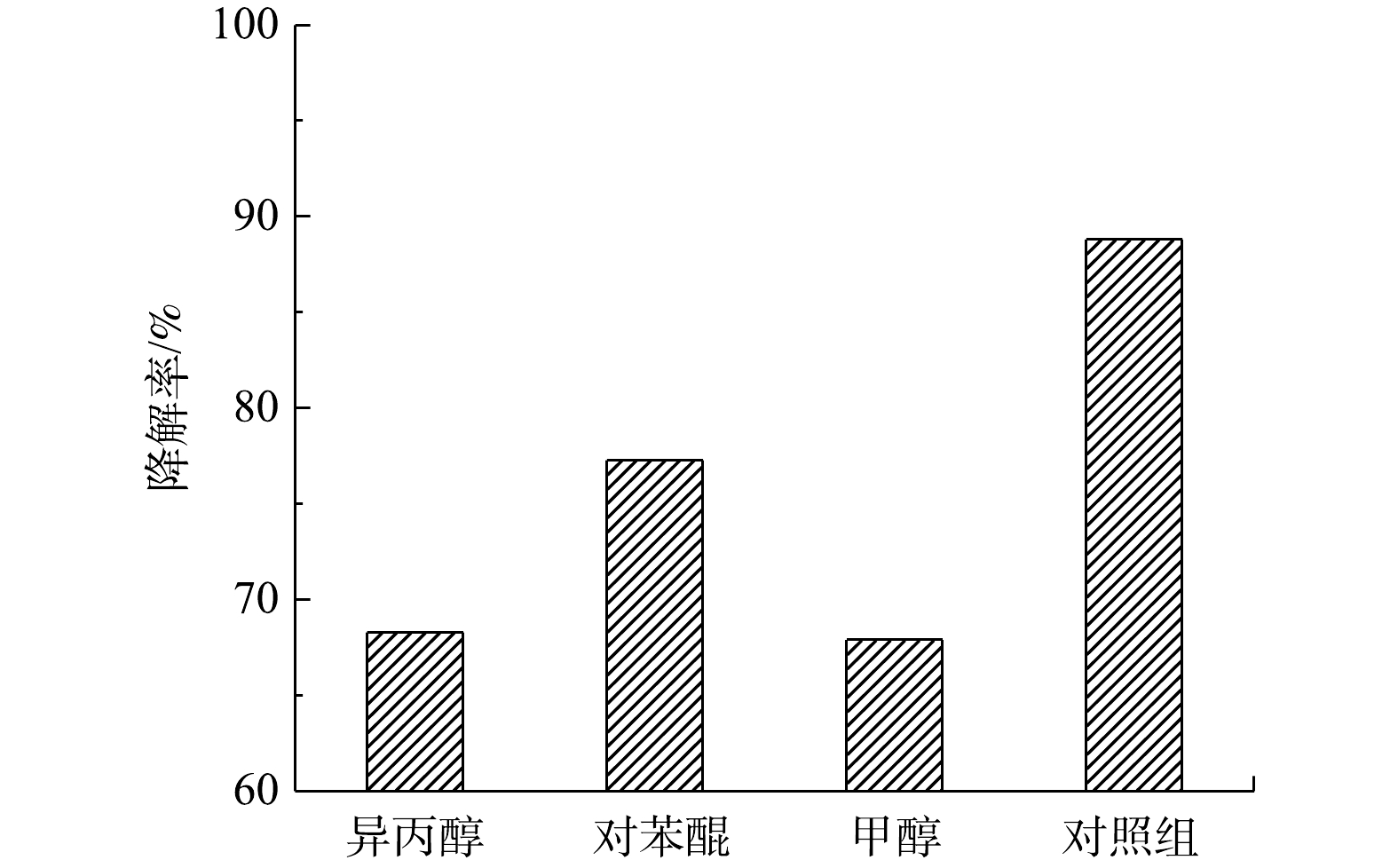
为了研究光催化反应中的主要活性物种以及降解过程的可能机理,用65 mmol·L−1异丙醇、3 mmol·L−1对苯醌、120 mmol·L−1甲醇分别淬灭活性物质羟基自由基·OH、超氧自由基·
O−2 、价带空穴h+。如图12所示,在添加不同类型的淬灭剂后,IL/GO/88A对TC的光催化降解均受到不同程度的抑制。这表明·OH、·O−2 、h+在反应系统中作为共氧化物种发挥了重要作用,其中,·OH、h+的贡献最大。根据上述实验结果,可提出IL/GO/88A复合材料增强的吸附-光催化协同机理。在协同过程中,IL/GO/88A吸附污染物使TC富集在光催化剂表面,在可见光照射下,IL/GO/88A的Fe—O团簇发生电子激发,光生电子e−在ILs的引导下迅速转移,同时产生相同数量的空穴h+,e−与材料表面吸附的O2进一步反应生成·OH、·O−2 以氧化TC,而具有强氧化性的h+则可以直接氧化TC。总体而言,较大的比表面积、可见光吸收的增加以及光生电子-空穴对的快速分离,均有助于光催化反应的进行。 -
表1列出了IL/GO/88A与先前报道的MOFs光催化剂对TC的降解比较。可以看出,考虑到催化剂投加量和可见光照射时间,IL/GO/88A对TC的降解效果相比于其他MOFs复合材料具有一定的优势。这表明利用ILs辅助制备IL/GO/88A复合材料用于光催化降解四环素是可行的。
2.1. 表征结果
2.2. 光催化性能分析
2.3. 光催化机理
2.4. 与其他MOFs光催化剂的比较
-
1)通过ILs辅助合成了IL/GO/88A复合光催化剂。ILs和GO的引入增大了光催化剂的比表面积,提高了其可见光利用率和电子传输性能。在可见光照射180 min后,IL/GO/88A对TC的光催化降解速率k 为0.007 88 min−1,分别是MIL-88A(Fe)(k = 0.002 62 min−1)和GO/88A(k = 0.003 21 min−1)的3.01倍和2.45倍。
2)催化剂投加量、溶液pH和TC初始浓度对IL/GO/88A光催化降解TC活性有较大影响。当IL/GO88A为0.30 g·L−1、pH为5.7、TC初始浓度为10 mg·L−1时,TC的光催化降解率可达到95.7%。
3)·OH、·
O−2 、h+对TC降解均有贡献,其中,·OH、h+是光催化过程中的主要活性物种。同时,该催化剂具有良好的可重复利用性和稳定性。相比其他MOFs复合材料,IL/GO/88A光催化降解TC表现出明显的优势。



 下载:
下载: