-
随着市政污水处理量逐年递增,导致污泥产量及污泥处理压力也迅速增加。目前污泥的主要处置方式有卫生填埋、焚烧、建材利用和土地利用等。然而,污泥中含有大量有机质、氮磷钾等营养物质,有利于其资源化、能源化处理。利用城市污水厂污泥制备污泥活性炭是上世纪80年代出现的一种新型污泥资源化利用途径[1]。相比于传统的污泥处理处置方法,市政污泥经高温碳化和活化制备污泥炭,在热解过程中能够杀死污泥中的病原体、固定污泥中的重金属和碳元素,具有良好的环境效益和经济效益[2-3]。在过去十几年里,污泥碳化制备生物炭应用于有机污染物的吸附与降解已取得一些研究进展[4-6]。
厌氧颗粒污泥法能有效处理高浓度有机废水,例如啤酒废水、制药废水和煤化工废水等,其工艺具有效率高、成本低、操作方便等优势[7-8]。厌氧颗粒污泥是微生物自絮凝的结果,为疏松结构且含大量的营养物质。由于厌氧颗粒污泥中含有大量的微生物,且种类丰富,故通过微生物新陈代谢作用可以将金属很好的分散在颗粒污泥中。以无定型污泥(活性污泥、脱水污泥等)作为底物制备污泥炭,其成型过程费用较高[9]。而颗粒污泥在厌氧反应过程中自然成型,并在热解后保持颗粒状态。因此,厌氧颗粒污泥是制备污泥炭的一种潜在原料[10],但还缺乏相关研究报道。
抗生素广泛应用于人类和动物的疾病预防与治疗,抗生素废水含有多种难降解且具有生物毒性的物质,污水处理厂对抗生素的最高转化率仅为81%,低浓度的抗生素也可能对环境造成潜在的影响。而催化湿式过氧化氢氧化技术(catalytic wet peroxide oxidation,CWPO)是一种处理难降解有机物废水的有效方法,其具有反应条件温和、试剂无毒[5]的特点。
本研究以厌氧颗粒污泥为原料制备污泥炭催化剂,以第1类头孢类抗生素——头孢氨苄为模型污染物,在CWPO体系中对其进行了降解实验,在此过程中考察了颗粒污泥炭的催化性能和稳定性,同时分析了颗粒污泥炭的理化性质,检测了中间产物并提出了可能的降解途径。本研究可为污泥的资源化、能源化利用和抗生素废水的高效治理提供参考。
全文HTML
-
厌氧颗粒污泥取自山西省某淀粉废水厂的废水处理厌氧反应器,粒径2~3 mm。依次用超纯水和乙醇冲洗颗粒污泥,再自然晾干,之后置于烘箱中105 ℃处理3 h,待冷却后,用管式炉进行炭化处理,实验装置示意图参考文献中的方法[11]建立。制备条件为:在80 mL·min−1的N2气氛下,以3 ℃·min−1速率升温至800 ℃,焙烧3 h,待冷却后,记为未改性颗粒污泥炭GSC-O。用53.4% (质量分数)的H3PO4在25 ℃对GSC-O进行改性24 h,之后用超纯水冲洗至中性,记为改性颗粒污泥炭GSC-P。
-
配置头孢氨苄废水模拟溶液100 mL,加入250 mL锥形瓶中,再投加颗粒污泥炭,置于设置好温度的水浴振荡器上,以120 r·min−1的速度振荡混合进行吸附实验。待吸附平衡后,加入一定的过氧化氢,进行CWPO催化降解反应,每隔一段时间进行取样并立即加入Na2SO3抑制反应进行,用0.45 μm滤膜过滤后分析头孢氨苄的转化率。分别考察温度、pH、过氧化氢投加量、催化剂投加量、反应物初始浓度和反应时间等因素对头孢氨苄降解过程的影响。反应条件为:温度60 ℃、pH为3、过氧化氢投加量为1.0 g·L−1、催化剂投加量为1.0 g·L−1、反应物初始浓度为100 mg·L−1和反应时间300 min。实验过程中,改变其中一种条件,研究其对头孢氨苄转化率和TOC去除率的影响。
-
使用元素分析仪(EURO EA3000)和电感耦合等离子体发射光谱法(ICP-OES,Horiba Jobin-Y von)测定颗粒污泥炭的元素组成;使用美国麦克ASAP 2460型物理吸附仪测定污泥炭的比表面积和孔容积;使用Rigaku Utima IV型X射线衍射仪(XRD)分析晶型结构;使用ZEISS MERLIN型扫描电子显微镜(SEM)结合X射线能谱(EDS)和元素面分布技术(EDS-mapping)观察颗粒污泥及颗粒污泥炭的表观形貌特征和元素组成;使用电子顺磁共振技术(EPR)技术并以5,5-二甲基-1-吡咯啉-N-氧化物(DMPO,C6H11NO)为自旋捕捉剂进行自由基检测;头孢氨苄采用液相色谱法(伍丰LC100高效液相色谱仪)进行测定,色谱柱:Bioband GP120-C18(250 mm×4.6 mm,5 μm),流动相A为甲醇,流动相B为超纯水,A∶B=40∶60 (v∶v),检测波长λ为254 nm,流速为1.0 mL·min−1,柱温为35 ℃,进样量20 μL;用Bruker Solarix 15T 傅立叶变换离子回旋共振质谱(FT-ICR MS)探测鉴定头孢氨苄降解中间产物,数据处理由软件DataAnalysis 4.2(Bruker, Daltonics GmbH, Bremen, Germany)完成。
1.1. 颗粒污泥炭的制备
1.2. 实验方法
1.3. 检测方法
-
1)颗粒污泥炭组成。由表1可知,颗粒污泥炭的产率较低,经过磷酸改性后,部分灰分被去除,产率进一步降低。颗粒污泥在灰分去除的同时形成多孔结构,颗粒污泥炭的比表面积由10.03 m2·g−1增加到96.21 m2·g−1。由此可见,经过改性后,颗粒污泥炭的比表面积和孔容积均得到改善。
颗粒污泥中金属类物质较丰富,因此,其热解产物污泥炭中金属种类较多。由表1可知,GSC-O和GSC-P的含铁量分别为6.06%和5.44%。与脱水污泥制备的污泥炭含铁量(0.978%)相比[12-13],颗粒污泥炭的含铁量很高,而Fe又是催化剂中重要的活性组分,这有利于后续进行的头孢氨苄降解实验。
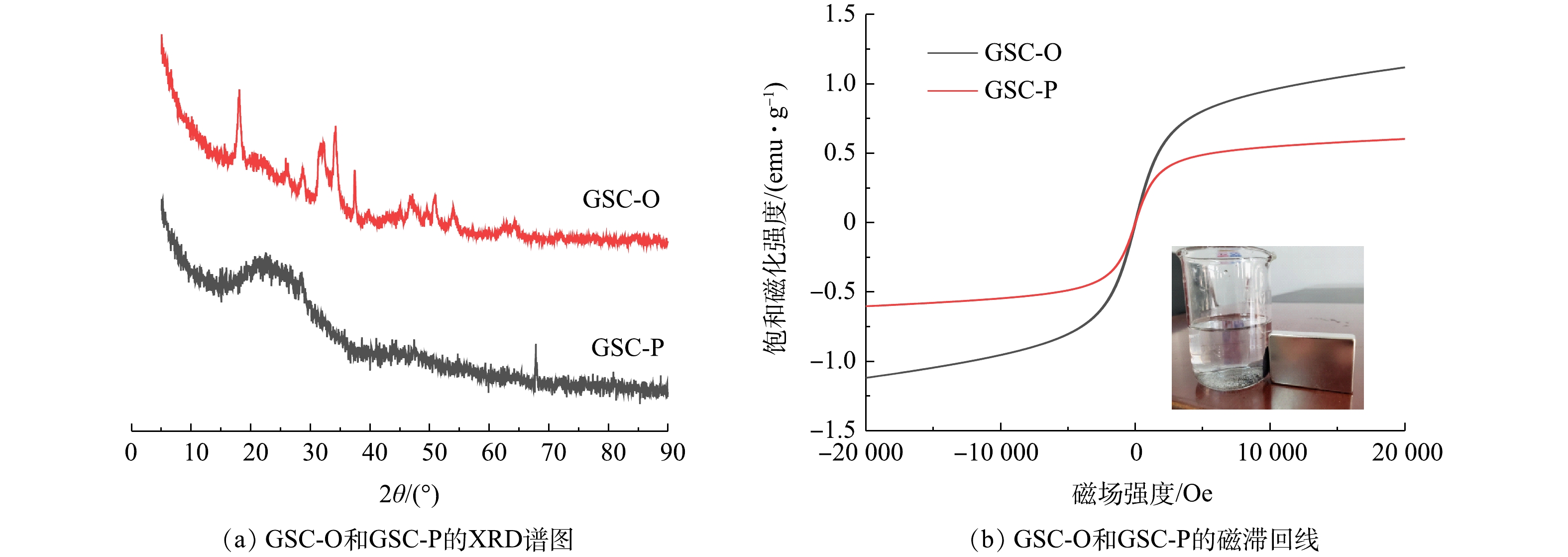
2)颗粒污泥炭表征。颗粒污泥炭的XRD分析结果如图1(a)所示。GSC-O具有一些明显的特征峰,位于28.73°、34.17°、47.25°和50.90°的峰属于Ca(OH)2(JCPDS 72-0156)的(100)、(011)、(012)和(110)晶面,位于32.24°、37.40°和53.93°的衍射峰是CaO(JCPDS 82-1690),位于18.29°、21.15°、37.07°和47.16°衍射峰是Fe3O4(磁性,JCPDS 79-0416)。TU等[14]的研究也表明污泥炭中存在Fe3O4,这是其具有磁性的重要原因。另外,位于31.13°和33.82°的峰是Fe2O3(JCPDS 40-1139)的晶面(113)和(116)。然而,GSC-P污泥炭的XRD谱图几乎没有明显特征峰,在13°~35°处的宽峰是一种典型的无定型结构[15]。这说明改性后污泥炭的晶体结构被破坏[14]。由图1(b)可知,虽然改性后磁性稍有降低,但GSC-P仍具有磁性,可被磁铁吸引,故GSC-P易实现回收和利用。
颗粒污泥及污泥炭的表面特征如图2所示。与颗粒污泥外部(图2(a))相比,颗粒污泥内部(图2(b))更粗糙且微生物更丰富。颗粒污泥经过高温热解后,微生物破壁死亡,胞内有机物转化为生物炭基体。如图2(c)所示,未改性颗粒污泥炭GSC-O样品表面较光滑、孔结构不发达。有研究[16]采用物理改性和化学改性法对热解后的污泥炭进行改性处理,以提高污泥炭催化性能。由图2(d)可见,改性颗粒污泥炭GSC-P表面粗糙、疏松多孔,从而增大颗粒污泥炭的比表面积和孔隙率,这与表1中的结果一致。虽然GSC-P的含铁量略低于GSC-O,但对比表2可知,GSC-P污泥炭表面上的铁含量为GSC-O的2倍,有利于催化过氧化氢分解产生羟基自由基,从而提高头孢氨苄的降解效率。
图3是颗粒污泥炭的C1s和Fe2p的XPS谱图。为了分析污泥炭表面官能团的存在形态和含量,对XPS谱图进行了分峰处理。C1s可分为3种峰:石墨态碳(C—C,284.80 eV),酚羟基及醚类碳(C—O,286.00~286.20 eV),羧基及酯类碳(C=O,287.30~287.70 eV)[16]。由图3(a)可见,GSC-O中的C—C含量很高,占87.70%,而经过磷酸改性得到的GSC-P中,部分石墨态碳被氧化,C—C含量降低为55.29%,生成了C—O(36.47%)和C=O(8.25%)等含氧结构。污泥炭表面存在4种结合形态的Fe,即FeO(710.00~710.01 eV)、α-Fe2O3(711.50~712.04 eV)、γ-Fe2O3(722.95~723.47 eV)和Fe3O4(725.43~725.45 eV)[17]。虽然经磷酸改性得到的GSC-P中铁含量略低于GSC-O(表1),但其表面铁含量较多(图2(f)),在XPS谱图中峰强度较大(图3(b)),且Fe(II)所占比例较大(26.21%),有利于提高催化剂的催化活性。
-
实验中探索了温度、pH、H2O2投加量、催化剂投加量和污染物浓度对头孢氨苄转化率的影响,结果如图4所示。前120 min进行吸附实验,不同条件下头孢氨苄的吸附去除率小于5%。颗粒污泥炭比表面积仅为10.03 m2·g−1和96.21 m2·g−1(表1),限制了其对污染物的吸附作用,因此,吸附作用对头孢氨苄的去除影响可忽略不计。
如图4(a)、图4(b)和图4(c)所示,随着温度的升高,2种催化剂对头孢氨苄的转化率逐渐升高。这是因为高温增加分子碰撞的概率,有利于·OH的产生[18]。当温度为60 ℃,GSC-P对头孢氨苄的转化率已高达90.2%,而GSC-O对头孢氨苄的转化率仅为23.9%。如图4(d)、图4(e)和图4(f)所示,随着pH由2升高至6,投加GSC-O和GSC-P的体系中头孢氨苄的转化率呈现下降趋势,说明酸性环境更有利于头孢氨苄的降解。当pH由4上升至6时,对于GSC-P催化剂,头孢氨苄的转化率由67.6%下降至43.7%。这是因为在酸性条件下,过氧化氢易分解产生羟基自由基,从而可促进头孢氨苄的降解[4, 19]。如图4(g)、图4(h)和图4(i)所示,当H2O2投加量为0.5 g·L−1时,GSC-O和GSC-P对头孢氨苄的转化率分别为19.5%和76.6%。当H2O2投加量增加为1.0 g·L−1时,GSC-P催化剂对头孢氨苄的转化率为90.2%,TOC去除率为53.9%,这是因为头孢氨苄降解生成了一些中间产物,并没有完全矿化。下文中的FT-ICR-MS结果也证实了这一结论。如图4(j)、图4(k)和图4(l)所示,随着GSC-P投加量增大,头孢氨苄的转化率增加。当GSC-P投加量达到1.0 g·L−1和1.5 g·L−1时,头孢氨苄转化率分别为90.2%和92.6%。这是因为催化剂投加量的提高,为反应提供更多的活性位点,促进·OH产生及头孢氨苄的降解[4]。如图4(m)、图4(n)和图4(o)所示,随着污染物浓度升高,头孢氨苄的转化率和TOC去除率降低。当污染物浓度为100 mg·L−1以下时,GSC-P对头孢氨苄的转化率高于89.6%,且TOC去除率大于53.5%。
-
降解过程的动力学分析结果如图5所示。头孢氨苄的催化降解过程分多个阶段,每个阶段均符合一级反应动力学特征。在120~150 min内,降解速率较快,符合一种一级反应动力学,头孢氨苄转化率约为45%;在150~270 min内,降解速率降低,进入另一种一级反应动力学,头孢氨苄转化率为89.6%;在270~300 min内,降解速率趋于平缓,头孢氨苄的转化率仅增加10%左右。
因此,综合考虑降解效率和经济等因素,在CWPO体系中,用磷酸改性的颗粒污泥炭GSC-P催化降解头孢氨苄对最佳条件为:温度为60 ℃、pH为3、过氧化氢投加量为1.0 g·L−1、催化剂投加量为1.0 g·L−1、反应物初始浓度为100 mg·L−1和反应时间300 min,此时,头孢氨苄的转化率为89.6%。而未改性颗粒污泥炭GSC-O对头孢氨苄的转化率仅为21%。由表1可知,GSC-O和GSC-P中的含铁量均较高,但GSC-O的催化活性却远低于GSC-P。由颗粒污泥炭的表征结果(表2和图3(b))可知,与GSC-O相比,GSC-P表面上的铁含量较多,且Fe(II)所占比例较大,表面疏松多孔,官能团如羟基、羧基等较丰富,这有利于提供催化反应的活性组分和活性位点。
-
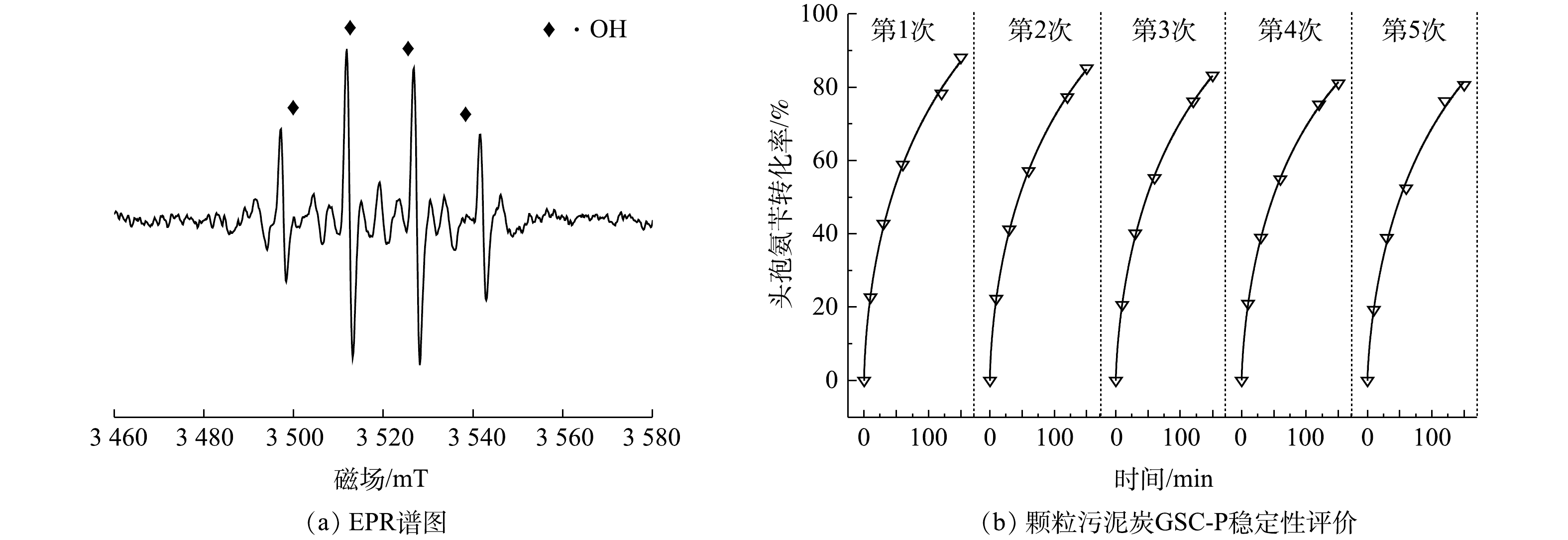
以DMPO为自旋捕捉剂进行自由基检测,结果如图6(a)所示。可见,DMPO-OH特征峰比例为1:2:2:1,证明为·OH[20]。在最佳条件下,对颗粒污泥炭GSC-P进行了5次循环利用,基于催化作用得到的头孢氨苄转化率结果如图6(b)所示。头孢氨苄的转化率稳定维持在80%~88%。颗粒污泥炭的铁溶出率仅为0.83%,远低于一些文献中铁的溶出率(2.77%~11.5%)[12, 14, 17, 21]。上述结果表明,经磷酸常温改性的颗粒污泥炭具有较高的催化活性,可以重复使用,是一种催化性能和稳定性较高的非均相CWPO催化剂。与粉末状污泥炭相比,颗粒污泥炭有一定形状且呈现磁性更易回收,可以重复使用,从而避免了二次污染[5]。
-
通过FT-ICR MS对头孢氨苄降解的中间产物进行了探测鉴定。根据检测的中间产物(表3),提出了降解途径。如图7所示,降解过程主要包括羟基化、去甲基化、脱羧和脱烷基等。头孢氨苄的甲基被·OH进攻氧化为羧基生成P1,再通过脱羧、羟基化使β-内酰胺环开环生成P2[22-23],P2上六元环连接的羧基碳失去1个氧原子得到P3,同时,P2通过脱羧和断键(C—N)反应生成P4和P5,之后生成苯甲酰甲酸(P6)、氨基乙酰胺(P7)、苯甲酸(P8)、丁烯(P9)、丁二酮(P10)等小分子有机物,由于TOC去除率为53.9%,所以部分中间产物进一步矿化生成无机小分子CO2、H2O等。HE等[24]的研究中也检测到P2、P3和P5。
2.1. 颗粒污泥性质
2.2. 催化降解过程中的影响因素
2.3. 反应动力学分析
2.4. 催化稳定性考察
2.5. 头孢氨苄的降解途径
-
1)以厌氧颗粒污泥制备颗粒污泥炭后,通过磷酸改性可以有效提高其催化活性。改性后催化剂表面铁含量增大,催化剂比表面积和孔容积增大,表面官能团较为丰富,有利于催化湿式过氧化氢氧化反应。
2)综合考虑降解效率和经济等因素,在CWPO体系中其对头孢氨苄的反应条件宜为:温度60 ℃、pH为3、过氧化氢投加量为1.0 g·L−1、催化剂投加量为1.0 g·L−1、反应物初始浓度为100 mg·L−1和反应时间300 min,在此条件下头孢氨苄的转化率为89.6%。
3) GSC-P催化稳定性较高,在反复使用5次后,活性组分Fe的溶出率很低,仅为0.83%,头孢氨苄的转化率稳定在80%~88%。颗粒污泥炭具有一定形状且呈现磁性易回收,可以重复使用,可避免二次污染。
4)头孢氨苄的降解是通过羟基化、去甲基化、脱羧和脱烷基等过程生成小分子有机物,再进一步矿化生成CO2和H2O等无机物。



 DownLoad:
DownLoad: