-
印染废水的化学成分复杂、色度大、毒性高、可生化性差[1-3],已成为威胁国家水环境安全的重要因素之一[4-5]。目前常用的印染废水处理方法有生物法、物理法和化学法[3-5]。化学法对染料废水的脱色和提高B/C有较好的效果,其中光催化氧化法能够有效的降解染料[6-7]。
传统的半导体电极在净化废水、解决环境污染等方面有很好的效果[7-9],但可见光吸收性能差,对利用太阳能催化降解污染物的应用造成了很大的限制。贵金属粒子由于其表面的等离子体共振效应而对可见光具有较好的吸收,由此相继开发出等离子体型光催化剂,如贵金属Ag[10-17]、Bi[18-19]和Pt[20]等。磷酸银(Ag3PO4)具有较好的可见光响应能力,但其对污染物的降解效率低,光生电子易与反应体系中溶解的Ag+反应生成Ag0,从而发生光腐蚀。氧化石墨烯(GO)的比表面积大,吸附污染物效率较高,导电性能较好,能够有效地促进光生电子-空穴对的分离,可提高催化活性[21-23]。因此,国内外学者将Ag3PO4和石墨烯进行复合改性[24-25],增大光催化剂的比表面积和提高光催化剂的导电性,以增强光催化活性和稳定性[26]。LIU等[27]运用吡啶辅助-水热法将Ag和Ag3PO4合成复合材料Ag/Ag3PO4,在可见光照射下降解甲基橙,降解效率高且甲基橙残留量低。陈晓娟[28]基于光生电子-空穴的迁移转化原理,构筑系列Ag3PO4基复合光催化剂,当Ag3PO4-GO复合光催化剂中GO含量为5%时,其光催化降解2,4-DCP的速率为0.060 0 min−1。
本研究针对Ag3PO4光稳定性差且难回收的问题,引入MgFe2O4磁性纳米材料,赋予光催化剂磁性,从而提高光催化剂的回收率。采用溶胶凝胶法制备了Ag3PO4/GO/MgFe2O4复合光催化剂,并对催化剂构型和可见光吸收性能进行了分析和表征;详细考察了不同实验条件对光催化降解RhB效果的影响,且评估了复合光催化剂的稳定性。
全文HTML
-
柠檬酸(C6H8O7·H2O)、九水合硝酸铁(Fe(NO3)3·9H2O)、聚乙二醇200(PEG 200)、六水合硝酸镁(Mg(NO3)2·6H2O)、硝酸银(AgNO3)、磷酸二氢钠(Na2HPO4)购自国药集团化学试剂有限公司,氨溶液(NH3·H2O)购自国药集团化学试剂有限公司,无水乙醇(C2H5OH)、罗丹明B(RhB)购自西龙化工有限公司,相关试剂均为分析纯,无需进一步纯化可直接使用。本实验中待降解染料废水为罗丹明B模拟废水。
-
1) MgFe2O4样品制备。将18.912 6 g柠檬酸固体溶于180 mL去离子水,再加入32.32 g Fe(NO3)3·9H2O、19 g聚乙二醇200、10.256 4 g Mg(NO3)2·6H2O,搅拌均匀。逐滴加入用25%的氨水,调节pH至6.7,搅拌15 h后置于电热恒温鼓风干燥箱中105 ℃下烘干15 h。烘干后的样品加入无水乙醇引燃,灼烧4 h后研磨成粉末状。研磨后的样品在轨道箱式炉中以5 ℃·min−1的升温速率升至600 ℃灼烧2 h。
2) Ag3PO4/GO/MgFe2O4光催化剂制备。将5.1 g AgNO3溶于180 mL的去离子水中,加入氧化石墨烯,超声1 h,在室温下搅拌下,再滴加氨水至沉淀刚好溶解为止。取出溶液,加入已经制备好的MgFe2O4样品0.059 g,用锡箔纸将其密封,再超声1 h后取出搅拌,逐滴滴加配置好的Na2HPO4溶液。滴定完毕后放入超声波仪中超声10 min,用去离子水将产物洗涤几次,经离心后在烘箱中干燥。烘干之后,取出研磨,得到Ag3PO4/GO/MgFe2O4光催化剂。
-
采用日本理学公司的Rigaku D/max2400/PC型XRD 衍射仪对光催化剂的晶体结构进行表征,辐射源为Cu-Ka,扫描速度为5(º)·min−1,扫描范围为5º~90º。采用日本日立公司的JSM-6701F 型冷场发射扫描电镜(FE-SEM)和美国Philips-FEI 公司的Tecnai-G2-F30 型高分辨透射电镜(TEM)对光催化剂的微观形貌和颗粒尺寸进行表征。采用美国珀金埃尔默公司的Perkinelmer Lambda 950型UV/Vis/NIR双光束分光光度计测定合成催化剂的紫外-可见漫反射光谱(UV-vis DRS)。采用美国Lake Shore公司的LAKESHORE-7304型振动样品磁强计(VSM)分析催化剂的磁性能。
-
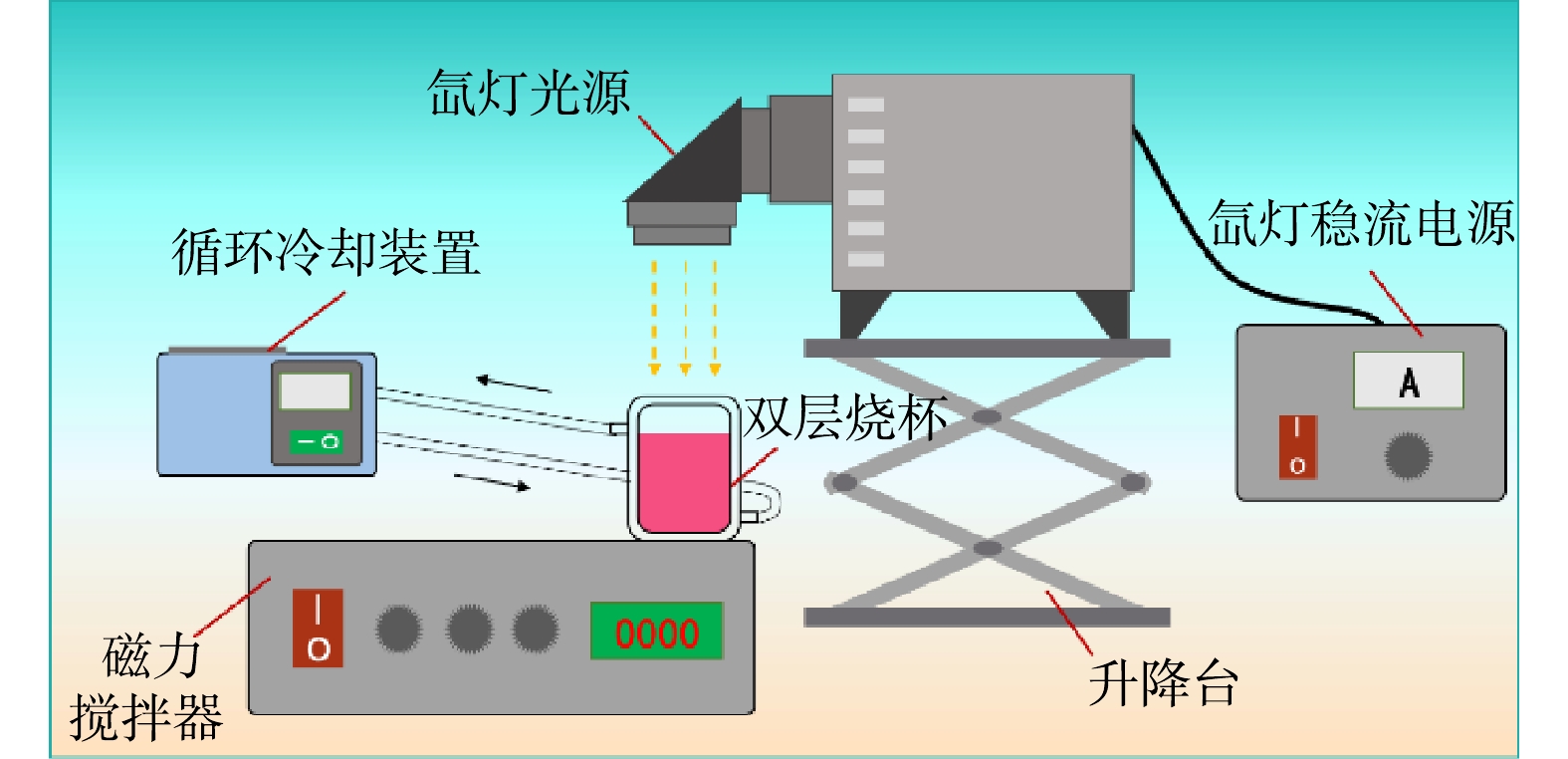
如图1所示,光催化剂活性的测试在自制具有冷却系统的玻璃反应器中进行,通过在反应器中光催化降解RhB(10 mg·L−1)进行评价。具体过程如下:将100 mL RhB(10 mg·L−1)溶液和20 mg光催化剂样品置于反应装置中,避光搅拌30 min达到吸附-脱附平衡。调整液面与光源距离为15 cm,在恒温搅拌下,打开光源(300 W氙灯光源,通过滤光片提供400~780 nm可见光),每隔一定时间间隔吸取1 mL样品,将样品通过0.45 μm滤器过滤进行分析,用紫外可见分光光度计测定滤液的吸光度,利用RhB的去除率以评价光催化剂降解有机污染物的光催化活性。
1.1. 材料
1.2. 光催化剂制备
1.3. 分析方法
1.4. 光催化活性测试
-
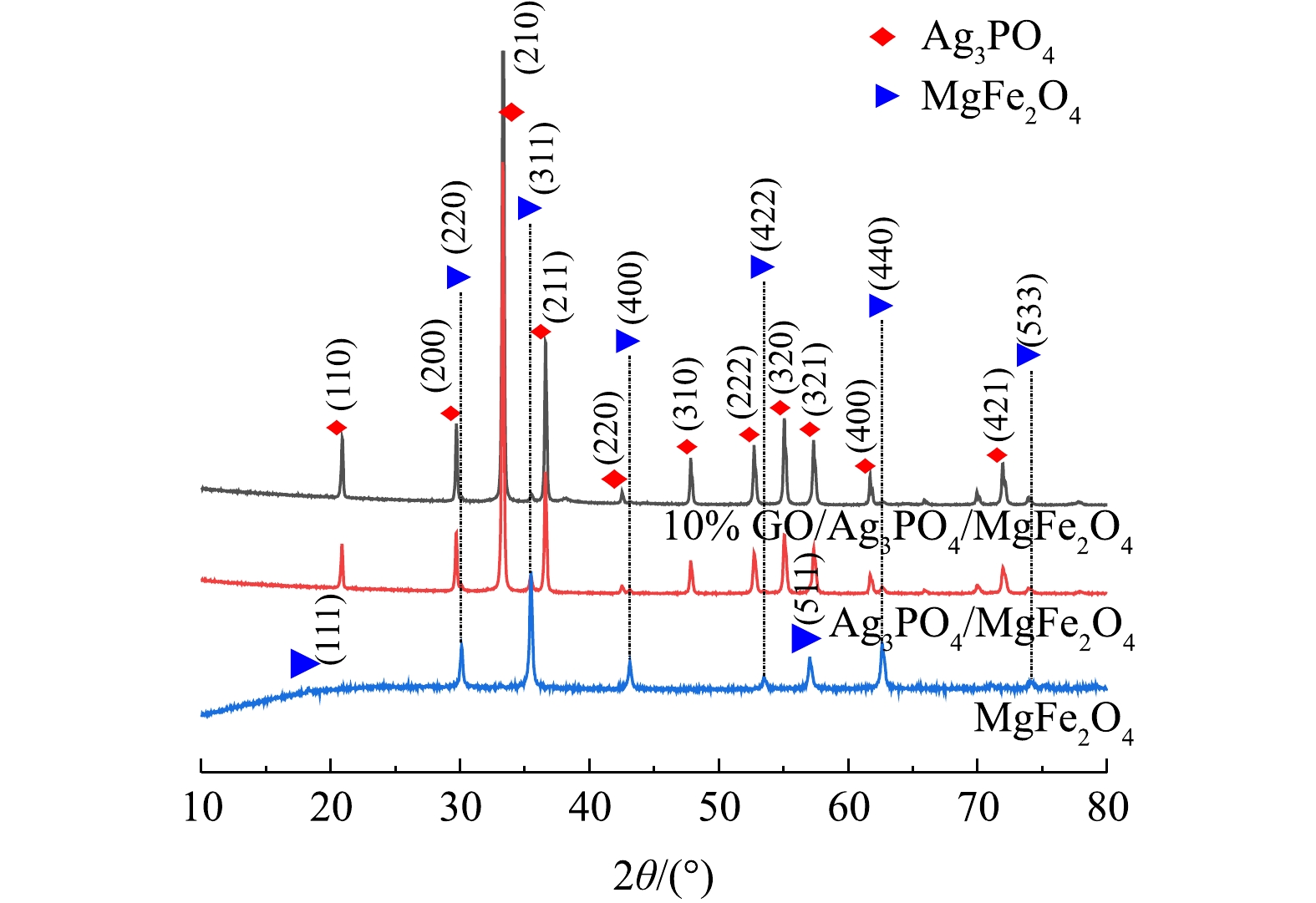
1) XRD表征。MgFe2O4、Ag3PO4/MgFe2O4和10% Ag3PO4/GO/MgFe2O4的XRD图谱如图2所示。在MgFe2O4对应的XRD图谱上出现了8个衍射峰,其位置符合MgFe2O4的标准PDF卡片(JCPDS 19-0629),表明该样品主要成分是MgFe2O4。对于10% Ag3PO4/GO/MgFe2O4样品来说,MgFe2O4衍射峰强度较低,这可能是由于氧化石墨烯低掺量比所致。此外,其XRD图谱中还出现了10个新衍射峰,衍射角2θ分别为20.91°、29.74°、33.34°、36.63°、47.87°、52.77°、55.11°、57.38°、61.75°、72.02°,其分别与Ag3PO4的(110)、(200)、(210)、(211)、(310)、(222)、(320)、(321)、(400)、(421)晶面(JCPDS 06-0505)对应,这表明10% Ag3PO4/GO/MgFe2O4样品中含有MgFe2O4和Ag3PO4。同时,可能在水热作用影响下,掺混的GO表面含氧官能团被还原,故在10% Ag3PO4/GO/MgFe2O4样品的图谱中,GO的(001)晶面衍射峰消失[29]。
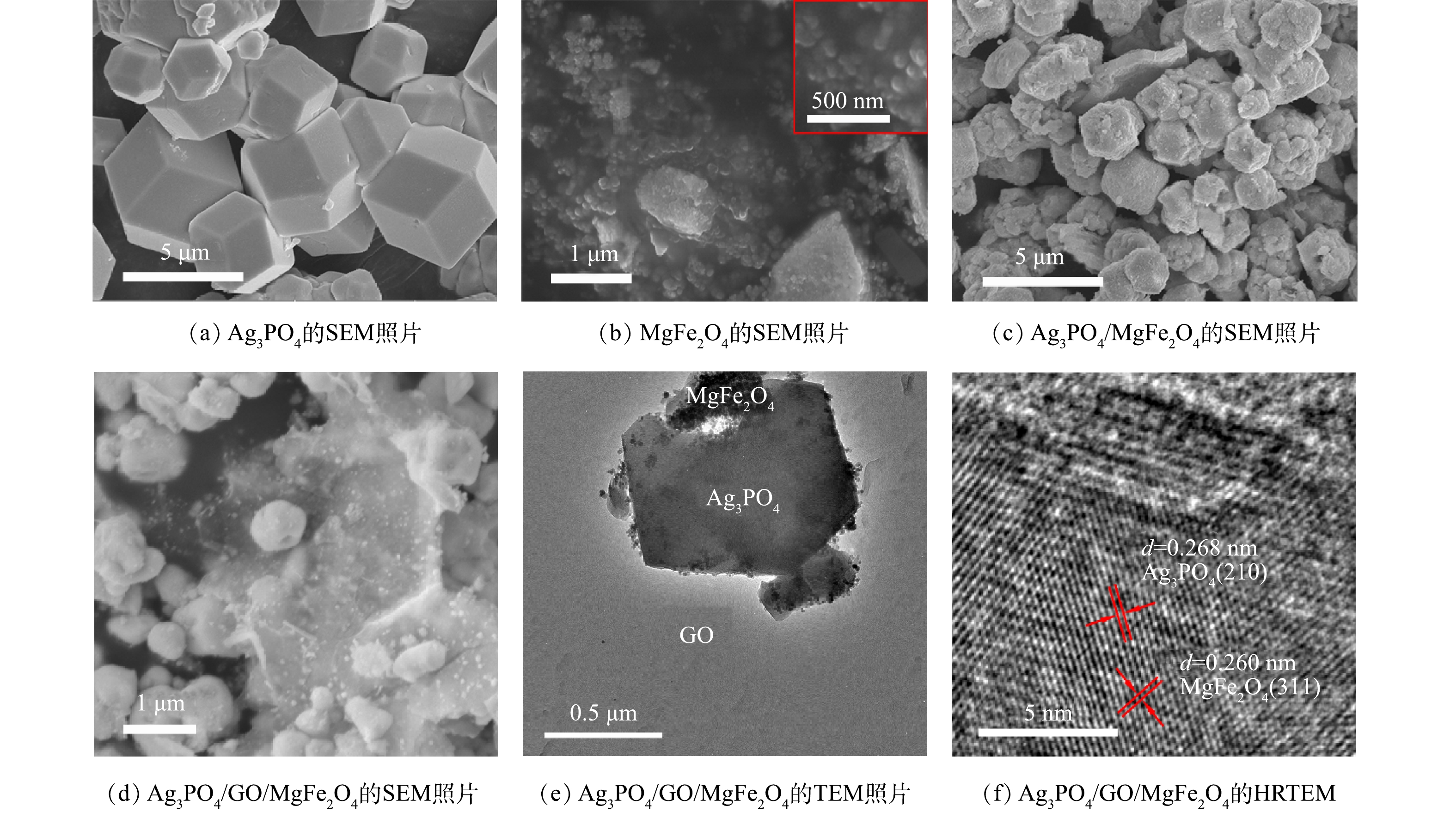
2) SEM、TEM表征。Ag3PO4、MgFe2O4、Ag3PO4/MgFe2O4和10% Ag3PO4/GO/MgFe2O4光催化剂的微观形貌如图3所示。图3(a)显示的Ag3PO4呈规则的立方体结构,粒径为4~5 μm。图3(b)中为自蔓延燃烧法制备的MgFe2O4样品的表面形貌,由具有不规则形态的颗粒组成,粒径为50~150 nm。图3(c)为合成的Ag3PO4/MgFe2O4光催化剂的表面形态,可以看出,MgFe2O4颗粒已成功负载到Ag3PO4上,而且分散均匀,粒径约为2~3 μm[30]。同时,Ag3PO4/MgFe2O4光催化剂的粒径小于Ag3PO4,这说明MgFe2O4的加入可以改变Ag3PO4颗粒的形成与生长[31]。图3(d)为10% Ag3PO4/GO/MgFe2O4光催化剂的表面形貌,呈不规则球形团聚体,粒径为1 μm左右。此外,由于Ag3PO4颗粒的生长和团聚受GO的结构空间抑制,使复合光催化剂的尺寸变小[32]。通过对磁性复合催化剂进行TEM 表征(图3(e))发现,MgFe2O4纳米粒子和Ag3PO4微粒均匀分散GO薄片的表面。为了进一步确认Ag3PO4与MgFe2O4的复合,对合成的磁性光催化剂进行了HR-TEM分析(图3(f))。结果表明,在光催化剂表面存在0.268 nm和0.254 nm不同的晶格条纹,分别对应于Ag3PO4立方体结构的(210)晶面[33]和尖晶石型MgFe2O4纳米粒子(311)晶面[34]。
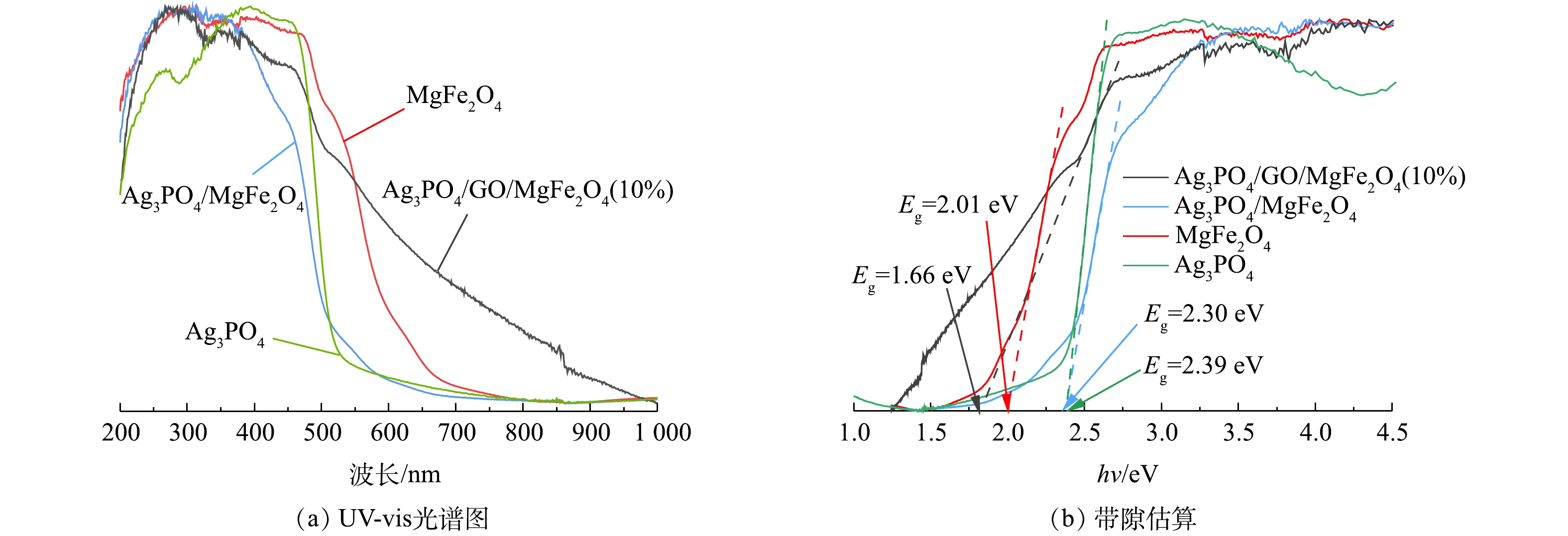
3) UV-vis分析。图4(a)为Ag3PO4、MgFe2O4、Ag3PO4/MgFe2O4和10% Ag3PO4/GO/MgFe2O4光催化剂的UV-vis DRS测试光谱图。由图4(a)可见,Ag3PO4/GO/MgFe2O4磁性光催化剂对可见光有较好的吸收,具有较好的响应性,进而可改善可见光的利用率,有利于提高光催化剂的光催化性能。利用Kubelka-Munk方程[35]对光催化剂的禁带宽度进行估算(图4(b)),估算结果得出,Ag3PO4、MgFe2O4、Ag3PO4/MgFe2O4和10% Ag3PO4/GO/MgFe2O4的Eg分别为2.39、2.01、2.30和1.66 eV。上述结果表明,磁性复合光催化剂的禁带宽度小于Ag3PO4。
-
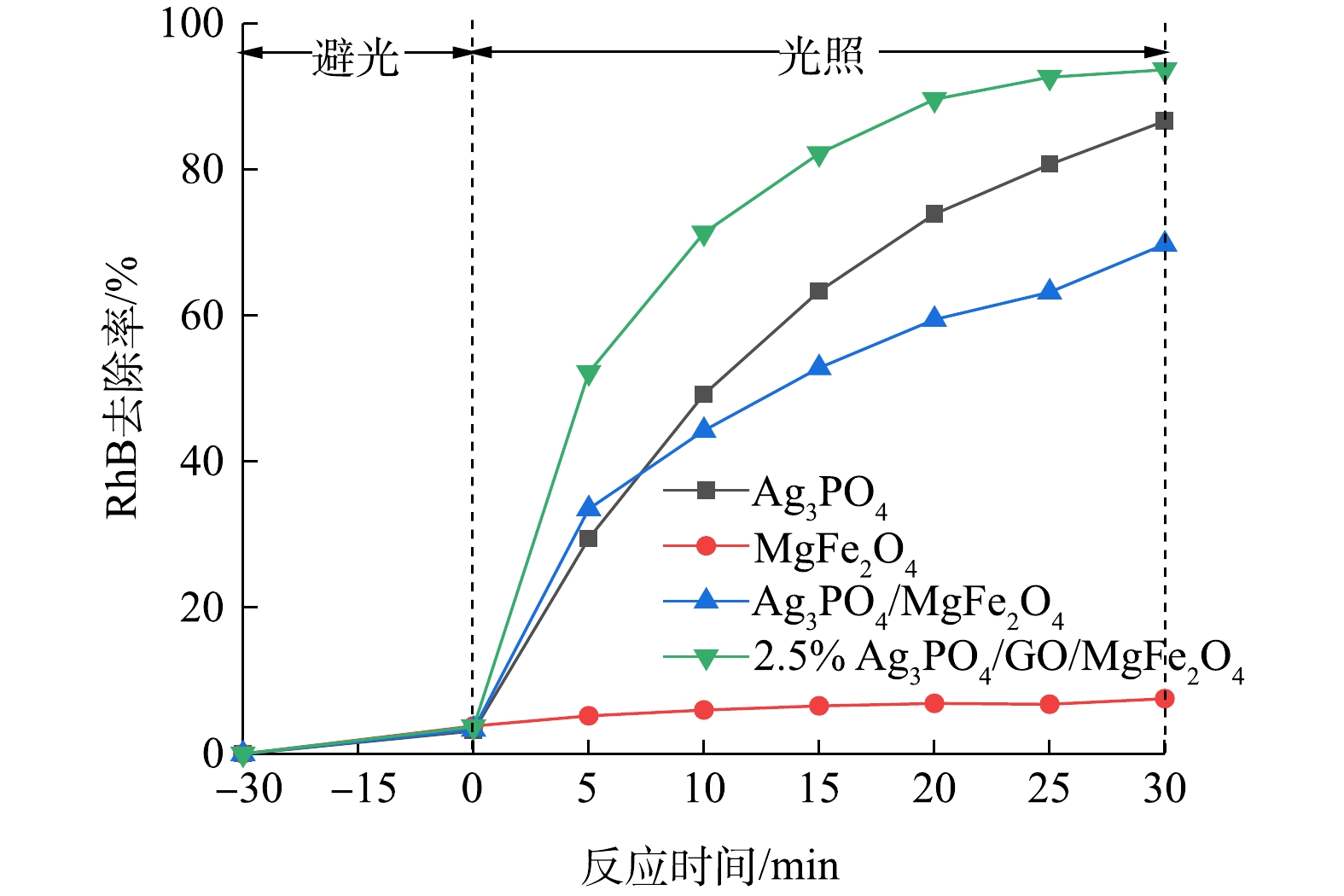
1)不同光催化剂对RhB去除率的影响。图5为Ag3PO4、MgFe2O4、Ag3PO4/MgFe2O4和2.5%Ag3PO4/GO/MgFe2O4光催化剂的光催化活性的测定结果。由图5可以看出,MgFe2O4的光催化活性最低,在光照30 min后对RhB的降解率为4.1%。要是因为生成的光生电子-空穴易重组,分离效率低,难以有效地降解污染物。当光照时间为30 min时,Ag3PO4/MgFe2O4的降解效率大于Ag3PO4,表明Ag3PO4和MgFe2O4的复合增强了光催化活性。当GO掺量为2.5%时,RhB的降解率达到93.4%,说明GO的掺杂增加了光催化剂的活性。
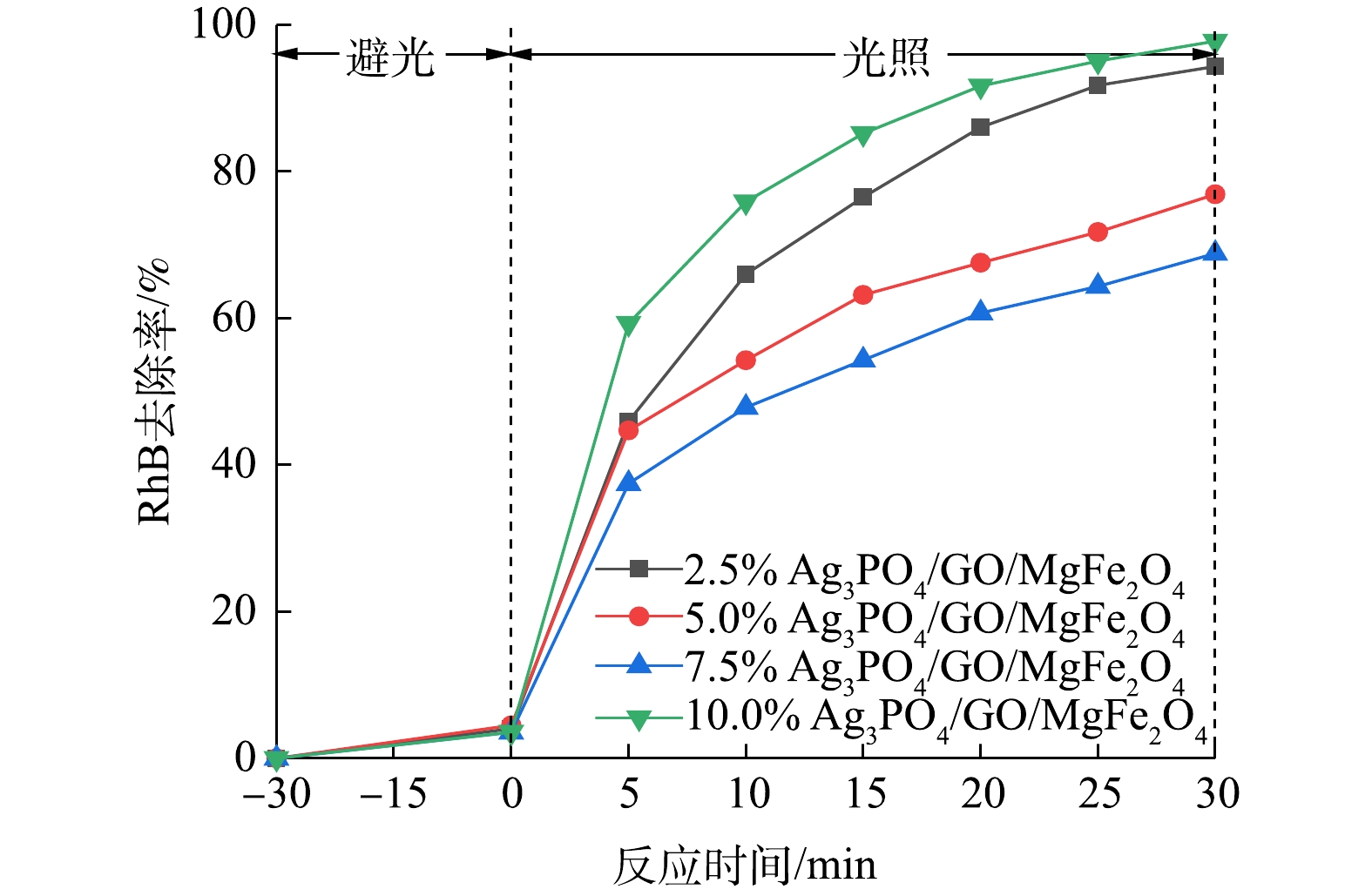
2)不同氧化石墨烯比例对RhB去除率的影响。光催化剂投量为200 mg·L−1时,不同GO掺量对光催化降解RhB的影响如图6所示。随着GO掺量的增加,降解效率呈先下降后上升的变化规律。GO掺量为10%时,30 min内RhB的降解效率为97.7%,远远优于GO质量分数为2.5%、5%、7.5%的光催化剂。由图6可以看出,在光催化剂Ag3PO4/MgFe2O4的基础上负载质量分数为10%的氧化石墨烯时,增加了光催化剂的比表面积,促进了光生电子与空穴的分离,进而提高了光催化剂的催化活性。
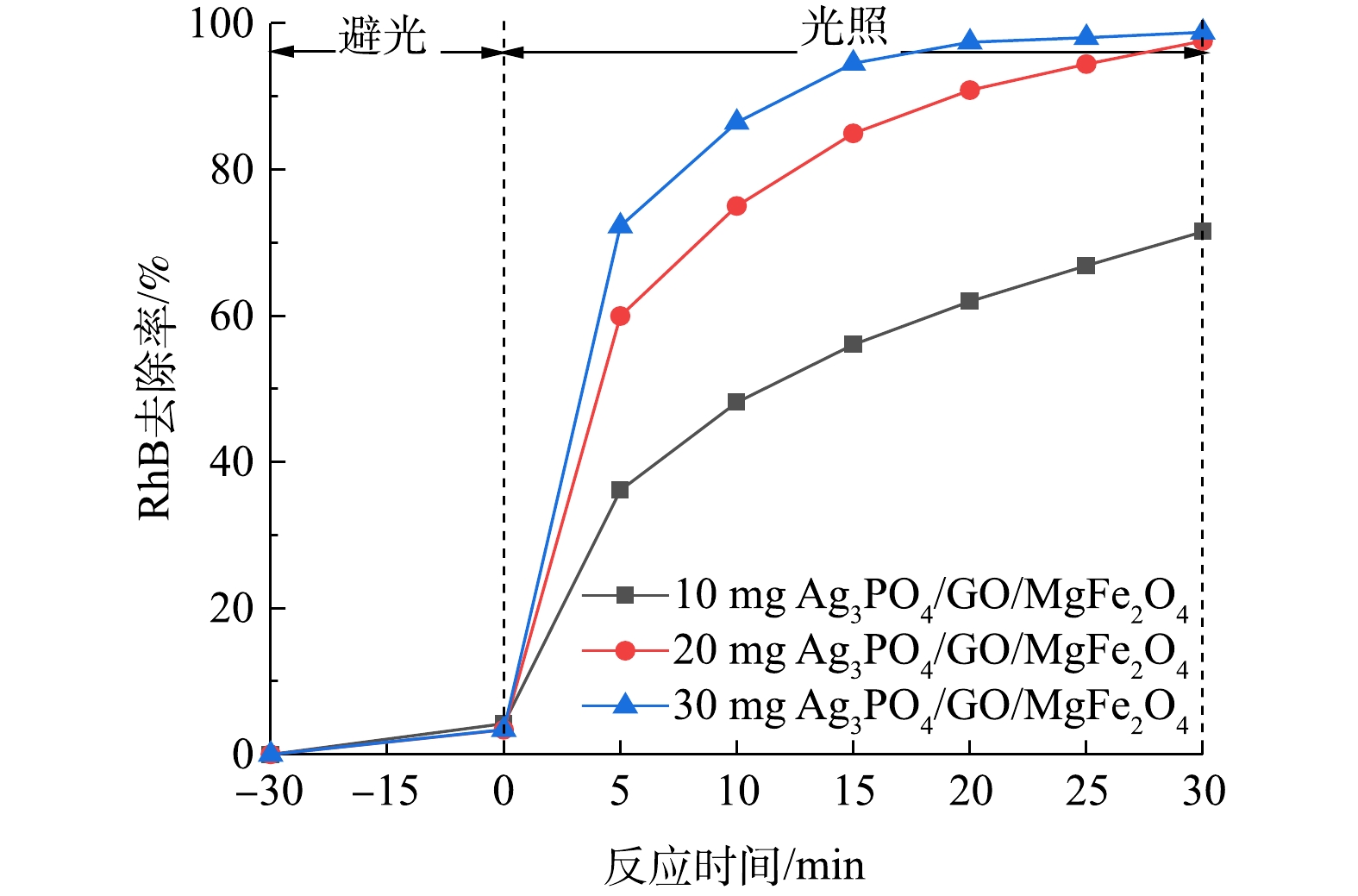
3)不同光催化剂用量对RhB去除率的影响。在GO掺杂比率为10%,300 W氙灯全波段辐射条件下,不同光催化剂用量对催化降解RhB的影响结果如图7所示。由图7可知,光催化剂投量为30 mg的RhB降解率明显好于投量为10 mg和20 mg时的RhB降解率。这表明在一定的投量范围内,更多的光催化剂投量可以增加RhB的降解率,其原因是增加催化剂投量可以增加反应体系中光催化剂的有效活性位点。但是,投入过量的光催化剂会增大光催化剂的回收难度,而且过多的光催化剂会阻碍光在溶液中的透过率,造成不必要的能耗损失。
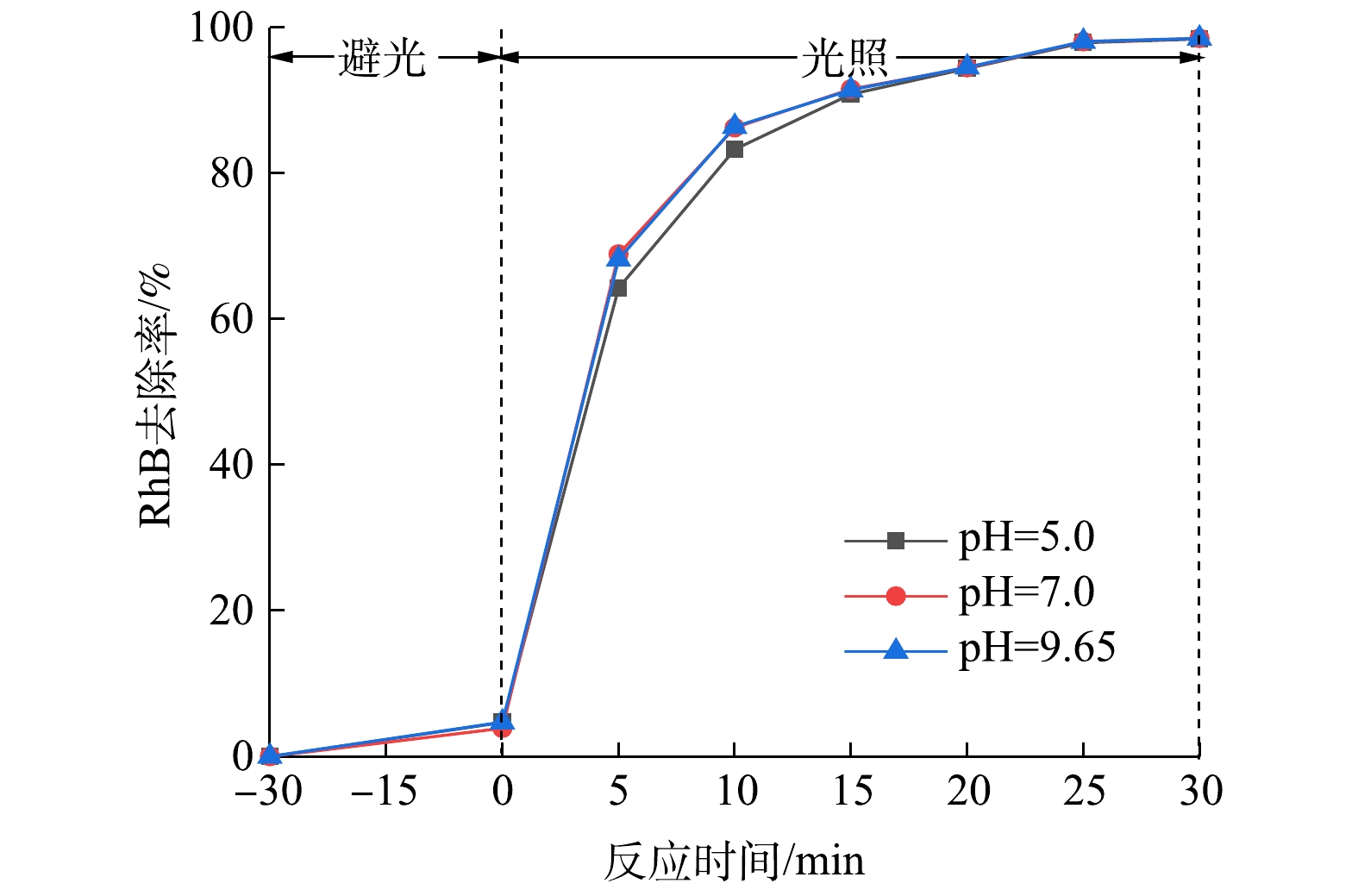
4)不同pH对RhB去除率的影响。溶液的pH会影响光催化剂表面和反应中间产物的电荷状态,降低光催化剂对光线的吸收,从而影响光催化剂的活性和表面活性物种的生成速率[36]。由图8可知,当10% Ag3PO4/GO/MgFe2O4投加量为30 mg时,不同pH对光催化降解RhB的影响不大,当pH在5~10时,光催化剂均可保证较高的光催化活性。因此,可以推测,在光催化降解过程中,pH并不是影响复合光催化剂Ag3PO4/GO/MgFe2O4在可见光下降解RhB的主要因素,反应过程中活性物种的生成及降解污染物的相关反应基本不受pH的影响。
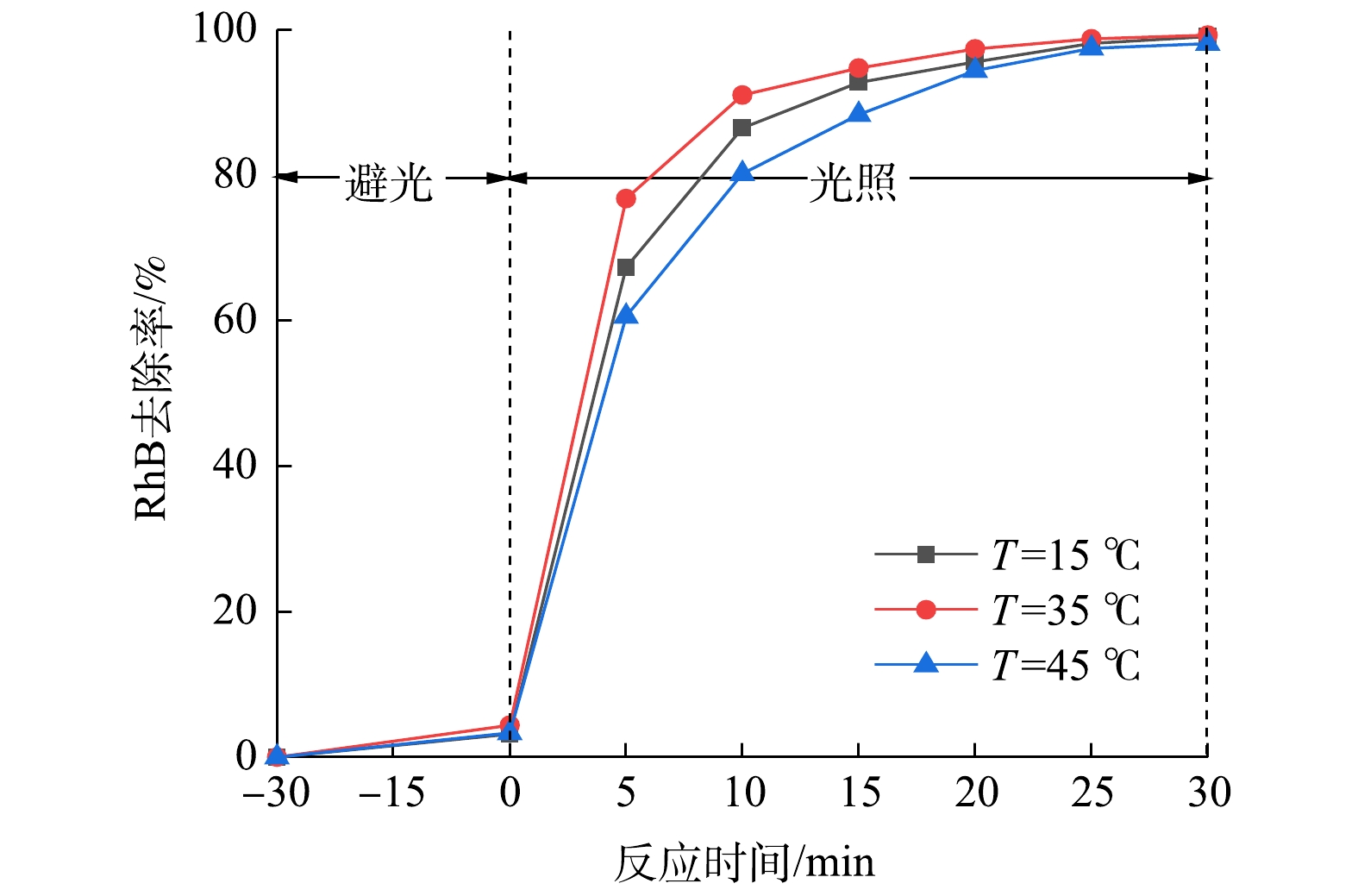
5)不同反应水温对RhB去除率的影响。反应水温会影响光催化体系中活性物种与污染物之间碰撞的剧烈程度,从而影响Ag3PO4/GO/MgFe2O4光催化体系对RhB的降解效果[37]。光催化剂的投加量为30 mg,溶液pH为5,不同反应水温对RhB降解率的影响如图9所示。Ag3PO4/GO/MgFe2O4光催化体系反应水温为15、35和45 ℃时对RhB的去除率分别为99.05%、99.24%、98.08%。其原因是随着反应水温的提高,反应体系中活性物种分子数量增加,污染物与活性物种之间的有效碰撞概率增加,导致反应速度加快;同时,随着反应水温的提高,光催化剂表面对RhB的吸附量也略有下降,从而导致反应水温变化对光催化体系中污染物去除率的变化影响不大。
-
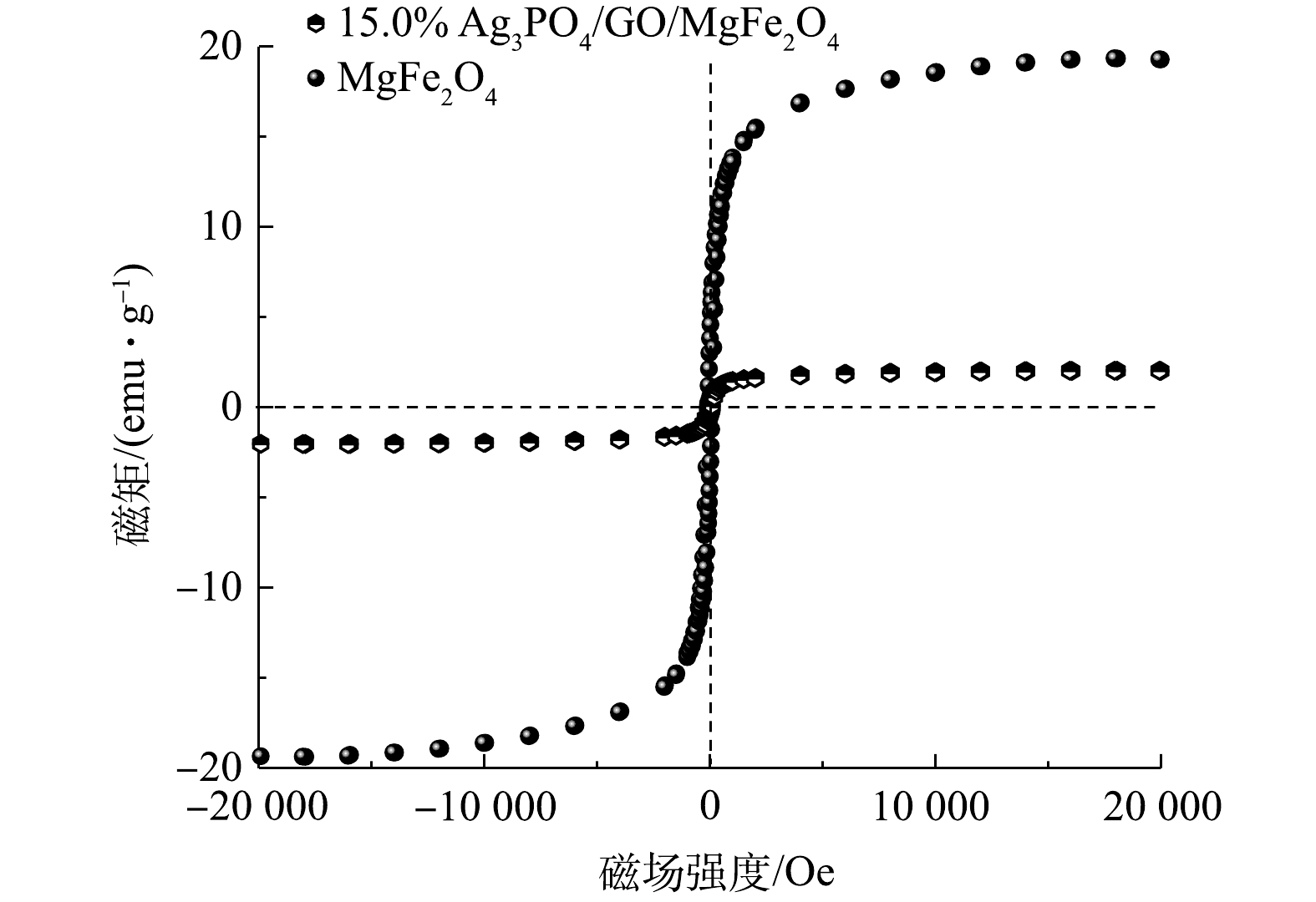
图10为MgFe2O4和15.0%Ag3PO4/GO/ MgFe2O4的磁性能测定结果。光催化剂的磁矩分别为19.3 emu·g−1和2.4 emu·g−1。Ag3PO4负载MgFe2O4后有一定的磁性,在光催化反应结束后,可通过外加磁力对光催化剂分离回收,使得合成的Ag3PO4/GO/MgFe2O4光催化剂不仅有很好的光催化活性,而且很容易进行分离回收利用,进而避免了二次污染。
-
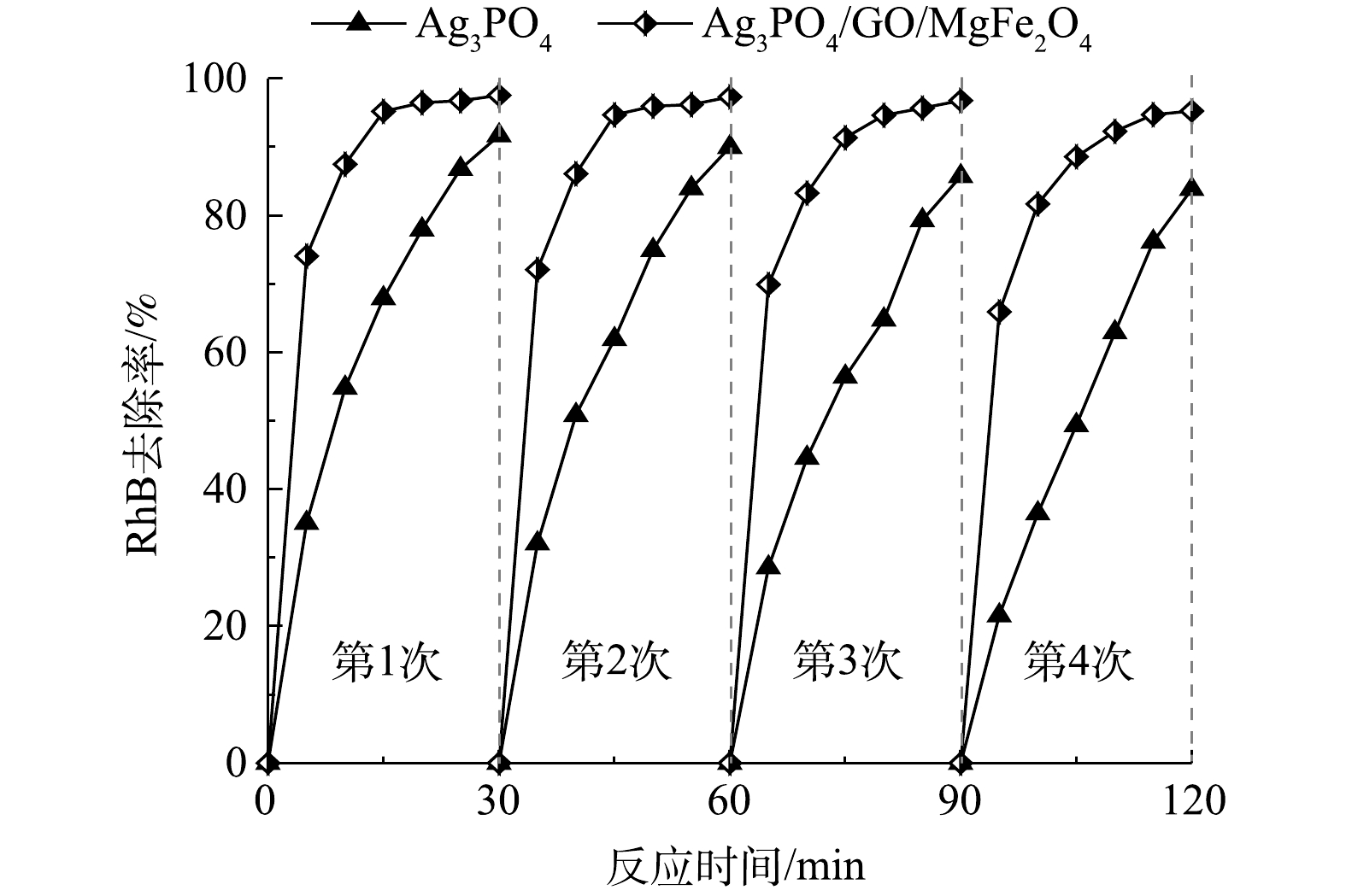
为了考察光催化剂的再生性能,对制备的Ag3PO4与10% Ag3PO4/GO/MgFe2O4光催化剂进行稳定性评价。图11为在相同反应条件下2种光催化剂重复循环降解4次的曲线。由图11可见,Ag3PO4光催化剂在使用4次后,对RhB的催化降解性能逐渐降低,稳定性能明显劣于Ag3PO4/GO/MgFe2O4光催化剂。Ag3PO4/GO/MgFe2O4光催化剂在循环使用4次后,30 min内对RhB的降解率仍能达到95%以上,说明该磁性复合光催化剂在多次使用后能保持相似的活性,具有很好的稳定性。
2.1. 光催化剂的基本性质
2.2. 光催化降解实验
2.3. 光催化剂磁性能测定
2.4. 光催化剂稳定性
-
1)通过在Ag3PO4/MgFe2O4表面负载氧化石墨烯,大大增加了光催化剂的比表面积,促进了电子-空穴对(e−-h+)的分离,进一步提高了Ag3PO4光催化活性和光催化稳定性,并抑制了Ag3PO4的光腐蚀。
2)当石墨烯掺杂量为10%、光催化剂投加量为30 mg、pH为5~10、300 W氙灯全波段辐射条件下,即可在30 min内基本实现RhB(10 mg·L−1)的完全降解;与单一Ag3PO4相比,Ag3PO4/GO/MgFe2O4复合光催化剂的光催化性能显著提高。
3)实验制备的Ag3PO4/GO/MgFe2O4复合光催化剂对RhB的光催化降解遵循异质结能带理论,具有良好的可回收性和稳定性,重复利用4次后,催化性能仍能达到95%以上。





 下载:
下载: