-
阻燃剂广泛应用于塑料、橡胶和纤维中,用来降低材料的可燃性[1]。2012年,中国有机磷阻燃剂产量超过17.9×104 t,在有机磷阻燃剂中,磷酸三苯酯(TPhP)是一种高产量产品,由于未与最终用途产品化学键合,故在水中可被检测到[2]。澳大利亚地表水中的TPhP浓度高达150 ng·L−1;在中国松花江,TPhP的浓度也可以达到65 ng·L−1;此外,在河南省的污水处理厂中检测到了TPhP[3-5]。在环境中出现的TPhP会通过饮食摄入和呼吸吸入,进而引起人类健康问题,有研究[6]表明,TPhP对胚胎发育、神经系统和免疫系统具有毒理作用。对于模型生物斑马鱼,当暴露于0.10 mg·L−1 TPhP时,对心脏发育具有巨大的毒性作用;通过对暴露于100 μg·L−1 TPhP的成年斑马鱼的生物蓄积和代谢研究,发现其体内TPhP浓度可高达3.12 μg·g−1[7-8]。因此,开发一种有效的材料以去除水中TPhP势在必行。
有研究[9]发现,尿素官能团化Fe3O4@LDH (urea-Fe3O4@LDH)对TPhP表现出了良好的去除效果,去除率可达90%以上,吸附容量高达589 mg·g−1,具有高效快速的吸附特性,吸附速率达到49.9 mg·(g·min)−1,且离子强度对吸附影响较小。而不同环境条件影响吸附过程中各物质的相互作用,故考察urea-Fe3O4@LDH去除TPhP受环境条件的影响情况势在必行。
天然水体中存在着悬浮颗粒物,地下水中悬浮固体的平均浓度约200 mg·L−1,在某些极端情况下,其可能接近500 mg·L−1。在这些悬浮颗粒中,大颗粒相对容易沉降,颗粒携带有机磷污染物形成沉积物,导致污染持续时间长;小颗粒容易在实际运动的水体或实验搅拌的条件下悬浮,从而与污染物更多接触,易影响污染物的去除[10-12]。水体中普遍存在的天然有机物(natural organic matter, NOM)可能改变吸附剂的表面电荷、亲疏水性和极性等性质,影响吸附剂在水体环境中的稳定性和对污染物的去除[13-14]。实际废水中一般有机和无机污染物共存,Br−作为一种无机污染物广泛存在,主要来自海水、地表水、工业废水和油田废水等,其存在极大地影响到人类的健康[15]。
实际水体中颗粒物的主要成分是黏土矿物,而富里酸(FA)、腐殖酸(HA)和可溶性微生物副产物(SMPs)是NOM的最重要组分。本研究利用高岭土模拟颗粒物,用富里酸、腐殖酸和牛血清白蛋白(BSA)模拟水体中存在的有机物,以及Br−作为无机污染物,考察了以上环境条件对urea-Fe3O4@LDH吸附TPhP的影响。利用urea-Fe3O4@LDH吸附剂的电荷特性和阴离子交换性,深入了解吸附过程中有机磷污染物的去除机理,以期为urea-Fe3O4@LDH在实际水体环境中去除有机磷污染物提供参考。
全文HTML
-
磷酸三苯酯(纯度>98%)、对甲苯基异氰酸酯和四氢呋喃(THF)购自麦克林生化科技有限公司,Fe3O4和3-氨丙基三乙氧基硅烷(APTES)购自阿拉丁生化科技股份有限公司,Na2CO3购自Sigma-Aldrich,Mg(NO3)2·6H2O、Al(NO3)3·9H2O、FA和HA购自福晨化学试剂厂,BSA购自上海金穗生物科技有限公司。
-
利用高效液相色谱HPLC测定溶液中的TPhP浓度,吸收波长为204 nm,流动相为甲醇溶液,体积分数为70%,流速为1.0 mL·min−1。用NOH等[16-17]开发的方法测量材料的零点电荷(pHPZC)。采用傅里叶变换红外分光光度计(FT-IR,Nicolet iS10,赛默飞世尔科技公司,美国)检测材料官能团。采用Zeta电位分析仪(ZS90,马尔文仪器有限公司,英国)测量材料电位。用紫外可见光分光光度计测量有机物在250 nm(E2)、365 nm(E3)、465 nm(E4)和665 nm(E6)处的吸光度。
-
将0.35 g的Fe3O4纳米颗粒超声分散在30 mL去离子水中,与15 mL的NaOH (0.76 g)和Na2CO3 (0.64 g)混合均匀,在搅拌下,滴加15 mL的Mg(NO3)2·6H2O (2.32 g)和Al(NO3)3·9H2O (1.13 g)混合溶液(Mg2+∶Al3+=3∶1),搅拌20 min。然后静置,取出上清液20 mL,并加入等量乙二醇,搅拌10 min,将混合物移入110 ℃反应釜中保持12 h。将得到的固体洗涤至中性,在60 ℃下干燥,研磨得到Fe3O4@LDH粉末。将Fe3O4@LDH分散在100 mL乙醇中,加入APTES (0.1 g),在80 ℃下回流10 h,抽滤、洗涤、干燥后得到NH2-Fe3O4@LDH。将得到的材料分散到THF中,加入对甲苯基异氰酸酯0.03 g,室温下,密闭搅拌24 h,用乙醇和水洗涤,于60 ℃下干燥,得到urea-Fe3O4@LDH。
-
取浓度为1.9 mg·L−1的TPhP溶液500 mL,加入10 mg·L−1吸附剂,以200 r·min−1搅拌6 h。配置TPhP和不同浓度的颗粒、FA、HA、BSA的混合溶液,采用0.1 mol·L−1 HCl和NaOH调节溶液pH。TPhP和Br−的混合体系中,保持TPhP浓度不变,Br−的初始浓度为10 mg·L−1,吸附剂投加量为100 mg·L−1。去除率和吸附量根据式(1)和式(2)进行计算。
式中:η为去除率;C0为初始溶液中TPhP的浓度,mg·L−1;Ct为时间t时TPhP的浓度,mg·L−1;t为反应时间,h;V为溶液的总体积,L;qt为时间t内单位质量吸附剂的吸附量,mg·g−1;m为吸附剂质量,g。
1.1. 药品及试剂
1.2. 分析测试仪器
1.3. 吸附剂的制备
1.4. 吸附剂的性能测试
-
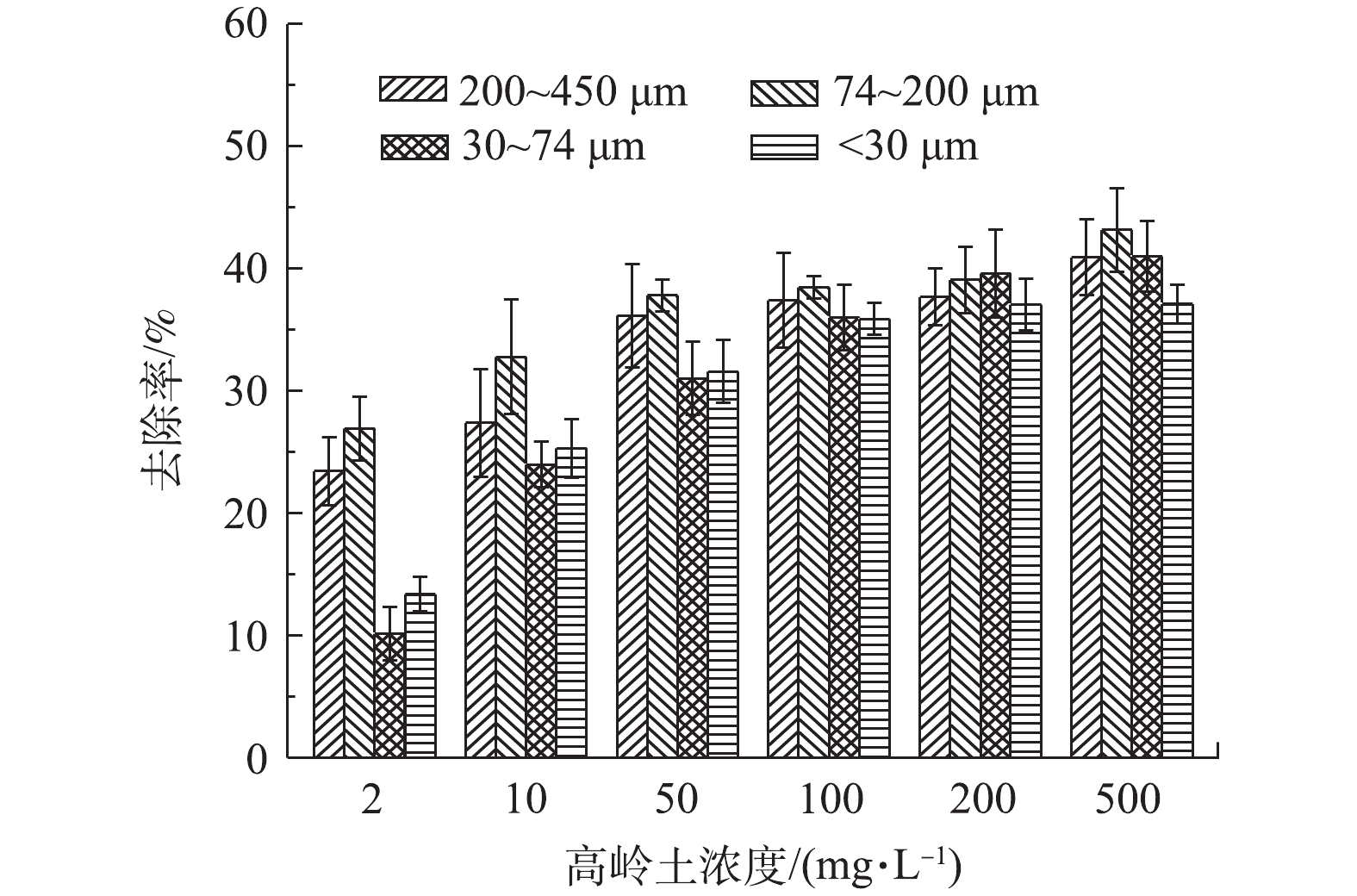
图1为不同浓度和粒径的高岭土对urea-Fe3O4@LDH吸附TPhP的影响结果。由图1可知,在相同颗粒粒径的条件下,高岭土浓度对urea-Fe3O4@LDH去除TPhP显示出一定规律性,呈现先轻微提高后降低的变化趋势。当颗粒粒径为200~450 μm时,高岭土浓度为0~100 mg·L−1,随着浓度的增大,urea-Fe3O4@LDH对TPhP的去除率从88%提高到98%;当高岭土浓度>100 mg·L−1时,TPhP去除率略有下降,由98%降低到78%。这是由于高岭土对TPhP有一定的吸附作用(图2)。当高岭土浓度增大至100 mg·L−1时,TPhP去除率增加到37%,随着高岭土浓度提高,高岭土和吸附剂共同作用去除TPhP,因此,导致TPhP的去除率有所提高。随高岭土浓度的继续增加,TPhP的去除率降低,这是因为高岭土减少了吸附剂与TPhP的接触概率。高岭土浓度>500 mg·L−1时,TPhP去除率明显降低。
当在同一高岭土浓度下,高岭土颗粒的粒径对urea-Fe3O4@LDH吸附TPhP有一定的影响。将高岭土颗粒粒径筛分成<30、30~74、74~200和200~450 μm,随着高岭土颗粒粒径的减小,吸附剂对TPhP的去除率有所降低。这是因为TPhP分子体积较大,由于空间效应,大颗粒的高岭土更容易吸附TPhP,故导致TPhP去除率有所升高;随着高岭土颗粒粒径的减小,其对TPhP的吸附减少,另一方面,高岭土减少了urea-Fe3O4@LDH和TPhP的碰撞概率,TPhP的吸附量降低,此时吸附剂和高岭土对TPhP的去除率降低。
-
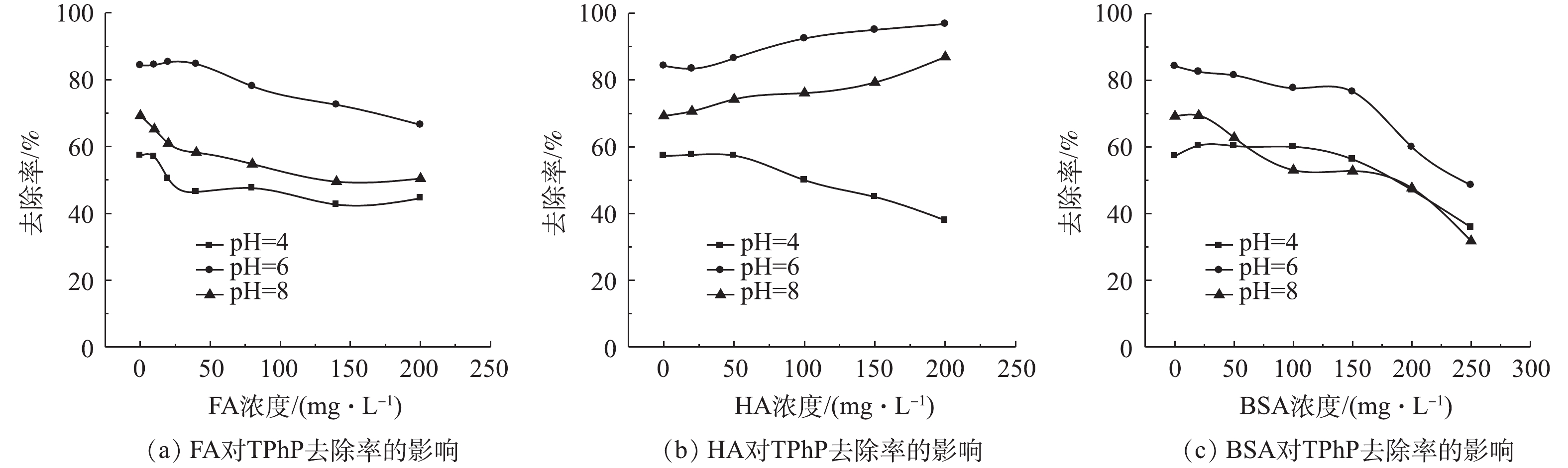
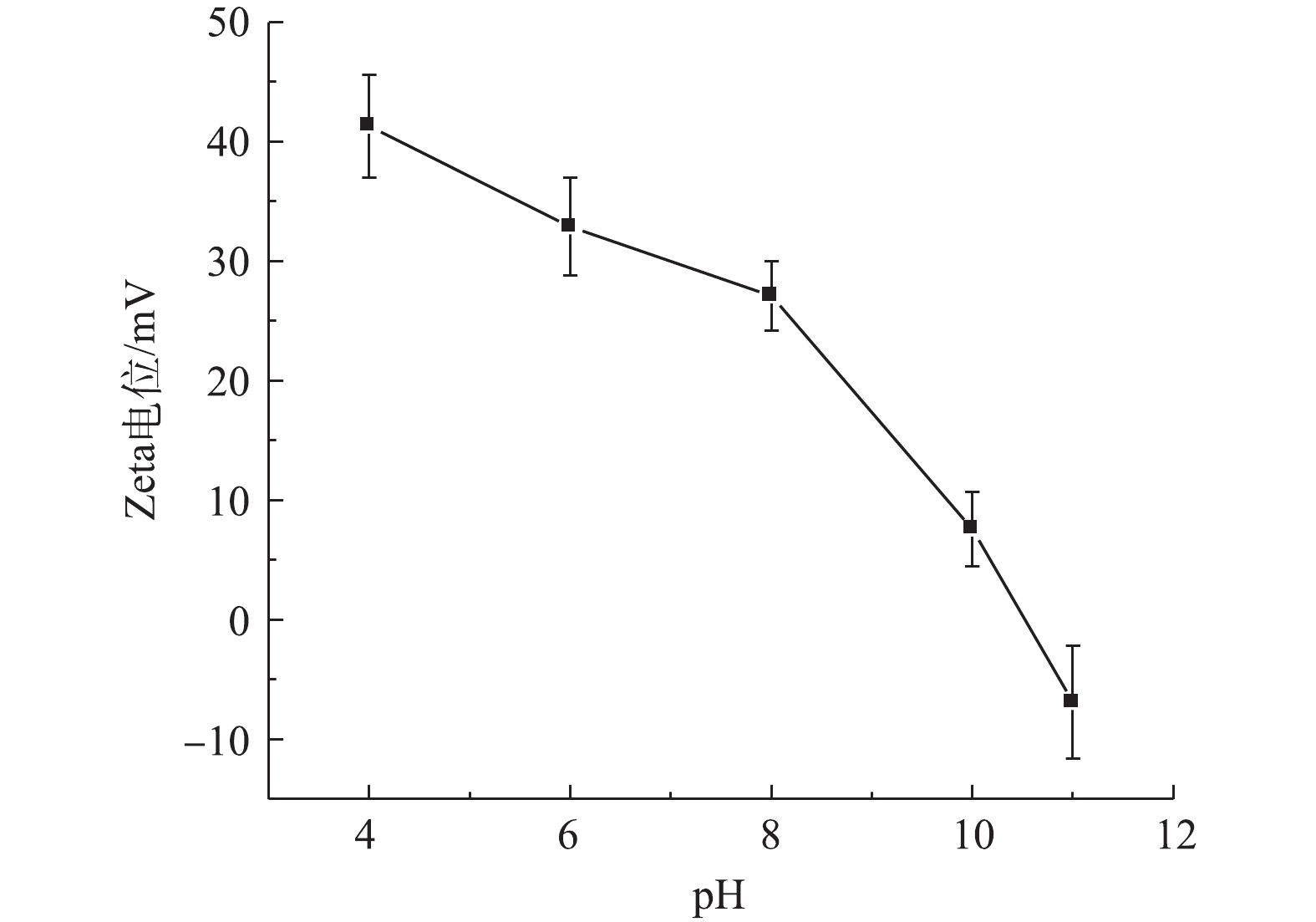
由于NOM的化学分子结构和性质不同,其与材料间的吸附和络合的相互作用均有所不同[18],因此,NOM的存在和不同分子质量有机物对urea-Fe3O4@LDH吸附TPhP具有一定的影响。图3为NOM中的FA、HA和BSA对urea-Fe3O4@LDH吸附TPhP的影响结果。如图3(a)所示,在相同pH条件下,当溶液中FA浓度由0 mg·L−1增大到200 mg·L−1时,urea-Fe3O4@LDH对TPhP的去除率降低。urea-Fe3O4@LDH吸附TPhP依靠静电相互作用,吸附剂的等电点为10.5,在pH为4~8时,其表面带有正电荷(pHpzc>pH),而FA中包含羧基、芳环、脂肪族链、和酚基等,这些酸性官能团的解离使FA带负电荷,故其可吸附到urea-Fe3O4@LDH表面,导致吸附剂表面电荷量减少,从而使得TPhP的去除率有所降低。在FA浓度相同的情况下,在pH=6时,urea-Fe3O4@LDH对TPhP的去除率高于pH=4和8时对应的去除率。由图4可知,pH在4~8时,urea-Fe3O4@LDH的Zeta电位为正,吸附剂表面带正电荷,且变化不大,因此,TPhP去除率变化主要是其基团所带电荷随pH变化所导致的。TPhP的磷酸基团中氧原子具有高的电子云密度,使得TPhP发生质子化[19],吸附剂与带正电荷的TPhP相互排斥;随着溶液pH的升高,质子化作用减弱,吸附剂表面与TPhP之间的排斥力减弱,带负电荷的氧和带正电荷的吸附剂表面之间的静电作用增强,故导致TPhP的去除率提高;继续增加溶液pH,TPhP中O=P基团带正电荷,吸附剂与TPhP中的磷酸基团发生排斥作用[20],最终导致TPhP的去除率有所降低。
图3(b)是HA对urea-Fe3O4@LDH吸附TPhP的影响结果。当pH=6和8时,随着HA浓度的增大,TPhP去除率亦随之提高。通过π-π相互作用,HA吸附在urea-Fe3O4@LDH材料表面,使吸附剂的疏水性增大,故使得疏水性较强的TPhP更易吸附在材料表面,从而有利于提高TPhP的去除率[21],此外,HA表面的芳环结构与TPhP间的π-π相互作用促进了TPhP吸附在HA上,同样可使TPhP去除率有所升高,此时HA和吸附剂均能够去除TPhP。WANG等[22]利用2种不同树脂在HA的存在下吸附有机磷阻燃剂,去除率随着HA浓度的增加基本保持恒定,其中TPhP去除率随HA浓度的增大而升高。PANG等[23]的研究发现,磁性MOF微球提取有机磷阻燃剂不受到HA的影响。然而,HA可以占据材料吸附位点,从而影响污染物的去除效果[24],因此,在pH=4时,随HA浓度升高,TPhP的去除率降低。此时质子化的TPhP带正电荷,不易吸附在带正电的urea-Fe3O4@LDH表面上,而HA中包含大量酸性位点,容易吸附在吸附材料上,占据了有效的吸附位点,随HA浓度升高,urea-Fe3O4@LDH对TPhP的去除率减少。HA对TPhP的去除率随其浓度升高趋于稳定,远低于urea-Fe3O4@LDH[9],故导致TPhP去除率降低。
图3(c)为使用BSA模拟SMPs的情况下,其对urea-Fe3O4@LDH吸附TPhP影响结果。在相同pH条件下,随着BSA浓度的增大,urea-Fe3O4@LDH对TPhP的去除率降低。这是由于BSA中含有大量氨基酸残基,可占据urea-Fe3O4@LDH表面的吸附位点;BSA带有负电荷时(pKa=4.7),容易被吸附在带正电的吸附剂表面,并且其浓度远高于TPhP,占据urea-Fe3O4@LDH的活性位点越多,TPhP的去除率越低。此外,BSA还有一定的絮凝作用,其能够促使吸附剂的沉淀,因而BSA对TPhP的去除率影响较大。TPhP的去除率在pH为6的条件下高于pH=4和8的情况下,此结果与FA、HA对TPhP去除率的影响结果一致。当溶液的pH=6和8时,BSA带负电荷,能吸附到材料表面,占据urea-Fe3O4@LDH吸附位点,导致TPhP去除率快速下降;当溶液的pH=4时,BSA表面带正电荷,占据吸附剂活性位点较少,从而使TPhP去除率下降相对较慢。
不同有机物的表面化学性质对去除率的影响不同,为研究其对吸附剂和有机磷污染物的作用,使用紫外线吸收率E2/E3和E4/E6作为有机物芳香性和极性的指标[25]。如表1所示,NOM的芳香性和极性对比为FA>HA>BSA,FA和HA表面含有更多的苯环等芳香基团和羧基、羟基等极性基团,因此,其能够通过π-π相互作用和静电相互作用吸附到urea-Fe3O4@LDH上。在有机物浓度低于50 mg·L−1时,FA、HA能够吸附到材料表面,进而通过苯环、羧基等官能团来吸附TPhP,使得TPhP去除率较为稳定,甚至略有增大,而组成BSA的氨基酸能占据部分吸附位点,但其对TPhP吸附能力弱,造成TPhP去除率略微降低。当溶液中有机物浓度较高时,urea-Fe3O4@LDH表面吸附更多有机物,吸附剂与TPhP接触率降低,尿素官能团对TPhP吸附能力减少,TPhP去除率降低。
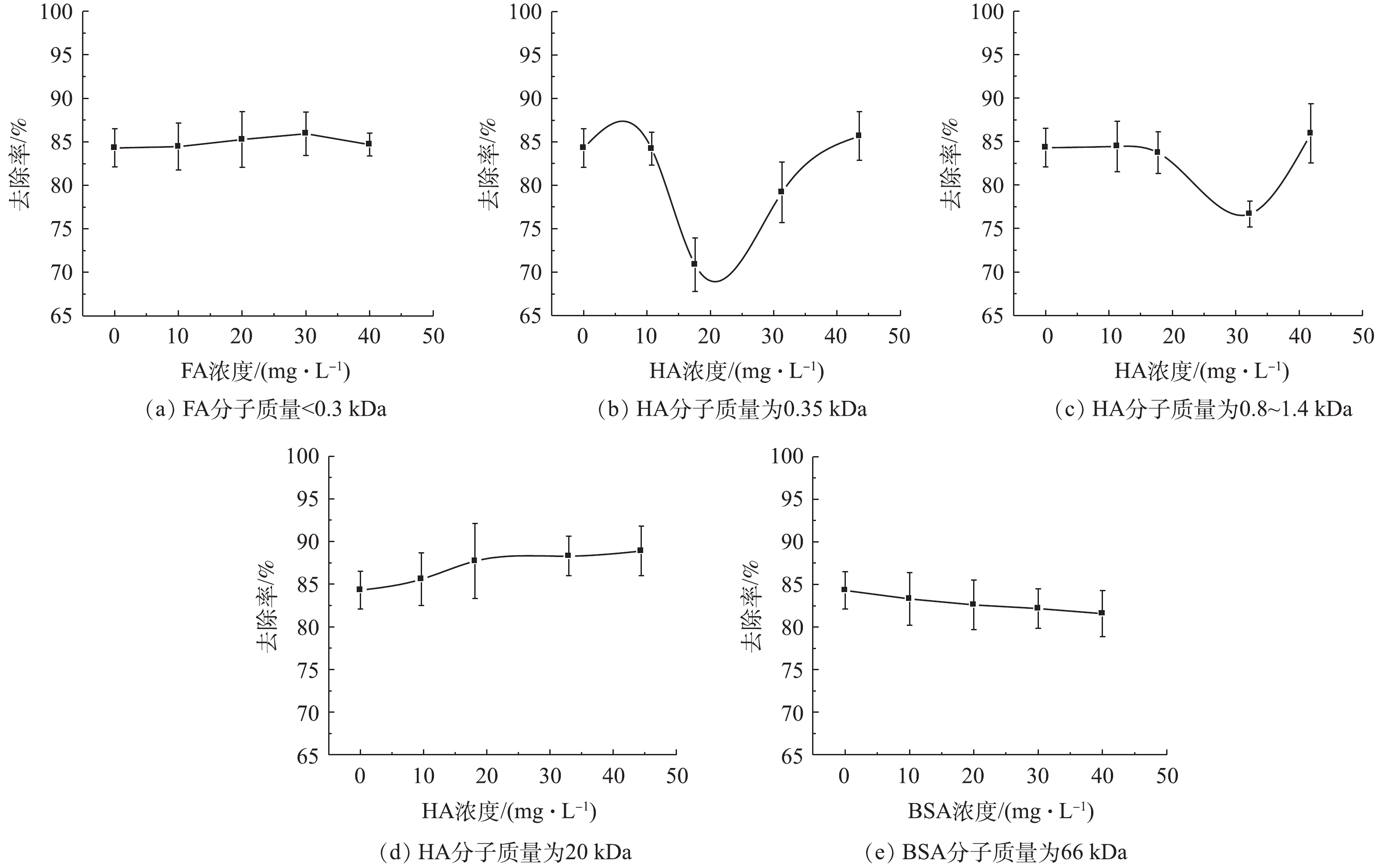
通常,FA的分子质量较小,小于0.3 kDa;HA的分子质量一般为几百到几万,将其划分为3.5、0.8~1.4和20 kDa;BSA分子质量约为66 kDa,不同分子质量的有机物在urea-Fe3O4@LDH孔道内扩散和吸附效果有所不同。图5为有机物分子质量对urea-Fe3O4@LDH吸附TPhP的影响结果。有机物浓度为0~40 mg·L−1时,有机物分子质量对urea-Fe3O4@LDH吸附TPhP的去除率影响不大,这是由于吸附主要依靠官能团而不是吸附剂的孔道。其中,不同分子质量HA对urea-Fe3O4@LDH吸附TPhP的效率有一定影响。当HA浓度为0~40 mg·L−1时,urea-Fe3O4@LDH对TPhP去除率略有降低,当HA浓度继续增加时,对TPhP的去除率增加。HA可占据urea-Fe3O4@LDH材料表面吸附位点和填充孔隙,抑制了污染物的去除,TPhP去除率降低;同时HA能够吸附有机磷化合物,污染物疏水性越强,与HA之间的吸附系数越大,结合能力随之越强,TPhP去除率上升。
-
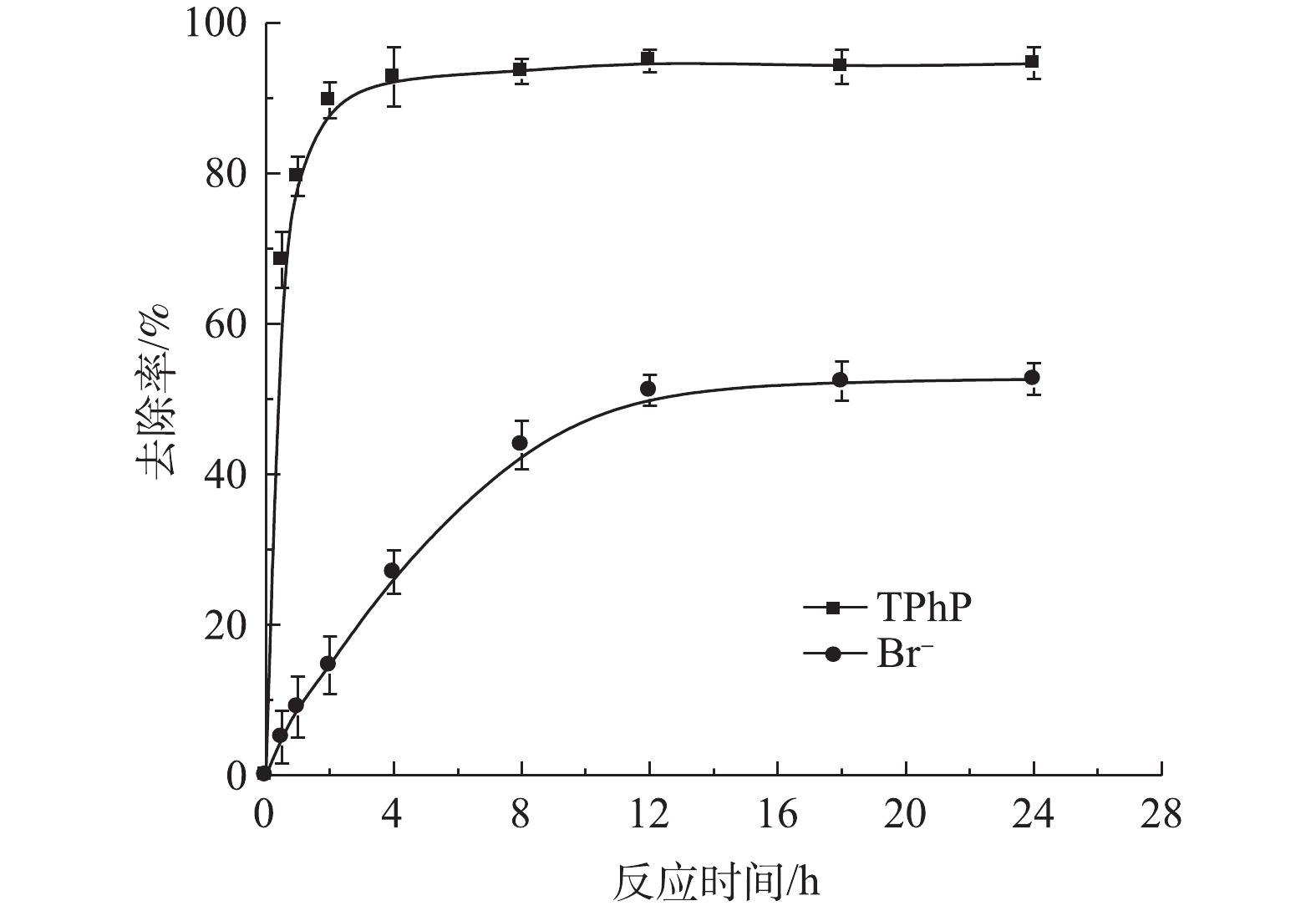
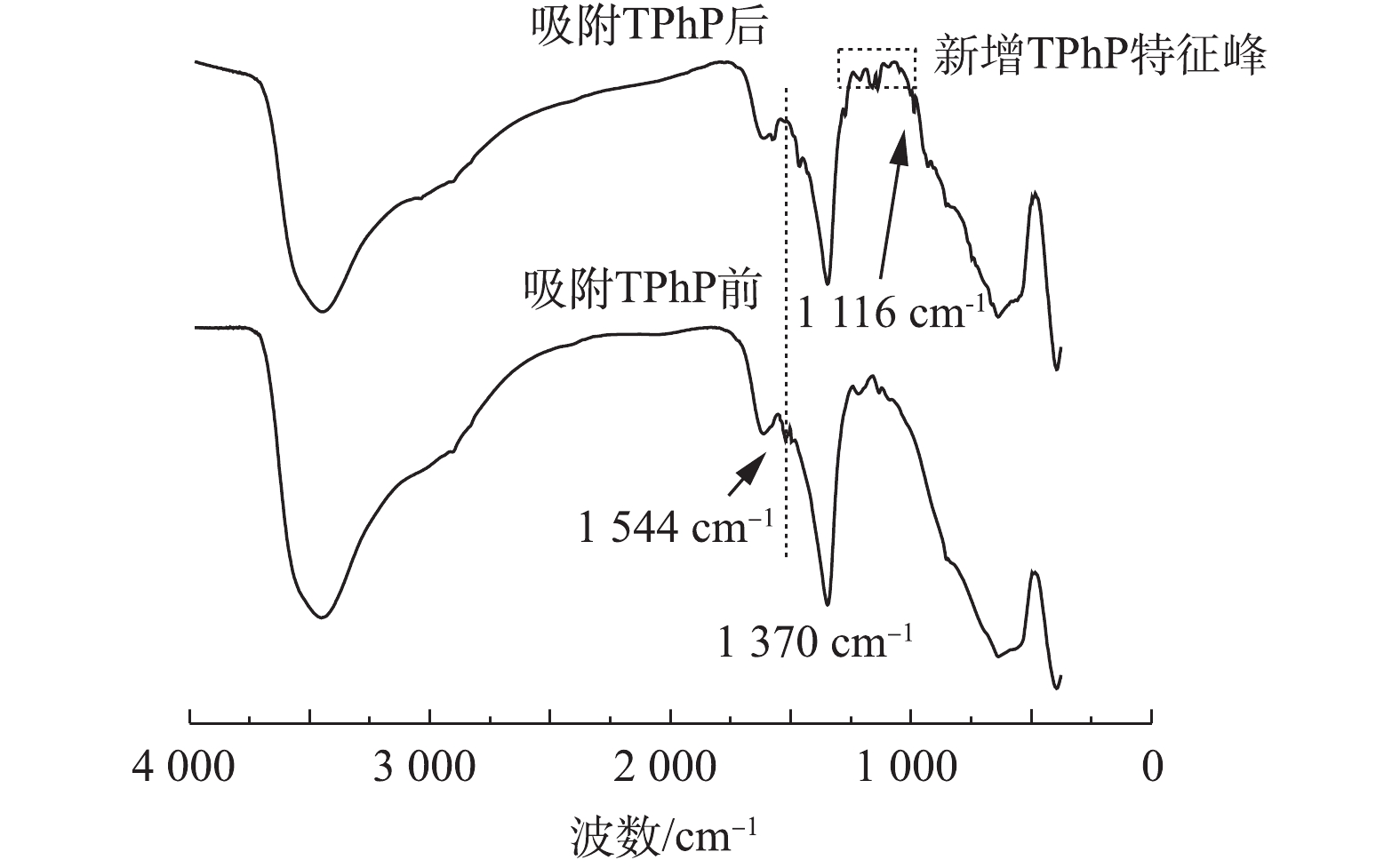
图6为在无机离子Br−存在的条件下,urea-Fe3O4@LDH对TPhP去除率的影响结果。结果表明,urea-Fe3O4@LDH对TPhP的去除率和速率并没有明显的影响,而且对Br−的去除率达到53%,因此,urea-Fe3O4@LDH可以同时吸附TPhP和无机阴离子Br−。图7为urea-Fe3O4@LDH吸附TPhP前后的FT-IR图谱。由图7可知,吸附TPhP后,材料表面出现其特征峰,在1 544 cm−1处的N—H峰有所减弱,在1 116 cm−1处的C—O峰附近出现N—O振动峰,这表明urea-Fe3O4@LDH和TPhP之间存在静电作用,在1 519 cm−1处的苯基在吸附后发生了变化,这是由于芳环间存在π-π相互作用。此外,在1 370 cm−1处的
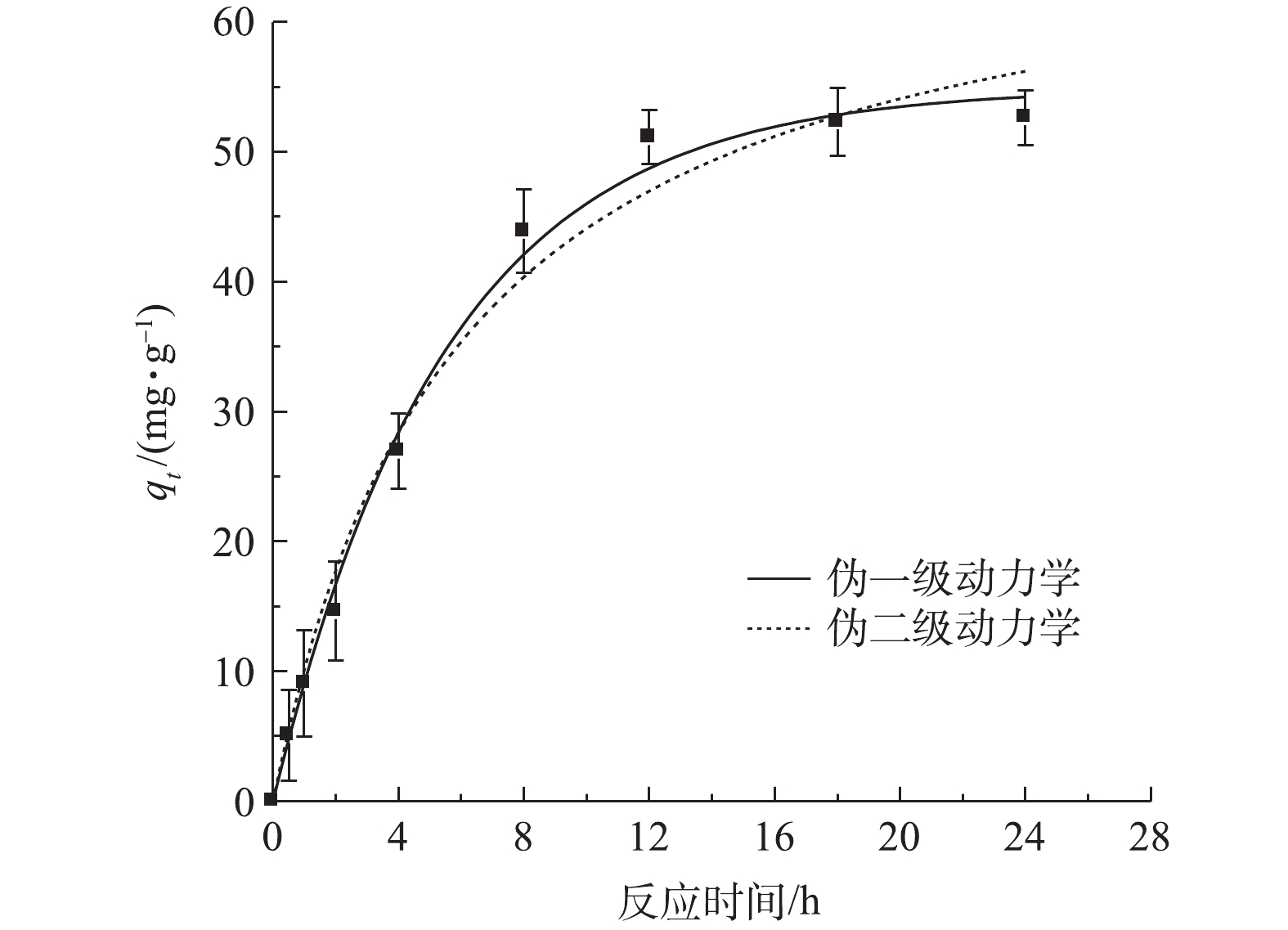
CO2−3 峰未变,表明TPhP没有进入LDH层间。加入Br−的浓度远高于TPhP,这表明TPhP和Br−在urea-Fe3O4@LDH表面没有发生竞争吸附,Br−可能通过离子交换进入层间,这是Br−吸附速率较慢的原因。urea-Fe3O4@LDH吸附Br−符合伪一级动力学方程(R2=0.995),Br−在吸附剂表面为单层吸附(图8)。通过动力学参数计算可知,Br−的最大吸附量为55 mg·g−1,吸附速率为11.9 mg·(g·h)−1。
2.1. 颗粒物的影响
2.2. 有机物的影响
2.3. 溴离子的影响
-
1)利用高岭土作为模拟颗粒物,当高岭土浓度为0~100 mg·g−1时,随着其浓度的升高,吸附剂对TPhP的去除率随之增加;当高岭土浓度>100 mg·g−1时,TPhP去除率略有下降;在同一浓度下,随着高岭土颗粒粒径的减小,TPhP去除率随之降低;高岭土对TPhP具有吸附作用,去除率最大能达到40%,且当颗粒大小为74~200 μm时,其对TPhP的吸附效果最好。
2)用FA、HA和BSA模拟水体中存在的有机物,在相同pH条件下,随着溶液中有机物浓度的增大,TPhP的去除率有所降低;在pH=6时,TPhP的去除率高于pH=4或8时对应的去除率;分子质量对TPhP去除率的影响不大。
3) urea-Fe3O4@LDH对TPhP的去除效率和速率没有受到Br−存在的影响,且能够同时吸附Br−,效率达到53%左右,吸附量达到55 mg·g−1;吸附剂去除Br−符合伪一级动力学方程,故为单层吸附。
4) urea-Fe3O4@LDH吸附主要依靠尿素官能团与TPhP间的静电作用和π-π作用;在一定浓度范围内,urea-Fe3O4@LDH去除TPhP受水体中颗粒物、有机物和无机阴离子影响较小,是一种去除废水中有机磷污染物尤其是TPhP的良好吸附剂。




 下载:
下载: