-
湖泊、水库是我国重要的饮用水源,且不少水体都存在不同程度的富营养化问题。当富营养水体发生“水华”时,造成水质恶化[1]。水源水中有害藻华(HABs)因其对水生态系统安全和人类健康产生了负面影响,引起了全世界的关注[2-3]。作为淡水中最普遍的蓝藻之一,铜绿微囊藻(Microcystis aeruginosa)具有生长繁殖的生态优势,在富营养化水体中成为优势藻种影响水质[4]。藻类种群密度在藻华季节的急剧增加严重影响了水处理过程的效率[5]。混凝作为一种常规处理方式,也常用于处理含藻水体工艺中[6]。原水水质是影响混凝效果的最主要因素之一,不同的原水水质,水中污染物含量、成分、pH、碱度等差异将直接影响混凝剂种类的选择和投加量[7-8]。藻华暴发将导致水体pH、碱度等异常升高,水体中的pH甚至可达到9~10,碱度会高于120 mg·L−1。而原水的碱度过高会对混凝过程产生不利影响,尤其是对于铝盐混凝剂,可能会产生出水余铝含量超标的问题[9-10]。
因此,本研究以氯化铝作为混凝剂,以铜绿微囊藻为研究对象,通过投加不同浓度的碳酸氢钠溶液来调节水样的碱度,考察了碱度对混凝去除藻细胞的性能影响,以期为处理富营养化水体和保证饮用水水质提供参考。
全文HTML
-
氯化铝(AlCl3·6H2O)、碳酸氢钠(NaHCO3)、硝酸钠(NaNO3)、氢氧化钠(NaOH)、盐酸(HCl)均购自国药集团化学试剂有限公司。
-
铜绿微囊藻(PCC7820),购自中国科学院水生生物研究所,采用BG11培养基进行培养,无菌条件下接种至锥形瓶中,放在人工气候培养箱中培养,培养条件为温度(25±1) ℃、光照强度2 000 lx、光暗比(L∶D)=12 h∶12 h。定期进行细胞计数,绘制生长曲线,待藻种达到稳定期后用于实验。
-
为了模拟蓝藻暴发时水体中的藻细胞浓度,控制水样藻细胞密度为1×106个·mL−1[11],并加入5. 0 mmol·L−1NaNO3提供离子强度。由于饮用水中只含有碳酸氢根碱度,故在此实验中只考虑调节碳酸氢根碱度[12-14]。配制0. 5 mol·L−1NaHCO3溶液,在500 mL稀释好的藻液中分别加入0、1.0、2.0、2.5、3.5、4.5 mL溶液,制成总碱度(以CaCO3计)分别为50、95、175、245、330、415 mg·L−1的水样,使用盐酸(0. 1 mol·L−1) 和氢氧化钠(0. 1 mol·L−1)溶液调节水样pH。
-
使用MY3000-6G智能型混凝搅拌仪(武汉梅宇有限公司)进行混凝实验,在200 r·min−1下快搅60 s,加混凝剂后,在200 r·min−1下快搅90 s,再在40 r·min−1下,慢搅10 min,静置沉淀30 min。在液面以下2.0 cm处取上清液,测定藻的吸光度及浊度。
利用马尔文激光粒度分析仪(Laser Particle Analyzer,Mastersizer2000,Malvern,UK)对混凝实验中絮体的形成过程进行在线监测,以D50代表絮体的平均粒径,并对已经形成的絮体进行破碎实验(5 min,200 r·min−1),随后进行絮体恢复实验(10 min,40 r·min−1),考察絮体的强度因子、恢复因子以及分形维数。
-
藻细胞密度采用UV-8500紫外/可见分光光度计(上海天美公司)测定;浊度采用浊度仪(2100N,Turbidimeter,HACH,USA)测定;将水样经0.45 μm水相滤头过滤后,采用总有机碳分析仪(Shimadzu,Japan)测定DOC;pH采用pH计(MP220,pH Meter,Mettler-Toledo,Switzerland)测定;出水余铝采用电感耦合等离子-原子发射光谱仪(ICP-OES OPTIMA-2000,PerknELMER,US)测定;于混凝快搅结束后取样,采用Zeta电位仪(zetasizer2000,Malvern,UK)测定Zeta电位;混凝静沉后的底部絮体经高速冷冻离心机(Aantij26XP,Beckman Coulter.Inc.USA)冷冻干燥后采用扫描电子显微镜(HITACHI SU8020 FE-SEM,Japan)测定。
1.1. 实验试剂
1.2. 藻种培养
1.3. 水样配制
1.4. 实验方法
1.5. 分析方法
-
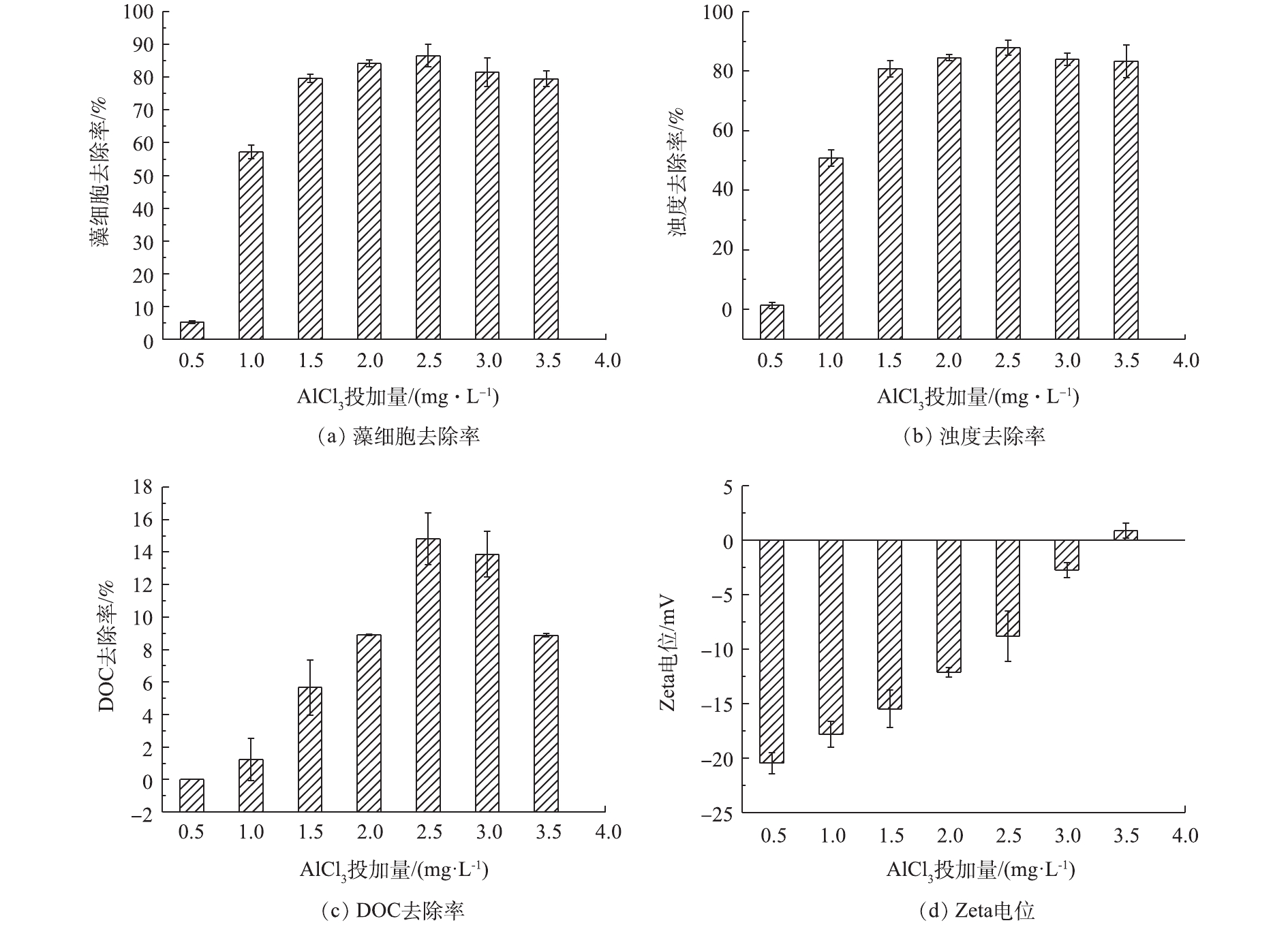
为探究混凝剂投加量对混凝效果的影响,考察了AlCl3(以Al计)投加量为0.5~3.5 mg·L−1、水样pH=7.5条件下藻细胞、浊度和DOC的去除率,结果见图1。由图1可见:当混凝剂投加量为0.5~2.0 mg·L−1时,藻细胞、浊度和DOC的去除率随着混凝剂投加量的增加而增加;当AlCl3投加量为2.5 mg·L−1时,藻细胞、浊度和DOC的去除率分别达到了86.52%、87.95%和14.80%;当投加量增大至3.5 mg·L−1时,藻细胞、浊度和DOC的去除率均有所降低。这主要是因为:当混凝剂投加量为0.5~2.0 mg·L−1时,投加量不足,导致电中和能力受到抑制,因此,藻细胞、浊度和DOC的去除率均不高;当混凝剂投加量过大时,体系中的胶体颗粒由于带有过高的正电荷而出现复稳现象,部分颗粒物难以聚集形成絮体而被去除,导致混凝效果下降。
-
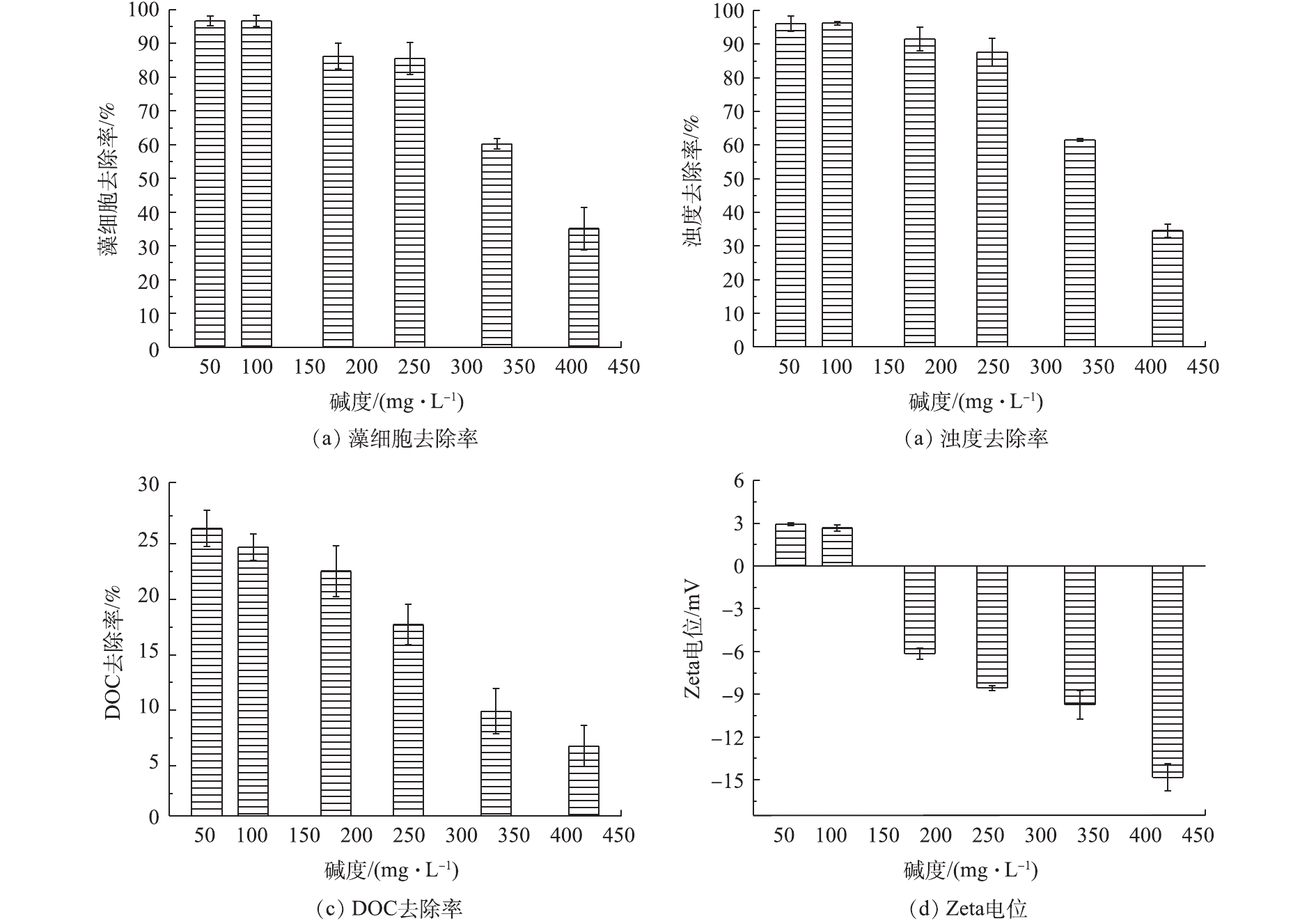
为探究碱度对混凝性能的影响,考察了各碱度含藻水样在AlCl3投加量为2.5 mg·L−1、pH=7.5条件下的藻细胞、浊度和DOC的去除率,结果见图2。由图2可知,随着碱度的增加,藻细胞、浊度、DOC的去除率以及Zeta电位值均呈下降趋势。藻细胞的去除率由96.65%降低至35.12%,浊度的去除率由96.12%降低至34.55%,有机物的去除率由24.61%降低至15.26%。同时也可以看到,随着碱度的增加,Zeta电位由2.67 mV降低至−14.83 mV。分析其原因在于,当水样pH=7.5、碱度为50~245 mg·L−1时,水样中主要存在Al(OH)3活性溶胶和一些具有较高聚合度的带正电水解产物,其可通过黏附架桥、网捕卷扫等作用达到较好的混凝效果[15],导致藻细胞、浊度和DOC的去除率较高。随着碱度的增加,负离子形态的
Al(OH)−4 占据优势,与带负电荷的铜绿微囊藻细胞之间产生了静电排斥,导致混凝效率下降[16],Zeta电位值也逐渐减小。 -
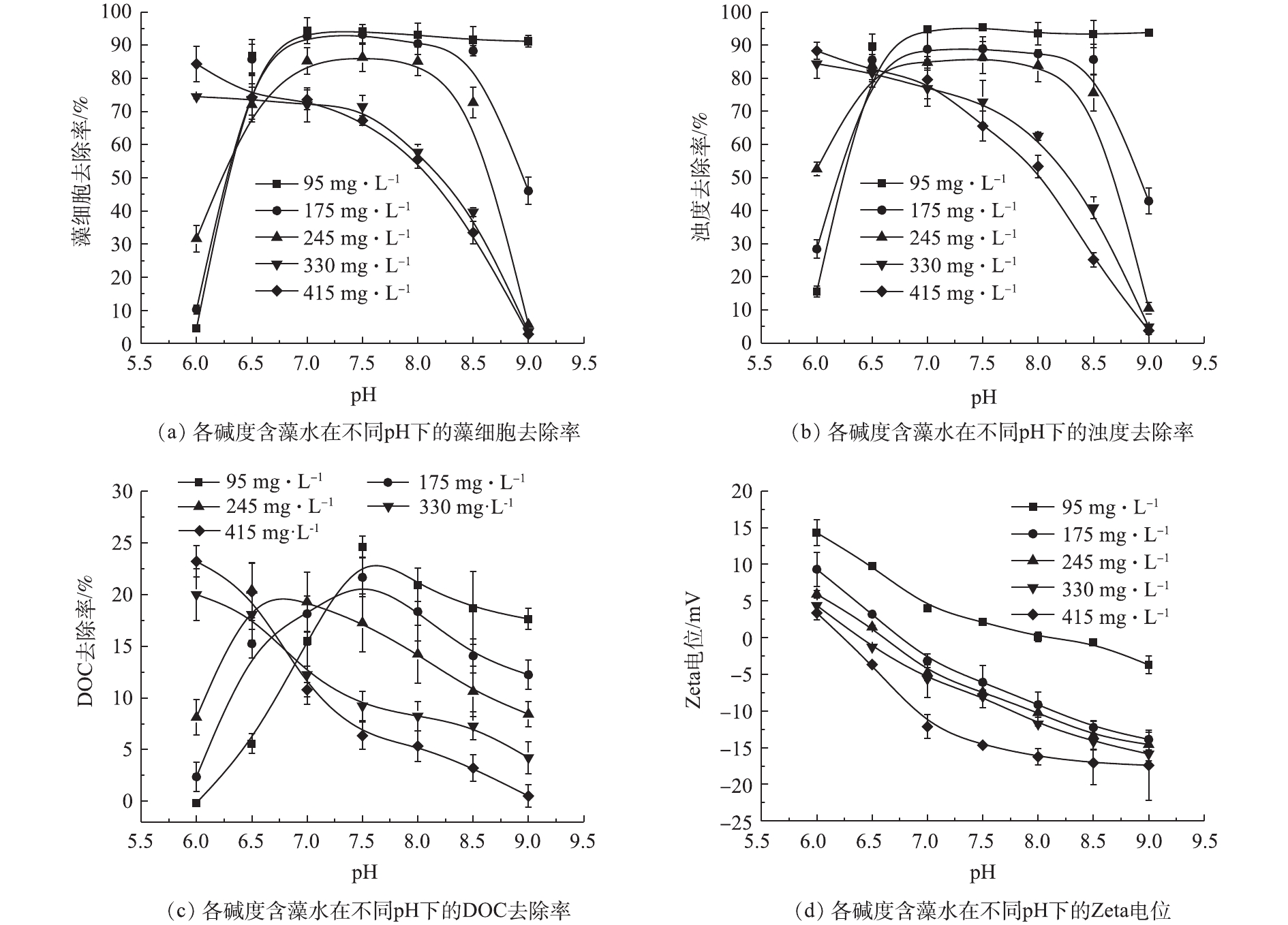
碱度和pH共同决定铝盐投加后的水解形态,进而影响混凝性能[17]。因此,可通过调整pH缓解碱度对混凝性能的影响,结果如图3所示。由图3可知:当碱度为95 mg·L−1和175 mg·L−1、pH=6.0时,藻细胞的去除率分别为4.59%和10.27%,在相同碱度条件下,当pH=6.5~8.5时,藻细胞的去除率在88.30%以上;当碱度为245 mg·L−1、pH=7.0~8.0时,藻细胞的去除率达到86.31%,当pH=6.0、9.0时,藻细胞的去除率均在31.64%以下;在碱度为330 mg·L−1和415 mg·L−1时,随着pH的增大,藻细胞的去除率呈下降的趋势,分别由74.45%和84.32%降低至3.56%和2.81%。浊度的去除率和藻细胞的去除效果一致。DOC的去除率虽然不高,在25%以下,但与藻细胞和浊度的去除率趋势一致。
分析原因在于,当水样的pH=6.0时,随着碱度的增加,Zeta电位绝对值逐渐减小,趋于等电点状态,体系的稳定性逐渐减弱,电荷排斥力逐渐下降,有助于絮体形成,有利于混凝过程进行,因此,导致藻细胞、浊度以及有机物的去除率增加。当水样的pH=6.5、碱度为95、175、245 mg·L−1时,混凝过程主要依靠吸附架桥和网捕卷扫协同作用,形成较大絮体,絮体沉降性好,去除率增加。当水样的pH=7~9、碱度为95 mg·L−1时,Zeta电位值趋近于0,体系趋于失稳状态,颗粒间的排斥力降低,藻细胞和浊度的去除率趋于稳定。而在其他各碱度下,随着pH的增大,铝盐混凝剂发生过度水解反应,生成的
Al(OH)−4 负离子增多,网捕卷扫作用减弱,且系统脱稳困难,不利于混凝过程的进行[18-20],所以藻细胞、浊度和DOC的去除率逐渐下降。在各碱度下,体系Zeta电位值均随pH的增大而降低,这是因为藻细胞本身的Zeta电位值会随着pH的增大而降低;水样pH对铝盐的水解形态分布也会影响体系的Zeta电位值;在相同pH下,碱度越高,带正电荷的铝盐水解产物越少,从而也会降低体系的Zeta电位值。综上所述,通过调节水样的pH,可以达到减缓碱度影响混凝过程的目的。当体系碱度较低时,可将pH调整至中性范围,以提高藻细胞的去除率;当体系碱度过高时,可以调节水样pH,从而使体系Zeta电位的绝对值趋近于0,打破体系稳定状态,进而有利于混凝过程的进行。
-
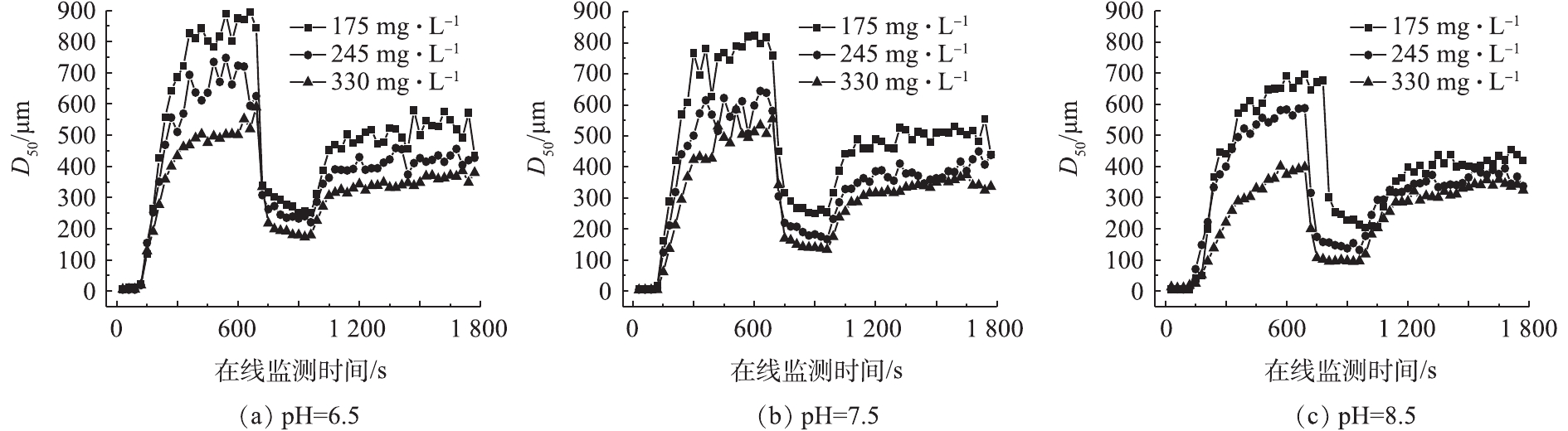
由于絮体的大小和强度对于分离过程有重要影响,因此,在大多数水处理过程中,絮体特性被当作是一个重要的操作参数[21],混凝过程絮体粒径变化情况如图4所示。
由图4可以看出,在相同pH条件下,随着碱度的增加,平衡时的絮体粒径逐渐减小。当pH=7.5、碱度为175、245和330 mg·L−1时,平衡时絮体粒径分别为811.02、633.28和540.62 μm。分析原因在于,在水样pH一定的条件下,碱度较低时,混凝过程依靠吸附架桥和网捕卷扫协同作用,形成粒径较大的絮体。随着碱度的增加,铝盐的水解产物Al(OH)3胶体更多转变为
Al(OH)−4 ,絮体间排斥能力增强,不利于颗粒聚集生成,故导致平衡时的絮体粒径减小。为了更好地了解不同条件下混凝形成絮体的特性,对强度因子(Sf)和恢复因子(Rf) (表1)以及分形维数(Df)[22](表2)进行了计算。
由表1可知,在相同pH条件下,随着碱度的增加,絮体的强度因子逐渐减小,恢复因子逐渐增大。当水样pH=7.5时,随着碱度的增加,絮体强度因子由35.97%降低至24.79%,恢复因子由35.31%增加至47.88%。强度因子逐渐减小的原因为:在相同pH条件下,碱度较低时,网捕卷扫在混凝机理上占主导地位,体系中有足够的Al(OH)3胶状沉淀,会促使形成比较密实的絮状结构[23],相应的强度因子就大;随着碱度的升高,铝盐水解生成的无定型Al(OH)3胶状沉淀逐渐转变为
Al(OH)−4 ,Al(OH)3胶状沉淀减少,导致网捕卷扫作用减弱,形成的絮体变得疏松,相应的强度因子就减小。恢复因子逐渐增大的原因在于,当网捕卷扫在混凝机理上占主导地位,破碎的絮体颗粒带正电荷[24],这些破碎的絮体颗粒可以继续吸附水样中的残余颗粒物。在水样pH一定的条件下,碱度较低时,藻细胞和有机物的去除率高,水样中残余颗粒物就少,在一定时间内与破碎的颗粒物碰撞概率小,因此,絮体恢复因子小;而碱度较高时,平衡时絮体粒径小,而且由于藻细胞去除率低,水样中还有大量的颗粒物,这就增大了与破碎絮体的碰撞概率,一定时间内破碎絮体吸附中和这些颗粒物使粒径再次增大,因此恢复因子也增大。由表2可以看出:在相同pH条件下,随着碱度的增加,平衡时絮体的分形维数逐渐减小;同一碱度下,随着pH的增加,平衡时絮体的分形维数也逐渐减小。当水样pH=7.5时,随着碱度的增加,平衡时絮体的分形维数由1.586减小到1.372;当碱度为245 mg·L−1时,随着pH的增加,平衡时絮体的分形维数由1.591降低至1.432。分析原因在于:当pH和碱度较低时,网捕卷扫占主导地位,形成的絮体结构更为致密,相应的分形维数就较高[25];随着碱度的增加,铝盐水解生成的Al(OH)3胶状沉淀逐渐转变为
Al(OH)−4 ,网捕卷扫作用减弱,胶体颗粒间的静电斥力增加,因此,形成的絮体结构变得疏松,相应的分形维数较低[26]。 -
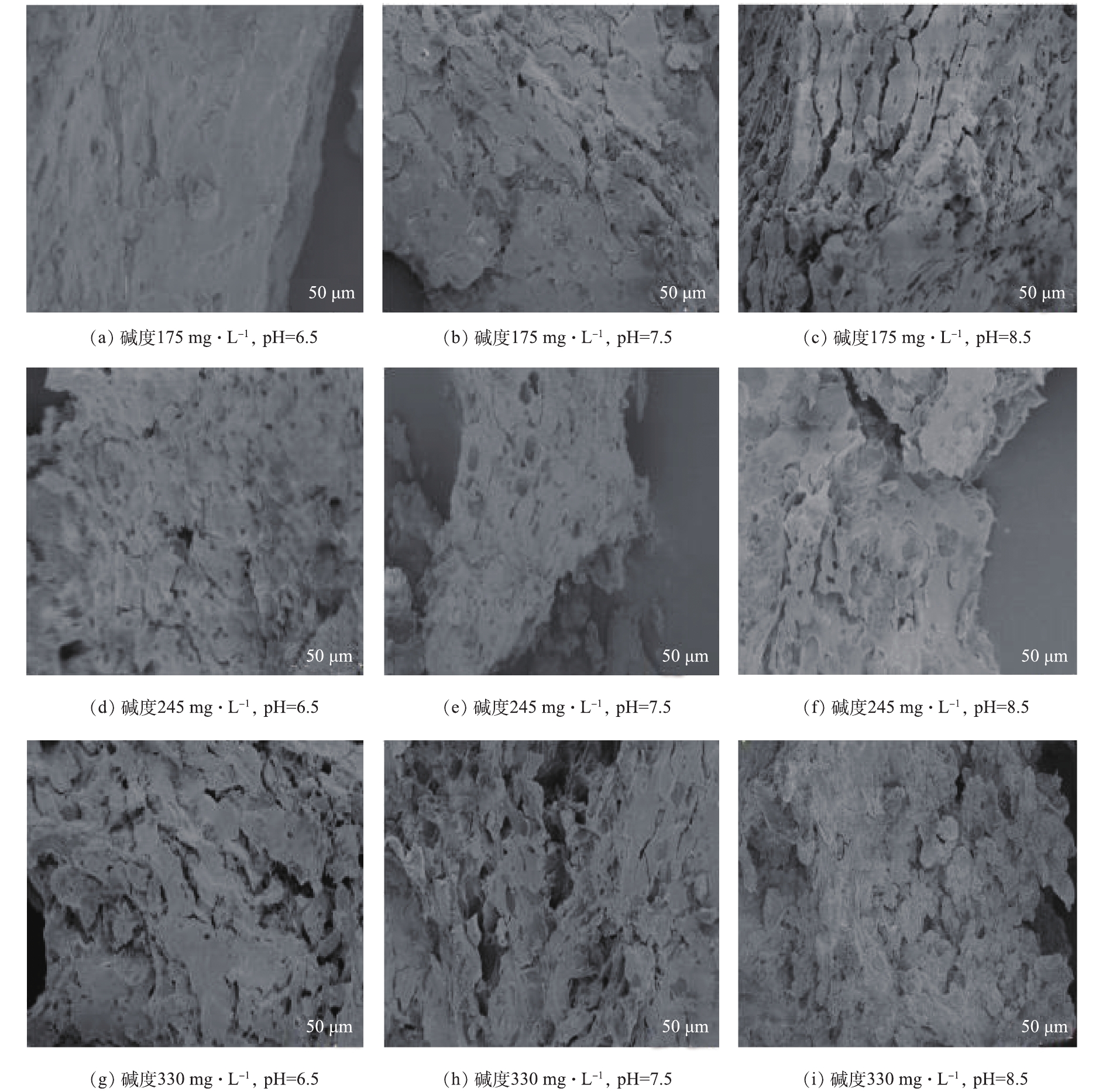
由图5可知,水样pH和碱度的不同导致混凝过程的作用机理不同,而不同混凝机理形成的絮体结构是有差异的。当水样pH和碱度较低时,形成的絮体更为致密,而当水样pH和碱度较高时,形成的絮体相对疏松。这主要是因为,在水样pH和碱度不同的情况下,形成的絮体粒径和分形维数不同。水样pH和碱度较低时,混凝过程依靠吸附架桥和网捕卷扫协同作用,形成絮体的分形维数大,絮体结构更为密实,絮体沉降性好,所以混凝效果较好;随着水样pH和碱度的增加,
Al(OH)−4 逐渐增多,致使网捕卷扫作用减弱,絮体间排斥能力增强,因此,分形维数逐渐减小,絮体结构越来越疏松,形成的絮体表面粗糙且多孔,而且絮体粒径小,沉降性差,导致混凝效果减弱。 -
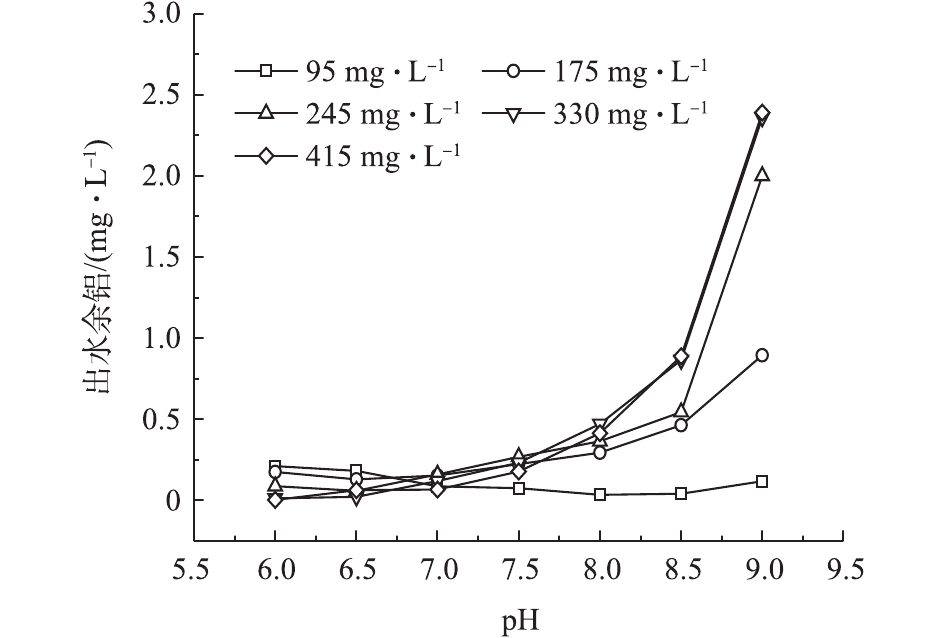
水样的碱度过高会导致使用铝盐混凝剂的残留铝含量增加,因此,实验对比了各碱度含藻水在不同pH条件下的出水余铝,结果如图6所示。可以看出:当碱度为95 mg·L−1、pH为6.0~8.0时,出水余铝从0.212 0 mg·L−1降低至0.035 3 mg·L−1。这是因为混凝效果逐渐增强,沉后出水中的溶解态铝含量降低,所以出水余铝减少。在其他碱度下,随着pH的增大,出水余铝均有所增加,且在相同pH下,随着碱度的增加,出水余铝也呈升高的趋势,当pH=9.0时,碱度为245、330和415 mg·L−1对应的出水余铝分别为2.030、2.360和2.390 mg·L−1。这是因为随着pH和碱度的升高,铝离子水解形成的 Al(OH)3胶状沉淀会溶解为负离子
Al(OH)−4 ,导致出水余铝含量升高。 -
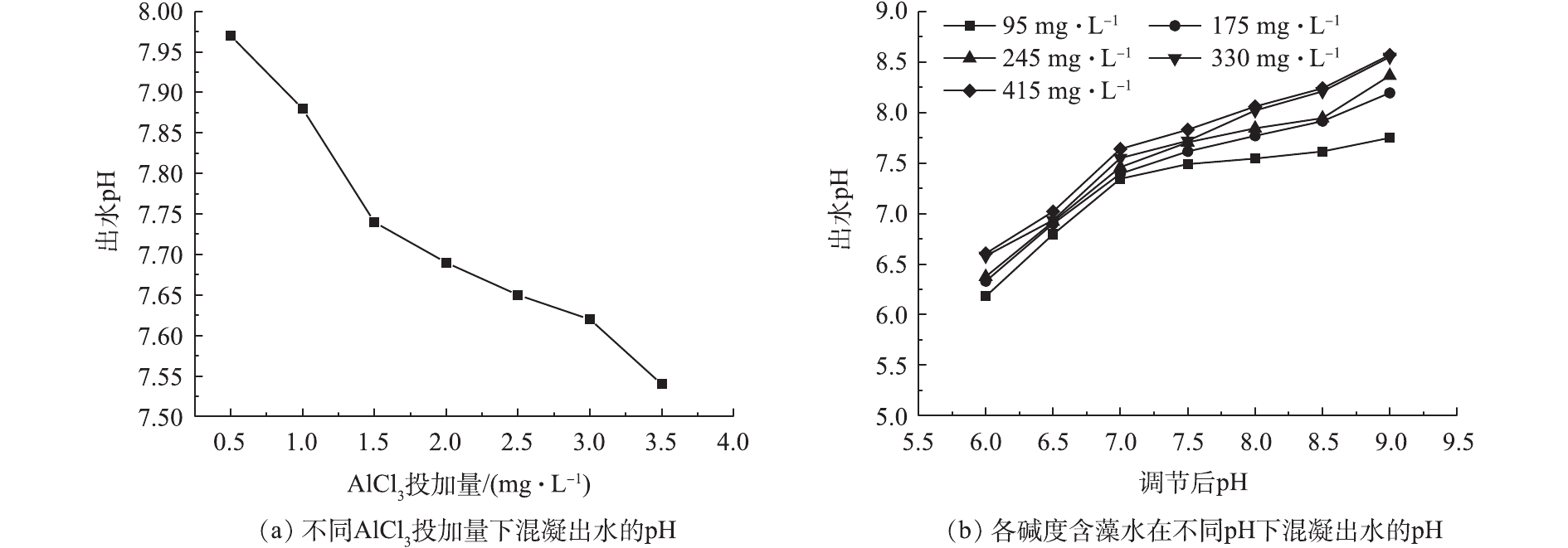
本研究对不同AlCl3投加量和各碱度含藻水在不同pH条件下混凝出水的pH进行测定,结果如图7所示。如图7(a)所示,当pH=7.5、碱度为245 mg·L−1时,随混凝剂投加量增大,出水pH由7.97降低至7.54。这是因为铝盐水解过程中会产生H+,H+的积聚会使体系的pH有所降低,虽然
HCO−3 能吸收H+,使体系pH波动减小,但是因为体系中HCO−3 的量是有限的,因此,无法中和过多的H+,所以随着混凝剂投加量的增加,体系出水pH有所降低。如图7(b)所示,在调节后的pH相同条件下,出水pH随着碱度的增大而增大,这是因为在体系中投加的铝离子的量是有限的,铝盐水解产生的H+也就有限,而HCO−3 浓度是增加的,其水解产生的OH−逐渐增多,但是没有足够的H+来中和,因此,反应后的出水pH随着碱度的增大而升高。
2.1. 混凝剂投加量对混凝性能的影响
2.2. 不同碱度对混凝性能的影响
2.3. pH对不同碱度含藻水混凝性能的影响
2.4. 絮体特性
2.5. 扫描电镜分析
2.6. 出水余铝
2.7. 反应体系出水pH
-
1)当水样pH=7.5、氯化铝投加量为2.5 mg·L−1、碱度为50、95 mg·L−1时,对于藻细胞和浊度的去除率均在96.50%左右,随着碱度的升高,对于藻细胞,浊度以及有机物的去除率均呈下降趋势,并且水样的Zeta电位值也逐渐降低。
2)通过调整水样pH,可有效缓解碱度对混凝过程的影响。当体系碱度较低时,可将pH调整至中性范围,提高藻细胞的去除效率,出水余铝含量也会降低;当体系碱度过高时,可通过调节pH使体系Zeta电位的绝对值趋近于0,打破体系稳定状态,从而有利于混凝过程的进行,出水残留铝含量也会有相应减少。
3)当水样pH≥6.5,碱度较低时,混凝过程依靠吸附架桥和网捕卷扫协同作用,形成的絮体粒径较大,而且絮体的结构较为致密,分形维数较大;随着pH和碱度的增加,絮体间排斥能力增强,不利于颗粒物的聚集生成,导致形成的絮体粒径较小,而且絮体结构比较疏松,分形维数逐渐减小。







 下载:
下载: