-
苯酚是一种对环境有污染的芳香族有机化合物,广泛存在于石油炼制、木材防腐、焦化等行业的废水中[1]。苯酚属于高毒物质,可以通过皮肤、黏膜、口腔进入人体内,低浓度苯酚可使细胞蛋白变性,高浓度可使蛋白质沉淀[2]。此外,苯酚被证明有致癌、致突变、致畸性等危害,被世界各国列为重点处理的有机污染物之一[3]。含酚废水的处理方法可分为物化法和生物法。其中,物化法具有成本高、容易造成二次污染等缺点。相比之下,生物法具有环境友好、成本低、不产生二次污染等优点[4-5]。微生物能利用酚类化合物作为碳源或能源,并将其代谢转化,因此,细菌[6]、真菌[7]、藻类[8]等去除酚类化合物得到了广泛研究。
苯酚降解细菌大多具有繁殖速度快、苯酚耐受性强和降解效率高等特点,常用于苯酚废水处理,并取得了良好的效果。但其在处理过程中存在一些限制,如曝气成本高、产生CO2等温室气体及难处理的污泥等[9]。藻菌共培养对废水中污染物的去除更加有效,且具有以下优势:微藻通过光合作用产生异养细菌降解有机物所需的O2,较细菌处理技术节省了曝气成本并减少污染物的挥发;微藻吸收利用细菌呼吸作用降解有机物时释放的CO2,进而减少温室气体排放;获得的微藻生物质可进一步加工制备高附加值产品,提高经济效益[10-13]。RYU等[14]利用四尾栅藻和活性污泥处理焦化废水,发现藻菌共培养可于144 h内完全降解初始浓度为24~120 mg·L−1的苯酚,而单藻体系在144 h时最高的降解率仅为27.3%(初始苯酚浓度24 mg·L−1)。MAZA-MÁRQUEZ等[15]利用斜生栅藻和小球藻与酚类物质降解菌Raoultella terrigena和Pantoea agglomerans,构建菌藻共生体系,可在48 h内去除99%以上的苯酚(50、100、150 mg·L−1)。
尽管藻菌共培养处理苯酚废水较细菌具有一定优势,但目前处理的苯酚浓度仍然较低。高浓度苯酚会对微生物产生毒害作用、抑制其活性功能,从而影响了该技术的实际应用。同时,大多研究关注藻菌共培养对含酚废水的处理效果,而对处理过程中微藻生长的变化关注较少,但微藻生物质的获得对提高该技术的经济性具有重要作用。简单芽胞杆菌已被证明是一种高效苯酚降解菌。袁利娟等[16]分离出1株高效苯酚降解菌JY01,其16S rDNA序列与 Bacillus simplex (AM9216370)的相似性为99.01%,在苯酚浓度为1 100 mg·L−1和1 300 mg·L−1时,该菌株30 h内可分别降解99.16%和74.76%的苯酚。本研究旨在构建小球藻与简单芽胞杆菌的共培养体系用于高浓度苯酚废水处理,探索其处理高浓度苯酚废水和积累微藻生物质的潜力,研究藻菌比、藻菌接种浓度和苯酚浓度对共培养体系苯酚降解能力和微藻生长的影响,以期为藻菌共培养处理苯酚废水同时积累微藻生物质的进一步研究提供参考。
全文HTML
-
小球藻接种于灭菌(121 ℃,30 min)的BG11培养基[17]中,于柱形光生物反应器(高40 cm、直径4.5 cm、体积为350 mL)中持续光照培养至对数增长期,光量子通量密度为150 μmol·(m2·s)−1,温度为(25±2) ℃,通入体积分数为1%的CO2加快微藻生长。
简单芽胞杆菌Bacillus simplex(NO.1.3471)购于中国微生物菌种保藏中心。细菌接种于灭菌的营养肉汤培养基(蛋白胨10 g·L−1、牛肉提取物3 g·L−1、氯化钠5 g·L−1、去离子水1 L),于250 mL锥形瓶内振荡培养至对数增长期,温度为(30±2) ℃、摇床转速为150 r·min−1。
实验所用废水为含苯酚模拟废水,即在灭菌BG11培养基内添加一定浓度苯酚。接种方法:对数期的藻液和菌液分别经4 000 r·min−1离心5 min和8 000 r·min−1离心10 min,并弃上清液后获得藻泥和菌泥,用灭菌水清洗3次去除残余培养基后,接种于苯酚模拟废水中。
-
为考察小球藻对苯酚的耐受性,设置了100、200、300、400、500、600 mg·L−1的苯酚浓度梯度,并设置1组不添加苯酚的对照。小球藻接种于模拟苯酚废水,于250 mL锥形瓶(工作体积为100 mL)中振荡培养,藻初始接种浓度(以干质量计)为0.2 g·L−1,光量子通量密度为110 μmol·(m2·s)−1,温度为(25±2) ℃,转速为150 r·min−1,每组实验3个重复。每日取样测定干质量浓度、叶绿素(a+b)含量、Fv/Fm (Fv/Fm表示PSⅡ的最大光化学量子产量,Fv为可变荧光,Fm为最大荧光产量)、苯酚浓度。
在小球藻初始接种浓度和苯酚浓度分别为0.2 g·L−1和400 mg·L−1条件下,考察不同藻菌比对苯酚降解和小球藻生长的影响,设置藻菌比(干质量比)为4∶1、2∶1、1∶1、1∶2、1∶4,并设置1组只接种小球藻不接种简单芽胞杆菌的处理组作为对照。
在初始藻菌比1∶1、苯酚浓度400 mg·L−1条件下,考察不同初始接种浓度对苯酚降解和小球藻生长的影响,设置小球藻和简单芽胞杆菌初始接种浓度为0.05、0.1、0.2、0.3、0.4 g·L−1。
在初始藻菌比为1∶1 (0.2 g·L−1∶0.2 g·L−1)条件下,考察不同苯酚浓度对苯酚降解和小球藻生长的影响,设置了200、300、400、500、600 mg·L−1的苯酚浓度梯度。在实验中,藻、菌接种于250 mL锥形瓶(体积为100 mL)中振荡培养,条件为110 μmol·(m2·s)−1、(25±2) ℃、150 r·min−1,每日取样测定苯酚浓度和叶绿素(a+b)含量,每组实验设3个重复。
-
微藻干质量浓度采用重量法[18]测定,醋酸纤维微孔滤膜(φ=0.45 μm),烘箱温度105 ℃;Fv/Fm值通过FMS-2便携脉冲调制式荧光仪[19]测定;藻液内叶绿素(a+b)含量采用分光光度法[20]测定,离心转速为13 400 r·min−1,提取试剂为甲醇,测定波长为652、665、750 nm。在藻菌共培养体系内,以叶绿素(a+b)含量变化评估微藻生长性能[21],并通过式(1)计算微藻比生长速率。
式中:μ为比生长速率,d−1;Nt和N0为培养时间为t和0时的叶绿素(a+b)含量,mg·L−1。
苯酚浓度的测定参照4-氨基安替比林分光光度法[22],苯酚去除率用式(2)进行计算。
式中:r为苯酚去除率;Ct和C0分别为培养时间为t和0时的苯酚浓度,mg·L−1。
1.1. 实验材料
1.2. 实验方法
1.3. 分析方法
-
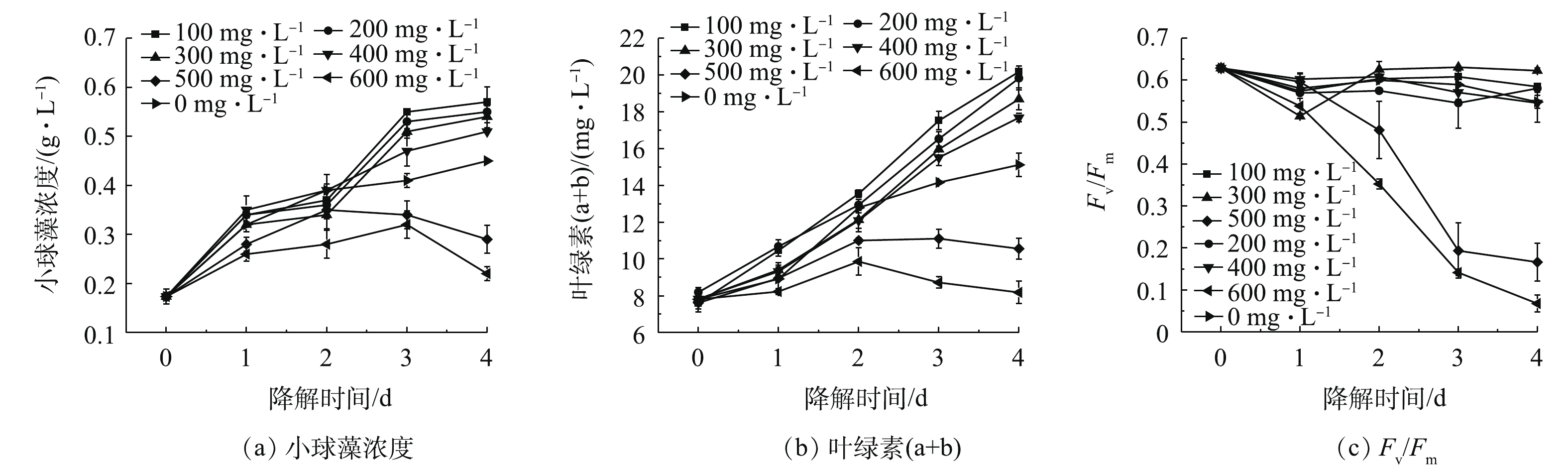
如图1(a)所示,在100、200、300、400 mg·L−1苯酚浓度下,小球藻在3~4 d时到达稳定期,4 d时小球藻浓度分别较对照组高26.67%、22.22%、20.00%、13.33%。这说明小球藻不仅能耐受较低浓度(≤400 mg·L−1)苯酚,并且低浓度苯酚能够促进小球藻生长。但随着苯酚浓度继续增加到500~600 mg·L−1,小球藻生长较对照组慢,小球藻浓度从2~3 d时开始下降,4 d时小球藻浓度分别较对照组低35.56%、51.11%,这说明高浓度苯酚抑制了小球藻的生长。任佳等[23]发现,低浓度苯酚(<100 mg·L−1)可以促进螺旋藻生长,而苯酚浓度升高(200、300 mg·L−1)对螺旋藻生长有抑制作用。苯酚可能起到类似双酚A的毒物兴奋效应,在低浓度下刺激小球藻生长,在高浓度下抑制小球藻生长[24]。
微藻叶绿素(a+b)的含量可以反映微藻的生长状况和细胞生存能力。如图1(b)所示,叶绿素(a+b)含量的变化趋势与小球藻浓度的变化趋势相一致。在4 d时,100~400 mg·L−1苯酚浓度下,叶绿素(a+b)含量较对照组高16.96%~33.46%;而在苯酚浓度为500、600 mg·L−1时,叶绿素(a+b)含量分别较对照组低30.14%、45.86%。RYU等[14]研究发现,四尾栅藻在60%、80%、100%的焦化废水下生长时,叶绿素a含量较20%、40%焦化废水下低,与本研究结果类似。这可能是由于在高浓度的苯酚条件下,会存在大量的活性氧,活性氧会攻击叶绿素a,同时抑制光合作用,对微藻的生长不利[25]。
Fv/Fm可以反映绿色植物潜在最大光合作用潜力。如图1(c)所示,在较低的苯酚浓度下(≤400 mg·L−1),Fv/Fm在0~1 d有小幅度的下降(4.21%~18.20%),然后迅速恢复并维持较高水平。这可能是由于在初始阶段微藻生长处于迟滞期,细胞光合活性较低;500、600 mg·L−1苯酚浓度条件下,Fv/Fm值在1 d后迅速降低,在4 d时分别降至0.17±0.05、0.07±0.02,较初始值分别下降了73.61%、89.29%。这说明此条件下小球藻光合活性急剧降低,高浓度苯酚对小球藻具有毒性效应。
-
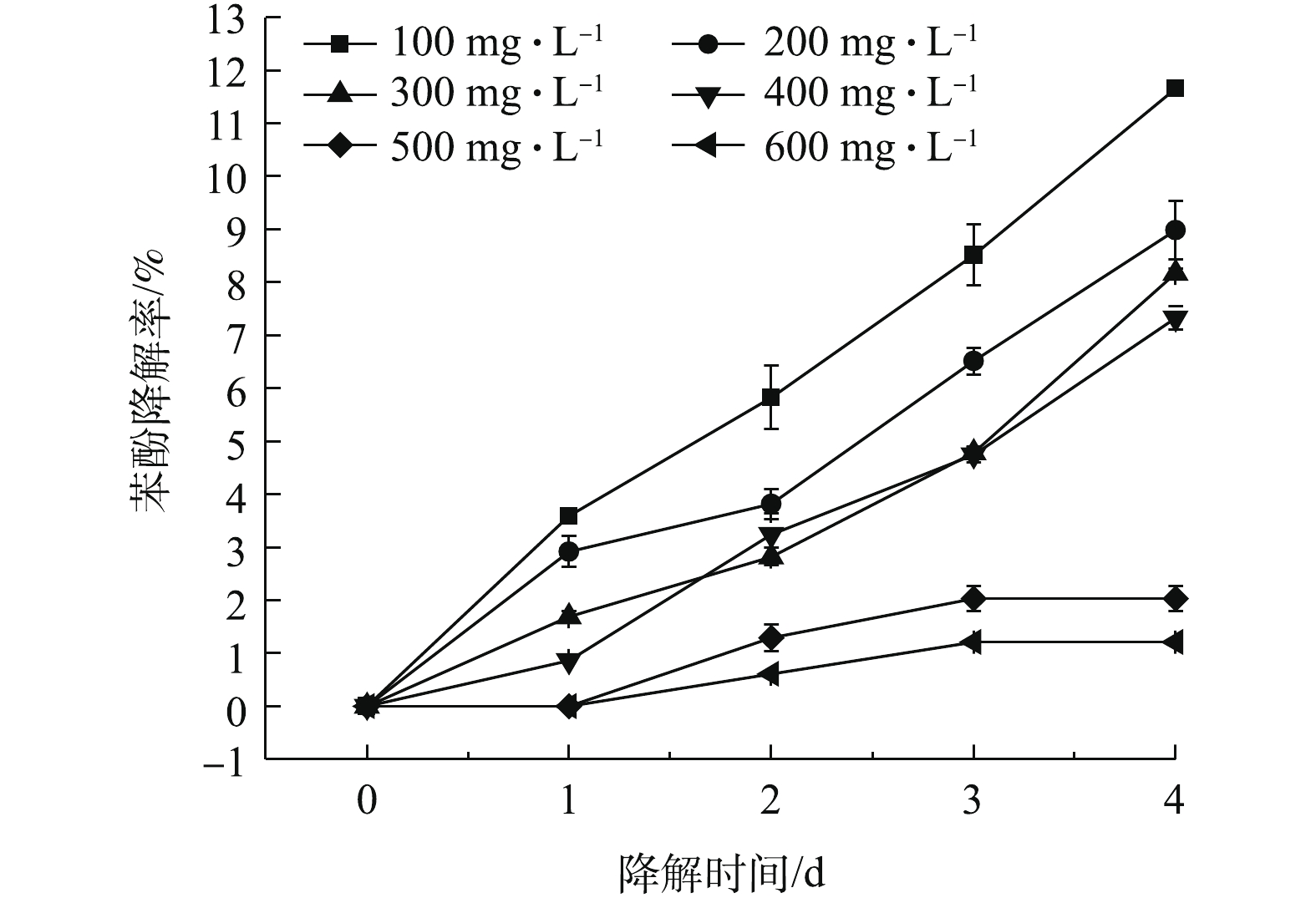
不同苯酚浓度下苯酚降解率随时间的变化如图2所示。4 d时,100、200、300、400、500、600 mg·L−1苯酚条件下,苯酚降解率分别为11.66%、8.99%、8.17%、7.33%、2.03%、1.21%。这说明小球藻对各浓度苯酚的去除能力较低,且随着苯酚浓度的增加,降解率降低。据SCRAGG等[26]报道,Chlorella VT-1在14 d内对100~400 mg·L−1苯酚的降解率仅为1%~10%,这与本研究中小球藻降解苯酚的能力相当。
-
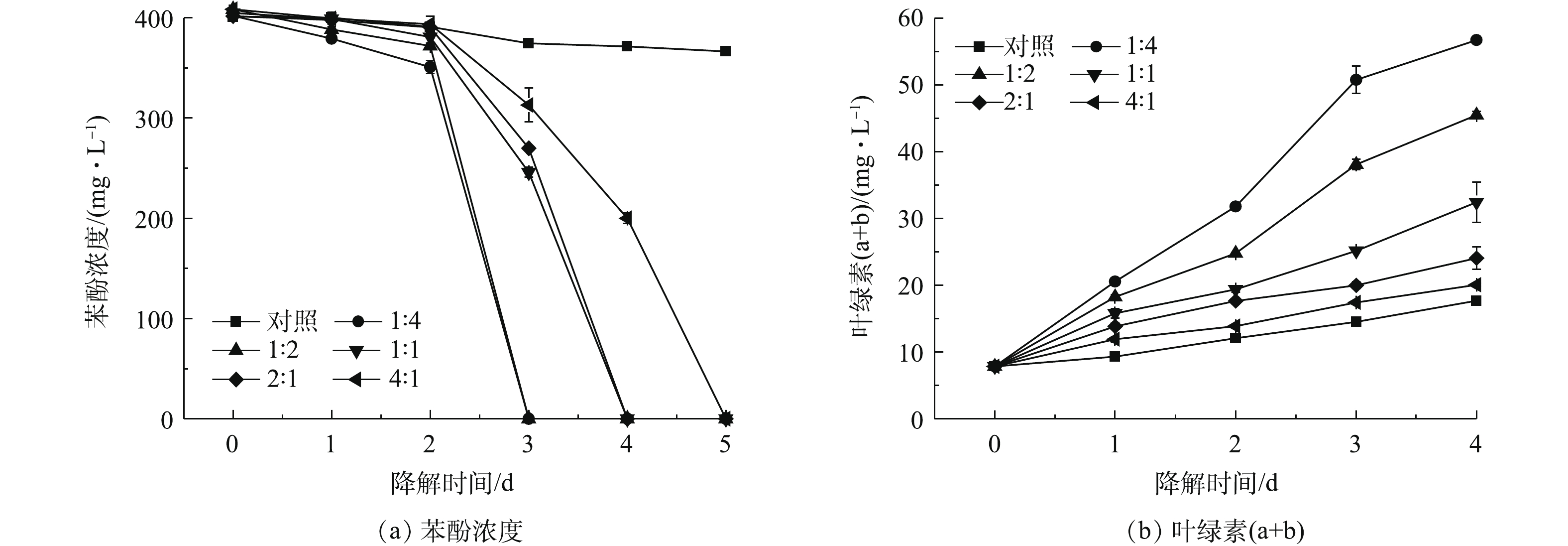
藻菌接种比例是影响藻菌体系的协同关系和污染物的去除率的关键因素之一[27-28]。在不同藻菌比下,苯酚浓度随时间的变化如图3(a)所示。可以看出,对照组苯酚降解能力较低,在3、4、5 d时,苯酚仅降解了6.63%、7.38%、8.62%。藻菌比为1∶4、1∶2、1∶1、2∶1、4∶1时,分别在3、3、4、4、5 d完全降解400 mg·L−1苯酚。这表明,接种简单芽胞杆菌明显提高了苯酚降解效率,且随着细菌添加量的增加,苯酚降解效率逐渐提高。由此可知,在共培养体系中苯酚降解主要是细菌的贡献。
叶绿素(a+b)含量随时间的变化如图3(b)所示。在初始小球藻接种浓度相同的情况下,藻菌比对小球藻叶绿素(a+b)含量的变化影响显著(P<0.05)。藻菌比为1∶4、1∶2、1∶1、2∶1、4∶1时,小球藻叶绿素(a+b)含量(4 d)分别较单藻组增加了2.21、1.57、0.83、0.36、0.14倍。可见,随着细菌浓度的增加,叶绿素(a+b)含量,即微藻生物量也随之增加。在藻菌共培养中,细菌促进微藻生长已有类似报道,如在20%和40%的焦化废水下,与活性污泥共培养的四尾栅藻叶绿素a含量分别较单藻高1.63倍和2倍[14]。在本研究中,藻菌比为1∶4、1∶2、1∶1时,藻菌组叶绿素(a+b)含量较单藻组可增加0.83~2.21倍。这说明此藻菌组合有收获微藻生物质的潜力。虽然高的菌藻接种比具有高的苯酚降解效率和微藻生物量,但高细菌浓度在实际应用中会增加细菌生产及后续处理成本,因此,本研究选择藻菌比1∶1进一步考察。
-
除藻菌接种比例外,微藻与细菌的初始接种浓度对苯酚降解也具有重要影响。在不同藻菌初始接种浓度下,苯酚浓度随时间的变化如图4(a)所示。可以看出,0.1~0.4 g·L−1的初始接种浓度可在4 d内完全降解苯酚,而0.05 g·L−1在5 d内完全降解苯酚。叶绿素(a+b)含量随时间的变化如图4(b)所示。可以看出,0.1、0.2、0.3、0.4 g·L−1的藻菌组随着培养时间的增加,各组叶绿素(a+b)含量增加,而0.05 g·L−1接种组内叶绿素(a+b)含量增加较少。4 d时,初始接种浓度为0.05、0.1、0.2、0.3、0.4 g·L−1条件下,各接种浓度下微藻比生长速率分别为0.22、0.39、0.41、0.25、0.24 d−1,初始接种浓度为0.2 g·L−1时具有最高的比生长速率。综合微藻生长和苯酚降解效率,在微藻和细菌接种浓度分别为0.2 g·L−1时具有最优效率。CHENG等[29]报道,在小型黄丝藻初始接种浓度为3 g·L−1、6 d时,模拟废水中250 mg·L−1苯酚的降解率为94.6%。LEE等[30]采用0.8 g·L−1的螺旋藻在6 d内可将400 mg·L−1苯酚降解97%左右。可见,本研究采用较低的微藻和细菌接种浓度,也可以达到较高的苯酚降解效率及微藻生物量。
-
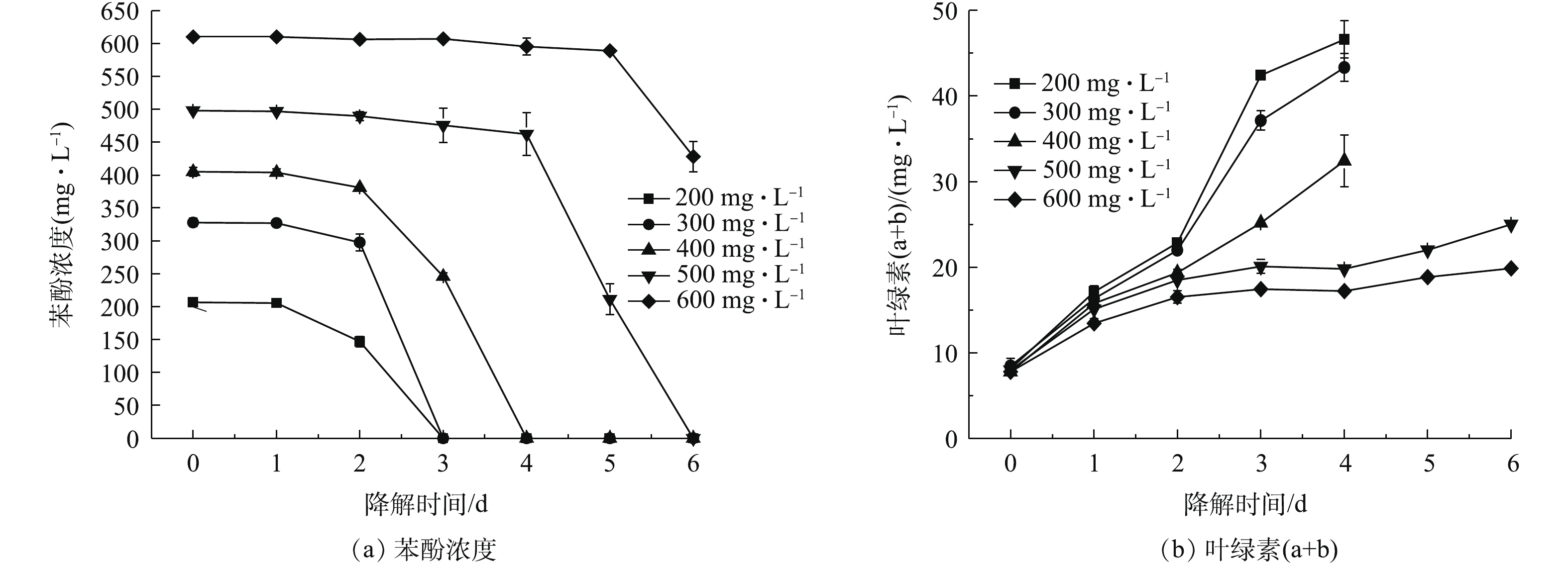
初始苯酚浓度对苯酚降解性能有重要影响,苯酚浓度越高,对微生物的毒性作用越大。选择藻菌比为1∶1、接种浓度为0.2 g·L−1作为苯酚降解体系,不同苯酚浓度对该体系苯酚降解性能的影响如图5(a)所示。可以看出,200、300、400、500 mg·L−1浓度的苯酚分别在3、3、4、5 d内被完全降解,600 mg·L−1的苯酚6 d被降解了29.8%。随着苯酚浓度的升高,苯酚完全降解时间增加。相比于单藻体系仅能耐受400 mg·L−1,其4 d苯酚降解率仅为7.33%,藻菌体系可完全降解400和500 mg·L−1苯酚,表现出较高的苯酚降解性能。不同苯酚浓度下叶绿素(a+b)含量的变化趋势如图5(b)所示。可以看出,在各苯酚浓度(200~600 mg·L−1)下,小球藻叶绿素(a+b)含量较初始接种值增加了1.54~4.71倍,这可能是由于细菌高的苯酚降解性能降低了苯酚对微藻的胁迫;同时,细菌降解苯酚产生CO2,为微藻生长提供碳源,提高微藻生物量,实现苯酚降解的同时积累微藻生物质[14-15]。近年来,部分微藻和藻菌共培养处理苯酚废水的研究结果如表1所示。可以看出,本研究在较低的藻菌接种浓度下,取得了较高的苯酚降解效率,并实现微藻生物质的多倍增长,这展现了藻菌共培养应用于处理苯酚废水同时积累微藻生物质的潜在优势。
2.1. 苯酚浓度对小球藻生长的影响
2.2. 小球藻的苯酚降解性能
2.3. 藻菌接种比例对小球藻生长和苯酚降解的影响
2.4. 藻菌初始接种浓度对小球藻生长和苯酚降解的影响
2.5. 初始苯酚浓度对小球藻生长和苯酚降解的影响
-
1) 本研究构建了小球藻与简单芽胞杆菌的共培养体系用于高浓度苯酚废水处理,该体系在处理高浓度苯酚废水同时达到了积累微藻生物质的目的。
2) 小球藻能耐受400 mg·L−1苯酚,但对100~600 mg·L−1的苯酚降解率仅为1.21%~11.66%。在考察藻菌比、藻菌初始接种浓度基础上获得藻菌共培养体系的最优藻菌接种浓度(0.2 g·L−1和0.2 g·L−1)。在此条件下,藻菌体系6 d内完全降解500 mg·L−1的苯酚,且在各苯酚浓度(200~600 mg·L−1)下,小球藻叶绿素(a+b)含量较初始接种值增加了1.54~4.71倍。
3) 小球藻和简单芽胞杆菌共培养可以提高苯酚降解能力并促进小球藻生长,在苯酚废水净化及资源化利用领域展现了一定的应用潜力。





 下载:
下载: