-
在水处理领域,基于硫酸根自由基(SO4·−)的高级氧化技术是近年来的一个研究热点. 与芬顿技术相比,SO4·−的氧化还原电位为2.5—3.1 V,高于羟基自由基(·OH,2.7 V),半衰期远比·OH长,pH的适应范围宽[1]. 固态过硫酸盐(PS)比液态过氧化氢(H2O2)要利于运输和存储,因此在处理水中污染物时具有更高的能效和实际操作优势. 过硫酸盐自身的氧化能力较弱,通常需要将其活化产生氧化性更强的活性物质(如SO4·−等自由基)来发挥氧化作用.
在活化过硫酸盐的方法中,碳基材料因其较大的比表面积、sp2和sp3杂化的碳骨架结构、良好的导电性能以及非金属特性而被广泛用于研究[2,3]. 目前,石墨烯、碳纳米管、活性炭、介孔炭和生物炭是备受关注的几种材料. 与其他碳材料相比,生物炭具有制备和原料来源上的"减碳"和成本优势,是一种环保的吸附剂和催化剂. 然而,与石墨化的碳纳米管和石墨烯相比,直接焙烧得到的生物炭主要由非晶碳组成,其催化活化过硫酸盐的能力有限,通常需要适当改性以提高其催化性能. Hou等将生物炭与适量氧化石墨烯复合,成功将材料活化过硫酸盐降解苯酚的去除率从原始生物炭的20%提高到100%[2]. 另一类活化过硫酸盐的优秀催化剂是过渡金属(如锰、铁、钴)及其氧化物[4]. 然而,这些金属及其氧化物容易团聚,活性位点难以充分利用. 将它们负载到生物炭上不仅可以提高活性金属及其氧化物的分散性和稳定性,还可以发挥生物炭自身的吸附能力,增强材料活化过硫酸盐的能力[5].
孔道结构和比表面积是影响碳材料活化过硫酸盐的另一个重要因素. 由于受到生物质原料的限制,生物炭的孔容和比表面积通常较低. 为了增加生物炭的孔道结构,可以采用CO2、氧气和水蒸气等氧化碳壁产生孔道,也可以通过化学活化剂(如氯化锌、磷酸、强碱等)活化新增孔容[6]. 近年来,高锰酸钾开始用于活化碳材料[7]. Hu等[8]利用高锰酸钾溶液对生物炭进行浸渍,显著提高了生物炭的比表面积. 此外,作为高锰酸钾的分解产物,锰氧化物活化过硫酸盐的性能优异,在自然界中的丰度高,对环境友好且生物毒性较低. 因此,利用高锰酸钾改性生物炭有望提高生物炭在高级氧化技术中的潜力.
垂序商陆是一种入侵植物,对本地物种和生态系统危害较大[9]. 此外,垂序商陆生物量大,生长快,适应性强,分布广[10]. 将垂序商陆转化成生物炭用于处理水环境中的污染物,对减污降碳意义较大. 本研究选择垂序商陆作为生物炭的原料,经高锰酸钾溶液处理,不同温度下焙烧制得锰改性生物炭,以水中常见污染物苯酚作为目标污染物,探讨该碳材料活化PDS降解苯酚的性能.
-
使用的生物质为垂序商陆茎秆部分,采自南京市浦口区龙王山脚;苯酚、过二硫酸钾(PDS)、甲醇(MeOH)、叔丁醇(TBA)、L-组氨酸(L-His)以及硫酸钠购于阿拉丁试剂有限公司;乙醇(EtOH)、氢氧化钠、盐酸、硫酸、氯化钠、硝酸钠、高锰酸钾以及二氧化锰购于国药集团化学试剂有限公司. 实验过程中所用试剂均为分析纯.
-
将洁净并风干的垂序商陆茎秆截成小段,超声清洗并烘干后,使用磨碎机研磨成粉. 粉碎后的生物质在氮气气氛下于700 ℃或850 ℃焙烧,保持2 h,自然冷却至室温,得到的生物炭分别标记为700BC和850BC,用乙醇和超纯水洗涤数次,烘干,研磨后备用.
用0.12 mol·L−1的KMnO4溶液浸泡研磨成粉的生物质24 h,过滤. 为了使KMnO4充分负载至生物质上,再用0.06 mol·L−1的KMnO4溶液二次浸泡24 h,过滤后水洗数次并真空干燥,在氮气氛围下碳化,碳化条件和后续洗涤步骤同上,制得的改性生物炭分别标记为Mn-700BC和Mn-850BC.
材料的形貌特征和物相分别通过扫描电镜(SEM,日本日立SU1510)和X射线衍射(XRD,日本岛津,XRD-6100)描述;材料表面的元素价态通过X射线光电子能谱(XPS,美国赛默飞Nexsa)进行分析;材料的表面官能团和碳结构分别利用傅立叶红外光谱(FT-IR,美国尼高力iS5)和拉曼光谱仪(美国赛默飞,DXR2)测试;材料的孔道信息在氮气吸附-脱附仪(美国麦克,ASAP2020)上采集.
-
将材料置于锥形瓶中,加入10 mL超纯水,在25 ℃下用磁力搅拌器搅拌30 min,使材料被水充分润湿后,加入40 mL一定浓度的苯酚溶液,使苯酚初始浓度为0.16 mmol·L−1,并调节初始pH=9. 待30 min达到表观吸附平衡后,加入适量PDS溶液,使PDS浓度为2 mmol·L−1. 在不同时间间隔里取样,过滤,定量滤液迅速与定量甲醇混合以抑制滤液中活性物质对苯酚的降解,混匀后用分光光度法在270 nm处测定苯酚浓度.
在评价材料活化降解苯酚的性能实验中,考察了苯酚初始浓度、PDS浓度、pH以及共存无机阴离子的影响. 此外,利用EtOH、TBA和L-His分别作为SO4·−和·OH、·OH、1O2的捕获剂,探讨这些活性组分在苯酚降解中的作用.
-
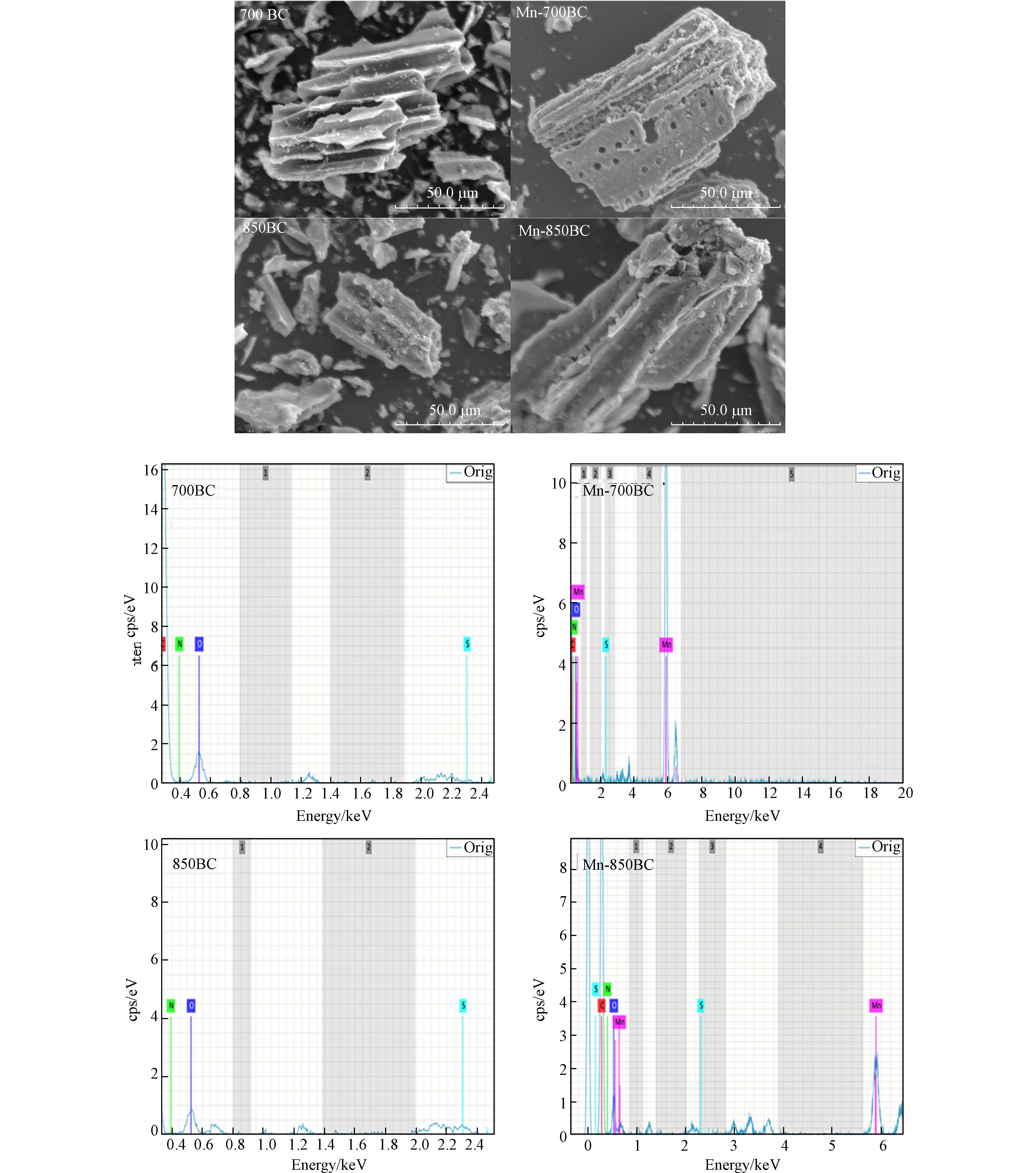
图1展示了700BC、Mn-700BC、850BC和Mn-850BC的SEM照片. 生物炭的外观形状大多不规则,Mn-700BC和Mn-850BC的形状相对于700BC和850BC没有发生明显变化,但表面出现了一些新的孔洞,这是因为在高温碳化时,碳壁在高锰酸钾活化下发生了二次裂解. EDS能谱(图1)分析证实Mn-700BC和Mn-850BC中存在锰的掺杂.
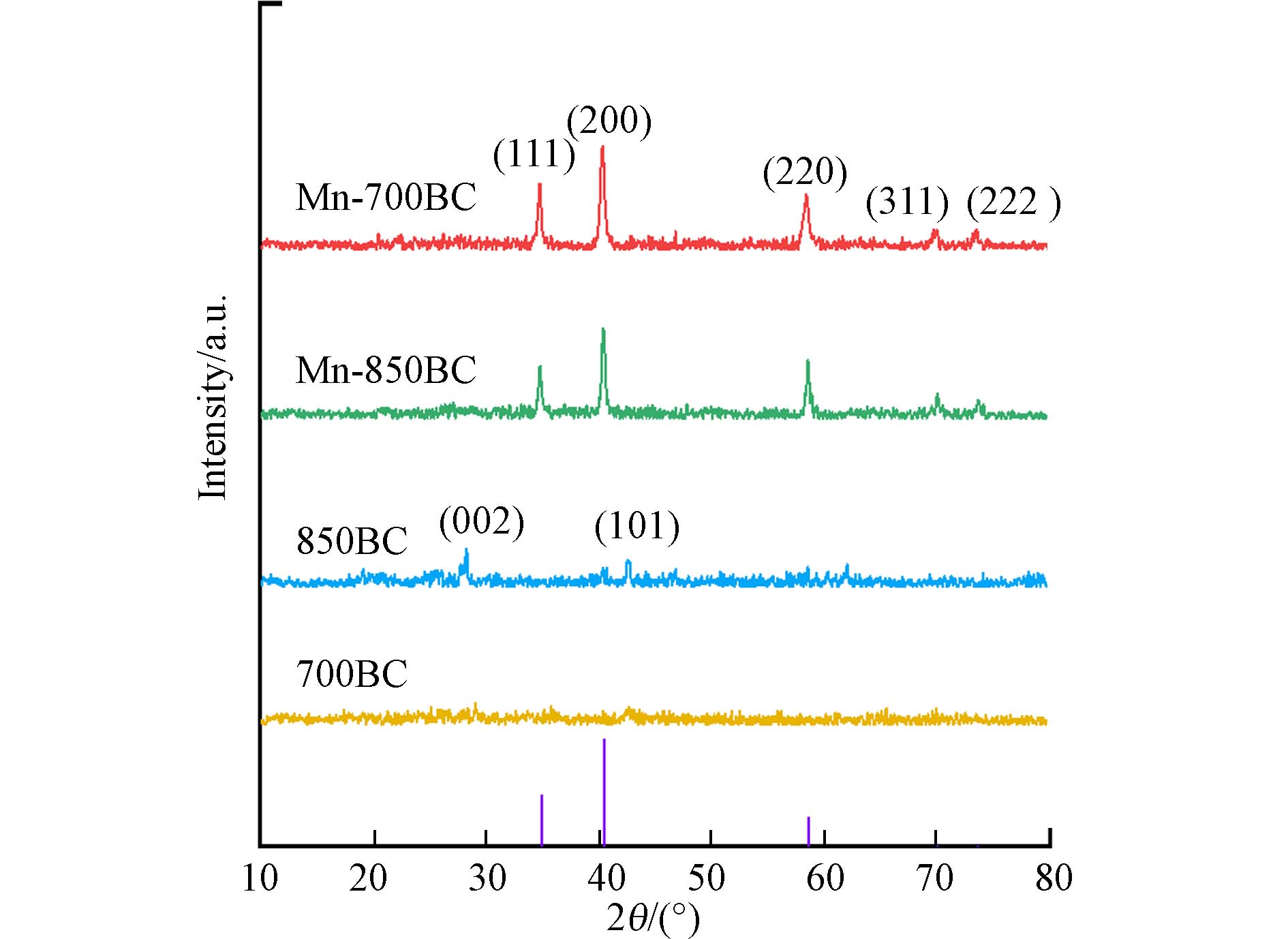
图2是700BC、Mn-700BC、850BC和Mn-850BC的XRD图谱. 从图2可以看出,原始生物炭没有出现金属元素锰等的衍射峰. 但是,Mn-700BC经过锰改性后,材料在34.91°、40.55°、58.72°、70.18°和73.79 °处均出现了衍射峰,对应于方锰矿MnO(JCPDSNo.07-0230). 由文献可知,二氧化锰会以无定形的形式存在于生物炭载体上[11]. 结合高锰酸钾的高温分解反应,推测改性生物炭上可能存在无定形二氧化锰. 对照700BC和850BC的图谱,发现850BC在2θ在26°和44°处各有1个衍射峰,是典型石墨晶面,说明850BC材料的石墨化程度高于700BC[10].
XPS结果(见图3)可证实上述锰氧化物的存在. 改性的两个材料均出现了Mn 2p能谱峰,642 eV附近的能谱峰揭示锰的氧化态为Mn2+/3+,642 eV和654 eV附近的能谱差值约为11.6 eV,说明材料中的锰具有Mn3+和Mn4+的混合价态,但以Mn4+居多[7,12]. C 1s在结合能为284.8 eV出现的能谱峰对应于C=C键[13]. O 1s能谱图中,结合能为532 eV附近和530 eV附近分别对应于缺陷氧羟基(C—OH)和晶格氧(Mn—O)的能谱峰[14 − 15].
从图4(a)的FT-IR光谱中可以发现,4种生物炭在1112 cm−1、1627 cm−1和3417 cm−1处均出现明显的吸收峰,对应于—C—O、羧基/酯基/醛基的C=O和—OH的伸缩振动峰[16 − 18]. 700BC和Mn-700BC在1410 cm−1处的—CH的弯曲振动峰比850BC和Mn-850BC明显,体现了碳化温度对官能团的影响,即低的碳化温度有利于保留材料表面的官能团[19].
4个材料的拉曼光谱(图4(b))均出现了D带和G带. 在1320 cm−1处的D带表示碳材料的缺陷和无序性,1580 cm−1处的G带反映了材料的石墨化程度. 相对于700BC,850BC的ID/IG值较大,石墨化结构的无序性增加,材料缺陷位点相对较多,即碳化温度的提高增加了材料的缺陷度[20],这和牛粪消化产物、苹果树修剪的废弃物所制备的生物炭结果相类似[21 − 22]. 此外,锰氧化物的掺入,也提高了材料的缺陷度.
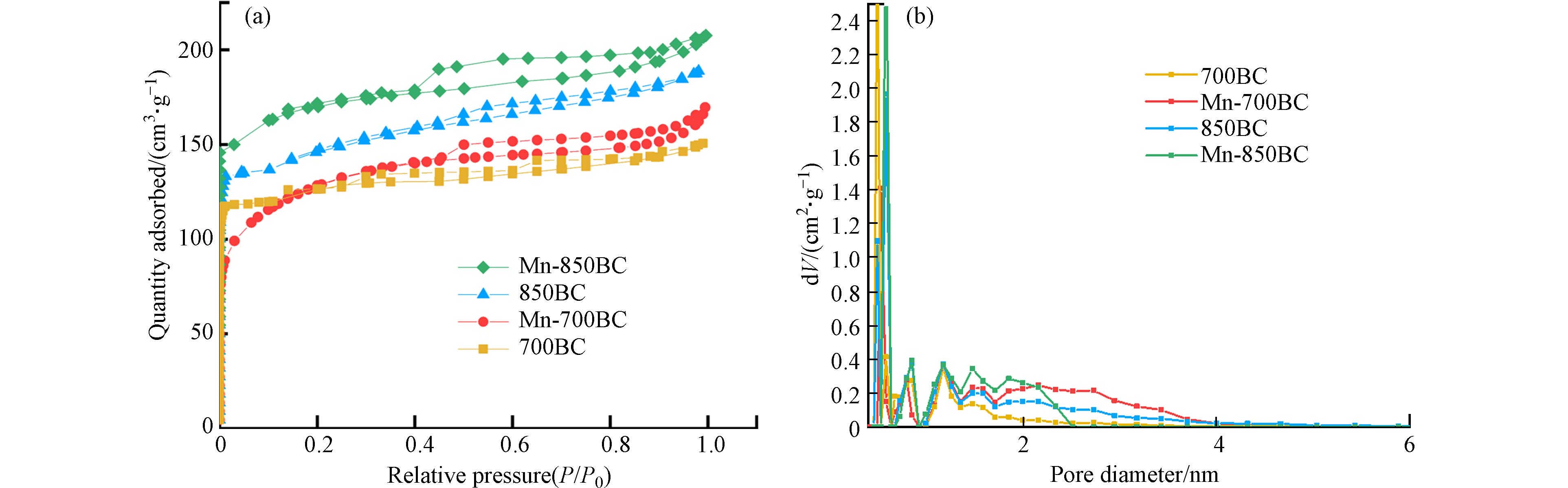
氮气吸附-脱附实验可以反映材料经改性后孔道的结构变化. 图5(a)是4种材料的氮气吸附-脱附等温线. 在压力很低时,4种材料的氮气吸附量急剧上升,说明它们均含有一定量的微孔孔道;随着压力的升高,氮气吸附量增长缓慢,在中等压力下,因毛细凝聚作用出现的滞后环反映出材料中还有中孔孔道,且改性材料的滞后环更明显,说明锰改性增加了材料的中孔比例,这和孔分布结果一致(图5(b)). 从表1可知,850 ℃下焙烧的生物炭,其孔容高于700 ℃的材料,说明提高温度有利于增加生物炭的孔道结构;同样,高锰酸钾和碳壁在高温下的反应也可在一定程度上增加材料的孔道,使Mn-850BC的比表面积高于850BC. 然而,Mn-700BC的比表面积略低于700BC的比表面积,这可能是锰氧化物颗粒堵塞了部分微孔孔道.
-
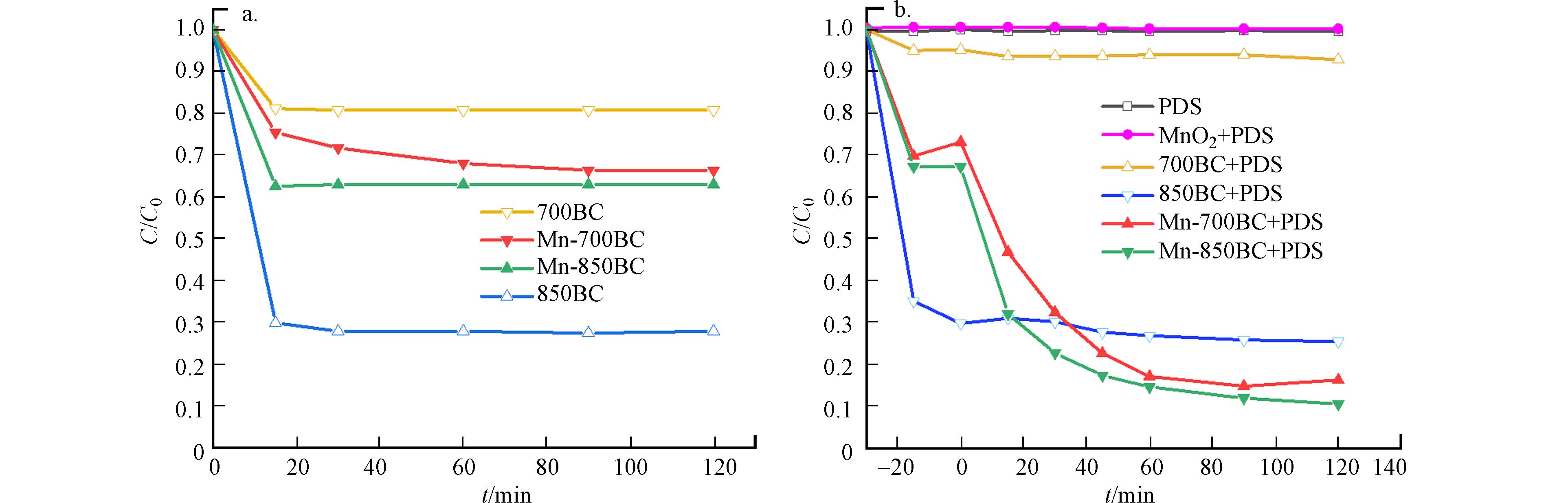
在评估材料的催化活性之前,首先考察了这些碳材料对苯酚的吸附情况,具体结果见图6(a). 在30 min内,苯酚在4种材料上基本能够达到吸附平衡,其中Mn-850BC、Mn-700BC、850BC和700BC对苯酚的去除率分别为38%、33%、73%和20%. 图6(b)展示了材料活化PDS降解苯酚的实验结果. 结果表明,锰改性生物炭降解苯酚的效果远优于商业MnO2,显示了这种锰改性方法具有一定的应用前景. 在材料吸附苯酚达到表观平衡后,加入PDS溶液,700BC/PDS几乎不能降解苯酚,850BC/PDS对苯酚的降解率约为5%,表明850BC石墨化的碳结构对活化PDS可能有一定贡献;而锰改性的两种材料Mn-700BC和Mn-850BC在吸附平衡后仍可降解约60%左右的苯酚,说明负载的锰氧化物是降解苯酚的重要成分,且主要通过活化PDS产生活性物质降解苯酚,它与苯酚之间直接的氧化还原不是主要降解路径. 此外,研究结果显示,β-MnO2等金属氧化物的添加大大加速了矿物、黏土等活化过硫酸盐产生SO4·−和·OH的速率,使苯的降解速率提高了两个数量级[23]. 除了可以产生SO4·−和·OH等活性物种,锰氧化物在活化过硫酸盐时,还可以在材料表面形成亚稳中间体,通过与过硫酸盐进一步反应生成1O2,以非自由基路径氧化污染物[24-25] .
-
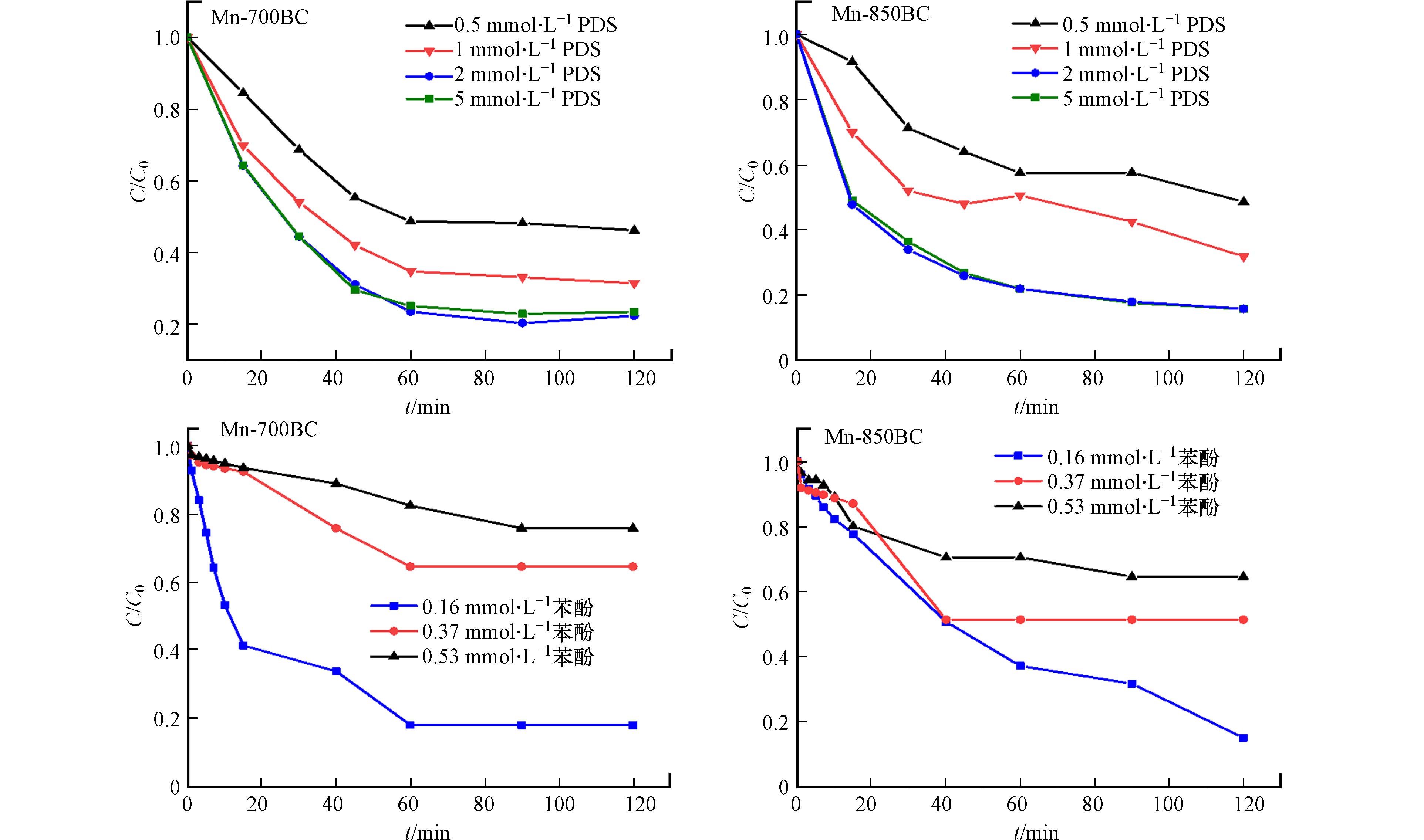
非均相催化反应中,反应组分浓度对降解速率的影响至关重要. 图7显示了在不同苯酚初始浓度及PDS浓度下锰改性碳材料活化降解苯酚的结果. 随着PDS浓度的增加,苯酚的降解速率逐渐增加,但浓度增加到5 mmol·L−1时,降解速率没有明显变化. 提高溶液中PDS含量可以加快苯酚的降解,但催化材料的活性位点数量有限,不能有效活化过多的PDS. 此外,过量的S2O82-会与逐渐增多的SO4·−反应,削弱对苯酚的降解[26]. 在苯酚初始浓度为0.16—0.53 mmol·L−1范围内,随着浓度的提高,苯酚的去除趋于缓慢. 用准一级动力学方程拟合,结果见表2. 拟合的相关系数R2均在0.94以上,说明苯酚的降解过程可以用准一级动力学方程描述. 浓度越高,速率常数k1值越小,反应速率越慢. 一般而言,非均相催化反应过程包括反应组分的扩散和吸附、表面反应、产物的脱附和扩散这些步骤. 在这些过程中,推测组分的内扩散和吸附过程对降解速率影响较大. 随着苯酚浓度的增加,前期占据于材料孔口附近的苯酚分子会阻碍后续分子进入孔道深处,扩散路径增加,使这些分子到达内表面位点的时间延长.
为了阐明上述推测,采用Langmuir-Hinshelwood模型来描述反应组分的吸附过程对催化反应速率的影响[27,28]. 该模型的简易方程为式(1, 2):
其中,r0为反应初活性,选择15 min以内的数据进行计算以避免降解中间产物或产物引起的竞争吸附. θs为苯酚或PDS在材料表面的覆盖率,k为反应速率常数,b为材料对苯酚或PDS的吸附平衡常数.
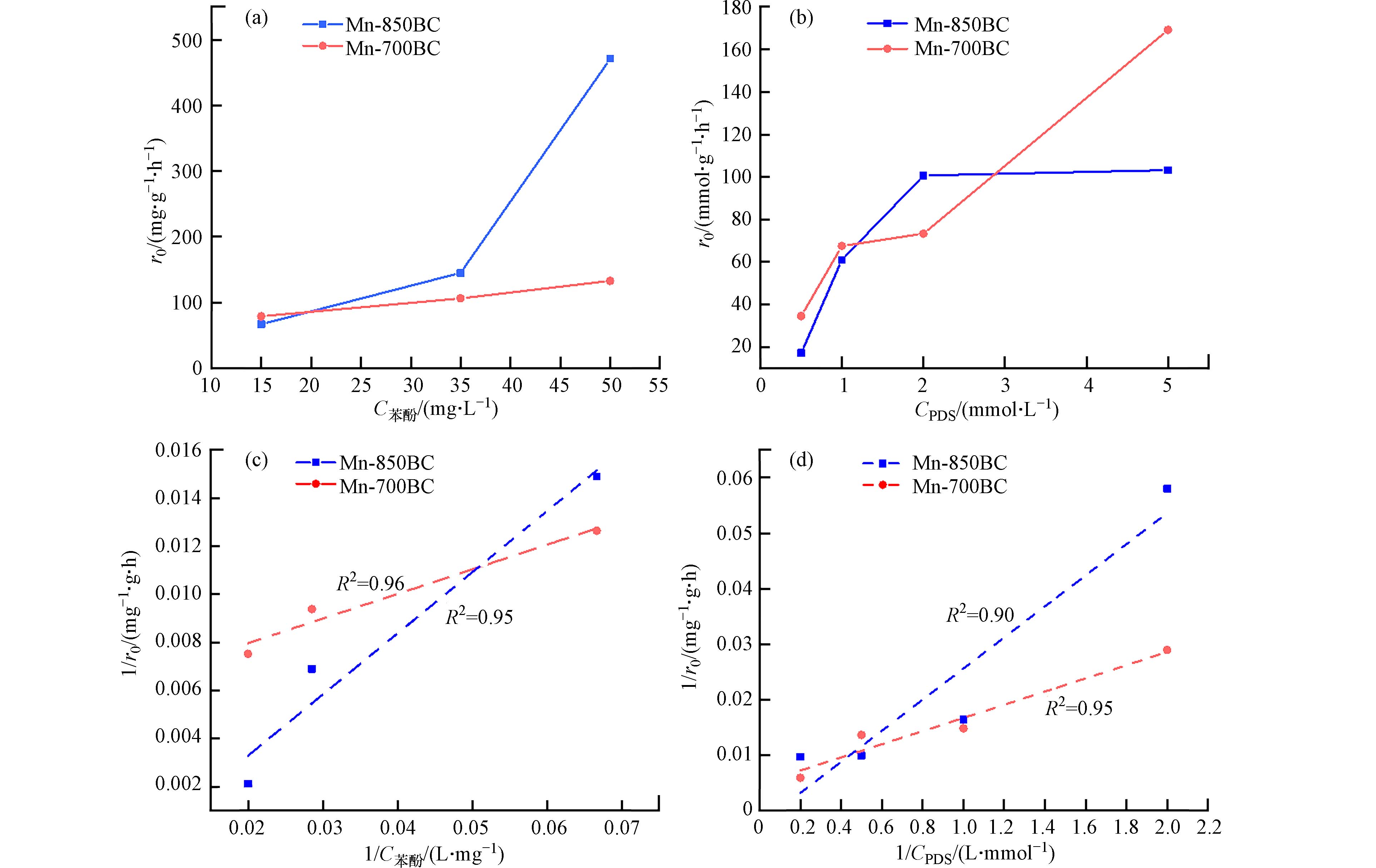
Langmuir-Hinshelwood模型认为,催化反应速率与吸附的反应组分浓度成比例关系. 由图8(a)可知,两个材料的反应初活性均随苯酚浓度的增加而增加,Mn-700BC和Mn-850BC的初活性分别由79.11 mg·g−1·h−1和67.15 mg· g·h−1增加到132.94 mg ·g−1·h−1和470.91 mg ·g−1·h−1. 相类似地,在实验范围内,提高PDS浓度同样使反应初活性增强. 将1/r0与1/C苯酚作图可知,两者具有良好线性关系(R2>0.95),说明锰改性碳材料活化PDS降解苯酚能够用Langmuir-Hinshelwood模型描述,吸附于材料表面的苯酚浓度是该催化反应的控制步骤. 同样地,1/r0与1/CPDS之间也存在较好的线性关系,吸附的PDS浓度在苯酚降解中也起重要作用.
-
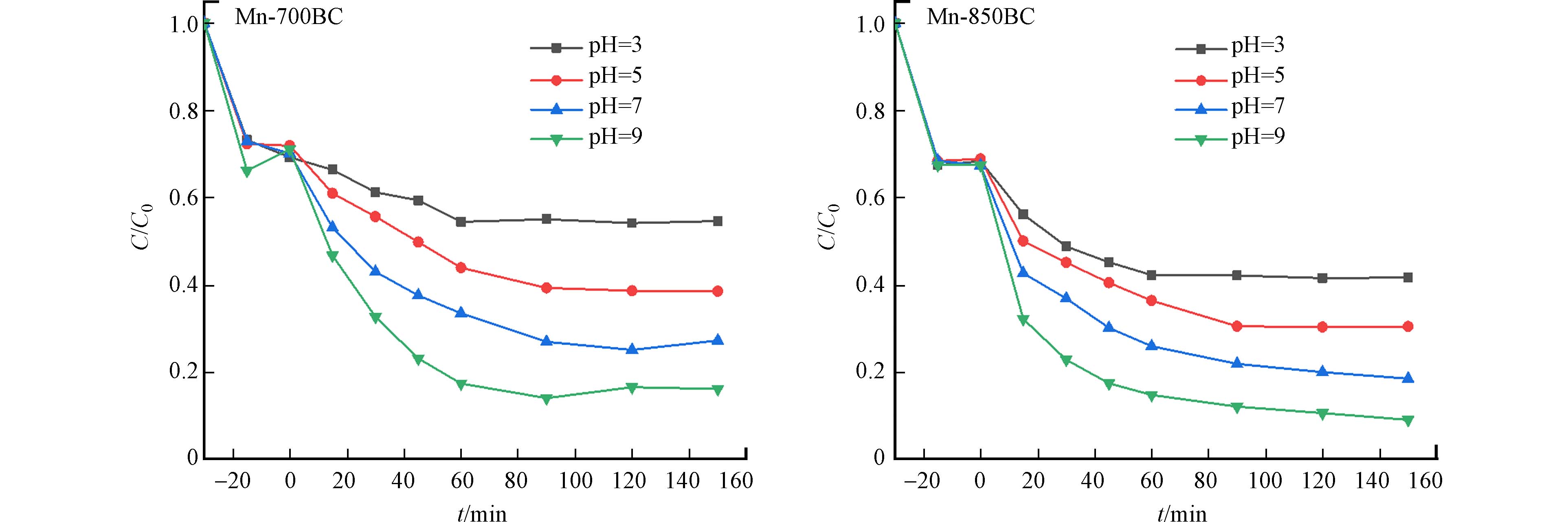
溶液pH可以改变材料表面的电荷特性以及污染物和活化剂PDS的化学特性,影响PDS与材料、苯酚与材料的相互作用,从而对活化和降解产生影响. 根据图9,在接近于实际水体的偏中性环境下,苯酚的去除率在80%左右,体现出这两种改性材料活化PDS降解苯酚的pH适应性. 在实验pH范围内,提高溶液pH有利于增强材料的活化效果. 这和生物炭负载γ-MnO2 复合材料活化过一硫酸盐降解对氯苯酚的结果相似[29]. 在酸性环境下,H+容易和S2O8−中的O—O基团形成氢键,不利于PDS与材料的接触,影响自由基的产生[30];其次,pH的提高能够增加过硫酸盐生成SO4·−和·OH的速率,Guan等通过表观摩尔吸收系数实验和半定量法测定两种自由基等方式验证了上述推测[31]. 同时,增加溶液中OH−的浓度,有利于材料表面羟基的形成,增加了电子转移的活性位点 [32].
天然水体中含有大量阴离子,如Cl−、SO42-、NO3−等,存在的这些阴离子对催化过程的影响不可避免. 本文研究了水体中主要阴离子与苯酚共存时的去除效果,结果见图10.
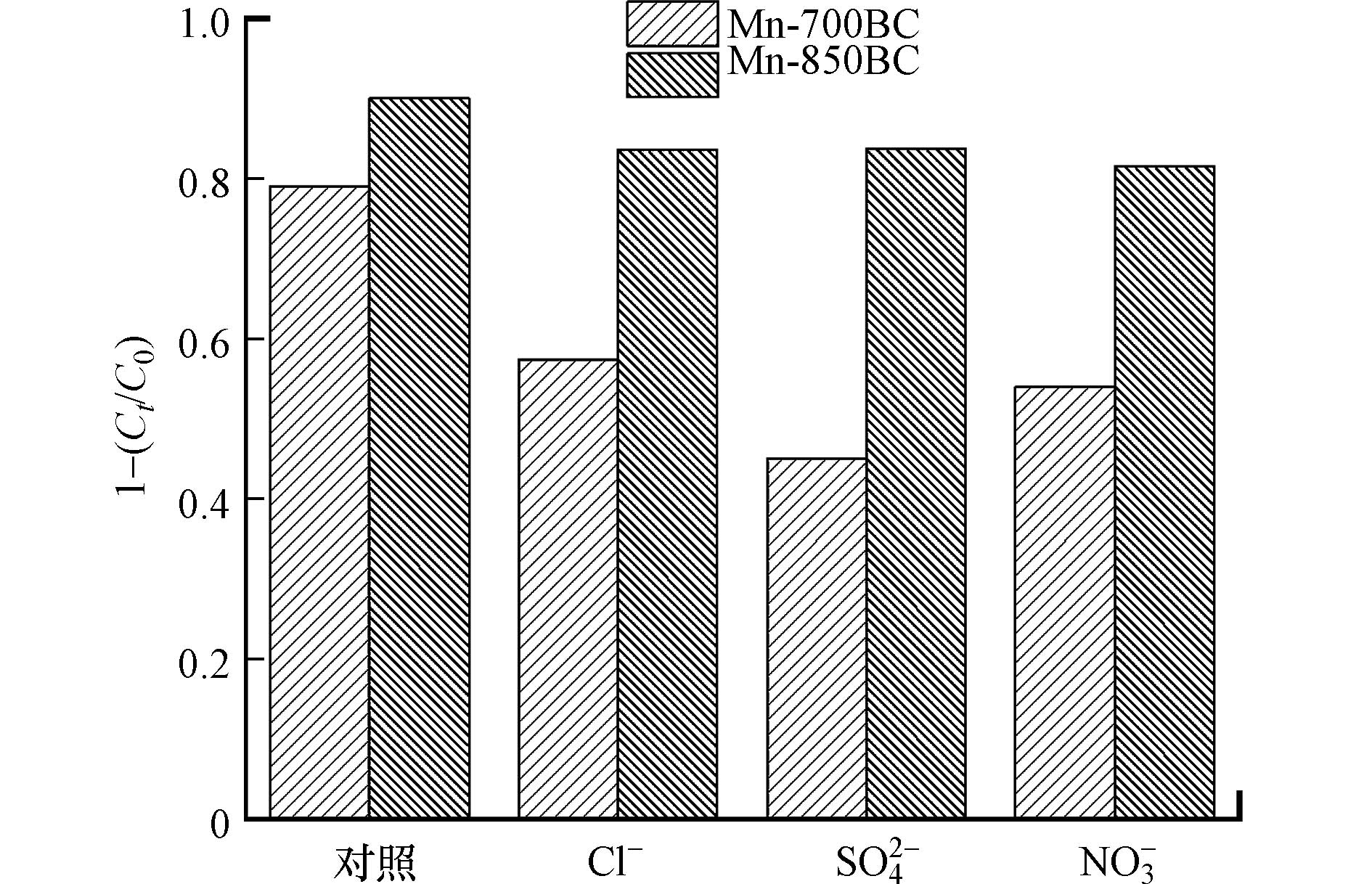
当Cl−、SO42-和NO3−浓度均为20 mmol·L−1时,对苯酚的降解均显示出一定的抑制作用,SO42-的抑制程度最明显. 例如,对于Mn-700BC,分别向溶液中加入Cl−、NO3−和SO42-时,与不含这些阴离子的对照样相比,苯酚的降解效率分别减少了27%、30%和39%. 由前述结果可知,吸附于材料表面的过硫酸根离子浓度是影响活化的重要因素,那么,共存的阴离子会与过硫酸根离子竞争材料表面的活性位点,影响PDS的活化[12]. SO42-的化合价比Cl−和NO3−高,与材料表面Mnx+的亲和力更大. 另一方面,这些阴离子可通过反应或非反应方式降低体系中活性物种的氧化能力. Cl−和NO3−会与苯酚竞争SO4·−和·OH,生成Cl·和Cl2−·、 ·NO3 和 ·NO2 [33],这些自由基的氧化能力比SO4·−和·OH低,从而降低了苯酚的降解效率;SO42-的加入,使体系中SO42-的浓度过高,降低了SO4·−/SO42-的氧化还原电位,在一定程度上阻碍了PDS的活化. 值得注意的是,Mn-700BC受抑制的程度高于Mn-850BC. 这可能跟材料的特性差异导致PDS活化路径不完全一致有关. 众多文献结果显示,阴离子主要影响以自由基氧化为主的活化降解过程,对电子转移介导的非自由基氧化路径影响较小[34 − 36]. 从红外光谱可以看到,Mn-700BC的含氧官能团比Mn-850BC丰富,有利于自由基的产生,但不利于PDS吸附于材料表面形成亚稳态复合体以氧化污染物,非自由基氧化作用较弱[19]. 除此以外,Mn-850BC的缺陷比Mn-700BC多,石墨化程度亦较高,这些都有利于提高基于电子转移的非自由基氧化的作用[21,37].
-
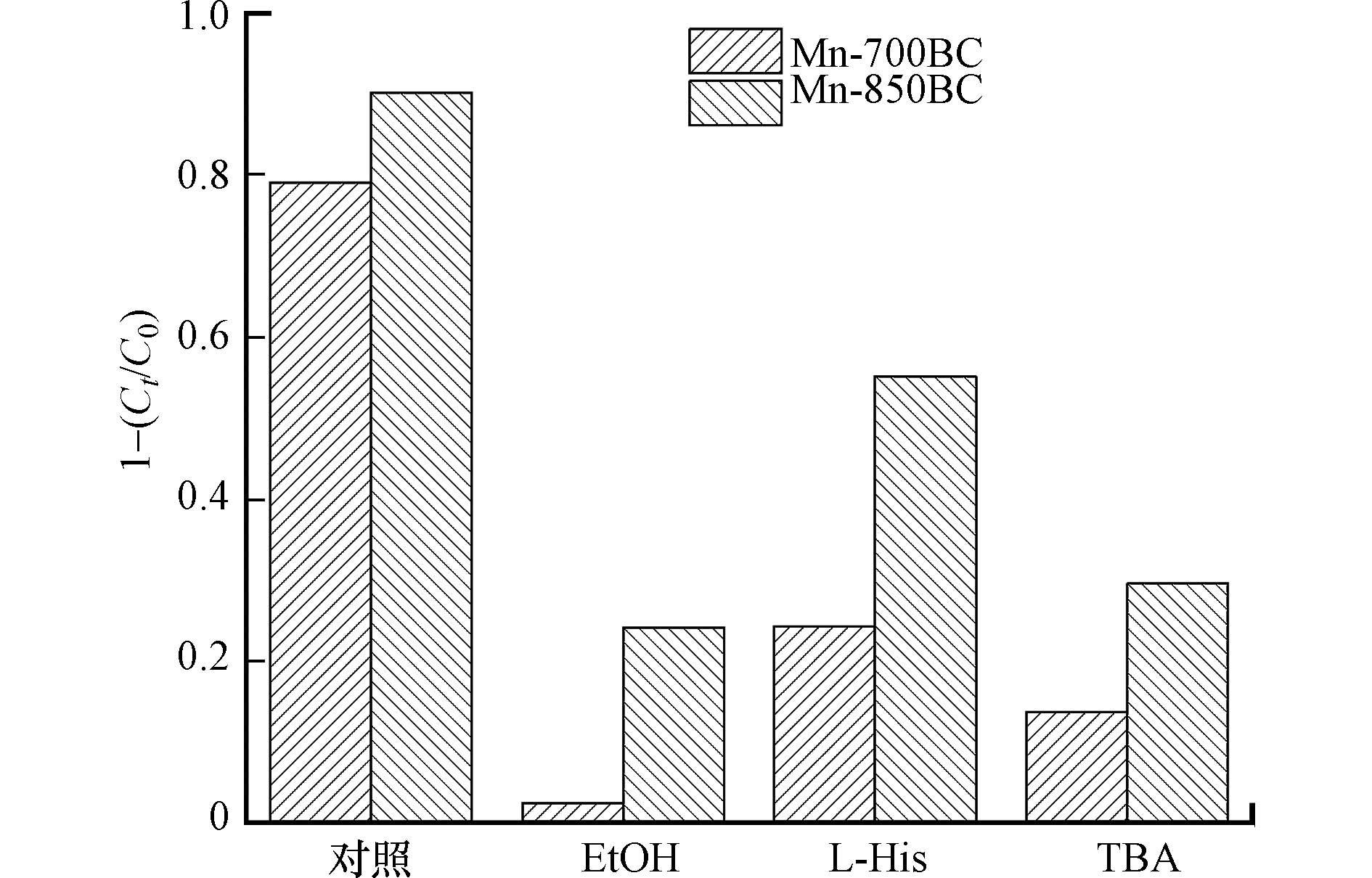
为了阐明该体系中的主要活性氧物质,我们探讨了活性氧捕获剂存在时苯酚的降解情况,具体结果如图11所示. 加入TBA后,Mn-700BC和Mn-850BC对苯酚的去除率分别从原来的84%和90%减少至14%和30%,这表明催化剂活化PDS产生了·OH,而·OH在氧化苯酚时发挥着重要作用. 乙醇作为·OH和SO4·−的淬灭剂,更大程度上抑制了苯酚的降解,意味着SO4·−和·OH同样参与了苯酚的降解过程. 在添加L-His后, Mn-700BC和Mn-850BC对苯酚的去除率降低至24%和55%,这表明体系中有1O2参与的非自由基途径存在. 添加淬灭剂后,Mn-700BC活化PDS降解苯酚的受抑制程度均高于Mn-850BC. 这可能是由于Mn-700BC的孔道和比表面积较低,活性位点相对较少,淬灭剂竞争活性物种更严重. 另外,由于C=O等官能团的差异,Mn-700BC活化PDS时自由基路径占比更大,体现出更强的受抑制程度.
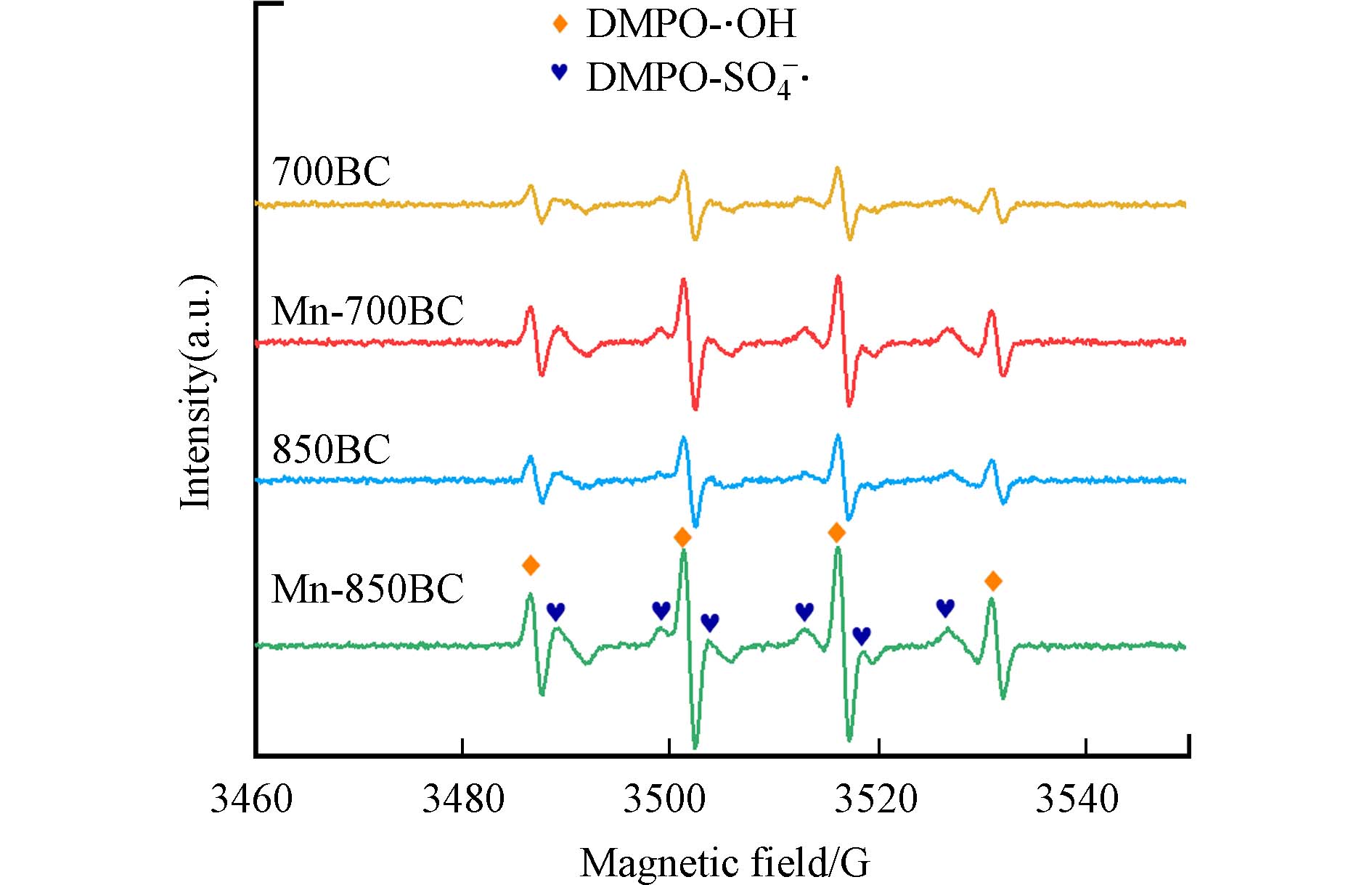
EPR分析进一步揭示了活化过程中产生的自由基. 采用甲基吡啶N-氧化物(DMPO)作为SO4·−和·OH自由基自旋捕获剂,所得波谱如图12所示. 在700BC、850BC、Mn-700BC和Mn-850BC中,观察到强度比例为1:2:2:1的DMPO-OH加成物峰,以及6个较弱的DMPO-SO4·−加成物峰,表明这些材料在活化PDS时产生了·OH和SO4·−[38]. 在其他锰氧化物与炭的复合材料活化过硫酸盐时,也常鉴定出·OH和SO4·−[39]. 这些加成物信号峰的强度大小顺序为Mn-850BC > Mn-700BC > 850BC > 700BC,反映出活化PDS产生的这些自由基的含量差异,这与之前的降解结果一致.
-
1)将垂序商陆茎末浸泡于高锰酸钾溶液再高温焙烧,成功将锰氧化物负载到生物炭上,且这种改性方式增加了生物炭的孔道结构. XRD和XPS结果显示,Mn以Mn(Ⅱ)、Mn(Ⅲ)和Mn(Ⅳ)混合价存在;随着焙烧温度的升高,材料的石墨化程度和缺陷增加,但表面含氧官能团含量降低.
2)锰改性生物炭活化PDS降解苯酚的效果显著优于未改性生物炭和商业二氧化锰,材料表面锰氧化物起主要活化作用;苯酚和PDS的初始浓度对改性材料活化降解苯酚的活性影响可用 Langmuir- Hinshelwood模型描述,说明其降解速率受PDS和苯酚的吸附控制;pH在3—9范围内时,提高溶液pH可提高苯酚的降解效率;溶液中共存阴离子Cl−、NO3−、SO42-均可在一定程度上抑制材料活化PDS降解苯酚的效果.
3)自由基捕获实验和EPR结果显示,在锰改性生物炭/过硫酸盐体系中,·OH和SO4·−等自由基路径是氧化苯酚的主要方式,同时还伴有1O2参与的非自由基路径.
锰改性生物炭活化过二硫酸盐降解水中苯酚
Study on catalytic peroxydisulfate activation on manganese modified biochars for phenol degradation
-
摘要: 废弃生物质的再利用对减污降碳有较大意义. 本文采用垂序商陆的茎为原料,经高锰酸钾溶液浸泡后,氮气保护下分别于700、850℃焙烧得到锰改性生物炭(Mn-700BC、Mn-850BC),并以同温度下未改性的生物炭(700BC、850BC)作为参照,探讨改性材料活化过二硫酸盐(PDS)降解苯酚的性能. SEM-EDS、XRD和XPS的结果表明,锰氧化物成功负载于生物炭上,锰的价态为Mn(Ⅱ)、Mn(Ⅲ)和Mn(Ⅳ)的混合价;850 ℃焙烧所得生物炭的石墨化程度、孔容及比表面积比700 ℃的生物炭高,官能团含量低. Mn-700BC和Mn-850BC对苯酚的去除率可达到89%和91%,且负载的锰氧化物对苯酚的降解起主要活化作用,碳的石墨化结构对活化有一定作用;EPR和自由基捕获实验结果显示,锰改性生物炭活化PDS降解苯酚以自由基氧化路径为主,同时伴有非自由基氧化途径. 苯酚和PDS初始浓度对催化反应初活性的影响可以用Langmuir-Hinshelwood模型描述,降解反应速率受苯酚及PDS的吸附控制. 在初始pH 3—9范围内,提高pH有利于苯酚的降解;共存的Cl−、NO3−、SO42-对苯酚降解均有一定抑制作用. 锰改性生物炭活化PDS降解苯酚效果优良,揭示了这种改性材料用于活化过硫酸盐处理水中有机物的潜在前景.Abstract: The reuse of biomass waste is helpful for pollution and carbon dioxide emissions. In this study, the stem of phytolacca americana L was chosen as the biomass, which was then soaked in potassium permanganate solution and calcined under N2 atmosphere at 700 oC or 850 oC. The obtained Mn-modified biochars (Mn-700BC and Mn-850BC) and the unmodified biochars (700BC and 850BC) as their references were used to evaluate their catalytic peroxydisulfate (PDS) activation performances for phenol degradation. The SEM-EDS, XRD and XPS results unveil that manganese oxides, mainly Mn(Ⅱ), Mn(Ⅲ) and Mn(Ⅳ), were loaded on the biochars successfully. The biochars prepared at 850 oC possess higher graphitization extent, bigger pore volume and larger specific surface area compared with those at 700 oC. However, the former materials have functional groups than the latter. Mn-700BC and Mn-850BC exhibited high phenol removal efficiencies of 89% and 91%, respectively. The loaded manganese oxides played a major role in the activation of phenol degradation, while the graphitic structure of carbon also played a certain role. Based on the results of radical tapping experiments and EPR spectra, the radical reaction pathway is the main mechanism, while the nonradical process is included for phenol degradation, too. The relationships between the initial concentration of phenol or PDS and the catalytic activity can be well described by Langmuir-Hinshelwood model, indicating that the adsorption of phenol or PDS is the rate-controlling step during PDS activation by Mn-modified biochars for phenol degradation. Increasing pH can enhance phenol degradation with pH ranging from 3 to 9. The coexistence of Cl−, NO3− and SO42- is harmful to phenol degradation. Its excellent catalytic performance of PDS activation for phenol degradation reveals that these Mn-modified biochars have a good potential prospect in activating PDS for aqueous organic pollutants removal.
-
Key words:
- manganese modification /
- biochar /
- persulfate /
- activation
-
在水处理领域,基于硫酸根自由基(SO4·−)的高级氧化技术是近年来的一个研究热点. 与芬顿技术相比,SO4·−的氧化还原电位为2.5—3.1 V,高于羟基自由基(·OH,2.7 V),半衰期远比·OH长,pH的适应范围宽[1]. 固态过硫酸盐(PS)比液态过氧化氢(H2O2)要利于运输和存储,因此在处理水中污染物时具有更高的能效和实际操作优势. 过硫酸盐自身的氧化能力较弱,通常需要将其活化产生氧化性更强的活性物质(如SO4·−等自由基)来发挥氧化作用.
在活化过硫酸盐的方法中,碳基材料因其较大的比表面积、sp2和sp3杂化的碳骨架结构、良好的导电性能以及非金属特性而被广泛用于研究[2,3]. 目前,石墨烯、碳纳米管、活性炭、介孔炭和生物炭是备受关注的几种材料. 与其他碳材料相比,生物炭具有制备和原料来源上的"减碳"和成本优势,是一种环保的吸附剂和催化剂. 然而,与石墨化的碳纳米管和石墨烯相比,直接焙烧得到的生物炭主要由非晶碳组成,其催化活化过硫酸盐的能力有限,通常需要适当改性以提高其催化性能. Hou等将生物炭与适量氧化石墨烯复合,成功将材料活化过硫酸盐降解苯酚的去除率从原始生物炭的20%提高到100%[2]. 另一类活化过硫酸盐的优秀催化剂是过渡金属(如锰、铁、钴)及其氧化物[4]. 然而,这些金属及其氧化物容易团聚,活性位点难以充分利用. 将它们负载到生物炭上不仅可以提高活性金属及其氧化物的分散性和稳定性,还可以发挥生物炭自身的吸附能力,增强材料活化过硫酸盐的能力[5].
孔道结构和比表面积是影响碳材料活化过硫酸盐的另一个重要因素. 由于受到生物质原料的限制,生物炭的孔容和比表面积通常较低. 为了增加生物炭的孔道结构,可以采用CO2、氧气和水蒸气等氧化碳壁产生孔道,也可以通过化学活化剂(如氯化锌、磷酸、强碱等)活化新增孔容[6]. 近年来,高锰酸钾开始用于活化碳材料[7]. Hu等[8]利用高锰酸钾溶液对生物炭进行浸渍,显著提高了生物炭的比表面积. 此外,作为高锰酸钾的分解产物,锰氧化物活化过硫酸盐的性能优异,在自然界中的丰度高,对环境友好且生物毒性较低. 因此,利用高锰酸钾改性生物炭有望提高生物炭在高级氧化技术中的潜力.
垂序商陆是一种入侵植物,对本地物种和生态系统危害较大[9]. 此外,垂序商陆生物量大,生长快,适应性强,分布广[10]. 将垂序商陆转化成生物炭用于处理水环境中的污染物,对减污降碳意义较大. 本研究选择垂序商陆作为生物炭的原料,经高锰酸钾溶液处理,不同温度下焙烧制得锰改性生物炭,以水中常见污染物苯酚作为目标污染物,探讨该碳材料活化PDS降解苯酚的性能.
1. 材料与方法(Materials and methods)
1.1 实验试剂与材料
使用的生物质为垂序商陆茎秆部分,采自南京市浦口区龙王山脚;苯酚、过二硫酸钾(PDS)、甲醇(MeOH)、叔丁醇(TBA)、L-组氨酸(L-His)以及硫酸钠购于阿拉丁试剂有限公司;乙醇(EtOH)、氢氧化钠、盐酸、硫酸、氯化钠、硝酸钠、高锰酸钾以及二氧化锰购于国药集团化学试剂有限公司. 实验过程中所用试剂均为分析纯.
1.2 材料的制备和表征
将洁净并风干的垂序商陆茎秆截成小段,超声清洗并烘干后,使用磨碎机研磨成粉. 粉碎后的生物质在氮气气氛下于700 ℃或850 ℃焙烧,保持2 h,自然冷却至室温,得到的生物炭分别标记为700BC和850BC,用乙醇和超纯水洗涤数次,烘干,研磨后备用.
用0.12 mol·L−1的KMnO4溶液浸泡研磨成粉的生物质24 h,过滤. 为了使KMnO4充分负载至生物质上,再用0.06 mol·L−1的KMnO4溶液二次浸泡24 h,过滤后水洗数次并真空干燥,在氮气氛围下碳化,碳化条件和后续洗涤步骤同上,制得的改性生物炭分别标记为Mn-700BC和Mn-850BC.
材料的形貌特征和物相分别通过扫描电镜(SEM,日本日立SU1510)和X射线衍射(XRD,日本岛津,XRD-6100)描述;材料表面的元素价态通过X射线光电子能谱(XPS,美国赛默飞Nexsa)进行分析;材料的表面官能团和碳结构分别利用傅立叶红外光谱(FT-IR,美国尼高力iS5)和拉曼光谱仪(美国赛默飞,DXR2)测试;材料的孔道信息在氮气吸附-脱附仪(美国麦克,ASAP2020)上采集.
1.3 苯酚降解实验
将材料置于锥形瓶中,加入10 mL超纯水,在25 ℃下用磁力搅拌器搅拌30 min,使材料被水充分润湿后,加入40 mL一定浓度的苯酚溶液,使苯酚初始浓度为0.16 mmol·L−1,并调节初始pH=9. 待30 min达到表观吸附平衡后,加入适量PDS溶液,使PDS浓度为2 mmol·L−1. 在不同时间间隔里取样,过滤,定量滤液迅速与定量甲醇混合以抑制滤液中活性物质对苯酚的降解,混匀后用分光光度法在270 nm处测定苯酚浓度.
在评价材料活化降解苯酚的性能实验中,考察了苯酚初始浓度、PDS浓度、pH以及共存无机阴离子的影响. 此外,利用EtOH、TBA和L-His分别作为SO4·−和·OH、·OH、1O2的捕获剂,探讨这些活性组分在苯酚降解中的作用.
2. 结果与讨论(Results and discussion)
2.1 材料的表征
图1展示了700BC、Mn-700BC、850BC和Mn-850BC的SEM照片. 生物炭的外观形状大多不规则,Mn-700BC和Mn-850BC的形状相对于700BC和850BC没有发生明显变化,但表面出现了一些新的孔洞,这是因为在高温碳化时,碳壁在高锰酸钾活化下发生了二次裂解. EDS能谱(图1)分析证实Mn-700BC和Mn-850BC中存在锰的掺杂.
图2是700BC、Mn-700BC、850BC和Mn-850BC的XRD图谱. 从图2可以看出,原始生物炭没有出现金属元素锰等的衍射峰. 但是,Mn-700BC经过锰改性后,材料在34.91°、40.55°、58.72°、70.18°和73.79 °处均出现了衍射峰,对应于方锰矿MnO(JCPDSNo.07-0230). 由文献可知,二氧化锰会以无定形的形式存在于生物炭载体上[11]. 结合高锰酸钾的高温分解反应,推测改性生物炭上可能存在无定形二氧化锰. 对照700BC和850BC的图谱,发现850BC在2θ在26°和44°处各有1个衍射峰,是典型石墨晶面,说明850BC材料的石墨化程度高于700BC[10].
XPS结果(见图3)可证实上述锰氧化物的存在. 改性的两个材料均出现了Mn 2p能谱峰,642 eV附近的能谱峰揭示锰的氧化态为Mn2+/3+,642 eV和654 eV附近的能谱差值约为11.6 eV,说明材料中的锰具有Mn3+和Mn4+的混合价态,但以Mn4+居多[7,12]. C 1s在结合能为284.8 eV出现的能谱峰对应于C=C键[13]. O 1s能谱图中,结合能为532 eV附近和530 eV附近分别对应于缺陷氧羟基(C—OH)和晶格氧(Mn—O)的能谱峰[14 − 15].
从图4(a)的FT-IR光谱中可以发现,4种生物炭在1112 cm−1、1627 cm−1和3417 cm−1处均出现明显的吸收峰,对应于—C—O、羧基/酯基/醛基的C=O和—OH的伸缩振动峰[16 − 18]. 700BC和Mn-700BC在1410 cm−1处的—CH的弯曲振动峰比850BC和Mn-850BC明显,体现了碳化温度对官能团的影响,即低的碳化温度有利于保留材料表面的官能团[19].
4个材料的拉曼光谱(图4(b))均出现了D带和G带. 在1320 cm−1处的D带表示碳材料的缺陷和无序性,1580 cm−1处的G带反映了材料的石墨化程度. 相对于700BC,850BC的ID/IG值较大,石墨化结构的无序性增加,材料缺陷位点相对较多,即碳化温度的提高增加了材料的缺陷度[20],这和牛粪消化产物、苹果树修剪的废弃物所制备的生物炭结果相类似[21 − 22]. 此外,锰氧化物的掺入,也提高了材料的缺陷度.
氮气吸附-脱附实验可以反映材料经改性后孔道的结构变化. 图5(a)是4种材料的氮气吸附-脱附等温线. 在压力很低时,4种材料的氮气吸附量急剧上升,说明它们均含有一定量的微孔孔道;随着压力的升高,氮气吸附量增长缓慢,在中等压力下,因毛细凝聚作用出现的滞后环反映出材料中还有中孔孔道,且改性材料的滞后环更明显,说明锰改性增加了材料的中孔比例,这和孔分布结果一致(图5(b)). 从表1可知,850 ℃下焙烧的生物炭,其孔容高于700 ℃的材料,说明提高温度有利于增加生物炭的孔道结构;同样,高锰酸钾和碳壁在高温下的反应也可在一定程度上增加材料的孔道,使Mn-850BC的比表面积高于850BC. 然而,Mn-700BC的比表面积略低于700BC的比表面积,这可能是锰氧化物颗粒堵塞了部分微孔孔道.
表 1 材料的孔结构参数Table 1. Pore structural parameters of the tested materials比表面积a/(m2·g−1) Specific surface area 总孔容b/(cm3·g−1)Total pore volume 微孔孔容c/(cm3·g−1) Mesopore volume 中孔孔容d/(cm3·g−1)Mesopore volume 700BC 423 0.22 0.18 0.04 Mn-700BC 412 0.25 0.13 0.12 850BC 512 0.29 0.21 0.08 Mn-850BC 570 0.33 0.22 0.11 a:Brunauer-Emmet-Teller(BET)方法计算 b:取相对压力为0.97处 c:t-plot方法 d:总孔容-微孔 2.2 材料活化PDS降解苯酚
2.2.1 不同材料活化PDS比较
在评估材料的催化活性之前,首先考察了这些碳材料对苯酚的吸附情况,具体结果见图6(a). 在30 min内,苯酚在4种材料上基本能够达到吸附平衡,其中Mn-850BC、Mn-700BC、850BC和700BC对苯酚的去除率分别为38%、33%、73%和20%. 图6(b)展示了材料活化PDS降解苯酚的实验结果. 结果表明,锰改性生物炭降解苯酚的效果远优于商业MnO2,显示了这种锰改性方法具有一定的应用前景. 在材料吸附苯酚达到表观平衡后,加入PDS溶液,700BC/PDS几乎不能降解苯酚,850BC/PDS对苯酚的降解率约为5%,表明850BC石墨化的碳结构对活化PDS可能有一定贡献;而锰改性的两种材料Mn-700BC和Mn-850BC在吸附平衡后仍可降解约60%左右的苯酚,说明负载的锰氧化物是降解苯酚的重要成分,且主要通过活化PDS产生活性物质降解苯酚,它与苯酚之间直接的氧化还原不是主要降解路径. 此外,研究结果显示,β-MnO2等金属氧化物的添加大大加速了矿物、黏土等活化过硫酸盐产生SO4·−和·OH的速率,使苯的降解速率提高了两个数量级[23]. 除了可以产生SO4·−和·OH等活性物种,锰氧化物在活化过硫酸盐时,还可以在材料表面形成亚稳中间体,通过与过硫酸盐进一步反应生成1O2,以非自由基路径氧化污染物[24-25] .
2.2.2 PDS和苯酚初始浓度的影响
非均相催化反应中,反应组分浓度对降解速率的影响至关重要. 图7显示了在不同苯酚初始浓度及PDS浓度下锰改性碳材料活化降解苯酚的结果. 随着PDS浓度的增加,苯酚的降解速率逐渐增加,但浓度增加到5 mmol·L−1时,降解速率没有明显变化. 提高溶液中PDS含量可以加快苯酚的降解,但催化材料的活性位点数量有限,不能有效活化过多的PDS. 此外,过量的S2O82-会与逐渐增多的SO4·−反应,削弱对苯酚的降解[26]. 在苯酚初始浓度为0.16—0.53 mmol·L−1范围内,随着浓度的提高,苯酚的去除趋于缓慢. 用准一级动力学方程拟合,结果见表2. 拟合的相关系数R2均在0.94以上,说明苯酚的降解过程可以用准一级动力学方程描述. 浓度越高,速率常数k1值越小,反应速率越慢. 一般而言,非均相催化反应过程包括反应组分的扩散和吸附、表面反应、产物的脱附和扩散这些步骤. 在这些过程中,推测组分的内扩散和吸附过程对降解速率影响较大. 随着苯酚浓度的增加,前期占据于材料孔口附近的苯酚分子会阻碍后续分子进入孔道深处,扩散路径增加,使这些分子到达内表面位点的时间延长.
为了阐明上述推测,采用Langmuir-Hinshelwood模型来描述反应组分的吸附过程对催化反应速率的影响[27,28]. 该模型的简易方程为式(1, 2):
stringUtils.convertMath(!{formula.content}) (1) stringUtils.convertMath(!{formula.content}) (2) 其中,r0为反应初活性,选择15 min以内的数据进行计算以避免降解中间产物或产物引起的竞争吸附. θs为苯酚或PDS在材料表面的覆盖率,k为反应速率常数,b为材料对苯酚或PDS的吸附平衡常数.
表 2 材料活化PDS降解苯酚的一级动力学拟合结果Table 2. Fitting results of phenol degradation using the first order kinetic model材料Materials 苯酚浓度/(mmol·L−1)Phenol concentration 表观速率常数k1 /(min−1)Apparent rate constant 相关系数R2Correlation coefficient Mn-700BC 0.16 0.0271 0.94 0.37 0.0070 0.98 0.53 0.0029 0.99 Mn-850BC 0.16 0.0188 0.97 0.37 0.0108 0.98 0.53 0.0089 0.97 Langmuir-Hinshelwood模型认为,催化反应速率与吸附的反应组分浓度成比例关系. 由图8(a)可知,两个材料的反应初活性均随苯酚浓度的增加而增加,Mn-700BC和Mn-850BC的初活性分别由79.11 mg·g−1·h−1和67.15 mg· g·h−1增加到132.94 mg ·g−1·h−1和470.91 mg ·g−1·h−1. 相类似地,在实验范围内,提高PDS浓度同样使反应初活性增强. 将1/r0与1/C苯酚作图可知,两者具有良好线性关系(R2>0.95),说明锰改性碳材料活化PDS降解苯酚能够用Langmuir-Hinshelwood模型描述,吸附于材料表面的苯酚浓度是该催化反应的控制步骤. 同样地,1/r0与1/CPDS之间也存在较好的线性关系,吸附的PDS浓度在苯酚降解中也起重要作用.
2.2.3 pH和共存阴离子的影响
溶液pH可以改变材料表面的电荷特性以及污染物和活化剂PDS的化学特性,影响PDS与材料、苯酚与材料的相互作用,从而对活化和降解产生影响. 根据图9,在接近于实际水体的偏中性环境下,苯酚的去除率在80%左右,体现出这两种改性材料活化PDS降解苯酚的pH适应性. 在实验pH范围内,提高溶液pH有利于增强材料的活化效果. 这和生物炭负载γ-MnO2 复合材料活化过一硫酸盐降解对氯苯酚的结果相似[29]. 在酸性环境下,H+容易和S2O8−中的O—O基团形成氢键,不利于PDS与材料的接触,影响自由基的产生[30];其次,pH的提高能够增加过硫酸盐生成SO4·−和·OH的速率,Guan等通过表观摩尔吸收系数实验和半定量法测定两种自由基等方式验证了上述推测[31]. 同时,增加溶液中OH−的浓度,有利于材料表面羟基的形成,增加了电子转移的活性位点 [32].
天然水体中含有大量阴离子,如Cl−、SO42-、NO3−等,存在的这些阴离子对催化过程的影响不可避免. 本文研究了水体中主要阴离子与苯酚共存时的去除效果,结果见图10.
当Cl−、SO42-和NO3−浓度均为20 mmol·L−1时,对苯酚的降解均显示出一定的抑制作用,SO42-的抑制程度最明显. 例如,对于Mn-700BC,分别向溶液中加入Cl−、NO3−和SO42-时,与不含这些阴离子的对照样相比,苯酚的降解效率分别减少了27%、30%和39%. 由前述结果可知,吸附于材料表面的过硫酸根离子浓度是影响活化的重要因素,那么,共存的阴离子会与过硫酸根离子竞争材料表面的活性位点,影响PDS的活化[12]. SO42-的化合价比Cl−和NO3−高,与材料表面Mnx+的亲和力更大. 另一方面,这些阴离子可通过反应或非反应方式降低体系中活性物种的氧化能力. Cl−和NO3−会与苯酚竞争SO4·−和·OH,生成Cl·和Cl2−·、 ·NO3 和 ·NO2 [33],这些自由基的氧化能力比SO4·−和·OH低,从而降低了苯酚的降解效率;SO42-的加入,使体系中SO42-的浓度过高,降低了SO4·−/SO42-的氧化还原电位,在一定程度上阻碍了PDS的活化. 值得注意的是,Mn-700BC受抑制的程度高于Mn-850BC. 这可能跟材料的特性差异导致PDS活化路径不完全一致有关. 众多文献结果显示,阴离子主要影响以自由基氧化为主的活化降解过程,对电子转移介导的非自由基氧化路径影响较小[34 − 36]. 从红外光谱可以看到,Mn-700BC的含氧官能团比Mn-850BC丰富,有利于自由基的产生,但不利于PDS吸附于材料表面形成亚稳态复合体以氧化污染物,非自由基氧化作用较弱[19]. 除此以外,Mn-850BC的缺陷比Mn-700BC多,石墨化程度亦较高,这些都有利于提高基于电子转移的非自由基氧化的作用[21,37].
2.3 活性氧的判定
为了阐明该体系中的主要活性氧物质,我们探讨了活性氧捕获剂存在时苯酚的降解情况,具体结果如图11所示. 加入TBA后,Mn-700BC和Mn-850BC对苯酚的去除率分别从原来的84%和90%减少至14%和30%,这表明催化剂活化PDS产生了·OH,而·OH在氧化苯酚时发挥着重要作用. 乙醇作为·OH和SO4·−的淬灭剂,更大程度上抑制了苯酚的降解,意味着SO4·−和·OH同样参与了苯酚的降解过程. 在添加L-His后, Mn-700BC和Mn-850BC对苯酚的去除率降低至24%和55%,这表明体系中有1O2参与的非自由基途径存在. 添加淬灭剂后,Mn-700BC活化PDS降解苯酚的受抑制程度均高于Mn-850BC. 这可能是由于Mn-700BC的孔道和比表面积较低,活性位点相对较少,淬灭剂竞争活性物种更严重. 另外,由于C=O等官能团的差异,Mn-700BC活化PDS时自由基路径占比更大,体现出更强的受抑制程度.
EPR分析进一步揭示了活化过程中产生的自由基. 采用甲基吡啶N-氧化物(DMPO)作为SO4·−和·OH自由基自旋捕获剂,所得波谱如图12所示. 在700BC、850BC、Mn-700BC和Mn-850BC中,观察到强度比例为1:2:2:1的DMPO-OH加成物峰,以及6个较弱的DMPO-SO4·−加成物峰,表明这些材料在活化PDS时产生了·OH和SO4·−[38]. 在其他锰氧化物与炭的复合材料活化过硫酸盐时,也常鉴定出·OH和SO4·−[39]. 这些加成物信号峰的强度大小顺序为Mn-850BC > Mn-700BC > 850BC > 700BC,反映出活化PDS产生的这些自由基的含量差异,这与之前的降解结果一致.
3. 结论(Conclusions)
1)将垂序商陆茎末浸泡于高锰酸钾溶液再高温焙烧,成功将锰氧化物负载到生物炭上,且这种改性方式增加了生物炭的孔道结构. XRD和XPS结果显示,Mn以Mn(Ⅱ)、Mn(Ⅲ)和Mn(Ⅳ)混合价存在;随着焙烧温度的升高,材料的石墨化程度和缺陷增加,但表面含氧官能团含量降低.
2)锰改性生物炭活化PDS降解苯酚的效果显著优于未改性生物炭和商业二氧化锰,材料表面锰氧化物起主要活化作用;苯酚和PDS的初始浓度对改性材料活化降解苯酚的活性影响可用 Langmuir- Hinshelwood模型描述,说明其降解速率受PDS和苯酚的吸附控制;pH在3—9范围内时,提高溶液pH可提高苯酚的降解效率;溶液中共存阴离子Cl−、NO3−、SO42-均可在一定程度上抑制材料活化PDS降解苯酚的效果.
3)自由基捕获实验和EPR结果显示,在锰改性生物炭/过硫酸盐体系中,·OH和SO4·−等自由基路径是氧化苯酚的主要方式,同时还伴有1O2参与的非自由基路径.
-
表 1 材料的孔结构参数
Table 1. Pore structural parameters of the tested materials
比表面积a/(m2·g−1) Specific surface area 总孔容b/(cm3·g−1)Total pore volume 微孔孔容c/(cm3·g−1) Mesopore volume 中孔孔容d/(cm3·g−1)Mesopore volume 700BC 423 0.22 0.18 0.04 Mn-700BC 412 0.25 0.13 0.12 850BC 512 0.29 0.21 0.08 Mn-850BC 570 0.33 0.22 0.11 a:Brunauer-Emmet-Teller(BET)方法计算 b:取相对压力为0.97处 c:t-plot方法 d:总孔容-微孔 表 2 材料活化PDS降解苯酚的一级动力学拟合结果
Table 2. Fitting results of phenol degradation using the first order kinetic model
材料Materials 苯酚浓度/(mmol·L−1)Phenol concentration 表观速率常数k1 /(min−1)Apparent rate constant 相关系数R2Correlation coefficient Mn-700BC 0.16 0.0271 0.94 0.37 0.0070 0.98 0.53 0.0029 0.99 Mn-850BC 0.16 0.0188 0.97 0.37 0.0108 0.98 0.53 0.0089 0.97 -
[1] XU Y, LIN H, LI Y K, et al. The mechanism and efficiency of MnO2 activated persulfate process coupled with electrolysis[J]. The Science of the Total Environment, 2017, 609: 644-654. doi: 10.1016/j.scitotenv.2017.07.151 [2] HOU X Z, DONG H R, LI Y J, et al. Activation of persulfate by graphene/biochar composites for phenol degradation: Performance and nonradical dominated reaction mechanism[J]. Journal of Environmental Chemical Engineering, 2023, 11(2): 109348. doi: 10.1016/j.jece.2023.109348 [3] 李珏秀, 施启旭, 赵锐, 等 锰基催化剂用于活化过硫酸盐降解有机废水的研究进展[J]. 环境化学, 2023, 42(11): 3861-3877 LI J X, SHI Q X, ZHAO R, et al. Research progress on manganese based catalysts for activating persulfate degradation of organic wastewater[J]. Environmental Chemistry, 2023, 42(11): 3861-3877(in Chinese)
[4] HOU J F, HE X D, ZHANG S Q, et al. Recent advances in cobalt-activated sulfate radical-based advanced oxidation processes for water remediation: A review[J]. The Science of the Total Environment, 2021, 770: 145311. doi: 10.1016/j.scitotenv.2021.145311 [5] 孙彬, 徐佳敏, 高仕谦, 等. 碳包覆中空磁性微球活化过硫酸盐降解水中甲氧苄啶[J/OL]. 环境化学, doi: 10.7524/j.issn.0254-6108.2023022604 SUN B, XU J M, GAO S Q, et al. Degradation of trimethoprim in water by persulfate activated based on carbon-coated hollow magnetic microspheres[J/OL]. Environmental Chemistry, doi: 10.7524/j.issn.0254-6108.2023022604
[6] 朱厚堃, 张琼元, 郑玉华, 等. 化学活化剂对活性炭制备影响的研究进展[J]. 天然气化工—C1化学与化工, 2022, 47(2): 25-34. ZHU H K, ZHANG Q Y, ZHENG Y H, et al. Research progress on influence of chemical activators on preparation of activated carbon[J]. Natural Gas Chemical Industry, 2022, 47(2): 25-34 (in Chinese).
[7] WANG H Y, GAO B, WANG S S, et al. Removal of Pb(Ⅱ), Cu(Ⅱ), and Cd(Ⅱ) from aqueous solutions by biochar derived from KMnO4 treated hickory wood[J]. Bioresource Technology, 2015, 197: 356-362. doi: 10.1016/j.biortech.2015.08.132 [8] HU S C, CHEN Y C, LIN X Z, et al. Characterization and adsorption capacity of potassium permanganate used to modify activated carbon filter media for indoor formaldehyde removal[J]. Environmental Science and Pollution Research International, 2018, 25(28): 28525-28545. doi: 10.1007/s11356-018-2681-z [9] 付俊鹏, 李传荣, 许景伟, 等. 沙质海岸防护林入侵植物垂序商陆的防治[J]. 应用生态学报, 2012, 23(4): 991-997. FU J P, LI C R, XU J W, et al. Prevention and control of invaded plant Phytolacca americana in sandy coastal shelter forests[J]. Chinese Journal of Applied Ecology, 2012, 23(4): 991-997 (in Chinese).
[10] 薛生国, 刘丰豪, 吴川, 等. 超富集植物垂序商陆的锰吸收动态研究[J]. 中南大学学报(自然科学版), 2012, 43(2): 424-428. XUE S G, LIU F H, WU C, et al. Kinetics of manganese uptake and accumulation in hyperaccumulator plant Phytolacca americana Linn[J]. Journal of Central South University (Science and Technology), 2012, 43(2): 424-428 (in Chinese).
[11] ZHU S M, XIAO P Y, WANG X, et al. Efficient peroxymonosulfate (PMS) activation by visible-light-driven formation of polymorphic amorphous manganese oxides[J]. Journal of Hazardous Materials, 2022, 427: 127938. doi: 10.1016/j.jhazmat.2021.127938 [12] LIANG J, LI X M, YU Z G, et al. Amorphous MnO2 modified biochar derived from aerobically composted swine manure for adsorption of Pb(II) and Cd(II)[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(6): 5049-5058. [13] YIN Y Y, GUO X T, PENG D. Iron and manganese oxides modified maize straw to remove tylosin from aqueous solutions[J]. Chemosphere, 2018, 205: 156-165. doi: 10.1016/j.chemosphere.2018.04.108 [14] LIAO M, WANG J W, YE L, et al. A deep-cycle aqueous zinc-ion battery containing an oxygen-deficient vanadium oxide cathode[J]. Angewandte Chemie (International Ed. in English), 2020, 59(6): 2273-2278. doi: 10.1002/anie.201912203 [15] SONG Z G, LIAN F, YU Z H, et al. Synthesis and characterization of a novel MnOx-loaded biochar and its adsorption properties for Cu2+ in aqueous solution[J]. Chemical Engineering Journal, 2014, 242: 36-42. doi: 10.1016/j.cej.2013.12.061 [16] 袁怡, 余恒超, 张亚新, 等. 生物炭对城乡多元有机固废蚯蚓堆肥产物应用于Cu(Ⅱ)吸附的研究[J]. 环境科学学报, 2023, 43(9): 211-220. YUAN Y, YU H C, ZHANG Y X, et al. Application of biochar to the adsorption of Cu(Ⅱ)from vermicomposting products of urban and rural multiple organic solid waste[J]. Acta Scientiae Circumstantiae, 2023, 43(9): 211-220 (in Chinese).
[17] OUYANG D, CHEN Y, YAN J C, et al. Activation mechanism of peroxymonosulfate by biochar for catalytic degradation of 1, 4-dioxane: Important role of biochar defect structures[J]. Chemical Engineering Journal, 2019, 370: 614-624. doi: 10.1016/j.cej.2019.03.235 [18] 燕翔, 杨建东, 韩丽, 等. 壳聚糖/磁性核桃壳生物炭的制备及其对高浓度Pb(Ⅱ)的吸附[J]. 中国有色冶金, 2023, 52(5): 135-145. YAN X, YANG J D, HAN L, et al. Preparation of chitosan supported magnetic walnut shell biochar and its adsorption properties on high concentration of Pb(Ⅱ)[J]. China Nonferrous Metallurgy, 2023, 52(5): 135-145 (in Chinese).
[19] AZARGOHAR R, NANDA S, KOZINSKI J, et al. Effects of temperature on the physicochemical characteristics of fast pyrolysis bio-chars derived from Canadian waste biomass[J]. Fuel, 2014, 125: 90-100. doi: 10.1016/j.fuel.2014.01.083 [20] DU N J, LIU Y, LI Q J, et al. Peroxydisulfate activation by atomically-dispersed Fe-Nx on N-doped carbon: Mechanism of singlet oxygen evolution for nonradical degradation of aqueous contaminants[J]. Chemical Engineering Journal, 2021, 413: 127545. doi: 10.1016/j.cej.2020.127545 [21] WANG Y S, SONG Y J, LI N, et al. Tunable active sites on biogas digestate derived biochar for sulfanilamide degradation by peroxymonosulfate activation[J]. Journal of Hazardous Materials, 2022, 421: 126794. doi: 10.1016/j.jhazmat.2021.126794 [22] KIM D G, KO S O. Effects of thermal modification of a biochar on persulfate activation and mechanisms of catalytic degradation of a pharmaceutical[J]. Chemical Engineering Journal, 2020, 399: 125377. doi: 10.1016/j.cej.2020.125377 [23] LIU H Z, BRUTON T A, DOYLE F M, et al. In situ chemical oxidation of contaminated groundwater by persulfate: Decomposition by Fe(III)- and Mn(IV)-containing oxides and aquifer materials[J]. Environmental Science & Technology, 2014, 48(17): 10330-10336. [24] ZHU S S, HO S H, JIN C, et al. Nanostructured manganese oxides: Natural/artificial formation and their induced catalysis for wastewater remediation[J]. Environmental Science:Nano, 2020, 7(2): 368-396. doi: 10.1039/C9EN01250H [25] SUN R Y, LIU J T, WU Y S, et al. Enhanced non-radical degradation of organic pollutants by peroxymonosulfate activation with Zr-Mn composite oxide[J]. Chemical Engineering Journal, 2023, 471: 144529. doi: 10.1016/j.cej.2023.144529 [26] SHAO F L, WANG Y J, MAO Y R, et al. Degradation of tetracycline in water by biochar supported nanosized iron activated persulfate[J]. Chemosphere, 2020, 261: 127844. doi: 10.1016/j.chemosphere.2020.127844 [27] HOU J F, LI H, TANG Y Q, et al. Supported N-doped carbon quantum dots as the highly effective peroxydisulfate catalysts for bisphenol F degradation[J]. Applied Catalysis B:Environmental, 2018, 238: 225-235. doi: 10.1016/j.apcatb.2018.07.032 [28] 张寅, 邵芸, 陈欢, 等. Pd/TiO2对水体中2, 4-二氯酚的催化加氢脱氯研究[J]. 环境科学, 2012, 33(1): 88-93. ZHANG Y, SHAO Y, CHEN H, et al. Catalytic hydrodechlorination of 2, 4-dichlorophenol over Pd/TiO2[J]. Environmental Science, 2012, 33(1): 88-93 (in Chinese).
[29] 姚宏嘉, 陈星, 张玉, 等. 生物炭负载γ-MnO2纳米复合材料活化过一硫酸盐降解对氯苯酚的性能及机理[J]. 环境工程学报, 2022, 16(6): 1833-1844. YAO H J, CHEN X, ZHANG Y, et al. Performance and mechanism of biochar doped γ-MnO2 nanocomposite activated peroxymonsulfate on 4-Chlorophenol degradation[J]. Chinese Journal of Environmental Engineering, 2022, 16(6): 1833-1844 (in Chinese).
[30] WANG Y, JI Q J, XU J X, et al. Activation of peroxydisulfate using N-doped carbon-encapsulated Ni species for efficient degradation of tetracycline[J]. Separation and Purification Technology, 2021, 276: 119369. doi: 10.1016/j.seppur.2021.119369 [31] GUAN Y H, MA J, LI X C, et al. Influence of pH on the formation of sulfate and hydroxyl radicals in the UV/peroxymonosulfate system[J]. Environmental Science & Technology, 2011, 45(21): 9308-9314. [32] CHEN J B, ZHOU X F, ZHU Y M, et al. Synergistic activation of peroxydisulfate with magnetite and copper ion at neutral condition[J]. Water Research, 2020, 186: 116371. doi: 10.1016/j.watres.2020.116371 [33] 徐梓淞, 宋雄伟, 黄闻宇, 等. 不同活化过硫酸盐体系的机理分析及不同无机阴离子的作用: 以两种有机染料为例[J]. 环境化学, 2022, 41(4): 1412-1424. doi: 10.7524/j.issn.0254-6108.2020122103 XU Z S, SONG X W, HUANG W Y, et al. Mechanism analysis of different activated persulfate systems and effects of different inorganic anions: A case study of two organic dyes[J]. Environmental Chemistry, 2022, 41(4): 1412-1424 (in Chinese). doi: 10.7524/j.issn.0254-6108.2020122103
[34] HUANG K Z, ZHANG H C. Galvanic oxidation processes (GOPs): An effective direct electron transfer approach for organic contaminant oxidation[J]. The Science of the Total Environment, 2020, 743: 140828. doi: 10.1016/j.scitotenv.2020.140828 [35] 冯勇, 李谕, 应光国. 基于过硫酸盐活化的微界面电子转移氧化技术[J]. 化学进展, 2021, 33(11): 2138-2149. FENG Y, LI Y, YING G G. Micro-interface electron transfer oxidation based on persulfate activation[J]. Progress in Chemistry, 2021, 33(11): 2138-2149 (in Chinese).
[36] GAO Y, ZHOU Y, PANG S Y, et al. Enhanced peroxymonosulfate activation via complexed Mn(II): A novel non-radical oxidation mechanism involving manganese intermediates[J]. Water Research, 2021, 193: 116856. doi: 10.1016/j.watres.2021.116856 [37] REN W, XIONG L L, NIE G, et al. Insights into the electron-transfer regime of peroxydisulfate activation on carbon nanotubes: The role of oxygen functional groups[J]. Environmental Science & Technology, 2020, 54(2): 1267-1275. [38] LIU J L, AN F X, ZHU C Y, et al. Efficient transformation of DDT with peroxymonosulfate activation by different crystallographic MnO2[J]. The Science of the Total Environment, 2021, 759: 142864. doi: 10.1016/j.scitotenv.2020.142864 [39] CHEN H Z, TIAN K, QING T P, et al. Efficient removal of tetrabromobisphenol A through persulfate activation by α-MnO2 nanofiber coated with graphene oxide[J]. Applied Surface Science, 2023, 641: 158445. doi: 10.1016/j.apsusc.2023.158445 期刊类型引用(0)
其他类型引用(1)
-






 下载:
下载: