-
氯酚(chlorophenols,CPs)是一类重要的有机化工原料,广泛应用于杀虫剂、除草剂和木材防腐剂的生产. 由于氯酚具有高毒性、持久性和“三致效应”(致癌、致畸、致突变)而被我国以及美国、欧盟等国家和地区列入优先控制污染物名单[1 − 2]. 氯酚污染物的毒性主要源于自身的芳环结构和氯原子取代基,而氯原子取代基是其主要毒性位点,氯原子数目越多毒性越高[3]. 此外,氯酚因氯原子的p轨道电子及苯环上的π电子易形成稳定的共轭体系而具有较强的化学稳定性. 高电负性的氯原子使苯环表现出难氧化的疏水性,抑制了苯环裂解酶的活性,提高了其抗生物降解能力,使其比普通的酚类污染物更难降解[4 − 5]. 因此,高效处理氯酚污染物的关键在于高毒性氯位点的精准去除. 目前, 去除氯酚污染物的主要处理方法有生物法、物理法和化学法等[6 − 8]. 生物法周期长且微生物受制于氯酚类污染物的高毒性难以发挥效用,物理法、化学法均存在使用成本高且能够引起二次污染等问题.
近年来,光催化技术因具有高效、环保、经济等优势在水污染控制领域显现出广阔的应用前景[9]. 有研究成果表明,光催化技术能够通过调控自由基与非自由基路径实现氯酚类污染物的深度处理[10],但自由基与非自由基反应的引发均需以过氧化物(过一硫酸盐、过二硫酸盐)为媒介,在面临复杂反应体系中由过氧化物引发的自由基与非自由基路径极易受反应体系中杂质污染物的干扰,且对氯酚污染物脱氯的靶向性较差及生成的中间副产物容易造成二次污染的问题. Chen等[11]构建了一种基于缓释碳耦合生物电化学系统,精准促进氯代烃的次序脱氯为乙烯,有效解决氯代烃降解不彻底导致的有毒有害中间产物积累的问题,实现了氯代烃的高效完全脱氯和脱毒. 因此,通过构建合适的光催化体系来实现氯酚污染物的精准脱氯,可减少次生风险及二次污染,将具有重要的环境意义,其中选择具有靶向性脱氯的光催化剂即成为了解决问题的关键.
共价三嗪框架(Covalent triazine-based frameworks,CTFs)是一类由芳香环和富氮三嗪环作为连接单元组成的二维层状聚合物[12]. 因高比表面积、可调的孔结构、合适的带隙和高化学稳定性,CTFs在吸附和光催化方面具有可开发的潜力[13]. 由于CTFs的特殊结构使其具有吸附剂的显著特征,引入的富电子三嗪环单元结构使其具有光催化反应能力,从而兼具吸附和光催化双功能的CTFs成为了去除水中有机污染物的理想催化剂[14 − 15].
本文采用1,4-苯二甲腈经离子热三聚合成具有共价三嗪框架的光催化剂CTF-1,测定其对4-氯酚(4-CP)、2,4-二氯酚(2,4-DCP)、2,4,6-三氯酚(2,4,6-TCP)和五氯酚(PCP)4种氯酚类污染物的光催化反应速率常数及脱氯效率,分析氯酚分子结构与脱氯降解效率的关系,并初步探索光催化脱氯降解机制,为光催化技术应用于卤代酚类废水的脱卤去除提供理论依据.
-
三氟甲烷磺酸(CF3SO3H,GR,TFMSA)和1,4-苯二甲腈(C8H4N2,AR)购自上海麦克林生化科技股份有限公司;三氯甲烷(CHCl3)、丙酮(CH3COCH3)、氯化锌(ZnCl2)、四氢呋喃(C4H8O)、氢氧化钠、盐酸等,购自西陇化工股份有限公司,均为AR; 4-氯酚(4-CP)、2,4-二氯酚(2,4-DCP)、2,4,6-三氯酚(2,4,6-TCP)、五氯酚(PCP)等,均为AR,购自上海化学试剂公司. 实验用水为去离子水.
-
对文献[16]报道的方法稍作改进后合成片层状CTF-1,实验步骤如下:首先,将40 mL三氟甲烷磺酸(TFMSA)加到含30 mL 三氯甲烷的干燥圆底三口烧瓶中,0 ℃及氩气氛围下搅拌. 然后,将4.26 g 1,4-苯二甲腈加入含200 mL的三氯甲烷溶液中,超声分散均匀后缓慢滴加到上述溶液中,并保持温度在40 ℃下快速搅拌48 h. 反应结束后将冷却至室温的溶液加入到含660 mL去离子水和34 mL25%氨水中,室温搅拌2 h,将生成的淡黄色沉淀物通过真空过滤方式从悬浮液中分离出来,先后用去离子水、乙醇和三氯甲烷洗涤3遍,在70 ℃下真空干燥过夜得到前驱体pre-CTF-1. 将pre-CTF-1与ZnCl2以1:8.5的物质的量比混合并研磨均匀,紧接着在400 ℃的管式炉中,氩气氛围下反应10 min. 反应结束待温度降至室温后将样品用去离子水和0.1 mol·L−1的稀盐酸分别搅拌清洗24 h,并先后用丙酮、四氢呋喃过滤洗涤以去除未反应完的ZnCl2. 最后将样品在70 ℃下真空干燥12 h,得到棕色粉末即为CTF-1.
-
研究中利用扫描电子显微镜(SEM,S-300N,日本Hitachi)、X-射线衍射仪(XRD,D8 Advance X,德国Bruker)、傅里叶变换红外光谱仪(FTIR,VERTEX-70,德国Bruker)和固体核磁共振光谱仪(SSNMR,Avance Ⅱ 600MHz,德国Bruker)分别对催化剂进行形貌和结构表征;利用比表面积与孔隙度分析仪(BET,TriStar Ⅱ 3020,美国Micromeritics)对催化剂的比表面积及孔分布进行分析;利用X射线光电子能谱仪(XPS,Axis Ultra DLD,日本岛津)分析催化剂表面化学组分.
-
光催化降解实验以300 W 氙灯(PLS-SXE300C,λ > 420 nm,北京泊菲莱)作为模拟太阳光光源,光强为100 mW·cm−2. 取200 mL初始浓度为10 mg·L−1的氯酚污染物水溶液于配有循环冷凝水的双层烧杯中,因PCP在pH > 10(25 ℃)时溶解性较好,为保持实验的一致性,溶液初始pH均调节为10 ± 0.1,通入冷凝水保持反应温度为(25 ± 1)℃,加入0.02 g催化剂,黑暗条件下充分搅拌60 min以达吸附平衡. 接着,打开氙灯进行光催化反应,设定一定时间间隔收集2 mL反应悬浮液,并用0.22 μm的过滤器滤除催化剂后转入棕色液相小瓶以进行后续分析及测定. 所有实验重复3次后取平均值. 氯酚污染物的降解率计算公式如下[17]:
式中,η为氯酚污染物降解率(%),C-60为氯酚污染物的初始浓度(mg·L−1),Ct为反应t时间(min)后的浓度(mg·L−1).
-
为探究不同活性物种对氯酚污染物降解的影响,在黑暗反应前向初始氯酚污染物水溶液(200 mL,10 mg·L−1)中加入不同的淬灭剂,然后再加入催化剂进行避光搅拌吸附,淬灭剂的加入量均为0.1 mmol,其余步骤同“1.4”节.
-
采用配有Agilent TC-C18色谱柱(150 mm × 4.6 mm,5 μm)的高效液相色谱仪(HPLC,LC-20AD,日本岛津)进行氯酚污染物的定量测定,检测器为紫外检测器. 流动相分别设置水相(纯水或稀释的乙酸、磷酸等)和有机相(甲醇或乙腈). 4种氯酚污染物检测方法如表1所示.
采用离子色谱仪(EOC IC,瑞士万通)分析氯酚污染物降解过程中生成的氯离子(Cl-),检测条件如下:流动相为3.5 mmol·L−1 NaCO3和 3.0 mmol·L−1 NaHCO3的混合淋洗液,设总流速为1.0 mL·min-l,进样体积为20 μL,阴离子色谱柱型号为Metrosep A Supp 5(150 mm × 4.0 mm). 利用总有机碳分析仪(Multi N/C 2100S,德国Analytik Jena)对光催化反应前后溶液中总有机碳(TOC)含量进行测定.
-
本文通过聚合法和熔盐法合成了结构有序的CTF-1,如图1a所示,并对CTF-1进行形貌和结构分析. 从图1b的SEM可以看出,CTF-1是由表面较平整的纳米片堆积而成的层状材料. 图1c的XRD衍射图显示CTF-1在7.4°、14.8°和25.8°处出现3个衍射峰,分别对应(1 0 0)、(2 0 0)和(0 0 1)晶面,其中(0 0 1)对应CTF-1的层间距,而(1 0 0)对应CTF-1的平面结构,这与文献报道一致[16],说明成功合成CTF-1材料. 由图1d的FTIR可知,1349.2 cm−1和1511.3 cm−1处的红外峰对应CTF-1三嗪环的特征峰,1671.6 cm−1和814.1 cm−1则归属于CTF-1苯环的特征峰[18]. 从图1e的固体核磁13C-NMR谱图观察到,CTF-1显示4个不同的13C特征信号峰,其中前3个分别归属于三嗪环中的碳原子(170 ppm)和桥联苯环中的2个碳原子(138 ppm和128 ppm),剩余的116 ppm处的峰则归属于末端未反应的腈基[16].
由图1f的N2吸附-脱附等温线可知,CTF-1的比表面积为15.93 cm2·g−1,孔径为20.16 nm. 以上表征结果说明本文合成的CTF-1为三嗪环和苯环交替连接形成的且具有一定孔道结构的片层堆积材料.
-
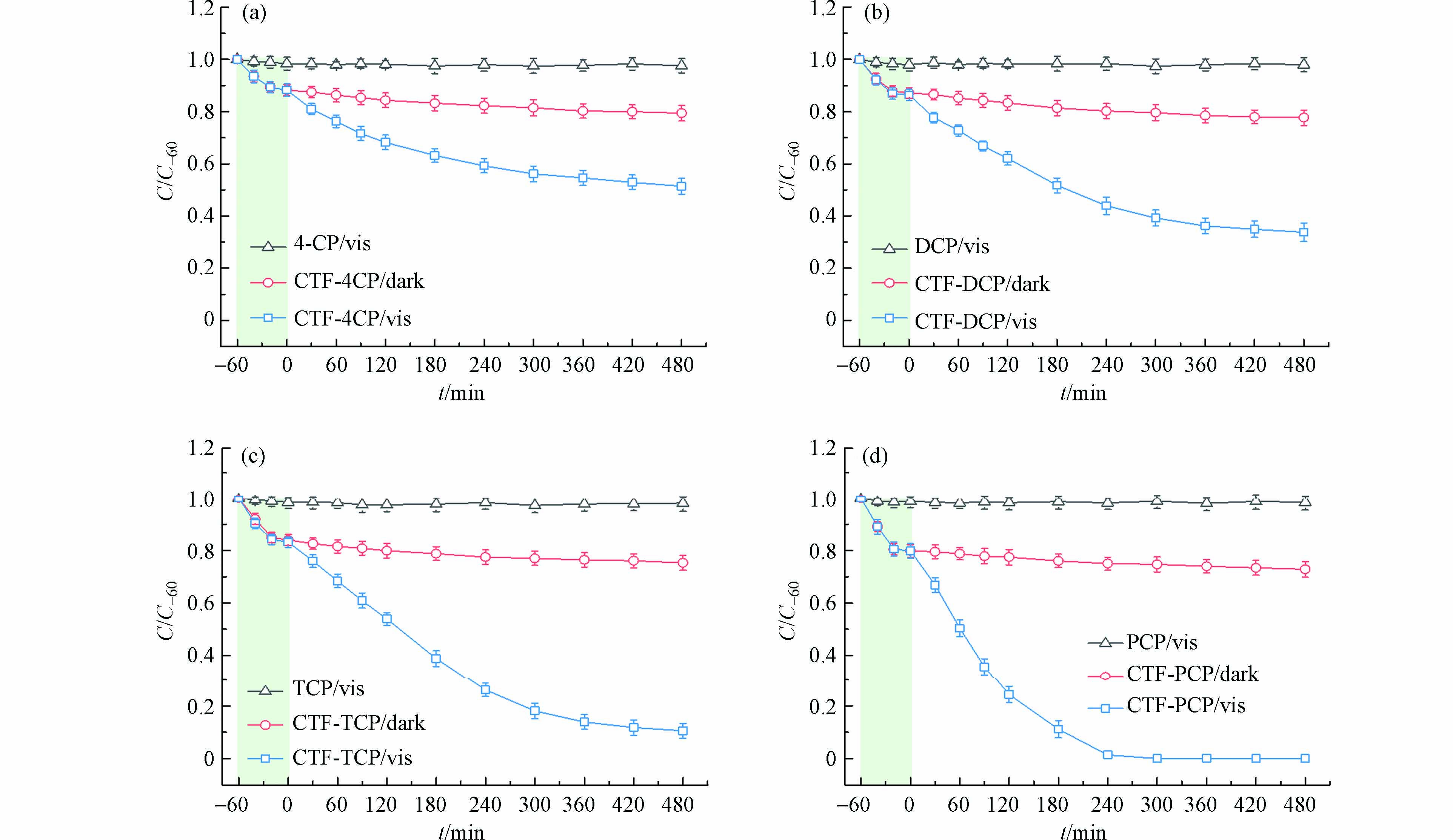
图2为CTF-1光催化降解氯酚污染物过程中氯酚含量随反应时间的变化曲线及氯酚污染物分别在光照条件和存在CTF-1的暗反应条件下的降解曲线图. 由图2可知,氯酚污染物在可见光照射下几乎不发生降解反应;随着CTF-1的引入,催化剂自身对氯酚类污染物存在吸附作用,避光反应60 min可达吸附平衡,且随着反应时间的延长,氯酚类污染物几乎不发生降解作用,证实CTF-1不会引发氯酚类污染物的降解;而当CTF-1与氯酚污染物达到吸附平衡后引入可见光,氯酚污染物发生降解反应,反应480 min后CTF-1对4-CP、2,4-DCP、2,4,6-TCP和PCP的降解率分别为49%、67%、89%和100%. 可以看出,随着氯原子取代数目的增多降解效率提高,相同条件下降解效率与氯原子数目呈正相关.
为了进一步了解CTF-1光催化降解氯酚污染物的降解效能,如图3a所示,利用准一级动力学模型对光催化降解反应进行拟合[17]:
式中,C0为避光搅拌60 min后达到吸附平衡时氯酚污染物的浓度(mg·L−1),C和k分别为光照反应时间t时(min)氯酚污染物的浓度(mg·L−1)和表观反应速率常数(min−1). 由图3a可知,PCP、2,4,6-TCP、2,4-DCP、4-CP的降解表观速率常数k差异较大,降解表观速率常数为:PCP(0.0159 min−1) > 2,4,6-TCP(0.0048 min−1) > 2,4-DCP(0.0021 min−1) > 4-CP(0.0011 min−1),这与降解速率结果一致,即氯原子数目越多的氯酚类污染物其表观速率常数越大.
为了进一步探究这种去除效果的差异性,本研究从催化材料本征的疏水性结构出发,解析污染物自身结构特性,以辛醇-水分配系数为恒量指标考察目标污染物的亲疏水性,通过调研文献可知4-CP、2,4-DCP、2,4,6-TCP、PCP的辛醇-水分配系数分别为2.35、3.08、3.69、5.01[19],即氯酚类污染物的疏水性与氯原子取代个数成正相关. 基于此,本研究构建了以氯酚类污染物的表观降解动力学常数与其辛醇-水分配系数的线性关系,如图3b所示,氯酚类污染物的表观速率常数与辛醇-水分配系数高度相关(P < 0.05),因此,初步推断CTF-1光催化降解氯酚类污染物的去除机制可能与氯酚类污染物自身亲疏水性相关.
-
为了进一步研究CTF-1光催化降解氯酚类污染物的去除机制,本研究监测了CTF-1光催化降解氯酚类污染物反应过程中氯离子的释放. 如图4所示,在没有CTF-1催化剂或无可见光照射的情况下,未观察到氯原子释放;在CTF-1作为催化剂的光催化过程中,不同氯原子数目的氯酚类污染物其脱氯率各不相同,反应480 min后,CTF-1光催降解PCP、2,4,6-TCP、2,4-DCP、4-CP体系中其氯离子浓度分别为6.665 mg·L−1、4.803 mg·L−1、2.951 mg·L−1和1.393 mg·L−1. 通过物料衡算可得10 mg·L−1的PCP、2,4,6-TCP、2,4-DCP、4-CP中氯离子质量浓度分别为6.6657 mg·L−1、5.3937 mg·L−1、4.3558 mg·L−1和2.7613 mg·L−1.
如图5所示,将检测值与理论值作比后,发现CTF-1光催化降解PCP、2,4,6-TCP、2,4-DCP、4-CP的脱氯率分别为100 %、88.6 %、66.5 %和47.0 %. 这一结果与CTF-1光催化降解氯酚类污染物的降解动力学结果一致. 由此推测基于CTF-1构建的光催降解氯酚类污染物体系,其反应机制均是针对氯酚类污染物上取代氯位点进行精准脱氯的过程.
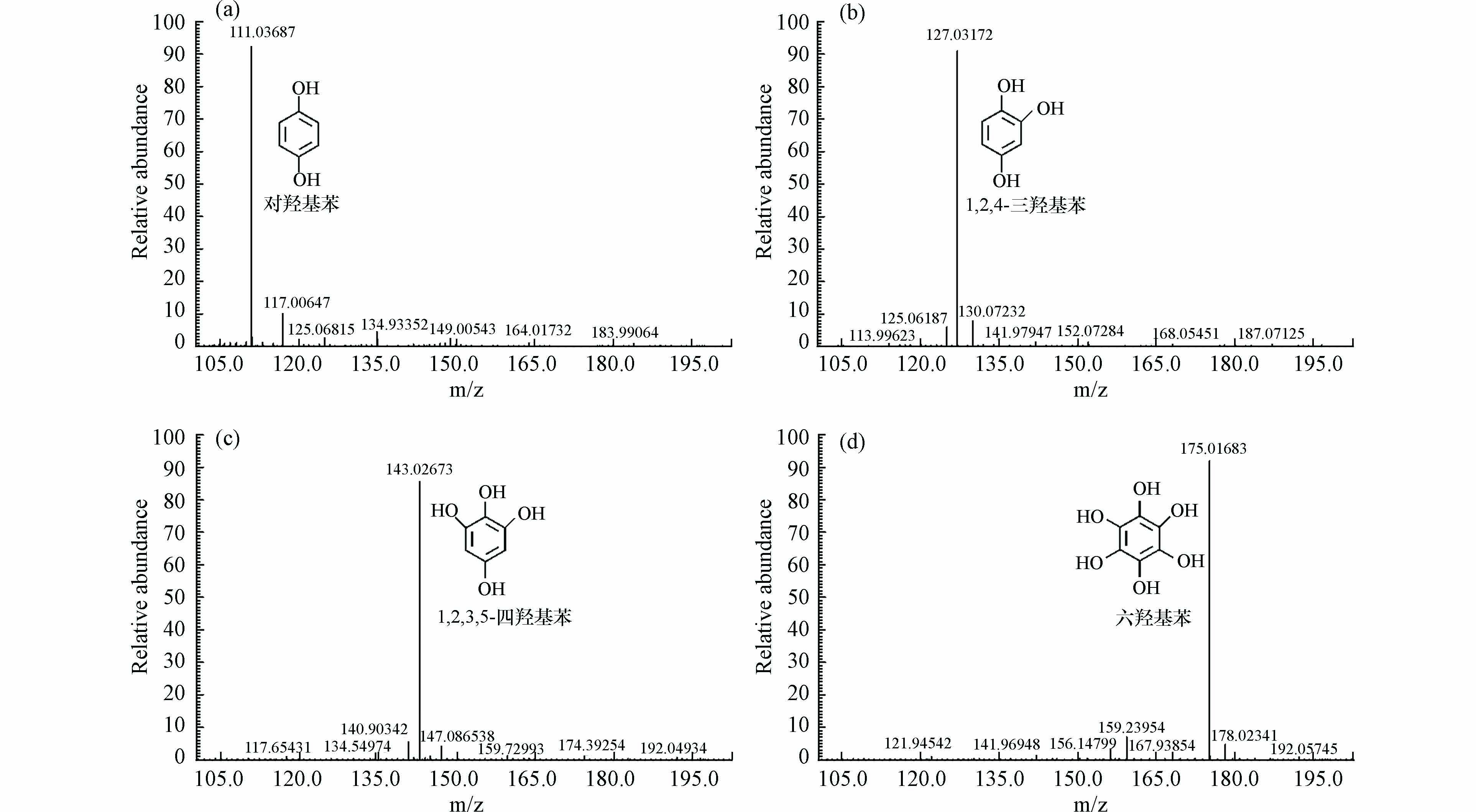
为了进一步测试氯酚污染物是否发生矿化生成CO2和水,对反应前后溶液中的TOC值进行测定. 从图6可知,4种氯酚污染物反应前后的TOC基本不变,体系不发生矿化. 同时,对4种氯酚污染物反应后的最终产物采用液相色谱-高分辨质谱法(LC-MS)进行测定,结果如图7所示. 由图7可知,在正离子模式质谱中检测到相应质子化产物[M+H]+峰,4-CP、DCP、TCP和PCP这4种氯酚反应产物的m/z分别为:111.03687、127.03172、143.02673和175.01683,分别对应:对羟基苯、1,2,4-三羟基苯、1,2,3,5-四羟基苯和六羟基苯. 以上结果说明构建的CTF-1光催化降解体系对氯酚污染物并未发生苯环的开环反应,而是针对氯酚污染物上氯位点作用,精准促进氯酚污染物脱氯,从而实现氯酚污染物的高效脱氯和脱毒.
-
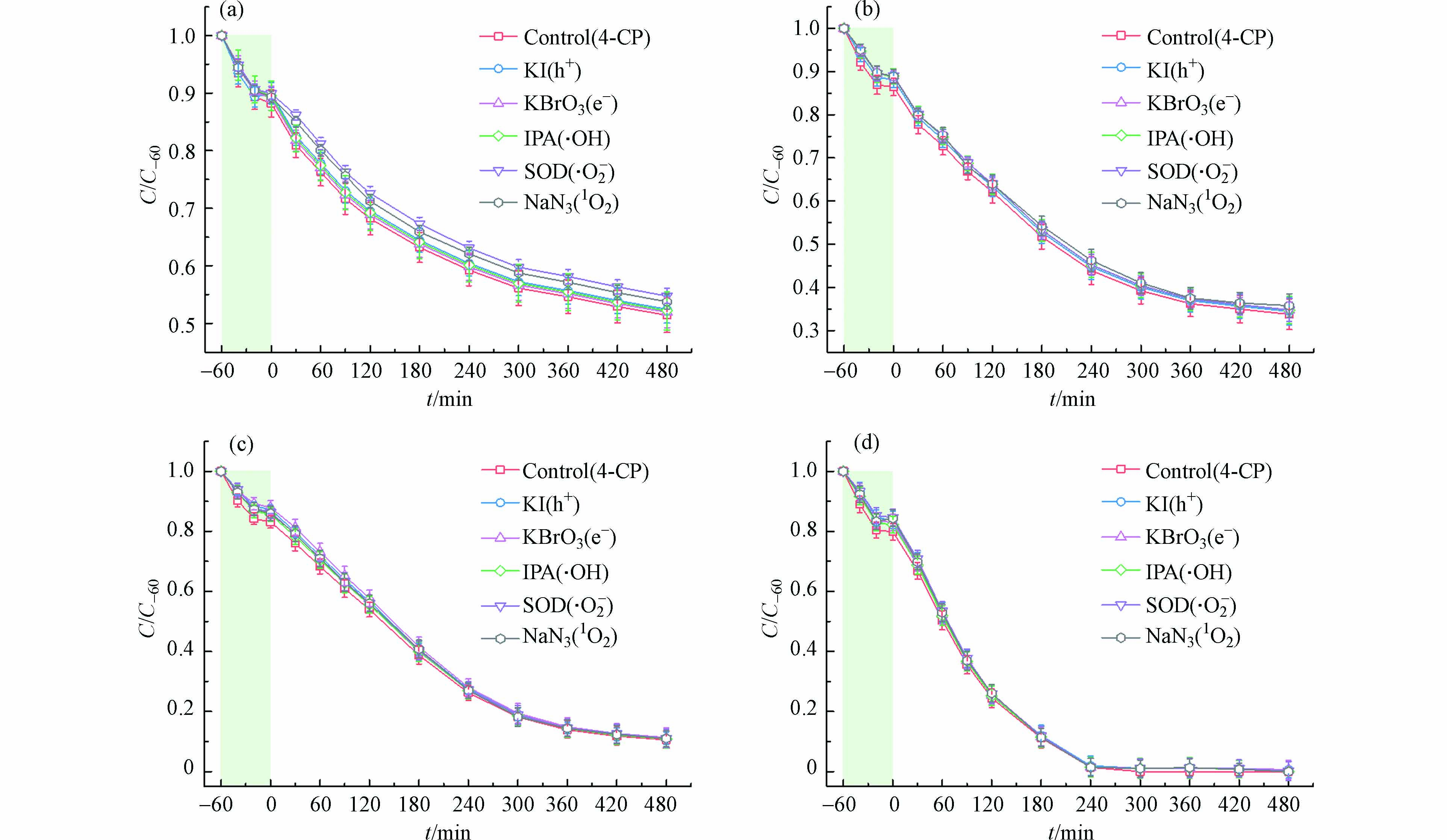
CTF-1作为一种典型的光催化剂,常规意义上其对于污染物的降解均是通过可见光激发产生光生电子和空穴,继而由空穴氧化引发自由基链式反应对目标污染物进行降解[20]. 基于反应过程中可能存在的活性物种(h+、e-、·OH、·O2- 和1O2),以碘化钾(KI)作为h+的淬灭剂,溴酸钾(KBrO3)作为e-的淬灭剂,异丙醇(IPA)作为·OH的淬灭剂,超氧化物歧化酶(SOD)作为·O2-的淬灭剂,叠氮化钠(NaN3)作为1O2的淬灭剂[21 − 22],进行淬灭实验. 如图8所示,加入淬灭剂后几乎不影响氯酚类污染物的降解,证实了在CTF-1光催化体系中氯酚类污染物的脱氯不是由自由基与非自由基路径产生的活性物种所引发.
-
经过一系列分析实验,推测光催化降解氯酚污染物的机制与光催化体系中CTF-1与氯酚污染物的相互作用有关. 为此,进行X射线光电子能谱(XPS)分析以了解催化剂表面化学组分及元素存在的化学状态. 从图9a可以看出,CTF-1在吸附氯酚前后均存在C、N和O元素,其中O元素为暴露在环境中不可避免的吸附空气中的水分和氧气[18]. 此外,观察到当吸附氯酚类污染物后CTF-1上出现氯元素的峰,说明氯酚类污染物吸附到CTF-1上,且随着氯原子取代数目的增多,氯峰强度增加. 图9b中Cl 2p的XPS光谱显示吸附氯酚污染物后在约为200.5 eV和202.1 eV处出现2个峰,分别归属于氯酚污染物C—Cl键的Cl 2p1/2和Cl 2p3/2,说明氯酚污染物吸附在CTF-1上;同时,观察到Cl 2p1/2和Cl 2p3/2的结合能随氯原子取代数目的增加而降低,即氯酚污染物的C-Cl键上Cl原子的电子云密度增加,且逐渐趋向离子化状态(NaCl). 由此,推测CTF-1光催化降解氯酚类污染物的机制如图10所示,光激发CTF-1产生电子空穴对,CTF-1与氯酚类污染物之间的相互作用促进电子由CTF-1传输到氯酚污染物上,氯原子作为电子聚集中心吸引来自CTF-1的电子,电子云密度增加,连续光照下产生的电子不断流向氯原子,使C—Cl键上电子云密度持续增加并趋向于离子化状态,此时,在水溶剂的作用下发生C—Cl键的断裂,从而完成氯酚污染物的脱氯降解.
总之,氯酚类污染物的降解效率与CTF-1与氯酚类污染物的相互作用强度有关,而氯酚类污染物与CTF-1之间的相互作用除了氯酚类污染物的苯环与CTF-1的π-π相互作用及静电作用外,主要与疏水作用有关. 氯原子数目越多,氯酚类污染物的疏水性越强,与CTF-1的结合力越强,越有利于电子的传输,C—Cl键的电子云密度增加越大,脱氯降解效率越高. 由于光激发产生的电子主要传输到吸电子氯原子上,因此,反应体系是以改变Cl上电子云密度来实现氯酚类污染物的脱氯降解,是一个针对氯位点完成的反应.
-
1)在可见光照射下,CTF-1对氯酚污染物进行光催化降解实验, 结果表明, CTF-1对氯原子数目最多的PCP的降解及脱氯效果最佳,且氯酚类污染物上氯原子数目与氯酚类污染物的降解效率、脱氯速率及表观速率常数k呈正相关,均遵循:PCP > 2,4,6-TCP > 2,4-DCP > 4-CP.
2)氯原子数目越多,氯酚类污染物的辛醇-水分配系数越大其疏水性越强,与CTF-1的作用力越强,对氯酚脱氯降解效果影响越大.
3)基于CTF-1构建的光催降解氯酚污染物体系,其反应机制是针对氯酚污染物上取代氯位点进行水解脱氯降解的过程,该工作对光催化降解卤代酚类污染物的脱卤降毒有一定的参考价值.
共价三嗪有机框架材料对水中氯酚类污染物的光催化脱氯降解
Photocatalytic dechlorination of chlorophenol pollutants in aqueous solution by covalent triazine organic framework material
-
摘要: 利用共价三嗪有机框架材料(CTF-1)对4-氯酚(4-CP)、2,4-二氯酚(2,4-DCP)、2,4,6-三氯酚(2,4,6-TCP)和五氯酚(PCP)等4种不同氯原子取代数目的氯酚类污染物进行光催化降解研究,探讨了底物结构对氯酚脱氯降解效率的影响及机制. 结果表明,氯酚脱氯降解过程明显受苯环氯原子取代数目的影响,氯原子数目越多,脱氯降解效率越高,氯原子数目与表观速率常数呈显著正相关,氯酚降解及脱氯速率均为:PCP >2,4,6-TCP >2,4-DCP >4-CP. 对CTF-1光催化降解氯酚机制研究表明,活性物种在反应中不起作用,体系反应机制为针对氯酚上取代氯位点进行水解脱氯过程. 本研究结果为深入揭示氯酚脱氯降解机制提供了理论依据,也为光催化技术处理卤代酚类废水提供了技术参考.Abstract: In this study, covalent triazine organic framework material (CTF-1) was applied to degrade four chlorophenols (CPs) with different chlorine atoms, including 4-chlorophenol (4-CP), 2,4-dichlorophenol (2,4-DCP), 2,4,6-trichlorophenol (2,4,6-TCP) and pentachlorophenol (PCP). The effect and mechanism of substrate structure on the dechlorination degradation efficiency of chlorophenols were explored. The dechlorination degradation process of CPs were significantly influenced by the purpose of selecting the number of chlorine atoms in the benzene ring. The more chlorine there was, the higher the dechlorination degradation efficiency. The number of chlorine atoms was significantly positively correlated with the apparent rate constant. The results showed that the degradation rate and dechlorination rate of CPs were as follows: PCP > 2,4,6-TCP > 2,4-DCP > 4-CP. The study on the mechanism of CTF-1 photocatalytic degradation of CPs showed that the active species do not play a role in the reaction, and the system reaction mechanism was the hydrolysis dechlorination process targeting the substituted chlorine sites on CPs. This study provided a theoretical basis for further revealing the degradation mechanism of CPs, as well as a technical reference for the treatment of halogenated phenol wastewater using photocatalytic technology.
-
Key words:
- CTF-1 /
- chlorophenols /
- photocatalytic degradation /
- dechlorination /
- chlorine atom numbers.
-
氯酚(chlorophenols,CPs)是一类重要的有机化工原料,广泛应用于杀虫剂、除草剂和木材防腐剂的生产. 由于氯酚具有高毒性、持久性和“三致效应”(致癌、致畸、致突变)而被我国以及美国、欧盟等国家和地区列入优先控制污染物名单[1 − 2]. 氯酚污染物的毒性主要源于自身的芳环结构和氯原子取代基,而氯原子取代基是其主要毒性位点,氯原子数目越多毒性越高[3]. 此外,氯酚因氯原子的p轨道电子及苯环上的π电子易形成稳定的共轭体系而具有较强的化学稳定性. 高电负性的氯原子使苯环表现出难氧化的疏水性,抑制了苯环裂解酶的活性,提高了其抗生物降解能力,使其比普通的酚类污染物更难降解[4 − 5]. 因此,高效处理氯酚污染物的关键在于高毒性氯位点的精准去除. 目前, 去除氯酚污染物的主要处理方法有生物法、物理法和化学法等[6 − 8]. 生物法周期长且微生物受制于氯酚类污染物的高毒性难以发挥效用,物理法、化学法均存在使用成本高且能够引起二次污染等问题.
近年来,光催化技术因具有高效、环保、经济等优势在水污染控制领域显现出广阔的应用前景[9]. 有研究成果表明,光催化技术能够通过调控自由基与非自由基路径实现氯酚类污染物的深度处理[10],但自由基与非自由基反应的引发均需以过氧化物(过一硫酸盐、过二硫酸盐)为媒介,在面临复杂反应体系中由过氧化物引发的自由基与非自由基路径极易受反应体系中杂质污染物的干扰,且对氯酚污染物脱氯的靶向性较差及生成的中间副产物容易造成二次污染的问题. Chen等[11]构建了一种基于缓释碳耦合生物电化学系统,精准促进氯代烃的次序脱氯为乙烯,有效解决氯代烃降解不彻底导致的有毒有害中间产物积累的问题,实现了氯代烃的高效完全脱氯和脱毒. 因此,通过构建合适的光催化体系来实现氯酚污染物的精准脱氯,可减少次生风险及二次污染,将具有重要的环境意义,其中选择具有靶向性脱氯的光催化剂即成为了解决问题的关键.
共价三嗪框架(Covalent triazine-based frameworks,CTFs)是一类由芳香环和富氮三嗪环作为连接单元组成的二维层状聚合物[12]. 因高比表面积、可调的孔结构、合适的带隙和高化学稳定性,CTFs在吸附和光催化方面具有可开发的潜力[13]. 由于CTFs的特殊结构使其具有吸附剂的显著特征,引入的富电子三嗪环单元结构使其具有光催化反应能力,从而兼具吸附和光催化双功能的CTFs成为了去除水中有机污染物的理想催化剂[14 − 15].
本文采用1,4-苯二甲腈经离子热三聚合成具有共价三嗪框架的光催化剂CTF-1,测定其对4-氯酚(4-CP)、2,4-二氯酚(2,4-DCP)、2,4,6-三氯酚(2,4,6-TCP)和五氯酚(PCP)4种氯酚类污染物的光催化反应速率常数及脱氯效率,分析氯酚分子结构与脱氯降解效率的关系,并初步探索光催化脱氯降解机制,为光催化技术应用于卤代酚类废水的脱卤去除提供理论依据.
1. 实验部分(Experimental section)
1.1 实验试剂
三氟甲烷磺酸(CF3SO3H,GR,TFMSA)和1,4-苯二甲腈(C8H4N2,AR)购自上海麦克林生化科技股份有限公司;三氯甲烷(CHCl3)、丙酮(CH3COCH3)、氯化锌(ZnCl2)、四氢呋喃(C4H8O)、氢氧化钠、盐酸等,购自西陇化工股份有限公司,均为AR; 4-氯酚(4-CP)、2,4-二氯酚(2,4-DCP)、2,4,6-三氯酚(2,4,6-TCP)、五氯酚(PCP)等,均为AR,购自上海化学试剂公司. 实验用水为去离子水.
1.2 CTF-1的合成
对文献[16]报道的方法稍作改进后合成片层状CTF-1,实验步骤如下:首先,将40 mL三氟甲烷磺酸(TFMSA)加到含30 mL 三氯甲烷的干燥圆底三口烧瓶中,0 ℃及氩气氛围下搅拌. 然后,将4.26 g 1,4-苯二甲腈加入含200 mL的三氯甲烷溶液中,超声分散均匀后缓慢滴加到上述溶液中,并保持温度在40 ℃下快速搅拌48 h. 反应结束后将冷却至室温的溶液加入到含660 mL去离子水和34 mL25%氨水中,室温搅拌2 h,将生成的淡黄色沉淀物通过真空过滤方式从悬浮液中分离出来,先后用去离子水、乙醇和三氯甲烷洗涤3遍,在70 ℃下真空干燥过夜得到前驱体pre-CTF-1. 将pre-CTF-1与ZnCl2以1:8.5的物质的量比混合并研磨均匀,紧接着在400 ℃的管式炉中,氩气氛围下反应10 min. 反应结束待温度降至室温后将样品用去离子水和0.1 mol·L−1的稀盐酸分别搅拌清洗24 h,并先后用丙酮、四氢呋喃过滤洗涤以去除未反应完的ZnCl2. 最后将样品在70 ℃下真空干燥12 h,得到棕色粉末即为CTF-1.
1.3 催化剂的表征
研究中利用扫描电子显微镜(SEM,S-300N,日本Hitachi)、X-射线衍射仪(XRD,D8 Advance X,德国Bruker)、傅里叶变换红外光谱仪(FTIR,VERTEX-70,德国Bruker)和固体核磁共振光谱仪(SSNMR,Avance Ⅱ 600MHz,德国Bruker)分别对催化剂进行形貌和结构表征;利用比表面积与孔隙度分析仪(BET,TriStar Ⅱ 3020,美国Micromeritics)对催化剂的比表面积及孔分布进行分析;利用X射线光电子能谱仪(XPS,Axis Ultra DLD,日本岛津)分析催化剂表面化学组分.
1.4 光催化降解实验
光催化降解实验以300 W 氙灯(PLS-SXE300C,λ > 420 nm,北京泊菲莱)作为模拟太阳光光源,光强为100 mW·cm−2. 取200 mL初始浓度为10 mg·L−1的氯酚污染物水溶液于配有循环冷凝水的双层烧杯中,因PCP在pH > 10(25 ℃)时溶解性较好,为保持实验的一致性,溶液初始pH均调节为10 ± 0.1,通入冷凝水保持反应温度为(25 ± 1)℃,加入0.02 g催化剂,黑暗条件下充分搅拌60 min以达吸附平衡. 接着,打开氙灯进行光催化反应,设定一定时间间隔收集2 mL反应悬浮液,并用0.22 μm的过滤器滤除催化剂后转入棕色液相小瓶以进行后续分析及测定. 所有实验重复3次后取平均值. 氯酚污染物的降解率计算公式如下[17]:
η=(C−60−Ct)/C−60 (1) 式中,η为氯酚污染物降解率(%),C-60为氯酚污染物的初始浓度(mg·L−1),Ct为反应t时间(min)后的浓度(mg·L−1).
1.5 活性物种分析实验
为探究不同活性物种对氯酚污染物降解的影响,在黑暗反应前向初始氯酚污染物水溶液(200 mL,10 mg·L−1)中加入不同的淬灭剂,然后再加入催化剂进行避光搅拌吸附,淬灭剂的加入量均为0.1 mmol,其余步骤同“1.4”节.
1.6 仪器分析方法
采用配有Agilent TC-C18色谱柱(150 mm × 4.6 mm,5 μm)的高效液相色谱仪(HPLC,LC-20AD,日本岛津)进行氯酚污染物的定量测定,检测器为紫外检测器. 流动相分别设置水相(纯水或稀释的乙酸、磷酸等)和有机相(甲醇或乙腈). 4种氯酚污染物检测方法如表1所示.
表 1 氯酚类污染物的液相检测方法Table 1. Liquid phase detection methods for chlorophenol pollutants污染物Pollutants 流动相Mobile phase (V:V) 流速/(mL·min−1)Flow rate 检测波长/nmDetection wavelength 柱温/℃Column temperature 4-CP 乙腈:水=50:50 1 280 35 2,4-DCP 甲醇:水=70:30 1 285 35 2,4,6-TCP 甲醇:水=70:30 1 290 35 PCP 甲醇:2%乙酸=80:20 0.8 300 35 采用离子色谱仪(EOC IC,瑞士万通)分析氯酚污染物降解过程中生成的氯离子(Cl-),检测条件如下:流动相为3.5 mmol·L−1 NaCO3和 3.0 mmol·L−1 NaHCO3的混合淋洗液,设总流速为1.0 mL·min-l,进样体积为20 μL,阴离子色谱柱型号为Metrosep A Supp 5(150 mm × 4.0 mm). 利用总有机碳分析仪(Multi N/C 2100S,德国Analytik Jena)对光催化反应前后溶液中总有机碳(TOC)含量进行测定.
2. 结果与讨论(Results and discussion)
2.1 CTF-1的结构及形貌分析
本文通过聚合法和熔盐法合成了结构有序的CTF-1,如图1a所示,并对CTF-1进行形貌和结构分析. 从图1b的SEM可以看出,CTF-1是由表面较平整的纳米片堆积而成的层状材料. 图1c的XRD衍射图显示CTF-1在7.4°、14.8°和25.8°处出现3个衍射峰,分别对应(1 0 0)、(2 0 0)和(0 0 1)晶面,其中(0 0 1)对应CTF-1的层间距,而(1 0 0)对应CTF-1的平面结构,这与文献报道一致[16],说明成功合成CTF-1材料. 由图1d的FTIR可知,1349.2 cm−1和1511.3 cm−1处的红外峰对应CTF-1三嗪环的特征峰,1671.6 cm−1和814.1 cm−1则归属于CTF-1苯环的特征峰[18]. 从图1e的固体核磁13C-NMR谱图观察到,CTF-1显示4个不同的13C特征信号峰,其中前3个分别归属于三嗪环中的碳原子(170 ppm)和桥联苯环中的2个碳原子(138 ppm和128 ppm),剩余的116 ppm处的峰则归属于末端未反应的腈基[16].
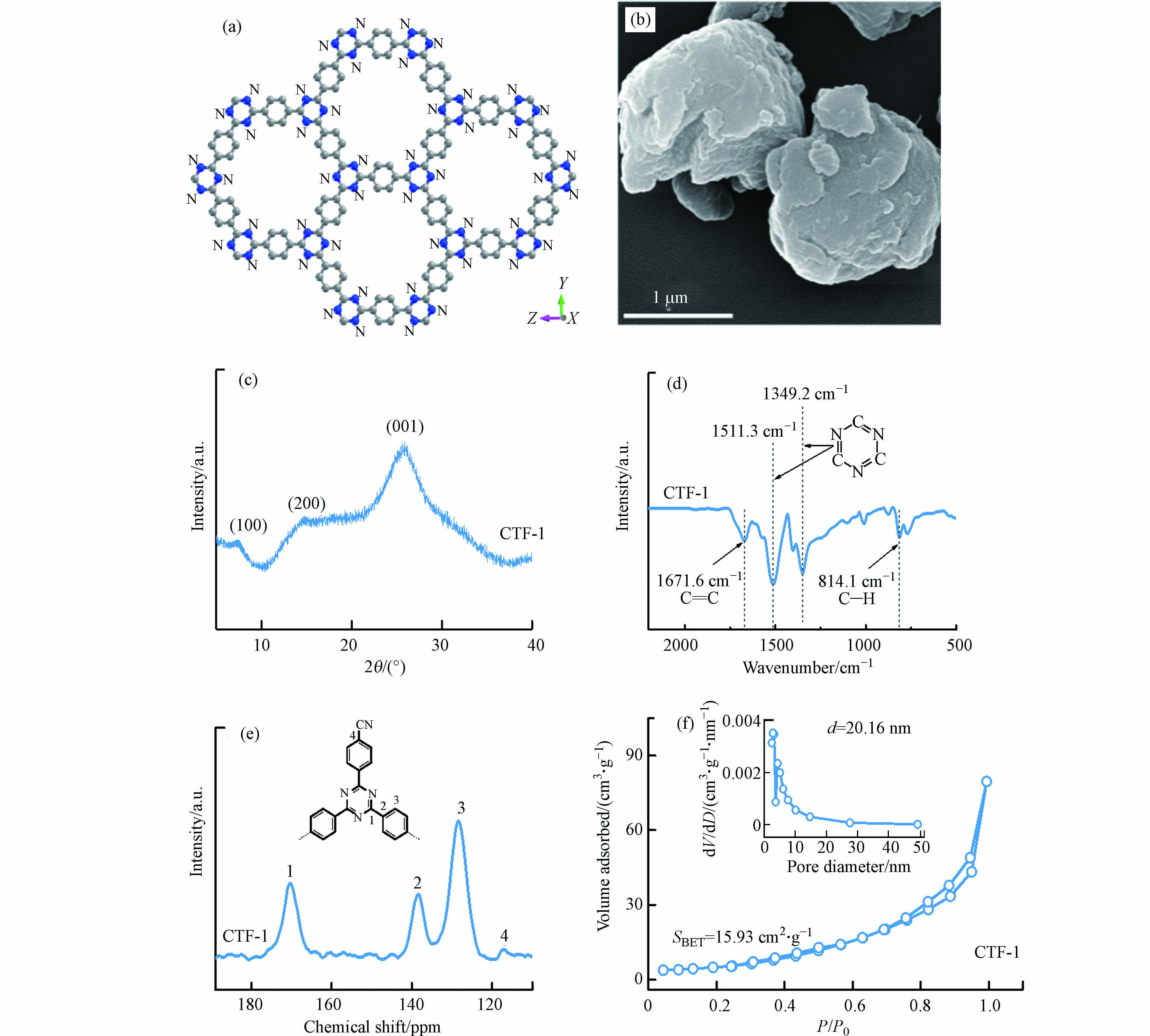 图 1 (a)CTF-1沿x轴方向的结构视图;CTF-1的SEM图(b)、XRD衍射图(c)、红外光谱图(d)、固体核磁13C NMR谱图(e)和N2吸附-脱附等温线图(插图:Barrett-Joyner-Halenda孔径分布)(f)Figure 1. (a) The view of the structure of CTF-1 along the x axis; (b) SEM image, (c) XRD pattern, (d) FTIR pattern, (f) Solid state MAS-13C-NMR spectra and N2 adsorption-desorption isotherm (inset: BJH pore size distribution) of CTF-1
图 1 (a)CTF-1沿x轴方向的结构视图;CTF-1的SEM图(b)、XRD衍射图(c)、红外光谱图(d)、固体核磁13C NMR谱图(e)和N2吸附-脱附等温线图(插图:Barrett-Joyner-Halenda孔径分布)(f)Figure 1. (a) The view of the structure of CTF-1 along the x axis; (b) SEM image, (c) XRD pattern, (d) FTIR pattern, (f) Solid state MAS-13C-NMR spectra and N2 adsorption-desorption isotherm (inset: BJH pore size distribution) of CTF-1由图1f的N2吸附-脱附等温线可知,CTF-1的比表面积为15.93 cm2·g−1,孔径为20.16 nm. 以上表征结果说明本文合成的CTF-1为三嗪环和苯环交替连接形成的且具有一定孔道结构的片层堆积材料.
2.2 光催化降解实验
2.2.1 氯酚类污染物降解效果
图2为CTF-1光催化降解氯酚污染物过程中氯酚含量随反应时间的变化曲线及氯酚污染物分别在光照条件和存在CTF-1的暗反应条件下的降解曲线图. 由图2可知,氯酚污染物在可见光照射下几乎不发生降解反应;随着CTF-1的引入,催化剂自身对氯酚类污染物存在吸附作用,避光反应60 min可达吸附平衡,且随着反应时间的延长,氯酚类污染物几乎不发生降解作用,证实CTF-1不会引发氯酚类污染物的降解;而当CTF-1与氯酚污染物达到吸附平衡后引入可见光,氯酚污染物发生降解反应,反应480 min后CTF-1对4-CP、2,4-DCP、2,4,6-TCP和PCP的降解率分别为49%、67%、89%和100%. 可以看出,随着氯原子取代数目的增多降解效率提高,相同条件下降解效率与氯原子数目呈正相关.
为了进一步了解CTF-1光催化降解氯酚污染物的降解效能,如图3a所示,利用准一级动力学模型对光催化降解反应进行拟合[17]:
 图 3 (a)可见光照射下(λ > 420 nm)CTF-1对4种氯酚类污染物的准一级动力曲线; (b)氯酚类污染物辛醇-水分配系数与表观速率常数的函数关系Figure 3. (a) Pseudo-first-order kinetics of four chlorophenols by CTF-1under visible light (λ > 420 nm) irradiation;(b) Relationship between octanol-water partition coefficient and apparent rate constant of chlorophenol pollutants
图 3 (a)可见光照射下(λ > 420 nm)CTF-1对4种氯酚类污染物的准一级动力曲线; (b)氯酚类污染物辛醇-水分配系数与表观速率常数的函数关系Figure 3. (a) Pseudo-first-order kinetics of four chlorophenols by CTF-1under visible light (λ > 420 nm) irradiation;(b) Relationship between octanol-water partition coefficient and apparent rate constant of chlorophenol pollutants−ln(C/C0)=kt (2) 式中,C0为避光搅拌60 min后达到吸附平衡时氯酚污染物的浓度(mg·L−1),C和k分别为光照反应时间t时(min)氯酚污染物的浓度(mg·L−1)和表观反应速率常数(min−1). 由图3a可知,PCP、2,4,6-TCP、2,4-DCP、4-CP的降解表观速率常数k差异较大,降解表观速率常数为:PCP(0.0159 min−1) > 2,4,6-TCP(0.0048 min−1) > 2,4-DCP(0.0021 min−1) > 4-CP(0.0011 min−1),这与降解速率结果一致,即氯原子数目越多的氯酚类污染物其表观速率常数越大.
为了进一步探究这种去除效果的差异性,本研究从催化材料本征的疏水性结构出发,解析污染物自身结构特性,以辛醇-水分配系数为恒量指标考察目标污染物的亲疏水性,通过调研文献可知4-CP、2,4-DCP、2,4,6-TCP、PCP的辛醇-水分配系数分别为2.35、3.08、3.69、5.01[19],即氯酚类污染物的疏水性与氯原子取代个数成正相关. 基于此,本研究构建了以氯酚类污染物的表观降解动力学常数与其辛醇-水分配系数的线性关系,如图3b所示,氯酚类污染物的表观速率常数与辛醇-水分配系数高度相关(P < 0.05),因此,初步推断CTF-1光催化降解氯酚类污染物的去除机制可能与氯酚类污染物自身亲疏水性相关.
2.2.2 氯酚类污染物脱氯效果
为了进一步研究CTF-1光催化降解氯酚类污染物的去除机制,本研究监测了CTF-1光催化降解氯酚类污染物反应过程中氯离子的释放. 如图4所示,在没有CTF-1催化剂或无可见光照射的情况下,未观察到氯原子释放;在CTF-1作为催化剂的光催化过程中,不同氯原子数目的氯酚类污染物其脱氯率各不相同,反应480 min后,CTF-1光催降解PCP、2,4,6-TCP、2,4-DCP、4-CP体系中其氯离子浓度分别为6.665 mg·L−1、4.803 mg·L−1、2.951 mg·L−1和1.393 mg·L−1. 通过物料衡算可得10 mg·L−1的PCP、2,4,6-TCP、2,4-DCP、4-CP中氯离子质量浓度分别为6.6657 mg·L−1、5.3937 mg·L−1、4.3558 mg·L−1和2.7613 mg·L−1.
如图5所示,将检测值与理论值作比后,发现CTF-1光催化降解PCP、2,4,6-TCP、2,4-DCP、4-CP的脱氯率分别为100 %、88.6 %、66.5 %和47.0 %. 这一结果与CTF-1光催化降解氯酚类污染物的降解动力学结果一致. 由此推测基于CTF-1构建的光催降解氯酚类污染物体系,其反应机制均是针对氯酚类污染物上取代氯位点进行精准脱氯的过程.
为了进一步测试氯酚污染物是否发生矿化生成CO2和水,对反应前后溶液中的TOC值进行测定. 从图6可知,4种氯酚污染物反应前后的TOC基本不变,体系不发生矿化. 同时,对4种氯酚污染物反应后的最终产物采用液相色谱-高分辨质谱法(LC-MS)进行测定,结果如图7所示. 由图7可知,在正离子模式质谱中检测到相应质子化产物[M+H]+峰,4-CP、DCP、TCP和PCP这4种氯酚反应产物的m/z分别为:111.03687、127.03172、143.02673和175.01683,分别对应:对羟基苯、1,2,4-三羟基苯、1,2,3,5-四羟基苯和六羟基苯. 以上结果说明构建的CTF-1光催化降解体系对氯酚污染物并未发生苯环的开环反应,而是针对氯酚污染物上氯位点作用,精准促进氯酚污染物脱氯,从而实现氯酚污染物的高效脱氯和脱毒.
2.3 光催化降解氯酚类污染物的机制
2.3.1 活性物种分析
CTF-1作为一种典型的光催化剂,常规意义上其对于污染物的降解均是通过可见光激发产生光生电子和空穴,继而由空穴氧化引发自由基链式反应对目标污染物进行降解[20]. 基于反应过程中可能存在的活性物种(h+、e-、·OH、·O2- 和1O2),以碘化钾(KI)作为h+的淬灭剂,溴酸钾(KBrO3)作为e-的淬灭剂,异丙醇(IPA)作为·OH的淬灭剂,超氧化物歧化酶(SOD)作为·O2-的淬灭剂,叠氮化钠(NaN3)作为1O2的淬灭剂[21 − 22],进行淬灭实验. 如图8所示,加入淬灭剂后几乎不影响氯酚类污染物的降解,证实了在CTF-1光催化体系中氯酚类污染物的脱氯不是由自由基与非自由基路径产生的活性物种所引发.
2.3.2 光催化降解机制
经过一系列分析实验,推测光催化降解氯酚污染物的机制与光催化体系中CTF-1与氯酚污染物的相互作用有关. 为此,进行X射线光电子能谱(XPS)分析以了解催化剂表面化学组分及元素存在的化学状态. 从图9a可以看出,CTF-1在吸附氯酚前后均存在C、N和O元素,其中O元素为暴露在环境中不可避免的吸附空气中的水分和氧气[18]. 此外,观察到当吸附氯酚类污染物后CTF-1上出现氯元素的峰,说明氯酚类污染物吸附到CTF-1上,且随着氯原子取代数目的增多,氯峰强度增加. 图9b中Cl 2p的XPS光谱显示吸附氯酚污染物后在约为200.5 eV和202.1 eV处出现2个峰,分别归属于氯酚污染物C—Cl键的Cl 2p1/2和Cl 2p3/2,说明氯酚污染物吸附在CTF-1上;同时,观察到Cl 2p1/2和Cl 2p3/2的结合能随氯原子取代数目的增加而降低,即氯酚污染物的C-Cl键上Cl原子的电子云密度增加,且逐渐趋向离子化状态(NaCl). 由此,推测CTF-1光催化降解氯酚类污染物的机制如图10所示,光激发CTF-1产生电子空穴对,CTF-1与氯酚类污染物之间的相互作用促进电子由CTF-1传输到氯酚污染物上,氯原子作为电子聚集中心吸引来自CTF-1的电子,电子云密度增加,连续光照下产生的电子不断流向氯原子,使C—Cl键上电子云密度持续增加并趋向于离子化状态,此时,在水溶剂的作用下发生C—Cl键的断裂,从而完成氯酚污染物的脱氯降解.
总之,氯酚类污染物的降解效率与CTF-1与氯酚类污染物的相互作用强度有关,而氯酚类污染物与CTF-1之间的相互作用除了氯酚类污染物的苯环与CTF-1的π-π相互作用及静电作用外,主要与疏水作用有关. 氯原子数目越多,氯酚类污染物的疏水性越强,与CTF-1的结合力越强,越有利于电子的传输,C—Cl键的电子云密度增加越大,脱氯降解效率越高. 由于光激发产生的电子主要传输到吸电子氯原子上,因此,反应体系是以改变Cl上电子云密度来实现氯酚类污染物的脱氯降解,是一个针对氯位点完成的反应.
3. 结论(Conclusion)
1)在可见光照射下,CTF-1对氯酚污染物进行光催化降解实验, 结果表明, CTF-1对氯原子数目最多的PCP的降解及脱氯效果最佳,且氯酚类污染物上氯原子数目与氯酚类污染物的降解效率、脱氯速率及表观速率常数k呈正相关,均遵循:PCP > 2,4,6-TCP > 2,4-DCP > 4-CP.
2)氯原子数目越多,氯酚类污染物的辛醇-水分配系数越大其疏水性越强,与CTF-1的作用力越强,对氯酚脱氯降解效果影响越大.
3)基于CTF-1构建的光催降解氯酚污染物体系,其反应机制是针对氯酚污染物上取代氯位点进行水解脱氯降解的过程,该工作对光催化降解卤代酚类污染物的脱卤降毒有一定的参考价值.
-
图 1 (a)CTF-1沿x轴方向的结构视图;CTF-1的SEM图(b)、XRD衍射图(c)、红外光谱图(d)、固体核磁13C NMR谱图(e)和N2吸附-脱附等温线图(插图:Barrett-Joyner-Halenda孔径分布)(f)
Figure 1. (a) The view of the structure of CTF-1 along the x axis; (b) SEM image, (c) XRD pattern, (d) FTIR pattern, (f) Solid state MAS-13C-NMR spectra and N2 adsorption-desorption isotherm (inset: BJH pore size distribution) of CTF-1
表 1 氯酚类污染物的液相检测方法
Table 1. Liquid phase detection methods for chlorophenol pollutants
污染物Pollutants 流动相Mobile phase (V:V) 流速/(mL·min−1)Flow rate 检测波长/nmDetection wavelength 柱温/℃Column temperature 4-CP 乙腈:水=50:50 1 280 35 2,4-DCP 甲醇:水=70:30 1 285 35 2,4,6-TCP 甲醇:水=70:30 1 290 35 PCP 甲醇:2%乙酸=80:20 0.8 300 35 -
[1] KEITH L, TELLIARD W. ES&T special report: Priority pollutants: I-a perspective view[J]. Environmental Science & Technology, 1979, 13(4): 416-423. [2] USEPA. Water quality criteria summary. Ecological risk assessment branch (WH-585) and human risk assessment branch (WH-550D) [R]. Washington DC, USA: Health and Ecological Criteria Division, 1991. [3] PAPAZI A, KARAMANLI M, KOTZABASIS K. Comparative biodegradation of all chlorinated phenols by the microalga Scenedesmus obliquus-the biodegradation strategy of microalgae[J]. Journal of Biotechnology, 2019, 296: 61-68. doi: 10.1016/j.jbiotec.2019.03.010 [4] PERA-TITUS M, GARCı́A-MOLINA V, BAÑOS M A, et al. Degradation of chlorophenols by means of advanced oxidation processes: A general review[J]. Applied Catalysis B: Environmental, 2004, 47(4): 219-256. doi: 10.1016/j.apcatb.2003.09.010 [5] AHLBORG U G, THUNBERG T M. Chlorinated phenols: Occurrence, toxicity, metabolism, and environmental impact[J]. Critical Reviews in Toxicology, 1980, 7(1): 1-35. doi: 10.3109/10408448009017934 [6] OLANIRAN A O, IGBINOSA E O. Chlorophenols and other related derivatives of environmental concern: Properties, distribution and microbial degradation processes[J]. Chemosphere, 2011, 83(10): 1297-1306. doi: 10.1016/j.chemosphere.2011.04.009 [7] de OLIVEIRA J C A, RODRIGUES P R M, de LUCENA S M P. Prediction of chlorophenols adsorption on activated carbons by representative pores method[J]. Environmental Science and Pollution Research, 2022, 29(53): 79866-79874. doi: 10.1007/s11356-022-18571-x [8] CHO Y C, HSU C C, LIN Y P. Integration of in situ chemical oxidation and permeable reactive barrier for the removal of chlorophenols by copper oxide activated peroxydisulfate[J]. Journal of Hazardous Materials, 2022, 432: 128726. doi: 10.1016/j.jhazmat.2022.128726 [9] SARAVANAN A, KUMAR P S, VO D V N, et al. Photocatalysis for removal of environmental pollutants and fuel production: A review[J]. Environmental Chemistry Letters, 2021, 19(1): 441-463. doi: 10.1007/s10311-020-01077-8 [10] ZADA A, KHAN M, KHAN M A, et al. Review on the hazardous applications and photodegradation mechanisms of chlorophenols over different photocatalysts[J]. Environmental Research, 2021, 195: 110742. doi: 10.1016/j.envres.2021.110742 [11] CHEN X Q, BAI C H, LI Z L, et al. Directional bioelectrochemical dechlorination of trichloroethene to valuable ethylene by introduction poly-3-hydroxybutyrate as a slow release carbon source[J]. Chemical Engineering Journal, 2023, 455: 140737. doi: 10.1016/j.cej.2022.140737 [12] SHEN Y, ZHU C, SONG S, et al. Defect-abundant covalent triazine frameworks as sunlight-driven self-cleaning adsorbents for volatile aromatic pollutants in water[J]. Environmental Science & Technology, 2019, 53(15): 9091-9101. [13] QIAN Z F, WANG Z J, ZHANG K A I. Covalent triazine frameworks as emerging heterogeneous photocatalysts[J]. Chemistry of Materials, 2021, 33(6): 1909-1926. doi: 10.1021/acs.chemmater.0c04348 [14] SUN M, HAN S, FENG J J, et al. Recent advances of triazine-based materials for adsorbent based extraction techniques[J]. Topics in Current Chemistry, 2021, 379(4): 24. doi: 10.1007/s41061-021-00336-8 [15] ZENG T, JIN S J, LI S Q, et al. Covalent triazine frameworks with defective accumulation sites: Exceptionally modulated electronic structure for solar-driven oxidative activation of peroxymonosulfate[J]. Environmental Science & Technology, 2022, 56(13): 9474-9485. [16] KUECKEN S, ACHARJYA A, ZHI L J, et al. Fast tuning of covalent triazine frameworks for photocatalytic hydrogen evolution[J]. Chemical Communications, 2017, 53(43): 5854-5857. doi: 10.1039/C7CC01827D [17] ZHU C, FANG Q L, LIU R L, et al. Insights into the crucial role of electron and spin structures in heteroatom-doped covalent triazine frameworks for removing organic micropollutants[J]. Environmental Science & Technology, 2022, 56(10): 6699-6709. [18] HUANG L M, WANG D K, ZENG H H, et al. Synergistically interactive P-Co-N bonding states in cobalt phosphide-decorated covalent organic frameworks for enhanced photocatalytic hydrogen evolution[J]. Nanoscale, 2022, 14(48): 18209-18216. doi: 10.1039/D2NR05076E [19] GARBA Z N, ZHOU W M, LAWAN I, et al. An overview of chlorophenols as contaminants and their removal from wastewater by adsorption: A review[J]. Journal of Environmental Management, 2019, 241: 59-75. [20] SAPUTRA E, PRAWIRANEGARA B A, SUGESTI H, et al. Covalent triazine framework: Water treatment application[J]. Journal of Water Process Engineering, 2022, 48: 102874. doi: 10.1016/j.jwpe.2022.102874 [21] 李鸿渐, 季秋忆, 朱诺亚, 等. 可见光下竹叶生物炭掺杂BiOBrxCl1-x光催化降解罗丹明B[J]. 环境化学, 2022, 41(10): 3390-3398. doi: 10.7524/j.issn.0254-6108.2021060801 LI H J, JI Q Y, ZHU N Y, et al. Photocatalytic degradation of rhodamine B by bamboo leaf biochar doped with BiOBrxCl1-x under visible light[J]. Environmental Chemistry, 2022, 41(10): 3390-3398 (in Chinese). doi: 10.7524/j.issn.0254-6108.2021060801
[22] CABEZUELO O, MARTINEZ-HAYA R, MONTES N, et al. Heterogeneous riboflavin-based photocatalyst for pollutant oxidation through electron transfer processes[J]. Applied Catalysis B: Environmental, 2021, 298: 120497. doi: 10.1016/j.apcatb.2021.120497 -




 DownLoad:
DownLoad: