-
铬金属是我国《重金属污染综合防治“十二五”规划》重点防控的重金属之一,在自然界中铬主要以三价铬Cr(Ⅲ)和六价铬Cr(Ⅵ)的形式存在. 三价铬Cr(Ⅲ)稳定、低毒,是动植物必需的微量元素之一,但是六价铬Cr(Ⅵ)具有致癌性和致畸性,可以通过食物链蓄积放大,引起人体多器官功能衰竭、坏死[1 − 3]. 根据研究报道,铬污染在世界十大最具毒性的污染问题中排名第三,根据我国《生活饮用水卫生标准》,饮用水中Cr(Ⅵ)的最大可接受限值为0.05 mg L−1 [4]. 目前常用的修复水中铬污染的方法主要有离子交换、过滤、电化学沉淀、活性炭吸附、生物修复、膜分离等,这些传统的去除方法效果差,成本较高、复杂,修复周期长[5 − 6],而纳米零价铁(nZVI)因其独特的物理化学性质、无毒和经济性,被认为是能够有效进行Cr(Ⅵ)治理和修复的材料[7 − 8]. 纳米零价铁是一种粒径在1—100 nm的颗粒,具有比表面积大、吸附性和还原性强等特点,可用于环境中的Cr(Ⅵ)污染的治理和修复[9 − 11].
nZVI作为一种广被研究和使用的环境纳米材料,早期研究集中在其性能及应用方面,主要进行溶液反应动力学、去除负荷、简单固相表征等研究,而对nZVI使用过程中的转化和最终归趋等科学问题尚未解决[12 − 13]. 研究纳米零价铁颗粒在水环境中的结构性质演变将有助了解nZVI去除重金属中的效能和环境归趋[14]. 基于此,本课题组前期研究了纳米零价铁在Cr(Ⅵ)水相中的结构性能演变,发现初始的溶液pH、Cr(Ⅵ)浓度、反应时间等均对其结构性能演变产生影响[15]. 研究复杂环境条件下nZVI在Cr(Ⅵ)水相中的晶相结构演变,同时探究其对其它污染物去除效能的影响,对于预测去除重金属之后的反应产物在环境中的赋存状态、最终迁移归趋等具有重要的意义.
本文主要研究了nZVI与不同浓度Cr(Ⅵ)(0—100 mg∙L−1)在不同环境条件下的反应特性,探究初始pH(2、3、4、5、7、9、11)、无机阴离子(Cl−、CO32−、SO42−、NO3−)、重金属离子(Co2+、Cd2+、Ni2+、Cu2+)共存条件下的Cr(Ⅵ)对nZVI晶相转化的影响. 采用电感耦合等离子体发射光谱仪(ICP-OES)跟踪反应中重金属离子浓度变化,X射线衍射仪(XRD)研究纳米零价铁在复杂环境条件中的晶相结构变化,扫描电子显微镜(SEM)、透射电子显微镜(TEM)、高分辨透射电镜的晶格衍射条纹、选区花样衍射对反应产物结构形貌、物相进行表征. 该研究为nZVI及其产物在环境中的迁移、转化、归趋等提供实验数据和理论支撑.
-
主要试剂:硼氢化钠(NaBH4,分析纯)购自美国西格玛奥德里奇有限公司,六水合氯化铁(FeCl3·6H2O,分析纯)、无水乙醇(C2H5OH,分析纯)、重铬酸钾(K2Cr2O7,优级纯)、氢氧化钠(NaOH,分析纯)、盐酸(HCl,分析纯)、微米零价铁(mZVI,分析纯)、纳米二氧化(nTiO2,分析纯)、聚合硫酸铁(PFS,分析纯)均购于中国国药控股股份有限公司.
主要仪器:pH/ORP计(PHSJ-4A,上海仪电科学仪器股份有限公司);电子天平(MS 105DU,梅特勒-托利多集团);真空干燥箱(DZF-6020,上海精宏实验设备有限公司);蠕动泵(YZ1515x,保定兰格恒流泵有限公司);电动搅拌机(D2004W,上海司乐仪器有限公司).
-
采用经典液相还原法[16]将NaBH4 (0.2 mol·L−1)逐滴加至FeCl3 (0.05 mol·L−1)溶液中制备得到nZVI,滴加完后继续搅拌20 min,整个过程均通氮气,其反应方程式为:
新鲜制备得到的nZVI使用去离子水和无水乙醇各进行多次洗涤,之后低温(4℃)保存于无水乙醇中并测量固含量以备使用.
-
纳米零价铁(nZVI)、微米零价铁(mZVI)、纳米二氧化钛(nTiO2)、聚合硫酸铁(PFS)4种材料投加到模拟电镀废水中(38.4 mg∙L−1)中反应2 h后,测定溶液中剩余铬的含量.
初始浓度为50、100、200 mg∙L−1 Cr(Ⅵ)溶液中,分别投加0.5、1、2 g·L−1的nZVI,搅拌、定时取样,反应完成后固液分离,固相物质保存在酒精中保存待测.
1 g∙L−1 nZVI投加到Cr(Ⅵ)浓度分别为10、20、50、100 mg∙L−1的Cr(Ⅵ)溶液,控制溶液初始pH为2—11,搅拌速率为310 r∙min−1,反应至2、6 h时测量溶液中铬的剩余浓度,并分离出固体保存. 上述方法获得的nZVI与新鲜的nZVI用于去除浓度均为100 mg·L−1的Cd2+、Cu2+废液,控制nZVI的投加量均为1 g·L−1,分别反应1、6 h后,收集水样检测溶液中Cd2+、Cu2+含量.
配置10 mg·L−1 Co2+、Cd2+、Ni2+、Cu2+与10、20、50、100 mg·L−1 Cr(Ⅵ)的双金属溶液及20、50、200 mg·L−1 SO42−、NO3−、Cl−、CO32−与20 mg·L−1 Cr(Ⅵ)的溶液,在500 mL烧杯中加入250 mL的混合溶液,投加1 g∙L−1的nZVI,控制pH为5左右,搅拌速率为310 r∙min−1,反应2 h后,取样分析.
-
采用电感耦合等离子体发射光谱仪(ICP-OES,Agilent 720ES)对水样中重金属含量进行测量. 将固体样品使用真空干燥箱(DZF-6020, 上海精宏实验设备有限公司)烘干后,采用扫描电子显微镜(SEM,TM3000,日本日立高新公司)、透射显微镜(TEM,EM-2100F,日本岛津公司)进行形貌结构分析.
X射线衍射仪(XRD, Bruker D8 Advance)对纳米零价铁的晶相结构进行分析,采用Cu Kα射线,LiF单色仪,测试电流在40 kV和40 mA条件下进行,扫描角度(2θ)为10°到90°,扫描速度10(°)·min−1.
-
为了比较不同水处理材料的去除效能,研究了nZVI、mZVI、nTiO2、PFS对于Cr(Ⅵ)的去除效率. 水处理材料的投加量分别为1、4、10 g·L−1,从图1a可以看出,nZVI具有比其他材料更高的去除效率,分别为88.0%、95.8%、98.4%,而mZVI对铬的去除率仅在10%—15%之间,其它两种材料去除效率更低. 因此nZVI是良好的重金属Cr(Ⅵ)的去除材料.
改变nZVI投加量对铬去除效率的研究表明(图1b),当投加量为2 g·L−1,反应10 min,溶液中的Cr(Ⅵ)完全去除,而投加量为1 g·L−1的nZVI完全去除Cr(Ⅵ)需要20 min,0.5 g·L−1的nZVI在20 min对Cr(Ⅵ)的去除率达到80%以上. 选择1 g·L−1 nZVI为投加量进行后续实验.
探究不同的Cr(Ⅵ)初始浓度对其去除效果的影响,Cr(Ⅵ)的初始浓度分别为50、100、200 mg·L−1,如图1c. 铬的初始浓度为50、100 mg·L−1时,在200 min时nZVI对铬的去除率接近100%,而铬初始浓度为200 mg·L−1时,去除效率只能达到60%左右. 铬的初始浓度越低,反应速率越快,去除效率越高. 这可能是由于纳米零价铁修复铬的过程一般在铁表面进行,主要以吸附还原为主,暴露在表面的nZVI被氧化成Fe2+和Fe3+,再进一步发生水解反应形成钝化膜,铬浓度较低时,形成的钝化膜不足以覆盖整个铁表面,反而形成原电池促进电子转移,当铬浓度较高时,铁表面形成的钝化层,阻止了nZVI与Cr6+之间的电子传递,导致反应速率降低[17].
图1d探究了溶液初始pH(2、3、4、5、7、9、11)对铬去除效果的影响,1 g·L−1 nZVI在pH=2下与100 mg·L−1 Cr(Ⅵ)液反应2 h,对Cr(Ⅵ)的去除率为57.9%,在pH=5时去除率为18.5%,故随着pH值逐渐变大,对铬的去除率逐渐下降. 这可能是因为在酸性条件下,有利于Fe(0)的溶解,nZVI具有更强的释放电子能力,电子易与Cr(Ⅵ)结合生成Cr(Ⅲ);在碱性条件下,nZVI表面的Fe2+/Fe3+易与OH−形成铁的氢氧化物阻止反应进行. 当Cr(Ⅵ)液的浓度为20、50 mg·L−1,pH在2—5之间时,nZVI对铬的去除效率没有明显变化,而pH>5时,去除效率开始明显下降,故本研究选择在pH=5进行相关实验研究.
-
图2为不同溶液初始pH的条件下nZVI与20 mg·L−1 Cr反应2 h后固体产物的XRD谱图. 当pH<5时,只能观察到γ-FeOOH的衍射峰. 当pH=5时,可以观察到γ-FeOOH和Fe(0)的衍射峰,铁氧化有所减缓. 随着pH逐渐升高,Fe(0)的峰开始出现. 在pH>7时,只能观察到Fe(0)的峰,表明酸性Cr溶液促进nZVI的腐蚀,主要腐蚀产物为γ-FeOOH[18].
在20 mg·L−1的铬液中nZVI反应2 h后样品的TEM图如图3. 从图3可以看出,不同的pH下,结构形貌表现出较大的差异:新鲜nZVI呈链球状,而不同pH条件下氧化后产物出现片状和针状结构. pH=3时,Cr-nZVI中有少量链球状结构存在. 随着pH升高,不规则的链球状结构逐渐增多,球型结构由边界模糊逐渐变得分明,根据文献知悉成分是铁氧化物和CrxFe1-x(OH)3 [19 − 20]. 根据XRD分析(图2),pH>7时,与Cr(Ⅵ)反应后Cr-nZVI主要是Fe(0)的形式.
nZVI的等电位点为8.2,当溶液pH小于7时,nZVI表面带正电荷[8],有利于吸附带有负电荷的Cr(Ⅵ);此外H+可以促进HCrO4−和CrO42−还原为Cr3+,且pH较低时,铁腐蚀会产生大量H+和Fe2+,可进一步促进Cr(Ⅵ)还原[21 − 22],如反应方程式(2—7). 当溶液由酸性变为碱性时,nZVI会在铁的表面生成铁氧化物或铁络合物,阻止电子传递,导致铁氧化速率下降[23].
-
为探究溶液中共存重金属对nZVI反应后晶相演变的影响,实验选取Co2+、Cd2+、Ni2+、Cu2+等4种重金属离子,研究nZVI在双金属(铬:20 mg·L−1,重金属:10 mg·L−1)体系中反应2 h的固体产物的晶相演变,XRD如图4a. 可以看出nZVI在单一铬金属中反应2 h后(pH=5),能够观察到Fe(0)和γ-FeOOH的衍射峰. 当含铬溶液中含有Cd、Co金属时,nZVI完全转化为纤铁矿;而Cr溶液中共存Ni和Cu金属时,nZVI晶相逐渐中转化为纤铁矿和针铁矿混合物. 同时,在含Cu2+的溶液中,能够观察到氧化亚铜晶相物质存在. nZVI在含铬-重金属溶液中反应2 h后表面形貌如图5所示,在未添加其它4种重金属离子时,与铬反应后固相产物主要呈球状、片状、针状结构,添加重金属后,固相产物链球状结构明显减少,片状和针状结构显著增多,重金属的存在对nZVI自身结构演变有巨大影响.
纳米零价铁具有类金属或类配体的配位性质,这与溶液的化学性质有关. 当溶液pH低于纳米零价铁的等电点(pHzpc=8.2)时[8],铁表面带正电荷,会吸引配体(如磷酸盐),当溶液pH高于纳米零价铁的等电点时,铁表面带负电荷,能与阳离子(如金属离子)形成表面络合物. 从经典的电化学来看,铁可以作为电子供体或还原剂,还原和沉淀活性较低的金属离子,铁表面结构也随之发生变化. Fe(Ⅱ) (−0.44 V)的标准电势低于镉Cd(Ⅱ) (−0.40 V)、Co(Ⅱ) (−0.28 V)、Cu(Ⅱ) (+0.34 V)、Ni(Ⅱ) (−0.24 V),因此这些重金属很容易被nZVI还原,如图4 b,nZVI与重金属之间形成了原电池腐蚀,nZVI表面氧化层能促进重金属吸附到nZVI表面,铁作为阳极,发生氧化反应失去电子,表面被腐蚀,重金属氧化还原电位较高,作为阴极,发生还原反应得到电子,最终形成铁氢氧化物沉淀或者重金属-铁络合物,铁晶相结构发生改变.
Cr-nZVI在不同重金属离子中的晶相转化与其氧化还原电位有关,不同重金属促进nZVI晶相转化的原理有差异. 由于Cu(Ⅱ) (+0.34 V)的标准还原电位远高于Fe(Ⅱ) (-0.44 V),在酸性条件下,能与铁形成原电池,铁作为正极发生强烈腐蚀,结构发生改变. 由于铜离子比较少,从而使得氧化亚铜的生成. nZVI与铜离子反应,会将铜离子还原成Cu和Cu2O [24 − 25],反应方程式如下:
如图4,加入铜离子后nZVI反应产物由γ-FeOOH、α-FeOOH、Cu2O和混合相,由于针铁矿的热稳定性更高,γ-FeOOH可能在Fe2+的作用下转化为α-FeOOH[19, 26].
由于Ni(Ⅱ) (-0.24 V)的标准还原电位略高于Fe(Ⅱ) (-0.44 V),因此nZVI对Ni(Ⅱ)的去除主要是通过还原和吸附的方式实现. 在反应初始阶段,Ni(Ⅱ)通过物理吸附被吸引到铁表面,通过化学吸附强烈结合,与nZVI表面的OH−形成微溶物,通过不断消耗nZVI氧化形成的OH−,促进nZVI表面腐蚀转化为铁(氢)氧化物,金属离子逐渐还原为金属镍[27],反应方程式如下:
Co((Ⅱ) (-0.28 V)的标准还原电位略高于Fe(Ⅱ) (-0.44 V),反应机制和Ni(Ⅱ)相似. 由于Cd(Ⅱ) (-0.40 V)的氧化还原电位与Fe(Ⅱ) (-0.44 V)接近,去除Cd的反应机制不涉及还原反应,主要通过吸附或形成表面配合物去除. Cd(Ⅱ)吸附到nZVI表面,与nZVI氧化产生的OH−形成Cd(OH)2沉淀,促进nZVI不断腐蚀氧化形成铁(氢)氧化物[28 − 29].
-
天然水体中往往存在Cl−、CO32−、SO42−、NO3−等离子,研究共存阴离子的影响对于nZVI应用于天然水体中铬污染修复具有重要意义. 本实验研究nZVI在浓度为(10、20、50、100 mg·L−1) Cr(Ⅵ)溶液中,同时共存阴离子浓度分别为20、50、200 mg·L−1,反应2 h的固相演变. nZVI在铬(10 mg∙L−1)与阴离子(50、200 mg∙L−1)反应2 h后物相图如图6a、b所示,4种阴离子对nZVI的晶相转化均有抑制作用,可以观察到Fe(0)和其它铁(氢)氧化物的衍射峰,其中CO32−抑制作用最明显,只能观察到Fe(0)的衍射峰. 4种阴离子起钝化作用,可能是形成的铁氧化物在沉降到nZVI时,会带入吸附的阴离子,从而阻塞了nZVI的还原位点,降低了nZVI的反应活性[30].
铬(20 mg∙L−1)与阴离子(20 mg∙L−1)反应2 h时(图6c),除CO32−外,其它3种离子的加入都在一定程度上促进Fe(0)转化为γ-FeOOH和γ-Fe2O3/Fe3O4,Fe(0)衍射峰减弱,能明显地观察到γ-FeOOH和 γ-Fe2O3/Fe3O4的衍射峰. 加入SO42−反应2 h后碱性增强,SO42−的存在可能破坏保护nZVI表面氧化膜,置换nZVI表面的OH−,与铁离子形成单配位基和双配位基复合物,从而加速铁的腐蚀[31 − 32]. NO3−存在下的溶液反应后pH为6.85,呈弱酸性,NO3−能被纳米零价铁还原(E0(NO3−/NO2−)=0.01 V),从而促进纳米零价铁的腐蚀,并对表面起到一定的剥蚀作用[33]. 当阴离子为50 mg∙L−1时(图6d),共存离子为Cl−和CO32−时,只发现Fe(0)的电子衍射峰,由于阴离子不存在的条件下产生γ-FeOOH衍射峰,说明此时共存Cl−和CO32−对nZVI的氧化有明显抑制作用,Cl−对nZVI晶相转化的影响与浓度有关,低浓度促进nZVI 腐蚀,高浓度起钝化作用.
当Cr≥50 mg∙L−1时,只能观察到Fe(0)的衍射峰,高浓度Cr抑制nZVI发生晶相转化,如图6e、f,根据Huang等研究,Cr(Ⅵ)浓度较低时(≤20 mg∙L−1),nZVI腐蚀速率加快,Cr(Ⅵ)浓度较高(Cr≥50 mg∙L−1)时,零价铁表面形成CrxFe1-xOOH或CrxFe1-x(OH)3钝化层,抑制nZVI腐蚀,因此铬浓度较高(Cr≥50 mg∙L−1)时,4种阴离子对nZVI的腐蚀及晶相转化作用不明显[34].
用透射电镜对nZVI在含有200 mg∙L−1 CO32−和Cl−的铬液(Cr: 10 mg∙L−1)反应2 h前后的固相产物进行了详细研究,如图7. 新鲜的纳米零价铁(图7a)呈链球状,核壳结构明显,纳米零价铁在不含阴离子的铬液(10 mg∙L−1)反应2 h后的形貌如图7b所示,呈链球状、片状和针状结构,结合图6a XRD,主要为Fe(0)和γ-FeOOH和γ-Fe2O3/Fe3O4. 在Cl−存在下反应后固体的低分辨率透射电镜图谱中观察到片层状结构(图7c),高分辨率的电子显微镜显示了晶格条纹间距为0.2501、0.2467、0.2059 nm(图7d),分别对应于Fe2O3的(110)面、γ-FeOOH的(130)面、Fe(110)面. 对在Cl−存在下反应后的固体进行选区电子衍射(图7e),可以观察到γ-FeOOH(040)晶面、Fe2O3(121)和(113)面、Fe(200)晶面、Fe3O4(554)晶面.
结合图6a、b,Cl−能够抑制Fe(0)转化为铁氧化物. 当铬浓度为20 mg∙L−1时,Cl−为20 mg∙L−1时能促进Fe(0)转化为γ-FeOOH和γ-Fe2O3/Fe3O4,Cl−促进nZVI发生腐蚀而发生晶相转化,通常有两个原因,一是Cl−能够击穿nZVI表面氧化膜,促进内部铁提供电子能力,进一步发生腐蚀[35];二是Cl−能促进铁的局部腐蚀,形成不规则的坑形,而铁表面的坑形提供了新的反应位点,促进nZVI氧化[36]. 而当铬浓度为20 mg∙L−1时,Cl−为50 mg∙L−1时能抑制Fe(0)转化为其它铁(氢)氧化物,如图6d,这可能是Cl−竞争性结合nZVI的表面位点,抑制nZVI发生氧化[37]. 因此Cl−对nZVI的腐蚀作用及晶相转化与浓度有关.
在CO32−存在下反应后固体的低分辨透射电镜图谱中观察到链球状结构(图7f),在高分辨率的图谱中观察到明显的核壳结构,选区电子衍射(图7e)可以观察到Fe(110)晶面,说明此时nZVI的晶体结构仍未发生转化,CO32−对nZVI的氧化有很强的抑制作用. 加入CO32−后,反应2 h后测得溶液pH达到9.6,这可能是CO32−使溶液pH升高,OH−与Fe2+/Fe3+形成了铁的氢氧化物附着在nZVI表面,形成钝化膜,CO32−也会在铁氧化物表面形成竞争吸附位点,抑制nZVI发生腐蚀[38]. 有关共存阴离子对于晶相结构转化的影响机理将在以后的工作中进一步的阐明.
-
(1) nZVI的投加量、铬的初始浓度、溶液初始pH对铬的去除效率有较大影响. 随着nZVI投加量的提高,对铬的去除率也提高;随着铬初始浓度的增加和溶液初始pH的增加,对铬的去除率逐渐下降. 对于不同去除重金属的材料(nZVI、mZVI、nTiO2、PFS),nZVI是最佳去铬的实验材料.
(2) pH对nZVI的结构演变影响很大. 不同pH条件下,nZVI氧化程度不同,酸性越强,对铬的去除率越高但nZVI的腐蚀程度也较大,主要转化为铁的氧化物和氢氧化物.
(3) 共存重金属离子影响Cr-nZVI的结构演变. 4种重金属离子(Co2+、Cd2+、Ni2+、Cu2+)均能够加速nZVI腐蚀,在铬浓度为20 mg∙L−1时,加入Co2+和Cd2+,Fe(0)转化为γ-FeOOH,加入Ni2+、Cu2+后,产生新的α-FeOOH.
(4) nZVI的晶相演变同时受到Cr离子的浓度和阴离子的浓度的影响. Cr浓度为10 mg∙L−1时,4种阴离子抑制nZVI氧化,Cr浓度为20 mg∙L−1时,共存的SO42−、NO3−促进Fe(0)转化为γ-FeOOH和γ-Fe2O3/Fe3O4,CO32−抑制nZVI发生氧化,低浓度(20 mg∙L−1)的Cl−促进nZVI氧化,高浓度(200 mg∙L−1)Cl−抑制nZVI氧化为γ-FeOOH和γ-Fe2O3、Fe3O4. CO32−对nZVI的腐蚀有极强的抑制作用,高浓度的Cr抑制nZVI发生晶相转化.
共存离子对纳米铁在Cr(Ⅵ)溶液中晶相转化的影响
Effect of coexisting ions on crystal phase transformation of nanoscale zero-valent iron in chromium-containing solutions
-
摘要: 纳米零价铁(nZVI)与水中六价铬[Cr(Ⅵ)]进行反应时,其本身的结构也发生变化,从而影响其效能和性质. 本文主要探究Cr(Ⅵ)溶液中共存重金属离子(Co2+、Cd2+、Ni2+、Cu2+)和阴离子(Cl−、CO32−、NO3−、SO42−)对nZVI晶相转化的影响. 研究发现,共存的重金属离子均能够促进nZVI氧化,促进nZVI转化为纤铁矿(γ-FeOOH)和针铁矿(α-FeOOH). 共存的阴离子对nZVI的晶相影响与铬和阴离子浓度有关. 当Cr为10 mg∙L−1时,4种阴离子均抑制nZVI的氧化作用;当Cr为20 mg∙L−1时,NO3−、SO42−促进nZVI氧化,CO32−抑制nZVI氧化,低浓度Cl−(20 mg∙L−1)促进nZVI晶相转化,高浓度Cl−(200 mg∙L−1)抑制Fe(0)转化为γ-FeOOH和γ-Fe2O3、Fe3O4的混合物;当Cr≥50 mg∙L−1时,nZVI氧化受到抑制,此时4种阴离子没有明显作用. 该研究对于探讨复杂环境条件下nZVI结构与去除效能之间的关系具有重要意义.Abstract: When nanoscale zero-valent iron (nZVI) reacts with hexavalent chromium [Cr(Ⅵ)] in water, its own structure also changes, which affects its efficacy and properties. In this paper, the effect of coexistence of heavy metal ions (Co2+, Cd2+, Ni2+, Cu2+) and anions (Cl−, CO32−, NO3−, SO42−) in Cr(Ⅵ) solution on the crystal phase transformation of nZVI was mainly investigated. Results showed that the coexistence of heavy metal ions can promote the oxidation of nZVI and promote the compositions of nZVI to lepidocrocite (γ-FeOOH) and goethite (α-FeOOH). The effect of coexisting anions on the crystal phase of nZVI is related to concentration of chromium and anions. When Cr was 10 mg∙L−1, the four anions all inhibited the oxidation of nZVI; when Cr was 20 mg∙L−1, NO3−, SO42− promoted the oxidation of nZVI, CO32− inhibited the oxidation of nZVI, low concentration of Cl−(20 mg∙L−1) promotes the crystal phase transformation of nZVI, high concentration of Cl−(200 mg∙L−1) inhibits the transformation of Fe(0) into the mixture of γ-FeOOH and maghemite/ magnetite(γ-Fe2O3/Fe3O4). When Cr(VI)≥50 mg∙L−1, the oxidation of nZVI is inhibited, and the four anions have no obvious effect at this time. This study had important significance for studying the relationship between the structure and removal efficiency of nZVI under complex environmental systems.
-
铬金属是我国《重金属污染综合防治“十二五”规划》重点防控的重金属之一,在自然界中铬主要以三价铬Cr(Ⅲ)和六价铬Cr(Ⅵ)的形式存在. 三价铬Cr(Ⅲ)稳定、低毒,是动植物必需的微量元素之一,但是六价铬Cr(Ⅵ)具有致癌性和致畸性,可以通过食物链蓄积放大,引起人体多器官功能衰竭、坏死[1 − 3]. 根据研究报道,铬污染在世界十大最具毒性的污染问题中排名第三,根据我国《生活饮用水卫生标准》,饮用水中Cr(Ⅵ)的最大可接受限值为0.05 mg L−1 [4]. 目前常用的修复水中铬污染的方法主要有离子交换、过滤、电化学沉淀、活性炭吸附、生物修复、膜分离等,这些传统的去除方法效果差,成本较高、复杂,修复周期长[5 − 6],而纳米零价铁(nZVI)因其独特的物理化学性质、无毒和经济性,被认为是能够有效进行Cr(Ⅵ)治理和修复的材料[7 − 8]. 纳米零价铁是一种粒径在1—100 nm的颗粒,具有比表面积大、吸附性和还原性强等特点,可用于环境中的Cr(Ⅵ)污染的治理和修复[9 − 11].
nZVI作为一种广被研究和使用的环境纳米材料,早期研究集中在其性能及应用方面,主要进行溶液反应动力学、去除负荷、简单固相表征等研究,而对nZVI使用过程中的转化和最终归趋等科学问题尚未解决[12 − 13]. 研究纳米零价铁颗粒在水环境中的结构性质演变将有助了解nZVI去除重金属中的效能和环境归趋[14]. 基于此,本课题组前期研究了纳米零价铁在Cr(Ⅵ)水相中的结构性能演变,发现初始的溶液pH、Cr(Ⅵ)浓度、反应时间等均对其结构性能演变产生影响[15]. 研究复杂环境条件下nZVI在Cr(Ⅵ)水相中的晶相结构演变,同时探究其对其它污染物去除效能的影响,对于预测去除重金属之后的反应产物在环境中的赋存状态、最终迁移归趋等具有重要的意义.
本文主要研究了nZVI与不同浓度Cr(Ⅵ)(0—100 mg∙L−1)在不同环境条件下的反应特性,探究初始pH(2、3、4、5、7、9、11)、无机阴离子(Cl−、CO32−、SO42−、NO3−)、重金属离子(Co2+、Cd2+、Ni2+、Cu2+)共存条件下的Cr(Ⅵ)对nZVI晶相转化的影响. 采用电感耦合等离子体发射光谱仪(ICP-OES)跟踪反应中重金属离子浓度变化,X射线衍射仪(XRD)研究纳米零价铁在复杂环境条件中的晶相结构变化,扫描电子显微镜(SEM)、透射电子显微镜(TEM)、高分辨透射电镜的晶格衍射条纹、选区花样衍射对反应产物结构形貌、物相进行表征. 该研究为nZVI及其产物在环境中的迁移、转化、归趋等提供实验数据和理论支撑.
1. 材料与方法 (Materials and methods)
1.1 实验材料及仪器
主要试剂:硼氢化钠(NaBH4,分析纯)购自美国西格玛奥德里奇有限公司,六水合氯化铁(FeCl3·6H2O,分析纯)、无水乙醇(C2H5OH,分析纯)、重铬酸钾(K2Cr2O7,优级纯)、氢氧化钠(NaOH,分析纯)、盐酸(HCl,分析纯)、微米零价铁(mZVI,分析纯)、纳米二氧化(nTiO2,分析纯)、聚合硫酸铁(PFS,分析纯)均购于中国国药控股股份有限公司.
主要仪器:pH/ORP计(PHSJ-4A,上海仪电科学仪器股份有限公司);电子天平(MS 105DU,梅特勒-托利多集团);真空干燥箱(DZF-6020,上海精宏实验设备有限公司);蠕动泵(YZ1515x,保定兰格恒流泵有限公司);电动搅拌机(D2004W,上海司乐仪器有限公司).
1.2 nZVI的制备
采用经典液相还原法[16]将NaBH4 (0.2 mol·L−1)逐滴加至FeCl3 (0.05 mol·L−1)溶液中制备得到nZVI,滴加完后继续搅拌20 min,整个过程均通氮气,其反应方程式为:
stringUtils.convertMath(!{formula.content}) (1) 新鲜制备得到的nZVI使用去离子水和无水乙醇各进行多次洗涤,之后低温(4℃)保存于无水乙醇中并测量固含量以备使用.
1.3 批次实验
纳米零价铁(nZVI)、微米零价铁(mZVI)、纳米二氧化钛(nTiO2)、聚合硫酸铁(PFS)4种材料投加到模拟电镀废水中(38.4 mg∙L−1)中反应2 h后,测定溶液中剩余铬的含量.
初始浓度为50、100、200 mg∙L−1 Cr(Ⅵ)溶液中,分别投加0.5、1、2 g·L−1的nZVI,搅拌、定时取样,反应完成后固液分离,固相物质保存在酒精中保存待测.
1 g∙L−1 nZVI投加到Cr(Ⅵ)浓度分别为10、20、50、100 mg∙L−1的Cr(Ⅵ)溶液,控制溶液初始pH为2—11,搅拌速率为310 r∙min−1,反应至2、6 h时测量溶液中铬的剩余浓度,并分离出固体保存. 上述方法获得的nZVI与新鲜的nZVI用于去除浓度均为100 mg·L−1的Cd2+、Cu2+废液,控制nZVI的投加量均为1 g·L−1,分别反应1、6 h后,收集水样检测溶液中Cd2+、Cu2+含量.
配置10 mg·L−1 Co2+、Cd2+、Ni2+、Cu2+与10、20、50、100 mg·L−1 Cr(Ⅵ)的双金属溶液及20、50、200 mg·L−1 SO42−、NO3−、Cl−、CO32−与20 mg·L−1 Cr(Ⅵ)的溶液,在500 mL烧杯中加入250 mL的混合溶液,投加1 g∙L−1的nZVI,控制pH为5左右,搅拌速率为310 r∙min−1,反应2 h后,取样分析.
1.4 材料分析和表征
采用电感耦合等离子体发射光谱仪(ICP-OES,Agilent 720ES)对水样中重金属含量进行测量. 将固体样品使用真空干燥箱(DZF-6020, 上海精宏实验设备有限公司)烘干后,采用扫描电子显微镜(SEM,TM3000,日本日立高新公司)、透射显微镜(TEM,EM-2100F,日本岛津公司)进行形貌结构分析.
X射线衍射仪(XRD, Bruker D8 Advance)对纳米零价铁的晶相结构进行分析,采用Cu Kα射线,LiF单色仪,测试电流在40 kV和40 mA条件下进行,扫描角度(2θ)为10°到90°,扫描速度10(°)·min−1.
2. 结果与讨论 (Results and discussion)
2.1 nZVI去除水中Cr(Ⅵ)的效能研究
为了比较不同水处理材料的去除效能,研究了nZVI、mZVI、nTiO2、PFS对于Cr(Ⅵ)的去除效率. 水处理材料的投加量分别为1、4、10 g·L−1,从图1a可以看出,nZVI具有比其他材料更高的去除效率,分别为88.0%、95.8%、98.4%,而mZVI对铬的去除率仅在10%—15%之间,其它两种材料去除效率更低. 因此nZVI是良好的重金属Cr(Ⅵ)的去除材料.
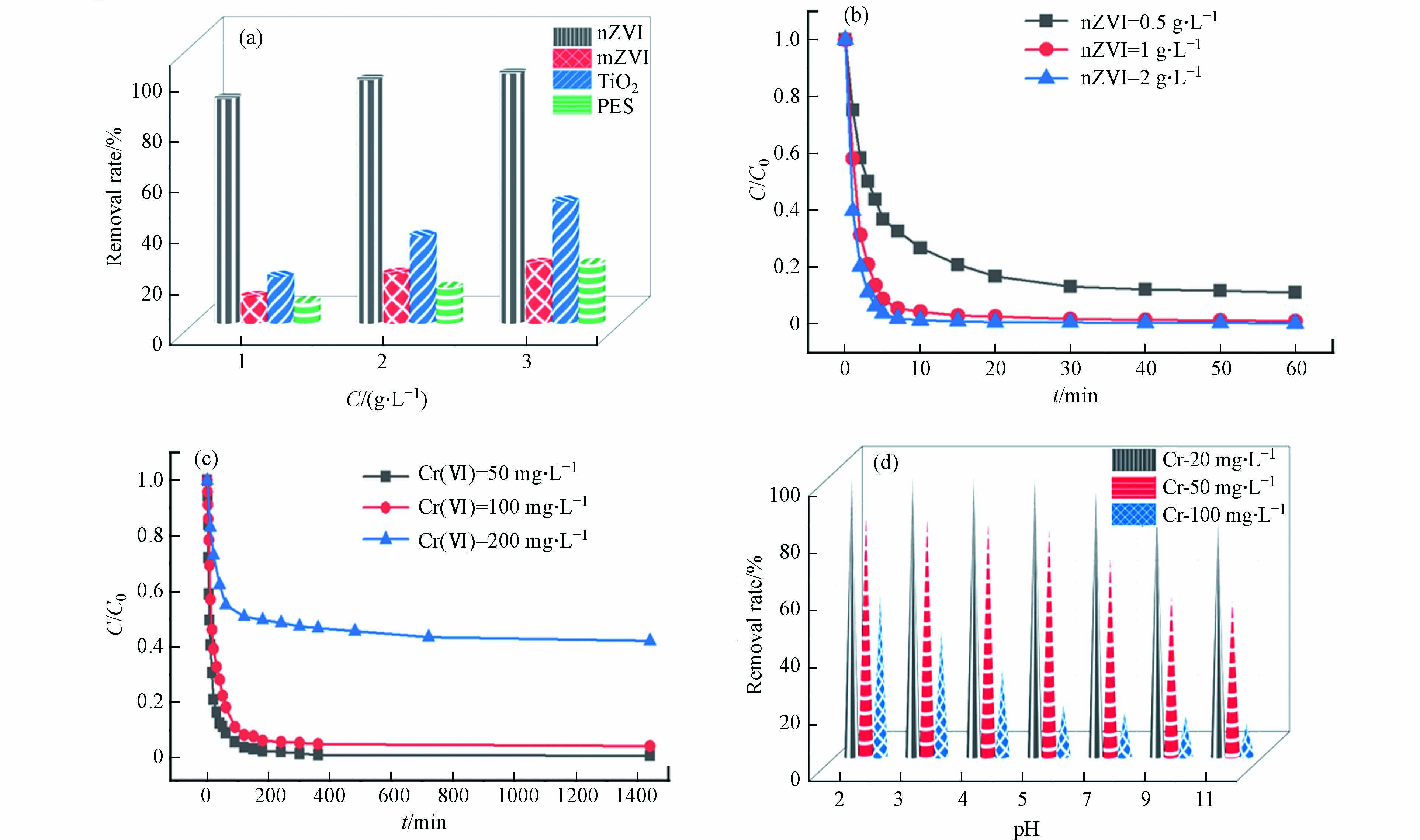 图 1 不同因素对铬去除效果的影响Figure 1. Effects of different factors on chromium removal(a)不同材料: nZVI、mZVI、nTiO2、PFS); 材料投加量: 1、4、10 g·L−1; 铬初始浓度为38.4 mg·L−1; (b) nZVI投加量: 0.5、1、2 g·L−1; (c) Cr(Ⅵ)的初始浓度50、100、200 mg·L−1; (d) 溶液初始pH: 2—11(a) different material: nZVI、mZVI、nTiO2、PFS; dosage of materials: 1、4、10 g·L−1; Initial chromium concentration: 38.4 mg·L−1; (b) dosage of nZVI: 0.5、1、2 g·L−1; (c) initial concentration of Cr(Ⅵ): 50、100、200 mg·L−1 ; (d) Initial pH of solution: 2—11
图 1 不同因素对铬去除效果的影响Figure 1. Effects of different factors on chromium removal(a)不同材料: nZVI、mZVI、nTiO2、PFS); 材料投加量: 1、4、10 g·L−1; 铬初始浓度为38.4 mg·L−1; (b) nZVI投加量: 0.5、1、2 g·L−1; (c) Cr(Ⅵ)的初始浓度50、100、200 mg·L−1; (d) 溶液初始pH: 2—11(a) different material: nZVI、mZVI、nTiO2、PFS; dosage of materials: 1、4、10 g·L−1; Initial chromium concentration: 38.4 mg·L−1; (b) dosage of nZVI: 0.5、1、2 g·L−1; (c) initial concentration of Cr(Ⅵ): 50、100、200 mg·L−1 ; (d) Initial pH of solution: 2—11改变nZVI投加量对铬去除效率的研究表明(图1b),当投加量为2 g·L−1,反应10 min,溶液中的Cr(Ⅵ)完全去除,而投加量为1 g·L−1的nZVI完全去除Cr(Ⅵ)需要20 min,0.5 g·L−1的nZVI在20 min对Cr(Ⅵ)的去除率达到80%以上. 选择1 g·L−1 nZVI为投加量进行后续实验.
探究不同的Cr(Ⅵ)初始浓度对其去除效果的影响,Cr(Ⅵ)的初始浓度分别为50、100、200 mg·L−1,如图1c. 铬的初始浓度为50、100 mg·L−1时,在200 min时nZVI对铬的去除率接近100%,而铬初始浓度为200 mg·L−1时,去除效率只能达到60%左右. 铬的初始浓度越低,反应速率越快,去除效率越高. 这可能是由于纳米零价铁修复铬的过程一般在铁表面进行,主要以吸附还原为主,暴露在表面的nZVI被氧化成Fe2+和Fe3+,再进一步发生水解反应形成钝化膜,铬浓度较低时,形成的钝化膜不足以覆盖整个铁表面,反而形成原电池促进电子转移,当铬浓度较高时,铁表面形成的钝化层,阻止了nZVI与Cr6+之间的电子传递,导致反应速率降低[17].
图1d探究了溶液初始pH(2、3、4、5、7、9、11)对铬去除效果的影响,1 g·L−1 nZVI在pH=2下与100 mg·L−1 Cr(Ⅵ)液反应2 h,对Cr(Ⅵ)的去除率为57.9%,在pH=5时去除率为18.5%,故随着pH值逐渐变大,对铬的去除率逐渐下降. 这可能是因为在酸性条件下,有利于Fe(0)的溶解,nZVI具有更强的释放电子能力,电子易与Cr(Ⅵ)结合生成Cr(Ⅲ);在碱性条件下,nZVI表面的Fe2+/Fe3+易与OH−形成铁的氢氧化物阻止反应进行. 当Cr(Ⅵ)液的浓度为20、50 mg·L−1,pH在2—5之间时,nZVI对铬的去除效率没有明显变化,而pH>5时,去除效率开始明显下降,故本研究选择在pH=5进行相关实验研究.
2.2 复杂环境条件下nZVI在Cr(Ⅵ)溶液中的晶相演变
2.2.1 Cr(Ⅵ)溶液中pH对nZVI晶相演变的影响
图2为不同溶液初始pH的条件下nZVI与20 mg·L−1 Cr反应2 h后固体产物的XRD谱图. 当pH<5时,只能观察到γ-FeOOH的衍射峰. 当pH=5时,可以观察到γ-FeOOH和Fe(0)的衍射峰,铁氧化有所减缓. 随着pH逐渐升高,Fe(0)的峰开始出现. 在pH>7时,只能观察到Fe(0)的峰,表明酸性Cr溶液促进nZVI的腐蚀,主要腐蚀产物为γ-FeOOH[18].
在20 mg·L−1的铬液中nZVI反应2 h后样品的TEM图如图3. 从图3可以看出,不同的pH下,结构形貌表现出较大的差异:新鲜nZVI呈链球状,而不同pH条件下氧化后产物出现片状和针状结构. pH=3时,Cr-nZVI中有少量链球状结构存在. 随着pH升高,不规则的链球状结构逐渐增多,球型结构由边界模糊逐渐变得分明,根据文献知悉成分是铁氧化物和CrxFe1-x(OH)3 [19 − 20]. 根据XRD分析(图2),pH>7时,与Cr(Ⅵ)反应后Cr-nZVI主要是Fe(0)的形式.
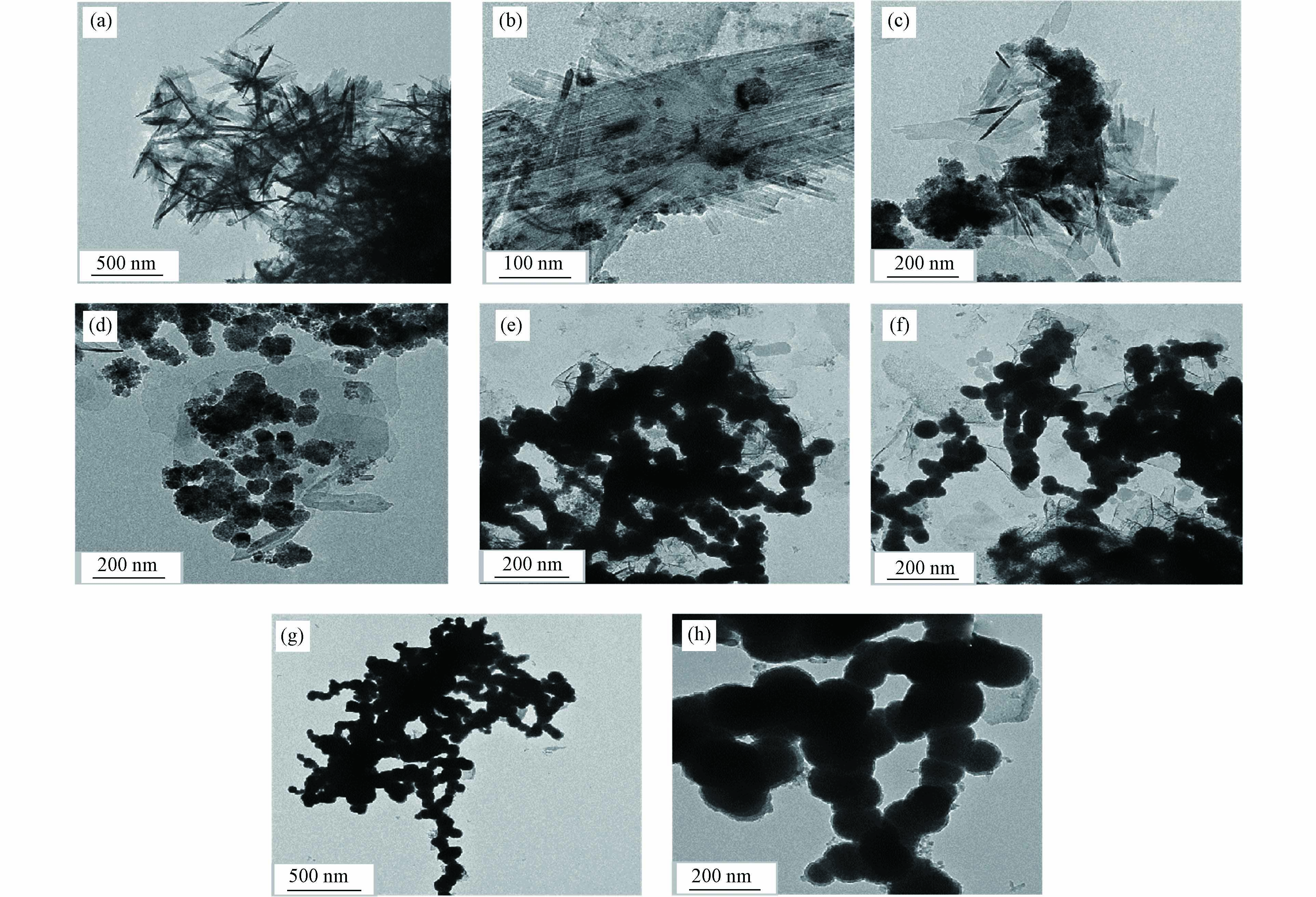 图 3 nZVI在不同初始pH的铬溶液(20 mg∙L−1)中氧化2 h的TEM图Figure 3. TEM images of nZVI oxidized for 2 h in chromium solution (20 mg∙L−1) at different initial pH(a) pH=2, (b) pH=3, (c) pH=4, (d) pH=5, (e) pH=7, (f) pH=9, (g) pH=11, (h) 新鲜nZVI(a) pH=2, (b) pH=3, (c) pH=4, (d) pH=5, (e) pH=7, (f) pH=9, (g) pH=11, (h) fresh nZVI
图 3 nZVI在不同初始pH的铬溶液(20 mg∙L−1)中氧化2 h的TEM图Figure 3. TEM images of nZVI oxidized for 2 h in chromium solution (20 mg∙L−1) at different initial pH(a) pH=2, (b) pH=3, (c) pH=4, (d) pH=5, (e) pH=7, (f) pH=9, (g) pH=11, (h) 新鲜nZVI(a) pH=2, (b) pH=3, (c) pH=4, (d) pH=5, (e) pH=7, (f) pH=9, (g) pH=11, (h) fresh nZVInZVI的等电位点为8.2,当溶液pH小于7时,nZVI表面带正电荷[8],有利于吸附带有负电荷的Cr(Ⅵ);此外H+可以促进HCrO4−和CrO42−还原为Cr3+,且pH较低时,铁腐蚀会产生大量H+和Fe2+,可进一步促进Cr(Ⅵ)还原[21 − 22],如反应方程式(2—7). 当溶液由酸性变为碱性时,nZVI会在铁的表面生成铁氧化物或铁络合物,阻止电子传递,导致铁氧化速率下降[23].
stringUtils.convertMath(!{formula.content}) (2) stringUtils.convertMath(!{formula.content}) (3) stringUtils.convertMath(!{formula.content}) (4) stringUtils.convertMath(!{formula.content}) (5) stringUtils.convertMath(!{formula.content}) (6) stringUtils.convertMath(!{formula.content}) (7) 2.2.2 Cr(Ⅵ)溶液中共存重金属对nZVI晶相演变的影响
为探究溶液中共存重金属对nZVI反应后晶相演变的影响,实验选取Co2+、Cd2+、Ni2+、Cu2+等4种重金属离子,研究nZVI在双金属(铬:20 mg·L−1,重金属:10 mg·L−1)体系中反应2 h的固体产物的晶相演变,XRD如图4a. 可以看出nZVI在单一铬金属中反应2 h后(pH=5),能够观察到Fe(0)和γ-FeOOH的衍射峰. 当含铬溶液中含有Cd、Co金属时,nZVI完全转化为纤铁矿;而Cr溶液中共存Ni和Cu金属时,nZVI晶相逐渐中转化为纤铁矿和针铁矿混合物. 同时,在含Cu2+的溶液中,能够观察到氧化亚铜晶相物质存在. nZVI在含铬-重金属溶液中反应2 h后表面形貌如图5所示,在未添加其它4种重金属离子时,与铬反应后固相产物主要呈球状、片状、针状结构,添加重金属后,固相产物链球状结构明显减少,片状和针状结构显著增多,重金属的存在对nZVI自身结构演变有巨大影响.
纳米零价铁具有类金属或类配体的配位性质,这与溶液的化学性质有关. 当溶液pH低于纳米零价铁的等电点(pHzpc=8.2)时[8],铁表面带正电荷,会吸引配体(如磷酸盐),当溶液pH高于纳米零价铁的等电点时,铁表面带负电荷,能与阳离子(如金属离子)形成表面络合物. 从经典的电化学来看,铁可以作为电子供体或还原剂,还原和沉淀活性较低的金属离子,铁表面结构也随之发生变化. Fe(Ⅱ) (−0.44 V)的标准电势低于镉Cd(Ⅱ) (−0.40 V)、Co(Ⅱ) (−0.28 V)、Cu(Ⅱ) (+0.34 V)、Ni(Ⅱ) (−0.24 V),因此这些重金属很容易被nZVI还原,如图4 b,nZVI与重金属之间形成了原电池腐蚀,nZVI表面氧化层能促进重金属吸附到nZVI表面,铁作为阳极,发生氧化反应失去电子,表面被腐蚀,重金属氧化还原电位较高,作为阴极,发生还原反应得到电子,最终形成铁氢氧化物沉淀或者重金属-铁络合物,铁晶相结构发生改变.
Cr-nZVI在不同重金属离子中的晶相转化与其氧化还原电位有关,不同重金属促进nZVI晶相转化的原理有差异. 由于Cu(Ⅱ) (+0.34 V)的标准还原电位远高于Fe(Ⅱ) (-0.44 V),在酸性条件下,能与铁形成原电池,铁作为正极发生强烈腐蚀,结构发生改变. 由于铜离子比较少,从而使得氧化亚铜的生成. nZVI与铜离子反应,会将铜离子还原成Cu和Cu2O [24 − 25],反应方程式如下:
stringUtils.convertMath(!{formula.content}) (8) stringUtils.convertMath(!{formula.content}) (9) 如图4,加入铜离子后nZVI反应产物由γ-FeOOH、α-FeOOH、Cu2O和混合相,由于针铁矿的热稳定性更高,γ-FeOOH可能在Fe2+的作用下转化为α-FeOOH[19, 26].
由于Ni(Ⅱ) (-0.24 V)的标准还原电位略高于Fe(Ⅱ) (-0.44 V),因此nZVI对Ni(Ⅱ)的去除主要是通过还原和吸附的方式实现. 在反应初始阶段,Ni(Ⅱ)通过物理吸附被吸引到铁表面,通过化学吸附强烈结合,与nZVI表面的OH−形成微溶物,通过不断消耗nZVI氧化形成的OH−,促进nZVI表面腐蚀转化为铁(氢)氧化物,金属离子逐渐还原为金属镍[27],反应方程式如下:
stringUtils.convertMath(!{formula.content}) (10) stringUtils.convertMath(!{formula.content}) (11) stringUtils.convertMath(!{formula.content}) (12) Co((Ⅱ) (-0.28 V)的标准还原电位略高于Fe(Ⅱ) (-0.44 V),反应机制和Ni(Ⅱ)相似. 由于Cd(Ⅱ) (-0.40 V)的氧化还原电位与Fe(Ⅱ) (-0.44 V)接近,去除Cd的反应机制不涉及还原反应,主要通过吸附或形成表面配合物去除. Cd(Ⅱ)吸附到nZVI表面,与nZVI氧化产生的OH−形成Cd(OH)2沉淀,促进nZVI不断腐蚀氧化形成铁(氢)氧化物[28 − 29].
2.2.3 共存阴离子对nZVI在Cr(Ⅵ)溶液中晶相演变的影响
天然水体中往往存在Cl−、CO32−、SO42−、NO3−等离子,研究共存阴离子的影响对于nZVI应用于天然水体中铬污染修复具有重要意义. 本实验研究nZVI在浓度为(10、20、50、100 mg·L−1) Cr(Ⅵ)溶液中,同时共存阴离子浓度分别为20、50、200 mg·L−1,反应2 h的固相演变. nZVI在铬(10 mg∙L−1)与阴离子(50、200 mg∙L−1)反应2 h后物相图如图6a、b所示,4种阴离子对nZVI的晶相转化均有抑制作用,可以观察到Fe(0)和其它铁(氢)氧化物的衍射峰,其中CO32−抑制作用最明显,只能观察到Fe(0)的衍射峰. 4种阴离子起钝化作用,可能是形成的铁氧化物在沉降到nZVI时,会带入吸附的阴离子,从而阻塞了nZVI的还原位点,降低了nZVI的反应活性[30].
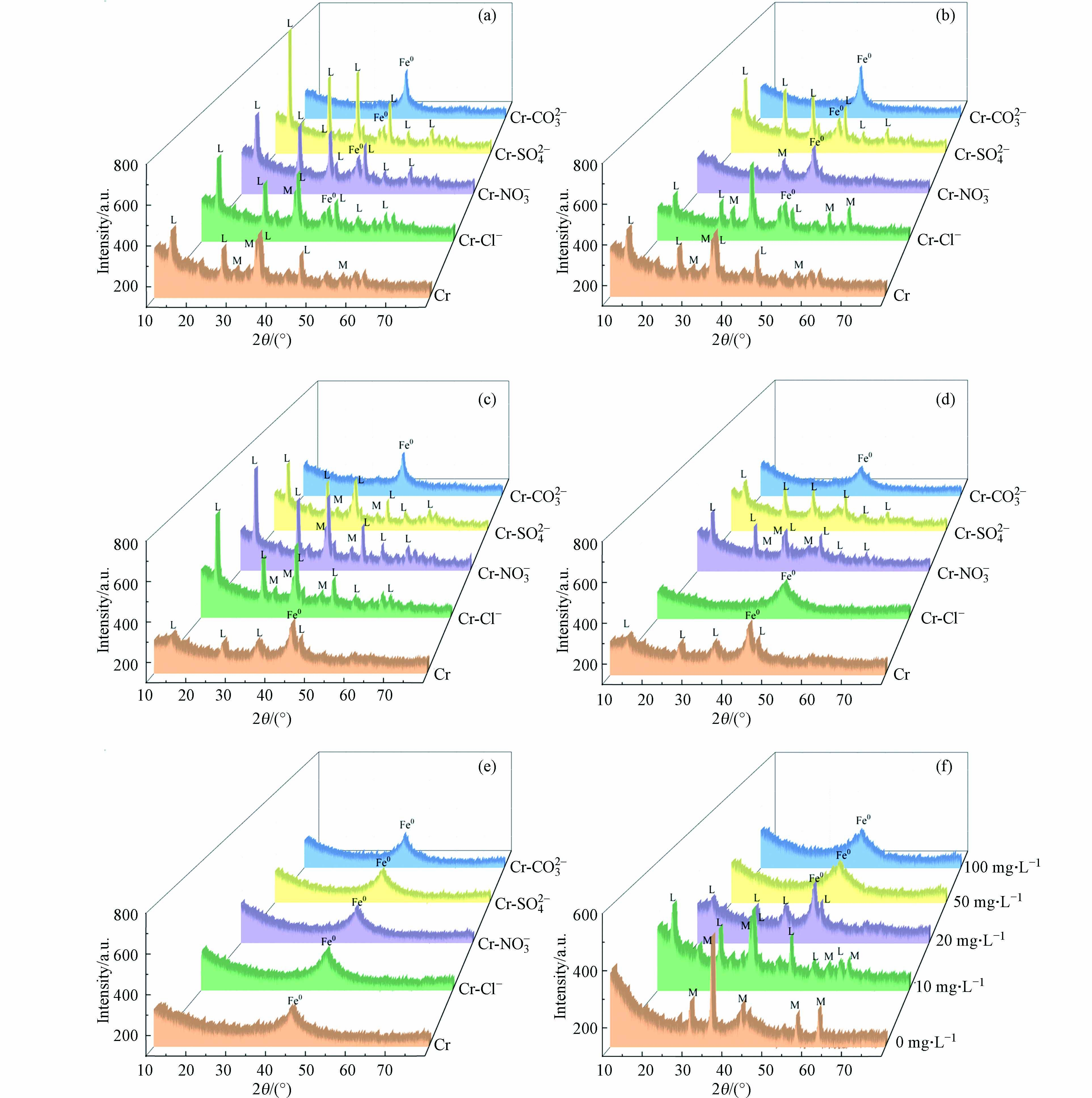 图 6 pH=5时nZVI在铬与阴离子混合液中反应2 h的XRDFigure 6. XRD patterns of nZVI reacting in a mixture of chromium and anion for 2 h (pH=5)(a) Cr: 10 mg∙L−1, 阴离子: 50 mg∙L−1, (b) Cr: 10 mg∙L−1, 阴离子: 200 mg∙L−1, (c) Cr: 20 mg∙L−1, 阴离子: 20 mg∙L−1, (d) Cr: 20 mg∙L−1, 阴离子: 50 mg∙L−1, (e) Cr: 50 mg∙L−1, 阴离子: 50 mg∙L−1, (f) Cr: 0、10、20、50、100 mg∙L−1 (L: γ-FeOOH, M: γ-Fe2O3/Fe3O4)(a) Cr: 10 mg∙L−1, anion: 50 mg∙L−1, (b) Cr: 10 mg∙L−1, anion: 200 mg∙L−1, (c) Cr: 20 mg∙L−1, anion: 20 mg∙L−1, (d) Cr: 20 mg∙L−1, anion: 50 mg∙L−1, (e) Cr: 50 mg∙L−1, anion: 50 mg∙L−1, (f) Cr: 0,10,20,50,100 mg∙L−1 (L: γ-FeOOH, M: γ-Fe2O3/Fe3O4)
图 6 pH=5时nZVI在铬与阴离子混合液中反应2 h的XRDFigure 6. XRD patterns of nZVI reacting in a mixture of chromium and anion for 2 h (pH=5)(a) Cr: 10 mg∙L−1, 阴离子: 50 mg∙L−1, (b) Cr: 10 mg∙L−1, 阴离子: 200 mg∙L−1, (c) Cr: 20 mg∙L−1, 阴离子: 20 mg∙L−1, (d) Cr: 20 mg∙L−1, 阴离子: 50 mg∙L−1, (e) Cr: 50 mg∙L−1, 阴离子: 50 mg∙L−1, (f) Cr: 0、10、20、50、100 mg∙L−1 (L: γ-FeOOH, M: γ-Fe2O3/Fe3O4)(a) Cr: 10 mg∙L−1, anion: 50 mg∙L−1, (b) Cr: 10 mg∙L−1, anion: 200 mg∙L−1, (c) Cr: 20 mg∙L−1, anion: 20 mg∙L−1, (d) Cr: 20 mg∙L−1, anion: 50 mg∙L−1, (e) Cr: 50 mg∙L−1, anion: 50 mg∙L−1, (f) Cr: 0,10,20,50,100 mg∙L−1 (L: γ-FeOOH, M: γ-Fe2O3/Fe3O4)铬(20 mg∙L−1)与阴离子(20 mg∙L−1)反应2 h时(图6c),除CO32−外,其它3种离子的加入都在一定程度上促进Fe(0)转化为γ-FeOOH和γ-Fe2O3/Fe3O4,Fe(0)衍射峰减弱,能明显地观察到γ-FeOOH和 γ-Fe2O3/Fe3O4的衍射峰. 加入SO42−反应2 h后碱性增强,SO42−的存在可能破坏保护nZVI表面氧化膜,置换nZVI表面的OH−,与铁离子形成单配位基和双配位基复合物,从而加速铁的腐蚀[31 − 32]. NO3−存在下的溶液反应后pH为6.85,呈弱酸性,NO3−能被纳米零价铁还原(E0(NO3−/NO2−)=0.01 V),从而促进纳米零价铁的腐蚀,并对表面起到一定的剥蚀作用[33]. 当阴离子为50 mg∙L−1时(图6d),共存离子为Cl−和CO32−时,只发现Fe(0)的电子衍射峰,由于阴离子不存在的条件下产生γ-FeOOH衍射峰,说明此时共存Cl−和CO32−对nZVI的氧化有明显抑制作用,Cl−对nZVI晶相转化的影响与浓度有关,低浓度促进nZVI 腐蚀,高浓度起钝化作用.
当Cr≥50 mg∙L−1时,只能观察到Fe(0)的衍射峰,高浓度Cr抑制nZVI发生晶相转化,如图6e、f,根据Huang等研究,Cr(Ⅵ)浓度较低时(≤20 mg∙L−1),nZVI腐蚀速率加快,Cr(Ⅵ)浓度较高(Cr≥50 mg∙L−1)时,零价铁表面形成CrxFe1-xOOH或CrxFe1-x(OH)3钝化层,抑制nZVI腐蚀,因此铬浓度较高(Cr≥50 mg∙L−1)时,4种阴离子对nZVI的腐蚀及晶相转化作用不明显[34].
用透射电镜对nZVI在含有200 mg∙L−1 CO32−和Cl−的铬液(Cr: 10 mg∙L−1)反应2 h前后的固相产物进行了详细研究,如图7. 新鲜的纳米零价铁(图7a)呈链球状,核壳结构明显,纳米零价铁在不含阴离子的铬液(10 mg∙L−1)反应2 h后的形貌如图7b所示,呈链球状、片状和针状结构,结合图6a XRD,主要为Fe(0)和γ-FeOOH和γ-Fe2O3/Fe3O4. 在Cl−存在下反应后固体的低分辨率透射电镜图谱中观察到片层状结构(图7c),高分辨率的电子显微镜显示了晶格条纹间距为0.2501、0.2467、0.2059 nm(图7d),分别对应于Fe2O3的(110)面、γ-FeOOH的(130)面、Fe(110)面. 对在Cl−存在下反应后的固体进行选区电子衍射(图7e),可以观察到γ-FeOOH(040)晶面、Fe2O3(121)和(113)面、Fe(200)晶面、Fe3O4(554)晶面.
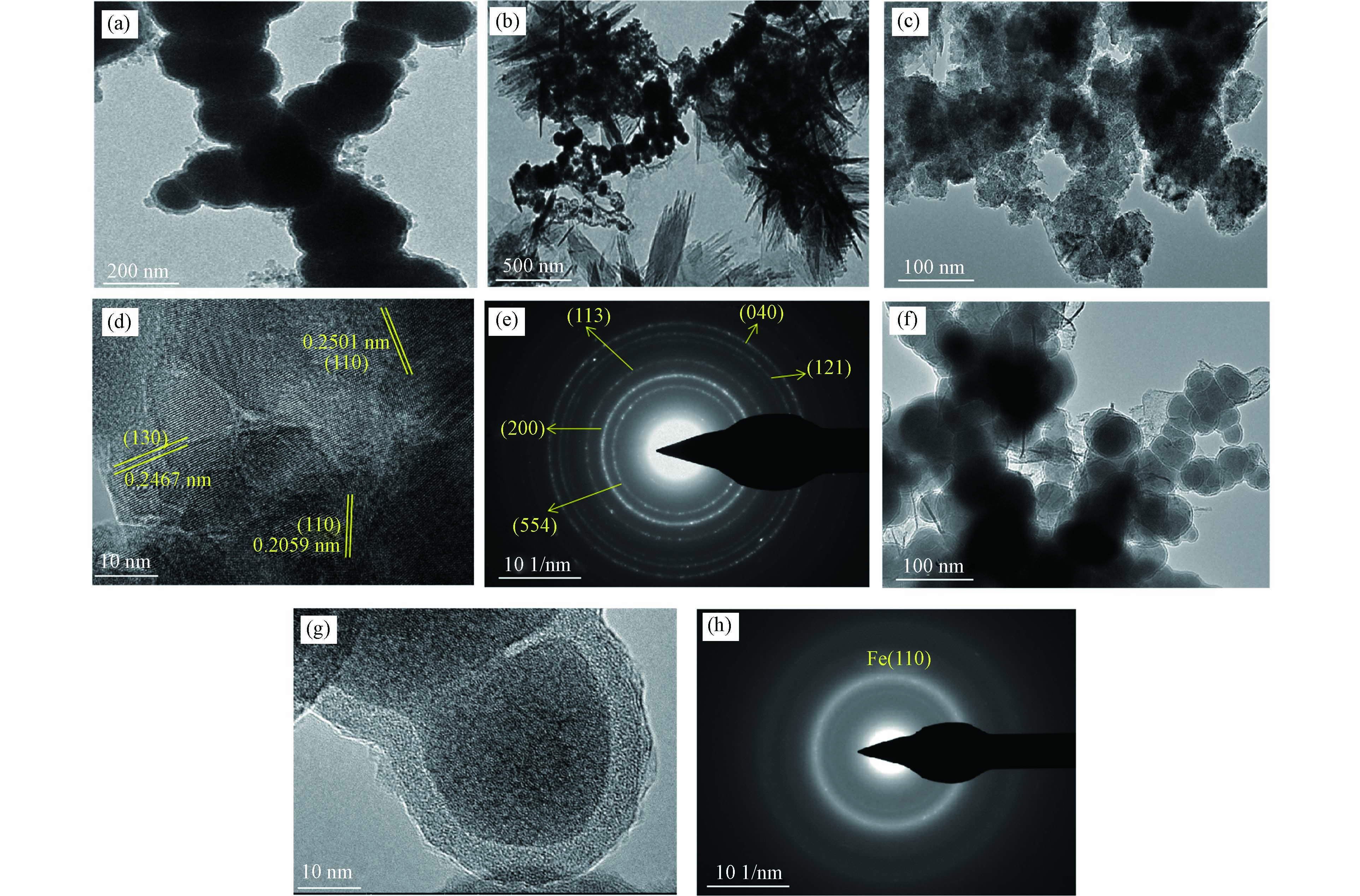 图 7 pH=5时nZVI在铬(10 mg∙L−1)与阴离子(200 mg∙L−1)的混合液中氧化2 h的TEM图Figure 7. TEM images of nZVI oxidized for 2 h in chromium solution (10 mg∙L−1) mixed with anion (200 mg∙L−1) (pH=5)(a) 仅有Cr, (b) 新鲜nZVI, (c) Cl−存在下的nZVI低分辨率TEM图, (d) Cl−存在下nZVI的高分辨率TEM图, (e) Cl−存在下nZVI的TEM选区衍射图, (f) CO32−存在下的nZVI低分辨率TEM图, (g) CO32−存在下nZVI的高分辨率TEM图, (h) CO32−存在下nZVI的TEM选区衍射图(a) only Cr, (b) fresh nZVI, (c) Low resolution TEM images (Cl−), (d) High resolution TEM images (Cl−), (e) TEM-SEAD images (Cl−), (f) Low resolution TEM images (CO32−), (g) High resolution TEM images (CO32−), (h) TEM-SEAD images (CO32−)
图 7 pH=5时nZVI在铬(10 mg∙L−1)与阴离子(200 mg∙L−1)的混合液中氧化2 h的TEM图Figure 7. TEM images of nZVI oxidized for 2 h in chromium solution (10 mg∙L−1) mixed with anion (200 mg∙L−1) (pH=5)(a) 仅有Cr, (b) 新鲜nZVI, (c) Cl−存在下的nZVI低分辨率TEM图, (d) Cl−存在下nZVI的高分辨率TEM图, (e) Cl−存在下nZVI的TEM选区衍射图, (f) CO32−存在下的nZVI低分辨率TEM图, (g) CO32−存在下nZVI的高分辨率TEM图, (h) CO32−存在下nZVI的TEM选区衍射图(a) only Cr, (b) fresh nZVI, (c) Low resolution TEM images (Cl−), (d) High resolution TEM images (Cl−), (e) TEM-SEAD images (Cl−), (f) Low resolution TEM images (CO32−), (g) High resolution TEM images (CO32−), (h) TEM-SEAD images (CO32−)结合图6a、b,Cl−能够抑制Fe(0)转化为铁氧化物. 当铬浓度为20 mg∙L−1时,Cl−为20 mg∙L−1时能促进Fe(0)转化为γ-FeOOH和γ-Fe2O3/Fe3O4,Cl−促进nZVI发生腐蚀而发生晶相转化,通常有两个原因,一是Cl−能够击穿nZVI表面氧化膜,促进内部铁提供电子能力,进一步发生腐蚀[35];二是Cl−能促进铁的局部腐蚀,形成不规则的坑形,而铁表面的坑形提供了新的反应位点,促进nZVI氧化[36]. 而当铬浓度为20 mg∙L−1时,Cl−为50 mg∙L−1时能抑制Fe(0)转化为其它铁(氢)氧化物,如图6d,这可能是Cl−竞争性结合nZVI的表面位点,抑制nZVI发生氧化[37]. 因此Cl−对nZVI的腐蚀作用及晶相转化与浓度有关.
在CO32−存在下反应后固体的低分辨透射电镜图谱中观察到链球状结构(图7f),在高分辨率的图谱中观察到明显的核壳结构,选区电子衍射(图7e)可以观察到Fe(110)晶面,说明此时nZVI的晶体结构仍未发生转化,CO32−对nZVI的氧化有很强的抑制作用. 加入CO32−后,反应2 h后测得溶液pH达到9.6,这可能是CO32−使溶液pH升高,OH−与Fe2+/Fe3+形成了铁的氢氧化物附着在nZVI表面,形成钝化膜,CO32−也会在铁氧化物表面形成竞争吸附位点,抑制nZVI发生腐蚀[38]. 有关共存阴离子对于晶相结构转化的影响机理将在以后的工作中进一步的阐明.
3. 结论 (Conclusion)
(1) nZVI的投加量、铬的初始浓度、溶液初始pH对铬的去除效率有较大影响. 随着nZVI投加量的提高,对铬的去除率也提高;随着铬初始浓度的增加和溶液初始pH的增加,对铬的去除率逐渐下降. 对于不同去除重金属的材料(nZVI、mZVI、nTiO2、PFS),nZVI是最佳去铬的实验材料.
(2) pH对nZVI的结构演变影响很大. 不同pH条件下,nZVI氧化程度不同,酸性越强,对铬的去除率越高但nZVI的腐蚀程度也较大,主要转化为铁的氧化物和氢氧化物.
(3) 共存重金属离子影响Cr-nZVI的结构演变. 4种重金属离子(Co2+、Cd2+、Ni2+、Cu2+)均能够加速nZVI腐蚀,在铬浓度为20 mg∙L−1时,加入Co2+和Cd2+,Fe(0)转化为γ-FeOOH,加入Ni2+、Cu2+后,产生新的α-FeOOH.
(4) nZVI的晶相演变同时受到Cr离子的浓度和阴离子的浓度的影响. Cr浓度为10 mg∙L−1时,4种阴离子抑制nZVI氧化,Cr浓度为20 mg∙L−1时,共存的SO42−、NO3−促进Fe(0)转化为γ-FeOOH和γ-Fe2O3/Fe3O4,CO32−抑制nZVI发生氧化,低浓度(20 mg∙L−1)的Cl−促进nZVI氧化,高浓度(200 mg∙L−1)Cl−抑制nZVI氧化为γ-FeOOH和γ-Fe2O3、Fe3O4. CO32−对nZVI的腐蚀有极强的抑制作用,高浓度的Cr抑制nZVI发生晶相转化.
-
-
[1] SHAO D D, CHEN C L, WANG X K. Application of polyaniline and multiwalled carbon nanotube magnetic composites for removal of Pb(II)[J]. Chemical Engineering Journal, 2012, 185/186: 144-150. doi: 10.1016/j.cej.2012.01.063 [2] ZHANG L, FU F L, TANG B. Adsorption and redox conversion behaviors of Cr(Ⅵ) on goethite/carbon microspheres and akaganeite/carbon microspheres composites[J]. Chemical Engineering Journal, 2019, 356: 151-160. doi: 10.1016/j.cej.2018.08.224 [3] YIN Z B, XU S, LIU S, et al. A novel magnetic biochar prepared by K2FeO4-promoted oxidative pyrolysis of pomelo peel for adsorption of hexavalent chromium[J]. Bioresource Technology, 2020, 300: 122680. doi: 10.1016/j.biortech.2019.122680 [4] XU H, GAO M X, HU X, et al. A novel preparation of S-nZVI and its high efficient removal of Cr(Ⅵ) in aqueous solution[J]. Journal of Hazardous Materials, 2021, 416: 125924. doi: 10.1016/j.jhazmat.2021.125924 [5] 杨艺琳, 周孜迈, 邓文娜, 等. 浮石负载纳米零价铁去除水相中的砷(Ⅴ)[J]. 环境化学, 2017, 36(3): 598-607. doi: 10.7524/j.issn.0254-6108.2017.03.2016051301 YANG Y L, ZHOU Z M, DENG W N, et al. Removal of arsenic(Ⅴ) from aqueous solutions using improved nanoscale zero-valent iron on pumice[J]. Environmental Chemistry, 2017, 36(3): 598-607 (in Chinese). doi: 10.7524/j.issn.0254-6108.2017.03.2016051301
[6] 廖聪坚, 赵晓蕾, 刘凯, 等. 电活性微生物激活生物质炭/零价铁协同钝化Cr(Ⅵ)及机制[J]. 环境科学, 2021, 42(9): 4520-4526. doi: 10.13227/j.hjkx.202010021 LIAO C J, ZHAO X L, LIU K, et al. Reactivation of passivated biochar/nanoscale zero-valent iron by an electroactive microorganism for cooperative hexavalent chromium removal and mechanisms[J]. Environmental Science, 2021, 42(9): 4520-4526 (in Chinese). doi: 10.13227/j.hjkx.202010021
[7] ZHANG W X. Nanoscale iron particles for environmental remediation: An overview[J]. Journal of Nanoparticle Research, 2003, 5(3): 323-332. [8] LI X Q, ELLIOTT D W, ZHANG W X. Zero-valent iron nanoparticles for abatement of environmental pollutants: Materials and engineering aspects[J]. Critical Reviews in Solid State and Materials Sciences, 2006, 31(4): 111-122. doi: 10.1080/10408430601057611 [9] 赵雅光, 万俊锋, 刘奉滨, 等. 零价铁(ZVI)治理水体砷污染研究进展[J]. 环境化学, 2013, 32(10): 1943-1949. doi: 10.7524/j.issn.0254-6108.2013.10.018 ZHAO Y G, WAN J F, LIU F B, et al. Application of zero-valent iron(ZVI)technology for arsenic removal from aqueous environment[J]. Environmental Chemistry, 2013, 32(10): 1943-1949 (in Chinese). doi: 10.7524/j.issn.0254-6108.2013.10.018
[10] CAO Z, LI H, LOWRY G V, et al. Unveiling the role of sulfur in rapid defluorination of florfenicol by sulfidized nanoscale zero-valent iron in water under ambient conditions[J]. Environmental Science & Technology, 2021, 55(4): 2628-2638. [11] DHAL B, THATOI H N, DAS N N, et al. Chemical and microbial remediation of hexavalent chromium from contaminated soil and mining/metallurgical solid waste: A review[J]. Journal of Hazardous Materials, 2013, 250/251: 272-291. doi: 10.1016/j.jhazmat.2013.01.048 [12] DONG H R, GUAN X H, LO I M C. Fate of As(V)-treated nano zero-valent iron: Determination of arsenic desorption potential under varying environmental conditions by phosphate extraction[J]. Water Research, 2012, 46(13): 4071-4080. doi: 10.1016/j.watres.2012.05.015 [13] PHENRAT T, LIU Y Q, TILTON R D, et al. Adsorbed polyelectrolyte coatings decrease Fe(0) nanoparticle reactivity with TCE in water: Conceptual model and mechanisms[J]. Environmental Science & Technology, 2009, 43(5): 1507-1514. [14] LIU J, LI R F, YAO Y H, et al. Fate and mechanistic insights into the transformation of aged nanoscale zerovalent iron (nZVIA) reacted with Cr(Ⅵ): Impact of aging time in oxic water[J]. ACS Earth and Space Chemistry, 2019, 3(7): 1288-1295. doi: 10.1021/acsearthspacechem.9b00095 [15] 刘爱荣, 戴雨薇, 夏泽阳, 等. 纳米零价铁在含Cr(Ⅵ)水相中的结构性能演变研究[J]. 环境科学学报, 2022, 42(9): 92-101. LIU A R, DAI Y W, XIA Z Y, et al. Evolution of nanoscale zero valent iron on structure and properties in Cr(Ⅵ)-containing aqueous phase[J]. Acta Scientiae Circumstantiae, 2022, 42(9): 92-101 (in Chinese).
[16] WANG C B, ZHANG W X. Synthesizing nanoscale iron particles for rapid and complete dechlorination of TCE and PCBs[J]. Environmental Science & Technology, 1997, 31(7): 2154-2156. [17] MANNING B A, KISER J R, KWON H, et al. Spectroscopic investigation of Cr(Ⅲ)- and Cr(Ⅵ)-treated nanoscale zerovalent iron[J]. Environmental Science & Technology, 2007, 41(2): 586-592. [18] LIU A R, LIU J, HAN J H, et al. Evolution of nanoscale zero-valent iron (nZVI) in water: Microscopic and spectroscopic evidence on the formation of nano- and micro-structured iron oxides[J]. Journal of Hazardous Materials, 2017, 322: 129-135. doi: 10.1016/j.jhazmat.2015.12.070 [19] LIU A R, LIU J, ZHANG W X. Transformation and composition evolution of nanoscale zero valent iron (nZVI) synthesized by borohydride reduction in static water[J]. Chemosphere, 2015, 119: 1068-1074. doi: 10.1016/j.chemosphere.2014.09.026 [20] FU F L, DIONYSIOU D D, LIU H. The use of zero-valent iron for groundwater remediation and wastewater treatment: A review[J]. Journal of Hazardous Materials, 2014, 267: 194-205. doi: 10.1016/j.jhazmat.2013.12.062 [21] ZHOU X B, JING G H, LV B H, et al. Highly efficient removal of chromium(Ⅵ) by Fe/Ni bimetallic nanoparticles in an ultrasound-assisted system[J]. Chemosphere, 2016, 160: 332-341. doi: 10.1016/j.chemosphere.2016.06.103 [22] 于新, 豆小敏, 张艳素, 等. 反应条件对零价铁去除As(Ⅲ)动力学的影响[J]. 环境化学, 2011, 30(5): 1011-1018. YU X, DOU X M, ZHANG Y S, et al. Effect of reaction conditions on the removal kinetics of as(Ⅲ) by zero-valent iron[J]. Environmental Chemistry, 2011, 30(5): 1011-1018 (in Chinese).
[23] LIU T Y, WANG Z L, YAN X X, et al. Removal of mercury (Ⅱ) and chromium (Ⅵ) from wastewater using a new and effective composite: Pumice-supported nanoscale zero-valent iron[J]. Chemical Engineering Journal, 2014, 245: 34-40. doi: 10.1016/j.cej.2014.02.011 [24] HAMDY A. Experimental study of the relationship between dissolved iron, turbidity, and removal of Cu(II) ion from aqueous solutions using zero-valent iron nanoparticles[J]. Arabian Journal for Science and Engineering, 2021, 46(6): 5543-5565. doi: 10.1007/s13369-020-05079-0 [25] QU G Z, ZENG D Y, CHU R J, et al. Magnetic Fe3O4 assembled on nZVI supported on activated carbon fiber for Cr(Ⅵ) and Cu(Ⅱ) removal from aqueous solution through a permeable reactive column[J]. Environmental Science and Pollution Research, 2019, 26(5): 5176-5188. doi: 10.1007/s11356-018-3985-8 [26] LIU A R, LIU J, PAN B C, et al. Formation of lepidocrocite (γ-FeOOH) from oxidation of nanoscale zero-valent iron (nZVI) in oxygenated water[J]. RSC Adv, 2014, 4(101): 57377-57382. doi: 10.1039/C4RA08988J [27] EFECAN N, SHAHWAN T, EROĞLU A E, et al. Characterization of the uptake of aqueous Ni2+ ions on nanoparticles of zero-valent iron (nZVI)[J]. Desalination, 2009, 249(3): 1048-1054. doi: 10.1016/j.desal.2009.06.054 [28] LI X Q, ZHANG W X. Sequestration of metal cations with zerovalent iron Nanoparticles-A study with high resolution X-ray photoelectron spectroscopy (HR-XPS)[J]. Journal of Physical Chemistry C, 2007, 111(19): 6939-6946. doi: 10.1021/jp0702189 [29] CALDERON B, FULLANA A. Heavy metal release due to aging effect during zero valent iron nanoparticles remediation[J]. Water Research, 2015, 83: 1-9. doi: 10.1016/j.watres.2015.06.004 [30] KIM H S, AHN J Y, KIM C, et al. Effect of anions and humic acid on the performance of nanoscale zero-valent iron particles coated with polyacrylic acid[J]. Chemosphere, 2014, 113: 93-100. doi: 10.1016/j.chemosphere.2014.04.047 [31] YU J, LIU W X, ZENG A B, et al. Effect of SO on 1, 1, 1-trichloroethane degradation by Fe(0) in aqueous solution[J]. Ground Water, 2013, 51(2): 286-292. [32] GUAN X H, SUN Y K, QIN H J, et al. The limitations of applying zero-valent iron technology in contaminants sequestration and the corresponding countermeasures: The development in zero-valent iron technology in the last two decades (1994–2014)[J]. Water Research, 2015, 75: 224-248. doi: 10.1016/j.watres.2015.02.034 [33] SUN Y K, LI J X, HUANG T L, et al. The influences of iron characteristics, operating conditions and solution chemistry on contaminants removal by zero-valent iron: A review[J]. Water Research, 2016, 100: 277-295. doi: 10.1016/j.watres.2016.05.031 [34] HUANG X Y, LING L, ZHANG W X. Nanoencapsulation of hexavalent chromium with nanoscale zero-valent iron: High resolution chemical mapping of the passivation layer[J]. Journal of Environmental Sciences, 2018, 67: 4-13. doi: 10.1016/j.jes.2018.01.029 [35] JOHNSON T L, FISH W, GORBY Y A, et al. Degradation of carbon tetrachloride by iron metal: Complexation effects on the oxide surface[J]. Journal of Contaminant Hydrology, 1998, 29(4): 379-398. doi: 10.1016/S0169-7722(97)00063-6 [36] HERNANDEZ R, ZAPPI M, KUO C H. Chloride effect on TNT degradation by zerovalent iron or zinc during water treatment[J]. Environmental Science & Technology, 2004, 38(19): 5157-5163. [37] HWANG Y, KIM D, SHIN H S. Inhibition of nitrate reduction by NaCl adsorption on a nano-zero-valent iron surface during a concentrate treatment for water reuse[J]. Environmental Technology, 2015, 36(9): 1178-1187. doi: 10.1080/09593330.2014.982723 [38] 杨文君, 郭迎庆, 杜尔登. 地下水中常见离子对纳米零价铁除Se(Ⅳ)动力学的影响[J]. 环境科学, 2014, 35(5): 1793-1797. doi: 10.13227/j.hjkx.2014.05.022 YANG W J, GUO Y Q, DU E D. Dynamic effects of commonly Co-existing anions on the removal of selenite from groundwater by nanoscale zero-valent iron[J]. Environmental Science, 2014, 35(5): 1793-1797 (in Chinese). doi: 10.13227/j.hjkx.2014.05.022
-




 下载:
下载:




