-
随着工业水平的不断进步,人类社会的发展对能源的需求量越来越高,但是化石燃料仍为主要的能源来源[1]. 到2040年,全球能源需求量将增长30%左右,表明CO2排放量将继续增长. 因此,有效利用CO2并开发新能源是本世纪面临的艰巨挑战. 通过直接利用可持续的太阳能,将CO2和H2O光催化还原为有用的太阳能燃料,是解决碳排放和能源短缺的一种极具前景的策略.
水滑石 (LDH) 具有高比表面积、结构可调性、酸碱可调性、记忆效应、热稳定性、无毒、价格低廉、光稳定性等优点,受到研究者的广泛关注. 研究表明,LDH的表面羟基还可以与价带空穴反应生成羟基自由基 (HO•),这可作为关键的中间物种参与氧化过程[2-3]. 此外,含Ti的LDH具有丰富的Ti–O表面缺陷,这些缺陷可作为光生载流子的有效捕获位点,促进了电子和空穴的分离[4-6],进而提高Ti基水滑石 (Ti-LDH) 的光催化性能. 近几年来,ZnTi-LDH在光催化领域展现出令人瞩目的潜能,被广泛应用于光催化领域. 例如,Xia等[7]合成了具有良好晶体结构的Fe3O4/ZnTi-LDH、CeO2/ZnTi-LDH和SnO2/ZnTi-LDH等3种复合材料,并将它们用于光催化降解酸性红14 (AR14),展现出较高的高催化活性. 但是,ZnTi-LDH可见光活性偏低,仍需对其做进一步的改性.
硫化处理是一种简单常用的改性方法,可对半导体的带隙宽度和电子和空穴的分离效率进行调控,有效的改善半导体的光催化性能. 例如,Du等[8]通过水热法合成了MoS2-CdS-TiO2催化剂用于光催化水分解. Zou等[9]采用湿法硫化制备了C/ZnS/ZnO空心球,用于光催化四环素的降解. 与C/ZnO相比,硫化后的C/ZnS/ZnO空心球光生电子和空穴的分离效率和可见光吸收性能显著提升. Ren等[10]以In金属有机框架作为前驱体制备了CdS/In2O3复合材料,其中CdS和In2O3纳米分子之间紧密相连形成异质结结构,促进了光生载流子的分离,进而提高其光催化水分解制氢效率. Yang等[11]采用水热法制备了核壳结构的In2S3/In2O3纳米材料,通过在In2O3进行硫化,可有效缩短其禁带宽度并提高其可见光利用率,进而有效提高其光催化水分解效率. 此外,硫化时间对催化剂的光催化活性也有较大的影响,当硫化30 min时,C/ZnS/ZnO样品在可见光下具有最佳的光降解活性. 但截至到目前为止,对LDH进行硫化处理后用于光催化H2O还原CO2的研究还未见报道.
为此,本文首先通过水热法制备了ZnTi-LDH,然后利用Na2S溶液对其进行硫化处理,并借助XRD、SEM、TEM、UV-Vis以及电化学工作站等对其晶体结构、形貌、光电性能等进行表征,探究硫化时间对光催化CO2还原性能的影响.
-
本文所用实验药品如表1所示.
-
采用水热法制备了ZnTi-LDH光催化剂,其步骤如下:将2.38 g Zn(NO3)2·6H2O和3.0 g尿素溶于70 mL去离子水中,随后将0.44 mL的TiCl4快速加入到上述混合溶液中,室温下剧烈搅拌30 min后将其置于100 mL水热釜中,在130 ℃下水热48 h. 最后,离心收集所得到的沉淀,去离子水洗涤4次后置于烘箱中50 ℃下干燥24 h.
-
称取200 mg制备的ZnTi-LDH样品,加入装有40 mL 0.1 mol·L−1 Na2S溶液的烧杯中,于60 ℃下分别硫化1 h、2 h和3 h后,得到所需样品. 将它们分别命名为ZnS/TiO2/S-1 h、ZnS/TiO2/S-2 h和ZnS/TiO2/S-3 h.
-
在光催化活性评价过程中,将催化剂负载于陶瓷基板上,其制备方法如下:首先将硅溶胶与拟薄水铝石粉按3∶1 (体积 (mL)∶质量 (g)) 的配比在烧杯中均匀混合,然后将其倒入长、宽、高分别为5.0、2.5、0.5 cm的矩形模具中,在室温下干燥24 h后置于马弗炉中700 ℃下煅烧4 h.
将50 mg催化剂样品在1 mL去离子水中超声分散1 h,然后用胶头滴管将所得悬浮液均匀滴于陶瓷基板表面,并在60 ℃下干燥2 h.
-
采用Bruker公司 (德国) 生产的D8-Focus X射线衍射仪 (XRD) 对催化剂的物相组成和结构进行分析,测试条件为:石墨单色化的铜靶,Cu Kα射线辐射波长0.15418 nm,管电压40 kV,管电流40 mA,扫描速度8 °·min−1,扫描范围2θ=20°—80°.
采用日立公司 (日本) 生产的加速电压为5 kV的S-4800 场发射扫描电子显微镜 (SEM) 测试催化剂的尺寸和表面形貌.
采用电子公司 (日本) 生产的加速电压为300 kV 的JEM-2100F 场发射透射电子显微镜 (TEM)观察测量催化剂晶粒的形貌和晶格间距. 制备方法如下:将催化剂充分研磨后,称取适量粉末放于0.5 mL样品管中,加入无水乙醇,超声处理至样品分散均匀,随后用胶头滴管取少许悬浮液滴于超薄碳膜上,待其自然晾干.
-
采用Perkin Elmer公司生产的紫外-可见分光光度计 (Lambda 750型) 对催化剂的吸光性能进行测试. 以BaSO4作为背景板,波长范围为200—800 nm.
采用上海辰华公司生产的CHI-660型电化学工作站对催化剂的光电性能进行表征. 测试条件:三电极系统,电解质溶液为0.1 mol·L−1的Na2SO4,工作电极为涂覆催化剂样品的ITO玻璃,对电极为铂电极,参比电极为Ag/AgCl电极. 工作电极的制备如下:称取0.4 mg样品放于离心管中,向其中滴加0.2 mL乙醇、0.2 mL水和20 μL萘酚,超声处理至样品分散均匀,随后用胶头滴管将悬浮液滴涂在ITO玻璃上,室温下自然晾干.
-
CO2光催化还原活性测试在总体积为300 mL的石英玻璃管循环体系中进行[12].
如图1所示,气体在进入反应体系之前需经净化,去除掉可能存在的微量CO,三通阀14接入管路3,经出气口15抽气进行检验. 当三通阀3和14接入管路01时为吹扫系统,接入管路01和02时为循环系统. 具体操作过程如下,将催化剂/陶瓷基板垂直放置在装有石英砂的石英反应器底部,通过CO2鼓泡将水带入反应器中参与反应. 光照之前,用CO2吹扫反应器1 h,去除反应器中的空气和催化剂表面可能吸附的有机物,随后将气路切换到循环系统,打开蠕动泵和氙灯,模拟光源采用装有AM 1.5 G滤光片的300 W氙灯 (PLS-SXE 300c氙灯) . 反应结束后,用注射器从13号取样口抽取0.1 mL气体,借助热导检测器进行气相色谱分析.
-
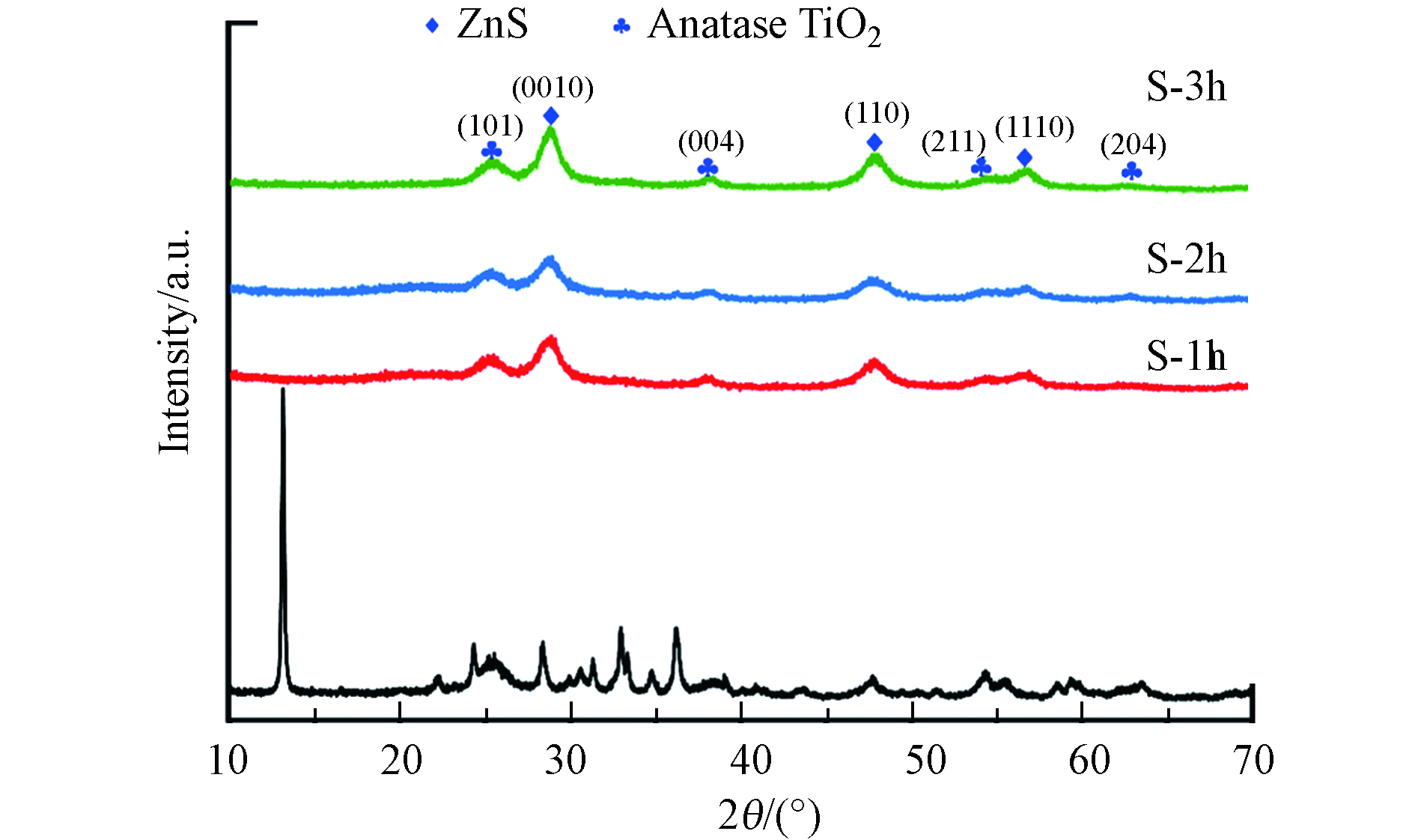
借助XRD对未硫化和硫化的ZnTi-LDH的晶体结构进行表征,结果如图2所示. 从图2可观察到,归属于ZnTi-LDH (003)、(006)、(009)、(100)、(101)、(012)、(110) 和 (113) 晶面的尖锐特征衍射峰[13],表明合成了结晶度较高的ZnTi-LDH. 此外,还可观察到归属于锐钛矿相TiO2 (101)、(004) 和 (211) 晶面的衍射峰. 借助0.1 mol·L−1的Na2S 对ZnTi-LDH进行硫化处理后,ZnTi-LDH的特征峰消失,并可观察到归属于立方相ZnS (0010)、(110) 和 (1110) 晶面的特征峰.
-
借助SEM和TEM对未硫化和硫化后的ZnTi-LDH的形貌、组成、尺寸大小等进行了研究,结果如图3所示. 由图3(a)中的SEM照片和图3(c)中的TEM照片可以看出,所合成的ZnTi-LDH具有由二维纳米薄片堆叠而成的片层结构. 借助Na2S对其进行硫化后,可观察到生成的ZnS/TiO2由纳米颗粒组成,且随着硫化时间的增加,纳米颗粒之间堆叠更加紧密(图3(d-f)). 这可能是由于硫化时间越长,生成的硫化物也越多,当硫化处理超过一定时间后,硫化物在相互作用下发生团聚或堆积. 为了进一步探究ZnS/TiO2的结构组成,借助高倍透射电镜 (HRTEM) 对其进一步探究. 从图3(g)可以清楚的观察到ZnS和TiO2的存在,其中晶格间距约为0.352 nm、0和0.239 nm的晶格条纹可分别归属于金红石相TiO2(101)面和ZnS(110)面. 此外,HRTEM图像还显示ZnS和TiO2之间存在紧密的界面接触,上述结果表明ZnTi-LDH硫化之后复合材料已成功制备.
-
图4(a)所示为未硫化和硫化的ZnTi-LDH紫外-可见漫反射光谱(UV-vis DRS). 从图4可看出,ZnTi-LDH样品只对波长小于400 nm处的光具有吸收带,这是由于在Zn原子、Ti原子及其配体形成的MO6八面体中发生了配体-金属之间的电荷转移 (LMCT)[14]. ZnTi-LDH经Na2S硫化后,其吸收带边明显发生了红移,在400—500 nm范围内产生了明显的光吸收,并且随着硫化时间的延长,ZnS/TiO2可见光吸收性能均逐渐增强. 这可归因于硫化后生成的ZnS和TiO2之间相互作用,减小了带隙宽度,降低了电子跃迁需要的能量,进而促进了配体-金属和金属-金属之间的电荷转移. 为了得到准确的禁带宽度,利用Kubelka-Munk公式[15]计算了未硫化和硫化的ZnTi-LDH的带隙宽度,(αhv)2 与hv的关系曲线如图4 (b) 所示. 从图4(b)和表2可以看出,ZnTi-LDH及ZnS/TiO2/S-1 h、ZnS/TiO2/S-2 h和ZnS/TiO2/S-3 h样品的带隙宽度分别为3.42、3.38、3.35、3.32 eV,表明硫化处理得到的ZnS/TiO2样品的带隙变窄. 且硫化时间越长,带隙宽度越窄,因此催化剂样品的光吸收性能越好. 但同时带隙变窄可能对电子和空穴的分离产生不利影响. 因此,需要做进一步的研究,以寻求最为合适的硫化时间.
采用电化学工作站对未硫化和硫化的ZnTi-LDH的光电化学性能做进一步的探究,结果如图5所示. 从瞬态光电流响应测试结果 (图5(a))可以发现,对ZnTi-LDH水滑石进行硫化处理后,光电流密度有所增加,这是由于硫化后带隙变窄,使得电子跃迁所需的能量降低,从而产生更多的光生电子和空穴. 但是,ZnTi-LDH在硫化1 h后具有最高的光电流密度,进一步增加硫化时间,光电流密度反而下降,这可归因于硫化时间变长后,带隙较窄,促进了光生电子和空穴的复合. 通过电化学阻抗谱图研究了未硫化和硫化处理的ZnTi-LDH与电解液之间的界面电荷转移电阻和分离效率,其结果如图5(b)所示. 由图5可知,ZnTi-LDH在未经硫化处理时,Nyquist曲线均呈现出较大曲率半径的圆弧,意味着较大的阻抗. 硫化处理后,圆弧的曲率半径有所降低,其中,ZnS/TiO2/S-1 h具有最小的阻抗,也即具备着最好的界面电荷传输速率与光生电子-空穴分离效率,这一结果与瞬态光电流响应结果相吻合.
为了进一步研究硫化对能带结构、载流子浓度和半导体类型的影响,利用Mott-Schottky曲线计算频率为10 kHz时的平带电势 (EFB) (式1).
其中,C为比容量,ε0是真空的介电常数,εr是半导体的介电常数,e是基本电荷,A是电极的有效面积,Nd是样品的电子载流子密度,E是外加电位,kb是玻尔兹曼常数,T是绝对温度. 图5(c)所示表明,未硫化和硫化处理的ZnTi-LDH的莫特肖特基曲线斜率均为正,表明ZnTi-LDH为n型半导体,且硫化并未改变其半导体类型. 借助公式(2)将电极电势转换为标准氢电极(NHE)电势,计算可得,ZnTi-LDH、ZnS/TiO2 /S-1 h、ZnS/TiO2 /S-2 h 和ZnS/TiO2 /S-3 h的EFB分别为−0.25、−0.77、−0.71、−0.47 eV.
众所周知,n型半导体的导带电势 (ECB) 比其平带势 (EFB) 负0.1或0.2 V,在本文中,取−0.2 V来计算硫化前后ZnTi-LDH样品的ECB值,结合带隙值,通过公式(3)计算出它们的价带电势(EVB),所有计算结果列于表2. 由表2可知,硫化处理可以明显的提高导带底的电子能级,从而有利于提高光生电子的还原能力.
-
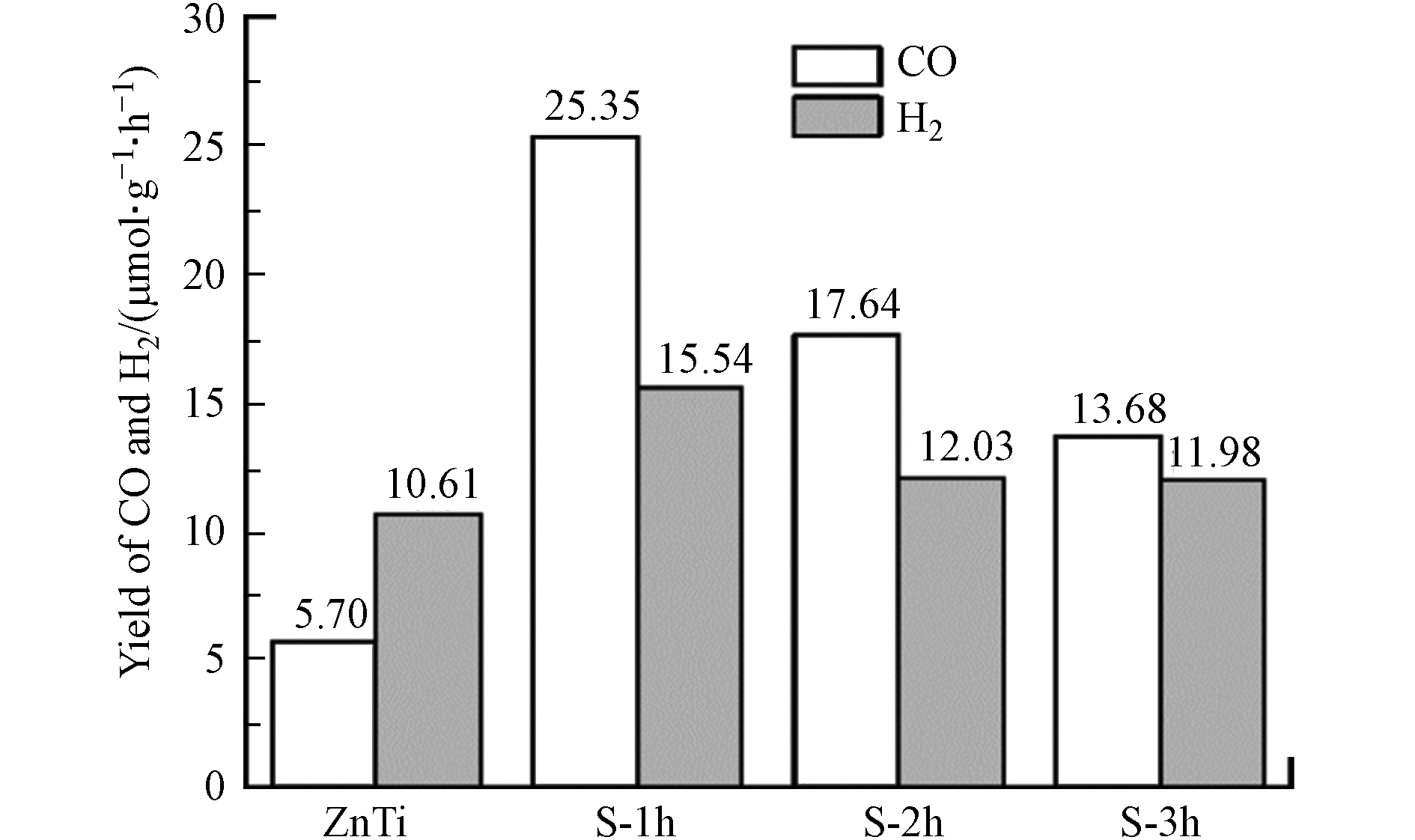
在200 ℃、模拟太阳光照射下以H2O为还原剂测试了未硫化和硫化处理的ZnTi-LDH光催化还原CO2的性能,结果如图6所示. 从图6可看出,纯的ZnTi-LDH光催化CO2还原为CO的产率仅为5.70 μmol·(g·h)−1,具有较低的光催化活性. 硫化处理得到的ZnS/TiO2光催化性能得到很大的提升,且其活性随硫化时间呈规律性变化,其中,ZnS/TiO2/S-1 h的光催化CO产率最高,为25.35 μmol·(g·h)−1,是纯ZnTi-LDH的4.4倍. 此外,对其副产物H2进行了检测. 结果显示,ZnS/TiO2/S-1 h的氢气产率最高,为15.54 μmol·(g·h)−1,是纯ZnTi-LDH的1.5倍.
为了进一步测试硫化处理的ZnTi-LDH的稳定性,以具有最高催化活性的ZnS/TiO2/S-1 h样品进行5次光催化稳定性测试,结果如图7所示. 图7结果显示,ZnS/TiO2/S-1 h在5次循环之后活性下降了18.2%,表明ZnS/TiO2/S-1 h具有相对较高的稳定性.
-
通过水热法制备了具有二维纳米片层结构的ZnTi-LDH,然后利用硫化钠溶液对其进行了硫化处理. 研究结果表明,硫化后的样品片层结构部分被破坏,形成了二维纳米片负载小颗粒的形貌,为光催化反应提供了更多的活性位点. 硫化后的样品,对可见光的吸收性能增强,光生载流子的分离效率提高,导带电子的还原能力增加. 活性测试结果证明,所有硫化后的样品的CO2还原活性均有所提升,而ZnS/TiO2/S-1 h样品具有最高的光催化活性,其CO和H2的产率分别为25.35 μmol·(g·h)−1和15.54 μmol·(g·h)−1,分别是ZnTi-LDH的4.4倍和1.5倍.
ZnS/TiO2催化剂光催化CO2的还原性能
The Study on photocatalytic CO2 reduction performance of ZnS/TiO2
-
摘要: 利用光催化技术将CO2转化为有用化学品或太阳能燃料是解决能源和环境问题的有效方法之一. 通过水热法制备了ZnTi-LDH,并通过0.1 mol·L−1 Na2S溶液对其进行硫化处理,制备了不同硫化时间的ZnS/TiO2光催化剂. 采用XRD、TEM、UV-vis DRS、化学工作站等方法,详细分析了硫化时间对光催化剂的组成、结构、光吸收性能和电化学质以及光催化H2O还原CO2性能的影响. 与ZnTi-LDH相比,硫化处理得到的ZnS/TiO2光催化CO2还原性能均有明显提高,其中ZnS/TiO2/S-1 h的样品具有最高的光催化活性,其CO和H2的产率分别为25.35 μmol·(g·h)−1和15.54 μmol·(g·h)−1,是未硫化样品的4倍和1.5倍. 硫化后样品光催化性能的改善可归因于,硫化样品较好的可见光吸收性能、较高的电子空穴分离效率以及其光电子还原能力的提高.Abstract: Converting CO2 to chemicals and solar fuels is an effective way to solve the energy shortage and environmental problems. In this work, ZnTi-LDH was prepared by hydrothermal method and sulfidized with different time by using 0.1 mol·L−1 Na2S solution, which was tested in photocatalytic CO2 reduction. The XRD, TEM, UV-vis DRS and chemical station were employed to investigate the effects of sulfidation time on the composition, structure, light absorption, electrochemical properties and photocatalytic activity for CO2 reduction. Compared with ZnTi-LDH, the photocatalytic CO2 reduction performance of ZnS/TiO2 obtained by the sulfidation treatment was significantly improved, and ZnS/TiO2/S-1h exhibited the highest photocatalytic activity with CO and H2 production rates of 25.35 μmol·(g·h)−1 and 15.54 μmol·(g·h)−1, respectively, which were 4 and 1.5 time higher than those of ZnTi-LDH. The enhancement of photocatalytic activity for sulfidized ZnTi-LDH was attributed to the better absorption capacity for visible light, the higher separation efficiency of photogenerated electrons an holes and the increase of reduction ability of photogenerated electrons.
-
Key words:
- photocatalytic CO2 reduction /
- ZnS/TiO2 /
- sulfidation treatment.
-
随着工业水平的不断进步,人类社会的发展对能源的需求量越来越高,但是化石燃料仍为主要的能源来源[1]. 到2040年,全球能源需求量将增长30%左右,表明CO2排放量将继续增长. 因此,有效利用CO2并开发新能源是本世纪面临的艰巨挑战. 通过直接利用可持续的太阳能,将CO2和H2O光催化还原为有用的太阳能燃料,是解决碳排放和能源短缺的一种极具前景的策略.
水滑石 (LDH) 具有高比表面积、结构可调性、酸碱可调性、记忆效应、热稳定性、无毒、价格低廉、光稳定性等优点,受到研究者的广泛关注. 研究表明,LDH的表面羟基还可以与价带空穴反应生成羟基自由基 (HO•),这可作为关键的中间物种参与氧化过程[2-3]. 此外,含Ti的LDH具有丰富的Ti–O表面缺陷,这些缺陷可作为光生载流子的有效捕获位点,促进了电子和空穴的分离[4-6],进而提高Ti基水滑石 (Ti-LDH) 的光催化性能. 近几年来,ZnTi-LDH在光催化领域展现出令人瞩目的潜能,被广泛应用于光催化领域. 例如,Xia等[7]合成了具有良好晶体结构的Fe3O4/ZnTi-LDH、CeO2/ZnTi-LDH和SnO2/ZnTi-LDH等3种复合材料,并将它们用于光催化降解酸性红14 (AR14),展现出较高的高催化活性. 但是,ZnTi-LDH可见光活性偏低,仍需对其做进一步的改性.
硫化处理是一种简单常用的改性方法,可对半导体的带隙宽度和电子和空穴的分离效率进行调控,有效的改善半导体的光催化性能. 例如,Du等[8]通过水热法合成了MoS2-CdS-TiO2催化剂用于光催化水分解. Zou等[9]采用湿法硫化制备了C/ZnS/ZnO空心球,用于光催化四环素的降解. 与C/ZnO相比,硫化后的C/ZnS/ZnO空心球光生电子和空穴的分离效率和可见光吸收性能显著提升. Ren等[10]以In金属有机框架作为前驱体制备了CdS/In2O3复合材料,其中CdS和In2O3纳米分子之间紧密相连形成异质结结构,促进了光生载流子的分离,进而提高其光催化水分解制氢效率. Yang等[11]采用水热法制备了核壳结构的In2S3/In2O3纳米材料,通过在In2O3进行硫化,可有效缩短其禁带宽度并提高其可见光利用率,进而有效提高其光催化水分解效率. 此外,硫化时间对催化剂的光催化活性也有较大的影响,当硫化30 min时,C/ZnS/ZnO样品在可见光下具有最佳的光降解活性. 但截至到目前为止,对LDH进行硫化处理后用于光催化H2O还原CO2的研究还未见报道.
为此,本文首先通过水热法制备了ZnTi-LDH,然后利用Na2S溶液对其进行硫化处理,并借助XRD、SEM、TEM、UV-Vis以及电化学工作站等对其晶体结构、形貌、光电性能等进行表征,探究硫化时间对光催化CO2还原性能的影响.
1. 实验部分(Experimental section)
1.1 实验药品
本文所用实验药品如表1所示.
表 1 原料试剂一览表Table 1. The list of materials and reagents试剂 Reagent 规格 Specifications 生产厂家 Manufacturer 硝酸锌(Zn(NO3)2·6H2O) 分析纯 天津市大茂化学试剂厂 硫化钠(Na2S·9H2O) 分析纯 天津市风船化学试剂科技有限公司 尿素 分析纯 上海阿拉丁生化科技股份有限公司 四氯化钛(TiCl4) 分析纯 上海阿拉丁生化科技股份有限公司 二氧化碳(CO2) ≥99.999% 天津联博化工股份有限公司 氩气(Ar) ≥99.999% 天津东祥特种气体有限公司 1.2 催化剂的制备
1.2.1 ZnTi-LDH的制备
采用水热法制备了ZnTi-LDH光催化剂,其步骤如下:将2.38 g Zn(NO3)2·6H2O和3.0 g尿素溶于70 mL去离子水中,随后将0.44 mL的TiCl4快速加入到上述混合溶液中,室温下剧烈搅拌30 min后将其置于100 mL水热釜中,在130 ℃下水热48 h. 最后,离心收集所得到的沉淀,去离子水洗涤4次后置于烘箱中50 ℃下干燥24 h.
1.2.2 ZnTi-LDH的硫化
称取200 mg制备的ZnTi-LDH样品,加入装有40 mL 0.1 mol·L−1 Na2S溶液的烧杯中,于60 ℃下分别硫化1 h、2 h和3 h后,得到所需样品. 将它们分别命名为ZnS/TiO2/S-1 h、ZnS/TiO2/S-2 h和ZnS/TiO2/S-3 h.
1.2.3 催化剂/陶瓷基板的制备
在光催化活性评价过程中,将催化剂负载于陶瓷基板上,其制备方法如下:首先将硅溶胶与拟薄水铝石粉按3∶1 (体积 (mL)∶质量 (g)) 的配比在烧杯中均匀混合,然后将其倒入长、宽、高分别为5.0、2.5、0.5 cm的矩形模具中,在室温下干燥24 h后置于马弗炉中700 ℃下煅烧4 h.
将50 mg催化剂样品在1 mL去离子水中超声分散1 h,然后用胶头滴管将所得悬浮液均匀滴于陶瓷基板表面,并在60 ℃下干燥2 h.
1.3 催化剂的表征
1.3.1 结构形貌表征
采用Bruker公司 (德国) 生产的D8-Focus X射线衍射仪 (XRD) 对催化剂的物相组成和结构进行分析,测试条件为:石墨单色化的铜靶,Cu Kα射线辐射波长0.15418 nm,管电压40 kV,管电流40 mA,扫描速度8 °·min−1,扫描范围2θ=20°—80°.
采用日立公司 (日本) 生产的加速电压为5 kV的S-4800 场发射扫描电子显微镜 (SEM) 测试催化剂的尺寸和表面形貌.
采用电子公司 (日本) 生产的加速电压为300 kV 的JEM-2100F 场发射透射电子显微镜 (TEM)观察测量催化剂晶粒的形貌和晶格间距. 制备方法如下:将催化剂充分研磨后,称取适量粉末放于0.5 mL样品管中,加入无水乙醇,超声处理至样品分散均匀,随后用胶头滴管取少许悬浮液滴于超薄碳膜上,待其自然晾干.
1.3.2 光电性能表征
采用Perkin Elmer公司生产的紫外-可见分光光度计 (Lambda 750型) 对催化剂的吸光性能进行测试. 以BaSO4作为背景板,波长范围为200—800 nm.
采用上海辰华公司生产的CHI-660型电化学工作站对催化剂的光电性能进行表征. 测试条件:三电极系统,电解质溶液为0.1 mol·L−1的Na2SO4,工作电极为涂覆催化剂样品的ITO玻璃,对电极为铂电极,参比电极为Ag/AgCl电极. 工作电极的制备如下:称取0.4 mg样品放于离心管中,向其中滴加0.2 mL乙醇、0.2 mL水和20 μL萘酚,超声处理至样品分散均匀,随后用胶头滴管将悬浮液滴涂在ITO玻璃上,室温下自然晾干.
1.4 光催化活性测试
CO2光催化还原活性测试在总体积为300 mL的石英玻璃管循环体系中进行[12].
如图1所示,气体在进入反应体系之前需经净化,去除掉可能存在的微量CO,三通阀14接入管路3,经出气口15抽气进行检验. 当三通阀3和14接入管路01时为吹扫系统,接入管路01和02时为循环系统. 具体操作过程如下,将催化剂/陶瓷基板垂直放置在装有石英砂的石英反应器底部,通过CO2鼓泡将水带入反应器中参与反应. 光照之前,用CO2吹扫反应器1 h,去除反应器中的空气和催化剂表面可能吸附的有机物,随后将气路切换到循环系统,打开蠕动泵和氙灯,模拟光源采用装有AM 1.5 G滤光片的300 W氙灯 (PLS-SXE 300c氙灯) . 反应结束后,用注射器从13号取样口抽取0.1 mL气体,借助热导检测器进行气相色谱分析.
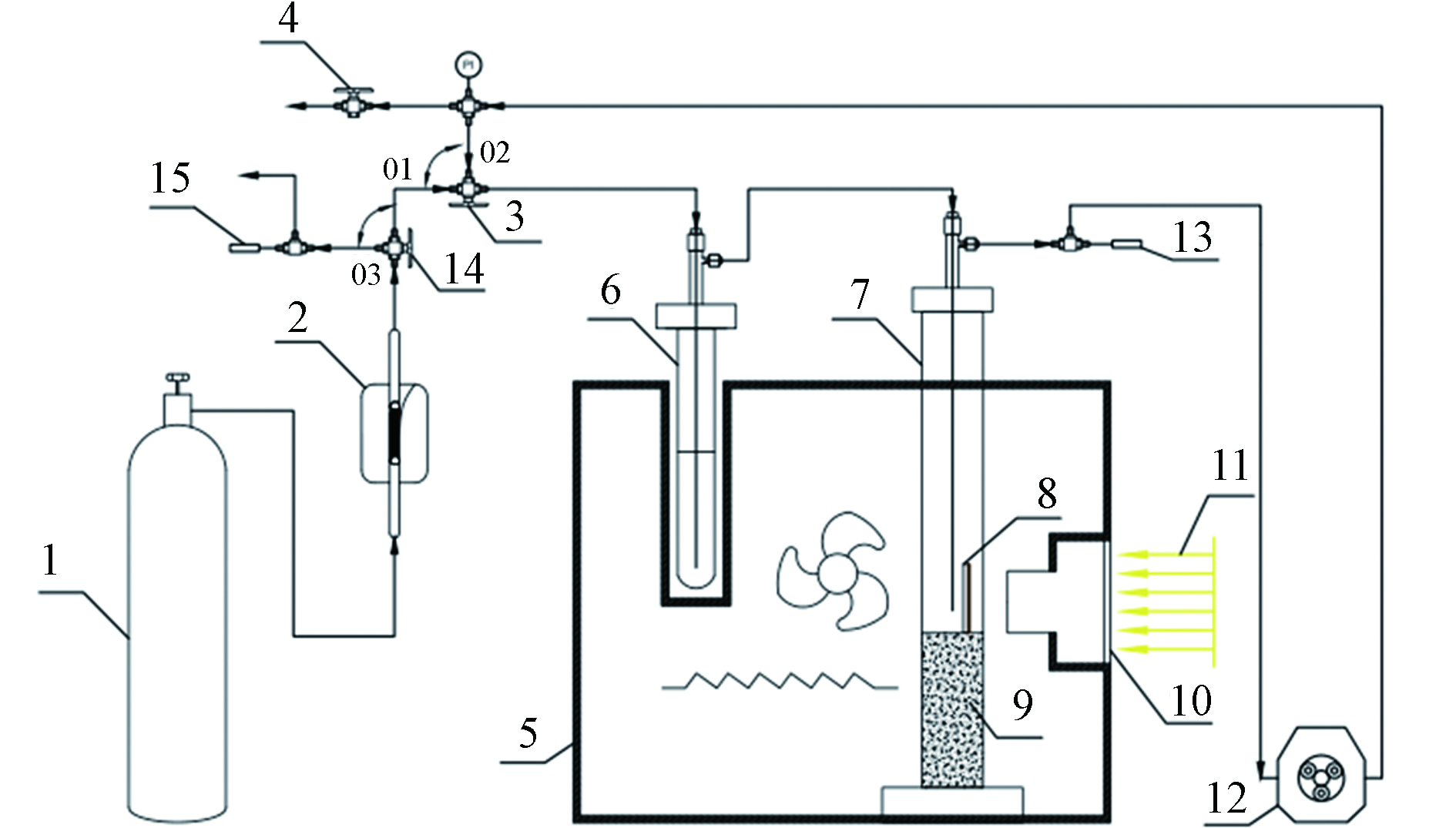 图 1 反应装置示意图Figure 1. Schematic diagram of experimental set-up for photocatalytic reduction of CO21.CO2钢瓶;2.气体净化器;3,14.三通阀;4.二通阀;5.加热炉;6.鼓泡管;7.反应器;8.陶瓷片; 9.石英砂;10.通光孔;11.氙灯光源;12.蠕动泵;13,15.取样口1.CO2 cylinder; 2.Gas purifier; 3,14.Three-way valve; 4.Two-way valve; 5.The heating furnace ;6.Water bubbler; 7.Photoreactor; 8.Ceramic chip; 9.Quartz sand; 10.The optical aperture;11.Xenon lamp light source; 12.Peristaltic pump; 13,15.Sampling port
图 1 反应装置示意图Figure 1. Schematic diagram of experimental set-up for photocatalytic reduction of CO21.CO2钢瓶;2.气体净化器;3,14.三通阀;4.二通阀;5.加热炉;6.鼓泡管;7.反应器;8.陶瓷片; 9.石英砂;10.通光孔;11.氙灯光源;12.蠕动泵;13,15.取样口1.CO2 cylinder; 2.Gas purifier; 3,14.Three-way valve; 4.Two-way valve; 5.The heating furnace ;6.Water bubbler; 7.Photoreactor; 8.Ceramic chip; 9.Quartz sand; 10.The optical aperture;11.Xenon lamp light source; 12.Peristaltic pump; 13,15.Sampling port2. 结果与讨论(Results and discussion)
2.1 XRD结构分析
借助XRD对未硫化和硫化的ZnTi-LDH的晶体结构进行表征,结果如图2所示. 从图2可观察到,归属于ZnTi-LDH (003)、(006)、(009)、(100)、(101)、(012)、(110) 和 (113) 晶面的尖锐特征衍射峰[13],表明合成了结晶度较高的ZnTi-LDH. 此外,还可观察到归属于锐钛矿相TiO2 (101)、(004) 和 (211) 晶面的衍射峰. 借助0.1 mol·L−1的Na2S 对ZnTi-LDH进行硫化处理后,ZnTi-LDH的特征峰消失,并可观察到归属于立方相ZnS (0010)、(110) 和 (1110) 晶面的特征峰.
2.2 形貌分析
借助SEM和TEM对未硫化和硫化后的ZnTi-LDH的形貌、组成、尺寸大小等进行了研究,结果如图3所示. 由图3(a)中的SEM照片和图3(c)中的TEM照片可以看出,所合成的ZnTi-LDH具有由二维纳米薄片堆叠而成的片层结构. 借助Na2S对其进行硫化后,可观察到生成的ZnS/TiO2由纳米颗粒组成,且随着硫化时间的增加,纳米颗粒之间堆叠更加紧密(图3(d-f)). 这可能是由于硫化时间越长,生成的硫化物也越多,当硫化处理超过一定时间后,硫化物在相互作用下发生团聚或堆积. 为了进一步探究ZnS/TiO2的结构组成,借助高倍透射电镜 (HRTEM) 对其进一步探究. 从图3(g)可以清楚的观察到ZnS和TiO2的存在,其中晶格间距约为0.352 nm、0和0.239 nm的晶格条纹可分别归属于金红石相TiO2(101)面和ZnS(110)面. 此外,HRTEM图像还显示ZnS和TiO2之间存在紧密的界面接触,上述结果表明ZnTi-LDH硫化之后复合材料已成功制备.
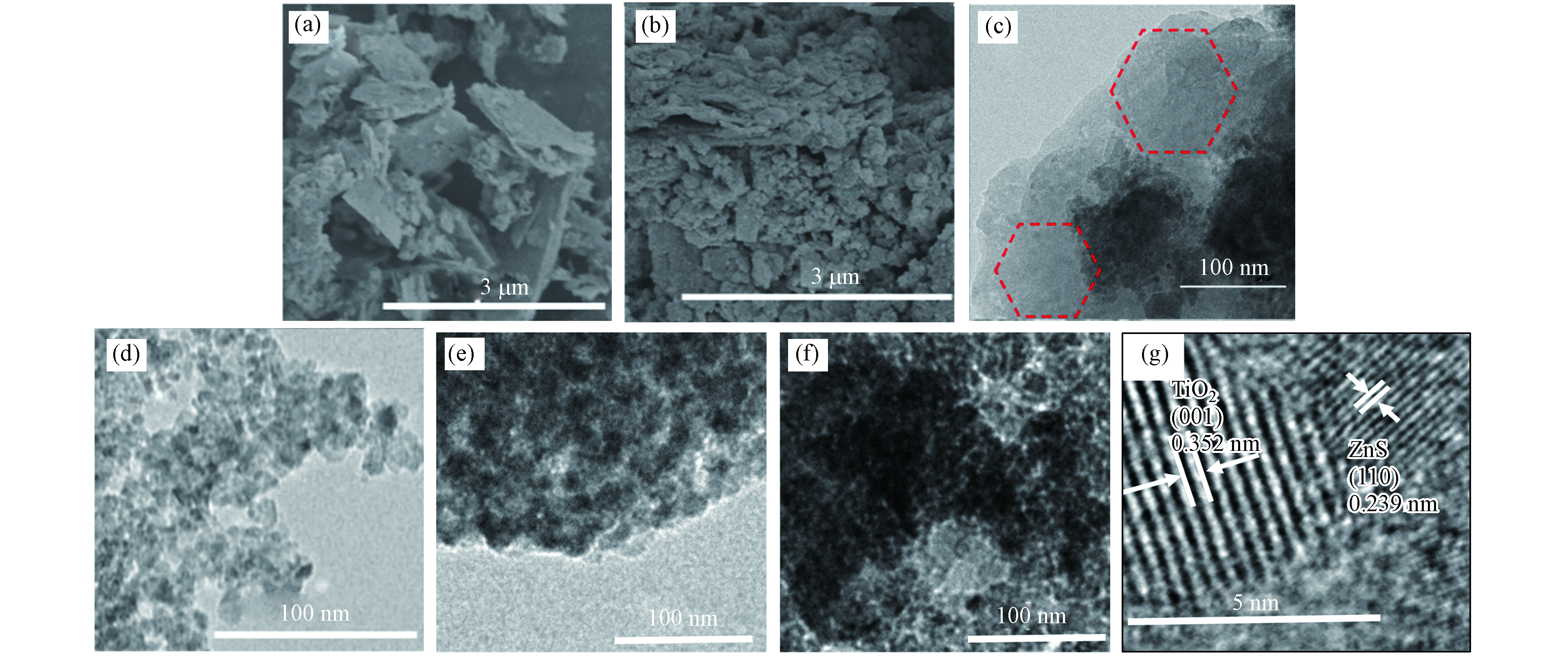 图 3 SEM照片(a) ZnTi-LDH, (c) ZnS/TiO2 /S-1h; TEM 照片(b) ZnTi-LDH, (d) ZnS/TiO2 /S-1h, (e) ZnS/TiO2 /S-2h, (f) ZnS/TiO2 /S-3h; HRTEM 照片(g) ZnS/TiO2 /S-1hFigure 3. SEM images of (a) ZnTi-LDH and (b) ZnS/TiO2/S-1h; TEM images of (c) ZnS/TiO2 and (d) ZnS/TiO2/S-1h, (e) ZnS/TiO2/S-2h, (f) ZnS/TiO2/S-3h; HRTEM image of (g) ZnS/TiO2/S-1h
图 3 SEM照片(a) ZnTi-LDH, (c) ZnS/TiO2 /S-1h; TEM 照片(b) ZnTi-LDH, (d) ZnS/TiO2 /S-1h, (e) ZnS/TiO2 /S-2h, (f) ZnS/TiO2 /S-3h; HRTEM 照片(g) ZnS/TiO2 /S-1hFigure 3. SEM images of (a) ZnTi-LDH and (b) ZnS/TiO2/S-1h; TEM images of (c) ZnS/TiO2 and (d) ZnS/TiO2/S-1h, (e) ZnS/TiO2/S-2h, (f) ZnS/TiO2/S-3h; HRTEM image of (g) ZnS/TiO2/S-1h2.3 光电性能表征
图4(a)所示为未硫化和硫化的ZnTi-LDH紫外-可见漫反射光谱(UV-vis DRS). 从图4可看出,ZnTi-LDH样品只对波长小于400 nm处的光具有吸收带,这是由于在Zn原子、Ti原子及其配体形成的MO6八面体中发生了配体-金属之间的电荷转移 (LMCT)[14]. ZnTi-LDH经Na2S硫化后,其吸收带边明显发生了红移,在400—500 nm范围内产生了明显的光吸收,并且随着硫化时间的延长,ZnS/TiO2可见光吸收性能均逐渐增强. 这可归因于硫化后生成的ZnS和TiO2之间相互作用,减小了带隙宽度,降低了电子跃迁需要的能量,进而促进了配体-金属和金属-金属之间的电荷转移. 为了得到准确的禁带宽度,利用Kubelka-Munk公式[15]计算了未硫化和硫化的ZnTi-LDH的带隙宽度,(αhv)2 与hv的关系曲线如图4 (b) 所示. 从图4(b)和表2可以看出,ZnTi-LDH及ZnS/TiO2/S-1 h、ZnS/TiO2/S-2 h和ZnS/TiO2/S-3 h样品的带隙宽度分别为3.42、3.38、3.35、3.32 eV,表明硫化处理得到的ZnS/TiO2样品的带隙变窄. 且硫化时间越长,带隙宽度越窄,因此催化剂样品的光吸收性能越好. 但同时带隙变窄可能对电子和空穴的分离产生不利影响. 因此,需要做进一步的研究,以寻求最为合适的硫化时间.
表 2 未硫化和硫化处理的ZnTi-LDH的禁带宽度和导价带位置Table 2. The band gap and the positions of the conduction band and valence band of ZnTi-LDH which was sulfated and unsulfated催化剂 Catalysts 禁带宽度/eV Band gap EFB/(V vs. NHE) ECB/(V vs. NHE) EVB/(V vs. NHE) ZnTi-LDH 3.42 −0.25 −0.45 2.97 ZnS/TiO2/S-1h 3.38 −0.77 −0.97 2.41 ZnS/TiO2/S-2h 3.35 −0.71 −0.91 2.44 ZnS/TiO2/S-3h 3.32 −0.47 −0.67 2.65 采用电化学工作站对未硫化和硫化的ZnTi-LDH的光电化学性能做进一步的探究,结果如图5所示. 从瞬态光电流响应测试结果 (图5(a))可以发现,对ZnTi-LDH水滑石进行硫化处理后,光电流密度有所增加,这是由于硫化后带隙变窄,使得电子跃迁所需的能量降低,从而产生更多的光生电子和空穴. 但是,ZnTi-LDH在硫化1 h后具有最高的光电流密度,进一步增加硫化时间,光电流密度反而下降,这可归因于硫化时间变长后,带隙较窄,促进了光生电子和空穴的复合. 通过电化学阻抗谱图研究了未硫化和硫化处理的ZnTi-LDH与电解液之间的界面电荷转移电阻和分离效率,其结果如图5(b)所示. 由图5可知,ZnTi-LDH在未经硫化处理时,Nyquist曲线均呈现出较大曲率半径的圆弧,意味着较大的阻抗. 硫化处理后,圆弧的曲率半径有所降低,其中,ZnS/TiO2/S-1 h具有最小的阻抗,也即具备着最好的界面电荷传输速率与光生电子-空穴分离效率,这一结果与瞬态光电流响应结果相吻合.
为了进一步研究硫化对能带结构、载流子浓度和半导体类型的影响,利用Mott-Schottky曲线计算频率为10 kHz时的平带电势 (EFB) (式1).
1/C=(2/εrε0eNdA2)[(E−EFB)−kbT/e] (1) E(NHE)=E(Ag/AgCl)+0.197 (2) EVB=ECB+Eg (3) 其中,C为比容量,ε0是真空的介电常数,εr是半导体的介电常数,e是基本电荷,A是电极的有效面积,Nd是样品的电子载流子密度,E是外加电位,kb是玻尔兹曼常数,T是绝对温度. 图5(c)所示表明,未硫化和硫化处理的ZnTi-LDH的莫特肖特基曲线斜率均为正,表明ZnTi-LDH为n型半导体,且硫化并未改变其半导体类型. 借助公式(2)将电极电势转换为标准氢电极(NHE)电势,计算可得,ZnTi-LDH、ZnS/TiO2 /S-1 h、ZnS/TiO2 /S-2 h 和ZnS/TiO2 /S-3 h的EFB分别为−0.25、−0.77、−0.71、−0.47 eV.
众所周知,n型半导体的导带电势 (ECB) 比其平带势 (EFB) 负0.1或0.2 V,在本文中,取−0.2 V来计算硫化前后ZnTi-LDH样品的ECB值,结合带隙值,通过公式(3)计算出它们的价带电势(EVB),所有计算结果列于表2. 由表2可知,硫化处理可以明显的提高导带底的电子能级,从而有利于提高光生电子的还原能力.
2.4 光催化活性测试
在200 ℃、模拟太阳光照射下以H2O为还原剂测试了未硫化和硫化处理的ZnTi-LDH光催化还原CO2的性能,结果如图6所示. 从图6可看出,纯的ZnTi-LDH光催化CO2还原为CO的产率仅为5.70 μmol·(g·h)−1,具有较低的光催化活性. 硫化处理得到的ZnS/TiO2光催化性能得到很大的提升,且其活性随硫化时间呈规律性变化,其中,ZnS/TiO2/S-1 h的光催化CO产率最高,为25.35 μmol·(g·h)−1,是纯ZnTi-LDH的4.4倍. 此外,对其副产物H2进行了检测. 结果显示,ZnS/TiO2/S-1 h的氢气产率最高,为15.54 μmol·(g·h)−1,是纯ZnTi-LDH的1.5倍.
为了进一步测试硫化处理的ZnTi-LDH的稳定性,以具有最高催化活性的ZnS/TiO2/S-1 h样品进行5次光催化稳定性测试,结果如图7所示. 图7结果显示,ZnS/TiO2/S-1 h在5次循环之后活性下降了18.2%,表明ZnS/TiO2/S-1 h具有相对较高的稳定性.
3. 结论(Conclusion)
通过水热法制备了具有二维纳米片层结构的ZnTi-LDH,然后利用硫化钠溶液对其进行了硫化处理. 研究结果表明,硫化后的样品片层结构部分被破坏,形成了二维纳米片负载小颗粒的形貌,为光催化反应提供了更多的活性位点. 硫化后的样品,对可见光的吸收性能增强,光生载流子的分离效率提高,导带电子的还原能力增加. 活性测试结果证明,所有硫化后的样品的CO2还原活性均有所提升,而ZnS/TiO2/S-1 h样品具有最高的光催化活性,其CO和H2的产率分别为25.35 μmol·(g·h)−1和15.54 μmol·(g·h)−1,分别是ZnTi-LDH的4.4倍和1.5倍.
-
图 3 SEM照片(a) ZnTi-LDH, (c) ZnS/TiO2 /S-1h; TEM 照片(b) ZnTi-LDH, (d) ZnS/TiO2 /S-1h, (e) ZnS/TiO2 /S-2h, (f) ZnS/TiO2 /S-3h; HRTEM 照片(g) ZnS/TiO2 /S-1h
Figure 3. SEM images of (a) ZnTi-LDH and (b) ZnS/TiO2/S-1h; TEM images of (c) ZnS/TiO2 and (d) ZnS/TiO2/S-1h, (e) ZnS/TiO2/S-2h, (f) ZnS/TiO2/S-3h; HRTEM image of (g) ZnS/TiO2/S-1h
表 1 原料试剂一览表
Table 1. The list of materials and reagents
试剂 Reagent 规格 Specifications 生产厂家 Manufacturer 硝酸锌(Zn(NO3)2·6H2O) 分析纯 天津市大茂化学试剂厂 硫化钠(Na2S·9H2O) 分析纯 天津市风船化学试剂科技有限公司 尿素 分析纯 上海阿拉丁生化科技股份有限公司 四氯化钛(TiCl4) 分析纯 上海阿拉丁生化科技股份有限公司 二氧化碳(CO2) ≥99.999% 天津联博化工股份有限公司 氩气(Ar) ≥99.999% 天津东祥特种气体有限公司 表 2 未硫化和硫化处理的ZnTi-LDH的禁带宽度和导价带位置
Table 2. The band gap and the positions of the conduction band and valence band of ZnTi-LDH which was sulfated and unsulfated
催化剂 Catalysts 禁带宽度/eV Band gap EFB/(V vs. NHE) ECB/(V vs. NHE) EVB/(V vs. NHE) ZnTi-LDH 3.42 −0.25 −0.45 2.97 ZnS/TiO2/S-1h 3.38 −0.77 −0.97 2.41 ZnS/TiO2/S-2h 3.35 −0.71 −0.91 2.44 ZnS/TiO2/S-3h 3.32 −0.47 −0.67 2.65 -
[1] GODIN J, LIU W Z, REN S, et al. Advances in recovery and utilization of carbon dioxide: A brief review [J]. Journal of Environmental Chemical Engineering, 2021, 9(4): 105644. doi: 10.1016/j.jece.2021.105644 [2] GUPTA S M, TRIPATHI M. A review of TiO2 nanoparticles [J]. Chinese Science Bulletin, 2011, 56(16): 1639-1657. doi: 10.1007/s11434-011-4476-1 [3] JO W K, KIM Y G, TONDA S. Hierarchical flower-like NiAl-layered double hydroxide microspheres encapsulated with black Cu-doped TiO2 nanoparticles: Highly efficient visible-light-driven composite photocatalysts for environmental remediation [J]. Journal of Hazardous Materials, 2018, 357: 19-29. doi: 10.1016/j.jhazmat.2018.05.038 [4] LI B, ZHAO Y F, ZHANG S T, et al. Visible-light-responsive photocatalysts toward water oxidation based on NiTi-layered double hydroxide/reduced graphene oxide composite materials [J]. ACS Applied Materials & Interfaces, 2013, 5(20): 10233-10239. [5] KHODAM F, REZVANI Z, AMANI-GHADIM A R. Enhanced adsorption of Acid Red 14 by co-assembled LDH/MWCNTs nanohybrid: Optimization, kinetic and isotherm [J]. Journal of Industrial and Engineering Chemistry, 2015, 21: 1286-1294. doi: 10.1016/j.jiec.2014.06.002 [6] GE L. Novel Pd/BiVO4 composite photocatalysts for efficient degradation of methyl orange under visible light irradiation [J]. Materials Chemistry and Physics, 2008, 107(2/3): 465-470. [7] XIA S J, ZHOU X B, SHI W, et al. Photocatalytic property and mechanism studies on acid red 14 by MxOy/ZnTi-layered double hydroxides (M = Fe, Sn, Ce) [J]. Journal of Molecular Catalysis A:Chemical, 2014, 392: 270-277. doi: 10.1016/j.molcata.2014.05.028 [8] DU J M, WANG H M, YANG M K, et al. Highly efficient hydrogen evolution catalysis based on MoS2/CdS/TiO2 porous composites [J]. International Journal of Hydrogen Energy, 2018, 43(19): 9307-9315. doi: 10.1016/j.ijhydene.2018.03.208 [9] ZOU Z M, YANG X Y, ZHANG P, et al. Trace carbon-hybridized ZnS/ZnO hollow nanospheres with multi-enhanced visible-light photocatalytic performance [J]. Journal of Alloys and Compounds, 2019, 775: 481-489. doi: 10.1016/j.jallcom.2018.10.116 [10] REN J T, YUAN K, WU K, et al. A robust CdS/In2O3 hierarchical heterostructure derived from a metal-organic framework for efficient visible-light photocatalytic hydrogen production [J]. Inorganic Chemistry Frontiers, 2019, 6(2): 366-375. doi: 10.1039/C8QI01202D [11] YANG X, XU J, WONG T, et al. Synthesis of In2O3-In2S3 core-shell nanorods with inverted type-I structure for photocatalytic H2 generation [J]. Physical Chemistry Chemical Physics, 2013, 15(30): 12688-12693. doi: 10.1039/c3cp51722e [12] WANG X N, JIANG Z L, CHEN H W, et al. Photocatalytic CO2 reduction with water vapor to CO and CH4 in a recirculation reactor by Ag-Cu2O/TiO2 Z-scheme heterostructures [J]. Journal of Alloys and Compounds, 2022, 896: 163030. doi: 10.1016/j.jallcom.2021.163030 [13] ZOU J H, WANG Z T, GUO W, et al. Photocatalytic selective oxidation of benzyl alcohol over ZnTi-LDH: The effect of surface OH groups [J]. Applied Catalysis B:Environmental, 2020, 260: 118185. doi: 10.1016/j.apcatb.2019.118185 [14] CIOCARLAN R G, WANG H, CUYPERS B, et al. ZnTi layered double hydroxides as photocatalysts for salicylic acid degradation under visible light irradiation [J]. Applied Clay Science, 2020, 197: 105757. doi: 10.1016/j.clay.2020.105757 [15] SUN D D, CHI D C, YANG Z K, et al. Mesoporous g-C3N4/Zn-Ti LDH laminated van der Waals heterojunction nanosheets as remarkable visible-light-driven photocatalysts [J]. International Journal of Hydrogen Energy, 2019, 44(31): 16348-16358. doi: 10.1016/j.ijhydene.2019.04.275 -




 DownLoad:
DownLoad: