-
我国是药物生产和使用大国. 药物主要作用为预防和治疗人类、动植物等疾病,调节生理机能,恢复机体正常等. 近年来,由于药物的生产,使用和排放缺乏长期有效的监管机制,导致在农作物土壤、地下水、地表水甚至饮用水水源中检测出了药物的存在[1-2],虽然检出浓度较低,在ng·L−1和μg·L−1之间,但对自然水体中的细菌产生抗性基因的风险不容忽视[3-4]. 药物毒性的累积性对人体的潜在伤害较为严重,例如饮用含有药物污染的地下水会影响人体的内分泌系统,长期饮用可导致癌症和畸形[5].
高级氧化技术(AOPs)是高效去除水体中痕量有机污染物的技术,其主要通过自由基及非自由基途径将污染物氧化甚至矿化[6]. 近年来,基于硫酸根自由基的高级氧化技术(SR-AOPs)受到广泛关注,相比于热活化、臭氧活化、金属活化产生活性基团等方式,UV活化效率高、副产物少且经济环保[7]. 同时,相比于传统的以·OH自由基(E0=1.9—2.7 V)[8]主导的高级氧化技术(HR-AOPs),
${\rm{SO}}_4^{-}\cdot $ 自由基(E0=2.5—3.1V)[9]以其高氧化电位、pH适应范围广、稳定性强,对污染物降解具有更广阔的应用前景.本论文选取可与组胺H2受体结合,产生抑酸作用,用于治疗消化性溃疡等疾病的受体拮抗剂(HRAs)[10](雷尼替丁(Ranitidine,RNTD)、尼扎替丁(Nizatidine,NZTD)、法莫替丁(Famotidine,FMTD)、西咪替丁(Cimetidine,CMTD)、罗沙替丁(Roxatidine,RXTD))为研究对象. 由于患者在给药后很大一部分HRAs以原结构排出至环境中,传统污水处理工艺无法有效去除HRAs[11-13],在地表水中检测到CMTD浓度达580 ng·L−1[14],在污水厂出水中检测到CMTD、FMTD和RNTD的浓度高达0—5.38 μg·L−1[11, 14]. HRAs在水体中的污染会对生态系统和公共卫生带来潜在风险[15],基于生物处理的传统污水处理工艺对HRAs的去除效果有限. 高级氧化被认为是去除水中有机微污染物的有效技术 [16],但是对HRAs类污染物的去除特性研究罕有报道. CMTD是第一代HRAs,污水厂出水中浓度甚至高达5380 ng·L−1[14],鉴于CMTD的高使用量和排放量,将其作为特征物质,通过建立拟稳态动力学模型,探究UV/PDS体系降解HRAs的机理,PDS投加量、不同pH条件、水体基质(Cl−、
${\rm{HCO}}_3^{-} $ 和NOM)对UV/PDS体系的影响,并评估在实际水样(地表水(SW)、实际废水(WW))中的应用. 通过比对实验值与模型预测值的差异,进一步评估UV/PDS体系稳态模型的适用性,明确UV/PDS降解HRAs的反应机理和应用前景. -
RNTD、NZTD、RXTD、CMTD、FMTD(~99.8%)(结构如表1所示),过二硫酸钠(PDS,99.9%)、双氧水(H2O2,质量分数30%)、氯化钠(NaCl,99%)、碳酸氢钠(NaHCO3,99.7%)、磷酸氢二钠(Na2HPO4,99.9%)、磷酸二氢钠(NaH2PO4,99.9%)、硫代硫酸钠(Na2S2O3,99.99%)、乙酸铵(CH3COONH4,99.9%)、乙酸钠(CH3COONa,99.9%)、腐殖酸(HA)、浓硫酸(H2SO4,98%)、乙醇(CH3CH2OH,99.5%)、叔丁醇(C4H10O,99.5%)等试剂均购自Sigma-Aldrich或ANPEL实验室技术(上海)有限公司. 甲醇、乙腈等为色谱级,实验溶液配置及反应用水均采用Milli-Q纯化系统(18.2 mΩ·cm)制备的超纯水.
-
本实验过程中主要使用的仪器设备为高效液相色谱(HPLC,1260,Agilent Technology,USA),紫外分光光度计(UV-1600PC,Shanghai Mapada Instruments),离子色谱仪(Thermo Dionex Aquion,赛默飞世尔科技有限公司),pH计(FE28K,Mettler Toledo),紫外反应器(内置一个4 W的低压紫外汞灯,输出波长为254 nm)等.
-
本实验使用的圆柱形石英反应器内置一个4 W的低压紫外汞灯,其光强根据草酸铁钾(K3Fe(C2O4)3·3H2O)法[17]测定为3.2×10−8 Einstein·L−1·s−1.
-
HRAs降解动力学实验在圆柱形石英反应器中进行,反应溶液置于石英杯中,磁力搅拌器维持反应溶液的均匀混合. 反应体系总体积为100 mL,10 mmol·L−1磷酸盐缓冲溶液维持体系pH 7,在0.01 mmol·L−1HRAs的溶液中加入0.2 mmol·L−1PDS,并于不同时刻取样1 mL过0.45 μm滤膜后加入至含有300 mmol·L−1 Na2S2O3的液相小瓶中. 样品在4 ℃下冰箱保存,24 h内进行液相分析,所有实验均进行两次以上.
-
UV/PDS体系中HRAs的降解主要是由直接光解和间接光解作用[18]. 如式(1)所示,kobs为拟一级降解速率常数(s−1)、kd和ki分别为直接和间接光解速率常数(s−1). ki可由kobs与kd的差值计算. 如式(2)所示,UV/PDS体系中
${\rm{SO}}_4^{-}\cdot $ 和·OH两种自由基主导了间接光解[19],k·OH/HRAs和$k_{{\rm{SO}}_4^{-}\cdot /{\rm{HRAs}}} $ 分别是HRAs和·OH与${\rm{SO}}_4^{-}\cdot $ 的二级速率常数. 无法通过竞争实验同时测定k·OH/HRAs和$k_{{\rm{SO}}_4^{-}\cdot /{\rm{HRAs}}} $ ,故本研究引入间接光解以·OH主导的UV/H2O2体系[18],此体系中HRAs的降解如式(3)所示.在UV/H2O2体系中,采用硝基苯(NB,kžOH/NB=3.0×109 L·mol−1·s−1)作为·OH的竞争化合物来测定k·OH/HRAs;在UV/PDS体系中,采用苯甲醚(AS,
$k_{{\rm{SO}}_4^{-}\cdot /{\rm{AS}}} $ =4.9×109 L·mol−1·s−1)作为${\rm{SO}}_4^{-}\cdot $ 的竞争化合物来测定$k_{{\rm{SO}}_4^{-}\cdot /{\rm{HRAs}}} $ [19-21].在UV/ H2O2体系中,NB的降解可以用式(4)表示.
其中,kobs,HRAs和kobs,NB是实验所得的拟一级降解速率常数(s−1);kd,HRAs和kd,NB是实验所得的拟一级直接光解速率常数(s−1);ki,HRAs和ki,NB为间接光解速率常数(s−1);kžOH,HRAs和kžOH,NB是HRAs和NB分别与·OH的二级速率常数(L·mol−1žs−1).
结合方程(3)和(4)得到方程(5):
式(5)可以进一步表示为式(6):
其中,ki,HRAs和ki,NB可以从式(7)和(8)中获得:
将ki,HRAs和ki,NB的计算值以及已知的kžOH,NB带入式(6)中,即可推导出HRAs和·OH的二级速率常数k·OH/HRAs.
UV/PDS体系,首先向反应体系中加入200 mmol·L−1叔丁醇(TBA,k·OH/TBA=(3.8—7.6)×108 L·mol−1·s−1)以淬灭反应体系中的·OH,之后可用上述方法测定
${\rm{SO}}_4^{-}\cdot $ 与HRAs的反应速率常数. UV/PDS体系中HRAs的降解可用式(9)表示,NB降解所测得的·OH浓度可用式(10)表示,${\rm{SO}}_4^{-}\cdot $ 和·OH引起的AS降解所测得的${\rm{SO}}_4^{-}\cdot $ 浓度可用式(11)表示,将式(10)和(11)带入式(9)中,可计算出${\rm{SO}}_4^{-}\cdot $ 与HRAs的二级速率常数$k_{{\rm{SO}}_4^{-}\cdot /{\rm{HRAs}}} $ . -
(1)模型的基本反应
${\rm{SO}}_4^{-}\cdot $ 的净生成率可用式(12)表示:式中,
$r_{{\rm{SO}}_4^{-}\cdot } $ 为${\rm{SO}}_4^{-}\cdot $ 的净生成率(mol·s−1);ΦPS为PS 的量子产率;PU-V为254 nm下的紫外线强度(3.2×10−8 Einstein·L−1·s−1);fPS是PS吸收光的分数;ε(λ)是PS在254 nm处的摩尔消光系数;C为PS的浓度(mol·L−1);α(λ) 是其他所有溶液成分在254 nm处的光吸收(cm−1);l是反应器的光程.UV/PDS降解HRAs过程中包含大量基元反应,·OH和
${\rm{SO}}_4^{-}\cdot $ 是过程中的主要反应物质,二级自由基如${\rm{O}}_2^{-}\cdot $ 、HO2·、${\rm{SO}}_5^{-}\cdot $ 等由于浓度极低,衍生自由基如Cl·、ClOH·−、${\rm{CO}}_3^{-}\cdot $ 等由于产生机理较为复杂,不包含在UV/PDS降解HRAs的建模过程中. 本模型中涉及的基本反应和速率常数如表2所示.(2)[
${\rm{SO}}_4^{-}\cdot $ ]ss,0和[·OH]ss,0的理论浓度估算和自由基分布UV/PDS降解HRAs拟稳态模型是在拟稳态假设基础上建立,假设
${\rm{SO}}_4^{-}\cdot $ ($r_{{\rm{SO}}_4^{-}\cdot } $ )和·OH(r·OH)的净生成速率为零,不随时间变化,拟稳态自由基浓度[${\rm{SO}}_4^{-}\cdot $ ]ss,[·OH]ss等于它们的初始浓度[${\rm{SO}}_4^{-}\cdot $ ]ss,0和[·OH]ss,0,表示为方程(13)和(14). 通过实验可知,反应过程的PDS浓度几乎不变(变化在10%以内). 此外,其他物种的拟稳态浓度也均为其初始浓度.式中,[Si]0是水体基质的初始浓度;ki是水体基质与·OH和
${\rm{SO}}_4^{-}\cdot $ 的速率常数. -
本实验为了探究UV/PDS体系在实际水样中的应用效果,从江苏太湖(N31°6′36″, E120°23′3″)采集地表水(SW),从上海市政污水处理厂的二级出水采集实际污水(WW). 水样储存于4 ℃的环境中,实验前用配备0.45 μm滤膜的玻璃砂芯抽滤装置过滤水样以去除颗粒物. 采用岛津总有机碳分析仪测定总有机碳含量(TOC). 用离子色谱测定了Cl−、
${\rm{NO}}_3^{-} $ 、${\rm{PO}}_4^{3-} $ 离子浓度,用电感耦合等离子体质谱测定了Fe、Mn元素的浓度,如表3所示. -
实验中RNTD、NZTD、FMTD、CMTD、RXTD、NB、AS浓度采用配备Agilent 1260色谱柱(ZORBAX SB-C18,2.4 mm×150 mm,5 μm)的高效液相色谱(HPLC,1260,Agilent Technology,USA)定量分析. 其中RNTD、NZTD、RXTD:流动相为乙腈和乙酸铵(50 mmol)溶液,V(乙腈)/V(乙酸铵)=20/80;FMTD:流动相为甲醇和水,V(甲醇)/V(水)=40/60;CMTD:流动相为甲醇和水,V(甲醇)/V(水)=45/55;NB、AS:流动相为0.1%(V/V)甲酸和甲醇,V(0.1%(V/V)甲酸)/V(甲醇)=45/55. RNTD、NZTD、FMTD、CMTD、RXTD、NB、AS的检测波长分别为242、315、208、270、218 、270、220 nm. 柱温均保持在35 ℃,等度洗脱.
-
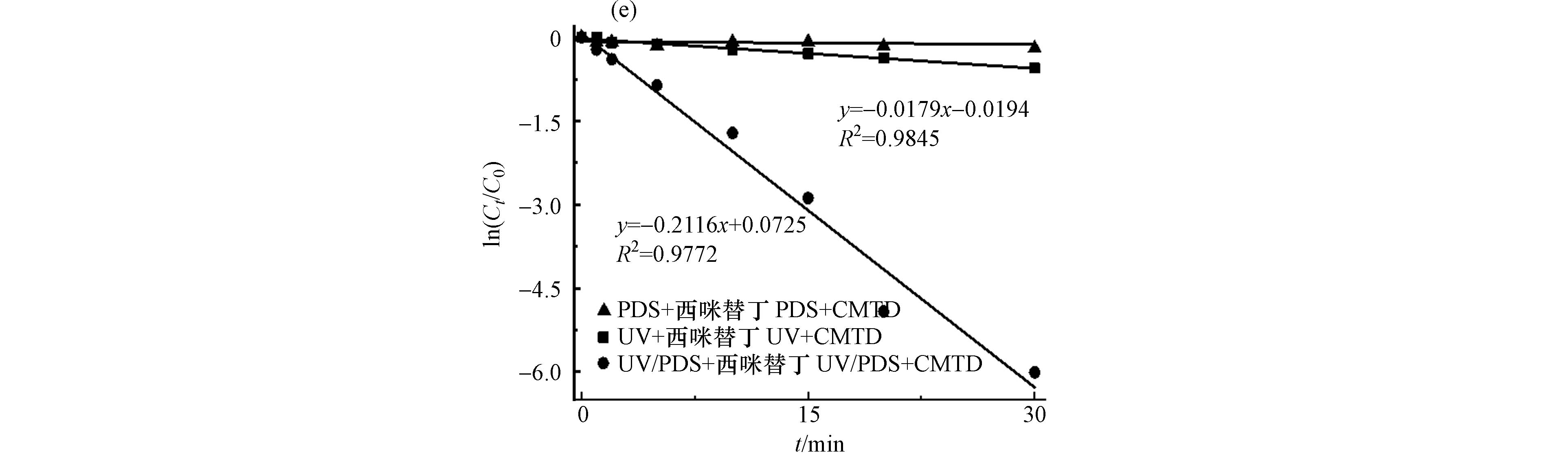
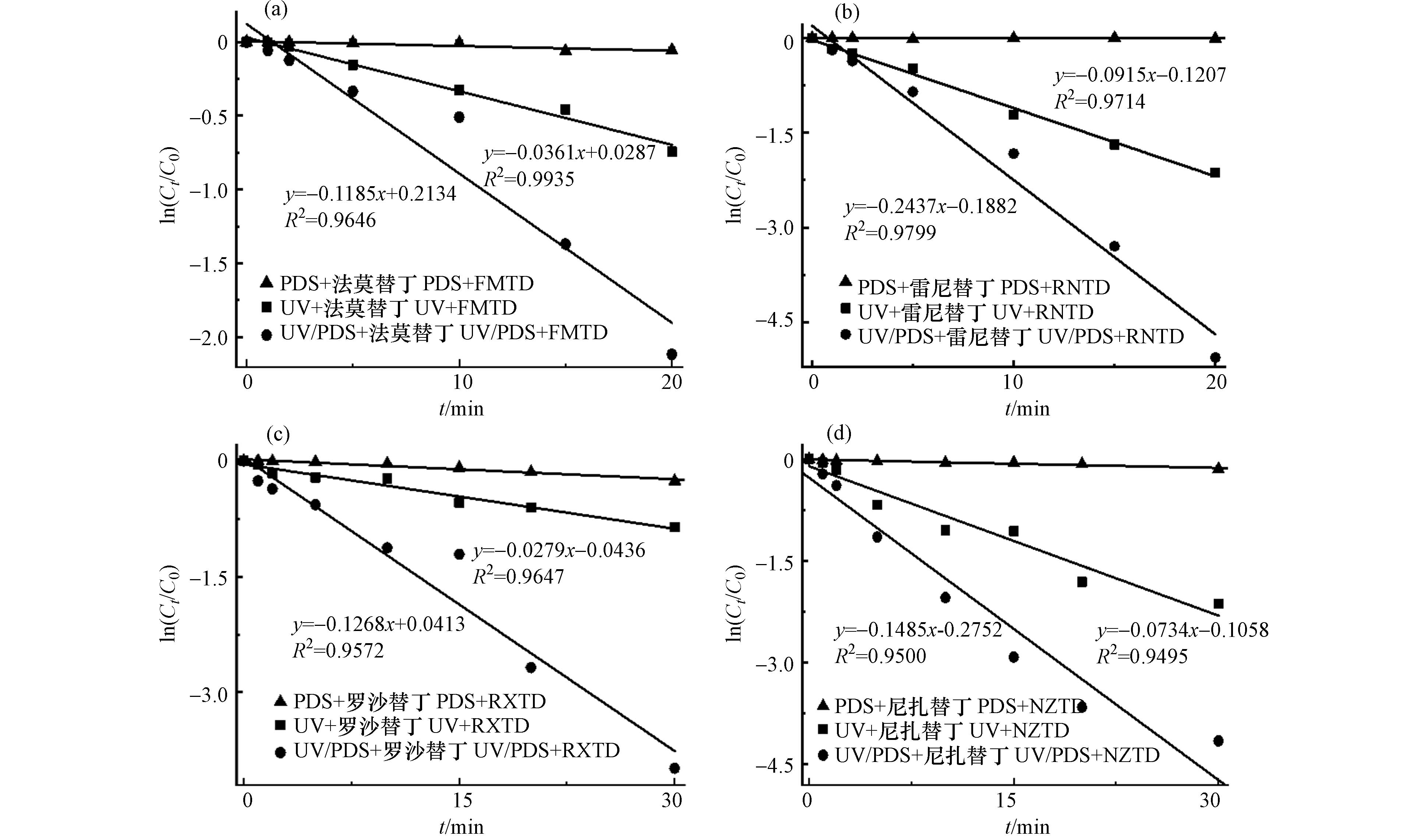
由实验可知,HRAs溶液在单独PDS处理条件下无明显降解,在单独紫外线照射下有一定的降解. 紫外线照射条件下加入PDS后,HRAs的降解显著增加,反应20 min后,FMTD、RNTD、RXTD、NZTD和CMTD的去除率分别为 87.96%、99.36%、93.13%、97.42%和99.27%. HRAs的降解如图1所示,在UV/PDS体系中,HRAs的降解ln([HRAs]/[HRAs]0)值和反应时间t表现出良好的线性关系(R2 > 0.95),说明反应遵循拟一级动力学.
实验表明HRAs的降解由直接光解和间接光解共同作用,直接光解是UV直接氧化HRAs,间接光解可能是由自由基氧化引起的. 已有相关研究表示在UV/PDS体系中,例如·OH和SO4-·可能是间接光解的主导自由基[18, 22],可与污染物快速反应.
-
本实验采用竞争动力学方法测定HRAs的二级反应速率常数k·OH/HRAs和
$k_{{\rm{SO}}_4^{-}\cdot /{\rm{HRAs}}} $ ,结果如表4所示. FMTD,RNTD,RXTD,NZTD和CMTD与·OH和${\rm{SO}}_4^{-}\cdot $ 的有较高的二级反应速率常数,k·OH/HRAs为(2.8—14.6)×109 L·mol−1·s−1,$k_{{\rm{SO}}_4^{-}\cdot /{\rm{HRAs}}} $ 为(0.81—8.10)×109 L·mol-1·s−1. HRAs的富电子部分,例如胺、双键和硫醚键被认为是易受·OH和${\rm{SO}}_4^{-}\cdot $ 攻击的反应位点[16,36]. CMTD的ki值相比其他HRAs较低,后续研究将以CMTD为特征污染物进行模型模拟. -
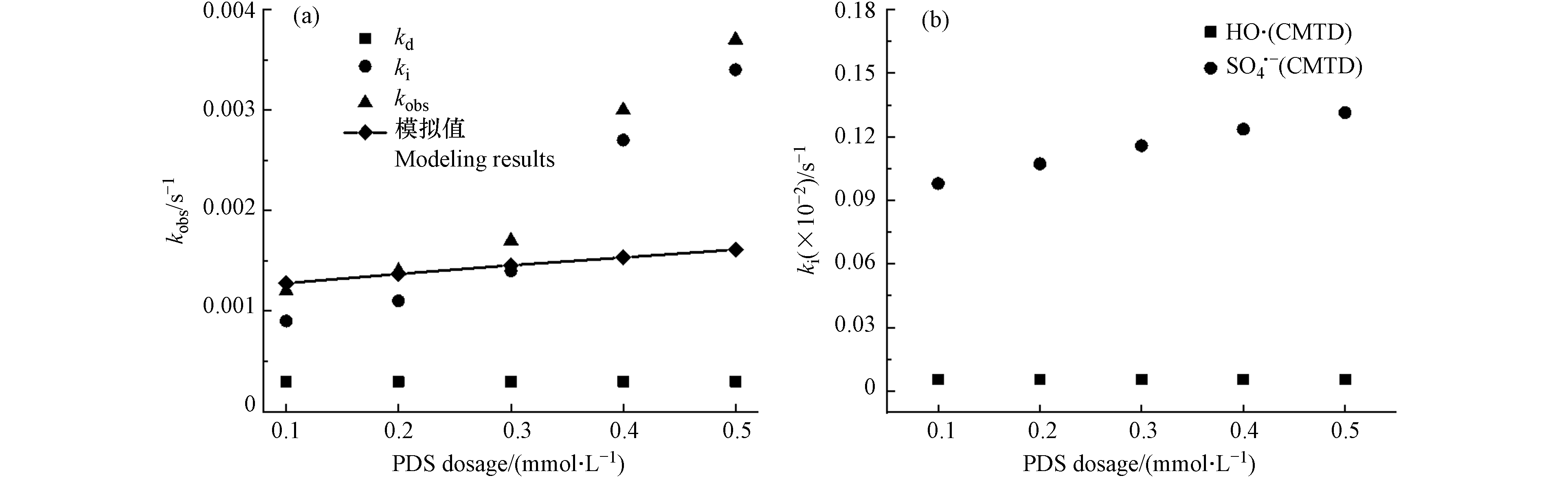
CMTD在不同PDS投加量的自由基浓度如表5所示. 在(0.1—0.5) mmol·L−1 PDS投加量下,·OH和
${\rm{SO}}_4^{-}\cdot $ 的浓度分别为(3.85—5.16) ×10−16 mol·L−1和(1.21—1.68) ×10−13 mol·L−1.得到的拟一级降解速率常数kobs、直接光解速率常数kd、间接光解速率常数ki和模型预测的kobs如图2所示. 图2(a)表示随着PDS投加量的增加,间接光解速率常数ki显著增加. 图2(b)表示模拟的
${\rm{SO}}_4^{-}\cdot $ 和·OH自由基降解CMTD的贡献值.PDS的浓度在0.1—0.3 mmol·L−1范围内时,模拟预测结果与实际值可较好的吻合,说明该模型在PDS的低浓度范围内有较好的应用. 而当PDS的浓度增加至0.4 mmol·L−1时,模拟预测结果与实际值有一定的偏差,表示模拟预测低估了实际降解的情况,这可能由于UV/PDS降解CMTD的研究不够全面,一些基元反应未包含在建模过程中所致.
${\rm{SO}}_4^{-}\cdot $ 可与OH−/H2O反应并生成·OH[9, 37],所以·OH浓度会随着PDS浓度的增长而增加,如式(15)和(16). 当PDS的浓度在0.1—0.5 mmol·L−1范围内,CMTD主要由间接光解去除,·OH的浓度几乎比${\rm{SO}}_4^{-}\cdot $ 低3个数量级,且k·OH/CMTD与$k_{{\rm{SO}}_4^{-}\cdot /{\rm{CMTD}}} $ 为同一数量级. 因此可推断kobs的主要部分为${\rm{SO}}_4^{-}\cdot $ 贡献的ki,CMTD主要被${\rm{SO}}_4^{-}\cdot $ 降解. -
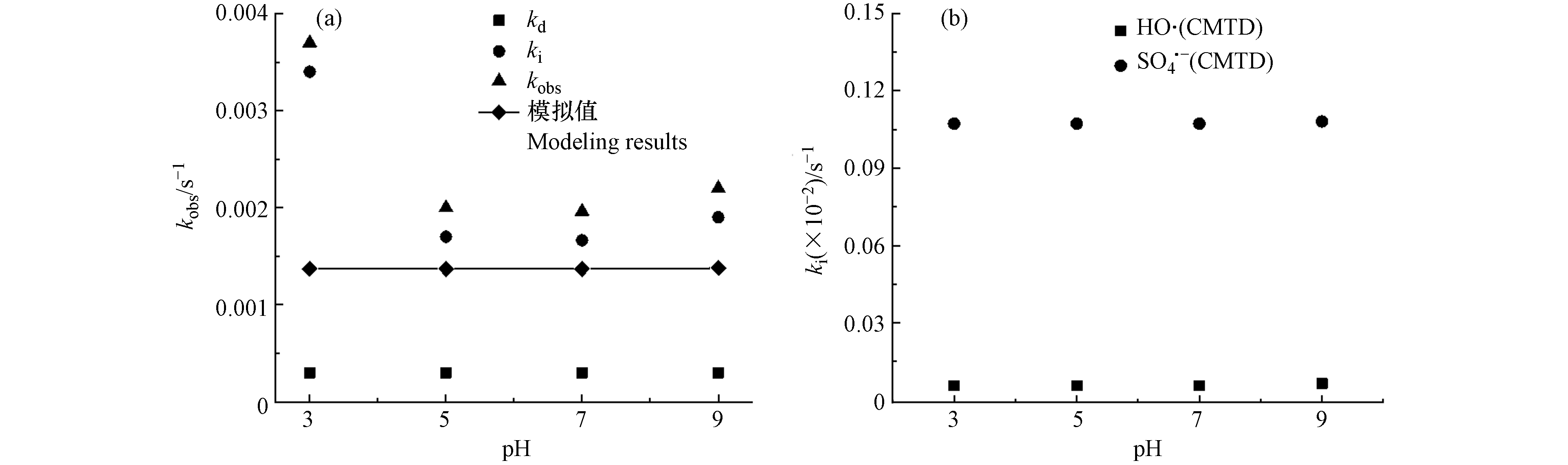
CMTD在不同pH条件下的自由基浓度如表6所示.
图3(a)中的点表示实验测得的kd、ki和kobs,实线表示模型预测的kobs. 图3(b)表示
${\rm{SO}}_4^{2-} $ 和·OH模拟的降解贡献值.酸性条件下的实验降解结果明显高于中性和碱性条件,表明酸性条件更有利于CMTD的降解. CMTD的pKa值为7.1[38],受pH影响较小. 此外,在酸性条件下,PDS被酸催化,体系中${\rm{SO}}_4^{2-} $ 产生量增加[39],从而促进了CMTD的降解. 而PDS被酸催化形成的${\rm{SO}}_4^{2-} $ 的机理未被详细阐明,故未包含在模型的基元反应中,导致了实验值与模型值的偏差. 在pH 5—9条件下,·OH和${\rm{SO}}_4^{2-} $ 的浓度略微增加,故ki无明显变化. 图(b)显示了在不同pH条件下,${\rm{SO}}_4^{2-} $ 自由基的ki值一直是kobs的主要部分,CMTD的降解主要由${\rm{SO}}_4^{2-} $ 自由基贡献. -
实验探究了CMTD在不同水基质(Cl−,
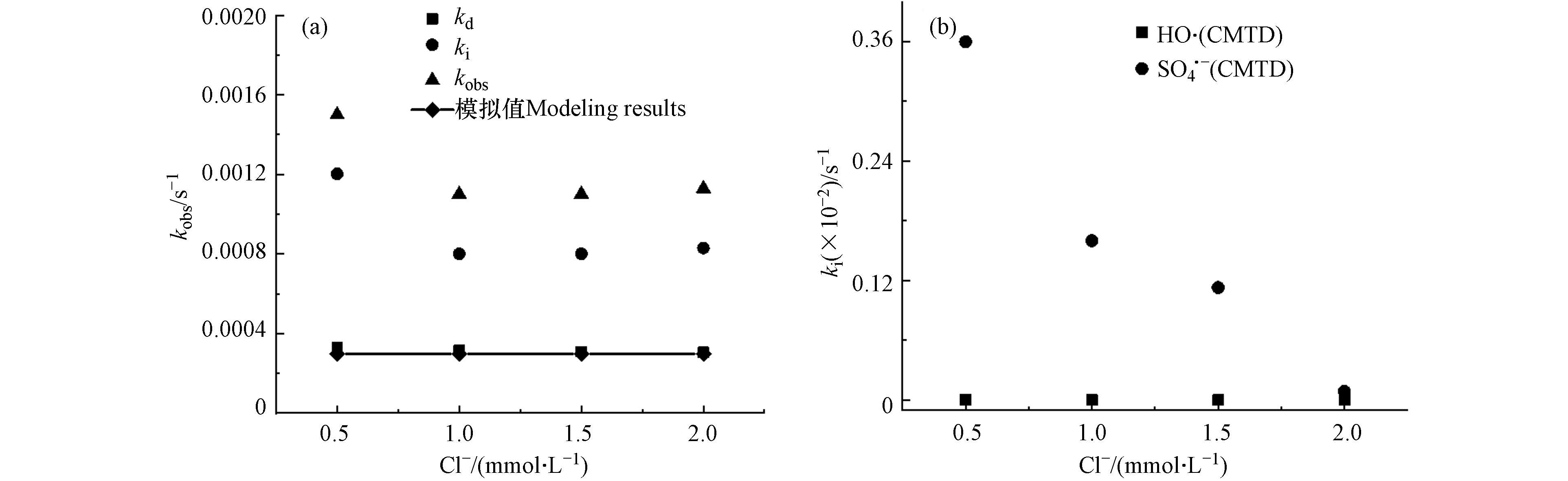
${\rm{HCO}}_3^{-} $ 和NOM)的降解情况,自由基浓度如表7、8、9所示,实验结果如图4、5、6所示.(1) Cl-的影响
CMTD在Cl-存在条件下自由基浓度如表7所示. 如图4所示,Cl−对CMTD降解的影响表现出显著的变化. Cl−浓度在0.5—1.0 mmol·L−1范围内,随着Cl−浓度的增加,kobs-CMTD下降. Cl−浓度在1.0—2.0 mmol·L−1范围内,随着Cl−浓度的增加,kobs-CMTD无明显变化. kobs-CMTD的模拟值明显小于实际值,这可能是因为Cl−可与
${\rm{SO}}_4^{-}\cdot $ 和·OH分别以4.7 × 108 L·mol−1·s−1和4.3 × 109 L·mol−1·s−1的速率常数反应,如式(17)—(19)所示[23, 30, 40]. Cl·、ClOH·−、${\rm{Cl}}_2^- \cdot $ 等活性氯物质(RCS)与污染物进行了反应,其中Cl·和${\rm{Cl}}_2^- \cdot $ 都是强氧化剂,氧化还原电位高达2.4 V和2.0 V[41]. RCS通过单电子氧化、夺氢或添加不饱和C—C键可以与富电子污染物CMTD发生反应[41]. 这些自由基与CMTD的反应未完全包含在模型中,导致了模型值与实验值的差异. 图(b)展示了随着Cl−浓度的增加,${\rm{SO}}_4^{-}\cdot $ 降解贡献的减少较为明显,这可能是由于${\rm{SO}}_4^{-}\cdot $ 自由基量比·OH高,Cl−与${\rm{SO}}_4^{-}\cdot $ 反应的消耗导致.(2)
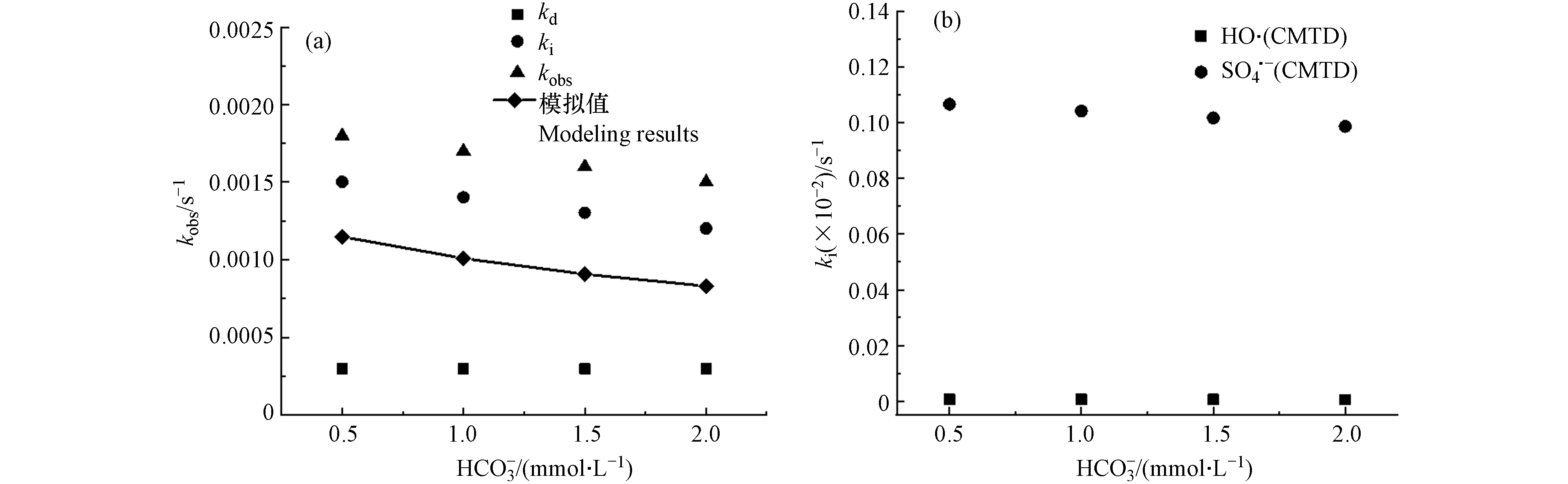
${\rm{HCO}}_3^{-} $ 的影响CMTD在
${\rm{HCO}}_3^{-} $ 存在条件下自由基浓度如表8所示. 如图5所示,随着${\rm{HCO}}_3^{-} $ 浓度从0.5—2.0 mmol·L−1,kobs-CMTD逐渐降低,这是由于${\rm{HCO}}_3^{-} $ 、${\rm{CO}}_3^{2-} $ 是·OH和${\rm{SO}}_4^{-}\cdot $ 的重要清除剂[42],${\rm{HCO}}_3^{-} $ 以3.6 × 106 L·mol−1·s−1和8.5 × 106 L·mol−1·s−1的速率常数分别与${\rm{SO}}_4^{-}\cdot $ 和·OH反应[8, 22],${\rm{CO}}_3^{2-} $ 以6.5 × 106 L·mol−1·s−1和4.2 × 108 L·mol−1·s−1分别与${\rm{SO}}_4^{-}\cdot $ 和·OH反应[8, 33],从而${\rm{SO}}_4^{-}\cdot $ 和·OH贡献率下降,且ki值降低. 但是在这里模拟值出现了偏离实验值的情况,且模拟值始终低于实验值. 这是由于模型中的${\rm{HCO}}_3^{-} $ 、${\rm{CO}}_3^{2-} $ 是作为自由基抑制剂考虑,而在实际反应中,${\rm{HCO}}_3^{-} $ 、${\rm{CO}}_3^{2-} $ 与·OH和${\rm{SO}}_4^{-}\cdot $ 反应生成${\rm{CO}}_3^{-} \cdot $ 自由基(E0=1.59 V)[24],氧化还原电位低于${\rm{SO}}_4^{-}\cdot $ 和·OH,但对富电子基团仍有一定的选择性,可部分氧化CMTD,从而造成了实验值与模拟值的偏差.(3) NOM的影响
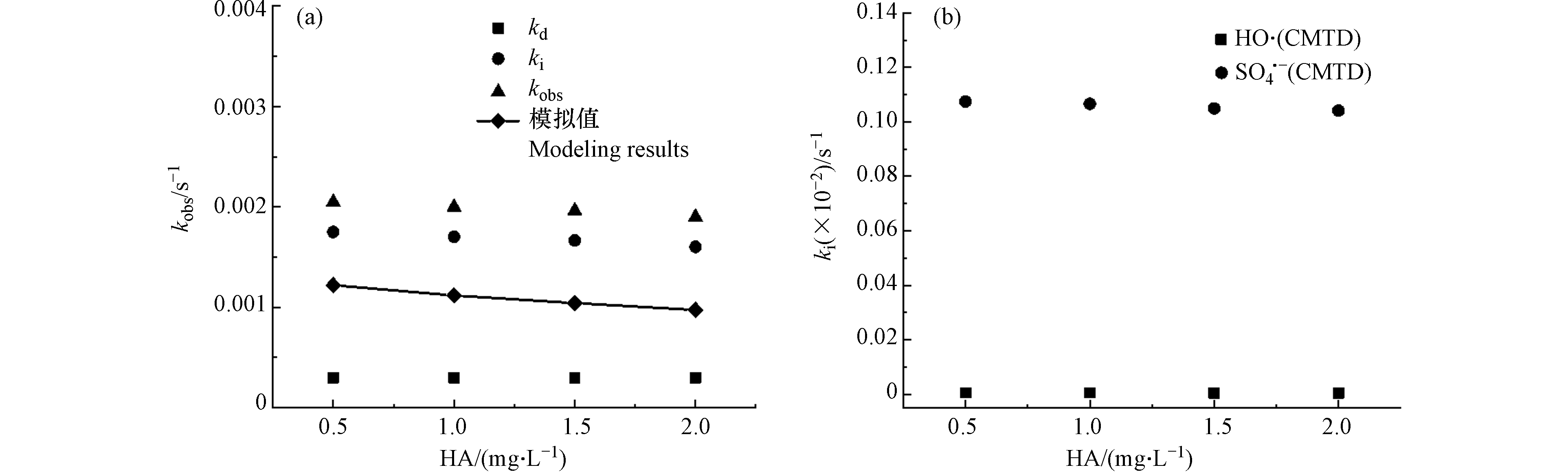
HA作为自然界中广泛存在的大分子有机物,本实验中用HA来研究NOM对HRAs降解速率的影响. CMTD在HA存在条件下自由基浓度如表9所示.
图6显示CMTD的kobs随着HA浓度的增加而逐渐下降,模拟预测值也相应下降. HA对降解反应的抑制作用可从以下方面考虑,一方面HA吸收过滤了紫外光,从而降低了PDS的活化[43];另一方面,HA作为自由基清除剂,与CMTD竞争
${\rm{SO}}_4^{-}\cdot $ [44]或·OH[45]自由基,从而降低了CMTD的降解效率. 然而模拟预测值低于实际值,这可能是由于NOM中的酚类[46]和醌类[47] 等含氧官能团也可以激活PDS以产生${\rm{SO}}_4^{-}\cdot $ ,这些活性物质同样贡献了CMTD的降解. 然而由于NOM存在下自由基形成的复杂性,本模型未包含其基元反应,导致了模拟预测值低于实际值.综上所述,CMTD在不同水基质(Cl−,
${\rm{HCO}}_3^{-} $ 和NOM)影响下,HRAs的间接降解受到抑制,${\rm{SO}}_4^{-}\cdot $ 比·OH具有更高的自由基浓度,且kžOH/CMTD与$k_{{\rm{SO}}_4^{-}\cdot/{\rm CMTD}} $ 为同一数量级,所以${\rm{SO}}_4^{-}\cdot $ 是间接光解的主导自由基. -
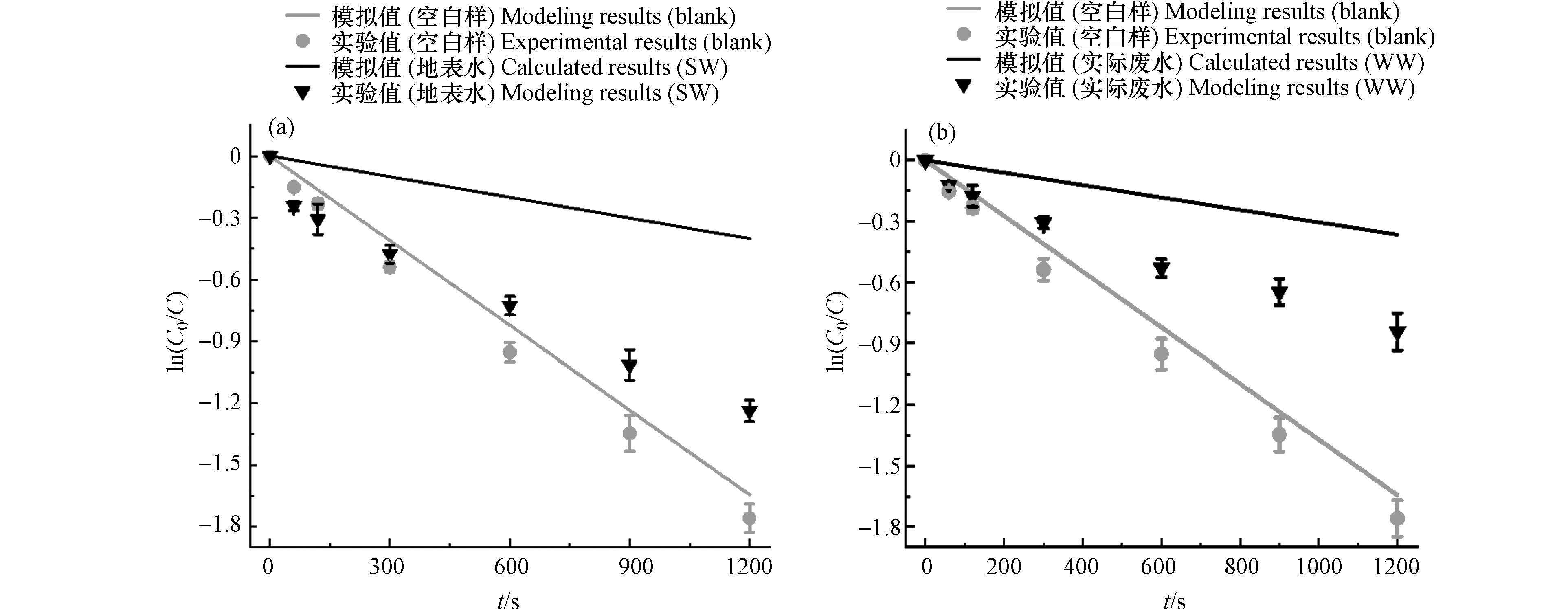
本实验探究了CMTD在不同水样下的降解情况,如图7所示. 图7(a)和图7(b)分别为污染物在地表水(SW)和实际废水(WW)及其空白样中的降解. 线和点分别表示水样中CMTD降解的模型预测值和实验值kobs.
SW和WW水样的各成分如表3所示,包括TOC、Cl−、
${\rm{NO}}_3^{-} $ 和${\rm{PO}}_4^{3-} $ . 为了评估在SW和WW水样中UV/PDS对HRAs的去除,重点考虑了Cl−,碱度,NOM的影响. 模型也包含了SW和WW在254 nm处的水基质吸附系数,分别为0.051 cm−1和0.086 cm−1.实验测得的CMTD降解拟一级速率常数如下:kobs, blank> kobs, SW > kobs, WW,这可能与水基质中复杂离子的光吸收和自由基清除效应有关. 模型预测值低估了实际水样中的降解,这是因为复杂物质与PDS反应产生的衍生自由基复杂多样,未全部包含在模型中,故导致了模型预测值与实际值的差异.
-
(1)UV/PDS体系可有效去除HRAs,反应20 min后,FMTD、RNTD、RXTD、NZTD和CMTD的去除率分别为 87.96%、99.36%、93.13%、97.42%和99.27%. CMTD的降解分为直接光解和间接光解,其中间接光解主导了CMTD的降解.
(2)拟稳态动力学模型研究中选取CMTD为研究对象,随着PDS用量的增加,间接光解的贡献增大,
${\rm{SO}}_4^{-}\cdot $ 是降解HRAs的主要自由基.(3)酸性条件有利于CMTD的降解,模型在中性和碱性条件下可以较好的模拟实验值.
(4)水体基质(Cl−,
${\rm{HCO}}_3^{-} $ 和NOM)添加在地表水和实际废水中,CMTD的降解均受到一定的抑制,出现模拟计算结果与实验值偏差较大的情况. 这是由于水体基质的复杂性,一方面降低体系的${\rm{SO}}_4^{-}\cdot $ 或·OH自由基,一方面生成衍生自由基有助于污染物降解.
紫外/过二硫酸盐对组胺H2受体拮抗剂的降解特性及自由基模拟
Degradation of histamine H2-receptor antagonists by UV/PDS: Kinetics and radical modeling
-
摘要: 研究了基于硫酸根的高级氧化技术UV/过二硫酸盐(UV/PDS)对水体中组胺H2受体拮抗剂(HRAs)的降解,并选取HRAs中的典型物质西咪替丁(CMTD)为目标污染物. 采用竞争动力学方法得到了HRAs和·OH及
${\rm{SO}}_4^{-}\cdot $ 反应的二级速率常数,k·OH/HRAs为(2.8—14.6)×109 L·mol−1·s−1,$k_{{\rm{SO}}_4^{-}\cdot /{\rm{HRAs}}} $ 为(0.81—8.10)×109 L·mol−1·s−1. 研究在实验基础上建立了UV/PDS的自由基拟稳态模型,模拟结果表明,UV/PDS对污染物的降解,其间接光解起主要作用,体系中·OH和${\rm{SO}}_4^{-}\cdot $ 是间接光解的主导自由基. 在(0.1—0.5) mmol·L−1 PDS投加量下,·OH和${\rm{SO}}_4^{-}\cdot $ 的浓度分别为(3.85—5.16) ×10−16 mol·L−1,(1.21—1.68) ×10−13 mol·L−1,${\rm{SO}}_4^{-}\cdot $ 对污染物的降解起主导作用. 酸性条件下自由基浓度相对更高,从而促进了CMTD的去除. 水体基质(Cl−、${\rm{HCO}}_3^{-} $ 和NOM)存在条件下,CMTD的降解受到一定的抑制,模拟结果表明自由基浓度显著降低;但是模拟结果与实验结果有一定偏差,主要是基质存在下生成了衍生自由基,由于衍生自由基的复杂性而未计入模型计算中导致. 在实际水样中应用的研究表明,UV/PDS可以有效降解地表水(SW)和实际废水(WW)中的CMTD,具有良好的应用前景.Abstract: The degradation of histamine H2-receptor antagonist (HRAs) by sulfate radical based advanced oxidation processes (SR-AOPs) of UV/peroxydisulfate (UV/PDS) was investigated in this study. Cimetidine (CMTD), a typical compound of HRAs, was selected as the target pollutant. The second-order rate constants between HRAs with ·OH and${\rm{SO}}_4^{-}\cdot $ were obtained by competition kinetic approaches, with kžOH/HRAs in the range of (2.8—14.6)×109 L·mol−1·s−1 and$k_{{\rm{SO}}_4^{-}\cdot /{\rm{HRAs}}} $ in the range of (0.81—8.10)×109 L·mol−1·s−1, respectively. Based on experimental results, a pseudo steady-state kinetic model was developed to calculate the radical concentration. The modeling results showed that CMTD degradation was mainly caused by indirect photolysis, which dominated by ·OH and${\rm{SO}}_4^{-}\cdot $ . With the dosage of (0.1—0.5) mmol·L−1 PDS, the ·OH and${\rm{SO}}_4^{-}\cdot $ concentrations were in the range of (3.85—5.16) ×10−16 mol·L−1 and (1.21—1.68) ×10−13 mol·L−1, respectively. Thereby,${\rm{SO}}_4^{-}\cdot $ was deemed as the major contributor to CMTD degradation. The radical concentration was relatively higher under acidic conditions, which promoted the degradation of CMTD. In the presence of water matrices, i.e., Cl−,${\rm{HCO}}_3^{-} $ and NOM, the degradation of CMTD was inhibited to some extent. Modeling results confirmed that the radical concentration decreased significantly. However, a certain deviation between modeling and experimental results was observed in the presence of water matrices, which might be caused by the secondary radicals derived from ·OH and${\rm{SO}}_4^{-}\cdot $ reacting with water matrices. Due to the complexity of secondary radicals, the reactions of derived radicals were not included in the model. The application of UV/PDS in real water samples showed that CMTD could be effectively degraded by UV/PDS in surface water (SW) and wastewater (WW), which is promising in water treatment.-
Key words:
- histamine H2-receptor antagonist /
- Cimetidine /
- UV/PDS /
- radical modeling.
-

-
表 1 HRAs的化学结构
Table 1. Chemical Structures of HRAs
污染物
Contaminants分子量
Molecular weight分子式
Molecular formula化学结构式
Chemical structural formula雷尼替丁 314.40 C13H22N4O3S 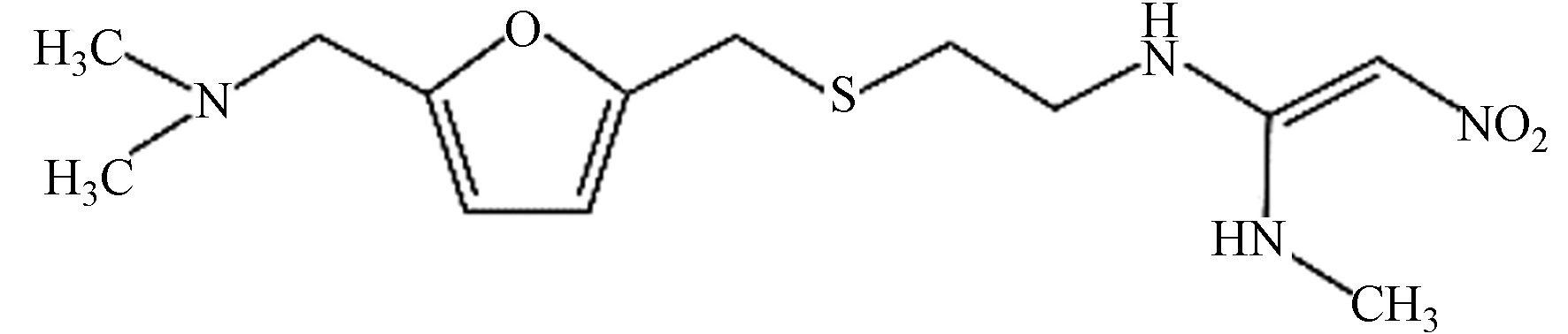
尼扎替丁 331.46 C12H21N5O2S2 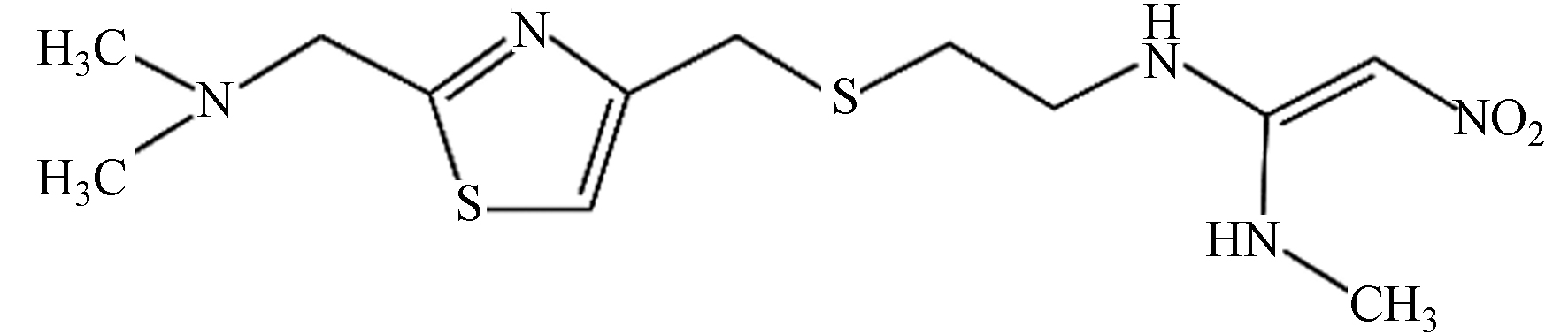
法莫替丁 337.45 C8H15N7O2S3 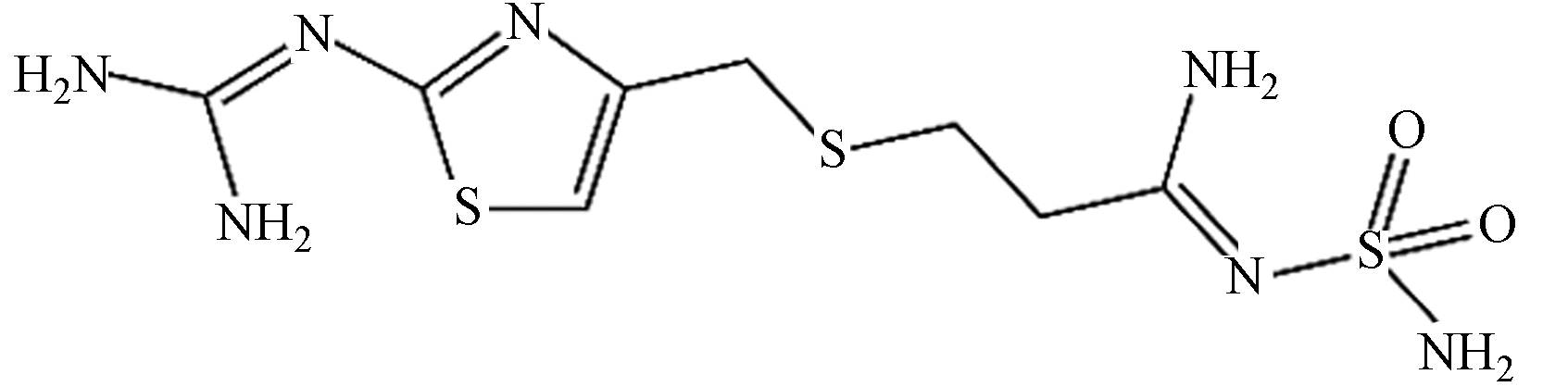
西咪替丁 252.34 C10H16N6S 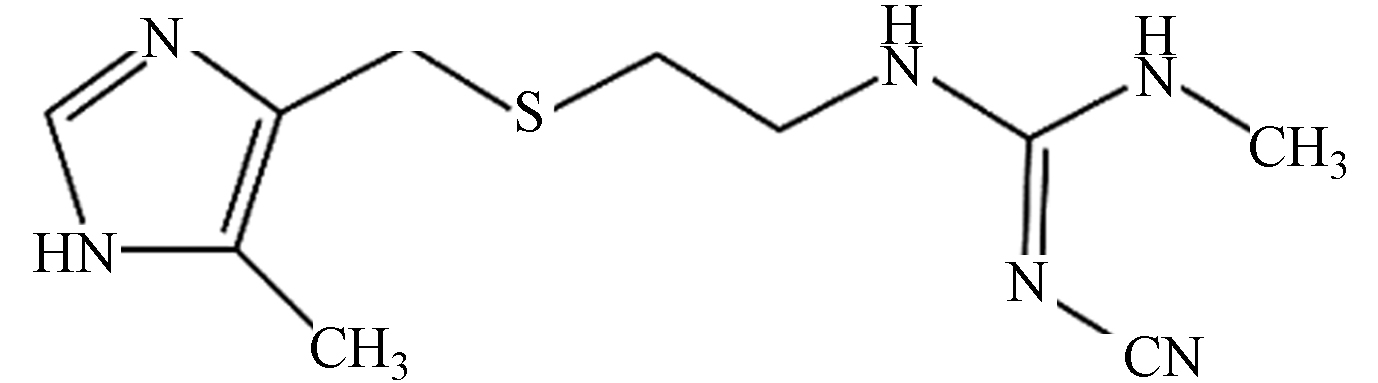
罗沙替丁 306.19 C17H26N2O3 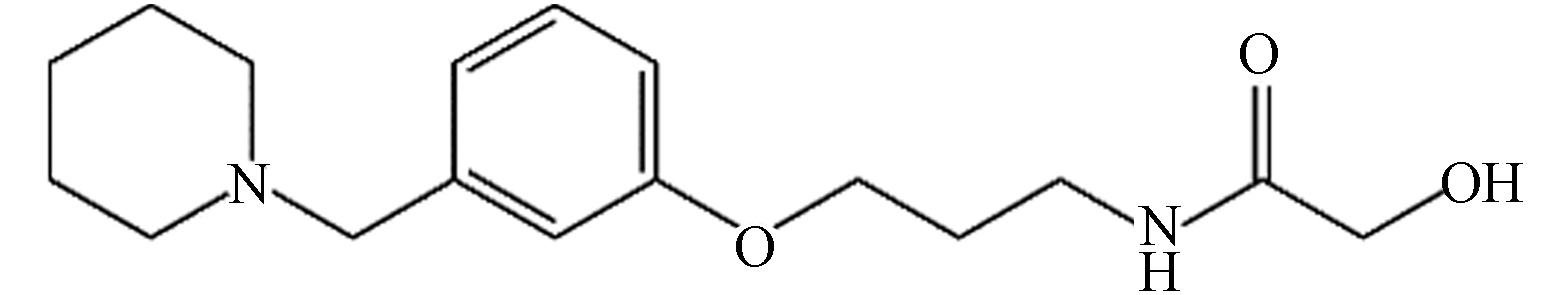
表 2 拟稳态模型中涉及的基本反应和速率常数
Table 2. Reactions and rate constants involved in the pseudo steady-state model
方程式
Equations速率常数
Rate constants参考文献
References1 ${\text{S} }_{\text{2} }{ {\text{O} }_{\text{8} } }^{2- }\text{+}\text{hv}\text{→2S} {\text{O} }_{\text{4} } ^{- }\cdot$ Φ254 = 0.70 mol·einstein−1 ${\epsilon }_{ {\mathrm{S} }_{2}{ {\mathrm{O} }_{8} }^{ {2-} } }^{254}$ [22-23] 2 $ \text{HRAs+}\text{hv}\text{→products} $ rUV,HRAs = -ΦHRAs·I·fHRAs·(1-e-A)
fHRAs = 2.303·b·εHRAs·CHRAs/A3 $\text{S} {\text{O} }_{\text{4} } ^{- }\cdot\text{+}{\text{S} }_{\text{2} } {\text{O} }_{\text{8} } ^{2- }\text{→}{\text{S} }_{\text{2} } {\text{O} }_{\text{8} } ^{- }\cdot \text{+S} {\text{O} }_{\text{4} } ^{2- }$ k1 = 6.62×105 L·mol−1·s−1 [24] 4 $ \text{Alkaline}\text{pH} $ $\text{S} {\text{O} }_{\text{4} } ^{-}\cdot \text{+H}{\text O} ^{-}\text{→HO}\cdot \text{+S} {\text{O} }_{\text{4} } ^{2- }$ k2 = 6.25×107 L·mol−1·s−1 [25] 5 $ \text{All pHs} $ $\text{S} {\text{O} }_{\text{4} } ^{-}\cdot \text{+}\left({\text{H} }_{\text{2} }\text{O}\right)\text{→HO}\cdot \text{+ HS} {\text{O} }_{\text{4} } ^{-}$ k2’[H2O] = 4.6×102 s−1 [26] 6 $\text{S} {\text{O} }_{\text{4} } ^{-}\cdot \text{+ S} {\text{O} }_{\text{4} } ^{-}\cdot \text{→}{\text{S} }_{\text{2} } {\text{O} }_{\text{8} } ^{2- }$ k3 = 8.1×108 L·mol−1·s−1 [24] 7 $ \text{HO}\cdot \text{+HO}\cdot \text{→}{\text{H}}_{\text{2}}{\text{O}}_{\text{2}} $ k4 = 5×109 L·mol−1·s−1 [27] 8 ${\text{S} }_{\text{2} } {\text{O} }_{\text{8} } ^{{2-} }\text{+HO}\cdot \text{→HS} {\text{O} }_{\text{4} } ^{-}\text{+ S} {\text{O} }_{\text{4} } ^{-}\cdot \text{+0.5}{\text{O} }_{\text{2} }$ k5 = 1.2×107 L·mol−1·s−1 [28] 9 $\text{S} {\text{O} }_{\text{4} } ^{-}\cdot \text{+ HO}\cdot \text{→HS} {\text{O} }_{\text{4} } ^{-}\text{+0.5}{\text{O} }_{\text{2} }$ k6 = 1.0×1010 L·mol−1·s−1 [28] 10 $\text{S} {\text{O} }_{\text{4} } ^{-}\cdot \text{+ R→products}$ $ \text{HO}\cdot \text{+ R→products} $ k7 = $k_{{\rm{SO}}_4^{-}\cdot /{\rm{HRAs}}} $
k7’ = k·OH/HRAs11 $\text{HS} {\text{O} }_{\text{4} } ^{-}\text{↔}{\text{H} }^{\text{+} }\text{+S} {\text{O} }_{\text{4} } ^{{2-} }$ pKa1 = 1.92 [29] 在Cl−存在条件下 12 $\text{S} {\text{O} }_{\text{4} } ^{-}\cdot \text{+ C}{\text{l} }^{-}\text{→S} {\text{O} }_{\text{4} } ^{{2-} }\text{+Cl}\cdot$ k8 = 4.7×108 L·mol−1·s−1 [30] 13 $\text{S} {\text{O} }_{\text{4} } ^{{2-} }\text{+Cl}\cdot \text{→S} {\text{O} }_{\text{4} } ^{-}\cdot \text{+ C}{\text{l} }^{-}$ k9 = 2.5×108 L·mol−1·s−1 [26] 14 $ \text{OH}\cdot \text{+ C}{\text{l}}^{-}\text{→ClO}{\text{H·}}^{-} $ k10 = 4.3×109 L·mol−1·s−1 [31] 15 $ \text{ClO}{\text{H·}}^{-}\text{→OH}\cdot \text{+ C}{\text{l}}^{-} $ k11 = 6.1×109 s−1 [31] 16 $ \text{Cl}\cdot \text{+}{\text{H}}_{\text{2}}\text{O→ClO}{\text{H·}}^{-}\text{+}{\text{H}}^{\text{+}} $ k12[H2O] = 1.3×103 s−1 [32] 17 $ \text{ClOH}{\text{·}}^{-}\text{+}{\text{H}}^{\text{+}}\text{→Cl}\cdot \text{+}{\text{H}}_{\text{2}}\text{O} $ k13 = 2.1×1010 L·mol−1·s−1 [31] 在 ${\rm{HCO}}_3^{-} $ 18 $\text{S} {\text{O} }_{\text{4} } ^{-}\cdot \text{+HC}{ {\text{O} }_{\text{3} } }^{-}\text{→C}{\text{O} }_{\text{3} }\cdot \text{+}{\text{H} }^{\text{+} }\text{+S} {\text{O} }_{\text{4} } ^{{2-} }$ k14 = 3.6×106 L·mol−1·s−1 [22] 19 $\text{S} {\text{O} }_{\text{4} } ^{-}\cdot \text{+C}{ {\text{O} }_{\text{3} } }^{2- }\text{→C} {\text{O} }_{\text{3} } ^{-}\cdot \text{+S} {\text{O} }_{\text{4} } ^{2- }$ k15 = 6.5×106 L·mol−1·s−1 [33] 20 $\text{HO}\cdot \text{+HC} {\text{O} }_{\text{3} } ^{-}\text{→C} {\text{O} }_{\text{3} } ^{-}\cdot \text{+}{\text{H} }_{\text{2} }\text{O}$ k16 = 8.5×106 L·mol−1·s−1 [8] 21 $\text{HO}\cdot \text{+C} {\text{O} }_{\text{3} } ^{2- }\text{→C} {\text{O} }_{\text{3} } ^{-}\cdot \text{+ O}{\text{H} }^{-}$ k17 = 4.2×108 L·mol−1·s−1 [8] 22 ${\text{H} }_{\text{2} }\text{C}{ {\text{O} }_{\text{3} } }^{\text{*} }\text{↔}{\text{H} }^{\text{+} }\text{+HC} {\text{O} }_{\text{3} } ^{-}$ pKa2 = 6.3 [34] 23 $\text{HC} {\text{O} }_{\text{3} } ^{-}\text{↔}{\text{H} }^{\text{+} }\text{+C} {\text{O} }_{\text{3} } ^{2- }$ pKa3 = 10.3 [31] 在NOM存在条件下 24 $\text{S} {\text{O} }_{\text{4} } ^{-}\cdot \text{+NOM→products}$ k18 = 2.35×107 L·molC−1·s−1 [35] 25 $ \text{HO}\cdot \text{+NOM→products} $ k19 = 3.0×108 L·molC−1·s−1 [20] 表 3 实际水样的表征
Table 3. Characteristics of the water samples
水样
Water samplespH TOC/(mg·L−1) Cl-/(mmol·L−1) NO3- /(mmol·L−1) PO43- /(mmol·L−1) Fe/(mmol·L−1) Mn/ (mmol·L−1) SW 6.57 0.79 0.39 0.0276 ND 3.35×10−6 6.74×10−6 WW 6.86 0.67 2.27 0.8009 ND ND 2.45×10−6 表 4 HRAs与·OH和
${\rm{SO}}_4^{-}\cdot $ Table 4. The second-order rate constants of ·OH and
${\rm{SO}}_4^{-}\cdot $ 组胺H2受体拮抗剂
HRAsk·OH/HRAs /(L·mol−1·s−1) $k_{{\rm{SO}}_4^{-}\cdot /{\rm{HRAs}}} $ FMTD 7.31×109 7.07×109 RNTD 2.80×109 2.46×109 RXTD 3.12×109 0.81×109 NZTD 3.54×109 0.85×109 CMTD 1.46×1010 8.10×109 表 5 不同PDS投加量下UV/PDS模型体系中·OH和
${\rm{SO}}_4^{-}\cdot $ Table 5. Concentration distribution of ·OH and
${\rm{SO}}_4^{-}\cdot $ [PDS]/(mmol·L−1) [·OH]/(mol·L−1) [ ${\rm{SO}}_4^{-}\cdot $ 0.1 3.85 × 10−16 1.21 × 10−13 0.2 4.20 × 10−16 1.34 × 10−13 0.3 4.54 × 10−16 1.45 × 10−13 0.4 4.86 × 10−16 1.57 × 10−13 0.5 5.16 × 10−16 1.68 × 10−13 注:([CMTD] = 0.01 mmol·L−1, PU-V = 3.2×10−8 Einstein·L−1·s−1). 表 6 不同pH条件下UV/PDS模型体系中·OH和
${\rm{SO}}_4^{2-} $ Table 6. Concentration distribution of ·OH and
${\rm{SO}}_4^{2-} $ pH [·OH]/(mol·L−1) [ ${\rm{SO}}_4^{2-} $ 3 4.14 × 10-16 1.35 × 10-13 5 4.14 × 10-16 1.35 × 10-13 7 4.20 × 10-16 1.34 × 10-13 9 1.03 × 10-16 1.34 × 10-13 注:([CMTD] = 0.01 mmol·L−1, [PDS] = 0.2 mmol·L−1, PU-V = 3.2×10−8 Einstein·L−1·s−1). 表 7 Cl−存在条件下UV/PDS模型体系中·OH和
${\rm{SO}}_4^{2-} $ Table 7. Concentration distribution of ·OH and
${\rm{SO}}_4^{2-} $ [Cl−]/(mmol·L−1) [·OH]/(mol·L−1) [ ${\rm{SO}}_4^{2-} $ 0.5 8.48 × 10-16 3.44 × 10-14 1.0 2.95 × 10-16 1.98 × 10-14 1.5 1.60 × 10-16 1.39 × 10-14 2.0 1.05 × 10-16 1.07 × 10-14 注:([CMTD] = 0.01 mmol·L−1, [PDS] = 0.2 mmol·L−1, PU-V = 3.2×10−8 Einstein·L−1·s−1, pH = 7). 表 8
${\rm{HCO}}_3^{-} $ ${\rm{SO}}_4^{-}\cdot $ Table 8. Concentration distribution of ·OH and
${\rm{SO}}_4^{-}\cdot $ ${\rm{HCO}}_3^{-} $ [ ${\rm{HCO}}_3^{-} $ [·OH]/(mol·L−1) [ ${\rm{SO}}_4^{-}\cdot $ 0.5 4.10 × 10-16 1.31 × 10-13 1.0 3.81 × 10-16 1.28 × 10-13 1.5 3.64 × 10-16 1.25 × 10-13 2.0 3.47 × 10-16 1.22 × 10-13 注:([CMTD] = 0.01 mmol·L−1, [PDS] = 0.2 mmol·L−1, PU-V = 3.2×10−8 Einstein·L−1·s−1, pH = 7). 表 9 HA存在条件下UV/PDS模型体系中·OH和
${\rm{SO}}_4^{-}\cdot $ Table 9. Concentration distribution of ·OH and
${\rm{SO}}_4^{-}\cdot $ [HA]/(mg·L−1) [·OH]/(mol·L−1) [ ${\rm{SO}}_4^{-}\cdot $ 0.5 3.84 × 10-16 1.32 × 10-13 1.0 3.53 × 10-16 1.31 × 10-13 1.5 3.25 × 10-16 1.29 × 10-13 2.0 3.05 × 10-16 1.28 × 10-13 注:([CMTD] = 0.01 mmol·L−1, [PDS] = 0.2 mmol·L−1, PU-V = 3.2×10−8 Einstein·L−1·s−1, pH = 7). -
[1] XIANG Y, WU H H, LI L, et al. A review of distribution and risk of pharmaceuticals and personal care products in the aquatic environment in China [J]. Ecotoxicology and Environmental Safety, 2021, 213: 112044. doi: 10.1016/j.ecoenv.2021.112044 [2] DING G Y, CHEN G L, LIU Y D, et al. Occurrence and risk assessment of fluoroquinolone antibiotics in reclaimed water and receiving groundwater with different replenishment pathways [J]. Science of the Total Environment, 2020, 738: 139802. doi: 10.1016/j.scitotenv.2020.139802 [3] LI Z, LI M, ZHANG Z Y, et al. Antibiotics in aquatic environments of China: A review and meta-analysis [J]. Ecotoxicology and Environmental Safety, 2020, 199: 110668. doi: 10.1016/j.ecoenv.2020.110668 [4] PERRY J A, WRIGHT G D. The antibiotic resistance “mobilome”: Searching for the link between environment and clinic [J]. Frontiers in Microbiology, 2013, 4: 138. [5] 胡譞予. 水环境中抗生素对健康的危害 [J]. 食品与药品, 2015, 17(3): 215-219. doi: 10.3969/j.issn.1672-979X.2015.03.022 HU X Y. Harm of antibiotics in aquatic environment on health [J]. Food and Drug, 2015, 17(3): 215-219(in Chinese). doi: 10.3969/j.issn.1672-979X.2015.03.022
[6] OTURAN M A, AARON J J. Advanced oxidation processes in water/wastewater treatment: Principles and applications. A review [J]. Critical Reviews in Environmental Science and Technology, 2014, 44(23): 2577-2641. doi: 10.1080/10643389.2013.829765 [7] GIANNAKIS S, LIN K Y A, GHANBARI F. A review of the recent advances on the treatment of industrial wastewaters by Sulfate Radical-based Advanced Oxidation Processes (SR-AOPs) [J]. Chemical Engineering Journal, 2021, 406: 127083. doi: 10.1016/j.cej.2020.127083 [8] BUXTON G V, GREENSTOCK C L, HELMAN W P, et al. Critical Review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (·OH/·O− in Aqueous Solution [J]. Journal of Physical and Chemical Reference Data, 1988, 17(2): 513-886. doi: 10.1063/1.555805 [9] NETA P, HUIE R E, ROSS A B. Rate constants for reactions of inorganic radicals in aqueous solution [J]. Journal of Physical and Chemical Reference Data, 1988, 17(3): 1027-1284. doi: 10.1063/1.555808 [10] GANELLIN C R, DURANT G J, EMMETT J C. Some chemical aspects of histamine H2-receptor antagonists [J]. Federation Proceedings, 1976, 35(8): 1924-1930. [11] RADJENOVIĆ J, PETROVIĆ M, BARCELÓ D. Fate and distribution of pharmaceuticals in wastewater and sewage sludge of the conventional activated sludge (CAS) and advanced membrane bioreactor (MBR) treatment [J]. Water Research, 2009, 43(3): 831-841. doi: 10.1016/j.watres.2008.11.043 [12] KOLPIN D W, FURLONG E T, MEYER M T, et al. Pharmaceuticals, hormones, and other organic wastewater contaminants in US streams, 1999-2000: A national reconnaissance [J]. Environmental Science & Technology, 2002, 36(6): 1202-1211. [13] CASTIGLIONI S, BAGNATI R, FANELLI R, et al. Removal of pharmaceuticals in sewage treatment plants in Italy [J]. Environmental Science & Technology, 2006, 40(1): 357-363. [14] CHOI K, KIM Y, PARK J, et al. Seasonal variations of several pharmaceutical residues in surface water and sewage treatment plants of Han River, Korea [J]. Science of the Total Environment, 2008, 405(1/2/3): 120-128. [15] HOPPE P D, ROSI-MARSHALL E J, BECHTOLD H A. The antihistamine cimetidine alters invertebrate growth and population dynamics in artificial streams [J]. Freshwater Science, 2012, 31(2): 379-388. doi: 10.1899/11-089 [16] QIAN Y J, HUANG J J, LIU X, et al. Rapid oxidation of histamine H2-receptor antagonists by peroxymonosulfate during water treatment: Kinetics, products, and toxicity evaluation [J]. Water Research, 2020, 185: 116278. doi: 10.1016/j.watres.2020.116278 [17] KUHN H J, BRASLAVSKY S E, SCHMIDT R. Chemical actinometry [J]. Pure and Applied Chemistry, 1989, 61(2): 187-210. doi: 10.1351/pac198961020187 [18] ZHANG R C, SUN P Z, BOYER T H, et al. Degradation of pharmaceuticals and metabolite in synthetic human urine by UV, UV/H2O2, and UV/PDS [J]. Environmental Science & Technology, 2015, 49(5): 3056-3066. [19] 李珂, 刘振鸿, 钱雅洁, 等. 基于硫酸根自由基的高级氧化对头孢氨苄的降解特性 [J]. 环境工程学报, 2019, 13(1): 40-48. doi: 10.12030/j.cjee.201808012 LI K, LIU Z H, QIAN Y J, et al. Cefalexin degradation by advanced oxidation process based on sulfate radical [J]. Chinese Journal of Environmental Engineering, 2019, 13(1): 40-48(in Chinese). doi: 10.12030/j.cjee.201808012
[20] CRITTENDEN J C, TRUSSELL R R, HAND D W, et al. MWH's water treatment[M]. Hoboken, NJ, USA: John Wiley & Sons, Inc. , 2012. [21] LIANG C J, SU H W. Identification of sulfate and hydroxyl radicals in thermally activated persulfate [J]. Industrial & Engineering Chemistry Research, 2009, 48(11): 5558-5562. [22] QIAN Y J, GUO X, ZHANG Y L, et al. Perfluorooctanoic acid degradation using UV-persulfate process: Modeling of the degradation and chlorate formation [J]. Environmental Science & Technology, 2016, 50(2): 772-781. [23] YANG Y, PIGNATELLO J J, MA J, et al. Comparison of halide impacts on the efficiency of contaminant degradation by sulfate and hydroxyl radical-based advanced oxidation processes (AOPs) [J]. Environmental Science & Technology, 2014, 48(4): 2344-2351. [24] HUIE R E, CLIFTON C L, NETA P. Electron transfer reaction rates and equilibria of the carbonate and sulfate radical anions [J]. International Journal of Radiation Applications and Instrumentation. Part C. Radiation Physics and Chemistry, 1991, 38(5): 477-481. doi: 10.1016/1359-0197(91)90065-A [25] HERRMANN H, REESE A, ZELLNER R. Time-resolved UV/VIS diode array absorption spectroscopy of SOx−(x=3, 4, 5) radical anions in aqueous solution [J]. Journal of Molecular Structure, 1995, 348: 183-186. doi: 10.1016/0022-2860(95)08619-7 [26] YU X Y, BAO Z C, BARKER J R. Free radical reactions involving Cl•, Cl2-•, and SO4-• in the 248 nm photolysis of aqueous solutions containing S2O82- and Cl- [J]. The Journal of Physical Chemistry A, 2004, 108(2): 295-308. doi: 10.1021/jp036211i [27] KAMEL D, SIHEM A, HALIMA C, et al. Decolourization process of an azoïque dye (Congo red) by photochemical methods in homogeneous medium [J]. Desalination, 2009, 247(1/2/3): 412-422. [28] CRIQUET J, LEITNER N K V. Degradation of acetic acid with sulfate radical generated by persulfate ions photolysis [J]. Chemosphere, 2009, 77(2): 194-200. doi: 10.1016/j.chemosphere.2009.07.040 [29] LIANG C J, LAI M C. Trichloroethylene degradation by zero valent iron activated persulfate oxidation [J]. Environmental Engineering Science, 2008, 25(7): 1071-1078. doi: 10.1089/ees.2007.0174 [30] YUAN R X, WANG Z H, HU Y, et al. Probing the radical chemistry in UV/persulfate-based saline wastewater treatment: Kinetics modeling and byproducts identification [J]. Chemosphere, 2014, 109: 106-112. doi: 10.1016/j.chemosphere.2014.03.007 [31] JAYSON G G, PARSONS B J, SWALLOW A J. Some simple, highly reactive, inorganic chlorine derivatives in aqueous solution. Their formation using pulses of radiation and their role in the mechanism of the Fricke dosimeter [J]. Journal of the Chemical Society, Faraday Transactions 1:Physical Chemistry in Condensed Phases, 1973, 69: 1597. [32] LIANG C J, WANG Z S, MOHANTY N. Influences of carbonate and chloride ions on persulfate oxidation of trichloroethylene at 20 ℃ [J]. Science of the Total Environment, 2006, 370(2/3): 271-277. [33] ZUO Z H, CAI Z L, KATSUMURA Y, et al. Reinvestigation of the acid-base equilibrium of the (bi)carbonate radical and pH dependence of its reactivity with inorganic reactants [J]. Radiation Physics and Chemistry, 1999, 55(1): 15-23. doi: 10.1016/S0969-806X(98)00308-9 [34] STUMM W, MORGAN J J. Aquatic chemistry: Chemical equilibria and rates in natural waters/-3rd ed[M]. Wiley, 1996. [35] XIE P C, MA J, LIU W, et al. Removal of 2-MIB and geosmin using UV/persulfate: Contributions of hydroxyl and sulfate radicals [J]. Water Research, 2015, 69: 223-233. doi: 10.1016/j.watres.2014.11.029 [36] RICKMAN K A, MEZYK S P. Kinetics and mechanisms of sulfate radical oxidation of β-lactam antibiotics in water [J]. Chemosphere, 2010, 81(3): 359-365. doi: 10.1016/j.chemosphere.2010.07.015 [37] FURMAN O S, TEEL A L, WATTS R J. Mechanism of base activation of persulfate [J]. Environmental Science & Technology, 2010, 44(16): 6423-6428. [38] LATCH D E, STENDER B L, PACKER J L, et al. Photochemical fate of pharmaceuticals in the environment: Cimetidine and ranitidine [J]. Environmental Science & Technology, 2003, 37(15): 3342-3350. [39] LIANG C J, WANG Z S, BRUELL C J. Influence of pH on persulfate oxidation of TCE at ambient temperatures [J]. Chemosphere, 2007, 66(1): 106-113. doi: 10.1016/j.chemosphere.2006.05.026 [40] DAS T N. Reactivity and role of SO5•- radical in aqueous medium chain oxidation of sulfite to sulfate and atmospheric sulfuric acid generation [J]. The Journal of Physical Chemistry A, 2001, 105(40): 9142-9155. doi: 10.1021/jp011255h [41] MÁRTIRE D O, ROSSO J A, BERTOLOTTI S, et al. Kinetic study of the reactions of chlorine atoms and Cl2•- radical anions in aqueous solutions. Ⅱ. Toluene, benzoic acid, and chlorobenzene [J]. The Journal of Physical Chemistry A, 2001, 105(22): 5385-5392. doi: 10.1021/jp004630z [42] LARSON R A, ZEPP R G. Reactivity of the carbonate radical with aniline derivatives [J]. Environmental Toxicology and Chemistry, 1988, 7(4): 265-274. doi: 10.1002/etc.5620070403 [43] LUO C W, JIANG J, MA J, et al. Oxidation of the odorous compound 2, 4, 6-trichloroanisole by UV activated persulfate: Kinetics, products, and pathways [J]. Water Research, 2016, 96: 12-21. doi: 10.1016/j.watres.2016.03.039 [44] GARA P M D, BOSIO G N, GONZALEZ M C, et al. A combined theoretical and experimental study on the oxidation of fulvic acid by the sulfate radical anion [J]. Photochemical & Photobiological Sciences, 2009, 8(7): 992. [45] WESTERHOFF P, MEZYK S P, COOPER W J, et al. Electron pulse radiolysis determination of hydroxyl radical rate constants with Suwannee River fulvic acid and other dissolved organic matter isolates [J]. Environmental Science & Technology, 2007, 41(13): 4640-4646. [46] AHMAD M, TEEL A L, WATTS R J. Mechanism of persulfate activation by phenols [J]. Environmental Science & Technology, 2013, 47(11): 5864-5871. [47] FANG G D, GAO J, DIONYSIOU D D, et al. Activation of persulfate by quinones: Free radical reactions and implication for the degradation of PCBs [J]. Environmental Science & Technology, 2013, 47(9): 4605-4611. -
















 下载:
下载: