-
近年来,挥发性有机化合物(VOCs)因其对空气污染的影响大而备受关注,如化学烟雾、雾霾等严重影响大气环境和人类健康[1]. 目前,美国环境保护署将超过300种化学物质列为挥发性有机化合物,其中大部分被认为是造成空气污染的主要污染物[2]. 在目前可以在工业上大规模应用的技术中,催化燃烧由于其转化效率高,能耗低而被认为是一种有前景的技术[3-5]. 用于催化氧化VOCs的催化剂中,75%是贵金属催化剂,一般认为比金属氧化物催化剂更有活性[6],但是贵金属元素的高价值和低储量限制了其实际应用. 近年来,部分研究以过渡金属混合氧化物作为替代贵金属催化剂的催化材料,因其中一些对卤素和硫等毒物表现出很高的选择性和抗性等原因而受到了广泛关注[7].
在处理废气的过程中,因为其中含有许多不同性质的有机污染物,这对催化剂的性能提出了更高的要求[8]. 整体式蜂窝催化剂促进活性组分的更高分散,成本低,热稳定性高[9]. 在固定床操作中,大量的球团或颗粒可能会由于烟气中的微粒而造成高流动阻力和堵塞问题. 相比之下,蜂窝状催化剂的压降低,耐磨性好,不易堵塞[10]. 在各种催化剂载体中,堇青石具有高机械稳定性和低热膨胀系数(CET)等特点而被广泛应用[11]. 但由于其表面光滑,活性组分难以固定,因此负载前的预处理是必要的. 催化剂载体常用的预处理方法有表面涂层法和化学处理法等,其中酸蚀在化学处理法中特别常见,包括无机酸(硝酸[11-13]、盐酸[12-13]、硫酸[14])和有机酸(柠檬酸[13]、草酸[10, 12-13]、甲酸、EDTA[13]等). 一方面酸蚀堇青石载体可以使Mg、Al等离子溶出,这可以产生更多的微孔与中孔,另一方面游离二氧化硅再沉淀使堇青石表面结构发生重组,同时微孔向中孔方向靠拢,中孔逐渐合并为大孔. Mccabe等[13]报道了在实验中当在质量浓度37%且煮沸的盐酸中连续酸蚀6 h比表面积达到最大,并表明酸处理使堇青石表面产生游离的二氧化硅,以结晶或无定形形式存在,使堇青石的表面形貌发生了一定程度的变化.
在加热方式上,目前催化燃烧装置多采用电加热对废气进行预热,但存在对催化剂加热慢、加热不均匀、设备能耗大等问题. 与催化氧化工艺中的传统电加热方法相比,微波加热可以快速、有选择性的加热催化剂上具有吸波能力的活性组分[15-18],同时微波加热降低了VOCs反应温度和活化能[19-20]. 本课题组之前的研究表明,与电炉加热相比,微波加热可以降低甲苯氧化温度和工艺能耗,并对催化剂结构与活性组分的分布几乎不产生影响[21-22].
根据已报道文献所知,催化剂预处理条件与催化剂性能密切相关,在酸蚀预处理的条件下,有关载体的负载性能及后续微波催化性能的报道较少. 有必要开展对载体酸蚀处理及后续负载性能及微波催化性能的系统研究. 本研究为制取具有高催化活性的堇青石催化剂,采用不同质量浓度的硝酸对堇青石载体进行预处理,随后负载Cu-Mn-Ce复合氧化物制成整体式催化剂,在微波条件下对气态甲苯进行催化氧化,研究了堇青石载体酸蚀预处理程度对催化剂催化性能的影响. 采用XRD、BET、SEM、XPS对负载催化剂进行表征,分析酸蚀预处理对于活性组分负载阶段的影响. 研究工作将为整体式催化剂载体酸蚀预处理提供理论参考.
-
通过切割商用蜂窝状堇青石单体获得直径28 mm、长度150 mm的圆柱体,然后超声清洗、干燥、并测试初始载体的吸水性能. 然后将样品浸泡在硝酸溶液中,酸处理条件和相应的催化剂代码如表1所示. 名称后面代码中的前两个数字表示酸的质量浓度,中间的两个数字表示酸处理温度,最后两个数字表示处理时间. 例如,用20% wt的硝酸在50 ℃下处理堇青石4 h制成CuMnCeOx/205004.然后用蒸馏水将所得酸蚀后载体彻底洗涤至中性,然后在105 ℃下保持1 h干燥,采用浸渍法制备了堇青石负载Cu-Mn-Ce催化剂:Ce(NO3)3·6H2O (99%), Mn(NO3)2 (50 %wt溶液),Cu(NO3)2·3H2O (99%). 浸渍完成后,载体在70 ℃下干燥过夜. 最后,将催化剂样品在450 ℃下煅烧5 h,自然冷却至室温. 堇青石载体的质量记为M1, M1:Cu:Mn:Ce的质量比为1:3.25%:3.25%:1.08%.
催化剂样品比表面积在液氮温度(-196 ℃)下使用氮气吸附-脱附法(BET)进行测定. 根据在473 K 预处理样品在77 K时的氮气吸附等温线确定比表面积,使用BET方程,以及比孔体积(Vs), P/P0=0.95. 孔径分布采用Barrett-Joyner-Halenda (BJH)模型计算孔隙分布(BET V-sorb 2800P,China) . 采用石墨单色仪和Cu Kα辐射(λ=0.154056 nm)的X射线衍射仪(Ultimany,Japan)在2θ范围10°—80°检测了催化剂的晶相,扫描速率为10(°)·min−1. 加速电压40 kV,加速电流40 mA.催化剂样品喷金后在JEOL JSM-6510LV(Japan)仪器上获得二次电子扫描图像. 催化剂表面活性组分的元素价态分析采用美国Thermo Fisher K-Alpha光谱仪采集非单色Cu Ka辐射的X射线光电子能谱,分析O 1s、Mn 2p、Ce 3d的光电子信号.
-
常压下在固定床石英管反应器(内径28 mm)中进行了对目标物甲苯的催化燃烧实验,实验装置由进气、微波源、尾气处理共3部分组成. 如图1所示.
将k型热电偶探头垂直插入床层,由温控仪显示床层温度. 由微量注射器注入的甲苯溶液与空气(经过干燥和净化)混合在1个三颈烧瓶中,该烧瓶放置在1个电热夹套上,在高温下保持恒定功率. 在固定床反应器中对模拟的甲苯废气进行微波催化氧化处理. 处理后的甲苯气体经有机溶剂和碱性溶液进一步净化后排放. 将进气(甲苯浓度为1.0 g·m−3)以2.0 L·min−1的流速(空速为1300 h−1)通入催化剂床层,通过调节微波功率使床层温度保持在150—200 ℃. 使用氢火焰离子化检测器的气相色谱系统(GC-FID, Agilent 6890N)对反应器出气进行分析,采用分流比50:1,进样量300 μL.所有实验数据均采用两次平行实验的综合数据.
-
通过不同条件的酸蚀条件考察堇青石载体吸水率及失重率的情况. 图2(a)通过在50 ℃,酸蚀时间4 h的情况下,通过改变硝酸浓度来改变酸蚀强度. 经20%、40%溶液处理后的失重率均为0.19%,这表明堇青石表面的Mg、Al离子有一定的溶出,同时堇青石载体表面的浮灰及杂质部分去除. 随着硝酸溶液浓度提升至60%,失重率下降至0.09%,表明在增加酸蚀浓度的同时载体表面的MgO、Al2O3基本反应完全,使得SiO2暴露,可能存在载体对溶液中的盐类的吸附导致失重率降低进一步吸附导致载体的失重率降低,这与梁文俊等[23]的研究结果基本保持一致. 同时3种处理条件下的堇青石的吸水率均无明显差异,这表明来自堇青石表面物质的吸水性能被完全去除,吸水性仅来自于堇青石本身. 图2(b)通过在50 ℃,硝酸浓度40% wt的条件下,通过改变酸蚀时间来改变酸蚀强度. 经4、8、16 h溶液处理后,失重率分别为0.19%、0.23%、0.53%且吸水率无明显差异. 这表明随着酸蚀时间的增加,溶液对堇青石内部基体的酸蚀效应进一步加剧,同时在长时间的酸蚀下伴随着堇青石表面的重组[13, 24],导致溶液可以进入短时间酸蚀下未曾进入的孔道内部,进而得到较高的失重率. 通过改变酸蚀浓度和酸蚀时间进而来改变酸蚀强度均未对堇青石载体的吸水性能造成明显差异,且失重率均维持在0.6%之下. 综上表明在50 ℃的酸蚀条件下,通过改变酸蚀浓度及时间并未对堇青石的机械强度造成明显影响,同时使堇青石的CuMnCeOx/405004比表面积及孔径分布发生一定变化,这有利于活性组分的负载以及催化剂处理VOCs时的吸附性能.
-
通过一系列实验研究了CuMnCeOx/堇青石催化剂在微波辐射下的催化氧化能力. 图3(a)显示了在6种不同条件下酸蚀堇青石载体并随后负载相同数量的活性组分催化剂的升温曲线(催化剂在恒定微波功率下的升温速率). 在微波辐照的情况下,介电极化导致负载金属氧化物的催化剂迅速升温[19, 25]. 与CuMnCeOx/405004相比,CuMnCeOx/NT在相同功率下床温差为40 ℃时,床温可在40 min内达到180 ℃,这可能有3点原因:(1)在酸蚀过程中提供部分吸波性能的Mg、Al氧化物以离子形态溶解在溶液中,同时酸处理降低了整体碱金属(Na和K等)含量[12], 降低了堇青石载体本身的吸波性能;(2)在CuMnCeOx/NT上生成的氧化物具有更多的晶体结构缺陷或更小的粒径,这意味着有更好的氧化还原能力和吸波性能[19]. 同时过渡金属氧化物、有变价离子共存的复合氧化物,或形成复合氧化物过程中有结晶转变的化合物,都可以通过电导损失或介质弛豫的方式将微波能转化为热能[15, 26],即微波辐射下的快速加热;(3)课题组之前的研究工作表明,经过不同强度酸蚀的堇青石载体,采用浸渍法负载活性组分的上载率维持在8%—9%,活性组分的脱落率维持在1%±0.05%[27]. 活性组分的实际上载率的差异也将会导致吸波性能略微的差异. 在催化活性的实验中,如图3(b)所示,CuMnCe氧化物在微波辐照下对微波能量的吸收较强,并在蜂窝催化剂表面形成热点,甲苯分子在这些热点迅速被氧化[28]. 在相同床层温度((150±8) ℃)、初始浓度(C0)为1 g·m−3、空速(GHSV)为1300 h−1的条件下测定了催化剂的催化活性. 在相同的反应条件下,CuMnCeOx/405004催化剂表现出最佳的活性,甲苯转化率接近80%. 因此,确定了最佳的载体酸蚀条件为CuMnCeOx/405004,故后续的催化剂活性实验和表征实验将围绕其展开.
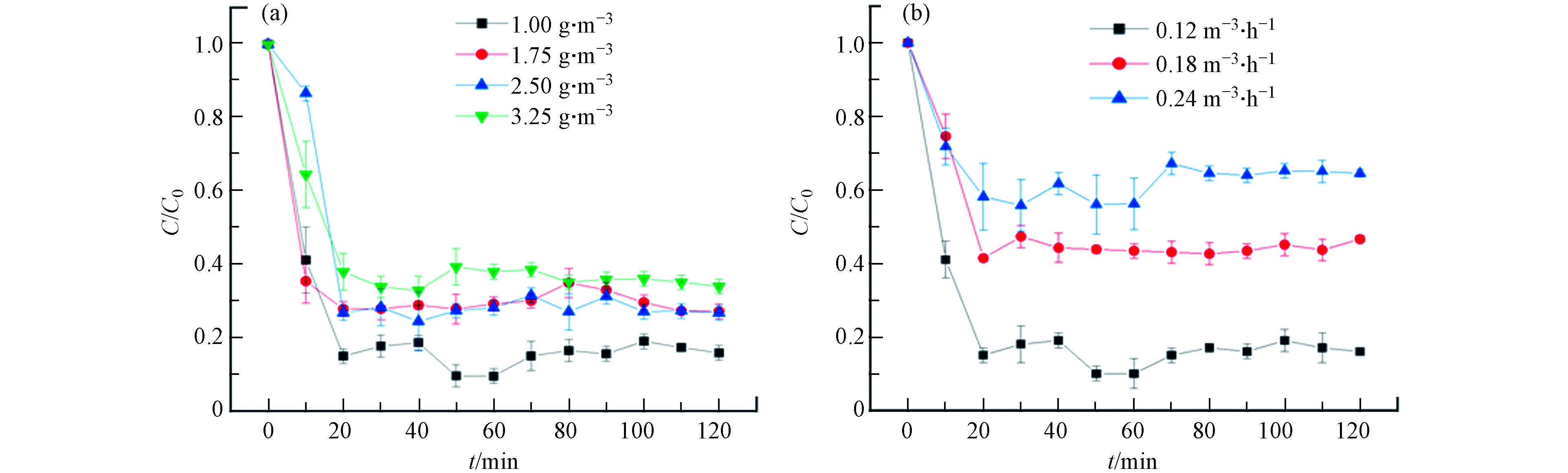
通过调节微波装置功率使催化剂床层温度恒定,图4(a)为T=200 ℃、Q=0.12 m3·h−1(GHSV=1300 h−1)条件下不同进气浓度下甲苯的脱除情况.
结果表明,在200 ℃时,甲苯的脱除率为85%,且随着原料气浓度的增加,甲苯的转化率逐渐降低. 当甲苯进气浓度达到3.25 g·m−3时,甲苯转化率降至60%. 进气初始浓度的增加意味着更多的甲苯会争夺催化剂表面有限的活性点位,这导致了催化效率的降低. 图4(b)为不同风量下(保持进气甲苯的浓度一定)的甲苯去除率,在0.12 m3·h−1的风量下,甲苯去除率为85%. 同时,甲苯转化率随风速的增大而减小. 当空气流量达到0.24 m3·h−1 (GHSV=2600 h−1)时,甲苯转化率降至55%. 根据Mars-van-Krevelen(MvK)模型[7], 随着空气流量的增加,甲苯分子在床上停留的时间越短,甲苯分子与氧(晶格氧或吸附氧)的接触时间越短. 同时,微波辐射的非热效应也造成甲苯分子的分解,非热效应可以看作是分子的搅动或搅拌,其中偶极子在微波中转动被分子中的键所阻挡,可能进而引起少量的甲苯分子的断键随后进一步转化分解[19].
-
酸蚀堇青石载体可以直接去除其表面的金属离子,在酸蚀开始阶段将产生微孔,但不会产生中孔,如要产生中孔堇青石表面结构必须重组,其中溶解的二氧化硅的再沉积涉及到这个过程[11, 13]. 从表2中可以看出,在相同的酸蚀时间条件下,BET比表面积随酸浓度增加有略微的增长,其中CuMnCeOx/405004相比于CuMnCeOx/205004的比表面积减少,这可能是由于活性组分在浸渍和烧结过程中在堇青石表面广泛分散,形成较小的活性组分颗粒,堵塞能提供比表面积较大的微孔,在孔表面或孔周围形成活性组分的晶相[12, 29]. 进一步增加酸液浓度,催化剂比表面积孔径孔容进一步增加这可能有两点原因:(1) 活性组分在大孔周围团聚,使原本可能堵塞的微孔和中孔暴露出来.
图5(a、b、c)分别为CuMnCeOx/205004、CuMnCeOx/405004 、CuMnCeOx/60500的表面形貌.
在图5(b)中活性组分的颗粒广泛分布在催化剂表面,且与其他两种催化剂相比粒径较小,能提供较大的接触面积,这是其甲苯转化率高的原因之一,同时图5(c)与其它两组催化剂相比,表面的活性组分发生了明显的聚集;(2)与堇青石载体本身相关. 在图5中,(d)、(e)、(f)为相对应的酸蚀后未负载前的表面形貌. 从图5(d)、(e)中可以看出随着溶液浓度的增大,堇青石的孔隙率明显提高,图5(f)进一步增加溶液浓度后孔隙率降低,孔径变大. 随着酸蚀强度的增加,Mg、Al离子浸出所产生的微孔,以及堇青石表面重组所表现的中孔合并为大孔会导致比表面积增加、孔径孔容的增大[11].表2中在相同的酸蚀浓度下,将CuMnCeOx/405004、CuMnCeOx/405008、CuMnCeOx/405016其三者的孔径分布进行比较,可以发现其三者比表面积呈现先减小后增大的趋势,但孔径孔容逐渐增大,这表明溶液可以进入短时间酸蚀下未曾进入的孔道内部,产生更多的微孔(这可能在负载过程中被堵塞导致比表面积降低),同时中孔向大孔方向移动.
图6显示了几中不同预处理条件催化剂的孔径分布.CuMnCeOx/405004在12—14 nm处以及20—30 nm处都展示了极好的孔隙率,同时,其平均孔径相对较低,这与SEM表征相符Williamse等[30]报道称,在用于废气流中的甲苯氧化中,具有更高结构孔隙率的介孔Ti-HMS催化剂上观察到较低的起燃温度,这说明结构孔隙率对催化剂性能有显著的正向影响. Lu等[31]表明,当活性组分浸渍在高介孔含量的碳载体上时,活性组分的高度分散导致催化活性的提高,促进了大分子和离子在载体表面的渗透. 综上CuMnCeOx/405004表现出良好的孔隙率,在吸附过程中催化剂可以更有效地吸附甲苯,也可以为催化燃烧提供更多的活性位点,进而可以提升催化剂的催化效率.
-
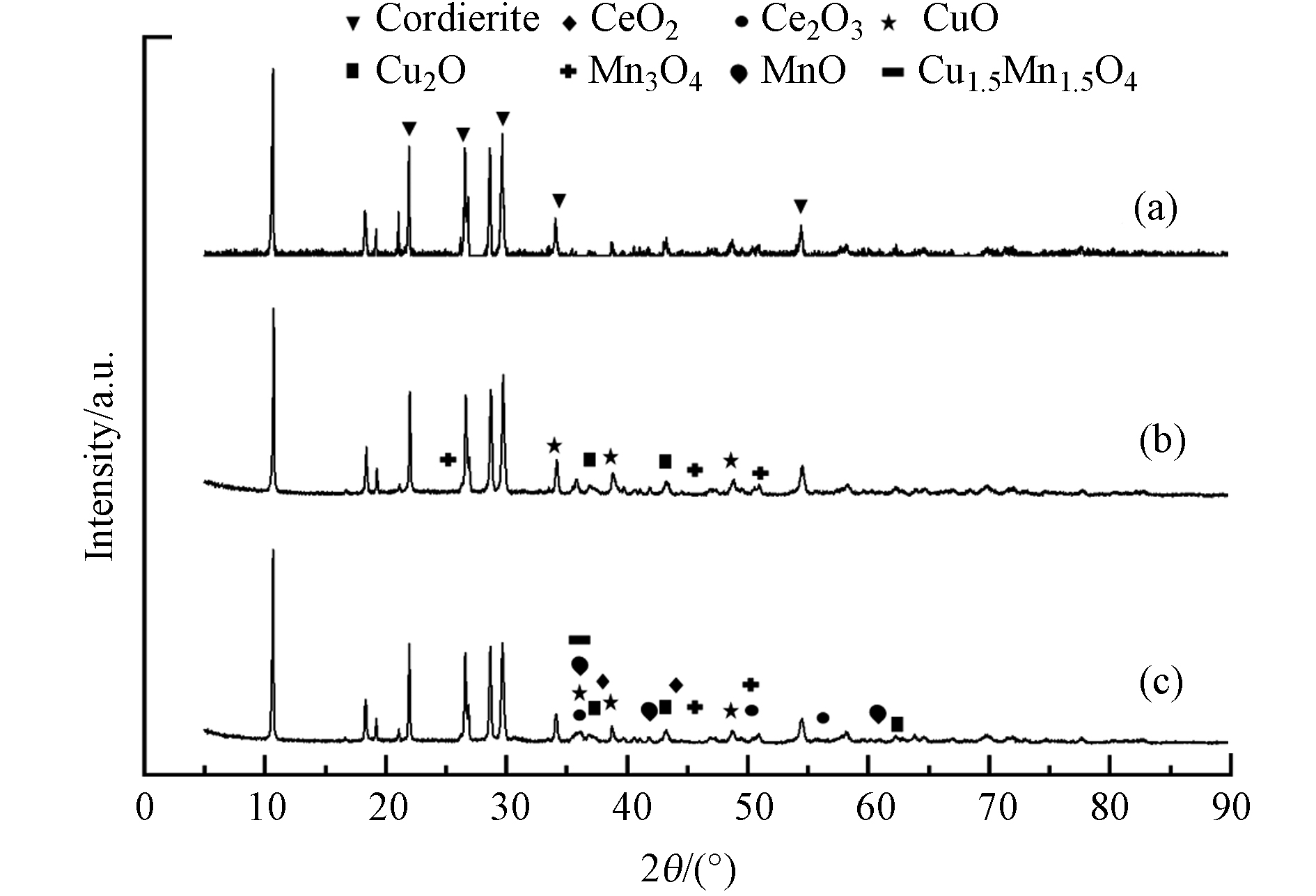
为了进一步了解负载后孔径差异对活性组分的影响,本研究测定了催化剂的XRD谱图,如图7所示.
尽管堇青石的衍射线存在,且与催化剂的大部分氧化物相的反射重叠并掩盖了绝大部分可能存在的氧化物的衍射峰,但在图7(XRD)中仍可以看到相对较弱活性组分的衍射峰,其中(a)为堇青石载体,(b)、(c)分别为CuMnCeOx/NT、CuMnCeOx/405004. 两种催化剂上都显示其表面存在铜锰铈的氧化物,其中CuMnCeOx/405004表面检出存在Cu1.5Mn1.5O4(ICCD PDFNO.02-70-0262),这些相对弱的衍射峰一定程度上说明3种氧化物以无定形态分散在整个催化剂表面,其中存在CuMnCe氧化物掺杂现象或是Cu、Mn的氧化物附着在Ce氧化物表面[32].Cu2+/Cu+、Mn4+/Mn2+、Ce4+/Ce3+之间的价态变化能引起电子的转移过程. 其他的研究者证实铜锰尖晶石在多种情况下都具有较高的活性[29],其中Cu1.5Mn1.5O4 是主要活性中心,MnOx既是活性中心也可以储存以及为活性中心传输氧[32],以完全氧化降解有机分子. 此外氧化铈的氧化还原性能及其晶格氧的高不稳定性是影响氧化铈催化反应活性的最重要因素[33],含铈过渡金属复合氧化物因其储氧容量大、氧空位丰富、Ce3+/Ce4+价态变化引起的氧化还原性能强而被认为可以有效提高催化性能[5].
-
利用XPS研究了催化剂表面元素组成和化学信息状态,CuMnCeOx/405004和CuMnCeOx/NT样品中Ce 3d、Mn 2p、O 1s电子能级的XPS谱图如图8所示.
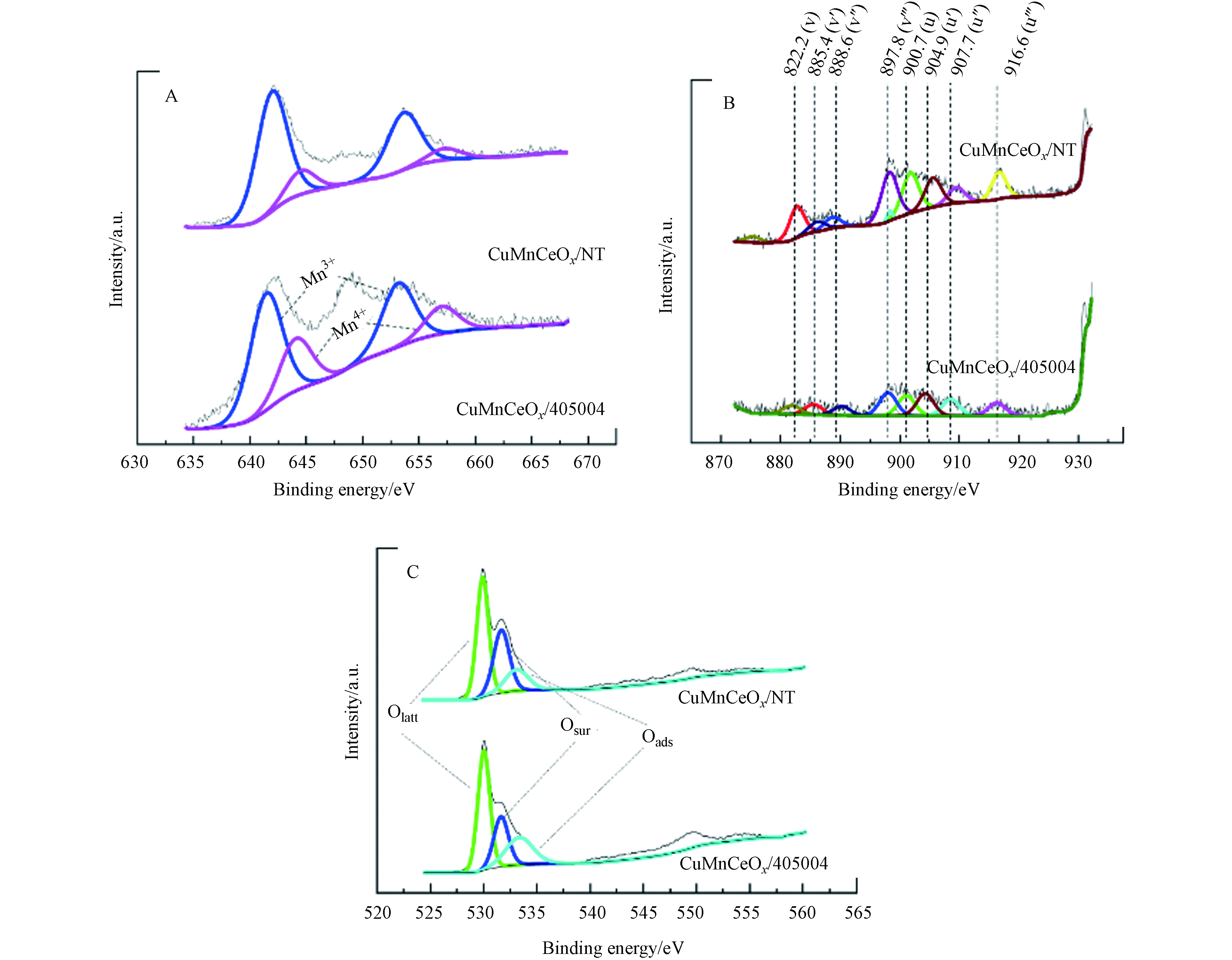
如表3所示, Cu、Mn的表面含量以及Mn、Ce、O的化学态及比例均以列出. 图8(A)为Mn 2p的XPS谱图,Mn 2p3/2和Mn 2p1/2的结合能与文献报道的相似[34-35]. 其中BE=653.6 eV和642.1 eV与BE=653.2 eV和641.5 eV分别属于CuMnCeOx/ NT与CuMnCeOx/405004的Mn3+,BE=657.1 eV和645.3 eV与BE= 657.1 eV和644.4 eV分别属于CuMnCeOx/NT与CuMnCeOx/405004的Mn4+. 从表3可以看出,CuMnCeOx/405004(1.72)与CuMnCeOx/NT(1.26)表面的铜锰比均高于理论值1.0. 这说明与铜离子相比,锰离子更容易迁移到铈的晶格中去,且在酸蚀预处理下,更能有效地将金属离子引入到铈的晶格中. Chi等[36]的研究结果表明,在铈表面的大量Mn4+离子可能增强Cu+和Cu2+之间的氧化还原偶联能力. CuMnCeOx/405004相比于CuMnCeOx/NT来说有着更多的Mn4+,这将可能有助于甲苯的氧化.
在图8(B)中,可以识别出自旋轨道双峰对产生的8个峰,其中6个峰分别为v(882.2 eV), v”(888.6 eV),v”’(897.7 eV),u(900.7 eV), u”(907.9 eV),u”’(916.6 eV)来自Ce4+,v’(885.4 eV)和u’(904.9 eV)来自Ce3+[37]. 如表3所示,经过酸蚀预处理过后Ce3+的含量更多,与氧空位存在相关的Ce3+离子在氧化机制中发挥了关键作用,参与甲苯的活化(表面氧空位)和氧向表面材料的迁移(次表面氧空位). López等人[38]合成了一系列具有不同理化性质的Ce基纳米棒和立方体,表明在对于甲苯的氧化过程中无论使用高负载的催化剂或是低负载的纳米棒Ce3+的浓度与催化氧化能力之间都有明确的线性关系. 同时由于Ce的氧化物晶相中部分位点被Cu或Mn取代,导致结晶度降低,产生更多的晶格缺陷和氧空位,有利于氧迁移率的提高[32],可以促进甲苯的催化氧化.
对催化剂进行了O 1s XPS分析, 如图8(C)所示.Bielański等[39]提出了催化剂表面的3种晶格氧:O22−、O−和O2−,O22−和O−是亲电氧种,容易参与氧化,而亲核氧(O2−)在选择性氧化中起重要作用. O1s XPS谱可分解为3个峰[32]. (1) 529—530 eV的晶格氧(Olatt);(2)表面氧(Osur)在530—532 eV,指缺陷氧或表面氧物种;(3)533—534 eV的吸附氧物种(Oads)和吸附的水物种作为在表面的污染物. 图8(C)表明对于CuMnCeOx/405004,晶格氧的结合能在529.9 eV,而CuMnCeOx/NT的结合能在529.8 eV,经酸蚀预处理后的催化剂晶格氧向更高的结合能方向移动. 这意味着当发生电子转移过程时,可以产生更多的活性氧物种. 表3列出了两种催化剂表面吸附氧和晶格氧各占百分比,众所周知,Osur/Olatt比值越高,物种供氧能力越强,在低温下相比于晶格氧来说可还原性越强,这意味着在催化甲苯活化实验中,CuMnCeOx/405004应该具有更高的甲苯降解效率,这与催化剂活性实验结论相印证.
-
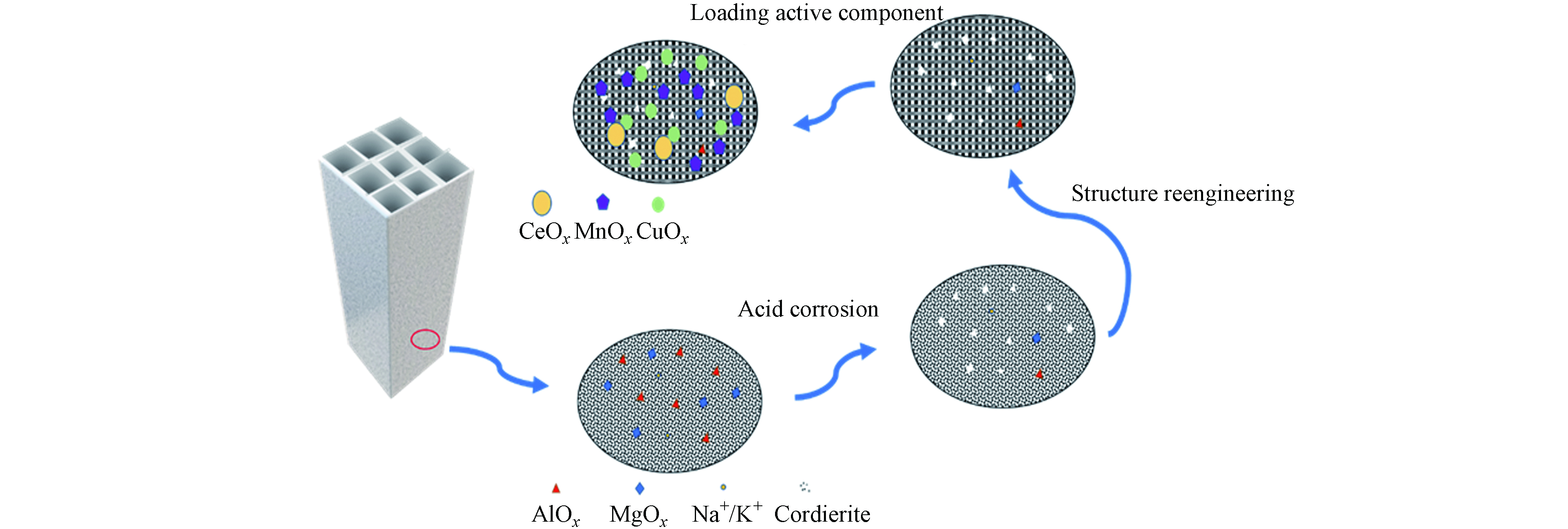
图9给出了堇青石酸蚀以及负载过程的机理. 酸处理可使Al、Mg离子和碱金属离子等从堇青石载体的结构中去除,同时去除表面上的浮灰或可以与酸发生相互作用的其他物质,并在堇青石表面形成非晶态的二氧化硅即游离二氧化硅. 酸蚀过程中会产生微孔和中孔即“增孔效应”,中孔的产生可能是由于堇青石结构的破坏和无定形二氧化硅的再沉淀,同时堇青石表面结构发生重组,同时微孔向中孔方向靠拢,中孔逐渐合并为大孔即酸蚀具有“扩孔效应”.
不同程度的酸蚀作用会导致堇青石的表面结构发生变化,控制酸蚀的强度可以获得具有理想表面结构的堇青石载体,这将有利于污染物的吸附. 在负载阶段,采用等体积浸渍法使Cu、Mn、Ce的3种离子负载到堇青石的孔隙之中,由于不同酸蚀强度造成载体表面结构的差异,进而影响氧化物晶相的形态和类型及比例的差异将影响晶格缺陷和氧空位的数量,即影响了氧迁移的效率.
-
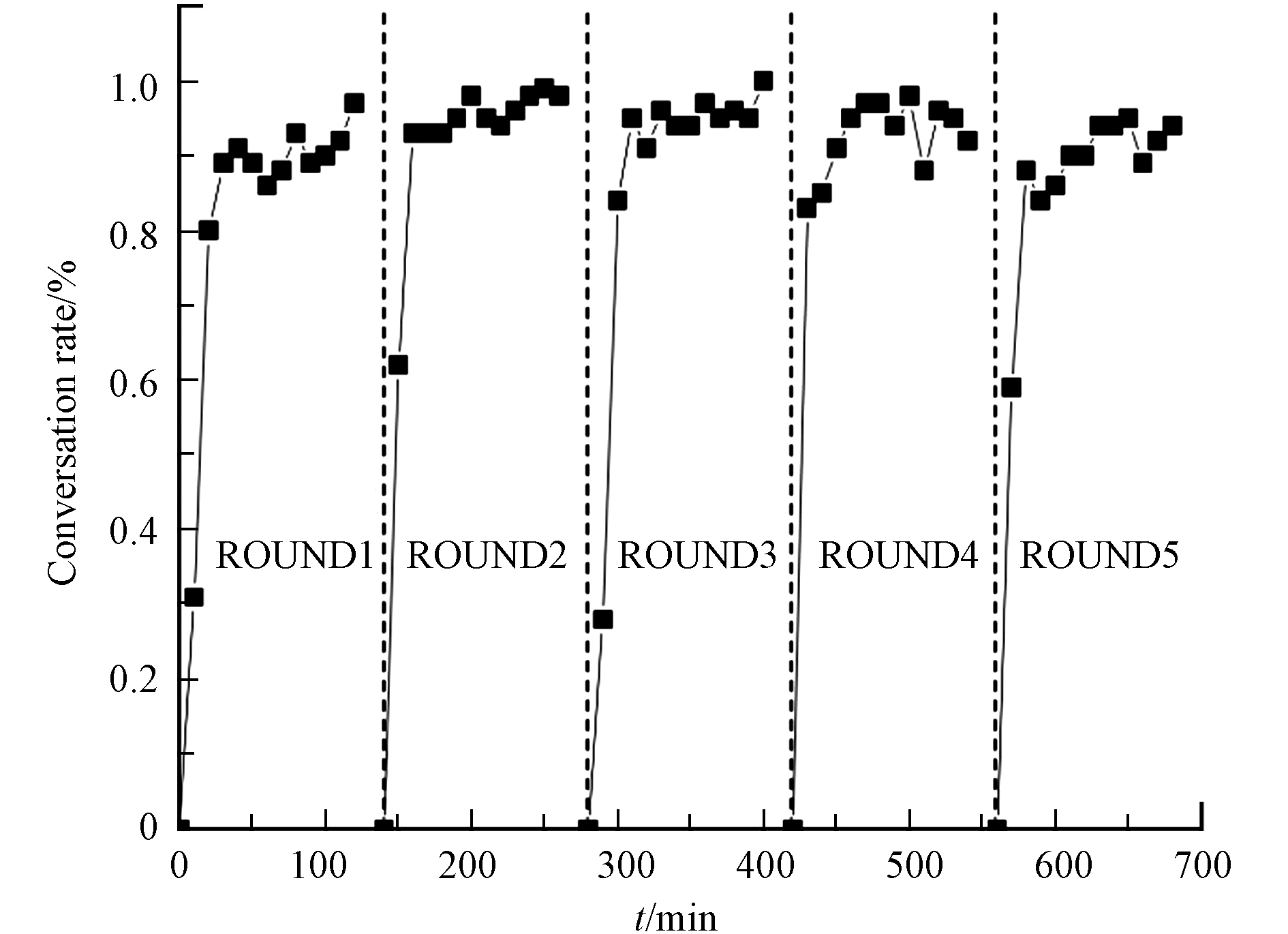
为结合实际需要,本研究检验了酸蚀预处理后所制得催化剂的稳定性,以CuMnCeOx/405004为目标催化剂. 图10中显示了连续5个周期内(每个周期120 min)的甲苯降解实验,并记录了降解效率. 结果表明,经过600 min的连续运行,甲苯的去除效率从95%下降至93%. 根据之前的实验表明,长时间的高温反应将会导致催化剂表面形貌发生变化或活性颗粒的发生团聚[15]. 本实验中催化剂在微波的照射下仍表现出了较高的催化活性.
-
在本工作中,蜂窝状堇青石采用不同浓度的硝酸进行预处理,50 ℃的酸蚀条件下,通过改变酸蚀浓度及时间并未对堇青石的机械强度造成明显影响,同时使堇青石载体表面结构发生一定变化. 堇青石载体负载CuMnCe氧化物后,以甲苯作为目标污染物确定了较优酸蚀条件,通过表征实验验证了酸蚀堇青石载体不仅会影响载体本身的表面形貌及孔径结构,同时酸蚀也会影响表面氧化物晶型种类及其含量比例和表面元素价态. 并在稳定性实验中验证了经过酸蚀后的催化剂的高效性和稳定性.
载体酸蚀预处理对CuMnCeOx/堇青石催化氧化甲苯性能的影响
Effects of acid etching pretreatment on toluene performance of CuMnCeOx/cordierite catalyst
-
摘要: 为解决蜂窝堇青石载体比表面积小、活性组分难以负载等问题,采用硝酸酸蚀堇青石载体并负载金属氧化物,制备了Cu-Mn-Ce/堇青石催化剂. 分析了不同酸蚀强度下催化剂对甲苯催化燃烧差异的原因,在40%wt硝酸溶液中、50 ℃下酸蚀4 h条件下得到的催化剂对甲苯的催化性能最佳,与其他催化剂相比,其表面平均孔径为38.31 nm并有更佳的孔隙率,且在12—14 nm处以及20—30 nm处有更佳的孔径分布,其上活性组分分布极为分散,具有更小的活性颗粒尺寸. 在与未经酸蚀预处理催化剂的比较中发现,经酸蚀预处理的催化剂具有更大的比表面积和多样的氧化物晶体类型,更高的Cu/Mn比例(1.72∶1.26),其中Mn4+(31.1%)、Ce3+(27.5%)及Osur(50.9%)在其占比均高于未经酸蚀预处理催化剂.Abstract: Cu-Mn-Ce/cordierite catalyst was prepared by etching cordierite carrier with nitric acid and loading metal oxides in order to solve the problems of low specific surface area of cordierite carrier and difficult loading of active components. The reasons for the difference in catalytic combustion of toluene under different acid etching intensities were analyzed. The catalyst obtained under the condition of 40% wt nitric acid solution and 4 h acid etching at 50 ℃ had the best catalytic performance for toluene. Compared with other catalysts, the average surface pore size of the catalyst was 38.31 nm and the porosity was better. Moreover, the pore size distribution at 12—14 nm and 20—30 nm is better, and the active component distribution on the pore size is very dispersed, and the active particle size is smaller. Compared with the catalyst without acid etching, the catalyst treated by acid etching has larger specific surface area, diversified oxide crystal types, higher Cu/Mn ratio (1.72:1.26). Mn4+(31.1%), Ce3+(27.5%) and Osur(50.9%) were higher than those without acid etching.
-
Key words:
- acid etching /
- cordierite /
- catalytic performance /
- toluene /
- microwave /
- characterization.
-
近年来,挥发性有机化合物(VOCs)因其对空气污染的影响大而备受关注,如化学烟雾、雾霾等严重影响大气环境和人类健康[1]. 目前,美国环境保护署将超过300种化学物质列为挥发性有机化合物,其中大部分被认为是造成空气污染的主要污染物[2]. 在目前可以在工业上大规模应用的技术中,催化燃烧由于其转化效率高,能耗低而被认为是一种有前景的技术[3-5]. 用于催化氧化VOCs的催化剂中,75%是贵金属催化剂,一般认为比金属氧化物催化剂更有活性[6],但是贵金属元素的高价值和低储量限制了其实际应用. 近年来,部分研究以过渡金属混合氧化物作为替代贵金属催化剂的催化材料,因其中一些对卤素和硫等毒物表现出很高的选择性和抗性等原因而受到了广泛关注[7].
在处理废气的过程中,因为其中含有许多不同性质的有机污染物,这对催化剂的性能提出了更高的要求[8]. 整体式蜂窝催化剂促进活性组分的更高分散,成本低,热稳定性高[9]. 在固定床操作中,大量的球团或颗粒可能会由于烟气中的微粒而造成高流动阻力和堵塞问题. 相比之下,蜂窝状催化剂的压降低,耐磨性好,不易堵塞[10]. 在各种催化剂载体中,堇青石具有高机械稳定性和低热膨胀系数(CET)等特点而被广泛应用[11]. 但由于其表面光滑,活性组分难以固定,因此负载前的预处理是必要的. 催化剂载体常用的预处理方法有表面涂层法和化学处理法等,其中酸蚀在化学处理法中特别常见,包括无机酸(硝酸[11-13]、盐酸[12-13]、硫酸[14])和有机酸(柠檬酸[13]、草酸[10, 12-13]、甲酸、EDTA[13]等). 一方面酸蚀堇青石载体可以使Mg、Al等离子溶出,这可以产生更多的微孔与中孔,另一方面游离二氧化硅再沉淀使堇青石表面结构发生重组,同时微孔向中孔方向靠拢,中孔逐渐合并为大孔. Mccabe等[13]报道了在实验中当在质量浓度37%且煮沸的盐酸中连续酸蚀6 h比表面积达到最大,并表明酸处理使堇青石表面产生游离的二氧化硅,以结晶或无定形形式存在,使堇青石的表面形貌发生了一定程度的变化.
在加热方式上,目前催化燃烧装置多采用电加热对废气进行预热,但存在对催化剂加热慢、加热不均匀、设备能耗大等问题. 与催化氧化工艺中的传统电加热方法相比,微波加热可以快速、有选择性的加热催化剂上具有吸波能力的活性组分[15-18],同时微波加热降低了VOCs反应温度和活化能[19-20]. 本课题组之前的研究表明,与电炉加热相比,微波加热可以降低甲苯氧化温度和工艺能耗,并对催化剂结构与活性组分的分布几乎不产生影响[21-22].
根据已报道文献所知,催化剂预处理条件与催化剂性能密切相关,在酸蚀预处理的条件下,有关载体的负载性能及后续微波催化性能的报道较少. 有必要开展对载体酸蚀处理及后续负载性能及微波催化性能的系统研究. 本研究为制取具有高催化活性的堇青石催化剂,采用不同质量浓度的硝酸对堇青石载体进行预处理,随后负载Cu-Mn-Ce复合氧化物制成整体式催化剂,在微波条件下对气态甲苯进行催化氧化,研究了堇青石载体酸蚀预处理程度对催化剂催化性能的影响. 采用XRD、BET、SEM、XPS对负载催化剂进行表征,分析酸蚀预处理对于活性组分负载阶段的影响. 研究工作将为整体式催化剂载体酸蚀预处理提供理论参考.
1. 实验部分(Experimental section)
1.1 催化剂制备与表征
通过切割商用蜂窝状堇青石单体获得直径28 mm、长度150 mm的圆柱体,然后超声清洗、干燥、并测试初始载体的吸水性能. 然后将样品浸泡在硝酸溶液中,酸处理条件和相应的催化剂代码如表1所示. 名称后面代码中的前两个数字表示酸的质量浓度,中间的两个数字表示酸处理温度,最后两个数字表示处理时间. 例如,用20% wt的硝酸在50 ℃下处理堇青石4 h制成CuMnCeOx/205004.然后用蒸馏水将所得酸蚀后载体彻底洗涤至中性,然后在105 ℃下保持1 h干燥,采用浸渍法制备了堇青石负载Cu-Mn-Ce催化剂:Ce(NO3)3·6H2O (99%), Mn(NO3)2 (50 %wt溶液),Cu(NO3)2·3H2O (99%). 浸渍完成后,载体在70 ℃下干燥过夜. 最后,将催化剂样品在450 ℃下煅烧5 h,自然冷却至室温. 堇青石载体的质量记为M1, M1:Cu:Mn:Ce的质量比为1:3.25%:3.25%:1.08%.
表 1 酸处理条件及相应的催化剂Table 1. Acid treatment conditions and corresponding catalysts催化剂 Catalyst 酸种类 Acid 酸浓度/% Acid concentration 处理时间/h Treatment time 处理温度/℃ Treatment temperature CuMnCeOx/205004 Nitric acid 20 4 50 CuMnCeOx/405004 40 4 CuMnCeOx/605004 60 4 CuMnCeOx/405008 40 8 CuMnCeOx/405016 40 16 CuMnCeOx/NTa — — 注:NTa是未经过酸蚀的堇青石载体. NTa means without acid treatment. 催化剂样品比表面积在液氮温度(-196 ℃)下使用氮气吸附-脱附法(BET)进行测定. 根据在473 K 预处理样品在77 K时的氮气吸附等温线确定比表面积,使用BET方程,以及比孔体积(Vs), P/P0=0.95. 孔径分布采用Barrett-Joyner-Halenda (BJH)模型计算孔隙分布(BET V-sorb 2800P,China) . 采用石墨单色仪和Cu Kα辐射(λ=0.154056 nm)的X射线衍射仪(Ultimany,Japan)在2θ范围10°—80°检测了催化剂的晶相,扫描速率为10(°)·min−1. 加速电压40 kV,加速电流40 mA.催化剂样品喷金后在JEOL JSM-6510LV(Japan)仪器上获得二次电子扫描图像. 催化剂表面活性组分的元素价态分析采用美国Thermo Fisher K-Alpha光谱仪采集非单色Cu Ka辐射的X射线光电子能谱,分析O 1s、Mn 2p、Ce 3d的光电子信号.
1.2 实验方案
常压下在固定床石英管反应器(内径28 mm)中进行了对目标物甲苯的催化燃烧实验,实验装置由进气、微波源、尾气处理共3部分组成. 如图1所示.
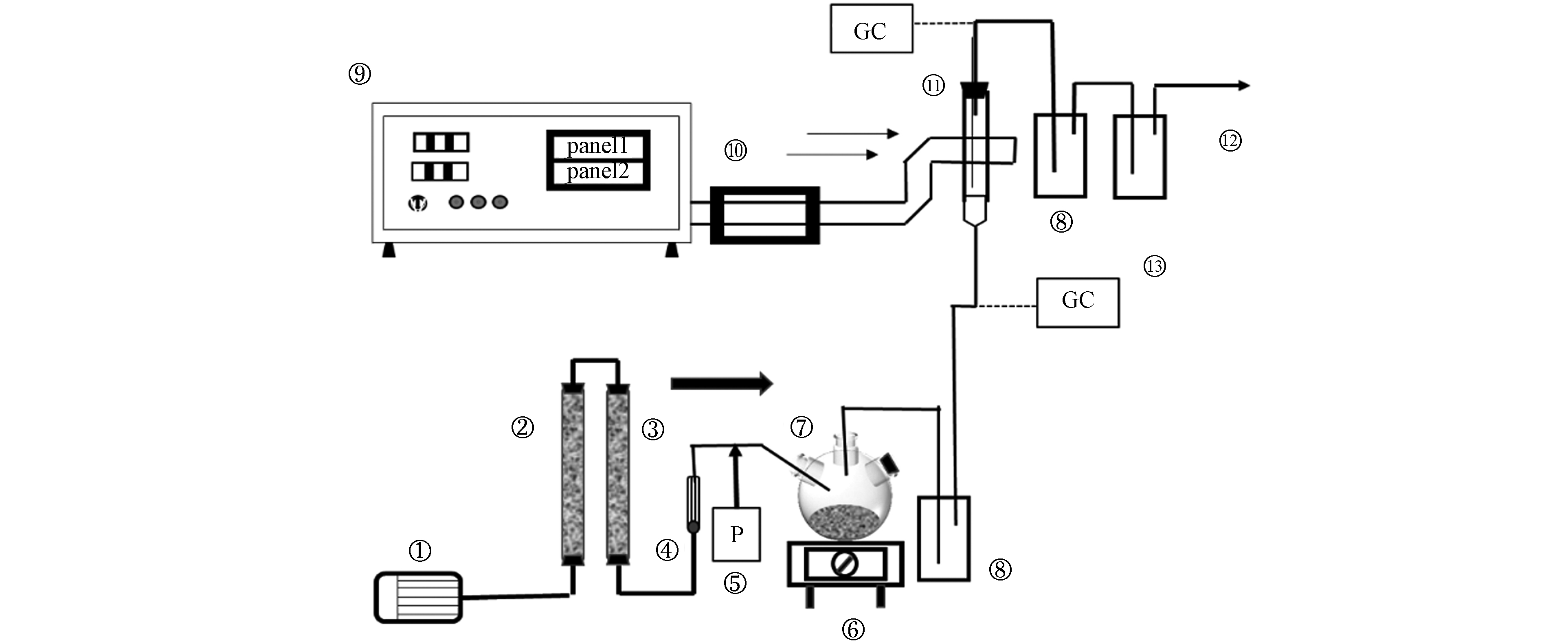 图 1 装置流程图Figure 1. Schematic of the experimental device flow1.空气泵; 2.变色硅胶; 3.活性炭; 4.流量计; 5.微量注射泵; 6.电加热套; 7.三颈烧瓶; 8.缓冲瓶; 9.微波装置; 10.水冷却系统;11.K型热电偶; 12.尾气净化系统; 13.气相色谱仪1. Air pump; 2. Color-changing silica gel; 3.Activated carbon; 4. Flow meter; 5. Microinjection pump; 6. Electric heating sleeve; 7. Three-neck flask; 8.Buffer bottle; 9.Microwave equipment; 10.Water cooling system; 11. Type K thermocouple; 12. Exhaust gas purification system; 13.Gas chromatograph
图 1 装置流程图Figure 1. Schematic of the experimental device flow1.空气泵; 2.变色硅胶; 3.活性炭; 4.流量计; 5.微量注射泵; 6.电加热套; 7.三颈烧瓶; 8.缓冲瓶; 9.微波装置; 10.水冷却系统;11.K型热电偶; 12.尾气净化系统; 13.气相色谱仪1. Air pump; 2. Color-changing silica gel; 3.Activated carbon; 4. Flow meter; 5. Microinjection pump; 6. Electric heating sleeve; 7. Three-neck flask; 8.Buffer bottle; 9.Microwave equipment; 10.Water cooling system; 11. Type K thermocouple; 12. Exhaust gas purification system; 13.Gas chromatograph将k型热电偶探头垂直插入床层,由温控仪显示床层温度. 由微量注射器注入的甲苯溶液与空气(经过干燥和净化)混合在1个三颈烧瓶中,该烧瓶放置在1个电热夹套上,在高温下保持恒定功率. 在固定床反应器中对模拟的甲苯废气进行微波催化氧化处理. 处理后的甲苯气体经有机溶剂和碱性溶液进一步净化后排放. 将进气(甲苯浓度为1.0 g·m−3)以2.0 L·min−1的流速(空速为1300 h−1)通入催化剂床层,通过调节微波功率使床层温度保持在150—200 ℃. 使用氢火焰离子化检测器的气相色谱系统(GC-FID, Agilent 6890N)对反应器出气进行分析,采用分流比50:1,进样量300 μL.所有实验数据均采用两次平行实验的综合数据.
2. 结果与讨论 (Results and discussion)
2.1 预处理条件对堇青石载体的影响因素研究
通过不同条件的酸蚀条件考察堇青石载体吸水率及失重率的情况. 图2(a)通过在50 ℃,酸蚀时间4 h的情况下,通过改变硝酸浓度来改变酸蚀强度. 经20%、40%溶液处理后的失重率均为0.19%,这表明堇青石表面的Mg、Al离子有一定的溶出,同时堇青石载体表面的浮灰及杂质部分去除. 随着硝酸溶液浓度提升至60%,失重率下降至0.09%,表明在增加酸蚀浓度的同时载体表面的MgO、Al2O3基本反应完全,使得SiO2暴露,可能存在载体对溶液中的盐类的吸附导致失重率降低进一步吸附导致载体的失重率降低,这与梁文俊等[23]的研究结果基本保持一致. 同时3种处理条件下的堇青石的吸水率均无明显差异,这表明来自堇青石表面物质的吸水性能被完全去除,吸水性仅来自于堇青石本身. 图2(b)通过在50 ℃,硝酸浓度40% wt的条件下,通过改变酸蚀时间来改变酸蚀强度. 经4、8、16 h溶液处理后,失重率分别为0.19%、0.23%、0.53%且吸水率无明显差异. 这表明随着酸蚀时间的增加,溶液对堇青石内部基体的酸蚀效应进一步加剧,同时在长时间的酸蚀下伴随着堇青石表面的重组[13, 24],导致溶液可以进入短时间酸蚀下未曾进入的孔道内部,进而得到较高的失重率. 通过改变酸蚀浓度和酸蚀时间进而来改变酸蚀强度均未对堇青石载体的吸水性能造成明显差异,且失重率均维持在0.6%之下. 综上表明在50 ℃的酸蚀条件下,通过改变酸蚀浓度及时间并未对堇青石的机械强度造成明显影响,同时使堇青石的CuMnCeOx/405004比表面积及孔径分布发生一定变化,这有利于活性组分的负载以及催化剂处理VOCs时的吸附性能.
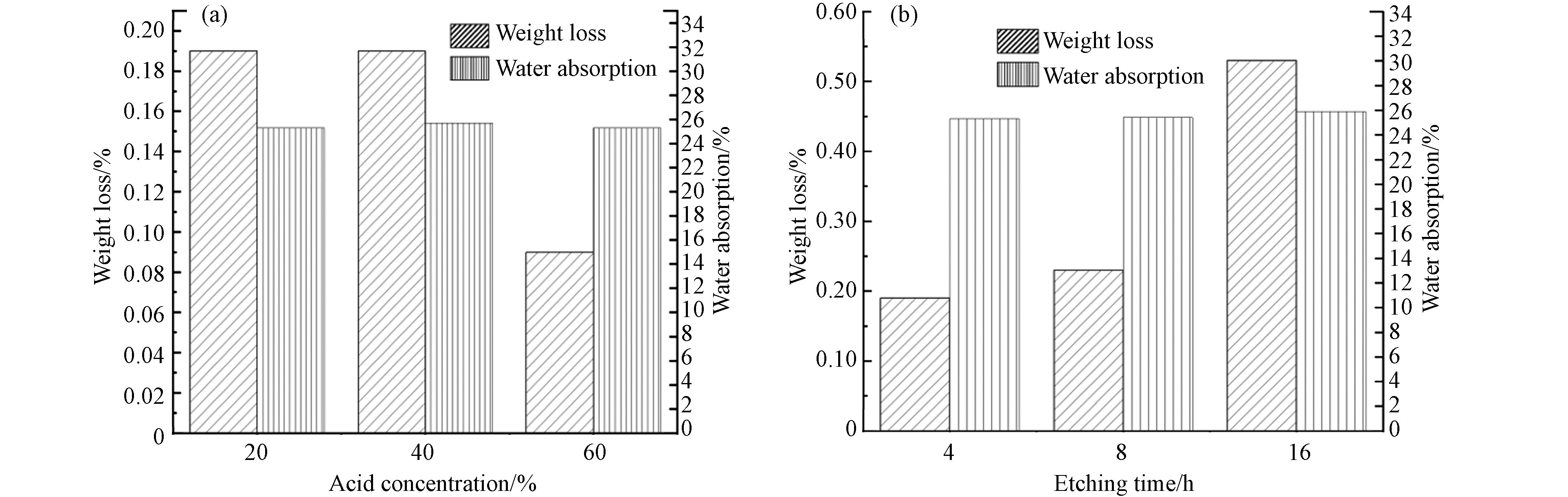 图 2 (a)在一定的酸蚀时间(4 h)及温度(50 ℃)条件下, 不同浓度酸处理下的堇青石失重率与吸水率变化;(b)在一定的酸浓度下(40%wt ), 不同酸蚀时间下堇青石的失重率与吸水率变化Figure 2. (a) The weight loss and water absorption of cordierite varied with different concentration of acid treatment under certain etching time (4 h) and temperature (50 ℃);(b) Changes of weight loss and water absorption of cordierite under certain acid concentration (40% wt) and different etching time
图 2 (a)在一定的酸蚀时间(4 h)及温度(50 ℃)条件下, 不同浓度酸处理下的堇青石失重率与吸水率变化;(b)在一定的酸浓度下(40%wt ), 不同酸蚀时间下堇青石的失重率与吸水率变化Figure 2. (a) The weight loss and water absorption of cordierite varied with different concentration of acid treatment under certain etching time (4 h) and temperature (50 ℃);(b) Changes of weight loss and water absorption of cordierite under certain acid concentration (40% wt) and different etching time2.2 催化剂活性测试
通过一系列实验研究了CuMnCeOx/堇青石催化剂在微波辐射下的催化氧化能力. 图3(a)显示了在6种不同条件下酸蚀堇青石载体并随后负载相同数量的活性组分催化剂的升温曲线(催化剂在恒定微波功率下的升温速率). 在微波辐照的情况下,介电极化导致负载金属氧化物的催化剂迅速升温[19, 25]. 与CuMnCeOx/405004相比,CuMnCeOx/NT在相同功率下床温差为40 ℃时,床温可在40 min内达到180 ℃,这可能有3点原因:(1)在酸蚀过程中提供部分吸波性能的Mg、Al氧化物以离子形态溶解在溶液中,同时酸处理降低了整体碱金属(Na和K等)含量[12], 降低了堇青石载体本身的吸波性能;(2)在CuMnCeOx/NT上生成的氧化物具有更多的晶体结构缺陷或更小的粒径,这意味着有更好的氧化还原能力和吸波性能[19]. 同时过渡金属氧化物、有变价离子共存的复合氧化物,或形成复合氧化物过程中有结晶转变的化合物,都可以通过电导损失或介质弛豫的方式将微波能转化为热能[15, 26],即微波辐射下的快速加热;(3)课题组之前的研究工作表明,经过不同强度酸蚀的堇青石载体,采用浸渍法负载活性组分的上载率维持在8%—9%,活性组分的脱落率维持在1%±0.05%[27]. 活性组分的实际上载率的差异也将会导致吸波性能略微的差异. 在催化活性的实验中,如图3(b)所示,CuMnCe氧化物在微波辐照下对微波能量的吸收较强,并在蜂窝催化剂表面形成热点,甲苯分子在这些热点迅速被氧化[28]. 在相同床层温度((150±8) ℃)、初始浓度(C0)为1 g·m−3、空速(GHSV)为1300 h−1的条件下测定了催化剂的催化活性. 在相同的反应条件下,CuMnCeOx/405004催化剂表现出最佳的活性,甲苯转化率接近80%. 因此,确定了最佳的载体酸蚀条件为CuMnCeOx/405004,故后续的催化剂活性实验和表征实验将围绕其展开.
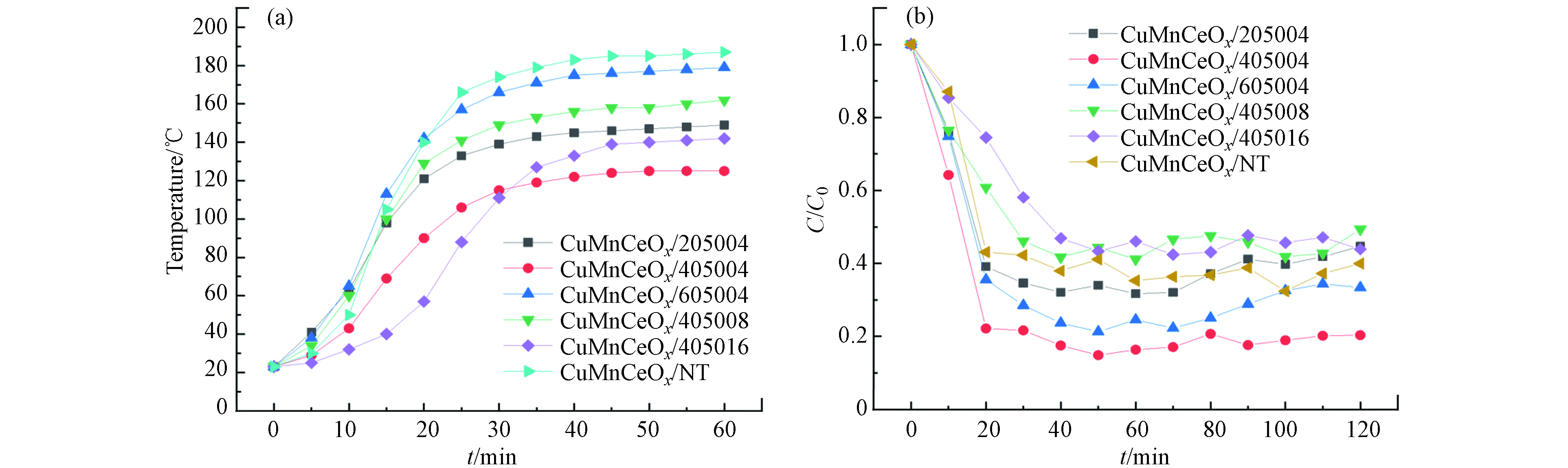 图 3 (a)在微波照射下的催化剂升温曲线(60 W); (b)催化氧化甲苯在相同的温度((150±8) ℃)Figure 3. (a) Temperature rise curve of catalyst under microwave irradiation (60 W); (b) Catalytic oxidation of toluene at the same temperature ((150±8) ℃)实验条件: GHSV = 1300 h−1, 甲苯浓度: 1 g·m−3Experimental conditions: GHSV = 1300 h−1, toluene concentration: 1 g·m−3
图 3 (a)在微波照射下的催化剂升温曲线(60 W); (b)催化氧化甲苯在相同的温度((150±8) ℃)Figure 3. (a) Temperature rise curve of catalyst under microwave irradiation (60 W); (b) Catalytic oxidation of toluene at the same temperature ((150±8) ℃)实验条件: GHSV = 1300 h−1, 甲苯浓度: 1 g·m−3Experimental conditions: GHSV = 1300 h−1, toluene concentration: 1 g·m−3通过调节微波装置功率使催化剂床层温度恒定,图4(a)为T=200 ℃、Q=0.12 m3·h−1(GHSV=1300 h−1)条件下不同进气浓度下甲苯的脱除情况.
结果表明,在200 ℃时,甲苯的脱除率为85%,且随着原料气浓度的增加,甲苯的转化率逐渐降低. 当甲苯进气浓度达到3.25 g·m−3时,甲苯转化率降至60%. 进气初始浓度的增加意味着更多的甲苯会争夺催化剂表面有限的活性点位,这导致了催化效率的降低. 图4(b)为不同风量下(保持进气甲苯的浓度一定)的甲苯去除率,在0.12 m3·h−1的风量下,甲苯去除率为85%. 同时,甲苯转化率随风速的增大而减小. 当空气流量达到0.24 m3·h−1 (GHSV=2600 h−1)时,甲苯转化率降至55%. 根据Mars-van-Krevelen(MvK)模型[7], 随着空气流量的增加,甲苯分子在床上停留的时间越短,甲苯分子与氧(晶格氧或吸附氧)的接触时间越短. 同时,微波辐射的非热效应也造成甲苯分子的分解,非热效应可以看作是分子的搅动或搅拌,其中偶极子在微波中转动被分子中的键所阻挡,可能进而引起少量的甲苯分子的断键随后进一步转化分解[19].
2.3 催化剂表面结构表征
酸蚀堇青石载体可以直接去除其表面的金属离子,在酸蚀开始阶段将产生微孔,但不会产生中孔,如要产生中孔堇青石表面结构必须重组,其中溶解的二氧化硅的再沉积涉及到这个过程[11, 13]. 从表2中可以看出,在相同的酸蚀时间条件下,BET比表面积随酸浓度增加有略微的增长,其中CuMnCeOx/405004相比于CuMnCeOx/205004的比表面积减少,这可能是由于活性组分在浸渍和烧结过程中在堇青石表面广泛分散,形成较小的活性组分颗粒,堵塞能提供比表面积较大的微孔,在孔表面或孔周围形成活性组分的晶相[12, 29]. 进一步增加酸液浓度,催化剂比表面积孔径孔容进一步增加这可能有两点原因:(1) 活性组分在大孔周围团聚,使原本可能堵塞的微孔和中孔暴露出来.
表 2 不同样品负载后的比表面积、孔体积和孔径Table 2. Specific surface area, pore volume and pore diameter of the different sample after loading催化剂Catalyst SBET/(m2·g−1) Vpore/(cm3·g−1) Dpore/nm (BET) CuMnCeOx/205004 2.78 0.033 49.41 CuMnCeOx/405004 2.75 0.032 38.31 CuMnCeOx/605004 3.00 0.063 75.14 CuMnCeOx/405008 2.41 0.033 49.05 CuMnCeOx/405016 4.53 0.065 58.37 CuMnCeOx/NT 2.02 0.027 36.23 图5(a、b、c)分别为CuMnCeOx/205004、CuMnCeOx/405004 、CuMnCeOx/60500的表面形貌.
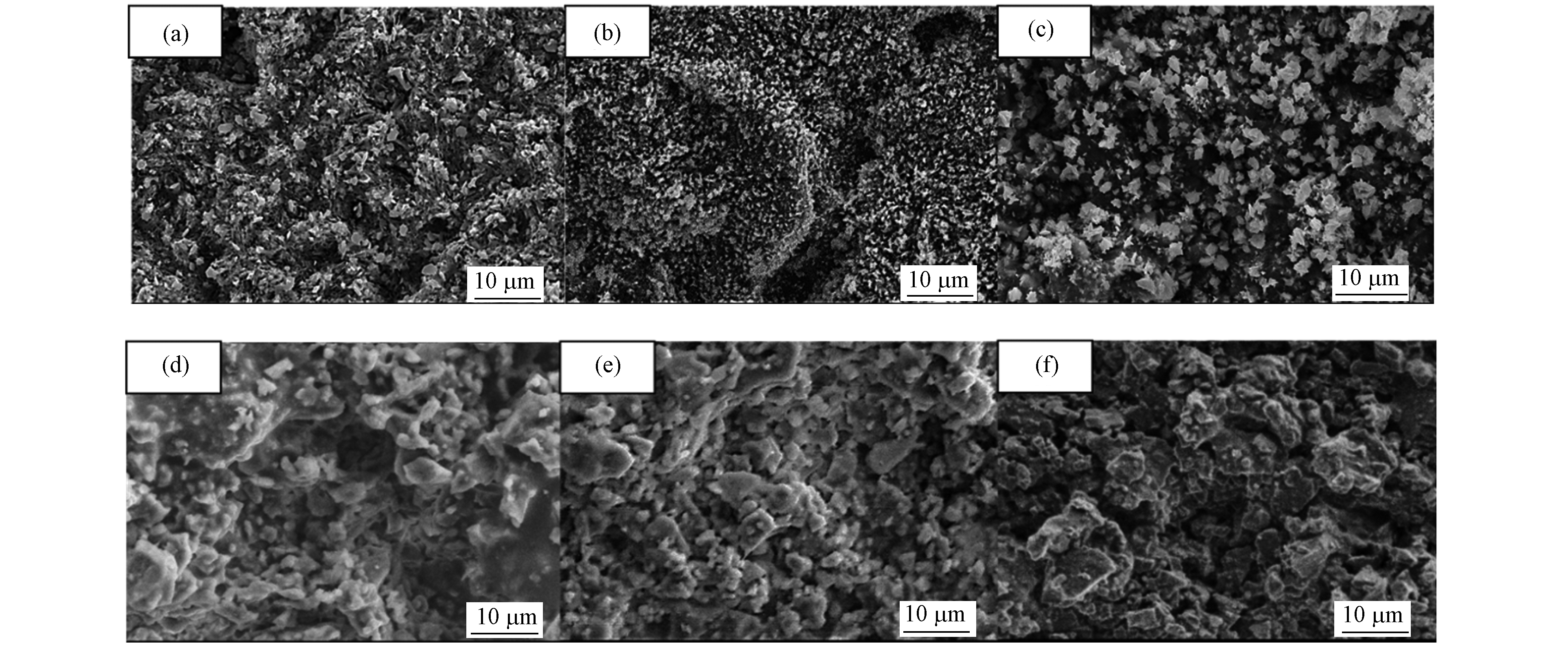 图 5 催化剂的电子扫描显微镜的图像Figure 5. Scanning electron microscope image of the catalyst(a) CuMnCeOx/205004; (b) CuMnCeOx/405004;(c) CuMnCeOx/605004;(d) 负载前CuMnCeOx/205004;(e) 负载前CuMnCeOx/405004;(f) 负载前CuMnCeOx/605004(a) CuMnCeOx/205004; (b) CuMnCeOx/405004;(c) CuMnCeOx/605004;(d) CuMnCeOx/205004 before loading;(e).CuMnCeOx/405004 before loading;(f) CuMnCeOx/605004 before loading
图 5 催化剂的电子扫描显微镜的图像Figure 5. Scanning electron microscope image of the catalyst(a) CuMnCeOx/205004; (b) CuMnCeOx/405004;(c) CuMnCeOx/605004;(d) 负载前CuMnCeOx/205004;(e) 负载前CuMnCeOx/405004;(f) 负载前CuMnCeOx/605004(a) CuMnCeOx/205004; (b) CuMnCeOx/405004;(c) CuMnCeOx/605004;(d) CuMnCeOx/205004 before loading;(e).CuMnCeOx/405004 before loading;(f) CuMnCeOx/605004 before loading在图5(b)中活性组分的颗粒广泛分布在催化剂表面,且与其他两种催化剂相比粒径较小,能提供较大的接触面积,这是其甲苯转化率高的原因之一,同时图5(c)与其它两组催化剂相比,表面的活性组分发生了明显的聚集;(2)与堇青石载体本身相关. 在图5中,(d)、(e)、(f)为相对应的酸蚀后未负载前的表面形貌. 从图5(d)、(e)中可以看出随着溶液浓度的增大,堇青石的孔隙率明显提高,图5(f)进一步增加溶液浓度后孔隙率降低,孔径变大. 随着酸蚀强度的增加,Mg、Al离子浸出所产生的微孔,以及堇青石表面重组所表现的中孔合并为大孔会导致比表面积增加、孔径孔容的增大[11].表2中在相同的酸蚀浓度下,将CuMnCeOx/405004、CuMnCeOx/405008、CuMnCeOx/405016其三者的孔径分布进行比较,可以发现其三者比表面积呈现先减小后增大的趋势,但孔径孔容逐渐增大,这表明溶液可以进入短时间酸蚀下未曾进入的孔道内部,产生更多的微孔(这可能在负载过程中被堵塞导致比表面积降低),同时中孔向大孔方向移动.
图6显示了几中不同预处理条件催化剂的孔径分布.CuMnCeOx/405004在12—14 nm处以及20—30 nm处都展示了极好的孔隙率,同时,其平均孔径相对较低,这与SEM表征相符Williamse等[30]报道称,在用于废气流中的甲苯氧化中,具有更高结构孔隙率的介孔Ti-HMS催化剂上观察到较低的起燃温度,这说明结构孔隙率对催化剂性能有显著的正向影响. Lu等[31]表明,当活性组分浸渍在高介孔含量的碳载体上时,活性组分的高度分散导致催化活性的提高,促进了大分子和离子在载体表面的渗透. 综上CuMnCeOx/405004表现出良好的孔隙率,在吸附过程中催化剂可以更有效地吸附甲苯,也可以为催化燃烧提供更多的活性位点,进而可以提升催化剂的催化效率.
2.4 XRD表征
为了进一步了解负载后孔径差异对活性组分的影响,本研究测定了催化剂的XRD谱图,如图7所示.
尽管堇青石的衍射线存在,且与催化剂的大部分氧化物相的反射重叠并掩盖了绝大部分可能存在的氧化物的衍射峰,但在图7(XRD)中仍可以看到相对较弱活性组分的衍射峰,其中(a)为堇青石载体,(b)、(c)分别为CuMnCeOx/NT、CuMnCeOx/405004. 两种催化剂上都显示其表面存在铜锰铈的氧化物,其中CuMnCeOx/405004表面检出存在Cu1.5Mn1.5O4(ICCD PDFNO.02-70-0262),这些相对弱的衍射峰一定程度上说明3种氧化物以无定形态分散在整个催化剂表面,其中存在CuMnCe氧化物掺杂现象或是Cu、Mn的氧化物附着在Ce氧化物表面[32].Cu2+/Cu+、Mn4+/Mn2+、Ce4+/Ce3+之间的价态变化能引起电子的转移过程. 其他的研究者证实铜锰尖晶石在多种情况下都具有较高的活性[29],其中Cu1.5Mn1.5O4 是主要活性中心,MnOx既是活性中心也可以储存以及为活性中心传输氧[32],以完全氧化降解有机分子. 此外氧化铈的氧化还原性能及其晶格氧的高不稳定性是影响氧化铈催化反应活性的最重要因素[33],含铈过渡金属复合氧化物因其储氧容量大、氧空位丰富、Ce3+/Ce4+价态变化引起的氧化还原性能强而被认为可以有效提高催化性能[5].
2.5 XPS表征
利用XPS研究了催化剂表面元素组成和化学信息状态,CuMnCeOx/405004和CuMnCeOx/NT样品中Ce 3d、Mn 2p、O 1s电子能级的XPS谱图如图8所示.
如表3所示, Cu、Mn的表面含量以及Mn、Ce、O的化学态及比例均以列出. 图8(A)为Mn 2p的XPS谱图,Mn 2p3/2和Mn 2p1/2的结合能与文献报道的相似[34-35]. 其中BE=653.6 eV和642.1 eV与BE=653.2 eV和641.5 eV分别属于CuMnCeOx/ NT与CuMnCeOx/405004的Mn3+,BE=657.1 eV和645.3 eV与BE= 657.1 eV和644.4 eV分别属于CuMnCeOx/NT与CuMnCeOx/405004的Mn4+. 从表3可以看出,CuMnCeOx/405004(1.72)与CuMnCeOx/NT(1.26)表面的铜锰比均高于理论值1.0. 这说明与铜离子相比,锰离子更容易迁移到铈的晶格中去,且在酸蚀预处理下,更能有效地将金属离子引入到铈的晶格中. Chi等[36]的研究结果表明,在铈表面的大量Mn4+离子可能增强Cu+和Cu2+之间的氧化还原偶联能力. CuMnCeOx/405004相比于CuMnCeOx/NT来说有着更多的Mn4+,这将可能有助于甲苯的氧化.
表 3 Mn 2P, Ce 3d, O 1sXPS谱图结合能的拟合结果Table 3. Fitting results of binding energies of Mn 2P, Ce 3d, O 1sXPS spectra催化剂Catalyst Cu/% Mn/% Cu/Mn Mn/% Ce/% O/% Mn3+ Mn4+ Ce3+ Ce4+ Osur Olat CuMnCeOx/405004 59.71 34.64 1.72 68.9 31.1 27.5 72.5 50.9 49.1 CuMnCeOx/NT 52.49 41.36 1.26 82.0 18.0 18.7 81.3 49.0 51.0 在图8(B)中,可以识别出自旋轨道双峰对产生的8个峰,其中6个峰分别为v(882.2 eV), v”(888.6 eV),v”’(897.7 eV),u(900.7 eV), u”(907.9 eV),u”’(916.6 eV)来自Ce4+,v’(885.4 eV)和u’(904.9 eV)来自Ce3+[37]. 如表3所示,经过酸蚀预处理过后Ce3+的含量更多,与氧空位存在相关的Ce3+离子在氧化机制中发挥了关键作用,参与甲苯的活化(表面氧空位)和氧向表面材料的迁移(次表面氧空位). López等人[38]合成了一系列具有不同理化性质的Ce基纳米棒和立方体,表明在对于甲苯的氧化过程中无论使用高负载的催化剂或是低负载的纳米棒Ce3+的浓度与催化氧化能力之间都有明确的线性关系. 同时由于Ce的氧化物晶相中部分位点被Cu或Mn取代,导致结晶度降低,产生更多的晶格缺陷和氧空位,有利于氧迁移率的提高[32],可以促进甲苯的催化氧化.
对催化剂进行了O 1s XPS分析, 如图8(C)所示.Bielański等[39]提出了催化剂表面的3种晶格氧:O22−、O−和O2−,O22−和O−是亲电氧种,容易参与氧化,而亲核氧(O2−)在选择性氧化中起重要作用. O1s XPS谱可分解为3个峰[32]. (1) 529—530 eV的晶格氧(Olatt);(2)表面氧(Osur)在530—532 eV,指缺陷氧或表面氧物种;(3)533—534 eV的吸附氧物种(Oads)和吸附的水物种作为在表面的污染物. 图8(C)表明对于CuMnCeOx/405004,晶格氧的结合能在529.9 eV,而CuMnCeOx/NT的结合能在529.8 eV,经酸蚀预处理后的催化剂晶格氧向更高的结合能方向移动. 这意味着当发生电子转移过程时,可以产生更多的活性氧物种. 表3列出了两种催化剂表面吸附氧和晶格氧各占百分比,众所周知,Osur/Olatt比值越高,物种供氧能力越强,在低温下相比于晶格氧来说可还原性越强,这意味着在催化甲苯活化实验中,CuMnCeOx/405004应该具有更高的甲苯降解效率,这与催化剂活性实验结论相印证.
2.6 堇青石酸蚀及负载过程
图9给出了堇青石酸蚀以及负载过程的机理. 酸处理可使Al、Mg离子和碱金属离子等从堇青石载体的结构中去除,同时去除表面上的浮灰或可以与酸发生相互作用的其他物质,并在堇青石表面形成非晶态的二氧化硅即游离二氧化硅. 酸蚀过程中会产生微孔和中孔即“增孔效应”,中孔的产生可能是由于堇青石结构的破坏和无定形二氧化硅的再沉淀,同时堇青石表面结构发生重组,同时微孔向中孔方向靠拢,中孔逐渐合并为大孔即酸蚀具有“扩孔效应”.
不同程度的酸蚀作用会导致堇青石的表面结构发生变化,控制酸蚀的强度可以获得具有理想表面结构的堇青石载体,这将有利于污染物的吸附. 在负载阶段,采用等体积浸渍法使Cu、Mn、Ce的3种离子负载到堇青石的孔隙之中,由于不同酸蚀强度造成载体表面结构的差异,进而影响氧化物晶相的形态和类型及比例的差异将影响晶格缺陷和氧空位的数量,即影响了氧迁移的效率.
2.7 稳定性实验
为结合实际需要,本研究检验了酸蚀预处理后所制得催化剂的稳定性,以CuMnCeOx/405004为目标催化剂. 图10中显示了连续5个周期内(每个周期120 min)的甲苯降解实验,并记录了降解效率. 结果表明,经过600 min的连续运行,甲苯的去除效率从95%下降至93%. 根据之前的实验表明,长时间的高温反应将会导致催化剂表面形貌发生变化或活性颗粒的发生团聚[15]. 本实验中催化剂在微波的照射下仍表现出了较高的催化活性.
3. 结论(Conclusion)
在本工作中,蜂窝状堇青石采用不同浓度的硝酸进行预处理,50 ℃的酸蚀条件下,通过改变酸蚀浓度及时间并未对堇青石的机械强度造成明显影响,同时使堇青石载体表面结构发生一定变化. 堇青石载体负载CuMnCe氧化物后,以甲苯作为目标污染物确定了较优酸蚀条件,通过表征实验验证了酸蚀堇青石载体不仅会影响载体本身的表面形貌及孔径结构,同时酸蚀也会影响表面氧化物晶型种类及其含量比例和表面元素价态. 并在稳定性实验中验证了经过酸蚀后的催化剂的高效性和稳定性.
-
图 2 (a)在一定的酸蚀时间(4 h)及温度(50 ℃)条件下, 不同浓度酸处理下的堇青石失重率与吸水率变化;(b)在一定的酸浓度下(40%wt ), 不同酸蚀时间下堇青石的失重率与吸水率变化
Figure 2. (a) The weight loss and water absorption of cordierite varied with different concentration of acid treatment under certain etching time (4 h) and temperature (50 ℃);(b) Changes of weight loss and water absorption of cordierite under certain acid concentration (40% wt) and different etching time
表 1 酸处理条件及相应的催化剂
Table 1. Acid treatment conditions and corresponding catalysts
催化剂 Catalyst 酸种类 Acid 酸浓度/% Acid concentration 处理时间/h Treatment time 处理温度/℃ Treatment temperature CuMnCeOx/205004 Nitric acid 20 4 50 CuMnCeOx/405004 40 4 CuMnCeOx/605004 60 4 CuMnCeOx/405008 40 8 CuMnCeOx/405016 40 16 CuMnCeOx/NTa — — 注:NTa是未经过酸蚀的堇青石载体. NTa means without acid treatment. 表 2 不同样品负载后的比表面积、孔体积和孔径
Table 2. Specific surface area, pore volume and pore diameter of the different sample after loading
催化剂Catalyst SBET/(m2·g−1) Vpore/(cm3·g−1) Dpore/nm (BET) CuMnCeOx/205004 2.78 0.033 49.41 CuMnCeOx/405004 2.75 0.032 38.31 CuMnCeOx/605004 3.00 0.063 75.14 CuMnCeOx/405008 2.41 0.033 49.05 CuMnCeOx/405016 4.53 0.065 58.37 CuMnCeOx/NT 2.02 0.027 36.23 表 3 Mn 2P, Ce 3d, O 1sXPS谱图结合能的拟合结果
Table 3. Fitting results of binding energies of Mn 2P, Ce 3d, O 1sXPS spectra
催化剂Catalyst Cu/% Mn/% Cu/Mn Mn/% Ce/% O/% Mn3+ Mn4+ Ce3+ Ce4+ Osur Olat CuMnCeOx/405004 59.71 34.64 1.72 68.9 31.1 27.5 72.5 50.9 49.1 CuMnCeOx/NT 52.49 41.36 1.26 82.0 18.0 18.7 81.3 49.0 51.0 -
[1] PARMAR G R, RAO N N. Emerging control technologies for volatile organic compounds [J]. Critical Reviews in Environmental Science and Technology, 2008, 39(1): 41-78. doi: 10.1080/10643380701413658 [2] TAYLOR S H. Preface: catalytic aspects of complete oxidation of volatile organic compounds [J]. Topics in Catalysis, 2009, 52(5): 457. doi: 10.1007/s11244-009-9179-3 [3] EVERAERT K, BAEYENS J. Catalytic combustion of volatile organic compounds [J]. Journal of Hazardous Materials, 2004, 109(1/2/3): 113-139. [4] HUANG H F, LIU Y Q, TANG W, et al. Catalytic activity of nanometer La1−xSrxCoO3 (x = 0, 0.2) perovskites towards VOCs combustion [J]. Catalysis Communications, 2008, 9(1): 55-59. doi: 10.1016/j.catcom.2007.05.004 [5] LU H F, ZHOU Y, HUANG H F, et al. In-situ synthesis of monolithic Cu-Mn-Ce/cordierite catalysts towards VOCs combustion [J]. Journal of Rare Earths, 2011, 29(9): 855-860. doi: 10.1016/S1002-0721(10)60555-8 [6] ERTL G, HK ZINGER, FS TH, J WEITKAMP, et al. Handbook of heterogeneous catalysis. 8 Volumes, 2nd Edition [M]. Wiley, 2008. [7] LEE J E, OK Y S, TSANG D C W, et al. Recent advances in volatile organic compounds abatement by catalysis and catalytic hybrid processes: A critical review [J]. Science of the Total Environment, 2020, 719: 137405. doi: 10.1016/j.scitotenv.2020.137405 [8] ARMOR J N. Environmental catalysis [J]. Applied Catalysis B:Environmental, 1994, 5(1/2): N7. [9] DENG L, HUANG C, KAN J W, et al. Effect of coating modification of cordierite carrier on catalytic performance of supported NiMnO3 catalysts for VOCs combustion [J]. Journal of Rare Earths, 2018, 36(3): 265-272. doi: 10.1016/j.jre.2017.07.015 [10] LIU Q Y, LIU Z Y, HUANG Z G, et al. A honeycomb catalyst for simultaneous NO and SO2 removal from flue gas: Preparation and evaluation [J]. Catalysis Today, 2004, 93/94/95: 833-837. [11] LIU Q C, HE Y Y, YANG J, et al. Modification of cordierite honeycomb ceramics matrix for DeNOx catalyst [J]. MRS Proceedings, 2012, 1449: (1):141-146. [12] LIU Q Y, LIU Z Y, HUANG Z G. CuO supported on Al2O3-coated cordierite-honeycomb for SO2 and NO removal from flue gas: effect of acid treatment of the cordierite [J]. Industrial & Engineering Chemistry Research, 2005, 44(10): 3497-3502. [13] SHIGAPOV A N, GRAHAM G W, MCCABE R W, et al. The preparation of high-surface-area cordierite monolith by acid treatment [J]. Applied Catalysis A:General, 1999, 182(1): 137-146. doi: 10.1016/S0926-860X(99)00003-4 [14] MADHUSOODANA C, DAS R, KAMESHIMA Y, et al. Characterization and adsorption behavior of ZSM-5 zeolite film on cordierite honeycombs prepared by a novel in situ crystallization method [J]. Journal of Porous Materials, 2001, 8(4): 265-271. doi: 10.1023/A:1013160914074 [15] BO L L, SUN S Y. Microwave-assisted catalytic oxidation of gaseous toluene with a Cu-Mn-Ce/cordierite honeycomb catalyst [J]. Frontiers of Chemical Science and Engineering, 2019, 13(2): 385-392. doi: 10.1007/s11705-018-1738-3 [16] BUCHELNIKOV V D, LOUZGUINE-LUZGIN D V, XIE G, et al. Heating of metallic powders by microwaves: Experiment and theory [J]. Journal of Applied Physics, 2008, 104(11): 113505. doi: 10.1063/1.3009677 [17] KHALED D E, NOVAS N, GAZQUEZ J A, et al. Microwave dielectric heating: Applications on metals processing [J]. Renewable and Sustainable Energy Reviews, 2018, 82: 2880-2892. doi: 10.1016/j.rser.2017.10.043 [18] MISHRA R R, SHARMA A K. Microwave-material interaction phenomena: Heating mechanisms, challenges and opportunities in material processing [J]. Composites Part A:Applied Science and Manufacturing, 2016, 81: 78-97. doi: 10.1016/j.compositesa.2015.10.035 [19] JACOB J, CHIA L H L, BOEY F Y C. Thermal and non-thermal interaction of microwave radiation with materials [J]. Journal of Materials Science, 1995, 30(21): 5321-5327. doi: 10.1007/BF00351541 [20] ROUSSY G, THIEBAUT J M, SOUIRI M, et al. Controlled oxidation of methane doped catalysts irradiated by microwaves [J]. Catalysis Today, 1994, 21(2/3): 349-355. [21] BO L L, LIAO J B, ZHANG Y C, et al. CuO/zeolite catalyzed oxidation of gaseous toluene under microwave heating [J]. Frontiers of Environmental Science & Engineering, 2013, 7(3): 395-402. [22] 卜龙利, 刘海楠, 王晓晖, 等. 不同加热方式下催化氧化甲苯的性能研究 [J]. 环境化学, 2013, 32(8): 1524-1531. doi: 10.7524/j.issn.0254-6108.2013.08.017 BU L L, LIU H N, WANG X H, et al. Study on the catalytic oxidation of toluene under different heating modes [J]. Environmental Chemistry, 2013, 32(8): 1524-1531(in Chinese). doi: 10.7524/j.issn.0254-6108.2013.08.017
[23] 梁文俊, 李庆磊, 任思达. 酸预处理对整体式催化剂载体性能的影响研究 [J]. 中国环境科学, 2020, 40(12): 5237-5245. doi: 10.3969/j.issn.1000-6923.2020.12.016 LIANG W J, LI Q L, REN S D. Influence of acid pretreatment on the performance of monolithic catalyst support [J]. China Environmental Science, 2020, 40(12): 5237-5245(in Chinese). doi: 10.3969/j.issn.1000-6923.2020.12.016
[24] BAI J H, GUO L C. Effects of chemical treatments on thermal expansion properties of cordierite ceramics [J]. Journal of Wuhan University of Technology(Materials Science), 2006, 21(3): 100-102. doi: 10.1007/BF02840892 [25] HOU T Q, WANG B B, JIA Z R, et al. A review of metal oxide-related microwave absorbing materials from the dimension and morphology perspective [J]. Journal of Materials Science:Materials in Electronics, 2019, 30(12): 10961-10984. doi: 10.1007/s10854-019-01537-0 [26] 段爱红, 毕先钧, 阚家德. 金属氧化物吸收微波辐射的能力与其结构的关系 [J]. 云南化工, 1998, 25(2): 34-36. DUAN A H, BI X J, KAN J D. Temperature rising behavior of metals oxide in microwave field [J]. Yunnan Chemical Technology, 1998, 25(2): 34-36(in Chinese).
[27] 宁轲, 卜龙利, 刘双, 等. 整体式催化剂活性组分负载策略及微波催化燃烧甲苯特性 [J]. 燃料化学学报, 2020, 48(9): 1140-1152. doi: 10.3969/j.issn.0253-2409.2020.09.014 NING K, BU L L, LIU S, et al. Loading strategy for the active components of monolithic catalyst and its influences on the microwave enhanced catalytic combustion of toluene [J]. Journal of Fuel Chemistry and Technology, 2020, 48(9): 1140-1152(in Chinese). doi: 10.3969/j.issn.0253-2409.2020.09.014
[28] ZHANG Y C, BO L L, WANG X H, et al. Study on catalytic oxidation of benzene by microwave heating [J]. Environmental Science, 2012, 33(8): 2759-2765. [29] WANG P, HE Y, YANG Z Q, et al. Experimental study of benzene catalytic combustion over Cu-Mn-Ce/Al2O3 particles [J]. ChemistrySelect, 2020, 5(3): 1122-1129. doi: 10.1002/slct.201902976 [30] WILLIAMS T, BELTRAMINI J, LU G Q. Effect of the preparation technique on the catalytic properties of mesoporous V-HMS for the oxidation of toluene [J]. Microporous and Mesoporous Materials, 2006, 88(1/2/3): 91-100. [31] LU C Y, WEY M Y, CHUANG K H. Catalytic treating of gas pollutants over cobalt catalyst supported on porous carbons derived from rice husk and carbon nanotube [J]. Applied Catalysis B:Environmental, 2009, 90(3/4): 652-661. [32] LU H F, KONG X X, HUANG H F, et al. Cu-Mn-Ce ternary mixed-oxide catalysts for catalytic combustion of toluene [J]. Journal of Environmental Sciences, 2015, 32: 102-107. doi: 10.1016/j.jes.2014.11.015 [33] WANG X Y, KANG Q, LI D. Low-temperature catalytic combustion of chlorobenzene over MnOx-CeO2 mixed oxide catalysts [J]. Catalysis Communications, 2008, 9(13): 2158-2162. doi: 10.1016/j.catcom.2008.04.021 [34] KAN J W, DENG L, LI B, et al. Performance of co-doped Mn-Ce catalysts supported on cordierite for low concentration chlorobenzene oxidation [J]. Applied Catalysis A:General, 2017, 530: 21-29. doi: 10.1016/j.apcata.2016.11.013 [35] DU J P, QU Z P, DONG C, et al. Low-temperature abatement of toluene over Mn-Ce oxides catalysts synthesized by a modified hydrothermal approach [J]. Applied Surface Science, 2018, 433: 1025-1035. doi: 10.1016/j.apsusc.2017.10.116 [36] HE C, YU Y K, SHEN Q, et al. Catalytic behavior and synergistic effect of nanostructured mesoporous CuO-MnOx-CeO2 catalysts for chlorobenzene destruction [J]. Applied Surface Science, 2014, 297: 59-69. doi: 10.1016/j.apsusc.2014.01.076 [37] DENG W, DAI Q G, LAO Y J, et al. Low temperature catalytic combustion of 1, 2-dichlorobenzene over CeO2-TiO2 mixed oxide catalysts [J]. Applied Catalysis B:Environmental, 2016, 181: 848-861. doi: 10.1016/j.apcatb.2015.07.053 [38] LÓPEZ J M, GILBANK A L, GARCÍA T, et al. The prevalence of surface oxygen vacancies over the mobility of bulk oxygen in nanostructured ceria for the total toluene oxidation [J]. Applied Catalysis B:Environmental, 2015, 174/175: 403-412. doi: 10.1016/j.apcatb.2015.03.017 [39] BIELAŃSKI A, HABER J. Oxygen in catalysis on transition metal oxides [J]. Catalysis Reviews, 1979, 19(1): 1-41. doi: 10.1080/03602457908065099 -




 DownLoad:
DownLoad: