-
工业化进程中会产生大量的重金属和有机染料,这是水体的主要污染源,也是全球面临的一个重要难题[1-3]. 重金属离子具有低生物降解性和高毒性,在环境中迁移速度快、分布广泛、长期累积,在通过食物链进入生物体内后,会极大的危害生物体的健康,表现出严重的生态危害[1- 2]. 有机染料含量高、毒性高、难以降解,在水环境中易消耗溶解氧,造成富营养化,危害水体中动植物的健康生长,严重破坏水体的自然生态链[3]. 因此,研究有效去除水中重金属与有机染料的材料与相关行为,对于保护水生态环境具有一定的参考价值和理论意义.
废水中污染物的去除已有多种方法,吸附法因其适应性广、操作简单、环保低成本而被广泛采用[1, 3- 4]. 自然资源因其可再生性,在吸附中常被作为一种载体,借助不同的改性方法制备功能不同的吸附材料,可显著提高其环境应用价值. 已有研究表明,天然类纤维材料可用于吸附废水中的重金属离子[5- 6]和有机染料[3, 7]. 黄麻是一种天然环保型材料,产量高且廉价易得,其表面有较多内孔、裂缝和不规则颗粒,比表面积较大,并含羟基和羧基等基团[8- 9],在制备吸附材料方面具有很好的应用前景. 然而,黄麻的活性组分含量不高,吸附能力和选择性较差,其吸附容量并不足以满足人们对黄麻的预期,限制其在环境中的应用[8, 10].
漆酶是一种具有催化性能的蛋白质,催化效率高、底物范围广,催化后的唯一产物是水[11-12],也能够吸附重金属、硫蛋白等物质,是一种有可能捕获金属的交联剂[11]. 漆酶能够结合介孔纳米纤维[13]、壳聚糖[14]、碳纳米管[15]等基质去除抗生素、农药、苯酚类、染料等. 本文前期使用漆酶改性黄麻对亚甲基蓝染料(MB)进行去除实验研究[16],结果显示漆酶改性黄麻对MB的去除包括黄麻吸附和漆酶酶解机制,去除率最高可达到95.12%. 但当前对漆酶结合生物质类材料对不同类型有机物、重金属去除的研究很少见到报道. 将漆酶与黄麻结合,把黄麻的吸附性能、漆酶对重金属的捕获性能/对有机物的酶解性能集于一体,来提高黄麻在重金属、染料废水中的去除效果,以期实现黄麻的增值性应用.
本文前期研究制备得到系列漆酶-黄麻复合材料(L-SJ),通过批处理法研究了黄麻(SJ)、漆酶-黄麻复合材料(L-SJ)对Cd2+和刚果红(CR)的去除行为,考察了漆酶浓度、温度、pH和离子强度对Cd2+和CR去除的影响,结合材料红外光谱图来探讨其去除机制,同时对比前期材料对MB去除的相关研究,探讨L-SJ在重金属、不同有机染料废水的去除能力,以期为漆酶-黄麻复合材料在重金属和有机染料废水处理中的应用提供理论依据.
-
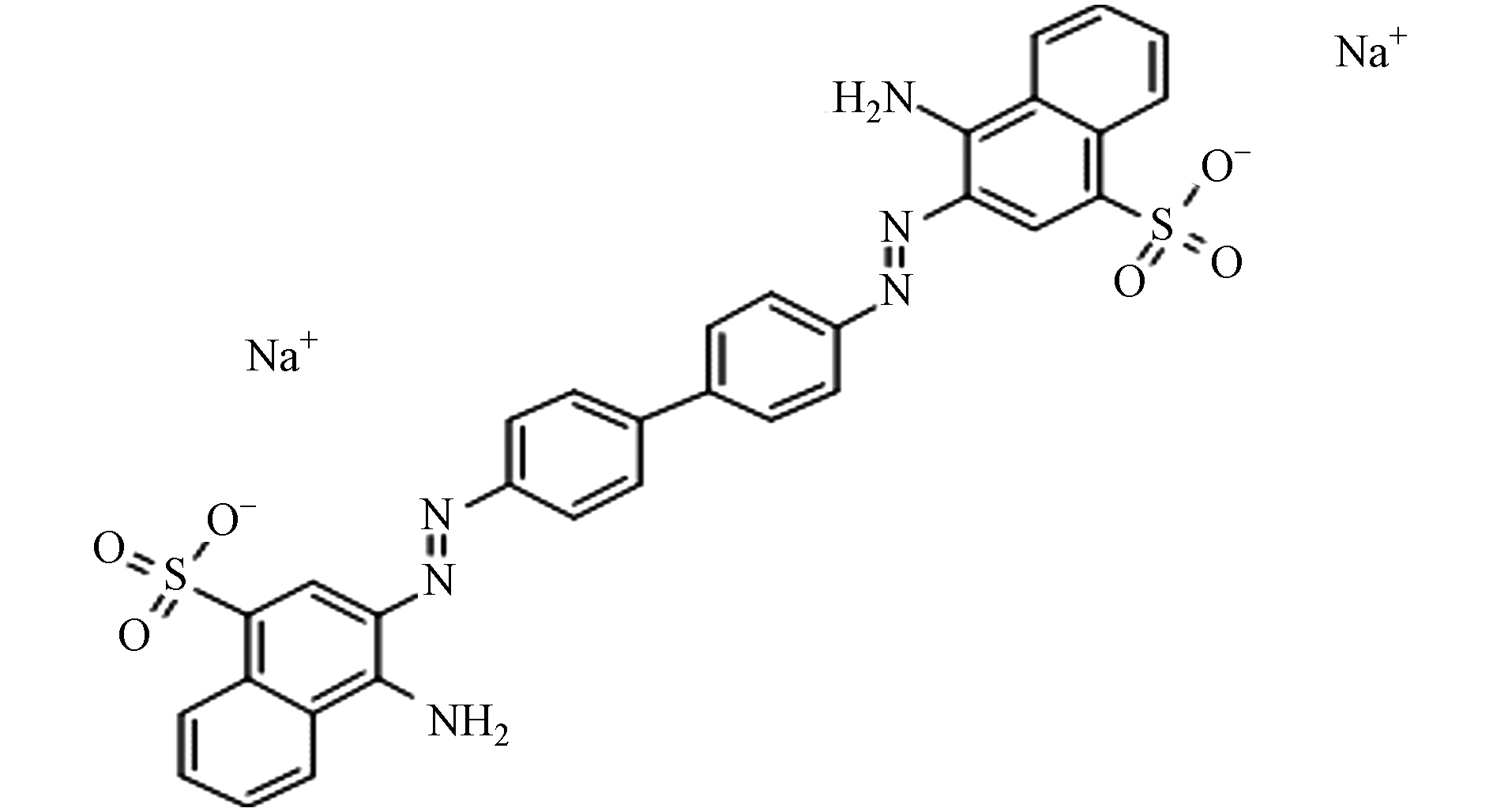
黄麻成分包括纤维素(60%)、木质素(12%)、半纤维素(14%)、水分(10%)及少量果胶、灰分和脂蜡质[8],选自江苏宿迁;漆酶(Laccase)产自米曲霉,其活性10000 U·L−1,购自西安欣禄生物科技有限公司;Cd2+,采用分析纯的3CdSO4·8H2O配制,购自天津天力化学试剂有限公司;刚果红(CR),分析纯,C32H22N6Na2O6S2,分子量为696.68,购自成都市科龙化工试剂厂,结构式如图1;漆酶-黄麻复合材料的制备同前文[16],分别得到0.1%漆酶-黄麻、0.5%漆酶-黄麻、1%漆酶-黄麻和5%漆酶-黄麻复合材料,以0.1%L-SJ、0.5%L-SJ、1%L-SJ和5%L-SJ表示.
-
以平衡吸附的方式研究了SJ、0.1%L-SJ、0.5%L-SJ、1%L-SJ和5%L-SJ在污染物浓度、温度、pH及离子强度因素下对Cd2+、CR去除效果的影响,吸附剂用量为5 g·L−1,每个处理均设2个重复. 实验设置如表1.
-
为确定漆酶效能,采用SJ、NaOH-SJ、戊二醛-SJ、0.1%L-SJ、0.1%L(D)-SJ、0.1%L(F)共6种材料进行实验. 其中,0.1%L(F)是0.1%体积浓度的游离漆酶(单位mL·L−1);NaOH-SJ是碱处理后的黄麻;戊二醛-SJ是戊二醛交联后的黄麻;0.1%L(D)-SJ是漆酶失活后的0.1%漆酶-黄麻.
-
以美国Nicolet仪器公司Nicolet 5 DX型傅里叶变换红外光谱仪(FTIR)测定SJ、戊二醛-SJ、5%L-SJ、SJ-CR、SJ-Cd2+、5%L-SJ-CR和5%L-SJ-Cd2+共7种材料的红外光谱图,其波数区间为600—4000 cm−1,其中戊二醛-SJ表示戊二醛交联后的SJ,SJ-CR和SJ-Cd2+分别表示处理CR和Cd2+后的SJ,5%L-SJ-CR和5%L-SJ-Cd2+分别表示处理CR和Cd2+后的5%L-SJ.
-
分别在9个50 mL的玻璃离心管中加入准确称取的0.1000 g黄麻供试样品,每个离心管中分别加入20 mL不同浓度的CR或Cd2+溶液,离心管口用封口膜密封后盖紧瓶盖,以100 r·min−1的转速反应120 min(实验表明已达去除平衡状态),后静置10 min,取上清液测定CR或Cd2+剩余浓度,差减法计算两者的去除量和去除率.
Cd2+测定方法为火焰光度法,仪器为日本日立的HITACHI Z-2000型原子吸收分光光度计,CR测定采用分光光度法,仪器为上海梅谱达的MAPADA UV-3200紫外可见分光光度计(500 nm波长).
-
Cd2+和CR的去除量(qe)和去除率(D)分别根据公式(1)、(2)计算:
式中,c0和ce分别表示CR或Cd2+初始浓度和平衡浓度(mg·L−1);V表示加入CR或Cd2+溶液体积(mL);m表示吸附剂质量(g);M表示CR或Cd2+相对分子质量(g·mol−1);qe表示CR或Cd2+去除量(mmol·kg−1);D为去除率(%).
-
采用Origin 8.5软件对Cd2+或CR的去除进行模型拟合及绘图,Langmuir模型的公式如下:
式中,ce表示CR或Cd2+的平衡浓度(mg·L−1);qe表示CR或Cd2+的去除量(mmol·kg−1);qL表示Langmuir等温吸附模型的饱和去除量(mmol·kg−1);KL表示Langmuir模型常数(L·mmol−1).
-
吸附热力学参数计算公式(4)、(5)、(6)如下:
式中,K是表观吸附平衡常数;
qe 、ce 同式(1),∆G是吸附自由能变(kJ·mol−1);ΔH 是焓变(kJ·mol−1);ΔS 是熵变(J·mol−1·K−1);R是气体常数(8.314 J·mol−1·K−1);T是开尔文温度(K). -
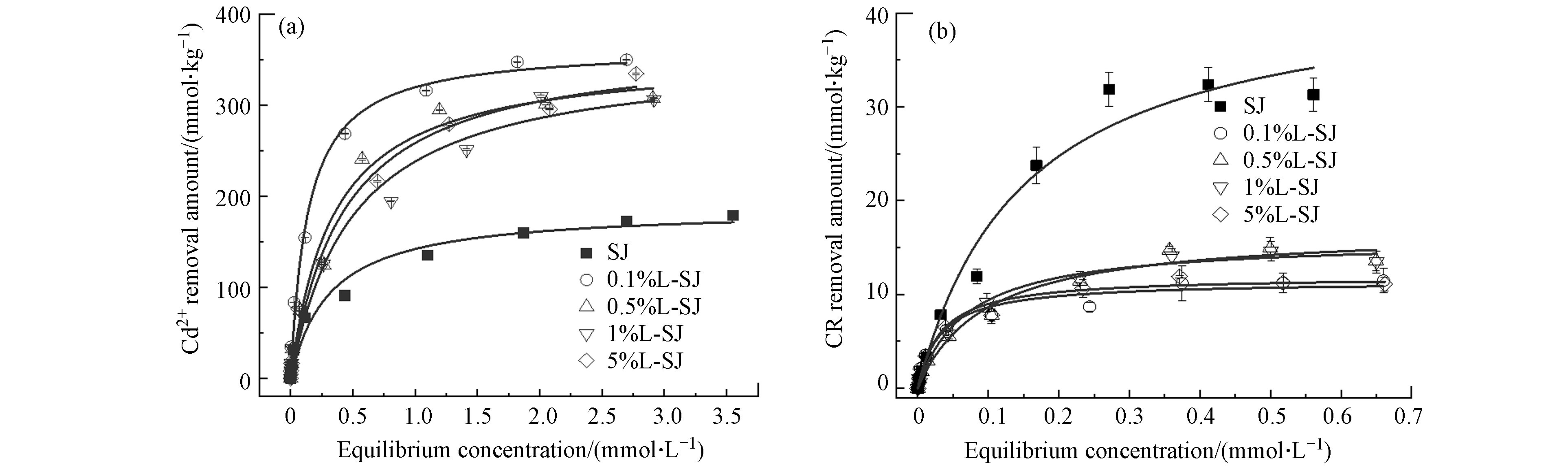
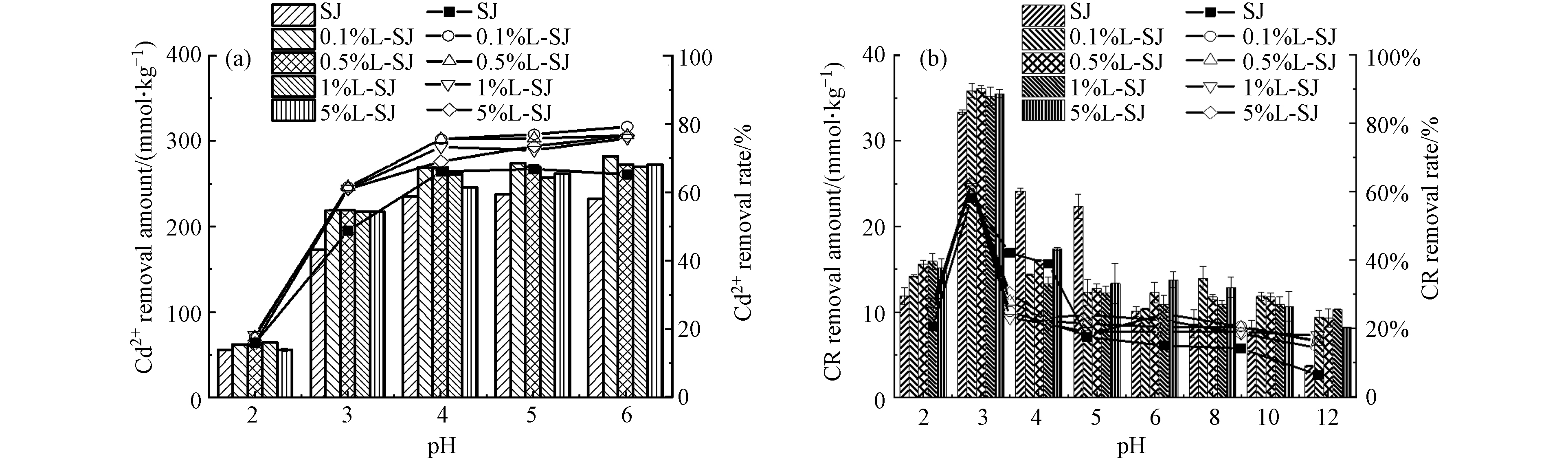
SJ、L-SJ对Cd2+和CR去除量曲线如图2. 由图2可见,SJ、L-SJ对Cd2+和CR的去除量均分别随Cd2+和CR平衡浓度的升高而增大,去除曲线均为“L”形,具有较好的一致性. 拟合决定系数显示Cd2+和CR的去除均适用于Langmuir模型,模型拟合结果见表2.
由图2(a)可以发现,不同漆酶负载的L-SJ对Cd2+的去除量均高于SJ,约为1.71—1.96倍,随给酶量的增加呈现先下降而后略有上升的趋势,顺序为0.1%L-SJ>5%L-SJ>0.5%L-SJ>1%L-SJ>SJ,4种复合材料之间,对Cd2+的去除量变化较小. 5种材料模型拟合得到的qL顺序,与实际去除量顺序一致,且数据较为接近,故SJ、L-SJ对Cd2+的拟合适用于Langmuir模型,表明单层吸附特性[17]. 对比5种材料KL发现,0.1%L-SJ的亲和系数最大,说明其对Cd2+具有最高亲和力.
如图2(b)所示,SJ、L-SJ对CR的去除量均随CR平衡浓度的增大而增加,去除曲线呈“L”形等温吸附线特征,同Cd2+变化一致. SJ对CR的去除量均高于不同负载率的L-SJ,去除量大小顺序为
SJ>>0.5%L-SJ≈1%L-SJ≈0.1%L-SJ≈5%L-SJ,去除量降低了0.64—0.67倍. 随漆酶给酶量的增加,不同漆酶处理的黄麻对CR去除量的差异较小. 复合材料不利于对阴离子染料CR的去除,这和前期对阳离子染料MB的去除结果不同,漆酶的负载有利于黄麻对MB的去除,随漆酶给酶量的增加,复合材料的去除率呈现先增加后减小的规律,说明复合材料对染料的去除受电荷类型的影响.
黄麻表面有较多内孔、裂缝和不规则颗粒,表面积较大,有利于Cd2+和CR的表面吸附,黄麻富含羟基和羧基等基团,表面具有负电荷吸附点位,有利于Cd2+的静电吸附和络合作用,这是黄麻对Cd2+和CR去除率较高的原因;随着NaOH对黄麻的预处理,其比表面积明显增大[18],—OH得到暴露[19],有利于对Cd2+的静电吸附和络合作用,也有利于对CR的表面吸附(氢键作用和π—π作用);当戊二醛在NaOH-SJ表面交联后,黄麻表面结合的—CHO可以与Cd2+发生络合反应,故提高对Cd2+的去除. 对CR而言,—CHO占据CR吸附点位,而CR的吸附机制以表面吸附为主,使得戊二醛交联后的黄麻对CR去除性能降低,戊二醛-SJ和5%L-SJ的—OH峰面积有所增大(图3),但CR去除量降低,验证了该观点;漆酶负载以后,漆酶表现出了对黄麻结构的改变:对木质素具有强化降解作用和聚合作用,使黄麻结构更为疏松,增加其比表面积,SEM结果[16]证实这一点. 漆酶在对木质素的作用过程中,伴随着重金属的吸附作用[19].
另外,漆酶是一种蛋白质,具有巯基、氨基、羟基、羧基等官能团,具有N、O、P、S等电负性较强的原子,通过为Cd2+提供电子发生螯合作用[12],故漆酶负载后的黄麻对Cd2+的去除性能优于原黄麻、NaOH-黄麻、戊二醛-黄麻. 适当的漆酶浓度(0.1%L)对Cd2+的去除效果最佳,因为漆酶浓度过高会造成拥挤或团聚,对酶的活性位点产生空间位阻[20]. 漆酶浓度过低会造成载体无效吸附[21]. 不同漆酶浓度的复合材料对CR的去除差异较小,这是因为复合材料对CR的去除主要以吸附为主,漆酶的降解作用占比较小.
漆酶-黄麻复合材料相较于黄麻而言,促进了对Cd2+的去除,而降低了对CR去除,主要是因为Cd2+和CR污染物性质的差异,Cd2+为正电荷(金属),CR为阴离子(有机物),对Cd2+的去除主要为—OH、—COOH等基团的静电引力和络合反应为主,漆酶螯合作用为辅,对CR的去除由于电荷斥力使得电性引力不是其作用形式,而主要以表面吸附为主,漆酶酶解作用为辅. 复合材料对Cd2+去除的正效应归因于NaOH预处理、戊二醛的增加和漆酶的负载. CR的去除负效应是戊二醛交联占据表面吸附点位,其下降程度远高于NaOH改性和漆酶负载的增加值.
-
图4为温度对Cd2+和CR去除性能的影响图,在3个实验温度梯度下,SJ与L-SJ对Cd2+和CR的去除变化趋势有所不同,Cd2+表现为随温度的不断升高,5种材料的去除性能降低,为放热反应特征,CR表现为随温度的升高,5种材料的去除性能增大,为吸热反应特征.
SJ、L-SJ去除Cd2+和CR的热力学参数见表3,ΔG均小于0,表明反应的自发性. 物理吸附自由能的绝对变化量小于化学吸附,物理吸附自由能范围在-20—0 kJ·mol−1,化学吸附则在−80—−400 kJ·mol−1,−80—−20 kJ·mol−1范围表示化学作用增大的物理吸附[22]. SJ和L-SJ对Cd2+的ΔG绝对值均小于20 kJ·mol−1,表明去除机制以物理吸附为主. SJ对CR的ΔG绝对值小于20 kJ·mol−1,L-SJ则在−80—−20 kJ·mol−1范围内,表明SJ对CR以物理吸附为主,L-SJ存在一定物理-化学综合作用.
ΔH表示吸附的吸/放热过程. 5种材料对Cd2+的去除过程其ΔH均小于0,为放热过程,而对CR的去除其ΔH均大于0,为吸热过程. ΔH的绝对值越小,吸附剂表面与吸附质之间的结合越松散[23]. 对比SJ、L-SJ的ΔH绝对值发现,漆酶负载后的L-SJ,与Cd2+/CR的结合更为紧密.
ΔS值代表系统混乱度增加或减小,SJ、L-SJ去除Cd2+和CR的ΔS均大于0,为熵增现象. 郝艳玲等[24]认为,ΔS变化是因为溶剂解吸、溶质吸附两者的共同作用,溶剂解吸熵增加,溶质吸附熵减小,因为固体表面与溶剂和溶质的作用程度不同、分子大小不同,在置换过程中,被置换的Cd2+/CR分子比水分子量少,使吸附熵为正值.
-
图5为pH对Cd2+和CR去除性能的影响,Cd2+和CR的去除性能呈现不同趋势. 如图5(a)可以发现,SJ随着pH的升高,对Cd2+的去除趋势为先增加后趋于平稳,在pH=5时,达到最佳去除性能,L-SJ随着pH的升高,对Cd2+的去除呈现不断增大的趋势,在pH=6时,Cd2+去除性能达到最大值;由图5(b)可以发现,SJ与L-SJ对CR的去除规律具有一致性,均随着pH的升高,去除性能先增加后降低,在pH=3时均达到最大值.
溶液pH会影响SJ、L-SJ的电荷数量和类型。SJ的pH PZC值为4.65[3],当溶液pH>pH PZC时,SJ荷净负电荷,当溶液pH<pH PZC时,SJ带净正电荷[7]。Cd2+在较低pH值时,H+会与Cd2+竞争吸附点位,与SJ、L-SJ产生静电斥力,使去除性能较低, 故SJ和L-SJ在较高pH条件下对Cd2+去除效果更佳.
CR在实验pH范围内,以阴离子形态存在(其pKa为0.45),但CR对pH值的变化较为敏感,在极酸条件下(pH=2)容易被质子化为正电荷,产生分子异构体[25],与同带正电荷的SJ、L-SJ产生斥力,同时大量的H+会竞争材料表面有限的吸附点位,使CR+难以被吸附,故在pH=2时,SJ、L-SJ的去除性能均较低;当pH=3时,CR的磺酸基团(—SO3Na),会水解成为负离子,与带正电的SJ、L-SJ发生表面吸附,故溶液在pH=3时,去除性能升高;当pH再增大(碱性)时,SJ、L-SJ的负电荷,与荷负电的CR发生静电排斥,与OH-竞争吸附,使CR去除性能不断降低.
-
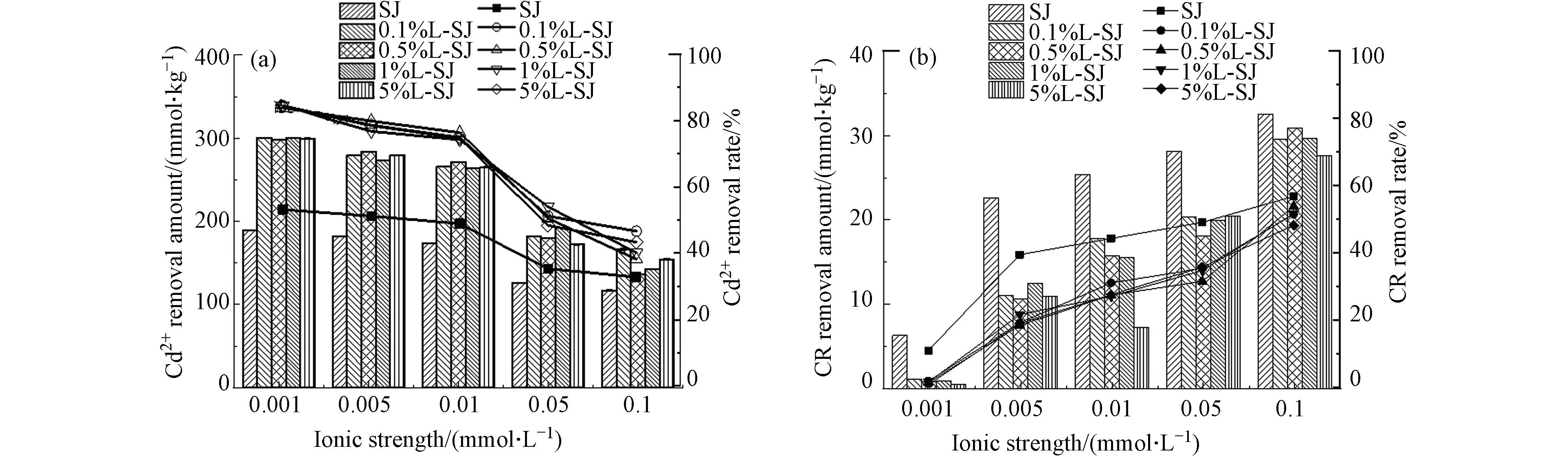
离子强度是影响Cd2+和CR去除的重要参数之一,如图6所示,Cd2+和CR的离子强度影响差异较大,SJ、L-SJ对Cd2+的去除均随离子强度的增加而降低,而对CR的去除呈相反趋势. Cd2+和CR在离子强度因素方面存在的巨大差异主要是因为两者性质的不同,Cd2+为重金属,K+可以与Cd2+形成竞争吸附[26],还可以降低Cd2+活度系数,这佐证了SJ、L-SJ对Cd2+的静电吸附机制. 化学吸附受离子强度的影响较小,但盐析作用提高了CR的疏水性,有助于CR与疏水性黄麻的表面结合,这一结果有力的支持了“CR吸附以表面吸附为主,具有物理-化学吸附特征”的结论.
-
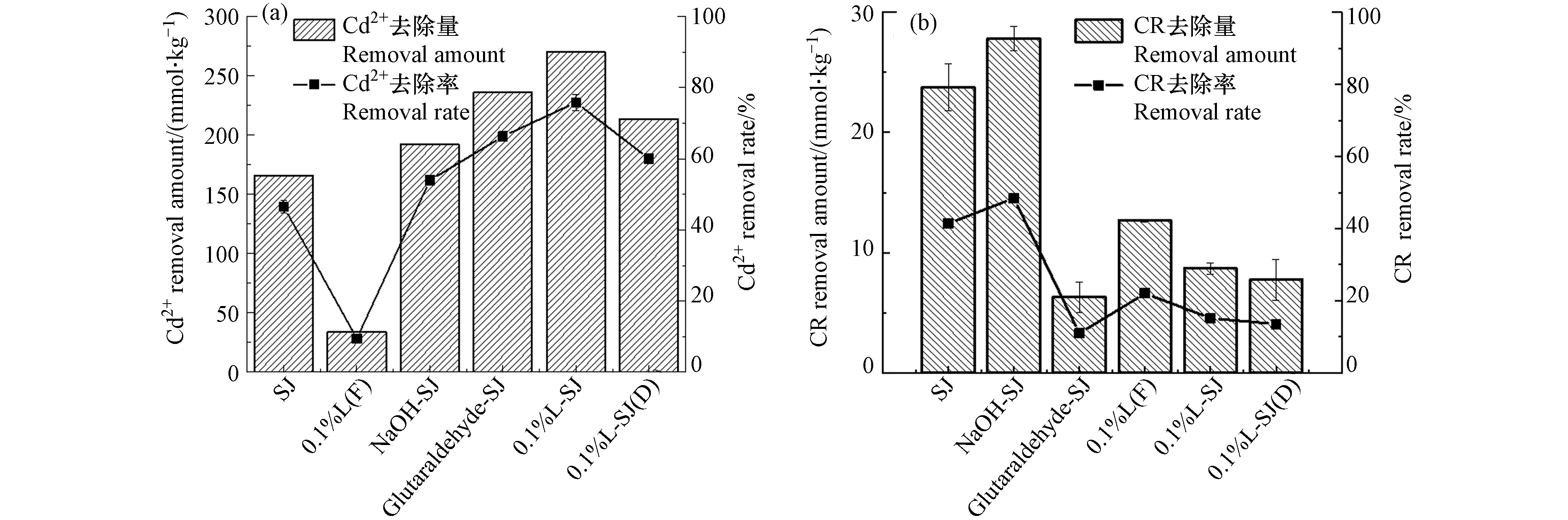
图7为SJ、NaOH-SJ、戊二醛-SJ、0.1%L-SJ、0.1%L(D)-SJ、0.1%L(F)对Cd2+和CR去除的漆酶效能分析图. Cd2+的去除率顺序是0.1%L-SJ>戊二醛-SJ>0.1%L(D)-SJ>NaOH-SJ>SJ>0.1%L(F),SJ的Cd2+去除率是46.52%,SJ经NaOH预处理后,其去除率增至53.91%,扫描电镜结果显示[16],碱处理后的纤维材料其孔隙率增加、表面积增大,碱处理增大对Cd2+的去除性能与静电吸附相关. 戊二醛交联后的黄麻,去除率增至66.27%,漆酶负载后的0.1%L-SJ,去除率达到最大值(75.79%),0.1%L-SJ的去除率减去戊二醛-SJ的去除率为9.52%,约等于0.1%L(F)去除率,0.1%L(D)-SJ(59.99%)的去除率减去0.1%L-SJ的去除率为15.80%,说明漆酶对Cd2+的去除率约为10%. NaOH预处理、戊二醛交联处理和漆酶的负载,均有效增加了对Cd2+的去除. 通过D SJ、D (NaOH-SJ)-SJ、D (戊二醛-SJ)- (NaOH-SJ)和D0.1%L-SJ- D(戊二醛-SJ)占 D 0.1%L-SJ的百分比作为贡献率,分别为61.38%、9.75%、16.31%和12.56%. 证实L-SJ对Cd2+的去除机制是黄麻吸附为主,漆酶作用为辅.
CR去除率的顺序为NaOH-SJ>SJ>0.1%L(F)>0.1%L-SJ>0.1%L(D)-SJ>戊二醛-SJ,去除率分别为48.45%、41.38%、21.57%、15.15%、13.50%、10.95%. 0.1%L(F)对CR的酶解率为21.57%,说明漆酶具有一定降解CR的能力,0.1%L-SJ的去除率减去戊二醛-SJ的去除率约为4.2%,0.1%L-SJ的去除率减去0.1%L-SJ(D)去除率为约1.65%,均证实了酶在CR去除中的作用. NaOH-SJ能够达到对CR的最高去除率,经戊二醛交联后,戊二醛-SJ去除性能显著降低,再经漆酶负载后,提高了其去除能力,但其增加量远低于戊二醛交联的降低量,总体显示出降低趋势. 该结果表明,L-SJ去除性能降低的原因是戊二醛的降低效应. 和Cd2+去除的结果相比较,L-SJ对CR的去除机制同样显示出以黄麻吸附作用为主,漆酶降解作用为辅的作用. 这也和L-SJ对CR、Cd2+去除随pH、温度和离子强度的变化而变化的趋势与SJ基本一致的结果相呼应.
-
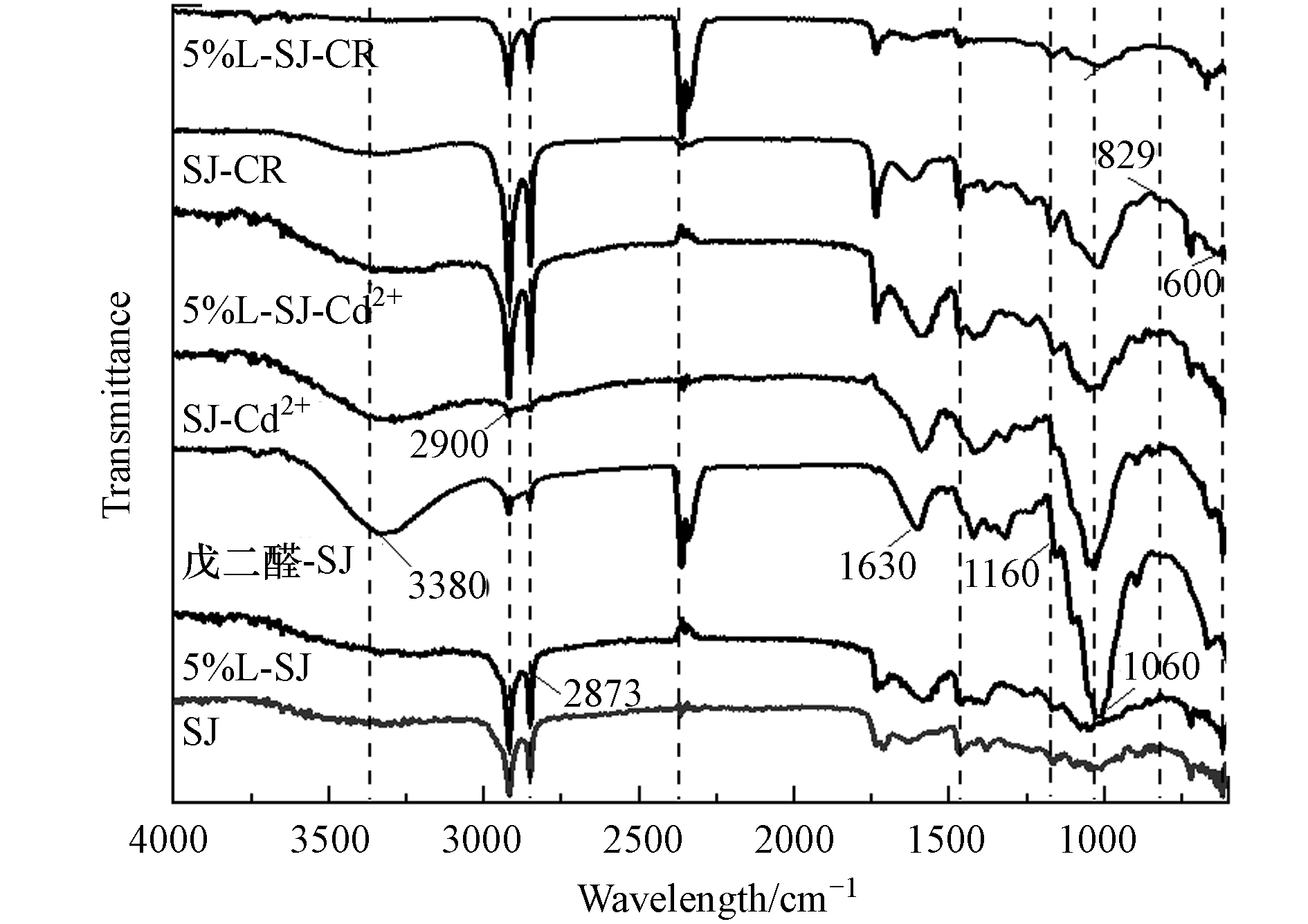
SJ、戊二醛-SJ、5%L-SJ、SJ-Cd2+、SJ-CR、5%L-SJ-Cd2+和5%L-SJ-CR的 7种材料红外光谱图见图3. 研究表明,SJ经漆酶负载后,基本结构未发生改变,但部分官能团的峰面积发生了改变[16].
分析SJ、SJ-Cd2+和5%L-SJ、5%L-SJ-Cd2+在—OH伸缩振动处的峰(3380 cm−1)发现,SJ-Cd2+和5%L-SJ-Cd2+峰面积均减小,表明—OH在Cd2+去除中发生静电作用或络合作用[27]. —COOH的C=O拉伸振动在1630 cm−1处发生蓝移现象,表明—COOH与Cd2+可能存在电性引力[28]. 有学者[29]认为,Cd2+可以与—OH和—COOH发生电性引力或配位作用,有利于对Cd2+吸附作用. 另外,程启明等[30]也证实花生壳去除Cd2+的去除机制,是Cd2+与花生壳表面的C=O、—OH官能团发生络合反应. 另外,其他峰值未发生较大变化,表明了SJ和5%L-SJ对Cd2+的去除机制,主要以静电作用或络合作用为主[18].
分析戊二醛-SJ、5%L-SJ在3380 cm−1、1060 cm−1处的—OH峰发现,戊二醛-SJ-CR的峰面积有所增大,—OH数量增多却降低了其对CR的吸附能力,表明其对CR的去除机制并非静电作用;SJ-CR、5%L-SJ-CR在829 cm−1处出现了CR中C=C芳香环的伸缩振动峰,表明CR在SJ和5%L-SJ表面吸附的存在[31]. SJ、5%L-SJ结构中的C=C官能团,均含有一个π电子轨道,可与CR分子结构显示含有丰富的苯环结构,易于形成π—π作用[31]. 分析SJ、L-SJ吸附Cd2+/CR前后的红外光谱图可以发现,图谱并没有发生较为明显的变化,表明SJ和5%L-L-SJ对CR的去除主要以物理吸附、π—π共轭的化学作用为主,这和吸附过程的温度效应相一致[18]. L-SJ对Cd2+和CR的机理图如图8所示.
-
(1)L-SJ对Cd2+和CR的去除曲线均符合Langmuir等温吸附模型,漆酶的负载增大了SJ对Cd2+的去除,去除量约增大1.71—1.96倍,Cd2+的去除正效应归因于NaOH预处理、戊二醛增加、漆酶负载与Cd2+的作用. 漆酶的负载降低了对CR的去除,降低了约0.64—0.67倍,主要是因为戊二醛交联占据表面吸附定位,其下降程度远高于NaOH改性和漆酶负载的增加值.
(2)SJ与L-SJ对Cd2+表现为放热反应,随pH的增大,SJ对Cd2+的去除性能先增大后趋于稳定,而L-SJ则不断增大,随离子强度的增大,SJ与L-SJ对Cd2+的去除性能不断下降. SJ与L-SJ对CR的去除随温度的升高而增大,随pH的增大呈现先增加后降低的趋势,随离子强度的增大其去除性能不断增大.
(3)L-SJ对Cd2+的去除机制以络合反应和静电引力为主,漆酶螯合作用为辅,对CR的去除以表面吸附为主.
黄麻固定化漆酶去除水中Cd2+和刚果红
The removal of Cd2+ and Congo red from water by immobilized laccase on jute
-
摘要: 本文通过酶固定化技术合成漆酶-黄麻复合材料(L-SJ),以Cd2+和刚果红(CR)为模型污染物,通过批处理法研究了黄麻和复合材料对Cd2+和CR的等温去除规律,考察了漆酶浓度、温度、pH和离子强度对Cd2+和CR去除的影响,并结合红外光谱图探讨其去除机制. 研究发现,黄麻和复合材料对Cd2+和CR的去除曲线均符合Langmuir等温吸附模型,复合材料对Cd2+的去除高于黄麻,增大约1.71—1.96倍,但降低了对CR的去除. 黄麻与复合材料对Cd2+的去除均表现为放热反应;随pH的增大,黄麻对Cd2+的去除先增大后趋于平稳,而复合材料则不断增大;随离子强度的增大,黄麻与复合材料对Cd2+的去除不断下降. 黄麻与复合材料对CR的去除随温度、离子强度的升高而增大,随pH的增大呈现先增加后降低的趋势. 漆酶效能实验发现,NaOH预处理、戊二醛改性和漆酶负载均增大了复合材料对Cd2+的去除,NaOH预处理和漆酶负载也增大了复合材料对CR的去除,但戊二醛交联后黄麻却降低了CR的去除能力,降低量远远高于NaOH改性和漆酶负载增加量. 复合材料对Cd2+的去除机理以静电引力和络合反应为主,漆酶作用为辅,对CR的去除以表面吸附为主,漆酶作用为辅.Abstract: In this paper, the laccase-jute composites (L-SJ) were synthesized by enzyme immobilization technology, Cd2+ and Congo red (CR) were used as model pollutants, the isothermal removal of Cd2+ and CR by jute and L-SJ were studied by batch process. Additionally, the effects of laccase concentration, temperature, pH and ionic strength on the removal of Cd2+ and CR were investigated. The Fourier Transform Infrared Spectroscopy was used for exploring the removal mechanism. The results showed that the removal curves of Cd2+ and CR by both jute and L-SJ fitted the Langmuir isotherm adsorption model. The removal of Cd2+ by L-SJ was higher than that of jute, increasing by about 1.71—1.96 times, but removal of CR was lower than jute. The removal of Cd2+ by both jute and composites was exothermic reaction.With the increase of pH, the removal of Cd2+ by jute inclined first and then stabilized, while which of L-SJ continued to increase. With the enhancement of ionic strength, the removal of Cd2+ by jute and L-SJ both continuously deceased. The removal of CR by jute and L-SJ increased with the rise of temperature and ionic strength, but increased first and then decreased with further increase in pH. The results of laccase efficiency experiment indicated that NaOH pretreatment, glutaraldehyde modification and laccase loading all enhanced the removal of Cd2+ by L-SJ. NaOH pretreatment and laccase loading also augmented the removal of CR by L-SJ. However, the negative effect of glutaraldehyde crosslinking on the removal of CR was much higher than the positive effect of NaOH modification and laccase loading. The removal of Cd2+ by L-SJ was mainly due to electrostatic attraction and complexation reaction, followed by the chelation of laccase, and the removal of CR was mainly due to surface adsorption, followed by the action of laccase.
-
Key words:
- immobilized laccase on jute /
- heavy metal /
- organic dyes /
- adsorption /
- enzymolysis.
-
2021年中国社会化石燃料的使用比重高达89%,二氧化碳(CO2)排放量高达7500万吨,随着化石燃料的大肆消耗和CO2排放量的逐年攀升,人类面临着温室效应和能源危机的双重挑战[1]. 在“碳中和”的大背景下,如何将CO2收集与资源化利用成为关键问题. 常见的CO2利用手段包括地质利用(CO2驱油提高采收率)、化工利用(以CO2为原料生产化学品或燃料)和生物利用(利用微藻类植物进行CO2生物转化)[2-3].
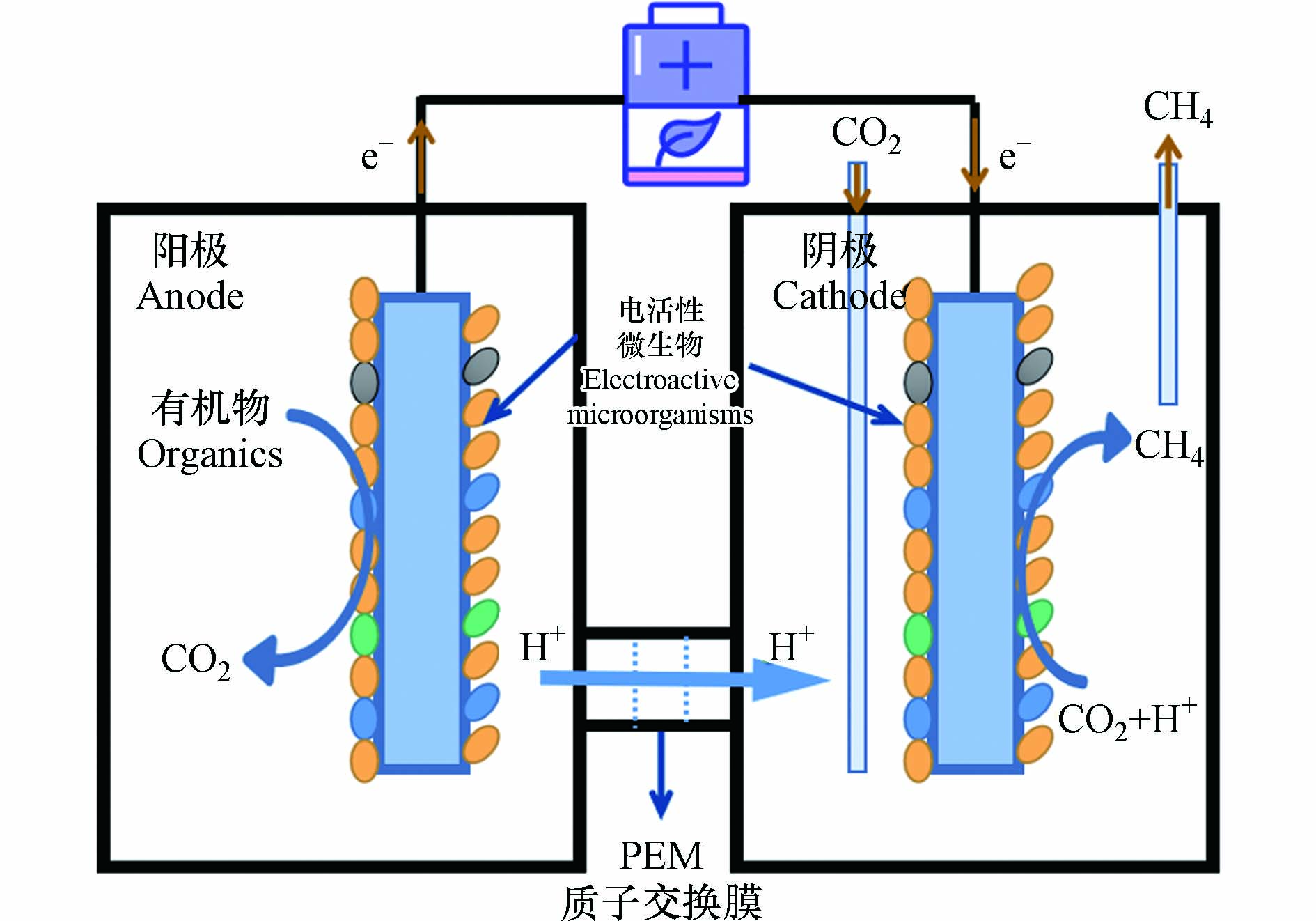
生物电化学系统(Bioelectrochemical systems,BES)是近年来发展起来的,结合了化工利用和生物利用的一项CO2资源化技术,其中微生物电解池(Microbial electrolysis cells,MEC)可有效利用CO2转化为低碳燃料[1, 3-4]. 甲烷(CH4)作为最简单的有机物且含碳量最少的烃,是当今主要的燃料和化工原料. 相较于MEC制氢气(H2)所面临的H2燃点范围宽,常温常压难储存且价格昂贵(每kg 60—70元)等挑战,利用MEC将CO2转化为低碳燃料CH4,不仅极大地降低了CO2的排放并促进碳循环,且CH4作为一种清洁的低碳燃料,价格低廉(每kg 20—30元)且便于运输,可缓解紧张的能源需求[2, 4]. 与传统的厌氧消化产甲烷相比,MEC辅助CO2电产甲烷可将有机物氧化和产甲烷过程分开进行,减少废水对产甲烷菌的冲击,提高甲烷产量[2]. 其原理如图1所示,MEC阳极通过多种氧化反应(析氧反应或有机物的氧化分解)提供质子,质子通过质子交换膜到达阴极,电活性微生物在生物阴极上完成CO2电甲烷化,HCO3-/CO2常常可以被微生物利用产生甲烷[5-6].
近年来关于MEC的研究如雨后春笋破土而出(图2),研究人员多分布在不同国家,但关于MEC的研究方向大多集中在制H2;随着“双碳”目标的提出,MEC精炼废物质能源被开发,相应的电极材料、反应器构造类型、能耗等成为了研究者们关注的问题[2, 5, 7]. 因此,本文全面回顾了MEC电极材料、电压和电产甲烷菌等对CO2电甲烷化效能的影响,并重点讨论阴极电活性功能菌和胞外电子传递机制,以期为MEC在未来应用方面的技术挑战提供理论支撑.
1. MEC性能的影响因素 (Factors affecting the performance of MEC)
MEC辅助CO2电产甲烷的效率受到多方因素的影响,为了获得更高的甲烷产率,需性能稳定的阴极催化剂、高效传递电子的电活性微生物和合适的外加电势等.
MEC阴极作为电活性微生物电子传递的主要介质和产甲烷菌附着的主要场所,是影响MEC性能的关键因素之一,MEC阴极材料一般具有良好的导电性、无微生物毒性、高比表面积、低过电位等[2-3]. 铂(Pt)是最早被用在MEC上的电极材料,其能量效率高达75%—80%,但由于Pt开采、提取过程中负面环境影响大,故亟需开发环境友好且效率与Pt相当的电极材料,碳基材料、金属基材料和复合材料近年来渐渐崭露头角[8-9]. 图3(a)总结了近5年MEC研究者利用不同电极材料所获得的最高甲烷产率和最大电流密度[2-4, 6-21](注:产甲烷率和电流密度均做归一化处理,气体体积按照标况下进行折算). 从数据统计看,最高甲烷产生速率为复合材料>金属基材料>碳基材料. 碳布、碳纸作为MEC生物阴极的基底可达到的最高甲烷产生速率仅为35—87 mL·L−1·d−1之间,低于碳毡和碳刷,可能是由于碳布这类平面电极比表面积低、微生物难以富集在材料内部所导致的,碳毡和碳刷则具有更大的比表面积,更有利于致密电活性生物膜的形成[2, 7]. 不锈钢和过渡金属(主要是镍(Ni))作为金属基材料则具有良好的导电性,其最高甲烷产生速率可达到135—350 mL·L−1·d−1,有研究证实不锈钢可引起电解液脱氢并释放H2,进而促进氢营养型产甲烷菌通过间接电子传递的方式产生CH4[9, 21]. 此外,Ni还是产甲烷菌的关键酶(甲基辅酶M)的组成金属元素,产甲烷菌相较于其他微生物更易占据Ni电极的阴极位点,进而提高甲烷产率[15]. Pt-碳毡和Pt-钛网等是常见的贵金属修饰复合阴极,但Pt修饰的贵金属电极也极易发生析氢反应产生H2,被氢营养型产甲烷菌利用,进而通过间接电子转移的方式刺激CO2快速向CH4转化[11-12, 16]. 电流密度可以侧面反映阴极单位表面积上单位时间内通过的电量,贵金属修饰的复合电极最大电流密度可达45—60 A·m−3,说明其导电性较好[2-4, 6-21]. 纳米管和纳米粒子等纳米材料通过电沉积、空气喷涂等方法修饰在碳基或金属基材料表面制备复合阴极材料,纳米修饰的复合材料通常具有良好的催化活性和稳定性,且多孔疏松的纳米形态有利于细胞的聚集和胞外聚合物的产生,其最高甲烷产生速率高达200—350 mL·L−1·d−1,纳米修饰的复合阴极材料也是近年来MEC阴极的研究热点[10, 14, 19],如磁铁矿/沸石纳米复合材料[22]、Ni/Co-NC纳米复合材料[23]、磁性GO/Fe3O4纳米复合材料[24]等,研究表明,纳米金属复合材料作为MEC阴极更有利于电活性微生物在其上形成厚且致密的生物膜,同时促进氢营养产甲烷通过直接电子转移的方式来来增强CO2到CH4的生物电化学还原作用[22-25].
电压调控对于MEC电产甲烷也尤为重要,图3(b)总结了近几年各个学者在不同电压下利用MEC产生的最高甲烷产生速率[5, 7, 9, 25-36]. −0.6—−1.0 V vs. AgCl是常见的阴极电压,其间的最高甲烷产生速率一般在15—100 mL·L−1·d−1之间[5, 7, 9, 25-36]. 阴极电位越负甲烷产量越高,可能是因为更负的电压提高了产电菌群的活性,并增强了其内部的电子传递效率,但是最佳的甲烷产量是在−0.9—−1.0 V vs. Ag/AgCl 的平衡电位下实现的,虽然研究者常通过施加更负电位的方法以克服CO2电甲烷生成的能垒,但研究表明电压高于−1.2 V时,产甲烷菌的生物膜会遭到破坏,生长活性和代谢速率会降低,所以MEC电压保持在一个合适的范围是非常重要的[5, 9, 28, 33].
除了电极材料和阴极电压外,MEC的结构也会间接影响微生物的电子传递性能及CH4产率[2, 10]. 图3(c)总结了近些年相关文献中,单室和双室MEC的最大产甲烷速率,单室MEC的最高甲烷产生速率一般在85—200 mL·L−1·d−1之间,而双室则在45—100 mL·L−1·d−1之间[3, 4, 7-8, 11-14]. 单室MEC由于无质子膜阻隔,阴阳两极间距较双室MEC更近,故内阻更小、物质间的传质阻力更低、电流密度更高,适合进行规模化CH4生产[13-14]. 但是单室MEC由于无质子膜阻隔,阴阳电解液相互接触,极易发生副反应,所以在单室MEC中如何实现目的产物CH4的高效定向转化是研究者们应该关注的重点问题[37].
此外,MEC中最大电流密度是CO2电甲烷化过程中相对电子传递效率的重要指标,与最高产甲烷速率之间也有一定关系,电流较高意味着更快的反应动力学,更低的生物膜电阻和电荷转移电阻,同时高电流响应意味着较好的生物膜活性和较低的电位损失[14, 16-17]. 从近5年的文献中可以发现,贵金属修饰的复合材料产生的最高电流密度最大(图3(a)),达到47—59 A·m−3之间,可能是贵金属修饰的复合电极更易生物膜的快速成型,从而产生更高的电流;较高的外加电压会产生较高的电流密度,MEC在−1.2 V vs. AgCl时的最高电流密度最大(图3(b)),达到7.5—11.4 A·m−3之间,可能是较高的电压输入给予MEC更高的电流流动;单室MEC的电流密度高于双室MEC(图3(c)),可能是单室MEC内阻小、传质阻力低[13-14].
2. MEC中电活性功能菌的特点及重要作用 (Characteristics and important roles of electroactive functional bacteria in MEC)
2.1 电活性功能菌的种类与形态特征
MEC辅助CO2电产甲烷的运行关键在于阴极腔室内的电活性功能微生物,它们承担着电子转移等重要工作,被誉为MEC的“心脏”. 电活性功能微生物可借助外源电势差突破超电势与内电阻的限制,摄取来自电极表面的电子,还原为CO2为低价态的有机物(如CH4),以及将氧化态物质还原成还原态无机物[38]. 为了提高产MEC产甲烷的性能,了解电活性功能微生物的类型、群落组成和微生物之间的相互作用也至关重要. 能够还原CO2产甲烷的微生物是一种重要的电活性功能微菌,在分类学上,属广古菌门(Euryarchaeota),其包括5个目(Methanobacteriales、Methanococcales、Methanomicrobiales 、Methanopyrales和 Methanosarcinales)、10个科(Methanobacteriaceae、Methanocaldococcaceae、Methanococcaceae、Methanocorpusculaceae、Methanomicrobiaceae、 Methanopyraceae、Methanosaetaceae 、Methanosarcinaceae、Methanospirillaceae、Methanothermaceae)及31个属,这些微生物对氧气极为敏感,因此大多是都需要在严格缺氧的环境中培养[39]. CO2电产甲烷菌常利用的底物类型为甲基类、H2和乙酸,对应的产甲烷菌分别可以被命名为甲基营养型(方程式1)、氢营养型(方程式2)和乙酸营养型(方程式3)产甲烷菌[39].
4CH3OH→3CH4+CO2+2H2O (1) 4H2+CO2→CH4+2H2O (2) CH3COO−+H+→CH4+CO2 (3) 产甲烷菌的主要生理特征(碳源、温度和pH范围)总结在表1中,大多数产甲烷菌是嗜温细胞,在pH值约为7时生长最佳[40]. 形态上,典型的产甲烷菌有甲烷杆菌属(Methanobacterium)、甲烷球菌属(Methanosarcina)和甲烷丝状菌属(Methanosaeta)(图4)[40-41]. 淡水沉积物、泥炭沼泽、稻田和污水消化池等为产甲烷菌最适宜生长的环境[42].
表 1 产甲烷菌的主要生理特征Table 1. Main physiological characteristics of the methanogenic bacteria产甲烷菌Methanogenic bacteria 碳源Carbon sources 温度范围/ °C Temperature range pH 参考文献References Methanosarcinales 乙酸盐,H2 + CO2,CO, 甲醇, 甲胺,甲硫基丙酸甲酯,二甲硫 1.0—70 4.0—10.0 [43-45] Methanomicrobiales H2 + CO2,甲酸盐,乙醇,2-丙醇,2-丁醇,环戊醇 15—60 6.1—8.0 [42, 46] Methanobacteriales H2 + CO2,CO,甲酸盐,C1-甲基化合物 20—88 5.0—8.8 [44] Methanococcales H2 + CO2,甲酸盐 < 20—88 4.5—9.8 [44, 46] Methanopyrales H2 + CO2 84—110 5.5—7.0 [47-48] Methanocellales H2 + CO2,甲酸盐 25—40 6.5—7.8 [44] 2.2 电活性功能菌的胞外传递机制
MEC中电子是如何从阴极电极表面转移用于CO2还原的过程受到学界的关注,一种解释是在MEC的阴极表面产生了H2,附着在阴极的电产甲烷菌以H2/CO2为底物合成CH4,还有一种解释是电产甲烷菌可以直接从阴极表面获得电子还原CO2产生CH4 [46-47]. 培养了一段时间后的MEC的阴极将会被一层厚厚的电活性生物膜覆盖,电活性生物膜的活性外层负责转移电子产生电流,死的内层将作为导电基质发挥作用[46, 49]. 这种电活性生物膜是MEC电产甲烷中微生物和电极之间电子转移过程的重要一环,可以使得电产甲烷菌之间通过有效的细胞“交流”促进细胞间的活动与代谢,利用阴极电极表面的电子产生CH4[50].
在MEC还原CO2产甲烷的体系中,电产甲烷微生物间通过种间电子传递的合作方式对底物及阴极表面的电子进行利用,形成复杂且高度组织化的多细胞和多物种结构,达到互营共生的效果. 一些胞外活性菌会先把大分子有机物分解为小分子有机物,电产甲烷微生物通过利用小分子产生甲烷[46, 49]. 在MEC生物阴极表面,根据电子传递路径的不同可以分为直接种间电子传递(direct interspecies electron transfer,DIET)和间接种间电子传递(mediated interspecies electron transfer,MIET)两种方式[50]. 在DIET中,微生物通过细胞表面的蛋白质(如c型细胞色素)或细胞附属物(如菌毛或纳米线)与不溶性电子受体(如电极)建立直接接触,在MIET中,微生物利用可溶性氧化还原活性化合物作为电子穿梭的媒介,以介导细胞表面暴露的导电蛋白和不溶性电子受体之间的电子转移[50, 51].
H2是最早被发现的能够进行间接种间电子传递的中间载体(图5a),且MIET过程中产甲烷菌的耗H2速率与NAD+/NADH、FAD/FADH2、Fd(ox)/Fd(red)和F420/F420-H2等多种内源性氧化还原介质有关[49]. 辅酶M和辅酶B形成混合二硫化物作为整个厌氧产甲烷呼吸链的电子受体,分子氢、还原型辅酶F420和还原型铁氧还蛋白作为电子供体;A1A0-ATP催化合成的驱动力型ATP合成酶、甲基转移酶和甲酰基甲烷呋喃脱氢酶是与产甲烷过程有关的能量转导酶. 其中,甲基转移酶是一种独特的、可逆的钠离子泵,它将甲基转移与Na+跨膜转运相结合,电子受体菌则利用电子供体菌提供的H2还原辅酶(F420)和铁氧还蛋白(Fd(ox)),进而将CO2还原成甲烷[52].
 图 5 细胞间的电子传递机制(a)通过可扩散分子(如H2和甲酸盐)[53],(b)通过电子穿梭(如黄素)[54],(c)通过导电菌毛[60],(d)通过细胞间的直接接触(如外膜c型细胞色素)[64],(e)通过导电材料(如活性炭、纳米磁铁矿)[61,66-67]Figure 5. Electron transfer mechanism between cells (a) via diffusible molecules (such as H2 and formate) [53], (b) via electron shuttle (such as flavin) [54], (c) via conductive pili [60], (d) via intercellular direct contact (e.g. outer membrane c-type cytochromes) [64], (e) via conductive materials (e.g. activated carbon, nanomagnetite) [61,66-67]
图 5 细胞间的电子传递机制(a)通过可扩散分子(如H2和甲酸盐)[53],(b)通过电子穿梭(如黄素)[54],(c)通过导电菌毛[60],(d)通过细胞间的直接接触(如外膜c型细胞色素)[64],(e)通过导电材料(如活性炭、纳米磁铁矿)[61,66-67]Figure 5. Electron transfer mechanism between cells (a) via diffusible molecules (such as H2 and formate) [53], (b) via electron shuttle (such as flavin) [54], (c) via conductive pili [60], (d) via intercellular direct contact (e.g. outer membrane c-type cytochromes) [64], (e) via conductive materials (e.g. activated carbon, nanomagnetite) [61,66-67]除H2外,甲酸也可作为电子载体介导MIET的发生,Rotaru等[53]在Pelobacter carbinolicus和Geobacter sulfurreducens共培养体系中发现P. carbinolicus和不能利用H2但能利用甲酸的G. sulfurreducens转基因菌株共培养时很容易生长,但若以乙醇作为电子供体、富马酸盐作为电子受体时,两菌没有互营共生且生长受到抑制,证明G. sulfurreducens的甲酸脱氢酶(fdnG)基因可有效弥补氢化酶的缺失,P. carbinolicus通过H2/甲酸盐的种间转移而不是DIET与G. sulfurreducens交换电子.
除H2和甲酸外,具有氧化还原性能的电子介质,如黄素、吩嗪和醌类等,也可介导电产甲烷微生物的MIET[54](图5b). Liu等[55]构建了一个含有S. oneidensis MR-1和可产生核黄素的枯草芽孢杆菌RH33联合体,发现RH33产生的高浓度核黄素可被S. oneidensis MR-1用于提高生物电. Engel等[56]联合培养G. sulfurreducens和S. oneidensis,发现混合培养优于单独培养,可能由于S. oneidensis代谢产生的氢化酶和黄素被G. sulfurreducens用于增强直接电子转移,促进该生物体能够在阳极表面形成更厚的生物膜,同时增加电流密度. 除核黄素之外,腐殖质也具有独特的电子转移能力,能在介导微生物种间电子转移方面发挥重要作用,G. metallireducens和S. alga可利用蒽醌-2,6-二磺酸盐作为电子受体进行厌氧呼吸[57]、维持细胞生长,研究显示,低剂量的蒽醌-2-磺酸盐(50 µmol·L−1)可得到最大的甲烷产量[58].
除了间接电子传递,近年来,研究者发现电活性微生物可通过菌体DIET传递电子,实现互营共生. Summers等[59]在G. metallireducens和G. sulfurreducens的共培养体系中首次证实了DIET的存在,微生物种间DIET机制通过导电菌毛[60]、导电材料[61]与功能蛋白复合物[62]3种方式实现.
导电菌毛是一类由电活性微生物合成、具有导电性的纤维状的蛋白质细丝,通过产甲烷微生物的导电菌毛,微生物胞内代谢产生的电子可以长距离输送到胞外受体或其他产甲烷微生物,实现了微生物-胞外环境的沟通交流[60](图5c). 微生物纳米导线最初通过导电探针原子力显微镜发现,G. sulfurreducens及S. oneidensis,蛋白PilA组成的Ⅳ型菌毛能够实现快速、长距离的电子传递,将电子从细胞表面转移到 Fe (Ⅲ) 表面. 近年来的研究表明,大部分的导电菌毛的主要成分为细胞色素OmcS、PilA蛋白起到调节omcS和其他多血红素细胞色素的分泌的作用[63]. 除了OmcS,常见的功能蛋白复合物还有血红素细胞色素c(MtrC和OmcA),可转运细胞间的蛋白质,实现细胞间的交流协作[64](图5d). 当G. sulfurreducens中血红素细胞色素c基因被敲除后,菌体生长受到抑制,这表明细胞色素所组成的跨膜电子通道主导了DIET过程[62]. 敲除MtrC和OmcA后的S. oneidensis MR-1 细胞,其在电极上的覆盖量和在电极上产生的电流比未敲除基因的细胞减少80%,此外,分离并纯化两种基因后发现,两种外膜细胞色素c可以结合金属氧化物如Fe2O3,并能将电子直接转移到Fe2O3的电极表面,Xiong等[65]还利用光学波导光模型和蛋白膜伏安法测得OmcA与Fe2O3的结合力为1.2—2.6 nmol·cm−2,总之,电活性菌外膜上的MtrC和OmcA可以相互接触通过其暴露在蛋白质表面的血红素将电子直接传导给胞外电子受体[60]. Zhang等在MEC电活性系统中检测到了产甲烷菌菌毛状的纳米线的存在,并认为其有电子转移的能力.
导电材料构建的“微生物-电极”的电子传递系统也是近年来DIET的研究热点,导电材料的加入可明显提升多种厌氧系统的效率并作为导体加强产甲烷微生物种间DIET[61,66-67](图5e). Liu等[61]通过在OmcS蛋白缺失的突变种G. sulfurreducens加入纳米磁铁矿使其弥补了细胞外电子交换中菌毛相关c型细胞色素的缺乏,恢复DIET的能力. Park等[66]将颗粒活性炭加入到厌氧消化的小瓶中使得总甲烷产量比对照组升高了75%,并通过宏基因组学证明了产甲烷总量与效率的提升是颗粒活性炭的加入改变了微生物群落结构并调整了DIET的相关基因的丰度两方面同时作用的结果. 此外,无导电菌毛或导电性能较差的菌株亦可以在导电材料表面与其他微生物建立联系,从而达到互营共生,Liu等[61]研究发现,磁铁矿纳米颗粒(20—50 nm)吸附在导电菌毛上,通过作为菌毛上OmcS的替代物,增强突变种Geobacter的DIET能力. 但有学者提出磁铁矿等导电材料只接受电子,却不会将电子传递给其他微生物[67],因此研究导电材料在电子传递中的作用时,还应该综合评估对产甲烷微生物的作用.
尽管,产甲烷微生物在碳氮磷的全球循环、环境污染物的修复以及各种生物能源策略中发挥着关键作用,已经有研究将电活性功能菌的电化学行为用于提高甲烷生产,减少启动时间,并提高厌氧消化系统的稳定性[68-69]. 在混合菌种共培养体系中平衡代谢过程的潜在驱动力可能与菌种之间的种内和种间的密切交流有关,然而电活性微生物之间传递偶联尚未得到广泛探索,因此了解并识别微生物通讯通信机制对于MEC电产甲烷的应用至关重要,并为工程系统的潜在应用开辟了无数可能性. 依靠微生物的本能来增强胞外电子传递可能是增强微生物电活性的一种有前途的方法,但仍需大量研究来解开电活性生物相互“通信”的过程.
3. 开发实用 MEC 技术的需求和挑战 (The needs and challenges of developing practical MEC technologies)
近年来,基于电催化耦合的技术近年来越来越多见,学者将多个处理方法有机融合到一起,达到耦合多个生化过程到一个装置、多工艺协同作用产甲烷的效果,如MEC-AD、MEC-UASB、M-MEC-AD等. 图6总结了部分典型MEC耦合其他系统产甲烷的工艺.
 图 6 近五年MEC耦合其他系统产甲烷示意图Figure 6. Schematic diagram of methane production from other systems coupled with MEC in the last five years(a)AD-MEC耦合系统系统用于食品加工废水处理[70],(b)MEC-UASB耦合系统治理Fischer-Tropsch废水[32],(c)MEC-UASB耦合系统治理乙醇废水[17],(d)MEC/AnMBR反应器组合处理生活污水[71],(e)MEC-AD-耦合磁铁矿处理废弃污泥[13],(f)MFCs-MEC耦合系统去除硫化物[4],(g)MFC-MEC-ABR耦合系统处理粪便废水[72](a) AD-MEC coupled system for food processing wastewater treatment [70],(b) MEC-UASB coupled system for Fischer-Tropsch wastewater treatment [32],(c) MEC-UASB coupled system for ethanol wastewater treatment [17]; (d) MEC/AnMBR reactor combination for domestic wastewater treatment [71],(e) MEC- AD-coupled magnetite to treat waste sludge [13],(f) MFCs-MEC coupled system to remove sulfide [4], (g) MFC-MEC-ABR coupled system to treat fecal wastewater [72]
图 6 近五年MEC耦合其他系统产甲烷示意图Figure 6. Schematic diagram of methane production from other systems coupled with MEC in the last five years(a)AD-MEC耦合系统系统用于食品加工废水处理[70],(b)MEC-UASB耦合系统治理Fischer-Tropsch废水[32],(c)MEC-UASB耦合系统治理乙醇废水[17],(d)MEC/AnMBR反应器组合处理生活污水[71],(e)MEC-AD-耦合磁铁矿处理废弃污泥[13],(f)MFCs-MEC耦合系统去除硫化物[4],(g)MFC-MEC-ABR耦合系统处理粪便废水[72](a) AD-MEC coupled system for food processing wastewater treatment [70],(b) MEC-UASB coupled system for Fischer-Tropsch wastewater treatment [32],(c) MEC-UASB coupled system for ethanol wastewater treatment [17]; (d) MEC/AnMBR reactor combination for domestic wastewater treatment [71],(e) MEC- AD-coupled magnetite to treat waste sludge [13],(f) MFCs-MEC coupled system to remove sulfide [4], (g) MFC-MEC-ABR coupled system to treat fecal wastewater [72]虽然近年来MEC相关的研究势如破竹,但MEC的大规模生应用仍面临诸多问题. 首先是成本问题,MEC的运行需要传质效率高的阴极导电材料,如活性炭颗粒(每t 2000—5000 元)、碳布(每m3 100—250 元)、生物炭(每t 1000—2000 元)、不锈钢(每m3 15—30 元)、钛网(每m3 80—120 元)、铂片(每g 300—400 元)等;虽然相较于其他材料而言,碳基材料成本较低,相关研究也比较充分,但其电催化性能不如金属电极. 金属基材料导电性能优越,但其表面光滑,不利于微生物的附着且高昂的价格也限制了其商业化的应用[4, 10, 14, 18]. 故而用复合材料修饰电极表面是近年来的研究热点,贵金属或纳米修饰的阴极材料不仅导电性好,且表面积大、易于微生物附着,但其制备过程需要用到PTFE等粘合剂,极易造成催化材料的活性位点被覆盖等问题,为此开发高性能、稳定和低成本的阴极材料以降低过电位和整体内阻并优化负载方法是未来MEC阴极材料的大势所趋[10, 14],如可以根据产甲烷菌的生理特性开发金属-纳米-碳基复合材料,并观察电活性微生物的原位长势及富集情况,改善其催化活性和电子传递方式,以提高产甲烷效果.
再者,现阶段单室MEC和双室MEC规模化放大生产中比对研究较少. 虽然单室MEC结构简单无质子膜阻隔,但阴阳电解液相互接触,极易发生副反应或短路现象,而大规模应用时副反应的发生将更加具有不可控性;双室MEC虽然有质子膜将阴阳极分离,但是极易造成阴阳两极的浓差极化,且双室MEC的阴阳两极相距较远,物质间传质阻力较大[4, 6-9, 11-15, 26-34, 37]. 故未来应通过实时监控、定点取样,推进MEC规模化生产应用的研究.
此外,生物阴极虽然结构简单,但阴极与产甲烷菌之间的电子传递和相互作用基质尚不明确,如不同导电材料的投加对于产甲烷MEC性能的影响、参与直接电子传递产甲烷代谢途径的酶除了还原酶Fdox、F420等是否还有未被发现的酶、不同种类的产甲烷菌生态位之间是否存在协同或竞争的关系、执行电子传递的主要控制基因又是什么等. 故未来应采用更加先进的技术手段(宏基因、宏蛋白和多组学生物技术等),分析MEC产甲烷阴极微生物群落结构的动态变化规律,对产甲烷菌的特性进行定向调控,优化MEC性能.
目前,MEC产甲烷的研究主要以小瓶实验为主,中试规模运行效能仍然未知. 在不少MEC耦合工艺的中试试验中,均检测到在长期运行期间由于膜污染、膜形变、废水中过量的生物质等原因,系统性能下降的情况[70-71],如Wang等[32]构建的中式规模的MEC-UASB产甲烷系统,在较低的HRT情况(HRT=2.7 h)下,VFAs积累较快,甲烷产量下降显著. 故而未来的研究中应该着重于MEC工业化长期化运行期间可能遇到的问题如膜污染、膜形变、废水中过量的生物质等问题,放大MEC反应器将是其未来研究方向的主导趋势,以推进MEC工程化应用.
4. 结语 (Conclusion)
为了实现低碳社会和可再生能源利用的目标,许多国家正进行各种技术和产业革命,发展绿色能源和低碳产业.
(1)甲烷是比较理想的清洁能源,通过微生物电解池(MEC)辅助CO2电甲烷化可以强化CO2的资源化,MEC也可以和相关技术联用,实现减碳降碳的同时对污染物进行减量化与无害化,应用前景广阔.
(2)现阶段的MEC电甲烷化依然存在长期批次试验不足、单次循环时间短、甲烷产量及纯度不高、产甲烷菌生长缓慢等问题. 故而开发连续的、系统的CO2电甲烷化反应器,优化生物阴极材料,调节微生物最适宜的环境条件迫在眉睫.
(3)未来的MEC电甲烷化研究应该将理论研究和实际应用进行更深入的结合,开发出:新型CO2电甲烷化生物反应器;克服CO2电甲烷化实验周期短、不稳定的难题;优化反应的条件(电极物理化学性质、工作温度、水力停留时间和有机负载率等);解析MEC中电活性功能微生物菌群、能量代谢理论体系及产甲烷菌的时空演替、种间信息交流方式,通过调控产甲烷菌电子传递的主控基因和胞外电子界面传导路径,提高CO2燃料化动力源的活力. 通过构建稳定、高效的CO2电甲烷化生物反应器,助力CO2减排与碳中和技术,以期为电甲烷化由基础走向工程提供新思路.
-
表 1 Cd2+(a)和CR(b)的实验设置
Table 1. Experimental setup of Cd2+ and CR
因素Factors 因素设置Factors set 控制因素Controlling factors 浓度 Cd2+和CR浓度均设为5、10、20、50、100、200、300、400、500 mg·L−1 温度30℃,pH 5,离子强度为0.005 mol·L−1 KCl溶液 温度 Cd2+和CR的温度均设为20、30、40℃ pH 5,离子强度为0.005 mol·L−1 KCl溶液,浓度均为200 mg·L−1 pH Cd2+的pH值为2、3、4、5、6,CR的pH值为2、3、4、5、6、8、10、12 温度为30℃,离子强度为0.005 mol·L−1 KCl溶液,浓度为200 mg·L−1 离子强度 Cd2+和CR的离子强度均设为0.001、0.005、0.01、0.05、0.1 mol·L−1 KCl 温度为30℃,pH值为5,浓度为200 mg·L−1 表 2 Cd2+(a)和CR(b)的Langmuir模型拟合结果1)
Table 2. Model fitting results of SJ and L-SJ for Cd2+ and CR
污染物Contaminants 材料Materials 拟合参数Fitting parameters qL/(mmol·kg−1) KL/(L·mmol−1) R2 Cd2+ SJ 186.82 3.18 0.9723** 0.1%L-SJ 364.53 7.19 0.9901** 0.5%L-SJ 355.88 2.96 0.9840** 1%L-SJ 357.41 2.00 0.9760** 5%L-SJ 370.01 2.32 0.9854** CR SJ 43.32 6.67 0.9748** 0.1%L-SJ 11.40 31.32 0.9635** 0.5%L-SJ 16.93 10.43 0.9755** 1%L-SJ 15.84 14.62 0.9878** 5%L-SJ 11.94 30.59 0.9803** 1)表中*表示达到显著水平,P<0.05,**表示达到极显著水平,P<0.01. 表 3 Cd2+和CR热力学参数表
Table 3. Thermodynamic parameters of adsorption of Cd2+ and CR
污染物Contaminants 材料Materials 20 °C 30 °C 40 °C △H/(kJ·mol−1) △S/(J·mol−1·K−1) K △G/(kJ·mol−1) K △G/(kJ·mol−1) K △G/(kJ·mol−1) Cd2+ SJ 228.27 −7.76 220.59 −8.02 197.22 −8.28 −5.69 26.10 0.1%L-SJ 665.80 −1.34 484.35 −1.38 459.52 −1.43 −14.63 4.46 0.5%L-SJ 617.16 −0.32 458.98 −0.33 416.33 −0.34 −15.50 1.01 1%L-SJ 584.31 −0.31 408.37 −0.32 381.82 −0.33 −15.39 0.99 5%L-SJ 609.72 −1.54 452.53 −1.59 425.00 −1.64 −14.23 5.12 CR SJ 132.79 −17.50 141.19 −18.09 152.57 −18.67 5.43 58.90 0.1%L-SJ 34.79 −61.27 35.70 −63.33 136.94 −65.39 53.09 206.36 0.5%L-SJ 30.13 −61.64 49.74 −63.72 118.41 −65.79 53.44 207.62 1%L-SJ 46.45 −43.56 51.03 −45.03 112.68 −46.50 34.40 146.72 5%L-SJ 35.02 −63.37 45.46 −65.50 144.09 −67.64 55.01 213.44 -
[1] NGAMBIA A, IFTHIKAR J, SHAHIB I I, et al. Adsorptive purification of heavy metal contaminated wastewater with sewage sludge derived carbon-supported Mg(II) composite[J]. Science of the Total Environment, 2019, 691: 306-321. [2] KUBIER A, PICHLER T. Cadmium in groundwater - A synopsis based on a large hydrogeochemical data set[J]. The Science of the Total Environment, 2019, 689: 831-842 [3] NIPA S T, RAHMAN M W, SAHA R, et al. Jute stick powder as a potential low-cost adsorbent to uptake methylene blue from dye enriched wastewater [J]. Desalination and Water Treatment, 2019, 153: 279-287. doi: 10.5004/dwt.2019.23767 [4] REN S, MENG Z F, SUN X X, et al. Comparison of Cd2+ adsorption onto amphoteric, amphoteric-cationic and amphoteric-anionic modified magnetic bentonites [J]. Chemosphere, 2020, 239: 124840. doi: 10.1016/j.chemosphere.2019.124840 [5] HUANG Q, HU D W, CHEN M X, et al. Sequential removal of aniline and heavy metal ions by jute fiber biosorbents: A practical design of modifying adsorbent with reactive adsorbate [J]. Journal of Molecular Liquids, 2019, 285: 288-298. doi: 10.1016/j.molliq.2019.04.115 [6] 温岚, 马建洪, 刘承斌. 一种新型多孔麻纤维重金属吸附剂制备与应用 [J]. 湖南大学学报(自然科学版), 2018, 45(6): 150-154. doi: 10.16339/j.cnki.hdxbzkb.2018.06.023 WEN L, MA J H, LIU C B. Preparation and application of a new porous fibre sorbent in heavy metal removal [J]. Journal of Hunan University (Natural Sciences), 2018, 45(6): 150-154(in Chinese). doi: 10.16339/j.cnki.hdxbzkb.2018.06.023
[7] GHOSH R K, RAY D P, DEBNATH S, et al. Optimization of process parameters for methylene blue removal by jute stick using response surface methodology [J]. Environmental Progress & Sustainable Energy, 2019, 38(5): 13146. [8] 邓灿辉, 粟建光, 陈基权, 等. 黄麻吸附材料的研究及应用前景 [J]. 中国麻业科学, 2017, 39(6): 306-311. doi: 10.3969/j.issn.1671-3532.2017.06.007 DENG C H, SU J G, CHEN J Q, et al. Research progress on removal of pollutant in wastewater with jute-based materials [J]. Plant Fiber Sciences in China, 2017, 39(6): 306-311(in Chinese). doi: 10.3969/j.issn.1671-3532.2017.06.007
[9] ROY A. Removal of color from real textile dyeing effluent utilizing tannin immobilized jute fiber as biosorbent: Optimization with response surface methodology [J]. Environmental Science and Pollution Research, 2021, 28(10): 12011-12025. doi: 10.1007/s11356-020-08820-2 [10] 杜兆林, 郑彤, 王鹏, 等. 微波辅助羧基改性黄麻吸附材料的制备工艺优化 [J]. 哈尔滨工业大学学报, 2017, 49(2): 54-61. doi: 10.11918/j.issn.0367-6234.2017.02.010 DU Z L, ZHENG T, WANG P, et al. Optimization of the microwave-assisted preparation process for the carboxyl modified jute fiber adsorbent [J]. Journal of Harbin Institute of Technology, 2017, 49(2): 54-61(in Chinese). doi: 10.11918/j.issn.0367-6234.2017.02.010
[11] KHOZANI M A, EMTIAZI G, AGHAEI S S, et al. Application of fungal laccase for heavy metals precipitation using tannin as a natural mediator [J]. International Journal of Environmental Science and Technology, 2021, 18(8): 2335-2344. doi: 10.1007/s13762-020-02992-7 [12] 张金帆. 利用生物固定化技术处理水体中Cd2+/孔雀石绿的研究[D]. 长沙: 长沙理工大学, 2017. ZHANG J F. Study on removal of Cd2+/malachite green in wastewater by biological immobilization technology[D]. Changsha: Changsha University of Science & Technology, 2017(in Chinese).
[13] LI Q Y, ZHOU Y L, MA K R, et al. A mesoporous SiO2/dense SiO2/Fe3O4 multiply coated hollow microsphere: Synthesis and application on papain immobilization [J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2016, 511: 239-246. [14] NGUYEN T A, FU C C, JUANG R S. Effective removal of sulfur dyes from water by biosorption and subsequent immobilized laccase degradation on crosslinked chitosan beads [J]. Chemical Engineering Journal, 2016, 304: 313-324. doi: 10.1016/j.cej.2016.06.102 [15] ZHANG W X, YANG Q, LUO Q H, et al. Laccase-Carbon nanotube nanocomposites for enhancing dyes removal [J]. Journal of Cleaner Production, 2020, 242: 118425. doi: 10.1016/j.jclepro.2019.118425 [16] 孙秀贤, 孟昭福, 曹雪雯, 等. 漆酶增强黄麻去除水中亚甲基蓝的研究 [J]. 农业环境科学学报, 2021, 40(7): 1548-1556. doi: 10.11654/jaes.2020-1434 SUN X X, MENG Z F, CAO X W, et al. Laccase mediated jute enhancement for the removal of methylene blue from water [J]. Journal of Agro-Environment Science, 2021, 40(7): 1548-1556(in Chinese). doi: 10.11654/jaes.2020-1434
[17] 王新欣, 孟昭福, 刘欣, 等. BS-18两性修饰膨润土对四环素和诺氟沙星复合污染的吸附 [J]. 环境科学, 2021, 42(5): 2334-2342. doi: 10.13227/j.hjkx.202009048 WANG X X, MENG Z F, LIU X, et al. Adsorption of BS-18 amphoterically modified bentonite to tetracycline and norfloxacin combined pollutants [J]. Environmental Science, 2021, 42(5): 2334-2342(in Chinese). doi: 10.13227/j.hjkx.202009048
[18] 胡静, 张杰, 王翠萍, 等. 改性麦壳对水中刚果红的吸附机理研究 [J]. 化工新型材料, 2015, 43(1): 163-165,172. HU J, ZHANG J, WANG C P, et al. Adsorption mechanism of modified wheat shell husk for Conge red from aqueous solutions [J]. New Chemical Materials, 2015, 43(1): 163-165,172(in Chinese).
[19] 赵雅兰, 易筱筠, 雷娟, 等. 基于镉吸附的花生壳酶改性研究 [J]. 矿物岩石地球化学通报, 2014, 33(2): 208-213. doi: 10.3969/j.issn.1007-2802.2014.02.008 ZHAO Y L, YI X Y, LEI J, et al. Research of Cd sorption by enzymatic modified peanut hulls [J]. Bulletin of Mineralogy, Petrology and Geochemistry, 2014, 33(2): 208-213(in Chinese). doi: 10.3969/j.issn.1007-2802.2014.02.008
[20] ABDEL-NABY M A. Immobilization of Paenibacillus macerans NRRL B-3186 cyclodextrin glucosyltransferase and properties of the immobilized enzyme [J]. Process Biochemistry, 1999, 34(4): 399-405. doi: 10.1016/S0032-9592(99)00017-5 [21] 孟庆辉. 漆酶在交联聚乙二醇二丙烯酸酯上的固定化及性能研究[D]. 哈尔滨: 哈尔滨工业大学, 2006. MENG Q H. Study of laccase immobilization on cross-linked polyglycol diacrylate and its performances[D]. Harbin: Harbin Institute of Technology, 2006(in Chinese).
[22] YU Y, ZHUANG Y Y, WANG Z H. Adsorption of water-soluble dye onto functionalized resin [J]. Journal of Colloid and Interface Science, 2001, 242(2): 288-293. doi: 10.1006/jcis.2001.7780 [23] SU J, LIN H F, WANG Q P, et al. Adsorption of phenol from aqueous solutions by organomontmorillonite [J]. Desalination, 2011, 269(1/2/3): 163-169. [24] 郝艳玲, 王远, 董良宇, 等. 坡缕石粘土对有机染料的吸附热力学研究 [J]. 岩石矿物学杂志, 2009, 28(6): 661-664. doi: 10.3969/j.issn.1000-6524.2009.06.026 HAO Y L, WANG Y, DONG L Y, et al. A thermodynamic study of the adsorption of organic dyes on palygorskite clay [J]. Acta Petrologica et Mineralogica, 2009, 28(6): 661-664(in Chinese). doi: 10.3969/j.issn.1000-6524.2009.06.026
[25] 岳新霞, 俸海凤, 林海涛, 等. 蔗渣基吸附剂的制备及对刚果红的吸附性能 [J]. 广西科技大学学报, 2017, 28(2): 119-125. doi: 10.16375/j.cnki.cn45-1395/t.2017.02.020 YUE X X, FENG H F, LIN H T, et al. Preparation of bagasse-based adsorbent and adsorption performance for Congo red [J]. Journal of Guangxi University of Science and Technology, 2017, 28(2): 119-125(in Chinese). doi: 10.16375/j.cnki.cn45-1395/t.2017.02.020
[26] 吴志坚, 刘海宁, 张慧芳. 离子强度对吸附影响机理的研究进展 [J]. 环境化学, 2010, 29(6): 997-1003. WU Z J, LIU H N, ZHANG H F. Research progress on mechanisms about the effect of ionic strength on adsorption [J]. Environmental Chemistry, 2010, 29(6): 997-1003(in Chinese).
[27] 梁学峰. 黏土矿物表面修饰及其吸附重金属离子的性能规律研究[D]. 天津: 天津大学, 2015. LIANG X F. Surface modification of clay minerals and their application for sorption of heavy metals[D]. Tianjin: Tianjin University, 2015(in Chinese).
[28] HIEW B Y Z, LEE L Y, LEE X J, et al. Utilisation of environmentally friendly okara-based biosorbent for cadmium(II) removal [J]. Environmental Science and Pollution Research, 2021, 28(30): 40608-40622. doi: 10.1007/s11356-020-09594-3 [29] 郑刘春. 玉米秸秆及其纤维素的改性和吸附水体镉离子的机理研究[D]. 广州: 华南理工大学, 2011. ZHENG L C. Studies on the modification of corn stalk (cellulose) and the mechanism of Cd (Ⅱ) adsorption[D]. Guangzhou: South China University of Technology, 2011(in Chinese).
[30] 程启明, 黄青, 刘英杰, 等. 花生壳与花生壳生物炭对镉离子吸附性能研究 [J]. 农业环境科学学报, 2014, 33(10): 2022-2029. doi: 10.11654/jaes.2014.10.020 CHENG Q M, HUANG Q, LIU Y J, et al. Adsorption of cadmium(Ⅱ)on peanut shell and its biochar [J]. Journal of Agro-Environment Science, 2014, 33(10): 2022-2029(in Chinese). doi: 10.11654/jaes.2014.10.020
[31] 曾伟. 改性氧化石墨烯材料制备及其对水中亚甲基蓝染料的吸附性能研究[D]. 长沙: 湖南大学, 2017. ZENG W. Modified graphene oxide material preparation and its adsorption properties of methylene blue in waster water[D]. Changsha: Hunan University, 2017(in Chinese).
期刊类型引用(0)
其他类型引用(1)
-






 下载:
下载: