-
抗生素的大量使用使得其会通过直接或间接途径进入水体引起污染. 四环素(TC)是水体检出浓度和检出频率最高的抗生素之一[1]. 水体中的四环素会诱导微生物逐渐产生抵抗力,造成抗药性菌群和抗性基因的产生[2]. 常规生物法、生物电化学法、高级氧化法、膜分离法、吸附法、微生物降解等是水体中四环素去除的主要方法[3-6] . 其中,高级氧化法(AOPs)已经被证明能有效的去除水中的四环素,因为具有高度活性的自由基的存在,包括硫酸根自由基(
SO−4⋅ )和羟基自由基(·OH).基于
SO−4⋅ 自由基的高级氧化技术是通过过二硫酸盐(PDS)或过一硫酸盐(PMS)的活化来降解水体中的污染物. 与·OH相比,SO−4⋅ 具有较长的半衰期 (30—40 μs 对比10−3 μs),更高的氧化还原电势 (2.5—3.1 V对比 1.8—2.7 V)、更宽pH的适应性[7]. 其中,PMS 过氧键两侧的电荷分布是不对称的,更容易受到各种亲核试剂的亲核攻击,PMS 能被过渡金属更有效地活化以生成SO−4⋅ . PMS 在含碳材料和贵金属催化剂氧化有机物方面也比PDS 更有效[8-11]. 催化剂的选择和制备是PMS活化技术能否成功应用的关键.研究显示,生物炭有作为高级氧化反应催化剂的潜力[12]. 但是原状生物炭的催化能力有限,需要对原状生物炭进行改性以进一步提升其对污染物的降解效果[13]. 其中,生物炭负载铁作为催化剂的研究报道最多,因为纳米铁氧化物能有效的启动催化降解反应[14]. 过渡金属活化PMS可以在常温常压下进行,且反应迅速、操作简便,被认为是活化效果最好的一种活化方式. 但同时在反应过程中也存在着过渡金属易团聚、重复利用性差和金属离子流失等问题[15]. 因此,如何改性提升生物炭的催化降解能力亟需进一步研究. 与此同时,氮改性生物炭也引起了更多关注,因为引入了含氮官能团和可以改变生物炭的结构[16]. 氮修饰生物炭更多的应用于生物炭的催化降解领域[17],因为氮具有局部未成对电子:(1) 改善周围碳原子的电子密度;(2)提高共轭增强sp2碳中π电子流;(3)进入更多的官能团和缺陷;(4)增加碳材料的表面亲水性能有效的结合极性吸附质. 因此,可以考虑铁氮共改性以提升生物炭的降解性能.
近年来,铁氮共改性生物炭在高级氧化领域的应用也逐渐引起了研究,作为催化剂被用于降解废水中有机污染物,包括酸性橙7 [18]、异丙甲草胺[19]、罗丹明B [20]、双酚A [21-22]、双酚F [23]和磺胺噻唑 [24]. 仅有Yu等[25]研究了铁氮改性生物炭活化过硫酸盐(PS)降解四环素的效果及机理. 上述研究均显示铁氮共改性碳材料可以有效的降解废水中的有机污染物. 然而,铁氮共改性生物炭活化PMS降解TC的研究报道还较少,降解的机理也尚不清楚.
因此,本研究以典型的农林废弃物—水稻秸秆为对象,选择氯化铁、尿素为改性剂,通过热解制备出铁氮改性生物炭,同时制备原状生物炭作为对照,对生物炭进行SEM、TEM、BET、EA、XPS、FTIR、Raman和VSM进行全面的表征,考察铁氮改性对生物炭理化性质的影响,探究对水体中四环素的降解行为及机理,以期为秸秆的资源化的利用及水体中四环素的去除提供理论依据和技术参考.
-
水稻秸秆来自安徽合肥郊区农田,将秸秆水洗去除灰尘,放入烘箱80℃烘干,之后用破碎机破碎后过40目筛,保存备用.
四环素(tetracycline,TC,购于上海源叶生物有限公司)、FeCl3·6H2O、尿素、甲醇、叔丁醇、糠醇、对苯醌、碘化钾、NaOH 、HCl均为分析纯,购于国药集团化学试剂有限公司.
-
将5 g水稻秸秆与0.1 mol·L−1 (FeCl3·6H2O)、0.1 mol·L−1尿素在100 mL水溶液中混合,放在磁力搅拌器上搅拌24 h后放入烘箱80 ℃烘干. 将烘干的混合固体盛于石英舟中,放置于管式马弗炉中(OTF-1200X-S,合肥科晶材料技术有限公司),700 ℃下热解(全程氮气保护),升温速率为5 ℃·min−1,保温2 h,待温度降至室温时,取出生物炭,对生物炭进行水洗,至洗脱接近中性后在烘箱中烘干. 最后进行球磨1 min后制得铁氮改性水稻秸秆生物炭(Fe-N-RSBC). 同时,制备原状水稻秸秆生物炭作为对照(RSBC).
-
(1)吸附体系:将100 mL含TC (浓度为50 mg∙L−1)的溶液置于锥形瓶中,然后加入0.02 g生物炭,将锥形瓶置于在摇床上,摇床转速为150 r·min−1. 分别在 0、5、10、20、30、40、50、60、90 min取样测定,并通过0.45 μm的膜过滤. 用紫外分光光度计(UV-5100B, 上海元析仪器有限公司)测量上清液的吸光度,波长设定为358 nm. TC浓度选择50 mg·L−1的原因是:有的药厂或畜禽养殖废水抗生素的浓度确实很高;凸显铁氮生物炭的吸附降解效果;便于仪器测定.
(2)PMS降解体系(没有添加生物炭):将100 mL含TC (浓度为50 mg·L−1)的溶液置于锥形瓶中,将锥形瓶置于在摇床上,摇床转速为150 r·min−1. 添加0.2 mL PMS (浓度为1 mmol·L−1),0、5、10、20、30、40、50、60、90 min取样测定 (预先加入1mL硫代硫酸钠作为淬灭剂),并通过0.45 μm的膜过滤. 用紫外分光光度计(UV-5100B, 上海元素仪器有限公司) 测量上清液的吸光度,波长设定为358 nm.
(3)生物炭催化降解体系(添加生物炭):将100 mL含TC (浓度为50 mg∙L−1)的溶液置于锥形瓶中,添加0.02 g生物炭,将锥形瓶置于在摇床上,摇床转速为150 r·min−1. 之后再添加0.2 mL PMS启动反应,在0、5、10、20、30、40、50、60、90 min取样测定(预先加入1mL硫代硫酸钠作为淬灭剂),并通过0.45 μm的膜过滤后测定.
(4)不同影响因素
溶液初始pH值:将100 mL含TC (浓度为50 mg∙L−1)的溶液置于锥形瓶中. 使用0.1 mol·L−1 NaOH或0.1 mol·L−1 HCl调整溶液的pH为3.0、5.0、7.0、9.0和11.0. 随后,0.02 g Fe-N-RSBC加入到溶液中,进行振荡,进行60 min预吸附后,随后加入0.2 mL PMS,启动降解实验,其它条件同上.
生物炭量:将100 mL含TC (TC浓度为50 mg∙L−1)的溶液置于锥形瓶中. 分别加入0.005、0.01、0.02、0.025 g Fe-N-RSBC加入到瓶中进行振荡,60 min预吸附之后,再加入0.2 mL PMS,启动降解实验,其它条件同上.
PMS量:将100 mL含TC(浓度为50 mg∙L−1)的溶液置于锥形瓶中. 随后,0.02 g Fe-N-RSBC加入到瓶中的溶液中,将锥形瓶置于150 r·min−1的摇床上,进行60 min预吸附,之后分别加入0.1、0.2、0.25、0.3 mL PMS,启动降解实验,其它条件同上.
TC初始浓度:将不同浓度的含TC(浓度为30、50 、75、100 mg∙L−1)溶液置于锥形瓶中. 随后,向锥形瓶的溶液中加入0.02 g Fe-N-RSBC,进行振荡,进行60 min预吸附之后,再加入0.2 mL PMS,进行降解实验,其它条件同上.
阴离子类型:将100 mL含阴离子的TC(浓度为50 mg∙L−1)的溶液置于锥形瓶中, 阴离子类型为Cl−、
H2PO−4 、NO−3 和CO2−3 ,浓度设定为50 mmol·L−1. 随后,向锥形瓶的溶液中加入0.02 g Fe-N-RSBC,进行60 min预吸附之后,再加入0.2 mL PMS,启动降解实验,其它条件同上. -
采样扫描电镜(SEM,SU8010, Hitachi, Japan)和透射电镜(TEM, TecnaiG2F20S-TWIN, 200KV)对生物炭的表面形貌和结构进行观察. 生物炭的晶体结构采用X射线衍射(XRD, Bruker D8 Advance, Germany)测定. 生物炭的比表面积、孔体积和孔尺寸采用比表面积和孔径分析仪(Micromeritics, Tristar II 3020, USA)测定. 采用元素分析仪( Elementar Vario EL cube,Germany) 测定秸秆生物炭中C、N、H和的含量. 灰分测定: 称取一定量的生物炭置于箱式马弗炉中700 ℃燃烧2 h,冷却至室温后称量,根据质量差计算灰分含量. 采用傅里叶变换红外光谱(FTIR,Nicolette is50,Thermo Fourier, USA )分析表征生物炭的官能团类型. 利用X光电子能谱分析表征(XPS, Thermo-VGScientific, ESCALAB250, USA)生物炭的表面元素及化学形态. 采用Zeta电位仪(DelsaMax PRO, Beckman Coulter, USA )测定生物炭的zeta电位. 采用Raman光谱进行表征(DXR Raman microscope; Thermo Fisher Scientific, Madison, WI, USA)生物炭的碳缺陷结构.
-
(1)活性氧化剂抑制实验
将100 mL含TC (50 mg·L−1)的溶液置于一系列锥形瓶中,之后分别加入0.02 g Fe-N-RSBC,添加甲醇(1 mol·L−1)、糠醇(20 mmol·L−1)、对苯醌(20 mmol·L−1)、叔丁醇(1 mol·L−1)、碘化钾(20 mmol·L−1)作为活性氧物种抑制剂. 将锥形瓶置于的摇床上振荡,进行60 min预吸附实验, 之后加入0.2 mL PMS启动降解实验,分别在0、5、10、20、30、40、50、60、90 min时取样(取样瓶中预先加入1 mL淬灭剂). 并通过0.45 μm的膜过滤后测定.
(2)EPR测定实验
在10 mL溶液中提前加入TEMP和DMPO,分别0.002 g Fe-N-RSBC,之后加入0.2 mL的PMS启动反应,振荡反应时间3 min,采用电子顺磁共振波谱仪(EPR)测定活性氧物种类型(JEOL JES-FA200,Tokyo, Japan).
-
将降解反应完成后的Fe-N-RSBC通过磁分离之后,水洗并烘干,开展再生利用实验,共进行3次. 再生利用降解的实验条件是:将100 mL含TC (浓度为50 mg∙L−1)的溶液置于锥形瓶中,添加0.02 g生物炭,进行振荡,之后再添加0.2 mL PMS,0、5、10、20、30、40、50、60、90 min取样测定 (预先加入1 mL硫代硫酸钠淬灭剂),并通过0.45 μm的膜过滤后测定.
为验证材料的适用性,选择代表性的阳离子染料亚甲基蓝和阴离子染料橙黄Ⅱ,探究Fe-N-RSBC催化PMS降解染料的效果,降解实验条件: 将100 mL含染料废水 (浓度为50 mg∙L−1)的溶液置于锥形瓶中,添加0.02 g Fe-N-RSBC,进行振荡预吸附60 min, 之后再添加0.2 mL PMS,0、5、10、20、30、40、50、60、90 min取样测定 (预先加入1 mL硫代硫酸钠淬灭剂),通过0.45μm的膜过滤后用分光光度计测定,亚甲基蓝的测定波长为665 nm,橙黄Ⅱ的测定波长为484 nm.
-
RSBC和Fe-N-RSBC的SEM、TEM和EDS mapping见图1.
由图1可见,原状秸秆生物炭中的存在无规则的杆状或梗状形态,但是也有一定的破碎,因为热解温度为700℃,秸秆中的大部分有机物都会热分解. Fe-N-RSBC中存在球状颗粒,但是可以看出明显孔隙结构更加发达,说明Fe、N能与秸秆组分之间反应从而促进秸秆热分解的更加彻底,使得生物炭更加碎片化. RSBC和Fe-N-RSBC的TEM见图1(c, d).
由TEM可见,RSBC性状不规则,呈现无定形形态,生物炭的碳层比较厚,有着明显的堆积. 黑色颗粒可能是生物炭中的矿物质组分. Fe-N-RSBC的碳层也比较薄,黑色颗粒为铁氧化物,铁氧化物分布在炭结构中,也有铁分布在炭的外围. HR-TEM可以看出存在微孔结构. 铁氮改性导致炭基质更加规则平整规则,降低了碳结构的堆积和褶皱的性状. 从 Fe-N-RSBC 的 EDS mapping 图可见,Fe 和 N 较为均匀的分布在生物炭的表面.
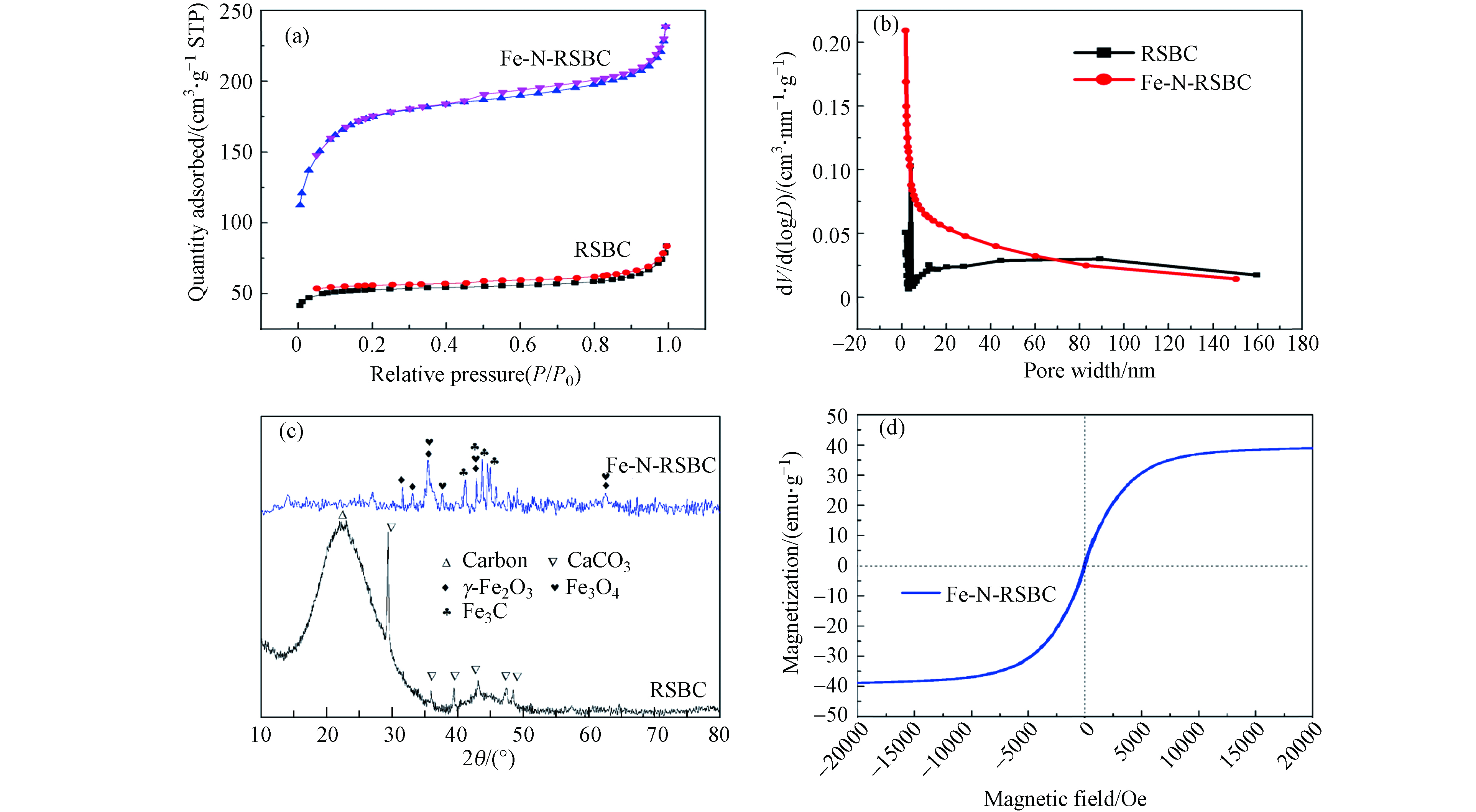
RSBC和Fe-N-RSBC的N2吸附-解吸曲线及孔径分布图见图2(a). RSBC及Fe-N-RSBC的N2吸附-解吸曲线都是典型的IV类型曲线,即高压处存在明显的滞后回线,说明都存在典型的介孔特性. 从孔径分布曲线(图2(b))可以看出,RSBC和Fe-N-RSBC的主要孔径分布在2—4 nm,也证实了RSBC和Fe-N-RSBC的介孔分布特性. 可以看出,Fe-N-RSBC的孔体积也大于RSBC.
RSBC和Fe-N-RSBC的XRD见图2(c). 如图2所示,RSBC在22°和44°附近分别是无定形碳和石墨碳结构[26]. 此外,RSBC中还含有CaCO3, 说明水稻秸秆中含有碱性元素. 铁氮改性之后生物炭中出现了γ-Fe2O3和Fe3O4,这也是导致铁改性生物炭具有磁性的原因. 铁氮改性之后出现还出现了Fe3C,说明氮的存在促进了铁的还原. 铁氮改性导致了铁进入了碳基质中形成Fe3C. 热解过程中铁的形态变化是:Fe3+→ Fe2O3→Fe3O4→Fe3C [27-28]. 可见,铁与氮的共改性会明显改性生物炭中碳的形态.
Fe-N-RSBC的磁滞曲线见图2(d),剩磁及矫顽力接近于零,判断Fe-N-RSBC为超顺磁性材料 [29]. Fe-N-RSBC的饱和磁化值38.99 emu·g−1,说明在外加磁场作用后,Fe-N-RSBC有良好的固液分离效果.
RSBC和Fe-N-RSBC的N2吸附-解吸曲线得到的比表面积和孔径参数见表1. 由表1可知,Fe-N-RSBC的比表面积达到606.62 m2·g−1, 微孔面积为341.29 m2·g−1 ,总孔体积为0.33 cm3·g−1,微孔体积为0.15 cm3·g−1,分别是原状生物炭的3.41倍、2.41倍、3.00倍和2.27倍. 铁氮改性之后平均孔径尺寸从2.48 nm降低2.21 nm. 从Vmicro/Vtotal比值可知,RSBC中以微孔和介孔为主,Fe-N-RSBC改性之后微孔所占比例降低到45.93%,介孔占据优势. 这与图2(b)一致. 生物炭比表面积和孔径参数与SEM和TEM的结果一致. 铁氮改性使得生物炭存在更多的介孔结构.
热解过程中,Fe、N能与生物炭中的碳组分发生热化学反应,促进了生物炭的中纤维素、半纤维素和木质素的分解. 铁氮改性增加生物炭比表面积的原因是 [30]:氮原子能掺杂到碳原子的网格之中,同时铁降低了碳基质在炭化过程中的伸缩;尿素的热分解能产生气体,这些气体会破坏碳结构从而制造更多的孔隙;氮的存在能促进铁与基质碳之间的反应从而形成更多的微孔. 同时,铁改性提高生物炭的比表面积和孔体积因为铁进入了介孔炭之中,从而降低了炭化过程中基质炭的伸缩;热解过程中铁的催化作用,能促进孔隙结构形成,特别是介孔结构;铁催化能将相邻的碳材料气化成低分子气体,如CH4和CO等;铁氧化物覆盖生物炭上也能创造更多的孔隙结构 [31-34].
RSBC和Fe-N-RSBC的元素含量、原子比、灰分含量及zeta电位见表2. 与RSBC相比,Fe-N-RSBC中的C含量显著降低,而N含量明显上升,H和O含量略微增加,灰分含量也显著增加,灰分增加主要是因为铁氧化物的存在. 此外,铁氮改性还导致了zeta电位的明显变化,从-43.89 mV变为-29.45 mV. 生物炭的原子比H/C、O/C和(N + O) / C分别指示生物炭的芳香性、亲水性和极性大小. H/C比值越小则芳香性越高,O/C和(O+N)/C比值越大,则亲水性和极性越大. 由表2可知, 铁氮改性之后生物炭的亲水性和极性均增加了,有利于与水体中极性污染物的结合.
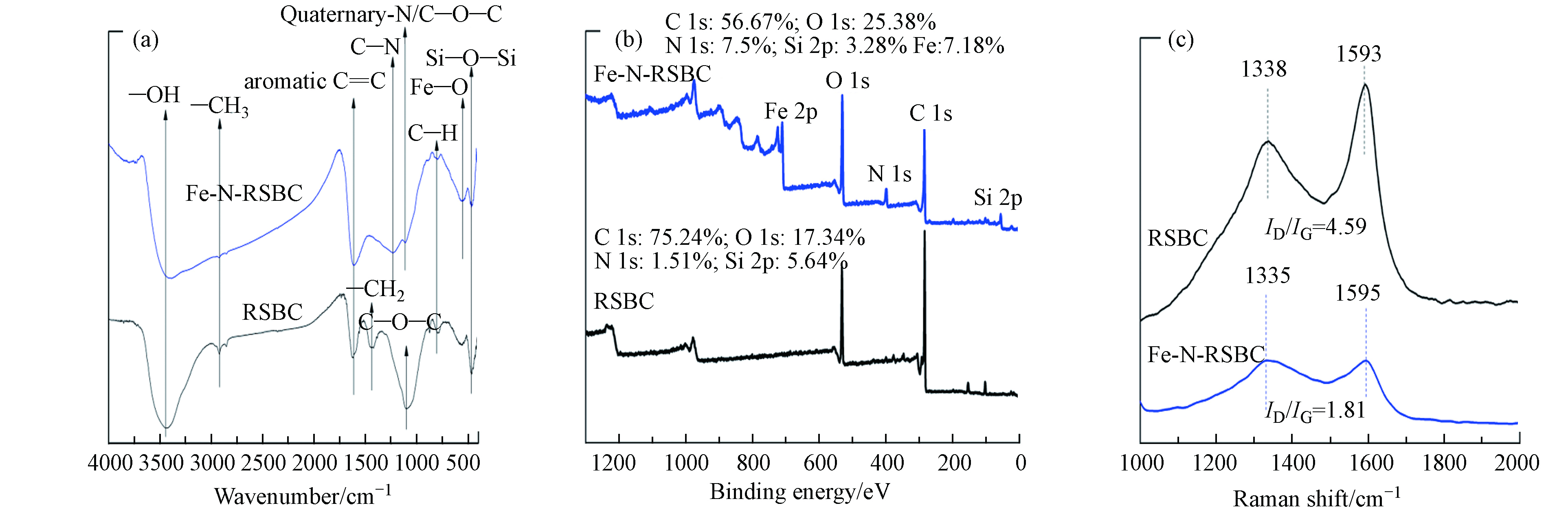
RSBC和Fe-N-RSBC的FTIR见图3(a). 由图3可知,原状生物炭中含有的官能团包括—OH、—CH3、芳香C=C环拉伸、—CH2、C—O—C、C—H和Si—O—Si等官能团 [35]. 铁氮改性之后生物炭的官能团包括—OH(3385 cm−1)、芳香C=C环拉伸(1610 cm−1)、C—N 拉伸(1230 cm−1)、Quaternary−N/C—O—C (1113 cm−1)、Fe—O (551 cm−1)、Si—O—Si (468 cm−1). 铁氮改性部分保留了生物炭的芳香结构(1617 cm−1),但是生物炭的芳香结构弱化了因为Fe和N已经嵌入到了生物炭的碳结构中,这与TEM、XRD、元素分析比H/C的结果是吻合的. 可见,改性导致铁氮已经嵌入到碳基质中,并改变了生物炭的含碳和含氧官能团.
RSBC和Fe-N-RSBC的XPS图谱见图3(b). RSBC的表面能检测到 C 1s、O 1s、N 1s和Si 2p的存在. Fe-N-RSBC中还能明显检测到Fe 2p的存在. 与RSBC相比,Fe和N改性之后导致了生物炭表面元素相对含量发生明显变化. Fe-N改性生物炭表面氮和氮的相对质量分数分别为6.13%和4.33%. Fe改性后碳含量的降低, 说明改性导致铁氮负载到生物炭上,同时铁和氮已经成功嵌入到碳骨架.
拉曼光谱可用于分析生物炭的碳结构缺陷. RSBC和Fe-N-RSBC的拉曼光谱见图3(c). 如图所示,生物炭的Raman光谱有两个明显的特征峰. D峰(1350 cm−1附近)是由碳层结构中的缺陷导致的,代表了碳材料中乱层结构以及不小于6 个环的多环芳烃,在1575 cm−1 附近出现的峰称为G 峰,代表稠芳环结构以及类石墨微晶的sp2杂化振动 [36]. ID/IG(面积之比)可以用来表示碳结构的石墨化程度,比值越大表明碳结构缺陷越大. 如图3(c)所示,RSBC的ID/IG值为4.59, 且存在明显的D和G峰. 铁氮改性之后ID/IG值变成1.81,因为铁氮改性会明显影响G峰,铁和氮会嵌入到石墨碳结构,从而破坏石墨碳结构,与TEM、BET和XRD的结果是一致的. 研究发现铁氮改性会破坏材料的碳格网络,产生更多的缺陷 [37]. 也有研究发现氮改性导致了石墨碳结构的破坏,因为氮原子嵌入到碳网格中,导致碳结构的缺陷 [38]. 此外,Raman分析还发现铁氮改性导致了D峰和G峰位置的偏移. 可见,铁氮改性会影响无定形的碳结构和石墨碳结构,特别是石墨碳结构.
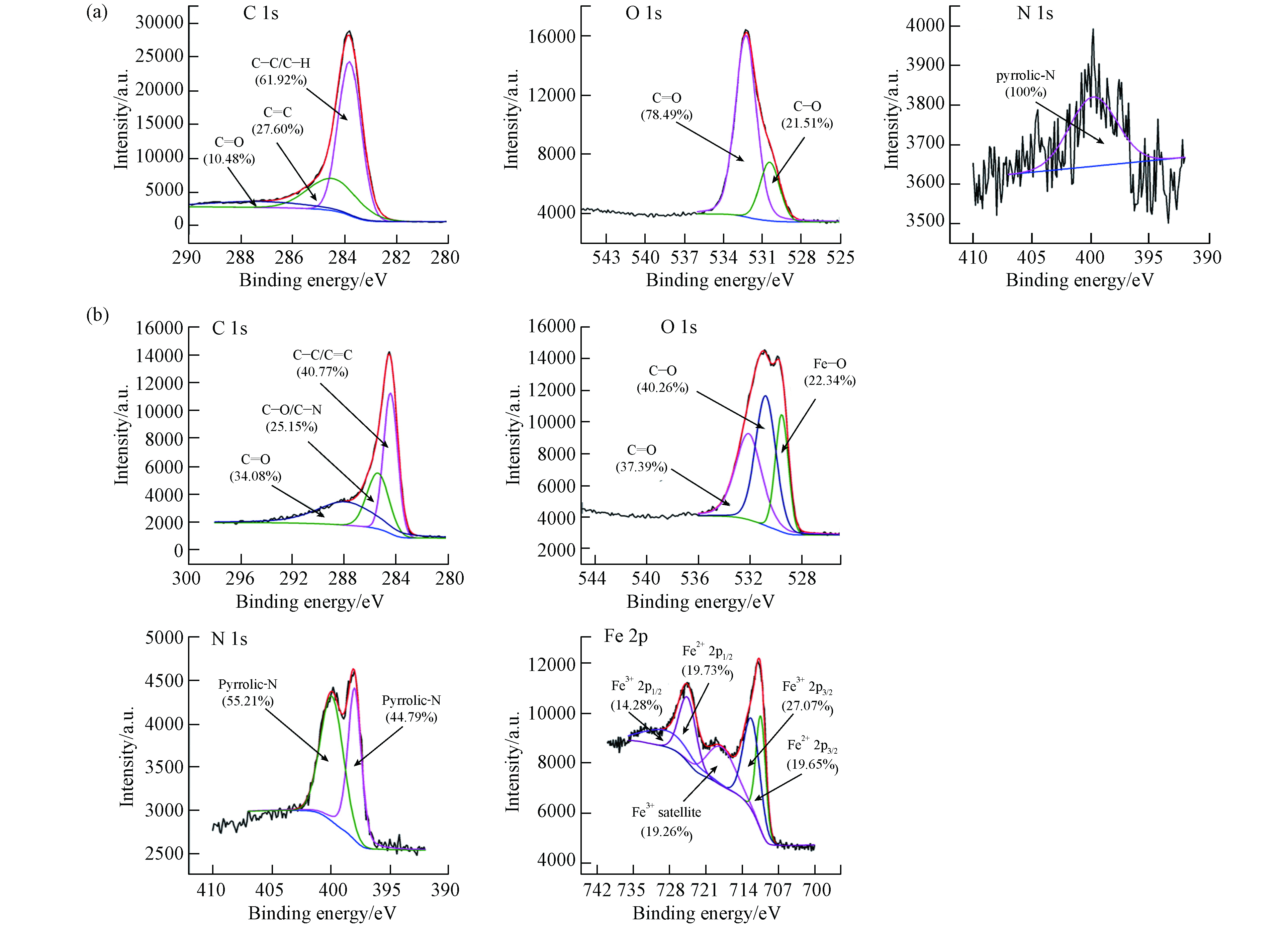
RSBC和Fe-N-RSBC的XPS分峰拟合见图4. RSBC的C分峰为283.8、284.5、287.3 eV, 分别对应C—C/C—H, C=C, C=O [39]. Fe-N-RSBC的C分峰为284.4 、285.37 、287.9 eV,分别对应C—C/C=C, C—O/C—N和C=O. RSBC中O以C—O和C=O形态存在. Fe-N-RSBC中O 分峰为C—O、C=O和Fe-O [40]. RSBC中N主要以pyrrolic-N形态存在. Fe-N-RSBC中N分峰为 pyrrolic-N和pyridinic-N. Pyridinic-N是形成是因为N取代了在石墨平面的C6环末端的C [41]. 可见,N掺杂到了碳结构中,这与XRD, Raman的结果相符合. Fe-N-RSBC中Fe 2p XPS分峰对应的是710.4、712.1、718、724.5、728.6 eV. Fe3+卫星峰的出现证实有Fe3+化合物的存在 [42],结合XRD结果,Fe2+和Fe3+的铁化合物为γ-Fe2O3、Fe3O4和Fe3C. 可见,铁氮改性导致了含碳、含氧、含氮和含铁官能团类型和所占比例的变化.
FeCl3改性生物炭的反应机理如下 [33]:
200—330 °C:
330—700 °C:
尿素热分解的组分可进一步与碳的官能团反应,从而取代碳层表面的羰基和羟基,形成含氮官能团,具体的反应如下 [43- 44]:
综上可见,铁氮改性会明显改变生物炭的理化性质,使得生物炭具有更大的比表面积和孔体积、更多的微孔体积,赋予更丰富的官能团,并引入了磁性组分,但是破坏了部分石墨碳结构.
-
不同体系下生物炭及PMS对四环素的去除见图5. 在90 min内,RSBC和Fe-N-RSBC对四环素的去除率为16%和50%(吸附过程),而PMS对四环素的去除率13%. 当PMS添加到RSBC,Fe-N-RSBC和四环素体系中,对四环素的去除率为59%和87%,其中前5 min的去除率分别为18%和80%. 可见Fe-N-RSBC催化PMS降解TC主要发生在初期阶段,因为存在大量的反应位点和活性氧化剂. 在吸附/降解体系中,150 min之内Fe-NRSBC/PMS对四环素的去除率可达87%.
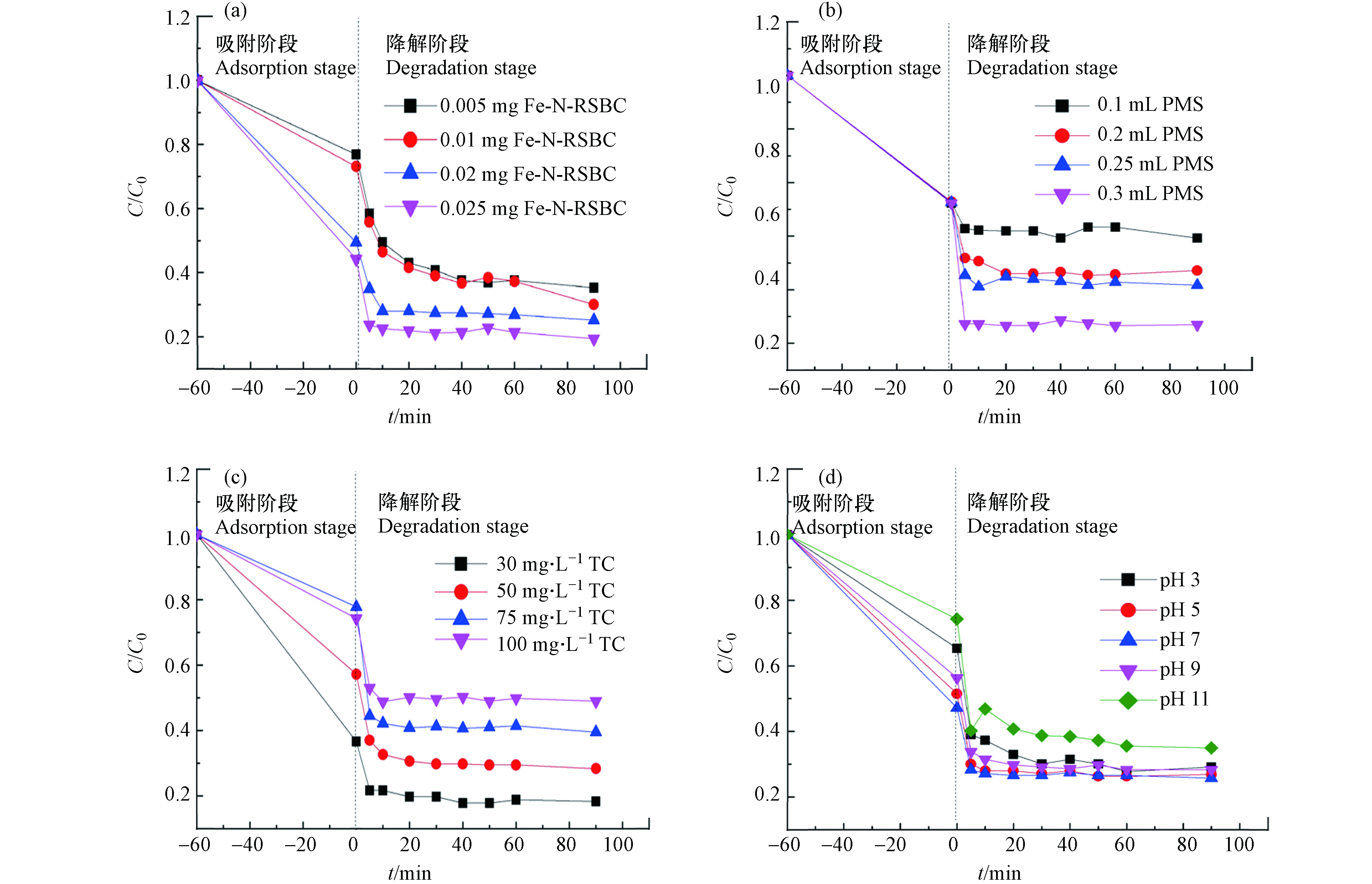
Fe-N-RSBC催化剂用量对四环素降解的影响见图6(a). 当加入PMS启动降解时,Fe-N-RSBC对TC的降解率为23%—43%. 添加量越大,对TC的去除率越高,当Fe-N-RSBC添加量为0.025 mg时,对TC的去除率可达80%以上. 显然, 催化剂用量提高可以提供更多的表面积和活性位点为TC的吸附和PMS活化;更高效的吸附可能导致降解加快;吸附的四环素迅速分解提供了更多的吸附位点激活PMS,从而促进TC的去除.
PMS用量对四环素降解的影响见图6(b). Fe-N-RSBC对TC在前60分钟的吸附率在50%. 当加入PMS启动降解时,Fe-N-RSBC对TC的降解率为13%—46%. PMS添加量越大,对TC的降解率越高,当PMS添加量为0.3 mL时,对TC的去除率可达95%以上. 因为,随着PMS浓度的增加,生物炭的表面会形成亚稳态PMS来降解污染物,或产生更多的活性氧物种(reactive oxidative species)来降解污染物.
四环素初始浓度对降解的影响见图6(c). 当加入PMS启动降解时,Fe-N-RSBC对TC的降解率为18%—37%. 初始浓度越低,对TC的去除率越高. 当TC浓度为30 mg∙L−1时,对TC的去除率可达80%以上. TC浓度越低,生物炭有更多的吸附位点来吸附四环素,加入PMS之后,也很快的能完成降解反应. TC浓度越高需要更多的活性氧物种来进行降解.
溶液初始pH对四环素降解的影响见图6(d). pH会影响Fe-N-RSBC对TC在前60分钟的吸附效果,pH为7时,吸附效果最好. 当加入PMS启动降解时,Fe-N-RSBC对TC的降解率为20%—40%,pH在3—9内下TC降解效果均较好. 碱性环境下降解效果略微下降的原因是:碱性条件下PMS降解效率降低可能是由于PMS在碱性条件下分解为
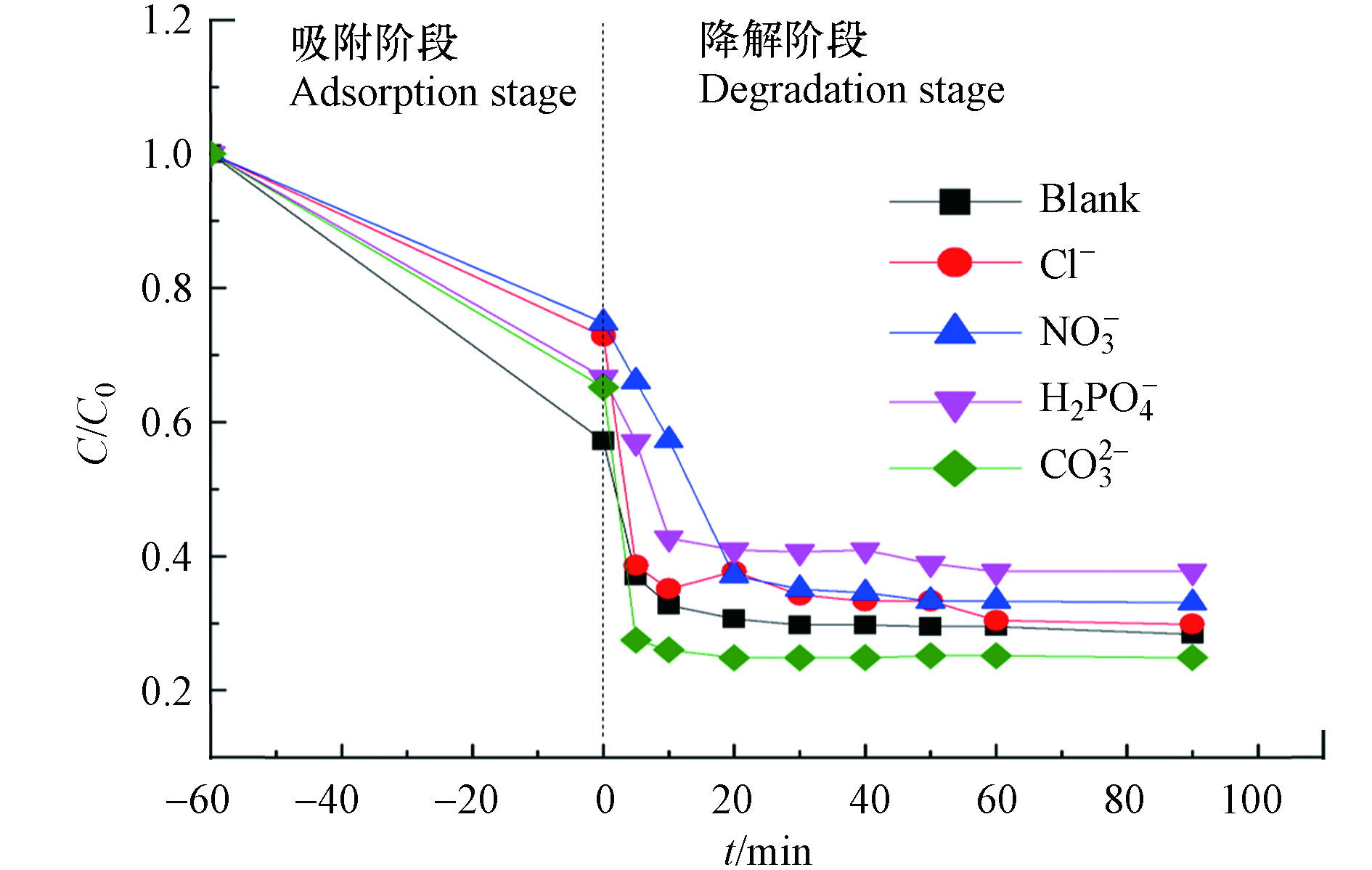
SO2−4 和O2 [45](见反应式8). 整体来说, 在较宽的pH的范围(3—9)内,Fe-N-RSBC可以有效的催化PMS降解废水中的TC.阴离子类型对Fe-N-RSBC催化PMS降解TC的影响见图7. 由图7可知,Cl−、
H2PO−4 和NO−3 会略微降低对TC的去除效果,而CO2−3 略微促进对TC的降解,阴离子的影响与自由基的弱化、阴离子与金属离子的络合和反应体系的酸碱环境改变有关 [20-23]. -
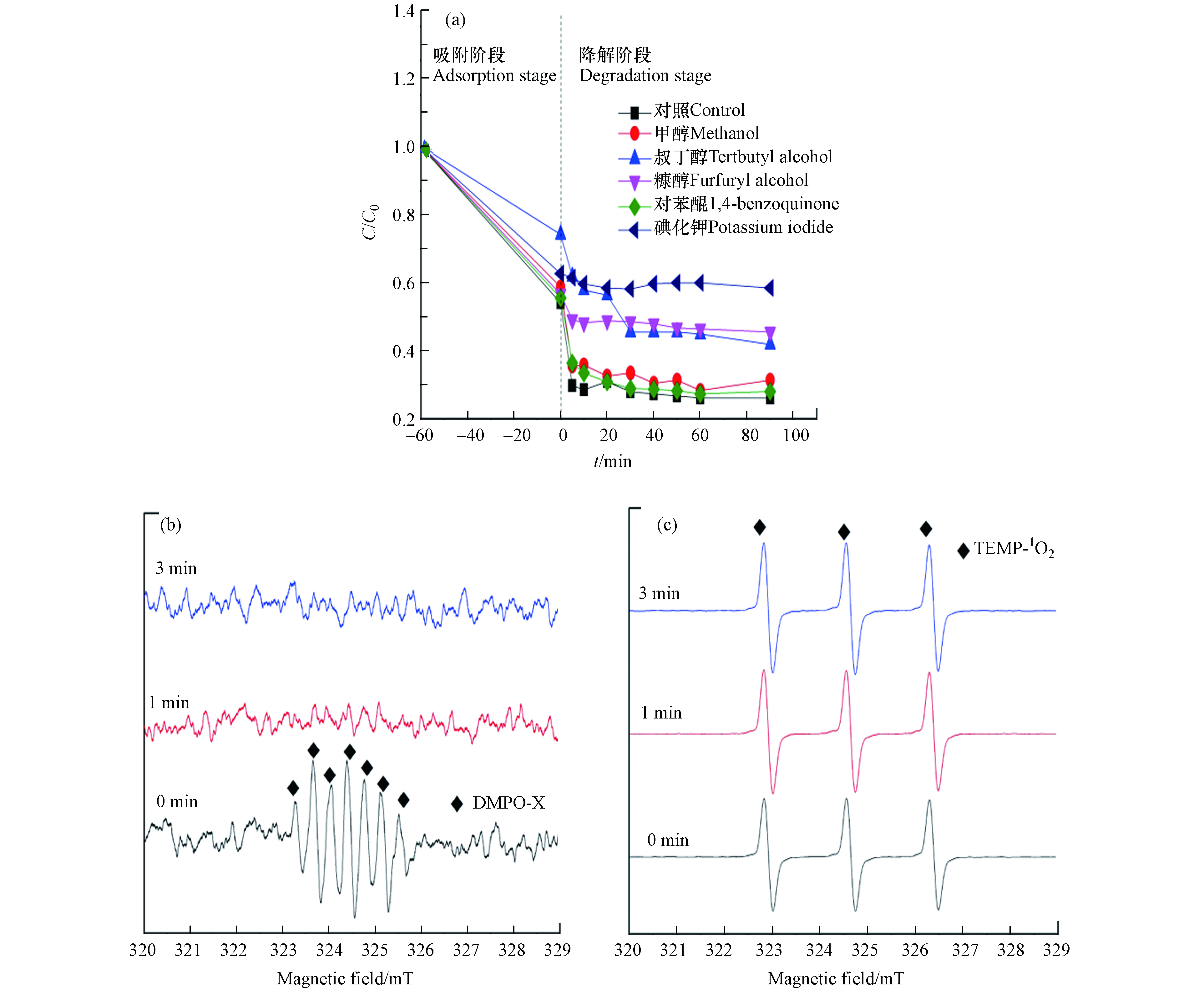
不同类型淬灭剂对Fe-N-RSBC催化PMS降解四环素的影响见图8(a). 甲醇可以淬灭∙OH和
SO−4⋅ ;叔丁醇可以淬灭∙OH;糠醇可以淬灭单线氧;对苯醌可以淬灭超氧自由基O⋅−2 ;KI主要用于清除表面结合的自由基 [18, 46]. 从添加PMS开始,Fe-N-RSBC对PMS的降解率为28%. 甲醇、叔丁醇、糠醇、对苯醌和碘化钾添加后,Fe-N-RSBC对PMS的降解率分别为27%、30%、14%、27%和4%. 但是,甲醇和叔丁醇的存在基本不会影响Fe-N-RSBC对TC的降解,说明降解体系基本没有∙OH和SO−4⋅ 的存在. 但是,碘化钾和糠醇的存在会显著抑制四环素的降解,说明单线氧(1O2)和表面结合的自由基在四环素降解中发挥重要作用.为进一步证实催化降解机理,采用EPR对催化降解过程中的自由基进行检测. 如图所示,EPR能检测到DMPO-X[图8(b))]和1O2[图8(c))]存在. DMPO-X的产生原因 [47-49]:由于DMPO的氧化出现了带有七元体的强峰DMPOX,这间接说明了在Fe-N-RSBC/PMS体系中产生的强氧化物种,如∙OH和
SO−4⋅ ,以及DMPO向DMPOX的转化. 但是在1 min和3 min检测不到DMPO-X的存在,说明DMPO-X不是主导的降解因子. 但是在1 min和3 min均检测到了1O2的存在,说明1O2在TC 的降解中发挥重要作用.1O2的来源可能是PMS的自降解、Fe-N-RSBC中铁的催化、Fe-N-RSBC中的官能团(如C=O,C=N,芳香基团和Fe-N-RSBC中的结构缺陷,具体反应公式所示 [8, 21, 24, 50]:
综上可见,Fe-N-RSBC降解TC的机理是包括自由基路径和非自由基路径,自由基途径涉及表面结合的自由基等,但是1O2非自由基作用占主要地位. 与其它材料相比[18-25],本研究制备的Fe-N-RSBC催化PMS降解TC的优势在:去除效果较好;受到干扰少;Fe和N能发挥协同作用.
-
图9(a)显示的是Fe-N-RSBC的再生利用结果. Fe-N-RSBC第1次、第2次和第3次对四环素的去除率分别为70%、53%和52%,去除效果降低可能与铁离子的流失、生物炭理化性质改变等有关. 为验证材料的适用性,选择代表性的阳离子染料亚甲基蓝和阴离子染料橙黄Ⅱ,探究Fe-N-RSBC催化PMS降解染料的效果,结果见图9(b). 可见,Fe-N-RSBC催化PMS对染料有着较好的效果,150 min内,对亚甲基蓝和橙黄Ⅱ的去除效果可达73%和95%,说明Fe-N-RSBC具有较好的适用性.
-
(1) 铁氮共改性可以明显改变生物炭的理化性质,使得生物炭的比表面积从177.7 m2·g−1增加到606.6 m2·g−1,孔体积从0.11 cm3·g−1增加到0.33 cm3·g−1,微孔体积从0.066 cm3·g−1增加到0.154 cm3·g−1,改性赋予更丰富的官能团,并引入磁性组分,Fe-N-RSBC的饱和磁化值在38.99 emu·g−1,但破坏了部分石墨碳结构.
(2) 铁氮改性生物炭可以有效催化PMS降解四环素,在预吸附60 min降解90 min体系中,Fe-N-RSBC催化PMS对TC的去除率可达87%(四环素浓度为50 mg·L−1, 固液比0.2 g·L−1, PMS浓度为1 mmol·L−1 , PMS添加量0.2 mL, 溶液pH=7).
(3) Fe-N-RSBC催化PMS降解TC的机理是包括自由基路径和非自由基路径,其中非自由基途径以1O2为主要活性氧物种.
铁氮生物炭的制备及催化过一硫酸盐(PMS)降解水体中四环素
Preparation of Fe-N modified biochar and its application in tetracycline degradation activated by peroxymonosulfate
-
摘要: 本文以水稻秸秆为对象,六水合氯化铁和尿素为改性剂,在700℃热解制备成铁氮改性秸秆生物炭(Fe-N-RSBC),同时制备原状生物炭(RSBC)作为对照. 采用SEM、TEM、EA、BET、XRD 、FTIR、Raman、XPS和VSM对生物炭的理化性质进行全面的表征. 研究铁氮改性生物炭催化过一硫酸盐(PMS)降解水体中的四环素(TC),考察溶液pH、固液比、PMS量、TC浓度和阴离子类型的影响. 采用淬灭实验和EPR测定揭示Fe-N-RSBC催化PMS降解TC的微观机制. 研究结果表明,铁氮共改性使得生物炭具有更大的比表面积和孔体积、更多微孔体积,赋予更丰富的官能团,并引入磁性组分,但破坏了部分石墨碳结构. Fe-N-RSBC可以有效的活化PMS降解溶液中的TC. 在吸附/降解体系中,150 min内,Fe-N-RSBC/PMS对TC的去除率可达87%(四环素浓度为50 mg∙L-1,固液比0.2 g∙L−1,PMS (浓度为1 mmol·L−1) 添加量0.2 mL,溶液pH=7),降解效果与pH、PMS量、催化剂量、TC初始浓度和阴离子类型有关. Fe-N-RSBC催化PMS降解TC的机理是包括自由基路径和非自由基路径,其中1O2非自由基作用占据主导地位. 因此,铁氮生物炭有作为催化剂降解废水中四环素的潜力.Abstract: Rice straw was mixed with FeCl3∙6H2O and urea to prepare a Fe-N modified biochar (Fe-N-RSBC). Meanwhile, pristine biochar (RSBC) was synthesized to serve as control. SEM, TEM, EA, BET, XRD, FTIR, Raman, XPS and VSM were used to characterize the physico-chemical properties of biochars. Furthermore, Fe-N-RSBC was applied for the TC degradation activated by peroxymonosulfate. The effect of factors on TC degradation were investigated, including pH, dosage, PMS amount, TC concentration and anion types. Quenching experiment and EPR was combined to reveal the degradation mechanisms. The results showed that Fe-N modification significantly affected the properties of biochar, involving increasing surface area and pore volume, creating more functional groups, destroying the graphited carbon structure, introducing more magnetic components. The Fe-N-RSBC effectively activated PMS to degrade TC in solution. The removal rate of TC reached 87% (TC: 50 mg∙L−1, catalyst: 0.2 g∙L−1, PMS: 0.2 mL (1 mmol·L−1), pH=7) under adsorption/degradation system in 150 min. The degradation effect was related to the pH, dosage, PMS amount, TC concentration and anion types. The degradation mechanisms included radical and non-radical pathways. The 1O2 played a key role for the TC degradation. Therefore, Fe-N-RSBC has the potential to act as a catalyst to degrade TC in wastewater.
-
Key words:
- rice straw /
- biochar /
- Fe-N modification /
- TC degradation /
- singlet oxygen
-
抗生素的大量使用使得其会通过直接或间接途径进入水体引起污染. 四环素(TC)是水体检出浓度和检出频率最高的抗生素之一[1]. 水体中的四环素会诱导微生物逐渐产生抵抗力,造成抗药性菌群和抗性基因的产生[2]. 常规生物法、生物电化学法、高级氧化法、膜分离法、吸附法、微生物降解等是水体中四环素去除的主要方法[3-6] . 其中,高级氧化法(AOPs)已经被证明能有效的去除水中的四环素,因为具有高度活性的自由基的存在,包括硫酸根自由基(
SO−4⋅ 基于
SO−4⋅ SO−4⋅ SO−4⋅ 研究显示,生物炭有作为高级氧化反应催化剂的潜力[12]. 但是原状生物炭的催化能力有限,需要对原状生物炭进行改性以进一步提升其对污染物的降解效果[13]. 其中,生物炭负载铁作为催化剂的研究报道最多,因为纳米铁氧化物能有效的启动催化降解反应[14]. 过渡金属活化PMS可以在常温常压下进行,且反应迅速、操作简便,被认为是活化效果最好的一种活化方式. 但同时在反应过程中也存在着过渡金属易团聚、重复利用性差和金属离子流失等问题[15]. 因此,如何改性提升生物炭的催化降解能力亟需进一步研究. 与此同时,氮改性生物炭也引起了更多关注,因为引入了含氮官能团和可以改变生物炭的结构[16]. 氮修饰生物炭更多的应用于生物炭的催化降解领域[17],因为氮具有局部未成对电子:(1) 改善周围碳原子的电子密度;(2)提高共轭增强sp2碳中π电子流;(3)进入更多的官能团和缺陷;(4)增加碳材料的表面亲水性能有效的结合极性吸附质. 因此,可以考虑铁氮共改性以提升生物炭的降解性能.
近年来,铁氮共改性生物炭在高级氧化领域的应用也逐渐引起了研究,作为催化剂被用于降解废水中有机污染物,包括酸性橙7 [18]、异丙甲草胺[19]、罗丹明B [20]、双酚A [21-22]、双酚F [23]和磺胺噻唑 [24]. 仅有Yu等[25]研究了铁氮改性生物炭活化过硫酸盐(PS)降解四环素的效果及机理. 上述研究均显示铁氮共改性碳材料可以有效的降解废水中的有机污染物. 然而,铁氮共改性生物炭活化PMS降解TC的研究报道还较少,降解的机理也尚不清楚.
因此,本研究以典型的农林废弃物—水稻秸秆为对象,选择氯化铁、尿素为改性剂,通过热解制备出铁氮改性生物炭,同时制备原状生物炭作为对照,对生物炭进行SEM、TEM、BET、EA、XPS、FTIR、Raman和VSM进行全面的表征,考察铁氮改性对生物炭理化性质的影响,探究对水体中四环素的降解行为及机理,以期为秸秆的资源化的利用及水体中四环素的去除提供理论依据和技术参考.
1. 材料与方法 (Materials and methods)
1.1 实验材料
水稻秸秆来自安徽合肥郊区农田,将秸秆水洗去除灰尘,放入烘箱80℃烘干,之后用破碎机破碎后过40目筛,保存备用.
四环素(tetracycline,TC,购于上海源叶生物有限公司)、FeCl3·6H2O、尿素、甲醇、叔丁醇、糠醇、对苯醌、碘化钾、NaOH 、HCl均为分析纯,购于国药集团化学试剂有限公司.
1.2 改性生物炭的制备
将5 g水稻秸秆与0.1 mol·L−1 (FeCl3·6H2O)、0.1 mol·L−1尿素在100 mL水溶液中混合,放在磁力搅拌器上搅拌24 h后放入烘箱80 ℃烘干. 将烘干的混合固体盛于石英舟中,放置于管式马弗炉中(OTF-1200X-S,合肥科晶材料技术有限公司),700 ℃下热解(全程氮气保护),升温速率为5 ℃·min−1,保温2 h,待温度降至室温时,取出生物炭,对生物炭进行水洗,至洗脱接近中性后在烘箱中烘干. 最后进行球磨1 min后制得铁氮改性水稻秸秆生物炭(Fe-N-RSBC). 同时,制备原状水稻秸秆生物炭作为对照(RSBC).
1.3 生物炭对四环素的降解试验
(1)吸附体系:将100 mL含TC (浓度为50 mg∙L−1)的溶液置于锥形瓶中,然后加入0.02 g生物炭,将锥形瓶置于在摇床上,摇床转速为150 r·min−1. 分别在 0、5、10、20、30、40、50、60、90 min取样测定,并通过0.45 μm的膜过滤. 用紫外分光光度计(UV-5100B, 上海元析仪器有限公司)测量上清液的吸光度,波长设定为358 nm. TC浓度选择50 mg·L−1的原因是:有的药厂或畜禽养殖废水抗生素的浓度确实很高;凸显铁氮生物炭的吸附降解效果;便于仪器测定.
(2)PMS降解体系(没有添加生物炭):将100 mL含TC (浓度为50 mg·L−1)的溶液置于锥形瓶中,将锥形瓶置于在摇床上,摇床转速为150 r·min−1. 添加0.2 mL PMS (浓度为1 mmol·L−1),0、5、10、20、30、40、50、60、90 min取样测定 (预先加入1mL硫代硫酸钠作为淬灭剂),并通过0.45 μm的膜过滤. 用紫外分光光度计(UV-5100B, 上海元素仪器有限公司) 测量上清液的吸光度,波长设定为358 nm.
(3)生物炭催化降解体系(添加生物炭):将100 mL含TC (浓度为50 mg∙L−1)的溶液置于锥形瓶中,添加0.02 g生物炭,将锥形瓶置于在摇床上,摇床转速为150 r·min−1. 之后再添加0.2 mL PMS启动反应,在0、5、10、20、30、40、50、60、90 min取样测定(预先加入1mL硫代硫酸钠作为淬灭剂),并通过0.45 μm的膜过滤后测定.
(4)不同影响因素
溶液初始pH值:将100 mL含TC (浓度为50 mg∙L−1)的溶液置于锥形瓶中. 使用0.1 mol·L−1 NaOH或0.1 mol·L−1 HCl调整溶液的pH为3.0、5.0、7.0、9.0和11.0. 随后,0.02 g Fe-N-RSBC加入到溶液中,进行振荡,进行60 min预吸附后,随后加入0.2 mL PMS,启动降解实验,其它条件同上.
生物炭量:将100 mL含TC (TC浓度为50 mg∙L−1)的溶液置于锥形瓶中. 分别加入0.005、0.01、0.02、0.025 g Fe-N-RSBC加入到瓶中进行振荡,60 min预吸附之后,再加入0.2 mL PMS,启动降解实验,其它条件同上.
PMS量:将100 mL含TC(浓度为50 mg∙L−1)的溶液置于锥形瓶中. 随后,0.02 g Fe-N-RSBC加入到瓶中的溶液中,将锥形瓶置于150 r·min−1的摇床上,进行60 min预吸附,之后分别加入0.1、0.2、0.25、0.3 mL PMS,启动降解实验,其它条件同上.
TC初始浓度:将不同浓度的含TC(浓度为30、50 、75、100 mg∙L−1)溶液置于锥形瓶中. 随后,向锥形瓶的溶液中加入0.02 g Fe-N-RSBC,进行振荡,进行60 min预吸附之后,再加入0.2 mL PMS,进行降解实验,其它条件同上.
阴离子类型:将100 mL含阴离子的TC(浓度为50 mg∙L−1)的溶液置于锥形瓶中, 阴离子类型为Cl−、
H2PO−4 NO−3 CO2−3 1.4 生物炭表征
采样扫描电镜(SEM,SU8010, Hitachi, Japan)和透射电镜(TEM, TecnaiG2F20S-TWIN, 200KV)对生物炭的表面形貌和结构进行观察. 生物炭的晶体结构采用X射线衍射(XRD, Bruker D8 Advance, Germany)测定. 生物炭的比表面积、孔体积和孔尺寸采用比表面积和孔径分析仪(Micromeritics, Tristar II 3020, USA)测定. 采用元素分析仪( Elementar Vario EL cube,Germany) 测定秸秆生物炭中C、N、H和的含量. 灰分测定: 称取一定量的生物炭置于箱式马弗炉中700 ℃燃烧2 h,冷却至室温后称量,根据质量差计算灰分含量. 采用傅里叶变换红外光谱(FTIR,Nicolette is50,Thermo Fourier, USA )分析表征生物炭的官能团类型. 利用X光电子能谱分析表征(XPS, Thermo-VGScientific, ESCALAB250, USA)生物炭的表面元素及化学形态. 采用Zeta电位仪(DelsaMax PRO, Beckman Coulter, USA )测定生物炭的zeta电位. 采用Raman光谱进行表征(DXR Raman microscope; Thermo Fisher Scientific, Madison, WI, USA)生物炭的碳缺陷结构.
1.5 机理实验
(1)活性氧化剂抑制实验
将100 mL含TC (50 mg·L−1)的溶液置于一系列锥形瓶中,之后分别加入0.02 g Fe-N-RSBC,添加甲醇(1 mol·L−1)、糠醇(20 mmol·L−1)、对苯醌(20 mmol·L−1)、叔丁醇(1 mol·L−1)、碘化钾(20 mmol·L−1)作为活性氧物种抑制剂. 将锥形瓶置于的摇床上振荡,进行60 min预吸附实验, 之后加入0.2 mL PMS启动降解实验,分别在0、5、10、20、30、40、50、60、90 min时取样(取样瓶中预先加入1 mL淬灭剂). 并通过0.45 μm的膜过滤后测定.
(2)EPR测定实验
在10 mL溶液中提前加入TEMP和DMPO,分别0.002 g Fe-N-RSBC,之后加入0.2 mL的PMS启动反应,振荡反应时间3 min,采用电子顺磁共振波谱仪(EPR)测定活性氧物种类型(JEOL JES-FA200,Tokyo, Japan).
1.6 生物炭的再生利用和适用性实验
将降解反应完成后的Fe-N-RSBC通过磁分离之后,水洗并烘干,开展再生利用实验,共进行3次. 再生利用降解的实验条件是:将100 mL含TC (浓度为50 mg∙L−1)的溶液置于锥形瓶中,添加0.02 g生物炭,进行振荡,之后再添加0.2 mL PMS,0、5、10、20、30、40、50、60、90 min取样测定 (预先加入1 mL硫代硫酸钠淬灭剂),并通过0.45 μm的膜过滤后测定.
为验证材料的适用性,选择代表性的阳离子染料亚甲基蓝和阴离子染料橙黄Ⅱ,探究Fe-N-RSBC催化PMS降解染料的效果,降解实验条件: 将100 mL含染料废水 (浓度为50 mg∙L−1)的溶液置于锥形瓶中,添加0.02 g Fe-N-RSBC,进行振荡预吸附60 min, 之后再添加0.2 mL PMS,0、5、10、20、30、40、50、60、90 min取样测定 (预先加入1 mL硫代硫酸钠淬灭剂),通过0.45μm的膜过滤后用分光光度计测定,亚甲基蓝的测定波长为665 nm,橙黄Ⅱ的测定波长为484 nm.
2. 结果与讨论 (Results and discussion)
2.1 生物炭的表征
RSBC和Fe-N-RSBC的SEM、TEM和EDS mapping见图1.
由图1可见,原状秸秆生物炭中的存在无规则的杆状或梗状形态,但是也有一定的破碎,因为热解温度为700℃,秸秆中的大部分有机物都会热分解. Fe-N-RSBC中存在球状颗粒,但是可以看出明显孔隙结构更加发达,说明Fe、N能与秸秆组分之间反应从而促进秸秆热分解的更加彻底,使得生物炭更加碎片化. RSBC和Fe-N-RSBC的TEM见图1(c, d).
由TEM可见,RSBC性状不规则,呈现无定形形态,生物炭的碳层比较厚,有着明显的堆积. 黑色颗粒可能是生物炭中的矿物质组分. Fe-N-RSBC的碳层也比较薄,黑色颗粒为铁氧化物,铁氧化物分布在炭结构中,也有铁分布在炭的外围. HR-TEM可以看出存在微孔结构. 铁氮改性导致炭基质更加规则平整规则,降低了碳结构的堆积和褶皱的性状. 从 Fe-N-RSBC 的 EDS mapping 图可见,Fe 和 N 较为均匀的分布在生物炭的表面.
RSBC和Fe-N-RSBC的N2吸附-解吸曲线及孔径分布图见图2(a). RSBC及Fe-N-RSBC的N2吸附-解吸曲线都是典型的IV类型曲线,即高压处存在明显的滞后回线,说明都存在典型的介孔特性. 从孔径分布曲线(图2(b))可以看出,RSBC和Fe-N-RSBC的主要孔径分布在2—4 nm,也证实了RSBC和Fe-N-RSBC的介孔分布特性. 可以看出,Fe-N-RSBC的孔体积也大于RSBC.
RSBC和Fe-N-RSBC的XRD见图2(c). 如图2所示,RSBC在22°和44°附近分别是无定形碳和石墨碳结构[26]. 此外,RSBC中还含有CaCO3, 说明水稻秸秆中含有碱性元素. 铁氮改性之后生物炭中出现了γ-Fe2O3和Fe3O4,这也是导致铁改性生物炭具有磁性的原因. 铁氮改性之后出现还出现了Fe3C,说明氮的存在促进了铁的还原. 铁氮改性导致了铁进入了碳基质中形成Fe3C. 热解过程中铁的形态变化是:Fe3+→ Fe2O3→Fe3O4→Fe3C [27-28]. 可见,铁与氮的共改性会明显改性生物炭中碳的形态.
Fe-N-RSBC的磁滞曲线见图2(d),剩磁及矫顽力接近于零,判断Fe-N-RSBC为超顺磁性材料 [29]. Fe-N-RSBC的饱和磁化值38.99 emu·g−1,说明在外加磁场作用后,Fe-N-RSBC有良好的固液分离效果.
RSBC和Fe-N-RSBC的N2吸附-解吸曲线得到的比表面积和孔径参数见表1. 由表1可知,Fe-N-RSBC的比表面积达到606.62 m2·g−1, 微孔面积为341.29 m2·g−1 ,总孔体积为0.33 cm3·g−1,微孔体积为0.15 cm3·g−1,分别是原状生物炭的3.41倍、2.41倍、3.00倍和2.27倍. 铁氮改性之后平均孔径尺寸从2.48 nm降低2.21 nm. 从Vmicro/Vtotal比值可知,RSBC中以微孔和介孔为主,Fe-N-RSBC改性之后微孔所占比例降低到45.93%,介孔占据优势. 这与图2(b)一致. 生物炭比表面积和孔径参数与SEM和TEM的结果一致. 铁氮改性使得生物炭存在更多的介孔结构.
表 1 生物炭的比表面积及孔隙参数Table 1. Surface area and pore parameters of biochars生物炭 Biochar BET /( m2·g−1 ) Smicro /( m2·g−1 ) Vtotal /( cm3·g−1) Vmicro /( cm3·g−1) Vmicro/Vtotal/% 孔径/nmPore size RSBC 177.71 141.62 0.11 0.066 59.60 2.48 Fe-N-RSBC 606.62 341.29 0.33 0.15 45.93 2.21 热解过程中,Fe、N能与生物炭中的碳组分发生热化学反应,促进了生物炭的中纤维素、半纤维素和木质素的分解. 铁氮改性增加生物炭比表面积的原因是 [30]:氮原子能掺杂到碳原子的网格之中,同时铁降低了碳基质在炭化过程中的伸缩;尿素的热分解能产生气体,这些气体会破坏碳结构从而制造更多的孔隙;氮的存在能促进铁与基质碳之间的反应从而形成更多的微孔. 同时,铁改性提高生物炭的比表面积和孔体积因为铁进入了介孔炭之中,从而降低了炭化过程中基质炭的伸缩;热解过程中铁的催化作用,能促进孔隙结构形成,特别是介孔结构;铁催化能将相邻的碳材料气化成低分子气体,如CH4和CO等;铁氧化物覆盖生物炭上也能创造更多的孔隙结构 [31-34].
RSBC和Fe-N-RSBC的元素含量、原子比、灰分含量及zeta电位见表2. 与RSBC相比,Fe-N-RSBC中的C含量显著降低,而N含量明显上升,H和O含量略微增加,灰分含量也显著增加,灰分增加主要是因为铁氧化物的存在. 此外,铁氮改性还导致了zeta电位的明显变化,从-43.89 mV变为-29.45 mV. 生物炭的原子比H/C、O/C和(N + O) / C分别指示生物炭的芳香性、亲水性和极性大小. H/C比值越小则芳香性越高,O/C和(O+N)/C比值越大,则亲水性和极性越大. 由表2可知, 铁氮改性之后生物炭的亲水性和极性均增加了,有利于与水体中极性污染物的结合.
表 2 元素组成、灰分含量及表面电荷Table 2. Element, ash content and zeta potential生物炭Biochar 元素/%Element H/C O/C (N + O) / C 灰分/%Ash Zeta 电位/mVZeta potential C N H O RSBC 56.97 0.71 1.63 6.70 0.03 0.12 0.13 33.99 -43.89 Fe-N-RSBC 23.74 4.35 1.68 7.07 0.07 0.30 0.48 63.16 -29.45 O%=100%-C-N-H-ash RSBC和Fe-N-RSBC的FTIR见图3(a). 由图3可知,原状生物炭中含有的官能团包括—OH、—CH3、芳香C=C环拉伸、—CH2、C—O—C、C—H和Si—O—Si等官能团 [35]. 铁氮改性之后生物炭的官能团包括—OH(3385 cm−1)、芳香C=C环拉伸(1610 cm−1)、C—N 拉伸(1230 cm−1)、Quaternary−N/C—O—C (1113 cm−1)、Fe—O (551 cm−1)、Si—O—Si (468 cm−1). 铁氮改性部分保留了生物炭的芳香结构(1617 cm−1),但是生物炭的芳香结构弱化了因为Fe和N已经嵌入到了生物炭的碳结构中,这与TEM、XRD、元素分析比H/C的结果是吻合的. 可见,改性导致铁氮已经嵌入到碳基质中,并改变了生物炭的含碳和含氧官能团.
RSBC和Fe-N-RSBC的XPS图谱见图3(b). RSBC的表面能检测到 C 1s、O 1s、N 1s和Si 2p的存在. Fe-N-RSBC中还能明显检测到Fe 2p的存在. 与RSBC相比,Fe和N改性之后导致了生物炭表面元素相对含量发生明显变化. Fe-N改性生物炭表面氮和氮的相对质量分数分别为6.13%和4.33%. Fe改性后碳含量的降低, 说明改性导致铁氮负载到生物炭上,同时铁和氮已经成功嵌入到碳骨架.
拉曼光谱可用于分析生物炭的碳结构缺陷. RSBC和Fe-N-RSBC的拉曼光谱见图3(c). 如图所示,生物炭的Raman光谱有两个明显的特征峰. D峰(1350 cm−1附近)是由碳层结构中的缺陷导致的,代表了碳材料中乱层结构以及不小于6 个环的多环芳烃,在1575 cm−1 附近出现的峰称为G 峰,代表稠芳环结构以及类石墨微晶的sp2杂化振动 [36]. ID/IG(面积之比)可以用来表示碳结构的石墨化程度,比值越大表明碳结构缺陷越大. 如图3(c)所示,RSBC的ID/IG值为4.59, 且存在明显的D和G峰. 铁氮改性之后ID/IG值变成1.81,因为铁氮改性会明显影响G峰,铁和氮会嵌入到石墨碳结构,从而破坏石墨碳结构,与TEM、BET和XRD的结果是一致的. 研究发现铁氮改性会破坏材料的碳格网络,产生更多的缺陷 [37]. 也有研究发现氮改性导致了石墨碳结构的破坏,因为氮原子嵌入到碳网格中,导致碳结构的缺陷 [38]. 此外,Raman分析还发现铁氮改性导致了D峰和G峰位置的偏移. 可见,铁氮改性会影响无定形的碳结构和石墨碳结构,特别是石墨碳结构.
RSBC和Fe-N-RSBC的XPS分峰拟合见图4. RSBC的C分峰为283.8、284.5、287.3 eV, 分别对应C—C/C—H, C=C, C=O [39]. Fe-N-RSBC的C分峰为284.4 、285.37 、287.9 eV,分别对应C—C/C=C, C—O/C—N和C=O. RSBC中O以C—O和C=O形态存在. Fe-N-RSBC中O 分峰为C—O、C=O和Fe-O [40]. RSBC中N主要以pyrrolic-N形态存在. Fe-N-RSBC中N分峰为 pyrrolic-N和pyridinic-N. Pyridinic-N是形成是因为N取代了在石墨平面的C6环末端的C [41]. 可见,N掺杂到了碳结构中,这与XRD, Raman的结果相符合. Fe-N-RSBC中Fe 2p XPS分峰对应的是710.4、712.1、718、724.5、728.6 eV. Fe3+卫星峰的出现证实有Fe3+化合物的存在 [42],结合XRD结果,Fe2+和Fe3+的铁化合物为γ-Fe2O3、Fe3O4和Fe3C. 可见,铁氮改性导致了含碳、含氧、含氮和含铁官能团类型和所占比例的变化.
FeCl3改性生物炭的反应机理如下 [33]:
200—330 °C:
FeCl3+2H2O→FeOCl∙H2O+2HCl (g) (1) FeOCl∙H2O+2HCl(g)→FeOOH+3HCl(g) (2) 330—700 °C:
2FeOOH→Fe2O3+H2O (3) 6Fe2O3+C→4Fe3O4+CO2 (4) 尿素热分解的组分可进一步与碳的官能团反应,从而取代碳层表面的羰基和羟基,形成含氮官能团,具体的反应如下 [43- 44]:
CO(NH2)2→NH3*/NH2*/NH*+HCNO (5) NH3/NH2*/NH*+(―C=O) →―CO-NH2 (6) NH3/NH2*/NH*+(―C-OH) →―(C-NH2)+H2O (7) 综上可见,铁氮改性会明显改变生物炭的理化性质,使得生物炭具有更大的比表面积和孔体积、更多的微孔体积,赋予更丰富的官能团,并引入了磁性组分,但是破坏了部分石墨碳结构.
2.2 铁氮生物炭催化PMS对四环素的降解
不同体系下生物炭及PMS对四环素的去除见图5. 在90 min内,RSBC和Fe-N-RSBC对四环素的去除率为16%和50%(吸附过程),而PMS对四环素的去除率13%. 当PMS添加到RSBC,Fe-N-RSBC和四环素体系中,对四环素的去除率为59%和87%,其中前5 min的去除率分别为18%和80%. 可见Fe-N-RSBC催化PMS降解TC主要发生在初期阶段,因为存在大量的反应位点和活性氧化剂. 在吸附/降解体系中,150 min之内Fe-NRSBC/PMS对四环素的去除率可达87%.
Fe-N-RSBC催化剂用量对四环素降解的影响见图6(a). 当加入PMS启动降解时,Fe-N-RSBC对TC的降解率为23%—43%. 添加量越大,对TC的去除率越高,当Fe-N-RSBC添加量为0.025 mg时,对TC的去除率可达80%以上. 显然, 催化剂用量提高可以提供更多的表面积和活性位点为TC的吸附和PMS活化;更高效的吸附可能导致降解加快;吸附的四环素迅速分解提供了更多的吸附位点激活PMS,从而促进TC的去除.
PMS用量对四环素降解的影响见图6(b). Fe-N-RSBC对TC在前60分钟的吸附率在50%. 当加入PMS启动降解时,Fe-N-RSBC对TC的降解率为13%—46%. PMS添加量越大,对TC的降解率越高,当PMS添加量为0.3 mL时,对TC的去除率可达95%以上. 因为,随着PMS浓度的增加,生物炭的表面会形成亚稳态PMS来降解污染物,或产生更多的活性氧物种(reactive oxidative species)来降解污染物.
四环素初始浓度对降解的影响见图6(c). 当加入PMS启动降解时,Fe-N-RSBC对TC的降解率为18%—37%. 初始浓度越低,对TC的去除率越高. 当TC浓度为30 mg∙L−1时,对TC的去除率可达80%以上. TC浓度越低,生物炭有更多的吸附位点来吸附四环素,加入PMS之后,也很快的能完成降解反应. TC浓度越高需要更多的活性氧物种来进行降解.
溶液初始pH对四环素降解的影响见图6(d). pH会影响Fe-N-RSBC对TC在前60分钟的吸附效果,pH为7时,吸附效果最好. 当加入PMS启动降解时,Fe-N-RSBC对TC的降解率为20%—40%,pH在3—9内下TC降解效果均较好. 碱性环境下降解效果略微下降的原因是:碱性条件下PMS降解效率降低可能是由于PMS在碱性条件下分解为
SO2−4 2HSO−5+2OH−→2SO2−4+O2+2H2O (8) 阴离子类型对Fe-N-RSBC催化PMS降解TC的影响见图7. 由图7可知,Cl−、
H2PO−4 NO−3 CO2−3 2.3 Fe-N-RSBC对四环素降解机理的探究
不同类型淬灭剂对Fe-N-RSBC催化PMS降解四环素的影响见图8(a). 甲醇可以淬灭∙OH和
SO−4⋅ O⋅−2 SO−4⋅ 为进一步证实催化降解机理,采用EPR对催化降解过程中的自由基进行检测. 如图所示,EPR能检测到DMPO-X[图8(b))]和1O2[图8(c))]存在. DMPO-X的产生原因 [47-49]:由于DMPO的氧化出现了带有七元体的强峰DMPOX,这间接说明了在Fe-N-RSBC/PMS体系中产生的强氧化物种,如∙OH和
SO−4⋅ 1O2的来源可能是PMS的自降解、Fe-N-RSBC中铁的催化、Fe-N-RSBC中的官能团(如C=O,C=N,芳香基团和Fe-N-RSBC中的结构缺陷,具体反应公式所示 [8, 21, 24, 50]:
Fe0+2HSO−5→Fe2++ 2SO·−4+2OH− (9) Fe2++HSO−5→Fe3++ SO·−4+ OH− (10) Fe3++HSO−5→Fe2++SO·−5+ H+ (11) HSO−5+e−→⋅OH+SO2−4 (12) HSO−5+e−→SO·−4+ OH− (13) Fe3++ e−→Fe2+ (14) O2+e−→O−·2 (15) 2O·−2+H+→1O2+H2O2 (16) SO·−4+OH−→⋅OH+SO2−4 (17) 2SO·−5→2SO·−4+O2 (18) ·OH+ O·−2→1O2+ OH− (19) HSO−5+SO2−5→SO2−4+HSO−4+1O2 (20) HSO−5→SO·−5+H++e− (21) 2SO·−5+H2O→1.51O2+ 2HSO−4 (22) SO·−5+ SO·−5→1O2+2SO−4 (23) 综上可见,Fe-N-RSBC降解TC的机理是包括自由基路径和非自由基路径,自由基途径涉及表面结合的自由基等,但是1O2非自由基作用占主要地位. 与其它材料相比[18-25],本研究制备的Fe-N-RSBC催化PMS降解TC的优势在:去除效果较好;受到干扰少;Fe和N能发挥协同作用.
2.4 Fe-N-RSBC再生利用和适用性实验
图9(a)显示的是Fe-N-RSBC的再生利用结果. Fe-N-RSBC第1次、第2次和第3次对四环素的去除率分别为70%、53%和52%,去除效果降低可能与铁离子的流失、生物炭理化性质改变等有关. 为验证材料的适用性,选择代表性的阳离子染料亚甲基蓝和阴离子染料橙黄Ⅱ,探究Fe-N-RSBC催化PMS降解染料的效果,结果见图9(b). 可见,Fe-N-RSBC催化PMS对染料有着较好的效果,150 min内,对亚甲基蓝和橙黄Ⅱ的去除效果可达73%和95%,说明Fe-N-RSBC具有较好的适用性.
3. 结论(Conclusion)
(1) 铁氮共改性可以明显改变生物炭的理化性质,使得生物炭的比表面积从177.7 m2·g−1增加到606.6 m2·g−1,孔体积从0.11 cm3·g−1增加到0.33 cm3·g−1,微孔体积从0.066 cm3·g−1增加到0.154 cm3·g−1,改性赋予更丰富的官能团,并引入磁性组分,Fe-N-RSBC的饱和磁化值在38.99 emu·g−1,但破坏了部分石墨碳结构.
(2) 铁氮改性生物炭可以有效催化PMS降解四环素,在预吸附60 min降解90 min体系中,Fe-N-RSBC催化PMS对TC的去除率可达87%(四环素浓度为50 mg·L−1, 固液比0.2 g·L−1, PMS浓度为1 mmol·L−1 , PMS添加量0.2 mL, 溶液pH=7).
(3) Fe-N-RSBC催化PMS降解TC的机理是包括自由基路径和非自由基路径,其中非自由基途径以1O2为主要活性氧物种.
-
表 1 生物炭的比表面积及孔隙参数
Table 1. Surface area and pore parameters of biochars
生物炭 Biochar BET /( m2·g−1 ) Smicro /( m2·g−1 ) Vtotal /( cm3·g−1) Vmicro /( cm3·g−1) Vmicro/Vtotal/% 孔径/nmPore size RSBC 177.71 141.62 0.11 0.066 59.60 2.48 Fe-N-RSBC 606.62 341.29 0.33 0.15 45.93 2.21 表 2 元素组成、灰分含量及表面电荷
Table 2. Element, ash content and zeta potential
生物炭Biochar 元素/%Element H/C O/C (N + O) / C 灰分/%Ash Zeta 电位/mVZeta potential C N H O RSBC 56.97 0.71 1.63 6.70 0.03 0.12 0.13 33.99 -43.89 Fe-N-RSBC 23.74 4.35 1.68 7.07 0.07 0.30 0.48 63.16 -29.45 O%=100%-C-N-H-ash -
[1] DAGHRIR R, DROGUI P. Tetracycline antibiotics in the environment: A review [J]. Environmental Chemistry Letters, 2013, 11(3): 209-227. doi: 10.1007/s10311-013-0404-8 [2] XU L Y, ZHANG H, XIONG P, et al. Occurrence, fate, and risk assessment of typical tetracycline antibiotics in the aquatic environment: A review [J]. Science of the Total Environment, 2021, 753: 141975. doi: 10.1016/j.scitotenv.2020.141975 [3] GOPAL G, ALEX S A, CHANDRASEKARAN N, et al. A review on tetracycline removal from aqueous systems by advanced treatment techniques [J]. RSC Advances, 2020, 10(45): 27081-27095. doi: 10.1039/D0RA04264A [4] HOMEM V, SANTOS L. Degradation and removal methods of antibiotics from aqueous matrices - A review [J]. Journal of Environmental Management, 2011, 92(10): 2304-2347. doi: 10.1016/j.jenvman.2011.05.023 [5] SAADATI F, KERAMATI N, GHAZI M M. Influence of parameters on the photocatalytic degradation of tetracycline in wastewater: A review [J]. Critical Reviews in Environmental Science and Technology, 2016, 46(8): 757-782. doi: 10.1080/10643389.2016.1159093 [6] 范世锁, 刘文浦, 王锦涛, 等. 茶渣生物炭制备及其对溶液中四环素的去除特性 [J]. 环境科学, 2020, 41(3): 1308-1318. doi: 10.13227/j.hjkx.201908179 FAN S S, LIU W P, WANG J T, et al. Preparation of Tea Waste Biochar and Its Application in Tetracycline removalfrom Aqueous Solution [J]. Environmental Science, 2020, 41(3): 1308-1318(in Chinese). doi: 10.13227/j.hjkx.201908179
[7] LEE J, von GUNTEN U, KIM J H. Persulfate-based advanced oxidation: Critical assessment of opportunities and roadblocks [J]. Environmental Science & Technology, 2020, 54(6): 3064-3081. [8] ZHAO C H, SHAO B B, YAN M, et al. Activation of peroxymonosulfate by biochar-based catalysts and applications in the degradation of organic contaminants: A review [J]. Chemical Engineering Journal, 2021, 416: 128829. doi: 10.1016/j.cej.2021.128829 [9] 梁锦芝, 许伟城, 赖树锋, 等. 磁性生物炭的制备及其活化过一硫酸盐的研究进展 [J]. 环境化学, 2021, 40(9): 2901-2911. doi: 10.7524/j.issn.0254-6108.2021022301 LIANG J Z, XU W C, LAI S F, et al. Research progress on preparation and peroxymonosulfate activation of magnetic biochar [J]. Environmental Chemistry, 2021, 40(9): 2901-2911(in Chinese). doi: 10.7524/j.issn.0254-6108.2021022301
[10] 彭建彪, 贺冰冰, 顾梦瑶, 等. 磁性氧化石墨烯活化过一硫酸盐去除水中阿托伐他汀 [J]. 环境化学, 2020, 39(10): 2869-2877. doi: 10.7524/j.issn.0254-6108.2020060702 PENG J B, HE B B, GU M Y, et al. Efficient removal of atorvastatin in aqueous solution via peroxymonosulfate activated by magnetic graphene oxide [J]. Environmental Chemistry, 2020, 39(10): 2869-2877(in Chinese). doi: 10.7524/j.issn.0254-6108.2020060702
[11] 刘萌, 胡莉敏, 张广山, 等. Co/Zn双金属氧化物活化过一硫酸盐降解双酚A的性能研究 [J]. 环境化学, 2018, 37(4): 753-760. doi: 10.7524/j.issn.0254-6108.2017081605 LIU M, HU L M, ZHANG G S, et al. Activation of peroxymonosulfate by the Co/Zn bimetallic oxide for the degradation of bisphenol A [J]. Environmental Chemistry, 2018, 37(4): 753-760(in Chinese). doi: 10.7524/j.issn.0254-6108.2017081605
[12] ZHAO Y L, YUAN X Z, LI X D, et al. Burgeoning prospects of biochar and its composite in persulfate-advanced oxidation process [J]. Journal of Hazardous Materials, 2021, 409: 124893. doi: 10.1016/j.jhazmat.2020.124893 [13] ZHOU X R, ZHU Y, NIU Q Y, et al. New notion of biochar: A review on the mechanism of biochar applications in advannced oxidation processes [J]. Chemical Engineering Journal, 2021, 416: 129027. doi: 10.1016/j.cej.2021.129027 [14] SONG G, QIN F Z, YU J F, et al. Tailoring biochar for persulfate-based environmental catalysis: Impact of biomass feedstocks [J]. Journal of Hazardous Materials, 2022, 424: 127663. doi: 10.1016/j.jhazmat.2021.127663 [15] NIDHEESH P V, GOPINATH A, RANJITH N, et al. Potential role of biochar in advanced oxidation processes: A sustainable approach [J]. Chemical Engineering Journal, 2021, 405: 126582. doi: 10.1016/j.cej.2020.126582 [16] YE S J, ZENG G M, TAN X F, et al. Nitrogen-doped biochar fiber with graphitization from Boehmeria nivea for promoted peroxymonosulfate activation and non-radical degradation pathways with enhancing electron transfer [J]. Applied Catalysis B:Environmental, 2020, 269: 118850. doi: 10.1016/j.apcatb.2020.118850 [17] LENG L J, XU S Y, LIU R F, et al. Nitrogen containing functional groups of biochar: An overview [J]. Bioresource Technology, 2020, 298: 122286. doi: 10.1016/j.biortech.2019.122286 [18] LI X, JIA Y, ZHOU M H, et al. High-efficiency degradation of organic pollutants with Fe, N co-doped biochar catalysts via persulfate activation [J]. Journal of Hazardous Materials, 2020, 397: 122764. doi: 10.1016/j.jhazmat.2020.122764 [19] LIU C, CHEN L W, DING D H, et al. From rice straw to magnetically recoverable nitrogen doped biochar: Efficient activation of peroxymonosulfate for the degradation of metolachlor [J]. Applied Catalysis B:Environmental, 2019, 254: 312-320. doi: 10.1016/j.apcatb.2019.05.014 [20] ZHU K, BIN Q, SHEN Y Q, et al. In-situ formed N-doped bamboo-like carbon nanotubes encapsulated with Fe nanoparticles supported by biochar as highly efficient catalyst for activation of persulfate (PS) toward degradation of organic pollutants [J]. Chemical Engineering Journal, 2020, 402: 126090. doi: 10.1016/j.cej.2020.126090 [21] XU L, FU B R, SUN Y, et al. Degradation of organic pollutants by Fe/N co-doped biochar via peroxymonosulfate activation: Synthesis, performance, mechanism and its potential for practical application [J]. Chemical Engineering Journal, 2020, 400: 125870. doi: 10.1016/j.cej.2020.125870 [22] LI Y, YANG T, QIU S H, et al. Uniform N-coordinated single-atomic iron sites dispersed in porous carbon framework to activate PMS for efficient BPA degradation via high-valent iron-oxo species [J]. Chemical Engineering Journal, 2020, 389: 124382. doi: 10.1016/j.cej.2020.124382 [23] WU S H, LIU H Y, YANG C P, et al. High-performance porous carbon catalysts doped by iron and nitrogen for degradation of bisphenol F via peroxymonosulfate activation [J]. Chemical Engineering Journal, 2020, 392: 123683. doi: 10.1016/j.cej.2019.123683 [24] CHEN L K, HUANG Y F, ZHOU M L, et al. Nitrogen-doped porous carbon encapsulating iron nanoparticles for enhanced sulfathiazole removal via peroxymonosulfate activation [J]. Chemosphere, 2020, 250: 126300. doi: 10.1016/j.chemosphere.2020.126300 [25] YU J F, TANG L, PANG Y, et al. Magnetic nitrogen-doped sludge-derived biochar catalysts for persulfate activation: Internal electron transfer mechanism [J]. Chemical Engineering Journal, 2019, 364: 146-159. doi: 10.1016/j.cej.2019.01.163 [26] LI Y C, XING B, WANG X L, et al. Nitrogen-doped hierarchical porous biochar derived from corn stalks for phenol-enhanced adsorption [J]. Energy & Fuels, 2019, 33(12): 12459-12468. [27] YAN X H, XU B Q. Mesoporous carbon material co-doped with nitrogen and iron (Fe–N–C): High-performance cathode catalyst for oxygen reduction reaction in alkaline electrolyte [J]. J Mater Chem A, 2014, 2(23): 8617-8622. doi: 10.1039/C3TA15300B [28] LIU J H, KANG X, HE X, et al. Temperature-directed synthesis of N-doped carbon-based nanotubes and nanosheets decorated with Fe (Fe3O4, Fe3C) nanomaterials [J]. Nanoscale, 2019, 11(18): 9155-9162. doi: 10.1039/C9NR01601E [29] DONG X P, CHEN H R, ZHAO W R, et al. Synthesis and magnetic properties of mesostructured γ-Fe2O3/carbon composites by a co-casting method [J]. Chemistry of Materials, 2007, 19(14): 3484-3490. doi: 10.1021/cm0709065 [30] AI T, JIANG X J, LIU Q Y, et al. Single-component and competitive adsorption of tetracycline and Zn(ii) on an NH4Cl-induced magnetic ultra-fine buckwheat peel powder biochar from water: Studies on the kinetics, isotherms, and mechanism [J]. RSC Advances, 2020, 10(35): 20427-20437. doi: 10.1039/D0RA02346A [31] RONG X, XIE M, KONG L S, et al. The magnetic biochar derived from banana peels as a persulfate activator for organic contaminants degradation [J]. Chemical Engineering Journal, 2019, 372: 294-303. doi: 10.1016/j.cej.2019.04.135 [32] WANG Y B, ZHAO H Y, ZHAO G H. Iron-copper bimetallic nanoparticles embedded within ordered mesoporous carbon as effective and stable heterogeneous Fenton catalyst for the degradation of organic contaminants [J]. Applied Catalysis B:Environmental, 2015, 164: 396-406. doi: 10.1016/j.apcatb.2014.09.047 [33] XU Z H, ZHOU Y W, SUN Z H, et al. Understanding reactions and pore-forming mechanisms between waste cotton woven and FeCl3 during the synthesis of magnetic activated carbon [J]. Chemosphere, 2020, 241: 125120. doi: 10.1016/j.chemosphere.2019.125120 [34] XU Y, LUO G Q, HE S W, et al. Efficient removal of elemental mercury by magnetic chlorinated biochars derived from co-pyrolysis of Fe(NO3)3-laden wood and polyvinyl chloride waste [J]. Fuel, 2019, 239: 982-990. doi: 10.1016/j.fuel.2018.11.102 [35] CHEN Y, DU L, LI S G, et al. Pyrolysis of antibiotic mycelial dreg and characterization of obtained gas, liquid and biochar [J]. Journal of Hazardous Materials, 2021, 402: 123826. doi: 10.1016/j.jhazmat.2020.123826 [36] TSANEVA V N, KWAPINSKI W, TENG X, et al. Assessment of the structural evolution of carbons from microwave plasma natural gas reforming and biomass pyrolysis using Raman spectroscopy [J]. Carbon, 2014, 80: 617-628. doi: 10.1016/j.carbon.2014.09.005 [37] OH W D, LISAK G, WEBSTER R D, et al. Insights into the thermolytic transformation of lignocellulosic biomass waste to redox-active carbocatalyst: Durability of surface active sites [J]. Applied Catalysis B:Environmental, 2018, 233: 120-129. doi: 10.1016/j.apcatb.2018.03.106 [38] MIAN M M, LIU G J. Activation of peroxymonosulfate by chemically modified sludge biochar for the removal of organic pollutants: Understanding the role of active sites and mechanism [J]. Chemical Engineering Journal, 2020, 392: 123681. doi: 10.1016/j.cej.2019.123681 [39] LI L, LAI C, HUANG F L, et al. Degradation of naphthalene with magnetic bio-char activate hydrogen peroxide: Synergism of bio-char and Fe-Mn binary oxides [J]. Water Research, 2019, 160: 238-248. doi: 10.1016/j.watres.2019.05.081 [40] LU Z W, LIU B C, DAI W L, et al. Carbon network framework derived iron-nitrogen co-doped carbon nanotubes for enhanced oxygen reduction reaction through metal salt-assisted polymer blowing strategy [J]. Applied Surface Science, 2019, 463: 767-774. doi: 10.1016/j.apsusc.2018.08.231 [41] LI Q, ZHU W L, FU J J, et al. Controlled assembly of Cu nanoparticles on pyridinic-N rich graphene for electrochemical reduction of CO2 to ethylene [J]. Nano Energy, 2016, 24: 1-9. doi: 10.1016/j.nanoen.2016.03.024 [42] LONG Y K, HUANG Y X, WU H Y, et al. Peroxymonosulfate activation for pollutants degradation by Fe-N-codoped carbonaceous catalyst: Structure-dependent performance and mechanism insight [J]. Chemical Engineering Journal, 2019, 369: 542-552. doi: 10.1016/j.cej.2019.03.097 [43] LI K X, CHEN W, YANG H P, et al. Mechanism of biomass activation and ammonia modification for nitrogen-doped porous carbon materials [J]. Bioresource Technology, 2019, 280: 260-268. doi: 10.1016/j.biortech.2019.02.039 [44] LI Z H, XING B, DING Y, et al. A high-performance biochar produced from bamboo pyrolysis with in situ nitrogen doping and activation for adsorption of phenol and methylene blue [J]. Chinese Journal of Chemical Engineering, 2020, 28(11): 2872-2880. doi: 10.1016/j.cjche.2020.03.031 [45] YANG Z, WANG Z W, LIANG G W, et al. Catalyst bridging-mediated electron transfer for nonradical degradation of bisphenol A via natural manganese ore-cornstalk biochar composite activated peroxymonosulfate [J]. Chemical Engineering Journal, 2021, 426: 131777. doi: 10.1016/j.cej.2021.131777 [46] 韩仪, 黄明杰, 周涛, 等. 氧化铜活化过硫酸盐的界面反应机理 [J]. 环境化学, 2020, 39(3): 735-744. doi: 10.7524/j.issn.0254-6108.2019110101 HAN Y, HUANG M J, ZHOU T, et al. Interfacial reaction mechanism of copper oxide activating persulfate [J]. Environmental Chemistry, 2020, 39(3): 735-744(in Chinese). doi: 10.7524/j.issn.0254-6108.2019110101
[47] YIN R L, GUO W Q, WANG H Z, et al. Enhanced peroxymonosulfate activation for sulfamethazine degradation by ultrasound irradiation: Performances and mechanisms [J]. Chemical Engineering Journal, 2018, 335: 145-153. doi: 10.1016/j.cej.2017.10.063 [48] CHEN X, ZHOU J, YANG H W, et al. PMS activation by magnetic cobalt-N-doped carbon composite for ultra-efficient degradation of refractory organic pollutant: Mechanisms and identification of intermediates [J]. Chemosphere, 2022, 287: 132074. doi: 10.1016/j.chemosphere.2021.132074 [49] ZHANG Z L, DING H, LI Y, et al. Nitrogen-doped biochar encapsulated Fe/Mn nanoparticles as cost-effective catalysts for heterogeneous activation of peroxymonosulfate towards the degradation of bisphenol-A: Mechanism insight and performance assessment [J]. Separation and Purification Technology, 2022, 283: 120136. doi: 10.1016/j.seppur.2021.120136 [50] DING Y B, WANG X R, FU L B, et al. Nonradicals induced degradation of organic pollutants by peroxydisulfate (PDS) and peroxymonosulfate (PMS): Recent advances and perspective [J]. Science of the Total Environment, 2021, 765: 142794. doi: 10.1016/j.scitotenv.2020.142794 期刊类型引用(2)
1. 周忠城,孙春暖,何子阳,宋小宇,吕强汝,赵健,邹海明. 2种磁性藻渣碳基功能材料的制备及其对养殖废水中抗生素去除效果. 安徽科技学院学报. 2025(02): 35-41 .  百度学术
百度学术
2. 贾华,牛建瑞,高玮,芮遨宇,余铭浩,陈梦,郭延凯. 电子转移主导的载钴生物炭活化PMS降解四环素. 环境科学学报. 2024(03): 51-60 .  百度学术
百度学术
其他类型引用(9)
-














 下载:
下载: