-
木犀草素(luteolin,LTL)是一种含有顺式二羟基结构的黄酮类化合物,广泛存在于植物、蔬菜和水果中,在废弃花生壳中具有较高的含量[1-2],花生壳作为一种常见的农业固体废弃物,通常被直接焚烧或者掩埋处理,从而导致资源浪费和环境污染[3]. LTL具有多种药理作用,如抗炎、抗癌、抗氧化等,对人体神经有一定的保护作用[4-6]. LTL的高医疗价值使其分离和提取具有重要意义. 硼亲和(boronate affinity,BA)材料是一种可以选择性地分离和富集顺式二羟基生物分子的功能材料,因硼酸配体可以在碱性溶液中与顺式二羟基形成稳定的共价五元或六元环状酯,而共价键可以在酸性溶液中可逆解离[7],被广泛应用于食品检测,生物分离等领域[8-10]. 但单个硼酸单体与含有顺式二羟基的化合物的结合强度相对较弱[11]. 因此,诸多学者在构建硼亲和功能化材料投入了相当大的努力[12-13].
由无机节点和有机配体组成的金属有机骨架材料(metal–organic frameworks,MOFs)具有高比表面积和可调孔径的特性,在分离领域具有广阔的前景[14-15]. 顾金楼课题组[16]通过金属配体片段共组装(MLFC)策略将3,5-二羧基苯硼酸作为配体片段引入了硼亲和功能组分,制备了MIL-100-B MOFs. 通过改变3,5-二羧基苯硼酸和1,3,5-苯三甲酸的配比调节MIL-100-B上硼酸的含量. UiO-66是一种典型的三维微孔金属有机骨架材料,以1,4-苯二甲酸(BDC)为有机配体,锆(Zr)为无机节点,具有优异的物理、化学和水热稳定性,其较大的比表面积也让UiO-66成为吸附分离材料研究热点之一[17-19].
本研究采用MLFC策略制备了硼亲和功能化UiO-66(UiO-66-BA)并用于对LTL的吸附分离. 引入与BDC结构相似的4-羧基苯硼酸(CPBA)作为硼亲和功能组分,与BDC作为混合有机配体. 研究改变CPBA/BDC的摩尔比对UiO-66-BA的晶体骨架结构和硼酸基团数量的影响. 结合场发射扫描电子显微镜(Field Emission Scanning Electron Microscope, FESEM)、X-射线粉末衍射图谱(X-ray diffraction, XRD)、Brunauer-Emmett-Teller (BET) 比表面积、孔容孔径和核磁共振等考查UiO-66-BA的理化性能,考察了溶液pH、初始浓度、吸附时间和吸附温度对UiO-66-BA吸附LTL性能的影响.
-
木犀草素(LTL,97%)购自上海安耐吉化学试剂有限公司;1,4-对苯二甲酸(BDC,99%)、4-羧基苯硼酸(CPBA,97%)、乙酸和N,N-二甲基甲酰胺(DMF)购自上海阿拉丁试剂有限公司;四氯化锆(ZrCl4,98%)、盐酸(HCl,37 %)、甲醇、乙醇和氢氧化钠(NaOH)购自国药集团化学试剂有限公司. 实验所用水均为去离子水.
-
采用MLFC策略,参考Erkartal等的方法并进行改进[20]:首先称取0.699 g ZrCl4,不同质量的BDC和CPBA(保证BDC和CPBA总摩尔量与ZrCl4摩尔量之比为1:1,具体质量与摩尔比见表1),然后将其加入到70 mL DMF中,滴加5 mL 乙酸,超声分散处理30 min后,将混合溶液放入聚四氟乙烯高压反应釜中,150℃下反应24 h. 待产物冷却至室温,离心收集固体产物,用DMF和乙醇分别清洗数次并在150℃真空烘箱内烘干24 h,得到的UiO-66和UiO-66-BA样品密封保存备用.
-
使用蔡司SUPRA-55场发射扫描电子显微镜(FESEM)对样品表面形貌进行分析;使用Bruker APEX II DUO X射线粉末衍射仪(XRD)对样品的晶体结构进行测定;使用Tristar3020气体吸附仪进行N2吸附-脱附研究,测定BET比表面积以及孔容孔径,其中样品在120℃条件下脱气24 h;使用Bruker 400M数字化核磁共振谱仪测定样品的1H NMR光谱,所用溶剂为1 mol·L−1 NaOH/D2O溶液;使用Thermo Scientific K-Alpha 测定样品的X-ray photoelectron spectroscopy (XPS)光谱.
-
实验用所制得的样品作为吸附剂吸附溶液中LTL,系统考察pH、溶液初始浓度、吸附温度以及吸附时间对吸附剂的吸附特性的影响. 通过紫外分光光度计(UV-2450,日本-岛津)在352 nm 处测定LTL的浓度,吸附量(Qe, mg·g−1)的计算公式如下所示:
其中,C0 (mg·L−1)为LTL的初始浓度;Ce (mg·L−1)为吸附后LTL的剩余浓度;V (mL)为LTL溶液体积,m (mg)为吸附剂的质量.
具体实验步骤如下:
(1)溶液初始pH的影响:首先分别称取10.0 mg 吸附剂加入到10 mL离心管中,随后加入10 mL pH 分别为5.5、7.0、8.5和10.0的初始浓度为20 mg·L−1 的LTL溶液,置于25℃恒温振荡器中吸附120 min.
(2)吸附等温线实验:分别称取10.0 mg UiO-66、UiO-66-BA-2、UiO-66-BA-4 加入到10 mL 离心管中,随后加入10 mL pH 为8.5、初始浓度分别为10、20、30、40、50、100 mg·L−1的LTL溶液,置于25℃和35℃恒温振荡器中吸附120 min.
(3)吸附动力学实验:分别称取10.0 mg UiO-66、UiO-66-BA-2、UiO-66-BA-4 加入到10 mL 离心管中,随后加入10 mL pH 为8.5、初始浓度为20 mg·L−1的LTL溶液,置于25℃恒温振荡器中分别吸附5、10、15、30、60、120、180、240、360 min.
(4)吸附再生实验:分别称取10.0 mg UiO-66、UiO-66-BA-2、UiO-66-BA-4 加入到10 mL 离心管中,随后加入10 mL pH 为8.5、浓度为20 mg·L−1的LTL溶液,置于25℃恒温振荡器中吸附120 min,过滤,通过紫外分光光度计测定吸附后剩余LTL浓度. 将吸附后的材料分散在体积比为1:1的甲醇/乙酸溶液中进行洗脱,然后用去离子水洗涤数次后收集,重复上述吸附实验4次,利用公式(1)计算材料的吸附容量,研究材料的吸附再生性能.
-
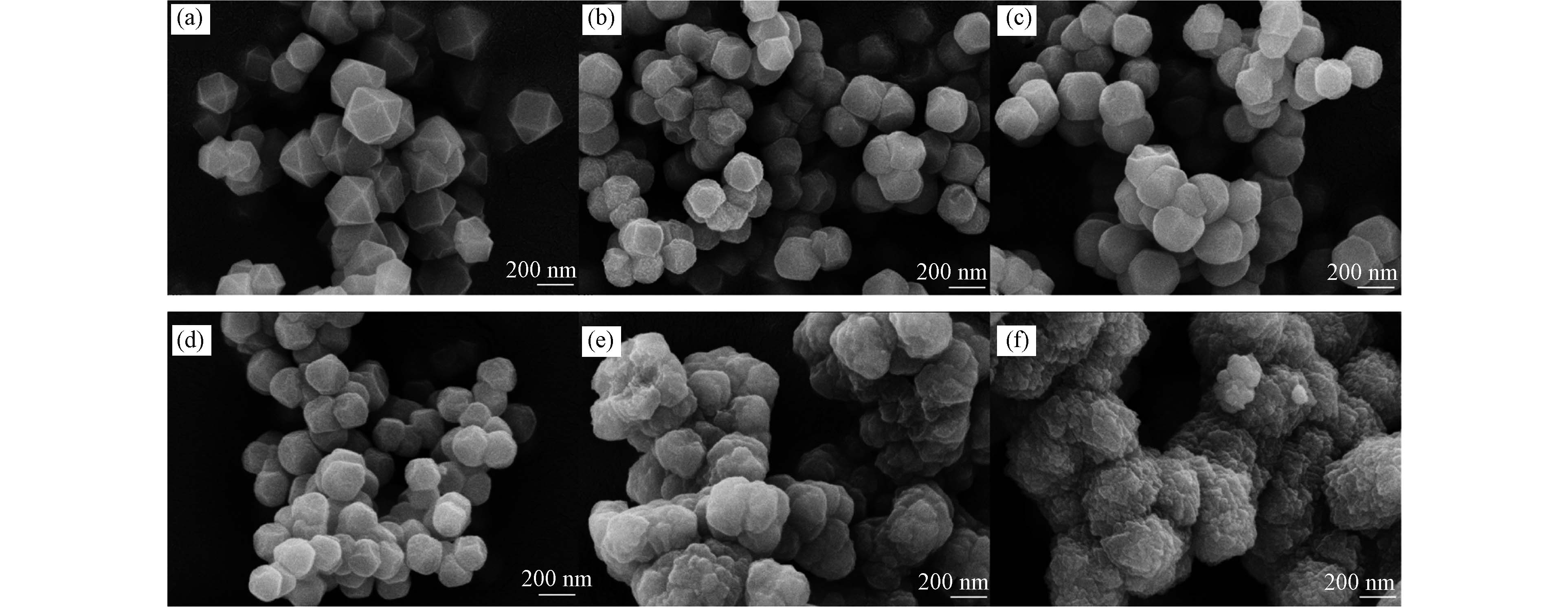
试验所制备样品的FESEM分析结果如图1所示. 从图1(a)可以看出,UiO-66颗粒边缘结构明显,形状规则,分散程度较好,具有均匀的尺寸和良好的晶体结构. 从图1(b)到(d)中可以发现,当CPBA的摩尔比在CPBA/BDC总摩尔量的30%以内时,所制备的UiO-66-BA-1、UiO-66-BA-2和UiO-66-BA-3有较明显的晶体结构,但伴随着轻微的团聚现象. 随着CPBA用量的增加,如图1(e)和(f)所示,UiO-66-BA-4和UiO-66-BA-5的晶体结构逐渐破坏,形貌逐渐变化为不规则的球形,尺寸大小不一,颗粒之间团聚现象加重.
-
为在不改变UiO-66晶体结构的前提下,将尽可能多的硼酸单体引入框架中,以不同的摩尔比例向材料中加入BDC和CPBA,不同比例样品的XRD分析结果如图2所示. UiO-66的XRD图谱上在2θ为7.25°和8.39°处显示出特征峰,这与Ediati等的研究结果一致[21]. 从分析结果可见,UiO-66-BA-1,UiO-66-BA-2和UiO-66-BA-3也保持了良好的晶体结构,表明适量的硼酸单体CPBA的引入使UiO-66仍可以很好地保持其晶体结构. 当CPBA/BDC的摩尔比达到0.4:0.6和0.5:0.5时,XRD的衍射峰变宽且减弱,分析可能是由于硼酸部分的不协调位点的增加导致材料的结晶度降低.
-
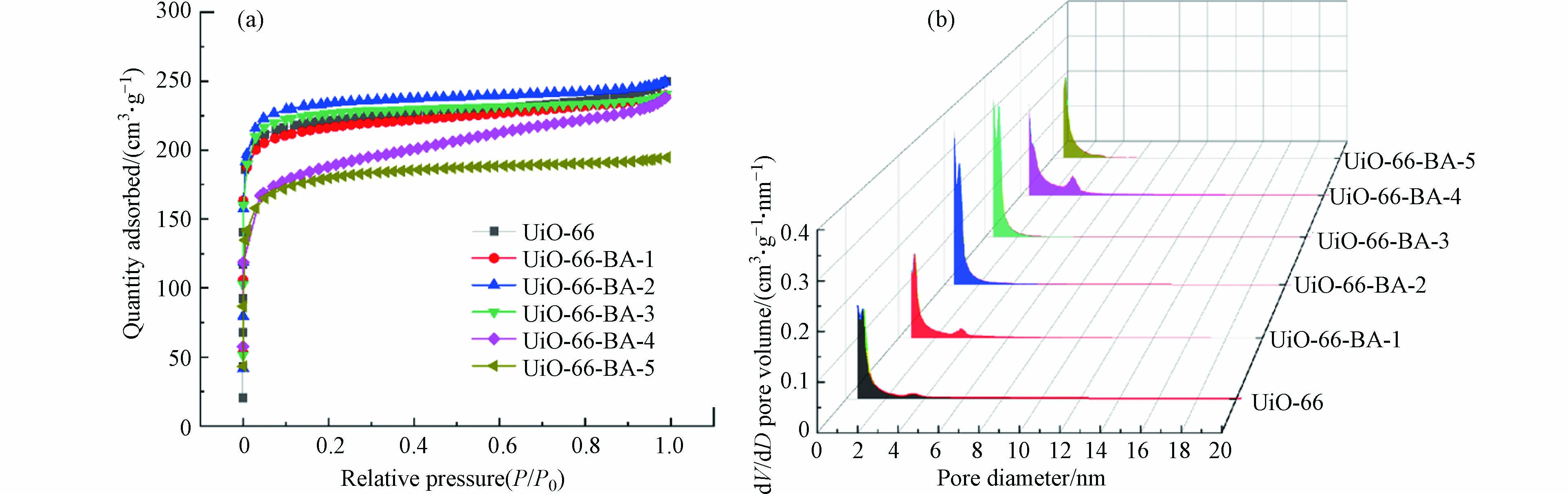
样品的BET和孔容孔径分析结果如图3和表2所示. 图3(a)所示制备样品的的氮气吸附-脱附曲线均为IUPAC分类的I型曲线,其吸附量在相对较低的压力下迅速增加,表明所制备样品均存在微孔结构[22]. 样品的孔容孔径分析结果如图3(b)所示,所制备UiO-66及UiO-66-BA样品含有大量的微孔. 表明CPBA的引入并没有消除UiO-66的骨架结构,且CPBA成功地被纳入到骨架中,而不是封闭在孔隙中. UiO-66的BET比表面积为734.35 m2·g−1,与Mohammadi的报告相似[23]. UiO-66-BA-1、UiO-66-BA-2和UiO-66-BA-3的BET比表面积与UiO-66相近,但是UiO-66-BA-4和UiO-66-BA-5的BET比表面积显著下降,这可能是由于CPBA缺陷配体的增加使它们无法支撑整个框架[24],这与XRD的表征结果一致.
-
图4为所制备样品的1H NMR图谱. 从图4(a)和(b)中可以看出,BDC苯环上4个H的1H NMR特征峰为7.63 ppm,而CPBA含有7.48 ppm和 7.35 ppm两个特征峰,分别对应于CPBA苯环上两个不对称的H(标记为Ha和Hb). 从图4(c)和(d)中可以发现,UiO-66-BA均含有BDC和CPBA的特征峰,且CPBA的特征峰的相对强度随着CPBA摩尔比例的增加而增强,表明了CPBA被成功地引入所制备的金属-有机骨架材料中去.
-
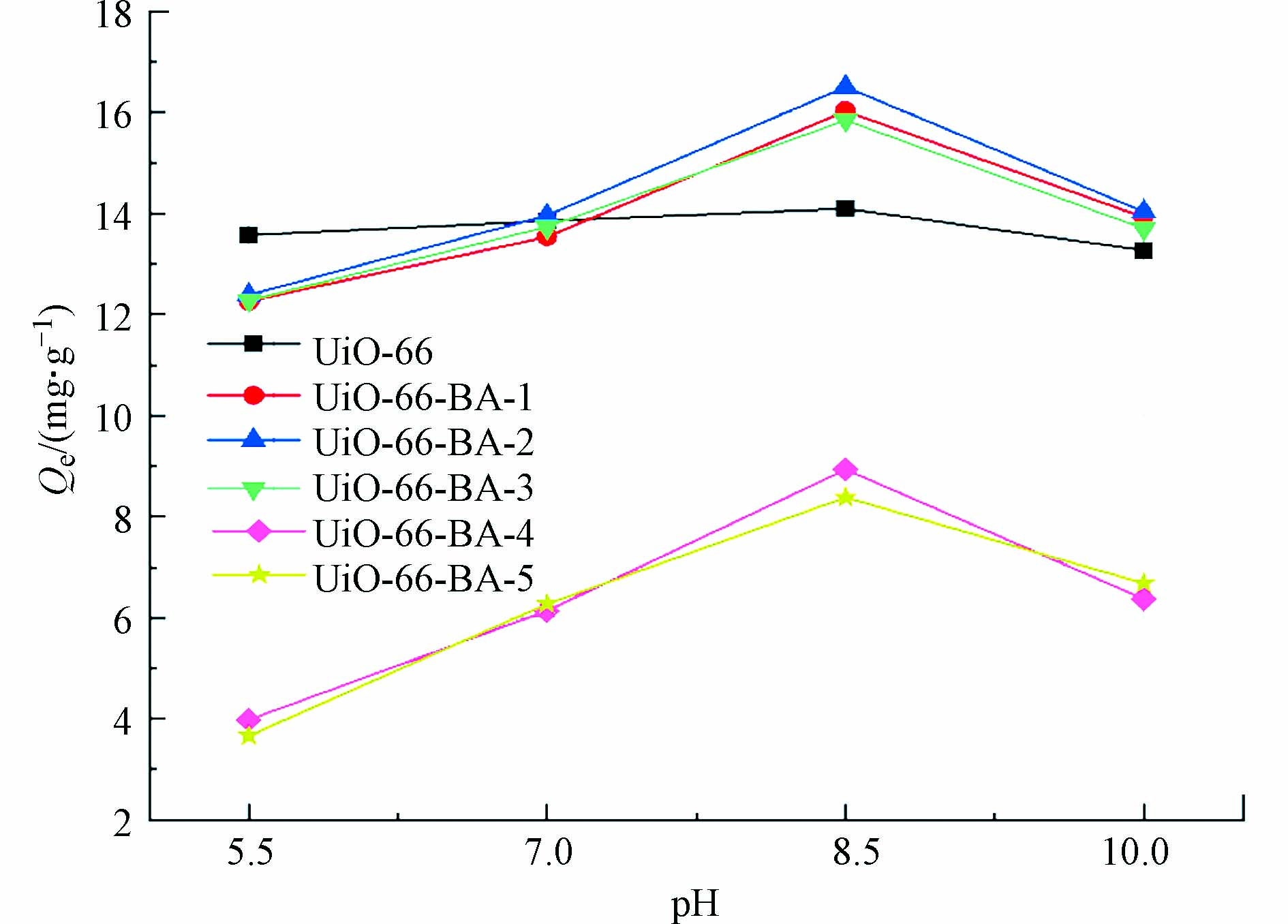
作为硼亲和作用最重要的参数之一,可以通过调控pH值来实现吸附/解吸. 本实验研究了在pH分别为5.5,7.0,8.5和10.0对所制备样品吸附能力的影响. 如图5所示,UiO-66在酸碱条件下对LTL的吸附都有着良好的效果,当pH=8.5时,UiO-66-BA-1、UiO-66-BA-2和UiO-66-BA-3的吸附能力达到最大,且相比较于UiO-66,吸附效果有着明显提升,分析是由于CPBA硼酸配体的引入与氢氧根发生络合反应,从而与LTL中顺式二羟基发生共价作用形成五元或者六元环酯,有利于其对LTL的吸附. 在酸性条件下,UiO-66-BA-1、UiO-66-BA-2和UiO-66-BA-3的吸附能力下降,分析是由于pH值低于硼酸基团的pKa,形成的五元环酯被解离[25]. 当pH = 10.0时,溶液中过多的氢氧根使得LTL的顺式二羟基不够稳定,导致其结合能力变弱[1]. 同时,UiO-66-BA-4和UiO-66-BA-5也遵循相同的规律,但由于自身框架结晶度的降低和BET比表面积的减小,导致它们的吸附能力降低.
-
结合理化表征和pH实验结果,选择UiO-66、UiO-66-BA-2和UiO-66-BA-4作为吸附剂,采用不同溶液初始浓度进一步研究其吸附LTL的性能. 利用Langmuir和Freundlich等温吸附模型[26]对所得实验数据进行拟合,两个模型的拟合方程如式(2)、(3)所示.
其中,Qm (mg·g−1)表示Langmuir方程拟合的吸附剂最大吸附量;KL (L·mg−1)表示Langmuir等温模型的亲和常数;KF (mg·g−1)表示Freundlich等温模型的吸附能力常数,1/n是吸附强度常数. 此外,使用分离因子RL来判断实验条件是否有利,其表达式如(4)所示:
其中,Cm (mg·g−1)表示LTL的最大初始浓度.
等温吸附实验及拟合结果如图6和表3所示. 图6中UiO-66、UiO-66-BA-2和UiO-66-BA-4的吸附平衡曲线表明,吸附剂的吸附能力在初始阶段均急剧增加,随后缓慢达到吸附平衡. UiO-66-BA-2的吸附能力明显优于UiO-66和UiO-66-BA-4,表明硼亲和吸附位点的增加可以有效提高UiO-66对LTL的吸附能力. 但是过量的硼酸基团使UiO-66的骨架结构受到影响,UiO-66-BA-4的吸附性能下降. 从表3中可以看出,Langmuir等温模型的相关系数(R2)高于Freundlich等温模型,表明吸附过程以单分子层吸附为主. 同时,RL值都在0—1之间,表明吸附条件都是有利的. 此外,吸附剂在35℃时的吸附能力大于25℃时的吸附能力,表明吸附剂对LTL的吸附属于吸热反应[27].
-
UiO-66、UiO-66-BA-2和UiO-66-BA-4在25℃ 时对LTL的吸附动力学实验结果如图7所示,在最初的60 min 内,吸附剂的吸附能力均迅速增加. 120 min 后,动力学曲线逐渐趋于平缓,吸附进入内层扩散阶段,基本达到吸附平衡. 从图7可见,UiO-66-BA-2的吸附能力优于UiO-66,表明pH = 8.5 时硼酸基团可以与LTL的顺式二羟基结合,从而增强吸附剂对LTL的吸附性能. 虽然UiO-66-BA-4含有较多的硼酸基团,但其骨架结构较差比表面降低,且单一的硼酸单体与顺式二羟基化合物结合能力较差[11],导致UiO-66-BA-4的吸附能力低于UiO-66.
采用准一级和准二级动力学方程对所得数据进行拟合,两个模型的方程如(5)和(6)所示,初始吸附速率h (mg ·g−1· min−1)的计算公式如(7)所示:
其中,Qt (mg g−1)表示在 t 时刻LTL的吸附量,k1 (L·min−1)和k2 (g·mg−1·min−1)分别表示准一级和准二级动力学模型的吸附速率常数.
表4列出了准一级和准二级动力学模型计算的相关参数,UiO-66-BA-2的初始吸附速率h值最高,意味着LTL溶液与UiO-66-BA-2之间的传质阻力最低[28]. 模型拟合相关系数(R2)表明准一级和准二级动力学模型都能很好地拟合UiO-66和UiO-66-BA-2的吸附过程,可见,UiO-66和UiO-66-BA-2对LTL的吸附过程包括物理吸附和化学吸附,其物理吸附主要为扩散作用和范德华力[29]. 但是更主要的速率控制步骤是LTL与硼酸有机配体之间的硼亲和力. UiO-66-BA-4对LTL的吸附过程更符合准二级动力学模型,表明吸附过程主要是化学吸附,即硼亲和作用.
-
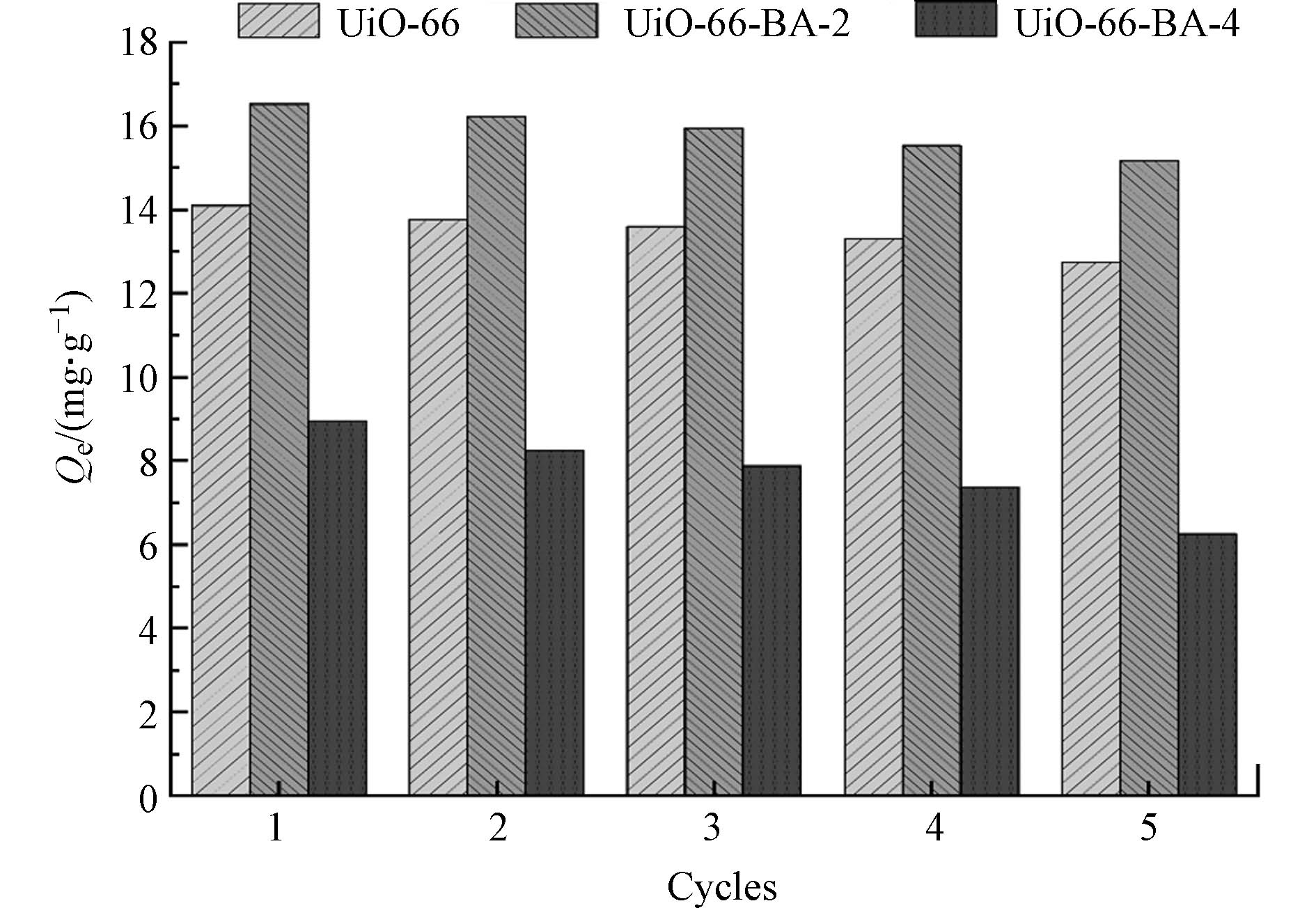
UiO-66,UiO-66-BA-2和UiO-66-BA-4的5次吸附-解吸再生实验结果如图8所示. 吸附在pH = 8.5条件下进行,解吸的洗脱液为体积比为1:1的甲醇/乙酸溶液. UiO-66-BA与LTL的结合的示意图如图9所示,在碱性条件下,LTL的顺式二羟基与UiO-66-BA的四面体硼离子形成五元或六元环酯(sp3),在酸性条件下,硼原子从sp3杂化状态转变为sp2杂化,从而使得形成的共价键解离,LTL释放到溶液中[30]. 图10为UiO-66,UiO-66-BA-2和吸附后UiO-66-BA-2的XPS谱图,由于B 1s (182.2 eV/184.8 eV) 的特征峰与Zr 3d (183.5 eV/185.9 eV)的重叠,且B 1s特征峰的灵敏度特别低[16],在图10(a)中无法很好地显示出B 1s的特征峰. 图10(b)中,UiO-66-BA-2以及吸附后的UiO-66-BA-2谱图中B元素特征峰的出现表明了CPBA成功地引入到了材料中,且图10(a)中可以发现,吸附后的UiO-66-BA-2谱图中的O和C元素的特征峰均明显变强,表明LTL与UiO-66-BA-2成功地结合.
经5次吸附-解吸后,UiO-66-BA-2的吸附能力降低8.28%. 而这种轻微的下降可能是由于UiO-66-BA-2的质量损失,且化学吸附导致了一些不可再生或无用的吸附位点的产生. 同时,UiO-66-BA-4的吸附能力损失较大(30.37%),是由于其框架结构不稳定和吸附位点的损失. 总体而言,UiO-66-BA-2具有良好的再生循环能力.
-
本研究选用了与BDC结构相似的CPBA作为硼亲和功能组分引入到UiO-66晶体骨架中,采用MLFC策略制备了硼亲和功能化MOFs,即UiO-66-BA. 研究不同的CPBA/BDC摩尔比对UiO-66的晶体骨架和吸附性能的影响,主要结论如下:
(1)不引入CPBA时,所制备的UiO-66具有良好的晶体结构,BET比表面积为734.35 m2·g−1,由于UiO-66在酸性或碱性溶液中较为稳定,使得pH对LTL的吸附性能影响较小. 经5次吸附循环后,UiO-66对LTL的吸附能力下降了9.62%,再生性能较好.
(2)当CPBA的摩尔比在CPBA/BDC总摩尔量的30%以内时,所制备的UiO-66-BA能保持良好的晶体骨架结构,其BET比表面积与UiO-66相近,对LTL的吸附性能显著提高. 当pH = 8.5,温度为35℃时,UiO-66-BA-2的吸附能力最大理论值为42.509 mg g−1;UiO-66-BA-2对LTL的吸附过程符合Langmuir等温模型,以单分子层吸附为主;吸附过程属于吸热反应;动力学实验表明最主要的速率控制步骤是LTL与硼酸有机配体之间的硼亲和力;且UiO-66-BA-2具有良好的再利用性能.
(3)当CPBA的摩尔量占比达到CPBA/BDC总摩尔量的40%和50%时,所制备的UiO-66-BA-4和UiO-66-BA-5的结晶度降低,BET比表面积减小,对LTL的吸附性能显著降低,再生性能也下降. 过量的CPBA不利于UiO-66骨架结构的稳定性.
硼亲和功能化MOFs的制备及其吸附木犀草素性能研究
Construction of boronate affinity functionalized MOFs for the adsorption and separation of Luteolin
-
摘要: 本研究通过金属配体片段共组装(MLFC)策略引入4-羧基苯硼酸(CPBA)作为硼亲和功能成分,和1,4-对苯二甲酸(BDC)作为混合有机配体,制备了硼酸亲和功能化MOFs(UiO-66-BA). 研究了不同CPBA/BDC的摩尔比对UiO-66-BA框架结晶度和对木犀草素(LTL)吸附性能的影响. 结果表明,当CPBA的摩尔比大于CPBA/BDC总摩尔量的30%时,会导致UiO-66-BA吸附剂的框架结晶度降低,BET比表面积减小,吸附性能下降,再生性能较差. 适量CPBA的添加(摩尔比≤30%)有利于UiO-66-BA保持良好的晶体结构,提高对LTL的吸附性能和吸附剂的再生能力. CPBA的摩尔比为20%时制得的UiO-66-BA-2在pH = 8.5、温度为35℃时对LTL的最大吸附量达到42.509 mg·g−1,吸附过程更符合Langmuir等温吸附模型和准二级动力学模型,吸附再生结果表明,UiO-66-BA-2具有良好的可重用性,经5次吸附-解吸附后吸附量下降8.28%. 由于存在硼酸识别位点,UiO-66-BA-2相比于UiO-66具有更高的吸附容量以及更快的吸附效率,为吸附分离木犀草素提供了一种新的思路.
-
关键词:
- 木犀草素 /
- 金属有机框架(MOFs) /
- UiO-66 /
- 硼亲和 /
- 吸附
Abstract: In this study, the boronate affinity functionalized MOFs (UiO-66-BA) was prepared by introducing 4-carboxyphenyboronic (CPBA) acid as a functional component through the metal–ligand–fragment coassembly (MLFC) strategy. The framework crystallinity and Luteolin (LTL) adsorption properties of the MOFs with different molar ratios of CPBA/1,4-dicarboxybenzene (BDC) were studied. The results showed that when the molar proportion of CPBA was greater than 30% of the total molarity of CPBA/BDC, the frame crystallinity and the Brunauer-Emmett-Teller (BET) specific surface area of the UiO-66-BA adsorbent were reduced. The adsorption performance and the regeneration performance of the UiO-66-BA were poor. The addition of an appropriate amount of CPBA (≤ 30%) was conducive to the UiO-66-BA to maintain a good crystal structure. The adsorption performance and regenerative capacity of the MOFs were improved. Among them, the equilibrium adsorption capacity of UiO-66-BA-2 reached 42.509 mg·g−1 when pH = 8.5 and the temperature was 35℃. The adsorption process of UiO-66-BA-2 was well fitted by the Langmuir equation and quasi-second-order kinetic equation. The results of regeneration experiment showed that UiO-66-BA-2 had excellent reusability and the adsorption amount drops by 8.28% after 5 adsorption-desorption. Compared with UiO-66, UiO-66-BA-2 has enhanced the binding properties to LTL due to the introduction of boronic acid, which provides a new idea for the adsorption and separation of LTL.-
Key words:
- luteolin /
- metal–organic frameworks (MOFs) /
- UiO-66 /
- boronate affinity /
- adsorption
-
酸性矿山废水( acid mine drainage, AMD )具有污染时间长、成分复杂、处理成本高等特点,国内处理AMD的工艺已发展得较为成熟,其中石灰、石灰石中和法在工程应用中最为广泛[1]。该类方法处理废水时会产生大量的沉渣,沉渣的成分主要为石膏和各类金属沉淀物。由于AMD的成分复杂,沉渣中的石膏晶体发育较差,沉渣中有价金属品位较低,利用难度较高[2]。因此,设计出一种能从AMD中回收高品质的石膏以及高品位金属沉渣的工艺具有现实意义。
大宝山位于韶关市曲江区沙溪镇、翁源县铁龙镇境内,矿种以硫矿为主。含硫化物的废矿石在硫杆菌的作用下,发生氧化产生AMD。AMD呈强酸性,含有大量有毒(类)金属元素,污染危害严重。近年,相关单位已开展多起针对大宝山AMD的修复工程,矿区下游的生态环境已得到明显改善;但尾矿库坝内的废水中仍含有高浓度的金属离子,是潜在的重金属污染源[3]。本研究使用的AMD均取自大宝山的尾矿废水,大宝山AMD中含有较高的SO42-、Fe2+/3+、Zn2+、Cu2+,采用石灰石中和法处理大宝山AMD时,回收的石膏不具备利用价值。原因是,废水中的各类金属离子会对中和石膏的结晶过程产生影响[4],导致其晶体发育较差,中和过程中废水中金属离子的沉淀也会导致石膏纯度降低。这类石膏杂质较多、游离水含量高、抗压性弱,难以用于工业生产[5]。
本研究以实现AMD的资源化处理为目标,采用氧化还原分步沉淀工艺,分别考察还原剂、回收石膏的工艺条件(石灰石投加次数、石灰石投加量、反应时间、搅拌速度)对回收石膏品质的影响;并采用正交实验优化回收石膏的工艺条件,对回收石膏的产量、纯度、成分进行分析;同时考察废水pH、曝气时间对废水中金属离子去除率的影响,确定分步回收金属的工艺条件,分析氧化还原-分步沉淀法处理AMD的经济效益,为AMD的资源化处理提供理论指导。
1. 实验部分
1.1 实验材料
实验原水取自韶关市大宝山AMD,废水pH为1.9~2.4,废水中金属离子主要为Fe2+、Fe3+、Cu2+、Zn2+,废水中Fe2+/3+、 Zn2+ 、Cu2+ 、Fe2+、 Ni2+、 SO42−的质量浓度分别为634~827、155~166、18~25、326~417、1.11~1.18、8 527~9 342 mg·L−1。实验所用的石灰石为工业级,盐酸羟胺、硫酸、氢氧化钠均为分析纯。
1.2 实验步骤与工艺流程
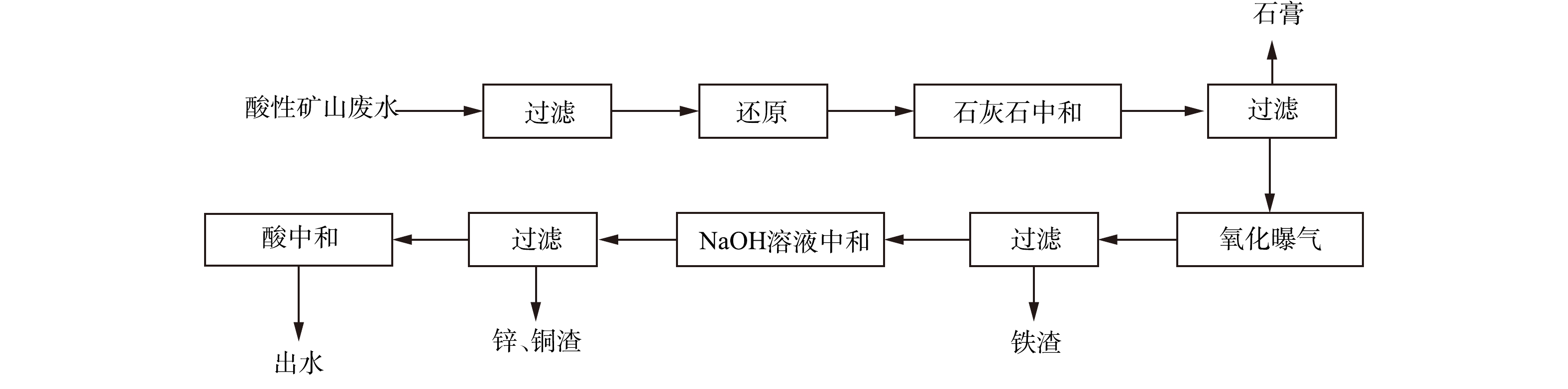
取2组500 mL实际废水,过滤除去悬浮物后,向其中一组加入一定量的盐酸羟胺,将废水中的Fe3+还原为Fe2+后;将一定量的石灰石分别加入2组废水中,反应45 min后,过滤得到沉渣,将沉渣干燥后,保存待测。 将废水中Fe3+还原后,将石灰石加入废水中,反应45 min后过滤,测量废水中金属离子浓度,再将滤渣干燥后,保存待测。 选取石灰石投加量为5~7 g·L−1,转速为150~250 r·min−1,投加3~5次、反应时间为30~60 min,设计L9(43)正交实验,并测定生成石膏的产量、纯度、游离水含量。在曝气回收废水Fe2+/3+实验中,往滤液中加入NaOH溶液,调节废水pH至某一值后曝气,曝气量为4 L·min−1,设置不同曝气时间,曝气后过滤,对沉渣进行分析,并测量废水中金属离子浓度。在NaOH溶液中和废水回收Zn2+、Cu2+实验中,向曝气后的滤液中加入NaOH溶液,继续调节废水pH至某一值,过滤后,将滤渣保存待测,并测量废水中金属离子浓度。氧化还原-分步沉淀工艺流程如图1所示。
1.3 分析检测方法
石膏游离水、结合水、纯度均按标准《石膏化学分析方法》 (GB/T 5484-2000) 的方法进行测定。废水中金属离子浓度用电感耦合等离子体质谱仪(美国PE公司NexION300D)进行测定。废水pH用PHS-3C 型酸度计进行测定。采用X射线荧光光谱仪(XRF光谱仪)分析沉渣中金属组成及含量。采用 Hitachi S-480扫描电子显微镜(SEM)分析石膏的形貌。采用X射线衍射(XRD)仪分析石膏的物相。采用热重分析(TG-DSC)仪分析石膏的质量随温度的变化情况。
2. 结果与讨论
2.1 还原剂对中和石膏的影响
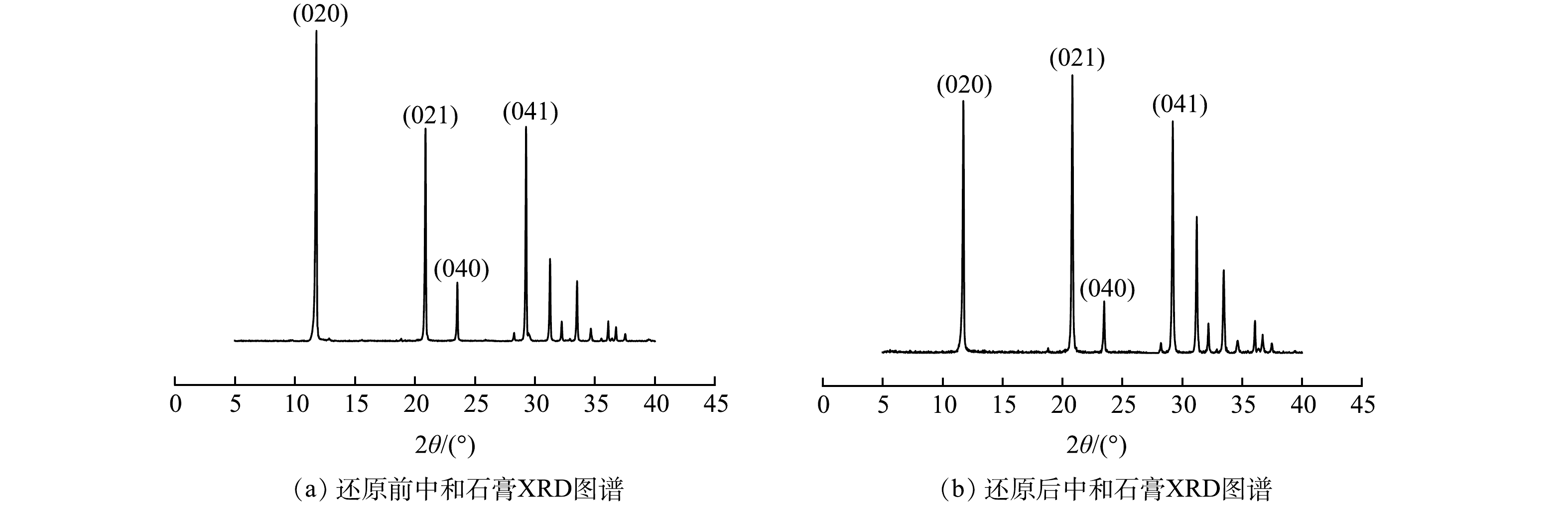
2组石膏的XRD结果如图2所示。图2(a)为0.6 g石灰石直接中和500 mL废水回收的石膏;图2(b)为投加0.1 g的盐酸羟胺将500 mL废水中的Fe3+还原后,用0.6 g石灰石中和废水回收的石膏。XRD结果表明,投加还原剂,将废水中的Fe3+还原为Fe2+后,并没有对沉渣中石膏晶体的矿物组成产生影响。沉渣中的水化产物主要为二水硫酸钙(PDF#33-0311),分别在2θ为11.617 °、20.722 °、23.380 °、29.103 °处出现了二水硫酸钙晶体的特征峰。
2组石膏的实物和形貌如图3所示。可以看出,图3(a)中的石膏为黄褐色,白度较低,当石膏含有杂质时,会呈现黄褐色、灰色等。SEM结果(图3(c))表明,石膏晶体呈颗粒状,粒径较小,晶体发育差。图3(b)中的石膏白度较高,颜色与天然石膏相近,所含杂质较少, SEM结果(图3(d))表明,晶体呈板柱状,与图3(a)石膏的晶体相比,粒径更大,晶体发育的更完整[6],这种石膏晶体抗压性更好,石膏游离水含量更低。
在石灰石中和废水的过程中,废水pH升高,废水中金属离子会形成氢氧化物沉淀,当废水中金属离子浓度低于10−5 mol·L −1 时,可视为该金属离子已完全沉淀[7]。根据氢氧化物的溶度积可分别计算出各金属离子开始沉淀和完全沉淀的理论pH。Fe(OH)2、Fe(OH)3、Cu(OH)2、Zn(OH)2的溶度积分别为8×10−16 、2.6×10−39、2.2×10−20、7.7×10−17,Fe3+开始沉淀的pH一般在2.11左右,完全沉淀的pH在3.5左右,而Fe2+、Cu2+、Zn2+开始沉淀的pH一般在6以上,完全沉淀的pH在9以上[8]。废水经过还原处理后,提高了石膏的纯度,减少了废水中Fe2+/3+的沉淀,提高了后续废水处理中Fe2+/3+的回收率。
2.2 石灰石投加量对石膏、废水pH及金属离子去除率的影响
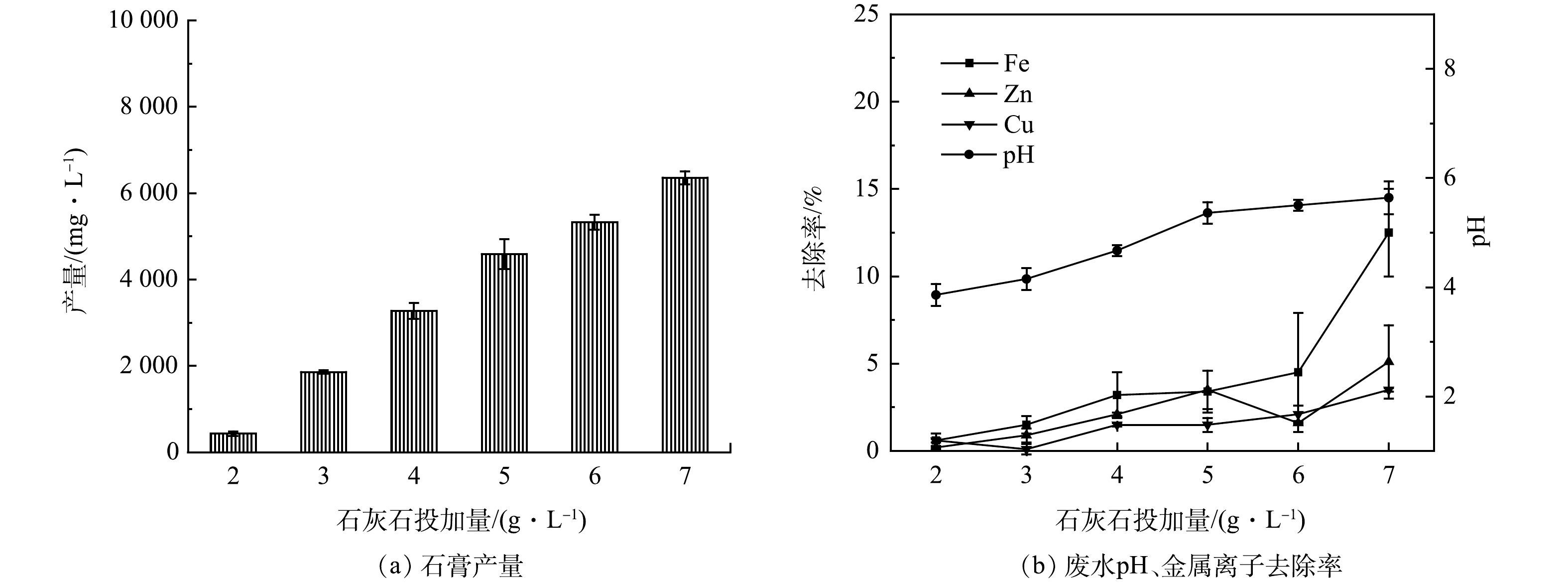
往AMD中投加0.2 g·L−1盐酸羟胺进行还原处理后,控制转速为150 r·min−1,反应时间为30 min,石灰石投加量对石膏产量及废水中金属离子去除率的影响如图4所示。可以看出,石灰石投加量增加,废水pH升高,石膏产量和废水中金属离子的去除率增加。当石灰石投加量为2 g·L−1时,废水pH为3.86,石膏产量为0.43 g·L−1,此时废水中的金属离子几乎不发生沉淀。石灰石投加量为7 g·L−1时,废水pH为5.64,石膏产量增加到6.36 g·L−1,废水中Fe2+/3+、Zn2+、Cu2+的去除率分别为12.5%、5.1%、3.5%。当废水pH > 5时,废水中金属离子的去除率陡增,故将石灰石的投加量控制在7 g·L−1以下,废水的pH在5左右时,回收石膏的产量较高,并且废水中金属离子去除率较低。
2.3 回收石膏工艺条件的优化
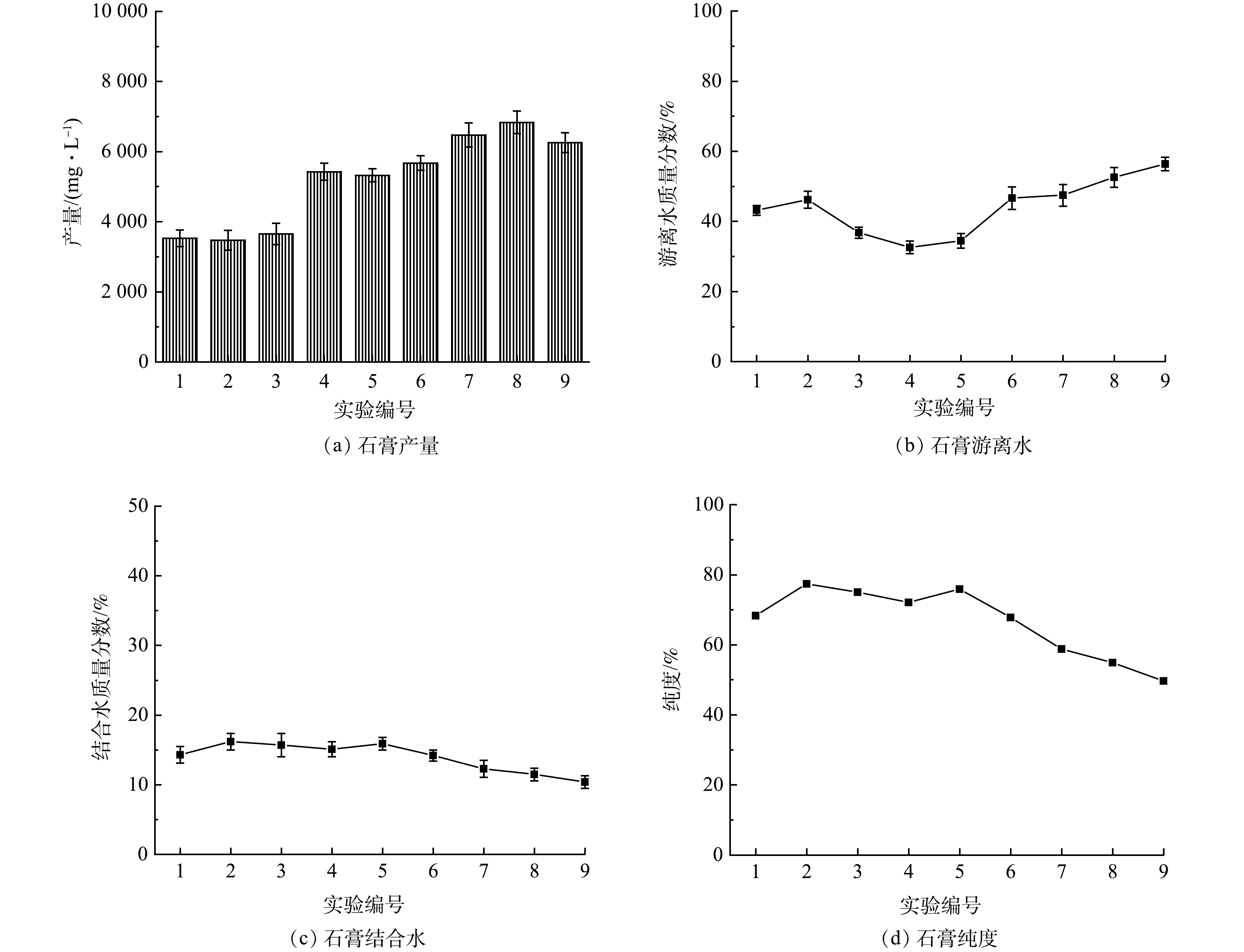
石灰石投加量为5~7 g·L−1,转速为150~250 r·min−1,投加3~5次、反应时间控制在30~60 min时,可从AMD中回收品质较高的石膏。选取石灰石投加量、转速、石灰石投加次数、反应时间4个因素,每个因素均匀选取3个水平,设计L9(43) 正交实验(表1)。对石膏的产量、纯度、游离水质量分数、形貌的结果进行综合分析,优化回收石膏的工艺条件。
表 1 正交实验Table 1. Orthogonal experiment序号 (A) (B) (C) (D) 投加量/ (g·L−1) 反应时间/min 搅拌速度/(r·min−1) 投加次数 1 5 30 150 4 2 5 45 200 5 3 5 60 250 6 4 6 30 200 6 5 6 45 250 4 6 6 60 150 5 7 7 30 250 5 8 7 45 150 6 9 7 60 200 4 为优化回收石膏的工艺条件,对9组正交实验生成石膏的产量、游离水质量分数、纯度进行分析,实验结果如图5所示。可以看出,编号1、2、3的实验中生成的石膏产量较低,为3.5~3.6 g·L−1,编号4、5、6、7、8、9的产量较高,为5.4~6.8 g·L−1,因此石灰石的投加量应控制在6 g·L−1以上。
编号2、3、4的实验中生成的石膏游离水质量分数较低,分别为36.8%、32.6%、34.5%。由正交实验极差结果(游离水质量分数)可知,影响石膏游离水质量分数的因素依次是石灰石投加量、搅拌速度、石灰石投加次数、反应时间,回收的石膏游离水质量分数最低的工艺参数为A2B1C3D3,即石灰石投加量为6 g·L−1、反应时间为30 min、转速为250 r·min−1、石灰石投加5次。
编号2、3、4、5的实验中生成的石膏结合水质量分数和纯度较高,石膏纯度为72.1%~77.4%。由正交实验极差结果(纯度)可知 ,影响石膏纯度的因素依次为石灰石投加量、搅拌速度、反应时间、石灰石投加次数,回收的石膏纯度最高的工艺参数为A1B2C3D2,即石灰石投加量为5 g·L−1、反应时间为45 min、转速为250 r·min−1、石灰石投加4次。
综合考虑石膏的纯度、产量、游离水质量分数的影响,确定回收石膏的最佳工艺参数:石灰石投加量为6 g·L−1、反应时间为45 min、转速为250 r·min−1、石灰石投加5次。该条件下回收石膏的游离水质量分数为44.6%、产量为5.4 g·L−1、品位为76.3%。
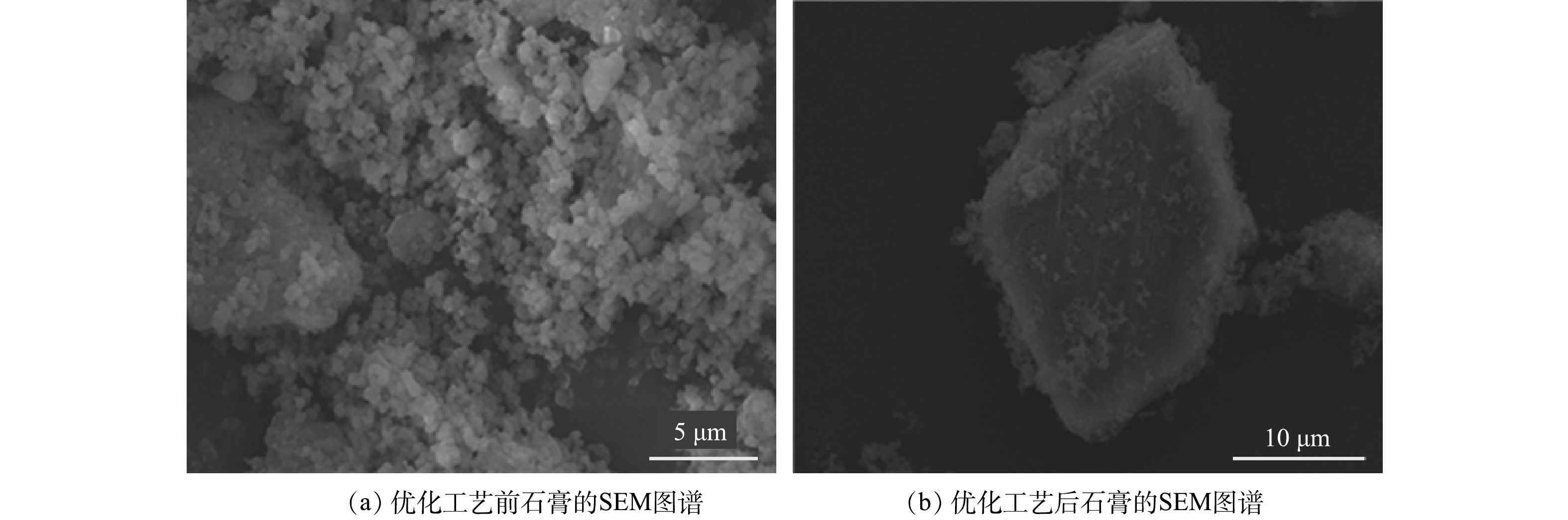
优化工艺前后回收的石膏分析结果见图6。优化工艺前的反应条件:石灰石一次性加入废水中、石灰石投加量为6 g·L−1、反应时间为45 min、转速为250 r·min−1;优化工艺后,先加入0.2 g·L−1的盐酸羟胺对废水进行还原处理,将6 g·L−1的石灰石间隔2 min分5次加入废水中,并控制反应时间为45 min、转速为250 r·min−1。由图6(a)可以看出,优化工艺前回收的石膏晶体是由许多细小的颗粒堆积生长,晶体粒径较小,表面空隙较多,晶体发育较差。这种石膏游离水含量较高,利用难度较高。而改良工艺后回收的石膏(图6(b)),形貌呈光滑的板状,晶体发育完整,晶体粒径明显增大。这种石膏游离水含量较低,抗压性更强,具备更高的回收价值[9]。
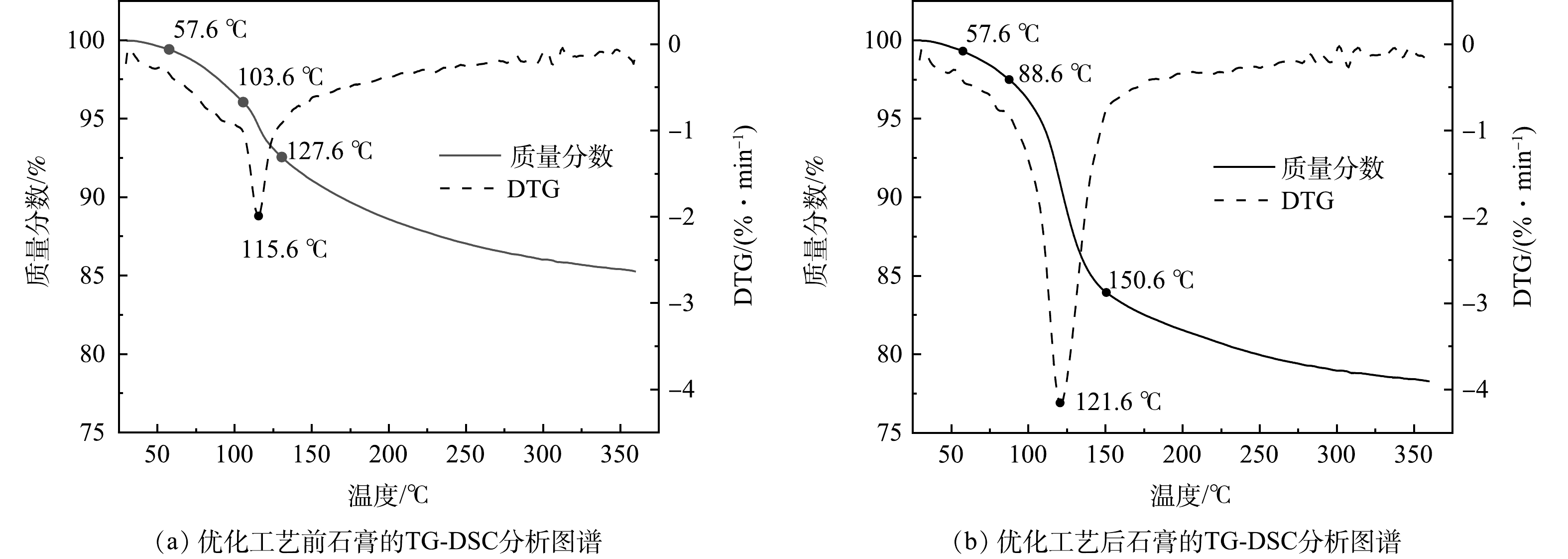
优化工艺前后回收的石膏TG-DSC分析结果如图7所示。由图7(a)可以看出,温度为57.6~103.6 ℃时,DTG曲线较为平缓,石膏重量损失较慢,主要是石膏的游离水;温度为103.6~127.6 ℃时,DTG曲线中出现了一个较大的吸热谷,石膏重量损失较快,石膏的结晶水开始减少,石膏逐渐转化为半水石膏;温度为127.6~303.6 ℃时,DTG曲线较为平缓,石膏重量损失较慢,半水石膏脱水为无水石膏;303.6 ℃以后,石膏重量基本无变化,较为稳定。根据式(1)可计算石膏纯度。
X1=4.7785c1 (1) 式中:X1为石膏纯度;c1为石膏结晶水质量分数。
可以看出,当石膏结晶水含量为10.27%左右时,由式(1)计算得出石膏的纯度为49.07%。
由图7(b)可以看出,温度为57.6~88.6 ℃时,DTG曲线较为平缓,石膏的游离水开始减少;温度为88.6~150.6 ℃时,DTG曲线中出现了一个较大的吸热谷,石膏重量损失较快,石膏的结晶水减少,逐渐转化为半水石膏;温度为150.6~309.6 ℃时,DTG曲线较为平缓,石膏重量损失较慢,半水膏脱水为无水石膏;309.6 ℃以后,石膏重量基本保持稳定。石膏结晶水质量分数为17.94%,石膏的纯度为85.72%。
工艺经过改良后,回收石膏的纯度提高了大约36.65%,极大地提高了石膏的利用价值,可代替天然石膏在某些领域的应用,如用于生产建筑石膏或生产水泥缓冲剂等对石膏品质要求较高的领域[10]。
2.4 pH和曝气时间对废水中Fe2+/3+、Zn2+、Cu2+去除率的影响
在曝气过程中,废水pH对各金属离子的去除率存在一定影响,Fe2+在废水中有3种存在形式,分别为 Fe2+、Fe(OH)+、Fe(OH)2。OH−的给电子性使中心原子更容易被氧化,因此,Fe(OH)2被氧化的速率常数比 Fe(OH)+高5个数量级[11]。提高废水pH,可加速Fe2+的氧化过程,缩短曝气时间,采用形成铁氧体和氧化沉淀的方式去除废水中的Fe3+/2+。但废水pH不宜过高,导致废水中Zn2+、Cu2+发生沉淀,沉渣中Fe的品位降低,以及后续处理中金属离子回收率降低[12]。
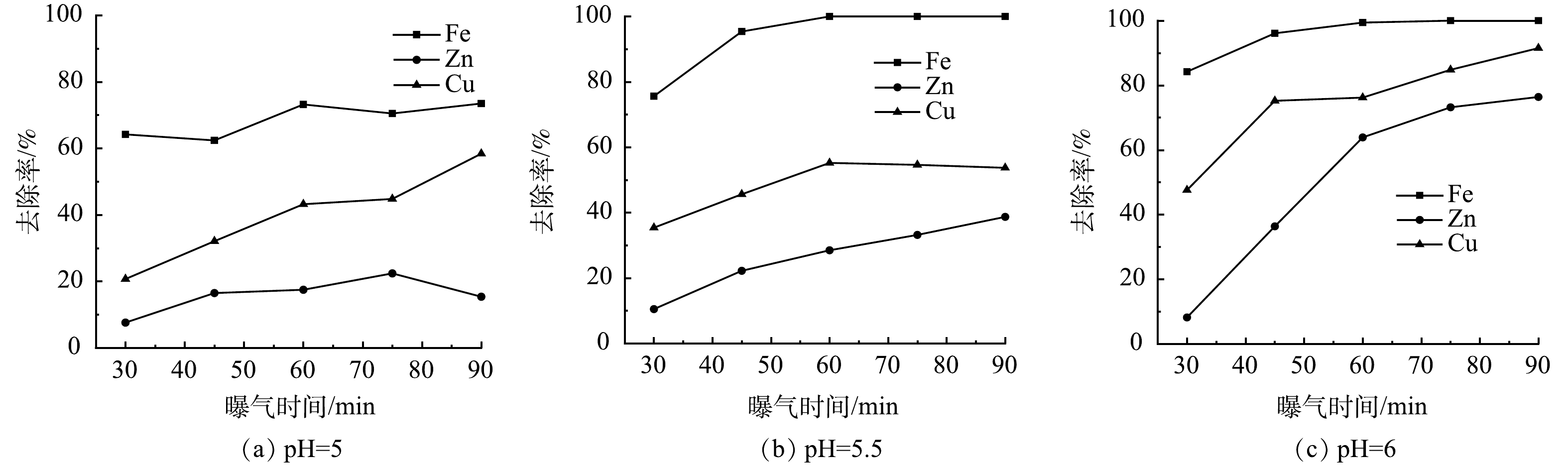
控制曝气过程中废水pH分别为5、5.5、6,反应时间对废水中金属离子浓度变化的影响如图8所示。可以看出,废水中各金属离子去除率随曝气时间的增加而增加。当废水pH为5,曝气时间为30 min时,废水中Fe2+/3+、Zn2+、Cu2+的去除率分别为64.2%、7.6%、20.7%,到90 min时,废水中Fe2+/3+、Zn2+、Cu2+的去除率分别增加到73.5%、15.4%、58.4%,废水中仍然存在部分Fe2+/3+;当废水pH为5.5,曝气时间为30 min时,废水中Fe2+/3+、Zn2+、Cu2+的去除率分别为75.6%、10.5%、35.4%,到45 min时,废水中Fe2+/3+的去除率增加到95.4%左右,废水中Zn2+、Cu2+的去除率分别为22.2%、45.6%左右,此时可实现废水中Fe2+/3+的分步回收;当废水pH为6,曝气时间为30 min时,废水中Fe2+/3+、Zn2+、Cu2+的去除率分别为82.4%、8.5%、43.4%,到45 min时,废水中Fe2+/3+的去除率增加到92.4%,但废水中Zn2+、Cu2+的去除率较高,分别为38.4%、75.3%左右。
在曝气过程中,控制废水的pH为5.5左右,曝气时间在45 min左右时,可实现废水中Fe2+/3+的分步回收,回收沉渣中Fe的品位较高。
在该反应条件下,1 t废水可产生沉渣铁渣1.53 kg(w(Fe)=35.4%),对沉渣进行XRF分析,沉渣中各金属的含量如表2所示。沉渣中含有多种金属,如Fe、Al、Mn、Ni、Zn、Cu等,其中Fe的含量最高,质量分数可达65.05%,元素占比为55.98%,说明在曝气过程中形成了以Fe为主要成分的沉渣。
表 2 沉渣中各元素含量Table 2. Contents of elements in sediment元素 质量分数/% 元素占比/% Na 6.038 12.629 Al 1.010 1.800 Si 5.256 8.999 S 3.986 6.980 Ca 1.400 1.680 Mn 1.316 1.152 Fe 65.050 55.979 Ni 0.111 0.091 Cu 1.321 0.999 Zn 14.512 9.691 总计 100.000 100.000 2.5 pH对废水中Fe2+/3+、Zn2+、Cu2+去除率的影响
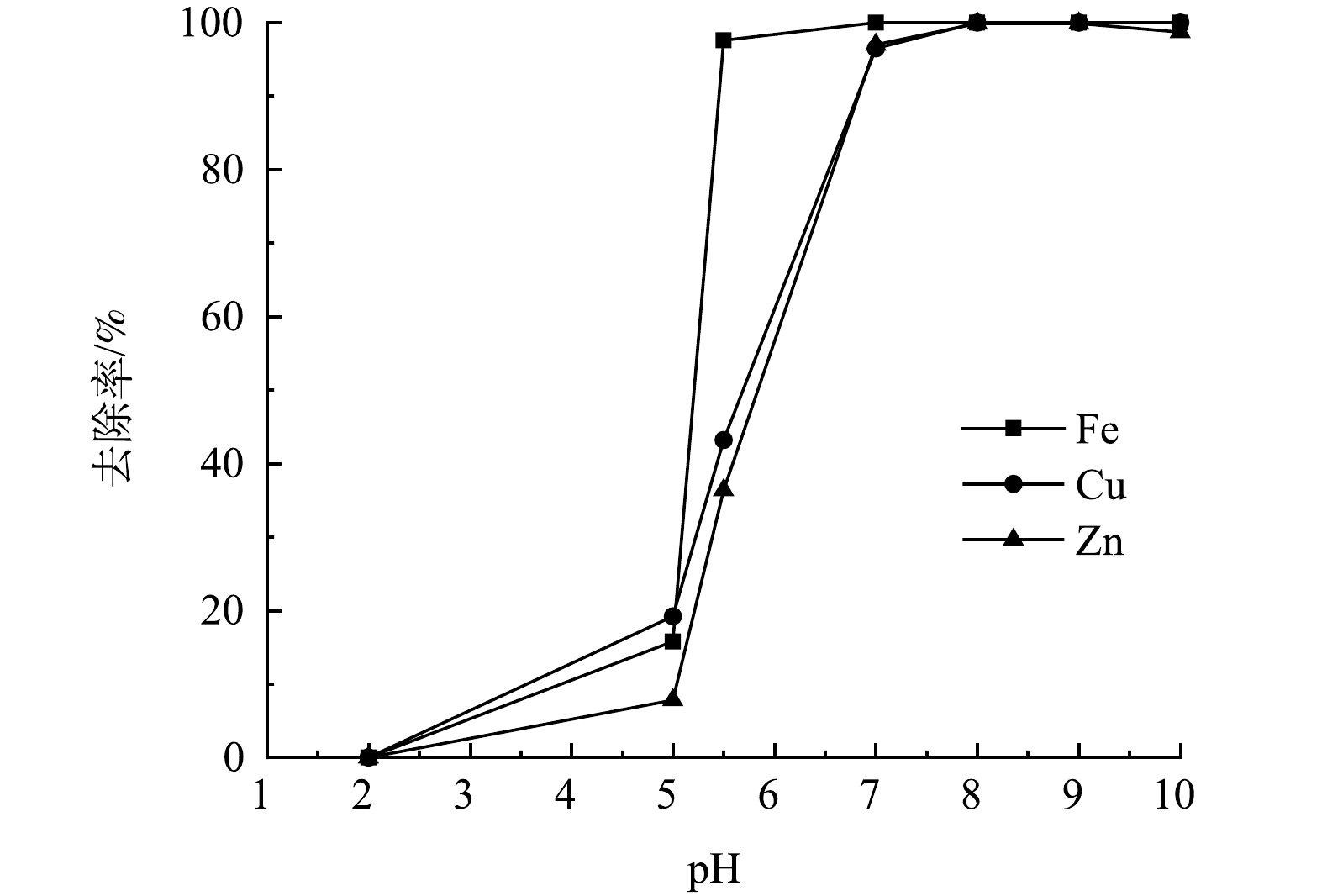
在整个工艺中,废水中金属离子浓度变化与pH关系如图9所示。将工艺分为3个阶段来分析pH对废水中Fe2+/3+、Zn2+、Cu2+去除率的影响。
第1阶段,在还原处理后的废水中投加石灰石,对废水进行中和处理并回收石膏。废水pH由2增至5时,由于废水中Fe3+被还原,在该pH范围内,废水中主要存在的Fe2+、Zn2+、Cu2+几乎不发生沉淀,且Fe2+氧化速率较低,废水中金属离子去除率较低,废水中Fe2+/3+、Zn2+、Cu2+的去除率分别为15.8%、7.8%、19.2%左右,该阶段废水中发生的反应见式(2)~式(6)。
CaCO3+2H++SO2−4=CaSO4+H2O+CO2↑ (2) Fe2++2OH−=Fe(OH)2↓ (3) Fe3++3OH−=Fe(OH)3↓ (4) Cu2++2OH−=Cu(OH)2↓ (5) Zn2++2OH−=Zn(OH)2↓ (6) 第2阶段,投加NaOH溶液,调节废水的pH至5.5并对废水进行曝气处理。采用铁氧体法合成Fe3O4,采用氧化沉淀法形成Fe(OH)3以回收废水中的Fe2+/3+。铁氧体法反应过程见式(7)。
Fe2++2Fe3++8OH−=Fe3O4↓+4H2O (7) 将溶液中的Fe2+氧化为Fe3+后,与废水中剩余的Fe2+、OH−结合生成Fe3O4[13],氧化沉淀法则是往废水中投加NaOH,在废水种形成Fe(OH)2,再通过曝气的方式,将Fe(OH)2氧化为Fe(OH)3,反应过程见式(8)。
4Fe2++8OH−+O2+2H2O=4Fe(OH)3↓ (8) 反应过程中生成的Fe(OH)3胶体能吸附废水中其他金属离子,这些金属离子嵌入到凝胶体结构中形成沉淀晶核,其后发生晶核生长,形成容易沉淀的大晶体从水中去除,在曝气过程中产生的大量铁凝胶为此提供了条件,故Zn2+、Cu2+的去除率也会增加[14]。该阶段废水中的Fe2+/3+基本完全去除,废水中Fe2+/3+、Zn2+、Cu2+的去除率分别为97.6%、36.4%、43.2%左右。
第3阶段,投加NaOH溶液,调节废水的pH至9.5,回收废水中Zn2+、Cu2+。废水的pH不宜过高,当废水中的pH上升到10以上时,废水中Zn2+的去除率降低,这是由Zn(OH)2是一种两性氢氧化物,会溶于碱,故回收废水的Zn2+的过程中,废水的pH应控制为9~9.5。
2.6 氧化还原-分步沉淀处理废水效果及资源化分析
废水经过氧化还原-分步沉淀工艺处理后,废水各阶段的金属离子浓度与pH如表3所示。可以看出,废水中Fe3+/2+、Zn2+、Cu2+已基本去除,废水中Fe2+/3+的回收率为84.3%,Zn2+、Cu2+的回收率为71.2%和46.2%左右,经过二段中和处理后废水中Fe3+/2+、Zn2+、Cu2+已低于国家污水综合排放标准(GB 8978-1996)。
表 3 废水各阶段金属离子质量浓度与pHTable 3. Metal ion concentration and pH of wastewater at each stage各阶段废水及污水排放标准 Fe2+/3+/(mg·L−1) Zn2+/(mg·L−1) Cu2+/(mg·L−1) pH 原水 643 161 26 1.9 一段中和后废水 542 148 21 4.9 曝气处理后废水 <0.1 115 12 5.5 二段中和废水 <0.1 <0.3 <0.1 9.5 污水综合排放标准 <0.5 <5 <2 6~9 本工艺所需药剂及费用如表4所示。按废水处理量为5×104 t·d−1计算,药剂费用20.5×104 元·d−1,废水的处理成本为4.1元·t−1,各药剂单价均参考工业级化学药品的价格。
表 4 药剂及费用Table 4. Pharmaceutical and pharmaceutical cost加药种类 投加量/(t·d−1) 加药位置 药剂费用/(104元·d−1) 石灰石 300 一级中和池 3 盐酸羟胺 10 还原池 13 1 mol·L−1NaOH 5 曝气池 1 石灰乳 37.5 二级中和池 1.5 1 mol·L−1 NaOH 10 三级中和池 2 注:药剂处理成本为4.1元·t−1,药剂处理费用总计20.5×104 元·d−1。 在本工艺处理废水过程中,产生的可回收资源主要有石膏、铁渣和锌渣,按废水处理量为5×104 t·d−1计算,本工艺可回收石膏270 t,石膏纯度可达76.3%以上,可回收含铁沉渣76.5 t,w(Fe)=35.4%,回收含锌沉渣70 t,w(Zn)=10.89%。在AMD处理过程中,可将回收沉渣中的金属提纯,也可将沉渣作为水泥混合生产材料,或者制备无机矿物纤维,具有较高的利用价值。可回收资源的产量和市场售价如表5所示,预计产品收入为18.94×104 元·d−1,处理废水收益为3.8 元·t−1。
表 5 可回收资源产量与售价Table 5. Recyclable resource yield and price可回收资源种类 产量/(t·d−1) 产品售价/(元·t−1) 产品收入/(104元·t−1) 石膏 270 360 9.72 含铁沉渣 76.5 720 5.508 含锌沉渣 70 450 3.15 含铜沉渣 70 80 0.56 注:收入为3.8元·t−1,总收入为18.94万元·d−1。 采取本工艺对废水进行处理时,废水药剂处理成本为4.1 元·t−1,废水产生可回收资源产品收入为3.8 元·t−1,可有效降低废水的处理成本。
3. 结论
1) 氧化还原-分步沉淀法通过将废水进行还原处理,再采用将石灰石进行多次中和的方式,可提高回收石膏的品质,回收石膏的工艺参数影响石膏的纯度、游离水含量、产量。正交实验确定回收石膏的最佳工艺参数:石灰石分5次投加、石灰石投加量为6 g·L−1、反应时间为45 min、转速为250 r·min−1。该条件下回收石膏的晶体为光滑的板状,晶体粒径大,石膏的纯度提高36.65%。
2) 回收石膏后,调节废水的pH至5.5,采用氧化沉淀法形成Fe(OH)3和铁氧体法形成Fe3O4的方式分步回收废水中的Fe2+/3+;继续调节废水pH至9.5,沉淀回收废水中的Zn2+、Cu2+,经处理后废水中各金属离子均低于国家污水综合排放标准(GB 8978-1996),废水中Fe2+/3+、Zn2+、Cu2+回收率分别为84.3%、71.2%、46.2%。
3) 氧化还原-分步沉淀法对废水进行有效处置的同时,可回收废水中的资源。该工艺可回收石膏5.4 kg·t−1,石膏纯度可达76.3%,回收铁渣1.53 kg·t−1,w(Fe)=35.4%,回收锌、铜渣1.4 kg,w(Zn)=10.89%、w(Cu)=0.1%,废水处理的药剂成本为4.1 元·t−1,废水产生可回收资源产品收入为3.8 元·t−1。该工艺可降低废水的处理成本,回收废水中的资源,实现环保与经济效益双赢的目标。
-
表 1 不同样品所用原材料的质量
Table 1. Mass of the raw materials used in the different samples
序号 No. ZrCl4/g BDC/g CPBA/g ZrCl4:BDC:CPBA摩尔比(ZrCl4:BDC:CPBA) 样品 Samples 1 0.699 0.498 0 1:1:0 UiO-66 2 0.699 0.448 0.050 1:0.9:0.1 UiO-66-BA-1 3 0.699 0.398 0.099 1:0.8:0.2 UiO-66-BA-2 4 0.699 0.349 0.149 1:0.7:0.3 UiO-66-BA-3 5 0.699 0.298 0.199 1:0.6:0.4 UiO-66-BA-4 6 0.699 0.249 0.249 1:0.5:0.5 UiO-66-BA-5 表 2 样品的BET比表面积和BJH孔结构参数
Table 2. BET surface area and BJH porous structure parameters of the samples
样品Samples BET比表面积/ (m2·g−1)SABET 总孔容/(m3·g−1)Total pore volume 平均孔径/nmMean pore diameter UiO-66 734.35 0.39 1.93 UiO-66-BA-1 728.01 0.37 1.82 UiO-66-BA-2 743.59 0.38 1.82 UiO-66-BA-3 727.42 0.37 1.84 UiO-66-BA-4 643.74 0.38 2.13 UiO-66-BA-5 624.69 0.30 1.72 表 3 吸附等温线拟合参数
Table 3. Isotherm parameters for adsorption
温度/℃T 吸附剂Adsorbent Langmuir Freundlich Qm/(mg·g−1) KL/(L·mg−1) RL R2 KF/(L·mg−1) 1/n R2 25 UiO-66 33.075 0.114 0.081 0.975 8.114 0.315 0.912 UiO-66-BA-2 40.397 0.162 0.058 0.984 11.070 0.308 0.907 UiO-66-BA-4 28.808 0.045 0.182 0.994 3.540 0.432 0.947 35 UiO-66 36.363 0.123 0.075 0.959 8.962 0.319 0.865 UiO-66-BA-2 42.509 0.209 0.046 0.987 13.226 0.282 0.899 UiO-66-BA-4 33.432 0.051 0.164 0.996 4.373 0.426 0.951 表 4 UiO-66、UiO-66-BA-2和UiO-66-BA-4吸附动力学拟合参数
Table 4. Correlation parameters of UiO-66、UiO-66-BA-2 and UiO-66-BA-4 adsorption kinetics
吸附剂Adsorbent 实验值/(mg·g−1)Qe, exp 准一级动力学模型Quasi-first-order dynamic model 准二级动力学模型Quasi-second-order dynamic model Qe, cal/(mg·g−1) k1/(L min−1) R2 Qe, cal/ (mg·g−1) k2/(g·mg−1·min−1) h R2 UiO-66 15.213 14.769 0.0303 0.975 16.698 0.0022 0.613 0.987 UiO-66-BA-2 17.948 17.259 0.0302 0.974 19.675 0.0019 0.736 0.987 UiO-66-BA-4 11.472 10.359 0.0200 0.917 12.097 0.0020 0.293 0.960 -
[1] LIU S C, LU G H, OU H X, et al. Boronate affinity imprinted hydrogel sorbent from biphasic synergistic high internal phase emulsions reactor for specific enrichment of Luteolin [J]. Journal of Colloid and Interface Science, 2021, 601: 782-792. doi: 10.1016/j.jcis.2021.05.165 [2] XIE Y, ZHANG T, CHEN Y L, et al. Fabrication of core-shell magnetic covalent organic frameworks composites and their application for highly sensitive detection of luteolin [J]. Talanta, 2020, 213: 120843. doi: 10.1016/j.talanta.2020.120843 [3] DEAN L L. Extracts of peanut skins as a source of bioactive compounds: Methodology and applications [J]. Applied Sciences, 2020, 10(23): 8546. doi: 10.3390/app10238546 [4] JIANG Z B, WANG W J, XU C, et al. Luteolin and its derivative apigenin suppress the inducible PD-L1 expression to improve anti-tumor immunity in KRAS-mutant lung cancer [J]. Cancer Letters, 2021, 515: 36-48. doi: 10.1016/j.canlet.2021.05.019 [5] ATTIQ A, JALIL J, HUSAIN K, et al. Luteolin and apigenin derived glycosides from Alphonsea elliptica abrogate LPS-induced inflammatory responses in human plasma [J]. Journal of Ethnopharmacology, 2021, 275: 114120. doi: 10.1016/j.jep.2021.114120 [6] DONG H, YANG X C, HE J P, et al. Enhanced antioxidant activity, antibacterial activity and hypoglycemic effect of luteolin by complexation with manganese(ii) and its inhibition kinetics on xanthine oxidase [J]. RSC Advances, 2017, 7(84): 53385-53395. doi: 10.1039/C7RA11036G [7] GUO B L, TONG Y K, ZHANG B Y, et al. Double affinity based molecularly imprinted polymers for selective extraction of luteolin: A combination of synergistic metal chelating and boronate affinity [J]. Microchemical Journal, 2021, 160: 105670. doi: 10.1016/j.microc.2020.105670 [8] LI D J, WANG N, WANG F F, et al. Boronate affinity-based surface-imprinted quantum dots as novel fluorescent nanosensors for the rapid and efficient detection of rutin [J]. Analytical Methods, 2019, 11(25): 3212-3220. doi: 10.1039/C9AY00787C [9] MOMPÓ-ROSELLÓ Ó, VERGARA-BARBERÁN M, LERMA-GARCÍA M J, et al. Boronate affinity sorbents based on thiol-functionalized polysiloxane-polymethacrylate composite materials in syringe format for selective extraction of glycopeptides [J]. Microchemical Journal, 2021, 164: 106018. doi: 10.1016/j.microc.2021.106018 [10] EKTIRICI S, GÖKTÜRK I, YıLMAZ F, et al. Selective recognition of nucleosides by boronate affinity organic-inorganic hybrid monolithic column [J]. Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences, 2021, 1162: 122477. doi: 10.1016/j.jchromb.2020.122477 [11] WANG H Y, BIE Z J, LÜ C, et al. Magnetic nanoparticles with dendrimer-assisted boronate avidity for the selective enrichment of trace glycoproteins [J]. Chemical Science, 2013, 4(11): 4298. doi: 10.1039/c3sc51623g [12] KIP Ç, DEMIR M C, YıLDıRıM D, et al. Highly porous, molecularly imprinted core–shell type boronate affinity sorbent with a large surface area for enrichment and detection of sialic acid isomers [J]. Journal of Inorganic and Organometallic Polymers and Materials, 2021, 31(7): 2806-2817. doi: 10.1007/s10904-021-01890-w [13] CHEN Y, ZHAO W M, QING C, et al. Boronate affinity mesoporous silica nanoparticle based selective enrichment for highly efficient analysis of ginsenosides [J]. Analytical Methods, 2019, 11(44): 5673-5679. doi: 10.1039/C9AY01913H [14] LIU C, WANG J, WAN J J, et al. MOF-on-MOF hybrids: Synthesis and applications [J]. Coordination Chemistry Reviews, 2021, 432: 213743. doi: 10.1016/j.ccr.2020.213743 [15] HONG D H, SHIM H S, HA J S, et al. MOF-on-MOF architectures: Applications in separation, catalysis, and sensing [J]. Bulletin of the Korean Chemical Society, 2021, 42(7): 956-969. doi: 10.1002/bkcs.12335 [16] ZHU X Y, GU J L, ZHU J Y, et al. Metal-organic frameworks with boronic acid suspended and their implication forcis-diol moieties binding [J]. Advanced Functional Materials, 2015, 25(25): 3847-3854. doi: 10.1002/adfm.201500587 [17] BAI Y, DOU Y B, XIE L H, et al. Zr-based metal-organic frameworks: Design, synthesis, structure, and applications [J]. Chemical Society Reviews, 2016, 45(8): 2327-2367. doi: 10.1039/C5CS00837A [18] CHENG Y F, LAI O M, TAN C P, et al. Proline-modified UIO-66 as nanocarriers to enhance Candida rugosa lipase catalytic activity and stability for electrochemical detection of nitrofen [J]. ACS Applied Materials & Interfaces, 2021, 13(3): 4146-4155. [19] ZHUANG S T, CHENG R, WANG J L. Adsorption of diclofenac from aqueous solution using UiO-66-type metal-organic frameworks [J]. Chemical Engineering Journal, 2019, 359: 354-362. doi: 10.1016/j.cej.2018.11.150 [20] ERKARTAL M, SEN U. Boronic acid moiety as functional defect in UiO-66 and its effect on hydrogen uptake capacity and selective CO2 adsorption: A comparative study [J]. ACS Applied Materials & Interfaces, 2018, 10(1): 787-795. [21] EDIATI R, AULIA W, NIKMATIN B A, et al. Chitosan/UiO-66 composites as high-performance adsorbents for the removal of methyl orange in aqueous solution [J]. Materials Today Chemistry, 2021, 21: 100533. doi: 10.1016/j.mtchem.2021.100533 [22] REGO R M, SRIRAM G, AJEYA K V, et al. Cerium based UiO-66 MOF as a multipollutant adsorbent for universal water purification [J]. Journal of Hazardous Materials, 2021, 416: 125941. doi: 10.1016/j.jhazmat.2021.125941 [23] MOHAMMADI A A, ALINEJAD A, KAMAREHIE B, et al. Metal-organic framework Uio-66 for adsorption of methylene blue dye from aqueous solutions [J]. International Journal of Environmental Science and Technology, 2017, 14(9): 1959-1968. doi: 10.1007/s13762-017-1289-z [24] CHAVAN S M, SHEARER G C, SVELLE S, et al. Synthesis and characterization of amine-functionalized mixed-ligand metal-organic frameworks of UiO-66 topology [J]. Inorganic Chemistry, 2014, 53(18): 9509-9515. doi: 10.1021/ic500607a [25] WANG Y C, LUO J, LIU X Y. Fluorescent molecularly imprinted nanoparticles with boronate affinity for selective glycoprotein detection [J]. Journal of Materials Chemistry B, 2020, 8(30): 6469-6480. doi: 10.1039/C9TB02648G [26] 曹小聪, 熊曾恒, 张鸣珊, 等. 锆基金属有机框架材料对酸性水中甲萘威的吸附 [J]. 环境化学, 2021, 40(11): 3627-3630. CAO X C, XIONG Z H, ZHANG M S, et al. Generated zirconium based metal organic framework materials for carbaryl adsorption in acidic aqueous solutions [J]. Environmental Chemistry, 2021, 40(11): 3627-3630(in Chinese).
[27] 杜明阳, 邹京, 豆俊峰, 等. 钾改性蒙脱石磁性微球对铯的吸附性能 [J]. 环境化学, 2021, 40(3): 779-789. DU M Y, ZOU J, DOU J F, et al. Adsorption properties of potassium modified montmorillonite magnetic microspheres for cesium [J]. Environmental Chemistry, 2021, 40(3): 779-789(in Chinese).
[28] ZHAO Y F, WANG D F, WEI W, et al. Effective adsorption of mercury by Zr(IV)-based metal-organic frameworks of UiO-66-NH2 from aqueous solution [J]. Environmental Science and Pollution Research, 2021, 28(6): 7068-7075. doi: 10.1007/s11356-020-11080-9 [29] AHMED I, JHUNG S H. Adsorptive desulfurization and denitrogenation using metal-organic frameworks [J]. Journal of Hazardous Materials, 2016, 301: 259-276. doi: 10.1016/j.jhazmat.2015.08.045 [30] YAO X, ZHANG S F, QIAN L W, et al. Dendrimer-assisted boronate affinity cellulose foams for the efficient and selective separation of glycoproteins [J]. Carbohydrate Polymers, 2021, 265: 118082. doi: 10.1016/j.carbpol.2021.118082 -




 下载:
下载: