-
土壤重金属污染是影响人类健康和生态环境质量的世界性问题[1-2]. 固定/稳定化和直接去除是重金属污染土壤修复的两种主要方法[1,3]. 修复技术主要有固定化、玻璃化、电动修复、植物修复和化学淋洗修复[1-3]. 土壤淋洗修复技术可以将重金属转移至液相以达到永久去除土壤中重金属的目的,是一种高效、低成本的方法,尤其适用于重度污染土壤[2,4-5]. 尽管淋洗剂(乙二胺四乙酸(EDTA)、谷氨酸N, N-二乙酸(GLDA)、乙二胺二琥珀酸(EDDS)和柠檬酸)对土壤中重金属去除效率高,但产生大量的淋洗废液中重金属主要以稳定的络合物形式存在,在较宽的pH范围内均有较高的稳定性,易造成二次污染问题[6]. 去除络合态重金属常用的方法有化学沉淀法、化学氧化法和离子交换法,但普遍存在产泥量大、处理条件复杂、费用较高等问题[6-8]. 与传统的化学处理方法相比,生物处理具有微生物来源广泛、适应性强、成本低、效率高、对环境友好等优点而有广阔应用前景[9].
近年来,学者们对硫酸盐还原菌(SRB)处理重金属污染废水进行了广泛的研究[9-11]. 研究表明,SRB可以去除传统废水中90%以上的Zn2+、Cu2+、Cd2+、Pb2+、Ni2+和Cr6+[9-11]. 一般来说,SRB去除废水中重金属离子主要通过硫化物沉淀和死菌体吸附[11-12]. 硫化物沉淀的形成过程分为两个阶段:(1)SRB利用硫酸盐作为电子受体氧化简单有机化合物生成碳酸氢根离子和硫化氢;(2)生物生成的硫化氢与游离重金属阳离子反应生成金属硫化物沉淀[9]. 此外,死菌体通过细胞壁上的官能团直接吸附重金属,有利于废水中重金属的去除[12].
虽然SRB对传统废水中的重金属离子具有很高的去除效率,但淋洗废液中的重金属主要以络合态形式存在[7],有研究报道SRB可以通过还原反应机理有效地去除Fe(Ⅲ)和Cr(Ⅵ)的络合形态[13-14],与高价态重金属相比,对于二价态重金属络合物去除的报道较少. Hakansson等[15]利用SRB产生的H2S处理络合态Pb和Cu的沉淀率达98%. 然而,据我们所知,二价重金属络合物的去除机理还不清楚. 不同络合剂如何影响SRB对络合态重金属的去除率,除形成金属硫化物沉淀外,细菌对络合态重金属的吸附效率几乎没有报道.
为了深入了解SRB对二价态重金属络合物的去除机理,利用不同络合剂形成Cu(Ⅱ)络合物,研究了分离SRB (Shewanella sp. JN01)对不同络合态Cu的去除效果及不同途径对菌株去除Cu(Ⅱ)络合物的贡献及其机理,以期达到淋洗废液的资源化再生和无害化处理提供科学依据.
-
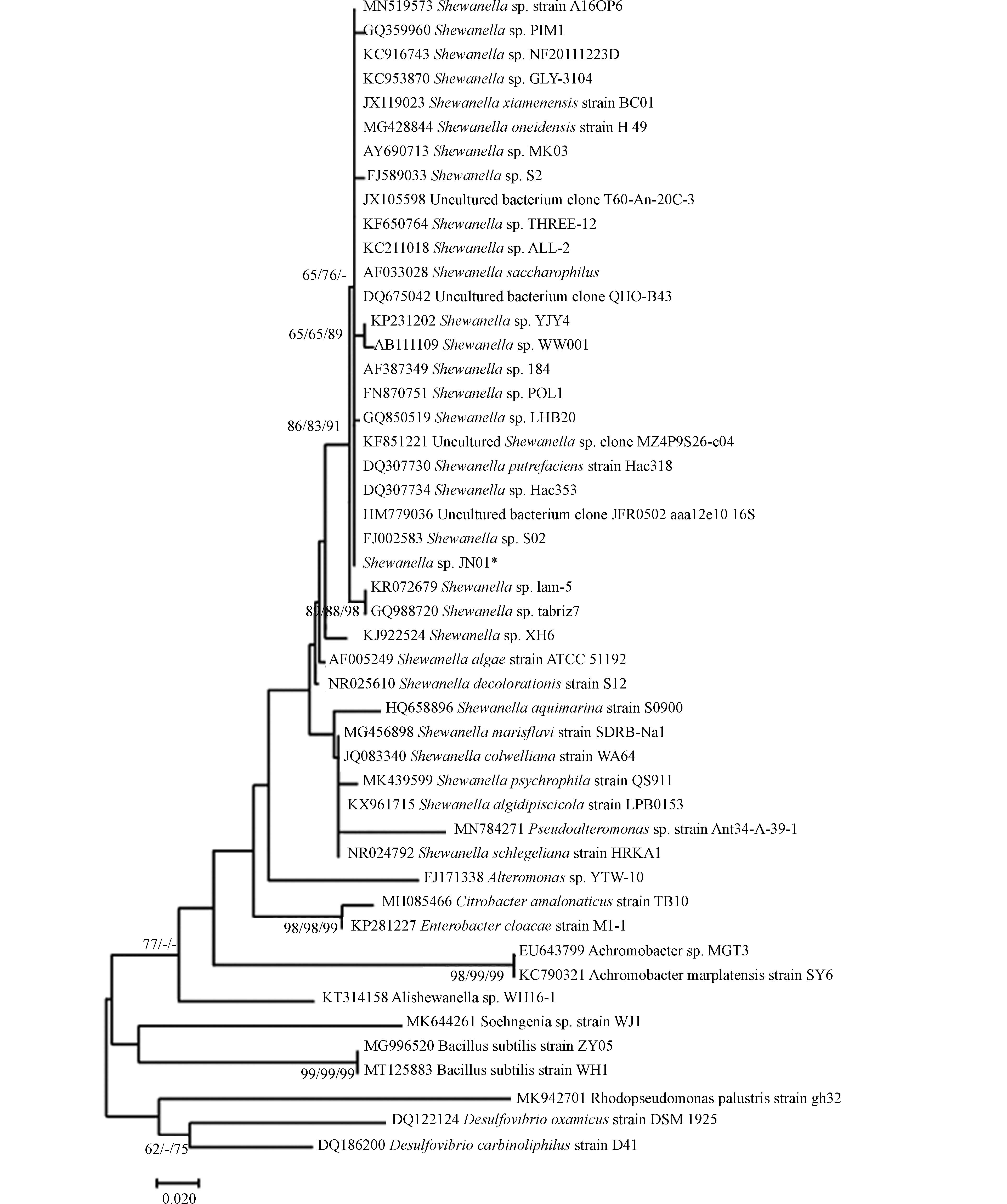
采集自山西省山阴县大营村(39°22′20.12″ N, 112°52′54.69″ E)地下井挖掘过程中21 m深的土柱. 将土柱置于手套箱(Whitley DG250,英国Don Whitley Scientific)中30 ℃下厌氧培养1周. 将土柱中央的土样置于无菌水中,用稀释涂布法富集培养菌株1周. 然后用富集培养基进一步的扩大培养. 每周更换新鲜培养基,连续培养4周后获得实验菌株. 将培养基(表1)调至pH=7.2,121 ℃高压灭菌30 min. 在富集培养基中加入2%的琼脂(W/V)制备固体培养基. 将纯化后的菌株JN01按平板划线法接种在固体培养基上. 使用DNA提取试剂盒(Sangon Biotech,生工生物工程(上海)股份有限公司)提取总DNA. 采用热循环仪(Bio-Rad,C1000 Touch,美国Bio-Rad公司)进行聚合酶链反应(PCR). 用细菌通用引物从总DNA中扩增16S rRNA基因. 采用上游引物(27F 5'TACGGYTACCTTGTTACGACTT3')和下游引物(1492R 5'AGAGTTTGATCCTGGCTCAG3')扩增菌株16S rRNA基因. PCR反应体系:2×Taq PCR Master 12.5 μL,DNA模板1 μL,上游引物2 µL,下游引物2 µL,dd H2O 7.5 μL. 扩增条件:95 ℃保持5 min;95 ℃保持1 min;54 ℃保持1 min;72 ℃保持2 min;72 ℃保持10 min;30次循环. 将16S rRNA基因序列在ClustalX 2.0[16]和GenBank核酸数据库中比对. 用Mega 6.0进行邻接法(NJ)、最大似然法(ML)和最大简约法(MP)分析.
-
所用试剂参照之前的研究[17]. 以5% V/V将SRB菌液分别接种至初始pH值为7.0的培养基中,加入高浓度Cu2+、EDTA、GLDA、CA和MC储备溶液. Cu2+浓度为50 mg·L−1. n (Cu2+): n (EDTA)分别为1:0、1:1、1:5、1:10和1:25. 当物质的量比超过1:25,混合溶液将会出现体积变化较大或EDTA溶解度较低. n (Cu2+): n (GLDA、CA或MC)分别为1:0、1:1、1:5、1:10、1:25、1:50、1:75和1:100. 处理1、3、5、7 d后,测定溶液中Cu2+浓度. 所有处理设置3组平行,并做空白对照.
死菌体吸附实验将厌氧培养24 h后的SRB悬浮液在121 ℃下灭菌30 min,然后混匀分装至装有不同络合态Cu溶液(C (Cu2+)=50 mg·L−1;n (Cu2+):n (络合剂)=1:10)的锥形瓶中,pH调至5.5. 于0、0.17、0.5、1、2、4、8、20 h后取样,测定溶液中Cu2+浓度. 所有处理设置3组平行,并做空白对照.
-
采用火焰原子吸收光谱仪(AAS,Z-2300,日本Hitachi)测定样品中Cu浓度;扫描电子显微镜(SEM-EDS,S-3400 N,日本Hitachi)和X射线衍射仪(XRD,Empyrean,荷兰PANalytical)对Shewanella sp. JN01处理不同络合态Cu的沉淀物进行表征.
-
采用Excel、SPSS 22.0和Origin 2018进行数据整理、统计分析及作图. 采用Duncan检验确定各处理之间的统计学差异(α = 0.05).
-
SRB与希瓦氏菌16S rDNA基因序列同源性达99%,构建系统发育树如图1所示,因此,分离菌株命名为Shewanella sp. JN01. Shewanella sp. JN01菌落形态在固体培养基上边缘规则,光滑圆润,中间呈凸起的黑色菌落(图2). Shewanella sp. JN01的SEM图表明,菌体为杆状、质地略光滑,聚集较多,宽×长约为0.5 μm×(2—5) μm(图2).
-
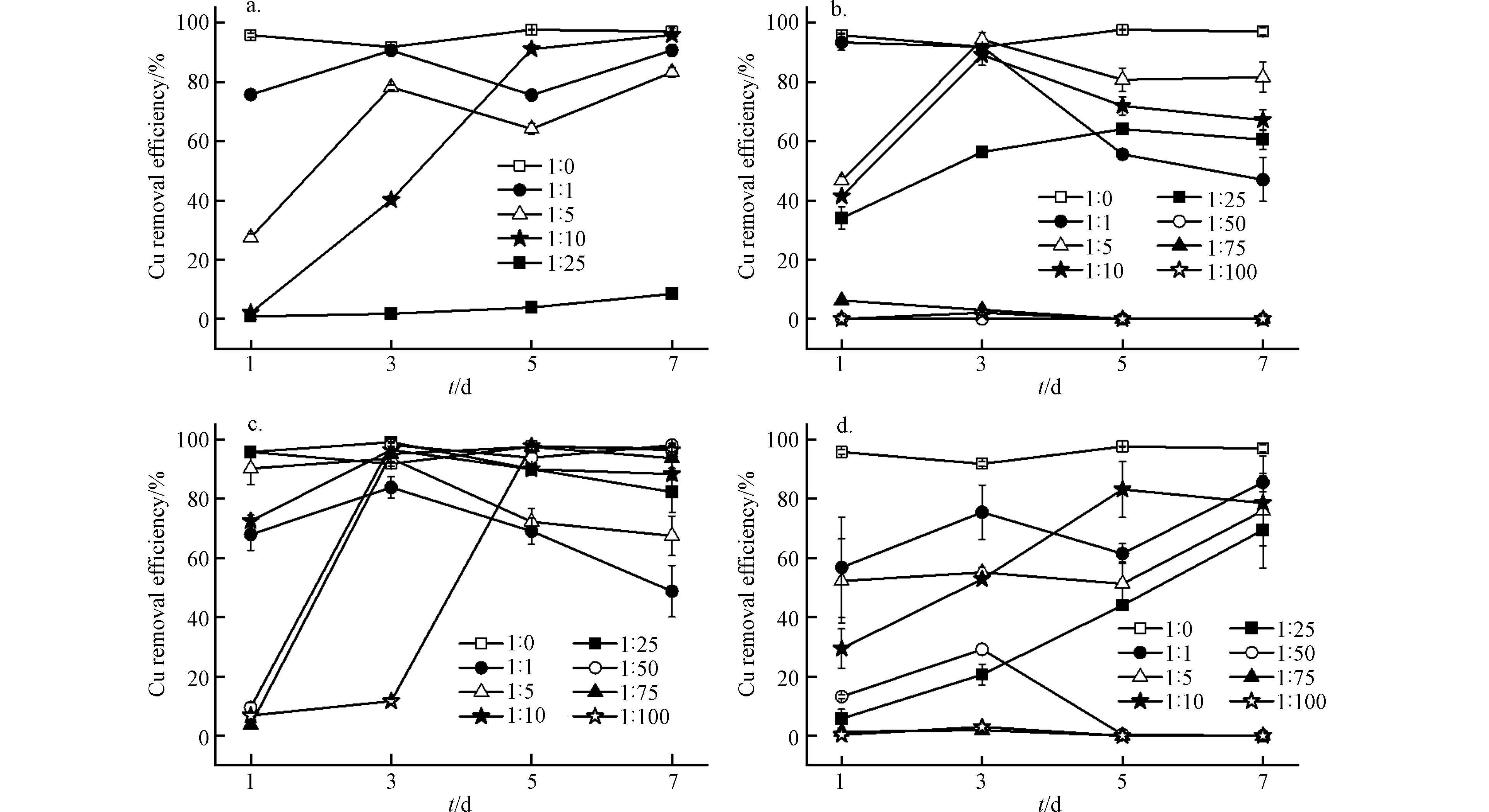
Shewanella sp. JN01对水溶液中不同络合态Cu (Cu-EDTA、Cu-GLDA、Cu-CA和Cu-MC)的去除率与反应时间、络合剂类型和Cu与络合剂的物质的量比有关(图3). 不同物质的量比下,Shewanella sp. JN01对Cu-EDTA和Cu-GLDA的去除率均低于Cu2+ (1:0)的去除率(图3). 与 Cu-EDTA 和 Cu-GLDA相比,游离Cu2+更易与H2S反应形成金属硫化物沉淀,并被细菌细胞活性成分吸附[18]. 在物质的量比较低的情况下(n (Cu2+): n (EDTA/GLDA)=1:1—1:10),Shewanella sp. JN01对Cu-EDTA和Cu-GLDA的最佳去除率一般在90%以上. 但是,随着EDTA与GLDA物质的量比增加(除n (Cu2+): n (GLDA)=1:25外),Shewanella sp. JN01对Cu-EDTA和Cu-GLDA的去除率显著降低至10%以下. 溶液中大量游离的EDTA和GLDA对Shewanella sp. JN01的毒性大于其络合形态,因为络合剂可通过抑制酶活性和改变细胞膜渗透压导致细胞死亡[19]. 此外,在Cu-EDTA和Cu-GLDA溶液中,EDTA和GLDA能抑制硫化物沉淀的形成,降低Cu2+的去除率[19-20]. 当n (Cu2+): n (EDTA/GLDA)=1:25时,Shewanella sp. JN01对Cu-GLDA的去除率为64.07%,而对Cu-EDTA的去除率小于10%,这可能是由于GLDA对微生物的毒性小于EDTA[5,21]. 同时,Cu-GLDA的稳定性(lg KCu-GLDA=13.03)低于Cu-EDTA(lg KCu-EDTA=18.80),表明Shewanella sp. JN01更易破络Cu-GLDA生成CuS沉淀[6,21].
Cu-CA的去除率随CA物质的量比的增加而变化,与Cu-EDTA和Cu-GLDA的去除率差异显著. 当n (Cu2+):n (CA)为1:1—1:25时,Cu-CA的最佳去除率由83.83%提高到99.11%. 随着CA物质的量比的进一步增加,Cu-CA的去除率由不足10%显著提高到97%以上,并保持相对稳定. 在CA的高物质的量比条件下,Shewanella sp. JN01对Cu-CA的高去除效率的延迟可能是由于CA浓度过高,Shewanella sp. JN01需要时间来适应其环境[18]. 与EDTA和GLDA不同,过量的CA并不会对Cu-CA的去除产生负面影响. 这很可能是由于Shewanella sp. JN01以CA为碳源,促进Shewanella sp. JN01的生长,从而去除Cu-CA[13].
Shewanella sp. JN01对Cu-MC的最佳去除率明显低于其他络合态Cu. 当n (Cu2+): n (MC)= 1:1—1:25时,Shewanella sp. JN01对Cu-MC的最佳去除率由85.50%降至69.40%. 当n (Cu2+): n (MC)= 1:50时,络合态Cu的去除率先显著升高后降低,5 d后Cu-MC的去除率为0. 这可能与Shewanella sp. JN01死后释放Cu有关. 尽管CA对Shewanella sp. JN01的生长没有明显抑制作用,但MC中的EDTA和GLDA会破坏其细胞结构的完整性[19]. Cu-CA在物质的量比为1:25和1:50时的去除率高于Cu-EDTA和Cu-GLDA,这说明MC对Shewanella sp. JN01的毒性作用低于EDTA和GLDA.
Cu与络合剂物质的量比较低时,Cu-GLDA和Cu-CA在3 d后的去除率下降,主要是受Cu和络合剂的胁迫所致. Shewanella sp. JN01细胞表面活性成分受损,导致累积的Cu再次释放[22]. 此外,细胞表面附着的硫化物沉淀,由于传质阻力增加,对菌株的代谢产生不利影响[23].
在一定的Cu与络合剂的物质的量比下,第7天的去除率基本稳定,Shewanella sp. JN01对不同络合态Cu的去除率为Cu-CA > Cu-MC > Cu-GLDA > Cu-EDTA. 这主要与他们的稳定常数有关,其稳定性为lg KCu-EDTA(18.80) > lg KCu-GLDA(13.03) > lg KCu-CA(5.95)[6,21,24]. 络合态Cu稳定越高,Shewanella sp. JN01破络难度越大,去除效果越差[15,18]. 结果表明,Cu-CA的去除率最高,尤其是在络合剂的物质的量比较高时. 与GLDA和EDTA相比,CA对菌株生长代谢和硫酸盐还原途径的抑制作用较小[13-14]. 此外,CA是小分子、可降解的络合剂,为微生物生长提供碳源[13,18]. 这进一步说明,MC对Shewanella sp. JN01的毒性较EDTA和GLDA温和,是因为MC中减少了EDTA和GLDA的用量,CA所占比例较大.
-
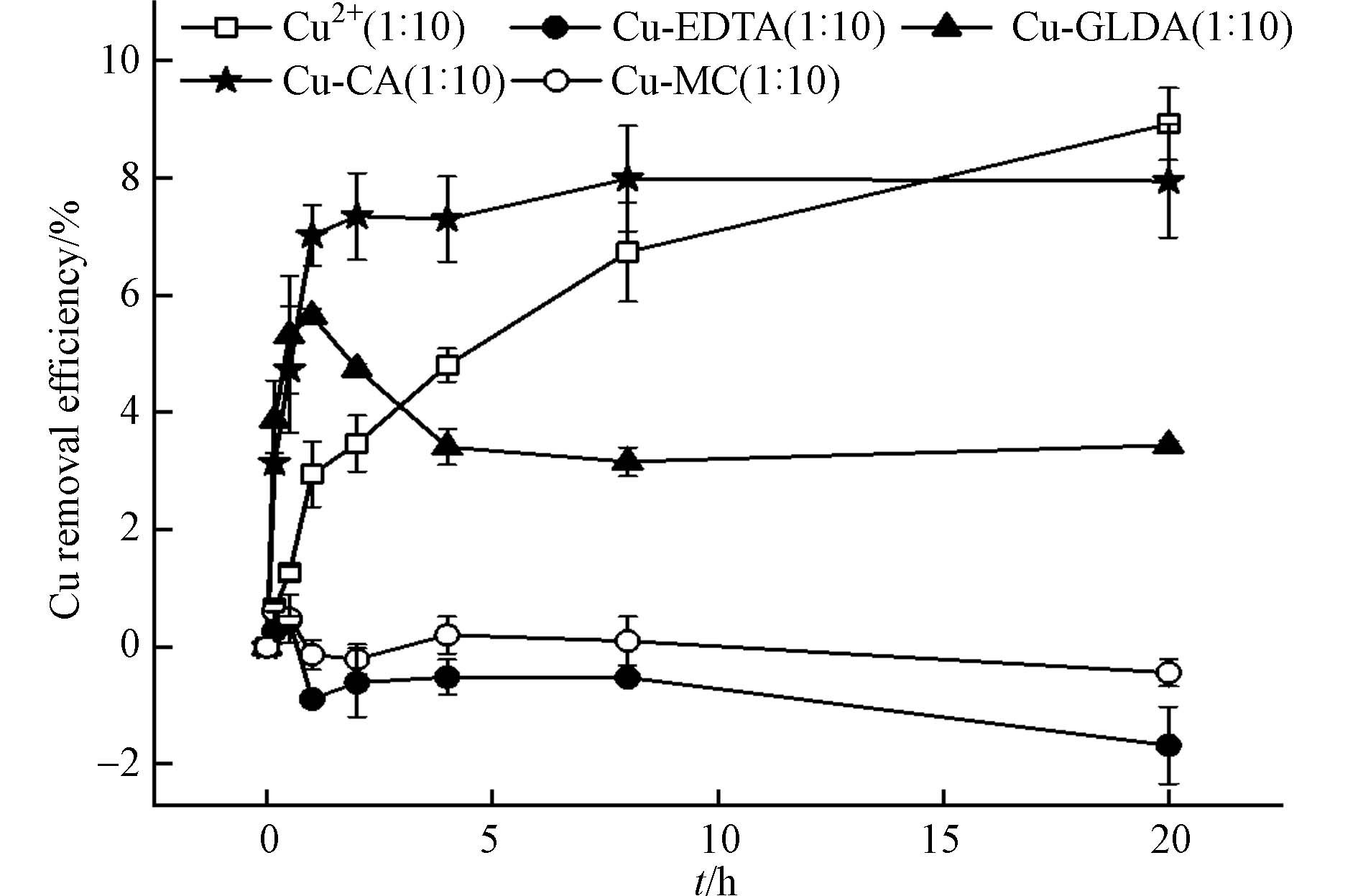
死菌体对络合态Cu的最佳去除率低于游离Cu2+ (图4). 死菌体对Cu2+的吸附率缓慢增加,20 h后达到8.92%. 死菌体对Cu2+的去除主要是由于Cu2+直接吸附在细胞壁上[18]. 高压灭菌后,许多带负电荷的官能团(如羟基、氨基或羧基)暴露在菌体表面,增加了对带正电荷离子如Cu2+的结合位点[22].
死Shewanella sp. JN01对络合态Cu的吸附去除率趋势与Cu2+明显不同(图4). 对于络合态Cu,死Shewanella sp. JN01对Cu-CA的吸附率在1 h内迅速增加,之后基本保持不变. 死菌体对Cu-CA的吸附去除率高达7.99%,与Cu2+的吸附率相当(P > 0.05). 这是因为CA是易降解的有机物,对细菌表面损害较小[13,18]. Cu-CA主要以CuL− (H3L代表柠檬酸)的形式存在,通过静电吸引与氨基结合[24-25]. 死菌体对Cu-GLDA的吸附率在1 h内达到最佳值(5.65%),随后逐渐下降后平稳;对Cu-EDTA的吸附率仅为0.44%;对Cu-MC的吸附率介于两者之间. Cu-EDTA的吸附去除率较差,这是由于Cu-EDTA在pH=5.5时主要以CuEDTA2-形式存在[6],CuEDTA2-络合物为六配位八面体结构,Cu2+被包裹于络合物内部,无法与吸附位点接触[26-27]. Cu-GLDA的吸附去除率高于Cu-EDTA,这是因为Cu-GLDA对死菌细胞的结合亲和力强[28].
Cu-EDTA和Cu-GLDA的吸附去除率达到最佳后下降,很可能是EDTA和GLDA的毒性作用使细胞壁破坏,导致吸附的络合态铜又释放回水溶液中[19,22]. 相比而言,络合态Cu经过破络后形成硫化物沉淀的途径去除率高(> 80%,图3),死菌体对络合态Cu的吸附去除率只占Shewanella sp. JN01对络合态Cu总去除率的一小部分(< 8%,图4).
-
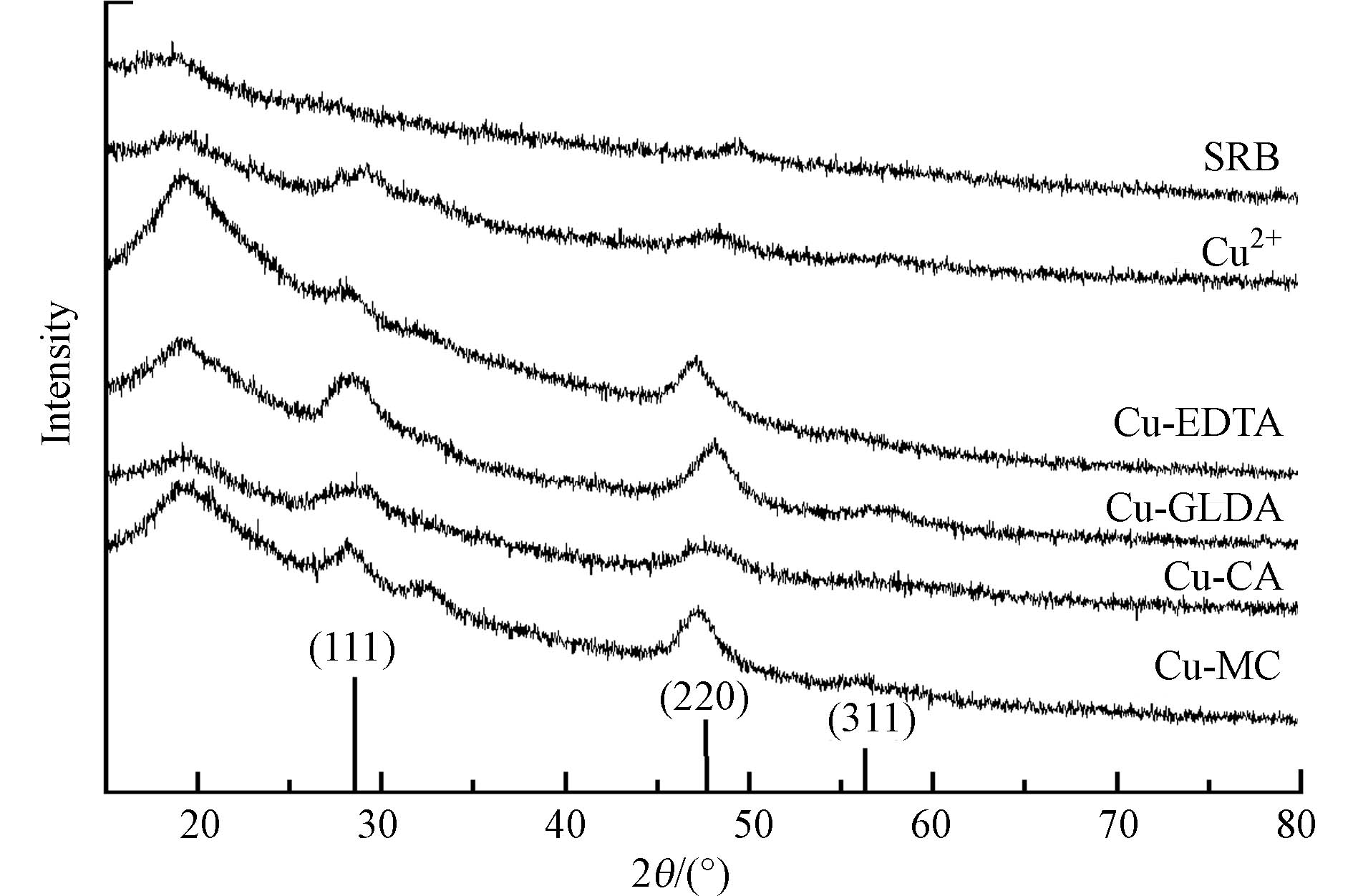
考虑到Shewanella sp. JN01去除络合态Cu的主要途径是先破络进而形成硫化物沉淀,实验收集并表征了Shewanella sp. JN01处理含Cu络合物水溶液后的沉淀. XRD衍射仪分析了所得沉淀物的晶体结构(图5). 沉淀物的无定型(2θ值为20°)很可能是由于菌体细胞中多糖、蛋白质和脂质的存在[12]. 在2θ值为28.68°、47.71°和56.62°处有较强的衍射峰,对照CuS标准图谱(PDF No.89-2073)中的(111)、(220)和(311)晶面. Shewanella sp. JN01只在47.71°处有微弱的峰. 然而,Cu2+处理后的沉淀物在28.68°和47.71°处的峰强度低于络合态铜,这可能是细菌在Cu2+处理过程中产生的硫化铜颗粒较小[15]. 此外,在金属离子的胁迫下,菌株分泌的代谢物能吸附铜离子并与铜离子络合,阻碍了结晶度高的硫化铜的形成[29]. 尽管培养基中存在磷酸盐,但对Cu的沉淀影响较小. 一方面,每个处理组接菌量均为5%,因此,每个实验组的磷酸盐含量相同;另一方面,磷酸根的浓度远远低于硫酸根(表1). 在XRD衍射图中也未检出Cu的磷酸盐沉淀物. 因此,培养基中磷酸盐对不同处理组Cu沉淀差异的影响很小.
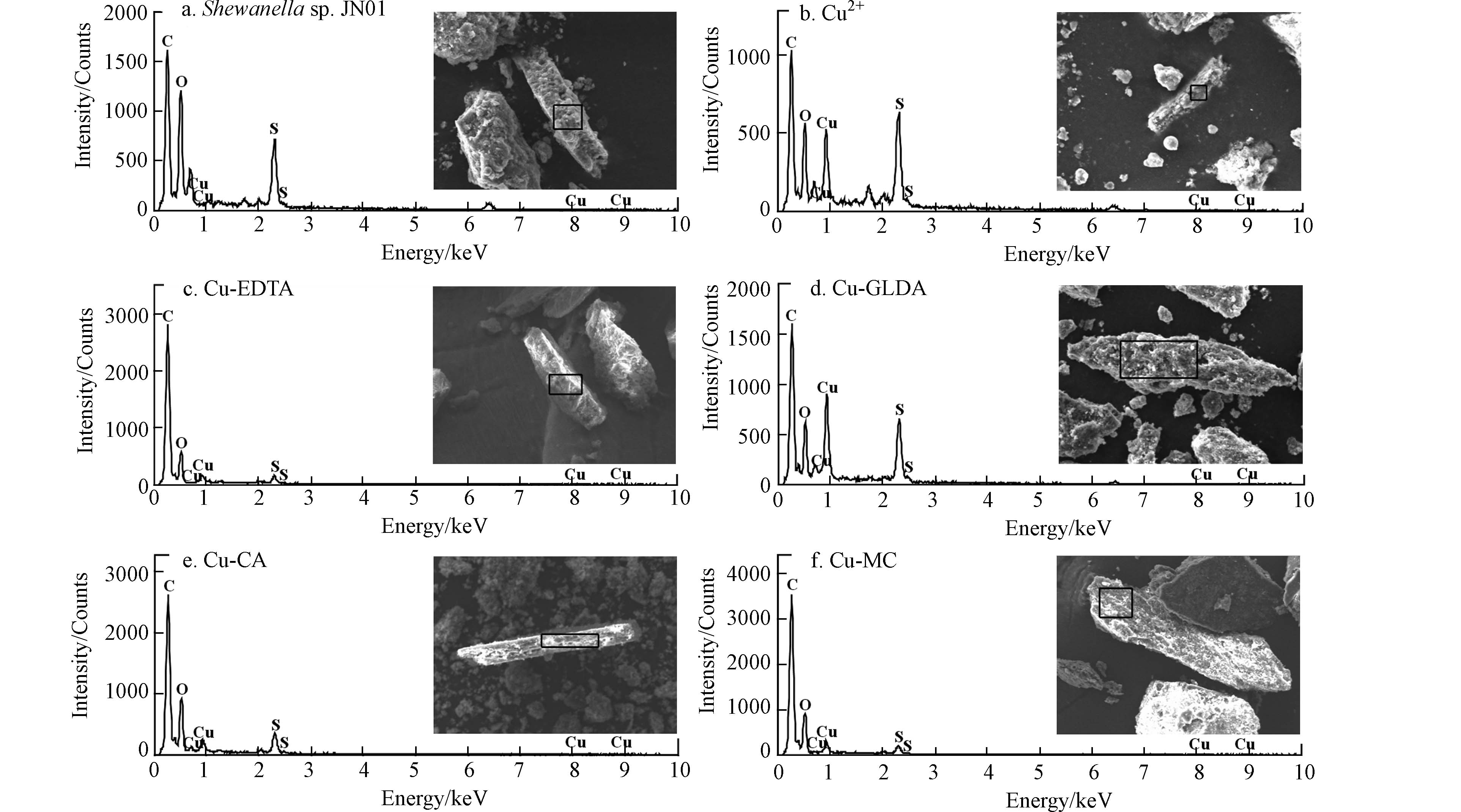
SEM图像进一步证实了Shewanella sp. JN01处理不同络合态Cu溶液后存在沉淀颗粒[12]. EDS结果表明,沉淀物中存在Cu和S,不同处理的Cu和S的含量变化较大(图6).
Shewanella sp. JN01沉淀物中O和S的原子比分别为29.85%和6.85%,其中未检测到Cu的含量. 加Cu2+处理后的沉淀物中O的原子比降低到19.45%,S和Cu的含量增加到9.07%和4.34%. 其中O的原子比下降很可能是由于Cu2+抑制了Shewanella sp. JN01的生长,因为沉淀物中O主要来自菌株的生长. S和Cu原子比的增加是由于加入CuSO4后,Shewanella sp. JN01在氧化还原反应中生成了CuS. 不同络合态Cu处理后的沉淀物中O、S和Cu的原子比分别为16.13%—23.28%、1.57%—6.64%和1.07%—5.38% (表2). O的含量与细菌活性密切相关,这在一定程度上可以解释菌株对Cu的去除效率随细菌活性的降低而降低[18]. 与其他Cu络合物相比,Cu-CA处理后的沉淀物中O原子比最高,说明柠檬酸可以作为碳源,提高细菌的代谢活性,从而产生更多的H2S,对Cu络合物去除率更高. 由于Cu和S的化学计量比为1:1,但S的原子比略高于Cu,这一结果很可能是由于Shewanella sp. JN01通过硫酸盐还原产生大量的硫化氢,使溶液中的Cu几乎都生成硫化铜沉淀被去除[30](图3). 不同络合态Cu处理后沉淀物中Cu和S的强峰表明,沉淀物中CuS是主要产物,这与XRD结果一致. 此外,无论溶液的pH值如何,CuS (Ksp=6.3×10−36)的溶解度低且稳定性高[31]. 实验结果表明,模拟淋洗废液中Cu络合物的去除机理主要是经Shewanella sp. JN01破络后生成硫化物沉淀.
-
SRB (Shewanella sp. JN01)对水溶液中的Cu络合物具有较高的去除效率,主要通过先破络后形成硫化物沉淀. Shewanella sp. JN01对不同Cu络合物的最佳去除率为Cu-CA (99.11%) > Cu2+ (97.69%) > Cu-EDTA (95.90%) > Cu-GLDA (94.22%) > Cu-MC (85.5%). 络合剂对Shewanella sp. JN01的抑制作用为EDTA > MC > GLDA > CA. 本研究的结果为从水溶液中去除Cu络合物提供了一种有效、节约成本和环境友好的方法. 然而,由于实际淋洗废液中所含物质比模拟淋洗废液更为复杂,因此有必要进一步探索SRB (Shewanella sp. JN01)对实际淋洗废液中多种重金属络合物的最佳去除效率.
Shewanella sp. JN01对水体系不同络合态Cu的去除效果及机理
Removal efficiency and mechanism of different kinds of copper complexes from aqueous system by Shewanella sp. JN01
-
摘要: 土壤淋洗废液处理难点在于废水中含有高浓度的稳定重金属络合物. 本研究分离了一株硫酸盐还原菌(Shewanella sp. JN01),探讨了其对模拟淋洗废液中不同络合态Cu (Cu-乙二胺四乙酸(Cu-EDTA)、Cu-谷氨酸N,N-二乙酸(Cu-GLDA)、Cu-柠檬酸(Cu-CA)和Cu-混合淋洗剂(Cu-MC))的去除效果及机理. 结果表明,活菌体和死菌体对不同络合态Cu的去除率分别大于80%和小于8%. 显然,死菌体细胞表面吸附作用对络合态Cu去除效率的贡献十分有限. 因此,Shewanella sp. JN01去除络合态Cu的主要机制是先破络,再形成CuS沉淀. Shewanella sp. JN01对不同络合态Cu的去除率为Cu-CA >Cu-MC >Cu-GLDA >Cu-EDTA,这一变化趋势与它们的稳定性常数和毒性的变化趋势相反,结果进一步证实了破络是微生物去除络合态重金属的限制性步骤. Shewanella sp. JN01能够有效去除土壤淋洗废液中重金属络合物,在淋洗废液再生利用方面具有潜在应用前景.
-
关键词:
- 土壤淋洗废液 /
- 络合态Cu /
- Shewanella sp. JN01 /
- 硫化物 /
- 沉淀.
Abstract: The difficulty in the treatment of soil washing effluent lies in its high concentrations of stable heavy metal complexes. This study isolated a strain of sulfate-reducing bacteria (Shewanella sp. JN01) to explore the removal efficiency and mechanism of different heavy metal complexes [Cu-ethylenediaminetetraacetic acid (Cu-EDTA), Cu-N, N-bis(carboxymethyl) glutamic acid (Cu-GLDA), Cu-citrate (Cu-CA) and Cu-mixed chelator (Cu-MC)] from the simulated soil washing effluent by Shewanella sp. JN01. The results showed that removal efficiencies of different Cu complexes by the alive and dead Shewanella sp. JN01 were above 80% and below 8%, respectively. Evidently, the contribution of sorption of Cu complexes by cell surface of the dead strain to its total removal efficiency was limited. Therefore, the dominant removal mechanism of Cu complexes by Shewanella sp. JN01 was related to dechelation first and then formation of CuS precipitation. The removal efficiencies of different Cu complexes by Shewanella sp. JN01 varied as the trend of Cu-CA > Cu-MC > Cu-GLDA > Cu-EDTA, which was opposite to the trend of their stability constants and toxicity. This finding further confirmed that the dechelation was a limiting step to remove heavy metal complexes by microorganisms. Collectively, the results of this study indicate that Shewanella sp. JN01 can effectively remove the heavy metal complexes from soil washing effluent, which has potential application prospects for recycling use of soil washing effluent.-
Key words:
- soil washing effluent /
- copper complexes /
- Shewanella sp. JN01 /
- sulfide /
- precipitation.
-
我国甘肃、新疆、青海等西北地区,干旱少雨,地表蒸发量大,属严重缺水区域[1]. 尤其是黄土塬上的偏远村镇,没有可利用的地表水和地下水,资源性缺水更加严重[2-3]. 这些村镇不具备修建大型水利工程的条件,收集雨水是当地村民唯一的饮水方式,而水窖是最常用的雨水储存设施[4].
与河、库等地表水相比,雨水是一种水质较好的优质水源[5],但在收集饮用时同样面临着一定的水质污染风险. 集流面材料是影响雨水安全集蓄的一个关键因素. 常见的集流面有瓦屋面、沥青屋面和混凝土地面等. 其中,沥青屋面释放的污染物最多,获得的水质最差;混凝土地面应用最多,释放的碱性物质会显著提高雨水pH[6]. 此外,水窖周围环境是影响窖水水质的另一重要因素. 已有研究表明,建在厕所和牲畜棚旁边的窖水含有更多的污染物质[7].
新污染物是指具有环境稳定性、生物累积性和生物毒性的有毒有害化学物质,主要包括持久性有机污染物、内分泌干扰物、抗生素和微塑料四大类. 不同于常规污染物,新污染物浓度水平一般较低,但由于生物累积和生物放大作用,对人体和生态系统潜在威胁较大.
邻苯二甲酸酯(PAEs)和全氟化合物(PFCs)是两类典型的新污染物. PAEs是一种环境激素类内分泌干扰物,能够抑制雄性激素的生成,对人类的呼吸系统、生殖系统和内分泌系统均有损害. PFCs广泛应用于聚四氟乙烯生产,是重要的防水防油材料,也是灭火泡沫的主要成分. PFCs对人体肝脏功能、脂肪代谢和遗传发育均有不良影响[8]. 已有研究表明,两种污染物广泛存在于全球大气、土壤及水环境介质中[9-12]. 可以推断,在西北高海拔黄土塬区村镇,PAEs和PFCs两种新污染物同样存在,窖水也面临着相应的的污染风险. 然而,针对西北村镇窖水这种分散型饮用水源,还未有新污染物方面的报道.
窖水作为西北高海拔村镇唯一可用的饮用水源,其水质安全对当地村民身体健康有着重要意义. 本研究基于西北黄土塬区村镇饮水现状,以我国甘肃某县为代表区域,采集冬夏两季窖水进行水质分析,研究了15种目标PAEs和17种PFCs在冬夏两季窖水中的浓度水平和组成,讨论了两种新污染物来源,并利用模型计算了采样点覆盖区域内人群通过饮用窖水摄入PAEs和PFCs的健康风险,为西北高海拔黄土塬区村镇窖水的饮用处理提供了基础数据支撑.
1. 材料与方法(Materials and methods)
1.1 研究区域及样品采集
研究区域所在县隶属甘肃省,为典型的黄土高原丘陵沟壑区,总体地势西北高、东南低,海拔1136—2089 m,常年干旱少雨,是典型的资源型缺水地区[13]. 该区以农业和畜牧业为主导产业,对水资源量的需求较高. 集雨水窖是当地村户最主要的饮用水渠道,雨季将雨水收集储存起来,仅依靠窖水的自然沉淀,未经其它处理,就直接进行生活饮用.
本次调研覆盖该县整个纵向区域,于2021年12月和2022年7月分两次,自北向南每间隔约10 km取水窖样品. 冬季窖水采样时距上次降雨约4个月,夏季窖水采样时正是雨天,具体采样点分布如图1所示,冬季共采集8个(a—h)窖水样品,所有窖水样品均进行了常规指标的检测,用于检测新污染物的窖水b、d、e、f用玻璃瓶密封,其它用聚乙烯瓶密封. 夏季共采集10个(1—10)窖水样品,所有样品均采用聚乙烯瓶密封. 1和a、2和b、4和c、6和d、7和f、8和g、9和h分别对应同一窖水采样点. 每批样品运回实验室后置于4℃冷库中保存,并在一周内完成所有样品的检测分析. 这些样品分布在不同的自然村落中,所有水窖材质均为混凝土水窖,集雨面均为混凝土地面,水窖8(g)是供水规模较大的集体水窖,其余各水窖皆为单户水窖.
1.2 药品及试剂
15种PAEs标准样品购自中国上海阿拉丁公司,如表1所列;17种PFCs标准样品,如表2所列,以及9种PFCs同位素内标(13C4PFBA、13C4PFHxA、13C4PFOA、13C4PFNA、13C4PFDA、13C4PFUdA、13C2PFDoA、18O2PFHxS 和13C4PFOS) 购自加拿大惠灵顿公司;色谱纯乙腈、色谱纯甲醇、色谱纯正己烷、色谱纯二氯甲烷均购自加拿大飞世尔科技公司;色谱级乙酸铵购自中国上海的麦克林公司;超纯水使用日本奥加诺株式会社的Milli-Q系统生成(电阻率>18.2 MΩ·cm).
表 1 检测的15种PAEs类型及化学式Table 1. The type and chemical formulas of 15 PAEs in this study化合物Compouds 英文名称English name 英文缩写Abbreviation 化学式Chemical formula CAS编号 CAS number 邻苯二甲酸二甲酯 Dimethyl phthalate DMP C10H10O4 131-11-3 邻苯二甲酸二乙酯 Diethyl phthalate DEP C12H14O4 84-66-2 邻苯二甲酸二异丁酯 Diisobutyl phthalate DIBP C16H20O4 84-69-5 邻苯二甲酸二丁酯 Dibutyl phthalate DBP C16H22O4 84-74-2 邻苯二甲酸二甲氧乙酯 Dimethylglycol phthalate DMEP C14H16O6 117-82-8 双(4-甲基-2-戊基)邻苯二甲酸酯 Bis(4-Methyl-2-pentyl)phthalate BMPP C20H30O4 146-50-9 双(2-乙氧基)邻苯二甲酸酯 Bis(2-ethoxyethyl) phthalate DEEP C16H22O6 605-54-9 邻苯二甲酸二戊酯 Di-n-pentyl phthalate DPP C18H26O4 131-18-0 邻苯二甲酸二己酯 Di-n-hexyl phthalate DNHP C20H30O4 84-75-3 邻苯二甲酸苄酯 Mono-Benzyl phthalate BBP C15H12O4 2528-16-7 双(2-正丁氧基乙酯)邻苯二甲酸酯 Bis(2-butoxyethyl) phthalate DBEP C20H30O6 117-83-9 邻苯二甲酸二环己基酯 Dicyclohexyl phthalate DCHP C20H26O4 84-61-7 邻苯二甲酸二辛酯 Di-n-octyl phthalate DNOP C24H38O4 117-84-0 双(2-乙基己基)邻苯二甲酸酯 Di(2-ethylhexyl)phthalate DEHP C24H38O4 117-81-7 邻苯二甲酸二异壬酯 Diisononyl phthalate DINP C26H42O4 68515-48-0 表 2 检测的17种PFCs类型及化学式Table 2. The type and chemical formulas of 17 PFCs in this study化合物Compouds 英文名称English name 英文缩写Abbreviation 化学式 Chemical formula CAS编号 Chemical formula 对应内标Inner standard 全氟丁酸 Perfluorobutanoic acid PFBA C3F7COOH 375-22-4 13C4PFBA 全氟戊酸 Perfluoropentanoic acid PFPeA C4F9COOH 2706-90-3 13C4PFOA 全氟己酸 Perfluorohexanoic acid PFHxA C5F11COOH 307-24-4 13C4PFHxA 全氟庚酸 Perfluoroheptanoic acid PFHpA C6F13COOH 375-85-9 13C4PFHxA 全氟辛酸 Perfluorooctanoic acid PFOA C7F15COOH 206-397-9 13C4PFOA 全氟壬酸 Perfluorononanoic acid PFNA C8F17COOH 375-95-1 13C4PFNA 全氟癸酸 Perfluorodecanoic acid PFDA C9F19COOH 335-76-2 13C4PFDA 全氟十一酸 Perfluoroundecanoic acid PFUnDA C10F21COOH 218-165-4 13C4PFUdA 全氟十二酸 Perfluorododecanoic acid PFDoDA C11F23COOH 307-55-1 13C2PFDoA 全氟十三酸 Perfluorotridecanoic acid PFTrDA C12F25COOH 276-745-2 13C2PFDoA 全氟十四酸 Perfluorotetradecanoic acid PFTeDA C13F27COOH 376-06-7 13C2PFDoA 全氟十六酸 Perfluorohexadecanoic acid PFHxDA C15F31COOH 67905-19-5 13C2PFDoA 全氟十八酸 Perfluorooctadecanoic acid PFODA C17F35COOH 16517-11-6 13C2PFDoA 全氟丁烷磺酸 Perfluorobutanesulfonic acid PFBS C4F9SO3H 375-73-5 18O2PFHxS 全氟己烷磺酸 Perfluorohexanesulfonic acid PFHxS C6F13SO3H 355-46-4 18O2PFHxS 全氟辛烷磺酸 Perfluorooctanesulfonic acid PFOS C8F17SO3H 1763-23-1 13C4PFOS 全氟癸烷磺酸 Perfluorodecanesulfonic acid PFDS C10F21SO3H 335-77-3 13C4PFOS 1.3 样品分析及实验方法
温度、溶解氧(DO)、pH、总溶解固体(TDS)、氧化还原电位(ORP)采用美国哈希公司的HQ40d多参数分析仪在现场测定;浊度采用哈希2100Q便携式浊度仪在现场测定;总有机碳(TOC)和总氮(TN)采用日本岛津公司的 TOC-L 总有机碳分析仪测定.
样品中的PAEs用C18固相萃取柱提取,富集倍数为100倍. 将500 mL各样品经0.45 μm尼龙滤膜过滤后置于1 L玻璃烧杯中备用. 萃取前,依次用5 mL甲醇、5 mL二氯甲烷、5 mL正己烷和5 mL超纯水活化C18小柱. 活化后,开始进行萃取,水样通过C18小柱的流速控制在每秒1滴. 水样全部通过小柱且淋洗完成后,利用真空泵抽干残余水分,然后用5 mL正己烷洗脱,取适量洗脱液,用外标法在日本岛津公司的TQ8030三重四极杆气相色谱质谱联用仪上进行检测. 色谱柱:Agilent DB-5MS UI 30 m×0.25 mm×0.25 μm, Intuvo 气相色谱柱 (货号:122-5532UI-INT);GC 部分:进样口温度 260℃,不分流进样,进样时间1 min;总流量50 mL·min−1,吹扫流量3 mL·min−1,柱流量1.2 mL·min−1,载气为恒压72.8 kPa,程序升温为: 初始温度60℃保持1 min,以10 ℃·min−1升至220 ℃保持1 min,再以7 ℃·min−1 升至260℃,保持10 min,总时间为33.71 min;MS部分:离子源温度230℃,接口温度为260℃,溶剂延迟时间7 min;各物质定性离子对和定量离子对分别为:DMP 163.0>133.1、163.0>77.1 ; DEP 177.0>149.0、177.0>93.1 ; DIBP 149.0>65.0、149.0>93.1;DBP 149.0>65.1、149.0>93.1;DMEP 149.0>65.1、149.0>93.1;BMPP 149.0>65.1、149.0>93.1;DEEP 176.0>149.0、176.0>104.1;DPP 149.0>65.1、149.0>93.1;DNHP 149.0>65.1、149.0>93.1;BBP 206.0>149.0、206.0>93.1;DBEP为193.0>149.0、193.0>93.1;DCHP 167.0>149.1、167.0>93.0;DNOP 149.0>65.1、149.0>93.1;DEHP 167.0>149.0、167.0>93.0;DINP 293.0>149.0、293.0>93.0.
样品中的全氟化合物(PFCs) 用WAX固相萃取柱提取. 样品各取400 mL加入2ng内标后不经过滤直接萃取,以检测窖水中PFCs的总浓度并考察浊度对PFCs浓度的影响. 萃取前,WAX小柱依次用4 mL甲醇、4 mL氨水甲醇和4 mL超纯水活化. 水样全部通过小柱且淋洗完成后,利用真空泵抽干残余水分,然后依次用4 mL甲醇、4mL 0.1% 氨水甲醇洗脱,将洗脱液氮吹至近干,用甲醇复溶至200 μL,浓缩倍数为2000倍,最后在电喷雾离子源负离子模式(ESI-)下采用内标法在美国安捷伦公司1290 Infinity HPLC系统与Agilent 6460三重四极杆质谱系统联用的高效液相色谱串联质谱仪上检测. 检测方法在本课题组已有研究中给出,采用其所用的定性定量参数,流速改为0.2 mL·min−1[14].
1.4 质量控制
为了保证测定结果的可靠,窖水样品中PAEs和PFCs的测定均设置有1个平行,1个方法空白、3个溶剂空白和1个现场空白(在现场用相同采样瓶,在相同时间装入娃哈哈纯净水),并进行了3个窖水样品和3个超纯水空白的加标回收.
PAEs的空白加标回收率为87.3%—103.7%,是在3份超纯水样品中加入1 μg·L−1混合标准样品测得的. PAEs的基质加标回收率为82.5%—101.6%,是在3份窖水样品中加入1 μg.L−1 混合标准样品测得的. 以信噪比S/N>3为检出限,PAEs检出限范围为0.09—0.19 μg·L−1,其中DIBP、DMEP、DBEP 3种物质的空白值达到了各自检出限分别为0.27、0.14、0.10 μg·L−1,在计算浓度时需进行差减扣除. 各物质相对标准偏差范围为1.27%—9.79%. PFCs检出限范围为0.01—0.09 ng·L−1,所有空白均未达到检出限. 各物质相对标准偏差范围为2.50%—12.82%. PFCs检测的空白加标回收率为80.5%—119.7%,是在3份超纯水样品中加入20 ng混合标准样品测得的. PFCs检测的基质加标回收率为75.3%—117.1%,是在3份窖水样品中加入20 ng混合标准样品测得的,检测中PAEs和PFCs的校准曲线回归系数(r2)均超过0.99.
1.5 健康风险评价模型
采用US EPA 提供的健康风险评价模型来评价窖水中PAEs,该评价模型包括致癌和非致癌两个部分[15-16]. 非致癌风险用风险值HI来表示,计算公式如下:
HI=CDIRfD (1) 式中,CDI是由于暴露造成的污染物长期日摄入剂量,
mg⋅(kg⋅d)−1 mg⋅(kg⋅d)−1 致癌风险则用R表示,是长期日摄入剂量与致癌斜率因子的乘积,计算公式如下:
R=CDI×SF (2) 式中,SF是致癌斜率因子,
(kg⋅d)⋅mg−1 饮用水途径暴露造成的污染物长期日摄入剂量计算公式如下:
CDI=C×U×EF×EDBW×AT (3) 式中,C为水中目标污染物的质量浓度,mg·L−1;U为日饮用水量,L·d−1;EF为暴露频率,d·a−1;ED为暴露持续时间,a;BW为平均体重,kg;AT为平均暴露时间,d.
以上公式中的参数取值来自文献[17]和中国人群暴露参数手册(成人卷)[18] : 夏、冬两季U分别取3.1 L·d−1和1.9 L·d−1(甘肃省);EF取365 d·a−1;ED对于非致癌物取30 a,对于致癌物取70 a;BW取61.8 kg(甘肃省); AT对于非致癌物取10950 d,对于致癌物取25550 d.
根据人群的PFCs饮水途径每日摄入量(estimated daily intake, EDI)来评估甘肃该县村民每日通过饮水摄入PFCs的健康风险. EDI计算公式如下:
EDI=Cf×VwaterBW 式中,Cf为窖水样品中PFCs的测定浓度,ng·L−1;Vwater为日饮水量,L·d−1;BW为平均体重,kg. 夏、冬两季Vwater分别取3.1 L·d−1和1.9 L·d−1(甘肃省);BW取61.8 kg(甘肃省).
把该县人群EDI值同美国卫生和公共服务部给出的PFOA、PFOS、PFHxS和PFNA人群每日可耐受摄入量参考值(分别为3、2、20 、3
ng⋅(kg⋅d)−1 2. 结果与讨论(Results and discussion)
2.1 窖水常规指标检测
冬季7个窖水样品的常规指标检测结果如表3所示,水温5.4—8.5℃,浊度1.8—29.5 NTU,pH 8.0—9.7,DO 4.9—8.8 mg·L−1,ORP 154.5—223.5 mV,TDS 59.6—110.0 mg·L−1,TOC 0.58—2.61 mg·L−1,TN 0.64—2.25 mg·L−1. 7个窖水样品pH均大于8,最高达到9.7. 不同窖水浊度差异较大,除了d、e、f 3个采样点窖水样品浊度低于《生活饮用水卫生标准》(GB5749-2022)中分散式供水所规定3 NTU,其它样品浊度均超出了标准中规定的限值.
表 3 冬季窖水样品常规指标检测结果Table 3. Test results of general parameters of cellar water samples in winter地点Position 温度/℃Temperature 浊度/NTUTurbidity pH 溶解氧/(mg·L−1)DO 氧化还原电位/mVORP 总溶解固体/(mg·L−1)TDS 总有机碳/(mg·L−1)TOC 总氮/(mg·L−1)TN a 8.5 11.7 9.7 6.9 154.5 61.4 0.69 0.99 b 7.8 29.5 8.0 7.7 207.0 92.4 2.61 2.25 c 7.9 4.2 8.8 6.2 223.5 67.7 1.02 0.78 d 6.1 1.8 9.0 4.9 207.7 59.6 0.58 1.00 e 5.8 3.2 8.6 5.6 196.3 62.4 0.62 1.02 f 6.2 2.3 8.4 5.2 213.3 65.6 0.73 1.23 g 5.4 6.1 8.6 8.8 185.4 110.0 1.62 0.64 h 7.2 5.3 8.3 5.9 218.6 68.0 0.98 0.68 夏季10个窖水样品的常规指标检测结果如表4所示,水温12.5—21.9℃,浊度4.7—58.5 NTU,pH 8.2—9.4,DO 2.9—7.6 mg·L−1,ORP 173.9—235.4 mV,TDS 46.4—238.0 mg·L−1,TOC 0.78—3.18 mg·L−1,TN 0.80—3.27 mg·L−1. 10个窖水样品pH均大于8,最高达到9.4.
表 4 夏季窖水样品常规指标检测结果Table 4. Test results of general parameters of cellar water samples in summer地点Position 温度/℃Temperatur 浊度/NTUTurbidity pH 溶解氧/(mg·L−1)DO 氧化还原电位/mVORP 总溶解固体/(mg·L−1)TDS 总有机碳/(mg·L−1)TOC 总氮/(mg·L−1)TN 1 18.8 30.6 8.8 5.9 193.2 51.3 1.91 2.07 2 21.3 58.5 9.4 6.3 211.7 223.0 3.18 3.27 3 12.5 4.7 9.0 7.6 176.5 62.9 0.78 1.38 4 17.0 18.8 8.5 6.0 204.0 60.0 1.39 0.98 5 21.9 14.1 8.5 4.8 188.2 68.7 1.19 0.80 6 20.2 11.8 8.4 6.5 226.7 61.6 1.18 1.97 7 19.3 15.1 8.6 5.1 189.7 51.7 1.34 0.99 8 21.6 19.6 8.5 4.3 235.4 238.0 1.56 2.45 9 19.5 8.8 8.3 2.9 228.0 46.4 1.22 2.05 10 18.2 19.0 8.2 5.6 173.9 53.7 1.51 1.68 夏季雨期采集的窖水样品TOC、TN、温度和浊度普遍高于冬季. 所有水窖样品浊度均超过GB5749-2022中分散式供水所规定限值,这是因为雨期水窖储水后没有经过自然沉淀,肉眼可见悬浮物较多. 同一采样点2(b)和8(g)样品浊度和TDS在各窖水样品中依然处于较高水平,实地调研发现2(b)水窖存在渗漏,8(g)水窖是集体水窖且位于道旁,两个水窖易受到周围环境的污染.
2.2 窖水中PAEs的浓度水平
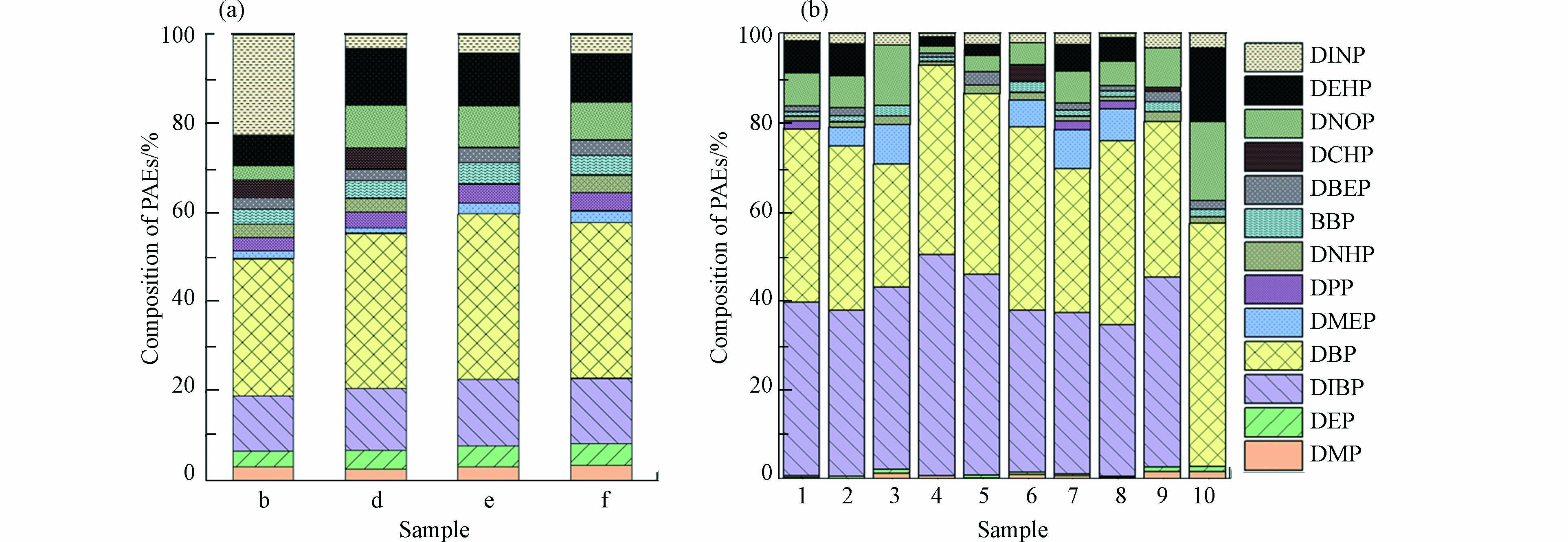
对冬季窖水样品b、d、e、f和夏季10个窖水样品进行PAEs组成及浓度的检测分析,结果如图2(a)、(b)所示. 15种目标PAEs中共有13种被检出,冬季样品总浓度平均值为3.12 μg·L−1. 其中DBP在各窖水样品中含量最高,平均值达到1.06 μg·L−1,其次是DIBP和DEHP,浓度平均值分别为0.43 μg·L−1和0.33 μg·L−1. 值得注意的是,b水窖中的DINP浓度较其它三口水窖高出约8倍,达到了0.84 μg·L−1. 夏季窖水样品总浓度平均值为4.56 μg·L−1,DBP和DIBP是各窖水样品的主要组成部分,浓度平均值分别为1.3μg·L−1和1.2 μg·L−1,两者占PAEs总量的64.5%—92.7%,高于冬季窖水两种物质比例43.5%—52.1%. 同一采样点2(b)、6(d)和7(f),夏季样品中DIBP浓度均显著高于冬季,而夏季样品中的DMP和DEP则略少于冬季. DIBP、DMP和DEP的LogP分别为4.46、1.64和2.70,与DMP和DEP相比,DIBP表现出更大的疏水性. 冬季窖水在长期自然沉淀过程中,疏水性更强的DIBP更易于随着颗粒物沉淀,而亲水性较强的DMP和DEP则在窖水中稳定存在,甚至会因颗粒物的释放,导致窖水中的溶解性PAEs含量上升,这可能是夏季窖水样品中DIBP浓度较冬季窖水低,而DMP和DEP浓度低于冬季的原因.
窖水中PAEs的可能来源:一是农村常使用塑料薄膜来建造温室大棚。塑料膜在使用及降解过程中,其含有的PAEs,最终可以水为介质进入环境中. 二是农村卫生状况相对较差,破损塑料膜、垃圾袋及包装纸等随意丢弃后,其中的PAEs可能通过降雨或径流进入到集雨水窖之中,带来水质风险[19].
通过与文献报道的其他水源中PAEs含量对比发现,一般地表水中的PAEs浓度范围波动较大,如三峡库区中6种优先控制PAEs(DMP、DEP、DBP、BBP、DEHP和DNOP)的总浓度为0.42—0.77 μg·L−1[20],远小于窖水中这6种PAEs的总浓度0.85—4.56 μg·L−1,而黄河甘肃兰州段干湿季PAEs的平均总浓度分别为3.24 μg·L−1和2.30 μg·L−1,与窖水中PAEs总浓度平均值相近[21]. 此外,窖水中的PAEs浓度普遍高于地下水,如东莞地区地下水中6种PAEs(DMP、DEP、DNBP、BBP、DEHP和DNOP)总浓度平均值为0.93 μg·L−1[22],湖北江汉地下水中PAEs总浓度平均值为0.98 μg·L−1[23],均小于此次窖水中的含量3.12 μg·L−1(冬季)和4.56 μg·L−1(夏季).
2.3 窖水中PFCs的浓度水平
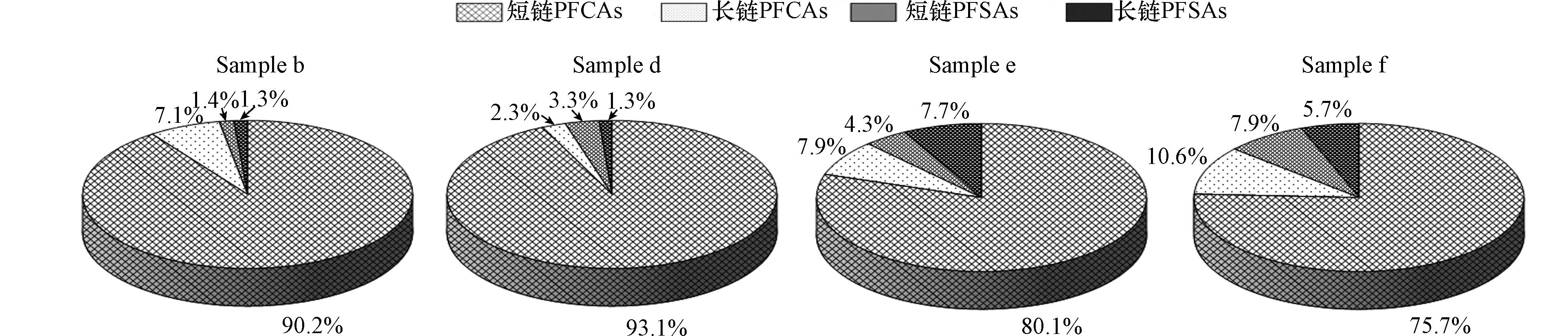
采用与PAEs测定相同的样品进行PFCs分析. 结果如图3和图4所示,所有窖水样品中,17种目标PFCs中共有14种被检出. 图3是冬季4个窖水样品PFCs组成情况,图4是夏季10个窖水样品PFCs组成情况,冬季PFCs总浓度范围为143.93—246.47 ng·L−1,夏季PFCs总浓度范围为275.90—405.51 ng·L−1,各窖水样品中的PFCs由PFCAs和PFSAs两部分组成. 其中PFCAs占窖水PFCs总量的80%以上,居于主体地位. PFCAs和PFSAs根据碳链长度可分为长链和短链,短链PFCAs占窖水PFCs总量的75%以上,全氟丁酸(PFBA)是短链PFCAs中含量最高的污染物,冬季窖水样品中平均浓度达到88.09 ng·L−1,而夏季窖水样品中平均浓度能达到214.98 ng·L−1.
同一采样点2(b)、6(d)和7(f),除PFPeA和PFHpA外,夏季雨期窖水PFCs浓度均高于冬季,尤其是PFOA和PFOS在所有PFCs中的浓度占比分别从6.7%和1.4%上升到了9.7%和15.1%. 这可能是由于夏季当地造纸、皮革和石油开采等氟化工产业扩大生产,提高产能,使得PFCs排放量上升,这些PFCs进入大气后随着降雨过程进入到水窖中,使得窖水PFCs浓度水平上升.
与地表水和地下水中的PFCs含量对比可知,相比于上海黄浦江和山东部分区域地表水中10种PFCs (PFBA、PFPeA、PFBS、PFHxA、PFHxS、PFHpA、PFOA、PFOS、PFNA、PFDA)总浓度(3.38—362.37 ng·L−1和35.71—1236.21 ng·L−1)[24-25],窖水10种PFCs总浓度121.82—395.16 ng·L−1处于中间水平,波动范围相对较小. 而地下水中的PFCs含量普遍较低,如天津市郊区和北京市部分地区地下水PFCs总浓度范围分别为0.32—8.30 ng·L−1和N.D.—165.80 ng·L−1[26-27],均低于窖水和地表水中的PFCs浓度. 可以发现,不同水环境中PFCs含量的差异与其所处区域周围工业活动以及水体自身特点密切相关. PFCs在工业发达地区水环境中检出率高,地表水作为工业废水处理后的受纳水体,易于受到污染. 而地下水由于经过渗流,水质一般较好. 水窖中的PFCs则可能来自于大气污染物湿沉降和集雨面径流,受间接污染.
2.4 窖水中新污染物的健康风险评估
根据美国环保署优先控制污染物名单和本次窖水检测出的污染物浓度水平,选择DMP、DEP、DIBP、DBP、DEHP和DNOP 等6种PAEs进行健康风险评价,除了对DEHP进行致癌风险评价外,其它5种PAEs均进行非致癌风险评价. 根据相关研究得到DMP、DEP、DIBP、DBP、DNOP等 5种PAEs的参考剂量RfD 分别为 0.1、0.8、0.098、0.1、0.02 mg·(kg·d)−1;DEHP的致癌斜率因子SF为0.014 (kg·d)·mg−1[28]. 将所有参数代入“1.5”中的公式(1)—(3),得到冬夏两季窖水中这6种PAEs的致癌和非致癌风险值. 非致癌风险值HI值小于1时,该污染物浓度不会存在非致癌风险,反之该污染物将存在非致癌健康风险. 致癌风险R值小于10−6 时,不会对人产生致癌风险;R为
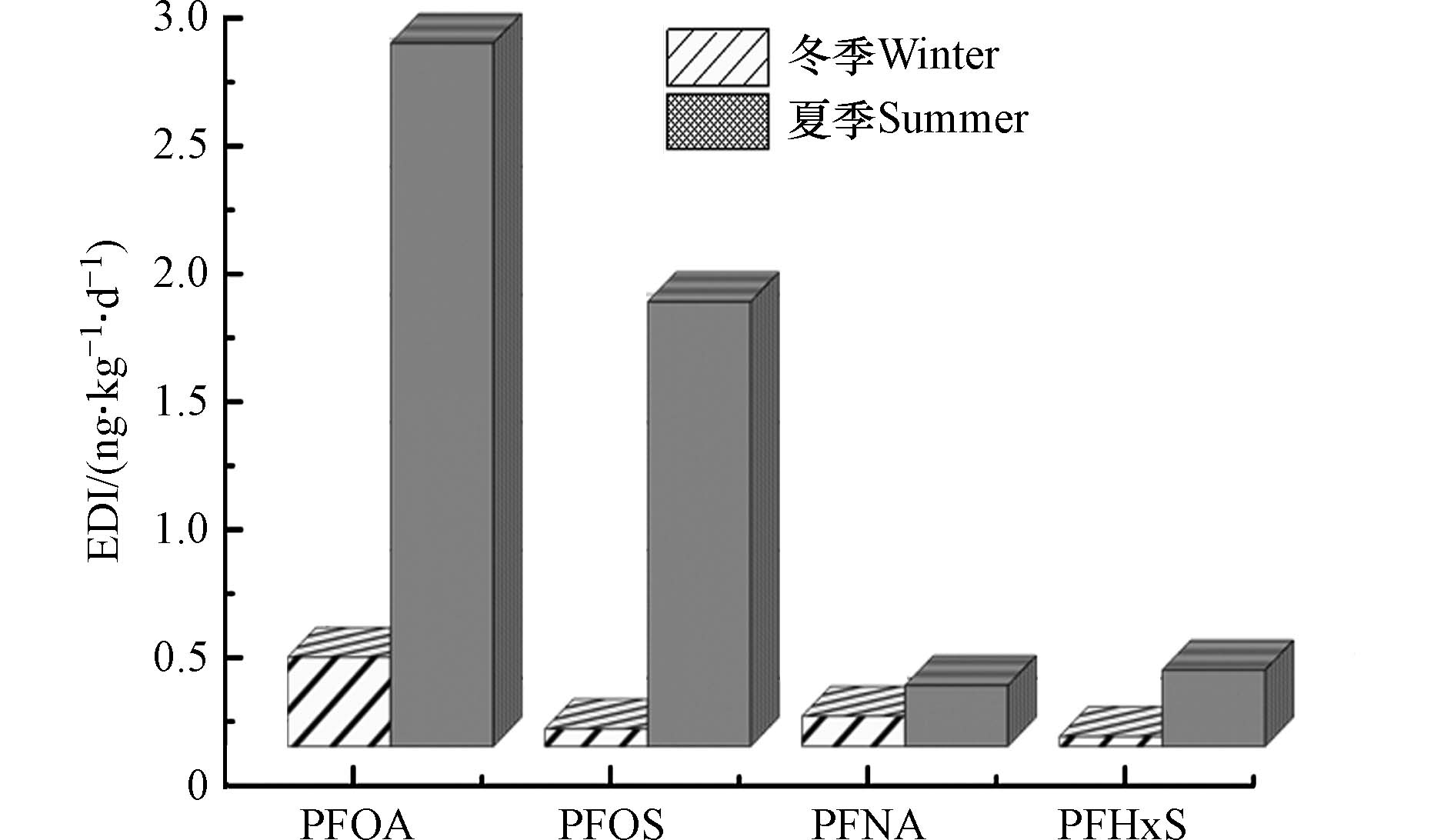
10−6—10−4 4.75×10−6— 5.11×10−4 1.11×10−7—1.86×10−7 1.11×10−7—1.35×10−3 3.39×10−8—2.99×10−7 根据美国卫生和公共服务部提出的人群饮用水途径全氟化合物每日耐受量和两季窖水中全氟化合物的检出情况,选择PFOA、PFOS、PFNA和PFHxS等 4种PFCs进行EDI值的计算,来评估健康风险水平. 经计算,冬季PFOA的EDI平均值为0.35
ng⋅(kg⋅d)−1 ng⋅(kg⋅d)−1 ng⋅(kg⋅d)−1 ng⋅(kg⋅d)−1 ng⋅(kg⋅d)−1 ng⋅(kg⋅d)−1 ng⋅(kg⋅d)−1 ng⋅(kg⋅d)−1 3. 结论(Conclusion)
(1)甘肃该县冬、夏两季的窖水均呈现弱碱性(8.0≤pH≤9.7),浊度普遍偏高,且夏季雨期窖水浊度显著高于冬季.
(2)15种目标PAEs中共有13种在窖水中检出,冬季窖水中PAEs总浓度平均值为3.12 μg·L−1,夏季为4.56 μg·L−1. DBP和DIBP是各窖水样品的主要组成部分. 夏季雨期窖水中PAEs总浓度平均值明显高于冬季.
(3)17种目标PFCs中共有14种在窖水中检出,冬季PFCs总浓度范围为143.93—246.47 ng·L−1,夏季PFCs总浓度范围为275.90—405.51 ng·L−1. 窖水样品中的PFCs由PFCAs和PFSAs两部分组成,短链PFCAs中的全氟丁酸(PFBA)在PFCs中含量最高. 夏季雨期窖水PFCs浓度水平显著高于冬季,尤其是PFOA和PFOS在所有PFCs中的浓度占比显著上升.
(4)该县两季窖水中PAEs无致癌风险和非致癌风险;PFCs中的PFOA、PFOS、PFNA和PFHxS的平均EDI值均小于参考值. 此外,夏季窖水中PFOA和PFOS的EDI值大于冬季,且个别窖水EDI值超过参考值,应该予以重视.
-
表 1 SRB富集培养基的组成
Table 1. Composition of SRB enrichment medium
药品名称Pharmaceutical ingredients 质量浓度/(g·L−1)Mass concentration K2HPO4 0.5 (NH4)2SO4 2.5 NaHCO3 0.5 CaCl2 0.2 MgSO4 1 乳酸钠sodium lactate 20 mL·L−1 L-抗坏血酸L-Ascorbic acid 0.1 L-半胱氨酸盐酸盐L-Cysteine hydrochloride monohydrate 0.5 酵母膏yeast extract 1.5 (NH4)2Fe(SO4)2·6H2O 0.5 表 2 不同处理中沉淀物中的原子比(Cu与络合剂物质的量比为1:10)
Table 2. The atomic ratios of precipitates from different treatments (molar ratio of Cu and complexing agent =1:10).
元素Element 原子比/%Atom Shewanella sp. JN01 Cu2+ Cu-EDTA Cu-GLDA Cu-CA Cu-MC C 63.30 67.13 78.21 71.85 72.00 75.19 O 29.85 19.45 19.03 16.13 23.28 21.69 S 6.85 9.07 1.70 6.64 3.36 1.57 Cu ND 4.34 1.07 5.38 1.36 1.56 ND.,未检出. ND., not detected. -
[1] LIU L W, LI W, SONG W P, et al. Remediation techniques for heavy metal-contaminated soils: Principles and applicability [J]. Science of the Total Environment, 2018, 633: 206-219. doi: 10.1016/j.scitotenv.2018.03.161 [2] WANG Z Z, WANG H B, WANG H J, et al. Effect of soil washing on heavy metal removal and soil quality: A two-sided coin [J]. Ecotoxicology and Environmental Safety, 2020, 203: 110981. doi: 10.1016/j.ecoenv.2020.110981 [3] GONG Y Y, ZHAO D Y, WANG Q L. An overview of field-scale studies on remediation of soil contaminated with heavy metals and metalloids: Technical progress over the last decade [J]. Water Research, 2018, 147: 440-460. doi: 10.1016/j.watres.2018.10.024 [4] 郭军康, 李艳萍, 李永涛, 等. 采用草酸和EDTA去除农田土壤中砷和镉污染 [J]. 环境工程, 2019, 37(5): 70-75. doi: 10.13205/j.hjgc.201905014 GUO J K, LI Y P, LI Y T, et al. Treatment of arsenic and cadmium in contaminated farmland soil with oxalic acid and EDTA [J]. Environmental Engineering, 2019, 37(5): 70-75(in Chinese). doi: 10.13205/j.hjgc.201905014
[5] WANG G Y, PAN X M, ZHANG S R, et al. Remediation of heavy metal contaminated soil by biodegradable chelator-induced washing: Efficiencies and mechanisms [J]. Environmental Research, 2020, 186: 109554. doi: 10.1016/j.envres.2020.109554 [6] GUAN W, ZHANG B F, TIAN S C, et al. The synergism between electro-Fenton and electrocoagulation process to remove Cu-EDTA [J]. Applied Catalysis B:Environmental, 2018, 227: 252-257. doi: 10.1016/j.apcatb.2017.12.036 [7] 郑雄开, 陶雪琴, 杜建军, 等. 模拟土壤淋洗废液中重金属的选择性去除与淋洗液的回收研究 [J]. 环境科学学报, 2020, 40(3): 995-1003. doi: 10.13671/j.hjkxxb.2019.0416 ZHENG X K, TAO X Q, DU J J, et al. Selective removal of heavy metals from simulated wastewater from leaching soil and recovery of eluent [J]. Acta Scientiae Circumstantiae, 2020, 40(3): 995-1003(in Chinese). doi: 10.13671/j.hjkxxb.2019.0416
[8] 薛璐璐, 袁翔, 朱梦羚, 等. 高级氧化法破络处理柠檬酸铜镍电镀废水 [J]. 净水技术, 2019, 38(3): 9-14,50. doi: 10.15890/j.cnki.jsjs.2019.03.003 XUE L L, YUAN X, ZHU M L, et al. Complex breakdown treatment for copper-nickel citrate electroplating wastewater by advanced oxidation process(AOP) [J]. Water Purification Technology, 2019, 38(3): 9-14,50(in Chinese). doi: 10.15890/j.cnki.jsjs.2019.03.003
[9] LI X, LAN S M, ZHU Z P, et al. The bioenergetics mechanisms and applications of sulfate-reducing bacteria in remediation of pollutants in drainage: A review [J]. Ecotoxicology and Environmental Safety, 2018, 158: 162-170. doi: 10.1016/j.ecoenv.2018.04.025 [10] KIEU H T Q, MÜLLER E, HORN H. Heavy metal removal in anaerobic semi-continuous stirred tank reactors by a consortium of sulfate-reducing bacteria [J]. Water Research, 2011, 45(13): 3863-3870. doi: 10.1016/j.watres.2011.04.043 [11] 董净, 代群威, 赵玉连, 等. 硫酸盐还原菌的分纯及对Cd2+钝化研究 [J]. 环境科学与技术, 2019, 42(5): 34-40. DONG J, DAI Q W, ZHAO Y L, et al. Isolation of sulfate-reducing bacteria and study on its passivation of Cd2+ [J]. Environmental Science & Technology, 2019, 42(5): 34-40(in Chinese).
[12] MOHAPATRA R K, PARHI P K, PANDEY S, et al. Active and passive biosorption of Pb(II)using live and dead biomass of marine bacterium Bacillus xiamenensis PbRPSD202: Kinetics and isotherm studies [J]. Journal of Environmental Management, 2019, 247: 121-134. [13] GU W Z, ZHENG D C, LI D P, et al. Integrative effect of citrate on Cr(Ⅵ) and total Cr removal using a sulfate-reducing bacteria consortium [J]. Chemosphere, 2021, 279: 130437. doi: 10.1016/j.chemosphere.2021.130437 [14] CASTRO L, BLÁZQUEZ M L, GONZÁLEZ F, et al. Anaerobic bioleaching of jarosites by Shewanella putrefaciens, influence of chelators and biofilm formation [J]. Hydrometallurgy, 2017, 168: 56-63. doi: 10.1016/j.hydromet.2016.08.002 [15] HÅKANSSON T, SJÖBERG S, MATTIASSON B. Treatment of metal ions and metal-chelate complexes in water with biologically produced H2S [J]. International Journal of Environment and Waste Management, 2012, 9(3/4): 330. doi: 10.1504/IJEWM.2012.046396 [16] LARKIN M A, BLACKSHIELDS G, BROWN N P, et al. Clustal W and Clustal X version 2.0 [J]. Bioinformatics (Oxford, England), 2007, 23(21): 2947-2948. doi: 10.1093/bioinformatics/btm404 [17] GUO X F, ZHAO G H, ZHANG G X, et al. Effect of mixed chelators of EDTA, GLDA, and citric acid on bioavailability of residual heavy metals in soils and soil properties [J]. Chemosphere, 2018, 209: 776-782. doi: 10.1016/j.chemosphere.2018.06.144 [18] PENG H L, LI D, YE J, et al. Biosorption behavior of the Ochrobactrum MT180101 on ionic copper and chelate copper [J]. Journal of Environmental Management, 2019, 235: 224-230. [19] CHEN M X, ZHANG Y, ZHOU J T, et al. Sulfate removal by Desulfovibrio sp. CMX in chelate scrubbing solutions for NO removal [J]. Bioresource Technology, 2013, 143: 455-460. doi: 10.1016/j.biortech.2013.06.037 [20] WANG Q W, CHEN J J, ZHENG A H, et al. Dechelation of Cd-EDTA complex and recovery of EDTA from simulated soil-washing solution with sodium sulfide [J]. Chemosphere, 2019, 220: 1200-1207. doi: 10.1016/j.chemosphere.2018.12.212 [21] WANG G Y, ZHANG S R, XU X X, et al. Heavy metal removal by GLDA washing: Optimization, redistribution, recycling, and changes in soil fertility [J]. Science of the Total Environment, 2016, 569/570: 557-568. doi: 10.1016/j.scitotenv.2016.06.155 [22] HUANG F, DANG Z, GUO C L, et al. Biosorption of Cd(Ⅱ) by live and dead cells of Bacillus cereus RC-1 isolated from cadmium-contaminated soil [J]. Colloids and Surfaces B:Biointerfaces, 2013, 107: 11-18. doi: 10.1016/j.colsurfb.2013.01.062 [23] SHAHSAVARI S, SETH R, CHAGANTI S R, et al. Inhibition of anaerobic biological sulfate reduction process by copper precipitates [J]. Chemosphere, 2019, 236: 124246. doi: 10.1016/j.chemosphere.2019.06.216 [24] GUZMAN J, SAUCEDO I, REVILLA J, et al. Copper sorption by chitosan in the presence of citrate ions: Influence of metal speciation on sorption mechanism and uptake capacities [J]. International Journal of Biological Macromolecules, 2003, 33(1/2/3): 57-65. [25] LU P J, HU W W, CHEN T S, et al. Adsorption of copper-citrate complexes on chitosan: Equilibrium modeling [J]. Bioresource Technology, 2010, 101(4): 1127-1134. doi: 10.1016/j.biortech.2009.09.055 [26] 袁媛, 刘自成, 李杰, 等. 新型生物质基复合水凝胶球珠高效吸附去除Ni-EDTA络合物的特性与机制 [J]. 离子交换与吸附, 2021, 37(1): 1-13. doi: 10.16026/j.cnki.iea.2021010001 YUAN Y, LIU Z C, LI J, et al. The removal of Ni-EDTA complex by a novel biomass based MCS/SA@PEI composite hydrogel beads [J]. Ion Exchange and Adsorption, 2021, 37(1): 1-13(in Chinese). doi: 10.16026/j.cnki.iea.2021010001
[27] ZHANG X L, HUANG P, ZHU S Y, et al. Nanoconfined hydrated zirconium oxide for selective removal of Cu(Ⅱ)-carboxyl complexes from high-salinity water via ternary complex formation [J]. Environmental Science & Technology, 2019, 53(9): 5319-5327. [28] PARSADOUST F, SHIRVANI M, SHARIATMADARI H, et al. Effects of GLDA, MGDA, and EDTA chelating ligands on Pb sorption by montmorillonite [J]. Geoderma, 2020, 366: 114229. doi: 10.1016/j.geoderma.2020.114229 [29] TYAGI S, MALIK W, ANNACHHATRE A P. Heavy metal precipitation from sulfide produced from anaerobic sulfidogenic reactor [J]. Materials Today:Proceedings, 2020, 32: 936-942. doi: 10.1016/j.matpr.2020.05.076 [30] BAI H, KANG Y, QUAN H E, et al. Treatment of copper wastewater by sulfate reducing bacteria in the presence of zero valent iron [J]. International Journal of Mineral Processing, 2012, 112/113: 71-76. doi: 10.1016/j.minpro.2012.06.004 [31] ZHANG M L, WANG H X, HAN X M. Preparation of metal-resistant immobilized sulfate reducing bacteria beads for acid mine drainage treatment [J]. Chemosphere, 2016, 154: 215-223. doi: 10.1016/j.chemosphere.2016.03.103 期刊类型引用(0)
其他类型引用(3)
-







 下载:
下载: