-
地下水是人类的主要水源之一。然而现代工业、农业的发展严重威胁地下水环境。据统计,2019年全国85.7%地下水水质为Ⅳ和Ⅴ类水[1],其中铁、锰和硝酸盐、亚硝酸盐是地下水污染的主要贡献指标,铁、锰的超标率为20%以上[2]。这些超标的污染物不同程度的对人体健康产生威胁,人体摄入的硝酸盐很容易转化为亚硝酸盐,通过血红蛋白不可逆地转化为高铁血红蛋白,从而抑制了体内的氧交换[3]。亚硝酸盐长时间暴露在体内会导致胃癌,高血压,冷球蛋白血症,先天性残疾和流产[4]。虽然微量的铁和锰是人体中不可缺少的元素,但是过量的铁和锰会引起帕金森氏病的发展并影响儿童的神经功能[5]。因此需要广泛地对地下水水质进行长期连续监测并扩大乡镇中以地下水为水源地的检测范围。目前水中硝酸盐、亚硝酸盐和重金属的常用的检测手段是离子色谱、火焰原子吸收光谱和电感耦合等离子体质谱等,这往往需要昂贵设备、专业操作人员和复杂前处理而降低检测速率,因此难以满足当前地下水快速检测要求。
相较于传统检测方法,基于光学传感器和电化学传感器检测方法则快速高效,且某些离子的检出限甚至低于专业的大型设备[6]。由于表面增强拉曼、纸基微流控等光谱技术和电化学方法对样品检测条件要求低、检测灵敏高效且分析检测设备便携,因此这些快速检测方法被广泛应用于营养盐、重金属离子检测中。
快速检测方法在环境中的应用的综述大多集中在某一类检测方法和重金属的检测,很少有关于地下水中主要无机污染物的快速检测方法的综述。由于纳米材料和微流控技术等快速检测方法在实际地下水检测中展现出巨大的应用潜力,因此本文综述了近10年来水中主要污染物硝酸盐、亚硝酸盐、铁和锰离子快速检测方法的研究进展,介绍各种快速检测方法的优势和局限性,指出在现场快速检测中可能面临的挑战,提出一种针对复杂水体的污染快速评估方案,并对今后的快速检测发展做出展望。
-
分光光度法是是当前使用最广泛的检测方法[7],主要是基于化学试剂与待测物质发生化学反应作用显色。按照亚硝酸盐的反应原理不同,可以分为Griess分光光度法、亚硝化分光光度法、催化分光光度法[8]。分光光度法中铁和锰的检测方法为邻菲啰啉分光光度法和高碘酸钾分光光度法。然而这些方法往往需要复杂的前处理,并且显色时间较长[9],离子干扰严重[10],并不适用于现场快速检测。
-
比色法是一种通过分析物与合适试剂发生特定反应而产生的颜色变化来检测分析物的技术[11-12]。比色法操作方便、成本低和响应快,以及小分子探针[13]和纳米材料高比表面积和光学特性促进了比色法在离子快速检测中的应用。其中,使用金属纳米材料对污染物通过比色法进行快速检测成为了当前的研究热点[14-15]。贵金属纳米颗粒与一般金属纳米颗粒相比,Au和Ag纳米颗粒具有表面等离子共振和距离相关的光学特性[16],并且贵金属纳米颗粒具有制备简单,良好的生物相容性,化学修饰简单的特点。其主要的检测原理如图1所示,经过化学修饰的贵金属纳米颗粒与检测离子间发生络合或者改变静电斥力而导致纳米颗粒的聚集状态和形貌尺寸的变化,从而引起纳米颗粒在溶液中颜色的变化,进而通过最大吸收波长的变化计算污染物的浓度。
Kumar等合成了酚醛螯合配体修饰的AgNPs比色传感器,加入NO2−使合成的AgNPs的棕黄色脱色,亚硝酸盐的检测限为0.1 μmol·L−1,然而检测时的加成反应合成酚类螯合配体反应时间较长,颜色变化不明显,肉眼较难识别[18]。Ibrahim等使用4-氨基-5-羟基萘-2,7-二磺酸单钠盐(AHNDMS)制备了银纳米颗粒(AgNPs),并使用氨基苯甲酸(PABA)进行了功能化,亚硝酸根离子的加入会使重氮基与芳香基团结合形成偶氮基团,进而引起AgNPs的聚集,使颜色由棕黄色变为紫红色。其肉眼可识别的浓度至0.016 mg·L−1[19]。
Au/Ag纳米颗粒比色法在铁的快速检测中也有着广泛的研究。Memon等合成了一种使用乙酰水杨酸修饰的金纳米颗粒,加入Fe3+后,纳米颗粒的尺寸增加,从而使溶液的颜色由粉红色变为蓝色,其定量限为0.17 μmol·L−1[20]。Lu等使用了另一种策略,在酸性条件下Fe2+与H2O2反应生成超氧化物自由基,该自由基纵向刻蚀金纳米颗粒使其粒径减小从而引起金纳米颗粒的吸收光谱和颜色的变化,其检测限为13.5 nmol·L−1,线性响应浓度范围为75—1000 nmol·L−1[21]。
吴等开发了一种基于银纳米颗粒的比色传感器,在pH 12.0的条件下Mn2+与使用焦磷酸钠和羟丙基甲基纤维素修饰的银纳米颗粒产生特异性聚集从而使溶液颜色由黄色变为棕色,检出限为20 nmol·L−1,线性范围为0.15—15 μmol·L−1,同时改变溶液酸碱度至pH 1.0可以同时检测Cu2+[22]。该方法合成法复杂,抗干扰能力较弱。与之相反,He等提出基于银纳米颗粒的抗聚集作用的比色传感器,在没有Mn2+的条件下L-精氨酸会使银纳米颗粒聚集,引入锰离子后,精氨酸与Mn2+络合阻止AgNPs的聚集,从而使颜色由无色转为黄色,检出限为20 nmol·L−1,线性范围为0—700 nmol·L−1和5—70 μmol·L−1。值得注意的是,该方法在实际水样中检测需要稀释以降低基质干扰[23]。
-
荧光光谱法因其高灵敏度,良好的选择性,极佳的检测限和简便的操作步骤亦被广泛地用于亚硝酸盐和重金属的检测中[24]。传统的硝酸盐和亚硝酸盐的荧光光谱法检测主要是利用亚硝酸根化学特异性的亚硝化和重氮化作用而引起的荧光信号增强而定量,铁和锰离子浓度的荧光检测原理是通过离子对有机荧光材料或纳米无机荧光材料产生的荧光信号的猝灭或增强来定量浓度。普通的检测试剂产生的荧光信号弱,离子干扰严重,选择性较差,只能进行单一目标检测。
虽然一些有机荧光染料如具有聚集诱导发射(AIE)效应的小分子荧光材料表现优异[25],但荧光纳米材料具有更高的荧光强度和光稳定性[26]。纳米荧光材料主要有金属纳米团簇(MNC)、荧光金属有机骨架(FMOF)和量子点(QDs)。MNC的稳定性较差,因此很容易聚合[27];FMOF具有相对较低的水热稳定性和化学稳定性,骨架易塌陷[28];QDs发射光谱宽,斯托克斯位移大,制备简单。在此重点综述量子点在荧光快速检测上的机理和应用。
自1980年代初发现量子点以来,它就引起了越来越多的关注[29]。QDs包括半导体量子点和碳量子点(CQD)。半导体量子点材料主要由分布在元素周期表中Ⅱ—Ⅵ、Ⅲ—Ⅴ或Ⅳ—Ⅵ组成且其粒径小于激子波尔半径[30],尺寸约为2—20 nm,因此由于量子限域效应使得连续能带结构分裂而产生荧光。此外,其窄发射的异常变化使得半导体量子点亦有具有多路复用的特点[30]。根据量子点表面电荷或配体成分的变化影响电子-空穴复合效率而影响的光致发光效能,可以通过量子点表面的离子或分子在的吸附或螯合作用而产生的荧光变化来检测污染物[31]。其猝灭方式如图2所示,荧光配体与分析物的结合、荧光物质的解离或团聚导致荧光猝灭。半导体量子点中研究较为广泛的是CdSe、ZnS、CdS、PbSe QDs等。为了进一步改善量子点缺陷,提高电子转移速率和光化学性能,一般通过配体交换、增加聚合物涂层等方式修饰量子点。Yang等通过引入杂化元素的方式经简单步骤合成三元ZnCdS量子点可检测NO2−,NO2−与量子点表面结合从而猝灭荧光信号,线性检测区间为180—700 μmol·L−1,LOD为0.780 μmol·L−1[32]。Ren等引入了聚合物涂层聚乙烯亚胺封装CdS量子点以实现对水中亚硝酸盐的检测,其检测原理为NO2−与聚乙烯亚胺通过静电吸引作用结合并发生电子转移从而引起静态猝灭,线性检测范围为0.1—100 μmol·L−1[33]。与之相似,Singh等通过水热法自下而上一步合成的1-半胱氨酸功能化蓝色荧光WS2量子点,在水溶液中表现出高稳定性、高溶解度和对Fe3+的高选择性,荧光的猝灭机制为Fe3+与量子点表面羟基络合而导致静态猝灭以及Fe3+与大量孤对电子氨基结合而抑制了Fe3+的3d轨道电子向量子点的转移而导致的动态猝灭,对Fe3+的检测限为1.32 μmol·L−1[34]。此外,He等通过经乳液自封装的具有蓝色荧光的两亲嵌段共聚物和两种类型的疏水半导体量子点ZnCdSe / ZnS QD合成了可以同时检测Hg2+、Cu2+和Fe3+离子的编码荧光传感器。Hg2+和Cu2+的荧光猝灭是与量子点表面产生离子交换,Fe3+是与表面活性剂表面络合而产生荧光猝灭[35]。此外,近年来钙钛矿量子点备受关注,在Cu2+[36],Yb3+[37]和有机氯农药等检测方面展现优秀的潜力,然而在检测亚硝酸盐和铁锰等离子方面鲜有报道。虽然半导体量子点有着优异特性,但是高毒性制约着其广泛的使用。
碳量子点(CQD)是一种直径小于10 nm的具有荧光特性的碳纳米粒子,首次在2004年纳米碳管纯化过程中发现[38]。相比于半导体量子点,碳量子具有低毒性,化学惰性,优异的生物相容性,激发光谱宽,发射光谱窄,抗光漂白性和成本低的特点[39]。CQD通常是准球形的纳米颗粒,包含无定形至纳米晶核,主要具有石墨或涡轮层碳或石墨烯通过类金刚石的sp3杂化碳插入物融合而成[40]。其荧光机制有两种,其中一种为共轭π域跃迁而产生的荧光,另一种是CQD表面缺陷。由于量子点本身的荧光信号较弱且不稳定,因此往往需要对其进行表面钝化以避免少量污染物对CQD污染而降低荧光强度[41]和化学修饰以增加激发能阱而增加荧光强度[42]。
Li等以传统的亚硝酸盐重氮化反应为基础,通过一锅法水热合成伯芳基胺碳量子点(PA-CDs)。PA-CDs表面伯烷基胺在酸性条件下重氮化后与亚硝酸盐发生偶氮反应,从而使处于激发态的PA-CDs中的电子向偶氮化合物转移,从而引起荧光猝灭。检测了24种阳离子和阴离子并未发现其对PA-CDs的干扰作用,通过自来水实际样品检测回收率为99.2%,其LOD为7.1 nmol·L−1,线性区间为0.05—1 μmol·L−1和1—50 μmol·L−1[43]。虽然该量子点检测灵敏,但是重氮化反应要求环境在pH 2的条件以及反应速率较慢,制约着实际的应用。Wu等提出了一种可同时多目标高效检测方法,通过Tb3+修饰碳量子点/3-氨基苯基硼酸实现了同时对Hg2+和NO2−的检测,其主要的原理是NO2−仅仅可以在波长373 nm处猝灭通过天线效应从碳量子点到Tb3+的能量转移而发射强的Tb3+特征荧光,Hg2+仅在波长为545 nm处对3-氨基苯基硼酸产生猝灭。亚硝酸盐的线性检测范围为5—1200 nmol·L−1[44]。
为了提高CQDs的检测的准确性和灵敏度,往往会采用双传感器检测或掺杂元素。Shi等合成了可以同时使用荧光和比色法的碳量子点。该碳点通过微波将柠檬酸(CA)、支化聚乙烯亚胺(BPEI)和硫氰酸钾(KSCN)反应制备,其主要的作用原理是Fe3+与碳点表面的官能团鳌合而产生荧光猝灭并显色,从而实现比色、荧光双传感器检测。其线性浓度范围为1—150 μmol·L−1,检出限为52 nmol·L−1[45]。 N[46]、B、S[47]和Mn[48]、Cd[49]等金属离子的掺杂可极大增强其量子产率等光学特性[50]。大多数的CQDs都会受到Fe2+,Cu2+的干扰,Wu等通过水热法合成了硼和氮共掺杂碳点通过荧光猝灭作用表现出良好的pH稳定性和对Fe3+的出色选择性,其线性范围在0.5—80 μmol·L−1和80—200 μmol·L−1,检出限为0.1 μmol·L−1[51]。Zhu等合成了可同时检测Hg2+和Fe3+的氮掺杂CQD,该NCQD以酒石酸和L-精氨酸为前驱体通过溶剂热法合成。Fe3+和Hg2+均可猝灭NCQD荧光,引入硫脲后Hg+与之络合并可增强荧光信号,Fe3+与硫脲并不发生鳌合反应,不改变荧光强度,因此通过硫脲可以同时区分Hg2+和Fe3+,该NCQD的LOD为0.5 μmol·L−1,线性区间为0—70 μmol·L−1。虽然硫脲的加入可以同时检测多种污染物,然而引入额外的试剂会使检测过程复杂化,并可能导致发射强度的波动并干扰痕量水平分析的准确性[52]。Bandi等在未加入任何掩蔽剂的情况下合成的NCQD可以实现Fe3+和Cu2+的同时检测,该量子点由邻苯二胺和2,5吡啶二羧酸作为前体一步水热法合成。由于Fe3+与NCQD表面的酚羟基或氨基络合而产生荧光猝灭且明显降低荧光寿命,Cu2+仅与氨基的特异性相互作用形成铜胺络合物但并未影响荧光寿命,基于此可以实现两种金属离子的同时检测。该量子点对Fe3+的线性响应范围为3—60 μmol·L−1和LOD为0.31 μmol·L−1[53]。
Hu等基于硫、氯和氮共掺杂的碳量子点(S, Cl, N- CQDs)来高效测定Mn (Ⅶ),其原理为由于内部滤光效应(IFE)和动态猝灭效应,Mn (Ⅶ)对S, Cl, N- CQDs产生荧光猝灭。检出限为12.5 nmol·L−1,线性区间为0.05—110 μmol·L−1[54]。Wang等通过表面修饰和N掺杂的方式合成了一种羧甲基壳聚糖-β-环糊精修饰的氮碳量子点(N-CQD / CCSCD)用于检测水中的Mn2+,由于Mn2+的螯合作用,N-CQD / CCSCD快速聚集从而引起荧光猝灭,其线性检测范围为0—21.1 μmol·L−1,LOD为5.3 nmol·L−1[55]。
-
表面增强拉曼(surface enhanced raman spectroscopy,SERS)是一种新兴的光谱技术,依靠光谱仪激发激光对目标分析物和特定底物之间的电子和化学相互作用来选择性增强目标分子的拉曼信号[56],从而进行检测。它具有检测快速、灵敏、破坏性小[57]、无需前处理的特点,被认为是一种有实际应用前景的技术[56]。Chen等开发了一种Fe3O4 @SiO2 / Au磁性纳米粒子用于检测水中亚硝酸盐[58]。在外部磁场和酸性介质中,亚硝酸盐离子触发共轭在Fe3O4@SiO2/Au MNPs上的4-氨基噻吩(4-ATP)分子而形成偶氮键,使SERS光谱上出现微量目标分子的拉曼指纹图谱,其检出限为13.69 μmol·L−1。Correa-Duarte等结合比色Griess反应和纳米技术设计出一种硝酸盐/亚硝酸盐检测的表面增强拉曼散射传感器。该方法主要是利用SERS/表面增强共振拉曼散射(SERRS)的优点,通过诱导形成均匀热点和彩色络合物与激光线共振,使硝酸盐/亚硝酸盐的检测限降低至0.4 pmol·L−1,最高至100 nmol·L−1 [6],虽然未进行在自然环境水体中的验证试验,但为SERS的开发提供了一种有效的思路。相比于如Pb2+、Hg2+、Cd2+等重金属离子,铁和锰离子的SERS的研究极少。Yan等提出了一种基于去铁胺功能化AgNPs用于检测水中的Fe3+的SERS方法,其检出限为0.2 μmol·L−1[59]。
对于地下水现场快速检测时往往需要便携式SERS仪器,由于小型化的体积使得这些系统目前仅支持近红外激光[60]。因此,为了适应现场快速检测,必须对SERS基板进行优化,以适用于近红外激光。此外,虽然SERS的灵敏度高,检测迅速,但是高昂的SERS费用限制了其广泛的应用,因此低成本和可扩展的检测平台的开发仍然是一个巨大的挑战。
-
试纸法是一种干化学法[61],其本质就是将检测试剂转移至滤纸上后干燥而成,使污染物与检测试剂间发生化学反应而显色,从而实现定性或半定量检测,因此具有携带方便、操作便捷、成本低廉的优点,但是每个试纸条检测的污染物种类单一,无法同时检测多种污染物。大致可分为显色型试纸、化学发光型试纸、免疫型试纸、微生物试纸。其中,显色型试纸被广泛应用与各类水体中的污染物检测。
Vellingiri等使用碱性品红沃顿试纸条用于检测地表水与地下水中的硝酸盐含量,其原理为亚硝酸盐与碱性品红中的二胺基团反应形成N-亚硝胺,从而使试纸条颜色由粉红色变为无色。加入吐温-80表面涂层剂后亚硝酸盐的裸眼检出限达到0.02 mg·L−1,检测浓度范围为0.02—9.2 mg·L−1 [62]。Aukema等开发了一种可同时检测水中的硝酸盐和亚硝酸盐微生物试纸。其原理是试纸上的大红肠杆菌还原硝酸盐为亚硝酸盐,亚硝酸盐与偶氮染料反应由棕色变成紫色,通过智能手机校准颜色并转化成硝酸盐浓度,亚硝酸盐检测则用没有细胞的另一端试纸条进行检测[63]。
Nawaz等制备了双传感器的试纸,以4,4′-亚甲基二苯基二异氰酸酯(MDI)为交联剂,通过化学键合1,10-菲咯啉-5-胺(Phen)到醋酸纤维素(CA)上,然后涂布在滤纸上。在CA的作用下,Fe2+可在2 s内迅速与之络合形成MDI-Phen-CA-Fe络合物,裸眼检测检测限为50 μg·L−1,使用荧光检测器检测的检出限为2.6 μg·L−1[64]。
关于试纸法检测Mn2+的相关研究较少,但总体而言试纸法可应用于地下水现场快速检测之中。由于试纸上的检测试剂有限,复杂水体对检测有一定影响,其灵敏度和检出限较低,因此试纸法更适用于要求不高的水体进行定性或半定量检测。可多污染物同时检测的纸基微流控传感器是试纸法的一个重要的拓展方向。
-
与试纸法不同,纸基微流控传感器可以同时检测多种污染物。纸基微流控传感器(μPAD)是一种新兴的以纸为基底通过化学修饰对目标物质分析的微流控平台[65]。它由已印刷图案的亲水性纸质底物组成,可作为多重分析物检测的平台。纸纤维在毛细作用下可驱动水溶液,通过沉积疏水性材料(例如光致抗蚀剂或蜡)来限制和引导微升体积的液体流动,从而在纸上形成通道,进而与特异性反应的检测剂反应来检测污染物。它具有成本低,可降解,易于修饰,节约样品,生物相容性好,可同时检测多个指标的特点[66],其中多指标检测的纸基传感器示意图如图3所示。Whitesides等介绍的基于纸基微流控传感器的制作方法,提供了一种革命性的方法来进行廉价且快速的分析检测[67],此后μPAD得到了迅速的发展。μPAD主要应用于医药领域,环境污染物的快速检测报道相对较少[68]。
目前纸基微流控传感器大部分基于比色法实现对污染物快速检测。为了实现准确检测污染物浓度,除了使用吸光度处理外,还可以将颜色强度转化为灰度或者转化为欧氏距离计算[69]。
ED为欧式几何距离,R/G/B分别为红、绿、蓝三色通道的颜色强度。Jayawardane等通过喷墨打印的方式合成可同时检测硝酸盐和亚硝酸盐的μPAD。亚硝酸盐直接通过Griess反应确定,而硝酸盐在固定有锌微粒的亲水通道中还原为亚硝酸盐,进而间接测定硝酸盐浓度[70]。Kamonet等使用4-(2-吡啶偶氮)-间苯二酚等络合剂通过蜡印印刷方式制备了可同时检测Mn2+、Cu2+、Co2+、Ni2+和Hg2+ μPAD,Mn2+的检测限为0.01 mmol·L−1[71]。Mentele等通过蜡印印刷技术制备了可同时检测Fe、Cu和Ni的多功能μPAD。μPAD往往需要前处理以保证检测的准确性,这一定程度上影响了使用的便捷性[72]。Moniz等设计了无需前处理的一种使用3-羟基-4-吡啶酮螯合剂对铁离子检测的μPAD。其线性检测区间为0.25—2.0 mg·L−1,LOD为55 μg·L−1[73]。另外多种传感器联用也是一种提高检测准确度的方法。Cate等通过蜡印印刷方法制造了可同时检测的Fe、Cu、Ni和Cr的μPAD[74]。Rattanarat等在此基础上,加入了电化学检测插层,实现了在纸基微流控平台上的多检测方式检验的突破,可同时检测Fe、Cu、Ni、Cr、Cd和Pb[75]。
此外,由于荧光的灵敏性,部分纸基微流控传感器亦基于荧光法实现对某些重金属[76]、酚类[77]的检测,但是与比色传感器现场拍照比色相比,这些大多需要实验室内设备测试,降低了便捷性[69]。为了增加现场适用性,Feng等开发了一种通过紫外光激发和具有可见光区荧光响应的μPAD[78]。数码相机拍摄前需要使用短波长滤光片(λ= 400—700 nm)排除激发波长干扰使用层序聚类分析(HCA)进行数据分析,该传感器可同时检测7种重金属离子。Li等通过Griess反应合成纳米棒-偶氮金纳米颗粒组装的亚硝酸根比色/荧光/表面增强拉曼散射(SERS)三重模式传感的新策略,这可以根据不同的污染物浓度检测要求来选择检测方式,比色,荧光,SERS的检测限分别达到50、10、0.8 nmol·L−1[79]。
由于大多纸基微流控传感器基于比色法,因此其灵敏度不及荧光法、化学发光法、SERS和电化学方法,但是其操作简单,结果读取方便,价格便宜,对于地下水现场检测要求不高的情况下可以满足需求。在实际的现场快速检测中务必要考虑结果的重现性,传感器的在复杂水样中的抗干扰性。
-
电化学快速检测的原理是电极将电流传递到水溶液中,并由于离子的存在而产生与溶液中的电化学反应相对应的一些有价值且可测量的电信号[80]。由于它具有快速响应,高灵敏度,易于操作和小型化便于携带的特点[81],从而使该技术有效且广泛用于水质检测中。根据工作原理不同,可以将电化学技术分为电位传感器,伏安传感器,场效应晶体管传感器[82],电化学生物传感器。其中伏安传感器更加灵敏且可同时检测多种离子,因此在现场水质检测中应用最广泛[80]。
伏安法的原理是施加电势以驱动电极/溶液界面上的化学反应(氧化/还原),从而导致检测过程中电流发生变化。其电化学工作电极(WEs)一般需要进行修饰以特异性测定目标污染物离子[83],其中电化学工作电极中的生物传感器、纳米材料、聚合物、金属氧化物等等研究较多。亚硝酸盐/亚硝酸盐对多种材料具有电活性,例如玻璃碳(GC)、铜、镍、铂、金、钻石、合金和TMO。碳糊电极已被用于硝酸盐和亚硝酸盐伏安传感器,硝酸盐的LOD分别为87 mmol·L−1和亚硝酸盐的LOD为0.625 μmol·L−1[71]。相比于传统电极,纳米材料修饰的电极显示出更高的灵敏度,这是因为其除了具有较高比表面积和更高活性位点外,它们还对检测物质显示出出色的吸附性能、导电性和电化学稳定性,甚至还可以作为电极催化剂,这有助于电极上亚硝酸盐更容易氧化。例如使用纳米碳管为基材并复合CuNPs[84]、AuNPs[85]、AgNPs[86]、PtNPs[87]、PbNPs[88]可以极大提升对硝酸盐和亚硝酸盐的检测能力。此外,二维材料氧化石墨烯或MXene与纳米颗粒或金属氧化物通过共价键或非共价键复合也增强了其电化学性能[89]。例如氧化石墨烯-聚苯胺-金纳米颗粒(GO-PANI-AuNPs)纳米复合材料对亚硝酸盐的线性检测范围为0.5 μmol·L−1—0.24 mmol·L−1和0.24—2.58 mmol·L−1,LOD为0.17 μmol·L−1[90]。
与硝酸盐/亚硝酸盐的电化学检测一样,伏安法测定铁离子和锰离子的性能在很大程度上取决于工作电极的性质。不同的材料修饰WEs,可以实现对金属离子的特定识别和浓缩。工作电极的无机材料修饰主要是金属纳米颗粒、金属氧化物、碳质纳米材料。Göde等制备了由杯芳烃修饰的还原氧化石墨烯(CA/RGO)电极可同时检测Fe3+ [91],Cd2+,Pb2+,其中Fe3+的LOD为0.02 nmol·L−1,线性检测区间为0.1—10 nmol·L−1,但是对于复杂的水质检测需要验证。George等通过β-环糊精介导的微波法合成Ag–Au双金属纳米颗粒修饰的金电极可同时检测水中的Mn2+和环丙沙星,Mn2+的LOD为0.82 nmol·L−1。工作电极的有机材料修饰主要是壳聚糖等[92]。Abdallah制备了用于检测Fe2+的碳纳米管-米氟沙星复合电位固体接触电极,LOD为4.8 nmol·L−1,线性范围为0.1—10 mmol·L−1[93]。Roushani等开发了一种使用多壁碳纳米管-壳聚糖-离子印迹聚合物离子液体(Mn(Ⅱ)-IIP/MWCNT/Chit/IL/GCE)纳米复合材料修饰的玻碳电极用于检测锰离子的化学传感器,LOD为0.15 μmol·L−1,线性检测范围为2.0—9.0 μmol·L−1[94]。
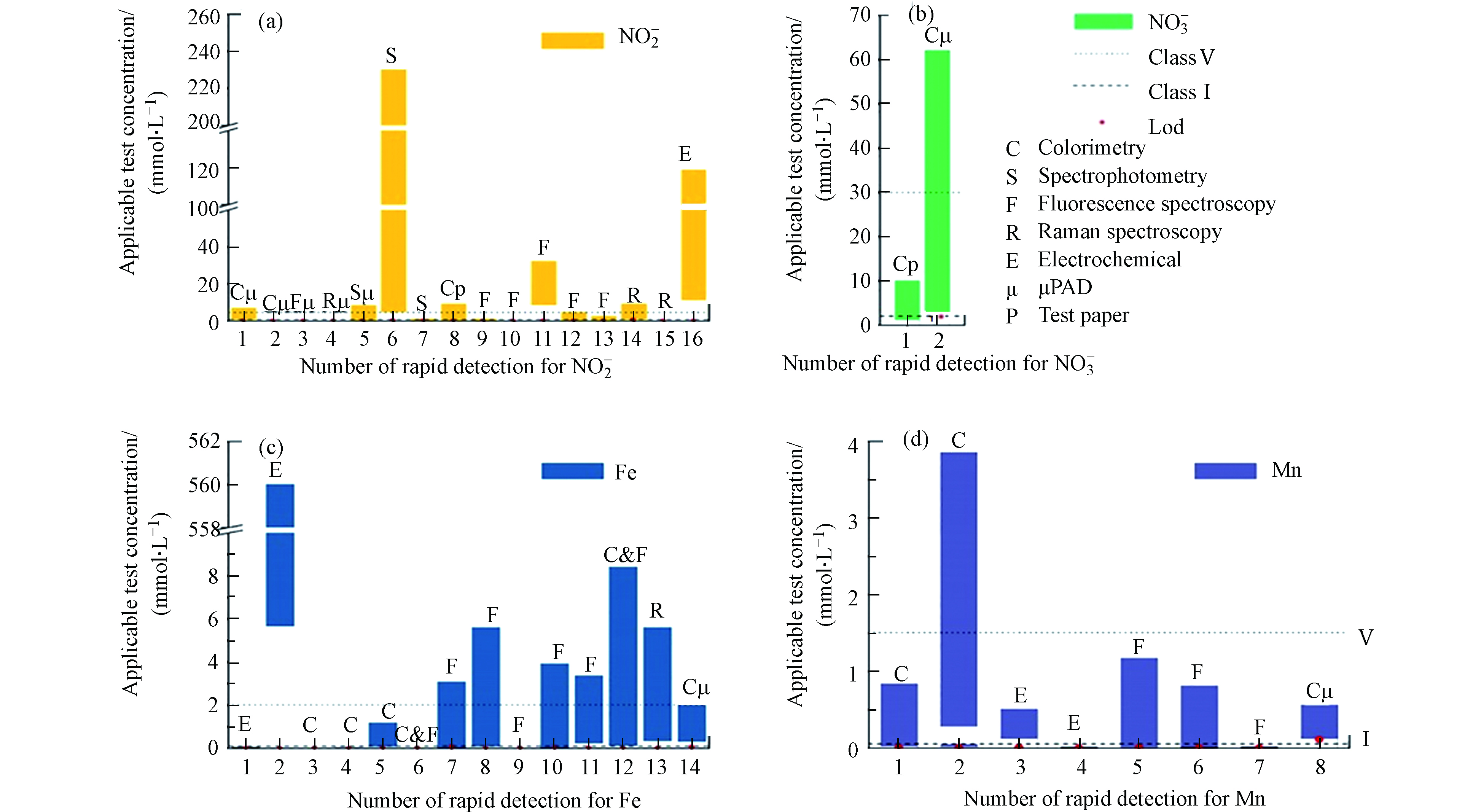
尽管电化学法在检测硝酸盐、亚硝酸盐、铁和锰离子方面应用广泛,但是离子干扰、电极中毒、有限的电极寿命问题依然是限制其在地下水现场快速检测的因素,因此开发绿色、寿命长且抗离子干扰的适宜复杂水体检测的电极是未来研究的方向。此外,如图4所示,电化学快速检测方法因其检测范围窄而难以应对污染场地的实际地下水检测。
-
区域性的场地污染对地下水安全构成严重威胁,而目前我国的场地污染较为严重。以垃圾填埋场为例,据统计,我国大部分垃圾填埋场地下水受到了不同程度的污染,并且已报道检出96种不同的污染物[95],污染的复杂性对现场快速检测方法的灵敏度和重现性等提出了巨大的挑战。在预警监测以及突发事故中往往需要对污染场地地下水的污染程度做出迅速评估,因此除了使用光、电等快速检测手段外,还需要加入统计学指标如根据其化学条件建立特征污染物与传统指标响应关系来快速评估污染物的浓度以增加监测数据的可信度,这可以被视为地下水污染物快速检测的补充。
在特定类型区域的地下水中往往水化学条件稳定,传统的检测指标如pH,溶解氧,电导率等与特征污染物有着良好的相关关系。在垃圾填埋场污染的地下水中,EC与Cl−呈显著线性正相关[96],R2为0.882,EC与大分子类富里酸和类胡敏酸呈显著线性正相关R2为0.901[97];在高尔夫球场的地下水中,氧化还原电位(ORP)与磷酸盐呈线性正相关,R2为0.913[98]。以因此在同类的污染场地中特征污染物与传统指标响应关系可以初步评估污染物的浓度。
-
本研究对光谱法和电化学法在地下水中主要污染物硝酸盐、亚硝酸盐、铁离子和锰离子的快速检测方法进行了综述,讨论了各类方法的优缺点,其不同的机制,线性检测区间和检测限列于表1和图4中。相比于常规检测手段,快速检测方法具有简单、快速、灵敏、经济的特点,因此快速检测方法在现场快速检测中有着广阔的应用前景。方法近年来发展迅速的纸基微流控技术可同时检测多种污染物,检测迅速,操作简单,并且可与SERS、荧光、比色联用,大大增加了检测的灵敏性和检测的多样性。而纳米技术尤其是金属纳米颗粒和碳纳米材料在快速检测中的广泛应用,显著增加了光学和电化学传感器的灵敏度和选择性,大幅降低检出限。不同的目标污染物有着不同的最适合检测方法,如纸基微流控技术对于硝酸盐和亚硝酸盐的快速检测线性检测区间较宽,并可同时检测这两种物质,铁的快速检测方式适宜使用荧光检测,锰的快速检测适宜使用比色和荧光技术。
然而,现阶段对地下水主要污染物营养盐和重金属的光/电化学快速检测仍存在一定的局限性。在实际应用角度而言,绝大部分的快速检测方法的检出限远远低于实际现场检测需求,大多数的线性检测区间较窄,远低于图4中标出的GB/T 14848—2017地下水Ⅴ类水的标准,因此对于实际场地应用需要面临稀释样品等问题,从而降低了现场快速检测效率和检测准确性。在检测原理上而言,大部分光学快速检测主要依靠污染物与检测剂反应而增强或减弱发光/显色/荧光或产生局部等离子体共振产生增强拉曼信号而检测污染物浓度,因此化学性质相似或者强化学键的竞争污染物会影响光学信号,从而影响检测准确性。此外,比色或荧光检测试剂往往使用有毒的化学试剂或重金属等污染环境,因此开发环境友好的检测剂和开发简单、快速的前处理工艺以降低其他物质干扰是将来的研究方向。电化学传感器主要受选择性和耐用性的制约,相似电位的有机物或离子被电极氧化从而影响选择性,电极材料溶出或生物污染影响电极耐用性。虽然纳米材料的应用提高了催化活性和稳定性,然而具有高比表面积和丰富的官能团的电极更易受到天然有机质的污染。因此在此基础上可以复合如蛋白质、DNA和有机小分子识别探针以降低溶出并增加选择性。此外,如图4大多数的电化学快速检测技术LOD较低,但检测区间短,无法满足实际场地中较宽浓度分布的检测需求。目前大多数快速检测依然停留在实验室阶段,研究中选择的实际水体大多使用较清洁的自来水等而忽视了成分复杂的实际受污染地下水,因此,开发具有较宽线性检测区间而非超低检测限的快速检测手段以及提高复杂水体的检测准确性和重复性是实现现场快速检测的关键。针对当前我国局部地下水严重污染现状,提出一种回归法快速评估污染物的方案以弥补当前快速检测的不足。
地下水主要无机污染物快速检测方法研究进展
Rapid detection method of main inorganic pollutants of groundwater: A review
-
摘要: 我国存在较为严重的地下水污染问题,因此需要对地下水中主要污染物进行简单连续监测。传统的检测方法费时费力并且检测仪器昂贵,难以满足当前广泛且频繁的地下水水质快速检测需求。发展一种经济高效地下水污染物检测方法对地下水水质监测和污染治理具有重要意义。近十几年来,基于光/电化学传感器技术发展迅速,纸基微流控、表面增强拉曼、纳米材料修饰的电化学传感器对地下水中的主要无机污染物检测展现出巨大的潜力。本文对基于光/电化学传感器的快速检测方法进行了综述并分析了各种传感器的优势和局限性,指出开发具有较宽线性检测区间而非超低检测限的快速检测手段以及提高复杂水体的检测准确性和重复性是实现现场快速检测的关键。此外提出了污染场地地下水可以以传统检测指标与特征污染物间的相关性作为地下水污染物的快速评估方法。Abstract: Groundwater have been seriously polluted in China. It is necessary to continuously monitor the major contaminants in groundwater. Traditional detection methods are time-consuming, laborious and expensive, expensive, which makes it difficult to test groundwater quality frequently and on a large scale. Therefore, it is essential to develop a cost-effective groundwater contaminant detection method for groundwater quality monitoring and pollution control. In recent decades, optical and electrochemical based sensors have been developed rapidly, in which paper-based microfluidic, surface-enhanced Raman, and nanomaterial-modified electrochemical sensors have shown great potential for the detection of major inorganic pollutants in groundwater. In this paper, rapid detection methods based on optical and electrochemical sensors are reviewed and the advantages and limitations of various sensors are evaluated. Finally, it is pointed out that developing rapid detection methods with wide linear detection intervals are more crucial than those with an ultra-low detection limits, which is the key to achieve rapid detection in the field. In addition, it is presented that the correlation between traditional detection indicators and characteristic contaminants can be used as a rapid assessment method for groundwater contaminants in groundwater at contaminated sites.
-
Key words:
- groundwater /
- rapid detection /
- heavy metals /
- nutrient /
- sensor
-
随着产业升级和城市扩张,大量工厂搬迁或废弃后遗留的场地存在土壤污染问题,需要进行土壤修复才能再次开发[1]。电阻加热技术具有对环境扰动小,受土壤异质性影响小,处理深度大等优点,尤其适合修复含有挥发性、半挥发性有机污染物的污染场地[2-5]。但在实际修复工程中,ERH技术的工程参数设计,例如电极间距、电场强度等,都会显著地影响场地电阻加热的实际效果[6],导致修复周期和成本控制的不确定性。修复场地的污染物分布、地下水流场和土壤特性等往往差异较大,但工程师只能根据已有工程经验和有限的取样勘探结果,进行原位加热工程的参数设计。若能够采用建模的方法,对场地条件下的加热过程进行预测,将有利于减少设计的盲目性,帮助缩短工程周期和控制修复成本。
目前,对于ERH技术的数值模型已经有了一定的探索和应用。HIEBERT等[7-8]开发了用于模拟单相电阻加热过程的二维有限差分模型,并研究了不同的横卧电极设置方式对非均质含油地层的加热效果的影响。CARRIGAN等[9]将改进的欧姆加热模型与非等温多孔流动和传输模型进行了耦合,研究了电极阵列的电相位如何影响电阻加热的均匀性。MCGEE等[10]进一步简化了模拟多相电阻加热的欧姆方程,并模拟了电阻加热从非均质油砂中回收沥青的过程。KROL等[11]考虑温度对密度、粘度、扩散系数的影响,建立了二维有限差分模型,模拟了电阻加热到50 ℃的情况下对地下水流动的影响,发现地下水流动方向和流速发生显著变化。许丹芸等[12]使用有限元方法模拟了电阻加热土壤过程。
尽管关于ERH技术的数值模型研究已有一定的开展,但一方面,以往的模型对电阻加热土壤过程中的水分蒸发缺乏关注和进一步的验证;另一方面,大部分模型是针对实际场地的验证评估,对如何运用模型指导ERH工艺参数的选取探讨不足。本研究使用COMSOL多物理场耦合软件,基于有限元计算方法开展原位电阻加热温度场模拟研究,建立了考虑土壤水分蒸发的模拟原位电阻加热温度场的数值模型。通过对比土柱装置小试实验和数值模拟的结果,验证了数值模型的准确性,并利用数值模型分析了场地尺度下电场强度、电极间距和地下水流动对电阻加热温度场的影响。本研究结果有助于预测修复周期和优化电极井布设,从而达到节约能源和降低修复成本的目的。
1. 数值模型与实验方法
1.1 控制方程及条件假设
本研究利用COMSOL的传热模块、电流模块、PDE模块以及电流和传热耦合的电磁热模块,构建土壤电阻加热模型。为简化模型概念,在模拟时做如下假设:1)忽略土壤和水在加热过程中密度、热容随温度的变化;2)将土体视为均质且各向同性的多孔介质,土壤初始温度均匀一致;3)忽略土体在加热过程中的热变形;4)忽略电极和土壤之间的接触电阻;5)由于实验土柱较短,需要考虑水分在低于沸点时的挥发[13],但在模拟大尺度的场地加热中,忽略水分的挥发;6)忽略水蒸气对热量传递的影响。
电阻加热土壤过程中能量的控制方程如式(1)所示。
stringUtils.convertMath(!{formula.content}) (1) 式中:ρeff为单元的有效密度,kg·m−3;Ceff为单元的有效热容,J·(kg·K)−1;T为温度,K;t为时间,s;λ为导热系数,W·(m·K)−1;σ为土壤电导率,S·m−1;E为电势梯度,V·m−1;mLG为水的气化速率,kg·(m³·s)−1;ΔHvap为水的潜热,J·kg−1;ρf为流体密度,kg·m−3;Cf为流体热容,J·(kg·K)−1;uf为流体流速,m·s−1。
土壤单元的有效密度ρeff和有效热容Ceff由土壤中固,液,气三相的体积分数决定,如式(2)~式(3)所示。
stringUtils.convertMath(!{formula.content}) (2) stringUtils.convertMath(!{formula.content}) (3) 式中:θ表示各相的体积分数;ρ为各相的密度,kg·m−3;C为各相的热容,J·(kg·K)−1;下标S,L,G表示固,液,气三相。
土壤含水量的控制方程如式(5)所示。
stringUtils.convertMath(!{formula.content}) (4) stringUtils.convertMath(!{formula.content}) (5) 式中:DL为导水系数,m2·s−1;α为比例常数,m2·s−1;θL*为残余饱和度。
液态水变为气态水的情况可分为2种,一种是低于水的沸点时的挥发,一种是到达沸点时的沸腾,用式(6)可以得到水的气化速率。需要注意,只有水的饱和蒸气压(p*)大于等于外部气压(pG)且含水量大于0时,沸腾才会发生。可以通过安托因方程[14](式(7))计算不同温度下水的饱和蒸气压,进而判断温度是否到达沸点。
stringUtils.convertMath(!{formula.content}) (6) stringUtils.convertMath(!{formula.content}) (7) 式中:A、B、C为经验常数;mvap为挥发速率,kg·(m³·s)−1;kvap为蒸发速率常数,s−1。
土壤单元的热导率会随着温度和含水量的变化发生极大的变化,在此使用TARNAWSKI等[15]推导出的经验公式,如式(8)~式(9)所示。
stringUtils.convertMath(!{formula.content}) (8) stringUtils.convertMath(!{formula.content}) (9) 式中:下标sat和dry分别表示饱和和干燥状态的土壤;a~g为经验常数;Sw为水饱和度。
电阻加热一般使用低频率电压(50~60 Hz),产生的电磁波长远大于系统的物理尺寸,位移电流可以忽略,因此可以假设电阻加热产生的电场为准静态电场,可以通过将欧姆定律代入电流连续性方程中来求解电势分布,电流连续性方程如公式(10)所示。
stringUtils.convertMath(!{formula.content}) (10) 土壤电导率则使用Archie定律进行计算[16-17],考虑温度对电导率的影响[18],如式(11)所示。
stringUtils.convertMath(!{formula.content}) (11) 式中: ψ为电势,V;φ为孔隙度;m、n和β为经验常数(m为胶结系数,n为饱和度系数,β为温度系数);σL为土壤溶液电导率,S·m−1。
模拟单相交流电加热时,其电势分布与直流电基本一致,可以设置2个电极分别为接地和施加的电压。但模拟三相交流电时,电极电势与直流电则完全不同,三相交流电的电极电势可以视为由虚部和实部组成[19],如式(12)所示。
stringUtils.convertMath(!{formula.content}) (12) 式中:ω为角频率,rad·s−1;t为时间,s;θ为相角,°(三相交流电分别为0 °、120 °和240 °);j为虚部;E0为正弦交流电振幅的绝对值,一般为电压的21/2倍,V。
1.2 几何模型和网格划分
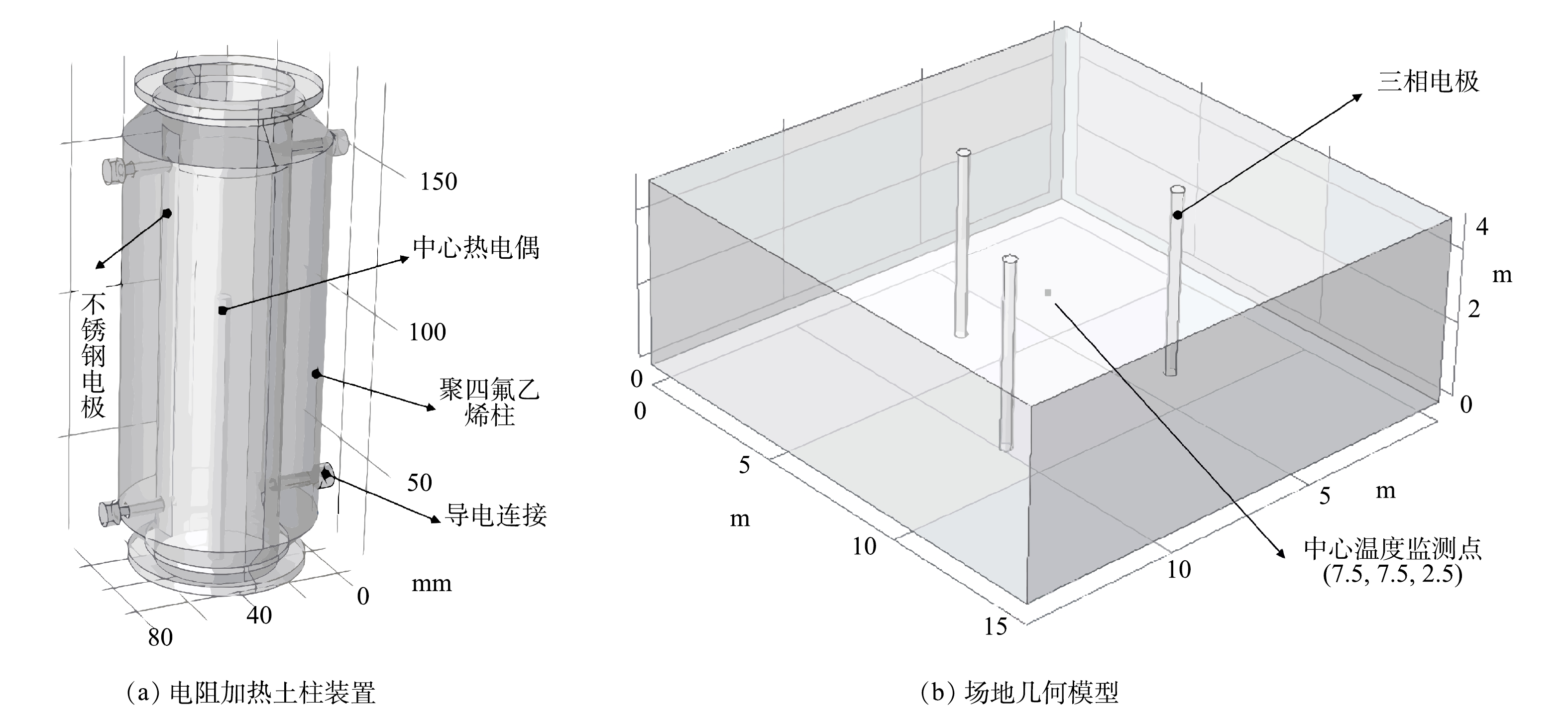
电阻加热土柱装置模型如图1(a)所示,装置高16 cm,内径4.5 cm。装置的罐体用不导电的聚四氟乙烯制成,内部装填细砂。装置2边为对称分布的用螺丝与螺母固定的不锈钢片电极,螺丝作为导电连接,电线连接到螺丝上以引入电压,热电偶从装置底部插入,用于监测土柱中心点的温度。使用Solidworks对电阻加热土柱装置进行建模,得到电阻加热土柱装置的几何模型,再导入到COMSOL中用于模拟,几何模型的网格划分均采用三角单元网格,单元大小选择细化。
为了模拟实际场地中电阻加热的过程,构建了图1(b)所示的三相电阻加热场地模型。场地模型为长宽15 m、高5 m的长方体,在场地中部按等边三角形放置3根半径20 cm、高5 m的电极,电极间距为6 m,并在3个电极构成的等边三角形中心点设置了温度监测点。
1.3 边界条件和参数设置
电阻加热土柱装置数值模拟的边界条件为:1)流动边界条件,所有边界均设置为0通量边界条件,这是因为已在方程中添加了描述水蒸发的汇项;2)温度边界条件,所有边界均设置为自然对流热通量边界;3)电势边界条件,2个电极分别设置为接地和电势,其他边界设置为电绝缘边界。土壤的初始温度、含水量、水的电导率和电极电压根据实测得到的初始值设置。对于各项参数的取值如表1所示。
表 1 数值模拟参数Table 1. Numerical simulation parameters模拟参数 取值 模拟参数 取值 液体密度,ρL 1 000 kg·m−3 温度系数,β 0.02 固体密度,ρS 2 650 kg·m−3 比例常数,α 5×10−6 m2·s−1 气体密度,ρG 1.9 kg·m−3 经验常数,A 8.07131 液体热容,CL 4 200 J·(kg·K)−1 经验常数,B 1 730.63 固体热容,CS 920 J·(kg·K)−1 经验常数,C 233.426 气体热容,CG 1 000 J·(kg·K)−11 胶结系数,m 1.44 湿导热系数,λsat 1.87 W·(m·K)−1 饱和度系数,n 2 干导热系数,λdry 0.23 W·(m·K)−1 水的潜热,ΔHvap 2 257.2 kJ·kg−1 孔隙度,φ 0.5 蒸发速率系数,kvap 1×10−6 s−1 电阻加热场地模拟的边界条件为:1)流动边界条件,模拟地下水位上涨时,底部边界设置为通量边界条件,其他边界设置为0通量边界条件;2)温度边界条件,地下水流入的边界设置为流入边界,流入温度10 ℃,其他边界设置为热绝缘边界;3)电势边界条件,3个电极分别设置为三相电势中的1相,其他边界设置为电绝缘边界。各项参数的设置与土柱装置实验相同。
1.4 实验条件
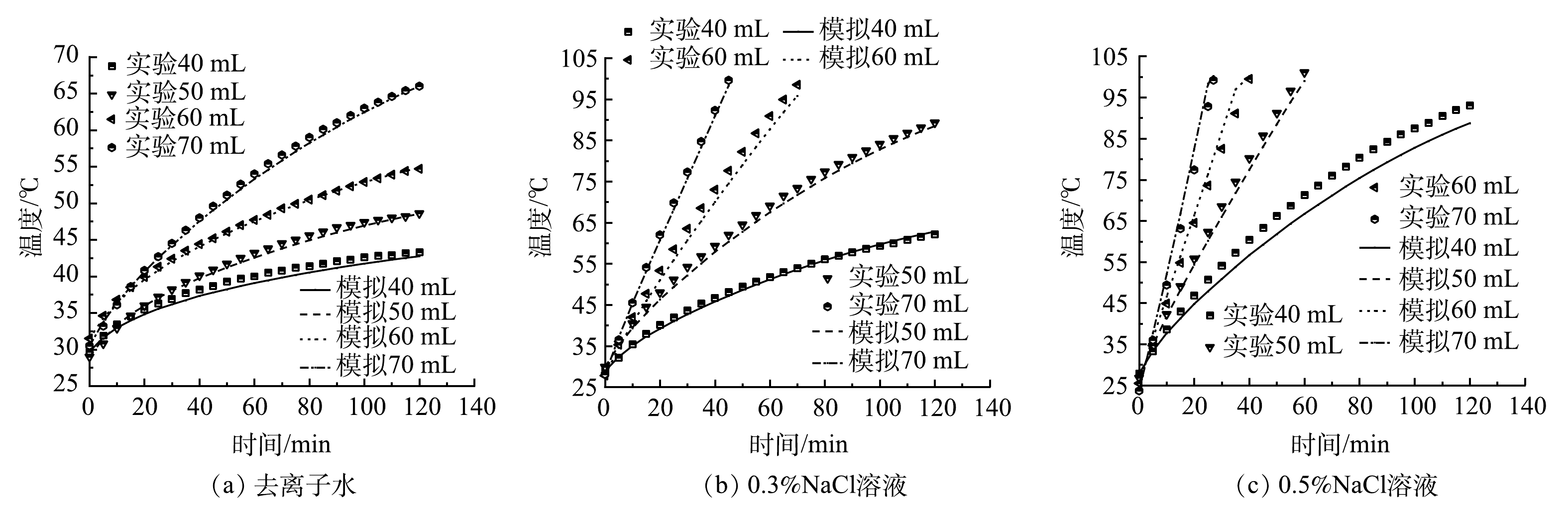
为验证模型在不同的土壤含水量和土壤溶液电导率时的准确性,使用电阻加热土柱装置进行了验证实验。由于装置较小,电场强度较大,故使用电导率较小的细砂充当模拟土壤。分别向300 g细砂中加入40、50、60、70 mL的去离子水,质量分数为0.3%的NaCl溶液和质量分数为0.5%的NaCl溶液,充分搅拌,并装填进装置中。为了保持砂的孔隙度一致,在装填时保证装填高度为15 cm。加入去离子水的砂在100 V的条件下进行电阻加热,加入NaCl溶液的砂在50 V的条件下进行电阻加热。由于模拟只探究电阻加热的升温过程,所以实验只进行到温度到达100 ℃就停止,未加热到100 ℃时则在加热2 h后停止。
为模拟实际工程环境下土壤内热量传递规律,用实际场地模型探究了地下水流动、电场强度和电极间距的变化对土壤温度变化的影响。模拟的工况如表2所示,其他参数与表1一致,土壤溶液电导率设为0.1 dS·m−1。
表 2 数值模拟工况Table 2. Numerical simulation conditions工况 电场强度/ (V·m−1) 初始水饱和度 地下水流速/ (m·s1) 电极间距/m 地下水涨速/ (m·d1) A-1 30 0.6 0 6 0 A-2 60 0.6 0 6 0 A-3 90 0.6 0 6 0 B-1 90/60 0.6 0 3 0 B-2 90/60 0.6 0 6 0 B-3 90/60 0.6 0 9 0 C-1 90 1 0.1 6 0 C-2 90 1 0.2 6 0 C-3 90 1 0.3 6 0 D-1 90 0.6 0 6 0.05 D-2 90 0.6 0 6 0.1 D-3 90 0.6 0 6 0.2 2. 结果与讨论
2.1 电阻加热土柱装置实验及模型验证
图2给出了不同含水率和电导率的情况下,土柱中心热电偶监测的温度变化。可以看出,随着含水量和土壤溶液电导率的增加,中心点加热到100 ℃所需要的时间不断减小。而当含水量较小或者土壤溶液电导率较小时,升温速率较小,甚至出现温度平台的情况。升温速率较小是由于此时土壤电导率较小[20],电流产生的焦耳热较小。升温速率减小则是因为,土柱中挥发掉的水份随着加热时间的增长逐渐变多[21],进一步降低了土壤电导率,使电流产生的焦耳热进一步减少。此外,由图2(a)可以看到,加入不同体积的去离子水后的细砂依然可以被加热,而去离子水的电导率几乎可以忽略不计。这说明,细砂中的离子溶解进入了去离子水中,提高了去离子水的电导率。
为验证模型的可靠性,将不同含水量和土壤溶液电导率的电阻加热实测值和模拟值进行了对比。利用均方误差MSE和平均相对误差MRE评价模拟值和实测值的差异(式(12)~式(13))。
stringUtils.convertMath(!{formula.content}) (13) stringUtils.convertMath(!{formula.content}) (14) 式中:n为实测数据个数;Mi、Si分别为第i个实测和模拟得到的数据。
图2(a)中给出的模拟值趋势线与实测值差异较小,实测值和模拟值均方误差为0.05~0.66,平均相对误差为0.42%~1.97%。较小的均方误差和平均相对误差表明,实测数据与模拟数据之间的偏差较小,模型具有较好的准确性。加入不同体积的0.3%NaCl(图2(b))和0.5%NaCl(图2(c))溶液,土壤溶液电导率随着NaCl溶液的体积和质量分数的增加而增大。对比实测和模拟预测结果,均方误差为0.38~12.29,平均相对误差为1.15%~5.32%。这表明,在较宽的土壤电导率范围内(加入70 mL的0.5%NaCl溶液时,土壤的电导率为1.1 dS·m−1),实测数据与模拟数据之间的偏差也较小,模型用于预测不同电导率的土壤加热过程是可靠的。误差产生的原因可能是,对土壤原有的电导率,实验过程中空气热对流导致的热量散失以及水分挥发速率的估值存在一定的偏差。
2.2 场地条件下电场强度和电极间距对电阻加热的影响
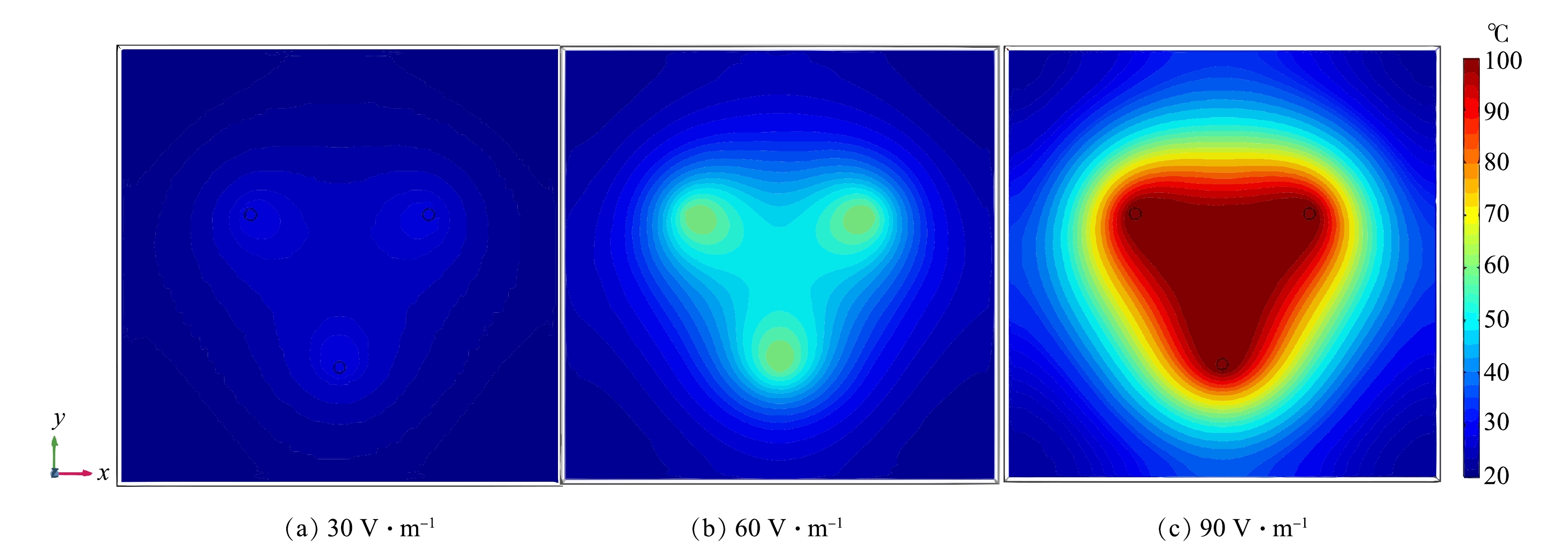
电场强度是影响电阻加热升温速率的重要因素,由电极电压和电极间距共同决定,为了考察场地尺度下的温度场以及各条件对温度场的影响,建立了图1(b)的场地模型。图3和图4分别展示了电极间距6 m时,30、60、90 V·m−1电场强度下加热70 d后,温度场的横截面以及位于3个电极中心点(见图1(b))温度的变化。从图3可以看出,电场强度越大,土壤升温速率越快,电极附近的升温更快,温度更高。这与MCGEE等[10]和HAN等[22]的研究结果是一致的。电流产生的焦耳热随电场强度的增大而增大,电场强度越大,土壤升温速率越快;电极附近的电流密度最高,产生的焦耳热最多,所以土壤升温速率更快。
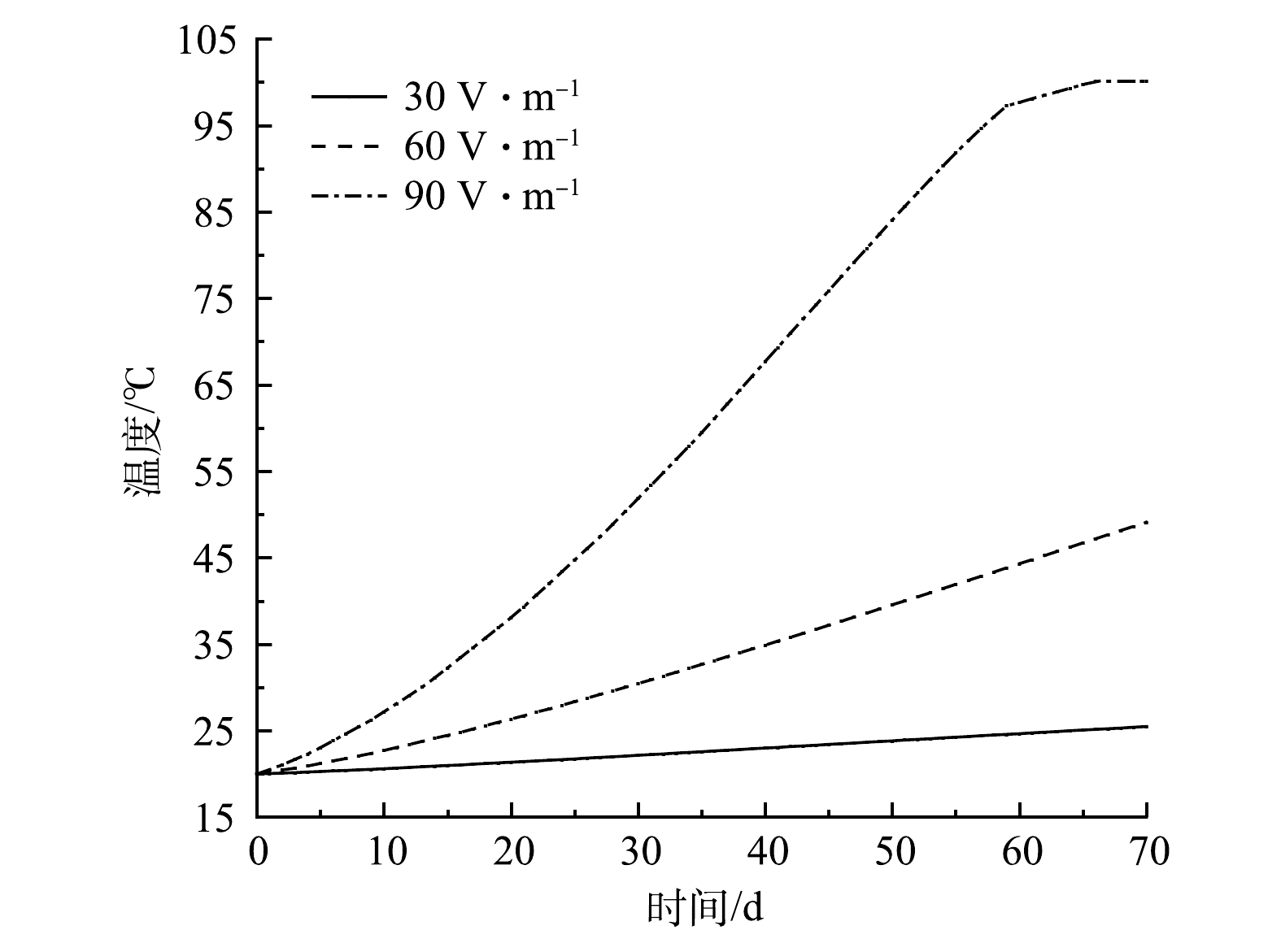
从图4可以看到,场地在加热90 d后,30、60和90 V·m−1电场强度获得的中心温度分别为25.5、49.1和100.0 ℃,中心点的平均升温速率分别为0.079,0.420和1.210 ℃·d−1。由式(1)可知,焦耳热与电场强度的平方呈正比,电场强度为30、60、90 V·m−1时中心点升温速率之比应为1∶4∶9,小于模拟得到的比值,即1∶5.3∶15.5。这说明,中心点的温度可能是电流焦耳热和外部热传导叠加共同决定。此外,电场强度为90 V·m−1时,当中心点温度达到97 ℃后,升温速率明显放缓。这是因为,此时的电极温度已经达到水的沸点,电极土壤水分蒸发带走了大量热量,导致土壤热导率和土壤电导率下降,从而使升温速率下降。
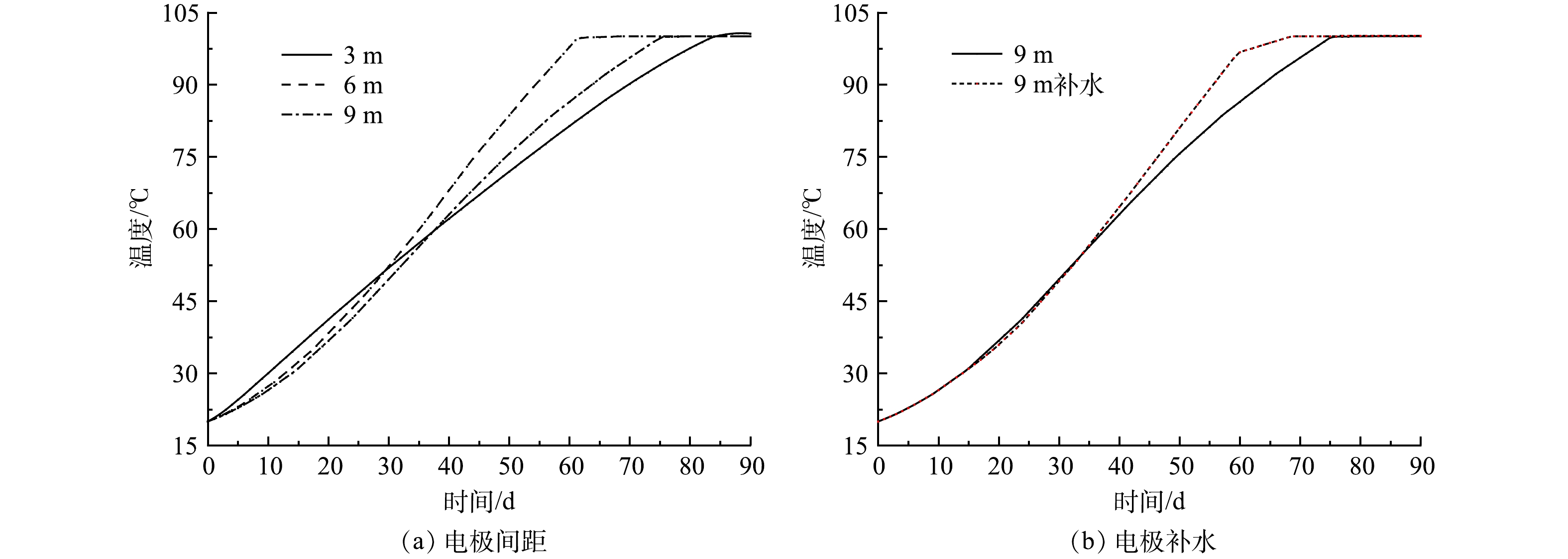
电极间距的设置决定了电极井的数量,会极大地影响修复场地的成本和热传导过程。为探究电极间距这一单因素对电阻加热过程的影响,在保持电场强度90 V·m−1不变的条件下,模拟电极间距3、6和9 m时中心温度的变化,结果如图5所示。从图5(a)可以看出,在电场强度为90 V·m−1,电极间距为3、6和9 m时,中心点的温度达到100 ℃的时间分别为84、62和75 d,达到100 ℃的能耗分别为10 418、23 375和51 311 kW·h。综上可知,6 m为最适宜的电极间距,此时中心点的升温速率最快,且相较于电极间距为3 m时场地修复需要布设的电极井数量更少,相较于电极间距为9 m时需要的能耗更少。从图5(a)还可以看出,随着电极间距的增大,中心点升温速率先增大后减小。这是因为,为了保证电场强度一定,电压随电极间距增大而增大,导致电极处的升温速率也随之增大,这一定程度上弥补了间距增大导致的中心点热传导距离增大的不足。但是,9 m间距下的电压增加,造成电极附近温度过早达到水的沸点,导致电极周围土壤水分过早蒸发,土壤热导率和电导率下降,升温速率略为下降。
为了考察电极附近土壤水分蒸发对中心点升温速率的影响,模拟了电场强度90 V·m−1、电极间距为9 m时,对电极附近补水的情况下,中心点温度的变化。从图5(b)可以看到,在加热40 d后,补水的情况下中心点温度明显高于不补水的情况,补水的情况下中心点的温度达到100 ℃的时间为68 d,比不补水的情况下早8 d。此结果表明,电极周围土壤水分过早蒸发是造成升温速率下降的原因之一,补水可以使土壤含水率增大,增加土壤的电导率和热导率,提高了升温速率。这与葛松等[3]的研究结果一致。监测电极的电流变化,并及时对电极附近进行补水,对于更快加热到目标温度至关重要。
2.3 场地条件下地下水流动对电阻加热的影响
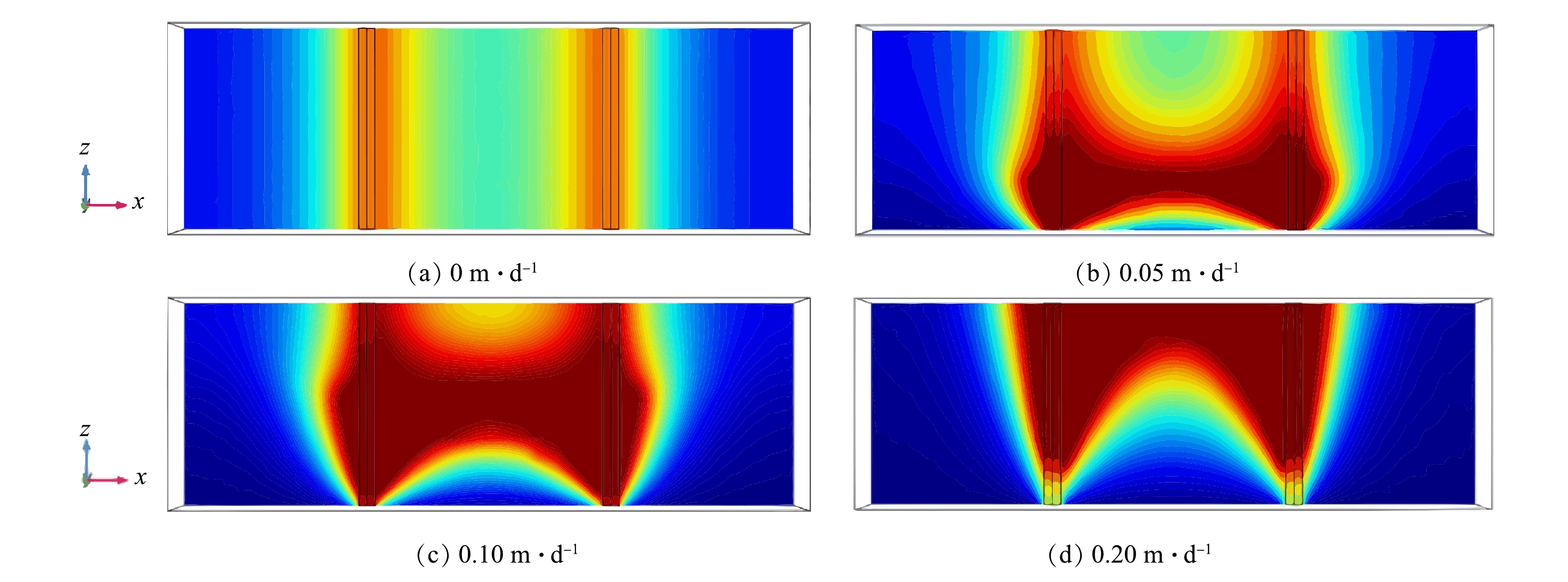
使用原位电阻加热的场地一般修复深度都较大,有必要考虑地下水流动和地下水位变化对温度场的影响。为进行预测,分别设定了0、0.1、0.2、0.3 m·d−1的地下水流速下加热30 d和0、0.05、0.10、0.20 m·d−1的地下水上涨速度下加热30 d的条件进行模拟,流入的地下水出的温度假定为10 ℃,温度场的变化结果如图6和图7所示。从图6可以看到,随着地下水流速的增加,场地左侧的温度逐渐降低到10 ℃,温度场右移的趋势越明显,中心点的最高温度从100.0 ℃降低到38.5 ℃。这说明,较高的地下水流速会将场地中的热量带向下游,对场地升温速率造成不利影响,与MUNHOLLAND等[23]在二维沙箱中得到的结果一致。实际工程中应尽量减小地下水流速,必要时可增加侧向的物理屏障来形成止水帷幕,或通过蒸汽注入等手段来增加流入地下水的温度。
从图7可以看到,随着地下水位上涨速度提升至0.1 m·d−1,场地下部和两侧的温度逐渐降低到10 ℃,但由不饱和区域变为饱和区域的土壤升温速率上升,中心点最高温度从51 ℃升高到100 ℃。随着地下水位上升速度达到0.2 m·d−1,相较于0.1 m·d−1时,温度场明显向上移动,中心点最高温度从100 ℃降低到54 ℃。这是因为,地下水位上涨可以起到一定的补水作用,提高不饱和区域土壤的电导率,从而提高土壤升温速率,但地下水上涨速度过快还是会导致流失的热量多于通过补水增加的焦耳热,使得场地温度从底部开始下降,不利于场地的修复。应当指出,由于实际场地地层的复杂性,地下水的流速和流向并不是均匀的。因此,对于非均质地层,可以建立相应的多模块的空间耦合模型,通过改变各地层模块的物理参数,从而实现数值模拟目标。
3. 结论
1)电阻加热温度场数值模型与土柱装置实验数据吻合度较好,模拟值和实测值均方误差为0.05~12.29,平均相对误差为0.42%~5.32%,数值模型具有较好的预测准确性。
2)场地模型研究发现,升温速率与电场强度成正比,电极处升温速率最快;电场强度为90 V·m−1时6 m为最适宜的电极间距,此时中心点升温速率最快,能耗相对较低,需要的电极井较少;模型考虑水分蒸发的情况下,电极周围土壤水分会更早蒸发,导致土壤升温速率下降,此时对电极附近补水可以显著增加土壤升温速率,故在实际工程中应监测电流和功率,并及时对电极附近进行补水。
3)场地地下水流动会带走热量,不利于场地的热修复。但对于不饱和场地,地下水位上涨速度小于等于0.1 m·d−1时可以起到补水作用,提高场地升温速率。故对于地下水流速过高的场地应采取水力平衡或设置止水帷幕等工程措施以缓解热量流失。
-
表 1 地下水主要无机污染物的快速检测方法
Table 1. Rapid detection method of main inorganic pollutants in groundwater
目标污染物Analyte 检测方法Method 传感器Sensors (data capture or material) 检测限Limit of Detection 线性区间Linear range 反应时间Reaction time 最佳pH pH of the best condition 环境样品检测Environmental sample test 同时检测物质Simultaneous detection 引用文献Reference 硝酸盐 比色法-试纸法 智能手机 1—10 mg·L−1 地下水 亚硝酸盐 [63] 硝酸盐 比色纸基微流控 比色 (image-J处理图像) 19 μmol·L−1 50–1000 μmol·L−1 5 min 自来水,池塘水 [70] 亚硝酸盐 1.0 μmol·L−1 10–150 μmol·L−1 5 min 亚硝酸盐 比色纸基微流控 比色 0.05 μmol·L−10.01 μmol·L−10.8 nmol·L−1 [79] 荧光 SERS 亚硝酸盐 分光光度法 0.39 μmol·L−1 1—180 μmol·L−1 1 min 自来水 [10] 亚硝酸盐 催化分光光度法 uv-vis分光光度计 4.6 μmol·L−1 100—5000 μmol·L−1 1.5 min 2 自来水 [8] 亚硝酸盐 分光光度法 uv-vis分光光度计 6.9 μg·L−1 16—1100 μg·L−1 35 min 9.4 自来水,河水 [19] 亚硝酸盐 比色-试纸法 裸眼 或 分光光度计 0.005 mg·L−1 0.005—9.2 mg·L−1 自来水、 [62] 亚硝酸盐 荧光光谱 CQD-Tb3+ 2.0 nmol·L−1 5—1200 nmol·L−1 7.5 地下水、湖水、雨水、自来水 Hg+ [44] 亚硝酸盐 荧光光谱 ZnCdS QDs 0.87 μmol·L−1 180—700 μmol·L−1 5 min [32] 亚硝酸盐 荧光光谱 聚乙烯亚胺-CdS QDs 0.05 μmol·L−1 0.1—100 μmol·L−1 7.4 自来水 [33] 亚硝酸盐 荧光光谱 PA-CDS 7.1 nmol·L−1 0.05—1 μmol·L−1和1—50 μmol·L−1 60 min 2 自来水 [43] 亚硝酸盐 SERS Fe3O4@ SiO2/Au NPs 13.69 μmol·L−1 10—200 μmol·L−1 几秒 无需处理 池塘水 [58] 亚硝酸盐 SERS AgNP @ ABT/ NA 0.4 pmol·L−1 0.4 pmol·L−1—100 nmol·L−1 [6] 亚硝酸盐 电化学伏安法 GO-PANI-AuNPs 0.17 μmol·L−1 0.5 μmol·L−1—0.24 mmol·L−1和 0.24—2.58 mmol·L−1 自来水、污水 [90] Fe3+ 电化学伏安法 杯芳烃-还原氧化石墨烯 0.02 nmol·L−1 0.1—10 nmol·L−1 [91] Fe2+ 电化学伏安法 碳纳米管-米氟沙星 4.8 nmol·L−1 0.1—10 mmol·L−1 5 s 3—9 自来水 [93] Fe2+ 比色法 亚苯基乙炔 0.02 μmol·L−1 4—9 自来水,地下水 Cd,Zn [13] Fe2+ 比色法 金纳米棒 13.5 nmol·L−1 0.075—1 μmol·L−1 8 min 6.6 [21] Fe3+ 比色法 ASA-AuNPs 0.051 μmol·L−1 0.3—21 μmol·L−1 河水 [20] Fe2+ 比色法 & 荧光法 裸眼 或 荧光分光光度计 2.6 μg·L−1 2s [64] Fe3+ 荧光光谱 WS2-QDs 1.32 μmol·L−1 0—55 μmol·L−1 [34] Fe3+ 荧光光谱 2QD@P-PEN 0.5—100 μmol·L−1 11 自来水 [35] Fe3+ 荧光光谱 席夫碱 0.163 μmol·L−1 [25] Fe3+ 荧光光谱 NCQDs 0.5 μmol·L−1 0—70 μmol·L−1 1 min 7 自来水、河水 Hg2+ [52] Fe3+ 荧光光谱 NCQDs 0.31 μmol·L−1 3—60 μmol·L−1 7.5 自来水 Cu2+ [53] Fe3+ 荧光和比色 CQD 52 nmol·L−1 1—150 μmol·L−1 5—11 [45] Fe3+ 拉曼光谱 便携式拉曼光谱 0.2 μmol·L−1 5—100 μmol·L−1 几秒 2 [59] Fe4+ 比色纸基微流控 3,4-HPO 55 μg·L−1 0.25—2.0 mg·L−1 15 min 8 自来水 [73] Mn2+ 比色法 uv-vis分光光度计 0.02 μmol·L−1 0.15— 15 μmol·L−1 12 自来水、湖水 Cu2+ [22] Mn2+ 比色法 AgNPs与精氨酸 0.02 μmol·L−1 0.02—0.7 μmol·L−1 和 5—70 μmol·L−1 9.4 自来水、湖水、河水 [23] Mn2+ 电化学伏安法 Mn(II)-IIP/MWCNT/Chit/IL/GCE 0.15 μmol·L−1 2.0—9.0 μmol·L−1 6 污水 [94] Mn2+ 电化学伏安法 Ag/Au NPs修饰的金电极 8.42 nmol·L−1 0.01—0.05 μmol·L−1 [92] Mn3+ 荧光光谱 N-CQDs/CCSCD 5.3 nmol·L−1 0—21.11 μmol·L−1 5 min 7 自来水、湖水 [55] Mn(VII) 荧光光谱 PTQA 1 ng·L−1 0.01—800 μg·L−1 5 min 1.3 地下水、海水、河水、自来水 [24] Mn(VII) 荧光光谱 S,Cl,N-CQDs 12.5 nmol·L−1 0.05—110 nmol·L−1 3 min 2 [54] Mn2+ 比色纸基微流控 比色(image-J处理图像) 0.002 mmol·L−1 0.002—0.01 mmol·L−1 11 池塘水,自来水 Cu,Co,Ni,Hg [71] -
[1] 中国生态环境状况公报[R]. 中国生态环境部, 2019. Bulletin of China’s ecological and environmental status in 2019 [R]. China’s Ministry of Ecology and Environment, 2019.
[2] 李圣品, 李文鹏, 殷秀兰, 等. 全国地下水质分布及变化特征 [J]. 水文地质工程地质, 2019, 46(6): 1-8. doi: 10.16030/j.cnki.issn.1000-3665.2019.06.01 LI S P, LI W P, YIN X L, et al. Distribution and evolution characteristics of national groundwater quality from 2013 to 2017 [J]. Hydrogeology & Engineering Geology, 2019, 46(6): 1-8(in Chinese). doi: 10.16030/j.cnki.issn.1000-3665.2019.06.01
[3] CHAN T Y. Food-borne nitrates and nitrites as a cause of methemoglobinemia [J]. The Southeast Asian Journal of Tropical Medicine and Public Health, 1996, 27(1): 189-192. [4] XIONG Y, WANG C J, TAO T, et al. A miniaturized fiber-optic colorimetric sensor for nitrite determination by coupling with a microfluidic capillary waveguide [J]. Analytical and Bioanalytical Chemistry, 2016, 408(13): 3413-3423. doi: 10.1007/s00216-016-9415-1 [5] RUGLESS F, BHATTACHARYA A, SUCCOP P, et al. Childhood exposure to manganese and postural instability in children living near a ferromanganese refinery in Southeastern Ohio [J]. Neurotoxicology and Teratology, 2014, 41: 71-79. doi: 10.1016/j.ntt.2013.12.005 [6] CORREA-DUARTE M A, PAZOS PEREZ N, GUERRINI L, et al. Boosting the quantitative inorganic surface-enhanced Raman scattering sensing to the limit: The case of nitrite/nitrate detection [J]. The Journal of Physical Chemistry Letters, 2015, 6(5): 868-874. doi: 10.1021/acs.jpclett.5b00115 [7] ZHAO Y, SHI R, BIAN X, et al. Ammonia detection methods in photocatalytic and electrocatalytic experiments: how to improve the reliability of NH3 production rates?[J]. Advanced Science, 2019, 6(8): 1802109. [8] ADEGOKE O, ZOLOTOVSKAYA S, ABDOLVAND A, et al. Rapid and highly selective colorimetric detection of nitrite based on the catalytic-enhanced reaction of mimetic Au nanoparticle-CeO2 nanoparticle-graphene oxide hybrid nanozyme [J]. Talanta, 2021, 224: 121875. doi: 10.1016/j.talanta.2020.121875 [9] ROMITELLI F, SANTINI S A, CHIERICI E, et al. Comparison of nitrite/nitrate concentration in human plasma and serum samples measured by the enzymatic batch Griess assay, ion-pairing HPLC and ion-trap GC-MS: The importance of a correct removal of proteins in the Griess assay [J]. Journal of Chromatography B, 2007, 851(1/2): 257-267. [10] LO H S, LO K W, YEUNG C F, et al. Rapid visual and spectrophotometric nitrite detection by cyclometalated ruthenium complex [J]. Analytica Chimica Acta, 2017, 990: 135-140. doi: 10.1016/j.aca.2017.07.018 [11] YU J J, QIN X C, WANG D, et al. Light-controlled configurable colorimetric sensing array [J]. Analytical Chemistry, 2019, 91(10): 6632-6637. doi: 10.1021/acs.analchem.9b00549 [12] FERNANDES G M, SILVA W R, BARRETO D N, et al. Novel approaches for colorimetric measurements in analytical chemistry - A review [J]. Analytica Chimica Acta, 2020, 1135: 187-203. doi: 10.1016/j.aca.2020.07.030 [13] ZHANG Y Y, CHEN X Z, LIU X Y, et al. A highly sensitive multifunctional sensor based on phenylene-acetylene for colorimetric detection of Fe2+ and ratiometric fluorescent detection of Cd2+ and Zn2+ [J]. Sensors and Actuators B:Chemical, 2018, 273: 1077-1084. doi: 10.1016/j.snb.2018.07.012 [14] PRIYADARSHINI E, PRADHAN N. Gold nanoparticles as efficient sensors in colorimetric detection of toxic metal ions: A review [J]. Sensors and Actuators B:Chemical, 2017, 238: 888-902. doi: 10.1016/j.snb.2016.06.081 [15] CHEN L F, TIAN X K, XIA D S, et al. Novel colorimetric method for simultaneous detection and identification of multimetal ions in water: Sensitivity, selectivity, and recognition mechanism [J]. ACS Omega, 2019, 4(3): 5915-5922. doi: 10.1021/acsomega.9b00312 [16] LI T H, LI Y L, ZHANG Y J, et al. A colorimetric nitrite detection system with excellent selectivity and high sensitivity based on Ag@Au nanoparticles [J]. The Analyst, 2015, 140(4): 1076-1081. doi: 10.1039/C4AN01583E [17] ZHAO X X, ZHAO H B, YAN L, et al. Recent developments in detection using noble metal nanoparticles [J]. Critical Reviews in Analytical Chemistry, 2020, 50(2): 97-110. doi: 10.1080/10408347.2019.1576496 [18] KUMAR V V, ANTHONY S P. Highly selective silver nanoparticles based label free colorimetric sensor for nitrite anions [J]. Analytica Chimica Acta, 2014, 842: 57-62. doi: 10.1016/j.aca.2014.06.028 [19] Ibrahim M H, Xue Z, Abdu H I, et al. Sensitive and selective colorimetric nitrite ion assay using silver nanoparticles easily synthesized and stabilized by AHNDMS and functionalized with PABA [J]. Nanoscale Advances, 2019, 1(3): 1207-1214. doi: 10.1039/C8NA00146D [20] MEMON S S, NAFADY A, SOLANGI A R, et al. Sensitive and selective aggregation based colorimetric sensing of Fe3+ via interaction with acetyl salicylic acid derived gold nanoparticles [J]. Sensors and Actuators B:Chemical, 2018, 259: 1006-1012. doi: 10.1016/j.snb.2017.12.162 [21] LU S M, ZHANG X, CHEN L, et al. Colorimetric determination of ferrous ion via morphology transition of gold nanorods [J]. Microchimica Acta, 2017, 185(1): 1-9. [22] WU G H, DONG C, LI Y L, et al. A novel AgNPs-based colorimetric sensor for rapid detection of Cu2+ or Mn2+ via pH control [J]. RSC Advances, 2015, 5(26): 20595-20602. doi: 10.1039/C5RA00001G [23] HE Y, ZHANG X H. Ultrasensitive colorimetric detection of manganese(II) ions based on anti-aggregation of unmodified silver nanoparticles [J]. Sensors and Actuators B:Chemical, 2016, 222: 320-324. doi: 10.1016/j.snb.2015.08.089 [24] AHMED M J, ISLAM M T, HOSSAIN F. A highly sensitive and selective spectrofluorimetric method for the determination of manganese at nanotrace levels in some real, environmental, biological, soil, food and pharmaceutical samples using 2-(α-pyridyl)-thioquinaldinamide [J]. RSC Advances, 2018, 8(10): 5509-5522. doi: 10.1039/C7RA12762F [25] HARATHI J, THENMOZHI K. AIE-active Schiff base compounds as fluorescent probes for the highly sensitive and selective detection of Fe3+ ions [J]. Materials Chemistry Frontiers, 2020, 4(5): 1471-1482. doi: 10.1039/C9QM00792J [26] 纪雪峰, 单斌, 王莎莎, 等. 荧光探针在水中重金属离子检测中的应用研究进展 [J]. 青岛理工大学学报, 2021, 42(16): 109-118. doi: 10.3969/j.issn.1673-4602.2021.01.018 JI X F, SHAN B, WANG S S, MA J P. Application research progress of fluorescent probe in the detection of heavy metal ions in water [J]. Journal of Qingdao University of Technology, 2021, 42(16): 109-118(in Chinese). doi: 10.3969/j.issn.1673-4602.2021.01.018
[27] ZHANG X S, LI C H, ZHAO S L, et al. S doped silicon quantum dots with high quantum yield as a fluorescent sensor for determination of Fe3+ in water [J]. Optical Materials, 2020, 110: 110461. doi: 10.1016/j.optmat.2020.110461 [28] CUI Y J, YUE Y F, QIAN G D, et al. Luminescent functional metal-organic frameworks [J]. Chemical Reviews, 2012, 112(2): 1126-1162. doi: 10.1021/cr200101d [29] ALIVISATOS A P. Semiconductor clusters, nanocrystals, and quantum dots [J]. Science, 1996, 271(5251): 933-937. doi: 10.1126/science.271.5251.933 [30] HIMMELSTOß S F, HIRSCH T. A critical comparison of lanthanide based upconversion nanoparticles to fluorescent proteins, semiconductor quantum dots, and carbon dots for use in optical sensing and imaging [J]. Methods and Applications in Fluorescence, 2019, 7(2): 022002. doi: 10.1088/2050-6120/ab0bfa [31] LOU Y B, ZHAO Y X, CHEN J X, et al. Metal ions optical sensing by semiconductor quantum dots [J]. Journal of Materials Chemistry C, 2014, 2(4): 595-613. doi: 10.1039/C3TC31937G [32] YANG M, YAN Y J, SHI H X, et al. A novel fluorescent sensors for sensitive detection of nitrite ions [J]. Materials Chemistry and Physics, 2020, 239: 122121. doi: 10.1016/j.matchemphys.2019.122121 [33] REN H H, FAN Y, WANG B, et al. Polyethylenimine-capped CdS quantum dots for sensitive and selective detection of nitrite in vegetables and water [J]. Journal of Agricultural and Food Chemistry, 2018, 66(33): 8851-8858. doi: 10.1021/acs.jafc.8b01951 [34] SINGH V K, MISHRA H, ALI R, et al. In situ functionalized fluorescent WS2-QDs as sensitive and selective probe for Fe3+ and a detailed study of its fluorescence quenching [J]. ACS Applied Nano Materials, 2019, 2(1): 566-576. doi: 10.1021/acsanm.8b02162 [35] HE X H, JIA K, BAI Y, et al. Quantum dots encoded white-emitting polymeric superparticles for simultaneous detection of multiple heavy metal ions [J]. Journal of Hazardous Materials, 2021, 405: 124263. doi: 10.1016/j.jhazmat.2020.124263 [36] LI Q N, ZHOU W L, YU L P, et al. Perovskite quantum dots as a fluorescent probe for metal ion detection in aqueous solution via phase transfer [J]. Materials Letters, 2021, 282: 128654. doi: 10.1016/j.matlet.2020.128654 [37] SHENG X X, LIU Y, WANG Y, et al. Cesium lead halide perovskite quantum dots as a photoluminescence probe for metal ions [J]. Advanced Materials, 2017, 29(37): 1700150. doi: 10.1002/adma.201700150 [38] XU X Y, RAY R, GU Y L, et al. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments [J]. Journal of the American Chemical Society, 2004, 126(40): 12736-12737. doi: 10.1021/ja040082h [39] DAS R, BANDYOPADHYAY R, PRAMANIK P. Carbon quantum dots from natural resource: A review [J]. Materials Today Chemistry, 2018, 8: 96-109. doi: 10.1016/j.mtchem.2018.03.003 [40] DEMCHENKO A P, DEKALIUK M O. Novel fluorescent carbonic nanomaterials for sensing and imaging [J]. Methods and Applications in Fluorescence, 2013, 1(4): 042001. doi: 10.1088/2050-6120/1/4/042001 [41] NICOLLIAN E H. Surface passivation of semiconductors [J]. Journal of Vacuum Science and Technology, 1971, 8(5): S39-S49. doi: 10.1116/1.1316388 [42] SHEN J H, ZHU Y H, CHEN C, et al. Facile preparation and upconversion luminescence of graphene quantum dots [J]. Chemical Communications (Cambridge, England), 2011, 47(9): 2580-2582. doi: 10.1039/C0CC04812G [43] LI W S, HUANG S P, WEN H Y, et al. Fluorescent recognition and selective detection of nitrite ions with carbon quantum dots [J]. Analytical and Bioanalytical Chemistry, 2020, 412(4): 993-1002. doi: 10.1007/s00216-019-02325-9 [44] WU H F, TONG C L. Dual-emission fluorescent probe for the simultaneous detection of nitrite and mercury(II) in environmental water samples based on the Tb3+-modified carbon quantum dot/3-aminophenylboronic acid hybrid [J]. Analytical Chemistry, 2020, 92(13): 8859-8866. doi: 10.1021/acs.analchem.0c00455 [45] SHI Y P, LIU J J, ZHANG Y, et al. Microwave-assisted synthesis of colorimetric and fluorometric dual-functional hybrid carbon nanodots for Fe3+ detection and bioimaging[J]. Chinese Chemical Letters, 2021 [46] HUANG H, WENG Y H, ZHENG L H, et al. Nitrogen-doped carbon quantum dots as fluorescent probe for “off-on” detection of mercury ions, l-cysteine and iodide ions [J]. Journal of Colloid and Interface Science, 2017, 506: 373-378. doi: 10.1016/j.jcis.2017.07.076 [47] MAGDY G, ABDEL HAKIEM A F, BELAL F, et al. Green one-pot synthesis of nitrogen and sulfur co-doped carbon quantum dots as new fluorescent nanosensors for determination of salinomycin and maduramicin in food samples [J]. Food Chemistry, 2021, 343: 128539. doi: 10.1016/j.foodchem.2020.128539 [48] SUN S J, GUAN Q W, LIU Y, et al. Highly luminescence manganese doped carbon dots [J]. Chinese Chemical Letters, 2019, 30(5): 1051-1054. doi: 10.1016/j.cclet.2019.01.014 [49] LIN L P, LUO Y X, TSAI P, et al. Metal ions doped carbon quantum dots: Synthesis, physicochemical properties, and their applications [J]. TrAC Trends in Analytical Chemistry, 2018, 103: 87-101. doi: 10.1016/j.trac.2018.03.015 [50] WANG Y, ZHANG Y, JIA M Y, et al. Functionalization of carbonaceous nanodots from Mn(Ⅱ) -coordinating functional knots [J]. Chemistry (Weinheim an Der Bergstrasse, Germany), 2015, 21(42): 14843-14850. [51] WU H, PANG L F, FU M J, et al. Boron and nitrogen codoped carbon dots as fluorescence sensor for Fe3+ with improved selectivity [J]. Journal of Pharmaceutical and Biomedical Analysis, 2020, 180: 113052. doi: 10.1016/j.jpba.2019.113052 [52] ZHU J T, CHU H Y, WANG T S, et al. Fluorescent probe based nitrogen doped carbon quantum dots with solid-state fluorescence for the detection of Hg2+ and Fe3+ in aqueous solution [J]. Microchemical Journal, 2020, 158: 105142. doi: 10.1016/j.microc.2020.105142 [53] BANDI R, DADIGALA R, GANGAPURAM B R, et al. N-Doped carbon dots with pH-sensitive emission, and their application to simultaneous fluorometric determination of iron(Ⅲ) and copper(Ⅱ) [J]. Microchimica Acta, 2019, 187(1): 1-10. [54] HU Q, LIU L F, SUN H J, et al. An ultra-selective fluorescence method with enhanced sensitivity for the determination of manganese (VII) in food stuffs using carbon quantum dots as nanoprobe [J]. Journal of Food Composition and Analysis, 2020, 88: 103447. doi: 10.1016/j.jfca.2020.103447 [55] WANG S, LIU J, ZHAO H H, et al. Carboxymethyl chitosan crosslinked β-cyclodextrin containing hydrogen bonded NC QDs nanocomposites to design fluorescence probes for manganese ion (Ⅱ) sensing [J]. Materials Science and Engineering:C, 2021, 119: 111556. doi: 10.1016/j.msec.2020.111556 [56] ONG T T X, BLANCH E W, JONES O A H. Surface Enhanced Raman Spectroscopy in environmental analysis, monitoring and assessment [J]. Science of the Total Environment, 2020, 720: 137601. doi: 10.1016/j.scitotenv.2020.137601 [57] SINGH P, SINGH M K, BEG Y R, et al. A review on spectroscopic methods for determination of nitrite and nitrate in environmental samples [J]. Talanta, 2019, 191: 364-381. doi: 10.1016/j.talanta.2018.08.028 [58] CHEN J H, PANG S, HE L L, et al. Highly sensitive and selective detection of nitrite ions using Fe3O4@SiO2/Au magnetic nanoparticles by surface-enhanced Raman spectroscopy [J]. Biosensors and Bioelectronics, 2016, 85: 726-733. doi: 10.1016/j.bios.2016.05.068 [59] YAN F, REDDY C V G, SHRESTHA Y K, et al. Correction: Determination of ferric ions using surface-enhanced Raman scattering based on desferrioxamine-functionalized silver nanoparticles [J]. Chemical Communications, 2018, 54(78): 11053. doi: 10.1039/C8CC90421A [60] WEI H R, HOSSEIN ABTAHI S M, VIKESLAND P J. Plasmonic colorimetric and SERS sensors for environmental analysis [J]. Environmental Science:Nano, 2015, 2(2): 120-135. doi: 10.1039/C4EN00211C [61] 王胜智, 张平, 谢思桃. 试纸法及其在水质检测领域的应用研究[J]. 给水排水, 2008, 44(增刊2): 216-220. WANG S Z, ZHANG P, XIE S T. Dipstick method and its application in the field of water quality detection [J]. Water & Wastewater Engineering, 2008, 44(Sup 2): 216-220(in Chinese).
[62] VELLINGIRI K, CHOUDHARY V, PHILIP L. Fabrication of portable colorimetric sensor based on basic fuchsin for selective sensing of nitrite ions [J]. Journal of Environmental Chemical Engineering, 2019, 7(5): 103374. doi: 10.1016/j.jece.2019.103374 [63] AUKEMA K G, WACKETT L P. Inexpensive microbial dipstick diagnostic fornitrate in water [J]. Environmental Science:Water Research & Technology, 2019, 5(2): 406-416. [64] NAWAZ H, TIAN W G, ZHANG J M, et al. Cellulose-based sensor containing phenanthroline for the highly selective and rapid detection of Fe2+ ions with naked eye and fluorescent dual modes [J]. ACS Applied Materials & Interfaces, 2018, 10(2): 2114-2121. [65] WANG Q H, YU L J, LIU Y, et al. Methods for the detection and determination of nitrite and nitrate: A review [J]. Talanta, 2017, 165: 709-720. doi: 10.1016/j.talanta.2016.12.044 [66] HUANGFU C X, ZHANG Y, JANG M, et al. A μPAD for simultaneous monitoring of Cu2+, Fe2+ and free chlorine in drinking water [J]. Sensors and Actuators B:Chemical, 2019, 293: 350-356. doi: 10.1016/j.snb.2019.02.092 [67] MARTINEZ A W, PHILLIPS S T, BUTTE M J, et al. Patterned paper as a platform for inexpensive, low-volume, portable bioassays [J]. Angewandte Chemie, 2007, 46(8): 1318-1320. doi: 10.1002/anie.200603817 [68] ZHANG D H, LI C C, JI D L, et al. Paper-based microfluidic sensors for onsite environmental detection: A critical review [J]. Critical Reviews in Analytical Chemistry, 2021: 1-40. [69] ZHANG Y, LI X, LI H, et al. Postage stamp-sized array sensor for the sensitive screening test of heavy-metal ions [J]. The Analyst, 2014, 139(19): 4887. doi: 10.1039/C4AN01022A [70] JAYAWARDANE B M, WEI S, MCKELVIE I D, et al. Microfluidic paper-based analytical device for the determination of nitrite and nitrate [J]. Analytical Chemistry, 2014, 86(15): 7274-7279. doi: 10.1021/ac5013249 [71] KAMNOET P, AEUNGMAITREPIROM W, MENGER R F, et al. Highly selective simultaneous determination of Cu(Ⅱ), Co(Ⅱ), Ni(Ⅱ), Hg(Ⅱ), and Mn(Ⅱ) in water samples using microfluidic paper-based analytical devices [J]. The Analyst, 2021, 146(7): 2229-2239. doi: 10.1039/D0AN02200D [72] MENTELE M M, CUNNINGHAM J, KOEHLER K, et al. Microfluidic paper-based analytical device for particulate metals [J]. Analytical chemistry, 2012, 84(10): 4474-4480. doi: 10.1021/ac300309c [73] MONIZ T, BASSETT C R, ALMEIDA M I G S, et al. Use of an ether-derived 3-hydroxy-4-pyridinone Chelator as a new chromogenic reagent in the development of a microfluidic paper-based analytical device for Fe(Ⅲ) determination in natural waters [J]. Talanta, 2020, 214: 120887. doi: 10.1016/j.talanta.2020.120887 [74] CATE D M, NANTHASURASAK P, RIWKULKAJORN P, et al. Rapid detection of transition metals in welding fumes using paper-based analytical devices [J]. The Annals of Occupational Hygiene, 2014, 58(4): 413-423. [75] RATTANARAT P, DUNGCHAI W, CATE D, et al. Multilayer paper-based device for colorimetric and electrochemical quantification of metals [J]. Analytical Chemistry, 2014, 86(7): 3555-3562. doi: 10.1021/ac5000224 [76] GUO X F, LIU C, LI N, et al. Ratiometric fluorescent test paper based on silicon nanocrystals and carbon dots for sensitive determination of mercuric ions [J]. Royal Society Open Science, 2018, 5(6): 171922. doi: 10.1098/rsos.171922 [77] CHANG J, LI H, HOU T, et al. Paper-based fluorescent sensor for rapid naked-eye detection of acetylcholinesterase activity and organophosphorus pesticides with high sensitivity and selectivity [J]. Biosensors and Bioelectronics, 2016(86): 971-977. [78] FENG L, LI H, NIU L Y, et al. A fluorometric paper-based sensor array for the discrimination of heavy-metal ions [J]. Talanta, 2013, 108: 103-108. doi: 10.1016/j.talanta.2013.02.073 [79] LI D, MA Y D, DUAN H Z, et al. Griess reaction-based paper strip for colorimetric/fluorescent/SERS triple sensing of nitrite [J]. Biosensors and Bioelectronics, 2018, 99: 389-398. doi: 10.1016/j.bios.2017.08.008 [80] BANSOD B, KUMAR T, THAKUR R, et al. A review on various electrochemical techniques for heavy metal ions detection with different sensing platforms [J]. Biosensors and Bioelectronics, 2017, 94: 443-455. doi: 10.1016/j.bios.2017.03.031 [81] JIANG C B, HE Y H, LIU Y. Recent advances in sensors for electrochemical analysis of nitrate in food and environmental matrices [J]. The Analyst, 2020, 145(16): 5400-5413. doi: 10.1039/D0AN00823K [82] CHEN X Y, ZHOU G H, MAO S, et al. Rapid detection of nutrients with electronic sensors: A review [J]. Environmental Science:Nano, 2018, 5(4): 837-862. doi: 10.1039/C7EN01160A [83] FEIER B, FLONER D, CRISTEA C, et al. Flow electrochemical analyses of zinc by stripping voltammetry on graphite felt electrode [J]. Talanta, 2012, 98: 152-156. doi: 10.1016/j.talanta.2012.06.063 [84] YANG S L, LIU X Y, ZENG X D, et al. Fabrication of nano-copper/carbon nanotubes/chitosan film by one-step electrodeposition and its sensitive determination of nitrite [J]. Sensors and Actuators B:Chemical, 2010, 145(2): 762-768. doi: 10.1016/j.snb.2010.01.032 [85] CUI Y P, YANG C Z, ZENG W, et al. Electrochemical determination of nitrite using a gold nanoparticles-modified glassy carbon electrode prepared by the seed-mediated growth technique [J]. Analytical Sciences:The International Journal of the Japan Society for Analytical Chemistry, 2007, 23(12): 1421-1425. doi: 10.2116/analsci.23.1421 [86] WANG Z F, LIAO F, GUO T T, et al. Synthesis of crystalline silver nanoplates and their application for detection of nitrite in foods [J]. Journal of Electroanalytical Chemistry, 2012, 664: 135-138. doi: 10.1016/j.jelechem.2011.11.006 [87] PHAM X H, LI C A, HAN K N, et al. Electrochemical detection of nitrite using urchin-like palladium nanostructures on carbon nanotube thin film electrodes [J]. Sensors and Actuators B:Chemical, 2014, 193: 815-822. doi: 10.1016/j.snb.2013.12.034 [88] WANG S Q, YIN Y M, LIN X Q. Cooperative effect of Pt nanoparticles and Fe(Ⅲ) in the electrocatalytic oxidation of nitrite [J]. Electrochemistry Communications, 2004, 6(3): 259-262. doi: 10.1016/j.elecom.2003.12.008 [89] MILHANO C, PLETCHER D. The electrodeposition and electrocatalytic properties of copper-palladium alloys [J]. Journal of Electroanalytical Chemistry, 2008, 614(1/2): 24-30. [90] CHEN G Z, ZHENG J B. Non-enzymatic electrochemical sensor for nitrite based on a graphene oxide-polyaniline-Au nanoparticles nanocomposite [J]. Microchemical Journal, 2021, 164: 106034. doi: 10.1016/j.microc.2021.106034 [91] GÖDE C, YOLA M L, YıLMAZ A, et al. A novel electrochemical sensor based on calixarene functionalized reduced graphene oxide: Application to simultaneous determination of Fe(Ⅲ), Cd(Ⅱ) and Pb(Ⅱ) ions [J]. Journal of Colloid and Interface Science, 2017, 508: 525-531. doi: 10.1016/j.jcis.2017.08.086 [92] GEORGE J M, PRIYANKA R N, MATHEW B. Bimetallic Ag-Au nanoparticles as pH dependent dual sensing probe for Mn(Ⅱ) ion and ciprofloxacin [J]. Microchemical Journal, 2020, 155: 104686. doi: 10.1016/j.microc.2020.104686 [93] ABDALLAH N A. Novel potentiometric solid-contact electrode for the determination of Fe2+ ions via MWCNTs-gemifloxacin composite [J]. Electroanalysis, 2021, 33(5): 1283-1289. doi: 10.1002/elan.202060319 [94] ROUSHANI M, SAEDI Z, HAMDI F, et al. Preparation an electrochemical sensor for detection of manganese (Ⅱ) ions using glassy carbon electrode modified with multi walled carbon nanotube-chitosan-ionic liquid nanocomposite decorated with ion imprinted polymer [J]. Journal of Electroanalytical Chemistry, 2017, 804: 1-6. doi: 10.1016/j.jelechem.2017.09.038 [95] HAN Z Y, MA H N, SHI G Z, et al. A review of groundwater contamination near municipal solid waste landfill sites in China [J]. Science of the Total Environment, 2016, 569/570: 1255-1264. doi: 10.1016/j.scitotenv.2016.06.201 [96] 雷抗. 垃圾填埋场地下水污染监测预警技术研究 : 以天津市某简易垃圾填埋场为例[D]. 北京: 中国地质大学(北京), 2018. LEI K. Study on monitoring and early warning technology applied to groundwater contamination in waste landfills —acase studyfor asimple landfill in Tianjin[D]. Beijing: China University of Geosciences, 2018(in Chinese).
[97] JIANG Y, LI R, YANG Y N, et al. Migration and evolution of dissolved organic matter in landfill leachate-contaminated groundwater plume [J]. Resources, Conservation and Recycling, 2019, 151: 104463. doi: 10.1016/j.resconrec.2019.104463 [98] 张云龙. 地下水典型污染源全过程监控及预警方法研究[D]. 成都: 成都理工大学, 2016. ZHANG Y L. Study on the whole-process monitoring and early warning of groundwater pollution of typical pollution sources[D]. Chengdu: Chengdu University of Technology, 2016(in Chinese).
-




 下载:
下载: