-
环境持久性自由基(environmental persistent free radicals,EPFRs)是一类有较长寿命的自由基,广泛存在于大气颗粒物[1-2]、土壤[3]、飞灰[4]、生物炭[5]、石油[6]、焦炭[7]等环境介质中. EPFRs具有较强的氧化活性,一旦进入肌体,会对人体健康造成不利影响.近年来,随着经济的快速发展,我国环境空气质量恶化,城市灰霾现象频发. PM2.5浓度升高是导致的城市灰霾的主要原因,PM2.5的来源和组成非常复杂,已成为现阶段大气环境化学研究的热点和难点[8-10].
PM2.5不仅恶化环境空气质量,还能危害人体健康. 这因为一方面PM2.5是一些有毒有害物质的载体,如含有一些有机物、金属等污染物,另一方面PM2.5是一些大气污染物的转化媒介[11-13],如PM2.5会改变一些气态污染物的大气迁移转化行为. 有关PM2.5的化学组分分析,现阶段大多集中在有机碳(organic carbon,OC)、元素碳(elementary carbon,EC)、水溶性离子
NO−3 、SO2−4 、Cl−、NH+4 、Na+、K+、Ca2+、Mg2+等)、金属元素(Al、Fe、Cu、Pb、V等)以及有机单体等,少量课题组开展了PM2.5中EPFRs的测定.这些研究结果表明,EPFRs浓度具有很强的季节分布特征,更易存在于超细颗粒物中,且EPFRs常伴含金属和芳香族有机物[14-15]。此外,EPFRs表现出较强的大气稳定性及生物毒性[16-19]. 虽然,这些研究使我们对PM2.5中EPFRs有了初步的认识,但对EPFRs的组成、来源认知较为匮乏,对EPFRs的大气迁移、转化规律的研究则更少.本研究在北京市怀柔区进行了大气环境PM2.5样品的采集,分析了PM2.5中EPFRs和共存的化学组分,探讨了EPFRs与共存化学组分的相关关系,并结合气态污染物和气象参数信息,研究了EPFRs及共存组分的变化规律和来源.
-
本研究采样点位于北京市中国科学院大学雁栖湖校区教一楼顶(东经116.69°,北纬40.40°,距地面约15 m,距国道G111约60 m). 采样点北距北京北四环约60 km,属于城市上风向,是典型的城市背景点. Digital DHA-80(500 L·min−1)采样器采集石英膜样品,采样时段为2018年1、4、7、10月,分别代表冬、春、夏和秋季,每个样品采集23 h(10:00—次日9:00). 采样期间每隔10 d采集1张空白样品,对采样全过程进行质量控制. 共收集105个样品. 所有石英采样膜在采样之前在550 ℃的马弗炉煅烧下5.5 h. 样品采集后铝箔包好后放入−20℃冰箱中直至分析.
-
从采样膜中切割28 mm×5 mm的样品并置于电子顺磁波谱仪(EPR)中,测定EPFRs的浓度. EPR仪器参数设置为:磁场强度3300-3450 G;检测时间60 s;扫描次数1次,微波强度8.0 mW,调制幅度2 G.选用标准样品(Mg2+和Cr3+的标准品,内径:3.0 mm,外径4.0 mm)校准绝对自旋量以及g因子. 测定EPFRs的谱峰宽得到线宽ΔHp-p,以此来表征自由基种类丰富度[10,12,20].
-
PM2.5中的有机碳(organic carbon, OC)和元素碳(elementary carbon, EC)用碳分析仪(Sunset Lab RT-4)来测定,升温程序采用美国职业安全与卫生研究所推荐的方法[12];水溶性离子(
NO−3 、SO2−4 、Cl−、NH+4 、Na+、K+、Ca2+、Mg2+)使用离子色谱分析仪(Thermo Fisher Scientific,型号ICS-1100);PM2.5样品经过微波消解、赶酸、定容后经ICP-MS(Thermo Fisher Scientific)用来测定样品中的金属元素. 检测方法为KED模式,驻留时间为0.02 s. 选用Rh(Sigma-Aldrich,美国)作为内标物质,使用Sigma-Aldrich 54704和Accustandard SQS-01-1配制标准曲线. -
计算金属元素的富集因子可用于判别PM2.5中金属元素的来源[14].富集因子的计算公式为:
式中,(Ci/Cn)Sample与(Ci/Cn)Crust为金属i、参比元素n分别在PM2.5中和地壳中的含量比. 本研究选用Al为参比元素,元素背景值取中国土壤元素含量的平均值[21],具体数值见表2.当EF>10,则元素i主要来自于人为污染源;当EF<1,则元素i主要来自于土壤扬尘等自然源;当1≤EF≤10,则认为自然源和人为源对元素i均有贡献.
-
由图1(a)可见,不同季节PM2.5中EPFRs的浓度和种类分布特征差异显著. 冬、春、夏、秋季的PM2.5中EPFRs的平均浓度分别为2.63×1013、2.92×1013、1.50×1013和2.13×1013 spins·m−3,其对应的单位质量PM2.5含有EPFRs的自旋值分别为11.11×1017、8.12×1017、4.04×1017及10.42×1017 spins. 该值与香烟中自由基浓度(6.175×1014 spins·cig−1)相比[20],计算得出怀柔在冬、春、夏、秋EPFRs日均暴露水平分别为0.85、0.94、0.48和0.68支香烟,则一年暴露水平是258支香烟,为西安地区EPFRs暴露水平的1/10[22]. 综合以往报道,不同区域大气颗粒物中EPFRs的浓度范围为1014—1020 spins·g−1,且存在很强的地域特征. 北京和重庆PM2.5中EPFRs的浓度约为1018—1019 spins·g−1[22],而西安约为1016—1018 spins·g−1[10]. 此外,不同社会功能区PM2.5中的EPFRs浓度有所差异,如Wang等在北京、广州等分别检测了农业区、居民区、商业区及工业区大气中EPFRs的浓度[23],结果表明EPFRs在居民区和商业区的浓度更高. 本次观测的EPFRs浓度比北京城市中心区的报道值(约1019 spins·g−1)低2—3个数量级[1],比同年西安市的报道值(约1018 spins·g−1)低1个数量级[22],这主要因为怀柔地区处于北京上风向,作为城市背景点,环境空气质量优于城市中心区. 在时间序列上,EPFRs的浓度呈现出冬、春季较高,秋季次之,夏季最低的分布特征,且同一季节内EPFRs的浓度呈现日变化幅度较大的分布特征,这表明PM2.5中EPFRs的生成、猝灭过程较为复杂,且本地排放以及气象条件的差异均会对EPFRs浓度产生影响. 春季时PM2.5中EPFRs的浓度最高,可能因为风起扬尘对EPFRs有较大贡献. Chen等[4]在分析济南、张北和二连浩特PM2.5中EPFRs时,发现EPFRs的浓度在沙尘暴天气时是非沙尘暴天气时2倍左右. 本研究春季Ca2+浓度是其它三季的2.2倍左右(见表1),而尘土中富含EPFRs[24],从而造成PM2.5中EPFRs的浓度最高;夏季时EPFRs浓度最低,可能因为夏季空气湿度较大,颗粒物水分含量的增加PM2.5中EPFRs的淬灭机率,且北京处于雨季,PM2.5的大气寿命较短;秋季时近地表昼夜温差大,易形成逆温现象,限制PM2.5的垂直扩散,延长了其大气寿命,可能造成EPFRs的积累和浓度抬升;冬季采暖期北京怀柔地区生物质和煤炭用量增加,可能导致EPFRs浓度处在较高的水平.
g因子(g-factors)是表征EPFRs种类的重要指标. 当g因子小于2.003时,EPFRs为碳中心自由基,如环戊二烯基;g因子大于2.004时,为氧中心自由基,如苯氧基,氯苯氧基,半醌基等;当g因子处于两值之间时,是碳氧混合为中心的复杂自由基或含有含氧官能团的碳中心自由基[22-23,30]. 由图1(c)所示,冬、春、秋三季的g因子平均值均为2.0038,变化范围为2.0027—2.0052;夏季为2.0035,变化范围为2.0021—2.0044. 该值表明大气中主要存在以碳氧混合为中心的复杂自由基或含有含氧官能团的碳中心自由基,如苯氧自由基或半醌自由基. 夏季比其它三季g因子平均值略低,但夏季g因子分布范围更广,可能因为夏季大气光化学反应活跃、大气热力交换剧烈等因素造成的自由基生消更快、本地和区域空气混合更迅速[31]. EPFRs的线宽ΔHp-p是表征自由基种类丰富度的重要参数[6]. 图1(d)显示夏季的ΔHp-p平均值和日变化幅度最大,表明夏季EPFRs的组成、来源更为复杂.来源对于EPFRs的种类影响非常显著,有研究报道了煤炭燃烧[23]、移动源[12,26]、扬尘[4,25]、光化学作用[16,27-28]、生物质燃烧[29]等源生成EPFRs的浓度及种类,结果表明来源的差异会对EPFRs生成和转化过程产生重要影响.
-
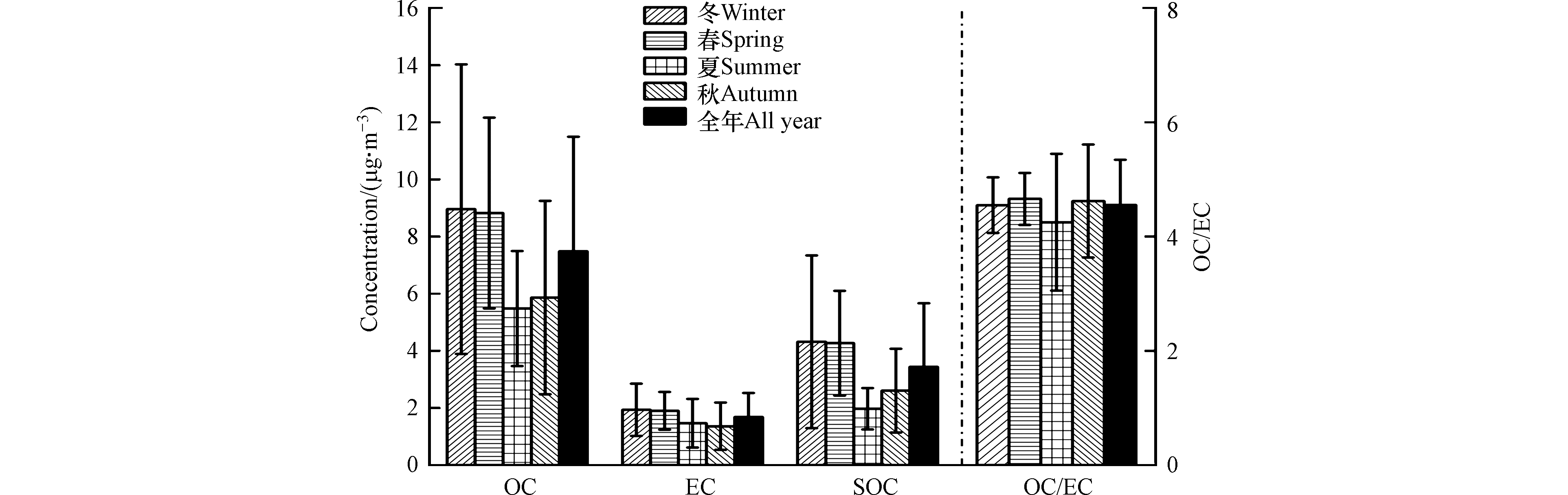
如图2所示,冬、春、夏及秋季的OC浓度平均值分别为8.96、8.82、5.48、5.86 μg·m−3;EC的浓度值为1.93、1.90、1.46、1.36 μg·m−3. OC和EC的质量浓度呈现冬≈春>秋≈夏的规律,表明PM2.5的燃烧排放强度在冬、春季较大;且OC和EC在四季均呈现较强的正相关关系(R2=0.88,P<0.001),表明OC、EC可能来源相同. 冬、春、夏和秋季OC/EC值分别为4.55、4.66、4.25及4.62. 尽管不同季节OC/EC值差别不大,但OC/EC日间变化幅度呈现夏>秋>春≈冬,这说明夏季PM2.5来源更为多样,这与EPFRs的线宽ΔHp-p在夏季最大相一致. 根据OC/EC值可以初步判断碳质组分的来源,不同燃烧排放源的OC/EC值差别较大,如燃煤、机动车和生物质燃烧源OC/EC分别为8.5—12、1.1和10—16.3[31-33]. 本次研究四季OC/EC均值在4.2—4.7,且冬、春、秋三季
NO−3 浓度均高于SO2−4 (夏季NO−3 的浓度较低,可能因为夏天气温较高导致其分解挥发),说明机动车排放对采样点PM2.5影响较为显著,可能采样点距国道G111较近有关;K+常作为生物质燃烧排放源的示踪物,表1所示K+浓度在夏季最低,这可能导致了OC/EC值在夏季略低. EC示踪法常用来计算二次有机碳(SOC)及一次有机碳(POC)的含量,计算公式如下:式中,(OC/EC)min为该采样时段OC/EC的最小值. 当OC/EC值远高于2,可认为二次污染过程对OC有显著贡献[31]. 经计算SOC的浓度呈现冬≈春>秋>夏的分布规律,这说明SOC在冬季生成较为显著;北京大学2016年冬季在怀柔的观测实验中也得出相似结论,其观察到冬季多次霾事件中均出现有自由基物质参与的光化学反应,反应速率与夏季光化学烟雾的速率高值相当,且这些反应能有效促进SOC的生成[34];另外冬、春季PM2.5大气寿命较长,夏季雨季PM2.5大气寿命较短,这也是导致SOC冬、春季浓度较高的原因之一.
-
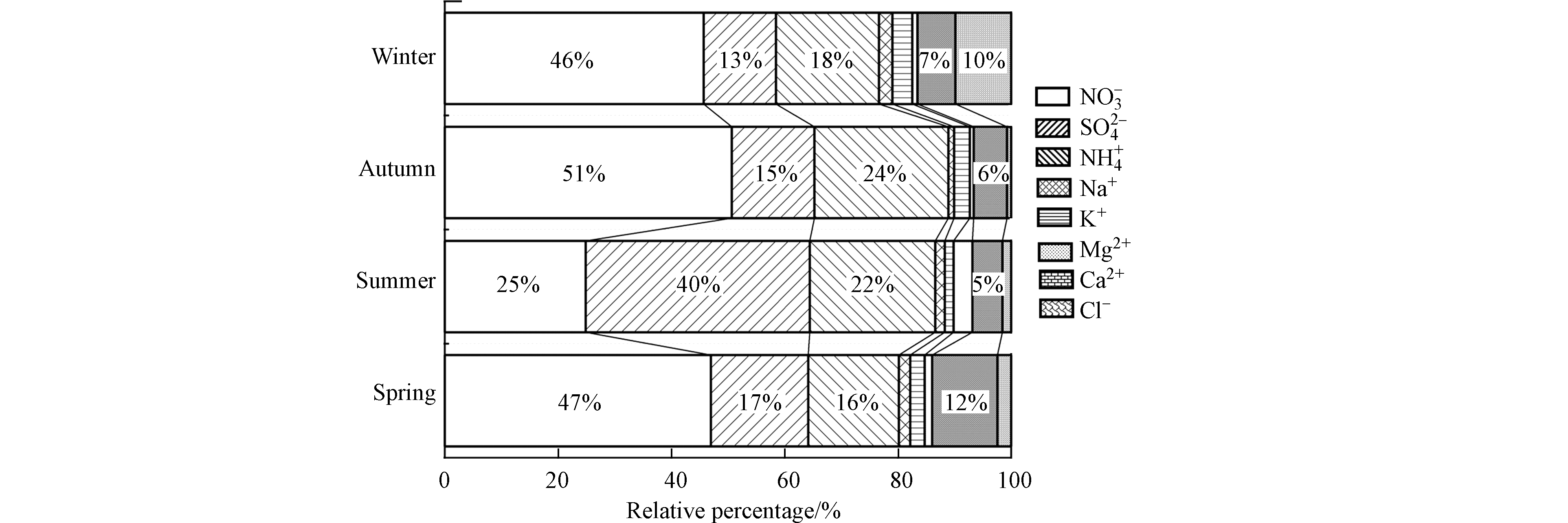
水溶性离子(Water-soluble ions)的四季浓度如表2所示,其中
NO−3 、SO2−4 、NH+4 质量浓度分别为(4.04±1.10)、(2.10±1.39)、(1.94±0.38) μg·m−3,分别占总离子浓度的41.68%、21.62%、19.95%.从时间序列来看,SNA/WSIIs的顺序为秋>夏>春>冬,且均达到75%以上. 此外,各类离子全年质量浓度高低顺序为NO−3 >SO2−4 >NH+4 >Ca2+>Cl−>K+>Na+>Mg2+,这与多数文献的报道值相符.由图3可见,
NO−3 、SO2−4 及NH+4 在各季的分布特征有显著区别. 其中,NO−3 浓度呈现春>秋>冬>夏的分布特征,NO−3 前体物NOx主要来自于移动源的排放,观测结果表明春季时NOx的二次转化效率较高;SO2−4 浓度呈现夏>春>秋>冬的分布特征,夏季观测期内,出现了不连续四天的SO2−4 浓度高值(>8 μg·m−3,而其余观测值均<2 μg·m−3),有可能是怀柔一些企业存在偷排现象;NH+4 浓度则呈现夏>秋>春≈冬的分布特征,这与SO2−4 的分布特征相似.此外,Ca2+是阳离子中浓度较高的一类离子,其主要来源于土壤沙尘、扬尘等[28].Ca2+在春季时的浓度显著高于其他三季,这与北方地区春季时风力较大、起尘较强相关.冬季时,K+和Cl−的浓度均有明显上升,这表明冬季时生物质燃烧和煤炭燃烧源均有增强[33]. -
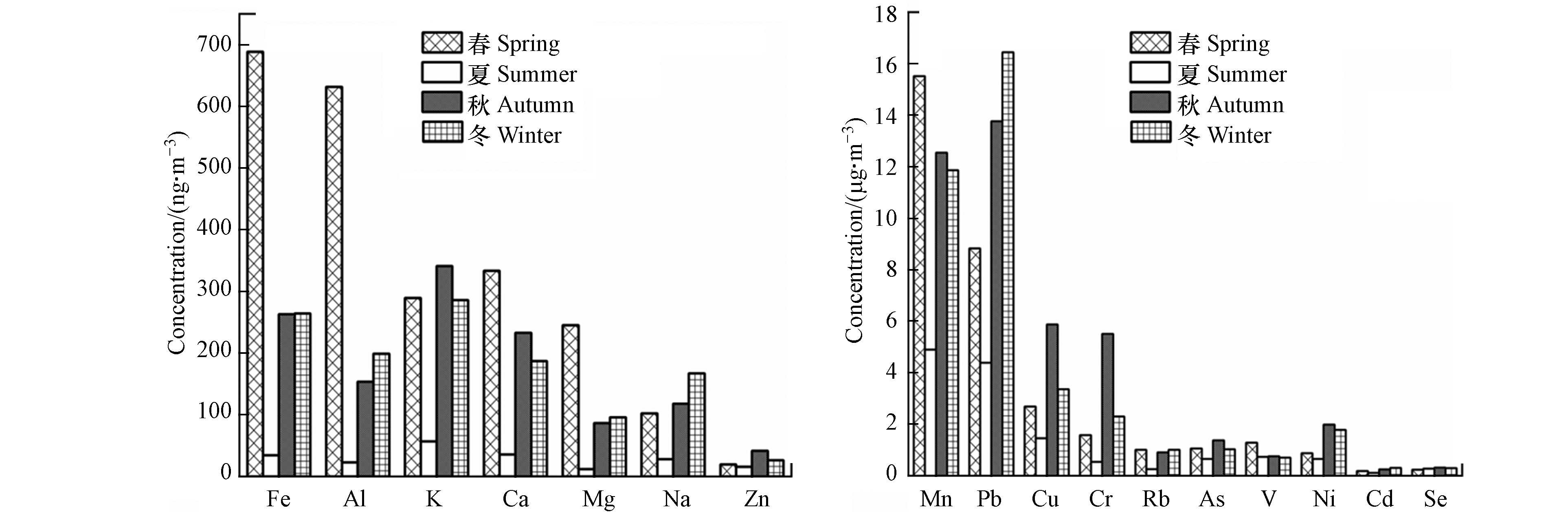
表2展示了采样期间PM2.5中不同金属的质量浓度,20种金属元素质量浓度总计为1275.63 ng·m−3,各金属含量大小为:Fe>Al>K>Ca>Mg>Na>Zn>>Mn>Pb>Cu>Cr>Rb>As>V>Ni>Cd>Se. 其中,Fe、Al、K、Ca、Mg、Na的含量较高,其质量浓度之和约占总浓度的95.4%. Cr、Ni的EF值稍大于10,受到人为源影响较轻;Zn、Cu、As及Pb的EF值在25—120之间,富集程度显现;Zn和Pb主要来自于机动车尾气或燃煤[35],Cu主要来自刹车片磨损或冶炼[36],As主要来自燃煤[37];Cd的富集程度较重,人为污染非常严重;大气中Cd主要来自于燃煤、石油燃烧、垃圾焚烧、机动车尾气等人为活动[38],本次观测Cd可能主要来自于燃煤或机动车尾气.
从图4怀柔区金属元素的季节变化可以看出,各金属元素浓度季节差异较大. 但总体均体现春>冬>秋>>夏的分布特征. 其中,地壳元素Fe、Al、Ca、Mg的春季浓度远高于其他季节,这表明春季风起扬尘作用贡献较大,PM2.5中含有更多的地壳元素,这也与春季时EPFRs浓度最高相符.
-
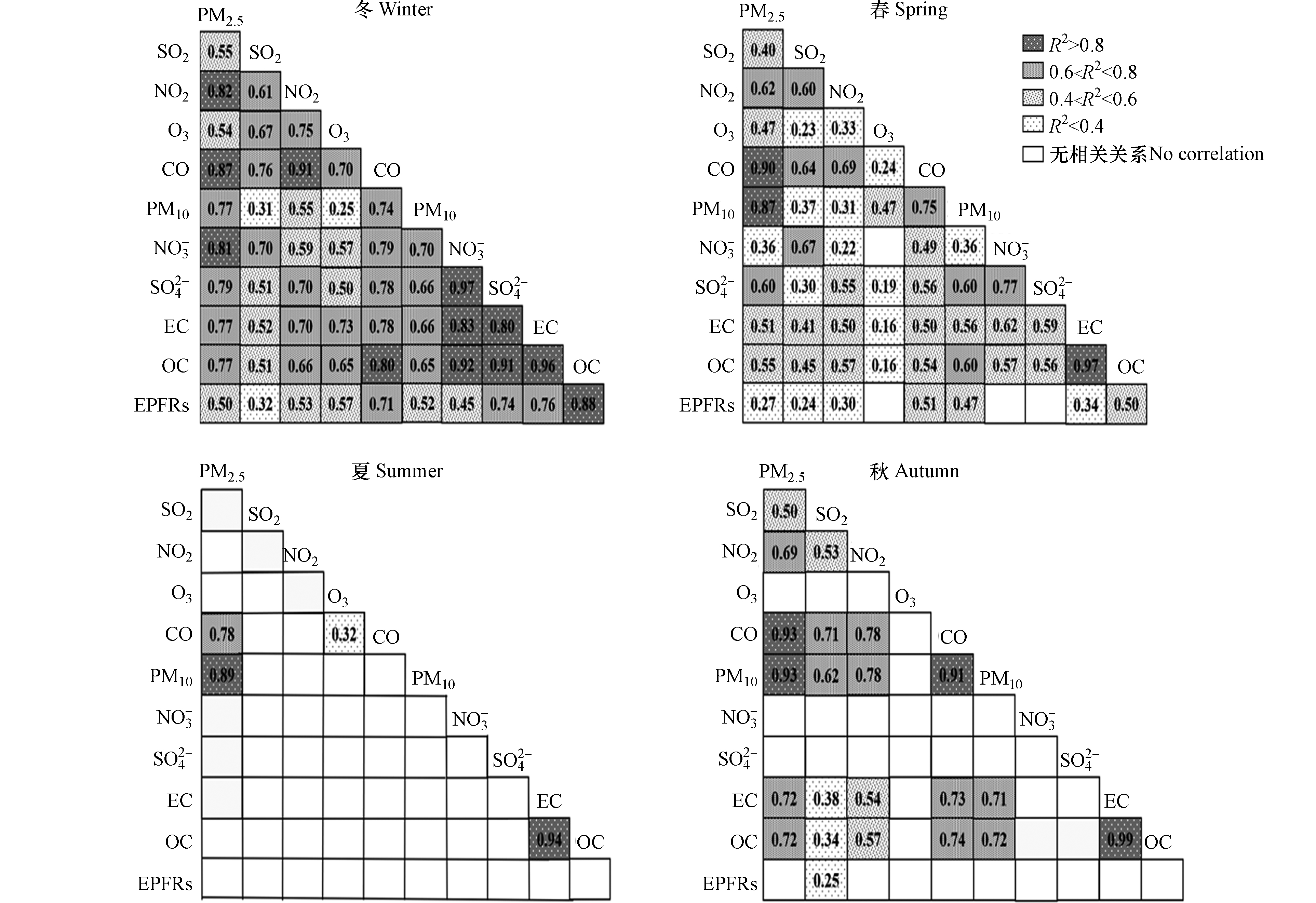
分析不同组分间的相关性可以更深入地了解EPFRs及共存组分的来源及转化过程. 如图5所示,冬季时,EPFRs与共存组分均呈现正相关,其中,EPFRs与OC、
SO2−4 的相关性最为显著,SO2−4 主要来源于燃煤过程,这说明冬季时PM2.5中部分EPFRs可能来自于燃煤排放源.此外,冬季时EPFRs和SOC和POC均呈线性相关关系,说明冬季EPFRs的来源不仅来自于一次直接排放源,二次化学反应生成也对其有重要贡献. 因PM2.5中OC与其共存主要组分(
SO2−4 、NO−3 等)有较强的相关性,且多类源均对OC有贡献,故选取POC、SOC,利用SPSS软件,建立POC和SOC与EPFRs的多元线性回归方程,进一步分析一次排放和二次生成对EPFRs的影响. 通过逐步回归法,并结合相关性系数、显著性水平及共线性诊断等参数分析法,进一步明确所建模型的统计学有效性. 最终得到方程如下:其中,CEPFRs表示EPFRs的浓度(spins·m−3),CSOC、CPOC分别表示SOC和POC的浓度(μg·m−3). 经计算,该方程R2=0.895,P<0.001,VIF<5. 其中,VIF为方差扩大因子(Variance Inflation Factor),当VIF<10时,表明因变量间不存在共线性. 因此,参数分析表明用SOC和POC表征EPFRs的来源具有统计学意义. 进一步将SOC和POC在冬季的平均质量浓度与方程系数相乘,得到SOC与POC对冬季EPFRs生成贡献比值为11∶4,且EPFRs与O3 (只来自于大气二次反应生成)仅在冬季时呈正相关(R2=0.57,P<0.001),表明冬季PM2.5中EPFRs可能更多源自于二次生成. 上文也提及EPFRs与OC、
SO2−4 的相关性最为显著,从图5中也可以看出EPFRs与CO、EPFRs与EC、OC与SO2−4 、OC与NO−3 以及OC与EC均呈现较强正相关关系,表明怀柔地区冬季机动车尾气和燃煤源排放及其排放污染物的大气二次转化,对PM2.5中EPFRs有显著贡献,李豪等也发现西安市冬季时煤炭燃烧源和交通源是PM2.5中EPFRs主要来源[22].春季时EPFRs与其他物质的相关性减弱,此时EPFRs与PM2.5的相关性低于与PM10的相关性,这表明春季时PM10中含有更多的EPFRs,同样可能来自于风起扬尘的贡献. 夏季时EPFRs与其他污染组分无相关关系,但与POC仍呈弱正相关(R2=0.32,P<0.001),这可能因为夏季气温高、湿度大,大气光化学反应活跃、大气热力交换剧烈,EPFRs的生消更为迅速.此外,分析常规污染气体与其他组分的相关性也可间接反映大气物理化学过程. NO2在冬、春两季和其他六种常规污染物及
NO−3 、EC、PM2.5、OC、EPFRs均呈正相关关系. 其中,NO2和CO、PM2.5的正相关关系尤为突出. 研究表明,城市大气中的NO2和CO均来自于移动源排放[9],这说明春冬时移动源排放可能是造成怀柔地区NO2及PM2.5污染的原因之一. 此外,NO2与二次污染物也呈现正相关关系,其中冬季和春季的相关关系较为显著,这说明此时的NO2较多的参与向SOA的转化过程,这与2.3节中关于春季时NOx的二次转化效率较高的讨论相符;秋季时两者呈弱正相关关系,夏季时则无相关性,这可能是由于夏季的NO2更多的参与了与O3、NO的光化学过程,并与挥发性有机物的作用生成了其他类型的有机污染物[9].SO2表现出与NO2相似的相关性特征,但SO2与其他物质的相关关系均表现出弱化的趋势. 与NO2不同的是,SO2与EPFRs在秋季是仍保持弱正相关关系. SO2主要来源于含硫燃料的燃烧,可能因为秋季时节(10月)采样点周围居民散煤使用造成的[21]. -
本文通过分析2018年北京怀柔PM2.5中EPFRs及化学物质的污染特征,发现EPFRs的四季平均浓度范围为1.50×1013—2.92×1013 spins·m−3,并呈现春>冬>秋>夏的分布特征,且种类主要为含氧碳中心自由基或碳中心、氧中心自由基混合态;冬季时机动车尾气和燃煤源直接排放及排放污染物的大气二次转化对PM2.5中EPFRs有显著贡献;春季时风起扬尘EPFRs浓度有较大影响;夏季尽管EPFRs的浓度最低,但是其物种分布最广;冬季时EPFRs与PM2.5中多种组分(OC、EC、
SO2−4 )、CO等相关性较好,而夏季EPFRs与PM2.5中组分(OC、EC、SO2−4 )、气态污染物均无相关性.
北京怀柔PM2.5中环境持久性自由基及共存健康风险物质的污染特征
Pollution characteristics of environmental persistent free radicals and coexisting health risk substances in PM2.5 in Huairou, Beijing
-
摘要: 北京怀柔科学城的建设加快了区域城市化的进程.为探究此过程对空气质量的影响,于2018年在怀柔地区采集了大气PM2.5样品,分析了PM2.5中环境持久性自由基(environmental persistent free radicals, EPFRs)及其共存组分的季节变化特征.结果表明,冬、春、夏、秋四季EPFRs的浓度数量级均为1013 spins·m−3,呈现春>冬>秋>夏的分布特征;冬、春、秋季的g因子均值为2.0038,夏季为2.0035,表明EPFRs主要是含氧的碳中心自由基或碳中心、氧中心自由基混合态;夏季g因子均值略低且分布广,可能因为夏季大气光化学反应活跃、大气热力交换剧烈.利用回归分析和物种示踪法,解析出冬季EPFRs主要源自机动车尾气和燃煤排放;春季风起扬尘对EPFRs浓度有显著影响. PM2.5中含碳物质(OC、EC)均呈现冬≈春>秋≈夏的分布特征,夏季OC/EC日间变化幅度最大;
NO−3 、SO2−4 及NH+4 是PM2.5中水溶性离子的主要成分,但三者的季节分布特征有显著差异;PM2.5中金属元素均呈现春>冬>秋>夏的分布特征,且少许金属的人为来源主要是燃煤和机动车尾气.Abstract: The construction of Beijing Huairou Science City has accelerated the process of regional urbanization. In order to explore the impact of this process on air quality, atmospheric PM2.5 samples were collected in Huairou in 2018, and environmental persistent free radicals in PM2.5 were analyzed (Environmental Persistent Free Radicals, EPFRs) and the seasonal variation characteristics of their coexisting components. The results show that the concentration of EPFRs in the four seasons of winter, spring, summer, and autumn are all 1013 spins·m−3, showing spring>winter>autumn>summer Distribution characteristics; the mean g-factor in winter, spring, and autumn is 2.0038 and 2.0035 in summer, indicating EPFRs mainly exist in the form of oxygen-containing carbon center free radicals or the mixture of carbon center and oxygen center free radicals; the mean value of g-factor in summer is slight low and wide distribution due to higher photochemical reaction rate and more frequent convection in summer. EPFRs in winter are mainly from vehicle exhaust and coal-burning emissions via regression analysis and chemical-tracer method; the dust raised by the spring wind has a significant impact on the concentration of EPFRs. PM2.5 Carbon-containing substances (OC, EC) all present the distribution characteristics of winter≈spring>autumn≈summer, and the OC/EC changes the most during the day in summer;NO−3 ,SO2−4 andNH+4 are the water-soluble ions in PM2.5 The main components, but the seasonal distribution characteristics of the three are significantly different; the seasonal trends of all metal elements in PM2.5 are spring>winter>autumn>summer, and the results of the enrichment factor show that the mainly anthropogenic sources of metals are coal-burning and vehicle exhaust. -
环境持久性自由基(environmental persistent free radicals,EPFRs)是一类有较长寿命的自由基,广泛存在于大气颗粒物[1-2]、土壤[3]、飞灰[4]、生物炭[5]、石油[6]、焦炭[7]等环境介质中. EPFRs具有较强的氧化活性,一旦进入肌体,会对人体健康造成不利影响.近年来,随着经济的快速发展,我国环境空气质量恶化,城市灰霾现象频发. PM2.5浓度升高是导致的城市灰霾的主要原因,PM2.5的来源和组成非常复杂,已成为现阶段大气环境化学研究的热点和难点[8-10].
PM2.5不仅恶化环境空气质量,还能危害人体健康. 这因为一方面PM2.5是一些有毒有害物质的载体,如含有一些有机物、金属等污染物,另一方面PM2.5是一些大气污染物的转化媒介[11-13],如PM2.5会改变一些气态污染物的大气迁移转化行为. 有关PM2.5的化学组分分析,现阶段大多集中在有机碳(organic carbon,OC)、元素碳(elementary carbon,EC)、水溶性离子
NO−3 SO2−4 NH+4 本研究在北京市怀柔区进行了大气环境PM2.5样品的采集,分析了PM2.5中EPFRs和共存的化学组分,探讨了EPFRs与共存化学组分的相关关系,并结合气态污染物和气象参数信息,研究了EPFRs及共存组分的变化规律和来源.
1. 材料和方法(Materials and methods)
1.1 大气PM2.5的采样
本研究采样点位于北京市中国科学院大学雁栖湖校区教一楼顶(东经116.69°,北纬40.40°,距地面约15 m,距国道G111约60 m). 采样点北距北京北四环约60 km,属于城市上风向,是典型的城市背景点. Digital DHA-80(500 L·min−1)采样器采集石英膜样品,采样时段为2018年1、4、7、10月,分别代表冬、春、夏和秋季,每个样品采集23 h(10:00—次日9:00). 采样期间每隔10 d采集1张空白样品,对采样全过程进行质量控制. 共收集105个样品. 所有石英采样膜在采样之前在550 ℃的马弗炉煅烧下5.5 h. 样品采集后铝箔包好后放入−20℃冰箱中直至分析.
1.2 EPFRs的浓度
从采样膜中切割28 mm×5 mm的样品并置于电子顺磁波谱仪(EPR)中,测定EPFRs的浓度. EPR仪器参数设置为:磁场强度3300-3450 G;检测时间60 s;扫描次数1次,微波强度8.0 mW,调制幅度2 G.选用标准样品(Mg2+和Cr3+的标准品,内径:3.0 mm,外径4.0 mm)校准绝对自旋量以及g因子. 测定EPFRs的谱峰宽得到线宽ΔHp-p,以此来表征自由基种类丰富度[10,12,20].
1.3 PM2.5的化学组分
PM2.5中的有机碳(organic carbon, OC)和元素碳(elementary carbon, EC)用碳分析仪(Sunset Lab RT-4)来测定,升温程序采用美国职业安全与卫生研究所推荐的方法[12];水溶性离子(
NO−3 SO2−4 NH+4 1.4 金属元素的富集因子(Enrichment Factors,EF)
计算金属元素的富集因子可用于判别PM2.5中金属元素的来源[14].富集因子的计算公式为:
EF=(Ci/Cn)Sample(Ci/Cn)Crust 式中,(Ci/Cn)Sample与(Ci/Cn)Crust为金属i、参比元素n分别在PM2.5中和地壳中的含量比. 本研究选用Al为参比元素,元素背景值取中国土壤元素含量的平均值[21],具体数值见表2.当EF>10,则元素i主要来自于人为污染源;当EF<1,则元素i主要来自于土壤扬尘等自然源;当1≤EF≤10,则认为自然源和人为源对元素i均有贡献.
2. 结果和讨论(Results and discussion)
2.1 EPFRs
由图1(a)可见,不同季节PM2.5中EPFRs的浓度和种类分布特征差异显著. 冬、春、夏、秋季的PM2.5中EPFRs的平均浓度分别为2.63×1013、2.92×1013、1.50×1013和2.13×1013 spins·m−3,其对应的单位质量PM2.5含有EPFRs的自旋值分别为11.11×1017、8.12×1017、4.04×1017及10.42×1017 spins. 该值与香烟中自由基浓度(6.175×1014 spins·cig−1)相比[20],计算得出怀柔在冬、春、夏、秋EPFRs日均暴露水平分别为0.85、0.94、0.48和0.68支香烟,则一年暴露水平是258支香烟,为西安地区EPFRs暴露水平的1/10[22]. 综合以往报道,不同区域大气颗粒物中EPFRs的浓度范围为1014—1020 spins·g−1,且存在很强的地域特征. 北京和重庆PM2.5中EPFRs的浓度约为1018—1019 spins·g−1[22],而西安约为1016—1018 spins·g−1[10]. 此外,不同社会功能区PM2.5中的EPFRs浓度有所差异,如Wang等在北京、广州等分别检测了农业区、居民区、商业区及工业区大气中EPFRs的浓度[23],结果表明EPFRs在居民区和商业区的浓度更高. 本次观测的EPFRs浓度比北京城市中心区的报道值(约1019 spins·g−1)低2—3个数量级[1],比同年西安市的报道值(约1018 spins·g−1)低1个数量级[22],这主要因为怀柔地区处于北京上风向,作为城市背景点,环境空气质量优于城市中心区. 在时间序列上,EPFRs的浓度呈现出冬、春季较高,秋季次之,夏季最低的分布特征,且同一季节内EPFRs的浓度呈现日变化幅度较大的分布特征,这表明PM2.5中EPFRs的生成、猝灭过程较为复杂,且本地排放以及气象条件的差异均会对EPFRs浓度产生影响. 春季时PM2.5中EPFRs的浓度最高,可能因为风起扬尘对EPFRs有较大贡献. Chen等[4]在分析济南、张北和二连浩特PM2.5中EPFRs时,发现EPFRs的浓度在沙尘暴天气时是非沙尘暴天气时2倍左右. 本研究春季Ca2+浓度是其它三季的2.2倍左右(见表1),而尘土中富含EPFRs[24],从而造成PM2.5中EPFRs的浓度最高;夏季时EPFRs浓度最低,可能因为夏季空气湿度较大,颗粒物水分含量的增加PM2.5中EPFRs的淬灭机率,且北京处于雨季,PM2.5的大气寿命较短;秋季时近地表昼夜温差大,易形成逆温现象,限制PM2.5的垂直扩散,延长了其大气寿命,可能造成EPFRs的积累和浓度抬升;冬季采暖期北京怀柔地区生物质和煤炭用量增加,可能导致EPFRs浓度处在较高的水平.
表 1 水溶性离子的四季浓度(μg·m−3)Table 1. Seasonal concentrations of water-soluble ions(μg·m−3)春Spring 夏Summer 秋Autumn 冬Winter 全年All year NO−3 5.02 2.59 4.76 3.82 4.04 SO2−4 1.84 4.12 1.37 1.07 1.38 NH+4 1.70 2.30 2.22 1.52 0.38 Na+ 0.22 0.18 0.09 0.20 0.05 K+ 0.28 0.16 0.26 0.29 0.05 Mg2+ 0.14 0.35 0.06 0.07 0.13 Ca2+ 1.23 0.55 0.55 0.56 0.33 Cl- 0.26 0.16 0.07 0.82 0.33 SNA/WSIIs 80.16% 86.59% 88.94% 76.76% 83.25% SNA., 离子 NO−3 SO2−4 NH+4 g因子(g-factors)是表征EPFRs种类的重要指标. 当g因子小于2.003时,EPFRs为碳中心自由基,如环戊二烯基;g因子大于2.004时,为氧中心自由基,如苯氧基,氯苯氧基,半醌基等;当g因子处于两值之间时,是碳氧混合为中心的复杂自由基或含有含氧官能团的碳中心自由基[22-23,30]. 由图1(c)所示,冬、春、秋三季的g因子平均值均为2.0038,变化范围为2.0027—2.0052;夏季为2.0035,变化范围为2.0021—2.0044. 该值表明大气中主要存在以碳氧混合为中心的复杂自由基或含有含氧官能团的碳中心自由基,如苯氧自由基或半醌自由基. 夏季比其它三季g因子平均值略低,但夏季g因子分布范围更广,可能因为夏季大气光化学反应活跃、大气热力交换剧烈等因素造成的自由基生消更快、本地和区域空气混合更迅速[31]. EPFRs的线宽ΔHp-p是表征自由基种类丰富度的重要参数[6]. 图1(d)显示夏季的ΔHp-p平均值和日变化幅度最大,表明夏季EPFRs的组成、来源更为复杂.来源对于EPFRs的种类影响非常显著,有研究报道了煤炭燃烧[23]、移动源[12,26]、扬尘[4,25]、光化学作用[16,27-28]、生物质燃烧[29]等源生成EPFRs的浓度及种类,结果表明来源的差异会对EPFRs生成和转化过程产生重要影响.
2.2 含碳物质
如图2所示,冬、春、夏及秋季的OC浓度平均值分别为8.96、8.82、5.48、5.86 μg·m−3;EC的浓度值为1.93、1.90、1.46、1.36 μg·m−3. OC和EC的质量浓度呈现冬≈春>秋≈夏的规律,表明PM2.5的燃烧排放强度在冬、春季较大;且OC和EC在四季均呈现较强的正相关关系(R2=0.88,P<0.001),表明OC、EC可能来源相同. 冬、春、夏和秋季OC/EC值分别为4.55、4.66、4.25及4.62. 尽管不同季节OC/EC值差别不大,但OC/EC日间变化幅度呈现夏>秋>春≈冬,这说明夏季PM2.5来源更为多样,这与EPFRs的线宽ΔHp-p在夏季最大相一致. 根据OC/EC值可以初步判断碳质组分的来源,不同燃烧排放源的OC/EC值差别较大,如燃煤、机动车和生物质燃烧源OC/EC分别为8.5—12、1.1和10—16.3[31-33]. 本次研究四季OC/EC均值在4.2—4.7,且冬、春、秋三季
NO−3 SO2−4 NO−3 SOC=OC−EC×(OC/EC)min POC=OC−SOC 式中,(OC/EC)min为该采样时段OC/EC的最小值. 当OC/EC值远高于2,可认为二次污染过程对OC有显著贡献[31]. 经计算SOC的浓度呈现冬≈春>秋>夏的分布规律,这说明SOC在冬季生成较为显著;北京大学2016年冬季在怀柔的观测实验中也得出相似结论,其观察到冬季多次霾事件中均出现有自由基物质参与的光化学反应,反应速率与夏季光化学烟雾的速率高值相当,且这些反应能有效促进SOC的生成[34];另外冬、春季PM2.5大气寿命较长,夏季雨季PM2.5大气寿命较短,这也是导致SOC冬、春季浓度较高的原因之一.
2.3 水溶性离子
水溶性离子(Water-soluble ions)的四季浓度如表2所示,其中
NO−3 SO2−4 NH+4 NO−3 SO2−4 NH+4 表 2 金属元素的全年浓度均值及EF值Table 2. The average annual concentration of metal elements and their EF values元素Elements 观测质量浓度C sample/(ng·m−3) 地壳含量[21]C crust/(mg·kg−1) 富集因子(EF) 春Spring 夏Summer 秋Autumn 冬Winter 全年All year Fe 312.40±272.99 ~29700 6.4 0.3 2.5 2.5 2.9 Al 251.62±263.94 ~69900 1 1 1 1 1 K 243.21±126.84 ~19300 4.2 0.8 4.9 4.1 3.5 Ca 197.04±123.93 ~15200 6.1 0.6 4.3 3.4 3.6 Mg 109.63±97.71 ~12700 5.4 0.3 1.9 2.1 2.4 Na 103.69±57.62 ~13100 2.2 0.6 2.5 3.5 2.2 Zn 25.69±11.39 102.6 52.5 42.5 112.3 71.0 69.5 Mn 11.20±4.49 705 6.1 1.9 4.9 4.7 4.4 Cu 3.34±1.86 23.6 31.6 17.2 69.1 39.5 39.4 Cr 2.48±2.14 68.1 6.4 2.2 22.5 9.4 10.1 Rb 0.79±0.36 99 2.8 0.7 2.5 2.8 2.2 As 1.02±0.3 9.7 30.1 18.5 39.4 29.2 29.3 V 0.87±0.28 79.2 4.5 2.6 2.6 2.4 3.0 Ni 1.32±0.66 29.0 8.4 6.2 19.0 17.0 12.6 Cd 0.21±0.08 0.074 672.0 421.2 905.1 1123.6 780.5 Pb 10.85±5.34 25.4 96.5 48.0 150.5 179.8 118.7 由图3可见,
NO−3 SO2−4 NH+4 NO−3 NO−3 SO2−4 SO2−4 NH+4 SO2−4 2.4 金属元素
表2展示了采样期间PM2.5中不同金属的质量浓度,20种金属元素质量浓度总计为1275.63 ng·m−3,各金属含量大小为:Fe>Al>K>Ca>Mg>Na>Zn>>Mn>Pb>Cu>Cr>Rb>As>V>Ni>Cd>Se. 其中,Fe、Al、K、Ca、Mg、Na的含量较高,其质量浓度之和约占总浓度的95.4%. Cr、Ni的EF值稍大于10,受到人为源影响较轻;Zn、Cu、As及Pb的EF值在25—120之间,富集程度显现;Zn和Pb主要来自于机动车尾气或燃煤[35],Cu主要来自刹车片磨损或冶炼[36],As主要来自燃煤[37];Cd的富集程度较重,人为污染非常严重;大气中Cd主要来自于燃煤、石油燃烧、垃圾焚烧、机动车尾气等人为活动[38],本次观测Cd可能主要来自于燃煤或机动车尾气.
从图4怀柔区金属元素的季节变化可以看出,各金属元素浓度季节差异较大. 但总体均体现春>冬>秋>>夏的分布特征. 其中,地壳元素Fe、Al、Ca、Mg的春季浓度远高于其他季节,这表明春季风起扬尘作用贡献较大,PM2.5中含有更多的地壳元素,这也与春季时EPFRs浓度最高相符.
2.5 相关性分析
分析不同组分间的相关性可以更深入地了解EPFRs及共存组分的来源及转化过程. 如图5所示,冬季时,EPFRs与共存组分均呈现正相关,其中,EPFRs与OC、
SO2−4 SO2−4 此外,冬季时EPFRs和SOC和POC均呈线性相关关系,说明冬季EPFRs的来源不仅来自于一次直接排放源,二次化学反应生成也对其有重要贡献. 因PM2.5中OC与其共存主要组分(
SO2−4 NO−3 CEPFRs=1010×(5.122×CSOC+1.768×CPOC+3.999) 其中,CEPFRs表示EPFRs的浓度(spins·m−3),CSOC、CPOC分别表示SOC和POC的浓度(μg·m−3). 经计算,该方程R2=0.895,P<0.001,VIF<5. 其中,VIF为方差扩大因子(Variance Inflation Factor),当VIF<10时,表明因变量间不存在共线性. 因此,参数分析表明用SOC和POC表征EPFRs的来源具有统计学意义. 进一步将SOC和POC在冬季的平均质量浓度与方程系数相乘,得到SOC与POC对冬季EPFRs生成贡献比值为11∶4,且EPFRs与O3 (只来自于大气二次反应生成)仅在冬季时呈正相关(R2=0.57,P<0.001),表明冬季PM2.5中EPFRs可能更多源自于二次生成. 上文也提及EPFRs与OC、
SO2−4 SO2−4 NO−3 此外,分析常规污染气体与其他组分的相关性也可间接反映大气物理化学过程. NO2在冬、春两季和其他六种常规污染物及
NO−3 3. 结论(Conclusion)
本文通过分析2018年北京怀柔PM2.5中EPFRs及化学物质的污染特征,发现EPFRs的四季平均浓度范围为1.50×1013—2.92×1013 spins·m−3,并呈现春>冬>秋>夏的分布特征,且种类主要为含氧碳中心自由基或碳中心、氧中心自由基混合态;冬季时机动车尾气和燃煤源直接排放及排放污染物的大气二次转化对PM2.5中EPFRs有显著贡献;春季时风起扬尘EPFRs浓度有较大影响;夏季尽管EPFRs的浓度最低,但是其物种分布最广;冬季时EPFRs与PM2.5中多种组分(OC、EC、
SO2−4 SO2−4 -
表 1 水溶性离子的四季浓度(μg·m−3)
Table 1. Seasonal concentrations of water-soluble ions(μg·m−3)
春Spring 夏Summer 秋Autumn 冬Winter 全年All year NO−3 5.02 2.59 4.76 3.82 4.04 SO2−4 1.84 4.12 1.37 1.07 1.38 NH+4 1.70 2.30 2.22 1.52 0.38 Na+ 0.22 0.18 0.09 0.20 0.05 K+ 0.28 0.16 0.26 0.29 0.05 Mg2+ 0.14 0.35 0.06 0.07 0.13 Ca2+ 1.23 0.55 0.55 0.56 0.33 Cl- 0.26 0.16 0.07 0.82 0.33 SNA/WSIIs 80.16% 86.59% 88.94% 76.76% 83.25% SNA., 离子 NO−3 SO2−4 NH+4 表 2 金属元素的全年浓度均值及EF值
Table 2. The average annual concentration of metal elements and their EF values
元素Elements 观测质量浓度C sample/(ng·m−3) 地壳含量[21]C crust/(mg·kg−1) 富集因子(EF) 春Spring 夏Summer 秋Autumn 冬Winter 全年All year Fe 312.40±272.99 ~29700 6.4 0.3 2.5 2.5 2.9 Al 251.62±263.94 ~69900 1 1 1 1 1 K 243.21±126.84 ~19300 4.2 0.8 4.9 4.1 3.5 Ca 197.04±123.93 ~15200 6.1 0.6 4.3 3.4 3.6 Mg 109.63±97.71 ~12700 5.4 0.3 1.9 2.1 2.4 Na 103.69±57.62 ~13100 2.2 0.6 2.5 3.5 2.2 Zn 25.69±11.39 102.6 52.5 42.5 112.3 71.0 69.5 Mn 11.20±4.49 705 6.1 1.9 4.9 4.7 4.4 Cu 3.34±1.86 23.6 31.6 17.2 69.1 39.5 39.4 Cr 2.48±2.14 68.1 6.4 2.2 22.5 9.4 10.1 Rb 0.79±0.36 99 2.8 0.7 2.5 2.8 2.2 As 1.02±0.3 9.7 30.1 18.5 39.4 29.2 29.3 V 0.87±0.28 79.2 4.5 2.6 2.6 2.4 3.0 Ni 1.32±0.66 29.0 8.4 6.2 19.0 17.0 12.6 Cd 0.21±0.08 0.074 672.0 421.2 905.1 1123.6 780.5 Pb 10.85±5.34 25.4 96.5 48.0 150.5 179.8 118.7 -
[1] YANG L, LIU G R, ZHENG M H, et al. Highly elevated levels and particle-size distributions of environmentally persistent free radicals in haze-associated atmosphere [J]. Environmental Science & Technology, 2017, 51: 7936-7944. [2] ARANGIO A M, TONG H J, SOCORRO J, et al. Quantification of environmentally persistent free radicals and reactive oxygen species in atmospheric aerosol particles [J]. Atmospheric Chemistry and Physics, 2016, 16: 13105-13119. doi: 10.5194/acp-16-13105-2016 [3] DELA C, COOK, ROBERT L, et al. Effect of low temperature thermal treatment on soils contaminated with pentachlorophenol and environmentally persistent free radicals [J]. Environmental Science & Technology, 2012, 46: 5971-5978. [4] CHEN Q C, WANG M M, SUN H Y, et al. Enhanced health risks from exposure to environmentally persistent free radicals and the oxidative stress of PM2.5 from Asian dust storms in Erenhot, Zhangbei and Jinan, China [J]. Environment International, 2018, 121: 260-268. doi: 10.1016/j.envint.2018.09.012 [5] RUAN X X, SUN Y Q, DU W M, et al. Formation, characteristics, and applications of environmentally persistent free radicals in biochars: A review [J]. Bioresource Technology, 2019, 281: 457-468. doi: 10.1016/j.biortech.2019.02.105 [6] KIRURI L W, DELLINGER B, LOMNICKI S, et al. Tar balls from deep water horizon oil spill: environmentally persistent free radicals (EPFR) formation during crude weathering [J]. Environmental Science & Technology, 2013, 47: 4220-4226. [7] INGRAM DJE, TAPLEY J G, JACKSON R, et al. Paramagnetic resonance in carbonaceous solids [J]. Nature, 1954, 174: 797-798. doi: 10.1038/174797a0 [8] GUAN L F, GENG X K, SHEN J M, et al. PM2.5 inhalation induces intracranial atherosclerosis which may be ameliorated by omega 3 fatty acids [J]. Oncotarget, 2018, 9(3): 3765-3778. doi: 10.18632/oncotarget.23347 [9] TALBI A, KERCHICH Y, KERBACHI R, et al. Assessment of annual air pollution levels withPM1, PM2.5, PM10 and associated heavy metals in Algiers, Algeria [J]. Environmental Pollution, 2018, 232: 252-263. doi: 10.1016/j.envpol.2017.09.041 [10] CHEN Q C, SUN H Y, MU Z, et al. Characteristics of environmentally persistent free radicals in PM2.5: Concentrations, species and sources in Xi'an, Northwestern China [J]. Environmental Pollution, 2019, 247: 18-26. doi: 10.1016/j.envpol.2019.01.015 [11] BALAKRISHNA, LOMINCLI, MCAVEY K M, et al. Environmentally persistent free radicals amplify ultrafine particle mediated cellular oxidative stress and cytotoxicity [J]. Particle and Fibre Toxicology, 2009, 6: 14. doi: 10.1186/1743-8977-6-14 [12] WANG Y Q, LI S P, WANG M M, et al. Source apportionment of environmentally persistent free radicals (EPFRs) in PM2.5 over Xi'an, China [J]. Science of the Total Environment, 2019, 689: 193-202. doi: 10.1016/j.scitotenv.2019.06.424 [13] 唐孝炎, 张远航, 邵敏. 大气环境化学[M]. 第二版. 北京: 高等教育出版社, 2006: 75-721. TANG X Y, ZHANG Y H, SHAO M, et al. Atmospheric environmental chemistry[M]. The Second Edition. Beijing: Higher Education Press, 2006 : 75-721 ( in Chinese) .
[14] FELD COOK, BOVENKAMP, LISA, et al. Effect of particulate matter mineral composition on environmentally persistent free radical (EPFR) formation [J]. Environmental Science & Technology, 2007, 51: 10396-10402. [15] GEHLING W, DELLIGINER B. Environmentally persistent free radicals and their lifetimes in PM2.5 [J]. Environmental Science & Technology, 2013, 47: 8172-8178. [16] DOU J, LIN P, KUANG B Y, et al. Reactive oxygen species production mediated by humic-like substances in atmospheric aerosols: enhancement effects by pyridine, imidazole, and their derivatives [J]. Environmental Science & Technology, 2015, 49: 6457-6465. [17] LAKEY, BERKEMEIER, TONG H J, et al. Chemical exposure-response relationship between air pollutants and reactive oxygen species in the human respiratory tract[J]. Scientific Reports2016, 6: 1-6. [18] KELLY, FUSSELL, JULIA C, et al. Size, source and chemical composition as determinants of toxicity attributable to ambient particulate matter [J]. Atmospheric Environment, 2012, 60: 504-526. doi: 10.1016/j.atmosenv.2012.06.039 [19] SARAVIA J, LEE G I, LOMNICKI S, et al. Particulate matter containing environmentally persistent free radicals and adverse infant respiratory health effects: A review [J]. Journal of Biochemical & Molecular Toxicology, 2013, 27(1): 56-68. [20] 中国环境监测总站. 中国土壤元素背景值[M]. 北京: 中国环境科学出版社, 1990. China Environmental Monitoring Station. Background value of soil elements in China[M]. Beijing: China Environmental Science Press, 1990(in Chinese).
[21] 李豪, 陈庆彩, 孙浩堯. 西安市PM2.5中环境持久性自由基污染特征 [J]. 中国环境科学, 2020, 40(3): 967-974. doi: 10.3969/j.issn.1000-6923.2020.03.005 Li H, CHEN Q C, SUN H Y. Characteristics of persistent free radical pollution in PM2.5 of Xi'an City [J]. China Environmental Science, 2020, 40(3): 967-974(in Chinese). doi: 10.3969/j.issn.1000-6923.2020.03.005
[22] WANG C, HUANG Y P, et al. Levels, spatial distribution, and source identification of airborne environmentally persistent free radicals from tree leaves[J]Environmental Pollution, 2020, 257: 1-10. [23] JIA H Z, LI S S, WU L, et al. Cytotoxic Free radicals on air-borne soot particles generated by burning wood or low-maturity coals[J]Environmental Science & Technology, 2020, 54: 5608-5618. [24] XU Y, YANG L L, WANG X P, et al. Risk evaluation of environmentally persistent free radicals in airborne particulate matter and influence of atmospheric factors[J]Ecotoxicology and Environmental Safety, 2020, 196: 1-9. [25] DELLINGER B, LONINICKI S, KHACHATRYAN L, et al. Formation and stabilization of persistent free radicals [J]. Proceedings of the Combustion Institute, 2007, 31: 521-528. doi: 10.1016/j.proci.2006.07.172 [26] 王庆良, 李倩倩, 童东革, 等. 光化学反应中自由基的作用及反应影响因素的研究进展 [J]. 环境化学, 2020, 39(2): 301-316. doi: 10.7524/j.issn.0254-6108.2019061802 WANG Q L, LI Q Q, TONG D G, et al. Research progress on the role of free radicals in photochemical reactions and reaction factors [J]. Environmental Chemistry, 2020, 39(2): 301-316(in Chinese). doi: 10.7524/j.issn.0254-6108.2019061802
[27] 杨莉莉, 郑明辉, 许杨, 等. 环境持久性自由基的污染特征与生成机理 [J]. 中国科学:化学, 2018, 48(10): 1226-1235. YANG L L, ZHENG M H, XU Y, et al. Pollution characteristics and generation mechanism of environmental persistent free radicals [J]. Science in China:Chemistry, 2018, 48(10): 1226-1235(in Chinese).
[28] 赵力, 梁妮, 张绪超, 等. 不同热处理条件对亚麻酸中持久性自由基产生的影响[J]. 环境化学, 2019, 38(6): 1207-1213. ZHAO L, LIANG N, ZHANG X C, et al. The effect of different heat treatment conditions on the generation of persistent free radicals in linolenic acid[J]Environmental Chemistry, 2019, 38(6): 1207-1213(in Chinese).
[29] 乔宝文, 刘子锐, 胡波, 等. 北京冬季 PM2.5中金属元素浓度特征和来源分析 [J]. 环境科学, 2017, 38(3): 876-883. QIAO B W, LIU Z R, HU B, et al. Concentration characteristics and source analysis of metal elements in PM2.5 in winter in Beijing [J]. Environmental Science, 2017, 38(3): 876-883(in Chinese).
[30] WATSON J G, CHOW J C, HOUCK J E. PM2.5 chemical source profiles for vehicle exhaust, vegetative burning, geological material, and coal burning in Northwestern Colorado during 1995[J]Chemosphere, 2001, 43 : 1141-1151. [31] ZHANG Y Y, SHAO M, ZHANG Y H, et al. Source profiles of particulate organic matters emitted from cereal straw burnings [J]. Environmental Science, 2007, 19: 167-175. doi: 10.1016/S1001-0742(07)60027-8 [32] ZHANG Y Y, SCHAUER J J, ZHANG Y H, et al. Characteristics of particulate carbon emissions from real-world Chinese coal combustion [J]. Environmental Science & Technology, 2008, 42: 5068-5073. [33] LU K D, FUCHS H, HOFZUMAHAUS, et al. Fast photochemistry in wintertime haze: consequences for pollution mitigation strategies [J]. Environmental Science & Technology, 2019, 53: 10676-10684. [34] 姬亚芹, 朱坦, 冯银厂, 等. 应用地质累积指数分析城市颗粒物源解析土壤风沙尘的污染 [J]. 农业环境科学学报, 2006, 25(4): 949-953. doi: 10.3321/j.issn:1672-2043.2006.04.026 JI Y Q, ZHU T, FENG Y C, et al. Pollution analysis of soil dusts of source apportionment using geo accumulation index (Igeo)in China [J]. Journal of Agro-Environment Science, 2006, 25(4): 949-953(in Chinese). doi: 10.3321/j.issn:1672-2043.2006.04.026
[35] TOSCANO G, MORET I, GAMBARO A, et al. Distribution and seasonal variability of trace elements in atmospheric particulate in the Venice Lagoon [J]. Chemosphere, 2011, 85(9): 1518-1524. doi: 10.1016/j.chemosphere.2011.09.045 [36] TIAN H Z, WANG Y, XUE Z G, et al. Trend and characteristics of atmospheric emissions of Hg, S and Se from coal combustion in China, 1980—2007 [J]. Atmospheric Chemistry and Physics, 2010, 10(23): 11905-11919. doi: 10.5194/acp-10-11905-2010 [37] 谭吉华, 段菁春. 中国大气颗粒物重金属污染、来源及控制建议 [J]. 中国科学院研究生院学报, 2013, 30(2): 145-155. TAN J H, DUAN J C. Pollution, sources and control recommendations of heavy metals in China's atmospheric particulate matter [J]. Journal of the Graduate School of the Chinese Academy of Sciences, 2013, 30(2): 145-155(in Chinese).
[38] 夏志勇, 侯鲁健, 高素莲, 等. 济南市PM2.5中金属元素的污染特征、潜在生态风险及来源分析 [J]. 生态环境学报, 2020, 29(5): 971-976. XIA Z Y, HOU L J, GAO SU L, et al. Pollution characteristics, potential ecological risks and source analysis of metal elements in PM2.5in Jinan City [J]. Journal of Eco-Environment, 2020, 29(5): 971-976(in Chinese).
-



















 下载:
下载: