-
高氯酸盐化学性质稳定,水溶性高,吸附性低,广泛存在于环境介质中。由于高氯酸盐具有与碘离子相似的离子半径和相同的电荷数,可以与碘离子竞争钠/碘同向转运体(NIS)的结合位点,从而抑制碘的吸收,干扰甲状腺激素的合成,引起机体代谢的紊乱[1-2]。欧洲食品安全局(EFSA)评估人体暴露高氯酸盐的风险,结果表明长期摄入高氯酸存在潜在风险,尤其是孕妇、胚胎、婴儿及碘缺乏的年轻群体最容易受到危害[3]。
我国是世界上最大的茶叶种植国,也是第二大茶叶出口国。2016年初中国出口欧盟的茶叶中频繁检出高含量的高氯酸盐,对中国茶叶出口产生极大负面影响。此前,美国环保局(US EPA)将高氯酸的参考剂量设定为0.0007 mg·(kg ·d)−1[4],欧盟建议成员国的干茶中
ClO−4 的参考限量暂定为0.75 mg·kg−1,欧洲食品安全局建议人体每日容许ClO−4 摄入量(tolerable daily intake,TDI)为0.0003 mg·(kg·d)−1[3,5]。现阶段,关于高氯酸盐的检测方法主要有离子色谱法、离子色谱串联质谱法(IC-MS/MS)、液相色谱串联质谱法(LC-MS/MS)等[6-9],所涉及的基质主要集中在水、土壤、底泥等环境样品和少量食品如奶粉、牛奶中[10-13]。针对茶叶样品中高氯酸盐分析的方法研究相对较少,并且多为LC-MS/MS方法[14],然而常见的C18色谱柱对高氯酸盐选择性差,高氯酸盐在其中保留较差或无保留,同时茶叶比较复杂的基质可能干扰高氯酸盐的准确测定,此时改善色谱分离和样品处理方法,提高高氯酸盐的提取率并且降低共存离子的干扰,就成为分析成败的关键。对于LC-MS/MS方法,多选择修饰离子交换基团的液相色谱柱来增强高氯酸盐的保留,以此改善高氯酸盐和基质的分离,前处理方法选择含乙酸溶液提取以防止在去除基质时高氯酸盐的损失[15-16]。相比之下,在各种环境基质高氯酸盐分析中具有广泛应用的IC-MS/MS法则具有更强的选择性,该方法通过电导检测的色谱图可以实时监测复杂基体样本中高氯酸盐与杂质离子的分离情况,减少在质谱检测时共淋洗物质对高氯酸盐信号的抑制,进而提高方法的抗干扰能力。在茶叶样品前处理方面,由于高氯酸盐属于无机阴离子,在水中溶解度高,因此选用高纯水提取茶叶中高氯酸盐。茶叶基质复杂,往往含有多种有机化合物和无机矿物元素,高纯水在提取高氯酸盐时也导致许多水溶性物质溶解在提取液中。因此,为了防止其它共存物质干扰检测结果,本研究首先用OnGuard Ⅱ RP柱处理,去除提取液中有机物,以达到离子色谱的进样要求,减少样品对色谱柱耐受的影响。然后选用对高氯酸盐专属性较好的离子色谱柱进行分离,良好的色谱分离极大减少了共存离子的干扰,提高了测定准确度。另外,用纯水直接对高氯酸盐进行提取亦可很好地模拟泡茶的过程,更能体现人体通过饮用茶水摄入高氯酸盐的真实水平。通过实验条件优化,建立了快速准确的茶叶样品中高氯酸盐的离子色谱-串联质谱分析方法,为茶叶检测分析提供方法学参考。
-
ICS2000型离子色谱仪(美国,Thermo Fisher),免化学试剂淋洗液自动发生器(美国,Thermo Fisher),AS自动进样器(美国,Thermo Fisher);IonPacAS19型阴离子分析柱(250 mm×2 mm)(美国,Thermo Fisher),IonPacAG19型保护柱(50 mm× 2mm)(美国,Thermo Fisher);IonPacAS20型阴离子分析柱(250 mm×2mm)(美国,Thermo Fisher),IonPacAG20型保护柱(50 mm×2 mm)(美国,Thermo Fisher);ASRS 3000 2 mm阴离子抑制器(美国,Thermo Fisher)。API 32000三重四极杆LC-MS/MS 系统(美国,Applied Biosystems)。METTLER TOLEDO 电子天平(美国,METTLER TOLEDO ),A11基本型研磨机(德国,IKA),KS4000 i control 温控摇床(德国,IKA),3-18k高速冷冻离心机(德国,Sigma),QL-901旋涡混合器(中国,其林贝尔)。
-
0.45 μm尼龙针头过滤器(中国,卅克),一次性无菌注射器(5 mL,江苏治宇医疗器械有限公司),OnGuard Ⅱ RP-1cc前处理柱(美国,Thermo Fisher)。超纯水为Milli-Q Advantage A10系统(美国,Millipore)于电阻率为18.2 MΩ·cm下提供,1000 mg·L−1的高氯酸盐标准品溶液(美国,Sigma,色谱纯),500 μg·L−1的18O4—高氯酸盐内标储备液(美国,Cambridge Isotope Laboratories,4 ℃冰箱保存),甲醇(美国,Thermo Fisher Scientific,色谱级)。
-
色谱系统:进样量为100 μL,流速为0.25 mL·min−1,采用EGC淋洗液(KOH)自动发生器控制淋洗液梯度,抑制器采用外接水模式,抑制电流25 mA。AS19和AS20色谱柱采用不同淋洗程序,柱温均为30 ℃。
AS19梯度淋洗程序:0—15 min,10 mmol·L−1;15—25 min,10—40 mmol·L−1;25—30 min,40 mmol·L−1;30.1—38 min,10 mmol·L−1。
AS20等度淋洗程序:0—22 min,35 mmol·L−1。
质谱条件:离子源为ESI源,在负离子模式下以MRM模式进行监测。气帘气(curtain gas):20 psi;碰撞气(collision gas):5 psi;离子喷雾电压(IonSpray Voltage):−4500 V。离子源温度(Temperature):500 ℃;雾化气(Gas 1):55 psi;加热气(Gas 2):55 psi。
检测的离子对及其对应质谱参数见表1。
-
高氯酸盐储备液:将高氯酸盐标准品溶液1000 mg·L−1用超纯水稀释至500 μg·L−1储存于4 ℃冰箱中保存备用。
高氯酸盐标准溶液的配制:用高氯酸盐储备液与18O4标记的高氯酸盐内标储备液配制高氯酸盐标准溶液,将高氯酸盐储备液用高纯水稀释至0.05、0.1、0.2、0.5、1、2、5、10、20、50 μg·L−1获得高氯酸盐标准溶液,其中18O4标记的高氯酸盐内标浓度均为5 μg·L−1。
-
首先将茶叶放入研磨机进行研磨,得到均匀粉末状的茶叶样品。称量0.5 g茶叶样品放入50 mL离心管中,加入超纯水浸泡茶叶数次,以萃取样品中的高氯酸盐。每次浸泡使用超纯水20 mL,浸泡一定时间后,以10000 r·min−1的速度离心5 min,取出上清液,最后将3次浸泡得到的上清液合并,得到高氯酸盐萃取液。取5 mL萃取液用0.45 μm尼龙滤膜过滤,再用OnGuard Ⅱ RP-1cc进行有机物的去除,弃掉前3—4 mL萃取液,收集1 mL萃取液为上机测试样品。OnGuard Ⅱ RP-1cc柱在使用前须用4 mL甲醇和10 mL超纯水进行活化。
-
准备28份茶叶样品,以每组7份平行样品分为4组,分别为原茶叶样品及低、中、高加标茶叶样品,其中低、中、高加标茶叶样品加入的高氯酸盐浓度为100、300、600 μg·kg−1。将4组样品同时进行上述“1.5”节前处理后进样分析,计算回收率。
-
高氯酸盐在水中稳定且溶解度高,同时考虑到饮茶的实际情况,因此选用高纯水浸泡茶叶以提取其中高氯酸盐。高纯水提取茶叶中的高氯酸盐也成功避免了溶剂峰对分析结果的干扰。本研究对不同浸泡条件(包括浸泡次数、浸泡时间、水的温度及是否振荡浸泡)进行优化,确定茶叶中高氯酸盐最佳萃取条件。
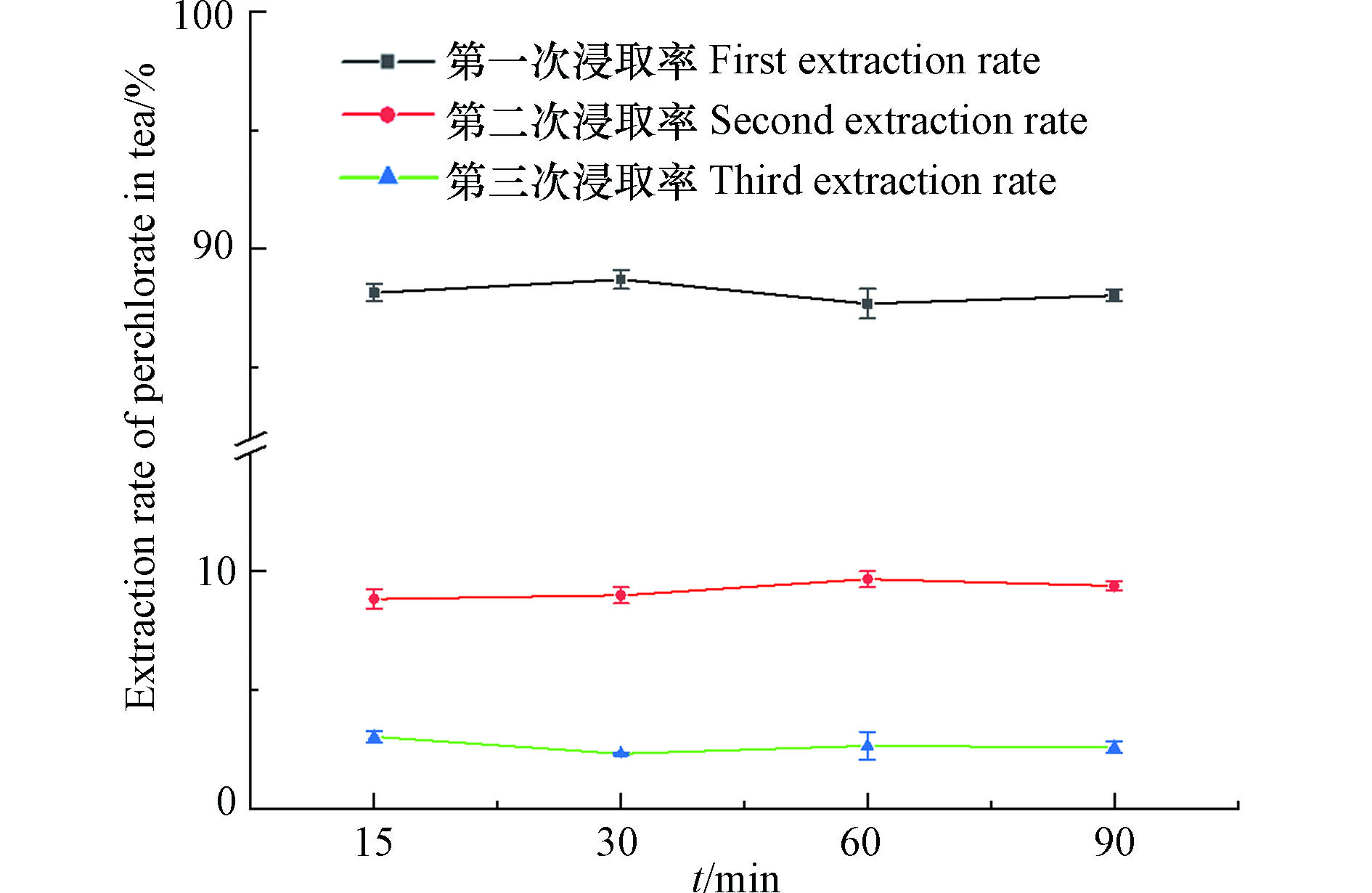
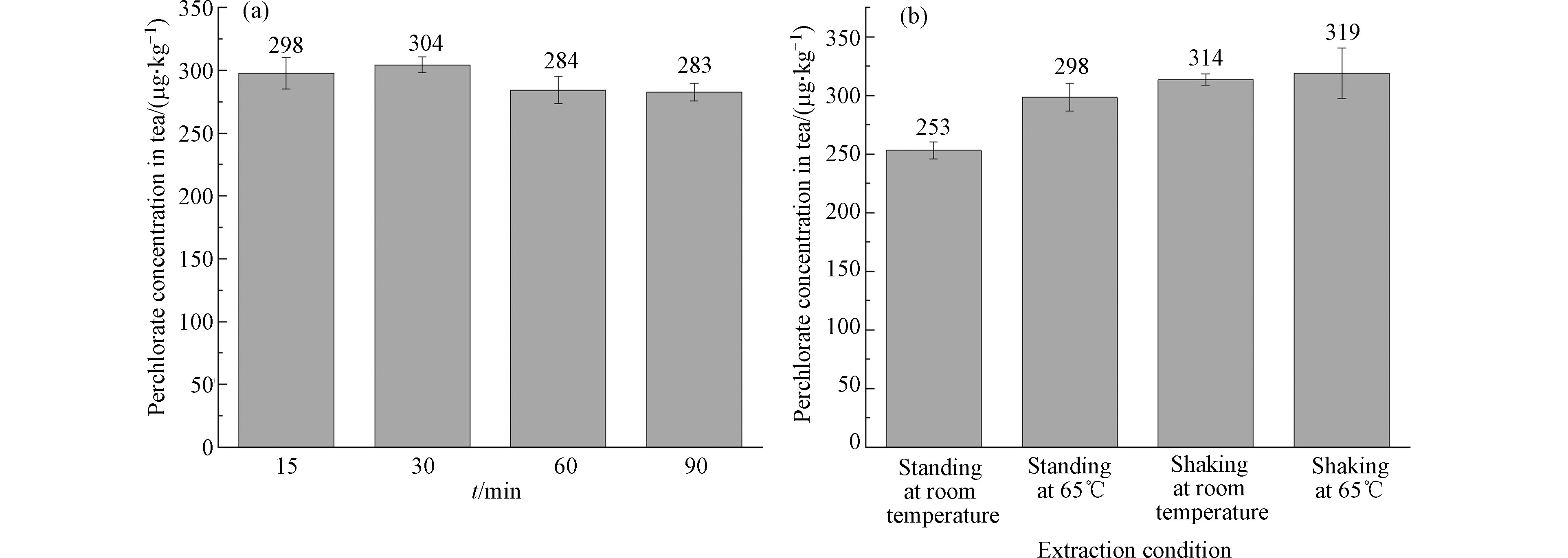
首先对茶叶浸泡次数和时间进行优化,将茶叶样品按上述“1.5节”方法进行前处理,每次使用20 mL高纯水,浸泡时间分为15、30、60、90 min,共浸泡3次,将每次浸泡的上清液进行处理后检测,根据3次提取量之和计算每次提取的效率。如图1所示,随着浸泡次数的增加,高氯酸盐的浸出量不断减少,第1次最高,浸出量占总浸出量的88%—89%,第3次浸泡提取时高氯酸盐浸出量仅为总浸出量的2.3%—3.0%,因此可认为茶叶经3次浸提后高氯酸盐浸出接近完全。不同浸泡时间下的高氯酸盐浸出量如图2(a)所示,这4组茶叶的高氯酸盐总浸出量范围为283—304 μg·kg−1,相对标准偏差为4.3%。上述结果表明浸泡时间对茶叶中高氯酸盐的萃取效率影响较小,最终选取15 min为提取时间。
最后考察水温和振荡浸泡对茶叶中高氯酸盐浸出量的影响,设置4组茶叶反复浸泡3次,4组茶叶分别选取室温振荡浸泡、室温静置浸泡、65 ℃振荡浸泡及65 ℃静置浸泡的方式,得到各自提取量结果,如图2(b)所示。由图2可知,静置条件下水温对高氯酸盐的浸出量有较大影响。振荡条件下,室温和65 ℃浸出的高氯酸盐量相差不大,但均优于静置提取的效率。因此,本研究最终选择在室温下振荡提取高氯酸盐。

-
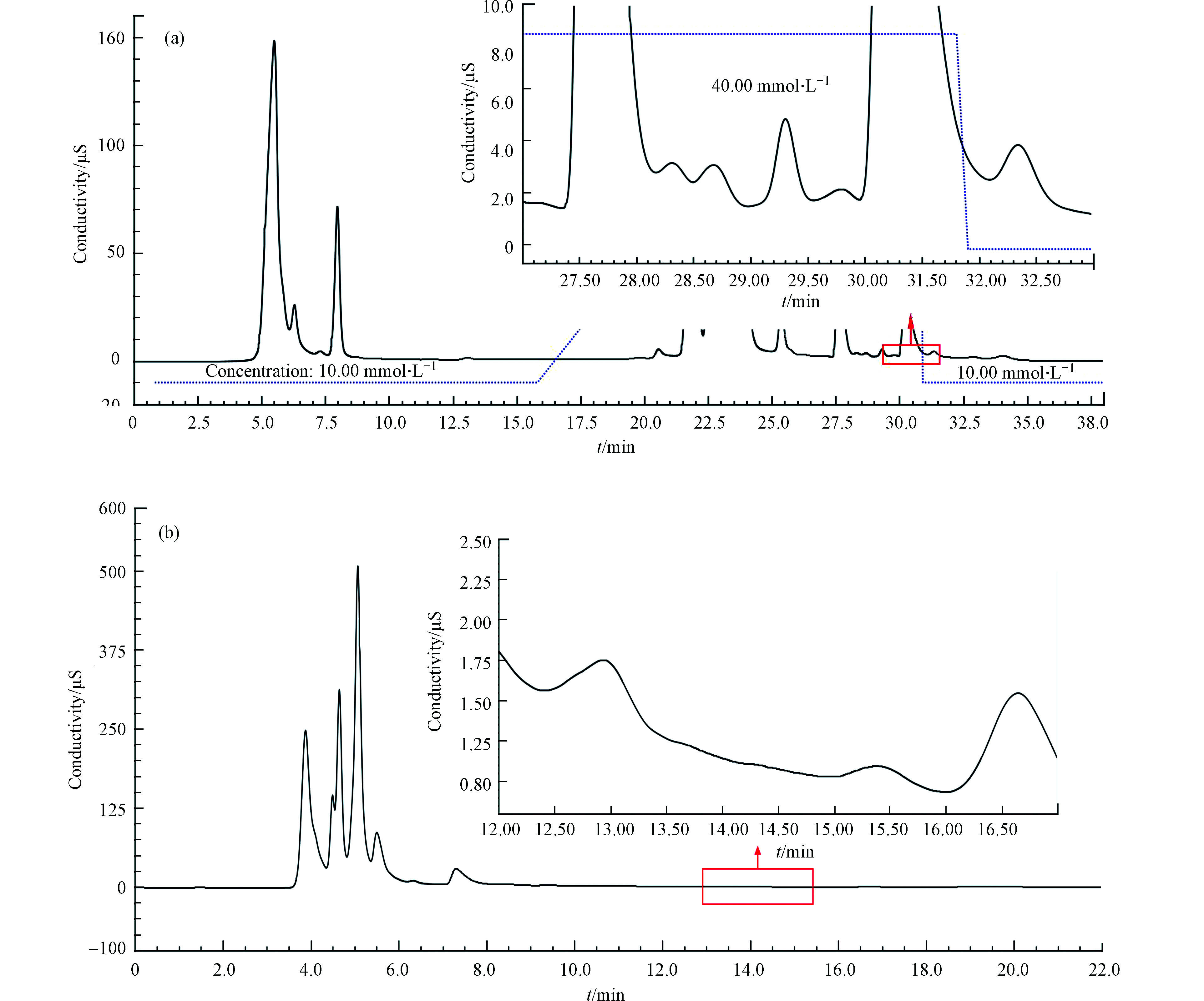
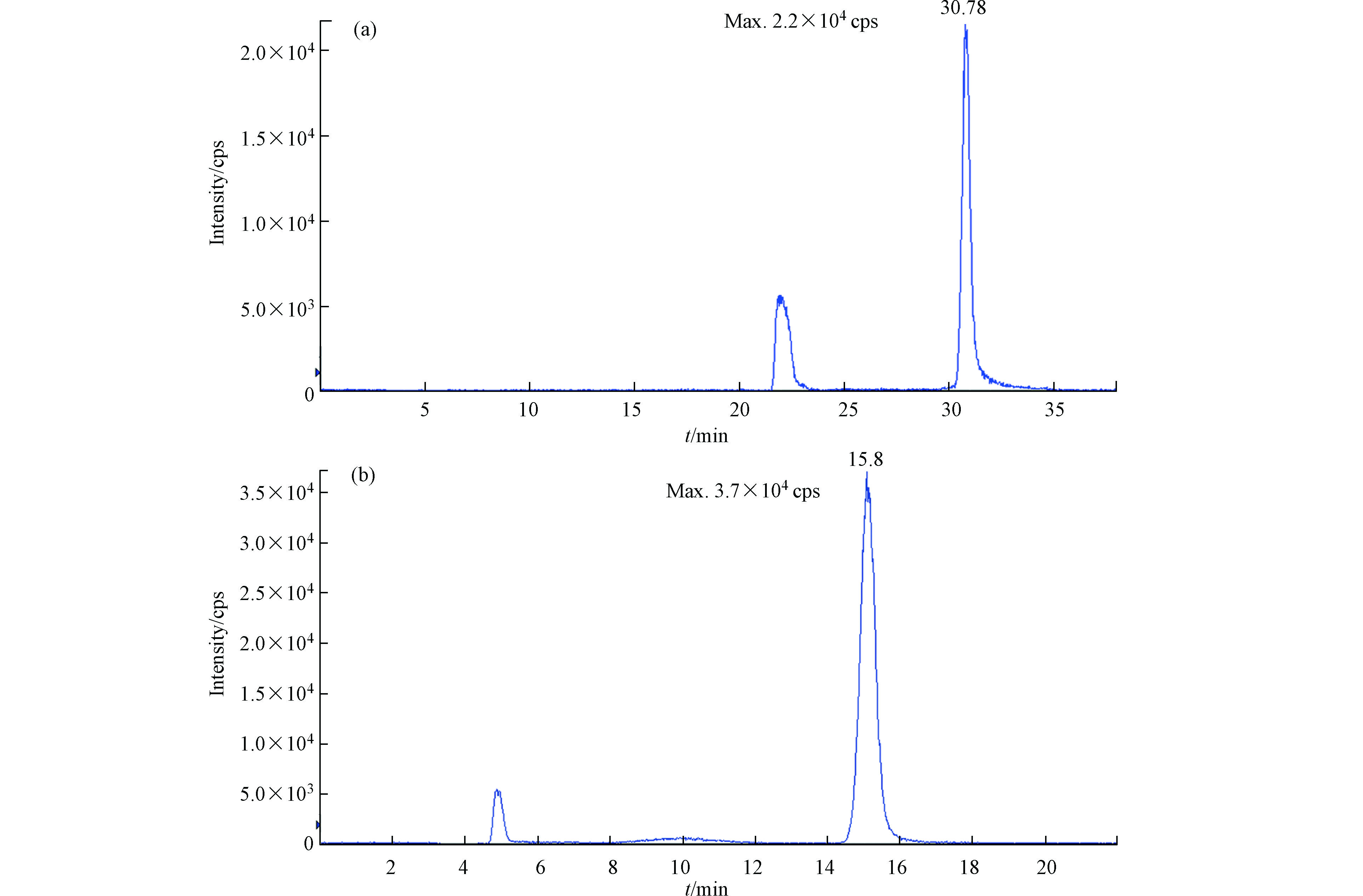
高氯酸根为疏水性强易极化的阴离子,本研究对AS19色谱柱和AS20色谱柱分离茶叶中高氯酸盐的效果进行比较,其中AS20色谱柱的亲水性较强,AS19色谱柱亲水性适中。图3为离子色谱电导检测时的色谱图,可以看出茶叶基质复杂,干扰较多。AS19分离时,高氯酸出峰的位置(30.78 min)有明显的干扰峰,而在AS20色谱柱中高氯酸盐(保留时间:15.08 min)和干扰离子却能实现很好的分离。另外,在AS20色谱柱中高氯酸盐的保留时间(15.08 min)是AS19色谱柱(30.78 min)的一半,意味着AS20对高氯酸盐的分离效率更高。从质谱检测的色谱图可知(图4),茶叶经AS19色谱柱分离的高氯酸盐响应(2.2×104 cps)明显低于AS20色谱柱(3.7×104 cps),进一步说明AS19色谱柱在分离茶叶样品时与高氯酸盐共淋洗的物质使质谱检测产生较明显的基体抑制效应。而AS20作为分离柱时,茶叶中与高氯酸盐共存的基质阴离子均可与高氯酸盐实现较好的分离,质谱的基质效应较小,高氯酸盐在质谱检测中响应较高。因此考虑到相较于AS19色谱柱,AS20色谱柱具有对高氯酸盐的分离效果更好,用时更短,效率更高的优点,最终选用AS20作为高氯酸盐的色谱分离柱。
-
将配制的一系列浓度(0.05—50 μg·L−1)高氯酸盐标准溶液进样分析后,以高氯酸盐的浓度为X轴,对应的高氯酸盐(98.8/82.8)与同位素标记的高氯酸盐(106.9/88.8)峰面积的比值为Y轴,绘制标准曲线为Y=0.0223X+0.000513,所得线性相关系数为R2=0.9998。以3倍和10倍信噪比确定仪器的检出限和定量限分别为0.014 μg·L−1和0.05 μg·L−1,转化成实际样品中高氯酸盐的检出限和定量限分别为1.7 µg·kg−1和6 µg·kg−1。
-
根据上述方法进行回收率实验,结果如表2所示。其中未加标茶叶样品中高氯酸盐浓度的相对标准偏差(RSD)为1.7%。3个加标水平的高氯酸盐回收率分别在94.6%—133%、101%—118%和101%—109%之间,说明该方法可用于茶叶中高氯酸盐的准确测定。
-
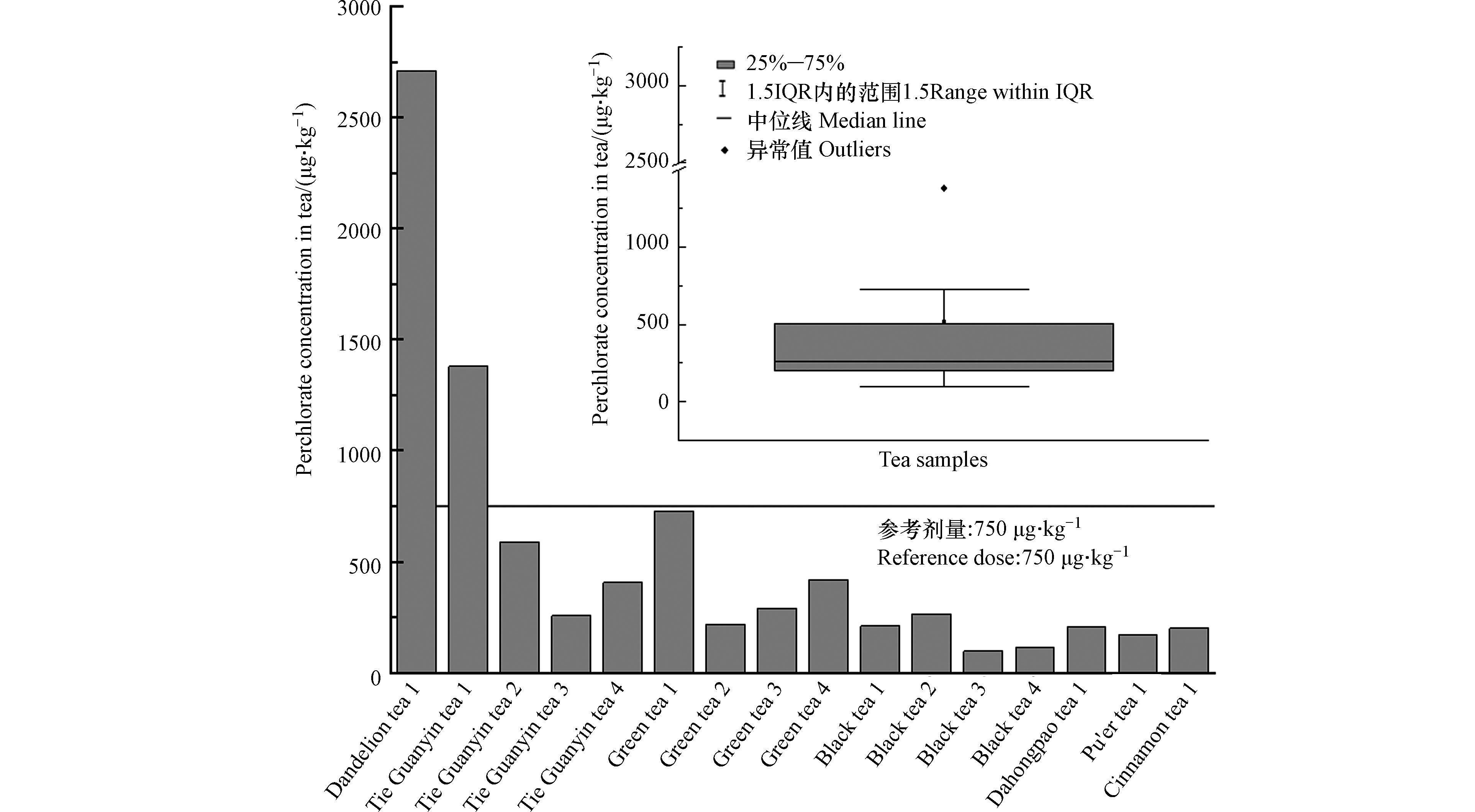
在超市随机选购16个品牌的茶叶样品,包括蒲公英茶(1种)、铁观音(4种)、绿茶(4种)、大红袍(1种)、普洱茶(1种)、肉桂茶(1种)。经上述前处理过程和检测后,发现所有样品中均检出高氯酸盐,浓度范围为97.0—2.71×103 μg·kg−1,如图5所示,其中4个样品浓度>500 μg·kg−1,2个样品浓度在300—500 μg·kg−1,10个茶叶样品高氯酸盐浓度<300 μg·kg−1。本研究购买的茶叶中,蒲公英茶所含高氯酸盐浓度最高,为2.71×103 μg·kg−1,其次为铁观音1样品,其中高氯酸盐浓度为1.38×103 μg·kg−1,这2个样品中高氯酸浓度超过了欧盟建议的干茶中
ClO−4 的参考限值750 μg·kg−1。根据《中国人群暴露参数手册》[17],人体中高氯酸盐的日均暴露量计算公式ADD=C×IR×EF×ED/(BW×AT),各参数意义及取值见表3。高氯酸盐是非致癌物质,用危害商(HQ)评价其健康风险。危害商的公式为HQ=ADD/RfD,其中RfD为高氯酸盐参考剂量。由于目前中国还没有与人体健康相关的高氯酸盐剂量的政策出台,本研究选取欧洲食品安全局(EFSA)建议的人体每日容许
ClO−4 摄入量0.0003 mg·(kg·d)−1作为参考剂量RfD。通过计算,人体通过饮茶摄入高氯酸盐的日均暴露量结果及危害商如表4所示。在检测的16个茶叶样品中,蒲公英茶1与铁观音1的危害商值大于1,其余茶叶样品均小于1。说明蒲公英茶1与铁观音1中高氯酸盐对人体可能产生一定的健康风险,其余健康风险较小。 -
本研究考察了不同浸提条件对茶叶中高氯酸盐的提取效率的影响,最终建立了去离子水振荡提取结合离子色谱-串联质谱联用分析茶叶中高氯酸盐的方法。对于茶叶中高氯酸盐的提取,最终选择以纯水在室温下振荡提取3次,每次浸泡15 min达到最佳提取效率。并且选用AS20色谱柱用于茶叶中高氯酸盐的分离。本研究建立的分析方法,操作简单、灵敏度高,可准确地测定茶叶中高氯酸盐的含量。方法应用于实际样品中高氯酸盐的分析得到了良好的结果,在分析的16个品牌茶叶样品中均检出了高氯酸盐的存在,并且发现长期饮用某些茶叶可能对人体健康存在一定的风险。
茶叶中高氯酸盐的离子色谱串联质谱分析
The determination of perchlorate in tea using IC-MS/MS
-
摘要: 本研究建立了茶叶中高氯酸盐的离子色谱串联质谱分析方法。对样品前处理方法进行了优化,结果表明用20 mL高纯水振荡提取15 min,重复3次,第3次高氯酸盐浸出量仅为总浸出量的2.3%—3.0%,因此可认为茶叶经3次浸提后高氯酸盐浸出接近完全。样品萃取液选用AS20色谱柱进行分离和电喷雾串联质谱(ESI-MS/MS)检测,样品在加标100、300、600 μg·kg−1的情况下,高氯酸盐回收率分别在94.6%—133%、101%—118%和101%—109%之间。方法在0.05—50 μg·L−1之间具有良好的线性关系,定量限为6 μg·kg−1。最后将方法用于不同类茶叶中高氯酸盐的分析,均检出不同浓度高氯酸盐的存在,浓度在97.0—2.71×103 μg·kg−1之间,通过茶叶摄入高氯酸盐对人体造成的暴露需引起人们的关注。Abstract: A method was established for the determination of perchlorate in tea using ion chromatography coupled with tandem mass spectrometry. The result showed that the amount of perchlorate after the third extraction process accounts only 2.3%—3.0% of the total amount of perchlorate by shaking extraction with 20 mL high purity water for 15 min and repeating for three times. Therefore, the perchlorate in tea was considered to be completely extracted after three times extraction. After extraction, the sample was separated using an AS20 column with the detection using electrospray tandem mass spectrometry (ESI-MS/MS). The recoveries of perchlorate were within the ranges of 94.6%—133%, 101%—118% and 101%—109% when the sample was spiked with the concentrations of 100, 300 and 600 μg·kg−1, respectively. The method showed a good linear range between 0.05—50 μg·L−1 with the limit of quantification at 6 μg·kg−1. Finally, the proposed method was successfully applied to determine perchlorate in different types of tea. The result showed that perchlorate could be detected in all samples with the concentrations between 97.0 μg·kg−1 and 2.71×103 μg·kg−1. The human exposure risks of perchlorate through tea intake needs to be concerned.
-
Key words:
- perchlorate /
- pretreatment /
- ion chromatography tandem mass spectrometry /
- tea
-
高氯酸盐化学性质稳定,水溶性高,吸附性低,广泛存在于环境介质中。由于高氯酸盐具有与碘离子相似的离子半径和相同的电荷数,可以与碘离子竞争钠/碘同向转运体(NIS)的结合位点,从而抑制碘的吸收,干扰甲状腺激素的合成,引起机体代谢的紊乱[1-2]。欧洲食品安全局(EFSA)评估人体暴露高氯酸盐的风险,结果表明长期摄入高氯酸存在潜在风险,尤其是孕妇、胚胎、婴儿及碘缺乏的年轻群体最容易受到危害[3]。
我国是世界上最大的茶叶种植国,也是第二大茶叶出口国。2016年初中国出口欧盟的茶叶中频繁检出高含量的高氯酸盐,对中国茶叶出口产生极大负面影响。此前,美国环保局(US EPA)将高氯酸的参考剂量设定为0.0007 mg·(kg ·d)−1[4],欧盟建议成员国的干茶中
ClO−4 ClO−4 在茶叶样品前处理方面,由于高氯酸盐属于无机阴离子,在水中溶解度高,因此选用高纯水提取茶叶中高氯酸盐。茶叶基质复杂,往往含有多种有机化合物和无机矿物元素,高纯水在提取高氯酸盐时也导致许多水溶性物质溶解在提取液中。因此,为了防止其它共存物质干扰检测结果,本研究首先用OnGuard Ⅱ RP柱处理,去除提取液中有机物,以达到离子色谱的进样要求,减少样品对色谱柱耐受的影响。然后选用对高氯酸盐专属性较好的离子色谱柱进行分离,良好的色谱分离极大减少了共存离子的干扰,提高了测定准确度。另外,用纯水直接对高氯酸盐进行提取亦可很好地模拟泡茶的过程,更能体现人体通过饮用茶水摄入高氯酸盐的真实水平。通过实验条件优化,建立了快速准确的茶叶样品中高氯酸盐的离子色谱-串联质谱分析方法,为茶叶检测分析提供方法学参考。
1. 材料与方法(Material and method)
1.1 仪器与设备
ICS2000型离子色谱仪(美国,Thermo Fisher),免化学试剂淋洗液自动发生器(美国,Thermo Fisher),AS自动进样器(美国,Thermo Fisher);IonPacAS19型阴离子分析柱(250 mm×2 mm)(美国,Thermo Fisher),IonPacAG19型保护柱(50 mm× 2mm)(美国,Thermo Fisher);IonPacAS20型阴离子分析柱(250 mm×2mm)(美国,Thermo Fisher),IonPacAG20型保护柱(50 mm×2 mm)(美国,Thermo Fisher);ASRS 3000 2 mm阴离子抑制器(美国,Thermo Fisher)。API 32000三重四极杆LC-MS/MS 系统(美国,Applied Biosystems)。METTLER TOLEDO 电子天平(美国,METTLER TOLEDO ),A11基本型研磨机(德国,IKA),KS4000 i control 温控摇床(德国,IKA),3-18k高速冷冻离心机(德国,Sigma),QL-901旋涡混合器(中国,其林贝尔)。
1.2 试剂与材料
0.45 μm尼龙针头过滤器(中国,卅克),一次性无菌注射器(5 mL,江苏治宇医疗器械有限公司),OnGuard Ⅱ RP-1cc前处理柱(美国,Thermo Fisher)。超纯水为Milli-Q Advantage A10系统(美国,Millipore)于电阻率为18.2 MΩ·cm下提供,1000 mg·L−1的高氯酸盐标准品溶液(美国,Sigma,色谱纯),500 μg·L−1的18O4—高氯酸盐内标储备液(美国,Cambridge Isotope Laboratories,4 ℃冰箱保存),甲醇(美国,Thermo Fisher Scientific,色谱级)。
1.3 色谱与质谱条件
色谱系统:进样量为100 μL,流速为0.25 mL·min−1,采用EGC淋洗液(KOH)自动发生器控制淋洗液梯度,抑制器采用外接水模式,抑制电流25 mA。AS19和AS20色谱柱采用不同淋洗程序,柱温均为30 ℃。
AS19梯度淋洗程序:0—15 min,10 mmol·L−1;15—25 min,10—40 mmol·L−1;25—30 min,40 mmol·L−1;30.1—38 min,10 mmol·L−1。
AS20等度淋洗程序:0—22 min,35 mmol·L−1。
质谱条件:离子源为ESI源,在负离子模式下以MRM模式进行监测。气帘气(curtain gas):20 psi;碰撞气(collision gas):5 psi;离子喷雾电压(IonSpray Voltage):−4500 V。离子源温度(Temperature):500 ℃;雾化气(Gas 1):55 psi;加热气(Gas 2):55 psi。
检测的离子对及其对应质谱参数见表1。
表 1 质谱检测条件Table 1. Experimental conditions of electrospray tandem mass spectrometry化合物Compound 离子对Q1/Q3 Q1/ Q3 解簇电压/V DP 入口电压/V EP 碰撞入口电压/V CE 碰撞出口电压/V CXP ClO4− 98.8/82.8* −45 −8.8 −35 −9 100.8/84.9 −45 −8.5 −35 −9 Cl18O4− 106.9/88.8* −50 −10 −35 −5 注:*定量离子对.*quantitative ion pair 1.4 标准溶液配制
高氯酸盐储备液:将高氯酸盐标准品溶液1000 mg·L−1用超纯水稀释至500 μg·L−1储存于4 ℃冰箱中保存备用。
高氯酸盐标准溶液的配制:用高氯酸盐储备液与18O4标记的高氯酸盐内标储备液配制高氯酸盐标准溶液,将高氯酸盐储备液用高纯水稀释至0.05、0.1、0.2、0.5、1、2、5、10、20、50 μg·L−1获得高氯酸盐标准溶液,其中18O4标记的高氯酸盐内标浓度均为5 μg·L−1。
1.5 茶叶样品的前处理
首先将茶叶放入研磨机进行研磨,得到均匀粉末状的茶叶样品。称量0.5 g茶叶样品放入50 mL离心管中,加入超纯水浸泡茶叶数次,以萃取样品中的高氯酸盐。每次浸泡使用超纯水20 mL,浸泡一定时间后,以10000 r·min−1的速度离心5 min,取出上清液,最后将3次浸泡得到的上清液合并,得到高氯酸盐萃取液。取5 mL萃取液用0.45 μm尼龙滤膜过滤,再用OnGuard Ⅱ RP-1cc进行有机物的去除,弃掉前3—4 mL萃取液,收集1 mL萃取液为上机测试样品。OnGuard Ⅱ RP-1cc柱在使用前须用4 mL甲醇和10 mL超纯水进行活化。
1.6 回收率实验
准备28份茶叶样品,以每组7份平行样品分为4组,分别为原茶叶样品及低、中、高加标茶叶样品,其中低、中、高加标茶叶样品加入的高氯酸盐浓度为100、300、600 μg·kg−1。将4组样品同时进行上述“1.5”节前处理后进样分析,计算回收率。
2. 结果与讨论(Results and discussion)
2.1 前处理条件的优化
高氯酸盐在水中稳定且溶解度高,同时考虑到饮茶的实际情况,因此选用高纯水浸泡茶叶以提取其中高氯酸盐。高纯水提取茶叶中的高氯酸盐也成功避免了溶剂峰对分析结果的干扰。本研究对不同浸泡条件(包括浸泡次数、浸泡时间、水的温度及是否振荡浸泡)进行优化,确定茶叶中高氯酸盐最佳萃取条件。
首先对茶叶浸泡次数和时间进行优化,将茶叶样品按上述“1.5节”方法进行前处理,每次使用20 mL高纯水,浸泡时间分为15、30、60、90 min,共浸泡3次,将每次浸泡的上清液进行处理后检测,根据3次提取量之和计算每次提取的效率。如图1所示,随着浸泡次数的增加,高氯酸盐的浸出量不断减少,第1次最高,浸出量占总浸出量的88%—89%,第3次浸泡提取时高氯酸盐浸出量仅为总浸出量的2.3%—3.0%,因此可认为茶叶经3次浸提后高氯酸盐浸出接近完全。不同浸泡时间下的高氯酸盐浸出量如图2(a)所示,这4组茶叶的高氯酸盐总浸出量范围为283—304 μg·kg−1,相对标准偏差为4.3%。上述结果表明浸泡时间对茶叶中高氯酸盐的萃取效率影响较小,最终选取15 min为提取时间。
最后考察水温和振荡浸泡对茶叶中高氯酸盐浸出量的影响,设置4组茶叶反复浸泡3次,4组茶叶分别选取室温振荡浸泡、室温静置浸泡、65 ℃振荡浸泡及65 ℃静置浸泡的方式,得到各自提取量结果,如图2(b)所示。由图2可知,静置条件下水温对高氯酸盐的浸出量有较大影响。振荡条件下,室温和65 ℃浸出的高氯酸盐量相差不大,但均优于静置提取的效率。因此,本研究最终选择在室温下振荡提取高氯酸盐。

2.2 色谱柱的选择
高氯酸根为疏水性强易极化的阴离子,本研究对AS19色谱柱和AS20色谱柱分离茶叶中高氯酸盐的效果进行比较,其中AS20色谱柱的亲水性较强,AS19色谱柱亲水性适中。图3为离子色谱电导检测时的色谱图,可以看出茶叶基质复杂,干扰较多。AS19分离时,高氯酸出峰的位置(30.78 min)有明显的干扰峰,而在AS20色谱柱中高氯酸盐(保留时间:15.08 min)和干扰离子却能实现很好的分离。另外,在AS20色谱柱中高氯酸盐的保留时间(15.08 min)是AS19色谱柱(30.78 min)的一半,意味着AS20对高氯酸盐的分离效率更高。从质谱检测的色谱图可知(图4),茶叶经AS19色谱柱分离的高氯酸盐响应(2.2×104 cps)明显低于AS20色谱柱(3.7×104 cps),进一步说明AS19色谱柱在分离茶叶样品时与高氯酸盐共淋洗的物质使质谱检测产生较明显的基体抑制效应。而AS20作为分离柱时,茶叶中与高氯酸盐共存的基质阴离子均可与高氯酸盐实现较好的分离,质谱的基质效应较小,高氯酸盐在质谱检测中响应较高。因此考虑到相较于AS19色谱柱,AS20色谱柱具有对高氯酸盐的分离效果更好,用时更短,效率更高的优点,最终选用AS20作为高氯酸盐的色谱分离柱。
2.3 方法性能
2.3.1 线性范围与检出限
将配制的一系列浓度(0.05—50 μg·L−1)高氯酸盐标准溶液进样分析后,以高氯酸盐的浓度为X轴,对应的高氯酸盐(98.8/82.8)与同位素标记的高氯酸盐(106.9/88.8)峰面积的比值为Y轴,绘制标准曲线为Y=0.0223X+0.000513,所得线性相关系数为R2=0.9998。以3倍和10倍信噪比确定仪器的检出限和定量限分别为0.014 μg·L−1和0.05 μg·L−1,转化成实际样品中高氯酸盐的检出限和定量限分别为1.7 µg·kg−1和6 µg·kg−1。
2.3.2 重复性与回收率
根据上述方法进行回收率实验,结果如表2所示。其中未加标茶叶样品中高氯酸盐浓度的相对标准偏差(RSD)为1.7%。3个加标水平的高氯酸盐回收率分别在94.6%—133%、101%—118%和101%—109%之间,说明该方法可用于茶叶中高氯酸盐的准确测定。
表 2 精密度与回收率实验结果Table 2. Experimental results of precision and recovery序号 Number 未加标茶叶中高氯酸盐浓度 /(μg·kg−1) Perchlorate concentration in unspiked tea 回收率/% Recovery 加标浓度100 μg·kg−1 Spiked concentration at 100 μg·kg−1 加标浓度300 μg·kg−1 Spiked concentration at 300 μg·kg−1 加标浓度600 μg·kg−1 Spiked concentration at 600 μg·kg−1 1 285 96.8 101 101 2 276 133 118 103 3 287 100 103 108 4 280 95.9 107 104 5 273 95.7 102 107 6 283 94.6 101 109 7 282 97.9 102 103 平均 281 102 104 105 RSD 1.7% 14% 5.7% 2.9% 2.4 实际样品测定
在超市随机选购16个品牌的茶叶样品,包括蒲公英茶(1种)、铁观音(4种)、绿茶(4种)、大红袍(1种)、普洱茶(1种)、肉桂茶(1种)。经上述前处理过程和检测后,发现所有样品中均检出高氯酸盐,浓度范围为97.0—2.71×103 μg·kg−1,如图5所示,其中4个样品浓度>500 μg·kg−1,2个样品浓度在300—500 μg·kg−1,10个茶叶样品高氯酸盐浓度<300 μg·kg−1。本研究购买的茶叶中,蒲公英茶所含高氯酸盐浓度最高,为2.71×103 μg·kg−1,其次为铁观音1样品,其中高氯酸盐浓度为1.38×103 μg·kg−1,这2个样品中高氯酸浓度超过了欧盟建议的干茶中
ClO−4 根据《中国人群暴露参数手册》[17],人体中高氯酸盐的日均暴露量计算公式ADD=C×IR×EF×ED/(BW×AT),各参数意义及取值见表3。高氯酸盐是非致癌物质,用危害商(HQ)评价其健康风险。危害商的公式为HQ=ADD/RfD,其中RfD为高氯酸盐参考剂量。由于目前中国还没有与人体健康相关的高氯酸盐剂量的政策出台,本研究选取欧洲食品安全局(EFSA)建议的人体每日容许
ClO−4 表 3 日均暴露量相关参数的意义及取值Table 3. Meaning and values of related parameters of average daily dose表 4 购买的16种茶叶中高氯酸盐对人体的日均暴露量(ADD)及危害商(HQ)Table 4. The average daily dose (ADD) and hazard quotient (HQ) of perchlorate in 16 kinds of tea purchased样品名称Samples name 茶叶中高氯酸盐浓度/(mg·kg−1) Perchlorate concentration in tea 日均暴露量/(mg·(kg·d)−1)Average daily dose (ADD) 危害商Hazard quotient (HQ) 蒲公英茶1 2.71 6.71×10−4 2.24 铁观音1 1.38 3.41×10−4 1.14 铁观音2 0.590 1.46×10−4 0.487 铁观音3 0.257 6.35×10−5 0.212 铁观音4 0.405 1.00×10−4 0.334 绿茶1 0.728 1.80×10−4 0.600 绿茶2 0.217 5.37×10−5 0.179 绿茶3 0.290 7.19×10−5 0.240 绿茶4 0.420 1.04×10−4 0.346 红茶1 0.210 5.20×10−5 0.173 红茶2 0.265 6.56×10−5 0.219 红茶3 0.970 2.40×10−5 0.0800 红茶4 0.115 2.85×10−5 0.0951 大红袍1 0.207 5.13×10−5 0.171 普洱茶1 0.169 4.19×10−5 0.140 肉桂茶1 0.200 4.96×10−5 0.165 3. 结论(Conclusion)
本研究考察了不同浸提条件对茶叶中高氯酸盐的提取效率的影响,最终建立了去离子水振荡提取结合离子色谱-串联质谱联用分析茶叶中高氯酸盐的方法。对于茶叶中高氯酸盐的提取,最终选择以纯水在室温下振荡提取3次,每次浸泡15 min达到最佳提取效率。并且选用AS20色谱柱用于茶叶中高氯酸盐的分离。本研究建立的分析方法,操作简单、灵敏度高,可准确地测定茶叶中高氯酸盐的含量。方法应用于实际样品中高氯酸盐的分析得到了良好的结果,在分析的16个品牌茶叶样品中均检出了高氯酸盐的存在,并且发现长期饮用某些茶叶可能对人体健康存在一定的风险。
-
表 1 质谱检测条件
Table 1. Experimental conditions of electrospray tandem mass spectrometry
化合物Compound 离子对Q1/Q3 Q1/ Q3 解簇电压/V DP 入口电压/V EP 碰撞入口电压/V CE 碰撞出口电压/V CXP ClO4− 98.8/82.8* −45 −8.8 −35 −9 100.8/84.9 −45 −8.5 −35 −9 Cl18O4− 106.9/88.8* −50 −10 −35 −5 注:*定量离子对.*quantitative ion pair 表 2 精密度与回收率实验结果
Table 2. Experimental results of precision and recovery
序号 Number 未加标茶叶中高氯酸盐浓度 /(μg·kg−1) Perchlorate concentration in unspiked tea 回收率/% Recovery 加标浓度100 μg·kg−1 Spiked concentration at 100 μg·kg−1 加标浓度300 μg·kg−1 Spiked concentration at 300 μg·kg−1 加标浓度600 μg·kg−1 Spiked concentration at 600 μg·kg−1 1 285 96.8 101 101 2 276 133 118 103 3 287 100 103 108 4 280 95.9 107 104 5 273 95.7 102 107 6 283 94.6 101 109 7 282 97.9 102 103 平均 281 102 104 105 RSD 1.7% 14% 5.7% 2.9% 表 3 日均暴露量相关参数的意义及取值
Table 3. Meaning and values of related parameters of average daily dose
表 4 购买的16种茶叶中高氯酸盐对人体的日均暴露量(ADD)及危害商(HQ)
Table 4. The average daily dose (ADD) and hazard quotient (HQ) of perchlorate in 16 kinds of tea purchased
样品名称Samples name 茶叶中高氯酸盐浓度/(mg·kg−1) Perchlorate concentration in tea 日均暴露量/(mg·(kg·d)−1)Average daily dose (ADD) 危害商Hazard quotient (HQ) 蒲公英茶1 2.71 6.71×10−4 2.24 铁观音1 1.38 3.41×10−4 1.14 铁观音2 0.590 1.46×10−4 0.487 铁观音3 0.257 6.35×10−5 0.212 铁观音4 0.405 1.00×10−4 0.334 绿茶1 0.728 1.80×10−4 0.600 绿茶2 0.217 5.37×10−5 0.179 绿茶3 0.290 7.19×10−5 0.240 绿茶4 0.420 1.04×10−4 0.346 红茶1 0.210 5.20×10−5 0.173 红茶2 0.265 6.56×10−5 0.219 红茶3 0.970 2.40×10−5 0.0800 红茶4 0.115 2.85×10−5 0.0951 大红袍1 0.207 5.13×10−5 0.171 普洱茶1 0.169 4.19×10−5 0.140 肉桂茶1 0.200 4.96×10−5 0.165 -
[1] PLEUS R C, COREY L M. Environmental exposure to perchlorate: A review of toxicology and human health [J]. Toxicology and Applied Pharmacology, 2018, 358: 102-109. doi: 10.1016/j.taap.2018.09.001 [2] STEINMAUS C M. Perchlorate in water supplies: Sources, exposures, and health effects [J]. Current Environmental Health Reports, 2016, 3(2): 136-143. doi: 10.1007/s40572-016-0087-y [3] EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on the risks to public health related to the presence of perchlorate in food, in particular fruits and vegetables [J]. EFSA Journal, 2014, 12(10): 3869. doi: 10.2903/j.efsa.2014.3869 [4] EPA. Perchlorate (ClO−4) and Perchlorate Salts[EB/OL]. [2020-9-19].https://cfpub.epa.gov/ncea/iris2/chemicalLanding.cfm?substance_nmbr=1007. [5] EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF). Scientific Opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs [J]. EFSA Journal, 2015, 13(1): 3978. doi: 10.2903/j.efsa.2015.3978 [6] LIU Y, SUN H, ZHOU L, et al. Quantitative determination and contamination pattern of perchlorate in tea by ultra performance liquid chromatography and tandem mass spectrometry [J]. Food Chemistry, 2019, 274: 180-186. doi: 10.1016/j.foodchem.2018.07.113 [7] BACKUS S M, KLAWUUN P, BROWN S, et al. Determination of perchlorate in selected surface waters in the Great Lakes Basin by HPLC/MS/MS [J]. Chemosphere, 2005, 61(6): 834-843. doi: 10.1016/j.chemosphere.2005.04.054 [8] ARIBI H E, BLANC Y J L, ATONSEN S, et al. Analysis of perchlorate in foods and beverages by ion chromatography coupled with tandem mass spectrometry (IC-ESI-MS/MS) [J]. Analytica Chimica Acta, 2006, 567(1): 39-47. doi: 10.1016/j.aca.2006.03.012 [9] 林立, 王海波, 史亚利. 二维离子色谱法同时测定环境水样中的碘离子、硫氰酸根和高氯酸根 [J]. 色谱, 2013, 31(3): 281-285. doi: 10.3724/SP.J.1123.2012.10034 LIN L, WANG H B, SHI Y L. Determination of iodide, thiocyanate and perchlorate ions in environmental water by two-dimensional ion chromatography [J]. Chinese Journal of Chromatography, 2013, 31(3): 281-285(in Chinese). doi: 10.3724/SP.J.1123.2012.10034
[10] 史亚利, 高健民, 李鑫, 等. 浏阳河水、底泥和土壤中高氯酸盐的污染 [J]. 环境化学, 2010, 29(3): 388-391. SHI Y L, GAO J M, LI X, et al. The investigation of perchlorate pollution level in Liu Yang River water, sediment and its nearby soil [J]. Environmental Chemistry, 2010, 29(3): 388-391(in Chinese).
[11] SHI Y L, ZHANG P, WANG Y, et al. Perchlorate in sewage sludge, rice, bottled water and milk collected from different areas in China [J]. Environment International, 2007, 33(7): 955-962. doi: 10.1016/j.envint.2007.05.007 [12] OH S, LEE J, MANDY P, et al. Analysis and exposure assessment of perchlorate in Korean dairy products with LC-MS/MS [J]. Environmental Health and Toxicology, 2011, 26: e2011011. doi: 10.5620/eht.2011.26.e2011011 [13] ZHOU X, LU X, WAN J, et al. Determination of chlorate and perchlorate in milk power by high-performance liquid chromatography-tandem mass spectrometry [J]. Chinese Journal of Chromatography, 2019, 37(10): 1064-1070. doi: 10.3724/SP.J.1123.2019.04052 [14] 曾艳, 郞红, 邵辉, 等. 茶叶中高氯酸盐检测方法研究进展[J]. 分析测试技术与仪器, 25(3): 175-181. ZENG Y, LANG H, SHAO H, et al. Advance in research of determination methods of perchlorate in tea[J]. Analysis and testing technology and instruments, 25(3): 175-181(in Chinese).
[15] 华永有, 李宇翔, 林宏琳, 等. 茶叶中高氯酸盐检测与污染情况分析 [J]. 海峡预防医学杂志, 2018, 24(01): 4-6. HUA Y Y, LI Y X, LIN H L, et al. Detection and analysis on contamination with perchlorate in tea [J]. Strait Journal of Preventive Medicine, 2018, 24(01): 4-6(in Chinese).
[16] 彭西甜, 夏珍珍, 胡西洲, 等. 分散固相萃取-超高效液相色谱-串联质谱法测定茶叶中的高氯酸盐 [J]. 分析科学学报, 2020, 36(2): 275-279. PENG X T, XIA ZZ, HU X Z, et al. Determination of perchlorate in tea by ultra-high performance liquid chromatography-tandem mass spectrometry coupled with dispersive solid phase extraction [J]. Journal of Analytical Science, 2020, 36(2): 275-279(in Chinese).
[17] 环境保护部. 中国人群暴露参数手册[M]. 北京: 中国环境出版社, 2013: 142-801. Ministry of Environmental Protection. Exposure factors handbooks of Chinese population[M]. Beijing: China Environment Press, 2013: 142-801(in Chinese).
[18] 屠幼英. 茶与健康[M]. 北京: 世界图书出版公司, 2011. TU Y Y, Tea and health[M]. Beijing: World Book Publishing Company, 2011(in Chinese).
-








 DownLoad:
DownLoad: