-
随着全球工业的发展,由石油、多环芳烃和苯酚等有机物导致的环境污染不断加剧。由于有机污染物通常具有难水溶性、化学稳定性、生物累积性和“三致”效应,对环境和人类健康产生灾难性的影响[1]。酶修复技术因具有可专一性高效降解某种或某类物质、降解大分子污染物时则不受跨膜运输、对环境营养的要求不高、不易受捕食和有毒物质影响等优势,在有机污染物治理领域表现出巨大的应用潜力[2-3]。
氧化还原酶是近年来研究最为广泛的酶系,主要包括过氧化物酶、氯过氧化物酶、木质素过氧化物酶、锰过氧化物酶、酪氨酸酶及漆酶等[3-4],其中漆酶(ECI.10.3.2)因具有底物谱宽泛、仅利用分子氧作为电子受体无需过氧化物和对环境友好等特点,被视为最具前景的污染治理酶制剂。漆酶是一种多铜氧化酶,在氧分子的参与下,能够催化多环芳烃、酚类和芳香胺类等其他富含电子的底物进行单电子氧化,形成醌或低聚物并将氧分子还原成水[5]。漆酶来源广泛,在原核生物、植物、真菌以及昆虫体内均有发现[6]。自漆酶发现以来,漆酶的研究一直是国内外学者研究的热点。
文章根据近几年国内外的研究成果,对漆酶的来源及性质、漆酶的结构及催化机制、漆酶催化氧化底物的作用方式、影响漆酶活性的因素及漆酶的应用研究现状进行了综述,并探讨了今后的发展方向,以期为我国开展漆酶的研究及工业化应用提供有益参考。
全文HTML
-
漆酶来源广泛,在植物、微生物及动物的分泌物中均有所发现。植物漆酶的来源主要包括漆树、拟南芥、松树、棉花、水稻和黄杨等,其中漆树漆酶是发现最早且植物漆酶中活性最高的酶[7]。微生物漆酶包括真菌漆酶与细菌漆酶两大类,其中能够分泌真菌漆酶的真菌主要分布在担子菌门、子囊菌门及半知菌类中[8];能够分泌细菌漆酶的细菌主要分布在放线菌门、异常球菌-栖热菌门、厚壁菌门、变形菌门以及蓝藻门中[9]。目前,对能够分泌动物漆酶的物种研究较少,已报道的相关动物包括麻蝇、绿头苍蝇、蚊子、烟草天蛾、猪肾及双翅目迁移类蝗虫等[10]。不同来源的漆酶催化部位的结构及理化性质具有较大差异。
漆酶理化性质的研究主要集中在分子量、最适温度、最适pH及米氏常数(Km)值几方面[11-12]。在一定的温度范围内,漆酶的活性随着反应温度的升高而增加。当温度超出一定范围,漆酶的酶活力会随着温度的升高而下降并逐渐失去活性[12]。漆酶的活性对pH值较为敏感,来源不同的漆酶pH值的耐受性及环境稳定性呈现显著的差异[12],通常微生物漆酶的最适pH在3.6~5.2之间,植物漆酶的最适pH在6.8~7.2之间[13]。Km值能够反映出酶对底物亲和力的大小,Km值越小,亲和力越大,酶对底物的氧化效率越高,以ABTS为底物时,氧化能力较强的漆酶Km值能够达到10~20 μmol/L[14]。
-
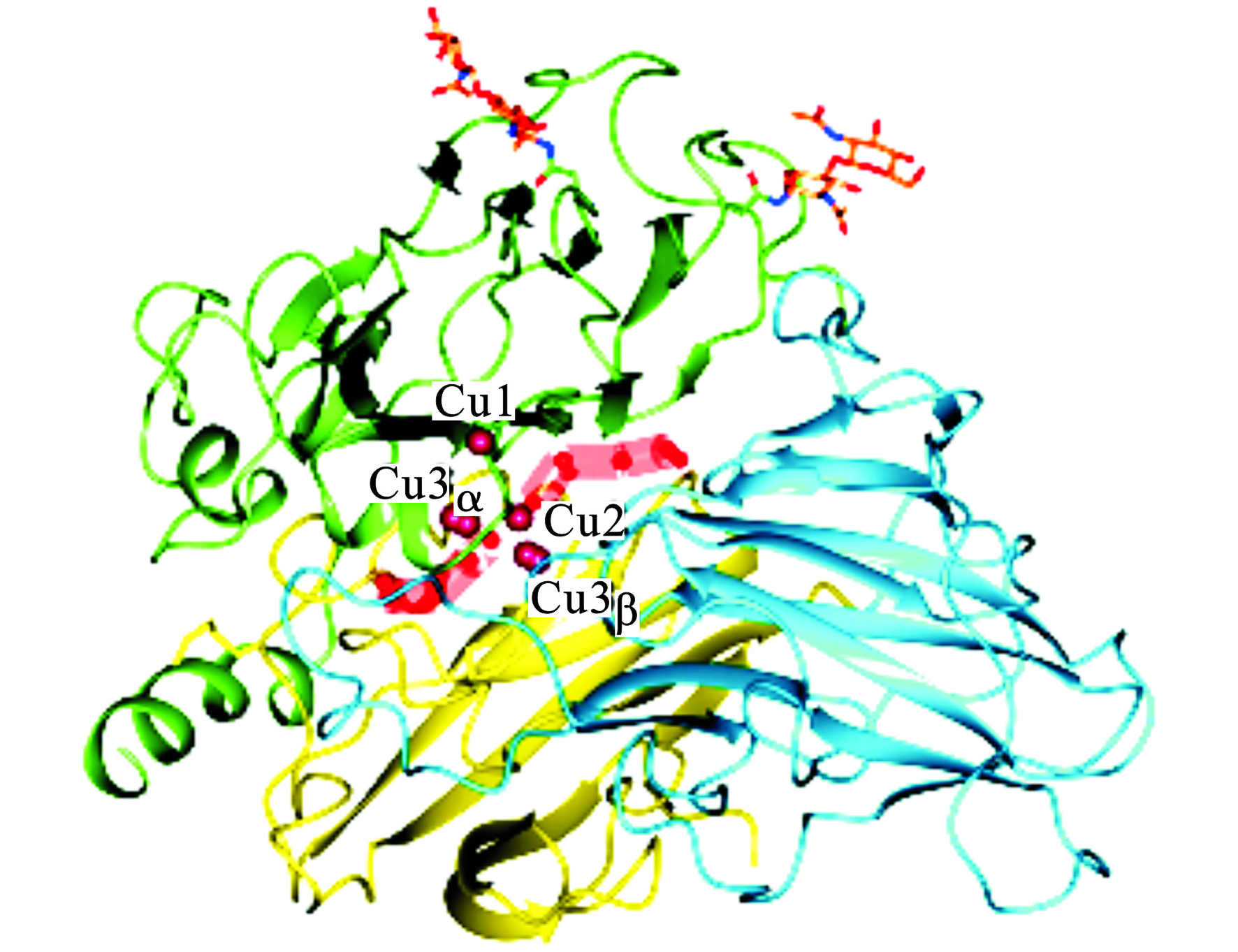
漆酶一般是由500~550个氨基酸组成的单体糖蛋白,因糖基化的程度不同,分子量一般也不同,通常在50~130 kDa之间[15-16]。漆酶是一种酸性蛋白,等电点一般在3~4之间[16]。根据漆酶的光谱学特性,漆酶的4个铜离子可以分成3大类见图1。
图1可见,1个I型铜离子(T1)、1个II型铜离子(T2)和2个III型铜离子(T3)[14-15]。T1型铜离子是底物的反应场所,底物在此附近被氧化,T1铜离子接受来自底物的电子。T2铜离子和T3铜离子组成三核铜簇中心,氧分子在此场所被氧化成水分子[17]。漆酶中的4个铜离子构成其活性中心,在漆酶的催化氧化反应中起着重要的作用。
-
漆酶催化氧化底物时,漆酶的来源不同、催化的底物不同,其催化氧化原理不同[17],见图2。
图2可见,漆酶与底物作用时,底物首先与T1铜离子先结合,产生的电子通过Cu→S(Cys)→N(His)途径向T3铜离子传递,最后T2铜原子被还原[18]。虚线表示的是溶剂或者氧气在漆酶中的传递通道(S/O通道),S/O通道的一边朝向三核簇的T3铜离子,另一边朝向三核簇的T2铜离子。氧分子通过S/O通道进入三核中心,得4个电子,被还原成水。漆酶催化氧化底物的反应过程大体上包括漆酶从底物中获取电子、酶分子内部的电子传递及氧分子得电子并还原成水3个步骤[18]。
2.1. 漆酶的结构
2.2. 漆酶的催化氧化机制
-
根据作用底物不同,漆酶催化的反应主要分为3种类型[19]:①直接氧化简单酚类或酚类衍生物;②在介体的协助下,氧化酚类或非酚类物质;③漆酶氧化产生的自由基发生偶联反应。
-
酚类或酚类衍生物在漆酶的作用下失去质子变成自由基[18],自由基不稳定,可被漆酶进一步氧化为醌类衍生物(图3a),或者通过亲核加成反应形成聚合物(图3b),从而进入第三类反应(偶联反应)。在反应足够长的时间后,漆酶催化氧化底物转化成一系列聚合物、寡聚体、二聚体等,从反应原理来看,第一类反应是第三类反应的前反应。但是,第一类反应也可能直接氧化底物,而不发生聚合反应。例如,某些酚类自由基经过非偶联反应,直接通过氧化开环或是脱羧基的作用,实现物质的降解[20]。
-
这类反应主要针对的是具有较高电势的还原态底物及较高空间位阻的高分子底物,漆酶催化氧化这类物质的反应,需要介体的参与才能实现[21]。最早使用的介体大多是含-NO、-HRNOH或-NOH等结构的物质,其中,最常用的是2’2-联氮双(3-乙基苯并噻唑啉-6-磺酸)二铵盐(ABTS)、N-羟基乙酰苯胺(NHA)、2,2,6,6-四甲基哌啶氧化物(TEMPO)和紫尿酸(VIO)等[22]。除上述介体外,一些木质素降解物,如乙酰香草酮、对香豆酸及香草醛等;一些无机物,如亚铁氰化物、多金属氧酸等也可以作为参与反应的电子介体[19]。
-
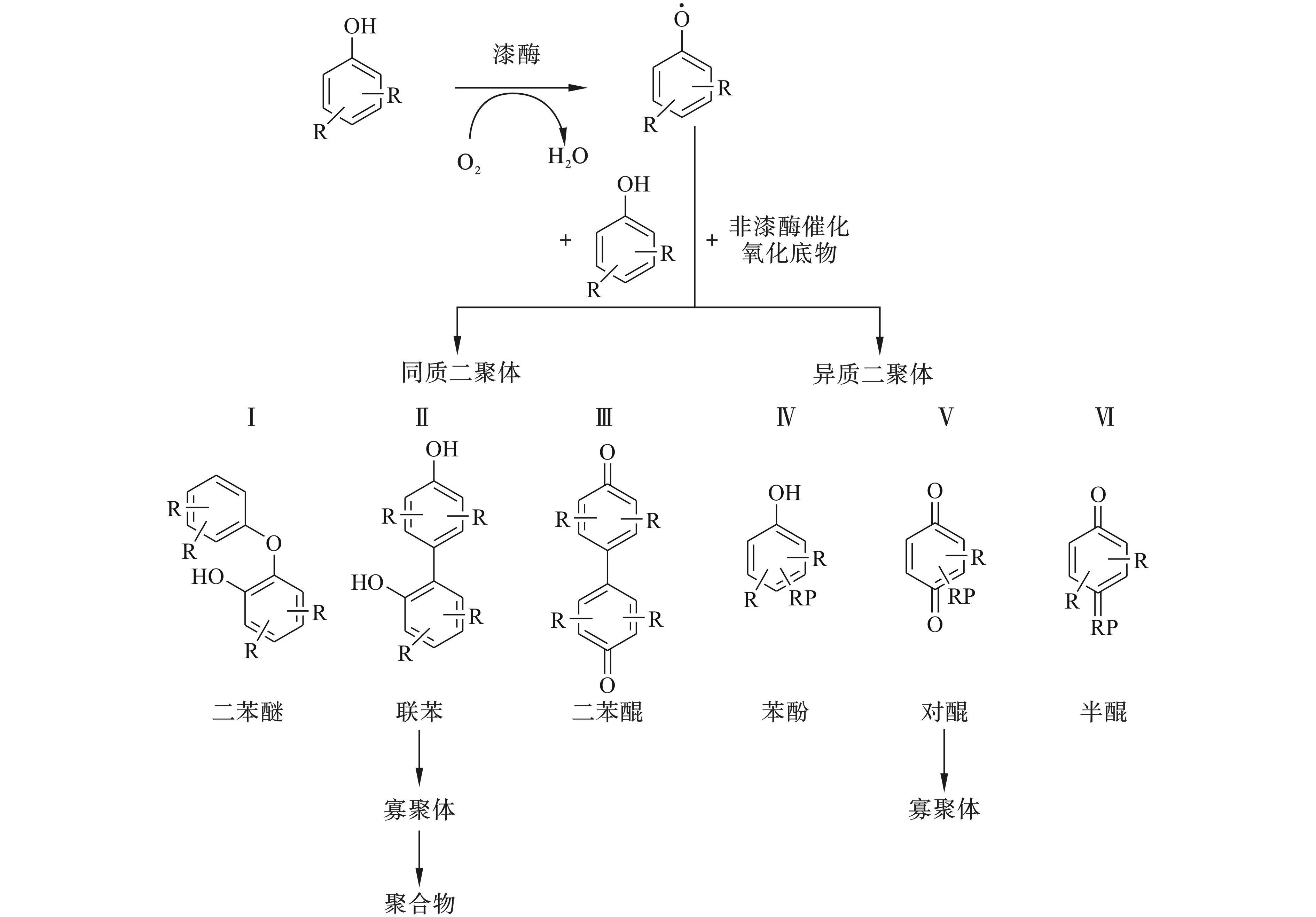
这类反应是植物细胞壁中木质素合成的重要机制,也是漆酶催化合成聚合物的基础。漆酶首先氧化底物形成酚类自由基或者是胺类自由基;其次,在非酶的作用体制下,发生自偶联反应形成二聚体;在反应时间足够长的情况下,还可能形成三聚体、寡聚体甚至是高聚体。
MIKOLASH和SCHAUER[23]把漆酶催化酚类底物合成二聚体分为6种类型见图4。漆酶催化酚类物质形成均质二聚体有3种途径:①单体之间通过醚键(C—O—C)形成二苯醚结构;②单体之间通过碳碳单键(C—C)形成联苯结构;③单体之间通过碳碳双键(C=C)形成二苯醌结构。同样,形成异质二聚体也有3种类型,即酚氧自由基与一些漆酶不能催化氧化的底物偶联形成苯酚、半醌和对醌3种结构。异质二聚体的形成有利于合成新的化学结构,因为自由基形成后,在没有酶的参与下能与异质分子通过C—C键、C—O键或C—N键等连接偶联。胺类物质和酚类物质合成聚合物的路径类似。
3.1. 漆酶氧化简单酚类或酚类衍生物
3.2. 漆酶-介体系统氧化酚类或非酚类等大分子物质
3.3. 漆酶氧化产生的自由基发生偶联反应
-
漆酶的主要来源是植物漆酶和真菌漆酶,尽管植物漆酶和真菌漆酶的结构相似,但是二者的性质差别显著。首先,植物漆酶和真菌漆酶的氧化还原能力不同,氧化物质的能力也不同[24]。例如,Trametes hirsuta漆酶的氧化还原电势约为780 mV,而漆树漆酶的氧化还原电势为420 mV,因此,漆树漆酶仅能用于氧化酚类而知,而不能用于催化聚苯胺(氧化还原电势为740 mV)的合成反应[25]。其次,不同来源的真菌漆酶氧化还原能力也不同。例如,FOROOTANFAR et al[26]考察不同来源的3种真菌漆酶对六种合成染料的去除效果,实验结果表明,Paraconiothyrium variabile漆酶对6种燃料的去除效果最好,Aspergillus oryzae漆酶的效果次之,Trametes versicolor漆酶的效果最好。一些常见菌株的氧化还原电势,见表1。
-
酚类底物的氧化,一般不需要介体的参与,漆酶可单独完成酚类物质的氧化。有时介体的加入不但不能引起底物的降解,反而会造成产率的下降[47]。然而,有时添加合适的介体可以有效地提高氧化聚合反应的选择性,使之定向的合成某些产物,减少副产物的形成[48]。
苯胺的聚合反应一般需要介体的参与,介体的添加可以加速漆酶催化合成苯胺的聚合物,得到分子量、电导率和溶解度不同的产品[25, 49]。例如,SHUMAKOVICH et al[25]研究了添加介体K4Mo(CN)6对其酶催化合成苯胺聚合物的影响,研究表明,添加介体后,聚合物的形成速度大大提高,基本在1 h内快速完成。
-
反应介质的pH对产物的影响比漆酶的来源更为显著。它不仅影响漆酶的活性[50]还对漆酶的氧化底物具有重要的影响。例如,LEONOWICZ et al[51]的研究表明,介质的pH范围在3.0~8.0时,漆酶氧化香酸的产物有4种,分别为对醌、醌二聚体、酚二聚体及醌三聚体,见图5(a);而pH在3.0~6.0之间时,仅检测到对醌和醌二聚体。同样的,当漆酶催化香草酸时,pH在3.0~6.0之间时,仅能合成2-甲氧基-1,4-苯醌一种产物,而pH在6.0~8.0之间时,可以合成C—C偶联二聚体和C—O—C偶联二聚体2种物质,见图5(b)。
-
漆酶催化的反应大多数都需要氧气的参与,氧分子的浓度对合成反应至关重要。现有的研究有通过搅拌增加溶解氧的浓度、还有利用合成体系里的溶解氧。如,ZHAO et al[52]研究了漆酶在不同氧气浓度的条件下漆酶对DDT的去除效果。实验结果表明,在N2、大气和O2存在的环境条件下,漆酶对DDT的去除效率分别为40.5%、48.5%和68.6%。
-
温度对漆酶的活性影响显著,来源不同的漆酶,催化不同反应底物,所需的最适温度均有所不同[53-54]。通常,在一定的温度范围内,随着反应体系温度的升高,漆酶的活性增加。但当温度超过最适温度后,漆酶的活性随着温度的升高而下降,直至失活。真菌漆酶的最适反应温度一般在40~60 ℃之间[14];细菌漆酶的最适反应温度一般在45~92 ℃之间[54]。
-
金属离子Mg2+、Zn2+、Mn2+、Ca2+、Cu2+、Fe3+、Fe2+、、K+、Ba2+、Al3+和Na+等对漆酶活性有较大的影响,但其对不同来源漆酶的影响有一定的差异,同一种金属离子对某些漆酶表现为抑制作用,而对另外一些漆酶则表现为激活作用[55-56]。例如,Mg2+、Mn2+和Zn2+对白腐菌Psathyrella candolleana漆酶的活性基本没有影响,而K+、Ba2+、Al3+、Fe2+、Na+、Ca2+、Cu2+和Fe3+等对该漆酶的活性表现出了明显的抑制作用,其中0.5 mol/L Fe2+的抑制作用最强[57]。
4.1. 漆酶的来源
4.2. 介体的影响
4.3. 介质的pH的影响
4.4. 溶解氧的影响
4.5. 温度的影响
4.6. 金属离子的影响
-
漆酶是一种底物利用范围广泛、高效和绿色的生物催化剂。漆酶的利用范围也十分广泛,尤其在有机污染物的去除方面有重要的应用价值。
-
白腐真菌Trametes hirsuta漆酶氧化烯烃,需要添加介体来引发反应。漆酶首先氧化介体,生成介体氧化物;之后介体氧化物氧化烯烃,生成相应的酮或醛[58]。其中,反应以羟基苯并三唑为介体取得最好效果,在20 ℃下,反应2 h后,可将脂肪多聚不饱和烃和芳香烯丙基醇全部氧化[58]。在介体的参与下,漆酶对多种烯烃,如烯丙基乙醚、环辛烯等,均具有一定的催化效果[58]。
-
在介体的参与下,漆酶可以催化氧化氯苯胺、二氯二苯并二恶英等联苯的氯化物类污染物[59]。漆酶对多氯联苯的催化速率与氯代基的个数有关,取代基个数越高,降解速率越低[60-61]。KORDON et al[62]利用漆酶处理氯化羟基联苯,结果表明,Myceliophthora thermophila漆酶对5-氯-2-羟基联苯、3,5-二氯-2-羟基联苯及2′, 5,5′三氯-2-羟基联苯的去除效率分别为100%、95.5%和0%。
-
漆酶可以直接催化氧化酚类和氯酚类污染物,如甲氧基酚、二氯苯酚等[63-64]。漆酶对氯酚及其衍生物的氧化能力主要取决于氯代基在苯环上的取代位置和取代数量,氯代基的数量越多,漆酶的转化能力越弱。例如,GAITAN et al[65]的研究表明,漆酶对2-CP, 2,4-DCP, 2,4,6-TCP和PCP的降解效率分别为100%、99%、82.1%和41.1%。
-
漆酶可以直接氧化多环芳烃类污染物,比如蒽、芴、菲和芘等,并且在介体的参与下,漆酶对多环芳烃的降解效率更高[66]。KOSCHOREECK et al[67]的研究表明单独的漆酶对蒽没有降解效果,而漆酶-ABTS体系对蒽的降解效率为83%。另外,介体不同漆酶催化氧化芳烃的能力也不同。例如,Trametes versicolor漆酶-HBT介体体系可以氧化苊和苊烯,而Trametes versicolor漆酶-ABTS介体体系对苊和苊烯没有明显的氧化作用[68]。
-
染料类污染物的来源不同,其组成不同,主要分为偶氮类燃料、靛蓝类染料、蒽醌类染料和三苯甲烷类染料,不同来源的染料,漆酶的作用机理差别很大[69]。漆酶可以直接氧化蒽醌类染料[70]和偶氮染料[71];而对于一些顽固性染料,漆酶通常需要在小分子介体的参与下,才可以达到高效的脱色效果。例如,CAMARERO et al[72]利用漆酶介体体系降解活性黑和天空蓝,结果表明,漆酶介体体系在30 min内对活性黑的降解率高达90%,对天空蓝的降解率也达30%,而单独的漆酶体系对这2种染料几乎没有降解作用。
5.1. 烯烃类污染物的去除
5.2. 联苯的氯化物类污染物的去除
5.3. 酚类和氯酚类污染物的去除
5.4. 多环芳烃类污染物的去除
5.5. 染料类污染物的去除
-
漆酶因其具有来源广泛、底物作用范围广和绿色无污染等优势,在有机物污染治理领域表现出巨大的应用潜力。然而,游离的漆酶具有易失活、变性及重复使用率低等缺陷,在实际环境中的应用受到限制。此外,尽管漆酶的来源广泛,但野生菌株的生长代谢受到多种因素的调控,产酶量往往难以达到较高的水平。为了进一步推进漆酶的工业化应用,在今后的研究中可以从以下几方面着手:1)开发新型的漆酶磁性纳米固定化材料,在保证漆酶活性的基础上,实现漆酶的重复利用;2)高效产漆酶基因工程菌的构建,以实现漆酶在异源受体上(酵母、大肠杆菌等)的高效表达。



 下载:
下载: