-
印染废水是典型的工业废水,其排放量占全球排放量的20%[1]。废水中有机污染物含量高,成分复杂,色泽高,有毒有害,若未经处理或处理不当后直接排放物将严重破坏生态环境,威胁人类健康[2]。目前印染废水处理方法包括物理法[3]、化学法[4]、生物法[2, 5]、光催化氧化法[6]和芬顿氧化法[7]等多种技术。其中芬顿氧化,尤其是基于H2O2的光芬顿氧化是迄今为止最重要的技术之一,其主要形成多种活性氧(reactive oxygen species,ROS),如过氧化氢自由基(·HO2)、超氧自由基(·O2 −)、羟基自由基(·OH)及单线态氧(1O2)作为氧化剂,将污染物大分子降解成CO2和H2O。
然而,经典的铁基均相芬顿反应存在pH适应范围窄、产生大量含铁污泥、Fe2+活化H2O2产生·OH的速率及Fe3+到Fe2+的转化效率均较低等问题[8]。为了克服这些缺点,基于金属-非金属复合材料的非均相类芬顿氧化工艺是研究的热点之一,其可以通过金属位点和非金属位点如(C-C,C-O,C-N)活化H2O2形成多种ROS。石墨碳氮化物(CN),由于共轭结构中氮和碳之间的共价键,使其具有高的化学稳定性[9],且原料廉价、合成方便。然而,由于CN表面积较小,导致电子-空穴对复合较高;由于层间堆叠导致在水中的分散性差[10]。目前,已经开发了多种方法修饰CN,例如缺陷工程[11]、构建异质结[12]、金属掺杂[13]等。其中,金属掺杂可以缩小其禁带宽度,将其光吸收范围扩展到可见光范围,提高其光催化性能。JIANG等[14]制备Fe掺杂CN材料,在LED灯条件下可以实现4 h内光催化降解4 mg·L−1的磺胺甲噁唑;铜基芬顿比铁基芬顿的pH适应范围更广,且·OH产生速率更快[15]。LI等[16]制备Cu掺杂CN材料,用于太阳光条件下活化H2O2,在pH= 4~8的较宽范围内使20 mg·L−1磺胺二甲氧嘧啶的降解率达到99%以上。而过渡金属钴(Co)具有高导电性,在光催化反应中,Co原子的3d轨道与C/O原子的2p轨道强烈杂化,因此,与g-C3N4的N/C原子键合的Co原子在光照射下可以充当电子收集中心[17]。双金属掺杂催化剂由于2种不同金属组分的协同作用,比单金属掺杂催化剂具有更低的离子浸出率、更好的催化性能和更高的选择性,因而受到广泛关注。有研究通过溶胶-凝胶法制备g-C3N4/ZnCo2O4纳米复合材料,并将其用于H2O2光催化降解RhB染料[18]。另一项研究中先通过水热法制备花状钴酸铜(CuCo2O4),煅烧退火法制备石墨氮化碳(g-C3N4),之后在溶剂中通过超声处理得CuCo2O4/g-C3N4复合材料,将其用于模拟太阳光照射下催化H2O2降解甲基橙(MO)染料[19]。然而,部分研究中材料的制备过程相对复杂,条件要求苛刻或者金属离子的溶出相对较高[7, 20]。在保证催化剂催化性能的前提下,从材料制备的难易程度及减少离子溶出方面考虑,亟需一种制备简单且不会引起二次污染的催化剂。
本研究采用浸渍法和热缩聚法制备Cu/Co共掺杂氮化碳复合材料,探索了复合材料对罗丹明B(RhB)的去除效果,研究了 pH、无机阴离子对该体系的影响及催化剂的稳定性。通过淬灭实验确定参与反应的主要活性物质。最后,结合表征提出H2O2活化和ROS产生的机理。本研究制备的多相类Fenton催化剂,克服了经典均相芬顿弊端,减少了金属离子溶出,可为光芬顿技术处理印染废水提供参考。
-
质量分数30%过氧化氢(H2O2)、乙二胺四乙酸二钠(C10H14N2Na2O8)、五水合硫酸铜(CuSO4·5H2O)、三聚氰胺(C3H6N6)、六水合硝酸钴(Co(NO3)2·6H2O)、罗丹明B(C28H31ClN2O3)、对苯醌(C6H4O2)、三聚氰酸(C3H3N3O3)、2-甲基咪唑(C4H6N2)、叔丁醇(C4H10O)、L-组氨酸(C6H9N3O2)均为分析纯;实验用水为去离子水。
-
CoCN制备参考文献并做修改[21],将5.14 g三聚氰胺和5.16 g三聚氰酸加入150 mL去离子水中,磁力搅拌4 h后加入0.564 2 g 2-甲基咪唑和0.25 g硝酸钴,搅拌1 h,静置沉淀6 h后,通过离心收集,去离子水洗涤3次(7 000 r·min−1,5 min)后,在70 ℃条件下干燥15 h,得到的样品记作ACo。将ACo置于氧化铝坩埚,用锡纸密封包裹后经管式炉煅烧(550 ℃,2.5 ℃·min−1,4 h)后得到CoCN。
CuCN制备参考文献并做修改[7],将5.044 8 g三聚氰胺和2.496 8 g硫酸铜加入60 mL去离子水中,磁力搅拌1 h,静置沉淀6 h,采取与上述ACo、CoCN相同的步骤得到ACu和CuCN。Cu/CoCN制备方法如下:称取2 gACo和一定量的ACu,使得ACu与ACo的质量比为0.1、0.3、0.5。将两者混合,经充分研磨、采取与上述相同的煅烧条件煅烧后得到复合催化剂Cu/CoCN(x),x=0.1、0.3、0.5。
-
将0.05 g催化剂分散于200 mLRhB溶液(50 mg·L−1),在无光照条件下搅拌30 min,使其达到吸附-脱附平衡,此时取样,作为初始值。之后,在氙灯(500 W)照射条件下,加入20 mmol· L−1 H2O2,每10 min取4 mL等分试样。样品离心(1000 0 r·min−1,10 min)后,取2 mL于10 mL比色管,定容后用UV-722 G分光光度计在554 nm处测定吸光度。将反应后的催化剂通过抽滤的方式收集,用去离子水和无水乙醇交替离心洗涤3次后置于60 ℃下干燥,以备循环实验使用。
-
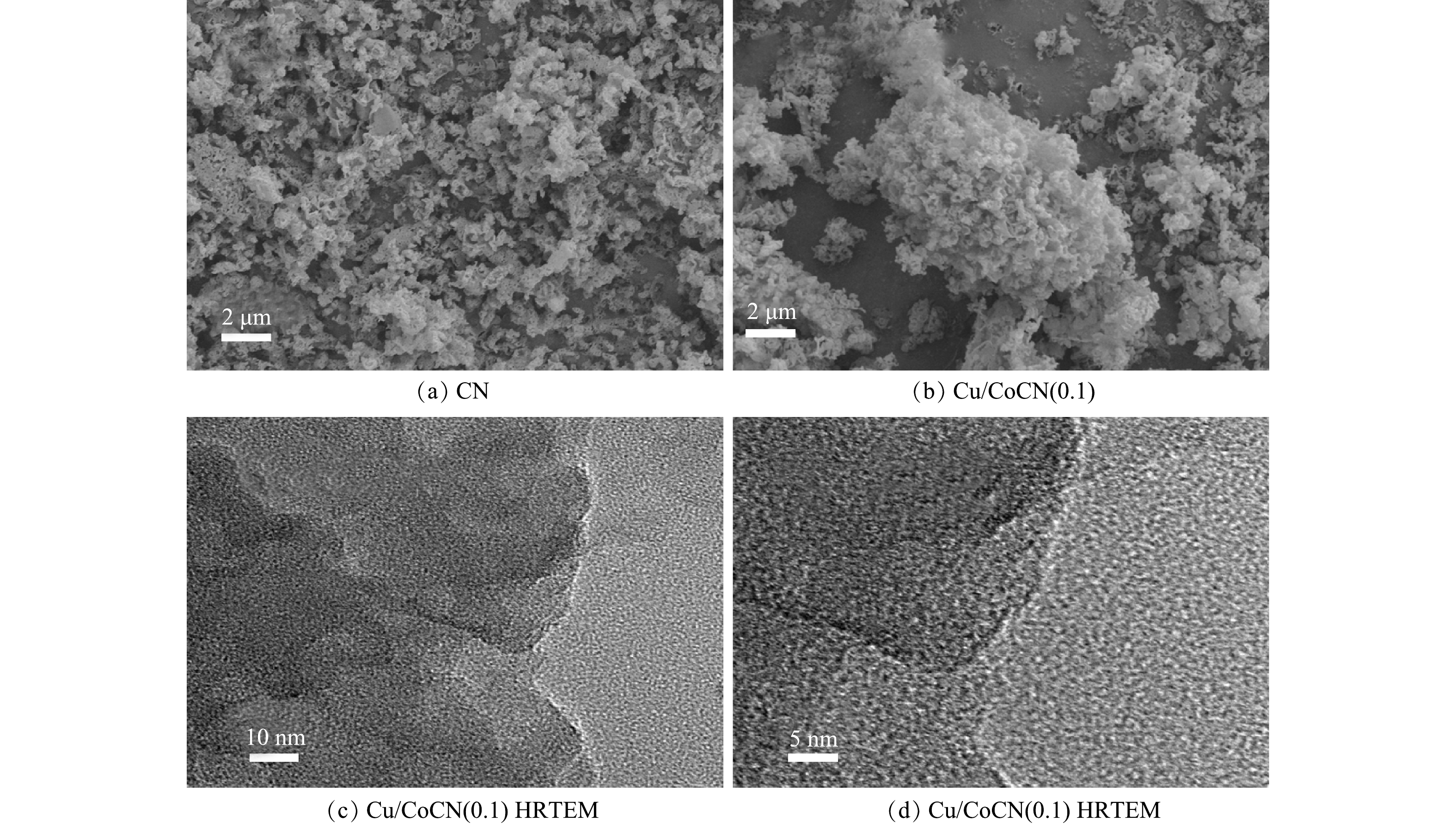
1) SEM分析。采用扫描电子显微镜(SEM)观察催化剂的微观形貌。在图1(a)中,单纯氮化碳CN呈疏松多孔结构,由图1(b)可见,Cu/CoCN(0.1)呈现出大大小小的块状结构且表面疏松。在Cu/CoCN(0.1)高倍透射电镜(HRTEM)图(图1(c)~(d))中没有发现任何晶格条纹,说明复合材料中不存在金属化合物。Cu/CoCN(0.1)具有的独特纳米片结构,不仅使得材料自身具有粘附性,而且可以提供更多的污染物接触位点,从而提高污染物去除效率。
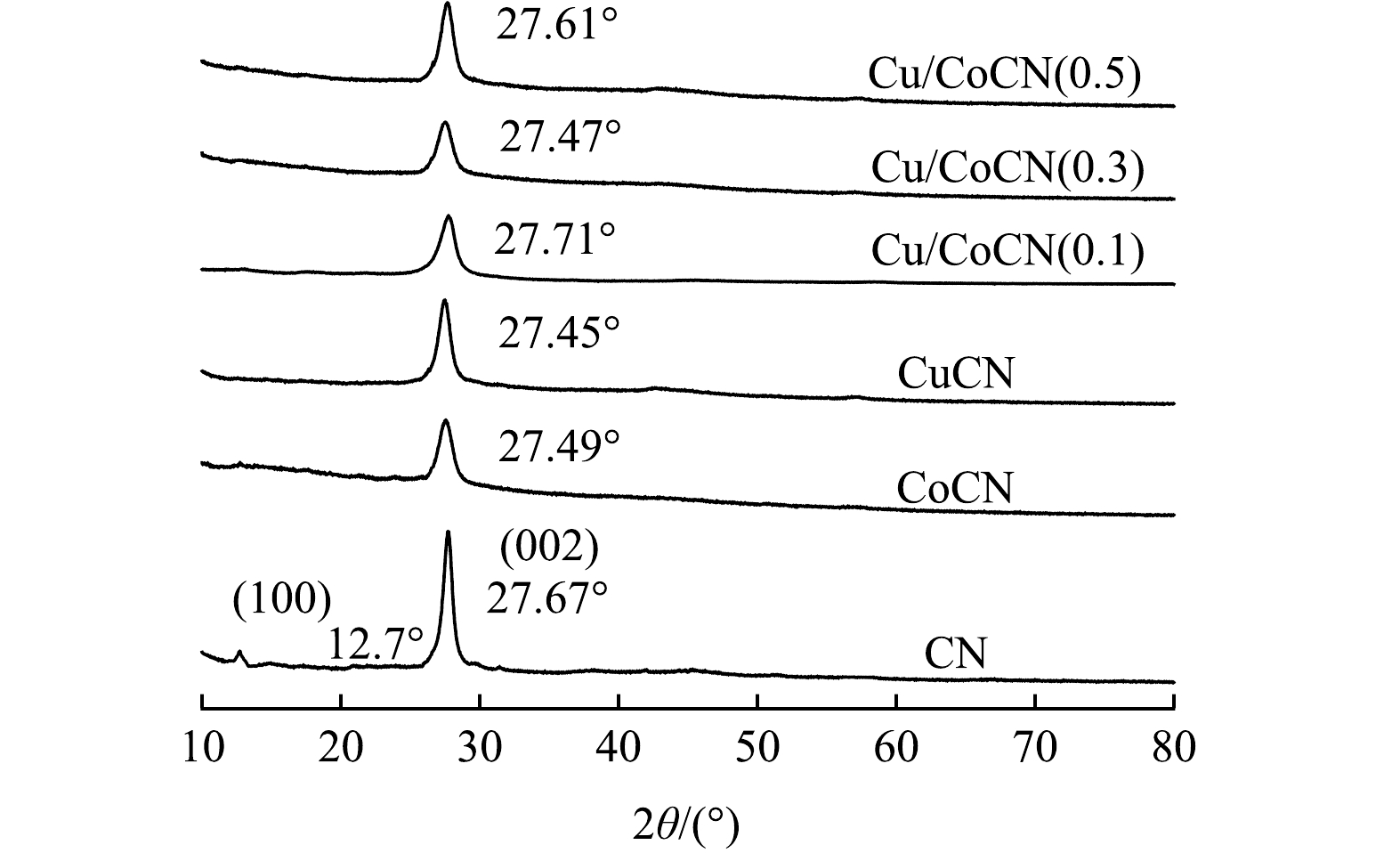
2) XRD分析。利用X射线衍射仪分析催化剂的晶体结构。如图2所示,对于CN样品,2θ=12.8°处的特征峰归属于(100)晶面,对应与平面内重复的3-s-三嗪单元[22];27.67°处的特征峰归属于CN(002)晶面,源于共轭芳香体系的层间堆积[23]。相比于CN、CoCN、CuCN 及Cu/CoCN(x)(x=0.1、0.3和0.5)样品在12.8°处的衍射峰的消失,在27.67°处的特征峰强度均变弱,说明Co、Cu的掺入,在一定程度上抑制了热缩聚过程中CN的结晶生长[24]。Cu/CoCN(0.1)样品中隶属于CN(002)晶面移动到更大的2θ角,表明材料的晶粒变大,晶面间距减小。此外,在所有复合材料的XRD谱图中未观察到任何钴化合物、铜化合物的衍射峰,可能是由于金属物质Co、Cu通过产生Cu-N、Co-N键成功地锚定到三嗪结构上[25-26]。
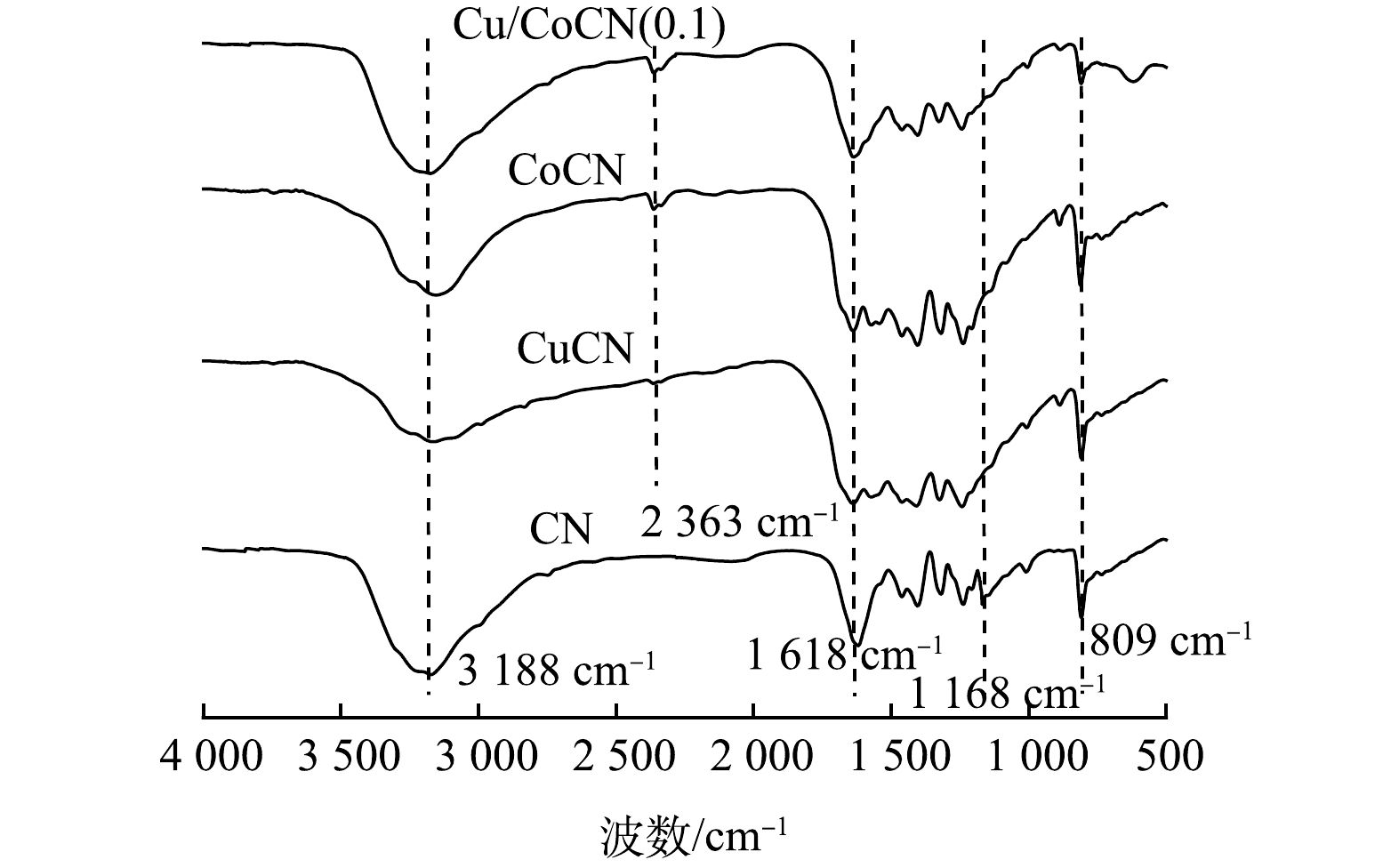
3) FT-IR 分析。如图3中CN的光谱所示,809 cm−1 处的峰对应于三-S-三嗪的面外弯曲振动[27]。在1 168~1 618 cm−1 的宽吸收峰归因于C=N和C—N杂环骨架的振动[28]。3 188 cm−1 附近较宽的特征吸收峰属于表面吸附H2O分子的伸缩振动[29]。此外,存在于复合材料2 363 cm−1 处的峰归因于叠氮化物基团的拉伸振动,表明结构N与掺杂金属之间能带形成[30]。CoCN、CuCN和Cu/CoCN(0.1)样品与CN的谱图非常相似,只是各吸收峰的峰强有所变弱,说明复合材料仍保持石墨相氮化碳的主体结构,这与XRD表征结果一致。
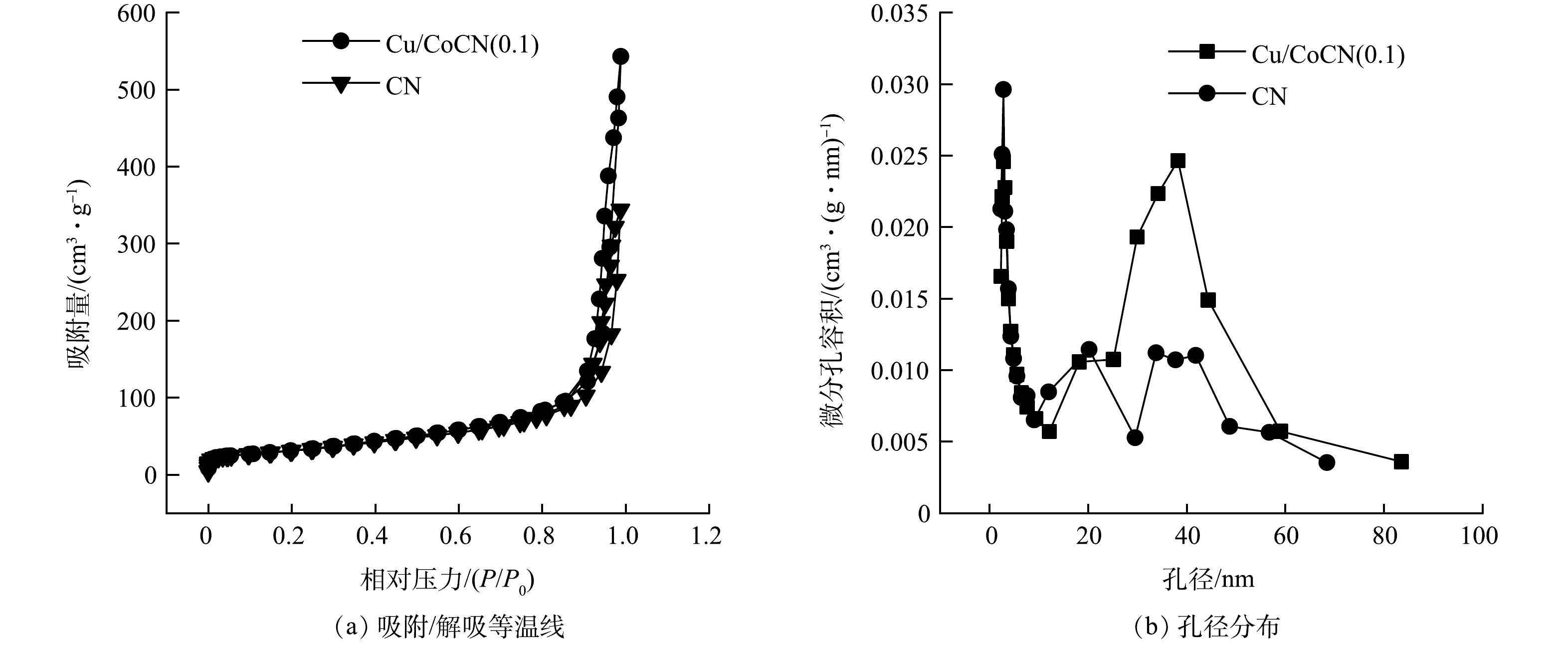
4) BET表征。由图4(a)可知,CN和Cu/CoCN(0.1)在相对压力为0.6~1.0的范围内出现典型的IV型等温线,同时呈现H3型回滞线,表明材料中存在介孔结构[31]。与CN相比,Cu/CoCN(0.1)的回滞环面积增大且吸附等温曲线上移,表明其比表面积增加。通过图4(b)可以看出,两种材料主要是介孔结构(2~50 nm),也有大孔的存在。与CN相比,Cu/CoCN(0.1)的孔体积更大,具体数据见表1。
5) UV-vis DRS表征。采用UV-vis DRS分析了CuCN、CoCN和Cu/CoCN(0.1)样品对光的吸收性能。如图5(a)所示,相比于CoCN,Cu/CoCN(0.1)对可见光的吸收向波长更长的方向移动,且对光的吸收强度有所增强;相比于CuCN,Cu/CoCN(0.1)的吸收曲线更为陡峭,这可能是由于Co、Cu双金属与CN之间存在电子转移,形成的Co-N、Cu-N键,降低了复合材料带隙。利用Kubelka-Munk函数(式(1))估算CN、CuCN、CoCN和Cu/CoCN(0.1)的跃迁带隙分别为2.63、1.60、2.51和2.36 eV。虽然CuCN的跃迁带隙1.6 eV小于Cu/CoCN(0.1)的2.36 eV,但CuCN中小的带隙会导致产生的光生电子-空穴对复合概率大大增加,从而不利于催化性能的提高;Cu/CoCN(0.1)中虽然带隙较大,但材料中的双金属可以通过接受CN上的电子,促进Cu2+/ Cu+、Co3+/ Co2+间的快速转换,从而降低光生电子-空穴对复合概率,有利于催化性能的提高。结合图5(b)可知,Cu/CoCN(0.1)禁带宽度介于CoCN和CuCN材料之间,亦说明了复合材料的成功耦合。Cu/CoCN(0.1)的禁带宽度为2.36 eV,将数据带入式(2)和式(3),得Cu/CoCN(0.1)的ECB和EVB分别为−1.11 eV和1.57 eV。
式中:α为吸光系数,L·g−1·cm;A为比例常数;h为普朗克常数,J·s;υ为光的频率,Hz;n与半导体类型相关。
式中:
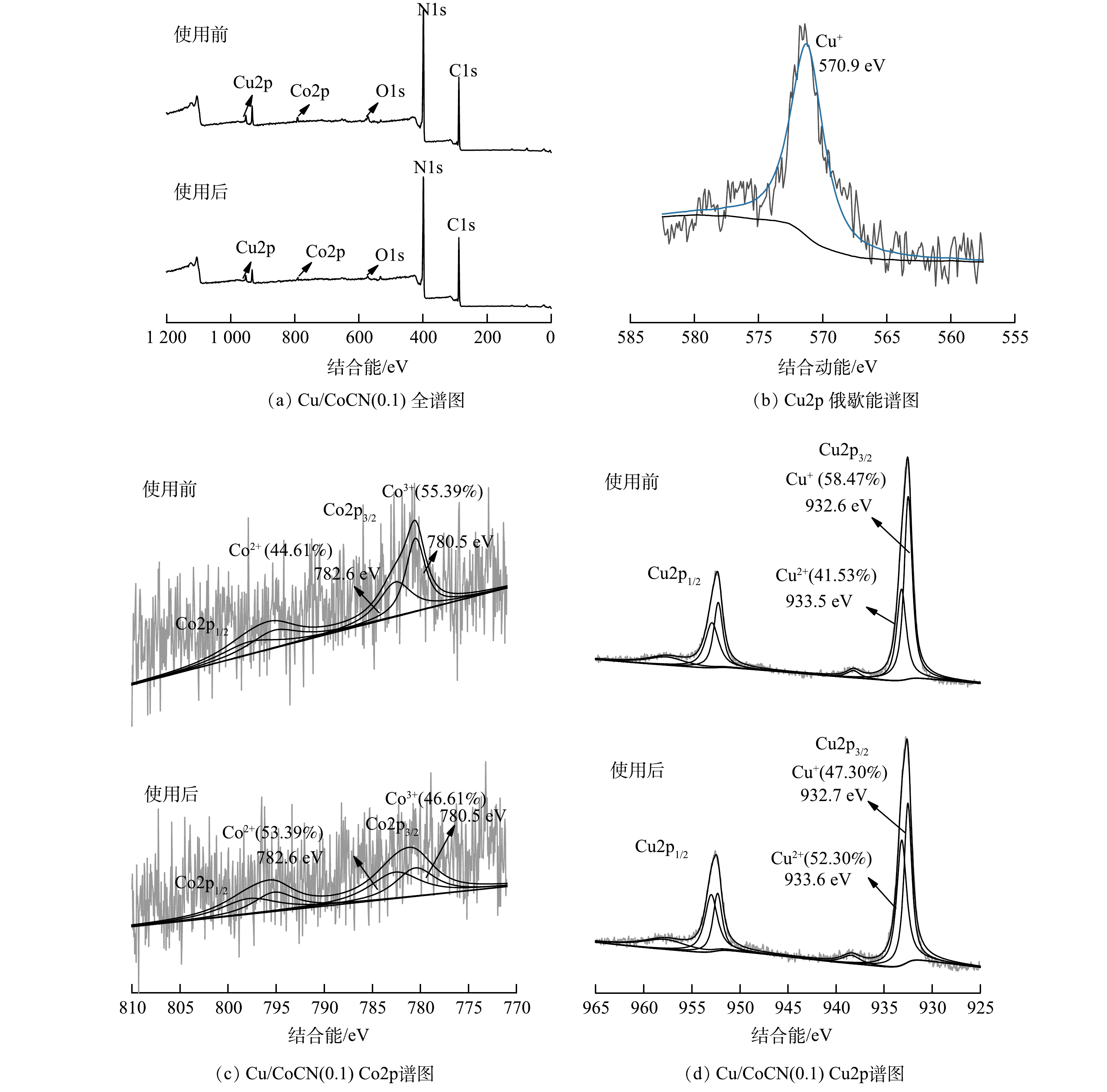
χ 为半导体的电负性,CN的电负性为4.73 eV[32];Ee为相对于氢标的自由电子能,约4.5 eV;Eg为催化剂的带隙,eV。6) XPS表征。通过 XPS 分析了样品的元素组成和化学状态。图6(a)显示Cu/CoCN(0.1)由Co、Cu、O、N、C元素组成。图6(c)是催化剂使用前后Co2p的高分辨能谱图,780.5 eV和782.6 eV分别对应于Co2p3/2 轨道中Co3+和Co2+;795.2 eV和797.8 eV分别对应于Co2p1/2 轨道中Co3+和Co2+,两轨道间约相差15.0 eV的结合能。图6(d)中高分辨率Cu2p XPS谱显示了归属于Cu0/Cu+(932.6和952.3 eV)和Cu2+(933.5和953.2 eV)的峰,表明Cu/CoCN(0.1)中存在Cu0/Cu+和Cu2+。Cu+和Cu0无法由结合能进行区分,因此,用Cu LMM XPS谱进一步确定材料中元素铜的化学价态[33]。Cu LMM光谱(图6(b))中仅观察到属于Cu+(570.9 eV)的强信号,说明材料中含有的低价态铜物种为Cu+[34]。为了更好地了解H2O2体系中Cu/CoCN(0.1)的活化过程,结合使用前后催化剂的图6(c)和图6(d)中Co2p和Cu2p XPS谱图中各价态金属含量变化进行分析。由图6(c)可见,使用前催化剂中Co2+和Co3+的含量分别为44.61%和55.39%,使用后Co2+和Co3+的含量分别为53.39%和46.61%;由图6(d)可见,使用前催化剂中的Cu+和Cu2+的含量分别为58.47%和41.53%,测得使用后Cu+和Cu2+的含量变为47.30%和52.30%。两者证实Cu和Co都参与了氧化和还原过程,且各价态金属含量变化较小,故可从侧面证明催化剂具有良好的循环利用性。
-
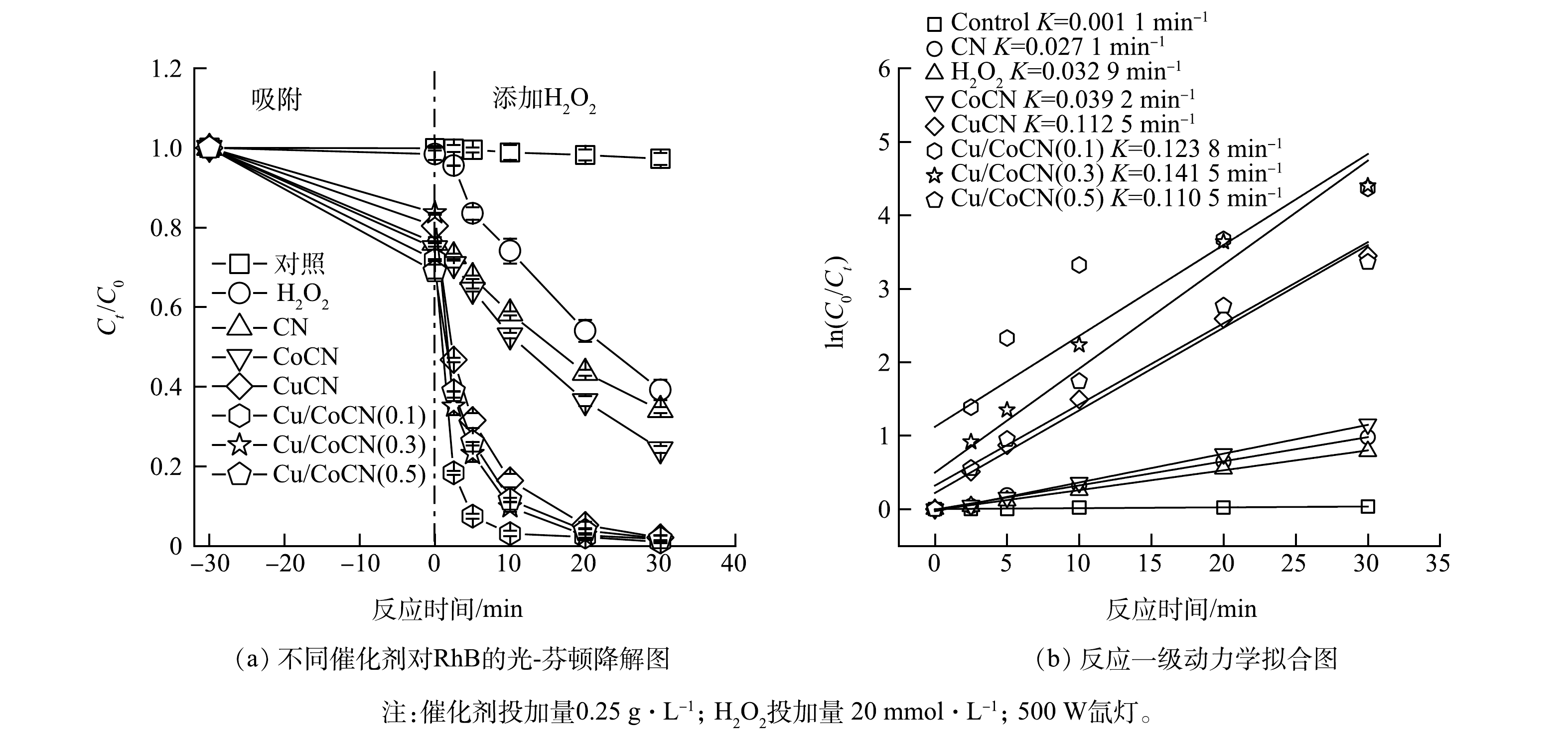
1)催化剂活化H2O2降解RhB性能比较。由图7可知,只添加 H2O2在10 min对RhB的降解率为23.69%,反应速率常数为0.032 8 min−1,因为氙灯中的紫外光能够活化H2O2产生·OH (式(4));与其他催化剂相比,Cu/CoCN(0.1)对H2O2的活化效果最好,反应10 min对RhB的降解率达到97.40%,比单纯的CoCN高出51.95%,反应速率常数最大,为0.141 5 min−1。虽然单纯的CuCN在光照条件下也能很好地活化H2O2降解污染物,但经过ICP-MS测试后,溶液中铜离子的浸出质量浓度为1.81 mg·L−1。相比之下,复合材料中铜离子的浸出质量浓度仅为0.12 mg·L−1,故复合催化剂Cu/CoCN更具有应用前景。
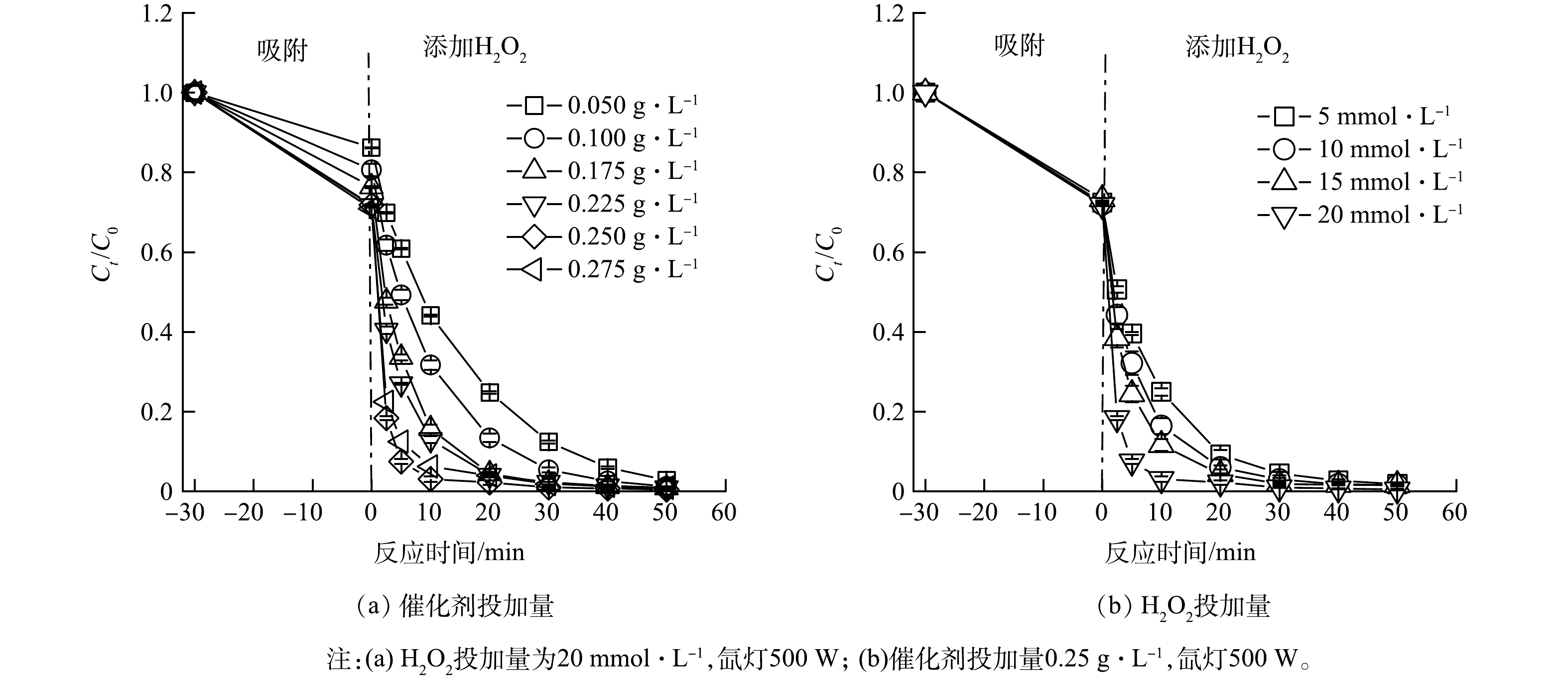
2)最佳催化剂和H2O2投加量确定。由图8(a)可知,催化效率随着催化剂投加量的增加表现为先增加后降低。原因在于适当的光催化剂增强可以提供更多的活性中心,促进活性物种的形成,从而提高催化效率。而过量的光催化剂会由于较高的浊度而影响透光率,进而影响催化效果。由图9(b)可知,随着在反应体系中加入H2O2,RhB的去除率显著提高,当 H2O2的投加浓度由5 mmol·L−1增加到 20 mmol·L−1时,反应 10 min对 RhB的降解率可分别达到 75.7%和 97.40%。如反应式(5)~(6)所示,添加过量的H2O2会对·OH 造成竞争性清除,进而生成氧化能力较弱的·OOH 自由基,这不利于污染物的降解。考虑到实际应用中的成本问题,后续研究中催化剂投加量和H2O2 浓度定为 0.25 g·L−1和 20 mmol·L−1。
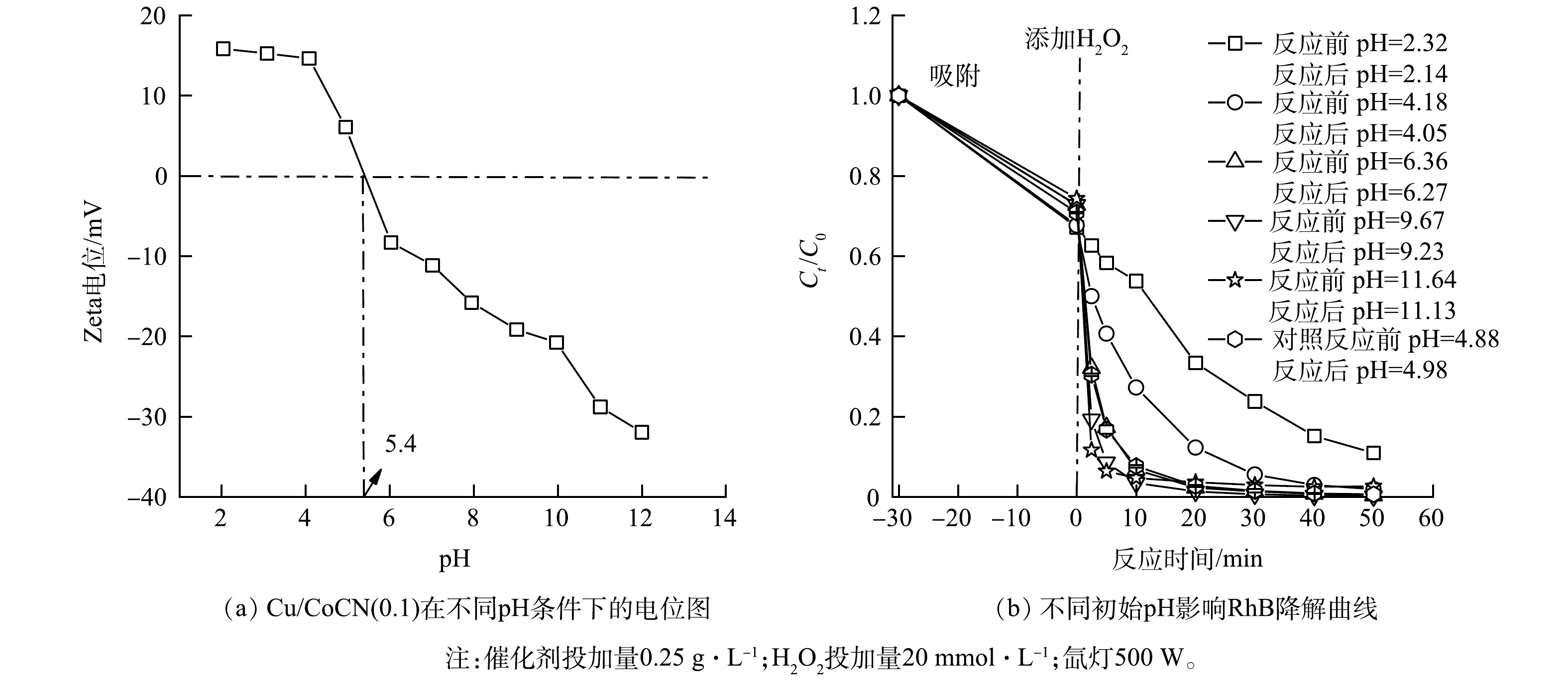
3)不同初始pH的影响。一方面由于传统的铁基均相 Fenton 反应需要在酸性pH条件(2.8~3)下进行,防止铁沉淀;另一方面溶液的pH可以通过改变材料的表面性质来影响催化性能[35],因此,研究催化剂在不同初始pH下的催化性能具有重要意义。如图9(a)所示,催化剂的等电点大约在5.4,因此,以溶液的pH=5.4为临界点,当溶液的pH低于5.4时,催化剂表面带正电,而当溶液的pH低于5.4时,催化剂表面带负电。pH的变化通过影响催化剂的表面所带电荷类型[36],从而影响吸附能力和电子转移能力,进而影响催化剂的催化活性。如图9(b)所示,在中性或弱碱性条件下,观察到良好的催化性能,证明Cu/CoCN(0.1)复合光催化剂可以克服传统Fe2+类Fenton过程窄pH范围的缺点。RhB是阳离子型染料,当反应体系初始pH=2.14时,Cu/CoCN(0.1)活化 H2O2降解 RhB 的性能明显减弱,原因一方面在于在极酸条件下(pH<3.00)H2O2稳定性较差,其会快速分解(式(7));另一方面在该溶液pH条件下,催化剂表面带正电,与阳离子染料RhB之间的静电排斥随着pH的降低而增强,从而导致降解率的下降;当反应体系pH在弱碱性条件时,催化剂催化性能相比于对照组得到提高,原因一方面是在室温条件下(25 ℃),H2O2的 pK1 =11.69,当溶液的pH高于11.69时,主要以过氧氢阴离子(HO2−)的形式存在,会促进各价态金属离子之间的相互转化,从而加快反应速率[37],如反应式(8)~(10)所示,且碱性介质可以作为一种均相催化剂,稳定 HO2− 中间体[38];另一方面是表面带负电荷的催化剂与污染物之间的静电吸引随着pH的增加而增强。基于上述原因,Cu/CoCN(0.1)在碱性条件下展现出优异的活化H2O2降解 RhB的能力。
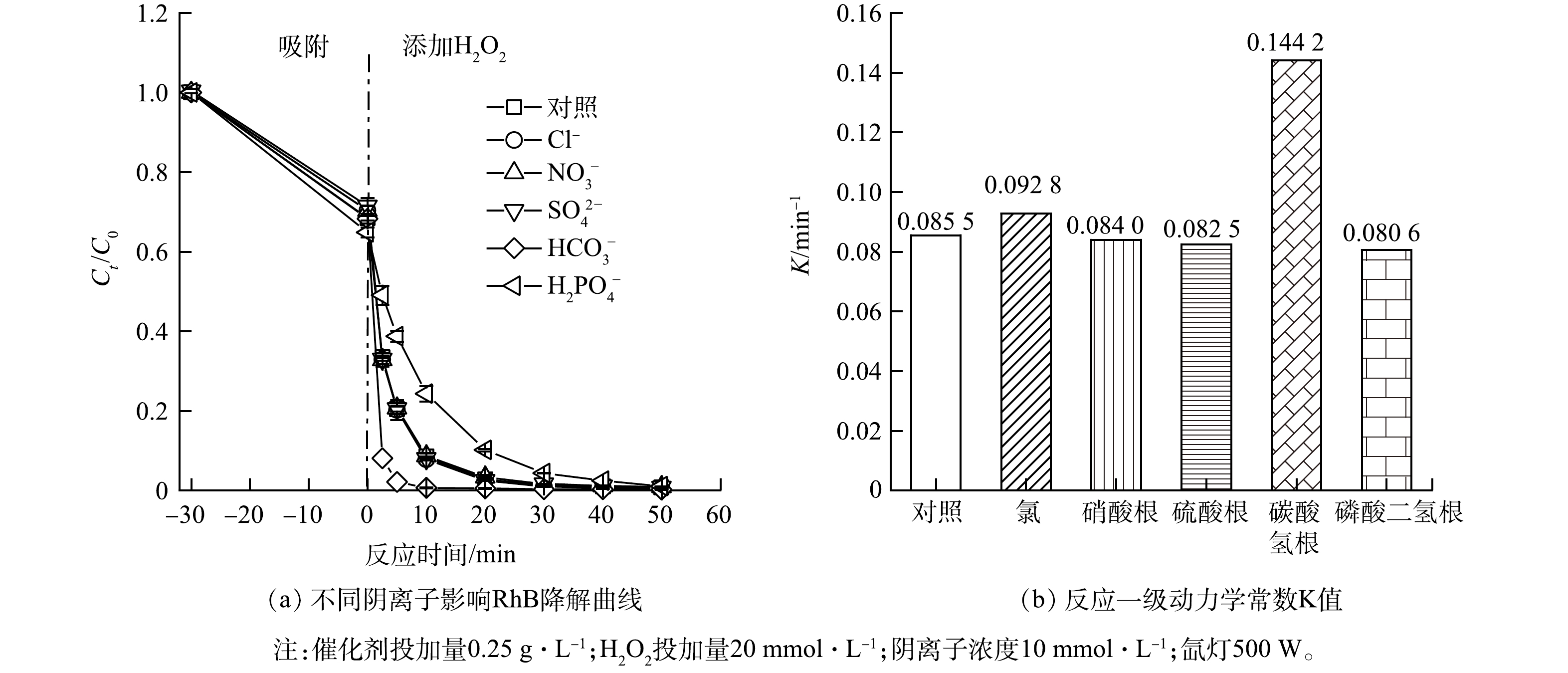
4)无机阴离子的影响。各类工业废水中含有的无机盐阴离子不仅会影响反应物种的转化[39],而且会抑制甚至损坏微生物的代谢功能,不利于有机污染物的去除[40]。选择常见的阴离子Cl−、NO3−、SO42-、HCO3−和 H2PO4−为研究对象,各种无机阴离子对反应体系降解 RhB 效果的影响结果如图10所示。某些阴离子具有独特的化学性质,可能会引起溶液pH的变化。由于HCO3−(pKa = 6.3)的水解,使溶液初始pH从4.85增加到8.38,而其它离子的加入对溶液的pH影响不大。添加不同阴离子后溶液的初始pH如表2所示。
与未添加无机阴离子的对照实验相比,H2PO4−对 RhB 的降解具有抑制作用。原因在于溶液在加入H2PO4−前后,pH在4.6附近,与上述不同初始pH的影响因素研究得到的结论相一致,即在酸性条件下,催化剂表面带正电,降低了催化剂与污染物之间的静电吸附,会抑制Cu/CoCN(0.1)的催化活性。亦有文献指出H2PO4−会与·OH发生反应,生成低氧化性的H2PO4·,从而对污染物的去除表现为抑制作用[41]。
在该体系下加入HCO3−后,降解曲线相比于对照组更为陡峭,说明反应速率加快,加入HCO3−有利于降解。产生促进作用的原因是溶液在加入HCO3−后,pH会增至8.38,与上述不同初始pH的影响因素研究得到的结论相一致,催化剂表面带负电,增强了催化剂与污染物之间的吸附作用,进而促进催化剂对污染物的降解。
加入Cl−、NO3−和SO42-离子对反应体系中 RhB 的降解影响不大。具体表现为实验组降解曲线与对照组降解曲线基本重合。产生该现象的原因是加入Cl−、NO3−和SO42-离子对溶液的pH无明显影响。其次,Cl−、NO3−和SO42-离子不与H2O2反应,即使Cl−会消耗·OH,式(11)~式(12),但式(11)和式(12)的反应速率相同,故Cl−对该体系的影响可忽略不计。NO3−和SO42-离子亦会与·OH发生反应,式(13)~式(14),但NO3·和SO4·自由基与·OH的氧化还原电位相当,因此NO3− 和SO42- 离子的引入对该体系无明显作用。
5)催化剂的稳定性评价。为了评价催化剂Cu/CoCN(0.1)能否应用于实际工程,在该体系下进行了循环实验,结果如图11(a)所示。在催化剂使用第4次时,经过50 min反应后RhB的去除率为91.6%,说明该催化剂在该体系下能够稳定活化H2O2降解污染物。如图11(b)所示,循环降解实验后溶液中铜离子的质量浓度均低于《城镇污水处理厂污染物排放标准》(GB 18918-2002)中铜最高允许排放质量浓度(0.5 mg·L−1),且铜的最高溶出质量浓度为0.17 mg·L−1,这低于之前相关文献报道[7];钴离子的质量浓度均低于《地表水环境质量标准》(GB 3838-2002)中集中式生活饮用水地表水水源地特定项目标准限值(1 mg·L−1),且钴离子的最高溶出质量浓度为0.11 mg·L−1。经四次循环使用,溶液中累积铜、钴离子的总质量浓度分别为0.56 mg·L−1和0.30 mg·L−1。
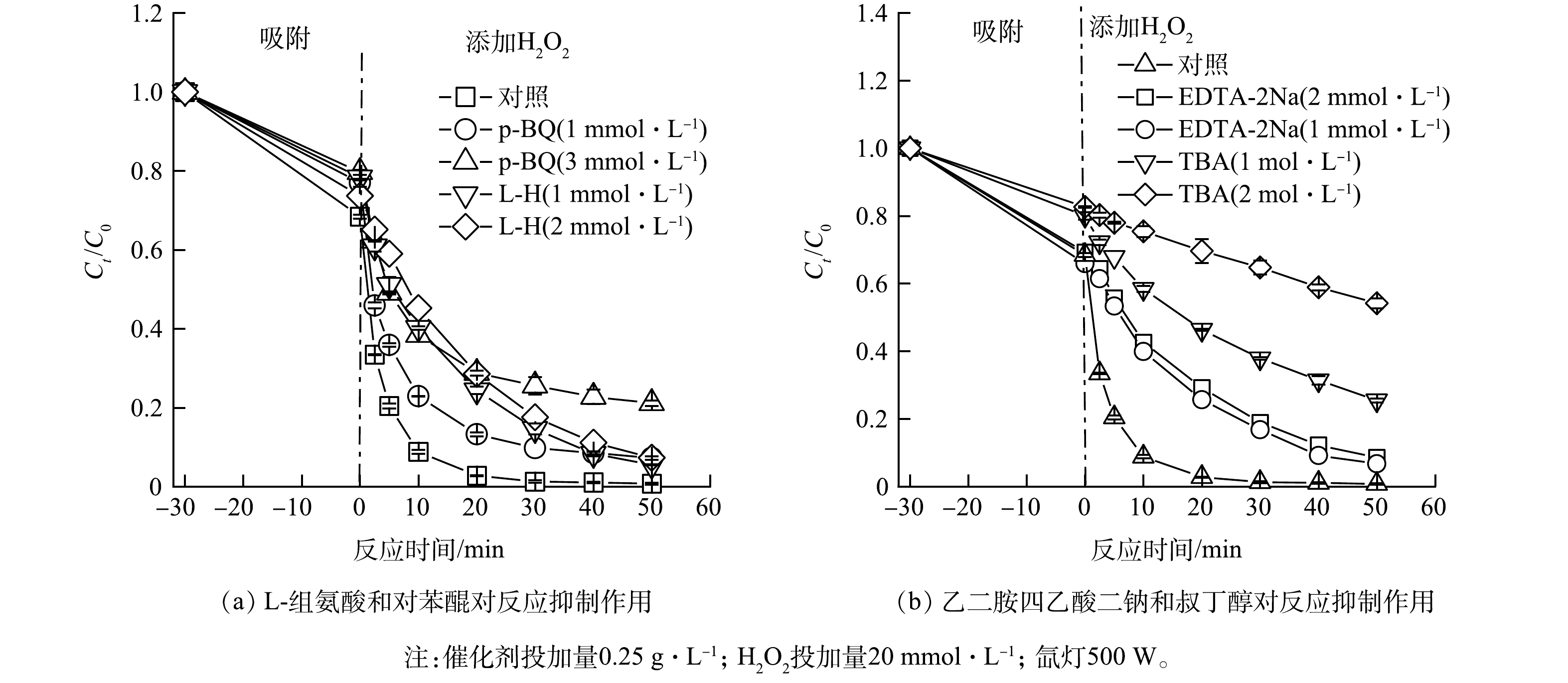
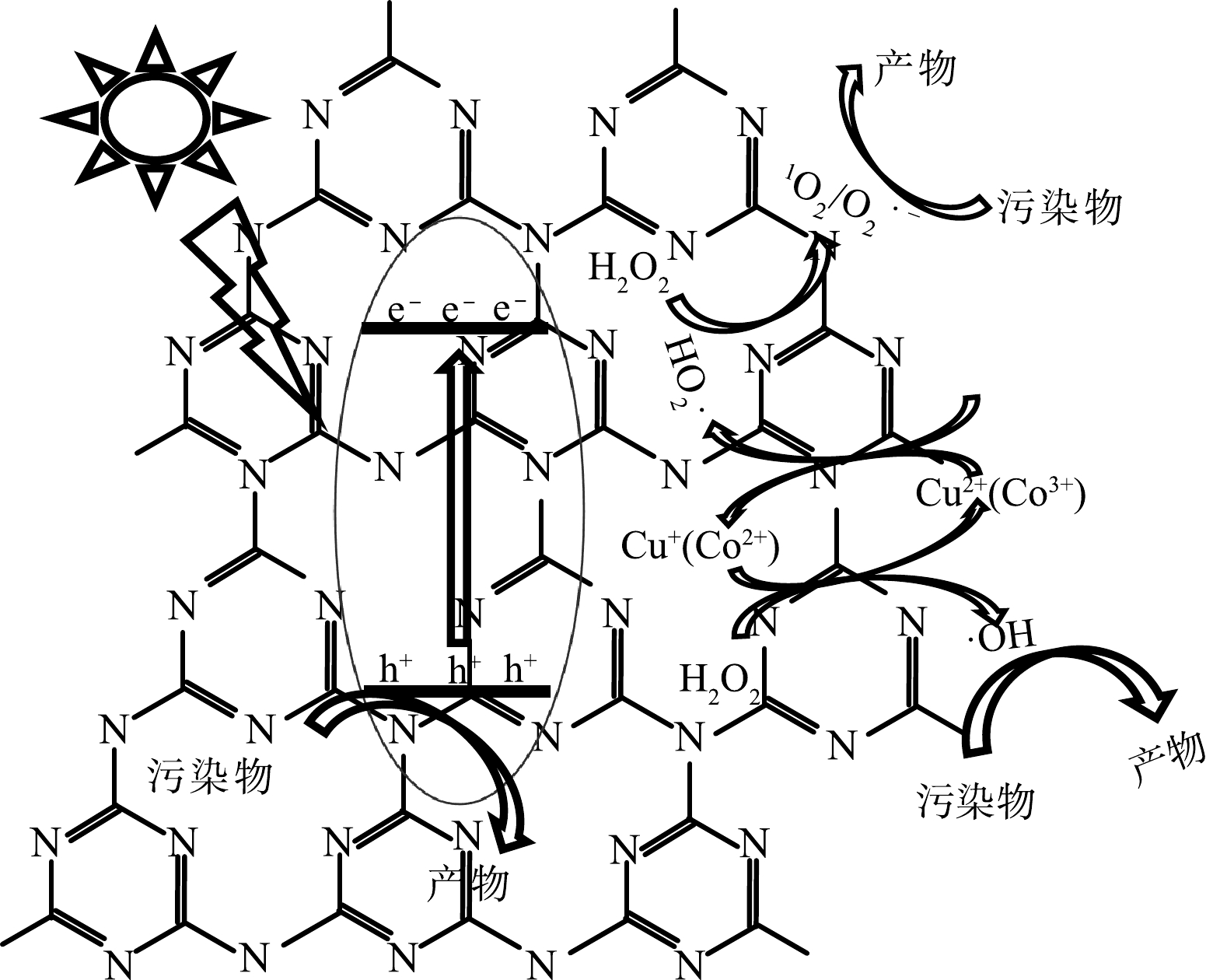
6)自由基淬灭实验。为探究Cu/CoCN(0.1)活化H2O2降解RhB的机理,采用自由基淬灭实验确定该体系中的活性物质。叔丁醇 (TBA)与·OH的反应速率k=3.8×108~7.6×108 mmol·(L·s)−1,常采用TBA作为·OH的淬灭剂[42]。乙二胺四乙酸二钠(EDTA-2Na)、L-组氨酸(L-H) 和对苯醌(p-BQ)分别作为hvB+、1O2 和
O2·− 的淬灭剂。如图12所示,Cu/CoCN(0.1)/H2O2体系在加入淬灭剂后,其对RhB 的降解均表现为不同程度的抑制。其中TBA的抑制作用最为明显,当反应体系中TBA的浓度从1 mmol·L−1增加到2 mmol·L−1,RhB 的降解率由74.4%降低到45.8%;p-BQ的抑制作用次之,表现为当反应体系中p-BQ的浓度从1 mmol·L−1增加到3 mmol·L−1,RhB的降解率由92.8%降低到79.2%;而当反应体系中加入1 mol·L−1 L-H与1 mmol·L−1EDTA-2Na时,50 min后RhB 的降解率仍为94.5%和91.5%,增加L-H与EDTA-2Na浓度到2 mmol·L−1,抑制作用并没有提高。因此,在该体系中发挥主要作用的自由基是·OH和O2·− ,少量的hvB+与1O2也可能参与其中。7)催化机理分析。结合表征分析和自由基淬灭实验结果,提出Cu/CoCN(0.1)活化H2O2降解污染物相应机理。首先,Cu/CoCN(0.1)在光照条件下产生
h+ 和e− (式(15)),但Cu/CoCN(0.1)的价带EVB电位(+1.57 eV)比OH−/·OH(+1.99 eV)低,无法产生·OH ,而Cu/CoCN(0.1)的导带ECB电位(−1.11 eV)比O2/O2·− 电位(−0.33 eV)高,可以产生O2·− (式(16));其次Cu2+/ Cu+、Co3+/ Co2+间的快速转换是类 Fenton 反应高效进行的基础,式(17)~式(21)。在该反应体系下,一方面高价态的Cu2+/Co3+可以接受CN上的电子,促进价态之间的转化;另一方面低价态的Cu+/Co2+与H2O2相互作用,产生·OH。·OH会引发一系列副反应产生其他活性物种,例如O2·− ,1O2 和HO2·等,均能够参与降解污染物(式(22)~式(25))。因此,Cu/CoCN(0.1)/ H2O2体系在光照射下的催化机理如图13所示。 -
1)通过浸渍法和热缩聚法将Co、Cu以Co—N、Cu—N键的形式稳定存在于CN中,当CuCN与CoCN的质量比为0.1时,催化剂Cu/CoCN(0.1)对RhB的降解效果最好,10 min内RhB的去除率为96.5%。
2)在该反应体系中,过渡金属一方面通过接受CN上的电子,促进Cu2+/ Cu+、Co3+/ Co2+间的快速转换,另一方面低价态的Cu+/Co2+与H2O2相互作用,产生·OH,表现出吸附-催化降解和光催化-Fenton氧化的多重协同作用。
3) Cu/CoCN(0.1)具有耐盐性、耐碱性、低的离子溶出性及良好的稳定性。
Cu/Co共掺杂氮化碳复合材料光-芬顿降解罗丹明B(RhB)
Photo-Fenton degradation of Rhodamine B (RhB) via Cu/Co bi-metal doped-graphitic carbon nitride composite
-
摘要: 针对金属-非金属复合材料制备条件苛刻、金属离子溶出较高的问题,通过简单的浸渍法和热缩聚法制备了钴、铜共掺杂石墨相氮化碳复合材料。以罗丹明B (RhB) 为污染物,研究了催化剂在模拟太阳光下活化H2O2降解RhB的性能。通过XRD、FTIR、XPS和BET等手段,表征分析了催化剂的物化性质,考察了催化剂用量、H2O2浓度、溶液初始pH以及共存无机阴离子对催化剂活化性能的影响。结果表明,Co、Cu以Co—N、Cu—N键的形式稳定存在于复合材料中,限制了钴、铜金属离子的溶出。当CuCN与CoCN的质量比为0.1,得到的复合材料Cu/CoCN(0.1)催化性能最佳。Cu/CoCN(0.1)在初始pH=5.0~12.0内均表现出良好的催化活性。当 Cu/CoCN(0.1)和H2O2的用量分别为0.25 g·L−1和20 mmol·L−1时,10 min对RhB的降解率达到96.5%。H2PO42- 的存在对RhB的去除有一定抑制作用,HCO3− 对RhB的去除有促进作用,而Cl−、SO42- 和NO3− 阴离子对RhB的去除无明显影响。Cu/CoCN(0.1)经4次循环使用后,对RhB的降解率仍能达到91.2%,且Co2+、Cu2+的总溶出质量浓度分别为0.30 mg·L−1和0.56 mg·L−1。自由基捕获实验结果表明,·OH和·O2−是降解过程中主要的活性物质。结合催化剂的表征分析和自由基捕获实验提出该催化剂对RhB的降解机理。本研究结果可为非均相类芬顿技术在印染废水处理中的应用提供理论参考。Abstract: In order to solve the problems of harsh preparation conditions and high dissolution of metal ions in metal-non-metal composites, cobalt and copper co-doped graphitic carbon nitride composites were prepared by the impregnation method and heat condensation polycondensation method. Rhodamine B (RhB) was used as a pollutant to study the performance of the catalyst on activating H2O2 under simulated sunlight and degrading RhB. The physicochemical properties of the catalyst were characterized by XRD, FTIR, XPS and BET. The effects of catalyst dosage, H2O2 concentration, initial pH of solution and coexistence of inorganic anions on the catalytic activation performance were investigated. The results show that Co and Cu existed stably in the composites with the form of Co-N and Cu-N bonds, and the special structure of carbon nitride restricted the dissolution of cobalt and copper metal ions. When the mass ratio of CuCN to CoCN was 0.1, the obtained composite Cu/CoCN(0.1) had the best catalytic performance. Within the initial pH=5.0~12.0 range, Cu/CoCN(0.1) showed a good catalytic activity. At Cu/CoCN(0.1) dosage of 0.25 g· L−1 and H2O2 dosage of 20 mmol·L−1, the degradation rate of RhB was 96.5% in 10 min. The presence of H2PO42- had a certain inhibitory effect on RhB removal, and HCO3− had a promoting effect on RhB removal, while Cl−, SO42- and NO3− had insignificant effects on RhB removal. After 4 cycles of use, the degradation rate of Cu/CoCN(0.1) could still reach 91.2%, and the total dissolution concentrations of Co2+ and Cu2+ were 0.30 and 0.56 mg·L−1, respectively. Free radical scavenging experiments showed that ·OH and ·O2− were the main active species. Through the characterization and analysis of the catalyst and free radical scavenging experiments, the degradation mechanism of RhB by the catalyst was proposed. The results of this study can provide a theoretical reference for the application of heterogeneous Fenton-like technology in the treatment of printing and dyeing wastewater.
-
Key words:
- bi-metal doped /
- composites /
- photo-Fenton /
- degradation of Rhodamine B
-
海产品作为水生物资源在解决人口激增、资源短缺与环境恶化等全球性问题中,扮演着越来越重要的角色[1]。为保证海产品的产品品质,国内外对海产品暂养环境的处理手段大多通过物理方法进行调控[2]。但在高密度的暂养条件下,海产品代谢的加快与暂养池设计的不合理,大量泡沫与污染物堆积于水体,并积累大量硝酸盐与悬浮颗粒物,致使海产品暂养环境恶化[3-5]。当总悬浮颗粒物浓度在44 mg·L−1时,会降低暂养水的洁净度[6];水体中氮磷等营养盐的动态失衡则会危及水生态系统的平衡,破坏其物质循环与能量流动,加之暂养池过滤效果差、脱氮除磷效果不佳等问题[7-8],最终造成暂养水体水质恶化。与此同时,暂养水体携带大量污染物,一旦进入受纳水体则严重破坏水域生境[9-10],危害水域生态的健康及物种的多样性。
养殖水体氮、磷含量过高易引发水环境污染,诱发海产品疾病的蔓延12]。目前,传统暂养水处理工艺逐渐被新型工艺取代,包括物理方法[13-14]、化学方法[15]和生物方法[16-17]。基于对海产品暂养水处理的更高的要求,驯化富集耐低温、嗜盐微生物菌种,并用于处理受纳水体,已逐渐成为当下水域生态和环境工程等领域的研究重点[18-20]。本研究通过构建低温条件下高盐微生物驯化系统,富集培养了低温耐盐菌种,分析了群落结构及物种多样性,并以玉米芯和玉米衣为碳源,探究了低温条件下暂养水处理与微生物驯化富集的耦合效应,可为低温菌种的筛选和水处理技术的拓展提供理论依据和科技支撑。
1. 材料与方法
1.1 实验试剂
本研究选用的试剂为KNO3、(NH4)2SO4、KH2PO4、NaNO2、C6H12O6、NaHPO4、MgSO4、7H2O、K2HPO4、CaCO3、Na3C6H5O7·2H2O5,以上试剂均为分析纯,来自于国药化学试剂有限公司;硝化菌实验样品采自上海海洋大学海参循环水养殖系统;反硝化菌实验样品采自上海海洋大学滨海基地池塘养殖底泥;玉米芯、玉米衣取自上海市宝山区罗南镇罗南新村农田。
1.2 实验装置
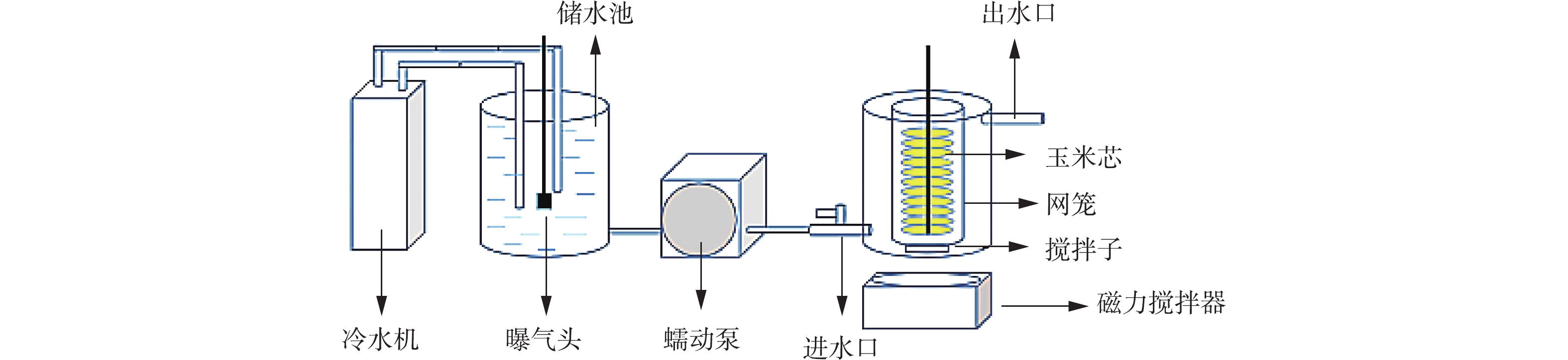
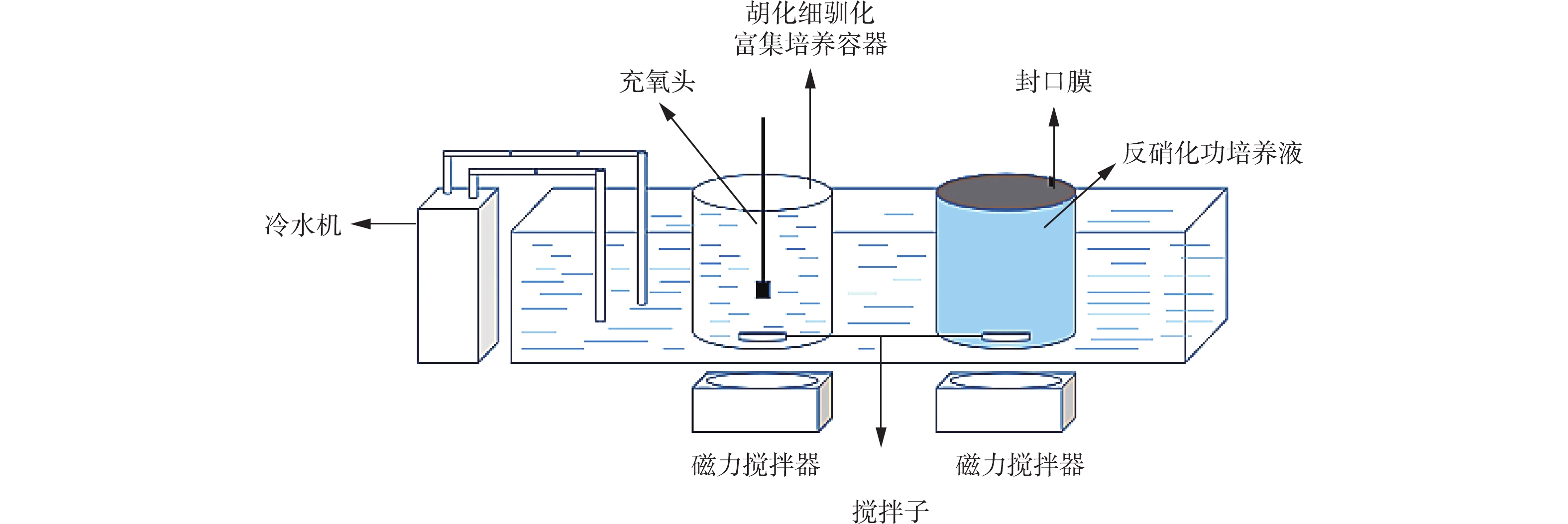
如图1所示,实验构建硝化反硝化菌培养容器,由冷水机、硝化菌驯化富集培养器、反硝化菌驯化富集培养器3部分组成,分别用以营造低温环境、驯化、富集培养耐低温、嗜盐菌种。玻璃容器有效容积为10 L底部置有磁力搅拌器。
在微生物驯化富集后,设计构建脱氮除磷装置,如图2所示。该装置由冷水机,储水池,蠕动泵与脱氮除磷反应器4部分组成。冷水机用以营造低温环境,储水池进水,将待处理水体经蠕动泵通入脱氮除磷反应器,反应器内置有经挂膜的玉米芯、玉米衣,对待处理水体进行脱氮除磷。
1.3 实验方法
将取自上海海洋大学海参循环水养殖系统的硝化菌实验样品与采自上海海洋大学滨海基地池塘养殖底泥的反硝化菌实验样品放置4 ℃冰箱保存备用。玉米芯、玉米衣加入去离子水浸泡4 h过滤清洗后,置于鼓风干燥箱中于50 ℃下,干燥12 h,取出置于干燥器中备用。
参考常规海产品循环暂养水水质指标,添加适量KNO3、(NH4)2SO4、KH2PO4配置实验用水,盐度27~30,水质指标保持pH为7.27±0.01,DO为(6.65±0.01) mg·L−1,TN为(41.62±0.11) mg·L−1,
NH+4 -N为(8.97±0.05) mg·L−1,NO−3 -N为(32.04±0.10) mg·L−1。实验采用10 L玻璃容器,加入培养液,硝化菌驯化培养基成分包含(NH4)2SO4、NaNO2;反硝化菌培养基成分包含KNO3、C6H12O6,NaHCO3调节pH至7~7.5,用NaCl调节盐度至27±0.5,曝气并用磁力搅拌器进行搅拌,DO保持4 mg·L−1以上,15 ℃恒温培养60 d[21]。
将装置洗净灭菌后,取适量经驯化培养的反硝化菌液(KNO3 4.00 g·L−1、Na3C6H5O7·2H2O 6.00 g·L−1、NaHPO4 1.00 g·L−1、MnSO4·7H2O 0.04 g·L−1、K2HPO4 1.00 g·L−1)于10 L玻璃容器中,参考邵留等[22]人工强化挂膜方式,将经洗涤和干燥的玉米芯、玉米衣、玉米芯和玉米衣3种载体形式分别置入反硝化菌液中,设置3组实验,充分浸泡3 d,保持容器的内部恒温15 ℃。将已经充分浸泡过反硝化菌的载体浸入富集培养好的硝化菌液((NH4)2SO4 2.00 g·L−1、MnSO4·4H2O 0.01 g·L−1、NaHPO4 0.25 g·L−1、MgSO4·7H2O 0.03 g·L−1、CaCO3 0.3 g·L−1、K2HPO4 0.75 g·L−1)中,经磁力搅拌器搅拌直至挂膜完成。使用NaHCO3溶液调节pH至7~7.5,富集培养60 d,保持15 ℃恒温进行富集培养,保证其增殖。
1.4 分析方法
高通量测序分析方法:取载体表层切片经EYELA东京理化冷冻干燥机(FDU-1200型 东京理化器械株式会社)低温处理,硝化菌液采用细菌通用引物,上游引物为5'-CARTGYCAYGTBGARTA-3',下游引物HQd5'-TWNGGCATRTGRCARTC-3'。反硝化菌液采用nrfA引物,上游引物为5'-CARTGYCAYGTBGARTA-3',下游引物为5'-TWNGGCATRTGRCARTC-3'。最后经16S rRNA基因文库测序分析微生物群落结构、丰度及其多样性[23]。高通量测序服务由上海派森诺生物科技股份有限公司提供(上海,中国)。
常规水质指标测定:采用国家水质标准[24]对TN、
NH+4 -N、NO−2 -N、NO−3 -N、DO、pH进行测定。2. 结果与讨论
2.1 微生物群落结构与多样性分析
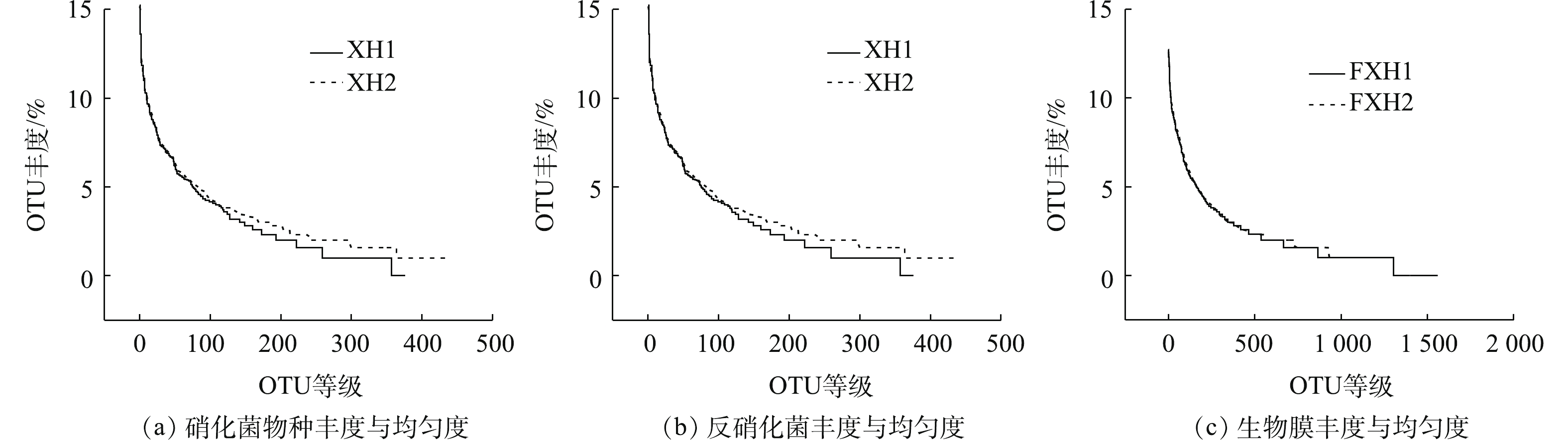
1)群落结构(丰度)。研究通过Rank-Abundance曲线检测载体表层微生物物种丰富和均匀度[25],如图3所示,A1、A2、A3分别代表实验初期(15 d)、中期(30 d)、末期(45 d)载体表层生物膜,XH1和XH2表示硝化菌物种丰度和均匀度;FXH1和FXH2表示反硝化菌物种丰度和均匀度。由图3可知,载体表层微生物的丰度大小序列为FXH1、FXH2>A2>A3>A1>XH1、XH2,载体所附着的反硝化菌的丰度高于硝化菌。但从均匀度方面来看,富集培养的硝化菌均匀度优于反硝化菌,这说明硝化菌种群的个体数目的分配状况对于反硝化菌来说相对合理;在载体均匀度方面,由A3>A2>A1的趋势可知,在低温、高盐条件下,反硝化菌富集培养效果比硝化菌富集培养效果更佳。
装置运行期间,即A2阶段(中期30 d)相较于A1阶段(初期15 d)和A3阶段(末期45 d),载体所附着的微生物膜的丰度状态处在较高的水平,表明装置在A2阶段(中期30 d)运行良好,且生物对于环境的适应性也在增强;A1阶段(初期15 d)处于装置运行的初始阶段,丰度处在较低的水平,其主要原因是:载体上的微生物菌群处于一个适应阶段,增长速度相对较慢,且对于低温高盐水体的实际适用性也需要一定周期来提升[26]其与JESSICA等[27]处理得到的丰度趋势相近;A3阶段(末期45 d)处在装置运行后期,其生物膜的丰度、均匀度从整体上略低于A2阶段(中期30 d),这是由于在一个运行周期内,载体上的微生物随时间的延长丰度开始降低,在一定水平达到的动态平衡,趋于低水平的稳态,与崔丙健等[28]对微生物菌落的解析相吻合。以上结果说明:本装置在微生物的驯化富集培养过程中具有较好的效果,并能够为其提供较好的好氧厌氧环境,从而达到成功驯化并富集的目的。
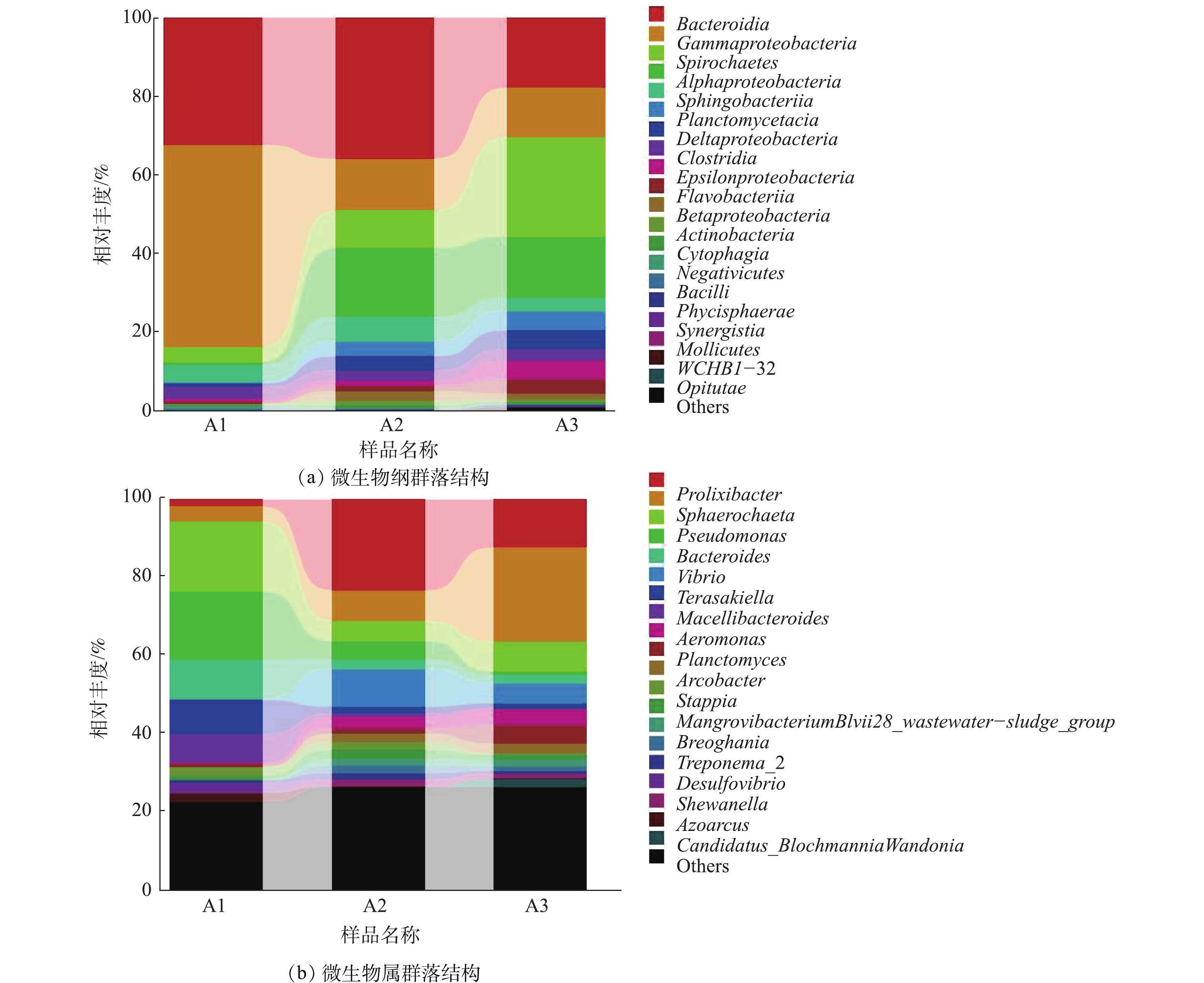
2)微生物多样性。图4为微生物纲水平和属水平群落结构,其优势菌种主要以α-变形杆菌纲(Alphaproteobacteria)、γ-变形菌纲(Gammaproteobacteria)、拟杆菌纲(Bacteroidia)、螺旋体纲(Spirochaete)、鞘脂杆菌纲(Sphingobacteriia)为主。A1表示实验初期载体表层生物膜的微生物数量占比,研究发现微生物主要以拟杆菌纲(Bacteroidia)、γ-变形菌纲(Gammaproteobacteria)为主,该阶段是微生物的自身因子对低温高盐环境的调控期间,周海红等[29]的研究中也证实了拟杆菌纲(Bacteroidia)与γ-变形菌纲(Gammaproteobacteria)生长的适应性与稳定性。伴随装置运行进入中后期,载体的相对丰度产生了明显的变化:从前期的拟杆菌纲(Bacteroidia)、γ-变形菌纲(Gammaproteobacteria)2大优势菌种更替为以拟杆菌纲(Bacteroidia)、γ-变形菌纲(Gammaproteobacteria)、螺旋体纲(Spirochaetes)、α-变形杆菌纲(Alphaproteobacteria)为主要的5大优势菌种;整个实验期间,检测出主要的属有7种,实验中期(A2)和后期(A3)的样品主要属的种类数量占比同实验前期有显著差异,载体表层所附着的菌群结构随着低温高盐水体的输入,使得优势菌门转变为变形菌门(Proteobacteria)、拟杆菌门(Bacteroidetes)、螺旋菌门(Spirochaetae)、浮霉菌门(Planctomycetes)。这些菌可从多种生境中分离获得,如海洋、超盐环境、碱性或酸性环境等,且具有很强的污水脱氮能力[30]。装置运行期间,3个样品中变形菌门的比例有所下降,从初始运行阶段的53.61%下降至10.55%,研究到后期仅有8.69%;假单胞菌属(Pseudomonas)作为反硝化菌的主要部分,其占比略高于其他菌属,与本实验前中期
NO−3 -N去除率略高于NH+4 -N与TN的去除率变化趋势相一致。2.2 微生物驯化富集培养与无机氮浓度变化关系
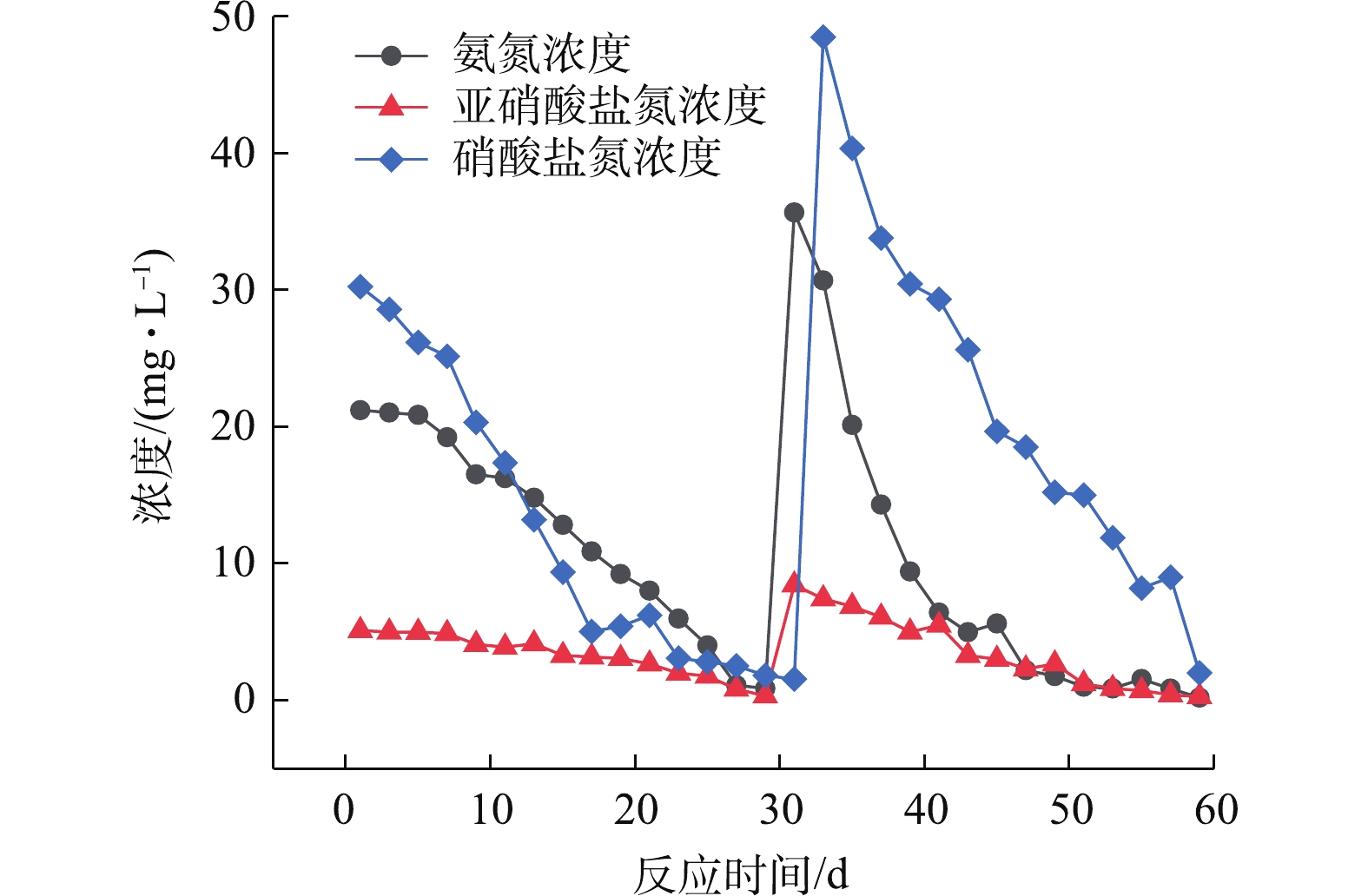
微生物经驯化培养,其数量级达到可投入使用的程度,再对其进行富集培养处理。图5分别为硝化菌在60 d内的培养中
NH+4 -N、NO−2 -N、NO−3 -N的变化情况。硝化菌驯化培养的1~5 d,NH+4 -N与NO−2 -N浓度变化不大,波动较小,表明硝化菌对新环境适应不足,与周海红等[30]的研究以及上文的纲水平、属水平变化相符。而NO−3 -N从第1天开始,呈现缓慢下降的趋势,第4天开始,曲线开始迅速下降,而后趋于动态的稳定趋势,表明反硝化菌在富集的同时消耗了大量的C6H12O6,并对NO−3 -N进行处理,致使NO−3 -N含量降低;在6~29 d,NH+4 -N的浓度开始降低,且下降加快,从初始的19.23 mg·L−1下降到0.8 mg·L−1,平均每天下降0.67 mg·L−1。NO−2 -N在实验前期也存在较长的缓冲时间,NO−3 -N在这个周期内的浓度降至1.01 mg·L−1,平均每天下降1.79 mg·L−1,在初始的30 d内,NO−2 -N的浓度从5.12 mg·L−1降至0.34 mg·L−1,平均每日降低0.31 mg·L−1。在第2个运行周期(31~60 d)内,硝化菌的活性随着环境的适应得到提升。第30天,加入适量(NH4)2SO4、NaNO2,使
NH+4 -N浓度提升至35.65 mg·L−1、NO−2 -N浓度达到8.45 mg·L−1。第31天,对反硝化菌培养液之中加入适量的KNO3、C6H12O6,提高培养液的NO−3 -N浓度至48.46 mg·L−1;在16~30 d,NO−3 -N浓度下降的速率加快,约为第1周期的2倍;在31~60 d,硝化菌驯化培养液,NH+4 -N的浓度总体呈现出下降速率增加的趋势,后趋于平稳。同第1运行周期(1~30 d)相比,第2运行周期NH+4 -N浓度下降的速率比第一运行周期增加近1倍,平均每天下降1.22 mg·L−1;NO−2 -N的浓度下降速率较第1个运行周期更快,平均每日下降浓度0.54 mg·L−1。在第1个运行周期内,由于对运行环境的适应性与对硝化菌生态因子调控的不足,使前期的
NH+4 -N与NO−2 -N去除效果不明显;在第2运行周期内,硝化菌的生态因子以及其耐受范围对于环境发生了适应性的变化,摄取了丰富的营养物质,加速了生长代谢,培养液中的NH+4 -N与NO−2 -N的消除速率也随之变快;反硝化菌在繁殖的同时,对与环境适应速度要优于硝化菌,且在第2周期的期末,NO−3 -N的浓度降低至2.01 mg·L−1,平均每天下降3.32 mg·L−1。结果表明,微生物驯化富集培养情况较为良好,与孟璇等[31]对微生物驯化富集的结果趋近,且要优于其近1个百分点。2.3 同属异构碳源条件下作用效果研究
微生物驯化富集培养后,按照图2的实验步骤,将硝化反硝化菌、玉米芯异构载体投入使用,并运行装置。玉米芯和玉米衣作为缓释碳源与载体,易于微生物的附着,应用于水处理领域可间接增大与水体接触面积、为微生物提供碳源,促进微生物的增殖,提高净化效率[32]。
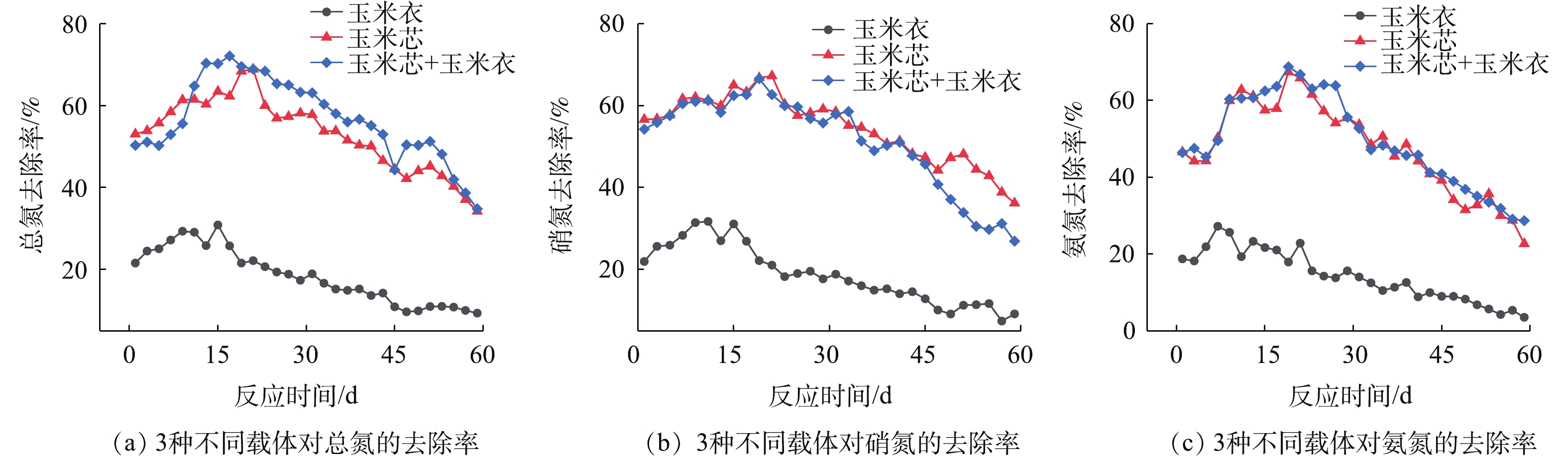
1)脱氮效果。在装置中置入玉米芯柱、玉米衣柱、玉米芯+玉米衣的组合(以下简称芯衣)3种不同组合的碳源载体来改变其来源与脱氮效果。在微生物脱氮运行时,玉米芯、玉米衣的碳源含量直接影响脱氮效率[33-34]。装置通过改变载体的方式来对低温海水暂养水进行处理。纵观3种碳源组合的处理效果可以发现,玉米芯对脱氮具有促进的效果。图6(a)表示TN的去除率,从初始阶段,玉米芯与芯衣对TN的去除具有良好效果,在17 d和21 d均达到最大值,分别为72.09%和68.88%。而玉米衣对TN的处理则处于一个较低的水平,维持在20%~30%,最高值为30.84%;图6(b)表示
NO−3 -N的去除率,前期与TN的处理效果较为接近,玉米芯和芯衣的NO−3 -N去除率最高分别可达66.56%和67.24%,而玉米衣对NO−3 -N的去除率也存在一个较低的水平,并在该低水平范围内波动,其最高值出现在17 d,为31.71%;图6(c)表示NH+4 -N的去除率,与TN、NO−3 -N的去除率相似,其最高值分别为68.76%(玉米芯)、67.38%(芯衣)和27.23%(玉米衣)。玉米芯自身构造特殊,其内外层分别形成厌氧/缺氧与好氧层,微生物附着后能够促进脱氮。本实验中,玉米芯表层附着的硝化菌消耗大量DO,致使玉米芯内部呈缺/厌氧环境,有利于反硝化菌的生长与繁殖;作为固相碳源,在水体中碳源含量较低时,玉米芯亦可作为固相碳源,释放一定量有机碳促进脱氮的进一步运行。装置运行期间总氮、硝氮与氨氮去除率分别达到(63.46±0.55)%、(65±0.63)%、(62.79±0.52)%。陈佼等[35]在利用玉米芯构造脱氮中将TN与
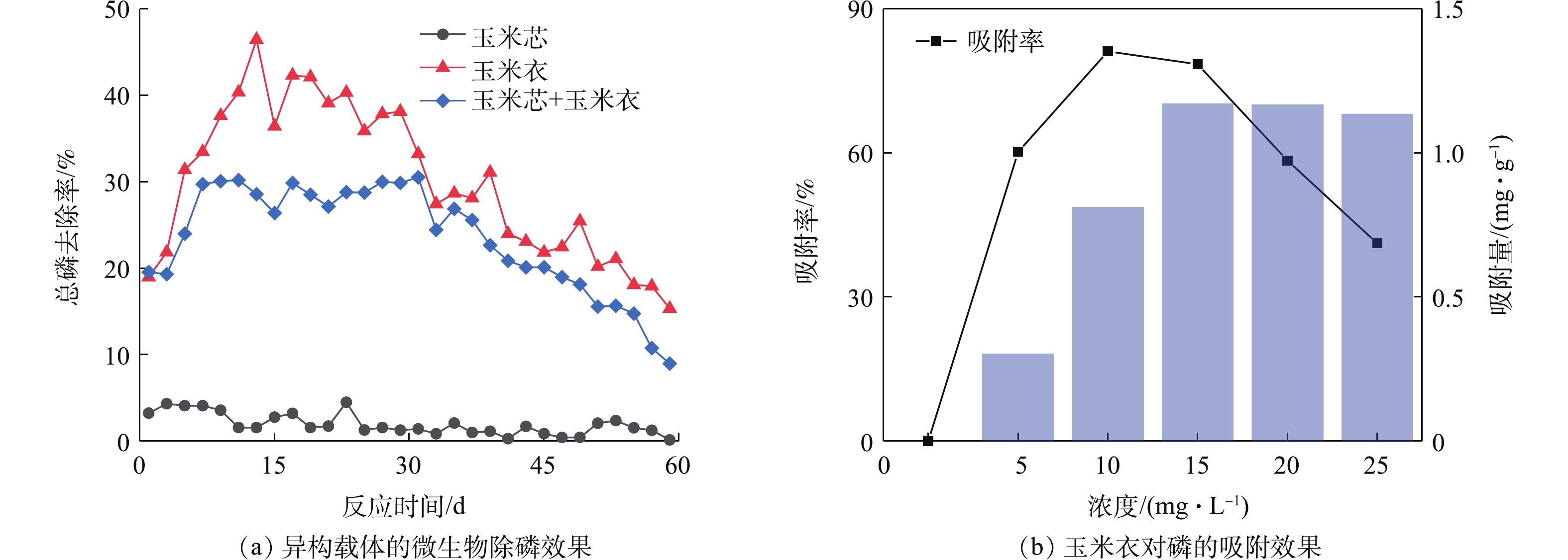
NO−3 -N的去除率提升较高,表明玉米芯作为碳源与载体的优越性,但其缺乏对NH+4 -N的去除率。2)除磷效果。在脱氮基础上,研究了不同碳源条件下,微生物对磷元素去除效果。构建装置(图2)。经60 d的周期实验,除磷效果如图7所示。对比玉米芯、玉米衣、芯衣在除磷效果方面的表现,玉米芯的除磷效果较差,最高仅达到4.49%,但玉米衣对除磷具有显著效果,高于芯衣,两者去除率分别为46.45%和30.51%,。说明玉米衣虽然脱氮效果不佳,但对磷元素的吸附和去除具有明显效果。针对玉米衣能够较好地去除磷元素这个特点,采用不同浓度的TP对其进行数据测定,磷元素吸附效果如图7所示。
随TP的浓度的增加,玉米衣对磷的吸附量呈先上升后缓降的趋势。当TP浓度达到10 mg·L−1时,玉米衣的吸附率达到最高值,为81.14%,但此时的吸附量仅为0.813 mg·g−1;当TP浓度达到15 mg·L−1时,玉米衣的吸附量达到最高为1.172 mg·g−1,吸附率为78.45 %,吸附率开始呈下降的态势,此时的吸附量趋于稳定。有研究[40]表明,在有限的玉米衣的量的情况下,其吸附量也有一个阈值,由实验结果可得,在TP的初始浓度在10 mg·L−1和15 mg·L−1时,玉米衣所呈现的效果较好,与唐婧等[36]所研究的磷吸附性能相吻合, 达到80%以上。
在对磷的去除情况下,玉米芯作为载体的实验组则表现出乏力的态势,玉米衣的除磷效果显著高于芯衣,去除率分别为46.45%和30.51%。实验表明,玉米芯对脱氮存在较好的效果,玉米衣则是对磷元素存在优良的去除效果,但囿于规模限制,结合实际微生物水处理性能,兼顾脱氮除磷除沫、载体材料回收利用及用量优化配比还有待研究。目前,用农业废弃物制备吸附剂是较热门的方向[37-39]。
除沫效果。在进行水处理的同时,水体中也会产生较多颗粒物,包括残饵、粪便、鱼体黏液和老化的生物絮体等,通常粒径分布在3~300 μm,而其中95%以上的颗粒物粒径小于20 μm,占颗粒物总质量的47%以上。养殖水中细微颗粒物在过滤时,气泡的细化和增加水力停留时间对颗粒物去除率有显著影响,特别是对粒径>50 μm的颗粒物。由表1可知,在水力停留时间为1.7 min的作用条件下,生物膜过滤对粒径为50~90 μm的颗粒物去除率相对较高,整个装置循环除沫率最高可达(82.14±0.23)%。
表 1 不同粒径颗粒与除沫、脱氮和除磷的效果Table 1. Effects of particle size on foam removal, nitrogen removal and phosphorus removal粒径/μm 除沫率% 脱氮率% 除磷率% <50 76.06±0.55 76.06±0.25 40.79±0.28 50~90 82.14±0.23 78.65±0.15 43.07±0.55 >90 79.36±0.15 76.34±0.55 40.05±0.17 3. 结论
1)微生物经富集培养后,利用高通量测序发现,其丰度、均匀度都有所提高。其中反硝化菌的多样性提升要优于硝化菌,且变形菌门(Proteobacteria)在适应、驯化、富集之中分别占据硝化菌96.40%、反硝化菌91.30%的丰度水平。
2)变形菌门(Proteobacteria)、拟杆菌门(Bacteroidetes)、螺旋菌门(Spirochaetae)、浮霉菌门(Planctomycetes)等此类污水脱氮菌,在低温高盐条件下具有较好的繁育与增殖能力,且微生物繁育程度越好,对有机物的降解速率越高。
3)微生物以玉米芯、玉米衣为载体和碳源,对TN去除率可达72.09%,并在此基础上,对除磷手段进行了拓展:玉米芯和玉米衣协同除磷,其去除率可达46.45%。为低温高盐暂养水处理提供了数据支撑。
4)本实验运用新型吸附剂材料玉米芯和玉米衣进行系统的脱氮除磷,为农用废弃物合理回收与再利用提供新的途径。但低温驯化富集培养的菌种在与异构碳源耦合后投入实验,其效果并非较为显著,可能是驯化、富集培养周期还不够长,还有待深入研究。
-
表 1 CN和Cu/CoCN(0.1)样品的比表面积和孔结构信息表
Table 1. Specific surface area and pore structure information of CN and Cu/CoCN (0.1) samples
催化剂 比表面积/(m2·g−1) 孔体积/(cm3·g−1) 孔径/nm CN 104.56 0.53 10.18 Cu/CoCN(0.1) 112.11 0.84 14.98 表 2 溶液中加入无机阴离子反应前后pH变化
Table 2. Changes in pH before and after the addition of inorganic anions to the solution
阴离子 加入阴离子后pH 氙灯/催化剂/过氧化氢 对照 4.85 5.05 Cl− 5.00 5.09 SO42- 5.02 5.07 HCO3− 8.38 9.04 NO3− 4.99 5.10 H2PO4− 4.63 4.68 -
[1] LI Y, CAO P, WANG S, et al. Research on the treatment mechanism of anthraquinone dye wastewater by algal-bacterial symbiotic system[J]. Bioresource Technology, 2022, 347: 126691. doi: 10.1016/j.biortech.2022.126691 [2] SONG Y, WANG L, QIANG X, et al. An overview of biological mechanisms and strategies for treating wastewater from printing and dyeing processes[J]. Journal of Water Process Engineering, 2023, 55: 104242. doi: 10.1016/j.jwpe.2023.104242 [3] PAN Z, SONG M, ZENG B, et al. Novel cetyltrimethylammonium bromide modified mixed adsorbent for efficient treatment of dyeing and printing wastewater[J]. Process Safety and Environmental Protection, 2023, 176: 560-567. doi: 10.1016/j.psep.2023.06.003 [4] TU Y, SHAO G, ZHANG W, et al. The degradation of printing and dyeing wastewater by manganese-based catalysts[J]. Science of the Total Environment, 2022, 828: 154390. doi: 10.1016/j.scitotenv.2022.154390 [5] LU X, WANG H, CHEN J, et al. Negatively charged hollow crosslinked aromatic polymer fiber membrane for high-efficiency removal of cationic dyes in wastewater[J]. Chemical Engineering Journal, 2022, 433: 133650. doi: 10.1016/j.cej.2021.133650 [6] LIU X, CHEN Z, DU W, et al. Treatment of wastewater containing methyl orange dye by fluidized three dimensional electrochemical oxidation process integrated with chemical oxidation and adsorption[J]. Journal of Environmental Management, 2022, 311: 114775. doi: 10.1016/j.jenvman.2022.114775 [7] YANG L, REN X, ZHANG Y, et al. One-step synthesis of a heterogeneous catalyst: Cu+-decorated triazine-based g-C3N4 nanosheet formation and catalytic mechanism[J]. Journal of Environmental Chemical Engineering, 2021, 9(4): 105558. doi: 10.1016/j.jece.2021.105558 [8] FANG G, DENG Y, HUANG M, et al. A mechanistic understanding of hydrogen peroxide decomposition by vanadium minerals for diethyl phthalate degradation[J]. Environmental Science & Technology, 2018, 52(4): 2178-2185. [9] HUANG H, JIANG L, YANG J, et al. Synthesis and modification of ultrathin g-C3N4 for photocatalytic energy and environmental applications[J]. Renewable and Sustainable Energy Reviews, 2023, 173: 113110. doi: 10.1016/j.rser.2022.113110 [10] WANG W, ZHOU C, YANG Y, et al. Carbon nitride based photocatalysts for solar photocatalytic disinfection, can we go further?[J]. Chemical Engineering Journal, 2021, 404: 126540. doi: 10.1016/j.cej.2020.126540 [11] LI J, HUANG J, ZENG G, et al. Efficient photosynthesis of H2O2 via two-electron oxygen reduction reaction by defective g-C3N4 with terminal cyano groups and nitrogen vacancies[J]. Chemical Engineering Journal, 2023, 463: 142512. doi: 10.1016/j.cej.2023.142512 [12] WANG Y, HE Y, CHI Y, et al. Construction of S-scheme pn heterojunction between protonated g-C3N4 and α-MnS nanosphere for photocatalytic H2O2 production and in situ degradation of oxytetracycline[J]. Journal of Environmental Chemical Engineering, 2023, 11(3): 109968. doi: 10.1016/j.jece.2023.109968 [13] SONG T, ZHANG X, MATRAS-POSTOLEK K, et al. Cobalt clusters on g-C3N4 nanosheets for enhanced H2/H2O2 generation and NO removal[J]. Journal of Environmental Chemical Engineering, 2022, 10(6): 108747. doi: 10.1016/j.jece.2022.108747 [14] JIANG Y, LIU Q, TAN K M, et al. Insights into mechanisms, kinetics and pathway of continuous visible-light photodegradation of PPCPs via porous g-C3N4 with highly dispersed Fe (III) active sites[J]. Chemical Engineering Journal, 2021, 423: 130095. doi: 10.1016/j.cej.2021.130095 [15] MANFRED G, EBERHARDT G, RAMIREZ E. Does the reaction of Cu+ with H2O2 give OH radicals: A study of aromatic hydroxylation[J]. Journal of Organic Chemistry, 1989, 54: 5922-5926. doi: 10.1021/jo00286a024 [16] LI X, GAN X. Photo-Fenton degradation of multiple pharmaceuticals at low concentrations via Cu-doped-graphitic carbon nitride (g-C3N4) under simulated solar irradiation at a wide pH range[J]. Journal of Environmental Chemical Engineering, 2022, 10(5): 108290. doi: 10.1016/j.jece.2022.108290 [17] NING S, XU H, QI Y, et al. Microstructure induced thermodynamic and kinetic modulation to enhance CO2 photothermal reduction: a case of atomic-scale dispersed Co–N species anchored Co@C hybrid[J]. ACS Catalysis, 2020, 10(8): 4726-4736. doi: 10.1021/acscatal.9b04963 [18] PALANIVEL B, HOSSAIN M S, REDDY I N, et al. Chemical oxidants (H2O2 and persulfate) activated photo-Fenton like degradation reaction using sol-gel derived g-C3N4/ZnCo2O4 nanocomposite[J]. Diamond and Related Materials, 2022, 130: 109413. doi: 10.1016/j.diamond.2022.109413 [19] JEGHAN S M N, DO J Y, KANG M. Fabrication of flower-like copper cobaltite/graphitic-carbon nitride (CuCo2O4/g-C3N4) composite with superior photocatalytic activity[J]. Journal of Industrial and Engineering Chemistry, 2018, 57: 405-415. doi: 10.1016/j.jiec.2017.08.049 [20] DONG Q, CHEN Y, WANG L, et al. Cu-modified alkalinized g-C3N4 as photocatalytically assisted heterogeneous Fenton-like catalyst[J]. Applied Surface Science, 2017, 426: 1133-1140. doi: 10.1016/j.apsusc.2017.07.254 [21] REN X, ZHANG Y, YANG L, et al. Degradation of ofloxacin by peroxymonosulfate activated with cobalt-doped graphitic carbon nitride: Mechanism and performance[J]. Inorganic Chemistry Communications, 2021, 133: 108863. doi: 10.1016/j.inoche.2021.108863 [22] CAO J, NIE W, HUANG L, et al. Photocatalytic activation of sulfite by nitrogen vacancy modified graphitic carbon nitride for efficient degradation of carbamazepine[J]. Applied Catalysis B:Environmental, 2019, 241: 18-27. doi: 10.1016/j.apcatb.2018.09.007 [23] GUAN C, JIANG J, PANG S, et al. Facile synthesis of pure g-C3N4 materials for peroxymonosulfate activation to degrade bisphenol A: Effects of precursors and annealing ambience on catalytic oxidation[J]. Chemical Engineering Journal, 2020, 387: 123726. doi: 10.1016/j.cej.2019.123726 [24] FENG Y, LIAO C, KONG L, et al. Facile synthesis of highly reactive and stable Fe-doped g-C3N4 composites for peroxymonosulfate activation: A novel nonradical oxidation process[J]. Journal of Hazardous Materials, 2018, 354: 63-71. doi: 10.1016/j.jhazmat.2018.04.056 [25] YANG D, JIANG T, WU T, et al. Highly selective oxidation of cyclohexene to 2-cyclohexene-1-one in water using molecular oxygen over Fe–Co–g-C3N4[J]. Catalysis Science & Technology, 2016, 6(1): 193-200. [26] DING Z, CHEN X, ANTONIETTI M, et al. Synthesis of transition metal‐modified carbon nitride polymers for selective hydrocarbon oxidation[J]. ChemSusChem, 2011, 4(2): 274-281. doi: 10.1002/cssc.201000149 [27] WANG H, BIAN Y, HU J, et al. Highly crystalline sulfur-doped carbon nitride as photocatalyst for efficient visible-light hydrogen generation[J]. Applied Catalysis B:Environmental, 2018, 238: 592-598. doi: 10.1016/j.apcatb.2018.07.023 [28] ZHANG L, DING N, HASHIMOTO M, et al. Sodium-doped carbon nitride nanotubes for efficient visible light-driven hydrogen production[J]. Nano Research, 2018, 11: 2295-2309. doi: 10.1007/s12274-017-1853-3 [29] ZHANG S, HU C, JI H, et al. Facile synthesis of nitrogen-deficient mesoporous graphitic carbon nitride for highly efficient photocatalytic performance[J]. Applied Surface Science, 2019, 478: 304-312. doi: 10.1016/j.apsusc.2019.01.270 [30] YAN W, YAN L, JING C. Impact of doped metals on urea-derived g-C3N4 for photocatalytic degradation of antibiotics: Structure, photoactivity and degradation mechanisms[J]. Applied Catalysis B:Environmental, 2019, 244: 475-485. doi: 10.1016/j.apcatb.2018.11.069 [31] DONG X, DUAN X, SUN Z, et al. Natural illite-based ultrafine cobalt oxide with abundant oxygen-vacancies for highly efficient Fenton-like catalysis[J]. Applied Catalysis B:Environmental, 2020, 261: 118214. doi: 10.1016/j.apcatb.2019.118214 [32] HAO R, WANG G, TANG H, et al. Template-free preparation of macro/mesoporous g-C3N4/TiO2 heterojunction photocatalysts with enhanced visible light photocatalytic activity[J]. Applied Catalysis B:Environmental, 2016, 187: 47-58. doi: 10.1016/j.apcatb.2016.01.026 [33] ZHANG H, TAN H-R, JAENICKE S, et al. Highly efficient and robust Cu catalyst for non-oxidative dehydrogenation of ethanol to acetaldehyde and hydrogen[J]. Journal of catalysis, 2020, 389: 19-28. doi: 10.1016/j.jcat.2020.05.018 [34] FANG M, XIA W, YAO T, et al. Boosting CO2 electroreduction to multi‐carbon products via oxygen‐rich vacancies and Ce4+‐O2‐‐Cu+ structure in Cu/CeO2 for Stabilizing Cu+[J]. ChemCatChem: e202301266. [35] GAO Y, YU G, LIU K, et al. Integrated adsorption and visible-light photodegradation of aqueous clofibric acid and carbamazepine by a Fe-based metal-organic framework[J]. Chemical Engineering Journal, 2017, 330: 157-165. doi: 10.1016/j.cej.2017.06.139 [36] DUNPHY GUZMAN K A, FINNEGAN M P, BANFIELD J F. Influence of surface potential on aggregation and transport of titania nanoparticles[J]. Environmental Science & Technology, 2006, 40(24): 7688-7693. [37] LI Y, CHEN M Y, LU B A, et al. Unravelling the role of hydrogen peroxide in pH-dependent ORR performance of Mn-NC catalysts[J]. Applied Catalysis B:Environmental, 2024, 342: 123458. doi: 10.1016/j.apcatb.2023.123458 [38] RAMASWAMY N, MUKERJEE S. Influence of inner-and outer-sphere electron transfer mechanisms during electrocatalysis of oxygen reduction in alkaline media[J]. The Journal of Physical Chemistry C, 2011, 115(36): 18015-18026. doi: 10.1021/jp204680p [39] HU L, ZHANG G, LIU M, et al. Enhanced degradation of Bisphenol A (BPA) by peroxymonosulfate with Co3O4-Bi2O3 catalyst activation: Effects of pH, inorganic anions, and water matrix[J]. Chemical Engineering Journal, 2018, 338: 300-310. doi: 10.1016/j.cej.2018.01.016 [40] SUI C, NIE Z, LIU H, et al. Singlet oxygen-dominated peroxymonosulfate activation by layered crednerite for organic pollutants degradation in high salinity wastewater[J]. Journal of Environmental Sciences, 2024, 135: 86-96. doi: 10.1016/j.jes.2023.01.010 [41] HUI W, DENG X, ZHU Y, et al. Insight for FeS2/MoS2@SiO2 nanoreactor with spatial separation of H2O2 activation sites and pollutant adsorption sites: Enhanced H2O2 activation efficiency and pollutant degradation performance in Fenton reaction[J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2023, 678: 132496. doi: 10.1016/j.colsurfa.2023.132496 [42] DING Y, ZHU L, WANG N, et al. Sulfate radicals induced degradation of tetrabromobisphenol A with nanoscaled magnetic CuFe2O4 as a heterogeneous catalyst of peroxymonosulfate[J]. Applied Catalysis B:Environmental, 2013, 129: 153-62. doi: 10.1016/j.apcatb.2012.09.015 -






 下载:
下载: