-
镉(cadmium)是一种高毒性的重金属元素,即使在低质量浓度下也会对水体生态环境健康构成威胁。陈荣钦[1]对沘江上游的镉元素含量进行了检测,发现镉的最大含量高达0.03 mg·L−1,其中28件检查样品中含有超标的Cd和Pb。镉还会通过食物链进入人体,对肾脏、骨骼、呼吸系统和免疫系统产生有害影响[2-4]。镉在人体内的生物半衰期长达10~30 a,可蓄积50 a之久[5],因此寻求高效解决水体镉污染问题的技术方法迫在眉睫。目前常用的方法主要包括化学沉淀法、离子交换法、膜过滤、生物法和吸附法[6]。其中,吸附法因其高效、简单操作、且相对低成本等优点而广泛受到关注。
生物炭因其发达的孔隙结构、丰富的表面官能团和独特的表面电子供受能力,而具有良好的重金属吸附能力[7-9]。姜禹奇等[10]在500 ℃下利用玉米秸秆制备的生物炭,其镉去除率可达64.43%。戴亮等[11]制备的污泥生物炭对水中Cd2+的最大吸附容量为27.27 mg·g−1,而CHEN等[12]制备的莲子壳生物炭在300 ℃和600 ℃下对溶液中Cd2+的吸附量分别为31.69 mg·g−1和51.18 mg·g−1。纳米零价铁(nanoscale zero-valent iron,nZVI)是一种新型吸附材料。由于其高比表面积、优异的吸附和反应性,被广泛应用于镉的去除。黄园英等[13]的研究表明,当纳米铁颗粒的投加量为1g·L−1,Cd2+的初始质量浓度为74.51 mg·L−1时,在24 h内,镉的去除率可达98.62%。尽管纳米零价铁在环境修复中表现出卓越的效果,但在实际应用中仍存在一些不足之处。纳米零价铁颗粒容易发生团聚,导致活性位点减少[14-15]。因此,研究人员利用生物炭负载纳米零价铁的方法来提高其去除污染物的能力。许亚琼等[16]制备的nZVI改性生物炭(nZVI-BC)对Cd2+的饱和吸附量达125.5 mg·g−1,为原始生物炭的5.3倍。张磊[17]制备的KMnO4改性磁性生物炭(KMBC)对Cd2+的最大吸附量为53.9 mg·g−1。可以看出,改性后的生物炭对Cd2+的吸附量均有提升。
常规制备生物炭负载零价铁的方法分为2步,先制备生物炭,再将生物炭分散在铁离子溶液中,通过NaBH4还原法制备生物炭负载零价铁,操作流程较复杂,NaBH4价格较高且有毒,NaBH4的反应速率极快,容易导致团聚和不规则颗粒[18]。因此,该方法不是环保有效的方法。
碳热法一步制备生物炭负载零价铁具有一定的研究前景。碳热法以无机碳作为还原剂,在高温/惰性气体保护下将Fe2+/Fe3+还原成Fe0。戴亮等[11]发现,碳热法制备的铁碳材料比还原法制备的铁碳材料具有更好的稳定性和还原性。孙国帅[19]利用碳热法制备的纳米零价铁(NZVI)对Cd2+的最大吸附量为87.715 mg·g−1。然而,碳热法一步制备的生物炭负载零价铁对水溶液中Cd2+的吸附效能与机制尚不清楚。因此,本研究拟通过共热解树叶粉末和少量Fe3O4,采用简易的高温碳还原一步法合成生物炭负载零价铁材料(FeNPs@BC),考察了FeNPs@BC对Cd2+的吸附性能,并采用XRD、SEM、FTIR等表征方法分析了FeNPs@BC对Cd2+的吸附机理。
-
实验所用原料树林落叶收集于东北农业大学,清洗后风干、粉碎并过100目筛备用。四氧化三铁(Fe3O4)购于天津博迪化工有限公司;氯化镉购于天津博迪化工有限公司;氢氧化钠购于天津凯通化学试剂有限公司;盐酸购于天津市富宇精细化工有限公司。
-
将树叶粉末与Fe3O4混合,混合的质量比例分别为5∶1、10∶1、15∶1、20∶1、25∶1和30∶1。将混合物转移到陶瓷方舟上,并压平,方舟置于管式炉的中心位置。在进行反应加热之前,使用氩气对反应器进行吹扫,持续时间为30 min以去除管式炉中的水分和氧气。氩气的流速为150 mL·min−1。随后,保持氩气流速不变,设定加热程序,加热速率为20 ℃·min−1,40 min后达到共热解的目标温度800 ℃,并保持该温度持续热解1 h。反应完成后,将管式炉冷却至室温,并将产物转移到棕色小瓶中,然后密封储存备用。此外,此外,还在相同的条件下使用相同的方法制备了单独的Fe3O4和BC,并将其储存备用,标记为热处理后的Fe3O4,单独落叶粉末制备的生物炭记为BC。在合成完成后,将产物用去离子水洗涤至中性,然后进行冷冻干燥,并通过100目筛筛选,最后将它们储存在棕色玻璃瓶中,以备后续实验使用。
-
采用扫描电子显微镜 (SEM)耦合X 射线能谱仪(EDS)观察 FeNPs@BC吸附Cd2+前后的微观表面,并分析其表面的元素组成; 应用 X 射线衍射(XRD)分析FeNPs@BC的晶体结构与组分;利用傅里叶变换红外光谱(FT-IR)表征吸附材料表面官能团的变化。
-
实验所需Cd2+溶液均由氯化镉与去离子水配置而成,溶液质量浓度为50 mg·L−1 ,吸附实验在 150 mL 锥形瓶中完成。加入一定量吸附剂开始吸附实验,在 25 ℃、150 r·min−1的条件下进行吸附,在15、30、45、60、120、180、240、360 min分别取点,溶液通过 0.45 μm 微孔膜过滤,滤液采用原子吸收光谱法测定残留Cd2+质量浓度 。考察溶液初始酸碱度影响:pH设置 为2.0、3.0、4.0、5.0、6.0;考察吸附剂投加量影响,吸附剂添加量设置为10、20、40、60、80 mg;探究50 mg·L−1 Mg2+、Cu2+、Pb2+共存对 Cd2+吸附量的影响;可重复性实验:吸附平衡后,将吸附Cd2+后的材料在纸过滤器中过滤,并在 80 °C 下完全干燥,进行4次吸附-再生循环实验。所有处理设置3个平行实验。
选择拟一阶(式(1))[20]、拟二阶(式(2))[21]、内扩散动力学模型(式(3))[22]、Banghum模型(式(4))[23]和Elovich模型(式(5))[23]对吸附动力学数据进行拟合。
式中:t为吸附时间,min;Qt和Qe分别代表吸附时间为t和吸附平衡时的吸附量,mg·g−1;k1为拟一级反应速率常数,min−1;k2为拟二级反应速率常数,g·(mg·min)−1;ki为粒子内扩散速率常数, mg·g−1·min−0.5;P为与边界层厚度相关的常数。Kb为吸附速率常数, mg·(g·min)−1;α 为初始吸附速率常数,mg·(g·min)−1;β 为解吸速率常数, mg·(g·min)−1。通过以lnQe-Qt对t作图,k1和Qe的值分别对应为斜率和截距;k2和Qe可以由t/Qt对t作图,通过拟合曲线的截距和斜率计算所得,α和β可以通过Qt对lnt作图,α和β分别对应斜率和截距。
吸附等温线Cd2+溶液分别设置为50、100、200、300、400、500 mg·L−1,实验分别在 298.15、308.15和 318.15 K 下进行,采用Langmuir(式(4))[24]、Freundlich(式(5))[25]和Temkin(式(6))[26]这3种吸附等温线模型对实验所得数据进行拟合。
式中:Ce为吸附平衡时溶液中CV的浓度;Qm为饱和吸附量,mg·g−1;KL为吸附系数,L·mg−1;KF和n均为常数,n>1时,吸附为易吸附过程;Kt和b均为常数。以Ce/Qe对Ce作图,Qm和 KL为的值分别对应为截距和斜率;KF和n的值可以通过lnQe对lnCe作图,通过拟合曲线的截距和斜率计算所得。
-
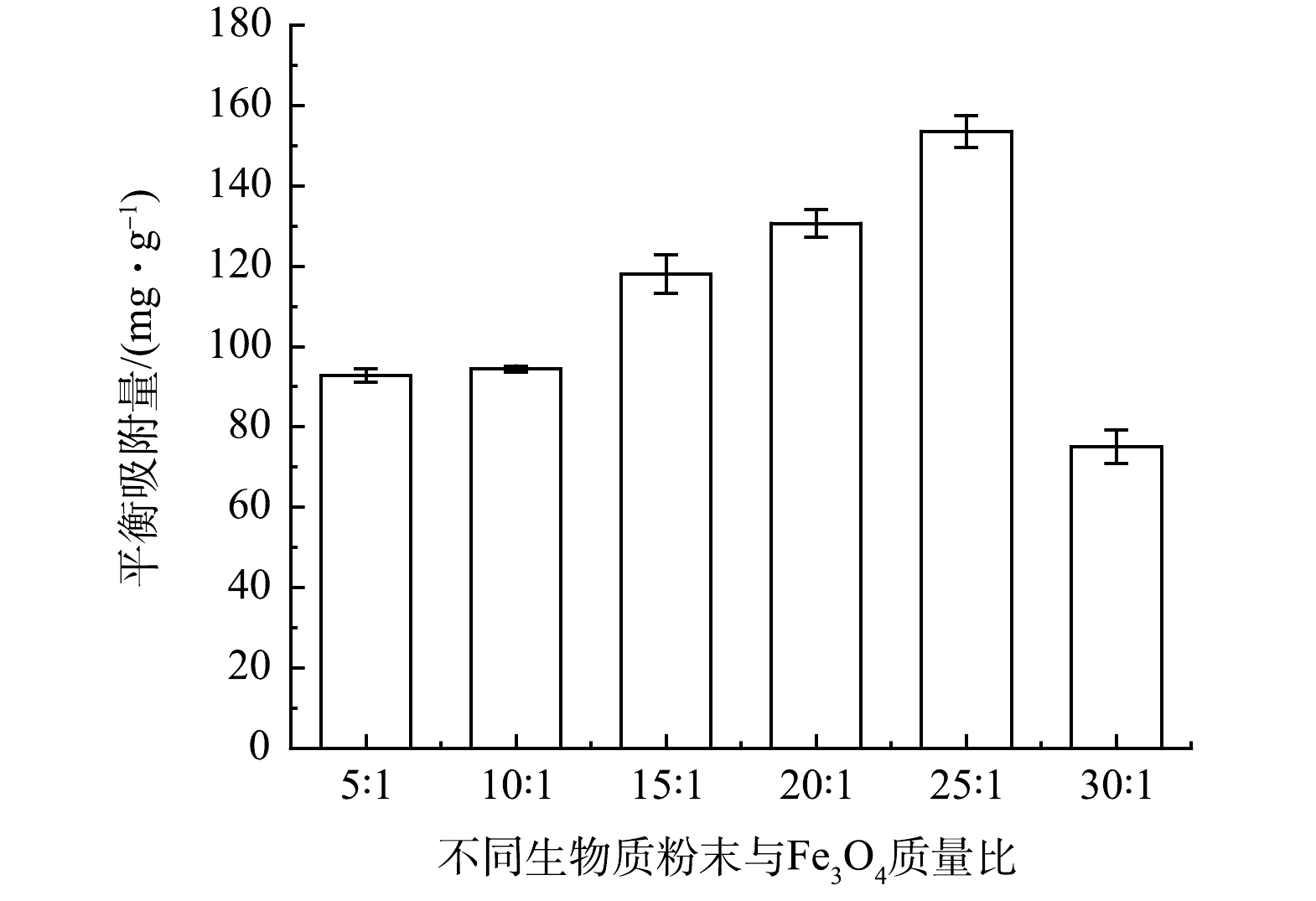
1)探究最佳吸附效果的原料比例。由图1可见,通过比较不同质量比例下生物质粉末与Fe3O4混合制备的FeNPs@BC材料对水溶液中Cd2+的吸附效果,发现当生物质与Fe3O4的比例为25∶1,FeNPs@BC材料表现出最佳吸附性能,可达到157.64 mg·g−1的吸附能力。因此,选择以25∶1的比例制备FeNPs@BC材料作为代表性吸附剂。
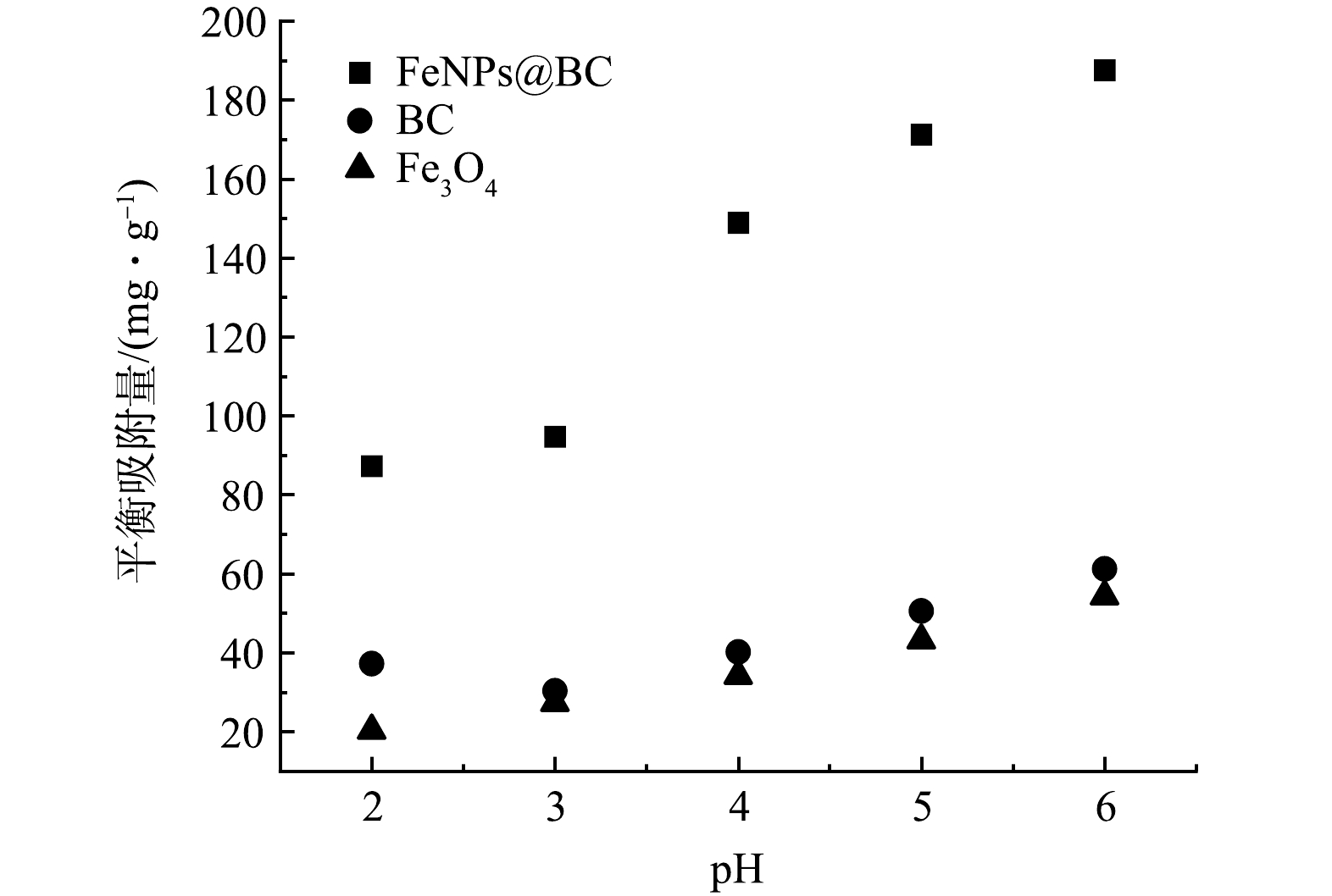
2)溶液的酸碱度对吸附效果的影响。溶液的初始酸碱度是影响重金属吸附过程的关键因素[27-28]。由图2可见,当溶液的 pH在 2~6时,FeNPs@BC 材料的吸附量随着 pH的升高而增加。在 pH从 2.0 上升到 6.0 的过程中,Cd2+ 的吸附量迅速增加,由 87.274 mg·g−1 增加到 158.6 mg·g−1。因此,强酸性条件不利于FeNPs@BC 对Cd2+ 的吸附,因为大量的 H+ 与 Fe0 反应,导致铁吸附剂失去活性[27]。相比之下,单独高温热解制备的 BC 和 Fe3O4 对环境酸碱度的影响主要体现在镉本身的形态上,对这2种吸附剂的吸附容量影响较小,其对溶液中的 Cd2+ 的吸附量基本维持在 40 mg·g−1 左右。总之,FeNPs@BC 材料在弱酸性环境下表现出较强的吸附能力,对 Cd2+ 的吸附效果显著,而酸碱性条件对 FeNPs@BC的吸附性能至关重要,强酸性环境会降低其吸附能力[29]。
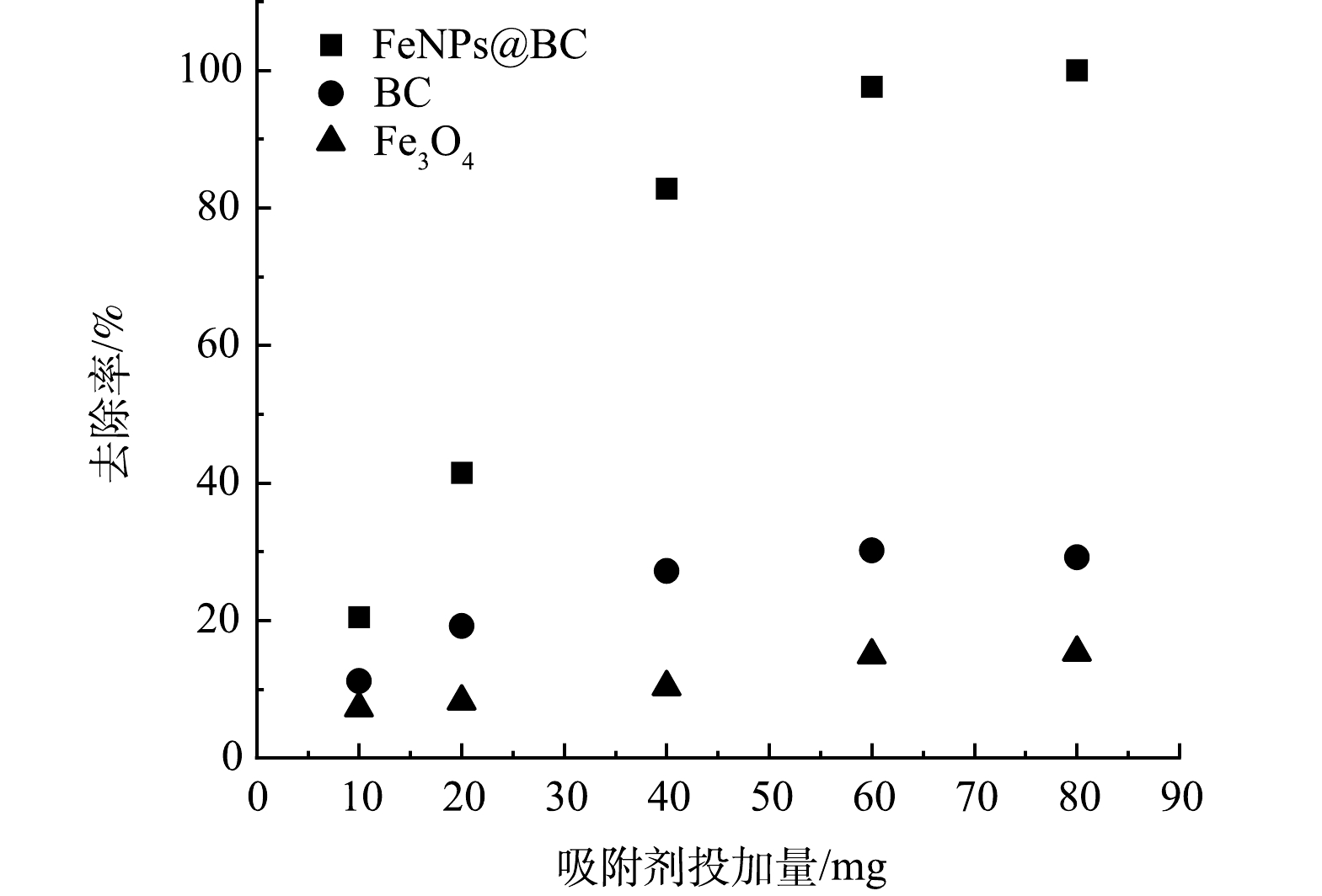
3) 吸附剂投加量对吸附效果的影响。如图3所示,吸附剂的投加量对Cd2+的去除率有一定的影响。当投加量小于50 mg时,随着FeNPs@BC投加量的增加,水溶液中 Cd2+ 的去除率迅速上升;而当投加量超过60 mg时,随着 FeNPs@BC 投加量的增加,Cd2+ 的去除率增加趋势逐渐减缓,相比之下,对于 BC 和 Fe3O4 材料,这种现象并不明显。这可能是因为 BC和 Fe3O4 对 Cd2+ 的吸附能力有限。
-
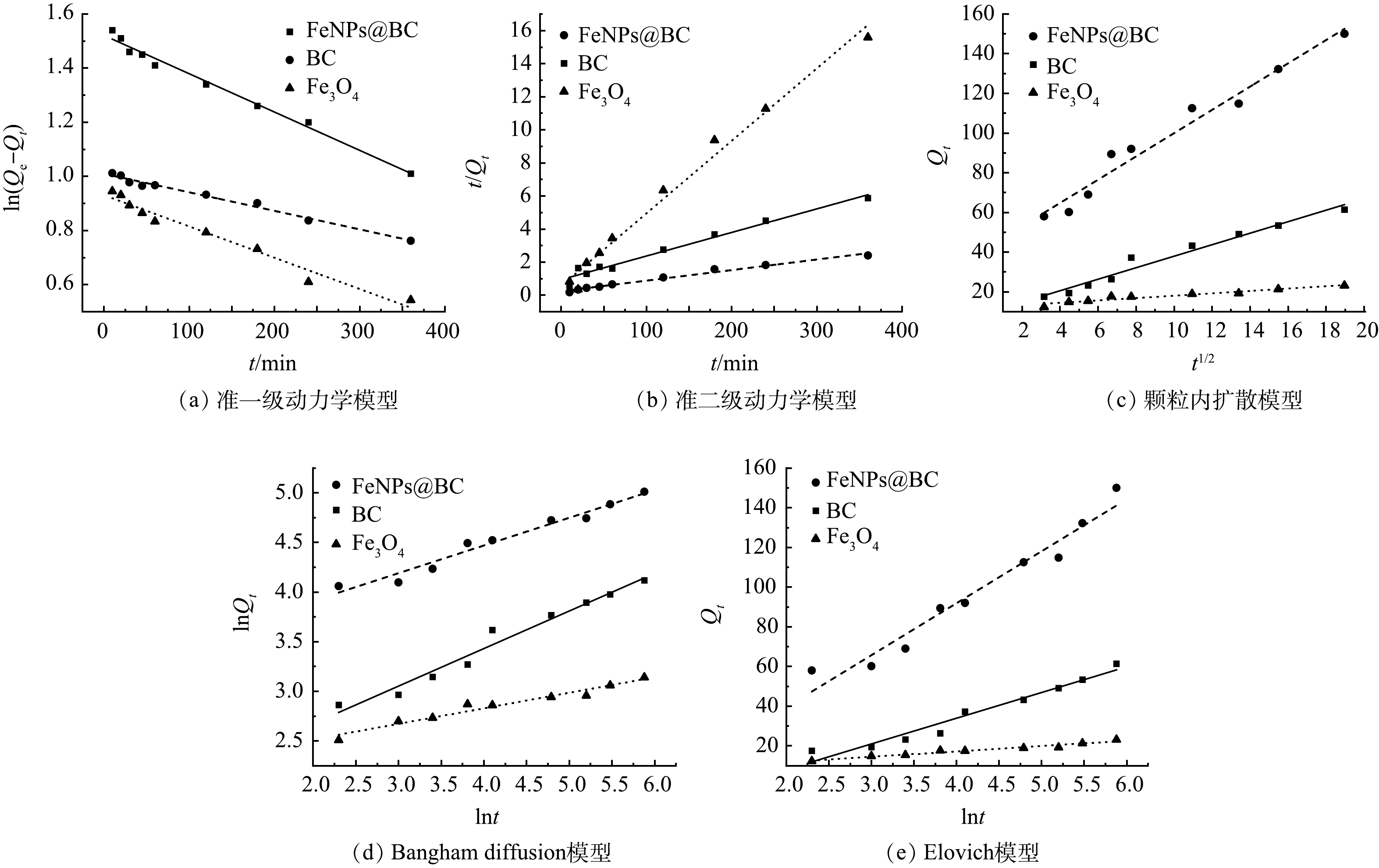
1)吸附动力学。使用准一级和准二级动力学模型(图4(a)~(b))对吸附动力学数据进行拟合,拟合参数见表1。对于Cd2+吸附,FeNPs@BC 和 Fe3O4 的准二级动力学模型在 Akaike 信息准则(AIC)上均低于准一级动力学模型。这表明准二级动力学模型更准确地描述了吸附过程,说明FeNPs@BC 和 Fe3O4 对 Cd2+ 的吸附主要是通过化学吸附进行的。同时,吸附动力学曲线表明FeNPs@BC对Cd2+ 的吸附能力高于BC 和 Fe3O4的吸附能力,表明了 FeNPs@BC 在 Cd2+ 吸附中的卓越性能。
颗粒内扩散模型的结果(图4(c)和表1)揭示了FeNPs@BC、BC 和 Fe3O4 对 Cd2+ 吸附与分子内扩散密切相关,这表现为相对较低的 P值,同时所有 R2 值均高于 0.92。在该模型中,P 值用于表示内扩散速率常数,较大的 P 值意味着吸附剂对 Cd2+ 的吸附更容易[13]。与 FeNPs@BC 和 Fe3O4 相比,BC 在 Cd2+ 吸附过程中的颗粒内扩散模型和 Bangham 扩散模型的 AIC 值最低,这表明 BC 在整个吸附过程中起着关键作用[30]。此外,这3个曲线均未经过原点,表明内扩散过程仅构成吸附过程的一部分,而非唯一的步骤。
此外,本研究还使用Elovich 模型对上述3种吸附剂的吸附数据进行了拟合。如图4(e) 和表1所示,Elovich 模型中的斜率越大,表示反应速率越快,而截距越大,表明吸附材料的扫气能力越强。结果显示,FeNPs@BC 的吸附强度最高,而 Fe3O4 的吸附速率最快。另外,根据 Bangham 扩散模型的理论,如图4(d) 和表1 所示,R2 值均小于 0.99,这表明实际的吸附情况无法通过通道扩散来完全描述。因此,采用 Elovich 模型对上述3种吸附剂的吸附数据进行了拟合。
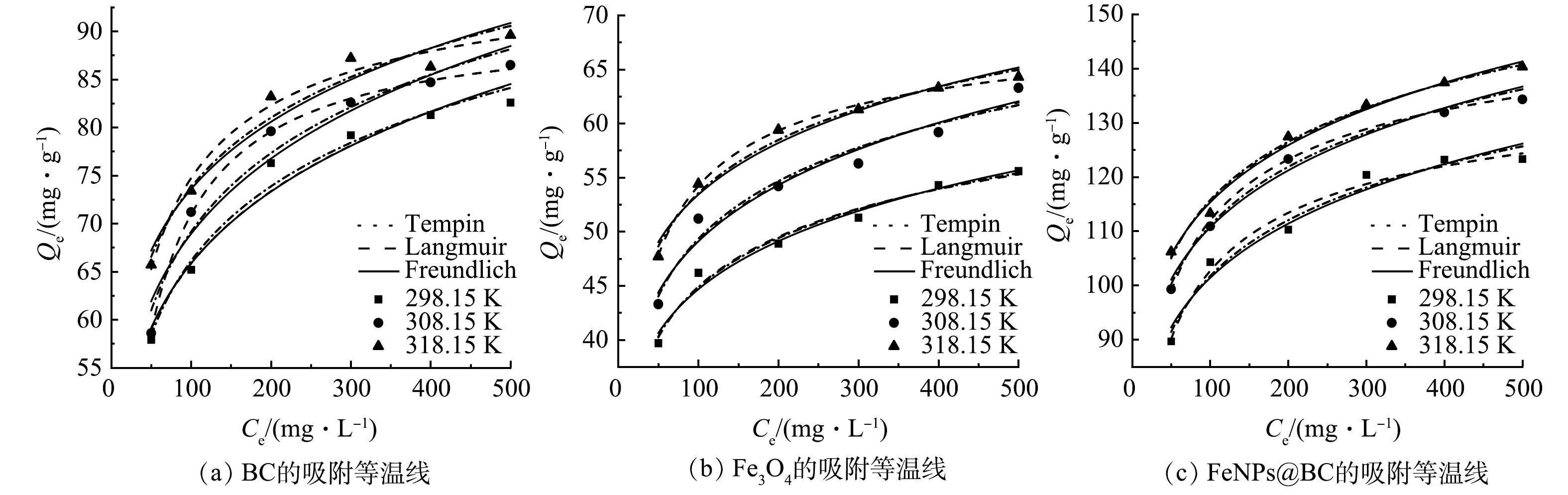
吸附等温线。为深入了解FeNPs@BC、BC和 Fe3O4 对 Cd2+ 吸附特性的影响,研究了初始质量浓度对吸附量的影响,采用了3种典型的吸附等温模型,包括 Freundlich 模型、Langmuir 模型和 Temkin 模型对吸附数据进行拟合。表2 列示了拟合得到的相关参数。图5(a)、图5(b) 和图5(c) 展示了在3个等温模型下,不同吸附剂的吸附容量(Qe,mg·g−1)与平衡溶液中溶质质量浓度(Ce,mg·L−1)之间的关系。值得注意的是,随着温度的升高,3种吸附剂对 Cd2+ 的吸附量增加。由表2 中可以看出,与 Temkin 模型和 Freundlich 模型相比,Langmuir 方程对不同温度下 FeNPs@BC 和 BC 吸附 Cd2+ 的过程具有更高的拟合度。这表明结合位点可以均匀分布在吸附剂表面,吸附过程为单层吸附。FeNPs@BC 材料的最大吸附量计算为145.208 mg·g−1(室温下)。此外,通过不同温度下的吸附 KL 值,可以得出 Cd2+ 在 FeNPs@BC 材料和 BC 表面上的吸附相对容易。与 Temkin 模型和 Langmuir 模型相比,Fe3O4 对 Cd2+ 的吸附更符合 Freundlich 模型,表明吸附过程伴随着多分子层吸附的发生。此外,0<1/n<1 表明Fe3O4 表面有利于Cd2+ 的吸附。
-
1)材料形貌及元素分析。FeNPs@BC吸附Cd2+后的表面形貌以及元素分析结果如图6所示。由图6(a)可以观察到BC表面光滑且含有天然大孔结构,这能为铁纳米颗粒提供更多的附着位点。FeNPs@BC材料表面有小颗粒分布,主要分布在BC的表面和空腔结构中(图6(b)~(c))。由图6(d)可以看出,吸附后的FeNPs@BC表面出现无规则絮状物。这可能是因为Cd已经吸附到材料的表面[31]。
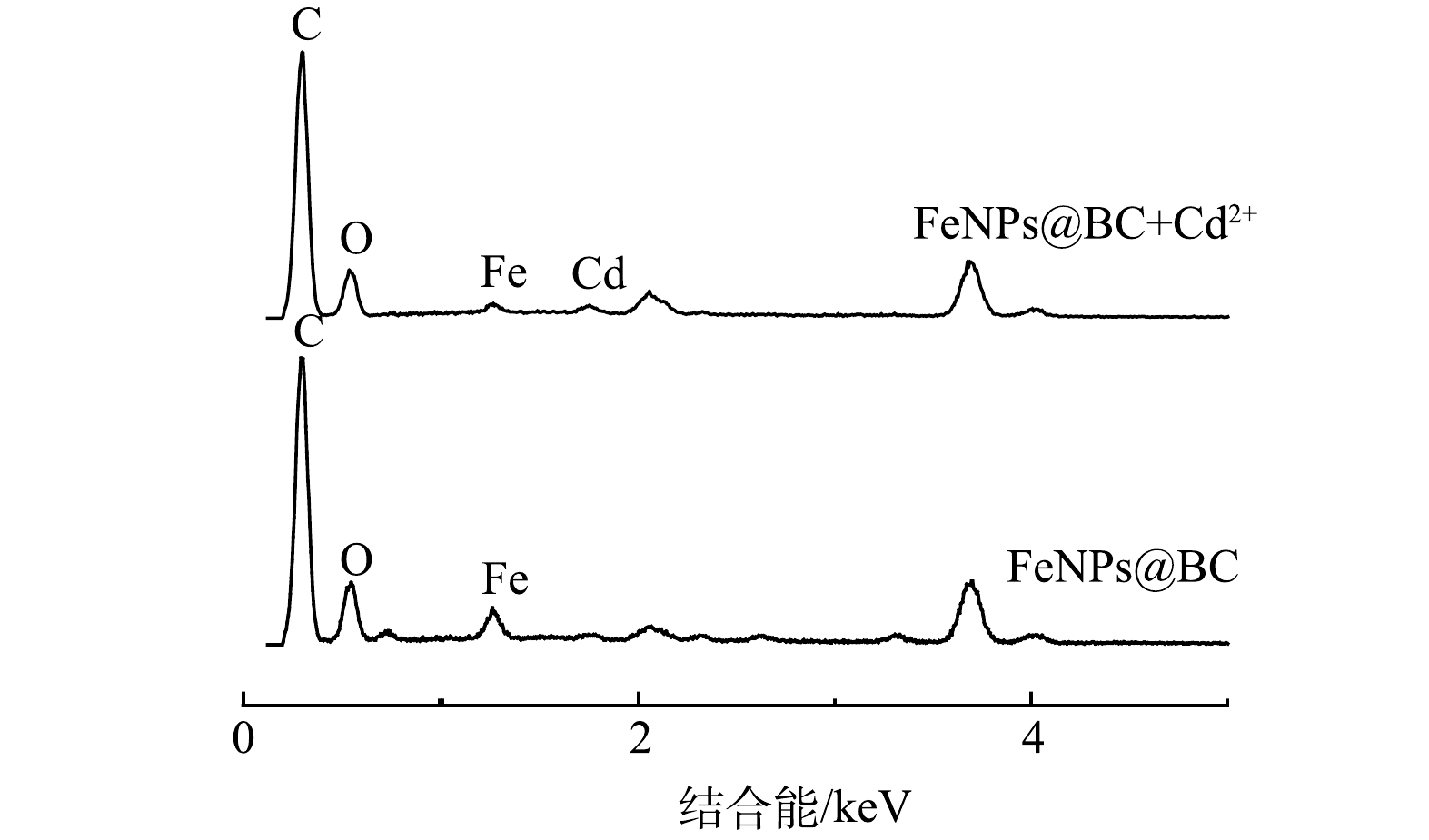
Cd2+吸收前的 FeNPs@BC 材料的主要表面元素如图7 所示。可见,材料表面主要有 C、O和Fe,对应的占比分别为 64.03%、25.87%和 10.10%。吸收 Cd2+后的 FeNPs@BC 材料的主要表面元素为 C、O、Fe 和 Cd,对应的占比分别为66.01%、31.45%、1.02%和 1.51%。这表明Cd被成功吸附。
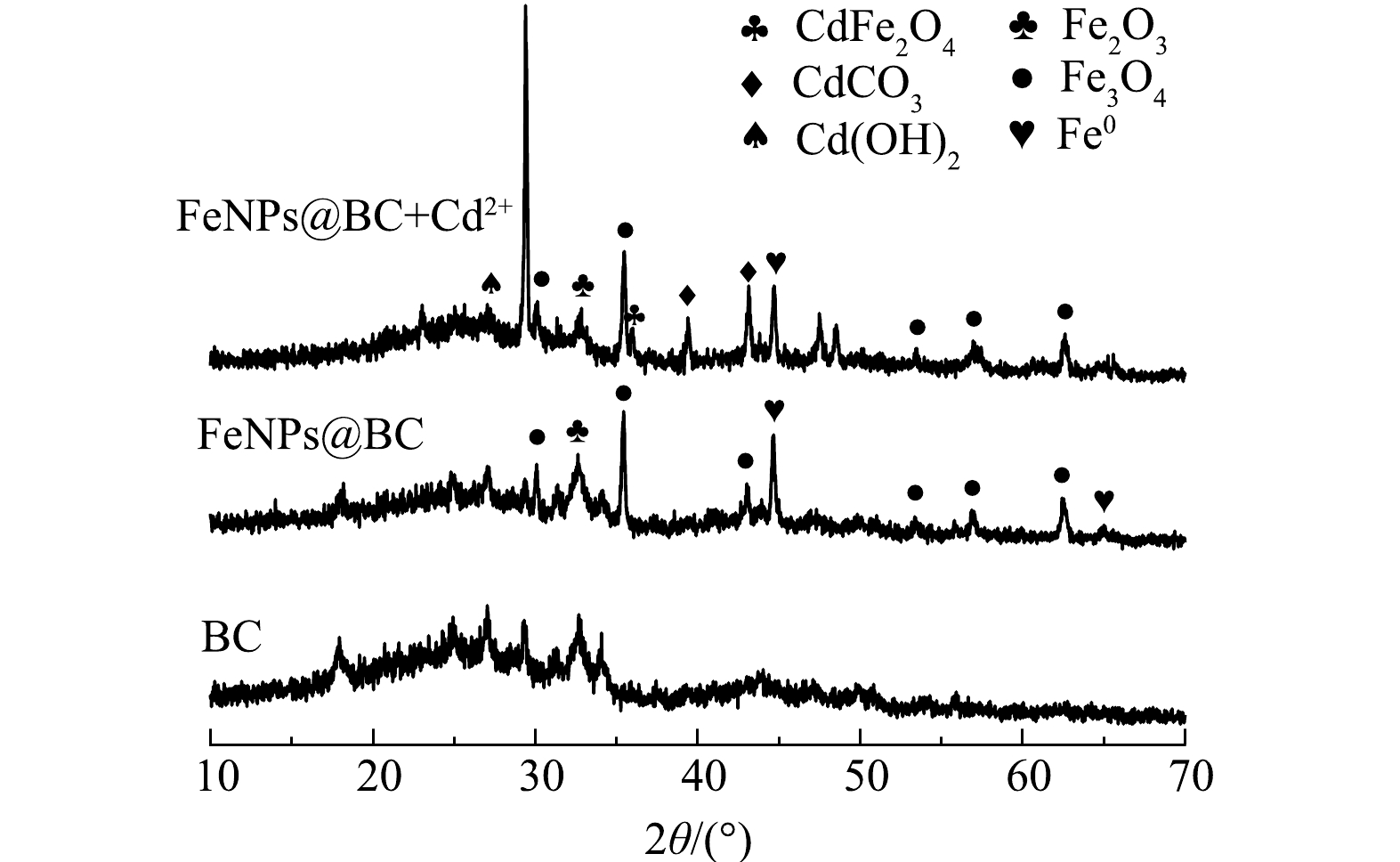
2)材料晶形结构分析。由图8可见,FeNPs@BC和吸附Cd2+后的FeNPs@BC在衍射角约为32.6°等处出现了Fe2O3的结晶峰,衍射角约为33.0°、43.1°和62.5°等处出现了Fe3O4的结晶峰。表明 Fe3O4 在 800 ℃时非常稳定。衍射角约为46.7°、和65.0°等处出现Fe0的结晶峰,这也与何[32]的研究相符合,Fe0物相的存在表明Fe0成功负载在了生物炭上。吸附后XRD图谱检测到了Cd(OH)2、CdCO3的特征峰,这两种化合物在之前许多关于磁性改性生物炭吸附重金属的研究中都很常见[33-35],此外还检测到了CdFe2O4的特征峰,这可能是氧化铁、含铁官能团以及矿物晶体,分别与镉离子发生交换、形成了配合物和沉淀[36]。吸附后Fe0特征峰强度减弱, 这说明Fe0可能与水或溶解氧反应生成铁氧化物[31]。
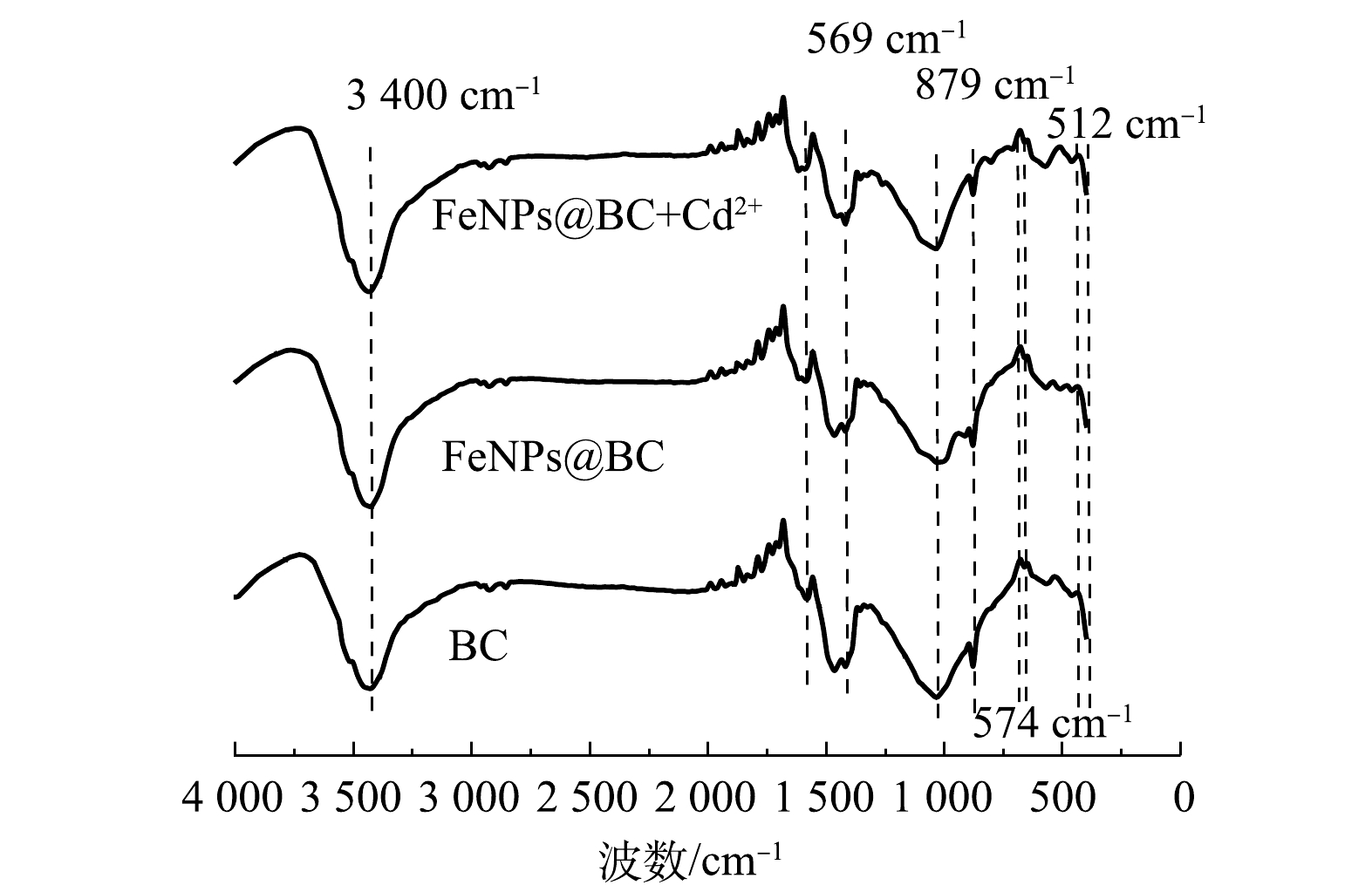
3)材料官能团及组成分析。由图9中的红外光谱可见,大约 3 400 cm−1 的波段归因于O—H 的振动。羟基可能有助于Cd2+的结合,因为其所对应的特征峰在Cd2+去除后变弱。铁基生物炭和吸附镉离子后的铁基生物炭的峰位差异主要出现在512、569 和879 cm−1位置,分别对应Fe—O键[37]、C—H键[35]、C=O键[38]。512、569、 879 cm−1的峰在吸附后消失,相关的功能基团参与了镉离子的吸附,导致了其红外光谱特征的改变。这可能意味着这些功能基团在镉离子的吸附过程中发挥了重要作用。新出现的574、712 cm−1的峰可能是因为吸附镉离子后Cd—O的产生从而产生了新的红外光谱特性。总体来看,铁基生物炭吸附镉离子后,红外光谱的改变反映了离子吸附过程中生物炭结构和化学性质的变化。
-
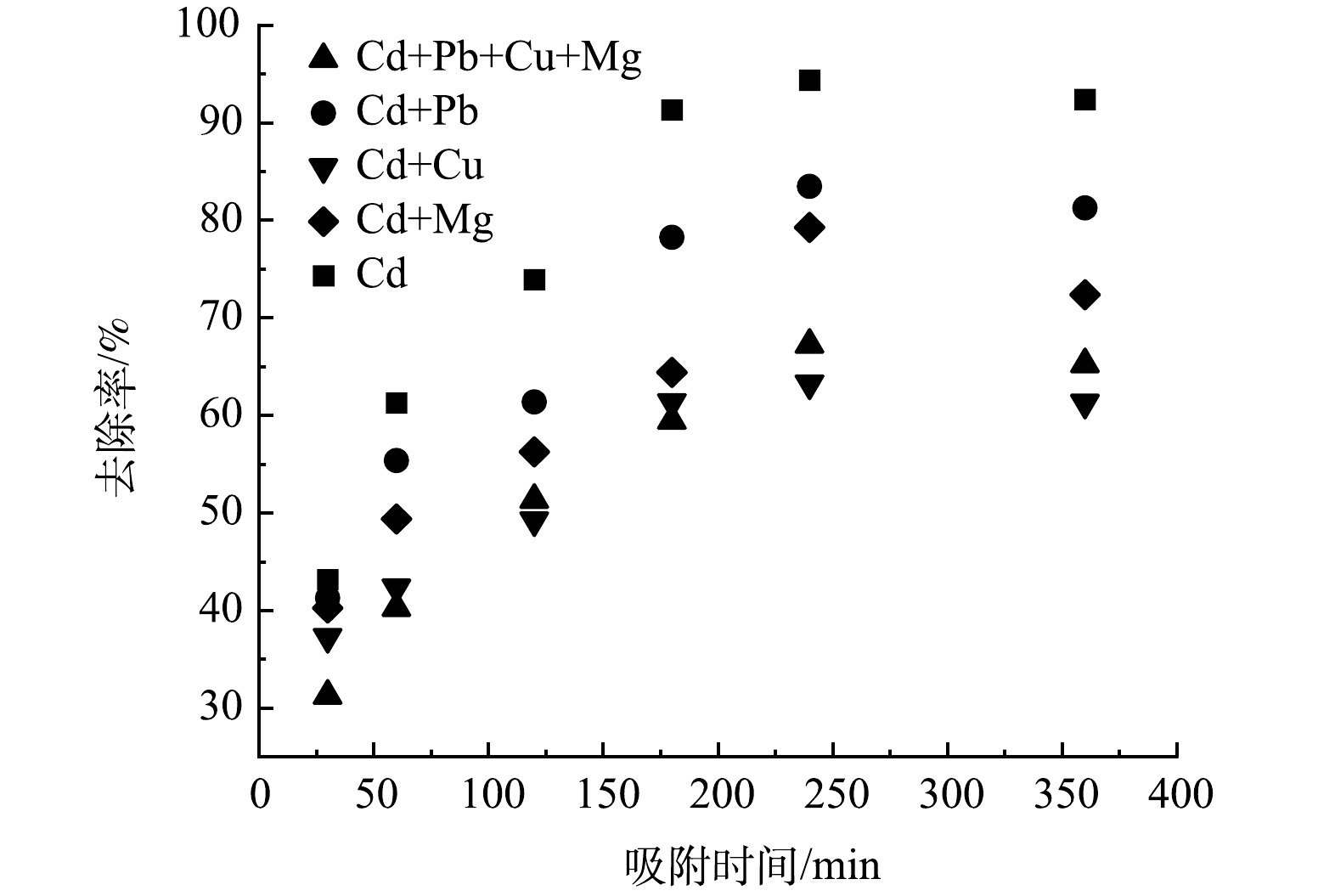
1)共存离子的影响。为了评价FeNPs@BC材料的实际应用效果,通过模拟真实水体中多种重金属污染物共存的状态,分别探讨了Mg2+、Cu2+、Pb2+和Cd2+共存对吸附的影响。如图10 所示,在Pb2+、Mg2+和 Cu2+存在的情况下,水溶液的Cd2+的去除率分别降低了18.7%、15.0%和 27.0%,在Cd2+与Pb2+、Mg2+和 Cu2+ 3种金属离子的混合体系中FeNPs@BC对Cd2+的去除率可达到61.27%,表明FeNPs@BC作为理想的 Cd2+吸附剂具有一定的特异性。
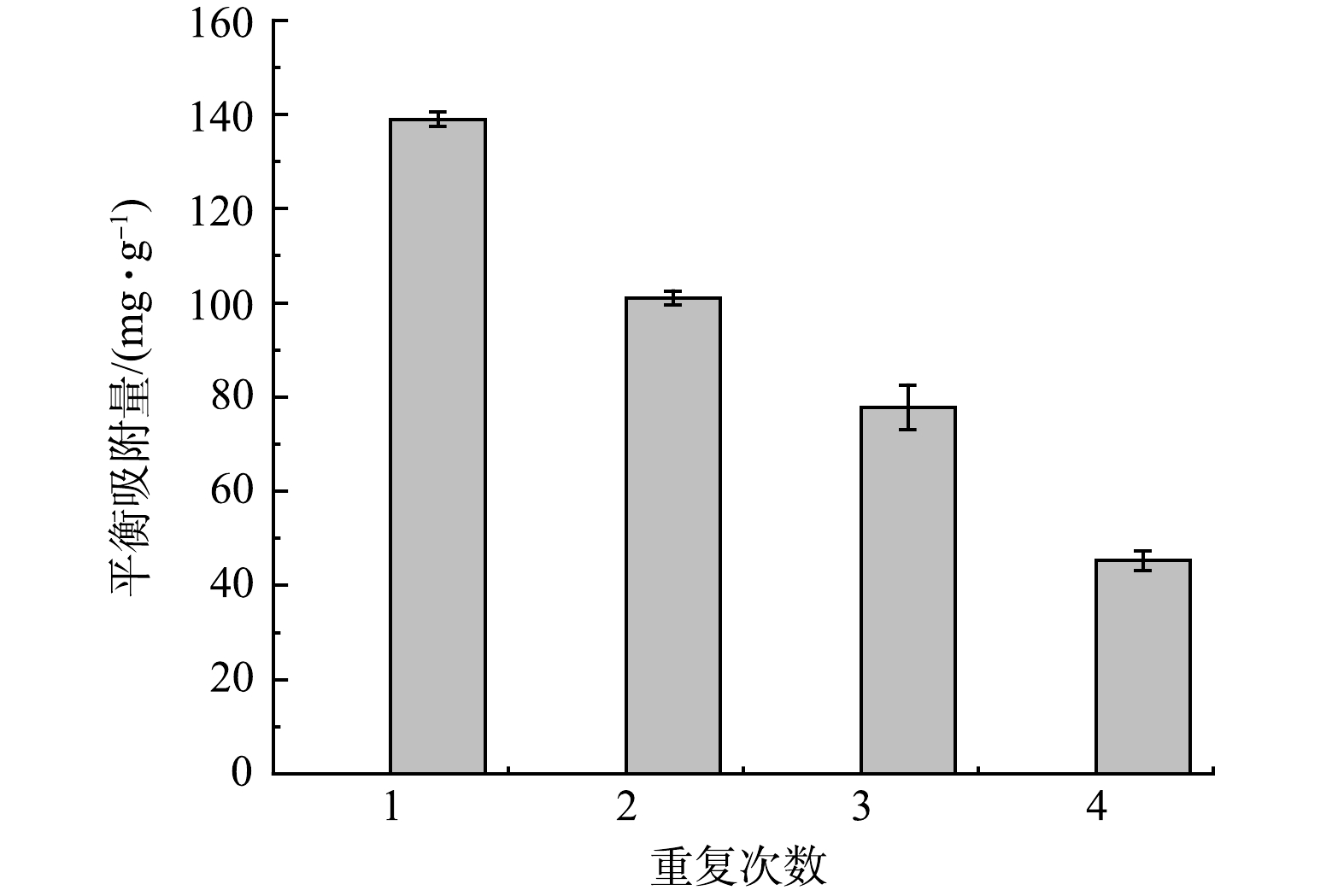
2)可重复性研究。FeNPs@BC 材料的可重复利用性与稳定性通过4次循环利用进行测试。如图11 所示,FeNPs@BC 材料重复利用第2、3和4次时, Cd2+的吸附量分别为 101.01、77.79和 45.28 mg·g−1。 Cd2+的吸附量随着循环次数的增加而显著降低。这可能是在吸附实验中Fe颗粒的浸出,导致吸附剂在4次再生后的吸附能力降低[39],但值得一提的是,第4次循环后,FeNPs@BC 材料对 Cd2+的吸附量仍保持在一定水平,其稳定性与可重复利用性仍然比一些负载铁纳米颗粒的活性炭材料更好[40]。
-
本研究通过碳热法制备的FeNPs@BC对水溶液中Cd2+最大吸附量为145.208 mg·g−1,如表3所示,与其他研究所报道的代表性的Cd2+的吸附材料相比,FeNPs@BC具有更优异的吸附能力。这可能是由于碳热法制备的负载铁纳米颗粒的生物炭具有良好的孔隙结构、丰富的表面活性官能团等有关。
-
1)本研究将树叶粉末与少量Fe3O4进行共热解,成功地经过高温碳还原法一步合成了含有纳米零价铁的铁/炭复合材料FeNPs@BC。
2)落叶生物质与Fe3O4的比例为25∶1时制备的材料对镉的吸附效果最佳。在pH为2~6内,pH越高,越有利于FeNPs@BC材料对Cd2+的吸附。
3) FeNPs@BC和Fe3O4对Cd2+的吸附符合拟二级动力学模型和Langmuir等温模型,主要是化学吸附过程,最大吸附容量可达145.208 mg·g−1(室温下)。
4)吸附机制包括:FeNPs@BC表面官能团与镉离子发生的离子交换以及铁氧化物、含铁官能团与镉离子形成配合物和沉淀。
生物炭负载铁纳米颗粒对水溶液中镉的吸附性能
Adsorption performance of cadmium in aqueous solution using biochar-supported iron composites
-
摘要: 通过高温共热解落叶粉末与Fe3O4的混合物,同步实现了生物质炭化和三价铁的还原,一步制备了含有零价铁的生物炭负载纳米铁颗粒(FeNPs@BC)。批量吸附实验结果表明:当落叶生物质粉末与Fe3O4的比例为25:1时,制备的复合材料对镉的吸附效果最佳。吸附动力学符合准二级动力学模型,吸附等温线符合Langmuir模型。这证明FeNPs@BC主要以化学吸附为主,室温下最大平衡吸附量可达145.208 mg·g−1。FeNPs@BC对水中Cd2+的吸附能力优越,制备工艺简单,为生物炭基材料去除污水中Cd2+可提供理论基础和可替代的吸附材料。Abstract: In this study, biomass carbonization and reduction of trivalent iron simultaneously occurred when leaf powder was co-pyrolyzed with a small amount of Fe3O4 at high temperature, then the biochar-supported iron nanoparticles (FeNPs@BC) containing zero-valent iron were prepared by a one-step method. The results of batch adsorption experiments showed that the optimal performance on cadmium adsorption occurred when the ratio of a leaf biomass to Fe3O4 was 25:1. The adsorption kinetics followed a pseudo-second-order model, while the adsorption isotherms conformed to the Langmuir model, suggesting that the adsorption of Cd2+ onto FeNPs@BC primarily dominated by a chemical one. The maximum equilibrium adsorption capacity at room temperature could reach 145.208 mg·g−1. FeNPs@BC displayed a superior adsorption capacity to Cd2+ in water. The preparation process is simple, it can provide a theoretical basis and an alternative adsorbent material for Cd2+ removal from wastewater by using biochar-based materials.
-
Key words:
- biochar /
- zero-valent iron /
- Cd2+ /
- adsorption
-
镉(cadmium)是一种高毒性的重金属元素,即使在低质量浓度下也会对水体生态环境健康构成威胁。陈荣钦[1]对沘江上游的镉元素含量进行了检测,发现镉的最大含量高达0.03 mg·L−1,其中28件检查样品中含有超标的Cd和Pb。镉还会通过食物链进入人体,对肾脏、骨骼、呼吸系统和免疫系统产生有害影响[2-4]。镉在人体内的生物半衰期长达10~30 a,可蓄积50 a之久[5],因此寻求高效解决水体镉污染问题的技术方法迫在眉睫。目前常用的方法主要包括化学沉淀法、离子交换法、膜过滤、生物法和吸附法[6]。其中,吸附法因其高效、简单操作、且相对低成本等优点而广泛受到关注。
生物炭因其发达的孔隙结构、丰富的表面官能团和独特的表面电子供受能力,而具有良好的重金属吸附能力[7-9]。姜禹奇等[10]在500 ℃下利用玉米秸秆制备的生物炭,其镉去除率可达64.43%。戴亮等[11]制备的污泥生物炭对水中Cd2+的最大吸附容量为27.27 mg·g−1,而CHEN等[12]制备的莲子壳生物炭在300 ℃和600 ℃下对溶液中Cd2+的吸附量分别为31.69 mg·g−1和51.18 mg·g−1。纳米零价铁(nanoscale zero-valent iron,nZVI)是一种新型吸附材料。由于其高比表面积、优异的吸附和反应性,被广泛应用于镉的去除。黄园英等[13]的研究表明,当纳米铁颗粒的投加量为1g·L−1,Cd2+的初始质量浓度为74.51 mg·L−1时,在24 h内,镉的去除率可达98.62%。尽管纳米零价铁在环境修复中表现出卓越的效果,但在实际应用中仍存在一些不足之处。纳米零价铁颗粒容易发生团聚,导致活性位点减少[14-15]。因此,研究人员利用生物炭负载纳米零价铁的方法来提高其去除污染物的能力。许亚琼等[16]制备的nZVI改性生物炭(nZVI-BC)对Cd2+的饱和吸附量达125.5 mg·g−1,为原始生物炭的5.3倍。张磊[17]制备的KMnO4改性磁性生物炭(KMBC)对Cd2+的最大吸附量为53.9 mg·g−1。可以看出,改性后的生物炭对Cd2+的吸附量均有提升。
常规制备生物炭负载零价铁的方法分为2步,先制备生物炭,再将生物炭分散在铁离子溶液中,通过NaBH4还原法制备生物炭负载零价铁,操作流程较复杂,NaBH4价格较高且有毒,NaBH4的反应速率极快,容易导致团聚和不规则颗粒[18]。因此,该方法不是环保有效的方法。
碳热法一步制备生物炭负载零价铁具有一定的研究前景。碳热法以无机碳作为还原剂,在高温/惰性气体保护下将Fe2+/Fe3+还原成Fe0。戴亮等[11]发现,碳热法制备的铁碳材料比还原法制备的铁碳材料具有更好的稳定性和还原性。孙国帅[19]利用碳热法制备的纳米零价铁(NZVI)对Cd2+的最大吸附量为87.715 mg·g−1。然而,碳热法一步制备的生物炭负载零价铁对水溶液中Cd2+的吸附效能与机制尚不清楚。因此,本研究拟通过共热解树叶粉末和少量Fe3O4,采用简易的高温碳还原一步法合成生物炭负载零价铁材料(FeNPs@BC),考察了FeNPs@BC对Cd2+的吸附性能,并采用XRD、SEM、FTIR等表征方法分析了FeNPs@BC对Cd2+的吸附机理。
1. 材料与方法
1.1 实验材料与仪器
实验所用原料树林落叶收集于东北农业大学,清洗后风干、粉碎并过100目筛备用。四氧化三铁(Fe3O4)购于天津博迪化工有限公司;氯化镉购于天津博迪化工有限公司;氢氧化钠购于天津凯通化学试剂有限公司;盐酸购于天津市富宇精细化工有限公司。
1.2 FeNPs@BC 的制备
将树叶粉末与Fe3O4混合,混合的质量比例分别为5∶1、10∶1、15∶1、20∶1、25∶1和30∶1。将混合物转移到陶瓷方舟上,并压平,方舟置于管式炉的中心位置。在进行反应加热之前,使用氩气对反应器进行吹扫,持续时间为30 min以去除管式炉中的水分和氧气。氩气的流速为150 mL·min−1。随后,保持氩气流速不变,设定加热程序,加热速率为20 ℃·min−1,40 min后达到共热解的目标温度800 ℃,并保持该温度持续热解1 h。反应完成后,将管式炉冷却至室温,并将产物转移到棕色小瓶中,然后密封储存备用。此外,此外,还在相同的条件下使用相同的方法制备了单独的Fe3O4和BC,并将其储存备用,标记为热处理后的Fe3O4,单独落叶粉末制备的生物炭记为BC。在合成完成后,将产物用去离子水洗涤至中性,然后进行冷冻干燥,并通过100目筛筛选,最后将它们储存在棕色玻璃瓶中,以备后续实验使用。
1.3 材料表征
采用扫描电子显微镜 (SEM)耦合X 射线能谱仪(EDS)观察 FeNPs@BC吸附Cd2+前后的微观表面,并分析其表面的元素组成; 应用 X 射线衍射(XRD)分析FeNPs@BC的晶体结构与组分;利用傅里叶变换红外光谱(FT-IR)表征吸附材料表面官能团的变化。
1.4 吸附实验
实验所需Cd2+溶液均由氯化镉与去离子水配置而成,溶液质量浓度为50 mg·L−1 ,吸附实验在 150 mL 锥形瓶中完成。加入一定量吸附剂开始吸附实验,在 25 ℃、150 r·min−1的条件下进行吸附,在15、30、45、60、120、180、240、360 min分别取点,溶液通过 0.45 μm 微孔膜过滤,滤液采用原子吸收光谱法测定残留Cd2+质量浓度 。考察溶液初始酸碱度影响:pH设置 为2.0、3.0、4.0、5.0、6.0;考察吸附剂投加量影响,吸附剂添加量设置为10、20、40、60、80 mg;探究50 mg·L−1 Mg2+、Cu2+、Pb2+共存对 Cd2+吸附量的影响;可重复性实验:吸附平衡后,将吸附Cd2+后的材料在纸过滤器中过滤,并在 80 °C 下完全干燥,进行4次吸附-再生循环实验。所有处理设置3个平行实验。
选择拟一阶(式(1))[20]、拟二阶(式(2))[21]、内扩散动力学模型(式(3))[22]、Banghum模型(式(4))[23]和Elovich模型(式(5))[23]对吸附动力学数据进行拟合。
stringUtils.convertMath(!{formula.content}) (1) stringUtils.convertMath(!{formula.content}) (2) stringUtils.convertMath(!{formula.content}) (3) stringUtils.convertMath(!{formula.content}) (4) stringUtils.convertMath(!{formula.content}) (5) 式中:t为吸附时间,min;Qt和Qe分别代表吸附时间为t和吸附平衡时的吸附量,mg·g−1;k1为拟一级反应速率常数,min−1;k2为拟二级反应速率常数,g·(mg·min)−1;ki为粒子内扩散速率常数, mg·g−1·min−0.5;P为与边界层厚度相关的常数。Kb为吸附速率常数, mg·(g·min)−1;α 为初始吸附速率常数,mg·(g·min)−1;β 为解吸速率常数, mg·(g·min)−1。通过以lnQe-Qt对t作图,k1和Qe的值分别对应为斜率和截距;k2和Qe可以由t/Qt对t作图,通过拟合曲线的截距和斜率计算所得,α和β可以通过Qt对lnt作图,α和β分别对应斜率和截距。
吸附等温线Cd2+溶液分别设置为50、100、200、300、400、500 mg·L−1,实验分别在 298.15、308.15和 318.15 K 下进行,采用Langmuir(式(4))[24]、Freundlich(式(5))[25]和Temkin(式(6))[26]这3种吸附等温线模型对实验所得数据进行拟合。
stringUtils.convertMath(!{formula.content}) (6) stringUtils.convertMath(!{formula.content}) (7) stringUtils.convertMath(!{formula.content}) (8) 式中:Ce为吸附平衡时溶液中CV的浓度;Qm为饱和吸附量,mg·g−1;KL为吸附系数,L·mg−1;KF和n均为常数,n>1时,吸附为易吸附过程;Kt和b均为常数。以Ce/Qe对Ce作图,Qm和 KL为的值分别对应为截距和斜率;KF和n的值可以通过lnQe对lnCe作图,通过拟合曲线的截距和斜率计算所得。
2. 结果与讨论
2.1 关键因素影响
1)探究最佳吸附效果的原料比例。由图1可见,通过比较不同质量比例下生物质粉末与Fe3O4混合制备的FeNPs@BC材料对水溶液中Cd2+的吸附效果,发现当生物质与Fe3O4的比例为25∶1,FeNPs@BC材料表现出最佳吸附性能,可达到157.64 mg·g−1的吸附能力。因此,选择以25∶1的比例制备FeNPs@BC材料作为代表性吸附剂。
2)溶液的酸碱度对吸附效果的影响。溶液的初始酸碱度是影响重金属吸附过程的关键因素[27-28]。由图2可见,当溶液的 pH在 2~6时,FeNPs@BC 材料的吸附量随着 pH的升高而增加。在 pH从 2.0 上升到 6.0 的过程中,Cd2+ 的吸附量迅速增加,由 87.274 mg·g−1 增加到 158.6 mg·g−1。因此,强酸性条件不利于FeNPs@BC 对Cd2+ 的吸附,因为大量的 H+ 与 Fe0 反应,导致铁吸附剂失去活性[27]。相比之下,单独高温热解制备的 BC 和 Fe3O4 对环境酸碱度的影响主要体现在镉本身的形态上,对这2种吸附剂的吸附容量影响较小,其对溶液中的 Cd2+ 的吸附量基本维持在 40 mg·g−1 左右。总之,FeNPs@BC 材料在弱酸性环境下表现出较强的吸附能力,对 Cd2+ 的吸附效果显著,而酸碱性条件对 FeNPs@BC的吸附性能至关重要,强酸性环境会降低其吸附能力[29]。
3) 吸附剂投加量对吸附效果的影响。如图3所示,吸附剂的投加量对Cd2+的去除率有一定的影响。当投加量小于50 mg时,随着FeNPs@BC投加量的增加,水溶液中 Cd2+ 的去除率迅速上升;而当投加量超过60 mg时,随着 FeNPs@BC 投加量的增加,Cd2+ 的去除率增加趋势逐渐减缓,相比之下,对于 BC 和 Fe3O4 材料,这种现象并不明显。这可能是因为 BC和 Fe3O4 对 Cd2+ 的吸附能力有限。
2.2 材料吸附性能
1)吸附动力学。使用准一级和准二级动力学模型(图4(a)~(b))对吸附动力学数据进行拟合,拟合参数见表1。对于Cd2+吸附,FeNPs@BC 和 Fe3O4 的准二级动力学模型在 Akaike 信息准则(AIC)上均低于准一级动力学模型。这表明准二级动力学模型更准确地描述了吸附过程,说明FeNPs@BC 和 Fe3O4 对 Cd2+ 的吸附主要是通过化学吸附进行的。同时,吸附动力学曲线表明FeNPs@BC对Cd2+ 的吸附能力高于BC 和 Fe3O4的吸附能力,表明了 FeNPs@BC 在 Cd2+ 吸附中的卓越性能。
表 1 吸附动力学模型参数Table 1. The parameters of adsorption kinetic model吸附剂 准一级动力学模型 准二级动力学模型 Qe /(mg·g−1) K1 /(g·(mg ·min)−1) R2 Qe /(mg·g−1) K1 /(g·(mg ·min)−1) R2 FeNPs@BC 155.781 0.001 4 0.967 153.642 0.0143 0.972 BC 71.608 0.000 7 0.984 72.593 0.006 0.981 Fe3O4 26.596 0.001 2 0.964 51.371 0.439 0.985 吸附剂 颗粒内扩散模型 Bangham diffusion模型 Elovich 模型 P R2 m Kb R2 α β R2 FeNPs@BC 14.643 0.964 3.581 28.594 0.967 23.218 0.038 0.953 BC 9.23 0.965 2.644 9.03 0.964 7.603 0.077 0.951 Fe3O4 3.231 0.913 6.412 6.796 0.95 4.803 0.37 0.955 颗粒内扩散模型的结果(图4(c)和表1)揭示了FeNPs@BC、BC 和 Fe3O4 对 Cd2+ 吸附与分子内扩散密切相关,这表现为相对较低的 P值,同时所有 R2 值均高于 0.92。在该模型中,P 值用于表示内扩散速率常数,较大的 P 值意味着吸附剂对 Cd2+ 的吸附更容易[13]。与 FeNPs@BC 和 Fe3O4 相比,BC 在 Cd2+ 吸附过程中的颗粒内扩散模型和 Bangham 扩散模型的 AIC 值最低,这表明 BC 在整个吸附过程中起着关键作用[30]。此外,这3个曲线均未经过原点,表明内扩散过程仅构成吸附过程的一部分,而非唯一的步骤。
此外,本研究还使用Elovich 模型对上述3种吸附剂的吸附数据进行了拟合。如图4(e) 和表1所示,Elovich 模型中的斜率越大,表示反应速率越快,而截距越大,表明吸附材料的扫气能力越强。结果显示,FeNPs@BC 的吸附强度最高,而 Fe3O4 的吸附速率最快。另外,根据 Bangham 扩散模型的理论,如图4(d) 和表1 所示,R2 值均小于 0.99,这表明实际的吸附情况无法通过通道扩散来完全描述。因此,采用 Elovich 模型对上述3种吸附剂的吸附数据进行了拟合。
吸附等温线。为深入了解FeNPs@BC、BC和 Fe3O4 对 Cd2+ 吸附特性的影响,研究了初始质量浓度对吸附量的影响,采用了3种典型的吸附等温模型,包括 Freundlich 模型、Langmuir 模型和 Temkin 模型对吸附数据进行拟合。表2 列示了拟合得到的相关参数。图5(a)、图5(b) 和图5(c) 展示了在3个等温模型下,不同吸附剂的吸附容量(Qe,mg·g−1)与平衡溶液中溶质质量浓度(Ce,mg·L−1)之间的关系。值得注意的是,随着温度的升高,3种吸附剂对 Cd2+ 的吸附量增加。由表2 中可以看出,与 Temkin 模型和 Freundlich 模型相比,Langmuir 方程对不同温度下 FeNPs@BC 和 BC 吸附 Cd2+ 的过程具有更高的拟合度。这表明结合位点可以均匀分布在吸附剂表面,吸附过程为单层吸附。FeNPs@BC 材料的最大吸附量计算为145.208 mg·g−1(室温下)。此外,通过不同温度下的吸附 KL 值,可以得出 Cd2+ 在 FeNPs@BC 材料和 BC 表面上的吸附相对容易。与 Temkin 模型和 Langmuir 模型相比,Fe3O4 对 Cd2+ 的吸附更符合 Freundlich 模型,表明吸附过程伴随着多分子层吸附的发生。此外,0<1/n<1 表明Fe3O4 表面有利于Cd2+ 的吸附。
表 2 Cd2+在 FeNPs@BC、BC 和 Fe3O4上等温吸附的拟合参数Table 2. Fitting parameters of Cd2+ isotherm adsorption on FeNPs@BC, BC and Fe3O4吸附剂 温度/℃ Langmuir模型 Freundlich模型 Temkin模型 Qm /(mg·g−1) KL /(L·mg−1) R2 Kf /(L·mg−1) 1/n R2 AT bT /(J·mol−1) R2 BC 25 96.985 0.129 0.983 32.251 0.175 0.96 3.699 194.26 0.975 35 91.482 0.044 0.999 33.762 0.155 0.934 3.508 116.58 0.958 45 99.711 0.143 0.971 40.23 0.131 0.944 4.106 189.16 0.959 Fe3O4 25 83.305 0.261 0.968 23.799 0.137 0.972 1.918 167.444 0.977 35 79.303 0.106 0.933 25.125 0.145 0.95 1.369 229.601 0.95 45 71.441 0.165 0.998 30.231 0.124 0.969 1.977 195.803 0.982 FeNPs@BC 25 145.208 0.182 0.967 54.282 0.136 0.954 1.565 858.704 0.966 35 158.193 0.201 0.992 60.574 0.131 0.973 2.272 959.448 0.984 45 173.29 0.192 0.986 64.467 0.126 0.989 2.523 942.428 0.988 2.3 材料表征与吸附机制
1)材料形貌及元素分析。FeNPs@BC吸附Cd2+后的表面形貌以及元素分析结果如图6所示。由图6(a)可以观察到BC表面光滑且含有天然大孔结构,这能为铁纳米颗粒提供更多的附着位点。FeNPs@BC材料表面有小颗粒分布,主要分布在BC的表面和空腔结构中(图6(b)~(c))。由图6(d)可以看出,吸附后的FeNPs@BC表面出现无规则絮状物。这可能是因为Cd已经吸附到材料的表面[31]。
Cd2+吸收前的 FeNPs@BC 材料的主要表面元素如图7 所示。可见,材料表面主要有 C、O和Fe,对应的占比分别为 64.03%、25.87%和 10.10%。吸收 Cd2+后的 FeNPs@BC 材料的主要表面元素为 C、O、Fe 和 Cd,对应的占比分别为66.01%、31.45%、1.02%和 1.51%。这表明Cd被成功吸附。
2)材料晶形结构分析。由图8可见,FeNPs@BC和吸附Cd2+后的FeNPs@BC在衍射角约为32.6°等处出现了Fe2O3的结晶峰,衍射角约为33.0°、43.1°和62.5°等处出现了Fe3O4的结晶峰。表明 Fe3O4 在 800 ℃时非常稳定。衍射角约为46.7°、和65.0°等处出现Fe0的结晶峰,这也与何[32]的研究相符合,Fe0物相的存在表明Fe0成功负载在了生物炭上。吸附后XRD图谱检测到了Cd(OH)2、CdCO3的特征峰,这两种化合物在之前许多关于磁性改性生物炭吸附重金属的研究中都很常见[33-35],此外还检测到了CdFe2O4的特征峰,这可能是氧化铁、含铁官能团以及矿物晶体,分别与镉离子发生交换、形成了配合物和沉淀[36]。吸附后Fe0特征峰强度减弱, 这说明Fe0可能与水或溶解氧反应生成铁氧化物[31]。
3)材料官能团及组成分析。由图9中的红外光谱可见,大约 3 400 cm−1 的波段归因于O—H 的振动。羟基可能有助于Cd2+的结合,因为其所对应的特征峰在Cd2+去除后变弱。铁基生物炭和吸附镉离子后的铁基生物炭的峰位差异主要出现在512、569 和879 cm−1位置,分别对应Fe—O键[37]、C—H键[35]、C=O键[38]。512、569、 879 cm−1的峰在吸附后消失,相关的功能基团参与了镉离子的吸附,导致了其红外光谱特征的改变。这可能意味着这些功能基团在镉离子的吸附过程中发挥了重要作用。新出现的574、712 cm−1的峰可能是因为吸附镉离子后Cd—O的产生从而产生了新的红外光谱特性。总体来看,铁基生物炭吸附镉离子后,红外光谱的改变反映了离子吸附过程中生物炭结构和化学性质的变化。
2.4 材料稳定性分析
1)共存离子的影响。为了评价FeNPs@BC材料的实际应用效果,通过模拟真实水体中多种重金属污染物共存的状态,分别探讨了Mg2+、Cu2+、Pb2+和Cd2+共存对吸附的影响。如图10 所示,在Pb2+、Mg2+和 Cu2+存在的情况下,水溶液的Cd2+的去除率分别降低了18.7%、15.0%和 27.0%,在Cd2+与Pb2+、Mg2+和 Cu2+ 3种金属离子的混合体系中FeNPs@BC对Cd2+的去除率可达到61.27%,表明FeNPs@BC作为理想的 Cd2+吸附剂具有一定的特异性。
2)可重复性研究。FeNPs@BC 材料的可重复利用性与稳定性通过4次循环利用进行测试。如图11 所示,FeNPs@BC 材料重复利用第2、3和4次时, Cd2+的吸附量分别为 101.01、77.79和 45.28 mg·g−1。 Cd2+的吸附量随着循环次数的增加而显著降低。这可能是在吸附实验中Fe颗粒的浸出,导致吸附剂在4次再生后的吸附能力降低[39],但值得一提的是,第4次循环后,FeNPs@BC 材料对 Cd2+的吸附量仍保持在一定水平,其稳定性与可重复利用性仍然比一些负载铁纳米颗粒的活性炭材料更好[40]。
2.5 FeNPs@BC与其他材料对Cd2+吸附能力的对比
本研究通过碳热法制备的FeNPs@BC对水溶液中Cd2+最大吸附量为145.208 mg·g−1,如表3所示,与其他研究所报道的代表性的Cd2+的吸附材料相比,FeNPs@BC具有更优异的吸附能力。这可能是由于碳热法制备的负载铁纳米颗粒的生物炭具有良好的孔隙结构、丰富的表面活性官能团等有关。
表 3 FeNPs@BC与其他材料吸附Cd2+的对比Table 3. Comparison of Cd2+ adsorption between FeNPs@BC and other materials3. 结论
1)本研究将树叶粉末与少量Fe3O4进行共热解,成功地经过高温碳还原法一步合成了含有纳米零价铁的铁/炭复合材料FeNPs@BC。
2)落叶生物质与Fe3O4的比例为25∶1时制备的材料对镉的吸附效果最佳。在pH为2~6内,pH越高,越有利于FeNPs@BC材料对Cd2+的吸附。
3) FeNPs@BC和Fe3O4对Cd2+的吸附符合拟二级动力学模型和Langmuir等温模型,主要是化学吸附过程,最大吸附容量可达145.208 mg·g−1(室温下)。
4)吸附机制包括:FeNPs@BC表面官能团与镉离子发生的离子交换以及铁氧化物、含铁官能团与镉离子形成配合物和沉淀。
-
表 1 吸附动力学模型参数
Table 1. The parameters of adsorption kinetic model
吸附剂 准一级动力学模型 准二级动力学模型 Qe /(mg·g−1) K1 /(g·(mg ·min)−1) R2 Qe /(mg·g−1) K1 /(g·(mg ·min)−1) R2 FeNPs@BC 155.781 0.001 4 0.967 153.642 0.0143 0.972 BC 71.608 0.000 7 0.984 72.593 0.006 0.981 Fe3O4 26.596 0.001 2 0.964 51.371 0.439 0.985 吸附剂 颗粒内扩散模型 Bangham diffusion模型 Elovich 模型 P R2 m Kb R2 α β R2 FeNPs@BC 14.643 0.964 3.581 28.594 0.967 23.218 0.038 0.953 BC 9.23 0.965 2.644 9.03 0.964 7.603 0.077 0.951 Fe3O4 3.231 0.913 6.412 6.796 0.95 4.803 0.37 0.955 表 2 Cd2+在 FeNPs@BC、BC 和 Fe3O4上等温吸附的拟合参数
Table 2. Fitting parameters of Cd2+ isotherm adsorption on FeNPs@BC, BC and Fe3O4
吸附剂 温度/℃ Langmuir模型 Freundlich模型 Temkin模型 Qm /(mg·g−1) KL /(L·mg−1) R2 Kf /(L·mg−1) 1/n R2 AT bT /(J·mol−1) R2 BC 25 96.985 0.129 0.983 32.251 0.175 0.96 3.699 194.26 0.975 35 91.482 0.044 0.999 33.762 0.155 0.934 3.508 116.58 0.958 45 99.711 0.143 0.971 40.23 0.131 0.944 4.106 189.16 0.959 Fe3O4 25 83.305 0.261 0.968 23.799 0.137 0.972 1.918 167.444 0.977 35 79.303 0.106 0.933 25.125 0.145 0.95 1.369 229.601 0.95 45 71.441 0.165 0.998 30.231 0.124 0.969 1.977 195.803 0.982 FeNPs@BC 25 145.208 0.182 0.967 54.282 0.136 0.954 1.565 858.704 0.966 35 158.193 0.201 0.992 60.574 0.131 0.973 2.272 959.448 0.984 45 173.29 0.192 0.986 64.467 0.126 0.989 2.523 942.428 0.988 -
[1] 陈荣钦. 沘江上游铅、镉元素迁移对沘江水体污染初探[J]. 云南地质, 1987(4): 332-338. [2] GODT J, SCHEIDIG F, GROSSE-SIETRUP C, et al. The toxicity of cadmium and resulting hazards for human health[J]. Occupational Medicine and Toxicology, 2006.1: 22. [3] MÉNDEZ-ARMENTA M, RÍOS C. Mini reviewcadmium neurotoxicity[J]. Environmental Toxicology and Pharmacology, 2007, 23: 350-358. [4] 崔玉静, 赵中秋, 刘文菊, 等 镉在土壤-植物-人体系统中迁移积累及其影响因子[J]. 生态学报, 2003(10): 2133-2143. [5] 李 婧, 周 艳, 陈 森, 等 我国土壤镉污染现状、危害及其治理方法综述[J]. 安徽农学学报 , 2015, 21(24): 104-107. [6] 张晓光. 我国当前污染水体修复方法研究[J]. 山西农经, 2020, 38(4): 93-95. [7] PARK J H, CHOPPALA G K, BOLAN N S, et al. Biochar reduces the bioavailability and phytotoxicity of heavy metals[J]. Plant and Soil, 2011: 439-451. [8] . 林雪原, 荆延德, 巩晨, 等 生物炭吸附重金属的研究进展[J]. 环境污染与防治, 2014, 36(5): 83-87. [9] 叶希青. 生物炭去除重金属离子及竞争吸附作用研究[D]. 武汉: 中国地质大学. 2020. [10] 姜禹奇, 都琳. 生物质炭修复重金属镉污染水体的研究[J]. 中国资源综合利用, 2021, 39(2): 201-204. [11] 戴亮, 任珺, 陶玲, 等. 不同热解温度下污泥基生物炭的性质及对Cd2+的吸附特性[J]. 环境工程学报, 2017, 11(7): 4029-4035. [12] CHEN Z, LIU T, TANG J, et al. Characteristics and mechanisms of cadmium adsorption from aqueous solution using lotus seedpod-derived biochar at two pyrolytic temperatures[J]. Environmental Science and Pollution Research, 2018, 25(12): 11854-11866. doi: 10.1007/s11356-018-1460-1 [13] 黄园英, 王倩, 汤奇峰, 等. 纳米铁去除水体中镉的反应动力学、吸附平衡和影响因素[J]. 生态环境学报, 2019, 28(10): 2053-2061. [14] LIU A, LIU J, HAN J, et al. Evolution of nanoscale zero-valent iron (nZVI) in water: microscopic and spectroscopic evidence on the formation of nano- and micro-structured iron oxides[J]. Journal of Hazardous Materials, 2017, 322: 129-135. doi: 10.1016/j.jhazmat.2015.12.070 [15] 谢武明, 毕小林, 黄子峻, 等. 纳米活性氧化铝负载磁性纳米零价铁对不同重金属的吸附机理[J]. 环境科学学报, 2020, 40(8): 2732-2740. [16] 许亚琼, 王雪佳, 李荣华, 等. 纳米零价铁改性生物炭对污染土壤中Cd稳定化效果及作用机制研究[J]. 农业环境科学学报, 2022, 41(11): 2478-2487. [17] 张磊. 改性生物炭吸附溶液中镉离子的性能研究[D]. 郑州: 郑州大学, 2022. [18] 吴凡. 液相还原法制备微米级银粉及其性能研究[D]. 杭州: 浙江科技学院, 2022. [19] 孙国帅. 碳热法负载纳米零价铁对Cr(Ⅵ)和Cd(Ⅱ)的去除研究[D]. 哈尔滨: 哈尔滨工业大学, 2015. [20] JAVADIAN H, ANGAJI M T, NAUSHAD M. Synthesis and characterization of polyaniline/γ-alumina nanocomposite: A comparative study for the adsorption of three different anionic dyes[J]. Journal of Industrial & Engineering Chemistry, 2014, 20(5): 3890-3900. [21] ANSARI R, KEIVANI M B, DELAVAR A F. Application of polyaniline nanolayer composite for removal of tartrazine dye from aqueous solutions[J]. Journal of Polymer Research, 2011, 18(6): 1931-1939. doi: 10.1007/s10965-011-9600-z [22] NETHAJI S, SIVASAMY A. Adsorptive removal of an acid dye by lignocellulosic waste biomass activated carbon: Equilibrium and kinetic studies[J]. Chemosphere, 2011, 82(10): 1367-1372. doi: 10.1016/j.chemosphere.2010.11.080 [23] LI P G, WANG J X, LI X T, et al. Facile synthesis of amino-functional large-size mesoporous silica sphere and its application for Pb2+ removal[J]. Journal of Hazardous Materials, 2019, 378: 120664. doi: 10.1016/j.jhazmat.2019.05.057 [24] WANG X Y, CAI J H, ZHANG Y J, et al. Heavy metal sorption properties of magnesium titanate mesoporous nanorods[J]. Journal of Materials Chemistry A, 2015, 3(22): 11796-11800. doi: 10.1039/C5TA02034D [25] XU Q H, WANG Y L, JIN L Q, et al. Adsorption of Cu (Ⅱ), Pb (Ⅱ) and Cr (Ⅵ) from aqueous solutions using black wattle tannin-immobilized nanocellulose[J]. Journal of Hazardous Materials, 2017, 339: 91-99. doi: 10.1016/j.jhazmat.2017.06.005 [26] CHEN X Y, HOSSAIN M F, DUAN C Y, et al. Isotherm models for adsorption of heavy metals from water : A review[J]. Chemosphere, 2022, 307: 135545. doi: 10.1016/j.chemosphere.2022.135545 [27] YAN Y Z, NAGAPP S, YOO J M, et al. Polyethyleneimine-grafted polysilsesquioxane hollow spheres for the highly efficient removal of anionic dyes and selective adsorption of Cr(VI)[J]. Journal of Environmental Chemical Engineering, 2021, 9(2): 104814. doi: 10.1016/j.jece.2020.104814 [28] YU H, WANG J, YU J X, et al. Adsorption performance and stability of the modified straws and their extracts of cellulose, lignin, and hemicellulose for Pb2+: pH effect[J]. Arabian Journal of Chemistry, 2020, 13(12): 9019-9033. doi: 10.1016/j.arabjc.2020.10.024 [29] JIANG Q, JIANG S, LI H, et al. A stable biochar supported S-nZVI to activate persulfate for effective dichlorination of atrazine[J]. Chemical Engineering Journal, 2022, 431: 133937. doi: 10.1016/j.cej.2021.133937 [30] BOPARAI H K, JOSEPH M, O’CARRALL D M. Kinetics and thermodynamics of cadmium ion removal by adsorption onto nano zerovalent iron particles[J]. Journal of Hazardous Materials, 2011: 458-465. [31] 韩涛. 污泥生物炭负载纳米零价铁对水中Cd2+的吸附性能研究[D]. 兰州: 兰州交通大学, 2022. [32] 何恬叶. 稳定化纳米零价铁生物炭对水中重金属的吸附[D]. 成都: 成都理工大学, 2019. [33] KHAN Z H, GAO M, QIU W, et al. Properties and adsorption mechanism of magnetic biochar modified with molybdenum disulfide for cadmium in aqueous solution[J]. Chemosphere, 2020, 255: 126995. doi: 10.1016/j.chemosphere.2020.126995 [34] WU J, HUANG D, LIU X, et al. Remediation of As(III) and Cd(II) co-contamination and its mechanism in aqueous systems by a novel calcium-based magnetic biochar[J]. Journal of Hazardous Materials, 2018, 348: 10-19. doi: 10.1016/j.jhazmat.2018.01.011 [35] YANG D, WANG L, LI Z, et al. Simultaneous adsorption of Cd(II) and As(III) by a novel biochar-supported nanoscale zero-valent iron in aqueous systems[J]. Science of the Total Environment, 2020: 134823. [36] HUANG F, ZHANG S M, WU R R, et al. Magnetic biochars have lower adsorption but higher separation effectiveness for Cd2+ from aqueous solution compared to nonmagnetic biochars[J]. Environmental Pollution, 2021: 116485. [37] TANG H X, STUMM W. The coagulating behaviors of Fe(III) polymeric species—I. preformed polymers by base addition[J]. Water Research, 1987, 21(1): 115-121. doi: 10.1016/0043-1354(87)90106-0 [38] YIN G, TAO L, CHEN X, et al. Quantitative analysis on the mechanism of Cd2+ removal by MgCl2-modified biochar in aqueous solutions[J]. Journal of Hazardous Materials, 2021: 126487. [39] 杨妍, 刘国涛, 余庆慧, 等. 多孔炭材料改性纳米零价铁的研究进展[J]. 化工进展, 2021, 40(S2): 198-202. [40] 江群. 玉米秸秆基生物炭及其负载nZVI去除水中阿特拉津研究[D]. 哈尔滨: 东北农业大学, 2020. [41] SHAO Y, TIAN C, YANG Y et al. Carbothermal synthesis of sludge iochar supported nanoscale zero-valent iron for the removal of Cd2+ and Cu2+: Preparation, performance, and safety risks[J]. International Journal of Environmental Research and Public Health, 2022: 16041. [42] CHEN L, LI F, WEI Y, et al. High cadmium adsorption on nanoscale zero-valent iron coated eichhornia crassipes biochar[J]. Environmental Chemistry Letters, 2019: 589-594. 期刊类型引用(2)
1. 包坤,刘昱镔,张文倩. 挺水植物秸秆对溶液中Cu~(2+)的吸附研究. 唐山学院学报. 2024(06): 1-9+33 .  百度学术
百度学术
2. 朱桐豆,王国庆,陈亚楠,杨蕾. 电石渣/铝酸盐水泥复合免烧填料的制备及除镉性能研究. 环境科学与技术. 2024(11): 152-161 .  百度学术
百度学术
其他类型引用(0)
-






 下载:
下载: