-
近年来,医药活性物质(pharmaceutical active substance, PhACs)的使用及其迁移转化受到人们的广泛关注[1]。据统计,每年大约60%~80%的PhACs不被动物或人类吸收而释放到环境中[2],这些PhACs能够对水生生物以及人体健康造成潜在的危害,甚至还可能诱导基因突变[3]。卡马西平(carbamazepine, CBZ)与双氯芬酸(diclofenac, DCF)是PhACs的典型代表[4],CBZ是一种用于控制癫痫发作和各种心理治疗的常用药物,DCF则是一种常用的止痛药和非甾体类抗炎药;2种PhACs在水环境中检出频率较高,譬如污水厂出水中CBZ、DCF的质量浓度分别达到6.3 µg·L−1和2.1 µg·L−1 [5-6],其特殊的结构性质而不能被传统的污水处理技术有效去除。因此,研究高效的非生物降解技术可有效减轻PhACs对生态水体以及人类健康的危害。
光催化是解决能源危机和环境污染问题的一种先进的高级氧化技术[7],半导体光催化剂可将紫外线及可见光能量转化为化学能,通过产生光生空穴(h+)、电子(e−)和活性氧物种(ROS)来非选择性地降解污染物[8]。硫化铟锌(ZnIn2S4)是一种高效光催化剂[9],其合适的带隙(2.10~2.48 eV)可吸收太阳光来进行PhACs的氧化还原反应;然而,ZnIn2S4耐光性较差、光生电子和空穴的复合率较高,因此,将贵金属钯(Pd)掺杂可有效阻止光生电子-空穴的复合,从而提高其光催化活性。BO等[10]采用水热合成法制备Pd掺杂ZnIn2S4粉末型催化剂并研究其光降解水中阿特拉津,证实太阳光照射1 h后阿特拉津的降解率达到了90%;田野等[11]对Pd掺杂ZnIn2S4光催化剂的含量进行了优化,发现太阳光下0.1% Pd-ZnIn2S4在90 min内可将50 mL水中100 µg·L−1的CBZ完全降解。但是,目前光催化材料大多为粉末态,在降解水中污染物时光催化材料的回收是制约光催化技术实际应用的一大难题。因此,将光催化剂固定在载体上是实现其重复利用和光催化技术实际应用的关键前提。
浮石是一种多孔结构、耐腐蚀和可浮于水面的火山喷出岩[12],它的多孔和无定形性质创造了一个大的表面积和骨架结构,其平均孔隙率高达90%;而且,浮石表面末端存在羟基和氧桥,可作为金属或金属氧化物的吸附点,因此,被认为是光催化剂的优良载体。唐西梅等[13]采用胶粘法制备Pd-ZnIn2S4/浮石负载型催化剂,太阳光下降解阿特拉津,300 min时降解率为61%;结果表明,胶粘法容易使光催化剂产生团聚现象,且光催化剂与载体之间连接较弱,反应过程中光催化剂脱落率较大。聚乙烯醇(polyvinyl alcohol , PVA)是一种天然高分子有机聚合物,PVA基体的碳链上含有丰富的羟基,能够使其他材料通过氢键与其结合,从而促进功能高分子材料中分子链的修饰和交联[14];此外,PVA具有高透光性、水溶性、热稳定性和耐腐蚀性,成为光电应用的良好基体[15-16]。然而,以浮石为载体,选用PVA作为水基粘合剂,负载0.1% Pd-ZnIn2S4光催化降解PhACs的研究目前尚未见报道。
因此,本研究采用水热合成法制备0.1% Pd-ZnIn2S4粉末态光催化剂,使用高分子聚合材料PVA作为水基粘合剂,通过超声浸渍法制备0.1% Pd-ZnIn2S4-PVA-浮石负载型催化剂,考察该催化剂分别在碘镓灯、太阳光下光催化降解CBZ、DCF的活性,通过催化剂表征、自由基淬灭实验以及EPR测试分析等探究其光催化降解CBZ机理。论文研究将为负载型光催化剂制备及其应用于生态水体中痕量PhACs的消除治理提供技术支持。
-
卡马西平(CBZ,99%)、双氯芬酸(DCF,99%),北京百灵威科技有限公司;硝酸四氨合钯([Pd(NH3)4])NO3)2),湖北七八九化学试剂厂;硫代乙酰胺(TAA)、硝酸锌(Zn(NO3)2·6H2O),天津市科密欧化学试剂有限公司;硝酸铟In(NO3)3,上海麦克林生化科技股份有限公司;聚乙烯醇(PVA)、对苯醌(PBQ)、异丙醇(IPA),上海阿拉丁生化科技股份有限公司;中性硅溶胶(ZS-30),浙江宇达化工有限公司;磷酸二氢铝(Al(H2PO4)3),石家庄市鑫盛化工有限公司;聚乙二醇400(PEG-400),天津市大茂化学试剂厂;实验所需化学试剂均为分析纯,无需处理可直接使用。浮石(粒径5~10 mm),山东济宁欣盛石材工艺品加工厂。
-
0.1%(质量百分比) Pd-ZnIn2S4粉末态光催化剂采用水热合成法制备,具体制备过程如下:首先,将4 μL的[Pd(NH3)4](NO3)2溶液、104.09 mg Zn(NO3)2·6H2O和210.58 mg In(NO3)3溶解 70 mL的去离子水中并超声助溶0.5 h;随后,将420.75 mg 的TAA加入到混合液中并超声0.5 h;然后,搅拌状态下滴加20%的HNO3调节溶液pH至2.5,将酸性混合液密封到聚四氟乙烯(PTFE)内胆的不锈钢反应罐中80 ℃下反应6 h;冷却至室温后打开反应罐,对反应液进行抽滤并对生成的Pd-ZnIn2S4黄色固体洗涤至中性,干燥研磨后过400目筛网即可获得0.1% Pd-ZnIn2S4粉末催化剂[10]。使用超纯水洗涤商业浮石2~3遍以除去表面浮灰,随后用10%的硝酸溶液浸泡浮石24 h以去除其表面及孔道内杂质,最后进行超声-超纯水洗涤直至溶液为中性,80 ℃下过夜烘干备用。
浮石负载型催化剂采用超声-浸渍法制备:首先取一定量超纯水,加入2 mL的5% PVA粘合剂(38 g水中加入2 g的PVA加热至93 ℃完全溶解再冷却后可得)[17]得到5%PVA水基粘合剂;随后加入0.1% Pd-ZnIn2S4粉末催化剂,超声30 min后得到0.1% Pd-ZnIn2S4-PVA水基悬浮液;最后,将预处理后的浮石放入0.1% Pd-ZnIn2S4-PVA水基悬浮液中超声浸渍,30 min后将浮石拿出,80 ℃下过夜烘干,冷却至室温后即得到0.1% Pd-ZnIn2S4-PVA-浮石负载型催化剂。重量法测得超声浸渍一次的光催化剂负载率为2%。其他制备条件不变,分别用ZS-30、Al(H2PO4)3以及PEG-400替换5%PVA粘合剂后可制得0.1% Pd-ZnIn2S4-ZS-30-浮石、0.1% Pd-ZnIn2S4-Al(H2PO4)3-浮石和0.%Pd-ZnIn2S4-PEG-400-浮石负载型催化剂,四种催化剂的照片如图1所示。
-
催化剂的晶形结构通过X射线衍射仪(XRD,D/MAX-2400,日本理学)进行测试分析;表面形貌和活性颗粒的大小与分布由扫描电子显微镜(SEM,JSM-6510LV,日本电子)予以观察;表面官能团通过傅里叶变换红外光谱仪(FTIR,BruKer)进行测试;UV-vis DRS光谱通过紫外/可见/近红外分光光度计(UV3600,日本岛津)予以测量;氧空位与自由基通过EPR电子顺磁共振仪(ZSXmicro-6/1,BruKer)进行测试分析。
-
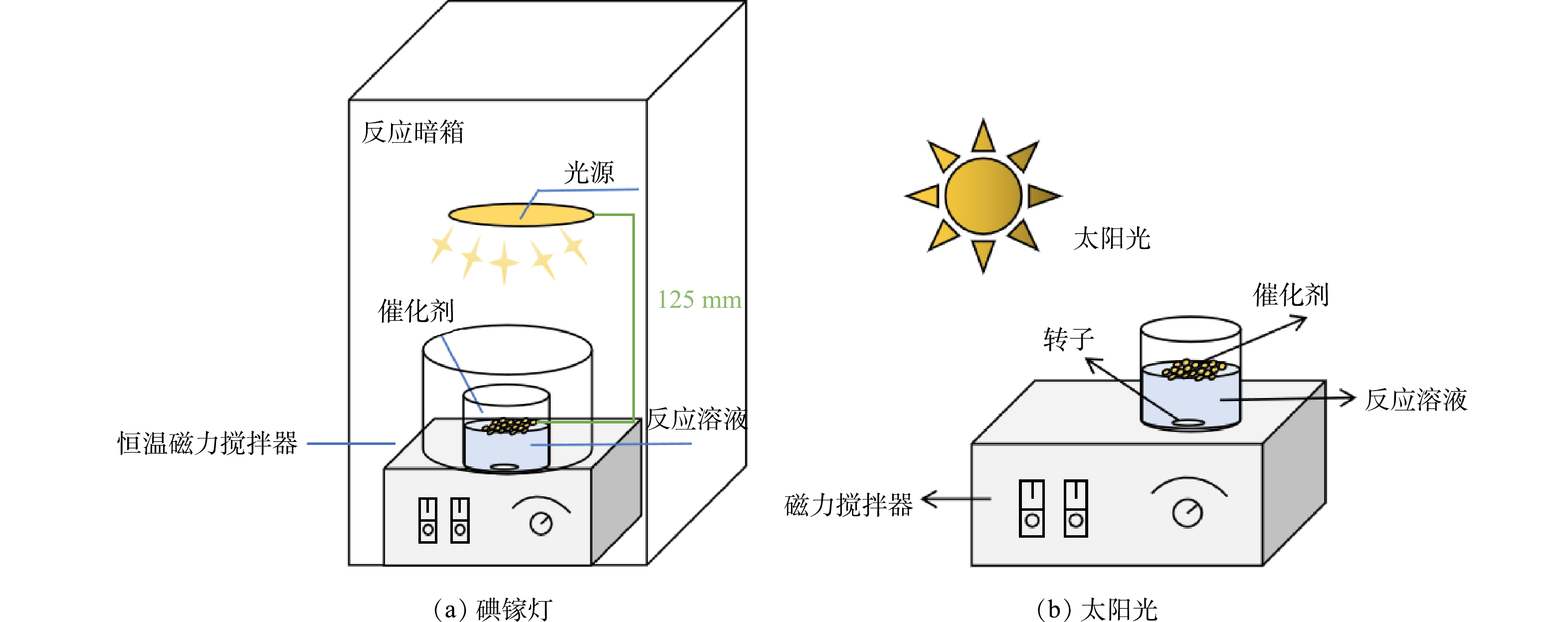
实际医药废水经处理后的排放质量浓度为100 mg·L−1 (TOC值) 左右[18],与其他污水混合后以500 µg·L−1进入污水处理厂,然后约80%的医药类污染物会被去除,污水厂出水中的医药类污染物浓度为µg·L−1级[19]。本研究结合水体中医药类污染物的实际浓度、检出药物及液相色谱的检测范围,确定以CBZ、DCF为目标污染物配置模拟医药废水,其初始质量浓度为100 µg·L−1。具体实验方案如下:提前配置1 g·L−1的CBZ、DCF储备液于冰箱内4 ℃下保存,实验前取一定体积的储备液逐级稀释配置成100 µg·L−1的溶液50 mL用于单组分实验;取质量浓度为100 µg·L−1的CBZ、DCF溶液各25 mL,形成50 mL混合液用于双组分实验。将一定量的0.1% Pd-ZnIn2S4-PVA-浮石负载型催化剂加入单组分或双组分溶液中,首先避光条件下暗反应30 min以消除催化剂吸附对PhACs光催化降解效果的影响,然后将反应装置分别置于碘镓灯、太阳光下进行光催化反应,反应装置如图2所示;不同光催化反应时间下取样1.5 mL并用0.22 µm有机膜过滤水样,使用高效液相色谱定量分析过滤后的水样CBZ、DCF浓度变化,以此计算CBZ、DCF的光催化降解效率。
本研究首先考察了在碘镓灯光照下不同粘结剂所制备0.1% Pd-ZnIn2S4-浮石负载型催化剂的活性,筛选出效果较好的2组催化剂;随后在碘镓灯以及太阳光照射下将2组粘合剂进行不同比例混合后选出催化活性最好的一组负载型催化剂,考察其降解单组分DCF以及CBZ和DCF双组分的活性;最后通过自由基淬灭实验以及EPR表征测试来探讨光催化过程中自由基的类别及其贡献程度,以此推测CBZ的光催化降解机理。论文中参数优化实验均重复开展3次,文中数据为3次实验测试的平均值,以此消除实验研究误差。
-
光催化实验中CBZ、DCF的浓度采用高效液相色谱(U3000,日本分光)定量分析,色谱柱为Agilent5 TC-C18柱(250 mm×4.6 mm, 5 µm),紫外检测波长分别为230 nm和276 nm,进样量100 μL,流动相分别为A:B=55%乙腈:45%超纯水和A:B=70%乙腈:30%冰醋酸水溶液(1%冰醋酸含量),流速分别为0.8 mL·min−1和0.7 mL·min−1。
负载型催化剂在实验过程中活性组分的脱落率由重量法进行分析,如式(1)所示。
式中:S为活性组分的脱落率,%;W1为浮石的质量,g;W2为负载型催化剂的质量,g;W3为超声30 min后负载型催化剂的质量,g。
-
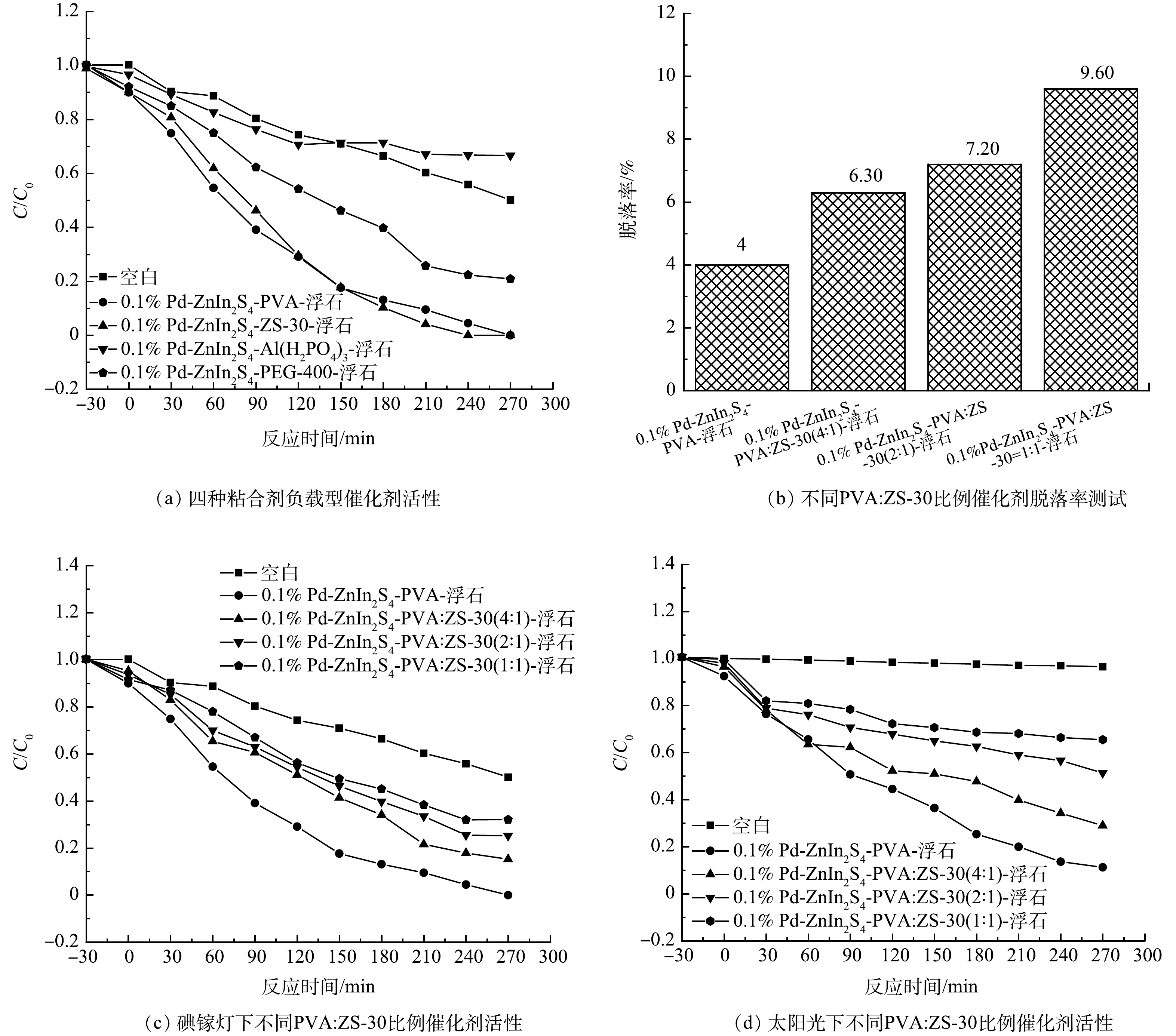
对4种水基粘合剂制备的0.1% Pd-ZnIn2S4-PVA-浮石、0.1% Pd-ZnIn2S4-ZS-30-浮石、0.1% Pd-ZnIn2S4-Al(H2PO4)3-浮石和0.1% Pd-ZnIn2S4-PEG-400-浮石负载型催化剂进行了碘镓灯下光催化降解水中CBZ的活性实验,实验条件为:CBZ初始质量浓度100 µg·L−1、催化剂投加量1.5 g·L−1和溶液体积50 mL,实验结果如图3所示。由图3(a)可见,碘镓灯下无催化剂的空白实验在270 min时对CBZ的降解率为50%,证实碘镓灯可直接光解CBZ;暗反应30min后,4种负载型催化剂对CBZ的降解率分别为10%、10%、13.38%和7.88%,说明吸附可去除10%左右的CBZ,这与之前BO等[10]的研究结果一致。光催化反应270 min后,4种催化剂对CBZ的降解率分别为100%、100%、33.38%和79.10%,可见0.1% Pd-ZnIn2S4-PVA-浮石和0.1% Pd-ZnIn2S4-ZS -30-浮石负载型催化剂的光催化CBZ效果最好,Al(H2PO4)3粘合剂对CBZ的降解起负作用,说明反应中Al(H2PO4)3会阻碍光催化反应的进行,PEG-400粘合剂则有一定的促进作用。
为了进一步证实PVA的粘合效果,且探讨PVA与ZS-30水基粘合剂的相互作用及其对光催化剂活性的影响,将PVA与ZS-30按照不同比例进行混合。研究发现,随着PVA的不断加入,明显观察到混合后的水基粘合剂较ZS-30粘合剂粘度更强,且溶液流动性降低。对所制备催化剂进行脱落率测定(图3(b))与活性实验(图3(c)~(d))。由图3(b)可知,超声0.5h后0.1% Pd-ZnIn2S4-PVA-浮石、0.1% Pd-ZnIn2S4-PVA:ZS-30(4:1)-浮石、0.1% Pd-ZnIn2S4-PVA:ZS-30(2:1)-浮石和0.1% Pd-ZnIn2S4-PVA:ZS-30(1:1)-浮石4种负载型催化剂的活性组分脱落率分别为4%、6.30%、7.20%和9.60%,随着PVA:ZS-30比例的不断增加,光催化剂脱落率逐渐升高。分析认为,ZS-30与PVA之间形成的Si-O-C键降低了PVA的粘结力,因此,单独的PVA具有更好的粘合作用与最小的活性组分脱落率。
由图3(c)可知,在碘镓灯条件下,0.1% Pd-ZnIn2S4-PVA-浮石、0.1% Pd-ZnIn2S4-PVA:ZS-30(4:1)-浮石、0.1% Pd-ZnIn2S4-PVA:ZS-30(2:1)-浮石和0.1% Pd-ZnIn2S4-PVA:ZS-30(1:1)-浮石4种催化剂对CBZ的降解率分别为100%、84.8%、74.8%和67.9%;太阳光照下(图3(d))4种催化剂对CBZ的降解率分别为88.8%、71%、48.6%和34.5%。结果表明,在以上2种光照条件下,4种催化剂的光催化活性顺序保持一致,0.1% Pd-ZnIn2S4-PVA-浮石催化剂活性最高,ZS-30粘合剂的加入降低了催化剂活性;4种催化剂在碘镓灯下的光催化活性均高于太阳光。分析认为,100 W碘镓灯光源距离反应液的液面近(125 mm,图2),实验时光强稳定在1 000 W·m−2,而且紫外线含量高;而太阳光的光强不稳定,由正午时分的1 200 W·m−2逐渐降至下午的800 W·m−2,在光照时紫外线含量低于碘镓灯。
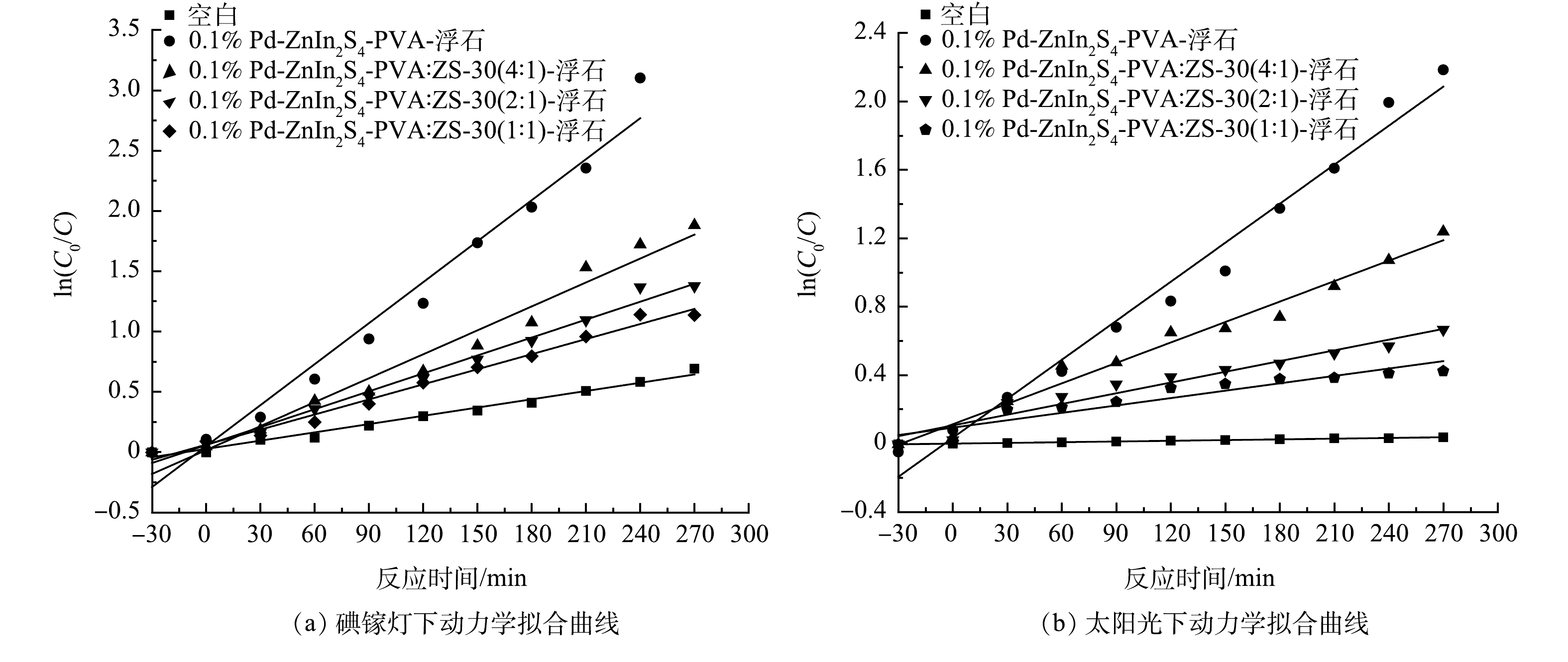
为了进一步分析催化剂活性和CBZ光降解速率的变化,以碘镓灯下0.1% Pd-ZnIn2S4-PVA:ZS-浮石催化剂光催化降解CBZ为例,根据一阶动力学方程拟合了不同比例PVA:ZS-30水基粘合剂制备的负载型催化剂光催化降解CBZ的动力学曲线,结果如图4所示,动力学方程及其相关参数见表1。基于图4的动力学拟合曲线和表1中相关系数(R2)大于0.97,证实0.1% Pd-ZnIn2S4-PVA:ZS-浮石催化剂光催化降解CBZ遵循伪一阶动力学,反应速率与反应物浓度的一次方成正比。由表1可以看出,在碘镓灯光照下0.1% Pd-ZnIn2S4-PVA-浮石可显著提高速率常数(K)且减少半衰期(t1/2),与不加催化剂相比,其K和t1/2值分别增加了4.93倍和降至20%;K值与t1/2的变化趋势与催化剂活性大小保持一致,明确量化了CBZ光解速度和降解时间,同时再一次说明了单独PVA粘合制备的催化剂活性高于PVA与ZS-30混合制备的催化剂。
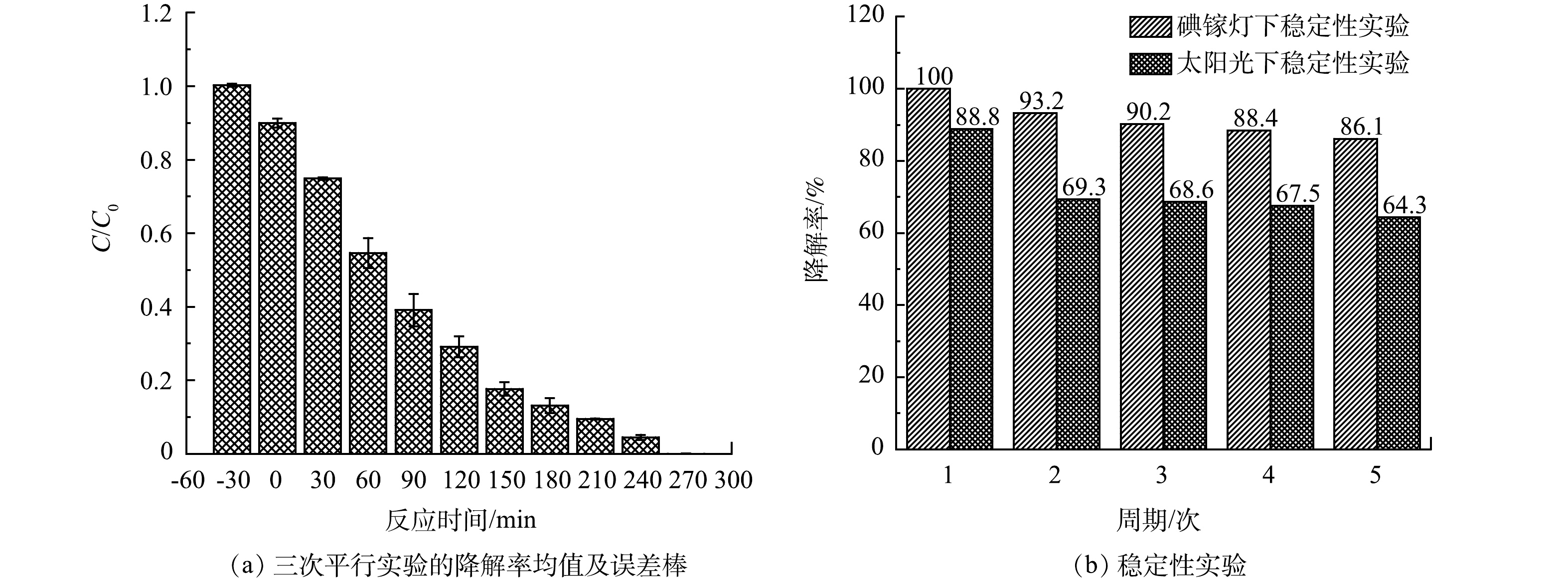
为确保实验数据的稳定性,对0.1% Pd-ZnIn2S4-PVA-浮石负载型催化剂在碘镓灯下开展3次重复实验,结果如图5(a)所示。由图5(a)可知,C/C0的标准差均小于0.05,误差范围小,证实了实验的准确性与稳定性。在碘镓灯及太阳光照射下对0.1% Pd-ZnIn2S4-PVA-浮石负载型催化剂开展5次稳定性实验测试,结果见图5(b)。由图5(b)可知,0.1% Pd-ZnIn2S4-PVA-浮石催化剂在碘镓灯下光催化CBZ的降解率由第1次的100%下降至第5次的86.1%;太阳光下对CBZ的降解率由第1次的88.8%下降至第5次的64.3%,稳定性实验中0.1% Pd-ZnIn2S4-PVA-浮石负载型催化剂的光催化剂活性略有降低。分析认为,CBZ光催化降解过程中会生成酸性中间产物,苯环最终被裂解生成各种短链脂肪酸[20-21],带正电荷的中间产物会吸附在带负电荷的0.1% Pd-ZnIn2S4-PVA上,使得催化剂的有效活性位点数量减少,光能利用率下降而造成电子-空穴对数量减少,从而导致催化剂的活性降低。稳定性测试表明,0.1% Pd-ZnIn2S4-PVA-浮石负载型催化剂经过5个周期的循环使用后,仍具有良好的光催化活性。
-
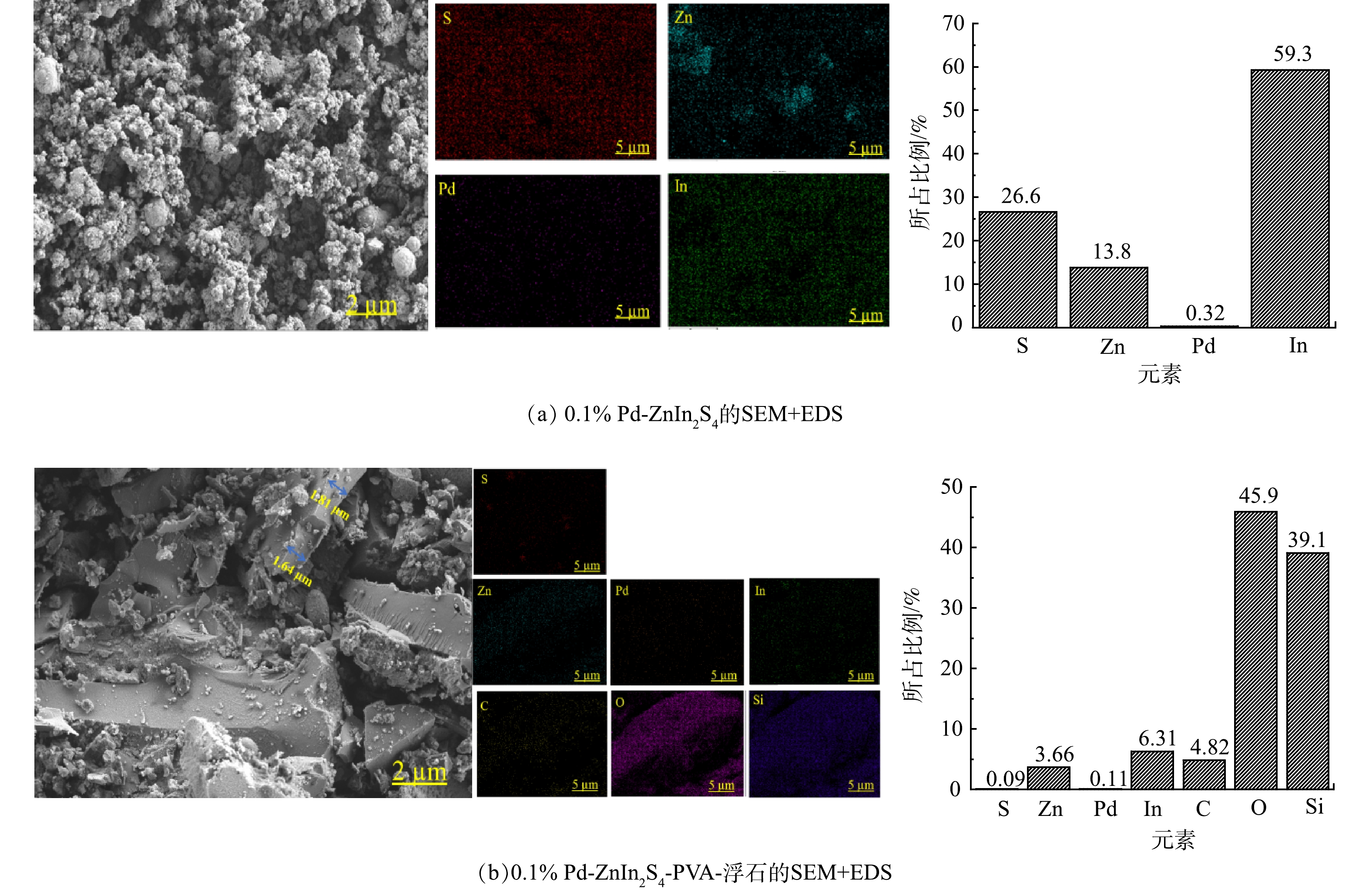
1) SEM+EDS表征。0.1% Pd-ZnIn2S4粉末催化剂的SEM照片如图6(a)所示。由图6(a)可知,0.1% Pd-ZnIn2S4光催化剂呈现大小不一的球状结构,与田野[11]XRD表征中粉末催化剂为六方晶相的结果相一致;EDS测试的In:S:Zn:Pd质量比为230:128:65:1,这与理论值197:90:47:1基本保持一致,其差别与EDS的半定量分析有关。将0.1% Pd-ZnIn2S4-PVA活性负载层从浮石表面刮下进行表征,由图6(b)能够观察到活性负载层的厚度约在1.64~1.81 µm,PVA将0.1% Pd-ZnIn2S4与浮石相互粘结;EDS谱图中除了S、Zn、Pd、In元素外,还存在一定量的C、O、Si等元素,这与PVA的掺入以及浮石自身的化学结构有关。
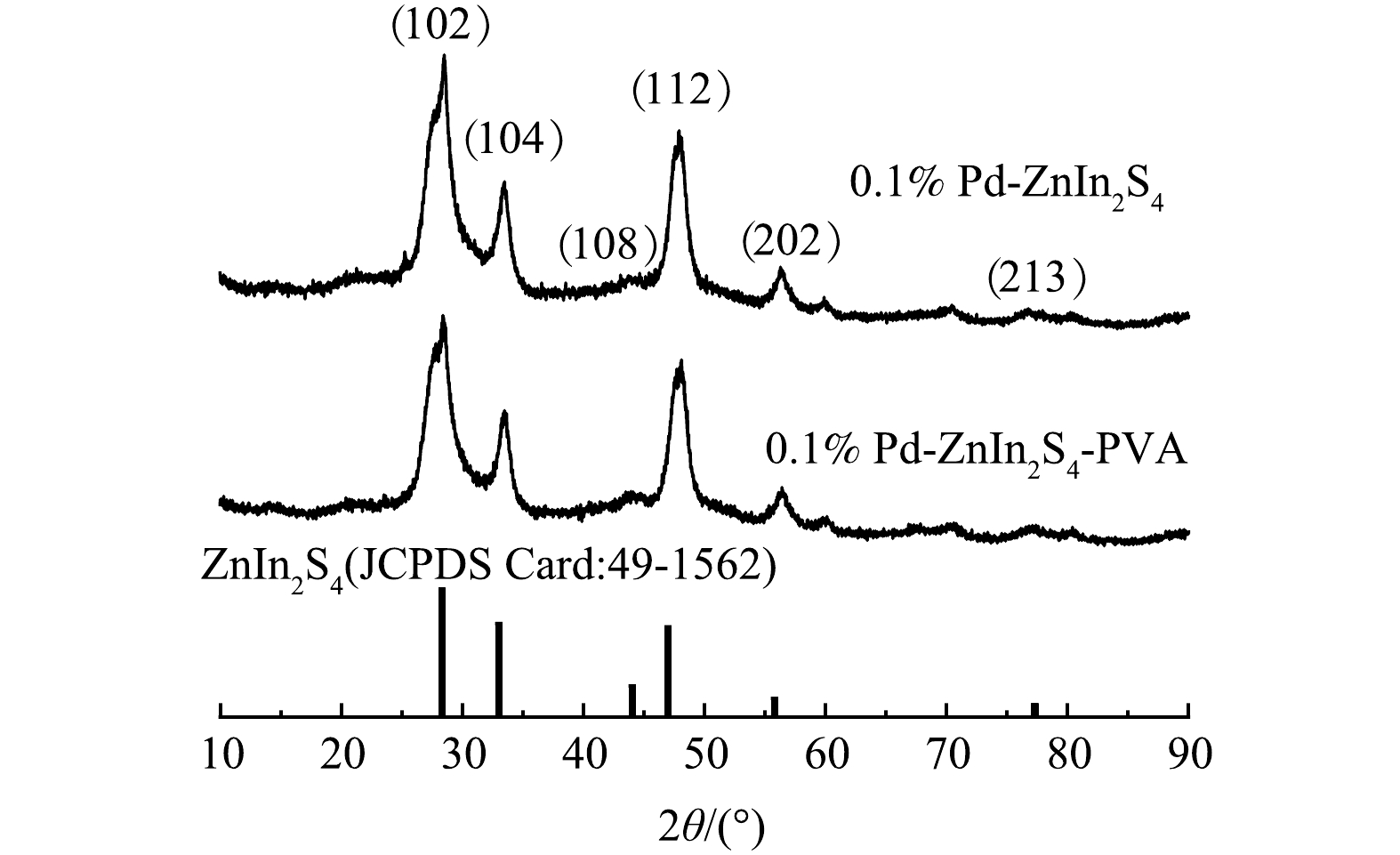
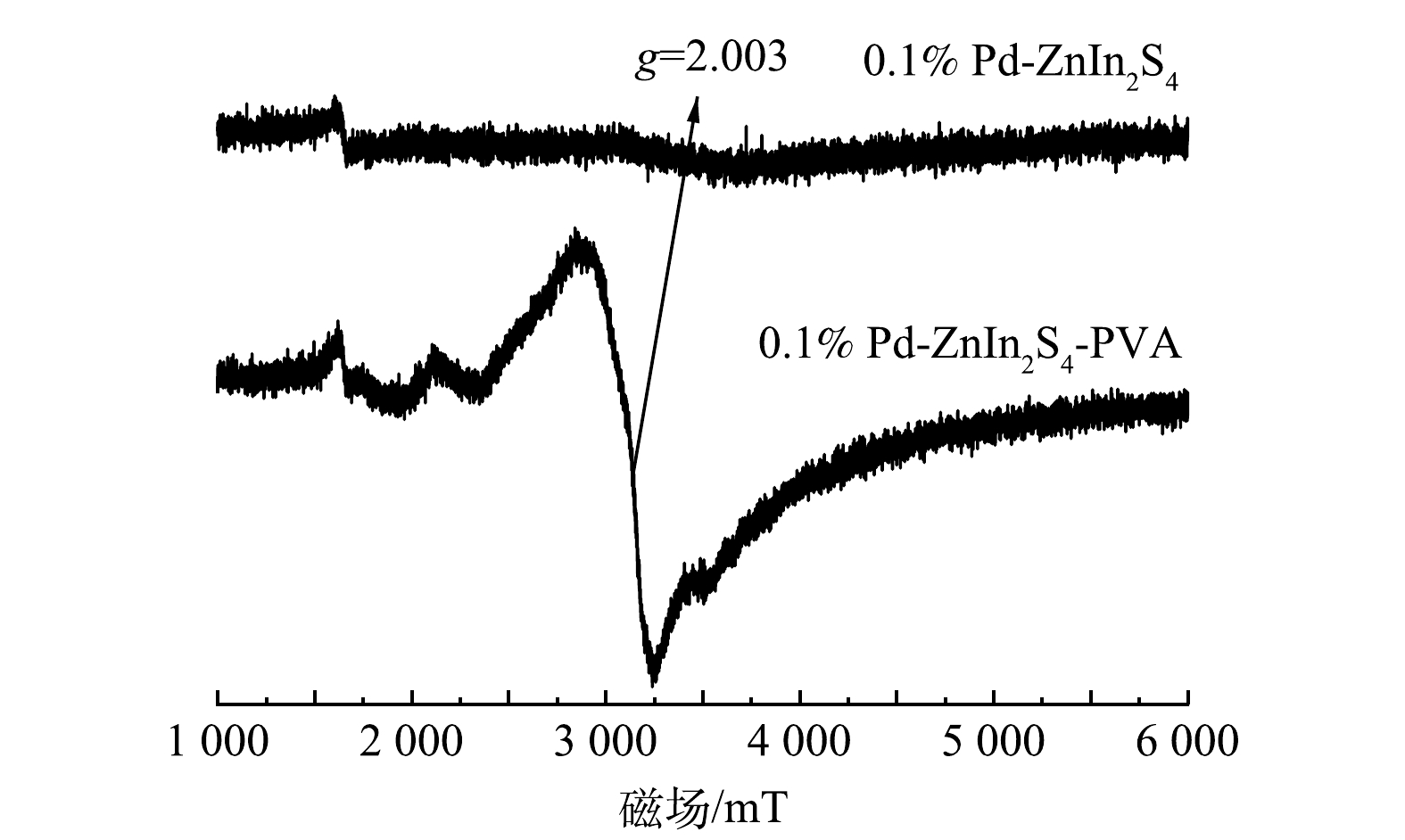
2) XRD表征。由0.1% Pd-ZnIn2S4和0.1% Pd-ZnIn2S4-PVA这2种催化剂的XRD谱图(图7)可知,六方晶相的ZnIn2S4的(102)、(104)、(108)、(112)、(202)和(213)晶面特征峰清晰可见,表明水热合成制备的0.1% Pd-ZnIn2S4光催化剂中存在ZnIn2S4晶体[22]。在0.1% Pd-ZnIn2S4-PVA图谱中,也可以观察到六方晶相结构,表明PVA的引入并未使材料改变六方晶相结构。利用Scherrer公式计算出0.1% Pd-ZnIn2S4和0.1% Pd-ZnIn2S4-PVA两种催化剂的晶粒尺寸分别为3.66 nm和3.53 nm,推测晶粒尺寸的减小与PVA中-OH对Pd-ZnIn2S4活性颗粒的分散作用有关;另外,峰强的轻微变化,推测与PVA和0.1% Pd-ZnIn2S4之间相互作用而产生了晶格缺陷有关。ZOU[23]等研究发现,由于晶格缺陷的引入,BiOCl-X的晶粒平均尺寸由28 nm减小为10 nm,其利用ESR技术对晶格缺陷进行了测量,观察到三维球形BiOCl-X在g=2.003处有一个宽峰,峰强增大表明了晶格缺陷的存在。
3) EPR测试分析。由于PVA与0.1% Pd-ZnIn2S4之间形成新的氢键,会引起材料的缺陷[24],因此,使用EPR对0.1% Pd-ZnIn2S4-PVA材料进行晶格缺陷测试(图8)。由图8可以看出,0.1% Pd-ZnIn2S4-PVA在g=2.003处有1个宽峰,并且峰强明显增高,证实了晶格缺陷的存在。RAO等[25]通过EPR发现纯PVA材料不含任何过渡金属杂质或其他顺磁中心(缺陷),这表明EPR信号的增强是因为0.1% Pd-ZnIn2S4与PVA之间相互作用而并非PVA本身信号引起的。此外,ZOU等[23]研究发现,晶格缺陷的存在使BiOCl-X (X=1,3,5)的ESR峰强增大,同时观察到材料颜色由白色逐渐变为灰褐色。而0.1% Pd-ZnIn2S4在加入PVA后催化剂表面颜色由深黄色变为浅棕色,这也进一步验证了催化剂表面存在缺陷,该结果与XRD分析结果相吻合。
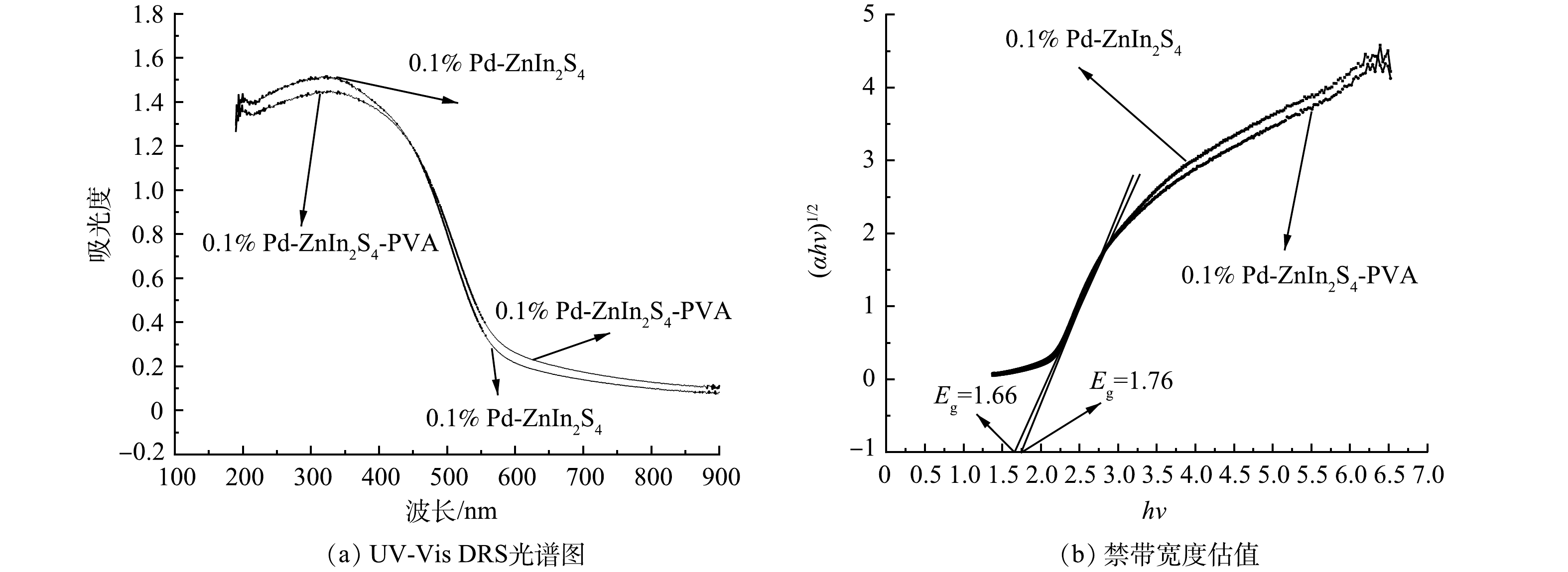
4) UV-Vis DRS分析。0.1% Pd-ZnIn2S4和0.1% Pd-ZnIn2S4-PVA的紫外-可见漫反射光谱图(UV-Vis DRS)(图9(a))可知,2种催化剂对紫外光和可见光均有吸收能力,且在紫外光区(200~400 nm)的吸收强于在可见光区域(400~800 nm),0.1% Pd-ZnIn2S4-PVA在可见光的吸光能力略强于0.1% Pd-ZnIn2S4;PVA的掺入使0.1% Pd-ZnIn2S4发生了轻微红移,这可能与催化剂缺陷引起的光响应红移有关[26-27]。图9(b)中外推直线(αhv)1/2与hv的交点,可得出0.1% Pd-ZnIn2S4和0.1% Pd-ZnIn2S4-PVA的带隙宽度分别为1.76 eV和1.66 eV;0.1% Pd-ZnIn2S4的带隙宽度与之前的研究结果一致[11],0.1% Pd-ZnIn2S4-PVA变窄的带宽有利于光照下电子跃迁而产生更多的电子-空穴对,从而提高其光催化活性。
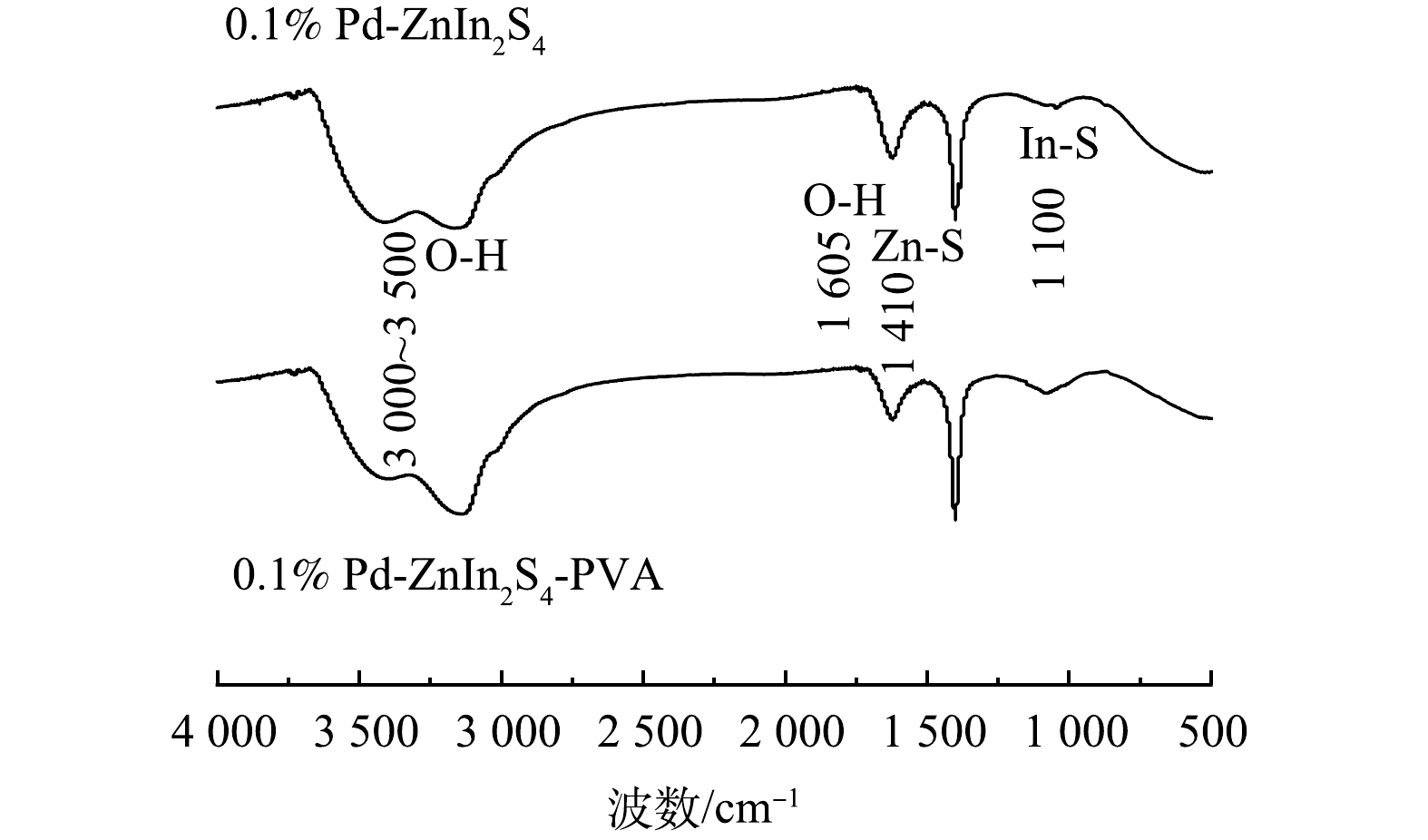
5) FTIR表征。0.1% Pd-ZnIn2S4和0.1% Pd-ZnIn2S4-PVA的傅里叶变换红外光谱(FTIR)如图10所示。可以看出,在3 000~3 500 cm−1内,PVA的加入增强了—OH对称拉伸振动,在2 800~3 000 cm−1 和1 300~1 500 cm−1处的吸收峰归因于—CH2拉伸和 CH/CH2变形振动;MUHAMMAD等[28]研究发现,—OH和—C—OH拉伸振动在氢键形成和界面相互作用中起着关键因素。这说明图谱的变化不是因为PVA本身,而是因为0.1% Pd-ZnIn2S4-PVA之间存在相互作用,进一步证实0.1% Pd-ZnIn2S4和PVA之间存在强烈的分子间氢键,氢键不仅使粉末催化剂的官能团发生一定程度的偏移,而且其强度也受到了一定程度上的影响[29]。在图10中 1 605 cm−1处存在—OH的拉伸振动,进一步说明氢键的存在,这与上述XRD、EPR的表征结果相呼应。1 410 cm−1和1 100 cm−1处分别出现Zn-S和In-S的振动峰[30-31],这与ZnIn2S4的六方晶相结构有关。
-
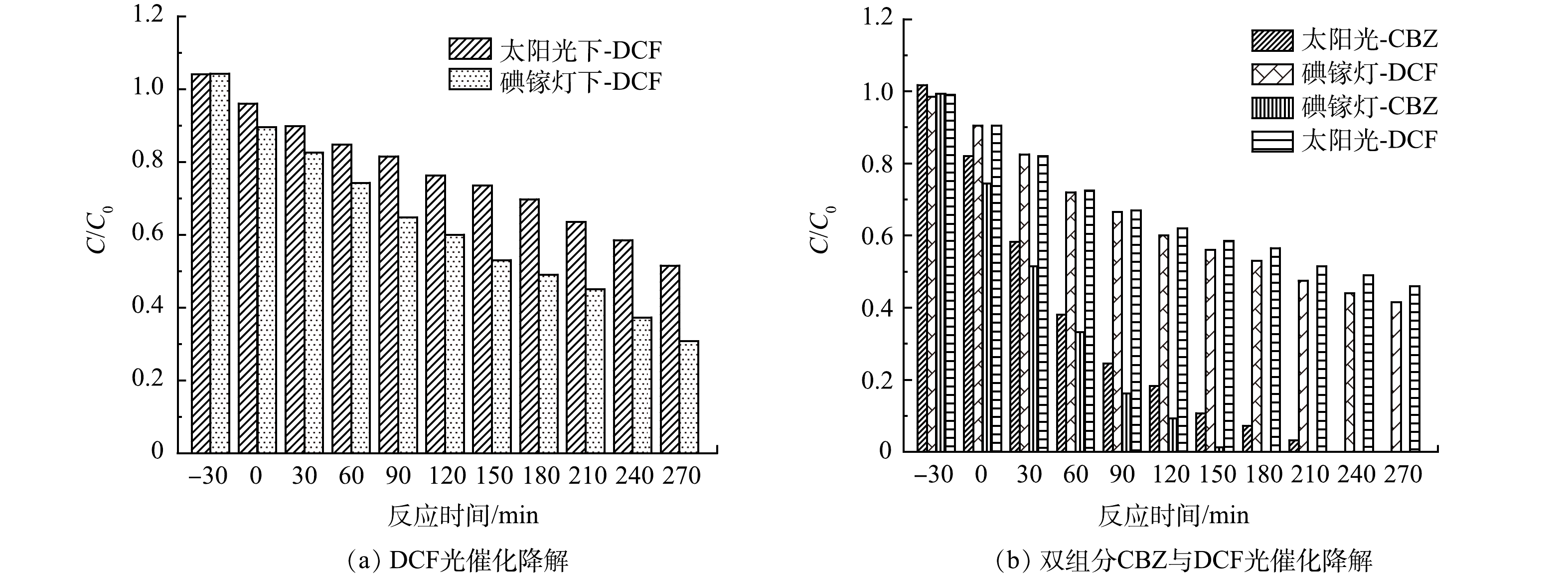
使用0.1% Pd-ZnIn2S4-PVA-浮石光催化降解单组分DCF、双组分的CBZ与DCF,实验条件同2.1、双组分溶液中CBZ和DCF的初始质量浓度均为50 µg·L−1,结果如图11所示。由图11(a)可见,在碘镓灯和太阳光照射下,初始质量浓度为100 µg·L−1的DCF的降解率分别为69.3%和48.5%。可以看出,碘镓灯下的降解效果好于太阳光,这与碘镓灯光强稳定、紫外光含量高有关。与图3(c)和图3(d)的CBZ光催化降解效率相比,DCF的降解效果弱于CBZ。由图11(b)中CBZ与DCF双组分的光催化降解结果可知,碘镓灯照射下光催化反应进行210 min时CBZ已经完全降解,相同条件下DCF反应270 min时降解率为58.5%;太阳光下光催化反应进行270 min时CBZ全部降解,此时DCF的降解率为54%。由此可见,对于双组分PhACs的光催化降解反应,碘镓灯下对PhACs的降解效果也好于太阳光,这与单组分的实验结果一致。对于同一体系中CBZ的降解效率强于DCF。分析认为:溶液初始pH对降解效率有影响,LEE等[32]使用类芬顿法降解CBZ和DCF时发现,溶液初始pH呈中性时有利于CBZ的降解,而呈酸性则有利于DCF降解。本实验中溶液初始pH为7.2,因此,在相同条件下0.1% Pd-ZnIn2S4-PVA-浮石对CBZ表现出良好的光催化降解效果。
-
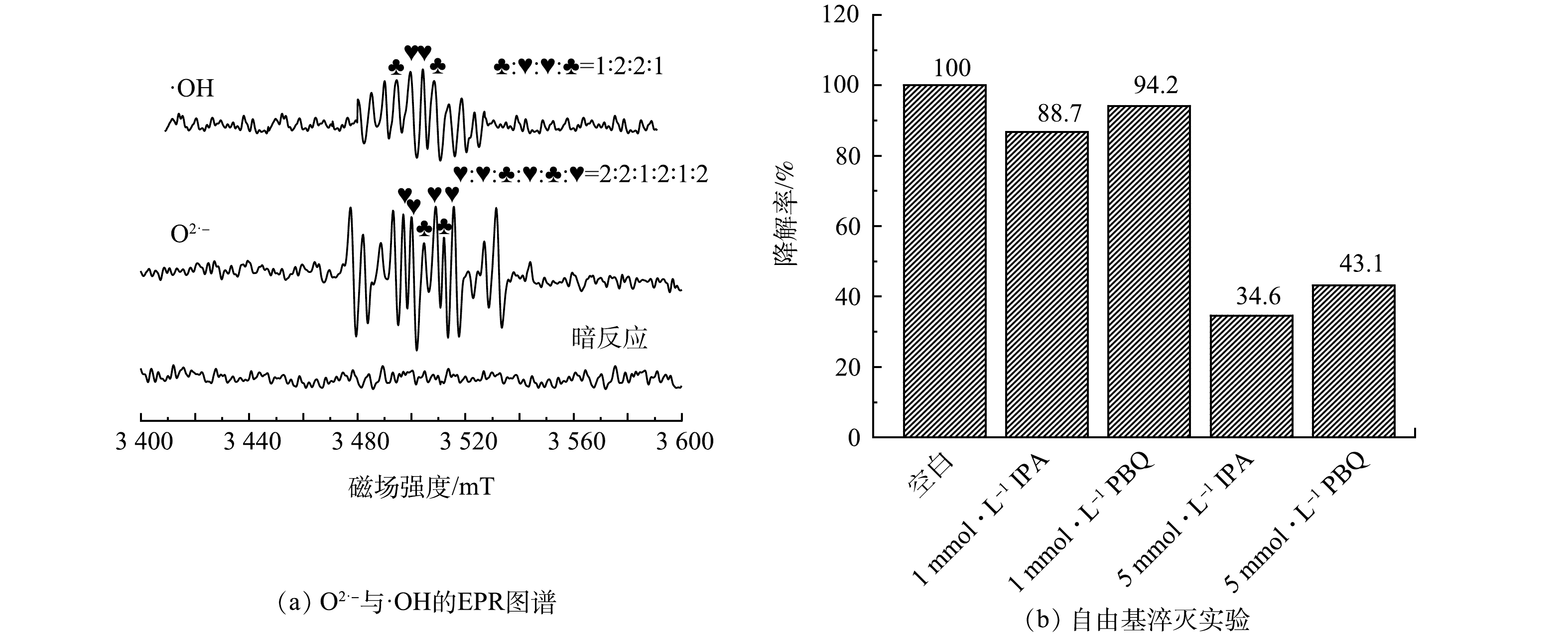
为验证0.1% Pd-ZnIn2S4-PVA-浮石负载型催化剂光催化降解CBZ过程中所产生的自由基及其作用,首先对自由基进行EPR测试,随后进行自由基捕获实验(图12)。由图12(a)可以看出,在以5,5-二甲基-1-吡咯啉-N-氧化物(DMPO)作为自由基陷阱条件下,催化剂在暗反应下没有显示出羟基(·OH)和超氧自由基(O2·−)的特征峰;打开光源后,可以明显看到具有1:2:2:1及2:2:1:2:1:2的与·OH和O2·−信号峰相对应的典型峰强度曲线,证实了光催化反应过程中·OH和O2·−的存在。实验使用1 mmol·L−1和5 mmol·L−1的PBQ和IPA分别捕获O2·−和·OH[33],由图12(b)可知,当PBQ和IPA投加量为1 mmol·L−1时,捕获剂对自由基影响较小,但可以看出·OH对光解的贡献程度大于O2·−;当PBQ投加量为5 mmol·L−1时,O2·−对CBZ降解率的抑制程度为56.9%;IPA投加量为5 mmoL·L−1时,·OH对CBZ降解率的抑制程度为65.4%。上述实验研究表明,在0.1% Pd-ZnIn2S4-PVA-浮石负载型催化剂光催化降解CBZ体系中·OH对光解的贡献程度大于O2·−。
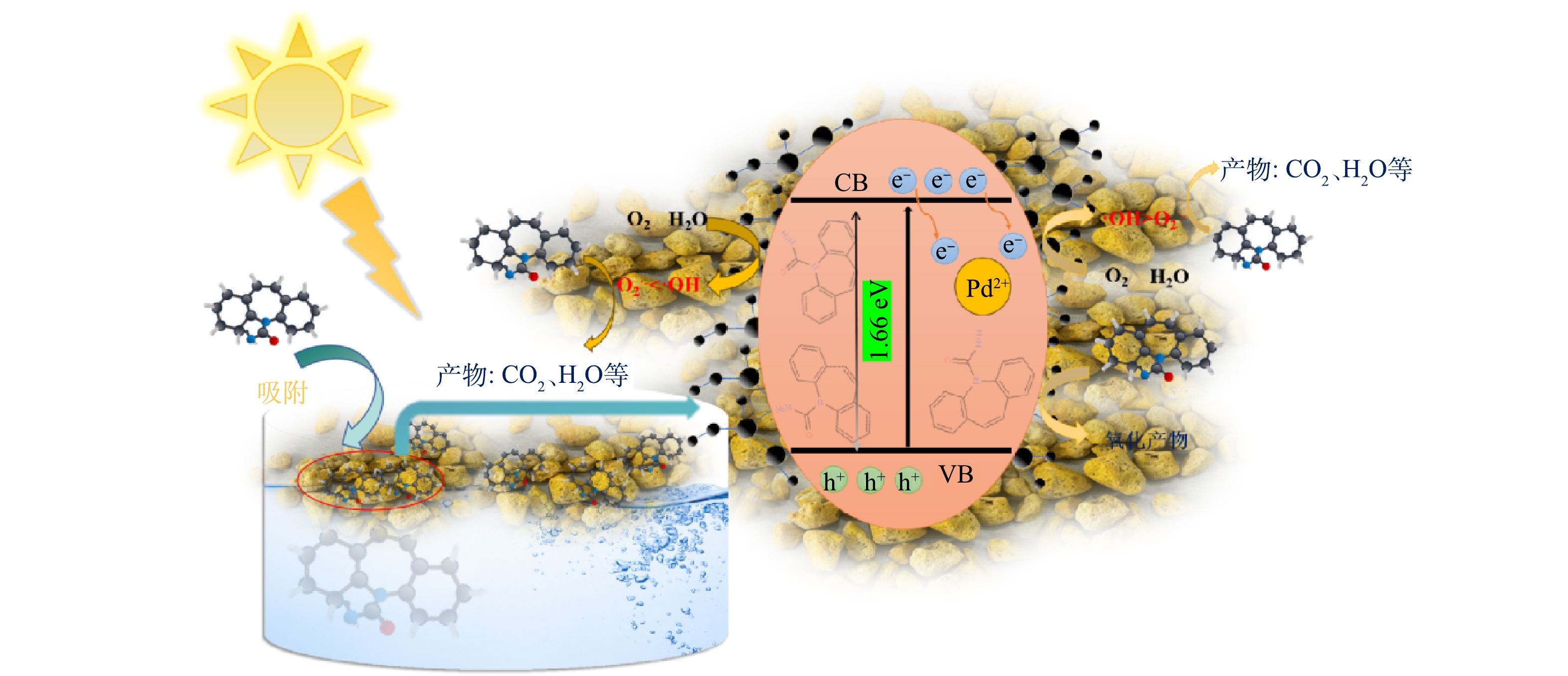
依据催化剂活性实验与表征测试结果,推测0.1% Pd-ZnIn2S4-PVA-浮石负载型催化剂光降解CBZ的机理(图13)。首先,PVA通过表面粘合力和分子间氢键与0.1% Pd-ZnIn2S4相结合,浮石则通过化学键将0.1% Pd-ZnIn2S4-PVA活性组分吸附于表面及孔隙内部。可见光照射下,浮在水面上的0.1% Pd-ZnIn2S4-PVA-浮石负载型催化剂吸收光能而发生电子跃迁,生成e−和h+;掺杂入ZnIn2S4晶体内的Pd2+可以截获一部分回流的电子,将他们转移到Pd2+的空轨道上[11],从而有效地抑制了电子-空穴对的复合;e−与Pd截留的e−和水中的氧分子(O2)以及水分子(H2O)反应生成活性物种O2·−和·OH等,·OH和O2·−能够立即氧化水中的CBZ而使其降解,降解过程中自由基的贡献程度为·OH>O2·−。此外,由于价带上的h+具有很强的氧化能力,其可会直接将一部分CBZ转换为氧化产物。
-
1) 通过PVA水基粘合剂将粉末态0.1% Pd-ZnIn2S4光催化剂粘附于多孔浮石上制备的0.1% Pd-ZnIn2S4-PVA-浮石负载型催化剂在碘镓灯和太阳光照射下可高效降解水体中的CBZ和DCF,碘镓灯光催化效果好于太阳光的原因归属于光强稳定和紫外线含量高,CBZ去除效率高于DCF则推测与反应液pH为中性有关。
2) 与直接光解相比,碘镓灯光照下0.1% Pd-ZnIn2S4-PVA-浮石光催化降解CBZ的速率常数增加了4.93倍,半衰期减至原来的20%;5个周期的循环使用证实了催化剂具有良好的催化活性和稳定性。
3) 光催化剂粘附层厚度约为1.64~1.81 µm,PVA的存在很好地分散了0.1% Pd-ZnIn2S4颗粒,晶格缺陷的存在有利于活性自由基的生成,而禁带宽度的变窄则有利于电子跃迁而生成更多的电子-空穴对,从而提高催化剂活性。
4) ·OH和O2·−自由基被检测证实存在于CBZ的光催化降解反应中,·OH对CBZ降解的贡献大于O2·−,CBZ在活性自由基和h+共同作用下被完全氧化降解。
Pd-ZnIn2S4-PVA-浮石负载型催化剂制备及其光催化降解水中PhACs性能
Preparation of Pd-ZnIn2S4-PVA-pumice supported catalyst and its performance on photocatalytic degradation of pharmaceuticals in water
-
摘要: 光催化是降解水体中痕量医药类物质卡马西平(CBZ)、双氯芬酸(DCF)等的有效技术,负载型光催化剂的开发可解决粉末催化剂不宜回收且易造成二次污染的问题。本研究采用水热合成-超声浸渍法制备浮石负载型催化剂,证实聚乙烯醇(PVA)粘合效果好于中性硅溶胶(ZS-30)、磷酸二氢铝(Al(H2PO4)3)和聚乙二醇400(PEG-400),超声振荡测得0.1% Pd-ZnIn2S4-PVA-浮石催化剂的脱落率为4%。在碘镓灯和太阳光照射下,1.5 g·L−1的0.1% Pd-ZnIn2S4-PVA-浮石催化剂对50 mL、初始质量浓度100 µg·L−1 CBZ的降解率为100%和88.8%,CBZ的光催化降解遵循伪一阶动力学。PVA与0.1% Pd-ZnIn2S4之间以氢键连接,并成功为负载型光催化剂引入了晶格缺陷,溶液中的CBZ和DCF被催化剂表面光照产生的·OH和O2·−自由基氧化降解。以上研究结果可为光催化技术净化水中痕量药物的实际应用提供参考。Abstract: Photocatalysis is an effective technology for the degradation of trace pharmaceuticals, such as carbamazepine (CBZ) and diclofenac (DCF), in aquatic environment. The development in supported photocatalyst can solve the problems of difficult recycling and easy to bring secondary pollution for powdery catalyst. In this study, a type of pumice supported catalyst was prepared by a hydrothermal synthesis and ultrasonic impregnation method, and the result confirmed that the adhesion of polyvinyl alcohol (PVA) was better than that of neutral silica sol (ZS-30), aluminium dihydrogen phosphate (Al(H2PO4)3) or polyethylene glycol 400 (PEG-400). An ultrasonic oscillating method determined the shedding rate of 0.1% Pd-ZnIn2S4-PVA-pumice catalyst was 4%. Under the irradiation of iodine gallium light or sunlight, 1.5 g·L−1 catalyst could result in 100% or 88.8% CBZ degradation efficiencies, respectively, with 50 mL reaction solution and CBZ initial concentration of 100 µg·L−1. The photocatalytic degradation of CBZ followed a pseudo first-order reaction kinetics. PVA connected with 0.1% Pd-ZnIn2S4 by hydrogen bond, and the crystal defects were successfully introduced onto the surface of the photocatalyst. CBZ and DCF in solution were oxidized by both ·OH and O2·− free radicals that produced on the catalyst surface under light irradiation. Above result can provide a reference for the real application of photocatalytic technology in the purification of aquatic environment that containing trace pharmaceuticals.
-
Key words:
- supported catalyst /
- photocatalysis /
- PhACs /
- mechanism /
- characterization
-
土壤是人类赖以生存的物质基础和不可或缺的自然资源,随着工业化、城镇化的加快推进,土壤重金属污染问题也日益突出。铅(Pb)作为土壤累积率最高的重金属元素,来源广泛且生态危害大,“三废”的排放、农药化肥的不合理施用、矿山开采及冶炼活动等都会造成土壤Pb污染,其中尤以铅锌矿的开采及冶炼过程最为严重[1]。进入土壤中的Pb在各粒级颗粒中的分布具有不均一性,其会优先依附于小颗粒土壤[2],粒径越细,对重金属的富集能力越强[3]。同时,细小的土壤颗粒在胶体共迁移的作用下更容易发生迁移,造成其他环境介质的污染[4-5]。另外,受土壤理化性质的影响,进入土壤中Pb的化学活性分布也具有高度不均一性,进而导致生物对Pb的吸收效率存在较大差异,因此,对生物体的影响程度也明显不同[6]。
重金属污染土壤的修复技术类型多样,不同修复方法的适用范围和修复效果也各不相同。其中,原位化学钝化法在修复时间和经济成本上能更好地满足重金属污染土壤的修复要求,其钝化效果及机理得到广泛研究。有机物料、石灰和磷酸盐来源广泛、价格低廉,且对重金属有着良好的钝化效果[7-8]。但上述3种钝化剂的钝化修复机理有很大差异,有机物料修复机理较复杂,其本身可以通过吸附、络合/螯合、氧化还原等方式降低重金属有效性,又可以通过影响土壤理化性质及土壤微生物的丰度与活性以间接减轻重金属的毒害性[9]。石灰与磷酸盐的钝化机理较单一,石灰主要通过提高土壤pH以促进Pb2+形成氢氧化铅及碳酸铅沉淀而降低其有效性[10];磷酸盐可以与Pb形成磷酸盐沉淀,并且当土壤中存有Cl−、F−等卤素离子时,可以形成非常稳定的磷铅矿类物质[11]。但土壤不是一个均质体,不同成分在土壤中的分布并不均匀[12]。土壤颗粒作为土壤最基本的组成部分,积极参与生态系统的地球化学过程,不同粒级土壤组分上存在不同的土壤固相组成,导致很多特定的反应或现象只出现在特定的粒径范围之内[13],因此,在研究钝化剂修复效果的同时,也应考虑重金属在土壤中不同粒级间的转化富集。虽然关于Pb污染土壤的化学钝化修复已有大量报道,但绝大多数研究均把土壤作为均质体,而钝化剂添加后对不同粒级土壤中Pb的迁移转化的相关研究较少,钝化剂修复Pb污染土壤的内在微观机制仍不明晰,钝化处理后的土壤对其他环境介质的影响尚未探究。
本研究以外源Pb污染的贵州黄壤为研究对象,以羊厩肥、石灰、磷酸盐为钝化材料,通过室内钝化培养实验,比较了不同钝化剂对外源Pb污染土壤的钝化修复效果,分析了不同钝化剂在不同添加量下在各个粒级土壤中对Pb的富集状况和形态分布的影响,探讨了不同钝化剂对Pb在不同粒级土壤中迁移转化规律的影响,以期为羊厩肥、石灰、磷酸盐在Pb污染土壤修复中的高效利用及修复后土壤的潜在生态风险管控提供参考。
1. 材料与方法
1.1 供试材料
供试土壤采自贵阳市花溪区某菜地(106°39′48″E,26°21′20″N),采样深度为0~20 cm,pH为6.46,田间持水量为30.40%,阳离子交换量为31.30 cmol·kg−1,有机质含量为36.89 g·kg−1,全氮含量为2.20 g·kg−1,全磷含量为0.58 g·kg−1,速效磷含量为41.32 mg·kg−1,全钾含量为12.46 g·kg−1,Pb含量为84.58 mg·kg−1。将采集的土壤于室内风干后过2 mm筛,以分析纯级Pb(NO3)2溶液为铅源加入到供试土壤中,使土壤Pb含量达到2 000 mg·kg−1,利用称重法加入超纯水,使土壤保持60%的田间持水量,60 d后风干,磨细过2 mm筛,制成模拟Pb污染土壤。
供试钝化剂分别为羊厩肥、石灰(Ca(OH)2)、磷酸盐(Ca(H2PO4)2·H2O)。其中,羊厩肥采自贵州修文某山羊养殖场,于自然条件下腐熟半年后,风干磨细过2 mm筛备用,其Pb含量为128.81 mg·kg−1;石灰及磷酸盐试剂均为分析纯。
1.2 实验设计
钝化实验设置3组,共12个处理,每个处理重复3次。依次对照空白处理(CK)添加,羊厩肥添加量为1%、2%、5%、10%(GM1、GM2、GM5、GM10);石灰添加量为1%、2%、5%、10%(L1、L2、L5、L10);磷酸盐添加量为1%、2%、5%、10%(P1、P2、P5、P10)。取300 g模拟Pb污染土壤置于500 mL塑料烧杯中,按上述比例将土壤与钝化剂充分混匀,于室温下保持60%田间持水量,钝化培养45 d后取样,自然风干后磨细备用。
1.3 不同土壤粒级分离
参照STEMMER等[14]和武天云等[15]的研究方法,首先对过2 mm筛的土样进行超声处理,再利用湿筛法分离出粗砂粒(2~0.2 mm),最后根据Stokes公式,使用离心法依次分离得到细沙粒(0.2~0.02 mm)、粉粒(0.02~0.002 mm)和黏粒(<0.002 mm)。为确保粒径分离的准确性,分离完成后,立即使用激光粒度仪(Mastersizer2000,英国Malvern公司)对各粒级土壤的粒径分布进行验证。将所得的各粒径土壤于50 ℃下烘干,磨细过0.149 mm筛备用。
1.4 分析方法
土壤理化性质测定参照文献中的方法[16],其中土壤pH使用超纯水浸提(水土比为2.5∶1),pH计(PHS-3C+,上海仪电科学仪器股份有限公司)测定;土壤有机质(SOM)采用重铬酸钾容量法测定;速效磷(Olsen-P)及水溶性磷(CaCl2-P)[17]分别采用pH为8.50的0.50 mol·L−1 NaHCO3及0.01 mol·L−1 CaCl2提取,全自动间断化学分析仪(CleverChem200+,德国DeChem-Tech Gmbh公司)测定。Pb全量[18]采用HCl-HNO3-HF-HClO4消解;植物有效态Pb(DTPA-Pb)[19]使用二乙烯三胺五乙酸浸提;土壤中各形态Pb的含量分布采用改进的BCR连续提取法[20]处理;均使用原子吸收光谱仪(GGX-800,北京海光仪器有限公司)测定。
1.5 数据处理
本研究所有实验数据均使用Excel 2016进行整理及计算,以平均值±标准差的形式作为分析结果;单因素方差分析(One-way ANONA)、Dancan多重比较法(差异性水平为P<0.05)及相关矩阵分析(Pearson)采用SPSS 22.0进行处理;实验数据绘图使用Origin 2017完成。
2. 结果与讨论
2.1 钝化剂添加对土壤理化性质的影响
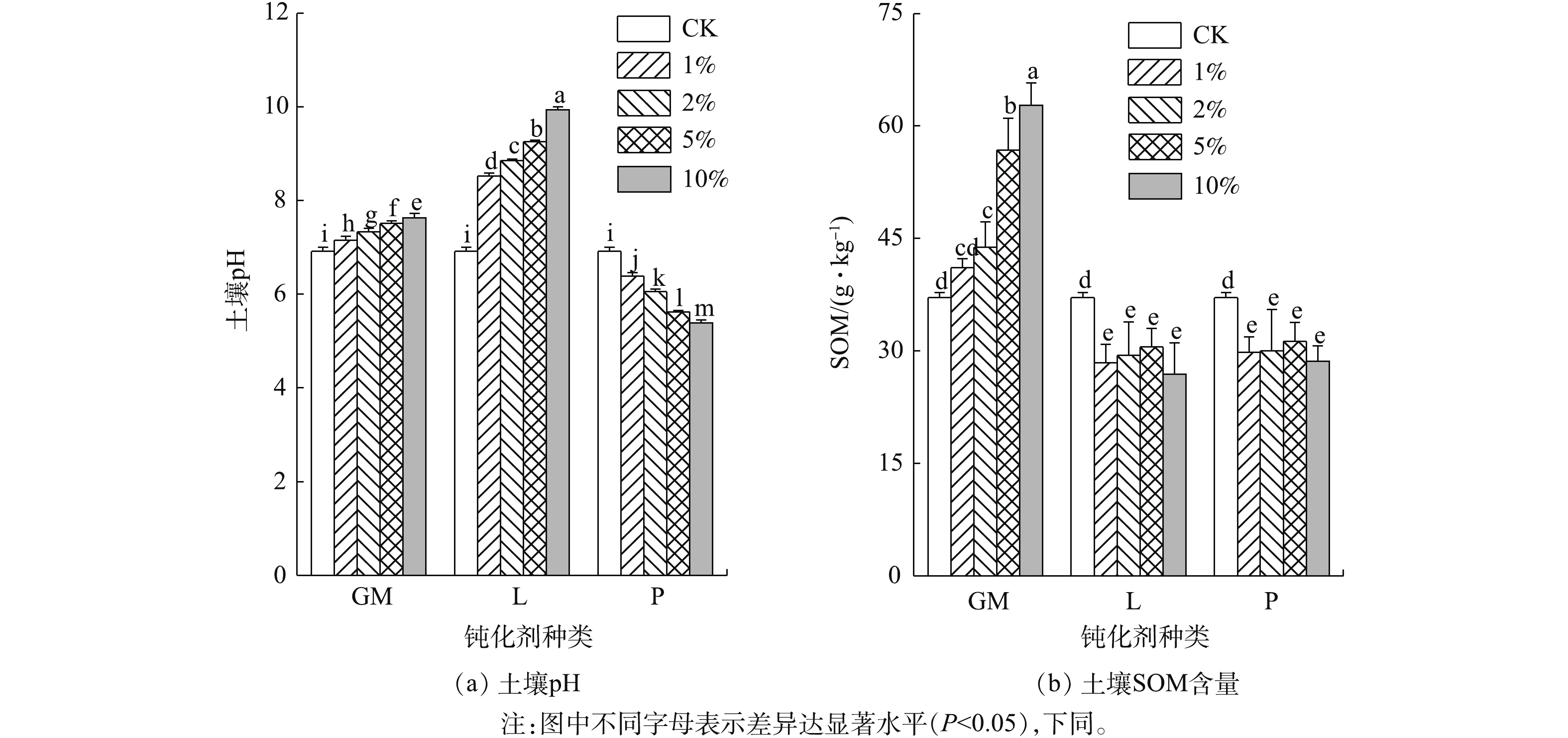
土壤pH与SOM是影响土壤中重金属有效态的重要因素。其中,pH可影响土壤中重金属的溶解-沉淀、吸附-解吸等反应过程,进而影响土壤中重金属的活性[21]。不同钝化剂处理下土壤pH及SOM含量见图1。由图1可知,羊厩肥添加可显著提升土壤pH,与CK相比,各添加量处理组分别提升了pH 0.22、0.43、0.59、0.72个单位,这可能是因为羊厩肥本身含有大量的盐基离子,致使土壤溶液的离子强度与阳离子交换量升高[22];石灰作为碱性钝化剂,对土壤pH有着显著的影响,所有石灰处理组的土壤pH均显著高于空白处理,石灰用量越大,pH增幅越高;磷酸盐施入土壤后可与土壤水溶液发生水解反应生成H3PO4,各添加量分别使土壤pH降低了0.54、0.85、1.25、1.48个单位,均达到显著水平。
SOM可强烈吸附重金属,与金属离子生成螯合物,且SOM具有还原性,能降低水溶态重金属的比例,减轻重金属的毒性[23]。与CK相比,羊厩肥添加均显著提升了SOM含量,GM10提升的幅度最大,为14.88 g·kg−1,GM1提升的幅度最小,为2.30 g·kg−1;石灰及磷酸盐处理均不同程度地降低了SOM的含量,与CK相比,均达到显著相关水平,这可能是由于石灰和磷酸盐的添加改善了土壤微生物的生存条件,使含碳有机物的转化速度加快,从而促进了SOM分解[24]。
磷是植物生长发育所必需的营养元素,土壤磷的存在形式多样,不同赋存形态的磷的移动性和植物可利用性也有很大差异。其中Olsen-P可直接被植物吸收,而CaCl2-P则被认为是土壤磷可被淋溶损失的部分[25]。图2为不同钝化剂处理对土壤Olsen-P和CaCl2-P含量的影响。由图2可知,3种钝化剂处理均显著提高了土壤Olsen-P含量,其中P10对Olsen-P提高量最大,为145.78 mg·kg−1,GM1最小,为24.93 mg·kg−1。羊厩肥既可直接供给磷素,又能促进相关酶和微生物的活性,进而增加Olsen-P含量[26];石灰可促使铁铝氧化物对磷的释放,以增加Olsen-P含量Olsen-P含量[27];磷酸盐属于易溶性磷肥,故对Olsen-P含量影响最大。对CaCl2-P而言,磷酸盐极大地提高了土壤中CaCl2-P的含量,各处理与CK间的差异均达到显著水平;羊厩肥与石灰虽提高了土壤中CaCl2-P的含量,但GM1、GM2、L1、L2和L10间的差异并不显著。
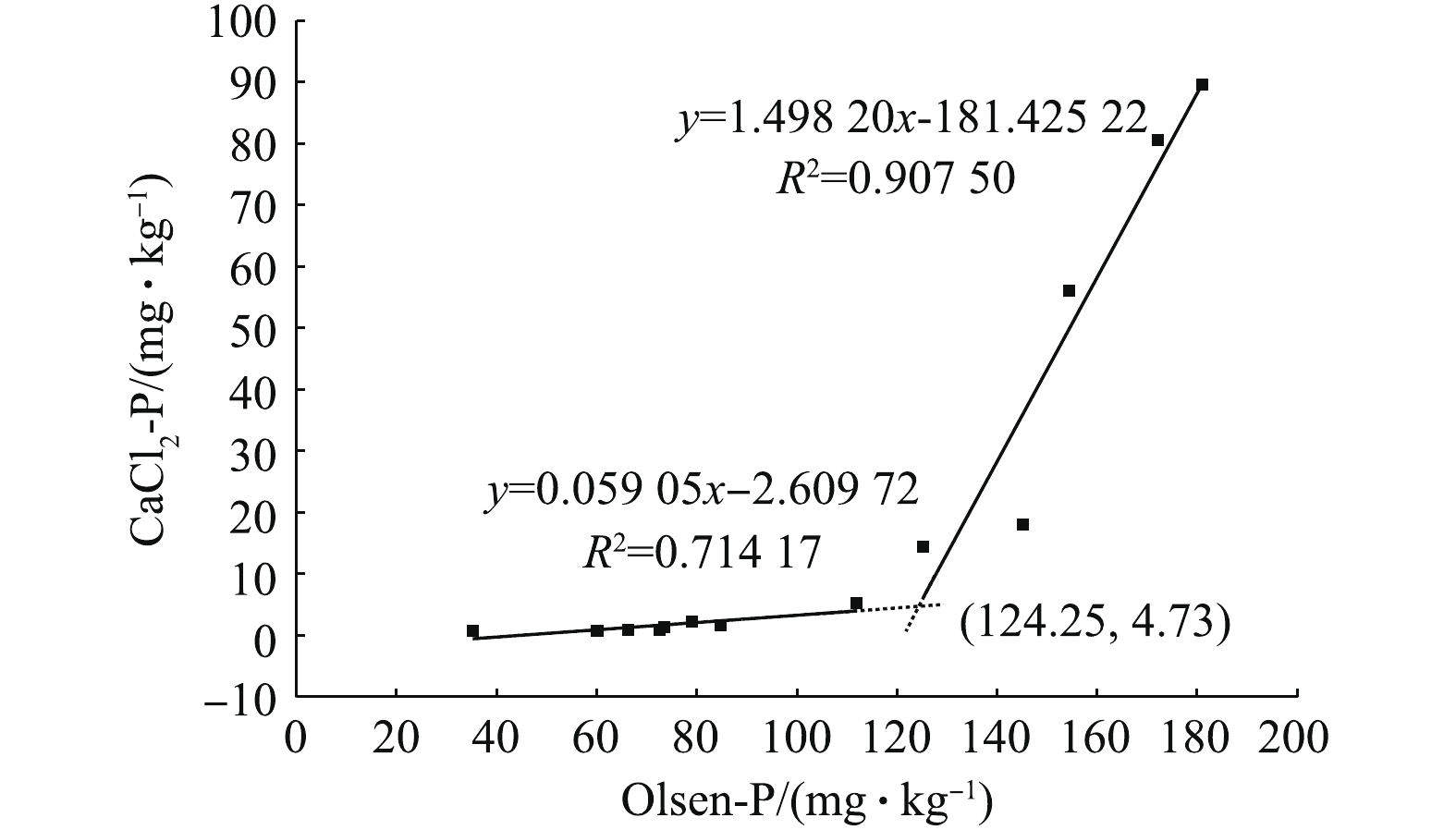
磷可降低土壤重金属的有效性,但土壤在磷素含量过高时,磷会通过淋溶/渗漏的形式向地表或地下水迁移,造成水体富营养化[28]。因此,在保证钝化修复效果的同时,避免其他营养元素的污染也是钝化修复亟待解决的问题。HESKETH等[29]提出的土壤磷淋溶临界值模型可有效评估不同处理对地下水体可能产生的影响。本研究中不同钝化剂处理后土壤Olsen-P与CaCl2-P的关系曲线如图3所示。2条直线相交点即为土壤磷淋溶临界点。结果表明,当土壤Olsen-P含量大于124.25 mg·kg−1时,土壤磷就会发生淋溶,即GM10及所有磷酸盐处理均会造成土壤磷素淋溶现象,使地下水产生富营养化的风险。
2.2 钝化剂添加对土壤Pb形态的影响
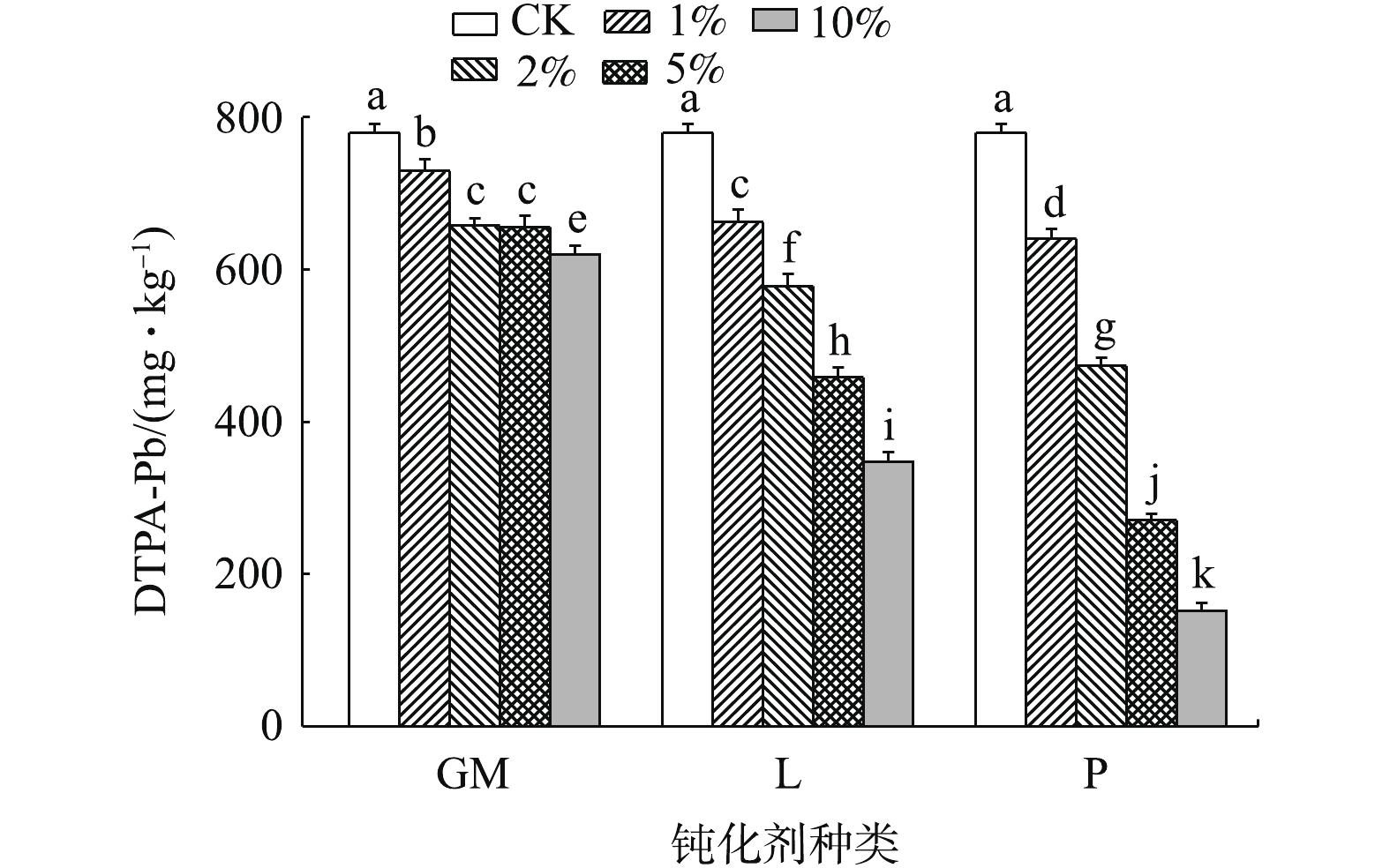
DTPA提取态重金属可直接被植物吸收利用,能直观反映土壤重金属对植物的毒害性,因此,有效降低土壤DTPA提取态重金属含量,对于评估原位钝化修复效果至关重要[30]。由图4可知,3种钝化剂在不同添加量下均显著降低了土壤DTPA-Pb含量,钝化效果随钝化剂添加量的增加而增强,但GM2与GM5之间差异不显著;在所有处理中,GM1降低幅度最小,为6.51%;P10降幅最大,高达80.53%。当钝化剂添加量相同时,对Pb的钝化效果为磷酸盐>石灰>羊厩肥,这与已有研究结果一致:ZENG等[31]通过对比分析氢氧化铅与磷酸铅的稳定性差异认为磷酸盐的钝化效果优于碱性材料;代允超等[32]研究发现酸性土壤添加石灰对于重金属的钝化效果优于有机物料。
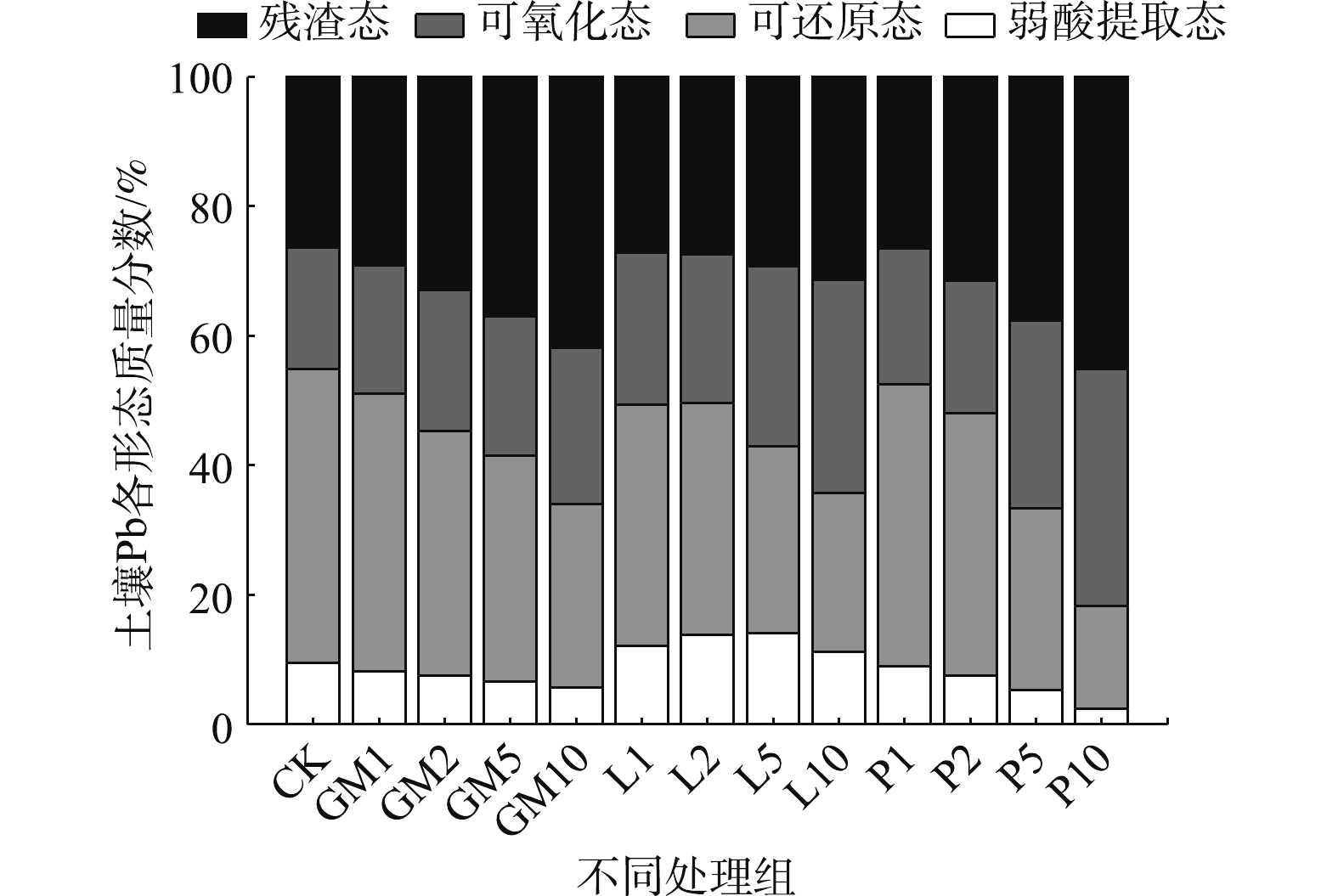
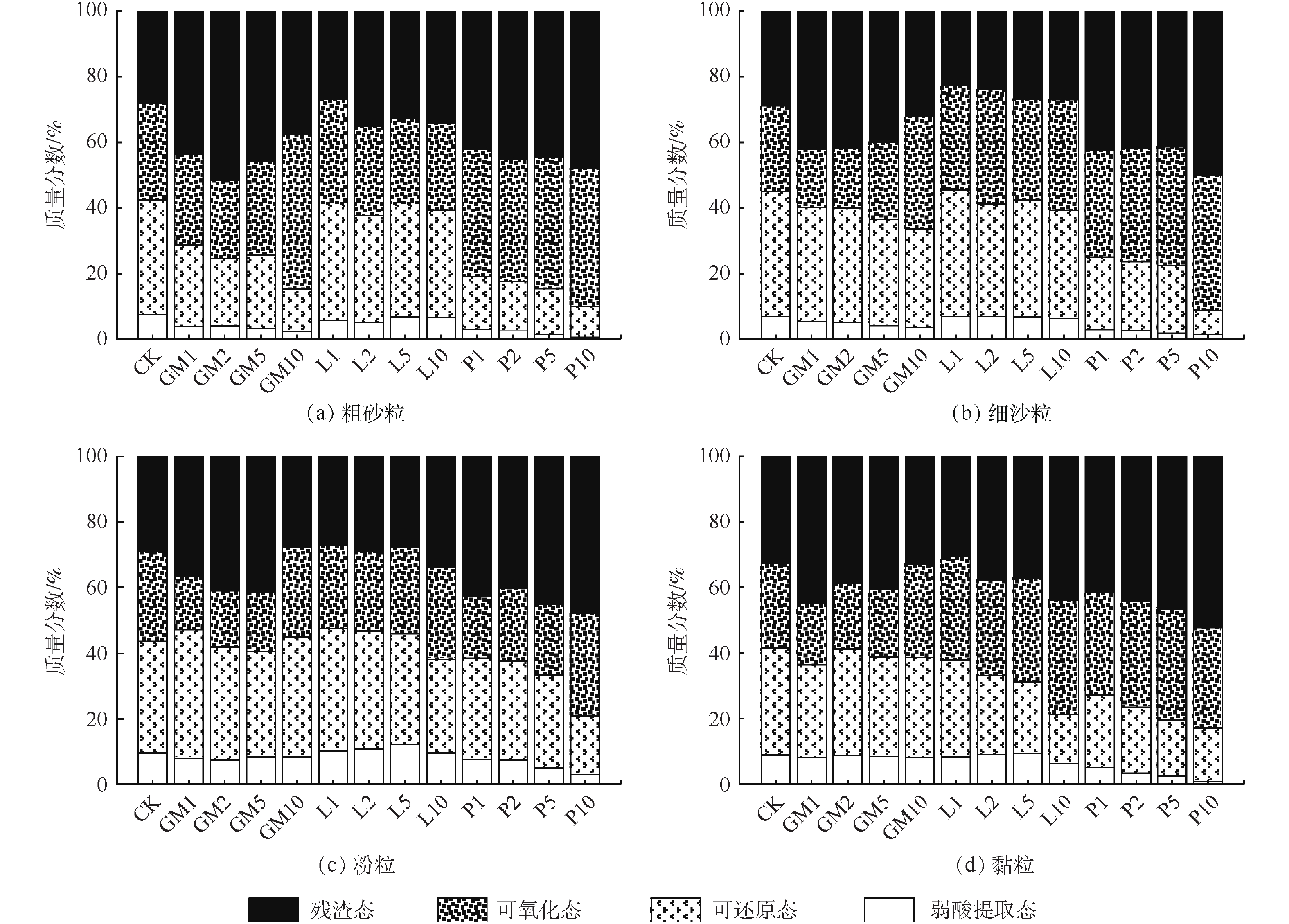
土壤不同形态重金属对生态环境的危害程度有所差异,钝化剂可通过改变重金属的赋存形态,以降低其对人体和生态环境的危害,因此,可以用形态分级方案来评价重金属污染土壤的钝化效果[33]。图5为不同钝化处理对土壤Pb形态分布的影响。由图5可知,与CK相比,羊厩肥与磷酸盐均降低了土壤中弱酸提取态Pb含量,降幅与添加量成正比,其中P1降幅最小为0.64%,P10降幅最大为7.05%。石灰处理均提高了土壤中弱酸提取态Pb含量,L5的增幅达4.51%,这可能是由于石灰处理增加了土壤中氢氧化铅和碳酸铅的含量。石灰添加可提高土壤pH,高pH会促进土壤中碳酸盐化合物和金属氢氧化物的生成[34],而上述2种物质在酸性条件极易分解[35]。各处理均降低了土壤中可还原态Pb含量,羊厩肥低添加量降幅适中而高添加量降幅较小,各处理降幅分别为2.47%、7.63%、10.52%和17.07%;石灰低添加量降幅最大但L10降幅适中,分别为8.12%、9.44%、16.49%和20.83%;磷酸盐与石灰相反,低添加量降幅较小而P10降幅最大,各添加量降幅分别为1.74%、4.83%、17.32%和29.52%。不同处理均提高了土壤可氧化态Pb含量,羊厩肥、石灰、磷酸盐在各添加量下增幅分别为1.17%~5.44%、4.22%~14.19%、2.35%~17.89%。对于残渣态Pb而言,羊厩肥与磷酸盐处理均不同程度的增加了其含量,其中P10增幅最大为18.69%,而石灰处理对土壤残渣态Pb含量基本无影响。
以上结果表明,羊厩肥与磷酸盐主要是通过将弱酸提取态与可还原态Pb转化为可氧化态与残渣态Pb,以降低土壤中Pb的活性;石灰处理大幅度降低了可还原态Pb含量,但是却被转化为弱酸提取态与可氧化态,残渣态含量基本无变化,虽然降低了土壤中Pb的活性,但其钝化效果的稳定性与长效性须进一步验证。
2.3 钝化剂添加对不同粒级土壤颗粒中Pb的影响
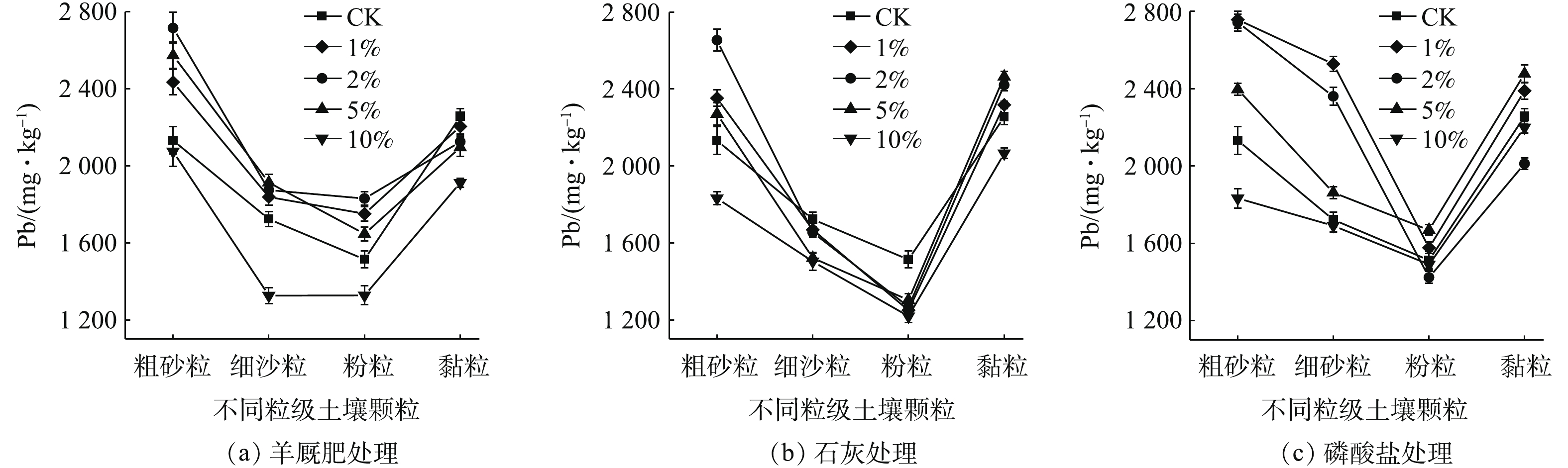
图6为不同钝化剂种类及添加量下各粒级颗粒中Pb的含量分布情况。由图6可知,Pb含量在土壤颗粒中呈双峰分布趋势,即粗砂粒和黏粒中的Pb含量较高,而细沙粒和粉粒中的Pb含量较低。黏粒比表面积较大,且黏土矿物和铁锰/铁铝氧化物含量较高,对Pb的吸附能力较强;粗砂粒富集Pb的原因较复杂,李恋卿等[36]认为这是由于有机质与其他物质通过复合作用而富集在团聚后的粗颗粒表面的结果,而QIAN等[37]则认为是粗砂粒中含有对重金属固持能力较强的粗矿物或重矿物。3种钝化剂10%添加量处理均降低了各粒级颗粒中Pb含量,这可能是因为钝化剂添加量较大,导致钝化剂在不同粒级颗粒中大量富集,而钝化剂本身Pb含量较低,进而使各粒级颗粒中Pb含量降低。1%、2%、5%羊厩肥添加量均不同程度提高了粗砂粒、细沙粒、粉粒中Pb含量,其中GM2处理对粗砂粒和黏粒中Pb增加量最大,分别为583.30 mg·kg−1和317.00 mg·kg−1,GM5对细沙粒中Pb含量增加量最大,为190.29 mg·kg−1;GM1、GM2和GM5均降低了黏粒中Pb含量,降低量分别为52.56、132.29和160.85 mg·kg−1。这可能是由于羊厩肥显著提高了土壤SOM量,但SOM在各粒级颗粒中的活性有所差异,砂粒中的活性最强,粉粒次之,黏粒最差[38],活性不同对污染物的吸附能力也有所差异,一般来说,活性越强,对污染物的吸附能力越好。与CK相比,1%、2%、5%石灰添加量均增加了粗砂粒中Pb含量,增加量分别为22.16、522.09和138.64 mg·kg−1,同时增加了黏粒中Pb含量,增加量与添加量成正比,L5处理增加量为208.32 mg·kg−1;石灰1%、2%、5%添加量却降低了细沙粒和粉粒中Pb含量。这可能是由于石灰添加增加了土壤颗粒表面负电荷,并生成了CaCO3和Ca(OH)2胶结物质引起的,黏粒比表面积大,持有的负电荷数量最高,因此,加大了对Pb2+的吸附,粗砂粒中的胶结物质与Pb2+进行交换吸附,以增加其Pb固持量[39-40]。相比于CK,磷酸盐增大了粗砂粒与细沙粒对Pb的富集,而对粉粒和黏粒中Pb含量影响无明显规律。
钝化剂添加不仅会影响Pb在各粒级颗粒中的再分配,还可能改变各粒级颗粒中Pb的形态分布,进而影响土壤Pb的有效性。由图7可知,CK处理下各粒级颗粒中Pb形态分布并无明显差异,表明外源Pb进入土壤后在各粒级颗粒中的分布无明显区别[41]。羊厩肥对粗砂粒中各形态Pb所占比例影响最大;而石灰对黏粒中Pb形态占比影响最大;磷酸盐处理下各粒级颗粒中Pb形态分布均由高毒性形态向低毒性形态转化的表现,这可能是由于易溶性磷酸盐与其他矿物晶体形成的光滑涂层,其可将Pb2+包缚在土壤颗粒表面所致[33]。
2.4 土壤与各粒级颗粒间Pb各形态含量相关分析
为了解土壤与各粒级颗粒Pb形态含量间可能存在的关系,对土壤与各粒级颗粒中Pb形态含量间做Pearson相关矩阵分析,其中易利用态为弱酸提取态与可还原态之和,难利用态为可氧化态与残渣态之和[42]。由表1可知,土壤DTPA-Pb含量与细沙粒、粉粒和黏粒中易利用态Pb含量均呈显著正相关关系,相关系数值排序为黏粒>细沙粒>粉粒,这说明土壤中植物有效态Pb含量可能来源于上述3部分中易利用态Pb。土壤弱酸提取态有着最高的生物毒性,各粒级颗粒中弱酸提取态含量均与其达到显著正相关关系,说明土壤弱酸提取态与各粒级弱酸提取态Pb有着密切的关系,本研究中土壤弱酸提取态Pb含量较低,在土壤DTPA-Pb中占比较小,残渣态在土壤中活性最低,因此,土壤DTPA-Pb含量主要受可还原态与可氧化态Pb控制[43]。土壤可还原态Pb含量与细沙粒、粉粒和黏粒中的可还原态呈极显著正相关,还与粉粒和黏粒中的可氧化态含量达到了显著负相关关系,表明钝化剂主要通过将细沙粒、粉粒和黏粒中的可还原态Pb转化为粉粒和黏粒中的可氧化态Pb,来降低土壤Pb的毒害性。
表 1 土壤与各粒级颗粒中Pb形态含量间的相关系数(n=13)Table 1. Correlation coefficients between Pb speciation concentrations in soil and in soil particle-size fractions(n=13)粒级 Pb形态 DTPA-Pb 弱酸提取态 可还原态 可氧化态 残渣态 粗砂粒 弱酸提取态 0.564* 0.890** 0.263 −0.473 −0.581* 可还原态 0.525 0.935** 0.234 −0.368 −0.498 可氧化态 −0.061 −0.369 0.238 0.013 0.354 残渣态 0.065 −0.305 0.475 −0.137 0.460 易利用态 0.537 0.935** 0.241 −0.390 −0.517 难利用态 0.017 −0.391 0.447 −0.090 0.492 细沙粒 弱酸提取态 0.642* 0.876** 0.318 −0.500 −0.438 可还原态 0.842** 0.702** 0.727** −0.751** −0.148 可氧化态 −0.524 −0.241 −0.206 0.309 −0.147 残渣态 −0.182 −0.575* 0.312 0.035 0.425 易利用态 0.828** 0.751** 0.673* −0.725** −0.203 难利用态 −0.360 −0.500 0.120 0.115 0.223 粉粒 弱酸提取态 0.723** 0.790** 0.428 −0.631* −0.335 可还原态 0.766** 0.222 0.765** −0.700** 0.316 可氧化态 −0.581* −0.470 −0.590* 0.485 −0.118 残渣态 −0.250 −0.649* 0.240 0.099 0.454 易利用态 0.817** 0.378 0.745** −0.740** 0.84 难利用态 −0.427 −0.774** 0.041 0.251 0.398 粘粒 弱酸提取态 0.720** 0.765** 0.317 −0.434 −0.216 可还原态 0.882** 0.354 0.692** −0.495 0.309 可氧化态 −0.671* 0.171 −0.606* 0.504 −0.738** 残渣态 −0.701** −0.396 −0.435 0.429 −0.047 易利用态 0.882** 0.522 0.604* −0.506 0.143 难利用态 −0.826** −0.172 −0.614* 0.555* −0.426 注:*为P<0.05,显著;**为P<0.01,显著。 3. 结论
1) 3种钝化剂在不同添加量下均能显著降低外源Pb污染土壤中DTPA-Pb含量,在所有处理中,GM1对DTPA-Pb含量降低幅度最小,为6.51%,P10降幅最大,高达80.53%,3种钝化剂在相同添加量下对外源Pb的钝化效果为磷酸盐>石灰>羊厩肥。形态分布显示,羊厩肥和磷酸盐通过将弱酸提取态与可还原态Pb转化为可氧化态与残渣态Pb,以降低土壤外源Pb有效性,石灰通过将可还原态Pb转化为可氧化态Pb,以降低其有效性。
2) 3种钝化剂均不同程度地提高了土壤Olsen-P含量,其中羊厩肥、石灰和磷酸盐处理下Olsen-P含量分别为60.12~145.12、66.30~84.72、125.29~180.98 mg·kg−1。土壤磷淋溶临界值模型显示,当土壤Olsen-P含量大于124.25 mg·kg−1时,土壤磷素就会向地下水淋溶。
3) 3种钝化剂对各粒级颗粒中Pb含量及形态分布的影响有所差异。羊厩肥可增加粗砂粒、细沙粒、粉粒中Pb含量,降低黏粒中Pb含量,对各粒级颗粒中Pb形态分布均有影响但粗砂粒影响最大。石灰会增加粗砂粒和黏粒中Pb含量,降低细沙粒和粉粒中Pb含量,并对黏粒中Pb形态分布产生较大影响。磷酸盐提高粗砂粒与细沙粒对Pb的富集而对粉粒和黏粒中Pb含量影响无明显规律,在磷酸盐作用下各粒级颗粒均明显出现高毒性向低毒性形态转化的现象。
4)相关性分析表明,土壤DTPA-Pb主要来源于细沙粒、粉粒和黏粒中易利用态Pb,钝化剂主要通过将细沙粒、粉粒和黏粒中的可还原态Pb转化为粉粒和黏粒中的可氧化态Pb,以降低土壤Pb的毒害性。
-
表 1 碘镓灯下不同比例0.1% Pd-ZnIn2S4-PVA:ZS-浮石催化剂光降解CBZ动力学参数
Table 1. Kinetic parameters of photocatalytic degradation of CBZ by 0.1% Pd-ZnIn2S4-PVA:ZS-pumice catalysts with different ratios under iodine gallium lamp
粘合剂 动力学方程 速率常数K/min−1 半衰期t1/2/min 相关系数R2 空白 y=0.002 29x+0.026 4 0.002 29 303 0.98 PVA y=0.011 30x+0.049 7 0.011 30 61.2 0.97 PVA:ZS(4:1) y=0.006 62x+0.001 7 0.006 62 105 0.97 PVA:ZS(2:1) y=0.004 95x-0.058 2 0.004 95 140 0.99 PVA:ZS(1:1) y=0.004 17x+0.061 5 0.004 17 166 0.99 -
[1] WANG J L, WANG S Z. Removal of pharmaceuticals and personal care products (PPCPs) from wastewater: A review[J]. Journal of Environmental Management, 2016, 182: 620-640. doi: 10.1016/j.jenvman.2016.07.049 [2] 严清, 张怡昕. 重庆主城区水域典型PPCPs污染水平及生态风险评估[J]. 环境科学研究, 2013, 26(11): 1179-1184. [3] 万众. 废水中典型药物化合物的类芬顿氧化特性及去除机理研究[D]. 北京: 清华大学, 2018. [4] GOBEL A, THOMSEN A, MCARDELL C S, et al. Occurrence and sorption behavior of sulfonamides, macrolides, and trimethoprim in activated sludge treatment[J]. Environmental Science & Technology, 2005, 39(11): 3981-3989. [5] ZHANG Y, GEISSEN S, GAL C. Carbamazepine and diclofenac: Removal in wastewater treatment plants and occurrence in water bodies[J]. Chemosphere, 2008, 73(8): 1151-1161. doi: 10.1016/j.chemosphere.2008.07.086 [6] 翟俊, 胡炜, 王泉峰, 等. 异化Mn(IV)还原耦合降解卡马西平及双氯芬酸[J]. 中国环境科学, 2021, 41(4): 1704-1710. [7] RUZIWA D T, OLUWALANA A E, MUPA M, et al. Pharmaceuticals in wastewater and their photocatalytic degradation using nano-enabled photocatalysts[J]. Journal of Water Process Engineering, 2023, 54: 103880. doi: 10.1016/j.jwpe.2023.103880 [8] LIU W, ZHANG W, LIU M, et al. Fabrication of niobium doped titanate nanoflakes with enhanced visible-light-driven photocatalytic activity for efficient ibuprofen degradation[J]. Chemistry Letters, 2019, 30(12): 2177-2180. [9] 毕洪飞, 刘劲松, 吴正颖, 等. 硫化铟锌的改性合成及光催化特性[J]. 化学进展, 2021, 33(12): 2334-2347. [10] BO L L, KIRIARACHCHI H D, BOBB J A, et al. Preparation, activity, and mechanism of ZnIn2S4-based catalysts for photocatalytic degradation of atrazine in aqueous solution[J]. Journal of Water Process Engineering, 2020, 36: 101334. doi: 10.1016/j.jwpe.2020.101334 [11] 田野. 硫化铟锌掺杂钯光催化降解水中卡马西平特性研究[D]. 西安: 西安建筑科技大学, 2022. [12] ASGARI G, ROSHANI B, GHANZADEH G. The investigation of kinetic and isotherm of fluoride adsorption onto functionalize pumice stone[J]. Journal of Hazardous Materials, 2012, 217-218: 123-132. doi: 10.1016/j.jhazmat.2012.03.003 [13] 唐西梅. Pd-ZnIn2S4负载型催化剂制备及其光催化降解水中阿特拉津特性研究[D]. 西安: 西安建筑科技大学, 2022. [14] LI Y, FANG X, WANG Y, et al. Highly Transparent and Water-Enabled Healable Antifogging and Frost-Resisting Films Based on Poly(vinyl alcohol)-Nafion Complexes[J]. Chemistry of Materials, 2016, 28(19): 6975-6984. doi: 10.1021/acs.chemmater.6b02684 [15] GAAZ T S, SULONG A B, AKHTAR M N, et al. Properties and Applications of Polyvinyl Alcohol, Halloysite Nanotubes and Their Nanocomposites[J]. Molecules, 2015, 20(12): 22833-22847. doi: 10.3390/molecules201219884 [16] SAINI I, SHARMA A, DHIMAN R, et al. Grafted SiC nanocrystals: For enhanced optical, electrical and mechanical properties of polyvinyl alcohol[J]. Journal of Alloys and Compounds, 2017, 714: 172-180. doi: 10.1016/j.jallcom.2017.04.183 [17] 盛野, 王洪艳. 改进聚乙烯醇基料耐水性的研究[J]. 化学世界, 2001, 12(6): 0367-6358. [18] 刘梦娇. 光活化过硫酸盐降解水中PhACs特性研究[D]. 西安: 西安建筑科技大学, 2023. [19] BO L L, FENG L, FU J T, et al. The fate of typical pharmaceuticals in wastewater treatment plants of Xi’an city in China[J]. Journal of Environmental Chemical Engineering, 2015, 3(3): 2203-2211. doi: 10.1016/j.jece.2015.08.001 [20] 谭娜, 卜龙利, 高波, 等. ZnIn2S4光催化降解水中痕量药物卡马西平的特性[J]. 环境工程学报, 2017, 11(1): 223-229. doi: 10.12030/j.cjee.201508197 [21] BO L L, HE K B, TAN N, et al. Photocatalytic oxidation of trace carbamazepine in aqueous solution by visible-light-driven Znln2S4: Performance and mechanism[J]. Journal of Environmental Management, 2017, 190: 259-265. doi: 10.1016/j.jenvman.2016.12.050 [22] CHEN Z, LI D, ZHANG W, et al. Low-temperature and template-free synthesis of ZnIn2S4 microspheres[J]. Inorganic Chemistry, 2008, 47(21): 9766-9772. doi: 10.1021/ic800752t [23] ZOU P, LI Z G, JIA P Q, et al. Enhanced photocatalytic activity of bismuth oxychloride by in-situ introducing oxygen vacancy[J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2021, 623: 126705. doi: 10.1016/j.colsurfa.2021.126705 [24] GUAN M, XIAO C, ZHANG J, et al. Vacancy associates promoting solar-driven photocatalytic activity of ultrathin bismuth oxychloride nanosheets[J]. Journal of the American Chemical Society, 2013, 135: 10410-10417. [25] RAO T R, OMKARAM I, BRAHMAM K V, Role of copper content on EPR, susceptibility and optical studies in poly(vinylalcohol) (PVA) complexed poly(ethyleneglycol) (PEG) polymer films[J]. Journal of Molecular Structure, 2013, 1036: 94-101. [26] MENG X C, ZHANG Z S. Bi2MoO6 co-modified by reduced graphene oxide and palladium (Pd2+ and Pd0) with enhanced photocatalytic decomposition of phenol[J]. Applied Catalysis B:Environmental, 2017, 209: 383-393. doi: 10.1016/j.apcatb.2017.01.033 [27] REDA M M, AHMED S. Palladium/zinc indium sulfide microspheres: Enhanced photocatalysts prepare methanol under visible light conditions[J]. Journal of the Taiwan Institute of Chemical Engineers, 2016, 65: 498-504. doi: 10.1016/j.jtice.2016.05.027 [28] MUHAMMAND A, MAZHAR A K, ZULFIQAR A R. Fabrication of reduced graphene oxide nanosheets doped PVA composite films for tailoring their opto-mechanical properties[J]. Applied Physics A:Materials Science and Processing, 2017, 424: 123. [29] SHI W L, CHEN Z Z, LU J L, et al. Construction of ZrC@ZnIn2S4 core-shell heterostructures for boosted near-infrared-light driven photothermal-assisted photocatalytic H2 evolution[J]. Chemical Engineering Journal, 2023, 474: 145690. doi: 10.1016/j.cej.2023.145690 [30] PENG X, LI J, YI L, et al. Ultrathin ZnIn2S4 nanosheets decorating PPy nanotubes toward simultaneous photocatalytic H2 production and 1, 4-benzenedimethanol valorization[J]. Applied Catalysis B-environmental, 2022, 300: 120737. doi: 10.1016/j.apcatb.2021.120737 [31] XU Z, SHI W L, SUN H R, et at. Carbon dots as solid-state electron mediator and electron acceptor in S-scheme heterojunction for boosted photocatalytic hydrogen evolution[J]. Applied Surface Science, 2022, 595: 153482. doi: 10.1016/j.apsusc.2022.153482 [32] LEE H J, LEE H S, LEE C H. Degradation of diclofenac and carbamazepine by the copper(II)-catalyzed dark and photo-assisted Fenton-like systems[J]. Chemical Engineering Journal, 2014, 245: 1385-8947. [33] QIAN J, XUE Y, AO Y H, et al. Hydrothermal synthesis of CeO2/NaNbO3 composites with enhanced photocatalytic performance[J]. Chinese Journal of Catalysis, 2018, 39: 682-692. doi: 10.1016/S1872-2067(17)62975-9 -




 下载:
下载: