-
目前,新污染物已被发现广泛存在于水体环境当中,由于其在环境中的持久性,并且具有致突变、致畸变和致癌的“三致”风险,受到了研究人员的高度关注。有研究表明,以布洛芬(ibuprofen IBP)、阿特拉津(atrazine ATZ)、乐果(dimethoate DIM)以及三氯乙酸(trichloroacetic acid TCAA)为代表的新型污染物多次在地表水中被检出[1-2]。在日常生活中,人们往往忽略这些微量有机污染物对健康产生的不利影响,但长期接触或摄入这类化合物会对人体产生难以预料的影响[3]。饮用水是人类接触新污染物的直接途径,在日常洗漱和饮用过程中都会直接接触和摄入未完全去除的微污染物。
因此,必须将微污染物充分去除后饮用。常规的传统工艺如混凝、沉淀、消毒等,虽然能去除水体的浊度和色度等,但却无法将水体中的有机物彻底截留或降解[4],对于微克、纳克级别物质的去除效率更是十分有限。虽然大量采用纳滤和反渗透膜可以截留水中的污染物,但也大大提高了水处理成本,截留后产生的浓水也会成为新的污染来源。针对这一现状,本研究结合“分质供水”理念,对人体直接接触和摄入的饮用水进行深度处理,在减少水处理成本的基础上提高家庭端水质。
光电氧化被认为是一种具有应用前景的水处理技术,通过施加外部偏压减少光生电子和空穴的复合,提高量子效率,可以在提高降解率的同时降低能耗,具有绿色、高效的特点。能高效分离光电子空穴和具有出色的光吸收特性的半导体电极是光电催化体系的首选电极,如TiO2、ZnO、BiVO4和MOFs电极。其中,TiO2基电极因其具有良好的光学性能和低毒性成为最常用的光电电极。研究者采用Ru0.3Ti0.7O2作为光电阳极,这种电极既具有RuO2的电化学特性,又具有TiO2的光催化特性,其矿化效率(38%)高于仅在电催化过程中的矿化效率(<10%)[5]。本研究选用具有良好光活性的TiO2-IrRu电极,钌铱金属由于其优异的导电性能和长时间的工作寿命而被作为一种电极涂层,以此来增加电极材料的稳定性和电催化活性。与传统光电阳极相比,该电极具有物化性质稳定、氧化能力强、安全无害的特点[6]。
目前,光电技术已被证实对低浓度的有机污染物也有非常高的降解效能[7-10],但由于能耗较高,不适用于大规模的饮用水处理。本研究提出将光电催化技术用于分质供水的末端水处理,选用性能稳定、无毒无害的TiO2-IrRu电极为光电催化阳极材料,采用自来水配置水样模拟实际水体,以4种污染物为模型污染物分析光电氧化系统的污染物降解情况和能耗,并通过布洛芬的降解路径,计算降解过程的毒性变化。
-
实验中以布洛芬(IBP,>98%,西安博涵化工科技有限公司)、阿特拉津(ATZ,>97%,广州科启化学技术有限公司)、三氯乙酸(TCAA,>99%,江苏艾康生物医药研发有限公司)、乐果(DIM,>98%,广州科启化学技术有限公司)为模型污染物,甲醇、乙酸铵、氯化钠、碳酸氢钠购自科密欧化学试剂有限公司(中国天津),腐殖酸购自国药集团化学试剂有限公司(中国上海),除甲醇为色谱纯外,其他药剂均为分析纯。
-
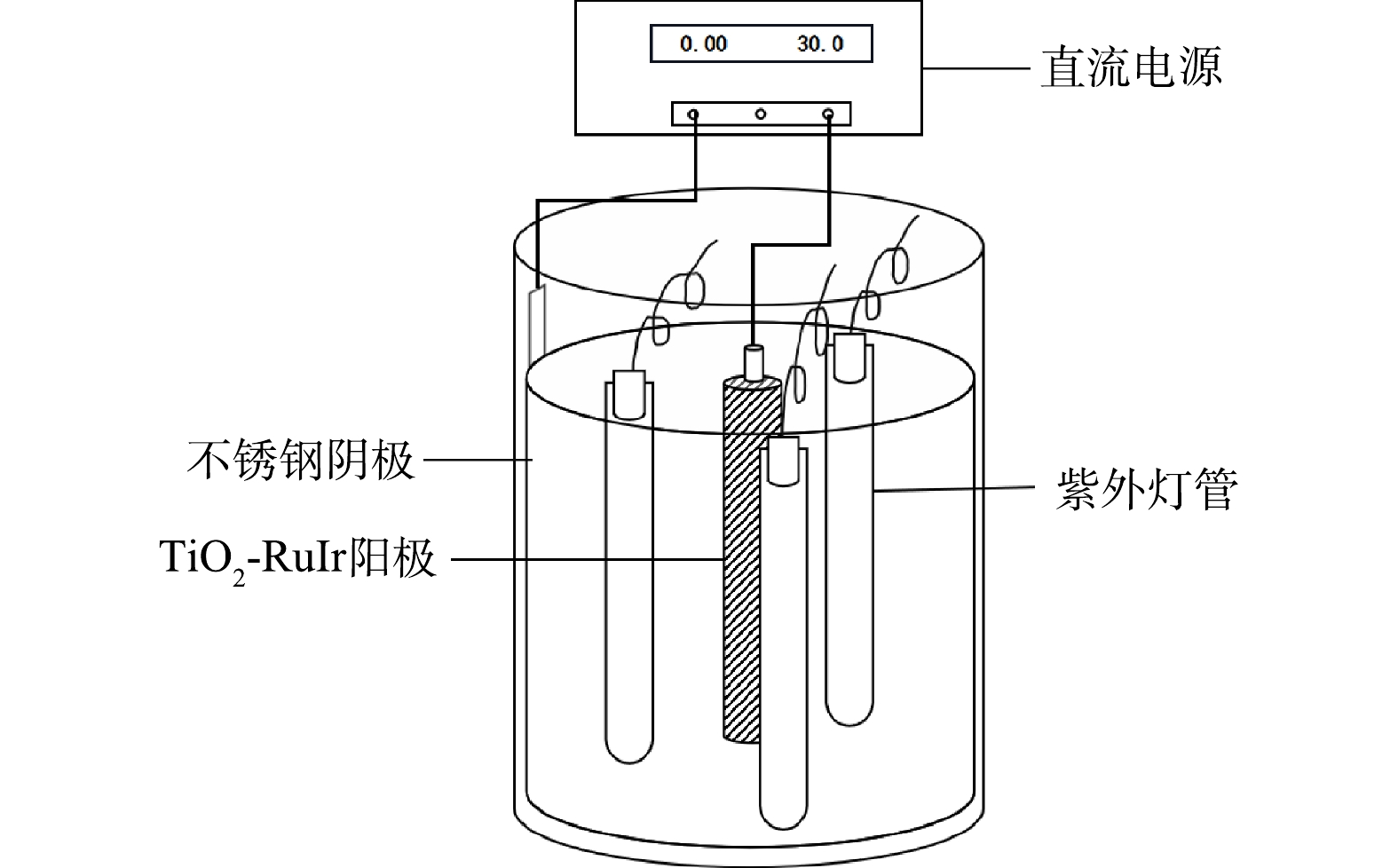
自制光电反应系统,采用3根ZW10D15Y(W)-Z212型低压汞灯、商业购买的TiO2-IrRu阳极及不锈钢阴极,具体结构如图1所示。实验中通过控制紫外灯管开关数来控制紫外光照射强度,使用1、2、3根灯管对应的光照强度分别为2 210、3 352、4 566 LUX。
-
考虑到实际应用的可能性以及稳定性,实验中的水样全部采用自来水配置,自来水水质如表1所示。根据《生活饮用水卫生标准》(GB 5749-2022)限值要求,IBP、ATZ、TCAA、DIM的初始质量浓度分别为80、50、200、60 µg·L−1。制备水样在光电反应器中进行反应后,样品经0.22 μm滤膜过滤后采用液质联用仪(Waters UPLC-TQD)检测4种模型污染物在降解过程中的浓度变化。采用Ultimate 3 000 UHPLC-Q Exactive液质联用仪分析IBP中间产物。
使用式(1)~(3)计算直接光解(UV)、电化学氧化(E)和光电氧化(E-UV)工艺将1 m3水中污染物浓度降低1个数量级的能耗需求[11]。
式中:E是工艺所需能耗,kWh·m−3;P是紫外线灯的功率,W;t是处理时间,min;C0和Ct分别是0和t时刻污染物质量浓度,µg·L−1;V是溶液体积,L;I是电流,A;U是施加电压,V。
-
Ti基电极是目前光电氧化中一种常用的电极,其表面添加的涂层材料很大程度上决定了电极性能。铅(PbO2)、锡(SnO2)、铱(IrO2)、钌(RuO2)等氧化物及其组合已被用于提高阳极的电催化活性、稳定性和能效[12]。SnO2等非活性阳极具有较高的析氧电位,但其使用寿命短[13],而PbO2电极具有低成本和高化学稳定性,但其析氧电位低[14]。目前,IrO2和RuO2的组合已被用于开发高效阳极,且已有研究证明TiO2-IrRu电极在光电降解有机物方面具有良好效果[15-16]。因此,本研究选用该电极作为光阳极。
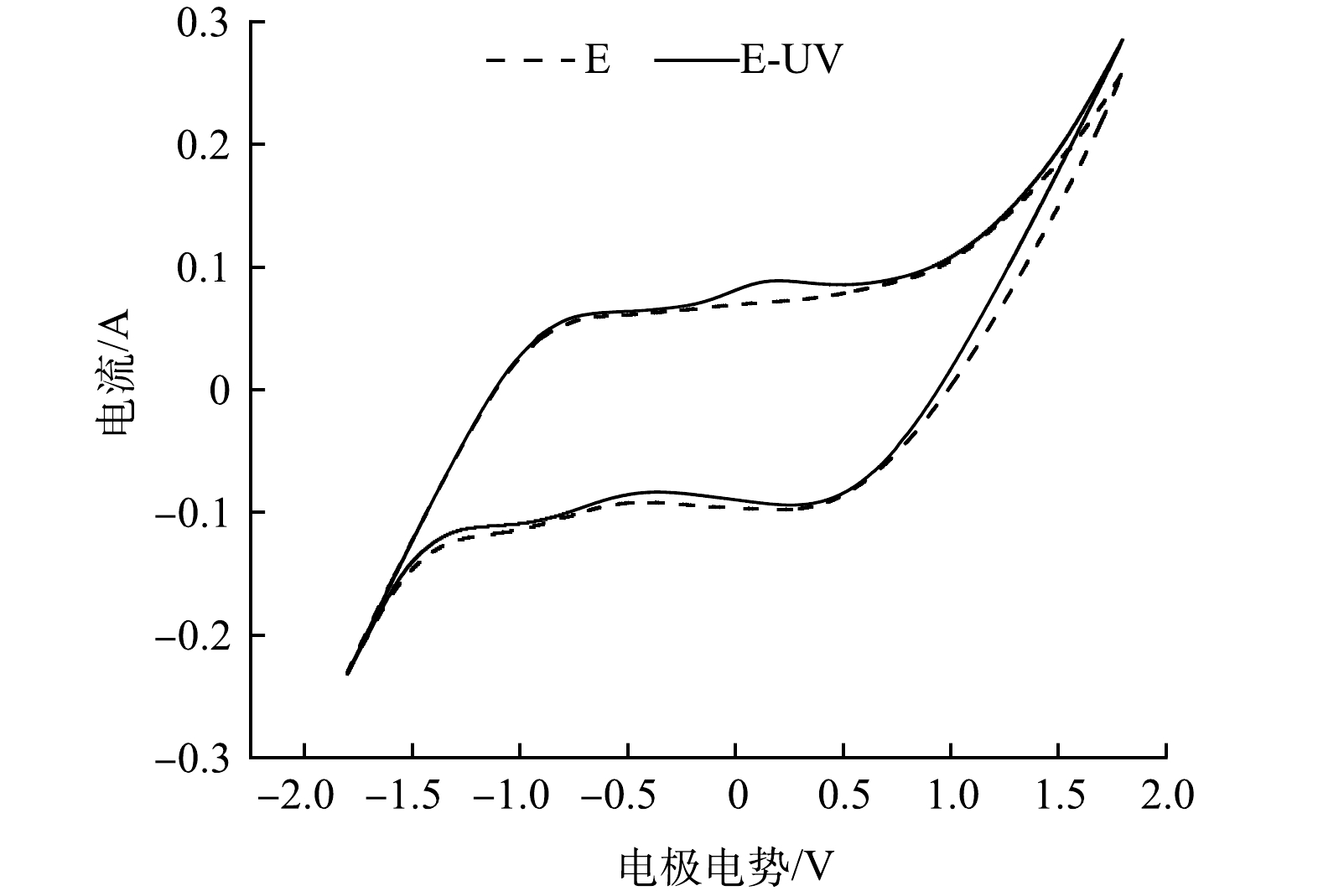
循环伏安扫描(CV)是对电极电化学性质的表征,为了探究实验所用的TiO2-IrRu电极在紫外照射下的性质,对其进行循环伏安扫描。为了避免电解液进入电极表面微观结构初期所带来的的不稳定曲线,选择循环40次后的CV曲线以最小化这种偏差。
图2为在0.05 mol·L−1的Na2SO4溶液中获得的CV曲线。结果表明,在光电体系中出现1个明显氧化峰,说明在该体系下发生了氧化反应。氧化峰的出现可以说明在光的照射下,电极对有机污染物的降解具有更好的电化学性能,与单纯的电化学工艺相比,光电工艺可以提供更多的降解路径,从而提高对有机污染物的氧化分解能力。
-
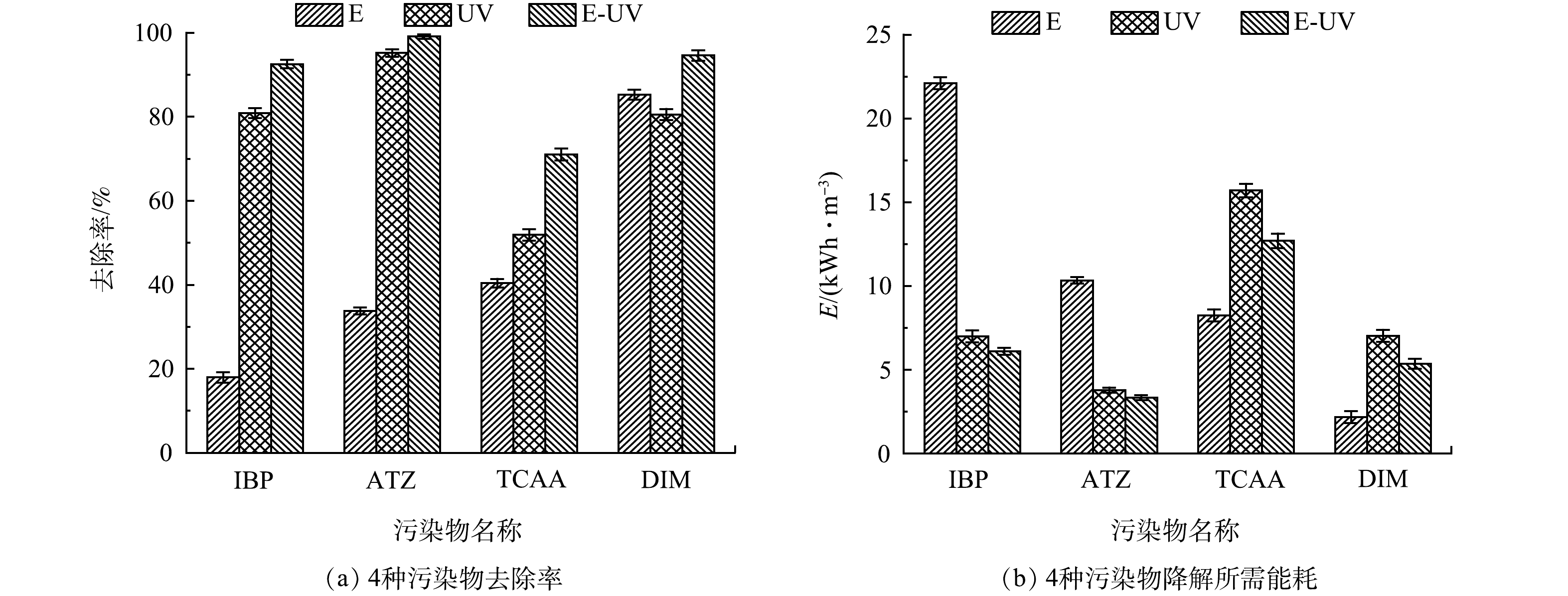
在光电反应器中,通过单独光照、单独电氧化和光电氧化来降解4种污染物。在电流为0.30 A、光照强度4 566 LUX的条件下,考察了15 min内4种污染物的降解效果。如图3(a)所示,虽然单独电氧化可较为有效地去除DIM,但电化学对IBP、ATZ和TCAA的去除率较低,IBP的降解率仅达到17.95%。在单独紫外照射中,TCAA的去除率也只有51.89%。与单独紫外线和电化学过程相比,光电耦合技术可以显著的提高4种污染物的降解率,ATZ的降解率可达到99.09%。以上结果表明,光电体系对污染物有更高效的去除效果。
在光电氧化降解有机污染物的过程中,不同类型的污染物对紫外光和电解有不同的反应速率。电化学氧化中主要通过电子的直接转移来降解污染物,由于IBP和ATZ在阳极表面的传质受限[17],这造成了单独电化学氧化对这两种物质的降解率和反应动力学常数都很低(表2)。紫外光解对TCAA的降解率较低主要是因为TCAA对紫外的吸收率很低,可以抵抗住紫外线的光解作用。根据实验结果,光电氧化技术能够提高4种物质的降解率,这主要是因为光、电之间的协同、互补作用,在光电氧化体系中存在多种降解途径,阳极直接氧化、自由基氧化以及紫外线的光解等。同时,体系中的阳极材料也提供了光催化氧化降解污染物的路径,施加的外部偏压更是减少了体系中光生电子空穴的复合,从而提高降解效能。
能耗是评价一个工艺的重要指标,决定了该工艺的经济性和应用前景,本研究中能耗包括紫外线的照射和电解,如图3(b)所示。虽然UV工艺可以以相对较小的能耗去除IBP和ATZ,但由于它们的电化学反应性较低,在单独电氧化中能耗较高。另一方面,虽然电化学工艺能以较低的能耗降解DIM,但是降解其他三种物质的能耗过高。与单独UV和电氧化相比,光电组合工艺能通过相对较少的能耗减少污染物。为了探究光电技术在实际应用方面的可能性,将其与其他技术进行对比,以西安市平均电价0.49 元·(kWh)−1计算,大致估算这些工艺处理1吨水所需要的价格,结果如表3所示。这些数据表明,光电氧化技术在能耗方面,可以成为水处理中消除微量有机污染物的一种有力竞争方法。
-
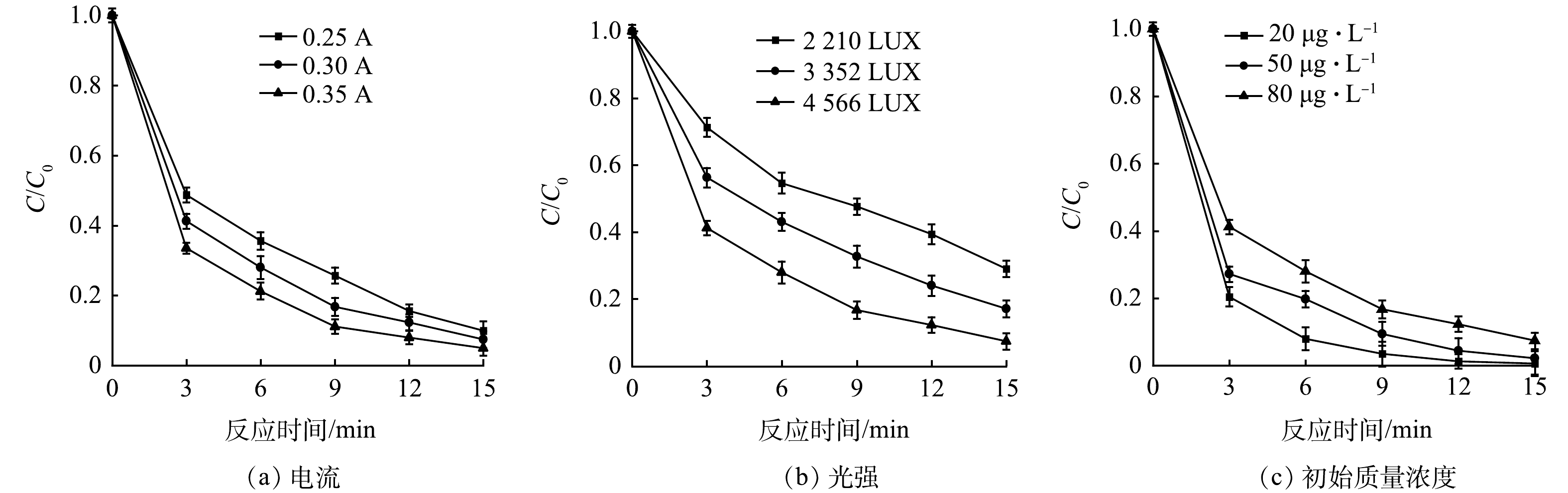
1)电流对光电氧化降解效果的影响。电流是光电反应中一个重要影响因素,在光照强度为4 566 LUX的情况下,探究不同电流对IBP降解情况的影响,结果如图4(a)所示。可见,当电流由0.25 A升至0.35 A的过程中,IBP的降解率逐渐升高,k值也随着电流的增加而升高(表4)。一方面,电流会直接影响电解过程中的电子转移,电流增大,电子转移速度加快,对IBP的直接电解作用增强;另一方面,对于光催化而言,电流的增加会减少光生电子-空穴对的复合,加速光电反应中电子的迁移,从而提高IBP的降解效率。此外,随着电流的增大,阳极表面会产生更多的·OH[21],IBP的降解速率也因此得到提高。由于采用自来水进行实验,受限于自来水中电解质浓度较低,可维持实验条件的电流区间较小,所设置的电流改变不大,对实验结果的影响并不明显,但并不能否认电流在光电体系中的重要性。另外,当电流过高时,析氧副反应将逐渐占据主导地位[22],影响降解反应的进行,导致污染物的去除效率变低,因此,在光电反应中选取合适的电流参数比较重要。
2)光强对光电氧化降解效果的影响。在IBP初始质量浓度不变,电流为0.30 A的情况下,通过控制紫外灯管的开关数研究不同光照强度对于光电反应的影响。根据图4(b)及表4可以发现,光强对于光电体系的影响十分显著,随着光强的增加,IBP的降解率提高了21.06%,并且动力学常数k值也有大幅度的提高。分析其原因,分子吸收光的本质是在紫外光辐射的作用下物质分子的能态发生了改变,由低能态被激发至高能态进而发生各种反应[23]。实际上,增加光强是通过提高单位反应体积内的光子流量来提高反应速率,光子流量的增加会使单位时间内被活化的IBP物质分子数增加,同时,光强增大会使电极表面获得更多能量,激发更多的光生空穴,进而提高降解效果。不可忽视的另一原因是紫外光的辐射强度与距离有关,光氧化只能在辐射点周围的一定范围内发生[24]。当只采用1根灯管时,紫外光的辐射体积有限,仅在灯管周围的IBP分子能够发生光氧化,而增加到3根灯管时,紫外光的辐射体积可以扩大到整个体系,使得水体中更多的IBP分子能够被照射,IBP的降解效果因此得到增强。
3)有机物初始质量浓度对光电氧化降解效果的影响。为了探究不同初始质量浓度下的污染物在光电体系中的降解情况,配制初始质量浓度为80、60、40 µg·L−1的IBP水样。在电流为0.30 A,光照强度为4 566 LUX的反应条件下,考察不同IBP初始质量浓度的有机物在光电体系中的去除情况,结果如图4(c)所示。可以看出,随着IBP初始质量浓度的增加,k值也逐渐减小。这表明IBP的降解效果随着质量浓度的增加而变差。导致上述现象的原因是,在一定的电流与光照条件下,阳极表面的电子转移与系统中的光子总量基本不变,随着IBP质量浓度的增加,阳极表面直接电解IBP的效率降低,并且单位分子得到的光子数也随之减少,直接光解效果变差。同样,光照射在电极上产生的电子空穴数量一定,当IBP分子数量增加时,光生空穴氧化有机物的能力也有所下降。同时,反应当中所生成的·OH恒定,IBP质量浓度越大,所消耗的·OH也越多,从而使得降解率下降。并且,初始质量浓度越高可能会在反应中生成更多的中间产物,这些产物会与IBP形成竞争关系,导致了降解效率下降。
-
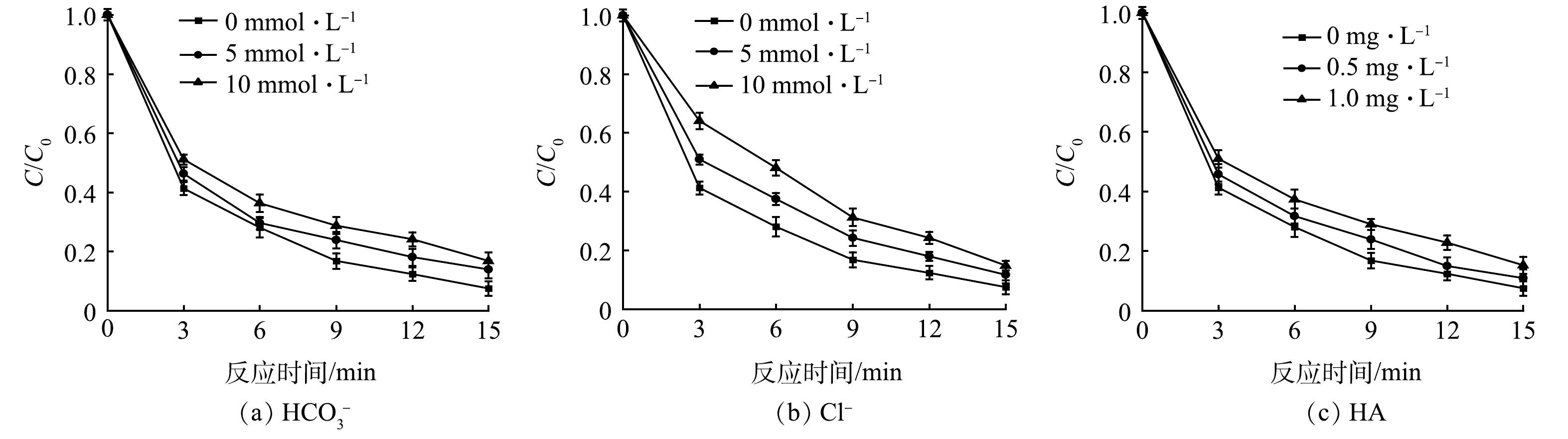
碱度和氯离子浓度是饮用水中的重要指标,以腐殖酸(HA)为代表的天然有机物也在地表环境中广泛存在,即使HA含量很低的情况下,也会在一定程度上影响反应的进行。因此,有必要探究水基质对光电氧化体系的影响,在IBP质量浓度为80 µg·L−1,电流为0.30 A,光强为4 566 LUX的条件下,水质参数对IBP降解的影响及动力学变化如图5和表5所示。
由图5可以看出,HCO3−、Cl−和HA都对IBP的降解产生了不同程度的抑制。随着这些物质浓度的增加,IBP的降解效果逐渐降低,动力学常数k值也变小。在光电反应中,IBP主要通过直接电解、直接光解和·OH的氧化3种降解途径,其中·OH的强大的氧化能力更是为IBP的降解提供了不可缺少的作用。有研究表明,HCO3−会和·OH生成CO3·−,与·OH的非选择性不同,CO3·−是一种选择性较强而氧化能力较弱的自由基。HA会产生轻微的光屏蔽效应[25],HA具有一定的色度,即使在低质量浓度下,其在光解中对紫外产生的光屏蔽作用也不可忽视,并且HA的添加会抑制·OH的生成[26]。Cl−作为水中一种常见的阴离子并会在光照条件下生成一系列氯自由基而被广泛关注,已有研究证明,活性氯自由基可以快速氧化多种微污染物,从而达到增强降解效果的目的[27]。但本研究发现随着Cl−浓度的增加,降解效果反而有所下降。这可能是IBP自身的性质所导致的,同时,Cl−也是·OH的有效清除剂。因此,实验中加入HCO3−、Cl−和HA后,随着·OH的淬灭,IBP的降解也受到抑制。
通常,在复杂的水质条件下,水中的离子会与污染物发生竞争从而导致降解效果变差。在本研究中,虽然HCO3−、Cl−和HA的加入在一定程度上削弱了光电体系的降解效能,但IBP的降解率仍能达到80%以上。由此可见,本研究的光电体系在不同的水质条件下,都表现出良好的适用性。
-
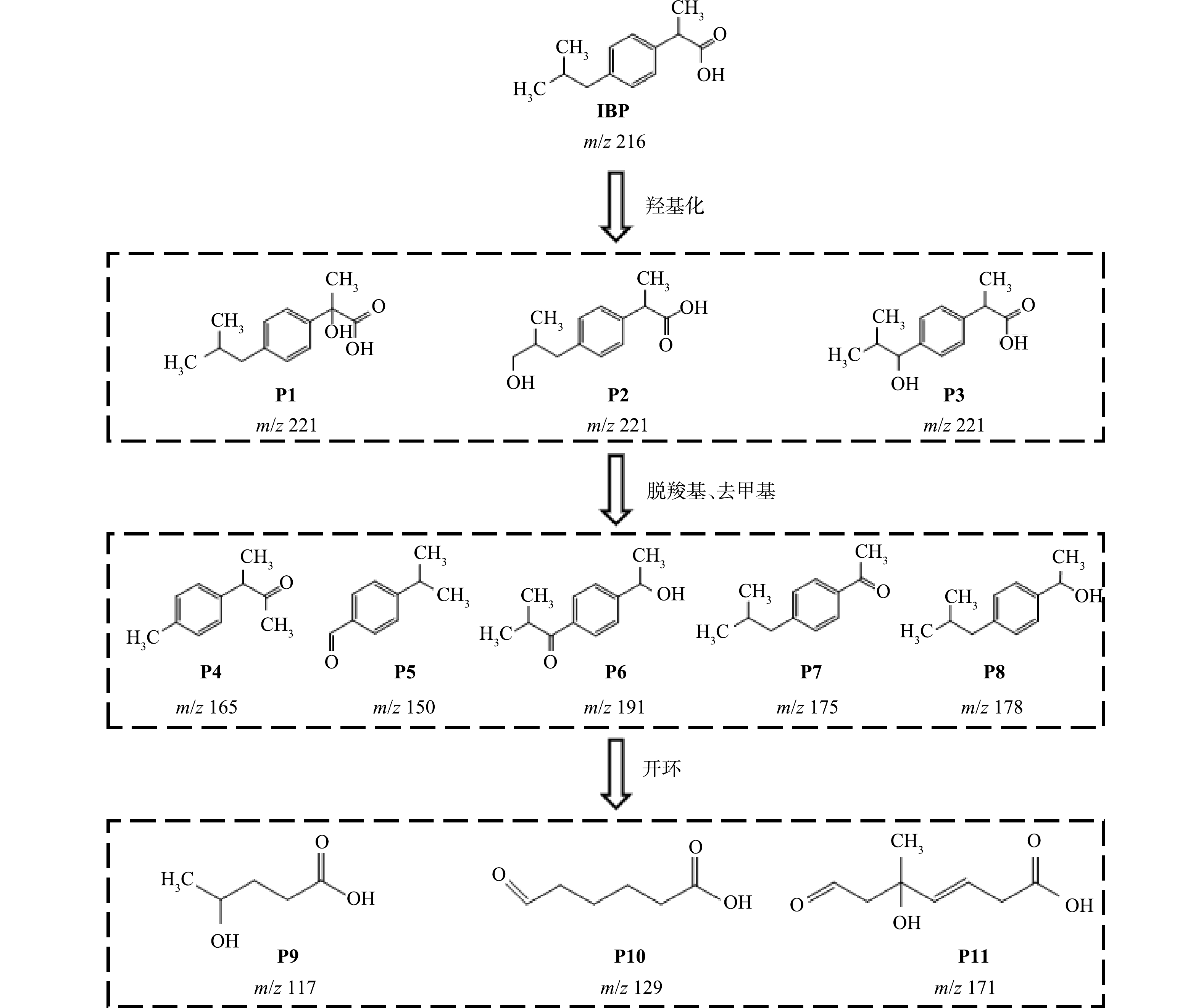
采用液质联用仪分析IBP的降解产物,在降解过程中检测到11种主要产物,根据分析结果结合相关文献[28]推测IBP可能的降解路径,如图6所示。IBP的降解由羟基化开始,首先生成了m/z=221的中间体,结合有关文献报道提出了P1、P2、P3三种羟基化布洛芬异构体。由于IBP中存在的弱C—H键可以促进紫外线辐射的直接吸收,这些产物会继续在·OH和直接光解的作用下经过脱羧、去甲基化等反应形成更小的芳香族中间产物,如4-异丁基苯乙酮、4-(3-甲基苯基)丁烷-2-酮、1-(4-异丁基苯基)乙醇等,接着打开苯环后形成小分子酸,最终小分子酸被进一步分解成CO2和H2O,IBP完成矿化。
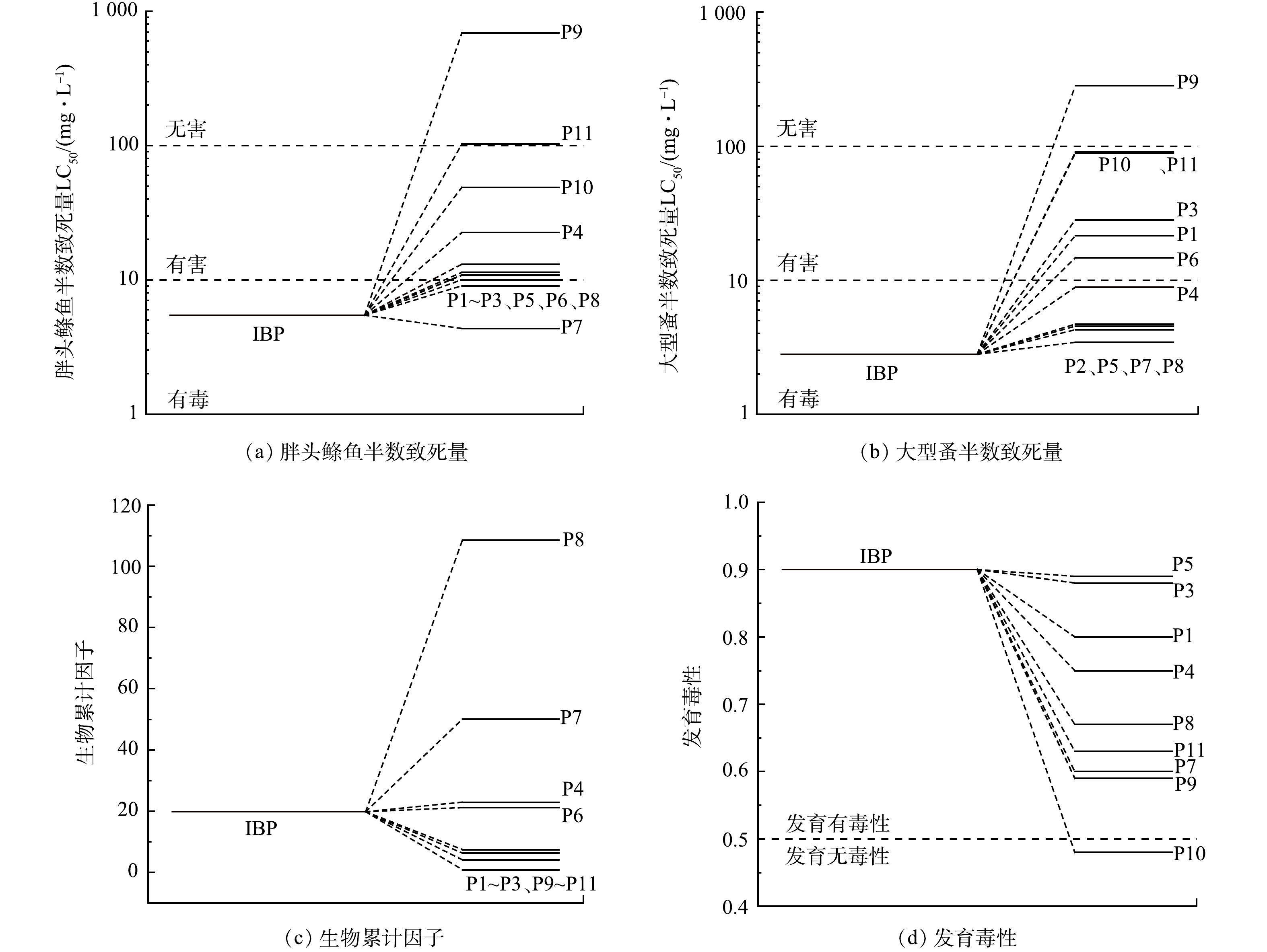
通过毒性评估软件工具(TEST)分析IBP及其降解中间产物的毒性[29]。采用定量构效关系(QSAR)法测定了胖头鲦鱼半数致死量(fathead minnow LC50)、大型蚤半数致死量(daphnia magna LC50)、生物因子(bioaccumulation factor)、发育毒性(developmental toxicity)。由图7(a)~(b)可看出,几乎所有的中间产物都具有较高的LC50值,说明在降解过程中急性毒性降低。由图7(c)可知,P7、P8的生物累计因子较大,虽然他们的发育毒性较小,但急性毒性均表现出较强毒性,因此,在降解过程中可能会生成比IBP毒性更强的物质。随着降解的进行,降解产物P9出现,其急性毒性和发育毒性明显降低,甚至达到无害程度,这就说明生成的毒性高的降解产物会随着反应的进行而被降解。此外,如图7(d)所示,光电体系使得IBP的发育毒性大大降低。综合上述毒性计算结果,推测在整个降解过程中,IBP在系统中的综合毒性有所降低。
-
1)相较于单独光氧化、电氧化,光电体系可显著提升污染物降解率,对多种污染物均体现出优异的降解能力;同时光电氧化可以降低能耗,有较好的实际应用潜力。
2)增加电流和光强可以提高光电氧化的降解效率,水体中的共存阴离子及天然有机物(HA)对光电体系降解污染物有一定抑制作用。在电流为0.30 A、3根灯管照射的最优工况下,初始质量浓度为20 µg·L−1的布洛芬降解率可达到99.37%,并且,自来水中HCO3−、Cl−和HA的浓度越低越好。光电体系有较强的抗水质干扰的能力。
3)检测出IBP降解过程中的11种中间产物,由此推测IBP在光电体系中可能的降解路径主要是通过羟基化、脱羧、去甲基化和开苯环进行降解。
4)通过毒性计算发现在光电体系的降解过程中,IBP的毒性显著降低。一些中间体表现出更高的毒性,但随着反应的进行,这些物质可以被继续降解,在整个降解过程中,IBP的综合毒性降低。
光电氧化降解饮用水中有机污染物的效能
Performance of photoelectro-oxidation process on the degradation of organic pollutants in drinking water
-
摘要: 针对地表水中普遍存在的有机污染物现状和常规水处理工艺无法去除有机污染物的问题,以紫外光电氧化技术为基础,提出了以“分质供水”为目标的末端深度水处理工艺。与单独电解和光解相比,电化学氧化与紫外耦合技术可以将布洛芬、阿特拉津、三氯乙酸、乐果的降解率在15 min内分别提高到92.5%、98.1%、71.0%和94.6%,并且能耗有所降低。电流和光强对光电氧化降解有机污染物有促进作用,但污染物初始质量浓度、Cl−、HCO3−和天然有机物(HA)和污染物去除率呈反比关系。在此基础上,以布洛芬为污染物代表,测定了布洛芬的中间产物,并计算了降解中间体的毒性变化,发现光电氧化明显降低了布洛芬的毒性。Abstract: In view of the present situation of organic pollutants in surface water and the problem that these organic pollutants cannot be effectively removed by the conventional water treatment technology, a deep end water treatment technology based on ultraviolet (UV) irradiation with electrochemical technology was proposed for dual water supply. Compared with electrolysis or UV irradiation alone, photoelectro-oxidation process coupled with UV irradiation could increase the degradation rates of ibuprofen, atrazine, trichloroacetic acid and dimethoate to 92.5%, 98.1%, 71.0% and 94.6% in 15 min, respectively, and could reduce the energy consumption. The increase of current and light intensity accelerated the degradation of organic pollutant by photoelectro-oxidation process. However, the initial concentration of pollutants、Cl−、HCO3− and organic matter (HA) were inversely proportional to the removal rates of these pollutants. Moreover, ibuprofen was taken as the representative pollutant, its intermediate products were determined and their toxicity changes were calculated. It was found that photoelectron-oxidation significantly reduced the toxicity of ibuprofen.
-
目前,新污染物已被发现广泛存在于水体环境当中,由于其在环境中的持久性,并且具有致突变、致畸变和致癌的“三致”风险,受到了研究人员的高度关注。有研究表明,以布洛芬(ibuprofen IBP)、阿特拉津(atrazine ATZ)、乐果(dimethoate DIM)以及三氯乙酸(trichloroacetic acid TCAA)为代表的新型污染物多次在地表水中被检出[1-2]。在日常生活中,人们往往忽略这些微量有机污染物对健康产生的不利影响,但长期接触或摄入这类化合物会对人体产生难以预料的影响[3]。饮用水是人类接触新污染物的直接途径,在日常洗漱和饮用过程中都会直接接触和摄入未完全去除的微污染物。
因此,必须将微污染物充分去除后饮用。常规的传统工艺如混凝、沉淀、消毒等,虽然能去除水体的浊度和色度等,但却无法将水体中的有机物彻底截留或降解[4],对于微克、纳克级别物质的去除效率更是十分有限。虽然大量采用纳滤和反渗透膜可以截留水中的污染物,但也大大提高了水处理成本,截留后产生的浓水也会成为新的污染来源。针对这一现状,本研究结合“分质供水”理念,对人体直接接触和摄入的饮用水进行深度处理,在减少水处理成本的基础上提高家庭端水质。
光电氧化被认为是一种具有应用前景的水处理技术,通过施加外部偏压减少光生电子和空穴的复合,提高量子效率,可以在提高降解率的同时降低能耗,具有绿色、高效的特点。能高效分离光电子空穴和具有出色的光吸收特性的半导体电极是光电催化体系的首选电极,如TiO2、ZnO、BiVO4和MOFs电极。其中,TiO2基电极因其具有良好的光学性能和低毒性成为最常用的光电电极。研究者采用Ru0.3Ti0.7O2作为光电阳极,这种电极既具有RuO2的电化学特性,又具有TiO2的光催化特性,其矿化效率(38%)高于仅在电催化过程中的矿化效率(<10%)[5]。本研究选用具有良好光活性的TiO2-IrRu电极,钌铱金属由于其优异的导电性能和长时间的工作寿命而被作为一种电极涂层,以此来增加电极材料的稳定性和电催化活性。与传统光电阳极相比,该电极具有物化性质稳定、氧化能力强、安全无害的特点[6]。
目前,光电技术已被证实对低浓度的有机污染物也有非常高的降解效能[7-10],但由于能耗较高,不适用于大规模的饮用水处理。本研究提出将光电催化技术用于分质供水的末端水处理,选用性能稳定、无毒无害的TiO2-IrRu电极为光电催化阳极材料,采用自来水配置水样模拟实际水体,以4种污染物为模型污染物分析光电氧化系统的污染物降解情况和能耗,并通过布洛芬的降解路径,计算降解过程的毒性变化。
1. 材料和方法
1.1 实验试剂
实验中以布洛芬(IBP,>98%,西安博涵化工科技有限公司)、阿特拉津(ATZ,>97%,广州科启化学技术有限公司)、三氯乙酸(TCAA,>99%,江苏艾康生物医药研发有限公司)、乐果(DIM,>98%,广州科启化学技术有限公司)为模型污染物,甲醇、乙酸铵、氯化钠、碳酸氢钠购自科密欧化学试剂有限公司(中国天津),腐殖酸购自国药集团化学试剂有限公司(中国上海),除甲醇为色谱纯外,其他药剂均为分析纯。
1.2 实验装置
自制光电反应系统,采用3根ZW10D15Y(W)-Z212型低压汞灯、商业购买的TiO2-IrRu阳极及不锈钢阴极,具体结构如图1所示。实验中通过控制紫外灯管开关数来控制紫外光照射强度,使用1、2、3根灯管对应的光照强度分别为2 210、3 352、4 566 LUX。
1.3 实验方法
考虑到实际应用的可能性以及稳定性,实验中的水样全部采用自来水配置,自来水水质如表1所示。根据《生活饮用水卫生标准》(GB 5749-2022)限值要求,IBP、ATZ、TCAA、DIM的初始质量浓度分别为80、50、200、60 µg·L−1。制备水样在光电反应器中进行反应后,样品经0.22 μm滤膜过滤后采用液质联用仪(Waters UPLC-TQD)检测4种模型污染物在降解过程中的浓度变化。采用Ultimate 3 000 UHPLC-Q Exactive液质联用仪分析IBP中间产物。
表 1 实验所用自来水水质参数Table 1. Water quality parameters of tap waterpH 浊度/NTU 电导率/(μS·cm−1) TOC/(mg·L−1) 总碱度/(mg·L−1) 总硬度/(mg·L−1) 6.8~7.2 0.2~0.3 170~230 1.2~1.8 100~110 100~120 使用式(1)~(3)计算直接光解(UV)、电化学氧化(E)和光电氧化(E-UV)工艺将1 m3水中污染物浓度降低1个数量级的能耗需求[11]。
stringUtils.convertMath(!{formula.content}) (1) stringUtils.convertMath(!{formula.content}) (2) stringUtils.convertMath(!{formula.content}) (3) 式中:E是工艺所需能耗,kWh·m−3;P是紫外线灯的功率,W;t是处理时间,min;C0和Ct分别是0和t时刻污染物质量浓度,µg·L−1;V是溶液体积,L;I是电流,A;U是施加电压,V。
2. 结果与讨论
2.1 电极表征
Ti基电极是目前光电氧化中一种常用的电极,其表面添加的涂层材料很大程度上决定了电极性能。铅(PbO2)、锡(SnO2)、铱(IrO2)、钌(RuO2)等氧化物及其组合已被用于提高阳极的电催化活性、稳定性和能效[12]。SnO2等非活性阳极具有较高的析氧电位,但其使用寿命短[13],而PbO2电极具有低成本和高化学稳定性,但其析氧电位低[14]。目前,IrO2和RuO2的组合已被用于开发高效阳极,且已有研究证明TiO2-IrRu电极在光电降解有机物方面具有良好效果[15-16]。因此,本研究选用该电极作为光阳极。
循环伏安扫描(CV)是对电极电化学性质的表征,为了探究实验所用的TiO2-IrRu电极在紫外照射下的性质,对其进行循环伏安扫描。为了避免电解液进入电极表面微观结构初期所带来的的不稳定曲线,选择循环40次后的CV曲线以最小化这种偏差。
图2为在0.05 mol·L−1的Na2SO4溶液中获得的CV曲线。结果表明,在光电体系中出现1个明显氧化峰,说明在该体系下发生了氧化反应。氧化峰的出现可以说明在光的照射下,电极对有机污染物的降解具有更好的电化学性能,与单纯的电化学工艺相比,光电工艺可以提供更多的降解路径,从而提高对有机污染物的氧化分解能力。
2.2 不同体系下污染物的降解对比
在光电反应器中,通过单独光照、单独电氧化和光电氧化来降解4种污染物。在电流为0.30 A、光照强度4 566 LUX的条件下,考察了15 min内4种污染物的降解效果。如图3(a)所示,虽然单独电氧化可较为有效地去除DIM,但电化学对IBP、ATZ和TCAA的去除率较低,IBP的降解率仅达到17.95%。在单独紫外照射中,TCAA的去除率也只有51.89%。与单独紫外线和电化学过程相比,光电耦合技术可以显著的提高4种污染物的降解率,ATZ的降解率可达到99.09%。以上结果表明,光电体系对污染物有更高效的去除效果。
在光电氧化降解有机污染物的过程中,不同类型的污染物对紫外光和电解有不同的反应速率。电化学氧化中主要通过电子的直接转移来降解污染物,由于IBP和ATZ在阳极表面的传质受限[17],这造成了单独电化学氧化对这两种物质的降解率和反应动力学常数都很低(表2)。紫外光解对TCAA的降解率较低主要是因为TCAA对紫外的吸收率很低,可以抵抗住紫外线的光解作用。根据实验结果,光电氧化技术能够提高4种物质的降解率,这主要是因为光、电之间的协同、互补作用,在光电氧化体系中存在多种降解途径,阳极直接氧化、自由基氧化以及紫外线的光解等。同时,体系中的阳极材料也提供了光催化氧化降解污染物的路径,施加的外部偏压更是减少了体系中光生电子空穴的复合,从而提高降解效能。
表 2 4种污染物动力学常数k值Table 2. Parameters of reaction kinetics of four pollutants污染物名称 k /min−1 E UV E-UV IBP 0.015 7 0.117 3 0.182 6 ATZ 0.034 6 0.219 2 0.368 0 TCAA 0.036 2 0.053 3 0.092 1 DIM 0.119 5 0.104 2 0.177 1 能耗是评价一个工艺的重要指标,决定了该工艺的经济性和应用前景,本研究中能耗包括紫外线的照射和电解,如图3(b)所示。虽然UV工艺可以以相对较小的能耗去除IBP和ATZ,但由于它们的电化学反应性较低,在单独电氧化中能耗较高。另一方面,虽然电化学工艺能以较低的能耗降解DIM,但是降解其他三种物质的能耗过高。与单独UV和电氧化相比,光电组合工艺能通过相对较少的能耗减少污染物。为了探究光电技术在实际应用方面的可能性,将其与其他技术进行对比,以西安市平均电价0.49 元·(kWh)−1计算,大致估算这些工艺处理1吨水所需要的价格,结果如表3所示。这些数据表明,光电氧化技术在能耗方面,可以成为水处理中消除微量有机污染物的一种有力竞争方法。
表 3 不同工艺能耗对比Table 3. Comparison of energy consumption of different processes2.3 不同实验参数对光电氧化降解饮用水中微量有机物的影响
1)电流对光电氧化降解效果的影响。电流是光电反应中一个重要影响因素,在光照强度为4 566 LUX的情况下,探究不同电流对IBP降解情况的影响,结果如图4(a)所示。可见,当电流由0.25 A升至0.35 A的过程中,IBP的降解率逐渐升高,k值也随着电流的增加而升高(表4)。一方面,电流会直接影响电解过程中的电子转移,电流增大,电子转移速度加快,对IBP的直接电解作用增强;另一方面,对于光催化而言,电流的增加会减少光生电子-空穴对的复合,加速光电反应中电子的迁移,从而提高IBP的降解效率。此外,随着电流的增大,阳极表面会产生更多的·OH[21],IBP的降解速率也因此得到提高。由于采用自来水进行实验,受限于自来水中电解质浓度较低,可维持实验条件的电流区间较小,所设置的电流改变不大,对实验结果的影响并不明显,但并不能否认电流在光电体系中的重要性。另外,当电流过高时,析氧副反应将逐渐占据主导地位[22],影响降解反应的进行,导致污染物的去除效率变低,因此,在光电反应中选取合适的电流参数比较重要。
表 4 不同实验参数下IBP的反应动力学k值Table 4. The parameters of IBP reaction kinetics under different experiment parametersmin−1 电流/A 光强/LUX 初始质量浓度/(µg·L−1) 0.30 0.30 0.35 2 210 3 352 4 566 20 40 80 0.156 2 0.182 6 0.217 0 0.083 0 0.122 0 0.182 6 0.359 6 0.259 9 0.182 6 2)光强对光电氧化降解效果的影响。在IBP初始质量浓度不变,电流为0.30 A的情况下,通过控制紫外灯管的开关数研究不同光照强度对于光电反应的影响。根据图4(b)及表4可以发现,光强对于光电体系的影响十分显著,随着光强的增加,IBP的降解率提高了21.06%,并且动力学常数k值也有大幅度的提高。分析其原因,分子吸收光的本质是在紫外光辐射的作用下物质分子的能态发生了改变,由低能态被激发至高能态进而发生各种反应[23]。实际上,增加光强是通过提高单位反应体积内的光子流量来提高反应速率,光子流量的增加会使单位时间内被活化的IBP物质分子数增加,同时,光强增大会使电极表面获得更多能量,激发更多的光生空穴,进而提高降解效果。不可忽视的另一原因是紫外光的辐射强度与距离有关,光氧化只能在辐射点周围的一定范围内发生[24]。当只采用1根灯管时,紫外光的辐射体积有限,仅在灯管周围的IBP分子能够发生光氧化,而增加到3根灯管时,紫外光的辐射体积可以扩大到整个体系,使得水体中更多的IBP分子能够被照射,IBP的降解效果因此得到增强。
3)有机物初始质量浓度对光电氧化降解效果的影响。为了探究不同初始质量浓度下的污染物在光电体系中的降解情况,配制初始质量浓度为80、60、40 µg·L−1的IBP水样。在电流为0.30 A,光照强度为4 566 LUX的反应条件下,考察不同IBP初始质量浓度的有机物在光电体系中的去除情况,结果如图4(c)所示。可以看出,随着IBP初始质量浓度的增加,k值也逐渐减小。这表明IBP的降解效果随着质量浓度的增加而变差。导致上述现象的原因是,在一定的电流与光照条件下,阳极表面的电子转移与系统中的光子总量基本不变,随着IBP质量浓度的增加,阳极表面直接电解IBP的效率降低,并且单位分子得到的光子数也随之减少,直接光解效果变差。同样,光照射在电极上产生的电子空穴数量一定,当IBP分子数量增加时,光生空穴氧化有机物的能力也有所下降。同时,反应当中所生成的·OH恒定,IBP质量浓度越大,所消耗的·OH也越多,从而使得降解率下降。并且,初始质量浓度越高可能会在反应中生成更多的中间产物,这些产物会与IBP形成竞争关系,导致了降解效率下降。
2.4 光电氧化降解饮用水中微量有机物的水体基质影响评价
碱度和氯离子浓度是饮用水中的重要指标,以腐殖酸(HA)为代表的天然有机物也在地表环境中广泛存在,即使HA含量很低的情况下,也会在一定程度上影响反应的进行。因此,有必要探究水基质对光电氧化体系的影响,在IBP质量浓度为80 µg·L−1,电流为0.30 A,光强为4 566 LUX的条件下,水质参数对IBP降解的影响及动力学变化如图5和表5所示。
表 5 不同水质参数下IBP的反应动力学k值Table 5. The parameters of IBP reaction kinetics under different water quality parametersmin−1 HCO3−/(mmol·L−1) Cl−/(mmol·L−1) HA/(mg·L−1) 0 5 10 0 5 10 0 0.5 1 0.182 6 0.146 8 0.127 7 0.182 6 0.148 3 0.124 9 0.182 6 0.158 3 0.131 5 由图5可以看出,HCO3−、Cl−和HA都对IBP的降解产生了不同程度的抑制。随着这些物质浓度的增加,IBP的降解效果逐渐降低,动力学常数k值也变小。在光电反应中,IBP主要通过直接电解、直接光解和·OH的氧化3种降解途径,其中·OH的强大的氧化能力更是为IBP的降解提供了不可缺少的作用。有研究表明,HCO3−会和·OH生成CO3·−,与·OH的非选择性不同,CO3·−是一种选择性较强而氧化能力较弱的自由基。HA会产生轻微的光屏蔽效应[25],HA具有一定的色度,即使在低质量浓度下,其在光解中对紫外产生的光屏蔽作用也不可忽视,并且HA的添加会抑制·OH的生成[26]。Cl−作为水中一种常见的阴离子并会在光照条件下生成一系列氯自由基而被广泛关注,已有研究证明,活性氯自由基可以快速氧化多种微污染物,从而达到增强降解效果的目的[27]。但本研究发现随着Cl−浓度的增加,降解效果反而有所下降。这可能是IBP自身的性质所导致的,同时,Cl−也是·OH的有效清除剂。因此,实验中加入HCO3−、Cl−和HA后,随着·OH的淬灭,IBP的降解也受到抑制。
通常,在复杂的水质条件下,水中的离子会与污染物发生竞争从而导致降解效果变差。在本研究中,虽然HCO3−、Cl−和HA的加入在一定程度上削弱了光电体系的降解效能,但IBP的降解率仍能达到80%以上。由此可见,本研究的光电体系在不同的水质条件下,都表现出良好的适用性。
2.5 IBP降解路径及毒性分析
采用液质联用仪分析IBP的降解产物,在降解过程中检测到11种主要产物,根据分析结果结合相关文献[28]推测IBP可能的降解路径,如图6所示。IBP的降解由羟基化开始,首先生成了m/z=221的中间体,结合有关文献报道提出了P1、P2、P3三种羟基化布洛芬异构体。由于IBP中存在的弱C—H键可以促进紫外线辐射的直接吸收,这些产物会继续在·OH和直接光解的作用下经过脱羧、去甲基化等反应形成更小的芳香族中间产物,如4-异丁基苯乙酮、4-(3-甲基苯基)丁烷-2-酮、1-(4-异丁基苯基)乙醇等,接着打开苯环后形成小分子酸,最终小分子酸被进一步分解成CO2和H2O,IBP完成矿化。
通过毒性评估软件工具(TEST)分析IBP及其降解中间产物的毒性[29]。采用定量构效关系(QSAR)法测定了胖头鲦鱼半数致死量(fathead minnow LC50)、大型蚤半数致死量(daphnia magna LC50)、生物因子(bioaccumulation factor)、发育毒性(developmental toxicity)。由图7(a)~(b)可看出,几乎所有的中间产物都具有较高的LC50值,说明在降解过程中急性毒性降低。由图7(c)可知,P7、P8的生物累计因子较大,虽然他们的发育毒性较小,但急性毒性均表现出较强毒性,因此,在降解过程中可能会生成比IBP毒性更强的物质。随着降解的进行,降解产物P9出现,其急性毒性和发育毒性明显降低,甚至达到无害程度,这就说明生成的毒性高的降解产物会随着反应的进行而被降解。此外,如图7(d)所示,光电体系使得IBP的发育毒性大大降低。综合上述毒性计算结果,推测在整个降解过程中,IBP在系统中的综合毒性有所降低。
3. 结论
1)相较于单独光氧化、电氧化,光电体系可显著提升污染物降解率,对多种污染物均体现出优异的降解能力;同时光电氧化可以降低能耗,有较好的实际应用潜力。
2)增加电流和光强可以提高光电氧化的降解效率,水体中的共存阴离子及天然有机物(HA)对光电体系降解污染物有一定抑制作用。在电流为0.30 A、3根灯管照射的最优工况下,初始质量浓度为20 µg·L−1的布洛芬降解率可达到99.37%,并且,自来水中HCO3−、Cl−和HA的浓度越低越好。光电体系有较强的抗水质干扰的能力。
3)检测出IBP降解过程中的11种中间产物,由此推测IBP在光电体系中可能的降解路径主要是通过羟基化、脱羧、去甲基化和开苯环进行降解。
4)通过毒性计算发现在光电体系的降解过程中,IBP的毒性显著降低。一些中间体表现出更高的毒性,但随着反应的进行,这些物质可以被继续降解,在整个降解过程中,IBP的综合毒性降低。
-
表 1 实验所用自来水水质参数
Table 1. Water quality parameters of tap water
pH 浊度/NTU 电导率/(μS·cm−1) TOC/(mg·L−1) 总碱度/(mg·L−1) 总硬度/(mg·L−1) 6.8~7.2 0.2~0.3 170~230 1.2~1.8 100~110 100~120 表 2 4种污染物动力学常数k值
Table 2. Parameters of reaction kinetics of four pollutants
污染物名称 k /min−1 E UV E-UV IBP 0.015 7 0.117 3 0.182 6 ATZ 0.034 6 0.219 2 0.368 0 TCAA 0.036 2 0.053 3 0.092 1 DIM 0.119 5 0.104 2 0.177 1 表 3 不同工艺能耗对比
Table 3. Comparison of energy consumption of different processes
表 4 不同实验参数下IBP的反应动力学k值
Table 4. The parameters of IBP reaction kinetics under different experiment parameters
min−1 电流/A 光强/LUX 初始质量浓度/(µg·L−1) 0.30 0.30 0.35 2 210 3 352 4 566 20 40 80 0.156 2 0.182 6 0.217 0 0.083 0 0.122 0 0.182 6 0.359 6 0.259 9 0.182 6 表 5 不同水质参数下IBP的反应动力学k值
Table 5. The parameters of IBP reaction kinetics under different water quality parameters
min−1 HCO3−/(mmol·L−1) Cl−/(mmol·L−1) HA/(mg·L−1) 0 5 10 0 5 10 0 0.5 1 0.182 6 0.146 8 0.127 7 0.182 6 0.148 3 0.124 9 0.182 6 0.158 3 0.131 5 -
[1] DAI G, WANG B, HUANG J, DONG R, et al. Occurrence and source apportionment of pharmaceuticals and personal care products in the Beiyun River of Beijing, China[J]. Chemosphere, 2015, 119: 1033-1039. doi: 10.1016/j.chemosphere.2014.08.056 [2] IKEHATA K, El-Din M G. Aqueous pesticide degradation by hydrogen peroxide/ultraviolet irradiation and Fenton-type advanced oxidation processes: A review[J]. Journal of Environmental Engineering and Science, 2006, 5(2): 81-135. doi: 10.1139/s05-046 [3] BOXALL A B A, RUDD M A, BROOKS B W, et al. Pharmaceuticals and personal care products in the environment: What are the big questions?[J]. Environmental health perspectives, 2012, 120(9): 1221-1229. doi: 10.1289/ehp.1104477 [4] 孙绪敏, 刘建广. 紫外高级氧化工艺控制消毒副产物生成研究进展[J]. 净水技术, 2022, 41(11): 7-15. [5] RAMOS L F, DA SILVA S W, SCHNEIDER D E, et al. Mineralization of erythromycin by UV-based and electro-oxidation processes[J]. Journal of Water Process Engineering, 2020, 33: 101039. doi: 10.1016/j.jwpe.2019.101039 [6] YAñEZ-RIOS A E, CARRERA-CRESPO J E, LUNA-SANCHEZ R M, et al. The influence of pH and current density on an UV254 photo-assisted electrochemical process generating active chlorine and radicals for efficient and rapid ciprofloxacin mineralization compared to individual techniques[J]. Journal of Environmental Chemical Engineering, 2020, 8(5): 104357. doi: 10.1016/j.jece.2020.104357 [7] YIN H, ZHANG Q, SU Y, et al. A novel UV based advanced oxidation process with electrochemical co-generation of chlorine and H2O2 for carbamazepine abatement: better performance, lower energy consumption and less DBPs formation[J]. Chemical Engineering Journal, 2021, 425: 131857. doi: 10.1016/j.cej.2021.131857 [8] 宋强, 曲久辉. 光电协同新技术降解饮用水中微量邻氯酚[J]. 科学通报, 2003, 48(3): 233-238. [9] LI C, WANG Y, WANG Y, et al. Electrochemical oxidation combined with UV irradiation for synergistic removal of perfluorooctane sulfonate (PFOS) in water[J]. Journal of Hazardous Materials, 2022, 436: 129091. doi: 10.1016/j.jhazmat.2022.129091 [10] ZHANG Y, WANG H, LI Y, et al. Removal of micropollutants by an electrochemically driven UV/chlorine process for decentralized water treatment[J]. Water Research, 2020, 183: 116115. doi: 10.1016/j.watres.2020.116115 [11] YU F, ZHOU M, YU X. Cost-effective electro-Fenton using modified graphite felt that dramatically enhanced on H2O2 electro-generation without external aeration[J]. Electrochimica Acta, 2015, 163: 182-189. doi: 10.1016/j.electacta.2015.02.166 [12] ZHANG W, GHALI E, HOULACHI G. Review of oxide coated catalytic titanium anodes performance for metal electrowinning[J]. Hydrometallurgy, 2017, 169: 456-467. doi: 10.1016/j.hydromet.2017.02.014 [13] XU A, DAI X, WEI K, et al. Preparation and characterization of a TiO2-NT/SnO2-Sb tubular porous electrode with long service lifetime for wastewater treatment process[J]. RSC Advances, 2017, 7(60): 37806-37814. doi: 10.1039/C7RA05127A [14] WANG W, DUAN X, SUI X, et al. Surface characterization and electrochemical properties of PbO2/SnO2 composite anodes for electrocatalytic oxidation of m-nitrophenol[J]. Electrochimica Acta, 2020, 335: 135649. doi: 10.1016/j.electacta.2020.135649 [15] HUANG X, WANG S, WANG G, et al. Kinetic and mechanistic investigation of geosmin and 2-methylisoborneol degradation using UV-assisted photoelectrochemical[J]. Chemosphere, 2022, 290: 133325. doi: 10.1016/j.chemosphere.2021.133325 [16] GONZAGA I M D, MORATALLA A, EGUILUZ K I B, et al. Outstanding performance of the microwave-made MMO-Ti/RuO2IrO2 anode on the removal of antimicrobial activity of Penicillin G by photoelectrolysis[J]. Chemical Engineering Journal, 2021, 420: 129999. doi: 10.1016/j.cej.2021.129999 [17] ZHANG Y, ZHAN J, WANG B, et al. Integration of ultraviolet irradiation with electrochemical chlorine and hydrogen peroxide production for micropollutant abatement[J]. Chemical Engineering Journal, 2022, 430: 132804. doi: 10.1016/j.cej.2021.132804 [18] GUO K H, WU Z H, YAN S W, et al. Comparison of the UV/ chlorine and UV/H2O2 processes in the degradation of PPCPs in simulated drinking water and wastewater: Kinetics, radical mechanism and energy requirements[J]. Water Research, 2018, 147: 184-194. doi: 10.1016/j.watres.2018.08.048 [19] 杨晓婷, 邱海燕, 谯梦丹等. Fenton氧化法与电絮凝法对水中盐酸四环素的去除[J]. 净水技术, 2022, 41(3): 90-99. [20] 郑雪. Fenton及电-Fenton降解盐酸四环素生产废水的研究[D]. 成都: 西南交通大学, 2021. [21] 贾伟建, 朱化雨, 王德生, 等. BDD阳极去除水中阿特拉津的特性及机理研究[J]. 工业水处理, 2022, 42(6): 174-179. [22] YU H, SUN X, ZHAO B, et al. Enhanced photoelectrocatalytic degradation of tetracycline using a bifacial electrode of nickel-polyethylene glycol-PbO2//Ti//TiO2-Ag2O[J]. Journal of Electroanalytical Chemistry, 2021, 893: 115319. doi: 10.1016/j.jelechem.2021.115319 [23] 邓南圣, 吴峰. 环境光化学[M]. 北京: 化学工业出版社 2003. [24] 陈宋义, 孙汝. 紫外灯辐射强度与电压, 温度及距离的关系[J]. 上海预防医学, 1999, 11(7): 309-311. [25] 李富华, 陈敏, 孔青青, 等. 水中几种无机离子对布洛芬光降解的影响[J]. 工业安全与环保, 2016, 42(10): 8-10. [26] FU Y, GAO X, GENG J, et al. Degradation of three nonsteroidal anti-inflammatory drugs by UV/persulfate: degradation mechanisms, efficiency in effluents disposal[J]. Chemical Engineering Journal, 2019, 356: 1032-1041. doi: 10.1016/j.cej.2018.08.013 [27] YANG X, SUN J, FU W, et al. PPCP degradation by UV/chlorine treatment and its impact on DBP formation potential in real waters[J]. Water Research, 2016, 98: 309-318. doi: 10.1016/j.watres.2016.04.011 [28] SRUTHI L, JANANI B, KHAN S S. Ibuprofen removal from aqueous solution via light-harvesting photocatalysis by nano-heterojunctions: A review[J]. Separation and Purification Technology, 2021, 279: 119709. doi: 10.1016/j.seppur.2021.119709 [29] CAI Z, HAO X, SUN X, et al. Highly active WO3@ anatase-SiO2 aerogel for solar-light-driven phenanthrene degradation: Mechanism insight and toxicity assessment[J]. Water Research, 2019, 162: 369-382. doi: 10.1016/j.watres.2019.06.017 -






 下载:
下载: