-
中国已成为最大的电解锰生产国,占世界电解锰生产能力和产量的98%以上[1]。目前,几乎大部分的电解锰渣都未经任何处理就被倾倒在填埋场,其主要污染物氨氮(NH4+-N)、可溶性锰(Mn2+)和重金属等会随着雨水的浸出、淋溶渗漏到周围的环境中,造成严重的土壤和水体污染[2-3],由电解锰渣引起的环境问题已引起社会的广泛关注。电解碳酸锰渣(electrolytic manganese carbonate residue,EMCR)是将菱锰矿中的固体碳酸锰转化为可溶性锰时排放的一种固体废弃物[4]。电解二氧化锰(electrolytic manganese dioxide,EMD)是现代碱性、锂和钠电池(包括电化学电容器和制氢)中阴极材料的关键成分,随着电池行业的快速发展,EMD的产能每年已达465 900 t,我国的产量约占64.02% [5]。而电解二氧化锰渣(electrolytic manganese dioxide residue,EMDR)是电解二氧化锰工业生产过程中产生的固体废物[6]。
目前,电解锰渣的综合利用主要集中于锰的回收、制备铺路砖体等建筑材料、用作土壤肥料等。如CHANG等[7]通过优化锰的微波浸出工艺,在温度76 ℃、时间55 min、H2SO4浓度为0.76 mol·L−1、柠檬酸的用量为3.51 mg·g−1的条件下微波辅助浸出锰。SHU等[8]开发了一种通过电还原从电解金属锰渣中浸出锰的改进工艺,电场可以改变电解金属锰渣粒子的表面电荷分布,还原高价锰。ZHANG等[9]将电解锰渣与赤泥(RM)和其他固体废物协同用作道路基层材料(RBM)。WANG等[10]根据电解锰渣的特点(即高石膏含量),开发了一种新型的电解金属锰渣活化胶凝材料(EGCH)。以上在工程实践中应用时易受到不可控的利用效率和潜在的再浸出环境风险的限制。而煅烧作为环保的无害化处理方式之一已广泛应用于固体废弃物的处理处置中[11–13]。电解锰渣的煅烧处理与其他方式相比有很多优点,在煅烧过程中电解锰渣中的有害物质会被去除,产生的气体可被回收制备硫酸,且电解锰渣含有大量的SiO2、AI2O3、Fe2O3等物质,经一定温度煅烧处理后可激发它们的潜在活性[14–16],后续可进行高效资源化利用。
但目前对于电解锰渣高温煅烧利用处置过程中污染物的形态分析研究较少。本研究对2种不同工艺产生的EMCR和EMDR在氮气气氛下经不同温度煅烧后残渣的矿物组成及微观组织形貌进行了分析,对排放烟气中N元素各化合态形式进行研究,并通过改进BCR连续提取法探究煅烧过程中两种电解锰渣残渣中Mn元素的形态变化特征,并通过风险评估代码(RAC)对样品中Mn元素进行环境风险评估。对电解锰渣煅烧处置过程开展性质分析,可清晰直观地掌握各温度段电解锰渣中污染物的存在形态,同时还将为2种电解锰渣经煅烧处置后的资源化再利用工作提供有效的数据支撑。
-
2种锰渣样品来自中国广西某企业采用四分法取样的新鲜锰渣,将样品经烘箱105 ℃下烘干至恒重,机械破碎后过100目筛网,装入封口袋保存备用。EMCR与EMDR的化学成分如表1所示。2种锰渣样品的主要成分为 SO3、SiO2、CaO、Fe2O3、Al2O3、MnO等,约占样品全部含量的90%。
-
将锰渣样品在管式炉中进行煅烧处理,设置5个煅烧温度(即500、800、1 000、1 100、1 200 ℃)。根据管式炉的升温限值,设定10 ℃·min−1的升温速率,待管式炉的温度达到实验设定温度后,迅速推动装有20 g锰渣样品的瓷舟向管式炉的中间移动,快速密封通入气氛,样品在管式炉内煅烧时间为20 min。
-
1) 表征分析。使用X射线荧光光谱仪(XRF,ARLADVANT XP+,瑞士arl公司)测定锰渣的化学成分组成。使用X射线衍射仪(XRD,Bruker-D8,德国BRUKER公司)测定锰渣的结晶相。使用扫描电镜能谱分析仪(SEM-EDX,HITACHI S-4800,日本日立公司)对锰渣微观结构和截面元素组成进行识别。采用热重分析仪(TG-DTG,SDT-Q600,美国TA仪器公司)对两种电解锰渣进行热重分析。
2) Mn元素形态分布的分析方法。重金属的总浓度测定在环境监测中具有重要价值,但在环境中的赋存形态与总量也有重要的研究意义,这将决定它们的毒性、流动性与利用度[17–19]。电解锰渣中主要重金属污染物是Mn元素,本研究采用改进BCR连续提取法[20],对锰渣样品中Mn元素的形态分布进行分析研究,BCR连续提取方案如表2所示。
提取步骤如下。
①可交换态。称取1 g样品于50 mL聚丙烯离心管中,加入0.11 mol·L−1醋酸提取液40 mL,室温下振荡16 h,之后用4 000 r·min−1转速的离心机离心分离20 min,将上层清液转移至比色管中,保存于4 ℃冰箱中待测。加入20 mL高纯水清洗残余物,振荡20 min,离心,弃去清洗液。
②可还原态。向第一步提取后的残余物中加入0.50 mol·L−1盐酸羟胺提取液40 mL,室温下振荡16 h,用4 000 r·min−1转速的离心机离心分离20 min,将上层清液转移至比色管中,保存于4 ℃冰箱中待测。加入20 mL高纯水清洗残余物,振荡20 min,离心,弃去清洗液。
③可氧化态。向第二步提取后的残余物中缓慢加入10 mL过氧化氢,每隔15 min振荡1次,室温下消解1 h,之后水浴加热到85 ℃消解1 h,打开离心管盖子,95 ℃加热至溶液近干,再加入10 mL过氧化氢重复上述过程1次。冷却后,加入1 mol·L−1醋酸铵提取液50 mL,室温下振满16 h,之后用4 000 r·min−1转速的离心机离心分离20 min,将上层清液转移至比色管中,保存于4 ℃冰箱中待测。加入20 mL高纯水清洗残余物,振荡20 min,离心,弃去清洗液。
④残渣态。向第三步提取后的残渣中加入配比为3∶5∶1∶5的盐酸、硝酸、高氯酸、氢氟酸,转移至聚四氟乙烯管中,之后加入5 mL硝酸,2 mL氢氟酸和2 mL过氧化氢,微波消解至固体消解完全,赶酸仪150 ℃赶至剩最后1滴,用去离子水定容至50 mL容量瓶,待测。
根据BCR连续提取结果,采用风险评估代码(RAC)对样品中重金属的生态风险进行评估。RAC是广泛采用的定量评估方法,用于综合评估样品中重金属元素的生态风险[21-22]。本研究用该方法评估经不同温度煅烧处理后的电解锰渣样品中Mn元素的风险等级。RAC值如式(1)所示。
根据RAC结果,可以将风险等级划分为5个等级,如表3所示。
3) N元素形态分布的分析方法。烟气中氨氮收集装置,由2个50 mL装有0.01 mol·L−1硫酸吸收液的洗气瓶串联组成,根据《水质 氨氮的测定 纳氏试剂分光光度法》(HJ 535-2009)[23]测定氨氮质量浓度;使用高温烟气分析仪直接测定烟气中除氨氮外N元素各化合态的质量浓度。
-
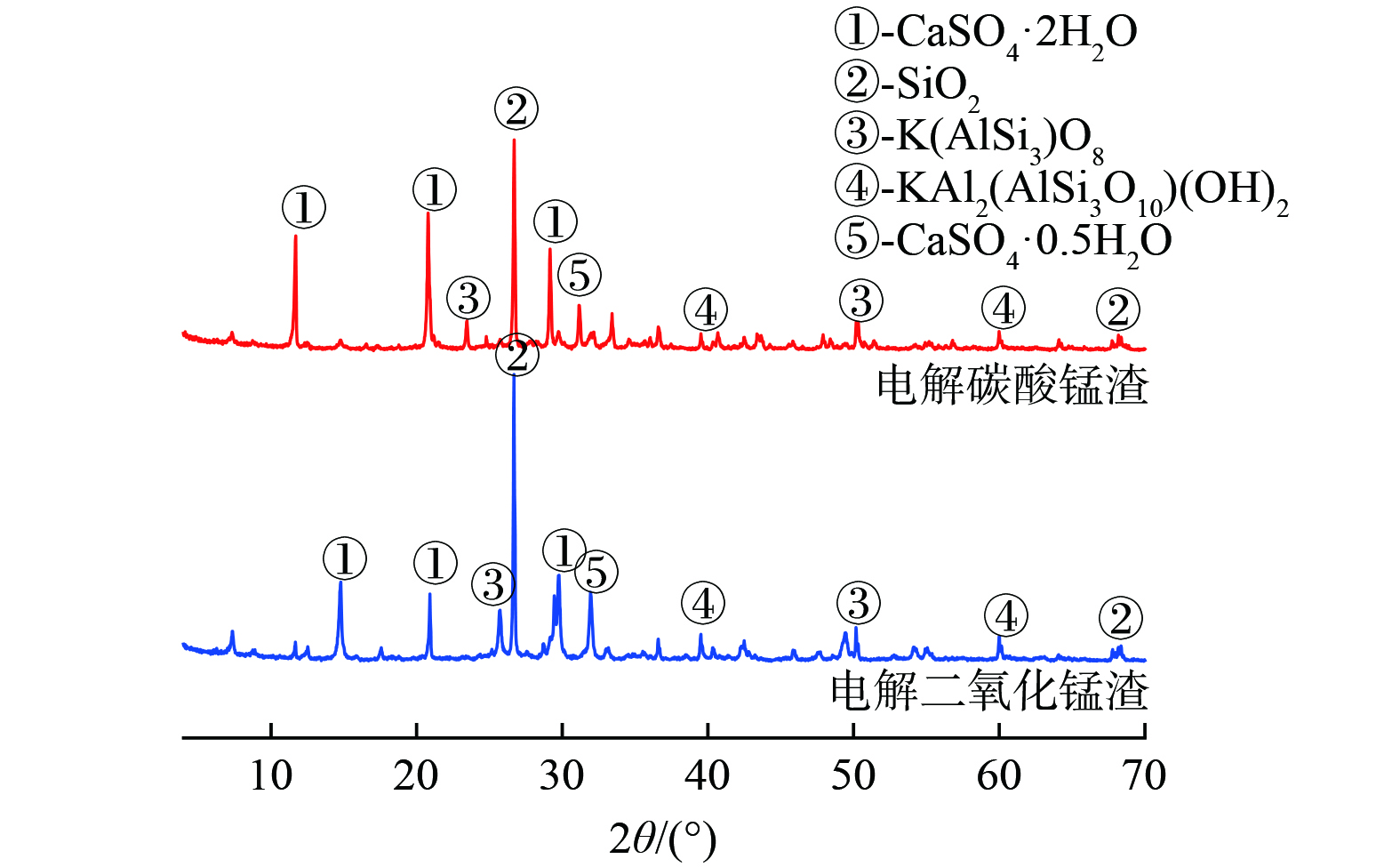
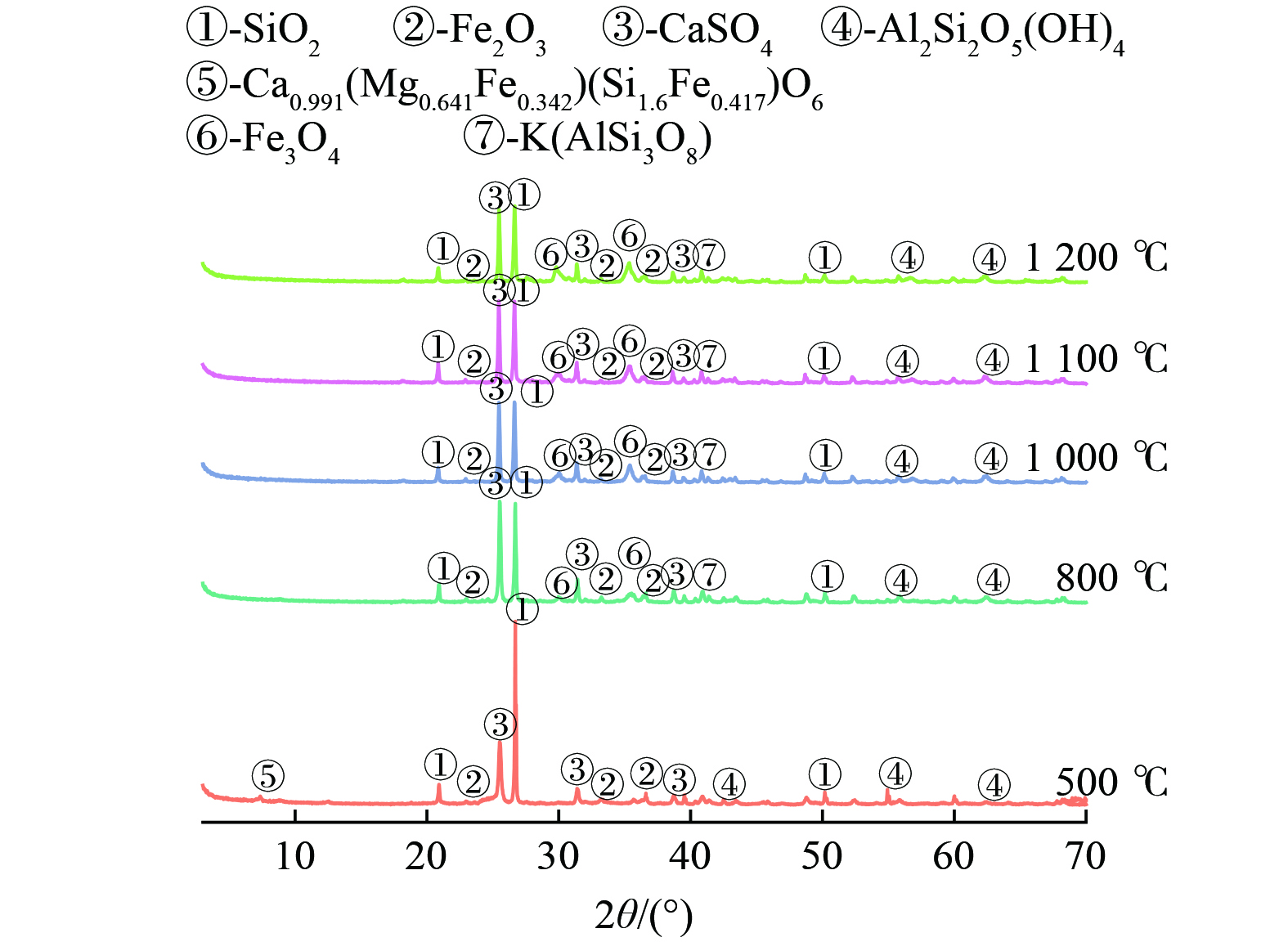
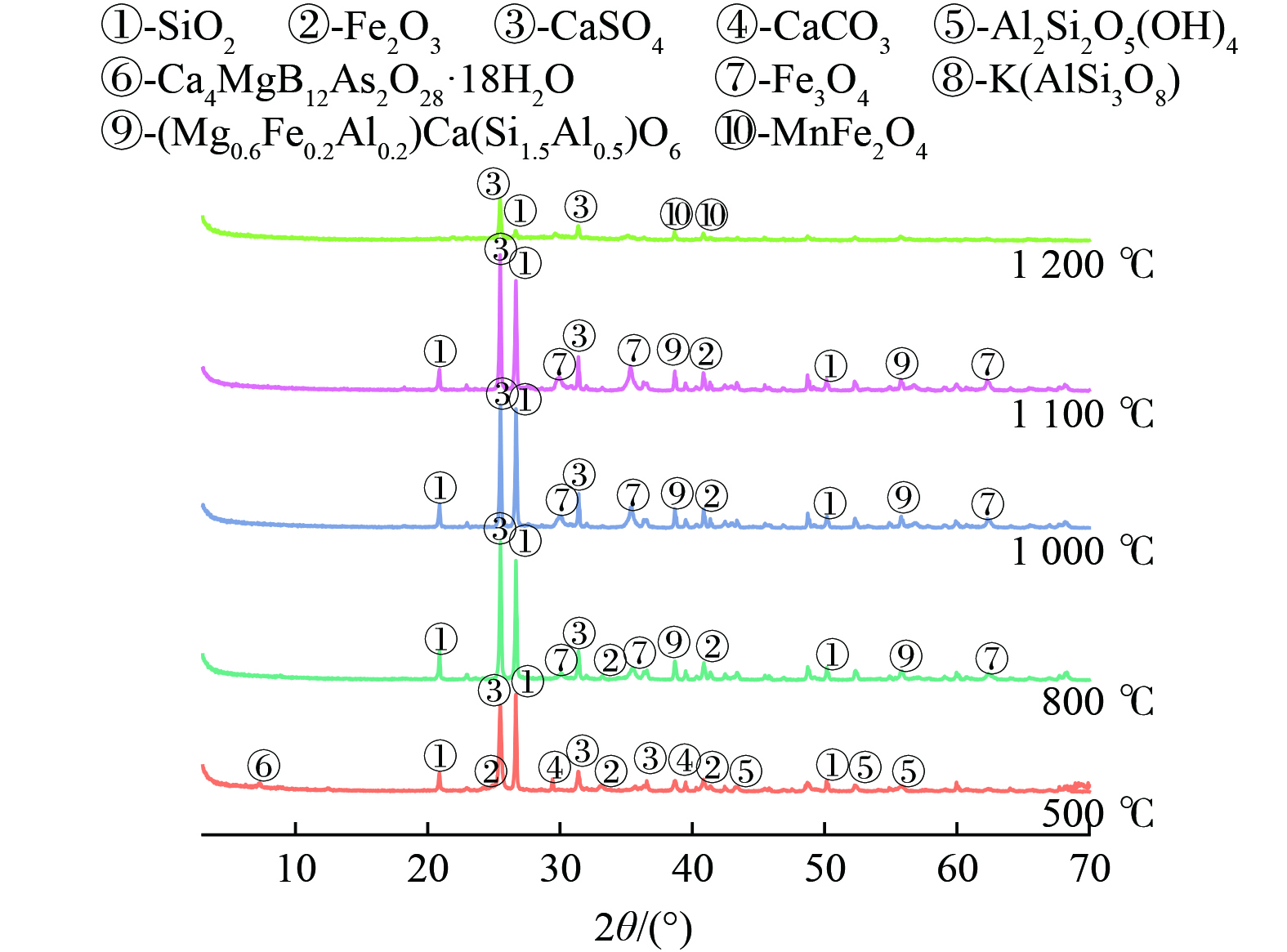
1) 物相组成分析。EMCR与EMDR的XRD图谱如图1所示。EMCR与EMDR的主要矿物成分包括石膏(CaSO4·2H2O)、石英(SiO2)、微斜长石(K(AlSi3)O8)、云母(KAl2(AlSi3O10) (OH)2)、烧石膏(CaSO4·0.5H2O)。其中,石英(SiO2)与石膏(CaSO4·2H2O)的特征谱线峰型尖锐,结晶形态较好,是EMCR与EMDR中的主要物相;其他矿物相的衍射峰强度较低,结晶度较低。
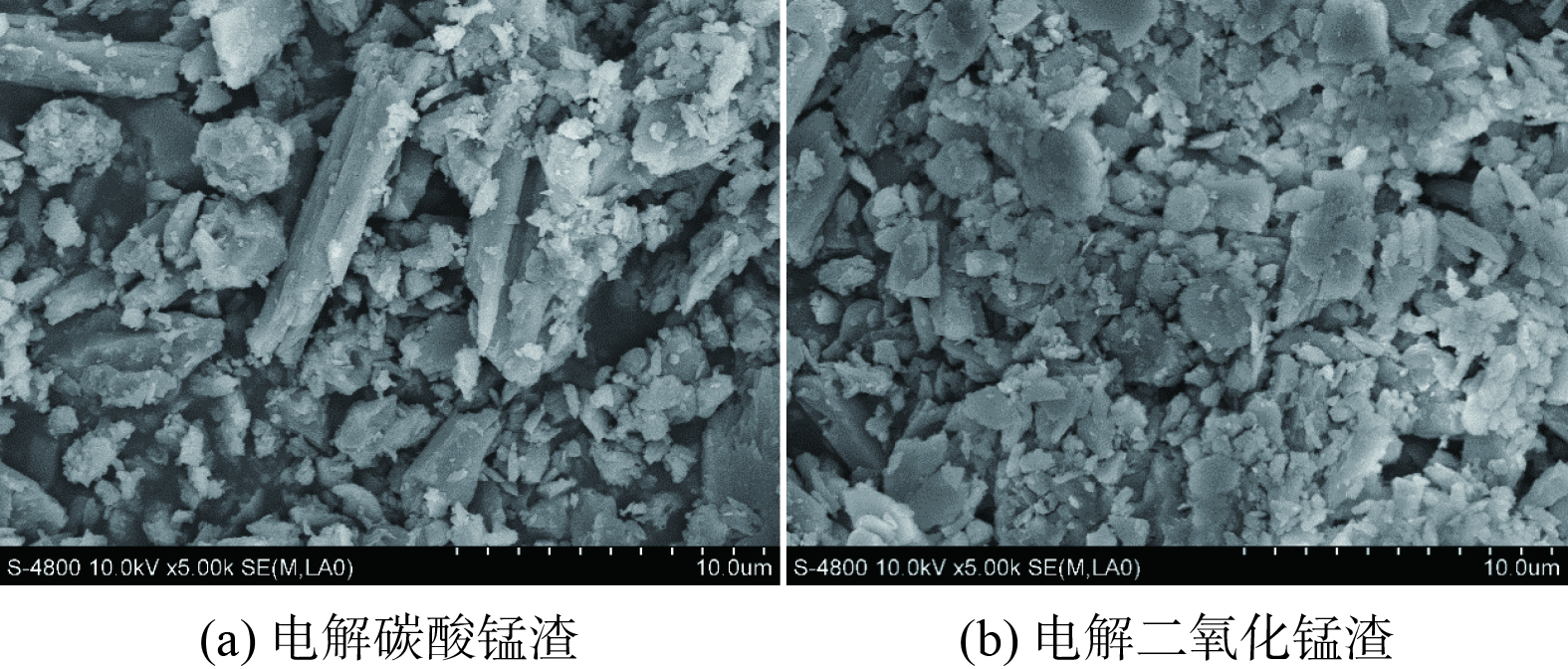
2) 微观结构分析。图2显示了EMCR与EMDR的扫描电子显微镜(SEM)结果。从图2(a)和图2(b)中可以看出, EMCR与EMDR的微观结构较为相似,均呈现出块状、 短柱状(CaSO4·2H2O)晶体颗粒在空间中杂乱无章的分布,整体结构较为疏松,其间填充了一些不规则绒球状(SiO2)颗粒,这些小颗粒间无明显粘结现象[24],这表明2种锰渣样品不具有活性。另外可以发现,图2(a)的柱状晶体颗粒较多,呈现更加疏松多孔的排布,其周围遍布的细小颗粒比图2(b)更稀少,孔隙度也更强,而图2(b)主要以块状颗粒为主,柱状颗粒较图2(a)相比明显较少。
3) 热重分析。图3是EMCR与EMDR在0~1 300 ℃的热重-热差分析图,图中的TG曲线代表热重,DTA曲线代表热差。在0 ℃~1 300 ℃温度范围内,EMCR的质量损失率为29.53%,EMDR的质量损失率为32.93%。
图3(a)显示EMCR在145、396、529、1 154 ℃附近存在4个明显的吸热峰;DTA曲线整体呈现出下凹形状,为锰渣矿物相变重结晶的结果。145 ℃的吸热峰主要由吸附水的解吸与CaSO4·2H2O脱水生成β-CaSO4·0.67H2O引起的[25],也就是CaSO4·2H2O为了给β-CaSO4·0.67H2O转换热,而吸收了很多热量,质量损失峰的产生也从图形上直观地反映了出来;EMCR中β-CaSO4·0.67H2O转换为CaSO4·0.5H2O,在晶体中逐步生成硫酸钙是396 ℃的吸热峰产生的主要原因,而曲线上吸热峰不特别显著主要是因为硫酸钙晶体转化是个渐进过程;温度为529 ℃的吸热峰是由CaSO4·0.5H2O全部转化为可溶解的CaSO4所引起;随着温度的不断上升,可溶解的CaSO4逐步转化为不可溶解的CaSO4,在温度达1 154 ℃时,无水型硫酸钙脱硫分解生成氧化钙并产生SO2气体[14],出现了晶型转变。
图3(b)显示EMDR在143、333、543、716、1 158 ℃附近存在5个明显的吸热峰。CaSO4·2H2O脱除结晶水是温度为143 ℃时出现较明显吸热峰的原因;当温度为333 ℃时,水基性矾[Al4SO4(OH)10·7H2O]脱除结晶水和羟基水,生成无定形的铝硫氧化物[26],黄钾铁矾[KFe3(SO4)2(OH)6]脱除羟基水,K2SO4和Fe2(SO4)3析出;当温度为543 ℃时,硫酸铁分解生成赤铁矿[15];当温度为716 ℃时,铝硫氧化物分解;当温度为1 158 ℃时,硫酸锰分解。
-
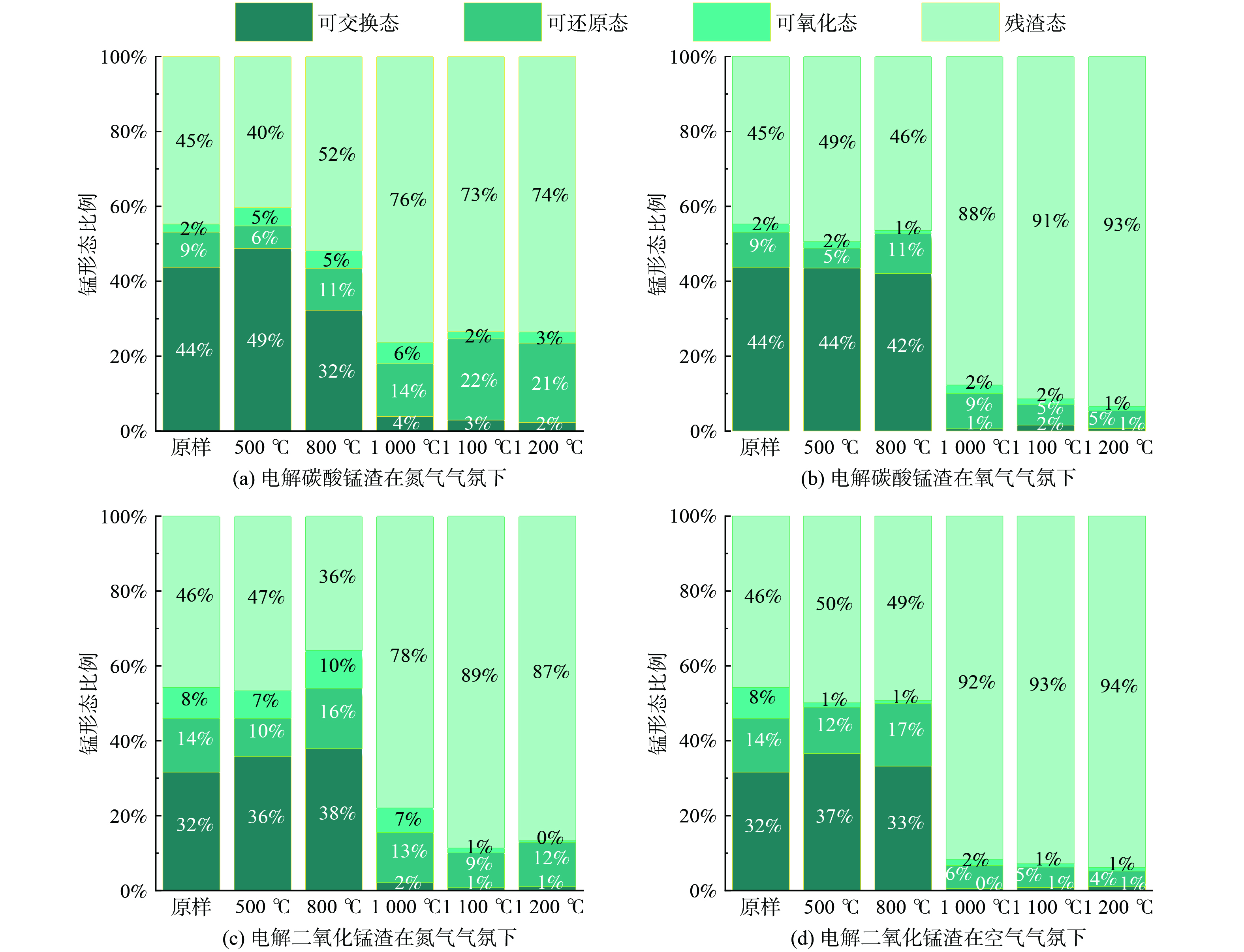
图4是EMCR与EMDR在氮气及空气气氛下经不同温度煅烧后样品中Mn元素的不同形态分布图。由图可知EMCR与EMDR原样中Mn元素主要以可交换态与残渣态的形态存在,EMDR原样中Mn元素的可氧化态与可还原态分布均大于EMCR,其可能的原因是用于生产的锰矿种类及工艺流程的不同。
EMCR在氮气气氛下随着煅烧温度的升高Mn元素的可交换态占比变化规律与在空气气氛下大致相同,表现为当温度在500 ℃至800 °C时可交换态占比在32%~49%之间,升温到1 000 ℃至1 200 ℃时可交换态占比降低至3%附近;可氧化态随温度变化不显著,但整体占比略高于空气气氛;残渣态随温度的升高到 1 000 ℃时增加幅度最大,之后变化不明显;氮气气氛下Mn元素的可还原态在整个煅烧过程中占比逐渐增加,这是因为在氮气气氛下促进了Mn元素还原态的生成,说明在氮气气氛下煅烧对Mn的包裹固封效果不佳,但Mn元素的可还原态占比要明显高于空气气氛,这有利于Mn的回收利用。在空气气氛下随着温度的升高Mn元素的可氧化态占比均较小,可还原态占比变化也没有显著差异;温度在500 ℃至800 °C时Mn元素的各形态与原样差别不大,总体表现为残渣态>可交换态>可氧化态>可还原态,升温到1 000 ℃至1 200 ℃时,Mn元素的形态基本以残渣态存在,占比达到了88%及以上,这表明煅烧温度在1 000 ℃以上时,EMCR的聚合度升高,生成了大量更复杂多样的硅铝酸盐矿物,加强了对金属Mn的包裹固封,重金属在高温下烧结时会固化[27]。
EMDR随着煅烧温度的升高在氮气与空气气氛下Mn元素的可交换态占比变化规律整体表现与EMCR大致相同;温度在500 ℃至1 000 ℃时可氧化态占比与原样相差不大,升温至1 100 ℃及1 200 ℃时降至1%附近;可还原态占比随温度的升高与原样相比变化不大,当温度为800 ℃时达到最大值16%,这说明在氮气气氛下EMDR经800 ℃煅烧处理有利于锰的回收,但效果没有EMCR好;残渣态占比整体上随温度的升高而增加,但略低于在空气气氛下煅烧,表明在氮气气氛下煅烧对Mn的包裹固封效果远没有在空气气氛下好,这与EMCR的变化规律相似。在空气气氛下随着煅烧温度的升高, Mn元素的可氧化态占比相较于原样降低效果明显;Mn元素的可还原态占比在温度升高到1 000 ℃至1 200 ℃时比低温段降低很多;在温度为1 000 ℃至1 200 ℃时Mn元素基本上以残渣态的形式存在,达到了92%以上,仅有少量以可还原态的形式存在。若将EMDR进行填埋处理,综合考虑经济效益,应将前期预处理煅烧温度控制在1 000 ℃。
对Mn进行的风险评估代码评估结果如图5所示,可以看出在2种气氛条件下2种锰渣原样与经500 ℃和800 ℃煅烧处理后Mn的RAC值处于高风险,经1 000、1 100、1 200 ℃煅烧处理的锰渣处于低风险,其中空气气氛条件下EMDR经1 000 ℃煅烧处理后属于无风险。结果显示,锰渣的煅烧处理温度应达到1 000 ℃以上时方可进行填埋等处理处置,此时电解锰渣中Mn无污染环境风险。
-
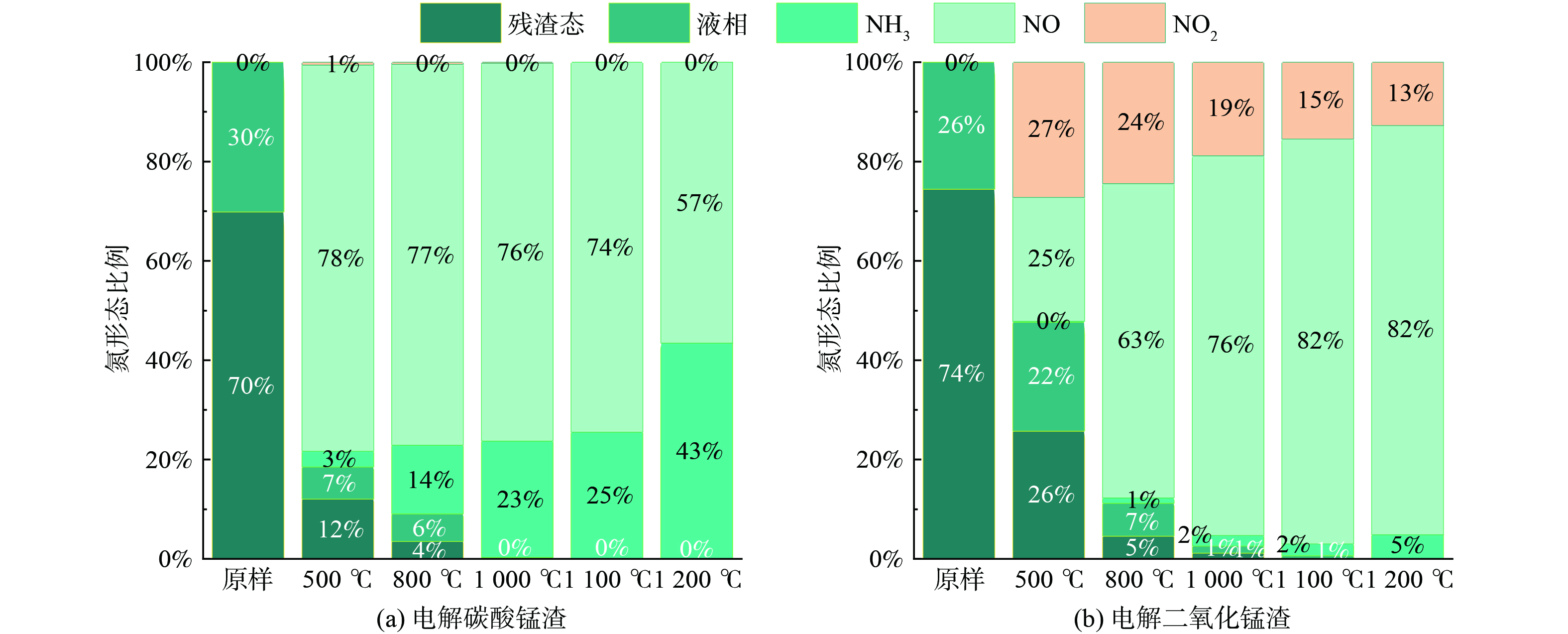
根据煅烧样品中氮元素含量、所测量液相中的总氮含量及通过高温烟气分析仪测定的氮元素含量,可以得到EMCR与EMDR在煅烧前后残渣、液相及气相中氮的分布情况,如图6所示。2种电解锰渣原样中的N元素形态大部分存在于残渣态中,少量存在于液相中。当煅烧温度为500 ℃时,EMCR中的N元素由残渣及液相通过挥发分逐步转化为NO、NO2及NH3,而EMDR中N元素则通过挥发分逐步转化为NO与NO2,挥发分通过热解产生,热解过程中析出的含氮气体浓度很大程度上受到原料中含氮化合物的特性影响。随着煅烧温度的升高,NH3中N元素的形态分布主要以NO及NH3的形式存在,其中,NO2转化为NO,这是由于NO和O2反应会生成NO2,此过程为放热反应,而升高温度有利于平衡向逆反应方向进行,同时NO的占比随着煅烧温度的升高而逐渐降低,NH3逐渐升高。随着温度升高至800 ℃~1 200 ℃时,EMCR中N元素形态分布整体以NO为主,存在少量NH3形式,这区别于EMDR,同时NO2存在逐步减少转化为NO,1 200 ℃时,NO2占比仍有13%,这说明通入空气气氛的高温反应下,EMDR中的NO2相比于EMCR不易于向NO转化。
-
1) 锰渣煅烧残渣组织结构的物相分析。在500、800、1 000、1 100、1 200 ℃温度下通入氮气气氛煅烧20 min的过程中EMCR内部成分发生了改变,物相分析结果如图7所示。与图1所示原渣的XRD图谱相比,经一定温度煅烧20 min后,2种锰渣均出现了赤铁矿(Fe2O3)、磁铁矿(Fe3O4)、辉石[Ca0.991(Mg0.641Fe0.342) (Si1.6Fe0.417)O6]等物质的特征峰。当温度为500 ℃时,EMCR中的铁相主要以赤铁矿的形式存在,温度为800 ℃时出现了磁铁矿的衍射峰,随着温度的升高至1 200 ℃时,赤铁矿的衍射峰逐渐减弱,磁铁矿的衍射峰逐渐增强,这表明随着温度的升高EMCR中的赤铁矿逐渐转化为磁铁矿。EMCR中石英相(SiO2)的衍射峰强度在温度500~800 ℃向800~1 200 ℃升温时逐渐减弱,并与其他重金属熔融向玻璃状硅酸盐转变,由此可知EMCR的活性最佳煅烧温度为800 ℃,这与WANG等[15]研究结果一致。EMCR中的硫酸盐在500 ℃时主要以硬石膏相(CaSO4)存在,当温度逐渐升高时,硫酸钙相衍射峰逐渐增强,为硬石膏析晶,当温度升高至1 000 ℃时,硬石膏衍射峰逐渐减弱,同时石英相的特征峰也逐渐减弱,出现了辉石(Ca0.991(Mg0.641Fe0.342)(Si1.6,Fe0.417)O6)的特征峰,这表明煅烧EMCR当温度达1 000 ℃时,硫酸钙分解,同时产生新晶体。而EMCR中锰相结晶度极低,随温度升高逐渐转化为残渣态[28]。
EMDR在500、800、1 000、1 100、1 200 ℃温度下通入氮气气氛煅烧20 min的物相分析结果如图8所示。EMDR中石英相(SiO2)的衍射峰强度在温度500~1 000 ℃向1 000~1 200 ℃升温时逐渐减弱,这表明EMDR的最佳煅烧处理温度为1 000 ℃,温度继续升高会降低其活性。当温度达1 200 ℃时石英相的衍射峰基本消失,说明随着煅烧温度升高EMDR中石英相逐步无定形化,并与其他重金属熔融向玻璃态硅酸盐转变。EMDR中硫酸钙相、赤铁矿、磁铁矿等随温度升高的变化状态与EMCR一致。当温度为500 ℃时EMDR中的铝相主要以高岭石(Al2(Si2O5)OH4)的形式存在,随着温度升高高岭石逐渐重结晶为微斜长石(K(AlSi3)O8),当温度达1 100~1 200 ℃时逐渐转化为辉石,且辉石衍射峰随着温度升高逐渐增强。
2) 锰渣煅烧残渣的微观组织形貌分析。EMCR在不同温度煅烧时的SEM-EDS图谱如图9所示。由图可知,不同煅烧温度下的EMCR主要由柱状、条状以及少量小颗粒组成。由于EMCR在煅烧后会与分解出的MgO及CaO等碱性氧化物反应生成难溶的Mn(OH)2 和MnO2[29-30],且经煅烧后会发生裂解产生大量疏松的孔隙,为Mn2+的附着提供了更多的着力点,使Mn2+吸附在多空介质中从而达到稳定化。对煅烧时的EMCR进行能谱分析,随着煅烧温度的升高EMCR中Mg、Mn和Fe元素占比在1 000 ℃达到峰值。800 ℃煅烧时Fe元素占比达到了52.40%,此时有利于Fe的回收利用,同时与前文对EMCR中铁相随温度升高过程中的变化分析基本吻合。
EMDR在不同温度煅烧时的SEM-EDS图谱如图10所示。EMDR主要由柱状、条状颗粒以及少量小颗粒组成。对煅烧后的EMCR进行能谱分析,结果显示Fe元素占比在1 100 ℃时达到了56.50%,同时Mn元素占比也达到最大,结合XRD图谱可知,此时生成了大量的锰铁矿,此时煅烧EMDR有利于Mn、Fe元素的回收利用,已有研究表明在合理条件下制备Fe-Mn双金属氧化物和工业废水处理剂,使废渣得到充分利用[31]。随着煅烧温度的升高Mg、Mn和Fe元素重量占比均比低温段占比多,且在1 200 ℃达到峰值,但Ca的元素重量占比在高温段要低于低温段。
-
1) 原始EMCR与EMDR的矿物成分主要包括石膏(CaSO4·2H2O)、石英(SiO2)、微斜长石(K(AlSi3)O8)、云母(KAl2(AlSi3O10) (OH)2)、烧石膏(CaSO4·0.5H2O)等。微观结构整体较为疏松,均呈现出块状、短柱状(CaSO4·2H2O)晶体颗粒在空间中杂乱无章的分布,其间填充了一些无明显粘结的不规则绒球状(SiO2)颗粒。在0 ℃~1 300 ℃温度范围内,EMCR的质量损失率为29.53%,EMDR的质量损失率为32.93%。
2) EMCR与EMDR中Mn元素在氮气气氛下煅烧时呈现出残渣态>可交换态>可还原态>可氧化态。两种锰渣经过高温煅烧后整体以残渣态的形式存在,经1 000、1 100 、1 200 ℃煅烧处理的锰渣处于低风险甚至无风险状态。
3) 原样EMCR与EMDR中的N元素形态大部分存在于残渣态中,少量存在于液相中。在空气气氛下进行煅烧处理EMCR中的N元素由残渣及液相逐步转化为NO、NO2及NH3,而EMDR中N元素则通过挥发分逐步转化为NO与NO2,随着温度升高EMCR中N元素形态分布整体以NO为主,存在少量NH3形式,而1 200 ℃时EMDR中NO2占比仍有13%。
4) 在氮气煅烧气氛下经一定温度煅烧20 min后,2种锰渣均出现了赤铁矿(Fe2O3)、磁铁矿(Fe3O4)、辉石[Ca0.991(Mg0.641Fe0.342) (Si1.6Fe0.417)O6]等物质的特征峰。在800 ℃时EMCR中石英相(SiO2)的衍射峰强度最佳,有利于活性发展。EMDR的最佳煅烧处理温度为1 000 ℃,铝相主要以高岭石(Al2(Si2O5)OH4)的形式存在,随着温度升高高岭石逐渐重结晶为微斜长石(K(AlSi3)O8),继而逐渐转化为辉石。由SEM-EDS图谱可知不同煅烧温度下的EMCR与EMDR主要由柱状、条状以及少量小颗粒组成。
电解锰渣煅烧时Mn、N元素形态分布与微观结构变化
Morphological distribution of Mn and N elements and changes in microstructure in electrolytic manganese residue during the calcination
-
摘要: 电解锰行业的发展会产生大量的电解锰渣,易导致严重的环境污染,高温煅烧已成为当前无害化处理电解锰渣的高效方法之一。为探究高温煅烧时电解锰渣中特征污染物的化学形态及物相组成,选取广西某电解锰企业经不同工艺产生的电解碳酸锰渣(EMCR)与电解二氧化锰渣(EMDR),通过其理化特性及Mn、N元素形态分布与微观结构变化分析,研究了不同温度煅烧处理对电解锰渣污染物的影响。结果表明,电解二氧化锰渣中Mn元素的可氧化态与可还原态分布均大于电解碳酸锰渣。当煅烧温度在1 000 ℃以上时,2种电解锰渣中Mn元素基本上以残渣态的形式存在,通过风险评估代码(RAC)结果可知样品处于低风险甚至无风险状态,可进行后续的处理处置。2种电解锰渣中N元素的形态随着温度的升高主要以NO形式为主。通过对煅烧过程中微观结构的变化分析可知,在800 ℃时电解碳酸锰渣中石英相(SiO2)的衍射峰强度最佳,有利于活性发展,此时的能谱分析结果显示Fe元素占比达到了52.40%,有利于Fe的回收利用,而电解二氧化锰渣的最佳煅烧处理温度显示为1 000 ℃。该研究结果可为2种电解锰渣经煅烧处置后的资源化再利用工作提供有效的数据支撑。Abstract: The development of the electrolytic manganese industry will generate a large amount of electrolytic manganese residue, which is prone to serious environmental pollution. High temperature calcination has become one of the efficient methods for harmless treatment of electrolytic manganese residue. To explore the chemical forms and phase composition of characteristic pollutants in electrolytic manganese residue during high-temperature calcination, electrolytic manganese carbonate residue(EMCR) and electrolytic manganese dioxide residue(EMDR) produced by a household appliance manganese removal enterprise in Guangxi through different processes were selected. The influence of calcination at different temperatures on the pollutants in electrolytic manganese residue was studied by analyzing its physicochemical propertie, including the morphological distribution of Mn and N elements, and the changes in microstructure. The results showed that the distribution of oxidizable and reducible states of Mn element in electrolytic manganese dioxide residue was greater than that in electrolytic manganese carbonate residue. When the calcination temperature was above 1 000 ℃, the Mn element in the two types of electrolytic manganese slag basically existed in the form of residue. The risk assessment code (RAC) results indicated that the sample was in a low-risk or even risk-free state, and can be subjected to subsequent treatment and disposal. The form of N element in the two types of electrolytic manganese residue was mainly in the form of NO with the increase of temperature. Through the analysis of the microstructure changes during the calcination process, it can be concluded that the diffraction peak intensity of quartz phase (SiO2) in electrolytic manganese carbonate residue was the best at 800 ℃, which was conducive to the development of activity. At this time, the energy spectrum analysis results showed that the proportion of Fe element reaches 52.40%, which was conducive to the recovery and utilization of Fe. The optimal calcination temperature for electrolytic manganese dioxide residue was shown to be 1 000 ℃. The research results will provide effective data support for the resource reuse of two types of electrolytic manganese slag after calcination treatment.
-
中国已成为最大的电解锰生产国,占世界电解锰生产能力和产量的98%以上[1]。目前,几乎大部分的电解锰渣都未经任何处理就被倾倒在填埋场,其主要污染物氨氮(NH4+-N)、可溶性锰(Mn2+)和重金属等会随着雨水的浸出、淋溶渗漏到周围的环境中,造成严重的土壤和水体污染[2-3],由电解锰渣引起的环境问题已引起社会的广泛关注。电解碳酸锰渣(electrolytic manganese carbonate residue,EMCR)是将菱锰矿中的固体碳酸锰转化为可溶性锰时排放的一种固体废弃物[4]。电解二氧化锰(electrolytic manganese dioxide,EMD)是现代碱性、锂和钠电池(包括电化学电容器和制氢)中阴极材料的关键成分,随着电池行业的快速发展,EMD的产能每年已达465 900 t,我国的产量约占64.02% [5]。而电解二氧化锰渣(electrolytic manganese dioxide residue,EMDR)是电解二氧化锰工业生产过程中产生的固体废物[6]。
目前,电解锰渣的综合利用主要集中于锰的回收、制备铺路砖体等建筑材料、用作土壤肥料等。如CHANG等[7]通过优化锰的微波浸出工艺,在温度76 ℃、时间55 min、H2SO4浓度为0.76 mol·L−1、柠檬酸的用量为3.51 mg·g−1的条件下微波辅助浸出锰。SHU等[8]开发了一种通过电还原从电解金属锰渣中浸出锰的改进工艺,电场可以改变电解金属锰渣粒子的表面电荷分布,还原高价锰。ZHANG等[9]将电解锰渣与赤泥(RM)和其他固体废物协同用作道路基层材料(RBM)。WANG等[10]根据电解锰渣的特点(即高石膏含量),开发了一种新型的电解金属锰渣活化胶凝材料(EGCH)。以上在工程实践中应用时易受到不可控的利用效率和潜在的再浸出环境风险的限制。而煅烧作为环保的无害化处理方式之一已广泛应用于固体废弃物的处理处置中[11–13]。电解锰渣的煅烧处理与其他方式相比有很多优点,在煅烧过程中电解锰渣中的有害物质会被去除,产生的气体可被回收制备硫酸,且电解锰渣含有大量的SiO2、AI2O3、Fe2O3等物质,经一定温度煅烧处理后可激发它们的潜在活性[14–16],后续可进行高效资源化利用。
但目前对于电解锰渣高温煅烧利用处置过程中污染物的形态分析研究较少。本研究对2种不同工艺产生的EMCR和EMDR在氮气气氛下经不同温度煅烧后残渣的矿物组成及微观组织形貌进行了分析,对排放烟气中N元素各化合态形式进行研究,并通过改进BCR连续提取法探究煅烧过程中两种电解锰渣残渣中Mn元素的形态变化特征,并通过风险评估代码(RAC)对样品中Mn元素进行环境风险评估。对电解锰渣煅烧处置过程开展性质分析,可清晰直观地掌握各温度段电解锰渣中污染物的存在形态,同时还将为2种电解锰渣经煅烧处置后的资源化再利用工作提供有效的数据支撑。
1. 材料与方法
1.1 实验原料
2种锰渣样品来自中国广西某企业采用四分法取样的新鲜锰渣,将样品经烘箱105 ℃下烘干至恒重,机械破碎后过100目筛网,装入封口袋保存备用。EMCR与EMDR的化学成分如表1所示。2种锰渣样品的主要成分为 SO3、SiO2、CaO、Fe2O3、Al2O3、MnO等,约占样品全部含量的90%。
表 1 电解碳酸锰渣与电解二氧化锰渣的化学成分组成Table 1. The chemical compositions of Electrolytic manganese carbonate residue and Electrolytic manganese dioxide residue% 样品种类 SO3 SiO2 CaO Fe2O3 Al2O3 MnO 电解碳酸锰渣 22.99 22.85 18.94 14.07 2.43 7.93 电解二氧化锰渣 34.58 18.96 14.96 7.80 9.46 4.66 1.2 实验方法
将锰渣样品在管式炉中进行煅烧处理,设置5个煅烧温度(即500、800、1 000、1 100、1 200 ℃)。根据管式炉的升温限值,设定10 ℃·min−1的升温速率,待管式炉的温度达到实验设定温度后,迅速推动装有20 g锰渣样品的瓷舟向管式炉的中间移动,快速密封通入气氛,样品在管式炉内煅烧时间为20 min。
1.3 分析方法
1) 表征分析。使用X射线荧光光谱仪(XRF,ARLADVANT XP+,瑞士arl公司)测定锰渣的化学成分组成。使用X射线衍射仪(XRD,Bruker-D8,德国BRUKER公司)测定锰渣的结晶相。使用扫描电镜能谱分析仪(SEM-EDX,HITACHI S-4800,日本日立公司)对锰渣微观结构和截面元素组成进行识别。采用热重分析仪(TG-DTG,SDT-Q600,美国TA仪器公司)对两种电解锰渣进行热重分析。
2) Mn元素形态分布的分析方法。重金属的总浓度测定在环境监测中具有重要价值,但在环境中的赋存形态与总量也有重要的研究意义,这将决定它们的毒性、流动性与利用度[17–19]。电解锰渣中主要重金属污染物是Mn元素,本研究采用改进BCR连续提取法[20],对锰渣样品中Mn元素的形态分布进行分析研究,BCR连续提取方案如表2所示。
表 2 BCR连续提取方案Table 2. BCR sequential extraction scheme提取步骤 (金属形态) 试剂 目标阶段 F1:可交换态 醋酸(0.11 mol·L−1) 酸溶性 F2:可还原态 盐酸羟胺(0.50 mol·L−1) 铁、锰氧化物 F3:可氧化态 过氧化氢→醋酸铵(1.00 mol·L−1) 有机物、化合物 F4:残渣态 硝酸+氢氟酸+过氧化氢 残余物质 提取步骤如下。
①可交换态。称取1 g样品于50 mL聚丙烯离心管中,加入0.11 mol·L−1醋酸提取液40 mL,室温下振荡16 h,之后用4 000 r·min−1转速的离心机离心分离20 min,将上层清液转移至比色管中,保存于4 ℃冰箱中待测。加入20 mL高纯水清洗残余物,振荡20 min,离心,弃去清洗液。
②可还原态。向第一步提取后的残余物中加入0.50 mol·L−1盐酸羟胺提取液40 mL,室温下振荡16 h,用4 000 r·min−1转速的离心机离心分离20 min,将上层清液转移至比色管中,保存于4 ℃冰箱中待测。加入20 mL高纯水清洗残余物,振荡20 min,离心,弃去清洗液。
③可氧化态。向第二步提取后的残余物中缓慢加入10 mL过氧化氢,每隔15 min振荡1次,室温下消解1 h,之后水浴加热到85 ℃消解1 h,打开离心管盖子,95 ℃加热至溶液近干,再加入10 mL过氧化氢重复上述过程1次。冷却后,加入1 mol·L−1醋酸铵提取液50 mL,室温下振满16 h,之后用4 000 r·min−1转速的离心机离心分离20 min,将上层清液转移至比色管中,保存于4 ℃冰箱中待测。加入20 mL高纯水清洗残余物,振荡20 min,离心,弃去清洗液。
④残渣态。向第三步提取后的残渣中加入配比为3∶5∶1∶5的盐酸、硝酸、高氯酸、氢氟酸,转移至聚四氟乙烯管中,之后加入5 mL硝酸,2 mL氢氟酸和2 mL过氧化氢,微波消解至固体消解完全,赶酸仪150 ℃赶至剩最后1滴,用去离子水定容至50 mL容量瓶,待测。
根据BCR连续提取结果,采用风险评估代码(RAC)对样品中重金属的生态风险进行评估。RAC是广泛采用的定量评估方法,用于综合评估样品中重金属元素的生态风险[21-22]。本研究用该方法评估经不同温度煅烧处理后的电解锰渣样品中Mn元素的风险等级。RAC值如式(1)所示。
RAC=F1F1+F2+F3+F4×100% (1) 根据RAC结果,可以将风险等级划分为5个等级,如表3所示。
表 3 风险评估代码(RAC)等级分类Table 3. Risk Assessment Code (RAC) Level ClassificationRAC 等级 风险等级 <1 1 无风险 1~10 2 低风险 10~30 3 中风险 30~50 4 高风险 50~100 5 超高风险 3) N元素形态分布的分析方法。烟气中氨氮收集装置,由2个50 mL装有0.01 mol·L−1硫酸吸收液的洗气瓶串联组成,根据《水质 氨氮的测定 纳氏试剂分光光度法》(HJ 535-2009)[23]测定氨氮质量浓度;使用高温烟气分析仪直接测定烟气中除氨氮外N元素各化合态的质量浓度。
2. 结果与讨论
2.1 2种电解锰渣原料特性
1) 物相组成分析。EMCR与EMDR的XRD图谱如图1所示。EMCR与EMDR的主要矿物成分包括石膏(CaSO4·2H2O)、石英(SiO2)、微斜长石(K(AlSi3)O8)、云母(KAl2(AlSi3O10) (OH)2)、烧石膏(CaSO4·0.5H2O)。其中,石英(SiO2)与石膏(CaSO4·2H2O)的特征谱线峰型尖锐,结晶形态较好,是EMCR与EMDR中的主要物相;其他矿物相的衍射峰强度较低,结晶度较低。
2) 微观结构分析。图2显示了EMCR与EMDR的扫描电子显微镜(SEM)结果。从图2(a)和图2(b)中可以看出, EMCR与EMDR的微观结构较为相似,均呈现出块状、 短柱状(CaSO4·2H2O)晶体颗粒在空间中杂乱无章的分布,整体结构较为疏松,其间填充了一些不规则绒球状(SiO2)颗粒,这些小颗粒间无明显粘结现象[24],这表明2种锰渣样品不具有活性。另外可以发现,图2(a)的柱状晶体颗粒较多,呈现更加疏松多孔的排布,其周围遍布的细小颗粒比图2(b)更稀少,孔隙度也更强,而图2(b)主要以块状颗粒为主,柱状颗粒较图2(a)相比明显较少。
3) 热重分析。图3是EMCR与EMDR在0~1 300 ℃的热重-热差分析图,图中的TG曲线代表热重,DTA曲线代表热差。在0 ℃~1 300 ℃温度范围内,EMCR的质量损失率为29.53%,EMDR的质量损失率为32.93%。
图3(a)显示EMCR在145、396、529、1 154 ℃附近存在4个明显的吸热峰;DTA曲线整体呈现出下凹形状,为锰渣矿物相变重结晶的结果。145 ℃的吸热峰主要由吸附水的解吸与CaSO4·2H2O脱水生成β-CaSO4·0.67H2O引起的[25],也就是CaSO4·2H2O为了给β-CaSO4·0.67H2O转换热,而吸收了很多热量,质量损失峰的产生也从图形上直观地反映了出来;EMCR中β-CaSO4·0.67H2O转换为CaSO4·0.5H2O,在晶体中逐步生成硫酸钙是396 ℃的吸热峰产生的主要原因,而曲线上吸热峰不特别显著主要是因为硫酸钙晶体转化是个渐进过程;温度为529 ℃的吸热峰是由CaSO4·0.5H2O全部转化为可溶解的CaSO4所引起;随着温度的不断上升,可溶解的CaSO4逐步转化为不可溶解的CaSO4,在温度达1 154 ℃时,无水型硫酸钙脱硫分解生成氧化钙并产生SO2气体[14],出现了晶型转变。
图3(b)显示EMDR在143、333、543、716、1 158 ℃附近存在5个明显的吸热峰。CaSO4·2H2O脱除结晶水是温度为143 ℃时出现较明显吸热峰的原因;当温度为333 ℃时,水基性矾[Al4SO4(OH)10·7H2O]脱除结晶水和羟基水,生成无定形的铝硫氧化物[26],黄钾铁矾[KFe3(SO4)2(OH)6]脱除羟基水,K2SO4和Fe2(SO4)3析出;当温度为543 ℃时,硫酸铁分解生成赤铁矿[15];当温度为716 ℃时,铝硫氧化物分解;当温度为1 158 ℃时,硫酸锰分解。
2.2 Mn元素形态分布分析
图4是EMCR与EMDR在氮气及空气气氛下经不同温度煅烧后样品中Mn元素的不同形态分布图。由图可知EMCR与EMDR原样中Mn元素主要以可交换态与残渣态的形态存在,EMDR原样中Mn元素的可氧化态与可还原态分布均大于EMCR,其可能的原因是用于生产的锰矿种类及工艺流程的不同。
EMCR在氮气气氛下随着煅烧温度的升高Mn元素的可交换态占比变化规律与在空气气氛下大致相同,表现为当温度在500 ℃至800 °C时可交换态占比在32%~49%之间,升温到1 000 ℃至1 200 ℃时可交换态占比降低至3%附近;可氧化态随温度变化不显著,但整体占比略高于空气气氛;残渣态随温度的升高到 1 000 ℃时增加幅度最大,之后变化不明显;氮气气氛下Mn元素的可还原态在整个煅烧过程中占比逐渐增加,这是因为在氮气气氛下促进了Mn元素还原态的生成,说明在氮气气氛下煅烧对Mn的包裹固封效果不佳,但Mn元素的可还原态占比要明显高于空气气氛,这有利于Mn的回收利用。在空气气氛下随着温度的升高Mn元素的可氧化态占比均较小,可还原态占比变化也没有显著差异;温度在500 ℃至800 °C时Mn元素的各形态与原样差别不大,总体表现为残渣态>可交换态>可氧化态>可还原态,升温到1 000 ℃至1 200 ℃时,Mn元素的形态基本以残渣态存在,占比达到了88%及以上,这表明煅烧温度在1 000 ℃以上时,EMCR的聚合度升高,生成了大量更复杂多样的硅铝酸盐矿物,加强了对金属Mn的包裹固封,重金属在高温下烧结时会固化[27]。
EMDR随着煅烧温度的升高在氮气与空气气氛下Mn元素的可交换态占比变化规律整体表现与EMCR大致相同;温度在500 ℃至1 000 ℃时可氧化态占比与原样相差不大,升温至1 100 ℃及1 200 ℃时降至1%附近;可还原态占比随温度的升高与原样相比变化不大,当温度为800 ℃时达到最大值16%,这说明在氮气气氛下EMDR经800 ℃煅烧处理有利于锰的回收,但效果没有EMCR好;残渣态占比整体上随温度的升高而增加,但略低于在空气气氛下煅烧,表明在氮气气氛下煅烧对Mn的包裹固封效果远没有在空气气氛下好,这与EMCR的变化规律相似。在空气气氛下随着煅烧温度的升高, Mn元素的可氧化态占比相较于原样降低效果明显;Mn元素的可还原态占比在温度升高到1 000 ℃至1 200 ℃时比低温段降低很多;在温度为1 000 ℃至1 200 ℃时Mn元素基本上以残渣态的形式存在,达到了92%以上,仅有少量以可还原态的形式存在。若将EMDR进行填埋处理,综合考虑经济效益,应将前期预处理煅烧温度控制在1 000 ℃。
对Mn进行的风险评估代码评估结果如图5所示,可以看出在2种气氛条件下2种锰渣原样与经500 ℃和800 ℃煅烧处理后Mn的RAC值处于高风险,经1 000、1 100、1 200 ℃煅烧处理的锰渣处于低风险,其中空气气氛条件下EMDR经1 000 ℃煅烧处理后属于无风险。结果显示,锰渣的煅烧处理温度应达到1 000 ℃以上时方可进行填埋等处理处置,此时电解锰渣中Mn无污染环境风险。
2.3 N元素形态分布分析
根据煅烧样品中氮元素含量、所测量液相中的总氮含量及通过高温烟气分析仪测定的氮元素含量,可以得到EMCR与EMDR在煅烧前后残渣、液相及气相中氮的分布情况,如图6所示。2种电解锰渣原样中的N元素形态大部分存在于残渣态中,少量存在于液相中。当煅烧温度为500 ℃时,EMCR中的N元素由残渣及液相通过挥发分逐步转化为NO、NO2及NH3,而EMDR中N元素则通过挥发分逐步转化为NO与NO2,挥发分通过热解产生,热解过程中析出的含氮气体浓度很大程度上受到原料中含氮化合物的特性影响。随着煅烧温度的升高,NH3中N元素的形态分布主要以NO及NH3的形式存在,其中,NO2转化为NO,这是由于NO和O2反应会生成NO2,此过程为放热反应,而升高温度有利于平衡向逆反应方向进行,同时NO的占比随着煅烧温度的升高而逐渐降低,NH3逐渐升高。随着温度升高至800 ℃~1 200 ℃时,EMCR中N元素形态分布整体以NO为主,存在少量NH3形式,这区别于EMDR,同时NO2存在逐步减少转化为NO,1 200 ℃时,NO2占比仍有13%,这说明通入空气气氛的高温反应下,EMDR中的NO2相比于EMCR不易于向NO转化。
2.4 煅烧过程中表征分析
1) 锰渣煅烧残渣组织结构的物相分析。在500、800、1 000、1 100、1 200 ℃温度下通入氮气气氛煅烧20 min的过程中EMCR内部成分发生了改变,物相分析结果如图7所示。与图1所示原渣的XRD图谱相比,经一定温度煅烧20 min后,2种锰渣均出现了赤铁矿(Fe2O3)、磁铁矿(Fe3O4)、辉石[Ca0.991(Mg0.641Fe0.342) (Si1.6Fe0.417)O6]等物质的特征峰。当温度为500 ℃时,EMCR中的铁相主要以赤铁矿的形式存在,温度为800 ℃时出现了磁铁矿的衍射峰,随着温度的升高至1 200 ℃时,赤铁矿的衍射峰逐渐减弱,磁铁矿的衍射峰逐渐增强,这表明随着温度的升高EMCR中的赤铁矿逐渐转化为磁铁矿。EMCR中石英相(SiO2)的衍射峰强度在温度500~800 ℃向800~1 200 ℃升温时逐渐减弱,并与其他重金属熔融向玻璃状硅酸盐转变,由此可知EMCR的活性最佳煅烧温度为800 ℃,这与WANG等[15]研究结果一致。EMCR中的硫酸盐在500 ℃时主要以硬石膏相(CaSO4)存在,当温度逐渐升高时,硫酸钙相衍射峰逐渐增强,为硬石膏析晶,当温度升高至1 000 ℃时,硬石膏衍射峰逐渐减弱,同时石英相的特征峰也逐渐减弱,出现了辉石(Ca0.991(Mg0.641Fe0.342)(Si1.6,Fe0.417)O6)的特征峰,这表明煅烧EMCR当温度达1 000 ℃时,硫酸钙分解,同时产生新晶体。而EMCR中锰相结晶度极低,随温度升高逐渐转化为残渣态[28]。
EMDR在500、800、1 000、1 100、1 200 ℃温度下通入氮气气氛煅烧20 min的物相分析结果如图8所示。EMDR中石英相(SiO2)的衍射峰强度在温度500~1 000 ℃向1 000~1 200 ℃升温时逐渐减弱,这表明EMDR的最佳煅烧处理温度为1 000 ℃,温度继续升高会降低其活性。当温度达1 200 ℃时石英相的衍射峰基本消失,说明随着煅烧温度升高EMDR中石英相逐步无定形化,并与其他重金属熔融向玻璃态硅酸盐转变。EMDR中硫酸钙相、赤铁矿、磁铁矿等随温度升高的变化状态与EMCR一致。当温度为500 ℃时EMDR中的铝相主要以高岭石(Al2(Si2O5)OH4)的形式存在,随着温度升高高岭石逐渐重结晶为微斜长石(K(AlSi3)O8),当温度达1 100~1 200 ℃时逐渐转化为辉石,且辉石衍射峰随着温度升高逐渐增强。
2) 锰渣煅烧残渣的微观组织形貌分析。EMCR在不同温度煅烧时的SEM-EDS图谱如图9所示。由图可知,不同煅烧温度下的EMCR主要由柱状、条状以及少量小颗粒组成。由于EMCR在煅烧后会与分解出的MgO及CaO等碱性氧化物反应生成难溶的Mn(OH)2 和MnO2[29-30],且经煅烧后会发生裂解产生大量疏松的孔隙,为Mn2+的附着提供了更多的着力点,使Mn2+吸附在多空介质中从而达到稳定化。对煅烧时的EMCR进行能谱分析,随着煅烧温度的升高EMCR中Mg、Mn和Fe元素占比在1 000 ℃达到峰值。800 ℃煅烧时Fe元素占比达到了52.40%,此时有利于Fe的回收利用,同时与前文对EMCR中铁相随温度升高过程中的变化分析基本吻合。
EMDR在不同温度煅烧时的SEM-EDS图谱如图10所示。EMDR主要由柱状、条状颗粒以及少量小颗粒组成。对煅烧后的EMCR进行能谱分析,结果显示Fe元素占比在1 100 ℃时达到了56.50%,同时Mn元素占比也达到最大,结合XRD图谱可知,此时生成了大量的锰铁矿,此时煅烧EMDR有利于Mn、Fe元素的回收利用,已有研究表明在合理条件下制备Fe-Mn双金属氧化物和工业废水处理剂,使废渣得到充分利用[31]。随着煅烧温度的升高Mg、Mn和Fe元素重量占比均比低温段占比多,且在1 200 ℃达到峰值,但Ca的元素重量占比在高温段要低于低温段。
3. 结论
1) 原始EMCR与EMDR的矿物成分主要包括石膏(CaSO4·2H2O)、石英(SiO2)、微斜长石(K(AlSi3)O8)、云母(KAl2(AlSi3O10) (OH)2)、烧石膏(CaSO4·0.5H2O)等。微观结构整体较为疏松,均呈现出块状、短柱状(CaSO4·2H2O)晶体颗粒在空间中杂乱无章的分布,其间填充了一些无明显粘结的不规则绒球状(SiO2)颗粒。在0 ℃~1 300 ℃温度范围内,EMCR的质量损失率为29.53%,EMDR的质量损失率为32.93%。
2) EMCR与EMDR中Mn元素在氮气气氛下煅烧时呈现出残渣态>可交换态>可还原态>可氧化态。两种锰渣经过高温煅烧后整体以残渣态的形式存在,经1 000、1 100 、1 200 ℃煅烧处理的锰渣处于低风险甚至无风险状态。
3) 原样EMCR与EMDR中的N元素形态大部分存在于残渣态中,少量存在于液相中。在空气气氛下进行煅烧处理EMCR中的N元素由残渣及液相逐步转化为NO、NO2及NH3,而EMDR中N元素则通过挥发分逐步转化为NO与NO2,随着温度升高EMCR中N元素形态分布整体以NO为主,存在少量NH3形式,而1 200 ℃时EMDR中NO2占比仍有13%。
4) 在氮气煅烧气氛下经一定温度煅烧20 min后,2种锰渣均出现了赤铁矿(Fe2O3)、磁铁矿(Fe3O4)、辉石[Ca0.991(Mg0.641Fe0.342) (Si1.6Fe0.417)O6]等物质的特征峰。在800 ℃时EMCR中石英相(SiO2)的衍射峰强度最佳,有利于活性发展。EMDR的最佳煅烧处理温度为1 000 ℃,铝相主要以高岭石(Al2(Si2O5)OH4)的形式存在,随着温度升高高岭石逐渐重结晶为微斜长石(K(AlSi3)O8),继而逐渐转化为辉石。由SEM-EDS图谱可知不同煅烧温度下的EMCR与EMDR主要由柱状、条状以及少量小颗粒组成。
-
表 1 电解碳酸锰渣与电解二氧化锰渣的化学成分组成
Table 1. The chemical compositions of Electrolytic manganese carbonate residue and Electrolytic manganese dioxide residue
% 样品种类 SO3 SiO2 CaO Fe2O3 Al2O3 MnO 电解碳酸锰渣 22.99 22.85 18.94 14.07 2.43 7.93 电解二氧化锰渣 34.58 18.96 14.96 7.80 9.46 4.66 表 2 BCR连续提取方案
Table 2. BCR sequential extraction scheme
提取步骤 (金属形态) 试剂 目标阶段 F1:可交换态 醋酸(0.11 mol·L−1) 酸溶性 F2:可还原态 盐酸羟胺(0.50 mol·L−1) 铁、锰氧化物 F3:可氧化态 过氧化氢→醋酸铵(1.00 mol·L−1) 有机物、化合物 F4:残渣态 硝酸+氢氟酸+过氧化氢 残余物质 表 3 风险评估代码(RAC)等级分类
Table 3. Risk Assessment Code (RAC) Level Classification
RAC 等级 风险等级 <1 1 无风险 1~10 2 低风险 10~30 3 中风险 30~50 4 高风险 50~100 5 超高风险 -
[1] XU F, JIANG L, DAN Z, et al. Water balance analysis and wastewater recycling investigation in electrolytic manganese industry of China — A case study[J]. Hydrometallurgy, 2014, 149: 12-22. doi: 10.1016/j.hydromet.2014.05.002 [2] YANG T, XUE Y, LIU X, et al. Solidification/stabilization and separation/extraction treatments of environmental hazardous components in electrolytic manganese residue: A review[J]. Process Safety and Environmental Protection, 2022, 157: 509-526. doi: 10.1016/j.psep.2021.10.031 [3] HE D, SHU J, WANG R, et al. A critical review on approaches for electrolytic manganese residue treatment and disposal technology: Reduction, pretreatment, and reuse[J]. Journal of Hazardous Materials, 2021, 418: 126235. doi: 10.1016/j.jhazmat.2021.126235 [4] DU B, ZHOU C, LI X, et al. A kinetic study of Mn(II) precipitation of leached aqueous solution from electrolytic manganese residues[J]. Toxicological & Environmental Chemistry, 2015, 97(3-4): 349-357. [5] BISWAL A, CHANDRA TRIPATHY B, SANJAY K, et al. Electrolytic manganese dioxide (EMD): a perspective on worldwide production, reserves and its role in electrochemistry[J]. RSC Advances, 2015, 5(72): 58255-58283. doi: 10.1039/C5RA05892A [6] ZHANG W, CHU Y C. Manganese metallurgy review. Part I: Leaching of ores/secondary materials and recovery of electrolytic/chemical manganese dioxide[J]. Hydrometallurgy, 2007, 89(3-4): 137-159. doi: 10.1016/j.hydromet.2007.08.010 [7] CHANG J, SRINIVASAKANNAN C, SUN X, et al. Optimization of microwave-assisted manganese leaching from electrolyte manganese residue[J]. Green Processing and Synthesis, 2019, 9(1): 2-12. doi: 10.1515/gps-2020-0001 [8] SHU J, LIU R, LIU Z, et al. Leaching of manganese from electrolytic manganese residue by electro-reduction[J]. Environmental Technology, 2017, 38(16): 2077-2084. doi: 10.1080/09593330.2016.1245789 [9] ZHANG Y, LIU X, XU Y, et al. Preparation and characterization of cement treated road base material utilizing electrolytic manganese residue[J]. Journal of Cleaner Production, 2019, 232: 980-992. doi: 10.1016/j.jclepro.2019.05.352 [10] WANG D, WANG Q, XUE J. Reuse of hazardous electrolytic manganese residue: Detailed leaching characterization and novel application as a cementitious material[J]. Resources, Conservation and Recycling, 2020, 154: 104645. doi: 10.1016/j.resconrec.2019.104645 [11] GUO Z, XU J, XU Z, et al. Performance of cement-based materials containing calcined coal gangue with different calcination regimes[J]. Journal of Building Engineering, 2022, 56: 104821. doi: 10.1016/j.jobe.2022.104821 [12] REN C, WANG W, HUA D, et al. Preparation and Properties of a Sulphoaluminate Magnesium-Potassium Phosphate Green Cementitious Composite Material from Industrial Solid Wastes[J]. Materials, 2021, 14(23): 7340. doi: 10.3390/ma14237340 [13] WU W, CHEN Z, HUANG Y, et al. Red mud for the efficient adsorption of U(VI) from aqueous solution: Influence of calcination on performance and mechanism[J]. Journal of Hazardous Materials, 2021, 409: 124925. doi: 10.1016/j.jhazmat.2020.124925 [14] LIU X ming, LI Y, ZHANG L ling, et al. Phase transitions relating to the pozzolanic activity of electrolytic manganese residue during calcination[J]. Journal of Shanghai Jiaotong University (Science), 2013, 18(1): 105-110. doi: 10.1007/s12204-013-1372-7 [15] WANG F, LONG G, BAI M, et al. Application of electrolytic manganese residues in cement products through pozzolanic activity motivation and calcination[J]. Journal of Cleaner Production, 2022, 338: 130629. doi: 10.1016/j.jclepro.2022.130629 [16] HOU P kun, QIAN J shi, WANG Z, et al. Production of quasi-sulfoaluminate cementitious materials with electrolytic manganese residue[J]. Cement and Concrete Composites, 2012, 34(2): 248-254. doi: 10.1016/j.cemconcomp.2011.10.003 [17] TOKALIOĞLU Ş, KARTAL Ş. Bioavailability of Soil‐Extractable Metals to Tea Plant by BCR Sequential Extraction Procedure[J]. Instrumentation Science & Technology, 2004, 32(4): 387-400. [18] NEMATI K, BAKAR N K A, ABAS Mhd R, et al. Speciation of heavy metals by modified BCR sequential extraction procedure in different depths of sediments from Sungai Buloh, Selangor, Malaysia[J]. Journal of Hazardous Materials, 2011: S0304389411006789. [19] JAMALI M K, KAZI T G, AFRIDI H I, et al. Speciation of heavy metals in untreated domestic wastewater sludge by time saving BCR sequential extraction method[J]. Environmental Letters, 2007, 42(5): 649-659. [20] TOKALIOLU E, KARTAL E, ELI L. Determination of heavy metals and their speciation in lake sediments by flame atomic absorption spectrometry after a four-stage sequential extraction procedure[J]. Analytica Chimica Acta, 2000, 413(1-2): 33-40. doi: 10.1016/S0003-2670(00)00726-1 [21] 聂霄悍, 雷学文, 刘磊, 等. 堆存陈化对电解锰渣重金属赋存形态及环境风险演化的影响[J/OL]. 中国环境科学.https://doi.org/10.19674/j.cnki.issn1000-6923.20230823.007. [22] 姜媛媛, 王彦, 段文焱, 等. 市政污泥热解过程中重金属迁移特性及环境效应评估[J]. 环境科学, 2021, 42(6): 2966-2974. doi: 10.13227/j.hjkx.202009078 [23] 中华人民共和国环境保护部. 水质 氨氮的测定 纳氏试剂分光光度法: HJ 535-2009[S]. 北京: 中国环境科学出版社, 2010. [24] CHEN M, WEI J, ZHANG R, et al. Analysis of basic physical and chemical characteristics of manganese slag before and after solidification and its feasibility as highway slope[J]. Materials, 2021, 14(19): 5530. doi: 10.3390/ma14195530 [25] KOLAITIS D I, FOUNTI M A. Development of a solid reaction kinetics gypsum dehydration model appropriate for CFD simulation of gypsum plasterboard wall assemblies exposed to fire[J]. Fire Safety Journal, 2013, 58: 151-159. doi: 10.1016/j.firesaf.2013.01.029 [26] HAN Y, CUI X, LV X, et al. Preparation and characterization of geopolymers based on a phosphoric-acid-activated electrolytic manganese dioxide residue[J]. Journal of Cleaner Production, 2018, 205: 488-498. doi: 10.1016/j.jclepro.2018.09.141 [27] ZHAN X, WANG L, WANG L, et al. Enhanced geopolymeric co-disposal efficiency of heavy metals from MSWI fly ash and electrolytic manganese residue using complex alkaline and calcining pre-treatment[J]. Waste Management, 2019, 98: 135-143. doi: 10.1016/j.wasman.2019.08.024 [28] DUAN N, CUI K, ZHU C, et al. Study on phase evolution and promoting the pozzolanic activity of electrolytic manganese residue during calcination[J]. Environmental Research, 2023, 227: 115774. doi: 10.1016/j.envres.2023.115774 [29] SHU J, LIU R, LIU Z, et al. Solidification/stabilization of electrolytic manganese residue using phosphate resource and low-grade MgO/CaO[J]. Journal of Hazardous Materials, 2016, 317: 267-274. doi: 10.1016/j.jhazmat.2016.05.076 [30] SHU J, LI B, CHEN M, et al. An innovative method for manganese (Mn2+) and ammonia nitrogen (NH4+-N) stabilization/solidification in electrolytic manganese residue by basic burning raw material[J]. Chemosphere, 2020, 253: 126896. doi: 10.1016/j.chemosphere.2020.126896 [31] XU L J, WANG X M, CHEN H C, et al. Mn forms and environmental impact of electrolytic manganese residue[J]. Advanced Materials Research, 2011, 183-185: 570-574. doi: 10.4028/www.scientific.net/AMR.183-185.570 期刊类型引用(2)
1. 吴再成,张国平,柳凤娟,陈京晶,毛宽. 电解锰渣对水中Sb(V)、As(V)的吸附特征研究. 地球与环境. 2025(01): 133-142 .  百度学术
百度学术
2. 赵博超,聂一凡,王雪婷,田向勤,田祎,潘涔轩. 不同制液工艺对锰矿锰浸出回收及钙镁铁迁移影响. 化工学报. 2024(S1): 292-299 .  百度学术
百度学术
其他类型引用(1)
-






 DownLoad:
DownLoad: