-
随着城镇化进程的推进,城镇生活、工厂和药企等污水排放量逐年提高,处理废水以减小对水资源污染迫在眉睫。废水氮磷会造成水体富营养化,溶解氧降低,甚至对生物有直接毒性,好氧颗粒污泥 (aerobic granular sludge,AGS) 脱氮除磷能力较絮状泥效率更高,更利于工程管理[1]。与活性污泥法相比,AGS具有生物量高,沉降性能好,抗冲击胁迫能力强等优点。可以预测,AGS大规模工业化应用是将来的发展方向[2]。但是,AGS的形成受到许多因素的综合影响,包括反应器的运行,颗粒化进程、进水水质等。其中,颗粒的形成与性质是一个重要的影响因素,因此,能够快速制备优异性能的AGS来脱氮除磷具有良好的使用前景[3]。
目前,关于好氧颗粒污泥快速颗粒化已经进行了大量研究。HAO等[4]发现,常见的阳离子包括Mg2+、Ca2+、Fe2+、Cu2+、Zn2+和K+可以通过静电相互作用影响EPS和细胞粘附,在促进颗粒化时,应当添加适合的离子种类和浓度。虽然那这种手段是经常使用的,然而,添加金属离子的作用是有限的,不足以加速造粒和提高颗粒的稳定性。因此需要与其他策略相结合,以提高AGS的造粒和稳定性。LONG等[5]发现,将25%成熟AGS与絮状活性污泥接种,在18 d内即可获得好氧颗粒污泥。但是AGS难以利用且资源稀缺。因此,用AGS作为核心来大规模加速造粒是不切实际的。添加载体能够为AGS的初始形成提供核心,加速微生物粘附,从而提高颗粒化速度。LIANG等[6]报道,投加3.5 g·L−1尺寸为 (0.2 ± 0.025) mm的颗粒活性炭,与空白组相比能够提前12 d得到成熟的AGS。载体的加入会影响造粒过程和颗粒的稳定性。该方法操作简单,性价比高。载体的加入可以显著加快AGS的制粒速度,增强AGS的稳定性,可以在实践中实现大规模应用。但是考虑到与活性污泥的生物相容性和亲和性,大量使用的成本,以及载体使用回收困难造成的二次环境污染等问题。通过菌种筛选,再次反投加回原体系促进好氧颗粒污泥颗粒化,是一种可行且有效的手段。
文献报道添加絮凝细菌[7],真菌[8]也可以加速活性污泥的造粒。在这些研究的基础上,真菌菌丝球 (丝状真菌的自固定化形式) 因其具有沉降速度快、易固液分离,高生物活性,吸附性能好、处理污染物能力高,能分泌各种功能酶等优异性能[9-10],作为AGS形成载体颇受青睐。CHEN等[11]利用塔宾曲霉构建了好氧颗粒污泥,加速颗粒化并且提高污染物去除效率。同时,好氧颗粒污泥尺寸影响了好氧-厌氧区结构分布,从而影响颗粒性能[12]。此外,粒径影响储存稳定性的影响也有报道[13]。因此,探究添加不同尺寸菌丝球载体对好氧颗粒污泥的形成以及污染物去除的影响有重要意义。
为了实现AGS的快速形成与优异去除氮磷性能的保持,并且探究菌丝球影响性能的机理,本研究从实验室保存污泥中筛选出1株能够自发成球的霉菌,并选取成熟大、中、小3种尺寸的曲霉菌丝球,通过定期检测培养过程中EPS分泌、颗粒化进程、污染物氮磷去除能力、以及菌群分布和演变情况的变化规律来揭示反投加霉菌形成不同粒径AGS过程中产生的影响以及原因,探索反投加真菌载体对AGS性能的影响规律,为AGS技术的发展进步提供理论支持。
-
实验中选用的真菌载体为实验室筛选在振荡碰撞环境中由孢子自发成球的真菌,经18S rDNA鉴定为曲霉属 (Aspergillus sp.) 。接种的初始污泥是实验室持续曝气培养的来自北京市奔驰汽车水厂中生活污水处理的好氧池的絮状污泥,初始污泥性能良好,未有膨胀等现象,SVI<200。实验中模拟废水配方见表1,除提供碳源、氮源外,还增加了微量元素。
-
本研究采用250 mL锥形瓶实验,曲霉-好氧颗粒污泥的培养环境通过恒温摇床 (上海知楚仪器有限公司,ZQZY-A8V) 保持120 r∙min−1,28 ℃。曲霉的接种、传代和纯化实验均在超净台内完成。
-
采用PDB与模拟废水配比1∶1的培养基,调节控制pH在3~6的酸性环境下,接种孢子,振荡培养箱28 ℃、转速180 r∙min−1条件下培养36 h,得到性能较好的菌丝球。
菌丝球与絮状污泥接种时质量比为1∶3,复合培养时振荡培养箱温度保持28 ℃,转速调整为120 r∙min−1。反应器运行按照进水-静置厌氧培养-好氧培养-沉降-出水的方式运行,1个运行周期为8 h,其中,进水5 min,静置105 min,好氧培养360 min,沉降5 min,出水5 min。进出水通过虹吸完成,保证均匀出水,防止污泥被带出损失。在培养过程中间隔取样分析。
-
采用光学显微镜 (SMZ-DV320,重庆奥特,中国) 对不同阶段的污泥形态进行观察和记录。采用重力沉降法测定了污泥颗粒的沉降速度。用国家统一的标准筛确定污泥粒度,然后用污泥干重在各粒度范围内的比例表征污泥粒度分布。MLSS和SVI采用标准方法测定[14]。根据文献,常规污染物的检测采用以下方法。化学需氧量 (COD) 用重铬酸钾法测定,氨氮用纳塞尔试剂分光光度法测定,总氮 (TN) 用过硫酸钾碱氧化法测定,总磷 (TP) 用抗坏血酸法测定[15-16],比耗氧速率 (SOUR) 采用把便携式溶氧仪 (METTLER TOLEDO,S4-Meter) 测定[16]。采用氢氧化钠法提取各组不同阶段污泥的EPS[17]。以牛血清蛋白和葡萄糖为标准,分别采用考马斯亮蓝法和苯酚-硫酸法测定蛋白质和多糖的含量[18]。
颗粒强度通过收集好氧颗粒污泥连续曝气6 h后未脱落的部分来计算,表征颗粒稳定性。
采用E.Z.N.A.组织DNA试剂盒 (Omega Biotek, Norcross, GA, USA) 从不同来源的絮凝剂和AGS样品 (每个样品约0.5 mL) 中提取全基因组。采用通用引物341F (CCTACGGGNGGCWGCAG) 和805R (GACTACHVGGGTATCTAATCC) 通过PCR纯化16S rDNA的V3和V4可变区,长度约为460 bp[19]。然后将样品送往Novogene (中国北京) 进行菌株鉴定和高通量测序。
-
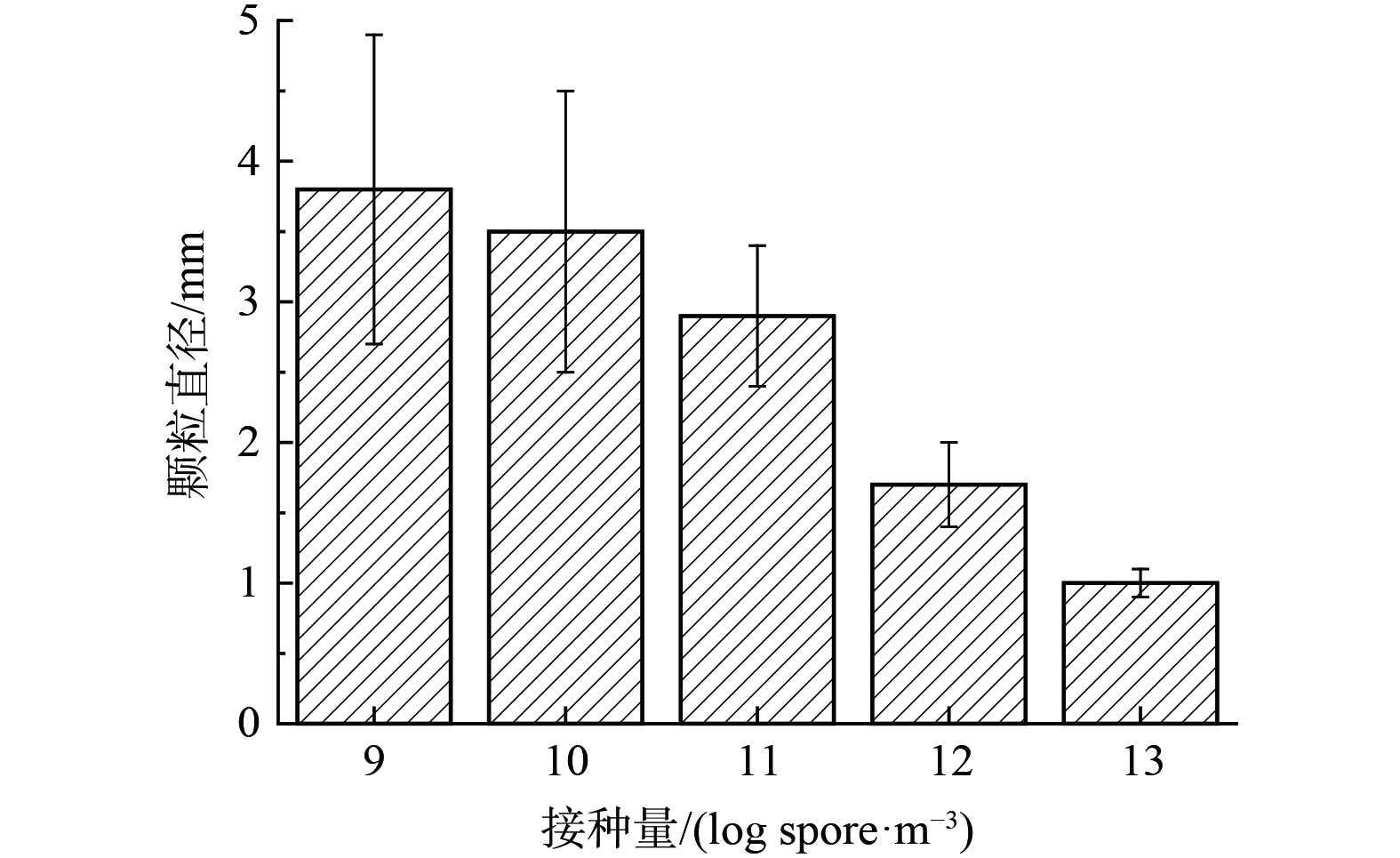
1) 接种量。图1所示为不同接种量下,菌丝球粒径分布。结果显示,接种量为109 cfu·(m3)−1时,粒径最大,为 (3.8±1.1) mm,与接种量1010 cfu·(m3)−1 ( (3.5±1.0) mm) 相差不大;当接种量增加至1011 cfu·(m3)−1时,粒径减小至 (2.9±0.5) mm;当接种量增加至1013 cfu·(m3)−1时,粒径最小,范围是 (1.0±0.1) mm。由此可以明显看出,随着接种量上升,粒径减小,并且颗粒范围逐渐减小,说明接种量越大,粒径分布越均匀。但是考虑到接种量太小,导致颗粒数量少而碰撞可能性降低,剪切力太小,造成菌丝球结构不紧密,菌丝外长。而量太大,孢子数太多,营养分配不均时会造成菌丝球内部发育不完整。因此,选择1011~1012 cfu·(m3)−1为最佳接种量。
2) pH。pH在真菌的培养中是一个非常重要的因素,营养物溶解、营养物质的传输、酶活性以及菌体表面的性质都受到pH的影响[20]。表2是在不同pH培养基环境中,菌丝球的生长情况。在pH为6.0时,形成的菌丝球形状规则,球状完整,且数量最多。当培养基pH为8.0时,菌丝球与菌丝团同时出现,并且菌丝含量很少。当培养基pH高至10.0时,曲霉则无法形成菌丝球。综上所述,培养基pH控制在弱酸性条件下,有利于刺激孢子萌发,并且更有利于形成菌丝球,但是如果是强酸性条件,则会造成菌丝过度液泡化,细胞肿胀导致菌丝无法生长。在文献中也有报道pH对菌丝球形态以及粒径都有较大的影响。LIU和WU[21]探究了pH对Cordyceps sinensis Cs-HK1形成菌丝球的影响中得到结论,当pH值 (4.0~5.0) 低于最佳pH值 (6.0) 时,菌丝球变得不均匀,或在pH值 (8~9) 较高时变成丝状。
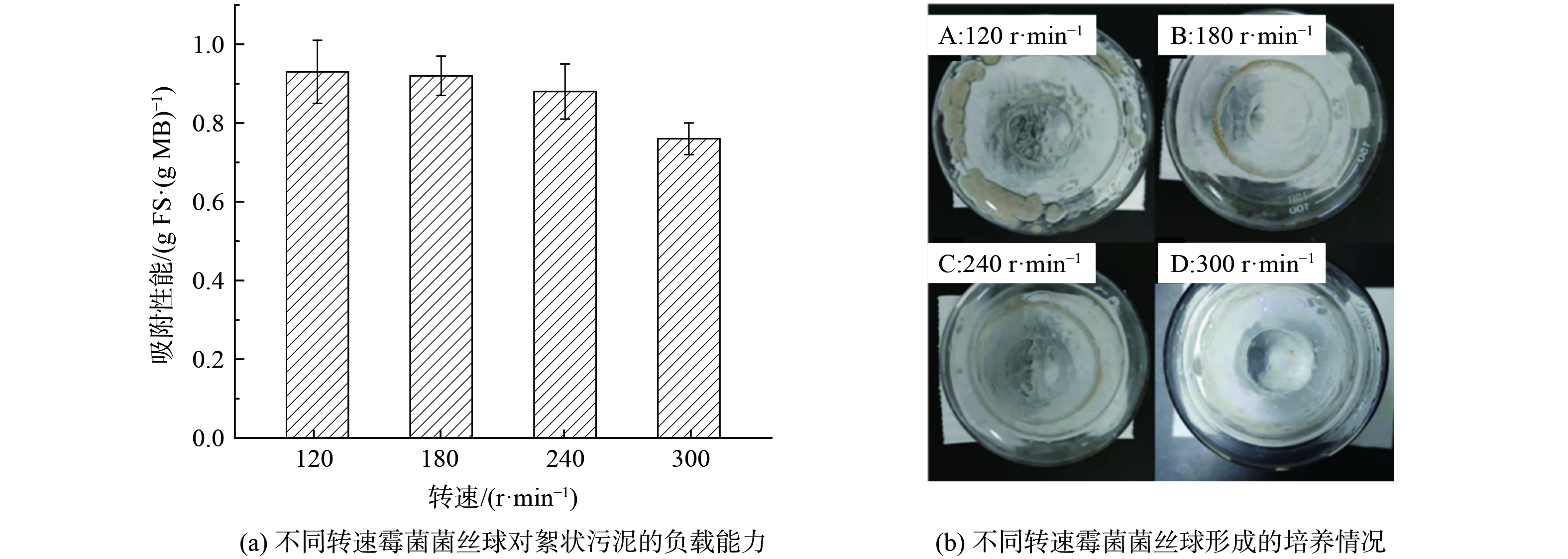
3) 转速。在真菌的培养过程中,传质与氧气传递有重要意义。一般情况下,孢子静置不会自发缠绕形成菌丝球,并且相对生物量很低,这可能是静置条件下碰撞、剪切、溶氧和传质效果都不好,会严重影响菌体生长。根据实验室经验,选取能够形成菌丝球的转速范围进行优化,分别是120、180、240和300 r·min-1。图2(a)结果表明,转速越高,霉菌菌丝球负载效果越差。转速为120 r·min-1时,负载絮状污泥量最多,高达0.93 g FS·(g MB)−1 (g FS·(g MB)−1表示每克霉菌菌丝球负载絮状泥质量) 。同时,转速为180和240 r·min-1时,吸附能力与120 r·min-1时相差不多,分别是0.92和0.88 g FS·(g MB)−1。
图2(b)为培养后瓶子情况,120 r·min-1转速下,由于转速较低导致菌丝球与容器底部粘连严重,极大降低了有效产出。因此,考虑到现实操作实践可能性与能耗问题,选择180 r·min-1为最适宜霉菌菌丝球形成的转速。
-
为了探究不同粒径曲霉-好氧颗粒污泥的颗粒化进程与颗粒性质,将曲霉菌丝球分成3个大小尺寸,分别是大尺寸L-AGS (3.0 mm<d≤5.0 mm) 、中等尺寸M-AGS (1.5 mm<d≤3.0 mm) 和小尺寸S-AGS (d≤1.5 mm) 。探究粒径对生物量、沉降性能、颗粒强度、比耗氧速率以及胞外多聚物分泌的影响。
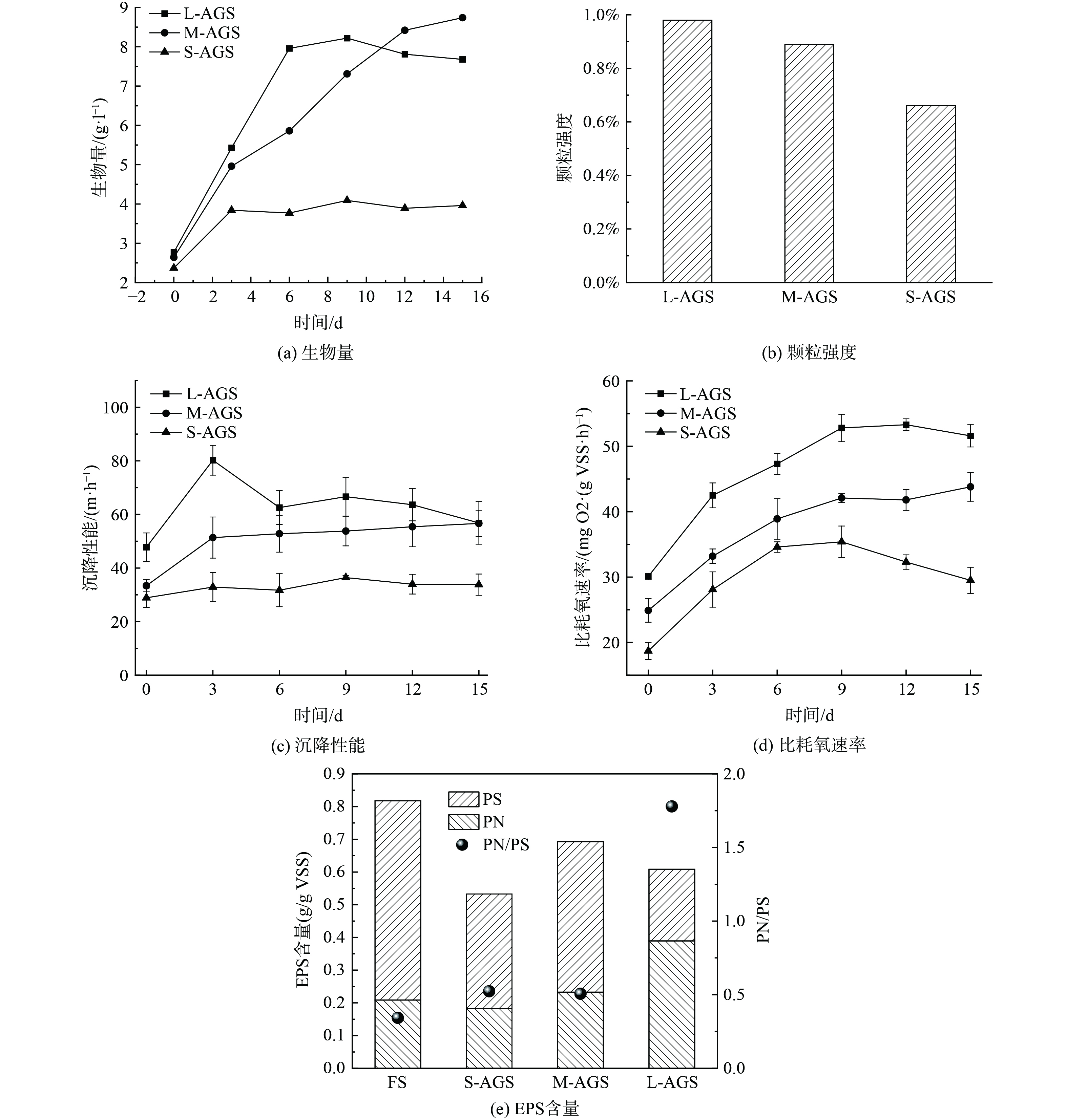
1) 生物量与沉降性能。图3所示为不同粒径好氧颗粒污泥随着培养时间进行,结构逐渐趋于稳定的颗粒化进程,其中图3(a)为混合液悬浮固体浓度 (MLSS) ,用来表示AGS生物量的浓度,MLSS数值越高,代表生物量越高[22],说明颗粒污泥处理废水时效率越高。从图中可以看出,S-AGS第3 d达到饱和并且与其他尺寸相比生物量最低,说明小尺寸AGS对微生物固定化效果最差。M-AGS在第15 d达到了生物量最大值,约为8.74 g·L−1,但是同时也可以观察到L-AGS在第9 d就达到了8.22 g·L−1,相比较而言,L-AGS生物量富集速度更快,虽然在培养后期会有一定降低,但是仍能保持较高水平。因此,L-AGS能够在较短时间内实现较高生物量负载,对颗粒化程度有很大提高。
图3(c)为培养过程中AGS沉降速率变化曲线,沉降速率越快,说明沉降性能越好,同时也反映了颗粒化程度越高。图中可以明显看出,L-AGS沉降速率远高于其他2组。一方面,可能由于自身重力影响加速了沉降;另一方面,结合生物量情况,也可以看出,L-AGS颗粒化性能更好,结构更加紧实,因此展现了更加优越的沉降性能。虽然在3 d时达到最大沉降速率后,速率下降,这可能是由于前期颗粒形成初期结构不稳定造成的,在之后的培养过程中,L-AGS保持60 m·h−1左右的稳定沉降速率。
研究结果表明,在培养过程中,L-AGS生物量更高,具有更快的沉降速率,因此颗粒化程度明显更高,结构更加紧实,是较理想的尺寸颗粒。
2) 颗粒强度与比耗氧速率。颗粒强度通过在曝气量为50 L·h−1持续6 h后,收集颗粒,与曝气前相比,颗粒的完整度。根据图3(b)可以看出,S-AGS颗粒完整度最差,仅有66%,可能由于尺寸太小导致结构不稳定,在长时间水力冲刷下,难以保持稳定,在应用中会造成丝状菌含量太高,影响出水水质,可能造成出水口堵塞。L-AGS能够保持高达98%的完整性,结果表明,菌丝球反投加后颗粒的稳定性与粒径有较大关系。菌丝球尺寸是通过接种孢子数量决定的,接种数量越少,菌丝球粒径越大,相同环境下,分配营养更充足,形成的菌丝缠绕结构更加致密,因此颗粒强度更高。
比耗氧速率 (SOUR) 在污泥处理废水中是一个关键指标,用来表征微生物活性,通过测试微生物的呼吸情况来反应污泥生理状态和基本代谢情况[23]。在培养过程中比耗氧速率变化如图3(d)所示,在培养第0 d时,大尺寸菌丝球即有最高比耗氧速率30.1 mg O2·(g VSS·h)−1,是小尺寸的2倍,可能由于大尺寸表面积更大,附着位点更多,表面分泌黏性物质更多,因此在表面附着更多活性污泥,为微生物提供更加合适的生长环境,因而大量消耗氧气,大量增长从而引起耗氧量增加[24],比耗氧速率更高。整体来看,SOUR L>M>S,与粒径有明显关系,小粒径在前期0~9 d比耗氧速率上升趋势至35.4 mg O2·(g VSS·h)−1后,可能由于颗粒强度太低发生崩解,微生物所处环境发生变化,导致活性下降,因此,通过结果可以看出,小尺寸菌丝球不能为絮状污泥附着提供稳定的环境,大尺寸颗粒稳定性更好,强度更高,抗冲击能力更强,能够为微生物生长以及形成内部厌氧外部好氧的结构提供支撑骨架。
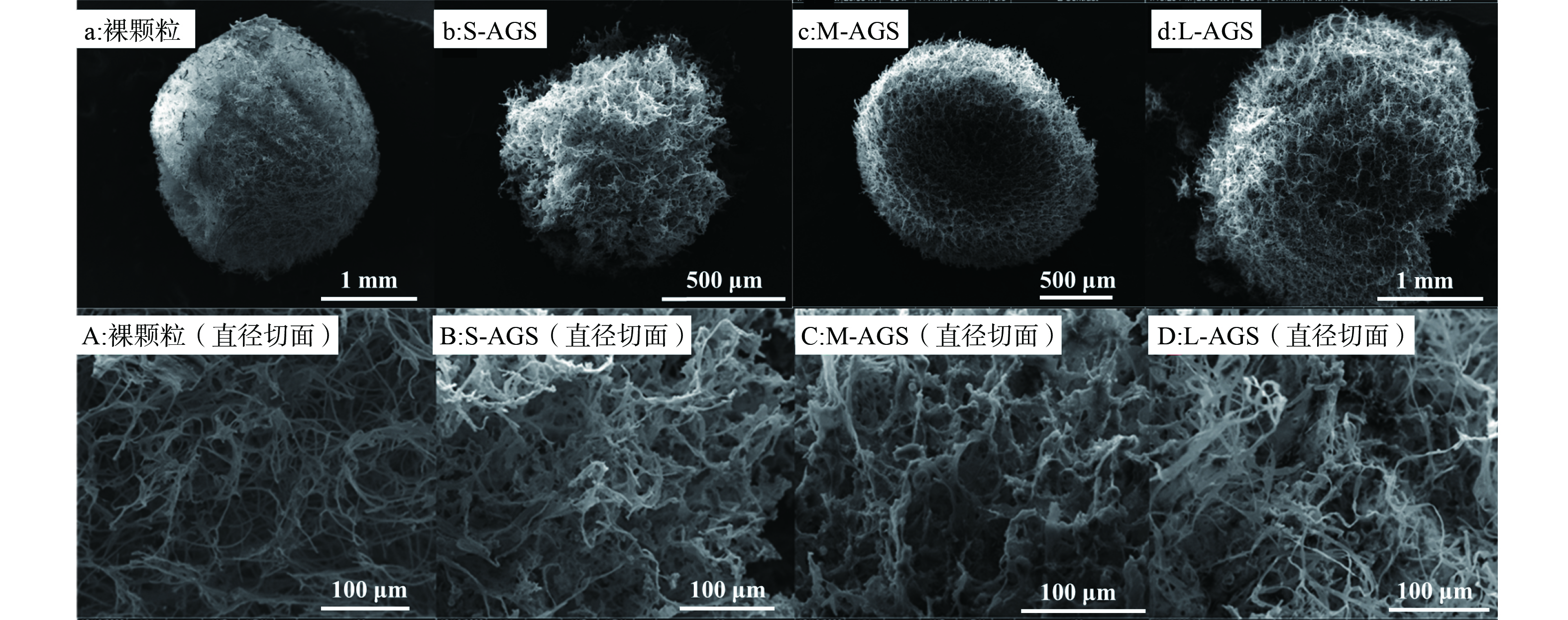
3) SEM表观结构。选取成熟时期的各个粒径好氧颗粒污泥制样,通过扫描电镜观察不同粒径整体与切面结构,图4(b)~(d)负载活性污泥的颗粒与图4(a)裸颗粒相比,明显可以看到表面负载了大量絮状污泥,并且都是球体,没有因为长期培养,菌丝球破损而导致破裂缺失。其中,M-AGS (c图) 表观更加平滑,菌丝外长的情况更少,表观来看这可能造成M-AGS性能更好,但是结合颗粒化程度、沉降速率、生物量和比耗氧速率来看,L-AGS具有更加优越的性能,这说明,正是L-AGS提供了适合微生物生长的内部环境,形成了稳定多菌结构。结合图4ABCD切面结构分析,L-AGS (图4D) 菌体在内部有大量的成团附着,不仅形成了厌氧-好氧结构,还有利于菌间交流与合作,并且与菌丝的紧密联系进一步加强了结构的稳定,为颗粒的完整性和高强度奠定了基础[25]。
4) 胞外聚合物的分泌。活性污泥周围的基质胞外聚合物 (EPS) 对其性质和功能有很大的影响,主要由蛋白质 (PN) 和多糖 (PS) 组成。细菌胞外聚合物 (EPS) 有助于活性污泥中生物菌团的形成,有助于其结构、表面电荷、沉降特性,并去除一些低浓度或大分子污染物[15, 26-27]。随着微生物群落的演化、环境条件的变化和微生物细胞的聚集,污泥EPS的组成、结构性质和功能会发生动态变化[28]。如图3(e)是絮状污泥以及不同粒径霉菌形成AGS的EPS分泌情况。据文献报道,环境条件的变化会影响微生物之间的信息传递,从而导致微生物产生一系列行为[29]。不同尺寸菌丝球的加入改变了环境造成菌群分泌EPS差异。整体来看,絮状污泥EPS含量明显高于霉菌负载,原因可能是霉菌占据了大部分比重,同时可能对微生物EPS分泌有抑制。添加霉菌菌丝球的实验组中,EPS含量M>L>S数值分别是0.53、0.69、0.61 g·(g VSS)−1。总的来说,添加霉菌菌丝球后,EPS分泌总量相差不大,但是L-AGS的PN/PS的比值高达1.7,是其他3组的3倍以上,PN的高含量在细胞交联中起着重要作用,有利于造粒和生物量积累[30],所以,推测L-AGS中的PN在颗粒化进程中发挥较大作用。
-
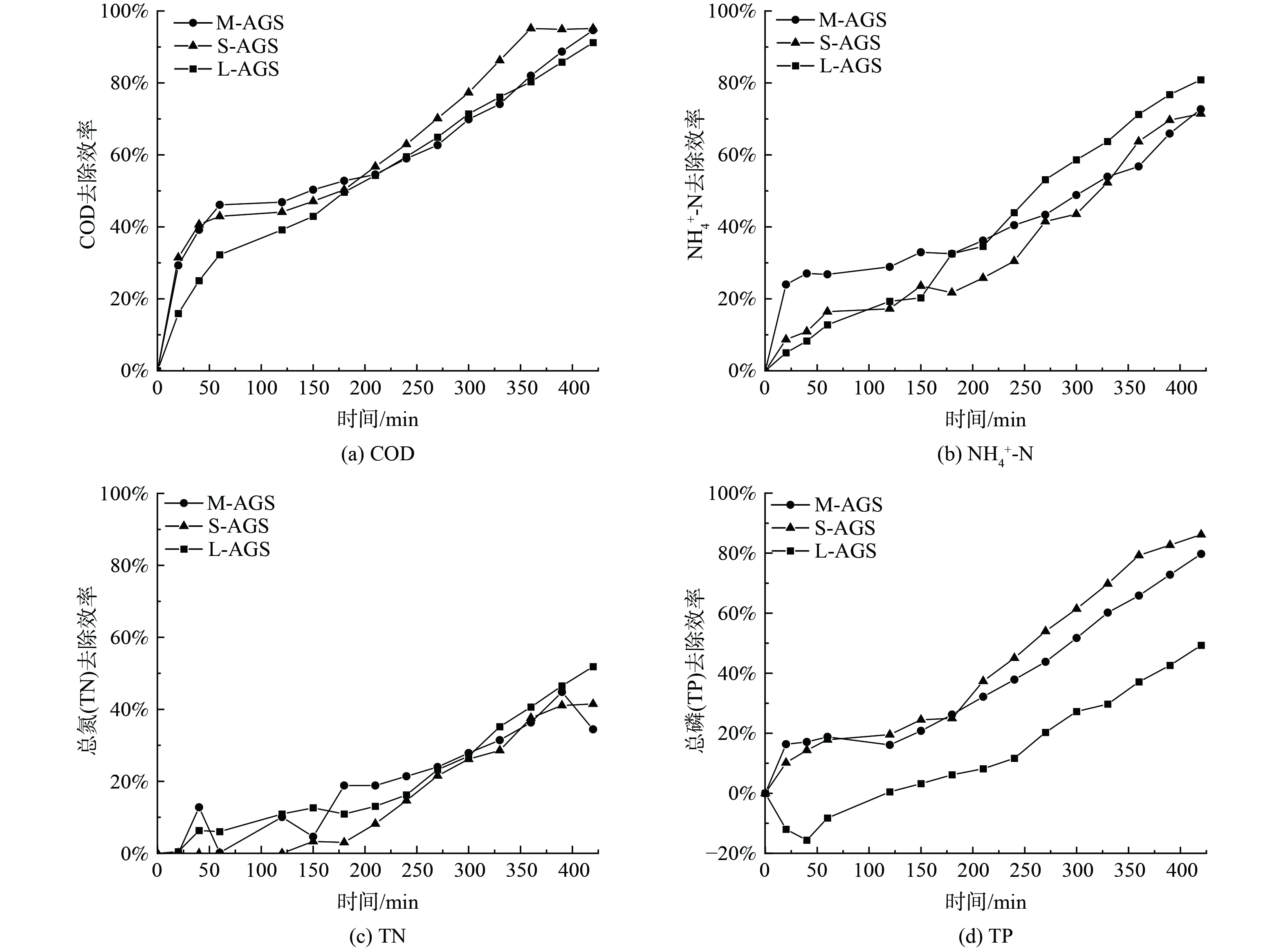
图5和图6分别是不同粒径的曲霉-好氧颗粒污泥废水处理的效果图。根据图5(a)可以看出,不同粒径颗粒污泥COD去除相差不大,6 h内均可达到90%以上,说明,曲霉-好氧颗粒污泥在形态上与絮状污泥更相近,因此较好的保持了COD去除能力。并且可以看出S-AGS对COD去除效果最好,可能是它松散的颗粒结构增大了与废水的接触面积,有利于处理效率提高。图5(b)为氨氮处理效率结果,其中L-AGS虽然前期启动较其他两组较慢,但是在颗粒稳定成熟后,去除效率最高,高达80.86%。推测可能是由于L-AGS颗粒结构更为丰富,形成相对较慢,但是颗粒化程度和水平更高。此外,菌丝球具有一定的生物活性和较强的吸附性能,能够通过生物降解和吸附的方式去除有机污染物[9]。
对TN的去除而言 (图5(c)) ,与氨氮去除相同,TN去除效果最好,但是整体不高,低于60%,这可能是在氮循环过程中的硝态化合物发生积累导致的。此外,S-AGS和M-AGS在总氮去除过程中,前期启动较慢且后期不稳定,这可能是由于系统本身不够成熟稳定的结果,同时也可能是菌群的不稳定造成的。总磷的去除效果见图5(d)所示,L-AGS去除远低于另外两种尺寸,且在前期释磷明显。根据磷的去除机理,好氧颗粒污泥在好氧阶段吸收磷,厌氧阶段会释放磷[31],因此推测S-AGS和M-AGS在好氧时吸收磷效率更好,可能由于L-AGS尺寸大,厌氧区更大,所以导致释磷更多,整体去除效果变差。
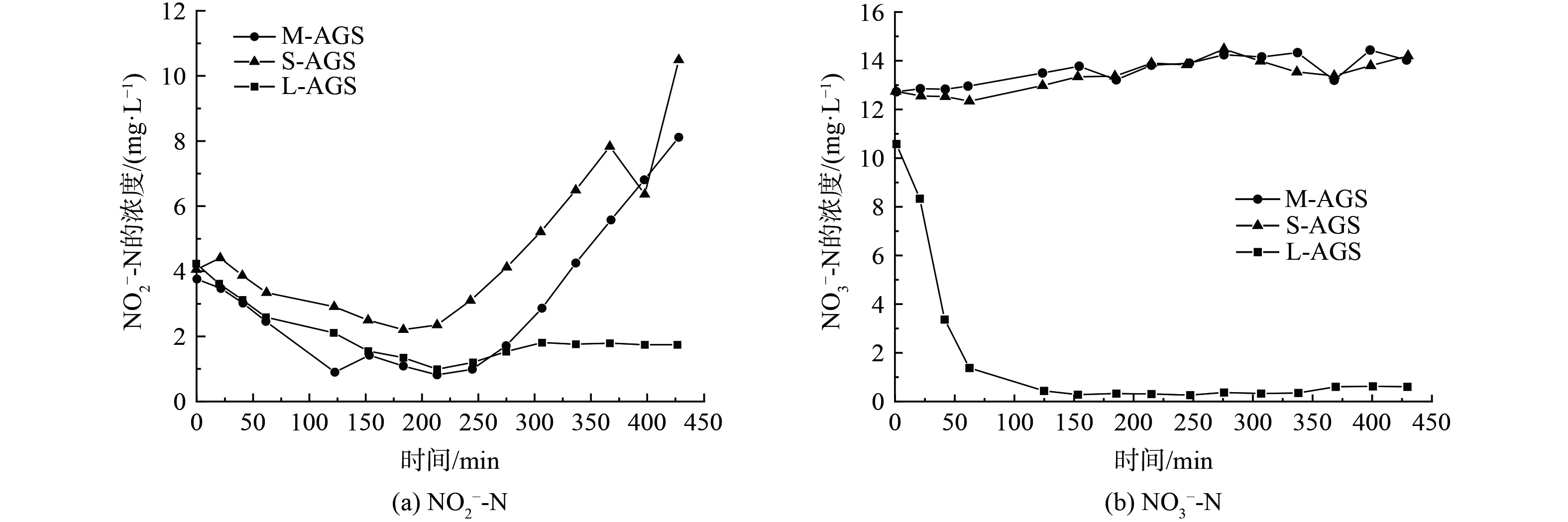
本研究还对氮去除中硝酸盐的去除与亚硝酸盐的积累情况做了探究,结果如图6所示。根据结果明显看出,L-AGS在硝酸盐去除和亚硝酸盐积累有明显优势。在210 min前,3组差异不大,但是在210 min后,S-AGS和M-AGS亚硝氮的积累迅速增多。S-AGS和M-AGS体系中亚硝酸盐的含量分别由初始时的4.05和3.76 mg·L−1上升至10.49和8.11 mg·L−1。结果表明,在氨氮的处理过程中,较大部分被氧化为亚硝酸盐,但是由于消耗亚硝酸盐菌群的匮乏或活性低,导致亚硝酸盐积累。相反,L-AGS保持稳定,层次结构更加丰富,提供了较大的厌氧区域,颗粒化程度更高,颗粒内部菌群结构丰富,进而强化对于硝酸盐的去除[32],L-AGS表现出更为优异的效果,几乎全部去除。
-
为了对比不同粒径霉菌添加后对脱氮除磷的去除效果,测试了脱氮反应比速率和除磷反应比速率。
1) 脱氮反应比速率。图7为不同粒径脱氮反应比速率结果,通过图7(a)可以看出,比氨氮化速率 (SAOR) L-AGS速率最高,但是3个组差异不大均有较好去除效果,说明氨氧化菌在3种尺寸都有较好生长,并且在L-AGS中生长最好且活性最高,为6.4 mg·(g MLSS·h)−1。图7(b)为比亚硝酸盐氧化速率 (SNOR) ,L-AGS表现出优越的结果,高达7.1 mg·(g MLSS·h)−1,主要依赖亚硝酸盐氧化菌在颗粒污泥好氧区进行,L-AGS的高速率可能依赖于它的表面积大因此与氧气接触面积以及物质交换能力高,同时,根据图3(d)比耗氧速率也能证明L-AGS的菌体活性更高,因此,亚硝酸盐氧化菌在L-AGS颗粒中有更高活性。在比硝酸盐还原速率 (SNRR,图7(c)) 和比亚硝酸盐还原速率 (SNIRR,图7(d)) 结果中,也能明显看出L-AGS的高去除速率,分别是12.8和5.1 mg·(g MLSS·h)−1。约为其他粒径的2倍,L-AGS由于颗粒尺寸大,内部拥有更大的厌氧区域,硝酸盐还原菌和亚硝酸盐还原菌有适合生存的缺氧环境,因而丰度会更高,活性更好,展现出更高的反应速率[32]。综合来看,与S-AGS和M-AGS相比,L-AGS在污染物脱氮方面具有更加优越的性能。
2) 除磷反应比速率。图7(ef)为不同粒径除磷反应比速率结果。其中,图7(e)为比吸磷速率,图7(f)为比释磷速率。根据图7(e)可以看出在好氧环境下吸磷过程中,S-AGS吸磷速率最快,L-AGS速率最慢,说明粒径越小,吸磷效果越好。释磷过程与吸磷速率相反,发生在厌氧环境,L-AGS释磷最慢,S-AGS最快,说明粒径越大,释磷效果越差。生物除磷是吸磷与释磷结合的好氧与厌氧综合的过程。因此综合两个过程来看,虽然L-AGS吸磷速率相较不高,但是它极差的释磷能力,仅有0.9 mg·(g MLSS·h)−1,因此,结合两个过程,与其他两个粒径相比,L-AGS除磷效果最好。
-
1) 多样性分析。为了揭示不同粒径霉菌菌丝球添加后对细菌群落的影响规律,通过高通量测序技术对各粒径污泥样品中细菌群落的演化进行分析。如表3所示,所有样品的覆盖率值均为1,因此测序结果可用作为实际的细菌群落样本[33]。污泥样品的alpha多样性反映了污泥的丰富度 (以Ace指数和Chao1指数为特征) 和多样性 (以Shannon指数和Simpson指数为特征) 。霉菌菌丝球的添加组,Ace和Chao1值都有提高,说明物种丰富度都有提升,霉菌菌丝球的添加有利于微生物丰富度。另外一组指数Simpson指数与微生物多样性呈负相关,Shannon指数呈正相关。其中,L-AGS的Simpson指数低于其他组为0.89,Shannon指数最高为5.10,说明L-AGS中微生物多样性更高,这样的结构更有利于微生物生长,能够形成更好的厌氧好氧分布环境,适合多种微生物的生长。
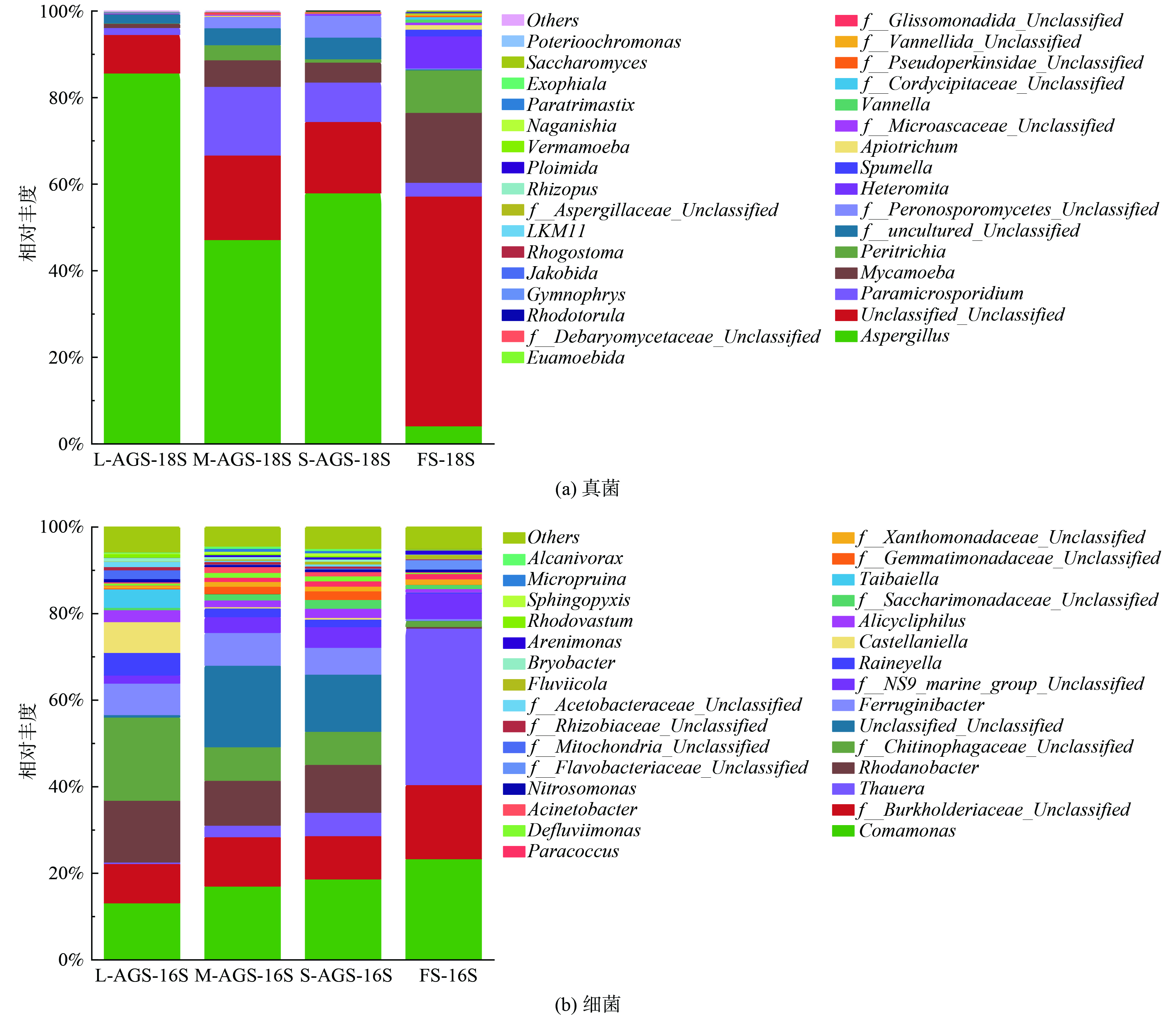
2) 真菌。通过18s RNA基因分析对真核微生物群落进行了特征分析 (图8(a)) 。总结了在属水平的最丰富的原生动物。可以明显看到,在每个反应器中都存在真菌Aspergillus,并且在L-AGS中丰度最高 (85.67%) ,同时,在絮状泥即筛选霉菌菌丝球来源的污泥中也存在4.17%,说明该菌来源,并且作为AGS核心为絮状泥生长提供位点,同时促进颗粒形成发育。虽然Aspergillus在L-AGS中占比较高,但是并没有造成污泥膨胀,反而促进了颗粒化与污染物去除。并且从原始污泥中筛选的菌丝球,投加回原体系能够减少生物入侵危害,同时也更有利于环境的适应。有报道表明,投加真菌菌丝球有利于颗粒化进程与污染物去除[11, 34]。
3) 细菌。图8(b)是不同粒径霉菌为核心好氧颗粒污泥在属水平上的群落结构。在絮状污泥FS中,丰度最高的菌种是Thauera (36.15%) ,此外Comamonas (23.37%) 和Burkholderiaceae (17.11%) 也占有较高比例。再添加霉菌菌丝球后,明显观察到Comamonas和Thauera丰度下降,并且L-AGS中含量最低,分别降低至13.2%和0.34%。这是两株EPS高产菌,EPS大量分泌有利于颗粒化进程加速以及毒性环境耐受性提高[35]。在L-AGS中这两种菌丰度大幅度降低表明霉菌菌丝球的添加帮助体系更快的适应冲击,不需要更多的EPS分泌菌来适应环境。同时也说明,在3个添加菌丝球的实验组中,L-AGS的添加更有利于AGS的形成与颗粒化。在4个反应器中,都存在大量乙酸盐去除菌Burkholderiaceae,此外,Rhodanobacter (14.26%) 在L-AGS中与其他几组相比有最高丰度。文献报道Burkholderiaceae和Rhodanobacter共存时在土壤中耐酸反硝化有重要作用[36]。与FS (0.37%) 相比,Rhodanobacter不仅在L-AGS中有大幅提升,在M-AGS (10.35%) 和S-AGS (11.07%) 中丰度有较大增加,表明霉菌菌丝球的添加可能加速了反硝化作用的启动,对污染物去除有促进作用。还有大量反硝化菌在L-AGS中分布,相关菌群丰度高达38.16%,远高于其他实验组,其中反硝化菌包括Ferruginibacter[37]、Castellaniella[38]等,说明L-AGS形成的好氧厌氧结构更加成熟,有利于反硝化菌生长。也正是因为这些菌占有较高丰度,使得L-AGS在硝酸盐和亚硝酸盐去除中展现优越的性能。
-
1) 霉菌菌丝球最适培养条件为,接种量1011~1012 cfu·(m3)−1,转速180 r·min-1,培养基弱酸性。
2) L尺寸霉菌菌丝球的添加促进颗粒化效果最好,形成的L-AGS强度最高,比耗氧速率最快,生物量最大,并且EPS中蛋白含量占比最高。是最佳的霉菌尺寸选择。
3) 在单位时间内的污染物去除效果上,L-AGS表现出最好的污染物综合去除能力。L-AGS体系6 h内的COD去除率为91.4%,氨氮去除率为77.2%,总氮去除率为52.8%,总磷的去除率为49.2%,硝酸盐的去除率为93.9%,反应结束时,体系中硝酸盐和亚硝酸盐的质量浓度仅为0.65和1.8 mg·L−1。
4) L-AGS形成的厌氧-好氧双层核壳结构最稳定,有利于脱氮除磷生物反应进行,微生物具有较高生物活性,SAOR为6.4 mg·(g MLSS·h)−1。SNOR高达7.1 mg·(g MLSS·h)−1,SNRR和SNIRR分别是12.8和5.1 mg·(g MLSS·h)−1。
5) 多种反硝化菌在L-AGS中生长,包括Ferruginibacter、Castellaniella等,为污染物去除奠定基础。
不同粒径曲霉-好氧颗粒污泥的微环境对脱氮除磷能力影响
Effects of different size Aspergillus-aerobic granular sludge microenvironment on nitrogen and phosphorus removal capacity
-
摘要: 针对目前实验室好氧颗粒污泥颗粒化慢,稳定性差等问题。以自发成球的曲霉为核心,开发了絮状污泥的快速颗粒化技术,提高体系运行稳定性。对形成的大 (3.0 mm<d≤5.0 mm) 、中 (1.5 mm<d≤3.0 mm) 、小 (d≤1.5 mm) 3种尺寸的曲霉-好氧颗粒污泥 (Aspergillus-AGS) 进行了性能对比,然后结合脱氮除磷性能实验监测数据,对曲霉投加后菌群结构的演变进行分析。大尺寸的Aspergillus-AGS是最优选,能促进胞外多聚物的分泌,从而有利于颗粒化性能提高,维持颗粒稳定结构,并且提高了污染物去除效率。在投加不同粒径的反应器中,通过菌群分析,曲霉Aspergillus作为优势载体菌种在对照组絮状污泥、大、中和小尺寸反应器中占比分别是4.17%、85.67%、47.20%和58.00%。此外,大尺寸颗粒反应器中,细菌种类NOB、PAO占比相比其他反应器明显更高,其中反硝化菌Ferruginibacter、Castellaniella等丰度较高,脱氮除磷有明显优势,L-AGS体系6 h内氨氮的去除率为77.2%,总氮去除率为52.8%,总磷的去除率为49.2%,硝酸盐的去除率为93.9%。Abstract: Aiming at the problems of slow granulation and poor stability of aerobic granular sludge in laboratory. Aspergillus, obtained by screening and separation in the laboratory, which can form into balls automatically in the process of oscillation culture, was added to the flocculent sludge system in reverse as a carrier to promote the granulation process. The performance of the large (3.0 mm < D ≤ 5.0 mm), medium (1.5 mm < D ≤3.0 mm), and small (D ≤1.5 mm) AGS were compared, and when combined with the monitoring experimental data of pollutant removal performance, and the evolution of bacterial community structure were analyzed. The results showed that the large size was the most optimal AGS size compared to the small and middle, which could promote the secretion of extracellular polymeric substances, thus improving the granulation performance, maintaining the stable structure of particles, and showing good comprehensive ability in the treatment of pollutants. In the reactors with different particle sizes, microbial community analysis showed that the fungus Aspergillus, as the dominant carrier of aerobic granular sludge, accounted for 4.17%, 85.67%, 47.20%, and 58.00% in the flocculent sludge, large, middle and small size reactors. In addition, the proportion of bacteria NOB and PAO in the large size adding reactor was significantly higher than those in the other two reactors. Denitrification bacteria such as Ferruginibacter and Castellaniella had high abundance and had obvious advantages in nitrogen and phosphorus removal. The removal rate of ammonia nitrogen, total nitrogen, total phosphorus and nitrate in L-AGS system within 6 h was 77.2%, 52.8%, 49.2% and 93.9%, respectively.
-

-
表 1 合成模拟废水成分表 (COD=1 000 mg·L−1)
Table 1. The table of synthetic simulated wastewater composition
组成 浓度/(mg·L−1) 组成 浓度/(mg·L−1) C6H12O6 468.72 CaCl2 166.51 CH3COONa 640.63 EDTA 50 NH4Cl 382.13 ZnSO4 2.2 KH2PO4 22 MnCl2·4H2O 5.06 K2HPO4 24 (NH4)6Mo7O2·4H2O 1.1 MgSO4 25.38 CuSO4·5H2O 1.57 FeSO4 20.34 CoCl2·5H2O 1.61 表 2 不同pH培养基环境中菌丝球生长情况表
Table 2. The table of mycelium pellet growth in different pH medium
pH 菌丝球生长情况 3.0 菌丝球、正常 6.0 菌丝球、较多 7.0 菌丝球、正常 8.0 菌丝团,菌丝球较少 10.0 没有形成 表 3 AGS细菌多样性指数的变化
Table 3. Changes of bacterial diversity index in AGS
样品 Ace Chao1 Shannon Simpson 平均 L-AGS 263.19 265.06 5.10 0.89 1 M-AGS 304.51 305.65 4.84 0.93 1 S-AGS 296.69 292.52 5.01 0.92 1 FS 241.34 240.05 4.19 0.95 1 -
[1] 郑丽颖, 温俊宝. 环丙沙星对好氧颗粒污泥同步脱氮除磷的影响[J]. 水处理技术, 2020, 46(10): 55-60. [2] HAN X S, JIN Y, YU J G. Rapid formation of aerobic granular sludge by bioaugmentation technology: A review[J]. Chemical Engineering Journal, 2022, 437: 134971. doi: 10.1016/j.cej.2022.134971 [3] 郭之晗, 徐云翔, 李天皓, 等. 好氧颗粒污泥长期稳定运行研究进展[J]. 化工进展, 2022, 41(5): 2686-2697. [4] HAO W, LI Y, LV J, et al. The biological effect of metal ions on the granulation of aerobic granular activated sludge[J]. Journal of Environmental Sciences (China), 2016, 44: 252-259. doi: 10.1016/j.jes.2015.10.031 [5] LONG B, YANG C Z, PU W H, et al. Rapid cultivation of aerobic granular sludge in a pilot scale sequencing batch reactor[J]. Bioresource Technology, 2014, 166: 57-63. doi: 10.1016/j.biortech.2014.05.039 [6] LIANG Z, TU Q, SU X, et al. Formation, extracellular polymeric substances, and structural stability of aerobic granules enhanced by granular activated carbon[J]. Environmental Science and Pollution Resesrch, 2019, 26(6): 6123-6132. doi: 10.1007/s11356-018-04101-1 [7] IVANOV V, WANG X H, STABNIKOVA O. Starter culture of Pseudomonas veronii strain B for aerobic granulation[J]. World Journal of Microbiology and Biotechnology, 2007, 24(4): 533-539. [8] WANG H L, YU G L, LIU G S, et al. A new way to cultivate aerobic granules in the process of papermaking wastewater treatment[J]. Biochemical Engineering Journal, 2006, 28(1): 99-103. doi: 10.1016/j.bej.2005.10.002 [9] WANG L, YU T, MA F, et al. Novel self-immobilized biomass mixture based on mycelium pellets for wastewater treatment: A review[J]. Water Environment Research, 2019, 91(2): 93-100. doi: 10.1002/wer.1026 [10] LI L, LIANG T, LIU W, et al. A comprehensive review of the mycelial pellet: Research status, applications, and future prospects[J]. Industrial & Engineering Chemistry Research, 2020, 59(39): 16911-16922. [11] CHEN Y Y, GE J Y, WANG S J, et al. Insight into formation and biological characteristics of Aspergillus tubingensis-based aerobic granular sludge (AT-AGS) in wastewater treatment[J]. Science of the Total Environment, 2020, 739: 140128. doi: 10.1016/j.scitotenv.2020.140128 [12] CHEN Y Y, GENG N F, HU T H, et al. Adaptive regulation of activated sludge's core functional flora based on granular internal spatial microenvironment[J]. Journal of Environmental Management, 2022, 319: 115714. doi: 10.1016/j.jenvman.2022.115714 [13] 张立楠, 张斌超, 刘祖文, 等. 粒径对好氧颗粒污泥储存稳定性的影响[J]. 化工进展, 2019, 38(7): 3450-3457. [14] 吴瑞馨, 赵彬, 陈宇航, 等. 高有机负荷对好氧颗粒污泥形成和稳定性能的影响[J]. 环境工程学报, 2023, 17(05): 1662-1673. [15] HOUGHTON J I, STEPHENSON T. Effect of influent organic content on digested sludge extracellular polymer content and dewaterability[J]. Water Research, 2002, 36: 3620-3628. doi: 10.1016/S0043-1354(02)00055-6 [16] APHA A. Standard methods for the examination of water and wastewater[J]. American Public Health Association Inc, Washington DC 1998. [17] DENG S, WANG L X, SU H J. Role and influence of extracellular polymeric substances on the preparation of aerobic granular sludge[J]. Journal of Environmental Management, 2016, 173: 49-54. [18] LIU Y, TAY J H. The essential role of hydrodynamic shear force in the formation of biofilm and granular sludge[J]. Water Research, 2002, 36: 1653-1665. doi: 10.1016/S0043-1354(01)00379-7 [19] TAKAHASHI S, TOMITA J, NISHIOKA K, et al. Development of a prokaryotic universal primer for simultaneous analysis of Bacteria and Archaea using next-generation sequencing[J]. PLoS One, 2014, 9(8): e105592. doi: 10.1371/journal.pone.0105592 [20] LIAO W, LIU Y, FREAR C, et al. A new approach of pellet formation of a filamentous fungus- Rhizopus oryzae[J]. Bioresource Technology, 2007, 98(18): 3415-3423. doi: 10.1016/j.biortech.2006.10.028 [21] LIU Y S, WU J Y. Effects of Tween 80 and pH on mycelial pellets and exopolysaccharide production in liquid culture of a medicinal fungus[J]. Journal of Industrial Microbiology & Biotechnology, 2012, 39(4): 623-628. [22] BEUN J J, VAN LOOSDRECHT M C M, HEIJNEN J J. Aerobic granulation in a sequencing batch airlift reactor[J]. Water Research:A Journal of the International Water Association, 2002, 36: 702-712. [23] 朱荣霞, 刘树信, 谭周亮, 等. 污水处理系统中比耗氧速率的测定及其应用[J]. 四川环境, 2022, 41: 279-285. [24] 李志华, 王晓昌, 王耀东. 含盐量对好氧颗粒污泥形成过程的影响[J]. 环境工程学报, 2008, 9: 1228-1230. [25] 耿明月. 菌丝球诱导形成好氧颗粒污泥的作用机制及运行特性研究[D]. 哈尔滨: 哈尔滨工业大学, 2022. [26] HOUGHTON J I, QUARMBY J, STEPHENSON T. Municipal wastewater sludge dewaterability and the presence of microbial extracellular polymer[J]. Water Science and Technology, 2018, 44: 373-379. [27] HUANGFU X L, XU Y, LIU C, et al. A review on the interactions between engineered nanoparticles with extracellular and intracellular polymeric substances from wastewater treatment aggregates[J]. Chemosphere, 2019, 219: 766-83. doi: 10.1016/j.chemosphere.2018.12.044 [28] JIN Y, XIONG W, ZHOU N, et al. Role of initial bacterial community in the aerobic sludge granulation and performance[J]. Journal of Environmental Management, 2022, 309: 114706. doi: 10.1016/j.jenvman.2022.114706 [29] LIN H, MA R, HU Y, et al. Reviewing bottlenecks in aerobic granular sludge technology: Slow granulation and low granular stability[J]. Environmental Pollution, 2020, 263: 114638. doi: 10.1016/j.envpol.2020.114638 [30] TAN C H, KOH K S, XIE C, et al. The role of quorum sensing signalling in EPS production and the assembly of a sludge community into aerobic granules[J]. Isme Journal, 2014, 8(6): 1186-1197. doi: 10.1038/ismej.2013.240 [31] ISLAM M S, ZHANG Y, DONG S, et al. Dynamics of microbial community structure and nutrient removal from an innovative side-stream enhanced biological phosphorus removal process[J]. Journal of Environmental Management, 2017, 198: 300-307. [32] BASSIN J, TAVARES D, BORGES R, et al. Development of aerobic granular sludge under tropical climate conditions: The key role of inoculum adaptation under reduced sludge washout for stable granulation[J]. Journal of Environmental Management, 2019, 230: 168-182. [33] WAN D, LI Q, LIU Y, et al. Simultaneous reduction of perchlorate and nitrate in a combined heterotrophic-sulfur-autotrophic system: Secondary pollution control, pH balance and microbial community analysis[J]. Water Research, 2019, 165: 115004. doi: 10.1016/j.watres.2019.115004 [34] SUN Y, ALI A, ZHENG Z, et al. Denitrifying bacteria immobilized magnetic mycelium pellets bioreactor: A new technology for efficient removal of nitrate at a low carbon-to-nitrogen ratio[J]. Bioresource Technology, 2022, 347: 126369. doi: 10.1016/j.biortech.2021.126369 [35] PENG H, GUO J B, LI H B, et al. Granulation and response of anaerobic granular sludge to allicin stress while treating allicin-containing wastewater[J]. Biochemical Engineering Journal, 2021, 169: 107971. doi: 10.1016/j.bej.2021.107971 [36] HETZ S A, HORN M A. Burkholderiaceae are key acetate assimilators during complete denitrification in acidic cryoturbated peat circles of the arctic tundra[J]. Frontiers in Microbiology, 2021, 12: 2307-2321. [37] WANG Z, YUAN S, DENG Z, et al. Evaluating responses of nitrification and denitrification to the co-selective pressure of divalent zinc and tetracycline based on resistance genes changes[J]. Bioresource Technology, 2020, 314: 123769. doi: 10.1016/j.biortech.2020.123769 [38] DENG L J, REN Y, WEI C H, et al. Biodegradation of pyrene by a novel strain of Castellaniella sp. under denitrifying condition[J]. Journal of Environmental Chemical Engineering, 2021, 9: 104970. doi: 10.1016/j.jece.2020.104970 -



 下载:
下载: