-
在我国的经济发展过程中,煤炭使用不可或缺,地位不可替代[1],但在使用时会造成大气污染,为了预防或减少煤燃料对环境和人体健康带来的危害,洗煤、洁净煤技术应运而生,煤的气化技术[2]为其中之一,但煤炭在气化过程中会产生大量的煤气化废水(coal gasification wastewater,CGW),该废水的主要特点是可生化性差,酚类物质含量高、毒性强、有机物浓度高、色度深,属于难处理工业废水[2-3]。
煤气化废水通常采用生物组合工艺(如厌氧、缺氧和好氧)处理[4]。但在生物处理厌氧段的废水存在可生化性差、处理效率低和出水毒性大等问题,导致后续处理工艺运行不稳和出水污染物浓度高。为了提高厌氧段的可生化性,提高污染物的去除效率,降低出水毒性(如降低酚类污染物含量),寻求高效且低成本的预处理方法势在必行。吸附法因其成本低、处理效果好、处理工艺简单、可实现废物资源化等优势,在工业废水的预处理中得到较多的研究,吸附法的使用吸附剂的选择尤为重要,直接影响吸附效果和使用成本。钠基膨润土(Na-Bentonite,Na-BBT)与粉煤灰(pulveized fuel ash,PFA)具有天然无害、价格低廉、来源丰富等优点,是一种潜在的廉价吸附剂[5-7],具有广阔的应用前景,被常用于工业废水处理[8-11]。罗秋艳等[12]使用Na-BBT吸附模拟酚类废水中的2,4,6-三氯苯酚,以物理吸附为主,吸附容量达到16.90 mg·g−1;BATABYAL[13]等用PFA吸附模拟废水中2,4-二甲基苯酚,结果表明,吸附过程符合准一级动力学模型。KHANSAA[14]用Na-BBT吸附处理橄榄油废水中的总酚类化合物,最大平衡吸附量为81.323 mg·g−1;赵伟高等[15]用PFA吸附处理焦化废水,以物理吸附为主,挥发酚的最大平衡吸附量为 39.5 mg·g−1。Na-BBT和PFA对酚类物质的吸附等温线均符合Langmuir模型[12-14]。目前,使用Na-BBT和PFA作为吸附材料对含酚废水吸附应用有一些研究,但在煤气化废水预处理领域还没有相关应用,尤其是对于复杂的、实际的煤气化废水中种类繁多的酚类化合物吸附去除效果研究较少,没有对其酚类物质的吸附去除机理进行深入研究。
鉴于此,本研究以实际煤气化废水厌氧段进水为研究对象,以Na-BBT和PFA作为吸附剂,对比分析了2种吸附剂对煤气化废水中主要的特征污染物(酚类物质)的吸附效果,探讨了煤气化废水的可生化性和毒性改变原因,并揭示了其吸附机理,以期为后续相关研究提供参考。
-
1)实验试剂:重铬酸钾(K2Cr2O7)、硫酸(H2SO4)、硫酸银(Ag2SO4)、硫酸汞(HgSO4)、硫酸亚铁铵((NH4)2Fe(SO4)2·6H2O)、盐酸(HCl)、碘化钾(KI)、碘化汞(HgI2)、氢氧化钾(KOH)、酒石酸钾钠(NaKC4H6O6)、硫代硫酸钠(Na2S2O3)、氢氧化钠(NaOH)、硼酸(H3BO3)、氯化铵(NH4Cl)、磷酸二氢钾(KH2PO4)、磷酸氢二钾(K2HPO4)、七水合磷酸氢二钠(Na2HPO4·7H2O)、七水合硫酸镁(MgSO4·7H2O)、氯化钙(CaCl2)、六水合氯化铁(FeCl3·6H2O)、亚硫酸钠(Na2SO3)、过硫酸钾(K2S2O8)、硝酸钾(KNO3)、硝酸(HNO3)、钼酸铵(NH4MoO4)、抗坏血酸(C6H8O6)、七水硫酸亚铁(FeSO4·7H2O)、五水硫酸铜(CuSO4·5H2O)、乙醚(C4H10O)、磷酸(H3PO4)、氯化钠(NaCl)、氯化镁(MgCl2)、氯化钙(CaCl2)、氯化汞(HgCl2)等购自国药集团化学试剂有限公司,以上药品均为分析纯。
2)吸附材料及水质指标。吸附剂Na-BBT和PFA,购自河南郑州华晶化工有限公司。所有吸附材料在使用前用纯水充分清洗去除杂质,然后在105 ℃的真空干燥箱(DZF-6020A,郑州宝晶电子科技有限公司)中干燥 6 h,得到的固体进一步研磨,筛分至 200 目。实验所采用的实际煤气化废水取自内蒙古大唐克旗煤制天然气有限责任公司厌氧工艺段进水,其中COD为3 302.5 mg·L−1 、五日生化需氧量(biochemical oxygen demand after 5 days, BOD5)为957.7 mg·L−1、BOD5/COD(B/C)为0.29、 总有机碳(total organic carbon, TOC)为1 395.3 mg·L−1、氨氮(NH3-N)为221.3 mg·L−1、总氮(total nitrogen, TN)为245.6 mg·L−1、总磷(total phosphorus, TP)为2.0 mg·L−1、总酚(total phenols, Tph)为554.8 mg·L−1、pH为7.7、电导率为1 856 μS·cm−1。
-
1)水质分析:水样指标测定均在25 ℃下。 COD 采用重铬酸盐法(HJ 828-2017);NH3-N 采用纳氏试剂分光光度法(HJ 535-2009);BOD5采用稀释与接种法(HJ 505-2009);TOC采用TOC-Lcph总有机碳析仪(ASK2-4,日本岛津制作所)测量;TN采用碱性过硫酸钾消解紫外分光光度法(HJ 636-2012);TP采用钼酸铵分光光度法(GB 11893-89);Tph采用溴化容量法(HJ 502-2009);pH使用pH仪(SG-2,美国哈希公司);电导率使用电导仪(S40d,美国哈希公司),均采用玻璃电极法。
2)Na-BBT 和PFA投加量对煤气化废水中BOD5、COD、NH3-N和Tph去除效果的影响以及分析B/C值的变化。在 25 ℃、 250 mL 煤气化废水中分别改变Na-BBT和PFA投加量(0.25、0.5、1、2、4、6、8、10 g·L−1),置于恒温摇床,设置180 r·min−1、吸附时间为 200 min,吸附后过0.45 μm滤膜,测定BOD5、COD、NH3-N和Tph值,计算其去除率和B/C值,确定吸附剂的投加量。
3)发光菌毒性分析:吸附剂Na-BBT 与PFA投加量为6 g·L−1,其他实验条件与投加量实验相同,采用多功能酶标仪(MDM9000,株式会社丽光制造所)对废水中发光强度(RLU)进行测试,采用水质急性毒性的测定发光细菌法(GB/T15441-1995),监测吸附前后煤气化废水发光菌生物急性毒性单元TU(Toxic Unit)值。
4)三维荧光分析。实验条件与发光菌毒性分析实验相同,实际煤气化废水和被吸附处理后的废水稀释1 500 倍至 TOC<1 mg·L−1,采用三维荧光光谱仪(F-7000,株式会社日立制造所)测试重复三次,样品的荧光光谱使用仪器内置软件处理,减去超纯水的空白信号,使用区域荧光积分方法(FRI)进行三维荧光分析,并用 Matlab2019b 处理和绘制数据。
5)GC-MS(Gaschromatography-mass spectrometry)分析。实验条件与发光菌毒性分析实验相同,采用气相色谱质谱仪(7890A 597C,美国安捷伦公司)对煤气化废水中酚类等特征污染物测试,监测煤气化吸附前后的特征污染物酚类、酮类物质变化。
6)吸附等温线。在吸附时间为 120 min,其余条件与投加量实验相同,进行吸附平衡实验,采用Langmuir(式(1))、Freundlich(式(2))和D-R(式(3))吸附等温线模型对 Na-BBT和PFA吸附过程进行拟合分析。
式中:qe为平衡吸附量,mg·g−1; ce为平衡时溶液中污染物的浓度,mg·L−1;qmax为最大吸附量,mg·g−1, KL为Langmuir系数; KF为Freundlich系数;n为Freundlich模型指数;β 为吸附能常数,mol2· kJ−1;R为一般气体常数,8.314 J· (mol·K)−1;T为绝对温度,K。
7)吸附动力学。吸附时间为 120 min,分别投加一定量Na-BBT和PFA(通过投加量实验结果得出最佳的投加量分别为2.0 g·L−1和0.5g·L−1),其余的条件与投加量实验相同,间隔一定时间进行取样,测定该时刻样品中 COD 和NH3-N去除率,采用 Lagergren 准一级和准二级动力学模型对 Na-BBT和PFA 吸附煤气化废水中COD 与NH3-N的过程进行吸附性能考察。
-
扫描电镜(SEM)分析采用扫描电镜(Zeiss SIGMA,德国卡尔蔡司公司)分析对吸附煤气化废水前后的吸附剂的表面形态变化情况。孔径与比表面积(BET)分析采用比表面积及孔隙分析仪(ASAP 2020,美国麦克仪器公司)测定吸附剂比表面积、总孔体积与孔径,在 180 ℃ 恒定温度下进行真空脱气,并进行多点 BET 分析,计算在 77.35 K 的高纯度液氮环境中的比表面积。傅立叶红外光谱分析(FT-IR)采用傅立叶红外光谱分仪(FTIR5700,美国赛默飞公司)对吸附煤气化废水前后吸附剂进行研究,扫描范围为 400~4 000 cm−1,步长为 4 cm−1,使用 OMNIC8.0 软件对原始光谱数据进行基线校正和归一化处理。X射线衍射分析(XRD)采用X射线衍射仪(XPert Pro,荷兰帕纳科公司)对吸附煤气化废水前后吸附剂进行研究其化学成分稳定性与晶形稳定性,扫描范围为 5°~80°。X射线荧光光谱(XRF)采用X射线荧光光谱仪(S4 Pioneer,德国布鲁克AXS有限公司)测定吸附煤气化废水前吸附剂主要化学氧化物。X-射线光电子能谱分析(XPS)测定采用X 射线光电子能谱分析仪(ESCALAB 250Xi,美国赛默飞公司)吸附煤气化废水前后吸附剂表面组成、价态变化以及元素成分。
-
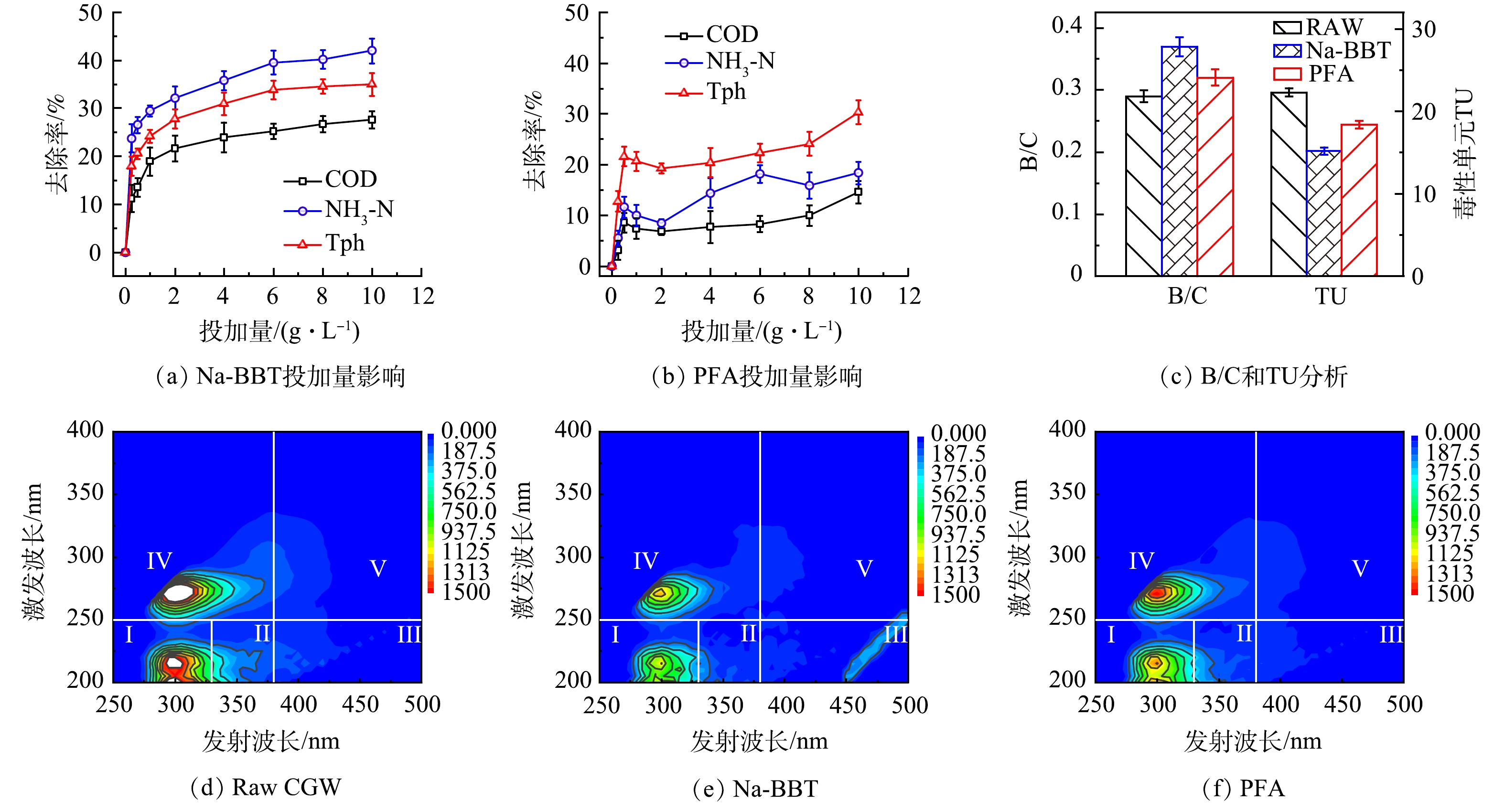
1)吸附剂Na-BBT 与PFA投加量对煤气化废水中COD、NH3-N和Tph去除效果的影响。由图1(a)可见,当吸附时间为 200 min, Na-BBT投加量为2.0 g·L−1时,COD 、NH3-N与Tph去除率分别达到 21.7%、32.2%和27.8%;当Na-BBT投加量为6.0 g·L−1时,COD 、NH3-N与Tph去除率分别达到 25.2%、39.6%和33.9%;随着投加量增加,去除率趋于平缓,在投加量为10 g·L−1时去除率分别为 27.6%、42.0%和34.9%。由图1(b)可知,当PFA投加量为0.5g·L−1时,COD 、NH3-N与Tph去除率分别达到 8.6%、11.6%和21.6%;当PFA投加量为6.0 g·L−1时,COD 、NH3-N与Tph去除率分别达到 8.3%、18.2%和22.3% ,随着投加量增加,污染物去除率稍有下降,之后再次上升并趋于平缓,当PFA投加量为10 g·L−1时去除率分别为14.5%、18.3%和30.3%。由图1(a)~(b)可见,Na-BBT去除效果比PFA好,投加量增加到一定程度后去除率上升不明显,但处理成本增加较多,需根据处理效果和处理成本的综合分析,选择适宜的投加量。
2)煤气化废水吸附前后的 B/C值和发光菌毒性(TU)分析。由图1(c)可见,未经预处理的煤气化废水B/C与TU 值分别为 0.29和22.3,属于难生物降解、高毒性废水[16]。经Na-BBT与PFA预处理后其B/C分别升至0.37和0.32;而生物急性毒性单元 TU 值分别降至15.2和18.4。这说明经预处理后,煤气化废水的可生化性得到提高,废水毒性得到降低。为了进一步分析煤气化废水可生化性差和生物毒性高的原因, 采用三维荧光光谱法对其预处理前后该废水中有机污染物的种类和组分进行分析。
3)三维荧光分析。根据不同种类有机物的荧光特点,对废水中的有机污染物进行定性分析,区域荧光积分法将三维荧光光谱划分为 5 个区域,5个区域分别为芳香类蛋白 I、芳香类蛋白 II、富里酸III、微生物代谢产物IV和腐殖酸V[17]。如图1(d)~(f)所示,煤气化废水荧光峰主要在 I 区和 IV 区。说明煤气化废水中有机污染物主要为芳香类蛋白和微生物代谢产物。微生物代谢产物也能代表类色氨酸、酪氨酸或者其残基[18]。如表1所示,经Na-BBT吸附后, I 区(芳香类蛋白 I)和 IV 区(微生物代谢产物)荧光强度分别降低了43.4% 和 45.2%, II 区的荧光强度(芳香类蛋白 II)降低 了43.2%;经PFA吸附后,Ⅰ和Ⅳ 区荧光强度分别降低了25.7%和30.5%,对 II 区的荧光强度仅降低了31.8%。表明Na-BBT对煤气化废水中芳香类蛋白和微生物代谢产物吸附效果较好,Na-BBT的吸附效果优于PFA,这与上述实验结果一致。
煤气化废水的三维荧光光谱主要荧光峰分布与酚类物质如苯酚、邻苯二酚等符合度较高,表明煤气化废水中主要有机污染物为酚类化合物,这与大部分研究一致[19-20]。为了进一步确定煤气化废水中酚类等特征污染物的去除效果,采用GC-MS对其进行分析。
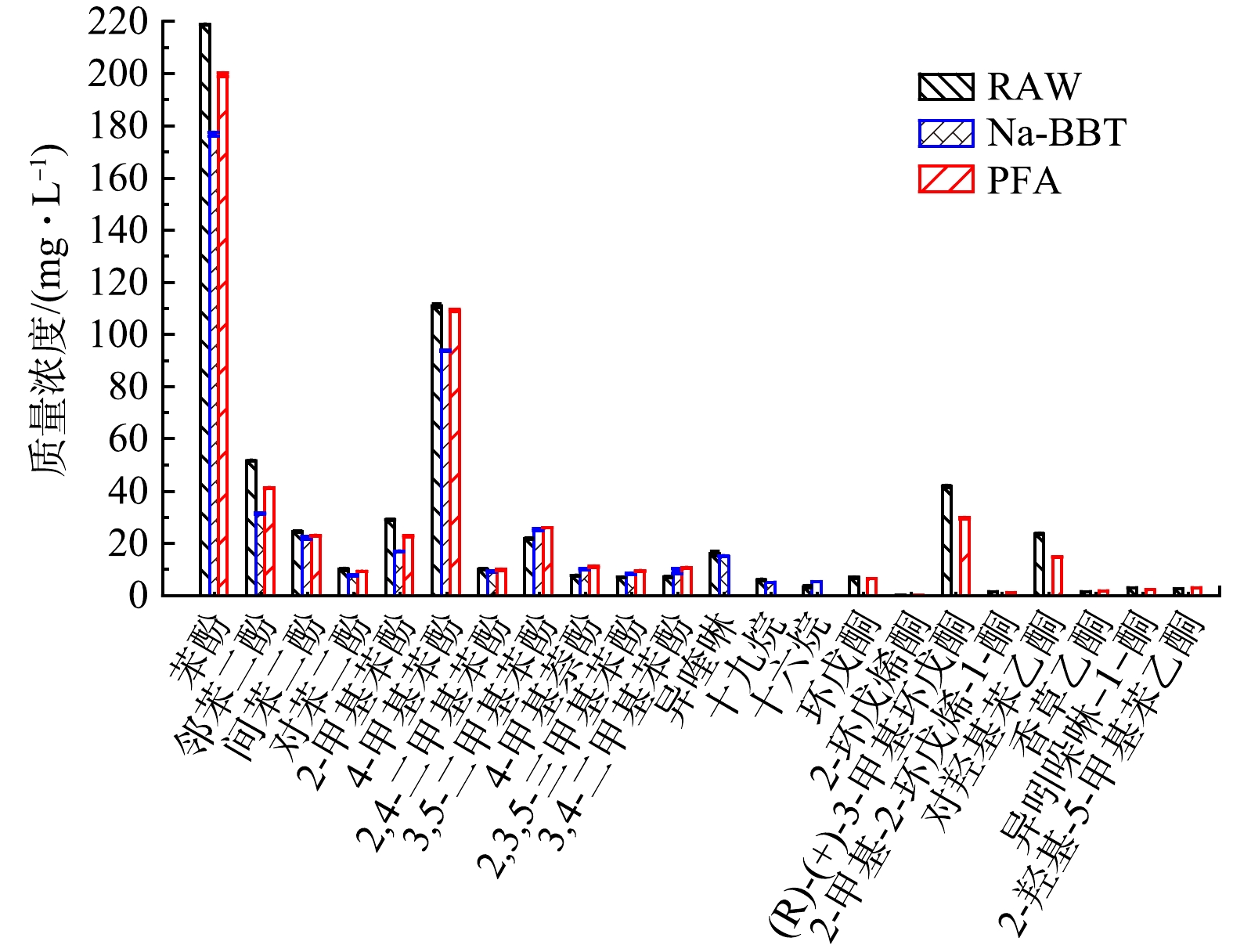
4) GC-MS分析。如图2所示,原煤气化废水酚类、酮类物质总占比分别为 65.5%和10.7%, 经Na-BBT、PFA吸附预处理后煤气化废水其酚类占比分别由原来的65.5%降低至 53.6%和62.0% 。酮类物质几乎被Na-BBT全部吸附,被PFA吸附占比由原来的10.7%降至7.8%。对于煤气化废水酚类和酮类含量较高的主要是苯酚、4-甲基苯酚与邻苯二酚和(R)-(+)-3-甲基环戊酮、对羟基苯乙酮,苯酚占比由原来的28.7%分别降至23.2%、26.2%;4-甲基苯酚由原占比的14.6%分别降至12.3%、14.3%;邻苯二酚由原占比的6.8%分别降至4.1%、5.4%;(R)-(+)-3-甲基环戊酮由原占比5.5%分别降至0.1%、3.9%;对羟基苯乙酮由原占比3.11%分别降至0.1%、1.9%,即Na-BBT和PFA主要吸附去除的是煤气化废水中苯酚、4-甲基苯酚、邻苯二酚、(R)-(+)-3-甲基环戊酮和对羟基苯乙酮等毒性物质。
-
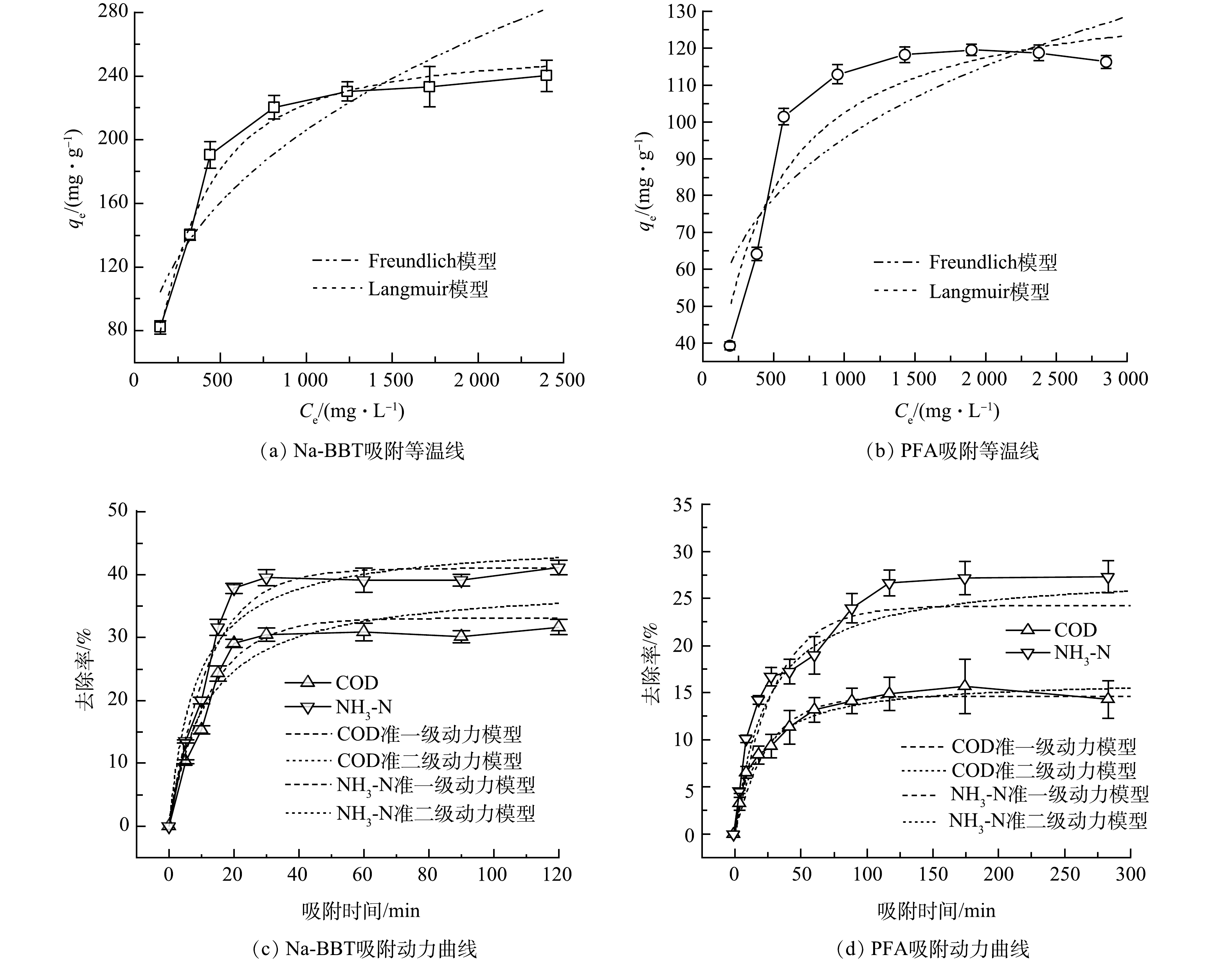
1)吸附等温线。吸附等温线结果如图3(a)、图3(b)和表2所示。由Na-BBT和PFA吸附煤气化废水中COD的R2 分析结果可知,Na-BBT、PFA对煤气化废水的吸附更符合 Langmuir 模型,结合前面对Na-BBT和PFA的实验分析可知,Na-BBT与PFA固体表面有大量的吸附活性中心,当其表面吸附活性中心全部被占满时,COD吸附量达到饱和值qe,遵循单分子层分布。由表2可见,Langmuir 吸附模型中Na-BBT、PFA的qmax分别为257.48 mg·g−1和137.46 mg·g−1,Na-BBT的吸附容量约为PFA的2倍。另外,通过 Dubinin-Radushkevich(D-R) 模型活性系数β计算了吸附能量 E,
E=1√2β , E 值可以在一定程度上判定吸附过程的理化属性。一般认为:E<8 kJ·mol−1,吸附以物理作用为主;8 kJ·mol−1<E<16 kJ·mol−1,吸附以离子交换作用为主;20 kJ·mol−1<E<40 kJ·mol−1,吸附以化学作用为主。Na-BBT和PFA的E值分别为0.017 kJ·mol−1和0.013 kJ·mol−1,可以初步判断Na-BBT和PFA是以物理吸附为主。2)吸附动力学分析。由图3(c)~(d)动力学拟合结果可知,随着吸附时间增加,Na-BBT与PFA吸附煤气化废水的 COD 和 NH3-N 速率迅速增加,分别在 20 min 和120min时达到吸附平衡。Na-BBT对 COD 和 NH3-N吸附率分别为 29.1% 和 37.8%;PFA对 COD 和 NH3-N 吸附率分别为 15.7% 和 27.1%。经Na-BBT吸附后煤气化废水的COD与 NH3-N准一级动力学R2分别为0.957 1和0.984 3; PFA吸附煤气化废水的COD和 NH3-N准一级动力学R2分别为0.970 2和0.984 0;Na-BBT吸附煤气化废水中COD和 NH3-N准二级动力学R2为0.900 9和 0.975 0;PFA吸附煤气化废水的COD和 NH3-N准二级动力学R2为0.942 2和0.963 1。Na-BBT与PFA吸附煤气化废水的过程更符合准一级动力学模型。准一级动力学模型的线性关系表明(Na-BBT 吸附时间<20 min 、PFA吸附时间<120min时),吸附速率与吸附剂浓度之间存在简单的比例关系,这种关系符合物理吸附过程中的Langmuir吸附模型,吸附为可逆过程,并且吸附位点间的相互作用较弱。因此,Na-BBT与PFA吸附煤气化废水中COD与 NH3-N,是以物理吸附为主,且Na-BBT的吸附速率远远大于PFA。
-
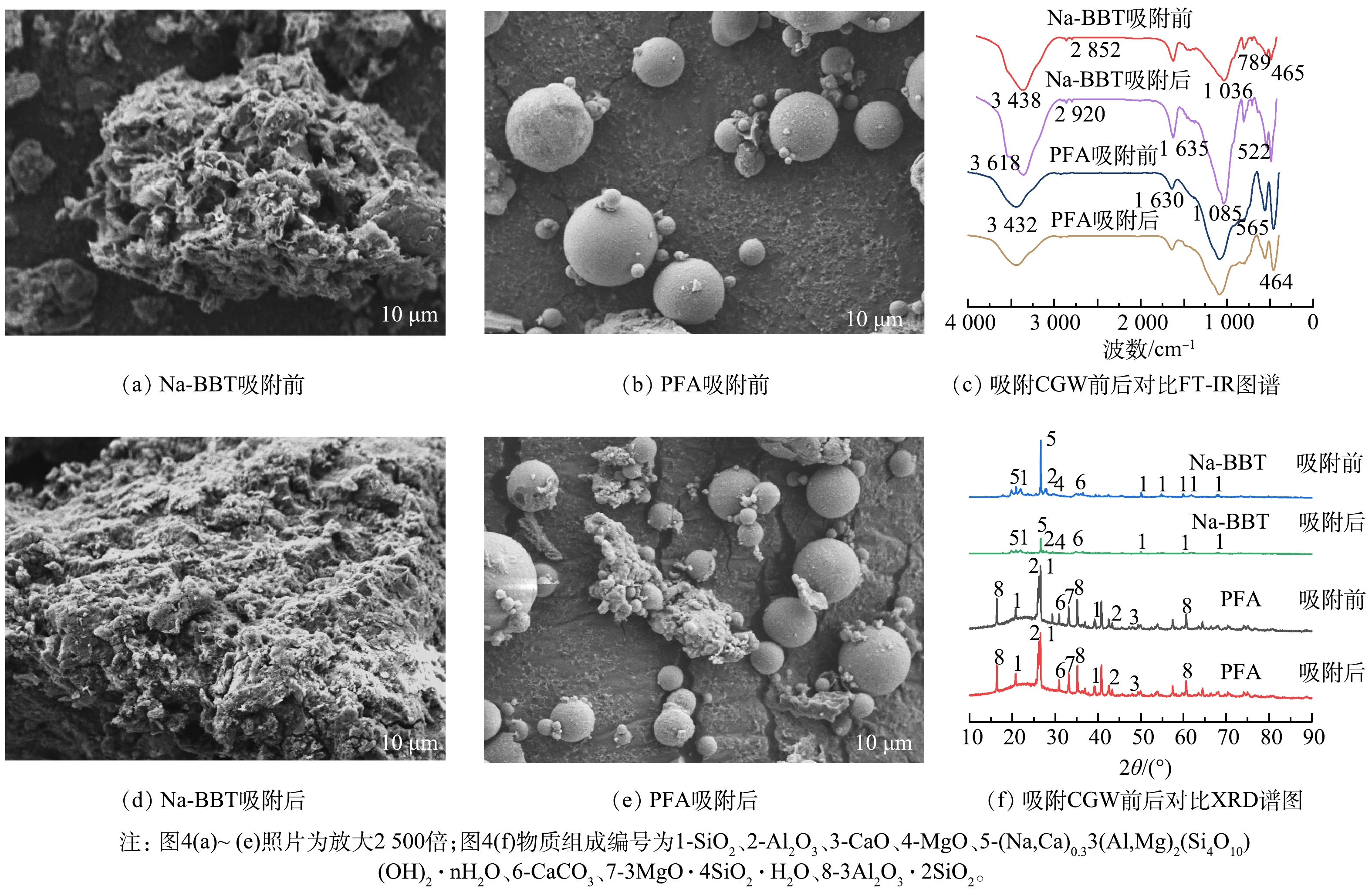
1)扫描电镜(SEM):由图4(a)可知,Na-BBT具有不规则的片层状,边缘有轻微卷曲,并且相互堆叠形成集合体[14],具有疏松多孔结构;由图4(b)可知,PFA以球状结构为主,其表面较粗糙,但Na-BBT具有更凹凸的表观形貌。吸附煤气化废水后,Na-BBT表面的部分孔隙被填充,片层状形态减少,但轮廓边界仍比较明显,表观形貌未有大的变化(图4(d));PFA表观形态亦未发生明显变化[16](图4e),其表面发生颗粒状物质团聚现象,这在一定程度上会影响其吸附效果[21]。
2)孔径与比表面积(BET)分析。Na-BBT、PFA的比表面积分别为365.0 m2·g−1、22.1 m2·g−1;总孔体积分别为0.15 cm3·g−1 、0.05 cm3·g−1;孔径分别为8.22 nm、8.32 nm。Na-BBT与PFA的孔径大小接近,但Na-BBT的比表面积和总孔体积远远大于PFA(分别相差16.5倍和3倍)。这也验证了SEM图像显示的Na-BBT表观形貌(存在较多微观的凹凸结构,更大的比表面积和总孔体积),可为Na-BBT吸附剂提供了更多的吸附位点,这也是Na-BBT的吸附容量大于PFA的原因(表2),而且从吸附动力学分析,Na-BBT的吸附速率也大于PFA(图3)。
3)傅立叶红外光谱(FT-IR)分析。由图4(c)可以看出,Na-BBT吸附煤气化废水前后在2 920 cm−1 出现了 —CH3的C-H反对称伸缩振动和2 852 cm−1 处出现了—CH3 的C—H 对称伸缩振动特征峰[22]。在3 438 cm−1和3 618 cm−1观察到其特征峰为游离的O—H。1 635 cm−1 处的峰值为弯曲振动峰,被认为是C=C和C=O的伸缩振动,说明存在含氧官能团。789 cm−1和 1 036 cm−1 分别对应Al—O和Si—O基团,形成Na-BBT的基本Si—O四面体骨架和 Al—O 八面体骨架[23]。Si—O—Mg 和 Si—O—Fe 的拉伸振动峰分别出现在522 cm−1 和465 cm−1 处。Na-BBT结构中形成了较强的Si—O键,由于 Na+ 部分进入Si—O 层,导致层间电荷减少,静电相互作用减弱,从而增强Si—O键能。而较强的Si—O键能增强了Na-BBT表面的吸附。对于PFA (图4(c)),464 cm−1 处的峰代表O—Si—O的弯曲振动,565 cm−1 处的峰归因于Fe—O,1 085 cm−1 处的宽带由对称和SiO4或AlO4四面体的不对称拉伸振动[24]。PFA 在1 630 cm−1 处的峰是由H2O中的O-H弯曲振动引起的,在3 432 cm−1 处的峰是由羟基或H2O中的O-H伸缩振动引起的,且吸附前较吸附后O—H的吸收峰强,表明PFA可以吸附煤气化废水中的酚类化合物[25-26]。Na-BBT与PFA吸附煤气化废水前后的峰形基本一致,表明吸附前后它们的其内部结构较为稳定,结构未发现明显破坏,也未发现有新物质的生成。
4) X 射线衍射(XRD)分析。由图4(f)可知,Na-BBT主要特征衍射峰是SiO2、Al2O3、MgO、(Na,Ca)0.33(Al,Mg)2(Si4O10)(OH)2·nH2O、CaCO3,而PFA的主要特征衍射峰为SiO2、Al2O3、CaO、CaCO3、3MgO·4SiO2·H2O、3Al2O3·2SiO2等,吸附煤气化废水后它们的特征衍射峰的种类和数量无明显变化,但吸附后其特征衍射峰强度有一定程度的减低,尤其是Na-BBT的特征衍射峰(Na,Ca)0.33(Al,Mg)2(Si4O10)(OH)2·nH2O强度降低明显,而PFA特征衍射峰降低不明显。
5) X射线荧光光谱(XRF):Na-BBT中氧化物主要为SiO2、MgO、Fe2O3、CaO、Al2O3、K2O、Na2O,分别占比59.1%、16.7%、3.7%、1.1%、3.2%、0.1%、6.6%;PFA主要氧化物为MgO、Na2O、Fe2O3、CaO、SiO2、Al2O3、K2O分别占比13.1%、1.3%、12.5%、11.6%、38.0%、20.7%、1.2%。
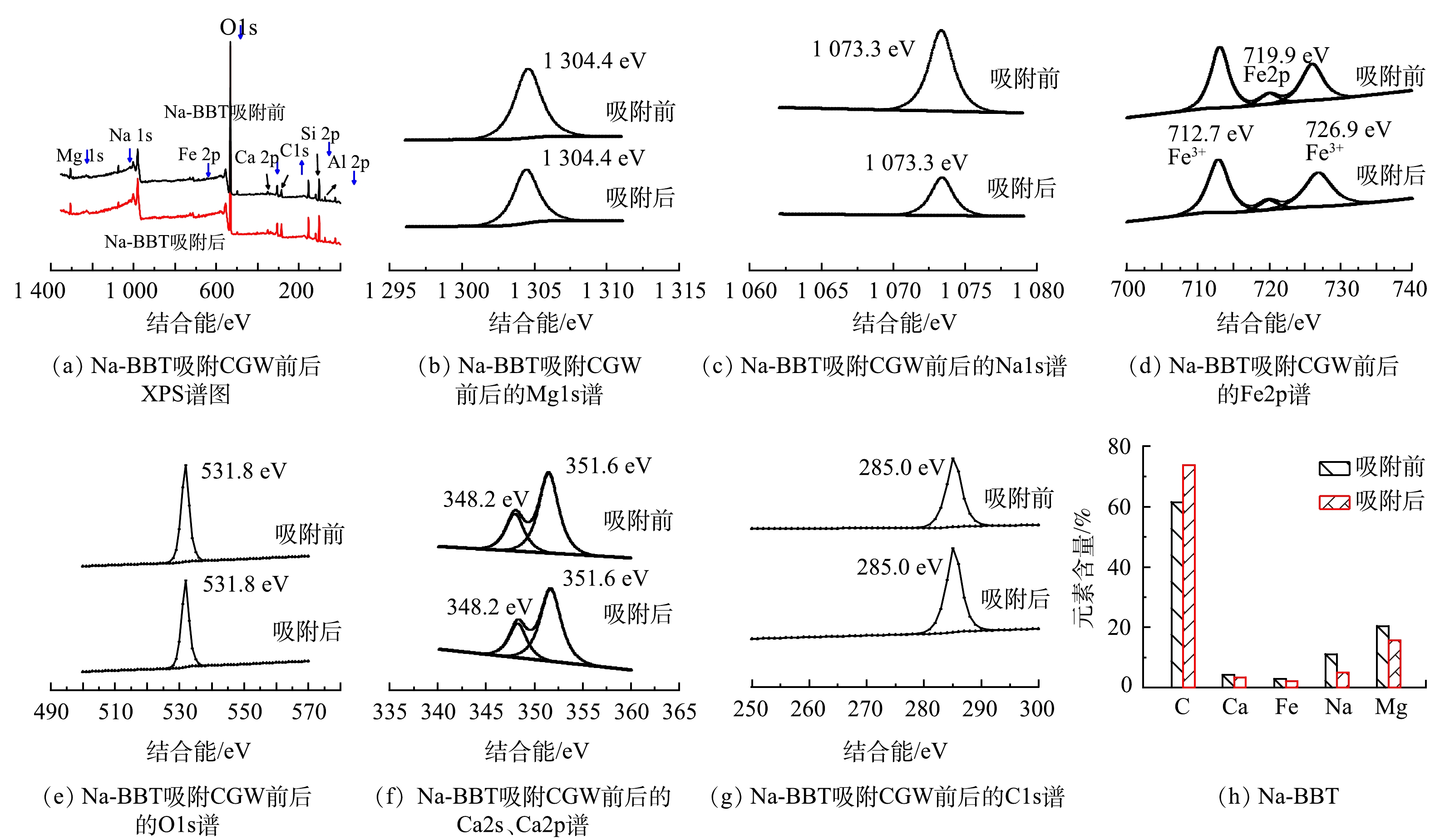
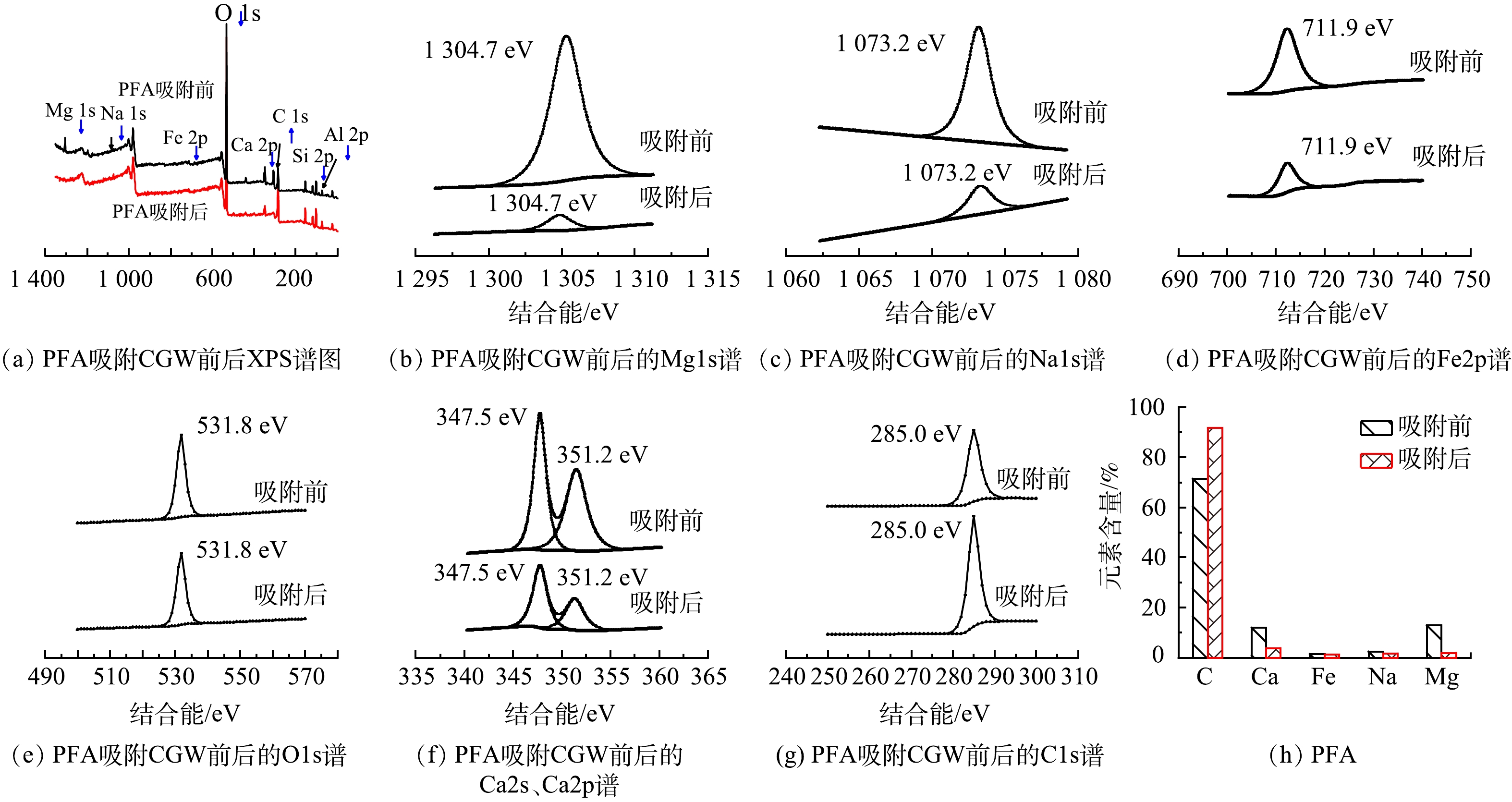
6) X射线电子能谱(XPS)分析。由图5(a)~(g)可见,Mg1s、Na1s、O1s和C1s的特征峰为单峰,分别为1 304.4、1 073.3、531.8和285.0 eV,而Ca和Fe特征峰为多峰,Ca1s和Ca2p的特征峰分别为348.2 eV和351.6 eV,Fe2p的特征峰分别为712.7 eV和726.9 eV,主要以三价铁的形式存在。吸附剂Na-BBT吸附后Mg、Na 、Fe、O、Ca等特征峰信号强度降低,C特征峰信号强度增加,吸附前后O 特征峰信号最强,但吸附后强度有所降低。由图5(h)可知,Na-BBT吸附煤气化废水后Mg、Na、Fe、Ca元素含量分别由原来的11.1%、20.5%、 2.7%、4.2% 降至5.0%、15.7%、2.2%、3.4%,Mg和Na含量降低明显;而 C元素含量由原来的61.4%增至73.7%。
由图6(a)~(g)发现,Mg1s、Na1s、Fe2p、O1s和C1s的特征峰为单峰,分别为1 304.7、1 073.2、711.9、531.8和285.0 eV,而Ca特征峰为多峰,Ca1s和Ca2p峰值分别为347.5 eV、351.2 eV。吸附剂PFA吸附后Mg、Na 、Fe、O、Ca等特征峰信号强度降低,C特征峰信号强度增加,吸附前后O 特征峰信号最强,但吸附后强度有所降低。由图6(h)可知,PFA吸附煤气化废水后Mg、Na、Fe、Ca元素含量分别由原来的12.9%、2.3%、 1.4%、11.9%降至1.9%、1.6%、1.1%、3.9%,Mg和Ca含量降低明显;而 C元素含量由原来的71.5%增至91.6%。
Na-BBT和PFA吸附煤气化废水后C1s的特征峰强度增大,C元素含量增加,推测原因是因为煤气化废水有机污染物(如酚类和酮类)被两种吸附剂吸附在吸附剂表面,导致吸附剂表面的C含量增加,而Mg、Na、Fe、Ca特征峰强度降低,且对应元素含量降低,推测煤气化废水中阳离子污染物(如NH4+)可通过与Mg2+、Na+、Ca2+等进行阳离子交换被固定在吸附质表面。但由于在XPS检测中,N元素通常以氮化物或氨基氮(NH4+)等形式存在,由于N原子的光电子能谱峰位分布在较低能量端,往往与C1s或O1s等元素的光电子峰重叠较为严重,单独测量和定量分析N峰值非常困难,曾对N峰进行过分析,但N峰值特别弱,所以没有添加N峰在XPS图中。但从图1(a)和图1(b)可以看出,在投加量为6 g·L−1 (XPS分析时吸附时用的投加量)时,Na-BBT、PFA的吸附去除率分别为39.6%、18.2%,推测氨氮的吸附去除机理:煤气化废水中的NH3-N主要以NH4+形式存在,被Na-BBT和PFA吸附在吸附剂表面之后,一部分NH4+进一步与Na-BBT和PFA吸附剂中的阳离子(如Mg2+ 、Na+、 Ca2+等)发生离子交换吸附,在低价离子中,NH4+通常具有很强的离子交换能力,且离子交换能力为NH4+ > Mg2+ > Ca2+ > Na+,这是因为NH4+的电荷较小,与水分子之间的水合作用相对较弱。因此,Na-BBT和PFA对煤气化废水中NH3-N的吸附除了物理吸附,还存在离子交换吸附[27]。
-
1)Na-BBT和PFA对煤气化废水中的COD、NH3-N与Tph等污染物的去除率虽然不高,但可提升煤气化废水的可生化性,降低其毒性。经Na-BBT和PFA吸附后,煤气化废水B/C由0.29分别提高至0.37和0.32;毒性单元 TU 值由22.3 分别降低至 15.2和18.4。Na-BBT和PFA主要吸附去除的是煤气化废水中的芳香类蛋白物质和微生物代谢产物,包括煤气化废水中苯酚、4-甲基苯酚、邻苯二酚、(R)-(+)-3-甲基环戊酮和对羟基苯乙酮等毒性物质。
2)通过吸附等温线分析可知Na-BBT和PFA吸附煤气化废水中COD的过程符合Langmuir 吸附模型,符合单分子层吸附;通过D-R 模型活性系数β计算的吸附能量 E(<0.02),可以初步判断Na-BBT与PFA是以物理吸附为主。Na-BBT与PFA吸附煤气化废水的COD与 NH3-N遵循准一级动力学模型,进一步验证Na-BBT和PFA吸附煤气化废水的COD与 NH3-N过程是物理吸附过程,且Na-BBT与PFA相比,具有更大的吸附容量和更快的吸附速率。
3)相比PFA,Na-BBT具有更大的比表面积和孔体积;吸附后Na-BBT和PFA的内部结构较为稳定,未发现明显破坏,也未发现有新物质的生成。2种吸附剂可吸附去除煤气化废水中一些酚类、酮类、氨氮等污染物,吸附过程主要受物理扩散控制,对有机物的吸附主要为表面和孔道的物理吸附;吸附后Na-BBT和PFA中Ca、Fe、Na、Mg 等元素含量降低,而C含量增加,可推测煤气化废水中NH3-N的去除,除了物理吸附,NH4+还可以与Mg2+、Na+、Ca2+等进行阳离子交换吸附。
4)Na-BBT和PFA可有效提高煤气化废水的可生化性,降低毒性,达到了预处理的目的,Na-BBT的吸附效果优于PFA,可优先考虑将Na-BBT用于煤气化废水的预处理,具有潜在的、广泛的、实际应用前景。
钠基膨润土、粉煤灰吸附预处理煤气化废水效果及机理
Adsorption effect and mechanism of sodium bentonite and pulveized fuel ash on pretreatment of coal gasification wastewater
-
摘要: 煤气化废水具有可生化性差,酚类物质含量高、毒性强的特点,导致生物处理效果不理想。以煤气化废水厌氧段进水为研究对象,分别以钠基膨润土(Na-BBT)与粉煤灰(PFA)作为吸附剂,探究其对煤气化废水中主要特征污染物的去除效果和吸附机理。结果表明:Na-BBT、PFA对煤气化废水中的化学需氧量(COD)、氨氮(NH3-N)与总酚(total phenols,Tph)有一定的去除效果,其可生化性得到提升,经过Na-BBT和PFA处理后,BOD5/COD由0.29分别提高至0.37和0.32;废水中的NH3-N由221.3 mg·L−1分别降至128.6 mg·L−1和180.8 mg·L−1,总酚由554.8 mg·L−1分别降至360.6 mg·L−1和386.7 mg·L−1。气相色谱质谱(GC-MS)分析结果表明,去除的主要物质为苯酚、4-甲基苯酚、邻苯二酚、(R)-(+)-3-甲基环戊酮和对羟基苯乙酮等毒性物质,废水毒性得到降低,毒性单元(toxic unit,TU)值由 22.3 分别降至 15.2和18.4。通过吸附等温和吸附动力学分析可知,Na-BBT和PFA对煤气化废水的吸附符合 Langmuir模型和准一级动力学模型;三维荧光光谱、孔径比表面积(BET)、扫描电镜(SEM)、红外光谱(FT-IR)、X 射线衍射(XRD)、X射线荧光光谱(XRF)和X射线电子能谱(XPS)等分析结果表明,Na-BBT和PFA对煤气化废水中污染物的吸附去除过程和机理主要受物理扩散控制,为表面和孔道的物理吸附,同时也存在离子交换吸附。以上结果表明Na-BBT和PFA作为吸附剂,可以有效提高煤气化废水的可生化性,降低毒性,将其用于煤气化废水的预处理,具有潜在和广泛的应用前景。Abstract: Coal gasification wastewater is characterized by poor biodegradability, high phenolic content and strong toxicity, which leads to its unsatisfactory biological treatment effect. In this study, the anaerobic section influent of coal gasification wastewater was taken as the research object, the removal effect and adsorption mechanism on the main characteristic pollutants were absorbed by Na-bentonite (Na-BBT) and pulveized fuel ash (PFA). The result showed that Na-BBT and PFA could remove COD, NH3-N and The total phenol (Tph) from coal gasification wastewater to a certain extent, and the biodegradability of wastewater could be improved because BOD5/COD increased from 0.29 to 0.37 and 0.32, respectively, NH3-N in wastewater decreased from 221.3 mg·L−1 to 128.6 mg·L−1 and 180.8 mg·L−1, and Tph decreased from 554.8 mg·L−1 to 360.6 mg·L−1 and 386.7 mg·L−1, respectively. Gas chromatography mass spectrometry (GC-MS) results indicated that the main toxic substances such as phenol, 4-methylphenol, catechol, (R)-(+)-3-methylcyclopentanone and p-hydroxyacetophenone were removed, and the toxicity of coal gasification wastewater was reduced because the toxic unit TU value decreased from 22.3 to 15.2 and 18.4, respectively. When Na-BBT and PFA removed the pollutants from coal gasification wastewater, the adsorption process was consistent with Langmuir model and quasi-first-order kinetic model through analysis of adsorption isotherms and adsorption kinetics. It was found that the adsorption process and mechanism of Na-BBT and PFA removed pollutants from coal gasification wastewater were mainly controlled by physical diffusion due to their surfaces and pores, also including ion exchange adsorption based on the analysis of three-dimensional fluorescence spectrum, pore specific surface area (BET), scanning electron microscope (SEM), infrared spectroscopy (FT-IR), X-ray diffraction (XRD) , X-ray fluorescence spectrum (XRF) and X-ray electron spectroscopy (XPS). The results showed that Na-BBT and PFA, as adsorbents, could effectively improve the biodegradability and reduce the toxicity of coal gasification wastewater, which had potential, extensive, and practical application prospects for the pre-treatment of coal gasification wastewater.
-
Key words:
- Na-bentonite /
- pulveized fuel ash /
- coal gasification wastewater /
- adsorption /
- mechanism
-
在我国的经济发展过程中,煤炭使用不可或缺,地位不可替代[1],但在使用时会造成大气污染,为了预防或减少煤燃料对环境和人体健康带来的危害,洗煤、洁净煤技术应运而生,煤的气化技术[2]为其中之一,但煤炭在气化过程中会产生大量的煤气化废水(coal gasification wastewater,CGW),该废水的主要特点是可生化性差,酚类物质含量高、毒性强、有机物浓度高、色度深,属于难处理工业废水[2-3]。
煤气化废水通常采用生物组合工艺(如厌氧、缺氧和好氧)处理[4]。但在生物处理厌氧段的废水存在可生化性差、处理效率低和出水毒性大等问题,导致后续处理工艺运行不稳和出水污染物浓度高。为了提高厌氧段的可生化性,提高污染物的去除效率,降低出水毒性(如降低酚类污染物含量),寻求高效且低成本的预处理方法势在必行。吸附法因其成本低、处理效果好、处理工艺简单、可实现废物资源化等优势,在工业废水的预处理中得到较多的研究,吸附法的使用吸附剂的选择尤为重要,直接影响吸附效果和使用成本。钠基膨润土(Na-Bentonite,Na-BBT)与粉煤灰(pulveized fuel ash,PFA)具有天然无害、价格低廉、来源丰富等优点,是一种潜在的廉价吸附剂[5-7],具有广阔的应用前景,被常用于工业废水处理[8-11]。罗秋艳等[12]使用Na-BBT吸附模拟酚类废水中的2,4,6-三氯苯酚,以物理吸附为主,吸附容量达到16.90 mg·g−1;BATABYAL[13]等用PFA吸附模拟废水中2,4-二甲基苯酚,结果表明,吸附过程符合准一级动力学模型。KHANSAA[14]用Na-BBT吸附处理橄榄油废水中的总酚类化合物,最大平衡吸附量为81.323 mg·g−1;赵伟高等[15]用PFA吸附处理焦化废水,以物理吸附为主,挥发酚的最大平衡吸附量为 39.5 mg·g−1。Na-BBT和PFA对酚类物质的吸附等温线均符合Langmuir模型[12-14]。目前,使用Na-BBT和PFA作为吸附材料对含酚废水吸附应用有一些研究,但在煤气化废水预处理领域还没有相关应用,尤其是对于复杂的、实际的煤气化废水中种类繁多的酚类化合物吸附去除效果研究较少,没有对其酚类物质的吸附去除机理进行深入研究。
鉴于此,本研究以实际煤气化废水厌氧段进水为研究对象,以Na-BBT和PFA作为吸附剂,对比分析了2种吸附剂对煤气化废水中主要的特征污染物(酚类物质)的吸附效果,探讨了煤气化废水的可生化性和毒性改变原因,并揭示了其吸附机理,以期为后续相关研究提供参考。
1. 材料与方法
1.1 实验材料
1)实验试剂:重铬酸钾(K2Cr2O7)、硫酸(H2SO4)、硫酸银(Ag2SO4)、硫酸汞(HgSO4)、硫酸亚铁铵((NH4)2Fe(SO4)2·6H2O)、盐酸(HCl)、碘化钾(KI)、碘化汞(HgI2)、氢氧化钾(KOH)、酒石酸钾钠(NaKC4H6O6)、硫代硫酸钠(Na2S2O3)、氢氧化钠(NaOH)、硼酸(H3BO3)、氯化铵(NH4Cl)、磷酸二氢钾(KH2PO4)、磷酸氢二钾(K2HPO4)、七水合磷酸氢二钠(Na2HPO4·7H2O)、七水合硫酸镁(MgSO4·7H2O)、氯化钙(CaCl2)、六水合氯化铁(FeCl3·6H2O)、亚硫酸钠(Na2SO3)、过硫酸钾(K2S2O8)、硝酸钾(KNO3)、硝酸(HNO3)、钼酸铵(NH4MoO4)、抗坏血酸(C6H8O6)、七水硫酸亚铁(FeSO4·7H2O)、五水硫酸铜(CuSO4·5H2O)、乙醚(C4H10O)、磷酸(H3PO4)、氯化钠(NaCl)、氯化镁(MgCl2)、氯化钙(CaCl2)、氯化汞(HgCl2)等购自国药集团化学试剂有限公司,以上药品均为分析纯。
2)吸附材料及水质指标。吸附剂Na-BBT和PFA,购自河南郑州华晶化工有限公司。所有吸附材料在使用前用纯水充分清洗去除杂质,然后在105 ℃的真空干燥箱(DZF-6020A,郑州宝晶电子科技有限公司)中干燥 6 h,得到的固体进一步研磨,筛分至 200 目。实验所采用的实际煤气化废水取自内蒙古大唐克旗煤制天然气有限责任公司厌氧工艺段进水,其中COD为3 302.5 mg·L−1 、五日生化需氧量(biochemical oxygen demand after 5 days, BOD5)为957.7 mg·L−1、BOD5/COD(B/C)为0.29、 总有机碳(total organic carbon, TOC)为1 395.3 mg·L−1、氨氮(NH3-N)为221.3 mg·L−1、总氮(total nitrogen, TN)为245.6 mg·L−1、总磷(total phosphorus, TP)为2.0 mg·L−1、总酚(total phenols, Tph)为554.8 mg·L−1、pH为7.7、电导率为1 856 μS·cm−1。
1.2 实验方法
1)水质分析:水样指标测定均在25 ℃下。 COD 采用重铬酸盐法(HJ 828-2017);NH3-N 采用纳氏试剂分光光度法(HJ 535-2009);BOD5采用稀释与接种法(HJ 505-2009);TOC采用TOC-Lcph总有机碳析仪(ASK2-4,日本岛津制作所)测量;TN采用碱性过硫酸钾消解紫外分光光度法(HJ 636-2012);TP采用钼酸铵分光光度法(GB 11893-89);Tph采用溴化容量法(HJ 502-2009);pH使用pH仪(SG-2,美国哈希公司);电导率使用电导仪(S40d,美国哈希公司),均采用玻璃电极法。
2)Na-BBT 和PFA投加量对煤气化废水中BOD5、COD、NH3-N和Tph去除效果的影响以及分析B/C值的变化。在 25 ℃、 250 mL 煤气化废水中分别改变Na-BBT和PFA投加量(0.25、0.5、1、2、4、6、8、10 g·L−1),置于恒温摇床,设置180 r·min−1、吸附时间为 200 min,吸附后过0.45 μm滤膜,测定BOD5、COD、NH3-N和Tph值,计算其去除率和B/C值,确定吸附剂的投加量。
3)发光菌毒性分析:吸附剂Na-BBT 与PFA投加量为6 g·L−1,其他实验条件与投加量实验相同,采用多功能酶标仪(MDM9000,株式会社丽光制造所)对废水中发光强度(RLU)进行测试,采用水质急性毒性的测定发光细菌法(GB/T15441-1995),监测吸附前后煤气化废水发光菌生物急性毒性单元TU(Toxic Unit)值。
4)三维荧光分析。实验条件与发光菌毒性分析实验相同,实际煤气化废水和被吸附处理后的废水稀释1 500 倍至 TOC<1 mg·L−1,采用三维荧光光谱仪(F-7000,株式会社日立制造所)测试重复三次,样品的荧光光谱使用仪器内置软件处理,减去超纯水的空白信号,使用区域荧光积分方法(FRI)进行三维荧光分析,并用 Matlab2019b 处理和绘制数据。
5)GC-MS(Gaschromatography-mass spectrometry)分析。实验条件与发光菌毒性分析实验相同,采用气相色谱质谱仪(7890A 597C,美国安捷伦公司)对煤气化废水中酚类等特征污染物测试,监测煤气化吸附前后的特征污染物酚类、酮类物质变化。
6)吸附等温线。在吸附时间为 120 min,其余条件与投加量实验相同,进行吸附平衡实验,采用Langmuir(式(1))、Freundlich(式(2))和D-R(式(3))吸附等温线模型对 Na-BBT和PFA吸附过程进行拟合分析。
Ceqe=Ceqmax+1KLqmax (1) lnqe=lnKF+1nlnCe (2) lnqe=lnqmax−βε2(ε=RTln(1+1Ce)) (3) 式中:qe为平衡吸附量,mg·g−1; ce为平衡时溶液中污染物的浓度,mg·L−1;qmax为最大吸附量,mg·g−1, KL为Langmuir系数; KF为Freundlich系数;n为Freundlich模型指数;β 为吸附能常数,mol2· kJ−1;R为一般气体常数,8.314 J· (mol·K)−1;T为绝对温度,K。
7)吸附动力学。吸附时间为 120 min,分别投加一定量Na-BBT和PFA(通过投加量实验结果得出最佳的投加量分别为2.0 g·L−1和0.5g·L−1),其余的条件与投加量实验相同,间隔一定时间进行取样,测定该时刻样品中 COD 和NH3-N去除率,采用 Lagergren 准一级和准二级动力学模型对 Na-BBT和PFA 吸附煤气化废水中COD 与NH3-N的过程进行吸附性能考察。
1.3 Na-BBT与PFA表征分析
扫描电镜(SEM)分析采用扫描电镜(Zeiss SIGMA,德国卡尔蔡司公司)分析对吸附煤气化废水前后的吸附剂的表面形态变化情况。孔径与比表面积(BET)分析采用比表面积及孔隙分析仪(ASAP 2020,美国麦克仪器公司)测定吸附剂比表面积、总孔体积与孔径,在 180 ℃ 恒定温度下进行真空脱气,并进行多点 BET 分析,计算在 77.35 K 的高纯度液氮环境中的比表面积。傅立叶红外光谱分析(FT-IR)采用傅立叶红外光谱分仪(FTIR5700,美国赛默飞公司)对吸附煤气化废水前后吸附剂进行研究,扫描范围为 400~4 000 cm−1,步长为 4 cm−1,使用 OMNIC8.0 软件对原始光谱数据进行基线校正和归一化处理。X射线衍射分析(XRD)采用X射线衍射仪(XPert Pro,荷兰帕纳科公司)对吸附煤气化废水前后吸附剂进行研究其化学成分稳定性与晶形稳定性,扫描范围为 5°~80°。X射线荧光光谱(XRF)采用X射线荧光光谱仪(S4 Pioneer,德国布鲁克AXS有限公司)测定吸附煤气化废水前吸附剂主要化学氧化物。X-射线光电子能谱分析(XPS)测定采用X 射线光电子能谱分析仪(ESCALAB 250Xi,美国赛默飞公司)吸附煤气化废水前后吸附剂表面组成、价态变化以及元素成分。
2. 结果与讨论
2.1 吸附预处理对煤气化废水有机污染物去除效果
1)吸附剂Na-BBT 与PFA投加量对煤气化废水中COD、NH3-N和Tph去除效果的影响。由图1(a)可见,当吸附时间为 200 min, Na-BBT投加量为2.0 g·L−1时,COD 、NH3-N与Tph去除率分别达到 21.7%、32.2%和27.8%;当Na-BBT投加量为6.0 g·L−1时,COD 、NH3-N与Tph去除率分别达到 25.2%、39.6%和33.9%;随着投加量增加,去除率趋于平缓,在投加量为10 g·L−1时去除率分别为 27.6%、42.0%和34.9%。由图1(b)可知,当PFA投加量为0.5g·L−1时,COD 、NH3-N与Tph去除率分别达到 8.6%、11.6%和21.6%;当PFA投加量为6.0 g·L−1时,COD 、NH3-N与Tph去除率分别达到 8.3%、18.2%和22.3% ,随着投加量增加,污染物去除率稍有下降,之后再次上升并趋于平缓,当PFA投加量为10 g·L−1时去除率分别为14.5%、18.3%和30.3%。由图1(a)~(b)可见,Na-BBT去除效果比PFA好,投加量增加到一定程度后去除率上升不明显,但处理成本增加较多,需根据处理效果和处理成本的综合分析,选择适宜的投加量。
2)煤气化废水吸附前后的 B/C值和发光菌毒性(TU)分析。由图1(c)可见,未经预处理的煤气化废水B/C与TU 值分别为 0.29和22.3,属于难生物降解、高毒性废水[16]。经Na-BBT与PFA预处理后其B/C分别升至0.37和0.32;而生物急性毒性单元 TU 值分别降至15.2和18.4。这说明经预处理后,煤气化废水的可生化性得到提高,废水毒性得到降低。为了进一步分析煤气化废水可生化性差和生物毒性高的原因, 采用三维荧光光谱法对其预处理前后该废水中有机污染物的种类和组分进行分析。
3)三维荧光分析。根据不同种类有机物的荧光特点,对废水中的有机污染物进行定性分析,区域荧光积分法将三维荧光光谱划分为 5 个区域,5个区域分别为芳香类蛋白 I、芳香类蛋白 II、富里酸III、微生物代谢产物IV和腐殖酸V[17]。如图1(d)~(f)所示,煤气化废水荧光峰主要在 I 区和 IV 区。说明煤气化废水中有机污染物主要为芳香类蛋白和微生物代谢产物。微生物代谢产物也能代表类色氨酸、酪氨酸或者其残基[18]。如表1所示,经Na-BBT吸附后, I 区(芳香类蛋白 I)和 IV 区(微生物代谢产物)荧光强度分别降低了43.4% 和 45.2%, II 区的荧光强度(芳香类蛋白 II)降低 了43.2%;经PFA吸附后,Ⅰ和Ⅳ 区荧光强度分别降低了25.7%和30.5%,对 II 区的荧光强度仅降低了31.8%。表明Na-BBT对煤气化废水中芳香类蛋白和微生物代谢产物吸附效果较好,Na-BBT的吸附效果优于PFA,这与上述实验结果一致。
表 1 荧光区域积分和占比Table 1. Integration and proportion of fluorescence region样品 区域积分/(×105) 区域占比/% I II III IV V I II III IV V 原水 1.75 0.44 0.25 2.10 0.48 55.4 22.3 5.2 13.6 3.4 Na-BBT 0.99 0.25 0.51 1.15 0.42 48.2 19.4 16.5 11.4 4.5 PFA 1.30 0.30 0.19 1.46 0.40 56.8 21.3 5.6 13.0 3.9 煤气化废水的三维荧光光谱主要荧光峰分布与酚类物质如苯酚、邻苯二酚等符合度较高,表明煤气化废水中主要有机污染物为酚类化合物,这与大部分研究一致[19-20]。为了进一步确定煤气化废水中酚类等特征污染物的去除效果,采用GC-MS对其进行分析。
4) GC-MS分析。如图2所示,原煤气化废水酚类、酮类物质总占比分别为 65.5%和10.7%, 经Na-BBT、PFA吸附预处理后煤气化废水其酚类占比分别由原来的65.5%降低至 53.6%和62.0% 。酮类物质几乎被Na-BBT全部吸附,被PFA吸附占比由原来的10.7%降至7.8%。对于煤气化废水酚类和酮类含量较高的主要是苯酚、4-甲基苯酚与邻苯二酚和(R)-(+)-3-甲基环戊酮、对羟基苯乙酮,苯酚占比由原来的28.7%分别降至23.2%、26.2%;4-甲基苯酚由原占比的14.6%分别降至12.3%、14.3%;邻苯二酚由原占比的6.8%分别降至4.1%、5.4%;(R)-(+)-3-甲基环戊酮由原占比5.5%分别降至0.1%、3.9%;对羟基苯乙酮由原占比3.11%分别降至0.1%、1.9%,即Na-BBT和PFA主要吸附去除的是煤气化废水中苯酚、4-甲基苯酚、邻苯二酚、(R)-(+)-3-甲基环戊酮和对羟基苯乙酮等毒性物质。
2.2 吸附机理分析
1)吸附等温线。吸附等温线结果如图3(a)、图3(b)和表2所示。由Na-BBT和PFA吸附煤气化废水中COD的R2 分析结果可知,Na-BBT、PFA对煤气化废水的吸附更符合 Langmuir 模型,结合前面对Na-BBT和PFA的实验分析可知,Na-BBT与PFA固体表面有大量的吸附活性中心,当其表面吸附活性中心全部被占满时,COD吸附量达到饱和值qe,遵循单分子层分布。由表2可见,Langmuir 吸附模型中Na-BBT、PFA的qmax分别为257.48 mg·g−1和137.46 mg·g−1,Na-BBT的吸附容量约为PFA的2倍。另外,通过 Dubinin-Radushkevich(D-R) 模型活性系数β计算了吸附能量 E,
E=1√2β 表 2 吸附等温线模型拟合参数Table 2. Fitting parameters of adsorption isotherm model吸附剂 Langmuir Freundlich D-R qmax/(mg·g−1) KL/(L·mg−1) R2 KF/( mg·L−1) 1/n R2 β qm/(mg·g−1) Na-BBT 257.48 0.039 8 0.979 9 17.19 2.78 0.851 3 1 678.2 10.46 PFA 137.46 0.002 9 0.909 2 14.74 3.698 0.762 3 3 019.9 7.78 2)吸附动力学分析。由图3(c)~(d)动力学拟合结果可知,随着吸附时间增加,Na-BBT与PFA吸附煤气化废水的 COD 和 NH3-N 速率迅速增加,分别在 20 min 和120min时达到吸附平衡。Na-BBT对 COD 和 NH3-N吸附率分别为 29.1% 和 37.8%;PFA对 COD 和 NH3-N 吸附率分别为 15.7% 和 27.1%。经Na-BBT吸附后煤气化废水的COD与 NH3-N准一级动力学R2分别为0.957 1和0.984 3; PFA吸附煤气化废水的COD和 NH3-N准一级动力学R2分别为0.970 2和0.984 0;Na-BBT吸附煤气化废水中COD和 NH3-N准二级动力学R2为0.900 9和 0.975 0;PFA吸附煤气化废水的COD和 NH3-N准二级动力学R2为0.942 2和0.963 1。Na-BBT与PFA吸附煤气化废水的过程更符合准一级动力学模型。准一级动力学模型的线性关系表明(Na-BBT 吸附时间<20 min 、PFA吸附时间<120min时),吸附速率与吸附剂浓度之间存在简单的比例关系,这种关系符合物理吸附过程中的Langmuir吸附模型,吸附为可逆过程,并且吸附位点间的相互作用较弱。因此,Na-BBT与PFA吸附煤气化废水中COD与 NH3-N,是以物理吸附为主,且Na-BBT的吸附速率远远大于PFA。
2.3 形貌和结构表征
1)扫描电镜(SEM):由图4(a)可知,Na-BBT具有不规则的片层状,边缘有轻微卷曲,并且相互堆叠形成集合体[14],具有疏松多孔结构;由图4(b)可知,PFA以球状结构为主,其表面较粗糙,但Na-BBT具有更凹凸的表观形貌。吸附煤气化废水后,Na-BBT表面的部分孔隙被填充,片层状形态减少,但轮廓边界仍比较明显,表观形貌未有大的变化(图4(d));PFA表观形态亦未发生明显变化[16](图4e),其表面发生颗粒状物质团聚现象,这在一定程度上会影响其吸附效果[21]。
2)孔径与比表面积(BET)分析。Na-BBT、PFA的比表面积分别为365.0 m2·g−1、22.1 m2·g−1;总孔体积分别为0.15 cm3·g−1 、0.05 cm3·g−1;孔径分别为8.22 nm、8.32 nm。Na-BBT与PFA的孔径大小接近,但Na-BBT的比表面积和总孔体积远远大于PFA(分别相差16.5倍和3倍)。这也验证了SEM图像显示的Na-BBT表观形貌(存在较多微观的凹凸结构,更大的比表面积和总孔体积),可为Na-BBT吸附剂提供了更多的吸附位点,这也是Na-BBT的吸附容量大于PFA的原因(表2),而且从吸附动力学分析,Na-BBT的吸附速率也大于PFA(图3)。
3)傅立叶红外光谱(FT-IR)分析。由图4(c)可以看出,Na-BBT吸附煤气化废水前后在2 920 cm−1 出现了 —CH3的C-H反对称伸缩振动和2 852 cm−1 处出现了—CH3 的C—H 对称伸缩振动特征峰[22]。在3 438 cm−1和3 618 cm−1观察到其特征峰为游离的O—H。1 635 cm−1 处的峰值为弯曲振动峰,被认为是C=C和C=O的伸缩振动,说明存在含氧官能团。789 cm−1和 1 036 cm−1 分别对应Al—O和Si—O基团,形成Na-BBT的基本Si—O四面体骨架和 Al—O 八面体骨架[23]。Si—O—Mg 和 Si—O—Fe 的拉伸振动峰分别出现在522 cm−1 和465 cm−1 处。Na-BBT结构中形成了较强的Si—O键,由于 Na+ 部分进入Si—O 层,导致层间电荷减少,静电相互作用减弱,从而增强Si—O键能。而较强的Si—O键能增强了Na-BBT表面的吸附。对于PFA (图4(c)),464 cm−1 处的峰代表O—Si—O的弯曲振动,565 cm−1 处的峰归因于Fe—O,1 085 cm−1 处的宽带由对称和SiO4或AlO4四面体的不对称拉伸振动[24]。PFA 在1 630 cm−1 处的峰是由H2O中的O-H弯曲振动引起的,在3 432 cm−1 处的峰是由羟基或H2O中的O-H伸缩振动引起的,且吸附前较吸附后O—H的吸收峰强,表明PFA可以吸附煤气化废水中的酚类化合物[25-26]。Na-BBT与PFA吸附煤气化废水前后的峰形基本一致,表明吸附前后它们的其内部结构较为稳定,结构未发现明显破坏,也未发现有新物质的生成。
4) X 射线衍射(XRD)分析。由图4(f)可知,Na-BBT主要特征衍射峰是SiO2、Al2O3、MgO、(Na,Ca)0.33(Al,Mg)2(Si4O10)(OH)2·nH2O、CaCO3,而PFA的主要特征衍射峰为SiO2、Al2O3、CaO、CaCO3、3MgO·4SiO2·H2O、3Al2O3·2SiO2等,吸附煤气化废水后它们的特征衍射峰的种类和数量无明显变化,但吸附后其特征衍射峰强度有一定程度的减低,尤其是Na-BBT的特征衍射峰(Na,Ca)0.33(Al,Mg)2(Si4O10)(OH)2·nH2O强度降低明显,而PFA特征衍射峰降低不明显。
5) X射线荧光光谱(XRF):Na-BBT中氧化物主要为SiO2、MgO、Fe2O3、CaO、Al2O3、K2O、Na2O,分别占比59.1%、16.7%、3.7%、1.1%、3.2%、0.1%、6.6%;PFA主要氧化物为MgO、Na2O、Fe2O3、CaO、SiO2、Al2O3、K2O分别占比13.1%、1.3%、12.5%、11.6%、38.0%、20.7%、1.2%。
6) X射线电子能谱(XPS)分析。由图5(a)~(g)可见,Mg1s、Na1s、O1s和C1s的特征峰为单峰,分别为1 304.4、1 073.3、531.8和285.0 eV,而Ca和Fe特征峰为多峰,Ca1s和Ca2p的特征峰分别为348.2 eV和351.6 eV,Fe2p的特征峰分别为712.7 eV和726.9 eV,主要以三价铁的形式存在。吸附剂Na-BBT吸附后Mg、Na 、Fe、O、Ca等特征峰信号强度降低,C特征峰信号强度增加,吸附前后O 特征峰信号最强,但吸附后强度有所降低。由图5(h)可知,Na-BBT吸附煤气化废水后Mg、Na、Fe、Ca元素含量分别由原来的11.1%、20.5%、 2.7%、4.2% 降至5.0%、15.7%、2.2%、3.4%,Mg和Na含量降低明显;而 C元素含量由原来的61.4%增至73.7%。
由图6(a)~(g)发现,Mg1s、Na1s、Fe2p、O1s和C1s的特征峰为单峰,分别为1 304.7、1 073.2、711.9、531.8和285.0 eV,而Ca特征峰为多峰,Ca1s和Ca2p峰值分别为347.5 eV、351.2 eV。吸附剂PFA吸附后Mg、Na 、Fe、O、Ca等特征峰信号强度降低,C特征峰信号强度增加,吸附前后O 特征峰信号最强,但吸附后强度有所降低。由图6(h)可知,PFA吸附煤气化废水后Mg、Na、Fe、Ca元素含量分别由原来的12.9%、2.3%、 1.4%、11.9%降至1.9%、1.6%、1.1%、3.9%,Mg和Ca含量降低明显;而 C元素含量由原来的71.5%增至91.6%。
Na-BBT和PFA吸附煤气化废水后C1s的特征峰强度增大,C元素含量增加,推测原因是因为煤气化废水有机污染物(如酚类和酮类)被两种吸附剂吸附在吸附剂表面,导致吸附剂表面的C含量增加,而Mg、Na、Fe、Ca特征峰强度降低,且对应元素含量降低,推测煤气化废水中阳离子污染物(如NH4+)可通过与Mg2+、Na+、Ca2+等进行阳离子交换被固定在吸附质表面。但由于在XPS检测中,N元素通常以氮化物或氨基氮(NH4+)等形式存在,由于N原子的光电子能谱峰位分布在较低能量端,往往与C1s或O1s等元素的光电子峰重叠较为严重,单独测量和定量分析N峰值非常困难,曾对N峰进行过分析,但N峰值特别弱,所以没有添加N峰在XPS图中。但从图1(a)和图1(b)可以看出,在投加量为6 g·L−1 (XPS分析时吸附时用的投加量)时,Na-BBT、PFA的吸附去除率分别为39.6%、18.2%,推测氨氮的吸附去除机理:煤气化废水中的NH3-N主要以NH4+形式存在,被Na-BBT和PFA吸附在吸附剂表面之后,一部分NH4+进一步与Na-BBT和PFA吸附剂中的阳离子(如Mg2+ 、Na+、 Ca2+等)发生离子交换吸附,在低价离子中,NH4+通常具有很强的离子交换能力,且离子交换能力为NH4+ > Mg2+ > Ca2+ > Na+,这是因为NH4+的电荷较小,与水分子之间的水合作用相对较弱。因此,Na-BBT和PFA对煤气化废水中NH3-N的吸附除了物理吸附,还存在离子交换吸附[27]。
3. 结论
1)Na-BBT和PFA对煤气化废水中的COD、NH3-N与Tph等污染物的去除率虽然不高,但可提升煤气化废水的可生化性,降低其毒性。经Na-BBT和PFA吸附后,煤气化废水B/C由0.29分别提高至0.37和0.32;毒性单元 TU 值由22.3 分别降低至 15.2和18.4。Na-BBT和PFA主要吸附去除的是煤气化废水中的芳香类蛋白物质和微生物代谢产物,包括煤气化废水中苯酚、4-甲基苯酚、邻苯二酚、(R)-(+)-3-甲基环戊酮和对羟基苯乙酮等毒性物质。
2)通过吸附等温线分析可知Na-BBT和PFA吸附煤气化废水中COD的过程符合Langmuir 吸附模型,符合单分子层吸附;通过D-R 模型活性系数β计算的吸附能量 E(<0.02),可以初步判断Na-BBT与PFA是以物理吸附为主。Na-BBT与PFA吸附煤气化废水的COD与 NH3-N遵循准一级动力学模型,进一步验证Na-BBT和PFA吸附煤气化废水的COD与 NH3-N过程是物理吸附过程,且Na-BBT与PFA相比,具有更大的吸附容量和更快的吸附速率。
3)相比PFA,Na-BBT具有更大的比表面积和孔体积;吸附后Na-BBT和PFA的内部结构较为稳定,未发现明显破坏,也未发现有新物质的生成。2种吸附剂可吸附去除煤气化废水中一些酚类、酮类、氨氮等污染物,吸附过程主要受物理扩散控制,对有机物的吸附主要为表面和孔道的物理吸附;吸附后Na-BBT和PFA中Ca、Fe、Na、Mg 等元素含量降低,而C含量增加,可推测煤气化废水中NH3-N的去除,除了物理吸附,NH4+还可以与Mg2+、Na+、Ca2+等进行阳离子交换吸附。
4)Na-BBT和PFA可有效提高煤气化废水的可生化性,降低毒性,达到了预处理的目的,Na-BBT的吸附效果优于PFA,可优先考虑将Na-BBT用于煤气化废水的预处理,具有潜在的、广泛的、实际应用前景。
-
表 1 荧光区域积分和占比
Table 1. Integration and proportion of fluorescence region
样品 区域积分/(×105) 区域占比/% I II III IV V I II III IV V 原水 1.75 0.44 0.25 2.10 0.48 55.4 22.3 5.2 13.6 3.4 Na-BBT 0.99 0.25 0.51 1.15 0.42 48.2 19.4 16.5 11.4 4.5 PFA 1.30 0.30 0.19 1.46 0.40 56.8 21.3 5.6 13.0 3.9 表 2 吸附等温线模型拟合参数
Table 2. Fitting parameters of adsorption isotherm model
吸附剂 Langmuir Freundlich D-R qmax/(mg·g−1) KL/(L·mg−1) R2 KF/( mg·L−1) 1/n R2 β qm/(mg·g−1) Na-BBT 257.48 0.039 8 0.979 9 17.19 2.78 0.851 3 1 678.2 10.46 PFA 137.46 0.002 9 0.909 2 14.74 3.698 0.762 3 3 019.9 7.78 -
[1] 陈进国. 空间场视角下煤炭能源基地的可持续发展评价与情景仿真研究[D]. 北京: 中国矿业大学, 2018. [2] 付强强. 煤气化废水水质分析及深度处理工艺研究[D]. 青岛: 青岛科技大学, 2016. [3] LIU Y,YANG J,et al. Modeling,simulation,and techno-economic analysis of Lurgi gasification and BGL gasification for coal-to-SNG[J]. Chemistry Engineering Research and Design, 2017, 117: 355-368. doi: 10.1016/j.cherd.2016.10.048 [4] 郑彭生,郭中权. 国内煤气化废水处理关键问题分析[J]. 水处理技术, 2018, 44(3): 17-20. doi: 10.16796/j.cnki.1000-3770.2018.03.004 [5] 纪华,夏立江,王峰,等. 改性膨润土对垃圾填埋场渗滤液吸附效果[J]. 环境工程学报, 2012, 6(3): 848-854. [6] 胡雪峰. 改性膨润土的制备及其吸附性能研究[D]. 武汉: 武汉理工大学, 2008. [7] 郑越,刘方,吴永贵. 粉煤灰对工业废水中氨氮的吸附性能研究[J]. 环境科学与技术, 2011, 34(1): 4-7. [8] 林俊雄,詹树林,方明晖,等. 三种吸附剂的改性与染料吸附特性比较研究[J]. 浙江大学学报 (工学版) , 2006(12): 2031-2036. [9] 王泽龙,李顺义,吴朕君. 膨润土改性和复配及在废水处理中的应用进展[J]. 工业水处理, 2022, 42(2): 11-18. [10] 史兵方,左卫元,仝海娟. 改性膨润土对水体中多环芳烃的吸附[J]. 环境工程学报, 2015, 9(4): 1680-1686. doi: 10.12030/j.cjee.20150426 [11] YAO Z,JI X,SARKER P,et al. A comprehensive review on the applications of coal fly ash[J]. Earth Science Reviews, 2015, 141(1): 105-121. [12] 罗秋艳,黄正根,范文哲,等. 复合改性膨润土对2,4,6-三氯苯酚和Pb2+的吸附行为研究[J]. 离子交换与吸附, 2019, 35(1): 20-29. [13] BATABYAL D,SAHU A,CHAUDHURI S K. Kinetics and mechanism of removal of 2,4-dimethyl phenol from aqueous solutions with coal fly ash[J]. Separations Technology, 1995, 5(4): 179-186. doi: 10.1016/0956-9618(95)00124-7 [14] KHANSAA A, ETHAR M. A. Effective approach of activated Jordanian Bentonite by sodium ions for total phenolic compounds removal from olive mill wastewater[J]. Journal of Chemistry, 2021: 16. [15] 赵伟高,谷启源,赵鹏,等. 粉煤灰对焦化废水中挥发酚的吸附机理研究[J]. 工业水处理, 2015, 35(7): 68-72. [16] WEIWEI M,YUXING H,CHUNYAN X,et al. Biotoxicity assessment and toxicity mechanism on coal gasification wastewater (CGW) :A comparative analysis of effluent from different treatment processes[J]. Science of the Total Environment, 2018, 637-638: 1-8. doi: 10.1016/j.scitotenv.2018.04.404 [17] XIAO,KANG,LIANG,et al. Fluorescence quotient of excitation-emission matrices as a potential indicator of organic matter behavior in membrane bioreactors[J]. Environmental science:Water Research & Technology, 2018, 4(2): 281-290. [18] CHEN W,PAUL W,LEENHEER J A,et al. Fluorescence Excitation - Emission Matrix Regional Integration to Quantify Spectra for Dissolved Organic Matter[J]. Environmental Science & Technology:ES& T, 2003, 37(24): 5701-5710. [19] ZENG F R,YONG-QING XI,ZHANG S Q,et al. Determination of phenol and hydroquinone in environmental water by fluorescence spectrometry[J]. Chinese Journal of Analysis Laboratory, 2007, 26(5): 76-79. [20] MARTIN,CHOI M F. Fluorescence quenching method for the determination of catechol with gold nanoparticles and tyrosinase hybrid system[J]. Chinese Chemical Letters, 2009, 21(3): 346-348. [21] 李杰松,黄璐. 改性膨润土对重金属的吸附性能研究[J]. 水处理技术, 2023, 49(5): 56-62. doi: 10.16796/j.cnki.1000-3770.2023.05.011 [22] 马清亮. 磁性膨润土改性吸附材料的制备及其应用研究[D]. 太原: 太原理工大学, 2016. [23] 李静. 有机膨润土的制备、表征及其对废水中酚类化合物的吸附研究[D]. 太原: 太原理工大学, 2013. [24] XH A,HZ A,GZ A,et al. Potential of removing Cd (II) and Pb (II) from contaminated water using a newly modified fly ash-ScienceDirect[J]. Chemosphere, 2020, 242: 125-148. [25] GARCÍA -LODEIRO I,FERNÁNDEZ -JIMÉNEZ A,BLANCO M T,et al. FTIR study of the sol–gel synthesis of cementitious gels:C-S-H and N-A-S-H[J]. Journal of Sol-Gel Science and Technology, 2008, 45(1): 63-72. doi: 10.1007/s10971-007-1643-6 [26] MOSTAFA N Y,KISHAR E A,ABO-El-ENEIN S A. FTIR study and cation exchange capacity of Fe3+ and Mg2+ substituted calcium silicate hydrates[J]. Journal of Alloys & Compounds, 2009, 473(1/2): 538-542. [27] 任晓宇. 粉煤灰基沸石的合成、生长机理及其吸附性能的研究[D]. 杭州: 浙江大学, 2020. -




 下载:
下载: