-
氯胺是一种常见的自来水消毒剂,分为一氯胺(NH2Cl)、二氯胺(NHCl2)和三氯胺(NCl3), 是氨分子中氢原子被氯原子取代后得到的衍生物。其中,NH2Cl在自来水的消毒中应用最为广泛[1-2],一般通过氨与次氯酸(HClO)在水溶液中反应制备(式(1))而成。相比于HClO,NH2Cl的稳定性更好,消毒能力更持久,含氯消毒副产物更少;但其消毒性能比HClO差,有更复杂的消毒副产物种类[3]。
除了直接用于自来水消毒外,NH2Cl还是高级氧化技术(advanced oxidation processes, AOPs)的常用氧化剂之一,其经过紫外(ultraviolet, UV)照射活化之后,能够产生羟基自由基(HO·)、氯自由基(Cl·)等自由基[4](式(2)~式(6)),基于NH2Cl的高级氧化技术具有选择性强,药品利用率高等优点。目前,UV/NH2Cl体系应用的领域包括饮用水、污水的杀菌消毒[5-6]以及水中微污染物的降解等[7-8]。LU等[9]研究表明,在中性条件下,中压紫外/氯胺体系能够有效降解水中的常见抗生素环丙沙星,去除率可达84.9%。另外一项研究使用了NH3/Cl2体系对水中的阿特拉津进行了降解[10],结果表明,体系中产生了HO·,活性氯自由基(reactive chlorine species, RCS)等活性组分,并成功实现了对阿特拉津的降解(降解率为50%)。
与基于紫外光UV的高级氧化系统相比,基于模拟太阳光的高级氧化系统被认为是一种更为节能环保的处理技术。在到达地球的太阳辐射光谱中,可见光(波长为λ,400<λ<760 nm)约占49%,红外光(760<λ<4 000 nm)约占46%,紫外光(λ<400 nm)约占4%[11]。近年来, 太阳光(solar)耦合自由氯(free available chlorine, FAC)体系在水处理领域的应用越来越受到关注,如消毒和药品和个人护理产品(pharmaceuticals and personal care products, PPCPs)的降解等[12]。HUA等[13]研究了太阳光/FAC系统对包括抗生素、非甾体抗炎药、β阻滞剂在内的24种典型PPCPs的降解效能,结果表明,当pH由6.0升至8.0,体系中HO·和·Cl稳态浓度下降,而氧化氯(ClO·)稳态浓度有显著上升,此时,有11种PPCPs的去除率增大。此外,由于臭氧(O3)的产生,溶解氧(dissolved oxygen, DO)的存在能够显著提升大部分PPCPs的降解效果。整体而言,目前对于Solar/FAC体系降解微污染物的反应动力学、反应机理以及消毒副产物的产生与演化等方面的研究已经十分深入,但对于太阳光/NH2Cl体系的相关研究仍然具有大片空白。相比于FAC,NH2Cl在水体中的存留时间更长,消毒效果也更持久。此外,城市污废水中通常含有较高浓度的NH4+-N,在对其进行处理与再利用的过程中也难免会产生NH2Cl。因此,有必要对太阳光/NH2Cl体系对污染物的降解效能、反应机理等进行深入的研究。
本研究选择阿司匹林(aspirin, ASA)和氟尼辛葡甲胺(flunixin meglumine, FMME)2种典型的PPCPs作为目标污染物,以硝基苯(nitrobenzene, NB)和苯甲酸(benzoic acid, BA)这2种常见的工业污染物作为指针物质,探究了太阳光/NH2Cl体系对有机污染物的降解效能及影响因素,深入解析了太阳光/NH2Cl体系中污染物的降解机理及活性因子的贡献率,最后对这一体系降解微污染物的经济性进行了评估。
-
主要药品试剂:阿司匹林(C9H8O4,分析纯),购自上海阿拉丁试剂有限公司;氟尼辛葡甲胺(C14H11F3N2O2C7H17NO5,纯度98.0%),购自上海源叶生物科技有限公司;硝基苯(C6H5NO2,纯度99.0%),购自上海阿拉丁试剂有限公司;苯甲酸(C7H6O2,纯度99.0%),购自国药集团化学试剂有限公司;三卤甲烷(trihalomethanes, THMs)标样(CHCl3,CHCl2Br、CHClBr2及CHBr3,色谱纯),购自美国o2si标准品公司。
主要仪器:高亮度平行光源系统(CHF-XM-500W);超高效液相色谱仪(B16CHA763G);总有机碳分析仪(TOC-L CPH);低温恒温循环器(MQA-30);紫外可见光分光光度计(DR6 000)等。
-
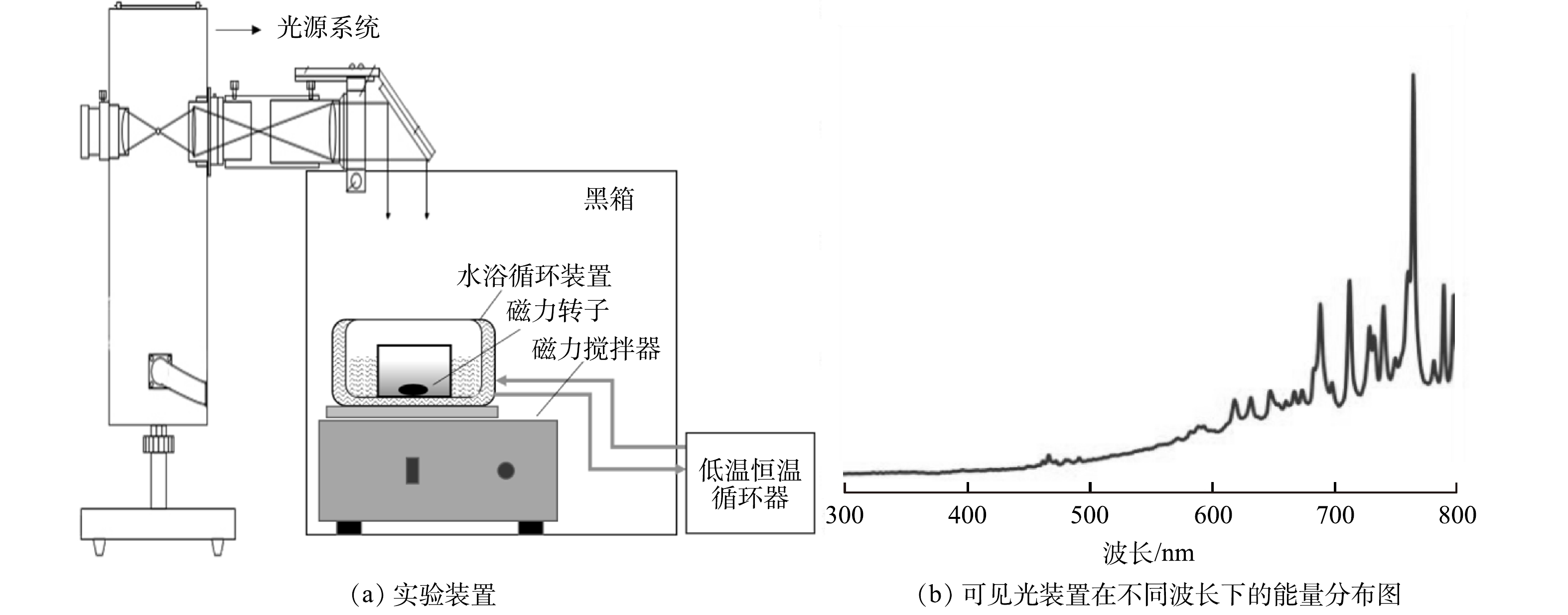
本研究使用的装置为特殊定制的光化学氧化反应实验装置(图1(a))。采用容量为50 mL的圆形反应皿作为反应器,置于低温恒温水浴加热器中。反应器内部安装高亮度平行光源以模拟可见光,其光谱见图1(b)。
污染物降解实验:若无特殊说明,则实验所用的污染物浓度均为10 μmol·L−1,投加NH2Cl初始浓度为0.2 mmol·L−1。NH2Cl采用现用现配的方式,在pH=8.5的条件下将Cl2与N的质量比为4:1的NaClO和NH4Cl溶液混合30 min后使用,然后将配制好的反应溶液置于装置中进行水浴控温,加入NH2Cl后打开灯罩开始反应,随后在取样时间检测污染物的浓度。
消毒副产物生成情况检测实验。取20 mL 4种污染物的混合溶液加入棕色管,并向其中加入相当于混合溶液的总有机碳(total organic carbon, TOC)总量5倍的NaClO溶液,反应24 h。随后加入200 μL 500 mmol·L−1抗坏血酸溶液,终止反应。加入约3.0 g无水硫酸钠和4.0 mL甲基叔丁基醚,振荡后静置10 min,从上层有机相取1.0 mL样品进行检测。
-
使用美国Waters公司ACQUITY UPLC H-Class超高效液相色谱仪来检测实验中目标污染物浓度,色谱柱为ACQUITY UPLC BEH 1.7 μm C18色谱柱(2.1 mm×100 mm),进样体积10 μL,柱温40 ℃。紫外检测器中ASA与BA的检测波长为230 nm,FMME和NB的检测波长为270 nm。NH2Cl浓度的检测采用N, N-二乙基-1, 4苯二胺分光光度法。TOC的检测采用日本Shimadzu公司的TOC-L CPH型TOC分析仪,使用680 ℃催化燃烧氧化-非色散红外探测法来测定反应溶液的TOC。使用气相色谱/电子捕获检测器GC/ECD测定溶液中的THMs浓度,参考美国环境保护署开发的方法(Method 551)。样品经过不分流进样器进入气相色谱仪,进样口温度为200 ℃,进样体积为2.0 μL,氮气流速恒定为1.0 mL·min−1,检测器ECD温度为290 ℃。
-
模拟太阳光装置的光量子产率可以通过式(7)进行计算,装置的有效光照强度可按照式(8)计算。本研究还采用竞争动力学方法计算目标污染物ASA和FMME与HO·的二级反应速率常数(式(9))。
式中:Ep0为光量子产率,Einstein·(m2·s)−1,本实验计算的是300~400 nm范围内可见光的光量子产率;kp,PNA为对硝基苯甲醚(p-nitroanisole, PNA)降解的拟一级反应速率,s−1,为6.8×10−5 s−1;ΦPNA/PYR是PNA在某一浓度吡啶(pyridine, PYR)存在下的量子产率系数,mol·Einstein−1,本实验的ΦPNA/PYR为5.44×10−4 mol·Einstein−1[14];fp,λ为模拟可见光装置的波长,本实验为300~400 nm,εPNA,λ表示在波长λ下PNA的摩尔吸光系数,m2·mol−1。综合计算,得到本研究中模拟太阳光装置的量子产率为1.14×10−4 Einstein·(m2·s)−1。
式中:E为装置的光照强度,W·cm−2;Ep0意义同式(7);L为阿伏伽德罗常数,6.02×1023 mol−1;h为普朗克常数,6.62×10−34 J·s;c为真空中的光速,3.0×108 m·s−1;fp,λ为模拟可见光装置的波长,nm,计算可得本实验装置的光照强度E为6.06 W·cm−2。
式中:[A]0和[A]t分别为目标污染物在0时刻和t时刻的浓度,mol·L−1;[B]0和[B]t分别为探针化合物在0时刻和t时刻的浓度,mol·L−1,kT为目标污染物与活性因子的二级反应速率常数,L·(mol·s)−1;kR为探针化合物与活性因子的二级反应速率常数,L·(mol·s)−1。
降解过程中生成消毒副产物(disinfection by-products, DBPs)的毒性可根据式(10)计算。
式中:cT表示消毒副产物的生物毒性;c(DBP)表示每种DBP的质量浓度,g·L−1;c50为半致死浓度,mol·L−1;M(DBP)为DBP的相对分子质量,g·mol−1。
使用单位数量级的等价电能消耗(electrical energy per order)来计算太阳光/NH2Cl体系降解污染物的处理成本,表示单位体积的溶液中目标污染物降解一个数量级(即降解90%)时所需的电耗成本(式(11)),其可分为光装置耗电量(Esolar,式(12))和氧化剂等价电能消耗(Eoxidant,式(13))两部分[15-16]。
式中:E为单位数量级的等价电能消耗,kWh·(m3·数量级) -1;P为模拟太阳光装置的功率,本研究中为0.5 kW;t为污染物降解数量级需要的反应时间,h;ci和cf分别为目标污染物的初始浓度和反应后浓度,mmol·L−1;V是溶液的总体积,取1 000 L;Oenergy表示生产单位质量的氧化剂的所消耗的等效电量,kWh·kg−1;coxidant为溶液中加入的氧化剂质量浓度,mg·L−1。江苏省2022年8月低于千伏电价为0.667 5元·kWh−1,NaClO和NH4Cl的市场价格分别为4.6元·kg−1和7.5元·kg−1,因此,NH2Cl的制备成本为27.1元·kg−1。计算得NH2Cl的等效电能消耗为40.60 kWh·kg−1。
-
使用浓度为10.0 mol·L−1的磷酸缓冲液调节反应体系的pH,考察pH对4种污染物的降解影响,4种污染物的初始浓度均为10.0 μmol·L−1,反应温度为25 ℃。首先考察在单独太阳光照和单独NH2Cl体系中4种污染物的降解情况(表1)。结果表明,在单独太阳光照条件下,4种污染物的去除率均小于5.0%;在单独NH2Cl条件下,除FMME外,3种污染物的去除率也均小于5.0%;FMME在碱性条件下几乎不发生反应(降解率<5.0%),而在酸性和中性条件下则会被NH2Cl氧化,计算贡献率时将考虑直接氧化因素。
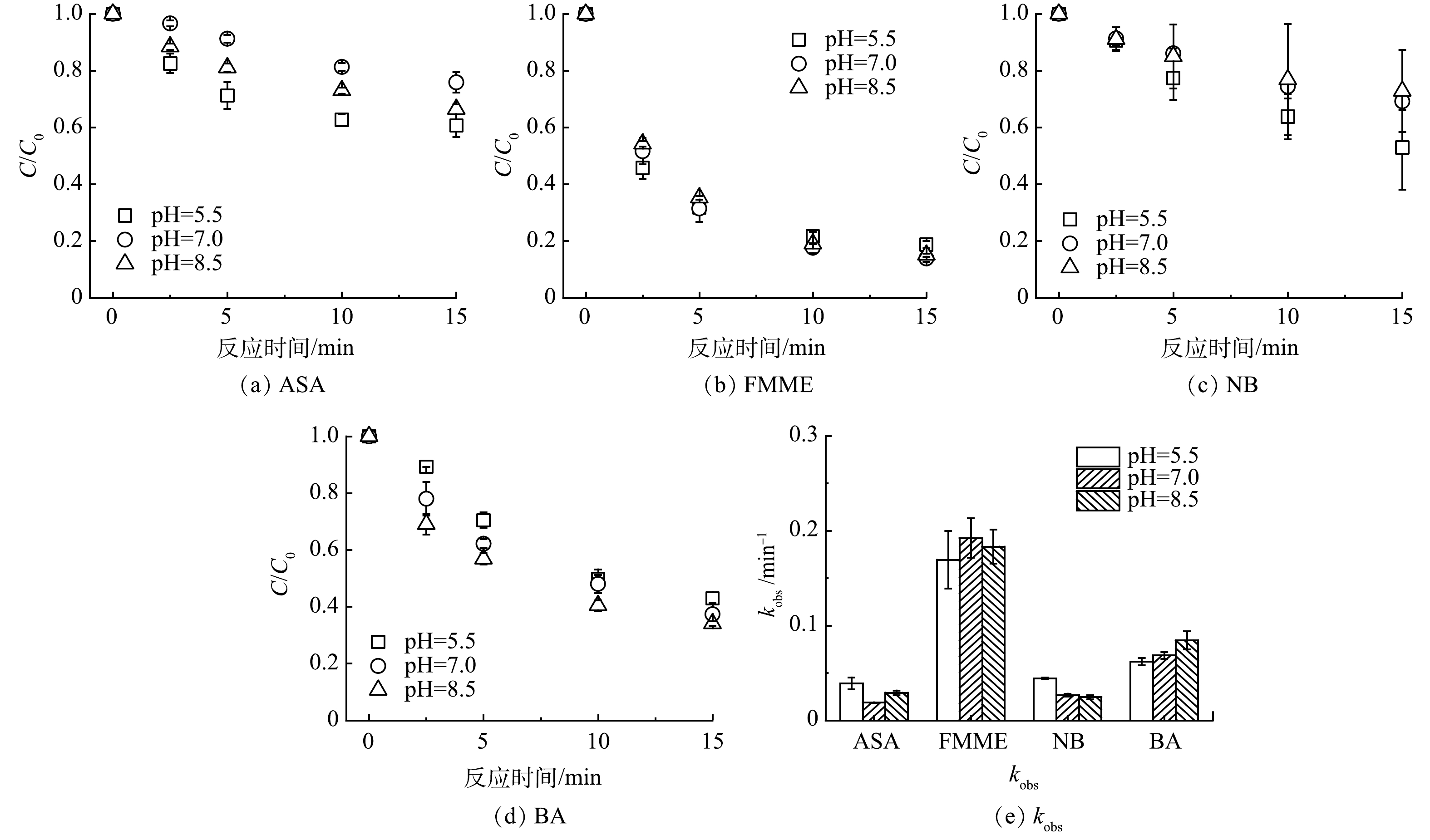
相较于单独太阳光照或单独NH2Cl体系,太阳光/NH2Cl体系在不同pH条件下均表现出较强的氧化性,4种污染物的降解效率均有明显提高(图2(a)~(d))。在pH=5.5时,ASA、FMME、NB和BA在反应15 min时的去除率分别为39.32%、81.20%、47.08%和57.02%,4种污染物的降解效率有一定的差别,是由于体系中产生的氧化性活性组分具有选择性。当pH上升至7.0时,4种污染物的去除率均发生了变化,分别为24.14%、86.13%、30.81%和62.63%;而在pH=8.5条件下,4种污染物的去除率则分别变为33.58%、84.89%、27.19%和65.89%。ASA的去除率随pH升高先减小后增大;不同pH条件下的FMME的15 min去除率变化很小(降解率接近5.0%);NB的去除率随pH的增大而降低,且当pH由5.5升至7.0时,去除率显著下降了16.27%;BA的去除率随pH的增大而增大,但变化幅度较小(降解率<10%)。
根据拟一级动力学模型计算公式,不同pH条件下污染物的降解符合拟一级反应动力学(图2(e)),在实验的pH范围内与时间呈良好的线性关系(R2>0.90)。太阳光/NH2Cl体系中ASA、FMME、NB和BA的表观速率常数分别为1.9~3.9×10−2、1.7~1.9×10−1、2.5~4.4×10−2、6.2~8.5×10−2 min−1,FMME降解速度最快,其次为BA、NB、ASA。4种污染物的kobs随pH的变化规律和其在15 min时的去除率变化规律基本一致。
太阳光/NH2Cl高级氧化体系中主要存在大量的HO·、RCS等活性因子。溶液pH对污染物降解效能的影响可归为以下几点:1) NH2Cl体系中会产生具强氧化性的HO·(式(2)~(5))[17],其对污染物有良好的降解效果。而随着pH的升高,HO·的浓度增加,污染物的降解也将得到促进;2) ASA、FMME、NB和BA的pKa值分别为3.5、5.8、4.0和4.2,随着pH的不断增大,污染物更多以离子形态存在,其电子密度更高,还原性更强[18],更容易被太阳光/NH2Cl体系中的HO·、·Cl等活性组分所去除;3)本实验的pH在5.5~8.5,使用磷酸缓冲液调节pH的过程中引入了H2PO4−和HPO42-,与HO·的二级反应速率分别为2.0×104 L·(mol·s)−1和1.5×105L·(mol·s)−1。根据磷酸根的电离常数计算得出,碱性环境中磷酸根的主要存在形式为HPO42-,其竞争HO·等活性因子的能力强于酸性条件下占主导的H2PO4−,因此,污染物的降解效能随着pH的增高而受到抑制。3种因素的综合作用结果表现为较高pH对污染物降解的促进作用。
除了HO·和RCS之外,太阳光/NH2Cl体系还会产生一类重要的自由基,如活性氮(reactive nitrogen species, RNS),其中包括·NO和·NO2等[19]。有研究[17]表明,UV/NH2Cl体系在碱性条件下会产生大量RNS,RNS与富电子基团具有更高的反应速率,因而随着pH的增加,部分污染物的降解效果也随之提升。
对太阳光/NH2Cl体系降解4种污染物15 min后的矿化情况进行检测。结果表明,在3种pH条件下的15 min去除率差异并不显著:pH=5.5时的去除率最高,为49.56%,pH=8.5时的去除率最低,为40.87%。表明在污染物的降解过程中生成了大量中间产物。与相关研究所得到的低于7%的去除率相比[20],本实验所得到的去除率较高,可能是由于本实验所研究污染物(BA、NB)的化学结构相对简单,不易生成中间产物。
-
本节实验通过水浴恒温装置控制反应器温度,使用磷酸盐缓冲液调节反应体系初始pH为7.0,探究反应温度对于太阳光/NH2Cl体系中污染物降解效能的影响。首先考察了在无光照条件下只改变温度时,单独NH2Cl条件下4种污染物的降解效能。结果表明,在单独NH2Cl氧化情况下,除FMME外,其他3种污染物的去除率均小于5.0%(表2),说明污染物不会在高温下自分解,也未被高温下的NH2Cl氧化。FMME的去除率较高(降解率为32.53%~40.44%),且随着温度升高而略有增大,在计算贡献率时将考虑直接氧化因素。
增加光照后,4种污染物的15 min去除率均有极大的提升(表3),且随温度升高而增大。当温度从10 ℃增加至55 ℃时,ASA、FMME、NB、BA的去除率分别从73.15%、50.16%、13.61%、17.69%提升至80.66%、61.17%、52.63%、28.75%。其中NB的去除率提升最大,达到了39.02%,而另外3种污染物的去除率的提升则并不显著(降解率为10%左右)。在UV/NH2Cl体系降解卡马西平的研究中,相似的结论也得到了验证:当温度从15 ℃上升至45 ℃时,卡马西平的去除率提高了20%左右[21]。
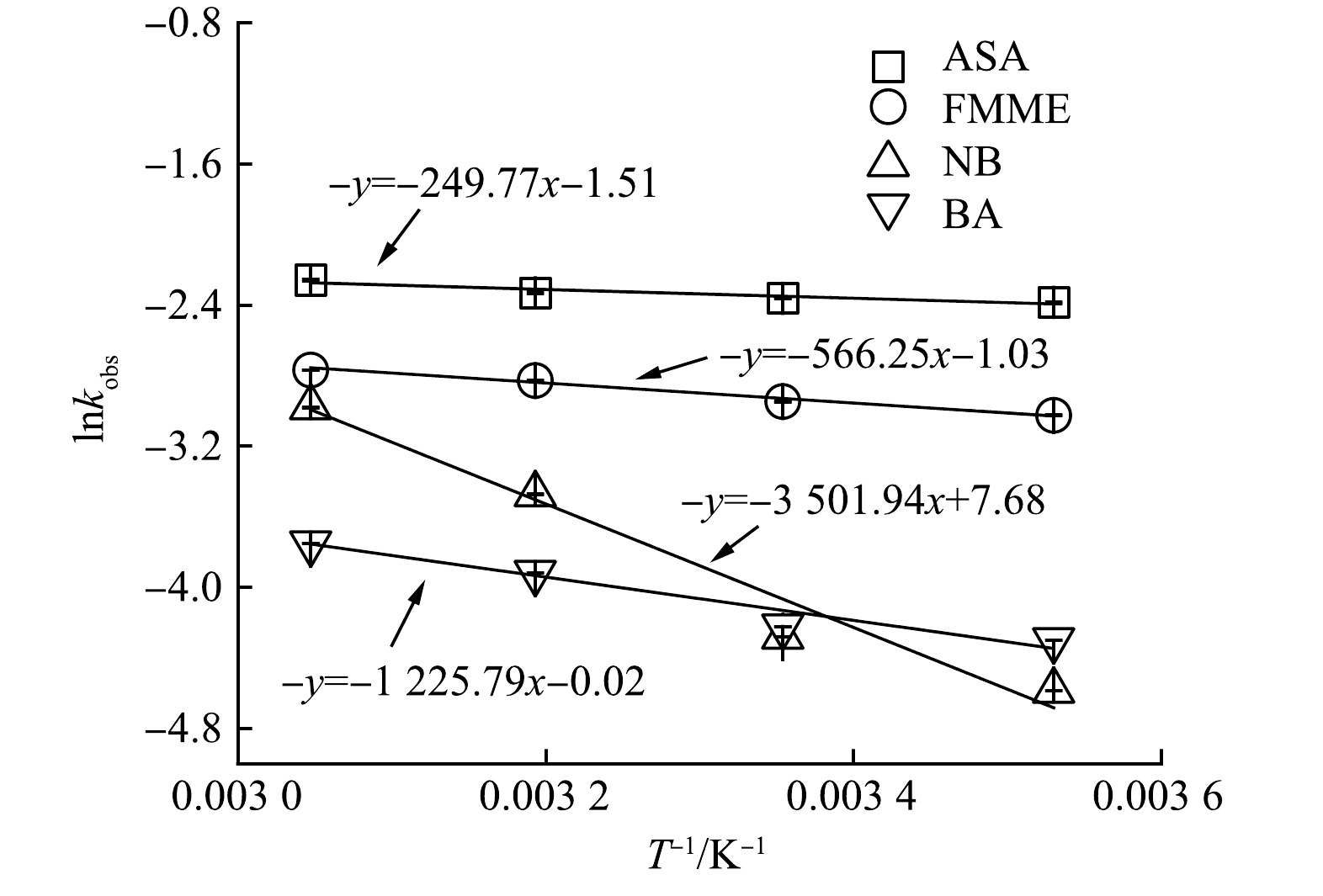
对不同温度下污染物的去除率进行拟合,污染物的降解符合拟一级反应动力学规律(表3)。在10~55 ℃下与时间呈良好的线性关系(R2>0.96)。4种污染物的kobs均随温度的升高而增大,在10 ℃条件下ASA、FMME、NB、BA 的kobs分别为9.2×10−2、4.8×10−2、1.0×10−2、1.4×10−2 min−1;而在55 ℃条件下,其kobs分别升高至10.4×10−2、6.3×10−2、5.1×10−2和2.3×10−2 min−1。使用阿仑尼乌斯公式拟合不同温度下太阳光/NH2Cl体系降解污染物kobs的变化规律,在本实验的温度范围内,lnkobs与1/T呈良好线性关系(R2>0.98)(图3)。可算得ASA、FMME、NB及BA在太阳光/NH2Cl体系中的平均化学反应活化能分别为2.08、4.71、29.12和10.19 kJ·mol−1。
-
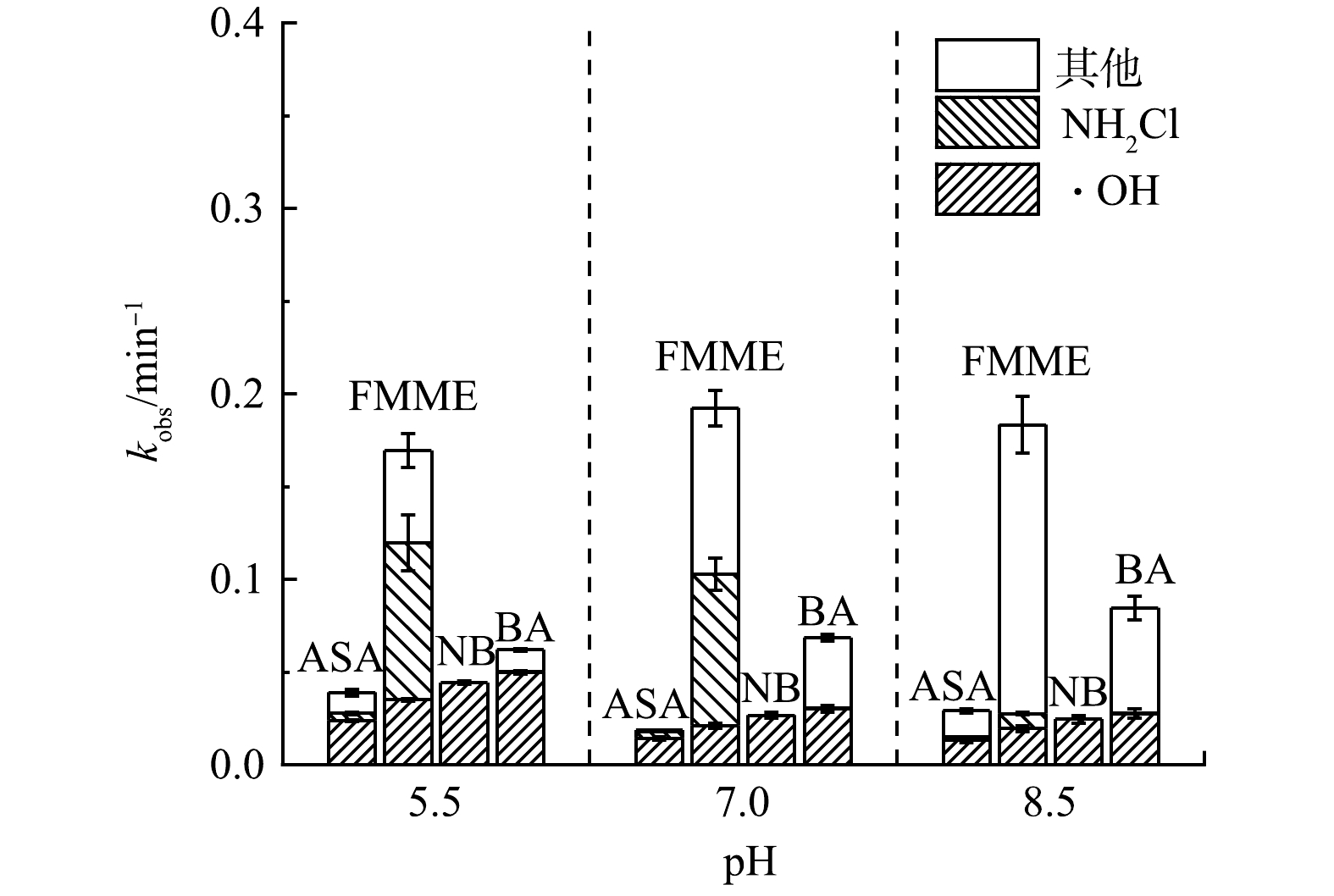
探针法是利用已知探针与某一活性自由基之间的二级反应速率常数,通过动力学方程来鉴定与计算系统中的活性因子的方法。HO·目前常用的探针为NB,因为NB选择性与系统中的HO·反应(kHO·-NB = 4.7×109 L·(mol·s)−1),而与RCS、RNS等自由基的反应速率低4个数量级以上,可以认为几乎不反应;BA与HO·之间的二级反应速率为5.3×109 L·(mol·s)−1,且也会与RCS快速反应,但一般认为难以与RNS发生反应。根据2.1节的实验,可以基本确定太阳光/NH2Cl体系中至少存在4种主要的活性组分,分别为NH2Cl、HO·、RCS、RNS[19]。本节实验利用NB的降解实验来分析HO·的贡献率[17]。NH2Cl的贡献率则通过单独NH2Cl体系的降解实验来计算。结果表明,单独NH2Cl体系中15 min时NB、BA的去除率小于1%,而ASA和FMME分别为3.2%和33.6%。因此,在太阳光/NH2Cl体系中前两者的降解没有NH2Cl参与,而后两者计算贡献情况时不能忽略NH2Cl的直接氧化作用。
太阳光/NH2Cl体系中HO·的稳态浓度可以通过NB的拟一级反应速率常数确定。2.1节的实验结果已经表明NB在太阳光/NH2Cl体系中的降解符合一级反应动力学,在pH=5.5时,NB的kobs为0.044 min−1,即0.73×10−3 s−1。可以计算出在pH=5.5条件下太阳光/NH2Cl系统中的羟基自由基稳态浓度[HO·]ss为1.57×10−13 mol·L−1。根据式(9)可计算得HO·与ASA和FMME的二级反应速率分别为2.53×109 L·(mol·s)−1和3.73×109 L·(mol·s)−1。因此可分别计算得到HO·对ASA和FMME的降解速率kHO·-ASA和kHO·-FMME,分别为3.97×10−4 s−1和5.86×10−4 s−1。其他pH条件下的速率常数可同理计算得到(表4)。
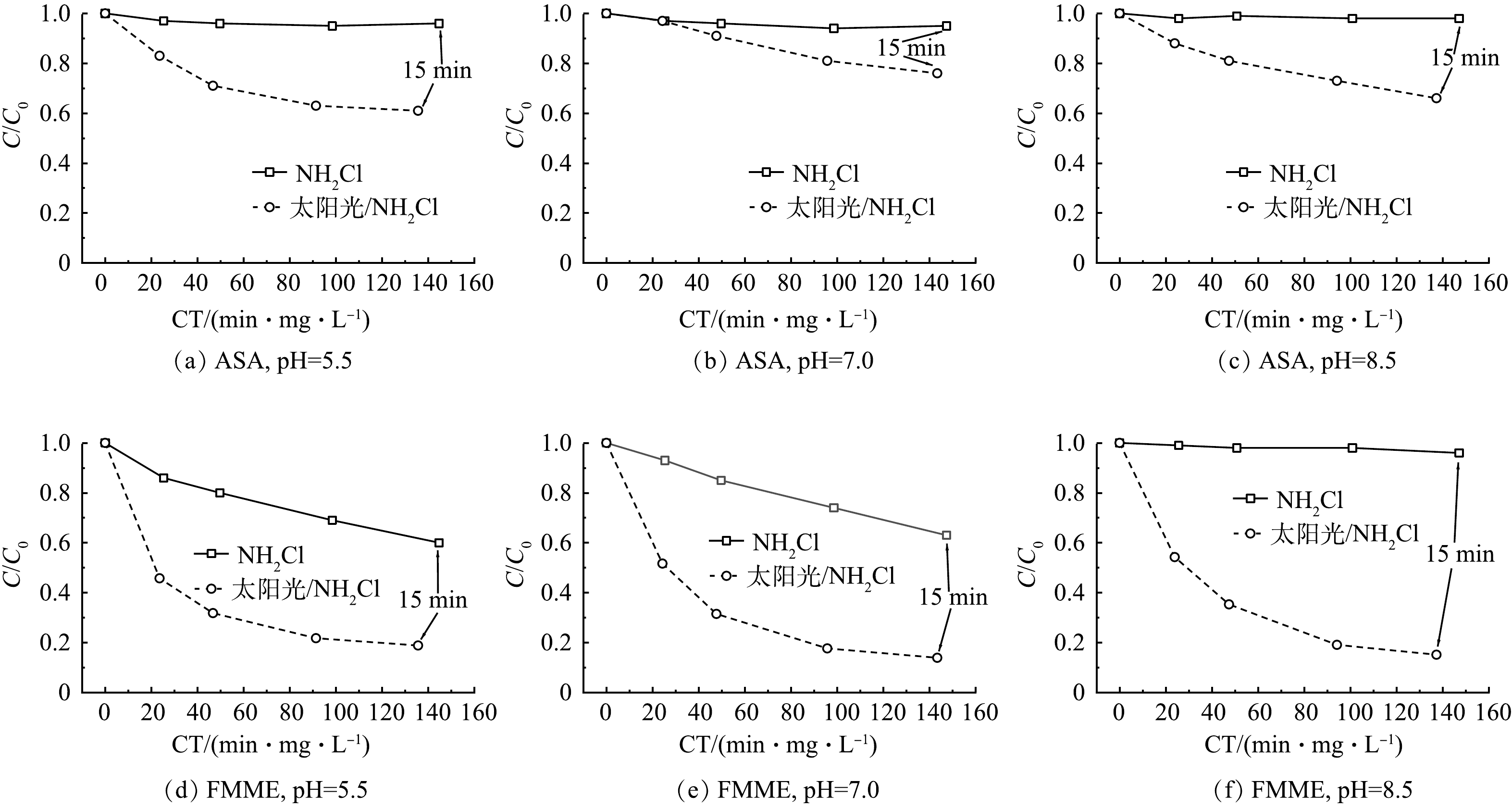
单独NH2Cl贡献率的测定结果见图4。由图4(a)可知,在pH为5.5时,太阳光/NH2Cl体系中反应15 min时NH2Cl的CT值为138.5 min·mg·L−1,且15 min时的ASA去除率为39.3%,而单独NH2Cl体系中对应情况下ASA的去除率为2.7 %,因此可以计算出NH2Cl在ASA去除中的贡献率为6.87%。同理可计算得其他条件下的NH2Cl对FMME、ASA降解的贡献率。
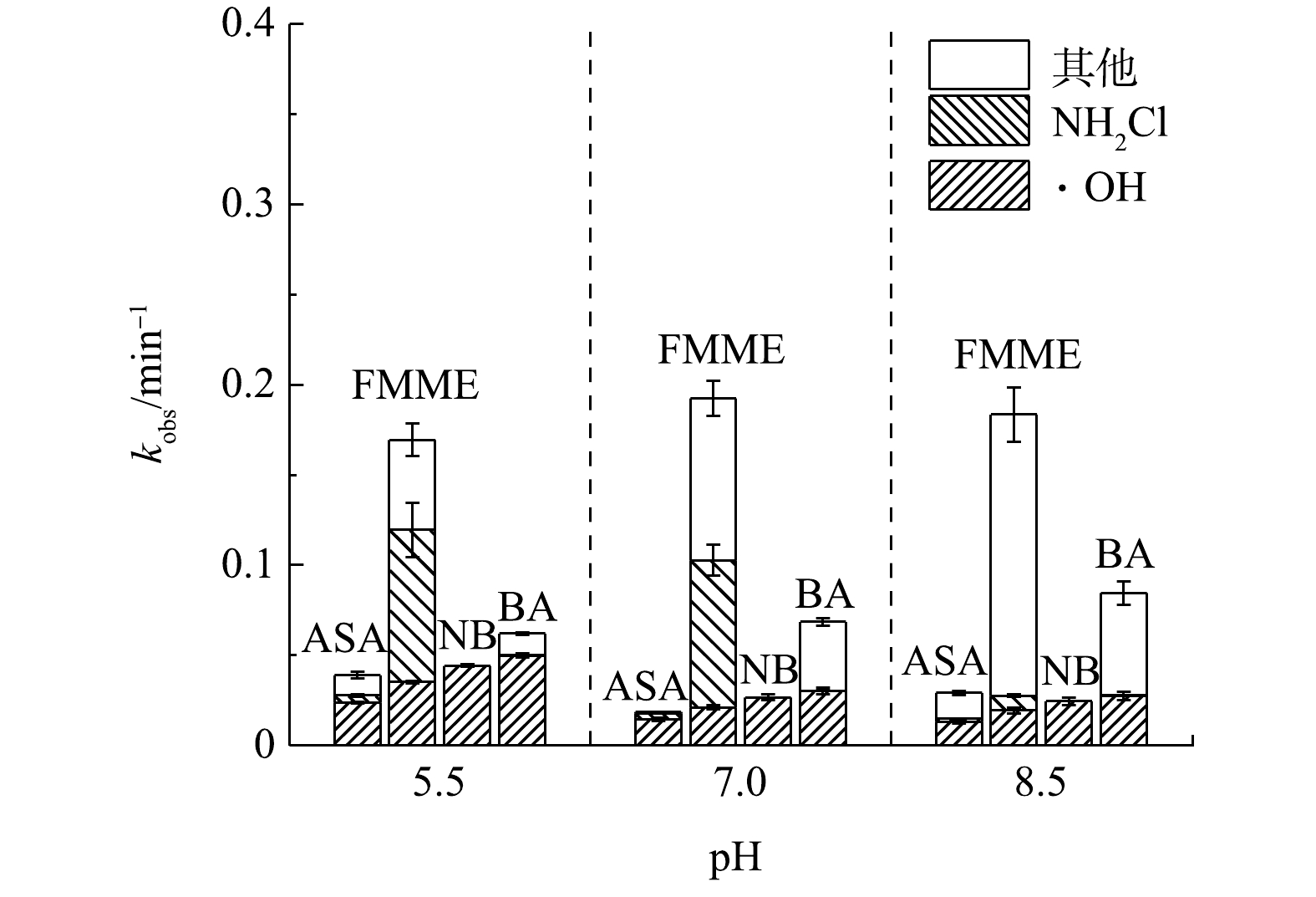
不同活性物质对4种目标污染物的降解贡献率分布情况如图5所示,在对FMME的降解中,随着pH的增大,HO·的清除作用导致其贡献率逐渐降低[19],由pH=5.5时的20.8% 降低至pH=8.5时的10.6%。NH2Cl的贡献率由pH=5.5时的43.9%下降至pH=8.5时的4.4%,其他活性物质(主要是RNS和RCS)对FMME的贡献率随着pH的增加呈增长趋势,从pH=5.5时的29.5%增长到了pH=8.5时的84.9%,这可能是因为RNS的大量产生,导致FMME的快速降解[17],WU等在使用UV/NH2Cl体系降解萘普生时,得出了类似的结论[19]。
在ASA的降解中,HO·的贡献率在pH为7.0时最大,占比达到76.8%,在碱性条件下HO·的贡献率降低至44.9 %,但相比其他活性组分而言,HO·仍为主要活性自由基。NH2Cl氧化对ASA的降解贡献率较低,在pH为5.5和8.5时贡献率低于7%,在中性pH下贡献率为24.1%。
-
本节选取了4种典型的DBPs(表5)作为代表,检测了有无太阳光照条件下,起始pH不同时NH2Cl降解ASA和FMME后DBPs的生成情况(图6)。在单独NH2Cl系统中,pH为7.0时THMs的总浓度最大,为11.0 μg·L−1,pH=8.5条件下THMs的总浓度最低。在全部的THMs中,CHCl2Br占比最大,CHCl3占比其次,而3种pH环境中并不产生CHBr3。该结果与相关文献报道基本一致[22],NH2Cl在酸性和中性条件下比在碱性条件下释放出更多的游离态氯,提高水中pH有利于提高NH2Cl生成量及稳定性,减慢HClO与三卤甲烷前体物的反应速度,从而减少水中三卤甲烷的生成量;同时,碱性条件下一些不稳定的THMs容易水解。实际应用中采用NH2Cl消毒工艺时应将pH控制在8.0以上[23]。
当体系中引入太阳光照之后,DBPs生成总量增大,但其生成的规律与单独NH2Cl体系类似:pH为7.0时THMs的总质量浓度最大,为16.0 μg·L−1,pH为5.0条件下总质量浓度其次,为14.1 μg·L−1。在全部的THMs中,CHCl2Br占比最大,CHCl3占比其次,而CHBr3仅在pH=5.5条件下有少量生成,pH=7.0和pH=8.5时则没有生成。有研究表明[24],随着pH从中性增大到碱性,THMs的生成会被显著抑制:在pH=8.5时,检测到的溴代三卤甲烷质量浓度相比酸性和中性时明显降低,CHBr3则几乎检测不到,这与本实验的结论基本一致。
-
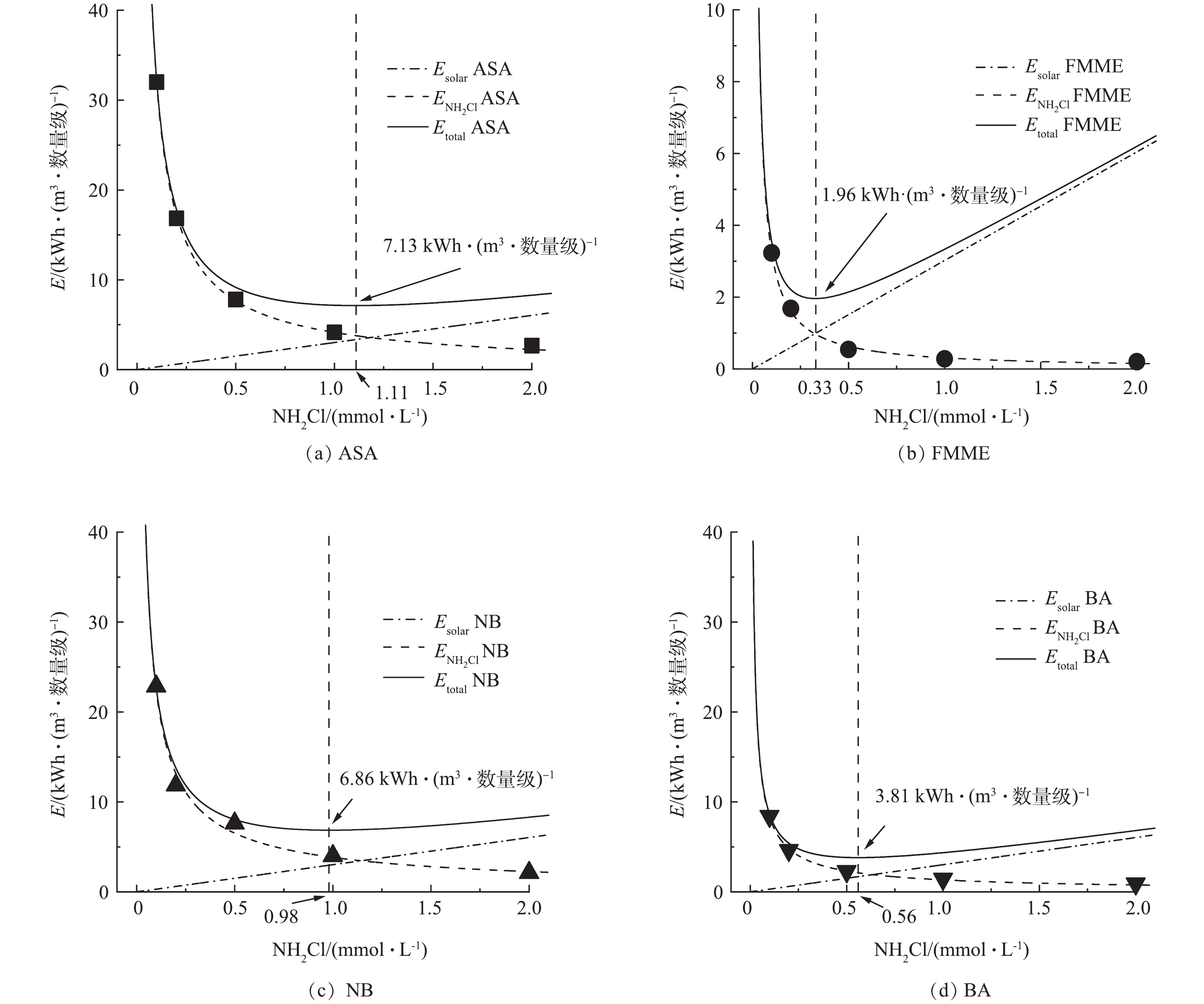
本节实验计算了不同初始NH2Cl浓度条件下(0.1~2 mmol·L−1),太阳光/NH2Cl体系降解污染物需要的总电能消耗Etotal。经过计算可发现(图7),氧化剂的投加量成本(
ENH2Cl )随着NH2Cl的投量增加而线性增加,而模拟太阳光装置的耗电量Esolar随着NH2Cl投加浓度的增加而逐渐下降。体系总电能消耗Etotal则随着NH2Cl投加浓度的增加先降低再升高,总电能消耗Etotal的最小值对应的NH2Cl投加浓度即为最经济的NH2Cl投量。4种污染物的最低总电能消耗不同,在本节实验条件下,太阳光/NH2Cl体系降解ASA、FMME、NB和BA的最低成本分别为7.13、1.96、6.86和3.81 kWh·(m3·数量级)−1,对应的最佳NH2Cl投量分别为1.11、0.33、0.98及0.56 mmol·L−1。在使用UV/H2O2降解水中阿莫西林的一项研究中[25],其降解成本换算为电能消耗为0.694 kWh·(m3·数量级)−1,远低于本研究的处理成本,推测是由于太阳光照对于NH2Cl的活化效果比UV要差,且NH2Cl的生产成本高于H2O2。 -
1)本实验选择的4种污染物ASA、BA、FMME和NB在单独太阳光照及单独NH2Cl体系中几乎均不发生降解(降解率<5%),仅FMME在中性及酸性条件下的单独NH2Cl体系发生了一定降解(降解率为36.53%~40.44%)。在太阳光/NH2Cl体系中,4种污染物的降解效能显著提升,pH的变化对污染物降解的影响是多方面的,因此,4种污染物降解效能随pH的变化并不相同。
2)太阳光/NH2Cl体系的降解能力受温度影响较为显著,当溶液温度由10 ℃升高到55 ℃时,4种污染物的降解效果显著提升;ASA、FMME、NB、BA的去除率分别由73.15%、50.16%、13.61%、17.69%提升到80.66%、61.17%、52.63%、28.75%。
3)太阳光/NH2Cl体系中,以RNS和RCS为主的其他活性组分和NH2Cl共同对FMME的降解有显著贡献(贡献率为79.27%~89.39%),HO·仅在中性和酸性条件下对FMME降解有明显的贡献。而在不同pH条件下的ASA的降解中,HO·始终为贡献最大的活性组分,NH2Cl的贡献较小。
4)相较于单独NH2Cl体系,太阳光/NH2Cl体系中产生的DBPs质量浓度和毒性方面均呈增大趋势,在酸性及中性条件下尤为显著;而碱性条件下产生的DBPs质量浓度有明显下降,实际应用中使用本体系消毒时应考虑将pH调至碱性。
太阳光/NH2Cl体系降解微污染物的效能及动力学
Performance and kinetics of Solar/NH2Cl system on micro-pollutant degradation
-
摘要: 利用太阳光和一氯胺(太阳光/NH2Cl)体系降解阿司匹林((aspirin, ASA)、氟尼辛葡甲胺(flunixin meglumine, FMME)、苯甲酸(benzoic acid, BA)以及硝基苯(nitrobenzene, NB)4种代表性微污染物,探究该体系对于微污染物的降解效能以及动力学特征。结果表明,单独太阳光以及单独NH2Cl体系对污染物几乎均无降解效果(降解率<5%),而太阳光/NH2Cl体系中污染物的降解效能显著提升,pH的改变对4种污染物降解效能的影响各不相同。在实验pH条件下,以活性氮自由基和活性氯自由基为主的其他活性组分和NH2Cl对FMME的降解起主要作用(贡献率为79.27%~89.39%),而羟基自由基(HO·)始终是ASA的降解中贡献最大的活性组分(贡献率为44.9%~76.8%)。在酸性环境中,太阳光/NH2Cl体系产生的消毒副产物质量浓度及毒性方面均大于单独NH2Cl体系,而在碱性条件下其产生的消毒副产物质量浓度则明显降低,实际中应用本体系进行消毒时应考虑将pH调至碱性。Abstract: In this study, the Solar/chloramine system was used to degrade four representative micro-pollutants, including aspirin (ASA), flunixin glucosamine (FMME), benzoic acid (BA), and nitrobenzene (NB), and the degradation efficiency and kinetic characteristics of the system were investigated. The experimental results showed that both Solar alone and NH2Cl alone systems led to low removal (the degradation rate<5%). However, the degradation of the pollutants in the Solar/NH2Cl system increased significantly, and pH variation had different effects on degradation of the four pollutants. Under the experimental pH conditions, NH2Cl and other active components such as reactive nitrogen species, reactive chlorine species contributed to most FMME degradation (the contribution rate was 79.27%~89.39%), while hydroxyl radicals (HO·) made a dominant contribution to the degradation of ASA (the contribution rate was 44.9%~76.8%). Under acidic conditions, the concentration and toxicity of disinfection by-products (DBPs) produced in Solar/NH2Cl system were higher than those in NH2Cl alone. Nevertheless, the concentration of DBPs in Solar/NH2Cl system decreased significantly under alkaline conditions. Thus, the pH should be adjusted to alkaline when this system is used to disinfect water in practice.
-
Key words:
- chloramine /
- micro-pollutants /
- kinetics /
- reactive species /
- disinfection byproducts
-
氯胺是一种常见的自来水消毒剂,分为一氯胺(NH2Cl)、二氯胺(NHCl2)和三氯胺(NCl3), 是氨分子中氢原子被氯原子取代后得到的衍生物。其中,NH2Cl在自来水的消毒中应用最为广泛[1-2],一般通过氨与次氯酸(HClO)在水溶液中反应制备(式(1))而成。相比于HClO,NH2Cl的稳定性更好,消毒能力更持久,含氯消毒副产物更少;但其消毒性能比HClO差,有更复杂的消毒副产物种类[3]。
NH3+HClO→NH2Cl+H2O (1) 除了直接用于自来水消毒外,NH2Cl还是高级氧化技术(advanced oxidation processes, AOPs)的常用氧化剂之一,其经过紫外(ultraviolet, UV)照射活化之后,能够产生羟基自由基(HO·)、氯自由基(Cl·)等自由基[4](式(2)~式(6)),基于NH2Cl的高级氧化技术具有选择性强,药品利用率高等优点。目前,UV/NH2Cl体系应用的领域包括饮用水、污水的杀菌消毒[5-6]以及水中微污染物的降解等[7-8]。LU等[9]研究表明,在中性条件下,中压紫外/氯胺体系能够有效降解水中的常见抗生素环丙沙星,去除率可达84.9%。另外一项研究使用了NH3/Cl2体系对水中的阿特拉津进行了降解[10],结果表明,体系中产生了HO·,活性氯自由基(reactive chlorine species, RCS)等活性组分,并成功实现了对阿特拉津的降解(降解率为50%)。
NH2Cl+hv→∙NH2+Cl∙ (2) ∙Cl+H2O→∙ClOH−+H+ (3) ∙Cl+OH−→∙ClOH− (4) ClOH−∙→∙OH+Cl− (5) ∙Cl+Cl−→Cl−2 (6) 与基于紫外光UV的高级氧化系统相比,基于模拟太阳光的高级氧化系统被认为是一种更为节能环保的处理技术。在到达地球的太阳辐射光谱中,可见光(波长为λ,400<λ<760 nm)约占49%,红外光(760<λ<4 000 nm)约占46%,紫外光(λ<400 nm)约占4%[11]。近年来, 太阳光(solar)耦合自由氯(free available chlorine, FAC)体系在水处理领域的应用越来越受到关注,如消毒和药品和个人护理产品(pharmaceuticals and personal care products, PPCPs)的降解等[12]。HUA等[13]研究了太阳光/FAC系统对包括抗生素、非甾体抗炎药、β阻滞剂在内的24种典型PPCPs的降解效能,结果表明,当pH由6.0升至8.0,体系中HO·和·Cl稳态浓度下降,而氧化氯(ClO·)稳态浓度有显著上升,此时,有11种PPCPs的去除率增大。此外,由于臭氧(O3)的产生,溶解氧(dissolved oxygen, DO)的存在能够显著提升大部分PPCPs的降解效果。整体而言,目前对于Solar/FAC体系降解微污染物的反应动力学、反应机理以及消毒副产物的产生与演化等方面的研究已经十分深入,但对于太阳光/NH2Cl体系的相关研究仍然具有大片空白。相比于FAC,NH2Cl在水体中的存留时间更长,消毒效果也更持久。此外,城市污废水中通常含有较高浓度的NH4+-N,在对其进行处理与再利用的过程中也难免会产生NH2Cl。因此,有必要对太阳光/NH2Cl体系对污染物的降解效能、反应机理等进行深入的研究。
本研究选择阿司匹林(aspirin, ASA)和氟尼辛葡甲胺(flunixin meglumine, FMME)2种典型的PPCPs作为目标污染物,以硝基苯(nitrobenzene, NB)和苯甲酸(benzoic acid, BA)这2种常见的工业污染物作为指针物质,探究了太阳光/NH2Cl体系对有机污染物的降解效能及影响因素,深入解析了太阳光/NH2Cl体系中污染物的降解机理及活性因子的贡献率,最后对这一体系降解微污染物的经济性进行了评估。
1. 材料和方法
1.1 药品与仪器
主要药品试剂:阿司匹林(C9H8O4,分析纯),购自上海阿拉丁试剂有限公司;氟尼辛葡甲胺(C14H11F3N2O2C7H17NO5,纯度98.0%),购自上海源叶生物科技有限公司;硝基苯(C6H5NO2,纯度99.0%),购自上海阿拉丁试剂有限公司;苯甲酸(C7H6O2,纯度99.0%),购自国药集团化学试剂有限公司;三卤甲烷(trihalomethanes, THMs)标样(CHCl3,CHCl2Br、CHClBr2及CHBr3,色谱纯),购自美国o2si标准品公司。
主要仪器:高亮度平行光源系统(CHF-XM-500W);超高效液相色谱仪(B16CHA763G);总有机碳分析仪(TOC-L CPH);低温恒温循环器(MQA-30);紫外可见光分光光度计(DR6 000)等。
1.2 实验方法
本研究使用的装置为特殊定制的光化学氧化反应实验装置(图1(a))。采用容量为50 mL的圆形反应皿作为反应器,置于低温恒温水浴加热器中。反应器内部安装高亮度平行光源以模拟可见光,其光谱见图1(b)。
污染物降解实验:若无特殊说明,则实验所用的污染物浓度均为10 μmol·L−1,投加NH2Cl初始浓度为0.2 mmol·L−1。NH2Cl采用现用现配的方式,在pH=8.5的条件下将Cl2与N的质量比为4:1的NaClO和NH4Cl溶液混合30 min后使用,然后将配制好的反应溶液置于装置中进行水浴控温,加入NH2Cl后打开灯罩开始反应,随后在取样时间检测污染物的浓度。
消毒副产物生成情况检测实验。取20 mL 4种污染物的混合溶液加入棕色管,并向其中加入相当于混合溶液的总有机碳(total organic carbon, TOC)总量5倍的NaClO溶液,反应24 h。随后加入200 μL 500 mmol·L−1抗坏血酸溶液,终止反应。加入约3.0 g无水硫酸钠和4.0 mL甲基叔丁基醚,振荡后静置10 min,从上层有机相取1.0 mL样品进行检测。
1.3 分析检测方法
使用美国Waters公司ACQUITY UPLC H-Class超高效液相色谱仪来检测实验中目标污染物浓度,色谱柱为ACQUITY UPLC BEH 1.7 μm C18色谱柱(2.1 mm×100 mm),进样体积10 μL,柱温40 ℃。紫外检测器中ASA与BA的检测波长为230 nm,FMME和NB的检测波长为270 nm。NH2Cl浓度的检测采用N, N-二乙基-1, 4苯二胺分光光度法。TOC的检测采用日本Shimadzu公司的TOC-L CPH型TOC分析仪,使用680 ℃催化燃烧氧化-非色散红外探测法来测定反应溶液的TOC。使用气相色谱/电子捕获检测器GC/ECD测定溶液中的THMs浓度,参考美国环境保护署开发的方法(Method 551)。样品经过不分流进样器进入气相色谱仪,进样口温度为200 ℃,进样体积为2.0 μL,氮气流速恒定为1.0 mL·min−1,检测器ECD温度为290 ℃。
1.4 计算方法
模拟太阳光装置的光量子产率可以通过式(7)进行计算,装置的有效光照强度可按照式(8)计算。本研究还采用竞争动力学方法计算目标污染物ASA和FMME与HO·的二级反应速率常数(式(9))。
E0p=kp,PNA2.303ΦPNA/PYR×Σ400300(fp,λ×εPNA,λ) (7) 式中:Ep0为光量子产率,Einstein·(m2·s)−1,本实验计算的是300~400 nm范围内可见光的光量子产率;kp,PNA为对硝基苯甲醚(p-nitroanisole, PNA)降解的拟一级反应速率,s−1,为6.8×10−5 s−1;ΦPNA/PYR是PNA在某一浓度吡啶(pyridine, PYR)存在下的量子产率系数,mol·Einstein−1,本实验的ΦPNA/PYR为5.44×10−4 mol·Einstein−1[14];fp,λ为模拟可见光装置的波长,本实验为300~400 nm,εPNA,λ表示在波长λ下PNA的摩尔吸光系数,m2·mol−1。综合计算,得到本研究中模拟太阳光装置的量子产率为1.14×10−4 Einstein·(m2·s)−1。
E=E0p∙Lhcfp,λ (8) 式中:E为装置的光照强度,W·cm−2;Ep0意义同式(7);L为阿伏伽德罗常数,6.02×1023 mol−1;h为普朗克常数,6.62×10−34 J·s;c为真空中的光速,3.0×108 m·s−1;fp,λ为模拟可见光装置的波长,nm,计算可得本实验装置的光照强度E为6.06 W·cm−2。
kTkR=ln([A]t/[A]0)ln([B]t/[B]0) (9) 式中:[A]0和[A]t分别为目标污染物在0时刻和t时刻的浓度,mol·L−1;[B]0和[B]t分别为探针化合物在0时刻和t时刻的浓度,mol·L−1,kT为目标污染物与活性因子的二级反应速率常数,L·(mol·s)−1;kR为探针化合物与活性因子的二级反应速率常数,L·(mol·s)−1。
降解过程中生成消毒副产物(disinfection by-products, DBPs)的毒性可根据式(10)计算。
cT=c(DBP)/M(DBP)c50 (10) 式中:cT表示消毒副产物的生物毒性;c(DBP)表示每种DBP的质量浓度,g·L−1;c50为半致死浓度,mol·L−1;M(DBP)为DBP的相对分子质量,g·mol−1。
使用单位数量级的等价电能消耗(electrical energy per order)来计算太阳光/NH2Cl体系降解污染物的处理成本,表示单位体积的溶液中目标污染物降解一个数量级(即降解90%)时所需的电耗成本(式(11)),其可分为光装置耗电量(Esolar,式(12))和氧化剂等价电能消耗(Eoxidant,式(13))两部分[15-16]。
Etotal=Esolar+Eoxident (11) Esolar=1000PtVlog(ci/cf) (12) Eoxident=Oenergycoxidentlog(ci/cf) (13) 式中:E为单位数量级的等价电能消耗,kWh·(m3·数量级) -1;P为模拟太阳光装置的功率,本研究中为0.5 kW;t为污染物降解数量级需要的反应时间,h;ci和cf分别为目标污染物的初始浓度和反应后浓度,mmol·L−1;V是溶液的总体积,取1 000 L;Oenergy表示生产单位质量的氧化剂的所消耗的等效电量,kWh·kg−1;coxidant为溶液中加入的氧化剂质量浓度,mg·L−1。江苏省2022年8月低于千伏电价为0.667 5元·kWh−1,NaClO和NH4Cl的市场价格分别为4.6元·kg−1和7.5元·kg−1,因此,NH2Cl的制备成本为27.1元·kg−1。计算得NH2Cl的等效电能消耗为40.60 kWh·kg−1。
2. 结果与讨论
2.1 pH对污染物降解效能的影响
使用浓度为10.0 mol·L−1的磷酸缓冲液调节反应体系的pH,考察pH对4种污染物的降解影响,4种污染物的初始浓度均为10.0 μmol·L−1,反应温度为25 ℃。首先考察在单独太阳光照和单独NH2Cl体系中4种污染物的降解情况(表1)。结果表明,在单独太阳光照条件下,4种污染物的去除率均小于5.0%;在单独NH2Cl条件下,除FMME外,3种污染物的去除率也均小于5.0%;FMME在碱性条件下几乎不发生反应(降解率<5.0%),而在酸性和中性条件下则会被NH2Cl氧化,计算贡献率时将考虑直接氧化因素。
表 1 不同pH条件下单独太阳光照和单独NH2Cl体系中污染物在15 min时的去除率Table 1. Degradation of contaminants in Solar alone and NH2Cl alone systems at different pH for 15 min污染物 15 min去除率/% 太阳光照(pH=7.0) NH2Cl(pH=5.5) NH2Cl(pH=7.0) NH2Cl(pH=8.5) ASA 3.95 3.91 4.54 1.97 FMME 0.30 40.44 36.53 3.78 NB 0.30 2.77 1.91 6.57 BA 0.12 0.66 0.35 0.15 相较于单独太阳光照或单独NH2Cl体系,太阳光/NH2Cl体系在不同pH条件下均表现出较强的氧化性,4种污染物的降解效率均有明显提高(图2(a)~(d))。在pH=5.5时,ASA、FMME、NB和BA在反应15 min时的去除率分别为39.32%、81.20%、47.08%和57.02%,4种污染物的降解效率有一定的差别,是由于体系中产生的氧化性活性组分具有选择性。当pH上升至7.0时,4种污染物的去除率均发生了变化,分别为24.14%、86.13%、30.81%和62.63%;而在pH=8.5条件下,4种污染物的去除率则分别变为33.58%、84.89%、27.19%和65.89%。ASA的去除率随pH升高先减小后增大;不同pH条件下的FMME的15 min去除率变化很小(降解率接近5.0%);NB的去除率随pH的增大而降低,且当pH由5.5升至7.0时,去除率显著下降了16.27%;BA的去除率随pH的增大而增大,但变化幅度较小(降解率<10%)。
根据拟一级动力学模型计算公式,不同pH条件下污染物的降解符合拟一级反应动力学(图2(e)),在实验的pH范围内与时间呈良好的线性关系(R2>0.90)。太阳光/NH2Cl体系中ASA、FMME、NB和BA的表观速率常数分别为1.9~3.9×10−2、1.7~1.9×10−1、2.5~4.4×10−2、6.2~8.5×10−2 min−1,FMME降解速度最快,其次为BA、NB、ASA。4种污染物的kobs随pH的变化规律和其在15 min时的去除率变化规律基本一致。
太阳光/NH2Cl高级氧化体系中主要存在大量的HO·、RCS等活性因子。溶液pH对污染物降解效能的影响可归为以下几点:1) NH2Cl体系中会产生具强氧化性的HO·(式(2)~(5))[17],其对污染物有良好的降解效果。而随着pH的升高,HO·的浓度增加,污染物的降解也将得到促进;2) ASA、FMME、NB和BA的pKa值分别为3.5、5.8、4.0和4.2,随着pH的不断增大,污染物更多以离子形态存在,其电子密度更高,还原性更强[18],更容易被太阳光/NH2Cl体系中的HO·、·Cl等活性组分所去除;3)本实验的pH在5.5~8.5,使用磷酸缓冲液调节pH的过程中引入了H2PO4−和HPO42-,与HO·的二级反应速率分别为2.0×104 L·(mol·s)−1和1.5×105L·(mol·s)−1。根据磷酸根的电离常数计算得出,碱性环境中磷酸根的主要存在形式为HPO42-,其竞争HO·等活性因子的能力强于酸性条件下占主导的H2PO4−,因此,污染物的降解效能随着pH的增高而受到抑制。3种因素的综合作用结果表现为较高pH对污染物降解的促进作用。
除了HO·和RCS之外,太阳光/NH2Cl体系还会产生一类重要的自由基,如活性氮(reactive nitrogen species, RNS),其中包括·NO和·NO2等[19]。有研究[17]表明,UV/NH2Cl体系在碱性条件下会产生大量RNS,RNS与富电子基团具有更高的反应速率,因而随着pH的增加,部分污染物的降解效果也随之提升。
对太阳光/NH2Cl体系降解4种污染物15 min后的矿化情况进行检测。结果表明,在3种pH条件下的15 min去除率差异并不显著:pH=5.5时的去除率最高,为49.56%,pH=8.5时的去除率最低,为40.87%。表明在污染物的降解过程中生成了大量中间产物。与相关研究所得到的低于7%的去除率相比[20],本实验所得到的去除率较高,可能是由于本实验所研究污染物(BA、NB)的化学结构相对简单,不易生成中间产物。
2.2 温度对污染物降解效能的影响
本节实验通过水浴恒温装置控制反应器温度,使用磷酸盐缓冲液调节反应体系初始pH为7.0,探究反应温度对于太阳光/NH2Cl体系中污染物降解效能的影响。首先考察了在无光照条件下只改变温度时,单独NH2Cl条件下4种污染物的降解效能。结果表明,在单独NH2Cl氧化情况下,除FMME外,其他3种污染物的去除率均小于5.0%(表2),说明污染物不会在高温下自分解,也未被高温下的NH2Cl氧化。FMME的去除率较高(降解率为32.53%~40.44%),且随着温度升高而略有增大,在计算贡献率时将考虑直接氧化因素。
表 2 不同温度下无光照单独NH2Cl体系中污染物的15 min去除率Table 2. Degradation of contaminants in NH2Cl alone system without solar irradiation at different temperature for 15 min污染物 去除率/% 10 ℃ 25 ℃ 40 ℃ 55 ℃ ASA 0.00 2.26 2.46 1.03 FMME 32.53 36.53 35.53 40.44 NB 0.00 0.00 2.87 3.20 BA 0.00 0.00 2.35 0.25 增加光照后,4种污染物的15 min去除率均有极大的提升(表3),且随温度升高而增大。当温度从10 ℃增加至55 ℃时,ASA、FMME、NB、BA的去除率分别从73.15%、50.16%、13.61%、17.69%提升至80.66%、61.17%、52.63%、28.75%。其中NB的去除率提升最大,达到了39.02%,而另外3种污染物的去除率的提升则并不显著(降解率为10%左右)。在UV/NH2Cl体系降解卡马西平的研究中,相似的结论也得到了验证:当温度从15 ℃上升至45 ℃时,卡马西平的去除率提高了20%左右[21]。
表 3 不同温度条件下太阳光/NH2Cl体系中污染物的15 min去除率及kobsTable 3. Degradation of contaminants and kobs in Solar/NH2Cl system at different temperature for 15 min污染物 去除率/% kobs/ min−1 10 ℃ 25 ℃ 40 ℃ 55 ℃ 10 ℃ 25 ℃ 40 ℃ 55 ℃ ASA 73.15 75.62 78.85 80.66 0.092 0.094 0.097 0.104 FMME 50.16 53.12 58.30 61.17 0.048 0.052 0.059 0.062 NB 17.69 19 .03 26.96 52.63 0.010 0.014 0.031 0.051 BA 13.61 20.21 25.25 28.75 0.014 0.015 0.020 0.023 对不同温度下污染物的去除率进行拟合,污染物的降解符合拟一级反应动力学规律(表3)。在10~55 ℃下与时间呈良好的线性关系(R2>0.96)。4种污染物的kobs均随温度的升高而增大,在10 ℃条件下ASA、FMME、NB、BA 的kobs分别为9.2×10−2、4.8×10−2、1.0×10−2、1.4×10−2 min−1;而在55 ℃条件下,其kobs分别升高至10.4×10−2、6.3×10−2、5.1×10−2和2.3×10−2 min−1。使用阿仑尼乌斯公式拟合不同温度下太阳光/NH2Cl体系降解污染物kobs的变化规律,在本实验的温度范围内,lnkobs与1/T呈良好线性关系(R2>0.98)(图3)。可算得ASA、FMME、NB及BA在太阳光/NH2Cl体系中的平均化学反应活化能分别为2.08、4.71、29.12和10.19 kJ·mol−1。
2.3 太阳光/NH2Cl体系活性组分解析
探针法是利用已知探针与某一活性自由基之间的二级反应速率常数,通过动力学方程来鉴定与计算系统中的活性因子的方法。HO·目前常用的探针为NB,因为NB选择性与系统中的HO·反应(kHO·-NB = 4.7×109 L·(mol·s)−1),而与RCS、RNS等自由基的反应速率低4个数量级以上,可以认为几乎不反应;BA与HO·之间的二级反应速率为5.3×109 L·(mol·s)−1,且也会与RCS快速反应,但一般认为难以与RNS发生反应。根据2.1节的实验,可以基本确定太阳光/NH2Cl体系中至少存在4种主要的活性组分,分别为NH2Cl、HO·、RCS、RNS[19]。本节实验利用NB的降解实验来分析HO·的贡献率[17]。NH2Cl的贡献率则通过单独NH2Cl体系的降解实验来计算。结果表明,单独NH2Cl体系中15 min时NB、BA的去除率小于1%,而ASA和FMME分别为3.2%和33.6%。因此,在太阳光/NH2Cl体系中前两者的降解没有NH2Cl参与,而后两者计算贡献情况时不能忽略NH2Cl的直接氧化作用。
太阳光/NH2Cl体系中HO·的稳态浓度可以通过NB的拟一级反应速率常数确定。2.1节的实验结果已经表明NB在太阳光/NH2Cl体系中的降解符合一级反应动力学,在pH=5.5时,NB的kobs为0.044 min−1,即0.73×10−3 s−1。可以计算出在pH=5.5条件下太阳光/NH2Cl系统中的羟基自由基稳态浓度[HO·]ss为1.57×10−13 mol·L−1。根据式(9)可计算得HO·与ASA和FMME的二级反应速率分别为2.53×109 L·(mol·s)−1和3.73×109 L·(mol·s)−1。因此可分别计算得到HO·对ASA和FMME的降解速率kHO·-ASA和kHO·-FMME,分别为3.97×10−4 s−1和5.86×10−4 s−1。其他pH条件下的速率常数可同理计算得到(表4)。
表 4 不同pH条件下HO·与FMME和ASA的速率常数Table 4. The rate constants of HO· reacting with FMME and ASA at different pHs s−1pH kobs-ASA kobs-FMME kOH-ASA kOH-FMME 5.5 0.65×10−3 2.82×10−3 3.97×10−4 5.86×10−4 7.0 0.31×10−3 3.21×10−3 2.38×10−4 3.51×10−4 8.5 0.49×10−3 3.06×10−3 2.20×10−4 3.25×10−4 单独NH2Cl贡献率的测定结果见图4。由图4(a)可知,在pH为5.5时,太阳光/NH2Cl体系中反应15 min时NH2Cl的CT值为138.5 min·mg·L−1,且15 min时的ASA去除率为39.3%,而单独NH2Cl体系中对应情况下ASA的去除率为2.7 %,因此可以计算出NH2Cl在ASA去除中的贡献率为6.87%。同理可计算得其他条件下的NH2Cl对FMME、ASA降解的贡献率。
不同活性物质对4种目标污染物的降解贡献率分布情况如图5所示,在对FMME的降解中,随着pH的增大,HO·的清除作用导致其贡献率逐渐降低[19],由pH=5.5时的20.8% 降低至pH=8.5时的10.6%。NH2Cl的贡献率由pH=5.5时的43.9%下降至pH=8.5时的4.4%,其他活性物质(主要是RNS和RCS)对FMME的贡献率随着pH的增加呈增长趋势,从pH=5.5时的29.5%增长到了pH=8.5时的84.9%,这可能是因为RNS的大量产生,导致FMME的快速降解[17],WU等在使用UV/NH2Cl体系降解萘普生时,得出了类似的结论[19]。
在ASA的降解中,HO·的贡献率在pH为7.0时最大,占比达到76.8%,在碱性条件下HO·的贡献率降低至44.9 %,但相比其他活性组分而言,HO·仍为主要活性自由基。NH2Cl氧化对ASA的降解贡献率较低,在pH为5.5和8.5时贡献率低于7%,在中性pH下贡献率为24.1%。
2.4 DBPs生成情况检测
本节选取了4种典型的DBPs(表5)作为代表,检测了有无太阳光照条件下,起始pH不同时NH2Cl降解ASA和FMME后DBPs的生成情况(图6)。在单独NH2Cl系统中,pH为7.0时THMs的总浓度最大,为11.0 μg·L−1,pH=8.5条件下THMs的总浓度最低。在全部的THMs中,CHCl2Br占比最大,CHCl3占比其次,而3种pH环境中并不产生CHBr3。该结果与相关文献报道基本一致[22],NH2Cl在酸性和中性条件下比在碱性条件下释放出更多的游离态氯,提高水中pH有利于提高NH2Cl生成量及稳定性,减慢HClO与三卤甲烷前体物的反应速度,从而减少水中三卤甲烷的生成量;同时,碱性条件下一些不稳定的THMs容易水解。实际应用中采用NH2Cl消毒工艺时应将pH控制在8.0以上[23]。
表 5 本实验选取的DBPs的毒性参数Table 5. Toxicity parameters of DBPs in this experiment物质名称 简称 半致死浓度/(mol·L−1) 三氯甲烷(CHCl3) TCM 9.62×10−3 二氯一溴甲烷(CHCl2Br) BDCM 11.5×10−3 一氯二溴甲烷(CHClBr2) DBCM 5.36×10−3 三溴甲烷(CHBr3) TBM 3.96×10−3 当体系中引入太阳光照之后,DBPs生成总量增大,但其生成的规律与单独NH2Cl体系类似:pH为7.0时THMs的总质量浓度最大,为16.0 μg·L−1,pH为5.0条件下总质量浓度其次,为14.1 μg·L−1。在全部的THMs中,CHCl2Br占比最大,CHCl3占比其次,而CHBr3仅在pH=5.5条件下有少量生成,pH=7.0和pH=8.5时则没有生成。有研究表明[24],随着pH从中性增大到碱性,THMs的生成会被显著抑制:在pH=8.5时,检测到的溴代三卤甲烷质量浓度相比酸性和中性时明显降低,CHBr3则几乎检测不到,这与本实验的结论基本一致。
2.5 经济性分析
本节实验计算了不同初始NH2Cl浓度条件下(0.1~2 mmol·L−1),太阳光/NH2Cl体系降解污染物需要的总电能消耗Etotal。经过计算可发现(图7),氧化剂的投加量成本(
ENH2Cl 3. 结论
1)本实验选择的4种污染物ASA、BA、FMME和NB在单独太阳光照及单独NH2Cl体系中几乎均不发生降解(降解率<5%),仅FMME在中性及酸性条件下的单独NH2Cl体系发生了一定降解(降解率为36.53%~40.44%)。在太阳光/NH2Cl体系中,4种污染物的降解效能显著提升,pH的变化对污染物降解的影响是多方面的,因此,4种污染物降解效能随pH的变化并不相同。
2)太阳光/NH2Cl体系的降解能力受温度影响较为显著,当溶液温度由10 ℃升高到55 ℃时,4种污染物的降解效果显著提升;ASA、FMME、NB、BA的去除率分别由73.15%、50.16%、13.61%、17.69%提升到80.66%、61.17%、52.63%、28.75%。
3)太阳光/NH2Cl体系中,以RNS和RCS为主的其他活性组分和NH2Cl共同对FMME的降解有显著贡献(贡献率为79.27%~89.39%),HO·仅在中性和酸性条件下对FMME降解有明显的贡献。而在不同pH条件下的ASA的降解中,HO·始终为贡献最大的活性组分,NH2Cl的贡献较小。
4)相较于单独NH2Cl体系,太阳光/NH2Cl体系中产生的DBPs质量浓度和毒性方面均呈增大趋势,在酸性及中性条件下尤为显著;而碱性条件下产生的DBPs质量浓度有明显下降,实际应用中使用本体系消毒时应考虑将pH调至碱性。
-
表 1 不同pH条件下单独太阳光照和单独NH2Cl体系中污染物在15 min时的去除率
Table 1. Degradation of contaminants in Solar alone and NH2Cl alone systems at different pH for 15 min
污染物 15 min去除率/% 太阳光照(pH=7.0) NH2Cl(pH=5.5) NH2Cl(pH=7.0) NH2Cl(pH=8.5) ASA 3.95 3.91 4.54 1.97 FMME 0.30 40.44 36.53 3.78 NB 0.30 2.77 1.91 6.57 BA 0.12 0.66 0.35 0.15 表 2 不同温度下无光照单独NH2Cl体系中污染物的15 min去除率
Table 2. Degradation of contaminants in NH2Cl alone system without solar irradiation at different temperature for 15 min
污染物 去除率/% 10 ℃ 25 ℃ 40 ℃ 55 ℃ ASA 0.00 2.26 2.46 1.03 FMME 32.53 36.53 35.53 40.44 NB 0.00 0.00 2.87 3.20 BA 0.00 0.00 2.35 0.25 表 3 不同温度条件下太阳光/NH2Cl体系中污染物的15 min去除率及kobs
Table 3. Degradation of contaminants and kobs in Solar/NH2Cl system at different temperature for 15 min
污染物 去除率/% kobs/ min−1 10 ℃ 25 ℃ 40 ℃ 55 ℃ 10 ℃ 25 ℃ 40 ℃ 55 ℃ ASA 73.15 75.62 78.85 80.66 0.092 0.094 0.097 0.104 FMME 50.16 53.12 58.30 61.17 0.048 0.052 0.059 0.062 NB 17.69 19 .03 26.96 52.63 0.010 0.014 0.031 0.051 BA 13.61 20.21 25.25 28.75 0.014 0.015 0.020 0.023 表 4 不同pH条件下HO·与FMME和ASA的速率常数
Table 4. The rate constants of HO· reacting with FMME and ASA at different pHs s−1
pH kobs-ASA kobs-FMME kOH-ASA kOH-FMME 5.5 0.65×10−3 2.82×10−3 3.97×10−4 5.86×10−4 7.0 0.31×10−3 3.21×10−3 2.38×10−4 3.51×10−4 8.5 0.49×10−3 3.06×10−3 2.20×10−4 3.25×10−4 表 5 本实验选取的DBPs的毒性参数
Table 5. Toxicity parameters of DBPs in this experiment
物质名称 简称 半致死浓度/(mol·L−1) 三氯甲烷(CHCl3) TCM 9.62×10−3 二氯一溴甲烷(CHCl2Br) BDCM 11.5×10−3 一氯二溴甲烷(CHClBr2) DBCM 5.36×10−3 三溴甲烷(CHBr3) TBM 3.96×10−3 -
[1] 孙坚伟. 紫外/氯胺消毒对供水系统中微生物数量分布的影响[J]. 中国给水排水, 2022, 38(9): 39-43. [2] 员建, 罗小平, 崔月娟, 等. 氯胺消毒对三卤甲烷生成影响因素研究[J]. 环境污染与防治, 2014, 36(7): 9-13. doi: 10.3969/j.issn.1001-3865.2014.07.003 [3] 马蓉, 吕锡武, 窦月芹. 氯胺消毒对管网中消毒副产物的控制[J]. 水处理技术, 2006, 32(7): 67-69. [4] 刘汝鹏, 郝玉友, 罗从伟, 等. 紫外/一氯胺降解水中氯霉素的性能与机理研究[J]. 中国给水排水, 2021, 37(9): 51-56. [5] 韩雪, 孙坚伟, 张力, 等. 紫外氯胺组合消毒供水系统中病毒微生物的分布特征[J]. 环境科学, 2021, 42(2): 860-866. [6] 张馨怡, 魏东斌, 杜宇国. 紫外-氯联合消毒处理及副产物生成特征研究进展[J]. 环境化学, 2018, 37(9): 1950-1560. [7] IBANEZ M, GRACIA-LOR E, BIJLSMA L, et al. Removal of emerging contaminants in sewage water subjected to advanced oxidation with ozone[J]. Journal of Hazardous Materials, 2013, 260: 389-398. doi: 10.1016/j.jhazmat.2013.05.023 [8] 朱永娟, 李健鹏, 孔德挺, 等. 紫外/氯胺高级氧化法降解水中四环素的研究[J]. 长春师范大学学报, 2022, 41(4): 74-78. [9] LU Z D, LING Y C, WANG X L, et al. Insight into the degradation of ciprofloxacin by medium-pressure UV-activated monochloramine process[J]. Science of the Total Environment, 2022, 832: 154850. doi: 10.1016/j.scitotenv.2022.154850 [10] YE B, LIU Z Y, ZHU X Q, et al. Degradation of atrazine (ATZ) by ammonia/chlorine synergistic oxidation process[J]. Chemical Engineering Journal, 2021, 415: 128841. doi: 10.1016/j.cej.2021.128841 [11] CHEN M, BLANKENSHIP R E. Expanding the solar spectrum used by photosynthesis[J]. Trends in Plant Science, 2011, 16(8): 427-431. doi: 10.1016/j.tplants.2011.03.011 [12] CHENG S S, ZHANG X R, SONG W H, et al. Photochemical oxidation of PPCPs using a combination of solar irradiation and free available chlorine[J]. Science of the Total Environment, 2019, 682: 629-638. doi: 10.1016/j.scitotenv.2019.05.184 [13] HUA Z C, GUO K H, KONG X J, et al. PPCP degradation and DBP formation in the solar/free chlorine system: Effects of pH and dissolved oxygen[J]. Water Research, 2019, 150: 77-85. doi: 10.1016/j.watres.2018.11.041 [14] LASZAKOVITS J R, BENG S M, ANDERSON B G, et al. p-Nitroanisole/Pyridine and p-Nitroacetophenone/Pyridine actinometers revisited: Quantum yield in comparison to ferrioxalate[J]. Environmental Science & Technology Letters, 2017, 4(1): 11-14. [15] ANIPSITAKIS G P, DIONYSIOU D D. Transition metal/UV-based advanced oxidation technologies for water decontamination[J]. Applied Catalysis B:Environmental, 2004, 54(3): 155-163. doi: 10.1016/j.apcatb.2004.05.025 [16] XIAO Y J, ZHANG L F, YUE J Q, et al. Kinetic modeling and energy efficiency of UV/H2O2 treatment of iodinated trihalomethanes[J]. Water Research, 2015, 75: 259-269. doi: 10.1016/j.watres.2015.02.044 [17] CHEN C Y, WU Z H, HUA Z C, et al. Mechanistic and kinetic understanding of micropollutant degradation by the UV/NH2Cl process in simulated drinking water[J]. Water Research, 2021, 204: 117569. doi: 10.1016/j.watres.2021.117569 [18] LEE Y, VON GUNTEN U. Quantitative structure–activity relationships (QSARs) for the transformation of organic micropollutants during oxidative water treatment[J]. Water Research, 2012, 46(19): 6177-6195. doi: 10.1016/j.watres.2012.06.006 [19] WU Z H, CHEN C Y, ZHU B Z, et al. Reactive nitrogen species are also involved in the transformation of micropollutants by the UV/monochloramine process[J]. Environmental Science & Technology, 2019, 53(19): 11142-1152. [20] XIANG Y Y, FANG J Y, SHANG C I. Kinetics and pathways of ibuprofen degradation by the UV/chlorine advanced oxidation process[J]. Water Research, 2016, 90: 301-308. doi: 10.1016/j.watres.2015.11.069 [21] BU L J, ZHOU S Q, ZHU S M, et al. Insight into carbamazepine degradation by UV/monochloramine: Reaction mechanism, oxidation products, and DBPs formation[J]. Water Research, 2018, 146: 288-297. doi: 10.1016/j.watres.2018.09.036 [22] 焦中志, 陈忠林, 陈杰, 等. 氯胺消毒对消毒副产物的控制研究[J]. 哈尔滨工业大学学报, 2005, 37(11): 1486-1488. [23] 焦中志, 陈忠林, 卢伟强, 等. 氯胺消毒对三卤甲烷类消毒副产物的控制研究[J]. 环境污染治理技术与设备, 2006, 7(6): 43-45. [24] 陈杰, 李星, 杨艳玲, 等. 预氯胺化控制消毒副产物技术研究[J]. 中国给水排水, 2005, 21(7): 5-8. [25] ZHANG Y Q, XIAO Y J, ZHONG Y, et al. Comparison of amoxicillin photodegradation in the UV/H2O2 and UV/persulfate systems: Reaction kinetics, degradation pathways, and antibacterial activity[J]. Chemical Engineering Journal, 2019, 372: 420-428. doi: 10.1016/j.cej.2019.04.160 期刊类型引用(0)
其他类型引用(1)
-






 下载:
下载: