-
氟是自然界广泛分布的元素之一,亦是人体必须的微量元素之一,但长期暴露于高氟环境中会对人体健康造成危害。自然界中高强度矿石开采尤其对含氟伴生矿石开采会形成高含氟排水或地下水,对当地居民生活用水安全构成威胁[1]。目前,有关地下水除氟技术主要包括吸附法[2]、化学沉淀法[3]、电渗析法[4]及电絮凝法[5]等,但上述方法均存在一定缺陷,如吸附法需消耗一定量化学药剂再生且吸附容量受pH影响较大;化学沉淀法药剂投加量大,产泥量大且难以深度除氟;电渗析法处理成本较高且产生一定量浓水。电絮凝除氟因占地面积小、成本低、除氟效率高,已成为国内外研究的热点,多数研究[5-6]集中于电极材料改性、参数调节控制、除氟机理及组合工艺等。有研究[7]表明,当氟离子质量浓度在3~5 mg·L−1时,在施加同等电量条件下,铝电极体系相比于铁电极体系具有更好的除氟效果;而当进水氟离子质量浓度在6 mg·L−1左右时,相同除氟效率下锌铝复合电极比单纯铝电极体系拥有更好的有机物和悬浮物去除效果[8]。另外,在铝电极体系中,当电流密度为4~15 mA·cm−2,氟离子进水质量浓度为4~6 mg·L−1时,氟离子去除率约在80%~99%,但当pH<5或pH>10时,铝电极体系所形成的氢化铝会发生水解,导致絮体吸附能力下降,除氟效率显著降低[9-10]。有研究在铝电极体系下通过引入钙、镁、氯离子来改变所形成絮体与氟离子的结合状态或提高离子强度以提高氟离子去除效率,并探讨此种情况下的除氟机理[11-12]。但上述研究多集中于低浓度含氟饮用水和地下水,含氟量在1~6 mg·L−1,而对于受外界环境污染的矿区高含氟地下水研究报道较少,并且对于高含氟情况下除氟过程和动力学模型仍需进一步研究。
本研究基于矿区高浓度含氟地下水,采用铝电极电絮凝进行除氟研究,在高氟浓度条件下考察了电流密度、极板间距、进水氟质量浓度及pH对除氟效果及电絮凝除氟动力学饱和常数的影响,建立了矿区高氟情况下地下水除氟动力学模型,以期为地下水除氟动力学过程研究及工程化应用提供参考。
-
本实验用水来自某矿区地下水,受煤矿和伴生矿开采影响,受污染地下水氟离子质量浓度在8.1~19.4 mg·L−1,其他指标检测结果如表1所示。
-
实验材料:氢氧化钠(NaOH)、盐酸(HCl)、氟化钠(NaF)均为分析纯;铝板(纯度为99.5%)由中海油天津化工研究设计院有限公司自行加工提供,尺寸为7.0 cm×4.5 cm×2.0 mm。
实验仪器:UTP-1305型直流电源;InPro3100型pH传感器;DF-101S型集热4磁力搅拌器;DR3900型哈希分光光度计;ME204E型电子天平;AUT83269型电化学工作站;JS94F型微电泳仪;D7advance型X射线衍射仪;S4TSTAR型X射线荧光光谱分析仪。
-
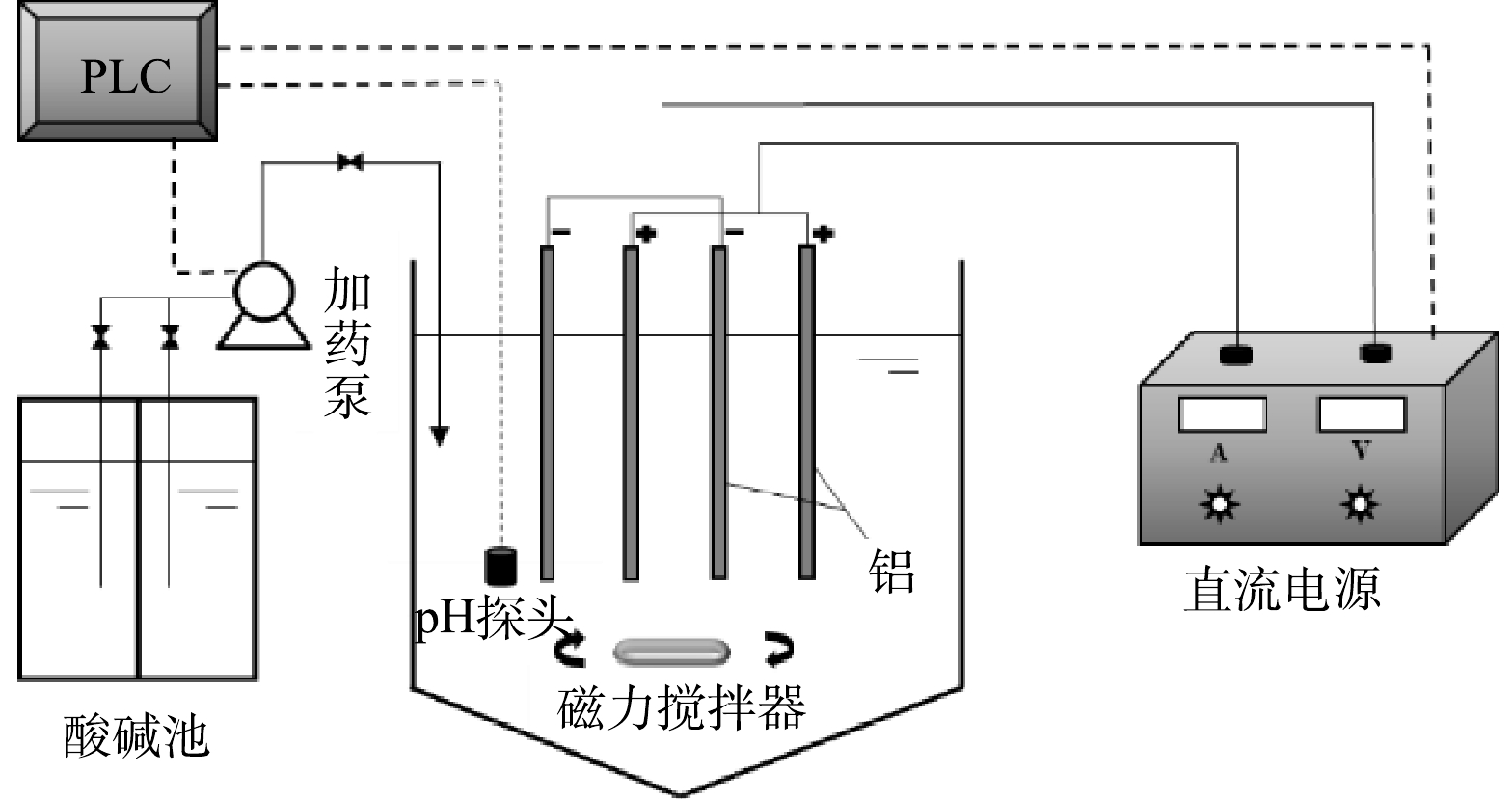
如图1所示,电絮凝反应器为有机玻璃材质,总有效容积为3 L,采用单极式铝电极布置,阴、阳极板有效放电面积均为0.03 m2,极板间距可根据需要调整,阴阳极间加载稳压直流电源,电流0~3 A连续可调,电压0~24 V自适应调节。电絮凝装置外部设有酸、碱池,分别配置有1.5 mol·L−1的HCl和1.5 mol·L−1的NaOH溶液,反应器内部设有pH探头,通过控制加入酸碱量保证反应器内维持设定pH,底部设置有磁力搅拌器和恒温控制器以维持反应器恒定温度和搅拌强度。
电絮凝反应器采用间歇批次处理矿区含氟地下水,分别考察电流密度J(150、300、450、600 A·m−3)、极板间距d(5、10、15、20 mm)、进水氟离子质量浓度C0(8~20 mg·L−1)和pH(5.0~6.0、6.0~7.0、7.0~8.0、8.0~9.0)对电絮凝除氟过程影响,其中,进水氟离子质量浓度实验采用NaF配置至所需浓度。每次实验前对电极进行打磨处理并用0.5%稀盐酸和去离子水冲洗后烘干备用。每批次电解反应时间控制在60 min,间隔10 min进行取样并用0.22 μm滤膜过滤后进行水样测定,滤膜表面含氟絮体烘干后可进行其它分析表征。
-
本实验氟离子质量浓度采用哈希分光光度法检测;电絮凝过程中产生的絮体物结构采用X射线衍射仪分析检测(XRD);含氟絮凝物元素分析采用X射线荧光光谱分析仪(XRF);pH由pH探头在线检测;电导率采用便携式电导仪检测。Tafel测试:以铝板为工作电极和对电极,饱和甘汞电极为参比电极,测试面积为1 cm×1 cm,测试溶液含12 mg·L−1氟化物,电导率2.24 mS·cm−1,pH=6.5。絮体颗粒ζ电位测定:含氟絮体颗粒的ζ电位测定取自不同pH下反应器内悬浮絮体,吸取少量液体在微电泳仪上测定;不含氟絮体颗粒ζ电位取自相同操作参数下处理非含氟水时反应器内悬浮絮体。
电絮凝反应器中溶出性Al3+浓度与外加直流电之间的关系如式(1)所示[5]。
式中:c(Al3+)为反应器内溶出Al3+质量浓度,mg·L−1;M为铝的相对摩尔质量,27 g·mol−1;I为阴、阳极间施加电流,A;t为电解时间,s;Z为溶出性铝的电荷数,取3;F为法拉第电解常数,96 485 C·mol−1;V为反应器有效容积,m3;K=M/ZF=9.328×10−5 g·C−1为常数。由式(1)可知,反应器内铝离子质量浓度与单位体积加载电流和电解时间直接相关,为便于研究本实验将单位体积加载电流定义为电流密度J,即J=I/V,A·m−3。因此,式(1)又可表示为式(2)。去除单位质量氟离子所需电耗根据式(3)计算。
式中:w为单位质量氟所需电耗,kWh·g−1; U为极间电压,V; C0为进水氟离子质量浓度,mg·L−1; Ct为t时刻氟离子质量浓度,mg·L−1。
-
在J=300 A·m−3,d=5 mm,pH=6.0~7.0,C0=12.10 mg·L−1条件下,反应器内氟离子质量浓度与去除率随时间变化状况如图2所示。由图2可知,经电絮凝反应60 min后,氟离子质量浓度由12.10 mg·L−1降至1.15 mg·L−1,去除率约为90.50%,且此时出水氟离子质量浓度不满足《生活饮用水卫生标准》(GB 5749-2022)有关氟化物的标准限值要求。另外,由式(2)可知,电絮凝时间的延长使溶出性铝离子质量浓度增加,能够形成更多的羟基铝化合物,可为氟离子结合提供更多的吸附活性位点并提高絮凝沉淀效果。此外,由图2可知,随着反应的进行,经核算单位时间去除氟离子质量浓度由0.43 mg·(L·min)−1降低为0.02 mg·(L·min)−1,即去除氟离子速率随氟离子质量浓度降低而降低,采用Origin9.1软件对电絮凝除氟过程拟合发现,整个除氟过程不同时刻氟离子质量浓度符合公式(4),遵循一级反应动力学过程。
式中:k为反应动力学饱和常数,min−1。其中,k=0.040 59 min−1,R2=0.990 6。由式(4)可知,在其他条件不变的情况下,当出水Ct=1.00 mg·L−1时,电絮凝理论反应时间T可由式(5)计算。
式中:T为理论反应时间,min。因此,当进水氟离子质量浓度C0=12.10 mg·L−1时,经电絮凝反应61.42 min后,出水氟离子质量浓度可小于1.00 mg·L−1,也即电絮凝理论反应时间T直接取决于进水氟离子质量浓度C0和反应动力学饱和常数k。结果表明,电流密度、极板间距及进水氟离子质量浓度均会对动力学饱和常数k产生影响,进而影响除氟过程和效果。因此,有必要探讨上述影响因素对除氟过程的影响。
-
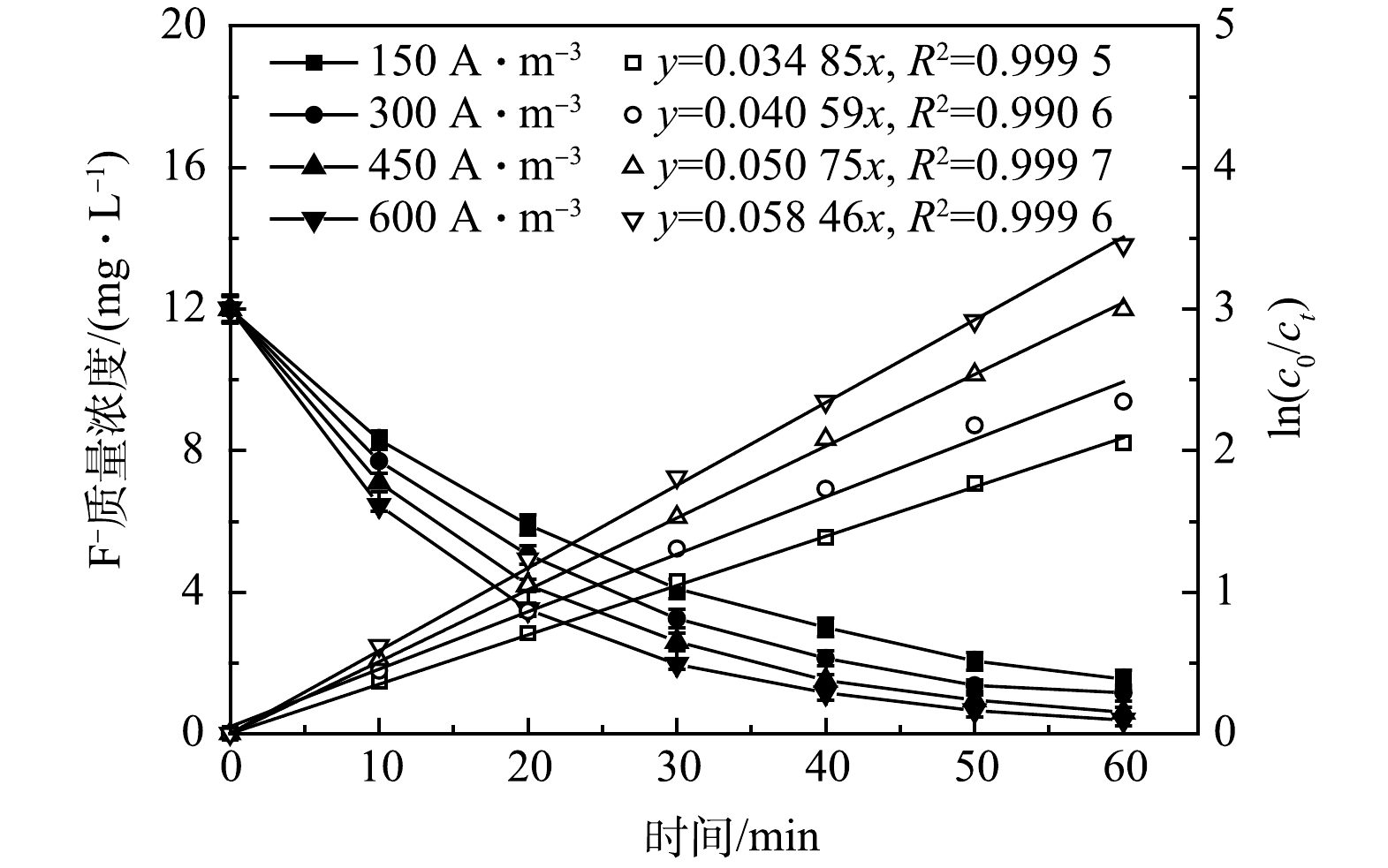
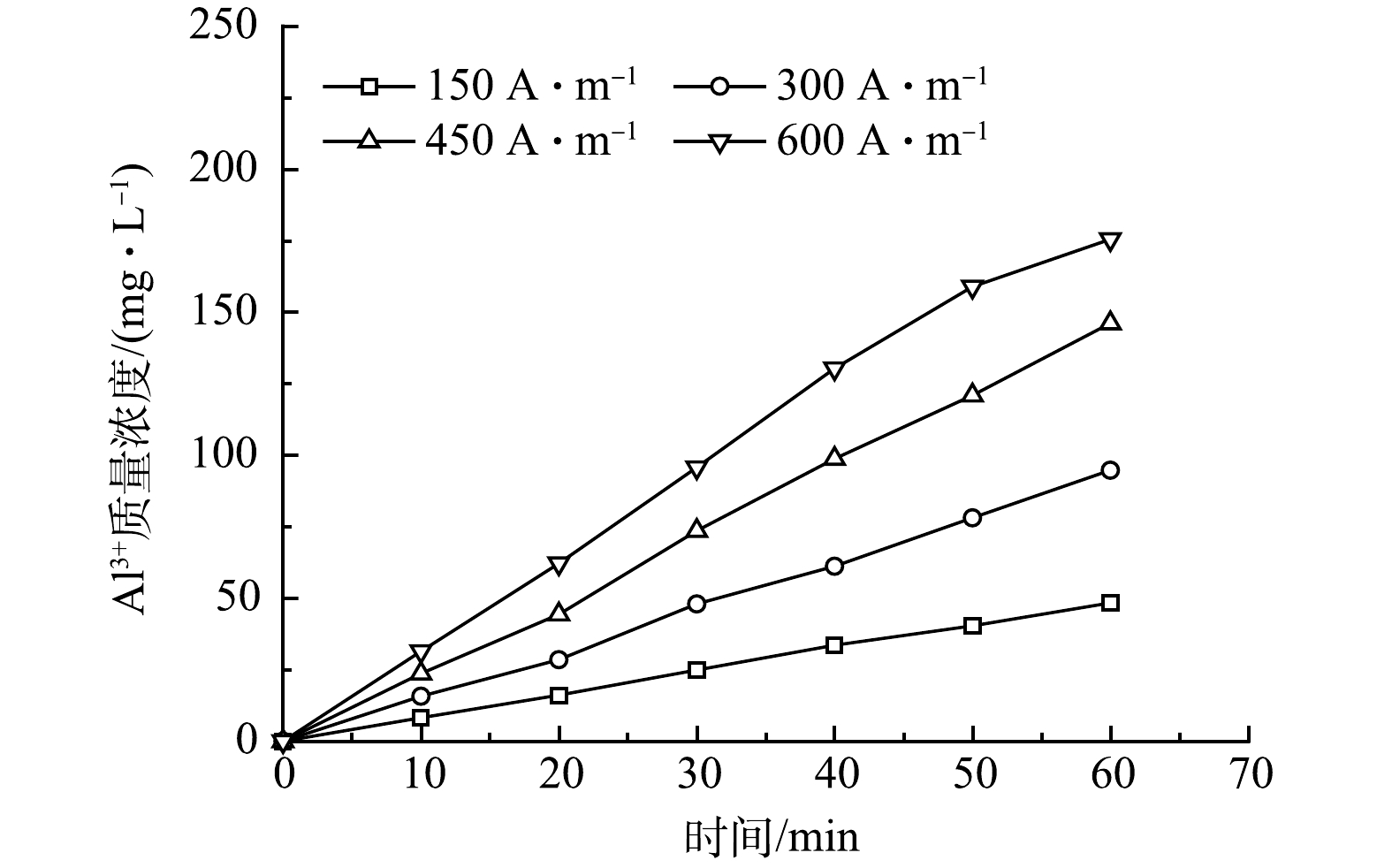
1)电流密度的影响。在C0=12.10 mg·L−1,pH= 6.0~7.0,d=5 mm,不同电流密度(150~600 A·m−3)下,电絮凝反应器内氟离子质量浓度随时间变化如图3所示。由图3可知,不同电流密度下氟离子质量浓度均随反应时间的延长而降低。当电流密度为150 A·m−3时,反应60 min后,最终出水氟离子质量浓度为1.54 mg·L−1,去除率约为87.27%;随着电流密度由300 A·m−3增加至600 A·m−3时,相同反应时间下出水氟离子质量浓度更低,在电流密度为150、300、600 A·m−3下,最终氟离子出水平均质量浓度分别为1.15、0.60和0.38 mg·L−1,总去除率分别达到90.50%、95.04%和96.86%。由此可见,增大电流密度可提高氟离子的去除率。另外,不同电流密度下氟离子去除过程均遵循一级动力学反应过程,并且随着电流密度由150 A·m−3增至600 A·m−3,动力学常数由0.034 85 min−1增至0.058 46 min−1,R2均大于0.99。而根据式(5)可知,在C0为12.10 mg·L−1, pH为6.0~7.0,d=5 mm的条件下,电流密度为150、300、450、600 A·m−3所需理论反应时间分别为71.54、61.42、49.13和42.65 min。因此,提高电流密度可提高电絮凝除氟速率,从而缩短除氟时间。
氟离子去除与铝离子质量浓度直接相关,而溶出性铝离子质量浓度取决于电流密度和反应时间(式(2)),为此对不同电流密度(150~600 A·m−3)下溶出性铝离子质量浓度随时间变化进行测定,结果如图4所示。由图4可知,不同电流密度下反应器内溶出性铝离子质量浓度均随反应时间增加而增加;随着电流密度由150 A·m−3增至600 A·m−3,经电解反应60 min后,溶出性铝离子质量浓度由48.37 mg·L−1增至175.48 mg·L−1,并且只有在电流密度为450 A·m−3和600 A·m−3条件下,出水氟离子质量浓度小于1.00 mg·L−1,满足GB 5749-2022标准要求。这说明随着电流密度的增大和电絮凝反应时间的延长,反应器内溶出性铝离子所形成的羟基铝化合物增加,从而为氟离子提供足够的活性位点提高吸附、络合及絮凝沉淀效果;但电流密度的增加和反应时间的延长势必加速阳极板的消耗且不利于缩小反应器容积和工程化实施。因此,本实验最佳电流密度为450 A·m−3。
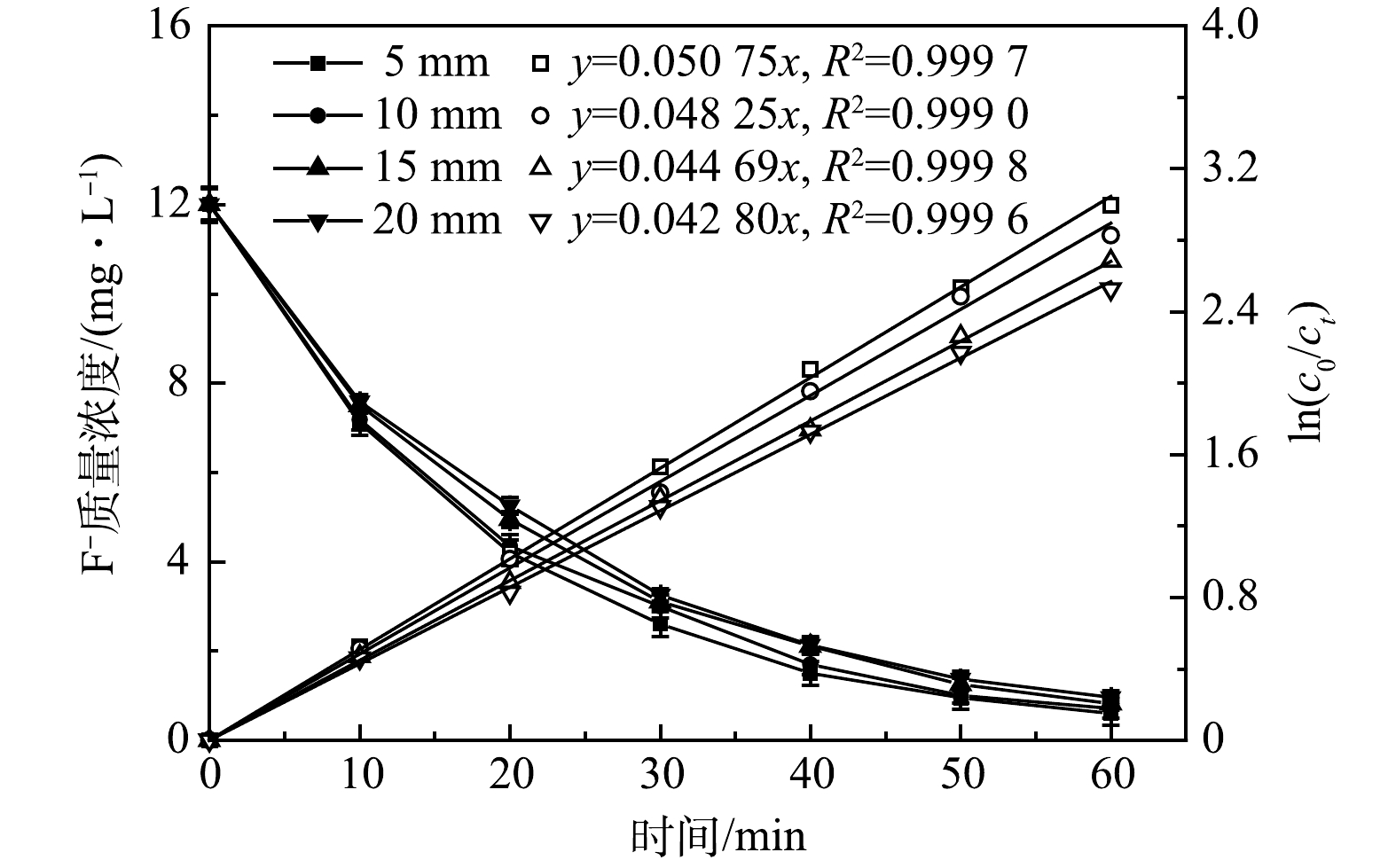
2)极板间距的影响。极板间距直接影响极间电压和反应器运行能耗,并且影响反应器内水力环境和传质过程,进而对除氟过程动力学产生影响。在J=450 A·m−3,C0=12.10 mg·L−1, pH=6.0~7.0,不同极间距(d=5~20 mm)下,氟离子质量浓度随时间变化状况如图5所示。 由图可知,不同极板间距下出水氟离子质量浓度均随反应时间延长而降低,而随着极间距由5 mm增至20 mm,出水氟离子质量浓度由0.60 mg·L−1小幅增至0.96 mg·L−1且均小于1.00 mg·L−1,满足GB 5749-2022中有关氟化物的标准限值要求,但除氟过程动力学常数却由0.050 75 min−1降至0.042 80 min−1,也即在相同电流密度下,极板间距虽然对除氟效果影响较小,但极板间距的增大促使除氟动力学常数降低。
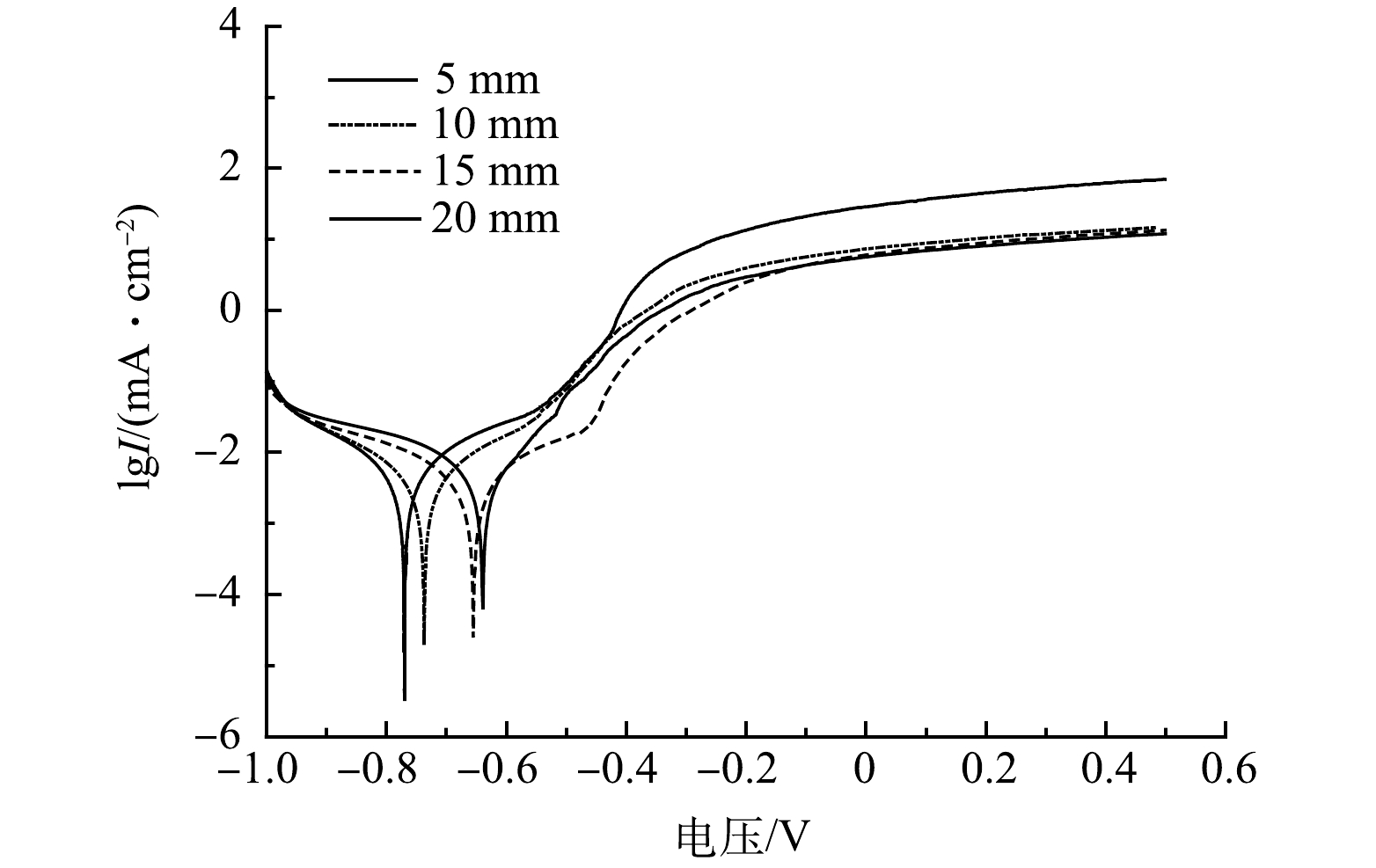
图6为不同极间距下铝电极的Tafel曲线,从其阳极分支开来看,不同极间距下均未出现钝化现象,但相同电压下电流密度随极间距的增大而减小,尤其当极间距大于5 mm时变化比较明显。这可能因为本实验处理含氟地下水电导率仅为1.65~2.42 mS·cm−1,较大的极板间距增加了离子迁移阻力,降低了氟离子与电絮凝絮体的相互作用;而较小的极板间距一方面增加了极板间电荷传导作用和氟离子向阳极板的迁移效率,另一方面阴极的析氢作用强化了极板间的气液扰动,促使氟离子与絮体的充分碰撞与吸附作用,这均有利于除氟效率的提高[13]。另外,在J=450 A·m−3,C0=12.10 mg·L−1, pH=6.0~7.0,当极间距为5、10、15、20 mm的条件下,槽电压分别达到3.79、4.86、5.93 和6.94 V,经式(3)核算得出,不同极间距下的电耗分别为0.149 6、0.193 7、0.238 7和0.282 9 kWh·g−1,也即极板间距由5 mm增至20 mm,去除单位质量的氟所需电耗增大了89.10%,这既不利于降低运行成本,也不利于缩小反应器体积。因此,本研究最佳极板间距设定为5 mm。
3)进水氟质量浓度的影响。进水氟质量浓度直接影响电絮凝时间或出水氟质量浓度(见公式4),甚至对除氟过程与效果产生影响。在J=450 A·m−3,d=5 mm,pH=6.0~7.0,不同进水氟质量浓度(8.00~20.00 mg·L−1)条件下,氟离子质量浓度随时间变化如图7所示。由图7可知,随着进水氟质量浓度由8.0 mg·L−1增至20.0 mg·L−1,经电絮凝60 min后,出水氟离子质量浓度由0.33 mg·L−1增至1.50 mg·L−1,氟离子去除率由95.86%降至92.50%,除氟过程动力学常数由0.053 34 min−1降至0.043 48 min−1,并且当进水氟质量浓度达到20.00 mg·L−1时,出水氟离子质量浓度出现了超标现象。
为进一步探究进水氟质量浓度对电絮凝除氟过程的影响,对反应器内含氟沉淀物中铝、氟元素进行了XRF分析,结果如表2所示。由表2可知,4种进水氟质量浓度下絮体中铝元素质量占比在33.9%~34.2%,相差基本不大,也即在相同电流密度下,阳极溶出性铝离子所形成的羟基铝聚合物量基本保持不变;但此时絮体中氟元素质量比由0.73%上升至2.69%,铝氟元素摩尔比由32.87:1降至8.89:1,但不同进水氟质量浓度下氟离子去除率并未明显下降,这说明在此条件下电絮凝反应器所形成的羟基铝聚合物提供的氟离子吸附活性位点充足,并且较高的进水氟质量浓度使吸附活性位点结合更加充分,从而提高了电絮凝溶出性铝离子利用效率[14];但进水氟质量浓度的增加势必造成羟基铝聚合物活性位点与氟离子结合竞争的加剧,造成反应速率下降,甚至容易引发出水氟离子质量浓度超标的风险。
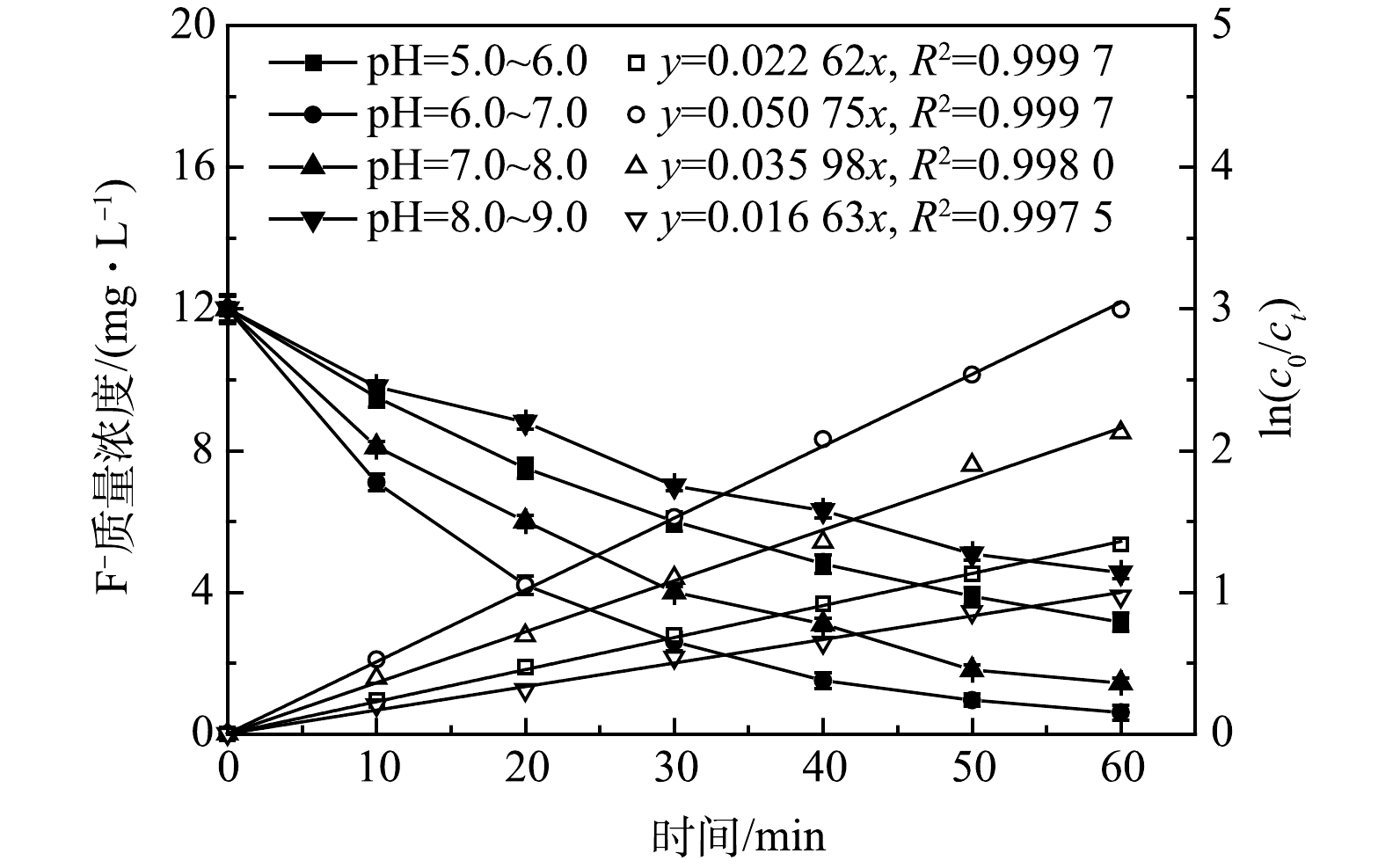
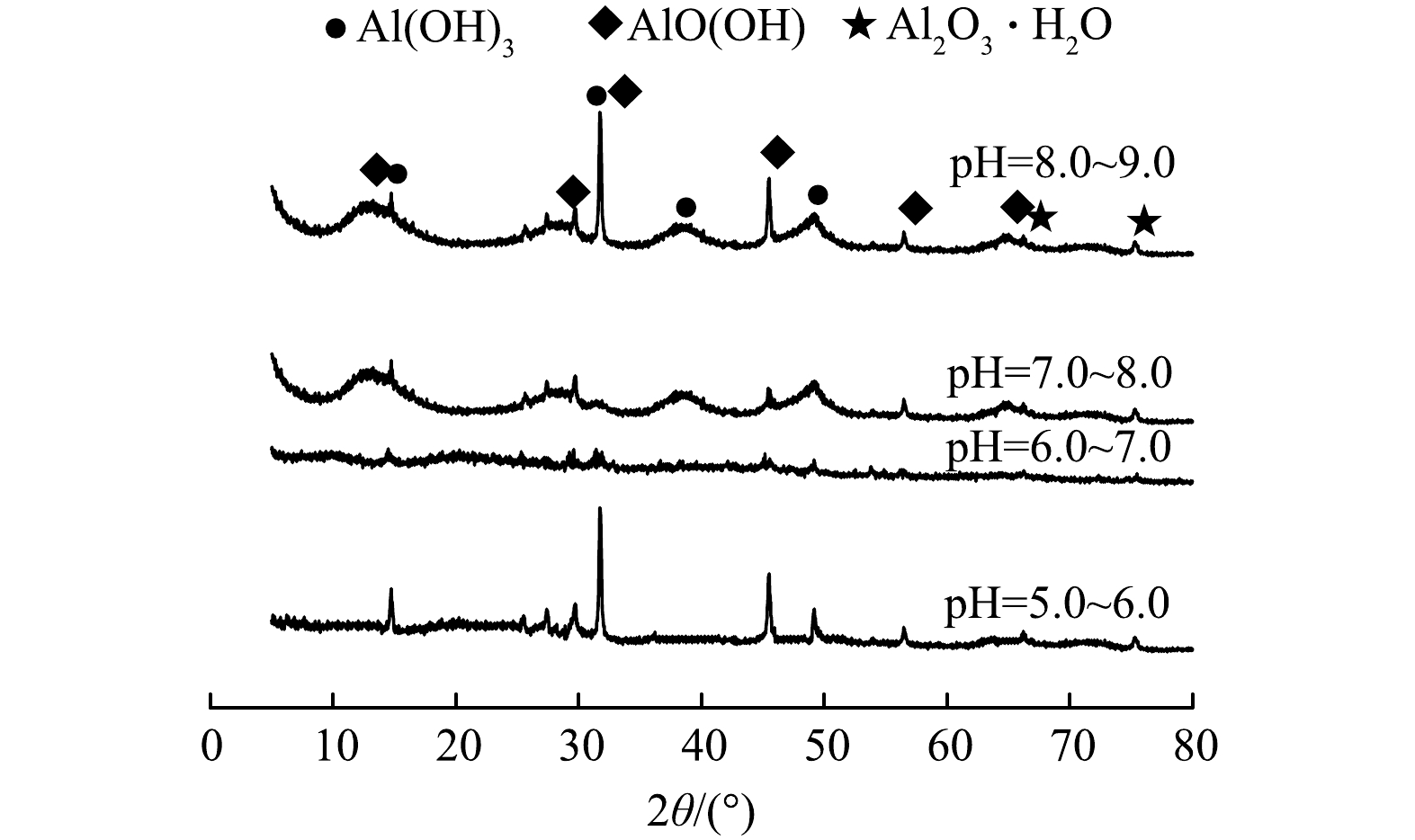
4) pH的影响。电絮凝过程中溶出性铝离子形成的羟基铝化合物形态以及电解过程中的析氢反应均与pH有密切关系,这些将直接影响电絮凝除氟效果。图8为在J=450 A·m−3,d=5 mm,C0=12.00 mg·L−1条件下,pH对氟离子质量浓度随时间变化的影响。由图8可知,不同pH下氟离子去除效果和除氟动力学常数均存在较大差异,其中,当pH=6.0~7.0时,经反应60 min后,出水氟离子质量浓度达到0.60 mg·L−1,除氟效果最好,除氟动力学常数为0.050 75 min−1;当pH=7.0~8.0时,氟离子去除效果次之,除氟动力学常数为0.035 98 min−1;当pH为5.0~6.0和8.0~9.0时,氟离子去除效果最差,除氟动力学常数分别为0.022 62 min−1和0.016 63 min−1,并且只有pH=6.0~7.0下出水氟离子质量浓度满足GB 5749-2022标准要求外,其他3种pH下出水氟离子质量浓度均出现了超标现象。
为探究其中原因,对不同pH下反应器内絮体进行XRD表征,结果如图9所示,经与标准谱图对比可知,该絮体主要为Al(OH)3、AlO(OH)及Al2O3·H2O等铝基化合物。当pH=5.0~6.0时,因反应器中OH−存在受到抑制,溶出性铝离子以Al3+,Al(OH)2+、Al(OH)2+等单体状态存在,絮体结晶程度较好,存在较为明显衍射峰,但此时絮体比表面积小,与氟离子吸附结合能力差,造成反应动力常数较低,氟离子去除效果较差;当pH=6.0~7.0时,溶出性铝离子以Al6(OH)153+、Al13(OH)345+、Al13O4(OH)247+等多核羟基铝化合物形式存在[10,15-17],絮体结晶程度差,属于无定型羟基铝化合物,此时絮体具有较大比表面积和较多的羟基活性位点,与氟离子吸附结合能力较强,吸附容量大,使除氟动力学常数较大,氟离子去除效果较好;当pH=7.0~8.0和8.0~9.0时,絮体XRD表征上出现了部分衍射峰,这可能因氢氧化铝絮体出现了部分水解[7],造成絮体与氟离子络合、吸附能力降低,除氟动力学常数出现下降,最终致使除氟效果下降。此外,对不同pH下电絮凝反应器内絮体颗粒ζ电位进行了测定(表3),由表3可知,相同pH下含氟絮体ζ电位要低于不含氟絮体,这主要因为絮体颗粒对F−、OH−及其它阴离子的吸附结合所致。另外,絮体颗粒ζ电位均随pH上升出现了不同程度下降,这显然不利于F−的吸附,但较低的pH又不利于无定型羟基铝化合物的形成。因此,电絮凝除氟最佳pH宜控制在6.0~7.0。
不仅如此,由表3可知,当pH=6.0~7.0时,由于絮体结构稳定,出水溶解性铝质量浓度为0.64 mg·L−1,明显大于0.20 mg·L−1,即不满足GB 5749-2022标准中有关铝的质量浓度要求,而其他pH条件下出水中溶解性铝质量浓度更高,这将对饮用水安全造成威胁,但通过电絮凝和膜过滤等组合工艺处理可实现对出水剩余铝离子的深度去除[9,18]。
-
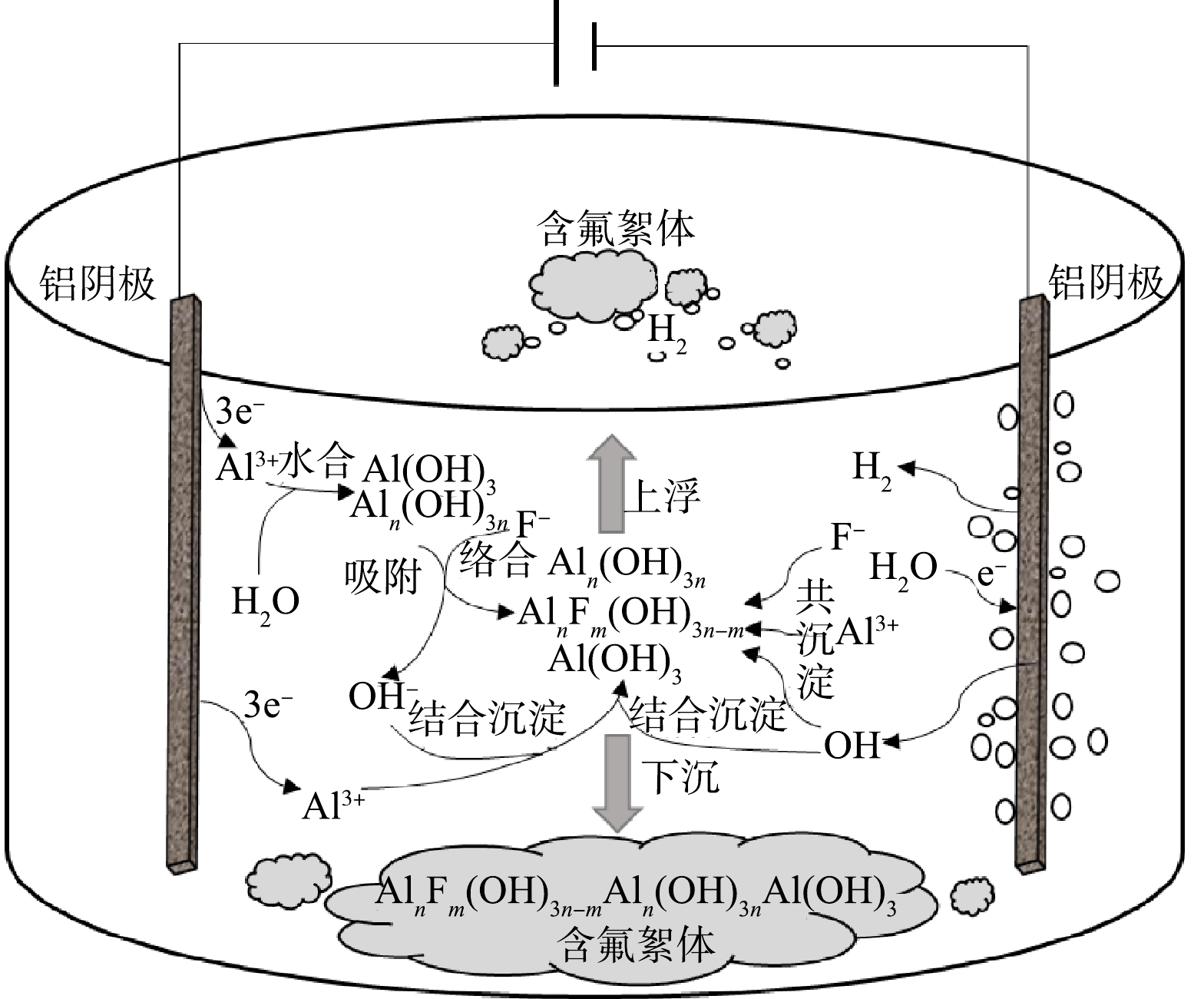
基于上述实验结果,以铝板作为电絮凝阴、阳极,如图10所示,其除氟机理主要包括以下3条路径:1)通过在铝阴、阳极间施加偏电压实现阳极氧化释放铝离子,使反应器内含有足够量溶解态铝离子;2)羟基铝化合物对氟的络合、吸附作用,溶解态铝离子在水解、聚合等作用下形成大量氢氧化铝絮体和无定型羟基铝化合物,包括Al6(OH)153+、Al13(OH)345+ 、Al13O4(OH)247+等,该类物质具有较大的比表面积,不仅为氟离子吸附、络合提供大量的活性位点,还可通过网捕、卷扫及共沉淀方式实现氟离子去除,最终形成含氟沉积物,具体见反应式(6)和式(7);3)阴极析氢产生的气浮作用,由于阴极析氢作用使电絮凝反应器产生一定量气体,通过气体扰动一方面促进极板间传质作用,增强氟离子与羟基铝化合物的结合,另一方面气泡粘附絮体上浮有利于实现含氟絮体的快速分离。
-
建立高含氟地下水电絮凝除氟过程模型不仅有利于除氟过程参数的选择与优化,而且可为地下水除氟工程化应用提供理论依据。结合上述实验结果对不同操作条件下电絮凝除氟动力学饱和常数进行了实验拟合分析,结果见表4。
上述实验结果可知,在特定pH下电絮凝除氟动力学饱和常数k直接取决于电流密度J、极板间距d和进水氟离子质量浓度C0。通过SPSS软件可根据控制因素J、d和C0对除氟动力学饱和常数K预测进行预测方程模拟分析,分析结果如表5所示。
根据表5可知,除氟动力学饱和常数K预测如式(8)所示,其中,R2=0.999;又根据式(4)和式(5)可知,电絮凝除氟过程中反应器内氟离子质量浓度随时间变化及理论反应时间T可采用式(9)和式(10)进行预测。
通过对电絮凝除氟动力学饱和常数k实验与K预测值之间进行拟合分析发现,k实验=1.013 9K预测(图11),R2=0.99,两者之间存在明显的线性关系且动力学饱和常数数值相差不大。
为进一步验证该模型的准确性,在pH=6.0~7.0条件下,选取电絮凝操作参数:电流密度300 A·m−3,极板间距为8 mm,进水氟质量浓度为14.00 mg·L−1,将实验数据与式(9)模型理论计算进行了对比,结果如图12所示。由图12可知,电絮凝除氟过程实验值与模型计算值非常接近,在此条件下,当出水氟离子质量浓度为1.00 mg·L−1时,所需电絮凝理论反应时间约为68.00 min,而根据式(10)模型预测值约为67.2 min。因此,通过分析得出,电絮凝除氟一级动力学模型与电絮凝实际除氟过程具有较好的拟合度,该模型可以准确预测在高含氟地下水条件下电絮凝除氟过程。
-
1)电絮凝对矿区高浓度含氟地下水中氟化物有较好去除率;最佳除氟参数:电流密度为450 A·m−3,极间距为5 mm,pH为6.0~7.0且电絮凝除氟过程受电流密度、极板间距、进水氟质量浓度及pH值影响;电絮凝除氟过程呈现一级反应动力学,理论除氟反应时间取决于矿区地下水氟离子质量浓度和除氟动力学饱和常数。
2)电絮凝反应动力学饱和常数随电流密度增大而增大,随极板间距和进水氟离子质量浓度增大而减小;通过对絮体XRD和XRF分析发现,在pH=6.0~7.0下,电絮凝产生絮体为无定型羟基铝化合物,除氟效果最好且较高的进水氟质量浓度使含氟絮体中铝氟比下降,溶出性铝离子利用效率更高。
3)建立了高含氟地下水电絮凝除氟动力学方程模型,即
Ct=C0e−10−5×(5.20J−55.50d−81.70C0+3956)t ,且实验结果与除氟模型预测结果有较好相符度。
基于电絮凝法处理矿区高浓度含氟地下水动力学分析
Kinetic analysis of treating high fluorine groundwater in mining area by electrocoagulation
-
摘要: 采用铝电极电絮凝体系对矿区高含氟地下水进行处理,研究了高氟浓度下电絮凝除氟过程,分别考察了电流密度、极板间距、进水氟质量浓度及pH对除氟效果和动力学常数的影响,并建立了电絮凝除氟动力学方程模型。结果表明:最佳除氟参数为电流密度为450 A·m−³,极间距为5 mm,pH为6.0~7.0;当进水氟质量浓度为12.1 mg·L−1时,经60 min电絮凝除氟后可使进水氟质量浓度由12.1 mg·L−1降为0.6 mg·L−1以下,整个除氟过程遵循一级反应动力学模型且除氟动力学常数取决于电流密度、极板间距和进水氟质量浓度。絮体结构与成分分析表明,在pH=6.0~7.0条件下,电絮凝体系中主要形成的无定型羟基铝化合物使除氟效果达到最好,较高的进水氟质量浓度有助于提高铝离子利用效率。Abstract: The defluoridation process with aluminum electrode electrocoagulation was used to treat high fluorine groundwater in the mining area. The effects of current density, electrode distance, initial influent fluoride concentration and pH values on the defluoridation effect and kinetic constant were investigated, respectively. The kinetic equation model of defluoridation was also established. The results showed that the fluoride concentration could be reduced from 12.1 mg·L−1 to 0.6 mg·L−1 after 60 minutes of electrocoagulation reaction at the optimal operational parameters: current density of 450 A·m−3, electrode distance of 5 mm and pH 6.0~7.0. The defluorination process followed the first-order reaction kinetic model, and the kinetic constant for defluoridation depended on the current concentration, electrode distance and initial fluoride concentration. The analysis of floc structure and composition showed that the amorphous hydroxy aluminum compound occurred in the electro-flocculation system at pH=6.0~7.0, which resulted in the best fluoride removal effect and high fluoride concentration in the influent water was conducive to the increase of the utilization efficiency of aluminum ions.
-
Key words:
- electrocoagulation /
- defluoridation /
- kinetic model /
- fluorine groundwater in mining area
-
电除尘器是工业烟气的主流除尘设备,在燃煤电厂的应用占比约为70% [1-3],烧结机机头的烟尘治理设备几乎全部为电除尘器[4-6]。随着燃煤电厂烟气超低排放的实施,湿式电除尘技术在燃煤电厂得到广泛应用。电除尘器主要分为电控和本体2个部分,近年来,针对燃煤电厂及非电行业的超低排放改造技术频有报道。在本体技术方面,超低排放技术包括低低温电除尘技术、湿式电除尘技术、颗粒团聚技术等[7-11]。在电源技术方面,朱法华等[12]分析了电除尘器高频电源节能减排的机理,介绍了国内外高频电源的研究与应用情况,并基于实际工程案例,介绍了高频电源的节能、减排幅度;李纪等[13]针对我国冶金转炉冶炼周期内工艺波动大、粉尘浓度及比电阻大等情况,提出了三相电源改造思路,提高了除尘器的除尘效率,并优化了电控性能;汤铭等[14]提出了一种低成本高压脉冲静电除尘电源,分析了该高压脉冲电源的稳态工作原理以及电场发生闪络时工作的情况;丁鑫龙等[15]通过实验方法,研究了脉冲电源技术对高比电阻粉尘的脱除特性;张滨渭等[16]研究发现,三相电源适合高粉尘负荷,高频电源在匹配良好条件下可实现较好的提效作用,而脉冲电源更多的研究是针对性地脱除细颗粒物和高比电阻粉尘。
按输出特性分类,电源可分为电压源和电流源,上述研究多针对干式电除尘器配套的电压源,对于湿式电除尘器配套高压恒流源的供电特性及对电除尘提效及能耗的分析,国内鲜有文献报道。电除尘器供电电源的工作状态直接影响除尘器的运行稳定性及除尘性能,对于湿式电除尘器而言,因其工作在饱和湿烟气状态,且存在喷淋冲洗环节,电场的放电状态变化大、干扰因素多,电源工作的稳定性至关重要。尤其是导电玻璃钢管式湿式电除尘器,鉴于其阳极管内壁材料的特殊性,必须尽量减少火花放电,防止电极灼伤甚至起火,保证设备安全、稳定运行。近年来,因火花控制不当等原因,山西、河南、山东等地频有导电玻璃钢管式湿式电除尘器着火事故报道。本研究通过实验室研究及现场实测相结合的手段,定量分析了导电玻璃钢管式湿式电除尘器的高压恒流源供电特性及其对电除尘提效、能耗的影响,为后续湿式电除尘器的性能提升及节能优化提供参考。
1. 实验部分
1.1 实验系统
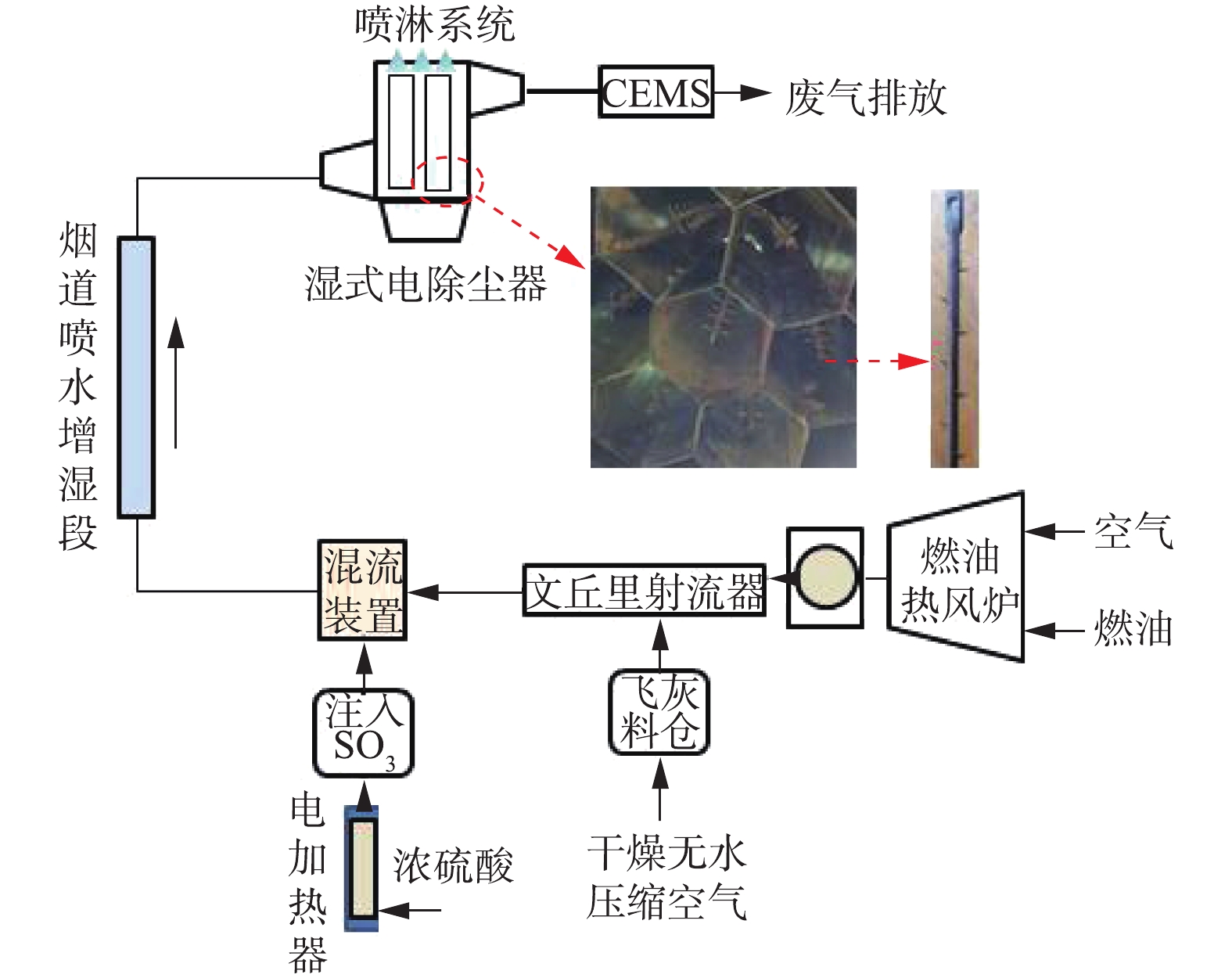
湿式电除尘器实验系统如图1所示,通过燃油热风炉产生高温烟气,设计烟气量为1×104 m3·h−1,炉膛出口烟气温度控制在70 ℃左右。通过飞灰料仓、文丘里射流器向实验系统内喷射燃煤飞灰。通过浓硫酸电加热方式产生气态SO3,以恒定流量均匀注入系统,并通过混流装置将其与烟气充分混合。通过向烟道内喷水增湿,使烟气达到湿饱和,并控制湿式电除尘器入口烟气温度在50 ℃左右。湿式电除尘器为导电玻璃钢管式湿式电除尘器,阳极板为正六边形(内切圆直径为φ300 mm),阳极管长度为4.7 m,湿式电除尘器的总集尘面积约为180 m2,阴极线为合金锯齿线,喷淋系统每次冲洗时间为5 min,冲洗水量约为0.2 t。湿式电除尘器的供电电源分别有72 kV/100 mA工频高压恒流源、恒压源和72 kV/200 mA高频高压恒流源,不同电源间可灵活切换。湿式电除尘器出口布置CEMS,用于监测出口烟气中的烟尘浓度,在实验期间,采用手工测试方法对CEMS进行数据校准。
1.2 工频电源实验
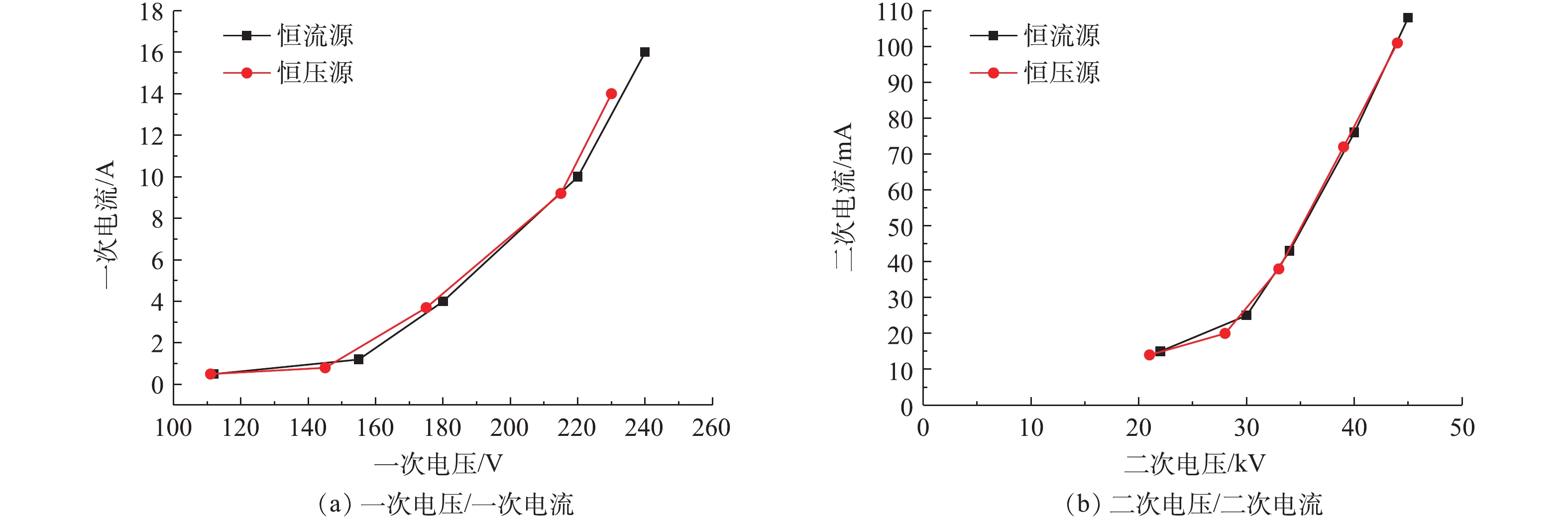
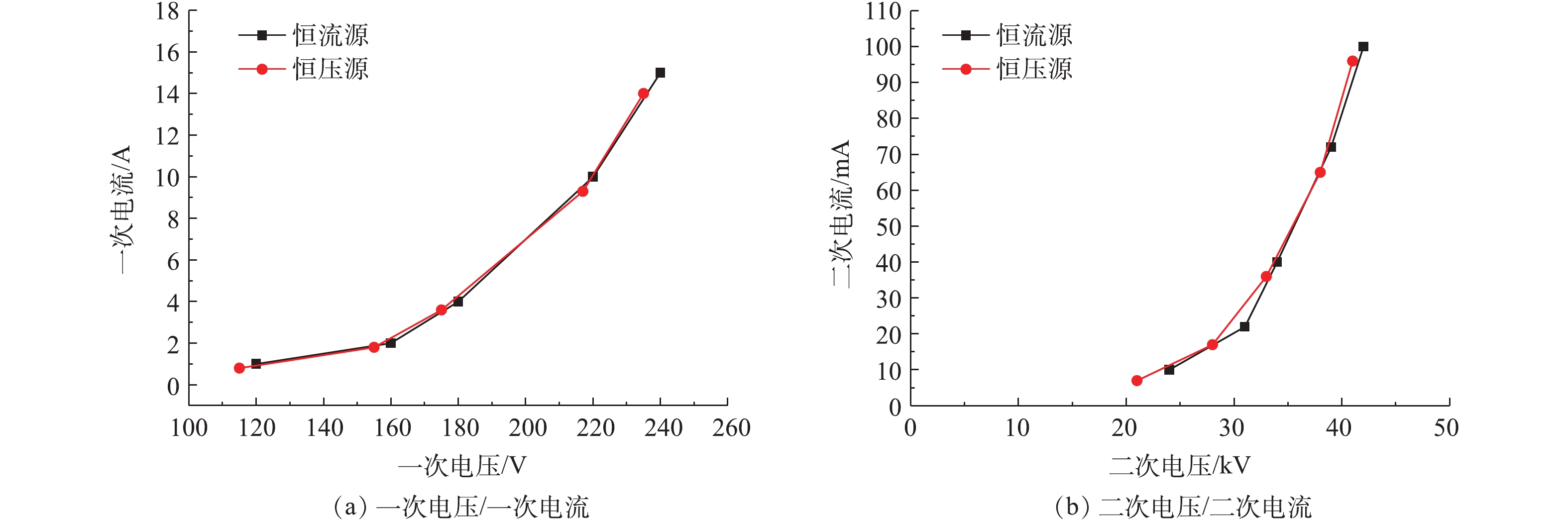
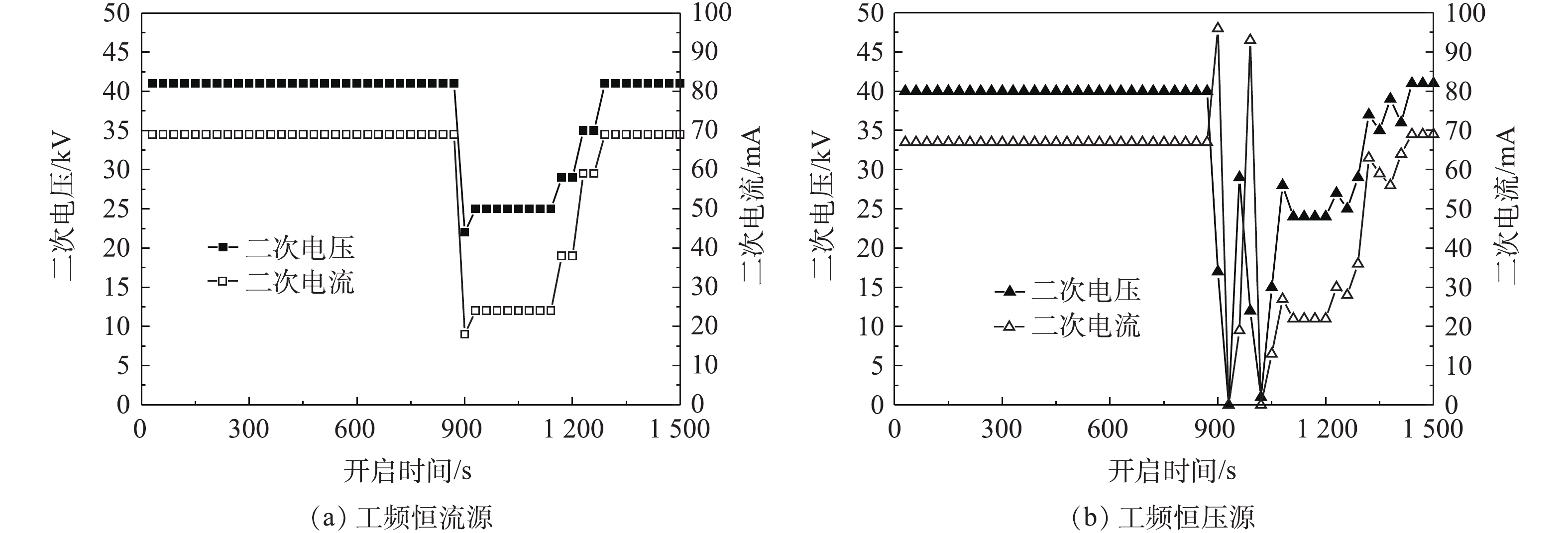
工频电源是目前电除尘器应用最为成熟和应用最多的电源[17-18]。工频恒压源输出电压恒定且可控,电流随负载变化;恒流源输出电流恒定且可控,电压随负载变化[19-21]。首先,参照行业标准《电除尘器设计、调试、运行、维护安全技术规范》(JB/T 6407-2017)的相关规定,分别在72 kV/100 mA工频高压恒流源和72 kV/100 mA工频高压恒压源供电情况下对湿式电除尘器进行空载升压实验,对应的一次电压/电流、二次电压/电流分别如图2(a)和图2(b)所示。在空载条件下,工频高压恒流源和工频高压恒压源的一次、二次电压/电流信号基本一致。
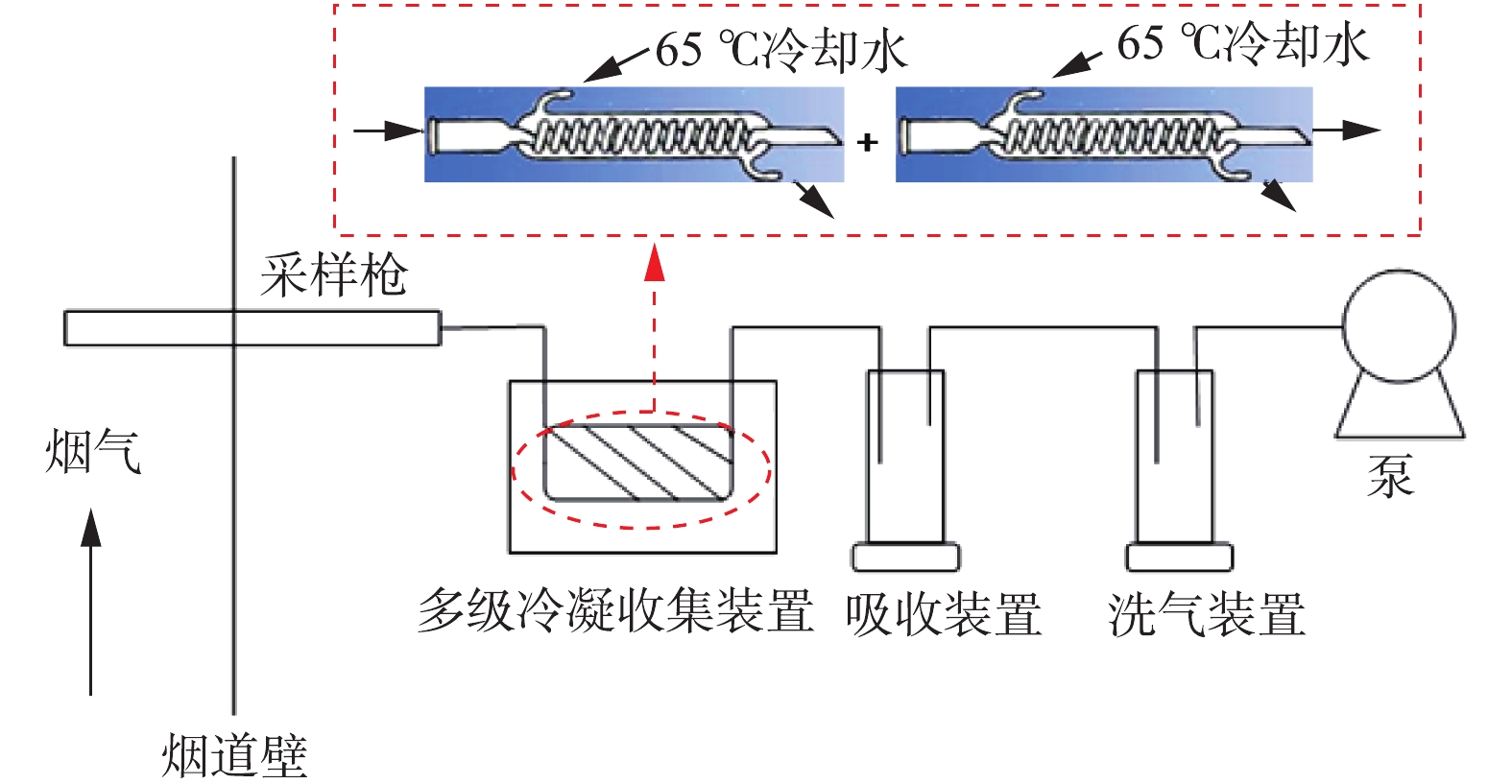
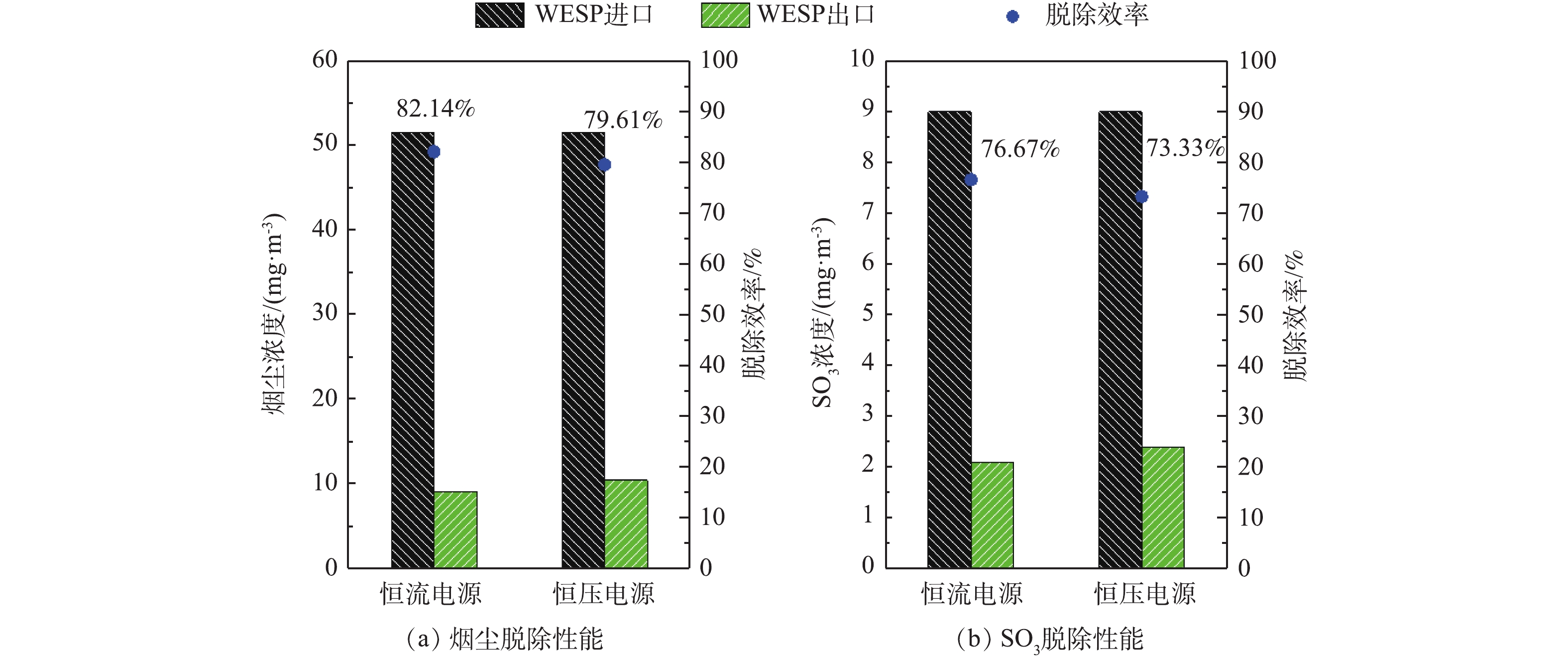
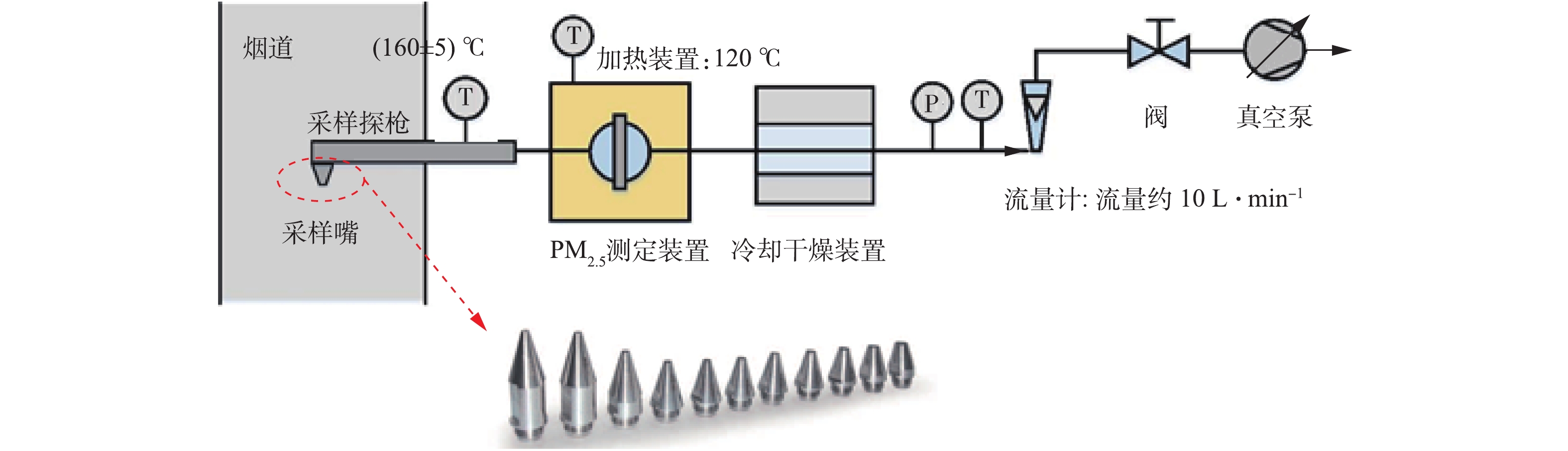
控制湿式电除尘器入口烟气温度为50 ℃,烟尘浓度为51.5 mg·m−3,SO3浓度为9 mg·m−3(大约为当前超低排放机组中湿法脱硫出口的SO3平均浓度[21])。烟尘浓度的测定采用ZR-D09A型一体化采样枪和ZR-3260型自动烟尘测试仪,测试方法符合行业标准《固定污染源废气低浓度颗粒物的测定重量法》(HJ 836-2017)的相关规定。SO3测定采用国家标准《燃煤烟气脱硫设备性能测试方法》(GB/T 21508-2008)所规定的控制冷凝法,采样系统如图3所示,水浴温度为65 ℃,多级冷凝装置为两级蛇形盘管,采样枪加热温度>280 ℃,抽气流量为20 L·min−1。采样后,用去离子水清洗蛇形盘管,之后用DR 6000型分光光度计测定溶液中的硫酸根,换算得到SO3浓度值。在上述带负载工况下,再次分别在72 kV/100 mA工频高压恒流源和72 kV/100 mA工频高压恒压源供电情况下对湿式电除尘器进行升压实验,对应的一次电压/电流、二次电压/电流分别如图4(a)和图4(b)所示。在负载条件下,工频高压恒流源和工频高压恒压源的一次、二次电压/电流信号一致性仍较好,且与空载升压时所示的运行电源参数相比差异不大。经测定,72 kV/100 mA工频高压恒流源和72 kV/100 mA工频高压恒压源供电情况下湿式电除尘器出口烟尘、SO3浓度及其脱除效率如图5所示,两者的污染物脱除性能也大致相当。
开启湿式电除尘器的喷淋系统,开启后约5 s后电场出现闪络,此时电源的二次电压、二次电流分别如图6(a)和图6(b)所示。对于工频恒流源来说,电源检测到火花放电后,自动下调电源运行参数,使得电流/电压稳定运行在相对较低的参数范围。虽然仍会有零星放电发生,但电源运行参数相对平稳,且喷淋系统关闭后,电源可自动回复到原设定参数运行。对于工频恒压源来说,在喷淋开启初期阶段,电场内频繁产生火花放电,电源运行参数不稳定,有一段明显的振荡区,且喷淋系统关闭后,其电源参数的回复过程也较恒流源慢一些。这是因为,恒流源输出特性受负载干扰产生的电流变量的约束,负载特性总能回到原来的平衡点,工作状态都是稳定的;恒压源输出存在不稳定的工作点,抗干扰能力差,喷淋系统开启后会使电除尘器进入负阻区,电流瞬间增大、电压下降,产生火花击穿,然后电源保护,停止供电,电压源既不能约束负载电压的减少又不能约束负载电流的增加,因而失去对负载的控制能力,造成电源运行参数振荡。
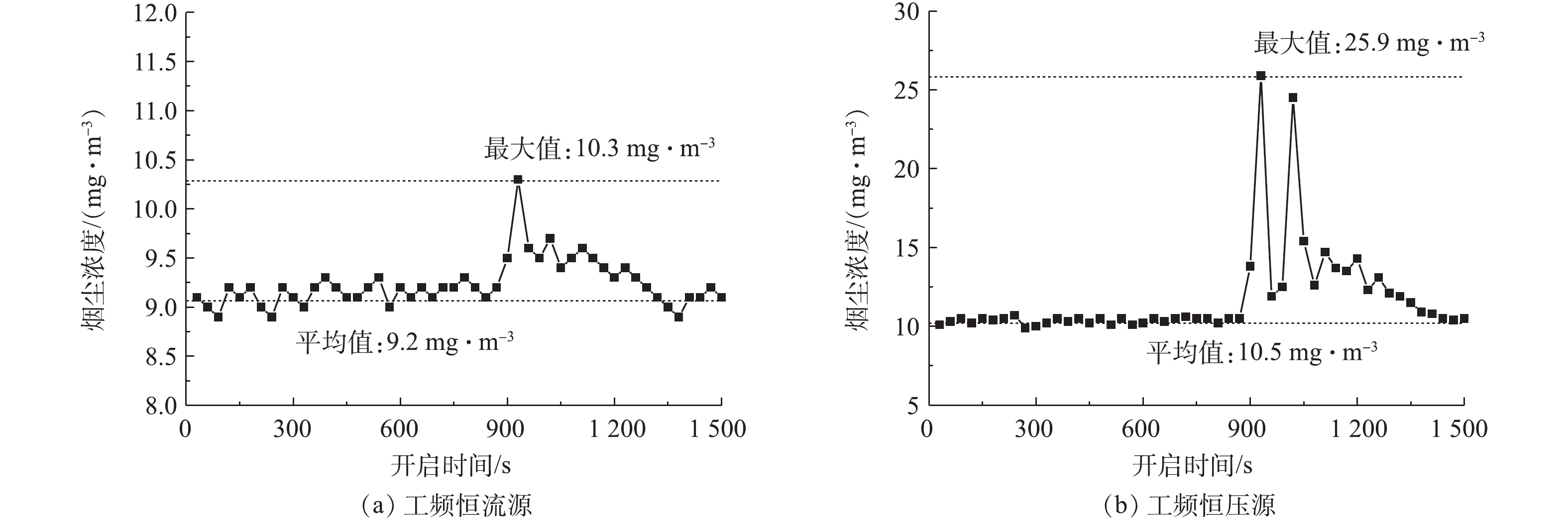
为研究不同电源供电特性对湿式电除尘器性能的影响,分别调取2种电源供电时湿式电除尘器出口CEMS测得烟尘浓度数据,显示喷淋系统开启前后湿式电除尘器出口烟尘浓度变化,结果如图7(a)和图7(b)所示。喷淋系统开启后,随着电源运行参数的降低,烟尘排放浓度均有不同程度的增加,其中,工频恒流源供电时,湿式电除尘器出口烟尘浓度最大值为10.3 mg·m−3,较喷淋前平均值(9.2 mg·m−3)增加了约12%;但恒压源存在一个电源参数振荡区,此时,出口烟尘浓度最大值达25.9 mg·m−3,较喷淋前平均值(10.5 mg·m−3)增加了约147%。因此,对于湿式电除尘器而言,应优先考虑采用抗干扰能力强的恒流源,尤其是导电玻璃钢管式湿式电除尘器,由于其阳极管内壁材料的特殊性,因此,必须尽量减少火花放电,防止电极灼伤甚至起火,保证设备安全、稳定运行。
1.3 高频电源实验
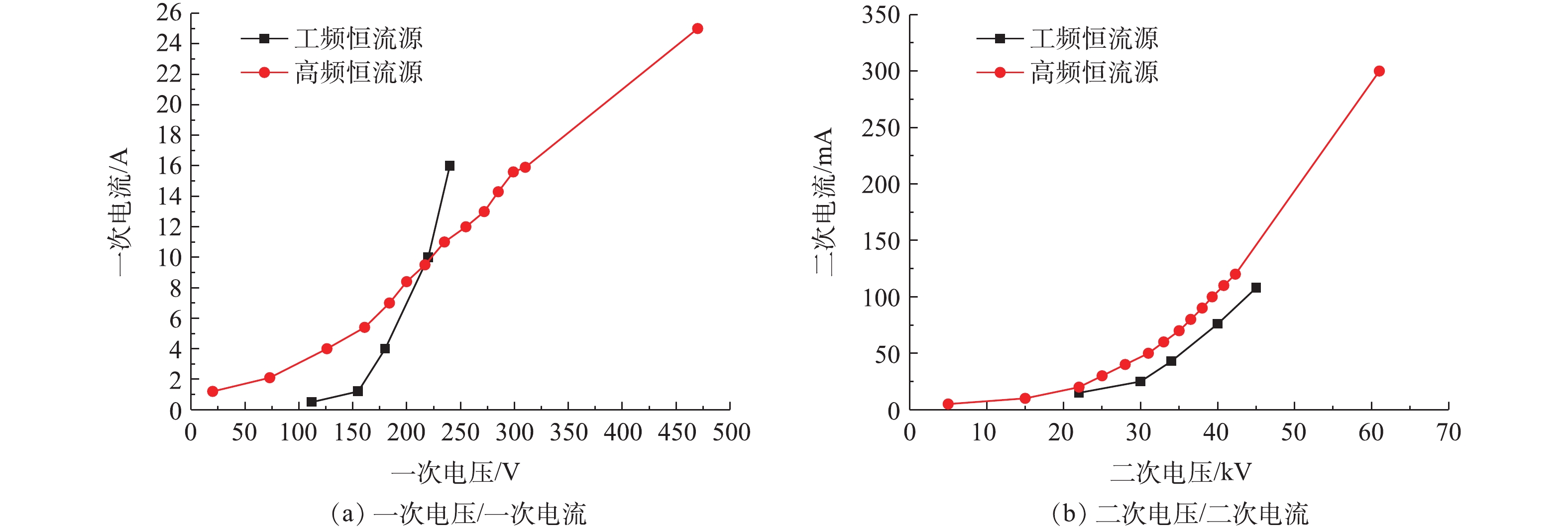
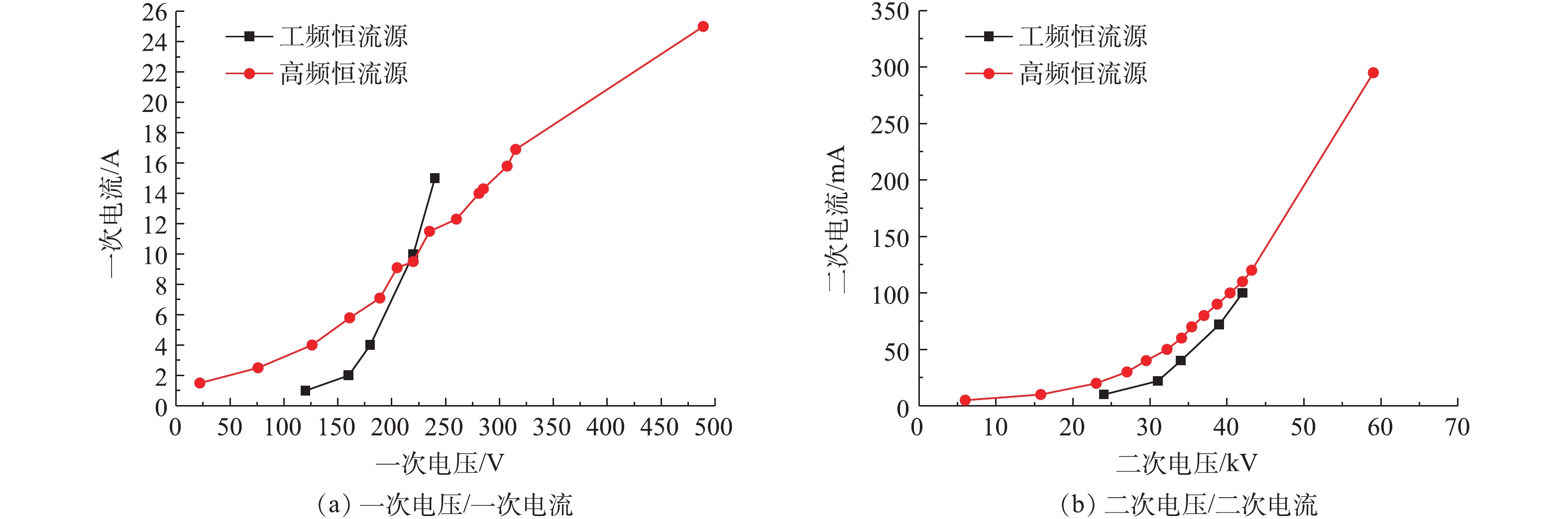
参照JB/T 6407-2017的相关规定,分别对72 kV/200 mA高频高压恒流源进行空载、负载升压实验,对应的一次电压/电流、二次电压/电流曲线及与工频恒流源对比分别如图8和图9所示。在负载条件下,高频电源的一次、二次电压/电流信号与空载升压时所示的运行电源参数相比差异不大。值得注意的是,空载实验前实际上也已通过湿烟气,只是空载时临时停掉了风机跟加灰装置,所以湿电场内的烟气仍基本处在湿饱和状态。推测是因湿电场内湿饱和烟气中水分子导电性能好,因此,运行电流较大,是否有烟气流动及飞灰加入,对升压实验的结果影响不大,这与某实际工程项目的通水升压实验/锅炉投运升压实验规律[18-19]一致。与工频恒流源相比,高频电源的功率因数更高,一般情况下,功率周数≥0.92,有效电能的转化率高,同样具有电除尘负载跟踪特性和火花抑制特性的自适应特点。因此,在相同的供电电压条件下,高频电源的运行电流更大,且在额定容量放开运行时,二次电压、二次电流可分别高达60 kV、300 mA,这更有利于湿式电除尘器的污染物脱除性能的提升。
为进一步分析高频与工频恒流源,对湿式电除尘器的提效特性,分别在相同供电电耗及高频恒流源最大电耗条件下,测定湿式电除尘器对烟尘及SO3的脱除性能。根据国家标准《电除尘器性能测试方法》(GB/T 13931-2017)的规定,采用三相有功电能表测定不同电源配置实验期间湿式电除尘器的电耗,分别记录电能表读数和测量时间,并参照式(1)计算湿式电除尘器电耗。
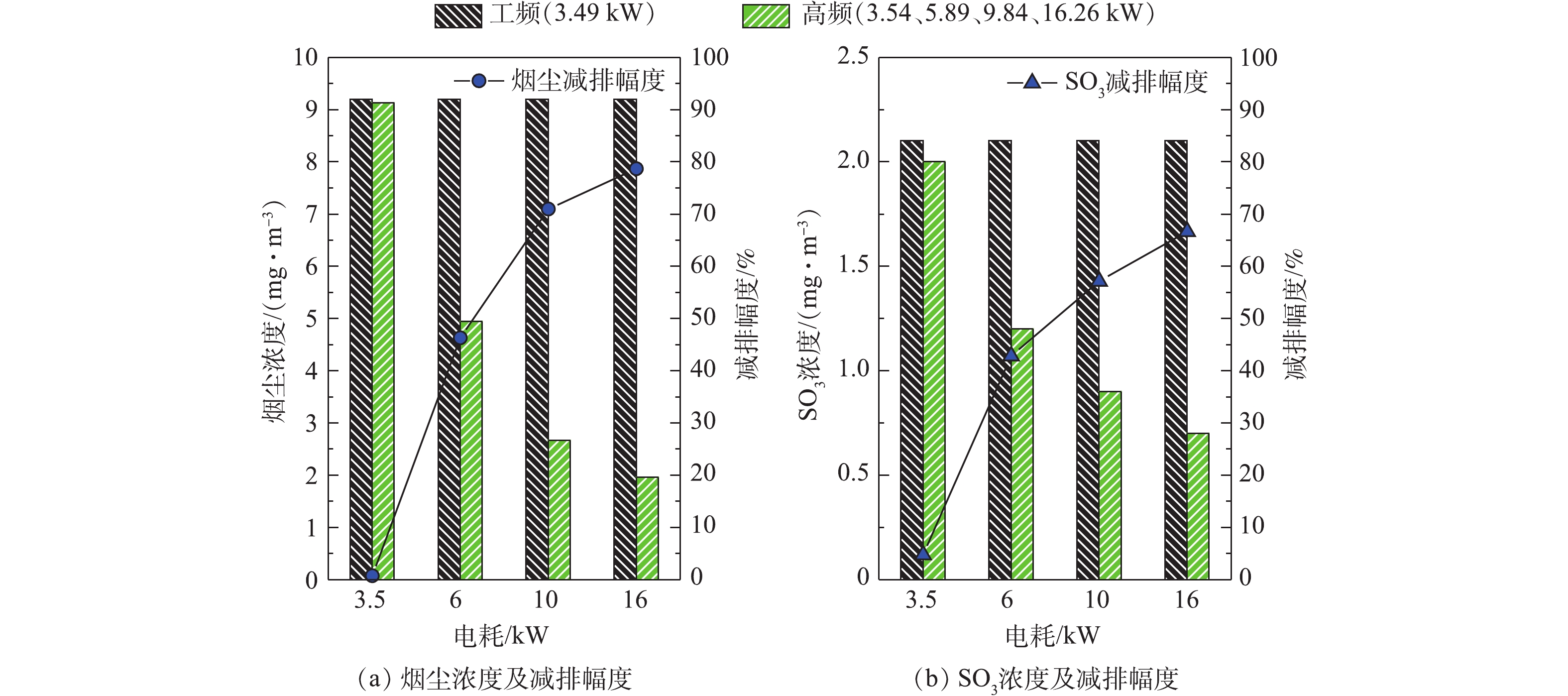
W=W2−W1t (1) 式中:
W 为湿式电除尘器电耗,kW;W2 为测量后电能表读数,kWh;W1 为测量前电能表读数,kWh;t 为测量时间,h。分别在工频恒流源电耗3.49 kW,高频恒流源电耗3.54、5.89、9.84和16.26 kW条件下,测定湿式电除尘器出口烟尘及SO3质量浓度,结果如图10所示。在供电电耗相当(工频3.49 kW、高频3.54 kW)的情况下,湿式电除尘器出口的烟尘、SO3浓度变化不大,可以认为两者具有相同的污染物脱除性能。分别将高频电源的电耗提高至5.89、9.84和16.26 kW,湿式电除尘器出口的烟尘、SO3浓度不断降低,与工频相比,烟尘的减排幅度分别为46.30%、70.98%、78.69%,SO3的减排幅度分别为42.86%、57.14%和66.67%。与烟尘的减排幅度相比,SO3减排幅度略小,这主要是因为此时SO3是以硫酸气溶胶颗粒的形式存在,粒径小(纳米级),驱进速度低,且荷电后的气溶胶颗粒还会在放电极周围产生空间电荷效应[20-23],影响电场放电。
另外,值得注意的是,随着供电电耗的增加,湿式电除尘器出口的烟尘、SO3浓度虽然不断降低,但减排幅度与电耗的增加并非呈线性关系,高频电源的供电电耗从3.54 kW增加至5.89 kW,仅增加了2.35 kW电耗,烟尘、SO3的减排幅度分别为46.30%、42.86%;但从9.84 kW增加至16.26 kW,电耗增加了6.42 kW,烟尘的减排幅度仅从70.98%增加至78.69%,增加了不足8个百分点,SO3的减排幅度仅从57.14%增加至66.67,增加了约9个百分点。因此,从节能角度来说,在满足5 mg·m−3超低排放要求的前提下,可适当减少湿式电除尘器的电能消耗,尤其是针对湿式电除尘器运行在2.5 mg·m−3甚至1 mg·m−3以下的工况,节能空间较大。该发现可为实际工程项目的节能优化运行提供有效的数据支撑。
2. 工程实测结果及分析
2.1 典型工程案例实测及分析
某660 MW机组锅炉为亚临界压力中间再热式直流炉,原配套双室四电场电除尘器出口烟尘浓度为35.7 mg·m−3,经石灰石-石膏湿法脱硫的协同除尘后仍无法满足超低排放要求,因此,在脱硫吸收塔出口烟气烟道上增设导电玻璃钢管式湿式电除尘器,分体(独立)布置,共布置4个电室,阳极采用正六边形导电玻璃钢,阴极线采用锯齿线型,喷淋系统采用间断冲洗方式,冲洗后的水进入吸收塔集水坑,作为脱硫部分用水。配套80 kV/1 600 mA高频高压恒流源。烟气量为2 127 660 m3·h−1,入口烟气温度为49~53 ℃,煤的水分、灰分、硫分含量分别为7.79%、16.59%、1.2%,低位发热量为21.4 kJ·g−1。
采用ZR-D09A型一体化采样枪、ZR-3260型自动烟尘测试仪、DEKATI PM2.5测定装置、DR 6000型分光光度计、ZR-D03A型高温采样枪等测试仪器分别测定湿式电除尘器进、出口的烟尘浓度、PM2.5浓度和SO3浓度等,并将三相有功电能表安装在湿式电除尘器除尘变出口母线处,用于读取并计算湿式电除尘器的电耗。
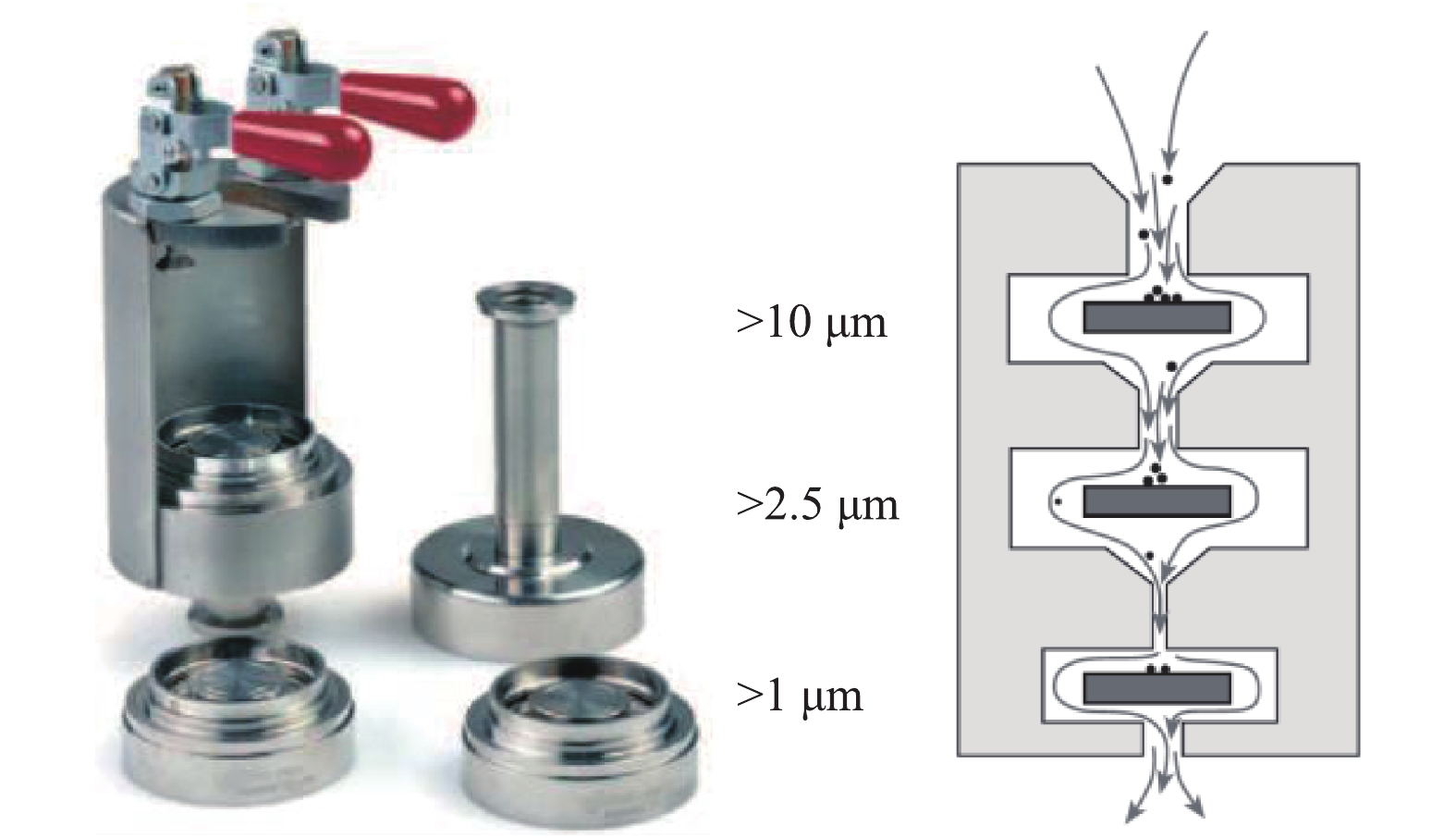
PM2.5测试采用DEKATI公司的PM2.5测试装置,测试方法参照行业标准《火电厂烟气中细颗粒物(PM2.5)测试技术规范重量法》(DL/T 1520-2016)中的规定,采样枪温度宜控制在(160 ±5)℃,PM2.5测定装置如图11所示。装置由三级撞击器组成,每级撞击器上布置滤膜,并涂上耐高温松脂,分别用于收集大于10、2.5、1 μm的颗粒,在最末级布置石英滤膜,石英滤膜对0.3 μm颗粒的拦截效率达99.9%,最末级撞击器和滤膜收集的颗粒累计为PM2.5,后二级撞击器和滤膜收集的颗粒累计为PM10。为防止液滴对颗粒分级及铝箔集尘的影响,对撞击器进行加热保温,温度为120 ℃。PM2.5的采样系统如图12所示。根据烟道流速、温度、压力等参数,选择合适的采样嘴及抽气流量,以保证各级撞击器收集的颗粒粒径在规定范围内。
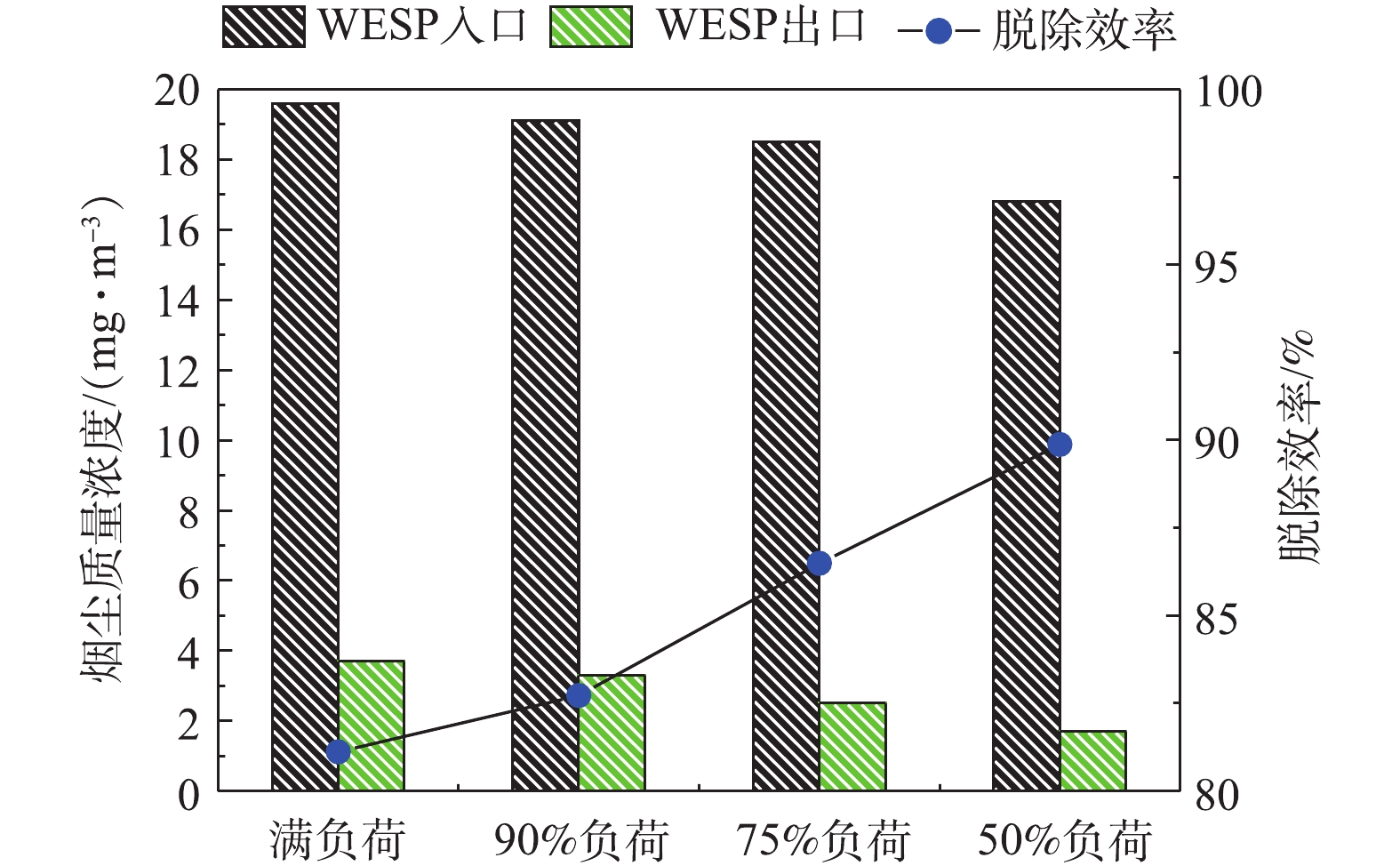
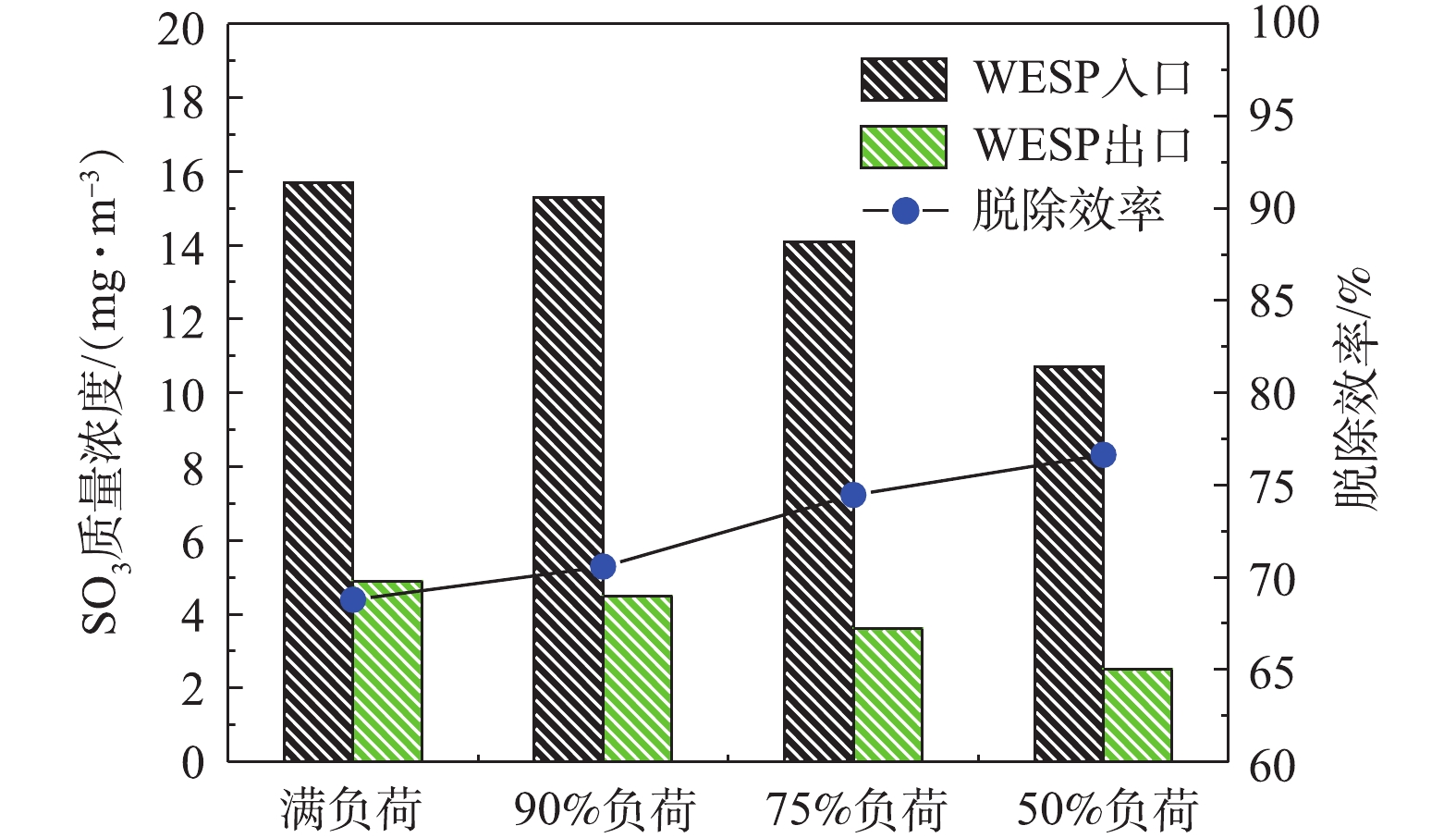
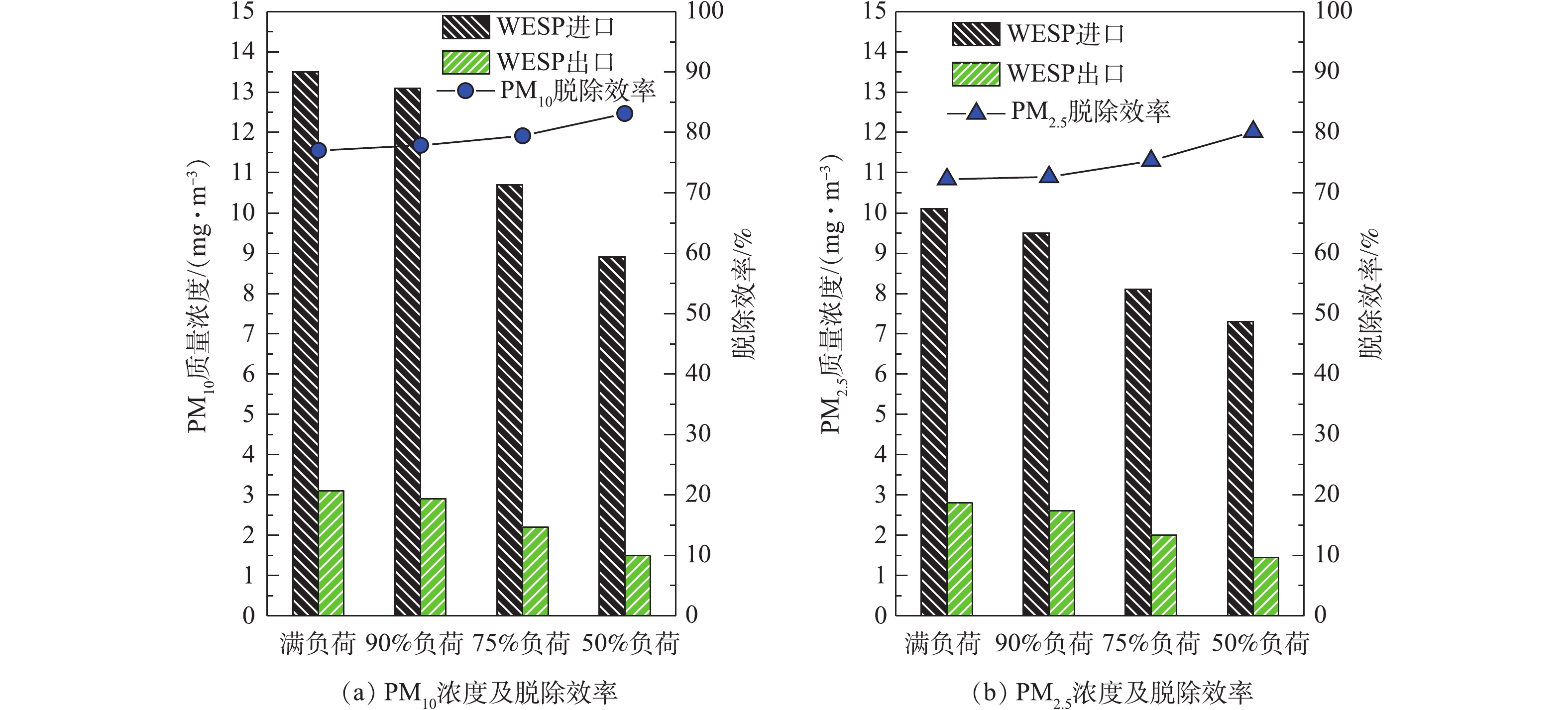
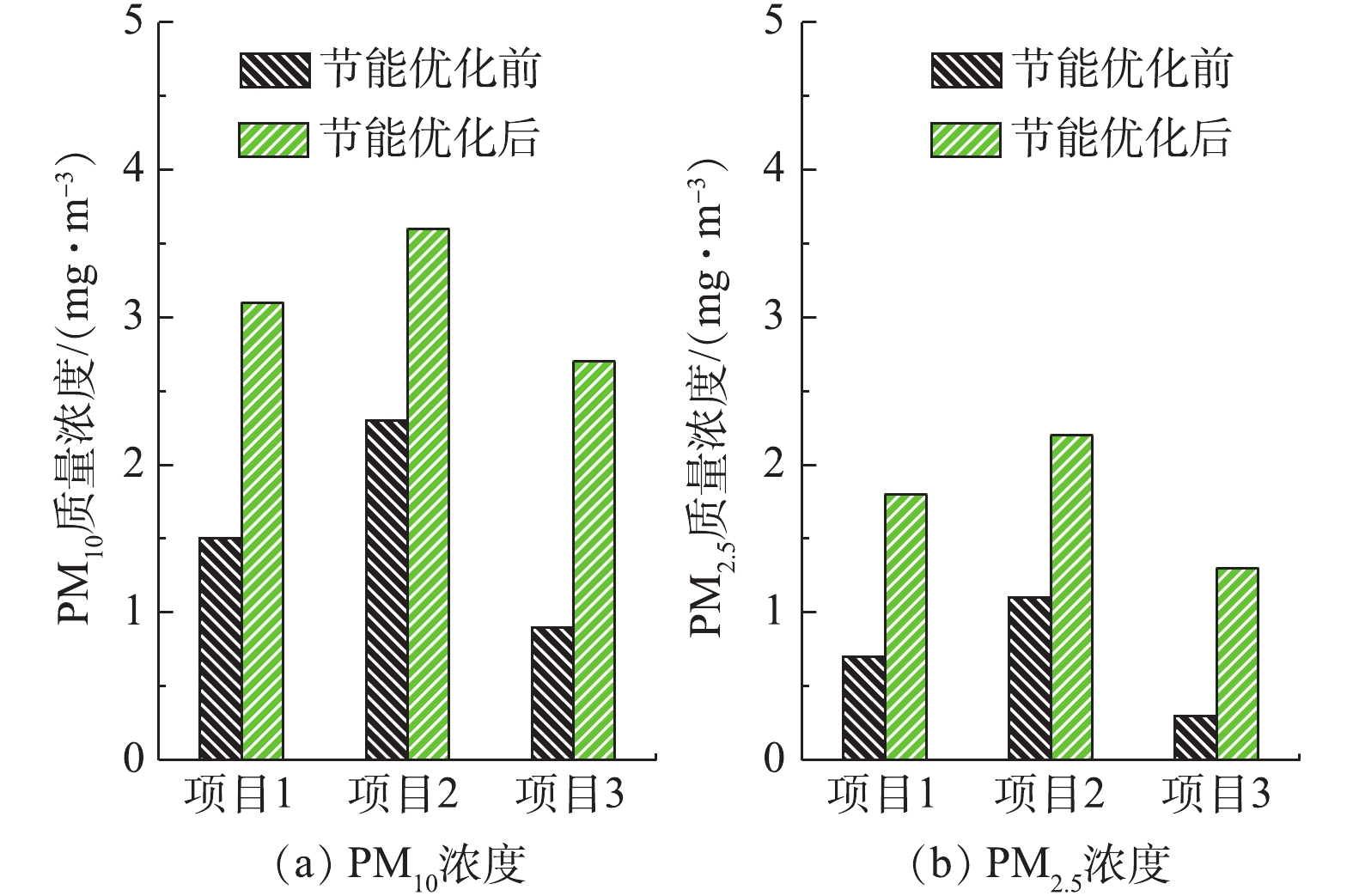
分别在满负荷、90%负荷、75%负荷、50%负荷条件下,测定湿式电除尘器对各污染物的脱除性能。烟尘测试结果如图13所示,随着机组负荷的降低,湿式电除尘器入口烟尘浓度有所降低,从19.6 mg·m-3降至16.8 mg·m−3,推测是因为负荷降低,烟气流速下降,前端电除尘器的除尘性能提升[24-25]所致。机组负荷降低,烟气流速下降,湿式电除尘器的除尘性能也得到提升,满负荷、90%负荷、75%负荷、50%负荷条件下湿式电除尘器的除尘效率分别为81.12%、82.72%、86.49%、89.88%。SO3测试结果如图14所示,随着负荷降低,湿式电除尘器入口的SO3浓度也有所下降,这主要是因为负荷降低后SCR脱硝的烟气温度降低,此处的SO2/SO3转化率减小[26-28]。同烟尘类似,烟气流速下降,湿式电除尘器对SO3气溶胶颗粒的脱除性能也得到提升,满负荷、90%负荷、75%负荷、50%负荷条件下湿式电除尘器对SO3的脱除效率分别为68.79%、70.59%、74.47%、76.64%,较烟尘的脱除效率要低一些。PM10/PM2.5测试结果如图15所示,随着负荷的降低,前端电除尘器对PM10/PM2.5的脱除性能提升,湿式电除尘器入口浓度均有所下降,同时,烟气流速下降,湿式电除尘器除尘也得到提升,满负荷、90%负荷、75%负荷、50%负荷条件下湿式电除尘器对PM10的脱除效率分别为77.04%、77.86%、79.44%、83.15%,对PM2.5的脱除效率分别为72.28%、72.63%、75.31%、80.14%。
为科学评价电除尘器的电耗水平,《高效能大气污染物控制装备评价技术要求第2部分:电除尘器》(GB/T 33017.2-2016)中给出了比电耗的概念,即处理单位工况烟气量所消耗的电量,计算方法如式(2)所示。
C=WQ (2) 式中:
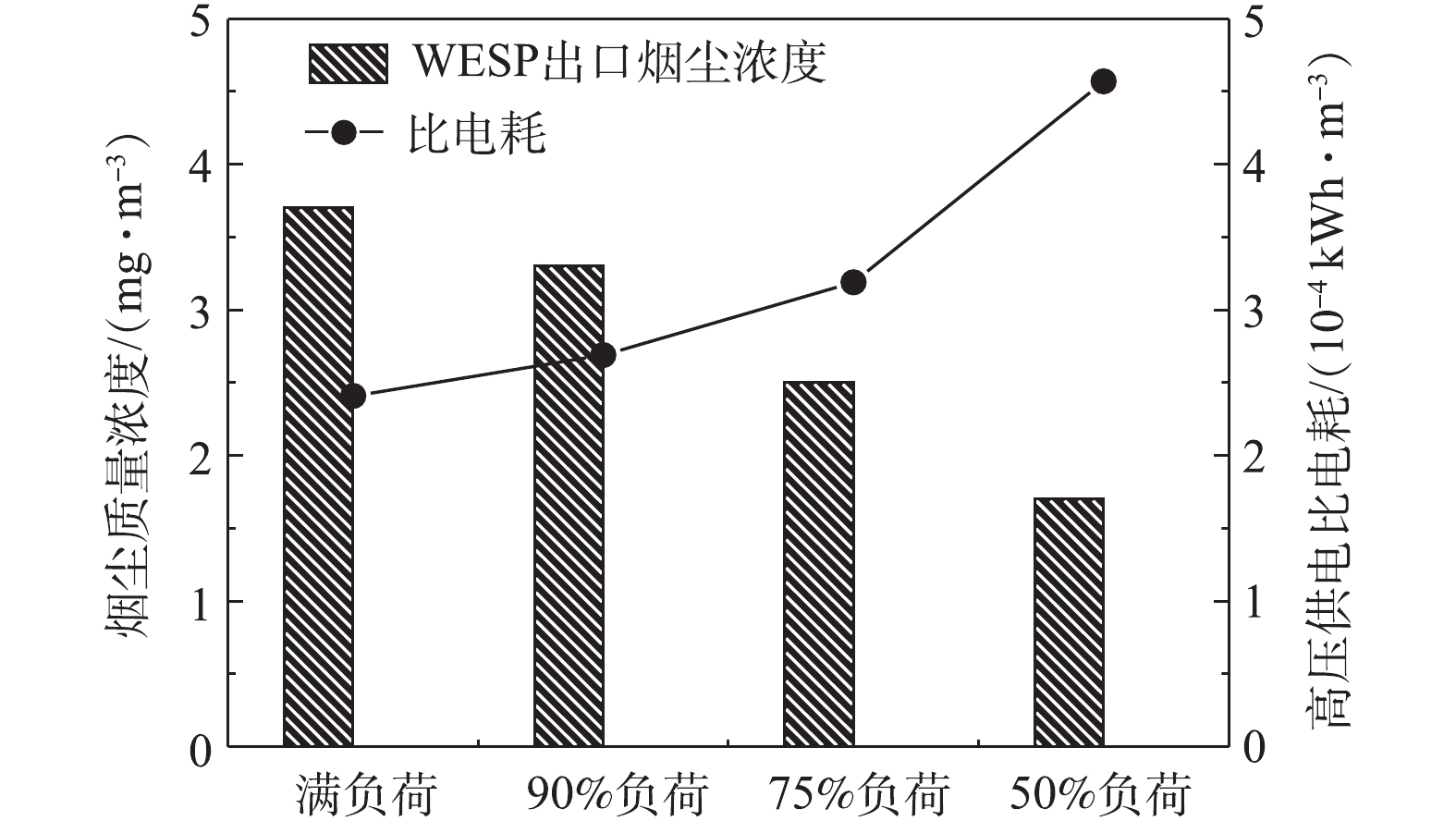
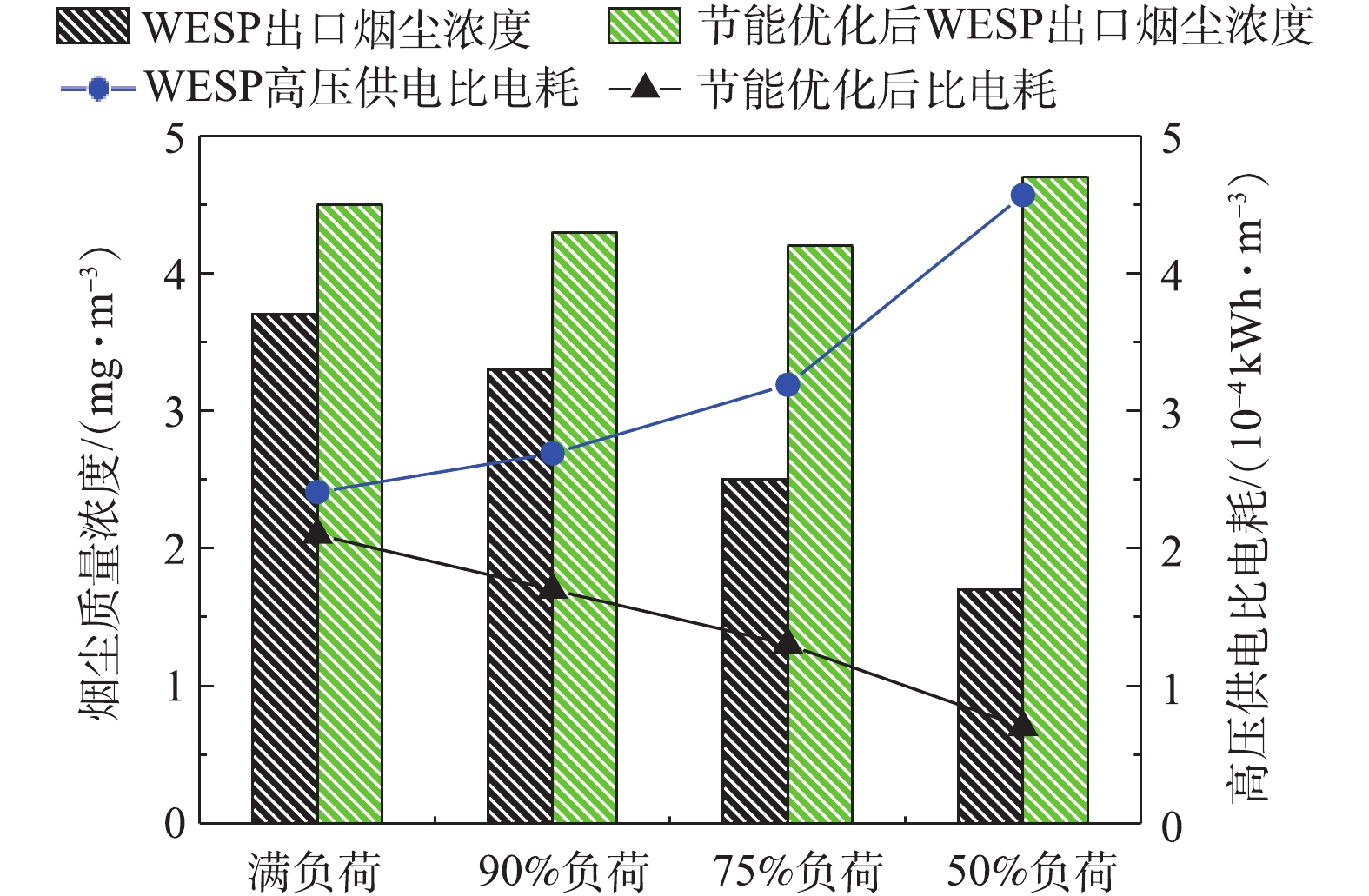
C 为湿式电除尘器比电耗,kWh·m−3;W 为湿式电除尘器的电耗,kW;Q 为进入湿式电除尘器入口的工况烟气量,m3·h−1。为对比不同负荷条件下湿式电除尘器的高压电耗,式(2)忽略了低压电耗、引风机阻力电耗等对比电耗的影响,湿式电除尘器的高压供电电耗采用三相有功电能表测定,经计算,不同负荷条件下,湿式电除尘器的高压供电比电耗如图16所示。随着负荷的降低,湿式电除尘器的高压供电比电耗大幅增加,从满负荷到50%负荷,比电耗从2.41×10−4 kWh·m−3升至4.57×10−4 kWh·m−3,有较大的节能空间。通过调整电源参数,控制湿式电除尘器出口烟尘浓度在4~5 mg·m−3,在满足5 mg·m-3超低排放要求的前提下,最大幅度地降低比电耗,实现节能最优化。节能优化后的比电耗结果如图17所示,湿式电除尘器的高压供电比电耗降幅显著,以50%负荷为例,节能优化后,比电耗从4.57×10−4 kWh·m−3降至0.7×10−4 kWh·m−3,节能优化后的比电耗下降达84.68%,即便是对于满负荷工况,烟尘浓度从3.7 mg·m−3增到4.5 mg·m−3,比电耗也下降了12.86%。该节能优化思路同样适用于其他工程项目及满负荷时烟尘排放远低于超低排放限值要求的工况。
2.2 其他工程项目的节能优化实验
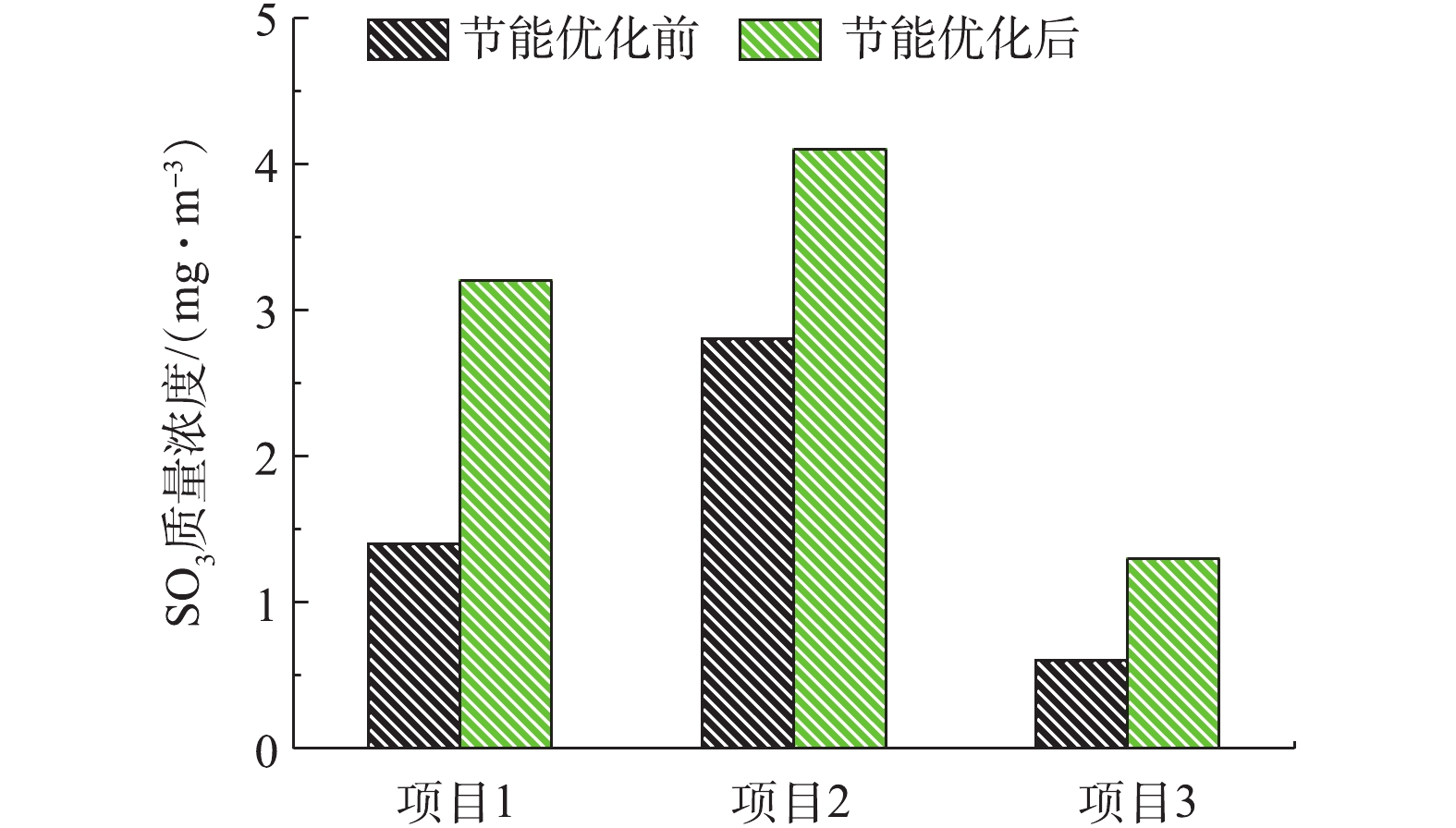
对其他3个导电玻璃钢湿式电除尘项目实施上述节能优化实验,相关数据如表1所示。在满负荷条件下,3个项目原烟尘排放浓度分别为1.9、2.7、1.2 mg·m−3,经节能优化,控制烟尘排放浓度在4.5 mg·m−3以内,此时比电耗下降幅度分别为32.65%、27.15%、41.64%。对应节能优化前后的SO3、PM10/PM2.5浓度测试结果分别如图18、图19所示。节能优化后,污染物排放浓度略有升高,但均在可承受范围内,如SO3浓度未超过5 mg·m−3,不会出现烟囱蓝烟拖尾的风险。值得注意的是,目前实际上有许多电厂的烟尘排放在2.5 mg·m−3甚至1 mg·m−3以下[29-37],此时的高压供电比电耗值较高,具有较大的节能优化空间,建议这类电厂在满足烟尘超低排放要求的前提下,适当降低电源运行参数,以达到节能的目的。
表 1 工程数据汇总Table 1. Project data summary序号 机组/MW 电源配置 设计出口烟尘浓度/(mg·m−3) 原排放浓度及电耗 节能优化后指标 比电耗下降幅度/% 烟尘浓度/(mg·m−3) 比电耗/(kWh·m−3) 烟尘浓度/(mg·m−3) 比电耗/(kWh·m−3) 1 300 72 kV/1 200 mA高频高压恒流源 <5 1.9 5.88 4.2 3.96 32.65 2 660 72 kV/1 200 mA高频高压恒流源 <5 2.7 4.31 4.1 3.14 27.15 3 1 000 80 kV/1 600 mA高频高压恒流源 <5 1.2 3.29 4.0 1.92 41.64 3. 结论
1)在正常工况下,工频高压恒流源和恒压源的空载/负载伏安特性曲线差别不大,两者的污染物脱除性能也大致相当。一旦喷淋系统开启,恒流源检测到火花放电后,自动下调电源运行参数,使电流/电压稳定运行在相对较低的参数范围,且运行相对平稳。恒压源则有一段明显的振荡区,抗干扰能力差。湿式电除尘器出口CEMS数据显示,喷淋系统开启后,工频恒流源供电的湿式电除尘器出口烟尘浓度最大值较喷淋前平均值增加了约12%;但恒压源因存在一个电源参数振荡区,出口烟尘浓度增加了约147%。因此,对于湿式电除尘器而言,应优先考虑抗干扰能力强的恒流源。
2)在供电电耗相当(工频3.49 kW、高频3.54 kW)的情况下,工频恒流源和高频恒流源供电的湿式电除尘器污染物脱除性能差异不大。但额定容量放开运行时,高频电源的运行电压/电流参数变大,其供电电耗分别提高至5.89、9.84、16.26 kW时,与工频相比,烟尘的减排幅度分别为46.30%、70.98%、78.69%,SO3的减排幅度分别为42.86%、57.14%、66.67%。
3)某660 MW机组典型工程的深度测试表明,随负荷的降低,湿式电除尘器的污染物脱除性能有所提升,在满负荷、90%负荷、75%负荷、50%负荷条件下,湿式电除尘器的除尘效率分别为81.12%、82.72%、86.49%、89.88%,SO3脱除效率分别为68.79%、70.59%、74.47%、76.64%,PM10脱除效率分别为77.04%、77.86%、79.44%、83.15%,PM2.5脱除效率分别为72.28%、72.63%、75.31%、80.14%。但随负荷的降低,湿式电除尘器高压供电比电耗大幅增加,从满负荷到50%负荷,比电耗从2.41×10-4 kWh·m-3升至4.57×10-4 kWh·m-3,有较大的节能空间。通过调整电源参数,控制湿式电除尘器出口烟尘浓度在4~5 mg·m-3,比电耗显著降低,满负荷的比电耗也下降了12.86%,50%负荷的比电耗下降达84.68%,实现了湿式电除尘器的节能优化运行。
4)根据本研究得到的节能优化思路,对其他3个工程项目实施运行优化,优化前烟尘排放浓度分别为1.9、2.7、1.2 mg·m−3,经节能优化,控制烟尘排放浓度在4.5 mg·m−3以内,比电耗下降幅度分别为32.65%、27.15%、41.64%。该思路同样适用于其他除尘项目及满负荷时烟尘排放远低于超低排放限值(5 mg·m−3)要求的工况,尤其是部分烟尘排放长期在2.5 mg·m−3甚至1 mg·m−3以下项目,建议这类电厂在满足烟尘超低排放要求的前提下,适当降低电源运行参数,以达到节能的目的。
-
表 1 受污染矿区含氟地下水水质特点
Table 1. Characteristics of fluorine-containing groundwater in contaminated mining area
质量浓度/(mg·L−1) pH 电导率/(mS·cm−1) F− Ca2+ Mg2+ Na+ Cl− SO42- 8.1~19.4 42~63 33~45 252~372 546~781 103~192 6.89~7.41 1.65~2.42 表 2 不同进水氟质量浓度下絮体中Al和F元素分析
Table 2. Al and F elements in flocs at different influent fluoride concentrations
进水氟质量浓度/( mg·L−1) 铝氟质量百分占比/% 铝氟摩尔比 F Al 8.00 0.73 34.1 32.87:1 12.00 1.68 34.2 14.33:1 16.00 2.21 33.9 10.79:1 20.00 2.69 34.0 8.89:1 表 3 pH对电絮凝反应器内絮体颗粒ζ电位及出水溶解性铝影响
Table 3. Influence of pH on the ζ potential of floc particles in EC reactor and dissolved aluminum in effluent
pH 絮体颗粒ζ电位/mV 出水溶解性铝质量浓度/( mg·L−1) 不含氟絮体 含氟絮体 5.0~6.0 28.14 22.02 2.89 6.0~7.0 18.15 12.13 0.64 7.0~8.0 9.06 3.12 1.67 8.0~9.0 −7.32 −11.51 3.76 表 4 不同操作条件下k实验结果
Table 4. Fitting results of Kobs under different operating conditions
电流密度/(A·m−3) 极间距/mm 进水氟质量浓度/(mg·L−1) k实验 150 5 8 0.037 74 300 5 12 0.040 59 450 5 16 0.047 28 600 5 20 0.051 31 150 10 12 0.032 04 300 10 8 0.042 57 450 10 20 0.041 43 600 10 16 0.051 85 150 15 16 0.025 77 300 15 20 0.030 42 450 15 8 0.047 99 600 15 12 0.052 59 150 20 20 0.019 92 300 20 16 0.031 09 450 20 12 0.042 27 600 20 8 0.052 90 表 5 预测方程统计结果
Table 5. Statistical results for the predictive equation
变量 未标准化系数 标准化系数 t 显著性 B 标准误差 Beta 电流密度 5.20×10−5 4.36×10−7 0.839 119.665 0 极板间距 −55.50×10−5 11.00×10−6 −0.357 −50.818 0 进水质量浓度 −81.70×10−5 16.00×10−6 −0.351 −49.995 0 (常量) 39.56×10−3 309.00×10−6 128.008 0 -
[1] 李果, 狄军贞, 吕情绪. 布尔台煤矿高氟地下水分布特征及形成机制研究[J]. 煤炭工程, 2022, 54(7): 122-128. [2] FAN X, PARKER D J, SMITH M D. Adsorption kinetics of fluoride on low cost materials[J]. Water Research, 2003, 37: 4929-4937. doi: 10.1016/j.watres.2003.08.014 [3] HU C Y, LO S L, KUAN W H. Effects of the molar ratio of hydroxide and fluoride to Al(III) on fluoride removal by coagulation and electrocoagulation[J]. Journal of Colloid and Interface Science, 2005, 283: 472-476. doi: 10.1016/j.jcis.2004.09.045 [4] AMOR Z, BARIOU B, MAMERI N, et al. Fluoride removal from brackish water by electrodialysis[J]. Desalination, 2001, 133: 215-223. doi: 10.1016/S0011-9164(01)00102-3 [5] EMAMJOMEH M M, SIVAKUMAR M. An empirical model for defluoridation by batch monopolar electrocagulation/flotation (ECF) process[J]. Journal of Hazardous Materials, 2006, 131: 118-125. doi: 10.1016/j.jhazmat.2005.09.030 [6] SANDOVAL M A, FUENTES R, THIAM A, et al. Arsenic and fluoride removal by electrocoagulation process: A general review[J]. Science of the Total Environment, 2021, 753: 1-26. [7] GOVINDAN K, RAJA M, MAHESHWARI S, et al. Comparison and understanding of fluoride removal mechanism in Ca2+, Mg2+, and Al3+ ion assisted electrocoagulation process using Fe and Al electrodes[J]. Journal of Environmental Chemical Engineering, 2015, 3(3): 1784-1793. doi: 10.1016/j.jece.2015.06.014 [8] 邵坚, 陆建红, 邹仲勋, 等. 锌铝电极电絮凝法处理高氟饮用水的研究[J]. 中国给水排水, 2009, 25(15): 100-102. doi: 10.3321/j.issn:1000-4602.2009.15.029 [9] ZUO Q, CHEN X, LI W, et al. Combined electrocoagulation and electroflotation for removal of fluoride from drinking water[J]. Journal of Hazardous Materials, 2008, 159(2-3): 452-457. doi: 10.1016/j.jhazmat.2008.02.039 [10] 陈聪聪, 钱光磊, 谢陈鑫, 等. 双铝电极电絮凝处理高含氟地下水的影响因素及动力学分析[J]. 环境工程学报, 2020, 14(5): 1216-1223. doi: 10.12030/j.cjee.201907180 [11] ZHAO H, ZHAO B, YANG W, et al. Effects of Ca2+ and Mg2+ on defluoridation in the electrocoagulation process[J]. Environmental Science & Technology, 2010, 44(23): 9112-9116. [12] HU C Y, LO S L, KUAN W H. Effects of co-existing anions on fluoride removal in electrocoagulation (EC) process using aluminum electrodes[J]. Water Research, 2003, 37(18): 4513-4523. doi: 10.1016/S0043-1354(03)00378-6 [13] MOUSSA D T, EL-NAAS M H, NASSER M, et al. A comprehensive review of electrocoagulation for water treatment: Potentials and challenges[J]. Journal of Environment Management, 2017, 186(1): 24-41. [14] THAKUR L S, MONDAL P. Simultaneous arsenic and fluoride removal from synthetic and real groundwater by electrocoagulation process: Parametric and cost evaluation[J]. Journal of Environmental Management, 2017, 190: 102-112. [15] LU J, Li Y, YIN M et al. Removing heavy metal ions with continuous aluminum electrocoagulation: A study on back mixing and utilization rate of electro-generated Al ions[J]. Chemical Engineering Journal, 2015, 267: 86-92. doi: 10.1016/j.cej.2015.01.011 [16] ZHU J, ZHAO H, NI J. Fluoride distribution in electrocoagulation defluoridation process[J]. Separation and Purification Technology, 2007, 56(2): 184-191. doi: 10.1016/j.seppur.2007.01.030 [17] OUAISSA Y A, CHABANI M, AMRANE A, et al. Removal of tetracycline by electrocoagulation: kinetic and isotherm modeling through adsorption[J]. Journal of Environmental Chemical Engineering, 2014, 2: 177-184. doi: 10.1016/j.jece.2013.12.009 [18] 梁言, 杨小明, 孙境求, 等. 电絮凝-超滤除氟控铝工艺参数优化[J]. 环境工程学报, 2018, 12(11): 3020-3027. doi: 10.12030/j.cjee.201805096 -




 下载:
下载: