-
随着石油工业、机械加工、食品加工与餐饮业的不断发展,含油废水的排放量日益增大。不经处理的含油废水直接排放会对周边环境造成严重的危害。含油废水中的油可分为浮油、分散油、乳化油和溶解油4种[1]。其中乳化油因其油滴尺寸较小,油水二元体系稳定性强,难以通过重力、气浮等方式实现油水分离,是最难处理的一类含油废水[2]。目前,乳化油废水处理方法包括:膜分离法、吸附法、絮凝法等[3-5]。其中的电絮凝法具有除油能力强、操作简便、自动化程度高、无需添加化学药剂等优点,是一种高效、经济、环保的含油污水处理方法[6]。电絮凝法的缺点在于废水处理过程需要消耗大量电能,导致其水处理成本相对较高。
为了降低废水处理过程的电能消耗,降低废水处理成本。近些年来提出了一种利用工作溶液盐/浓差能驱动的逆电渗析(reverse electrodialysis,RED)技术来处理各种有机/无机废水的技术[7-12]。工作溶液盐/浓差能即可来自于自然界(海水/盐湖水与入海/入盐湖的河水之间的盐差),也可以来自盐/海水分离副产物(浓盐/海水与自然盐/海水之间的盐差),还可以通过废热转换(溶液热分离)获得[13]。
1954年PATTLE[14]首次提出利用自然界盐差能的RED发电技术以来,对该技术的研究逐渐深入,各国学者发表了大量有关RED技术的研究论文。RED电堆/反应器的结构和工作原理类似。他们都是由端板、阴/阳电极,交错布置的阴/阳离子交换膜(AEM/CEM)及隔垫所构成。当浓/稀盐溶液分别流经由膜隔垫所隔两电极会产生得失电子的氧化还原反应。电子通过外部电路从阳极流向阴极,从而在外电路中产生电流。发电用的RED电堆与水处理用的RED反应器不同之处在于:电极液在RED电堆内作可逆的闭式循环,而废水作为电极液流经RED反应器电极流道并因电极的氧化还原反应生成各种反应物来降解废水中的污染物。不同的电极材料和废水成分在RED反应器电极氧化还原反应过程中会生成不同的反应物。理论上而言,若RED反应器阳极选用铁或铝作为牺牲阳极时,RED反应器可以产生电絮凝效果来处理一些难以生化降解的有机或无机废水[15]。
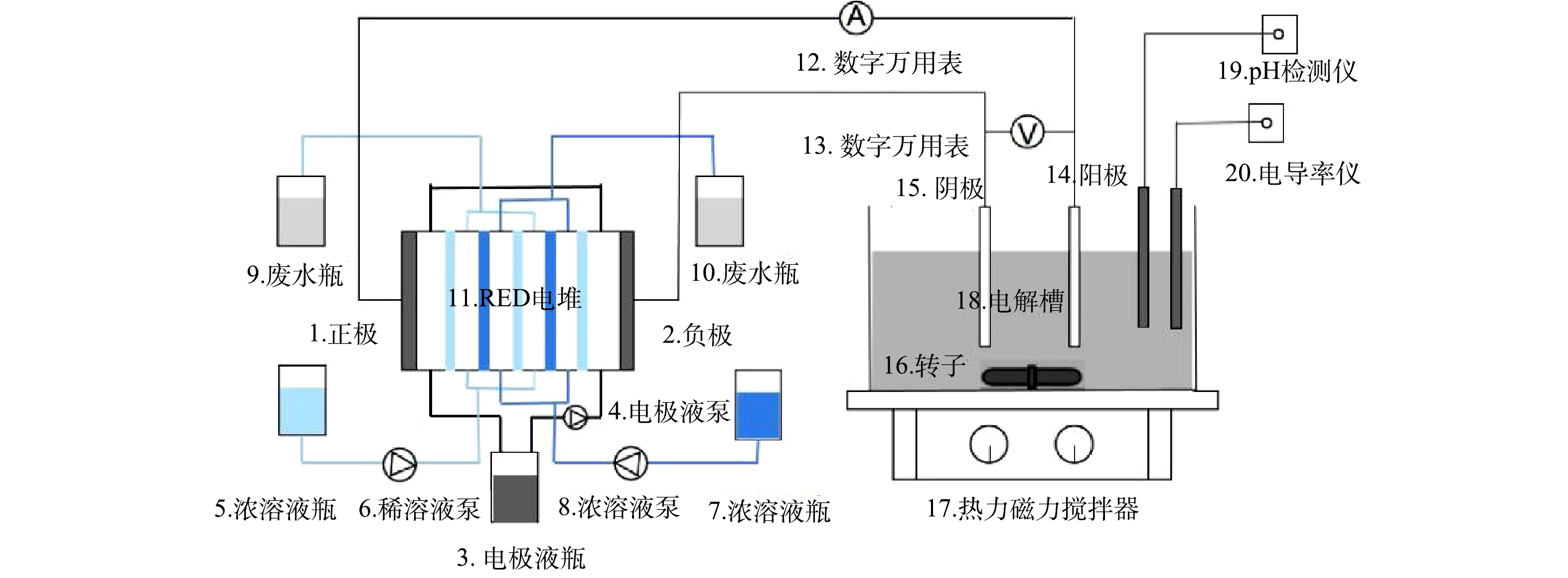
但实际上,因在使用过程中牺牲阳极会被不断消耗而需要定期换新,导致需要不断拆装RED反应器造成使用不便。另外,在处理含油废水过程时电极表面易被油膜污染,减弱含油废水的处理效果。为此,本课题组提出了一种如图1所示的RED电堆与常规电絮凝器耦合的乳化油废水处理系统。该系统由工作溶液的盐差能驱动RED电堆发电,电絮凝器作为负载连接在电堆的外部电路中。由于盐差能驱动的RED电堆属于内生电源。在RED电堆结构参数确定的条件下,其输出电参数(电压与电流)与外部电路负载(电絮凝器)电阻有关。而电极材料、电极间隙、处理时间、阳极钝化、含油废水参数(初始pH、电导率和温度)等变化会影响电絮凝器电阻,进而影响其工作效率。因此,本研究采用单因素法,在RED电堆结构和操作参数不变的条件下,考察电絮凝器电极材料、电极间距、支撑电解质浓度、含油废水初始pH及温度对耦合系统的乳化油废水处理效果的影响。
-
逆电渗析电堆与电絮凝器耦合系统合处理乳化油废水实验系统流程如图1所示。由图1可见,RED电堆与电絮凝器构成一串联电路。实验所用的RED电堆和电絮凝器均为自制。RED电堆由离子交换膜、丝网隔垫及电极构成,离子交换膜有40张CEM以及41张AEM(最外两侧膜采用AEM),均购自日本富士公司,型号为type 10,厚度为0.12 mm;丝网隔垫厚度为0.38 mm,孔隙率为80%;2个10 cm×10 cm×1.5 cm的钛镀钌铱板构成电极。电絮凝器由有机玻璃(透明亚克力板)制成,电解槽尺寸为20 cm×10 cm×15 cm。槽内壁设有0.3 cm宽的竖直凹槽,用来放置电极板(10 cm×10 cm×0.3 cm)。凹槽下端距电解槽底面1 cm,电极板实际有效浸没面积为90 cm2。电解槽放置在磁力搅拌器上,转子以400 r·min−1的速度不断搅拌电解槽内的含油废水,以使其保持油水均匀状态。选择这个搅拌速度值,是因为它促进了胶体和不稳定物质之间的相遇,而不会在所使用的实验装置内造成任何可察觉的聚合体破裂[16]。
-
人工配置浓度分别为0.03 mol·L−1和3 mol·L−1的稀/浓NaCl水溶液作为工作溶液并储存在相应的玻璃瓶内(5和7)。0.1 mol·L−1 的铁氰化钾水溶液作为电极液储存在避光的玻璃瓶(3)内。2台蠕动泵(6和8)泵送稀/浓溶液工作溶液流经RED电堆(11)。在稀/浓溶液盐差的作用下,浓溶液中的盐分跨膜迁移到稀溶液中,使得浓溶液浓度降低,稀溶液浓度升高。流出RED电堆的稀/浓溶液分别储存在其相应的玻璃瓶内(9和10)。一台蠕动泵(4)泵送电极液,并在RED阴/阳极回路内作循环流动,以将电荷快速转移到RED正/负极。RED电堆输出电能驱动电絮凝器工作。RED阴/阳(正/负)极与电絮凝器对应的阳/阴极用铜导线相接,构成外部电路。利用数字万用表(12和13)在线检测RED电堆输出电流和电压。RED电堆的理论开路电压可按能斯特(Nernst)方程(式(1))计算[13]。离子活度系数可用扩展的 Debye-Hückel方程(式(2))表示。
式中:U为理论开路电压,V;
α 为离子交换膜的选择性透过系数;Nm为膜对(membrane pair)数;z为离子价数;R为通用气体常数,8.314 J·(mol·K)−1;T为温度,K;F为法拉第常数,96 485 C·mol−1;γ 为离子活度系数;C为浓度,mol·L−1。下标CEM、AEM分别代表阴、阳离子交换膜;CS和DS分别为浓和稀溶液。式中:z为离子化合价;A为有效水合离子半径(
ANa+=450pm,ACl−=300pm );Λ 为离子浓度,mol·L−1。由于流经RED电堆的浓、稀溶液浓度会发生变化,因此RED电堆理论开路电压需按浓、稀溶液平均浓度计算。本次实验固定RED电堆结构和操作参数[浓溶液浓度为3 mol·L−1,稀溶液浓度恒定为0.03 mol·L−1,过膜流速均为0.35 cm·s−1(稀/浓溶液流率均为0.134 4 L·min−1)]不变,计算得理论开路电压为6.335 V。RED电堆输出(端)电压为式(3)计算值,在RED输出电能的驱动下,电絮凝器的阳极和阴极发生的电化学反应如式(4)~式(6)所示
式中:Ri为RED电堆的内电阻,Ω,其由膜电阻,稀/浓溶液流道电阻和电极液流道及电极电阻所组成。
反应生成的单体和聚合氢氧化物作为絮凝剂具有高吸附性能,它与废水中分散的颗粒和溶解的污染物具有很强的亲和力。因此,可以通过絮凝作用去除废水中的污染物。絮凝后的产物可以通过漂浮或沉淀的方法从水相中分离出来。阴极还原反应所产生的氢气气泡可促进污染物聚结过程,有助于采用浮选去除乳化油废水中的油组份。氢气泡尺寸越小,絮凝体的截留面积越大,污染物与水的分离效果也越好[17]。此外,电场也被证明是一种有效的破乳方法。在电场力的驱动下,油滴沿着电场的方向运动,然后聚集并破乳[18]。油滴在电场中极化,导致相邻油滴之间的静电吸引力增强[19]。
-
以自制乳化油废水作为研究对象。乳化油制备过程为:每升水中含40 g15#机油(购自德国德殻石化(中国)发展有限公司)并添加8 g十二烷基苯磺酸钠作为稳定剂。采用磁力搅拌器以1 000 r·min−1的速度搅拌30 min,形成稳定的油水乳状液。去除表面浮油后的乳化油再作进一步稀释。稀释后取2 L含油量为1 g·L−1的含油废水进行实验研究。利用氢氧化钠和硫酸来调节含油废水的初始pH,并通过添加支撑电解质(Na2SO4)来调节含油废水的电导率。
实验中各种溶液配制所需的溶剂水为去离子水,所用的盐/电解质购自天津大茂化学试剂厂,为分析纯级,其所含杂质可以忽略。
实验所用的仪器有,紫外可见分光光度计(UV1780,日本岛津有限公司);蠕动泵(BT100-2J,保定兰格恒流泵有限公司);蠕动泵(KCP PRO-2-N16,卡默尔流体科技(上海)有限公司);电子天平(JJ1023BC,G电子秤有限公司);数字万用表(KEITHLEY 2110-220,美国泰克科技有限公司);低速离心机(LSC-20,上海秋佐科学有限公司);热力磁力搅拌器(EMS-9A,天津欧诺仪器股份有限公司);电导率仪(FE38,梅特勒托利多科技(中国)有限公司);pH测试仪(PXSJ-226,上海雷磁仪器有限公司)。
-
每次实验前,铁或铝阳极先要在稀硫酸溶液中浸泡15 min,捞出后用砂纸打磨再用去离子水冲洗,去除表面氧化层。在实验过程中,含油废水处理时长为60 min,每隔10 min抽取电解槽(18)中的水样。对所有水样进行离心处理,将电絮凝过程中形成的絮凝物从水相中分离出来。在每份水样中取1 mL加入氯化钠进行破乳。氯化钠投加量为总体积的5%。然后用10 mL石油醚萃取水样中的油以便测量。测量时通过紫外可见分光光度仪测试水样的吸光度,通过依据绘制的标准曲线反推其浓度。按式(7)计算除油率。为了探索操作参数变化对含油废水处理效果的影响,采用单一变量法进行实验。相同的实验重复2次。
式中:C0为含油废水中油初始质量浓度,mg·L−1;Ct为絮凝处理t时间后测得含油废水中油质量浓度(乳化后的含油废水紫外光吸收波长峰值λ=225 nm),mg·L−1。
-
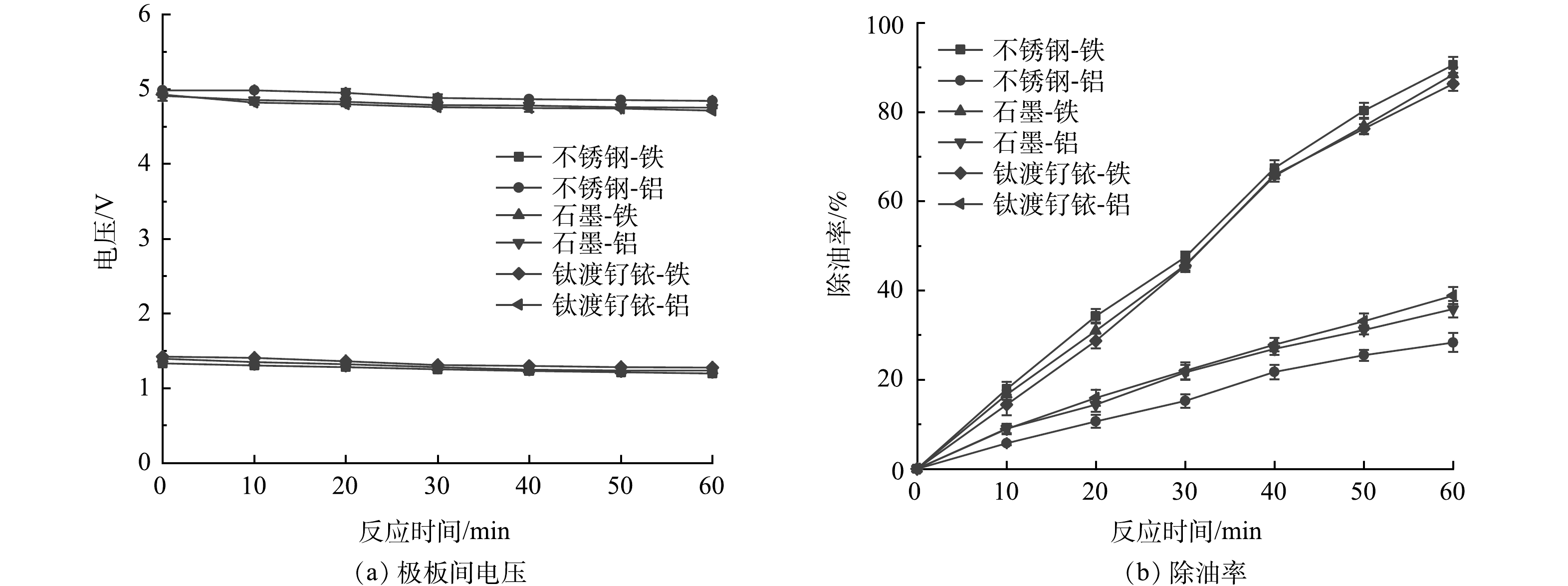
电絮凝器电极选用不锈钢-铁、不锈钢-铝、石墨-铁、石墨-铝、钛镀钌铱-铁、钛镀钌铱-铝6种阴-阳极材料组合,用以考察不同阴-阳材料组合对乳化油废水去除效果的影响。图2给出了不同电极组合下极板间电压及除油率随时间的变化关系。其中,实验条件为:极板间距1 cm、含油废水中添加0.1 mol·L−1硫酸钠,pH为7.8 (pH不为7的原因是配备的乳化油中添加了支撑电解质Na2SO4,SO42-离子可以少量结合H+离子,间接引起pH升高),温度20 ℃。
由图2(a)可见,采用铝阳极时极板间电压远高于采用铁阳极,而RED的输出电压与电絮凝器采用何种阳极材料关联不大。此外,实验还发现采用铝阳极时串联电路的电流远低于采用铁阳极时的电流,因此,虽然采用铝阳极时极板间电压较高,但采用铁阳极絮凝时,RED电堆可以输出更大的电流。
根据法拉第电解定律,通过的总电荷越多,电极产生的混凝剂也越多。一方面,电流密度越大,产生的絮凝剂越多[20];另一方面,电流密度越大,阴极还原生成的氢气气泡量越大且气泡尺寸越小[21],导致气泡密度的增加,更多的小气泡也为油微粒附着于絮凝剂提供了更大的表面积,使得油水分离效率提高。
由图2(b)可见,采用铁阳极对乳化油废水的处理效果要优越于铝阳极。一方面是因为采用铁阳极可以获得较大的电流密度,有利于产生更多的絮凝剂;另一方面,铝阳极氧化过程中易形成致密的表面氧化层,导致电极电阻增大。由于表面氧化层在pH为4~10内的水溶液中非常稳定,氧化层后的铝不易被氧化成铝离子,使得铝离子与氢氧根结合生成的絮凝剂Al(OH)3量减小,絮凝效率变差。
从实验结果可以发现,采用不锈钢-铁电极组合处理含油废水的除油率最高,石墨-铁电极以及钛镀辽铱-铁电极组合处理效果次之,采用铝阳极组合电极处理含油废水的除油率最差。因此,后续均采用不锈钢-铁电极组合进行实验。
-
图3给出了不同极板间距下极板间电压及除油率随时间的变化关系。除电极间距外,其他操作参数与电极材料实验时的操作条件相同。由图3(a)可见,随着时间的延长,极板间电压均会降低。其原因在于,电解过程中电极的氧化还原反应均产生阴、阳离子且溶于废水中,会使废水的电导率逐渐增大,废水的电阻减小,极板间电压降低。由图3(a)还可见,随着极板间距的增大,电极间的电压随之增大。这是由于在相同的废水电导率下,极板间距越大,废水的电阻就越大。因此,用来克服溶液间的电阻的电压也就越大。
由图3(b)可见,当极板间距由1 cm增大至2.5 cm时,系统的除油率降低。其原因在于,絮凝器电极电阻增大,导致电流密度降低。然而,当极板间距由0.5 cm增大至1 cm时,系统的除油率反而上升。其原因在于,当絮凝器电极间距较小时,适当增大电极间距有利于改善电极间溶液的混合状态,减轻极板附近的浓差极化现象,进而有利于金属离子的溶出,形成絮凝体。
由于需要处理的乳化油废水电导率较低,采用较小的极板间距可以降低用于克服溶液电阻所损失的电压。但极板间距过小时,电极氧化还原反应过程的浓差极化现象较为严重。通过实验发现,对于所研究的RED-EC耦合废水处理系统,采用1 cm的极板间距较为合适。因此,后续实验中极板间距均为1 cm。
-
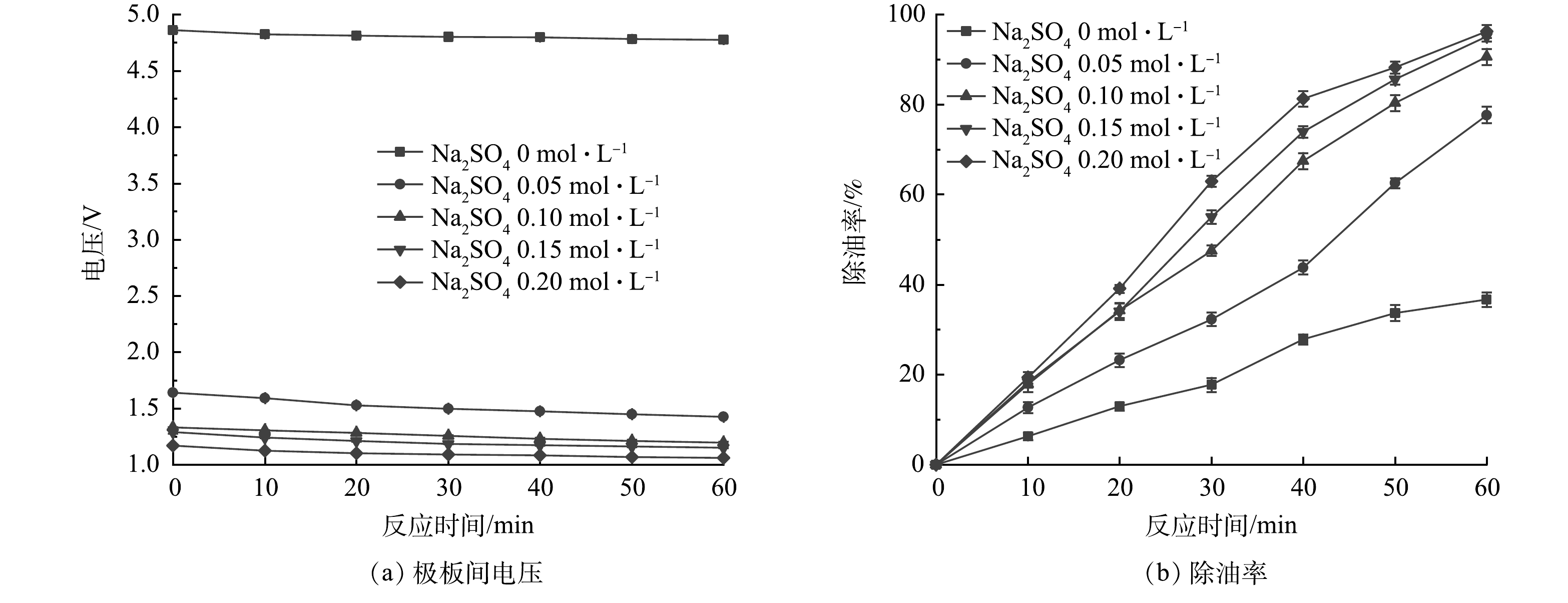
支撑电解质浓度变化会影响废水电导率变化,进而会影响电絮凝器电极间废水电阻值,从而影响RED电堆的输出电流和系统的除油率。为了考察支撑电解质浓度变化对除油率的影响,在模拟乳化油废水中添加不同浓度的硫酸钠。图4给出了不同支撑电解质浓度下,极板间电压及除油率随时间的变化关系(除废水中支撑电解质浓度外,其他操作参数与电极材料实验时的操作参数条件相同)。
比较图4(a)和图4(b)可见,当模拟乳化油废水中不添加支撑电解质(硫酸钠浓度为0 mol·L−1)时,极板电压很高,RED电堆的输出电流很小,除油率较低,仅为36.7%。其原因在于,配制的不含支撑电解质的模拟乳化油废水的电导率很低,溶液电阻很大,导致极板间废水所损失的电压很大,RED电堆输出电流减小,除油率降低。当向模拟乳化油废水加入少量支撑电解质(硫酸钠浓度为0.05 mol·L−1)后,废水的电导率会迅速增加,废水的电阻迅速降低,电极电压也随之降低,RED电堆的输出电流随之增加,除油率也随之增大。如果继续增加废水中的支撑电解质浓度,尽管废水电导率会增大,但增大的幅度逐渐变小,对电絮凝器电极电压的影响也会逐渐降低,除油率增速逐渐减小。因此,在采用电絮凝法处理含油废水时,可根据废水的实际电导率来添加支撑电解质量。
-
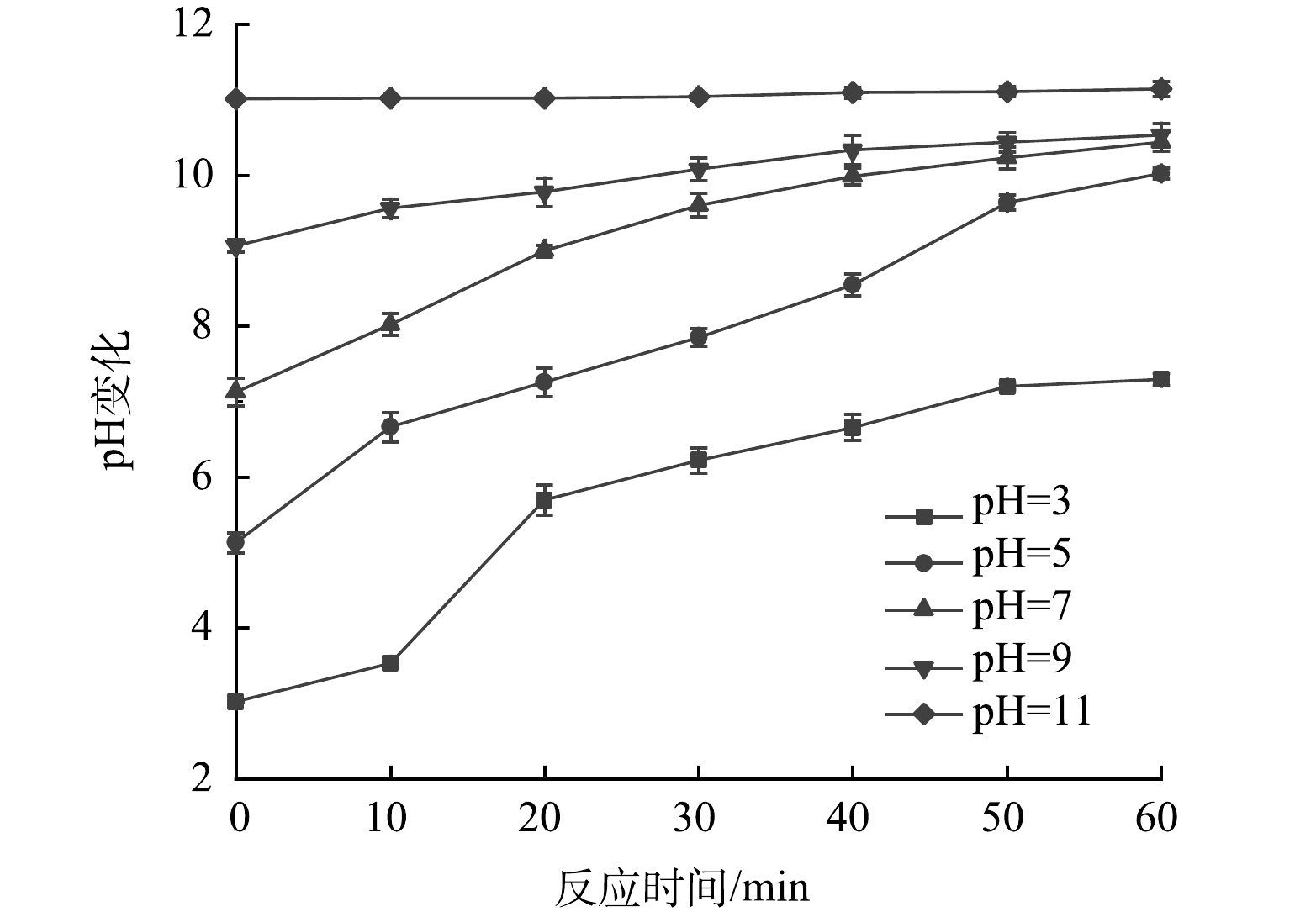
由于含油废水初始pH会影响影响金属氢氧化物(絮凝剂)的形成。因此,该值变化对电絮凝过程会产生较大的影响[22]。为了探索不同初始pH对实验系统除油率的影响,在其它操作参数不变的情况下,通过添加氢氧化钠或硫酸调节废水初始pH(初始pH为3~11),考察初始pH对RED-EC耦合废水处理系统的除油率的影响关系。
图5给出了在不同初始pH条件下,含油废水pH随处理时间的变化情况。由图5可见,当含油废水初始pH较低时,随着处理时间的增加,废水的pH也会随之增加。其原因是由于在电絮凝过程中阴极还原反应产生了氢氧根(OH−)((5))。但随着含油废水初始pH的增大,随着处理时间而变化的废水pH增速逐渐减小。当初始pH为11时,废水的pH不再随处理时间的变化而变化,几乎保持恒定。
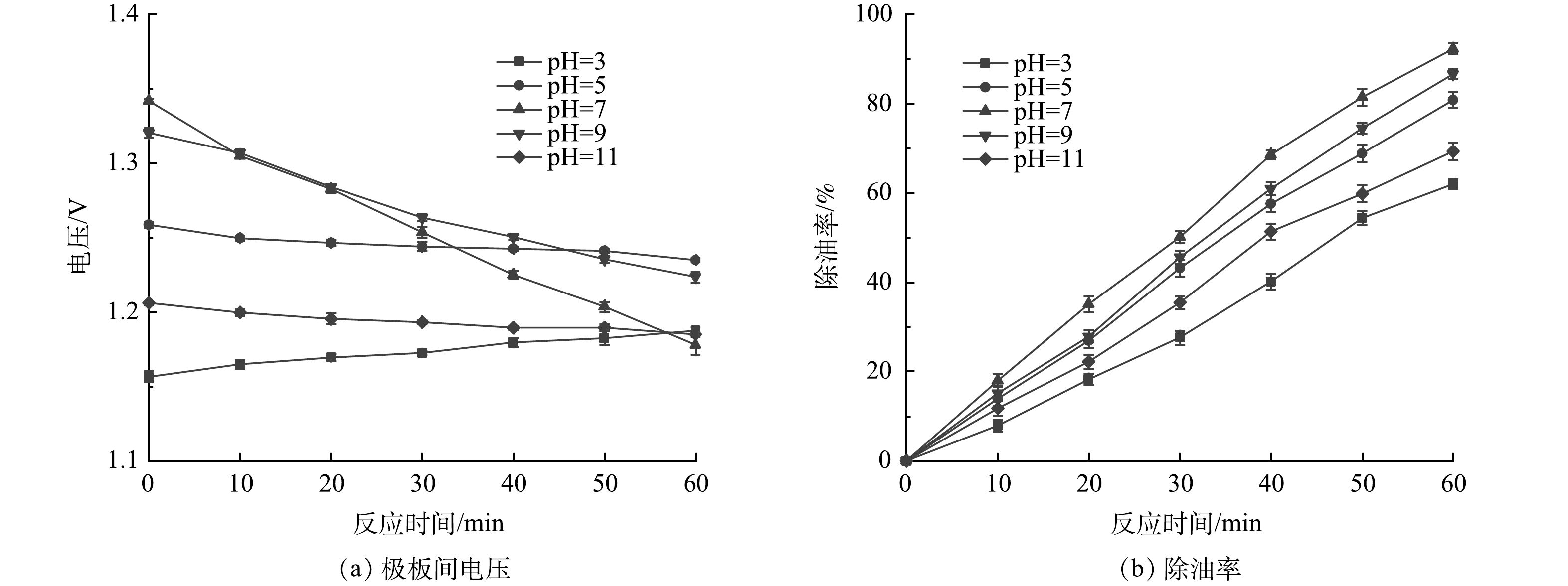
图6给出了不同初始pH条件下,极板间电压及除油率随时间的变化关系。由图6(a)可见,当初始pH偏离7(中性)越多,电极电压相对越低,相应的RED电堆输出电流也就相对越大。其原因在于,添加的酸或碱都属于强电解质,其浓度越高废水的电导率越大,极板间溶液电阻越小,极板间电压越小。当废水初始pH为3时,极板间电压随着处理时间的延长而增大。其原因在于,电絮凝反应产生的部分OH−与酸中的H+中和成水,废水的pH升高(图5),电导率降低所致。而当废水初始pH为中性或弱碱性时,极板间电压随着处理时间的延长而减小,原因在上述分析中已有阐述。
由图6(b)可见,当废水初始pH为中性时,耦合系统的除油率最高。而初始pH太低或太高均对耦合系统的除油率不利。影响耦合系统电絮凝除油效果是多方面的。从预防电极钝化的思路考虑,当pH较低时,电极氧化膜易于溶解,使得电极钝化现象得到延缓。而从絮凝的角度看,中性偏碱性条件更有利于Fe(OH)3絮体形成,以达到较好的絮凝效果[23]。正是由于这两种相互矛盾关系的影响,初始pH在5~9内含油废水经耦合系统处理60 min后的除油率可达到80%以上。
-
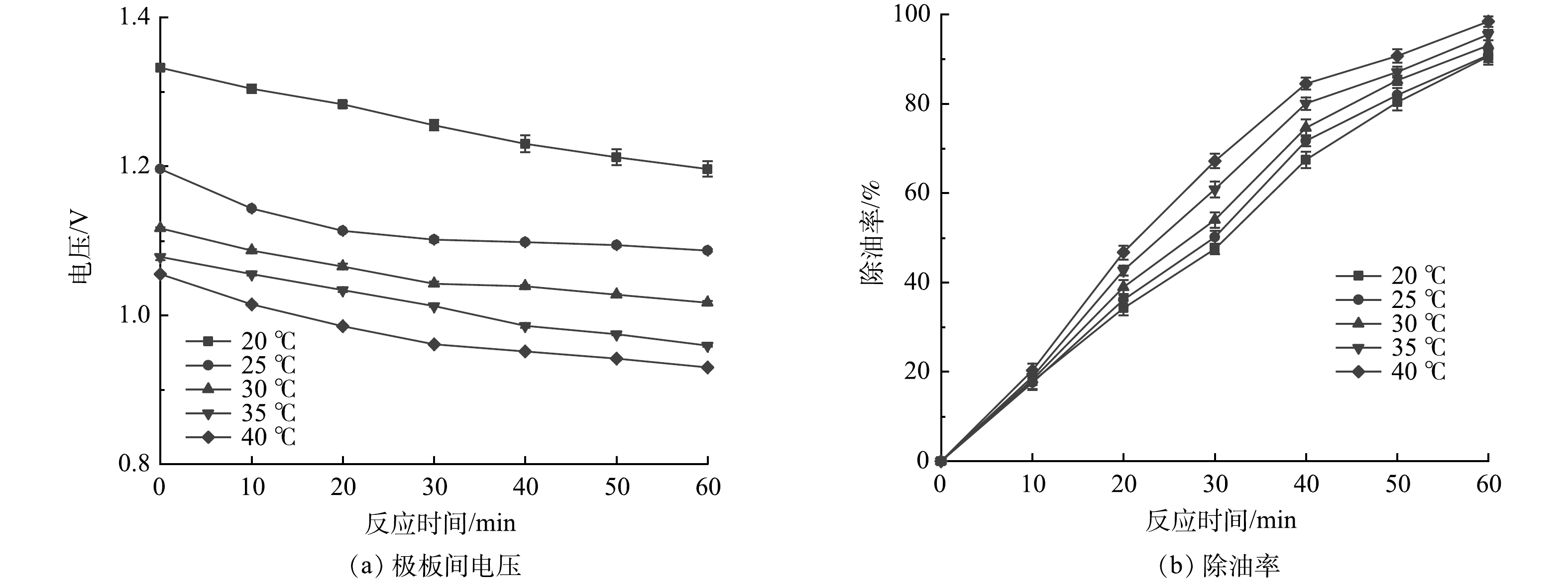
电絮凝处理含油废水时,废水温度变化会对除油率产生影响。在电极为不锈钢-铁电极、极板间距1 cm、支撑电解质浓度0.1 mol·L−1、pH为7.8的条件下,不同温度下极板间电压及除油率随时间的变化情况如图7所示。由图7(a)可见,随着电解槽内的含油废水温度升高,电极板间的工作电压降低,RED电堆输出电流增加。其原因在于,随着废水温度的升高,溶解于废水中的金属盐溶质“颗粒”的布朗运动强度增加,溶液电导率增大,电极板间的溶液电阻减小,使得电极电压降低。PAZENKO等[24]研究了废水温度对电絮凝法处理含油废水的影响,得出温度越高,电导率越高,能耗越低的结论。由图7(b)可见,在相同的处理时间内,耦合系统对含油废水的除油率随温度的升高而增加,验证了PAZENKO文献[24]得出的结论。因此,在有条件时,适当提高含油废水温度有利于提高耦合系统处理效率。
-
1)与采用铝阳极材料相比,采用铁阳极材料的耦合废水处理系统具有更高的除油率。
2)极板间距和含油废水参数(电导率、初始pH和温度)变化会对耦合废水处理系统的除油率产生影响。过大或过小的极板间距均对系统的除油率不利,在所研究的系统中,电絮凝器极板间距为1 cm时最佳;当废水的电导率很低时,系统的除油率很小,适当增加废水的电导率可以迅速提高系统的除油率;中性或微碱性条件下系统的除油率较高;温度越高,系统的除油率也越高。
3)采用不锈钢-铁电极,1 cm电极间距,0.1 mol·L−1支撑电解质(Na2SO4)浓度,pH 7.8和40 ℃的实验条件下,经耦合废水处理系统处理60分钟后的含油废水除油率可达到98.39%。
逆电渗析电堆与电絮凝器耦合系统处理乳化油废水
Treatment of emulsified oil wastewater by the coupling system of reverse electrodialysis stack and electrocoagulation
-
摘要: 通过对由逆电渗析(RED)技术与电絮凝(EC)技术结合构成由盐差能驱动的RED-EC耦合废水处理系统处理模拟乳化油废水的实验研究,探讨了电絮凝器电极材料、极板间距、支撑电解质浓度、废水初始pH及温度变化对乳化油废水除油率的影响。结果表明,与采用铝阳极材料相比,采用铁阳极材料的耦合废水处理系统具有更高的除油率。极板间距和含油废水参数(电导率、初始pH和温度)变化会对耦合废水处理系统的除油率产生影响。过大或过小的极板间距均对系统的除油率不利,在所研究的系统中,电絮凝器极板间距为1 cm时最佳。当废水的电导率很低时,系统的除油率也较低,适当增加支撑电解质可以迅速提高系统的除油率。中性或微碱性条件下系统的除油率较高。温度越高,系统的除油率也越高。在实验范围内,对总量为2 L、质量浓度为1 g·L−1模拟乳化油废水经60 min絮凝处理后,除油率可达98.39%。Abstract: Based on an experimental study using the RED-EC coupling system powered by SGE energy to treat simulated emulsified oil wastewater, the impacts of the electrode material, plate spacing, supporting electrolyte concentration, initial pH and temperature of the wastewater on the removal rate were investigated. The results show that the coupled wastewater treatment system using iron anode material had higher oil removal rate than the aluminum anode material. The oil removal rate of coupled wastewater treatment system was affected by the variations of plate spacing and oily wastewater parameters (conductivity, initial pH and temperature). Too large or too small plate spacing was bad for the oil removal rate of the system, in the studied system, the best plate spacing of the electrocoagulation was 1 cm.When the conductivity of wastewater was very low, the oil removal rate of the system was also low, and appropriate addition of supporting electrolytes could quickly increase the oil removal rate of the system. The oil removal rate was higher under neutral or slightly alkaline conditions. The higher the temperature, the higher the oil removal rate of the system. In the experimental range, the oil removal rate of 2 L simulated emulsified oil wastewater with a mass concentration of 1 g·L−1 could reach 98.39% after 60 min electrocoagulation treatment .
-
Key words:
- reverse electrodialysis /
- eletrocoagulation /
- emulsified oil wastewater /
- removal rate
-
在地球生物化学循环中,微生物对有机物分解、转化和营养物质再利用起着关键作用[1]。在细菌含量丰富、物质交换频繁的高活性沉积物中,细菌控制着营养元素的形态和去向[2],因此,研究沉积物中细菌群落结构的组成对阐明湖泊的物质循环及其生态系统功能具有重要的指导意义。
有关微生物多样性研究,已有较多文献报道。薛银刚等[3]发现,不同湖区表层沉积物细菌的物种丰富度和均匀度有差异,表现为水草区>湖心区>河口区;不同湖区优势细菌群落组成存在差异。赵大勇等[4]通过T-RFLP技术比较了太湖梅梁湾不同深度沉积物中细菌群落结构组成及多样性,结果表明,相邻近的沉积物分层中细菌群落结构的相似度较高;随着沉积物深度的增加,沉积物中优势菌属发生了一定的变化;表层沉积物与底层沉积物的细菌群落结构相差较大。寄博华等[5]通过高通量测序技术考察了滇池斗南湿地菖蒲、芦苇和美人蕉3种代表性挺水植物根区沉积物的细菌群落结构特征,结果表明,相同植物不同采样区沉积物细菌群落结构较相似,但不同植物区菌群结构差异较大。不同的人类活动对湖泊沉积物细菌群落组成有一定影响,JIN等[6]以鄱阳湖为研究对象,发现在干扰区,细菌群落多样性高于未干扰区,但主要的细菌门相似。WAN等[7]研究了5种不同营养状态湖泊底泥于冬季和夏季的细菌群落,结果表明,细菌群落的时空变化较为明显,夏季细菌多样性高于冬季,且随湖区营养状况的下降而增加。
白洋淀作为雄安新区的重要生态水体,其生态环境状况受到社会各界广泛关注。2016—2018年夏秋季,河北省监测白洋淀淀区及入淀河流水质为劣Ⅴ类,由营养盐超标导致的富营养化程度加剧[8]。自雄安新区成立以来,白洋淀流域实施了一系列污染综合整治措施,基本上遏制了水体污染加重趋势[9]。目前,富营养化湖泊底泥微生物多样性的研究已有一些报道,但多见于研究典型富营养化湖泊垂直向或水平向的微生物群落结构特征及其与环境因子之间的相关性。本研究以白洋淀6个不同富营养化程度的湖区为研究对象,应用高通量测序技术研究不同富营养化水平湖泊底泥中细菌群落结构和多样性。本研究获得的结果有利于认识白洋淀沉积物中微生物生长状况、影响因素及其与富营养化程度的关系。
1. 材料与方法
1.1 研究区域与样品采集
于白洋淀选取6个采样点位(图1),由北向南依次为白沟引河入淀口、王家寨、鸳鸯岛、府河入淀口、枣林庄、采蒲台采样点。选择水流平缓且远离岸边的位点,于2018年8月在各采样位点分别采集表层0 ~ 5 cm沉积物样品,每个采样点取3个平行样并充分混合,将混合样品放入灭菌的聚乙烯自封袋并尽快运回实验室,在−80 ℃冰箱保存。取一小部分沉积物新鲜样品用于测定氨氮(
NH+4 -N)、硝氮(NO−3 -N);一部分样品自然风干后研磨,用于测定总氮(TN)、总磷(TP)及总有机碳(TOC)含量。上覆水样品于水面下约80 cm处采集,尽快运回实验室用于上覆水化学需氧量(CODCr)、高锰酸盐指数(CODMn)、TN、NH+4 -N、NO−3 -N、TP及TOC含量测定。1.2 水质、沉积物理化指标测定
应用水质多功能测定仪现场测定各采样点水体的温度(T)、溶解氧(DO)和pH,其余指标均在实验室测定。上覆水CODcr采用快速消解分光光度法(HJ/T 399-2007)[10]测定;TN采用碱性过硫酸钾消解紫外分光光度法(HJ 636-2012)[11]测定;
NH+4 -N采用纳氏试剂分光光度法(HJ 535-2009)[12]测定;NO−3 -N采用紫外分光光度法(HJ/T 346-2007)[13]测定;TP采用钼酸铵分光光度法(GB 11893-89)[14]测定。沉积物TN采用凯氏法(HJ 717-2014)[15]测定;NH+4 -N和NO−3 -N采用氯化钾溶液提取-分光光度法(HJ 634-2012)[16]测定;TP采用碱熔-钼锑抗分光光度法(HJ 632-2011)[17]测定。TOC含量采用总有机碳分析仪(HJ/T 104-2003)[18]测定。1.3 综合营养状态指数的计算
评价因子选择透明度(SD)、叶绿素a含量(Chla)、TN、TP、CODMn共5个指标进行评价。综合营养状态指数TLI(∑)[7]计算公式为:
TLI(∑)=∑Wj×TLI(j) (1) 式中:TLI(∑)为综合营养状态指数;Wj为第j种参数的营养状态指数的相关权重;TLI(j)代表第j种参数的营养状态指数。贫营养TLI(∑)<30;中营养30≤TLI(∑)≤50;富营养TLI(∑)>50;轻度富营养50<TLI(∑)≤60;中度富营养60<TLI(∑)≤70;重度富营养TLI(∑)>70。
1.4 MiSeq高通量测序
在对样品预处理之后,通过E.Z.N.A.WaterDNAKit(OTUMEGA,美国)试剂盒,进行不同富营养化水平水体底泥样品基因组DNA提取、测定浓度,并将提取的DNA样品置于−80 ℃冷冻储存。采用16S rRNA通用引物对341F(CCTACGGGNGGCWGCAG), 805R(GACTACHVGGGTATCTAATCC)进行V3-V4区目的基因PCR扩增。在Miseq平台上对16SrRNA的V3-V4高变区进行高通量测序。每个样品均重复测定3次。来自该研究的所有RNA-seq数据可通过NCBI序列阅读档案(SRA)下载(http://www.ncbi.nlm.nih.gov/sra),登录号为SRP268032。
1.5 测序数据分析
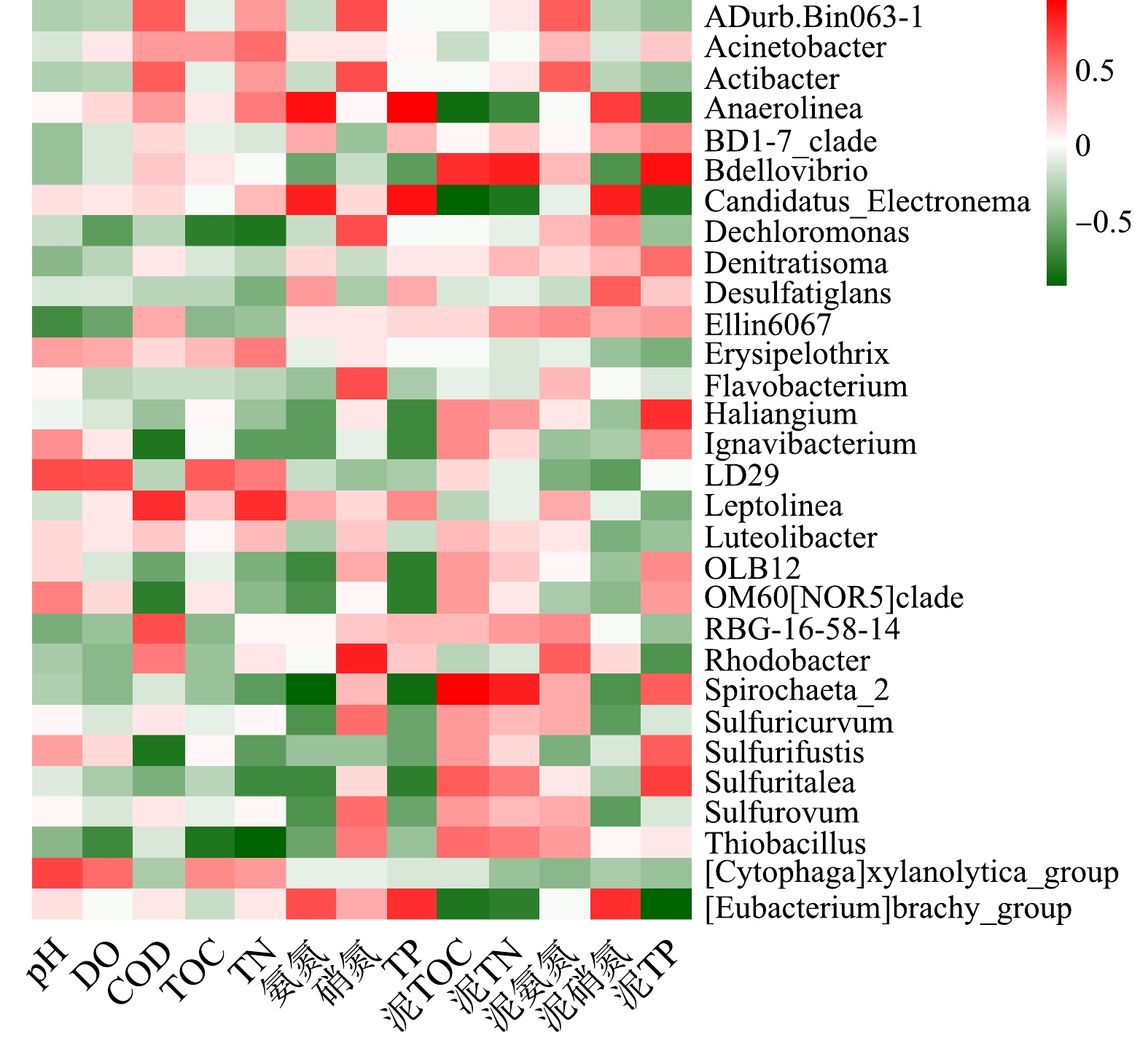
将Miseq测序所得的原始数据进行拼接,同时对拼接后的序列进行质控和过滤,得到优化序列。将相似性>97%的优化序列划分为一个操作分类单元(OperationalTaxonomicUnits,OTUs)。计算Chao1指数、observed_species指数评估菌群丰度;用香农指数(Shannon)、辛普森指数(Simpson)和PD_whole_tree指数表征菌群的多样性。基于R语言的ggplot2包绘制物种丰度柱状图。使用1-cor函数计算样本间的相关距离,并基于R的pheatmap包绘制样本间距离热图;计算菌属和环境因子之间的Spearman相关性系数,并基于R的pheatmap包绘制相关性热图。
2. 结果与讨论
2.1 水体水质分析
各采样位点水体富营养化评价结果如表1所示。白沟引河入淀口、府河入淀口、鸳鸯岛是重度富营养化采样点,其综合营养状态指数TLI为78.12~81.33;王家寨、枣林庄、采蒲台是中度富营养化采样点,其TLI为65.05 ~ 67.76。
表 1 白洋淀水体富营养化评价结果Table 1. Eutrophication evaluation results of Baiyangdian Lake评价区域 SD/m Chla/(μg·L−1) CODMn/(mg·L−1) TN/(mg·L−1) TP/(mg·L−1) TLI/(∑) 营养程度 白沟引河入淀口 0.75 41.30 12.12 4.658 11.18 79.73 重度 府河入淀口 0.60 39.71 16.53 4.037 3.71 78.12 鸳鸯岛 0.75 29.69 11.59 3.849 33.55 81.33 王家寨 1.35 26.40 8.25 3.006 1.30 66.56 中度 枣林庄 1.25 23.97 13.00 3.815 0.73 67.76 采蒲台 0.80 20.08 7.72 3.949 0.47 65.05 表2为不同采样位点的温度、pH、DO、Chla量。图2为各采样位点上覆水和沉积物的氮磷水平。夏季白洋淀各采样点水体温度变化不明显,维持在29.5 ~ 32.2 ℃。除采蒲台以外,其余采样点的上覆水DO含量相对较低,在3.13~5.60 mg·L−1,这是因为富营养化严重的湖区藻类等水生植物大量繁殖,阻碍了空气中氧气进入水体,也与水体中好氧微生物的分解消耗有关。各采样点pH为6.85 ~ 8.13,整体上呈中性偏碱性。Chla变化规律为重度富营养化区域>中度富营养化区域,其浓度代表湖泊藻类含量,是评价水体富营养化程度的重要指标,白洋淀水体中Chla含量均达到10 μg·L−1以上。
表 2 不同富营养化水平采样点的环境与水质参数Table 2. Environmental parameters of sampling points at different eutrophication levels富营养化水平 采样点 T/℃ pH DO/mg·L−1 Chla/μg·L−1 重度 白沟引河入淀口 31.00 7.26 5.60 41.30 府河入淀口 32.00 6.85 4.05 39.71 鸳鸯岛 29.50 7.05 4.95 29.69 中度 王家寨 30.10 6.92 3.42 26.40 枣林庄 31.90 6.85 3.13 23.97 采蒲台 32.20 8.13 7.48 20.08 由图2可知,重度富营养化采样点上覆水的TOC、TN和TP含量高于中度富营养化采样点,而沉积物TOC、TN和TP含量低于中度富营养化采样点。鸳鸯岛和采蒲台上覆水TP含量分别为最高和最低,其沉积物TP含量也分别为最低和最高。白沟引河入淀口的上覆水TN含量最高和最低分别出现在白沟引河入淀口和王家寨,他们沉积物TN含量分别为最低及最高。枣林庄采样点的上覆水
NH+4 -N含量最低,NO−3 -N含量最高,沉积物NH+4 -N含量最高。采蒲台采样点的上覆水NO−3 -N、沉积物NH+4 -N和NO−3 -N含量均最低。府河入淀口采样点的上覆水和沉积物中均有较高的NH+4 -N含量,这可能和保定市区生活污水排放有关[8]。2.2 微生物α多样性分析
本研究测序共获得204 320条序列,共检测到16 005个OTUs。单个测样点OTUs数为2 455~2 895个。不同富营养化水平水体底泥细菌群落α多样性指数如表3所示。Observed_species和chao1指数用来评估样本中被检测到的OTUs量,而Shannon指数、Simpson指数以及PD_whole_tree综合考虑群落中物种的丰富度和均匀度。不同采样点的α多样性指数表明,中度富营养化区域细菌群落的丰富度和均匀度均高于重度富营养化区域,因此可认为,富营养化水体沉积物中细菌群落多样性随营养水平升高而降低。WAN等[7]对太湖夏季综合营养状态指数为48.6~61.3的5个湖区沉积物的微生物多样性进行了研究,结果表明,细菌群落多样性随湖区营养程度减轻而增加,本研究结果与之一致。在极度贫营养条件下,水体中微生物种类相对较少,随着营养水平的逐渐升高,微生物由于营养物质等生存条件的改变,多样性增加,超富营养和接近超富营养湖区的水体中细菌群落多样性表现为减少的趋势19]。
表 3 不同富营养化水平湖泊底泥细菌群落α多样性指数Table 3. Diversity indexes of bacterial communities in lake sediments at different eutrophication levels富营养化程度 采样点 observed_species chao1 PD_whole_tree Shannon Simpson 重度 白沟引河入淀口 2389 3050 227 9.255 0.995 府河入淀口 2265 2927 221 8.886 0.991 鸳鸯岛 2307 2993 221 8.623 0.986 中度 王家寨 2668 3468 262 9.352 0.994 枣林庄 2701 3438 262 9.272 0.992 采蒲台 2620 3364 261 9.343 0.994 2.3 细菌群落结构组成及功能
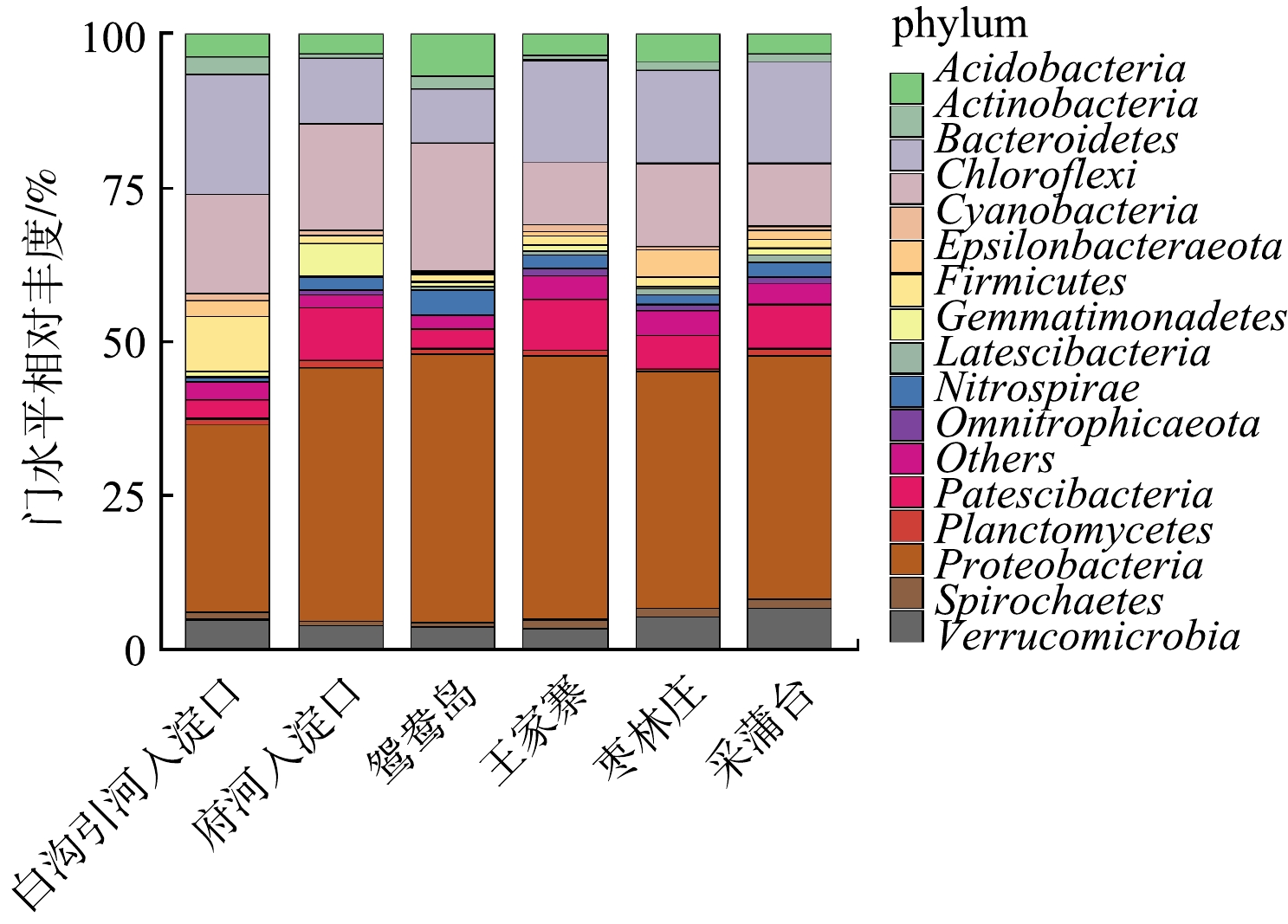
将白洋淀不同富营养化水平底泥样品进行数据库比对,同时分析各个水平上的细菌群落结构组成。将门水平上相对丰度≥1%的菌种绘制柱形图,结果如图3所示。白洋淀沉积物细菌群落结构在门分类水平上具有较高的多样性,各采样点位细菌组成差异不大,优势菌门为变形菌门(Proteobacteria) 31%~43%、绿弯菌门(Chloroflexi) 10%~21%、拟杆菌门(Bacteroidetes) 9%~20%、浮霉菌门(Patescibacteria) 3%~9%、疣微菌门(Verrucomicrobia) 3%~7%、酸杆菌门(Acidobacteria) 3%~7%、硝化螺旋菌门(Nitrospirae) 1%~4% 等;在大多数沉积物样品中,变形菌门(34%~47%)、酸杆菌门(3%~8%)、绿弯菌门(3%~10%)和拟杆菌门(3%~13%)为优势种类[20-21]。Nitrospirae也多次被发现在淡水沉积物中为优势菌群[22]。在变形菌门中发现的主要菌种有α
-变形菌纲(α-Proteobacteria)1.47% ~ 6.11%、γ -变形菌纲(γ-Proteobacteria)12.80% ~ 32.99% 和δ –变形杆菌纲(δ –Proteobacteria)8.50% ~ 11.73%。白沟引河入淀口的α-Proteobacteria丰度和δ -Proteobacteria高于其它采样点,分别为6.11%、11.73%,而γ-Proteobacteria丰度却远低于其它采样点,为12.80%。有研究[23]表明,α-Proteobacteria、β-Proteobacteria 和 γ-Proteobacteria中含有许多反硝化细菌,可进行自养或异养反硝化,δ -Proteobacteria包含了以其他细菌为食的细菌,对沉积物氮、磷、硫和有机质循环有重要作用[24]。据报道[25],Chloroflexi为光能自养型细菌,可在各种条件下较好生存,其多数种类是涉及有机污染物降解的细菌,白洋淀水体不同程度的富营养化状态使得Chloroflexi具有显著生存优势,成为白洋淀水体底泥的第二大优势菌门。Chloroflexi在重度富营养化采样点白沟引河入淀口、府河入淀口、鸳鸯岛的占比分别为16%、17%、21%,而在中度富营养化采样点王家寨、枣林庄、采蒲台的占比分别为10%、14%、10%,这间接说明了重度富营养化区域的COD含量高于中度富营养化区域,和水质分析结果一致。Verrucomicrobia是革兰氏阴性细菌,在海洋动物、南极沿岸沉积物和海水等环境中存在[26],在厌氧条件下进行亚硝化作用。 除此之外,放线菌门(Actinobacteria)、厚壁菌门(Firmicutes)、芽单胞菌门(Gemmatimonadetes)等的平均相对丰度也达到1%以上。据报道[27],Actinobacteria和Acidobacteria是重要的根际细菌,鸳鸯岛采样点的Acidobacteria相对丰度远高于其它采样点,为6.76%,Actinobacteria的相对丰度为2.26%,仅次于白沟引河入淀口,这可能与鸳鸯岛是白洋淀旅游区,种植了大量观赏性植物有关。有研究[28]称Gemmatimonadetes是一类光合细菌,能利用水体中氮源,在氮循环过程中起重要作用;本研究Gemmatimonadetes在府河入淀口采样点占比为5.26%,在除采蒲台(1.38%)以外的其它采样点占比均<1%,因此推断,这可能与府河入淀口氮含量高有关。Firmicutes可参与多种物质和元素的降解和转化[29],Firmicutes在白沟引河入淀口采样点的相对丰度为9.01%,在其它采样点的相对丰度为1.21% ~ 2.52%。
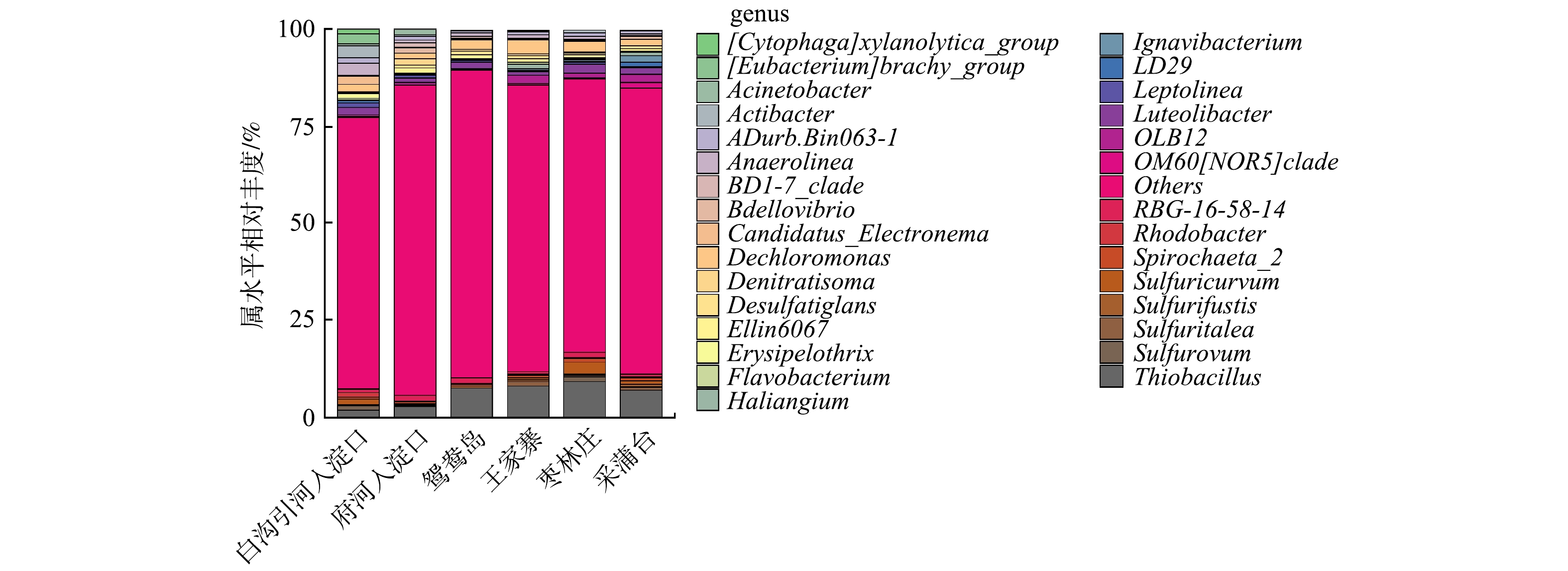
由图4可知,不同富营养化水平采样点属水平上细菌群落结构有差异,且物种丰度小于1%的菌属较多。这也说明不同富营养化水平底泥的物种丰富度较高,与α多样性较高一致。有研究[30]表明,这些沉积物细菌群落的丰富性和多样性使沉积物在营养盐循环、有机物降解、重金属形态转化等方面起着重要的生态功能。重度富营养化水体底泥的优势菌属是硫杆菌属(Thiobacillus)、脱氯单胞菌属(Dechloromonas)、厌氧绳菌属(Anaerolinea)、Luteolibacter、RBG-16-58-14;中度富营养化水体底泥的优势菌属是硫杆菌属(Thiobacillus)、脱氯单胞菌属(Dechloromonas)、OLB12、Luteolibacter、Sulfuricurvum。
Thiobacillus是白洋淀水体底泥中相对丰度最高的菌属,一些种类的Thiobacillus可直接将有机硫分解为无机硫。多数Thiobacillus可利用H2、硫化物等为电子供体,以硝态氮、亚硝态氮为电子受体实现反硝化及硫酸盐的还原,生成H2S;另外,还原态硫化物(H2S、
S2O2−3 )或单质硫也可被Thiobacillus氧化为硫酸。因此,硫杆菌属对白洋淀底泥氮硫污染物去除有一定积极作用,并且参与了白洋淀水体的自净过程。但要防止富余的H2S气体与水体中Fe2+、Mn2+等形成金属硫化物,被水体中悬浮颗粒吸附而悬浮在水体中形成水体的黑度;剩余H2S气体容易随水体上升,形成难闻气味[31]。Dechloromonas是白洋淀水体底泥的第二大优势菌属,是一种反硝化细菌,在王家寨采样点的相对丰度最高,为3.45%,这可能是由于王家寨上覆水和底泥中均有较高的硝氮含量。不动杆菌属(Acinetobacter)在白沟引河入淀口的相对丰度最高,为3.14%,具有反硝化、异养硝化功能。Spirochaeta_2是一种厌氧细菌。黄杆菌属(Flavobacterium)在白洋淀水体底泥的相对丰度为0.09% ~ 0.41%,Flavobacterium大多为具有脱氮与除磷功能的反硝化菌和异养硝化-好氧反硝化菌[32]。2.4 β多样性分析
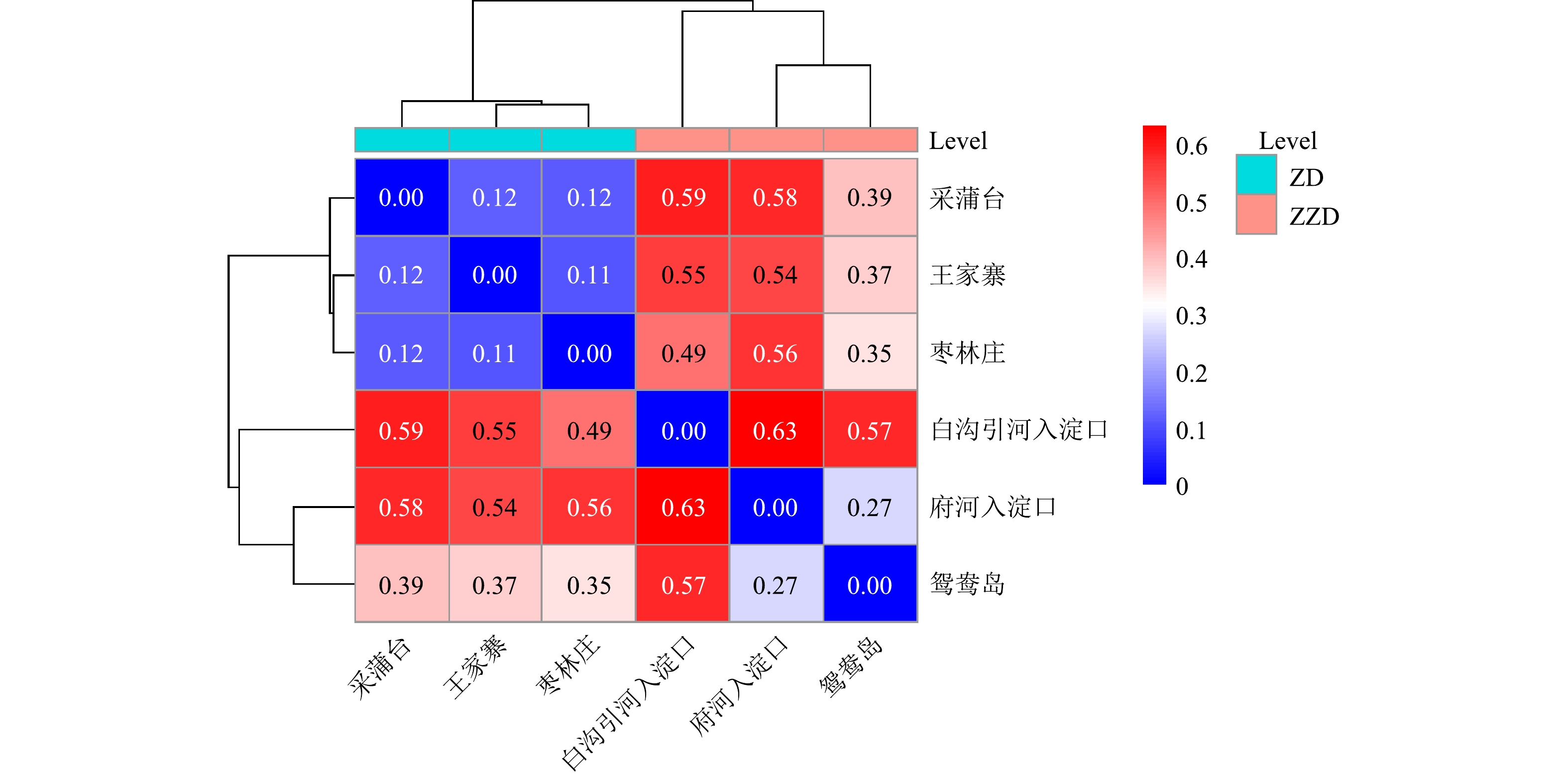
β多样性可反映不同富营养化程度组沉积物样品细菌群落结构差异。从样本相关距离热图(图5)可以看出,样本分为两大簇,一簇为重度富营养化区域白沟引河入淀口、府河入淀口、鸳鸯岛;另一簇为中度富营养化区域枣林庄、王家寨、采蒲台。这说明湖泊富营养化水平对沉积物细菌群落结构有一定影响,重度和中度富营养化水平水体沉积物细菌群落结构有明显差异,同一营养水平下的底泥细菌群落结构更相似。中度富营养化采样点王家寨和枣林庄的距离最近,为0.11,说明王家寨和枣林庄的细菌群落具有较高的相似度;府河入淀口和白沟引河入淀口的距离最远,为0.63。二者虽然都属于重度富营养化区域,但其细菌群落结构差异明显,可能和不同地理位置、人文条件及有机物组分差异有关。
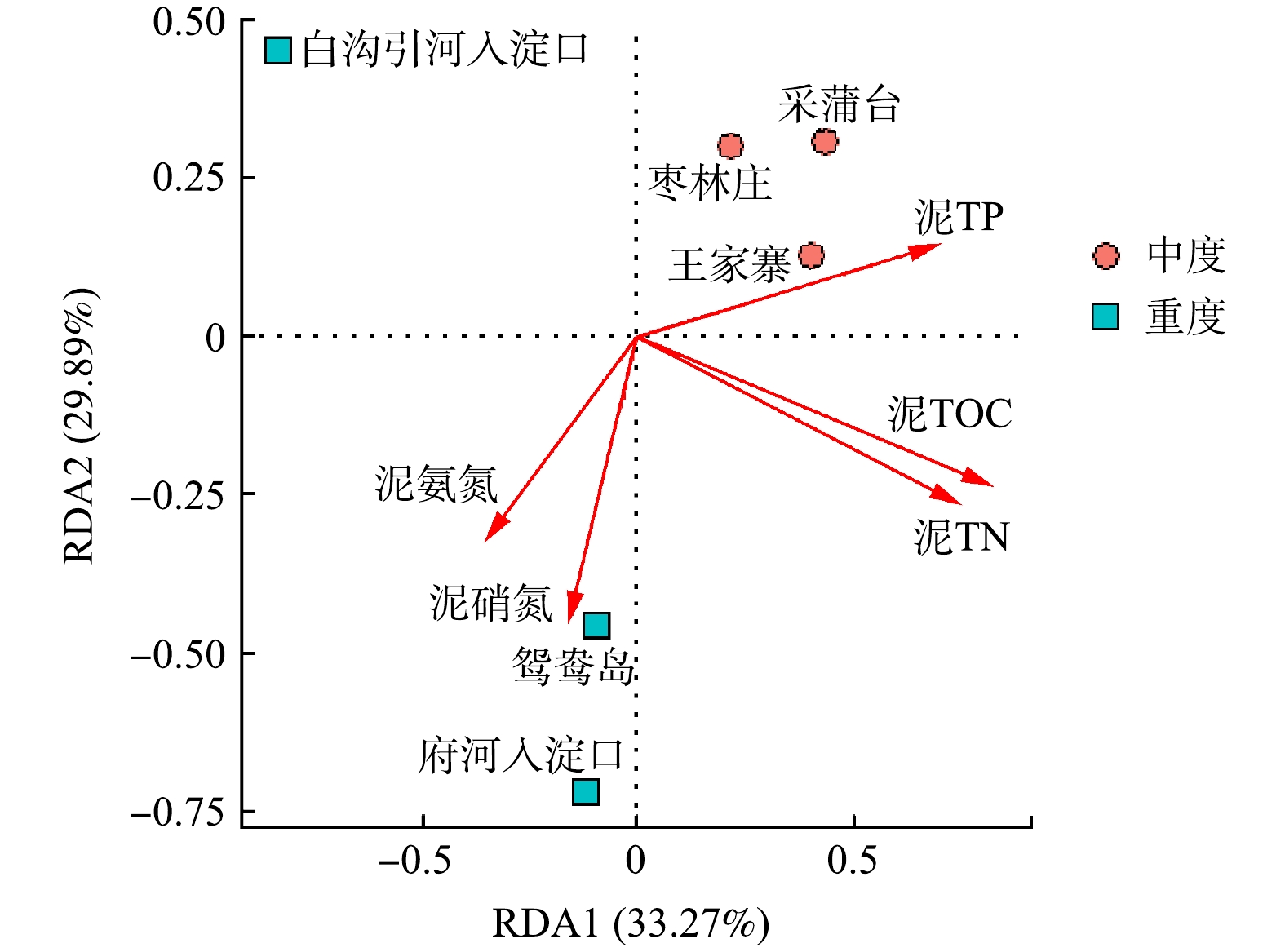
β多样性用于反映不同样品间细菌群落的差异性,冗余分析则可解释这种差异在多大程度上来源于环境因子的影响[5]。对各采样点底泥样品所有检测到的OTU和沉积物性质之间做冗余分析(图6)。RDA分析结果显示,RDA1和RDA2共同解释了总体变化的63.16%,表明沉积物5个化学指标对细菌群落多样性及组成有一定影响。泥氨氮、泥硝氮与第一主轴呈负相关,对重度富营养化采样点菌群影响大,泥TOC、泥TN、泥TP与第一主轴呈正相关,对轻度富营养化采样点的菌群影响较大。
2.5 细菌群落结构与环境因子相关性
环境因子对底泥细菌群落结构有重要影响。选取表层底泥细菌物种相对丰度>1%的菌属,对5个沉积物化学指标(泥TOC、泥TN、泥氨氮、泥硝氮、泥TP),8个上覆水水质指标(pH、DO、CODCr、TOC、TN、
NH+4 -N、NO−3 -N、TP)进行Spearman相关性分析(p<0.05),显著性水平为并作出热图,结果如图7所示。由图7可知,上覆水pH、DO、CODCr、TOC、TN、NH+4 -N、NO−3 N和TP、底泥TOC、TN、NH+4 -N、NO−3 N和TP对沉积物细菌群落结构均有一定影响,但氮磷营养物质对白洋淀富营养化水体底泥细菌群落结构影响显著。优势菌属Thiobacillus、Dechloromonas、OLB12、Sulfuricurvum、Anaerolinea的相对丰度受多种因素影响;而RBG-16-58-14等受环境因素影响很小。Anaerolinea和上覆水TP、上覆水NH+4 -N、泥NO−3 N显著正相关,相关系数分别为0.94、0.89、0.71,而和泥TOC、泥TP、泥TN显著负相关,相关系数分别为−0.89,−0.75,−0.71,而由细菌群落结构分析和水质调查结果得知,重度富营养化区域Anaerolinea的相对丰度明显高于中度富营养化区域,其TP、NH+4 -N、泥NO−3 -N含量均高于中度富营养化区域,泥TOC、泥TP、泥TN含量低于中度富营养化区域。该结论再次验证了细菌种群丰度与环境因子之间的相关性关系。上覆水TP和NH+4 -N、底泥TP和NO−3 -N对白洋淀水体沉积物中的细菌群落结构有较大影响,磷含量是白洋淀水体底泥细菌群落结构的制约因素。3. 结论
1)不同富营养化水平水体底泥α多样性存在差异。重度富营养化水体沉积物细菌群落的丰富度和均匀度均高于中度、富营养化水体沉积物;污染水体沉积物细菌群落多样性随营养水平升高而下降。
2)不同富营养化水体沉积物中细菌群落在门分类水平上没有明显差异,在属水平上有差异。重度富营养化区域的优势菌属为Thiobacillus、Dechloromonas、Anaerolinea、Luteolibacter、RBG-16-58-14,它们主要与含碳氮污染物的降解和循环有关;中度富营养化水体底泥的优势物种为Thiobacillus、Dechloromonas、OLB12、Luteolibacter、Sulfuricurvum,这些微生物大多参与了硫循环活动。
3)从样本距离热图可看出,重度和中轻度富营养化采样点细菌群落结构有差异,同一营养水平水体的沉积物中细菌群落结构相似性高。底泥氨氮和硝氮对重度富营养化采样点细菌群落影响大,沉积物TOC、TN和TP对中度富营养化采样点细菌群落影响大。
4)上覆水TP和
NH+4 -N、底泥TP和NO−3 -N对白洋淀水体底泥属水平细菌群落结构影响大。磷含量是白洋淀水体底泥细菌群落结构的制约因素。 -
-
[1] 郭研, 肖尊东. 乳化状含油废水难处理的物化原因探析[J]. 吉林省教育学院学报, 2015(1): 149-150. [2] DUAN C T, ZHU T, GUO J, et al. Smart enrichment and facile separation of oil from emulsions and mixtures by superhydrophohie/superoleophilie particles[J]. ACS Applied Materials & Interfaces, 2015, 7(19): 10475-10481. [3] 张玲玲, 陈强, 殷梦辉, 等. 膜分离技术在乳化态含油废水处理中的应用研究进展[J]. 应用化工, 2021, 50(10): 2791-2796. doi: 10.3969/j.issn.1671-3206.2021.10.035 [4] 陈文博, 耿安朝, 廖德祥. 吸附法分步处理含油废水研究[J]. 科技咨询导报, 2012(22): 14-14. [5] 商连. 絮凝剂处理含乳化油废水试验研究[D]. 西安: 长安大学, 2005. [6] 崔明玉, 王栋, 曹同川. 絮凝-电气浮法处理乳化油废水[J]. 环境技术, 2005, 23(2): 29-31. [7] 徐士鸣, 吴曦, 冷强. 一种利用低品位热能氧化降解有机废水的方法: CN201711384061.2[P]. 2018-05-29. [8] 王偲雪, 徐士鸣, 吴曦, 等. 溶液浓差能驱动的REDR阴/阳极独立环路降解废水中的苯酚[J]. 环境工程学报, 2021, 15(3): 886-897. [9] 徐士鸣, 徐志杰, 吴曦, 等. 溶液浓差能驱动的逆电渗析有机废水氧化降解机理研究[J]. 环境科学学报, 2018, 38(12): 4642-4651. [10] 冷强, 徐士鸣, 吴曦, 等. 逆电渗析反应器降解模拟甲基橙染料废水实验[J]. 环境科学学报, 2021, 41(8): 3157-3165. [11] 徐士鸣, 冷强, 吴曦, 等. 逆电渗析反应器阴、阳极联合降解酸性橙Ⅱ实验研究[J]. 环境科学学报, 2019, 39(7): 2163-2171. [12] XU S M, LENG Q, JIN D X, et al. Experimental investigation on dye wastewater treatment with reverse electrodialysis reactor powered by salinity gradient energy[J]. Desalination, 2020, 49: 114541. [13] 徐士鸣, 吴曦, 吴德兵, 等. 从吸收制冷到逆向电渗析发电-溶液浓差能应用新技术[J]. 制冷技术, 2017, 37(2): 8-13. [14] PATTLE R E. Production of electric power by mixing fresh and salt water in the hydroelectric pile[J]. Nature, 1954, 174(4431): 660. doi: 10.1038/174660a0 [15] ZHANG W H, ZHOU Y, HU C Z, et al. Electricity generation from salinity gradient to remove chromium using reverse electrodialysis coupled with electrocoagulation[J]. Electrochimica Acta, 2021, 379: 138153. doi: 10.1016/j.electacta.2021.138153 [16] TROMPETTE J L, VERGNES H, COUFORT C. Enhanced electrocoagulation efficiency of lyophobic colloids in the presence of ammonium electrolytes[J]. Colloids and Surfaces A, 2008, 315: 66-73. doi: 10.1016/j.colsurfa.2007.07.024 [17] CHEN X M, CHEN G H, PO L Y. Separation of pollutants from restaurant wastewater by electrocoagulation[J]. Separation and Purification Technology, 2000, 19: 65-76. doi: 10.1016/S1383-5866(99)00072-6 [18] HU J L, CHEN J Q, ZHANG X, et al. Dynamic demulsification of oil-in-water emulsions with electroco alescence: Diameter distribution of oil droplets[J]. Separation and Purification Technology, 2021, 254: 117631. doi: 10.1016/j.seppur.2020.117631 [19] DUTCHER C S, WOEHL T J, TALKEN N H, et al. Hexatic-to-disorder transition in colloidal crystal near electrodes: Rapid annealing of polycrystalline domains[J]. Physical Review Letters, 2013, 111: 128302. doi: 10.1103/PhysRevLett.111.128302 [20] Moussa D T, El-Naas M H, Nasser M, et al. A comprehensive review of electrocoagulation for water treatment: Potentials and challenges[J]. Journal of Environmental Management, 2017, 186: 24-41. [21] KOBYA M, DEMIRBAS E, CAN O T, et al. Treatment of levafix orange textile dye solution by electrocoagulation[J]. Journal of Hazardous Materials B, 2006, 132: 183-188. doi: 10.1016/j.jhazmat.2005.07.084 [22] DUAN J M, GREGORY J. Coagulation by hydrolysing metal salts[J]. Advances in Colloid and Interface Science, 2003, 100: 475-502. [23] GHERNAOUT D, BADIS A, KELLIL A, et al. Application of electrocoagulation in Escherichia coli culture and two surface waters[J]. Desalination, 2008, 219: 118-125. doi: 10.1016/j.desal.2007.05.010 [24] PAZENKO T Y, KHALTURINA T I, KOLOVA A F, et al. Electrocoagulation treatment of oil-containing wastewaters[J]. Journal of Applied Chemistry of the USSR, 1986, 58: 11. -





 下载:
下载: