-
随着城市化进程的不断推进,城镇污水处理厂产生了大量剩余污泥,而剩余污泥通常具有较高的含水率 (97%~99%) ,这不仅造成运输困难,也增加了对其处置的难度和成本[1]。此外,污泥中含有大量的病原体、有机物和重金属等毒害物质,随意处置会造成环境污染和生态失衡。对污泥进行减量化脱水能节省后续处理成本,避免二次污染[2]。因此,寻求一种经济高效的污泥脱水处理工艺具有重要意义。
有研究发现,胞外聚合物 (EPS) 是阻碍污泥脱水的重要因素之一[1]。EPS主要是由蛋白质、多糖和核酸等组成的高分子聚合物,其中含有大量结合水。EPS质量浓度会影响剩余污泥的生物絮凝和泥水分离效能,因此,有效处理EPS是改善污泥脱水性能的关键因素[3]。近年来,超声波[4]、电化学法[5]、紫外[6]、酸碱消化[7]和高级氧化[8]等方法被广泛应用于强化污泥脱水性能。其中,高级氧化技术是解决污水脱水问题的有效方法之一。
次氯酸钠 (NaClO) 是一种水处理中广泛使用的强氧化剂。与传统芬顿法中的H2O2相比,NaClO具有水亲和力强、不易腐蚀金属、便于运输、易于制备以及储存安全等优势,具有十分广阔的市场前景和研究价值。BEHIN等[9]利用Fe2+活化NaClO处理工业废水中的芳香化合物,表现出良好的处理效果。ZHAO等[10]研究发现,Fe2+/NaClO工艺可以去除垃圾渗滤液中难降解组分。JESSIELEENA等[11]研究了Fe2+/NaClO工艺在提高制革厂污泥脱水性、有机物降解性和铬浸出性方面的效果,但缺乏对污泥内部各类型结合水的迁移转化与污泥亲疏水性结构、官能团的关联性探究。
本研究拟对Fe2+/NaClO工艺强化剩余污泥脱水性能的可行性进行探究,并考察pH、Fe2+和NaClO投加量对污泥脱水性能的影响。同时,通过对比分析Fe2+/NaClO处理前后污泥结合水质量分数、粒径分布、絮体结构、表面官能团以及EPS的组成与分布等理化性质的变化,拟阐明该工艺实现污泥脱水的作用机理。本研究结果可为污水厂剩余污泥脱水减量化提供参考。
-
本实验中使用的剩余活性污泥取自于杭州市七格污水处理厂四期的二沉池,该污水处理厂采用“改良型AAO+反硝化深床滤池”处理工艺,设计日处理污水规模3×105 t,取泥时采用20目筛子进行筛分,以去除砂石、碎粒等不均匀物质;经筛分后的污泥静置2 h,除去上清液后运回实验室,储存在4 ℃冰箱中。经测试,剩余活性污泥基本指标为:含水率98.63%±0.01%、pH 6.99±0.07、污泥比阻 (13.47±0.85)×1012 m·kg−1、挥发性固体量 (VS) (12.73±0.47) g·L−1、总悬浮固体量 (TSS) (20.58±1.27) g·L−1、平均粒径 (D50) (28.19±0.03) μm。
-
次氯酸钠 (NaClO,化学纯) 、七水硫酸化亚铁 (FeSO4·7H2O,分析纯) 、酒石酸钾钠 (C4H4KNaO6·4H2O、≥30%) 、福林酚 (生物纯) 、牛血清白蛋白 (BSA,生物纯) 、硫酸 (H2SO4,分析纯) 。
-
分3组探讨实验参数对污泥脱水性能的影响。NaClO投加量影响实验在溶液pH=5,Fe2+投加量为48.61 mg·g−1,NaClO投加量分别为9.76、19.52、29.28、39.04和48.80 mg·g−1下进行。Fe2+投加量影响实验在溶液pH=5,NaClO投加量为39.04 mg·g−1,Fe2+投加量分别为16.20、32.41、48.61、64.82和81.02 mg·g−1下进行。在不同初始pH影响实验中,NaClO和Fe2+投加量分别为39.04和48.61 mg·g−1,调节pH分别为3.0、4.0、5.0、6.0、7.0和8.0。
采用浓度为1 mol·L−1的H2SO4将污泥混合液pH调至5.0,将100 mL污泥倒入250 mL锥形瓶中,先后加入FeSO4·7H2O和NaClO,摇晃均匀后用封口膜封口,放入恒温振荡器中 (250 r·min−1,25 ℃) 反应40 min,通过测定含水率和污泥比阻考察污泥脱水性能。在此基础上提取EPS,通过分析多糖、蛋白质质量浓度和三维荧光激发光谱的变化探究Fe2+/NaClO体系强化污泥脱水的作用机理。所有测试实验均重复进行3次。
污泥脱水试验完成后,对含水污泥样品进行抽滤得到污泥固体样品,取3 mL污泥固体样品倒入5 mL离心管中,放至冰箱预冷冻至固体状态;再将预冷冻后的待测样品放入真空冷冻离心机中在-55 ℃下冷冻干燥36 h,得到粉末状污泥样品。
-
1) 污泥脱水性能测定。污泥比阻 (SRF) 和含水率 (Wc) 是表征污泥脱水性能的重要指标,污泥比阻采用真空过滤法完成,含水率采用标准方法测定,如式 (1)~式 (3) 所示。
式中:P为过滤压强,Pa,;A为过滤面积,m2;μ为动力粘度,1×10−3 Pa·s;b为t/V与V的线性斜率,s·m−6;C为滤过单位面积体积的滤液在过滤介质上截留的干固体重量,g;T为温度, ℃;m0为坩埚重量,g;m1为烘干滤纸重量,g;m2为坩埚、滤纸和泥饼抽滤后的总重量,g;m3为坩埚、滤纸和泥饼烘干后的总重量,g。
2) EPS的提取与测定。采用改良的热提取法[12],分为提取溶解型EPS (S-EPS) 、松散附着型EPS (LB-EPS) 和紧密黏附型EPS (TB-EPS) 。提取S-EPS时,首先取35 mL污泥放入50 mL的离心管内,经高速离心 (4 000 r·min−1,10 min) 处置后将离心后上清液倒出,再经0.45 μm滤膜过滤后收集。提取LB-EPS时,首先将0.05%的NaCl溶液 (70 ℃) 加入离心管中,使污泥样品体积恢复至35 mL,再使用涡旋振荡器震荡2 min,以相同条件离心处理并取上清液,最后经0.45 μm滤膜过滤后收集。提取TB-EPS时,首先将0.05%的NaCl溶液 (70 ℃) 加入离心管中,使污泥样品体积恢复至35 mL,再使用涡旋振荡器震荡2 min,然后放入60 ℃的水浴中保持30 min,再以相同条件离心处理15 min,后经0.45 μm滤膜过滤后收集。提取完的各层EPS保存在4 ℃冰箱中待分析。蛋白质和多糖质量浓度的测定分别采用Lowry法[13]和苯酚-硫酸法[14]。
3) 其它指标。采用差示扫描量热仪 (瑞士梅特勒SERIES 2000,瑞士Mettler Toledo公司) 分析测试污泥处理前后结合水质量分数的变化;采用纳米粒度仪 (NanoZS 90型,英国Marvern有限公司) 测定污泥上清液颗粒的Zeta电位;通过自动激光粒度分析仪 (LAP-W2000H,中国厦门易仕特仪器有限公司) 测定污泥样品的粒径分布;采用傅里叶变换红外光谱仪 (Nicolet 6700,美国Thermo公司) 分析检测污泥样品中的蛋白质二级结构和官能团的变化规律;通过扫描电子显微镜 (Gemini 50,美国Zeiss公司) 观察污泥颗粒表面微观形貌结构;采用X-射线光电子谱仪 (XPS,Thermo Kalpha,美国Thermo Fisher Scientific公司) 分析污泥表面官能团的元素形态变化。
-
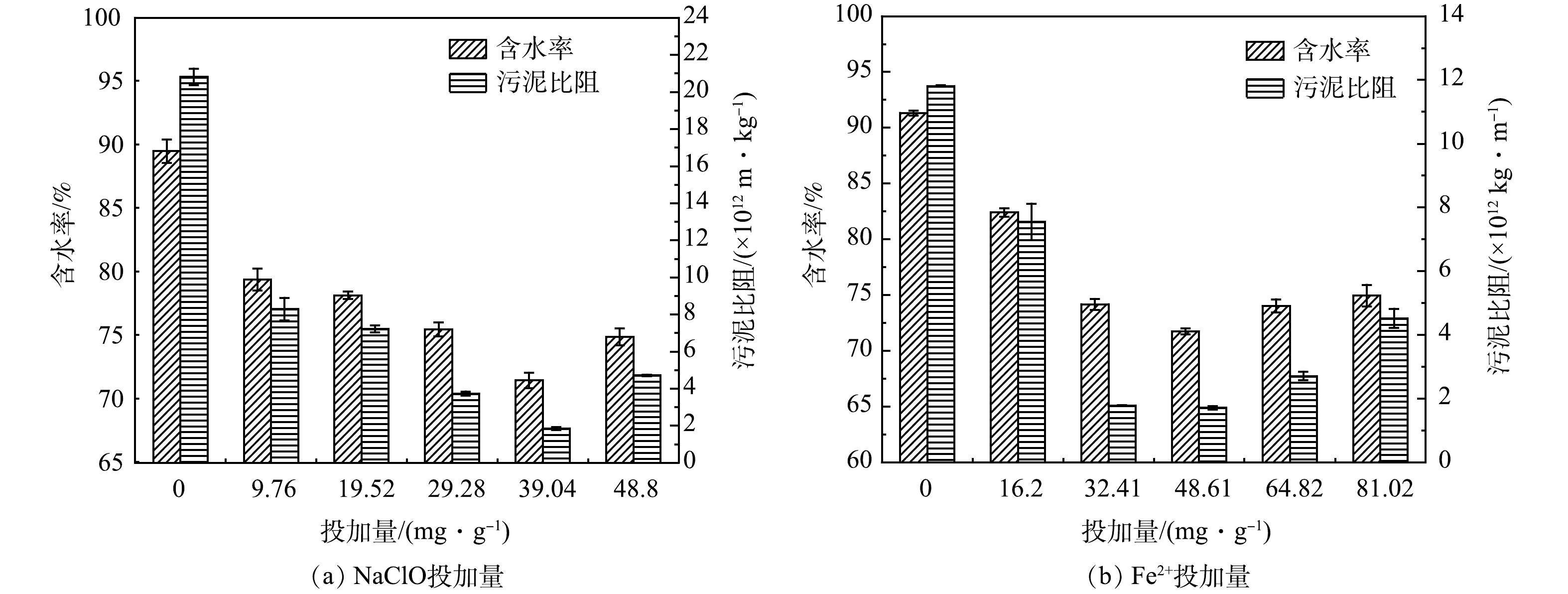
当污泥液初始pH为5.00,Fe2+投加量为48.61 mg·g−1,NaClO投加量对污泥脱水性能的影响如图1 (a) 所示。当NaClO投加量从0增加至39.04 mg·g−1时,污泥比阻和含水率分别从20.81×1012 kg·m−1和89.51%降至1.85×1012 kg·m−1和71.48%,这是由于在酸性条件下,NaClO转化生成反应活性更高的HClO,其氧化能力对污泥絮体的破坏也有一定的贡献。此外,Fe2+活化HClO产生的羟基自由基 (·OH) 和氯自由基 (·Cl) 可以有效破坏EPS结构,造成污泥絮体的崩解和细胞的裂解,促使结合水转化为游离水并转移至液相中,进而在机械脱水过程中更易除去[15],如式 (4) 和 (5) 所示。然而,随着NaClO投加量增加至48.80 mg·g−1时,污泥脱水性能没有进一步改善反而恶化,归因于过量ClO−会消耗·OH并生成氧化能力相对较弱的氯氧自由基 (ClO·) ,如式 (6) 所示,降低了体系氧化能力从而导致污泥脱水性能恶化。BEHIN等[9]在探究类芬顿工艺去除模拟工业废水中的污染物时,同样发现过量的ClO−会造成自由基淬灭,抑制工业废水中COD的去除。
图1 (b) 展示了Fe2+投加量对于污泥脱水性能的影响。当pH=5,NaClO=39.04 mg·g−1时,随着Fe2+投加量从0增加至48.61 mg·g−1,污泥比阻和含水率分别从11.81×1012 kg·m−1和91.32%降低到1.71×1012 kg·m−1和71.74%。结果表明,污泥脱水性能随着Fe2+投加量的增加而提高。这是因为,一方面Fe2+浓度的提高促进Fe2+/NaClO体系中·OH的释放,导致EPS的氧化,弱化污泥的持水能力;另一方面,反应过程中原位生成的Fe3+可以起到有效絮凝作用,改善污泥的固液分离性能,如式 (4) 和式 (7) 所示。当Fe2+投加量从48.61 mg·g−1进一步增加至81.02 mg·g−1时,污泥脱水性能也出现一定恶化。一方面,过量的Fe2+会生成更多的Fe3+,这对污泥的絮凝是有利的;另一方面,过量的Fe2+会淬灭·OH,导致该体系氧化能力变弱。结果表面单一絮凝作用对污泥脱水性能的改善并不明显,氧化降解EPS和裂解细胞在结合水的释放中起关键作用。
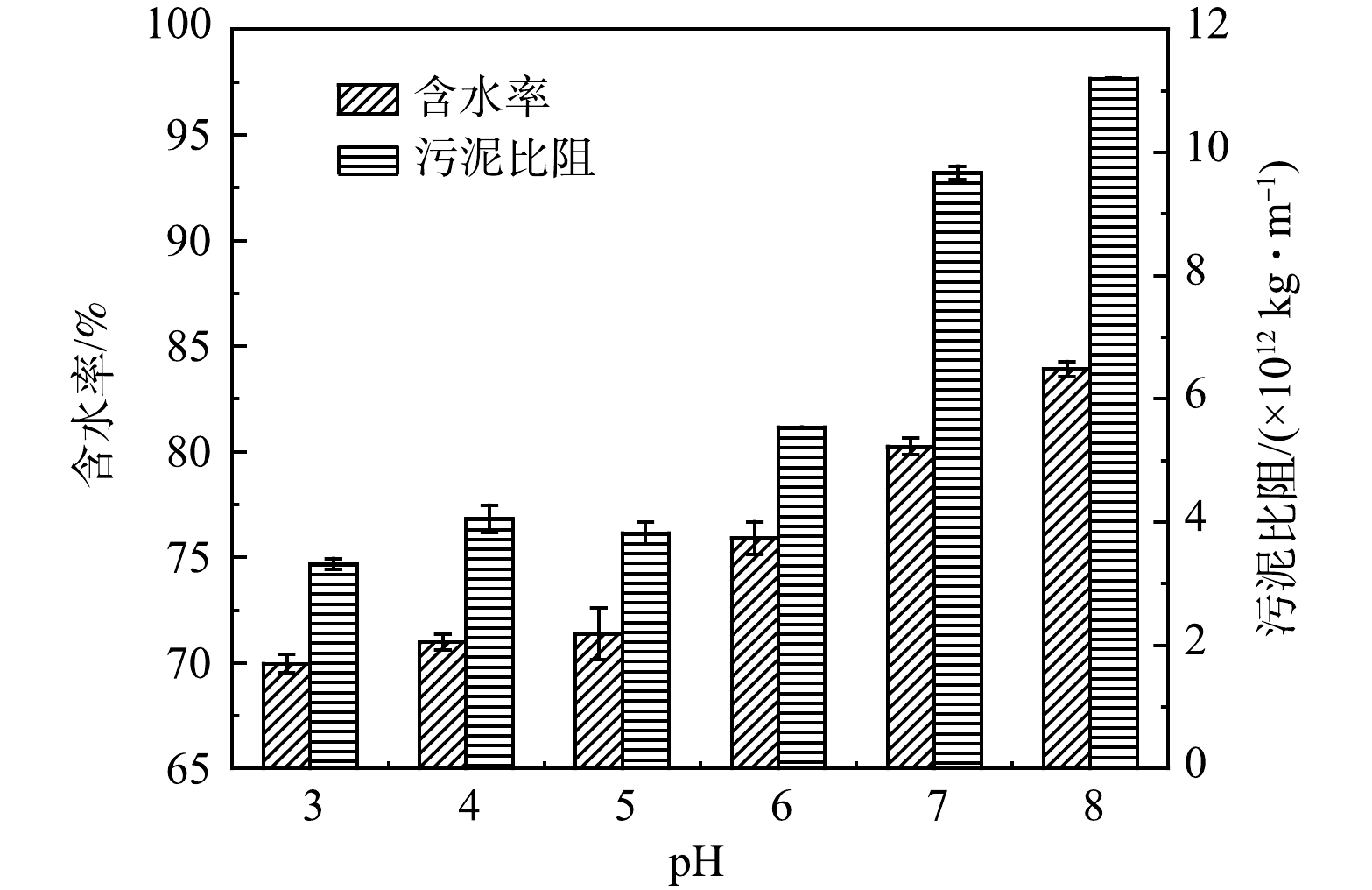
如图2所示,实验探究了6组pH (3.0、4.0、5.0、6.0、7.0、8.0) 对Fe2+/NaClO工艺改善污泥脱水性能的影响。结果表明,Fe2+/NaClO工艺在pH范围为3.0~5.0时脱水性能达到最佳,在此范围内,污泥比阻和含水率范围分别降至 (3.32~3.82)×1012 kg·m−1和69.97%~71.38%,这说明Fe2+/NaClO工艺在酸性环境中脱水性能更好。主要由于酸性条件下NaClO转化生成氧化活性更高的HClO,有助于破坏污泥细胞和释放结合水,并且H+能使污泥EPS表面的阴离子官能团 (羰基、羧基、羟基等) 质子化,从而促进污泥胶体颗粒脱稳聚集,提高沉降性能[16]。但当pH从6.0上升到8.0时,污泥比阻和含水率分别从5.54×1012 kg·m−1和75.91%迅速增至11.20×1012 kg·m−1和83.91%,污泥脱水性能恶化趋势明显。这是因为,pH升高会导致体系中Fe2+易于OH−反应生成Fe(OH)2沉淀,使得其对NaClO的催化活性下降,从而减少了·OH的生成,减弱了Fe2+/NaClO工艺的氧化能力[9]。结果表明,Fe2+/NaClO工艺的pH作用范围较为广泛,该工艺在pH=3~5范围内对污泥脱水性能的强化作用最佳。
-
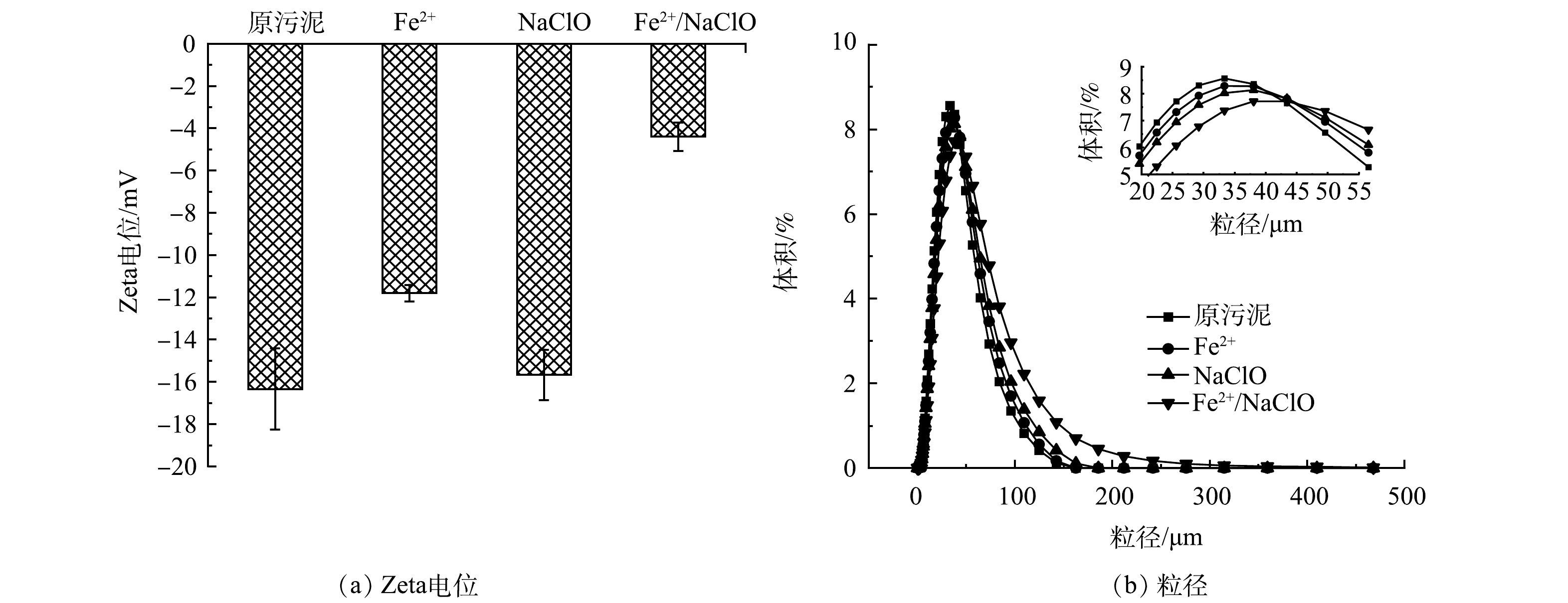
1) Zeta电位与粒径分布。不同调理工艺对污泥Zeta电位的影响如图3 (a) 所示,原污泥的Zeta电位值为-16.33 mV,其表面负电性是由于EPS表面的阴离子官能团电离造成的,其电负性较强,使得污泥颗粒间的静电斥力较大,阻碍了污泥的有效沉降[17]。在pH=5时,经Fe2+、NaClO和Fe2+/NaClO工艺处理后,污泥的Zeta电位从−16.33 mV分别增至−11.80、−15.67和−4.40 mV。其中,Fe2+/NaClO工艺处理后的Zeta电位绝对值最低,这表明Fe2+/NaClO体系中生成的强氧化性的活性物种可将EPS表面的阴离子官能团氧化,同时生成的Fe3+可起到电中和的作用,共同影响污泥颗粒的表面负电性,减弱静电斥力,从而提高污泥絮体密实度[18]。污泥粒径在不同处理工艺下的变化情况如图3 (b) 所示,污泥的粒径分布通常被认为是影响污泥脱水性能的一个关键因素。经调理后的各污泥样品D50大小依次为:Fe2+/NaClO (35.46 μm) >Fe2+ (30.98 μm) >NaClO (29.62 μm) >原污泥 (28.19 μm) 。粒径的变化结果与Zeta电位的结果相一致,较低的Zeta电位能够促使胶体颗粒聚集成大颗粒,从而提高污泥的脱水性能。然而,RUAN等[19]的研究结果发现,热活化过硫酸盐工艺调理污泥时发现预处理后的污泥粒径减小。这可能是因为在Fe2+/NaClO工艺氧化污泥过程中,·OH并非直接破坏污泥大分子物质的长链骨干 (如蛋白质中的-CO-NH-) ,而是被氧化降解的中间产物在Fe3+的絮凝作用下更容易聚合,使污泥粒径变大,从而表现出更加优异的固液分离性能[20]。
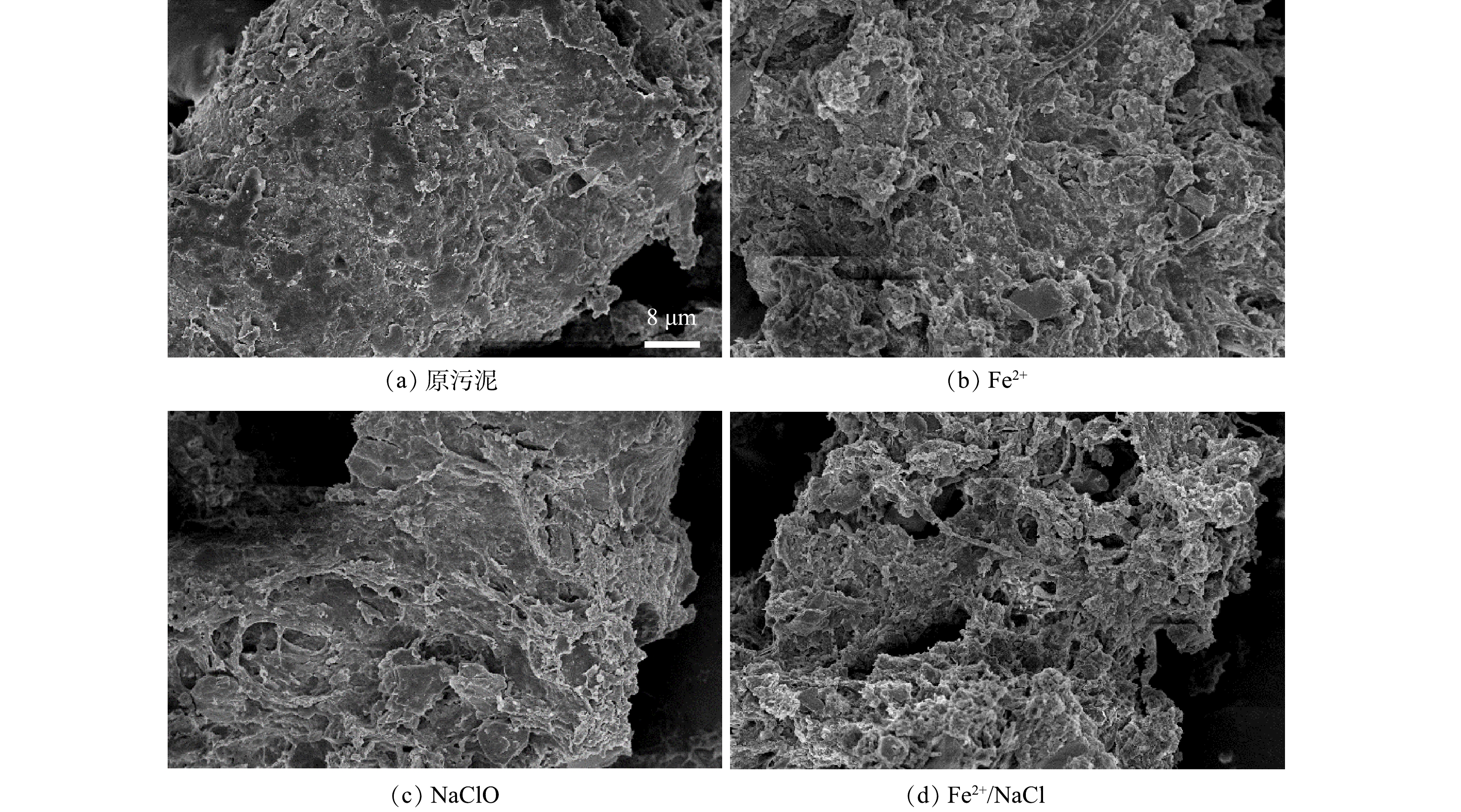
2) 污泥的表面微观形态。如图4所示,原污泥的表面相对光滑且较为密实,孔隙较小,限制了内部结合水的排出。经Fe2+和NaClO处理后的污泥絮体表面呈现出松散的孔隙结构,有利于水分的流动和排出。一方面,EPS的结构被破坏,网状结构中的间隙水得到释放;另一方面,EPS表面的亲水官能团被氧化降解,有机物和水相的水合作用被弱化,结合水与固相污泥间的氢键相互作用被减弱,进而导致结合水更易从形成的孔隙中排出[1,8,19]。DAI等[20]以改性磷石膏预处理污泥时也发现,污泥絮体形成的孔隙结构增多有利于结合水的释放。因此,Fe2+/NaClO工艺影响了污泥絮体的空间结构,使污泥由致密结构转变为多孔隙松散结构,从而改善污泥的脱水性能。
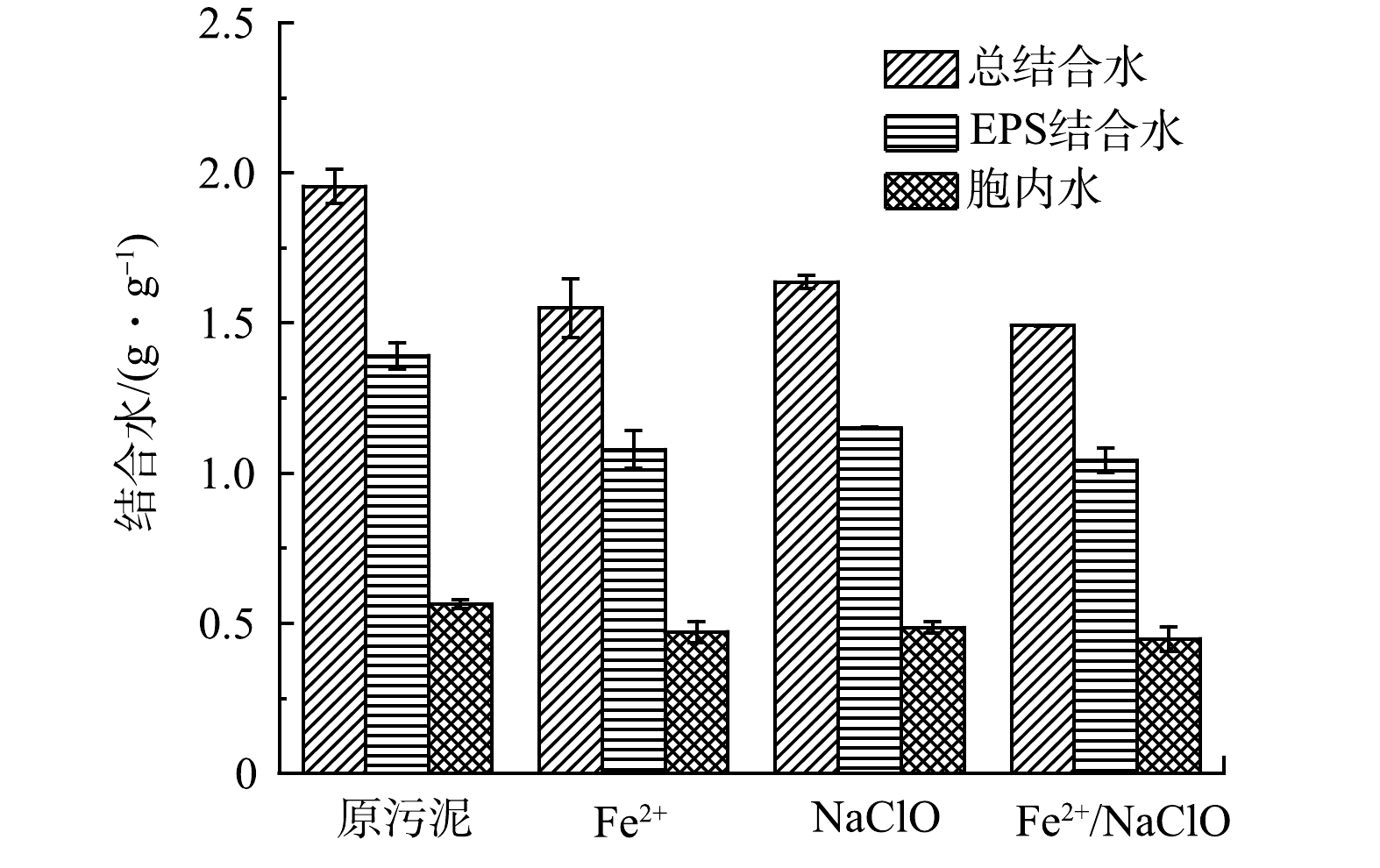
3) 结合水的变化。污泥的EPS具有复杂的网状结构,其中有机相使部分水嵌入生物聚合物的网状结构中,从而改变这部分水分的迁移性质,传统机械脱水过程难以将其除去,此部分水被定义为结合水[21]。本实验将总结合水分为胞内水和EPS结合水,分析了不同预处理工艺对各类型结合水转移的影响。图5展示了不同预处理工艺后污泥的总结合水、胞内水和EPS结合水质量分数的变化。Fe2+/NaClO工艺处理后,各类型结合水质量分数均大幅降低。Fe2+、NaClO和Fe2+/NaClO处理后总结合水质量分数 (以干泥固体计) 由1.95 g·g−1分别降低至1.55、1.64和1.49 g·g−1。这是因为,Fe2+/NaClO体系中产生的自由基破坏了污泥絮体结构,降低了污泥表面的亲水性,促进了结合水向游离水的转化,从而提高了污泥脱水性能。LIU等[22]研究也发现,自由基可以破坏污泥絮体周围复杂的胶体结构,释放大量结合水。此外,EPS结合水的质量分数明显高于胞内结合水质量分数,且Fe2+/NaClO工艺对于去除EPS结合水的效果最为明显。该结果表明,EPS作为污泥细胞的保护层,是污泥具有较高持水能力的主要因素,在氧化过程中更容易受到强氧化性自由基的破坏,因而仅有少部分的细胞发生裂解并释放胞内水。因此,在Fe2+/NaClO体系中,发生了大量的EPS降解和少部分的细胞裂解,并将EPS结合水和胞内结合水转化为自由水,实现了剩余污泥的深度脱水。
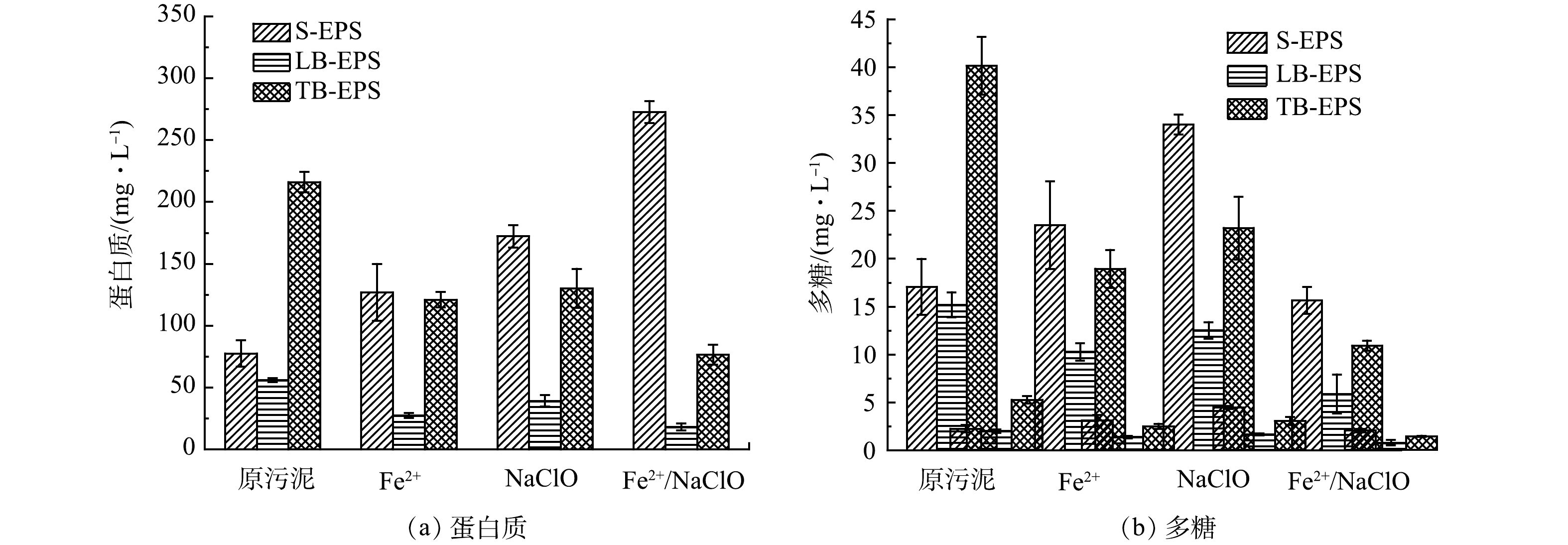
4) 不同工艺对EPS组分的影响。EPS基质是由不同微生物分泌物、细胞裂解副产物和介质中的有机物聚集而成的复杂聚合物。有研究表明,EPS的主要成分是蛋白质和多糖,其对污泥的亲疏水性、表面电荷、絮体结构和脱水性能有着显著影响[1]。因此,本实验通过分析不同处理工艺对污泥EPS各组分质量浓度的变化,探究强化污泥深度脱水的潜在机制。图6 (a) 展示了不同工艺处理前后各EPS中蛋白质的质量浓度变化。如图所示,原污泥的蛋白质主要集中在TB-EPS中,经Fe2+和NaClO单独处理后,TB-EPS的蛋白质质量浓度由216.07 mg·L−1分别降低至120.94和130.20 mg·L−1,而S-EPS中的蛋白质质量浓度从77.57 mg·L−1分别增加至127.03和172.29 mg·L−1。这是因为,TB-EPS中的不溶性有机质被降解成可溶性有机质,并转移至S-EPS中。上述变化趋势在Fe2+/NaClO工艺处理后的污泥中最为明显,表明Fe2+/NaClO与单独Fe2+和NaClO处理相比对TB-EPS的破坏程度更大,导致TB-EPS中更多的蛋白质发生降解,从而导致EPS结合水的释放[23]。图6 (b) 呈现了不同工艺处理前后各EPS中多糖质量浓度的变化。如图所示,TB-EPS中的多糖变化趋势与蛋白质保持一致,而Fe2+/NaClO处理后S-EPS中多糖质量浓度反而降低。这可能是因为蛋白质相比多糖更容易被氧化,多糖从TB-EPS增溶至S-EPS后又被·OH矿化成无机物,因此S-EPS中的多糖质量浓度并没有显著增加[24]。综上,经Fe2+/NaClO处理后LB-EPS的蛋白质和多糖质量浓度变化较小,TB-EPS和S-EPS的蛋白质和多糖质量浓度变化较大。因此,TB-EPS的破坏和S-EPS的增加可以有效改善污泥脱水性能,而LB-EPS对污泥脱水性能的影响不大[25]。此外,多糖的质量浓度普遍小于蛋白质,且工艺处理前后变化较小,这说明蛋白质质量浓度的变化在污泥脱水性能中起主导作用[26]。
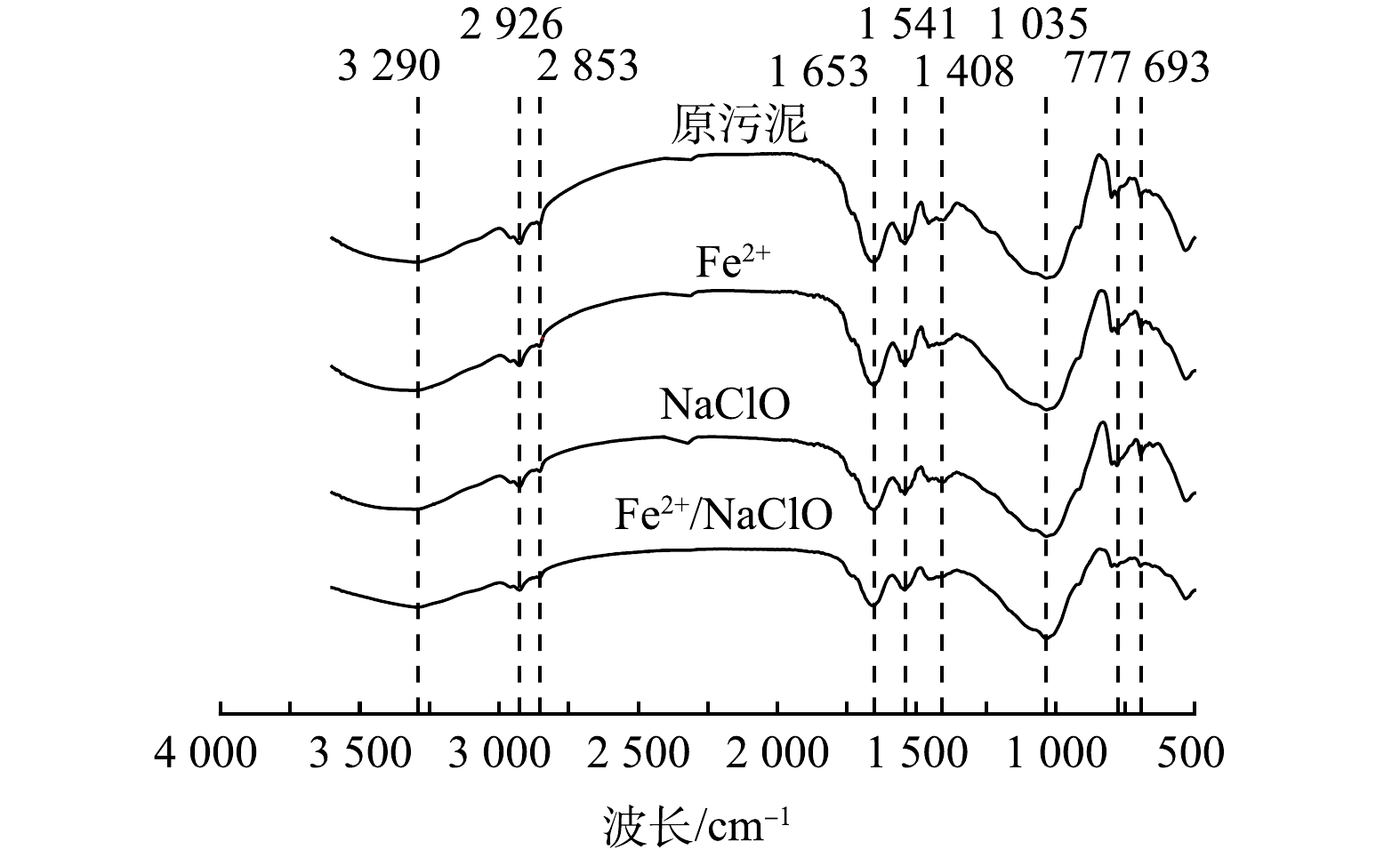
5) 表面官能团分析。上述研究表明,污泥的脱水性能与EPS中的亲水性蛋白质和多糖有关。采用FTIR进一步探究了污泥表面的亲疏水性官能团变化,官能团特征峰反映出污泥的主要成分为碳氢化合物、蛋白质、多糖和芳香族氨基酸。如图7所示,3 290 cm−1处的峰值与碳氢化合物中羟基官能团O-H键的伸缩振动有关;1 653 cm−1的峰值对应蛋白质中酰胺Ⅰ区C-O和C-N的伸缩振动;1 541 cm−1的峰值对应蛋白质酰胺Ⅱ区-CO-NH-中N-H和C-N的伸缩振动;1 408 cm−1的峰值对应质子化羧基中C=O的对称伸缩振动;1 035 cm−1的峰值对应多糖中C-OH和C-O-C的伸缩振动;777 cm−1和693 cm−1的峰值对应芳香族氨基酸的C-C和C-OH的环振动[27]。Fe2+/NaClO工艺处理后的污泥所呈现出的特征峰表现出更高的透射率,表明Fe2+/NaClO可显著降低污泥中此类官能团的质量分数。这是因为,在弱酸性条件下,H+的存在会引起羧基官能团的质子化;而且,反应过程产生的强氧化性的活性物种会破坏蛋白质酰胺Ⅰ区中的N-H和C-N基团,导致蛋白质二级结构发生变化,进一步影响蛋白质的空间结构,使更多的疏水位点暴露,降低污泥表面亲水性,从而提高其脱水性能[28]。此外,芳香族氨基酸中C-C和C-OH基团的环振动表明,氧化活性物种可引起芳香环的羟基化作用,有利于EPS的破坏和结合水的释放。
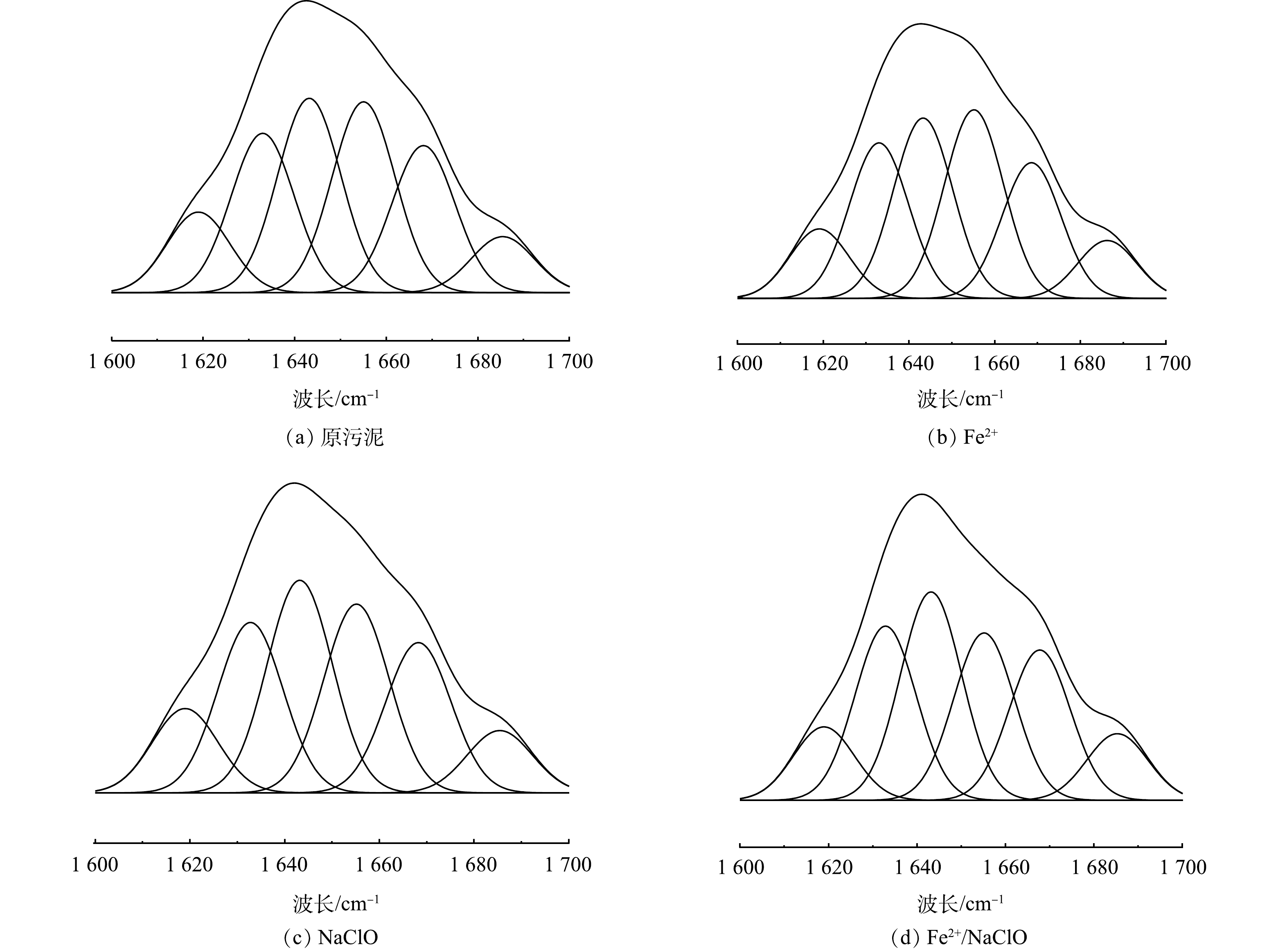
为了进一步分析污泥脱水性能与污泥表面亲疏水性的相关性,通过分析对酰胺I区得到蛋白质二级结构的质量分数。如图8所示,在1 600-1 700 cm−1范围内表现出6个峰值,同时表1列出了各种蛋白质二级结构的质量分数。经Fe2+/NaClO工艺处理后,α-螺旋的质量分数24.37%降至21.90%,[α-螺旋/(β-折叠+无规则卷曲)]的比值从67.07%降至52.62%,此结果会引起蛋白质结构的松散,暴露更多的疏水点位,从而促进污泥脱水性能[29]。此外,α-螺旋影响蛋白质二级结构的关键是氢键的断裂,这是由于氢键能够维持羰基氧 (位于肽键号N上) 和酰胺氢 (位于肽键号N+4上) 之间的稳定性。而且,蛋白质中S–S键的断裂,使得α-螺旋可以转化为β-折叠,这可能会改变蛋白质的二级结构质量分数变化[30]。
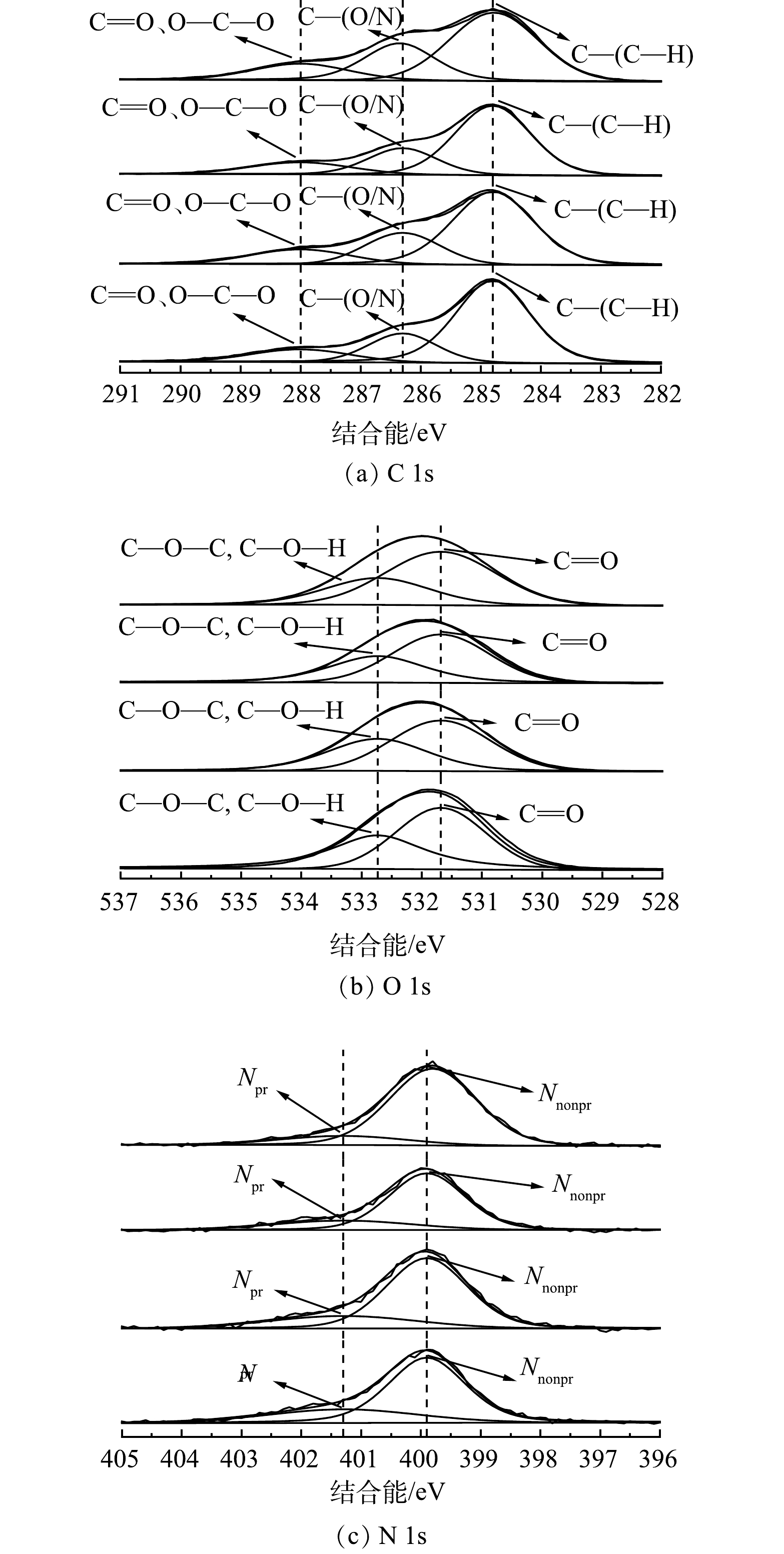
通过XPS对不同处理工艺对污泥表面C、N、O元素变化的影响进行分析。如图9 (a) 所示,C 1s高分辨率光谱显示了3种主要含碳物种的特征峰,C 1s在284.8 eV的峰代表脂质或氨基酸侧链中的C-(C, H),在286.3 eV的峰代表醚、乙醇或胺中的C-(O/N),在286.3 eV的峰代表羧酸酯,羰基,酰胺,缩醛或半缩醛中的C=O、O-C-O[31-32]。有研究表明,脂肪族碳质量分数与污泥表面疏水性质有关,其质量分数越高,表面相对疏水性越强[33]。由表2可知,经过Fe2+/NaClO处理后,脂肪族碳质量分数由57.09%增加至67.21%。这说明,污泥表面疏水性显著提高,而酰胺和羰基等亲水性物质显著减少,进一步证实了之前的结论。由图9 (b) N 1s高分辨率光谱可知,经过Fe2+/NaClO处理后的质子态氮 (Npr 401.3 eV) 质量分数减少,非质子态氮 (Nnonpr 399.0 eV) 质量分数增加。Nnonpr中的胺和酰胺与蛋白质二级结构中β-折叠的C=O拉伸以及蛋白质羧基的COO-和C-O的拉伸有关[34]。因此,Nnonpr质量分数的减少可以显著减弱蛋白质的强度和刚度,导致蛋白质结构的稳定性下降而更容易被破坏,从而改善污泥脱水性能。如图9 (c) 所示,Fe2+/NaClO工艺处理后污泥中O 1s的C=O质量分数下降,C-O-C和C-O-H质量分数上升,这与红外光谱图中C-OH、C-O-C和C=O基团的对称伸缩振动变化一致。
-
1) Fe2+/NaClO工艺能显著强化污泥脱水性能,且适用的pH范围宽泛。当初始pH为5.0,Fe2+和NaClO投加量分别为48.61和39.04 mg·g−1时,污泥含水率和污泥比阻分别从91.32%和11.81×1012 m·kg−1降至71.74%和1.71×1012 m·kg−1,总结合水质量分数下降了23.59%。
2) Fe2+/NaClO工艺通过提高污泥的Zeta电位,并减小污泥胶体颗粒间的静电斥力,增大污泥粒径,且反应过程中原位生成的Fe3+会附着于污泥表面,在污泥絮体间形成一种多孔隙通道的结构,结合水通过絮体中的孔隙通道排出,转变为自由水,促进了污泥的固液分离。
3) Fe2+/NaClO工艺处理污泥的过程中,在酸性条件下会形成HClO,既能裂解部分细胞,还能与Fe2+反应生成氧化活性物种,氧化降解TB-EPS和LB-EPS中的蛋白质和多糖,引起蛋白质结构的松散,导致更多的表面疏水位点暴露;该工艺还可以氧化表面亲水性含O、N类官能团,提高污泥表面疏水性,改善污泥脱水性能。
Fe2+联合NaClO强化污泥脱水性能与作用机理
Performance and Mechanisms of Fe2+ Combined with NaClO for Enhanced Sludge Dewatering
-
摘要: 为了强化污泥脱水性能,采用亚铁离子 (Fe2+) 活化次氯酸钠 (NaClO) 氧化工艺改善其脱水效果,考察了Fe2+/NaClO体系中Fe2+和NaClO投加量、初始pH对污泥脱水性能的影响,并探究强化污泥深度脱水的作用机制。研究表明,初始pH为5.0、Fe2+和NaClO投加量 (以总悬浮固体计) 分别为48.61和39.04 mg·g−1时,污泥的脱水性能最佳,污泥含水率和比阻分别由91.32%和11.81×1012 m·kg−1降低至71.74%和1.71×1012 m·kg−1。Fe2+/NaClO氧化体系使胞外聚合物 (EPS) 降解和部分细胞裂解,反应生成的强氧化性的·OH会降解松散附着型EPS (LB-EPS) 和紧密黏附型EPS (TB-EPS) 中的蛋白质和多糖,改变蛋白质二级结构,导致蛋白质结构松散,暴露了更多的疏水位点,同时减少污泥表面的亲水性官能团,释放EPS结合水。Fe3+使污泥絮体颗粒在絮凝形成松散、多孔的絮体结构,有利于胞内结合水的排出,从而改善污泥脱水性能。本研究结果可为有效去除污泥中的结合水和提高污泥的脱水性能提供参考。Abstract: The application of ferrous iron (Fe2+)-activated sodium hypochlorite (NaClO) could improve the waste-activated sludge dewatering. The impacts of Fe2+ dosages, NaClO dosages, and initial pH values on sludge dewatering performance were explored. In addition, the mechanisms of enhancing deep sludge dewatering were investigated. The results indicated that the water content and specific resistance to filtration of sludge decreased from 91.32% and 11.81×1012 m·kg−1 to 71.74% and 1.71×1012 m·kg−1, respectively, when Fe2+ dosage, NaClO dosage, and initial pH were fixed at, 48.61 mg·g−1, 39.04 mg·g−1 and 5.0. The oxidation of Fe2+/NaClO system resulted in the destruction of extracellular polymeric substances (EPS) and partial cell lysis. Meanwhile, the strongly oxidizing ·OH degraded the proteins and polysaccharides in TB-EPS/LB-EPS, and altered protein secondary structure, resulting in loosening of protein structure and exposure of additional hydrophobic sites. In addition, this process lowered hydrophilic functional groups on the sludge surface and released EPS-bound water. Simultaneously, Fe3+ induced the sludge floc particles to form a loose and porous structure, which aided in the outflow of intracellularly bound water, thus enhancing the dewatering performance of the sludge. This result has developed a novel and cost-effective method for rapidly removing bound water from sludge and enhancing sludge dewatering performance.
-
Key words:
- ferrous iron /
- sodium hypochlorite /
- sludge dewatering /
- oxidation /
- enhanced coagulation
-
近年来,随着新能源产业的迅速发展,锂电池的产量大幅度增加,废旧锂电池也随之大量产生[1]。在锂电池湿法回收过程中,通常采用黄铁矾法实现其中铁元素的脱除,进而产生铁矾渣固体废弃物[2]。铁矾渣主要成分黄钠铁矾 (NaFe3(SO4)2(OH)6) 是一种硫酸盐复盐,通常含有少量的镍钴锰等重金属元素,直接填埋潜在环境风险较大。利用铁矾渣为原料烧制陶粒产品是一种可以固定重金属元素,实现资源化利用的有效途径。但由于其含硫较高,高温过程易分解产生SO2,存在着后续环境处理成本较高的问题[3-6]。
目前,高温固硫的研究主要集中在高温反应中固硫和烟气脱硫[7-9]方面。在高温反应中,固硫是在高温烧结前采取措施减少高温过程含硫物质的分解[10],主要技术手段是在高温过程前或者高温过程中添加一定量的固硫剂,使高温时产生的气态硫化物与固硫剂中的有效成分 (CaO、MgO等) 发生反应,使其转变成硫酸盐等形式留在渣中,进而有效减少SO2和SO3的释放,从而达到固硫的效果。其反应温度一般控制在800~1 250 ℃,常用固硫剂有钙基固硫剂、钠基固硫剂和镁基固硫剂[11-13]。烟气脱硫是利用固相吸收剂、水或碱性溶液与含硫烟气充分接触,生成对应的硫酸盐等化合物,从而减少烟气中SO2的排放,目前此方法存在脱硫设备易腐蚀且副产石膏量大[14-17]的问题。陶粒通常在900~1 300 ℃烧制形成[18],然而黄钠铁矾在高温过程中易分解产生SO2,如式(1)所示[19],该过程会提高后端脱硫处理成本。因此,通过研究在陶粒的烧制过程中添加固硫剂降低生产过程中SO2的排放可有效减小后续脱硫设备规模,以降低生产成本。
4NaFe3(SO4)2(OH)6→2Na2SO4+6Fe2O3+6SO2↑+3O2↑+12H2O↑ (1) 通过对不同的固硫剂在高温下可能的反应进行热力学模拟,筛选出高温过程对铁矾渣可能存在固硫效果的Ca/Na/Mg基固硫剂,并利用铁矾渣-固硫剂混合焙烧的实验方式验证固硫剂在1 100 ℃条件下焙烧对铁矾渣的固硫效果,以筛选出固硫效果较佳的固硫剂,从而进一步验证固硫剂掺量对陶粒密度、吸水率和抗压强度的影响。同时,通过XRD和TG-FTIR分析,明确不同烧成反应物的矿相、反应热力学和气相产物的变化,从而明确固硫机理。对固硫陶粒进行了毒性浸出分析,检验其环境安全性。
1. 材料与方法
1.1 原料及仪器
本研究制备陶粒的主要原料包括页岩、铁矾渣和含碳化硅废料分别来自湖南某锂电回收企业和陶瓷企业;固硫剂筛选考察实验分别采用碳酸钙 (CaCO3、氧化钙 (CaO) 、氢氧化钙 (Ca(OH)2) 、硅酸钙 (CaSiO3) 、碳酸钠 (Na2CO3) 、碳酸氢钠 (NaHCO3) 、氢氧化钠 (NaOH) 、硅酸钠 (Na2SiO3) 、偏铝酸钠 (NaAlO2) 、氧化镁 (MgO) 和氢氧化镁 (Mg(OH)2) ,均为分析纯。
主要使用的设备及分析仪器:X 射线衍射仪 (Smartlab,日本株式会社理学公司);X射线荧光光谱仪(AXIOS,荷兰帕纳科公司);烧结炉 (佛山市科顺龙电控有限公司);万能试验机 (E100,济南试金集团有限公司);热重红外联用 (TENSOR III,德国布鲁克公司;STA449 F5,德国耐驰公司)。
1.2 实验及固硫率计算方法
1) 固硫率的计算方法。固硫率采用硫质量守恒的算法,根据XRF测定焙烧前样品硫的质量分数,称取一定质量的样品,将称取质量的样品进行焙烧后再次称重,并对焙烧后的样品测定硫的质量分数具体计算如式 (2)~式 (3)所示。
Xn=xn∗mnxns∗mns (2) X∗=Xn−XT1−XT (3) 式中:Xn为硫残留率;X*为固硫率;XT为未添加固硫剂的样品硫残留率;xn为烧制样品含硫质量分数;mn为烧制样品质量,g;xns为未烧样品含硫质量分数;mns为未烧样品质量,g。
2) 混合焙烧实验。称取一定量的铁矾渣,经过对铁矾渣硫含量的计算,分别按照摩尔比Ca/S=2.35、Na/S=2.35和Mg/S=2.35称取相应物质的质量,将称取的各物质利用球磨机充分混合5 min后称重,计算出混合物体系含硫质量分数,取一定量混合物在1 100 ℃的条件下灼烧30 min后再次称重,根据式 (2)~式 (3)计算固硫率,最终固硫率取3次稳定实验结果的平均值。
3) 陶粒配方及制备方法。将页岩、铁矾渣和碳化硅废料原料在 (105±2) ℃烘干至恒重,经破碎和球磨机球磨30 min后过100目 (150 μm) 标准筛,按照页岩和铁矾渣的质量分数为92.5%和7.5%,在页岩和铁矾渣总质量基础上掺加0.8%含碳化硅废料,利用球磨机混合5 min后加入适量水,利用成球设备制备生料球。将滚好的生料球在 (105±2) ℃烘干至恒重后,在500 ℃下预热30 min得到陶粒熟料,预热好的陶粒熟料直接放入1 100 ℃的电炉中烧制一定时间后取出冷却。
4) 陶粒物理性能测试方法。抗压强度采取一种已经报道的方法[20],将陶粒样品垂直放置于压力机承压板之间,以一定升压速度施加载荷,压至破坏时得到破坏荷载Pc,每组样品测试10颗陶粒,取其平均值,得到陶粒的强度S。测试方法原理如式(4)所示。
S=2.8PcπX2 (4) 式中:S为陶粒的单颗粒强度,MPa;Pc为陶粒破坏时的荷载,N;X为陶粒在起始受力时与压力机承压板接触点间的垂直距离,mm。
陶粒的表观密度和吸水率按照《轻集料及其试验方法》[21]测定。
2. 结果与讨论
2.1 原料的化学成分及矿相结构
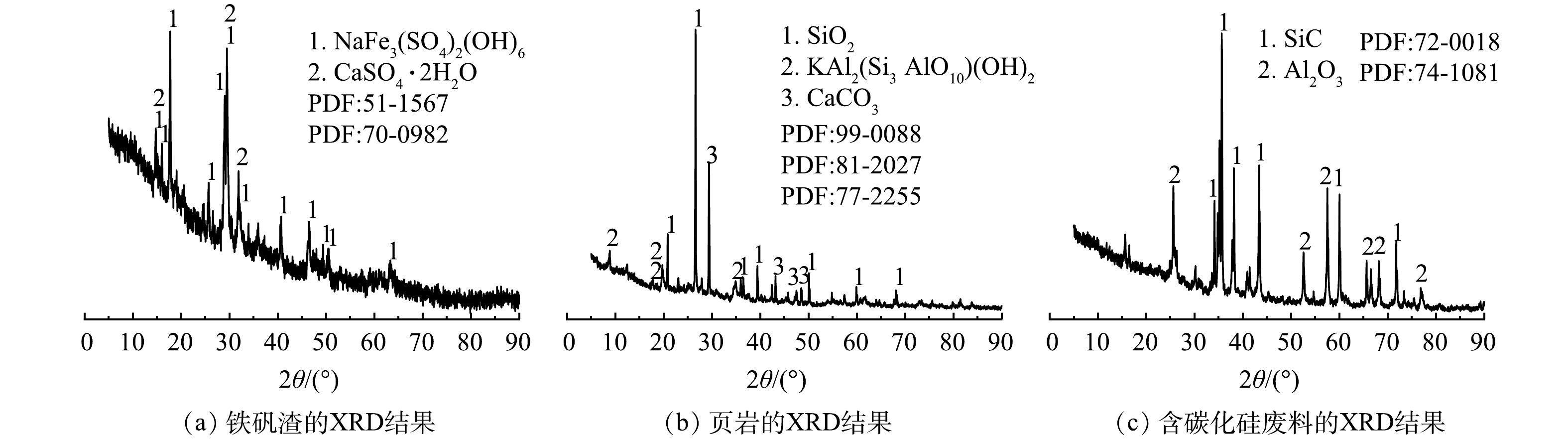
将烘干至恒重的原料进行XRD和XRF测试。所使用的原料主要化学成分如表1所示,由表可知,铁矾渣中硫、铁、铝和钠含量较高,其中同时含有镍、钴、锰和铜等金属元素,烧失率为22.60%;页岩富含铝、硅、钙等元素,其中SiO2、Al2O3和CaO的质量分数分别为53.79%、18.04%和11.93%,烧失率为14.60%;陶粒成孔剂采用含有碳化硅成分的电子陶瓷行业研磨抛光产生的废料,其中Al2O3和SiC的质量分数分别为49.41%和43.34%,烧失率为2.80%。由图1可知,铁矾渣主要矿相为黄钠铁矾 (NaFe3(SO4)2(OH)6) 和CaSO4·2H2O,根据铁矾渣产生过程推测含铝物相为无定型的氢氧化铝;页岩的主要矿相成分是白云母石,石英和方解石;含碳化硅废料的主要矿相是碳化硅和氧化铝。
表 1 原料化学成分Table 1. Chemical composition of raw materials% (质量分数) 供试原料 SO3 Fe2O3 Al2O3 Na2O CaO NiO SiO2 Co3O4 TiO2 MnO K2O SiC LOI 铁矾渣 26.82 26.55 20.28 11.96 6.95 1.21 0.60 0.58 0.44 0.27 — — 22.60 页岩 0.11 7.78 18.04 0.30 11.93 — 53.79 — 0.78 0.13 3.47 — 14.60 含碳化硅废料 0.08 0.61 49.41 0.18 0.35 — — 0.15 — 0.16 43.34 2.80 2.2 固硫剂筛选及效果验证
常用固硫剂有很多种,碱金属固硫剂是其中最重要的一类,其中钙基应用最为广泛[7, 22-25],部分研究人员利用富含碱金属氧化物的固废作为固硫剂对反应产生二氧化硫进行固定[9, 26],结合文献和理论分析,通过对钙基固硫剂,钠基固硫剂和镁基固硫剂3类可能具有固硫效果的固硫剂进行理论和实验验证。其中,钙基固硫剂选择CaCO3、CaO、Ca(OH)2和CaSiO3,钠基固硫剂选择Na2CO3、NaHCO3、NaOH、Na2SiO3和NaAlO2,镁基固硫剂选择MgO和Mg(OH)2。
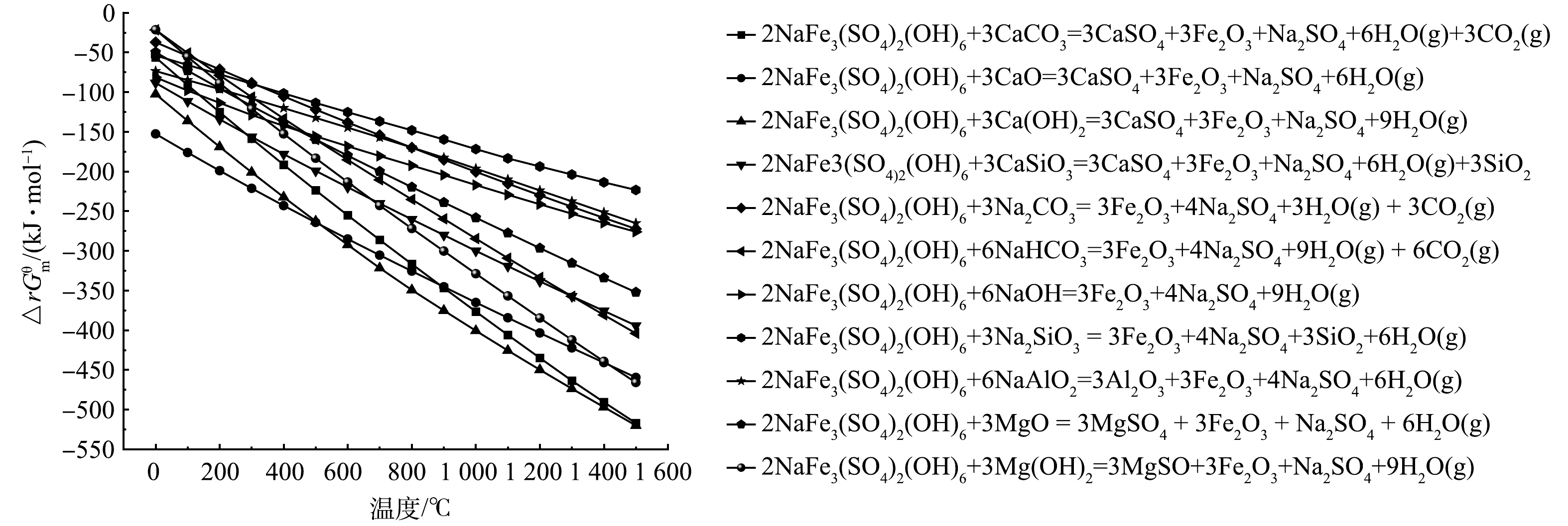
本研究铁矾渣中主要成分是黄钠铁矾,是受热分解产生二氧化硫的主要物质,利用HSC 6.0对各种固硫剂与黄钠铁矾可能发生的反应进行热力学模拟计算,可能发生的固硫反应在不同温度下反应的吉布斯自由能结果如图2所示。
模拟结果如图2所示,理论上,所有固硫剂在温度范围内△rGθm<0,即1 500 ℃以下选择的固硫剂均有可能发生固硫反应,且反应温度越高,△rGθm越小,反应趋向于正向发生。
以不同固硫剂与黄钠铁矾在高温下反应热力学计算结果为依据,进铁矾渣和固硫剂混合焙烧实验,并按照式 (2) 和式 (3) 所述方式进行固硫率计算,结果见表2。除9、10和11组外,其余固硫剂在1 100 ℃实验条件下均有固硫效果,其中添加钙基固硫剂1、2、3和4组的固硫率均大于75%,相较于钠基固硫剂的有更好的固硫效果;而添加钠基固硫剂的5、6、7和8组的固硫率均低于70%,其中Na2SiO3的固硫率最高为68.36%;添加NaAlO2、MgO和Mg(OH)2固硫率低于0,即促进了硫的排放。后续实验选择固硫率最高的CaO作为铁矾渣轻质固硫陶粒的系统实验用固硫剂。
表 2 固硫率的实验结果Table 2. Experimental results of sulfur-retention rate组别 固硫剂 硫残留率/% 固硫率/% 对照 未添加 71.21 — 1 CaCO3 93.89 78.78 2 CaO 99.99 99.97 3 Ca(OH)2 96.09 86.41 4 CaSiO3 99.90 99.66 5 Na2CO3 85.87 50.92 6 NaHCO3 88.80 61.11 7 NaOH 85.06 48.09 8 Na2SiO3 90.89 68.36 9 NaAlO2 69.57 -5.71 10 MgO 63.34 -27.34 11 Mg(OH)2 45.47 -89.42 2.3 固硫陶粒的固硫率及工艺条件优化
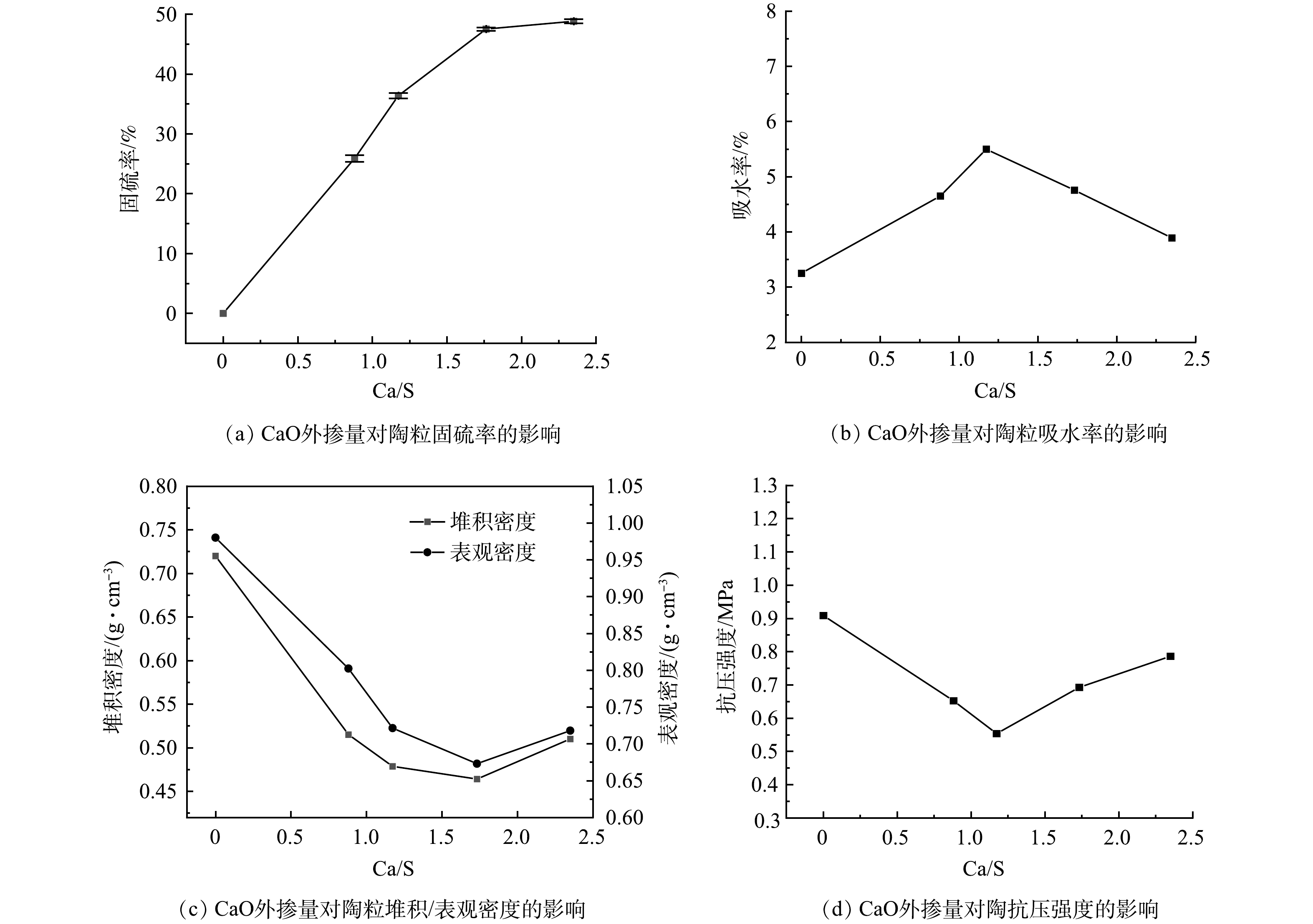
采用CaO作为陶粒的固硫剂,根据体系不同Ca/S (摩尔比) 外掺固硫剂制备铁矾渣固硫陶粒。图3(a)~图3(d)分别是CaO外掺量对陶粒固硫率、吸水率、堆积/表观密度、和抗压强度的影响,其中外掺Ca/S的摩尔比为0.88、1.17、1.76和2.35。由图3(a)可知,随着CaO掺加量的增加,固硫率增大,当Ca/S为2.35时,固硫率达到48.8%,当Ca/S小于1.76时,固硫率上升较为明显,当Ca/S大于1.76时,固硫率基本保持恒定。由图3(b)~图3(d)可知,铁矾渣固硫轻质陶粒的吸水率随着CaO的掺加量增加呈现先增加后减小的趋势,抗压强度、堆积密度和表观密度均表现出先减小后增大的趋势;当Ca/S达到1.17后,吸水率达到最高为5.50%,抗压强度达到最低0.55 MPa,堆积密度为0.48 g·cm−3,表观密度为0.72 g·cm−3。
图4为不同CaO掺量下的陶粒截面照片。由图可知,随着CaO掺量的增加截面孔数明显减少,同时截面孔径不断增大。其主要原因可能是,由于CaO掺量的增加,即碱金属氧化物的占比增加,导致陶粒烧制过程中,在较低的温度即可达到熔融状态。因此,在相同的温度的条件下,熔融的液相表面张力降低,内部更易形成气泡,导致陶粒内部孔隙变多;当气泡达到一定数量,距离表面较近的气泡较大且容易破裂,易形成更多的开孔,从而导致吸水率的增加,堆积密度、表观密度和强度降低。但随着CaO掺量的继续增加,陶粒表面熔融相张力更低,更易形成平滑的玻璃相表面,封闭了表面的孔道,导致吸水率降低;同时,内部熔融相在烧结过程中难以包裹产生的气体,即陶粒内部和表面过熔,孔结构被破坏,导致堆积密度、表观密度和抗压强度的提高[27-29]。
2.4 固硫机理研究
1) XRD结果分析。图5为不同掺加CaO固硫陶粒和未掺加固硫剂的陶粒的XRD结果,Ca/S摩尔比分别为0.88、1.18、1.76和2.35。由图5可知,陶粒中主要矿相特征峰有SiO2、CaO·Al2O3·2SiO2、CaSO4和K2O·Al2O3·6SiO2,固硫陶粒的主要矿相种类基本一致;随着CaO掺量的增加,当Ca/S摩尔比达到1.76时,出现了明显的K2SO4特征峰,且Ca/S摩尔比怎加到2.35时特征峰明显增强,同时SiO2的峰也逐渐增强。这说明,CaO的增加,使体系中产生了更多的K2SO4和SiO2。
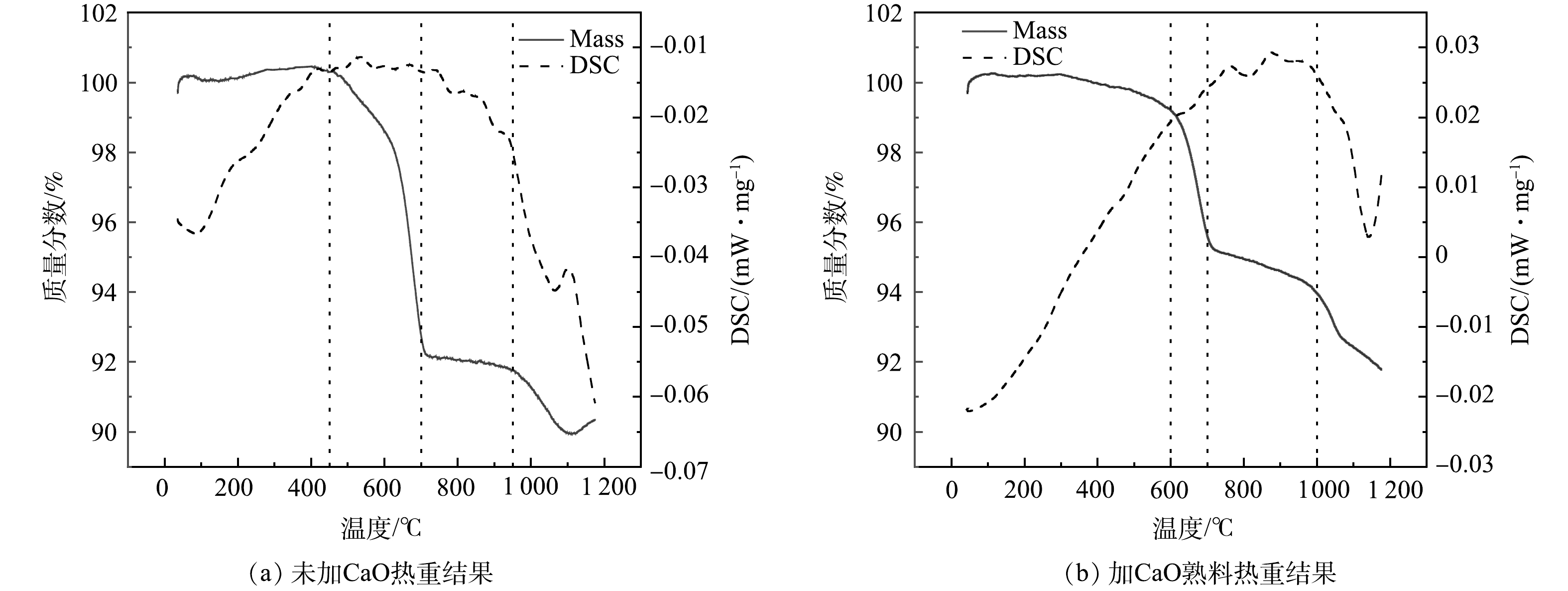
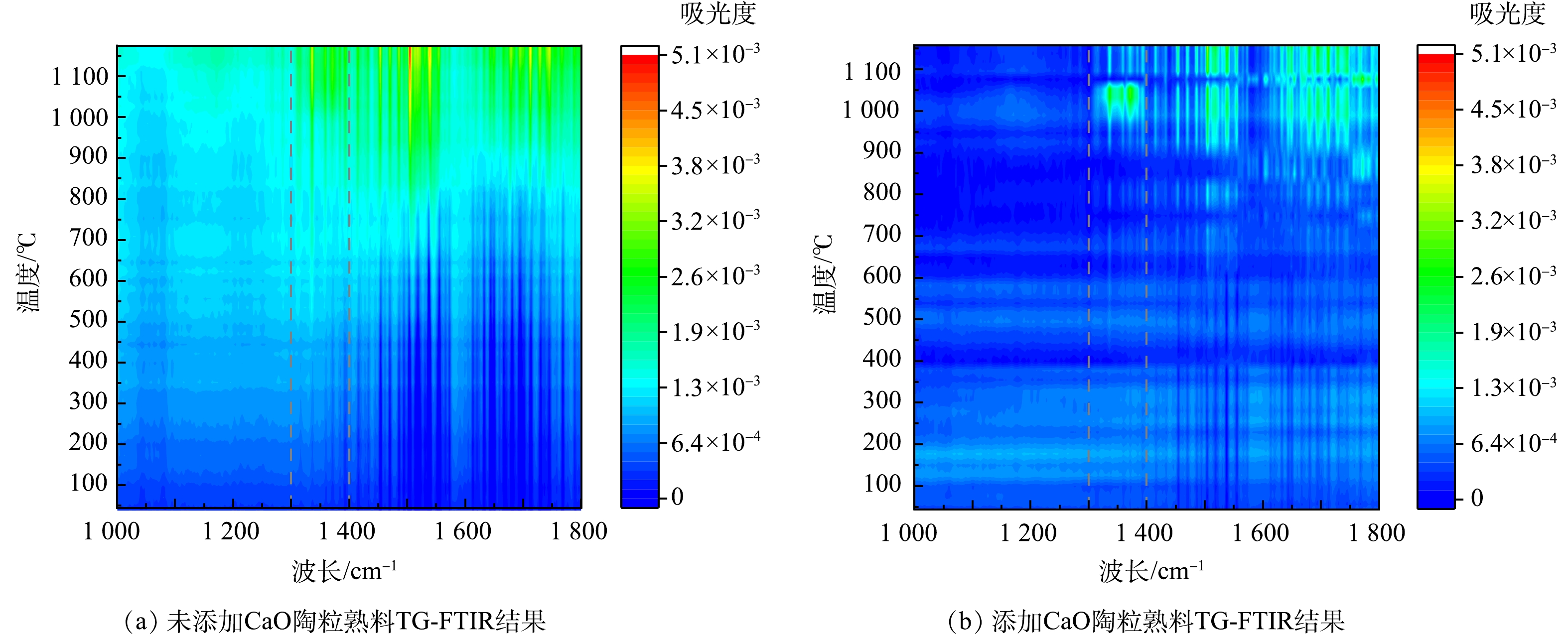
2) TG-DSC-FTIR分析。图6和7所示分别为利用TG-DSC-FTIR研究未掺加CaO的陶粒熟料及添加CaO且Ca/S=2.35的陶粒熟料在常温至1 200 ℃的质量损失变化规律,对固硫陶粒熟料升温过程产生的气体进行红外分析。由图6(a)可知,在温度上升过程中,2次失重分别在450和950 ℃附近发生;随着温度升高,失重速度逐渐增加,陶粒烧制过程中总共失重达到了超过10%。由图6(b)可知,添加CaO后,2次失重分别在600和1 000 ℃左右发生,整个过程总失重不超过7%;在1 100~1 150 ℃范围内,失重由3%降低至2%以下。二氧化硫所对应的红外波长范围是1 300~1 400 cm−1附近,不同体系下存在偏移。根据图7加固硫剂熟料产生气体红外结果可知,CaO的加入有效的提高了分解产生SO2的温度,在0~1 000 ℃的升温过程中,当未加入CaO的样品达到在500 ℃附近开始有SO2产出,达到800 ℃时SO2产生量增加,并随着温度升高逐渐加强,结果与热失重结果相对应。加入CaO的样品主要在1 000 ℃后产生SO2,相较于未掺加CaO的结果,吸光度在1 100~1 150 ℃温度明显降低。可见,CaO的加入,SO2的溢出量在升温过程减少,硫元素在升温过程中被有效固定下来。
有研究表明,钾长石可在高温过程与CaSO4反应转化成其他含钙矿相并生成K2SO4[30-31],因此推测陶粒的固硫机理如下:首先NaFe3(SO4)2(OH)6与CaO反应,生成相应的固硫产物CaSO4;生成的CaSO4可继续和陶粒中的K2O·Al2O3·6SiO2反应,生成了CaO·Al2O3·2SiO2和K2SO4,并且随着CaO的增加,K2SO4特征峰逐渐增强,体系中K2SO4含量的增加印证了这一观点,反应方程式如式式 (5)~式 (6)所示:
2NaFe3(SO4)2(OH)6+3CaO=3CaSO4+3Fe2O3+Na2SO4+6H2O↑ (5) K2O·Al2O3·6SiO2+CaSO4=K2SO4+CaO·Al2O3·2SiO2+4SiO2 (6) 2.5 毒性浸出分析
采用《固体废物浸出毒性浸出方法醋酸缓冲溶液法》[32]的方法对原料及固硫陶粒进行毒性浸出实验,实验对象包括铁矾渣、页岩及Ca/S=2.35掺加量CaO的固硫陶粒,并根据《危险废物鉴别标准浸出毒性鉴别》[33]中规定的鉴别浸出毒性,结果如表3所示。
表 3 浸出毒性测试结果(Ni/Co/Mn/Cu/Zn/Cr/Se/Cd/Pb/Ag/Ti/Zr)Table 3. Test results of extraction toxicity (Ni/Co/Mn/Cu/Zn/Cr/Se/Cd/Pb/Ag/Ti/Zr)mg·L−1 被检元素 检测样品 GB5085.3-2007 铁矾渣 页岩 陶粒 固硫陶粒 Ni 19.819 0.083 0.008 0.010 5 Co 12.580 0.035 0.007 0.008 * Mn 5.715 15.143 0.051 0.046 * Cu 0.181 0.001 0.000 0.107 100 Zn 0.054 0.014 0.003 0.050 100 Cr 0.011 0.017 0.000 0.005 5 Se — 0.098 0.000 0.042 1 Cd — 0.009 0.000 0.000 1 Pb — — — — 5 Ag — — — — 5 Ti — — — — * Zr — — — — * 注:GB 5085.3—2007未规定的数据以“*”表示;数据未检出以“—”表示。 如表3所示,铁矾渣中浸出的可被检测元素有Ni、Co、Mn、Cu、Zn和Cr。Ni、Co和Mn的浸出量分别达到了19.819、12.580和5.715 mg·L−1,其中Ni超过标准近4倍,浸出量较高。页岩中检出元素有Ni、Co、Mn、Cu、Zn、Cr、Se和Cd,其中Mn的浸出量较高,达到15.143 mg·L−1。固硫陶粒的检出元素的浸出浓度低于铁矾渣和页岩检出元素的浸出浓度,并且小于《危险废物鉴别标准浸出毒性鉴别》[33]所规定的元素浓度,对环境没有污染风险。
3. 结论
1) 固硫剂验证实验中,钙基固硫剂相较于钠基固硫剂对铁矾渣有更好的固硫效果,其中氧化钙的固硫率较优;固硫陶粒实验中,当固硫剂掺加量达到Ca/S=2.35,固硫率达到48.8%。
2) 由于固硫剂添加导致碱金属含量增加,熔融温度降低烧制过程中陶粒性能发生改变,其中吸水率和抗压强度先增加后减少,堆积密度和表观密度先减少后增加;固硫陶粒毒性浸出符合《GB 5085.3-2007危险废物鉴别标准浸出毒性鉴别》中的规定,无环境污染风险。
3) 固硫剂可有效提高SO2开始分解的温度,并减少反应失重;CaO反应过程中与黄钠铁矾发生反应生成CaSO4,部分CaSO4会和K2O·Al2O3·6SiO2继续反应,生成钙长石和K2SO4。
-
表 1 不同蛋白质二级结构的占比
Table 1. Percentage of different protein secondary structures
% (质量分数) 反应体系 聚集链 β-折叠 无规则卷曲 α-螺旋 3-旋转螺旋 反平行β-折叠/聚集链 α-螺旋/(β-折叠+无规则卷曲) 原污泥 7.74 16.31 20.02 24.37 20.94 10.62 67.07 Fe2+ 4.46 17.59 23.25 22.62 21.62 10.27 55.39 NaClO 5.96 16.93 22.34 23.08 21.35 10.33 58.76 Fe2+/NaClO 4.64 18.41 23.21 21.90 21.59 10.25 52.62 表 2 不同工艺C 1s、O 1s和N 1s在不同结合能下的峰面积比例
Table 2. Proportions of peak areas of different processes C 1s, O 1s, and, N 1s under different binding energies
元素 反应体系 C 1s/eV峰面积/% O 1s/eV峰面积/% N 1s/eV峰面积/% 284.8 286.3 288.0 531.7 532.7 399.9 401.3 原污泥 57.09 27.48 15.43 86.03 13.97 66.26 33.74 Fe2+ 63.74 22.34 13.92 78.11 21.89 60.04 39.96 NaClO 62.90 22.91 14.19 77.03 22.97 57.87 42.13 Fe2+/NaClO 67.21 20.49 12.30 72.81 27.19 55.00 45.00 -
[1] WU B R, DAI X H, CHAI X L. Critical review on dewatering of sewage sludge: Influential mechanism, conditioning technologies and implications to sludge re-utilizations[J]. Water Research, 2020, 180: 115912. doi: 10.1016/j.watres.2020.115912 [2] QI Y, THAPA K B, HOADLEY A F A. Application of filtration aids for improving sludge dewatering properties–A review[J]. Chemical Engineering Journal, 2011, 171(2): 373-384. doi: 10.1016/j.cej.2011.04.060 [3] KIM M S, LEE K M, KIM H E, et al. Disintegration of Waste Activated Sludge by Thermally-Activated Persulfates for Enhanced Dewaterability[J]. Environmental Science & Technology, 2016, 50(13): 7106-7115. [4] GAO J L, LIU Y, YAN Y X, et al. Promotion of sludge process reduction using low-intensity ultrasonic treatment[J]. Journal of Cleaner Production, 2021: 325. [5] WANG G J, GE D D, BAI L, et al. Insight into the roles of electrolysis-activated persulfate oxidation in the waste activated sludge dewaterability: Effects and mechanism[J]. Journal of Environmental Management, 2021, 297: 113342. doi: 10.1016/j.jenvman.2021.113342 [6] ZHANG Y P, LI T T, TIAN J Y, et al. Enhanced dewaterability of waste activated sludge by UV assisted ZVI-PDS oxidation[J]. Journal of Environmental Sciences, 2022, 113: 152-164. doi: 10.1016/j.jes.2021.06.010 [7] 汪辉, 肖庆聪, 赵阳, 等. 基于不同破解方法的市政污泥厌氧消化产气量优化[J]. 环境工程学报, 2017, 11(1): 572-577. doi: 10.12030/j.cjee.201508213 [8] LING X, DENG J, YE C, et al. Fe(II)-activated sodium percarbonate for improving sludge dewaterability: Experimental and theoretical investigation combined with the evaluation of subsequent utilization[J]. Science of The Total Environment, 2021, 799: 149382. doi: 10.1016/j.scitotenv.2021.149382 [9] BEHIN J, AKBARI A, MAHMOUDI M, et al. Sodium hypochlorite as an alternative to hydrogen peroxide in Fenton process for industrial scale[J]. Water Research, 2017, 121: 120-128. doi: 10.1016/j.watres.2017.05.015 [10] ZHAO X, WEI X Y, XIA P F, et al. Removal and transformation characterization of refractory components from biologically treated landfill leachate by Fe2+/NaClO and Fenton oxidation[J]. Separation and Purification Technology, 2013, 116: 107-113. doi: 10.1016/j.seppur.2013.05.030 [11] JESSIELEENA A A, M P, MP S. Comparative study of Fenton, Fe2+/NaOCl and Fe2+/(NH4)2S2O8 on tannery sludge dewaterability, degradability of organics and leachability of chromium[J]. Journal of Hazardous Materials, 2021, 402: 123495. doi: 10.1016/j.jhazmat.2020.123495 [12] WEI H, HU P, LI A M, et al. Evaluation of acidification and oxidation of sludge to improve the effect of a starch-based flocculant on the dewaterability of sewage sludge[J]. Journal of Environmental Management, 2019, 231: 405-412. [13] FRøLUND B, PALMGREN R, KEIDING K, et al. Extraction of extracellular polymers from activated sludge using a cation exchange resin[J]. Water Research, 1996, 30(8): 1749-1758. doi: 10.1016/0043-1354(95)00323-1 [14] DUBOIS M, A E, HAMILTON J K, et al. Calorimetric Dubois Method for Determination of Sugar and Related Substances[J]. Analytical Chemistry, 2002, 28: 350-356. [15] YANG P, LI D, ZHANG W, et al. Study of sludge conditioning using organic acids chelated ferrous ion catalyzed NaClO oxidation: Evolution of extracellular polymeric substances and floc structure[J]. Journal of Environmental Management, 2021, 280: 111757. doi: 10.1016/j.jenvman.2020.111757 [16] DEBORDE M, VON GUNTEN U. Reactions of chlorine with inorganic and organic compounds during water treatment-Kinetics and mechanisms: a critical review[J]. Water Research, 2008, 42(1/2): 13-51. [17] XU Q Y, WANG Q D, ZHANG W J, et al. Highly effective enhancement of waste activated sludge dewaterability by altering proteins properties using methanol solution coupled with inorganic coagulants[J]. Water Research, 2018, 138: 181-191. doi: 10.1016/j.watres.2018.03.038 [18] BAI L, WANG G J, GE D D, et al. Enhanced waste activated sludge dewaterability by the ozone-peroxymonosulfate oxidation process: Performance, sludge characteristics, and implication[J]. Science of The Total Environment, 2022, 807(Pt 3): 151025. [19] RUAN S Y, DENG J, CAI A H, et al. Improving dewaterability of waste activated sludge by thermally-activated persulfate oxidation at mild temperature[J]. Journal of Environmental Management, 2021, 281: 111899. doi: 10.1016/j.jenvman.2020.111899 [20] DAI Q X, MA L P, REN N Q, et al. Investigation on extracellular polymeric substances, sludge flocs morphology, bound water release and dewatering performance of sewage sludge under pretreatment with modified phosphogypsum[J]. Water Research, 2018, 142: 337-346. doi: 10.1016/j.watres.2018.06.009 [21] LEE D J. Moisture distribution and removal efficiency of waste activated sludges[J]. Water Science and Technology, 1996, 33(12): 269-272. doi: 10.2166/wst.1996.0347 [22] LIU C G, WU B R, CHEN X E. Ultrasound enhanced zero-valent iron-activated peroxymonosulfate oxidation for improving dewaterability of aerobically digested sludge[J]. Chemical Engineering Journal, 2020, 392: 124850. doi: 10.1016/j.cej.2020.124850 [23] ZHEN G Y, LU X Q, SU L H, et al. Unraveling the catalyzing behaviors of different iron species (Fe2+ vs. Fe0) in activating persulfate-based oxidation process with implications to waste activated sludge dewaterability[J]. Water Research, 2018, 134: 101-114. doi: 10.1016/j.watres.2018.01.072 [24] ZHANG D X, WANG Y L, GAO H Y, et al. Variations in macro and micro physicochemical properties of activated sludge under a moderate oxidation-in situ coagulation conditioning: Relationship between molecular structure and dewaterability[J]. Water Research, 2019, 155: 245-254. doi: 10.1016/j.watres.2019.02.047 [25] LIANG J L, ZHANG S W, HUANG J J, et al. Mechanism of zero valent iron and anaerobic mesophilic digestion combined with hydrogen peroxide pretreatment to enhance sludge dewaterability: Relationship between soluble EPS and rheological behavior[J]. Chemosphere, 2020, 247: 125859. doi: 10.1016/j.chemosphere.2020.125859 [26] XIAO K K, PEI K Y, WANG H, et al. Citric acid assisted Fenton-like process for enhanced dewaterability of waste activated sludge with in-situ generation of hydrogen peroxide[J]. Water Research, 2018, 140: 232-242. doi: 10.1016/j.watres.2018.04.051 [27] SHCHUKAREV A, GOJKOVIC Z, FUNK C, et al. Cryo-XPS analysis reveals surface composition of microalgae[J]. Applied Surface Science, 2020, 526: 146538. doi: 10.1016/j.apsusc.2020.146538 [28] LI Y F, ZHU Y Q, WANG D B, et al. Fe(II) catalyzing sodium percarbonate facilitates the dewaterability of waste activated sludge: Performance, mechanism, and implication[J]. Water Research, 2020, 174: 115626. doi: 10.1016/j.watres.2020.115626 [29] YOU G X, WANG P F, HOU J, et al. Insights into the short-term effects of CeO2 nanoparticles on sludge dewatering and related mechanism[J]. Water Research, 2017, 118: 93-103. doi: 10.1016/j.watres.2017.04.011 [30] WU B R, NI B J, HORVAT K, et al. Occurrence State and Molecular Structure Analysis of Extracellular Proteins with Implications on the Dewaterability of Waste-Activated Sludge[J]. Environmental Science & Technology, 2017, 51(16): 9235-9243. [31] DING P F, SONG W F, YANG Z H, et al. Influence of Zn(II) stress-induction on component variation and sorption performance of extracellular polymeric substances (EPS) from Bacillus vallismortis[J]. Bioprocess and Biosystems Engineering, 2018, 41(6): 781-791. doi: 10.1007/s00449-018-1911-6 [32] HE C S, DING R R, CHEN J Q, et al. Interactions between nanoscale zero valent iron and extracellular polymeric substances of anaerobic sludge[J]. Water Research, 2020, 178: 115817. doi: 10.1016/j.watres.2020.115817 [33] NAKAO R, RAMSTEDT M, WAI S N, et al. Enhanced biofilm formation by Escherichia coli LPS mutants defective in Hep biosynthesis[J]. PLoS One, 2012, 7(12): e51241. doi: 10.1371/journal.pone.0051241 [34] ZHANG X R, SUN J, LIU X X, et al. Production and flocculating performance of sludge bioflocculant from biological sludge[J]. Bioresource Technology, 2013, 146: 51-56. doi: 10.1016/j.biortech.2013.07.036 -




 DownLoad:
DownLoad: