-
铊是一种稀有的剧毒重金属元素,毒性高于铅、汞、镉等有毒物质,世界卫生组织关于铊的环境卫生标准规定,一般人群铊的总摄入量不超过5 μg,致死剂量为8~12 μg·g−1,铊对人体的急性毒性剂量为6~40 mg·kg−1 BW[1]。铊污染主要来源于工业排放,全世界每年用于工业生产的铊达到1.5×105 t左右,向环境中释放的铊达到2 000~5 000 t[2-3]。此外,尾矿、冶炼废弃物、含铊矿石等含铊物质经地表径流、淋滤、大气降水进入环境,以及钢铁厂等企业含铊废水的超标排放等,导致铊污染突发事件时有发生,给下游饮用水安全造成了严重威胁[4-5]。如广东韶关冶炼厂排放含铊污水造成了严重的水体铊污染事件、广西贺江铊污染事件、四川广元段的嘉陵江铊污染事件等。而且,2021年1月20日嘉陵江流域再次发生铊污染事件。因此,含铊废水的高效治理与防控刻不容缓。
水中铊主要以Tl(I)和Tl(III)的无机形式存在,Tl(I)比Tl(III)更稳定和可溶[6]。与其它除铊技术相比,吸附法因其高效、经济、操作简便而被认为是最有前景的铊去除方法。已有不同类型吸附剂被开发并用于去除水中的铊污染物,包括腐殖质[7-8]、锯末[9]、活性炭[10-11]、多壁碳纳米管[12]、钛纳米管[13]、纳米Al2O3[14]和二氧化钛[15-16]等。然而,这些吸附剂的分离回收常采用离心机或过滤器,需要消耗能量且处理困难,使其在实际应用过程中受到限制。相比之下,在外加磁场作用下,磁性吸附剂可以简单地从处理水中分离出来,大大降低了操作能耗。为了提高吸附性能,通常将典型磁性材料 (例如Fe3O4和Fe2O3) 与对目标污染物具有强而特殊亲和力的吸附材料相结合[17-18],进而开发出磁性吸附剂用于去除铊、砷[17]、镉[18]和汞[19]等有毒物质。
本研究通过化学共沉淀法制备Fe3O4颗粒,结合水热反应、溶胶凝胶等改性方法,制备磁性钛铁纳米颗粒 (TFNPs) 、四氧化三铁/二氧化钛核壳颗粒 (Fe3O4@TiO2) 和还原氧化石墨烯负载四氧化三铁/二氧化钛 (rGO-Fe3O4@TiO2) 复合磁性材料,并进一步优化TFNPs、Fe3O4@TiO2和rGO-Fe3O4@TiO2磁性材料的制备方法,探讨TFNPs、Fe3O4@TiO2和rGO-Fe3O4@TiO2磁性材料吸附、吸附氧化去除铊的性能,通过电化学手段,从微观水平阐明磁性材料中rGO对去除铊的促进机制。
全文HTML
-
本研究中使用的所有化学品均为分析纯级,主要有硝酸铊 (TINO3) 、正钛酸四丁酯 (C16H36O4Ti,TBOT) 、乙醇 (C2H6O) 、叔丁醇 (C4H10O) 、氯化铁六水合物 (FeCl3·6H2O) 、硫酸亚铁七水合物 (FeSO4·6H2O) 、硼氢化钾 (KBH4) 、盐酸 (HCl) 、双氧水 (H2O2) 、氢氧化钠 (NaOH) 、硝酸 (HNO3) 、过硫酸钾 (K2S2O8,PS) ;所有溶液均采用超纯水配制。
-
TFNPs是以TBOT和Fe3O4粉末为原料,其制备方法参考已有研究[20]。通过调节Fe3O4、TBOT的加入量和水热反应温度、时间等条件,制备最优的TFNPs磁性吸附材料。为减少实验次数,采用均匀实验设计方法进行实验设计[21],筛选具有代表性的Fe3O4、TBOT的加入量和水热反应温度、时间。均匀设计表用Un(qs)表示,其中U代表均匀设计,n代表要做的实验次数,q代表每个因素有q个水平,s代表因子个数。本研究主要考察的因子为Fe3O4加入量、TBOT加入量、水热反应温度和水热反应时间,实验次数一般为因子个数的3倍,采用均匀设计表 (U12[44]) 构建实验方案,如表1所示。以对Tl(I)的吸附量为优化指标筛选最佳吸附材料,具体的实验条件为:Tl(I)的初始质量浓度为10 mg·L−1、吸附剂用量为0.1 g·L−1、温度为 (25±1) ℃、pH为7、吸附时间为4 h。
-
Fe3O4@TiO2的制备已有研究[22]。Fe3O4粉末投加量会直接影响TiO2壳状结构的厚度和致密性,进而影响材料的吸附容量。通过改变Fe3O4粉末和TBOT投加量,可以对核壳结构吸附材料结构与性能进行有效调控。采用表2优化Fe3O4粉末、TBOT的加入量,构建最优的Fe3O4@TiO2核壳磁性吸附材料,以对Tl(I)的吸附量为优化指标。具体吸附实验条件为:Tl(I)的初始质量浓度为10 mg·L−1、溶液pH为7.0、Fe3O4@TiO2用量为0.1 g·L−1、吸附时间为0.5 h。
考察TBOT的加入量时,Fe3O4粉末加入量为50 mg。
-
rGO-Fe3O4@TiO2磁性材料的制备参考已有研究[23],采用表3优化rGO-Fe3O4和TBOT的加入量,构建最优的rGO-Fe3O4@TiO2磁性材料,以对Tl(I)的吸附量为优化指标。具体吸附实验条件为:Tl(I)的初始质量浓度为10 mg·L−1、溶液pH为7.0、吸附剂用量为0.1 g·L−1、吸附时间为0.5 h。
考察TBOT的加入量时,rGO-Fe3O4粉末加入量为5 mg。
-
采用铊离子储备液 (100 mg·L−1) 配置一系列浓度的铊离子工作溶液 (0.5、1、5、10、20、30和50 mg·L−1) ,用针管吸取1~2 mL溶液测定不同工作溶液的初始铊浓度。每个浓度的铊离子工作溶液,用量筒准确移取50 mL,转移至玻璃瓶中,调整溶液pH为7,吸附剂投加量为0.1 g·L−1。将所有玻璃瓶放置在超声仪器中超声3 min,然后放置在摇床摇晃24 h,摇床温度分别设置为25、35和45 ℃,分别在30 min、1.5 h、4 h和8 h校准pH至7。吸附24 h后,用针管吸取1~2 mL溶液,过0.45 μm滤膜,过滤溶液加到2 mL离心管中,采用逐级稀释方法,将样品进行不同倍数的稀释,确保样品浓度符合ICP-MS进样要求,待测。
吸附热力学模型如式(1)~式(3)所示。
式中:
ΔG 为Gibbs自由能,kJ·mol−1;ΔH 为焓变,J·(mol·K)−1;ΔS 为熵变,k·mol−1;KT 为无量纲参数;55.5 为水的摩尔浓度,mol·L−1;204.38为铊的摩尔分子量,g·mol−1;R为气体常数,8.314×103 kJ·(mol·K)−1;T为绝对温度,K。 -
取50 mL Tl(I)反应溶液 (8.9 mg·L−1) ,移至玻璃瓶中,加入10 mmol PS,调整溶液pH=8,分别添加0.2 g·L−1的Fe3O4@TiO2和rGO-Fe3O4@TiO2。超声10 min后,放置于160 r·min−1的水浴振荡器中反应,分别在30 min、1 h、2 h和4 h校准pH=7,反应24 h后,取样过0.45 µm滤膜后测定反应后溶液中Tl的浓度。
-
将10 mg rGO-Fe3O4@TiO2纳米颗粒用甲醇固定在Pt电极上,干燥后,作为工作电极,与甘汞和铂丝构成三电极体系,分别测定Tl(I)溶液 (8.5 mg·L−1) 和Tl(I)/PS (10 mg·L−1、10 mmol·L−1) 混合溶液条件下的循环伏安特性曲线。
采用相同三电极体系,打开电流模式,依据循环伏安特性曲线测定结果,分别将工作电极电位设置为相应的氧化峰电位和还原峰电位,测定氧化电流-时间曲线和还原电流-时间曲线,计算电子供给容量 (EDC) 和电子接受容量 (EAC) 。
-
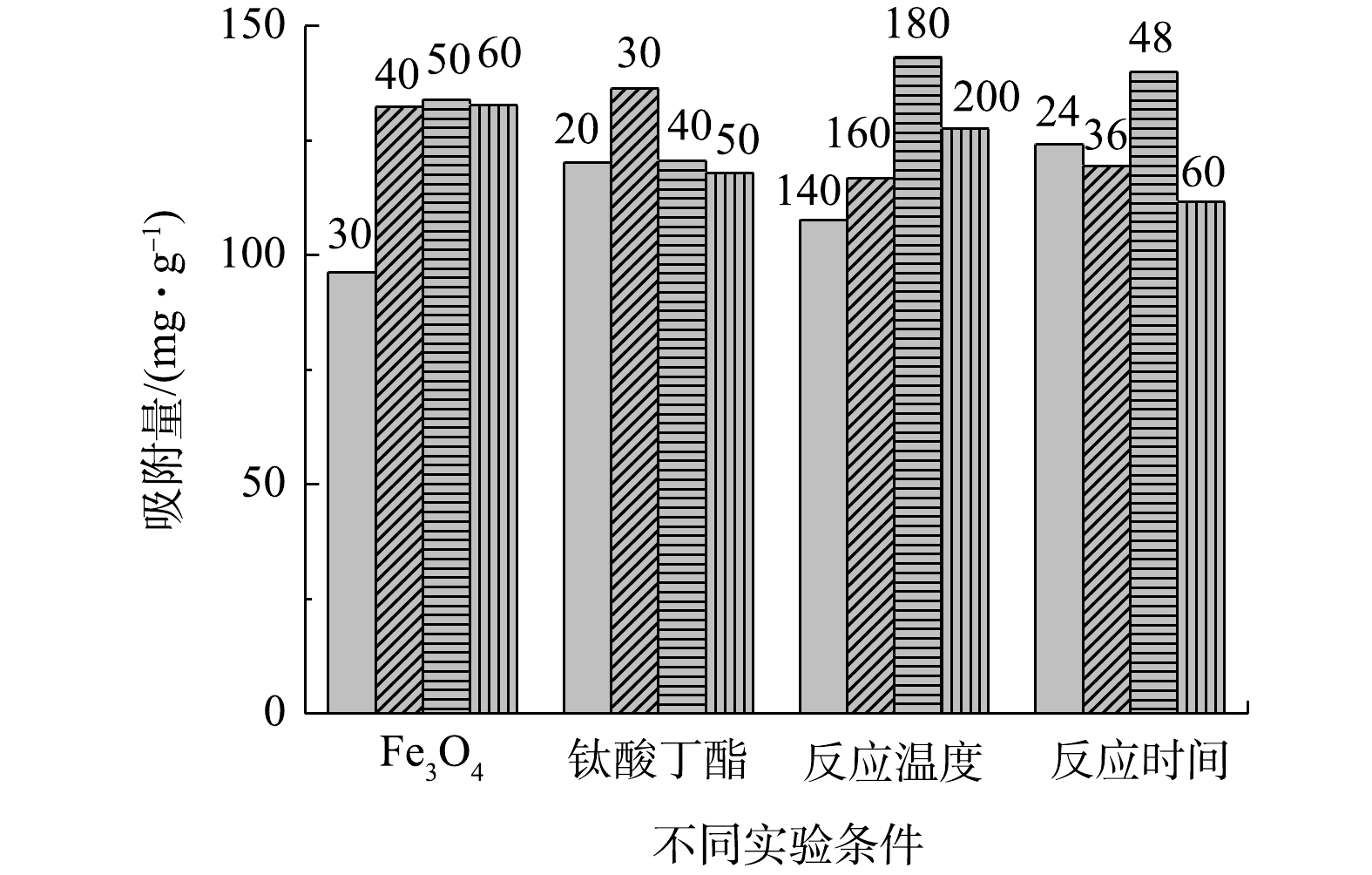
1) TFNPs磁性材料制备条件的优化。4因素4水平条件下合成的TFNPs吸附Tl(I)的效果如图1所示,在实验条件范围内,Fe3O4加入量为50 mg、TBOT加入量为30 mL、水热反应温度为180 ℃和水热反应时间为48 h时,TFNPs吸附Tl(I)的效果最佳,确定此为制备TFNPs的最佳条件。
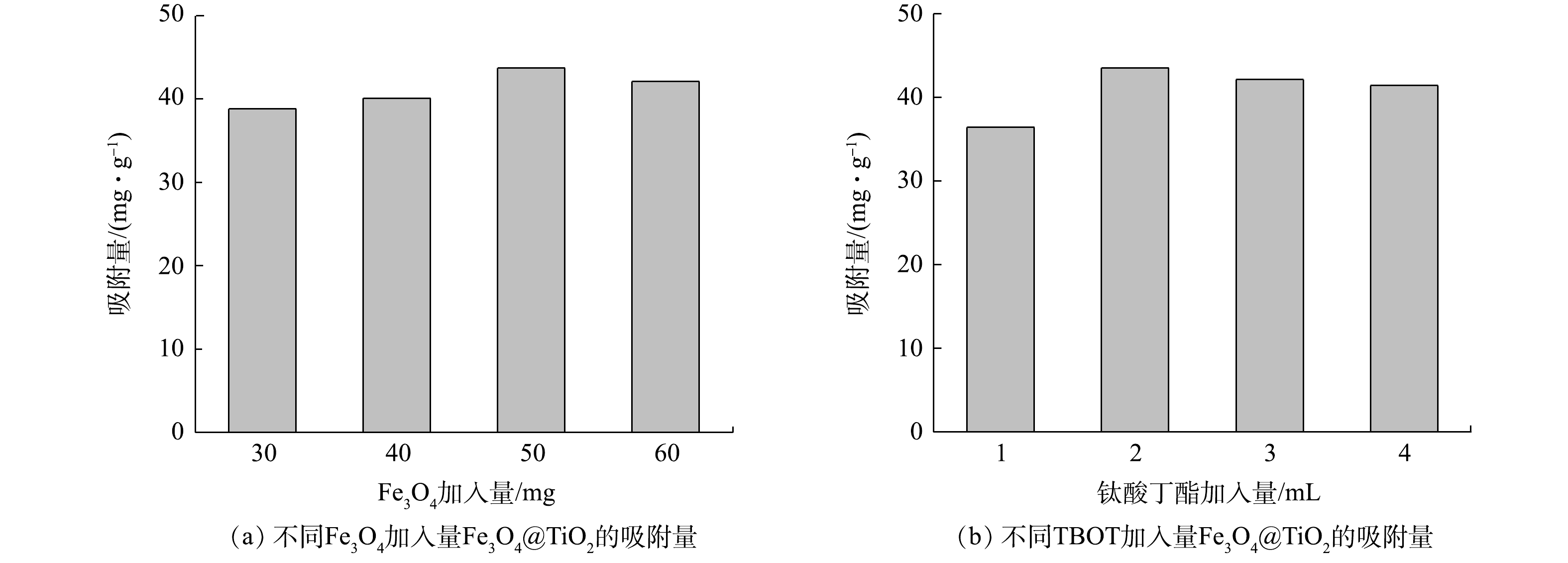
2) Fe3O4@TiO2核壳磁性材料制备条件的优化。改变Fe3O4粉末加入量的吸附结果如图2 (a) 所示,Fe3O4粉末加入量为50 mg时吸附效果最好,其次是60 mg。如图2 (b) 所示,TBOT的加入量为2 mL时吸附效果最好,确定Fe3O4粉末和TBOT的最佳加入量分别为50 mg和2 mL。
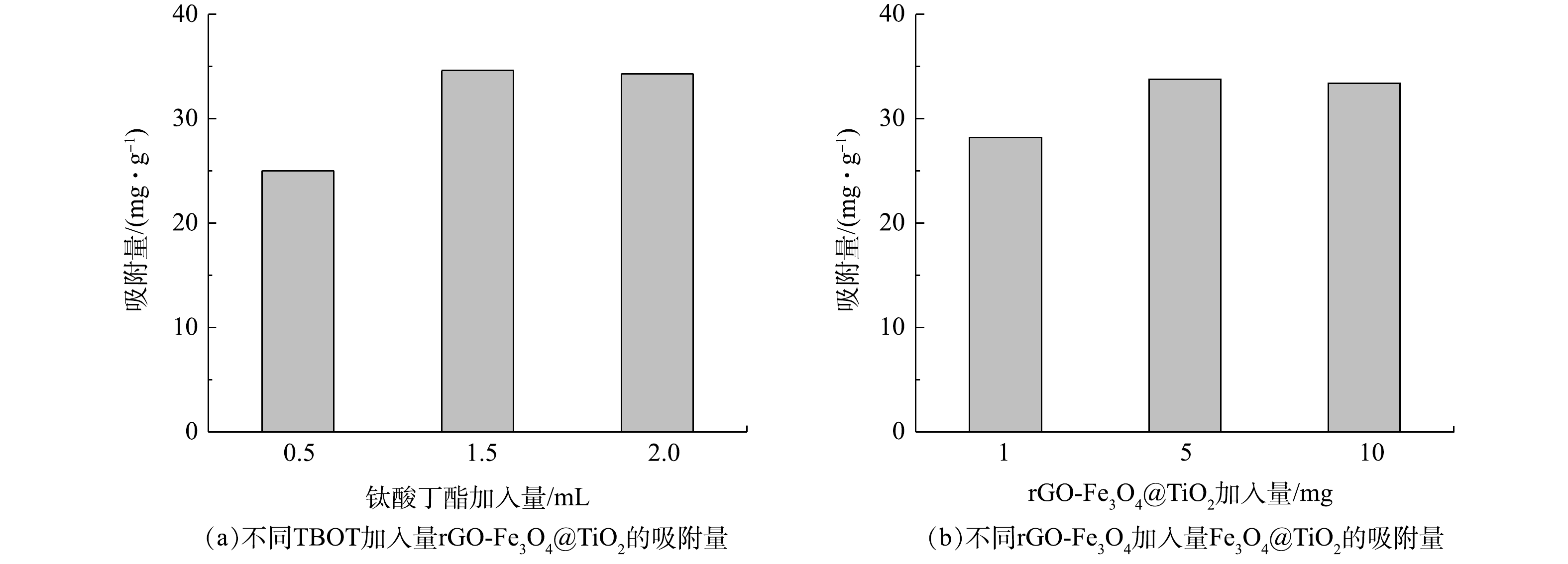
3) rGO-Fe3O4@TiO2磁性材料制备条件的优化。在rGO-Fe3O4粉末加入量为5 mg条件下,改变TBOT投加量对材料吸附性能的影响结果如图3 (a) 所示,TBOT的加入量为1.5 mL和2.0 mL时,吸附材料性能更优。如图3 (b) 所示,将rGO-Fe3O4粉末加入量从1 mg提高至5 mg,吸附容量能够提高19.8%,继续提高rGO-Fe3O4粉末加入量至10 mg,吸附容量没有明显变化。确定TBOT和rGO-Fe3O4粉末的最佳加入量分别为1.5 mL和5 mg。
-
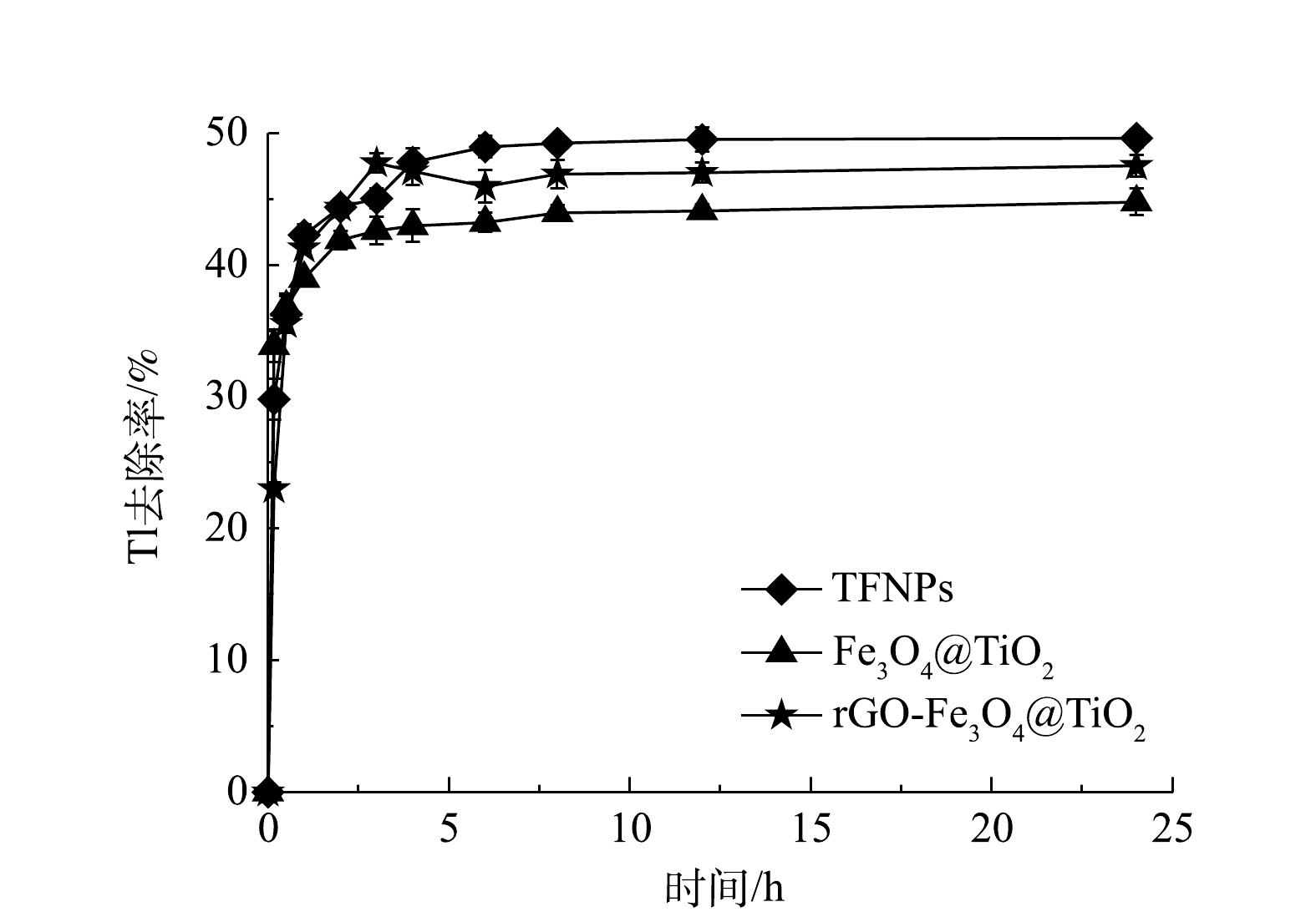
在pH=7.0、Tl(I)的初始浓度为10 mg·L−1、吸附剂量为0.1 g·L−1和T=(25±1)℃时,TFNPs、Fe3O4@TiO2和rGO-Fe3O4@TiO2对Tl(I)的吸附去除率如图4所示。10 min内,TFNPs、Fe3O4@TiO2和rGO-Fe3O4@TiO2对Tl(I)的去除率分别为29.98%、33.87%和23.01%,6 h后分别达到48.95%、43.23%和45.95%。3种磁性复合材料吸附除铊性能处于相同水平,但制备TFNPs的TBOT添加量 (10 mL) 远高于rGO-Fe3O4@TiO2 (1.5 mL) 。考虑到材料制备前驱体使用量,本研究将考察单位Ti条件下的吸附容量,即吸附位点的利用效率。
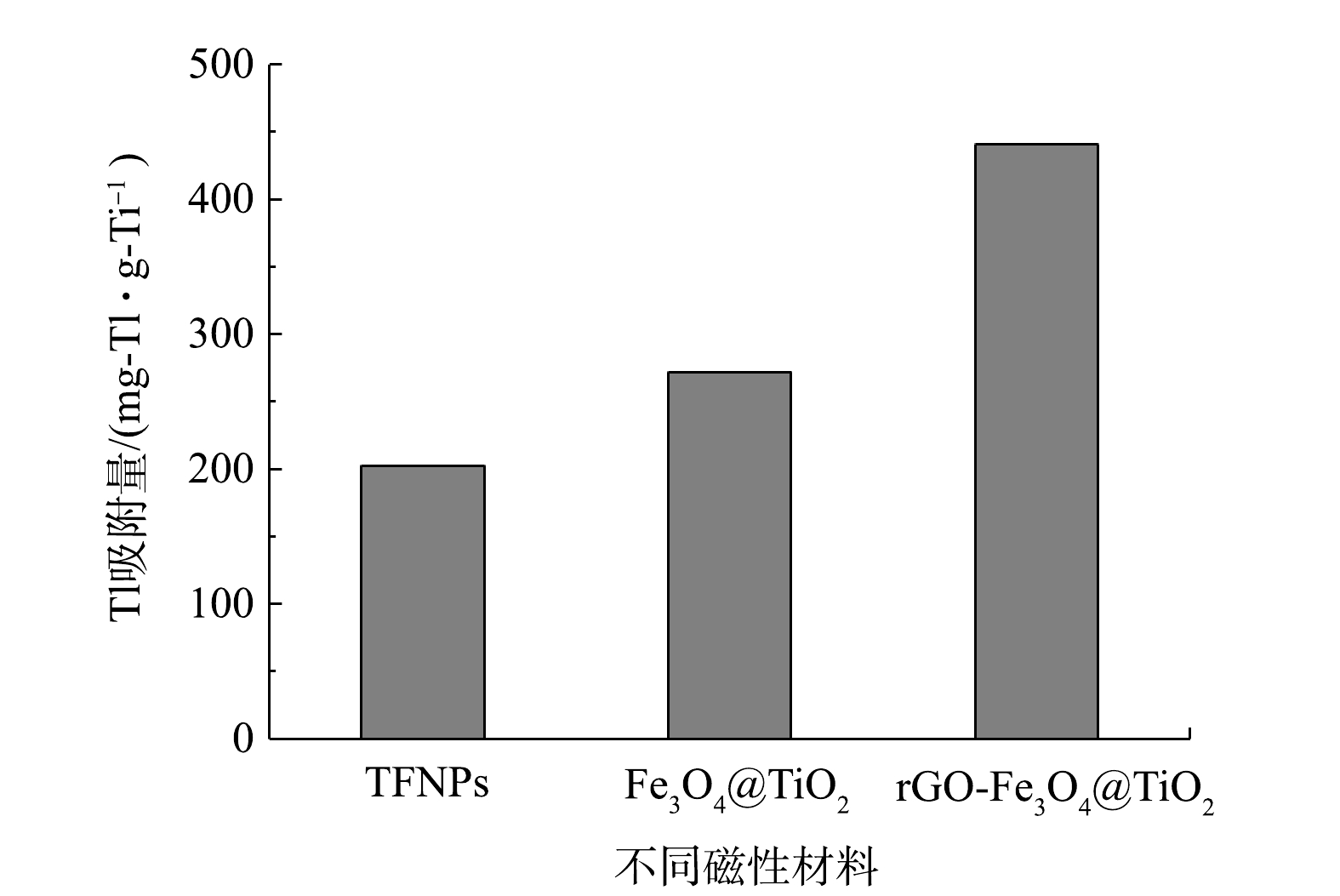
由于TFNPs、Fe3O4@TiO2和rGO-Fe3O4@TiO2磁性材料制备过程中TBOT添加量不同,而钛原子表面羟基 (Ti-OH) 是Tl离子吸附的主要功能基团,根据已有XPS分析结果[20,22,23 ],计算3种磁性材料在单位Ti含量条件下的Tl(I)吸附容量。如图5所示,10 min内,rGO-Fe3O4@TiO2相应单位Ti含量的Tl(I)吸附量 (97.3 mg-Tl·g-Ti−1) 是TFNPs (62.9 mg-Tl·g-Ti−1) 的1.55倍,是Fe3O4@TiO2 (68.6 mg-Tl·g-Ti−1) 的1.42倍。TFNPs、Fe3O4@TiO2和rGO-Fe3O4@TiO2单位Ti含量条件下Tl(I)最大吸附容量,分别为200、271.8和440 mg-Tl·g-Ti−1。Fe3O4@TiO2的单位Ti吸附容量高于TFNPs的1.36倍,而rGO-Fe3O4@TiO2的单位Ti吸附容量高于TFNPs的2.2倍。这主要与吸附材料结构特征直接相关[24-25],利用rGO纳米片作为模板,制备rGO-Fe3O4@TiO2磁性材料提高了TiO2的利用效率,最大程度上发挥所负载TiO2吸附Tl(I)的性能;而TFNPs通过将TiO2和Fe3O4颗粒在水热反应过程中集聚在一起,无次序的堆积,且相互挤压,使其部分活性位点被覆盖。同时,Fe3O4颗粒的晶体结构可能会受到干扰,材料的磁性会减弱。与TFNPs相比,Fe3O4@TiO2的核壳结构能够将吸附材料的活性中心更充分地暴露在磁性吸附剂的外表面上,降低了Tl污染物在吸附剂孔道内扩散阶段对吸附过程的影响,Tl污染物在该表面上吸附速度会更快[26]。但由于纳米TiO2粒子之间的范德华静电引力的作用,粒子表面相互靠在一起,使总表面积和表面自由能下降,TiO2粒子从高分散态变为团聚体,仍然会在一定程度降低了纳米颗粒的实际应用效果[27-29]。因此,综合考虑制备试剂的用量、单位吸附容量,rGO-Fe3O4@TiO2是快速、高效去除水中Tl(I)的最佳吸附剂。
与已报道的其他材料的吸附性能相比 (如表4所示) 。钛纳米管吸附量最大,达到709.2 mg·g−1,但需调节pH至5,而在铊泄漏造成的地表水污染情况时,水体pH一般在7左右。在中性情况下 (pH=7) ,FeOOH负载MnO2、过氧化钛、二氧化钛对Tl(I)的吸附量较大,分别为450、412和258 mg·g−1,均高于rGO-Fe3O4@TiO2的吸附性能,但过氧化钛和二氧化钛属于常规纳米吸附剂,需要借助离心或膜截留等方式进行回收再利用,高能耗在很大程度上,限制了其推广应用。rGO-Fe3O4@TiO2对Tl(I)的吸附量高于普鲁士蓝藻酸盐胶囊、聚丙烯酰胺膨润土等吸附剂,分别高于TFNPs、Fe3O4@TiO2磁性吸附剂的1.27、1.40倍[20, 22]。因此,rGO-Fe3O4@TiO2可作为一种磁选性能好且能有效去除铊污染物的吸附材料。
-
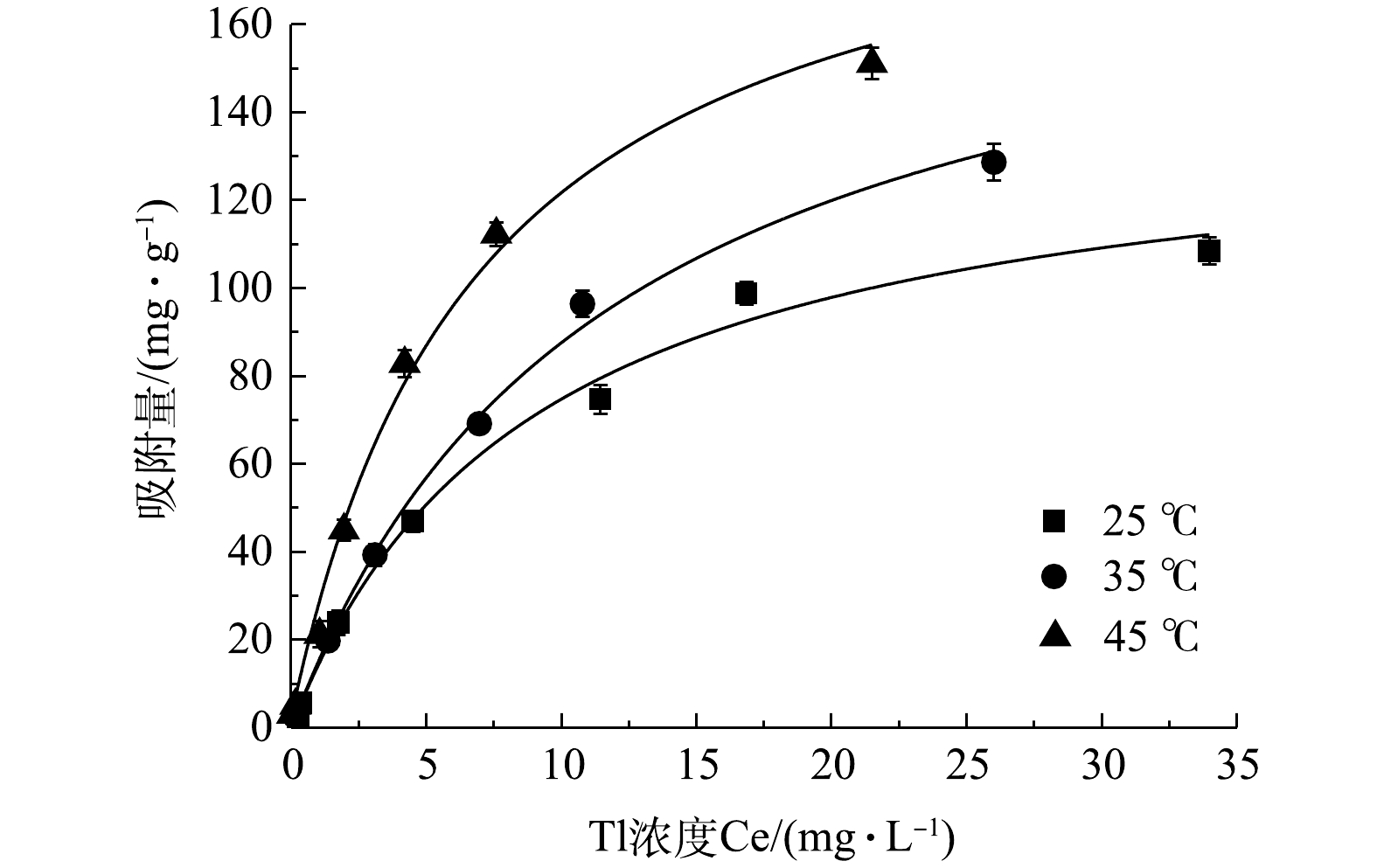
在pH=7.0,吸附剂量为0.1 mg·L−1和不同温度条件下的吸附热力学过程及Langmuir模型模拟数据如图6所示,依据式 (1)~式 (3) 计算的相关热动力学参数见表5。在温度为25、35和45 ℃条件下,rGO-Fe3O4@TiO2吸附去除Tl(I)过程的Gibbs自由能分别为−34.8、−36.3和−37.8 kJ·mol−1。这表明rGO-Fe3O4@TiO2吸附去除Tl(I)过程是自发进行的,且温度升高有助于提高材料的吸附性能[38]。相应焓变和熵变分别为10.19 kJ·(mol·K)−1和0.15 kJ·mol−1。这表明,Tl(I)在rGO-Fe3O4@TiO2磁性材料表面的吸附过程为吸热反应,且吸附后会导致固液界面无序性增加[39]。
-
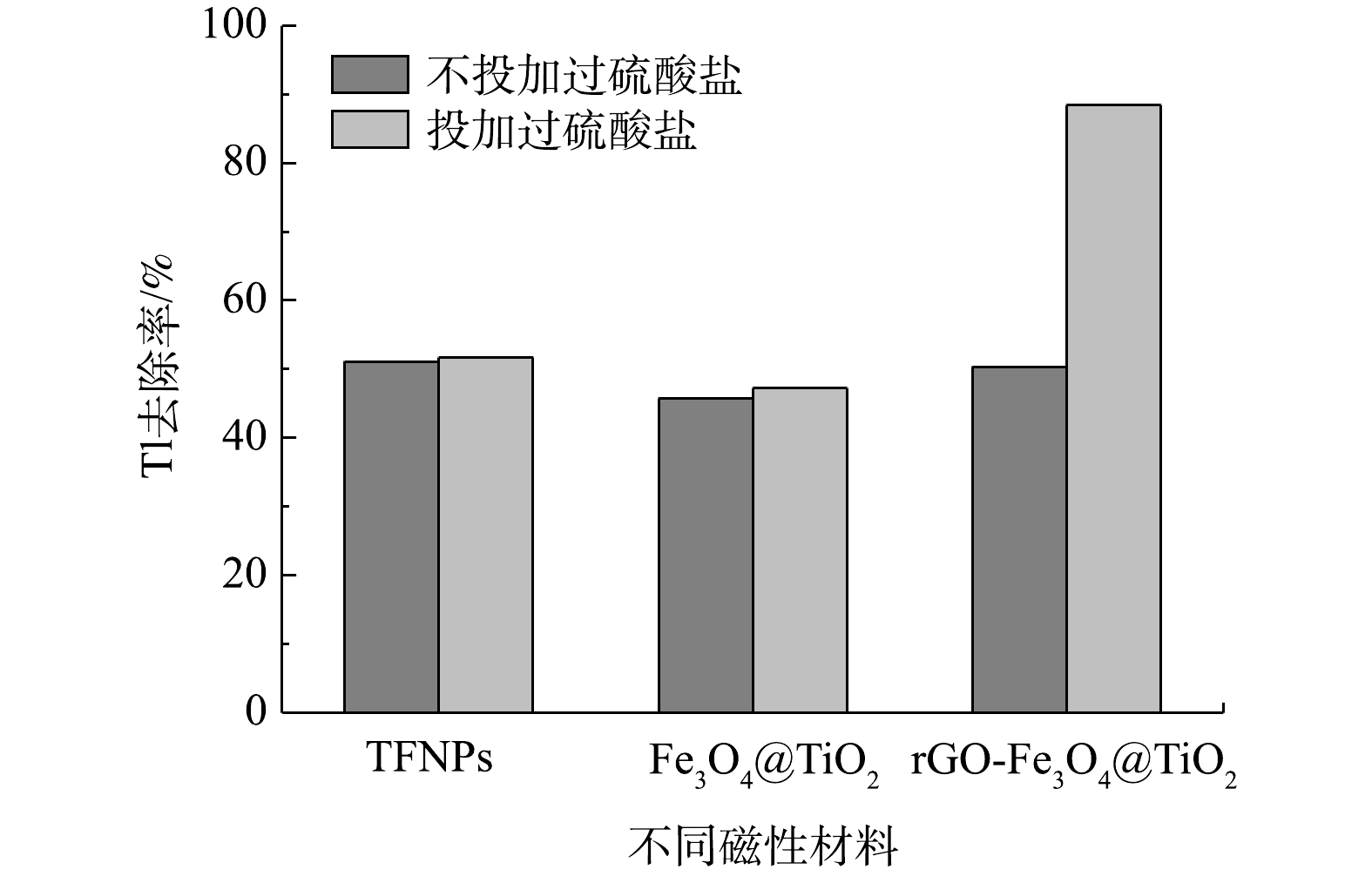
3种磁性吸附材料TFNPs、Fe3O4@TiO2和rGO-Fe3O4@TiO2中的Fe3O4颗粒具有一定的PS活化能力,将其与PS耦合去除铊污染物,可以有效结合吸附和氧化技术,实现铊污染物的快速、高效去除[40]。如图7所示,在Tl(I)质量浓度为8.9 mg·L−1、PS浓度为10 mmol·L−1、磁性材料投加量为0.2 g·L−1和pH=8条件下反应24 h后,TFNPs、Fe3O4@TiO2和rGO-Fe3O4@TiO2对铊离子的去除率分别为51.1%、45.7%和50.3%,而TFNPs/PS、Fe3O4@TiO2/PS和rGO-Fe3O4@TiO2/PS耦合体系对Tl的去除率分别为51.7%、47.2%和88.4%。与TFNPs、Fe3O4@TiO2和rGO-Fe3O4@TiO2吸附去除率相比,TFNPs和Fe3O4@TiO2磁性材料在PS存在条件下对Tl的去除效率略有提高,而rGO-Fe3O4@TiO2磁性材料在PS存在条件下对Tl的去除率提高了75.7%。这说明,rGO-Fe3O4@TiO2磁性材料能够有效活化PS,提高了反应体系对Tl的去除效能[41]。
-
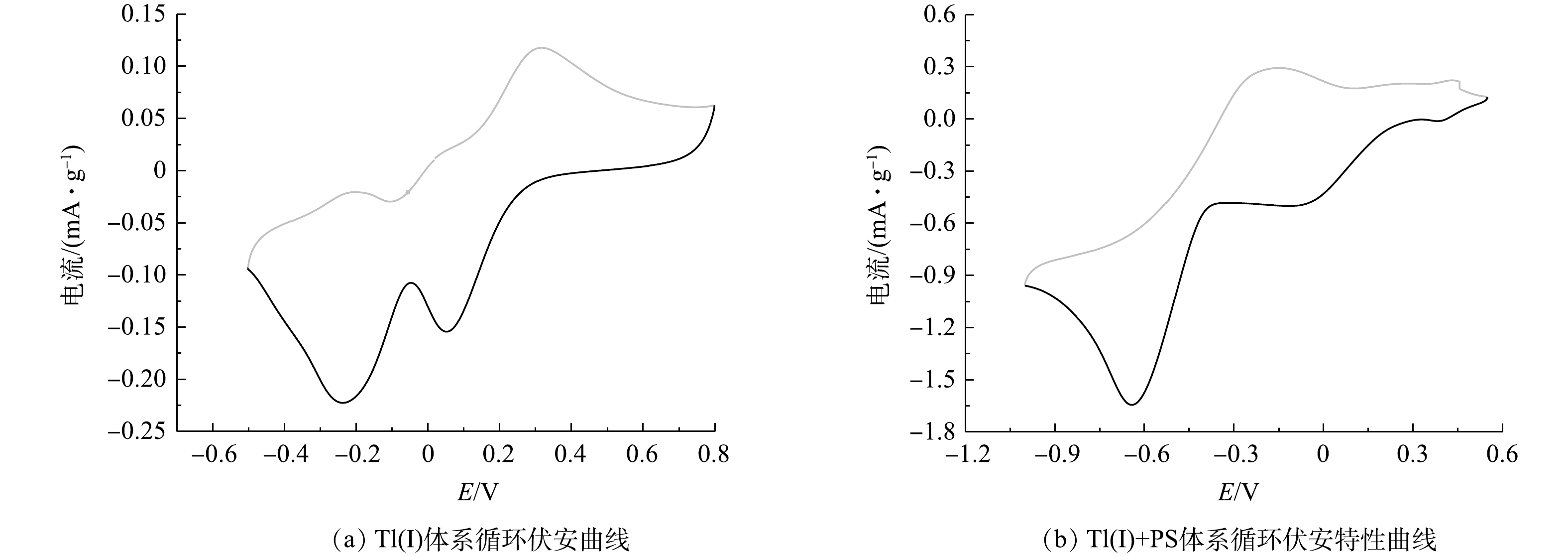
为探究rGO-Fe3O4@TiO2磁性材料能够更有效活化PS的内在机制,采用循环伏安特性曲线和电子交换容量进一步分析rGO-Fe3O4@TiO2磁性材料在反应过程中的电子传递性能,将rGO-Fe3O4@TiO2粉末固定在Pt电极上,作为工作电极。在Tl(I)溶液中的伏安特性曲线如图8 (a) 所示,呈现出明显的氧化还原峰,氧化峰位于0.31 V,还原峰位于0.06 V,2者具有较好的对称性,显示出一定的可逆性,在−0.20 V出现较弱的氧化峰,在−0.23 V出现较强的还原峰。这表明,rGO-Fe3O4@TiO2纳米颗粒自身具有较强的氧化还原能力。在Tl(I)和PS体系中的伏安特性曲线如图8 (b) 所示,出现2组氧化还原峰,分别在0.45 V和−0.18 V出现氧化峰,在−0.06 V和−0.63 V出现还原峰。与Tl(I)体系相比,PS存在条件下反应体系的氧化还原能力得到显著提高[42]。
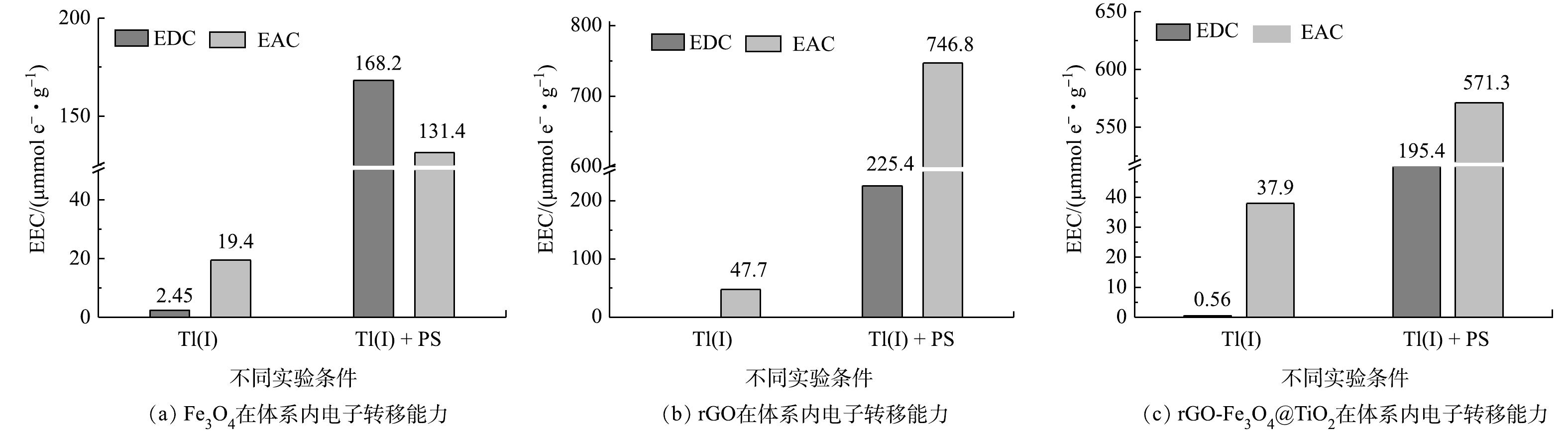
有研究表明,rGO-Fe3O4@TiO2/PS耦合体系中主要是rGO和Fe3O4共同活化PS产生自由基将Tl(I)氧化为Tl(III)[43]。为进一步明确Fe3O4、rGO和rGO-Fe3O4@TiO2在Tl溶液和Tl/PS混合溶液条件下的电子转移能力即电子交换容量 (EEC) ,采用计时电流法评估不同材料 (包括Fe3O4、rGO和rGO-Fe3O4@TiO2) 在Tl溶液和Tl/PS混合溶液条件下的电子转移能力,以揭示rGO-Fe3O4@TiO2/PS耦合处理含铊污水的电子传输规律。如图9所示,Fe3O4、rGO和rGO-Fe3O4@TiO2在Tl(I)溶液体系中电子转移能力较弱,其EDC分别为2.45、0和0.56 μmol-e−·g−1,而EAC分别为19.4、47.7和37.9 μmol-e−·g−1。在Tl(I)/PS混合溶液体系中,3种材料的电子转移能力均显著提高,EDC分别为168.2、225.4和195.4 μmol-e−·g−1,EAC测定值分别为131.4、746.8和571.3 μmol-e−·g−1。这表明,PS存在条件下大量增加了反应体系的电子,rGO在Tl溶液和Tl/PS溶液中具有较强的电子转移能力,这主要是由于其自身含有的功能基团和碳骨架会促进电子的转移[44]。rGO-Fe3O4@TiO2的电子转移能力介于Fe3O4和rGO之间,主要是由于其表面锚定的Fe3O4@TiO2减小了rGO的电子转移能力。这表明,rGO在rGO-Fe3O4@TiO2/PS耦合体系中能够作为电子穿梭体介导电子的转移[45],具有优异的电子转移能力。
-
1) 采用水热法、溶胶凝胶法、rGO模板法分别制备TFNPs、Fe3O4@TiO2和rGO-Fe3O4@TiO2磁性复合材料,优化其制备条件获得性能最佳的吸附材料。以rGO纳米片作为模板材料能够解决TiO2颗粒在废水中团聚的问题,加强吸附材料对重金属离子的吸附亲和力。rGO-Fe3O4@TiO2单位Ti含量条件下Tl(I)最大吸附容量可达到440 mg-Tl·g-Ti−1,吸附过程为吸热反应,温度升高有助于提高材料的吸附性能。
2) rGO具有优异的电子转移能力,结合rGO-Fe3O4@TiO2磁性材料的高吸附性能,能够在材料周边形成局部高浓度,极大提高了电子传递给目标污染物的效率,进而强化电子利用效率,使rGO-Fe3O4@TiO2磁性材料在PS存在条件下对Tl的去除效率提高了75.7%。




 下载:
下载: