-
近年来,药物和个人护理产品(pharmaceuticals and personal care products, PPCPs)在环境中的频繁出现引起了越来越多的关注。PPCPs可对水生生物和人类造成慢性毒性和内分泌紊乱,甚至引起致病菌耐药性的发生,对人体健康和生态系统构成潜在的严重威胁。布洛芬(ibuprofen, IBP)是目前世界上应用最多的一种非甾体抗炎药,也是在城镇污水处理厂二级出水中检出频率最高的一种PPCPs[1]。因此,探索从水环境中去除以布洛芬为代表的PPCPs技术已经成为环境领域的研究热点[2-3]。
目前, PPCPs的去除技术主要有生物技术[4-5]、臭氧氧化技术[6-8]、膜技术[9-11]、活性炭吸附技术[12-13]等。生物技术对PPCPs的降解效果并不稳定,这种现象与微生物的降解特性和PPCPs的结构有关[14]。膜技术与臭氧氧化技术对PPCPs的去除效果受PPCPs的种类和浓度影响[15]。活性炭可有效去除部分PPCPs,但对于极性化合物的去除效果有限,并且其他竞争物质的出现也会对活性炭的吸附能力产生影响[16]。
电催化强化技术在难降解废水的深度处理中表现出高效的污染物去除能力。CHEN等[17]通过O3+H2O2强化电催化体系对地表水中的氧氟沙星进行降解,发现O3单独和O3+H2O2强化电催化体系均可快速氧化氧氟沙星,与单独臭氧氧化(30%)相比,O3+H2O2体系显著提高了氧氟沙星的矿化率(55%)。BAVASSO等[18]采用O3强化电催化对利尿酮进行了去除,结果表明,在200 mA电流和酸性pH条件下,在2 h内可实现利尿酮的快速降解和矿化,这与羟基自由基的大量产生密不可分。SANTANA-MARTINEZ等[19]评价了H2O2强化电催化法的氧化效率,结果表明,在pH为3、电流密度为60 mA·cm−2、H2O2流速为4.7 L·min−1和电解质浓度为0.05 mol·L−1的条件下,苯酚的最高矿化率约为75%,其出水的原始毒性显著降低。综上所述,电催化强化对水体中的PPCPs类污染物具有高效的去除效率,然而电催化强化体系对布洛芬的深度去除效果和机制研究还很缺乏。因此,本研究设计了3种电催化强化处理体系,考察了H2O2浓度、O3投加量、腐殖酸等因素对IBP去除效果的影响,基于布洛芬的降解产物,阐明了IBP的降解机理。本研究以期为电催化强化体系去除以布洛芬为代表的PPCPs类污染物提供参考。
-
实验中所用到的布洛芬为分析纯,购于西格玛公司,实验中使用的其他试剂(如硫酸钠、磷酸氢二钠、硫酸、碳酸钙、过氧化氢等)均为分析纯购于国药公司,腐殖酸购买于西格玛公司,醋酸铵为色谱纯,购自于英国Alfa Aesar公司。实验中所需溶液均由密理博的高纯水系统产生的高纯水(阻抗18.2 MΩ)配制,实验中布洛芬溶液的质量浓度为10 mg·L−1。
-
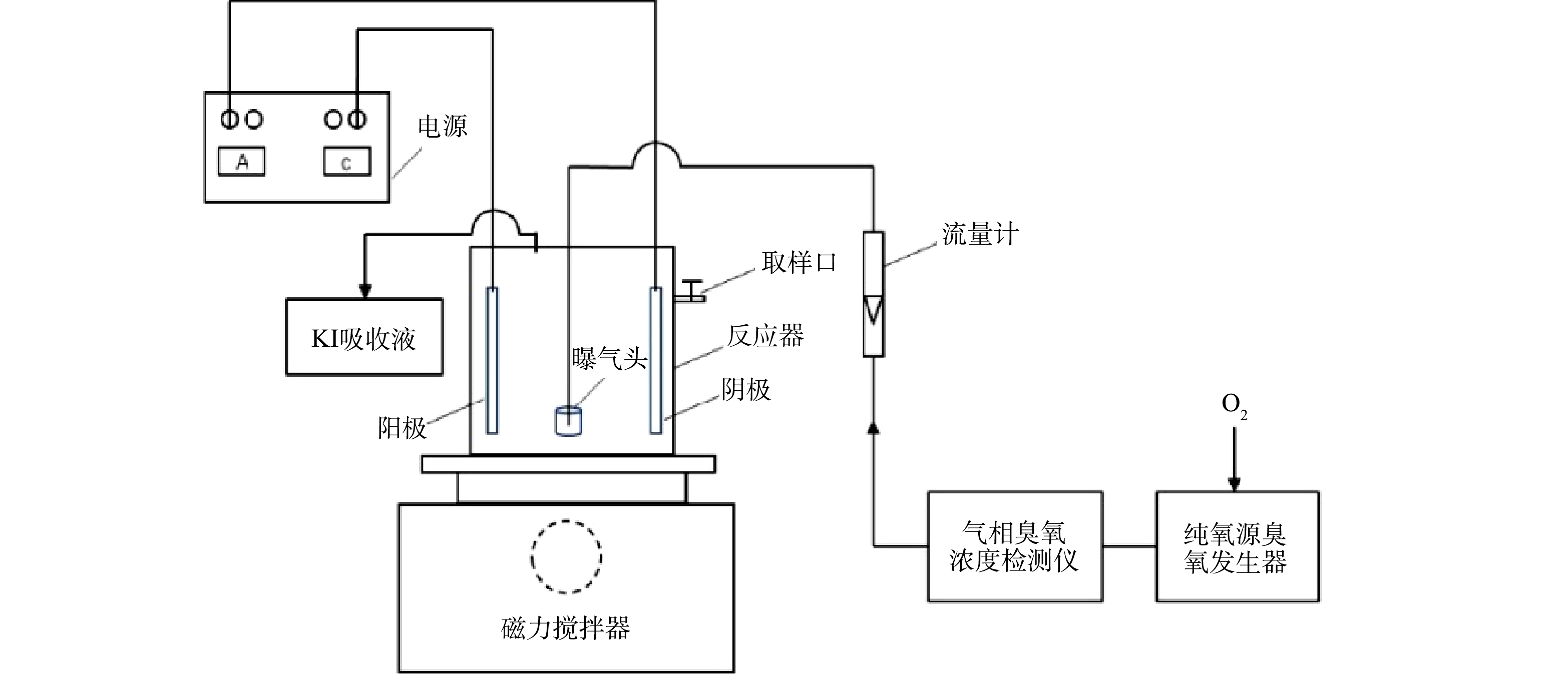
电催化及其强化的实验均在自行设计的玻璃容器中进行,容器有效容积为500 mL。实验中采用的阳极为钛镀钌铱电极,阴极为钛电极,电极间采用单极式连接方式,电极间距为4 mm,电极的有效面积为25 cm2(图1)。在处理中,每批次处理量为 200 mL,运行时间为0~30 min。电流由恒流恒压电源(大华,MC-100/5)控制,输出电压为0~50 V,输出电流为0~5 A,电流密度为30 mA·cm−2。所有实验均重复3次。对于电催化强化实验,则为在电催化反应基础上分别加入H2O2、O3和O3+H2O2,从而形成3种强化方式,单独电催化、H2O2强化电催化、O3强化电催化、O3+H2O2强化电催化以下分别简称为E0、E1,E2和E3。
-
水样的荧光图谱采用Hitachi F-4600 (日本Hitachi公司)荧光色谱仪进行分析。分析仪激发光源为150 w氙弧灯,PMT电压为700 V,信噪比为110;激发波长为5 nm, 发射波长为5 nm; 响应时间设为自动,扫描速度为12 000 nm·min−1,激发波长为200~500 nm,发射波长为220~600 nm。布洛芬质量浓度通过Waters高效液相色谱仪测试,色谱柱为Agilent TC-C18,柱温为30 ℃,检测波长220 nm,流动相为75%甲醇和25%超纯水,流动相流速1 mL·min−1,进样体积50 μL。水样中有机物表观分子质量分布采用尺度排阻色谱-紫外分析仪测定(SEC-UV)。色谱柱采用 TSK-GEL G3000 PWxl型柱子(日本Tosoh Bioscience),流动相为1.2 g·L−1的磷酸二氢钠和2.5 g·L−1磷酸二氢钾的混合溶液,流速为1.0 mL·min−1。布洛芬在反应过程中的中间产物采用超高效液相色谱串联飞行时间质谱进行检测(UPLC/Q-TOF-MS)(UPLC,Ultimate 3200,Dionex,USA,micrOTOF III,Bruker,Germany),流动相为水(2 mmol·L−1醋酸铵)和乙腈,采用Waters Cortecs C18 色谱柱(1.7 μm,50 mm × 2.1 mm),流速为 0.4 mL· min−1,洗脱从10%的乙腈开始,持续2 min,然后在23 min内增加至60%的乙腈洗脱液,保持5 min。反应过程中产生的羟基自由基(·OH)采用Waters高效液相色谱仪测定,·OH的捕获剂为对-氯苯甲酸(0.5 μmol·L−1),色谱柱为Agilent TC-C18,检测波长为 254 nm,柱温为30 ℃;流动相为75%甲醇和25%超纯水,流速为1 mL· min−1。
-
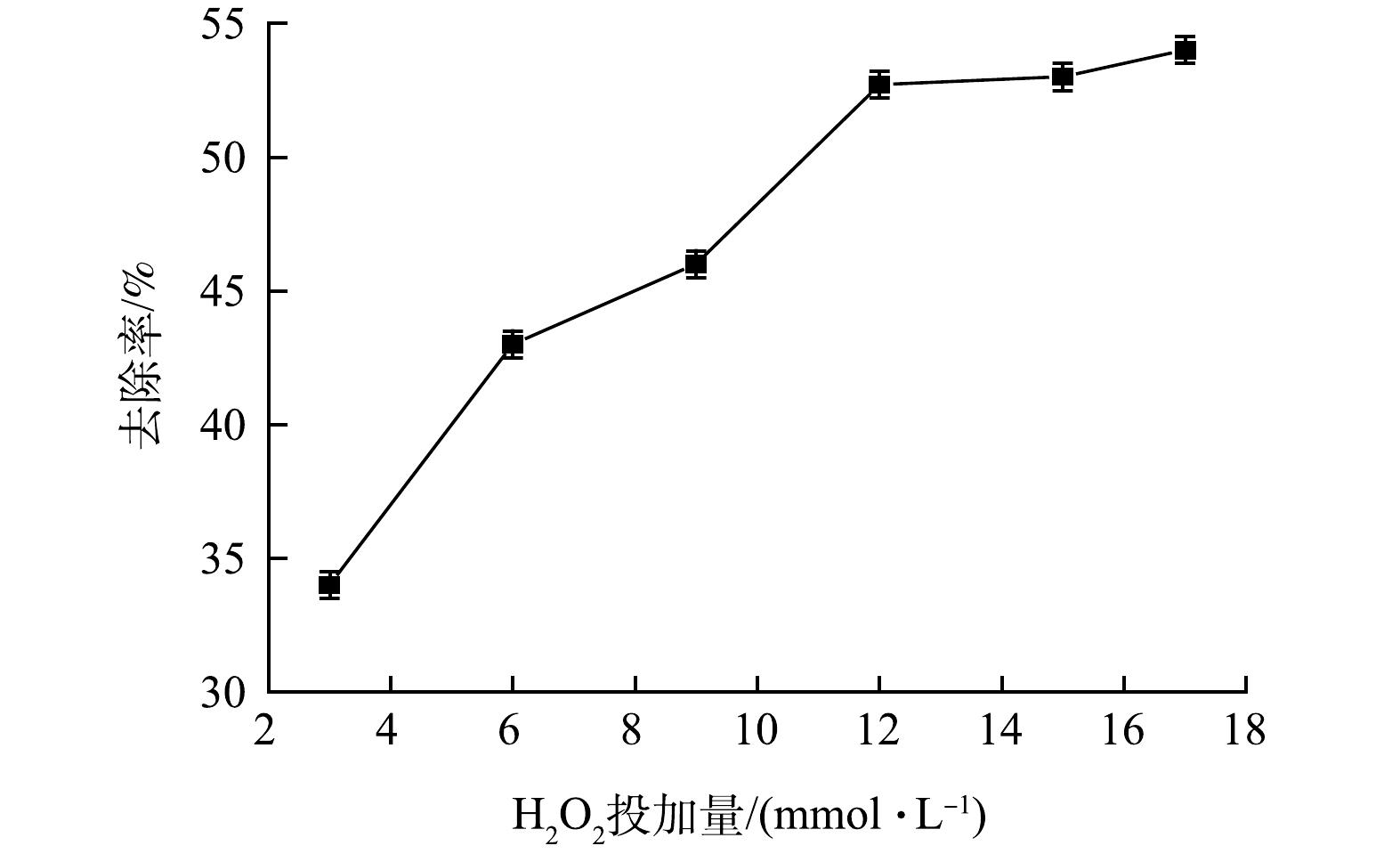
为考察H2O2电催化强化体系对布洛芬的去除影响,分别投加3、6、9、12、15和17 mmol·L−1的H2O2,反应30 min结束后,计算布洛芬的去除率。由图2可见,随着H2O2投加量的提高,布洛芬的去除率显著升高。在H2O2为3 mmol·L−1时,布洛芬的去除率为34.1%,其去除率低于单独电催化时的去除率(46.2%);而在在H2O2为17 mmol·L−1时,布洛芬的去除率为54.1%,升高了58.6%。此外,从布洛芬的去除率变化趋势可以看出,H2O2添加并不能持续有效提高布洛芬的去除率。这是因为布洛芬在H2O2投加量为12、15和17 mmol·L−1条件下的去除率基本保持不变。添加H2O2有利于·OH的生成,但其生成效率受多方面影响。反应过程中H2O2的添加有利于·OH的生成速率增加, H2O2在反应中易分解生成·OH(式(1)),但随着H2O2浓度的增加,·OH的生成速率也会受到影响。这是因为H2O2会抑制·OH的生成(式(2)~式(4))。所以过量的H2O2不仅会增加运行成本,也不能高效去除布洛芬。结果表明,在单独电催化中投加3 mmol·L−1 H2O2所获得的布洛芬去除率相比单独电催化的去除率低,可能因为H2O2浓度的增加抑制了· OH的生成,从而影响了布洛芬的降解。
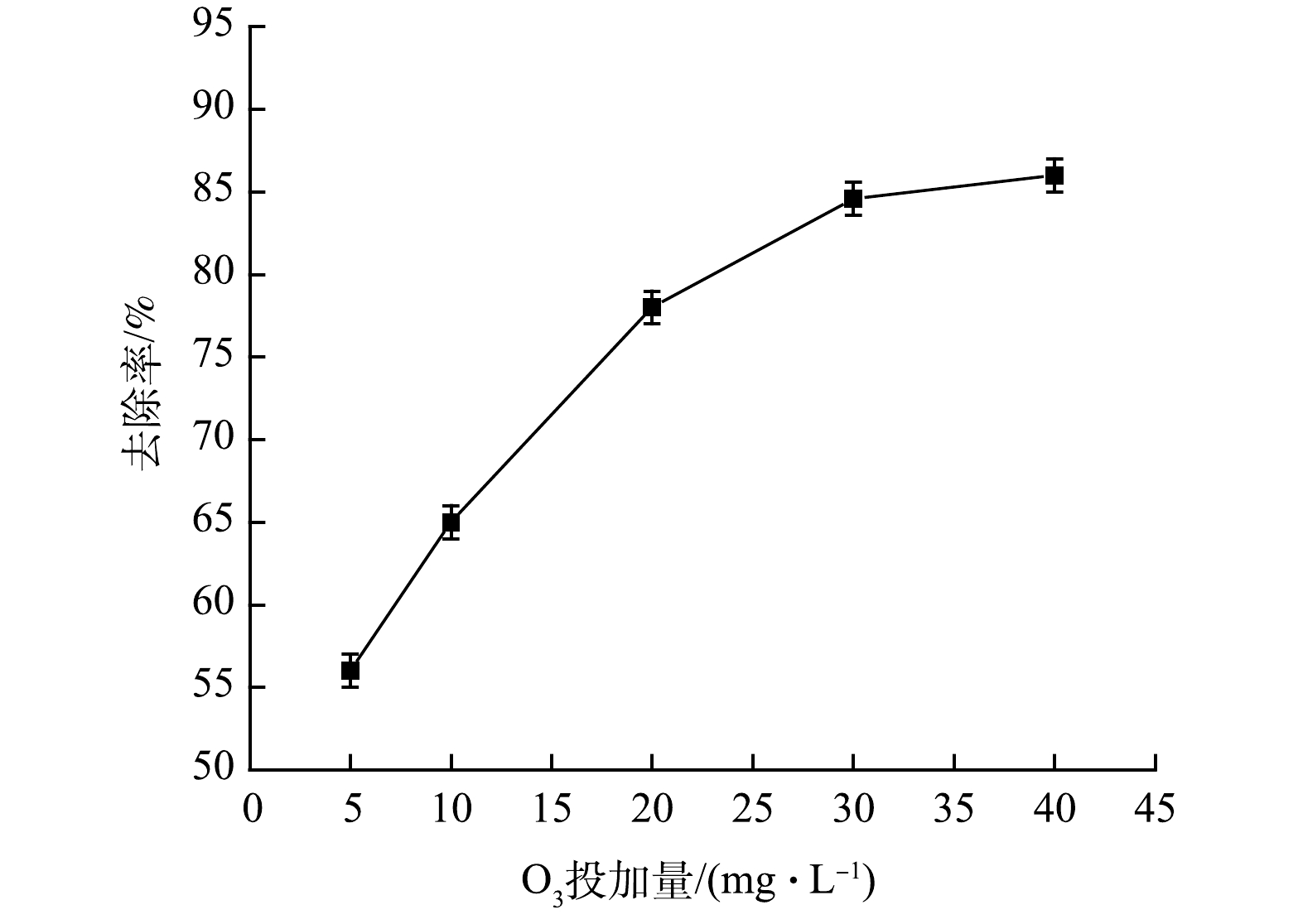
图3反映了在O3的质量浓度分别为5、10、20、30和40 mg·L−1、O3流量为0.8 mL·min−1,反应时间为30 min的条件下,O3电催化强化体系对布洛芬的去除效果。由图3可见,O3电催化强化体系对布洛芬去除效果较为显著。在O3加入量由5 mg·L−1增加到30 mg·L−1时,布洛芬的去除率由56%提高到84.5%。这是因为O3是氧的同素异形体,是一种较强的氧化剂,其氧化还原电位高达2.07 V,可通过断链、开环等一系列反应降解去除污染物;同时,在电催化过程中,O3的添加可促进反应过程中有效产生·OH,进而对布洛芬进行有效去除(式(5)~式(7))。
为了对比3种电催化强化体系对布洛芬的去除效果,分别考察了17 mmol·L−1 H2O2、30 mg·L−1 O3 (流量为0.8 mL·min−1)和17 mmol ·L−1H2O2+ 30 mg·L−1 O3 (流量为0.8 mL·min−1)3个体系对布洛芬的去除情况。如图4所示,随着反应时间的延长,布洛芬的去除率先快速上升,然后持续增加,3种强化方式在前10 min处理中的去除率上升较快,分别达到31.6% (E1)、45.1%(E2)和55.8%(E3)。在30 min后,对布洛芬的去除率顺序为为E3>E2>E1,其去除率分别为93.2%、84.5%和52.7%,高于电催化(E0)时46.2%的去除率。
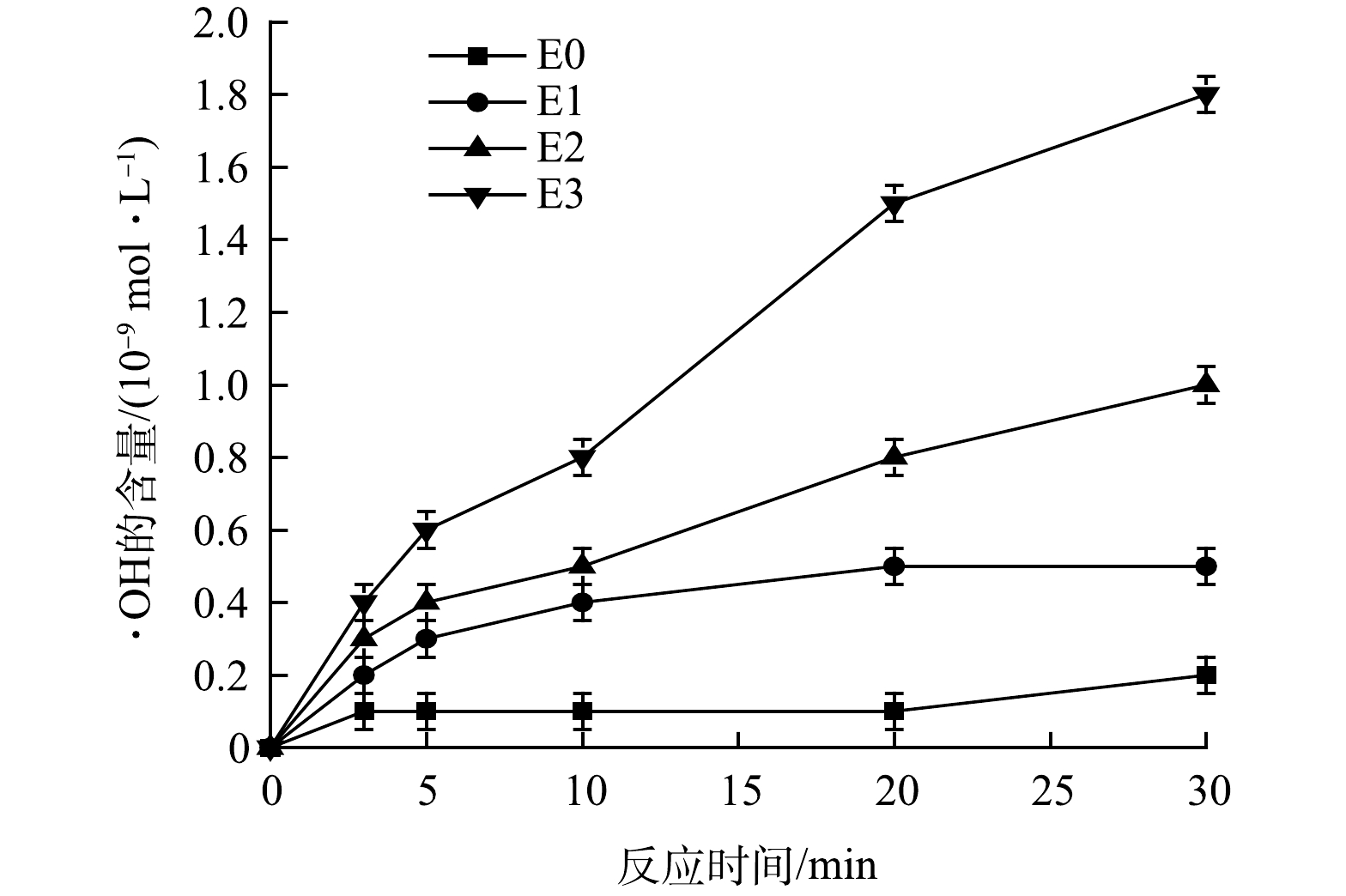
图5所示的电催化及电催化强化体系中·OH的生成量,可以很好的解释上述布洛芬去除率的差异。由图5可知,E3在这几种电催化强化过程中的·OH生成量最高,并且其生成量随时间增加呈线性增长趋势,表明在该过程中·OH浓度基本保持稳定。其中,E0体系中·OH的生成量最低,在反应30 min后,·OH仅为0.13×10−9 mol·L−1。从电催化反应机理的角度来看,通常是通过电催化的电极材料表面直接氧化水中的有机污染物,同时电极材料通过电化学作用产生具有强氧化能力的自由基基团(羟基自由基(·OH)、超氧自由基(·O2)、H2O2等)间接氧化水中的有机污染物,最终达到降解去除污染物的目的。在O3和H2O2同时添加体系中,反应过程中H2O2与通入的 O3 发生反应(式(8))[20],可高效生成·OH (图5),对布洛芬进行深度降解。有研究[21]表明,O3 + H2O2强化电催化体系比单独的臭氧氧化和单独电催化体系对有机污染物具有更高效的去除效率。图4中E3对布洛芬的高效去除也进一步证实了这一点。
-
1)腐殖酸的质量浓度影响分析。污水处理厂的二级出水以及地表水环境中均包含大量的天然有机物质(natural organic matter, NOM),NOM不仅会影响电催化强化体系的催化效果,也会影响布洛芬的去除。以不同质量浓度的腐殖酸(humic acid, HA)为代表物质,考察HA对布洛芬去除效果的影响。电催化强化实验采用图4中的实验参数(17 mmol·L−1 H2O2、30 mg·L−1 O3 (流量为0.8 mL·min−1)和17 mmol ·L−1H2O2+ 30 mg·L−1 O3 (流量为0.8 mL·min−1)),反应时间为30 min。
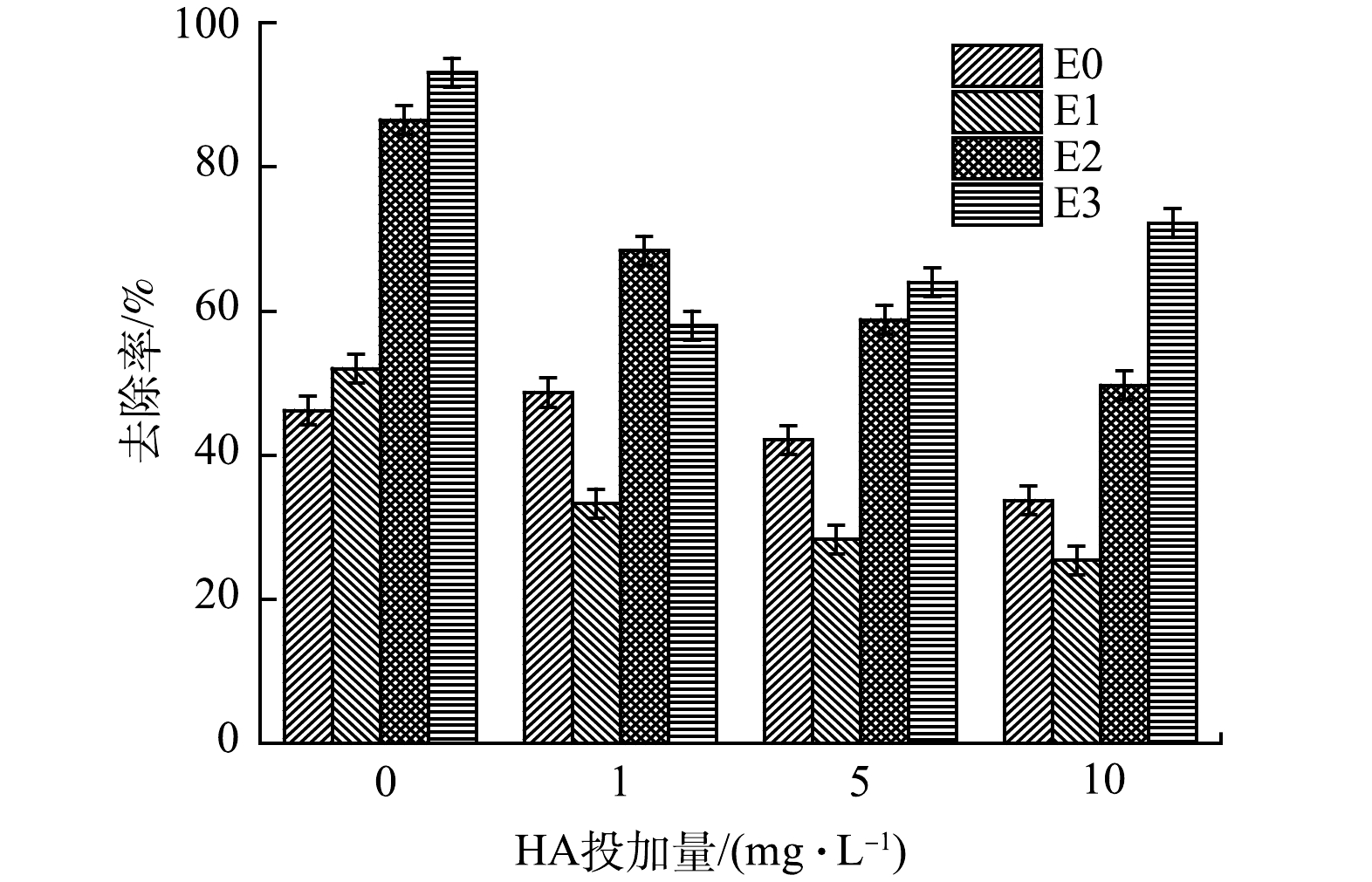
由图6可见,HA的添加降低了电催化强化体系对布洛芬的去除效率。当HA的质量浓度为从1 mg·L−1增加到10 mg·L−1时,E1~E3中布洛芬的去除率均低于不添加HA的溶液,例如,E1在HA质量浓度为1 mg·L−1时的去除率为33.3%,而在10 mg·L−1质量浓度下的去除率为25.4%。这是由于反应过程中HA的添加会限制·OH的生成,减弱H2O2和O3的强化作用,从而抑制布洛芬的去除[22]。此外,布洛芬的去除率在HA质量浓度为10 mg·L−1时相比1 mg·L−1有显著的升高。这是因为HA会促进臭氧的自由基链式反应从而强化了布洛芬的去除[23]。布洛芬的去除受到以上2种因素共同作用。
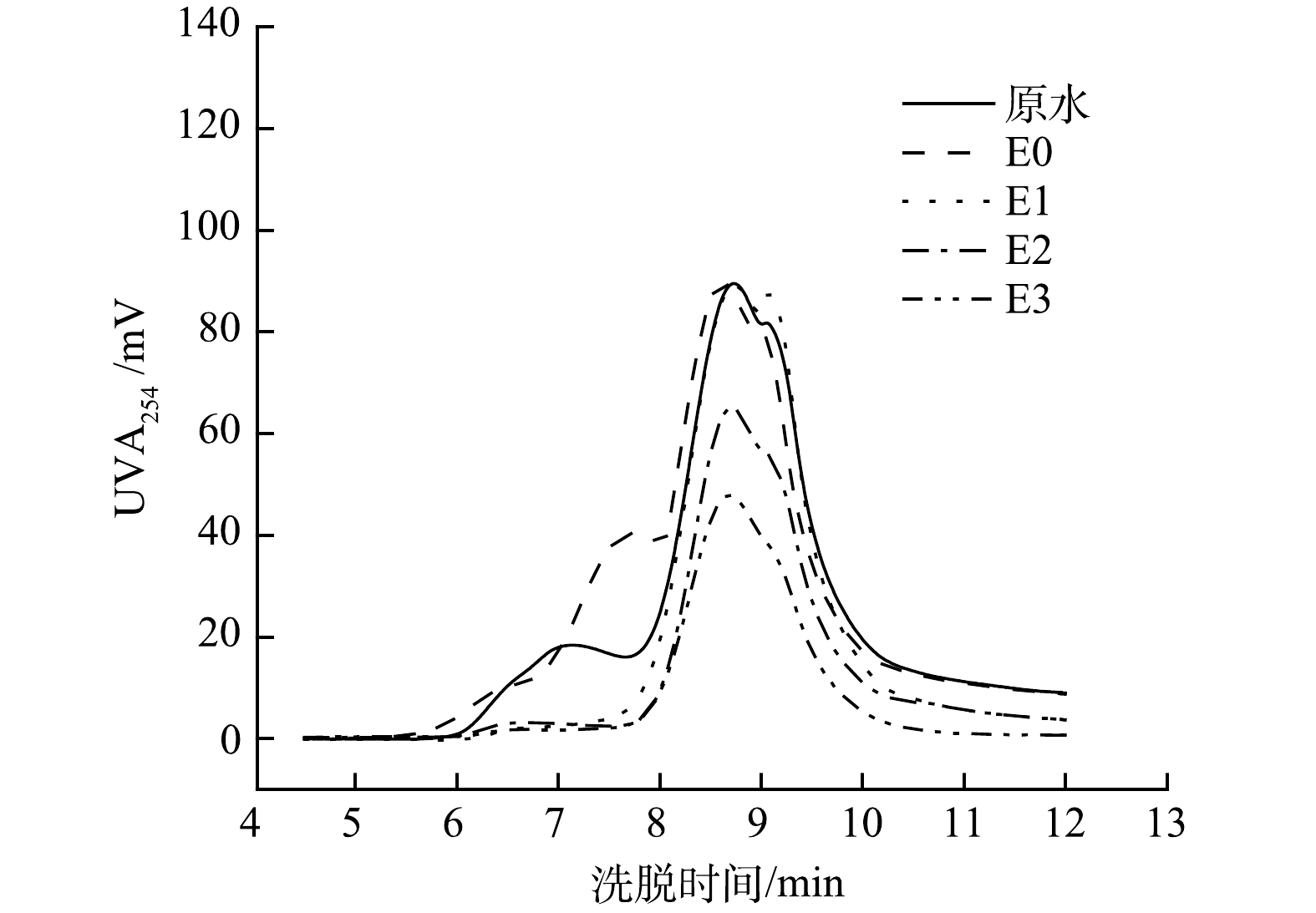
2)分子质量分析。作为一个重要的水处理参数,有机污染物的分子量分布对研究有机污染物的特性以及布洛芬在电催化强化过程中的降解及去除机理具有重要作用。根据尺度排阻色谱-紫外分析结果,本研究的水样中主要含有2种分子质量的有机污染物。由图7可见,这2种有机物的保留时间分别为7.1 min和8.6 min,参照聚乙二醇和聚氧化乙烯标准物质的出峰时间,2种污染物的名义分子质量分别为28 800 Da和2 900 Da。结果表明,电催化强化体系对这2种有机污染物去除效果不同,其中单独电催化体系对2 900 Da的污染物没有去除作用,而对28 800Da的污染物可进行有效降解。在7.7 min 处有新的紫外吸收峰出现,所对应的化合物分子质量为11 150 Da。该结果表明,单独的电催化体系(E0)可使得28 800 Da的有机污染物发生部分降解,同时也产生了新的污染物。相比单独电催化(E0)的去除,电催化强化对这2种污染物均可有效去除。E3、E2和E1体系可完全去除28 800 Da的污染物,E3和E2对2 900 Da的污染物的去除率则为53.1%和41.2%,而E1对此污染物并没有去除作用。由此可见,电催化强化体系对大分子和小分子污染物均有较好的去除作用,并且E3的强化作用有利于小分子污染物的去除。该结果也进一步证明E3在3种电催化强化体系中的氧化作用最强,不仅能高效去除布洛芬(图4),对其他有机污染物也具有高效的降解;同时结合图7结果,该部分研究说明腐殖酸中分子质量为28 800 Da和2 900 Da的污染物降低了布洛芬的去除效率。
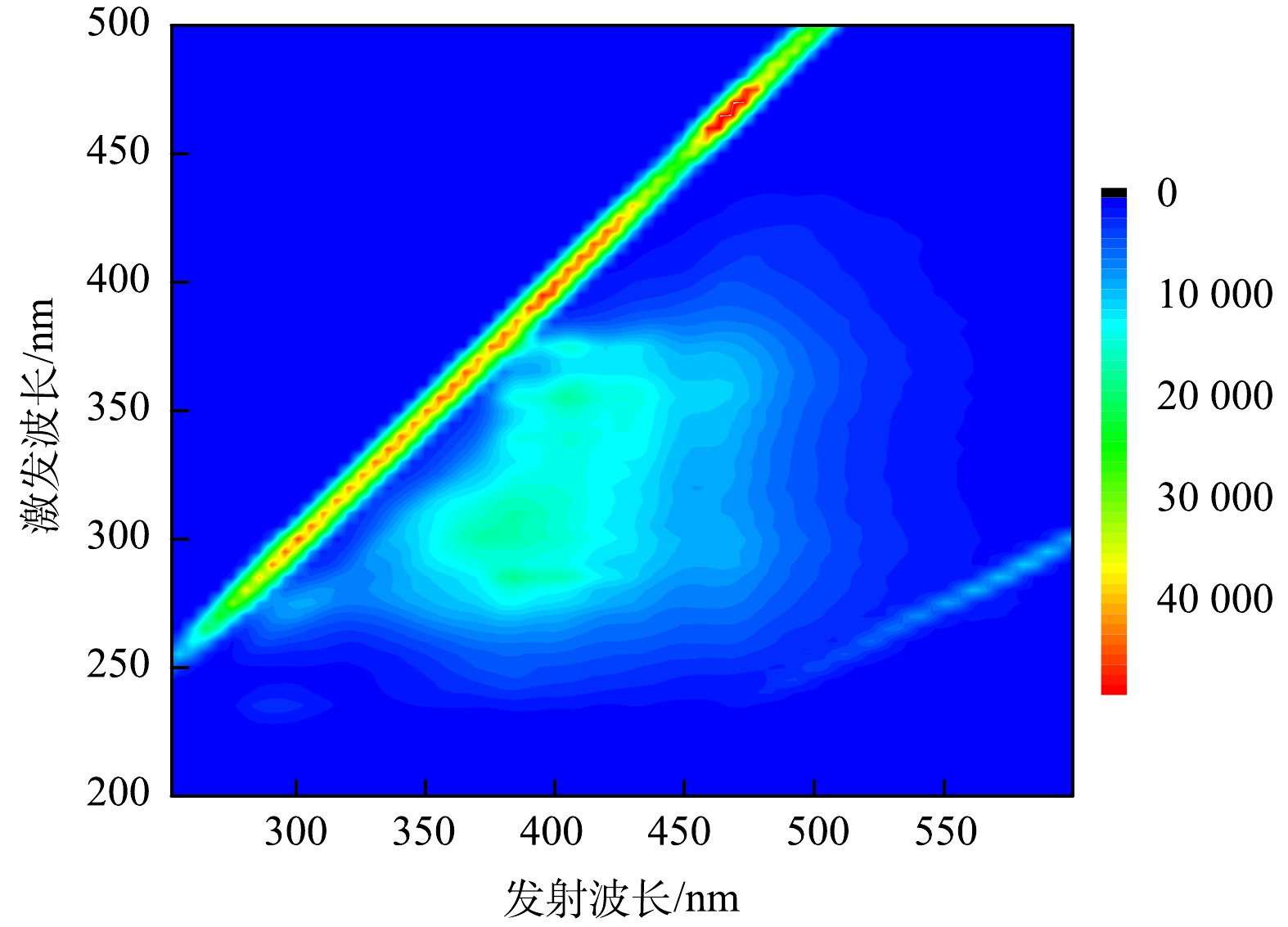
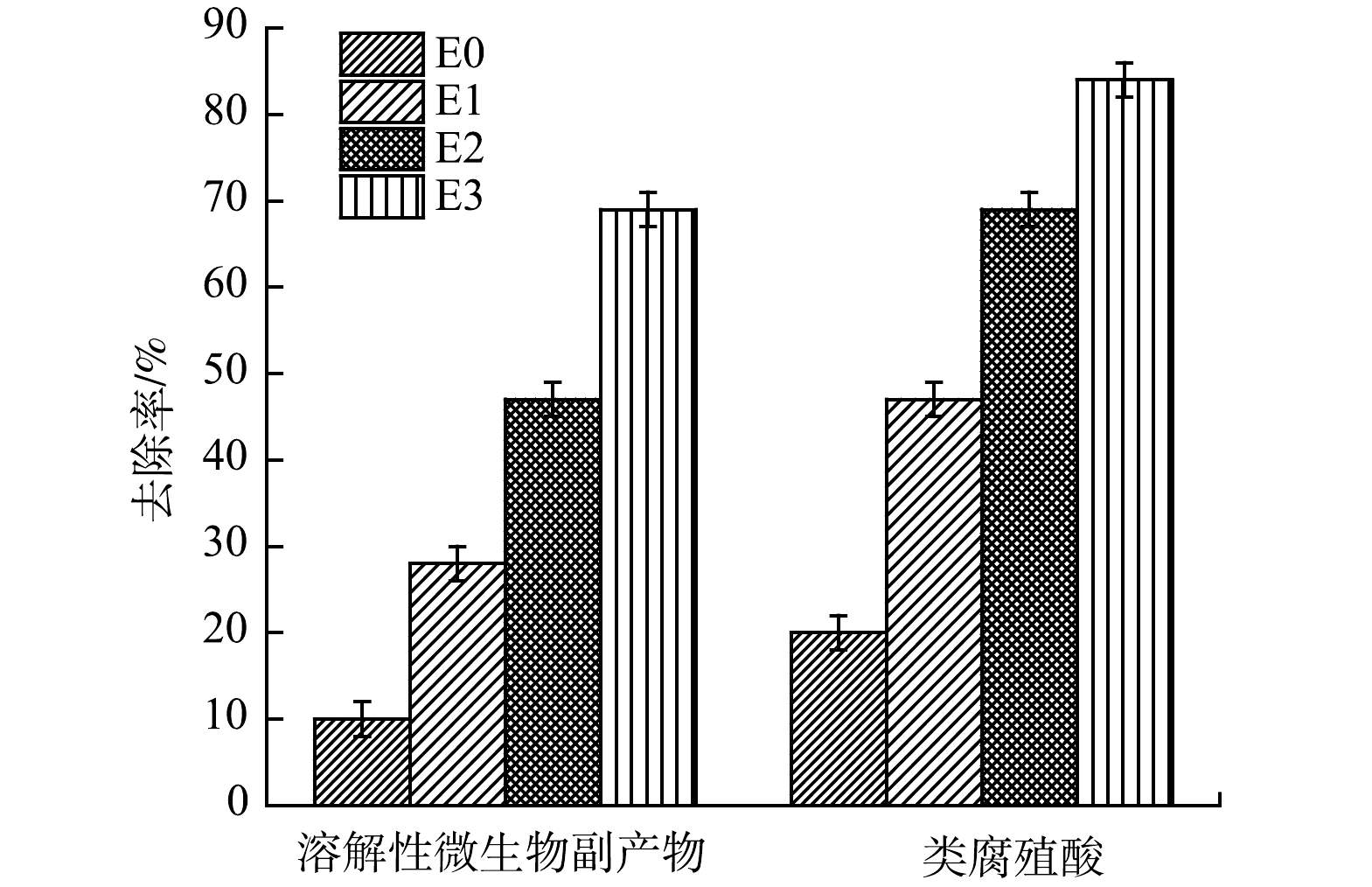
3)荧光光谱分析。荧光光谱分析对于研究布洛芬在电催化强化过程中的去除效果及降解机制具有重要作用。由图8可以发现,水样中主要包含2个特征荧光峰,对应的是溶解性微生物副产物和类腐殖酸化合物。这2种有机物也是限制布洛芬去除的主要原因。根据图中荧光强度的变化分析得出,溶解性微生物副产物和类腐殖酸化合物被有效去除,而且类腐殖酸化合物比溶解性微生物副产物更容易被去除。由图9可以看出,经30 min 处理后,E1、E2和 E3对类腐殖酸化合物的去除率分别为47.1%、69.2%和84.1%。这与氧化作用对有机污染物的选择性降解有直接的联系,在类似研究[24]中也证实了电化学氧化对类腐殖酸的去除率要高于溶解性微生物副产物。
-
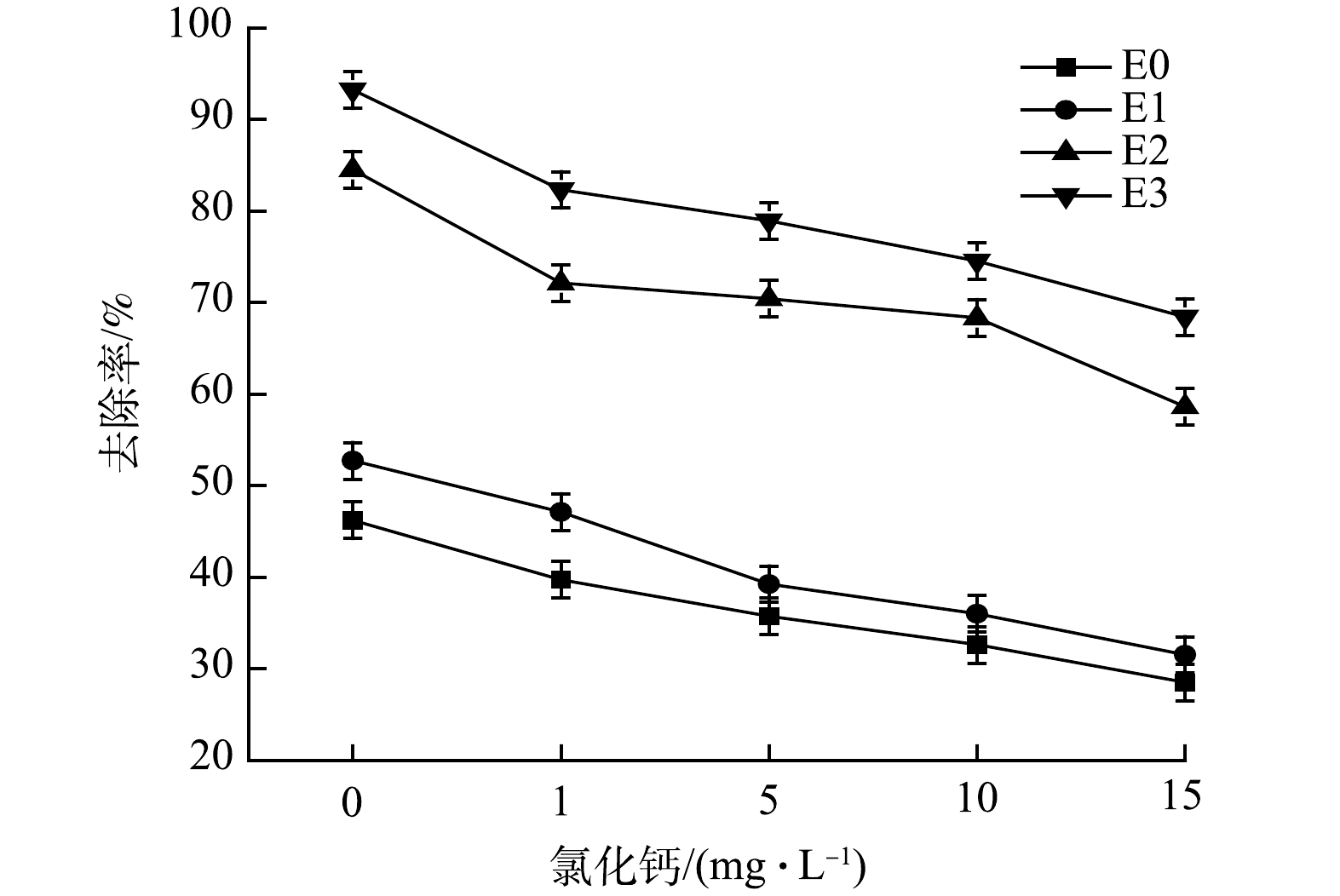
硬度普遍存在于地表水和废水中,而过高的硬度会降低电催化体系对布洛芬的去除效率,并且增加运行成本[25]。硬度对电催化强化布洛芬去除率的影响结果如图10所示。硬度对布洛芬的去除具有显著的抑制作用,随着硬度的增加,3种电催化强化体系的去除率都受到影响。在没有硬度的干扰下,E3对布洛芬的去除率为93.2%,而在15 mg·L −1 CaCO3的质量浓度下,布洛芬的去除率为68.4%,下降了26.6%;而E2和E1的去除率也相应的下降了30.6%和40.2%。基于电催化过程的反应原理,水中的钙离子会在外加电场的作用下向阴极迁移,使得阴极溶液中碳酸钙的浓度达到过饱和,容易在极板附着形成沉淀,从而降低了反应的催化效率[26-27],最终导致布洛芬去除率的下降。
-
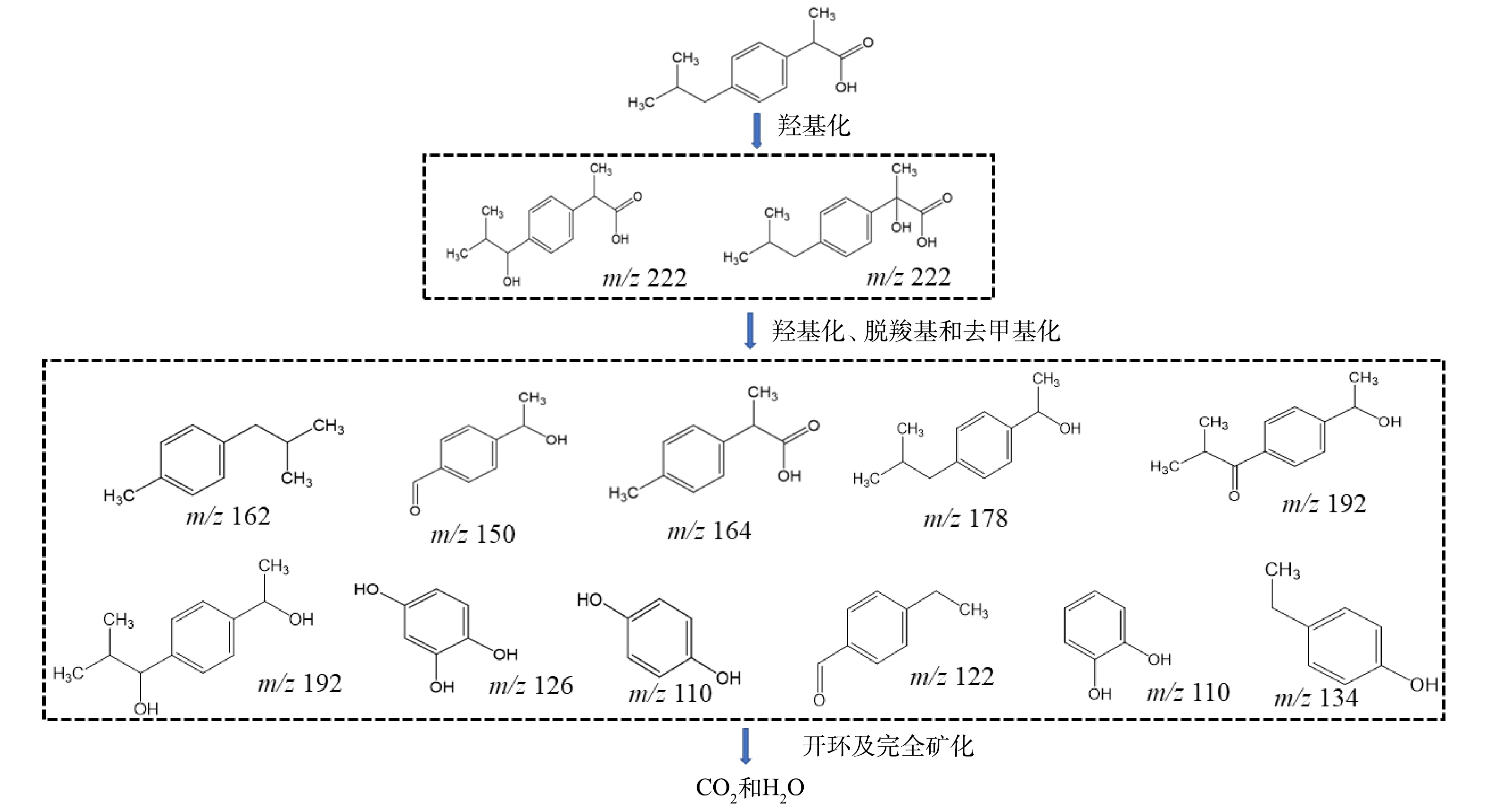
采用高效液相色谱串联飞行时间质谱(UPLC/Q-TOF-MS)分析了电催化强化反应过程中布洛芬的降解物。由表1可知,布洛芬在降解过程中共产生了13种中间产物,其他研究也证实了这些降解产物是电催化降解过程中的主要产物[28]。根据以上的降解产物提出了布洛芬的降解路径(图11)。由于反应过程中存在大量的中间体以及反应机制的复杂性,因此,该降解路径分析仅提供可能的理论参考。首先,在电催化强化反应过程中产生的·OH在不同位置攻击布洛芬,最终形成羟基化布洛芬异构体。然后羟基化的布洛芬进一步通过脱羧、脱甲基等一系列反应,生成了不同的小分子降解产物,反应过程中生成的·OH最终对苯环进行开环,氧化污染物为CO2和H2O,完成布洛芬的矿化作用[29]。
-
1)电催化强化可显著提高对布洛芬的去除率,在30 min的反应过程中,布洛芬的去除率从高到低依次为O3 + H2O2强化电催化> O3强化电催化> H2O2强化电催化,其去除率分别达到93.2%、84.5%和52.7%,高于单独电催化时的去除率。
2)有机物和硬度的存在均降低了电催化强化对布洛芬的去除率,其中类腐殖酸和溶解性微生物副产物是限制布洛芬去除的主要原因。3种电催化强化体系可彻底去除分子质量为28 800 Da的污染物,O3+H2O2体系和O3体系对分子质量为2 900 Da的污染物的去除率分别为53.1%和41.2%,而H2O2强化电催化对该有机污染物并没有去除效果。
3)布洛芬在降解过程中产生了13种中间产物,反应过程中产生的·OH从不同位置攻击布洛芬,形成羟基化布洛芬异构体,然后羟基化的布洛芬进一步通过脱羧、脱甲基等一系列反应,最终将污染物氧化为CO2和H2O。
电催化强化对布洛芬的去除效果及机制
Removal effect and mechanism of ibuprofen by enhanced electrocatalysis
-
摘要: 对比研究了O3 + H2O2电催化、 O3电催化和H2O2电催化这3种电催化强化体系对水中难降解污染物布洛芬的去除效果,并且进一步阐明了布洛芬的降解机制。结果表明:经30 min反应后,3种电催化强化体系对布洛芬的去除率分别达到93.2%、84.5%和52.7%,均高于在单独电催化条件下的去除率(46.2%)。尺度排阻色谱-紫外分析结果表明3种电催化强化体系对大分子(28 800 Da)和小分子(2 900 Da)有机污染物均有较好的去除作用。腐殖酸的存在会显著降低布洛芬的去除率。荧光光谱分析结果表明,类腐殖酸和溶解性微生物副产物是限制布洛芬去除的主要原因。硬度离子的存在对布洛芬去除影响较大,在CaCO3的质量浓度为15 mg·L −1时,O3 + H2O2体系对水中布洛芬的去除率相比去离子水溶液时下降了26.6%。研究阐明了布洛芬的降解路径,布洛芬在降解过程中产生了13种中间产物,反应中通过脱羧、脱甲基等一系列反应,最终将污染物氧化为CO2和H2O。Abstract: In this study, the performance of O3+H2O2 enhanced electrocatalysis, O3 enhanced electrocatalysis and H2O2 enhanced electrocatalysis on ibuprofen removal from water was compared, and the degradation mechanism was analyzed. The results showed that after 30 min reaction, the removal rates of ibuprofen by these three types of enhanced electrocatalysis were 93.2%, 84.5% and 52.7%, respectively, which were higher than 46.2% by electrocatalysis alone. The three enhanced electrocatalysis systems had good removal effects on macromolecular (28 800 Da) and small molecular (2 900 Da) pollutants detected by size exclusion chromatography-UV. Humic acid in water significantly reduced the removal effect of ibuprofen. Fluorescence spectrum analysis showed that the humic acid like and soluble microbial by-products were the main reason for restricting ibuprofen removal. The hardness of water significantly reduced the removal efficiency of ibuprofen. The removal rate of ibuprofen by O3+H2O2 enhanced electrocatalysis decreased by 26.6% at the 15 mg·L−1 CaCO3 in comparison with that in deionized aqueous background solution. The degradation path of ibuprofen was clarified, 13 intermediate products were produced during the degradation process of ibuprofen. In the reaction, the ibuprofen was finally oxidized to CO2 and H2O through a series of reactions such as decarboxylation and demethylation.
-
Key words:
- electrocatalysis /
- ibuprofen /
- organic matters /
- degradation /
- molecular weight
-
近年来,药物和个人护理产品(pharmaceuticals and personal care products, PPCPs)在环境中的频繁出现引起了越来越多的关注。PPCPs可对水生生物和人类造成慢性毒性和内分泌紊乱,甚至引起致病菌耐药性的发生,对人体健康和生态系统构成潜在的严重威胁。布洛芬(ibuprofen, IBP)是目前世界上应用最多的一种非甾体抗炎药,也是在城镇污水处理厂二级出水中检出频率最高的一种PPCPs[1]。因此,探索从水环境中去除以布洛芬为代表的PPCPs技术已经成为环境领域的研究热点[2-3]。
目前, PPCPs的去除技术主要有生物技术[4-5]、臭氧氧化技术[6-8]、膜技术[9-11]、活性炭吸附技术[12-13]等。生物技术对PPCPs的降解效果并不稳定,这种现象与微生物的降解特性和PPCPs的结构有关[14]。膜技术与臭氧氧化技术对PPCPs的去除效果受PPCPs的种类和浓度影响[15]。活性炭可有效去除部分PPCPs,但对于极性化合物的去除效果有限,并且其他竞争物质的出现也会对活性炭的吸附能力产生影响[16]。
电催化强化技术在难降解废水的深度处理中表现出高效的污染物去除能力。CHEN等[17]通过O3+H2O2强化电催化体系对地表水中的氧氟沙星进行降解,发现O3单独和O3+H2O2强化电催化体系均可快速氧化氧氟沙星,与单独臭氧氧化(30%)相比,O3+H2O2体系显著提高了氧氟沙星的矿化率(55%)。BAVASSO等[18]采用O3强化电催化对利尿酮进行了去除,结果表明,在200 mA电流和酸性pH条件下,在2 h内可实现利尿酮的快速降解和矿化,这与羟基自由基的大量产生密不可分。SANTANA-MARTINEZ等[19]评价了H2O2强化电催化法的氧化效率,结果表明,在pH为3、电流密度为60 mA·cm−2、H2O2流速为4.7 L·min−1和电解质浓度为0.05 mol·L−1的条件下,苯酚的最高矿化率约为75%,其出水的原始毒性显著降低。综上所述,电催化强化对水体中的PPCPs类污染物具有高效的去除效率,然而电催化强化体系对布洛芬的深度去除效果和机制研究还很缺乏。因此,本研究设计了3种电催化强化处理体系,考察了H2O2浓度、O3投加量、腐殖酸等因素对IBP去除效果的影响,基于布洛芬的降解产物,阐明了IBP的降解机理。本研究以期为电催化强化体系去除以布洛芬为代表的PPCPs类污染物提供参考。
1. 实验材料和方法
1.1 试剂与材料
实验中所用到的布洛芬为分析纯,购于西格玛公司,实验中使用的其他试剂(如硫酸钠、磷酸氢二钠、硫酸、碳酸钙、过氧化氢等)均为分析纯购于国药公司,腐殖酸购买于西格玛公司,醋酸铵为色谱纯,购自于英国Alfa Aesar公司。实验中所需溶液均由密理博的高纯水系统产生的高纯水(阻抗18.2 MΩ)配制,实验中布洛芬溶液的质量浓度为10 mg·L−1。
1.2 实验装置
电催化及其强化的实验均在自行设计的玻璃容器中进行,容器有效容积为500 mL。实验中采用的阳极为钛镀钌铱电极,阴极为钛电极,电极间采用单极式连接方式,电极间距为4 mm,电极的有效面积为25 cm2(图1)。在处理中,每批次处理量为 200 mL,运行时间为0~30 min。电流由恒流恒压电源(大华,MC-100/5)控制,输出电压为0~50 V,输出电流为0~5 A,电流密度为30 mA·cm−2。所有实验均重复3次。对于电催化强化实验,则为在电催化反应基础上分别加入H2O2、O3和O3+H2O2,从而形成3种强化方式,单独电催化、H2O2强化电催化、O3强化电催化、O3+H2O2强化电催化以下分别简称为E0、E1,E2和E3。
1.3 分析方法
水样的荧光图谱采用Hitachi F-4600 (日本Hitachi公司)荧光色谱仪进行分析。分析仪激发光源为150 w氙弧灯,PMT电压为700 V,信噪比为110;激发波长为5 nm, 发射波长为5 nm; 响应时间设为自动,扫描速度为12 000 nm·min−1,激发波长为200~500 nm,发射波长为220~600 nm。布洛芬质量浓度通过Waters高效液相色谱仪测试,色谱柱为Agilent TC-C18,柱温为30 ℃,检测波长220 nm,流动相为75%甲醇和25%超纯水,流动相流速1 mL·min−1,进样体积50 μL。水样中有机物表观分子质量分布采用尺度排阻色谱-紫外分析仪测定(SEC-UV)。色谱柱采用 TSK-GEL G3000 PWxl型柱子(日本Tosoh Bioscience),流动相为1.2 g·L−1的磷酸二氢钠和2.5 g·L−1磷酸二氢钾的混合溶液,流速为1.0 mL·min−1。布洛芬在反应过程中的中间产物采用超高效液相色谱串联飞行时间质谱进行检测(UPLC/Q-TOF-MS)(UPLC,Ultimate 3200,Dionex,USA,micrOTOF III,Bruker,Germany),流动相为水(2 mmol·L−1醋酸铵)和乙腈,采用Waters Cortecs C18 色谱柱(1.7 μm,50 mm × 2.1 mm),流速为 0.4 mL· min−1,洗脱从10%的乙腈开始,持续2 min,然后在23 min内增加至60%的乙腈洗脱液,保持5 min。反应过程中产生的羟基自由基(·OH)采用Waters高效液相色谱仪测定,·OH的捕获剂为对-氯苯甲酸(0.5 μmol·L−1),色谱柱为Agilent TC-C18,检测波长为 254 nm,柱温为30 ℃;流动相为75%甲醇和25%超纯水,流速为1 mL· min−1。
2. 结果与讨论
2.1 电催化强化体系对布洛芬的去除
为考察H2O2电催化强化体系对布洛芬的去除影响,分别投加3、6、9、12、15和17 mmol·L−1的H2O2,反应30 min结束后,计算布洛芬的去除率。由图2可见,随着H2O2投加量的提高,布洛芬的去除率显著升高。在H2O2为3 mmol·L−1时,布洛芬的去除率为34.1%,其去除率低于单独电催化时的去除率(46.2%);而在在H2O2为17 mmol·L−1时,布洛芬的去除率为54.1%,升高了58.6%。此外,从布洛芬的去除率变化趋势可以看出,H2O2添加并不能持续有效提高布洛芬的去除率。这是因为布洛芬在H2O2投加量为12、15和17 mmol·L−1条件下的去除率基本保持不变。添加H2O2有利于·OH的生成,但其生成效率受多方面影响。反应过程中H2O2的添加有利于·OH的生成速率增加, H2O2在反应中易分解生成·OH(式(1)),但随着H2O2浓度的增加,·OH的生成速率也会受到影响。这是因为H2O2会抑制·OH的生成(式(2)~式(4))。所以过量的H2O2不仅会增加运行成本,也不能高效去除布洛芬。结果表明,在单独电催化中投加3 mmol·L−1 H2O2所获得的布洛芬去除率相比单独电催化的去除率低,可能因为H2O2浓度的增加抑制了· OH的生成,从而影响了布洛芬的降解。
H2O2+H++e−⇌⋅OH+H2O (1) H2O2+⋅OH→H2O+HO2⋅ (2) H2O2+2e−→2OH− (3) 2H2O2→O2+2H2O (4) 图3反映了在O3的质量浓度分别为5、10、20、30和40 mg·L−1、O3流量为0.8 mL·min−1,反应时间为30 min的条件下,O3电催化强化体系对布洛芬的去除效果。由图3可见,O3电催化强化体系对布洛芬去除效果较为显著。在O3加入量由5 mg·L−1增加到30 mg·L−1时,布洛芬的去除率由56%提高到84.5%。这是因为O3是氧的同素异形体,是一种较强的氧化剂,其氧化还原电位高达2.07 V,可通过断链、开环等一系列反应降解去除污染物;同时,在电催化过程中,O3的添加可促进反应过程中有效产生·OH,进而对布洛芬进行有效去除(式(5)~式(7))。
O2⋅−+O3→O2+O3⋅− (5) O3⋅−→O⋅−+O3 (6) O⋅−+H2O⇌⋅OH+OH− (7) 为了对比3种电催化强化体系对布洛芬的去除效果,分别考察了17 mmol·L−1 H2O2、30 mg·L−1 O3 (流量为0.8 mL·min−1)和17 mmol ·L−1H2O2+ 30 mg·L−1 O3 (流量为0.8 mL·min−1)3个体系对布洛芬的去除情况。如图4所示,随着反应时间的延长,布洛芬的去除率先快速上升,然后持续增加,3种强化方式在前10 min处理中的去除率上升较快,分别达到31.6% (E1)、45.1%(E2)和55.8%(E3)。在30 min后,对布洛芬的去除率顺序为为E3>E2>E1,其去除率分别为93.2%、84.5%和52.7%,高于电催化(E0)时46.2%的去除率。
图5所示的电催化及电催化强化体系中·OH的生成量,可以很好的解释上述布洛芬去除率的差异。由图5可知,E3在这几种电催化强化过程中的·OH生成量最高,并且其生成量随时间增加呈线性增长趋势,表明在该过程中·OH浓度基本保持稳定。其中,E0体系中·OH的生成量最低,在反应30 min后,·OH仅为0.13×10−9 mol·L−1。从电催化反应机理的角度来看,通常是通过电催化的电极材料表面直接氧化水中的有机污染物,同时电极材料通过电化学作用产生具有强氧化能力的自由基基团(羟基自由基(·OH)、超氧自由基(·O2)、H2O2等)间接氧化水中的有机污染物,最终达到降解去除污染物的目的。在O3和H2O2同时添加体系中,反应过程中H2O2与通入的 O3 发生反应(式(8))[20],可高效生成·OH (图5),对布洛芬进行深度降解。有研究[21]表明,O3 + H2O2强化电催化体系比单独的臭氧氧化和单独电催化体系对有机污染物具有更高效的去除效率。图4中E3对布洛芬的高效去除也进一步证实了这一点。
O3+H2O2→⋅OH+O2⋅−+H++O2 (8) 2.2 有机物对布洛芬去除影响
1)腐殖酸的质量浓度影响分析。污水处理厂的二级出水以及地表水环境中均包含大量的天然有机物质(natural organic matter, NOM),NOM不仅会影响电催化强化体系的催化效果,也会影响布洛芬的去除。以不同质量浓度的腐殖酸(humic acid, HA)为代表物质,考察HA对布洛芬去除效果的影响。电催化强化实验采用图4中的实验参数(17 mmol·L−1 H2O2、30 mg·L−1 O3 (流量为0.8 mL·min−1)和17 mmol ·L−1H2O2+ 30 mg·L−1 O3 (流量为0.8 mL·min−1)),反应时间为30 min。
由图6可见,HA的添加降低了电催化强化体系对布洛芬的去除效率。当HA的质量浓度为从1 mg·L−1增加到10 mg·L−1时,E1~E3中布洛芬的去除率均低于不添加HA的溶液,例如,E1在HA质量浓度为1 mg·L−1时的去除率为33.3%,而在10 mg·L−1质量浓度下的去除率为25.4%。这是由于反应过程中HA的添加会限制·OH的生成,减弱H2O2和O3的强化作用,从而抑制布洛芬的去除[22]。此外,布洛芬的去除率在HA质量浓度为10 mg·L−1时相比1 mg·L−1有显著的升高。这是因为HA会促进臭氧的自由基链式反应从而强化了布洛芬的去除[23]。布洛芬的去除受到以上2种因素共同作用。
2)分子质量分析。作为一个重要的水处理参数,有机污染物的分子量分布对研究有机污染物的特性以及布洛芬在电催化强化过程中的降解及去除机理具有重要作用。根据尺度排阻色谱-紫外分析结果,本研究的水样中主要含有2种分子质量的有机污染物。由图7可见,这2种有机物的保留时间分别为7.1 min和8.6 min,参照聚乙二醇和聚氧化乙烯标准物质的出峰时间,2种污染物的名义分子质量分别为28 800 Da和2 900 Da。结果表明,电催化强化体系对这2种有机污染物去除效果不同,其中单独电催化体系对2 900 Da的污染物没有去除作用,而对28 800Da的污染物可进行有效降解。在7.7 min 处有新的紫外吸收峰出现,所对应的化合物分子质量为11 150 Da。该结果表明,单独的电催化体系(E0)可使得28 800 Da的有机污染物发生部分降解,同时也产生了新的污染物。相比单独电催化(E0)的去除,电催化强化对这2种污染物均可有效去除。E3、E2和E1体系可完全去除28 800 Da的污染物,E3和E2对2 900 Da的污染物的去除率则为53.1%和41.2%,而E1对此污染物并没有去除作用。由此可见,电催化强化体系对大分子和小分子污染物均有较好的去除作用,并且E3的强化作用有利于小分子污染物的去除。该结果也进一步证明E3在3种电催化强化体系中的氧化作用最强,不仅能高效去除布洛芬(图4),对其他有机污染物也具有高效的降解;同时结合图7结果,该部分研究说明腐殖酸中分子质量为28 800 Da和2 900 Da的污染物降低了布洛芬的去除效率。
3)荧光光谱分析。荧光光谱分析对于研究布洛芬在电催化强化过程中的去除效果及降解机制具有重要作用。由图8可以发现,水样中主要包含2个特征荧光峰,对应的是溶解性微生物副产物和类腐殖酸化合物。这2种有机物也是限制布洛芬去除的主要原因。根据图中荧光强度的变化分析得出,溶解性微生物副产物和类腐殖酸化合物被有效去除,而且类腐殖酸化合物比溶解性微生物副产物更容易被去除。由图9可以看出,经30 min 处理后,E1、E2和 E3对类腐殖酸化合物的去除率分别为47.1%、69.2%和84.1%。这与氧化作用对有机污染物的选择性降解有直接的联系,在类似研究[24]中也证实了电化学氧化对类腐殖酸的去除率要高于溶解性微生物副产物。
2.3 硬度对布洛芬去除影响
硬度普遍存在于地表水和废水中,而过高的硬度会降低电催化体系对布洛芬的去除效率,并且增加运行成本[25]。硬度对电催化强化布洛芬去除率的影响结果如图10所示。硬度对布洛芬的去除具有显著的抑制作用,随着硬度的增加,3种电催化强化体系的去除率都受到影响。在没有硬度的干扰下,E3对布洛芬的去除率为93.2%,而在15 mg·L −1 CaCO3的质量浓度下,布洛芬的去除率为68.4%,下降了26.6%;而E2和E1的去除率也相应的下降了30.6%和40.2%。基于电催化过程的反应原理,水中的钙离子会在外加电场的作用下向阴极迁移,使得阴极溶液中碳酸钙的浓度达到过饱和,容易在极板附着形成沉淀,从而降低了反应的催化效率[26-27],最终导致布洛芬去除率的下降。
2.4 布洛芬的降解机理
采用高效液相色谱串联飞行时间质谱(UPLC/Q-TOF-MS)分析了电催化强化反应过程中布洛芬的降解物。由表1可知,布洛芬在降解过程中共产生了13种中间产物,其他研究也证实了这些降解产物是电催化降解过程中的主要产物[28]。根据以上的降解产物提出了布洛芬的降解路径(图11)。由于反应过程中存在大量的中间体以及反应机制的复杂性,因此,该降解路径分析仅提供可能的理论参考。首先,在电催化强化反应过程中产生的·OH在不同位置攻击布洛芬,最终形成羟基化布洛芬异构体。然后羟基化的布洛芬进一步通过脱羧、脱甲基等一系列反应,生成了不同的小分子降解产物,反应过程中生成的·OH最终对苯环进行开环,氧化污染物为CO2和H2O,完成布洛芬的矿化作用[29]。
表 1 高效液相色谱串联飞行时间质谱检测到布洛芬在反应过程的中间产物Table 1. Intermediate products of ibuprofen in the reaction process detected by high performance liquid chromatography-time-of-flight mass spectrometry序号 名称 化学式 m/z 保留时间/min 1 2-(3-羟基-4-异丁基苯基)丙酸 C13H18O3 222 2.5 2 2-羟基-2-(4-异丁基苯基)丙酸 C13H18O3 222 6.6 3 4-(1-羟乙基)苯甲醛 C9H10O2 150 13.9 4 2-(4-甲基苯基)丙酸 C9H8O3 164 8.2 5 1-(4-异丁基苯基)乙醇 C11H13O2 178 11.7 6 1-(4-(1-羟乙基)苯基)-2-甲基丙烷-1-酮 C12H16O2 192 2.7 7 4-(1-羟基-2-甲基丙基)苯乙酮 C12H16O2 192 14 8 1,2,4-苯三酚 C6H6O3 126 1.7 9 邻苯二酚 C6H6O2 110 2.1 10 4-乙基苯酚 C8H10O 122 2.4 11 对苯二酚 C6H6O2 110 1.8 12 4-乙基苯甲醛 C9H10O 134 3.5 13 1-乙基-4-(2-甲基丙基)苯 C12H18 162 3.9 3. 结论
1)电催化强化可显著提高对布洛芬的去除率,在30 min的反应过程中,布洛芬的去除率从高到低依次为O3 + H2O2强化电催化> O3强化电催化> H2O2强化电催化,其去除率分别达到93.2%、84.5%和52.7%,高于单独电催化时的去除率。
2)有机物和硬度的存在均降低了电催化强化对布洛芬的去除率,其中类腐殖酸和溶解性微生物副产物是限制布洛芬去除的主要原因。3种电催化强化体系可彻底去除分子质量为28 800 Da的污染物,O3+H2O2体系和O3体系对分子质量为2 900 Da的污染物的去除率分别为53.1%和41.2%,而H2O2强化电催化对该有机污染物并没有去除效果。
3)布洛芬在降解过程中产生了13种中间产物,反应过程中产生的·OH从不同位置攻击布洛芬,形成羟基化布洛芬异构体,然后羟基化的布洛芬进一步通过脱羧、脱甲基等一系列反应,最终将污染物氧化为CO2和H2O。
-
表 1 高效液相色谱串联飞行时间质谱检测到布洛芬在反应过程的中间产物
Table 1. Intermediate products of ibuprofen in the reaction process detected by high performance liquid chromatography-time-of-flight mass spectrometry
序号 名称 化学式 m/z 保留时间/min 1 2-(3-羟基-4-异丁基苯基)丙酸 C13H18O3 222 2.5 2 2-羟基-2-(4-异丁基苯基)丙酸 C13H18O3 222 6.6 3 4-(1-羟乙基)苯甲醛 C9H10O2 150 13.9 4 2-(4-甲基苯基)丙酸 C9H8O3 164 8.2 5 1-(4-异丁基苯基)乙醇 C11H13O2 178 11.7 6 1-(4-(1-羟乙基)苯基)-2-甲基丙烷-1-酮 C12H16O2 192 2.7 7 4-(1-羟基-2-甲基丙基)苯乙酮 C12H16O2 192 14 8 1,2,4-苯三酚 C6H6O3 126 1.7 9 邻苯二酚 C6H6O2 110 2.1 10 4-乙基苯酚 C8H10O 122 2.4 11 对苯二酚 C6H6O2 110 1.8 12 4-乙基苯甲醛 C9H10O 134 3.5 13 1-乙基-4-(2-甲基丙基)苯 C12H18 162 3.9 -
[1] FALAHI O, ABDULLAH S, HASAN H, et al. Simultaneous removal of ibuprofen, organic material, and nutrients from domestic wastewater through a pilot-scale vertical sub-surface flow constructed wetland with aeration system[J]. Journal of Water Process Engineering, 2021, 43: 102214. doi: 10.1016/j.jwpe.2021.102214 [2] OBA S, IGHALO J, ANIAGOR C, et al. Removal of ibuprofen from aqueous media by adsorption: A comprehensive review[J]. Science of the Total Environment, 2021, 780: 146608. doi: 10.1016/j.scitotenv.2021.146608 [3] KRISHNAN R, MANIKANDAN S, SUBBAIYA R, et al. Removal of emerging micropollutants originating from pharmaceuticals and personal care products (PPCPs) in water and wastewater by advanced oxidation processes: A review[J]. Environmental Technology & Innovation, 2021, 23: 101757. [4] GUO M, FENG Y, LI X, et al. Enhanced degradation of pharmaceuticals and personal care products (PPCPs) by three-dimensional electrocatalysis coupled biological aerated filter[J]. Journal of Environmental Chemical Engineering, 2021, 9(5): 106035. doi: 10.1016/j.jece.2021.106035 [5] LI J, HAN X, BRANDT B, et al. Physico-chemical and biological aspects of a serially connected lab-scale constructed wetland-stabilization tank-GAC slow sand filtration system during removal of selected PPCPs[J]. Chemical Engineering Journal, 2019, 369: 1109-1118. doi: 10.1016/j.cej.2019.03.105 [6] LEE C, HOWE K, THOMSON B. Ozone and biofiltration as an alternative to reverse osmosis for removing PPCPs and micropollutants from treated wastewater[J]. Water Research, 2012, 46(4): 1005-1014. doi: 10.1016/j.watres.2011.11.069 [7] ESPLUGAS S, BILA D, KRAUSE L, et al. Ozonation and advanced oxidation technologies to remove endocrine disrupting chemicals (EDCs) and pharmaceuticals and personal care products (PPCPs) in water effluents[J]. Journal of Hazardous Materials, 2007, 149(3): 631-642. doi: 10.1016/j.jhazmat.2007.07.073 [8] QUERO-PASTOR M, GARRIDO-PEREZ M, QUIROGA J. Ozonation of ibuprofen: A degradation and toxicity study[J]. Science of the Total Environment, 2014, 466-467: 957-964. doi: 10.1016/j.scitotenv.2013.07.067 [9] XU R, ZHANG P, WANG Q, et al. Influences of multi influent matrices on the retention of PPCPs by nanofiltration membranes[J]. Separation and Purification Technology, 2019, 212: 299-306. doi: 10.1016/j.seppur.2018.11.040 [10] ZHANG L, SHA J, SUN G, et al. Vacancy engineering and constructing built-in electric field in Z-scheme full-spectrum-Response 0D/3D BiOI/MoSe2 heterojunction modified PVDF membrane for PPCPs degradation and anti-biofouling[J]. Chemical Engineering Journal, 2021, 414: 128867. doi: 10.1016/j.cej.2021.128867 [11] YANG Y, OK Y, KIM K, et al. Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: A review[J]. Science of the Total Environment, 2017, 596-597: 303-320. doi: 10.1016/j.scitotenv.2017.04.102 [12] GERRITY D, SNYDER S. Review of Ozone for Water Reuse Applications: Toxicity, Regulations, and Trace Organic Contaminant Oxidation[J]. Ozone-Science & Engineering, 2011, 33(4): 253-266. [13] YI C, QIN W, WEN X. Renovated filter filled with poly-3-hydroxybutyrateco-hydroxyvalerate and granular activated carbon for simultaneous removal of nitrate and PPCPs from the secondary effluent[J]. Science of the Total Environment, 2020, 749: 141494. doi: 10.1016/j.scitotenv.2020.141494 [14] BOYD G R, ZHANG S Y, GRIMM D A. Naproxen removal from water by chlorination and biofilm processes[J]. Water Research, 2005, 39(4): 668-676. doi: 10.1016/j.watres.2004.11.013 [15] KUJAWSKA A, KIELKOWSKA U, ATISHA A, et al. Comparative analysis of separation methods used for the elimination of pharmaceuticals and personal care products (PPCPs) from water: A critical review[J]. Separation and Purification Technology, 2022, 290: 120797. doi: 10.1016/j.seppur.2022.120797 [16] VIENI N M, HARKKI H, TUHKANEN T, et al. Occurrence of pharmaceuticals in river water and their elimination a pilot-scale drinking water treatment plant[J]. Environmental Science & Technology, 2007, 41(14): 5077-5084. doi: 10.1021/es062720x [17] CHEN H, WANG J. Degradation and mineralization of ofloxacin by ozonation and peroxone (O3/H2O2) process[J]. Chemosphere, 2021, 269: 128775. doi: 10.1016/j.chemosphere.2020.128775 [18] BAVASSO I, MONTANARO D, PALMA L, et al. Electrochemically assisted decomposition of ozone for degradation and mineralization of Diuron[J]. Electrochimica Acta, 2020, 331: 135423. doi: 10.1016/j.electacta.2019.135423 [19] SANTANA-MARTINEZ G, ROA-MORALES G, GOMEZ-OLIVAN L, et al. Downflow bubble column electrochemical reactor (DBCER): In-situ production of H2O2 and O3 to conduct electroperoxone process[J]. Journal of Environmental Chemical Engineering, 2021, 9(4): 105148. doi: 10.1016/j.jece.2021.105148 [20] SLJUKIC B, BANKS C E, COMPTON R G. An overview of the electrochemical reduction of oxygen at carbon-based modified electrodes[J]. Journal of the Iranian Chemical Society, 2005, 2: 1-25. doi: 10.1007/BF03245775 [21] BAKHEET B, QIU C, YUAN S, et al. Inhibition of polymer formation in electrochemical degradation of p-nitrophenol by combining electrolysis with ozonation[J]. Chemical Engineering Journal, 2014, 252: 17-21. doi: 10.1016/j.cej.2014.04.103 [22] YONG E, LIN Y. Kinetics of natural organic matter as the initiator, promoter, and inhibitor and their influences on the removal of ibuprofen in ozonation[J]. Ozone-Science & Engineering, 2013, 35: 472-481. [23] LAJEUNESSE A, BLAIS M, BARBEAU B, et al. Ozone oxidation of antidepressants in wastewater treatment evaluation and characterization of new by-products by LC-Q-TOF-MS[J]. Chemistry Central Journal, 2013, 15: 7. [24] CHANG C, CHEN T, CHIN C, et al. Enhanced electrochemical degradation of ibuprofen in aqueous solution by PtRu alloy catalyst[J]. Chemosphere, 2017, 175: 76-84. doi: 10.1016/j.chemosphere.2017.02.021 [25] RAPHAEL R, ADISA A. Life cycle environmental impacts of advanced wastewater treatment techniques for removal of pharmaceuticals and personal care products (PPCPs)[J]. Journal of Environmental Management, 2018, 215: 258-272. [26] AMARASOORIYA A, KAWAKAMI T. Removal of fluoride, hardness and alkalinity from groundwater by electrolysis[J]. Groundwater for Sustainable Development, 2019, 9: 100231. doi: 10.1016/j.gsd.2019.100231 [27] 张霄磊. 电化学法在钢铁企业循环冷却水处理中的研究[J]. 给水排水, 2014, 50(1): 256-261. doi: 10.3969/j.issn.1002-8471.2014.z1.079 [28] SOLTANI R, MASHAYEKHI M. Decomposition of ibuprofen in water via an electrochemical process with nano-sized carbon black-coated carbon cloth as oxygen-permeable cathode integrated with ultrasound[J]. Chemosphere, 2018, 194: 471-480. doi: 10.1016/j.chemosphere.2017.12.033 [29] AMBULUDI S, PANIZZA M, OTURAN N, et al. Kinetic behavior of anti-inflammatory drug ibuprofen in aqueous medium during its degradation by electrochemical advanced oxidation[J]. Environmental Science and Pollution Research, 2013, 20(4): 2381-2389. doi: 10.1007/s11356-012-1123-6 -






 下载:
下载: