-
氧氟沙星(OFX)作为第3代氟喹诺酮类抗生素因其良好的抑菌性能和较少的不良反应被广泛用作人畜抑菌药[1-2]。近年来,由于OFX的广泛生产和过度滥用,大量含有OFX的废水未经处置排放到水环境中,对生态环境和人类健康构成严重威胁[3]。因此,开发高效先进的处理技术以将其从水环境中去除至关重要。迄今为止,处理含有OFX废水的主要方法包括生物降解法[4],膜分离法[5],吸附法[6]和高级氧化技术(AOPs)。生物降解法处理效果不显著且耗时慢,膜分离和吸附技术不仅无法彻底矿化污染物还会造成二次污染,限制了其实际应用。AOPs在反应过程中可以产生高效活性氧物种,能将大部分有机污染物矿化成CO2和H2O[7],被认为是降解水中OFX类抗生素的最有效方法之一。
Fenton技术作为AOPs中最有效的方法之一,可以通过Fe2+活化H2O2产生高活性·OH而迅速破坏难降解有机污染物的分子结构,从而使其得以高效去除[8]。然而传统均相Fenton技术存在pH适用范围窄、水中的铁离子难以回收和易产生铁泥的缺点[9]。近年来,异相类Fenton技术以固相铁基催化剂代替可溶性Fe2+离子,可以克服均相Fenton反应的弊端,被认为是一种更有效的处理技术。目前应用于异相类Fenton技术的铁基催化剂主要是单金属含铁化合物,比如零价铁(Fe0)[ 10-11]、赤铁矿(α-Fe2O3)[12]、磁赤铁矿(γ-Fe2O3)[13]、磁铁矿(Fe3O4)[14]、针铁矿(α-FeOOH)[15]、黄铁矿(FeS2)[16]和氧基氯化铁(FeOCl)[17]。单金属铁基化合物的催化活性低和稳定性差,不利于其大规模应用。而多金属固体催化剂因不同金属间的协同作用可以有效提高催化活性。常见的多金属固相催化剂可以分为以下4类:尖晶石型铁氧体(AB2O4),如铁酸铜(CuFe2O4) [18]、铁酸锰(MnFe2O4)[19]和钴酸锰(MnCo2O4)[20]等;铜铁矿型氧化物(ABO2),如CuFeO2[9]、CuMnO2[21]等;新型多金属材料如多金属氧酸盐(POMs)[22]和多金属有机框架材料(MOFs)[23]等;多金属复合催化剂,如双金属复合催化剂[24]、金属/金属氧化物复合催化剂[25]、非金属/金属氧化物材料[26]等。已有研究表明,CuFeO2结构中的Cu+活化H2O2产生·OH的氧化速率常数(1.0×104 mol−1·s−1)比Fe2+活化H2O2产生·OH的速率常数更快(63~76 mol−1·s−1),同时Fe3+被H2O2还原成Fe2+(4.6×102 mol−1·s−1)比Cu2+被H2O2还原为Cu+(0.001~0.01 mol−1·s−1)具有更快的反应速率[27]。此外,CuFeO2结构中的Fe3+可与Cu+金属之间产生协同作用,有助于加快Fe2+的生成,使催化剂呈现出较高的催化活性。上述分析可知,CuFeO2作为一种成本低、毒性小的双金属异相类Fenton催化剂在难降解有机污染物处理方面显示出良好的潜力。然而,采用溶胶-凝胶法制备CuFeO2过程中需使用大量有机溶剂,增加了制备成本。固相烧结法制备CuFeO2时所需温度一般为1 100~1 200 °C 及以上,能耗高且反应过程中会产生大量的杂质。
为了降低CuFeO2的制备能耗和减少杂质,本文采用低温水热法,以三水合硝酸铜和九水合硝酸铁分别作为铜源和铁源,成功制备了高纯度3R型CuFeO2催化剂,利用各种表征手段对其形貌及晶型结构进行分析。以所制3R型CuFeO2为异相催化剂,考察了其活化H2O2降解OFX的效能和机制。深入探讨了催化剂投加量、H2O2浓度、溶液pH、共存阴离子等参数对OFX降解效果的影响;研究了所制3R型CuFeO2异相催化剂活化H2O2降解OFX的循环稳定性;通过自由基捕获实验和电子自旋共振(ESR)光谱鉴定了CuFeO2/H2O2类Fenton体系降解OFX的主要活性物质,通过分析反应前后CuFeO2的X射线光电子能谱进一步揭示CuFeO2活化H2O2的机理,根据质谱法鉴定的降解中间物质,提出了可能的OFX降解路径。
-
准确称取3.06 g Cu(NO3)2·3H2O和6.06 g Fe(NO3)3·9H2O将其溶于30 mL去离子水中,室温下磁力搅拌10 min以获得Fe:Cu摩尔比1:1的黄绿色盐溶液,待其完全溶解后加入0.5 g无水葡萄糖充当还原剂,室温下继续磁力搅拌15 min,即配制完成所需A液。称取5 g NaOH溶于30 mL去离子水中,室温下磁力搅拌至完全溶解,将配制好的碱溶液缓慢加入到A液中,室温下剧烈搅拌30 min得到B液。将B液缓慢倒入不锈钢反应釜(100 mL)的白色内衬中,在180 ℃下保持24 h,待黑色产物冷却至室温后分别用去离子水和乙醇对其进行多次清洗,并于60 ~70 ℃下干燥,得到CuFeO2催化剂,并将其装袋备用。
-
采用D8 ADVANCE型X-射线多晶衍射仪(XRD)对所制备CuFeO2的晶型结构进行分析。采用Zeiss sigma 500型扫描电子显微镜(SEM)和能量色散X射线能谱仪(EDS)以及Tecnai G2 f20 s-twin型FEI透射电子显微镜(TEM)对所制备CuFeO2的微观形貌进行观察。以MgKα射线(1253.6 eV)作为激发源,采用Thermo Fisher Scientific公司所产ESCALAB 250型XPS仪器分析所制备CuFeO2元素组成、结构以及化合价态。
-
在常温条件下进行异相类Fenton催化降解OFX的实验,称取适量的CuFeO2置于含有100 mL浓度为10 mg·L−1 的OFX溶液的锥形瓶中,通过0.2 moL·L−1的HCl或NaOH溶液调节溶液pH。在降解反应开始前,将添加CuFeO2的降解溶液搅拌处理60 min,以达到CuFeO2催化剂和OFX之间的吸附-解吸平衡。随后,用移液枪向锥形瓶中加入一定体积的H2O2启动异相类Fenton反应,反应过程中在一定时间间隔内取样,并用0.45 µm的滤膜过滤,采用高效液相色谱(HPLC-Ultimate 3000)仪在294 nm波长下测定溶液中残留OFX的浓度;采用电子自旋共振波谱仪(ESR,FA200)检测降解过程中的活性自由基;采用Waters XEVO G2-S TOF型液相色谱-质谱联用仪(HPLC-MS)分析CuFeO2/H2O2异相类Fenton体系下OFX的降解产物。分别利用式(1)和式(2)计算OFX的降解效率和拟一级动力学反应速率常数。
式中:C0为OFX的初始质量浓度,mg·L−1;C为异相类Fenton降解时间为t时OFX的质量浓度,mg·L−1; t为降解时间,min;k为一级动力学反应速率常数,min−1。
-
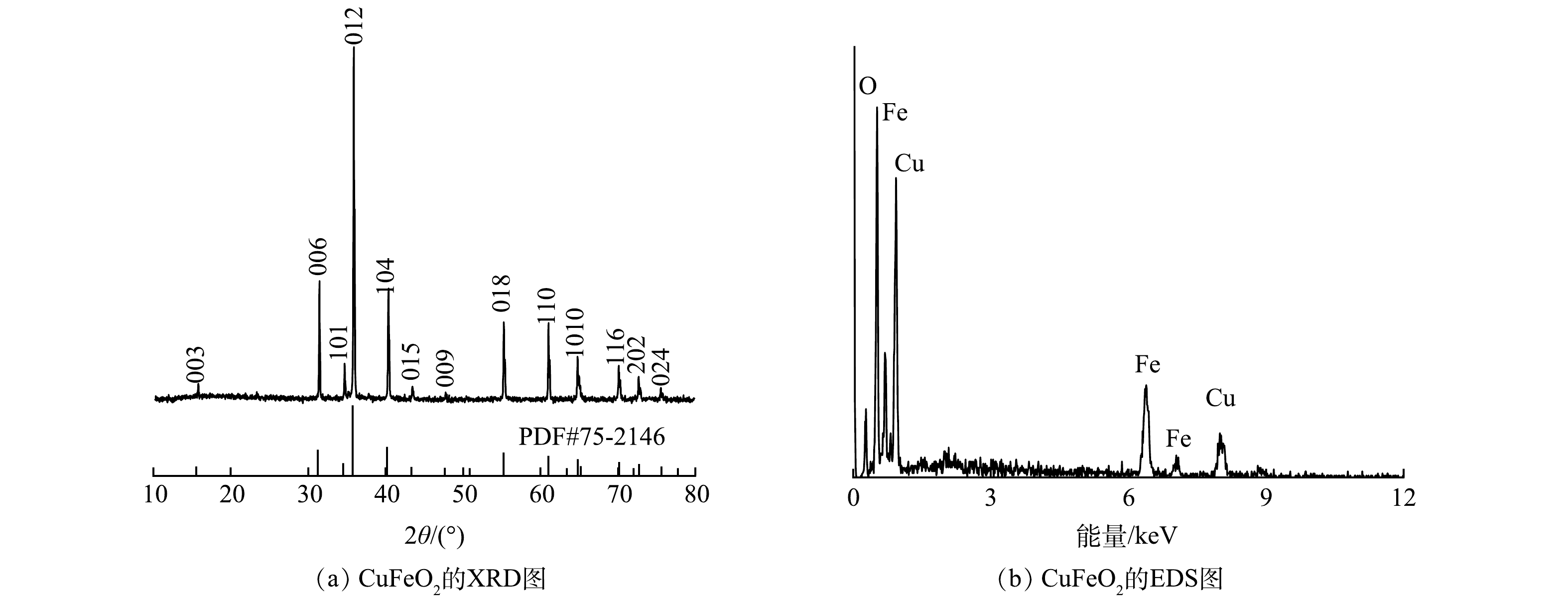
由图1(a)可见,CuFeO2在2θ为15.5°、31.1°、35.6°、40.2°、43.3°、47.6°、50.8°、55.2°、61.0°、64.8°、65.1°、70.1°、72.72°和75.6°处出现的特征峰与R-3m相CuFeO2(PDF#75-2416)在(003)、(006)、(101)、(012)、(104)、(015)、(009)、(107)、(018)、(110)、(1010)、(116)、(202)和(024)晶面上的布拉格反射有关。由图1(b)可见,CuFeO2中的Cu、Fe和O原子比大致接近1:1:2。这与其化学式中Cu、Fe和O的理论比值一致,进一步证实纯晶相CuFeO2的成功制备。由图2(a)可见,铜铁盐溶液经过碱处理和180°C水热反应后生成了斜方六面体结构的CuFeO2,其表面光滑均匀、棱角分明,粒径为2~5 μm,大小不一的斜方六面体结构CuFeO2颗粒之间发生团聚作用,形成较大的团簇物。TEM图(图2(b))也清晰展现了微米级CuFeO2的斜方六面体结构形态和团聚状态。由图2(c)可见,在CuFeO2的HRTEM谱图中晶格间距为0.287 nm,对应于CuFeO2的(006)晶面。CuFeO2的元素映射图证实了Cu、Fe和O均匀分布在催化剂表面(图2(d))。
-
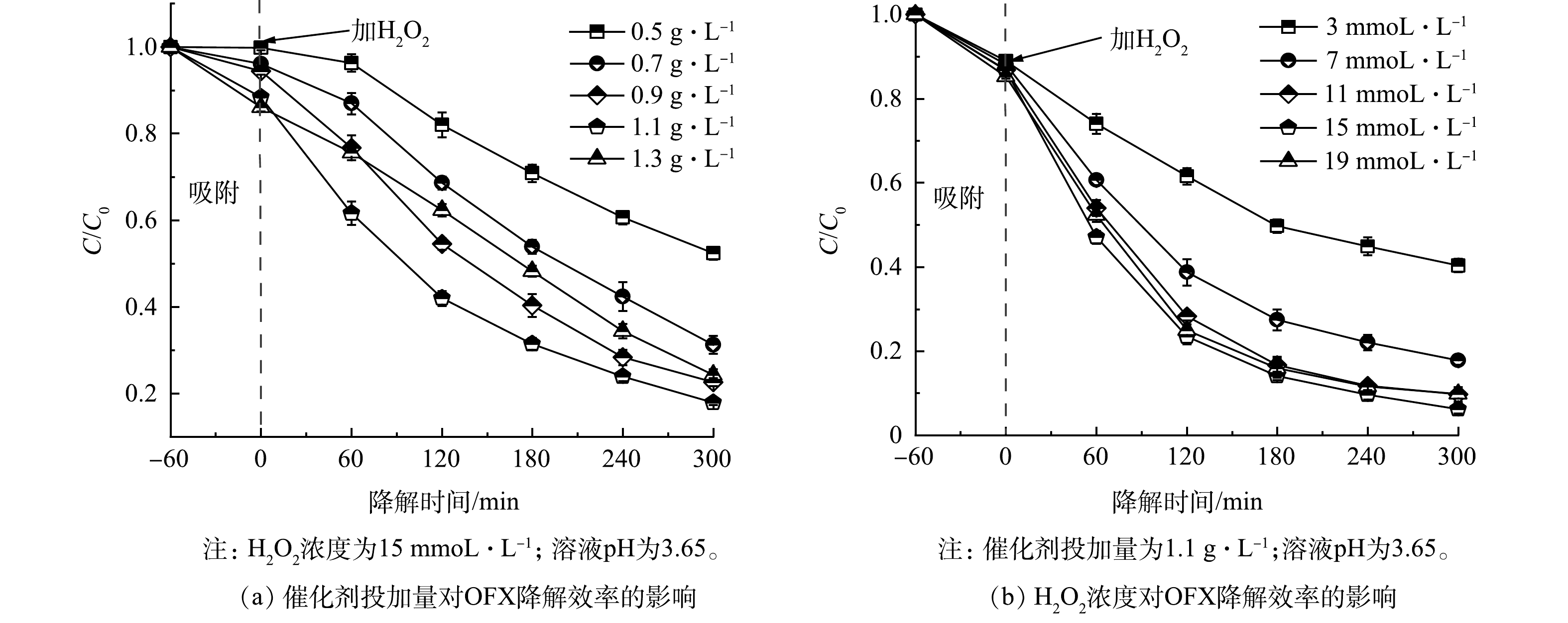
1)催化剂投加量的优化。在常温下以10 mg·L−1的OFX和15 mmoL·L−1的H2O2下考察了催化剂投加量对CuFeO2在异Fenton体系下降解OFX的影响,通过单因素实验找出降解性能最佳的催化剂投加量。如图3(a)所示,当纯3R型CuFeO2催化剂的投加量从0.5 g·L−1增加到1.1 g·L−1时,CuFeO2/H2O2类Fenton体系对OFX的降解率由47.6%增至82.1%,这是由于随着催化剂用量增多,吸附和催化H2O2的活性位点数目也随之增加,促使H2O2分解产生更多的活性自由基。然而,随着CuFeO2催化剂投加量进一步增加至1.3 g·L−1,OFX的去除率出现轻微下降,其降解率由82.1%变成了75.7%。尽管CuFeO2催化剂的投加量增加会使活性位点增加,但受到H2O2浓度的限制,使得过量的催化剂活性位点得不到较好地利用[28]。此外,过量CuFeO2催化剂的投加还会导致溶液中活性自由基的不良消耗,抑制了OFX的降解。因此,本研究选定1.1 g·L−1作为异相类Fenton催化降解OFX的最适宜CuFeO2催化剂投加量。
2) H2O2浓度的优化。在CuFeO2催化剂投加量为1.1 g·L−1时,考察了H2O2浓度对异相类Fenton催化降解OFX的影响。由图3(b)可见,当H2O2浓度从3 mmoL·L−1增加到15 mmoL·L−1时,OFX的降解率从59.6%增加至93.8%。这可能是H2O2浓度增加会产生更多的活性自由基,促进了OFX的降解。然而,随着H2O2浓度进一步增加到19 mmoL·L−1,OFX的降解率下降至90.2%。这说明在适当的范围内增加H2O2浓度可促进活性自由基的产生,然而过量的H2O2会通过式(3)和式(4)发生副反应,从而导致·OH的不良消耗,产生了比·OH的氧化能力更低的·O2H和·O2−[29],从而抑制了OFX的降解。因此,本研究选定为15 mmoL·L−1作为异相类Fenton催化降解OFX的最佳H2O2浓度。
-
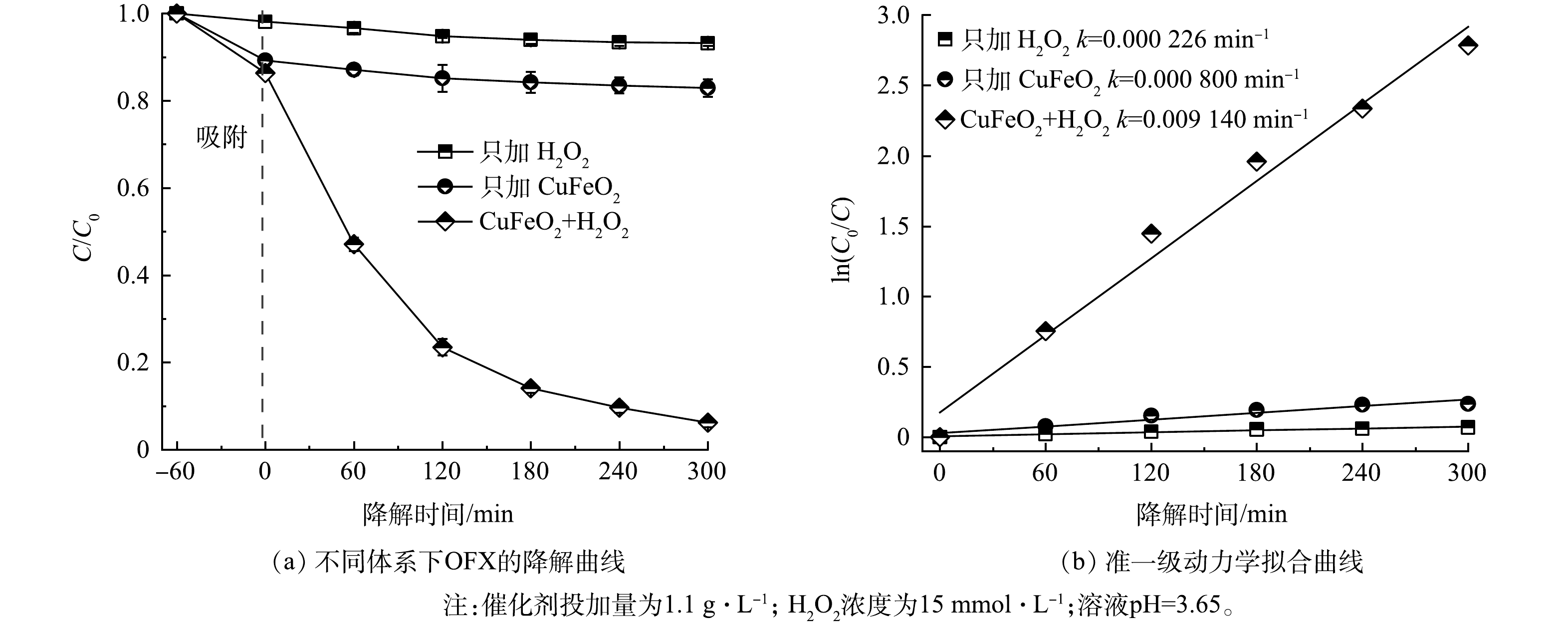
为了评价CuFeO2对H2O2的活化性能,在最佳降解条件下研究了不同体系对OFX的降解效果并对相应的动力学进行了分析。由图4(a)可见,在未调节降解溶液初始pH的条件下,反应300 min后,15 mmoL·L−1的H2O2对10 mg·L−1的OFX氧化去除效率仅为6.6%,主要是由于H2O2具有较低的氧化还原电位(E0 =1.77 eV)对有机污染物的降解效率有限[29]。CuFeO2催化剂对OFX的吸附去除结果表明,反应60 min后,CuFeO2对OFX的吸附达到饱和,随着吸附时间的进一步增加,水中OFX的浓度几乎保持不变,CuFeO2对OFX的去除率仅为21.4%。这主要是因为CuFeO2固有的磁性使其易团聚,减少了CuFeO2颗粒与OFX分子在水中接触的概率,限制了CuFeO2对OFX的吸附效果。当H2O2和CuFeO2共存时,OFX的降解率增加到93.8%,表明CuFeO2能够有效活化H2O2产生自由基,促进了OFX的降解。通过准一级动力学方程进一步分析OFX的降解过程。由图4(b)可见,CuFeO2催化剂活化H2O2降解OFX的反应速率常数(0.009 14 min−1)分别是H2O2氧化(0.000 226 min−1)和CuFeO2吸附(0.000 800 min−1)的40.4倍和11.4倍。此外,CuFeO2催化剂活化H2O2降解OFX的效率优于文献报道的其他铁基氧化物(MnFe2O4[30]和Fe-Cu@MPSi[31]),进一步说明CuFeO2可以作为一种有效的异相类Fenton催化剂活化H2O2降解有机污染物。
-
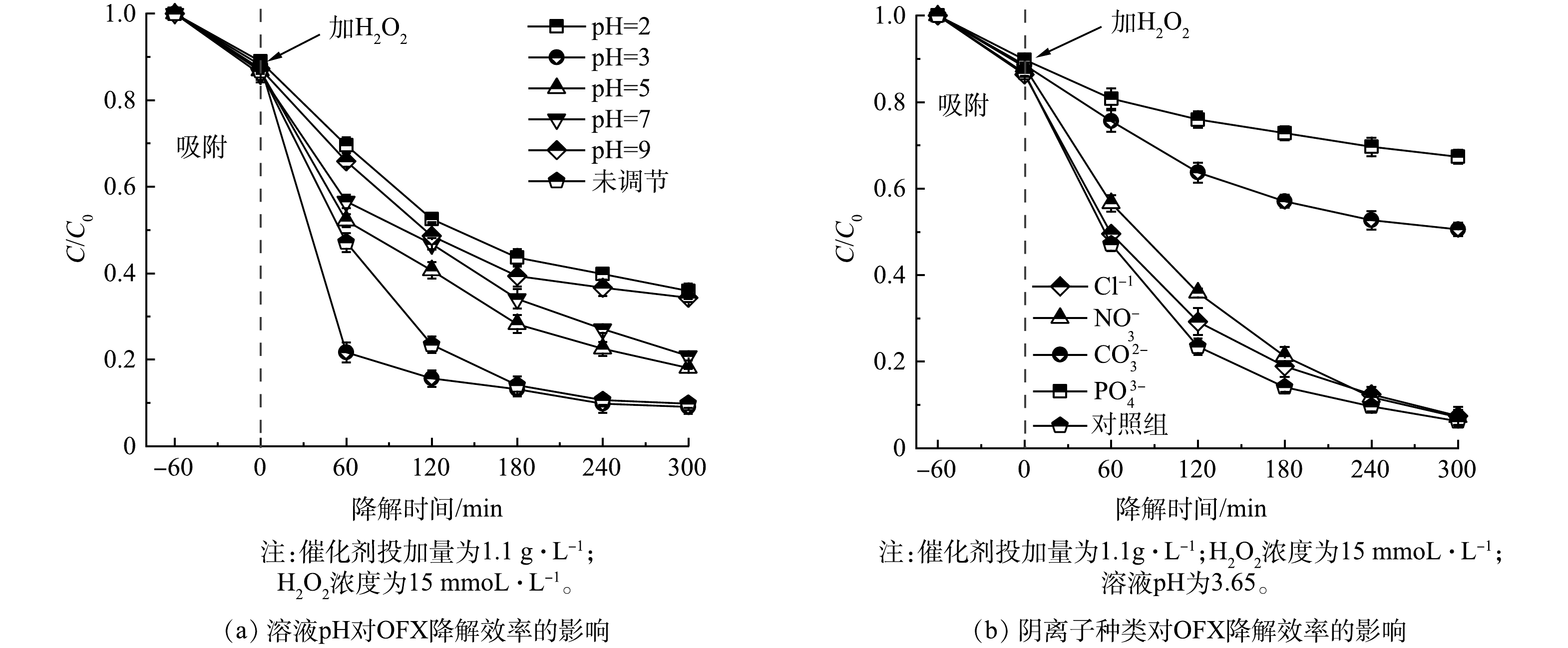
1)溶液pH的影响。本研究在CuFeO2添加量为1.1 g·L−1和H2O2浓度为15 mmoL·L−1条件下,研究了初始pH对CuFeO2/H2O2类Fenton体系降解OFX的影响。由图5(a)可见,在未调节溶液初始pH(pH=3.65)时,反应300 min后,CuFeO2活化H2O2对OFX的降解效率高达93.8%。相较于初始溶液pH=3.65,当溶液pH调节至3时,降解60 min后,OFX的降解效率明显增加,主要是在溶液pH为3时,溶液中含有足够的H+,可以促进CuFeO2对H2O2的活化,使得短时间内产生大量可以降解OFX的活性自由基。随着反应时间的进行,溶液中产生大量中间产物会与OFX发生竞争反应,导致OFX降解效率没有明显增加,降解反应300 min后,OFX的降解率为94.1%,这与pH=3.65时的降解率基本一致。随着溶液pH降低至2时,OFX的降解率仅为64.6%。这主要是因为溶液中过多的H+可通过式(5)将H2O2转化成更加稳定的H3O2+,不利于CuFeO2对H2O2的活化。此外,过量的H+会通过式(6)导致活性自由基的不良消耗,进一步抑制了OFX的降解。当溶液初始pH增加至5、7和9时,OFX的降解率分别为81.1%、79.9%和65.7%。主要是因为随着溶液pH的增加溶液中OH−会加速H2O2和活性自由基的不良消耗,从而降低OFX的降解率[32]。由上述研究结果可知,在强酸、中性和碱性条件下,CuFeO2活化H2O2可抑制对OFX的降解,尽管溶液pH为3时比未调节pH时OFX的降解效率会有所提升,但在调节溶液pH过程中需要添加大量的酸,不仅增加的处理成本,还存在二次污染。因此,采用CuFeO2作为异相催化剂活化H2O2降解OFX时,没有必要调节溶液pH。
2)溶液中阴离子的影响。含有OFX的实际水环境中通常存在一些无机阴离子,可能会对OFX的降解过程产生干扰作用。因此,本研究评价了水中无机阴离子(Cl−、NO3−、PO43-和CO32-)对CuFeO2活化H2O2降解OFX的影响,实验过程中无机阴离子的浓度均为1 mmoL·L−1。由图5(b)可见,与不加阴离子的对照组相比,尽管Cl−的添加可以捕获·OH生成·ClOH−(式(7)),但·ClOH−可通过式(8)快速解离成·OH,因此,溶液中Cl−对OFX的降解没有明显影响。此外,·OH和NO3−之间的反应速率常数较慢,NO3−很难捕获体系内的·OH导致NO3−的添加不会影响OFX的降解。然而,当溶液中加入CO32−后,OFX的降解效率从对照组的93.8%明显降低至49.4%,这主要是因为CO32-可通过式(9)捕获溶液中·OH产生氧化能力较弱的·CO3−。当溶液中加入PO43-后,OFX的降解效率抑制效果最明显,降解效率仅为39.6%,主要是因为PO43-不仅可以将溶液中的·OH转化成氧化能力较弱的·PO43-(式(10)),还能与催化剂表面金属离子发生络合反应占据活性位点,不利于H2O2的活化,进而降低了OFX的降解效率[1]。
-
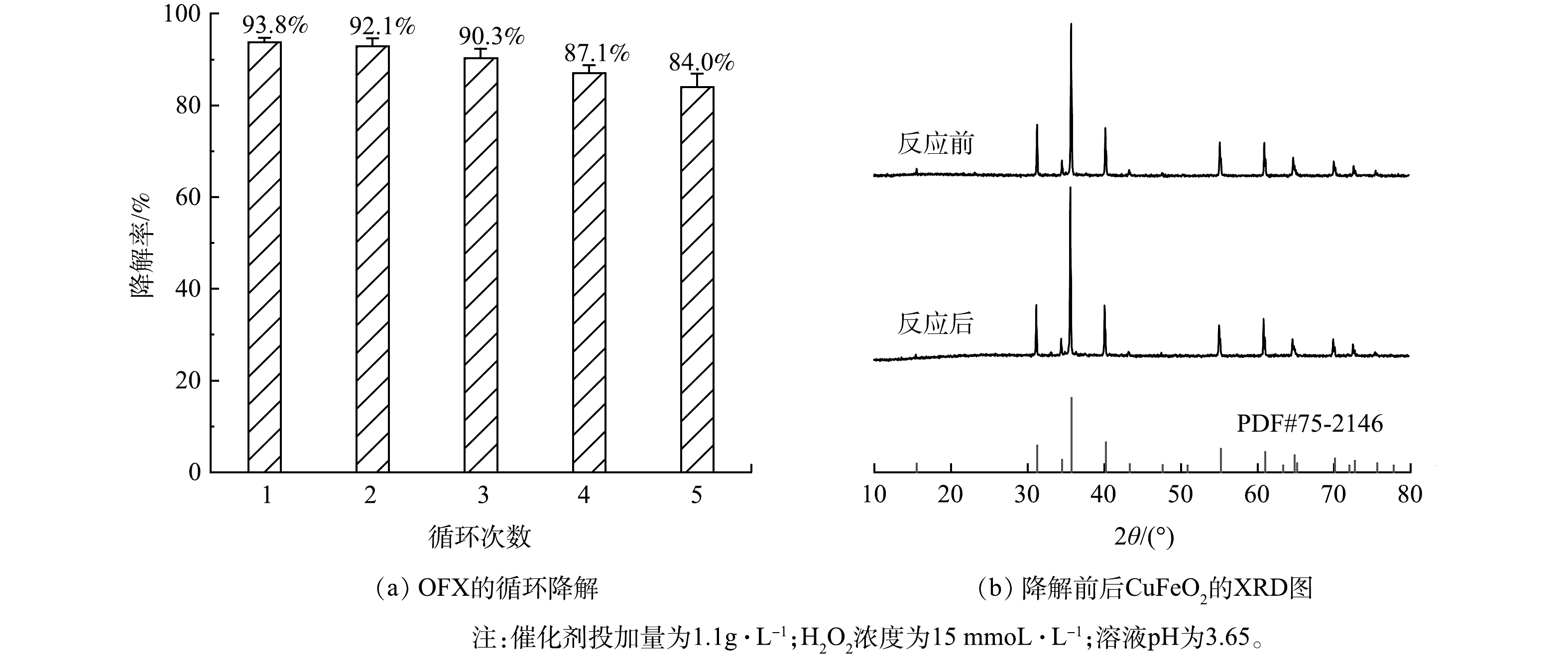
异相催化剂的稳定性是评估其在实际应用过程中的重要指标。本研究在CuFeO2添加量为1.1 g·L−1,H2O2浓度为15 mmoL·L−1和未调节溶液pH的条件进行了连续5次异相类Fenton催化降解OFX的实验,评价CuFeO2活化H2O2的稳定性。每次完成降解实验后,经抽滤、洗涤和烘干回收CuFeO2催化剂用于后续降解实验。由图6(a)可见,经过1 500 min的5次连续循环降解实验, OFX的降解率从最初的93.8%降低至84.0%。表明CuFeO2作为异相催化剂在活化H2O2过程中具有良好的稳定性。为了进一步探讨CuFeO2催化剂的稳定性,通过测定反应前后CuFeO2的XRD谱图对降解前后催化剂的晶型结构进行分析。由图6(b)可见,经5次连续循环降解实验,在CuFeO2的XRD谱图中的特征峰几乎没有发生变化,表明在异相Fenton反应过程中,CuFeO2仍能保持原有晶型结构。结果表明,CuFeO2作为异相催化剂在长期活化H2O2降解OFX的实验中可以保持较高的催化活性,对其实际应用具有重要的意义。
-
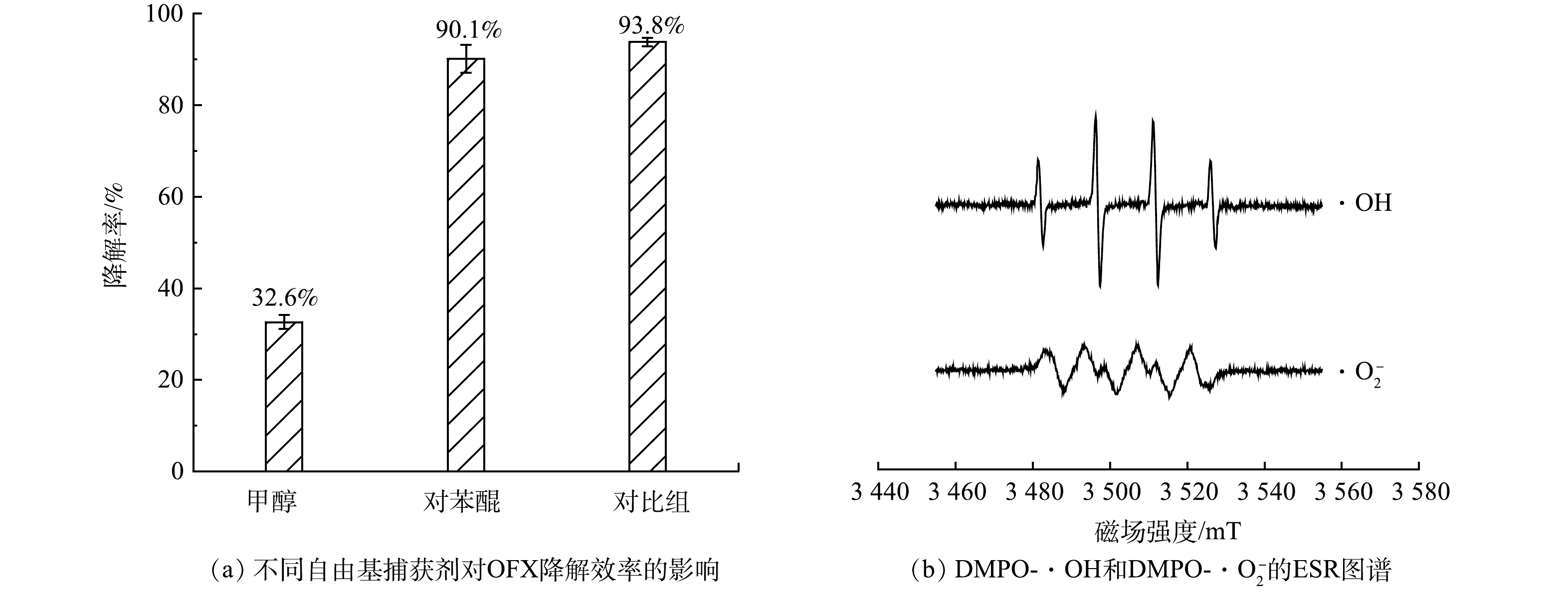
1)反应体系中活性自由基的鉴定。为了鉴定CuFeO2活化H2O2降解OFX的主要活性自由基,本研究分别采用浓度为50 mmoL·L−1的甲醇和对苯醌作为·OH和·O2−的捕获剂。由图7(a)可见,与不加捕获剂的对照组相比,甲醇的添加明显抑制了OFX的降解,而对苯醌的添加对OFX降解没有明显影响。当添加甲醇后,反应300 min后,OFX的降解效率由对照组的93.8%下降到61.2%,而对苯醌添加后OFX的降解效率仅下降了2.7%,表明·OH是异相类Fenton催化降解OFX的主要活性自由基。为了进一步探究CuFeO2/H2O2体系声称活性自由基的情况,以二甲基吡啶N-氧化物(DMPO)为捕获剂分别在水溶液和甲醇溶液中测定CuFeO2活化H2O2产生的活性自由基。ESR谱图(图7(b))显示在CuFeO2/H2O2体系中可以检测到了强度比为1:2:2:1的DMPO-·OH加合物的特征峰和强度比为1∶1∶1∶1的DMPO-·O2加合物的特征峰,表明CuFeO2活化H2O2可以生成·OH和·O2−。然而在相同反应时间内,DMPO-·OH特征峰的强度比DMPO-·O2特征峰强度大,表明CuFeO2活化H2O2产生的·OH是主要活性自由基,这与自由基捕获实验的结果一致。
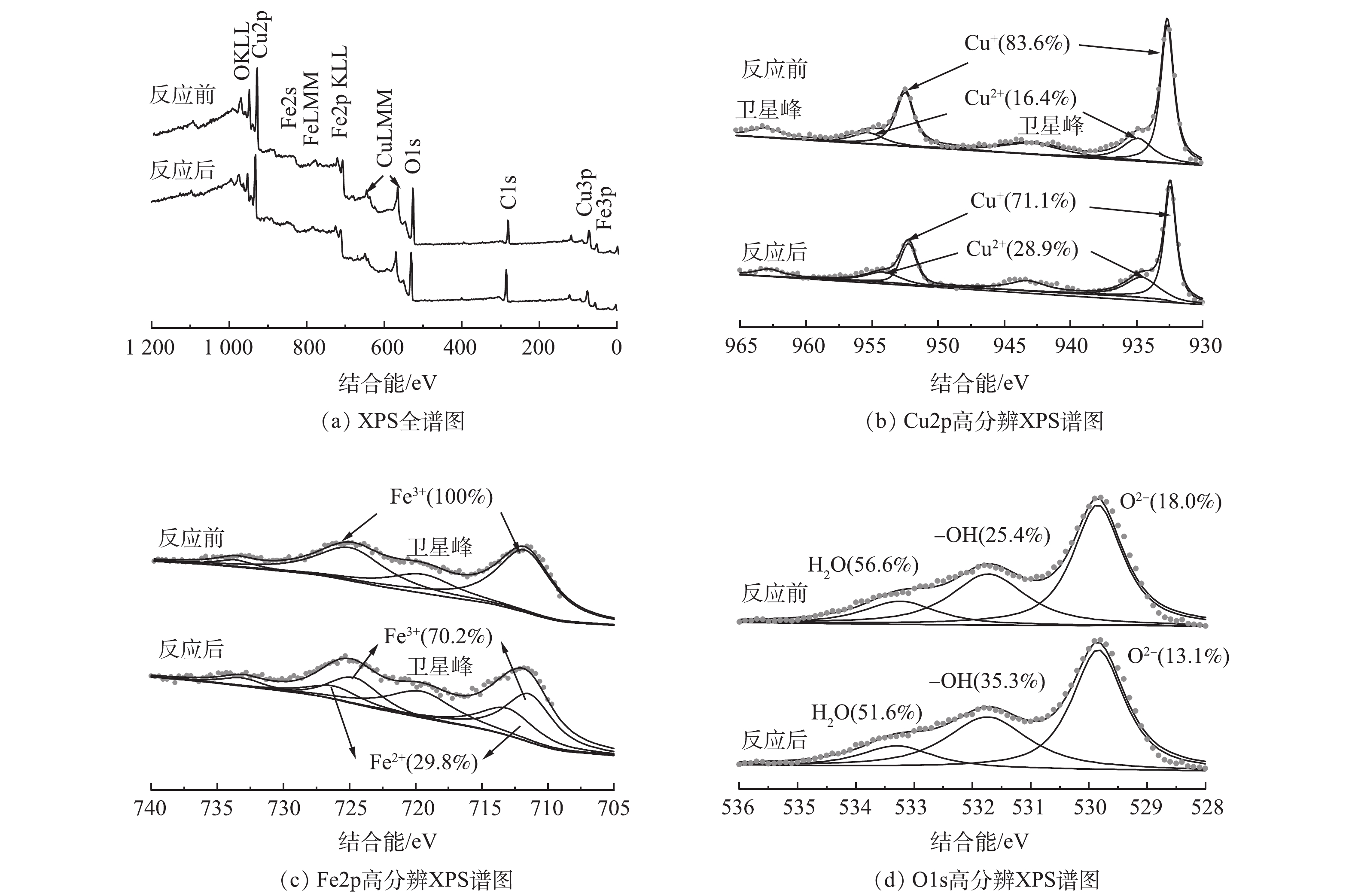
2)反应活性位点鉴定。为了进一步探讨CuFeO2活化H2O2生成活性自由基的反应位点,本文采用XPS测试技术对降解前后CuFeO2的表面元素价态组成以及各元素相对含量进行了分析。由图8(a)可知,反应前后CuFeO2催化剂的XPS总谱图特征峰无明显变化,表明经异相类Fenton反应后,CuFeO2表面元素组成未发生明显变化,均由C,O,Cu,Fe元素组成,进一步证明了CuFeO2的稳定性。其中CuFeO2表面的C元素可能是催化剂在测试过程中表面吸附的CO2。由图8(b)可见,Cu2p的高分辨率XPS谱显示出5个峰,其中在结合能为932.4 eV和952.3 eV处出现的强峰以及943.7 eV处的卫星峰均属于Cu+。然而在结合能为954.5 eV和934.9 eV出现的属于Cu2+的弱特征峰可能是因为水热过程中Cu+被少量氧化导致的。经降解反应后CuFeO2内Cu+的相对含量由83.6%降至77.1%,同时Cu2+的相对含量从16.4%增至28.9%,表明在异相类Fenton过程中Cu+可以被氧化成Cu2+。Fe2p高分辨XPS谱图中显示在结合能为724.9 eV和711.7 eV的强特征峰以及在结合能为719.5 eV和733.6 eV处的卫星峰均属于Fe3+,经降解反应后Fe2p高分辨XPS谱谱图出现两个属于Fe2+的新峰(图8(c))。经降解反应前后在CuFeO2 内Fe3+的相对含量由100.0%降至70.2%,相应的Fe2+相对含量增至29.8%,表明反应过程中Fe3+可以被还原成Fe2+。Cu2p和Fe2p高分辨XPS谱图结果表明CuFeO2表面的Cu和Fe是其活化H2O2的主要反应活性位点。在O1s高分辨XPS谱图中(图8(d))可以观察到结合能在533.4、531.4和529.7 eV的3个特征峰,分别归属于催化剂表面吸附的H2O,表面羟基(-OH)和CuFeO2结构中的晶格氧(O2-)。经降解反应后,催化剂表面-OH由25.4%增至35.3%,而O2-和吸附的H2O相对含量分别由56.6%和18.0%降至51.6%和13.1%。表面-OH含量的增加可能因为Cu和Fe可以作为Lewis 位点与表面-OH结合形成Cu-OH和Fe-OH质子酸位点,有助于活化H2O2产生活性基团[28],而O2-含量的降低可能是因为其可能参与了催化剂表面金属的氧化还原反应变成了O2。
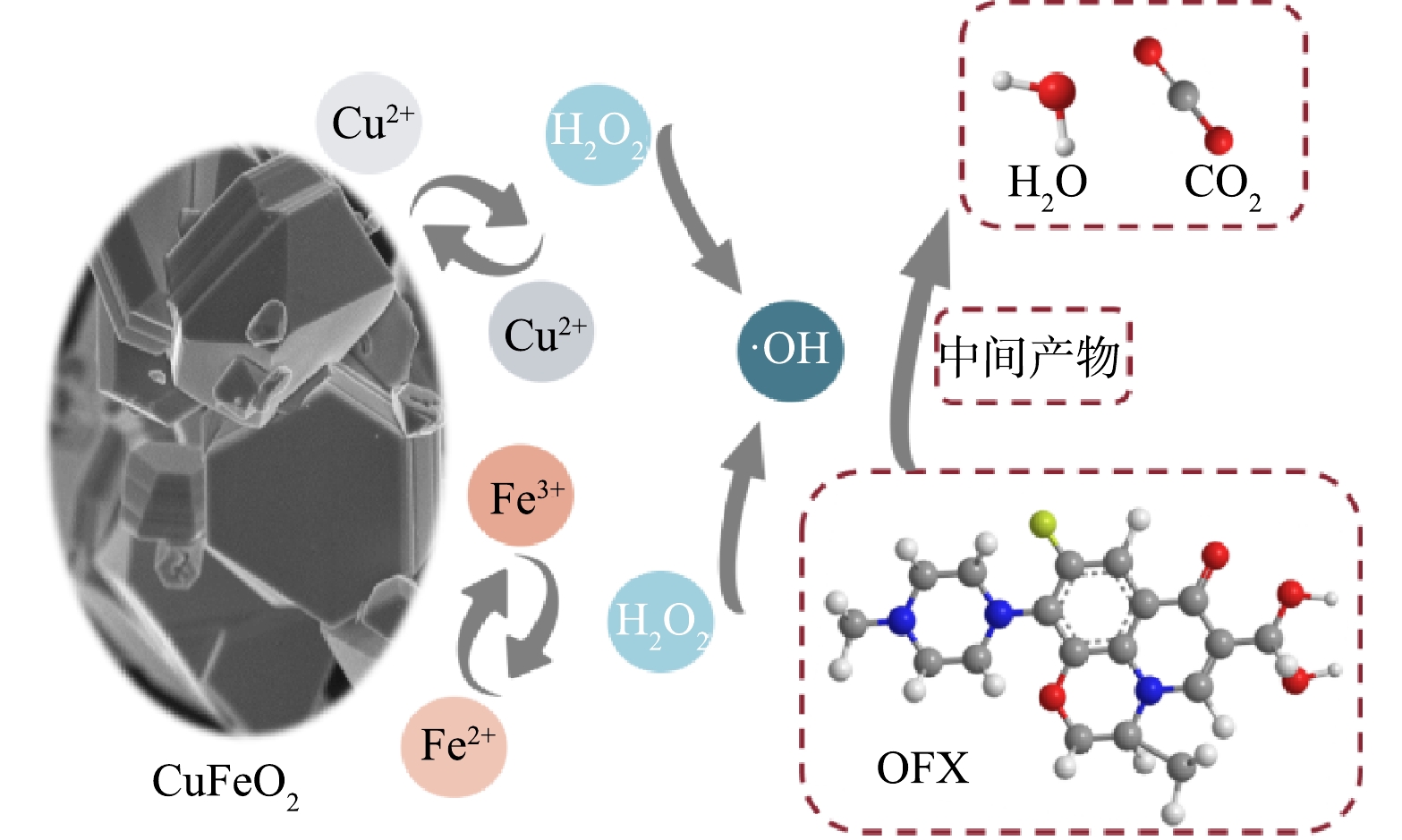
3)反应机理推测。基于自由基捕获实验、ESR分析和CuFeO2反应前后的XPS谱图,提出了CuFeO2活化H2O2降解OFX的可能机理(图9),该反应过程主要发生在CuFeO2表面的Cu和Fe活性位点上。当CuFeO2添加到水中后,其表面的Cu和Fe作为Lewis位点通过式(11)反应生成≡Cu+/Cu2+/Fe3+-OH络合物,其中≡Cu+-OH可以通过式(12)与H2O2反应生成·OH和≡Cu2+-OH,生成的≡Cu2+-OH可经式(13)被H2O2还原成≡Cu+-OH并生成·O2−。类似地,CuFeO2表面的≡Fe3+-OH在反应过程中可以与H2O2反应生成≡Fe2+-OH并生成·O2−(式(14))。此外,≡Fe3+-OH还可以经式(15)被表面O2-被还原成≡Fe2+-OH,这是导致反应后CuFeO2表面O2-含量降低的主要原因。随后,CuFeO2催化剂表面形成的≡Fe2+-OH可进一步活化H2O2生成·OH和≡Fe3+-OH(式(16))。由于Cu2+/Cu+的氧化还原电位(0.17 V)低于 Fe3+/Fe2+的氧化还原电位(0.77 V),因此,CuFeO2表面的≡Cu+-OH可以通过式(17)将≡Fe3+-OH还原成≡Fe2+-OH。通过催化剂表面的≡Cu+-OH/≡Cu2+-OH和≡Fe2+-OH/≡Fe3+-OH的氧化还原循环可以促进活化H2O2生成活性自由基。最后形成的活性自由基可以将OFX降解成中间产物、CO2和H2O(式(18))。
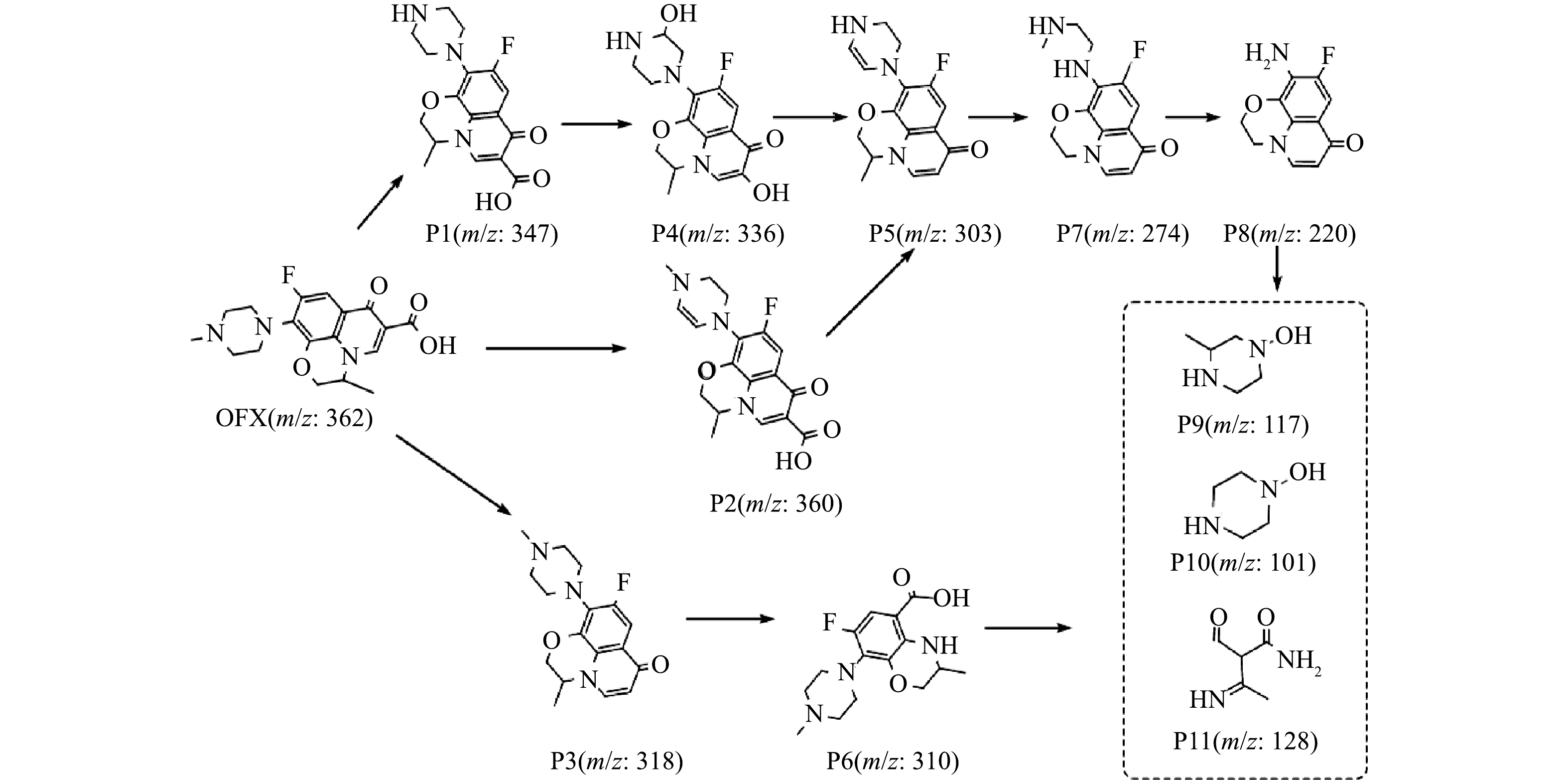
4)反应中的降解产物及降解途径。为阐明OFX在CuFeO2/H2O2体系内的降解路径,通过液相色谱-质谱联用仪(LC-MS)测定了降解的过程中生成的中间产物。由图10可见,由于OFX分子(m/z=362)中C-N的键能较低,在活性自由基的攻击下,可通过脱除甲基生成中间产物P1(m/z=347),生成的P1进一步通过脱除羧基,然后经羟基化反应产生P4(m/z=336)。此外,OFX分子可以通过脱除2个氢产生C=C键形成中间产物P2(m/z=360)。形成的P2和P4可分别通过水解反应和脱水反应生成中间产物P5(m/z=303)。由于哌嗪环具有较高的电子密度很容易受到·OH的攻击,从而使得P5通过哌嗪环不同程度地裂解得到中间产物P7(m/z=274)和中间产物P8(m/z=220)[33]。OFX分子还可以通过脱除羧基形成中间产物P3(m/z=318),随后通过C=C键裂解,脱烷基和羟基化反应进一步生成中间产物P6(m/z=310)。生成的P6和P8在活性自由基的攻击下进一步裂解为更小分子的P9(m/z=117)、P10(m/z=101)和P11(m/z=128)。生成的部分中间产物经进一步异相类Fenton催化降解反应转化成CO2、H2O和无机离子。
-
1)以三水合硝酸铜和九水合硝酸铁分别作为铜源和铁源,通过水热法成功制备了具有3R相和斜方六面体结构的CuFeO2催化剂,其表面光滑均匀、棱角分明,粒径为2~5 μm,且CuFeO2颗粒团聚成较大的团簇物。
2)当催化剂投加量为1.1 g·L−1、H2O2浓度为15 mmoL·L−1以及溶液pH为3.65时;CuFeO2催化剂活化H2O2降解OFX的速率常数分别是H2O2氧化和CuFeO2吸附的40.4倍和11.4倍;体系中Cl−和NO3−的添加对OFX降解无明显影响,而PO43−和CO32-的添加可抑制的OFX降解。
3)经5次连续降解后,CuFeO2/H2O2体系对OFX的降解率仅降低了8.1%;降解反应后CuFeO2仍能保持原有的晶型结构,表明CuFeO2活化H2O2过程中具有良好的稳定性。
4) CuFeO2/H2O2体系中有·OH和·O2−自由基,以·OH为主要活性自由基;降解反应前后的XPS谱图揭示了CuFeO2中的Fe和Cu双活性位点在异相类Fenton反应中起着重要的作用,其通过加速Fe3+/Fe2+和Cu+/Cu2+的氧化还原循环,促进活性自由基的生成,有利于OFX的降解。
异相类Fenton催化剂CuFeO2的制备及其对水中氧氟沙星的降解性能
Preparation of CuFeO2 as heterogeneous Fenton-like catalyst and its degradation performance on ofloxacin in the water
-
摘要: 以三水合硝酸铜、九水合硝酸铁分别作为铜源和铁源,通过简单的一步水热法合成纯3R相结构CuFeO2催化剂。通过X射线衍射仪、扫描电镜、透射电镜和高分辨透射电镜对其晶格结构和形貌进行了表征,构建了CuFeO2/H2O2体系对目标污染物氧氟沙星(OFX)进行降解,考察了不同催化剂投加量、H2O2浓度、溶液pH等参数对OFX降解效果的影响。结果表明,在催化剂投加量为1.1 g ·L−1、H2O2浓度为15 moL·L−1以及pH=3.65的条件下,反应300 min后,CuFeO2/H2O2体系对10 mg·L−1 OFX的降解率高达93.8%;此外,CuFeO2催化剂在活化H2O2降解OFX过程中具有良好的稳定性,经5次降解后的降解率仅降低了8.1%;自由基捕获实验和电子自旋共振波谱测试结果表明CuFeO2/H2O2体系中·OH是主要的活性自由基;降解反应前后CuFeO2的X射线光电子能谱表征结果表明,Cu+和Fe3+的相对含量均有不同程度的降低,表明CuFeO2中的Fe和Cu双活性位点在异相类Fenton反应中均起着重要的作用。最后,通过质谱鉴定了OFX的降解产物,并在此基础上提出了可能的降解路径。Abstract: The CuFeO2 catalyst with pure 3R phase structure was synthesized via a simple one-step hydrothermal method using copper nitrate trihydrate and iron nitrate as copper and iron sources, respectively. The lattice structure and morphology were characterized by X-ray diffractometer, scanning electron microscopy, transmission electron microscopy and high-resolution transmission electron microscopy. The CuFeO2/H2O2 system was constructed for the degradation of ofloxacin (OFX) as the target pollutant. To explore the optimum operating parameters, the effects of catalyst dosage, H2O2 concentrations, solution pH values and coexistence ion on OFX degradation were discussed. The results showed that the degradation efficiency of OFX (10 mg·L−1) by CuFeO2/H2O2 system was 93.8% after 300 min at catalyst dosage of 1.1 g·L−1, H2O2 concentration of 15 moL·L−1 and pH=3.65. In addition, the prepared CuFeO2 catalyst exhibited good stability, and the degradation efficiency after five successive degradation cycles only decreased by 8.1%. Based on the free radical scavenging experiments and electron spin resonance spectrometer results, ·OH was the major active free radicals in CuFeO2/H2O2 system for OFX degradation. The X-ray Photoelectron Spectrometer spectra of CuFeO2 before and after the degradation reaction revealed that the relative contents of Cu+ and Fe3+ decreased in varying degrees, indicating that the Fe and Cu as the double catalytic active sites in CuFeO2 played an important role in the heterogeneous Fenton-like reaction. A possible degradation pathway of OFX was proposed based on the intermediates identified by HPLC-MS spectra.
-
Key words:
- heterogeneous catalyst /
- CuFeO2 /
- Fenton-like reaction /
- ofloxacin /
- degradation performance
-
抗生素应用于多个领域,主要涉及医药和畜牧饲料行业。由于抗生素的滥用,导致环境中抗生素污染问题普遍存在[1-3],目前,在水环境[4-6]、土壤[7-9]、水产动物[10]和植物[11]中均检测到了多种抗生素。青霉素G(PCN)是由青霉菌产生的一种β-内酰胺类水溶性抗生素[12],其可阻止肽聚糖的产生从而破坏细菌细胞壁的合成[13],是最具抗菌活性的抗生素,现已被广泛用于治疗人类和动物的疾病中[14]。PCN具有难以降解且含有生物毒性的特性,传统水处理方法难以完全对其产生作用,如果直接将其排放到水环境中,将会对生态环境以及人类构成较大威胁[15-16],因此,探索去除水环境中PCN的新方法十分必要。
O3氧化是一种清洁的水处理技术,且具有无二次污染和经济可行等特点[17],可作为强氧化剂,对污水中的难降解有机物进行降解[18]。有学者用O3氧化降解垃圾渗滤液[19]、有机氯农药[20]和布洛芬[21]等难降解有机物,结果表明,降解效果均十分明显。有研究[22-24]表明,将H2O2与O3联合时,H2O2会促进HO·的产生,从而使O3的利用率以及降解效果均可得到显著提升。陈炜鸣等[23]在采用O3降解垃圾渗滤液浓缩液的过程中,发现添加0.13 mol·L−1 H2O2能显著提升有机物的去除效果,且O3利用率提升了22.29%,同时废水可生化性得到了明显改善,BOD5/COD值由0.01提高到0.43。LI等[25]采用O3预处理氢化可的松制药废水,在H2O2/O3的摩尔比为0.3的条件下,反应15 min后,COD去除率可达67%,COD去除率相对于单一O3氧化体系提升了23%,证明添加适量H2O2可显著提高降解效果。虽然众多研究已经证明了O3和O3/H2O2法对难降解有机物的降解效果显著,但目前许多研究倾向于对工艺条件的优化,而对降解过程中的中间产物分析和降解规律的研究却相对较少。
基于此,本研究以难降解有机物PCN为目标,对其在O3/H2O2体系中的降解规律及其相关的机理进行研究,对降解过程中的中间产物及可能的降解路径进行探讨,并根据实验数据对降解动力学过程进行分析,为该法处理水中PCN的工程应用提供参考。
1. 实验材料与方法
1.1 实验试剂
实验试剂包括PCN(1 650 U·mg−1,阿拉丁)、H2O2(分析纯)、淀粉(分析纯)、甲酸(色谱级)、乙腈(色谱级)、NaOH(分析纯)、Na2S2O3(分析纯)、KI(分析纯)。
1.2 实验设备与仪器
自制反应器、微波快速消解COD测定仪(GZ-WXJ-Ⅲ)、pH计(pHS-3C)、液相色谱仪(Agilent-1200,美国Agilent公司)、液质联用色谱仪(WATERS TQD,美国waters公司)、精密分析天平(FA1004)、傅里叶红外光谱仪器(Nicolet Nexus 410,美国Nicolet公司)、真空冷冻干燥机(LFD-56D10S)等。
自制有机玻璃材质反应器高度为200 mm,内径为90 mm,O3由臭氧发生器(JZ110B-SJG)供给,采用微孔石英砂芯底层曝气,通过转子流量计控制流量,同时O3产量使用碘量法进行测量。利用2个串联的吸收瓶组成尾气收集装置,对溢出尾气进行收集,定时在反应器中部取样。
1.3 实验方法
将PCN溶液加入反应器中均匀混合,在通入O3前,加入适量H2O2并控制反应温度和pH,待臭氧发生器稳定工作后,调节气体流量为1.2 L·min−1(臭氧产量为492 mg·h−1),反应开始后按时取样,然后用Na2S2O3终止反应。样品经0.22 μm滤膜过滤后,测定其COD和ρ(PCN)。每次均设计重复实验,每个样品都进行平行测定,然后取其均值。
1.4 分析方法
使用HPLC对PCN的降解产物进行检测。具体实验条件为:Hypersil BDS C18色谱柱;流动相为超纯水(含0.1%甲酸)∶乙腈=50∶50(体积比);流速为1.0 mL·min−1;柱温为30 ℃;进样量为20 μL[26]。质谱检测采用电喷雾电离源,在负离子模式下进行检测,扫描的质荷比m/z为100~700。O3气相浓度采用碘量法(CJ/T 3028.2-1994)测定,COD采用重铬酸钾快速消解法进行测定。
2. 结果与讨论
2.1 pH对PCN和COD去除率的影响
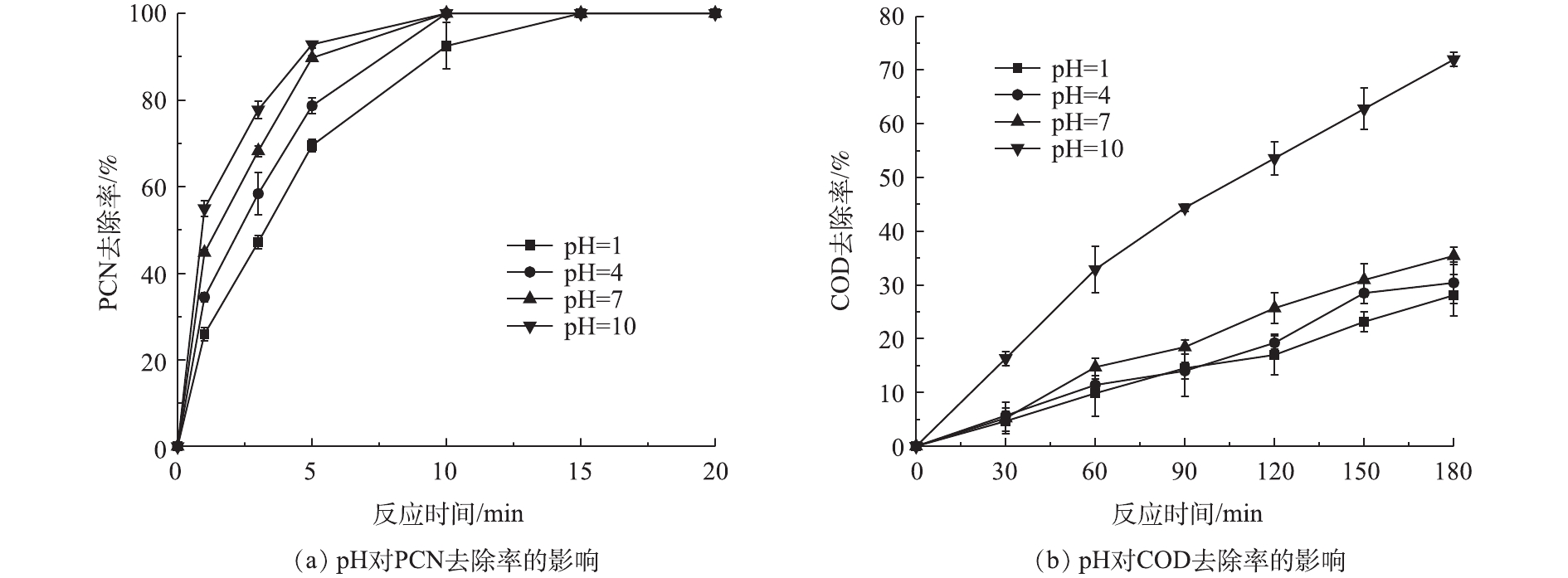
在温度为20 ℃、ρ(PCN)为25 mg·L−1、O3气体流量为1.2 L·min−1、H2O2投加量为7.84 mmol·L−1的反应条件下,考察pH对PCN和COD去除效果的影响,结果如图1所示。由图1可知,在不同pH下,COD和PCN的去除效果差异明显,在酸性和中性条件下,COD去除效果相对较差,PCN去除速率缓慢,当pH升高时,反应去除速率也相应加快;在碱性反应环境下,去除效果显著提升,在反应5 min后,PCN去除率为92.5%,在反应3 h后,COD去除率为71.9%。这是因为pH会影响O3/H2O2体系中HO·的产生效率,在酸性条件下,主要是O3分子的氧化,而在碱性情况下,溶液中OH-会促进HO·的生成,此时主要以HO·氧化为主,反应速率得到了提升,具体反应如式(1)~式(3)所示。
O3+OH−→HO−2+O2 (1) H2O2↔HO−2+H+ (2) O3+HO−2→HO⋅+O−2+O2 (3) 此外,在碱性环境中,H2O2更容易离解生成
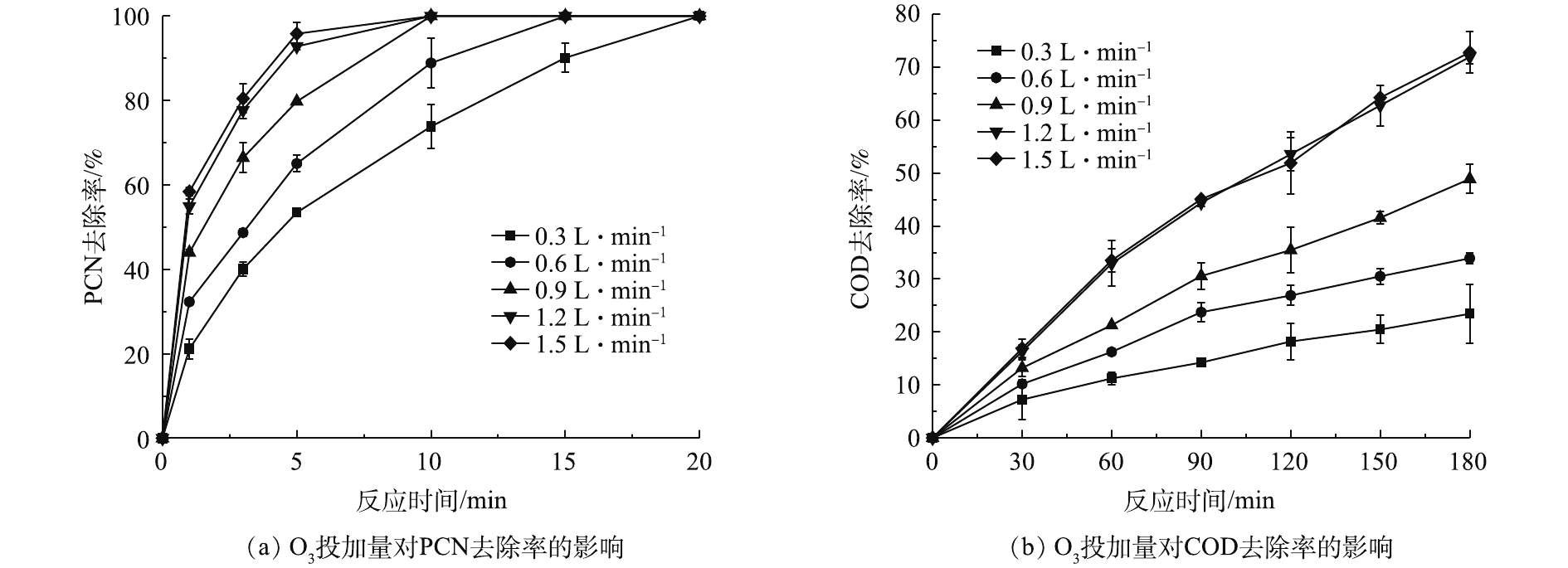
HO−2 ,而HO−2 又是HO·的诱发剂,所以可促进HO·的生成,进而加快氧化速率[23]。2.2 O3投加量对PCN和COD去除率的影响
在温度为20 ℃、ρ(PCN)为25 mg·L−1、pH=10,H2O2投加量为7.84 mmol·L−1的反应条件下,考察O3投加量对PCN和COD去除效果的影响,结果如图2示。由图2可知,O3投加量对去除PCN和COD的影响较大,当流量由0.3 L·min−1(O3产量为123 mg·h−1)升至1.5L·min−1(O3产量为615 mg·h−1)时,随着O3投加量的不断增加,PCN和COD的去除率也不断提升,当O3流量为1.5 L·min−1时,PCN和COD去除效果达到最佳。在反应5 min后,PCN去除率为95.83%,在反应3 h后,COD去除率为72.8%。由图2还可看出,在1.2 L·min−1(O3产量为492 mg·h−1)和1.5 L·min−1反应条件下,PCN和COD的去除效果无明显差异,PCN和COD的去除率增幅明显降低,原因可能是,当水中O3溶解度达到最大时,O3的利用率将会降低,未参加反应的O3分子将会直接从液相转移至气相中,故导致无法继续提高降解效能。所以本实验最佳O3流量设定为1.2 L·min−1,以避免造成O3的浪费。
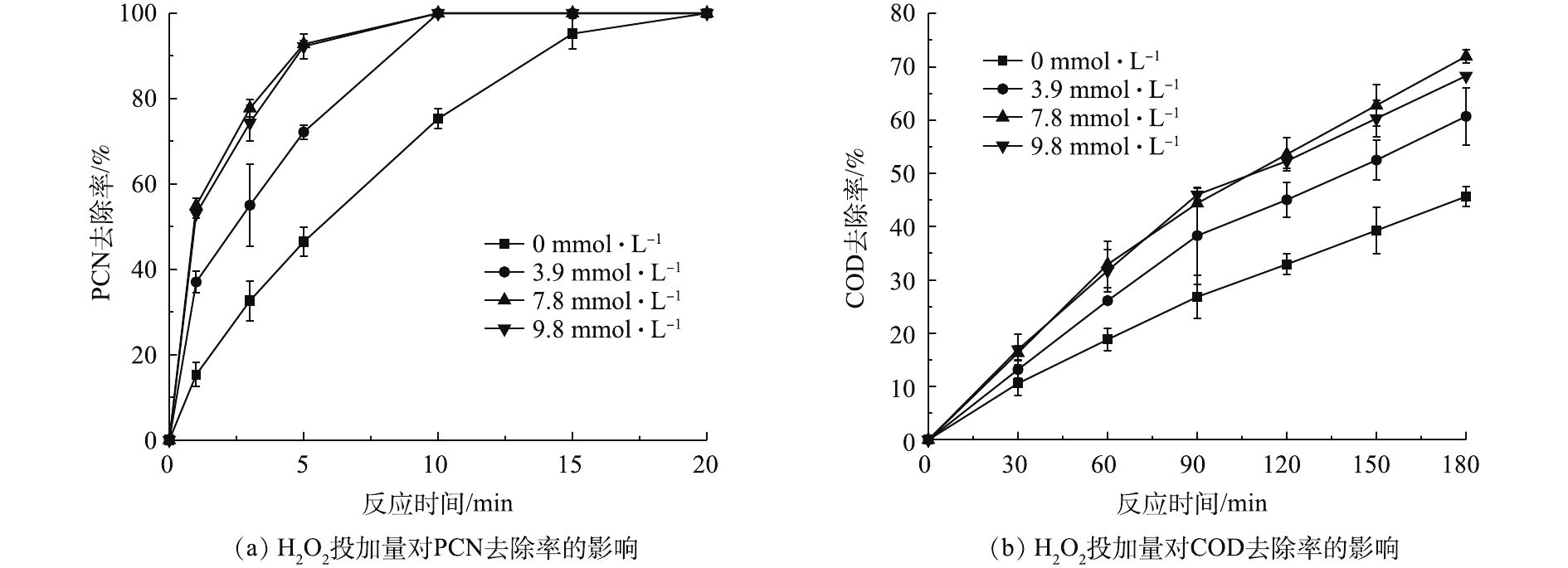
2.3 H2O2投加量对PCN和COD去除率的影响
在温度为20 ℃、ρ(PCN)为25 mg·L−1、pH=10、O3气体流量为1.2 L·min−1的条件下,考察H2O2投加量对PCN和COD去除效果的影响,结果如图3所示。由图3可知,在O3/H2O2体系氧化PCN的过程中,PCN能在较短时间内快速被氧化成中间产物,而中间产物的氧化速率则较为缓慢,但H2O2的促进效果明显。当H2O2的投加量由0升至7.84 mmol·L−1时,PCN和COD的去除率也相应随之升高。在反应5 min后,PCN去除率为100%,增幅为37.4%;在反应3 h后,COD去除率为71.9%,增幅为26.3%。相比于单独的O3体系,添加双氧水能显著提升COD和PCN的去除率,这是由于O3和H2O2之间存在协同机制,适量双氧水可促进氧化过程中HO·的生成,从而提升反应效果[3]。具体反应如式(4)所示。
O3+2H2O2→2HO⋅+2O2 (4) 由图3可看出,当H2O2投加量大于7.84 mmol·L−1时,COD去除率略微下降。这可能是由于反应体系中多余的H2O2成为了HO·的捕获剂,从而降低了HO·氧化有机物的效率[22-23]。具体反应机理如式(5)所示。
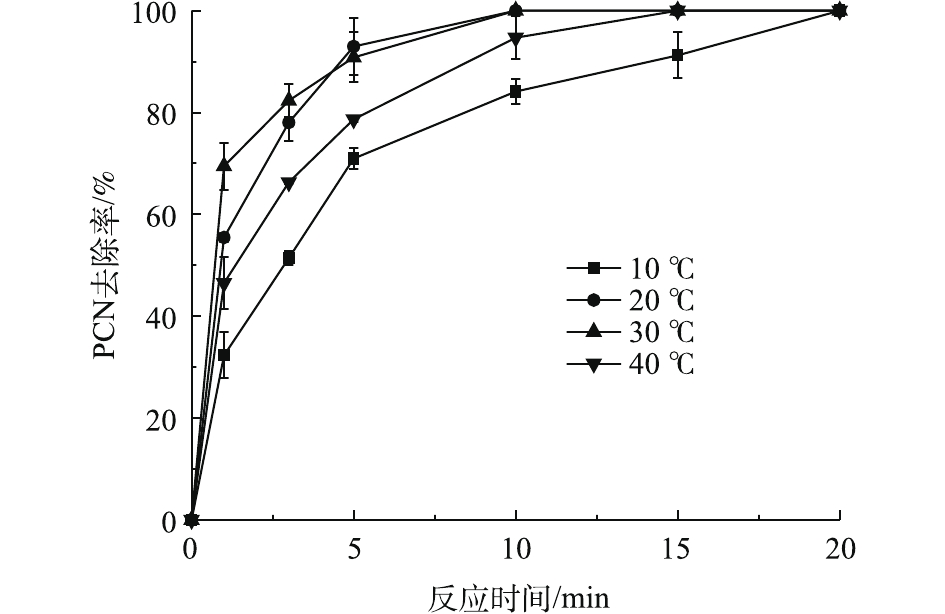
H2O2+2HO⋅→2H2O+O2 (5) 2.4 温度对PCN去除率的影响
在ρ(PCN)为25 mg·L−1、pH为10、H2O2投加量为7.84 mmol·L−1、O3气体流量为1.2 L·min−1的条件下,考察温度对PCN去除效果的影响,结果如图4所示。由图4可知:在10~30 ℃时,随着温度的上升,PCN去除速率也逐渐加快,去除速率由8.11 mg·(L·min)−1增至17.34 mg·(L·min)−1;但当温度为40 ℃时,PCN去除速率明显降低。原因可能是:当反应温度升高时,加快了分子之间的运动,加速了HO·的生成和O3在水中的扩散速率,从而提升了PCN去除速率。但当温度继续升高时,H2O2的自分解效果加剧,且O3在溶液中的溶解度也有所降低,导致去除速率明显减缓。
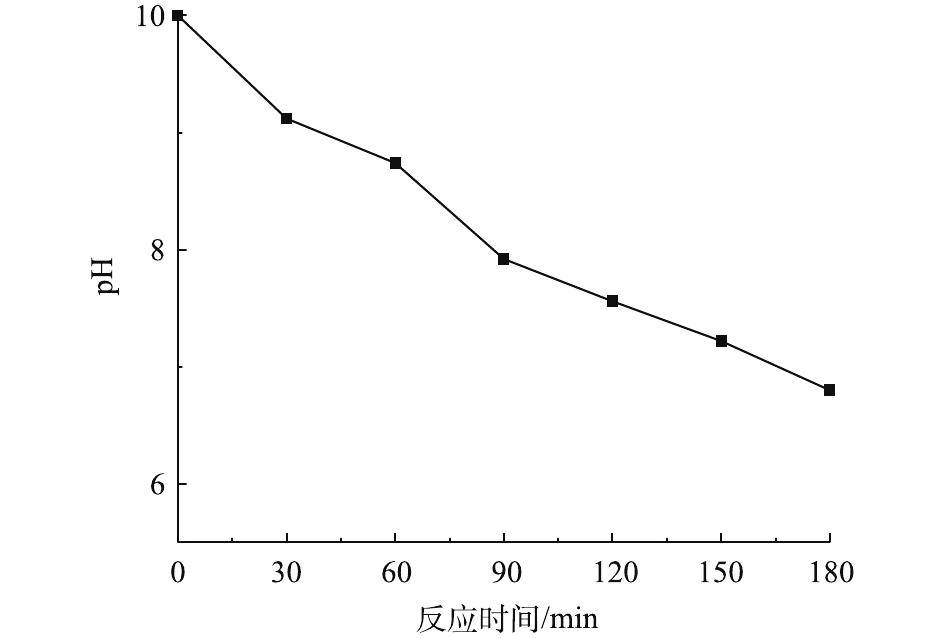
2.5 反应体系中pH的变化
在温度为20 ℃、ρ(PCN)为25 mg·L−1、pH为10,O3气体流量为1.2 L·min−1、H2O2投加量为7.84 mmol·L−1的条件下,考察O3/H2O2反应体系中pH的变化趋势,结果如图5所示。由图5可知,在O3氧化PCN的过程中,反应体系pH随反应时间的延长呈下降趋势,在反应3 h后,pH由10下降至6.8,最终反应溶液呈弱酸性,这可能是由于在降解过程中产生了酸性中间产物,从而导致pH的下降。这与红外光谱和LC-MS的分析结论相一致。体系pH的降低不利于反应的进行,这可能也是反应过程中反应速率均呈先快后慢的变化趋势的原因。
2.6 表观动力学方程的建立
通过大量的实验得出最优pH和温度,研究O3、H2O2和PCN初始浓度对氧化过程中PCN浓度衰减的影响,结果如表1所示,降解动力学方程见式(6)。
表 1 不同反应物的初始浓度对反应速率的影响Table 1. Effect of initial concentration of different reactants on reaction rate序号 反应物初始浓度/(mg·L−1) T/K 初始速率/(mg·(L·min)−1) 拟合方程 PCN O3 H2O2 1 25 8.2 266.4 303.15 13.87 y=0.358 9x−2.901 9R2=0.996 1 2 50 8.2 266.4 303.15 17.76 3 75 8.2 266.4 303.15 20.22 4 100 8.2 266.4 303.15 23.29 5 25 2.05 266.4 303.15 5.31 y=0.697 9x−1.756 4R2=0.997 6 6 25 4.1 266.4 303.15 8.25 7 25 6.15 266.4 303.15 11.4 8 25 8.2 266.4 303.15 13.87 9 25 8.2 66.6 303.15 8.84 y=0.323 3x−3.701 1R2=0.999 8 10 25 8.2 133.2 303.15 11.12 11 25 8.2 199.8 303.15 12.6 12 25 8.2 266.4 303.15 13.87 13 25 8.2 266.4 283.15 8.11 — 14 25 8.2 266.4 293.15 13.87 15 25 8.2 266.4 303.15 17.34 −dCPCN/dt=k0exp(−Ea/RT)⋅CαPCN⋅CβO3⋅CγH2O2 (6) 式中:α、β、γ分别为PCN、O3、H2O2的反应级数;CPCN、CO3、CH2O2分别为PCN、O3、H2O2的初始浓度,mol·L−1;Ea为反应活化能,kJ·mol−1;k0为指前因子,mol·(L·s)−1;R为气体常数,取值8.314 J·(mol·K)−1;T为反应温度,℃。
根据表1的数据并结合表观动力学计算原理,可计算出PCN、O3和H2O2反应物的反应级数,其数值分别为α=0.367、β=0.697 3、γ=0.323 3。
由于总反应速率常数k=k0exp(-Ea/RT),两侧一起取对数可得式(7)。据T和k相应值可得图6。计算得到Ea=27.59 kJ·mol−1,k0=0.052 mol·(L·s)−1,因此,得出总动力学方程,见式(8)。
lnk=−(Ea/R)(1/T)+lnk0 (7) −dCPCN/dt=0.052exp(−27594.9/RT)⋅C0.367PCN⋅C0.6973O3⋅C0.3233H2O2 (8) 本动力学模型是依据反应物初始浓度对降解速率的影响而建立的,对于整个降解过程而言,模型可能会高估反应速率。由式(8)可知,O3的反应级数为0.697 9,高于PCN (0.358 9)和H2O2 (0.335 4)的反应级数,说明降解过程中O3初始浓度对反应速率的影响最大。原因可能是,在O3氧化降解PCN的过程中,存在O3分子直接氧化和HO·氧化2种氧化方式,反应过程中只要有O3就能氧化有机物,而H2O2与O3反应只能加快HO·的生成。此外,反应活化能 (Ea=27.59 kJ·mol−1)较低,说明该反应容易发生。
2.7 PCN降解前后红外光谱分析
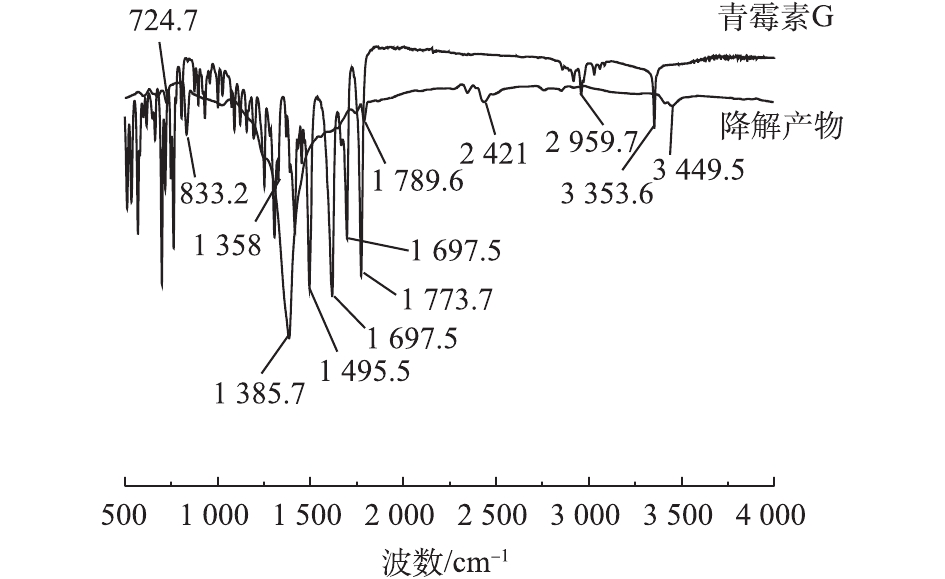
将PCN溶液及其氧化降解的最终产物进行冷冻干燥后进行红外光谱检测,结果如图7所示。在PCN红外光谱图中,1 773.7 cm−1为—COOH中的C=O的伸缩振动峰,3 353.6 cm−1为—COOH中的O—H的伸缩振动峰;1 495.5、1 617.9和2 959.7 cm−1为苯环结构对应的吸收峰,650~1 000 cm−1为苯环上的C—H取代伸缩振动峰;而1 697.5 cm−1为酰胺结构的C=O的伸缩振动;1 307 cm−1处为—(CH3)2的吸收峰。
由图7可知,PCN在氧化前后的谱图有着明显差异,在1 450~1 620 cm−1和3 000 cm−1处苯环骨架吸收峰消失,这说明氧化破坏了PCN的苯环结构。在2 421 cm−1处出现了新的吸收峰,这说明在氧化过程中可能有含叁键或者累积双键的物质产生。在1 697.5 cm−1处的酰胺结构吸收峰消失不见,说明氧化反应破坏了PCN的抑菌结构β-内酰胺环,从而使PCN的抑菌性减弱[27-28]。在1 385.7 cm−1处的峰强度有明显增大,这说明原—(CH3)2结构仍存在,吸收峰在3 449.5 cm−1处出现,有可能是伯胺官能团的不对称伸缩振动与—COOH上O—H的伸缩振动,说明最终产物中可能含有胺类化合物。在1 789.5 cm−1和833.2 cm−1处分别出现羧酸的C=O的伸缩振动和O—H的弯曲振动,这表明最终产物中可能含有酸类化合物,这是导致反应中pH下降的原因。
2.8 PCN降解产物分析
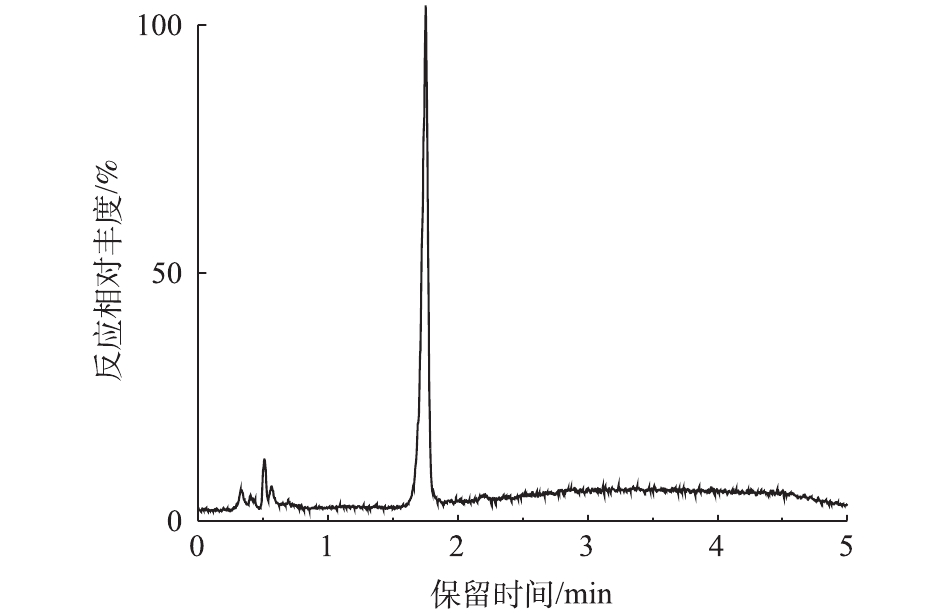
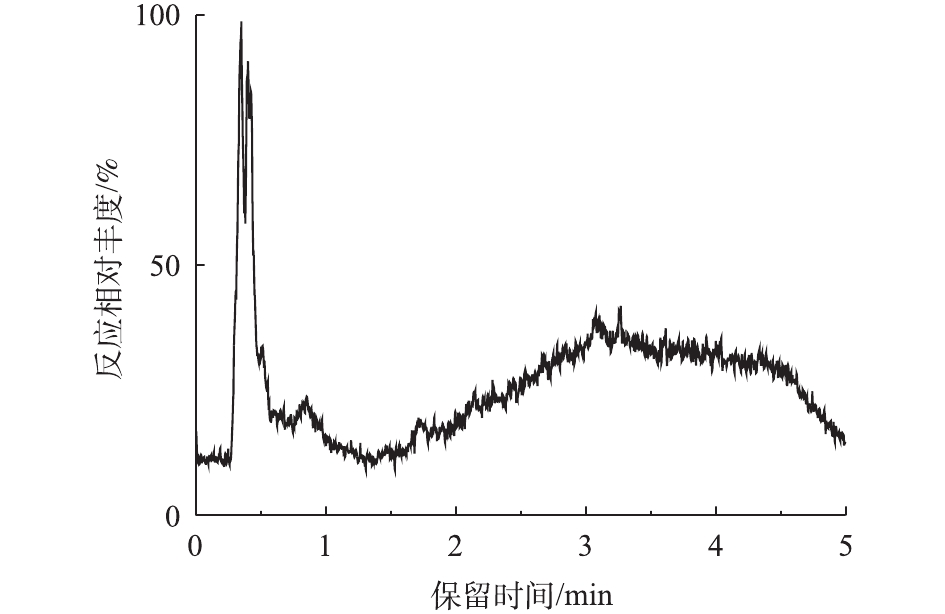
对PCN的降解产物进行LC-MS检测,PCN及其降解产物的总离子流图如图8和图9所示。结果表明,PCN及其降解产物得到了较好的分离,降解后没有检测到PCN的出峰,说明PCN已被完全降解,离子流图显示了PCN降解产物的变化;综合FT-IR的表征结果,对降解产物进行了质谱分析,推测出PCN降解产物可能的分子结构(表2)。
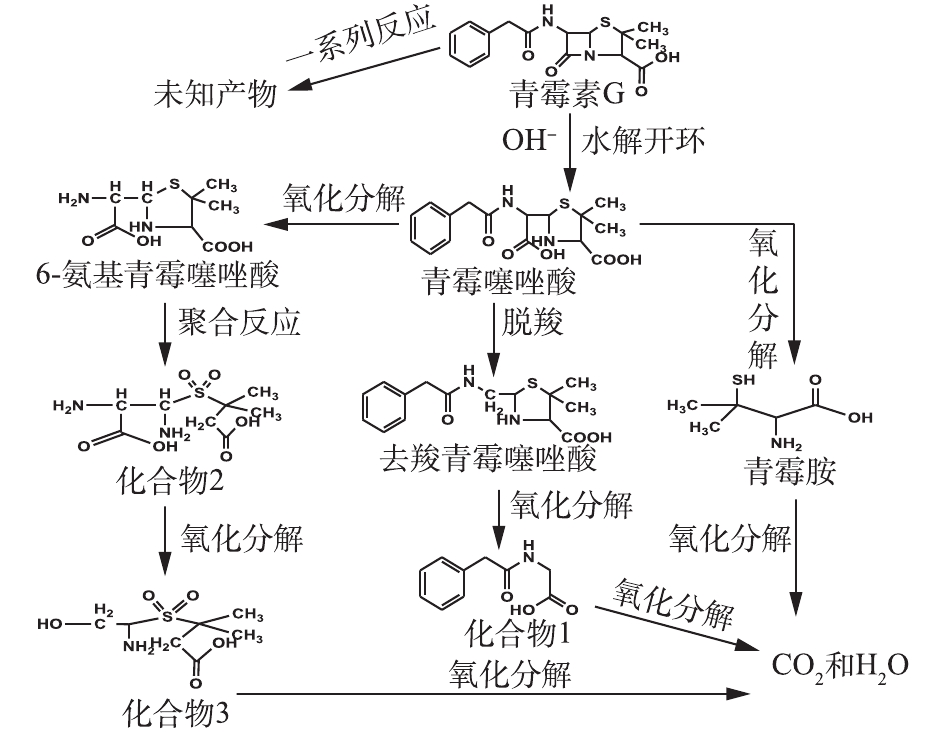
表 2 PCN及其降解产物的质谱数据Table 2. Mass spectrometry data of PCN and its degradation products物质 分子式 保留时间/min 离子质荷比 青霉噻唑酸 C16H21N2O5S 0.841 352 青霉素钠 C16H18N2O4S 1.753 334 去羧青霉素噻唑酸 C15H20N2O3S 0.505 308 6-氨基青霉噻唑酸 C8H14N2O4S 0.407 234 青霉胺 C5H11NO2S 0.488 149 化合物1 C10H11NO3 0.515 193 化合物2 C8H16N2O6S 0.488 267 化合物3 C7H15NO5S 0.339 225 在O3降解PCN的过程中,可能有HO·氧化以及水解等非常复杂的反应存在。在碱性条件下,PCN的β-内酰胺环容易水解打开生成青霉噻唑酸;经脱酸反应后,可能生成去羧青霉噻唑酸;同时在HO·的强氧化能力下,青霉噻唑酸可能进一步被氧化降解成6-氨基青霉噻唑酸、青霉胺和其他未知产物;中间产物也可能最终矿化成为CO2和H2O。根据中间产物分析,推测PCN可能的降解路径如图10所示。
根据LC-MS对产物的分析结果,并结合红外光谱表征结果可知,PCN降解前后的官能团结构发生了较大的变化,氧化使PCN的β-酰胺环被破坏,这也解释了PCN及其降解产物的抑菌性消失或者减弱的原因。
3. 结论
1) O3和H2O2有显著的协同作用,能明显加快反应速率,显著提升COD和PCN的去除率。在初始ρ(PCN):25 mg·L−1,pH=10、O3投加量为1.48 g·L−1、H2O2投加量为7.84 mmol·L−1、温度为20 ℃的条件下,反应10 min后,PCN被完全去除,反应3 h后,COD去除率为71.9%。这说明O3/H2O2体系能有效氧化降解PCN和降解过程中产生的中间产物。
2)通过数据的拟合,得到了O3/H2O2降解PCN的反应动力学方程,O3的反应级数为0.697 3,高于PCN(0.367)和H2O2(0.323 3)的反应级数,说明在降解过程中,O3初始浓度对反应速率的影响最大;此反应的活化能(Ea=27.59 kJ·mol−1)较低,说明此反应容易发生。
3)根据LC-MS和红外光谱检测结果得出,PCN分子结构在降解前后发生了明显变化,PCN的抑菌结构β-内酰胺环被破坏。此外,降解产物中含有酸性物质,这会导致反应体系pH下降,从而不利于O3反应的进行。
-
-
[1] TIAN Y, HE X, ZHOU H, et al. Efficient fenton-like degradation of ofloxacin over bimetallic Fe-Cu@Sepiolite composite[J]. Chemosphere, 2020, 257: 127209. doi: 10.1016/j.chemosphere.2020.127209 [2] CAO D Q, YANG W Y, WANG Z, et al. Role of extracellular polymeric substance in adsorption of quinolone antibiotics by microbial cells in excess sludge[J]. Chemical Engineering Journal, 2019, 370: 684694. [3] ROBLES-JIMENEZ L E, ARANDA-AGUIRRE E, CASTELAN-ORTEGA O A, et al. Worldwide traceability of antibiotic residues from livestock in wastewater and soil: A systematic review[J]. Animals, Multidisciplinary Digital Publishing Institute, 2022, 12(1): 60. [4] LIU Y, LIU X, LU S, et al. Adsorption and biodegradation of sulfamethoxazole and ofloxacin on zeolite: Influence of particle diameter and redox potential[J]. Chemical Engineering Journal, 2020, 384: 123346. doi: 10.1016/j.cej.2019.123346 [5] YANG Z, GUO H, YAO Z, et al. Hydrophilic silver nanoparticles induce selective nanochannels in thin film nanocomposite polyamide membranes[J]. Environmental Science & Technology, 2019, 53(9): 53015308. [6] LIANG Y, ZHANG Q, LI S, et al. Highly efficient removal of quinolones by using the easily reusable MOF derived-carbon[J]. Journal of Hazardous Materials, 2022, 423: 127181. doi: 10.1016/j.jhazmat.2021.127181 [7] WANG J, CHEN H. Catalytic ozonation for water and wastewater treatment: Recent advances and perspective[J]. Science of Total Environment, 2020, 704: 135249. doi: 10.1016/j.scitotenv.2019.135249 [8] QIN Q, LIU Y, LI X, et al. Enhanced heterogeneous Fenton-like degradation of methylene blue by reduced CuFe2O4[J]. RSC Advances, 2018, 8(2): 10711077. [9] XIN S, LIU G, MA X, et al. High efficiency heterogeneous Fenton-like catalyst biochar modified CuFeO2 for the degradation of tetracycline: Economical synthesis, catalytic performance and mechanism[J]. Applied Catalysis B:Environmental, 2021, 280: 119386. doi: 10.1016/j.apcatb.2020.119386 [10] SEGURA Y, MARTíNEZ F, MELERO J A, et al. Enhancement of the advanced Fenton process (Fe0/H2O2) by ultrasound for the mineralization of phenol[J]. Applied Catalysis B:Environmental, 2012, 113/114: 100106. [11] YUAN Y, LAI B, TANG Y Y. Combined Fe0/air and Fenton process for the treatment of dinitrodiazophenol (DDNP) industry wastewater[J]. Chemical Engineering Journal, 2016, 283: 15141521. [12] XIAO C, LI J, ZHANG G. Synthesis of stable burger-like α-Fe2O3 catalysts: Formation mechanism and excellent photo-Fenton catalytic performance[J]. Journal of Cleaner Production, 2018, 180: 550559. [13] ZHAN J, LI M, ZHANG X, et al. Aerosol-assisted submicron γ-Fe2O3/C spheres as a promising heterogeneous Fenton-like catalyst for soil and groundwater remediation: Transport, adsorption and catalytic ability[J]. Chinese Chemical Letters, 2020, 31(3): 715720. [14] TOLBA A, GAR Alalm M, ELAMADONY M, et al. Modeling and optimization of heterogeneous Fenton-like and photo-Fenton processes using reusable Fe3O4-MWCNTs[J]. Process Safety and Environmental Protection, 2019, 128: 273283. [15] WU P, ZHOU C, LI Y, et al. Flower-like FeOOH hybridized with carbon quantum dots for efficient photo-Fenton degradation of organic pollutants[J]. Applied Surface Science, 2021, 540: 148362. doi: 10.1016/j.apsusc.2020.148362 [16] LIU W, WANG Y, AI Z, et al. Hydrothermal synthesis of FeS2 as a high-efficiency Fenton reagent to degrade alachlor via superoxide-mediated Fe(II)/Fe(III) cycle[J]. ACS Applied Materials & Interfaces, 2015, 7(51): 2853428544. [17] ZHANG J, ZHAN M, ZHENG L, et al. FeOCl/POM heterojunctions with excellent Fenton catalytic performance via different mechanisms[J]. Inorganic Chemistry, 2019, 58(1): 250258. [18] DING R R, LI W Q, HE C S, et al. Oxygen vacancy on hollow sphere CuFe2O4 as an efficient Fenton-like catalysis for organic pollutant degradation over a wide pH range[J]. Applied Catalysis B:Environmental, 2021, 291: 120069. doi: 10.1016/j.apcatb.2021.120069 [19] WEI Z, HUANG S, ZHANG X, et al. Hydrothermal synthesis and photo-Fenton degradation of magnetic MnFe2O4/rGO nanocomposites[J]. Journal of Materials Science:Materials in Electronics, 2020, 31(7): 51765186. [20] HASSANI A, ÇELIKDAG G, EGHBALI P, et al. Heterogeneous sono-Fenton-like process using magnetic cobalt ferrite-reduced graphene oxide (CoFe2O4-rGO) nanocomposite for the removal of organic dyes from aqueous solution[J]. Ultrasonics Sonochemistry, 2018, 40: 841852. [21] XIONG D, GAO H, DENG Y, et al. Impact of Mg doping on the optical and electrical properties of p-type CuMnO2 ultrathin nanosheets[J]. Journal of Materials Science:Materials in Electronics, 2020, 31(7): 54525461. [22] ZHANG L, ZENG H, ZENG Y, et al. Heterogeneous Fenton-like degradation of 4-chlorophenol using a novel Fe(III)-containing polyoxometalate as the catalyst[J]. Journal of Molecular Catalysis A:Chemical, 2014, 392: 202207. [23] CHENG M, LAI C, LIU Y, et al. Metal-organic frameworks for highly efficient heterogeneous Fenton-like catalysis[J]. Coordination Chemistry Reviews, 2018, 368: 8092. [24] LUO M, BOWDEN D, BRUMBLECOMBE P. Catalytic property of FeAl pillared clay for Fenton oxidation of phenol by H2O2[J]. Applied Catalysis B:Environmental, 2009, 85(3): 201206. [25] TIAN Y, ZHOU M, PAN Y, et al. MoS2 as highly efficient co-catalyst enhancing the performance of Fe0 based electro-Fenton process in degradation of sulfamethazine: Approach and mechanism[J]. Chemical Engineering Journal, 2021, 403: 126361. doi: 10.1016/j.cej.2020.126361 [26] WANG F, YU X, GE M, et al. Facile self-assembly synthesis of γ-Fe2O3/graphene oxide for enhanced photo-Fenton reaction[J]. Environmental Pollution, 2019, 248: 229237. [27] FENG Y, LIAO C, SHIH K. Copper-promoted circumneutral activation of H2O2 by magnetic CuFe2O4 spinel nanoparticles: Mechanism, stoichiometric efficiency, and pathway of degrading sulfanilamide[J]. Chemosphere, 2016, 154: 573582. [28] DU Z, LI K, ZHOU S, et al. Degradation of ofloxacin with heterogeneous photo-Fenton catalyzed by biogenic Fe-Mn oxides[J]. Chemical Engineering Journal, 2020, 380: 122427. doi: 10.1016/j.cej.2019.122427 [29] LIU Y, ZHANG J, SHENG C, et al. Simultaneous removal of NO and SO2 from coal-fired flue gas by UV/H2O2 advanced oxidation process[J]. Chemical Engineering Journal, 2010, 162(3): 10061011. [30] QIN H, CHENG H, LI H, et al. Degradation of ofloxacin, amoxicillin and tetracycline antibiotics using magnetic core–shell MnFe2O4@C-NH2 as a heterogeneous Fenton catalyst[J]. Chemical Engineering Journal, 2020, 396: 125304. doi: 10.1016/j.cej.2020.125304 [31] ZHENG C, YANG C, CHENG X, et al. Specifically enhancement of heterogeneous Fenton-like degradation activities for ofloxacin with synergetic effects of bimetallic Fe-Cu on ordered mesoporous silicon[J]. Separation and Purification Technology, 2017, 189: 357365. [32] YU Y, HUANG F, HE Y, et al. Heterogeneous fenton-like degradation of ofloxacin over sludge derived carbon as catalysts: Mechanism and performance[J]. Science of the Total Environment, 2019, 654: 942947. [33] CHEN X, ZHANG M, QIN H, et al. Synergy effect between adsorption and heterogeneous photo-Fenton-like catalysis on LaFeO3/lignin-biochar composites for high efficiency degradation of ofloxacin under visible light[J]. Separation and Purification Technology, 2022, 280: 119751. doi: 10.1016/j.seppur.2021.119751 -




 下载:
下载: