-
进入河流湖泊的重金属污染物,其中约90%的重金属可通过吸附、絮凝、络合、共沉淀等作用方式沉积于河湖底泥表面并不断积累[1]。底泥中沉积的重金属不仅对水生生物及沿河居民的饮用水安全造成了严重威胁,还容易重新释放至上覆水体,引发“二次污染”,最终危害人类健康[2]。其中,镉(Cd)是一种移动性较强、毒性较大的重金属[3]。
综合处理效率、修复成本及操作难易程度等问题的考虑,稳定化技术逐渐在底泥重金属修复中呈现出较大潜力[4]。对于该修复技术来说,稳定试剂的选择非常关键。目前,利用铁系纳米材料作为稳定试剂来修复底泥重金属污染问题已成为研究的热点[5]。其中,纳米零价铁(nZVI)凭借其独特的结构性质,如吸附还原活性强、粒径小、反应快速等特性,已被广泛应用于废水、地下水、土壤及底泥重金属的稳定修复[6-7]。但nZVI在实际应用中存在易团聚、不稳定、易被氧化等问题,进而限制了其在底泥重金属污染修复领域的进一步发展[8]。生物炭(biochar,BC)是由生物质在低氧条件下通过热解制备得到的碳质材料,在贮存碳汇、土壤肥力改善、污染物固定化和废物处理等诸多方面都发挥着重要作用[9]。BC具有多孔性、碱性、强吸附性、高比表面积、高阳离子交换量及丰富的含氧官能团等特性,对重金属具有较强的吸附和固定能力[10]。因此,将nZVI负载到BC固体载体上构建nZVI/BC复合材料,预计能减少团聚、增强nZVI的稳定性,发挥出两者的优势,实现重金属的有效稳定。基于nZVI/BC能产生多方面的有益效应,目前已有一些关于利用nZVI/BC复合材料去除(或固定)水体或土壤中的重金属和有机污染物的批量实验研究,而将该复合材料体系应用于湖泊底泥重金属修复的研究尚鲜见报道。
与水体、土壤介质不同的是,重金属在水生底栖生物条件下的迁移过程更为复杂,其迁移转化直接涉及到水体环境生态安全[11],研究修复过程中底泥重金属形态的变化是判断nZVI/BC稳定效果的重要指标。此外,重金属的浸出毒性、生物可利用性及上覆水中溶解态重金属质量浓度的变化可作为评价修复底泥中重金属环境效应的综合指标[12-13],对深入理解nZVI/BC修复重金属污染底泥的潜在环境风险具有重要科学意义。基于上述研究,本研究采用液相还原和原位沉积法制备了nZVI/BC复合材料,并对其进行了分析和表征,考察了其对湖泊底泥中Cd的固定效果,并进一步探讨了nZVI/BC对底泥Cd污染的修复机理。
-
实验所需试剂主要包括:三氯化铁(FeCl3·6H2O)、硼氢化钠(NaBH4)、无水乙醇(CH3CH2OH)、冰醋酸(CH3COOH)、盐酸羟胺(NH2OH·HCl)、乙酸铵(CH3COONH4)、过氧化氢(H2O2)、甘氨酸(C2H5NO2)、硫酸亚铁(FeSO4·7H2O)均为分析纯并采购于国药集团化学试剂有限公司;实验用水均为超纯水(18.25 MΩ·cm)。
实验所需仪器主要包括:真空干燥箱(DZK-6020,上海仪电科学仪器股份有限公司);恒温水浴振荡箱(HH-80,常州市万丰仪器制造有限公司);电感耦合等离子体质谱仪(Optima 7300 DV,美国PerkinElmer公司);便携式pH计(PHS-3D,上海雷磁仪器有限公司);场发射扫描电子显微镜(SEM,S-4800,日本Hitachi公司);透射电子显微镜(TEM,Tecnai 12,荷兰Philips公司);傅里叶红外光谱(FTIR,Nicolet 5700,美国Thermo Nicolet公司);X射线能谱仪(EDS,Quanta 250,美国FEI公司);X射线光电子能谱(XPS,K-Alpha,美国赛默飞世尔科技公司)。
-
nZVI的制备:将100 mL、4.83 g的FeCl3·6H2O水溶液转移至500 mL的三口烧瓶中,在通氮气的条件下搅拌30 min,然后逐滴加入等体积2.10 g NaBH4溶液,滴加完毕后再搅拌30 min,待反应完全后,用无水乙醇洗涤3次,然后用磁铁收集沉淀物,60 ℃真空干燥8 h,碾磨成粉末,保存备用。
BC的制备:将小麦秸秆放入石英舟内压实,然后置于管式炉,加热前向管内通入氮气5 min以排出管内空气,以5 ℃·min−1的速度升温至600 ℃,保温2 h。然后待管式炉温度降至近50 ℃后取出石英舟,将舟内生物炭碾磨成粉末状,保存备用。
nZVI/BC的制备:将2 g BC和100 mL、2.4156 g的FeCl3·6H2O水溶液转移至500 mL的三口烧瓶中,在通氮气的条件下搅拌30 min,然后逐滴加入等体积1.15 g NaBH4溶液,滴加完毕后再搅拌30 min,待反应完全后,用无水乙醇洗涤3次,然后用磁铁收集沉淀物,60 ℃真空干燥8 h,碾磨成粉末,保存备用。nZVI与BC质量比为1∶4,该质量比是所制备nZVI/BC材料中实际nZVI与BC的质量比。
-
采集太湖梅梁湾西北角和东北角入湖河口区域的表层底泥,采样深度约为20 cm。去除其中的石块、树枝、塑料垃圾等杂物后,将底泥样品置于冷冻真空干燥箱中进行干燥,研磨过筛(2 mm)后备用。底泥的pH为7.53,含水率为65.32%,有机质含量为17.72 g·kg−1,阳离子交换量为13.62 cmol·kg−1。底泥样品中Cd、Pb、Zn、Cu、Cr的含量分别为0.46、26.14、53.21、20.81、47.22 mg·kg−1。
-
实验共设置4组:C(对照组,不添加材料)、2% nZVI(nZVI添加量2%)、2% BC(BC添加量2%)、2% nZVI/BC(nZVI/BC添加量2%),每组设3个平行样。称取一定质量的底泥样品与材料置于50 mL离心管,按照与底泥体积质量比5 mL:1 g的比例添加超纯水,混合均匀后,然后置于恒温培养箱中24 ℃静置培养。分别在第1、7、14、28、42、56 d取样测定修复过程中Cd的形态、浸出毒性、生物可利用性、上覆水中溶解态Cd质量浓度及底泥的pH和有机质。
-
底泥Cd形态的测定采用改进的三步连续提取法(BCR法)[12],即分为弱酸提取态(以CH3COOH提取)、可还原态(以NH2OH·HCl提取)、可氧化态(以H2O2和CH3COONH4提取)、残渣态(以HNO3-HF-HClO4提取)。Cd的浸出毒性采用醋酸缓冲溶液法测定[12],生物可利用性采用生理提取实验测定[14]。上覆水中溶解态Cd质量浓度采用虹吸法提取,通过电感耦合等离子体质谱仪(Optima 7300 DV,美国PerkinElmer公司)测定Cd的质量浓度。pH采用便携式pH计测定,有机质采用重铬酸钾容量法-稀释热法测定[15]。数据分析采用Excel和SPSS软件,作图采用Origin软件。
-
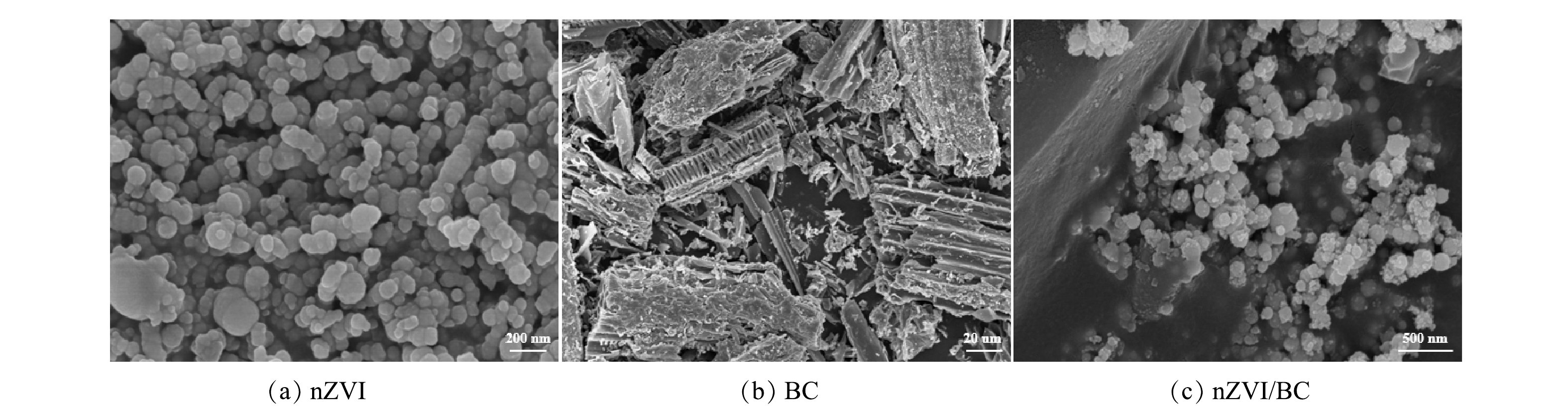
1) SEM分析。由图1(a)可以看出,nZVI为球状并聚集在一起。这主要是由于其自身的磁性引力所致[15]。由图1(b)可以看出,BC具有丰富的孔结构,孔隙结构可为重金属提供更多的吸附位点[16]。图1(c)为nZVI/BC复合材料的SEM图。在图1(c)可以观察到nZVI附着在BC表面,且呈现出较好的分散性。这说明BC作为支撑载体,有效降低了nZVI的团聚。
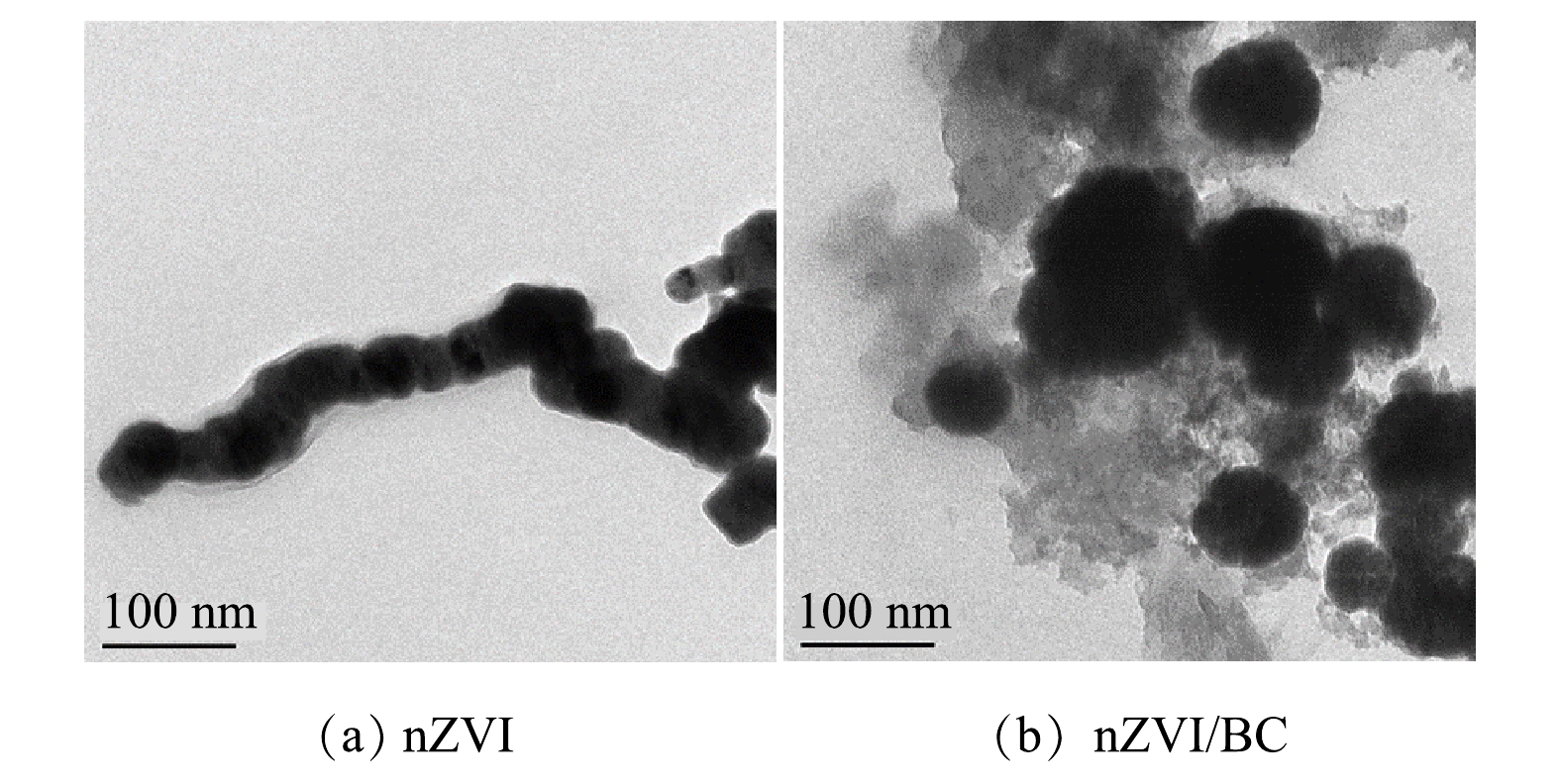
2) TEM分析。由图2(a)可以看出,nZVI颗粒粒径为50 nm左右,且呈现出链条状,体现了nZVI易团聚的特点。这与SEM的表征结果一致。由图2(b)可以看出,nZVI颗粒被固定在BC表面,nZVI的颗粒粒径分布在50~120 nm,可以看出其分散性明显优于单独的nZVI。
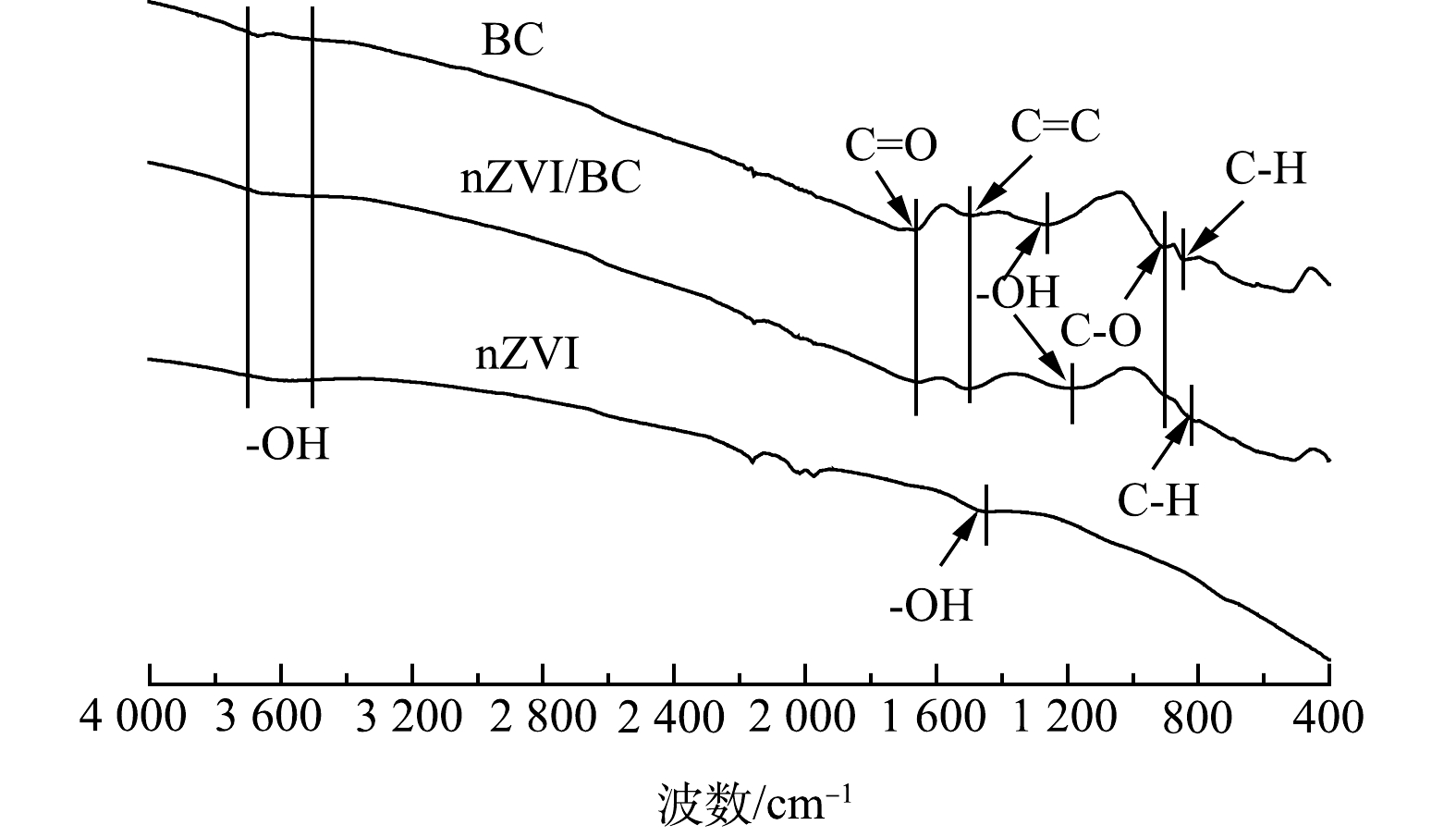
3) FTIR分析。由图3可知,nZVI、BC和nZVI/BC在3 500~3 700 cm−1处均出现了—OH的吸收峰,对nZVI来说是其表面吸附水的O—H基团的弯曲振动[17],对BC来说是其表面存在的羟基形成的[18]。此外,位于1 461 cm−1处的吸收峰为-OH的伸缩振动峰,说明nZVI表面发生了羟基化反应(FeOOH) [18]。由图3可见,波数为1 643、1 500、1 258、910和845 cm−1处分别对应C=O、C=C、—OH、C—O和C—H的伸缩振动峰。这些特征峰是由于BC中存在的羧酸、醇、酚、醚和酯等引起的[19-20],表明BC表面含有丰富的含氧官能团。在nZVI/BC的FTIR图谱中可以看到BC结构中特有的C=O、C=C和C—O键,且观察到—OH和C—H的吸收峰发生偏移。这说明nZVI与BC中的官能团之间发生反应。
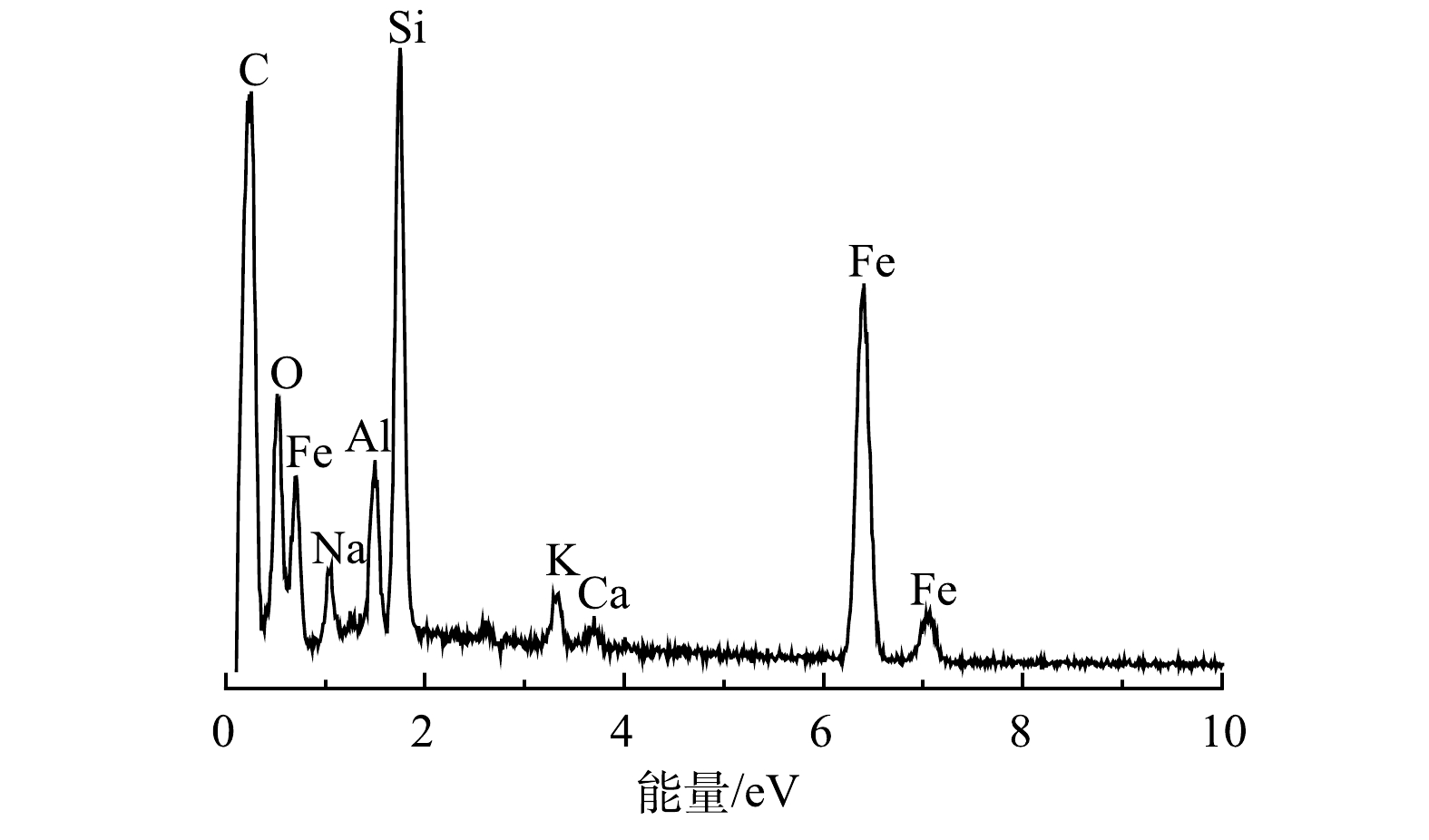
4) EDS分析。由图4可见,nZVI/BC表面除了含有C(54.96%)、O(15.07%)、Fe(18.39%)3种主要元素,还含有Na(1.92%)、Al(1.86%)、Si(6.72%)、K(0.73%)、Ca(0.34%)。这些元素属于生物质中的碱金属离子(灰分),在热解过程中会转化为氧化物、氢氧化物和碳酸盐[21]。
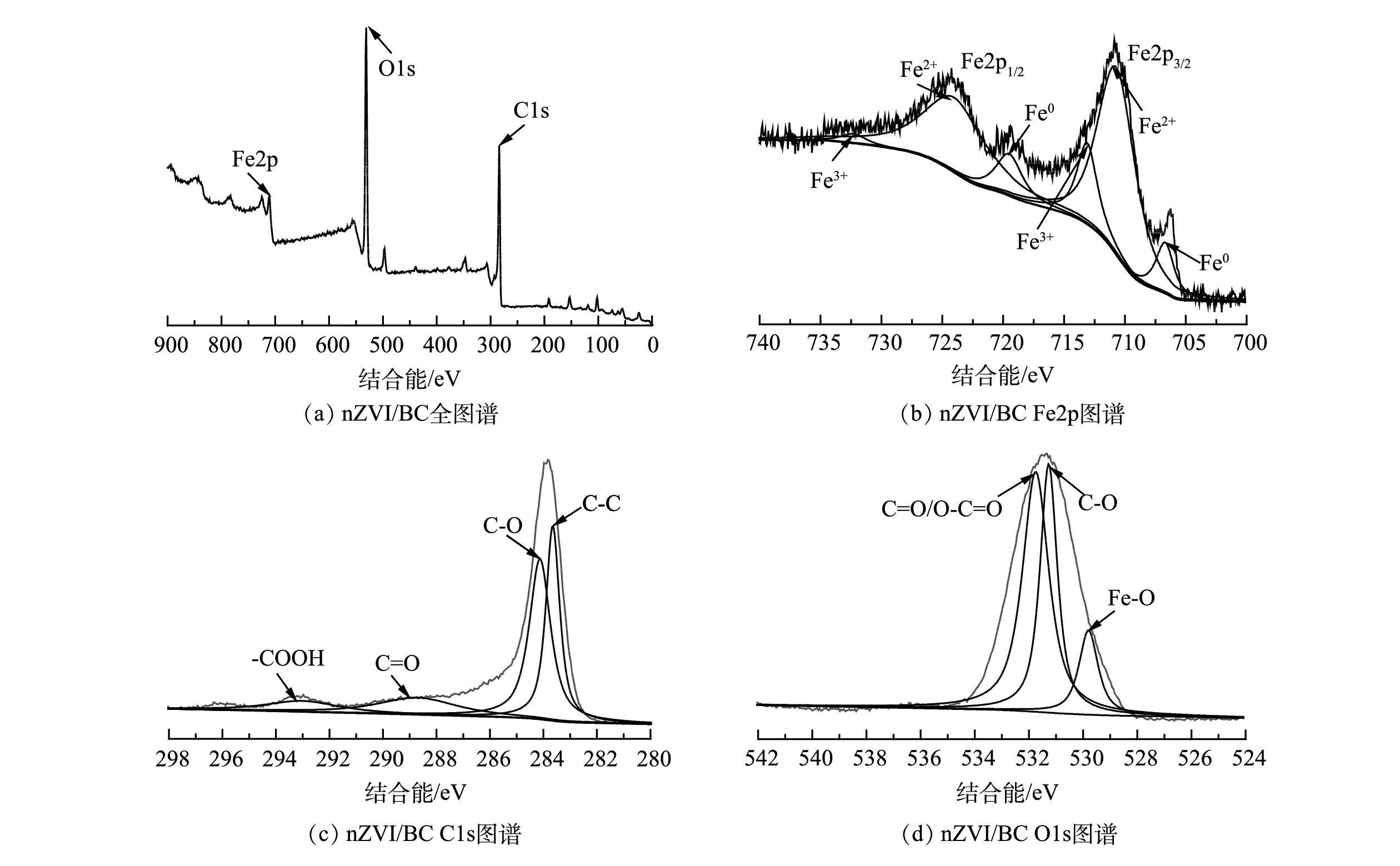
5) XPS分析。由图5(a)可见,nZVI/BC表面含有Fe、C、O 3种元素。为了进一步确定这3种元素的化学状态,分别对其进行分峰。图5(b)中Fe元素主要存在Fe2p3/2和Fe2p1/2轨道特征峰,其中结合能位于706.5 eV和720.0 eV处的特征峰对应Fe0。这说明nZVI的存在[22]。710.1 eV和724.5 eV处为Fe2+特征峰;713.0 eV和731.2 eV处为Fe3+的特征峰。Fe2+和Fe3+的存在说明在制备材料或存储的过程中nZVI不可避免的被氧化,这也与nZVI本身独特的核壳结构有关[23]。由图5(c)可见,结合能位于293.5、288.8、284.4和283.9 eV处的特征峰分别对应BC中的—COOH、C=O、C—O和C—C官能团[23-24]。如图5(d)所示,O1s被分为3个子峰,531.8、531.1和529.4 eV处的特征峰值分别归因于C=O/O—C=O、C—O和Fe—O的存在[15,25]。这表明nZVI/BC复合材料表面含有大量含氧官能团,并存在一些铁氧化物,这与FTIR的结果一致。以上结果表明nZVI/BC复合材料成功合成。
-
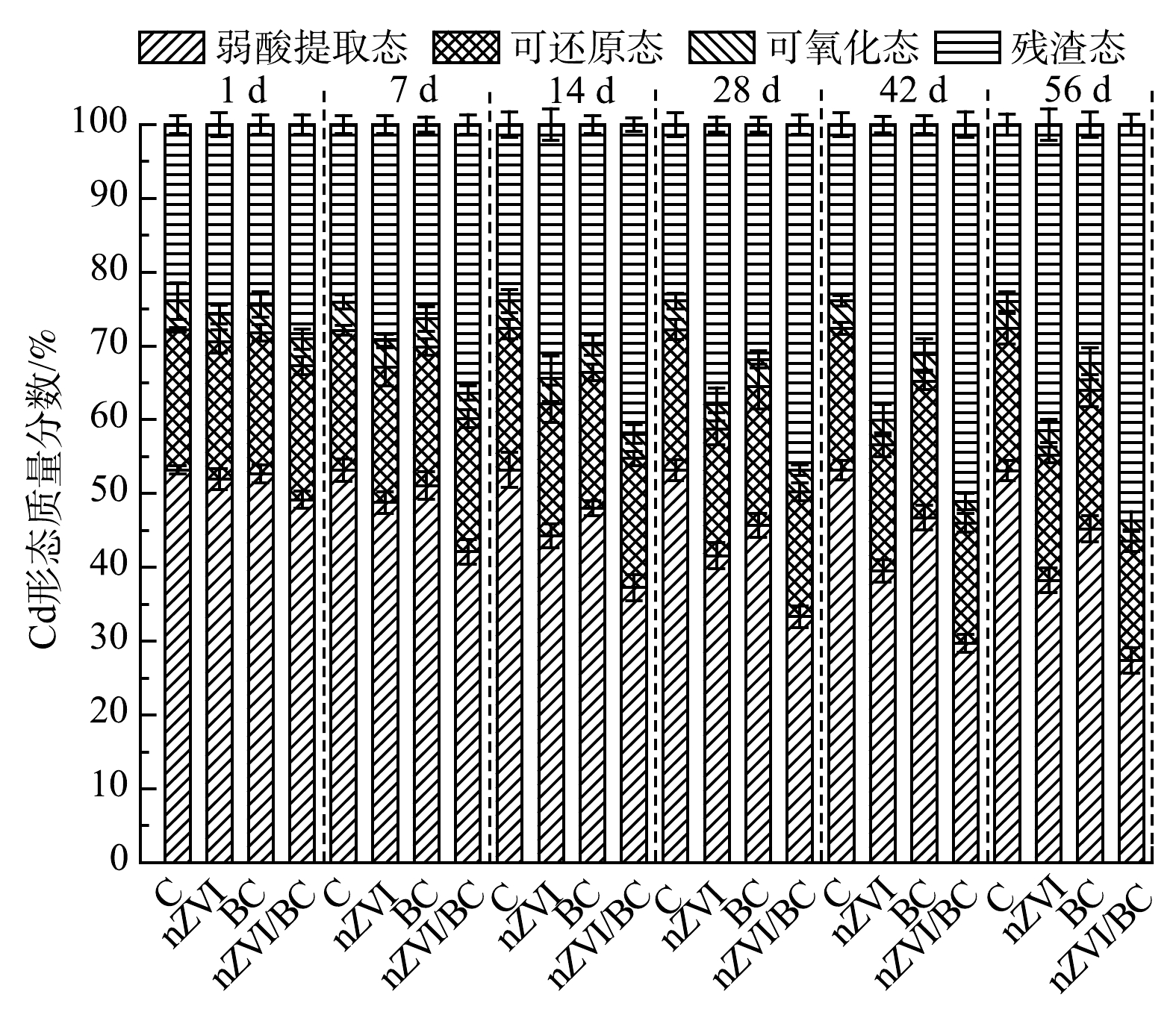
底泥中重金属的毒性和生态风险主要取决于重金属的形态而不是总量,改进的BCR提取法将重金属的形态归为4种,其毒性和移动性大小顺序为弱酸提取态>可还原态>可氧化态>残渣态[12]。由图6可见,原始底泥样品中Cd的弱酸提取态、可还原态、可氧化态和残渣态的质量分数分别为53.19%、19.01%、3.88%和23.92%。根据风险评估准则(RAC),弱酸提取态质量分数高于50%的重金属,属于第5等级,说明底泥中的Cd具有极高风险[26]。对照组中Cd的形态在整个修复过程中仅有轻微波动,侧面说明重金属在自然条件下很难自行稳定。随着修复时间的延长,各处理组中Cd的残渣态质量分数逐渐增加,其余3种形态的质量分数逐渐降低并趋于稳定。56 d后,nZVI、BC和nZVI/BC处理组中Cd的残渣态质量分数与对照组相比分别增加了17.53%、8.44%和29.73%,Cd的弱酸提取态质量分数分别降低了19.41%、7.91%和25.77%。相比于弱酸提取态,Cd的可还原态(Cd与铁锰氧化物结合)和可氧化态(Cd与硫化物和有机物结合)的质量分数的降低程度较小。这主要归因于以下2点:一方面是由于这些结合形态的Cd相对较为稳定;另一方面是可还原态和可氧化态Cd需要先经过强酸的提取,转化为酸溶态,进而才能被材料固定[15]。56 d后,相比于对照组,nZVI/BC处理组中Cd的可还原态和可氧化态质量分数分别降低了3.07%和0.89%。以上结果表明,nZVI、BC和nZVI/BC 3种材料均能使Cd从不稳定的形态逐渐转化成稳定的形态,有效降低了Cd的移动性[14]。其中,nZVI/BC对Cd的稳定效果最好。
目前,有研究采用其他材料修复底泥中的Cd。WEN等[27]采用改性沸石来稳定底泥中的Cd,一定时间后Cd的残渣态质量分数增加了8.3%。LI等[14]利用氧化铁对Cd污染的底泥进行修复56 d后,Cd的最大残渣态质量分数增加了14.94%。ZHANG等[28]发现纳米羟基磷灰石能高效固定底泥中的Cd,且经过14 d的修复,Cd的残渣态质量分数从29.1%(无添加)增加至41.8%(10%添加)。与以上研究相比,本研究所制备的nZVI/BC对底泥中Cd的稳定效果更好。
-
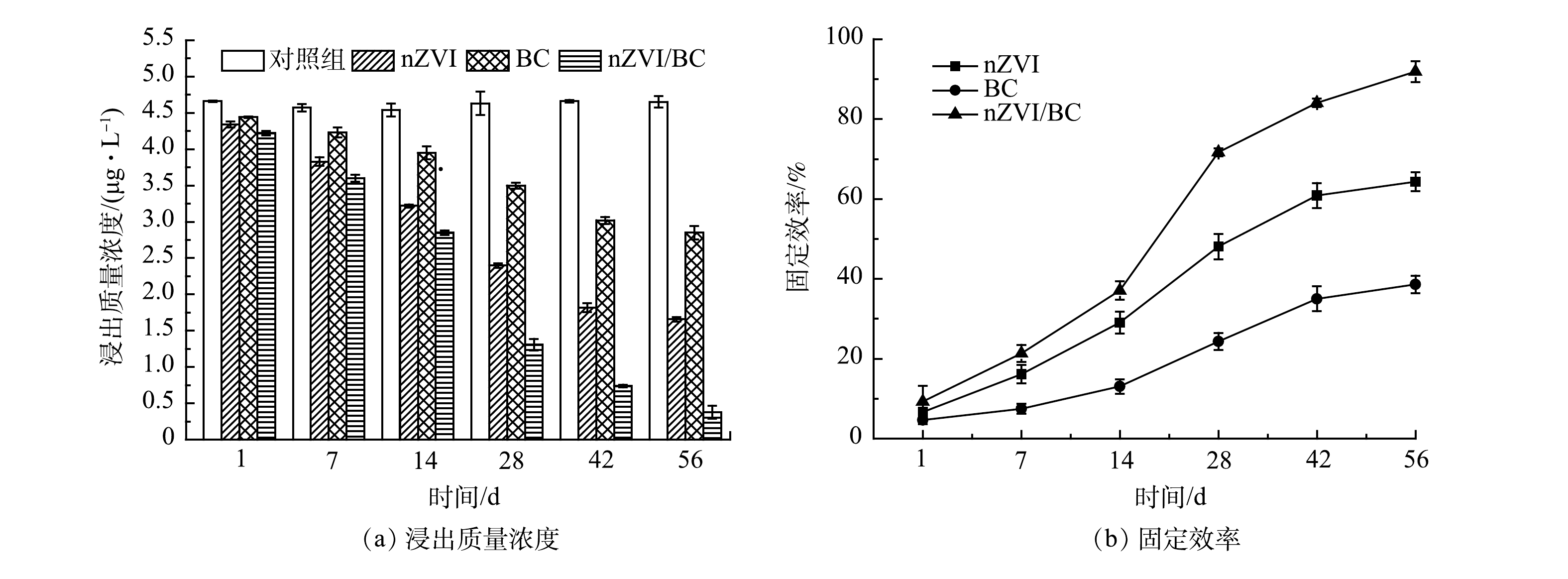
浸出毒性实验(TCLP)可直接反映出底泥Cd的溶出性能[12]。nZVI、BC和nZVI/BC添加后底泥Cd浸出质量浓度的变化及其对Cd的固定效率如图7所示。由图7(a)可以看出,经过材料的修复,底泥样品中Cd浸出质量浓度均呈逐渐降低的趋势。56 d后,nZVI、BC和nZVI/BC处理组中Cd浸出质量浓度分别为1.66、2.85和0.38 μg·L−1,较对照组中Cd浸出质量浓度分别减少了2.99、1.80和4.27 μg·L−1。根据浸出质量浓度,评估了材料对Cd的固定效率。由图7(b)可见,随着时间的延长,3种材料对Cd的固定效率均逐渐增加,其顺序为nZVI/BC>nZVI>BC。56 d后,nZVI、BC和nZVI/BC对Cd的固定效率分别达到了64.31%、38.60%和91.83%。以上结果表明,3种材料均能有效降低Cd的浸出质量浓度并高效固定Cd,进而降低了底泥中Cd的溶出性和环境风险。
-
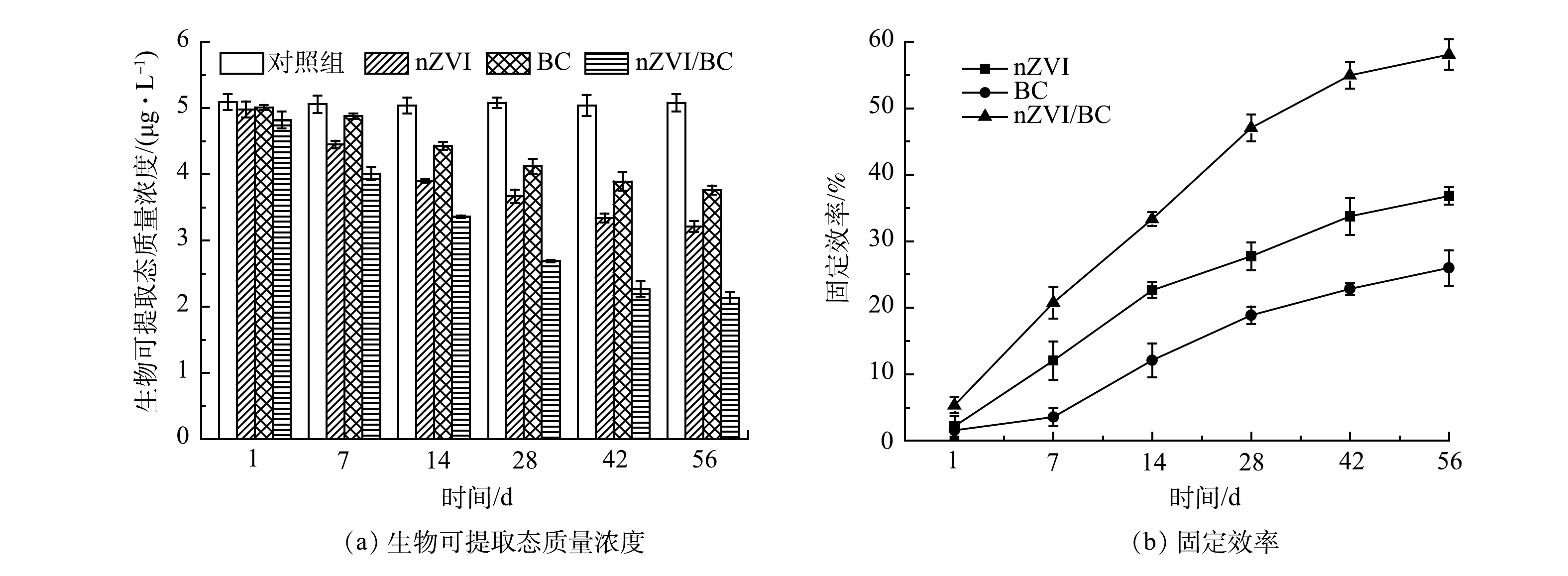
生理提取实验(PBET)主要用于评估重金属的生物可利用性,即指重金属进入生物体内并被利用的难易程度,可反映出重金属对生物体的毒性大小[14]。从图8(a)可以看出,对照组中Cd的生物可提取态质量浓度基本维持在5.06 μg·L−1,各处理组中Cd的生物可提取态质量浓度在整个修复过程中均呈逐渐降低的趋势。由图8可见,56 d后,nZVI、BC和nZVI/BC处理组中Cd的生物可提取态质量浓度由5.08 μg·L−1分别降低到3.21、3.76和2.13 μg·L−1,其固定效率分别为36.81%、25.98%和58.07%。其中nZVI/BC对Cd的固定效果最好,固定效率的大小与TCLP结果一致。以上结果表明,材料的添加有效降低了Cd生物可利用性,有助于减少Cd对底栖生物体的毒害作用。此外,也可以发现Cd的生物可提取态质量浓度要大于浸出质量浓度,这主要是由于PBET所用提取液的pH更低,Cd更容易被提取出来,使得Cd的生物可提取态质量浓度要高一些。这与CHOU等[29]研究结果一致。
-
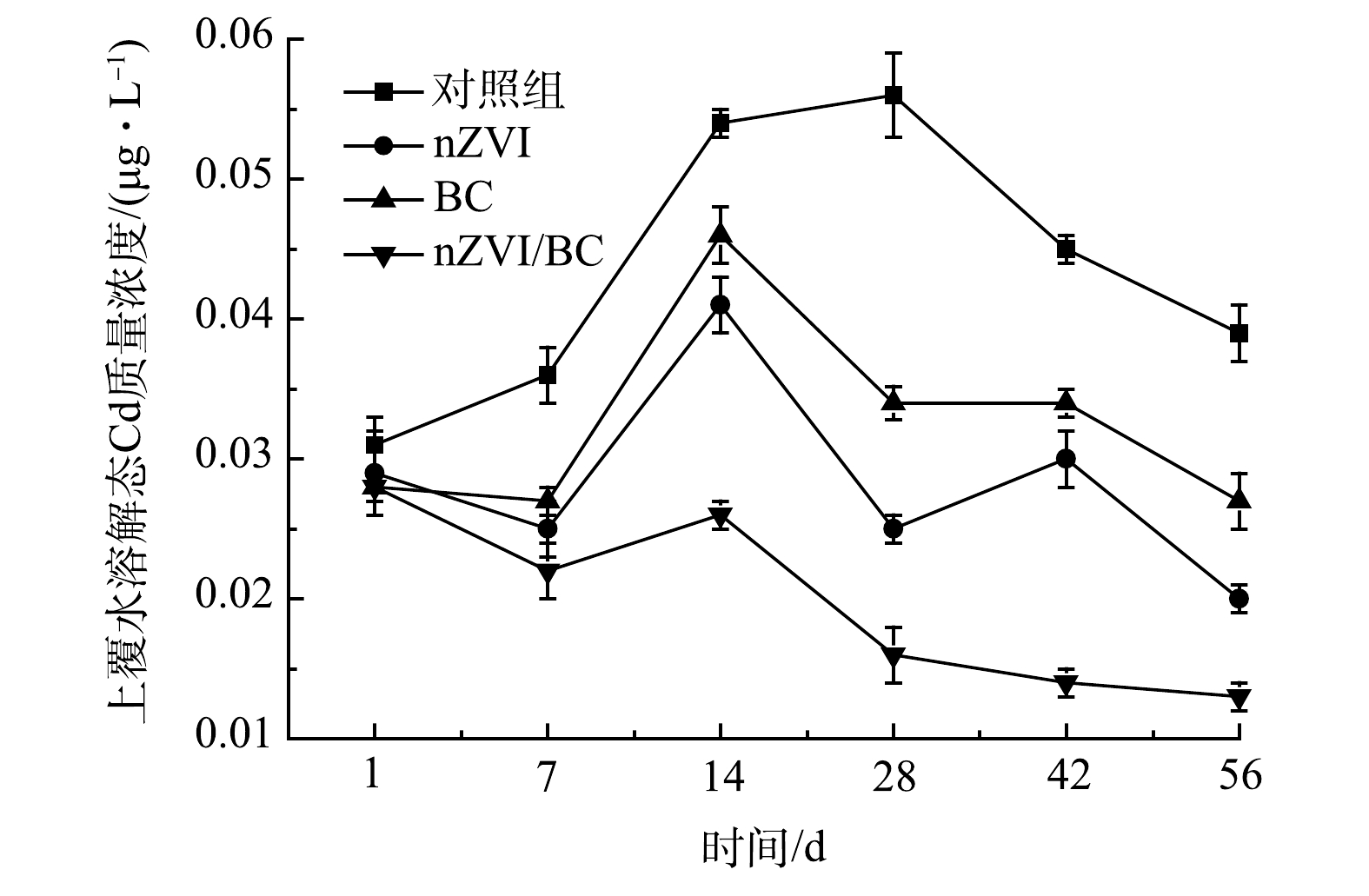
上覆水中溶解态重金属质量浓度的变化直接影响水体生态系统的健康[30]。修复过程中上覆水溶解态Cd质量浓度的变化如图9所示。可以看出,对照组上覆水中溶解态Cd质量浓度在整个修复过程中呈现先上升后降低的趋势。其原因可能是底泥作为主要的内源污染,本身就有向水体释放污染物的趋势。此外,对照组和处理组的底泥样品在实验设置时均进行了摇晃,造成Cd的释放。nZVI、BC和nZVI/BC处理组上覆水中溶解态Cd质量浓度虽在14 d时也有一定上升,但在整个修复过程中其浓度均低于对照组。14 d后各处理组上覆水中溶解态Cd质量浓度逐渐下降,可能是位于泥水界面处的材料对上覆水中溶解态Cd的吸附络合作用[13]。此外,悬浮于上覆水中的溶解态Cd一般与泥沙颗粒吸附在一起,随着修复时间的延长,因其密度大于水,进而会逐渐沉降下来[1]。56 d后,与对照组相比,nZVI、BC和nZVI/BC分别使上覆水中溶解态Cd质量浓度降低48.05%、29.87%和67.53%,说明3种材料均对底泥Cd释放起到抑制作用,其中nZVI/BC对底泥Cd释放抑制效果最好,同时,降低上覆水中溶解态Cd质量浓度也能有效缓解其对水体环境的生态毒性。
-
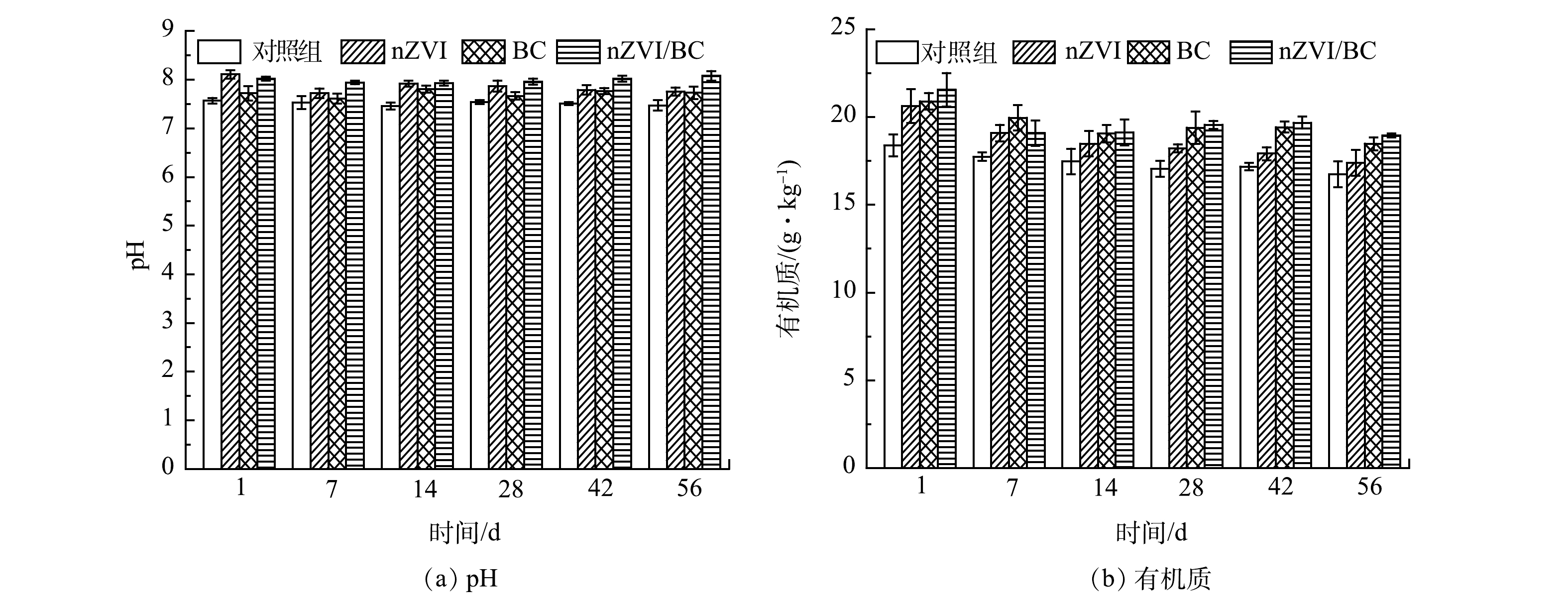
pH对重金属的移动性具有一定影响,如较低的pH会减少底泥颗粒所带的负电荷,从而降低其对Cd的吸附能力,进而增加底泥中Cd的流动性[14]。如图10(a)所示,对照组pH在整个修复过程中有轻微波动,基本维持在7.50左右,且各处理组的pH均高于对照组。56 d后,nZVI、BC和nZVI/BC处理组pH由7.47分别上升至7.76、7.73和8.08。有研究表明,当环境中pH≥8时,Cd的羟基物质开始形成,如CdOH+、Cd2(OH)3+和Cd(OH)2沉淀[31]。此时,nZVI/BC材料表面带有更多的负电荷,Cd羟基物质会被吸附在BC或铁的氧化壳表面,进而降低了Cd的移动性[31]。通过上述结果可以发现,材料的添加均增加了底泥样品pH,其中nZVI/BC在提高底泥pH作用上效果更为明显。nZVI使底泥pH增加的原因在于本身的氧化所致,Fe0可与H2O和O2反应生成Fe2+和OH−[5-6];BC增加底泥pH的原因是由于BC具有丰富的碱性基团和碳酸盐,可以中和底泥酸度,进而导致底泥的pH增加[16,21]。此外,底泥pH的升高与有机质的微生物降解过程也有关,因有机质被微生物分解后会释放出二氧化碳和水,也就是碳酸,这样就会有少量的氢离子流失,进而造成pH上升[36]。pH的升高能增加底泥样品颗粒或矿物表面的电负性,可促进Cd的吸附络合,从而提高对Cd的吸附性能[32]。
有机质可以与重金属形成络合物进而降低重金属的可迁移性,同时,有机质也是微生物生命活动所需的能量和养分来源[15]。从图10(b)可以看出,对照组和各处理组有机质的含量在整个修复过程中逐渐降低并趋于稳定,且各处理组有机质含量均高于对照组。56 d后,对照组、nZVI、BC和nZVI/BC处理组有机质含量分别为16.73、17.38、18.47和18.94 g·kg−1,相比于对照组,nZVI/BC处理组的有机质含量高出1.13倍。相较于对照组,nZVI处理组有机质含量的增加可能是nZVI释放出的Fe离子与底泥固相中的有机质结合形成水溶性化合物所致[33]。BC由小麦秸秆这种有机物质高温裂解制备,本身具有丰富的碳,因而可通过碳封存来增加底泥有机质含量[34]。此外,也有研究表明,BC自身结构中含有部分溶解性有机质[37]。随着修复时间的延长,由于nZVI、BC和nZVI/BC对Cd较好的稳定效果,将可移动态的Cd逐步转化为残渣态,降低了Cd的生物可利用性,促进了某些细菌菌群的增长,进而加大了对有机质的分解利用[15]。以上结果表明,材料的添加均增加了底泥样品中水溶性有机质的含量,有利于重金属的稳定。
-
nZVI、BC和nZVI/BC的添加促使Cd由不稳定形态转化为稳定形态,均增加了Cd的残渣态质量分数,降低了Cd的迁移性。同时,3种材料的添加均降低了Cd的浸出毒性、生物可利用性和上覆水中溶解态Cd的质量浓度,表明nZVI、BC和nZVI/BC对底泥中Cd具有较好的固定效果。本研究中,3种材料对Cd的固定效率大小均为nZVI/BC>nZVI>BC。材料对Cd的稳定机制可能分为2个方面。一方面,游离的Cd被吸附在材料表面(nZVI、BC或nZVI/BC),并与nZVI表面的铁氢氧化物(FeOOH)发生络合反应(FeOCdOH)进而被固定[17]。FRANCIS等[35]发现Cd与FeOOH的结合力很强,几乎没有Cd能被提取出来。此外,BC的多孔结构和丰富的含氧官能团也对Cd具有较强吸附络合能力,如—OH等基团能增加BC表面的负电荷,增强其对Cd的吸附性能,C=O可与Cd发生络合反应[30]。另一方面,材料的添加增加了底泥样品的pH和有机质,相应的原因已在2.6小节进行了分析,pH和有机质的增加也可促进底泥中Cd的稳定。
-
1)制备所得的nZVI/BC复合材料与nZVI相比,分散性更好,且BC具有丰富的孔隙结构和大量含氧官能团,对湖泊底泥中重金属Cd具有更好的稳定性能。
2) nZVI、BC和nZVI/BC的添加均可显著降低底泥中Cd的弱酸提取态质量分数及增加残渣态质量分数,Cd的可还原态和可氧化态质量分数降低的程度较小。其中,nZVI/BC处理组中Cd的弱酸提取态质量分数最大降低了25.77%,表明nZVI/BC对Cd具有较好的稳定效果,可促使Cd向稳定形态的转化。
3) nZVI/BC的添加可有效降低底泥中Cd的浸出质量浓度、生物可提取态质量浓度和上覆水中溶解态Cd质量浓度,进而可降低底泥中Cd的环境风险及对底栖生物体和水体环境的生态毒性。
4) nZVI、BC和nZVI/BC的添加均可增加底泥pH和有机质含量,其中nZVI/BC处理组中pH和有机质含量分别增加了0.61和2.21 g·kg−1。材料对Cd的稳定机制包括材料本身对Cd的吸附络合作用及底泥pH和有机质的增加。
生物炭负载纳米零价铁对湖泊底泥镉污染的修复效果
Remediation effect of cadmium pollution in lake sediment by biochar-supported nanoscale zero-valent iron
-
摘要: 制备了生物炭负载纳米零价铁(nZVI/BC)复合材料,采用SEM、TEM、FTIR、EDS、XPS等手段对其表面形貌、粒径大小、官能团结构、表面元素及化学形态进行了表征和分析。通过镉(Cd)形态、浸出毒性、生物可利用性、上覆水中溶解态Cd质量浓度及底泥理化性质等指标评价了nZVI/BC对湖泊底泥中Cd的修复效果。结果表明,相比于对照组,经56 d修复后nZVI/BC处理组中Cd的残渣态质量分数增加了29.73%,有效降低了Cd的移动性;Cd的浸出质量浓度从4.65 μg·L−1降至0.38 μg·L−1,Cd的生物可提取态质量浓度从5.08 μg·L−1降至2.13 μg·L−1,其浸出质量浓度和生物可提取态质量浓度分别下降了91.83%和58.07%。同时,经过56 d的修复,nZVI/BC使上覆水中溶解态Cd的质量浓度比对照组降低了67.53%。此外,nZVI/BC的添加提高了底泥的pH和有机质,根据这2个指标的变化进一步分析了nZVI/BC复合材料对Cd的稳定机制。以上研究结果可为重金属污染底泥的修复提供参考。Abstract: Biochar-supported nano zero-valent iron (nZVI/BC) composites were prepared, and its surface morphology, particle size, functional group structure, surface elements and chemical morphology were characterized and analyzed by SEM, TEM, FTIR, EDS and XPS. The remediation effect of cadmium (Cd) in lake sediment by nZVI/BC was evaluated by Cd speciation, leaching toxicity, bioavailability, dissolved mass concentration of Cd in overlying water and physical and chemical properties of sediment. The results showed that after 56 d remediation, the residual mass speciation of Cd in nZVI/BC treatment group increased by 29.73% in comparison with the control group, which effectively reduced the mobility of Cd. The Cd leaching mass concentration decreased from 4.65 μg·L−1 to 0.38 μg·L−1, and the Cd bioextractable mass concentration decreased from 5.08 μg·L−1 to 2.13 μg·L−1, and the leaching mass concentration and bioextractable mass concentration of Cd decreased by 91.83% and 58.07%, respectively. At the same time, after 56 d remediation, nZVI/BC reduced the mass concentration of dissolved Cd in the overlying water by 67.53% in comparison with the control group. Besides, the addition of nZVI/BC increased the pH and organic matter of the sediment. According to the changes of these two indexes, the stability mechanism of nZVI/BC composites to Cd was further analyzed. The above results can provide a reference for remediation of heavy metal contaminated sediment.
-
Key words:
- nanoscale zero-valent iron /
- biochar /
- lake sediment /
- cadmium speciation /
- pollution remediation /
- mechanism analysis
-
-
[1] SUN X, FAN D, LIU M, et al. Budget and fate of sedimentary trace metals in the Eastern China marginal seas[J]. Water Research, 2020, 187: 116439. doi: 10.1016/j.watres.2020.116439 [2] CHEN J, LIU J, HONG H, et al. Coastal reclamation mediates heavy metal fractions and ecological risk in saltmarsh sediments of northern Jiangsu Province, China[J]. Science of the Total Environment, 2022, 825: 154028. doi: 10.1016/j.scitotenv.2022.154028 [3] LI Y, CHEN M, GONG J, et al. Effects of virgin microplastics on the transport of Cd (II) in Xiangjiang River sediment[J]. Chemosphere, 2021, 283: 131197. doi: 10.1016/j.chemosphere.2021.131197 [4] GHOSH U, LUTHY R G, CORNELISSEN G, et al. In-situ sorbent amendments: a new direction in contaminated sediment management[J]. Environmental Science & Technology, 2011, 45(4): 1163-1168. [5] CAI C Y, ZHAO M H, YU Z, et al. Utilization of nanomaterials for in-situ remediation of heavy metal(loid) contaminated sediments: A review[J]. Science of the Total Environment, 2019, 662: 205-217. doi: 10.1016/j.scitotenv.2019.01.180 [6] ZOU Y, WANG X, KHAN A, et al. Environmental remediation and application of nanoscale zero-valent iron and its composites for the removal of heavy metal ions: A Review[J]. Environmental Science & Technology, 2016, 50(14): 7290-7304. [7] ZHOU S, NI X, ZHOU H L, et al. Effect of nZVI/biochar nanocomposites on Cd transport in clay mineral-coated quartz sand: Facilitation and rerelease[J]. Ecotoxicology and Environmental Safety, 2021, 228: 112971. doi: 10.1016/j.ecoenv.2021.112971 [8] FAN H, REN H, MA X, et al. High-gravity continuous preparation of chitosan-stabilized nanoscale zero-valent iron towards Cr(VI) removal[J]. Chemical Engineering Journal, 2020, 390: 124639. doi: 10.1016/j.cej.2020.124639 [9] YU J, DEEM L M, CROW S E, et al. Comparative metagenomics reveals enhanced nutrient cycling potential after two years of biochar amendment in a tropical oxisol[J]. Applied and Environmental Microbiology, 2019, 85: 2957. [10] LEE H S, SHIN H S. Competitive adsorption of heavy metals onto modified biochars: Comparison of biochar properties and modification methods[J]. Journal of Environmental Management, 2021, 299: 113651. doi: 10.1016/j.jenvman.2021.113651 [11] WANG M M, ZHU Y, CHENG L R, et al. Review on utilization of biochar for metal-contaminated soil and sediment remediation[J]. Journal of Environmental Sciences, 2018, 63: 156-173. doi: 10.1016/j.jes.2017.08.004 [12] WAN J, ZHANG C, ZENG G M, et al. Synthesis and evaluation of a new class of stabilized nano-chlorapatite for Pb immobilization in sediment[J]. Journal of Hazardous Materials, 2016, 320: 278-288. doi: 10.1016/j.jhazmat.2016.08.038 [13] ZHAO Q, LI X M, XIAO S T, et al. Integrated remediation of sulfate reducing bacteria and nano zero valent iron on cadmium contaminated sediments[J]. Journal of Hazardous Materials, 2021, 406(11): 124680. [14] LI X C, YANG Z Z, ZHANG C, et al. Effects of different crystalline iron oxides on immobilization and bioavailability of Cd in contaminated sediment[J]. Chemical Engineering Journal, 2019, 373: 307-317. doi: 10.1016/j.cej.2019.05.015 [15] XUE W J, CAO S, ZHU J, et al. Stabilization of cadmium in contaminated sediment based on a nanoremediation strategy: Environmental impacts and mechanisms[J]. Chemosphere, 2022, 287(3): 132363. [16] WANG S S, ZHAO M Y, ZHOU M, et al. Biochar-supported nZVI (nZVI/BC) for contaminant removal from soil and water: A critical review[J]. Journal of Hazardous Materials, 2019, 373: 820-834. doi: 10.1016/j.jhazmat.2019.03.080 [17] HUANG D L, XUE W J, ZENG G M, et al. Immobilization of Cd in river sediments by sodium alginate modified nanoscale zero-valent iron: Impact on enzyme activities and microbial community diversity[J]. Water Research, 2016, 106: 15-25. doi: 10.1016/j.watres.2016.09.050 [18] TANG J C, ZHAO B B, LYU H H, et al. Development of a novel pyrite/biochar composite (BM-FeS2@BC) by ball milling for aqueous Cr(VI) removal and its mechanisms[J]. Journal of Hazardous Materials, 2021, 413: 125415. doi: 10.1016/j.jhazmat.2021.125415 [19] 李长欣, 吕严凤, 张梦迪, 等. 热解条件对茶叶渣生物炭特性及镉污染土壤钝化效果的影响[J]. 环境工程学报, 2017, 11(12): 6504-6510. doi: 10.12030/j.cjee.201703050 [20] GAO L, LI Z H, YI W M, et al. Quantitative contribution of minerals and organics in biochar to Pb(II) adsorption: Considering the increase of oxygen-containing functional groups[J]. Journal of Cleaner Production, 2021, 325: 129328. doi: 10.1016/j.jclepro.2021.129328 [21] YANG Y Y, YE S J, ZHANG C, et al. Application of biochar for the remediation of polluted sediments[J]. Journal of Hazardous Materials, 2021, 404: 124052. doi: 10.1016/j.jhazmat.2020.124052 [22] TANG L, FENG H P, TANG J, et al. Treatment of arsenic in acid wastewater and river sediment by Fe@Fe2O3 nanobunches: The effect of environmental conditions and reaction mechanism[J]. Water Research, 2017, 117: 175-186. doi: 10.1016/j.watres.2017.03.059 [23] WANG J, DENG Z L, FENG T, et al. Nanoscale zero-valent iron (nZVI) encapsulated within tubular nitride carbon for highly selective and stable electrocatalytic denitrification[J]. Chemical Engineering Journal, 2021, 417: 129160. doi: 10.1016/j.cej.2021.129160 [24] FAN Y X, HUANG L L, WU L G, et al. Adsorption of sulfonamides on biochars derived from waste residues and its mechanism[J]. Journal of Hazardous Materials, 2020, 406: 124291. [25] KHAN Z H, GAO M L, WU J J, et al. Mechanism of As(III) removal properties of biochar-supported molybdenum- disulfide/iron-oxide system[J]. Environmental Pollution, 2021, 287: 117600. doi: 10.1016/j.envpol.2021.117600 [26] 罗松英, 邢雯淋, 梁绮霞, 等. 湛江湾红树林湿地表层沉积物重金属形态特征、生态风险评价及来源分析[J]. 生态环境学报, 2019, 28(2): 348-358. [27] WEN J, YI Y J, ZENG G M. Effects of modified zeolite on the removal and stabilization of heavy metals in contaminated lake sediment using BCR sequential extraction[J]. Journal of Environmental Management, 2016, 178: 63-69. doi: 10.1016/j.jenvman.2016.04.046 [28] ZHANG Z Z, LI M Y, CHEN W, et al. Immobilization of lead and cadmium from aqueous solution and contaminated sediment using nano-hydroxyapatite[J]. Environmental Pollution, 2010, 158(2): 514-519. doi: 10.1016/j.envpol.2009.08.024 [29] CHOU J D, WEY M Y, LIANG H H, et al. Biotoxicity evaluation of fly ash and bottom ash from different municipal solid waste incinerators[J]. Journal of Hazardous Materials, 2009, 168(1): 197-202. doi: 10.1016/j.jhazmat.2009.02.023 [30] 孟梅, 华玉妹, 朱端卫, 等. 生物炭对重金属污染沉积物的修复效果[J]. 环境化学, 2016, 35(12): 2543-2552. doi: 10.7524/j.issn.0254-6108.2016.12.2016042803 [31] BOPARAI H K, JOSEPH M, O’CARROLL D M. Cadmium (Cd2+) removal by nano zerovalent iron: surface analysis, effects of solution chemistry and surface complexation modeling[J]. Environmental Science and Pollution Research, 2013, 20(9): 6210-6221. doi: 10.1007/s11356-013-1651-8 [32] SHEN B B, WANG X M, ZHANG Y, et al. The optimum pH and Eh for simultaneously minimizing bioavailable cadmium and arsenic contents in soils under the organic fertilizer application[J]. Science of the Total Environment, 2019, 711: 135229. [33] ZHOU D M, JIN S Y, WANG Y J, et al. Assessing the impact of iron-based nanoparticles on pH, dissolved organic carbon, and nutrient availability in soils[J]. Journal of Soil Contamination, 2012, 21(1): 101-114. doi: 10.1080/15320383.2012.636778 [34] 汤家喜, 李玉, 朱永乐, 等. 生物炭与膨润土对辽西北风沙土理化性质的影响研究[J]. 干旱区资源与环境, 2022, 36(3): 143-150. [35] FRANCIS A J, DODGE C J. Anaerobic microbial remobilization of toxic metals coprecipitated with iron oxide[J]. Environmental Science & Technology, 1990, 24(3): 373-378. [36] CHEN Y, JIANG X, XIAO K. Enhanced volatile fatty acids (VFAs) production in a thermophilic fermenter with stepwise pH increase-Investigation on dissolved organic matter transformation and microbial community shift[J]. Water Research, 2017, 112: 261-268. doi: 10.1016/j.watres.2017.01.067 [37] CEN R, FENG W, YANG F, et al. Effect mechanism of biochar application on soil structure and organic matter in semi-arid areas[J]. Journal of Environmental Management, 2021, 286: 112198. doi: 10.1016/j.jenvman.2021.112198 -




 下载:
下载: